Chapter 7 Diseases of the liver and pancreas
DISEASES OF THE LIVER–INTRODUCTION 383
PRINCIPLES OF HEPATIC DYSFUNCTION 383
MANIFESTATIONS OF LIVER AND BILIARY DISEASE 384
SPECIAL EXAMINATION OF THE LIVER 387
PRINCIPLES OF TREATMENT IN DISEASES OF THE LIVER 391
DIFFUSE DISEASES OF THE LIVER 391
FOCAL DISEASES OF THE LIVER 395
DISEASES OF THE PANCREAS 396
Diseases of the liver – introduction
Primary diseases of the liver, with the exception of the fat cow syndrome of cows in early lactation, seldom occur in farm animals except as a result of poisoning. Liver metabolism in late pregnancy and early lactation in dairy cows is under a great deal of stress. The metabolic demands at these times are much increased and require that the liver synthesize more glucose from noncarbohydrate precursors, metabolize butyrate and, because the cow is so often in negative energy balance, mobilize body fat, resulting in an increase in deposition of fat in the liver; a fatty liver and the fat cow syndrome may result (see Ch. 28).
Secondary disease of the liver, arising as part of a generalized disease process or by spread from another organ, occurs more commonly. In primary hepatic disease the clinical manifestations are caused solely by the lesions in the liver while in secondary involvement the syndrome may include clinical signs unrelated to the hepatic lesions. This chapter is devoted to a consideration of primary diseases of the liver and to those aspects of other diseases in which manifestations of hepatic involvement occur.
Diseases of the liver are in general neglected by agricultural animal clinicians and clinical descriptions of them are meager.
Principles of hepatic dysfunction
DIFFUSE AND FOCAL HEPATIC DISEASE
The liver has a large reserve of function and approximately three-quarters of its parenchyma must be rendered inactive before clinical signs of hepatic dysfunction appear. Diffuse diseases of the liver are more commonly accompanied by signs of insufficiency than are focal diseases, which produce their effects either by the toxins formed in the lesions or by pressure on other organs, including the biliary system. The origin of a toxemia is often difficult to localize to the liver because of the physical difficulty of examining the organ.
Diffuse diseases of the liver can be classified as hepatitis and hepatosis according to the pathological change that occurs, and the classification also corresponds roughly with the type of causative agent. Clinically the differences between these two diseases are not marked, although some assistance can be obtained from clinicopathological examination.
HEPATIC DYSFUNCTION
There are no specific modes of hepatic dysfunction. The liver has several important functions and any diffuse disease of the organ interferes with most or all of the functions to the same degree. Variations occur in the acuteness and severity of the damage but the effects are the same and the clinical manifestations vary in degree only. The major hepatic functions that, when disordered, are responsible for clinical signs include:
• The maintenance of normal blood glucose levels by providing the source as glycogen
• The formation of some of the plasma proteins
• The formation and excretion of bile salts and the excretion of bile pigments
• The formation of prothrombin
• The detoxification and excretion of many toxic substances, including photodynamic agents.
The clinical signs produced by interference with each of these functions are dealt with under manifestations of hepatic dysfunction. A rather special aspect is the role of the liver in the genesis of primary ketosis of cattle.
PORTAL CIRCULATION
The portal circulation and the liver are mutually interdependent, the liver depending upon the portal vein for its supply of nutrients and the portal flow depending upon the patency of the hepatic sinusoids. The portal flow is unusual in that blood from the gastrosplenic area and the lower part of the large intestine passes to the left half of the liver and the blood from the two intestines to the right half, without mixing of the two streams in the portal vein. The restriction of toxipathic hepatitis to one half of the liver and the localization of metastatic abscesses and neoplasms in specific lobes results from the failure of portal vein blood from different gut segments to mix. The localization of toxipathic hepatitis may be because of selective distribution of the toxin or of protective metabolites. The passage of blood from the portal circuit through the liver to the caudal vena cava is dependent upon the patency of the hepatic vascular bed, and obstruction results in damming back of blood in the portal system, portal hypertension, interference with digestion and absorption, and in the final stages the development of ascites.
Manifestations of liver and biliary disease
JAUNDICE
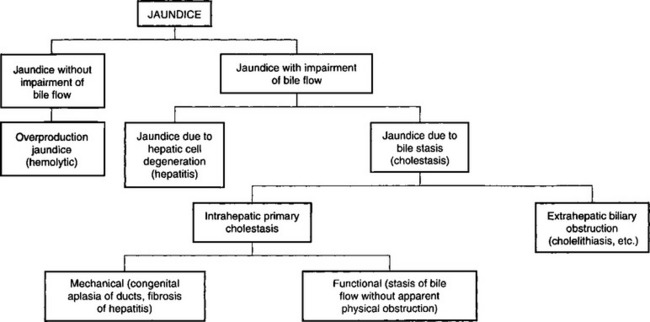
Jaundice is a clinical sign that often arises in diseases of the liver and biliary system but also in diseases in which there are no lesions of these organs. It does not always occur and may be conspicuously absent in acute hepatitis. Although jaundice is a result of the accumulation of bilirubin, the staining is much more pronounced with conjugated (direct) bilirubin than with unconjugated (indirect) bilirubin. Thus the jaundice is more intense in cases of obstructive and hepatocellular jaundice than in hemolytic jaundice. The levels of bilirubin in blood also affect the intensity of the jaundice, the obstructive form often being associated with levels of bilirubin that are ten times higher than those commonly seen in hemolytic anemia. The staining of jaundice is due to staining of tissues, especially elastic tissue, and not to accumulation in tissue fluids, so that it is best detected clinically in the sclera, and jaundice that may be detectable easily at necropsy may not be visible on clinical examination. Many classifications have been suggested but the simplest is that proposed by Popper and Schaffner, illustrated in Figure 7.1.
The primary differentiation has to be made between jaundice with and without impairment of bile flow. Some indication of the type of jaundice can be derived from clinical examination. Thus jaundice is usually much more severe when impairment of flow occurs and when bile pigments are absent from the feces. However, obstructive jaundice can occur with only partial occlusion of hepatic flow provided at least half the bile flow is obstructed. In such cases jaundice may occur even though bile pigments are still present in the feces. With lesser obstruction the portion of the liver and biliary tract that is functioning normally excretes the extra load of bile pigments. The only accurate basis for the differentiation between jaundice with impaired bile flow and jaundice without impaired flow is the examination of the urine for the presence of bilirubin and urobilinogen and the determination of the relative amounts of conjugated and unconjugated bilirubin present in the serum. Unconjugated (indirect) bilirubin that has not passed through hepatic cells is not excreted by the kidney, so that in hemolytic jaundice the indirect bilirubin content of serum is increased markedly and, although the urine contains an increased amount of urobilinogen, no bilirubin is present. In those cases in which jaundice is caused by impairment of bile flow there is a marked increase in the serum level of conjugated (direct) bilirubin, and the bilirubin content of the urine is greatly increased. The amount of urobilinogen varies depending on whether any bilirubin reaches the intestine to be metabolized to urobilinogen and reabsorbed. In complete extrahepatic biliary obstruction urobilinogen is not present in the urine.
OVERPRODUCTION OR HEMOLYTIC JAUNDICE
Hemolytic jaundice is common in animals and may be associated with bacterial toxins, invasion of erythrocytes by protozoa or viruses, inorganic and organic poisons and immunological reactions. Diseases in which bacterial toxins cause intravascular hemolysis are bacillary hemoglobinuria of cattle and leptospirosis, although the mechanism by which hemolysis is produced in the latter disease does not seem to have been accurately determined. The common protozoan and viral diseases in which hemolysis occurs include babesiosis, anaplasmosis, eperythrozoonosis and equine infectious anemia. Chronic copper poisoning, selenium poisoning in sheep, phenothiazine poisoning in horses, pasturing on rape and other cruciferous plants and bites by some snakes are other common causes. Postparturient hemoglobinuria has an uncertain etiology but is usually attributed to a deficiency of phosphorus in the diet and the feeding of cruciferous plants. Isoimmunization hemolytic anemia of the newborn is caused by an immunological reaction between the sensitized cells of the newborn and antibodies in the colostrum of the dam. The occurrence of acute hemolytic anemia and jaundice in calves that drink large quantities of cold water may also be of the nature of an immunological response.
Neonatal jaundice is relatively common in babies and is regarded as a benign condition. It is rarely, if ever, observed clinically in newborn animals but may be noticeable at necropsy. Although it is generally stated that the jaundice is hemolytic and results from the destruction of excess erythrocytes when postnatal life begins, it appears more probable that it is due to retention of bile pigments because of the immaturity of the hepatic excretion mechanism. It does occur in foals and is an important differential diagnosis from isoerythrolysis.
Hemolytic jaundice is characterized clinically by a moderate degree of yellowing of the mucosae, and by the presence of hemoglobinuria in severe cases. Clinicopathological findings indicate the presence of anemia, an increase in urobilinogen and an absence of bilirubin in the urine, and a preponderance of indirect bilirubin in the serum.
JAUNDICE DUE TO HEPATIC CELL DEGENERATION
The cause may be any of those diffuse diseases of the liver that cause degeneration of hepatic cells, which are listed under hepatitis. Because there is only partial obstruction of biliary excretion, the changes in serum and urine lie between those of hemolytic jaundice and extrahepatic biliary obstruction. Serum levels of total bilirubin are increased because of retention of direct bilirubin, which also passes out in the urine, causing an elevation of urine levels. The urobilinogen levels in the urine also rise.
EXTRAHEPATIC BILIARY OBSTRUCTION
Obstruction of the bile ducts or common bile duct by biliary calculi or compression by tumor masses is a rare occurrence in farm animals. Commonly listed causes are obstruction of the common duct by nematodes and inflammation of the bile ducts by extension from an enteritis or by infestation with trematodes.
A significant number of pigs die with biliary obstruction and purulent cholangitis secondary to invasion of the ducts by Ascaris lumbricoides. Parasitic cholangitis and cholecystitis also occur due to fascioliasis and infestation with Dicrocoelium dentriticum. In horses an ascending cholangitis may develop from a parasitic duodenal catarrh and cause signs of biliary obstruction.
Obstruction is usually complete and results in the disappearance of bile pigments from the feces. Serum levels of conjugated bilirubin rise, causing a marked elevation of total bilirubin in the serum. Excretion of the conjugated bilirubin in urine occurs on a large scale but there is no urobilinogen because of the failure of excretion into the alimentary tract. Partial obstruction of the common bile duct or occlusion of a number of major bile ducts may cause variations in serum and urine similar to those observed in complete obstruction, except that the feces do contain bile pigments and urobilinogen appears in the urine. In this circumstance it is difficult to differentiate between partial extrahepatic biliary obstruction and jaundice caused by hepatic cell degeneration (see above).
JAUNDICE DUE TO INTRAHEPATIC PRIMARY CHOLESTASIS
The mechanical stasis of biliary flow caused by fibrous tissue constriction and obliteration of the small biliary canaliculi may occur after hepatitis and in many forms of fibrosis. Cholelithiasis, the formation of biliary calculi, is frequently reported as a cause of cholestasis in humans and has been reported in horses1 and cattle.2 Functional stasis is a major problem in hepatic disease in humans but has not been defined in animals. In both instances the defect is the same as in extrahepatic biliary obstruction and the two diseases cannot be differentiated by laboratory tests.
NERVOUS SIGNS (HEPATIC ENCEPHALOPATHY)
These are common with any severe hepatocellular insufficiency or major circulatory bypass of the liver. Terminally, hepatic coma may occur. The biochemical and anatomical basis for these signs is not well understood. Many factors, including hypoglycemia and failure of normal hepatic detoxification mechanisms, leading to the accumulation of excess amino acids and ammonia, or of acetylcholine, and the liberation of toxic breakdown products of liver parenchyma, have all been suggested as causes and it is probable that more than one factor is involved.
One of the primary effects of severe, acute liver damage is a precipitate fall in blood glucose accompanied by nervous signs, including hyperexcitability, convulsions and terminal coma. If the hepatic damage occurs more slowly the hypoglycemia is less marked and less precipitous and is accompanied by: inability to perform work, drowsiness, yawning and lethargy. With persistent hypoglycemia, structural changes may occur in the brain (hypoglycemic encephalopathy) and these may be the basis for the chronically drowsy animals or dummies.
However, hypoglycemia does not always occur in acute hepatitis and cannot be considered to be the only or even the most important factor in producing the cerebral signs.
High blood levels of ammonia occur in pyrrolizidine poisoning in sheep, and are reflected in the development of spongy degeneration in the brain and the clinical signs of hepatic encephalopathy.
Status spongiosa has also been reproduced experimentally in sheep and calves by the intravenous infusion of ammonia. This role of ammonia as a cerebrotoxicant can be important in hepatopathies in which the detoxicating function of the liver is lost, and also in congenital defects of hepatic vasculature in which blood is bypassed around the liver. In the latter case ammonia and similar toxic byproducts of protein degradation in the large intestine avoid the detoxication filter of the liver. Blood ammonia levels are increased and sulfobromophthalein sodium (BSP) dye clearance is delayed.
The most common cause of hyperammonemia and encephalopathy in the horse is a depression of hepatic function due to acute or chronic liver disease. The severity of encephalopathy clinically correlates well with the degree of hepatic functional compromise but only poorly with the degree of hyperammonemia. Other factors, such as hypokalemia, alkalosis, short-chain volatile fatty acids, and false and true neurotransmitters, may be important in the pathogenesis of hepatic coma in cattle and horses.3,4
Neurological disease was reported in a 13-year-old horse with hyperammonemia and no gross or histological evidence of disease.5 The ammonia level was 475 μmol/L (normal 7–60 μmol/L). Neurological signs included apparent blindness, ataxia, falling, dysphagia, bruxism, circling, head pressing, muscle fasciculations, yawning and depression.
Plasma ammonia levels are also significantly elevated in cattle with hepatic disease. Clinical signs of hepatic encephalopathy such as blindness, head pressing, excitability, ataxia and weakness, together with fever and jaundice, are grave prognostic signs.4
An intrahepatic porto-systemic shunt causing hepatoencephalopathy has been reported in a 3-month-old goat.6
EDEMA AND EMACIATION
Failure of the liver to anabolize amino acids and protein during hepatic insufficiency is manifested by tissue wasting and a fall in plasma protein. This may be sufficiently severe to cause edema because of the lowered osmotic pressure of the plasma. Hepatic edema is not usually very marked and is manifested most commonly in the intermandibular space (bottle jaw). If there is obstruction to the portal circulation, as may occur in hepatic fibrosis, the edema is much more severe but is largely limited to the abdominal cavity.
DIARRHEA AND CONSTIPATION
In hepatitis, hepatic fibrosis and obstruction or stasis of the biliary system, the partial or complete absence of bile salts from the alimentary tract deprives it of the laxative and mildly disinfectant qualities of these salts. This, together with the reflex effects from the distended liver in acute hepatitis, produces an alimentary tract syndrome comprising anorexia, vomition in some species and constipation punctuated by attacks of diarrhea. The feces are pale in color and, if there is an appreciable amount of fat in the diet, there is steatorrhea.
PHOTOSENSITIZATION
Most photosensitizing substances, including phylloerythrin, the normal breakdown product of chlorophyll in the alimentary tract, are excreted in the bile. In hepatic or biliary insufficiency excretion of these substances is retarded and photosensitization occurs.
HEMORRHAGIC DIATHESIS
In severe diffuse diseases of the liver there is a deficiency in prothrombin formation and a consequent prolongation of the clotting time of the blood. Abnormality of the prothrombin complex is not the only defect, deficiencies of fibrinogen and thromboplast also occurring. Prothrombin and other factors in the prothrombin complex depend upon the presence of vitamin K for their formation and an absence of bile salts from the intestine retards the absorption of this fat-soluble vitamin. Parenteral administration of vitamin K is advisable before surgery is undertaken in patients with severe hepatic dysfunction.
ABDOMINAL PAIN
Two mechanisms cause the pain in diseases of the liver: distension of the organ with increased tension of the capsule, and lesions of the capsule. Acute swelling of the liver occurs as a result of engorgement with blood in congestive heart failure and in acute inflammation. Inflammatory and neoplastic lesions of the capsule, or of the liver parenchyma just beneath the capsule, cause local irritation to its pain end organs. The pain is usually subacute, causing abnormal posture, particularly arching the back, and disinclination to move. Tenseness of the abdominal wall and pain on deep palpation over the liver area may also be detected in the majority of cases.
ALTERATION IN SIZE OF THE LIVER
Great variation in the size of the liver is often seen at necropsy but clinical detection is not easy unless the liver is grossly enlarged. This is most likely to occur in advanced congestion of the liver due to congestive heart failure, in some plant poisonings in horses and when multiple abscesses or neoplastic metastases occur. In acute hepatitis the swelling is not sufficiently large to be detected clinically and in terminal fibrosis the liver is much smaller than normal.
Atrophy of the right lobe of the liver occurs in the horse and may be related to chronic distension of adjacent segments of the intestinal tract.7 The normal equine liver is anatomically bisected into two approximately equal halves by the umbilical interlobar fissure; and additional interlobular fissures divide the liver into four distinct lobes in the foal: right, left, quadrate and caudate. In horses with right lobe atrophy, the capsule of the right lobe is wrinkled and thick when atrophy is severe. In clinically normal horses, the right lobe constitutes half of the total liver weight while the right lobe in horses with atrophy ranges from 11.0–38.8% of the total liver weight.7 This is thought to be due to long-term, insidious compression of this portion of the liver by abnormal distension of the right dorsal colon and base of the cecum.
DISPLACEMENT OF THE LIVER
The liver may be displaced from its normal position and protrude into the thoracic cavity through a diaphragmatic hernia, causing respiratory distress and abnormal findings on percussion of the chest. Torsion of a lobe of the liver has been recorded in aged sows in the early part of lactation.8 Inappetence, uneasiness and unwillingness to suckle the young were followed by severe, prolonged vomiting, acute abdominal pain and dyspnea. The twisted lobe was greatly increased in size and in one case the capsule was ruptured, leading to severe internal hemorrhage.
RUPTURE OF THE LIVER
Rupture of the liver is an occasional accident in animals, occurring usually as a result of trauma. In most instances rupture results in death from hemorrhage, although small breaks in the capsule may heal. Horses used for the production of serum frequently develop hepatic amyloidosis, presumably as a reaction to repeated injection of foreign protein, and the death rate from rupture of the liver is relatively high in this group.9 Amyloidosis is essentially a space-occupying lesion, which results in a liver with a friable texture. The amyloid masses exert pressure on liver cell cords and sinusoids, gradually causing pressure atrophy, ischemic degeneration and necrosis of hepatic parenchyma.
A high prevalence of liver rupture is recorded in newborn lambs of the North Country Cheviot breed. Losses resulting from the condition were 12.5% of all neonatal deaths in purebred lambs, and varied from 6.4–24.7% on individual farms. The lambs are stillborn, or are born alive but become anemic and weak and die within 12 hours of birth from internal hemorrhage. It is thought that the cause of the fatal anemia is an inherited short sternum, which exposes the liver to compression and rupture of its capsule. Vitamin E deficiency in the ewes and lambs may also be a factor.10
BLACK LIVERS OF SHEEP
Dark brown to black pigmentation of the liver and kidneys occurs commonly in sheep in certain parts of Australia. No illness is associated with the condition but the livers are not used for human consumption for esthetic reasons and extensive financial loss may result. Commonly referred to as ‘melanosis’, the pigmentation has been determined to be the result of deposition of the pigment lipofuscin at various stages of oxidation. Areas in which the disease occurs carry many mulga trees (Acacia aneura), the leaves of which are fed to sheep in drought times.
The above condition should not be confused with the black livers found in a mutant strain of Corriedales in California. In these mutant sheep there is photosensitization following retention of phylloerythrin. The darkening of the liver is due to melanin.
Special examination of the liver
When disease of the liver is suspected after a general clinical examination, special techniques of palpation, biopsy and biochemical tests of function can be used to determine further the status of the liver.
PALPATION AND PERCUSSION
In cattle, the liver is well concealed by the rib cage on the right-hand side and its edge cannot be palpated. A general impression of the size of the liver can be obtained by percussion of the area of liver dullness but accurate definition is not usually attempted. Deep percussion or palpation to detect the presence of hepatic pain can be carried out over the area of liver dullness in the posterior thoracic region on the right-hand side. Percussion over the entire area is necessary, as the pain of a discrete lesion may be quite localized.
If the liver is grossly enlarged in cattle, its edge can be felt on deep palpation behind the costal arch and the edge is usually rounded and thickened compared to the more defined edge of the normal liver. In cattle, the liver may be enlarged and palpable in advanced right-sided congestive heart failure, multiple liver abscesses and diffuse hepatitis. This type of palpation is relatively easy in ruminants but is unrewarding in horses and pigs because of the thickness of the abdominal wall and the shortness of the flank.
BIOPSY
Biopsy of the liver has been used extensively as a diagnostic procedure in infectious equine anemia, poisoning by Crotalaria spp. and other species of plants, and experimental work on copper and vitamin A deficiency. The technique requires some skill and anatomical knowledge.
The most satisfactory instrument is a long, small-caliber trocar and cannula to which is screwed a syringe capable of producing good negative pressure. The sharp point of the instrument is introduced in an intercostal space on the right-hand side (the number depending on the species) and advanced across the pleural cavity so that it will reach the diaphragm and diaphragmatic surface of the liver at an approximately vertical position. The point of insertion is made high up in the intercostal space so that the liver is punctured at the thickest part of its edge. For example, in cattle the biopsy is made in the 11th intercostal space at a point on an imaginary line between the right elbow and tuber coxa. The instrument is rotated until the edge of the cannula approximates the liver capsule; the trocar is then withdrawn, the syringe is attached and strong suction is applied; the cannula is twisted vigorously and advanced until it reaches the visceral surface of the liver. If its edge is sufficiently sharp the cannula will now contain a core of liver parenchyma and if the instrument is withdrawn with the suction still applied a sample sufficient for histological examination and microassay of vitamin A, glycogen or other nutrient is obtained.
Details of the technique for cattle,11,12 sheep13 and horses14 are available. A disposable biopsy needle suitable for use in animals is available and a needle has also been designed that includes a device that ensures that the core of tissue in the cannula is in fact detached from the parenchyma of the organ.14
A system to score liver biopsies as a prognostic aid in horses with suspected liver disease is highly reliable.15 Horses with scores of 0 or 1 were equally likely to survive up to 6 months with a combined mortality of 4%. Horses with biopsy scores between 2 and 6 had a combined mortality of 33% and were at a 12-fold increased risk of nonsurvival within 6 months compared to horses with a biopsy score of 0. Horses with biopsy scores between 7 and 14 had a combined mortality of 86% and were at a 46-fold increased risk of nonsurvival compared to horses with a biopsy score of 0. The evidence indicates that liver biopsy is the one antemortem test of greatest value in the absence of noninvasive tests that are able to reliably distinguish horses with significant liver disease from those without. Examination of liver biopsies may establish the presence of liver disease, provide a specific diagnosis, guide therapeutic choice and also help determine prognosis in cases of suspected liver disease.
Multiple liver biopsies can be done safely in neonatal calves from 4–28 days of age.16
The major deficiency of the method lies in the small sample that is obtained, and unless the liver change is diffuse the sample may not be representative. The procedure has been repeated many times on one animal without injury. The principal danger is that if the direction of the instrument is at fault it may approach the hilus and damage the large blood vessels or bile ducts. If the liver is shrunken or the approach too caudal no sample is obtained. Fatal hemoperitoneum may result if a hemorrhagic tendency is present and peritonitis may occur if the liver lesion is an abscess containing viable bacteria. Biliary peritonitis results if a large bile duct is perforated. It seems possible that the technique could precipitate a fatal attack of ‘black disease’, but many thousands of biopsies are performed without such an incident.
Compared to the human patient, who can voluntarily restrain respiratory movements, the animal patient will traumatize its diaphragm and liver if the needle is not withdrawn quickly.
MEDICAL IMAGING OF THE LIVER
ULTRASONOGRAPHY
Ultrasonography of the liver is now being used as an aid to diagnosis of diseases of the liver of large animals. A complete ultrasonographic assessment of the liver can provide detailed information about the size, position and parenchymal pattern of the liver. Ultrasonographic examinations of the liver of normal cattle17 and sheep18 have now been described and represent the basis for use of the technique in diagnosis of liver disease. In cattle, the liver, caudal vena cava, portal vein and gallbladder can be visualized.17 Ultrasonography is the only practical method for the diagnosis of thrombosis of the caudal vena cava.19
Ultrasonographic technique
Examination of the liver of cattle is done with a 3.5 MHz linear transducer on the right side of the abdomen while the cows are standing. The hair is clipped and the skin shaved between the sixth intercostal space and a hand’s breadth behind the last rib. After application of transmission gel to the transducer the cows are examined, beginning caudal to the last rib and ending at the sixth intercostal space. Each intercostal space is examined dorsally to ventrally, with the transducer held parallel to the ribs. The texture and the visceral and diaphragmatic surface of the liver are scanned, and the hepatic and portal veins, caudal vena cava and biliary system are examined.20 Breed and age of cow does not influence the ultrasonographic appearance of the liver, particularly position, size, and vasculature of the liver and gallbladder.21 During pregnancy, the diameter of the caudal vena cava increases slightly and that of the portal vein decreases. Ultrasonography has been used to detect thrombosis of the caudal vena cava in a cow with ascites22 and cholelithiasis in horses.23
Percutaneous ultrasound-guided cholecystocentesis in cows is an excellent method of obtaining samples of bile for demonstration of Fasciola hepatica and Dicrocoelium dendriticum eggs and for determination of bile acids. The procedure is done on the right side in the ninth, 10th or 11th intercostal space.24
Percutaneous ultrasound-guided portocentesis in cows is an excellent method for measuring the composition of hepatic portal blood and comparing it with peripheral blood.25
Ultrasonography and digital analysis can be used for the diagnosis of hydropic degeneration of the liver of cows instead of biochemical analysis.8 Diffuse hepatocellular disease such as fatty liver in dairy cows can also be detected and evaluated.26 Ultrasonography has been used to evaluate the liver–kidney contrast in the diagnosis of fatty liver infiltration in dairy cattle.27
Cholestasis
Cholestasis in cows can be diagnosed using ultrasonography (Fig. 7.2) to visualize dilatation of the extrahepatic and intrahepatic bile ducts, and dilatation of the gallbladder.28 The presence of jaundice and bilirubinuria combined with the ultrasonographic findings supports the diagnosis of jaundice due to obstruction.29
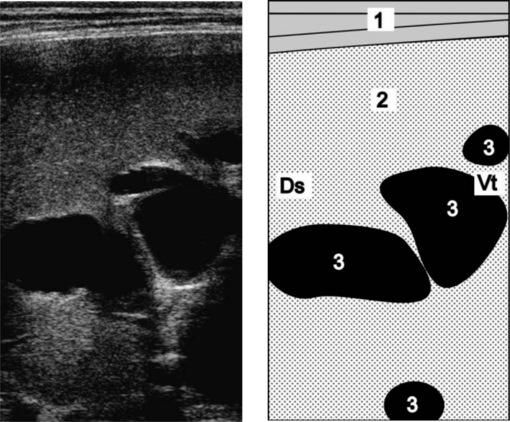
Fig. 7.2 Ultrasonogram and schematic of the liver in a cow with obstructive cholestasis due to fasciolosis. The intrahepatic bile ducts are dilated. Normally, they are not visible. The ultrasonogram was obtained from the 11th intercostal space of the right side using a 5.0 MHz linear transducer. 1 = Lateral abdominal wall; 2 = Liver; 3 = Dilated intrahepatic bile ducts. Ds, Dorsal; Vt, Ventral.
(Reproduced with kind permission of U. Braun.)
Hepatic abscesses
Hepatic abscesses in cows and feedlot cattle can be visualized using ultrasonography.20,30 The abscesses may vary in location from the caudodorsal aspect of the liver in the 11th and 12th intercostal spaces to the cranioventral aspect of the liver in the sixth, seventh and eighth intercostal spaces.20 Those on the left side of the liver cannot be detected. The medical imaging of the abscesses was more diagnostic than laboratory evaluation of liver tests, which was not useful.30 The diameter of the abscesses in cows may vary in size from 5–15 cm and the presence of the abscesses can be confirmed by centesis and aspiration of the contents.
LABORATORY TESTS FOR HEPATIC DISEASE AND FUNCTION
Hepatic disease is difficult to diagnose based on clinical findings alone and the use of laboratory tests is necessary. The results and interpretation of such tests, however, depend on the nature of the lesion, the duration and severity of the disease, and species variations. Specific tests that identify the exact nature of the lesion are not available, and a combination of tests is usually necessary to make a diagnosis. For example, it is suggested that testing for serum bile acids, arginase and gamma-glutamyl transferase (GGT) gives a sensitive indicator of cholestasis and/or hepatocellular necrosis, and a liver biopsy would form the minimum combination of tests for the diagnosis and prognosis of hepatic disease in the horse.31,32 Total serum bile acids, plasma glutamate dehydrogenase, GGT and liver biopsy are useful in the horse with liver disease.33 Based on experimentally induced liver disease in cattle, it is suggested that the serum activities of sorbitol dehydrogenase (SDH), GGT and aspartate aminotransferase (AST, formerly known as SGOT), and the BSP clearance test, provide sensitive indicators of hepatocellular injury in cattle.
All laboratory tests are aids to diagnosis and must be carefully interpreted in conjunction with clinical and other available data. This is particularly important in the laboratory investigation of liver disease in the horse.34 No one test will provide sufficient information, and a combination of tests is necessary. Depending on the time of sampling in relation to the pathological processes developing and the presence of complicating or secondary pathology, there may be elevations in alkaline phosphatase (ALP) and GGT. A horse with chronic hepatic lesions may have a leukocytosis and neutrophilia, hypoalbuminemia, hyperbetaglobulinemia, increased ALP and GGT and, depending on other factors, there may be increases in AST, SDH, total lactate dehydrogenase and others. None of these individual tests are specific for hepatic disease and there is no direct relationship between the magnitude of the serum enzyme level and the degree of liver injury. For these reasons, it is often necessary to take a liver biopsy.
The laboratory tests for the diagnosis of hepatic disease and to evaluate hepatic function in farm animals can be divided into those that measure:
• Excretory rate of parenterally administered substances such as BSP
• Ability of the liver to remove substances from the serum and detoxify them
• Serum levels of liver enzymes that increase following hepatic injury
• Indirect assessment of hepatic function such as blood glucose, serum proteins, clotting factors and urinalysis.
HEPATIC FUNCTION
The sulfobromophthalein sodium clearance test has been used in cattle, sheep and horses, and although little information is available the test appears to have diagnostic value. The single injection technique has the advantage of being noninvasive, repeatable and suitable for conscious animals.35 The time required by the normal liver to reduce the plasma concentration of BSP to half the initial concentration is taken as the standard BSP half-life and in cattle is 2.5–5.5 minutes, in sheep 2.0 minutes36 and in normal horses about 2.0 minutes.35 All horses with confirmed liver disease have a reduction in plasma BSP clearance against time.35 The results are modified by the ability of the liver to excrete BSP via the biliary system and to store it in hepatocytes. Factors other than liver disease that increase the half-life significantly are starvation in horses, competition with bilirubin for excretory capacity, and youth, foals less than 6 months of age having a significantly slower clearance time. Precise timing of samples is needed because of the rapid excretion rate.
In sheep, where severe hepatic dysfunction is accompanied by a steep rise in blood ammonia levels, and where this is reflected in the development of spongy degeneration in the brain, the level of glutamine in the cerebrospinal fluid is also elevated. Glutamine is a byproduct of the metabolism of ammonia in brain cells. Acute ammonia toxicity is manifested by tetany, ataxia and pulmonary edema, and affected animals are likely to die before the effects of subacute poisoning, hepatic encephalopathy, are seen.
ICTERIC INDEX
Measurement of the icteric index of plasma, by comparing its color with a standard solution of potassium dichromate, cannot be considered to be a liver function test but it is used commonly as a measure of the degree of jaundice present. The color of normal plasma varies widely between species depending upon the concentration of carotene. Horse, and to a less extent cattle, plasma is quite deeply colored, but sheep plasma is normally very pale. The color index needs to be corrected for this factor before the icteric index is computed. Hyperbilirubinemia occurs in many diseases of cattle and in most cases is related to a failure of the liver to remove unconjugated bilirubin from the serum rather than to a failure of the liver to excrete conjugated bilirubin.37 The cause may be associated with anorexia, which resembles the hyperbilirubinemia associated with fasting in sick horses.
Adult cattle with hepatic disease do not consistently have high serum bilirubin concentrations and visible jaundice does not occur frequently in cattle with hyperbilirubinemia. Total bilirubin concentrations in adult cattle should be 0.4 mg/dL but young healthy calves may have mean concentrations 0.87 mg/dL and even higher, up to 1.7 mg/dL.38 The use of high bilirubin concentrations as an indicator of liver disease in calves is unreliable because the sensitivity is only about 66%. This is similar to results in adult cattle in which serum bilirubin concentrations are neither a specific nor a sensitive test for chronic liver disease.
Persistent hyperbilirubinemia has been reported in a healthy Thoroughbred horse that was not related to feed intake and not associated with increased hemolysis or acquired hepatic disease.39 Inappropriate conjugation of bilirubin rather than any abnormality in bilirubin uptake or excretion is considered a possibility similar to the human syndrome associated with a familial deficiency of bilirubin–uridine diphosphate glucuronyl transferase.
SERUM HEPATIC ENZYMES
The determination of serum levels of hepatic enzymes is used commonly for the detection and evaluation of hepatic disease. The interpretation of elevated values of enzymes in plasma is dependent not only on the tissue and site of origin but also on the half-time of clearance of the enzyme.
• Sorbitol dehydrogenase (also called l-iditol dehydrogenase (ID)) is almost completely selective as an indicator of liver damage and is the preferred test for hepatic damage in sheep and cattle
• Lactate dehydrogenase (LDH) is abundant in liver, kidney, muscle and myocardium
• Aspartate aminotransferase or l-alanine aminotransferase (ALT, previously known as SGPT) are of some value as an indicator of liver damage because of their high content in liver but are generally considered to be too nonspecific to be of great diagnostic value
• Arginase is a specific indicator of hepatic disease because it is not found in appreciable quantities in other organs. Arginase has a short blood half-life, which makes it useful for the diagnosis of acute hepatic disease but not for less severe forms31
• Gamma-glutamyl transferase is an enzyme widely distributed in a variety of equine tissues. Specific activity of GGT in the horse is highest in the kidney, pancreas and liver. Serum GGT activity is used as a diagnostic criterion for hepatobiliary diseases in cattle, sheep and horses. In the horse, increases in serum GGT may be associated with hepatocellular damage and liver necrosis in a variety of natural and experimentally induced liver diseases. These include bile duct ligation, carbon disulfide toxicity, carbon tetrachloride toxicoses cholestasis, iron hepatoxicosis, Senecio poisoning40 and hyperlipidemia in ponies. GGT is a sensitive indicator of liver damage in horses affected with pyrrolizidine alkaloids in the early stages of the disease but values do not correlate with the increase in the severity of the lesions observed on liver biopsy samples collected later in the chronic phase of the disease.40 GGT has sufficient sensitivity (75%) and specificity (90%) to function as a primary screening test for subclinical liver disease in horses exposed to pyrrolizidine alkaloids. In horses that had consumed hay contaminated with Senecio vulgaris, the GGT values fluctuated widely: some horses with high levels did not die, whereas others had values slightly above reference values at the initial sample collection and died. GGT is a practical routine test for the evaluation of liver amyloidosis status in serum-producing horses.41 In foals during the first month of life values were 1.5–3 times higher than the upper physiological reference values for healthy adult horses.42 In neonatal foals, the serum ALP, GGT and SDH activities were increased during the first 2 weeks of life43
• Glutamate dehydrogenase (GD) occurs in high concentration in the serum of ruminants and horses with liver disease
• Ornithine carbamoyl-transferase (OCT) levels are also elevated even in chronic diseases, but only when there is active liver necrosis and not when the lesions are healing
• Alkaline phosphatase levels are used as a test of hepatic excretory function in the horse and are of value in that species but variations in normal cattle have such a wide range that results are difficult to interpret. Of the tests available for testing of biliary obstruction the serum ALP test is preferred. However, there is a similar response to damage in other tissues.
Hepatic enzyme profile according to species
The serum hepatic enzymes considered to be most useful as an aid in the diagnosis of liver disease in the different species are as follows.
Cattle
In adult cattle, GGT, ALP, SDH,AST and GD are most useful in identifying animals with chronic hepatic disease.38 The dehydrogenases (SDH and GD) have the shortest half-lives in serum and may not increase in cattle with chronic liver disease.
In the early stages of hepatic dysfunction in cattle, SDH is the most efficient and sensitive test. In the later stages when tests of biliary excretion are more applicable, estimations of serum bilirubin and BSP test are indicated.
Calves
In neonatal calves under 6 weeks of age, none of the common tests for assessment of liver damage or function in adult cattle are useful for detection of hepatic disease.38 The serum activity of most enzymes, total bilirubin concentration and sulfobromophthalein sodium clearance half-time are significantly higher in newborn calves than in 2-week-old calves.38 In calves less than 6 weeks of age with suspected liver disease, several tests should be used to assess liver damage, which includes GD activity and total serum bile acid concentration. The concentrations of direct bilirubin may be of more value than determination of total bilirubin for assessing liver damage. It is suggested that percutaneous liver biopsy may provide the most information.
Horses
The clinicopathological features of primary liver disease in the horse have been examined in several case studies.15,33,34,44-46 Total serum bile acids, GD, GGT and liver biopsy are helpful in studying different types of hepatic disease in the horse.3 In one series of primary hepatic disease all horses had high activities of serum GGT and most had high activities of serum GD and high concentrations of bile acids.44 Horses that were euthanized or died had significantly higher concentrations of GGT, GD and bile acids than survivors. Horses with signs of hepatic encephalopathy had plasma ammonia levels greater than 90 μmol/L but this was not correlated with the clinical severity of the disease. Half of the cases with hepatic encephalopathy were hyperglycemic, none was hypoglycemic, and none had abnormally low levels of plasma urea.44
In a series of 82 cases in horses, 61 were confirmed to have significant liver disease and 12 were not.46 Only serum concentrations of GGT, globulins and ALP were found to be significantly different between the two groups of horses.
Clinical and ultrasonographic data were found, when present, to be good indicators of the presence of liver disease.
The single positive test results of greatest diagnostic value were the presence of hepatic encephalopathy, increased GGT, hypoalbuminemia, increased ALP, increased total bile acids and increased total bilirubin. Increased AST and increased GD were also good diagnostic value but only when used in combination with the above tests. No single combination or sequential test was able to fully discriminate between horses with and without biopsy-confirmed liver disease and reliance on the use of noninvasive tests for the prediction of the presence or absence of significant liver disease may lead to frequent diagnostic errors. Certain positive results did reliably predict the presence of liver disease but negative test results were invariably unsatisfactory predictors of absence of liver disease.
In the early stages of hepatic dysfunction, SDH is preferred. Plasma ammonia concentrations may be significantly elevated compared to clinically normal horses but are not always accompanied by a decline in plasma urea concentration. A fall in plasma glucose concentration represents a poor prognosis.
The most useful noninvasive prognostic test in cases of suspected liver disease in adult horses is the severity of clinical signs.34
SERUM BILE ACIDS
The concentration of total serum bile acids has been reported as a sensitive and specific indicator of hepatobiliary disease in humans and animals.47 Abnormalities of bile acid metabolism may be detectable in animals with liver disease that have little evidence of hepatic dysfunction as determined by other common liver function tests. Bile acids are the end-products of the metabolism of cholesterol by the liver. They are excreted in the bile and reabsorbed from the intestine either unchanged or after further transformation by bacterial action. In experimental chronic copper poisoning in sheep, the total bile acid concentration in the plasma is a more sensitive indicator of hepatic damage than the concentration of plasma bilirubin or the activity of transaminases. The rise in total serum bile acid concentration usually correlates well with the severity of liver disease. In cattle, there is extreme variability among all types and ages of animals and the variation is even greater in beef cattle than in dairy cattle.48 Values for calves 6 weeks of age and for 6-month-old heifers are significantly lower than values for lactating dairy cows.
The 5th–95th percentile range of values (μmol/L) were:
In order to be specific for liver damage in cattle, the value determined for a single sample would have to be more than 126 μmol/L in beef cattle, or more than 88 μmol/L in lactating dairy cattle. There are hour-to-hour fluctuations in serum bile acid concentrations in cattle, which makes interpretation difficult.49 Feeding practices and stage of lactation can also affect the serum bile acid concentrations.
The serum bile acid concentrations in dairy cattle with hepatic lipidosis were compared with liver fat content and sulfobromophthalein (BST) half-life.47 Because of the large variability in serum bile acid concentrations in fed cows and the lack of correlation of measured values with liver fat content, bile acid determinations are not reliable as an indicator of subclinical hepatic lipidosis.47
In cattle, total serum bile acids are more specific and sensitive indicators of a wide variety of hepatic disease and are significantly correlated with the degree of illness compared to other tests of hepatic function. Some diurnal variations in total serum bile acids occur in normal cattle. In horses, total serum bile acid concentrations are also a sensitive indicator of several hepatic diseases and are most useful when combined with other tests of hepatic disease.41
Blood ammonia levels
The microbial deamination of amino acids in the intestinal tract is the major source of ammonia which is absorbed by the intestine into portal venous blood and converted into urea by the liver. The concentration of blood ammonia can be an indication of functional hepatic mass. Generally, plasma ammonia concentration is a sensitive and specific indicator of hepatic disease in the horse, although it may fluctuate widely even on the same day and the concomitant low plasma urea concentration anticipated because of the liver’s reduced synthetic ability is often not apparent.3
In cattle with hepatic disease, plasma ammonia levels are significantly elevated compared to normal animals but not always accompanied by a decline in plasma urea concentrations. In healthy cattle, the plasma ammonia:urea concentration ratio is 9:1 and the plasma ammonia:glucose concentration 11:1. In hepatic disease, a plasma ammonia:glucose ratio 40:1 or plasma ammonia:urea ratio 30:1, particularly with a rising total ketone body concentration and a declining glucose concentration, represents a guarded prognosis.4
Most cases of portosystemic shunts are accompanied by marked increases in blood ammonia levels.50
Careful handling of the blood samples is critical to obtain reliable results. Blood samples with species and preferably age-matched controls should be collected, transported on ice, and evaluated immediately.
West HJ. The evaluation of hepatobiliary disease in horses and cattle. FRCVS thesis, London, 1994.
McGorum BC, Murphy D, Love S, Milne EM. Clinicopathological features of Equine primary hepatic disease: a review of 50 cases. Vet Rec. 1999;145:134-139.
Pearson EG. Liver disease in the mature horse. Equine Vet Educ. 1999;11:87-96.
1 Johnston JK, et al. J Am Vet Med Assoc. 1989;194:405.
2 West HJ, Hogg R. Vet Rec. 1988;122:251.
3 West HJ. Equine Vet J. 1996;28:146.
4 West HJ. Vet Res Commun. 1997;21:169.
5 Mair TS, Jones RD. Vet Rec. 1995;137:642.
6 Humann-Ziehank E, et al. Small Rumin Res. 2001;42:157.
7 Jakowski RM. J Am Vet Med Assoc. 1994;204:1057.
8 Acorda JA, et al. Vet Radiol Ultrasound. 1995;36:322.
9 Abdelkader SV, et al. J Comp Pathol. 1991;105:203.
10 Smart ME. Compend Contin Educ Pract Vet. 1985;7:5327.
11 Green LE, et al. Vet Rec. 1995;136:197.
12 Buckley WT, et al. Can J Anim Sci. 1986;66:1137.
13 Harvey RB, et al. Cornell Vet. 1984;74:322.
14 Simpson JW. Vet Rec. 1985;117:639.
15 Durham AE, et al. Equine Vet J. 2003;35:534.
16 Swanson KS, et al. J Anim Sci. 2000;78:2459.
17 Braun U. Am J Vet Res. 1990;51:1522.
18 Braun U, Hausammann K. Am J Vet Res. 1992;53:198.
19 Braun U, et al. Vet Rec. 2002;150:209.
20 Braun U, et al. Vet Rec. 1995;137:284.
21 Braun U, Gerber D. Am J Vet Res. 1994;55:1201.
22 Braun U, et al. Schweiz Arch Tierheilkd. 1992;134:235.
23 Reef VB, et al. J Am Vet Med Assoc. 1990;196:1836.
24 Braun U, Gerber D. Am J Vet Res. 1992;53:1079.
25 Braun U, et al. Vet Rec. 2000;147:623.
26 Acorda JA, et al. Vet Radiol Ultrasound. 1994;35:196.
27 Acorda JA, et al. Vet Radiol Ultrasound. 1994;35:400.
28 Braun U, et al. Vet Rec. 1995;137:537.
29 Braun U, et al. Schweiz Arch Tierheilkd. 1994;136:275.
30 Liberg P, Jonsson G. Acta Vet Scand. 1993;34:21.
31 Hoffmann WE, et al. Am J Vet Res. 1987;48:1343.
32 Pearson EG. Equine Vet Educ. 1999;11:87.
33 West HJ. Equine Vet J. 1996;28:146.
34 Durham AE, et al. Equine Vet J. 2003;35:542.
35 West HJ. Res Vet Sci. 1988;44:343.
36 West HJ. Res Vet Sci. 1989;46:258.
37 McSherry BJ, et al. Can J Comp Med. 1984;48:237.
38 Pearson EG, et al. J Am Vet Med Assoc. 1995;207:1466.
39 Divers TJ, et al. Cornell Vet. 1993;83:237.
40 Curran JM, et al. Aust Vet J. 1996;74:236.
41 West HJ. Res Vet Sci. 1989;46:264.
42 Patterson WH, Brown CM. Am J Vet Res. 1986;47:2461.
43 Bauer JE, et al. Am J Vet Res. 1989;50:2037.
44 McGorum BC, et al. Vet Rec. 1999;145:134.
45 Smith MRW, et al. Equine Vet J. 2003;35:549.
46 Durham AE, et al. Equine Vet J. 2003;35:554.
47 Garry FB, et al. J Vet Intern Med. 1994;8:432.
48 Craig AM, et al. Am J Vet Res. 1992;53:1784.
Principles of treatment in diseases of the liver
In diffuse diseases of the liver no general treatment is satisfactory and the main aim should be to remove the source of the damaging agent. The most that can be attempted in acute hepatitis is to tide the animal over the danger period of acute hepatic insufficiency until the subsidence of the acute change and the normal regeneration of the liver restores its function. Death may occur during this stage because of hypoglycemia, and the blood glucose level must be maintained by oral or intravenous injections of glucose. Because of the danger of guanidine intoxication an adequate calcium intake should be insured by oral or parenteral administration of calcium salts.
There is some doubt as to whether protein intake should be maintained at a high level, as incomplete metabolism of the protein may result in toxic effects, particularly in the kidney. However, amino acid mixtures, especially those containing methionine, are used with apparently good results. The same general recommendations apply in prevention as in the treatment of acute diffuse liver disease. Diets high in carbohydrate, calcium and protein of high biological value and a number of specific substances are known to have a protective effect against hepatotoxic agents.
In chronic, diffuse hepatic disease fibrous tissue replacement causes compression of the sinusoids and is irreversible except in the very early stages, when removal of fat from the liver by the administration of lipotrophic factors including choline and maintenance on a diet low in fat and protein may reduce the compressive effects of fibrous tissue contraction. A high-protein diet at this stage causes stimulation of the metabolic activity of the liver and an increased deposit of fat, further retarding hepatic function.
Local diseases of the liver require surgical or medical treatment depending upon the cause, and specific treatments are discussed under the respective diseases.
Diffuse diseases of the liver
HEPATITIS
The differentiation of hepatic diseases into two groups of hepatitis and hepatosis has not achieved general acceptance and nonspecific terms such as hepatic injury have been suggested to avoid the connotation of inflammation associated with the word hepatitis. To facilitate ease of reading, the word hepatitis is used throughout this chapter to include all diffuse, degenerative and inflammatory diseases that affect the liver. It is used here also to include the common pathological classification of cirrhosis. Clinically the syndrome caused by fibrosis of the liver is the same as that caused by hepatitis and the etiology is the same, the only difference being that the onset of the disease is slower and less acute than in hepatitis.
ETIOLOGY AND EPIDEMIOLOGY
Although there is an extensive list of causes of hepatitis there are still a number of unknown factors. At least there are many sporadic cases of hepatic insufficiency, especially in horses, in which the cause is not determined. In most cases the clinical disease has an acute onset and a fatal outcome but the lesion is of a much longer duration.
In a case-control study of cases of equine hepatic disease admitted to the Liphook Equine Hospital in the UK, ponies were more likely to develop hepatic disease than light riding horses but neither age nor gender were significant factors.1 Overall the case fatality was low (25.9%); horses with unclassified hepatopathies had the lowest fatality rate and horses with cholangiohepatitis, pyrrolizidine alkaloid toxicity and chronic active hepatitis had significantly higher fatality rates by comparison. None of age, breed or gender had any detectable effect on outcome.
In a series of 50 cases of equine primary hepatic disease in England, 37 cases were in ponies;2 25 cases were caused by pyrrolizidine alkaloid toxicity and 11 cases classified as undifferentiated nonmegalocytic cirrhosis on the basis of histopathological findings.
The literature on primary liver disease in the horse has been reviewed.3
Toxic hepatitis
The common causes of toxic hepatitis in farm animals are:
• Inorganic poisons – copper, phosphorus, arsenic, possibly selenium
• Organic poisons – carbon tetrachloride, hexachloroethane, Gossypol, creosols and coal tar pitch, chloroform and copper diethylamine quinoline sulfonate.
Ferrous fumarate administered in a digestive inoculate to newborn foals is also recorded as a cause.4
Poisonous plants
• Weeds, including Senecio, Crotalaria, Heliotropium, Amsinckia and Tribulus spp., Encephalartos lanatus and Trachyandra spp.
• Pasture and cultivated plants – Panicum effusum, lupins, alsike clover,5 water-damaged alfalfa hay6
• Trees and shrubs – lantana (Lantana camara); yellow wood (Terminalia oblongata); ngaio tree (Myoporum laetum); Australian boobialla (Myoporum tetrandrun); seeds of cycads (Zamia spp.)
• Fungi – Pithomyces chartarum, Aspergillus flavus, Penicillium rubrum, Phomopsis leptostromiformis, Fusarium spp., Myrothecium spp., Periconia spp.
• Algae – the slow death factor
• Insects – ingestion of sawfly larvae (Lophyrotoma interrupta).
Toxemia perfusion hepatitis
Moderate degrees of hepatitis occur in many bacterial infections regardless of their location in the body and the hepatitis is usually classified as toxic; whether the lesions are caused by bacterial toxins or by shock, anoxia or vascular insufficiency is unknown. Hepatic failure may occur in dairy cattle following mastitis or metritis; it is thought that the hepatic dysfunction may have been the result of endotoxemia.7 The same position applies in hepatitis associated with extensive tissue damage occurring after burns, injury and infarction.
Infectious hepatitis
Diffuse hepatic lesions in animals are rarely associated with infectious agents. The significant ones are:
• The virus of Rift Valley fever
• Bacillus piliformis, associated with Tyzzer’s disease in foals
• The equid herpesvirus 1 of viral rhinopneumonitis as a cause of abortion in horses
• Deltaproteobacterium associated with epizootic abortion of cattle in California
• Postvaccinal hepatitis of horses, also known as serum hepatitis, idiopathic acute hepatic disease, Theiler’s disease and acute liver atrophy, is the most common cause of acute hepatic failure in the horse.8 The disease is commonly associated with the administration of biologics of equine origin, usually tetanus antitoxin.9
A series of four fatal cases of serum hepatitis associated with the administration of commercial plasma in the horse has been reported.10 The prevalence in one veterinary teaching hospital has been recorded as 0.4%.10
A large number of cases of equine hepatic encephalopathy occurred in France between 1992 and 199711 and the cause remains unknown.
• Severe cases of equine viral arteritis manifest signs of hepatitis
• Systemic mycoses, e.g. histoplasmosis, may be accompanied by multiple granulomatous lesions of the liver
• Other diseases in which hepatic lesions may be common at necropsy, but in which there are no overt signs of clinical disease during life. Some of these are infectious equine anemia, salmonellosis, septicemic listeriosis, leptospirosis in aborted foals12
• Infectious necrotic hepatitis associated with Clostridium novyi has been described in a 9-year-old mare.13
Nutritional hepatitis (trophopathic hepatitis)
Selenium and vitamin E deficiency are factors in dietary hepatic necrosis in pigs. A multiple dietary deficiency has also been suggested as the cause of a massive hepatic necrosis observed in lambs and adult sheep on trefoil pasture in California. Hepatic lipidosis and hyperlipemia occurs most commonly in pregnant Shetland pony mares on a falling plane of nutrition.16 The fat cow syndrome occurs in beef and dairy cattle in late pregnancy or within days after parturition and is associated with excess energy intake in pregnancy followed by a sudden mobilization of body depot fat in late pregnancy or at the onset of lactation.17 The fatty infiltration of the liver that occurs in most dairy cattle in late pregnancy and early lactation is functional and reversible and related to the metabolic demands of those periods in the production cycle.17
White liver disease is a well-identified clinical entity occurring in young sheep in the warmer parts of New Zealand. The cause is unknown but the disease affects only cobalt-deficient sheep. The disease occurs on leafy pastures with lots of leaf litter, and in spring and early summer. Affected sheep show photosensitivity, anorexia, weight loss, sometimes jaundice and blindness. At necropsy there is a very much enlarged, light-colored, fatty liver. Most deaths occur in a chronic phase after the acute signs have passed. A similar disease, suspected to be caused by a mycotoxin, has been observed in Norway.
Idiopathic hepatosis and cirrhosis
Hepatic cirrhosis and hemochromatosis in horses has been recorded.18 There is cirrhosis with increased iron stores in the parenchymal cells of the liver. Hepatic fatty cirrhosis (hard yellow liver) in sheep and cattle has occurred in isolated areas of western and southern Texas during years of maximal rainfall.19 The cause is unknown but the high incidence during periods of heavy rainfall suggests the possibility of either a mycotoxin or nutritional deficiency.20
Congestive hepatopathy
Increased pressure in the sinusoids of the liver causes anoxia and compression of surrounding hepatic parenchyma. Congestive heart failure is the common cause and leads to centrilobular degeneration.
Inherited hepatic insufficiency occurs in Southdown and Corriedale sheep (see Ch. 34, Inherited photosensitization).
PATHOGENESIS
Hepatitis may be associated with a number of agents but the clinical effects are approximately the same in all instances as described under clinical manifestations earlier in the chapter. The usual lesion in toxipathic hepatitis is centrilobular and varies from cloudy swelling to acute necrosis with a terminal veno-occlusive lesion in some plant poisonings. If the necrosis is severe enough or repeated a sufficient number of times, fibrosis develops. The effects of endotoxin on the liver include multifocal hepatocellular necrosis, decreased hepatic gluconeogenesis and decreased hepatic blood flow.7 It is possible that endotoxin may cause the Kupffer cells to release lysosomal enzymes, prostaglandins and collagenase, which can damage hepatocytes. Endotoxin not detoxified by the Kupffer cells may interact directly with the hepatocytes, causing lysosomal damage and decreased mitochondrial function, leading to necrosis. In infectious hepatitis the lesions vary from necrosis of isolated cells to diffuse necrosis affecting all or most of the hepatic parenchyma.
Serum hepatitis in the horse is characterized by severe central lobular necrosis following the administration of biologics of equine origin such as tetanus antitoxin, commercial equine plasma and other products.10
In parasitic hepatitis the changes depend upon the number and type of migrating parasites. In massive fluke infestations sufficient damage may occur to cause acute hepatic insufficiency, manifested particularly by submandibular edema. In more chronic cases extension from a cholangitis may also cause chronic insufficiency.
Trophopathic hepatitis is characterized by massive or submassive necrosis. Hepatic lipidosis is characterized by fatty infiltration of hepatocytes progressing to development of fatty cysts.
Congestive hepatitis is characterized by dilatation of central veins and sinusoids with compression of the parenchymal cells. Hepatic fibrosis develops particularly if there is massive hepatic necrosis that destroys entire lobules. Degeneration is not possible, as it is when the necrosis is zonal, and fibrous tissue replacement occurs. Thus fibrosis is a terminal stage of hepatitis that may have developed acutely or chronically and is manifested by the same clinical syndrome as that of hepatitis except that the signs develop more slowly. Fibrosis may also develop from a cholangitis.
The term cirrhosis has been avoided because it carries connotations from human medicine that may be misleading when applied to animals. Hepatic fatty cirrhosis occurs in sheep and cattle and is characterized at necropsy by ascites, hydropericardium and acquired hepatic vascular shunts.20 There is progressive fatty change of the liver leading to cirrhosis. Fibrosis begins in the periacinar zone associated with ruptured fatty cysts and continues until there is widespread bridging periacinar fibrosis. No lesions of hepatic encephalopathy occur.20
In portosystemic vascular anomalies the increased levels of ammonia, short-chain fatty acids and amino acids in the peripheral circulation are the cause of the depression and neurological abnormalities that are typical of hepatic encephalopathy.22 These high levels of metabolites are the result of failure of the hepatic metabolism and detoxification of substances absorbed from the intestines, which are normally delivered to the liver via the portal vein before they enter the peripheral circulation.
Liver disease and liver failure
The liver has vast reserves of function, an almost embryonic capacity to regenerate itself, and it can perform adequately despite often extensive pathological damage to its integrity. This is best exemplified in liver abscesses in cattle, where rarely is clinical disease evident in the presence of large abscesses.
Liver disease is usually diagnosed by identifying clinical signs produced by failure of some of its functions.23 There is often liver disease prior to failure of function and laboratory tests may detect disease before there is actual failure. The liver has a reserve of about 70–80% and this must be compromised before some of its functions fail. Some functions fail before others, which explains the progression of clinical signs.
Intravascular hemolysis in equine liver disease
Intravascular hemolysis with prominent hemoglobinuria has occurred in horses with severe and advanced liver disease.24 Neutrophil hypersegmentation of undetermined cause was present in one horse with liver disease and intravascular hemolysis.
CLINICAL FINDINGS
The cardinal signs of hepatitis are anorexia, mental depression – with excitement in some cases, muscular weakness, jaundice and in the terminal stages somnolence, recumbency and coma with intermittent convulsion. Hemoglobinuria is also a variable sign in horses. The hemolytic crisis with which it is associated is always a precursor to a fatal outcome. Animals that survive the early acute stages may show photosensitization, a break in the wool or hair leading to shedding of the coat and susceptibility to metabolic strain for up to a year.
The clinical findings of hepatic disease in the horse are generally nonspecific but the most useful noninvasive prognostic test in cases of suspected liver disease in adult horses is the severity of clinical signs.25 Regardless of the cause, consistent clinical findings include weight loss, anorexia, dullness and depression. Other findings include jaundice, tachycardia, intermittent fever, abdominal pain, ventral body wall edema, clotting deficiency, muscle fasciculations and diarrhea or constipation.16 Jaundice is a constant feature in acute hepatic necrosis. Dysphagia, photosensitization, encephalopathy and hemorrhages tend to occur terminally, particularly in horses with cirrhosis. In chronic liver disease, the course is several months.
The initial anorexia is often accompanied by constipation and punctuated by attacks of diarrhea. The feces are lighter in color than normal and if the diet contains much fat there may be steatorrhea.
In a series of 50 cases of primary hepatic disease in horses, the following occurrence of clinical signs was observed (%): dull demeanor (68); anorexia (56); abdominal pain (50); encephalopathy (50); weight loss (50); jaundice (42); abnormal intestinal motility (42); abnormal fecal consistency (28): dehydration (18); photosensitization (16); bilateral laryngeal paralysis (14); clinical coagulopathy (10); dermatitis and pruritus (8); peripheral edema (6); oral ulceration (6); tenesmus (4); penile prolapse (2); and rectal impaction (2).2
The nervous signs are often pronounced and vary from ataxia and lethargy with yawning, or coma, to hyperexcitability with muscle tremor, mania, including aggressive behavior, and convulsions. A characteristic syndrome is the dummy syndrome, in which affected animals push with the head, do not respond to normal stimuli and may be blind. There may be subacute abdominal pain, usually manifested by arching of the back, and pain on palpation of the liver. The enlargement of the liver is usually not palpable.
Jaundice and edema may or may not be present and are more commonly associated with the less acute stages of the disease. Photosensitization may also occur but only when the animals are on a diet containing green feed and are exposed to sunlight. A tendency to bleed more freely than usual may be observed. In chronic hepatic fibrosis the signs are similar to those of hepatitis but develop more slowly and persist for longer periods, often months. Ascites and the dummy syndrome are more common than in hepatitis.
Serum hepatitis (Thelier’s disease) is the most common cause of acute hepatic failure in the horse.9 Typically, clinical findings become apparent several weeks after administration of tetanus antitoxin. Lactating mares appear to be at a higher risk than other horses but this may be due to the administration of the antitoxin to mares at the time of parturition. In a group of affected horses, the illness may begin with an unexplained death in a horse after a short illness. Clinical findings include sudden anorexia, marked lethargy, stiff gait, subcutaneous edema of the distal aspects of all four limbs and body wall, blindness, head-pressing, circling, bruxism, abdominal pain, tachycardia, icterus and a marked reduction in gastrointestinal sounds. Death in a few days is common.9
Serum hepatitis following the transfusion of commercial plasma into horses may cause severe unresponsive colic, lethargy and sudden death 41–60 days later.10 Severe encephalopathy has also been described.
Hepatic disease in cattle is characterized by weight loss, dullness and depression.26 Signs of hepatic encephalopathy include blindness, head pressing, excitability, ataxia and weakness. The presence of fever and jaundice represents a poor prognosis.
Hepatic fatty cirrhosis in ruminants in Texas is characterized by failure to gain weight, progressive emaciation, loss of wool crimp, ascites, depression, head-pressing, and walking with the head held high. In the terminal stages, animals become immobile and die in a state of coma.7 Morbidity may reach 80–100% and mortality varies from 10–60%. Mortality increases during each succeeding month following October, climaxes in January and February, and then decreases in the months thereafter.
Portosystemic shunts
In young animals with portosystemic shunts the clinical findings include stunted growth, ascites and variable neurological abnormalities resulting from hepatic encephalopathy. Calves and foals may be a few weeks to a few months of age before they are presented for examination. Apparent cortical blindness, circling and dementia are common. Persistent tenesmus is common in calves.13 Recurrent episodes of unexplained neurological clinical findings in a young foal suggest the presence of a portosystemic shunt. A tentative diagnosis may be made using clinicopathological results but a definitive diagnosis requires portovenography.22 Blood ammonia levels are markedly increased and serum bile acids are also increased but the serum levels of hepatic derived enzymes may be normal.21
CLINICAL PATHOLOGY
The clinicopathological features of primary liver disease have been examined2,27 and are summarized in the section dealing with laboratory tests for hepatic disease and function.
Scoring liver biopsies of the horse with suspected liver disease is highly predictive of the severity of the lesion and of prognosis.28
In a series of 82 cases in horses, 61 were confirmed to have significant liver disease and 12 were not.29 Only serum concentrations of GGT, globulins and ALP were found to be significantly different between the two groups of horses.
Clinical and ultrasonographic data were found, when present, to be good indicators of the presence of liver disease.
The single positive test results of greatest diagnostic value were the presence of hepatic encephalopathy, increased GGT, hypoalbuminemia, increased ALP, increased total bile acids and increased total bilirubin. Increased AST and increased GD were also good diagnostic value but only when used in combination with the above tests. No single combination or sequential test was able to fully discriminate between horses with and without biopsy-confirmed liver disease and reliance on the use of noninvasive tests for the prediction of the presence or absence of significant liver disease may lead to frequent diagnostic errors. Certain positive results did reliably predict the presence of liver disease but negative test results were invariably unsatisfactory predictors of absence of liver disease.
The most useful noninvasive prognostic test in cases of suspected liver disease in adult horses is the severity of clinical signs.25 A significantly poorer prognosis was found in association with clinical signs suggestive of liver disease, presence of hepatic encephalopathy, ultrasonographic abnormalities, increased globulins, increased total bile acids, increased ALP, increased GGT, erythrocytosis, leukocytosis, low serum albumin and low serum urea. The literature on liver disease in the mature horse has been reviewed.23
NECROPSY FINDINGS
The liver in hepatitis is usually enlarged and the edges swollen but the appearance of the hepatic surface and cross-section varies with the cause. In acute toxic and trophopathic hepatitis the lobulation is more pronounced and the liver is paler and redder in color. The accentuation of the lobular appearance is caused by engorgement of the centrilobular vessels or centrilobular necrosis. There may be accompanying lesions of jaundice, edema and photosensitization. In infectious hepatitis the lesions are inclined to be patchy and even focal in their distribution. Parasitic hepatitis is obviously traumatic, with focal hemorrhages under the capsule and the necrosis and traumatic injury definable as tracks. Congestive hepatitis is marked by severe enlargement of the liver, a greatly increased content of blood and marked accentuation of the lobular pattern caused by vascular engorgement and fatty infiltration of the parenchyma. In hepatic fibrosis the necropsy findings vary widely depending on the causative agent, the duration of its action and on its severity. The liver may be grossly enlarged or be much reduced in size with marked lobulation of the surface.
Hepatic encephalopathy associated with portosystemic shunt is characterized by spongiform changes and gliosis of white matter in all levels of the brain.30 The liver may be of normal size or small and firm, with a prominent reticular pattern visible on the capsular and cut surfaces,22 and the portal veins may be absent.
TREATMENT
The principles of treatment of hepatitis have already been outlined. Results are seldom good. Protein and protein hydrolysates are probably best avoided because of the danger of ammonia intoxication. The diet should be high in carbohydrate and calcium and low in protein and fat, but affected animals are usually completely anorectic. Because of the failure of detoxification of ammonia and other nitrogenous substances by the damaged liver and their importance in the production of nervous signs, the oral administration of broad-spectrum antibiotics has been introduced in humans to control protein digestion and putrefaction. The results have been excellent with neomycin and chlortetracycline, the disappearance of hepatic coma coinciding with depression of blood ammonia levels. Purgation and enemas have also been used in combination with oral administration of antibiotics but mild purgation is recommended to avoid unnecessary fluid loss. Supplementation of the feed or periodic injections of the water-soluble vitamins are desirable. Hepatic fibrosis is considered to be a final stage in hepatitis and treatment is not usually undertaken.
Hepatitis is easily misdiagnosed as an encephalopathy unless jaundice or photosensitization is present. The nervous signs are suggestive of:
Congestive hepatitis is usually not manifested by nervous signs and, being a secondary lesion in congestive heart failure, is usually accompanied by ascites and edema in other regions and by signs of cardiac involvement. Hepatic fibrosis may produce ascites without evidence of cardiac disease.
Acute diseases affecting the alimentary tract, particularly engorgement on grain in cattle and horses, may be manifested by signs of nervous derangement resembling those of acute hepatic dysfunction but the history and clinical examination usually suggest a primary involvement with the alimentary tract. Anorexic hepatic insufficiency may be mirrored by an adenocarcinoma of the pancreas, which is unlikely to be diagnosed during life.
Ross MA. The relationship of hepatic drug metabolism to hepatotoxicity with some examples in sheep. Vet Annu. 1982;22:129-134.
McGorum BC, Murphy D, Love S, Milne EM. Clinicopathological features of Equine primary hepatic disease: a review of 50 cases. Vet Rec. 1999;145:134-139.
Pearson EG. Liver disease in the mature horse. Equine Vet Educ. 1999;11:87-96.
Olsman AF, Sloet van Oldruitenborgh-Oosterbaan MM. Primary liver disease in the horse. Tijdschr Diergeneeskd. 2004;129:510-522.
1 Smith MRW, et al. Equine Vet J. 2003;35:549.
2 McGorum BC, et al. Vet Rec. 1999;145:134-139.
3 Olsman AF, Sloet van Oldruitenborgh-Oosterbaan MM. Tijdschr Diergeneeskd. 2004;129:510.
4 Mullaney TP, Brown CM. Equine Vet J. 1988;20:119.
5 Nation PN. Can Vet J. 1991;32:602.
6 Putnam MR, et al. J Am Vet Med Assoc. 1986;189:77.
7 Sweeney RW, et al. J Vet Intern Med. 1988;2:80.
8 Messer NT, Johnson PJ. J Am Vet Med Assoc. 1994;204:1934.
9 Messer NT, Johnson PJ. J Am Vet Med Assoc. 1994;204:1790.
10 Aleman M, et al. J Vet Intern Med. 2005;19:120.
11 Zentara S, et al. Vet Rec. 1994;134:18.
12 Wilkie IW, et al. Can Vet J. 1988;29:1003.
13 Sweeney HJ, Greig A. Equine Vet J. 1986;18:150.
14 Buergelt CD, Greener EC. J Vet Diagn Invest. 1995;7:102.
15 Davis CR, et al. J Parasitol. 1999;85:965.
16 West HJ. Equine Vet J. 1996;28:148.
17 West HJ. Bovine Pract. 1990;25:127.
18 Pearson EG, et al. J Am Vet Med Assoc. 1994;204:1053.
19 Helman RG, et al. J Am Vet Med Assoc. 1993;202:129.
20 Helman RG, et al. Vet Pathol. 1995;32:635.
21 Fortier LA, et al. Vet Surg. 1996;25:154.
22 Hillyer MH, et al. Vet Rec. 1993;132:457.
23 Pearson EG. Equine Vet Educ. 1999;11:87-96.
24 Ramaiah SK, et al. J Vet Intern Med. 2003;17:360.
25 Durham AE, et al. Equine Vet J. 2003;35:542.
26 West HJ. Vet Res Commun. 1997;21:169.
27 West HJ. Equine Vet J. 1996;28:146.
28 Durham AE, et al. Equine Vet J. 2003;35:534.
Focal diseases of the liver
HEPATIC ABSCESS
Local suppurative infections of the liver do not cause clinical signs of hepatic dysfunction unless they are particularly massive or extensively metastatic. They do cause significant losses in feedlot and grain-fed cattle because of the frequency of rumenitis in those cattle leading to hepatic abscess formation and the rejection of the affected livers at the abattoir. In feedlot cattle in North America the incidence of liver abscesses averages about 16% but ranges from about 8% to 40% or even higher, up to 78%.1
In a 2-year study of bovine hepatic abscessation in 10 abattoirs in Ireland, the livers of 6337 12–16-month-old heifers were examined.2 The frequency of gross lesions was 5.8%, of which 1.9% had abscesses. Only 1.17 had scarring, and 0.7% telangiectasis. Of the livers with abscesses, 44% had a single large abscess, 35% had a single small abscess and 19% had more than two abscesses; in 16% the abscesses were resolving and in 8.3% the abscesses were ruptured. A total of 43% of the livers with abscesses had adhesions to the diaphragm and diaphragmatic lung lobes, 2.5% had adhesions to other abdominal organs, 10% also had scarring and 1.7% also had lesions attributable to the liver fluke. Clinical signs attributable to the abscesses were observed in only one animal.
Cattle started on feed in November and January in North America, and cattle housed in confinement or outside without overhead shelter, had higher incidences of liver abscesses.1 Virginiamycin at 16.5–19.3 mg/kg DM in the diet reduced the incidence of liver abscesses in feedlot cattle.3 Occasional cases occur in dairy cows and cause severe illness.4 The toxemia of traumatic hepatitis is usually due to toxins from Arcanobacterium pyogenes, Streptococcus and Staphylococcus spp. and Fusobacterium necrophorum, which are implanted in the lesions by the perforating foreign body; of the F. necrophorum isolates most will be of the A biotype, although the B biotype does occur usually in combination with other bacteria.5
Hepatic abscesses in goats have been described.6 Out of 658 necropsies of goats, 2.5% had hepatic abscesses. The abscesses occurred most commonly in adults. All the affected animals were in poor bodily condition. The organisms isolated included Corynebacterium pseudotuberculosis (58.9%), Escherichia coli (11.8%), Corynebacterium spp. (11.8%), Mannheimia haemolytica (5.0%), Proteus sp. (5.9%) and Staphylococcus aureus (5.9%).
Omphalophlebitis, ruminal parakeratosis or rumenitis may also lead to hepatic invasions by F. necrophorum or other organisms and abscessed livers are common in cattle fed heavily on concentrates. Black disease is a profound toxemia caused by the liberation of potent exotoxin from C. novyi. Clostridium sordellii is associated with hepatic abscesses in neonatal lambs and bacillary hemoglobinuria by a toxin from Clostridium haemolyticum with focal hepatic necroses. A focal bacterial hepatitis, identified as ‘Tyzzer’s disease’ and associated with Bacillus piliformis, and yersiniosis associated with Yersinia pseudotuberculosis are listed elsewhere. Occasional cases of strangles that develop bacteremic spread may also develop hepatic abscesses, as may septicemia in lambs associated with Histophilus somni.
The fungus Mortierella wolfii has been isolated from a liver abscess in a cow in Australia.7 The liver abscess was grossly indistinguishable from other common bacterial abscesses, such as those associated with A. pyogenes or F. necrophorum.
The clinical signs of these specific diseases are included under the discussion of each disease and the only finding common to all is local pain on palpation or percussion over the liver.
A most important relationship is that between liver abscess and caudal vena caval syndrome. Sudden death, or any death, of cattle due to pulmonary hemorrhage should be examined with this possibility in mind. Liver abscesses have been produced experimentally in cattle by injecting F. necrophorum into the hepatic portal vein. They are characterized by an elevation of blood levels of sialic acid and mucoprotein. High concentration of alpha-1 acid glycoprotein (1-AG) present in naturally occurring cases is correlated with sialic acid concentration.8
Hepatic abscesses in horses
Hepatic abscesses in horses are characterized clinically by a history of weight loss, fever, inappetence and depression. The etiology and pathogenesis are unknown. Clinicopathological abnormalities are consistent with a diagnosis of chronic bacterial infection such as leukocytosis with a mature neutrophilia, thrombocytosis, hyperglobulinemia, hypoalbuminemia and a markedly decreased albumin-to-globulin concentrations ratio.9 Ultrasonography is a useful diagnostic aid. Secondary immune-mediated complications may develop in young adult horses with hepatic abscesses. The prognosis is very unsatisfactory, in spite of intensive antibiotic and supportive therapy, and euthanasia is recommended.
TELANGIECTASIS OF THE BOVINE LIVER (‘SAWDUST LIVER’)
Telangiectasis of the bovine liver accounts for about 10% of all bovine liver condemnations in federally inspected slaughter facilities in the USA.10 Condemnation is based on aesthetics. The lesion is usually easily diagnosed by inspectors but its biological significance is undetermined. Affected livers contain more bacteria than normal livers but telangiectasis cannot be linked to an infectious process. In some affected livers, E. coli O157 was present, which is a potential zoonotic risk.10 It is believed that E. coli O157 in meat at slaughter is an external contaminant and, experimentally in cattle, there is no evidence that the organism translocates from the alimentary tract to deeper tissues. The morphological, immunohistochemical and ultrastructural studies of pretelangiectasis and telangiectasis of the bovine liver have been examined.11
TUMORS OF THE LIVER
Metastatic lesions of lymphomatosis in calves are the commonest neoplasms encountered in the liver of animals, although primary adenoma, adenocarcinoma and metastases of other neoplasms in the area drained by the portal tract are not uncommon, especially in ruminants. For the most part, they produce no signs of hepatic dysfunction but they may cause sufficient swelling to be palpable, and some abdominal pain by stretching of the liver capsule. Primary tumors of the gallbladder and bile ducts also occur rarely and do not generally cause clinical signs. A primary hepatic fibrosarcoma in a goat has caused loss of body weight, although appetite was maintained, anemia and jaundice.12 Hepatic biliary cystadenoma has been described in a 10-year-old horse.13 It is regarded as a morphological variant of biliary cystadenoma of domestic animals.
A series of 66 primary hepatic tumors of cattle has been examined and classified using modern criteria.14 Fifty hepatocellular tumors (10 adenomas and 40 carcinomas), 10 cholangiocellular tumors, two cavernous hemangiomas, two hemangioendothelial sarcomas, one fibroma and one Schwannoma were diagnosed. An association with cirrhosis was not found. A bile duct hamartoma in a calf has been reported.15
1 Harman BR, et al. J Anim Sci. 1989;67:311.
2 O’Sullivan EN. Vet Rec. 1999;145:389.
3 Rogers JA, et al. J Anim Sci. 1995;73:9.
4 Cahill LW. Bovine Pract. 1993;27:171.
5 Scanlan CM, Hathcock CL. Cornell Vet. 1983;73:288.
6 Rosa JS, et al. Br Vet J. 1989;145:73.
7 Uzal FA, et al. Vet Rec. 1999;145:260.
8 Motoi Y, et al. Am J Vet Res. 1992;53:574.
9 Sellon DC, et al. J Am Vet Med Assoc. 2000;216:882.
10 Stotland EL, et al. J Am Vet Med Assoc. 2001;219:36.
11 Marcato PS, et al. J Comp Pathol. 1998;119:95.
12 Higgins RJ, et al. Vet Rec. 1985;116:444.
13 Salvaggio A, et al. Vet Pathol. 2003;40:114.
DISEASES OF THE BILIARY SYSTEM
Cases of biliary tract disease with clinical manifestations are uncommon in food animals and horses. Occasional cases of cholangitis occur in cattle and horses. Associated clinical signs include fever, pain over the liver, jaundice and photosensitization. There is usually an accompanying leukocytosis and a left shift. In horses a sequel to cholangitis may be a diffuse bacterial hepatitis with signs of hepatic insufficiency. Septic cholangiohepatitis and cholangiocarcinoma have been recorded in a horse.1
Concretions in the biliary system of cattle are usually a sequel to fascioliasis.2 Mild cases show anorexia and pain over the liver. Severe cases show recurrent attacks of severe abdominal pain, alimentary tract stasis and pain on percussion over the liver. Jaundice occurs only in the terminal stages of fatal cases and is accompanied by recumbency, depression and coma.3 The frequency of pigment gallstones is high in sheep and associated with high total bilirubin concentration in the bile.4 Other causes of biliary tract disease include gallbladder empyema and a bile duct carcinoma. In the latter case there was severe loss of body weight and signs referable to metastases in other organs but there were no clinical or postmortem signs of biliary malfunction. Biliary atresia in young foals is manifested by an early period of normality for 2–3 weeks after birth followed by the development of listlessness, anorexia, the passage of gray, pasty feces and jaundice. Death occurs about a week later.
Obstructive cholelithiasis in horses may cause intermittent colic or continuous pain and sometimes jaundice. A series of 10 cases in horses is reported.5 Clinical findings included fever, icterus, mild intermittent colic and weight loss. Laboratory findings included leukocytosis, hyperproteinemia and hyperfibrinogenemia. GGT and lactate dehydrogenase were also elevated.
Cholangiohepatitis in horses
A series of nine cases was reported of cholangiohepatitis and cholelithiasis in mature horses with a median age of 13 and a range of 4–18 years.6 Clinical signs that prompted referral of each horse to a veterinary teaching hospital included anorexia, depression, weight loss, colic, intermittent fever and icterus. In all horses, the GGT and ALP were elevated, and there was hyperbilirubinemia. Transabdominal ultrasonography was used to evaluate the size and nature of the liver and to obtain liver biopsy for histopathology and culture. The ultrasonographic findings included increased hepatic echogenicity, hepatomegaly, enlarged distended bile ducts and occasional calculi as the salient features. Neutrophilic cholangiohepatitis consistent with an infectious cause was a feature of biopsy material from each horse.
The etiology and pathogenesis of cholangiohepatitis and cholelithiasis in horses is uncertain. Retrograde bacterial infection from the small intestine is considered probable. Culture of liver biopsy material yielded E. coli and Bacteroides vulgatus from only a small number of affected animals. Long-term parenteral Gram-negative antibiotics daily for a median of 51 days (range 17–124) was associated with survival in 7/9 horses. Supportive intravenous fluid therapy is also necessary. Progress can be monitored by evidence of clinical improvement and declining levels of GGT.
Cholangiohepatitis in a 2-month-old calf was characterized clinically by depression, fever and diarrhea.7 There was marked leukocytosis and neutrophilia. The GGT and ALP were markedly elevated and total bilirubin was elevated. Ultrasonographic examination of the liver revealed gross abnormality and liver biopsy results indicated neutrophilic hepatitis and multifocal hyperplasia of the biliary epithelium suggesting cholangiohepatitis. Culture of liver tissue yielded E. coli sensitive to amikacin, cefazolin and ceftiofur. Supportive fluid therapy and antibiotics may be successful.
Suppurative cholangiohepatitis and cholelithiasis associated with enteritis has been described occurring in adult horses.8 Clinical findings included nonresponsive colic, fever, depression, severe abdominal pain, tachycardia, dehydration, gastric fluid accumulation and absence of abdominal sounds over all four quadrants. Distended loops of small intestine were palpable on rectal examination and the peritoneal fluid was serosanguinous. Azotemia, hyperbilirubinemia, increased ALP and GGT were present and persisted for several days. Cases were associated with severe inflammation of the small intestine and hypotensive shock.
Cholangiohepatitis and pancreatitis secondary to gastroduodenal ulceration in a 2-month-old foal was characterized clinically by colic unresponsive to surgical treatment.9 At necropsy, gastric ulceration, segmental duodenal stenosis and severe chronic cholangiohepatitis and pancreatitis were present.
Choledocholithiasis attributable to a foreign body in a horse is recorded.10 Clinical signs suggestive of biliary disease in adult horses may be due to neoplasia of the pancreas (see below). A case of congenital hepatic fibrosis in a newborn calf is recorded.11
1 Durando MM, et al. J Am Vet Med Assoc. 1995;206:1018.
2 West HJ, Hogg R. Vet Rec. 1988;122:251.
3 Lechtenberg KF, et al. Am J Vet Res. 1988;49:58.
4 Catallini A, et al. Am J Vet Res. 1991;52:2043.
5 Johnston JK, et al. J Am Vet Med Assoc. 1989;194:405.
6 Peek SF, Divers TJ. Equine Vet J. 2000;32:301.
7 Coombs DK, et al. Vet Rec. 2002;150:551.
8 Davis JL, Jones SL. J Vet Intern Med. 2003;17:583.
9 Buate M. Can Vet J. 2003;44:746.
Diseases of the pancreas
Pancreatic disease in large animals is rare and only a few comments are presented here.
DIABETES MELLITUS
Lesions of the pancreas causing diabetes mellitus are recorded in cows1 and horses and donkeys.2 The clinical syndrome in horses includes weight loss, polydipsia, polyuria, intense hyperlipidemia and high blood levels of cholesterol, triglycerides and glucose. Clinical observations suggest that the disease is most likely to occur in old horses and may be due to pancreatic injury related to migration of strongyle larvae.3 Diabetes mellitus resulting from pancreatic beta-cell failure is rare in the horse but has been reported in a domesticated Spanish Mustang.4 In cows there is afebrile emaciation, polydipsia, ketonuria, glucosuria and hyperglycemia.
PANCREATIC ADENOCARCINOMA
The pancreatic duct of the horse is anatomically close to the common bile duct and it is not unexpected that a tumor mass should cause a syndrome of biliary duct pathology,5,6 although there is a surprising absence of jaundice at some stages of the disease. There is emaciation, concomitant moderate abdominal pain and variable fecal texture up to diarrhea. GGT and blood ammonia levels are greatly increased.
PANCREATIC ADENOMA
Convulsions due to hypoglycemia have been recorded in a pony with a pancreatic adenoma.7 It is assumed that the hypoglycemia resulted from hyperinsulinism generated by the beta-cell adenoma.
PANCREATITIS
Pancreatitis is rare in farm animals. Inflammatory and degenerative changes are detected post mortem in some cattle but are rarely diagnosed clinically because of a lack of clinical and laboratory findings. Ultrasonographic imaging of experimentally induced pancreatitis in cattle has been described.8
1 Mostaghni K, Ivoghli B. Cornell Vet. 1977;67:24.
2 Moore JN, et al. Endocrinology. 1979;104:576.
3 Bulgin MS, Anderson BC. Compend Contin Educ Pract Vet. 1983;5:S482.
4 Johnson PJ, et al. J Am Vet Med Assoc. 2005;226:584.
5 Kerr OM, et al. Equine Vet J. 1982;14:338.
6 Church S, et al. Equine Vet J. 1987;19:77.