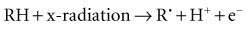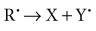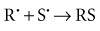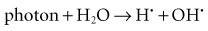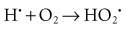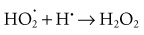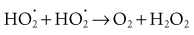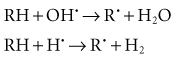Radiobiology
Radiobiology is the study of the effects of ionizing radiation on living systems. This discipline requires studying many levels of organization within biologic systems spanning broad ranges in size and temporal scale. The initial interaction between ionizing radiation and matter occurs at the level of the electron within the first 10−13 second after exposure. These changes result in modification of biologic molecules within the ensuing seconds to hours. In turn, the molecular changes may lead to alterations in cells and organisms that persist for hours, decades, and possibly even generations. These changes may result in injury or death.
Radiation Chemistry
Radiation acts on living systems through direct and indirect effects. When the energy of a photon or secondary electron ionizes biologic macromolecules, the effect is termed direct. Alternatively, a photon may be absorbed by water in an organism, ionizing some of its water molecules. The resulting ions form free radicals (radiolysis of water) that in turn interact with and produce changes in biologic molecules. Because intermediate changes involving water molecules are required to alter the biologic molecules, this series of events is termed indirect.
DIRECT EFFECT
In direct effects, biologic molecules (RH, where R is the molecule and H is a hydrogen atom) absorb energy from ionizing radiation and form unstable free radicals (atoms or molecules having an unpaired electron in the valence shell). Generation of free radicals occurs in less than 10−10 second after interaction with a photon. Free radicals are extremely reactive and have very short lives, quickly reforming into stable configurations by dissociation (breaking apart) or cross-linking (joining of two molecules). Free radicals play a dominant role in producing molecular changes in biologic molecules.
Because the altered biologic molecules differ structurally and functionally from the original molecules, the consequence is a biologic change in the irradiated organism. Approximately one third of the biologic effects of x-ray exposure result from direct effects. However, direct effects are the most common outcome for particulate radiation such as neutrons and α particles.
RADIOLYSIS OF WATER
Because water is the predominant molecule in biologic systems (about 70% by weight), it frequently participates in the interactions between x-ray photons and biologic molecules. A complex series of chemical changes occurs in water after exposure to ionizing radiation. Collectively these reactions result in the radiolysis of water.
Although the radiolysis of water is complex, on balance water is largely converted to hydrogen and hydroxyl free radicals. When dissolved oxygen is present in irradiated water, hydroperoxyl free radicals may also be formed:
Hydroperoxyl free radicals contribute to the formation of hydrogen peroxide in tissues:
Both peroxyl radicals and hydrogen peroxide are oxidizing agents and are the primary toxins produced in the tissues by ionizing radiation.
INDIRECT EFFECTS
Indirect effects are those in which hydrogen and hydroxyl free radicals, produced by the action of radiation on water, interact with organic molecules. The interaction of hydrogen and hydroxyl free radicals with organic molecules results in the formation of organic free radicals. About two thirds of radiation-induced biologic damage results from indirect effects. Such reactions may involve the removal of hydrogen:
The OH· free radical is more important in causing such damage.
Organic free radicals are unstable and transform into stable, altered molecules as described in the earlier section in this chapter on direct effects (p. 18). These altered molecules have different chemical and biologic properties from the original molecules.
Both direct and indirect effects are completed within 10−5 second. The resulting damage may take hours to decades to be come evident.
CHANGES IN DEOXYRIBONUCLEIC ACID
Damage to a cell’s deoxyribonucleic acid (DNA) is the primary cause of radiation-induced cell death, heritable (genetic) mutations, and cancer formation (carcinogenesis). Radiation-induced changes in protein, lipids, and carbohydrates after low or moderate doses (up to 10 Gy) of radiation are so slight that they do not contribute to radiation effects.
Radiation produces a number of different types of alterations in DNA, including the following:
• Breakage of one or both DNA strands
• Cross-linking of DNA strands within the helix to other DNA strands or to proteins
The most important of these types of damage are single- and double-strand breakage. Most single-strand breakage is of little biologic consequence because the broken strand is readily repaired by using the intact second strand as a template. However, misrepair of a strand can result in a mutation and prevent cell division. If germ line cells are involved, this may lead to heritable effects. If somatic cells are involved, this may also lead to cancer. Double-strand breakage occurs when both strands of a DNA molecule are damaged. If the damaged sites on each strand are far apart, they are readily repaired. However, if the breaks are at the same location or within a few base pairs, then repair is complicated by the lack of an intact template strand and misrepair is common. Double-strand breakage is believed to be responsible for most cell killing, carcinogenesis, and heritable effects.
Deterministic and Stochastic Effects
Radiation injury to organisms results from either the killing of large numbers of cells (deterministic effects) or sublethal damage to individual cells that results in cancer formation or heritable mutation (stochastic effects). The differences between deterministic and stochastic effects are shown in Table 2-1.
TABLE 2-1
Comparison of Deterministic and Stochastic Effects of Radiation
| DETERMINISTIC EFFECTS | STOCHASTIC EFFECTS | |
| Examples | Mucositis resulting from radiation therapy to oral cavity Radiation-induced cataract formation |
Radiation-induced cancer Heritable effects |
| Caused by | Killing of many cells | Sublethal damage to DNA |
| Threshold dose? | Yes: sufficient cell killing required to cause a clinical response. | No: even one photon could cause a change in DNA that leads to a cancer or heritable effect. |
| Severity of clinical effects and dose | Severity of clinical effects is proportional to dose. The greater the dose the greater the effect. | Severity of clinical effects is independent of dose. All-or-none response; an individual either has effect or does not. |
| Probability of having effect and dose | Probability of effect independent of dose. All individuals show effect when dose is above threshold. | Frequency of effect proportional to dose. The greater the dose the greater the chance of having the effect. |
Deterministic Effects on Cells
EFFECTS ON INTRACELLULAR STRUCTURES
The effects of radiation on intracellular structures result from radiation-induced changes in their macromolecules. Although the initial molecular changes are produced within a fraction of a second after exposure, cellular changes resulting from moderate exposure require a minimum of hours to become apparent. These changes are manifest initially as structural and functional changes in cellular organelles. The changes may cause cell death.
Nucleus
A wide variety of radiobiologic data indicate that the nucleus is more radiosensitive (in terms of lethality) than the cytoplasm, especially in dividing cells. The sensitive site in the nucleus is the DNA within chromosomes.
Chromosome Aberrations
Chromosomes serve as useful markers for radiation injury. They may be easily visualized and quantified, and the extent of their damage is related to cell survival. Chromosome aberrations are observed in irradiated cells at the time of mitosis when the DNA condenses to form chromosomes. The type of damage that may be observed depends on the stage of the cell in the cell cycle at the time of irradiation.
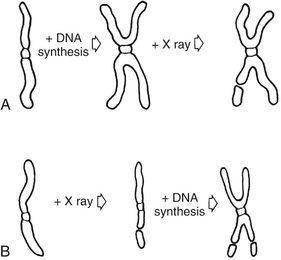
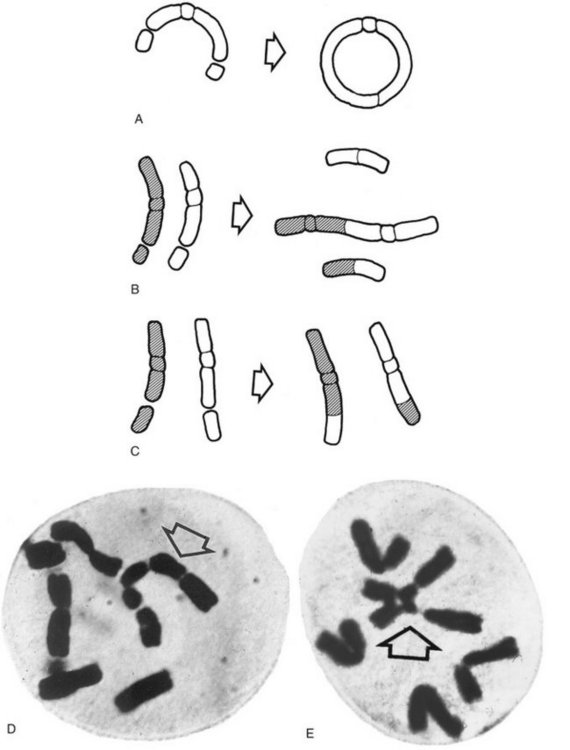
Figure 2-1 shows the stages of the cell cycle. If radiation exposure occurs after DNA synthesis (i.e., in G2 or mid and late S), only one arm of the affected chromosome is broken (chromatid aberration) (Fig. 2-2, A). However, if the radiation-induced break occurs before the DNA has replicated (i.e., in G1 or early S), the damage manifests as a break in both arms (chromosome aberration) at the next mitosis (Fig. 2-2, B). Most simple breaks are repaired by biologic processes and go unrecognized. Figure 2-3 illustrates several common forms of chromosome aberrations resulting from incorrect repair. Formation of rings (Fig. 2-3, A) and dicentrics (Fig. 2-3, B) are lethal as the cell cannot complete mitosis. Translocations (Fig. 2-3, C) result in unequal distribution of chromatin material to daughter cells or they prevent completion of a subsequent mitosis. Chromosome aberrations have been detected in peripheral blood lymphocytes of patients exposed to medical diagnostic procedures. Moreover, the survivors of the atomic bombings of Hiroshima and Nagasaki have demonstrated chromosome aberrations in circulating lymphocytes more than two decades after the radiation exposure. The frequency of aberrations is generally proportional to the radiation dose received.
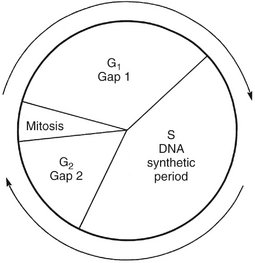
FIG. 2-1 Cell cycle. A proliferating cell moves in the cycle from mitosis to gap 1 (G1) to the period of DNA synthesis (S) to gap 2 (G2) to the next mitosis.
EFFECTS ON CELL REPLICATION
Radiation is especially damaging to rapidly dividing cell systems, such as skin and intestinal mucosa and hematopoietic tissues. Irradiation of such cell populations will cause a reduction in size of the irradiated tissue as a result of mitotic delay (inhibition of progression of the cells through the cell cycle) and cell death (usually during mitosis). Reproductive death in a cell population is loss of the capacity for mitotic division. The three mechanisms of reproductive death are DNA damage, bystander effect, and apoptosis.
Deoxyribonucleic Acid Damage
Cell death is caused by damage to DNA, which in turn causes chromosome aberrations, which cause the cell to die during the first few mitoses after irradiation. It is the rate of cell replication in various tissues, and thus the rate of reproductive death, that accounts for the varying radiosensitivity of tissues. When a population of slowly dividing cells is irradiated, larger doses and longer time intervals are required for induction of deterministic effects than when a rapidly dividing cell system is involved.
Bystander Effect
Cells that are damaged by radiation release into their immediate environment molecules that kill nearby cells. This bystander effect has been demonstrated for both α particles and x rays and causes chromosome aberrations, cell killing, gene mutations, and carcinogenesis.
Apoptosis
Apoptosis, also known as programmed cell death, occurs during normal embryogenesis. Cells round up, draw away from their neighbors, and condense nuclear chromatin. This characteristic pattern, different from necrosis, can be induced by radiation in both normal tissue and in some tumors. Apoptosis is particularly common in hemopoietic and lymphoid tissues.
Recovery
Cell recovery from DNA damage and the bystander effect involves enzymatic repair of single-strand breaks of DNA. Because of this repair, a higher total dose is required to achieve a given degree of cell killing when multiple fractions are used (e.g., in radiation therapy) than when the same total dose is given in a single brief exposure. Damage to both strands of DNA at the same site is usually lethal to the cell.
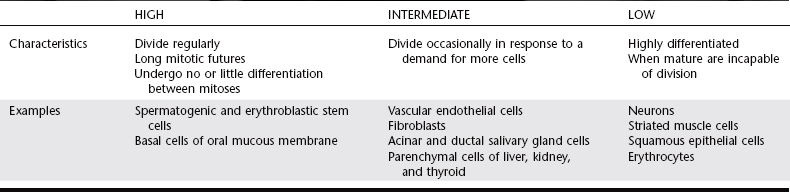
RADIOSENSITIVITY AND CELL TYPE
Different cells from various organs of the same individual may respond to irradiation quite differently. This variation was recognized as early as 1906 by the French radiobiologists Bergonié and Tribondeau. They observed that the most radiosensitive cells have the following characteristics:
Mammalian cells may be divided into three broad categories of radiosensitivity as shown in Table 2-2.
Deterministic Effects on Tissues and Organs
The radiosensitivity of a tissue or organ is measured by its response to irradiation. Loss of moderate numbers of cells does not affect the function of most organs. However, with the loss of large numbers of cells, all affected organisms display an observable result. The severity of this change depends on the dose and thus the amount of cell loss. The following discussion pertains to the effect of irradiation of tissues and organs when the exposure is restricted to a small area. Moderate doses to a localized area may lead to repairable damage. Comparable doses to the whole animal may result in death from damage to the most radiation-sensitive systems.
SHORT-TERM EFFECTS
The short-term effects of radiation on a tissue (effects seen in the first days or weeks after exposure) are determined primarily by the sensitivity of its parenchymal cells. When continuously proliferating tissues (e.g., bone marrow, oral mucous membranes) are irradiated with a moderate dose, cells are lost primarily by reproductive death, bystander effect, and apoptosis. The extent of cell loss depends on damage to the stem cell pools and the proliferative rate of the cell population. The effects of irradiation on such tissues become apparent quickly as a reduction in the number of mature cells in the series. Tissues composed of cells that rarely or never divide (e.g., neurons or muscle) demonstrate little or no radiation-induced hypoplasia over the short term. The relative radiosensitivities of various tissues and organs are shown in Box 2-1.
LONG-TERM EFFECTS
The long-term deterministic effects of radiation on tissues and organs (seen months and years after exposure) are a loss of parenchymal cells and replacement with fibrous connective tissue. These changes are caused by reproductive death of replicating cells and by damage to the fine vasculature. Damage to capillaries leads to narrowing and eventual obliteration of vascular lumens. This impairs the transport of oxygen, nutrients, and waste products and results in death of all cell types dependent on this vascular supply. Thus both dividing (radiosensitive) and nondividing (radioresistant) parenchymal cells are replaced by fibrous connective tissue, a progressive fibroatrophy of the irradiated tissue.
MODIFYING FACTORS
The response of cells, tissues, and organs to irradiation depends on exposure conditions and the cell environment.
Dose
The severity of deterministic damage seen in irradiated tissues or organs depends on the amount of radiation received. Very often a clinical threshold dose exists below which no adverse effects are seen. In all individuals receiving doses above the threshold level, the amount of damage is proportional to the dose.
Dose Rate
The term dose rate indicates the rate of exposure. For example, a total dose of 5 Gy may be given at a high dose rate (5 Gy/min) or a low dose rate (5 mGy/min). Exposure of biologic systems to a given dose at a high dose rate causes more damage than exposure to the same total dose given at a lower dose rate. When organisms are exposed at lower dose rates, a greater opportunity exists for repair of damage, thereby resulting in less net damage. Although the total dose of diagnostic exposures is low, they are given at a high dose rate compared with background exposure.
Oxygen
The radioresistance of many biologic systems increases by a factor of 2 or 3 when the exposure is made with reduced oxygen (hypoxia). The greater cell damage sustained in the presence of oxygen is related to the increased amounts of hydrogen peroxide and hydroperoxyl free radicals formed. This is important clinically because hyperbaric oxygen therapy may be used during radiation therapy of tumors having hypoxic cells.
Linear Energy Transfer
In general, the dose required to produce a certain biologic effect is reduced as the linear energy transfer (LET) of the radiation is increased. Thus higher-LET radiations (e.g., α particles) are more efficient in damaging biologic systems because their high ionization density is more likely than x rays to induce double-strand breakage in DNA. Low-LET radiations such as x rays deposit their energy more sparsely, or uniformly, in the absorber and thus are more likely to cause single-strand breakage and less biologic damage.
Radiotherapy in the Oral Cavity
The oral cavity is irradiated during radiation therapy of radiosensitive oral malignant tumors, usually squamous cell carcinomas. Radiation therapy for malignant lesions in the oral cavity is usually indicated when the lesion is radiosensitive, advanced, or deeply invasive and cannot be approached surgically. Combined surgical and radiotherapeutic treatment often provides optimal treatment. Increasingly, chemotherapy is being combined with radiation therapy and surgery.
Fractionation of the total x-ray dose into multiple small doses provides greater tumor destruction than is possible with a large single dose. Fractionation characteristically also allows increased cellular repair of normal tissues, which are believed to have an inherently greater capacity for recovery than tumor cells. Fractionation also increases the mean oxygen tension in an irradiated tumor, rendering the tumor cells more radiosensitive. This results from killing rapidly dividing tumor cells and shrinking the tumor mass after the first few fractions, reducing the distance that oxygen must diffuse from the fine vasculature through the tumor to reach the remaining viable tumor cells. The fractionation schedules currently in use have been established empirically.
RADIATION EFFECT ON ORAL TISSUES
The following sections describe the complications (deterministic effects) of a course of radiotherapy on the normal tissue of the oral cavity (Fig. 2-4). Typically 2 Gy is delivered daily, bilaterally through 8- × 10-cm fields over the oropharynx, for a weekly exposure of 10 Gy. This continues typically for 6 to 7 weeks until a total of 64 to 70 Gy is administered.
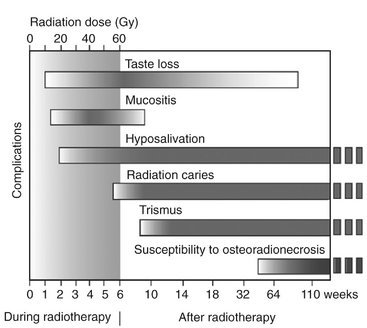
FIG. 2-4 Oral complications. Typical time course of complications seen during and after a course of radiation therapy to the head and neck. Shaded area in first 6 weeks represents accumulated dose. Shading within bars indicates severity of complication. Those changes persisting after 2 years pose lifelong risks. (Adapted from Kielbassa AM, Hinkelbein W, Hellwig E et al: Radiation-related damage to dentition, Lancet Oncol 7:326-335, 2006.)
Cobalt is often the source of γ radiation; however, on occasion small implants containing radon or iodine 125 are placed directly in a tumor mass. Such implants deliver a high dose of radiation to a relatively small volume of tissue in a short time. Recently a three-dimensional technique called intensity-modulated radiotherapy (IMRT) has been used to control the dose distribution with high accuracy.
Oral Mucous Membrane
The oral mucous membrane contains a basal layer composed of rapidly dividing, radiosensitive stem cells. Near the end of the second week of therapy, as some of these cells die, the mucous membranes begin to show areas of redness and inflammation (mucositis). As the therapy continues, the irradiated mucous membrane begins to separate from the underlying connective tissue, with the formation of a white to yellow pseudomembrane (the desquamated epithelial layer). At the end of therapy the mucositis is usually most severe, discomfort is at a maximum, and food intake is difficult. Good oral hygiene minimizes infection. Topical anesthetics may be required at mealtimes. Secondary yeast infection by Candida albicans is a common complication and may require treatment.
After irradiation is completed, the mucosa begins to heal rapidly. Healing is usually complete by about 2 months. Later the mucous membrane tends to become atrophic, thin, and relatively avascular. This long-term atrophy results from progressive obliteration of the fine vasculature and fibrosis of the underlying connective tissue. These atrophic changes complicate denture wearing because they may cause oral ulcerations of the compromised tissue. Ulcers may also result from radiation necrosis or tumor recurrence. A biopsy may be required to make the differentiation.
Taste Buds
Taste buds are sensitive to radiation. Doses in the therapeutic range cause extensive degeneration of the normal histologic architecture of taste buds. Patients often notice a loss of taste acuity during the second or third week of radiotherapy. Bitter and acid flavors are more severely affected when the posterior two thirds of the tongue is irradiated and salt and sweet when the anterior third of the tongue is irradiated. Taste acuity usually decreases by a factor of 1000 to 10,000 during the course of radiotherapy. Alterations in the saliva may partly account for this reduction, which may proceed to a state of virtual insensitivity. Taste loss is reversible and recovery takes 60 to 120 days.
Salivary Glands
The major salivary glands are at times unavoidably exposed to 20 to 30 Gy during radiotherapy for cancer in the oral cavity or oropharynx. The parenchymal component of the salivary glands is rather radiosensitive (parotid glands more so than submandibular or sublingual glands). A marked and progressive loss of salivary secretion (hyposalivation) is usually seen in the first few weeks after initiation of radiotherapy. The extent of reduced flow is dose dependent and reaches essentially zero at 60 Gy. The mouth becomes dry (xerostomia) and tender, and swallowing is difficult and painful. Patients with irradiation of both parotid glands are more likely to complain of dry mouth and difficulty with chewing and swallowing than are those with unilateral irradiation. Various saliva substitutes are available to help restore function. Use of IMRT has helped to spare the contralateral salivary glands and thus minimize the loss of salivary function.
The reduced volume of saliva in patients receiving radiation therapy that includes the major salivary glands is altered from normal. Because serous cells are more radiosensitive than mucous cells, the residual saliva is more viscous than usual. Further, the small volume of viscous saliva that is secreted usually has a pH value 1 unit below normal (i.e., an average of 5.5 in irradiated patients compared with 6.5 in unexposed individuals). This pH is low enough to initiate decalcification of normal enamel. In addition, the buffering capacity of saliva falls as much as 44% during radiation therapy. If some portions of the major salivary glands are spared, dryness of the mouth usually subsides in 6 to 12 months because of compensatory hypertrophy of residual salivary gland tissue. Reduced salivary flow that persists beyond a year is unlikely to show significant recovery.
Histologically, an acute inflammatory response may occur soon after the initiation of therapy, particularly involving the serous acini. In the months after irradiation the inflammatory response becomes more chronic, and the glands demonstrate progressive fibrosis, adiposis, loss of fine vasculature, and concomitant parenchymal degeneration (Fig. 2-5), thus accounting for the xerostomia.
Teeth
Children receiving radiation therapy to the jaws may show defects in the permanent dentition such as retarded root development, dwarfed teeth, or failure to form one or more teeth (Fig. 2-6). If exposure precedes calcification, irradiation may destroy the tooth bud. Irradiation after calcification has begun may inhibit cellular differentiation, causing malformations and arresting general growth. Such exposure may retard or abort root formation, but the eruptive mechanism of teeth is relatively radiation resistant. Irradiated teeth with altered root formation still erupt. In general, the severity of the damage is dose dependent.
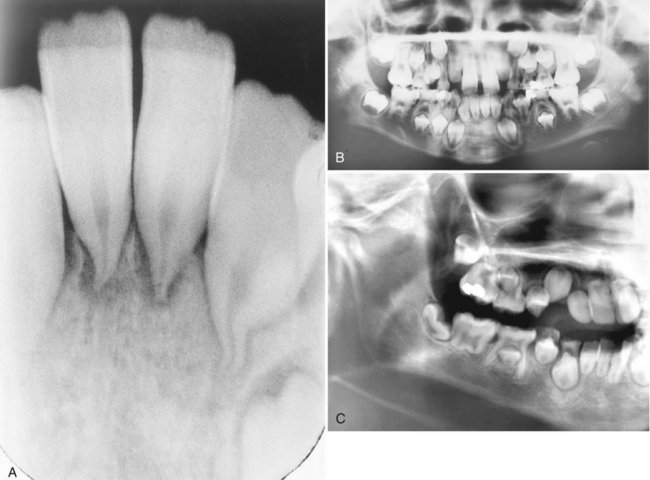
FIG. 2-6 Dental abnormalities after radiotherapy in two patients. The first, a 9-year-old girl who received 35 Gy at the age of 4 years because of Hodgkin’s disease, had severe stunting of the incisor roots with premature closure of the apices at 8 years (A) and retarded development of the mandibular second premolar crowns with stunting of the mandibular incisor, canine, and premolar roots at 9 years (B). The other patient (C), a 10-year-old boy who received 41 Gy to the jaws at age 4 years, had severely stunted root development of all permanent teeth with a normal primary molar. (A and B, Courtesy Mr. P.N. Hirschmann, Leeds, United Kingdom. C, Courtesy Dr. James Eischen, San Diego, Calif.)
Adult teeth are resistant to the direct effects of radiation exposure. Pulpal tissue demonstrates long-term fibroatrophy after irradiation. Radiation has no discernible effect on the crystalline structure of enamel, dentin, or cementum, and radiation does not increase their solubility.
Radiation Caries
Radiation caries is a rampant form of dental decay that may occur in individuals who receive a course of radiotherapy that includes exposure of the salivary glands. After radiotherapy that includes the major salivary glands, the microflora undergo a pronounced change, rendering them acidogenic in the saliva and plaque. Patients receiving radiation therapy to oral structures have increases in Streptococcus mutans, Lactobacillus, and Candida. Caries results from changes in the salivary glands and saliva, including reduced flow, decreased pH, reduced buffering capacity, increased viscosity, and altered flora. The residual saliva in individuals with xerostomia also has a low concentration of Ca+2 ion. This results in greater solubility of tooth structure and reduced remineralization. Finally, because of the reduced or absent cleansing action of normal saliva, debris accumulates quickly. Irradiation of the teeth by itself does not influence the course of radiation caries.
Clinically, three types of radiation caries exist. The most common is widespread superficial lesions attacking buccal, occlusal, incisal, and palatal surfaces. Another type involves primarily the cementum and dentin in the cervical region. These lesions may progress around the teeth circumferentially and result in loss of the crown. A final type appears as a dark pigmentation of the entire crown. The incisal edges may be markedly worn. Combinations of all these lesions develop in some patients (Fig. 2-7). The histologic features of the lesions are similar to those of typical carious lesions. It is the rapid course and widespread attack that distinguish radiation caries.
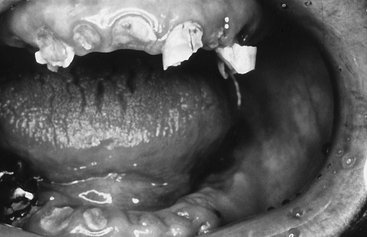
FIG. 2-7 Radiation caries. Note the extensive loss of tooth structure in both jaws resulting from radiation-induced xerostomia.
The best method of reducing radiation caries is daily application for 5 minutes of a viscous topical 1% neutral sodium fluoride gel in custom-made applicator trays. Use of topical fluoride causes a 6-month delay in the irradiation-induced elevation of S. mutans. Avoidance of dietary sucrose, in addition to the use of a topical fluoride, further reduces the concentrations of S. mutans and Lactobacillus. The best result is achieved from a combination of restorative dental procedures, excellent oral hygiene, a diet restricted in cariogenic foods, and topical applications of sodium fluoride. Patient cooperation in maintaining oral hygiene is extremely important because radiation caries is a lifelong threat. Teeth with gross caries or periodontal involvement are often extracted before irradiation.
Bone
Treatment of cancers in the oral region often includes irradiation of the mandible or maxilla. The primary damage to mature bone results from radiation-induced damage to the vasculature of the periosteum and cortical bone, which are normally already sparse. Radiation also acts by destroying osteoblasts and, to a lesser extent, osteoclasts. Subsequent to irradiation, normal marrow may be replaced with fatty marrow and fibrous connective tissue. The marrow tissue becomes hypovascular, hypoxic, and hypocellular. In addition, the endosteum becomes atrophic, showing a lack of osteoblastic and osteoclastic activity, and some lacunae of the compact bone are empty, an indication of necrosis. The degree of mineralization may be reduced, leading to brittleness, or little altered from normal bone. When these changes are so severe that bone death results and the bone is exposed, the condition is termed osteoradionecrosis.
Osteoradionecrosis is the most serious clinical complication that occurs in bone after irradiation. The decreased vascularity of the mandible renders it easily infected by microorganisms from the oral cavity. This bone infection may result from radiation-induced breakdown of the oral mucous membrane, by mechanical damage to the weakened oral mucous membrane such as from a denture sore or tooth extraction, through a periodontal lesion, or from radiation caries. This infection may cause a nonhealing wound in irradiated bone that is difficult to treat (Fig. 2-8). It is more common in the mandible than in the maxilla, probably because of the richer vascular supply to the maxilla and the fact that the mandible is more frequently irradiated. The higher the radiation dose absorbed by the bone, the greater the risk for osteoradionecrosis.
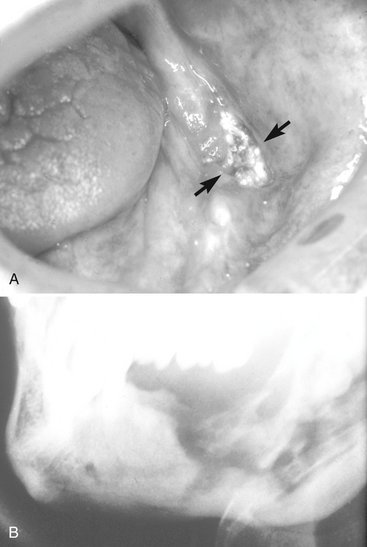
FIG. 2-8 Osteoradionecrosis. A, Area of exposed mandible after radiotherapy. Note the loss of oral mucosa. B, Destruction of irradiated bone resulting from the spread of infection.
Patients should be referred for dental care before undergoing a course of radiation therapy to minimize radiation caries and osteoradionecrosis. Radiation caries can be minimized by restoring all carious lesions before radiation therapy and initiating preventive techniques of good oral hygiene and daily topical fluoride. The risk for osteoradionecrosis and infection can be minimized by removing all teeth with extensive caries or with poor periodontal support (allowing sufficient time for the extraction wounds to heal before beginning radiation therapy) and adjusting dentures to minimize the risk of denture sores. Removal of teeth after irradiation should be avoided when possible. When teeth must be removed from irradiated jaws, the dentist should use atraumatic surgical technique to avoid elevating the periosteum and provide antibiotic coverage.
Often patients who have had radiation therapy require a radiographic examination to supplement clinical examinations. Radiographs are especially important to detect caries early. The amount of radiation from such diagnostic exposures is negligible compared with the amount received during therapy and should not serve as a reason to defer radiographs. Whenever possible, it is desirable to avoid taking radiographs during the first 6 months after completion of radiotherapy, however, to allow time for the mucous membrane to heal.
Musculature
Radiation may causes inflammation and fibrosis resulting in contracture and trismus in the muscles of mastication. Usually the masseter or pterygoid muscles are involved. Restriction in mouth opening usually starts about 2 months after radiotherapy is completed and progresses thereafter. An exercise program may be helpful in increasing opening distance.
Deterministic Effects of Whole-Body Irradiation
The acute radiation syndrome is a collection of signs and symptoms experienced by persons after acute whole-body exposure to radiation. Information about this syndrome comes from animal experiments and human exposures in the course of medical radiotherapy, atom bomb blasts, and radiation accidents. Individually, the clinical symptoms are not unique to radiation exposure, but taken as a whole, the pattern constitutes a distinct entity (Table 2-3).
TABLE 2-3
| DOSE (Gy) | MANIFESTATION |
| 1 to 2 | Prodromal symptoms |
| 2 to 4 | Mild hematopoietic symptoms |
| 4 to 7 | Severe hematopoietic symptoms |
| 7 to 15 | Gastrointestinal symptoms |
| 50 | Cardiovascular and central nervous system symptoms |
Prodromal Period
Within the first minutes to hours after exposure to whole-body irradiation of about 1.5 Gy, an individual may have anorexia, nausea, vomiting, diarrhea, weakness, and fatigue. These early symptoms constitute the prodromal period of the acute radiation syndrome. Their cause is not clear but probably involves the autonomic nervous system. The severity and time of onset may be of significant prognostic value because they are dose related: the higher the dose, the more rapid the onset and the greater the severity of symptoms.
Latent Period
After the prodromal reaction comes a latent period of apparent well-being during which no signs or symptoms of radiation sickness occur. The extent of the latent period is also dose related. It extends from hours or days after supralethal exposures (greater than approximately 5 Gy) to a few weeks after exposures of about 2 Gy.
Hematopoietic Syndrome
Whole-body exposures of 2 to 7 Gy cause injury to the hematopoietic stem cells of the bone marrow and spleen. The high mitotic activity of these cells makes bone marrow a highly radiosensitive tissue. Doses in this range cause a rapid fall in the numbers of circulating granulocytes, platelets, and finally erythrocytes (Fig. 2-9). Although mature circulating granulocytes, platelets, and erythrocytes are radioresistant because they are nonreplicating cells, their paucity in the peripheral blood after irradiation reflects the radiosensitivity of their precursors. The rate of fall in the circulating levels of a cell depends on the life span of that cell in the peripheral blood. Granulocytes, with short lives in circulation, fall off in a few days, whereas red blood cells, with long lives in circulation, fall off slowly.
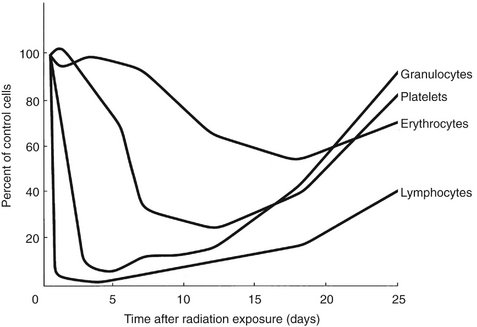
FIG. 2-9 Radiation effects on blood cells. When whole-body exposure inhibits the replacement of circulating cells by stem cell proliferation, the duration of the circulating cells survival is largely determined by their life span.
The clinical signs of the hematopoietic syndrome include infection (from lymphopenia and granulocytopenia), hemorrhage (from loss of platelets), and anemia (from erythrocyte depletion). The probability of death is low after exposures at the low end of this range but much higher at the high end. When death results from the hematopoietic syndrome, it usually occurs 10 to 30 days after irradiation.
Gastrointestinal Syndrome
The gastrointestinal syndrome is caused by whole-body exposures in the range of 7 to 15 Gy, which causes extensive damage to the gastrointestinal system in addition to the hematopoietic damage described previously. Exposure in this dose range causes considerable injury to the rapidly proliferating basal epithelial cells of the intestinal villi and leads to rapid loss of the epithelial layer of the intestinal mucosa. Because of the denuded mucosal surface, there is loss of plasma and electrolytes, loss of efficient intestinal absorption, and ulceration of the mucosal lining with hemorrhaging into the intestines. These changes are responsible for the diarrhea, dehydration, and loss of weight. Endogenous intestinal bacteria readily invade the denuded surface, producing septicemia.
At about the time that developing damage to the gastrointestinal system reaches a maximum, the effect of bone marrow depression is beginning to be manifested. The result is a marked lowering of the body’s defense against bacterial infection and a decrease in effectiveness of the clotting mechanism. The combined effects of damage to these hematopoietic and gastrointestinal stem cell systems cause death within 2 weeks from fluid and electrolyte loss, infection, and possibly nutritional impairment. Thirty of the firefighters at the accident site at Chernobyl, Ukraine, died in the first few months of the hematopoietic or gastrointestinal syndrome.
Cardiovascular and Central Nervous System Syndrome
Exposures in excess of 50 Gy usually cause death in 1 to 2 days. The few humans who have been exposed at this level showed collapse of the circulatory system with a precipitous fall in blood pressure in the hours preceding death. Autopsy revealed necrosis of cardiac muscle. Victims also may show intermittent stupor, incoordination, disorientation, and convulsions suggestive of extensive damage to the nervous system. Although the precise mechanism is not fully understood, these latter symptoms most likely result from radiation-induced damage to the neurons and fine vasculature of the brain.
Management of Acute Radiation Syndrome
The presenting clinical problems govern the management of different forms of acute radiation syndrome. Antibiotics are indicated when the granulocyte count falls. Fluid and electrolyte replacement is used as necessary. Whole blood transfusions are used to treat anemia, and platelets may be administered to arrest thrombocytopenia. Bone marrow grafts are indicated between identical twins because there is no risk for graft-versus-host disease.
RADIATION EFFECTS ON EMBRYOS AND FETUSES
The effects of radiation on human embryos and fetuses have been studied in animals and in women exposed to diagnostic or therapeutic radiation during pregnancy and those exposed to radiation from the atomic bombs dropped at Hiroshima and Nagasaki. Embryos and fetuses are considerably more radiosensitive than adults because most embryonic cells are relatively undifferentiated and rapidly mitotic.
Exposures in the range of 2 to 3 Gy during the first few days after conception are thought to cause undetectable death of the embryo. The cells in the embryo are dividing rapidly and are highly sensitive to radiation. Lethality is common and many of these embryos fail to implant in the uterine wall. The first 15 weeks includes the period of organogenesis when the major organ systems form. The most common abnormalities among the Japanese children exposed early in gestation were reduced growth that persists through life and reduced head circumference (microcephaly), often associated with mental retardation. Other abnormalities included small birth size, cataracts, genital and skeletal malformations, and microphthalmia. The period of maximal sensitivity of the brain is 8 to 15 weeks after conception. The frequency of severe mental retardation after exposure to 1 Gy during this period is about 43%. These effects are deterministic in nature and are believed to have a threshold of about 0.1 to 0.2 Gy. This threshold dose is 400 to 800 times higher than the exposure from a dental examination (0.25 mGy from a full-mouth examination when a leaded apron is used).
LATE EFFECTS
A number of late deterministic effects have been found in the survivors of the atomic bombing of Hiroshima and Nagasaki.
Growth and Development
Children exposed in the bombings showed impairment of growth and development. They have reduced height, weight, and skeletal development. The younger the individual was at the time of exposure, the more pronounced the effects.
Cataracts
The threshold for induction of cataracts (opacities in the lens of the eye) ranges from about 0.6 Gy when the dose is received in a single exposure to more than 5 Gy when the dose is received in multiple exposures over a period of weeks. These doses are much larger than those from dental radiography. Most affected individuals are unaware of their presence.
Life Span Shortening
The survivors of the atomic bombings show a clear decrease in median life expectancy with increasing radiation dose. The reduction ranges from 2 months up to 2.6 years by dose group, with an overall mean of 4 months. Survivors demonstrate increased frequency of heart disease, stroke, and diseases of the digestive, respiratory, and hematopoietic systems.
Stochastic Effects
Stochastic effects result from sublethal changes in the DNA of individual cells. The most important consequence of such damage is carcinogenesis. Heritable effects, although much less likely, can also occur.
CARCINOGENESIS
Radiation causes cancer by modifying DNA. The most likely mechanism is radiation-induced gene mutation. Most investigators think that radiation acts as an initiator, that is, it induces a change in the cell so that it no longer undergoes terminal differentiation. Evidence also exists that radiation acts as a promoter, stimulating cells to multiply. Finally, it may also convert premalignant cells into malignant ones, for instance, conversion of proto-oncogenes to oncogenes. Gene mutations may also involve a loss of function in the case of tumor-suppressor genes. Data on radiation-induced cancers come primarily from populations of people who have been exposed to high levels of radiation; however, in principle, even low doses of radiation may initiate cancer formation in a single cell.
Estimation of the number of cancers induced by radiation is difficult. Radiation-induced cancers are not distinguishable from cancers produced by other causes. This means that the number of cancers can be estimated only as the number of excess cases found in exposed groups compared with the number in unexposed groups of people. The group of individuals most intensively studied for estimating the cancer risk from radiation is the Japanese atomic bomb survivors. The cases of more than 120,000 individuals have been followed since 1950, of whom 91,000 were exposed. The incidences of deaths from leukemias and solid cancers are shown in Tables 2-4 and 2-5. The risk for most solid cancers increases linearly with dose.
TABLE 2-4
Cancer Mortality Rate in 86,611 Atomic Bomb Survivors Having 47,685 Deaths from All Causes (1950-2000)
| LEUKEMIAS | SOLID CANCERS | |
| Deaths | 296 | 10,127 |
| Radiation induced | 93 | 479 |
Data adapted from Preston DL, Pierce DA, Shimizu Y et al: Effect of recent changes in atomic bomb survivor dosimetry on cancer mortality risk estimates, Radiat Res 162:377-389, 2004.
TABLE 2-5
Comparison of Radiation-Induced Leukemias and Solid Tumors
| FEATURE | LEUKEMIAS | SOLID TUMORS |
| Onset | 2-3 years after exposure | 10 or more years after exposure |
| Peak incidence | 5-7 years after exposure, rarely occur more than 15 years after exposure | Elevated risk remains for the rest of the exposed individual’s life |
| Demographics | The risk from exposure during childhood is about twice as great as the risk during adulthood. All forms except chronic lymphocytic leukemia. | The risk from exposure during childhood is about twice as great as the risk during adulthood. The number of cancers induced by radiation is most likely a multiple of their spontaneous frequency (see Box 2-2). |
Most individuals in these studies received exposures far in excess of the diagnostic range. Thus the probability that a cancer will result from a small dose can be estimated only by extrapolation from the rates observed after exposure to larger doses (see Chapter 3). Box 2-2 shows the radiosensitivity of various tissues in terms of susceptibility to radiation-induced cancer. The following discussion of radiation carcinogenesis pertains primarily to those organs exposed in the course of dental radiography.
Leukemia
The incidence of leukemia (other than chronic lymphocytic leukemia) rises after exposure of the bone marrow to radiation. Atomic bomb survivors and patients irradiated for ankylosing spondylitis show a wave of leukemias beginning soon after exposure and peaking at around 7 years. For individuals exposed under age 30 years, the risk for development of leukemia ceases after about 30 years. For individuals exposed as adults, the risk persists throughout life. Leukemias appear sooner than solid cancers because of the higher rate of cell division and differentiation of hematopoietic stem cells compared with the other tissues. Persons younger than 20 years are more at risk than adults are.
Evidence also exists for a slightly increased risk for childhood cancer, both leukemia and solid tumors, after diagnostic irradiation in utero. The level of the risk is uncertain but thought to increase the absolute risk by about 0.06% per 0.1 Gy.
Thyroid Cancer
The incidence of thyroid carcinomas (arising from the follicular epithelium) increases in humans after exposure. Only about 10% or less of individuals with such cancers die from their disease. The best-studied groups are Israeli children irradiated to the scalp for ringworm; children in Rochester, New York, irradiated to the thymus gland; and survivors of the atomic bombs in Japan. Susceptibility to radiation-induced thyroid cancer is greater early in childhood than at any time later in life, and children are more susceptible than adults. Females are two to three times more susceptible than males to radiogenic and spontaneous thyroid cancers. The fallout from the accident at the Chernobyl nuclear power plant, primarily iodine 131, is thought to have caused about 4000 cases of thyroid cancer in children and 15 fatalities.
Esophageal Cancer
Data pertaining to esophageal cancer are relatively sparse. Excess cancers are found in the Japanese atomic bomb survivors and in patients treated with x radiation for ankylosing spondylitis.
Brain and Nervous System Cancers
Patients exposed to diagnostic x-ray examinations in utero and to therapeutic doses in childhood or as adults (average midbrain dose of about 1 Gy) show excess numbers of malignant and benign brain tumors. Additionally, a case-control study has shown an association between intracranial meningiomas and previous medical or dental radiography. The strongest association was with a history of exposure to full-mouth dental radiographs when younger than 20 years. Because of their age, it is likely that these patients received substantially more exposure than is the case today with contemporary imaging techniques.
Salivary Gland Cancer
The incidence of salivary gland tumors is increased in patients treated with irradiation for diseases of the head and neck, in Japanese atomic bomb survivors, and in persons exposed to diagnostic x radiation. An association between tumors of the salivary glands and dental radiography has been shown, the risk being highest in persons receiving full-mouth examinations before the age of 20 years. Only individuals who received an estimated cumulative parotid dose of 0.5 Gy or more showed a significant correlation between dental radiography and salivary gland tumors.
Cancer of Other Organs
Other organs such as the skin, paranasal sinuses, and bone marrow (with respect to multiple myeloma) also show excess neoplasia after exposure. However, the mortality and morbidity rates expected after head and neck exposure are much lower than for the organs described previously.
HERITABLE EFFECTS
Heritable effects are changes seen in the offspring of irradiated individuals. They are the consequence of damage to the genetic material of reproductive cells. The basic findings of radiation-induced heritable effects are listed in Box 2-3. At low levels of exposure, such as encountered in dentistry, they are far less important than carcinogenesis.
Effects on Humans
Our knowledge of heritable effects of radiation on humans comes largely from the atomic bomb survivors. To date, no such radiation-related genetic damage has been demonstrated. No increase has occurred in adverse pregnancy outcome, leukemia or other cancers, or impairment of growth and development in the children of atomic bomb survivors. Similarly, studies of the children of patients who received radiotherapy show no detectable increase in the frequency of genetic diseases. These findings do not exclude the possibility that such damage occurs but do show that it must be at a very low frequency.
Doubling Dose
One way to measure the risk from genetic exposure is by determining the doubling dose, which is the amount of radiation a population requires to produce in the next generation as many additional mutations as arise spontaneously. In humans, the genetic doubling dose is estimated to be approximately 1 sievert (Sv). Because the average person receives far less gonadal radiation, radiation contributes relatively little to genetic damage in populations. For comparison, the background dose is about 0.003 Sv per year and the gonadal dose to males from a full-mouth radiographic examination is about 0.001 Sv or less. This exposure is contributed largely by the maxillary views, which are angled caudally. The dose to the ovaries is about 50 times less, in the range of 0.00002 Sv.
Bushong, SC. Radiologic science for technologists: physics, biology, and protection, ed 7. St. Louis: Mosby; 2001.
Gusev, I, Guskova, A, Mettler, F. Medical management of radiation accidents, ed 2. Boca Raton, Fla: CRC; 2001.
Hall, EJ, Giaccia, AJ. Radiobiology for the radiologist, ed 6. Philadelphia: Lippincott Williams & Wilkins; 2006.
Steel, GG. Basic clinical radiobiology, ed 3. London: Hodder Arnold; 2002.
United Nations Scientific Committee on the Effects of Atomic Radiation. Hereditary effects of radiation. http://www.unscear.org/unscear/en/publications/2001.html, 2001.
Dahllof, G. Craniofacial growth in children treated for malignant diseases. Acta Odontol Scand. 1998;56:378.
Kielbassa, AM, Hinkelbein, W, Hellwig, E, et al. Radiation-related damage to dentition. Lancet Oncol. 2006;7:326–335.
ORAL SEQUELAE OF HEAD AND NECK RADIOTHERAPY
Chung, EM, Sung, EC. Dental management of chemoradiation patients. J Calif Dent Assoc. 2006;34:735–742.
Sciubba, JJ, Goldenberg, D. Oral complications of radiotherapy. Lancet Oncol. 2006;7:175–183.
Teng, MS, Futran, ND. Osteoradionecrosis of the mandible. Curr Opin Otolaryngol Head Neck Surg. 2005;13:217–221.