Radiation Safety and Protection
Dentists must be prepared to intelligently discuss with patients the benefits and possible hazards involved with the use of x rays and to describe the steps taken to reduce the hazard. This chapter considers sources of exposure, estimates of risks from dental radiography, and means to minimize exposure from dental examinations.
Sources of Radiation Exposure
The general population is exposed to radiation from natural and man-made sources (Table 3-1). Understanding these exposure sources provides a useful framework for considering dental exposure.
TABLE 3-1
Average Annual Effective Dose of Ionizing Radiation
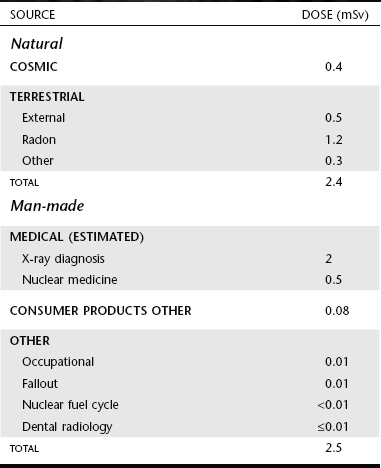
Natural exposures, consumer products and other from National Council on Radiation Protection and Measurements: NCRP Reports 93, 1987; 94, 1987. Medical exposures are estimated in recent unpublished data.
NATURAL RADIATION
All life on earth has evolved in a continuous exposure to natural radiation (Fig. 3-1 and Table 3-1). Background radiation from cosmic and terrestrial sources yields an average annual effective dose of about 2.4 millisieverts (mSv) worldwide and 3.0 mSv in the United States because of higher radon levels.
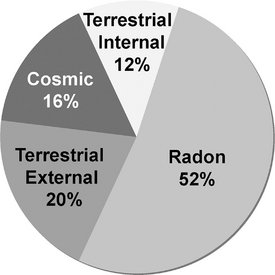
FIG. 3-1 Sources of global background radiation contribute 2.4 mSv per year. Most exposure comes from radon, but there are significant contributions from cosmic and terrestrial sources including external from the soil and building materials and from ingested radionuclides. (Data modified from UNSCEAR 2000.)
Cosmic Sources
Cosmic radiation includes energetic subatomic particles, photons from the sun and supernova, and to a lesser extent, the particles and photons (secondary cosmic radiation) generated by the interactions of primary cosmic radiation with atoms and molecules of the earth’s atmosphere. Exposure from cosmic radiation is primarily a function of altitude, almost doubling with each 2000-meter (m) increase in elevation, because less atmosphere is present to attenuate the radiation. At sea level the exposure from cosmic radiation is about 0.24 mSv per year; at an elevation of 1600 m (approximately 1 mile, the elevation of Denver, Colorado), it is about 0.50 mSv per year. The global average is 0.4 mSv per year, about 16% of natural exposure.
Cosmic radiation also includes exposure resulting from airline travel. As more people travel frequently above the protection of the earth’s atmosphere, cosmic radiation becomes a more significant contributor to exposure. An airline flight of 5 hours in the middle latitudes at an altitude of 12 km may result in a dose equivalent of about 25 μSv.
Terrestrial Sources
Exposure from terrestrial sources comes from external sources such as soil and from internal sources, including radon and other radionuclides that are inhaled or ingested.
External Radiation.: Exposure from terrestrial sources comes from radioactive nuclides in the soil, primarily potassium 40 and the radioactive decay products of uranium 238 and thorium 232. Most of the γ radiation from these sources comes from the top 20 cm of soil. Indoor exposure from radionuclides is very close to that occurring outdoors because the shielding provided by structural materials balances the exposure from radioactive nuclides contained within these shielding materials. The average terrestrial exposure rate is about 0.5 mSv per year, or approximately 20% of the average annual background exposure.
Radon.: Radon, a decay product in the uranium series, is estimated to be responsible for approximately 52% of the radiation exposure of the world’s population. As such, it is the largest single contributor to natural radiation (1.2 mSv). Radon is a gas (radon 222) that enters homes and buildings and by itself does little harm. However, radon decays to form solid products that emit α particles (porion 218, porion 214, lead 214, and bismuth 214). These decay products become attached to dust particles that can be inhaled and deposited on the bronchial epithelium in the respiratory tract. Exposure to this quantity of radiation may cause as many as 10,000 to 20,000 lung cancer deaths per year in the United States, mostly in smokers.
Other Internal.: Other sources of internal terrestrial exposure are radionuclides that are taken up from the external environment by ingestion. The greatest internal exposure comes from the ingestion of uranium and thorium and their decay products, primarily potassium 40 but also rubidium 87, carbon 14, tritium, and others. The total exposure from ingestion and inhalation other than radon is estimated at 0.3 mSv per year, about 12% of natural-origin exposure.
MAN-MADE RADIATION
Humans have contributed many additional sources of radiation to the environment (Fig. 3-2). These may be categorized into three major groups: medical diagnosis and treatment, consumer and industrial products and sources, and other minor sources. Recent estimates suggest that medical exposure in the developed countries has grown rapidly in recent decades, particularly computed tomography (CT) of the chest and abdomen and increased use of cardiac nuclear medicine studies. It is estimated that the average doses from medical exposures are comparable to natural background exposure.
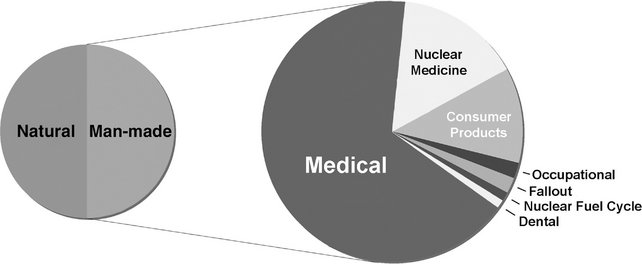
FIG. 3-2 Sources of man-made radiation in the United States. The average person in the United States receives about as much radiation from man-made sources as from natural background exposure. Most man-made exposure comes from medical x-ray examinations, particularly CT, with significant contributions from nuclear medicine examinations, primarily cardiac imaging, and consumer products. Exposures from dental examinations and from occupational, fallout, and nuclear power sources are small.
Medical Diagnosis and Treatment
Well over one billion medical x-ray examinations are performed annually worldwide. This source of exposure contributes the large majority of exposures from man-made sources. Although sources in this group include radiation therapy and nuclear medicine, diagnostic medical exposure is the largest contributor, contributing most of this source. Dental x-ray examinations are responsible for less than 1% of the average annual exposures from man-made sources.
Consumer and Industrial Products
Consumer and industrial products contain some of the most interesting and unsuspected sources. This group includes the domestic water supply, tobacco products, combustible fuels, dental porcelain, television receivers, pocket watches, smoke alarms, and airport inspection systems but contributes only a small proportion of the total average annual man-made exposure.
Other Man-made Sources
Individuals who work at medical and dental x-ray facilities, mining or milling, or with nuclear weapons are occupationally exposed to additional radiation exposure. Another source is the nuclear fallout from the nuclear weapons testing in the 1950s and early 1960s. Of these sources, strontium 90 and iodine 131 are the most important. Because of its chemical similarity to calcium, strontium 90, a β emitter, is readily assimilated in the bones and teeth of children and young adults. Iodine 131, a γ emitter, accumulates in the thyroid gland. Fallout is no longer considered a significant source of exposure to the public because of the cessation of atmospheric testing of nuclear weapons.
Nuclear power (which contributes only about 0.01 mSv to the average annual exposure) is another man-made source of particular concern to the public. However, nuclear power and support facilities, in normal operation, add only about 10% of that contributed by the release of naturally occurring radionuclides from the combustion of coal, natural gas, and oil. In spite of this low contribution to the average annual exposure made by nuclear power, accidents have occurred. Between 1945 and 1987, 284 nuclear reactor accidents, excluding Chernobyl, were reported in several countries, resulting in the exposure of more than 1300 people, with 33 fatalities. The public was not directly affected in the majority of these accidents. The nuclear accident at Chernobyl in the Ukraine in 1986 made clear that the use of nuclear power facilities carries the real potential of causing considerable harm if not properly controlled. In that event, 29 persons in the immediate vicinity of the plant died of acute radiation injury in the first months after exposure. The long-term risk to the general population includes thyroid tumors that have resulted in 15 known fatalities.
Dose and Risk in Radiography
This section considers governmental dose limits on individuals who are occupationally exposed and members of the general population, amounts of radiation received by patients in dental and medical radiography, and the estimated risks from these exposures.
DOSE LIMITS
Recognition of the harmful effects of radiation and the risks involved with its use led the National Council on Radiation Protection and Measurements (NCRP) and the International Commission on Radiological Protection (ICRP) to establish guidelines for limitations on the amount of radiation received by both occupationally exposed individuals and the public. Since their establishment in the 1930s, these dose limits have been revised downward several times. These revisions reflect the increased knowledge concerning the harmful effects of radiation and the increased ability to use radiation more efficiently. The current occupational exposure limits have been established to ensure that no individuals will have deterministic effects and that the probability for stochastic effects is as low as reasonably and economically feasible (Table 3-2). Note that there are no limits on the exposure a patient can receive from diagnostic or therapeutic exposures.
TABLE 3-2
Recommended Annual Limits for Human Exposure to Ionizing Radiation
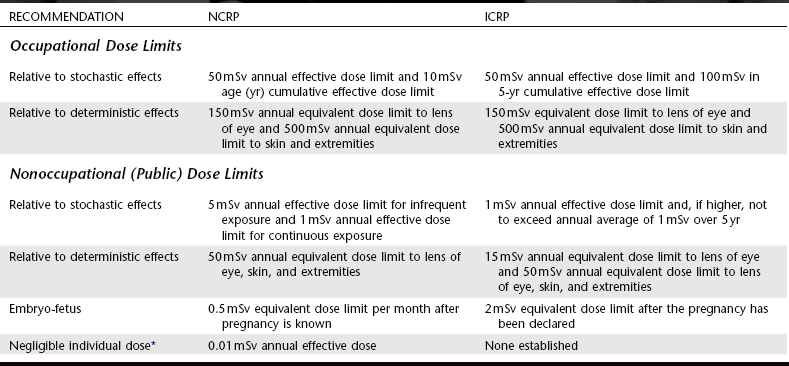
*That dose below which any effort to reduce the radiation exposure cannot be justified.
From National Council on Radiation Protection and Measurements: NCRP Report 116, 1993, and International Commission on Radiological Protection: Radiation protection, ICRP Publication 60, 1990.
Dose limits from man-made sources for members of the general public, not occupationally exposed, have been established at 10% of that of occupationally exposed individuals. The negligible individual dose, established by the NCRP, is considered to be the dose below which any effort to reduce the radiation exposure may not be cost-effective. In spite of the NCRP’s endorsement of the nonthreshold hypothesis for purposes of radiation safety, it is thought that the impact on society of radiation exposure of this magnitude is negligible.
Dentists and their staff are occupationally exposed workers and are allowed to receive up to 50 mSv of whole-body radiation exposure per year (Table 3-2). Although this is considered to present only a minimal risk, every effort should be made to keep the dose to all individuals as low as practical. As a profession we do rather well. The average dose for individuals occupationally exposed in the operation of dental x-ray equipment is far less than the limit: 0.2 mSv, or 0.4% of the allowable exposure.
PATIENT EXPOSURE AND DOSE
Patient dose from dental radiography is usually reported as the amount of radiation received by a target organ. Although the actual exposures may vary considerably, Table 3-3 shows typical doses from various examinations. The equivalent exposure from natural and man-made background sources is shown. It may be seen that dental exposures are a small fraction of the annual average background exposure. The most radiosensitive target organs commonly studied include bone marrow, thyroid gland, brain, and salivary glands. The mean active bone marrow dose is an important measurement because bone marrow is the target organ thought responsible for radiation-induced leukemia. Particular concern has been expressed over exposure of the thyroid because this gland has one of the highest radiation-induced cancer rates. There are also reports of brain and salivary gland tumors after therapeutic and diagnostic x-ray examinations.
TABLE 3-3
Effective Dose from Diagnostic X-Ray Examinations and Equivalent Background Exposure
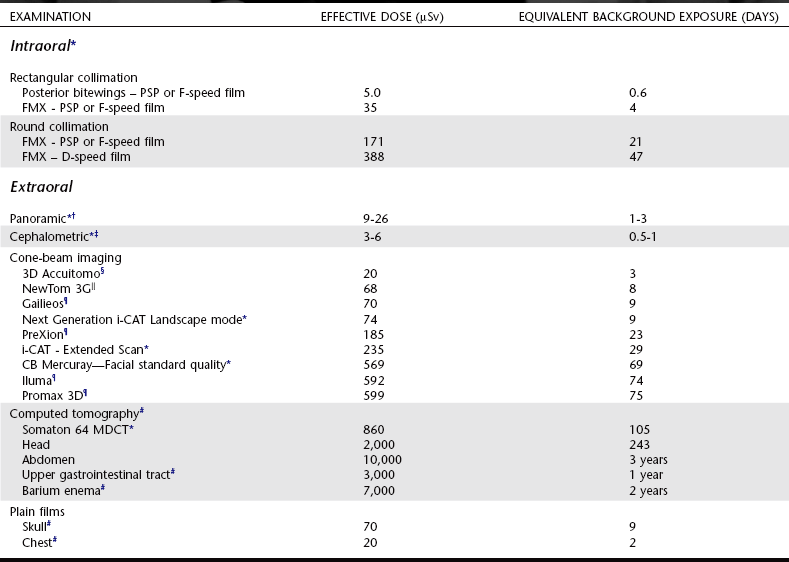
*Ludlow J: Personal communication, 2008.
†Lecomber AR, Yoneyama Y, Lovelock DJ et al: Comparison of patient dose from imaging protocols for dental implant planning using conventional radiography and computed tomography, Dentomaxillofac Radiol 30:255-259, 2001.
‡Gijbels F, Sanderink G, Wyatt J et al: Radiation doses of indirect and direct digital cephalometric radiography, Br Dent J 197:149-152, 2004.
§Hirsch E, Wolf U, Heinick F et al. Radiation doses from the cone beam CT-veraviewepocs 3D. 16th Int. Conf. Dentomaxillofacial Radiology, abstract OP-54, Beijing, 2007.
||Ludlow JB, Davies-Ludlow LE, Brooks SL et al: Dosimetry of 3 CBCT devices for oral and maxillofacial radiology: CB Mercuray, NewTom 3G and i-CAT, Dentomaxillofac Radiol 35:219-226, 2006.
¶Ludlow J, Davies-Ludlow LE, Mol A: Dosimetry of recently introduced CBCT Units for Oral and Maxillofacial Radiology. 3D. 16th Int. Conf. Dentomaxillofacial Radiology, abstract OP-53, Beijing, 2007.
#Directorate-General for the Environment of the European Commission: Referral guidelines for imaging, Radiation Protection Report 118, European Commission, Luxembourg, 2000, and Shrimpton PC, Hillier MC, Lewis MA et al: National survey of doses from CT in the UK: 2003, Br J Radiol 79:968-980, 2006.
RISK ESTIMATES
The primary risk from dental radiography is radiation-induced cancer. The actual risk for cancer being induced in humans as a result of exposure to low doses of radiation is difficult to estimate for a number of reasons:
• The data for the cancer risk from radiation exposure involve exposures many times larger than those involved with dental radiology. Estimating cancer risk in dentistry thus requires extrapolating from high doses down to the low-dose range. A linear extrapolation is considered to be most appropriate, but the accuracy of this assumption is not known.
• Cancer is a common disease, accounting for about 20% of all deaths. It is estimated that in the United States in 2007 more than 1,400,000 new cases of cancer were diagnosed and that more than 560,000 people will die from this disease. The very low incidence that may result from dental exposure is impossible to detect by direct measurement.
• Radiation-induced cancers are clinically and histologically indistinguishable from cancers induced by other causes.
• The time between radiation exposure and the development of cancer may be years to decades, during which time individuals may be subjected to many other carcinogens.
In spite of these difficulties, the ICRP has developed an estimate that includes the probability for the induction of both fatal and nonfatal cancer and hereditary effects in an exposed population. On the basis of this estimate, 33 μSv for a full-mouth x ray delivered to 1,000,000 people would result in about two additional cancer deaths over the lifetime of the exposed individuals. This would be in addition to the 200,000 that would occur spontaneously. Such a calculation assumes a linear dose-response relationship and no threshold dose below which no risk exists. These assumptions may be in error and, if so, they most likely overestimate the actual risk.
This degree of risk from exposure to ionizing radiation may be expressed in multiple ways. It is helpful to compare the risk from dental exposures with that of equivalent natural exposures. Equivalent natural exposure is calculated as the product of the effective dose resulting from a specific radiographic examination and the average daily effective dose (8 μSv) delivered by natural sources. The dentist may point out to the patient that with optimal intraoral radiographic technique (E- or F-speed film or digital imaging, along with rectangular collimation) the days of equivalent exposure are only about 4 days for a full-mouth examination. For another example, the dependence of physical location within the United States on exposure to natural radiation may be used. The effective dose resulting from cosmic radiation in Denver is 0.24 mSv (240 μSv), higher than the average of the United States because of its high elevation and reduced atmospheric protection. This means that a person living in an average location in the United States who had one complete mouth survey and one panoramic image made by optimized techniques every year (total effective dose for these examinations = 42 μSv, see Table 3-3) would incur less than one fifth the risk for a person living in Denver who was not exposed to dental radiography. Put another way, if a person living in an average location in the United States had 14 complete mouth surveys (238 μSv) made by optimized techniques every year, he or she would incur only the same risk as a person living in Denver who was not exposed to dental radiography. There is no known risk from living in Denver pertaining to cosmic radiation.
Everyone is subject to risks in everyday life. Newspapers and news magazines occasionally publish articles dealing with the level of such risks. In consideration of the potential risk associated with dental radiography, it might be good to keep in mind that the average person’s risk for death as a result of an accident while a patient in hospital is about 230 per million; choking to death, 13 per million; and dying in a boating accident, 4.6 per million. The risk from each of these events is greater than the risk from intraoral radiographic procedures.
In addition, people needlessly expose themselves to x radiation. There is a current trend for having full-body CT scans in hopes of detecting early signs of cancer, coronary artery disease, and other abnormalities. What most people do not realize is that a combined CT scan of the chest and abdomen delivers an E dose equal to almost 1000 chest radiographs. Furthermore, this increased exposure is often incurred for what is considered to be an unnecessary test: there is insufficient scientific evidence to justify CT screening for patients with no symptoms or family history suggesting disease.
Although the risk involved with dental radiography is certainly small in comparison with many other risks that are a common part of everyday life such as smoking or consumption of fatty foods, no basis exists to assume that it is zero. Although diagnostic radiation appears to be a weak carcinogen, the risk is increased because of the large number of people exposed. Practitioners must ensure that their patients avoid even the smallest unnecessary dose of radiation.
Reducing Dental Exposure
There are three guiding principles in radiation protection; the first is the principle of justification. In making dental radiographs this principle obligates the dentist to do more good than harm. In radiology this means the dentist should identify those situations where the benefit to a patient from the diagnostic exposure exceeds the low risk of harm. In practice this principle influences what patients we select for radiographic examinations and what examinations we choose. These matters are considered in Chapter 15, Guidelines for Prescribing Dental Radiographs.
The second guiding rule is the principle of optimization. This principle holds that dentists should use every means to reduce unnecessary exposure to their patient and themselves. This philosophy of radiation protection is often referred to as the principle of ALARA (As Low As Reasonably Achievable). ALARA holds that exposures to ionizing radiation should be kept as low as reasonably achievable, economic and social factors being taken into account. The means to accomplish this end are considered later in this chapter.
The third principle is that of dose limitation. Dose limits are used for occupational and public exposures to ensure that no individuals are exposed to unacceptably high doses. There are no dose limits for individuals exposed for diagnostic or therapeutic purposes.
The dentist in each facility is responsible for the design and conduct of the radiation protection program. In this section, methods of exposure and dose reduction are described that can be used in dental radiography. Each subsection begins with a recommendation of the American Dental Association (ADA) Council on Scientific Affairs. This is followed by a discussion of ways in which these recommendations can be satisfied.
PATIENT SELECTION CRITERIA
Dentists should not prescribe routine dental radiographs at preset intervals for all patients. Instead, they should prescribe radiographs after an evaluation of the patient’s needs that includes a health history review, a clinical dental history assessment, a clinical examination, and an evaluation of susceptibility to dental diseases. (ADA, 2006)
Radiographic selection criteria are clinical or historical findings that identify patients for whom a high probability exists that a radiographic examination will provide information affecting their treatment or prognosis. These criteria satisfy the principle of justification and are considered in Chapter 15.
CONDUCTING THE EXAMINATION
When the decision has been made that a radiographic examination is justified (patient selection), the way in which the examination is conducted, the principle of optimization, greatly influences patient exposure to x radiation. The conduct of the examination may be divided into choice of equipment, choice of technique, operation of equipment, and processing and interpreting the radiographic image.
Film and Digital Imaging
Currently, intraoral dental x-ray film is available in three speed groups: D, E, and F (Chapter 5). Clinically, film of speed group E is almost twice as fast (sensitive) as film of group D and about 50 times as fast as regular dental x-ray film (Fig. 3-3). The current F-speed films require about 75% the exposure of E-speed film and only about 40% that of D-speed. Faster films are desirable from the standpoint of exposure reduction. Multiple studies have found that F-speed film has the same useful density range, latitude, contrast, and image quality as D- and E-speed films and can be used in routine intraoral radiographic examinations without sacrifice of diagnostic information. Current digital sensors offer equal or greater dose savings than F-speed film and comparable diagnostic utility.
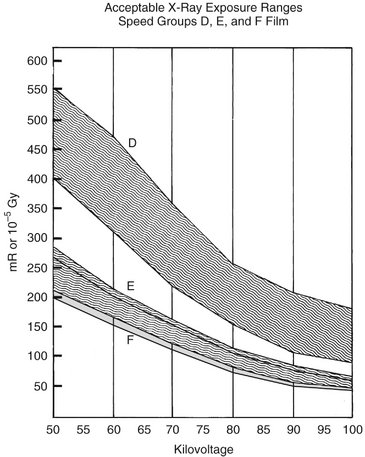
FIG. 3-3 Relationship between surface exposures delivered to a patient by exposure of groups D, E, and F (in light gray) intraoral films and diagnostic density at various kilovoltages. Note substantial overlap between E- and F-speed films. (Modified from HHS Publication [Food and Drug Administration] 85-8245, 1985.)
Intensifying Screens and Film or Digital Imaging
Rare-earth intensifying screens are recommended … combined with high-speed film of 400 or greater. (ADA, 2006)
Contemporary intensifying screens used in extraoral radiography use the rare earth elements gadolinium and lanthanum (see Chapter 5). These rare earth phosphors emit green light on interaction with x rays. Compared with the older calcium tungstate screens, rare earth screens decrease patient exposure by as much as 55% in panoramic and cephalometric radiography.
Unlike digital intraoral imaging, there is no significant dose reduction to be gained by replacing extraoral screen-film systems with digital imaging. Image resolution with digital systems is comparable to that obtained with rare earth screens matched with appropriate film.
Source-to-Skin Distance
Use of long source-to-skin distances of 40 cm, rather than short distances of 20 cm, decreases exposure by 10 to 25 percent. Distances between 20 cm and 40 cm are appropriate, but the longer distances are optimal. (ADA, 2006)
Two standard focal source-to-skin distances have evolved over the years for use in intraoral radiography, one 20 cm (8 inches) and the other 41 cm (16 inches). Use of the distance results in a 32% reduction in exposed tissue volume because the x-ray beam is less divergent (Fig. 3-4). One study reported a 30% decrease in the effective dose resulting from the use of a 30-cm distance instead of a 20-cm distance for a simulated 19-image complete mouth survey. Another study of patient exposures from intraoral radiographic examinations comparing a 40-cm distance with a 20-cm distance found a 38% to 45% decrease in thyroid exposure.
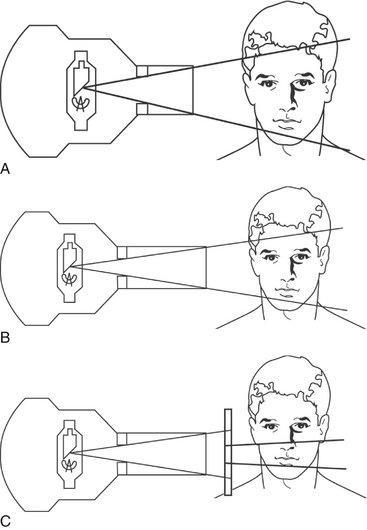
FIG. 3-4 Effect of source-to-skin distance and collimation on the volume of tissue irradiated. A larger volume of irradiated tissue results from A (with shorter source-to-skin distance) than from B (in which the longer source-to-skin distance produces a less divergent beam). In C the collimator between the round PID and the patient produces the effect of a rectangular PID on the tube housing or a rectangular collimating face shield on the film-holding instrument. This rectangular collimator (close to the patient in C) results in a smaller, less divergent beam and a smaller volume of tissue irradiated than in A or B.
The use of a longer source-to-object distance also results in a smaller apparent focal spot size and thereby theoretically increases the resolution of the radiograph (see Chapter 4). The clinical significance of the effect of focal spot size on image resolution, however, has been questioned.
Rectangular Collimation
Since a rectangular collimator decreases the radiation dose by up to fivefold as compared with a circular one, radiographic equipment should provide rectangular collimation for exposure of periapical and bitewing radiographs. (ADA, 2006)
The federal government requires that the x-ray beam used in intraoral radiography be collimated so that the field of radiation at the patient’s skin surface is “contained in a circle having a diameter of no more than 7 cm ( inches)” when the x-ray tube is operated above 50 kilovolts peak (kVp). In view of the dimensions of No. 2 intraoral film (3.2 × 4.1 cm) or digital sensor, a field size of this magnitude is almost three times that necessary to expose the image. Consequently, limiting the size of the x-ray beam even more than required by law may significantly reduce patient exposure. This results in not only decreased patient exposure but also increased image quality (Fig. 3-4). Additionally, the amount of radiation scatter generated is proportional to the area exposed. If scatter radiation is decreased, image fog is decreased and image quality is increased.
inches)” when the x-ray tube is operated above 50 kilovolts peak (kVp). In view of the dimensions of No. 2 intraoral film (3.2 × 4.1 cm) or digital sensor, a field size of this magnitude is almost three times that necessary to expose the image. Consequently, limiting the size of the x-ray beam even more than required by law may significantly reduce patient exposure. This results in not only decreased patient exposure but also increased image quality (Fig. 3-4). Additionally, the amount of radiation scatter generated is proportional to the area exposed. If scatter radiation is decreased, image fog is decreased and image quality is increased.
There are several means to limit of the size of the x-ray beam. First, a rectangular position-indicating device (PID) may be attached to the radiographic tube housing (Fig. 3-5). Use of a rectangular PID having an exit opening of 3.5 × 4.4 cm (1.38 × 1.34 inches) reduces the area of the patient’s skin surface exposed by 60% over that of a round (7 cm) PID (see Fig. 3-4, C). This reduction in beam size, however, may make aiming the beam difficult. To avoid the possibility of unsatisfactory radiographs (cone cutting), a film-holding instrument that centers the beam over the film or sensor is recommended (Fig. 3-6).

FIG. 3-5 A rectangular PID mounted on an x-ray machine provides a means to limit the shape of the x-ray beam to just larger than the film or digital sensor, thus minimizing the volume of tissue exposed. These devices are attached to an x-ray tube head to limit the size of the beam. They should be used with an external guide ring connected to the film or sensor to avoid cone cutting. (Courtesy Margraf Dental Manufacturing, Inc., www.margrafdental.com.)

FIG. 3-6 XCP film-holding instrument. The aiming ring aligns a circular aiming cylinder from an x-ray machine with the sensor to assure that the image plane is perpendicular to the central ray and in the middle of the beam. Note notches to align rectangularly collimated aiming devices such as shown in Figures 3-5 or 3-7. Sensor is in plastic bag to prevent contamination from saliva. (Courtesy Dentsply Rinn, http://www.rinncorp.com/.)
Alternatively, film- and sensor-positioning devices with rectangular collimators may be used with round aiming cylinders (Figs. 3-7 and 3-8). These holders reduce patient exposure to the same degree as rectangular PIDs. In a study reviewing the effective dose delivered during complete mouth examinations made with film holders using round and rectangular collimation, rectangular collimation reduced the patient dose from intraoral examinations by about 60%.

FIG. 3-7 Rectangular collimation. An alternative means of limiting the size of an x-ray beam to a rectangle is to insert the device shown here into the end of a circular aiming cylinder that restricts the beam field to a rectangle.

FIG. 3-8 Rectangular collimation. Another means to collimate a round beam to a rectangle is to place a metallic shield in the path of the beam, thus limiting the size of the exposure field to an area just larger than the film or sensor. JADRAD Dental X-Ray Shield is illustrated. (Courtesy JADRAD Dental Diagnostics.)
Filtration
The x-ray beam emitted from the radiographic tube consists of not only high-energy x-ray photons, but also many photons with relatively lower energy (see Chapter 1). Low-energy photons, which have little penetrating power, are absorbed mainly by the patient and contribute nothing to the information on the image. The purpose of filtration is to remove these low-energy x-ray photons selectively from the x-ray beam. This results in decreased patient exposure with no loss of radiographic information.
When an x-ray beam is filtered with 3 mm of aluminum, the surface exposure is reduced to about 20% of that with no filtration. In light of this and other information, the federal government has designated the specific amount of filtration, expressed as minimum half-value layer, required for dental x-ray machines operating at various kilovoltages. Practically, these requirements can be met by having 1.5 mm Al total filtration when operation from 50 to 70 kVp and with 2.5 mm Al total filtration when operating above 70 kVp.
Leaded Aprons and Collars
If all of the NCRP recommendations are followed rigorously, the use of a leaded apron on patients is not required. However, if any of the recommendations is not implemented, then a leaded apron should be used. Thyroid shielding with a leaded thyroid shield or collar is strongly recommended for children and pregnant women, as these patients may be especially susceptible to radiation effects. (ADA, 2006)
The function of leaded aprons and thyroid collars (Fig. 3-9) is to reduce radiation exposure of the gonads and thyroid gland. The NCRP 2003 recommendations referred to by the ADA are principally those already described, namely, use of patient selection criteria, fast receptors, and rectangular collimators. The NCRP and ADA think that leaded aprons are not necessary because it is far more important in patient protection to place emphasis on reducing exposure of the primary beam to facial structures than to reduce the already very slight gonadal exposure. Recent research has shown that the risk of heritable effects from dental exposure is essentially insignificant (Chapter 2). Note, however, that most states currently require the use of leaded aprons.

FIG. 3-9 Leaded apron with a thyroid collar. Children are more sensitive to radiation than adults; thus the use of leaded aprons with thyroid collars is especially valuable. (Courtesy Dentsply Rinn.)
There is, however, reason to be concerned about radiation exposure to the thyroid gland. Multiple studies, including those performed after the explosion of the Chernobyl reactor, have shown that the thyroid gland in children is especially sensitive to radiation. Accordingly, it is entirely appropriate to protect the thyroid glands of children during radiographic examinations. Again, the use of fast receptors and rectangular collimation is the best way to accomplish this aim, but thyroid collars further reduce dose to this organ.
Film and Sensor Holders
Film holders that align the film precisely with the collimated beam are recommended for periapical and bitewing radiographs. (ADA, 2006)
Film or digital sensor holders should be used when intraoral radiographs are made because they improve the alignment of the film, or digital sensor, with teeth and x-ray machine. Their use results in a significant reduction in unacceptable images. The use of film and sensor holders allows the operator to control the position and alignment of the film or sensor with respect to the teeth and jaws. This is especially important when the paralleling technique (Chapter 4) and digital imaging (Chapter 7) are used. In these cases it is often desirable to position the receptor away from the teeth so as to get the best image and reduce patient discomfort. This requires the use of a film or sensor holder. Most such devices have an external guide that shows the operator where to align the aiming cylinder (PID). As a result, the x-ray beam is properly directed toward the receptors. This greatly reduces the chance of the beam partially missing the image receptor (a “cone-cut”) and also reduces image distortion (Chapter 4).
The decision as to which technique is used should be based on the diagnostic quality of the resultant radiographs, the efficiency of using radiation, and the convenience of the technique. The more efficient the technique, the fewer radiograph retakes will be required, along with less patient exposure. A study of comparative efficiencies of the bisection and parallel techniques found that the number of nondiagnostic radiographs was reduced by more than half when intraoral complete mouth examinations were made with the paralleling technique.
Kilovoltage
The operating potential of dental X-ray machines must range between 50 and 100 kilovolt peak but should range between 60 and 80 kVp. (ADA, 2006)
As the kVp is lowered, the mean energy of the beam decreases. This results in (1) an image with greater contrast (assuming that exposure time is increased), (2) a beam with more low-energy photons that carry the potential for risk but are not useful in making an image, and (3) reduced beam intensity requiring increased exposure time, thus increasing the risk of the patient moving and blurring the image. Although image diagnosis may be improved slightly with increased image contrast (low kVp) images, the patient dose is somewhat reduced with higher kVp exposures. The best balance is to use 60 to 80 kVp.
The availability of constant-potential (fully rectified), high-frequency or direct current (DC) dental x-ray units has made possible the production of radiographs with lower kilovoltage and at reduced levels of radiation. The surface exposure required to produce a comparable radiographic density using a constant-potential unit is approximately 25% less than that of a conventional self-rectified unit operating at the same kilovoltage. Currently several manufacturers produce DC units.
Milliampere-Seconds
The operator should set the amperage and time settings for exposure of dental radiographs of optimal quality. (ADA, 2006)
Of the three technical conditions (tube voltage, filtration, and exposure time), exposure time is the most crucial factor in influencing diagnostic quality. In terms of exposure, optimal image quality means that the radiograph is of diagnostic density, neither overexposed (too dark) nor underexposed (too light). Both overexposed and underexposed radiographs result in repeat exposures, thereby leading to needless additional patient exposure. Image density is controlled by the quantity of x rays produced, which in turn is best controlled by the combination of milliamperage and exposure time, termed milliampere-seconds (mAs) (Chapter 1). Typically, a radiograph of correct density will demonstrate very faint soft tissue outlines. Dentin will have an optical density of about 1.0. If your x-ray machine has a variable milliampere control, it should be set at the highest choice. Proper exposure times should be determined empirically by using optimal film processing conditions (Chapter 6) or manufacturer’s recommendations for digital sensors. A chart showing optimal exposure times for each region of the arch in children and adults should be mounted by each x-ray machine. Because film-processing conditions are standardized, the only decision the dentist or the assistant needs to make is to select the proper exposure time.
Film Processing
Radiographs should not be overexposed and then underdeveloped, because this practice results in greater exposure to the patient and dental health care worker and can produce images of poor diagnostic quality. Dental radiographs should not be processed by sight, and manufacturers’ instructions regarding time, temperature and chemistry should be followed. (ADA, 2006)
A major cause of unnecessary patient exposure is the deliberate overexposure of films compensated by underdevelopment of the film. This procedure results in both needless exposure of the patient and in films that are of inferior diagnostic quality (because of incomplete development). On the other hand, a properly exposed radiograph is of no value if all its diagnostic information is lost as a result of poor processing procedures. One dental insurance carrier reported that some 6% of the dental radiographs it received were not readable because of improper processing. Another study of 500 panoramic radiographs found that the average film contained at least one processing error. Time-temperature processing, in an adequately equipped and maintained darkroom, is the best way to ensure optimal film quality (see Chapter 6). To help ensure optimal image quality, the dental assistant should follow the film manufacturer’s recommendation for processing solutions, not the solution manufacturer’s directions.
The use of machines to process dental x-ray film has become widespread. More than 90% of dentists surveyed have reported using dental film processors. Automatic film processors should be used in a darkroom. Although some units have daylight loaders, allowing film to be placed in the machine in room light, such loaders are difficult to keep clean and free of contamination. Film processors, however, can actually increase patient exposure if not correctly maintained. Approximately 30% of all films retaken because of incorrect film density were directly related to processor variability. The introduction of a comprehensive maintenance program was found to reduce this retake rate significantly, resulting in a substantial savings in both patient exposure and operating costs.
Interpreting the Images
The dentist should view radiographs under appropriate conditions for analysis and diagnosis. (ADA, 2006)
Radiographs are best viewed in a semidarkened room with light transmitted through the films; all extraneous light should be eliminated. In addition, radiographs should be studied with the aid of a magnifying glass to detect even the smallest change in image density. A variable-intensity light source should also be available. Similarly, digital images are best interpreted on a computer screen in a darkened environment.
The diagnostic accuracy of radiographic caries diagnosis is only about 70% or less. This fact should stimulate individuals to place a greater emphasis on careful radiographic interpretation. Failure to diagnose problems is an increasing source of liability claims.
PROTECTING PERSONNEL
Operators of radiographic equipment should use barrier protection when possible, and barriers should contain a leaded glass window to enable the operator to view the patient during exposure. When shielding is not possible, the operator should stand at least two meters from the tube head and out of the path of the primary beam. (ADA, 2006)
The methods of dose reduction discussed thus far have emphasized the effect on patient exposure. It should be apparent, however, that any procedure or technique that reduces radiation exposure to the patient also reduces the possibility of operator or office personnel exposure. In addition to those mentioned, several other steps can be taken to reduce the chance of occupational exposure.
Perhaps the single most effective way of limiting occupational exposure is the establishment of radiation safety procedures that are understood and followed by all personnel. Such written procedures are currently mandated by several states. First, every effort should be made so that the operator can leave the room or take a position behind a suitable barrier or wall during exposure of the image. Dental operatories should be designed and constructed to meet the minimal shielding requirement of the NCRP. This will require consultation with a qualified expert. This recommendation states that walls must be of sufficient density or thickness that the exposure to nonoccupationally exposed individuals (e.g., someone occupying an adjacent office) is no greater than 100 mGy per week. In most instances, it is not necessary to line the walls with lead to meet this requirement. Walls constructed of gypsum wallboard (drywall or sheet rock) are adequate for the average dental office.
If leaving the room or making use of some other barrier is impossible, strict adherence to what has been termed the position-and-distance rule is required: The operator should stand at least 6 feet (2 m) from the patient, at an angle of 90 to 135 degrees to the central ray of the x-ray beam (Fig. 3-10). When applied, this rule not only takes advantage of the inverse square law to reduce x-ray exposure to the operator but also take advantage of the fact that in this position the patient’s head absorbs most scatter radiation. All practitioners should check their state’s regulations for use of ionizing radiation regarding operator position during x-ray exposures.
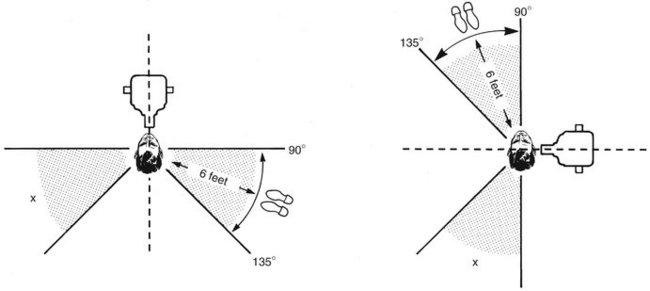
FIG. 3-10 Position-and-distance rule. If no barrier is available, the operator should stand at least 6 feet from the patient, at an angle of 90 to 135 degrees to the central ray of the x-ray beam when the exposure is made.
Second, the operator should never hold films or sensors in place. Film or sensor-holding instruments should be used (see Rectangular Collimation, p. 37). If correct film placement and retention are still not possible, a parent or other individual responsible for the patient should be asked to hold the sensor in place and, of course, be afforded adequate protection with a leaded apron. Under no circumstances should this person be one of the office staff.
Third, neither the operator nor patient should hold the radiographic tube housing during the exposure. Suspension arms should be adequately maintained to prevent housing movement and drift.
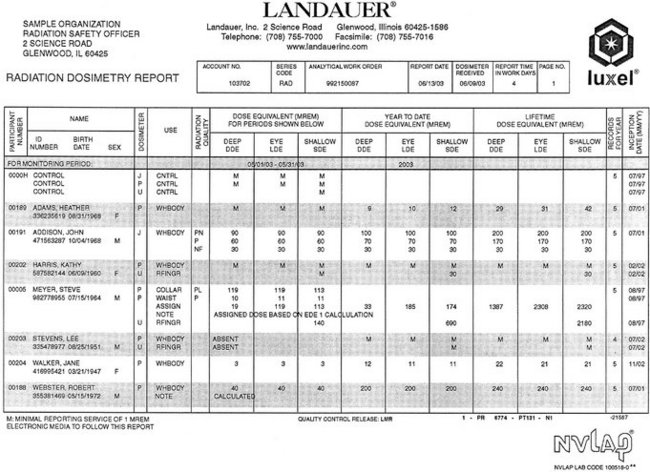
The best way to ensure that personnel are following office safety rules such as those described previously is with personnel-monitoring devices. Commonly referred to as film badges, these devices provide a useful record of occupational exposure. Their use is not only recommended but also required by law in certain states. Several companies in the United States offer dosimetry monitoring services. For a reasonable charge, these services provide badges that contain either a piece of sensitive film or a radiosensitive crystal (thermoluminescent dosimeter) and a printed report of accumulated exposure at regular intervals (Fig. 3-11). These reports indicate any undesirable change in work habits and help remove any apprehension office staff members may have about the possibility of exposure to x rays.
QUALITY ASSURANCE
Quality assurance protocols for the X-ray machine, imaging receptor, film processing, dark room, and leaded aprons and thyroid collars should be developed and implemented for each dental health care setting. (ADA, 2006)
Quality assurance may be defined as any planned activity to ensure that a dental office will consistently produce high-quality images with minimum exposure to patients and personnel (see Chapter 8). Studies have indicated that dentists may be needlessly exposing their patients to compensate for improper exposure techniques, film processing practices, and darkroom procedures. One study reported that only 33% of panoramic radiographs that accompanied biopsy specimens were of acceptable diagnostic quality. However, when demands were placed on dentists to improve their techniques, the number of unsatisfactory radiographs was significantly reduced. Two studies by a dental insurance carrier demonstrated that after claims were rejected for unsatisfactory radiographs and the dentist was made aware of the errors and ways in which they could be corrected, the number of satisfactory radiographs submitted doubled. This suggests that when the dentist is presented with guidelines for quality assurance, along with proper motivation, patient exposure can be dramatically reduced.
Currently some states require dental offices to establish written guidelines for quality assurance and to maintain written records of quality assurance tests. Regardless of requirements, each dental office should establish maintenance and monitoring procedures as outlined in Chapter 8.
CONTINUING EDUCATION
Practitioners should remain informed about safety updates and the availability of new equipment, supplies and techniques that could further improve the diagnostic quality of radiographs and decrease radiation exposure. (ADA, 2006)
Those who administer ionizing radiation must become familiar with the magnitude of exposure encountered in medicine, dentistry, and everyday life; the possible risks associated with such exposure; and the methods used to affect exposure and dose reduction. Although this chapter presents some of this information, acquiring knowledge and developing and maintaining skills is a life-long process.
American Dental Association Council on Scientific Affairs. The use of dental radiographs: update and recommendations. J Am Dent Assoc. 2006;137:1304–1312.
Code of Federal Regulations 21, Subchapter J: Radiological health, part 1000, Office of the Federal Register, General Services Administration, Washington, DC, 1994.
Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiations. Health risks from exposure to low levels of ionizing radiation: BEIR VII. Washington, DC: National Academy Press; 2006.
Hall, EJ, Giaccia, AJ. Radiobiology for the radiologist, ed 6. Baltimore: Lippincott Williams & Wilkins; 2006.
Horner, K, Rushton, VE, Walker, A, et al. European guidelines on radiation protection in dental radiology: the safe use of radiographs in dental practice. Radiat Protect. 2004;136:1–115.
National Council on Radiation Protection and Measurements, Control of radon in houses. NCRP Report 103. Bethesda, Md: National Council on Radiation Protection and Measurements; 1989.
National Council on Radiation Protection and Measurements, Quality assurance for diagnostic imaging. NCRP Report 99. Bethesda, Md: National Council on Radiation Protection and Measurements; 1990.
National Council on Radiation Protection and Measurements, Limitation of exposure to ionizing radiation. NCRP Report 116. Bethesda, Md: National Council on Radiation Protection and Measurements; 1993.
National Council on Radiation Protection and Measurements, Dental x-ray protection. NCRP Report 145. Bethesda, Md: National Council on Radiation Protection and Measurements; 2003.
CRCPD Publication E-03-6. Nationwide Evaluation of X-Ray Trends (NEXT), tabulation and graphical summary of the 1999 dental radiography survey. Bethesda, Md: Center for Devices and Radiological Health, U.S. Food and Drug Administration; 2003.
Preston, RJ. Radiation biology: concepts for radiation protection. Health Phys. 2005;88:545–556.
Sources and effects of ionizing radiation. New York: UNSCEAR, UN Publication; 2000;volume 1. sources
Wall, BF, Kendall, GM, Edwards, AA, et al. What are the risks from medical X-rays and other low dose radiation? Br J Radiol. 2006;79:285–294.