HEMORRHAGIC BOWEL SYNDROME IN CATTLE (JEJUNAL HEMORRHAGE SYNDROME)
Hemorrhagic bowel syndrome, also known as jejunal hemorrhage syndrome, is a recently recognized disease of cattle characterized clinically by a syndrome similar to obstruction of the small intestine causing abdominal distension, dehydration and shock due to necrohemorrhagic enteritis affecting primarily the small intestine. At necropsy there is segmental necrohemorrhagic enteritis of the small intestine and large intraluminal blood clots. In spite of intensive medical and surgical therapy, the prognosis is unsatisfactory and the case fatality rate is almost 100%.
ETIOLOGY
The etiology is unknown. C. perfringens type A has been isolated from the intestines of naturally occurring cases but its significance is uncertain. Because C. perfringens type A can be found in the intestinal tracts of healthy cattle and is able to proliferate quickly after death, the role of the organism in the pathogenesis of hemorrhagic jejunitis is uncertain.
EPIDEMIOLOGY
The disease occurs sporadically, primarily in mature lactating dairy cows in North America.1,2 Individual cases have occurred in beef cows.3 In Germany, the disease occurs in Simmental cattle.4
The morbidity is low but the case fatality rate is almost 100%.3
Investigations of herds with cases have failed to identify any reliable possible risk factors.2 Most cases occur in lactating dairy cows in the first 3 months of lactation. In a single dairy herd, 22 cases occurred in a period of 4 years. Affected cows ranged from 2–8 years of age and the time since parturition ranged from 9–319 days.
A mail and conference survey of dairy cattle veterinarians in Minnesota found that the disease occurred with greatest frequency in lactating dairy cows in early lactation under a wide variety of management systems, in varying herd sizes and in both free-stall and tie-stall housing systems.1 The incidence appeared to be higher in herds of more than 100 cows and in herds using total mixed rations.
As part of the National Animal Health Monitoring System’s Dairy 2002, information was collected about hemorrhagic jejunitis in dairy cattle in the USA.5 The disease was observed in 9.1% of herds within the previous 5 years and in 5.1% of herds during the preceding 12 months. Risk factors found to be associated with the disease during the preceding 12 months were large herd size, administration of bovine somatotrophin and routine use of milk urea nitrogen concentration to determine ration composition. Use of pasture as part of the lactating cow ration during the growing season was associated with decreased odds of the disease in herds with a rolling herd average milk production of 20 000 lb or less, whereas in herds with higher milk production, use of pasture was not associated with the occurrence of the disease. For individual cows with signs consistent with the disease, the third lactation was the median of the parity distribution and the medial time between parturition and the onset of clinical signs was 104 days. In summary, management practices implemented to achieve high milk production may increase the risk of developing the disease in dairy cattle. Increased consumption of high-energy diet seems to be the most plausible common pathway of all the risk factors that have been described.5
Feeding rations high in soluble carbohydrates has been suggested as a possible risk factor by providing the intestinal environment for C. perfringens type A to proliferate and produce enterotoxins, similar to the situation that may cause hemorrhagic enteritis, abomasitis and abomasal ulceration in calves.6
PATHOGENESIS
The primary lesion is an acute localized necrotizing hemorrhagic enteritis of the small intestine leading to the development of an intraluminal blood clot, which causes a physical obstruction of the intestine, and ischemia and devitalization of the wall of the affected segment of the intestine.6 The lesion is similar to hemorrhagic enterotoxemia associated with C. perfringens in young rapidly growing calves, lambs or piglets.
There is gastrointestinal stasis with accumulation of intestinal gas and fluids proximal to the obstructed intestine, resulting in distended loops of intestine, hypochloremia, hypokalemia, dehydration and varying degrees of anemia. The serum biochemistry changes are those of an obstruction of the upper small intestine and sequestration of abomasal secretions, with resultant hypokalemia and hypochloremia. The hemorrhagic enteritis is progressive, with the ischemia and necrosis extending through the intestinal wall, and within 24–48 hours there is marked fibrinous peritonitis, dehydration, continued electrolyte imbalance, marked toxemia and death.
CLINICAL FINDINGS
Common historical findings include sudden anorexia and depression, marked reduction in milk production, abdominal distension, weakness progressing to recumbency, bloody to dark-red feces or dry scant feces, dehydration and abdominal pain, including bruxism, vocalization, treading and kicking at the abdomen.6 Sudden death without prior clinical findings has been reported.6
On clinical examination there is depression, dehydration, the body temperature may be normal to slightly elevated, the heart rate is increased to 90–120 beats/min, the mucous membranes are pale and the respiratory rate is increased. The abdomen is usually distended moderately over the right side. The rumen is usually atonic. Fluid-splashing sounds are commonly audible by succussion over the right abdomen. In some cases, a ping can be elicited over the right abdomen.
On rectal examination, the feces are black–red, jelly-like and sticky, and smell like digested blood.4 On deep palpation of the right abdomen, distended loops of intestine may be palpable, some of which are firm (those loops containing the blood clot) while others may be resilient, representing loops of intestine proximal to the blood clot obstruction that contain excessive fluid and gas and in which the intestine is in a state of ileus.
The course of the disease in most cases is 2–4 days. Even with intensive fluid and electrolyte therapy, affected animals continue to worsen progressively, become weak, recumbent and die, or euthanasia is chosen.
On laparotomy, the abomasum is commonly distended with fluid. Up to 60–100 cm of small intestine may be distended and firm to touch, with a markedly dark red to purplish hemorrhagic serosal surface covered with fibrin tags. The mesenteric band may be too tense to allow exteriorization of the affected intestine. Manipulation of the affected intestine may lead to its rupture because of its thin and fragile intestinal wall due to ischemia and devitalization. The small intestine proximal to the affected segment is usually distended with fluid and gas and compressible; that distal to the affected segment is usually relatively empty.
CLINICAL PATHOLOGY
Hematology
The hemogram is variable and not diagnostic. Leukocytosis and mature neutrophilia with increased band neutrophils and increased fibrinogen concentrations are common but neutropenia with a left shift may also occur.7 The PCV and plasma protein concentrations are variable.
Serum biochemistry
Metabolic alkalosis with compensatory respiratory acidosis, hypokalemia and hypochloremia are common, which is consistent with abomasal outflow obstruction due to the obstruction caused by the clotted blood or ileus.7
NECROPSY FINDINGS
The abdomen is moderately distended as a result of marked dilatation of the small intestine, which is dark red, hemorrhagic and commonly covered by fibrinous exudate. The affected segment of intestine, especially the jejunum and ileum, may be 1 m or more in length and contains a firm blood clot, adherent to the mucosa, which is necrotic and hemorrhagic over the entire length of the affected portion.
Histologically, there is multifocal submucosal edema and neutrophil infiltration, segmental necrosis, ulceration, and mucosal and transmural hemorrhage (hematoma) of the jejunum. Frequently, the epithelium is completely sloughed and, in the area of attachment of the blood clot, the mucosa is absent.7 Extensive fibrin and neutrophil infiltration occur on the serosal surface and fibrinous peritonitis is common.
C. perfringens type A has been isolated from the intestinal contents of typical cases but its significance is unknown.
TREATMENT
No specific treatment is available. For valuable animals, intensive fluid and electrolyte therapy is indicated. Because of the possibility of clostridial infection, penicillin is indicated if treatment is attempted. Laparotomy and resection of the affected segment of the intestine and anastomosis is indicated but has been unsuccessful to date.
The disease must be differentiated from other causes of acute physical or functional obstruction of the small intestine causing distended loops of intestine, fluid-splashing sounds on ballottement of the abdomen and dehydration and electrolyte imbalances. These include intussusception, cecal dilatation and volvulus and diffuse peritonitis (causing ileus). In ileal impaction in mature cows, distended loops of intestine are palpable on rectal examination but on laparotomy the abnormalities consist of ileal impaction and distended loops of intestine which are amenable to treatment.
Diseases causing melena and dysentery include bleeding abomasal ulcers, acute salmonellosis and coccidiosis.
Transabdominal ultrasonography (Fig. 6.8) can be used to detect ileus of the small intestine and distension of loops of small intestine with homogeneous echogenic intraluminal material compatible with intraluminal hemorrhage and clot formation.2
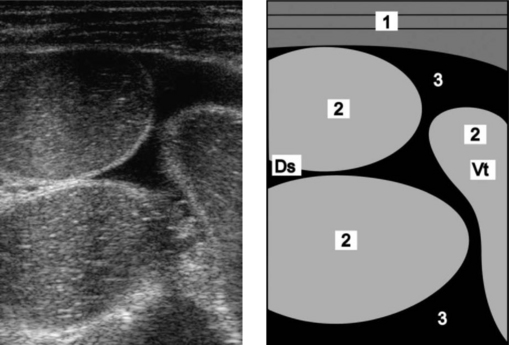
Fig. 6.8 Ultrasonogram and schematic of the abdomen in a cow with ileus due to obstruction of the jejunum with coagulated blood (hemorrhagic bowel syndrome). The jejunal loops are dilated and there is anechoic fluid (transudate) between the dilated loops. The ultrasonogram was obtained from the right abdominal wall caudal to the last rib using a 5.0 MHz-linear scanner. 1 = Lateral abdominal wall; 2 = Dilated jejunal loops; 3 = Anechoic fluid between the jejunal loops. Ds, Dorsal; Vt, Ventral.
(Reproduced with kind permission of U. Braun.)
1 Godden S, et al. Bovine Pract. 2001;35:97.
2 Dennison AC, et al. J Am Vet Med Assoc. 2002;221:686.
3 Abutarbush SM, et al. Can Vet J. 2004;45:48.
4 Von Rademacher G, et al. Tierarztl Umsch. 2002;57:399.
5 Berghans RD, et al. J Am Vet Med Assoc. 2005;226:1700.
INTESTINAL OBSTRUCTION IN SHEEP
Intestinal obstructions are not commonly observed in sheep unless a series of them causes a noticeable mortality. Some notable occurrences have been:
• Heavy infestation with nodular worm (Oesophagastomum columbianum) leading to high prevalence of intussusception occlusion by adhesion
• High incidence of intussusception in traveling sheep for no apparent reason
• Cecal torsion (red-gut) in sheep grazing lush pastures of alfalfa or clover in New Zealand. Affected lambs survive only a few hours and up to 20% of a flock are affected. The outstanding postmortem lesion is a distended, reddened cecum and/or colon that has undergone torsion. The rumen is smaller and the large intestine larger than normal because of the high digestibility of the diet. All ages, except sucking lambs, are affected and the mortality rate may be as high as 20%. Sheep that are seen alive have a distended abdomen, show abdominal pain and have tinkling sounds on auscultation of the right flank.
TERMINAL ILEITIS OF LAMBS
This disease causes poor growth in lambs 4–6 months old. The circumstances usually suggest parasitism or coccidiosis. The terminal 50–75 cm of the ileum is thickened and resembles the classical lesion of Johne’s disease. Chronic inflammation is evident and there are some shallow ulcers in the epithelium. The terminal mesenteric lymph node is enlarged. Histopathological examination of affected ileal wall shows mucosa thickened by epithelial hyperplasia, leukocytic infiltration and connective tissue infiltration. The cause is unknown, and the course of the disease has not been identified because most affected lambs are likely to be culled for ill-thrift.