Shock and resuscitation
The pathophysiology of shock
The term ‘shock’ can be defined as acute circulatory failure of sufficient magnitude to compromise tissue perfusion, which if untreated, proceeds rapidly to irreversible organ damage and death of the patient.
Hypoxia in the shocked patient compounds the problem of cellular oxygen delivery. It may be caused by mechanical airways obstruction, impaired gas exchange (e.g. pneumonia or pulmonary embolism) or hypoventilation (e.g. respiratory depression due to opioids) for example.
There are several mechanisms of shock:
• Cardiogenic shock occurs when the pump function of the heart is impaired
• Septic shock arises as a result of microvascular changes and cardiac depression caused by systemic inflammation
• Anaphylactic shock is an acute hypersensitivity reaction to an allergen
The classical symptoms and signs of shock include hypotension, hyperventilation, a rapid weak pulse, cold clammy cyanotic skin and oliguria. Mental changes also occur, most commonly anxiety, confusion and combativeness. Investigations reveal metabolic acidosis, low oxygen saturation and low central venous pressure. Notably, in septic shock there is peripheral vasodilatation rather than vasoconstriction.
Shock has been described as progressing through three stages. In stage I, there are attempts at compensation with skin and splanchnic vasoconstriction. Symptoms and signs are minimal but recognisable. In stage II, decompensation occurs, with body mechanisms unable to sustain tissue perfusion despite working at full capacity. Urgent intervention is needed at this stage. By stage III the changes are essentially irreversible, with prolonged shock having caused severe damage to major organs. Successful treatment depends crucially on early recognition of shock and its precursors, prompt diagnosis and treatment of the underlying cause and effective support of vital organ function.
Early recognition of shock
When surgical patients deteriorate catastrophically, it is often found on retrospective examination of charts that vital signs had been deteriorating for some time, but that clinical staff had failed to respond. Early recognition and intervention is crucial because failure of one organ leads to synergistic failure of other organs and an escalating risk of irreversible damage and death.
To help recognise these patients early, structured scoring systems have been developed, seeking to emulate the simplicity, reliability and clinical value of the Glasgow Coma Scale (Table 16.1, p. 219). These generally employ routinely recorded physiological data and most are modifications of the Early Warning Score (Table 4.1). These have proved extremely useful for spotting those at risk of deterioration needing urgent medical attention and have become part of standard care for surgical patients.
Table 4.1
Modified Early Warning Score (MEWS)*
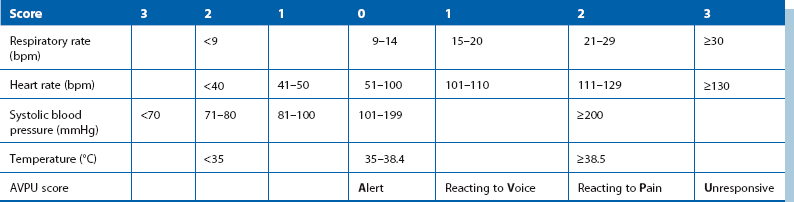
*MEWS is one form of bedside scoring that can help early identification of patients likely to need urgent assessment (score 3 or more). A score of 5 or more indicates that the patient is likely to require critical care, usually in a high-dependency or intensive care unit
Types of shock
Hypovolaemic shock (preload insufficiency): Preload is defined as the rate of venous return of blood to the heart. Preload insufficiency reduces the diastolic filling pressure and volume and leads to low cardiac output. The underlying problem may be inadequate total blood volume and underfilling of the venous compartment, i.e. absolute hypovolaemia (hypovolaemic shock) or else may be relative hypovolaemia (distributive shock) caused by an increase in capacity of the venous compartment or capillary beds relative to blood volume. Hypovolaemia is responsible for the majority of cases of shock encountered in hospital. Figure 15.1 (p. 200) shows the changes in vital signs associated with increasing amounts of blood loss.
The main causes of fluid loss leading to hypovolaemic shock are:
• ‘Revealed’ haemorrhage, e.g. massive haematemesis from peptic ulcer, deep lacerations, large haematemeses (vomiting of blood), continued loss from a wound drain indicating internal bleeding
• ‘Concealed’ haemorrhage, e.g. intra-abdominal bleeding from ruptured spleen or aortic aneurysm, haemorrhage from a duodenal ulcer into small intestine, intramuscular blood loss from fractures
• Extensive burns, resulting in massive loss of serum into blisters or from the skin surface
• Severe vomiting or diarrhoea, or prolonged fluid loss from a small bowel fistula or ileostomy
• Excessive urinary fluid loss, e.g. diabetic ketoacidosis, recovering acute tubular necrosis
• Sequestration of fluid in bowel caused by bowel obstruction
• Massive loss of fluid into interstitial tissues (‘third space losses’) as occurs in sepsis
• Major accumulation of fluid in the peritoneal cavity, e.g. acute pancreatitis
Distributive shock: Relative hypovolaemia occurs if there is inappropriate expansion of the circulatory capacity in relation to blood volume; it may result from failure of normal peripheral resistance and/or venodilatation of large veins. Peripheral resistance normally maintains cardiac afterload and is controlled by the tone of smooth muscle arteriolar and capillary sphincters. About 80% of capillaries are normally closed, and any mechanism that causes inappropriate opening greatly expands circulatory capacity. In septic shock, both arteriolar dilatation and an increase in venous volume play a part.
Septic shock: Septic shock is impaired tissue perfusion in the context of an inflammatory response. The aetiology is frequently infective but not always; it may be traumatic or surgical, or it may involve local inflammation, infection, severe burns or the presence of necrotic tissue, e.g. a gangrenous leg. If immune responses escape local control, this spill over provokes a complex cellular response and mediator cascade that leads to progressive clinical manifestations including the systemic inflammatory response syndrome (SIRS) and, later, multiple organ dysfunction (MODS). Mediator responses involve the complement system, acute phase proteins and cytokines (particularly TNF-alpha and the interleukins IL1-beta and IL6); once triggered, the inflammatory response cascade is difficult to control or suppress.
Septic shock is a combination of distributive shock and organ dysfunction induced by mediators of the host inflammatory response (e.g. cytokines, complement) and sometimes directly by bacterial toxins, especially certain staphylococci or Gram-negative bacilli, e.g. from a colonic anastomotic leak. Bacterial toxins and cell wall components activate defensive mechanisms and the net result of this immune burst is that oxygen usage declines, metabolic acidosis develops and multiple organ dysfunction ensues. Failure of oxygen usage is the result of cardiorespiratory impairment, microcirculatory imbalance and, at cellular level, mitochondrial dysfunction.
Septic shock itself can be thought of in three phases. Initially there is extensive vasodilatation, causing relative hypovolaemia. In phase 2 there is widespread endothelial damage causing greatly increased capillary permeability and massive fluid leakage into the interstitial space. This manifests clinically as inadequate blood pressure in the presence of normal or increased cardiac output; until phase 3, when depression of myocardial contractility ensues.
The inflammatory burst also upsets normal blood coagulation by downregulating normal anticoagulants such as alpha-1-antitrypsin, and stimulating procoagulants such as tissue factor as well as inhibiting fibrinolysis. The result may be disseminated intravascular coagulation with microvascular occlusion, large vessel thrombosis and ischaemia, all contributing to organ dysfunction.
Toxic shock syndrome is a particular form of septic shock associated with staphylococcal or streptococcal infection associated with the use of super-absorbent tampons.
Pump failure (cardiogenic shock): Cardiogenic shock describes a drastic reduction in cardiac output resulting from any form of ‘pump failure’ caused by direct myocardial damage, mechanical abnormality or malfunction of the heart. This most commonly arises from an acute myocardial infarction or an acute ventricular arrhythmia. Myocardial infarction may cause ischaemia or infarction of papillary muscles which produces acute mitral regurgitation. Another cause is when a large pulmonary embolus obstructs blood flow through the lungs and causes secondary cardiac failure. Other causes include cardiac (pericardial) tamponade and tension pneumothorax.
Anaphylactic shock: Anaphylactic shock is a generalised form of type I hypersensitivity reaction. The stimulatory antigen binds with antibodies attached to the surface of mast cells, triggering degranulation and release of histamine and other vasoactive amines. The predominant effect is extensive dilatation of the venous compartment and rapid movement of fluid into the tissues. The systemic effects are compounded by hypoxia due to bronchoconstriction and often laryngeal oedema.
In surgical practice, anaphylactic shock usually results from drug administration, particularly via the intravenous route. Antibiotics, particularly penicillins, and radiological contrast are the most common culprits. Anyone administering a drug must ensure the patient is not sensitive. Anaphylactic reactions may also occur after intravenous injections of radiological contrast media. Insect bites (wasps, bees and hornets) and ingested nuts are also important causes and may be encountered in the emergency department.
Clinical features of shock
The essential feature of any type of shock is a precipitate fall in arterial blood pressure with systolic pressure dropping by at least 40 mmHg from usual levels. The immediate homeostatic response is intense sympathetic activity and catecholamine release. The heart rate increases dramatically in an attempt to increase cardiac output. Except in septic shock, there is intense cutaneous and visceral vasoconstriction to restore intravascular volume by increasing peripheral resistance. Sudomotor activity causes profuse sweating. Hypoxic tissues revert to anaerobic respiration, producing lactic acid sufficient to cause a metabolic acidosis and compensatory tachypnoea. The clinical picture is a cold, pale, clammy, hypotensive patient with a rapid thready pulse and increased respiratory rate.
Septic shock presents a contrasting clinical picture in which cytokine-mediated peripheral vasodilatation is unresponsive to circulating catecholamines. The patient's skin is flushed and hot and cardiac output is increased to fill the dilated periphery. The pulse is typically ‘bounding’ in quality. Temperature may be above normal or below normal (‘cold sepsis’).
In all forms of shock, the circulatory system cannot support the main organ systems without treatment, and organs fail (i.e. decompensate) one by one in a synergistic manner. Pulmonary failure leads to acute respiratory distress syndrome (ARDS), and cerebral hypoxia soon causes confusion and eventually coma. Inadequate renal perfusion causes oliguria which, if not rapidly corrected, leads to acute tubular necrosis and renal failure. Cerebral ischaemia causes confusion and, if shock persists, reduced coronary flow and heart failure cause death. In septic shock, organ damage is exacerbated by an intense inflammatory burst and deterioration is inevitable unless the source of infection can be rapidly eliminated and effective support instituted.
Specific treatments for shock
Hypovolaemic shock: Identifying the cause of the fluid loss is the top priority. Immediate measures should be taken to control blood loss, e.g. pressure on a swab over a bleeding wound, endoscopic injection of bleeding peptic ulcer. Fluid replacement should be equivalent to estimated fluid loss but adjusted according to the response of pulse rate, blood pressure, observed JVP (or central venous pressure) and urine output. If available, transoesophageal ultrasound helps assess stroke volume and hence preload adequacy. Small falls in stroke volume of 15–20 ml can radically affect splanchnic flow. Where possible, fluids of similar composition to those lost should be used: whole blood for haemorrhage, colloids after major burns. Fluids should be titrated in rapid boluses, e.g. 250 ml at a time, and the response observed.
Cardiogenic shock: The management of cardiogenic shock is best reviewed in a medical textbook; the management of pulmonary embolism (which may present as cardiogenic shock) is discussed in Chapter 12, p. 168. Fluid overload is a significant hazard in cardiogenic shock.
Septic shock: (see SIRS and MODS, Ch. 3, p. 51)
Systemic sepsis leading to septic shock usually originates from a specific focus of infection or bacterial translocation from the patient's own intestine. In general surgical practice, septic shock most commonly results from faecal peritonitis following large bowel perforation or anastomotic breakdown. Infection in sites unrelated to the primary surgical pathology, such as bladder, chest or ‘line’ (usually central venous cannula) infection, are often the cause. Debilitated patients, uncontrolled diabetics and infants are particularly vulnerable to acute sepsis; a clear source of infection is less often found. Gangrene of a leg is also a potent cause. The damaging effects of the underlying poor organ perfusion are increased by direct and indirect bacterial exotoxic and endotoxic tissue damage.
Treatment of septic shock is urgent and involves fluid resuscitation, oxygenation, administration of appropriate antibiotics and the tracing and eliminating of the source of infection. Blood cultures must be taken and intravenous broad-spectrum antibiotics administered on a ‘best-guess’ basis. Because of interstitial losses, plasma expanders are often needed in large volumes, but should still be titrated against clinical parameters (e.g. pulse, CVP). Volume expansion helps to sustain cardiac output and tissue perfusion but addition of inotropes which induce peripheral vasoconstriction, in particular noradrenaline (norepinephrine), may be required. Corticosteroids are known to stabilise cell membranes and low dose administration has been shown in some clinical trials to prolong survival in patients with refractory shock.
If the diagnosis of septic shock is correct, resuscitative measures should produce dramatic improvement in the patient's condition within 1–2 hours. By that time, the patient should be ready for immediate operation if an abscess, bowel perforation or other surgically remediable cause needs treatment. It is important to emphasise that the source of infection must be urgently eliminated if the septic cascade is to be reversed.
Disseminated intravascular coagulation: A major problem in sepsis is generalised activation of the clotting cascade causing disseminated intravascular coagulation (DIC). This exhausts the supply of platelets and clotting factors V, VIII and fibrinogen (consumption coagulopathy) and activates intrinsic fibrinolytic mechanisms. DIC manifests as spontaneous bleeding or bruising and uncontrollable haemorrhage from operation sites. Diagnosis is made by finding low fibrinogen levels and high levels of D-dimers, cleaved from fibrin by plasmin and providing evidence of fibrin lysis. Treatment includes managing the initiating cause, giving intravenous heparin to arrest the coagulation process and transfusing appropriate clotting factors, e.g. fresh-frozen plasma, cryoprecipitate.
Anaphylactic shock: Immediate treatment of anaphylactic shock includes securing the airway and giving oxygen, laying the patient flat and raising the feet and administering 500 mg of intramuscular adrenaline (epinephrine), i.e. 0.3 ml of 1 : 1000 solution. This dose may be repeated at 5 minute intervals if necessary. An antihistamine (e.g. chlorphenamine 10–20 mg) should be given by slow intravenous injection and continued for up to 48 hours to stabilise mast cells. Hydrocortisone 100–300 mg should also be given intravenously but takes several hours to block histamine receptors and so should not be regarded as contributing to emergency treatment. Intravenous fluids may also be needed to treat hypovolaemia.
Resuscitation of the ‘collapsed’ non-trauma patient
Principles of managing shock by resuscitation: In the collapsed trauma patient, initial resuscitation focuses on the airway and replacement of blood loss. In contrast, shocked non-trauma patients often have multiple co-morbidities, both medical and surgical, which combine to produce the similar clinical picture of shock. Whatever the cause, the aim is to restore tissue perfusion and oxygenation by resuscitative measures while making the diagnosis and before specific treatment begins.
Early management, ideally within 24 hours of acute deterioration, can be summarised as follows:
• Clinical volume assessment—dryness of mouth, peripheral perfusion, pulse rate, blood pressure (compared with normal), jugular venous pressure observation, peripheral and pulmonary oedema (lung bases), measured urinary output, fluid balance charts and trends in body weight
• Treat the cause of shock—this can shut down the inflammatory response. Treatment may mean an operation to exteriorise a leaking anastomosis (i.e. bring the bowel ends to the skin surface) or to excise infected and necrotic tissue or drain pus
• Treat infection—if infection is apparent or suspected, institute antibiotic therapy using potent agents chosen on clinical suspicion until definitive microbiological results
• Support vital organs during the time it takes surgery or antibiotics to work. Airway and breathing support (e.g. oxygen administration, intubation, ventilation) benefits most patients, but the critical concern is to optimise the cardiovascular and haemodynamic system, aiming for a central venous pressure between 8 and 12 mmHg, a mean arterial pressure over 65 mmHg and a urine output of at least 0.5 ml/kg/hour
• Monitor and assess the response—clinically, by urine output and, ideally by transoesophageal Doppler assessment of stroke volume
A scheme for managing the acutely ill or shocked patient
This scheme is given in note form as an aide-mémoire for clinicians.
Recognition of the acutely unwell patient
(see Table 4.1)
The cardinal signs in a patient likely to need critical care in a high-dependency or intensive care unit include:
Sequence for action in the patient at risk
In summary this includes the following steps, which are detailed below:
A: Initial assessment The aim is rapidly to establish the urgency and severity of the situation. If vital signs are unsatisfactory and the patient unresponsive, a cardiac arrest or ‘crash call’ may be needed to bring more hands to assist. If an early warning score (EWS) is above a predetermined threshold, consultation is needed to arrange transfer to a critical care unit.
History: As much history should be gleaned as time permits, e.g. the context of the acute deterioration, medical state before deterioration, past medical history including allergies, and recent treatments including operations. Check drug charts.
Vital signs: Nurses can help gather information while assessment continues; vital signs should be monitored at frequent intervals. Observations include respiratory rate, temperature, pulse rate, systolic blood pressure and level of consciousness (the AVPU descending scale is simple and reproducible—A means the patient is fully alert, V means responds to voice, P means responds to pain and U is unresponsive).
Initial tests include oxygen saturation, blood glucose estimation, blood gas analysis and ECG for evidence of myocardial infarction. Note that new onset atrial fibrillation or flutter on ECG is often an indicator of sepsis. A bounding pulse and flushed extremities may suggest septic shock.
Examination—the initial survey:
Abdomen including rectal and/or vaginal examination if necessary:
B: Broad diagnosis Priorities for immediate treatment and further investigation need to be decided following initial assessment, if necessary from a single major finding. Priorities change as evidence is collected and depending on the response to treatment.
• Respiratory—upper airways obstruction, e.g. thyroid enlargement/haemorrhage into nodule
• Vascular events—abdominal aortic aneurysm rupture, aortic dissection, pulmonary embolism, acute coronary syndromes including MI, stroke and lower limb gangrene (usually causes insidious rather than acute development of shock)
a. blood loss—upper or lower gastrointestinal bleeding, intra- or retroperitoneal bleed
b. gastrointestinal obstruction including strangulation—gastric, small bowel or large bowel, hernia, volvulus
c. generalised peritonitis—perforation of an abdominal viscus (appendix, peptic ulcer, diverticular disease); acute pancreatitis
d. abdominal colic—ureteric, biliary, intestinal
e. intra-abdominal infection—gastroenteritis, acute appendicitis, diverticulitis, cholecystitis; urinary tract infection
f. obstetric and gynaecological—ruptured ectopic pregnancy/ovarian cyst/pregnancy/salpingo-oophoritis
• Other infections—infected central venous line, abscesses, cellulitis, diabetic foot, limb gangrene, gastroenteritis including antibiotic-associated colitis
• Metabolic—surgical disease can precipitate or be complicated by hypoglycaemia in diabetics; consider Addison's disease (adrenal insufficiency) if the patient is hypotensive
C: Immediate care Inform a senior doctor about the urgency of the case if appropriate.
Oxygen: Immediately secure a mask delivering 100% oxygen. The comatose patient may need to be intubated and positive pressure ventilation commenced.
Fluid management: Haemodynamic optimisation is very important and is based on clinical signs and monitoring of central venous pressure and urine output.
• Take venous blood for haemoglobin, haematocrit, urea and electrolytes, glucose, amylase and blood grouping/ordering of blood for transfusion if necessary. Take an arterial sample for blood gas estimation and acid–base status; get blood cultures if septic shock is suspected
• Set up an intravenous infusion and administer i.v. fluids/drugs, guided by vital signs and findings. For example, if the patient is hypotensive, give crystalloid or colloid solutions (at least 1 litre rapidly); if cardiogenic shock is likely, the circulating volume must not be expanded rapidly
E: Investigation to narrow the diagnosis Consider the most likely diagnosis and quickest route to confirming the initial ‘best guess’: