NATURALLY OCCURRING COMBINED ABNORMALITIES OF FREE WATER, ELECTROLYTE AND ACID–BASE BALANCE
These abnormalities are seldom primary and usually secondary to a serious disease state such as abomasal volvulus, rumen overload or acute intestinal obstruction – diseases that are in themselves life-threatening. Fluid and electrolyte abnormalities are also life-threatening and simple correction of the primary abnormality, for example removal of a large section of a horse’s small intestine, is valueless unless the dehydration, hyponatremia and acidosis are also corrected. The variation that can occur in these naturally occurring errors of fluid, electrolyte and acid–base balance is what makes their diagnosis and treatment so difficult. If it were possible to have instant clinicopathological advice on what the abnormalities were, and how they were progressing as determined by constant laboratory monitoring, there would be little challenge in it. However, under normal clinical circumstances these services are not readily available and it is necessary to have an understanding of the basic physiology and pathology of these diseases to be able to predict by clinical examination and examination of the history, the likely deficiencies and imbalances and their degrees of severity.
In the preceding paragraphs the individual abnormalities of fluid and electrolyte homeostasis were described. In most naturally occurring diseases, the abnormalities are complex. In example, the probable events in a case of acute diarrhea are set out diagrammatically in Figure 2.8. It is important to remember that the variation in fluid and electrolyte imbalance is dynamic as a result of the compensatory changes occurring in various organs, especially the respiratory and circulatory systems and the kidneys. It is this volatility which makes clinical pathological monitoring so important. Some generalizations on the dynamics of fluid and electrolyte status are as follows:
• The body water and electrolytes are maintained at a homeostatic level by the buffering system of the blood, the lungs and the kidney
• In disturbances of body water and electrolytes, the changes that occur are also dynamic, and there is constant reaction by the homeostatic mechanism to restore the water and electrolyte relationship to normal
• With some exceptions, it is unusual to find an uncompensated alkalemia or acidemia. A partial compensation in the opposite direction of the primary acid–base imbalance is usually in progress and it is important to determine the nature of the primary disturbance for the selection of rational therapy
• Often, the nature of the primary disturbance can be determined from a consideration of the history and the clinical findings
• The dehydration caused by deprivation of water and electrolytes (lack of water or inability to drink) is mild and animals may appear only mildly dehydrated even after several days of water deprivation. The feces are hard and dry, the rumen contents are firm and dry and urine volume is considerably decreased
• With the exception of clinical dehydration, the clinical findings of electrolyte and acid–base imbalances are not characteristic
• Without laboratory evaluation, the nature and degree of electrolyte and acid–base imbalance must be assumed and estimated based on the history of the affected animal and the changes that are most likely to have occurred.
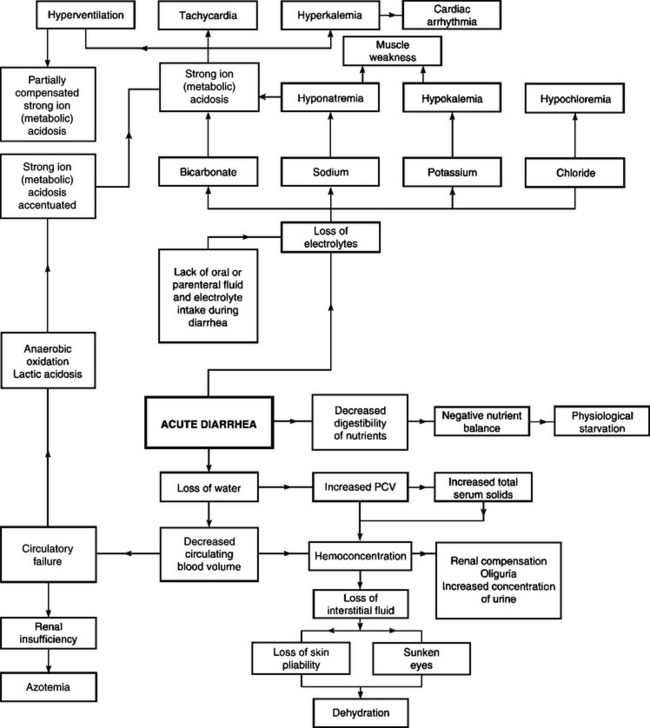
Fig. 2.8 The interrelationships between the changes in body water, electrolytes and acid–base balance that can occur in diarrhea.
NATURE OF THE DISEASE AND HISTORY
The history of the case, the length of time the animal has been affected and the tentative diagnosis will provide a clinical assessment of the possible nature and degree of electrolyte and acid–base imbalance. Animals affected with acute diarrhea due to infectious enteritis are likely to be in a state of metabolic acidosis and hyponatremia. In intestinal obstruction of the horse, there are varying degrees of dehydration and metabolic acidosis. Obstruction of the upper intestinal tract, or abomasal stasis, is characterized by varying degrees of dehydration, and metabolic alkalosis with hypochloremia and hypokalemia. A combination of the clinical assessment and the available laboratory evaluation will allow the clinician to make the most rational approach to treatment.
The information on the duration of illness must be accurate or it will be misleading. The sequence of clinical findings in the history may indicate the trend in severity. Animals that have had a profuse watery diarrhea for 18–24 hours may be severely acidemic. Acute intestinal obstruction in cattle is not as severe as in the horse. Acute gastric or intestinal rupture in the horse or in cattle is usually rapidly fatal. Acidosis in grain overload in cattle may be fatal in 24–48 hours; acidosis in the horse with grain overload may be much more rapidly fatal as electrolyte disturbances are more severe in the horse.
CLINICAL FINDINGS
Dehydration is usually obvious clinically and determination of the PCV and total serum solids will improve the assessment.
A normal temperature is not a good prognostic guide but a subnormal temperature suggests a worsening situation.
A gradually progressive tachycardia indicates that the patient is deteriorating. In general, in the horse, a heart rate up to 60 beats/min suggests a minor lesion (but not always), a heart rate between 60–80 beats/min is in the danger area, 80–100 beats/min is serious; more than 100 beats/min is commonly premortal (except in intestinal tympany that may be relieved).
A cold clammy skin that remains tented for more than 30 seconds suggests severe dehydration. Cyanosis of the oral mucous membranes and a capillary refill time of more than 4 s suggests a poor prognosis, as does rapid respiration (three to four times normal) with intermittent hyperpnea and apnea.
Muscular tremors and leg buckling are grave signs in the horse and are commonly followed by collapse and death. The inability of any dehydrated animal to stand (other reasons being eliminated) is ominous. Severe depression and dullness are commonly observed in acute conditions, and coma is usually terminal.
Metabolic acidosis is characterized by varying degrees of mental depression, weakness and ataxia. Some of the depression and weakness will be due to dehydration. In newborn animals with metabolic acidosis associated with diarrhea, a failure to suck and the lack of a suck reflex are common.
CLINICAL PATHOLOGY
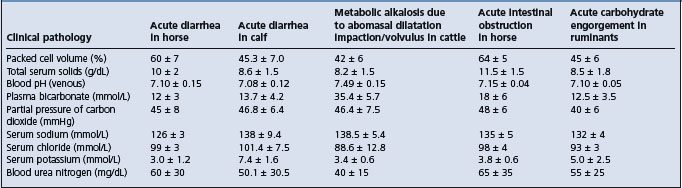
Some representative laboratory values in examples of body water and electrolyte disturbances are given in Table 2.1.
Packed cell volume and total serum solids
The PCV and the total serum proteins or total serum solids will indicate the severity of water loss. Anemic animals and those affected with diseases causing hypoproteinemia may provide misleading values.
The normal range depends on the age and species of animal, previous excitement and the presence of anemia or hypoproteinemia. A packed cell volume of 30–40% is considered normal; between 40% and 50%, fluid therapy may or may not be necessary; between 50% and 60%, fluids are necessary for recovery and above 60% intensive fluid therapy is necessary and the prognosis is unfavorable. A total serum solids of 6.0–7.5 g/dL is usually considered normal; at 8–10 g/dL fluids are needed and the prognosis is favorable and above 12 g/dL the prognosis is unfavorable.
Blood pH and blood gases
Sample collection and analysis
A useful screening test for acid–base status in animals without evidence of respiratory disease is the total CO2. Total CO2 is defined as the amount of total carbon dioxide in plasma that can be liberated with a strong acid, and can be calculated from the results of routine blood gas analysis as: total CO2 = [HCO3−] + dissolved CO2 + [H2CO3]. The [HCO3−] is calculated using the Henderson–Hasselbalch equation, the dissolved CO2 is equal to S × Pco2, whereas [H2CO3] is negligible.
Many automatic serum biochemical analyzers directly measure total CO2 (instead of calculating its value from the results of blood gas analysis) but for total CO2 measurement it is important that blood collection tubes are completely filled before serum is harvested: failure to completely fill the blood tubes promotes escape of CO2 from serum into the partial vacuum above, thus resulting in measured total CO2 values that underestimate true serum total CO2.1 Because changes in total CO2 reflect changes in actual [HCO3−], total CO2 can never provide an independent measure of the nonrespiratory component of an acid–base disturbance. Total CO2 does, however, provide a useful screening test for the presence of acid– base disturbances in domestic animals without clinical evidence of respiratory disease. In the absence of respiratory disease, a decrease in total CO2 indicates a metabolic acidosis, whereas an increase in total CO2 indicates metabolic alkalosis. Total CO2 has historically been measured using the Harleco apparatus,2 although this methodology is no longer used due to the availability of point-of-care analyzers.
If the primary clinical interest is acid– base assessment, then a jugular venous blood sample should be anaerobically obtained in a 3 mL plastic syringe that has been previously coated internally with sodium heparin (by drawing sodium heparin into the syringe barrel and then expelling all heparin from the syringe into the barrel before blood collection). Three mL of air should then be drawn into the syringe and forcibly expelled; this process is repeated three times. Evacuating the syringe in this manner ensures that minimal heparin is retained to dilute the blood sample but a sufficient quantity is still present to prevent coagulation.3 After blood collection, the air bubbles should be removed from the blood in the syringe, the end should be corked to prevent loss of CO2 and addition of O2 to the blood sample and the syringe should be placed on ice (4°C) until analysis. This will minimize any time-related changes in pH, Pco2 and base excess that occur when blood is held at room temperature (20°C), particularly in blood samples with high white blood cell concentrations. The change in pH, Pco2 and base excess per hour at 22–24°C are –0.024, +2.5 and –0.5 respectively.3 A portable blood gas analyzer for equine venous blood is available and provides reproducible and acceptable analysis.4
If the primary interest is evaluation of the respiratory system, an arterial blood sample should be obtained in the same manner but the sample should be kept at body temperature (preferable) or room temperature before blood gas analysis, which should be performed as soon as possible. This is because keeping 3 mL plastic syringes on ice (4°C) facilitates oxygen diffusion through the plastic syringe barrel, causing an increased Po2.5
Use of point-of-care clinical analyzing systems has greatly facilitated routine evaluation of acid–base status in domestic animals. Thorough assessment of acid– base status requires blood gas analysis and serum biochemical analysis, with blood samples being obtained from a major vein or any artery. If serum total protein, albumin and phosphate concentrations are approximately normal, then acid–base status should be evaluated using blood pH, Pco2 and extracellular base excess concentration. This is the traditional Henderson–Hasselbalch approach. The presence of unidentified anions should be investigated by calculating the anion gap. If serum total protein, albumin, and phosphate concentrations are markedly abnormal, then acid–base status should be evaluated using blood pH, Pco2, measured [SID+] and [ATOT]. This is the simplified strong ion approach. The presence of unidentified strong ions should be investigated by calculating the SIG.
Blood pH and acid–base interpretation
Normal blood pH varies from 7.35 to 7.45 (venous blood). The degree of acidemia encountered includes moderate acidemia (pH 7.30–7.25), severe acidemia (pH 7.25–7.20), grave (and commonly fatal except in neonates) acidemia (pH 7.10–7.00). Horses with volvulus or strangulation of the intestines generally have blood lactate levels over 75 mg/dL (8.2 mmol/L) whereas cases of impaction have levels of 5–9 mg/dL (0.55–1.0 mmol/L). The normal value is 6.0 mg/dL (0.78 mmol/L) with a range of 4–12 mg/dL (0.44–1.33 mmol/L). The survival rate in a series fell from 85% to 0% as the lactate concentration increased from 75 to 155 mg/dL (8.3 to 17.2 mmol/L).
Serum electrolytes
Serum electrolyte concentrations indicate the severity of the electrolyte losses and the necessity for replacement with either balanced electrolyte solution or specific electrolyte solution. Serum concentrations of sodium, chloride and potassium are usually determined. The total deficit for each electrolyte can be estimated using the standard formula presented under calculation of electrolyte requirements.
Serum electrolyte concentrations depend on the initial cause and the severity of the disease. For example, in most cases of acute diarrhea there is hyponatremia and metabolic acidosis, which are usually marked in the horse with acute diarrhea. The serum levels of chloride may be normal or subnormal in acute diarrhea. The serum levels of potassium will be below normal initially but as acidosis develops and becomes severe, hyperkalemia may occur. In diseases causing abomasal atony there will be hypochloremic, hypokalemic and metabolic alkalosis.
Water and electrolyte abnormalities are classified into three types based on the measurement of electrolytes and osmolality:
• Hypertonic dehydration (true dehydration/desiccation): osmolality greater than 300 mosmol/kg (300 mmol/kg), associated with water deprivation, some acute gastrointestinal problems and some types of diarrhea
• Hypotonic dehydration (acute desalting water loss): osmolality less than 260 mosmol/kg (260 mmol/kg), associated with acute diarrhea, particularly secretory diarrheas, such as salmonellosis
• Isotonic dehydration: normal electrolyte and osmolality levels, as in horses losing electrolytes and water in almost equal proportions.
Urea nitrogen and creatinine
Plasma urea nitrogen and plasma creatinine are metabolic breakdown constituents that can be used to assess the degree of dehydration and to distinguish between prerenal, renal and postrenal uremia. The plasma urea nitrogen and creatinine concentration will be elevated, depending on the severity of the dehydration and decrease in circulating blood volume. Following treatment with fluids and electrolytes in prerenal uremia, the levels of plasma urea and creatinine will decline.
Total leukocyte and differential counts
A marked leukopenia and neutropenia with a degenerative left shift carries an unfavorable prognosis. A regenerative left shift with a neutrophilia is a favorable prognosis. A marked lymphopenia indicates severe stress and the prognosis may be unfavorable.
Blood glucose
Blood glucose concentration can be determined using conventional laboratory techniques, which require the submission of heparinized blood samples as soon as possible to avoid erroneous results due to hemolysis or erythrocyte glycolysis. A quantitative, rapid method of determining blood glucose concentrations in mature cattle and calves is available and the results correlate with the conventional, laboratory-based method.6 The laboratory-based plasma glucose levels were 10–15% higher than the blood glucose levels determined by the rapid field method. The field method is based on the glucose oxidase reaction and uses impregnated test strips and a pocket-sized, digital readout reflectance meter to measure colorimetric change.
Anion, strong ion and osmolal gaps
Acid–base balance has traditionally been evaluated by using the Henderson– Hasselbalch equation to characterize four primary acid–base disturbances (i.e. respiratory acidosis and alkalosis, metabolic acidosis and alkalosis) and by calculating the anion gap to estimate the unmeasured anion concentration. Evaluation of the anion gap has become routine in many medical institutions. The calculation takes little time, is essentially without cost and is valuable in assessing a variety of clinical conditions in which electrolyte imbalances occur.
The anion gap (AG) represents the difference between the concentration of unmeasured anions [UA] and unmeasured cations [UC] in serum, which can be expressed in the equation:7
[Na+] + [K+] + [UC] = [Cl−] + [HCO3−] + [UA],
[UA] − [UC] = AG = ([Na+] + [K+]) − ([Cl−] + [HCO3−]).
A change in [UA] or [UC] will cause a change in the AG. Under normal circumstances, approximately two-thirds of the AG originates from the net negative charge of serum proteins, and the remainder represents the serum concentration of phosphate and strong anions, such as lactate, sulfate, β-OH butyrate, aceto-acetate and anions associated with uremia.7 The normal range for AG depends on the age and species. The normal range for 2–3-week-old foals is 9–22 mEq/L which is higher than that for 2-year-old horses (range 8–13 mEq/L). The range of AG (mean ± 2 SD) for adult animals varies for different species: 8–13 mEq/L (horse), 14–20 mEq/L (cow) and 17–29 mEq/L (sheep). AG values greater than 30 mEq/L have been seen in critically ill cattle, with the increase being attributed to an increase in blood lactate and ketoacid concentration as well as to anions associated with uremia.
A potentially valuable clinical use for the AG is in estimating the plasma l-lactate concentration, which provides information about the adequacy of oxygen delivery to the tissues, thereby providing a means for assessing the severity of cardiovascular or pulmonary dysfunction, monitoring the response to treatment and formulating a prognosis for survival. The normal plasma l-lactate concentration is generally considered to be less than 1.5 mmol/L. Increases in plasma l-lactate concentration have been categorized as mild (2.5–4.9 mmol/L), moderate (5.0–9.9 mmol/L) and severe (≥10 mmol/L), with l-lactate concentrations greater than 10 mmol/L being associated with a high mortality in humans, pigs and horses.7
Because lactate determinations may not be available in some laboratories, calculation of the AG can be considered a ‘poor man’s plasma lactate’. The correlation between AG and l-lactate concentrations is excellent in horses with intestinal disease. The AG of neonatal calves with experimental diarrhea was 28.6 ± 5.6 mEq/L, and the blood lactate concentration ranged from 1.1–2.9 mmol/L; the AG was significantly correlated with serum phosphate and creatinine concentration. The AG of adult cattle with abomasal volvulus was 20.5 ± 7.8 mEq/L and the blood l-lactate concentration ranged from 0.6–15.0 mmol/L. The AG in adult cattle is only moderately correlated with l-lactate concentrations and is similarly correlated with serum phosphate and creatinine concentrations in neonatal calves and adult cattle, as well as with serum albumin and total protein concentrations in adult cattle. Anion gap determination is of limited usefulness in predicting blood l-lactate concentration in sick cattle, whereas the correlation between AG and serum concentration in sick cattle suggests that an increased AG should suggest the potential presence of uremic anions.7
In summary, the determinants and utility of the anion gap in predicting hyperlactatemia are as follows.7
• The AG in critically ill cattle is influenced by at least three factors: blood l-lactate concentration and the serum concentrations of phosphate and creatinine
• There is a substantial quantity of unmeasured anions in sick cattle (approximately 7 mEq/L), which implies that either unidentified cations or anions other than chloride, bicarbonate, l-lactate, pyruvate, β-OH butyrate or phosphate are present in critically ill cattle or that the formula used to assign protein charge was inaccurate
• The correlation coefficient between AG and blood l-lactate concentration is similar to that observed in human patients and less than that seen in sick horses
• The AG appears to predict blood l-lactate concentration more accurately in neonatal calves with experimental diarrhea than that in adult cattle with spontaneously occurring abomasal volvulus.
Strong ion gap
The strong ion gap represents the concentration of unmeasured strong ions in plasma and is more specific in detecting the presence of unmeasured strong ions in plasma than the anion gap. The SIG concept is a logical extension of the AG concept and was developed using the strong ion difference approach in order to express SIG in terms of other factors: SIG = ATOT/(1 + 10(pKa−pH)) – AG, where SIG represents the difference between unmeasured strong cation concentration and unmeasured strong anion concentration in plasma or serum.8 Calculation of the SIG requires species-specific values for the total plasma concentration of nonvolatile weak acids (ATOT; i.e. the total concentration of plasma nonvolatile buffers; albumin, globulin and phosphate) and the negative logarithm to the base 10 (pKa) of the effective dissociation constant (Ka) for plasma nonvolatile buffers. Values for ATOT and pKa have been determined for the plasma of horses (ATOT, 15.0 mmol/L = 0.22 mmol/g of total protein or 0.47 mmol/g of albumin; pKa, 6.66) and calves (ATOT, 23.1 mmol/L = 0.41 mmol/g of total protein or 0.75 mmol/g of albumin; pKa, 7.08).9,10
The normal SIG value is –5 to +5 mEq/L. An increase in SIG to above 5 mEq/L (a rare occurrence) therefore reflects an increase in unmeasured strong cations or a decrease in unmeasured strong anions. A decrease in SIG to below –5 mEq/L (a common occurrence) reflects a decrease in unmeasured strong cations or, more likely, an increase in unmeasured strong anions.
The SIG offers a more accurate approach to identifying unmeasured strong ions in plasma than does the AG. The critical difference between the AG and SIG is that the SIG provides an estimate of the difference between unmeasured strong cations and strong anions, whereas AG provides an estimate of the difference between unmeasured cations and anions (including strong ions and nonvolatile buffer ions such as albumin, globulins, and phosphate). A change in SIG therefore provides a more specific method for detecting a change in unmeasured strong ions (such as lactate) than a change in AG.
Osmolal gap
Evaluation of the osmolal gap is a means of detecting an increased amount of abnormal osmotically active solute in the blood. The osmolal gap is the difference between the measured plasma osmolality and the osmolality calculated from the plasma concentration of normally measured solutes. Sodium and potassium and their associated anions, along with glucose and urea, constitute the majority of normal osmotically active solutes. The following formula is recommended, although many clinicians disregard the contribution of serum urea nitrogen (SUN) because it is an ineffective osmole that easily crosses cell membranes:
1.86 × ([Na+] + [K+]) + (glucose/18) + (SUN/2.8) + 8.6.
Examination of the triad of calculated osmolality, measured osmolality and the osmolal gap is beneficial in the diagnosis and prognosis of a number of diseases.
The effects of acidemia on the anion gap and electrolytes can vary depending on the cause of the acidosis and the species involved. Experimentally in horses, the infusion of l-lactic acid and d- and l-lactic acid results in acidosis with a high anion gap.11 An infusion of hydrochloric acid causes metabolic acidosis with a decreased anion gap. Saline infusions cause mild acidosis with no significant change in anion gap. The plasma potassium was decreased by the infusions of the organic acids but not by hydrochloric acid. Hypophosphatemia occurred with the saline and hydrochloric acid infusions but not with the organic acids. These results indicate that large changes in plasma potassium and serum inorganic phosphate can occur in acidosis in the horse and are probably not the direct result of acidemia. High-intensity exercise in the horse results in a progressive rise in plasma potassium and lactate.12
Arterial blood pressure
Arterial blood pressure and central venous pressure are not measured routinely but are occasionally measured in referral centers where the technical assistance and instrumentation are readily available. Mean arterial blood pressure provides a rough guide for the presence and severity of terminal shock but not for the severity or extent of the initiating lesion.
Jugular or central venous pressure
This is more useful as a monitor during fluid replacement. Normal pressure is 2–10 cmH2O (0.3–1.0 kPa), referenced to the point of the shoulder (scapulohumeral joint). Below 2 cmH2O (0.3 kPa) requires fluid therapy; above 15 cmH2O (1.5 kPa) indicates cardiac failure and volume overload.
Total body water
Total body water can be measured in horses before and after exercise using orally administered deuterium oxide followed by a series of blood samples taken for analysis.13 Mean total body water content is about 62%. It is not determined clinically.
PRINCIPLES OF FLUID AND ELECTROLYTE THERAPY
The most important principle is to prevent or minimize dehydration and electrolyte loss whenever possible. This means the provision of an adequate water supply, adequate drinking space and a continuous supply of salt and the necessary minerals. The next most important principle is to treat potential losses of fluid and electrolytes as quickly as possible to minimize the degree of dehydration and acid–base imbalance that may occur in animals with diseases in which losses are occurring.
The major therapeutic objectives are to correct the abnormalities that already exist and to monitor and provide maintenance therapy until the animal has recovered. Correction of the abnormalities may require 4–6 hours and maintenance therapy may be necessary for 2–4 days, depending on the cause of the disease. There are at least four possible abnormalities that could exist at the same time and must be corrected:
The two major problems are to determine the nature and degree of the abnormalities present and to decide which fluid and electrolyte replacement solution should be used.
The ideal situation would be to make both a clinical and laboratory evaluation of the animal as described above. The history and the diagnosis will suggest the possibility of acidemia or alkalemia and the electrolyte imbalances that are likely to be present. The degree of dehydration can usually be recognized clinically. Severe dehydration and acidemia should be treated as quickly as possible. A summary of the disturbances of fluid and electrolyte balance that occur in some common diseases of cattle and horses, and the suggested fluid therapy, is presented in Table 2.2.
Table 2.2 Summary of disturbances of body water, electrolytes and acid–base balance in some common diseases of cattle and horses, and suggested fluid therapy.
| Disease | Major abnormalities and deficits | Fluid and electrolyte requirements |
|---|---|---|
| Neonatal calf diarrhea (including piglets and lambs) | Metabolic acidosis, low plasma bicarbonate, severe dehydration, loss of sodium, hyperkalemia when acidosis severe | Equal mixtures of isotonic saline and isotonic sodium bicarbonate with 5% dextrose. |
| Balanced electrolytes too, IV and PO. See Colibacillosis, Ch. 18, for details | ||
| D-lactic acidosis (carbohydrate engorgement of ruminants) | Metabolic acidosis, low plasma bicarbonate, severe dehydration | Sodium bicarbonate initially followed by balanced electrolytes, IV. See Acute carbohydrate engorgement of ruminants, Ch. 6, for details |
| Acute diffuse peritonitis | Dehydration. Slight metabolic alkalosis due to paralytic ileus | Balanced electrolyte solutions in large quantities IV for hydration and maintenance |
| Right-side dilatation/abomasal volvulus of cattle, abomasal impaction (dietary or vagal nerve injury) | Metabolic alkalosis, marked hypochloremia, hypokalemia, severe dehydration | Balanced electrolyte solutions or high-potassium and chloride-acidifying solution, IV. May give acidifying solutions orally. See Right-side displacement of abomasum Ch. 6, for details; can also use mixture of 2 L of isotonic saline (0.9%), 1 L isotonic potassium chloride (1.1%) and 1 L isotonic dextrose (5%) |
| Peracute coliform mastitis | Severe dehydration, mild electrolyte deficits including mild hypocalcemia. Metabolic acidosis if diarrhea present | Balanced electrolyte solutions IV in large quantities for hydration and maintenance for 24–48 hours (100–150 mL/kg B W/24 h) |
| Acute diarrhea in the horses (enteric salmonellosis | Severe dehydration, marked hyponatremia, metabolic acidosis. Hypokalemia occurs following bicarbonate therapy | Hypertonic sodium bicarbonate (5%) 3–5 L 500 kg BW followed by high-sodium, high-potassium alkalinizing solution to correct hypokalemia following bicarbonate therapy. All by the IV route |
| Acute grain engorgement in the horse | Metabolic acidosis, dehydration and shock | Hypertonic sodium bicarbonate (5%) 3–5 L/500 kg BW followed by balanced electrolytes IV |
| Water and electrolyte deprivation. Esophageal obstruction in horses | Moderate dehydration | Balanced electrolytes IV. When obstruction relieved, provide electrolyte solution orally |
| Acute intestinal obstruction | Metabolic acidosis or alkalosis dependent on level of obstruction. Severe dehydration in horse, moderate in cow | Isotonic sodium bicarbonate initially, 3–5 L/500 kg BW followed by balanced electrolytes IV. Horses may develop hypokalemia following bicarbonate therapy and must be given potassium chloride |
Calculation of electrolyte requirements
The electrolyte deficits can be estimated using the serum electrolyte values of the affected animal. The total deficit of the electrolyte in milliequivalents (mEq) is the product of the deficit of the electrolyte in mEq per liter (ΔmEq/L) and the distribution space for the electrolyte. For sodium, chloride and bicarbonate, the distribution space is the extracellular fluid volume, which approximates 30% of BW in normally hydrated adults and 50% in normally hydrated neonates. In other words, for sodium, chloride and bicarbonate, the total milliequivalent deficit = (ΔmEq/L) × (estimated euhydrated body weight in kg) × (0.3 or 0.5).
There is less certainty about the size of the potassium space because potassium is mainly an intracellular ion.
Types of intravenous fluid
Fluids are categorized on the basis of their physical nature (crystalloid or colloid) and osmolarity (hypotonic, isotonic or hypertonic). Isotonic or slightly hypotonic crystalloid solutions are most commonly administered parenterally, although under specific circumstances hypertonic crystalloid solutions or isotonic colloid solutions are preferred.
Crystalloid solutions
A crystalloid is a substance that forms a true solution and is capable of being crystallized. Examples of crystalloid solutions are Ringer’s solution, lactated Ringer’s solution, acetated Ringer’s solution, 0.9% NaCl, 7.2% NaCl (hypertonic saline), 1.3% NaHCO3, 8% NaHCO3, calcium gluconate and 50% dextrose. Sodium chloride is the classic crystalloid solution, as table salt (NaCl) exists as a crystal but dissolves completely when placed in water. Because crystalloids dissolve completely in water, crystalloid solutions containing sodium distribute throughout the entire extracellular fluid space and are therefore not confined to the intravascular space. Sodium-containing crystalloid solutions are always indicated in hypovolemia (circuit problem) but are contraindicated in congestive heart failure (pump problem) because they provide an additional sodium load, and animals with heart failure have already retained too much sodium. Sodium-containing crystalloid solutions are also contraindicated in the presence of severe hypoalbuminemia because sodium-containing crystalloids will further decrease plasma albumin concentration and oncotic pressure, resulting in movement of fluid into the interstitial spaces and exacerbating tissue edema.
Crystalloid solutions are characterized in terms of the number of molecules (numerator) per volume of solution (denominator). The number of molecules is expressed in moles (abbreviated as mol), where 1 mol of compound is equivalent to the molecular weight of the compound in grams (formula weights for NaCl, NaHCO3 and KCl are 58.5 g, 85 g and 74 g respectively). Because body fluids are dilute, we express moles as millimoles (mmol = mol/1000) to facilitate readability.
Crystalloid solutions are commonly expressed in terms of the number of charged components (numerator) per volume of solution (denominator). The number of charged components is expressed in equivalents (abbreviated as Eq), where 1 Eq is the number of each charged component that combines with or replaces 1 mol of hydrogen ion (this means that Eq is always a positive number). Because body fluids are dilute, equivalents are expressed as milliequivalents (mEq = Eq/1000). To calculate the number of mEq from mmol, we simply multiply the number of millimoles by the valence (charge), whereby: mEq/L = (mmol/L) × valence. For instance, 1 mmol of NaCl in solution provides 2 mEq: 1 mEq of Na+ (1 × 1) and 1 mEq of Cl− (1 × 1), assuming that NaCl acts as a strong electrolyte in water (i.e. it completely dissociates into Na+ and Cl− in water). In comparison, 1 mmol of CaCl2 in solution provides 4 mEq: 2 mEq of Ca2+ (1 × 2) and 2 mEq of Cl− (2 × 1), and 1 mmol of dextrose provides 0 mEq, because dextrose does not dissociate into charged components in water.
The principal reason we define constituents of plasma in terms of mEq instead of mmol is because electroneutrality must be preserved at all times; the difference between the charge assigned to all strong cations (Na+, K+, Ca2+, Mg2+) and strong anions (Cl−, lactate, sulfate, ketoacids, non-esterified fatty acids, etc.) in plasma is called the strong ion difference and this factor independently and directly alters blood pH and therefore acid–base status. The normal SID of plasma is approximately 40 mEq/L, although there are species differences in the actual value. Electrolyte solutions with an effective SID of more than 40 mEq/L are therefore alkalinizing because they create a strong ion alkalosis. Electrolyte solutions with an effective SID = 0 are acidifying because they create a strong ion acidosis. Electrolyte solutions of intermediate SID may be alkalinizing or acidifying, depending upon the change in plasma SID relative to the decrease in plasma protein concentration (which is alkalinizing) (Table 2.3).
Table 2.3 Summary of effective strong ion difference (SID) and osmolarity of parenterally administered crystalloid solutions.
| Solution | Effective SID (mEq/L) | Osmolarity (mosmol/L) |
|---|---|---|
| Hypertonic solutions (>312 mosmol/L) | ||
| Alkalinizing | ||
| 8.4% NaHCO3 | 1000 | 2000 |
| 5.0% NaHCO3 | 595 | 1190 |
| 10% NaH2PO4 | 145 | 1150 |
| Acidifying | ||
| 50% dextrose | 0 | 2500 |
| 7.2% NaCl | 0 | 2460 |
| 25% magnesium sulfate | 0 | 2028 |
| 23% calcium borogluconate | 0 | 1069 |
| Isotonic solutions (300 to 312 mosmol/L) | ||
| Alkalinizing | ||
| Tromethamine | 210 | 300 |
| 1.3% NaHCO3 | 155 | 310 |
| Carbicarb | 75 | 300 |
| McSherry’s solution | 54 | 312 |
| Darrow’s solution | 53 | 312 |
| Acidifying | ||
| Ringer’s solution | 0 | 309 |
| 0.9% NaCl | 0 | 308 |
| 1.15% KCl | 0 | 308 |
| Hypotonic solutions (<300 mosmol/L) | ||
| Alkalinizing | ||
| Acetated Ringer’s | 27 | 294 |
| Lactated Ringer’s | <14 | 275 |
| Acidifying | ||
| 5% dextrose | 0 | 250 |
The effective SID is the difference between the strong cation and strong anion concentration after metabolizable anions (such as lactate or acetate) have been completely metabolized to produce bicarbonate. Electrolyte solutions with an effective SID of more than 27 mEq/L are alkalinizing because they create a strong ion alkalosis. Electrolyte solutions with an effective SID = 0 are acidifying because they create a strong ion acidosis.
Isotonic, hypertonic, and hypotonic crystalloid solutions
The tonicity of the solution is an important clinical issue. Complete understanding of the tonicity concept requires differentiation of two terms, osmolality and osmolarity. Osmolality is the number of dissolved particles per kilogram of solution and is expressed as mosmol/kg of solution. The normal plasma osmolality in large animals is approximately 285 mosmol/kg, and plasma osmolality is aggressively defended by increasing water intake (osmolality >285 mosmol/kg) or promoting free water excretion (osmolality <285 mosmol/kg). The correct term in plasma and extracellular fluid is osmolality, because this factor is measured in the laboratory; however, frequently the term osmolarity is used because 1 kg of plasma approximates 1 L of plasma and because osmolarity can be easily calculated from the concentration of electrolytes in the fluid solution. Osmolarity is the number of particles per liter of solution and is expressed as mosmol/L of solution.
One kg (1 L) of plasma from an adult large animal has two components, 70 g of protein and 930 g of plasma water. Accordingly, the osmolality of normal plasma (285 mosmol/kg) is equivalent to a plasma water osmolarity of 306 mosmol/L ((285 mosmol/kg)/(0.93 L/kg)). Ringer’s solution, 0.9% NaCl and 1.3% NaHCO3 are therefore considered isotonic solutions because they distribute in plasma water and have calculated osmolarities of 309 mosmol/L, 308 mosmol/L and 310 mosmol/L respectively.
The normal plasma osmolarity for large animals is 306 mosmol/L; solutions are defined as isotonic (300–312 mosmol/L), hypertonic (>312 mosmol/L) or hypotonic (<300 mosmol/L). Using this categorization, it is readily apparent that some routinely used crystalloid solutions are hypotonic; in particular, lactated Ringer’s solution (275 mosmol/L) is mildly hypotonic and 5% dextrose (250 mosmol/L) is moderately hypotonic, although, as glucose is metabolized, 5% dextrose becomes an increasingly hypotonic solution. Erythrocytes are resistant to increases in plasma osmolarity, whereas they are susceptible to mild decreases in osmolarity; this is the basis of the red blood cell fragility test whereby red blood cell suspensions are placed in solutions of decreasing osmolarity. Because of hypotonic-induced hemolysis, parenterally administered fluids should be isotonic or hypertonic.
Hypotonic crystalloid solutions
Lactated Ringer’s solution is a balanced, polyionic, alkalinizing, hypotonic (275 mosmol/L), crystalloid solution containing physiological concentrations of Na+, K+, Ca2+, Cl− and lactate (CH3CH(OH)COO−). Lactated Ringer’s solution alkalinizes because lactate is predominantly metabolized to the bicarbonate ion, whereby:
CH3CH(OH)COO− + 3O2 → 2CO2 + 2H2O + HCO3−.
The lactate in lactated Ringer’s is a racemic equimolar mixture of l-lactate and d-lactate; in healthy animals l-lactate is rapidly metabolized; however, animals have negligible d-lactate dehydrogenase activity, leading to slow clearance of d-lactate, which is primarily through the urinary system. dl-lactate solutions such as lactated Ringer’s therefore have approximately half the alkalinizing ability of l-lactate solutions. The effective SID of lactated Ringer’s solution is less than 14 mEq/L because l-lactate can also be used in gluconeogenesis instead of bicarbonate production. Lactated Ringer’s solution is the standard intravenous fluid for neonates and adult horses because these animals tend to get acidemic when inappetent. However, lactated Ringer’s solution is theoretically inferior to acetated Ringer’s solution, because critically ill animals may have increased blood lactate concentrations and it is incongruous to add lactate in this situation.
Acetated Ringer’s solution is a balanced, polyionic, alkalinizing, hypotonic (294 mosmol/L), crystalloid solution. Commercially available formulations of acetated Ringer’s solution contain physiological concentrations of Na+, K+, Mg2+, Cl−, acetate (CH3COO−) and gluconate (CH2(OH){CH(OH)}4COO−); the gluconate is problematic because calves (and presumably all large animals) slowly metabolize gluconate.14 Acetated Ringer’s solution alkalinizes because acetate is metabolized to the bicarbonate ion, whereby:
CH3COO− + 2O2 → CO2 + H2O + HCO3−.
The strong ion approach to acid–base balance states that acetated Ringer’s solution is alkalinizing because it contains a metabolizable strong anion (acetate) that, when metabolized, increases the SID.
Five percent dextrose is 250 mosmol/L as administered, but plasma osmolarity decreases as the glucose is metabolized, leaving free water. Because 5% dextrose has no sodium to expand the extracellular volume and has much less energy content than 50% dextrose on a volume basis, the only application of 5% dextrose is to provide free water or as a vehicle for pharmacological agents.
Isotonic crystalloid solutions
Ringer’s solution is a balanced, polyionic, nonalkalinizing, isotonic, crystalloid solution that contains physiological concentrations of Na+, K+, Ca2+, and Cl−. This solution is mildly acidifying because its effective SID = 0 mEq/L. Addition of a fluid with a SID of 0 mEq/L to plasma (normal SID ≈ 40 mEq/L) will decrease plasma SID and therefore directly and independently decrease plasma pH because a 1 mEq/L decrease in SID decreases plasma pH by approximately 0.016. Ringer’s solution is the standard intravenous fluid for adult ruminants because these ruminants tend to get alkalemic when inappetant.15
Isotonic saline (0.9% NaCl solution) is an isotonic crystalloid solution that has little merit in the routine treatment of sick ruminants, principally because ruminants usually develop hypocalcemia and hypokalemia when inappetent. Accordingly, the use of 0.9% NaCl should be confined to horses, the irrigation of surgical sites and wounds, or as a vehicle for adding other electrolytes and dextrose. Like Ringer’s solution, 0.9% NaCl is mildly acidifying because effective SID = 0 mEq/L.
Isotonic sodium bicarbonate (1.3% NaHCO3 solution) is an alkalinizing isotonic crystalloid solution that is used to treat severe acidemia (indicated whenever blood pH <7.20 as a result of metabolic acidosis). This solution is alkalinizing because it buffers hydrogen ion: HCO3− + H+  CO2 + H2O, and because it increases SID (effective SID = 155 mEq/L). Sodium bicarbonate is superior to sodium l-lactate and sodium acetate for the treatment of metabolic acidosis because it provides an immediate source of bicarbonate. On theoretical grounds, sodium bicarbonate (NaHCO3) should not be used to treat severe respiratory acidosis because additional CO2 generated may worsen the respiratory acidosis.
CO2 + H2O, and because it increases SID (effective SID = 155 mEq/L). Sodium bicarbonate is superior to sodium l-lactate and sodium acetate for the treatment of metabolic acidosis because it provides an immediate source of bicarbonate. On theoretical grounds, sodium bicarbonate (NaHCO3) should not be used to treat severe respiratory acidosis because additional CO2 generated may worsen the respiratory acidosis.
Tromethamine (Tham, tris-hydroxymethyl aminomethane, 300 mmol/L) is an isotonic solution of an organic amine that is a safe and effective buffer.16 After administration, 70% of the neutral compound (CH2OH)3C-NH2 in tromethamine is immediately protonated to the strong cation (CH2OH)3C-NH3+ in plasma, with the net equation being:
(CH2OH)3C-NH2 + H+  (CH2OH)3C-NH3+.
(CH2OH)3C-NH3+.
The remaining 30% of the administered tromethamine remains unprotonated and can therefore cross cell membranes and potentially buffer the intracellular compartment. Tromethamine therefore provides an alternative alkalinizing agent to sodium bicarbonate; however, tromethamine does not currently appear to offer any important clinical advantages over sodium bicarbonate in spontaneously breathing animals.
Isotonic formulations are available for intravenous administration with or without electrolytes; administration of tromethamine without electrolytes leads to hyponatremia and it would appear preferable to administer tromethamine in conjunction with electrolytes.
Carbicarb is an isotonic buffer (300 mosmol/L) made from equimolar disodium carbonate (Na2CO3) and sodium bicarbonate; carbonate avoids generation of CO2 when buffering acidemic blood:17
CO32− + H+  HCO3−.
HCO3−.
Carbicarb was suspected to decrease the incidence and magnitude of hypercapnia when rapid alkalinization was needed in animals with mixed metabolic and respiratory acidosis. Despite numerous studies comparing Carbicarb to sodium bicarbonate, the potential clinical advantages of Carbicarb have only been demonstrated in animals being ventilated or with extremely limited ventilatory ability. Carbicarb has been administered intravenously to diarrheic calves; however, these studies have failed to identify a clinically important advantage over conventional isotonic sodium bicarbonate administration.18 Accordingly, there does not appear to be a compelling reason to prefer Carbicarb to isotonic sodium bicarbonate when rapid alkalinization of conscious animals is required.
Darrow’s solution is an isotonic polyionic solution formulated by Darrow in 1946 for use in human infants; the solution has been administered to calves.19,20 Compared to other isoosmotic polyionic solutions, Darrow’s solution is hyponatremic, hyperkalemic and hyperlactatemic and does not contain calcium or magnesium. As such, Darrow’s solution is not recommended for administration to large animals.
McSherry’s balanced electrolyte solution is an isotonic polyionic solution formulated by McSherry and Grinyer in 1954 for intravenous and intraperitoneal administration to dehydrated diarrheic calves.21 On theoretical grounds, this is an excellent parenteral fluid for resuscitating dehydrated diarrheic calves that deserves more frequent use. Unfortunately, commercial formulations are currently unavailable.
Hypertonic crystalloid solutions
Fifty percent dextrose is 2500 mosmol/L (approximately eight times normal osmolarity). Fifty percent dextrose solutions are commonly administered to ruminants with ketosis or hypoglycemia and produce a transient increase in cardiac contractility.22 Some commercially available formulations in Europe contain an equimolar mix of dextrose and fructose, although the addition of fructose does not appear to produce a more sustained increase in plasma glucose concentration than that produced by glucose alone.23
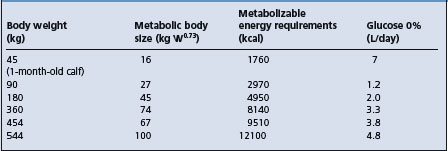
The necessity for glucose in fluid therapy has been controversial. Hypoglycemia occurs commonly in septicemic neonates and calves with diarrhea but is uncommon in most other common diseases in which there is an acute fluid and electrolyte disturbance. Dextrose will promote the movement of extracellular potassium into the cell, will provide metabolic water and is a source of carbohydrate. If glucose is indicated, large quantities of parenteral glucose are necessary to meet the maintenance energy requirements and every effort must be made to restore the animal’s appetite and to provide the necessary requirements through dietary intake. The energy requirements for maintenance are calculated on the basis of metabolic body size, kg0.73, which is a measure of the fasting metabolism in an animal not eating and not doing any muscular work. If 1 g of dextrose given intravenously will provide 5 kcal (2.1 kJ) of energy, the approximate amounts of dextrose solution needed to meet the energy needs for maintenance in cattle are shown in Table 2.4. Table 2.4 comprises a rough estimate of the requirements and should be used as a general guideline only. Every effort should be made to supply the energy needs through oral intake of energy-containing foods.
NaCl 7.2% (Hypertonic saline) is 2460 mosmol/L (approximately eight times normal osmolarity) and is used for the rapid resuscitation of animals with hypovolemia. Hypertonic saline should be administered at 4–5 mL/kg BW intravenously over 4–5 min (1 (ml/kg BW)/min). Faster rates of administration lead to hemodynamic collapse due to vasodilation and decreased cardiac contractility, whereas slower rates of administration provide no advantages over isotonic crystalloid solutions. Like high-volume 0.9% NaCl, small-volume hypertonic saline consistently induces a mild strong ion acidosis as its effective SID = 0 mEq/L. In general, the decrease in pH following hypertonic saline administration is less than 0.08 pH units and rapidly dissipates with time.23 The effect of hypertonic saline on acid–base balance is therefore clinically inconsequential.
The use of small volumes (4–5 mL/kg BW) of hypertonic saline solution, ranging in concentration from 7.0% to 7.5%, has been extensively evaluated for the treatment of various forms of hemorrhagic, septic and endotoxic shock.24 Plasma volume is increased by the movement of free water from the intracellular space, thereby increasing cardiac output, mean arterial blood pressure, systemic oxygen delivery and glomerular filtration rate. Total peripheral vascular resistance and pulmonary vascular resistance decrease, and mean circulatory filling pressure increases. Urine output is restored and acid–base equilibrium returns towards normal in conjunction with improved tissue perfusion.
Hypertonic saline solution is widely used for the treatment of dairy cattle with endotoxic shock and endotoxemia associated with coliform mastitis. Affected cows are given 2 L of hypertonic saline (4–5 mL/kg BW) intravenously, followed by immediate access to drinking water and other supportive therapy. The small volume of hypertonic saline followed by the oral water load increases circulatory volume rapidly, induces slight metabolic acidosis, increases renal perfusion and glomerular filtration rate, and induces homeostatic changes in serum calcium and phosphorus.25 In experimental endotoxin-induced mastitis of cattle, small volumes of hypertonic saline given intravenously (7.5%, 5 mL/kg BW) resulted in expanded plasma volume and increased the cows’ voluntary water intake by about 12 times compared to cows treated with isotonic saline.26 The rapid intravenous administration of hypertonic saline can successfully, but only transiently, resuscitate calves in experimental endotoxic shock.27 Hypertonic saline (7.2%NaCl, 2400 mosmol/L), 4 mL/kg BW intravenously over 4 min can be safely administered to endotoxic calves.28 On a comparative basis, the rapid infusion of large-volume isotonic saline is superior to small-volume hypertonic saline for initial resuscitation of experimentally induced acutely endotoxemic calves.27
Hypertonic saline has been associated with greater and more prolonged improvement in cardiopulmonary function and survival in horses with experimentally induced hemorrhagic and endotoxemic shock and in halothane-induced hypotension in horses.29 When given intravenously to normal conscious horses at 5 mL/kg BW, there are increases in plasma osmolality and serum sodium and chloride but clinically normal horses rapidly regulate variable sodium loads.30
Sodium bicarbonate 8.4% is 2000 mosmol/L (approximately seven times normal osmolarity). This solution is used for rapid alkalinization, particularly in the presence of severe acidemia (pH < 7.20). The solution osmolarity was selected because it provides 1 mEq of HCO3−/mL of solution, which facilitates calculation of the volume to be administered. The speed of intravenous administration of 8.4% sodium bicarbonate should not exceed 1 (ml/kg BW)/min. There is one report of the intravenous administration of 8.4% sodium bicarbonate to normovolumic calves with experimentally induced mixed respiratory and metabolic acidosis; the study found that rapid administration of NaHCO3 (5 mL/kg intravenously over 5 min) rapidly corrected the metabolic acidosis, increased blood pH and improved cardiovascular status without inducing paradoxical cerebrospinal fluid acidosis,31 suggesting that this treatment may be of value in treating dehydrated diarrheic calves. Efficacy studies in calves with naturally acquired diarrhea appear indicated. Hypertonic solutions of sodium bicarbonate are highly effective for the initial treatment of acidosis associated with d-lactic acidosis in calves, acute diarrhea in calves31 and strong ion (metabolic) acidosis in newborn calves.32
Sodium bicarbonate 5% is 1190 mosmol/L (approximately four times normal osmolarity). This solution is also used for rapid alkalinization in the presence of severe acidemia (pH < 7.20). The speed of intravenous administration of 5.0% sodium bicarbonate should not exceed 2 (ml/kg)/min. Three to five L of 5% sodium bicarbonate may be necessary as initial therapy to correct the severe hyponatremia and strong ion (metabolic) acidosis that occurs in the horse with acute diarrhea. Following this initial treatment, hypokalemia characterized by muscular weakness commonly occurs, which can be treated using a high sodium, high potassium, alkalinizing solution.
Calcium gluconate 23% or calcium borogluconate are 1069 mosmol/L (approximately three and a half times normal osmolarity). Calcium borogluconate is the standard treatment for milk fever (hypocalcemia) in cattle. d-gluconate is an aldose sugar produced by oxidation of d-glucose and is the preferred salt for calcium-containing parenteral solutions because it does not cause tissue necrosis as severe as does CaCl2. Calcium gluconate should not be added to sodium bicarbonate solutions because a white precipitate (CaCO3) forms immediately that interferes with normal fluid administration. Likewise, calcium gluconate should not be administered with tetracycline antibiotics because a yellow precipitate forms.
Colloid solutions
A colloid is a substance that is too large to pass through a semipermeable membrane. Examples of colloid solutions administered to ruminants are whole blood, stroma-free hemoglobin, plasma, dextrans, hydroxyethyl starches and gelatins. As a group, colloid solutions are excellent for sustained expansion of plasma volume, which is in marked contrast to the effect of crystalloid solutions. Colloid solutions are contraindicated in congestive heart failure because these animals have increased plasma volume. Colloid solutions are also contraindicated in the presence of oliguric or anuric renal failure because the sustained volume overload may lead to pulmonary edema.
Whole blood is the perfect balanced colloid/crystalloid solution, with great O2-carrying capacity. It has a short shelf life (< 24 h at 4°C) and is expensive to obtain. Whole blood administration runs the risk of disease transmission and allergic reactions; the latter are extremely rare in ruminants with the first blood transfusion but common enough in horses for blood typing or cross-matching to be required. Excellent descriptions for collecting, storing and administering blood are available elsewhere (Chapter 9).33
Stroma-free hemoglobin is a blood substitute containing a purified hemoglobin glutamer-200 solution (13 g hemoglobin/dL) derived from cattle blood. A commercially available solution has a 2-year shelf life at 20°C, an osmolarity of 300 mosmol/L and an oncotic pressure of 43 mmHg; the solution is therefore isotonic but hyperoncotic. Stroma-free hemoglobin solutions are excellent at increasing oxygen delivery and carrying capacity, while providing similar plasma volume expansion to dextrans and hydroxyethyl starches. The major theoretical concerns regarding administration of stroma-free hemoglobin solutions are potent vasoconstriction34 and hemoglobinuric nephrosis. Some of the original experimental studies examining the effects of stroma-free hemoglobin administration were completed in sheep35,36 and there are occasional reports of its successful administration to critically ill horses in a clinical situation. It is likely that the high cost of this product will minimize its administration to large animals.
Plasma (fresh or frozen) is an excellent balanced colloid/crystalloid solution. Compared with blood, plasma has a much longer shelf life (at least 1 year at −20°C) but is more expensive to obtain. Details for collection, harvesting, storing and administering plasma are available elsewhere,33 and bovine, equine and New World camelid plasma is commercially available. Like blood, administration of plasma runs the risk of disease transmission and allergic reactions, although these risks are less than with blood transfusion.
Plasma is routinely administered to foals with inadequate transfer of passive immunity. Hyperimmune plasma is occasionally administered to neonatal foals and adult horses with Gram-negative septicemia and endotoxemia. There appears to be only one report documenting the efficacy of plasma administered to neonatal calves with diarrhea, and these calves were probably colostrum-deprived. The 14-day survival rate in diarrheic calves that received 600–800 mL of bovine plasma (5 g protein/dL) and electrolytes intravenously was 93% (37/40), which was significantly greater than the survival rate of calves receiving intravenous electrolytes alone (54%, 7/13).37 Another study failed to identify a beneficial effect of blood transfusion in treating diarrheic calves.38 Because blood is cheaper to obtain than plasma, whole blood transfusions are usually administered when a neonatal ruminant needs plasma.
Dextran preparations (such as Dextran-70) are high-molecular-weight glucose polymers obtained by bacterial fermentation of sucrose; the fermentation metabolites then undergo acid hydrolysis and fractionation. The molecular weight of dextran can therefore be ‘selected’, and two dextran products, Dextran-70 (mean molecular weight 70 000) and Dextran-40 (mean molecular weight 40 000) are commercially available. Because the molecular weight of Dextran-70 is similar to albumin (molecular weight 65 000), there is limited diffusion of dextran into the interstitial space and Dextran-70 therefore acts clinically as a plasma volume expander; this is in contrast to isotonic crystalloid solutions, which act as extracellular fluid volume expanders. Dextran-70 has been the most widely used dextran formulation in large animals and is therefore the recommended product for administration. Dextran-70 is supplied as a 6% concentration in 0.9% NaCl; this provides a hyperoncotic but isotonic solution. Reported administration rates of Dextran-70 are 5–40 (mL/kg)/h, but it is safer to administer Dextran-70 at less than 20 (mL/kg)/h. One mL of Dextran-70 expands the plasma volume by 0.8–1.2 mL, but 50% of the administered dose is gone by 24 hours. Dextran administration runs the risk of exacerbating pre-existing coagulopathies, although the clinical significance of dextran-induced prolongation of activated partial thromboplastin time (APTT) by decreasing factor VIII:C is probably minimal. The risk of coagulopathy is dependent upon the administration rate, total dose administered (20 mL/kg is maximum 24 h dose in humans) and the molecular weight of dextran. The deleterious effects of dextrans are usually associated with large doses or prolonged administration.
The use of hypertonic saline–dextran solution (4 mL/kg, 2400 mosmol/L sodium chloride in 6% Dextran-70 administered intravenously once over 4 min) combined with an isotonic oral alkalinizing solution containing sodium chloride (3.22 g/L), potassium chloride (1.12 g/L), sodium acetate trihydrate (4.76 g/L) and glucose anhydrous (16.22 g/L), providing 300 mosmol/kg of water and administered at 55 mL/kg BW, was superior to either solution alone for the treatment of experimentally induced hypovolemic diarrhea in calves.39 The combined treatment resulted in immediate and sustained increases in plasma volume, cardiac output and stroke volume, thereby improving tissue perfusion. Rapid and sustained rehydration after the combined treatment was indicated by improvement in hydration and clinical depression scores and decreases in hematocrit, blood lactate concentration and serum creatinine, albumin and phosphate concentrations. Resuscitation with oral electrolyte solution alone was slower but was complete within 24 hours. Resuscitation with the hypertonic saline–dextran solution alone resulted in only transient benefit.
The administration of hypertonic saline–dextran solution (7.2% NaCl solution with 6% dextran at the rate of 4 mL/kg BW, intravenously during a 4 min period, combined with oral administration of isotonic electrolyte solution at the rate of 50–60 mL/kg BW) provided a rapid and effective method for resuscitating severely dehydrated calves with experimentally induced diarrhea40 or with naturally acquired diarrhea.41
Hydroxyethyl starch preparations (hetastarch, pentastarch) Two hydroxyethyl starch preparations are currently commercially available; hetastarch and pentastarch. Hetastarch is a high-molecular-weight glucose polymer (mean molecular weight 450 000) that is chemically synthesized from amylopectin, producing a highly branched glucose polymer with a structure similar to that of glycogen. Because the molecular weight of hetastarch is much greater than that of albumin, hetastarch decreases endothelial permeability by sealing separations of endothelial cells. Hetastarch is hydrolyzed in blood by α-amylase, and the addition of hydroxyethyl groups slows hydrolysis and therefore prolongs the duration of plasma volume expansion. Hetastarch is supplied as a 6% concentration in 0.9% NaCl; this provides a hyperoncotic but approximately isotonic solution. Reported administration rates are 5–40 (mL/kg BW)/h but, like Dextran-70, it is safer to administer hetastarch at less than 20 (mL/kg BW)/h. Like Dextran-70, hetastarch administration also runs the risk of exacerbating pre-existing coagulopathies. The risk of coagulopathy is dependent upon the administration rate and total dose administered (20 mL/kg BW is the maximum 24 h dose in humans).
Pentastarch has a mean molecular weight of 280 000 and is available as a 10% solution. Pentastarch has two important advantages over hetastarch: it has less exacerbating effect on pre-existing coagulopathies and the rate of elimination is faster. Pentastarch has rarely been administered to large animals.
Gelatins (modified bovine collagens) are available for veterinary use. The formulation uses gelatin with a mean molecular weight of 30 000 and is a 5.6% suspension in NaCl. Compared to dextrans and hydroxyethyl starches, gelatins have a shorter plasma half-life but appear to have less effect on coagulation. In general, gelatins have not been evaluated as completely as dextrans and hydroxyethyl starches and, on this basis, are not currently preferred.
Practical administration of electrolyte solutions
Under ideal conditions, with laboratory evaluation of the animal, the deficits can be accurately assessed and fluids containing the deficient electrolytes can be formulated. However, under most practice conditions this is not possible and polyionic crystalloid solutions are in general use. These usually contain sodium, potassium, chloride and calcium or magnesium at a concentration similar to the electrolyte composition of extracellular fluid; the solutions may also contain lactate or acetate as bicarbonate precursors. Dextrose may be added to the solution to make an initial mildly hypertonic solution.
Polyionic crystalloid solutions are safe and can be used in large quantities without inducing electrolyte disturbances provided that circulating blood volume and renal function have been restored and are maintained. They can be used for most situations of dehydration and moderate acidemia or alkalemia and moderate electrolyte imbalances. They are not usually adequate for the treatment of severe acidemia or alkalemia, or severe hyponatremia, hypokalemia or hypochloremia.
For the treatment of severe acidemia or alkalemia, and severe hyponatremia, hypokalemia and hypochloremia, specific electrolyte solutions are necessary. Generally, they consist of a mixture of the common simple solutions with supplemented electrolytes to correct some major abnormality. These are considered necessary to correct abnormalities quickly that could not be corrected using balanced electrolyte solutions. These solutions are summarized in Tables 2.3 and 2.5. Many intravenous solutions for fluid therapy in calf diarrhea are available and it is recommended that they should contain 150 mmol/L of sodium, 5 mmol/L of potassium and about 50 mmol/L of a mixture of bicarbonate and precursors.42
Table 2.5 Composition (mmol/L) and indications for use of electrolyte solutions used in fluid therapy.
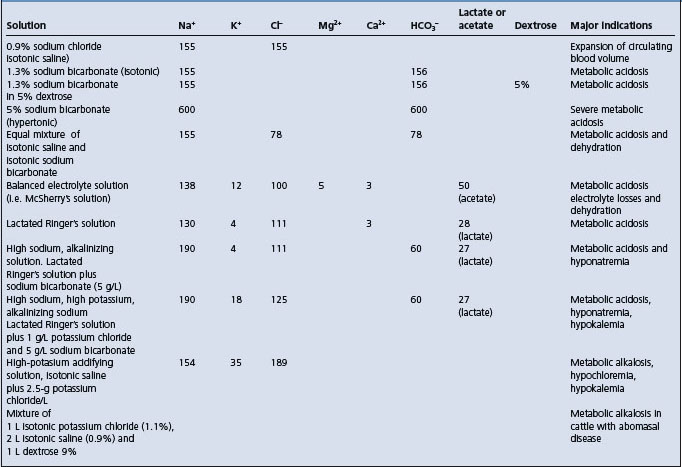
When acidemia is not present it is not necessary to use a fluid containing bicarbonate.43
Mature cattle affected with metabolic alkalosis associated with diseases of the abomasum are usually hypokalemic, hypochloremic and dehydrated. For such cases, a balanced electrolyte solution containing sodium, chloride and potassium is satisfactory. A solution containing sodium (135–155 mEq/L), chloride (150–170 mEq/L) and potassium (10–20 mEq/L) is effective.43 In recently calved dairy cattle, calcium borogluconate is commonly added to the mixture.
Solutions containing potassium have been recommended for the treatment of the potassium depletion that occurs in calves with acute diarrhea and in inappetent ruminants and horses. However, in calves with severe acidemia and hyperkalemia, it is important to expand circulating blood volume, restore renal function and correct the strong ion (metabolic) acidosis before providing additional potassium, which may be toxic. Solutions containing potassium may be indicated following correction of the acidosis and dehydration. However, if the animal’s appetite is returned to normal, the oral potassium intake will usually correct any existing deficiencies.
For the treatment of hypochloremic, hypokalemic, metabolic alkalosis, acidifying solutions can be used but preferably only if constant laboratory evaluation of the animal is possible. Without laboratory evaluation, the use of Ringer’s solution, 0.9% NaCl or hypertonic saline for correction of strong ion (metabolic) alkalosis in adult cattle is recommended, along with the oral administration of potassium in animals that are inappetent. In experimentally induced hypochloremic hypokalemic metabolic alkalosis in 40–50 kg BW sheep, replacement of the chloride deficit using 2 L of hypertonic saline (1.8% sodium chloride) was effective in returning plasma sodium and chloride concentrations to normal within 12 hours, and the plasma potassium concentrations and acid–base balance returned to normal within 36 hours of treatment without providing potassium.44 Small volumes of hypertonic saline are also effective for the treatment of experimentally induced hypochloremic, hypokalemic metabolic alkalosis in sheep.45
In summary, four different kinds of solutions are used in large animal practice:
• Polyionic crystalloid solutions, such as lactated Ringer’s solution and acetated Ringer’s solution, are indicated for dehydration and moderate degrees of acid–base and electrolyte imbalance
• Hypertonic saline solution and an oral water load represent a practical and inexpensive alternative to parenteral administration of large fluid volumes
• Hypertonic or isotonic sodium bicarbonate, such as 8.4%, 5.0% (hypertonic) or 1.3% (isotonic) solutions of sodium bicarbonate, are used for severe strong ion (metabolic) acidosis and hyponatremia
• Chloride-containing acidifying solutions, such as Ringer’s solution, are used for treatment of strong ion (metabolic) alkalosis.
Because cost is a major consideration in large animal fluid therapy, it may not be possible to use sterile solutions. Most of the above solutions can be formulated using the necessary salts mixed with distilled water, boiled water or ordinary tap water and are therefore prepared inexpensively.
Quantity of fluids required and routes of administration
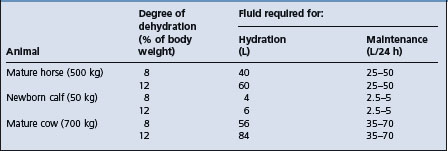
The amount of fluid required depends on the degree of dehydration (an estimate of the volume losses which have already occurred), the continuous losses which are occurring during treatment, and the maintenance requirements of the animal during treatment presuming its dietary intake of water, electrolytes and nutrients is minimal. The fluids are usually given in two stages:
• Hydration therapy in the first 4–6 hours at a rate of 100–150 mL/kg BW intravenously
• Maintenance therapy (a combination of continuous losses and maintenance requirements) in the next 20–24 hours, depending on the severity and the course of the disease, at 60–80 mL/kg BW/24 hours intravenously (approximately 3–4 mL/kg BW/hour). In some cases of profuse diarrhea, the continuous losses and maintenance requirements will be about 150 mL/kg BW over a 24-hour period. The daily maintenance water requirements of adult horses range from 54–83 mL/kg BW, with a mean of 64 mL/kg BW.46
Some examples of the large quantities of fluid required for hydration and maintenance therapy in cases of acute diarrhea are outlined in Table 2.6.
Parenteral fluid therapy
The total amount of the estimated necessary hydration therapy should be given intravenously using indwelling intravenous catheters in the first 4–6 hours in order to expand and maintain circulating blood volume. If acidemia or alkalemia is present, it also should be treated immediately. Thus the most important abnormalities – decreased circulating blood volume and acid–base imbalance – are treated first. Restoring circulating blood volume will restore renal function, which will assist in correcting acid–base and electrolyte balance. The immediate correction of acidemia will return the tissues to their normal physiological activity. The intravenous route is preferred for hydration therapy and for the correction of severe acid–base and electrolyte imbalances. All other routes (intraperitoneal, subcutaneous and oral) are unsatisfactory in the presence of decreased circulating blood volume.
During the intravenous administration, the animal must be monitored for clinical and laboratory evidence of improvement or deleterious effects. A favorable response is indicated by urination within 30–60 minutes, an improvement in mental attitude and some evidence of hydration. Unfavorable responses include dyspnea because of pre-existing pneumonia or pulmonary edema because of too rapid administration, failure to urinate because of renal failure or paralysis of the bladder, and tetany because of the excessive administration of alkali. Unusual responses such as sweating, trembling and depression within several hours following the intravenous administration of electrolytes or other substances such as commercial amino-acids may occur if the infusion is contaminated during administration.47 If a laboratory is available, the determination of PCV, bicarbonate and blood pH will provide an excellent monitoring system during the administration of the fluids.
Rate of administration
The rate of administration will depend on the size of the animal, the severity of the illness, the type of fluids being administered and the response of the animal to the fluids. In calves, isotonic saline (0.9% NaCl) and sodium bicarbonate solutions can be given at the rate of 1–3 L/h; in a mature horse, fluids may be given at the rate of 10–12 L/h. Hypertonic solutions such as 5% sodium bicarbonate can be given to a mature horse at the rate of 3–5 L/h, followed by balanced electrolytes at 10–12 L/h. Solutions containing added potassium should be given cautiously, at the rate of 3–5 L/h. In a cow with severe dehydration and acidosis due to carbohydrate engorgement, fluids may be given at the rate of 10–12 L/h.
Adverse reactions in all species include sudden muscle weakness (suggests hypokalemia) and sudden tachycardia and hyperventilation, which suggest overhydration. When these occur the fluids should be stopped and the clinical findings assessed. If laboratory assistance is available, the determination of blood pH and bicarbonate may provide an explanation for the reaction.
Intravenous catheters and complications
The administration of large quantities of fluids intravenously to farm animals is best done with an indwelling jugular vein flexible catheter (10–14-gauge) that is appropriately secured to the animal’s neck to prevent withdrawal from the vein. Standard aseptic technique must be used. A plastic, spring-like, coiled tube and suitable rubber tubing are used to deliver the fluids from large 20–25 L plastic containers. The coiled plastic tubing allows the animal to lie down or stand up without disrupting the catheter and tubing.48 The use of a drip chamber in the rubber tubing system assists in determining the flow rate, which can be adjusted with a clamp. With a 12-gauge catheter, 25–30 L of fluids can be delivered as hydration therapy to a mature horse or cow.
Auricular vein of cattle
The short neck, thick skin and, in some breeds, pendulous dewlap of cattle make it difficult to introduce and secure indwelling jugular catheters for long-term use. The auricular vein of adult cattle can be successfully catheterized with an over-the-needle, 14-gauge catheter, 5 cm long, permitting 20 L of rehydration solution to be delivered over 4 hours.49
Cecal catheters in horses
Percutaneous cecal catheters have been used to deliver fluid solutions in ponies.50 The advantages include less cost but complications include peritonitis, diarrhea, laminitis and hypocalcemia.
Thrombophlebitis
Long-term jugular vein catheterization (over a period of a few days) in adult cattle and particularly horses can result in thrombophlebitis, suppurative phlebitis, and catheter sepsis. Inspection of the affected jugular vein reveals swelling, firmness and moderate pain. Careful digital and visual inspection are necessary to determine the patency of the vein; in about 50% of cases the vein is completely thrombosed and occluded and cannot be used for intravenous administration for 2–3 weeks. The extent and severity of the thrombophlebitis can be determined by ultrasonography of the neck and patency of the vein can be assessed by compressing the vein with the transducer head.51
The development of thrombophlebitis is dependent on the method used for skin preparation and the catheterization technique. Careful preparation of the skin and aseptic technique during insertion and placement of the catheter are crucial in preventing this complication.52 Heparin subcutaneously, 150 IU/kg BW immediately after insertion of the catheter and repeated every 12 hours, has been used prophylactically52 but this is not deemed necessary with good technique. Alternating catheters between jugular veins every 48–72 hours is standard practice in equine fluid therapy but despite this precaution complications occur in 20–50% of horses whose jugular veins are catheterized for 48 hours.53 By using catheters made of materials that are less thrombogenic, inserting them in an aseptic manner and observing simple management practices, the duration of catheter survival increases to about 14 days. The least reactive catheter is Silastic, followed by polyurethane; polytetrafluoroethylene causes most reaction. Catheters that are soft are superior to stiff and rigid ones.
A retrospective study of the risk factors associated with vein thrombosis in horses treated with intravenous fluids in a veterinary teaching hospital found that the use of carboy fluids, diarrhea and fever were related; the incidence was lower in horses that had general anesthesia, surgery and received antimicrobial agents.54 A variety of aerobic bacteria were cultured from about 50% of the intravenous catheters removed from horses.55 Bacteria were isolated from 7% of skin swabs taken from the area around the catheter after surgical preparation with iodine soap and before and after removal of the catheter. However, there was no correlation between bacterial culture and the condition.
Oral fluid therapy
Whenever possible, the oral route can be used to deliver the maintenance requirements. Provided there are no abnormalities of the digestive tract that interfere with oral administration or the absorption of the fluids, the oral route is preferred for maintenance therapy. In ruminants such as adult cattle, rumen function must be present for significant absorption of fluids and electrolytes. The oral administration of large quantities of fluid to cattle with rumen atony results in sequestration of the fluid in the rumen and the development of metabolic hypochloremic, hypokalemic alkalosis.
Oral fluid therapy in calves and adult cattle
For diarrheic calves, the total 24-hour maintenance requirement is calculated and given orally in divided doses every 2–4 hours. Compared to parenteral therapy, there is less danger from overhydration and electrolyte toxicity, and in acute diarrhea the maintenance of oral fluid and electrolyte intakes will replace continuous losses that are occurring during the diarrhea. Livestock owners should be informed of the value of providing newborn animals affected with diarrhea associated with dehydration, depression, inactivity or failure to suck with oral fluids and electrolytes as soon as possible and of the value of continuing this treatment until the animal has returned to normal. Oral electrolyte solutions and water should be made available at all times to animals affected with diarrhea and other diseases in which there are continuous losses of fluid and electrolytes. The exception is cattle affected with carbohydrate engorgement, in which the water supply should be restricted to one-half or less until the animals begin to eat.
Calves with dehydration and diarrhea absorb electrolyte solutions almost as effectively as healthy calves. The important principle underlying the efficacy of oral fluid therapy is the use of low concentrations of glucose (about 2%) to promote sodium absorption from the intestine.56 Water follows passively and, because sodium is the osmotic skeleton of the extracellular fluid, fluid is held predominantly where it is needed in the extracellular space, including plasma. Amino acids, such as glycine, also act like glucose to promote sodium absorption. In enterotoxigenic colibacillosis in calves, the glucose and amino acid cotransport mechanisms for sodium transport into epithelial cells are intact.57 Thus, water and salt, together with glucose and glycine, facilitate the absorption of sodium and water in calves with diarrhea.
A high-calorie hypertonic oral rehydration solution containing glutamine was more effective in correcting plasma, extracellular fluid and blood volume than conventional solutions with a lower calorie content and without glutamine.58 Glutamine also promotes enteric sodium uptake and may be important in sustaining villus form and function. The higher-calorie solution (7.5% glucose) also sustains blood glucose levels at a higher level than conventional solutions. An effective oral fluid should also contain or yield sufficient bicarbonate to correct metabolic acidosis.
A variety of oral and parenteral electrolyte replacement solutions are available commercially.59 Most preparations are in the form of powders to be mixed with water. They contain sodium, chloride, potassium, glucose, glycine and bicarbonate or its precursors (acetate or propionate).
The sodium bicarbonate included in oral fluids for the acidosis in calf diarrhea is usually effective directly and quickly. There is speculation that sodium bicarbonate may interfere with milk clotting in the abomasum, which requires an acidic environment for the action of rennin.60 There is no direct evidence that oral fluids containing sodium bicarbonate interfere significantly with clotting of milk in the abomasum. Nevertheless, some oral electrolyte solutions for diarrheic calves contain acetate, propionate or citrate, which, when absorbed, act as bicarbonate precursors;57 it should be noted that gluconate is not metabolized in calves and probably not in other large animals. Because of their acidic pH, it is claimed that these fluids do not interfere with abomasal clotting of milk but, as already stated, there is no direct evidence to support the claim and no evidence that the final outcome in naturally occurring cases of diarrhea in calves is superior when oral fluids containing metabolizable bases (rather than bicarbonate) are used. Furthermore, the oral fluids containing the base precursors are most effective in diarrheic calves with a blood pH over 7.2 (see Colibacillosis of calves, Ch. 18).
The alkalinizing effects of commercial oral electrolyte solutions have been compared in healthy calves.61,62 A sodium bicarbonate-rich solution induced the best alkalinization effect. The preparation should not be mixed with milk but given one hour before or after feeding milk because any fluid substance added to milk changes the physicochemical characteristics of milk and may interfere with optimum clotting of milk by rennin in the abomasum, although the clinical significance of this effect remains uncertain. Bicarbonate-containing oral electrolyte solutions will restore acid–base imbalance in calves with viral-induced diarrhea much more effectively than solutions without bicarbonate.
The continued feeding of milk to diarrheic calves while they are receiving oral fluids and electrolytes is controversial.61 It has been conventional to withhold milk from diarrheic calves for 1–2 days and gradually reintroduce milk over the next few days when there is evidence of recovery. An extreme practice was to totally deprive the calf of milk until the diarrhea ceased. The rationale was that the ability of the calf’s intestine to digest milk was impaired. It is known that lactose digestion is impaired in the rotavirus and coronavirus diarrheas of young calves. It was also thought that the presence of milk in the intestine would provide a substrate for continued growth of enteric pathogens.
Another different practice was to continue feeding milk to diarrheic calves because it resulted in more rapid recovery from diarrhea, less debilitation, continued weight gain and improved circulating plasma volume. This is based on the premise that continuous feeding provides the intestinal mucosa with nutrients.
In experimentally induced diarrhea in calves, the continued feeding of milk during the course of the diarrhea sustained growth, resulted in greater fat stores, facilitated regeneration of the intestinal mucosa and resulted in less thymic atrophy than calves deprived of milk.61 However, the number of calves in the experiment was small and extrapolation of the results to the naturally occurring disease is not yet warranted. Whole milk and an acidic oral fluid therapy given to calves with naturally occurring diarrhea did not adversely affect the calves or prolong or worsen the diarrhea, and promoted body weight gain.63 However, none of the calves was severely dehydrated or acidemic and treatment was begun very early in the stage of diarrhea (see also E. coli in Ch. 18).
Oral fluid therapy in horses
Intravenous fluid and electrolyte therapy has been used extensively for the treatment of dehydration and electrolyte disturbances in the horse with diarrhea. However, oral fluid therapy, as used in calves, has not been employed to the same extent. It may be an effective, practical and economical method of rehydration of horses with diarrhea that has not yet been fully explored.64,65
In the horse with acute diarrhea, several factors contribute to the nature of the fluid and electrolyte losses. There are increases in fecal sodium and water loss but the fecal potassium excretion may remain unchanged.64 Experimentally induced diarrhea (castor oil) in adult horses results in dehydration, metabolic acidosis and large fecal losses of sodium and urinary losses of potassium.66 Plasma volume decreased while horses were clinically dehydrated. The lack of feed intake, which affects primarily the potassium intake, can result in losses of 2500–3000 mmol of potassium per day. Although urinary water and potassium losses are reduced, potassium depletion continues; thus potassium losses are very high and need to be replaced, especially in the anorexic horse. The large potassium deficit in diarrheic horses should also be considered when formulating the composition of oral fluids. Administration of 30–40 g potassium chloride or, if chloride administration is inappropriate, 30–40 g potassium bicarbonate in 2–4 L of water given by nasogastric tube several times daily to an inappetent horse with diarrhea can complement intravenous fluid therapy and replace the potassium deficit.
The optimum electrolyte composition of oral fluids and the amount to be used have not yet been determined for the horse. The amount given depends on the degree of dehydration. Dehydration in horses becomes clinically apparent when about 5% of body weight has been lost. In a 500 kg horse, assuming 90% water loss, the fluid deficit is about 23 L.64 Abdominal discomfort may occur following the nasogastric tube administration of a series of 8–10 L doses of oral rehydration fluid.67 The administration of large amounts may result in rapid transit through the stomach and intestines and decreased absorption. A slower rate of administration, such as 8–10 L every few hours, may be tolerated more effectively and the transit time in the intestine may be decreased, enhancing absorption. Volumes of 6–8 L can be given by nasogastric tube as often as every 15–20 minutes by funnel; as much as 20–30 L is possible during the first hour and 40 L is possible during a 2-hour period.68 Oral fluids may also be administered through a small-diameter indwelling nasogastric tube, as is used for prolonged enteral nutrition of horses with dysphagia.65
Commercially available oral electrolyte solutions are inadequate for horses because the concentrations of sodium and potassium are too low to adequately replace losses. When treating horses with acute diarrhea, the ratio of sodium to chloride ions in the oral solution should be approximately 1.4:1, and the need for glucose in an oral rehydration solution for adult horses has not been clearly demonstrated. One formulation contained 5.27 g of NaCl, 0.37 g of KCl and 3.78 g NaHCO3 per liter of tap water; this produced a suitable electrolyte composition for oral administration (Na 135 mmol/L; K 5 mmol/L; Cl 95 mmol/L; HCO3 45 mmol/L).69
Oral administration of bicarbonate will result in a pronounced alkalemia within 3–6 hours, with the maximum change in pH occurring at a sodium bicarbonate dose of 1 g/kg BW (which represents 40% of normal extracellular sodium). Doses above this level do not induce additional alkalinization, presumably because of limited absorption of bicarbonate from the intestinal tract. The oral administration of sodium bicarbonate to normal mature resting horses without ad libitum access to water induces metabolic alkalosis, hypernatremia, hypokalemia and hyperosmolality for at least 8 hours.70 The oral doses were 0.25, 1 and 1.5 g/kg BW in 3 L water; the intravenous dose was 0.25 g/kg BW in 3 L water. The effects were dose-dependent: in the horses given the 1 and 1.5 g/kg BW oral doses, the hypercapnia persisted for 12 hours, whereas hypercapnia lasted 2 hours in horses given the 0.25 g/kg BW dose orally or intravenously. The effects of these large doses of sodium bicarbonate on the renal function of horses indicated increases in urine flow, fractional clearance of electrolytes and bicarbonate, electrolyte-free water reabsorption, urine concentrations of sodium and bicarbonate, urine excretion, clearance of sodium and bicarbonate, urine pH and anion gap.70
The temperature or glucose concentration of the fluid does not appear to be important, as the rate of fluid absorption was similar in dehydrated horses administered an oral rehydration solution at 5°C, 21°C or 37°C or containing glucose at 0%, 2.5% or 3.5%.71 The tonicity of the oral rehydration solution is of minor clinical importance; however, oral administration of hypertonic solutions (628 mosmol/kg BW) to dehydrated horses caused a transient increase in plasma protein concentration that was attributed to movement of water into the bowel lumen.71 A practical limitation of oral rehydration solutions in horses is that they should be ingested voluntarily rather than by nasogastric intubation. This limitation has led to recent interest in the oral administration of pastes.
The oral administration of an electrolyte paste has been shown to be effective in correcting mild to moderate dehydration in horses, provided animals are monitored to ensure that they drink water.72 Oral electrolyte pastes may be formulated as follows: 30 g of 1:1 mixture of sodium chloride and potassium chloride, potassium chloride and sodium bicarbonate, or potassium chloride and potassium carbonate, and administered every 6 hours; 120 g of the latter mixture provides 1400 mmol or more of potassium in a 24-hour period.65 Administration of higher doses of oral pastes (0.5 g of NaCl/kg BW, 0.5 g of KCl/kg BW or a mixture of 0.25 g of NaCl/kg BW and 0.25 g of KCl/kg BW) to dehydrated horses induced a transient period of hyperhydration and apparent plasma volume expansion that lasted 12 hours.72 Although the absorbed electrolytes from an oral paste are subsequently eliminated via the urine, this treatment is potentially of benefit in horses with disease processes associated with ongoing fluid losses, such as diarrhea.
There is no published information on the use of oral fluid therapy in horses that are diarrheic as a result of disease of the small intestine such as enteritis, or proximal enteritis (duodenitis). It would seem unlikely that oral fluid therapy would be indicated or effective for anterior duodenitis. In horses with colitis, the small intestinal absorptive capacity is probably intact and oral fluid therapy prior to transport of the horse to a clinical center for intensive fluid therapy may delay the onset of more serious complications. Horses with mild dehydration can be rehydrated effectively with oral fluid therapy. Horses treated with oral fluid therapy must be monitored clinically, and the hematocrit, total plasma protein concentration and serum electrolytes should be measured.
Oral fluid therapy in horses with impaction of the large colon provides an effective and inexpensive treatment and should be regarded as the initial treatment of choice. In general, 6–8 L of water can be administered by nasogastric tube and funnel (gravity flow) every 15–20 minutes;68 the administered fluid is rapidly transported to the large intestine. It is generally recommended that the osmolality of the fluids should be isotonic, ranging from 280–360 mosmol/L; the upper range of tonicity which is safe to administer is unknown. Oral administration of 60 L of lactated Ringer’s solution or an isotonic solution over 12 hours was superior in hydrating the contents of the right dorsal colon when compared to intravenous administration of an equivalent volume of lactated Ringer’s solution or enteral administration of 1 g/kg BW of MgSO4.7H2O (Epsom’s salts) or anhydrous Na2SO4as a 1 L solution.69,73 Moreover, enteral administration of Epsom’s salts has been associated with hypermagnesemia, and anhydrous Na2SO4 has been associated with hypocalcemia.73
Fluid and electrolyte therapy in newborn piglets and lambs
The most common cause of fluid and electrolyte imbalance in newborn piglets and lambs is acute neonatal diarrhea. There is severe dehydration, acidemia, hyponatremia and, in some cases, hyperkalemia due to the acidosis. Balanced electrolyte solutions or isotonic saline and sodium bicarbonate initially followed by balanced electrolytes are indicated and successful. These are given subcutaneously or intraperitoneally at the rate of 15 mL per piglet every 2 hours plus the same amount orally. The safe amount of sterilized porcine serum or saline and 5% dextrose that can be given to piglets is equivalent to about 8% BW intraperitoneally, in two divided doses given 8 hours apart. Lambs are also treated subcutaneously (30–40 mL) and orally (50–100 mL) every 2 hours.
Parenteral nutrition
Parenteral nutrition is used to provide adequate nutrition intravenously, as long as necessary, when feeding by the gastrointestinal tract is impractical, inadequate or impossible. The term parenteral nutrition is preferred to total parenteral nutrition because the complete nutritional requirements of large animals are either not completely known or not addressed by intravenous fluid administration. It should be recognized that enteral nutrition represents state-of-the-art medicine because enteral nutrition supports the repair, maintenance and growth of the gastrointestinal tract to a much greater extent than does parenteral nutrition. It should also be recognized that parenteral nutrition should only be contemplated after at least 5 days of inappetence.
The technique is used to supply the nutrient requirements, most importantly protein, of the animal until it returns to normal. In calves affected with persistent diarrhea due to chronic disease of the alimentary tract, or that cannot or will not eat, total intravenous feeding may be indicated.74 High concentrations of glucose, protein hydrolysates, lipid emulsions and electrolytes are given by continuous slow intravenous infusion over a period of several days. Some encouraging results in calves have been published but the cost-effectiveness of the technique has not been examined.74
Parenteral nutrition is an acceptable method of maintaining nutrition in the healthy horse over a period of 10 days.75 Body weight was maintained at 94% of initial values without clinical evidence of dehydration. No problems were encountered with the long-term intravenous catheterization. The total daily amounts given are calculated on the basis of daily caloric requirement. The intravenous catheter must be inserted down into the cranial vena cava, where a large volume of blood will dilute the hypertonic concentration of the solution. The potential problems associated with parenteral nutrition include difficulty in the maintenance of a steady intravenous drip, hypertonicity of the solutions used, venous thrombosis, excessive diuresis, catheter sepsis and bacterial contamination of the solutions.
McGinness SG, Mansmann RA, Breuhaus BA. Nasogastric electrolyte replacement in horses. Comp Cont Educ Pract Vet. 1996;18:942-950.
Ecke P, Hodgson DR, Rose RJ. Review of oral rehydration solutions for horses with diarrhea. Aust Vet J. 1997;75:417-420.
Schott HC. Oral fluids for Equine diarrhea: an underutilized treatment for a costly disease. Vet J. 1998;155:119-121.
Schott HC, Hinchcliff KW. Treatments affecting fluid and electrolyte status during exercise. Vet Clin North Am Equine Pract. 1998;14:175-204.
Berchtold J. Intravenous fluid therapy of calves. Vet Clin North Am Food Anim Pract. 1999;15:505-532.
Constable PD. Clinical assessment of acid-base status: Strong ion difference theory. Vet Clin North Am Food Anim Pract. 1999;15:447-471.
Constable PD. Hypertonic saline. Vet Clin North Am Food Anim Pract. 1999;15:559-585.
Naylor JM. Oral electrolyte therapy. Vet Clin North Am Food Anim Pract. 1999;15:487-504.
Roussel AJ. Fluid therapy in mature cattle. Vet Clin North Am Food Anim Pract. 1999;15:545-558.
Constable PD. Clinical assessment of acid-base status: Comparison of the Henderson-Hasselbalch and strong ion approaches. Vet Clin Path. 2000;29:115-128.
Kudnig ST, Mama K. Perioperative fluid therapy. J Am Vet Med Assoc. 2002;221:1112-1121.
Constable PD. Fluids and electrolytes. Vet Clin North Am Food Anim Pract. 2003;19:1-40.
1 James KM, et al. Am J Vet Res. 1997;58:343.
2 Groutides C, Michell AR. Vet Rec. 1990;126:29.
3 Siggaard-Andersen O. Scand J Clin Lab Invest. 1961;13:196.
4 Mitten LA, et al. J Vet Intern Med. 1995;9:353.
5 Wu EY, et al. J Appl Physiol. 1997;82:196.
6 Roeder BL, et al. J Am Vet Med Assoc. 1996;208:707.
7 Constable PD, et al. J Vet Intern Med. 1997;11:71.
8 Constable PD, et al. Am J Vet Res. 1998;59:881.
9 Constable PD. J Appl Physiol. 1997;83:297.
10 Constable PD, et al. J Vet Intern Med. 2005;19:581.
11 Gossett KA, et al. Am J Vet Res. 1990;51:1375.
12 Harris P, Snow DH. Equine Vet J. 1988;20:109.
13 Andrews FM, et al. Am J Vet Res. 1997;58:1060.
14 Naylor JM, Forsyth GW. Can J Vet Res. 1986;50:509.
15 Roussel AJ, et al. J Am Vet Med Assoc. 1998;212:1769.
16 Nahas GG. Pharmacol Rev. 1962;14:447.
17 Rhee KH, et al. Chest. 1993;104:913.
18 Berchtold J. Vet Clin North Am Food Anim Pract. 1999;15:505.
19 Groutides CP, Michell AR. Res Vet Sci. 1990;49:292.
20 Watt JG. J Am Vet Med Assoc. 1967;150:742.
21 McSherry BJ, Grinyer I. Am J Vet Res. 1954;15:535.
22 Constable PD, et al. Am J Physiol. 1994;267:H667.
23 Goetsch DD, et al. Am J Vet Res. 1956;17:213.
24 Muir WMIII. Cornell Vet. 1990;80:7.
25 Roeder BL, et al. Am J Vet Res. 1997;58:549.
26 Tyler JW, et al. Am J Vet Res. 1994;55:278.
27 Constable PD, et al. Am J Vet Res. 1991;52:981.
28 Constable PD, et al. Am J Vet Res. 1991;52:990.
29 Schmall LM, et al. Equine Vet J. 1990;22:278.
30 Bertone JJ, Shoemaker KE. Am J Vet Res. 1992;53:1844.
31 Berchtold J, et al. J Vet Intern Med. 2005;19:240.
32 Bleul U, et al. Vet Rec. 2005;156:202.
33 Hunt E. Wood B Vet Clin North Am Food Anim Pract. 1999;15:559.
34 Muir WW, et al. Vet Surg. 2000;29:449.
35 Vlahakes GJ, et al. J Thorac Cardiovasc Surg. 1990;100:379.
36 Slanetz PJ, et al. Surgery. 1994;115:246.
37 Watt JG. Vet Rec. 1965;77:1474.
38 Buntain BJ, Selman IE. Vet Rec. 1980;107:245.
39 Constable PD, et al. Am J Vet Res. 1996;57:97.
40 Walker PG, et al. J Am Vet Med Assoc. 1998;213:113.
41 Senturk S. J Vet Med. 2003;50:57.
42 Groutides CP, Michell AR. Res Vet Sci. 1990;49:292.
43 Roussel AJ. Vet Clin North Am Food Anim Pract. 1990;6:111.
44 Fubini SL, et al. Am J Vet Res. 1991;52:1898.
45 Ward JL, et al. Am J Vet Res. 1993;54:1160.
46 Groenendyk S, et al. Equine Vet J. 1988;20:189.
47 Young DR, et al. J Am Vet Med Assoc. 1989;195:340.
48 Grove-White D. In Pract. 1994;16(5):263.
49 Roussel AJ, et al. J Am Vet Med Assoc. 1996;208:905.
50 Mealey RH, et al. J Vet Intern Med. 1995;9:347.
51 Pusterla N, Braun U. Vet Rec. 1995;137:431.
52 Pusterla N, Braun U. Vet Rec. 1996;139:287.
53 Spurlock SL, et al. J Am Vet Med Assoc. 1990;196:425.
54 Traub-Dargatz JL, Dargatz DA. J Vet Intern Med. 1994;8:264.
55 Ettlinger JJ, et al. Vet Rec. 1992;130:248.
56 Michell AR, et al. Br Vet J. 1992;148:507.
57 Naylor JM. Vet Clin North Am Food Anim Pract. 1990;6:51.
58 Brooks HW, et al. Vet J. 1997;153:163.
59 Frederick GS, et al. Vet Clin North Am Food Anim Pract. 1990;6:149.
60 Naylor JM. J Am Vet Med Assoc. 1992;201:1026.
61 Heath SE, et al. Can J Vet Res. 1989;53:477.
62 Naylor JM, et al. Can Vet J. 1990;31:753.
63 Garthwaite BD, et al. J Dairy Sci. 1994;77:835.
64 Ecke P, et al. Aust Vet J. 1997;75:417.
65 Schott HCII. Vet J. 1998;155:119.
66 Ecke P, et al. Vet J. 1998;155:149.
67 Ecke P, et al. Vet J. 1998;155:161.
68 McGuiness SG, et al. Comp Cont Educ Pract Vet. 1996;18:942.
69 Lopes MA, et al. Equine Vet J. 2002;34:505.
70 Rivas LJ, et al. Am J Vet Res. 1997;58:658-664.
71 Sosa Leon LA, et al. Equine Vet J. 1995;20:140.
72 Schott HC, et al. Am J Vet Res. 2002;63:19.
73 Lopes MA, et al. Am J Vet Res. 2004;65:695.
74 Sweeney RW, Divers TJ. Vet Clin North Am Food Anim Pract. 1990;6:125.