Pancreatitis
Introduction
Pancreatitis is a common inflammatory disorder of the pancreas characterised by abdominal pain. Most cases present in an acute form known as acute pancreatitis and attacks range in severity from mild to severe. Severe attacks can be life-threatening, with a mortality of around 20%.
A small proportion of patients suffer a persistent form known as chronic pancreatitis; this is much more common in men and is often associated with high alcohol intake.
Acute Pancreatitis
Aetiology and epidemiology of acute pancreatitis
Gallstones and alcoholism together account for about 80% of acute pancreatitis worldwide. These and the other main causes of acute pancreatitis are listed in Table 25.1 (see also Boxes 25.1 and 25.2). Opie first pointed out the relationship between gallstones and pancreatitis in 1901 based on autopsy evidence. Small gallstones may cause transient obstruction as they pass through the ampulla via the bile ducts, or larger stones may impact at the lower end of the common bile duct, resulting in pancreatic duct obstruction. Beyond this, the precise mechanism of gallstone pancreatitis remains obscure.
Table 25.1
Aetiology of acute pancreatitis
| Condition | Frequency |
| Obstruction | |
| Gallstones | 30–70% of cases |
| Congenital abnormalities: pancreas divisum with accessory duct obstruction; choledochocoele; duodenal diverticula | 5% of cases |
| Ampullary or pancreatic tumours | 3% of cases |
| Abnormally high pressure in the sphincter of Oddi (over 40 mmHg) | 1–2% of cases |
| Ascariasis (second most common cause in endemic areas, e.g. Kashmir) | Depends on locality |
| Drugs and toxins | |
| Alcohol excess | 30–70% of cases |
| Drugs: (‘SAND’—Steroids and sulphonamides, Azathioprine (and 6-mercaptopurine), NSAIDs, Diuretics such as furosemide and thiazides, and didanosine); also antibacterials such as metronidazole and tetracycline, H2 blockers and many other classes of drug | 1–2% |
| Scorpion venom | Very rare |
| Snake bites | Very rare |
| Iatrogenic and traumatic causes | |
| Following endoscopic retrograde cholangio-pancreatography (ERCP) or endoscopic sphincterotomy | 2–6% of patients having the procedure |
| Following cardiopulmonary bypass | 0.5–5% of patients having bypass |
| Blunt pancreatic trauma, usually due to motor vehicle accidents | Very rare |
| Repeated marathon running | Very rare |
| Metabolic causes | |
| Hypertriglyceridaemia (> 11 mmol/L) | 2% of cases |
| Hypercalcaemia | Rare |
| Hypothermia | Rare |
| Pregnancy | Rare |
| Infection | |
| AIDS: secondary infection with cytomegalovirus and others | About 10% in patients with AIDS |
| Other viruses: mumps, chickenpox, Coxsackie viruses, hepatitis A, B and C | Very rare |
| Idiopathic pancreatitis | |
| No definable cause after thorough diagnostic evaluation including ERCP; research studies show about two-thirds of ‘idiopathic’ cases have gallstone microlithiasis | 10–12% of cases |
The proportion of acute pancreatitis caused by alcoholism varies from country to country; for example, about two-thirds of patients in the UK have a gallstone aetiology whereas in parts of the USA and continental Europe about two-thirds have an alcohol aetiology. Longstanding high intake for at least 2 years is usually required to cause alcoholic pancreatitis; the mean daily intake of pure alcohol in subjects of one study was 140 g, compared with 40 g in controls. Occasionally acute pancreatitis can result from a single session of heavy drinking—students finishing exams beware! The mechanism of alcoholic pancreatitis is unknown but is probably the toxic effect of alcohol on the pancreas in genetically predisposed individuals.
In developed countries, the annual incidence of acute pancreatitis requiring hospital admission is about 1 : 2000 population and mortality is between 2% and 6%. About 15% fall into the category of severe pancreatitis. Women are affected more than men, but men are more likely to suffer recurrent attacks. The peak incidence is between 50 and 60 years. The reported incidence appears to have risen by a factor of 10 over the last 40 years, with only part of this attributable to improved diagnosis. Other factors are unknown.
Pathophysiology of acute pancreatitis
Acute pancreatitis is characterised by the sudden onset of diffuse inflammation of the pancreas. A range of diverse factors initiate disturbances of cellular metabolism, chiefly concerned with membrane stability. This leads to inappropriate activation of zymogens (pre-enzymes) within the pancreas. Activation of trypsin is probably the key initiating event and this overwhelms intrinsic antitrypsin activity, leading to interstitial oedematous pancreatitis. Fortunately, the extent and severity of inflammation remain mild and self-limiting in most patients and systemic effects are mild. In the least severe cases, there is minimal peritoneal exudation and no pancreatic changes detectable on contrast-enhanced CT scanning. In more severe disease, the pancreas becomes swollen and oedematous but remains viable. If laparotomy is inadvertently performed at this stage, clear, non-infected peritoneal fluid is found, with whitish patches on great omentum and mesentery representing areas of fat saponification (‘fat necrosis’). If it is extensive, calcium becomes sequestered in these areas and this is incriminated in the drop in blood calcium characteristic of severe acute pancreatitis.
As severity increases, trypsin and other enzymes cause increasingly extensive local damage as well as activation of complement and cytokine systems leading on to systemic inflammatory response syndrome (SIRS) and organ failure. Manifestations include shock, acute respiratory distress syndrome (ARDS), renal failure and disseminated intravascular coagulation (see Ch. 2). At this stage, acute peripancreatic fluid collections become detectable on CT. The most severe pancreatitis is associated with pancreatic necrosis. Ischaemia within the gland plus reperfusion injury are likely mechanisms in transforming acute oedematous pancreatitis into this necrotising disease. Complications are common and mortality in this group (even without infection) is as high as 10%.
A substantial proportion of these patients develop infection of the necrotic pancreas, usually with Gram-negative organisms translocated from bowel. This occurs within 2 weeks of the onset and greatly increases mortality. Pancreatic abscess formation is a different phenomenon, developing later and having a somewhat better prognosis.
In patients dying of acute necrotising pancreatitis, the peritoneal cavity becomes filled with dark, blood-stained inflammatory exudate containing fine lipid droplets. This is known as acute haemorrhagic pancreatitis. The peritoneal surface is grossly inflamed and semi-digested, and the pancreas is a necrotic mass.
Clinical features of acute pancreatitis (see Box 25.3)
Acute pancreatitis presents as a patient with an acute abdomen who has severe abdominal pain coming on suddenly and continuously from the outset. Initially it is poorly localised in the central and upper abdomen and is often described by the patient as ‘going through to the back’. Vomiting may be an early feature. In the early stages, the patient is restless and constantly changes posture in the search for a comfortable position. Pain is most often relieved by leaning forward in the ‘pancreatic position’. With the onset of chemical peritonitis, movement becomes increasingly painful and the patient lies very still. Clinical signs depend on the severity of the inflammatory process and the stage it has reached.
Investigation of suspected pancreatitis
Acute pancreatitis must be excluded in any adult presenting with acute abdominal pain, and in any child with peritonitis not readily attributable to appendicitis. The British Society of Gastroenterology (BSG) and other organisations have produced comprehensive guidelines on the management of acute pancreatitis: (http://www.bsg.org.uk/pdf_word_docs/pancreatic.pdf).
Plasma amylase: Amylase is one of the enzymes released into the circulation in pancreatitis; measurement is a simple laboratory investigation and is generally a reliable diagnostic test. A level above 1000 i.u. /ml is usually regarded as diagnostic of acute pancreatitis but levels are often lower in alcoholic pancreatitis, particularly during recurrent attacks. Any inflammatory upper abdominal condition near the pancreas (e.g. cholecystitis, perforated peptic ulcer or strangulated bowel) can cause a moderate rise in amylase, although this rarely reaches 1000 i.u. /ml. Levels rise rapidly at the outset of an attack, and readings of 10 000 i.u./ml or more may be recorded on admission to hospital. However, levels fall as the patient recovers so a diagnosis of pancreatitis should not rely on an arbitrary threshold; rather the amylase level at any moment should be interpreted in relation to how much time has elapsed since the onset.
The peak amylase level is not an indicator of disease severity, but persistently raised levels over several days warn of developing complications. Note that false negative amylase results may occur in lipaemic serum. In this case, true results can be obtained on diluted specimens.
On rare occasions, plasma amylase may be normal in acute pancreatitis. This occurs where most of the gland has been destroyed by severe pancreatitis. It may also occur if a patient presents several days into an attack, by which time the amylase may have returned to normal. If pancreatitis is strongly suspected but the amylase is normal, the diagnosis may be confirmed by appropriate radiological imaging (see next section).
Plasma lipase estimation has been recommended for diagnosing acute pancreatitis as it is more sensitive than amylase and has a longer half-life, so it is easier to detect later in the course of the disease. It is not as widely used but is often useful in difficult or late-presenting cases. In addition, elevated alanine aminotransferase (ALT) levels are highly specific for gallstone pancreatitis.
Once the patient is in the recovery phase, fasting bloods should be taken for calcium levels and for plasma lipids, particularly triglycerides. Viral antibody titres may be useful in cases of idiopathic pancreatitis.
Imaging: Plain X-rays of chest (erect) to look for free gas under the diaphragm, and abdomen (supine) are usually performed during the initial investigation. The latter may show a featureless ‘ground-glass’ appearance. Bowel gas tends to be absent except perhaps for a ‘sentinel loop’ of dilated adynamic small bowel in the centre. Note, however, that these signs are not specific for pancreatitis and are often absent. Rarely, radiopaque gallstones are visible.
An ultrasound scan of the biliary tree is essential but good images are not always obtained early; a delay of 48–72 hours may improve image quality. The goal is to look for small calculi in the gall bladder or bile ducts typically responsible for gallstone pancreatitis. If no definite cause for the pancreatitis is found, a repeat ultrasound should be performed following recovery.
CT scanning has a limited role in the diagnosis of acute pancreatitis. Initially, it is indicated only when clinical and biochemical findings are equivocal, particularly if amylase is normal and intra-abdominal pathology such as perforation or infarction needs to be excluded. CT scans performed too early cannot predict the final severity and are unlikely to influence management during the first week. In severe pancreatitis, contrast-enhanced CT is valuable for demonstrating necrosis, but this cannot be identified until at least 4 days after the onset of symptoms.
Endoscopy: In patients with severe acute gallstone pancreatitis, urgent ERCP and sphincterotomy should be carried out within 72 hours of the onset of pain. This is especially important in patients with cholangitis, jaundice or a dilated common bile duct. ERCP also has an important diagnostic role once the patient has fully recovered from the acute attack if a cause for pancreatitis is not evident from the history or initial imaging studies. In this group, a cause can be found in about 50%, e.g. small pancreatic or periampullary tumours, pancreatic duct stricture, gallstones, congenital pancreas divisum or a high-pressure sphincter of Oddi.
Clinical classification
To provide early warning of severity, each patient with acute pancreatitis is sorted into one of two categories, mild or severe. This provides an indicator of prognosis within the first 48 hours, and is central to formulating a management strategy. Categorisation is based on thoroughly tested scoring systems originally developed by Ranson in USA (see Box 25.4) and modified by Imrie (Glasgow criteria—see Table 25.2). If three or more of the factors listed are present, the patient is diagnosed as having severe pancreatitis and should be admitted to an intensive care or high-dependency unit for careful monitoring. The more adverse factors present, the worse the prognosis. Even if a patient is initially placed in the mild group, continued observation is essential as deterioration to severe pancreatitis can occur at any time. The clinical features of acute pancreatitis are summarised in Box 25.3.
Table 25.2
A mnemonic (‘PANCREAS’) for remembering the modified Glasgow scoring system of severity prediction in acute pancreatitis (After E M Moore, with permission*)
| Mnemonic letter | Criterion | Positive when |
| P | PaO2 | < 8 kPA or 60 mmHg |
| A | Age | > 55 years |
| N | Neutrophil count | > 15 × 109/L |
| C | Calcium (blood) | < 2 mmol/L |
| R | Raised plasma urea | > 16 mmol/L |
| E | Enzyme (plasma lactate dehydrogenase, LDH) | > 600 i.u./L |
| A | Albumin (plasma) | < 32 g/L |
| S | Sugar (plasma glucose) | > 10 mmol/L |
*E Moore A useful mnemonic for severity stratification in acute pancreatitis. Ann R Coll Surg Engl 2000; 82: 16–17
Mild acute pancreatitis: Mild attacks are common. The patient looks generally well with minimal systemic features. Nevertheless, there is often considerable pain. The abdomen is usually distended and diffusely tender but with little guarding. Bowel sounds are absent as a result of inflammatory ileus. The patient may be mildly jaundiced from periampullary oedema. The differential diagnosis includes biliary colic, acute cholecystitis, an acute exacerbation of a peptic ulcer or even a perforation of a peptic ulcer. Lower lobe pneumonia or an inferior myocardial infarction may sometimes present like this. Sometimes the pancreatitis diagnosis is made after plasma amylase is unexpectedly found to be elevated.
Severe acute pancreatitis: In a severe attack the patient looks apathetic, grey and shocked and there are typical abdominal signs of generalised peritonitis, i.e. extreme tenderness, guarding and rigidity. In this case, the differential diagnosis includes other major abdominal catastrophes, especially faecal peritonitis from perforated large bowel and concealed haemorrhage from a leaking aortic aneurysm or ruptured ectopic pregnancy. Massive bowel infarction due to arterial occlusion may present like this but abdominal signs are less marked. An important early and dangerous complication of severe acute pancreatitis is acute respiratory distress syndrome (ARDS).
Management of acute pancreatitis
Mild attacks: The management of acute pancreatitis has been the subject of several international consensus conferences, the most important of which took place in Atlanta in 1992. This codified many definitions and circumstances within acute pancreatitis. Another important consensus meeting was in Washington in 2004. Numerous sets of guidelines are available; in the UK, the most used are published by BSG, updated in 2005.
Mild attacks require no further emergency investigation once diagnosed on plasma amylase, and are managed by fluid resuscitation and analgesia: recovery is usually rapid. These patients need no dietary restriction. Later management is aimed at treating predisposing factors. Gallstones should be sought by ultrasonography; if present, cholecystectomy is the definitive treatment and is ideally performed on the same admission or at worst 2–4 weeks after recovery. Ductal stones should be removed endoscopically before discharge from hospital. Alcohol abuse must be discouraged.
Severe attacks: A severe attack is defined by referring to a specified list of criteria which should be evaluated on admission and over the next 48 hours (see Table 25.2 and Box 25.4). Patients with severe pancreatitis may die early in the attack because of profound systemic toxaemia (SIRS), shock and multiple organ dysfunction syndrome (MODS); see Chapter 3, p. 51. ARDS develops rapidly with little warning but a deteriorating arterial PO2 may herald its onset. This is an indication for urgent ventilatory support before the condition becomes established.
Even when pancreatitis is severe, supportive measures are still the mainstay of treatment. These include oxygen supplementation and carefully planned intravenous fluid resuscitation. A nasogastric tube is passed to aspirate the stomach only if gastroparesis causes troublesome vomiting. Enteral nasogastric or nasojejunal feeding has been reported to significantly decrease morbidity. If enteral feeding is not tolerated because of ileus, then total parenteral nutrition may be necessary.
Gross fluid and electrolyte disturbances as well as hypocalcaemia are also likely to occur. Fluid balance in the shocked patient is complicated by massive losses of protein-rich fluid into the peritoneal cavity and interstitially (‘third space’). This sequestration of fluid needs to be countered by large amounts of intravenous fluids, carefully monitored by measuring central venous pressure and hourly urine output. Any patient with severe pancreatitis, or anyone with acute pancreatitis who develops signs of serious deterioration, should be admitted to an intensive care unit for close monitoring and vigorous treatment of cardiovascular, pulmonary, renal and septic complications. Box 25.5 lists recommended investigations to guide management.
There is probably little benefit in measuring plasma amylase again once the diagnosis is made. C-reactive protein (CRP) is more useful as it is a good indicator of systemic inflammation and hence developing necrosis and other complications. If the CRP is elevated > 150 after 48 hours, complications are more likely. Biochemical estimations, particularly liver transaminases and bilirubin, are charted regularly, looking chiefly for evidence of biliary obstruction; renal function tests are performed for evidence of acute renal insufficiency.
Prophylactic parenteral antibiotics are no longer recommended as there is no evidence that they reduce either infective complications or mortality.
Endoscopy and surgery in severe acute pancreatitis: All patients suspected of having or proven to have a gallstone aetiology should undergo urgent therapeutic ERCP, which should take place within 72 hours of the onset of pain. This applies whether severe pancreatitis is predicted or confirmed. All of these patients require sphincterotomy of the sphincter of Oddi whether or not stones are found in the common bile duct. If stones are seen or if cholangitis or jaundice is present, biliary stenting is usually required.
There is no role for surgery during the acute attack but in patients with stones, laparoscopic cholecystectomy with operative cholangiography should be performed before discharge from hospital. This is because deferring cholecystectomy until months later increases the risk of another attack. In the small group of critically ill patients with infected necrotic tissue and infected peripancreatic fluid collections, surgical debridement with or without continuous peritoneal irrigation is unavoidable, but mortality remains high.
Complications of acute pancreatitis
Mortality: About 15% of patients admitted to hospital with acute pancreatitis have severe disease, which carries a high risk of potential complications. However, about a further 10% initially diagnosed with mild pancreatitis deteriorate markedly during admission and become severe. In severe pancreatitis, mortality is 10–30%, i.e. 2–5% of all cases, and obese patients have a higher mortality. About half of those that die, do so within the first week, usually of ARDS and pulmonary failure. Other early life-threatening complications are associated with multiple organ dysfunction (MODS). Manifestations include cardiovascular collapse aggravated by fluid shifts, renal insufficiency made worse by hypotension, and disseminated intravascular coagulopathy. If death occurs after the first week, infective complications of pancreatic necrosis added to existing organ failure are the usual cause.
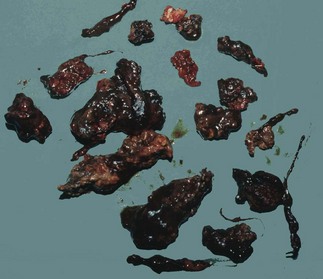
Pancreatic necrosis and infection: In severe pancreatitis, pancreatic and peripancreatic necrosis may manifest during the first 2 weeks of the attack (see Fig. 25.1). Necrosis is identified using intravenous contrast-enhanced CT scanning in which necrotic pancreas does not opacify, having lost its blood supply. Infection of devitalised pancreatic and peripancreatic tissues occurs in about one-third of patients with severe pancreatitis, and is often lethal. Infection may be suspected on CT by gas bubbles in pancreatic fluid collections but can only be confirmed by percutaneous aspiration (with microscopy and culture of the aspirate), usually performed under CT guidance. In proven infected cases, operative debridement, drainage and sometimes continuous peritoneal irrigation may be necessary, along with aggressive organ support.
Fluid collections around the pancreas: During the initial attack, acute peripancreatic fluid collections may develop. Most resolve spontaneously, but for those that do not, CT-guided percutaneous drainage is valuable. Fluid collections persisting for longer than 6 weeks are termed pancreatic pseudocysts (see below). Late in the course of the disease, a pancreatic abscess may appear. This is a well-localised collection of pus within the gland, and contrasts with infected necrotising pancreatitis which appears earlier and is not localised.
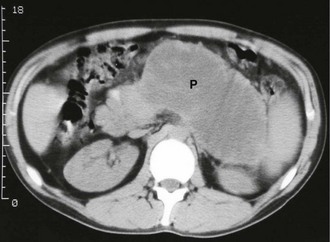
Pancreatic pseudocyst: A pancreatic pseudocyst is a collection of pancreatic enzymes, inflammatory fluid and necrotic debris, usually encapsulated within the lesser sac. Pseudocysts appear in 1–8% of cases of acute pancreatitis and are less likely to resolve spontaneously than the early acute fluid collections. A pseudocyst is not a true cyst (i.e. there is no epithelial lining) although the surrounding tissues become thickened by the inflammatory response. Pseudocysts may occur after even a moderate attack of pancreatitis and sometimes reach the size of a football! An upper abdominal mass may be palpable. If a pseudocyst is suspected, CT scanning is the investigation of choice (see Fig. 25.2).
Management varies according to the size of the cyst. Larger cysts, especially those larger than 10 cm, are unlikely to resolve. Those around 6 cm can be safely observed for up to 6 months, provided they have a typical appearance on CT scanning and are asymptomatic. If the cyst has failed to resolve by then, operative intervention should be considered. Operation, either by laparoscopy or an open approach, involves ‘marsupialising’ the pseudocyst into the posterior wall of the stomach. This can be performed after about 6 weeks, when the wall of the pseudocyst has ‘matured’ enough to hold sutures.
Pancreatic abscess: Pancreatic abscesses occur in 1–4% of cases of acute pancreatitis. Certain patients remain systemically well despite pancreatic necrosis, with an illness that may grumble on for several weeks. There is a recurrent high swinging fever indicating the presence of an abscess. By that time, the necrotic pancreas is likely to have formed a discrete grey mass lying free within the pancreatic bed and bathed in pus. Pus may extend widely in retroperitoneal tissues. Surgery is then required to remove the necrotic tissue and drain the abscesses.
Complications of severe acute pancreatitis: Severe acute pancreatitis can cause wide-ranging complications in almost every body system:
• Multi-organ failure may lead to renal failure, respiratory failure, cardiac failure, and haematological and coagulation disorders
• Direct pressure effects, inflammation and hypotension may cause portal vein thrombosis
• Local pressure plus hypotension may cause bowel ischaemia—the transverse colon is commonly affected because of the position of the middle colic artery
• Pseudoaneurysms may form in vessels such as the splenic artery because of inflammatory damage to the arterial wall and fluid collections around them
• Internal pancreatic fistulae may form, particularly if necrosis causes disruption of the pancreatic duct or the wall of a pseudocyst. The result may be pancreatic ascites, mediastinal pseudocysts, enzymatic mediastinitis or pancreatic pleural effusions
Recurrent and Chronic Pancreatitis
Some patients suffer recurrent attacks, usually resulting from alcohol abuse or gallstone disease. The first and second attacks may be severe, but attacks after that almost never have lethal complications. The patient is entirely well between attacks. Attacks vary in severity in different patients but are rarely extreme.
Chronic pancreatitis
Some patients suffer persistent and severe upper abdominal pain, similar in character to a prolonged attack of acute pancreatitis. These patients do not develop the other features of acute pancreatitis and may not have elevated amylase levels. The pain is so severe and so persistent as to drive some patients to suicide. Carcinoma of the pancreas and chronic pancreatic inflammation should both be considered in patients with this pattern of pain. Inflammatory swelling of the pancreatic head occasionally causes obstructive jaundice but carcinoma in this position is a far more common cause of jaundice.
Despite the pain, there are usually no abnormal abdominal signs. Plasma amylase may be moderately elevated on occasions; the diagnosis of chronic pancreatitis may, however, be missed if raised amylase is not detected (because tests are not done at an appropriate time or the patient never has elevated levels). There is a danger these patients may be dismissed as suffering from psychosomatic pain.
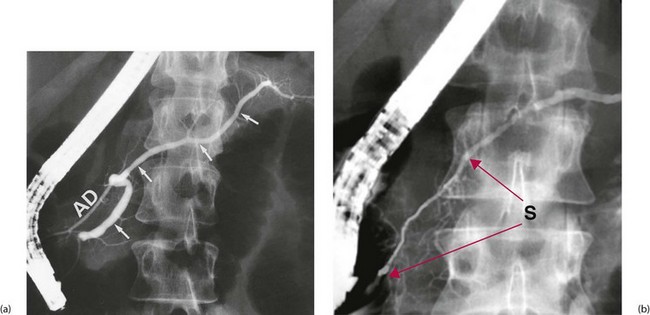
In chronic pancreatitis, ultrasound or CT scans may show glandular swelling (sometimes difficult to differentiate from pancreatic carcinoma) and a dilated pancreatic duct. If ERCP is performed, the pancreatic duct system may look normal or else may be distorted and irregular in calibre, confirming chronic inflammation and fibrosis (see Fig. 25.3). Sometimes pancreatic duct stones are shown.
Chronic pancreatitis may cause years of misery, perhaps eventually ‘burning out’ as the gland atrophies completely. It is important to make the diagnosis early so pain can be relieved. In the long term, malabsorption or diabetes is more likely to develop than after acute pancreatitis.
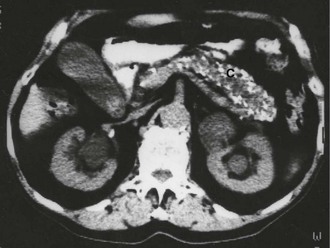
Pancreatic calcification seen on abdominal X-rays is diagnostic of chronic pancreatitis but is a rare finding and is sometimes found in asymptomatic patients (see Fig. 25.4). X-rays are therefore of little clinical value.
Treatment of chronic pancreatitis is far from satisfactory. Surgery is only useful if structural abnormalities can be found. Procedures include removal of pancreatic duct stones, partial pancreatectomy of body and tail for duct stenosis, sphincteroplasty of the pancreatic duct opening, or occasionally total pancreatectomy. Chemical coeliac ganglion blockade provides useful (and often permanent) pain relief, but does nothing to prevent inflammation. If there are multiple duct strictures, the pancreatic duct can be surgically split along its whole length and the side of a loop of jejunum sutured to it to allow unrestricted drainage. Interventional endoscopy can be employed for dilatation and stenting of isolated pancreatic duct strictures.