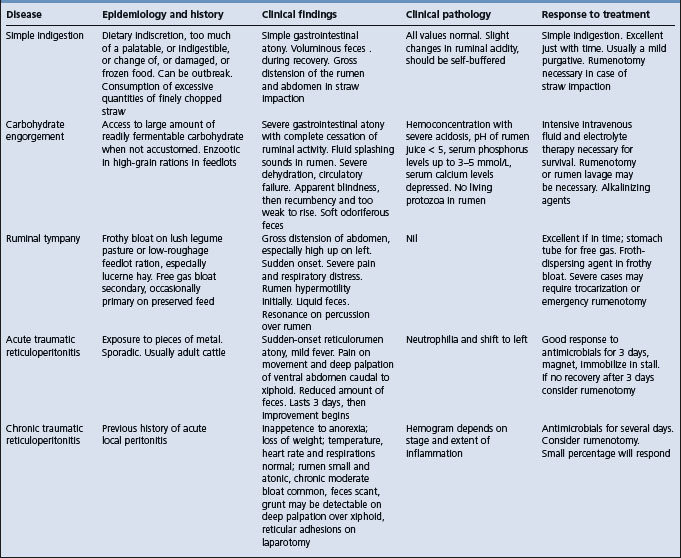
Chapter 6 Diseases of the alimentary tract – II
Diseases of the forestomach of ruminants
Forestomach motility of ruminants, especially cattle, is of major concern to the veterinarian. Evaluation of forestomach motility is an integral part of the clinical examination and differentiation of forestomach abnormalities into primary and secondary causes and is essential for diagnosis and accurate therapy. Application of the knowledge of the physiology of normal reticulorumen motility can improve the diagnosis, prognosis and therapy for diseases of the forestomach.1,2 A brief review of the clinical aspects of the motility of the reticulorumen is presented here.
ANATOMY AND PHYSIOLOGY
The ruminant forestomach compartments, consisting of the reticulum, rumen and omasum, is like a fermentation vat. The animal exerts some control over the fermentation process by selecting the feed, adding a buffer-like saliva, and providing continual agitation and mixing with specialized contractions of the forestomach. Reticulorumen motility insures a consistent flow of partially digested material into the abomasum for further digestion.
The forestomach can be divided into primary structures: the reticulorumen and the omasum; they are functionally separated by a sphincter: the reticulo-omasal orifice. The reticulorumen of an adult cow occupies almost the entire left half of the abdominal cavity and has a capacity of up to 90 kg of digesta. Because of its large size and ease of clinical examination, rumen motility is considered to represent digestive functions in the ruminant.
Both parasympathetic and sympathetic nerves supply the reticulorumen but only the former nerves stimulate motility. Parasympathetic innervation occurs through the vagus nerve, which is predominantly sensory from the forestomach. Sympathetic innervation to the forestomach consists of numerous fibers from the thoracolumbar segment; these fibers join at the celiac plexus to form the splanchnic nerve. The splanchnic nerve can inhibit motility, but normally there is little or no tonic sympathetic drive to the forestomach.
RETICULORUMEN MOTILITY
Four different specialized contraction patterns can be identified in the forestomach:
• Secondary or eructation cycle
• Rumination (associated with cud chewing and associated with the primary cycle)
• Esophageal groove closure (associated with sucking of milk).
It is important for the clinician to understand the motility pattern of each cycle. Specific diseases of the forestomach have characteristic alterations in motility, which aid in the diagnosis and prognosis.
Primary contraction cycle
The primary cyclic activity results in the mixing and circulation of digesta in an organized manner. The primary contraction in cattle begins with a biphasic contraction of the reticulum. The first reticular contraction forces ingesta dorsal and caudad into the rumen, as does the much stronger second reticular contraction. The dorsal ruminal sac then begins to contract as the ventral sac relaxes, thereby causing digesta to move from the dorsal to the ventral sac. Sequential contractions of the caudoventral, caudodorsal and ventral ruminal sacs force digesta back into the reticulum and cranial sac. After a brief pause the contraction sequence is repeated. During each reticular contraction fluid and food particles, particularly heavy grain, pass into the reticulo-omasal orifice and into the omasum and abomasum.
Reticulorumen motility results in stratification of ruminal contents, with firmer fibrous material floating on top of a more fluid layer. Solid matter remains in the rumen until the particle size is sufficiently small (1–2 mm in sheep,2–4 mm in cattle) to pass through the reticulo-omasal orifice. The size of digested plant fragments in ruminant feces can therefore be considered an indirect measurement of forestomach function.
Identification of ruminal contractions requires both auscultation and observation of the left paralumbar fossa. Sound is produced when fibrous material rubs against the rumen during contraction. Only slight sound is produced when the rumen contains small quantities of fibrous material.
External palpation of the rumen is valuable in determining the nature of ruminal contents. The normal rumen feels doughy in the dorsal sac and more fluid ventrally; the difference in consistency is attributable to stratification of ruminal contents. Very liquid ruminal contents that splash and fluctuate on ballottement (fluid-splashing sounds) are suggestive of lactic acidosis, vagal indigestion, ileus or prolonged anorexia.
Rumen hypomotility or hypermotility is usually associated with a change in the type of sounds heard during auscultation, with gurgling, bubbling or distant rustling sounds replacing the normal crescendo– decrescendo crackling sounds. The rumen can be examined and evaluated using a combination of auscultation and simultaneous ballottement or percussion, by palpation through the left flank and by rectal examination. Inspection and laboratory analysis of rumen contents is also possible.
Control of primary contractions
The primary contraction cycle of the reticulorumen is a complex and organized contraction initiated, monitored and controlled by the gastric center in the medulla oblongata. These cycles are mediated by the vagus nerve. The reticulorumen is under extrinsic nervous control compared to the remainder of the gastrointestinal tract. It is also affected by hormones and smooth muscle tone.
The gastric center is bilaterally paired and located in the dorsal vagal nucleus in the medulla. The gastric center has no spontaneous rhythm of its own but acts as a processor and integrator of afferent information. Various excitatory and inhibitory inputs are brought together to determine both the rate and strength of contraction.
Ruminal atony
Ruminal atony, seen in lactic acidosis and endotoxemia, can be attributed to one or more of the following factors:
• Direct depression of the gastric center, usually associated with generalized depression and severe illness (toxemia)
• Absence of excitatory inputs to the gastric center
• Increase in excitatory inhibitory inputs to the gastric center
• Failure of vagal motor pathway (Table 6.1).
Table 6.1 Effects of some common clinical excitatory and inhibitory influences on primary cycle movements of the reticulorumen
| Clinical afferent input | Clinical findings and responses to treatment | |
|---|---|---|
| Excitatory inputs | ||
| Low threshold reticular tension receptors | ||
| Increased reticular tension | Increases frequency, duration and amplitude of primary cycle contractions and mixing promotes fermentation | |
| After feeding | ||
| Mild ruminal tympany | ||
| Decreased reticular tension | Decreases frequency, duration and amplitude of primary cycle contractions and decreases fermentation | |
| Starvation | ||
| Anorexia | ||
| Lesions of medial wall of reticulum | Cause hypomotility of rumen contractions and may be explanation for atony in some cases of | |
| Chronic induration and fibrosis due to traumatic reticuloperitonitis | vagus indigestion. Some cases are characterized by erratic hypermotility | |
| Acid receptors in abomasum | Increase primary cycle movements, which increases flow of ruminal contents into abomasum to maintain optimum volume and to decrease acidity | |
| Increases in abomasal acidity following emptying of organ | ||
| Buccal cavity receptors | Increased reticulorumen activity | |
| Following eating | ||
| Inhibitory inputs | ||
| High-threshold reticular tension receptors | ||
| Peak of reticular contraction | Depression of primary cycle movements, ruminal hypomotility, depression of fermentation because of failure of mixing | |
| Severe ruminal tympany | ||
| Ruminal impaction with forage, hay, straw (not necessarily grain overload) | ||
| Abomasal tension receptors | ||
| Impaction, distension or displacement of abomasum | Abomasal impaction, dilatation and torsion may result in complete ruminal stasis. Left-side displacement of abomasum usually does not cause clinically significant hypomotility | |
| Pain | ||
| Visceral pain due to distension of abomasum or intestines. Severe pain from anywhere in body | Moderate to total inhibition of reticulorumen movements possible with visceral pain. The degree of inhibition from pain elsewhere will vary | |
| Depressant drugs | ||
| Anesthetics, central nervous system depressants | Inhibition of primary and secondary cycle movements and of eructation, resulting in ruminal tympany | |
| Prostaglandin E | ||
| Changes in rumen content | ||
| Marked decrease (< 5) or increase (> 8) in pH of ruminal fluid. Engorgement with carbohydrates or protein-rich feeds. | Inhibition of primary and secondary cycle movement and lack of fermentation. Cud transfer promotes return to normal activity | |
| Absence of protozoa in ruminal acidosis and in lead and other chemical poisoning | ||
| Changes in body water, electrolytes and acid–base balance | ||
| Hypocalcemia | Inhibition of primary and secondary cycle movements and of eructation, resulting in ruminal tympany which responds to treatment with calcium | |
| Dehydration and electrolyte losses, acidosis, alkalosis | ||
| Peritonitis | ||
| Traumatic reticuloperitonitis | Inhibition of primary and secondary cycle movements and of eructation, resulting in ruminal tympany. Return of primary movements is good prognostic sign. Lesions must heal without involvement of nerve receptors or adhesions that will interfere with normal motility | |
| Toxemia/fever | ||
| Peracute coliform mastitis | Inhibition of primary and secondary cycle movements, which return to normal with treatment of toxemia | |
| Acute bacterial pneumonia | ||
| Ruminal distension | ||
| Early ruminal tympany | Increased frequency of secondary cycle movements and of eructation | |
| Covering of cardia (fluid or form) | ||
| Ruminal tympany | Cardia does not open; failure of eructation, resulting in ruminal tympany. Clearance of cardia results in eructation | |
| Recumbent animal | ||
Most of the sensory inputs are transmitted to gastric centers in the dorsal vagal nerve nuclei from which the efferent outputs originate and pass down the vagal motor nerve fibers.
Source: modified from Leek BF. Vet Rec 1969; 84:238.
Hypomotility
Hypomotility is a reduction in the frequency or strength of extrinsic contractions, or both, and usually is caused by either a reduction in the excitatory drive to the gastric center or an increase in inhibitory inputs.
Properties of contractions
The frequency of primary contractions is determined from information accumulated during the quiescent phase of motility. Frequency provides a rough estimate of the overall health of a ruminant. In cows, the frequency of primary contractions averages 60 cycles per hour but decreases to 50 cycles per hour during rumination and even lower when the cow is recumbent. Feeding increases the rate to up to 105 cycles per hour. Because of this variability, the clinician should auscultate the rumen for at least two minutes before determining the frequency of contractions.
The strength and duration of each contraction are determined by information obtained just before and during the contraction and are therefore more dependent on the nature of the forestomach contents than is frequency of contraction. The strength of contraction is subjectively determined by observing the movement of the left paralumbar fossa and assessing the loudness of any sounds associated with ruminal contraction.
The distinction between frequency and strength is important clinically, particularly in reference to therapy of reticulorumen hypomotility. When feed is withheld from sheep for 4 days, the rate of forestomach contractions remains unchanged but the strength of contractions progressively decreases because of changes in ruminal contents.
Extrinsic control of primary contractions
Excitatory inputs to the gastric center
Tension and chewing movements are two major excitatory inputs to the gastric center. Low-threshold tension receptors deep in the circular smooth muscle layer detect reticulorumen distension. The greatest density of receptors is found in the medial wall of the reticulum and dorsal ruminal sac. These low-threshold tension receptors send afferent impulses along the dorsal or ventral vagus nerve to the gastric center, where they excite extrinsic reticulorumen contractions. Prolonged anorexia, leading to a smaller reticulorumen volume, decreases this excitatory input. Feeding increases reticulorumen volume, thus leading to a prolonged increase in forestomach motility.
Buccal receptors, which are stimulated during feeding, are also excitatory to the gastric center. These are mechanoreceptors, and their effect is mediated by the trigeminal nerve. This reflex increases the rate of primary contractions only but is short-lived and wanes with time. The stimulatory response of feeding also has a higher brain center component: the sight of feed can increase the frequency of primary contractions by 50% during a period of 4–5 minutes. Rumination, in comparison with feeding, is accompanied by a lower than normal primary contraction rate.
Other relatively minor excitatory inputs to the gastric center include milking, environmental cold and a decrease in abomasal pH. Milking or udder massage of dairy goats markedly increases the frequency and strength of primary contractions. In a cold environment, the ruminant increases the frequency of forestomach contractions, thereby maximizing the fermentation rate and helping to maintain body temperature.
Inhibitory inputs to the gastric center
The four most important inhibitory inputs to the gastric center are fever, pain, moderate to severe rumen distension and increased ruminal volatile fatty acid concentrations.
Fever
Fever has been associated with decreased rumen motility. Endogenous pyrogens may cause prolonged forestomach hypomotility or atony often seen in cattle with endotoxemia due to bacterial infections. Pyrogens directly affect the gastric center in the hypothalamus, and opioid receptors mediate their action.
Endotoxemia
Endotoxemia is common in cattle and often associated with fever, anorexia and rumen atony. Inhibition of forestomach motility during endotoxemia is thought to be a combination of two different pathways: a prostaglandin-associated mechanism and a temperature-independent mechanism. The former can be attenuated by administration of nonsteroidal anti-inflammatory drugs (NSAIDs). Therapy for endotoxin-induced hypomotility or atony includes the use of antimicrobials for the underlying cause of the inflammation and NSAIDs for the effects of the endotoxemia.
Pain
Pain may be associated with rumen hypomotility or atony. Painful stimuli act directly on the gastric center, although modification of reticulorumen motility in response to painful stretching of viscera can be partially attributed to catecholamine release. The sympathetic nervous system response to pain can also stimulate splanchnic motor nerves, thereby directly inhibiting reticulorumen motility.
Because of their stoic nature, the only clinical evidence of pain in ruminants may be anorexia and depressed forestomach motility. Prostaglandins have been implicated in increasing the sensitivity to pain both locally and centrally, and NSAIDs are indicated for alleviation of pain associated with inflammation. Other analgesics are of limited usefulness in the treatment of pain-induced forestomach hypomotility. Xylazine, an excellent sedative-analgesic for ruminants, causes a dose-dependent inhibition of reticulum contractions.
Distension of forestomach
Moderate to severe forestomach distension exerts an inhibitory influence on reticulorumen motility. Epithelial receptors located in the ruminal pillars and papillae of the reticulum and cranial rumen sac respond to mechanical stimulation (stretch)as well as changes in ruminal volatile fatty acid concentration. These receptors, also known as high-threshold tension receptors, are stimulated continuously during severe rumen distension. The opposing actions of low- and high-threshold tension receptors help to control the fermentation process and maintain an optimum reticuloruminal volume. A good example of their activities is the motility changes evident with some forms of vagus indigestion.
Ruminal volatile fatty acids
The ruminal volatile fatty acid concentration also influences forestomach motility. Epithelial receptors detect the concentration of nondissolved volatile fatty acids in ruminal fluid, which is normally high enough to produce a tonic inhibitor input to the gastric center. Volatile fatty acids in the reticulorumen exist in both the dissociated and nondissociated forms, with the degree of ionization being governed by the rumen pH and the pKa of each particular acid. Ruminal atony in animals with lactic acidosis results from elevated levels of nondissociated volatile fatty acids in ruminal fluid, with the decrease in rumen pH changing more of the volatile fatty acids into a nondissociated form. Systemic acidosis does not appear to contribute to ruminal atony, although increased volatile fatty acid concentrations in the abomasum may reduce forestomach motility.
Abomasal disease
Diseases of the abomasum influence forestomach motility. Abomasal distension may contribute to the decreased forestomach motility often observed with abomasal volvulus, impaction or right-sided dilatation. Abomasal tension receptors detect overfilling and reflexly decrease reticuloruminal movements, thus reducing the rate of flow of ingesta into the abomasum. Ruminal hypomotility is not always observed in left-side displacement of the abomasum even though appetite may be decreased.
Effect of depressant drugs
General anesthetics and other depressant drugs acting on the central nervous system also inhibit reticulorumen motility by a direct effect on the gastric center.
Intrinsic control of primary contractions
The contribution of intrinsic smooth muscle tone to forestomach motility is not well understood. Intrinsic contractions are involved in maintaining normal reticulorumen tone, directly influencing the discharge of low-threshold tension receptors to the gastric center. Calcium is required for smooth muscle contraction and hypocalcemia will usually cause ruminal atony. The administration of calcium borogluconate to cattle, sheep and goats with hypocalcemia will restore rumen motility and eructation commonly occurs after the intravenous administration of the calcium.
Treatment of forestomach hypomotility
Anorexia and forestomach hypomotility usually exist together. Reduced feed intake reduces the two primary drives for reticulorumen activity: moderate forestomach distension and chewing activity. A wide variety of drugs have been used for many years to induce forestomach motility with the aim of stimulating anorexic cattle with forestomach hypomotility to begin eating. Most if not all of these drugs have been unsuccessful. Ruminatorics such as nux vomica, ginger, gentian and tartar given orally have not been effective. Parasympathomimetics, such as neostigmine or carbamylcholine, should not be used to treat forestomach atony. Neostigmine requires vagal activity to be effective and therefore cannot incite normal primary contractions in atonic animals. Neostigmine may increase the strength of a primary contraction without altering rhythm or coordination. Carbamylcholine causes hypermotility in sheep but the contractions are uncoordinated, spastic and functionless.
Any effective drug must be able to induce forestomach motility in a coordinated sequence so that the ingesta moves through the reticulo-omasal orifice, into the omasum, out of the omasum, and into the abomasum, and out of the abomasum into the small intestine. This means that there must be a coordinated sequence of contractions and relaxations of sphincters. Experimentally, metoclopramide increases the rate of ruminal contractions and therefore might be beneficial in rumen hypomotility or motility disturbances associated with vagal nerve damage.
Secondary cycle contraction and eructation
Secondary cycles are contractions that involve only the rumen and are associated with the eructation of gas. They occur independently of the primary cycle contractions and usually less frequently, about once every 2 minutes. The contraction rate depends on the gas or fluid pressure in the dorsal sac of the rumen. Secondary cycles can be inhibited by severe distension of the rumen.
Normally, the dorsal sac of the rumen contains a pocket of gas composed of CO2,, N2 and CH4. Gas is produced at a maximum rate of 1 L per minute in cattle, with the rate depending on the speed of microbial degradation of ingesta. Eructation occurs during both primary and secondary contraction cycles but most gas is removed during the latter. Eructation is capable of removing much larger quantities of gas than is produced at the maximum rates of fermentation and therefore free gas bloat does not occur because of excessive gas production but rather from insufficient gas elimination.
Ruminal contractions are essential for eructation. Tension receptors in the medial wall of the dorsal ruminal sac initiate the reflex by means of the dorsal vagus nerve. Contractions begin in the dorsal and caudodorsal ruminal sacs and spread forward to move the gas cap ventrally to the cardia region. Contraction of the reticuloruminal fold is necessary to stop fluid from moving forward to the reticulum and covering the cardia. Receptors in the cardia region detect the presence of gas; the cardia remains firmly closed if fluid or foam (as in frothy bloat) contacts it. Injury to the dorsal vagal nerve decreases the efficiency of eructation but either the ventral or dorsal vagus nerve alone can initiate enough eructation activity to prevent bloat.
Despite the presence of normal secondary contractions, eructation may not occur in recumbent animals when the cardia is covered with fluid. Bloat is often observed in ruminants in lateral recumbency. Eructation occurs after the animal stands or attains sternal recumbency as fluid moves away from the cardia. Bloat can also result from peritonitis, abscesses or masses that distort the normal forestomach anatomy and preventing active removal of fluid from the cardia region. Esophageal obstructions associated with intraluminal, intramural or extraluminal masses are a common cause of free gas bloat. Passage of a stomach tube usually identifies these abnormalities, and forestomach motility is unimpaired unless the vagal nerve is damaged.
Bloat is often observed in cattle with tetanus. Distension of the rumen is usually not severe and can be accompanied by strong and regular ruminal contractions. Because the ruminant esophagus is composed of striated muscle throughout its length, tetanus-associated bloat may be due to spasm of the esophageal musculature.
Persistent mild bloat is often observed in ruminants that have rumen atony or hypomotility secondary to systemic disease. Although the fermentation rate is lower than normal in these cases, ruminal contractions are not strong enough to remove all the gas produced. The bloat usually requires no treatment and resolves with return of normal forestomach motility.
Secondary contractions cannot be distinguished from primary contractions by auscultation of the left paralumbar fossa only, unless a synchronous belch of gas is heard. However, primary contractions can be identified by simultaneous palpation of the left paralumbar fossa and auscultation with the stethoscope over the left costochondral junction between the seventh and eighth ribs. Reticular contractions indicating the beginning of a primary contraction can be heard followed by contraction of the dorsal sac and lifting of the paralumbar fossa.
Secondary contractions are relatively autonomous and are not subject to the same central excitatory and/or inhibitory influences as are primary contractions. Agents that inhibit reticulorumen motility by a central action have a lesser effect on eructation than on primary contraction cycles. However, high doses of xylazine can inhibit secondary contractions and the duration of inhibition is dose-dependent.
No drugs are yet available to improve secondary contractions as a means of treating bloat. Severe bloat usually arises from mechanical or diet-related causes, and therapy should be directed specifically to those causes.
Rumination
Rumination is a complex process and consists of:
Rumination is initiated by the rumination center close to the gastric center in the medulla oblongata. Rumination allows further physical breakdown of feed with the addition of large quantities of saliva and is an integral part of ruminal activity. The time devoted to rumination is determined by the coarseness of ruminal contents and the nature of the diet. Rumination usually commences 30–90 minutes after feeding and proceeds for 10–60 minutes at a time, resulting in up to 7 hours per day spent on this activity.
The epithelial receptors located in the reticulum, esophageal groove area, reticulorumen fold and ruminal pillars detect coarse ingesta and initiate rumination. The receptors can be activated by increases in volatile fatty acid concentration, stretching and mechanical rubbing.
An intact dorsal or ventral vagus nerve is necessary for regurgitation to proceed. Regurgitation is associated with an extra contraction of the reticulum immediately preceding the normal reticular biphasic contraction of the primary cycle. The glottis is closed, and an inspiratory movement lowers the intrathoracic pressure. The cardia then relaxes, and the distal esophagus fills with ingesta. Reverse peristalsis moves the bolus up to the mouth, where it undergoes further mastication.
The usual causes for a reduction or absence of rumination are:
• Reticulorumen hypomotility or atony
• Central nervous system depression
• Liquid ruminal contents such as a high-concentrate diet with no coarse fiber
Other less common causes include chronic emphysema (difficulty in creating a negative thoracic pressure) and extensive damage to the epithelial receptors that incite the reflex, as occurs in rumenitis.
Reticulorumen motility is required for rumination to proceed. The extra reticular contraction is not essential for regurgitation because fixation or removal of the reticulum does not prevent rumination from occurring. Rumination can be easily inhibited by higher brain centers, as disturbance of a ruminating cow often stops the process and is absent when animals are stressed or in pain. Milking commonly elicits rumination in cows and goats.
Pharmacologic stimulation of regurgitation is not attempted.
Esophageal groove closure
The esophageal groove reflex allows milk in the sucking preruminant to bypass the forestomach, and directs milk from the esophagus along the reticular groove and omasal canal into the abomasum. Milk initiates the reflex by chemical stimulation of receptors in the oral cavity, pharynx and cranial esophagus. Once the reflex is established in neonatal ruminants, sensory stimuli (visual, auditory, olfactory) can cause esophageal groove closure without milk contacting the chemoreceptors. This occurs in calves teased with milk or given water in an identical manner to which the calf previously received milk. The esophageal groove reflex continues to operate during and after the development of a functional rumen, provided the animal continues to receive milk.
Liquid administered to calves with an esophageal feeder (tube) does not cause groove closure. In calves younger than 3 weeks of age, overflow of liquid from the rumen into the abomasum begins when 400 mL of liquid are given. Thus if the goal of oral feeding is to insure that fluid administration by esophageal tube rapidly enters the abomasum, more than 400 mL of liquid must be given.
Closure of the esophageal groove in cattle younger than 2 years of age can be induced by solutions of sodium chloride, sodium bicarbonate or sugar. From 100–250 mL of 10% solution of sodium bicarbonate induces esophageal groove closure in 93% of cattle immediately and it lasts for 1–2 minutes. Any other oral solution administered during this time is directed into the abomasum to avoid dilution in the rumen. Closure of the groove may be used to treat abomasal ulcers if magnesium hydroxide or kaolin– pectin solutions are given orally immediately after a sodium bicarbonate solution.
RUMINANT GASTROINTESTINAL DYSFUNCTION
The clinical findings which suggest primary ruminant gastrointestinal dysfunction include the following:
• Inappetence to anorexia, failure to ruminate
• Dropping regurgitated cuds occurs occasionally and is associated with straw impaction of the rumen, vagus indigestion, esophageal dilatation and rumenitis
• Visible distension of the abdomen, which may be asymmetrical or symmetrical, dorsal or ventral or both. Distension of the left dorsal abdomen because of ruminal tympany is most common
• The abdomen may appear gaunt or empty
• The rumen may feel abnormal on palpation through the left paralumbar fossa. It may feel more doughy than normal, distended with gas, fluid filled, or it may not be palpable
• Ruminal atony or hypermotility observed visually and detectable on auscultation and palpation
• Abdominal pain, usually subacute and characterized by humping of the back, reluctance to move or acute colicky signs of kicking at the abdomen and stretching. Pain may also be detectable on deep palpation of the abdomen if there is peritonitis, either local or diffuse
• Abnormal feces. The feces may be absent, reduced in amount or voluminous, and the composition may be abnormal. In carbohydrate engorgement the feces are usually increased in amount and are sweet–sour smelling. In most other diseases of the ruminant stomachs the feces are reduced in amount (scant), are pasty and foul-smelling and appear overdigested because of the increased transit time in the alimentary tract. A complete absence of feces for 24–48 hours is not uncommon with diseases of the ruminant stomach and may be confused with an intestinal obstruction or the earliest stages of hypocalcemia in a recently calved mature cow
• The temperature, heart rate and respirations are variable and may be within normal ranges. With an inflammatory lesion such as acute peritonitis, a fever is usually present. In acute diffuse peritonitis with toxemia, the temperature may be normal or subnormal; in subacute and chronic peritonitis the temperature is usually normal. In most other diseases of the ruminant stomachs except carbohydrate engorgement and abomasal torsion, where dehydration, acidosis and gastric infarction occur, vital signs may be within the normal range.
The differential diagnosis of the diseases associated with gastrointestinal dysfunction in cattle is summarized in Table 6.2.
In contrast with most other parts of the ruminant alimentary tract, and with the stomach of nonruminants, specific lesions of the mucosa of the forestomachs are uncommon. Penetration of the reticular wall by metallic foreign bodies is a common disease and is dealt with below under the heading of traumatic reticuloperitonitis, but it is the peritonitis that causes interference with ruminal motility. Rarely, there are actinomycotic or neoplastic lesions at the fundus of the reticulum that interfere with the proper functioning of the esophageal groove and lead to a syndrome of vagus indigestion described later. Rumenitis does occur commonly but only as a secondary change in acute carbohydrate engorgement and it is this that has such damaging effects on gut motility and fluid and electrolyte status and eventually kills most cows. The rumenitis may have a long-term effect on ruminal motility but its main significance is as a portal for infection leading to the development of hepatic abscesses. Ingested animal hairs, plant spicules and fibers are also credited with causing rumenitis but no clinical signs have been associated with the lesions. Because of the high prevalence of rumenitis lesions in cattle on heavy concentrated feed, especially when the feed is awned barley, the awns have been incriminated as traumatic agents. In acute arsenic poisoning there is an early postmortem dehiscence of the ruminal mucosa but no apparent lesions during life.
Other lesions of the forestomachs are parakeratosis, discussed below, and villous atrophy, sometimes encountered in weanling ruminants on special diets low in fiber, even succulent young pasture, but these are not known to influence stomach function or motility. The factors that principally affect ruminal motility are those chemical and physical characteristics of its contents that are dealt with in simple indigestion and acute carbohydrate engorgement. Lesions in, and malfunctioning of, the abomasum are much more akin to abnormalities of the stomach in monogastric animals.
Some of the physiological factors that affect reticulorumen function and the clinical factors which cause reticulorumen dysfunction are summarized in Table 6.1. When reticulorumen hypomotility is present the problem is to decide if the cause is directly associated with the forestomach and abomasum, or both, or other parts of the alimentary tract, or if the cause is due to an abnormality of another system. Differentiation requires a careful clinical examination, including simple laboratory evaluation of the rumen contents.
The factors that affect the motility of the rumen are presented in the section on simple indigestion, as are the principles of treatment in cases of ruminal atony.
Constable PD, Hoffsis GF, Rings DM. The reticulorumen: normal and abnormal motor function. Part I. Primary contraction cycle. Compend Contin Educ Pract Vet. 1990;12:1008-1014.
Constable PD, Hoffsis GF, Rings DM. The reticulorumen: normal and abnormal motor function. Part II. Secondary contraction cycles, rumination, and esophageal groove closure. Compend Contin Educ Pract Vet. 1990;12:1169-1174.
Special examination of the alimentary tract and abdomen of cattle
When gastrointestinal dysfunction is suspected, a complete special clinical and laboratory examination may be necessary to determine the location and nature of the lesion. A systematic method of examination is presented here.
HISTORY
A complete history, with as much detail as is available, should be obtained. The stage of the pregnancy–lactation cycle, days since parturition, the nature of the diet, the speed of onset and the duration of illness may suggest diagnostic possibilities. An accurate description of the appetite will suggest whether the disease is acute or chronic. The previous treatments used and the response obtained should be determined. Any evidence of abdominal pain and its characteristics should be determined. The nature and volume of the feces may suggest enteritis or alimentary tract stasis.
SYSTEMIC STATE, HABITUS AND APPETITE
The vital signs indicate the severity of the disease and suggest whether it is acute, subacute or chronic. In acute intestinal obstruction, abomasal torsion, acute diffuse peritonitis and acute carbohydrate engorgement, the heart rate may be 100–120/min and dehydration is usually obvious. Pallor of the mucous membranes is an indicator of alimentary tract hemorrhage, especially if there is concurrent melena. If cattle with any of the above diseases are recumbent and unable to stand, the prognosis is usually unfavorable. A marked increase in the rate and depth of respirations associated with alimentary tract disease usually indicates the presence of fluid or electrolyte disturbances and possible subacute pain. Grunting or moaning suggests abdominal pain associated with distension of a viscus or acute diffuse peritonitis.
The appetite and the presence or absence of rumination are very reliable indicators of the state of the alimentary tract, including the liver. Complete anorexia persisting for more than 3–5 days is unfavorable. The return of appetite and rumination with chewing of the cud following medical or surgical treatment for alimentary tract disease is a favorable prognostic sign. Persistent inappetence suggests a chronic lesion, usually with an unfavorable prognosis.
ORAL CAVITY AND ESOPHAGUS
The oral cavity is easily examined by inspection and manual palpation with the aid of a suitable mouth speculum. The patency of the esophagus is determined by passage of a stomach tube into the rumen through the oral cavity, with the aid of a cylindrical metal speculum, or through the nasal cavity.
INSPECTION OF THE ABDOMEN
The contour or silhouette of the abdomen should be examined from the rear, and each lateral region viewed from an oblique angle. Examination of the contour can assist in determining the cause of abdominal distension. Abdominal distension may be unilateral, bilaterally symmetrical or asymmetrical or more prominent in the dorsal or ventral half. Recognition of the anatomical region of maximum distension suggests diagnostic possibilities, which are set out in Figure 6.1. The differential diagnosis of abdominal distension of cattle is summarized in Table 6.3.
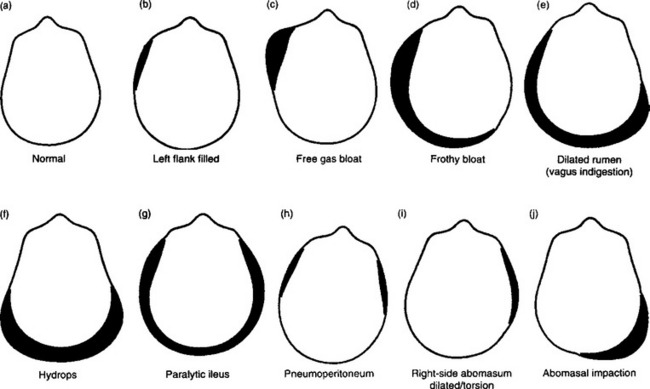
Fig. 6.1 Silhouettes of the contour of the abdomen of cattle, viewed from the rear, with different diseases of the abdominal viscera. (After Stober M, Dirksen G. Bovine Pract 1977; 12:35–38.)
Table 6.3 Differential diagnosis of abdominal distension in cattle
| Cause | Major clinical findings and methods of diagnosis |
|---|---|
| Distension of rumen | |
| Acute ruminal tympany | Marked distension of left abdomen, less of right. Very tense distended left paralumbar fossa, dull resonance on percussion. Pass stomach tube and attempt to relieve gas or froth |
| Vagus indigestion | Marked distension of left abdomen, less of right ‘papple-shaped’ abdomen. Fluctuating rumen on palpation. Excessive rumen activity or complete atony. Large L-shaped rumen on rectal examination. Pass large-bore stomach tube to remove contents to aid in diagnosis |
| Grain overload | Moderate distension of left flank, less of right. Rumen contents are doughy or fluctuate. Fluid-splashing sounds may be audible on ballottement. Rumen static and systemic acidosis. Rumen pH below 5 |
| Simple indigestion | Moderate distension of left flank; rumen pack easily palpable and doughy. Contractions may be present or absent depending on severity. Systemically normal. May be dropping cuds |
| Distension of abomasum | |
| Right displacement of abomasum and torsion (volvulus) | Right flank and paralumbar fossa normal to severely distended. Ping. Rectal palpation of fluctuating or tense viscus in right lower quadrant |
| Abomasal impaction | Right lower flank normal to moderately distended. Doughy viscus palpable caudal to costal arch. Rectal palpation feel doughy viscus in right lower quadrant |
| Left displacement of abomasum | Abdomen usually gaunt. Occasionally distended left paralumbar fossa due to displaced abomasum. Ping on percussion over upper aspects of ribs 9–12 |
| Abomasal trichobezoars | Older calves (2–4 months). Right lower flank distended. Fluid-splashing sounds. Painful grunt on deep palpation. |
| Confirm by laparotomy and abomasotomy | |
| Distension of intestines | |
| Enteritis | Slight to moderate distension of right abdomen. Fluid-rushing and splashing sounds on auscultation and ballottement. |
| Diarrhea and dehydration | |
| Intestinal obstruction | Slight to moderate distension of right abdomen. Fluid tinkling, percolating and splashing sounds on auscultation and ballottement. May palpate distended loops of intestine or intussusception rectally. Scant dark feces. Paracentesis abdominis |
| Paralytic ileus | Slight to moderate distension of right abdomen. Tinkling sounds on auscultation. Tympanitic ping on percussion. |
| Loops of distended intestine palpable per rectum. Scant feces but recover if no physical obstruction | |
| Cecal dilatation and torsion | Right flank may be normal or moderately distended. Ping present in right paralumbar fossa. Palpate movable blind end cecum on rectal examination. Confirm by laparotomy |
| Enlargement of uterus | |
| Physiological | Gross distension of both flanks, especially right. Normal pregnancy with more than one fetus. May palpate rectally |
| Pathological | |
| Hydrops amnion | Gradual enlargement of lower half of abdomen in late gestation. Flaccid uterus, fetus and placentomes are easily palpable per rectum |
| Hydrops allantosis | Gradual distension of lower half of abdomen in late gestation. Palpable uterus rectally, cannot palpate placentomes or fetus |
| Fetal emphysema | History of dystocia or recent birth of one calf, twin in uterus and emphysematous. Diagnosis obvious on vaginal and rectal examination |
| Fluid accumulation in peritoneal cavity | |
| Ascites | |
| Congestive heart failure, ruptured bladder | Bilateral distension of lower abdomen. Positive fluid waves. Paracentesis abdominis. May feel enlarged liver behind right costal arch |
| Pneumoperitoneum | |
| Perforated abomasal ulcer, postsurgical laparotomy | Not common. Bilateral distension of dorsal half of abdomen. Ping both sides |
DISTENSION OF THE ABDOMEN
The cause of distension of the abdomen of cattle is determined by a combination of the following examinations:
• Inspection of the contour or silhouette of the abdomen to determine the region of maximum distension
• If necessary, relief of rumen contents with a stomach tube to determine if the distension is due to an enlarged rumen. The ruminal contents can also be examined grossly at the same time
• Percussion or ballottement and simultaneous auscultation to detect fluid-splashing sounds indicating the presence and location of gas- and fluid-filled viscera
• Rectal examination to feel any obvious enlargements or abnormalities
• Abdominocentesis to determine the nature and amount of peritoneal fluid, which may indicate the presence of ischemic necrosis of intestines or peritonitis
• Trocarization of severely gas-filled distended regions, such as an abomasal volvulus in a calf.
LAVAGE OF DISTENDED RUMEN
In adult cattle presented with severe abdominal distension due to gross distension of the rumen it is difficult, if not impossible, to assess the status of the abdomen. To determine if the rumen is distended and/or to relieve the pressure, a large-bore stomach tube should be passed into the rumen. In vagus indigestion, the rumen may be grossly distended with fluid contents that will gush out through a large-bore tube. In some cases 100–150 L of rumen contents may be released. If no contents are released the contents may be frothy or mushy and the rumen end of the tube will plug almost instantly. Rumen lavage may then be attempted using a water hose to deliver 20–40 L of water at a time, followed by back drainage by gravity flow. After the rumen is partially emptied it is usually possible to more accurately assess the rumen and the abdomen.
LEFT SIDE OF ABDOMEN AND RUMEN
Inspection and palpation
The primary and secondary cycle contractions of the reticulorumen are identified by simultaneous auscultation, palpation and observation of the left paralumbar fossa and the left lateral abdominal region. During contractions of the rumen there is an alternate rising and sinking of the left paralumbar fossa in conjunction with abdominal surface ripples. The ripples reflect reticulorumen contractions and occur during both the primary (or mixing) cycle contraction and the secondary (or eructation) cycle contractions.1 As the left paralumbar fossa rises during the first part of the primary cycle contraction there are two horizontal ripples that move from the lower left abdominal region up to the paralumbar fossa. When the paralumbar fossa sinks, during the second part of the primary cycle, the ripple moves ventrally and fades out at the lower part of the left abdominal region. Similar ripples follow up and down after the rising and sinking of the paralumbar fossa associated with the secondary cycle movements.
In vagus indigestion, there may be three to five vigorous incomplete contractions of the reticulorumen per minute. These contractions may not be audible because the rumen contents are porridge-like and do not cause the normal crackling and rustling sounds of the rumen containing coarse fibrous ingesta. However, the contractions are visible and palpable as waves of undulations of the left flank. If reticulorumen motility is assessed only on the basis of inspection and palpation, the results will be misleading.
Nature of rumen contents
The nature of the rumen contents can be assessed by palpation of the rumen through the left paralumbar fossa. In the roughage-fed animal, the rumen contents are doughy and pit on pressure. In cattle that have consumed large quantities of unchopped cereal grain straw, the rumen is large and the contents feel very firm but not hard; they always pit on pressure. In the dehydrated animal the contents may feel almost firm. In the grain-fed animal the contents may be soft and porridge-like. When the rumen contains excessive quantities of fluid, the left flank fluctuates on deep palpation. In the atonic rumen distended with excess gas the left flank will be tense, resilient and tympanitic on percussion.
In mature cattle that have been anorexic for several days, the rumen may be smaller than normal and the dorsal sac will be collapsed (rumen collapse). There will be a ‘pung’ (low-pitched ping) in the left upper abdomen extending dorsally to the transverse processes of the lumbar vertebrae, lack of abdominal distension, absence of fluid upon succession of the area of the ping, and on rectal palpation the dorsal sac of the rumen will feel collapsed.2
Auscultation of the rumen and left flank
In the normal animal on a roughage diet there are two independent contraction sequences of the reticulorumen. The primary cycle recurs approximately every minute and consists of a diphasic contraction of the reticulum followed by a monophasic contraction of the dorsal ruminal sac and then by a monophasic contraction of the ventral ruminal sac. These movements are concerned primarily with ‘mixing’ the rumen contents and with assisting the passage of rumen contents into the omasum.
The secondary cycle movements occur at intervals of about 2 minutes and are confined to the rumen and consist of a contraction of the dorsal sac followed by a contraction of the ventral sac. The former causes the fluid contents of the dorsal sac to be forced ventrally and the gas layer to be forced cranially to the region of the cardia where eructation takes place. Contractions of the dorsal and ventral sacs cause undulations of the left paralumbar fossa and lower flanks that are readily visible and palpable.
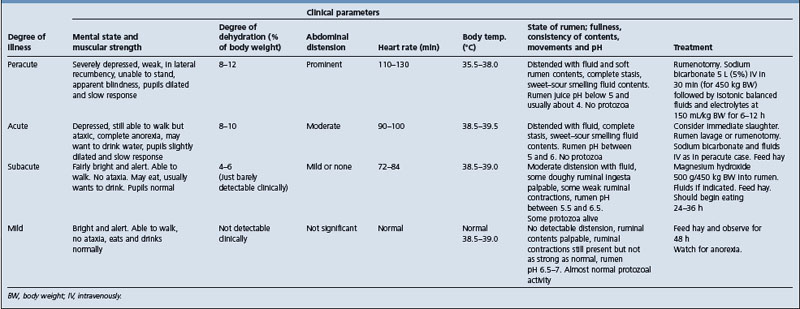
The clinical recognition of the presence or absence of either the primary cycle or secondary cycle contractions or both may aid in determining the cause and severity of the disease and the prognosis. These are outlined in Table 6.1.
Auscultation of rumen
To auscultate the rumen, the stethoscope is placed in the middle of the left paralumbar fossa. After two complete contractions have occurred, the stethoscope is moved cranially in the fossa and cranial to the fossa over the dorsal third of the 10th–13th ribs to determine if rumen contractions are audible in the region, which commonly becomes occupied with a left-side displacement of the abomasum. In the normal animal, ruminal contractions are audible in this region.
The type, strength and frequency of rumen movements should be noted. The rumen sounds of the normal animal consuming roughage are rasping, rustling, exploding and booming-crackling sounds. When the rumen contains less coarse roughage or primarily grain, the sounds may be much less distinct but still possess a crackling characteristic.
Fluid-tinkling or fluid-splashing sounds. The presence of fluid-tinkling or fluid-splashing sounds over the left paralumbar fossa, usually along with an atonic rumen, suggests the presence of excessive quantity of liquid contents in the rumen, and that the coarse ingesta is not floating on the fluid layer of the rumen contents as in the normal animal. Fluid-splashing sounds suggest diseases such as grain overload, or an atonic rumen associated with prolonged anorexia (chronic diffuse peritonitis, abomasal or omasal impaction). Fluid-splashing and -tinkling sounds can also be elicited by ballottement and simultaneous auscultation of the left lower flank in left-side displacement of the abomasum, because of its liquid contents. To assist in the differential diagnosis, the outline of the rumen can be auscultated and percussed to observe a much wider area of metallic sound than is normally expected in left-side displacement of the abomasum.
In vagus indigestion with an enlarged hypermotile rumen, the contractions of the rumen occur more frequently than normal, at 3–6/min, and are easily visible as prominent abdominal ripples over the left flank. But characteristically, the ruminal sounds are usually not audible or barely so because the rumen contents are homogeneous and porridge-like as a result of prolonged maceration in the rumen. The absence of coarse fiber in the ingesta and the lack of coordinated reticulorumen primary and secondary contractions minimizes the intensity of the ruminal sounds. The lack of effective secondary cycle contractions and eructation results in frothy bloat. Complete atony and gross distension of the rumen is characteristic of advanced vagus indigestion.
Percussion and simultaneous auscultation of the left paralumbar fossa over an area extending from the mid-point of the ninth rib to the 13th rib is used to detect the presence of a ‘ping’ or high-pitched metallic tympanic sound associated with left-side displacement of the abomasum. Percussion is performed with a flick of the flexed finger or most reliably with a percussion hammer. The causes of ‘pings’ on percussion of the left abdomen in mature cattle include left-side displacement of the abomasum, atonic rumen and, rarely, pneumoperitoneum. The tympanic sound associated with an atonic rumen is lower-pitched than that associated with a left-side displacement of the abomasum and may be called a ‘pung’.
For special investigations of reticulorumen motility radiotelemetry capsules can be placed in the rumen.3
RIGHT SIDE OF ABDOMEN
The contour of the right side of the abdomen should be examined by inspection for evidence of distension, which may be due to a viscus filled with fluid, gas or ingesta, ascites or a gravid uterus. In severe distension of the rumen, the ventral sac may also distend the lower half of the right flank.
A combination of deep palpation, ballottement and simultaneous percussion and auscultation, and succussion (slightly rocking the animal from side to side) is used to detect the presence of viscera that are distended with gas and/or fluid, or ingesta.
The causes of ‘pings’ audible on auscultation and percussion over the right abdomen include:
• Right-sided dilatation and volvulus of the abomasum
• Cecal dilatation and torsion
• Gas-filled descending colon and rectum in a cow with persistent tenesmus
• Intestinal tympany of unknown etiology
• Torsion of the root of the mesentery in young calves
• Intussusception causing intestinal tympany
• Postpartum intestinal tympany, which occurs in the postparturient cow (for the first few days following parturition).
The causes of fluid-splashing sounds on ballottement and auscultation of the right flank include:
Palpation of a firm viscus in the right flank caudal or ventral to the right costal arch may be due to:
• Enlarged ventral sac of the rumen, which extends over to the right abdominal wall
• Enlargement of the liver. The liver must be grossly enlarged before it is palpable caudal to the right costal arch.
A rectal examination is necessary to identify the distended viscus associated with these abnormal sounds, and often a laparotomy is required.
EXAMINATION OF RUMEN FLUID
Examination of the rumen fluid is often essential to establish an accurate diagnosis of diseases of the forestomach. Rumen fluid can be obtained with a stomach tube passed into the rumen, the fluid being withdrawn with the vacuum of a stomach pump. The major difficulty is avoiding contamination of the sample with saliva, which can be avoided if a free flow of fluid is obtained. Specialized stomach tubes are available that are weighted and can be directed into the ventral sac to collect up to 500 mL of fluid.4 Rumen fluid samples can also be obtained by percutaneous aspiration of the ventral sac of the rumen on the lower left ventrolateral abdominal quadrant, horizontal with the patella and 20 cm caudal to the last rib. The site is prepared, xylazine sedation given and a 12–15 cm 16-gauge needle is thrust firmly and quickly perpendicular to the skin into the rumen. Rumen fluid is withdrawn with a syringe and pH is measured immediately with a portable pH meter or wide-range pH paper (pH values of 2–12).
ANALYSIS OF RUMEN FLUID
The color, depending on the feed to a limited extent, will be a green, olive green or brown green. At pasture, the color is very green; with root crops the color tends to be gray; and with silage or straw the color is mostly yellow-brown. The color of the rumen contents is milky-gray in grain overload and greenish-black in cases where rumen stasis is of long duration and where putrefaction is occurring within the rumen.
The consistency of the rumen fluid is normally slightly viscid, and watery rumen contents are indicative of inactive bacteria and protozoa. Excess froth is associated with frothy bloat as in primary ruminal tympany or vagus indigestion. The odor is normally aromatic and, although somewhat pungent, not objectionable to the nose. A moldy, rotting odor usually indicates protein putrefaction, and an intensely sour odor indicates an excess of lactic acid formation, due to grain or carbohydrate engorgement.
The pH of the rumen fluid varies according to the type of feed and the time interval between the last feeding and taking a sample for pH examination. The normal range, however, is between 6.2 and 7.2. High pH values (8–10) will be observed when putrefaction of protein is occurring in the rumen or if the sample is mixed with saliva. Low pH values (4–5) are found after the feeding of carbohydrates. In general, a value below 5 indicates carbohydrate engorgement and this pH level will be maintained for 6–24 hours after the animal has actually consumed the carbohydrate diet.
For experimental purposes, continuous monitoring of the pH of the rumen contents is possible with a pH probe containing a commercial microelectrode and a reference-electrode with a pressure-equalizing system placed in the reticulum.5 By feeding diets with changing composition it is possible to provoke marked changes in rumen pH. The probes are programmed to sample pH and temperature every 30 seconds.
Microscopic examination of a few drops of rumen fluid on a glass slide with a low-power field will reveal the level of protozoon activity. Normally 5–7 protozoons are active per low-power field. In lactic acidosis the protozoa are usually absent or a few dead ones are visible. The rumen fluid can be stained with Gram stain to determine the predominant bacterial flora, which are normally Gram-negative but in grain overload become Gram-positive.
Chloride concentration can be determined by centrifuging the fluid and analyzing the supernatant for chloride levels. These are normally 10–25 mEq/L in cattle and <15 mEq/L in sheep. Elevated rumen chloride concentrations result from abomasal reflux, ileus or high salt intake.
RECTAL PALPATION OF ABDOMEN
Some of the specific abnormalities of the digestive tract, which are commonly palpable on rectal palpation, include the following, which relates to Figure 6.2 (a–l), illustrating the abnormalities through a transverse section of the abdomen.
(b) L-shaped rumen: occurs commonly in vagus indigestion and other diseases of the rumen characterized by gradual distension of the rumen
(c) Cecal torsion: commonly palpable as long distended organ, usually movable, may feel the blind end
(d) Abomasal torsion: commonly palpable as tense viscus in lower right half of abdomen
(e) Abomasal impaction: not usually palpable in late pregnancy
(f) Left-side displacement of the abomasum: usually cannot palpate the displaced abomasum but can often feel rumen, which is usually smaller than normal
(g) Intussusception: not always palpable, dependent on location of intussusception and the size of the animal
(h) Mesenteric torsion: usually palpable
(i) Intestinal incarceration: commonly palpable
(j) Peritonitis: only palpable if peritoneum of posterior aspect of abdomen affected
(k) Lipomatosis: commonly palpable as ‘lumps’ in the abdomen and pelvic cavity
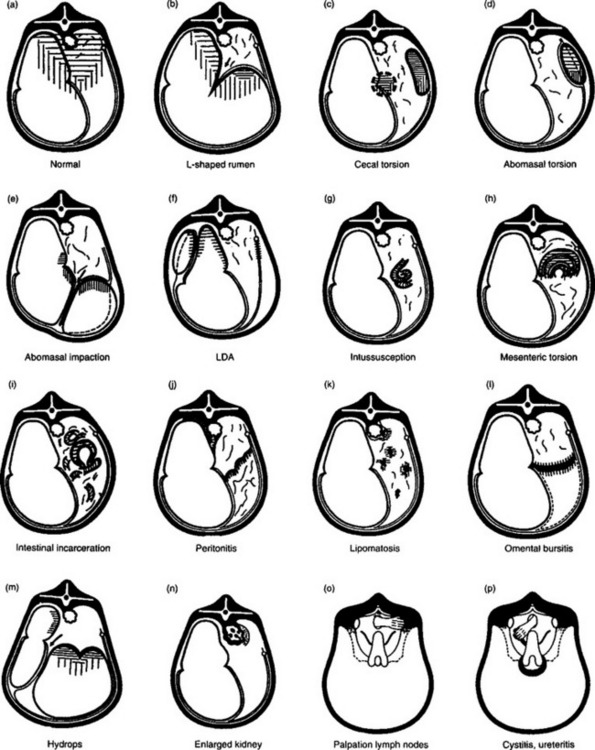
Fig. 6.2 Schematic illustration of the rectal findings in cattle affected with different diseases of the abdominal viscera. (After Stober M, Dirksen G. Bovine Pract 1977; 12:35–38.)
In Figure 6.2 (m–p) are included for the differential diagnosis of the diseases each represents.
As part of the differential diagnosis of digestive tract disease in the postparturient cow, the uterus should be examined carefully for evidence of retained placenta and metritis. Both vaginal and rectal examinations should be performed. The toxemia caused by retained fetal membranes and postpartum metritis may cause anorexia, rumen stasis, paralytic ileus, scant feces and sometimes an idiopathic postpartum ‘ping’ in the right flank, all of which may be misinterpreted as a primary digestive tract disease.
GROSS EXAMINATION OF FECES
The gross appearance of the feces of cattle is not only an indicator of disease of the digestive tract but can provide valuable clues for the differential diagnosis of disease elsewhere.
AMOUNT
In adult cattle, the passage of ingesta through the digestive tract takes 1.5–4 days. Mature cattle generally pass some feces every 1.5–2 hours, amounting to a total of 30–50 kg/day in 10–24 portions.
A reduction in the bulk of feces can be due to a decrease in feed or water intake or a retardation of the passage through the alimentary tract. In diarrhea, the feces are passed more frequently and in greater amounts than normal and contain a higher water content (>90%) than normal.
ABSENCE OF OR SCANT FECES
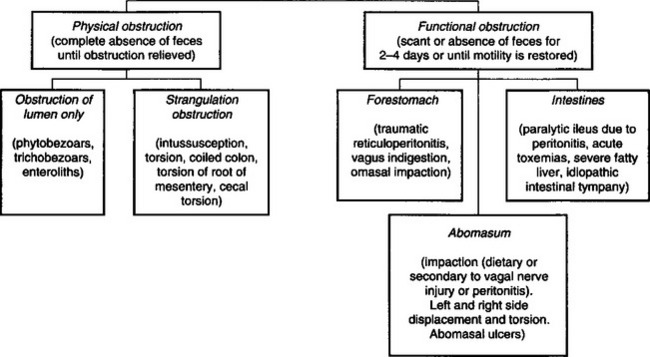
Failure to pass any feces for 24 hours or more is abnormal and the continued absence of feces may be due to a physical intestinal obstruction. However, in many cases the intestine is not physically obstructed but rather there is a functional obstruction. Diseases causing disturbances of motility of the rumen and abomasum often result in a relative absence of feces. Paralytic ileus of the intestines due to peritonitis or idiopathic intestinal tympany also result in a marked reduction in feces, sometimes a complete absence, for up to 3 days. The marked reduction of feces that occurs in functional obstruction is a major source of diagnostic confusion because it resembles physical obstructions of the intestines. The causes of physical and functional obstruction of the alimentary tract of cattle are summarized in Figure 6.3.
COLOR
The color of the feces is influenced by the nature of the feed, the concentration of bile in the feces and the passage rate through the digestive tract. Calves reared on cows’ milk normally produce gold-yellow feces, which become pale brown when hay or straw is eaten. The feeding of milk substitutes adds a gray component to a varying degree.
The feces of adult cattle on green forage are dark olive-green, on a hay ration more brown-olive, while the ingestion of large amounts of grain produces gray-olive feces. A retardation of the ingesta causes the color to darken. The feces become ball-shaped and dark brown with a shining surface due to the coating with mucus. Diarrheic feces tend to be paler than normal because of their higher water content and lower concentration of bile.
The presence of large amounts of bile produces a dark olive-green to black-green color such as in cattle with hemolytic anemia. In cattle with obstruction of the common bile duct, the feces are pale olive-green because of the absence of bile pigments.
Blood in the feces may originate from the following locations:
• Hemorrhage into the abomasum: acute hemorrhage usually appears as black, tarry feces (melena); chronic hemorrhage as occult blood
• Hemorrhagic enteritis of small intestines: the feces are uniformly dark red
• Hemorrhagic enteritis of the large intestines: in the cecum or colon, blood appears evenly distributed throughout the feces (dysentery); in the rectum, blood appears as streaks or chunks of frank blood unevenly distributed throughout the feces (hematochezia)
• ‘Occult blood’ is not visible grossly; the color of the feces may be normal or dark. An occult blood test (Hemetest tablets) is required to determine its presence. Occult blood occurs most commonly when there are only small quantities of blood in the alimentary tract, as with minimal hemorrhage insufficient to result in melena. It also may be due to the swallowing of blood coughed up from pulmonary hemorrhage.
ODOR
Fresh bovine feces are not normally malodorous. Objectionable odors are usually due to putrefaction or fermentation of ingesta, usually associated with inflammation. For example, the feces in cattle with salmonellosis may be fetid while in advanced pericarditis with visceral edema due to passive congestion the feces are profuse but not odoriferous.
CONSISTENCY
The consistency of the feces is dependent on the water content, the type of feed and the length of time the ingesta has remained in the digestive tract. Normally, milk-fed calves excrete feces of a medium to firm porridge-like consistency. After transition to a plant diet, the first solid particles begin to appear. Normal bovine feces are of a medium porridge-like consistency. A moderate thickening leads to the passage of fecal disks of a more solid consistency and severe dehydration causes the formation of firm balls of feces arranged in facets inside the rectum, the surfaces of which are dark and coated with mucus. The feces of cows with left-side displacement of the abomasum are commonly pasty in appearance. Sticky and tenacious feces are commonly seen in obstruction of the forestomachs (vagus indigestion, chronic peritonitis).
DEGREE OF DIGESTION
The proportion of poorly digested plant particles in the feces is dependent on the duration and adequacy of rumination and the rate of passage of ingesta through the forestomach and abomasum. The length of time the ingesta is in the postruminal digestive tract seems to have no appreciable influence on its digestion. Inadequate digestion indicates failure in rumination and/or accelerated passage of ingesta through the forestomach. Thus in some cattle with acute traumatic reticuloperitonitis, the feces may contain small walnut-sized chunks of undigested plant fibers that have escaped the cellulose digestive processes of the forestomachs. The presence of large numbers of kernels of grain in the feces is associated with the ingestion of large quantities of unprocessed grain such as whole wheat or barley.
OTHER SUBSTANCES IN THE FECES
Mucus
The presence of excessive mucus on the surface of feces suggests increased transit time of the ingesta in the large intestine. The presence of a plug of mucus in the rectum is suggestive of a functional obstruction (paralytic ileus). In enteritis, large quantities of clear, watery mucus may be passed, which sometimes clot to form gelatinous masses.
DETECTION OF ABDOMINAL PAIN
Cattle with acute local or diffuse peritonitis may grunt spontaneously with almost every expiration; this is usually exaggerated in the recumbent position. However, grunting may also be caused by severe pneumonia, pleurisy and severe pulmonary emphysema. Careful auscultation and percussion of the lungs is therefore necessary to exclude the presence of pulmonary disease.
Not all grunts occur spontaneously. Deep palpation of the cranial part of the abdomen using the closed hand or knee is often necessary to elicit a grunt in cattle. Auscultation over the trachea is often necessary to hear the grunt. The grunt is best elicited if pressure is applied to the abdomen at the end of inspiration and the beginning of expiration. The inspiratory and expiratory sounds are noted for 6–8 respirations by auscultation over the trachea and then, without warning to the animal, firm palpation is applied to the abdomen. A grunt indicates the presence of a peritoneal lesion (stretching or inflammation of the peritoneum regardless of cause). The absence of a grunt does not preclude the presence of a peritoneal lesion. In acute traumatic reticuloperitonitis the grunt may be present for only 3–5 days after the initial penetration of the reticulum.
A rigid bar or wooden pole may be necessary to apply pressure in large cattle (large cows and bulls). The bar is held by two people in a horizontal position just behind the xiphoid sternum while a third person auscultates over the trachea when the bar is lifted firmly up into the abdomen. Simultaneous auscultation over the trachea insures that the grunt is heard. Several attempts should be made to elicit a grunt before concluding the absence of one. The ventral aspect and both sides of the abdomen should be examined beginning at the level of the xiphoid sternum and moving caudally to approximately the umbilicus. This will insure that the cranial and caudal aspects of the abdomen are examined for the presence of points of abdominal pain.
Pinching of the withers is also used to elicit a grunt. In the average-sized cow, pinching of the withers causes the animal to depress its back. In an animal with a painful lesion of the peritoneum, depression of its back will commonly result in a grunt, which is clearly audible by auscultation over the trachea and is often audible without the use of the stethoscope.
The term anterior abdominal pain is used to characterize the pain associated with several diseases of anterior abdomen of cattle, which would include traumatic reticuloperitonitis, hepatic abscesses, abomasal ulcers and intestinal obstruction. The differential diagnosis of the anterior abdominal pain would include diseases that cause thoracic pain such as pleuritis, pericarditis and severe pulmonary disease.6
CLINICAL EXAMINATION OF THE DIGESTIVE TRACT AND ABDOMEN OF THE CALF
Clinical examination of the digestive tract and abdomen of the calf may be more difficult than in the adult animal. The rumen in the preruminant calf is not yet functional, and thus cannot be used as an indicator of the state of the alimentary tract as in adult cattle. Also, rectal examination is not usually possible until the animal is about 10–12 months of age, depending on the breed. A digital examination of the rectum of young calves is useful to determine the nature and amount of feces. This may provide an indication of the presence of impending diarrhea. A complete absence of feces suggests the presence of an acute intestinal obstruction, acute diffuse peritonitis or atresia coli.
The oral cavity of the calf is easily examined and should be part of the clinical examination of every sick calf.
ABDOMINAL DISTENSION IN CALVES
Abdominal distension occurs commonly in calves under 2 months of age. If the distension is symmetrical it may be difficult to determine if it originates in the rumen, abomasum, intestines or peritoneal cavity.
Examination of the abdomen of the young calf includes inspection of the contour of the abdomen to determine the maximum area of any distension, deep palpation and ballottement of each flank to determine the presence of fluid-splashing sounds that indicate a fluid-filled viscus, and percussion and auscultation to determine the presence of a gas-filled viscus. Placing the calf’s hindquarters on the ground and allowing the viscera to move to the caudal part of the abdomen may allow visual inspection and palpation of a distended abomasum below the xiphoid sternum. With the calf in lateral recumbency, careful palpation and simultaneous auscultation may reveal the location of the distended viscus. However, it is often necessary to do an exploratory laparotomy to determine the cause. A stomach tube should always be passed into the rumen to relieve any pressure caused by the accumulation of gas or fluid. In the case of severe distension of the abdomen accompanied by severe abdominal pain (kicking, bellowing, rolling, getting up and lying down) it may be necessary to relieve pressure with a large-gauge needle (12–14-gauge, 75–100 mm; 3–4 in). The most common cause of severe abdominal distension in a young calf that can be relieved by trocarization is abomasal torsion.
Abdominocentesis is easily done in the calf and at least three punctures should be attempted before concluding the absence of fluid. To avoid puncture of the abomasum, sites that are caudal to the umbilicus are used. (See Abdominocentesis in Ch. 5.)
The differential diagnosis of the common causes of abdominal distension in the calf is set out in Table 6.4.
Table 6.4 Differential diagnosis of diseases of the digestive tract and abdomen of young calves presented with distension of the abdomen
| Disease | History, clinical and laboratory findings, treatment |
|---|---|
| Abomasal torsion (volvulus) | Always acute to peracute, 1 week to 6 months of age, acute abdominal pain, bellowing, up and down, severe tight distension of abdomen, loud ping and fluid-splashing right side, emergency surgery necessary; recovery about 50% if recognized and corrected early |
| Abomasal dilatation (fluid, milk, hair balls and often abomasal ulcers) | Chronic or acute onset, calves 1–6 months of age, history of abnormal feces, may be unthrifty, mild to moderate abdominal distension and pain, fluid-splashing sounds over right flank, dehydration, negative peritoneal fluid, laparotomy and abomasotomy required |
| Perforated abomasal ulcers | Acute onset, sudden collapse, calves 2 weeks to 3 months, hand-fed or nursing calves, weakness, recumbency, tachycardia, mild to moderate abdominal distension, mild or no abdominal pain, abdominal splinting occasionally, positive paracentesis, feces variable. Laparotomy required; survival about 25% |
| Torsion of root of mesentery | Sudden onset, found in state of collapse, abdominal pain common, moderate abdominal distension, distended loops of intestine visible and palpable over right flank, bloodstained peritoneal tap, fluid-splashing sounds on palpation and auscultation, scant feces, emergency surgery |
| Acute diffuse peritonitis (not due to perforated abomasal ulcer) | Usually in calves under 3 weeks of age. Toxemia, temperature variable, weak, may be grunting, splinting of abdominal wall, mild abdominal distension, scant feces, fluid-splashing sounds over right flank (due to paralytic ileus), positive paracentesis, commonly associated with enteric colibacillosis, polyarthritis and umbilical and urachal abscess. Exploratory laparotomy. Prognosis poor |
| Atresia coli | Calf usually under 10 days of age, progressive distension of abdomen, bright and alert for first few days then becomes depressed, no feces only thick mucus from rectum, insertion of tube into rectum may lead to blind end but often blind end is near spiral colon. Surgery indicated but often unrewarding |
| Intussusception | May have history of diarrhea, now scant bloodstained feces, depressed, will not suck or drink, dehydrated, contour of abdomen may appear normal or slightly distended, fluid-splashing sounds and small ‘ping’ may be audible, bloodstained peritoneal fluid, presurgical diagnosis often difficult, surgery necessary. Recovery rate good if diagnosis early |
| Peracute to acute enteritis | Usually in calves under 3 weeks of age, acute onset of abdominal pain (kicking, stretching), won’t suck or drink, may not yet appear dehydrated, temperature variable, mild to moderate abdominal distension, fluid-splashing sounds on auscultation and succussion of abdomen, continuous loud peristaltic sounds on auscultation, diarrheic feces may not be present on first examination, digital examination of rectum may stimulate defecation of foul-smelling, soft, watery feces, peritoneal tap negative |
| Omphalitis, omphalophlebitis, umbilical abscess | Single calf, usually 2–6 weeks of age. May be unthrifty, chronic toxemia. Large, painful swelling of umbilicus that may be obvious externally or deep palpation dorsal to umbilicus reveals firm swellings directed towards liver or bladder. Surgical excision required |
| Gastrointestinal tympany of dietary origin | Calves under 10 days of age. Nursing calves sucking good cows. May be due to ingestion of excessive quantities of milk and excessive gas formation in abomasum and large intestine. Abdominal pain (kicking at abdomen), and pain on palpation of abdomen. Marked to severe abdominal distension. At laparotomy there is gaseous distension of the abomasum and cecum. Recovery is usually good |
| Intestinal hairball | Calves 3–8 weeks of age. Sudden onset of failure to suck. Normal vital signs. Total absence of feces. Slight to moderate distension of the abdomen, fluid-splashing sounds over right abdomen, normal peritoneal fluid. Will remain anorexic, and fail to pass any feces for up to several days. Hemogram normal. Metabolic alkalosis with hypokalemia, and hypochloremia may occur. Laparotomy and surgical removal of hairball required |
LAPAROSCOPY
Endoscopy of the abdomen through the right paralumbar fossa, left paralumbar fossa7 and cranioventral midline provides a safe alternative to exploratory celiotomy in cattle.8 Feed and water are withheld for 24 hours and the animals are sedated with acepromazine for both right and left paralumbar fossa laparoscopies and xylazine for the cranioventral approach. For laparoscopy through the fossae, the sites are prepared aseptically and a 2 cm incision is made through the skin and abdominal musculature after infiltration with 2% lidocaine. Each incision is made 8 cm ventral to the tip of the transverse process of the third lumbar vertebra and 5 cm caudal to the caudal aspect of the last rib. The laparoscope is introduced by standard technique and carbon dioxide gas is used to insufflate the abdominal cavity, after introduction of the trocar and cannula and prior to introduction of the laparoscope. The abdominal cavity is insufflated to a pressure of 20–24 mmHg. Each examination is completed by directing the laparoscope cranially then moving counterclockwise to examine the caudal portion of the abdomen. After the laparoscopy, the abdomen is passively deflated through the cannula and the skin is closed with sutures.
Cranioventral laparoscopy is performed with the animal positioned in dorsal recumbency. The incision for entry is made on the midline, through the linea alba, 10 cm caudal to the xiphoid process. Examination of the cranioventral portion of the abdomen is begun at the central aspect of the diaphragm then circularly moving the laparoscope counterclockwise.
Right paralumbar fossa laparoscopy provides excellent viewing of the caudal and right cranial portions of the abdomen for evaluation of diseases involving the right kidney, liver, diaphragm, small intestine, cecum, colon, reproductive tract and cranial part of the pelvic canal. Inadvertent penetration of the greater omentum or mesoduodenum may be avoided by careful placement of the trocar and periodic examination with the laparoscope to assess proper positioning of the cannula. Left paralumbar fossa laparoscopy provides excellent viewing of the left cranial portion of the abdomen and is appropriate for evaluation of diseases involving the left kidney, rumen, spleen and diaphragm.8
The cranioventral midline laparoscopy provides excellent visibility of the cranioventral portion of the abdomen. It allows evaluation of diseases involving the abomasum, liver, reticulum, spleen and diaphragm.8
DIAGNOSTIC IMAGING
Radiography of the cranial abdomen and reticulum of mature cattle is now being performed more frequently. Radiological examination of the reticulum with the animal in dorsal recumbency (dorsal reticulography) is an accurate diagnostic method for the evaluation of cattle with suspected traumatic reticuloperitonitis, and the techniques used are presented under that heading.
Ultrasonography is a suitable method for investigation of reticular contractions in healthy ruminants and in cattle for the diagnosis of traumatic reticuloperitonitis.9 In contrast to radiography, ultrasonography provides more precise information about the contour of the reticulum and reticular motility. It is an ideal diagnostic aid for the examination of gastrointestinal diseases of cattle including left and right displacement of the abomasum, abnormal motility of the small and large intestines, and cecal dilatation.9 It is done on the standing nonsedated animal using a 3.5 MHz linear transducer. The techniques used are presented under that heading.
INTERPRETATION OF CLINICAL FINDINGS
A guide to the interpretation of the clinical findings associated with diseases of the digestive tract and abdomen of cattle is summarized in Table 6.5. In conjunction with the history and the laboratory findings, a differential diagnosis list can be generated.
Table 6.5 Pathogenesis and interpretation of clinical findings associated with diseases of the digestive tract and abdomen of cattle
| Clinical findings | Pathogenesis, interpretation |
|---|---|
| Anorexia, inappetence | Toxemia, distension of intestines and stomachs, enteritis, peritonitis |
| Scant feces, includes small-volume diarrhea | Reduced feed intake, functional obstruction of forestomachs and abomasum, paralytic ileus, strangulation obstruction or obstruction of lumen of intestine with phytobezoar or trichobezoar |
| Large-volume diarrhea | Profuse, watery diarrhea usually associated with enteritis, simple indigestion or carbohydrate engorgement |
| Dehydration | Failure to drink adequate amounts of water (due to toxemia or lesions of oral cavity), malabsorption due to enteritis, diseases of the forestomachs interfering with absorption of water, e.g. vagus indigestion |
| Tachycardia | Toxemia, acid–base imbalance, abdominal pain, distension of intestines |
| Polypnea | Acid–base imbalance (torsion of the abomasum, severe enteritis, vagus indigestion), distension of the abdomen due to gas- or fluid-filled intestines |
| Weakness and recumbency Colic (abdominal pain) | Toxemia, severe dehydration, severe distension of abdomen, peritonitis |
| Sudden onset of distension of forestomachs, abomasum or intestines. Stretching of mesenteric bands. Strangulation of intestine in mesenteric tear or scrotal hernia | |
| Grunting with every respiration | Diffuse peritonitis (also pleuritis, pulmonary emphysema and advanced pneumonia), distension of stomachs or intestines |
| Presence of grunt on deep palpation of ventral abdominal wall | Presence of peritoneal lesion (stretching of the peritoneum, inflammation, edema, recent adhesions) |
| Abdominal distension | Most commonly due to gas- or fluid-filled intestines and/or forestomachs and abomasum. Rarely due to pneumoperitoneum. Also due to ascites and hydrops allantois/amnion |
| Rumen distension | May be distended with gas, fluid or ingesta. Primary dietary ruminal tympany and grain overload. Secondary ruminal tympany due to peritonitis, vagus indigestion |
| Rumen stasis | Toxemia, metabolic (hypocalcemia), fever, ruminal acidosis, distension of omasum or abomasum, peritonitis, vagal nerve injury |
| Hyperactive rumen | Early stages of primary dietary ruminal tympany; vagal nerve injury |
| Acidic rumen pH | Ruminal acidosis associated with carbohydrate engorgement; almost no other cause known |
| Alkaline rumen pH | Ruminal alkalosis associated with accidental consumption of high-protein diet, urea poisoning |
| Reduced or absent rumen protozoon activity | Ruminal acidosis (lactic acid inactivates protozoa); primary starvation lasting more than 2–3 days; ingestion of lead, arsenic and other poisonous substances |
| Abnormal foul-smelling rumen contents | Putrefaction of rumen contents in static and defaunated rumen |
| Presence of ‘ping’ or ‘pung’ over left flank | Left displacement of abomasum (ping), atonic rumen with a gas cap (pung), pneumoperitoneum (rarely) |
| ‘Ping’ over right flank | Right-side dilatation displacement and torsion of the abomasum, cecal dilatation and torsion, torsion of the spiral colon, gas in distended colon and rectum |
| Presence of low-pitched ‘pings’ not clearly distinct over right flank | Tympany of right paralumbar fossa in recently calved cows (2–3 days). |
| Gas in distended colon and rectum. Fluid- and gas-filled intestines with enteritis | |
| Distended upper right flank | Dilatation and torsion of abomasum. Cecal dilatation and torsion. Torsion of spiral colon |
| Distended lower right flank | Impaction of the abomasum. Enlarged L-shaped rumen and distension of ventral sac to the right flank. Advanced pregnancy |
| Fluid-splashing sounds on ballottement of abdomen or succussion | Fluid-filled intestines or forestomachs or abomasum. Usually associated with enteritis, paralytic ileus, or obstruction. Fluid-splashing sounds are rarely due to fluid in the peritoneal cavity. Percolating fluid sounds audible over right flank are common in cattle with acute intestinal obstruction |
| Dropping cuds | Cattle rarely regurgitate uncontrollably (dropping cuds). It is usually associated with chronic inflammatory lesions of the reticulum and cardia resulting in lack of control of regurgitation and a larger than normal bolus of rumen contents being regurgitated that cannot be controlled by the animal. Also occurs in certain heavy-metal poisonings such as arsenic poisoning. Cattle affected with straw impaction of the rumen will also drop large, dry, fibrous cuds |
EXPLORATORY LAPAROTOMY (EXPLORATORY CELIOTOMY)
An exploratory laparotomy can usually assist in the diagnosis of diseases of the digestive tract or abdomen. Identification and evaluation of the abnormality allows for a more accurate diagnosis, prognosis and rational treatment. However, because a properly done laparotomy is time-consuming and expensive, the veterinarian would like to minimize the number of laparotomies in which no significant lesions are present. The challenge is, therefore, to improve the accuracy of diagnosis and to evaluate the prognosis as much as possible before doing a laparotomy unnecessarily.
There are some well-recognized diseases in which, if a clinical diagnosis can be made, a laparotomy is indicated (Table 6.6). (In some cases slaughter for salvage may be more economical.)
Table 6.6 Diseases of the digestive tract and abdomen of cattle in which a laparotomy is indicated if the diagnosis can be made
| Disease | Major clinical findings |
|---|---|
| Left displacement of the abomasum (LDA) | ‘Ping’ over ribs 9–12 and other well-recognized findings |
| Right displacement (RDA) and torsion of the abomasum | Distension of upper right flank, ‘ping’ on percussion over ribs 9–12, viscus palpable per rectum |
| Cecal dilatation and torsion | Distension of upper right flank, ‘ping’ in right paralumbar fossa, long cylindrical mass palpable per rectum |
| Torsion of spiral colon | Distension of upper right flank, ‘ping’, distended loops of intestine easily palpable |
| Intussusception | Abdominal pain, absence of feces, distended loops of intestine, palpable intussusception |
| Phytobezoars or trichobezoars | Scant feces, subacute abdominal pain, distended loops of intestine and hard lumps palpable rectally |
| Severe life-threatening ruminal tympany | Severe distension of rumen, skin over rumen cannot be picked up, animal grunting, is lying down, mouth breathing, cannot relieve with stomach tube or trocar |
| Unidentifiable lumps palpable on rectal examination, i.e. fat necrosis | Chronic gastrointestinal atony, scant feces, large hard lumps palpable per rectum |
| Peracute grain overload | Weakness, recumbency, dehydration, tachycardia, rumen pH 5 (see Table 29.1 for guidelines in the treatment of grain overload) |
Other than the rumenotomy for the treatment of grain overload and the cesarean section, the most common indication for a laparotomy in cattle is for the surgical correction of displacement or obstruction of parts of the digestive tract (i.e. abomasal displacement, abomasal dilatation and volvulus, intussusception and volvulus, torsion of the root of the mesentery, torsion of spiral colon, cecal dilatation and torsion). If any of these diagnoses can be made, a laparotomy or slaughter is indicated.
In other cases, the diagnosis may be suspected, but is not obvious and the indications for a laparotomy, slaughter, euthanasia or conservative medical treatment are not clear. The major question is, ‘Under what conditions is a laparotomy indicated if the history and clinical and laboratory findings suggest an obstruction (strangulation obstruction or functional) but the obstruction cannot be located on clinical examination?’
Some examples of diseases that may elude diagnosis before laparotomy and that are or may be amenable to surgical correction include the following.
INTUSSUSCEPTION AND OTHER STRANGULATION OBSTRUCTIONS OF THE SMALL INTESTINES
An intussusception may be located in the anterior part of the abdomen and not palpable per rectum. A clinical history of acute onset of colic, absence of feces and serosanguineous exudate on peritoneal tap are indications for a laparotomy. However, phytobezoars and trichobezoars can cause acute intestinal obstruction which may not be palpable rectally and which becomes progressively more severe with time, and only minimal, if any, changes may occur in the peritoneal fluid. A progressively worsening systemic state warrants a laparotomy.
‘ATYPICAL’ LEFT-SIDE DISPLACEMENT OF THE ABOMASUM (ATYPICAL LDA)
A small percentage of cases are difficult to detect on auscultation and percussion. When the typical LDA ‘ping’ cannot be detected after several examinations over a period of a few days, a presumptive diagnosis may be made on the basis of ketosis in a recently calved cow (within the last week), the presence of rumen contractions, but reduced intensity, normal vital signs (unless fatty liver is present) and spontaneous fluid-gurgling sounds audible over the left flank or fluid-splashing sounds on ballottement and auscultation of the lower left flank.
TRAUMATIC RETICULOPERITONITIS
In traumatic reticuloperitonitis with a persistently penetrating foreign body, conservative medical treatment of immobilization in a stanchion, antimicrobials and a magnet may be unsuccessful even after several days of antimicrobial therapy. Diagnosis depends on continued anorexia, mild fever, grunt, rumen stasis, a hemogram indicating infection and peritoneal fluid containing exudate.
The guidelines for the indications of an exploratory laparotomy when a tentative diagnosis is not made are set out in Table 6.7.
Table 6.7 Clinical and laboratory indications for an exploratory laparotomy in cattle when the diagnosis is not obvious
| Parameter/criterion | Significance and interpretation of criteria |
|---|---|
| History | Does the history suggest an acute surgically correctable condition? |
| Abdominal distension | Laparotomy indicated if distension of abdomen caused by distension of abomasum, cecum or intestines with fluid and gas |
| Volume and nature of feces | Scant or absence of feces for more than 36–48 h indicates a physical or functional obstruction. In functional obstruction (i.e. peritonitis) some dark feces are usually present. In physical obstruction (intussusception) feces are very scant and dark red due to leakage of blood into intussusceptum. Laparotomy indicated unless can determine that cause of absence of feces is not surgically correctable (diffuse peritonitis or impaction of abomasum or omasum) |
| Rectal findings | Distended viscera other than rumen (abomasum, cecum, small and large intestines) warrant laparotomy. Palpable ‘bread and butter’ fibrinous inflammation in caudal part of abdomen suggests acute diffuse peritonitis and laparotomy would not be rewarding |
| Peritoneal fluid and hemogram | Bloodstained peritoneal exudate and a degenerative left shift in the leukocyte count suggest leakage of the intestinal wall and warrants laparotomy if history and clinical findings suggest a strangulation obstruction |
| Abdominal pain (colic) and grunting | Behavioral and postural signs of acute abdominal pain (colic) such as kicking at the belly, stretching the body, suggest acute distension of the stomachs or intestines with fluid and gas. Spontaneous grunting with each respiration, which usually becomes pronounced in sternal recumbency, or the presence of a grunt on deep palpation of the abdomen suggests inflammation or stretching of the peritoneum |
Braun U, editor. Atlas und Lehrbuch der Ultraschall-diagnostik beim Rind. Berlin: Parey Buchverlag. 1997:1-279.
Braun U. Ultrasonography in gastrointestinal disease in cattle. Vet J. 2003;166:112-124.
Cockcroft P, Jackson P. Clinical examination of the abdomen in adult cattle. In Pract. 2004; June:304-317.
Ivany JM, Rings DM, Anderson DE. Reticuloruminal disturbances in the bovine. Bovine Pract. 2002;36:56-64.
Radostits OM. Clinical examination of the alimentary system: Ruminants. In: Radostits OM, Mayhew IGJ, Houston DM, editors. Veterinary clinical examination and diagnosis. London: WB Saunders; 2000:409-468.
1 McCarthy PH. Am J Vet Res. 1981;42:255.
2 Rebhun WC. Cornell Vet. 1987;77:244.
3 Kath GS, et al. Am J Vet Res. 1985;46:136.
4 Geishauser T. Bovine Pract. 1994;28:109.
5 Enemark JMD, et al. J Vet Med A. 2003;50:62.
6 Henninger RW, Mullowney PC. Compend Contin Educ Pact Vet. 1984;6:S453.
7 Wilson AD, Ferguson JG. Aust Vet J. 1984;25:229.
Diseases of the rumen, reticulum and omasum
SIMPLE INDIGESTION
ETIOLOGY
The disease is common in dairy cattle and stall-fed beef cattle because of the variability in quality and the large amounts of feed consumed. It is not commonly observed in pastured beef cattle or sheep because they are less heavily fed. The common causes are dietary abnormalities of minor degree including indigestible roughage, particularly when the protein intake is low, moldy, overheated and frosted feeds, and moderate excesses of grain and concentrate intake.
Etiology Excessive feed intake (grain, silage); indigestible roughage
Epidemiology Usually in hand-fed dairy cattle and stall-fed beef cattle
Signs Inappetence, drop in milk production, lack of rumination, rumen usually full and reticulorumen contractions decreased or absent, vital signs are normal. Spontaneous recovery in 12–24 hours
Clinical pathology None needed except to rule out differential diagnoses. Lesions not fatal
Diagnostic confirmation Spontaneous recovery
Differential diagnosis list Early parturient hypocalcemia, acetonemia, traumatic reticuloperitonitis, carbohydrate engorgement, left-side displacement of the abomasum, right-side dilatation of abomasum, abomasal volvulus, vagus indigestion, phytobezoars, secondary ruminal atony in toxemia
Control Feeding management and provision of digestible feeds
Cases occur under excellent feeding regimens and are usually attributed to overfeeding with grain. Although the difference between simple indigestion and carbohydrate engorgement (grain overload) is one of degree, their separation can be justified by the marked clinical difference between the two syndromes. Gross overfeeding usually occurs when cattle or sheep gain accidental access to large quantities of grain or are suddenly introduced to high-grain diets in feedlots. Indigestion is more common when heavily fed cows are fed a little more concentrate than they can digest adequately. A sudden change to a new source of grain, especially from oats to wheat or barley, may have the same effect.
Indigestible roughage may include straw, bedding or scrub fed during drought periods. It is probable that limitation of the available drinking water may contribute to the occurrence of the disease during dry seasons. Depraved appetite may also contribute to the ingestion of coarse indigestible material. Although good-quality ensilage cannot be considered an indigestible roughage, cases of indigestion can occur in cattle that are allowed unlimited access to it. This is most likely to happen in heavy-producing cows running outside in cold weather whose hay and grain rations are limited. It is not uncommon for large Holstein cows to eat 45–50 kg of ensilage daily in such circumstances and the high intake of acetate and acetic acid may be sufficient to depress their appetite. Prolonged or heavy oral dosing with antimicrobials may cause indigestion due to inhibition of the normal ruminal flora. An unusual circumstance is the feeding of a special diet to produce milk, and dairy products, with a high content of polyunsaturated fats for special diets in humans. Fats in the diet are protected against hydrogenation in the rumen by a coating of formalin. The efficiency and safety of the diet depends on a thorough mixing of the formalin with the concentrates. If this is not done the free formalin causes severe rumenitis.
PATHOGENESIS
Primary atony caused by dietary abnormality is difficult to explain. Changes in the pH of its contents markedly affect the motility of the rumen and in cases caused by overeating on grain an increase in acidity is probably of importance. High-protein diets, including the feeding of excessively large quantities of legumes or urea, also depress motility because of the sharp increase in alkalinity that results. Atony that occurs after feeding on damaged feeds may have the same basis or be due to other unidentified agents in the food. The simple accumulation of indigestible food may physically impede ruminal activity. Putrefaction of protein may also play a part in the production of atony. The toxic amides and amines produced may include histamine, which is known to cause ruminal atony when given intravenously and to be reversed by the administration of antihistamine drugs. Histamine may contribute to the ruminal atony that occurs in allergy, or after heavy grain feeding, but the absorption of histamine from the forestomachs in any circumstances is probably very limited.
A marked fall in milk yield occurs, caused probably by the sharp decrease in volatile fatty acid production in a hypotonic reticulorumen. Rumen contractions appear to play the same role as hunger contractions in simple stomachs and the decreased food intake is probably due to the ruminal atony.
CLINICAL FINDINGS
A reduction in appetite is the first clinical finding, followed closely in milking cows by a slight drop in milk production. Both occur suddenly; the anorexia may be partial or complete but the fall in milk yield is relatively slight. The animal’s posture is unaffected but there is mild depression and dullness. Rumination ceases and the ruminal movements are depressed in frequency and amplitude and sometimes are almost absent. The rumen may be larger than normal if the cause is sudden access to an unlimited supply of palatable feed. There may be moderate tympany, especially with frozen or damaged feeds or in allergy, but the usual finding is a firm, doughy rumen without obvious distension. The feces are usually reduced in quantity and are drier than normal on the first day. However, 24–48 hours later the animal is commonly diarrheic; the feces are softer than normal, voluminous and commonly malodorous.
There is no systemic reaction and the heart rate, temperature and respirations are usually within normal ranges. Pain cannot be elicited by deep palpation of the ventral abdominal wall, although cows that have consumed an excessive quantity of a highly palatable feed such as silage, after not having had any for a long period of time, will have a grossly distended rumen, and mild abdominal discomfort may be present for several hours. The discomfort usually resolves when the rumen movements return to normal and the rumen returns to its normal size. Most cases recover spontaneously or with simple treatments in about 48 hours.
CLINICAL PATHOLOGY
Examination of the urine for ketone bodies is usually necessary to differentiate indigestion from acetonemia.
Two simple laboratory tests have been introduced to assess the activity of the ruminal microflora. The sediment activity test is carried out on aspirated ruminal fluid strained to remove coarse particles. The strained fluid is allowed to stand in a glass vessel at body temperature and the time required for flotation of the particulate material is recorded. The time in normal animals varies between 3 minutes, if the animal has just been fed, and 9 minutes, if the last feeding has occurred some time previously. Settling of the particulate material indicates gross inactivity, less severe degrees being manifested by prolongation of the time required for flotation. The cellulose digestion test is also performed on aspirated rumen fluid and depends upon the time required to digest a thread of cotton. A bead is tied to the end of the thread to indicate when separation occurs. Digestion times in excess of 30 hours indicate abnormality.
The rumen juice can be examined for pH using wide-range indicator paper. Values between 6.5 and 7.0 are considered normal. In cattle on grain diets, the pH may range from 5.5–6.0 normally but in cattle that have been on roughage diets such low values should arouse suspicion of lactic acidosis and careful monitoring is necessary.
NECROPSY FINDINGS
The disease is not a fatal one.
Simple indigestion must be differentiated from all the diseases of the forestomachs and abomasum in which ruminal atony is a common clinical finding, and from diseases of other body systems that cause secondary ruminal atony:
• Acetonemia: the appetite and milk production decrease over a few days, there is ketonuria and the rumen contractions are present but weaker than normal
• Traumatic reticuloperitonitis: there is a sudden onset of anorexia and agalactia, a mild fever, a painful grunt on deep palpation of the xiphoid sternum, and the rumen is static with an increase in the size of the gas cap
• Carbohydrate engorgement: characterized by depression, dehydration, tachycardia, staggering, recumbency, diarrhea and ruminal stasis with the presence of fluid-splashing sounds, and the pH of the ruminal fluid is usually below 6 and commonly down to 5
• Left-side displacement of the abomasum (LDA): usually occurs within a few days after parturition and the rumen is usually smaller than normal, the contractions are usually reduced in amplitude, there is a ping on percussion over the lower left flank, and ketonuria
• Right-side dilatation of abomasum: occurs most commonly in dairy cows 2–4 weeks post partum, there is inappetence, reduced feces, ruminal atony, reduced milk production and a ping over the right flank, and a distended viscus is palpable per rectum in the lower right quadrant
• Abomasal volvulus: anorexia, depression, reduced feces, dehydration, tachycardia, a ping over the right flank and a distended viscus in the lower right quadrant are common
• Vagal indigestion: characterized by gradual distension of the abdomen due to distension of the rumen over a period of several days, progressive dehydration and scant feces. Initially there is hypermotility of the rumen and the development of secondary frothy bloat. This is commonly followed by ruminal atony
• Phytobezoars: cause inappetence to anorexia, scant feces, and on rectal examination distended loops of intestine and the firm masses may be palpable
• Secondary ruminal atony: occurs in many diseases in which septicemia or toxemia (coliform mastitis) are present but there are usually additional clinical findings to indicate their presence
• Ruminal atony with mild bloat is common in the early stages of hypocalcemia, which may last for 6–18 hours, and is usually accompanied by anorexia and a decreased amount of feces. The ruminal motility and appetite return to normal following treatment with calcium borogluconate
• The rumen is also atonic in allergic and anaphylactic states and returns to normal following treatment.
TREATMENT
Spontaneous recovery
Most cases of simple indigestion recover spontaneously. Small quantities of fresh, good-quality, palatable hay should be provided several times daily to encourage eating and to stimulate reticulorumen motility. Because anorexia and forestomach hypomotility usually exist together the objective is to stimulate both appetite and motility. Reduced feed intake reduces the two primary drives for reticulorumen activity: moderate forestomach distension and chewing activity.
Rumenatorics
A wide variety of oral preparations containing rumenatorics were available for many years and is was conventional to administer these to stimulate reticulorumen motility and to stimulate appetite. These preparations contained nux vomica, ginger and tartar emetic in powder form to be added to water and pumped into the rumen. However, there is no evidence that they are effective and they are not recommended.1,2 The routine use of magnesium hydroxide for rumen disorders is not recommended unless there is evidence of ruminal acidosis.
Magnesium hydroxide is a potent alkalinizing agent for use in ruminants as an antacid and mild laxative. It can significantly decrease rumen microbial activity and should be used only in cattle with rumen acidosis and not for symptomatic therapy of idiopathic rumen disorders or hypomagnesemia. The oral administration of boluses of magnesium hydroxide (162 g) or a powdered form (450 g) dissolved in 3.5 L of water daily for 3 days resulted in a significant increase in rumen pH after 48 and 24 hours, respectively.3 Both the boluses and the powder forms of magnesium hydroxide decreased rumen protozoal numbers and increased methylene blue reduction times compared with baseline values. There was no change in blood pH, bicarbonate or base excess values.
Parasympathomimetics
These agents have also been used to stimulate reticulorumen activity but have the disadvantage of inducing undesirable side effects and being very transitory in effect. Large doses depress reticulorumen activity but small doses repeated at short intervals increase ruminal activity and promote vigorous emptying of the colon in normal animals. The normal flow of rumen contents from the reticulorumen to the abomasum is the result of a complex of synchronized contractions and relaxations of various parts of the forestomachs, orifices and abomasum occurring simultaneously. One of the major limitations of injectable parasympathomimetics used as rumenatorics is that they do not provide these synchronized movements and therefore little movement of ingesta can occur. Carbamylcholine chloride, physostigmine and neostigmine are most commonly used. Neostigmine is the most effective at a dose of 2.5 mg/45 kg body weight (BW). Carbamylcholine acts on the musculature only and causes uncoordinated and functionless movements. These drugs are not without danger, especially in very sick animals or those with peritonitis, and are specifically contraindicated during late pregnancy.
Experimentally, metoclopramide increases the rate of ruminal contractions and therefore might be beneficial in rumen hypomotility or motility disturbances associated with vagal nerve damage.1,2
Epsom salts (0.5–1.0 kg per adult cow) and other magnesium salts are reasonably effective and have the merit of simplicity and cheapness.
Alkalinizing and acidifying agents
If an excessive quantity of grain is the cause of the simple indigestion, the use of alkalinizers, such as magnesium hydroxide, at the rate of 400 g per adult cow (450 kg BW), is recommended when the rumen contents are excessively acid. Magnesium oxide or hydroxide should be used only if ruminal acidosis is present. The administration of 400 g of magnesium oxide to normal, mature, nonfasted cattle weighing 450 kg can cause metabolic alkalosis and electrolyte disturbances for up to 24 hours following treatment. A sample of rumen fluid can be readily obtained and the pH determined. If the rumen contents are dry, 15–30 L of water should be administered by stomach tube.
Acetic acid or vinegar, 5–10 L, is used when the rumen contents are alkaline as a result of the ingestion of high-protein concentrates.
Reconstitution of ruminal microflora
In cases of indigestion that have run a course of more than a few days, and in animals that have been anorexic for prolonged periods, there will be significant loss of ruminal microflora, especially if there have been marked changes in pH. Reconstitution of the flora by the use of cud transfers from normal cows is highly effective. An abattoir is the best source of rumen contents (especially rumen fluid) but it can be obtained from live animals by reaching into the mouth during rumination when the bolus is regurgitated. Rumen fluid may also be removed by siphoning from the rumen with a stomach tube or by vacuum withdrawal with a special pump. Best results are obtained if 20–30 L of water is pumped into the rumen and then allowed to siphon by gravity flow (rumen lavage). The rumen fluid to be transferred should be strained and administered as an oral drench or by stomach tube. Repeated dosing is advisable. The infusion will keep for several days at room temperature. Commercial products comprising dried rumen solids are available and provide some bacteria and substrate for their activity.
When affected animals resume eating they are best tempted by good, stalky meadow or cereal hay. Good-quality alfalfa (lucerne) or clover hay, green feed and concentrate may be added to the diet as the appetite improves.
Constable PD, Hoffsis GF, Rings DM. The reticulorumen: normal and abnormal motor function. Part I. Primary contraction cycle. Compend Contin Educ Pract Vet. 1990;12:1008-1014.
Constable PD, Hoffsis GF, Rings DM. The reticulorumen: normal and abnormal motor function. Part II. Secondary contraction cycles, rumination, and esophageal groove closure. Compend Contin Educ Pract Vet. 1990;12:1169-1174.
RUMEN IMPACTION IN SHEEP WITH INDIGESTIBLE FOREIGN BODIES
Rumen impaction in sheep with indigestible foreign bodies has been described in a semi-arid region of Nigeria.1 The sheep had visited refuse dumps around a town. Only certain breeds of sheep, the Yankasa, Uda and their crossbreeds, were found feeding on refuse dumps. Rumen-indigestible foreign bodies were present in 19.3% of the sheep slaughtered in the local abattoir. The foreign bodies were polythene/cellophane materials, ropes, dry seeds, caked sand, metallic objects, paper, fiber and hair balls. The polythene/cellophane materials were present in 81.6% of the sheep. Clinically, the rumen impaction was characterized by emaciation, abdominal distension and symmetry, lack of feces in the rectum, foamy salivation, recumbency and inappetence.
At necropsy, the foreign bodies were usually loosely matted together and impacted with rumen ingesta.
Hyperglycemia, alkalosis, hyponatremia, hypochloridemia, hypocalcemia, hypoproteinemia and hypoalbuminemia occurred in some cases. The impaction was related to the sheep scavenging on refuse dumps and the blood biochemical changes, along with the clinical signs, might be of some diagnostic significance.
INDIGESTION IN CALVES FED MILK REPLACERS (RUMINAL DRINKERS)
A form of indigestion known as ruminal drinking occurs in veal calves and is characterized clinically by recurrent ruminal tympany, inappetence, unthriftiness and the production of clay-like feces.1 The disease occurs most commonly in calves 5–6 weeks after being placed on a milk diet and being fed with a bucket.
The cause is insufficient closure of the reticular groove while drinking milk. The ingested milk enters the rumen in large quantities instead of flowing directly into the abomasum. The experimental intraruminal administration of milk to calves at 6 weeks of age induces changes in the rumen similar to those seen in spontaneous cases of the disease.2 The pH of the rumen decreases and lactate concentrations increase rapidly. The daily oral administration of untreated whole milk via stomach tube into calves 5–23 days of age results in a d-lactic metabolic acidosis within a few days.3 The onset of ruminal acidosis occurred quickly and mean pH values fell from 6.7 to 4.9 after the first feeding. In the following days the rumen pH values varied between 4 and 5. During ruminal acidosis, both l- and d-lactic acid are produced abundantly by bacterial fermentative activity. Both isomers of lactic acid are absorbed from the rumen, or from the intestines, where they exert an acidotic effect. The l-lactate can be metabolized quickly by the body and does accumulate despite the continuous influx into the blood. However, d-lactate cannot be metabolized at the same rate because of a lack of specific metabolic pathway, and it accumulates with the consequence of the risk of hyper-d-lactatemia.3
There is marked ruminal hyperkeratosis. Villous atrophy occurs in proximal jejunum accompanied by a reduction in brush border enzyme activities.4 Clinical recovery occurs within several days after returning to normal feeding practices, with restoration of villous length and brush border enzyme activities in 3–4 weeks.
On clinical examination the temperature, heart rate and respiratory rates are within normal range. The abdominal contour is increased in size, especially over the ventral half of the abdomen. Distension is more obvious on the left side. Ballottement of the left abdominal wall commonly reveals fluid-splashing sounds.5,6 Auscultation of the left paralumbar fossa while the calf is drinking reveals loud fluid-splashing sounds. Large volumes of foul- or acid-smelling, grayish-white fluid can be siphoned off from the rumen. Examination of the rumen contents after calves have consumed milk reveals the presence of a casein clot. Radiological examination reveals that ingested milk enters the rumen and reticulum and is only slowly moved on to the abomasum.
Affected calves remain unthrifty while they continue to drink milk. Esophageal groove reflex dysfunction may be a complication in some milk-fed calves affected with diarrhea.5 Weaning on to hay and concentrates returns the calf to normal very quickly. Rumen movements, via eructation reflex, and ruminations become normal within 1–2 weeks.
The administration of colostrum and other fluids to calves using an esophageal feeder does not induce the esophageal groove reflex. However, colostrum and other fluids administered directly into the rumen with a feeder does move from the forestomachs into the abomasum within 3 hours.7 Feeding colostrum to newborn calves by means of an esophageal feeder is a labor-saving and effective method of obtaining optimum levels of serum immunoglobulins. This is particularly useful in large dairy herds because colostrum can be given to calves immediately after birth.
At necropsy the rumen is enlarged and there are varying degrees of hyper- and parakeratosis. Villous atrophy is prominent in the small intestine, which is partially restored to normal when the reticular groove reflex is restored.8
Affected calves can be treated by inducing them to suck on the herdsman’s fingers while they are being fed a small quantity of cows’ whole milk or milk replacer.
1 Breukink H, et al. Vet Q. 1988;10:126.
2 Van Buisman WK, et al. J Anim Physiol Anim Nutr. 1990;63:255.
3 Gentile A, et al. J Vet Med A. 2004;51:64.
4 Van Buisman WK, et al. Vet Res Commun. 1990;14:129.
5 Dirksen G, Din L. Bovine Pract. 1989;24:53. 54–60
6 Dirksen G, Garay F. Compend Contin Educ Pract Vet. 1987;9:140. 173
ACUTE CARBOHYDRATE ENGORGEMENT OF RUMINANTS (RUMINAL LACTIC ACIDOSIS, RUMEN OVERLOAD)
ETIOLOGY
The sudden ingestion of toxic doses of carbohydrate-rich feed, such as grain, is the most common cause of the acute form of the disease.1,2 Less common causes include engorgement with apples, grapes, bread, baker’s dough, sugar beet, mangels, sour wet brewers’ grain that was incompletely fermented in the brewery, and concentrated sucrose solutions used in apiculture. Subacute ruminal acidosis (SARA) in dairy cattle is a disorder of ruminal fermentation in dairy cattle caused by the ingestion of large amounts of concentrates and inadequate amounts of fiber administered in order to increase milk production in early lactation.3,4
Etiology Sudden ingestion of large amounts of highly fermentable carbohydrates
Epidemiology Accidental consumption by ruminating cattle of excessive quantities of highly digestible feeds such as cereal grains, corn, baker’s bread, grapes, apples and the like. Subacute ruminal acidosis is considered an important problem in dairy herds. In beef and lamb feedlots the rapid introduction of high-level grain diets is a major risk factor. Outbreaks occur when animals gain access to a large quantity of grain. High mortality rate when large quantity of grain ingested
Signs Anorexia, depression, dehydration, ruminal stasis, profuse diarrhea with sweet–sour odor of feces, which may contain undigested kernels, weakness and ataxia leading to recumbency. Rumen may or may not feel full but atonic and fluid-splashing sounds audible on ballottement. Laminitis, mycotic rumenitis are complications
Clinical pathology Ruminal fluid pH below 5, rumen protozoa absent or inactive in rumen fluid; hemoconcentration, blood lactate increased, hypocalcemia
Lesions Acute congested and inflamed rumenitis, sloughing ruminal mucosa; mycotic inflammation and necrosis of forestomach and fungal hepatitis if disease lasts several days
Diagnostic confirmation Ruminal fluid pH below 5
Differential diagnosis list Simple indigestion, parturient hypocalcemia, peracute coliform mastitis, acute diffuse peritonitis
Treatment Triage to determine which animals need medical treatment, rumen lavage or rumenotomy. Correct ruminal and systemic acidosis with alkalinizing agents parenterally or orally depending on severity. Fluid and electrolyte therapy as necessary. Restore forestomach and intestinal motility by providing palatable hay
Control Prevent accidental access to grain. Gradual introduction to high-level grain diets in feedlots. Total mixed rations containing chopped roughage and grain to insure controlled intake of carbohydrates. Careful feeding management of dairy cattle during late pregnancy and early lactation. Use of ionophores in feed alter rumen metabolism and potentially can control ruminal acidosis
EPIDEMIOLOGY
Occurrence
All types of ruminant cattle and sheep are susceptible but the disease occurs most commonly in feedlot cattle and dairy cattle fed on high-level grain diets. The disease also occurs in lamb feedlots and has been recorded in goats, wild deer and farmed ungulates.
Previous diet and change of ration
Because the type and level of ration consumed by a ruminant affects the numbers and species of bacteria and protozoa in the rumen, a change from one ration to another requires a period of microbial adaptation, which is a variable interval of time before stabilization occurs. Animals being fed a low-energy ration are most susceptible to a rapid change to a high-energy ration because satisfactory adaptation cannot occur quickly enough. This results in the rapid onset of abnormal fermentation.
Accidental consumption of excess carbohydrates
The disease occurs commonly following accidental consumption of toxic amounts of grain by cattle gaining sudden access to large quantities of grain. A single animal or a group of hungry cows may break into a grain storage bin or find a large supply of unprotected grain, as not uncommonly happens on a mixed cattle–grain farm. Another common occurrence is when cattle are left under the care of an assistant who, being unaware of the feeding schedule, gives the cattle an unaccustomed quantity of grain. Outbreaks have occurred in dairy herds following malfunction of automatic feeders, which delivered many times more than the usual amount of grain. In a similar outbreak, recently calved cows consumed an excessive amount of feed delivered by an automatic feeder but not eaten by other cows because of hot weather.
Outbreaks have occurred when cattle have been turned into unripe, green corn standing in the field, when cattle or sheep have been placed on stubble fields in which considerable grain lost by the harvester was available on the ground, and following the irregular feeding of large quantities of other less common animal feeds and byproducts, such as bread, baker’s dough and wet brewers’ grain. Problems usually arise with these feeds when a larger than usual amount is fed to cattle either for the first time or because the usual supplementary feed is in short supply.
Dairy cattle herds
Subacute ruminal acidosis occurs in dairy cattle herds fed high-grain, low-fiber rations in early lactation.3,5 It is considered of major economic importance because of the possible association with laminitis in dairy herds.2,6
The transition from the pregnant, nonlactating state to the nonpregnant, lactating state is the period during which the majority of metabolic diseases occur in the dairy cow. During this period, which ranges from 3 weeks before until 3 weeks after calving, the cow is changed from a high-fiber, low-concentrate diet to a diet that is higher in concentrate feeds and lower in fiber. Cows that have not adapted to these high-grain diets are particularly susceptible to ruminal acidosis, Subacute ruminal acidosis is characterized by repeated bouts of depressed rumen pH between 5.2 and 5.6. The abnormality often results from a large intake of rapidly fermentable carbohydrates that leads to the accumulation of organic acids in the rumen. Up to 20% of commercial dairy farm cows in early to mid-lactation have a rumen pH of less than 5.5, indicative of subacute ruminal acidosis.6 The economic losses associated with SARA have been estimated at $1.12 per cow per day.7
Field observations suggest that periparturient cows are at risk of subacute ruminal acidosis because of the time required for the rumen microflora and papillae to adapt to increased intakes of concentrates immediately before parturition and during early lactation when feed intake increases rapidly to meet the energy needs of high-producing dairy cows. The adaptation of the ruminal microflora and papillae from a system appropriate for forage to a system capable of utilizing high-energy lactation rations requires a gradual change during a period of 3–5 weeks.6
The need for individual cows to adapt to high-energy rations and the common practice of feeding dairy cows as groups results in periparturient cows being at risk of developing subacute ruminal acidosis. For practical reasons, as total mixed rations have become more common, many dairy herds limit the number of rations to a single dry-cow ration and a single lactating-cow ration, because of the time and labor required to mix each ration. This system has made it difficult to introduce concentrates to individual cows in the first few weeks after calving. If the dry-cow ration has not resulted in adaptation of the ruminal microflora required for high-energy rations, acidosis may occur when the cow is fed the lactating-group ration. The net energy of a ration can be safely increased in 10% increments. For example, a change from an energy density of 0.70 Mcal/lb NE1 (net energy, lactation) to 0.77 Mcal/lb NE1 would be considered safe. The National Research Council recommends that dry-cow total mixed rations have 0.57 Mcal/lb NE1 and that a high-production lactation cow ration have 0.78 Mcal/lb NE1.8 Using the 10% guideline for gradual energy change would require at least two intermediate rations.5
Dairy producers attempt to minimize the negative energy balance of lactating cows in early lactation by maximizing concentrate intake early in the period after parturition. The early lactation period is a high-risk period for lactating dairy cows if they are fed rations as separate components, for three reasons:
• Concentrates are consumed by the cow in preference to forage
• Forage consumption is not usually measured on an individual cow basis and is commonly assumed to approximate the herd average
• Dry matter intake of periparturient cows is lower than commonly thought and is very dynamic through this period.5
Thus high-producing lactating dairy cows consuming large quantities of high-energy grains are susceptible to subacute ruminal acidosis during early lactation.9
Field recommendations for feeding component-fed concentrates during the first 3 weeks of lactation are usually excessive.3,5 Feeding excessive quantities of concentrate and insufficient forage results in a fiber-deficient ration likely to cause subacute acidosis. The same situation may occur during the last few days before parturition if the ration is fed in separate components; as dry matter intake drops before calving, dry cows will preferentially consume too much concentrate and insufficient fiber, and develop acidosis.
Subacute ruminal acidosis may also be caused by formulation of rations that contain excessive amounts of rapidly fermentable carbohydrates, a deficiency of fiber, or errors in delivery of the rations. Recommendations for the fiber content of dairy rations are available in the National Research Council (Nutrient Requirements of Dairy Cattle).8 Dry-matter content errors in total mixed rations are commonly related to a failure to adjust for changes in moisture content of forages.5
In a survey in Denmark, dairy cattle practitioners were asked to retrospectively report on the occurrence and relative importance of SARA in dairy herds compared to the actual number of cases reported to the national computer-based dairy herd health recording system.10 The most common diagnoses believed to occur were ketosis (26%), rumen acidosis (22%), abomasal disorders (16%), subclinical hypocalcemia (15%) and milk fever (15%). Subclinical rumen acidosis was considered to be a commonly occurring underlying condition with significant importance as a cause of reduced appetite, and inadequate feeding strategies were given as the main cause. However, according to the national dairy health recording system, SARA was rarely reported as a diagnosis. The practitioners were reluctant to imply that feeding management was a problem. The clinical signs of SARA were unclear to the practitioners, and the diagnostic tests necessary, such as rumenocentesis, were considered time consuming and unreliable because of the small size of the herds.10
Feedlot cattle
The occurrence of grain overload in feedlot cattle, however, has gained the most attention, presumably because of its economic impact. Digestive disorders account for approximately 25–35% of deaths in feedlot cattle and may contribute to decreased performance and efficiency of production.11 The economics of feedlot beef production dictate that cattle should gain weight at their maximum potential rate and this usually involves getting them on to a full feed of a high concentration of grain quickly. Economics also favor the processing of grain by one of several methods available that will increase the availability of starch and thereby increase the rate of degradation in the rumen. All these factors set the stage for a high incidence of grain overload in feedlot cattle.12
There are some critical periods during which grain overload occurs in feedlot cattle. When starting cattle on feed, animals with previous experience of eating grain will commonly consume a toxic dose if offered a ration with a high percentage of grain. The disease occurs commonly in feedlot cattle in which the total daily feed intake has been brought up to what is considered the same feed on an ad libitum basis; they gorge themselves. When increasing the concentration of grain in the ration from one level to another, if the increment is too high the total amount of grain consumed by some cattle will be excessive. Rapid changes in barometric pressures may affect the voluntary intake of cattle. A rapid change to cold weather may result in a moderate increase in feed intake in animals that are fed ad libitum and outbreaks of grain overload may occur. When rain is involved and feed becomes wet and possibly even moldy, feed intake will drop, but when fresh dry feed is offered again there may be a marked increase in feed intake that results in grain overload.
The disease also occurs when cattle that have been on a high-level grain ration (full feed) have become hungry because they have been out of feed for 12–24 hours as a result of a breakdown in the feed mill or handling facilities. Offering an unlimited supply of feed to these cattle will often result in severe cases of grain overload. In large feedlots, where communications can be a problem, the accidental feeding of a high-level grain ration to cattle that are on a high-level roughage ration is a common cause of the disease.
The ruminal lesions of rumenitis and ruminal hyperkeratosis, which are commonly present in feedlot cattle at slaughter, are thought to be associated with the continuous feeding of grain. These lesions are often remarkable at slaughter in well-nourished cattle and their effect on live weight gain and feed conversion is not known.
Beef breeding herds
Cows in beef cow–calf herds may develop acute ruminal acidosis if offered a high-energy grain ration during the winter feeding period without a period of adjustment.
Lamb feedlots and liquid-fed calves
Outbreaks of the disease occur in lamb feedlots in which lambs are started on a high-level grain ration without a period of adjustment. The disease is not as common in lambs as in cattle, perhaps because lambs are usually fed on oats.
Rumenitis and metabolic acidosis have also been reported when newborn calves were force-fed liquid feeds or nutrient– electrolyte solutions containing easily digestible carbohydrates.13
Morbidity and case fatality rates
Outbreaks of the disease occur in cattle herds kept on grain farms and in feedlots. Depending on the species of grain, the total amount eaten and the previous experience of the animals, the morbidity will vary from 10–50%. The case fatality rate may be up to 90% in untreated cases, while in treated cases it still may be up to 30–40%.
Types and toxic amounts of feeds
Wheat, barley and corn grains are the most toxic when ingested in large quantities. Oats and grain sorghum are least toxic. All grains are more toxic when ground finely or even crushed or just cracked – processes that expose the starch component of the grain to the ruminal microflora. The experimental feeding of unprocessed barley to cattle did not result in rumenitis, whereas feeding rolled barley was associated with ruminal lesions. An unrestricted supply of stale bread can cause outbreaks.
The amount of a feed required to cause acute illness depends on the species of grain, previous experience of the animal with the grain, its nutritional status and body condition score, and the nature of the ruminal microflora. Dairy cattle accustomed to high-level grain diets may consume 15–20 kg of grain and develop only moderate illness, while beef cows or feedlot cattle may become acutely ill and die after eating 10 kg of grain to which they are unaccustomed. Amounts of feed that are lethal range from 50–60 g of crushed wheat/kg BW in undernourished sheep to 75–80 g/kg BW in well-nourished sheep, and in cattle doses ranging from 25–62 g/kg BW of ground cereal grain or corn produced severe acidosis.
PATHOGENESIS
The details of the pathogenesis of ruminant lactic acidosis have been reviewed.1 A summary of the events that occur in the rumen and the systemic effects on the animal is presented here. The disease is a good example of metabolic acidosis in ruminants.
Changes in rumen microflora
The ingestion of excessive quantities of highly fermentable feeds by a ruminant is followed within 2–6 hours by a marked change in the microbial population in the rumen. There is an increase in the number of Streptococcus bovis, which utilize the carbohydrate to produce large quantities of lactic acid. In the presence of a sufficient amount of carbohydrate (a toxic or a lethal amount) the organism will continue to produce lactic acid, which decreases the rumen pH to 5 or less, which results in the destruction of the cellulolytic bacteria and protozoa. When large amounts of starch are added to the diet, growth of S. bovis is no longer restricted by energy source and it multiplies faster than any other species of bacteria.
Volatile fatty acids and lactic acid in the rumen
The concentration of volatile fatty acids increases initially, contributing to the fall in ruminal pH. The low pH allows lactobacilli to use the large quantities of carbohydrate in the rumen to produce excessive quantities of lactic acid, resulting in ruminal lactic acidosis. Both D and L forms of the acid are produced, which markedly increases ruminal osmolality, and water is drawn in from the systemic circulation, causing hemoconcentration and dehydration. Ruminal osmolality increases from a normal of 280 mosmol/L to almost 400 mosmol/L.1
Some of the lactic acid is buffered by ruminal buffers but large amounts are absorbed by the rumen and some moves into and is absorbed further down the intestinal tract. Lactate is a 10 times stronger acid than the volatile fatty acids, and accumulation of lactate eventually exceeds the buffering capacity of rumen fluid. As the ruminal pH declines, the amplitude and frequency of the rumen contractions are decreased and at about a pH of 5 there is ruminal atony. The increased ruminal levels of unassociated volatile fatty acids may be more important than increased lactic acid or increased hydrogen ion concentration in causing ruminal atony. Experimentally, increased molar concentration of butyrate, not the lactic acid, causes ruminal stasis.1 Inhibition of ruminal activity may also be due to lactic acid entering the duodenum and exerting a reflex inhibitory action on the rumen. Experimentally, ruminal atony occurs in sheep within 8–12 hours after grain engorgement but the precise pathophysiological mechanism for loss of forestomach motility is uncertain. The diarrhea is considered to be due to the reduction in net absorption of water from the colon.
Systemic lactic acidosis
The absorbed lactic acid is buffered by the plasma bicarbonate buffering system. With nontoxic amounts of lactic acid, the acid–base balance is maintained by utilization of bicarbonate and elimination of carbon dioxide by increased respirations. In those which survive the acute form of the disease, this compensatory mechanism may overcompensate, resulting in alkalosis. In severe cases of lactic acidosis the reserves of plasma bicarbonate are reduced, the blood pH declines steadily, the blood pressure declines, causing a decrease in perfusion pressure and oxygen supply to peripheral tissues and resulting in a further increase in lactic acid from cellular respiration. Lactic acid given intravenously to cattle causes hypertension, increased responses to norepinephrine, slight bradycardia and slight hyperventilation.
Both d- and l-lactic acids are produced. The l-lactic acid is utilized much more rapidly than the d-isomer which accumulates and causes a severe d-lactic acidosis. If the rate of entry of lactic acid into body fluids is not too rapid, compensatory mechanisms are able to maintain the blood pH at a compatible level until the crisis is over, and recovery is usually rapid. This may explain the common observation that feedlot cattle may be ill for a few days after being introduced to a grain ration but quickly recover, while in other cases when the rate of entry is rapid the compensatory mechanisms are overcome and urgent treatment is necessary.
In experimental lactic acidosis using sucrose in sheep, feed intake does not resume until rumen pH has returned to 6.0 or higher and lactic acid is no longer detectable in the rumen. Renal blood flow and glomerular filtration rate are also decreased, resulting in anuria. Eventually there is shock and death. All these events can occur within 24 hours after engorgement of a lethal dose of carbohydrate; with toxic doses the course of events may take 24–48 hours.
Chemical and mycotic rumenitis
The high concentration of lactic acid in the rumen causes chemical rumenitis, which is the precursor for mycotic rumenitis in those that survive; this occurs about 4–6 days later. The low pH of the rumen favors the growth of Mucor, Rhizopus and Absidia spp. which invade the ruminal vessels, causing thrombosis and infarction. Inoculation of Absidia corymbifera orally into sheep with experimental ruminal acidosis produced with barley causes desquamation of the superficial layers of the mucosae and focal necrosis from lamina propria to muscular layers. Severe bacterial rumenitis also occurs. Widespread necrosis and gangrene may affect the entire ventral half of the ruminal walls and lead to the development of an acute peritonitis. The damage to the viscus causes complete atony and this, together with the toxemia resulting from the gangrene, is usually sufficient to cause death. Mycotic omasitis and rumenitis may also occur without a history of grain engorgement in cattle. Anorexia and forestomach atonicity associated with a primary illness in other body systems may predispose the mucosae to fungal infection because of abomasal reflux of acid and the prolonged use of antimicrobials.
Chronic rumenitis and ruminal hyperkeratosis are common in cattle fed for long periods on grain rations, and the lesions are attributed to the chronic acidosis, but it is possible that barley awns and ingested hair may contribute to the severity of the lesions.
Hepatic abscesses
In uncomplicated chemical rumenitis, the ruminal mucosa sloughs and heals with scar tissue and some mucosal regeneration. Hepatic abscesses commonly occur as a complication as a result of a combination of rumenitis caused by lactic acidosis and allowing Fusobacterium necrophorum and Arcanobacter (Corynebacterium) pyogenes to enter directly into ruminal vessels and spread to the liver, which may have also undergone injury from the lactic acidosis. Severe diffuse coagulation necrosis and hyperplasia of the bile duct epithelium and degeneration of renal tubules may also be present histologically.
In cattle being placed on a grain ration, even with control of the daily intake, hepatic cell damage and liver dysfunction occur even though dietary adaptation may have occurred in 2–3 weeks. The biochemical profile indicates that complete metabolic adaptation requires at least 40 days following the start of grain feeding.
Laminitis
Laminitis occurs in acute, subclinical and chronic forms associated with varying degrees of severity of ruminal acidosis. The association between acidosis and laminitis appears to be associated with altered hemodynamics of the peripheral microvasculature. Vasoactive substances (histamine and endotoxins) are released during the decline of rumen pH and the bacteriolysis and tissue degradation. These substances cause vasoconstriction and dilation, which injure the microvasculature of the corium. Ischemia results, which causes a reduction in oxygen and nutrients reaching the extremities of the corium. Ischemia causes physical degradation of junctures between tissues that are structurally critical for locomotion. The insidious rotation of the distal phalanx (pedal bone) can result in permanent anatomical change. Manifestations of subclinical laminitis are sole hemorrhages and yellowish discoloration. Other clinical manifestations include double soles, heel erosion, dorsal wall concavity and ridging of the dorsal wall.2
Other toxic substances produced
Several toxic substances other than lactic acid have been suggested as contributory to the disease. Increased concentrations of histamine have been found in the rumen of experimentally engorged cattle, but its possible role in the disease remains unknown. Histamine is not absorbed from the rumen except at abnormally high pH values, but is absorbed from intestinal loops. Laminitis occurs in some cases of rumen overload but the pathogenesis is unknown.
Other substances that have been recovered from the rumen in grain overload include a suspected endotoxin, ethanol and methanol. In experimental lactic acidosis induced in cattle with 70 g barley/kg BW, endotoxin and arachidonic acid metabolites are produced and may be important. However, the role of the endotoxin is uncertain. Endotoxin administered into the intestine of lactic acidotic sheep is not absorbed. Clostridium perfringens and coliform bacteria have also been found in increased numbers but their significance is uncertain. The electrolyte changes that occur include a mild hypocalcemia due to temporary malabsorption, loss of serum chloride due to sequestration in the rumen, and an increase in serum phosphate due to renal failure.
Experimental lactic acidosis
The disease can be reproduced in cattle and sheep with a variety of grains, fruits, sugars and pure solutions of lactic acid. The oral administration of sucrose at 18 g/kg BW to goats can cause lactic acidosis. In cattle the sucrose is used to induce rumen lactic acidosis experimentally.14 The severity of the experimental disease and the magnitude of the pathophysiological changes vary depending on the substance used, but changes similar to the natural disease occur.
Lesions in the brain have been recorded in the experimental disease in sheep and naturally occurring cases in cattle, but their pathogenesis and significance are uncertain. There are detectable changes in the cellular and biochemical composition of the cerebrospinal fluid, which suggests that the blood–brain barrier may be affected. Experimentally, sublethal doses of volatile fatty acids, lactate and succinate have an effect on liver function. Toxic and lethal doses of butyrate can cause sudden flaccid paralysis and death from asphyxia.
Adaptation to grain-based diets in beef cattle
The health and ruminal variables during adaptation to grain-based diets in beef cattle have been examined experimentally. Successive diets with forage-to-grain ratios of 75:25 (diet 1), 50:50 (diet 2), 25:75 (diet 3) and 10:90 (diet 4) were each fed for 7 days. The health variables such as rectal temperature, heart rate, respiratory rate, rumen motility rates, fecal consistency, demeanor, blood pH, and blood glucose and L+-lactate concentrations remained within reference range limits throughout the adaptation period. Blood pH continually decreased during feeding of the four diets. The pH of the ruminal contents decreased progressively from 6.8 to 5.3. By the end of the period, the ruminal contents were acidic (pH < 5.5) and, on the basis of the amounts of ruminal glucose and dl-lactate, it was concluded that ruminal microbial equilibrium had not yet been achieved. In addition, an increase in the heart and respiratory rates in animals fed diets 2 and 4 indicated stress. During normal fermentation, glucose is not detectable in ruminal fluid because its production is closely linked with its assimilation. In general, changing from a high-roughage to low-roughage diet is stressful for cattle and their resident ruminal microflora.
Subacute ruminal acidosis (dairy cattle)
The pathogenesis of SARA in lactating dairy cows is not as well understood as acute ruminal acidosis associated with the sudden ingestion of large amounts of readily fermentable carbohydrates, for example, most commonly in beef cattle that gain accidental access to large quantities of grain. In early-lactating dairy cows, SARA is usually caused by the consumption of diets with high levels of rapidly fermentable carbohydrates and/or marginal, often deficient, levels of physically active fiber.8
The biochemical changes that occur in lactating dairy cows in early lactation that are affected with SARA have not been examined in detail. In SARA, fermentation of nonstructural carbohydrates leads to the production of large quantities of volatile fatty acids and lactate, which accumulate in the rumen and subsequently decrease rumen pH. It has been difficult to reproduce SARA in early-lactation dairy cows even with diets such as high-moisture corn, cracked dried corn grain and rolled barley.7 These feeds did not induce SARA, either because of an inability of the feeds to depress the rumen pH rapidly enough or because of the cow’s refusal to consume them.
Wheat/barley pellets were readily consumed by lactating dairy cows and did result in a sustained reduction in rumen pH.7 When cows with experimental SARA are given a choice between alfalfa hay and alfalfa pellets, cows will choose the alfalfa hay more strongly, which implies that dairy cows would increase their dietary preference for a feed of longer particle size when given the appropriate choice during a bout of SARA.7 As intake of long hay will result in more saliva production and rumen buffering than intake of pelleted alfalfa, this indicates that cows select feeds with high rumen buffering capacity in an attempt to prevent SARA. When cows with SARA were offered sodium bicarbonate ad libitum, they did not select the compound in order to attenuate the ruminal acidosis.15 When cows with SARA were offered a choice between two test pellets, one containing 4% sodium bicarbonate and the other 4.5% sodium chloride, the intake of the sodium bicarbonate pellets increased over time, but the intake of sodium chloride pellets remained unaltered.16
There is some evidence that lactic acid is not the causal reason for the prolonged reduction in pH of the ruminal contents. Studies have shown only low lactate levels between 0.45 mmol/L and 0.74 mmol/L in cows with suspected SARA. Excessive volatile fatty acid production may be a more important contributor to SARA in lactating dairy cows.
The induction of SARA by excess feeding of wheat/barley pellets reduces the rumen digestion of neutral detergent fiber from grass hay, legume hay and corn silage.17 It is thought that SARA affects the productivity of dairy cows by reducing the fiber digestion, because low pH negatively affects cellulolytic bacteria. The induction of SARA in lactating dairy cows by replacing 25% of the total mixed ration intake with pellets consisting of 50% wheat and 50% barley reduced the in-situ dry matter and neutral detergent fiber digestion of mixed hay. Disappearance of neutral detergent fiber was reduced from 39.5% to 30.9%.18
In experimentally induced SARA, lipopolysaccharide concentration in the rumen increases during periods of grain feeding compared with times when only hay is fed.19 The concentration of serum amyloid-A and serum haptoglobin indicate a systemic inflammatory response.
Rumen pH drops considerably in dairy cows after calving when the diet is changed. Monitoring rumen pH throughout the transition period of dairy cows in which the concentrate to forage ration was changed from 70:30 to 55:45 at calving found that 1 week prior to calving the average daily pH was 6.83, average daily time with rumen pH below 6 was 25.5 minutes and average daily time with rumen pH below 5.6 was 5.6 minutes. During the first week after calving, average daily pH was 6.51, and average daily time with rumen pH below 6 and 5.6 were 312 and 59.6 minutes respectively.18 The drop in rumen pH is associated with an increase in the rate of production of volatile fatty acids, which temporarily increases the concentration of volatile fatty acids in the rumen, until the absorptive capacity of the rumen mucosa for volatile fatty acids has been increased.
The pathogenesis of rumenitis, hepatic abnormalities and laminitis associated with SARA is considered to be similar to those described above for acute ruminal acidosis.
CLINICAL FINDINGS
Speed of onset and severity
The speed of onset of the illness varies with the nature of the feed, being more rapid with ground feed than with whole grain. The severity increases with the amount of feed eaten. If cattle are examined clinically within a few hours after engorgement, the only abnormalities that may be detectable are a distended rumen and abdomen, and occasionally some abdominal discomfort, evidenced by kicking at the belly. In the mild form, affected cattle are anorexic and still fairly bright and alert, and the feces may be softer than normal. Rumen movements are reduced but not entirely absent. Affected cattle do not ruminate for a few days but usually begin to eat on the third or fourth day without any specific treatment.
In outbreaks of the severe form, within 24–48 hours some animals will be recumbent, some staggering and others standing quietly alone. Most affected cattle are anorexic, apathetic and depressed. Teeth grinding may occur in about 25% of affected sheep and goats. Once they are ill they usually do not drink water, but cattle may engorge themselves on water if it is readily available immediately after consuming large quantities of dry grain. In an outbreak, inspection of the feces on the ground will usually reveal many spots of soft to watery feces.
Individual animals
Depression, dehydration, inactivity, weakness, abdominal distension, diarrhea and anorexia are typical. The temperature is usually below normal, 36.5–38.5°C (98–101°F), but animals exposed to the sun may have temperatures up to 41°C (106°F). In sheep and goats, the rectal temperatures may be slightly higher than normal. The heart rate in cattle is usually increased and continues to increase with the severity of the acidosis and circulatory failure. In general, the prognosis is better in those with heart rates below 100/min than those with rates up to 120–140/min. In sheep and goats, the heart rate may be higher than 100/min. The respirations are usually shallow and increased up to 60–90/min. A mucopurulent discharge is common because animals fail to lick their nares.
Diarrhea is almost always present and usually profuse, and the feces are light-colored with an obvious sweet–sour odor. The feces commonly contain an excessive quantity of kernels of grain in grain overload, and pips and skins when grapes or apples have been eaten. An absence of feces is considered by some veterinarians as a grave prognostic sign but diarrhea is much more common. The dehydration is severe and progressive. In mild cases, the dehydration is about 4–6% BW, and with severe involvement up to 10–12% BW. Anuria is a common finding in acute cases and diuresis following fluid therapy is a good prognostic sign.
Careful examination of the rumen is important. The rumen contents palpated through the left paralumbar fossa may feel firm and doughy in cattle that were previously on a roughage diet and have consumed a large amount of grain. In cattle that have become ill on smaller amounts of grain, the rumen will not necessarily feel full but rather resilient because the excessive fluid contents are being palpated. Therefore, the findings on palpation of the rumen may be deceptive and a source of error. The primary contractions of the reticulorumen are usually totally absent, although low-pitched tinkling and gurgling sounds associated with the excessive quantity of fluid in the rumen are commonly audible on auscultation of the rumen. The ruminal fluid is a milky green to olive brown color and has a pungent acid smell. Collection of a sample of ruminal fluid in a glass beaker will reveal an absence of foam. The pH of the rumen fluid is usually below 5.
Severely affected animals have a staggery, drunken gait and their eyesight is impaired. They bump into objects and their palpebral eye preservation reflex is sluggish or absent. The pupillary light reflex is usually present but slower than normal. Acute laminitis may be present and is most common in cases that are not severely affected and appear to be good treatment risks. Affected animals are lame in all four feet, shuffle while they walk slowly and may be reluctant to stand. The lameness commonly resolves if the animals recover from the acute acidosis. Evidence of chronic laminitis may develop several weeks later.
Recumbency usually follows after about 48 hours but may occur earlier. Affected animals lie quietly, often with their heads turned into the flank, and their response to any stimulus is much decreased so that they resemble parturient paresis. A rapid onset of recumbency suggests an unfavorable prognosis and the necessity for urgent treatment, because death may occur in 24–72 hours after the ingestion of the feed. Evidence of improvement during this time includes a fall in heart rate, rise in temperature, return of ruminal movement and passage of large amounts of soft feces.
The clinical findings described above are most common but when a group of animals have been exposed to overfeeding there are all degrees of severity from simple indigestion, cases of which recover spontaneously, to the severe cases that need intensive therapy. The prognosis varies with the severity, and the clinical variables that are useful in deciding on a course of treatment or action are summarized in Table 6.8.
Mycotic rumenitis
Some animals appear to recover following treatment but become severely ill again on the third or fourth day. Mycotic rumenitis is common in these animals and is characterized by a fluid-filled atonic rumen, dehydration in spite of fluid therapy, diarrhea, anorexia, weakness leading to recumbency and death in 2–3 days due to acute diffuse peritonitis.
Complications
Chronic laminitis may occur several weeks or months later. This is particularly important in dairy cattle herds affected with subacute acidosis.5
Abortions may occur 10 days to 2 weeks later in pregnant cattle that survive the severe form of the disease.
Subacute ruminal acidosis in dairy cattle
Subacute ruminal acidosis (SARA) is being recognized with increased frequency in dairy herds.3-5 However, the case definition is not yet well described. Clinical findings include laminitis, intermittent diarrhea, suboptimal appetite or cyclic feed intake, a high herd culling rate, loss of body condition in spite of adequate energy intake, liver abscesses, and hemoptysis and epistaxis associated with venal caval thrombosis and pulmonary hemorrhage. Milk-fat depression and suboptimal milk production in the second- and subsequent-lactation cows compared to the first-lactation cows may occur.3
A decrease in dry matter intake is commonly reported in herds with SARA.4 The causes of a lowered dry matter intake are uncertain but may be related to weaker rumen motility during low pH phases, bacterial endotoxins and changes in the osmolarity of the rumen contents.
The laminitis is characterized by ridges in the dorsal hoof wall, sole ulceration, white line lesions, sole hemorrhages and misshapen hooves.20 It is suggested that when the incidence of laminitis exceeds 10% of the herd, it should be considered a herd problem related to the feeding program.
CLINICAL PATHOLOGY
The severity of the disease can usually be determined by clinical examination, but field and laboratory tests are of some additional value.
Ruminal fluid pH
The pH of the ruminal fluid obtained by stomach tube or by rumenocentesis through the left paralumbar fossa can be measured in the field using wide-range pH (2–12) indicator paper. The ruminal fluid must be examined immediately because the pH will increase upon exposure to air. Cattle that have been fed a roughage diet will have a ruminal pH of 6–7; for those on a grain diet it will be 5.5–6. A ruminal pH of 5–6 in roughage-fed cattle suggests a moderate degree of abnormality but a pH of less than 5 suggests severe grain overload and the need for energetic treatment. Feedlot cattle that have been on grain for several days or weeks and are affected with grain overload usually have a pH below 5.
Rumenocentesis has become a commonly used diagnostic test for subacute ruminal acidosis.13,20 A hypodermic needle of 1.6 (outer diameter) × 130 mm (length) is inserted into the ventral rumen and rumen contents aspirated with a syringe. Landmarks for the puncture site are the left side, on a horizontal line level with the top of the patella about 15–20 cm posterior to the last rib. The hair of the site is clipped and prepared using a standard scrub. The cow is restrained in a stanchion or head-gate and one assistant elevates the tail of the cow while another assistant inserts a ‘nose leader’ and pulls the cow’s head to the right side. The needle will usually become obstructed by ingesta, which is cleared by forcing a small amount of air or fluid back through the needle. When the needle becomes obstructed it is important to avoid creating a negative pressure within the syringe, as carbon dioxide will leave the fluid and increase the pH. Typically, 3–5 mL of rumen fluid can be collected with minimal difficulty.
The pH is measured immediately using a pH meter with a digital readout. Samples should be collected when the pH is likely to be near the lowest point of the day. If the ration is fed as separate components, rumenocentesis should be performed 2–4 hours after the cows are fed the primary concentrate of the day. If the ration is fed as a total mixed ration, the samples should be collected 4–8 hours later. A pH of 5.5 is recommended as the cut-point between normal and abnormal.20 At least 12 or more cows should be sampled from any group in which acidosis is suspected. If 30% of 10 or more sampled cows are below 5.5, the group is classified as in a state of ruminal acidosis. A subsample of 12 cows from a herd or diet group and a critical number of three cows with a ruminal pH less than or equal to 5.5 may effectively differentiate between herds with 15% or less or greater than 30% prevalence of cows with a low ruminal pH.13
Ruminal protozoa
Microscopic examination of a few drops of ruminal fluid on a glass slide (with a coverslip) at low power will reveal the absence of ruminal protozoa, which is a reliable indicator of an abnormal state of the rumen, usually acidosis. The predominantly Gram-negative bacterial flora of the rumen is replaced by a Gram-positive one.
Serum biochemistry
The degree of hemoconcentration, as indicated by hematocrit, increases with the amount of fluid withdrawn from the extracellular fluid space into the rumen. The hematocrit rises from a normal of 30–32% to 50–60% in the terminal stages and is accompanied by a fall in blood pressure. Blood lactate and inorganic phosphate levels rise and blood pH and bicarbonate fall markedly. In almost all cases there is a mild hypocalcemia, which is presumably due to a temporary malabsorption. Serum levels may drop to between 6–8 mg/dL (1.5–2 mmol/L).
The serum enzyme activities of cattle fed on barley for several months has been measured and suggest that hepatocellular damage occurs during the early stages of feeding grain but that recovery occurs after about 1 month.
NECROPSY FINDINGS
In acute cases where the animal dies in 24–48 hours the contents of the rumen and reticulum are thin and porridge-like and have a typical odor suggestive of fermentation. The cornified epithelium may be mushy and easily wiped off, leaving a dark, hemorrhagic surface beneath. This change may be patchy, caused probably by the production of excess lactic acid in pockets where the grain collects, but is generally restricted to the ventral half of the sacs. Abomasitis and enteritis are also evident in many cases. The abomasum may contain large quantities of grain. There is a pronounced thickening and darkening of the blood and the visceral veins stand out prominently.
In cases that have persisted for 3–4 days the wall of the reticulum and rumen may be gangrenous. This change is again patchy but may be widespread. In affected areas the wall may be three or four times the normal thickness, show a soft black mucosal surface raised above surrounding normal areas and have a dark red appearance visible through the serous surface. The thickened area is very friable and on cutting has a gelatinous appearance. Histological preparations show infiltration of the area by fungal mycelia and a severe hemorrhagic necrosis. A fungal hepatitis is common in those with fungal rumenitis. In the nervous system, in cases of 72 hours or more duration, demyelination has been reported. A terminal ischemic nephrosis is present in varying degrees in most fatal cases of more than several days’ duration.
If the examination takes place less than an hour after death, estimation of ruminal pH may be of value in confirming the diagnosis but after 1 hour the pH of the rumen contents begins to increase and its measurement may not be reliable. A secondary enteritis is common in animals that have been ill for several days.
When outbreaks of the disease with an appropriate history are encountered, the diagnosis is usually readily obvious and confirmed by the clinical findings and examination of the ruminal fluid for pH and rumen protozoa.
When the disease occurs in a single animal without a history of engorgement, the diagnosis may not be readily obvious. The anorexia, depression, ruminal stasis with gurgling fluid sounds from the rumen, diarrhea and a staggery gait with a normal temperature are characteristics of rumen overload.
Acute and subacute carbohydrate engorgement must be differentiated from:
• Simple indigestion. The consumption of large quantities of palatable feed, such as ensiled green feed offered to cattle for the first time, may cause simple indigestion, which may resemble grain overload. The rumen is full, the movements are reduced in frequency and amplitude, there may be mild abdominal pain due to the distension, but the ruminal pH and protozoan numbers and activity are normal
• Parturient paresis. Severe cases that are recumbent may resemble parturient paresis, but in the latter the feces are usually firm and dry, marked dehydration does not occur, the absolute intensity of the heart sounds is reduced and the response to calcium injection is favorable
• Toxemias. Common toxemias of cattle that may resemble ruminal overload include peracute coliform mastitis and acute diffuse peritonitis, but clinical examination will usually reveal the cause of the toxemia
• Subacute ruminal acidosis must be differentiated from diseases of dairy cows in early lactation in which there is reduced appetite and milk production. These include simple indigestion, left-side displacement of the abomasum, ketosis and other causes of suboptimal milk production in dairy cows in early lactation.21 Feeding management problems such as poor-quality forage or poor feeding bunk management are common causes of suboptimal performance in lactating dairy cows that are not affected with SARA
TREATMENT
The principles of treatment are:
• Correct the ruminal and systemic acidosis and prevent further production of lactic acid
• Restore fluid and electrolyte losses and maintain circulating blood volumes
There are at least two common clinical situations encountered. One is when cattle have been found accidentally eating large quantities of grain, are not yet ill and all appear similar clinically except for varying degrees of distension depending on the amount each animal has consumed. In the other situation, the engorgement occurred 24–48 hours previously and the animals have clinical evidence of lactic acidosis.
When cattle are found engorging themselves, the following procedures are recommended:
• Prevent further access to feed
• Do not provide any water for 12–24 hours
• Offer a supply of good-quality palatable hay equal to one-half of the daily allowance per head
• Exercise all animals every hour for 12–24 hours to encourage movement of the ingesta through the digestive tract.
Those cattle that have consumed a toxic amount of grain will show signs of anorexia, inactivity and depression in approximately 6–8 hours and should be identified and removed from the group for individual treatment. Those cattle that did not consume a toxic amount are usually bright and alert and will usually begin eating hay if it is offered. Not all cattle found engorging themselves with grain will have consumed a toxic dose and careful monitoring over a 24–48-hour period will usually distinguish between those that need treatment and those that do not.
After 18–24 hours, those cattle that have continued to eat hay may be allowed free access to water. Those with clinical evidence of grain overload must be identified and treated accordingly. They will engorge themselves with water if allowed free access to it. The rumen becomes grossly distended with fluid and affected cattle may die 18–24 hours later from electrolyte disturbances and acid– base imbalance.
In certain situations, if feasible and warranted by economics, such as when finished beef cattle have accidentally engorged on grain, emergency slaughter may be the most economical course of action.
Triage
The recommendations for treatment given in Table 6.8 are guidelines. In an outbreak, some animals will not require any treatment while severely affected cases will obviously need a rumenotomy. For those that are not severely affected, it is often difficult to decide whether to treat them only medically with antacids orally and systemically or to do a rumenotomy. Each case must be examined clinically and the most appropriate treatment selected. The degree of mental depression, muscular strength, degree of dehydration, heart rate, body temperature, and rumen pH are clinical parameters that can be used to assess severity and to determine the treatment likely to be most successful.
Rumenotomy
In severe cases, in which there is recumbency, severe depression, hypothermia, prominent ruminal distension with fluid, a heart rate of 110–130/min and a rumen pH of 5 or below, a rumenotomy is the best course of action. The rumen is emptied, washed out with a siphon and examined for evidence of and the extent of chemical rumenitis, and a cud transfer (10–20 L of rumen juice) is placed in the rumen along with a few handfuls of hay. The rumenotomy will usually correct the ruminal acidosis and an alkalinizing agent in the rumen is not necessary. A large quantity of the lactic acid and its substrate can be removed. The oral or intraruminal administration of compounds such as magnesium oxide or magnesium hydroxide to cattle following complete evacuation of the rumen may cause metabolic alkalosis for up to 24–36 hours. Not all of the feed consumed will be removed because considerable quantities may have moved into the omasum and abomasum, where fermentation may also occur. The major disadvantages of a rumenotomy are time and cost, particularly when many animals are involved.
Intravenous sodium bicarbonate and fluid therapy
The systemic acidosis and the dehydration are treated with intravenous solutions of 5% sodium bicarbonate at the rate of 5 L for a 450 kg animal given initially over a period of about 30 minutes. This will usually correct the systemic acidosis. This is followed by isotonic sodium bicarbonate (1.3%) at 150 mL/kg BW intravenously over the next 6–12 hours. Cattle that respond favorably to the rumenotomy and fluid therapy will show improved muscular strength, begin to urinate within 1 hour and attempt to stand within 6–12 hours.
Rumen lavage
In less severe cases, in which affected cattle are still standing but are depressed, their heart rate is 90–100/min, there is moderate ruminal distension and the rumen pH is between 5 and 6, an alternative to a rumenotomy is rumen lavage if the necessary facilities are available. A large 25–28 mm inside-diameter rubber tube is passed into the rumen and warm water is pumped in until there is an obvious distension of the left paralumbar fossa; the rumen is then allowed to empty by gravity flow. The rumen can be almost completely emptied by 10–15 irrigations. With successful gastric lavage, alkalinizing agents are not placed in the rumen but the systemic acidosis is treated as described above.
Intraruminal alkalinizing agents
In moderately affected cases, the use of 500 g of magnesium hydroxide per 450 kg BW, or magnesium oxide in 10 L of warm water pumped into the rumen and followed by kneading of the rumen to promote mixing will usually suffice.
Magnesium hydroxide is a potent alkalinizing agent for use in ruminants as an antacid and mild laxative. It can significantly decrease rumen microbial activity and should be used only in cattle with rumen acidosis and not for symptomatic therapy of idiopathic rumen disorders or hypomagnesemia.22 The oral administration of boluses of magnesium hydroxide (162 g) or a powdered form (450 g) dissolved in 3.5 L of water daily for 3 days resulted in a significant increase in rumen pH after 48 and 24 hours, respectively. Both the boluses and the powder forms of magnesium hydroxide decreased rumen protozoal numbers and increased methylene blue reduction times compared with baseline values. There was no change in blood pH, bicarbonate or base excess values. Serum magnesium values were significantly increased in cows receiving the powder.
Ancillary therapy
Ancillary treatment has included antihistamines for laminitis, NSAIDs for shock therapy, thiamin or brewer’s yeast to promote the metabolism of lactic acid, and parasympathomimetics to stimulate gut motility. Their efficacy has been difficult to evaluate and it is unlikely that any of them would be of much value. Calcium borogluconate is used widely because there is a mild hypocalcemia and a beneficial but temporary response does occur, but it is of doubtful value.
Orally administered antimicrobials including penicillin and the tetracyclines have been used to control growth of the bacteria that produce lactic acid, but appear to be of limited value.
Monitor response to therapy
Regardless of the treatment used, all cases must be monitored several times daily until recovery is obvious, for evidence of unexpected deterioration. Following treatment, cattle should begin eating hay by the third day, some ruminal movements should be present, large quantities of soft feces should be passed and they should maintain hydration. In those that become worse, the heart rate increases, depression is marked, the rumen fills with fluid and weakness and recumbency occur. During treatment, the water supply should be restricted because some cattle, either immediately after they have engorged themselves or once they become ill, appear to have an intense thirst and will drink excessive quantities of water and die precipitously within a few hours.
The fungal rumenitis that may occur about 3–5 days after engorgement is best prevented by early effective treatment of the ruminal acidosis.
CONTROL AND PREVENTION
Cattle can be started, grown and finished on high-level grain rations successfully, providing they are allowed a gradual period of adaptation during the critical period of introduction. The important principle of prevention is that the ruminant can adapt to an all-concentrate ration. For animals that have just arrived in the feedlot, the length of the adaptation period required will depend on the immediate nutritional history of the animals, their appetite and the composition of the ration to be used.
Total mixed rations
One of the safest procedures is to feed a milled mixed ration, consisting of 50–60% roughage and 40–50% grain, as the starting ration for 7–10 days and monitor the response. If results are satisfactory, the level of roughage is decreased by 10% every 2–4 days down to a level of 10–15% roughage, with the remainder grain and vitamins–mineral–salt supplement. The use of roughage–grain mixtures insures that cattle do not engorge themselves on grain, and adaptation can occur in about 21 days.
Small incremental increases in concentrate
Another method is to begin with small amounts of concentrate 8–10 g/kg BW, which is increased every 2–4 days by increments of 10–12%. A source of roughage is supplied separately. The disadvantages of this system are that hungry or dominant cattle may eat much more than their calculated share and there is no assurance that sufficient roughage will be consumed. In this system, on a practical basis, the cattle are usually fed twice daily and brought up to a daily intake of concentrate that satisfies their appetite and then the concentrate ration is offered free-choice from self-feeders. Unless there is sufficient feeding space in the self-feeders, competitive and dominant animals will often overeat and careful monitoring is necessary.
Feedlot starter rations
Feedlot starter rations consisting of a mixture of roughage and grain, offered free-choice along with hay and gradually replaced by a finishing ration have successfully adapted cattle in 10 days. The starter ration contains about 2500 kcal (10 460 kJ) DE (digestible energy) per kg of feed. The finishing ration contains about 3100 kcal (12 970 kJ), and controlling the rate of increase of DE concentration of the ration was a major factor in getting cattle on feed.
A comparison of the effect of rapid or gradual grain adaptation on subacute acidosis and feed intake by feedlot cattle indicates a range of individual responses to grain challenge and current management strategies for preventing acidosis in pens of cattle are based on responses of the most susceptible individuals.23 Using this approach requires consideration of individual animal responses. The data suggest that most cattle can be rapidly adapted to high-grain diets in few incremental steps; minimizing acidosis in the most susceptible individuals requires decreasing the pace of grain adaptation for the entire group.
Dietary buffers
The incorporation of buffers, such as sodium bicarbonate, into the ration of feedlot cattle has been studied extensively but to date the results are inconclusive and reliable recommendations cannot be made. A level of 2% dietary sodium bicarbonate, sodium bentonite or limestone provided some protection from acidosis during the early adaptation phase of high-concentrate feeding; but they were no more effective than 10% alfalfa hay. Buffers have been most effective in reducing acidosis early in the feeding period and have little or no effect later. They may be associated with an increased incidence of urinary calculi, bloat and vitamin deficiencies. The experimental results to date are conflicting. Some trials indicate that buffers maintain a Gram-negative rumen flora in sheep fed grain compared to a shift to Gram-positive rumen flora in animals not fed buffers. Liveweight performance is also improved in some trials but not in others fed 0.75, 1.0 or 2.25% of diet as sodium bicarbonate.
The potential efficiency of products for the control of ruminal acidosis has been examined through the measurement of the increase in buffer capacity and acid-consuming capacity.24 Sodium bicarbonate provided the highest increment in buffering capacity and acid-consuming capacity compared to calcium carbonate. Magnesium oxide provided higher acid-consuming capacity but had no effect on buffer capacity.
Dietary supplementation of sodium bicarbonate at a level of 1.5% for 90 days in high-concentrate diets fed to lambs improved cellulose digestibility, ciliate protozoal number, ruminal pH and total nitrogen concentration, resulting in improved growth of lambs maintained on a high-concentrate diet.25
Ionophores
The ionophores salinomycin, monensin and lasalocid have been compared for their protective effects, and salinomycin is more effective than the other two; monensin also shows some promise. Laidlomycin propionate does not prevent ruminal acidosis but may reduce the severity of ruminal acidosis during adaptation to a 100% concentrated diet. Monensin supplementation did not affect dry matter intake, milk yield and composition, and ruminal pH characteristics in experimentally induced SARA.16 The rates of ruminal forage fiber degradability were similar between control and monensin-treated cows; however, monensin supplementation increased total digestive tract fiber digestion, especially at postruminal sites. Thus monensin could be used for the improvement of nutrient digestion during grain-induced SARA in dairy cows.26
Subacute ruminal acidosis in dairy cattle
The basic principles of preventing SARA in dairy herds include:
• Limiting the intake of rapidly fermentable carbohydrates
• Providing adequate ruminal buffering
• Allowing for ruminal adaptation to high-grain diets.27
Prevention of subacute ruminal acidosis includes proper adaptation of rumen papillae during the prepartum period, adequate intake of forage in early lactation, and adequate fiber nutrition throughout lactation. Successful management of energy balance through the periparturient transition period depends on providing adequate energy density in the prepartum diet. Increasing energy density of the prepartum diet also promotes dry matter intake before and after calving. The energy density in the prepartum diet should be 1.54–1.63 Mcal/kg NEl.
Dry cows should be fed according to their needs; cows in the early and middle portion of the dry period (far-off cows) and cows in the final 3 weeks prior to calving (pre-fresh cows) have different nutritional requirements in order to achieve optimal milk production and maintain the health and fertility of early-lactation cows.
Prepartum diets should be offered, starting at least 3 weeks prior to calving. Because of the different calving dates of dry cows fed in groups, the use of a prepartum diet over a prepartum feeding period of 21 days will usually allow each cow to consume the diet for a minimum of 5 days. The nutrient requirements for pre-fresh dry cows is controversial. The National Research Council does not provide recommendations for pre-fresh cows and it is recommended that a dairy cattle nutritionist be consulted for formulation of such rations. In general, a pre-fresh diet will provide about 0.50–0.75% BW per day as concentrates. Pre-fresh diets should be similar to early lactation diets so that the transition occurs effectively. The forages fed in the pre-fresh diet should be similar to those fed in early lactation.
Dairy cows are usually fed total mixed rations, where the concentrates and forages are mixed and fed as a total ration, or separate component rations in which the concentrates and forage are fed independently. In herds using separate component diets, the concentrates in the pre-fresh diet should be gradually introduced over a period of 3–5 days, and preferably fed individually. Forages should also be fed individually so that intake can be evaluated.
Limiting the intake of rapidly fermentable carbohydrates
As a guideline, cows should not receive more than 8–12 lb (3–5 kg) of dry matter from grain in the first week after calving.27 Grain feeding should then increase by about 0.25–0.50 lb (110–220 g) per cow per day until peak grain feeding is reached at 6–8 weeks post calving.
The physical form of the feed ingredients is as important as their chemical composition in determining how rapidly and completely they are fermented in the rumen. Grains that are finely ground, steam-flaked, extruded and/or very wet will ferment more rapidly and completely in the rumen than unprocessed or dry grains. Starch from wheat or barley is more rapidly and completely fermented than starch from corn (maize). Corn silage that is very wet, finely chopped or kernel-processed is also a greater risk for SARA than drier, coarsely chopped, or unprocessed corn silage. Particle size analysis of grains is a useful adjunct test when assessing the risk for SARA in a dairy herd. Grain particle size length can be determined using metal sieves.
Providing adequate ruminal buffering
Ruminal buffering includes dietary and endogenous buffering.27
Dietary buffering is the inherent buffering capacity of the diet and is dependent on cation–anion difference (DCAD). Diets high in sodium and potassium relative to chloride and sulfur have higher DCAD concentrations, tend to support higher ruminal pH, and increase dry matter intake and milk yield. Optimal DCAD for early lactation diets is approximately +400 mEq/kg of (Na + K) – (Cl + S). Mid-lactation cows have an optimal DCAD of +275 to +400 mEq/kg. Formulating diets with a high DCAD requires the addition of buffers such as sodium bicarbonate. Alfalfa forages have a higher DCAD than corn (maize) silage, depending on the mineral composition of the soil. Concentrate feeds typically have a low or negative DCAD, which adds to their already high potential to cause ruminal acidosis because of their high fermentable carbohydrate content.
Endogenous buffers are produced by the cow and secreted into the rumen via saliva. The amount of physical fiber in the diet determines the extent of buffer production by the salivary glands. Coarse, fibrous feeds contain more effective fiber and stimulate more saliva production during eating than do finely ground feeds or fresh pasture. Coarse, fibrous feeds also make up the mat layer of the rumen, which is the stimulus for rumination. Fiber particles must be at least 4 cm in length in order to contribute to mat layer formation. Rumination promotes much chewing activity and the secretion of large amounts of saliva into the rumen. Ruminal pH increases during bouts of rumination.
The ability of a diet and feeding program to promote maximal amounts of ruminal buffering must be evaluated in herds with SARA. Wet chemistry analysis of a carefully collected total mixed ration bunk sample can be used to determine the actual DCAD of the diet actually consumed by the cows. Diets with measured DCAD values below +275–400 mEq/kg of (Na + K) – (Cl + S) should be supplemented with additional buffers to provide more Na or K relative to Cl and S.
Endogenous buffering can be estimated by observing the number of cows ruminating (a goal is at least 40% of cows ruminating at any given time) and by measuring the particle length of the total mixed ration actually consumed by the cows using the Pennsylvania State Forage Particle Separator.27 Diets with less than 7% long particles render cows at increased risk of SARA, especially if the diets are also borderline or low in chemical fiber content. Diets with excessive (over 15%) long forage particles can paradoxically increase the risk of SARA if the long particles are unpalatable and sortable. Sorting of the long particles occurs soon after delivery of the feed, resulting in the cows consuming a diet low in physically effective fiber after feeding. The diet consumed later in the feeding period is then excessively high in physically effective fiber and low in energy. Socially dominant cows are particularly susceptible to SARA in this situation because they are likely to consume more of the fine total mixed ration particles soon after delivery of the feed. Cows lower on the social order then consume a very low-energy diet. Limiting feed bunk space to less than 75 cm per cow exacerbates the effect of total mixed ration sorting in a group of cows.
Allowing for ruminal adaptation to high-grain diets27
Cows in early lactation are susceptible to SARA if they are poorly adapted for the lactation diet. Ruminal adaptation to diets high in fermentable carbohydrates depends on microbial adaptation (particularly the lactate-utilizing bacteria, which grow more slowly than the lactate-producing bacteria) and the length of the ruminal papillae (longer rumen papillae promote greater volatile fatty acid absorption and thus lower ruminal pH).
In herds with total mixed rations, the pre-fresh diets can be offered to pre-fresh cows as they approach calving, usually with success. With total mixed rations, cows cannot eat excessive quantities of concentrate at the expense of forage. Cows that have become adapted on a well-formulated pre-calving total mixed ration during the prepartum period can go directly on to the high-producing lactating total mixed ration after calving without any further adaptation.
In summary, one of the most challenging aspects of diet formulation for lactating dairy cows is balancing for carbohydrates. Adequate effective fiber must be provided to stimulate chewing and secretion of salivary buffers. However, effective fiber is more filling than other nutritional components of the diet and the filling effect often limits the energy intake of high-producing cows. Therefore, diets for high-producing cows should be balanced to provide adequate effective fiber with the least filling effect. A balance must also be attained for ruminal carbohydrate fermentation, which is desirable to provide nutrients for microbial growth and protein. However, the fermentability of the diet must be limited to prevent excessive production of acids of fermentation.28
Feeding management in early lactation
This consists of ensuring that concentrates are introduced gradually, and preferably at the same rate as dry matter intake increases in the first 6 weeks of lactation. Formulation strategies for feeding concentrates in the first 6 weeks of lactation without compromising fiber nutrition have been developed. Weekly dry matter predictions were used and the proper increase in concentrate feeding is only 0.9–1.6 kg/week. At the same time, it is necessary to insure that cows receive adequate dietary energy to prevent primary acetonemia.
Routine monitoring of the dry-matter content of feed ingredients is an important strategy in preparing total mixed rations for dairy cattle. Electronic silage testers are available and recommended.
Ionophores, such as monensin sodium, alter rumen metabolism and have the potential to control ruminal acidosis in dairy cattle, increase milk production, modify milk composition and improve health. Monensin alters the volatile fatty acid profile in the rumen towards increased propionate production, which induces glucogenesis. Milk production is increased but the percentage of milk fat is depressed, which is effective in reducing the incidence of ketosis. Monensin decreases the population of S. bovis in the rumen, resulting in a reduction in the production of lactic acid; it increases the clearance of lactate from the rumen and increases ruminal pH. This has the potential to reduce the incidence of subacute ruminal acidosis in dairy cattle and the sequelae of rumenitis, laminitis and hepatic abscessation. Monensin also decreases ruminal methanogenesis, ruminal ammonia and blood levels of ketone bodies. Thus monensin has the potential to improve health of dairy cows and prevent ruminal acidosis during the transition period of the periparturient cow as described above. Ionophores have not yet been approved for use in lactating dairy cows in North America but extensive studies are under way.
Vaccination against lactic acidosis
Some preliminary research has investigated the immunization of cattle against lactic-acid-producing bacteria, S. bovis and Lactobacillus. Immunization induced high levels of persistent saliva antibody responses against S. bovis and Lactobacillus, which reduced the risk of lactic acidosis in cattle.29
Galyean ML, Rivera JD. Nutritionally related disorders affecting feedlot cattle. Can J Anim Sci. 2003;83:13-20.
Kleen JL, Hooijer GA, Rehage J, Noordhluizen JPTM. Subacute ruminal acidosis (SARA): a review. J Vet Med A. 2003;50:406-414.
Loerch SC, Fluharty FL. Physiological changes an digestive capabilities of newly received feedlot cattle. J Anim Sci. 1999;77:113-1119.
National Research Council. Nutrient requirements of dairy cattle, 7th ed. Washington DC: National Academy Press, 2001.
Nocek JE. Bovine acidosis: implications on laminitis. J Dairy Sci. 1997;80:1005-1028.
Nordlund K. Sore feet, sour rumens, clinical quandaries. In: Proceedings of the 33rd Annual Meeting of the American Association of Bovine Practitioners. Opelika, AL: AABP; 2000:58-64.
Nordlund K. Factors that contribute to subacute ruminal acidosis. Available on line at: http://www.vetmed.wisc.edu/dms/fapm/fapmtools/2nutr/sarafacters.pdf. In: Preconvention Seminar 7: Dairy Herd Problem Investigation Strategies. American Association of Bovine Practitioners 36th Annual Conference, September 15–17 2003, Columbus, OH.
Nordlund K. Herd-based diagnosis of subacute ruminal acidosis. Available on line at: http://www.vetmed.wisc.edu/dms/fapm/fapmtools/2nutr/sara2aabp.pdf. In: Preconvention Seminar 7: Dairy Herd Problem Investigation Strategies. American Association of Bovine Practitioners 36th Annual Conference, September 15–17 2003, Columbus, OH
Oetzel GR. Clinical aspects of ruminal acidosis in dairy cattle. In: Proceedings of the 33rd Annual Meeting of the American Association of Bovine Practitioners. Opelika, AL: AABP; 2000:46-53.
Oetzel GR. Introduction to ruminal acidosis in dairy cattle. Available on line at: http://www.vetmed.wisc.edu/dms/fapm/fapmtools/2nutr/sara1aabp.pdf. In: Preconvention Seminar 7: Dairy Herd Problem Investigation Strategies. American Association of Bovine Practitioners 36th Annual Conference, September 15–17 2003, Columbus, OH.
Oetzel GR. Nutritional management and subacute ruminal acidosis. Available on line at: http://www.vetmed.wisc.edu/dms/fapm/fapmtools/2nutr/sara3aabp.pdf. In: Preconvention Seminar 7: Dairy Herd Problem Investigation Strategies. American Association of Bovine Practitioners 36th Annual Conference, September 15–17 2003, Columbus, OH.
Owens FN, Secrist DS, Hill WJ, Gill DR. Acidosis in cattle: a review. J Anim Sci. 1998;76:275-286.
1 Owens FN, et al. J Anim Sci. 1998;76:275.
2 Nocek JE. J Dairy Sci. 1997;80:1005.
3 Oetzel GR. Proceedings of the 33rd Annual Meeting of the American Association of Bovine Practitioners. Opelika, AL: AABP, 2000;46.
4 Kleen JL, et al. J Vet Med A. 2003;50:406-414.
5 Nordlund K. Proceedings of the 33rd Annual Meeting of the American Association of Bovine Practitioners. Opelika, AL: AABP, 2000;58.
6 Oetzel GR. http://www.vetmed.wisc.edu/dms/fapm/fapmtools/2nutr/sara1aabp.pdf, 2003.
7 Keunen JE, et al. J Dairy Sci. 2002;85:3304.
8 National Research Council. Nutrient requirements of dairy cattle, 7th ed. Washington, DC: National Academy Press, 2001.
9 Nordlund K. http://www.vetmed.wisc.edu/dms/fapm/fapmtools/2nutr/sarafacters.pdf, 2003.
10 Enemark JMD, Jorgensen RJ. Vet Q. 2001;23:206.
11 Galyean ML, Rivera JD. Am J Anim Sci. 2003;83:13.
12 Loerch SC, Fluharty FL. J Anim Sci. 1999;77:1113.
13 Garrett EF, et al. J Dairy Sci. 1999;82:1170.
14 Ortolani EL. Vet Hum Toxicol. 1995;37:462.
15 Keunen JE, et al. J Dairy Sci. 2003;86:954.
16 Cumby JL, et al. Can J Anim Sci. 2001;81:149.
17 Krajcarski-Hunt H, et al. J Dairy Sci. 2002;85:570.
18 Plaizer JC, et al. Can J Anim Sci. 2001;81:421.
19 Gozho GN, et al. J Dairy Sci. 2005;88:1399.
20 Nordlund K. http://www.vetmed.wisc.edu/dms/fapm/fapmtools/2nutr/sara2aabp.pdf, 2003.
21 Steen A. Acta Vet Scand. 2001;42:219.
22 Smith GW, Correa MT. J Vet Intern Med. 2004;18:109.
23 Bevans DW, et al. J Anim Sci. 2005;83:1116.
24 Zammarreno AM, et al. J Sci Food Agric. 2003;83:1607.
25 Santra A, et al. Small Rumin Res. 2003;47:203.
26 Osborne JK, et al. J Dairy Sci. 2004;87:1840.
27 Oetzel GR. http://www.vetmed.wisc.edu/dms/fapm/fapmtools/2nutr/sara3aabp.pdf, 2003.
28 Allen M. Proceedings of the 33rd Annual Meeting of the American Association of Bovine Practitioners. Opelika, AL: AABP, 2003;1.