Anal and perianal disorders
Introduction
Anal and perianal disorders make up about 20% of general surgical outpatient referrals. These conditions can be distressing or embarrassing and patients often tolerate symptoms for a long time before seeking medical advice. The common anal symptoms are summarised in Box 30.1 and interpretation is discussed in Chapter 18.
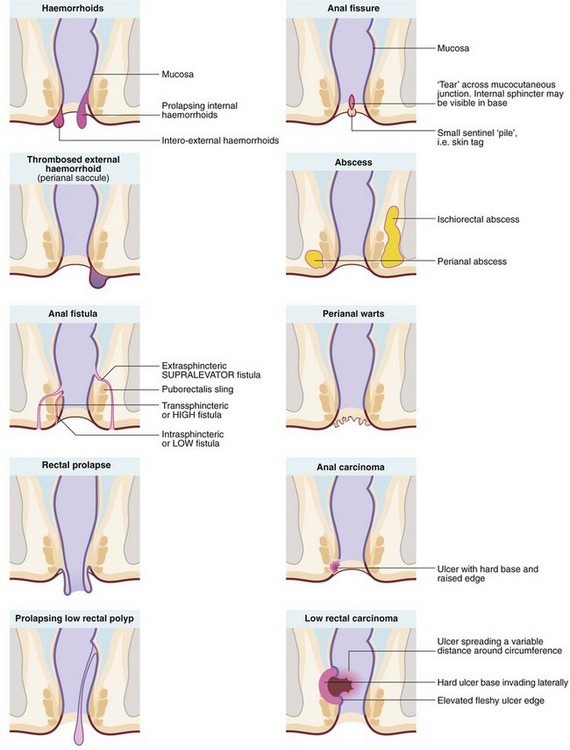
The range of anal and perianal disorders is illustrated in Figure 30.1. Haemorrhoids and other common benign conditions must be distinguished from rectal carcinoma and the rare anal carcinoma. Most anal and perianal conditions can be treated on an outpatient basis, although abscesses, and haemorrhoids that have become strangulated or thrombosed may present as surgical emergencies.
Anatomy of the anal canal
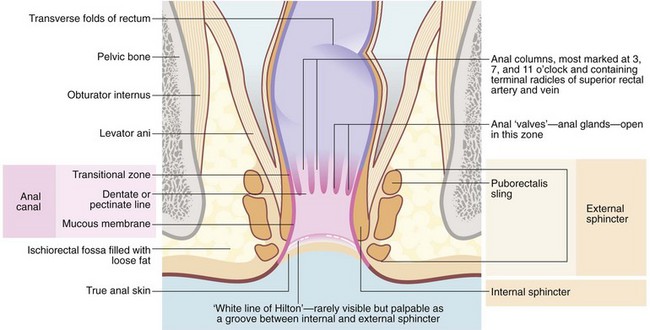
At the anal verge outside the anal canal, there is normal skin composed of stratified squamous epithelium with skin appendages—sweat glands, hair follicles and sebaceous glands. The anal canal proper is about 4 cm long, extending from the lower to the upper border of the internal sphincter (see Fig. 30.2). There are three zones, each with different lining epithelium:
• The lowest or distal zone lies between the squamous–mucocutaneous junction and the level of the anal valves at the dentate (pectinate) line. This is lined by non-keratinising squamous epithelium without skin appendages or glands; the epithelium contains some melanocytes. This area is exquisitely sensitive, for example to injection
• The anal transitional zone (ATZ). This lies between the zone of squamous epithelium below and the columnar mucosal zone above, and extends a distance varying between 0.3 and 2 cm. It consists of transitional epithelium resembling urothelium, 4–9 cell layers thick. Anal glands are present in the submucosa but there is minimal mucin production. A unique type of anal carcinoma develops from it with a viral aetiology
• The upper part of the anal canal is lined by rectal mucosa. On proctoscopic inspection, it is a dark reddish-blue where it overlies the submucosal venous plexus, becoming the typical pink of colorectal mucosa more proximally. This area of mucosa is relatively insensitive
The mucosa of the upper part of the anal canal is thrown into 6–10 longitudinal folds, the columns of Morgagni, each containing a terminal branch of the superior rectal artery and vein. The folds are most prominent in the left lateral, right posterior and right anterior sectors where the vessels form prominent anal cushions. These are important in fine control of continence. They may become pathologically enlarged to form haemorrhoids, which are complex collections of arterioles, arteries, venules, venous saccules and connective tissue. The anal columns are not readily visible on proctoscopy but the transition between glandular rectal mucosa and anal skin is clearly visible. The lymphatics of the upper anal canal drain to the pelvic and abdominal lymph node chain, whereas the lower part of the anal canal drains to inguinal lymph nodes.
The anal sphincter mechanism has three constituents: the internal sphincter, the external sphincter and the puborectalis muscle. The internal sphincter represents a downward but thickened continuation of the rectal wall musculature. The encircling external sphincter and the puborectalis sling (part of levator ani) arise from the pelvic floor. Continence is maintained principally by the anal sphincters squeezing the three anal cushions together to occlude the lumen. Continence is assisted by the rectum forming a compliant reservoir to accumulate faeces.
Haemorrhoids
Haemorrhoids (piles) are extremely common, affecting nearly half of the population at some time. Men tend to suffer more often and for longer periods, whereas women are particularly susceptible in late pregnancy and the puerperium.
Pathogenesis of haemorrhoids
Constipation and pregnancy are the most common triggers for development of haemorrhoids. Lack of fibre in the modern Western diet is a likely factor. Straining during constipation raises intra-abdominal pressure which obstructs venous return, causing the venous plexuses to engorge. The bulging mucosa is then dragged distally by the hard stool. Furthermore, persistent straining causes the pelvic floor to sag downwards, extruding the anal mucosa and causing a small degree of prolapse. Haemorrhoids are usually located in the 3, 7 and 11 o'clock positions when viewed with the patient in the supine lithotomy position. These correspond to the anatomical positions of the anal cushions. The venous component causes a problem only if it becomes thrombosed to form a thrombosed external venous saccule (sometimes inaccurately labelled a perianal haematoma).
In pregnancy-related haemorrhoids, venous engorgement and mucosal prolapse are probably the main mechanisms. Progesterone mediates venous dilatation, and the fetus obstructs pelvic venous return.
Classification of haemorrhoids
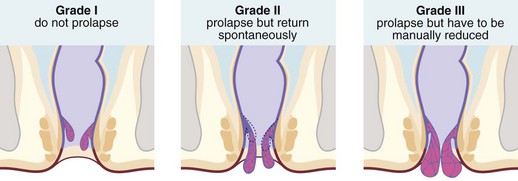
Haemorrhoids (piles) are classified into first, second and third degrees according to the extent of prolapse through the anal canal. First degree (or grade I) piles never prolapse; second degree (grade II) piles prolapse during defaecation and then return spontaneously; third degree (grade III) piles remain outside the anal margin unless replaced digitally (Fig. 30.3). Most haemorrhoids can be described as ‘internal’ because they are covered by glandular mucosa. Large neglected haemorrhoids may extend beneath the stratified squamous epithelium so their lower part becomes covered by skin. These are correctly described as ‘intero-external’ haemorrhoids, or more commonly ‘external piles’.
Symptoms and signs of haemorrhoids
Haemorrhoids often produce symptoms intermittently. Attacks last from a few days to a few weeks, often with complete freedom from trouble between times. Episodes of constipation are often a precipitating factor.
Any haemorrhoid may bleed from stool trauma during defaecation. Bleeding from the arterial component of the anal cushion results in the characteristic bright red rectal bleeding. Large haemorrhoids may prolapse and then thrombose, causing acute severe pain if venous return is obstructed by sphincter tone. Longstanding haemorrhoids eventually atrophy, probably by thrombosis and fibrosis, leaving small skin tags at the anal margin.
The common chronic or intermittent symptoms of haemorrhoids are:
• Perianal irritation and itching (pruritus ani) caused by mucus leakage. Scratching exacerbates the problem
• Rectal bleeding (fresh blood, on the paper or separate from stool)
• Mucus leakage due to imperfect closure of the anal cushions
• Mild incontinence of flatus also due to imperfect closure of the anal cushions
Most patients reaching the surgeon have tried various anaesthetic or soothing creams and suppositories, either self-administered or prescribed by the family practitioner. The usual reasons for referral are persistent symptoms or the need to exclude malignancy as a cause of bleeding.
On examination, external piles or skin tags may be visible in the anal area. Digital examination is essential to exclude carcinoma and provides a useful measure of anal tone. Haemorrhoids are not palpable unless they are large since the contained blood empties with finger pressure. Proctoscopy is needed to demonstrate internal piles, which are seen bulging into the lumen as the proctoscope is withdrawn. Sigmoidoscopy, rigid or ideally flexible, is important in patients over 40 years if there is a history of bleeding or any symptoms suspicious of malignancy; occasionally a rectal polyp will be diagnosed in this way. Since haemorrhoids are so common, they can mask an unrelated diagnosis of cancer.
Acute presentations of haemorrhoids
Thrombosed or strangulated haemorrhoids present with acute severe pain and many patients are admitted to hospital as emergencies. These complications are common in the late stages of pregnancy and soon after delivery. The diagnosis of thrombosed haemorrhoids is usually obvious on inspection as an oedematous, congested purplish mass at the anal margin. Tight spasm of the anal sphincter makes digital rectal examination extremely painful. Strangulated haemorrhoids are even more painful, and the strangulated mass may become necrotic or even ulcerated. Symptomatic relief is provided by several days of bed rest and application of ice packs and topical anaesthetic gel; this conservative treatment may be the best that can safely be offered in late pregnancy. Some surgeons favour urgent haemorrhoidectomy for thrombosed or strangulated piles, accepting the slightly higher risk of complications in exchange for a more rapid recovery and a shorter hospital stay. Prophylactic antibiotics should be given to cover the operation because of the risk of infection in necrotic tissue.
Conservative management and prevention of haemorrhoids
The most important means of preventing and treating haemorrhoids is avoiding constipation and ensuring a bulky stool. This is best achieved by taking a diet high in fibre. The patient should be advised to always heed the call to evacuate. This appears to be associated with a reflex release of lubricating mucus which may be absent later. Sufferers should be strongly encouraged to avoid straining and to spend minimal time defaecating. A prolonged ritual often leads to further straining at the end of defaecation when a mild prolapse can be experienced as incomplete evacuation of faeces. In many patients, these simple measures are enough to relieve symptoms. Note that repetitive straining occasionally leads to the formation of a ‘solitary ulcer’ on the anterior wall of the proximal anal canal, which may be clinically indistinguishable from a malignant ulcer. With third degree haemorrhoids, symptoms can often be relieved by the patient replacing the prolapsing haemorrhoids digitally after defaecation.
Creams, suppositories and other topical preparations available with or without prescription are very widely used. Some contain local anaesthetic agents or steroids. They are useful for temporary symptomatic relief but do nothing to treat the underlying condition. Overuse may cause allergic reactions, maceration of the perianal skin and secondary infections.
Surgical treatments for haemorrhoids
Injection of sclerosants or banding
First degree haemorrhoids which do not regress with dietary change and avoiding straining, and most second degree haemorrhoids, can be treated on an outpatient basis by sclerosant injections or banding.
In sclerotherapy, with the aid of a proctoscope, 1–3 ml of a mildly irritant solution of 5% phenol in oil is injected submucosally around the pedicles of the three major haemorrhoids in the insensitive upper anal canal. This provokes a fibrotic reaction, effectively obliterating the haemorrhoidal vessels and causing atrophy of the haemorrhoids (see Fig 30.4). Injections are painless if the needle is placed correctly; direct injection into the haemorrhoid would be extremely painful. Sclerotherapy is usually repeated on 2–3 occasions at intervals of 4–6 weeks. Note: sclerotherapy is not suitable for patients with nut allergies because of the nut origin of the carrier oil.
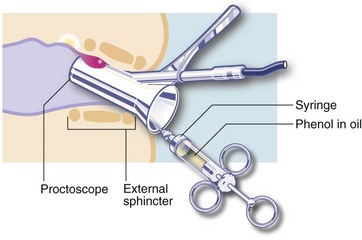
Fig. 30.4 Sclerotherapy or injection of heamorrhoids
A mildly irritant solution of 5% phenol in oil is injected submucosally around the pedicle of the haemorrhoid
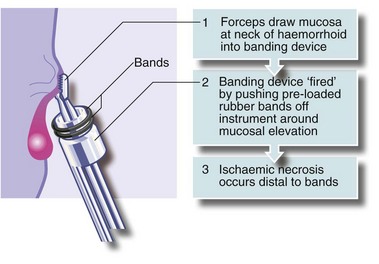
An alternative treatment is banding. A cone of mucosa just above the haemorrhoidal neck is drawn into a banding instrument, often by suction, and tight elastic bands released around the base of the cone, constricting the haemorrhoidal vessels (see Fig. 30.5). Importantly, the bands are not placed around the stalks of prolapsing haemorrhoids; this would be unbearably painful because of the somatic innervation of anal skin. The result of banding is that the haemorrhoid gradually shrinks. The bands separate with time and are passed.
Haemorrhoidectomy
Haemorrhoidal excision is indicated for third degree haemorrhoids and for lesser degrees when other treatments have failed. The most common operation is that described by Milligan and Morgan in which the haemorrhoidal masses are excised with overlying mucosa and some skin (Fig. 30.6). This leaves skin and mucosal defects which heal by secondary intention and wound contraction. A skin bridge must be preserved between each wound to prevent the serious late complication of anal stenosis. Stapled haemorrhoidectomy enjoyed some popularity for large haemorrhoids, particularly when mucosal prolapse is a feature. It aims to restore the anatomy of the anal cushions by excising a ring of low rectal mucosa, including the engorged necks of the piles. The metal staple line remains permanently in situ, palpable digitally and sometimes causing pain. This operation requires great skill and occasionally results in serious complications; this fact plus the residual staple line are contributing to a decline in interest in the operation.
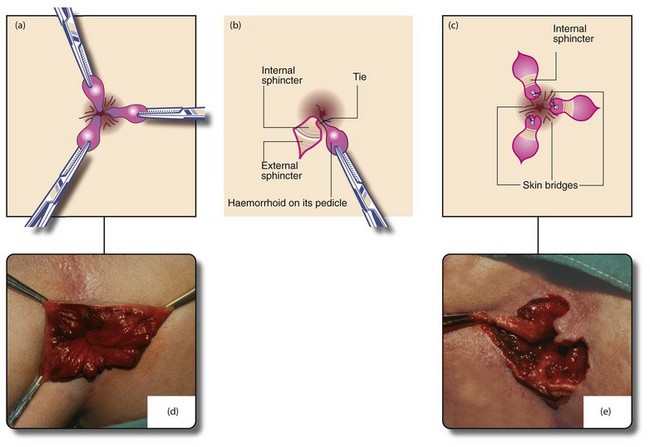
Fig. 30.6 Milligan–Morgan open haemorrhoidectomy
(a) and (d) Identification of the main haemorrhoids; the external part of each is clamped with a haemostat and retracted outwards. (b) Scissors are used to incise the skin around the external haemorrhoid, excess skin being excised at the same time. The haemorrhoid is then raised on its pedicle by dissecting it from the external and internal sphincters. The pedicle is transfixed and ligated at its base with an absorbable suture. The skin is not closed. (c) and (e) The process is repeated for the other primary haemorrhoids, ensuring that skin bridges are preserved at the anal margin between the areas resected or anal stenosis will occur as healing proceeds (‘If it looks like a dahlia, it’s a failure’). The completed haemorrhoidectomy has a ‘cloverleaf’ appearance (‘if it looks like a clover, it’s all over’). After haemostasis is ensured, wounds are dressed with a non-adherent dressing (e.g. Mepitel) and a surgical pad applied, held in place by elasticated net underpants
Before haemorrhoidectomy, stool softeners such as bulking agents and gentle laxatives should be given to avoid constipation afterwards. The painful early postoperative period can be greatly eased by caudal analgesia given at operation.
Haemorrhoidal artery ligation operation (HALO)
This is a promising new procedure without an incision. It involves locating the artery supplying each haemorrhoid using ultrasound, then encircling it with a stitch via the insensitive lower rectal mucosa to cut off its blood supply. Over the following few days the haemorrhoids shrink, bleeding and local symptoms abate, although skin tags remain.
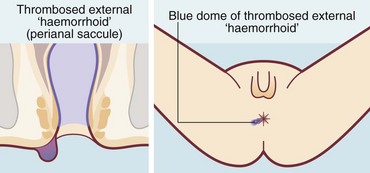
Thrombosed external haemorrhoids
A thrombosed external haemorrhoid or thrombosed external venous saccule is an acutely painful anal condition (Fig. 30.7). The onset is sudden and, if untreated, there is persistent pain lasting 1–2 weeks, worse on defaecation. On examination, a blue-black hemispherical bulge is seen in the skin near the anal margin. This is sometimes called a perianal haematoma but this is inaccurate. The condition can occur in patients with haemorrhoids but is usually seen in isolation.
Most thrombosed external haemorrhoids subside over a few days and patients need only oral analgesia. If pain is severe or prolonged, the thrombosis may be incised and drained under local anaesthesia; some surgeons favour this as first line therapy.
Anal fissure
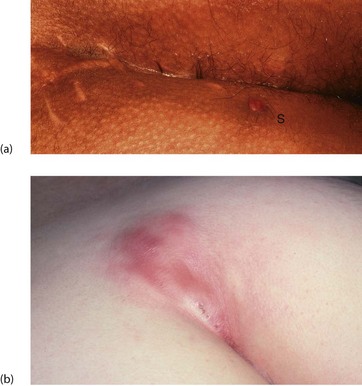
An anal fissure is a longitudinal tear in the mucosa and skin of the anal canal, sometimes caused by passing a large, constipated stool. The tear is nearly always in the posterior midline of the anal margin. The fissure causes acute pain during defaecation and sphincter spasm, both of which persist for an hour or longer. There is often a small amount of fresh bleeding at defaecation. The result is fear of defaecation and this aggravates the constipation. This history alone is diagnostic of an anal fissure. On inspection, the fissure is concealed by the anal spasm but a small skin tag (sentinel pile) may be seen at the superficial end of the fissure (see Fig. 30.8). Rectal examination is extremely painful and rarely possible unless the fissure has become chronic.
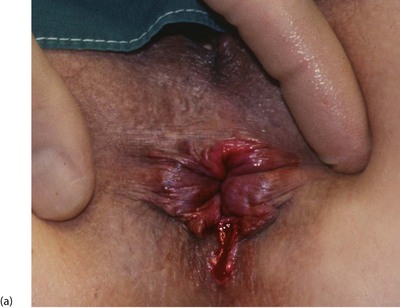
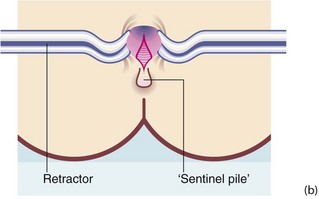
Fig. 30.8 Anal fissure
(a) Chronic anal fissure with a ‘sentinel pile’. Simple fissures are typically posteriorly located, as in this patient. (b) Explanatory diagram
Patients sometimes tolerate the pain of an acute fissure by using local anaesthetic creams and then present much later with a chronic anal fissure, prevented from healing by sphincter spasm and repeated tearing open of the fissure during passage of stools.
Management of anal fissure
Anal fissure can be managed conservatively or operatively. Modern conservative treatment involves the use of topical glyceryl trinitrate (GTN) ointment 0.2–0.4%, applied three times a day for a month. This relaxes the sphincter spasm and increases blood supply to the fissure, allowing healing. Patients need to be warned that it may cause headaches. This treatment can cure most anal fissures. For the rest, diltiazem ointment, a calcium channel blocker, may be successful. Injection of botulinum toxin into the sphincter complex is another way to cause a temporary ‘chemical sphincterotomy’.
Surgery in the form of lateral submucous (internal) sphincterotomy brings more immediate relief, but there is a 10–15% incidence of incontinence of flatus following this procedure. Surgeons tend to be reluctant to offer sphincterotomy to women because their sphincters are shorter and less robust, and because occult sphincter injury from childbirth may already have occurred. However, if conservative treatments fail and the patient is suitably informed of the risks, an internal anal sphincterotomy may be offered. This operation involves dividing about 1 cm of the lower rim of the internal sphincter via a small lateral incision. For chronic refractory fissures, anal advancement flap operations are sometimes performed; this avoids the sphincter muscle damage caused by sphincterotomy. Lord's anal stretch, which involved manual dilatation of the sphincter, led to unacceptable rates of incontinence due to sphincter damage and has long been abandoned.
Anorectal abscesses
Pathophysiology and clinical features
Abscesses in the anorectal area are common surgical emergencies. They present with constant and often severe perineal pain and local tenderness.
Anorectal abscesses begin as acute purulent infections of anal glands. These lie in the intersphincteric space between the internal and external sphincters and drain into tiny pits, the anal crypts near the dentate line. The ducts are very narrow and duct obstruction may be what initiates the infection. Rarely, an abscess remains confined between the sphincters and an intersphincteric abscess results. The only symptom may be chronic anal pain, and there is often little to find on clinical examination. The only clue may be localised tenderness on rectal palpation.
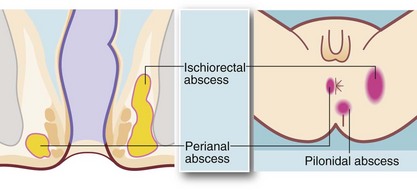
From the intersphincteric plane, infection tends to spread in one or more of three directions (see Fig. 30.9):
• Downwards between the sphincters towards the anal verge, forming a perianal abscess. This is the most common presentation and accounts for 80% of anorectal abscesses. The patient presents acutely with a painful, tender, red swelling close to the anal verge
• Outwards through the external sphincter into the loose fibro-fatty tissue of the ischiorectal fossa, forming an ischiorectal abscess. There is little barrier to spread of infection once it has entered this space and a neglected or inadequately treated abscess may become enormous. Ischiorectal abscesses make up about 15% of anorectal abscesses. The patient presents with perineal pain and systemic signs of infection. There is tenderness over the ischiorectal fossa lateral to the anus but there may be no visible redness or swelling; rectal palpation reveals a tender mass lateral to the rectum
• Upwards between the sphincters to form a supralevator abscess, involving the pararectal tissues above the pelvic floor. These make up less than 5% of anorectal abscesses and present with systemic signs of infection, rectal pain and difficulty in micturition. On rectal examination, a tender mass is often palpable near the tip of the finger
Treatment of anorectal abscesses
If perianal infection is seen very early, oral antibiotic treatment may abort it. Antibiotics used in this way by general practitioners, coupled with early referral, has reduced the number and severity of cases reaching the surgeon. However, once an abscess is diagnosed, surgical drainage is needed; antibiotics are only indicated in addition when there is spreading infection. Drainage is performed after careful examination (EUA) to determine the extent of the abscess under regional or general anaesthesia. Perianal and ischiorectal abscesses are drained via the perianal skin, ensuring all loculations are broken down. An intersphincteric abscess is drained via an internal sphincterotomy. A swab of the pus is sent for microbiological diagnosis to differentiate infection by skin pathogens (e.g. Staphylococcus) which occur spontaneously, from infections of bowel origin (e.g. E. coli) which suggest an underlying fistula. Large ischiorectal abscesses require packing or placement of a drain to keep the neck of the cavity open whilst granulation tissue gradually fills the space from its depths. Further examinations under anaesthesia after a few days are usually planned to ensure complete drainage and to inspect for fistulae. Supralevator abscesses are more complicated and require complex staged surgical procedures.
Incising a perianal abscess results in complete resolution in about 50% of cases; the other half develop an anal fistula (see below). The fistula is usually undetectable at the time of drainage but is recognised by a persistent discharge near the skin incision for several weeks afterwards (see Fig. 18.6).
Differential diagnosis of abscesses in the perianal area includes:
• Crohn's disease—may cause multiple abscesses and complex fistulae (see Ch. 28) and must be excluded
• Hidradenitis suppurativa—originates in perianal apocrine glands in the skin; it is easily distinguished from deeper perianal abscesses by careful inspection and palpation. There may be multiple infected glands in the natal cleft, groins and sometimes axillae
• Pilonidal abscess—occurs in the skin of the natal cleft (see Fig. 30.9) but may mimic a true perianal abscess if near the anal margin; careful examination shows no communication with the anal canal and often the presence of embedded hairs. Treatment is by incision and drainage but further procedures may be required to treat the associated pilonidal sinus (see p. 393)
Anal fistula
Anal fistulae usually develop as a complication of perianal, ischiorectal or supralevator abscesses. A fistula is an abnormal connection between two epithelial surfaces and consists of a chronically infected tract which may eventually become epithelialised. It extends from an internal opening at the level of the dentate line, and passes through the site of previous abscess to an external opening on the perianal skin near the old drainage scar. The communication between abscess cavity and bowel is established by spontaneous discharge of the enlarging abscess into the bowel before surgical drainage or after incomplete surgical drainage. To minimise the risk of fistula, any abscess in the anal region should be drained early and thoroughly.
The patient with a fistula typically complains of intermittent discharge of mucus or pus in the perianal region, often faecally stained. On examination, a small papilla of granulation tissue is seen on the skin within 2–3 cm of the anal margin (see Fig. 18.6). Pus may be expressed from it by compressing the underlying tract digitally between papilla and anus. This clinical picture is diagnostic of an anal fistula but this apparently trivial skin lesion is often dismissed as a pustule or an incompletely healed perianal abscess.
Most anal fistulae are simple and relatively superficial, with the internal opening located in a crypt at the level of the dentate line (well below puborectalis), most often in the posterior midline. These are known as low anal fistulae (Fig. 30.10). For successful treatment, it is essential to locate the internal opening so the entire tract can be dealt with.
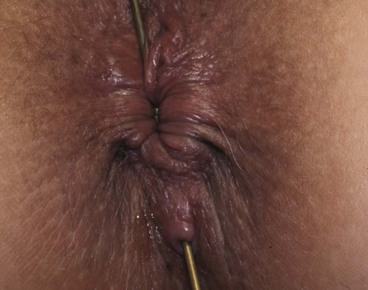
Fig. 30.10 Anal fistula
A probe has been passed from the skin surface through a low anal fistula to emerge in the anal canal at the level of the dentate line. Treatment consisted simply of cutting down onto the probe, laying open the fistula along its length. The wound was left to heal by secondary intention
Goodsall's rule helps to predict the course of a low fistula:
• If the external opening is in front of an imaginary transverse line across the anus, the fistula is likely to have a short direct tract to the anal canal
• If the external opening is behind the transverse line, the tract is likely to have a curved course towards an internal opening in the posterior midline
Assessment and treatment of fistulae requires general or regional anaesthesia. Examination under anaesthetic (EUA) is performed first. A malleable probe is gently manipulated through the fistula to try to demonstrate the internal orifice. If this is not found, hydrogen peroxide can be gently injected into the external opening. Provided the fistula is superficial and involves less than half of the sphincter bulk, treatment is by laying open the entire tract by cutting down on to the probe with diathermy, transecting the anal margin and opening the whole length of the fistula. This is known as fistulotomy and involves dividing some of the internal and external sphincter. The wound heals gradually by secondary intention. There should be no loss of faecal continence, but flatus may be less well controlled. Attempts have been made to deal with some fistulae by using tissue glues or fibrin packs but results so far are unimpressive.
If the fistula involves more than half the length of the anal sphincter complex, surgical treatment is difficult and highly specialised because of the need to retain the functional integrity of the sphincters to preserve continence. Where complex fistulae are suspected, the anatomy can be defined well by magnetic resonance imaging (MRI) so that careful staged surgery and/or appropriate conservative measures can be planned.
In many of the more severe cases, primary surgical cure is not attempted and infection is controlled long-term by placing a soft Seton or thread through the tract and out through the anus, where it is tied loosely to form a ring. This maintains free drainage of pus and reduces the risk of abscess formation, whilst the Seton goes largely unnoticed by the patient. After a long period of quiescence, the Seton may be removed with the hope that the tract will close. In the worst cases, where there is extensive destructive involvement of the anal sphincters in the infective process, the only surgical cure is perineal excision of the whole anal canal and lower rectum, and a permanent end colostomy. In exceptional cases, patients may choose this option to improve their quality of life.
Anal fistulae sometimes occur as a manifestation of Crohn's disease. Such fistulae tend to be multiple and in the most extreme cases form a ‘pepper-pot’ perineum (see Fig. 28.8, p. 371).
Pilonidal sinus and abscess
These conditions arise from the skin of the natal cleft rather than the anus. As the name implies, pilonidal sinuses, cysts and abscesses contain ‘a nest of hairs’. They are common in young adults, particularly hirsute men, and are found at the upper end of the natal cleft. Here, between the buttocks, there is often a congenital dimple or pit. Fragments of hair falling from the back or head accumulate in this nidus. The hairs slowly work their way into the dermis, with the cuticular scales on the hairs acting like barbs of an arrow. The process is encouraged by the massaging effect of sitting for long periods; pilonidal sinus is thus common in truck and tractor drivers. Sinuses also occur between the hairdresser's fingers from implantation of their clients' hair (see below).
Pilonidal sinuses tend to run a long indolent course with chronic or intermittent purulent discharge to the skin surface via one or more sinuses. Periodic acute exacerbations may progress to abscesses (Fig 30.11).
Pilonidal abscess
The mass of hairs and other skin debris in a pilonidal sinus excites a foreign-body inflammatory reaction, often resulting in a mildly or intermittently discharging sinus. If the cavity becomes secondarily infected, an abscess develops and causes acute pain and swelling. Pilonidal abscesses are often multilocular. They sometimes drain spontaneously but rarely heal completely. Many require surgical drainage because of pain.
Treatment of pilonidal sinus
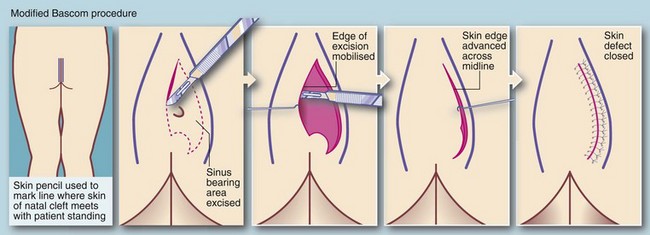
Definitive treatment aims to eliminate the nidus of hairs and associated cystic cavities, chronic abscesses and sinuses. At operation, obvious plugs of hair are first removed and then the sinus network is explored with probes. In the past, surgical excision left large tissue defects extending to the sacral fascia which took months to heal. Currently the favoured surgical treatment is the Bascom ‘cleft lift’ procedure which may have the secondary advantage of flattening the cleft to minimise recurrence (see Fig. 30.12).
Despite surgery, pilonidal lesions commonly recur, although the Bascom procedure is promising in this respect. Recurrence may also be reduced by careful attention to hygiene. Daily baths and regular shaving of the area are recommended.
Rectal prolapse
Rectal prolapse is a herniation of the rectum through the pelvic floor, so the mucosa and muscle wall effectively intussuscept through the anal canal (see Fig. 30.13). It is mainly seen in young children and the elderly.
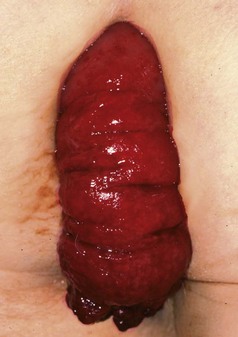
Fig. 30.13 Rectal prolapse
This complete rectal prolapse was in an 80-year-old woman. It emerged spontaneously whenever she stood, causing considerable discomfort and inconvenience, to say the least!
In childhood, prolapse usually occurs around the age of 2 years. It tends to occur during toilet training and causes parental anxiety. Parents should be reassured that the prolapse will return spontaneously after defaecation or if not, gentle manipulation using water-soluble lubricant jelly may be required. These children should be given a high-fibre diet and taught not to strain during defaecation. More sophisticated treatment is rarely required.
In the elderly, rectal prolapse initially occurs only with defaecation and retracts spontaneously. Sometimes the patient has to reduce the prolapse manually, often with little complaint. At a later stage, prolapse may occur when the patient merely stands up. This can lead to incontinence because of dilatation of the internal anal sphincter. The patient becomes reluctant to leave home and often becomes socially isolated and is then likely to require surgical treatment.
Management of rectal prolapse
Rectal prolapse can be treated by abdominal or perineal procedures, or a combination of both. The abdominal operations, which may be performed laparoscopically, include two main types:
• Suture fixation rectopexy, where the rectum is mobilised and the mesorectum sutured to the sacral promontory and presacral fascia
• Resection rectopexy, where the rectum is mobilised and sutured in the same way, but a sigmoid colectomy is also performed to try to prevent the constipation that often accompanies suture fixation alone
The most popular perineal procedure is Delorme's operation, which is appropriate for most elderly patients because of its low morbidity and mortality. It involves excising redundant rectal mucosa, plicating the rectal wall and replacing the prolapsed rectum. More radical abdominal procedures or any procedure involving an anastomosis (e.g. Altemeier's perineal sigmoidectomy) is usually contraindicated because of greater risk.
Faecal incontinence (Table 30.1)
The process of maintaining continence is complex, involving higher behavioural control, sensory and motor pathways and the anal sphincter mechanisms. In addition, the rectal reservoir must function effectively. The continence mechanism has evolved to cope principally with semi-solid faeces and may fail if the stool is fluid. Incontinence presents in varying degrees: first for flatus, then for fluid and finally for solids as control is progressively lost. Declining mobility may also be a factor: mild incontinence that would otherwise be manageable may become a problem where debility and immobility impair the patient's ability to move to the toilet when required.
Table 30.1
| Underlying problem | Disorders |
| Anorectal incontinence—pudendal neuropathy (previously known as ‘idiopathic faecal incontinence’), anal sphincter and pelvic floor damage | Obstetric damage, operative damage, radiation damage, rectal prolapse, high anal fistula |
| Colorectal disease | Inflammatory bowel disease; polyps and tumours in rectum and anal canal |
| Faecal quality | Diarrhoea from any cause including infective; faecal impaction with overflow diarrhoea and incontinence |
| Rectal reservoir and sensation | Inflammatory bowel disease |
| Brain and higher cerebral functioning | Neurological disorders—dementias, psychological disturbances, impaired consciousness |
| Sensorimotor pathways | Spinal injury; neurological disorders |
| Mobility and access to toilet | Enforced bed rest or impaired mobility |
Incontinence is socially debilitating. It is surprisingly common but often concealed. It particularly affects some younger parous women and many elderly people. Social embarrassment forces patients to alter their lifestyles so that they never stray far from a lavatory, using constipating agents like loperamide or simply staying at home all day. Assessment of the severity of incontinence should include enquiring about these coping strategies, as patients or carers may be too embarrassed to volunteer them. Standard rating scales can be used to assess incontinence and compare treatments, e.g. the Cleveland Clinic faecal incontinence scale.
Young faecally incontinent patients are mostly female and usually suffer from anorectal incontinence. In the elderly, the aetiology is usually multifactorial.
Anorectal incontinence
The main functional abnormality in anorectal incontinence is weakness of the external anal sphincter and pelvic floor muscles. This is sometimes due to direct injury from trauma or surgery but most cases were labelled idiopathic. It is now recognised that the most important cause of sphincter dysfunction in women is obstetric injury. Repeated childbirth, episiotomies or difficult forceps deliveries increase the risk. The mechanism is probably via traumatic pudendal neuropathy leading to atrophy of sphincteric and pelvic floor muscles. Even chronic straining at stool may cause pudendal neuropathy. In old age, degenerative changes in the spinal cord appear to be the principal cause of muscle atrophy.
If sphincters have been physically damaged, surgical sphincter repair may be undertaken but results are not always predictable or long-lasting. Continuous sacral nerve stimulation, where an implanted ‘pacemaker’ promotes increased sphincter tone, can be attempted. As a last resort, a colostomy may allow the patient a better quality of life.
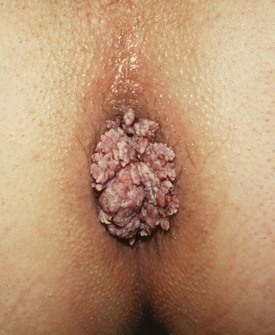
Anal warts (condylomata accuminata)
Warts in the perianal region (see Fig. 30.14) have a viral aetiology (human papilloma-virus (HPV) types 6 and 11) and are generally transmitted by sexual activity. Just as cervical cancer is linked to specific strains of HPV infection, anal warts indicate an increased risk of anal canal carcinoma by virtue of their common aetiology. Immune suppression, for example in patients with organ transplants or with HIV infection, can lead to rapidly developing anal warts and progression to malignant change.
In small numbers, anal warts can be treated by topical applications of podophyllin. When large numbers are present, surgical excision under general anaesthetic is the only practical option. This involves meticulous excision of each individual wart by electrocautery. The normal skin between the warts is carefully preserved to avoid delayed healing or the disastrous complication of anal stenosis. Carefully mapped biopsies can also be undertaken to monitor for dysplastic change. In the future, prevention will come from human papilloma virus (HPV) vaccines in both sexes, shown to prevent papillomavirus-induced cervical cancer, genital warts, and some oral cancers.
Squamous cell carcinoma of the anus
The annual incidence of anal cancer in women and the general population is about 1 : 100 000; however, in men who have sex with men and who are HIV negative, the incidence markedly increases to 35 : 100 000 and it doubles if the men are HIV positive. The sharp increase of mainly non-keratinising cancers arising in the transitional zone in these groups is largely due to heightened rates of infection with HPV and the effects of immunosuppression fostering its progress. An effective vaccine has now been developed for HPV and, if administered in the early teens, should prevent infection and its consequences.
Clinical features
The symptoms of anal carcinoma are similar to those of haemorrhoids and other familiar benign anal conditions, namely fresh rectal bleeding, anal pain, discomfort and discharge. Later, incontinence can result from involvement of the anal sphincter. The patient may ignore the symptoms for a period and the doctor may initially overlook the diagnosis. On digital rectal examination, a localised firm or hard ulcer or a growth with an irregular surface and edge may be palpable and there may be surrounding woody induration. The lesion is usually visible on proctoscopy and the diagnosis confirmed by biopsy. Palpation of the groins may reveal hard, matted involved inguinal nodes. Anal canal carcinoma also metastasises to intra-abdominal superior rectal nodes, reflecting the mixed drainage of the anal canal.
Chemoradiotherapy has now largely superseded abdomino-perineal excision because of demonstrably better cure rates for non-surgical therapy.
Other rare anal neoplasms
The anal canal is the third most common site for malignant melanomas after skin and the eye. These cause non-specific anal symptoms of discomfort and bleeding and diagnosis may thus be delayed. These melanomas are usually non-pigmented and biopsy evidence is needed to make a firm diagnosis. Treatment outcomes are poor. Adenocarcinoma of the rectum may extend distally into the anal region, and rarely basal cell carcinomas may occur here.
Proctalgia fugax
Proctalgia fugax is a neuropathic type of pain which often manifests as brief episodes of severe lancinating pain in the perineum with sphincter spasms. It occurs at unpredictable times but often at night. It usually occurs independently of conditions such as anal fissure, complicated piles and anal or rectal neoplasms; the patient may be convinced that cancer is the cause and may suspect that the doctor thinks it is ‘all in the mind’. The cause is unknown but is believed not to be psychological. Whilst it is difficult to cure, reassurance and recommending ice packs or warm baths may help. If this fails, therapy with amitriptyline or gabapentin may control symptoms.
Pruritus ani
Anal itching can be distressing. It is generally caused by mucus leakage and may occur without significant haemorrhoids; even a minute quantity of leaking mucus or faecal material causes profound irritation. Patients generally try to self-medicate with various creams but these tend to increase skin maceration and worsen the problem. Pruritus can be helped greatly if all topical creams are stopped and the perineum is washed and dabbed dry after defaecation using plain water. Even soap can aggravate the symptoms.