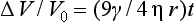Ceramics
CLASSIFICATION OF DENTAL CERAMICS
SINTERED ALL-CERAMIC MATERIALS
Leucite-Reinforced Feldspathic Porcelain
Heat-Pressed All-Ceramic Materials
GENERAL APPLICATIONS OF CERAMICS IN RESTORATIVE DENTISTRY
MECHANICAL AND THERMAL PROPERTIES OF DENTAL CERAMICS
The term ceramic refers to any product made essentially from a nonmetallic inorganic material usually processed by firing at a high temperature to achieve desirable properties. The more restrictive term porcelain refers to a specific compositional range of ceramic materials made by mixing kaolin, quartz, and feldspar, and firing at high temperature. Dental ceramics for ceramic-metal restorations belong to this compositional range and are commonly referred to as dental porcelains.
The laboratory portion of a ceramic restoration is usually made in a commercial dental laboratory by a skilled technician working with specialized equipment to the shape and shade specifications provided by the dentist. Skilled technicians and artisans are also employed by the manufacturers of artificial denture teeth to produce the many forms, types, and shades necessary in this application of porcelain.
HISTORICAL BACKGROUND
Dental ceramics were first used in dentistry in the late 1700s. Porcelain jacket crowns were developed in the early 1900s. They consisted of feldspathic or aluminous porcelain baked on a thin platinum foil and can be considered the ancestors of all-ceramic crowns. However, because of their low strength, porcelain jacket crowns were limited to anterior teeth. The poor match in thermal expansion (and contraction) between framework alloys and veneering ceramics, which often led to failures and fractures upon cooling, stimulated the development of leucite-containing feldspathic porcelains in the 1960s. The problem was solved by mixing controlled amounts of high-expansion leucite with feldspar glass at the manufacturing stage. This allowed the adjustment of the coefficient of thermal expansion of feldspathic porcelains to very narrow specifications. This invention led to considerable improvement in the reliability of ceramic metals and allowed ceramic materials to be veneered on a metal framework. During cooling, the thermal contraction of the metal framework is slightly higher than that of the veneering ceramic, thus placing the internal surface of the ceramic in compression. Because ceramics are stronger in compression than in tension, this property is used to advantage to provide increased resistance to shattering.
The end of the twentieth century saw the introduction of several innovative systems for fabricating all-ceramic dental restorations. The first was a castable glass-ceramic system in which the restoration was cast using the lost-wax technique and later heat-treated to promote its transformation into a glass-ceramic. This castable system was later abandoned because of processing difficulties and the high incidence of fractures. Ceramics for slip-casting, heat-pressing, and machining were developed concurrently within the past 20 years. New materials for all-ceramic restorations are introduced every year and attest to the increasing popularity of ceramics in dentistry.
CLASSIFICATION OF DENTAL CERAMICS
Dental ceramics can be classified according to their fusion temperature, application, fabrication technique, or crystalline phase (Table 18-1).
TABLE 18-1
Classification of Dental Ceramic Materials with Examples of Products and their Manufacturers

FUSION TEMPERATURE
This classification dates back to the early 1940s. The high-fusing ceramics have a fusing range from 1315° to 1370° C; the medium-fusing ceramics, from 1090° to 1260° C; and the low-fusing ceramics, from 870° to 1065° C. It was employed more intensively with the earlier dental ceramic compositions, which belong to the triaxial porcelain compositions. The term triaxial means the composition has three major ingredients: quartz (or flint), feldspar, and clay (or kaolin). The fusion temperature is dictated by the relative amounts of these three ingredients. It is interesting to note that these temperature ranges vary from textbook to textbook and are not continuous. This is related to the fact that the processing of ceramic materials was initially monitored using pyrometric ceramic cones of specific composition. These cones are placed in the furnace and bend under their own weight when the temperature is reached. The medium- and high-fusing ceramics are used for denture teeth. Ultra-low-fusing dental ceramics with firing temperatures below 870° C have been recently introduced.
The medium- and low-fusing porcelain powders are usually modified by the manufacturer with chemicals or fluxes (boron oxide or alkali carbonates) of low melting temperature. They are melted together (prefused) and reduced to powder form. The addition of fluxing agents results in narrower fusing ranges and increases the tendency for the porcelain to slump during repair or when making additions, staining, or glazing. Prefusing and regrinding, however, increase the homogeneity of the powder, which may be an advantage in the handling and fusing operations.
High-fusing porcelains are considered superior in strength, insolubility, translucency, and maintenance of accuracy in form during repeated firings. Recent tests of low-fusing products, however, indicate that they are essentially as strong as the high-fusing types, and their solubility and translucency are adequate. The principal practical advantage of high-fusing porcelain is therefore its ability to be repaired, added to, stained, or glazed without distortion.
APPLICATIONS
Ceramics have three major applications in dentistry: (1) ceramics for ceramic-metal crowns and fixed partial dentures, (2) all-ceramic crowns, inlays, onlays, and veneers, when esthetics is a priority, and (3) ceramic denture teeth.
FABRICATION TECHNIQUE
This classification is summarized in Table 18-1, which also includes examples of commercial ceramics and their manufacturers. One of the most common fabrication techniques for dental ceramics is called sintering. Sintering is the process of heating the ceramic to ensure densification. This occurs by viscous flow when the firing temperature is reached. Ceramics fired to metals are processed by sintering, whereas all-ceramic materials encompass a wider range of processing techniques, including slip-casting, heat-pressing, and machining.
CRYSTALLINE PHASE
Regardless of their applications or fabrication technique, after firing, dental ceramics are composed of two phases: a glassy (or vitreous) phase surrounding a crystalline phase. Depending on the nature and the amount of crystalline phase present, the mechanical and optical properties of dental ceramics vary widely. Increasing the amount of glassy phase lowers the resistance to crack propagation but increases translucency. Materials for all-ceramic restorations have increased amounts of crystalline phase (between 35% and 99%) for better mechanical properties. Table 18-1 lists the various crystalline phases found in dental ceramics.
CERAMIC-METAL RESTORATIONS
Ceramic-metal restorations consist of a cast metallic framework (or core) on which at least two layers of ceramic are baked. The first layer applied is the opaque layer, consisting of ceramic, rich in opacifying oxides. Its role is to mask the darkness of the oxidized metal framework to achieve adequate esthetics. As the first layer, it also provides ceramic-metal bond. The next step is the buildup of dentin and enamel (most translucent) ceramics to obtain an esthetic appearance similar to that of a natural tooth. Opaque, dentin, and enamel ceramics are available in various shades. After building up or modeling the porcelain powders, the ceramic-metal crown is sintered in a porcelain furnace.
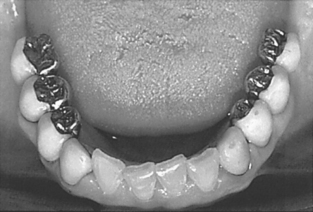
Fig. 18-1 shows a three-unit ceramic-metal fixed partial denture. When fabricated by skilled technicians, these restorations can provide excellent esthetics, along with good strength because of the alloy framework. The alloys used for casting the substructure are usually gold-based containing tin and indium. Gold-palladium, silver-palladium, and nickelchromium alloys were initially developed as lower-cost alternatives. However, the recent steep increase in the price of palladium has changed the palladium-containing alloys into a higher-cost alternative.
It is essential that the coefficient of thermal expansion of the veneering ceramic be slightly lower than that of the alloy to ensure that the ceramic is in slight compression after cooling. This will establish a better resistance to crack propagation of the ceramic-metal restoration. The ceramic-metal systems are described in detail in Chapter 19.
COMPOSITION AND MANUFACTURE
The quality of any ceramic depends on the choice of ingredients, correct proportioning of each ingredient, and control of the firing procedure. Only the purest ingredients are used in the manufacture of dental porcelains because of the stringent requirements of color, toughness without brittleness, insolubility, and translucency, as well as the desirable characteristics of strength and thermal expansion. In many instances, the manufacturer must formulate a product that is a compromise.
In its mineral state, feldspar, the main raw ingredient of classical high-fusing dental porcelains for ceramic-metal restorations, is crystalline and opaque with an indefinite color between gray and pink. Chemically it is designated as potassium aluminum silicate, with a composition of K2O · Al2O3 · 6SiO2. Feldspar melts incongruently at about 1150° C, forming leucite and molten glass.
Iron and mica are commonly found as impurities in feldspar. It is particularly important to remove the iron, because metallic oxides act as strong coloring agents in porcelain. To remove iron, each piece of feldspar is broken with a steel hammer, and only the uniformly light-colored pieces are selected for use in the porcelain. These pieces are ground in mills to a fine powder. The final particle size is carefully controlled by a screening process to remove coarser particles and a flotation process to remove the excessively fine particles. The dry powder is then slowly vibrated down inclined planes equipped with a series of narrow ledges formed by induction magnets. In this way the remaining iron contaminants are separated and removed, and the feldspar is made ready for use.
MANUFACTURE
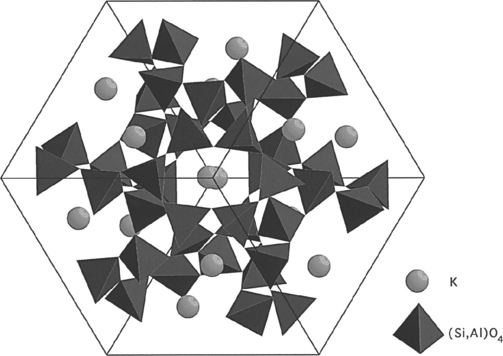
Nowadays, many dental porcelain manufacturers buy feldspar as powder already screened and cleaned from impurities to their specifications. Other raw materials used in the manufacture of dental porcelains are various types of SiO2 in the form of fine powder, alumina and hydrated alumina, as well as alkali and alkaline earth carbonates as fluxes. During the manufacturing process, the ground ingredients are carefully mixed together and heated to about 1200° C in large crucibles. As mentioned earlier, feldspar melts incongruently at about 1150° C to form a glassy phase with an amorphous structure, as illustrated in Fig. 18-2, and a crystalline (mineral) phase consisting of leucite (KAlSi2O6 or K2O · Al2O3 · 4SiO2). The crystalline structure of leucite is tetragonal (Fig. 18-3).
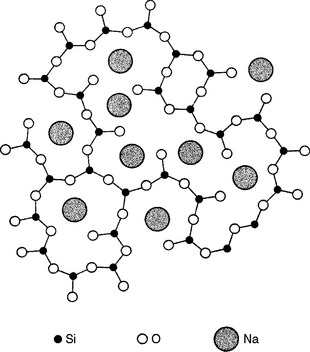
FIGURE 18-2 Two-dimensional structure of sodium silicate glass. (Modified from Warren BE, Biscoe J: J Am Ceram Soc 21:259, 1938.)
The mix of leucite and glassy phase is then cooled very rapidly (quenched) in water which causes the mass to shatter in small fragments. The product obtained, called a frit, is ball-milled to achieve proper particle size distribution. Coloring pigments in small quantities are added at this stage, to obtain the delicate shades necessary to mimic natural teeth. The metallic pigments include titanium oxide for yellow-brown shades, manganese oxide for lavender, iron oxide for brown, cobalt oxide for blue, copper or chromium oxides for green, and nickel oxide for brown. In the past, uranium oxide was used to provide fluorescence; however, because of the small amount of radioactivity, lanthanide oxides (such as cerium oxide) are being substituted for this purpose. Tin, titanium, and zirconium oxides are used as opacifiers.
After the manufacturing process is completed, feldspathic dental porcelain consists of two phases. One is the vitreous (or glass) phase, and the other is the crystalline (or mineral) phase. The glass phase formed during the manufacturing process has properties typical of glass, such as brittleness, nondirectional fracture pattern, translucency, and high surface tension in the fluid state. The crystalline phase is leucite, a potassium aluminosilicate with high thermal expansion (>20 × 10−6/° C). The amount present (10% to 20%) controls the thermal expansion coefficient of the porcelain. Leucite also contributes strength to the ceramic; high-leucite ceramics are approximately twice as strong as those containing low concentrations. The microstructure of conventional feldspathic porcelain is shown in Fig. 18-4; the glassy phase has been lightly acid-etched to reveal the leucite crystals.

FIGURE 18-4 Scanning electron micrograph showing the microstructure of feldspathic porcelain for ceramic-metal restorations. GM, Glassy matrix; LC, leucite crystal.
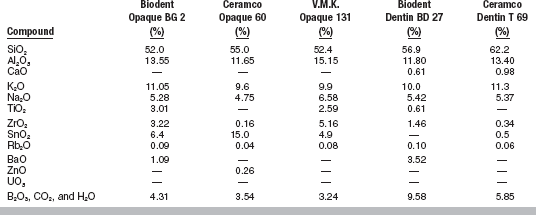
Typical compositions for opaque and dentin porcelain powders are given in Table 18-2.
PROCESSING
The production of a satisfactory ceramic-metal restoration requires careful attention to the principles and details of the operation.
Porcelain Application and Condensation
After the tooth has been prepared, an elastomeric impression is made, and a working model or die is formed of a suitable die material. A wax framework is fabricated and the wax is cut back by 1 mm in the esthetic areas to ensure enough space for porcelain application. The framework is cast by the lost-wax technique. It is extremely important that all sharp angles be rounded to avoid stress concentrations and wedge effects in the fired porcelain. After careful cleaning of the metal framework, a thin layer of opaque porcelain is applied and baked. Dentin porcelain powder, in the shade selected for the body or dentin portion, is mixed with modeling liquid (mainly distilled water) to a creamy consistency and is applied on the opaque layer, with allowances made for shrinkage. To produce minimum shrinkage and a dense, strong porcelain, it is important to achieve a thorough condensation of the particles at this stage. Various means of condensation may be employed. The vibration method is particularly efficient in driving the excess water toward the surface. The wet porcelain mix is applied with a spatula and vibrated gently until the particles settle together. The excess water is then removed with a clean tissue or an absorbent medium. A cross-section of a ceramic-metal crown is shown in Fig. 18-5, right.
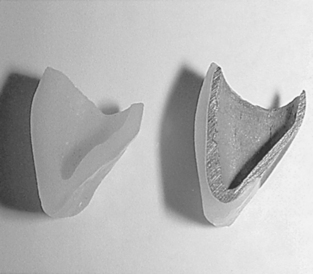
FIGURE 18-5 Cross-sectional micrograph of a ceramic-metal crown (right). Cross-sectional view of an all-ceramic crown (left).
Other methods of condensation include the spatulation and brush techniques. The spatulation method consists of smoothing the wet porcelain with a suitable spatula until the excess water is brought to the surface, where it is absorbed with a tissue. The brush or capillary attraction method depends on the action of dry porcelain powder to remove the excess water by capillary attraction. The dry powder is applied with a brush to a small area of the wet porcelain mass, and as the water is withdrawn toward the dry area, the wet particles are pulled closely together. This process is repeated as dry powder is placed on the opposite side of each new addition of the wet mix.
Drying
After the porcelain mix has been applied and condensed, the restoration is placed in an open preheated porcelain furnace to be dried. This drying stage, which lasts between 5 and 8 minutes, is a very important step; it ensures that any remaining excess water is removed from the porcelain mix. During the drying stage, the water diffuses toward the surface and then evaporates. If the piece is dried too quickly, the water evaporation rate becomes greater than the diffusion rate and the unfired porcelain can undergo spontaneous breakage. After the mass is placed in the furnace, remaining free and combined water is eliminated in various stages of heating until a temperature of 480° C is reached.
Firing/Sintering
Porcelain restorations may be fired either by temperature control alone or by controlled temperature and a specified time. In the first method the furnace temperature is raised at a constant rate until a specified temperature is reached. In the second method the temperature is raised at a given rate until certain levels are reached, after which the temperature is maintained for a measured period until the desired reactions are completed.
Either method gives satisfactory results, but the time and temperature method is generally preferred because it is more likely to produce a uniform product. Porcelain is a poor thermal conductor, and thus too rapid heating may overfuse the outer layers before the inner portion is properly sintered.
As the temperature is raised, the particles of porcelain fuse by sintering. Sintering is the process responsible for the fusion of the porcelain particles to form a continuous mass. During this densification, the volume change, ΔV, in the early stages is related to the surface tension of the porcelain, γ, viscosity, η, the particle radius, r, and sintering time, t, as shown in the following equation:
As this equation indicates, the lower the viscosity and the finer the particle size, the greater the rate of densification. A minimum number of three firing operations are needed in the fabrication of a ceramic-metal restoration: one for the opaque portion, one for the dentin and enamel portion, and another for the stain and glaze. However, due to the shrinkage associated with the sintering process, it is necessary to oversize the porcelain buildup. For this reason, only experienced operators can complete a porcelain restoration in only three firings.
During the second firing, the dentin and enamel portion, which is formed about 13% oversize, is heated to the biscuit (or bisque) bake. This temperature is 56° C below the fusing temperature of the porcelain. Virtually all the shrinkage takes place during this firing.
The presence of gas bubbles (pores) has always been a problem in the production of dental porcelain. The extent to which pores may exist in air-fired porcelain is shown in Fig. 18-6, A. The specimen shown was ground and polished, and the round black spots are the cross-sectioned pores. It has been calculated that air-fired porcelain contains as much as 6.3% voids. This not only results in undesirable roughness and pits when a porcelain crown must be ground, but also exerts an even more undesirable effect on the strength and optical properties of the porcelain.
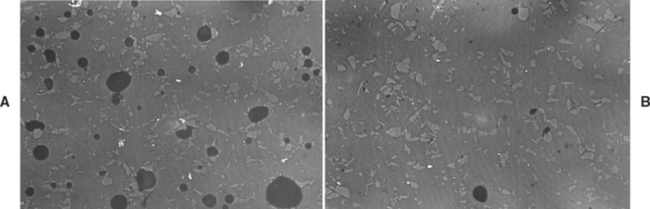
FIGURE 18-6 A, Microscopic view of air-fired porcelain, showing porosity. B, Microscopic view of vacuum-fired porcelain showing minimal porosity. (Courtesy JO Semmelman, York, PA, 1959, Dentsply International.)
Pores are caused by air trapped during the sintering process. Air spaces become spherical under the influence of surface tension and expand with increased temperature. Porcelains for ceramic-metal restorations are fired under vacuum. As the porcelain furnace door closes, the pressure is lowered to 10 MPa (0.1 atmosphere). The temperature is raised until the firing temperature is reached; the vacuum is then released and the furnace pressure returns to 100 MPa (1 atmosphere). The increase in pressure from 10 to 100 MPa helps compress and close the residual pores. This occurs by viscous flow at the firing temperature. The result is dense, relatively pore-free porcelain, as illustrated in Fig. 18-6, B. Studies have shown that sintering under vacuum reduces the amount of porosity to 0.56% (from 5.6%) in air-fired dental porcelains. Vacuum firing improves the translucency, decreases the surface roughness of feldspathic porcelains, and increases impact strength about 50%.
An alternate method uses the principle of diffusion to secure improved density in fused porcelain. A diffusible gas such as helium may be introduced to the furnace at low pressure during the sintering (densification) stage. The helium gas (instead of air) is therefore entrapped in the interstitial spaces, and because its molecular diameter is smaller than the porcelain lattice, it diffuses outward under the pressure of the shrinking porcelain.
Before the development of these improved firing methods, fine texture and translucency were not possible at the same time. Maximum translucency was obtained only by use of coarse powders that trapped only a few relatively large pores, but which also produced an undesirable granular appearance. Fine-grained powders developed a better texture but increased the opacity because of the large number of small pores. Vacuum firing achieved both fine texture and translucency in ceramic-metal restorations; however, another property was still missing. Human enamel exhibits a specific optical property called opalescence: a scattering effect that makes it appears bluish when viewed in reflected light and orange when viewed in transmitted light. Studies of pigment particle size and dispersion have made it possible to produce partially opalescent restorations that compare favorably with natural teeth.
Glazing
After the porcelain is cleaned and any necessary stains are applied, it is returned to the furnace for the final glaze firing. Usually, the glazing step is very short; when the glazing temperature is reached, a thin glassy film (glaze) is formed by viscous flow on the porcelain surface. Overglazing is to be avoided, because it gives the restoration an unnatural shiny appearance and causes loss of contour and shade modification. Glazing temperatures and times vary with the type and brand of porcelain employed.
Cooling
It is commonly accepted that the cooling stage is a critical one in the fabrication of ceramic-metal restorations and that extreme (too fast or too slow) cooling rates should be avoided. Too-rapid cooling of the outer layers may result in surface crazing or cracking; this is also called thermal shock. Very slow cooling (e.g., in a furnace) as well as multiple firings, might induce the formation of additional leucite and increase the overall coefficient of thermal expansion of the ceramic, and may also result in surface cracking and crazing. Slow cooling is preferred, and is accomplished by gradual opening of the porcelain furnace.
ALL-CERAMIC RESTORATIONS
Materials for all-ceramic restorations use a wide variety of crystalline phases as reinforcing agents and contain up to 99% by volume of crystalline phase. The nature, amount, and particle size distribution of the crystalline phase directly influence the mechanical and optical properties of the material. The match between the refractive indexes of the crystalline phase and glassy matrix is an important factor for controlling the translucency of the porcelain.
As mentioned earlier, several processing techniques are available for fabricating all-ceramic restorations: sintering, heat-pressing, slip-casting, and machining. Fig. 18-5, left, illustrates the cross-section of an all-ceramic crown.
SINTERED ALL-CERAMIC MATERIALS
Two main types of all-ceramic materials are available for the sintering technique: alumina-based ceramic and leucite-reinforced ceramic.
Alumina-Based Ceramic
Aluminous core ceramic is a typical example of strengthening by dispersion of a crystalline phase. Alumina has a high modulus of elasticity (350 GPa) and relatively high fracture toughness (3.5 to 4 MPa · m0.5), compared to feldspathic porcelains. Its dispersion in a glassy matrix of similar thermal expansion coefficient leads to a significant strengthening of the core. It has been proposed that the excellent bond between the alumina and the glass phase is responsible for this increase in strength compared with leucite-containing ceramics. The first aluminous core porcelains contained 40% to 50% alumina by weight. The core was baked on a platinum foil and later veneered with matched-expansion porcelain. Aluminous core ceramic is now baked directly on a refractory die. Aluminous core porcelains have flexural strengths of about 139 MPa and shear strengths of 145 MPa.
Leucite-Reinforced Feldspathic Porcelain
Feldspathic porcelain containing up to 45% by volume tetragonal leucite is available for fabricating all-ceramic sintered restorations. Leucite acts as a reinforcing phase; the greater leucite content (compared with conventional feldspathic porcelain for ceramic-metal restorations) leads to higher flexural strength (104 MPa) and compressive strength. The large amount of leucite in the material also contributes to a high thermal contraction coefficient. In addition, the large mismatch in thermal contraction between leucite (20 to 25 × 10−6/° C) and the glassy matrix (8 × 10−6/° C) results in the development of tangential compressive stresses in the glass around the leucite crystals upon cooling. These stresses can act as crack deflectors and contribute to increased resistance of the weaker glassy phase to crack propagation.
Sintered all-ceramic restorations are slowly being replaced by heat-pressed all-ceramic restorations that simplify the processing steps.
Heat-Pressed All-Ceramic Materials
Heat-pressing relies on the application of external pressure to sinter and shape the ceramic at high temperature. Heat-pressing is also called hightemperature injection molding. It is used in dentistry to produce all-ceramic crowns, inlays, onlays, veneers, and more recently, fixed partial dentures. Heat-pressing classically helps avoid large pores and promotes a good dispersion of the crystalline phase within the glassy matrix. The mechanical properties of many ceramic systems are maximized with high density and small crystal size.
Leucite-Based Ceramic
Leucite-based ceramics are available for heatpressing. Leucite (KAlSi2O6 or K2O · Al2O3 · 4SiO2) is used as a reinforcing phase in amounts varying from 35% to 55%. Ceramic ingots are pressed between 1150° and 1180° C (under a pressure of 0.3 to 0.4 MPa) into a refractory mold made by the lost-wax technique (Fig. 18-7). This temperature is held for about 20 minutes in a specially designed automatic press furnace. The ceramic ingots are available in different shades. The final microstructure of these heat-pressed ceramics consists of leucite crystals, 1 to 5 μm, dispersed in a glassy matrix (Fig. 18-8, A). Two techniques are available: a staining technique and a layering technique involving the application of veneering ceramic. The two techniques lead to comparable mean flexural strength values for the resulting ceramic composite. To ensure compatibility with the thermal expansion coefficient of the veneering ceramic, the thermal expansion coefficient of the core material for the veneering technique (14.9 × 10−6/° C) is lower than that of the core material for the staining technique (18 × 10−6/° C). The flexural strength of these ceramics (120 MPa) is about double that of conventional feldspathic porcelains. The main disadvantages are the initial cost of the equipment and relatively low strength compared with other all-ceramic systems.

FIGURE 18-7 The Empress high-temperature injection molding systems for all-ceramic restorations. (Courtesy Ivoclar Inc., Amherst, NY.)
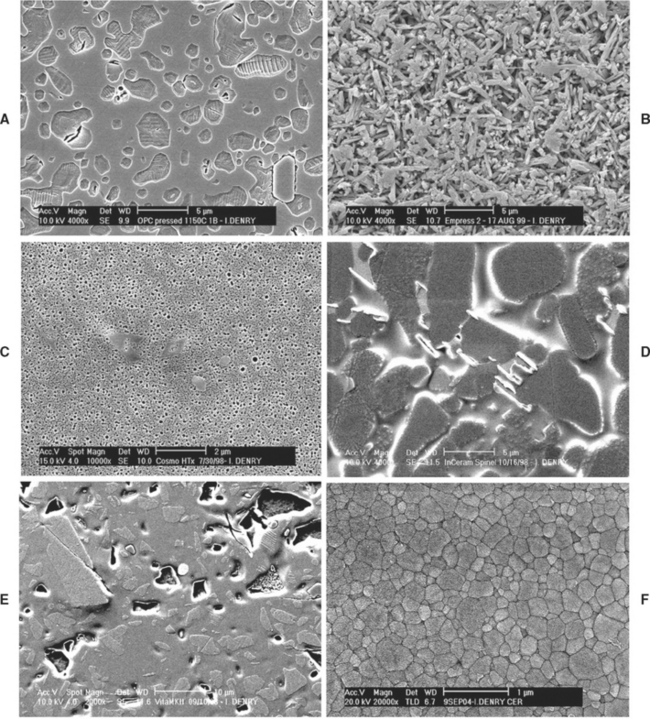
FIGURE 18-8 Scanning electron micrographs showing the microstructure of selected all-ceramic materials (polished and etched surfaces). A, Leucite-reinforced pressable ceramic; B, lithium disilicate pressable ceramic; C, lithium phosphate pressable ceramic; D, slip-cast alumina/spinel ceramic; E, feldspar-based machinable ceramic; F, zirconia-based machinable dental ceramic.
Lithium Disilicate–Based Materials
In recent years, new all-ceramic materials for heat-pressing have become available. These materials contain lithium disilicate (Li2Si2O5) as a major crystalline phase. They are heat-pressed in the 890° to 920° C temperature range, using the same equipment as for the leucite-based ceramics. The heat-pressed restoration is later veneered with ceramics of matching thermal expansion. The final microstructure consists of about 60% prismatic lithium disilicate crystals (0.5 to 5 μm long) dispersed in a glassy matrix (Fig. 18-8, B). The main advantage of the lithium disilicate–containing ceramics is their good flexural strength (350 MPa) and fracture toughness (3.2 MPa · m0.5), which extend their range of applications. The fabrication of fixed partial dentures is theoretically possible with these materials.
Lithium Phosphate–Based Ceramic
A ceramic material that can be heat-pressed onto a zirconia endodontic post has also been introduced in recent years. This ceramic contains lithium phosphate (Li3PO4) as a major crystalline phase. The recommended heat-pressing temperature is 900° C. Pressing can be done in a conventional heat-pressing furnace. The microstructure of this material is shown in Fig. 18-8, C, and consists of spherical lithium phosphate crystals uniformly dispersed in a glassy matrix.
The advantages of heat-pressed ceramics include good esthetics for the leucite-reinforced ceramics, high strength (but higher opacity) for the lithium disilicate–based ceramics and ability to use the well-known lost-wax technique. Processing times are short, and margin accuracy is within an acceptable range.
SLIP-CAST ALL-CERAMIC MATERIALS
Slip-casting involves the condensation of an aqueous porcelain slip on a refractory die. The porosity of the refractory die helps condensation by absorbing the water from the slip by capillary action. The piece is then fired at high temperature on the refractory die. Usually the refractory die shrinks more than the condensed slip so that the piece can be separated easily after firing. The fired porous core is later glassinfiltrated, a unique process in which molten glass is drawn into the pores by capillary action at high temperature. Materials processed by slip-casting tend to exhibit reduced porosity, fewer defects from processing, and higher toughness than conventional feldspathic porcelains.
Alumina-Based Materials
An alumina-based slip is applied to a gypsum refractory die designed to shrink during firing. The alumina content of the slip is more than 90%, with a particle size between 0.5 and 3.5 μm. After firing for 4 hours at 1100° C, the porous alumina coping is shaped and infiltrated with a lanthanum-containing glass during a second firing at 1150° C for 4 hours. After removal of the excess glass, the restoration is veneered using matched-expansion veneering ceramic. This processing technique is unique in dentistry and leads to a high-strength material due to the presence of densely packed alumina particles and the reduction of porosity. The flexural strength of this slip-cast alumina material is around 450 MPa. Because of the high strength of the core, short span anterior fixed partial dentures can be made using this process.
Spinel- and Zirconia-Based Materials
Two modified ceramic compositions for this technique have been recently introduced. One contains a magnesium spinel (MgAl2O4) as the major crystalline phase with traces of alpha-alumina, which improves the translucency of the final restoration. The microstructure of this ceramic is shown in Fig. 18-8, D. The second material contains tetragonal zirconia and alumina. The spinel-based ceramic has a lower modulus of rupture than the alumina-based material, whereas the zirconia-based material has a reported flexural strength neighboring 600 MPa.
The main advantage of slip-cast ceramics is their high strength; disadvantages include high opacity (with the exception of the spinel-based materials) and long processing times.
MACHINABLE ALL-CERAMIC MATERIALS
Machinable ceramics can be milled to form inlays, onlays, and veneers using special equipment. One system uses CAD/CAM (computer assisted design/computer assisted machining) technology to produce restorations in one office visit. After the tooth is prepared, the preparation is optically scanned and the image is computerized. The restoration is designed with the aid of a computer, as shown in Fig. 18-9. The restoration is then machined from ceramic blocks by a computer-controlled milling machine. The milling process takes only a few minutes. Although convenient, the CAD/CAM system is very expensive and its marginal accuracy is poor, with gap values of 100 to 150 μm. Bonding of the restorations with resin cements may help compensate for some of the problems of poor marginal fit. The recently introduced version of the CAD/CAM software (Cerec 3D, Sirona Dental Systems, LLC) allows complete tridimensional visualization of the projected restoration with “virtual seating” capabilities. The various surfaces of the virtual restoration can be modified in all three dimensions prior to machining.
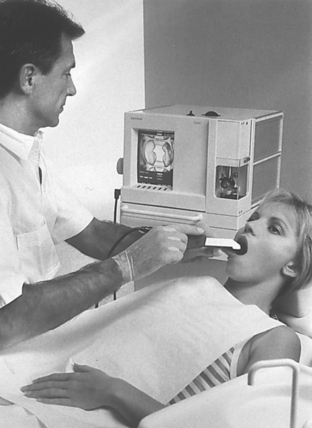
FIGURE 18-9 The Cerec chairside CAD/CAM system for porcelain inlay fabrication. (Courtesy Siemens Corp., Bad Soligman, Germany, 1995.)
Another system for machining ceramics is to form inlays, onlays, and veneers using copy milling. In this system, a hard resin pattern is made on a traditional stone die. This handmade pattern is then copied and machined from a ceramic block using a pantographic device similar in principle to those used for duplicating house keys. Again, marginal accuracy is a concern and there are high equipment costs.
Several machinable ceramics are presently available for use with these systems. One contains a potassium feldspar as a major crystalline phase dispersed in a glassy matrix (see Fig. 18-8, E). It is available in several shades. Its flexural strength can be ranked as moderate (105 MPa).
Pre-sintered slip-cast alumina blocks can be machined using the copy-milling system to generate copings for crowns and fixed partial dentures. The alumina copings are further infiltrated with glass, resulting in a final marginal accuracy within 50 μm.
A more recent system involves an industrial CAD/CAM process to produce crowns. The die is mechanically scanned by the technician and the data are sent to a workstation where an enlarged die is milled using a computer-controlled milling machine. This enlargement is necessary to compensate for the sintering shrinkage. Aluminum oxide powder is then compacted onto the die and the coping is milled before sintering at very high temperature (>1550° C). The coping is further veneered with an aluminous ceramic with matched expansion.
In 2002, a zirconia-based machinable ceramic was introduced on the dental market. The material consists of tetragonal zirconia polycrystals stabilized by addition of 3 mole percent yttrium (3Y-TZP). Partially sintered blanks are machined by CAD/CAM and later sintered at 1350° C for 2 hours. The machined restorations are oversized to compensate for the large shrinkage (20% to 25%) that occurs during the sintering process. The microstructure of this ceramic consists of densely packed tetragonal zirconia crystals with an average particle size of 0.2 to 0.4 μm (see Fig. 18-8, F). This material has the highest flexural (900 MPa) and highest fracture toughness (>5 MPa · m0.5) of all currently available dental ceramics.
As mentioned earlier, the main advantage of the machining process in making dental ceramic restorations is the ability to accomplish the restoration in one office visit. This is not true for the last system reviewed in which the restoration is fabricated in a laboratory. High equipment costs, poor marginal accuracy (compared to gold restorations) of the machining process, and the high opacity of the ceramic materials available constitute the main disadvantages of this process. Strength remains a concern for all the feldspar-containing machinable ceramics, and posterior fixed partial dentures are now possible with zirconia-based machinable ceramics.
GENERAL APPLICATIONS OF CERAMICS IN RESTORATIVE DENTISTRY
Ceramics are probably the best material available for matching the esthetics of a complex human tooth. They are used for ceramic-metal crowns, fixed partial dentures, and all-ceramic restorations, and to fabricate denture teeth. However, ceramics are brittle and fragile in tension, and the quality of the final product is very technique-sensitive, in terms of strength and esthetics.
CERAMIC-METAL CROWNS AND FIXED PARTIAL DENTURES
Ceramic is widely used as the veneering material in ceramic-metal crowns and fixed partial dentures. This development was the result of successfully matching the coefficients of thermal expansion of porcelain with alloys and achieving a proper bond. The glazed surface produces a restoration that is color-stable, tissue-friendly, biocompatible, and chemically durable and has low thermal diffusivity. Ceramic-metal restorations are still widely used; their failure rate at 10 years is considerably lower than most all-ceramic crown systems. It should be noted, however, that no long-term survival data are available for some of the newest all-ceramic systems.
ALL-CERAMIC CROWNS, INLAYS, ONLAYS, AND VENEERS
Ceramics have been used to fabricate jacket crowns since the early 1900s. At that time feldspathic porcelain was used in the fabrication. Aluminareinforced ceramics were later introduced to improve the mechanical properties. In the past 20 years, novel materials and techniques for the fabricating all-ceramic restorations have been introduced. As reviewed earlier in this chapter, they include slip-cast, machined, and heat-pressed all-ceramic materials. These new materials and techniques have widened the range of applications of all-ceramic materials, and in some cases made their processing easier.
Ceramic inlays and onlays are becoming increasingly popular as an alternative to posterior resin composites. They have better abrasion resistance than posterior composite resins and therefore are more durable. However, occlusal adjustments are more difficult and can lead to subsequent wear of the opposing tooth if not properly polished. The marginal gap is greater than with gold inlays or onlays. These restorations are not indicated when high occlusal loads are expected.
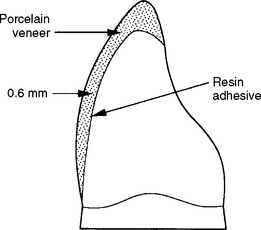
A ceramic esthetic veneer (laminate veneer) is a layer of ceramic bonded to the facial surface of a prepared tooth to cover an unsightly area (Fig. 18-10). Ceramic veneers are custom made and are fabricated in a dental laboratory. Initially, ceramic veneers were made of feldspathic porcelain and sintered. Currently, most ceramic veneers are fabricated by heat-pressing, using either a leucite-reinforced or lithium disilicate ceramic. To obtain sufficient adhesion, the tooth enamel is etched with phosphoric acid and the bonding surface of the ceramic is etched with 5% to 9% hydrofluoric acid gel and treated with a silane coupling agent. Resin composites specifically formulated for bonding to ceramic are used as the adhesive.
MECHANICAL AND THERMAL PROPERTIES OF DENTAL CERAMICS
The properties of dental ceramics depend on their composition, microstructure, and flaw population. The nature and amount of reinforcing crystalline phase present dictate the material’s strength and resistance to crack propagation as well as its optical properties.
Ceramics are brittle and contain at least two populations of flaws: fabrication defects and surface cracks. The fracture mechanisms involve crack propagation from these flaws.
Fabrication defects are created during processing and consist of inclusions at the condensation stage or voids generated during sintering. Inclusions are often linked to improper cleaning of the metal framework or use of unclean instruments. Porosity on the internal side of clinically failed glass-ceramic restorations has been identified as the fracture initiation site. Microcracks also develop upon cooling in feldspathic porcelains and can be due to thermal contraction mismatch between the leucite crystals and the glassy matrix or to thermal shock if the porcelain is cooled too rapidly.
Surface cracks are induced by machining or grinding. The average natural flaw size varies from 20 to 50 μm. Usually, failure of the ceramic originates from the most severe flaw.
Dental ceramics are subjected to repeated (cyclic) loading in a humid environment (chewing), conditions that are ideal for the extension of the preexisting defects or cracks. This phenomenon, called slow crack growth, can contribute to a severe reduction of the survival probability of ceramic restorations.
TOUGHENING METHODS
Toughening methods for dental ceramics can be intrinsic, such as crystalline reinforcement or transformation toughening, or extrinsic, including chemical strengthening or glazing.
The principle of crystalline reinforcement is to increase the resistance of the ceramic to crack propagation by introducing a dispersed crystalline phase with high toughness. Crystals can also act as crack deflectors when their coefficient of thermal expansion is greater than that of the surrounding glassy matrix, placing them under tangential compressive stresses.
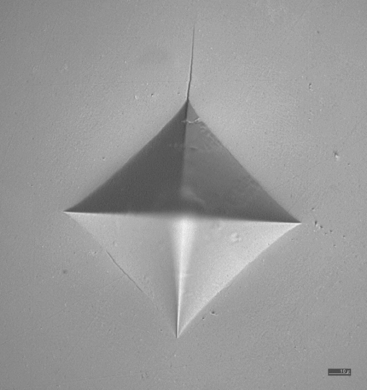
Transformation toughening is found in ceramics containing tetragonal zirconia. Zirconia (ZrO2) exists under several crystallographic forms. The monoclinic form is stable at all temperatures below 1170° C. The tetragonal form is stable between 1170° and 2370° C. However, this form can be stabilized to room temperature by doping with various oxides, such as yttrium oxide. The newest machinable zirconia-based dental ceramics contain tetragonal zirconia stabilized with 3 mole percent yttrium (3Y-TZP). Under stress, transformation from the metastable tetragonal phase to the stable monoclinic phase occurs in zirconia. This transformation is also called stress-induced and is accompanied by a volume increase leading to a closing of the cracks in the transformed zone. This transformation is responsible for the excellent mechanical properties of 3Y-TZP. Fig. 18-11 shows a Vickers indentation in a zirconia-based dental ceramic under a 98.1-N load. Only one short crack can be seen emanating from one corner of the indentation.
Chemical strengthening is based on the creation of a compressive layer at the surface of the ceramic after ion exchange, usually done in a molten salt bath. The principle is to exchange small alkali ions such as sodium in the glassy matrix network for larger ions such as potassium, diffusing from the molten salt bath. This diffusion-based ion exchange is usually performed at temperatures between 450° and 480° C, which preclude any stress relaxation from occurring and ensure that the surface of the ceramic is placed under compression, thereby offering a greater resistance to crack propagation. This technique has long been used in the glass industry and was popular in dentistry in the early 1990s. However, the thickness of this exchanged layer is only about 140 μm in the best-case scenario. The strengthening obtained is therefore lost after any adjustment or in case of occlusal wear of the restoration.
As mentioned earlier, glazing is the final step in the fabrication of ceramic-metal restorations. This standard technique, also called self-glazing, does not significantly improve the flexural strength of feldspathic dental ceramics. A low-expansion glass called glaze can also be applied at the surface of the ceramic, then fired to high temperature. Upon cooling, this glaze layer is placed under compression from the greater contraction of the underlying ceramic. This layer is also known to reduce depth and width of the surface flaws, thereby improving the overall resistance of the ceramic to crack propagation.
TEST METHODS
Numerous test methods are available to evaluate the mechanical properties of ceramics. Studies of the influence of test method on the failure stress of brittle dental materials have shown that important test parameters are the specimen thickness, contact zone at loading, homogeneity and porosity of the material, and loading rate. For this reason, discrepancies exist among the published values of mechanical properties for a given material.
Sometimes, researchers use devices that try to simulate dental morphology. However, the experimental variables can become extremely complex and difficult to reproduce in this type of testing. Finite element analysis (FEA) constitutes another approach to the simulation of clinical conditions. Failure predictions of ceramic inlays by the FEA technique have successfully matched fractographic analyses of clinically failed restorations.
Fractography is well established as a failureanalysis technique for glasses and ceramics. It has been recognized as a powerful analytical tool in dentistry. The in vivo failure stress of clinically failed all-ceramic crowns can be determined using fractography.
COMPARATIVE DATA
The flexural strengths of dental ceramics are summarized in Table 18-3. Feldspathic porcelains for ceramic-metal restorations have a mean flexural strength of about 70 MPa. This value is lower than those listed for all-ceramic materials; however, because ceramic-metal restorations are supported by a metallic framework, their probability of survival is usually higher.
TABLE 18-3
Flexural Strength of Selected Dental Ceramics
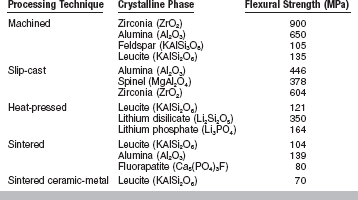
From Seghi R, Sorensen J: Int J Prosthodont 8:239, 1995; Seghi RR, Daher T, Caputo A: Dent Mater 6:181, 1990; Höland W, Beall G. Glass-ceramic technology, Westerville, OH, 2002, The American Ceramic Society.
Among the currently available all-ceramic materials, machined zirconia ceramics exhibit the highest values (900 MPa), followed by slip-cast ceramics (378 to 604 MPa), and lithium disilicate heat-pressed ceramics (350 MPa). The flexural strength of leucite-reinforced heat-pressed ceramics is around 120 MPa. As mentioned previously, the nature and amount of the crystalline phase present in the ceramic material strongly influences the mechanical properties of the final product.
The shear strength of feldspathic porcelain is 110 MPa, and the diametral tensile strength is lower at 34 MPa. The compressive strength is about 172 MPa, and the Knoop hardness is 460 kg/mm2.
Fracture toughness is also an important property of ceramics; it is a measure of the energy absorbed by the material as it fails. The fracture toughness of conventional feldspathic porcelains is very similar to that of soda lime glass (0.78 MPa · m0.5). Lithium disilicate heat-pressed ceramics have a fracture toughness about four times that of sodalime glass and double that of leucite-reinforced ceramics.
The elastic constants of dental ceramics are of interest as they are needed in the calculations of both flexural strength and fracture toughness. Poisson’s ratio lies between 0.21 and 0.26 for dental ceramics. The modulus of elasticity is about 70 GPa for feldspathic porcelain, 110 GPa for lithium disilicate heat-pressed ceramics, and 210 GPa for zirconia machinable ceramics and reaches 350 GPa for alumina-based ceramics.
The amount of shrinkage in ceramic bodies depends on the average pore size, which is directly related to the particle size distribution. The smaller pores obtained from a broader particle size distribution generate higher capillary pressure, leading to more shrinkage. The linear firing shrinkage of feldspathic porcelains has been reported to be about 14% for low-fusing porcelain (ceramic-metals) and 11.5% for high-fusing porcelain (denture teeth). Overglazed porcelain has a greater percentage of shrinkage for both the low- and high-fusing types, and the volumetric shrinkage shows even greater differences of approximately 8%. Several studies have shown the low-fusing type to have a volumetric shrinkage of from 32% to 37% and the high-fusing porcelain as much as 28% to 34%. The medium-fusing porcelain has shrinkage between the high- and low-fusing types. As pointed out earlier in this chapter, precise control of the condensation and firing technique is required to compensate for such shrinkage during the construction of the porcelain restoration. The greatest dimensional change will occur where porcelain is thickest.
Shrinkage remains an issue for all-ceramic materials with the exception of machined ceramics from fully sintered ceramic blocks and heat-pressed ceramics. Shrinkage of the veneering ceramics applied on all-ceramic cores has to be carefully compensated for during porcelain buildup. The large shrinkage of machined zirconia restorations during the subsequent sintering at very high temperature is compensated for by computerized enlargement from the wax pattern.
The density of fully sintered feldspathic porcelain is around 2.45 g/cm3 and will vary with the porosity of the material. The density of ceramic materials also depends on the amount and nature of crystalline phase present. The theoretical density of zirconia dental ceramics is 6.10 g/cm3, assuming that the material is pore free. A density of 99% and above is commonly reached for these materials.
The thermal properties of feldspathic porcelain include a conductivity of 0.0030 cal/sec/cm2 (° C/cm), a diffusivity of 0.64 mm2/sec, and a linear thermal coefficient of expansion of 12.0 × 10−6/° C between 25 and 500° C. This coefficient is about 10 × 10−6/° C for aluminous ceramics and lithium disilicate ceramics, 10.5 × 10−6/° C for zirconia-based ceramics, and 14 to 18 × 10−6/° C for leucite-reinforced ceramics.
OPTICAL PROPERTIES OF DENTAL CERAMICS
Color matching is a critical problem in replacing portions of natural teeth. Porcelain, being partially amorphous in structure, does not resemble crystalline enamel completely. As a result, various kinds of light are reflected and absorbed in different manners by the tooth tissue and porcelain, and restorations viewed from an angle may not appear the same as they do when viewed from the front. The cementing medium is an important factor in the final appearance of an all-ceramic restoration. Because of its opacity, an aluminous all-ceramic restoration may be cemented with a wide range of luting agents, but not with resin-modified glass ionomer cements (associated with fracture). However, more translucent all-ceramic restoration such as a leucite-reinforced heat-pressed crown or veneer, or a machined inlay or veneer, usually requires the use of translucent resin luting agents that are available in different shades.
The colors of commercial premixed dental porcelain powders are in the yellow to yellow-red range. Because the range of colors of natural teeth is much greater than the range available in a kit of premixed porcelains, modifier porcelains are also supplied for adjustments. These modifiers are strongly pigmented porcelains usually supplied in blue, yellow, pink, orange, brown, and gray. The dental technician may add the modifier porcelain to the opaque and body porcelains during the building of the crown. Extrinsic surface staining, another way of changing the color of a dental porcelain crown, involves the application of highly pigmented glazes. The main disadvantages of surface staining are a lowered durability (a result of solubility) and the reduction of translucency.
Translucency is another critical property of dental ceramics. The translucency of opaque, dentin (body), and enamel (incisal) porcelains differs considerably. Opaque porcelains have very low translucency, allowing them to mask metal substructure surfaces. Dentin porcelain translucency values range between 18% and 38%, as seen in Table 18-4. Enamel porcelains have the highest values of translucency, ranging between 45% and 50%. The translucency of materials for all-ceramic restorations varies with the nature of the reinforcing crystalline phase. Alumina- and zirconia-based systems are opaque, whereas leucite-reinforced systems are more translucent. The translucency of spinel-based systems is comparable with that of lithium disilicate–based systems and intermediate between alumina-based and leucite-reinforced systems.
TABLE 18-4
Percent Light Transmission of 1-mm Thick Dentin Porcelains

Adapted from Brodbelt RHW, O’Brien WJ, Fan PL: J Dent Res 59:70, 1980.
Because dental enamel is fluorescent under ultraviolet light, uranium oxide had been added to produce fluorescence with porcelain. However, because of the low but detectable radioactivity of uranium, newer formulations contain rare earth oxides (such as cerium oxide) to produce fluorescence.
Because the outer layers of a ceramic crown are translucent, the apparent color is affected by reflectance from the inner opaque or core ceramic. For ceramic-metal restorations, color mixing results from combining the light reflected from the inner, opaque porcelain surface and the light transmitted through the body porcelain. The thickness of the body porcelain layer determines the color obtained with a given opaque porcelain. This thickness effect may be minimized if the body porcelain and the opaque porcelain are the same color as that of some commercial systems.
PROPERTIES OF PORCELAIN DENTURE TEETH
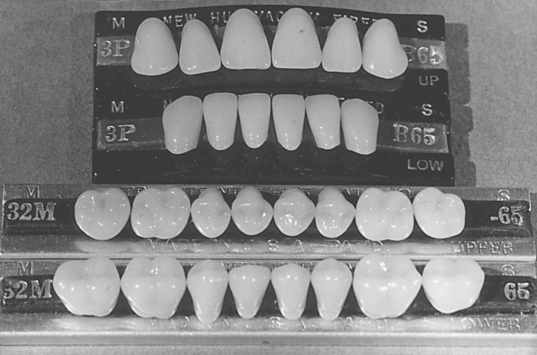
Porcelain denture teeth are commercially manufactured and their composition and properties set them apart from ceramics for fixed restorations. Individual or complete sets of teeth of excellent quality are available in a wide range of shapes and shades from a number of manufacturers. They have been used for complete dentures. The anterior teeth have one or two gold-covered pins to provide retention to the denture base. The posterior teeth have diatoric holes located centrally in the underside of the teeth for retention to the denture base. A typical set of porcelain teeth is shown in Fig. 18-12.
The ceramic composition of porcelain denture teeth belongs to the triaxial porcelain compositions (quartz, clay, kaolin). The teeth are made in split molds, fired under vacuum, and slowly cooled to prevent crazing.
The main advantages of porcelain denture teeth are their superior esthetics, resistance to abrasion, and excellent shade stability. One disadvantage is the difficulty of polishing the surface after occlusal adjustment, resulting in significant wear of the opposing teeth.
The physical properties of tooth porcelain are perhaps best shown when compared with the properties of plastic teeth and, when possible, with those of natural teeth. These properties, however, are often so radically different that they cannot be measured with the same equipment or compared quantitatively. The hardness values of porcelain compared with plastic and natural tooth structures are shown in Box 4-12.
It is evident from these values that the two standard tooth-replacement materials are quite different in hardness. Porcelain is harder than enamel, whereas the best plastic teeth are softer than dentin.
In regard to abrasion resistance, dental porcelain has been clinically evaluated as being equal to or slightly more wear resistant than natural tooth structure. At present, clinical data represent the only measure of abrasion believed to be of real significance. On this basis, porcelain has been estimated to have from 10 to 20 times the abrasion resistance of plastic teeth.
Porcelain is resistant to the action of solvents, with only hydrofluoric acid known to have any significant effect on it. The cross-linked plastics are relatively craze resistant and are immune to reasonable amounts of ordinary solvents. They may, however, be softened to some extent by a number of organic solvents.
Water bleaching and sunlight have no effect on porcelain, but repeated cycles of drying and water immersion may cause whitening and loss of color in plastic teeth. Continued exposure to ultraviolet light may cause a slight yellowing. When plastic is softened by solvents, organic dyes may penetrate the outer layer and cause discoloration.
The flexural strength of dental plastics is unquestionably superior to that of the dental porcelains. The pin anchorage and the strength of the porcelain that surrounds it are the determining factors in the basic strength of a porcelain tooth. Retention is adequate for most service conditions.
The high impact strength of plastic gives it a definite advantage. Vacuum-firing has improved the fracture resistance of dental porcelain by about 50%, but this does not preclude the possibility of fracture from sudden shock.
In dimensional stability or permanence of form, plastic is less acceptable than porcelain. The superior strength of plastic is indicated by its ability to cushion the impact blow of mastication and avoid fracture caused by brittleness. Unfortunately, plastic does not return to its original form each time it yields, and there is cumulative loss of dimension; this loss is described as flow. Porcelain, like tooth enamel, is incapable of cold flow.
It is often necessary to “spot” grind porcelain denture teeth or to heat them in a flame momentarily during the waxing of a denture. Overheating during these procedures may cause a small area to expand and become too large to be compatible with the remainder of the tooth. This may result in immediate breakage, or perhaps only in cracking the tooth, so that failure occurs in service. It is therefore advisable to keep a porcelain tooth wet during all grinding operations and to avoid rapid heating or cooling.
Both porcelain and plastic withstand heating well enough for the usual dental procedures. Plastic teeth will withstand temperatures of approximately 200° C, and porcelain is unaffected by temperatures in excess of 1100° C if the heat is applied slowly.
A direct and more complete comparison of the characteristic properties of porcelain and plastic teeth is discussed under “Properties of Dental Plastics” in Chapter 21.
Anusavice, KJ, Gray, A, Shen, C. Influence of initial flaw size on crack growth in air-tempered porcelain. J Dent Res. 1991;70:131.
Anusavice, KJ, DeHoff, PH, Hojjatie, B, Gray, A. Influence of tempering and contraction mismatch on crack development in ceramic surfaces. J Dent Res. 1989;68:1182.
Asaoka, K, Nuwayama, N, Tesk, JA. Influence of tempering method on residual stress in dental porcelain. J Dent Res. 1992;71:1623.
Baran, GR, O’Brien, WJ, Tien, TY. Colored emission of rare earth ions in a potassium feldspar glass. J Dent Res. 1977;56:1323.
Barreiro, MM, Riesgo, O, Vicente, EE. Phase identification in dental porcelains for ceramo-metallic restorations. Dent Mater. 1989;5:51.
Brodbelt, RHW, O’Brien, WJ, Fan, PL. Translucency of dental porcelain. J Dent Res. 1980;59:70.
Denry, IL, Rosenstiel, SF, Holloway, JA, Niemiec, MS. Enhanced chemical strengthening of feldspathic dental porcelain. J Dent Res. 1993;72:1429.
Denry, IL. Recent advances in ceramics for dentistry. Crit Rev Oral Bio Med. 1996;7(2):134.
Denry, IL, Holloway, JA, Colijn, HO. Phase transformations in a leucite-reinforced pressable dental ceramic. J Biomed Mater Res. 2001;54:351.
Dong, JK, Luthy, H, Wohlwend, A, et al. Heat-pressed ceramics: technology and strength. Int J Prosthodont. 1992;5:9.
Farah, JW, Powers, JM. All-ceramic restorations. Dent Advis. 2004;21(9):1.
Filser, F, Kocher, P, Gauckler, LJ. Net-shaping of ceramic components by direct ceramic machining. Assembly Automation. 2003;23(4):382.
Garvie, RC, Hannink, RH, Pascoe, RT. Ceramic steel? Nature. 1975;258:703.
Gray, HS. The porcelain jacket crown. NZ Dent J. 1963;59:283.
Hodson, JT. Some physical properties of three dental porcelains. J Prosthet Dent. 1959;9:235.
Höland, W, Beall, G. Glass-ceramic technology. Westerville, OH: The American Ceramic Society, 2002.
Jones, DW, Wilson, HJ. Some properties of dental ceramics. J Oral Rehabil. 1975;2:379.
Kelly, JR, Giordano, R, Pober, R, et al. Fracture surface analysis of dental ceramics: clinically failed restorations. Int J Prosthodont. 1990;3:430.
Kelly, JR, Nishimura, I, Campbell, SD. Ceramics in dentistry: historical roots and current perspectives. J Prosthet Dent. 1996;75:18.
Kelly, JR, Tesk, JA, Sorensen, JA. Failure of all-ceramic fixed partial dentures in vitro and in vivo: analysis and modeling. J Dent Res. 1995;74:1253.
Kingery, WD, Bowen, HK, Uhlmann, DR. Introduction to ceramics, ed 2. New York: John Wiley & Sons, 1976.
Kosmac, T, Oblak, C, Jevnikar, P, et al. Strength and reliability of surface treated Y-TZP dental ceramics. J Biomed Mater Res. 2000;53(4):304.
Lawn, BR, Pajares, A, Zhang, Y, et al. Materials design in the performance of all-ceramic crowns. Biomaterials. 2004;25(14):2885.
Leone, EF, Fairhurst, CW. Bond strength and mechanical properties of dental porcelain enamel. J Prosthet Dent. 1967;18:155.
Mackert, JR, Jr., Rueggeberg, FA, Lockwood, PE, et al. Isothermal anneal effect on microcrack density around leucite particles in dental porcelains. J Dent Res. 1994;73:1221.
Mackert, JR, Jr., Twiggs, SW, Evans-Williams, AL. Isothermal anneal effect on leucite content in dental porcelains. J Dent Res. 1995;74:1259.
May, K, Russell, M, Razzoog, M, Lang, B. Precision of fit: the Procera AllCeram crown. J Prosth Dent. 1998;80:394.
McLaren, EA, Sorensen, JA. High-strength alumina crowns and fixed partial dentures generated by copy-milling technology. Quint Dent Technol. 1995;18:31.
McLean, JW. A higher strength porcelain for crown and bridge work. Br Dent J. 1965;119:268.
McLean, JW. The alumina reinforced porcelain jacket crown. J Am Dent Assoc. 1967;75:621.
McLean, JW, Hughes, TH. The reinforcement of dental porcelain with ceramic oxides. Br Dent J. 1965;119:251.
Meyer, JM, O’Brien, WJ, Yu, R. Sintering of dental porcelain enamels. J Dent Res. 1976;55:696.
Milleding, P, Örtengren, V, Karlsson, S. Ceramic inlay systems: some clinical aspects. J Oral Rehabil. 1995;22:571.
Mora, GP, O’Brien, WJ. Thermal shock resistance of core reinforced all-ceramic crown systems. J Biomed Mater Res. 1994;28:189.
Morena, R, Lockwood, PE, Fairhurst, CW. Fracture toughness of commercial dental porcelains. Dent Mater. 1986;2:58.
O’Brien, WJ. Ceramics. Dent Clin North Am. 1985;29:851.
O’Brien, WJ. Recent developments in materials and processes for ceramic crowns. J Am Dent Assoc. 1985;110:547.
O’Brien WJ, Craig RG, eds. Proceedings of conference on recent developments in dental ceramics, Ceramic Engineering and Scientific Proceedings. Columbus, Ohio: American Ceramic Society, 1985.
Paravina RD, Powers JM, eds. Esthetic color training in dentistry. St. Louis: Elsevier Mosby, 2004.
Piché, PW, O’Brien, WJ, Groh, CL, et al. Leucite content of selected dental porcelains. J Biomed Mater Res. 1994;28:603.
Pröbster, L, Diehl, J. Slip-casting alumina ceramics for crown and bridge restorations. Quint Int. 1992;23:25.
Rekow, ED. A review of the developments in dental CAD/CAM systems. Curr Opin Dent. 1992;2:25.
Seghi, R, Sorensen, J. Relative flexural strength of six new ceramic materials. Int J Prosthodont. 1995;8(3):239.
Sherrill, CA, Jr., O’Brien, WJ. The transverse strength of aluminous and feldspathic porcelains. J Dent Res. 1974;53:683.
Thompson, JY, Anusavice, KJ, Naman, A, et al. Fracture surface characterization of clinically failed all-ceramic crowns. J Dent Res. 1994;73:1824.
Vaidyanathan, TK, Vaidyanathan, J, Prasad, A. Properties of a new dental porcelain. Scanning Microscopy. 1989;3:1023.
Vines, RF, Semmelman, JO. Densification of dental porcelain. J Dent Res. 1957;36:950.
Weinstein M, Katz S, Weinstein AB: Fused porcelain-to-metal teeth. US Patent No. 3,052,982, September 11, 1962.
Zhang, Y, Lawn, BR. Long-term strength of ceramics for biomedical applications. J Biomed Mater Res. 2004;69B(2):166.