TRAUMATIC RETICULOPERITONITIS
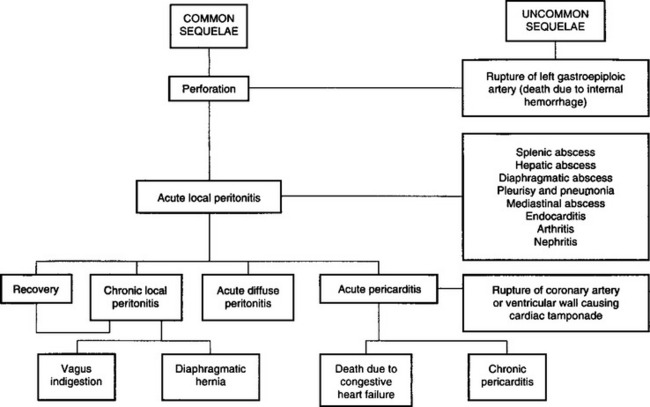
Perforation of the wall of the reticulum by a sharp foreign body initially produces an acute local peritonitis, which may spread to cause acute diffuse peritonitis or remain localized to cause subsequent damage, including vagal indigestion and diaphragmatic hernia. The penetration of the foreign body may proceed beyond the peritoneum and cause involvement of other organs resulting in pericarditis, cardiac tamponade, pneumonia, pleurisy and mediastinitis, and hepatic, splenic or diaphragmatic abscess. These sequelae of traumatic perforation of the reticular wall are set out diagrammatically in Figure 6.4.
This complexity of development makes diagnosis and prognosis difficult, and the possibility that a number of syndromes may occur together further complicates the picture. All these entities except endocarditis are dealt with together here, even though many of them are diseases of other systems.
ETIOLOGY
Traumatic reticuloperitonitis is caused by the penetration of the reticulum by metallic foreign objects that have been ingested in prepared feed. Baling or fencing wire that has passed through a chaff-cutter, feed chopper or forage harvester is one of the most common causes. In one series of 1400 necropsies, 59% of lesions were caused by pieces of wire, 36% by nails and 6% by miscellaneous objects. The metal objects may be in the roughage or concentrate or may originate on the farm when repairs are made to fences, yards and in the vicinity of feed troughs.
The wire from motor vehicle radial tires may be the cause.1-3 Used tires are commonly used to hold down plastic sheeting over silage piles. The wire is gradually released from the tires, which are in a state of deterioration, and is mixed with the feed supply, or the tires may be inadvertently dropped into a feed mixer wagon and become fragmented, mixing the pieces of wire throughout the ration.
Etiology Penetration of reticulum by metallic foreign objects such as nails and pieces of wire, including tire wire, that were ingested by the animal and located in the reticulum
Epidemiology Most common in adult dairy cattle fed prepared feeds
Signs Sudden anorexia and fall in milk yield, mild fever, ruminal stasis and local pain in the abdomen. Rapid recovery may occur, or the disease may persist in a chronic form or spread widely to produce an acute, diffuse peritonitis
Clinical pathology In acute local peritonitis, neutrophilia and regenerative left shift; in chronic form, leukopenia and degenerative left shift. Peritoneal fluid contains marked increase in nucleated cells and total protein. Plasma protein concentration increased. Radiography and ultrasonography of abdomen
Lesions Localized reticuloperitonitis and varying degrees of locally extensive fibrinous adhesions. Abnormal peritoneal fluid. Abscesses and adhesions possible throughout the peritoneal cavity
Diagnostic confirmation Reticuloperitonitis and metallic foreign body
• Acute local traumatic reticuloperitonitis must be differentiated from: simple indigestion, acute carbohydrate engorgement, acute intestinal obstruction, abomasal volvulus, pericarditis, acute pleuritis, perforated abomasal ulcer, postpartum septic metritis, pyelonephritis acute hepatitis, acetonemia
• Acute diffuse or generalized peritonitis must be differentiated from those diseases causing severe toxemia or acid–base imbalance, dehydration, and shock which include the following: carbohydrate engorgement, acute intestinal obstruction, advanced vagus indigestion, abomasal volvulus, perforated abomasal ulcer, and miscellaneous causes of generalized peritonitis
• Chronic traumatic reticuloperitonitis must be differentiated from early stages of vagus indigestion, hepatic abscessation, traumatic splenitis, chronic pneumonia and pleuritis, and miscellaneous causes of chronic peritonitis such as peritoneal abscesses secondary to intraperitoneal injections
Treatment Antimicrobials daily for several days, reticular magnet and immobilization in stall to promote adhesions. Rumenotomy to remove foreign body if medical treatment unsuccessful or in valuable animal
Control Prevent exposure of cattle to metallic foreign objects that can be ingested. Feed processing equipment should be equipped with magnets to remove metallic foreign bodies
In an abattoir survey of the gastrointestinal tract of 1491 slaughter cows in Denmark, foreign bodies were found in 16% of the cows. Of 286 foreign bodies, 11% were tire wires, 14% fencing wires, 5% screws, 9% nails, 37% mixed pieces of metal, 2% copper and 22% remnants of boluses containing antiparasitic drugs.3 A significant association was found between the type of foreign body and the presence of lesions, and a significant association between the cross-section of the foreign body and the presence of lesions. There was also an association between the end shape of the foreign body and the presence of lesions. Tire wire was the most common traumatizing foreign body, as 81% of all lesions were associated with tire wires.
EPIDEMIOLOGY
Occurrence
Adult dairy cattle are most commonly affected because of their more frequent exposure but cases occur infrequently in yearlings, beef cattle, dairy bulls, sheep and goats. In the series of 1400 referred to above, 93% were in cattle over 2 years old and 87% were in dairy cattle. In the Danish abattoir survey of cows (see Etiology), foreign body lesions were present in 10% of the cows.3 Magnets, one or two, were found in only 7% of the cows. All magnets collected iron filings and fencing wire (30%), and ‘other pieces of metal’ (39%) were the predominant contents of the magnet. There were no lesions in 97% of the cows with magnets, and a significant association was found between the use of magnets and the absence of lesions.
The disease is much more common in cattle fed on prepared feeds, especially those fed inside for part of the year. It is almost unknown in cattle fed entirely on pasture. Accordingly, it is much more common in the winter months in the northern hemisphere. The incidence is low in sheep and goats.
The incidence is usually sporadic but outbreaks have occurred when sources of wire have become mixed into feed supplies, as in the case of perforation of the alimentary tract by pieces of tire wire.2 Over a period of 6 months, 30% of 170 lactating dairy cows in one herd exhibited clinical signs suggestive of hardware disease associated with the ingestion of tire wire in the feed supplies.
Risk factors
There are few studies of the epidemiology of traumatic reticuloperitonitis. The effects of 23 veterinary diagnoses, host characteristics and production were examined on the risks of ruminal acidosis and traumatic reticuloperitonitis.4 The lactational incidence risk for the disease in Finnish Ayrshire dairy cattle was 0.6%,4 which is similar to observations made in Holstein–Friesian cows.5 The risk of the disease in the former study increased with early metritis, nonparturient paresis, ketosis, acute and chronic mastitis, and foot and leg problems. It is unknown how metritis and mastitis could be risk factors for traumatic reticuloperitonitis. The median day of occurrence was on 113 days after calving, which makes it unlikely that calving was a risk factor. Similarly, dystocia was not found to be a risk factor.
When several or more cases occur in a cluster outbreak, the nature of the feed supply should be considered as a risk factor. The use of used tires to secure plastic sheeting over silage piles may be an important risk factor.
Economic importance
The disease is economically important because of the severe loss of production it causes and the high mortality rate. Many cases go unrecognized and many more make spontaneous recoveries. In industrialized countries, metallic foreign bodies may be present in the reticulum in up to 90% of normal cattle and residual traumatic lesions may be present in as many as 70% of dairy cows. Among the clinically affected animals, about 25% develop incurable complications. The other 75% can be expected to recover completely with conservative treatment or routine surgical intervention.
PATHOGENESIS
Ingestion of foreign body
Lack of oral discrimination by cattle leads to the ingestion of foreign bodies that would be rejected by other species. Swallowed foreign bodies may lodge in the upper esophagus and cause obstruction or in the esophageal groove and cause vomiting, but in most instances they pass to the reticulum. Radiological examination of goats that have been fed foreign bodies experimentally indicate that they may first enter various sacs of the reticulorumen before reaching the reticulum. Many lie there without causing harm but the honeycomb-like structure of the reticulum provides many sites for fixation of the foreign body, and contractions of the reticulum are sufficient to push a sharp-pointed object through the wall.
Penetration of reticulum
Most perforations occur in the lower part of the cranial wall of the reticulum but some occur laterally in the direction of the spleen and medially towards the liver.
If the reticular wall is injured without penetration to the serous surface no detectable illness occurs, and the foreign body may remain fixed in the site for long periods and gradually be corroded away.A piece of wire can disappear in 6 weeks but certain nails last much longer and are unlikely to corrode away in less than 1 year. The ease with which perforation occurs has been illustrated by the artificial production of the disease. Sharpened foreign bodies were given to 10 cows in gelatin capsules. Of 20 pieces of wire and 10 nails, 25 were found in the reticulum. Of the 20 pieces of wire 18 had perforated or were embedded in the wall or plicae. Only one of the nails was embedded. Complete perforations were caused by 13 foreign bodies and incomplete by six. All cows suffered at least one perforation, showed clinical signs of acute local peritonitis and recovered after surgical removal of the foreign bodies.
Many foreign bodies may not remain embedded but are commonly found free in the reticulum if surgery is carried out about 72 hours after illness commences. This may be due to necrosis around the penetrating object and the reticular contractions moving the foreign body back into the reticulum. Objects that are deeply embedded or have kinks, barbs or large diameters tend to remain in situ and cause persistent peritonitis.
Acute local peritonitis
The initial reaction to perforation is one of acute local peritonitis and, in experimentally induced cases, clinical signs commence about 24 hours after penetration. The peritonitis causes ruminal atony and abdominal pain. If the foreign body moves back into the reticulum spontaneous recovery may occur.
Resolution of acute fibrinous local peritonitis is characterized by the development of fibrous adhesions, which gradually become long, stringy strands over a period of weeks and months; motility of the reticulum is restored and the animal may recover fully. Followup ultrasonographic examinations of cows with traumatic reticuloperitonitis in which rumenotomies were done found that the adhesions disappeared in most of the animals by 6 months.6
Depending on the severity of the local peritonitis, the ventral aspect of the reticulum becomes adherent to varying degrees to the abdominal floor and diaphragm. This results in decreased reticular motility. Ultrasonography of cows with traumatic reticuloperitonitis reveals that the biphasic contractions of the reticulum are slower than normal or indistinct and the number of contractions are reduced.7 Reticular abscesses are common complications and may be located between the reticulum and the ventral body wall, between the reticulum and the right thoracic wall and between the reticulum and the spleen.8 Persistent local peritonitis with or without abscesses results in reduced reticulorumen motility, inappetence to anorexia, a capricious appetite (may eat hay not concentrate), chronic ruminal tympany, persistent mild fever, abdominal pain on deep palpation, and changes in the hemogram and feces. Immobilization of the reticulum impairs the clearance function of the reticulum, which results in the passage of poorly comminuted feces characterized by an increased proportion of large particles.9
Generalized peritonitis and extension of disease
Spread of the inflammation causing generalized or diffuse peritonitis may occur in cows that calve at the time of perforation and in cattle that are forced to exercise. Immobility is a prominent clinical finding and may be a protective mechanism so that adhesions are able to form and localize the peritonitis. Animals made to walk or transported long distances frequently suffer relapses when these adhesions are broken during body movements. Generalized peritonitis results in toxemia, alimentary tract stasis, dehydration and shock.
During the initial penetration of the reticulum, the foreign body may penetrate beyond the peritoneal cavity and into the pleural or pericardial sacs. This may occur more commonly in cows in advanced pregnancy than in nonpregnant cows, because of the gravid uterus, although this is uncertain. Complications such as pericarditis occur most commonly in cows after the sixth month of pregnancy.
Details of the pathogenesis of the more common complications are presented under traumatic pericarditis, vagus indigestion, diaphragmatic hernia and traumatic abscess of the spleen and liver. Less common sequelae include rupture of the left gastroepiploic artery causing sudden death due to internal hemorrhage and the development of a diaphragmatic abscess, which infiltrates tissues to the ventral abdominal wall at the xiphoid process, rupturing to the exterior and sometimes discharging the foreign body. Hematogenous spread of infection from a diaphragmatic abscess or chronic local peritonitis is a common cause of endocarditis and its sequelae of polysynovitis and arthritis, nephritis and pulmonary abscessation. Penetration into the pleural cavity causes acute suppurative pleurisy and pneumonia. In rare cases the infection is localized to the mediastinum causing abscessation, which causes pressure on the pericardial sac and congestive heart failure. Rarely, the foreign body penetrates to the abomasum, causing abomasitis, pyloric stenosis and abomasal ulceration. Even more rarely, puncture of the reticular vein by a migrating metal wire may lead to fatal hemorrhage causing sudden death.10
CLINICAL FINDINGS
Acute local peritonitis
Characteristically, the onset is sudden with complete anorexia and a marked drop in milk yield, usually to about a third or less of the previous milking. These changes occur within a 12-hour period and their abrupt appearance is typical. Subacute abdominal pain is common in most cases. The animal is reluctant to move and does so slowly. Walking, particularly downhill, is often accompanied by grunting. Most animals prefer to remain standing for long periods and lie down with great care; habitual recumbency is characteristic in others. Arching of the back occurs in about 50% of cases, along with the appearance of tenseness of the back and the abdominal muscles so that the animal appears gaunt or ‘tucked-up’. Defecation and urination cause pain and the acts are performed infrequently and usually with grunting. This results in constipation, scant feces and in some cases retention of urine. Rarely, acute abdominal pain with kicking at the belly and stretching occurs. In others there is recumbency and reluctance to stand.
A moderate systemic reaction is common in acute localized peritonitis. The temperature ranges from 39.5–40°C (103–104°F), rarely higher, the heart rate is about 80/min and the respiratory rate about 30/min. Temperatures above 40°C (104°F) accompanied by heart rates greater than 90/min suggest severe complications. The respirations are usually shallow and, if the pleural cavity has been penetrated, are painful and accompanied by an audible expiratory grunt.
Rumination is absent and reticulorumen movements are markedly depressed and usually absent. The rumen may appear to be full because of the presence of a free-gas bloat with moderate distension of the left paralumbar fossa. On palpation of the fossa, the ruminal gas cap is usually larger than usual and the rumen contents more doughy than normal. Deep palpation of the gas cap in the fossa may be required to feel the rumen pack below the gas cap.
Pain can be elicited by deep palpation of the abdominal wall just caudal to the xiphisternum. Palpation is done using short, sharp pushes with the closed fist or knee over an imaginary band about 20 cm wide covering the ventral third of the abdomen from the left to the right side with the cranial border of the band being the point just caudal to the xiphisternum. This area should be probed with at least six deep palpations on both sides of the abdomen while listening with a stethoscope over the trachea for evidence of a grunt. Pinching the withers to cause depression of the back and eliciting a grunt is also an effective diagnostic aid, except in large adult cows and bulls; for these the sharp elevation of a solid rail held horizontally under the abdomen is a useful method for eliciting a grunt. A positive response to any of these tests is a grunt of pain, which may be audible some distance away but is best detected by auscultation of the trachea. Rarely, a grunt may also be audible by auscultation over the trachea when infrequent reticulorumen contractions occur.
The course of acute local peritonitis is short and the findings described above are most obvious on the first day; in most cases they subside quickly and may be difficult to detect by the third day. In these cases, in addition to persistent anorexia and ruminal atony, the most constant finding is the abdominal pain, which may require deep palpation for its demonstration. In cases that recover spontaneously or respond satisfactorily to conservative treatment there may be no detectable signs of illness by the fourth day.
Chronic local peritonitis
In chronic peritonitis the appetite and milk yield do not return to normal after prolonged therapy with antimicrobials. The body condition is usually poor, the feces are reduced in quantity and there is an increase in undigested particles. In some cases, the temperature may be within the normal range, which makes the diagnosis uncertain. A persistent slightly elevated temperature is supportive evidence of the presence of a chronic inflammatory lesion. The grunt test may be positive or negative; often it is uncertain. The gait may be slow and careful and, occasionally, grunting may occur during rumination, defecation and urination. Rumination activities are infrequent, the rumen is usually smaller than normal, chronic moderate bloat is common and there is ruminal atony or some moderate reticulorumen activity.
Reticular abscesses in cows are characterized by poor body condition, a relatively full rumen but with reduced ruminal contractions or almost complete ruminal atony, persistent mild bloat, an arched back with a tense abdomen and a grunt indicating abdominal pain, and undigested particles in the feces. Most have a clinical history of not responding to prolonged therapy with antimicrobials. These can be diagnosed with radiography and ultrasonography.
Rectal examination
Rectal examination of cattle with acute or local traumatic reticuloperitonitis may cause a painful grunt when the animal strains during the examination. The feces are usually dry and firm and covered by a thin coating of mucus because of prolonged retention. In acute localized peritonitis the rumen may feel larger than normal and the gas cap is easily palpable. In acute and chronic generalized peritonitis, fibrinous adhesions may be palpable between the rumen and the left abdominal wall or between loops of intestine, or in the pelvic cavity.
Acute diffuse (generalized) peritonitis
Acute diffuse peritonitis is characterized by the appearance of profound toxemia within a day or two of the onset of local peritonitis. Alimentary tract motility is reduced, mental depression is marked and the temperature is elevated or subnormal in severe cases, especially those that occur immediately after calving. The heart rate increases to 100–120/min and a painful grunt may be elicited by deep digital palpation at almost any location over the ventral abdominal wall. This stage is usually followed by rapid collapse and peripheral circulatory failure and an absence of pain responses. Terminally, recumbency and depression are common.
Sudden death
There is a record of sudden death in a 20-month-old pregnant heifer in which the reticular vein was punctured by a migrating piece of metal wire, causing fatal hemorrhage into the reticulum. At necropsy, a large blood clot was present in the reticulum, the rumen contents were red brown and no reticular adhesions were present.10
Iatrogenic reticulitis
There is a record of iatrogenic reticulitis that occurred as a result of the oral administration of intraruminal anthelmintic boluses, which may have lodged in the reticulum and become filled with other foreign objects ingested by the animal, resulting in a syndrome similar to acute traumatic reticuloperitonitis.11 Inappetence, reduced milk production, reduced reticulorumen motility, abdominal pain and scant feces were present. On exploratory rumenotomy the reticulum contained two cylindrical boluses filled with stones, nuts and bolts. Removal of the boluses was followed by prompt recovery.
CLINICAL PATHOLOGY
Hemogram
The total and differential leukocyte counts provide useful diagnostic and prognostic data. The differential leukocyte count is usually considerably more indicative of acute peritonitis than the total count.
In acute local peritonitis a neutrophilia (mature neutrophils above 400/μL) and a left shift (immature neutrophils above 200/μL) are common. This is a regenerative left shift. Both the neutrophilia and the left shift will be increased on the first day and will last for up to 3 days, when in uncomplicated cases the count begins to return to normal. In chronic cases the levels do not return completely to normal for several days or longer periods and there is usually a moderate leukocytosis, neutrophilia and a monocytosis.
In acute diffuse peritonitis a leukopenia (total count below 4000/μL) with a greater absolute number of immature neutrophils than mature neutrophils (degenerative left shift) occurs, which suggests an unfavorable prognosis if severe. The degree of lymphopenia (lymphocyte count below 2500–3000/μL) is an indication of a stress reaction to inflammation.
Plasma protein and fibrinogen
There is a significant difference in total plasma protein levels between cattle with traumatic reticuloperitonitis and those with other diseases of the gastrointestinal tract that might be confused with the former.12 The mean plasma protein concentrations, measured before surgery, were 88 ± 13 g/L for traumatic reticuloperitonitis and 77 ± 12 g/L for controls. In severe diffuse peritonitis the fibrinogen levels may be increased up to 10–20 g/L.12
Cut-off points for total plasma protein (TPP) and plasma fibrinogen (PF) were determined to differentiate between traumatic reticuloperitonitis and other gastrointestinal diseases with similar clinical findings.13 There was moderate negative dependence between sensitivities of TPP and PF at the 8.82 g/dL and 766 mg/dL cut-off points, and mild negative dependence between their specificities at the 7.78 g/dL and 691 mg/dL cut-off points, respectively.13 Acceptable accuracy (98% or 86% specificity with 62% or 88% sensitivity, respectively) was obtained with serial interpretation of the tests.
Abdominocentesis and peritoneal fluid
Abdominocentesis and analysis of peritoneal fluid can be a valuable diagnostic aid. The best site for abdominocentesis is uncertain because the rumen occupies a large portion of the ventral abdominal wall and avoiding penetration of it is difficult. Cattle have a low volume of peritoneal fluid and failure to obtain a sample is not unusual. Empirically, the best sites are those in which, on an anatomical basis, there are recesses between the forestomachs, abomasum, diaphragm and liver. These are usually 10–12 cm caudal to the xiphisternum and 10–15 cm lateral to the midline. A blunt-ended teat cannula is recommended but with care and caution a 16-gauge 5 cm hypodermic needle may also be used. The hair of the site is clipped, the skin is prepared aseptically and a local anesthetic is applied. The skin in incised with a stab scalpel and the cannula is pushed carefully and slowly through the abdominal wall. The latter will twitch and a ‘pop’ will be felt when the peritoneum is punctured. When the cannula is in the peritoneal cavity the fluid may leak out without the aid of a vacuum. If it does not, a syringe may be used to apply a vacuum while the needle is manipulated in an attempt to locate some fluid.
If no fluid can be obtained, a trocar and cannula 80 mm long and with a 4 mm internal diameter can be used with success. The trocar and cannula are inserted into the abdomen, the trocar is removed and an 80 cm long 10 French gauge infant feeding tube is inserted into the abdomen through the cannula, leaving about 10–20 cm outside. The tube acts as a wick and within several minutes fluid can be collected into vials. At least three different sites should be attempted to obtain peritoneal fluid. Peritonitis in cattle is characterized by a marked fibrinous response and localization of a lesion, and the amount of exudative fluid available at the abdominocentesis sites may be minimal. Thus the failure to obtain fluid does not preclude the presence of peritonitis.
Laboratory evaluation of peritoneal fluid consists of determinations of total white blood cell count, differential cell count, total protein and culture for pathogens. The interpretation of the analysis of the peritoneal fluid can be unreliable because to date only a few correlations have been made between the laboratory findings and the presence or absence of peritoneal lesions. A nucleated cell count above 6000 cells/μL and total protein above 3 g/dL is consistent with the diagnosis of peritonitis in 80% of cases. Using a differential cell count, a relative neutrophil count more than 40% and a relative eosinophil count less than 10% was frequently associated with the diagnosis of peritonitis.
METAL DETECTION
Metal detectors were used at one time to aid in the diagnosis of traumatic reticuloperitonitis. Ferrous metallic foreign bodies can be detected with metal detectors but the instruments are of limited use because most normal dairy cows are positive for metal over the reticular area.
LAPAROSCOPY
Right flank laparoscopy using a flexible fiberoptic laparoscope, 14 mm diameter and 1120 mm working length, is a reliable diagnostic aid for the presence of traumatic reticuloperitonitis.
RADIOGRAPHY OF CRANIAL ABDOMEN AND RETICULUM
Radiological examination of the reticulum with the animal in dorsal recumbency (dorsal reticulography) is an accurate diagnostic method for the evaluation of cattle with suspected traumatic reticuloperitonitis.14 However, the lack of adequate radiographic equipment in private veterinary practices precludes its routine use. Also, the technical difficulties of positioning the animal and the increased potential for personnel exposure associated with manual restraint suggests that it may not be practical except for valuable animals that may warrant referral to a veterinary medical center.
The cranioventral abdomen of cattle can be evaluated using two cranial abdominal and one caudal thoracic radiographs. An X-ray machine with a capacity of 1000–1250 mA and 150 kV is necessary, which is usually only available in veterinary teaching hospitals. However, such techniques may be appropriate in valuable animals in which an accurate diagnosis and prognosis for surgical treatment may be desirable.15 In a consecutive series of standing lateral cranial abdominal radiographs, the sensitivity and specificity for detecting traumatic reticuloperitonitis or pericarditis was 83% and 90%, respectively.16 These values are higher than those achieved with dorsal recumbency. In standing lateral radiographs, an enlarged reticulum was associated with a final diagnosis of vagal indigestion. Alteration in reticulodiaphragmatic separation does not correlate with any specific disease process. The presence of focal perireticular gas collections and reticular foreign bodies greater than 1 cm in length unattached to a magnet were good indicators of traumatic reticuloperitonitis. Radiography is best suited for identification of radiodense foreign bodies in and outside the reticulum (these cannot be visualized ultrasonographically).14
Features found to be reliable in the diagnosis of traumatic reticuloperitonitis using lateral radiographs of the reticulum include:
• Atypically positioned foreign bodies
• Abnormal gas shadows in the region of the reticulum
• Depressions in the cranioventral margin of the reticulum.17
The reticulum is commonly markedly displaced caudally from the diaphragm or dorsally or caudodorsally from the ventral abdominal wall. Space-occupying masses of the density of soft tissue, with or without gas inclusions, gas shadows and gas– fluid interfaces in the region of the reticulum, were highly predictive of peritonitis (specificity 97%, positive predictive value 96%).
ULTRASONOGRAPHY OF THE RETICULUM
Ultrasonography is a suitable method for investigation of reticular contractions in healthy ruminants and in cattle for the diagnosis of traumatic reticuloperitonitis.18,19 The literature on the use of ultrasonography as a diagnostic aid in gastrointestinal disease in cattle has been reviewed.20 The reader is referred to an excellent atlas and textbook on the use of ultrasonography in cattle.19
The reticulum and adjacent organs of cows can be examined with ultrasonography using a 3.5 MHz linear transducer applied to the ventral midline of the thorax over the sixth and seventh intercostal spaces and from the left and right sides of the midline.21,22 It may not be possible to image the reticulum in large cows in good body condition because of the high proportion of fat in the muscle layers. In older cows, calcification of the xiphisternum may interfere with imaging. The most common reason for being unable to visualize the reticulum in sick animals is the displacement of the reticulum by a markedly distended rumen or by space-occupying lesions such as abscesses and fibrin-containing effusions. The pattern, number, amplitude and duration of the interval between contractions can be visualized.21 The contour of reticulum, the reticular contractions and the organs adjacent to the reticulum can be imaged. The biphasic reticular contractions can be visualized at the rate of 4 during a 4-minute period.21,22 During the first incomplete contraction, the reticulum contracts by a mean of about 7.2 cm and during the second contraction the reticulum disappears from the screen.
Ultrasonography for traumatic reticuloperitonitis
In contrast to radiography, ultrasonography provides more precise information about the contour of the reticulum and reticular motility.7,22 In cattle with traumatic reticuloperitonitis, ultrasonography can be used to identify morphological changes in the region of the cranial, ventral or caudal reticular wall.20 The caudoventral reticular wall is the most frequently affected, often in association with the craniodorsal blind sac of the rumen. The changes in the contour of the reticulum depend on the severity of the inflammatory changes.
The reticulum can be visualized in more than 90% of cows in spite of interference by the ribs and sternum. In cows with disturbed reticular motility, biphasic contractions are slower than normal, or indistinct, and the number of contractions is reduced. Fibrinous material appears as echogenic deposits, sometimes accompanied by hypoechogenic fluid. Reticular abscesses have an echogenic capsule with a hypoechogenic center. Involvement of the spleen, omasum, liver and abomasum may also be imaged. Neither magnets nor foreign bodies can be visualized by ultrasonography.22
Reticular activity is almost always affected in cattle with traumatic reticuloperitonitis. The frequency, amplitude or velocity of contractions, singly or combined, may be abnormal. The frequency can be reduced from 3 to 2, 1 or no contractions per 3 minutes. The reduction in the amplitude of contractions varies: when formation of adhesions is extensive, reticular contractions appear indistinct. Although the pattern of biphasic contraction is often maintained, the reticulum contracts only 1–3 cm. The velocity of reticular contractions may be normal but can be markedly reduced. In cattle with reticulo-omasal obstruction due to a foreign body, the frequency of reticular contractions may be increased.23
Reticular abscesses associated with traumatic reticuloperitonitis can be visualized by ultrasonography8 (Fig. 6.5). The amplitude of reticular contractions is reduced, the reticulum is displaced from the ventral body wall, and the abscesses have hypoechogenic centers and echogenic capsules.
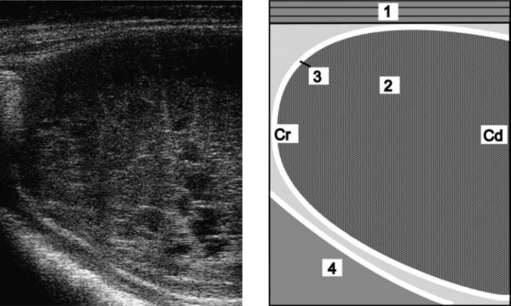
Fig. 6.5 Ultrasonogram and schematic of a reticular abscess in a cow with chronic traumatic reticuloperitonitis. The abscess is between the reticulum and the ventral abdominal wall. The ultrasonogram was obtained from the sternal region with a 5.0 MHz-linear transducer. 1 = Ventral abdominal wall; 2 = Abscess; 3 = Capsule of the abscess; 4 = Reticulum. Cr, Cranial; Cd, Caudal.
(Reproduced with kind permission of U. Braun.)
Peritoneal effusion is visible as an accumulation of fluid without an echogenic margin and restricted to the reticular area. Depending on the fibrin and cell content, the fluid may be anechoic or hypoechogenic. Fibrinous deposits are easily identified in the fluid and bands of fibrin are sometimes seen within the effusion. Occasionally, the peritoneal effusion is considerable and extends to the caudal abdomen.
The spleen, particularly its distal portion, is often affected. Fibrinous changes are frequently seen as echogenic deposits of varying thickness, often surrounded by fluid, between the spleen and reticulum or rumen. The spleen may be covered by fibrinous deposits. Occasionally, one or more splenic abscesses are visible, and the vasculature may be dilated, indicating splenitis.20
Ultrasonography and radiography of cattle with traumatic reticuloperitonitis
These two techniques have been compared in cows with traumatic reticuloperitonitis. The major advantages of radiography are that metallic foreign bodies can be visualized and their position determined. It has a specificity of 82%, a positive predictive value of 88% and a sensitivity of 71%.24 Abnormal gas shadows or gas–fluid interfaces observed on radiographs are highly diagnostic for the disease and have a specificity of 97% and positive predictive value of 88%. However, they are seldom seen on radiographs and their sensitivity is only 19%. The position of the reticulum is a good criterion for the diagnosis of traumatic reticuloperitonitis, with a specificity of 80% and a positive predictive value of 82%. Thick-walled changes or abscessation should be suspected when the reticulum is displaced caudodorsally from the sternum. Changes in the contour of the reticulum such as indentations are highly suggestive of inflammation, with a specificity of 95% and positive predictive value but a low sensitivity of only 34%.
The major advantage of ultrasonography is being able to visualize and assess reticular motility.19,22,24 Even in the presence of severe adhesions and abscessation, the reticulum may maintain its basic contractile rhythm, but much reduced. Abscesses have an echogenic capsule of varying width and a central cavity filled with hypoechogenic material. Purely fibrous deposits are echogenic, and fibrinous deposits containing an accumulation of fluid from inflammatory processes are echogenic interspersed with hypoechogenic accumulations of fluid (Fig. 6.6).24 Radiography and ultrasonography complement each other and the combined results can be used to decide whether an exploratory laparotomy is indicated, if the animal should be treated conservatively with antibiotics, or if it should be slaughtered for salvage.22
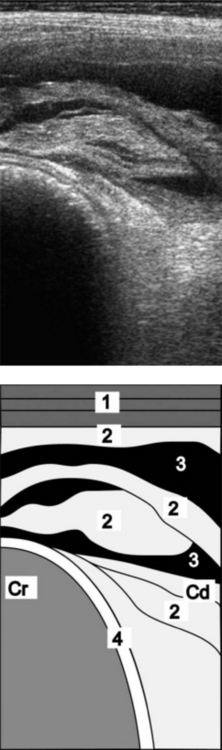
Fig. 6.6 Ultrasonogram and schematic of the reticulum in a cow with chronic traumatic reticuloperitonitis. The reticulum is covered with fibrinous deposits. The ultrasonogram was obtained from the sternal region with a 5.0 MHz-linear scanner. 1 = Lateral abdominal wall; 2 = Fibrinous deposits; 3 = Anechoic fluid; 4 = Reticulum. Cr, Cranial; Cd, Caudal.
(Reproduced with permission from U. Braun.)
NECROPSY FINDINGS
Localized traumatic reticuloperitonitis is characterized by varying degrees of locally extensive fibrinous adhesions between the cranioventral aspects of the reticulum and the ventral abdominal wall and the diaphragm. Adhesions and multiple abscesses may extend to either side of the reticulum involving the spleen, omasum, liver, abomasum and ventral aspects of the rumen. Large quantities of turbid, foul-smelling peritoneal fluid may be present, containing fibrinous clots. Some cases of reticular abscesses are solitary and there are adhesions between the reticulum, diaphragm and ventral body wall, which are strictly localized. The size of the abscess varies. It may be from 5–10 cm in diameter or else a single one may be irregularly shaped and measure 30 × 10 × 10 cm, along with multiple smaller ones measuring around 3 × 3 × 3 cm.24 The foreign body can usually be found perforating the cranioventral aspect of the reticulum, although it may have fallen back into the reticulum, leaving only the perforation site and its surrounding inflammation as evidence of the site of penetration. A reticular magnet with many pieces of metallic foreign bodies stuck to it may be present in the reticulum, the mucosa of which is usually normal.
In acute diffuse peritonitis a fibrinous or suppurative inflammation may affect almost the entire peritoneal cavity with extensive fibrinous adhesions of various stages of development involving the forestomach, abomasum, small and large intestines, liver, bladder, reproductive tract and pelvic cavity. Large quantities of turbid, foul-smelling fluid containing clots of fibrin are usually present. Loops of intestine and omenta are commonly stuck together by thick layers of fibrin.
Typical acute traumatic reticuloperitonitis is characterized by a sudden onset of complete anorexia, marked drop in milk production, mild fever, ruminal atony, pain on deep palpation of the ventral abdomen, an elevated leukocyte count with a left shift in the hemogram and a peritoneal fluid sample that indicates inflammation.
However, the times at which cases of traumatic reticuloperitonitis are seen varies from day 1, when the syndrome is typical, to day 3 or 4, by which time the acuteness has subsided so much clinically that confusion with other diseases is a significant possibility. The sudden onset of anorexia and marked drop in milk production will usually be noted in lactating dairy cattle but not in dry dairy cattle or beef cattle, including mature bulls whose feed intake and behaviors are not monitored daily. In these animals the clinical findings can change in a few days and be characterized by anorexia to inappetence, normal temperature, ruminal hypotonicity or atony and no evidence of abdominal pain on deep palpation of the abdomen.
The clinician must review the history carefully, conduct a thorough clinical examination and attempt to intensify the diagnostic efforts on those abnormalities that are present.
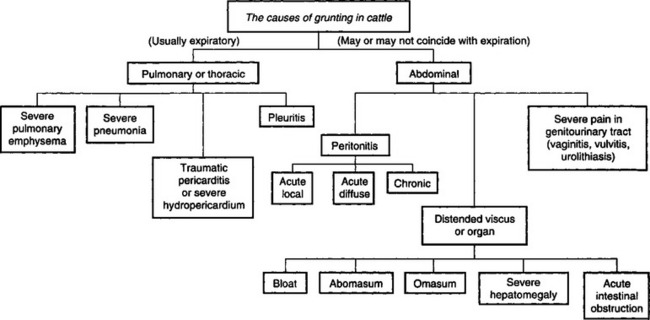
The differential diagnosis of gastrointestinal dysfunction of cattle is summarized in Table 6.2. An algorithm for the causes of grunting in cattle is shown in Fig. 6.7.
Acute local traumatic reticuloperitonitis
Acute local traumatic reticuloperitonitis must be differentiated from those diseases in which sudden anorexia, sudden drop in milk production, ruminal atony, abdominal pain and abnormal feces are common. They include the following:
• Simple indigestion characterized by sudden anorexia or inappetence, normal mental state, full rumen but atonic, perhaps uncomfortable if ingested large quantities of palatable feed like fresh silage, normal vital signs, abnormal feces and spontaneous recovery in 24 hours are typical
• Obstruction of reticulo-omasal orifice with a foreign body such as a roll of polyethylene twine causes intermittent inappetence, a slightly enlarged rumen with normal motility, slight reduction in the amount of feces, a decrease in milk yield for 24–48 hours followed by a return to normal and then subsequent relapses. A grunt is not present, the temperature, heart and respiratory rates are normal and the hemogram is normal. Obstruction of the reticulo-omasal orifice with foreign bodies such as rope can cause distension and hypermotility of the rumen and persistent vomiting.23 A rumenotomy must be done to make the diagnosis
• Acute carbohydrate engorgement characterized by sudden anorexia, diarrhea and dehydration, weakness, tachycardia, staggering, ruminal distension and atony, fluid-splashing sounds in the rumen with a rumen pH of less than 5 and a history of access to grain
• Acute intestinal obstruction characterized by sudden anorexia, mild abdominal pain perhaps with kicking at the abdomen and stretching, ruminal atony, mild dehydration, scant feces or complete absence of feces, straining on rectal examination, dark blood-stained feces and perhaps distended loops of intestine palpable on rectal examination
• Abomasal volvulus (following right-side dilatation) characterized by anorexia, dehydration, tachycardia, distended right abdomen, ping audible over right flank, distended viscus palpable on rectal examination. Usually in lactating dairy cows a few weeks after parturition and following the clinical findings of right-side dilatation of the abomasum that lasts several days culminating in the volvulus but may occur spontaneously in some cows with no immediate history of previous illness
• Pericarditis. Continued high fever, toxemia, anorexia, tachycardia and muffled heart sounds suggest pericarditis, which is marked by markedly elevated total leukocyte and neutrophil counts. In pericarditis, the heart sounds are muffled and the typical to and fro fluid-splashing sounds are audible. The jugular veins are engorged and other signs of congestive heart failure such as anasarca are present. Pericardiocentesis to obtain foul-smelling, turbid fluid is diagnostic
• Acute pleuritis is characterized by a fever, toxemia, anorexia, painful respirations that may be accompanied by a grunt, pain on digital palpation of intercostal spaces, ruminal atony, and abnormal and muffled lung sounds. Fluid on thoracentesis
• Perforated abomasal ulcer causes acute local peritonitis characterized by marked pain on palpation over a much larger area of the abdominal wall and in the early stages is most marked on the right-hand side. If, as is usual, the peritonitis becomes diffuse the syndrome cannot be distinguished clinically from that caused by traumatic reticuloperitonitis. Extension from a metritis to involve the peritoneum is suggested by other signs of the primary disease
• Postpartum septic metritis occurs a few days after parturition and is characterized by anorexia, fever, tachycardia, ruminal hypotonicity to atony, reduced amount of feces and foul-smelling vaginal discharge, and retained placenta may be present. Very important to examine the uterus vaginally for the presence of the placenta, which may be protruding through the cervix
• Acute local peritonitis due to penetration of the uterine wall by a catheter or of the rectal wall by a foreign body thrust sadistically into the rectum may be difficult to differentiate unless the painful area of the peritoneum can be determined. Acute local peritonitis can be differentiated from indigestion, acute ruminal impaction and acetonemia by the presence of fever, local abdominal pain and the abrupt fall in milk yield and appetite
• Pyelonephritis is occasionally accompanied by mild abdominal pain but can be distinguished by the presence of pus and blood in the urine
• Acute hepatitis or severe hepatic abscess is characterized by anorexia, fever, decreased ruminal movements, reluctance to move, a painful grunt on deep palpation over the cranial aspects of the right lower flank, icterus if obstruction of the bile ducts has occurred, and a poor response to therapy. A marked neutrophilia is typical of hepatic abscessation secondary to traumatic reticuloperitonitis
• Acetonemia. Traumatic reticuloperitonitis usually causes a secondary acetonemia when it occurs during early lactation and the presence of ketonuria should not be used as the sole basis for differentiation of the diseases. Differentiation may be extremely difficult if the peritonitis is of 3–4 days’ duration. Response to treatment may also serve as a guide. The history is often helpful; the appetite and milk yield fall abruptly in traumatic reticuloperitonitis but slowly over a period of several days and not to the same degree in acetonemia
Acute diffuse or generalized peritonitis
Acute diffuse peritonitis is characterized by anorexia, fever, toxemia, tachycardia, dehydration, weakness leading to recumbency, distended abdomen, ruminal atony, spontaneous grunting or a grunt on deep palpation over the abdomen, fluid-splashing sounds and pings on auscultation and percussion or ballottement of the abdomen due to ileus, scant feces, perhaps palpable fibrinous adhesions on rectal palpation, profuse quantities of abnormal peritoneal fluid and marked changes in the hemogram. It must be differentiated from those diseases causing severe toxemia or acid–base imbalance, dehydration and shock, which include: carbohydrate engorgement, acute intestinal obstruction, advanced vagus indigestion, abomasal volvulus, perforated abomasal ulcer and miscellaneous causes of generalized peritonitis.
Chronic reticuloperitonitis
The clinical findings of chronic traumatic reticuloperitonitis are not typical. Each chronic case may have a different combination of clinical findings, which makes the diagnosis uncertain. The clinical findings that may be present include inappetence to anorexia, mild fever, loss of body condition, lack of rumination, ruminal hypotonicity to atony, moderate bloat, scant feces containing increased amounts of undigested feed particles, possibly a grunt on deep palpation of abdomen, and changes in the hemogram. The presence of abnormal peritoneal fluid is highly supportive. It must be differentiated from early stages of vagus indigestion, hepatic abscessation, traumatic splenitis, chronic pneumonia and pleuritis, and miscellaneous causes of chronic peritonitis such as peritoneal abscesses secondary to intraperitoneal injections.
TREATMENT
Two methods of treatment are in general use: conservative treatment with or without the use of a magnet, and rumenotomy. Both have advantages and each case must be considered when deciding on the form of treatment to be used.
Conservative medical therapy
Conservative treatment comprises immobilization of the animal, administration of antimicrobials for the inflammation and the oral administration of a magnet to immobilize the foreign body. The cow is tied, stanchioned or confined in a box stall for several days. Immobilization of the animal facilitates the formation of adhesions.
Antimicrobials
Penicillin or broad-spectrum antimicrobials given parenterally daily for 3–5 days are widely used with empirical success. Because of the high probability that a mixed gastrointestinal flora is the cause of the lesion it is more rational to use a broad-spectrum antimicrobial such as the tetracyclines or trimethoprim-potentiated sulfonamides rather than penicillin, which is commonly used because of cost and a short withdrawal period in the event that the animal does not respond favorably in a few days. For lactating dairy cattle, those antimicrobials with a short milk withdrawal period are desirable. However, there are no published clinical trials to indicate the preferential value of any particular antimicrobial. The general effect appears to be good and a high rate of recovery is recorded with antimicrobials parenterally combined with immobilization provided treatment is begun in the early stages of the disease. Cows past their sixth month of pregnancy are unlikely to recover completely and commonly relapse.
Rumenotomy
Surgical removal of the foreign body through a rumenotomy incision is widely used as a primary treatment. It has the advantage of being both a diagnostic procedure in the first instance and a satisfactory treatment. The recovery rate varies, depending on when the surgery is done relative to the time of initial penetration, but is approximately the same as that obtained with the conservative treatment described above. In both instances 80–90% of animals recover compared with about 60% in untreated animals. Failure to improve is usually due to involvement of other organs or to the development of locally extensive peritonitis and reticular abscesses associated with persistent penetration of the foreign body or, uncommonly, generalized peritonitis.
Based on follow-up ultrasonography of cows that had surgery for traumatic reticuloperitonitis, the inflammatory adhesions resolved and disappeared in the majority of animals by 6 months.6 As a consequence, reticular function normalizes. In animals with severe adhesions, there is a marked disturbance of digesta passage and, in these animals, extensive abscesses are present.
Persistent penetration by the foreign body necessitates removal for optimum results but a rumenotomy is necessary to determine the extent of the lesion. Radiography and ultrasonography as described above may assist in determining the presence and location of the foreign body. A single preoperative dose of antimicrobial such as potassium penicillin G at 10 million IU given intravenously is recommended to avoid complications after a rumenotomy in cattle.
The recovery rate after surgery is likely to be much lower if only complicated cases are selected for rumenotomy and conservative treatment is given to the early mild cases. In one series the recovery rate in the cases treated conservatively was 84% and in those difficult cases treated surgically it was 47%.
Drainage of reticular abscesses
Reticular abscesses may be drained through an ultrasound-guided transcutaneous incision.8
Choice of treatment
The choice of treatment is largely governed by economics and the facilities and time available for surgery. A rumenotomy, satisfactorily performed, is the best treatment but is unnecessary in many cases because of the tendency of the foreign body to return to the reticulum. A commonly used practice is to treat the animal conservatively for 3 days and if marked improvement has not occurred by that time to consider a rumenotomy. A rumenotomy is highly desirable in cows in the last 3 months of pregnancy if severe sequelae are to be avoided. Movement of the cow during the early stages of the disease is undesirable because of the risk of disrupting the adhesions that localize the infection.
Cases of chronic traumatic reticuloperitonitis are best treated by rumenotomy because of the probability that the foreign body is still embedded in the wall. Acute diffuse peritonitis is highly fatal but if detected early daily treatment with broad-spectrum antimicrobials may be effective.
PREVENTION
All processed feed should be passed over magnets to remove metallic material before being fed to cattle. The use of synthetic string instead of wire has resulted in a major decrease in the incidence of the disease.
Reticular magnets
Small cylindrical or bar magnets, 7.5 cm long by 1.0–2.5 cm diameter with rounded ends, are used to prevent the disease but are also used in acute cases to minimize penetration of the foreign body. When given orally to normal healthy animals the magnets locate in the reticulum within a few days, where they remain indefinitely and maintain their magnetic pull. The magnets attract foreign bodies, which then do not penetrate the reticular wall as easily as when they are free. The extensive prophylactic use of these magnets in a dairy herd has reduced the incidence of the disease and its complications by 90–98%. The magnets are given to herd replacement heifers at 18 months to 2 years of age as part of a herd health program.
The effects of magnets in traumatic reticulitis was examined in the Danish study of cows at slaughter (see under Etiology).3 Two magnets tested were cylindrical cage magnets with different fields of magnetic force. Magnet I had a magnetic force of attraction of 110 mT; magnet II had a force of 210 mT. Magnets were found in only 7% of the cows. There were no lesions in 97% of the cows with magnets. Magnet II was superior to magnet I in attracting all types of foreign bodies, including tire wires. Thus the prophylactic use of magnets should be promoted to reduce the occurrence of foreign body lesions.3
It is unlikely that magnets will extract a firmly embedded foreign body from the wall of the reticulum but loosely embedded ones with long free ends may be returned to the reticulum and loose foreign bodies will be immobilized. The position of the foreign body within the reticulum greatly influences the efficacy of treatment with a magnet. A foreign body at an angle to the ventral aspect of the reticulum of more than 30° is less likely to become attached to a magnet than a foreign body situated horizontally on the ventral aspect of the reticulum.25 There have been only a few reports of physical injury to the wall of the reticulum being caused by the magnets or the foreign bodies that may be attached to them. A compass can be used to locate the presence and position of the magnet.
Braun U. Atlas und Lehrbuch der Ultraschall-diagnostik beim Rind. Berlin: Blackwell Wissenschafts-Verlag, 1997.
Braun U. Ultrasonography in gastrointestinal disease in cattle. Vet J. 2003;166:112-124.
Braun U. Ultrasound as a decision-making tool in abdominal surgery in cows. Vet Clin North Am Food Anim Pract. 2005;21:33-53.
1 Harwood D. Vet Rec. 2004;154:574.
2 Monies R. Vet Rec. 2004;154:735.
3 Cramers T, et al. Vet Rec. 2005;157:287.
4 Grohn YT, Bruss ML. J Dairy Sci. 1990;73:655.
5 Dohoo IR, et al. Prev Vet Med. 1984;2:655.
6 Herzog K, et al. Dtsch Tierarztl Wochenschr. 2004;111:57.
7 Braun U, et al. Vet Rec. 1993;133:416.
8 Braun U, et al. Vet Rec. 1998;142:184.
9 Rehage J, et al. J Am Vet Med Assoc. 1995;207:1607.
10 Hailat N, et al. Can Vet J. 1993;34:698.
11 Sheldon IM. Vet Rec. 1995;136:126.
12 Ward JL, Ducharme NG. J Am Vet Med Assoc. 1994;204:874.
13 Jafarzadeh SR, et al. Prev Vet Med. 2004;65:1.
14 Farrow CS. Vet Clin North Am Food Anim Pract. 1999;15:397.
15 Fubini SL, et al. J Am Vet Med Assoc. 1990;197:1060.
16 Partington BP, Biller DS. Vet Radiol. 1991;32:155.
17 Braun U, et al. Vet Rec. 1993;132:103.
18 Kaske M, et al. J Vet Med A. 1994;41:748.
19 Braun U. Atlas und Lehrbuch der Ultraschall-diagnostik beim Rind. Berlin: Blackwell Wissenschafts-Verlag, 1997.
20 Braun U. Vet J. 2003;166:112.
21 Braun U, Gotz M. Am J Vet Res. 1994;55:325.
22 Braun U. Vet Clin North Am Food Anim Pract. 2005;21:33-53.
23 Braun U. Vet Rec. 2002;150:580.