Chapter 28 Diseases associated with arthropod parasites
Gasterophilus spp. infestation (stomach bot)
Oestrus ovis infestation (nasal bots)
Hypoderma spp. infestation (warble flies)
CUTANEOUS MYIASIS 1589
KED AND LOUSE INFESTATIONS 1596
TICK INFESTATIONS 1599
MISCELLANEOUS FLIES, MIDGES, AND MOSQUITOES 1603
MITE INFESTATIONS 1606
GASTEROPHILUS SPP. INFESTATION (STOMACH BOT)
Infestations with larvae of Gasterophilus spp. have a widespread distribution. They cause a chronic gastritis and a loss of condition in infested horses, donkeys, and mules. Reduced performance is often attributed to this infestation. On rare occasions they cause perforation of the stomach and death.
Epidemiology
Eggs are laid on hair of the body or around the lips; eggs hatch spontaneously or are stimulated to hatch by oral grooming, larvae penetrate oral mucosa or external epithelium of cheek and migrate to inner regions of mouth, congregate at epithelial surface around teeth for 6–10 weeks before migration to the stomach and intestine. Larvae attach in stomach or intestine and remain there for some months before being passed in the feces. One species attaches near the rectum. Larvae pupate and adults emerge after 3–5 weeks. Adults only live a few days and are mainly active in the summer, the fly surviving as larvae in the stomach over the colder months.
ETIOLOGY
Five species of flies of are known to parasitize domestic equids; Gasterophilus nasalis, G. intestinalis, G. haemorrhoidalis, G. pecorum, and G. inermis. Their larvae are the ‘stomach bots’ of horses, donkeys, and mules. Three species, G. intestinalis, G. nasalis, and G. haemorrhoidalis, are the most important and have a world wide distribution. The later larval stages inhabit the stomach and duodenum. These creamy pink larvae are thick, segmented, and about 5–15 mm long. The adult flies are golden brown, hairy, and about the size of a bee with two wings and vestigial mouth parts.
LIFE CYCLE AND EPIDEMIOLOGY
Flies do not feed and only live a few days.1 They are active during the summer months and there may be overlap among the species in their periods of activity. In areas with mild winters the flies may be active throughout the year. In colder regions fly activity ceases with the first frost and there is usually only a single generation per year. In these regions the second and third instars remain in the stomach over the winter.
Eggs are attached to hairs while the fly hovers close to the horse. Fecundity is roughly correlated to the size of the fly. G. haemorrhoidalis matures about 50–200 eggs, G. nasalis 300–500 eggs, and G. intestinalis up to 1000.1 Eggs of the various species are laid in specific locations and are attached in a specific manner, allowing identification of eggs to species. The eggs are laid on the horse’s coat except for G. pecorum which lays up to 2000 eggs in batches of 100–200 on pasture plants. The eggs of G. pecorum and G. haemorrhoidalis are dark brown; the eggs of the others are yellow and are readily visible glued to the hairs, usually one to a hair. The eggs of G. intestinalis, the most common fly, are laid on the front legs, particularly the lower parts; those of G. nasalis in the intermandibular area; the others species’ eggs are laid on the cheeks and lips.
The eggs are ready to hatch in about 2–10 days and the first instars enter the mouth either by host biting or licking or by subcutaneous migration from the cheeks into the oral cavity. The eggs of G. intestinalis and G. pecorum require a stimulus, provided by licking (moisture) or rubbing (friction), before they will hatch. The larvae penetrate oral mucosa, migrate to inner surfaces and emerge in the inter-dental spaces. The larvae of G. intestinalis penetrate the anterior end of the tongue and burrow in the buccal mucosa for about 3–4 weeks before invading pockets between the teeth or between the gum and molars.2 G. nasalis may also accumulate in pockets alongside the molars and cause mouth irritation. G. haemorrhoidalis can penetrate the skin of the cheek and after wandering in the tissues of the mouth may attach in the pharynx. The second instar of G. intestinalis may also attach for a few days to the pharynx and the sides of the epiglottis before passing to the stomach. The first instars of G. pecorum burrow into the mucous membranes of the hard palate, cheek and tongue where they develop into second instars. They then move to the pharynx where they develop into the third instar.3 Occasional larvae migrate to abnormal sites including the brain, the cranial sinuses, the heart, and lungs.
Third instars of G. intestinalis are found attached to the mucosa, usually in bunches, at the junction of the glandular and non-glandular portion of the stomach, where they become attached to the mucosa. G. nasalis larvae are found in the pyloric region of the stomach and the duodenum. G. pecorum larvae may be found in the pharynx and upper part of the esophagus and in the fundus of the stomach. G. haemorrhoidalis larvae are found in the tongue, the pharynx, and the gastric fundus.
In the host, two molts are made and the larvae pass out in the droppings 10–12 months after infestation, usually in the spring and early summer. Some larvae may attach temporarily to the rectal mucosa on their way through. The larvae migrate into the ground, pupate, and adult flies emerge after 3–5 weeks to recommence the late summer attacks on horses.
PATHOGENESIS
The adult fly causes considerable annoyance when ovipositing. The droning noise and the sudden attacks to lay eggs causes head tossing and running in the host. G. nasalis is particularly troublesome as it darts at the lips and throat.
There is some doubt as to the importance of the lesions associated with the larvae. At the sites where they adhere there is an area of thickening and inflammation and in rare cases gastric perforation occurs. It is probable that there is some chronic gastritis and interference with digestion in most infestations. G. intestinalis, the most common species, attaches to the squamous epithelium and this has a relatively slight impact on digestion in the horse. However, the ulceration, edema, and abscessation associated with this species cannot be overlooked and one must expect some effect from such lesions although it is difficult, in practice, to separate these findings from those associated with a concurrent worm burden. Occasional perforation of the gut has been documented.4 The larvae do not remove sufficient blood to cause anemia, feeding mostly on tissue exudate. In rare cases pleurisy may occur following perforation of the esophagus close to the cardia.5 In very heavy infestations with G. pecorum the presence of large numbers of larvae (100–500) on the soft palate and base of the tongue can cause stomatitis and some deaths. Migration of first instars in the tongue and interdental gingiva and the aggregation of larvae in periodontal pockets may produce irritation or pain and may prevent foals eating.
CLINICAL FINDINGS
A non-specific syndrome of unthriftiness, poor coat, occasional mild colic, and lack of appetite, plus bad temper and unwillingness to work is usually ascribed to bot infestations. Adult flies frighten horses by their hovering, darting flight, especially around the head of the horse, and may be a cause of shying and balking.
CLINICAL PATHOLOGY
The eggs on the hairs can be seen by direct inspection but the presence of larvae in the stomach and intestines can only be detected after treatment with a suitable boticide.
NECROPSY FINDINGS
A few larvae are present in the stomach of most horses at necropsy but clinical illness is usually associated with very large numbers. The areas of larval attachment are pitted and the gastric wall thickened. There may be an adhesive peritonitis with attachment and abscessation of the spleen over such areas.
The syndrome produced is not sufficiently characteristic to make antemortem diagnosis possible and bot infestations are commonly associated with helminth infestations which produce most of the signs observed. A tentative diagnosis of infestation of the gums can be made by signs of pain on mastication and the presence of bot-fly eggs on the horse at that time. A variety of serologic tests, including an ELISA, have been evaluated5 and found to be generally specific and sensitive. There has been no further development of a practical test. Endoscopy using a video gastroscope has been applied to the diagnosis of gasterophilosis, although its use has been confined to use in drug efficacy studies.6
TREATMENT
Many of the organophosphates are effective. Trichlorfon 40 mg/kg is effective and is usually given with a benzimidazole to control strongyles as a common broad-spectrum mixture in the horse. It can also be used as a paste. Ivermectin 0.2 mg/kg has high efficacy against the oral and gastric Gasterophilus larvae7 as well as all gastrointestinal nematodes, including migrating and hypobiotic large strongyles, microfilaria of Onchocerca cervicalis and larval stages of Habronema spp. and Draschia megastoma in the skin. Ivermectin has gained high acceptance in horse practice. Moxidectin 2% oral gel has been shown to have excellent efficacy.6,8
CONTROL
Treatment should preferably be administered after fly activity has ceased and the larvae have reached the stomach but before gastric damage has occurred. In most districts two doses should be given in winter, or in late winter and early spring. In foals showing pain on mastication, treatment with ivermectin paste or dichlorvos should be given as needed throughout the fly season.
The use of repellents or agents to kill the larvae in manure has not been successful, nor has bathing the affected hairs to encourage mass hatching and death of the larvae. The use of fringes, veils and tassels on the head harness helps protect horses against fly worry but is of little use in preventing bot infestation.
1 Cope SE, Catts EP. J Med Entomol. 1991;28:67.
2 Cogley TP. Vet Parasitol. 1989;31:317.
3 Zayed AA, et al. Vet Med J Giza. 1993;41:53.
4 Cogley TP, Cogley M. Vet Parasitol. 1999;86:127.
5 Escartin-Pena M, Bautista-Garfias CR. Med Vet Entomol. 1993;7:233.
6 Reinemeyer CR, et al. Vet Parasitol. 2000;88:287.
7 van-der Kolk JH, et al. Tijdschr Diergeneeskd. 1989;114:769.
OESTRUS OVIS INFESTATION (NASAL BOTS)
Infestation of sheep and goats with larvae of the nasal bot fly has a serious effect on the productivity and welfare of both sheep and goats. Adult activity induces stress responses and significant behavioral change. Larval infestation induces moderate to severe pathology that reduces productivity.
Similar flies are known to affect horses, donkeys, and mules (Rhinoestrus spp.) in the Mediterranean region and to affect camels (Cephenemyia titillator) in Africa as well as Australia. Wild ungulates are affected by nasal bots (e.g. Cephenemyia spp.). Very little is known about the pathology and impact of these later groups of flies, but similarities in life history suggest their affects will be similar to that discussed below.
Etiology
One species, Oestrus ovis, which inhabits the nasal passages and sinuses of sheep and goats.
Epidemiology
Larvae are sprayed onto the nares of hosts by passing females. Flies are active during spring and summer, inducing behavioral changes in hosts under attack. In temperate climates there is only a single generation per year, but in warmer climates two generations are known. First instars in the nasal passages undergo hypobiosis during winter or hot summer when survival of pupae or adults is low, resuming development when conditions are more favorable.
Clinical signs
Shortly after arrival of the larvae an increase in nasal discharge and sneezing are evident. As the infestations develop the amount of discharge increases and the nostrils may become caked with dust and debris forcing the infested animals to breath through their mouth.
Clinical pathology
Changes are noted to the mucosa of the ethmoid and sinus regions. Inflammation of these surface tissues is evident and increases as the larvae become mature. Changes to the epithelial structure are noted including the erosion of the surface ciliary covering and a breakdown in epithelial cell integrity. Abrasive action of the body armature as well as the activity of proteolytic enzymes excreted/secreted by the larvae are responsible for the pathology. Secondary effects Include the induction of lung lesions and the activation of latent ‘orf’ infections. Diagnostic confirmation. Behavioral changes during fly season and nasal discharge.
ETIOLOGY
A single species, known as the sheep nose bot, affects sheep and goats in most regions, but is particluarly significant in the Mediteranean basin, central America southern Africa, and eastern Europe. The larvae inhabit the nasal passages and sinuses, eventually being expelled through the nares. Goats are less dramatically affected than sheep. The slightly dorso-ventrally flattened, segmented larvae are light cream in color, but as they reach maturity dark bands appear on each segment.
LIFE CYCLE AND EPIDEMIOLOGY
The adult fly is stout, mottled gray in color, and about 1 cm long. Its mouthparts are rudimentary and it does not feed. In North America, flies emerge in the late spring, mate, and the females begin larviposition activities approximately 2–3 weeks later.1 Adult flies attempting to deposit larvae on the nares annoy the sheep and cause them to bunch or seek shelter. Stamping of the feet and shaking of the head are common. Sheep may bunch together and press their heads into the fleece of others. Fly activity occurs primarily during the warmer parts of the day, but still may result in the loss of a good deal of grazing time. Behavioral changes in goats are less dramatic,2 presumably because of their browsing habit.2
Larval development takes place within the dorsal turbinates and frontal sinuses. The period of development can vary from 3 weeks to several months after which they migrate to the nostrils. Larvae feed on the mucosal secretions and cells eroded from the mucosal epithelium. The larvae are thick, yellow-white in color and when mature there is a dark dorsal band on each segment. The ventral surface has rows of small spines on each segment. Mature larvae exit the host, usually during a bout of sneezing, and actively burrow beneath the upper layers of soil and ground litter. Pupation occurs at these locations and development of the adults requires 4–5 weeks, but may take longer at low temperatures.3 In temperate areas there may be one or two generations per year but several generations may be completed in hot areas. O. ovis are adapted to the various climates prevailing wherever sheep and goats are kept. When winters are cold, the larvae can overwinter by remaining dormant in the first instar (hypobiosis), but in warmer climates development may continue throughout the winter. In those regions where summer temperatures are extreme the larvae will also undergo hyopobiosis.
O. ovis are an important zoonosis as the females may larviposit in the eye, nose or on the lips of humans. In some countries ophthalmomyiasis or infection of the upper respiratory tract is a common occurrence.
PATHOGENESIS
The stress of the larviposition attacks can be significant with reduced grazing time and over-heating resulting from bunching. Herdsmen find the animals are more nervous and difficult during the fly activity periods.
Larvae induce a gradually increasing rhinitis and sinusitis as the infestation persists. Marked changes in the structure of the epithelial tissues are noted with a marked cellular degeneration and a loss of the ciliary layer. The changes are a result of both mechanical activity of the larval spines and mouthhooks as well as the effect of proteolytic enzymes excreted or secreted.4 Varying degrees of mucous discharge are observed in the later stages of the infestation. This can lead to the nostrils being occluded by adherent straw and dust.
CLINICAL FINDINGS
Early in the infestation there is a distinct rhinitis accompanied by a muco- to muco-purulent discharge. Later as larvae mature, a sinusitis is evident. Presence of mature larvae in nasal cavities may induce excessive sneezing which assists larval exit.
Activity of the larvae in the nasal cavities and the changes they induce lead to an increased incidence of secondary pathology. The number and severity of lung abscess are more significant in nose bot infested sheep.5 The presence of bots also is correlated with increased carcinomas6 and may lead to reactivation of latent ‘orf’ symptoms.
The behavioral changes during fly activity, including bunching and burying of noses in neighbors’ fleece is a reliable indicator of fly attack. Nasal discharge and excessive sneezing are highly suggestive, but not definitive. Infested sheep and goats develop some level of immunity form exposure to larval antigens. An ELISA for detection of antibodies to larvae secretions has been developed.7
1 Cepeda-Palacios R, Scholl PJ. J Med Entomol. 1999;36:435.
2 Hoste H, et al. Vet Parasitol. 2001;101:127.
3 Biggs HC. Preventive Vet Med. 1999;33:267.
4 Tabouret G, et al. Vet Parasitol. 2003;114:305.
5 Dorchies Ph, et al. Vet Rec. 1993;133:325.
6 Bergeaud, et al. Rev de Méd Vét. 1994;145:863.
7 Tabouret G, et al. Vet Res. 2003;34:231.
8 Dorchies Ph, et al. Vet Rec. 1993;135:600.
9 Roncalli RA. Vet Med. 1984;79:1095.
HYPODERMA SPP. INFESTATION (WARBLE FLIES)
Infestations of cattle with the larvae of Hypoderma spp. cause serious damage to hides and carcasses, as well as production losses. Occasional deaths from anaphylactic shock or toxemia and damage to the central nervous system or esophagus. Several other flies with very similar life histories similarly affect goats (Przhevalskiana silenus), and semi-domestic reindeer (Hypoderma tarandi) in addition to affecting the well-being of wild ruminants.
Etiology
Hypoderma bovis and H. lineatum in cattle, H. sinense in cattle and yaks, H. diana in deer, H. tarandi in reindeer and caribou, Przhevalskiana silenus in goats. Horses are occasionally affected.
Epidemiology
Eggs attached to hair in spring to late summer, larvae penetrate skin and migrate to esophagus (H. lineatum) or spine (H. bovis) where they stay for 6–7 months; they then move to subdermal tissue along the back and after 2–3 months emerge from the breathing hole, fall to the ground, pupate and emerge as adult flies 3–5 weeks later.
Clinical signs
Poor growth and production. Larvae in the back causes obvious swellings while larvae in the spinal cord may cause posterior paralysis. Treatment of larvae while they are in the esophagus may cause massive edema, and edema and paraplegia may occur if animals are treated when larvae are in the spinal canal.
ETIOLOGY
There are two species which specifically parasitize cattle: Hypoderma bovis and H. lineatum. A third species, H. sinense, affects cattle and yaks in central Asia.1 The adult flies are robust and hairy, about the size of a bee (12–18 mm long), are yellow-orange in color and have two wings. They are not easily seen because of the rapidity of their flight. Repeated infestation results in an acquired immunity that results in older animals being less severely affected than younger animals.
Horses are occasionally infected with Hypoderma species of cattle. The larvae are found in subcutaneous cysts on the back, but have not been reported to complete development. This location causes problems if they are in the saddle region.
Losses to the cattle industry associated with warble fly have not been estimated recently, but in 1965 the loss was estimated to be US$192 million per annum in the United States, and in 1976 approximately CAN$100 million in Canada. In 1982 the cost of warble fly was estimated as £35 million for Great Britain, but the parasite has now been eradicated from the UK and Ireland. Advent of the macrocyclic lactone endectocides have greatly reduced the prevalence of the cattle species in North America, but they persist in localized areas.2
Hypoderma tarandi, H. acteon, and H. diana infect reindeer/caribou and deer respectively. H. diana is found throughout Europe in several deer species but may also occur in sheep. H. actaeon, also found throughout Europe, is known only from the red deer. These species do not undergo deep tissue migrations that characterize the lifecycle of the cattle species.
Przhevalskiana silenus is similar to the above and parasitizes goats in the Mediterranean basin, parts of eastern Europe as well as in Pakistan and India. This species also does not have a deep tissue migration and larvae tend to develop subcutaneously very near the site of initial skin penetration. The losses resulting from this parasite are significant and result from reductions in carcass quality and reduced animal health.
The larvae of Dermatobia hominis, a small (12 mm long) related fly, parasitize a wide variety of hosts and cause major economic losses to cattle production in South America. They also affect man and are a major zoonosis for travelers in the region. Mature larvae are about 2.5 cm long and develop in a subcutaneous cyst that can be quite painful. Female Dermatobia oviposit on zoophilous, ‘porter’ flies such as mosquitoes and stable flies which they catch ‘on the wing’. The eggs are transported to mammalian hosts where they hatch in response to increased temperature as the fly lands. Larvae penetrate the skin, but do not migrate. Treatment and control measures are the same as for H. bovis.
LIFE CYCLE AND EPIDEMIOLOGY
Warble flies are common parasites of cattle in the northern hemisphere, including North America, Europe, and Asia. Precise distribution of these parasites have been changing recently with the widespread use of macrocyclic lactone endectocides and the adoption of eradication programs in many European countries. Infestations south of the equator are rare and are the result of imported cattle, although endemic cases have occurred in Chile.
Adult flies are active in the spring to late summer, H. lineatum usually appearing 3–4 weeks before H. bovis. H. lineatum attaches up 600 eggs, in strings of 5–25, to hairs on the legs or lower parts of the body while H. bovis attaches eggs, one at a time, to hairs on the rump and upper parts of the hindleg. The oviposition flight of H. bovis, darting in to lay each egg, will terrorize cattle. Eggs hatch in 4–6 days. The larvae penetrate the skin using protease enzymes and migrate through connective tissues to reach the esophagus (H. lineatum) or the epidural fat in the spine (H. bovis) where they stay, feeding and growing, for 2–4 months. They subsequently continue their migration to reach the subdermal tissue of the back in the early spring. Here they make a breathing hole and become encased in a granulomatous cyst. They complete development in 1–2 months, passing through second and third instars and emerge through the hole, fall to the ground and pupate. Adult flies emerge some 3–5 weeks later. The fully developed larvae are thick and long (25–30 mm), light cream in color, but darkening to almost black as mature third instars. A single animal may have up to 300 larvae each developing with granulomatous cysts, with breathing holes, under the skin of the back.
The timing of the life cycle, i.e. the period when grubs are present in the animals and the time at which the flies are present in large numbers, varies with the climate and is of importance in a control program. H. lineatum generally is 1–2 months ahead of H. bovis and where the two flies are present both ‘grub’ and ‘fly’ seasons may be very long. In the southern United States the ‘fly season’ is February and March; in Canada it is June to August. The period when grubs are present in the back is December in the south and February to May in Canada. In Europe the larvae begin to move to the back in January to July.
PATHOGENESIS
Migrating first instars cause little damage as they use their proteolytic enzymes to migrate through connective tissue. The enzymes, however, have an anti-inflammatory effect, partially through cleavage of complement components.3 Larvae maturing under the skin of the back form holes in the skin and the reaction of the host encloses each grub within a granulomatous cyst. On rare occasions an anaphylactic reaction may occur in a sensitized animal as the result of death of migrating larvae; chance migration into the brain may also occur. Intracranial myiasis due to H. bovis has also been recorded in the horse. Treatment of animals when the first instars are in the esophagus may cause a massive inflammatory edema which may prevent feeding and swallowing of saliva; eructation may stop and bloating may occur. Treatment of H. bovis while it is in the spinal canal may also cause edema and mild to severe paraplegia.
CLINICAL FINDINGS
If the fly population is heavy, cattle at pasture may be worried by their attacks which disrupt grazing and breeding behavior. Avoidance behavior, called gadding, may result in injury as cattle run into fences and other natural obstructions. Heavy infestations with larvae are commonly associated with poor growth, condition and production but such heavy infestations are often complicated by other forms of mismanagement including malnutrition and parasitic gastroenteritis. Immunosuppression results from the effect of larval secretions. Infected cattle milk poorly and a considerable increase in milk production and milk fat occurs after treatment.
The presence of the subcutaneous larvae causes obvious swelling with pain on touch. The swellings are usually soft and fluctuating. There may be as many as 200–300 such lesions on the back of one animal.
With involvement of the spinal cord there is a sudden onset of posterior paralysis without fever and without other systemic signs. The suddenness of onset and the failure of the disease to progress usually suggest traumatic injury. A similar disease can occur in horses and is reputed to be more common in horses than in cattle.
CLINICAL PATHOLOGY
An ELISA which detects antibodies to the secreted enzymes of H. lineatum and H. bovis has been developed. It has been used in the eradication program in Great Britain4 and in France.5 In addition, an antigen capture ELISA, used to detect the presence of circulating quantities of the predominant larval enzyme has been developed.6 This will be useful in differentiating active from cleared infestations and will be useful in detailed surveillance programs.
NECROPSY FINDINGS
The first instars, migrating within connective tissue, are usually surrounded by a zone of yellow-green discoloration. Later larval stages lie in a subcutaneous, granulomatous cyst which may contain a pale fluid. Rarely the cyst will contain a large amount of purulent discharge.
• No other disease causes the characteristic swellings on the back
• The differential diagnosis of posterior paralysis and anaphylaxis are discussed in detail under the respective headings of disease of the spinal cord and anaphylaxis
• The clinical signs of organophosphorus poisoning usually occur within 12–24 hours following application of the compound
• Posterior paralysis due to destruction of the larvae in the epidural space usually occurs approximately 72 hours after application of the organophosphorus insecticide.
TREATMENT
Organophosphorus compounds
Organophosphorus insecticides kill migrating larvae and can be applied by spray or a ‘pour-on’ technique, by individual oral dosing or by mixing in the feed. They are highly effective, but unless used in strict accordance with the recommendations of the manufacturer damaging side-effects may occur.
The time of their administration is regulated by the stage of larval development and their location within the host. Hence the timing varies with climate. The emphasis is to deliver treatment shortly after all eggs have hatched and larvae are small and their death releases the least amount of enzymes into the host system. If treatment is delayed until these larvae have reached their maximum size, the sudden release of large amounts of proteolytic enzymes causes tissue damage that results in severe swelling. This swelling and inflammation affect the function of the esophagus (H. lineatum) resulting in bloat or swelling around the spinal chord leading to hind quarter paralysis (H. bovis). Severity of the reaction also appears to be related to the number of larvae present. These insecticides are not effective against second and third instars within the subcutaneous cysts.
Application in the form of a spray should insure that the skin is wetted by using a pressure spray (20–30 kg/cm2), and spraying the flat body surfaces such as the neck, back, shoulders, sides, and thighs. Use of a low pressure spray or wash should be followed by vigorous use of a curry comb or brush. Pour-on applications should be made carefully along the midline in a thin stream which penetrates the hair coat.
Although these compounds disappear very quickly from tissues, recommendations are that they should not be administered to milking animals, and beef animals should not be slaughtered for at least 60 days after dosing. Milking cattle with back lesions can be treated with derris dust (containing rotenone) but this must be brushed in vigorously or sprayed under pressure so that the material penetrates to the larvae.
Toxic effects of organophosphates have been described elsewhere. The pitfalls in their use in warble control are related chiefly to the time of administration.
Macrocyclic lactone compounds
All larval stages of cattle grubs are very sensitive to these endectocides. Their widespread use in nematode control programs plays a major role in controlling warble flies. They remain effective for about 4 weeks.2
Manual removal
When small numbers of cattle are affected with relatively few warble grubs, manual removal of the larvae is practised. Incomplete removal or breaking the larvae during removal may cause a severe systemic reaction. This reaction and the one which sometimes occurs after systemic treatment of cattle infected with warble fly have been ascribed to anaphylaxis. However, there is evidence that it is due directly to toxins liberated from dead warble maggots and that phenylbutazone may control this toxin.3 The clinical signs include dullness, salivation, lacrimation, dyspnea, wrinkling of skin on the side of the neck, and edema under the jaw.
CONTROL
While rotenone has been used successfully to kill the larvae in the subcutaneous tissues in the back, this technique does not prevent damage to the hide and has been largely superseded by the use of organophosphate insecticides and the macrocyclic lactone endectocides. In general, systemic treatments are given in the autumn (between September 15 and November 30) and in the spring after March 1.
Warble fly has been eradicated in Norway, Sweden, Denmark, Malta, Ireland, and Great Britain. Eradication programs have commenced in France. A joint Canadian–US study using sterile male Hypoderma species eradicated these species from the test area, but the difficulty of mass producing flies, in the absence of an in vitro rearing system, makes this technique impractical for large scale warble fly control.7
Vaccination of cattle using crude larval extracts has reduced both the number of warbles in the back and the number of larvae that could pupate.8 Results of vaccination studies with recombinant antigens have been variable,9 but show promise although there has been no commercial development.
1 Otranto, et al. J Parasitol. 2004;90:958.
2 Colwell DD. Vet Parasitol. 2000;94:127.
3 Boulard C, Benacharif F. J Immunol. 1984;6:459.
4 Tarry DW, et al. Vet Rec. 1992;131:310.
5 Boulard C, Villejoubert C. Vet Parasitol. 1991;39:171.
6 Panadero-Fontan R, et al. Vet Parasitol. 2002;108:85.
7 Kunz SE, et al. J Med Entomol. 1990;27:523.
8 Baron RW, Weintraub J. Vet Parasitol. 1986;21:43.
9 Colwell DD. Mange and myiasis of livestock. COST. 2002;833:7.
Cutaneous myiasis
Cutaneous infestation by fly larvae or maggots (known as myiasis) causes serious loss to the livestock industries across the world. Losses include mortality, increased morbidity and reduced production of meat, milk, and fiber. The disease is associated with larvae of flies in two major dipteran families, Calliphoridae and Sarcophagidae.
Two types of cutaneous myiasis can be distinguished; primary, in which the fly larvae are obligate parasites feeding on living tissues and secondary, in which the larvae feed primarily on necrotic tissues and only secondarily invade uninjured tissue. Clearly, primary myiasis is most significant to animal health and therefore the most costly, not only in terms of mortality, morbidity and reduced productivity, but in terms of cost of control. However, it may be difficult to differentiate primary from secondary myiasis because the larvae are superficially similar.
Three primary fly strike disease states, resulting from the activities of different species, are well known and described. Blowfly strike by calliphorids such as Lucillia cuprina and L. sericata is a major problem, particularly for sheep producers, in Australia, New Zealand, and Great Britain. The second group are the screwworms, Cochliomyia hominovorax (in the New World) and Chrysomyia bezziana (in southern Europe, Africa, and Asia) which are of importance across the livestock species and result in great costs for control. The sarcophagid (flesh fly) Wohlfahrtia magnifica causes traumatic myiasis in a wide range of livestock species, but has most impact in goat and sheep production. This species occurs in southern Europe, particularly the Mediterranean and the steppe regions of the continent. Because of differences in the nature of the disease state and control practices for each of these three groups they will be dealt with as separate entities.
BLOW FLY STRIKE
The cost of blow fly control and production losses in Australia for 1985 have been estimated as $200 million. A review of blow fly strike in Australia has been published and is given here as a review reference. Blow fly strike is a very important cause of losses in sheep in most countries where large numbers are kept. In bad years many sheep may die (up to 30% of a flock) and the expense of controlling the flies and failure of wool to grow after recovery may be a serious strain on the local sheep economy. Merino sheep, especially those with heavy skin wrinkles, are by far the most susceptible breed.
Etiology
Lucilia cuprina and L. sericata are the most important primary flies while other calliphorids act as secondary invaders.
Epidemiology
Fly numbers depend on temperature. Flies are attracted to wool that has been wetted or to areas affected by fleece rot, mycotic dermatitis, diarrhea or urine staining. Incidence of strike is positively correlated with fly numbers, rainfall, humidity, cloud cover, and pasture growth. Covert strikes provide larvae for future generations.
Clinical signs
Sheep are restless, bite at the affected area, and wriggle their tails. Affected area is moist and malodorous, body temperatures may reach 42°C, pulse and respiratory rates increase.
Lesions
Moist, malodorous areas containing active larvae. Predisposing diseases such as dermatophilosis, fleece rot, parasitic gastroenteritis, and footrot are easily identified.
Control
Cyromazine or triflubenzuron protect for 10–12 weeks while organophosphates kill larvae and protect for 2–3 weeks. Breeding for resistance and control of predisposing diseases is important. Conduct mules operation on lambs at marking, cut tails to the correct length, institute a proper gastrointestinal nematode control program, and give a mid-season crutching to control breech strike.
ETIOLOGY
Despite there being a large number of species capable of causing the disease, grouped geographically below, there are two species that are the primary agents of blow fly strike: Lucilia cuprina and L. sericata.
• Australia: Lucilia cuprina, L. sericata, (Calliphora stygia, C. novica, C. augur, C. hilli, C. albifrontalis, Chrysomyia rufifacies, Ch. varipes)
• Lucilia sericata, L. cuprina, (Calliphora stygia)
• Great Britain and northern Europe: Lucilia sericata (Calliphora erythrocephala, C. vomitoria, Phormia terraenovae)
LIFE CYCLE AND EPIDEMIOLOGY
The primary agents of fly strike are obligate parasites. Lucilia cuprina is overwhelmingly important in the initiation of strike in sheep from Australia, and South Africa. L. cuprina was introduced to New Zealand in 1988 and fly strike is now a major disease in that country. In northern Europe the primary agent of fly strike is Lucilia sericata although there are some other minor species that have been reared from struck sheep.1
In sheep, the incidence varies widely depending largely on the climate, warm humid weather being most conducive to a high incidence. In summer rainfall areas fly strike may be seen most of the year, being limited only by dry winter conditions; while in winter rainfall areas it is usually too cold in the winter and too dry in the summer for outbreaks to occur. Under these conditions abnormally heavy summer or autumn rains may be necessary before an outbreak will occur.
The fly population
Primary flies are of particular importance as these initiate the strike and provide suitable conditions for subsequent invasion by secondary flies. These latter flies are not of economic importance but may infest wool matted with dried exudate or feed on necrotic tissue surrounding healing strike. In warm areas pupal development may continue throughout the year but as soil temperatures fall an increasing number of larvae fail to pupate and larvae may overwinter until the following spring. In the spring adult flies emerge and after one or two breeding cycles, numbers build up to a peak in summer. Numbers may remain high if climatic conditions are suitable, moisture being of prime importance, but may fall dramatically in hot dry conditions. An increase in numbers may occur again in the autumn.
All adult flies require carbohydrate and water, but females require protein for ovarian development. The flies are attracted to sheep that have undergone prolonged wetting so that bacterial decomposition of the skin has occurred. The association of fly strike with fleece rot, mycotic dermatitis, diarrhea, urine staining, and footrot is related to the excessive moisture deposited on the skin or to the production of serous exudates. Fractions of Pseudomonas aeruginosa infected fleece have been shown to stimulate oviposition.2Lucilia cuprina deposit eggs in batches of up to 300, the actual number depending on the fly’s size and its ability to locate sufficient protein for egg development. Similarly, L. sericata deposit eggs in batches of approximately 200. The average female longevity in the field in Australia is about 2 weeks and females rarely live long enough to mature more than two or three batches of eggs. In the UK mean female longevity is shorter at 5 days.
The eggs hatch in 12–24 hours and the first instars feed on protein-rich serous exudate that has been provoked by bacterial damage or some other irritation. Larval mouthhooks and enzymes present in the saliva and excreta will further digestion of the skin. Large groups of larvae, particularly second and third instars, further damage of the skin which extends the lesion and insures a continuing supply of food. The second and third instars are 6–12 mm long, thick, yellow and white in color and move actively. Larvae reach maturity after approximately 72 hours. They leave the feeding lesion, fall to the ground, wander briefly and then burrow into the earth to pupate. The length of the life cycle is highly temperature dependent; it be completed in as little as 8 days but may require up to 6 weeks in temperate regions such as the UK.2 Eggs and larval stages are highly susceptible to desiccation and morality will be high if the relative humidity in the fleece falls below 60%. In temperate climates such as the UK, as photoperiods decline the larvae that have left the host, will burrow into the ground and cease development, thereby overwintering as mature larvae.
Several generations of primary flies are necessary before numbers are high enough to cause severe outbreaks and therefore warm humid weather must persist for some time before severe outbreaks occur. The incidence of body strike increases with the increase in the number of gravid flies and is positively correlated with rainfall, cloud cover, and rate of pasture growth.3 Other primary flies are not as effective as L. cuprina in initiating a strike, and in Australia at least 85–90% of all primary strikes are due to L. cuprina. Larvae of primary flies, other than L. cuprina, and secondary flies develop in carrion or in rotting vegetation and their main role is to invade and extend the primary strike. Ch. rufifacies is the most important secondary fly in Australia. It requires higher temperatures than the other flies, is found later in the season and is the first to disappear as temperatures fall.
Detailed population models have been developed for the strike by Lucilia spp. in Australia4 and northern Europe5 and both have been used to predict onset of fly strike in sheep populations. The latter model has been extensively validated and is sufficiently accurate to establish an early warning system for alerting producers of the impending onset of fly strike and thus allowing well timed prophylactic treatments.
Distribution of fly strike in flocks is highly aggregated with a small number of sheep having high numbers of larvae in lesions, a moderate number of sheep with low numbers of larvae and the majority of sheep being unstruck.6 In part this is a result of the attractiveness of already struck sheep to ovipositing flies, although other factors such as innate attractiveness play a role.
Susceptibility of sheep
By far the most common site for fly strike is the breech: infestation occurs here because of soiling and excoriation by soft feces and the urine of ewes. Lush pasture, parasitic gastroenteritis and fleece length are predisposing factors but individual sheep are predisposed because of the conformation of this part of their anatomy. Excessive wrinkling of the skin on the back of the thighs and the perineum, a narrow perineum and crutch and an excessively long or short tail favor continuous soiling of the area and encourage ‘crutch or breech strike’ or ‘tail strike’. Less common sites for infestation are around the prepuce (‘pizzle strike’), on the dorsum of the head when there is excessive folding of the skin (‘poll strike’), and along the dorsum of the body (‘body strike’) in wet seasons when fleece rot or dermatophilosis is common. Sheep grazing on tall, dense pasture are commonly affected by body strike because of the way in which the wet plants keep the fleece on the lower part of the body wet. Wounds, especially castration incisions, docking wounds and head wounds on rams caused by fighting, are also likely to provide good sites for blow fly strike. Young sheep are more susceptible.
PATHOGENESIS
The first instars feed on the exudate produced by the bacterial infection on the skin, but the larvae also produce excretory/secretory enzymes which may cause some skin degradation after egg hatch and provide soluble molecules on which the first instars can feed.7,8 Later instars can cause severe skin damage to provide themselves with food. Larvae may also migrate from the original area of strike, along the surface of skin to establish additional focal lesions.
Many primary strikes remain small and are unnoticed by the farmer.6 Such covert strikes may outnumber overt strikes and are important as a source of future generations of flies. Once the initial strike is made, the site becomes suitable for the secondary flies that now invade and extend the lesion. The effects of strike include toxemia due to absorption of toxic products of tissue decomposition, loss of skin and subsequent fluid loss, and secondary bacterial invasion.
CLINICAL FINDINGS
Individual sheep may be ‘struck’ at any time provided they are in a susceptible condition. Massive outbreaks tend to be confined to periods of humid, warm weather and are therefore in temperate areas usually limited in length to relatively short periods of 2–3 weeks, but in sub-tropical areas characterized by summer rainfall severe strikes may occur over many months.
The clinical effects of ‘blowfly’ strike vary with the site affected but all struck sheep have a basic pattern of behavior caused by the irritation of the larvae. The sheep are restless, moving about from place to place with their heads held close to the ground and they become anorexic. They tend to bite or kick at the ‘struck’ area and continually wriggle their tails.
If the area is large there is an obvious odor and the wool can be seen to be slightly lifted above normal surrounding wool. The affected wool is moist and usually brown in color although in wet seasons when fleece rot is prevalent other colors may be evident. In very early cases the maggots may still be in pockets in the wool and not yet in contact with the skin. When they have reached the skin it is inflamed and then ulcerated and the maggots begin burrowing into the subcutaneous tissue.
Three days after the primary oviposition feed intake is reduced, rectal temperature rises to about 42°C (108°F) and pulse and respiratory rates increase. Some sheep may die. The wool may be too hot to handle as a result of the inflammation caused by the mass of maggots that can be seen when the wool over the strike is opened. When primary strikes are invaded by secondary flies, particularly Ch. rufifacies, the affected area is extended and the maggots may burrow deeply into the tissues. Affected sheep may lose their fleece over the affected area and may suffer a break in the remaining fleece. Tracts of discolored wool may lead to other affected areas of skin. As the struck area extends a scab forms over the center, the wool falls out and the maggots are active only at the periphery.
CLINICAL PATHOLOGY AND NECROPSY FINDINGS
A clinical examination is all that is necessary to make the diagnosis but identification of the flies responsible may be important if epidemiology is being considered. Identification of larvae should be carried out by a specialist. Molecular techniques for accurate identification are available from specialists.9 Preservation of the larval stages is critical to these techniques and larvae should be rapidly frozen or preserved in 70% ethanol. Fly trapping may not correlate with larval findings as not all flies are equally attracted by commonly used baits.
Attention will be drawn to affected sheep by their foot stamping, tail twitching, and biting at the affected part. Affected sheep can easily be diagnosed by finding the moist, malodorous, maggot-infested area. Many covert strikes may be present without producing clinical signs. Predisposing diseases such as footrot, wound infections, dermatophilosis and diarrhea resulting from parasitic gastroenteritis are usually easily detected and fleece rot is indicated by matting of the wool and discoloration.
TREATMENT
A local dressing containing a larvicide and an antiseptic is applied. The prevention of reinfestations is also an important aim and as repellents have been largely unsatisfactory, it has become apparent that the larvicide in a suitable dressing must be one with maximum retention in the treated area. Modern dressings usually contain organophosphate insecticides but most products do not kill all the organophosphate resistant larvae and some perform poorly even against susceptible larvae. Variations in effectiveness with the same compound sold by different companies is probably related to formulation.10 Ivermectin at 0.03 mg/kg applied by hand jetting is highly effective in killing all larval stages.11 Powder and liquid dressings containing diazinon, tetrachlorfenvinphos, propetamphos, and ivermectin are most generally favored.
Cyromazine, an insect growth regulator, is now widely used. It is particularly active against second and third instars, but is slow-acting and so live larvae may be seen in the fleece for some days after treatment.
CONTROL
Practical control of crutch strike in extensive farming areas depends on the use of the Mules operation to extend the bare areas around the perineum and tail, good worm control to prevent contamination of the perineal region, correct tail length and a midseason crutching. Control of strike in other situations is based on insecticidal treatment and treatment of wounds as they occur. Under conditions of extensive sheep raising, such as occur in Australia and South Africa and where climatic conditions are conducive to the development of the disease, the control of blow fly strike is a major undertaking and an extensive bibliography on the subject is available. Only a summary can be presented here.
The subject can be divided into three phases: reduction in fly numbers; prediction of fly waves followed by prophylactic crutching and application of larvicides; and reduction in susceptibility of sheep.
Reduction of fly numbers
Reducing the fly population has been of limited value as there are usually enough flies present to strike all susceptible sheep if suitable conditions are present. However, if the primary fly responsible for initiating strikes can be controlled the importance of secondary flies is greatly reduced. The measures used include trapping, early treatment of clinical cases and the proper disposal of carcasses and wool waste. Biological control by the use of insects parasitizing blow flies has been generally unsuccessful. Trapping, provided the traps are carefully looked after and satisfactory baits are used, can reduce the number of blow flies.12 Recent work with synthetic attractants have shown reduction of strikes by up to 55%,13 which suggests this approach will make a good adjunct to the overall fly management practices.
It is important to identify clinically affected sheep, particularly those affected early in the season, and to treat the infestations. If these early season strikes are not treated they serve to multiply the fly population and outbreaks will occur later in the season. When affected areas are clipped, the clippings should be disposed of and larvae on the sheep destroyed with a suitable dressing.
Control by genetic means offers promise for long-term control. Chromosome translocation to produce reduced fertility in male flies and lethal mutants, such as flies with yellow or white eyes which will be blind under field conditions and die, has been reported.14 This technique is logistically and economically feasible and more cost-effective than the irradiated male technique used for screwworm control, as only two or three early-season releases of males would be required rather than continuous release. Future development of transgenic males that would be useful in male-only sterile insect releases also shows some promise.
Prediction of fly waves
Sporadic cases of body strike may occur in sheep at any time and cannot reasonably be prevented, but if the environmental circumstances conducive to high fly populations and high susceptibility of sheep are recognized fly ‘waves’ can be predicted and short-term prophylactic measures taken. Warm, showery weather extending over several weeks allows several generations to be completed and sufficient flies to be available to cause an outbreak of strike. Once sufficient flies are present, an outbreak of cutaneous myiasis may occur whenever the sheep become susceptible. Warm humid weather, rain over 2–3 days, or grazing in long wet grass, may provide suitable conditions for the sheep to become susceptible to body strike. Sheep with yellow fleece, i.e. high suint content, comprised of pointed, thin staples less tightly packed and with a low wax content, would be most susceptible. Measurement of simple fleece values has been suggested as a means of predicting the susceptibility of flocks to fleece rot and flystrike. Sheep with long fleeces are more susceptible and the time at which shearing is carried out may exert an influence on the frequency and severity of outbreaks.
Outbreaks of breech strike will occur if the sheep have diarrhea, or if ewes have urine splashing onto the breech area because the tails are too long. If an outbreak is predicted or has begun, ‘crutching’ and the prophylactic application of larvicides will reduce the severity of the infestation. ‘Crutching’ refers to clipping of the wool around the breech or crutch of the sheep to avoid it becoming wet with urine and feces and providing a focus of attraction for flies. It is carried out routinely before lambing and immediately prior to a strike wave but provides protection for no more than 6 weeks. All the wool from above the tail, to the posterior aspect of the thighs and down to the hocks, must be removed. Because of the labor and loss of wool involved most sheep farmers depend on prophylactic dressing with a larvicide.
Predictive models incorporating climatic and production components have been developed in the UK. These are used to give producers warning of impending fly strike (see above)
Treatment and prevention
Prophylactic treatment with a larvicide has been a major part of blow fly control for many years but the preparations used and the methods of their administration have undergone many important changes. The triazine, Vetrazin, gives 8–10 weeks of protection and can be applied by jetting, as a pour-on15 or as a intraruminal slow-release bolus.16 Triflubenzuron, another insect growth regulator, also gives good protection.17 Their action is specific to dipteran larvae. It should be noted that they are slow to kill as they affect the development of the subsequent life cycle stage. Diazinon, tetrachlorfenvinphos, propetamphos, fenthion ethyl, coumaphos, and other organophosphorus insecticides are widely used, but the period of protection gained by their use has markedly declined as the resistance to these compounds has increased. Further, the organophosphorus insecticides may be degraded by Pseudomonas aeruginosa. However, most fly waves are of short duration, and if the insecticide is applied thoroughly at the time an outbreak commences, these compounds may still give sufficient protection to minimize losses.
Three avermectins and the synthetic pyrethroids act as oviposition suppressants. Deltamethrin is more active than the avermectins but the avermectins also produce significant mortality in the adult flies. They can be applied to the fleece as pour-ons or by spray races to prevent egg-laying.
The methods of application include dipping, jetting, and tip spraying. Dipping has the advantage of thorough wetting but requires high equipment and labor costs. Jetting is still recommended for crutch strike and if the jetting piece is combed through the wool from the poll to the rump with the solution at high pressure (500–900 kPa), good control of body strike will also be achieved. Tip spraying, which is the deposition of a higher concentration of insecticide onto the tip of the fleece, is only of use with dieldrin or aldrin as it relies on the ability of these compounds to diffuse down the wool fibers. The latter compounds are now banned in most countries. Tip spraying is not effective with organophosphates.
It is recommended that sheep should be treated early in the spring. This protects sheep against the early season strikes, many of which are covert, and prevents the build-up of fly populations in spring and early summer which leads to outbreaks.
Reduction in susceptibility of sheep
The primary method for reduction of sheep susceptibility to fly strike is the Mules operation. This technique, originally developed to remove the wrinkled region of the breech has been modified to address concerns regarding animal welfare which must be balanced against the impact of fly strike. The technique is still recommended in the recently established codes of practice. Other good management practices are essential to prevention of fly strike. These include management of gastrointestinal nematodes to prevent scouring, tails cut to the correct length and a midseason crutching to reduce soiling that predisposes animals to strike. Pizzle strike will be reduced by the use of testosterone implants and by pizzle dropping (surgical separation of the preputial sheath from the belly) although this procedure may cause some difficulty at shearing unless the shearers are warned. Ringing (shearing of the pizzle area) will give 6–8 weeks protection. Fleece rot occurs most commonly on the withers of sheep, and the conformation that allows accumulation of moisture and the development of fleece rot and fly strike have been shown to be hereditary. Sheep with these faults should be culled. Although control is mainly a matter of management, in periods particularly suitable for fly strike the periodic application of an insecticide is still essential.
The modified Mules operation is best performed at marking (tailing and castration) as the lambs heal more quickly and suffer little, if any, setback in growth. Further, minimum death rates due to fly strike and maximum wool weights are obtained when sheep are mulesed as lambs. The technique has been developed extensively in Australia and is not described in detail here. In the hands of an experienced technician the time required to improve the perineal topography need be no longer than 1–2 minutes. The improvement is permanent and reduces crutch strike by 80–90%. Mulesing is accompanied by some pain, but current data unequivocally establish the positive health and welfare benefits conferred upon sheep in the Australian environment. The protection gained by mulesing surpasses that afforded by breeding and is immediate and permanent.
The Mules operation is often supplemented by including a tail-strip operation in which a thin strip of woolled skin is removed from each side of the tail to above its butt. This results in a reduction of the amount of wool on the tail and less chance of fecal and urinary contamination. Since the introduction of elastic rings for castration and removal of the tail, a tendency has developed to remove the tail at the butt. This allows wool to grow in around the anus where it may become soiled and struck and, when combined with the Mules operation which slightly everts the vulva, has caused a dramatic increase in carcinomas of the mucocutaneous junction in the vulva. Tail stripping is important when tails are left at the longer recommended length, that is to the tip of the vulva. Docking so that the tail is of the correct length and so that a flap of ventral wool-less skin is left to seal over the stump is important. The latter can be effected by pushing the skin back with the back of the docking knife before severing the tail.
Removal of the wool and skin wrinkles from the breech of sheep by surgical means or by selective breeding reduces the susceptibility of sheep to crutch strike and when combined with:
Alternatives to mulesing are seen as highly desirable, but remain elusive. A breeding program aimed at the selection of plain-bodied animals suggests itself as a suitable control measure, but progress would be slow.
Vaccination of sheep with proteases and extracts of peritrophic matrix from the larvae causes retardation of larval growth and in some cases leads to larval mortality.18 In practice it is difficult to envisage sufficient antibody being secreted through the skin to protect against the initial strike. However, it could reduce the number of third instars leaving the sheep and reduce subsequent fly numbers. Enhanced approaches utilizing recombinant proteins delivered cutaneously have shown additional promise.19 Vaccination against Ps. aeruginosa to protect against fleece rot and the likelihood of body strike has given encouraging results.20
1 Wall R, et al. Med Vet Entomol. 1992;6:177.
2 Wardhaugh HG, Morton R. Am J Agric Res. 1990;41:1155.
3 Watts JE, et al. Aust Vet J. 1981;57:450.
4 Ward MP. Prev Vet Med. 2001;49:115.
5 Wall R, et al. Med Vet Entomol. 2002;16:335.
6 Fenton A, et al. Med Vet Entomol. 1999;13:453.
7 Casu RE, et al. Int J Parasitol. 1996;26:623.
8 Young AR, et al. Int J Parasitol. 1996;26:245.
9 Stevens J, Wall R. Biochem Sys Ecol. 1997;25:81.
10 Levot GW, Barchia I. Aust Vet J. 1995;72:245.
11 Thompson DR, et al. Aust Vet J. 1994;71:42.
12 Dymock JJ, Forgie SA. Am J Exp Agric. 1995;35:699.
13 Ward MP, Farrell R. Prev Vet Med. 2003;59:21.
14 Foster GG, et al. Aust J Biol Res. 1985;38:275.
15 Lonsdale B, et al. Vet Rec. 1990;126:207.
16 Anderson N, et al. Res Vet Sci. 1989;46:131.
17 Hughes PB, Levot GW. Vet Parasitol. 1987;24:275.
18 East IJ, Eisemann CH. Immunol Cell Biol. 1993;71:453.
SCREWWORM (COCHLIOMYIA HOMINIVORAX AND CHRYSOMYIA BEZZIANA)
Cutaneous myiasis associated with the screwworm maggots has been a cause of great financial loss in livestock in the western hemisphere, Africa, and Asia. Deaths may be heavy in groups of livestock which are at range and seen infrequently.
Etiology
Cochliomyia hominovorax in the New World (New World Screwworm) and Chrysomyia bezziana (Old World Screwworm) in Africa and Asia.
Epidemiology
Eggs laid in fresh wounds. Flies most active at 20–30°C. Disease spread by dispersal of flies or transport of infested animals.
Clinical signs
Larvae invade the tissue producing characteristic large lesions containing mature larvae and foul smelling brown exudate.
Diagnostic confirmation
Rows of spines are present on the anterior part of each segment of the third instar.
ETIOLOGY
Larvae of the flies Cochliomyia hominivorax and Chrysomyia bezziana cause myiasis or ‘screwworm disease’ of animals. The flies are typical blow flies, C. hominivorax (New World Screwworm) being blue-green with an orange head; Ch. bezziana (Old World Screwworm) is of similar coloring. C. hominivorax occurs in the Americas, Ch. bezziana in the Persian Gulf, Africa, and Asia. The occurrence of Ch. bezziana in Papua New Guinea provides a constant threat to livestock on the Australian mainland. A similar fly is Callitroga (Cochliomyia) macellaria which is not a true ‘screw-fly’ in that the larvae feed only on carrion or necrotic tissues.
EPIDEMIOLOGY
The screwworm maggots are obligatory parasites with no host-specificity. Thus, all domestic and wild, mammals, marsupials, and birds are potential hosts. Females are attracted to fresh wounds where they will oviposit. The navel of a newborn animal is a favored site, but fresh accidental or surgical wounds, such as those produced by castration, docking, and dehorning, are readily infested. Wounds which have already been infested are markedly attractive to the flies because of their odor. In bad seasons the flies will lay eggs on minor wounds such as areas of excoriation, tick bites, running eyes, peeling brands, and on the perineum soiled by vaginal and uterine discharges in animals which have recently given birth. Injury is not necessarily a prerequisite for screwworm strike in sheep, which can be struck in the intact infraorbital fossa and vulva. Wool loss and tenderness may occur and the remaining fleece may be stained.
The development of the fly is favored by hot, humid weather. The optimum temperature range for C. bezziana is 20–30°C (68–86°F). Below this the flies become sluggish and at 10°C (50°F) and below the flies will not move. Temperatures above 30°C (86°F) can be tolerated provided shade is available. C. hominovorax is active all year in areas where temperatures exceed 16°C and disperses rapidly from these areas as the temperature increases in the neighboring colder areas. The disease can be spread either by migration of flies or their carriage in livestock ships or commercial aircraft,1 by shipment of infested cattle or other livestock, and by movement of affected wildlife. The mean distance that C. bezziana can travel and deposit eggs is 11 km. The maximum distance is 100 km, but long distances are probably wind assisted. In the new environment the flies may die out if the climate is unsuitable or persist to set up a new enzootic area. Persistence of the fly in an area may depend upon persistence in wildlife or in neglected domestic animals, although the latter do not usually survive unattended for more than about 2 weeks.
In many enzootic areas it is common for the fly to persist in neighboring warmer areas during winter, returning to its normal summer habitat as the temperature rises. This pattern is exemplified by the introduction of screwworms into the southeastern United States in 1933 where they had not previously occurred. The flies died out in most areas in winter but persisted in southern Florida. In succeeding summers migrations of flies northwards caused outbreaks. The disease has since been eradicated from the area.
The disease is of importance in tropical and subtropical areas of Africa, Asia, North and South America, especially Central America, the Caribbean islands, Mexico, and American states bordering on Mexico. The prevalence of the fly in enzootic areas places severe restriction on the times when prophylactic surgical operations can be carried out.
The potential worldwide geographical distribution and abundance of Ch. bezziana has been assessed using a computer program. The differences in the observed global distribution and the potential predicted distribution indicate the areas at risk of colonization.2
LIFE CYCLE
The screwworm flies have a typical fly lifecycle with eggs, three larval instars, and a pupal stage. Females lay 150–500 white eggs in shingle-like clusters at the edges of fresh wounds. Larvae hatch in about 12 hours and penetrate the tissues surrounding the wound. The larvae preferentially feed on fresh, living tissue which is digested by regurgitation of a wide variety of salivary enzymes. Oviposition by other screwworm flies is encouraged by the presence of larvae already in the wound. The larvae feed as a group and at their time of maturation will have created a deep lesion 10–12 cm in diameter. Larval development is complete in 5–7 days, after which they leave the wound and fall to the ground. These mature third instars burrow into the upper soil layers and pupate. On the ground, pupal development is highly temperature dependent requiring from 3–60 days. Emerging flies commence egg-laying in about 1 week, having completed the life cycle, under optimum environmental conditions, in less than 3 weeks. There may be 15 or more generations per year.
The temperature sensitivity of the pupal stage, which is unable to survive freezing for more than short periods, limits the distribution of this parasite. As with all flies pupal development is highly temperature regulated. The screwworm pupal development is inhibited at soil temperatures below 15°C (60°F). Temperatures below this point for more than 2 months cause death of the pupa. Thus the occurrence of the disease is limited to warm climates. Pupae are also affected by the moisture content of the soil. The emergence of adults is reduced when the moisture content is more than 50%, while temporary floods can drown pupae.
PATHOGENESIS
Following invasion of the wound a cavernous lesion is formed, characterized by progressive liquefaction, necrosis and hemorrhage. Anemia and decreased total serum protein results from hemorrhage into the wound. Secondary bacterial infection, toxemia, and fluid loss contribute to the death of the animal. Surviving calves frequently develop infectious polyarthritis.
CLINICAL FINDINGS
The young larvae invade the nearby healthy tissues vigorously and do not feed on necrotic superficial tissue. A profuse brownish exudate, composed of larval excreta, and host fluids, pours from the wound and an objectionable odor is apparent. This is highly attractive to other flies and multiple infestations of a single wound may occur within a few days. The resulting tissue damage may be so extensive that the animal is virtually eaten alive. Affected animals show irritation in the early phase of the infestation and by day 3 show pyrexia. Animals do not feed but wander about restlessly, seeking shade and shelter.
CLINICAL PATHOLOGY
It is imperative to differentiate screwworm infestation from infestation with other fly larvae. The appearance and smell of the wound are significant but careful examination of the larvae is necessary to confirm the diagnosis. Mature larvae are 1–2 cm long and pink in color; they are pointed anteriorly and blunt posteriorly; two dark lines are visible reaching from the blunt posterior to the middle of the body and they have rows of dark fine spines on the anterior part of each segment. Specimens forwarded to a laboratory for identification should be preserved in 70% alcohol.
NECROPSY FINDINGS
Superficial examination of infested wounds is usually sufficient to indicate the cause of death.
TREATMENT
Affected wounds should be treated with a dressing containing an efficient larvicide and preferably an antiseptic. The larvicide should be capable of persisting in the wound for some time to prevent reinfestation. A number of proprietary preparations and some of the newer insecticides have been compared.3 Lindane 3% and coumaphos 3% were the most effective but fenchlorphos 2.5%, diazinon 1.5%, chlorfenvinphos 0.05% and fenthion-methyl 0.2% were also very efficient. Stirofos (15%) and dichlorvos (20%) give season-long protection in the ears of cattle against C. hominivorax and Amblyomma maculatum.4 An ointment or gel base is preferred so that as much of the active ingredient as possible is left in the site. It should be liberally and vigorously applied with a paint brush to insure that larvae in the depths of the wound are destroyed. To avoid reinfestation in extensive lesions or in bad seasons the treatment should be repeated twice weekly.
When large numbers of animals are affected and individual treatment is impractical, spraying with a 0.25% solution of coumaphos, chlorfenvinphos, or fenchlorphos, using a power sprayer, is recommended. The spray is directed forcibly into wounds and, except for young calves, applied generally over the body to provide protection for about 2 weeks. Young calves may show signs of toxicity if sprayed too liberally and application should be restricted to the belly. These sprays can be used to protect animals which are not infested but are exposed to considerable risk or are to be shipped to free areas. In the latter situation dusts are also available if spraying is undesirable in cold weather. A pyrophyllite dust containing 5% coumaphos and 2% mineral oil is effective as a protectant if applied at the rate of 60–180 g per animal. Residual protection lasts for 3–7 days.
Thirteen acaricides, commonly used for Boophilus microplus control, have been tested against Ch. bezziana larvae. While they are not sufficiently active to use as a primary treatment, their continued use for tick control would reduce screwworm populations.
Ivermectin 200 mg/kg given subcutaneously kills all Ch. bezziana larvae up to 2 days old and many older larvae. It provides residual protection for 16–20 days. Bull calves treated with ivermectin at the time of castration were completely protected against strike.5 A preliminary study showed that closantel at 15 mg/kg body weight was effective with a residual protection of 8–15 days.6 Doramectin 200 mg/kg subcutaneously caused complete expulsion of C. hominovorax larvae within 8 days.7 Prophylactic use of ivermectin and doramectin significantly reduced occurrence of screwworm strike in cattle.8 Fipronil had a prophylactic effect, reducing occurrence of screwworm infestations in cattle and providing efficacious treatment in those that did become infested.9
CONTROL
The eradication of screwworm by genetic means, chemical control, trapping techniques and lures, and dispersal of flies has been reviewed.10 In an enzootic area the incidence of the disease can be kept at a low level by the general institution of measures designed to break the life cycle of the fly. Surgical procedures should be postponed where possible until cold weather. In the warm months all wounds including shearing cuts must be immediately dressed with one of the preparations described under treatment. All range animals should be inspected twice weekly and affected animals treated promptly. Infestation of fresh navels is common and newborn animals should be treated prophylactically. If possible the breeding program should be arranged so that parturition occurs in the cool months. The routine use of ivermectin for internal parasite control provides protection for about 2 weeks.5
In the United States, the Caribbean, and central America an eradication program has been successfully carried out against C. hominivorax using the sterile insect technique (SIT). Huge numbers of pupae are mass reared on semi-artificial media and exposed to the sterilizing effects of cobalt 60. The resulting sterile male flies are released over large areas, primarily by aerially drops, where they compete with wild males for available females which mate only once. C. hominivorax has now been eliminated from the United States, the Caribbean, and all of Central America, up to the Darien Gap in Panama.11 C. hominivorax appeared in Libya in 1988, apparently with a load of sheep transported from South America, but has been eradicated using sterile male flies from the USA.12
Attractants may also be used to reduce the fly population. A chemical bait has been developed, and when combined with an insecticide forms a screwworm adult suppression system (SWASS) which reduces the fly population and the incidence of strikes. An examination of the efficacy of various methods of baiting showed that polythene sachets containing sworm-lure 2, a pungent mixture of 11 chemicals, attracted flies (not C. bezziana) for at least 2 weeks and was as efficient as jar baits.13 This result needs confirming in a screwworm endemic country.
1 Rajapaksa N, Spradbery JP. Aust Vet J. 1989;66:94.
2 Sutherst RW, et al. Med Vet Entomol. 1989;3:273.
3 Spradbery JP, et al. Aust Vet J. 1991;68:338.
4 Gladney WJ. J Econ Entomol. 1976;69:757.
5 Spradbery JP, et al. Aust Vet J. 1985;62:311.
6 Spradbery JP, Owen I. Aust Vet J. 1990;67:340.
7 Soerensen B, et al. Unimax-Ciencias. 1994;3:19.
8 Benitez-Usher C, et al. Vet Parasitol. 1997;72:217.
9 Lima WS, et al. Vet Parasitol. 2004;125:373.
10 Spradbery JP, Evans K. Agric Zool Rev. 1994;6:1.
11 Chaudhury MF. Vet Parasitol. 2004;125:99.
WOHLFAHRTIOSIS (FLESH FLY)
Cutaneous infestation by larvae of the sarcophagid fly, Wohlfahrtia magnifica, has become a major disease of domestic livestock, including birds managed extensively (e.g. geese) in the Mediterranean basin, eastern Europe, and western regions of China.1 The disease is particularly significant for sheep in these regions where it is more prevalent than strike by the calliphorid fly, Lucilia sericata.
Other species of this genus are known from North America, but they do not infest domestic species. They are predominantly reported from very young rodents and birds, although there are occasional reports from infants.2 Mortality of infested hosts tends to be very high.
LIFE CYCLE AND EPIDEMIOLOGY
Larvae of this species are obligatory parasites developing only in the living flesh of warm-blooded vertebrates. They are not host specific. Adults are typical for this group of flies, being dark gray in color with three distinct black stripes on the thorax where the wings are attached.
Female flesh flies, which are active during the warm parts of the day, deposit first instar larvae on the host, usually in small groups of 15–20. Each female may produce up to 170 larvae. Completion of the three larval stages takes from 5 to 7 days after which the mature third instars leave the lesion and fall to the ground where they pupate. Development of the fly within the pupa is regulated by temperature and may require between 7 and 21 days.
Larvae are usually deposited near small wounds (bites of blood-feeding arthropods are sufficient to attract the larvae), but the favored sites appear to be the genitalia.3 Irritation of the vulva associated with the use of vaginal sponges for estrus synchronization may be a predisposing factor in sheep.
Flies are active between April and October with several generations being produced. Little information is available on overwintering. Wildlife are suspected as being reservoir hosts, but little information is available on which are the most important.
PATHOGENESIS
Larvae have well developed mouthhooks which are used to abrade the skin surface and with the aid of a wide variety of salivary enzymes they quickly produce a dramatic lesion. Lesions increase in size as the larvae grow and require additional fresh tissue. Each animal may have one or more focal lesions, each packed with larvae. In severe cases several lesions may coalesce into one larger site.
Animals are often struck multiple times during a season, suggesting the absence of protective immunity. This adds to the impact of this disease as animals must be constantly monitored.
CLINICAL PATHOLOGY AND NECROPSY FINDINGS
A clinical examination is all that is necessary to make the diagnosis. Larvae can be distinguished from those of the screwworm or the strike flies by the presence of a large posterior cavity surrounded by a number of prominent tubercles. However, specific identification should be done by a specialist. Larvae should be preserved in 70% ethanol.
Affected animals are clearly stressed, showing restlessness and anorexia.3 Lesions formed at the vulva or prepuce are the most significant causing great discomfort and dysfunction. Lightly infested animals shown no impairment of productivity.3 Infested animals develop strong antibody responses to salivary secretions, particularly of the third instars.4
TREATMENT
There are currently no products specifically registered for management of this disease. Evaluations of several drugs and treatment approaches have been made. Of particular interest is the equivocal results of trials with macrocyclic lactones. In sheep, ivermectin and moxidectin had no effect on existing infestations and no prophylactic effect5 or only short protection against early instars.6 In contrast, doramectin provided complete prophylactic protection for 21 days and significant reductions for 40 days.7 The pyrethroid, cypermethrin, was less effective.7
The insect growth regulator dicyclanil has also been evaluated and shown to reduce prevalence of infestation in sheep.3 The reduction not only occurred in treated animals, but was seen in untreated herdmates possibly as a result of the overall reduction in fly numbers.
Ked and louse infestations
Infestations with these insects cause irritation resulting in skin or wool damage. Blood loss may occur with some species.
SHEEP KED (MELOPHAGUS OVINUS)
This flat brown wingless fly, about 6–7 mm in length, was found in sheep throughout the world, but is now rarely reported in many countries. For example it wasn’t mentioned in a review of livestock ectoparasites of Europe and the Mediterranean,1 although anecdotal evidence suggests it may be present in isolated pockets associated with organic production.2 In Australia it is now rarely seen. The ked may transmit Trypanosoma melophagium and Rickettsia melophagi, harmless blood parasites of the sheep. Staining of the wool by the feces of the ked reduces its value and gives it a peculiar musty odor. Heavy infestations cause skin blemishes which are costly to the leather industry. Sheep in poor condition suffer most from infestations. Goats may also be infested.
LIFE CYCLE
Keds live their entire life cycle on the host. Adults of both sexes are blood feeders and although the degrees of infestation usually encountered cause only irritation with resulting scratching, biting, and damage to the fleece, very heavy infestations may cause severe anemia. Spread is generally the result of direct contact between hosts. A recent review2 suggests this exchange is primarily between dams and their offspring and that it is predominantly the newly emerged adults that migrate to new hosts. Larvae develop within the female one at a time and are deposited on the host as mature third instars which pupate within a few hours. The female ked lives for 4–5 months and may lay up to 10–15 larvae, so build-up of infection is slow. The larvae are attached to the wool fiber some distance above the skin and many larvae and pupae are removed at shearing. The young ked usually emerges in 20–22 days but this period may be prolonged for up to 35 days in winter. The complete life cycle takes 5–6 weeks under optimal conditions. Heavy infestations usually occur in winter months and they decline in the summer. The parasite is mainly seen in colder, wetter areas and infestations may be lost when sheep are moved to hot dry districts. Resistance is acquired in time and resistant sheep grow better and produce more wool.
A seasonal pattern of infestation occurs. Keds are sensitive to hot, dry weather and numbers decrease markedly over the summer. Populations increase slowly over the autumn and winter. While keds that have been dislodged from the host can live for up to 2 weeks if in mild moist conditions, most die in 3–4 days and probably do not play a part in reinfesting sheep.
CONTROL
At shearing a large proportion of adults and pupae will be removed. This can provide effective control on adult sheep particularly where a combination of hot conditions and a short fleece will kill most of the remaining keds. However, some may remain alive in protected places such as the ventral neck and breech regions and on younger stock. If treatment is carried out within the next 2–4 weeks eradication will be achieved as long as all sheep are included and the insecticide used has a residual protection longer than the time taken for the last pupae to hatch.
Keds are particularly susceptible to organophosphates and most of those used to eliminate lice will also remove keds. They can also be used in higher dose rates in pour-on applications. Diazinon used as a pour-on removed all keds and prevented re-establishment for 9 weeks.3 The synthetic pyrethroids are also active against keds; deltamethrin, cyhalothrin and cypermethrin are used. Cyfluthrin 2 mg/kg pour-on in sheep also eradicates ked and protects for 50 days. Amitraz will kill adult keds but has little residual action and is therefore usually combined with another compound to provide sufficient residual action to eliminate infections. Ivermectin given at the standard anthelmintic dose will also eliminate keds. Closantel which is mainly used against Haemonchus or Fasciola is effective against keds.
LOUSE INFESTATIONS (PEDICULOSIS)
Lice infestations are common throughout the world. The species are host-specific and are divided into biting and sucking lice.
Epidemiology
Transmission from host to host. Lice show a marked seasonal periodicity rising from low numbers after summer to a peak in the following late spring. Foot lice infested from pasture.
Clinical signs
Irritation which causes rubbing, damage to the fleece or skin, and loss of milk production. Some species cause anemia. Foot lice cause stamping.
Diagnostic confirmation
Lice can be seen on careful inspection. Preferred site varies with host and species of louse.
Differential diagnosis
In sheep must be differentiated from Psorergates, ked and Psoroptes infections. In other animals separate from allergic dermatitis.
Control
Pour-on and injectable treatments control lice on cattle, horses, sheep, and pigs. Good husbandry practices, will reduce infestations. Plunge or shower dips used on sheep, all sheep should be treated and sheep must be thoroughly wetted. Treatment should follow shearing which removes many lice; sheep in short wool are also easier to wet.
ETIOLOGY
Sucking lice – Linognathus vituli (long-nosed sucking louse), Solenopotes capillatus (small blue sucking louse), Haematopinus eurysternus (short-nosed sucking louse), H. quadripertusus (tail louse), H. tuberculatus (buffalo louse)
Chewing lice – Damalinia (=Bovicola) bovis
Sucking lice – Linognathus ovillus (sucking face louse), L. africanus, L. stenopsis (sucking goat louse), L. pedalis (sucking foot louse)
Sucking lice – Linognathus stenopsis (sucking blue louse), L. africanus
Chewing lice – Damalinia caprae, D. limbata, D. crassiceps
Sucking lice – Haematopinus suis
LIFE CYCLE AND EPIDEMIOLOGY
Sucking lice
All life cycle stages are found on the host. Both sexes are obligate blood feeders, taking small meals from capillaries in the upper skin. Survival off the host is limited although some species, such as the foot lice of sheep, may survive away from the host for up to 2 weeks. Females lay 2–6 eggs per day which are attached to individual hair shafts. Eggs complete embryonation and hatch within 8–11 days of deposition. Lice have three nymphal stages, which bear a morphological similarity to the sexually mature adult stage. Each nymphal stage will take 2–4 days to complete. Louse development rate, at all stages, is highly temperature dependent and requires a narrow temperature range. Temperatures above 41°C and 46°C are lethal for eggs and adults, respectively, of Linognathus vituli. Optimal development takes place between 33°C and 37°C. Lice therefore show a seasonal periodicity with very low numbers in the summer when conditions are hot. Populations begin to increase with cooler fall temperatures, reaching maximum levels in late winter.
Chewing lice
All life cycle stages are found on the host. Lice feed on dead skin cells, hair, and oil secretions which they abrade from the surface using their chewing mouthparts. There may be some abrasion of the upper skin layers and there has been demonstration that sheep develop antibodies to salivary sections of Damalinia (=Bovicola) bovis.1 Sex ratios are highly female biased and there are suggestions that parthenogenesis occurs in some species. Females deposit <1 egg per day. Embryonation is completed in 7–10 days producing nymphs which molt three times before reaching sexual maturity. As with the sucking lice development is highly regulated by temperature with a narrow range for optimal development and survival. Chewing lice can survive off the host for up to 2 weeks
Transmission of both types of lice occurs by direct contact but inert objects such as blankets, grooming tools and harness may remain infective for several days.2 Sheep may become infested with foot lice from the pasture. Young pigs may become infected some 10 hours after birth. Newborn calves also rapidly acquire infestations from their dams.
CLINICAL FINDINGS AND DIAGNOSIS
Sucking lice
All species cause irritation of the skin and stimulate scratching, rubbing and licking leading to restlessness, damage to hair coat or fleece and hides and loss of milk production. These behavioral changes3 result in reduced efficiency, particularly in feedlot cattle.
Lice appear to be present on a large proportion of cattle,4,5 but measurement of their impact on productivity has produced equivocal results. It is often believed that infestation has little or no effect on weight gains and hematological values.6,7 However, there appears to be a synergistic effect between louse infestations and the presence of gastrointestinal nematodes that does have an impact on weight gain.8 Anemia is rare but has been described for heavy infestations of H. eurysternus. Treatment, however, may be warranted to reduce the damage to hides and prevent damage to fences and other fixtures. Hairballs may be present in infested calves due to continual licking.
The pig louse spreads swinepox and while weight loss may not occur, even with heavy burdens, some pigs develop an allergic dermatitis and the consequent rubbing leads to skin lesions.
Foot lice of sheep are believed to live on blood. Light infestations may not cause clinical signs, but moderate to severe infestations cause stamping and biting the affected parts. Lice cause goats to rub or to bite their coat, which becomes matted and damaged. Angora goats can damage the hair shaft and lose their coats. Signs of infestation are restlessness, hair loss, and decreased milk production. In horses H. asini is the more serious species as it removes blood and may cause some anemia.
Chewing lice
Sheep body lice cause irritation and rubbing. The wool loses its brightness, becomes cotted and more yellow. There is evidence that a pelt defect called cockle is associated with infestation with body lice.9 The quantity and quality of the fleece is reduced and losses up to AUS$3.20 per infested sheep have been measured.
Chewing lice on cattle also cause an increase in rubbing and licking which contributes to reduction of efficiency and damage to facilities. Hair loss has been attributed to this infestation, but it is a controversial association as many other causes are likely.
Diagnosis of lice on cattle and horses requires close visual inspection with particular attention being paid to known predilection sites.10 These include the head, the sides of the neck, the dewlap, the escutcheon, and tail switch. Effective diagnosis requires that hair be parted and skin examined at several locations at each of the predilection sites. Use of a supplementary light source and restraint of the animal is very helpful.
Chewing lice of cattle, sheep, and horses are recognized by their rounded head and light brown color. These lice are highly mobile and will move away from inspection sites. Their eggs are difficult to see unless on dark haired cattle or horses. Sucking lice are recognized by their gray or blue-gray color and their pointed head. They tend to remain fixed to the skin.
Chewing lice may congregate on the dorsal surface and flanks, while sucking lice are found on the head and in the long hair of the mane and tail but, in heavy winter infestations, lice may be found on any part of the body. In sheep with long wool, greatest numbers of D. ovis may be seen on the midside, particularly the shoulders, from where they spread to the back and rump. After shearing, small residual infestations may be found on the ventral neck. Foot lice are usually found in clusters on those parts covered with hair, mainly on the lower limbs, but in heavy infestations they can be found in clusters above the hock, on the scrotum, in the belly wool, and more rarely on the face.
TREATMENT AND CONTROL
Self-grooming and grooming by headmates effectively regulates louse populations on most hosts, but the effectiveness is limited when hair coat or fleece become too long for the tongue surface to effectively remove the lice and eggs. Similarly, shearing is an important factor in reducing body lice populations on sheep. Between 30 and 50% of the population is removed with the fleece and those remaining are subjected to a more variable microclimate. Populations are at their lowest 30–60 days after shearing. Reversing temperature gradients as sheep move in and out of shade, and very wet conditions, will also reduce lice numbers.
Body lice of sheep are relatively easy to eradicate if a clean muster is achieved, if the sheep are thoroughly treated and reinfestation is avoided. However, in practice, failure to eradicate commonly occurs due to the inability to thoroughly wet the fleece because of poor maintenance of dips or poor formulation of products, or because the lice are resistant to the chemical used. The most difficult problem when attempting to eradicate lice from flocks over a large area is the diagnosis of lice in lightly infested flocks. Dipping clean sheep is wasteful but if lightly infested flocks are not dipped the infestation will build up and may cause serious economic loss in the next year. Techniques have been devised to test for lice by digesting the wool and examining the residue for lice, but the delays inherent in such a system often mean that by the time the farmer obtains the results, the optimum time to treat sheep has passed. On-farm tests have been examined but none are yet sufficiently accurate.11
Affected sheep can be effectively treated in a plunge or shower dip with organophosphate insecticides (diazinon, coumaphos, chlorfenvinphos, carbophenothion, propetamphos), synthetic pyrethroids, or carbamates. The synthetic pyrethroids, cypermethrin and cyhalothrin, have been shown to be effective in sheep and give good residual protection. Products formulated as emulsifiable concentrates wet the fleece better and give better results than wettable powders.12 Cypermethrin, alphamethrin, and deltamethrin are marketed as pour-ons for sheep and goats. They must be used immediately after shearing. A small proportion of sheep show irritation after application and some may show fleece damage. Further, most of the chemical that is applied remains in the tip of the fleece and grows out with the wool and so is not in contact with the lice.13
The spread of synthetic pyrethroid following backline treatment of sheep is slower than in cattle and horses and takes some days to reach maximum concentrations along the midside. However, the ease of application, low capital outlay required, and the need for only one muster has led to rapid acceptance of backline treatments. It is important that the pour-on is applied along the spine from the poll to the tail. Failures to eradicate occur if the sheep are not cleanly shorn, and in large-bodied sheep with extensive neck folds which are difficult to shear cleanly. The presence of unshorn lambs, cotted or Dermatophilus-affected fleeces, wrinkly sheep or inexpert shearers make backline pour-on application an inappropriate method. Heavy rain following application may also cause failure. Some lice remain alive for up to 6 weeks after backline treatment and so sheep are infective for this time. Jetting races have been used to treat sheep because of their ease of use and the speed with which sheep can be treated. However none of the machines marketed in Australia is able to eradicate lice even from sheep with short wool.14
The manufacturer’s recommendations should be accurately followed, particularly when using shower dips. The most common cause of failure when using shower dips is poor maintenance of the pump so that insufficient volume of dip wash is applied from the overhead sprays. Blocked jets, incorrect speed of rotation of the top spray and leaving sheep in the shower for insufficient time are also common faults.
Infested long-woolled sheep can be jetted to reduce the population until the sheep can be shorn and dipped. Treatment of sheep in long wool leads to residue problems in the wool presented for sale. Every effort should be made to treat sheep properly in the first 2 weeks after shearing to insure eradication of lice and to present wool without insecticidal residues. Cyhalothrin has been shown to eradicate body lice from long-woolled sheep when applied by jetting at 20 ppm, but in field use eradication is rarely achieved. Phoxim 125 mg/L has also been reported to eliminate lice in long-woolled sheep, while 250 mg/L gave at least 4 months of protection against reinfestation. An ivermectin 0.03% jetting fluid was reported to have high efficacy in treating lice in sheep with 3–9 months wool, but failed to eradicate. High concentrations of cyhalothrin (1500 ppm) and diazinon (36000 ppm) in 100 mL applied to sheep has also proved effective but requires a practical method of application. No treatment is known that can eradicate lice from long-woolled sheep under field conditions. Following treatment of foot lice, sheep should be moved to a paddock that has been free of sheep for a month.
Treatment of goats has not been studied extensively and the treatments used on sheep and cattle are thought to be effective in goats. Lactating goats should not be treated.
Organophosphates (e.g. fenthion, famphur, chlorpyrifos, temephos, methidathion, fenchlorphos, phosmet) and pyrethroids (e.g. deltamethrin and flumethrin) have been used in pour-on application on cattle.12,13 While pour-on applications are easy to use, none will kill all lice; they are expensive compared with sprays if large numbers are to be treated, and in many countries they should not be used on lactating cows. Similarly, organophsphates (diazinon, coumaphos, ethion) and pyrethroids (cypermethrin) or combinations (bromophos-ethyl combined with chlorfenvinphos) are used as sprays or dips for cattle. Macrocyclic lactone based products (ivermectin, moxidectin, doramectin and eprinomectin) are available as pour-on or injectable formulations for cattle and have shown excellent efficacy against both sucking and chewing lice. Persistence of activity is one of the exceptional benefits of these products.14,15
Fenthion 2% has been widely used on horses in Australia with good results although coat color changes and, rarely, hair loss does occur. A single subcutaneous injection of 0.2 mg/kg ivermectin will remove sucking lice but is not completely effective against chewing lice. Moxidectin, a compound with ivermectin-like activity, performs similarly.12 Treatment of horses with a shampoo containing 1% selenium sulfide three times at 10 day intervals eradicated lice; most horses showed a marked improvement after treatment.13
Sheep lice have been shown to quickly develop insecticide resistance and strains of D. ovis which are resistant to the chlorinated hydrocarbon insecticides are common in the United Kingdom, while strains of lice resistant to the synthetic pyrethroids have followed inappropriate backline use of these compounds.14 Resistance management strategies that involve rotational use of the various insecticide/parasiticide active ingredient categories should be adopted wherever practical. For example, if pyrethroid-resistant lice are present an organophosphate or macrocyclic based product should be used. Subsequent treatments should involve another change of compound class.
Treatments should be timed to coincide with the beginning of population growth (i.e. autumn or early winter). Extremely early treatments often result in spring outbreaks that result from very small residual populations on a few animals. When products with short residual activity are used (e.g. organophosphates) two treatments separated by 10–14 days are required. The second treatment will kill any newly hatched nymphs. Products with persistent activity in excess of 21 days (e.g. macrocyclic lactones) do not require a second application.
Effective management of lice in a herd requires that new animals be isolated for a period of time sufficient for all lice to be eliminated by treatment. The introduction of one or two lousy sheep to a flock, such as occurs when stray infested sheep enter a clean mob, leads to a slow build-up of infestation.
1 James PJ. Moon Int J Parasitol. 1998;28:419.
2 Crawford S, et al. Vet Parasitol. 2001;94:205.
3 Weeks, et al. Vet Rec. 1995;137:33.
4 Colwell, et al. Can Vet J. 2001.
5 Milnes, Green. Vet Rec. 1999;145:357.
6 Bailey PJ, et al. Aust J Exp Agric Anim Husb. 1984;24:140.
7 Nelson WA. Exp Parasitol. 1970;28:263.
8 Devaney, et al. J Econ Entomol. 1992;85:144.
9 Heath ACG, et al. Vet Parasitol. 1995;59:53.
10 Watson, et al. Vet Parasitol. 1997;69:283.
11 Morcombe PW, et al. Aust Vet J. 1996;73:170.
12 Johnson PW, et al. Int J Parasitol. 1995;25:1457.
13 Wilkinson FC. Aust Adv Vet Sci. 1986;VOL?:130.
Tick infestations
Etiology
Many species of ticks act as vectors of disease or cause death from anemia; others cause paralysis. Heavy burdens cause loss of production.
Epidemiology
Life cycles vary widely both in the number of hosts required and the host specificity. Animals are infested by larval or nymphal states on the ground.
Clinical pathology
Ticks obvious on clinical examination. Blood smears for tick fevers (Babesia, Theileria, and Anaplasma).
Tick infestations are of great importance in the production of animal diseases. In addition to their role as vectors of infectious diseases, as outlined below, heavy infestations can cause direct losses. Many are active blood feeders and may cause death from anemia. Some species cause tick paralysis and it is possible that other ticks may elaborate toxins other than those causing paralysis.1 Heavy tick burdens cause sufficient irritation and stress such that affected animals become anorexic which may lead reduced productivity. One tick, Boophilus microplus, is reported to affect in excess of 75% of the world cattle population.2 The economic impact has been estimated at $7 (US) per animal per year and in Brazil with the fifth largest cattle herd, the losses are estimated at $2billion per year.3
The life cycles of the ticks vary widely. Some species pass their entire life on the one host, others pass different stages of the cycle on successive hosts, and others are parasitic only at certain stages. The eggs are laid in the soil and larvae attach themselves to a passing host on which they may develop through one or more nymphal stages before becoming adults. Adult females engorge on blood or lymph and drop to the ground to lay their eggs. One-host ticks are more easily controlled than those which pass part of their life cycles away from the host. A list of the single and multiple host ticks is shown in Table 28.1.
Table 28.1 Single and multiple host ticks
Although many ticks favor a particular host they are usually not completely host-specific and many parasitize a wide variety of animals. In the limited space available here the species are listed according to whether they cause worry only or transmit infectious diseases of large domestic animals.
For more detailed information on transmission of infectious diseases and the biology and distribution of ticks the papers by Neitz4 and Theiler1 should be consulted, while the role of ticks in disease is covered well by Sonenshine.5
Ticks causing paralysis
Paralysis is not uncommon in young domestic animals which are heavily infested with ticks. A review lists 31 species in seven genera of ixodid ticks and seven species in three genera of argasid ticks as being implicated in tick paralysis.6 The most important species are given in Table 28.2. Details of the clinical syndromes are provided in Chapter 32. Recovery is usual in early7 mild cases if the ticks are removed but antiserum against some (e.g. Ixodes holocyclus) is available.
Table 28.2 Ticks reported to cause paralysis
| Animal | Tick | Country |
|---|---|---|
| Sheep, calves, goats | Dermacentor andersoni | United States, Canada |
| D. occidentalis | United States | |
| Calves, lambs, foals, goats | Ixodes holocyclus | Australia |
| Sheep, goats, calves | I. pilosus | South Africa |
| Sheep, goats, calves, antelopes | I. rubicundus | South Africa |
| Lambs | Rhipicephalus evertsi | South Africa |
| Calves, sheep, goats | Haemaphysalis punctata | South Africa, Europe, Japan |
| Sheep | Ornithodorus lahorensis | Central Asia |
| Sheep | Hyalomma aegyptium | Yugoslavia |
| Sheep, goats | Ixodes ricinus | Crete, Israel |
| Cattle, sheep, goats | Amblyomma cajannense | Central, South America |
| Cattle | Rhipicephalus evertsi | Africa |
Ticks which transmit protozoan diseases
Ticks are the most important vectors of many protozoan diseases, the protozoan in most instances surviving from generation to generation of ticks by infecting their eggs. Where control of these diseases is to be undertaken it is necessary to know which ticks are vectors, how many hosts the tick parasitizes during a life cycle and which animals can act as host. Much of the information on these points is fragmentary and only a summary is presented in Table 28.3.
Table 28.3 Ticks reported to transmit protozoan disease11
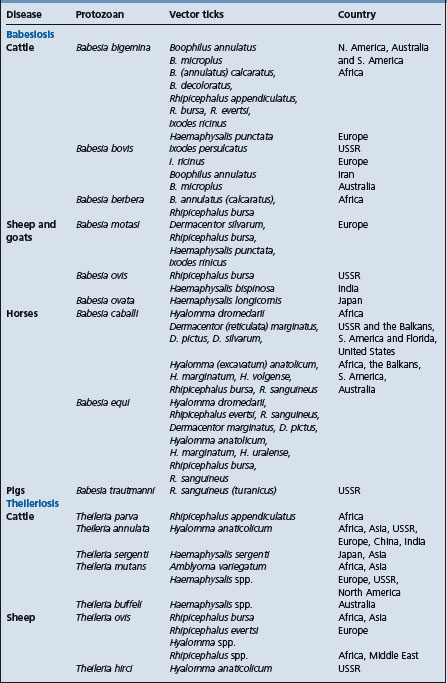
Bacterial, viral and rickettsial diseases transmitted by ticks
The transmission of diseases associated with these agents may be effected by means other than ticks. Anaplasma marginale can be spread by biting flies if large numbers are present when the animals are experiencing a heavy parasitemia. Outbreaks of anaplasmosis can also occur following the use of unclean instruments for dehorning, vaccination, castration or blood sampling, and is easily caused by blood transfusions. The ticks involved more commonly in transmitting bacteria, viruses, and rickettsia are given in Table 28.4. Transmission of Anaplasma may be transovarially, one stage becoming infected and a subsequent stage passing the infection to a new host, or ticks may transmit infection within the one stage if they detach and feed on a new host.
Table 28.4 Diseases associated with bacteria, viruses, and rickettsia and reported to be transmitted by ticks22
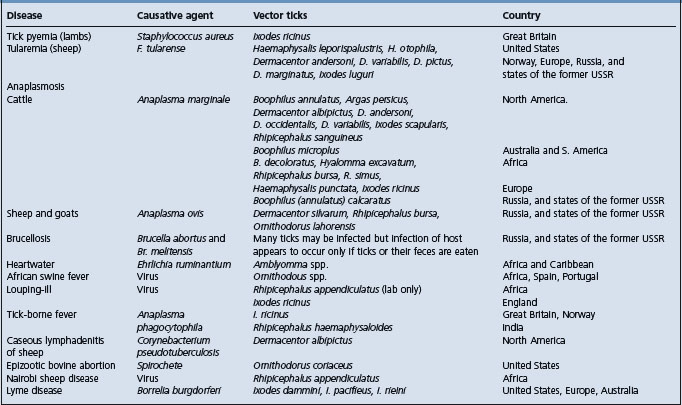
Ticks which cause direct losses
Ticks cause damage to hides and loss of production, anemia and death when they are present in large numbers. They also cause greater morbidity and mortality during periods of drought, as well as delays in fattening resulting in animals held longer before they can be sold. A list of ticks which have this effect on production but which are not known to cause paralysis or transmit infectious diseases in farm animals, is given below.
• Otobius megnini – the ‘spinose ear tick’ of the United States and Canada
• Amblyomma americanum – the ‘Lone Star tick’ of the United States
• A. maculatum, – the ‘Gulf Coast tick’ of the United States
• Margaropus winthemi – of South America and Africa
• Ornithodorus moubata – of Africa and southeast Asia
TREATMENT AND CONTROL OF TICK INFESTATIONS
Four methods are now available to control ticks:
Crude vaccines made from extracts of semi-engorged adult female B. microplus give effective immunity. Antibody destroys cells lining the tick’s gut and allows blood to escape into the hemocele, some ticks die and the fertility of those remaining is reduced by up to 70%. The fertility of males is also reduced.7 A recombinant vaccine based on a membrane bound glycoprotein Bm86 has been isolated and shown to be as effective as the native antigen, and to be effective against acaricidal resistant ticks.8 Its major effect is a progressive control in tick numbers in successive generations through a decrease in their reproductive capacity.9 Because the vaccine acts against an antigen in the tick’s gut to which cattle are never exposed, they must be given booster injections at regular intervals.
This was the first recombinant parasite vaccine sold commercially and is marketed in Australia as Tickgard. A similar product, using the same antigen produced in a eukaryotic expression system, was produced in Cuba and has been trialed in other countries.10 A second antigen has now been added to the vaccine (Tickgard 2). This significantly enhances efficacy and does not impair the response to Bm86. Addition of a saponin adjuvant has greatly increased the efficacy of the vaccine.2
Although vaccines offer long term control, they need to be used with pasture management, dips and tick resistant cattle as part of an integrated pest management control system. Certain Stylosanthes spp., tropical legumes, can kill or immobilize larval ticks and the use of these plants may simultaneously improve pasture quality and reduce the pasture contamination of larval ticks if high legume to grass ratios are achieved. Brachiaria brizantha has also been shown to be lethal to Boophilus larvae.11
Integrated management of ticks requires the use of several complementary approaches to reduce populations below acceptable thresholds. One component of these strategies is the development of acaricidal pathogens that may augment other approaches such as vaccination and selective acaricide application. Fungal pathogens are under evaluation for use in this type of program, in particular Metarhizium anisopliae and Beauvaria bassiana.12,13
Choice of acaricide
Individual animals can be effectively treated by the application of any one of a number of acaricides applied either as a spray or by dipping. The choice of acaricide depends largely on three factors:
• The persistence of the compound on the skin and hair coat
• The likelihood of residues toxic to man appearing in the milk or meat
• Whether or not the ticks in the area have developed resistance to the particular acaricide.
The same criteria apply in control as in treatment except that cost becomes a limiting factor when large numbers of animals require frequent treatments and it is obvious in some circumstances that the effect of tick infestation on Brahman-cross steers is insufficiently great to warrant treatment. It is impossible to make specific recommendations on methods of application and the most efficient insecticide to use because these vary widely between species of ticks. However, whenever possible, treatment should be given systematically in a program based on the life cycle and epidemiology of the tick. A number of treatments may be used early in the tick season to prevent the increase in tick numbers. Care must be taken in areas where tick fevers also occur, not to disrupt the transmission of the tick fever organisms and leave the cattle susceptible to later infection.
Amitraz, a formamidine, and the synthetic pyrethroids have been used widely in Australia and have proved to be efficient, active against organophosphate resistant strains and safe. In a study in the USA 0.025% amitraz applied as a whole body spray or by dipping gave 86.0–99.8% control respectively.14 Ticks resistant to DDT are also resistant to the synthetic pyrethroids, and to overcome this the pyrethroids can be combined with an organophosphate. Successful combinations in Australia are cypermethrin plus chlorfenvinphos and deltamethrin plus ethion. A synthetic pyrethroid, flumethrin, has been marketed by itself at higher use concentrations for both plunge dipping and as a pour-on treatment. As a 1% pour-on 1 mL per 10 kg body weight gave 97% efficacy while 0.0033% as a spray gave 99% and acted more quickly.15 The efficacy of synthetic pyrethroid impregnated eartags has been reported but these are likely to lead to resistance. Cyhalothrin also controls multiresistant strains and is used in plunge dips.
Bioassay results show lambdacyhalothrin to be as effective as cyhalothrin as a whole body spray, although the 1% pour-on was less than 50% effective.16 Resistance to all pyrethroids has been reported.17 Three pyrethroid acaricides have been shown to markedly reduce the hatching of eggs. Permethrin 0.1% or cypermethrin and cyfluthrin 0.05% could be useful in cleansing and disinfecting premises.18
The organophosphates as a group are effective but strains resistant to many of them have appeared. Other drugs in current use include dioxathion, diazinon, carbophenothion, coumaphos, ethion, bromophos-ethyl, chlorpyrifos, and phosmet. Pour-on applications of chlorpyrifos and phosmet have been tested but were not as effective as spray applications. Addition of acaricides to the feed has also been tried but has not been successful, while eartags impregnated with tetrachlorvinphos did not give satisfactory control and increased the risk of resistance developing to the drug.19 Ivermectin given subcutaneously gives satisfactory control of Boophilus microplus for 21 days following an initial lag period of 2 days. As little as 0.015 mg/kg per day gives complete control and raises the possibility of a slow-release subcutaneous implant. Two treatments of 0.2 mg/kg at 4-day intervals is considered satisfactory in cleansing cattle under field conditions.20 However, ivermectin may not be effective against Ixodes ricinus21 but a slow release bolus active for 90 days did give good control of a variety of ticks.19 If given topically 0.5 mg/kg was required to give the efficiency achieved by 0.2 mg/kg subcutaneously.22
Moxidectin 200 mg/kg subcutaneously at 4-week intervals or 500 mg/kg as a pour-on along the back gives good protection against B. microplus resistant to organophosphorous insecticides and DDT, and each treatment gave a rapid knockdown effect on populations of buffalo fly after treatment.23 Doramectin 200 mg/kg is highly efficacious in removing B. microplus and preventing re-establishment.24 Closantel 22.2 mg/kg orally to cattle disrupted the life cycle of Rhipicephalus appendiculatus; those that oviposited laid few eggs and most of these did not hatch. Few larvae or nymphs molted.25
Ticks in the ear of horses should be treated by the insertion of a few drops of an oily acaricidal preparation.
Preparations vary in the duration of the protection they afford and local conditions of rainfall and tick population must be taken into account when determining the time intervals between sprayings or dippings. A special case is that of young lambs which are exposed to tick pyemia. Sprays, dips, and ointments are too toxic and the most effective procedure is the application of a liquid emulsion cream containing the insecticide to the wool-less parts of the body. Chlorpyrifos 0.48 kg/ha markedly reduces the number of ticks on the pasture, but is too expensive for routine use.
Resistance to chemical acaricides has become a major issue for the effective management of one cattle tick, Boophilus microplus. In many cases cross-resistance between chemical families occurs, further complicating the use of rotational scheme aimed at managing the development and degree of resistance. The development of strains of this tick resistant to macrocylic lactone has been reported.26
CONTROL AND ERADICATION
In most countries all that is attempted is reduction of the tick population by periodic dipping or spraying. Complete eradication is extremely difficult because of the persistence of ticks, especially multihost ticks, on wild fauna, and the ability of adult ticks to live for very long periods away from a host. On the other hand, continuous treatment to restrain the tick population is highly conducive to the development of resistance, a problem which has become apparent in many tick areas. Boophilus annulatus was eradicated from the southeastern United States by a program of continuous dipping at short intervals of all livestock in the area. B. microplus was also eradicated from Florida by a similar procedure but 20000 deer, the important alternate host in the area, had to be slaughtered. Concern has been expressed that deer and other wildlife species may threaten efforts to prevent B. microplus and B. annulatus becoming re-established in southern USA after they are introduced from Mexico.27 Attempts to eradicate other single-host ticks in other countries have not been generally successful.
Although both dipping and spraying are recommended for the control of ticks, complete wetting of the animals, which can only be effected by dipping, is essential if eradication is to be undertaken. This adds another impediment to eradication plans because of the cost of constructing proper dips and yards. When one considers that dipping may have to be carried out every 14 days for 15 months, that every animal in the eradication area must be dipped, and that a strict quarantine of the area must be maintained, it is obvious that eradication cannot be undertaken lightly. The use of pour-on applications, which allow a longer period between treatments, and of ivermectin, will necessitate a review of control and eradication techniques.
Measures other than the application of insecticides used in the control of tick infestation include burning of pasture, removal of native fauna, plowing of fields, and rotational grazing. So little is known of the bionomics of specific ticks in specific areas that these measures have been largely unsuccessful and it is impossible to provide details for their proper implementation.1
In those areas where the epidemiology is known it has been shown that in regions with a cold winter the females stop laying eggs, and that the development of eggs is prolonged. This results in few larvae being available in the spring, and if repeated treatments are given at this time, pasture contamination will remain low for some months. In hot tropical areas where the required temperatures for tick breeding are always present, the dry period may cause mortality by desiccation.
Pasture spelling and rotational grazing have been shown to be capable of greatly reducing the tick population on farms in some areas. If cattle are placed on spelled pastures early in winter when the ticks are producing few or no progeny and then alternated at 4-monthly intervals, the tick population can be controlled with a markedly lower number of treatments. The practicability of the procedure depends upon a full-scale financial assessment of the increased weight gains relative to the costs of management. Duration of the spelling period varies between 2 and 3 months in summer to 3–4 months in the winter, but these intervals need to be determined for each district. In practice, pasture spelling is rarely used.
It is possible to reduce the impact of ticks and tick-borne diseases by the introduction of Brahman and Brahman-cross cattle which are more resistant than British breeds. The resistance has been shown to be largely acquired, and is mainly expressed against the larvae in the first 24 hours after attachment.28 In Australia the possibility that B. microplus might escape from its control area because of increased resistance to acaricides has been realized. For this reason a great deal of attention is being paid to the possibility of selecting cattle for tick resistance. In most tick-infested areas, cattle should have up to 50% Bovis indicus breeding, as this allows a reduction in the frequency of treatments. Penalties such as reduced liveweight gains, late maturity and poor temperament become evident when cattle have more than 50% B. indicus. With successive infestations cattle differ in their response to Boophilus microplus. Thus there is increased irritation and more licking28 and a decrease in the number of ticks carried. Resistance to ticks has been shown to be heritable29 and can be increased by breeding from cows and bulls selected for resistance. Selection for tick resistance does not affect milk production.30
Other special cases include Otobius megnini, the nymphs of which drop off to molt and lay eggs in protected spots, necessitating the spraying of buildings, fence posts, feed troughs, and tree trunks in feedlots where heavy infestations are most common. Ornithodorus spp. ticks are difficult to control because the nymphs and adults attach to feed for brief periods only. Where ticks which cause paralysis are common it may be necessary to apply an insecticide as a dust and dip at short intervals.
1 Theiler G. J South Afr Vet Med Assoc. 1959;30:195.
2 Sales-Junior PA, et al. Vet Immunol Immunopath. 2005;107:281.
3 Grissi IA. Hora Vet. 2002;21:8.
4 Neitz WD. Onderstepoort J Vet Res. 1956;27:115.
5 Sonenshine DE. The biology of ticks, vol 2. 1993. New York: Oxford University Press.
6 Murnaghan MF, O’Rourke FJ. Bettini S, editor. Handbook of experimental pharmacology, 48. Berlin: Springer-Verlag. 1978:419.
7 Willadsen P, et al. J Immunol. 1989;143:1346.
8 Tellam RL, et al. Vaccination against ticks. In: Yong WK, editor. Animal parasite control utilising biotechnology. Boca Raton: CRC Press; 1992:303.
9 Willadsen P, et al. The role of vaccination in current and future strategies for tick control. In: Resistencia y control en garrapatas y moseas de importancia veterinaria. Seminario Internacional de Parasitologia Animal Acapulco, 1995; p. 88.
10 Rodriguez M, et al. Vaccine. 1995;13:1804.
11 Barros ATM, et al. Pesq Vet Brazil. 1989;9:17.
12 Kirkland BH, et al. Biol Cont. 2004;31:414.
13 Guedes-Frazzon AP, et al. Vet Parasitol. 2000;94:117.
14 Ahrens EH, et al. J Econ Entomol. 1989;82:850.
15 Ahrens EH, et al. J Econ Entomol. 1988;81:1133.
16 Davey RB, et al. J Econ Entomol. 1993;85:3286.
17 Nolan J, et al. Aust Vet J. 1989;66:179.
18 Davy RB. J Agric Entomol. 1995;12:67.
19 Owen LG. Aust Vet J. 1985;62:24.
20 Nolan J, et al. Aust Vet J. 1985;62:386.
21 de C Giles MB. Vet Rec. 1986;118:82.
22 Cram LT, et al. Vet Parasitol. 1988;29:341.
23 Sibson GJ. Aust Vet J. 1994;71:381.
24 Gonzales JC, et al. Vet Parasitol. 1993;49:197.
25 Kariuki DP, Mbogo SK. Bull Anim Health Prod Afr. 1988;36:59.
26 Martins JR, Furlong J. Vet Rec. 2001;149:64.
27 George JE. J Agric Entomol. 1990;7:119.
28 Wagland BM. In: Ticks and tick-borne diseases. Proc 56th Ann Gen Mtg Aust Vet Assoc, Townsville, 1980; p. 55.
Miscellaneous flies, midges, and mosquitoes
Although these insects differ quite markedly they are dealt with together because they exert similar deleterious effects. Their activity causes stress and induces behavioral changes and in many cases are important vectors for a variety of parasites and infectious diseases.
STABLE FLIES
The stable fly, Stomoxys calcitrans, has a cosmopolitan distribution, occurring in most countries. Other species, including S. nigra, occur in South Africa. S. calcitrans is about the size of a house fly, is gray in color. These flies are the most economically important species affecting confined livestock in North America. Flies rest on fences and structural surfaces in a characteristic head upwards position and can readily be recognized by the prominent, forward-directed, pointed proboscis between short palps. Stable flies of both sexes are blood feeders, attacking particularly horses, cattle, to a lesser extent pigs and people. Bites are quite painful and often bleed freely when fresh. The flies are intermittent feeders, spending only short periods on the host, while most of their time is spent resting on fences and building sides. Eggs are laid in high moisture areas of rotting hay or straw, along the edge of silage pits, on the edges of manure pack of feedlots and compost piles. Mature larvae leave the high moisture sites to pupate in drier sites nearby. Development times are regulated by temperature, with higher temperatures resulting in more rapid development. A complete life cycle will require 3–4 weeks in summer. In termperate climates flies exhibit a distinct seasonality with peak populations in mid to late summer.1 Larvae will overwinter in warmer areas of silage piles. The flies are highly mobile traveling up to 20 km in search of suitable hosts.
Feeding activity by the flies results in stress to the animals and reduced efficiency through reductions in feeding time. When large numbers of flies are present the animals will bunch to reduce biting rates. At high temperatures the bunching may result in cattle overheating. A localized sensitivity of the forelimbs of cattle may develop and result in the formation of intradermal blisters which coalesce to form bleeding sores. With very heavy infestations some deaths may occur. Populations can be assessed by counting the number of flies on the front legs of cattle. When the average number exceeds 5–10 per animal significant losses are occurring and population management is required.
Stomoxys calcitrans are mechanical vectors for anthrax, infectious equine anemia, bovine virus, diarrhea virus, and surra. They are intermediate hosts for the nematode Habronema majus which is reputed to be a cause of allergic dermatitis in horses in Japan.
Effective management of stable flies requires removal of high moisture, rotting organic matter from the environment.2 Edges of silage pits, manure, and compost piles should be kept dry and manure-contaminated bedding should be removed regularly. Insecticide treatments must be applied to all exterior surfaces (i.e. barn sides, fences, and exterior of feed bunks). Diflubenzuron 0.5 g/m2 sprayed in a cow barn completely inhibited breeding of the house fly and stable fly.3 Spraying of fixtures and walls, particularly sunlit walls where the flies often remain unnoticed, with long-acting compounds such as tetrachlorvinphos, diazinon, crotoxyphos, and propoxur reduces infestations for 2 weeks or longer.
Application of insecticides or repellants directly on animals is generally impractical because of the short duration of efficacy. Low frequency of insecticidal application, when necessary, slows the development of insecticide resistance. Crotoxyphos and methoxychlor can also be sprayed onto cattle giving about 4 days of protection. Fenchlorphos can be used but requires daily spraying or daily or alternate daily wipe-on application for good control. Permethrin applied as a microencapsulated formulation gave longer protection than an emulsifiable concentrate.4 Affected horses can be treated locally with an analgesic cream, and if the irritation is severe they can be tranquilized with acetylpromazine.
HORSE FLIES; MARCH FLIES OR BREEZE FLIES (TABANUS SPP.); DEER FLIES (CHRYSOPS, HAEMATOPOTA, AND PANGONIA SPP.)
These large, robust blood feeding flies are widespread in both temperate and tropical regions. Only the females take blood meals, but the bites are savage and cause significant distress to large animals, particularly horses and cattle. These flies can act as mechanical vectors of diseases associated with viruses (equine infectious anemia, bovine leukosis, vesicular stomatitis, hog cholera), bacteria (anthrax, tularemia), and trypanosomes (surra). Eggs are laid on the leaves of plants growing in or near standing water. The larval and pupal stages occur in the water or mud and the life cycle takes 4–5 months to complete. The flies are active in summer and attack animals principally on the legs and ventral abdomen. Duration of activity can be relative short (i.e. 3–4 weeks), but stress on the animals can be very high during that time. Fly attacks lead to bunching of animals5 with the attendant likelihood of overheating and in some cases resulting in animals stampeding through fences. Control is difficult unless wet areas can be drained or livestock kept away from these areas where the flies are most active. Repellents have been used and are reasonably effective in horses subject to fly-worry. The use of o-diethyl toluamide (DEET) affords protection for only a few days and is costly, but its use in milking cattle gives increased milk yield and butter fat. Permethrin, a synthetic pyrethroid, used as a spray or as a dust on cattle and horses, killed 90% of flies for about 2 weeks after treatment. Synthetic pyrethroid impregnated ear tags give very little protection against these flies.
BUFFALO FLIES; HORN FLIES (HAEMATOBIA SPP.)
These small (6 mm) grayish flies have distinct geographical distributions, H. irritans exigua in Australia and South-east Asia, H. irritans irritans throughout North and South America and Hawaii, and H. minuta in Africa. Haematobia irritans irritans is common in Europe where it causes few problems This species was transported to North America in the late 1800s, where it rapidly established and spread. It has subsequently moved into South America where it has also become a major problem. They have similar life cycles and habits. Both sexes of these flies are obligate blood feeders, primarily attacking pastured cattle and water buffalo. They do not survive off the host, other than for short periods. They are not known as vectors for any disease agents other than the nematodes Stephanofilaria spp. They cause significant reductions in productivity of pastured cattle through induction of stress, changes in grazing patterns and, in extreme cases, blood loss. Burdens of 200–500 flies will reduce weight gains of beef cattle (up to 14% reduction)6 and milk yield of dairy cows. Heavy infestations (over 1000 flies) can cause serious loss of condition and rarely, deaths. Control results in higher feed efficiency, increased growth rate and increased calf weaning weights.
The flies congregate chiefly on the withers, shoulders and flanks as well as around the horns and eyes. Flies take numerous (15–20) small blood meals per day. In North America feeding often takes place on the ventral midline and several 2–5 cm diameter feeding lesions are often observed. Zebu cattle are less affected by the flies than British breeds and although they may carry large populations of flies, they show fewer feeding lesions.
The flies are easily recognized by the way in which the wings are held at rest, slightly divergent and angled upwards away from the body. Adult flies stay on the host most of the time, unless disturbed. Females leave the host, as feces are passed, to deposit eggs around edges of the freshly deposited dung. Larvae develop within the dung pat, feeding primarily on bacteria. Development is regulated by environmental temperatures and the larvae are stimulated to enter diapause (arrested development) if temperatures become too low.7 Mature larvae exit the dung to pupate in the dry soil below and around the pat. A complete life cycle may require up to 3 weeks under optimal environmental conditions. Thus, at higher temperatures in excess of 15 generations may be produced in a single season. In more temperate climates such as Canada and the upper United States, only five generations may occur.
While adults rarely leave the host except for oviposition the newly emerged flies of H. irritans irritans will travel up to 20 km in search of new hosts. They may be dispersed also by prevailing strong winds, and they are carried long distances by the movement of cattle to new pastures. The distribution of H. irritans exigua is controlled by environmental factors, particularly temperature and humidity. Below 21°C (70°F) the flies become sluggish and at 5°C (41°F) they become comatose.
Infestations have been controlled by traps, insecticide sprays, back rubbers, dust bags, or eartags impregnated with insecticides. Traps have been designed for use with dairy cattle that walk through them on their way to and from the dairy. The flies, dislodged by gauze strips, are retained in the trap and killed when they rest on the insecticide-coated walls. Traps are rarely used today but recent work with modified traps has given 80–90% control.8
Back rubbers consist of absorbent material, impregnated with insecticide or oil, wrapped around a cable or chain suspended from a central pole and attached to ground level supports or as a cable suspended a little over a meter above the ground between two posts 4–5 m apart. Cattle quickly learn to use rubbers to dislodge flies and their coats become smeared with insecticide. Ethion 1% in fuel oil is commonly used against H. irritans exigua while coumaphos 1 or 2% has been shown to be effective against horn flies. Insecticide-impregnated eartags attached to back rubbers and dust bags controlled horn fly for about 6 weeks, while fenvalerate tags were still effective 18 weeks after application.9
Eartags impregnated with organophosphorus compounds and synthetic pyrethroids have been widely used, but resistance has built up to levels that make this technique ineffective.10 Discontinuing the use of pyrethroid impregnated ear-tags for one season does not allow substantial reduction in resistance to occur.9 Recent work reported that ear-tags impregnated with 20% diazinon gave 90–100% control of H. irritans exigua in dairy and beef herds and allowed better weight gains,11 but if continued will lead to resistance as resistance to diazinon has been reported in eastern Canada and in the United States. Eartags impregnated with compounds of both classes have been effective in managing increases in pyrethroid resistance. Current recommendations for use of impregnated eartags note that tags should be applied to the cows (because they harbor the most flies and present the largest surface area for exposure to the insecticide) at the maximum recommended rate. While this is less convenient it helps to avoid one of the leading causes of insecticide resistance which is the dilution of the insecticide as it spreads from calves to cows. Flies can also be controlled by dipping, but this technique is rarely used solely for flies. Organophosphorus compounds have a residual protection of only a few days and products are combined with synthetic pyrethroids to extend the protective period. In areas where cattle ticks require regular treatment adequate control of flies may be gained incidentally, but if treatments are not effective cattle can be oversprayed with pyrethroids.
Macrocyclic lactone endectocides are highly effective against larval horn flies,12 as well as larvae of face flies, stable flies, and house flies, often killing larvae for periods in excess of 8 weeks. However, in terms of practical control, where flies immigrate from surrounding herds, the duration of efficacy is not more the 2 weeks.13 In addition, the macrocyclic lactones generally cause significant reductions of non-target insects in the dung community, many of which are beneficial as they are natural enemies of the horn fly and buffalo fly.13 The various macrocyclic lactone products have differential effects on flies and other dung insects and it appears that moxidectin has the least impact.14
Pour-on formulations of pyrethroids are highly effective as evidenced by application of 1% cyfluthrin.15 Insect growth regulators (e.g. Diflubenzuron) applied as a bolus give 80% control of the immature stages of the face fly-horn fly in the manure for at least 20 weeks but reduced the number of dung beetles for 7 weeks. A 3% methoprene bolus was also active against flies but had no apparent effect on the dung beetles.16
HORSE LOUSE FLIES (HIPPOBOSCA EQUINA, H. RUFIPES, AND H. MACULATA)
The horse louse fly, Hippobosca equina, is a common parasite of horses and cattle in many tropical countries. It is a flat, glossy, reddish-brown fly, slightly bigger than a housefly. These flies are blood feeders and live most of the time on the host, particularly on the perineum and between the hindlegs. Female flies deposit single mature third instars which pupate in dry humus; the puparia mature to adult flies. The flies appear to cause little annoyance in horses which are accustomed to them but horses experiencing them for the first time manifest fright and irritation. Being blood feeders they may act as mechanical vectors for infectious diseases. Topical spraying of susceptible areas of the body with chlorinated hydrocarbons appears to keep these parasites in check. Local application of 0.2% coumaphos solution quickly kills flies but only gives protection for 3 days. H. maculata was controlled for 1 year by mass application of 0.005% deltamethrin to cattle and horses.17
BITING MIDGES
These tiny flies (1–3 mm long) are members of the family Ceratopogonidae, the important genus being Culicoides. These flies are blood feeders and as such induce stress in hosts and, can transmit infectious diseases such as bluetongue in sheep, horse sickness, ephemeral fever in cattle. They are also intermediate hosts for nematodes of the genus Onchocerca. Because of their importance as vectors of arboviruses,18 studies have been done on their feeding habits. Cattle and sheep are the most common hosts attacked but some species also feed on birds or dogs. Hypersensitivity to the bites of Culicoides brevitarsis results in an allergic dermatitis (Queensland itch or sweet itch) in horses in Australia and North America and is discussed elsewhere. Cattle also show considerable irritation during attacks by large numbers of midges. They react with vigorous stamping of the feet, switching of the tail and continuous movement.
The flies are plentiful in the warmer months and are most active at dawn and dusk. Because of their small size they are capable of being carried long distances by wind. Control of the flies is virtually impossible and most measures to reduce their importance are based on preventing access of the flies to the animals. Repellents, especially dimethyl phthalate or o-diethyl toluamide (DEET), are effective on a short-term basis. Antihistamines can be used regularly but are too expensive for general use. Keeping horses away from areas where the flies are present in large numbers is advisable. Backline pour-on treatment of horses with 40 mL of a 4% high CIS permethrin three times weekly gave a good response in 86% of horses.19 Ivermectin at the recommended dose of 0.2 mg/kg would not produce the serum concentration that would have noticeable effects on blood-feeding C. variipennis.20
BLACK FLIES; BUFFALO GNATS; SANDFLIES
These small gray to black flies (5 mm) are members of the family Simuliidae and include a number of species and genera. The important flies appear to be Cnephia pecuarum which is common in the southern states of the United States, Simulium arcticum in northern Canada, Austrosimulium pestilens and A. bancrofti in Australia, and Simulium ornatum in Great Britain. These very small flies occur in most parts of the world. With the exception of S. arcticum and two or three other species common in northern regions of North America black flies are primarily a concern in tropical regions.
Female flies are voracious blood feeders. They are active in the summer months when large numbers emerge from streams and rivers where they have spent their larval and pupal stages.
Austrosimulium pestilens has adapted to reach large numbers, mate, and oviposit within a very short time to utilize the flood situations that occur in northern Australia. The flies congregate in swarms and attack all animals, causing much worry and annoyance. They tend to bite animals around the legs, on the belly and around the head, causing wheals and papules. The annoyance may be so intense that animals stampede or mill about and young animals may be injured or even trampled to death and are frequently separated from their dams. Cattle may spend much of their time wallowing in mud or kicking up dust to keep the flies away. Herding of cattle onto bare areas reduces fly attacks as the flies commonly rest in tall grass, but this reduces feeding. The cause of death is unknown although swelling of the throat causing suffocation, anaphylaxis or direct toxicity are suspected. Filarid worms of Onchocerca spp. are transmitted by these flies and their role as an intermediate host of nematodes has been discussed.21
A similar situation occurs in northern Canada where large numbers of Simulium arcticum have caused severe stress and occasional deaths of cattle introduced into the area of the Athabasca River and similar regions in the province of Saskatchewan. When black fly populations are extreme, previously unexposed cattle develop symptoms of shock resulting from blood loss and cumulative effects of the fly salivary secretions.
Because the larval stages of these flies are passed in flowing streams, large-scale control measures must be directed at killing the larvae at this stage. Aerial distribution of DDT has been effective when added to streams and water supplies or control can be effected by adding insecticides into rivers and canals. However, rapid reinfestation occurs with increased rate of water flow after heavy rains. Annual injection of methoxychlor upstream from major larval sites proved effective in reducing black fly populations, but off-target effects were undesirable.22 For less ambitious control programs, efforts should be directed towards keeping flies away from animals by the application of repellents or the use of smudge fires. Repellents are of limited use but alcoholic or aqueous solutions and dusts of permethrin, cypermethrin and resmethrin applied to the whole body repelled black flies for some days.23
MOSQUITOES
A number of mosquitoes including Psorophora, Aedes, Mansonia, Culex, and Anopheles spp. are important parasites of domestic animals. When the blood feeding females are present in large numbers they cause stress to animals and have been known to kill young pigs and puppies by the severe anemia they produce. Although such occurrences are rarely recorded the blood loss that can occur in severe infestations is surprising. The stress associated with mosquito attack is sufficient to cause reductions in efficiency, even in mature large animals.
Their most important role is as vectors of disease. Culex tarsalis, Aedes dorsalis, and A. nigromaculis transmit equine encephalomyelitis. Culex tritaeniorhyncus is the principal vector of Japanese B encephalitis in Japan. Various Culex species vector Western Equine Encephalitis, Eastern Equine Encephalitis, and West Nile Virus. These viruses can have serious effects on unprotected horses and are transmissible to humans via mosquito bites. Vaccines are available to protect against all of these arboviruses. Psorophora confinnis is instrumental in spreading the eggs of Dermatobia hominis, the tropical warble fly; and Mansonia spp. transmit Rift Valley fever. The filarid worm Setaria digitata is also spread by mosquitoes.
Control over a large area must include drainage of collections of still surface water or destruction of the larvae by the addition of any one of a number of insecticides, particularly DDT or Abate. For small groups of animals protection from the attacks of mosquitoes can only be satisfactorily effected by mosquito-proof screens. Temporary protection by repellents such as dimethyl phthalate is partial only. Permethrin, 100 mL of a 0.5% emulsion, applied with an electrostatic sprayer provided greater than 70% protection for at least 72 hours.
HOUSE FLIES (MUSCA DOMESTICA)
The common house fly has a worldwide distribution and achieves veterinary importance because it is capable of transmitting, in a mechanical manner, the causative bacteria of many infectious diseases. It is often cited as a means whereby anthrax, erysipelas, and brucellosis are spread but its importance in this regard is largely unproven. House flies are intermediate hosts for the larvae of Habronema muscae and Draschia megastoma.
The eggs are laid in decaying organic matter of any kind. Larval development is temperature dependent and a life cycle may be completed in 12–14 days so that in warm, wet summers the fly population may increase very rapidly, causing annoyance to livestock and farm workers.
House fly population management requires frequent and thorough removal of manure and other rich organic matter. In dry weather the manure can be spread thinly on fields but a more dependable method is to place it in a special fly trap, e.g. Baber’s fly traps, from which larvae and adult flies cannot escape. Chemical treatments to control flies require application to resting sites on buildings and other facilities, or the placement of baits containing methomyl, propoxur, naled, or dichlorvos at appropriate locations. Development of insecticide resistance can occur rapidly and there are numerous examples of resistance to multiple classes of insecticide at a single location. Rotational use of insecticide classes is absolutely essential in the management of resistance.
Management of house fly populations can be augmented through release of parasitic wasps (Family Pteromalidae) that kill pupae. These tiny wasps (1–2 mm long) actively search for the fly pupae and lay one or more eggs inside. The developing wasps devour the fly within the pupa. They have been found useful adjuncts to other fly control measures when used in confined facilities such as hog barns.24 Inundative releases at feedlots, where thousands of wasps are released at regular intervals throughout the fly season, has shown some efficacy25 but requires an integrated approach with good manure management and selective application of insecticides.
To reduce the fly population in buildings is an important procedure in public health work and many measures are recommended. It is not possible to give details of them here because so many factors have to be taken into consideration, including toxicity of the products used for man and animals, development of resistance to the insecticides, and contamination of food products such as milk by the insecticides.
BUSH FLIES (MUSCA VETUSTISSIMA)
These flies occur commonly in Australia, in drier areas, and are a cause of stress to livestock in the summer months. Bush flies die out in southern Australia each winter but breeding continues in the north and the regular northern winds that commence about September each year blow flies southwards and repopulate the areas that are now suitable for breeding. Musca vetustissima occur in very large numbers and during the day congregate around the eyes, on the lips, on any visible mucous membrane and on wounds to obtain moisture. They are thought to carry contagious ophthalmia of sheep, infectious keratoconjunctivitis of cattle and contagious ecthyma of sheep, to delay the healing of wounds, to contribute to the lesions produced by buffalo flies (Haematobia irritans exigua) and to act as intermediate hosts for the larvae of Draschia megastoma, Habronema muscae, and Thelazia spp. Control of the fly population is virtually impossible in the areas where it occurs but individual animals may be protected by repellents such as dimethyl phthalate or o-diethyl toluamide (DEET). Sprays containing 1% of dichlorvos or crotoxyphos are effective but must be applied daily. Fenvalerate and cypermethrin give excellent relief and lasting protection against the related Musca autumnalis. Dung beetles, introduced from Africa, breakup dung pats which will aid in reducing fly numbers.
FACE FLIES (MUSCA AUTUMNALIS)
This small fly, indigenous to Europe and Asia, first appeared in North America in 1952 and is now present over large areas of eastern Canada and northeastern and north-central United States. The flies resemble the house fly but are slightly larger. They congregate on the face of cattle, feeding on nasal and lacrimal secretions and saliva. Very large numbers cause a certain amount of stress, cause petechiation in the eye, and are thought to be instrumental in transmitting infectious keratoconjunctivitis (pinkeye) of cattle. Face flies are vectors for the eyeworms, Thelazia spp. which infest the conjunctival sacs and lacrimal ducts of domestic animals.
Flies oviposit on fresh cattle manure where larval development takes place. As with all flies development is temperature dependent. In temperate latitudes the flies will over-winter as adults, resting inside homes and other farm structures.
Fly numbers are greatest in summer and cattle are worried particularly when outdoors. Repellents have been extensively used but are not highly successful. Self-applied or hand-applied dusts containing organophosphate insecticides are more extensively used. A dose of 10 mL per animal of 1% cyfluthrin applied as a pour-on reduced fly numbers by 90% and treatment was effective for about 4 weeks.14 Fenvalerate and cypermethrin give immediate relief, and a lasting reduction in fly numbers when used on fly breeding sites.26 Reduction of face fly populations on cattle can be achieved through use of synthetic pyrethroids impregnated eartags, but their use is complicated by the presence of insecticide resistant horn flies. Diflubenzuron boluses give 80% control of the immature stages of M. autumnalis in the manure for up to 20 weeks.15
HEAD FLIES (HYDROTOEA IRRITANS)
This small fly, similar in appearance to the house fly but having an olive abdomen and yellow wing bases, is found in the United Kingdom and Europe. It is a non-biting muscid fly that swarms around animals and man from late June to September. Breeding is in soil and litter and there is only one life cycle per year. The lesions on sheep are self-inflicted trauma in attempts to alleviate fly irritation. Sores are often large, open, and may be made more severe by bacterial invasion. The wounds may predispose to blowfly strike by Lucilia sericata. The pathogens of summer mastitis of cattle can be spread mechanically by muscid flies, and Areanobacterium pyogenes has been shown to persist in H. irritans for up to 4 days.27
Control is difficult and is similar to that used for the other non-biting muscid fly, M. autumnalis. Eartags impregnated with 8.5% cypermethrin or 10% permethrin reduce the severity of fly damage in sheep, and tagged ewes give protection to their lambs. However, it is likely that resistance will quickly occur in the same manner as in the face fly. Pour-on applications of synthetic pyrethroids are easier to apply, are cheaper and leave a higher concentration than a spray or an eartag.28 Cyfluthrin 1% applied as a pour-on at a dose rate of 10 mL per animal reduced fly numbers by 90% and gave 4 weeks protection.14 Crotoxyphos cream is effective but at least two applications are needed. Head-caps are most effective but are tedious to apply.
1 Lysyk TJ. J Med Entomol. 1995;32:508.
2 Thomas GD. J Econ Entomol. 1996;89:411.
3 Demeny A. Parasitol Hung. 1989;22:87.
4 Meyer JA, Hunter JS. Med Vet Entomol. 1991;3:359.
5 Ralley W, et al. Can J Zool. 1993;71:725.
6 Haufe WO. Can J Anim Sci. 1982;62:567.
7 Lysyk TJ. Environ Entomol. 1992;21:1134.
8 Sutherst RW, Tozer RS. Am J Agric Res. 1995;46:269.
9 Harvey TL, et al. J Econ Entomol. 1983;76:96.
10 Cilek JE, et al. J Econ Entomol. 1991;84:756.
11 Mwangala FS, Galloway TD. Can Entomol. 1993;125:839.
12 Floate KD, et al. Med Vet Entomol. 2001;15:117.
13 Lysyk TJ, Colwell DD. J Econ Entomol. 1996;89:1513.
14 Floate KD, et al. Bull Entomol Res. 2002;92:471.
15 Leibisch G, et al. Proc 18th World Buiatrics Congr Bologna, Italy. 1994;1:765.
16 Fincher GT. Environ Entomol. 1991;20:77.
17 Parashar BD, et al. Med Vet Entomol. 1991;5:363.
18 Cybinski DH, Muller MJ. Am J Zool. 1990;38:25.
19 Stevens DP, et al. Vet Rec. 1988;122:308.
20 Holbrook TR, Mullens BA. Proc Am Mosquito Control Assoc. 1994;10:70.
21 Poinar PO. Bull WHO. 1977;55:509.
22 Haufe WO, Croome GCR. Alberta Environ Spec Pub. 1980.
23 Shemanchuk JA. Pesticide Sci. 1981;12:412.
24 Weintraub J. Research Highlights — 1984 Ag Canada Res Stn. 1985:26.
25 Floate KD. Can Entomol. 2003;135:599.
26 Supperer R, Heimbucher J. Wien Tierarztl Monatsschr. 1982;69:229.
Mite infestations
HARVEST MITES (CHIGGER MITES)
Infestations with trombidiform mites cause dermatitis in all species. Except for Psorergates ovis, P. bos, and Demodex spp. they are all harvest or grain mites. These mites are primarily predatory on other arthropods associated with harvested grain and infesting animals only secondarily and usually transiently. It is usually the larval stages which are found feeding on animals while the nymphs and adults are free-living.
The larvae of Pyemotes ventricosus, Neotrombicula autumnalis, Eutrombicula alfreddugesi, E. splendens, E. batatas, Trombicula spp., and some species of Leptotrombidium and Schoengastia are parasitic on man and most animals, causing dermatitis and, in man, transmitting ricketsial diseases. Nymphs and adults are free-living predators feeding mainly on arthropods in grain and hay. The larvae are most active in the autumn at harvest time and may cause dermatitis in animals grazing at pasture or those confined in barns and being fed newly harvested grain.
Horses and cattle are usually affected on the face and lips, which, in white-faced horses, may suggest a diagnosis of photosensitization, and about the feet and lower limbs, especially in the flexures. Affected areas are itchy and scaly but, with rubbing, small fragile scabs and absence of hair may become apparent. Infestation of horses with Trombicula sarcina causes a severe pruritus and yearlings show irritation by lip-biting their legs and rubbing against stable walls. Stamping is uncommon, and usually occurs when yearlings are stabled on fresh, contaminated bedding.1 Sheep, when first affected, stamp their feet repeatedly and bite their legs. The skin at the heels, coronet, and pasterns, and sometimes the shank, becomes erythematous and weeps fluid. The mites detach after 3–5 days and leave a small ulcerated area. In light infestations the mites may be confined to the area between the accessory digits, but in heavy infestations the skin over the whole of the lower limbs may be swollen and thickened. The infestation is self-limiting and treatment is not usually necessary but the legs can be washed in 0.25% maldison. Area control of the mite may be obtained by the use of chlorpyrifos either as 0.5% granules, 1.1 kg/ha, or the 22.4% spray at 1.6 kg/ha.2
Infestation with Tyroglyphus spp. in pigs appears to be manifested by itchiness and the development of fragile scabs about 3 cm in diameter scattered over the body. Unlike the thick scabs of sarcoptic mange, the skin beneath appears normal. The infestations occur in pigs eating dry ground grain from automatic feeders, lesions appearing several weeks after the dry feeding is begun and disappearing spontaneously about 3 weeks later. No treatment is necessary although spraying with malathion is usually recommended. Affected pigs show no ill-effects but the lesions may be mistaken for those of swinepox or sarcoptic mange. The ingestion of large numbers of mites appears to have no ill-effects.
ITCHMITEs (PSORERGATES OVIS, P. BOS)
The ‘itchmite’ has been recorded as a parasite of sheep in Australia, New Zealand, South Africa, the United States, Argentina, and Chile. Psorergates bos has also been recorded from cattle in the UK.1
LIFE CYCLE AND EPIDEMIOLOGY
The entire lifecyle of this mite, eggs, larvae, two nymphal stages, and adults, takes place entirely on the host. In sheep the cycle takes 4–5 weeks. All stages occur in the superficial layers of the skin. The adults are extremely small and can be seen only with the aid of a microscope. Only the adults are mobile on the skin surface and they effect spread of the disease by direct contact. In sheep this often occurs between recently shorn animals when contact is close and prolonged such as when shorn sheep are packed in yards after shearing, or from ewe to lamb while suckling. Mite feeding activity, in addition to excreta causes skin irritation leading to rubbing and biting of the affected parts (principally the sides, flanks, and thighs) and raggedness, sometimes shedding, of the fleece. Wool over these areas becomes thready and tufted and contains dry scales.2
PATHOGENESIS
The skin shows no gross abnormality other than an increase in scurf. Histologically there is hyperkeratosis, desquamation, and increased numbers of mast cells.3 The irritation appears to be a hypersensitivity and results in biting and chewing of the fleece on the flanks and rump behind a line approximately from the elbow to the hips. In the individual sheep and in flocks the disease spreads slowly so that it may be several years before clinical cases are observed and an appreciable number are visibly affected. The incidence of clinical cases in a neglected flock may be as high as 15%. Sheep on poor nutrition have significantly higher mite populations, more scurf and greater fleece derangement.3 Affected sheep may become tolerant after 1–2 years and show no signs, even though they remain infested.
Amongst sheep, Merinos are most commonly affected. The highest incidence is observed in this breed, particularly in areas where the winter is cold and wet. There is a marked seasonal fluctuation in the numbers of mites; the numbers are very low in summer, commence to rise in the autumn, and peak numbers are found in the spring. Spring or summer shearing exacerbates the decline in numbers. Clinically, the disease resembles louse infestation, but may be distinguished on the smaller proportion of the flock affected (10–15%), the less severe irritation and tendency of the sheep to bite those areas it can reach. Hence lesions are confined to parts of the flank and the hindquarters and the wool tufts have a chewed appearance.
CLINICAL FINDINGS
Diagnosis depends on finding the mites in a skin scraping. The selection of sheep with excess scurf and fleece derangement increases the chance of finding mites and in the absence of lice, ked, and grass seed infestation, about 75% of such sheep prove positive for P. ovis. The wool should be clipped as close as possible, the skin smeared lightly with oil and scraped over an area of about 25 cm2. The mites have a seasonal incidence and may be very difficult to find in summer and autumn. For best results the scraping should be made on the ribs or shoulder in winter or spring. Scrapings are usually teased out in oil and examined microscopically without digestion. A number of scrapings may be needed from each sheep before mites can be demonstrated. Because of the difficulty of finding mites in summer and autumn, sheep dipped at that time cannot be said to be free of infestation until they prove negative on skin scraping in the following spring, when mite numbers should be at the highest levels.
TREATMENT AND CONTROL
There is no compound available that will eradicate itchmite after a single treatment. Arsenic, lime sulfur, or finely divided sulfur have been used and markedly reduce the number of mites. Because the mites are slow to build up, dipping every second year will mask the signs of infestation. However, arsenic is no longer used in most countries. Finely divided rotenone by itself or mixed with the synergist piperonyl butoxide reduces the mite population. It is usually combined with an organophosphate to include lice and ked control in the one product. Phoxim, an organophosphorus compound, has good activity but two dippings 1 month apart are necessary to eradicate infestations. Amitraz causes a marked reduction in mites that will be maintained for some months.
A single subcutaneous injection of 0.2 mg/kg ivermectin freed sheep of mites up to 56 days post-treatment.4 However these sheep would have to be examined over a longer period to insure eradication. Other macrocyclic lactone products, in various formulations, have been shown to have good efficacy.
DEMODECTIC MANGE (FOLLICULAR MANGE)
Mites of Demodex spp. infest hair follicles of all species of domestic animals. The disease causes little concern but in cattle and goats there may be significant damage to the hide and, rarely, death that may result from gross secondary bacterial invasion.
ETIOLOGY
Mites infesting the different host species are considered to be specific and are designated as Demodex bovis for cattle, D. ovis for sheep, D. caprae for goats, D. equi for horses, and D. phylloides for pigs.
Demodicosis may occur in farm animals of any age, especially those in poor condition but most cases in cattle occur in adult dairy cattle in late winter and early spring. This differs from the well-known condition in the dog which occurs in young, immunodeficient animals.
LIFE CYCLE AND EPIDEMIOLOGY
The entire life cycle is spent on the host. Adult mites invade the hair follicles and sebaceous glands which become distended with mites and inflammatory material. The life cycle consists of the egg, larval, and two nymphal stages. The disease spreads slowly and transfer of mites is thought to take place by contact, probably early in life. Calves can acquire mites from an infected dam in half a day.1 However in horses grooming instruments and rugs may transmit infection.
PATHOGENESIS
Invasion of hair follicles and sebaceous glands leads to chronic inflammation, loss of the hair fiber and in many instances the development of secondary staphylococcal pustules or small abscesses. It is these foci of infection which cause the small pinholes in the hide which interfere with its industrial processing as well as reducing the value dramatically. In most farm animals the lesions are difficult to see externally and only the advanced ones will be diagnosed.
CLINICAL FINDINGS
The important sign is the appearance of small (3 mm diameter) nodules and pustules which may develop into larger abscesses, especially in pigs and goats. The small lesions can be seen quite readily in short-coated animals and on palpation feel like particles of bird-shot in the hide. In severe cases there may be a general hair loss and thickening of the skin in the area, but usually there is no pruritus and hair loss is insufficient to attract attention. The contents of the pustules are usually white in color and cheesy in consistency. In large abscesses the pus is more fluid. In cattle and goats the lesions occur most commonly on the brisket, lower neck, forearm, and shoulder, but also occur on the dorsal half of the body, particularly behind the withers. Larger lesions are easily visible but very small lesions may only be detected by rolling a fold of skin through the fingers. In horses the face and around the eyes are predilection areas. Demodicosis in pigs usually commences on the face and spreads down the ventral surface of the neck and chest to the belly. There is little irritation and the disease is observed mainly when the skin is scraped at slaughter. The disease may be especially severe in goats, spreading extensively before it is suspected and in some instances causing deaths. Severe cases in goats commonly involve several skin diseases such as mycotic dermatitis, ringworm, besnoitiosis and myiasis. Demodicosis is rare in sheep. In this species pustules and scabs appear on the coronets, nose, tips of the ears, and around the eyes, but clinical signs are not usually seen and mites may be found in scrapings from areas of the body not showing lesions.
CLINICAL PATHOLOGY
The characteristically elongated mites are usually easy to find in large numbers in the waxy material which can be expressed from the pustular lesions. They are much more difficult to isolate from squamous lesions. Lesions in hides can be detected as dark spots when a fresh hide is viewed against a strong light source. However, lesions may not be readily seen until the hair has been removed and the skin has been soaking for some time.
• The commonest error is to diagnose the disease as a non-specific staphylococcal infection
• In cattle and goats the disease often passes unnoticed unless the nodules are palpated
• Deep-seated ringworm in horses has much in common with demodicosis
• A satisfactory diagnosis can only be made by demonstration of the mite.
TREATMENT AND CONTROL
Repeated dipping or spraying with the acaricides recommended for other manges is usually carried out but is more to prevent spread than cure existing lesions. Ivermectin which does not eradicate the infection in dogs, possibly because of the difficulty in getting the acaricide to the mite, has been reported to cure 98% of beef bulls when used at 0.3 mg/kg.2 Ivermectin in a premix, fed for 7 consecutive days has been reported to clear the infestation in pigs.3
SARCOPTIC MANGE (BARN ITCH)
Sarcoptic mange occurs in a wide variety of host species causing a severe puritic dermatitis. While in most countries it has been a major problem and was a reportable disease the advent of macrocyclic lactone endectocides has reduced the incidence of disease dramatically.
ETIOLOGY
The causative mite, Sarcoptes scabiei, is usually considered to have a number of varieties each generally specific to a particular host species. Morphological, immunological and molecular research confirms the close relationship among the varieties,1 but do not explain the biological differences, particularly with respect to host specificity. Because host specificity is not strict and transference from one host species to another can occur there is some concern when attempting to control the disease.
Animals in poor condition appear to be most susceptible, but conditions, especially overcrowding, in which sarcoptic mange occurs often go hand in hand with poor feeding and general poor husbandry. The disease is most active in cold, wet weather and spreads slowly during the summer months.
LIFE CYCLE AND EPIDEMIOLOGY
Female mites form shallow burrows in the lower stratum corneum of the skin in which they deposit eggs. Development for both sexes includes a larval stage, two nymphal stages prior to molting to the adult. All life cycle stages, except the eggs, can be found moving on the skin surface and are thus easily transferred to other hosts. The normal exfoliation of the skin eventually exposes the tunnels exposing eggs as well. The life cycle from egg to egg takes 10–13 days.
Although direct contact between hosts is the most effective method of transmission inert materials such as bedding, blankets, grooming tools, and clothing may act as carriers. Adult mites do not usually survive for more than a few days away from the host but in optimum laboratory conditions they may remain alive for up to 3 weeks. In pigs adult sows are often the source of infestation for young pigs even though they show no signs of the disease. Large numbers of mites can often be found in the ears of normal sows and the mites are transmitted soon after farrowing. Significant scratching does not occur until a hypersensitivity develops some 8–10 weeks later and may continue until slaughter.2 A small proportion of young pigs do not develop a hypersensitivity and these become chronically affected.2
Amongst domestic species pigs are most commonly affected, but it is an important disease in cattle and camels and occurs in sheep. It has been a notifiable disease in most countries, because of its severity, but a decline in prevalence accompanying the advent of new therapeutics has resulted in the removal of this requirement in some countries. People handling infested animals may become infected but lesions will disappear if further contact is prevented.
Infested animals develop protective immunity3 and are able to clear challenge infestations rapidly. A proportion of infested hosts do however remain chronically infested and mite populations may show a post-partum recrudescence thereby facilitating transfer to the susceptible offspring.
PATHOGENESIS
Young animals, in particular piglets, become infected in the first few weeks of life and develop a hypersensitivity within 8–10 weeks. This allergic phase lasts for 8–9 months4 and during this time affected animals are constantly itchy. The disease, if untreated, progress to a localized crust formation characteristic of a chronic hyperkeratotic state.
Many infestations in pigs have little or no effect on weight gain although there is some controversy5 and treatments improve productivity (see below). There are suggestions in other hosts of reduced feed efficiency. In some pigs the loss of condition, production and vitality may be severe, and the appearance of affected animals is esthetically displeasing. Erythema, papules and intense pruritis may be seen. Few mites may be necessary to cause a reaction in a previously sensitized animal. A chronic condition is uncommon but is seen in pigs with an immunodeficiency.
In cattle and camels, severe hypersensitivity lesions occur and often lead to death. Sheep initially show an intense pruritus and rub the affected part against fences or bite at the skin. Later papules and vesicles occur and the skin becomes thickened, covered with pale scabs and the hair is lost.6
CLINICAL FINDINGS
Early lesions are characterized by the presence of small red papules and general erythema of the skin. The affected area is intensely itchy and frequently excoriated by scratching and biting. Loss of hair, thick brown scabs overlying a raw surface, and thickening and wrinkling of surrounding skin soon follow. In pigs the lesions commence on the trunk, in sheep and goats on the face, in cattle on the inner surface of the thighs, the underside of the neck and brisket and around the root of the tail, and in horses and camels on the head and neck. Except in sheep where the lesions do not spread to the woolled skin, lesions become widespread if neglected and such animals may show systemic effects including emaciation, anorexia, and weakness, and in neglected cases death may occur.
The course of sarcoptic mange is rather more acute than in the other forms of mange and may involve the entire body surface of cattle in a period as short as 6 weeks.
CLINICAL PATHOLOGY
Necropsy examinations are not usually undertaken. Deep scrappings which draw blood are required for accurate diagnosis and must be taken from the edges of any evident lesions (scrappings taken from the central portions of lesions are very often negative). Examination of scrapings either directly or after digestion in 10% potassium hydroxide will reveal mites and/or eggs. When practical multiple scrapings from affected animals should be taken. Examination of the ear wax of pigs often shows mites when none can be seen in scrapings.
Change in behavior, a result of the intense pruritis, have been used in swine as an initial diagnostic tool. An increase in the rubbing index is indicative of infestation, but other clinical confirmation is required.7
An ELISA for detection of antibodies to Sarcoptes scabiei has been developed.8 The test has high specificity and moderate sensitivity, being more sensitive in young animals undergoing their first infestation. It has been shown to work well in herd level eradication programs and functions afterward as an effective surveillance tool.9
• Sarcoptic mange is the only mange which occurs in pigs. It can be confused with infestation with Tyroglyphus spp. mites or lice, or with swinepox, parakeratosis, infectious dermatitis, pityriasis rosea, and ringworm. In most of these diseases there are clinical features which are characteristic and final diagnosis can be made on the presence or absence of the mite
• The same comments apply to the differentiation in cattle of sarcoptic mange from chorioptic and psoroptic mange and from chlorinated naphthalene poisoning and ringworm
• Horses may be affected by psoroptic or chorioptic mange but the lesions are most common at the base of the mane and tail and at the back of the pastern respectively
• Infestation with the trombidiform mites and photosensitization may resemble sarcoptic mange
TREATMENT AND CONTROL
Macrocyclic lactone endectocides (including ivermectin, eprinomectin, moxidectin, and doramectin) are the preferred products for treatment of sarcoptic mange. Use of these products in pour-on or injectable formulations are highly efficacious when used at the label recommended dose. Because of the residual activity of these compounds10 retreatment is not usually necessary although moxidectin given subcutaneously at 0.2 mg/kg to infested sheep resulted in a rapid clinical improvement but did not eliminate the mites. Two doses 10 days apart resulted in negative skin scrapings by 14 days post-treatment.11 A single injection to cattle eliminated the mites by day 14.12 The resolution of the lesions may take considerable time, but should not be misconstrued as product failure.
Prefarrowing treatment with ivermectin to prevent transmission to the newborn piglets improves weight gains and early feed conversion.
If other treatments are used they must be thoroughly applied so that all parts of the skin, especially under the tail, in the ears and between the legs are wetted by the acaricide. Although buildings, bedding, and other inert materials do not support the mite for more than a few days they should also be treated unless they can be left in a dry state for 3 weeks.
Treatments should be repeated three times at 7-day intervals. For sows this should commence 3 weeks before farrowing. Special attention should be paid to the ears. Trichlorfon, maldison (0.5%), diazinon (0.02%), coumaphos (0.05–0.1%), fenchlorphos, chlorfenvinphos, amitraz (0.1%), and phoxim (0.025%) have been used. All animals should be treated. Phosmet 20% applied as a pour-on at weaning eliminated Sarcoptes and allowed 12% increase in live weight gain.13
1 Zahler M, et al. Int J Parasitol. 1999;29:759.
2 Davis DP, Moon RD. J Med Entomol. 1990;27:727.
3 Arlian LG, et al. Vet Parasitol. 1996;62:133.
4 Sheahan BJ. Vet Rec. 1974;94:202.
5 Arends JJ, et al. J Anim Sci. 1990;68:1495.
6 Abu-Samra MT, et al. Ann Trop Med Parasitol. 1981;75:639.
7 Hollanders W, et al. Vet Parasitol. 1995;58:117.
8 Hollanders W, et al. Vet Parasitol. 1997;69:117.
9 Jacobson M, et al. Vet Parasitol. 1999;81:249.
10 Arends JJ, et al. Vet Parasitol. 1999;582:71.
11 Corba J, et al. Vet Parasitol. 1995;56:339.
PSOROPTIC MANGE (SHEEP SCAB, BODY MANGE, EAR MANGE)
Psoroptic mange is of greatest importance in sheep, in which it causes sheep scab, but it is also responsible for body mange in cattle and horses and ear mange in horses, sheep, goats, and rabbits. The disease is a major animal welfare concern.1
ETIOLOGY
The various species of Psoroptes have now been reduced to two or three species.2 Based on molecular evidence3 P. ovis, P cuniculi, and P. cervinus are identical despite differences in morphology and biology. It is clear that P. ovis from cattle and sheep are identical4 although cross-transmission is not always successful.5 P. equi occurs on horses, donkeys, and mules in Great Britain and P. natalensis on cattle and the water buffalo. The ear mites are all P. cuniculi and recent work has suggested this is a variant of P. ovis adapted to the aural environment. P. cervinus assumes a dual role, being an ear mite of the American bighorn and a body mite of the wapiti.
LIFE CYCLE AND EPIDEMIOLOGY
Psoroptic mange is a major disease in sheep which was once virtually eliminated in most progressive countries where wool production is an important industry. With the cessation of organophosphate dips in the UK there has been a resurgence of the problem. The disease in cattle was widespread in the United States but has now largely been brought under control. It can spread rapidly and cause serious losses in cattle if neglected as shown by the serious losses that can occur in feedlots. The ear manges cause irritation and, in horses, a touchiness around the head.
Psoroptic mites abrade the surface and feed on lipid exudate, bacteria, and skin debris.6 Erythrocytes are not normally a constituent of the diet and may be accidentally ingested when host scratching results in skin breakage.6 They cause the formation of scabs, under which they live. The eggs are laid on the skin at the edge of a scab and hatch in 1–3 days, although this is prolonged if eggs are not in contact with the skin. There are the usual larval and nymphal stages and the whole life cycle is complete in 10–11 days.7 All stages are capable of survival away from the host for up to 10 days and under optimum conditions adult females may survive for 3 weeks.
Optimum conditions for development include high humidity and cool temperatures. Thus the disease is most active in autumn and winter months. This is a result of not only increased activity of the mites but also the more rapid development in housed animals and the tendency for the disease to be most severe in animals in poor condition. When conditions are adverse, as in summer, mites survive in sheep in protected parts in the perineum, in the inguinal and interdigital regions, the infraorbital fossae, inside the ear and the scrotum. Spread occurs from sheep to sheep but transmission from infected premises and by passive spread of pieces of wool also occurs.
While P. ovis has been shown to survive for up to 17 days away from the host,8 no natural transmission has taken place where premises have been rested for more than 10 days.5 Premises left free of sheep for at least 2 weeks can be assumed to be safe.5 If cattle are housed in stanchions that prevent grooming infestation will be more severe.7 Although individual mites survive for only 4–6 weeks the disease is continuous and a very rapid increase in mite numbers may occur.7
The life cycle of the other species is thought to be similar. Spread of ear mite in horses can occur by grooming or by the use of infected harness.
PATHOGENESIS
The mite migrates to all parts of the skin and prefers areas covered with hair or wool. Salivary secretions and mite excreta contain proteinases that result in a severe allergic pruritis. The exudation of serum accumulates to form a crust. In cattle the mites are most active at the edge of the crust and the lesion spreads peripherally. Infested calves have lower weight gains, lower feed conversion and lower energy retention than non-infested calves.7 In sheep the mites are more generally distributed and bacterial invasions of the skin are more common.4
CLINICAL FINDINGS
Sheep
Cutaneous lesions may occur on any part of the body but characteristically in badly affected sheep they are most obvious on the sides. Very early lesions are small (6 mm diameter) papules which ooze serum. Attention may be attracted to the area by raggedness of the wool caused by biting and scratching. In older lesions thin yellow crusts are present and the wool commences to shed. The wool may contain large masses of scab material which bind the fibers together in a mat. Under suitable conditions the infestation spreads rapidly and in 6–8 weeks three-quarters of the body may be affected.
In a typical outbreak of sheep scab many animals are affected and show itchiness and shedding of the fleece. Some become markedly emaciated and weak, and deaths may occur. However, it is possible to have the disease in a flock at a very low level of incidence and with minimal lesions. This usually occurs when the sheep are highly resistant because of good nutrition, or climatic conditions are adverse for mite development, or treatment has been carried out but has been incomplete. In such cases there may be little or no clinical evidence of the disease and a careful search for latent cases may be necessary. This is facilitated by packing the animals into a confined space, so that the mites become active, and watching for signs of itchiness.
Behavioral changes in infested sheep are dramatic with sheep biting at the affected areas, rubbing or scratching. In addition infested sheep exhibited stereotypic behaviors typical of animals under stress.1 These changes combine to reduce productivity. Animals exhibiting these changes should be carefully examined by palpating the surface of the skin in search of papules and scabs. Special attention should be paid to the ears, the base of the horns, the infraorbital fossa and the perineal and scrotal areas in rams.
Goats
Lesions can vary from a dry crusty scab on the external ear canal with no clinical signs to severe lesions covering much of the body and causing death. However, it is commonly an ear mite, feeding on whole blood, and causing the production of scabs which vary from a single layer lining the large sulcus at the base of the concha to abundant laminated scab formation occluding the meatus. In severe cases the poll may be affected, and scabs may also be found on the pasterns. Female goats serve as the source of infection for the kid; mites may be found by 5 days and clinical signs are seen by the 3rd week of life.9 Raillietia may also be found in the ear of goats but Raillietia caprae is easily differentiated microscopically as all legs are on the anterior part of the body.
Horses
P. equi causes the production of large, thick crusts on those parts of the body carrying long hair, the base of the mane and the root of the tail, and hairless areas such as the udder, prepuce, and axilla. Affected parts are itchy, the hair is lost and with constant rubbing the surrounding skin becomes thickened. P. cuniculi infestations in horses cause severe irritation in the ear accompanied by discharge, shaking of the head, rubbing of the head, and tenderness of the poll.
Cattle
Typical lesions appear first on the withers, neck, and around the root of the tail. In severe cases they may spread to the rest of the body. The lesions are intensely itchy. They commence as papules but soon are covered with a scab which enlarges peripherally and coalesces with other lesions so that very large areas of skin may become involved. The hair is lost, the skin becomes thickened, wrinkled, and covered with scabs. Badly affected animals becomes weak and emaciated, and may die.
CLINICAL PATHOLOGY
The mites can be easily demonstrated in scrapings taken from the edges of the lesions. Examination is facilitated by prior digestion of the scraping in warm, 10% potassium hydroxide solution.
An ELISA has been developed for diagnosis of Psoroptes infestation in sheep.10 It has been applied to monitoring of infestations as part of efficient control programs.11
• Severe cases of psoroptic mange in sheep are similar to mycotic dermatitis except that there is no itching in the latter. Disease causing itchiness such as scrapie, ked, and louse infestations and infestations with Psorergates ovis and harvest mites do not have typical cutaneous lesions and the latter group can usually be detected by examination for the causative parasites
• In horses attention is drawn to the condition because of the horse rubbing its head, by swelling around the base of the ear, or by resentment to the bridle passing over the ears. In some horses the affected ear may droop.
TREATMENT AND CONTROL
Macrocyclic lactone endectocides are used most frequently for control of psoroptic scabies. Cattle treated with ivermectin must be separated from non-infested cattle for between 9 and 14 days, otherwise spread and re-infection12 may occur. In sheep two treatments of ivermectin 0.2 mg/kg subcutaneously are necessary to eliminate infestations.13
Moxidectin applied as a 0.5% pour-on at 0.5 mg/kg to cattle is effective against P. ovis as well as lice and Chorioptes bovis14 and was equally effective against P. ovis as 0.2 mg/kg by subcutaneous injection.15 In sheep, although a single subcutaneous dose of 0.2 mg/kg moxidectin gave a rapid clinical improvement, two doses 7 days apart were necessary to eliminate mites.16 In large scale field use, sheep receiving a single injection in the autumn remained free of the infestation throughout the winter, while two injections 10 days apart were effective in treating outbreaks.17
Doramectin injectable at 200 μg/kg was highly effective in eliminating mites in scrapings of infested cattle.18 The same treatment was found to protect cattle from infestation for up to 3 weeks.18
If sheep are to be dipped, it is important to wet the skin thoroughly and pay special attention to severe cases where mites are likely to be present in inaccessible sites on the body. Thus a plunge dip is almost essential and the sheep must be kept immersed in the dipping fluid for at least one minute. Prior shearing may be advisable but may lead to further spread of the infestation. Care must be taken to insure that the concentration of the acaricide in the dip is maintained, especially when large numbers of sheep are being treated. Badly affected animals should be set aside and inaccessible sites including ears, horn bases, and perineum treated manually with the dipping fluid. Dipped sheep should not be returned to their pastures, nor to the barn unless the latter has been thoroughly cleaned and sprayed with the dipping fluid.
Diazinon (0.01%), propetamphos (0.0125%), and flumethrin (0.055%) will all eliminate P. ovis from sheep with a single dipping and will give at least 4 weeks of protection.19 Coumaphos (0.1%), phoxim (0.05%), and amitraz (0.05%) require two treatments at 7–10 day intervals to eliminate infestations. The synthetic pyrethroids are variable in their efficacy. Flumethrin, used as a non-stripping dipping compound, eradicated P. ovis from sheep when used at 55 ppm and gave at least 7 weeks of protection.20 Fenvalerate (0.05%) used twice will also clear infested cattle, but another synthetic pyrethroid, cypermethrin, when used at 150 ppm did not eliminate infestations after three treatments.21
In horses, affected ears should be cleaned of all wax and ear preparations containing benzene hexachloride should be used at weekly intervals. Local treatment of diazinon or propetamphos could also be used. Benzyl benzoate is a safe and effective treatment when given every 5 days for three treatments. Ivermectin is highly effective against P. equi.22
Eradication of sheep scab on an area basis is usually undertaken by quarantine and compulsory treatment of all susceptible animals in the area at the same time. Now that there are effective treatments that do not require dipping, eradication of scab from areas should be more easily accomplished. The necessity to dip all animals in the area during a short period presents difficulties and the cost of construction of dips and lack of desire to dip in cold climates are other obstructing factors. The use of pour-ons or injections is an attractive alternative to autumn dipping, and has the added advantage of providing helminth control in late season lambs and in ewes. Further, even pregnant animals can be yarded and treated by subcutaneous injection or pour-on as long as care is taken in the yards. Where it is desired to keep the disease at a low level short of eradication, the disease is made notifiable, movement of stock is restricted and infested farms are quarantined.
1 Cork MJ, Broom DM. Vet Parasitol. 1999;83:291.
2 Bates PG. Vet Parasitol. 1999;83:201.
3 Zahler M, et al. Int J Parasitol. 1998;28:1713.
4 Losson, et al. Vet Parasitol. 1999;83:219.
5 O’Brien DJ, et al. Vet Res Comm. 1994;18:113.
6 Rafferty DE, Gray JS. J Parasitol. 1987;73:901.
7 Guillot FS. J Med Entomol. 1981;18:44.
8 Strong KL, Halliday RB. Exp Appl Acarol. 1992;15:153.
9 Heath ACG, et al. NZ Vet J. 1989;37:56.
10 Ochs H, et al. Vet Parasitol. 2001;96:233.
11 Falconi F, et al. Vet Parasitol. 2002;109:119.
12 Lonneux J-F, et al. Vet Parasitol. 1997;69:319.
13 Bates PG. Vet Rec. 1994;134:334.
14 Losson B, Lonneux J-F. Vet Parasitol. 1996;63:119.
15 Lonneux J-F, et al. Vet Parasitol. 1997;49:67.
16 Parker LD, et al. Vet Parasitol. 1999;83:301.
17 O’Brien DJ, et al. Vet Rec. 1994;139:437.
18 Clymer BC, et al. Vet Parasitol. 1997;72:79.
19 Kirkwood AG, Bates PG. Aust Vet J. 1987;41:42.
20 Kirkwood AG, Bates PG. Vet Rec. 1987;120:197.
21 Palmer CR, van Amelsfoort A. J South Afr Vet Assoc. 1983;54:99.
CHORIOPTIC MANGE (TAIL MANGE, LEG MANGE, SCROTAL MANGE)
Chorioptic mange is the commonest form of mange in cattle and horses. While the primary effect on cattle is esthetic damage there are production effects in dairy animals.1,2 In horses leg mange is a source of annoyance and inefficiency at work. In sheep it affects the scrotum and may cause a decrease in fertility.1
ETIOLOGY
Chorioptic mites were formerly named according to the host species but those on cattle, horses, goats, and sheep are now considered to be one species, Chorioptes bovis. Another species, C. texanus, has been reported on goats, cattle, and Canadian reindeer. In cattle, the mites are much more active in the latter part of the winter and tend to disappear in cattle at pasture. This diminution in activity is not noted in cattle kept housed in the summer.
LIFE CYCLE AND EPIDEMIOLOGY
Chorioptes bovis feed on the skin surface, abrading the upper layers with their mouthparts and contaminating the area with salivary secretions and excreta. Developmental stages are similar to that of Psoroptes and a complete cycle, from egg to adult, requires approximately 3 weeks. The number of parasites is influenced by temperature and humidity; the mite populations beginning to increase on sheep in early autumn, numbers reach a peak in late autumn or early winter and decline in spring. In cattle the cycle is longer, peak numbers occurring in late winter and early spring and declining in summer. Transmission is probably effected by direct contact in most instances although in animals housed in barns, grooming tools may be an additional method of spreading the disease. Infestation of bedding is not a common method of transmission.
In horses, the parasites occur almost entirely in the long hair on the lower parts of the legs and are rarely found on other parts of the body. In cattle the disease is most evident in the winter, lesions occurring most commonly on the perineum, and back of the udder, extending in severe cases to the backs of the legs and over the rump. In the summer months the mites persist in the area above the hooves, particularly the pasterns of the hind leg. In sheep, lesions are confined to the wool-less areas, chiefly the lower parts of the hindlegs and scrotum. Rams are more heavily infected than ewes and probably infect ewes while copulating. Lactating ewes probably act as the source of infection for lambs.
PATHOGENESIS
The mites cause an allergic, exudative dermatitis; the yellowish serous exudate coagulates and breaks as the hair grows so that small scabby lesions are seen on the hair. In horses the mites cause severe irritation and itchiness. The initial lesion in cattle is a small nodule which exudes serum causing matting of the hair. In severe cases these coalesce to form heavy scabs and cause thickening and wrinkling of the skin. Mites can be isolated from many animals which show no clinical evidence of the disease. While most cases do not cause any symptoms, a rapidly spreading syndrome characterized by coronitis, intense irritation and a marked fall in milk production has been reported. C. bovis is a common parasite of sheep in the United States, New Zealand, and Australia, and causes an allergic exudative dermatitis on the scrotum of rams. This may cause a rise in temperature of the scrotal contents and a severe testicular degeneration if the lesion has an area greater than 10 cm2.1
CLINICAL FINDINGS
The first sign in horses is usually violent stamping of the feet and rubbing of the back of the hind pasterns on wire, rails, or stumps. This is most evident during periods of rest and at night. Examination of the area is difficult because of the long hair present and the horses may resent manipulation. In cases of long duration the skin is seen to be swollen, scabby, cracked, and usually greasy; small amounts of serous exudate may be attached to most hair in the affected area.
Cattle show little evidence of cutaneous irritation but the small crusty scabs (3 mm diameter) on the escutcheon, udder, and thighs are unsightly. Although the mites appear to cause little trouble in the summer, occasional animals are seen which have thick, crusty scabs on the skin, just above the coronets and around the muzzle.
The main lesion in sheep is seen on the scrotum of rams where an allergic dermatitis results in the production of a yellowish serous exudate over areas from a few millimeters to several centimeters.1
CLINICAL PATHOLOGY
Scrapings from the affected areas usually contain large numbers of mites.
• Greasy heel in horses resembles chorioptic mange except that pain is more evident in the former and itchiness in the latter. It has been suggested that the two diseases are etiologically related
• The lesions in cattle may go unnoticed but are not likely to be mistaken for those of any other disease with the possible exception of other manges. The presence of chorioptic mites in footrot and mucosal disease lesions may be purely coincidental, but cases of chorioptic mange which have lesions around the coronet and muzzle may be mistaken for one of the erosive diseases
• Sheep with itchy, scabby legs may be infested with other forms of mange or have contagious ecthyma or strawberry footrot.
TREATMENT AND CONTROL
The macrocyclic lactone endectocides have shown efficacy against Chorioptes spp., but eradication of the parasites from a herd is difficult. Moxidectin 0.5 mg/kg applied as a pour-on eliminated C. bovis as well as sucking lice and Psoroptes ovis.3 When given as a single injection of 0.2 mg/kg there was a marked decline in the number of mites but few cattle were cleared of infection.4 Doramectin has high efficacy at the label rate in cattle, but a single treatment did not clear mites from all of the trial animals.5 Treatment with eprinomectin at recommended rates was completely effective, but mites persisted for at least 14 days.2
Two doses of 2 mg/kg flumethrin applied to the whole body 1 week apart eliminates mites from cattle, while treatment of 2 mg/kg was successful if applied to the caudal region.6 Fenvalerate 0.05% also killed all mites while amitraz 0.05% removed 98%.7 Phoxim 0.05% and 0.1% used twice at 10-day intervals has also eradicated the infection from cattle.8 Other compounds if used repeatedly will reduce mite numbers but recrudescences may occur. Ivermectin 0.2 mg/kg given subcutaneously on two occasions reduced but did not eliminate the infestation on cattle. A single treatment of infested horses with ivermectin paste did not remove all mites but when combined with hair removal, washing encrusted areas with oil of salicylic acid and the later removal of crusts with a stiff brush, eradication was achieved.9
1 Rhodes AP. Aust Vet J. 1976;52:250.
2 Barth D, et al. Am J Vet Res. 1997;58:1257.
3 Losson B, Lonneux JF. Vet Parasitol. 1996;63:119.
4 Losson B, Lonneux JF. Vet Parasitol. 1993;51:113.
5 Losson B, et al. Vet Rec. 1998;142:18.
6 Liebisch A. Vet Med Rev. 1986;1:17.
7 Wright FC, et al. Am J Vet Res. 1988;49:903.