Diagnosis and management of common postoperative problems
Introduction
Despite good preoperative assessment, surgical and anaesthetic technique and perioperative management, unexpected symptoms or signs arise after operation that may herald a complication. Detecting these early by regular monitoring and surgical review means early treatment can often forestall major deterioration. This chapter uses a problem-orientated approach to help junior (and more senior) doctors deal with such problems. The management of more serious complications is described in the next chapter.
Managing problems such as pain, fever or collapse requires correct diagnosis then early treatment. Determining the cause can be challenging, particularly if the patient is anxious, in pain or not fully recovered from anaesthesia. It is vital to see and assess the patient and if necessary, arrange investigations, whatever the hour, when deterioration suggests potentially serious but often remediable complications. Consider also whether and when to call for senior help.
Postoperative pain
Some types of wound are more painful, for example vertical abdominal incisions and skin graft donor sites. It is better to prevent pain pre-emptively than react to established pain.
Methods of management
Postoperative pain can be minimised by preoperative counselling, perioperative measures and postoperative analgesia. Counselling lets the patient know the probable extent of pain, the plans for pain relief and the likely degree of mobility after operation. During the operation pre-emptive analgesia ensures that pain does not become established.
• Long-acting analgesic drugs given intravenously
• Local anaesthetic infiltration into the wound edges at the end of the operation with a long-acting agent, such as bupivacaine
• Regional nerve blocks (e.g. intercostal nerves for upper abdominal surgery using a transversus abdominis plane (TAP) block)
• Epidural analgesia using local anaesthetic and often morphine, during and after abdominal and pelvic surgery. These do not influence the rate of anastomotic leakage
• Non-steroidal analgesics given before the patient awakes by suppository or intravenous injection. These must not be given to patients with known allergy to aspirin or other NSAIDs, a history of severe asthma or angio-oedema, bleeding disorders, renal impairment, hypovolaemia or pregnancy. Mild asthma is not a contraindication. It is also unwise to use these in operations with a high risk of haemorrhage
Analgesia for minor and intermediate surgery
Patients vary greatly in their pain tolerance and need for analgesics. For minor and intermediate surgery, pre-emptive analgesia usually means simple analgesic tablets are sufficient. However, anxiety, exhaustion and sleep deprivation may reduce pain tolerance and the amount of analgesia must be adapted to individual need.
Analgesia for major surgery and trauma
(see Box 11.1)
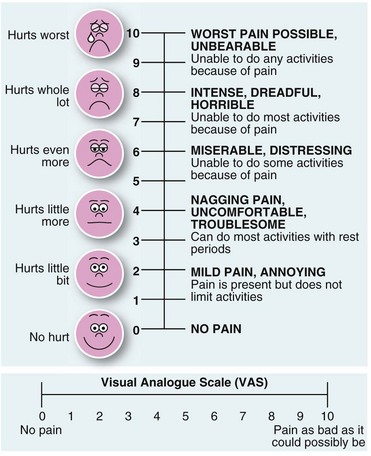
Many hospitals now provide an acute pain service, run by anaesthetists and specialist nurses. This team can plan individual analgesic strategies and help deal with pain problems as they arise. True objective rating of pain is difficult but some form of visual analogue scale chart can be helpful (Fig. 11.1).
Following major abdominal and perineal operations epidural analgesia using local anaesthetic drugs and morphine can be invaluable. A single dose can provide anaesthesia for the operation, e.g. transurethral prostatectomy, plus several hours of complete postoperative analgesia. For more extensive surgery, an epidural cannula can be left in situ to allow ‘topping-up’ to extend postoperative analgesia. These patients need careful observation for signs of toxicity, severe hypotension or respiratory depression. Note that moderate hypotension is merely an indication of satisfactory sympathetic blockade.
For major surgery and trauma where epidural analgesia is inappropriate, the analgesic dose needs to be enough to eliminate the pain without causing dangerous side-effects, and to be given often enough for continuous pain relief. Effective pain control can be achieved by allowing patients to give themselves small intravenous increments of opiates using a patient-controlled analgesia (PCA) device (Fig. 11.2). This allows presetting of the incremental dose (often 1 mg of morphine), with a 5 minute lockout to prevent it being given too frequently, as well as control of the total dose. Continuous effective pain relief is thus easily achieved and the total dose used is often less than with intermittent injections. This technique causes minimal sedation and respiratory depression whilst maintaining excellent continuous analgesia, although it can cause opiate-induced nausea.
Excessive postoperative pain
If the pain is not controlled by an apparently adequate dose and frequency of analgesia, complications should be suspected. The dose should first be reviewed in relation to the expected severity of pain and the weight of the patient.
• Local postoperative complications should be considered. Wound pain may be caused by pressure from a haematoma. In limb trauma, bleeding into or inflammatory oedema in a fascial compartment must be diagnosed before ischaemia ensues (‘compartment syndrome’). Wound pain increasing after the first 48 hours may be caused by infection. The wound is unusually tender even before redness and induration develop and there is usually a pyrexia. Other complications with lower limb pain include deep vein thrombosis and acute ischaemia. Lastly, major co-morbid conditions may be the cause of pain, for example myocardial ischaemia, or a fractured neck of femur may follow falling out of bed
• Major complications in the operation area. After an abdominal operation, excessive pain can be caused by intra-abdominal complications. These include haemorrhage, anastomotic leakage, biliary leakage, abscess formation, gaseous distension due to ileus or air swallowing, intestinal obstruction, urinary retention and bowel ischaemia, any of which is likely to require reoperation. Constipation may also cause late postoperative pain
As a rule, serious complications cause deterioration in the patient's general condition, whereas the patient remains well with less serious complications such as urinary retention or constipation.
Pyrexia (see Fig. 11.3)
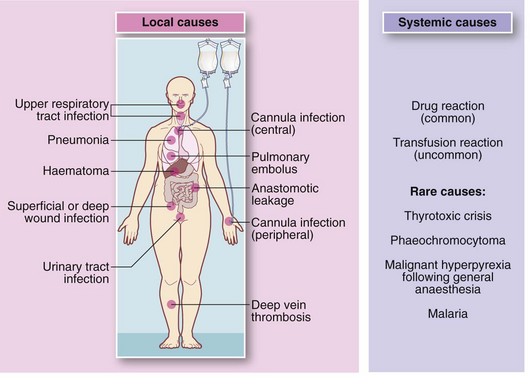
Fever is a common postoperative observation not always caused by infection. Pyrexia within 48 hours is usually caused by basal lung atelectasis and should be treated with physiotherapy and mobilisation. After this period, a search should be made for a focus of infection. The common ones are superficial or deep wound infection, chest infection (pneumonia), urinary tract infection and infection of an intravenous cannula site. If there is a central venous line, infection should always be suspected in unexplained pyrexia. Unfortunately, this can only be diagnosed by removing the line and culturing the tip for organisms. Blood cultures alone are often positive but do not reveal the source of infection. Patients usually recover spontaneously once the central line is removed.
Common non-infective causes of pyrexia include transfusion reactions, wound haematomas, deep venous thrombosis and pulmonary embolism. Pyrexia is sometimes the only sign of an idiosyncratic or allergic drug reaction. A rare cause is malignant hyperpyrexia following general anaesthesia.
Tachycardia
Tachycardia (rapid heart rate) may simply indicate pain or anxiety but it is also a feature of infection, circulatory disturbances and thyrotoxicosis. Mild tachycardia may be a sign of incipient hypovolaemic shock as a result of haemorrhage or dehydration. It may also herald cardiac failure. Tachycardia may be a sign of recent onset atrial fibrillation or flutter; this is confirmed by electrocardiography, and may indicate the patient has suffered a myocardial infarction. In bowel surgery patients, this is often a sign of anastomotic leakage, presumably mediated by cytokines released as a result of the leakage.
Cough, shortness of breath and tachypnoea
These symptoms are often associated with an overt respiratory problem such as acute bronchopneumonia, aspiration of gastric contents, lobar collapse, pneumothorax or an exacerbation of a pre-existing chronic lung disorder. Clinical examination and chest X-ray will rapidly diagnose most of them.
Shortness of breath and rapid shallow breathing are a feature of alveolar collapse (atelectasis), which may not be detected by clinical examination or chest X-ray. Atelectasis usually responds to chest physiotherapy. Abdominal distension may also cause rapid shallow breathing by inhibiting diaphragmatic movement. Shortness of breath and tachypnoea may be early features of cardiac failure or fluid overload but there are usually other clues such as tachycardia and basal crepitations.
A sudden onset of shortness of breath and tachypnoea, often with collapse, may indicate pulmonary embolism. This must be recognised, investigated and treated vigorously. Acute respiratory distress syndrome may occur in chest trauma, acute pancreatitis or systemic sepsis and should be anticipated in these patients. Finally, respiratory symptoms may be due to hyperventilation induced by pain, anxiety or hysteria.
Collapse or rapid general deterioration
The doctor on call is commonly asked to deal with a patient who has ‘collapsed’ or ‘gone off’ in a non-specific way. To make matters more difficult, the patient is often under the care of another surgical team. The more serious possibilities are summarised in Box 11.2.
In practice, the problem is tackled in the following order, which usually leads to a logical diagnosis:
• Brief history of the collapse and postoperative course to date.
• Rapid clinical appraisal—check Airway, Breathing and Circulation, then changes in conscious state and neurology
• Chart review reveals changes in temperature, pulse, blood pressure and respiratory rate. Check urinary output and fluid balance
• Reason for admission and preoperative state
• Pre-existing co-morbid conditions
• Nature and extent of surgical operation, including any operative problems
• Likely extent of perioperative blood and other fluid losses (including sequestration in bowel)
• Adequacy of fluid replacement
• Drug therapy—prescribed drugs? Have important drugs been given or omitted?
• Detailed physical examination
• Check blood glucose using reagent strips
• Special tests as suggested by clinical findings, e.g. ECG, chest X-ray, serum electrolyte estimation, arterial blood gas analysis, full blood count, urinalysis
Nausea and vomiting
Nausea and vomiting are common postoperative problems. The usual causes are side-effects from drugs used for general anaesthesia or postoperative analgesia, particularly opiates. Anti-emetics are usually given with opiates ‘as required’ for the early postoperative period but may have been missed. Nausea and sometimes vomiting later in the postoperative period may be caused by drugs. The worst offenders are erythromycin and metronidazole given orally, and digoxin overdosage (particularly in the elderly and in chronic renal failure).
Bowel obstruction causing nausea and vomiting
Sustained vomiting 48 hours or more after operation is usually caused by mechanical obstruction or by adynamic bowel (see Ch. 12, p. 172). Adynamic small bowel (ileus) is common after bowel operations and is difficult to differentiate from adhesional obstruction, but if it persists beyond 5 days after surgery, obstruction is more likely than ileus.
Mechanical obstruction, usually of small bowel, can follow any abdominal operation. Early obstruction due to fibrinous adhesions occurs within 4 days of operation and may respond to conservative treatment but often requires a further operation.
Finally, faecal impaction is a common problem in the elderly or immobile patient and may cause vomiting.
Systemic disorders causing nausea and vomiting
Electrolyte disturbances, uraemia, hypercalcaemia and other systemic disorders may cause vomiting via central effects. Centrally-mediated vomiting also occurs with raised intracranial pressure. This must be considered following head injuries, neurosurgical operations or in patients with cerebral metastases.
Haematemesis
Elderly postoperative patients sometimes produce a small quantity of ‘coffee ground’ vomitus positive for blood on ‘stick’ testing. This usually results from trivial bleeding from mild, stress-related gastritis or reflux oesophagitis and rarely indicates major haematemesis. The patient should be closely observed for signs of internal bleeding, and antacid preparations such as a proton pump inhibitor given.
Occasionally, a major upper GI haemorrhage occurs in the postoperative patient. If there has been forceful vomiting, a Mallory–Weiss tear at the oesophago-gastric junction may be the cause. Major bleeding may also arise from exacerbation of a peptic ulcer or even oesophageal varices. Seriously ill patients and the victims of burns and head injuries are susceptible to acute stress ulceration, which may cause catastrophic gastrointestinal haemorrhage (see Ch. 21, p. 298).
Other disorders of bowel function
Transient diarrhoea frequently follows abdominal operations, and should be regarded as normal in the recovery phase following bowel resections or operations to relieve intestinal obstruction, once any ileus has resolved.
Diarrhoea may also complicate antibiotic therapy. Several days after starting treatment, loose, frequent stools are passed for a short period, probably as a result of bacterial or fungal overgrowth. Less commonly, antibiotic-associated diarrhoea may develop, often due to overgrowth of Clostridium difficile which can be highly infective. If it progresses to pseudomembranous colitis or toxic megacolon it can become life-threatening. This is characterised by severe and persistent diarrhoea, sometimes containing blood (see Chs 3 and 12, p. 170).
After surgery of the abdominal aorta, blood-stained diarrhoea may occur a few days postoperatively. This may indicate large-bowel ischaemia due to ligation of the inferior mesenteric artery. This is a dangerous complication and requires urgent investigation and surgical exploration.
Constipation
Constipation is common after surgery. The causes include restriction of oral fluids and fibre, difficulty or reluctance in using a bed pan, slow recovery of normal peristalsis, the use of opiate analgesia and general lack of mobility. Anal pain is a powerful disincentive to defaecation following anal surgery. Constipation causes great distress, especially in the elderly. It should be anticipated and prevented if practicable by prescribing bulk-forming agents (e.g. methylcellulose or ispaghula husk preparations), stool softeners (e.g. docusate), osmotic laxatives (e.g. lactulose) or gentle stimulant laxatives (e.g. Senokot). Rectal preparations such as glycerine suppositories or enemas can be used if there is still no progress.
Impacted faeces in any patient may result in overflow incontinence; thus any patient with abnormal bowel function must undergo digital rectal examination to exclude faecal impaction.
Poor urine output
Low urinary output or complete failure to pass urine is a frequent postoperative problem. The most common cause is urinary retention, usually occurring in males. It is readily diagnosed if there is a palpable suprapubic mass which is dull to percussion.
Acute retention needs to be distinguished from true oliguria resulting from poor renal perfusion or acute renal failure; retention can readily be confirmed by ultrasound examination or by passing a urinary catheter.
Pathophysiology
Postoperative retention is much more common in men, particularly when there is prostatic hypertrophy. Patients with bladder outflow obstruction symptoms (‘prostatism’) are at risk of developing acute retention, although young males can also be affected.
Acute postoperative urinary retention seems to result from a combination of the following factors:
• Pre-existing bladder outlet obstruction
• Difficulty in passing urine in the supine position
• Embarrassment at passing urine without sufficient privacy
• Accumulation of a large volume of urine during the operation and recovery, causing overfilling of the bladder
• Transient disturbance of the neurological control of voiding by general or spinal anaesthesia
• Pain from an abdominal or inguinal wound inhibiting normal contraction of the abdominal musculature and relaxation of the bladder neck
• Problems after certain operations which predispose to acute retention, e.g. abdomino-perineal resection of rectum or (bilateral) inguinal hernia repair
• Constipation—gross faecal loading is common in the elderly in hospital and is probably the most frequent cause of acute retention (and faecal incontinence)
Management of postoperative urinary retention
Most postoperative acute retention can be managed conservatively, bearing in mind the precipitating factors. If the problem is dealt with early, the patient is less likely to require catheterisation, which should be avoided if possible. However, if there is a history suggesting bladder outlet obstruction or previous prostatectomy, catheterisation is more likely to be needed. The first step is to ensure there is adequate analgesia and this may be sufficient to enable the patient to pass urine. The next step is to help the patient out of bed to use a commode at the bedside or a urine bottle.
If these measures fail, the patient should be wheeled into a bathroom for privacy and left alone for a while, if fit enough. The familiar sound of a tap left running often encourages micturition. If the patient still does not pass urine, encourage a bowel movement by means of a glycerine suppository. Defaecation is usually accompanied by bladder neck relaxation and micturition.
Catheterisation
If conservative measures fail, catheterisation is usually necessary. In females, bladder drainage and immediate removal of the catheter is done. In males, the catheter is usually left in situ until the following morning or until the patient is well. In patients with prostatic obstruction, a suprapubic catheter is a better option as it avoids urethral trauma and can easily be clamped to check if normal micturition has returned. Recurrent retention is usually caused by bladder outlet obstruction and is managed as described in Chapter 35.
Diminished urine production
A degree of reduced urine output after operation is normal and results from increased release of ADH and aldosterone caused by surgical stress. Oliguria requiring attention in an adult is defined as less than 0.5 ml per kg per hour.
Low urine output is most often caused by reduced renal perfusion, resulting from relative hypovolaemia or low cardiac output. This leads to diminished glomerular filtration and enhanced tubular reabsorption. The usual reason is inadequate replacement of perioperative fluid deficit. Potentially more serious causes of reduced renal perfusion include cardiac failure and acute myocardial infarction. Acute renal insufficiency should only be diagnosed if poor renal perfusion (prerenal failure) can be excluded.
A urinary catheter should be inserted to ensure the bladder is emptying and to enable hourly measurement of output. When retention has been excluded and true oliguria or anuria is diagnosed, the problem is to differentiate between acute tubular necrosis and reduced renal perfusion. Poor renal perfusion may be due to hypotension during the operation or hypovolaemia. If untreated, this may progress to acute kidney injury and in advanced cases, acute cortical necrosis. Renal insufficiency from other causes, in particular systemic sepsis or drug toxicity, should also be considered.
If hypovolaemia is suspected, an intravenous fluid challenge of 250 ml of crystalloid solution should be given over 15 minutes or so, while monitoring JVP and urine output (Fig. 11.4). This can be repeated after 30 minutes. If this restores output, under-hydration is confirmed and fluid balance corrected to prevent acute tubular necrosis.
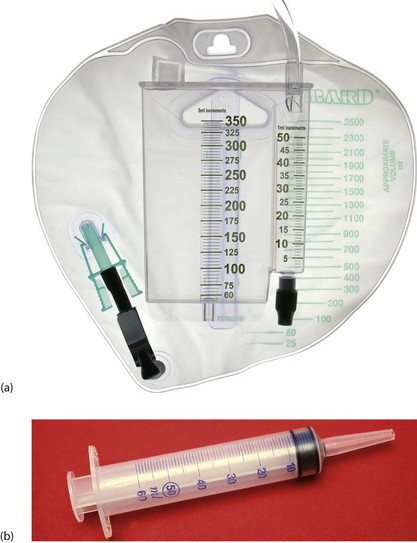
Fig. 11.4 Urine burette and bladder syringe
(a) Urine burette for precise measurement of urine output. Urine first enters the narrow compartment on the right to enable small quantities to be measured. It is then tipped into the main container to measure running totals. (b) Bladder syringe to flush urinary catheters suspected of blockage with debris. The nozzle is shaped to fit the end of a Foley urinary catheter. Note that full sterile precautions must be employed to reduce the risk of urinary infection
If the patient is hypotensive, the cause (cardiac failure or hypovolaemia, for example) must be identified and treated. A bladder ultrasound scan will show urine is being produced; a urinary catheter may be needed. If urine output is still poor, an oesophageal Doppler should be placed for monitoring stroke volume to allow goal-directed fluid therapy. Failing this, a central venous line can be used to monitor CVP. If simple measures fail, acute kidney damage must be suspected and investigated.
Lastly, bilateral ureteric obstruction should be considered (or unilateral if only one kidney is present). This is rare as a cause of postoperative low urine output. It can be diagnosed by renal ultrasound which will reveal bilateral hydronephrosis and an empty bladder.
Changes in mental state
Marked mental changes may occur early after operation and are most common in older patients. These changes are loosely called ‘confusion’. The elderly are vulnerable to dementia and cerebrovascular insufficiency so may have a low tolerance of systemic insults that interfere with cerebral equilibrium. Common phenomena include clouding of consciousness, perceptual disturbances, incoherent speech and agitation or destructive behaviour, such as pulling out cannulas or catheters. Other features are loss of orientation, apathy and stupor, and stereotypical movements such as plucking at the bedclothes.
Factors which predispose to postoperative mental changes in the elderly include:
• Disorientation brought about by rapid changes of environment (from ward to operating theatre or ward to ward for example)
• Hypoxia (e.g. from pneumonia or cardiac failure)
• Infection (especially of the urinary tract)
In addition, pain, anxiety and sleep deprivation may precipitate confusion.
Other causes
Pronounced alterations in behaviour, particularly in younger patients, may indicate alcohol withdrawal or craving for drugs such as cocaine or heroin. The history is concealed at admission. Patients with a recent history of head injury may behave abnormally if hypoxic, or if intracerebral bleeding develops.
Jaundice
Jaundice may develop several days after operation in a patient with no history of biliary disease. In these patients, the cause is usually a prehepatic or hepatic disorder. Causes of prehepatic jaundice include large blood transfusions, absorption of large haematomas or emergence of a haemolytic disorder such as thalassaemia or sickle-cell trait (exacerbated by postoperative hypoxia, dehydration or hypothermia).
Hepatic causes are less common. They include cholestasis (caused by infection near the liver or drug idiosyncrasy), hepatitis and liver cell toxicity from drug idiosyncrasy and visceral ischaemia caused by shock.
Causes related to biliary or liver surgery
Patients having biliary tract or liver surgery may become jaundiced after operation. The most likely cause is obstruction of the extrahepatic bile ducts due to retained stone, unrecognised surgical trauma or inadvertent duct ligation. Other causes include infection such as ascending cholangitis, and systemic absorption of an intra-abdominal collection of bile (biliary peritonitis).