Chapter 8 Diseases of the cardiovascular system
Principles of circulatory failure
The primary function of the cardiovascular system is to ensure an adequate circulation of blood so that nutrients are delivered, waste products are removed and a homeostatic milieu is maintained at the organ and cellular level. An inadequate circulation interferes with nutrient delivery and waste product removal, and ultimately leads to circulatory failure, the primary concept in diseases of the cardiovascular system.
The two functional units of the cardiovascular system are the heart and the blood vessels; these two units are best characterized as a pump (the heart) and a circuit (the blood vessels and blood). The pump and circuit may fail independently of each other, giving rise to two forms of circulatory failure – heart failure and circuit failure. In heart failure the primary problem is inadequate pump performance, whereas in circuit failure the deficiency is in the vascular system, which fails to return an adequate volume of blood to the heart. Circuit failure can also result from decreased circulating blood volume.
HEART FAILURE
The failure of the heart as a pump can result from a defect in filling of the heart, an abnormality in the generation or conduction of the electrical wave of depolarization, an abnormality in contractile function, excessive workload or a combination of one or more abnormalities.
It is usual to divide heart failure into two types, acute heart failure and chronic (congestive) heart failure. However, a complete range of syndromes occurs and some of them do not fit neatly into one or other category. Circulatory equilibrium is not maintained when cardiac output is deficient. If this develops sufficiently slowly, compensatory mechanisms, plus the failure of the heart itself as a pump, result in an increase in venous pressure and congestive heart failure. If on the other hand there is an acute reduction of cardiac output, as is caused by sudden cessation of the heart beat, the effect is to deprive tissues of their oxygen supplies and the syndrome of acute heart failure develops.
Heart failure can be left-sided, right-sided or both left- and right-sided. Left-sided heart failure causes an increase in left ventricular end diastolic pressure, mean left atrial pressure and pulmonary venous pressure. Depending upon the magnitude and rate of the increase in pressure, left-sided heart failure results in interstitial edema in the lungs and, if severe enough, pulmonary edema, dyspnea and death. Right-sided heart failure causes an increase in right ventricular end-diastolic pressure, mean right atrial pressure and jugular venous pressure. Depending upon the magnitude and rate of the increase in pressure, right-sided heart failure results in symmetric venous distension (most readily detected in the jugular veins), an increase in pleural, pericardial and abdominal fluid (ascites), and hepatomegaly.
CIRCUIT FAILURE
In circuit failure the effective blood volume is decreased because of loss of fluid from the vascular system (hypovolemic shock) or by pooling of blood in peripheral vessels and increased capillary permeability (maldistributive shock). The failure of venous return results in incomplete filling of the heart and a reduction in cardiac output, although there is no primary defect in pump performance. The effects of circuit failure are the same as those of chronic (congestive) heart failure in that the supply of nutrients to the tissues and the removal of waste products from the tissues are reduced.
CARDIAC RESERVE AND COMPENSATORY MECHANISMS IN HEART FAILURE
The normal heart has the capacity to increase its output severalfold in response to normal physiological demands created by exercise and to a lesser extent by pregnancy, lactation, digestion and hot ambient temperatures. Collectively, these compensatory responses comprise the cardiac reserve. Similar compensatory responses are utilized by the failing heart in an attempt to maintain cardiac output. Cardiac reserve and its response in heart failure have not been studied extensively in large domestic animals and consequently its description must rely heavily on studies on cardiac failure in small domestic animals and studies of the effect of exercise on cardiovascular performance in the horse.1-6 Clinical observations on cardiac insufficiency and cardiac failure in large animals suggest that the processes are very similar to those in small animals and humans.
The major mechanisms whereby the blood flow to an organ can be increased are:
• Redistribution of blood flow to vital organs, or organs with particularly high metabolic requirements.
All of these mechanisms act synergistically and are interrelated. Heart rate and stroke volume are the determinants of cardiac output (cardiac output is the product of heart rate and stroke volume).
CARDIAC RESERVE AND HEART RATE
There is a great deal of cardiac reserve in the heart rate, and an elevation of heart rate alone is a significant factor in increasing cardiac output in the exercising horse. There is a limitation to heart rate reserve because with increasing heart rates there is a decrease in diastolic filling time, and stroke volume falls at excessive heart rates. Effective heart rate reserve can be increased with exercise training, and maximum heart rate in trained exercising horses is six to seven times resting values.7 This large increase in heart rate reflects the metabolic scope of trained horses. In contrast, cattle can only increase their heart rate to two to four times their resting values. An increase in heart rate is also used to maintain cardiac output by the failing heart. With cardiac insufficiency in the horse and the cow it is rare for the heart rate to exceed 120/min, and rates higher than this are frequently due to tachyarrhythmias that require immediate treatment.
CARDIAC RESERVE AND STROKE VOLUME
Stroke volume is variable and depends upon the amount of shortening that the myocardial fibers can attain when working against arterial pressure. It is determined by an interplay of four factors:
• Ventricular distending or filling pressure (preload)
• Contractility of the myocardium (inotropic state)
• The tension that the ventricular myocardium must develop during contraction and early ejection (afterload)
An increase in ventricular distending pressure (end-diastolic pressure or volume) will increase ventricular end-diastolic fiber length, which, by the Frank–Starling mechanism and stretch-dependent calcium sensitization, will result in increased stroke work and a larger stroke volume. Ventricular distending pressure is influenced by atrial contraction and is greatly augmented by increased venous return associated with exercise and increased sympathetic activity. Contractility is most influenced by adrenergic activity and circulating catecholamines. An increase in stroke volume is achieved primarily by an increase in the ejection fraction and a reduction in the end-systolic volume but can also be achieved by a decrease in afterload, which is primarily a function of aortic or pulmonary impedance (the resistance and reactance of the vasculature to ejection).
CARDIAC RESERVE AND MIXED VENOUS OXYGEN TENSION
In normal animals at rest, the oxygen tension of mixed venous blood is above 40 mmHg (5.3 kPa), which represents a considerable reserve. Increased extraction of oxygen from the blood by various tissues, with a subsequent decrease in mixed venous oxygen tension and a corresponding increase in arterial venous oxygen difference, occurs during exercise and in pump and circuit failure.4 In uncompensated heart failure, where stroke volume is reduced, the mixed venous oxygen tension falls below 40 mmHg, reaching 15–25 mmHg in severe shock states, and the arterial venous oxygen difference is large. There is also a redistribution of blood flow to vital organs. In the horse the splenic storage capacity for erythrocytes is large and the spleen may contain one-third of the total red cell volume. Maximal emptying of the spleen under adrenergic activity can significantly influence the oxygen-transporting capacity of the blood and, in the horse, the splenic reservoir contributes significantly to cardiovascular reserve.
CARDIAC RESERVE AND AUTONOMIC NERVE ACTIVITY
It is evident that increased sympathetic nerve activity also plays a significant role in compensating for the failing ventricle, but one that is not readily determined clinically. An increase in sympathetic activity acts to augment cardiac output by increasing the heart rate, by improving the contractility of the myocardium and by augmenting venous return to the heart. Autonomic nerve activity also regulates blood flow to more essential organs even when faced with insufficient cardiac output.
CARDIAC RESERVE IN CARDIAC INSUFFICIENCY
In cardiac insufficiency the principal defect is in the contractile state of the myocardium, and ventricular performance at any given end-diastolic volume or pressure is diminished. In early failure, cardiac output may still be maintained in the normal range by an increase in filling pressure and, through utilization of stretch-dependent calcium sensitization and the Frank–Starling principle, the ventricles can eject a normal stroke volume despite the depression in contractility. Thus, early in the course of cardiac failure, the end-diastolic pressure may be elevated only during periods with heavy demands on the heart, such as during exercise. However, as myocardial function becomes increasingly impaired, this mechanism is increasingly utilized for lesser work demands until end-diastolic pressure is elevated even at rest or with normal activity.
Ventricular filling pressure is augmented by increased venous return associated with contraction of the venous capacitance vessels under increased sympathetic tone, and by an increase in blood volume as the result of salt and water retention by the kidney. Decreased renal perfusion results in the release of renin by the juxtaglomerular cells in the kidney and the activation of the renin–angiotensin– aldosterone system. Renin causes the conversion of angiotensinogen to angiotensin I and angiotensin I in turn is converted to angiotensin II in the lungs. Angiotensin II is a powerful vasoconstrictor and promotes the effect of norepinephrine. Angiotensin II also stimulates the release of aldosterone from the adrenal cortex, which acts to increase sodium retention by the kidney with consequent expansion of the interstitial fluid and blood volumes.
Although the increase in ventricular end-diastolic pressure acts to maintain cardiac output, it is associated with a marked increase in systemic or pulmonary venous pressure, producing secondary effects that result in many of the clinical abnormalities associated with congestive heart failure. Where the contractile state of the heart is markedly reduced, the increased end-diastolic pressure is unable to maintain normal stroke volume, even at normal activity, and cardiac output is reduced even at rest – the state of uncompensated heart failure, which is clinically manifest as pump failure.
MEASUREMENT OF CARDIAC RESERVE
From a clinical standpoint it would be desirable to be able to detect incipient cardiac insufficiency at a very early stage.
A clinical estimation of cardiac reserve based on physical examination is important when a prognosis is to be made for an animal with heart disease. Some of the important criteria used in making this assessment include the heart rate, the intensity of the heart sounds, the size of the heart, the characteristics of the pulse and the tolerance of the animal to exercise. A resting heart rate above normal indicates loss of cardiac reserve. The absolute intensity of the heart sounds suggests the strength of the ventricular contraction, soft sounds suggesting weak contractions and sounds that are louder than normal suggesting cardiac dilatation and possibly hypertrophy, although this is a very crude and insensitive measure. The interpretation of variation in intensity must be modified by recognition of other factors, such as pleural and pericardial effusion, that interfere with audibility of the heart sounds.
Pulse characteristics are of value in determining the cardiac reserve but they are greatly affected by factors other than cardiac activity. An increased amplitude of the pulse occurs when the cardiac stroke volume is increased, but a decreased amplitude may result from reduced venous return as well as from reduced contractile power of cardiac muscle.
Exercise tolerance is an excellent guide to cardiac reserve and the least expensive and most practical method for quantifying cardiovascular reserve. Exercise tolerance is best assessed by measuring the maximum heart rate attained after a standard exercise test, and the speed with which the heart rate returns to normal.1,3,4
1 Flaminio MJBF, et al. Vet Clin North Am Equine Pract. 1996;12:565.
2 El Aguera EI, et al. J Equine Vet Sci. 1995;15:532.
3 Physick-Sheard PW. Vet Clin North Am Equine Pract 1. 1985;2:383.
4 Evans DL, Rose RJ. Pflugers Arch. 1988;411:316.
5 Hinchcliff KW, et al. Am J Physiol. 1990;258:R1177.
CARDIAC ENLARGEMENT
The ratio of heart weight to body weight is greater in athletic animals than in nonathletic animals, and the heart:weight ratio in horses can be modestly increased during training as a result of physiological hypertrophy. Cardiac enlargement is also a compensatory response to persistent increased workloads that are associated with cardiovascular disease. The heart may respond by dilatation, hypertrophy or a combination of both.
Cardiac hypertrophy (concentric hypertrophy) is the usual response to an increased pressure load, and there is hypertrophy of individual fibers with an increase in the number of contractile units (sarcomeres) and an increase in total muscle mass. However, cardiac hypertrophy is usually accompanied by decreased capillary density and increased intercapillary distance and, in states of cardiac insufficiency, coronary blood flow reserve places limitations on this compensatory mechanism.
Cardiac dilatation (eccentric hypertrophy) is the usual response to an increased volume load and probably results from fiber rearrangement. Contractions occurring in a dilated chamber can eject a larger volume of blood per unit of myocardial shortening. However, the limitation to this compensatory mechanism is evident in the law of Laplace, which shows that in the dilated chamber greater myocardial wall tension is required to produce an equivalent elevation of intrachamber pressure during ejection.
The significance of finding cardiac enlargement on clinical examination is that it indicates the presence of a significant volume or flow load on the heart, or the presence of myocardial disease and a reduction of cardiac reserve. The detection of cardiac enlargement on physical examination is aided by careful auscultation of the heart, palpation of the apex beat and rarely by thoracic percussion. A palpable and audible increase in the apex beat and area of audibility, backward displacement of the apex beat, increased visibility of the cardiac impulse at the base of the neck and behind the elbow and increased area for the cardiac shadow during thoracic percussion are all suggestive of cardiac enlargement. Care must be taken that the abnormalities observed are not due to displacement of the heart by a space-occupying lesion of the thorax such as thymic lymphosarcoma, or to collapse of the ventral part of the lung and withdrawal of lung tissue from the costal aspects of the heart. Echocardiography should be used to quantify the magnitude of the enlargement whenever the results of physical examination suspect the presence of cardiac enlargement.
Manifestations of circulatory failure
The manifestations of circulatory failure depend on the rapidity of its onset, the magnitude of its severity, and on its duration. Chronic (congestive) heart failure and acute heart failure are discussed below.
CHRONIC (CONGESTIVE) HEART FAILURE
Etiology Diseases of the endocardium, myocardium and pericardium that interfere with the flow of blood into or away from the heart, or that impair myocardial function, may result in congestive heart failure
Clinical findings Generalized venous distension and edema in right-sided failure. Pulmonary edema and respiratory distress in left-sided failure
Clinical pathology Increased serum concentration of cardiac troponin I, a cardiac-specific enzyme
Necropsy findings Subcutaneous edema, ascites, hydrothorax and hydropericardium; enlargement and engorgement of the liver with right-sided failure. Pulmonary edema with left-sided failure
Diagnostic confirmation Clinical
Treatment Treatment of specific cause, often unsuccessful. Diuretics, salt restriction, minimize activity, possibly digoxin
ETIOLOGY
Causes of chronic (congestive) heart failure can be broadly characterized as follows.
Pressure load
Pressure loads occur with lesions that produce an obstruction to outflow such as aortic or pulmonary valve stenosis, where the heart is required to perform more work to eject an equivalent amount of blood. Pressure loads are not necessarily associated with lesions in the heart. For example, pulmonary hypertension, such as occurs in high altitude disease of cattle due to an increase in pulmonary vascular resistance, may result in cardiac insufficiency. In general, the left ventricle can tolerate a pressure load to a much greater extent without overt signs of cardiac insufficiency than the right ventricle.
Volume load
Volume loads (flow loads) occur commonly with both acquired and congenital heart defects. In aortic valve insufficiency and mitral valve insufficiency the volume of blood delivered to the tissues does not differ significantly from normal. However, in order to achieve a normal cardiac output, the forward stroke volume of the ventricle is markedly increased and the heart is much more inefficient for the same amount of effective work. In a similar manner a patent ductus arteriosus or an interventricular septal defect with a large left-to-right shunt of blood can place a considerable flow load on the left ventricle. In general, the right ventricle is more capable of sustaining a flow load than the left ventricle.
Pumping defects (systolic failure)
Cardiac insufficiency may occur without any increase in workload if there is a primary weakness in the myocardium or defect in its rhythmic and coordinated contraction. Myocarditis, cardiomyopathy and neoplasms of the heart, especially bovine viral leukosis lesions of the right atrium, are the common causes. Arrhythmias are a rare cause of congestive heart failure but a common cause of acute heart failure.
Filling defects (diastolic failure)
Pericardial diseases such as pericarditis and pericardial tamponade can result in cardiac insufficiency by interfering with diastolic filling. Filling of the ventricle is determined by the complex interaction of a number of factors, including mean circulatory filling pressure, mean right atrial pressure, stiffness of the ventricular chamber (which is determined, in part, by mean arterial blood pressure) and the pressure gradient across the ventricular wall. The latter is markedly affected by increases in pericardial fluid pressure that are present in pericarditis and pericardial tamponade.
PATHOGENESIS
Cardiac reserve and compensatory mechanisms in heart failure are described in the preceding section. In the early stages of cardiac disease circulatory equilibrium may be maintained. However, cardiac reserve is reduced and the animal is not able to cope with circulatory emergencies as well as a normal animal. This is the stage of waning cardiac reserve in which the animal is comparatively normal at rest but is incapable of performing exercise – the phase of poor exercise tolerance – or responding appropriately to a physiological stressor such as late gestation or being housed in hot ambient temperatures. Congestive heart failure develops when these compensatory mechanisms reach their physiological limit and the heart is unable to cope with the circulatory requirement at rest.
Failure may manifest as primarily being right-sided, left-sided or both left- and right-sided. Many of the clinical signs that appear during the development of cardiac insufficiency, as well as those associated with decompensated heart failure, are the consequence of congestion or edema due to increased venous hydrostatic pressure. A decreased cardiac output also contributes to the clinical signs by the production of tissue hypoxia.
Right-sided congestive heart failure
Venous congestion is manifest in the systemic circulation. The increase in mean right atrial pressure increases the mean capillary pressure and the net force for filtration of fluid across the capillary bed is therefore greatly increased. This results in the production of edema in dependent subcutaneous body areas and in body cavities. In the kidneys the increase in hydrostatic pressure is offset by the reduced flow of blood and urine output is reduced. The increased back pressure to the glomerulus causes increased permeability and escape of plasma protein into the urine. Venous congestion in the portal system is an inevitable sequel of hepatic congestion and is accompanied by impaired digestion and absorption and terminally by diarrhea.
Left-sided congestive heart failure
Increased pulmonary venous pressure results in venous congestion, decreased compliance of the lung and an increase in respiratory rate, an increase in the work of breathing, and exercise intolerance. Similarly, bronchial capillary congestion and edema result in encroachment on airways and a decrease in ventilatory efficiency. Where venous hydrostatic pressure is exceptionally high, the net force for filtration of fluid across the pulmonary capillary bed is greatly increased. This can result in pulmonary edema, with the presence of fluid around the septal vessels and in the alveolar spaces accompanied by marked impairment of gas exchange. The development of clinically detectable pulmonary edema depends to some extent on the rapidity of the onset of cardiac failure. In chronic failure syndromes, the development of a capacious lymphatic drainage system limits the occurrence of clinical pulmonary edema and, in large animals, pulmonary edema is usually limited to acute heart failure where there is a relatively sudden onset of a volume load on the left ventricle.
CLINICAL FINDINGS
The specific findings on auscultation and other examinations are described under the specific causes of congestive cardiac failure.
In the very early stages when cardiac reserve is reduced but decompensation has not yet occurred there is respiratory distress on light exertion. The time required for return to the normal respiratory and pulse rates is prolonged. In affected animals there may be evidence of cardiac enlargement and the resting heart rate is moderately increased. There may be a loss of body weight.
Right-sided congestive heart failure
The heart rate is increased and there is venous distension and subcutaneous edema. The superficial veins are engorged. In ruminants there is subcutaneous edema occurring in the brisket region, under the jaw and along the ventral midline, and ascites as indicated by the presence of an abdominal fluid wave on ballottement with palpation and less frequently by the presence of abdominal distension with a pear-shaped abdomen. Ascites needs to be differentiated from other causes of abdominal distension, and the detection by palpation per rectum of viscera floating in a fluid medium and the presence of a fluid wave on abdominal ballottement are highly suggestive of ascites. Care must be taken to differentiate ascites from uroabdomen and hydrops conditions of the uterus. Hydrothorax and hydropericardium may also be clinically detected in animals with ascites. In horses, edema is initially more prominent in the pectoral region between the front limbs, the ventral abdominal wall, the prepuce and the limbs. Ruminants and camelids do not get edema in their legs in right-sided heart failure because their comparatively thicker skin acts as an antigravity suit (‘G’ suit), minimizing the extent of hydrostatic pooling of blood in the limbs.
The liver is enlarged and, in cattle, may be palpable, protruding beyond the right costal arch with a thickened and rounded edge. In both horses and cattle liver enlargement may be detected by ultrasound examination. The respiration is deeper than normal and the rate may be slightly increased. Urine flow is usually reduced and the urine is concentrated and contains a small amount of protein. The feces are usually normal at first but in the late stages diarrhea may be evident. Body weight may increase because of edema but the appetite is poor and condition is lost rapidly. Epistaxis may occur in the horse but is rare in other species. The attitude and behavior of the animal is one of listlessness and depression; exercise is undertaken reluctantly and the gait is shuffling and staggery through weakness.
Left-sided congestive heart failure
The heart rate is increased and there is an increase in the rate and depth of respiration at rest, with cough, the presence of crackles (discontinuous sounds) at the base of the lungs and increased dullness on percussion of the ventral borders of the lungs. Terminally there is severe dyspnea and cyanosis.
The prognosis in congestive heart failure varies to a certain extent with the cause but in most cases in large animals it is poor to grave. The possibility of recovery exists with an arrhythmia, pericardial tamponade or pericarditis, but when the epicardium, myocardium or endocardium are involved complete recovery rarely if ever occurs, although the animal may survive with a permanently reduced cardiac reserve. Uncomplicated defects of rhythm occur commonly in the horse and these defects are more compatible with life than are extensive anatomical lesions.
CLINICAL PATHOLOGY
Clinicopathological examinations are usually of value only in differentiating the causes of congestive heart failure and in differentiating from other diseases. Aspiration of fluid from accumulations in any of the cavities may be thought necessary if the origin of the fluid is in doubt.1 The fluid is an edematous transudate except in pericardial tamponade (serosanguinous) or pericarditis (effusion) when it may be septic or nonseptic.2 In most cases protein is present in large amounts because of leakage of plasma from damaged capillary walls. Proteinuria is often present because of pressure-induced damage to the glomerulus. The serum concentration of cardiac tropinin I provides an excellent cardiac biomarker in large animals, providing a sensitive and persistent indicator of cardiac injury.3
NECROPSY FINDINGS
Lesions characteristic of the specific cause are present and may comprise abnormalities of the endocardium, myocardium, pericardium, lungs or large vessels. Space-occupying lesions of the thorax may constrict the cranial vena cava and interfere with venous return. The lesions that occur in all cases of congestive heart failure, irrespective of cause, are: pulmonary congestion and edema if the failure is left-sided; anasarca, ascites, hydrothorax and hydropericardium and enlargement and engorgement of the liver, with a ‘nutmeg’ pattern of congested red centers of liver lobules surrounded by paler fatty peripheral regions, if the failure is right-sided. It is important to characterize the heart failure as being left-sided, right-sided or both left- and right-sided at necropsy, because this information will help in prioritizing the likely cause.
TREATMENT
The treatment of animals with clinical signs of congestive heart failure due to pericarditis or pericardial tamponade focuses on removing the pericardial fluid and preventing its return. In animals with pump failure, the treatment of congestive heart failure initially focuses on the reduction of the effects of increased preload by administering diuretic agents and restricting sodium intake, reducing the demands on cardiac output by restricting activity, and improving contractility by the administration of positive inotropic agents such as cardiac glycosides.
Diuretics
Diuretic treatment, furosemide, acetazolamide or chlorothiazide, is an important component of treatment in that it mobilizes and eliminates excess body fluids. Furosemide is most commonly used because it is the most potent diuretic available, is inexpensive and pharmacokinetic parameters have been determined for large animals. Furosemide should be administered at an initial intravenous dose of 0.25–1.0 mg/kg for horses and 2.5–5.0 mg/kg for cattle for the treatment of congestive heart failure,4,5 Multiple doses of furosemide will induce a hypokalemic, hypochloremic metabolic alkalosis, so it is important to monitor serum potassium and chloride concentrations during treatment. Access to free salt should be stopped, although it is usually impractical to formulate a salt-restricted diet.
Stall rest
Stall rest in a thermoneutral environment is also an important treatment requirement. Parturition may be electively induced in late gestation in order to prevent in-utero fetal hypoxia and abortion, and to decrease the additional demand placed by placental blood flow on the cardiac output.
Cardiac glycosides
Digoxin is the most commonly used cardiac glycoside. In horses it can be administered either intravenously or orally but in ruminants it must be given intravenously or after induction of esophageal groove closure because digoxin is destroyed in the rumen. Digoxin should not be given intramuscularly in any species as it causes severe muscular necrosis and this is also reflected in erratic plasma digoxin concentrations following intramuscular administration. Treatment with digoxin results in an increase in cardiac contractility and a decrease in heart rate with increased myocardial oxygen consumption, increased cardiac output and a decrease in cardiac size.4 The improvement in cardiac output promotes diuresis and the reduction and elimination of edema.
The half-life of digoxin in the horse is 17–23 hours.6,7 and a plasma therapeutic range for digoxin of 0.5–2.0 ng/mL has been suggested.6 Pharmacokinetic studies suggest that therapeutic but nontoxic plasma concentrations of digoxin in the horse will be achieved by an initial intravenous loading dose of 1.0–1.5 mg/100 kg followed by a maintenance dose of 0.5–0.75 mg/100 kg every 24 hours.7 In the horse the bioavailability of powdered digoxin given orally is low, being less than 20% of the administered dose. An oral loading dose of 7 mg/100 kg, followed by a daily oral maintenance dose of 3.5 mg/100 kg is suggested by pharmacokinetic studies.6
The half-life of digoxin in cattle is 5.5–7.2 hours,8,9 requiring more frequent dosing than in horses, and an initial intravenous loading dose of 2.2 mg/100 kg followed by 0.34 mg/100 kg every 4 hours has been suggested.8 An alternative is to give digoxin as a continual infusion at 0.086 mg/100 kg.5 There is no established dose for digoxin administration in sheep but the half-life is similar to that in cattle.10
No dosing regimen is absolute and the dose may need adjustment based on clinical response, evidence of toxicity, or by measuring the plasma digoxin concentration. Dose rates other than those above have been used successfully.11,12 Toxicity with digoxin treatment is reported and may occur because the clearance of digoxin in some animals with congestive heart failure differs from that of normal animals on which the suggested doses have been based.13
If treated animals are not eating, the daily oral administration of KCl (cattle 100 g, horses 30 g) is recommended5 and it is recommended that serum potassium concentrations be monitored because the toxic effects of digoxin are impacted by the serum potassium concentration. Because of the necessity for frequent dosing in cattle and the ineffectiveness of oral treatment, digoxin therapy has major limitations in ruminants, especially since the primary pathology that leads to congestive heart failure in cattle is commonly not correctable. Unless myocardial damage is transient, administration of the digoxin in all species will probably have to be continued for life, and this is rarely practical.
Muir MW, McGuirk SM. Pharmacology and pharmacokinetics of drugs to treat cardiac disease in horses. Vet Clin North Am Equine Pract. 1985;1:335-352.
Muir MW, McGuirk S. Cardiovascular drugs. Their pharmacology and use in horses. Vet Clin North Am Equine Pract. 1987;3:37-57.
McGuirk SM. Treatment of cardiovascular disease in cattle. Vet Clin North Am Food Anim Pract. 1991;7:729-746.
1 Milne MH, et al. Vet Rec. 2001;148:341.
2 Jesty SA, et al. J Am Vet Med Assoc. 2005;226:1555.
3 Phillips W, et al. J Vet Intern Med. 2003;17:597.
4 Muir MW, McGuirk S. Vet Clin North Am Equine Pract. 1987;3:37.
5 McGuirk SM. Vet Clin North Am Food Anim Pract. 1991;7:729.
6 Button C, et al. Am J Vet Res. 1980;41:1388.
7 Brumbaugh GW, et al. J Vet Pharmacol Ther. 1983;6:163.
8 Koritz GD, et al. J Vet Pharmacol Ther. 1983;6:141.
9 Garry FB, Klee W. Tierarztl Umsch. 1990;45:750.
10 Dix LP, et al. Am J Vet Res. 1985;46:470.
11 Staudacher G. Berl Munch Tierarztl Wochenschr. 1989;102:1.
12 Stewart GA, et al. Aust Vet J. 1990;67:187.
13 Peardon EG, et al. Compend Contin Educ Pract Vet. 1987;2(1):1.
ACUTE HEART FAILURE
ETIOLOGY
Acute heart failure can occur when there is a severe defect in filling, when there is failure of the heart as a pump, either due to severe tachycardia, bradycardia or arrhythmia, and where there is a sudden increase in workload. The sudden occurrence of tachyarrhythmias in association with excitement and severe enough to cause acute heart failure presumably results from the exacerbating influence of catecholamines.1,2 These are released in association with episodes of excitement and act to heighten the discharge potential of ectopic excitatory foci associated with myocardial disease.
Etiology Sudden onset of a severe arrhythmia, rupture of a heart valve or vessel, pericardial tamponade
Clinical findings Sudden loss of consciousness, falling with or without convulsions, severe pallor of the mucosae and either death or complete recovery from the episode
Clinical pathology Increased serum cardiac troponin I concentrations, but clinical course usually too short for examination
Diagnostic confirmation Clinical
Necropsy findings Pulmonary congestion and edema. Findings related to specific cause
Acute heart failure can also occur in the absence of primary cardiac disease under the influence of pharmacological agents that affect cardiac conduction. These are associated with the ingestion of certain poisonous plants.
The many causes of acute heart failure are listed in greater detail under myocardial diseases. Some examples are as follows:
Arrhythmias and cardiac arrest may occur during the induction of anesthesia with barbiturates in the horse and may also occur without premonitory signs in horses under halothane anesthesia.
PATHOGENESIS
With excessive tachycardia the diastolic period is so short that filling of the ventricles is impaired and cardiac output is grossly reduced. In ventricular fibrillation no coordinated contractions occur and no blood is ejected from the heart. The cardiac output is also seriously reduced when the heart rate slows to beyond a critical point because cardiac output is the product of heart rate and stroke volume, and stroke volume cannot be markedly increased. In all these circumstances there is a precipitate fall in cardiac output and a severe degree of tissue ischemia. In peracute cases the most sensitive organ, the brain, is affected first and the clinical signs are principally neurological. Pallor is also a prominent sign in acute heart failure because of the reduction in blood flow.
In less acute cases respiratory distress is more obvious because of pulmonary edema and although these can be classified as acute heart failure they are more accurately described as acute congestive heart failure.
CLINICAL FINDINGS
The acute syndrome may occur while the animal is at rest but commonly occurs during periods of excitement or activity. The animal usually shows dyspnea, staggering and falling, and death often follows within seconds or minutes of the first appearance of signs. There is marked pallor of the mucosae. Although clonic convulsions may occur they are never severe and consist mainly of sporadic incoordinate movements of the limbs. Death is usually preceded by deep, asphyxial gasps. If there is time for physical examination, weakness or absence of a palpable pulse and bradycardia, tachycardia or absence of heart sounds are observed. The specific findings in the heart and vascular system depend upon the arrhythmia and are detailed in the section on arrhythmias later in this chapter.
Horses with sudden onset of tachyarrhythmias due to atrial fibrillation or multiple ventricular extrasystoles, or with rupture of the aortic or mitral valve chordae show a syndrome where sudden onset of respiratory distress is the prominent manifestation. However, examination of the heart will allow a diagnosis of the underlying cause.
Acute heart failure is the cause of death in a significant proportion of horses that die suddenly and unexpectedly during training or racing.3 The diagnosis is based primarily on the findings of significant pulmonary hemorrhage and edema, although myocardial pathology is absent in most cases. Severe arrhythmic disturbances secondary to pre-existing myocardial injury and the concurrent presence of catecholamines, hyperkalemia and metabolic acidosis are likely causes.
CLINICAL PATHOLOGY
In general, there is insufficient time available in which to conduct laboratory tests before the animal dies. The demonstration of elevated serum troponin I concentrations, a sensitive and specific marker of myocardial damage, strongly supports the presence of myocardial disease. Laboratory tests may also be used to elucidate the specific etiology.
NECROPSY FINDINGS
In typical acute cases engorgement of visceral veins may be present if the attack has lasted for a few minutes but there may be no gross lesions characteristic of acute heart failure. Microscopic examination may show evidence of pulmonary congestion and early pulmonary edema. In more prolonged cases, venous engorgement with pulmonary congestion and edema are evident along with hydrothorax but these are more accurately described as acute congestive heart failure. The primary cause may be evidenced by macroscopic or microscopic lesions of the myocardium.
Acute heart failure should always be a major consideration as a cause of sudden and unexpected death in large animals, especially when death is associated with exertion or excitement. Acute heart failure may be mistaken for primary disease of the nervous system but is characterized by excessive bradycardia or tachycardia, pallor of mucosae, weakness or absence of the pulse and the mildness of the convulsions. Epilepsy and narcolepsy are usually transient and repetitive and have a characteristic pattern of development.
TREATMENT
Treatment of acute heart failure is not usually possible or practical in large animals because of the short course of the disease. Deaths due to sudden cardiac arrest or ventricular fibrillation while under anesthesia can be avoided to a limited extent in animals by external or internal cardiac compression or electrical conversion–stimulation but these techniques are generally restricted to sophisticated institutional surgical units. Also, the electrical energy required for defibrillation of animals larger than a sheep or goat is beyond the capabilities of conventional defibrillators unless the paddles are placed directly across the pericardium or transvenous electrodes are used. Intracardiac injections of very small doses of epinephrine in conjunction with external cardiac compression by jumping up and down on the thorax with the knees can be tried, with occasional success.
Special examination of the cardiovascular system
The more commonly used techniques of examination of the heart and pulse are described in Chapter 1. A more detailed clinical examination of the system that gives greater attention to nuances of location and intensity of heart sounds and arterial and venous pulse characteristics is conducted whenever cardiovascular disease is suspect.
Special techniques of examination are also available which may be of value in some cases. With the exception of jugular venous pressure measurement, assessment of exercise intolerance, electrocardiography and indirect methods for measuring arterial blood pressure, many of these techniques have limited application in general practice as they require sophisticated and expensive equipment. The use of specialized diagnostic equipment is generally confined to teaching hospitals and investigative units.
PHYSICAL EXAMINATION
In the examination of animals suspected to have heart disease, it is important to determine the rate, rhythm and intensity of the individual heart sounds and the rate, rhythm and amplitude of the arterial pulse, examine for the presence of venous pulsation at the jugular inlet, and identify the point of maximal intensity and timing of murmurs within the cardiac cycle.
HEART SOUNDS
In the horse it is not uncommon to hear four heart sounds on auscultation, whereas two to three heart sounds are heard in ruminants and camelids.
First heart sound
The first heart sound (S1) signals the onset of ventricular systole, is synchronous with the apex beat and is temporally associated with closure of the mitral and tricuspid valves. The area for maximal audibility of the mitral valve in the horse is on the left fifth intercostal space, at a level midway between a horizontal line drawn through the point of the shoulder and one drawn at the sternum at the caudal edge of the triceps muscle. With cattle, sheep, goats and swine the sound is located at a similar level but at the fourth intercostal space. The area for maximal audibility of the tricuspid valve is on the right side of the chest slightly ventral to the equivalent level for the mitral valve and at the fourth intercostal space in the horse; and at the level of the costochondral junction at the third intercostal space for the other species.
Second heart sound
The second heart sound (S2) is associated with aortic and pulmonic valve closure and is synchronous with the end of systole and the beginning of cardiac diastole. The aortic component is most audible just ventral to a horizontal line drawn through the point of the shoulder and in the left fourth intercostal space in horses and the left third in the other species. The pulmonic component is most audible ventral and anterior to the aortic valve area in the left third intercostal space in horses and the left second or third intercostal space close to the costochondral junction in the other species. These two components of the second heart sound have the same temporal occurrence on auscultation but tonal differences can frequently be detected at the two areas of maximal audibility. Splitting of the second sound in the horse can be detected on phonocardiographic examination but cannot be detected on auscultation and there is no respiratory-associated splitting, as occurs with some other species.
Third heart sound
The third heart sound (S3) is associated with rapid filling of the ventricle in early diastole and is heard as a dull thudding sound occurring immediately after the second sound. It is usually most audible on the left side just posterior to the area of maximal audibility of the first heart sound. However it is frequently heard over the base and also over the area of cardiac auscultation on the right side. Phonocardiographically there are two components to this heart sound but these are not usually detectable on clinical auscultation.
The third heart sound is very common in horses and can be detected in the majority of fit racing animals. It is more audible at heart rates slightly elevated above resting normal. The third heart sound is very common in slightly excited cattle (heart rates 80–100 beats/min) but becomes more difficult to hear when the heart rate exceeds 100 beats/min.
Fourth heart sound
The fourth heart sound (S4) is associated with atrial contraction. It is also called the ‘a’ sound. It occurs immediately before the first heart sound and is a soft sound most audible over the base of the heart on the left- and right-hand side. It is also common in horses but its clear separation from the first heart sound is dependent upon the length of the P–R interval, which varies between horses. At resting heart rates the S4 sound is detectable on clinical examination in at least 60% of horses.
The interval between the S4 and S1 frequently varies in the same horse at rest in association with variation in the P–Q interval and results in a clear separation in some beats with slurring of the two sounds together in other beats. The fourth heart sound or a split S1 is also commonly heard in young cattle, but phonocardiographic studies have not been undertaken.
Sequence of heart sounds
The sequence of heart sound occurrence is thus 4–1–2–3. The intensity of the third and fourth sounds is less than that of the first and second and the complex can be described as du LUBB DUP boo. In some horses, the third or fourth sound may be inaudible so that 1–2, 4–1–2 and 1–2–3 variations occur. The name gallop rhythm is frequently applied when these extra sounds occur. Gallop rhythms also occur in cattle and may be due to the occurrence of a fourth or third sound or to true splitting of the components of the first heart sound. In sheep, goats and pigs only two heart sounds are normally heard. The occurrence of a third or fourth heart sound in horses and cattle is not an indication of cardiovascular abnormality, as it is in other species.
Variation in heart sound intensity
Change in the intensity of the generation of sound by the heart or change in the transmission of the sounds between the heart and the stethoscope can result in variation in the intensity of heart sounds normally heard on auscultation.
• A decrease in the intensity of heart sound generation occurs in disease where there is poor venous return and decreased strength of cardiac contractility, such as in terminal heart failure, in hypocalcemia in cattle or in circulatory failure in all species
• Conversely the intensity of the heart sounds may increase with anemia, cardiac hypertrophy and metabolic diseases such as hypomagnesemia. However, the intensity of the heart sounds is most often increased by sympathetic activation as a result of exercise, fear and excitement.
Muffling of the heart sounds suggests an increase in tissue and tissue interfaces between the heart and the stethoscope. This can be due to a shift in the heart due to displacement by a mass, changes in the pericardium (increased fluid or fibrous tissue), changes in the pleural space or increased subcutaneous fat. Heart sounds are detectable by auscultation on the left side in animals of all condition scores but heart sounds may become inaudible on the right side where the condition score approaches 5/5.
Heart rate
The relative temporal occurrence and the intensity of the third and fourth heart sounds changes with heart rate. At moderately elevated heart rates the third heart sound becomes more audible. At faster heart rates the third sound may merge and sum with the fourth sound or the fourth sound may merge with the first sound if the P–R interval decreases. During periods of a rapid change in heart rate, such as during the increase in rate that occurs following sudden noise or similar stimuli in excitable horses or the subsequent decrease in rate, the variation in the occurrence and the intensity of the third and fourth sound coupled with the variation in intensity of the first and second sound during this change can give the impression of a gross arrhythmia. Such impressions should be ignored if they occur only at times of rapid change of rate that is obviously induced by external influences and if there is no arrhythmia at the resting rate or the intervening stable elevated rate. Examination of the pulse during these periods of rapid change is also of value.
Variations in the intensity of the individual heart sounds or complete absence of some of them can occur in conduction disturbances and arrhythmic heart disease and can provide valuable clinical information. In several of these disturbances there is variation in the intensity of the first and third heart sounds associated with variation in the time of the preceding diastolic period and variations in diastolic filling. The intensity of the first heart sound may also vary with variations in the P–R interval or where there is complete atrioventricular dissociation. In several of the arrhythmias there is absence of one or more of the heart sounds. These findings are detailed below under the specific abnormalities.
EXAMINATION OF THE ARTERIAL PULSE
In arrhythmic heart disease the arterial pulse should be examined in more detail than that applied during routine clinical examination.
Pulse rate
The pulse rate should be examined over a period to determine if there is any sudden change in rate such as can occur with a shift in pacemaker to an irritable myocardial focus. At some stage during the examination of animals with tachyarrhythmias the heart rate and pulse rate should be taken synchronously to determine the presence of a pulse deficit (auscultation of S1 but a weak or absent S2 accompanied by a weak or absent pulse). A convenient artery for this purpose is located on the posterior medial aspect of the radius and carpus in the horse and cow. However, the best artery to determine the pulse rate, rhythm and amplitude is the descending aorta; this artery should be palpated during rectal examination in horses and cattle.
Pulse rhythm
Pulse rhythm is carefully examined. When a ‘dropped pulse’ or arrhythmia is detectable in the pulse the basic underlying rhythm should be established in order to determine if the heart is under regular pacemaker influence. This is best done by mentally or physically tapping out the basic rhythm of the heart and continuing this rhythm when irregularity occurs. With conditions such as second-degree heart block where there is a basic underlying rhythm initiated by the sinoatrial node, it is possible to tap through the irregularity and re-establish synchrony with the pulse. However, in conditions such as atrial fibrillation where there is no regular pacemaker it is not possible to establish any basic rhythm. This examination of rhythm can alternatively be conducted by auscultation and allows an immediate categorization of the arrhythmia into one of the two basic group, those superimposed on a regular pacemaker influence (occasionally irregular) and those in which there is no regular pacemaker (irregularly irregular).
Amplitude
The amplitude of the pulse should also be carefully examined. Variations in pulse amplitude are associated with those arrhythmias that produce a variation in diastolic filling period within the heart. The extreme of this is a pulse deficit (decrease in intensity or absence of a pulse associated with heart sounds).
EXAMINATION OF THE JUGULAR VEIN
In the normal adult horse and cow, the jugular vein will be distended with blood some 5–8 cm above the level of the base of the heart when the animal is standing with its head in a normal, nonfeeding, alert position. There is a rapid but minor fall in the level of jugular distension associated with the fall of blood into the ventricle during the period of rapid filling during ventricular diastole followed by a slower rise in the level of jugular filling to its original point. Superimposed on this, and immediately preceding the fall, is a small wave or retrograde distension associated with atrial contraction (‘a’ wave) and a second smaller retrograde wave (‘c’ wave) associated with bulging of the atrial ventricular valves into the atrium during ventricular systole. These pulsations can be observed in most horses and cattle by careful observation of the jugular vein at its entrance into the thorax and can be timed in conjunction with auscultation of the heart.
Observation of the presence or absence of the atrial ‘a’ wave is an aid in the clinical differentiation of first- and second-degree heart block. Cannon atrial waves occur periodically in complete heart block when atrial contractions occur against a closed atrioventricular valve. An accentuated ‘c’ wave occurs with tricuspid valve insufficiency.
MEASUREMENT OF JUGULAR VENOUS PRESSURE
The jugular veins are symmetrically distended in chronic (congestive) right-sided heart failure. This distension is accompanied by an increased jugular venous pressure that can be subjectively assessed by palpation or objectively determined by measuring jugular venous pressure.
This underutilized technique can be easily and rapidly performed. The equipment required is a 14–16-gauge needle attached to a three-way stopcock. A 20 mL syringe containing heparinized 0.9% NaCl is attached directly opposite the needle, and a flexible rigid wall fluid administration line is attached to the remaining port on the three-way stopcock. The stopcock is turned so that the needle is in the off position, the needle is threaded down the jugular vein towards the heart, the syringe is pushed to fill the first 10 cm of the flexible fluid line with heparinized 0.9% NaCl, and the stopcock is turned so that the syringe is in the off position.1 Blood will flow into the flexible tube and the vertical distance (in cm) between the top of the column of 0.9% NaCl supported by the jugular venous pressure and the point of the shoulder (scapulohumeral joint), which approximates the position of the right atrium,2 is a direct measure of jugular venous pressure.
EXERCISE TOLERANCE
Dyspnea, fatigue and a prolonged elevation in heart rate following exercise are signs suggestive of cardiac insufficiency. Frequently, animals with suspect cardiac disease are exercised in an attempt to elicit these signs and to get an estimate of exercise tolerance.1,2 In most practice situations the assessment of exercise tolerance is subjective. There is obviously a considerable difference in the amount of exercise that a beef bull and a trained racehorse can tolerate under normal conditions, and the amount of exercise given to any one animal is determined by the clinician’s judgment. The rate of fall in heart rate following exercise and the time required to reach resting levels depend upon the severity of the exercise, even in fit horses. Heart rate falls rapidly over the first minute and then more slowly over the ensuing 10–15-minute period.
More objective tests have been developed for the horse, which include evaluation by means of telemetry from horses timed over a measured distance on race tracks3 or the use of a treadmill1,4 to provide a defined amount of exercise. The amount and intensity of exercise can be varied by the speed and incline of the treadmill and by the duration of the exercise period. The treadmill allows the recording of a variety of cardiorespiratory measurements in the exercising horse5,6 and can be used for evaluating the significance of cardiopulmonary disease and for establishing the cause of poor racing performance.
There are many noncardiac causes of exercise intolerance and, in a report on the evaluation of 275 horses, 84% were found to have more than one problem leading to poor athletic performance.7
Criteria for cardiovascular performance in endurance rides are described and the rapidity of heart rate decline following completion of each section of the ride can be used for field assessment of this function.1,8,9
1 Parente EJ. Vet Clin North Am Equine Pract. 1996;12:421.
2 Mitten LA. Vet Clin North Am Equine Pract. 1996;12:473.
3 Gati L, Holmes JR. Equine Vet Educ. 1990;2:28.
4 Scheffer CWJ, et al. Vet Rec. 1995;137:371.
5 Seeherman HJ, Morris EA. Equine Vet J Suppl. 1990;9:20.
6 Evans DL, Rose RJ. Equine Vet J. 1988;20:94.
7 Morris EA, Seeherman HJ. Equine Vet J. 1991;23:169.
ELECTROCARDIOGRAPHY
The electrocardiogram (ECG) provides a record and measure of the time varying potential difference that occurs over the surface of the body as the result of electrical activity within the heart. This is associated with depolarization and repolarization of the myocardium. At any one instant during depolarization and repolarization there are generally several fronts of electrical activity within the heart. However, at the body surface the potential difference is generally the sum of this activity and at any one instant the electrical activity in the heart registers as a single dipole vector that has polarity, magnitude and direction.
The polarity is determined by the charge on the surface of the cells while the magnitude and direction is determined by the mass of muscle being depolarized or repolarized and the sum of the instantaneous vectors. Thus a wave of depolarization or repolarization over a muscle mass such as the atria or the ventricles is presented at the body surface as a sequence of instantaneous vectors with changing magnitude and direction.
THE ELECTROCARDIOGRAPH
The electrocardiograph is used to detect these characters. In simple terms it can be considered as a voltmeter consisting of two input terminals, an amplifier to allow the recording of low input signals and a galvanometer with an attached recording device such as a heated stylus on heat sensitive paper or an ink pen or ink squirter. When a potential difference exists across the input terminals (electrodes), current flows through the coils of the electromagnet suspended between the poles of the permanent magnet to cause a deflection of the recording pen. The electrocardiograph can therefore detect the polarity of the cardiac electrical vectors and by calibration of the machine and appropriate placement of electrodes on the body surface it can detect their magnitude and direction.
Calibration of most electrocardiographs is such that an input of 1 mV produces a 1 cm deflection of the recording pen. Recording speeds are generally 25 or 50 mm/s. In recording an ECG, certain standard electrode positions are used for recording.
• A lead is the recording or circuit between two recording points. Depending upon the wiring within the electrocardiograph the same potential difference across a lead could result in an upward or downward deflection of the recording pen
• In order to allow standard recording and comparison between recordings the polarity of the electrodes for standard leads has been established by convention and the leads are always recorded at these polarities
• The electrodes of a lead are commonly called positive or negative
• A positive electrode in a lead is one that, when electrically positive relative to the other, due to a potential difference between them, yields an upward or positive deflection of the recording pen.
DEPOLARIZATION AND REPOLARIZATION
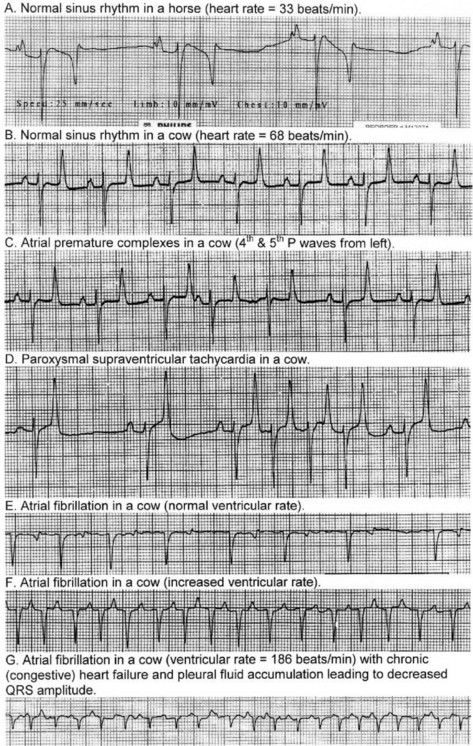
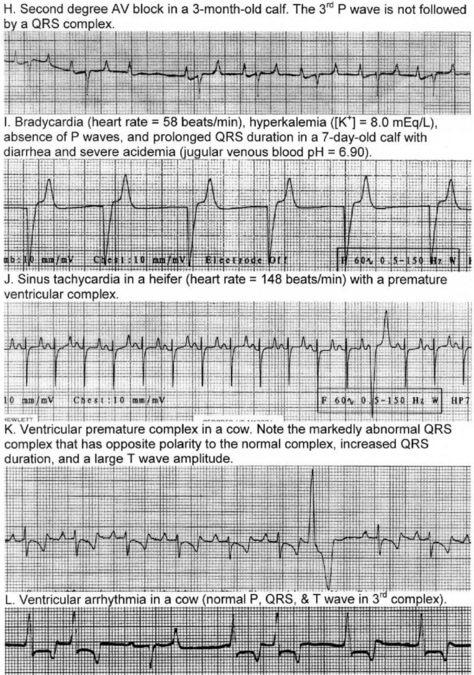
In the normal heart, depolarization and repolarization of the myocardium occurs in a definite pattern and sequence and the electrocardiography can be used to measure and time these events. Thus discharge of the sinoatrial node results in a wave of depolarization over the atria to produce a P wave in the ECG. The delay in conduction at the AV node is registered by no electrical activity at the body surface and an isoelectric P–R interval on the ECG (isoelectric means zero voltage difference between the two leads). Depolarization of the ventricles occurs with several sequential fronts to produce the QRS complex, which is followed by another isoelectric period before repolarization represented by the T wave.
In dogs, cats and humans the electrocardiogram can be used to assess the cardiac rhythm and the size of the cardiac chambers. However, the order of ventricular activation in horses, cattle, sheep and swine differs from that of humans and dogs in that ventricular depolarization is represented by only two fronts of activity. Depolarization of a large proportion of the myocardial mass in large animals is not recognized by the surface electrocardiogram because the Purkinje fibers penetrate much more deeply in these species and depolarization occurs over multiple minor fronts that tend to cancel out, rather than over a large single front as in dogs. For this reason, the detection of chamber enlargement by vector analysis of the electrocardiogram is, in general, not possible in large animals. Consequently, electrocardiography is confined to a simple base– apex lead system to examine for conduction disturbances and arrhythmias, which are detected by measurement of the various waveforms and intervals in the ECG that represent depolarization and repolarization in the heart, and by observation of their absence or abnormality.
LEAD SYSTEMS
The base–apex lead system provides the best method for electrocardiography in large animals, with the only exception being fetal electrocardiography. All other lead systems are clinically superfluous or inferior, or have only a research application.
Traditional lead systems are based on Einthoven’s triangle as used in humans, and the standard bipolar limb leads (I, II and III) and the augmented unipolar limb leads (aVR, aVL, aVF) are commonly used in conjunction with an exploring unipolar chest lead. Variations in the position of the feet may produce changes in ECG waveforms with this lead system and recordings should be taken with the animal standing square or with the left front foot set slightly in advance of the right front foot. This lead system is quite satisfactory for the detection of conduction disturbances and arrhythmic heart disease but is subject to movement artefact. There are, however, deficiencies associated with its use for the detection of change in the magnitude and direction of electrical vectors in the heart of large animals.1,2 Nevertheless, traditional lead systems have been used extensively for this purpose.
Vector-based lead systems. There have been several studies to determine if it is possible to detect changes in cardiac chamber size in large animals. Many of these have examined alternative lead systems, recognizing that the standard limb leads are not particularly suited for detection of vector changes associated with changes in chamber dimensions. The standard limb leads are primarily influenced by vectors in the frontal plane (longitudinal and transverse) whereas early and late forces in the myocardium are significantly directed in the vertical direction. Furthermore, the heart is not electrically equidistant from the electrodes of each lead and distortion of recorded vector loops can result.1,3 A partial correction of these deficiencies can be made by recording a lead using an exploring electrode at the V10 position over the dorsal spinous processes in addition to the standard limb leads. However, for proper representation of the vector changes associated with electrical activity within the heart, completely different electrode placement is required. A number of systems have been proposed. The electrode placement varies and is quite complicated but electrocardiographic studies using these methods are available for horses,3-5 cattle,6,7 pigs8 and sheep.9 In general, a three lead system consisting of leads I, aVf and V10 provides semiorthogonal axes suitable for three-dimensional reconstruction of depolarization and repolarization.
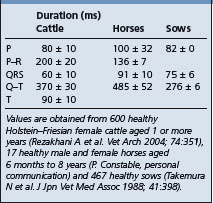
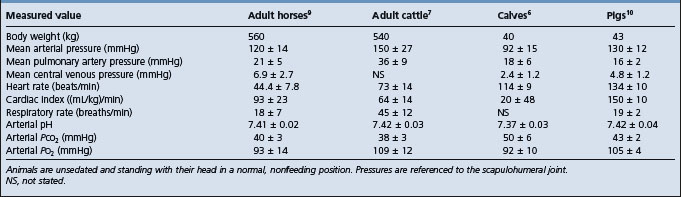
The base–apex lead system is most commonly used as it records the major electrical forces in the heart of large animals with consistently clear and large-amplitude waveforms. Animal movement also has minimal effect on the quality of the ECG. The most commonly used bipolar lead placement in horses and cattle consists of two electrodes, one positive and one negative, in a format called the base–apex lead. The positive electrode of lead I (left arm) is attached to the skin of the left thorax at the fifth intercostal space immediately caudal to the olecranon, and the negative electrode (right arm) is placed on the jugular furrow in the caudal third of the right neck. This is the most common lead placement, although some investigators place the negative electrode on the left side of the neck instead of the right side. With sheep, where wool interferes with placement on the neck, the negative electrode can be placed on the midline of the poll. When using the base–apex lead system, the ground electrode is placed remote from the heart, and the location of the ground is not important. The electrodes are usually placed using alligator clips and a 70% isopropyl alcohol or gel contact, although disposable human stick-on type electrodes can be used in horses after clipping of the skin and cleaning with alcohol before application of the gel. In order to ensure good adherence to the skin, the skin should be shaved and cleaned with alcohol prior to the application of the gel. The ECG is recorded with the animal in a standing position with minimal restraint. Normal values for cattle, horses, and pigs are summarized in Table 8.1.
FETAL ELECTROCARDIOGRAPHY
The fetal ECG may be recorded, and can be of value in determining if the fetus is alive, the presence of a singleton or twins, and as a monitor for fetal distress during difficult or prolonged parturition. A modified bipolar lead system is required, with the RA electrode being placed on the right ventral abdomen and the LA electrode below placed on the ventral midline in front of the udder. The ground lead can be situated anywhere. The bipolar lead should be recorded using increased sensitivity with meticulous attention to obtaining the best electrical connection to the skin. The animal needs to be electrically isolated (standing on a rubber mat) and muscular activity must be minimized.
Fetal electrocardiography has been used in cattle to monitor fetal viability, but the fetal ECG signal is very weak and suffers from interference from the maternal ECG, the electromyogram and motion artifacts caused by gastrointestinal movement.10 For these reasons, the position of the bipolar recording leads on the abdomen should be moved to provide the optimal recording site for each cow. Digital processing of the fetal ECG signal can assist in detection of fetal heart rate10,11 at more than 157 days of gestation. Fetal heart rates for calves tend to decrease with advancing gestation, approximating 140 beats/min from 160–190 days of gestation and 120 beats/minute at 250–280 days of gestation.10
The foal fetal heart rate decreases logarithmically from approximately 110 beats/min at 150 days before term to 75 beats/min near to term.12 Continued monitoring traces may be needed to assess fetal distress. Fetal heart rate and heart rate variability has also been measured as an indicant of hypoxia and fetal distress during parturition in cattle.13,14 Cardiac arrhythmia is common at the time of birth and is believed to result from the transient physiological hypoxemia that occurs during the birth process.15 Following birth and during early growth of the foal there are age-dependent increases in the electrocardiographic intervals and changes in the orientation of the mean electrical axis.16
OTHER USES OF THE ELECTROCARDIOGRAM
• Changes in the electrocardiogram occur with some electrolyte imbalances in large animal species
• There is an approximately linear correlation between the heart-rate-corrected Q–T interval and plasma ionized calcium concentration in cattle, with elongation of the interval in hypocalcemic and shortening in hypercalcemic states
• Decreased amplitude and flattening of the P wave, widening of the QRS complex and an increased symmetry and amplitude of the T wave are seen with hyperkalemia18
• Estimates of heart size of the horse have been made from measurements of the QRS duration on the electrocardiogram and the resultant heart score is used to assess potential racing performance19
• Exercise and postexercise electrocardiograms frequently deliver information additional to that of the resting ECG, and can be recorded by radiotelemetry or Holter monitor systems20-22
• Heart rate variability has received recent interest as a research method to evaluate the relative contributions of sympathetic and parasympathetic tone to the cardiovascular system. Heart rate variability has been assessed in cattle using time domain23 and frequency domain procedures.24
Hamlin RL, Smith CR. Categorization of common domestic mammals based upon their ventricular activation process. Ann NY Acad Sci. 1965;12:195-203.
Kanagawa H, Too K, Kawata K, Ono H. Fetal electrocardiogram in dairy cattle II. Diagnosis for twin pregnancy. Jpn J Vet Res. 1965;13:111-119.
Too K, Kanagawa H, Kawata K. Fetal electrocardiogram in dairy cattle. I Fundamental studies. Jpn J Vet Res. 1965;13:71-83.
Robertson SA. Practical use of the ECG in the horse. In Pract. 1990;12:59-67.
Amory H, et al. Bovine vector cardiography: a comparative study relative to the validity of four tridimensional systems. J Vet Med A. 1992;39:453-469.
1 Nielsen K, Vibe Petersen G. Equine Vet J. 1980;12:81.
2 Fregin GF. Vet Clin North Am Equine Pract. 1985;1:419.
3 Miller PJ, Holmes JR. Res Vet Sci. 1984;36:370.
4 Miller PJ, Holmes JR. Res Vet Sci. 1984;37:334.
5 Deegen E, Reinhard HJ. Dtsch Tierarztl Wochenschr. 1974;81:257.
6 Schultz RA, Pretorius PJ. Am J Vet Res. 1972;39:209.
7 Amory H, et al. J Vet Med A. 1993;40:81.
8 Thielscher HH. Zentralbl Vet Med. 1969;16A:370.
9 Torio R. Small Rumin Res. 1997;24:239.
10 Chen W, et al. Anim Sci J. 2002;73:545.
11 Chen W, et al. Anim Sci J. 2004;75:471.
12 Matsui K, et al. Jpn J Vet Sci. 1985;47:597.
13 Jonker FH, et al. Am J Vet Res. 1996;57:1373.
14 Steffen S, et al. Schweiz Arch Tierheilkd. 1995;137:432.
15 Yamamoto K, et al. Equine Vet J. 1992;23:169.
16 Lombard CW, et al. Equine Vet J. 1984;16:342.
17 Stewart JH, et al. Equine Vet J. 1984;16:332.
18 Spier SJ, et al. J Am Vet Med Assoc. 1990;197:1009.
19 Blakely JA, Blakely AA. N Z Vet J. 1995;43:57.
20 Jacobson LH, Cook CJ. Vet J. 1998;155:205.
21 Scheffer CJW, Sloet van Oldruitenborgh-Oosterbaan M. Vet Q. 1996;18:2.
22 Scheffer CJW, et al. Vet Rec. 1995;137:371.
SERUM CARDIAC TROPONIN I CONCENTRATION
The serum concentration of cardiac tropinin I provides an excellent cardiac biomarker in large animals, providing a sensitive and persistent indicator of cardiac injury.1 Troponin I, T and C are components of the tropomyosin– troponin complex in cardiac and skeletal muscle, with cardiac troponin I and T having different amino-acid sequences at the N-terminal end compared to skeletal muscle tropinin I and T. This means that an immunoassay directed at the N-terminal end will be able to differentiate between cardiac and skeletal muscle isoforms and therefore the site of injury.2 Myocardial tissue from horses, cattle, sheep and pigs has high reactivity for cardiac troponin I when tested using a human immunoassay, and this reactivity is selective for the myocardium, being more than 1000-fold higher in cardiac tissue than in skeletal muscle.1 Cardiac troponin I has greater myocardial selectivity than cardiac troponin T, and is therefore preferred as a biomarker of cardiac injury.1,2 3–8% of cardiac troponin I and T are found in the myocardial cytosol; damage to the myocardial cell membrane causes cytosolic tropinin I and T to escape into the interstitial fluid, thereby increasing serum cardiac troponin I concentrations.
Serum activities of cardiac isoenzymes of creatine kinase (creatine kinase isoenzyme MB (CK-MB)) and lactate dehydrogenase (isoenzymes 1 and 2) have been used in the past as indices of cardiac disease in horses. However, only 1.5% of the total CK activity in the equine heart is attributable to CK-MB (compared to 20% in the human heart);3 therefore CK-MB is an insensitive indicator of cardiac disease in the horse. Isoenzymes of lactate dehydrogenase suffer from a similar lack of specificity for cardiac disease. Cardiac troponin I is the preferred biomarker for detecting and quantifying cardiac disease in animals,4 and healthy horses have cardiac troponin I concentrations below 0.11 ng/mL using the human immunoassay.1,5,6 Healthy neonatal foals have cardiac troponin I concentrations of less than 0.49 ng/mL.7 Healthy cattle have cardiac troponin I concentrations below 0.04 ng/mL.8
1 O’Brien PJ, et al. Clin Chem. 1997;43:12.
2 Cornelisse CJ, et al. J Am Vet Med Assoc. 2000;217:231.
3 Argiroudis SA, et al. Equine Vet J. 1982;14:317.
4 Wallace KB, et al. Toxicol Pathol. 2004;32:106.
5 Smith GW, et al. Am J Vet Res. 2002;63:538.
6 Phillips W, et al. J Vet Intern Med. 2003;17:597.
PHONOCARDIOGRAPHY
Phonocardiography allows the recording and measurement of heart sounds. A special microphone is placed directly over the various areas of the thorax used for heart auscultation and the heart sounds are recorded graphically on moving paper or on an oscilloscope. Prior to recording, the heart sounds are usually passed through high-pass, low-pass or band-pass filters to allow better discrimination of the individual sounds and to allow a crude frequency examination. Phonocardiograms are usually recorded in conjunction with an electrocardiogram and chamber pressure measurements, which permits timing of their occurrence in relationship to the electrical activity within the heart.
Phonocardiograms can provide considerable information on heart sounds additional to that acquired by stethoscopic examination. In the horse, up to 11 sound events can be detected in each cardiac cycle and figures of the occurrence and duration of normal heart sounds in large animals are available.1-3 In conjunction with an electrocardiogram, the phonocardiogram can be used to measure systolic time intervals, which may be altered in congenital and acquired cardiovascular abnormalities.4
Phonocardiograms have been infrequently used for the characterization and timing of murmurs in animals with cardiovascular disease, especially at fast heart rates where simple stethoscopic examination may not allow this. However, phonocardiography has been rarely used as a clinical diagnostic tool, and the widespread availability of echocardiographs make the clinical application of phonocardiography less likely in the future.
CARDIAC OUTPUT
There are several techniques available for the measurement of cardiac output but the one almost universally applied in large animals is the indicator dilution technique using thermodilution (injection of iced 5% dextrose) or indicator dyes such as Evans blue, indocyanine green or lithium chloride.1,2 With dye dilution, an exact amount of dye is injected into the jugular vein or pulmonary artery via a catheter and the serial collection of blood samples is performed from a suitable proximally located artery that has been catheterized. Cardiac output is most commonly measured using thermodilution3 but can also be calculated from a dye dilution curve by determining the mean concentration of the dye and the time taken for one circulation through the heart.1 Automated cardiodensitometers are also available for this estimation. Cardiac output is expressed as liters per minute and is usually corrected to cardiac index on the basis of weight or body surface area.
Most domestic animals have a cardiac index of 100 (mL/kg body weight (BW))/min at rest. The cardiac index for horses, sheep and cattle at rest has been determined as 86 ± 13, 131 ± 39 and 113 ± 11 (mL/kg)/min, respectively. Stroke volume can also be calculated from the measured cardiac output and simultaneously determined heart rate, whereby stroke volume = cardiac output/heart rate. In general the normal variation between animals in indexes of cardiac output is too great to allow it to be used as a diagnostic measure in individual animals suspected to have cardiac disease. Measures of cardiac output are used in experimental studies, where the effects of certain procedures can be followed within the same animal. Indicator dilution curves using dyes or thermodilution methodology can be used to detect the presence of intracardiac defects such as septal defects and to quantify their significance.
Doppler echocardiography can be used to estimate cardiac output and gives values equivalent to those obtained by thermodilution techniques.4,5
MEASUREMENT OF ARTERIAL BLOOD PRESSURE
Blood pressure may be determined directly by arterial puncture and pressure measurement but this is impractical in clinical cases. The development of simple methods for the indirect determination of arterial blood pressure has proved difficult in large animals because of the paucity of suitably located arteries where a pressure cuff can be applied and because there are problems in detecting pulse return by simple auscultatory or palpatory methods.
In the horse a simple and relatively inexpensive method uses oscillometric sphygmomanometry to detect arterial pulsations and therefore simultaneously determine heart rate and mean arterial pressure.1-4 For adult horses, the optimal cuff width for the oscillometric method is approximately 20–35% of the tail circumference,1,2 when the cuff is applied snugly to the base of the tail and the ventral coccygeal artery pressure is monitored. The mean tail circumference of an adult horse is 22 cm, therefore the optimal cuff width for horses is 5–8 cm for oscillometric pressure measurement. Because the oscillometric units were designed for use in humans, the software programs often have difficulty in measuring arterial pressure when the heart rate is less than 40 beats/min2 and when arrhythmias or arterial hypotension are present3, which minimizes the clinical utility of these units in trained or sick horses. The units are also susceptible to motion of the tail and it is therefore preferable to keep the tail still during recording. Other methods of indirect pressure measurement in the horse (modified auscultatory technique, ultrasonic Doppler methodology) appear less accurate than the oscillometric sphygmomanometry. Moreover, oscillometric techniques offer the advantage of providing systolic, diastolic and mean arterial blood pressures, whereas other indirect methods do not provide mean arterial pressure.3
Systolic and diastolic blood pressure of a large series of trained Thoroughbred horses were 112 ± 16 mmHg (14.9 ± 2.1 kPa) and 77 ± 14 mmHg (10.2 ± 1.9 kPa) respectively.1 Equivalent values have been recorded in other breeds. These values are coccygeal uncorrected values and can be corrected to the correct reference level (scapulohumeral joint, which is equivalent to the right atrium) by adding 0.7 mmHg (0.09 kPa) for every centimeter in height between the scapulohumeral joint and the tail if the coccygeal artery was the recording site for indirect pressure measurement. Posture of the horse is important, as lowering the head significantly lowers systolic, diastolic and pulse pressure.
Hypertension has been found in association with epistaxis, laminitis in horses and painful fractures of the distal bones of the limb. Systolic blood pressure is often also elevated in obstruction of the large intestine in horses. Blood pressure measurements are of value in the assessment of the degree of shock and possibly may prove of value in the differential diagnosis of conditions such as acute salmonellosis and in assessing the prognosis of colic. Mean arterial pressure is considered the true driving pressure for blood flow and organ perfusion, therefore determination of mean arterial pressure provides one index of perfusion. However, its is important to recognize that mean arterial pressure is poorly correlated with cardiac output.
Blood pressure readings can be obtained by equivalent techniques from the tails of cattle. However, because of anatomical differences these do not always correlate well with true blood pressure. Pressures have been observed to be 100–140 mmHg (13.3–18.6 kPa) systolic and 50–85 mmHg (6.7–11.3 kPa) diastolic.
ECHOCARDIOGRAPHY
Echocardiography has provided a relatively simple and noninvasive method for the examination of the heart that can give considerable information on its function. In echocardiography, high-frequency sound waves are pulsed through tissues at known velocities. When the sound waves encounter an acoustic tissue interface, echoes are reflected back to a transducer and recorded in a number of different modalities. The modalities have become increasingly sophisticated and they have largely replaced traditional invasive evaluations of cardiac function such as cardiac catheterization. The newer technologies are expensive and are currently limited to teaching hospitals and referral clinics.
Echocardiography will allow the measurement of cardiac chamber size, wall thickness, global and regional wall movement and valve structure and function.1-3 Functional indices can be calculated that will allow the determination of the presence of hypertrophy or dilatation of areas of the heart and the percentage of wall thickening.4 The fractional shortening of the left ventricle can be used as a sensitive index of left ventricular contraction. Quantitative studies are available for the horse,3,5-11 sheep,12 pigs2,13 and cattle.1,4,14-16 The measurement of the ratio of cardiopulmonary blood volume to stroke volume, determined from a radiocardiogram following the injection of technetium-99 m pertechnetate, has proved a more sensitive test for cardiac insufficiency in horses than measurements of cardiac output.17 Measurements of cardiac and individual chamber dimensions, vessel diameters and flow rates can be used to assess normality, indexes of contractility and effects of cardiac lesions on cardiac response and function.5,18-21 They can also be used to predict the type of lesion likely to result in these changes.19
Valvular defects and endocarditis may be diagnosed by imaging abnormal valve motion, incompetent valve orifices or vegetative masses associated with the valves22 and tumor masses in the heart can be detected.23 Similarly, the severity of valvular regurgitation can be quantified.24 Echocardiography can be of considerable value in the diagnosis of congenital cardiovascular defects1 and the injection of echogenic materials such as microbubble-laden saline may aid in the detection of shunts.2,19 Echocardiography can also be used to determine the presence and extent of pleural and pericardial effusion. In the examination of the vascular system, ultrasound is capable of the early detection of iliac thrombosis in horses and is more sensitive than manual palpation per rectum.3
There is a long-held belief that horses with a large heart relative to their body size have greater athletic capacity.25 An accurate and noninvasive method for determining heart weight therefore has potential utility as one method for predicting racing success. Echocardiography provides a useful estimate of heart weight that may compliment electrocardiographically determined heart score (calculated from the QRS duration) in the prediction of athletic performance. The thickness of the interventricular septum in diastole provides an accurate prediction of heart weight;26 the predictive accuracy was such that echocardiography using this measurement has utility as an index of subsequent athletic performance27 and has been used in North America, Europe and Australia in such a manner.
Reef VB. Advances in echocardiography. Vet Clin North Am Equine Pract. 1991;7:435-450.
Bonagura JD. Echocardiography. J Am Vet Med Assoc. 1994;204:516-522.
Marr CM. Equine echocardiography — sound advice at the heart of the matter. Br Vet J. 1994;150:527-545.
Voros K. Quantitative two-dimensional echocardiography in the horse: a review. Acta Vet Hung. 1997;45:127.
Braun U, et al. Echocardiography of the normal bovine heart: technique and ultrasonographic appearance. Vet Rec. 2001;148:47. 41
1 Reef VB. Equine Vet J Suppl. 1996;19:97.
2 Kvart C, et al. Equine Vet J. 1985;17:361.
3 Reef VB. J Am Vet Med Assoc. 1987;190:286.
4 Braun U, et al. Am J Vet Res. 2005;66:962.
5 Tucker RL, et al. J Equine Vet Sci. 1995;15:404.
6 Reef VB. Vet Clin North Am Equine Pract. 1991;7:435.
7 Young LE, Scott GR. Equine Vet J. 1998;30:117.
8 Blissitt KJ. Equine Vet J. 1997;29:18.
9 Seeherman HJ, Morris EA. Equine Vet J Suppl. 1990;9:20.
10 Harkins JD, Kamerling SG. J Equine Vet Sci. 1991;11:237.
11 Voros K, et al. Equine Vet J. 1991;233:461.
12 Moses BL, Ross JN. Am J Vet Res. 1987;48:1313.
13 Gwathmey JD, et al. Am J Vet Res. 1989;50:192.
14 Amory H, et al. Am J Vet Res. 1992;53:1540.
15 Pipers FS, et al. Bovine Pract. 1978;13:114.
16 Amory H, Lekeux P. Vet Rec. 1991;128:349.
17 Guthrie AJ, et al. J S Afr Vet Med Assoc. 1991;62:43.
18 Young LE, Scott GR. Equine Vet J. 1998;30:117.
19 Bonagura JD. J Am Vet Med Assoc. 1994;204:516.
20 Marr CM. Br Vet J. 1994;150:527.
21 Slater JD, Herrtage ME. Equine Vet J Suppl. 1996;19:28.
22 Reef VB, et al. Equine Vet J. 1998;30:18.
23 Braun U, et al. Schweiz Arch Tierheilkd. 1995;137:187.
24 Hagio M, Otsuka H. Am J Vet Sci. 1987;49:1113.
25 Buhl R, et al. J Am Vet Med Assoc. 2005;226:1881.
CARDIAC CATHETERIZATION
The measurement of pressure within the various chambers of the heart and in the inflow and outflow vessels can provide diagnostic information in both acquired and congenital heart disease in large animals. Generally, pressure is determined by means of fluid-filled catheters introduced into these areas and connected to an external pressure transducer. These systems are generally satisfactory for the measurement of pressure and the detection of changes with abnormality. However, because of their transmission characteristics, they are less suitable for the precise timing of pressure events, and high-fidelity catheter tip manometers should be used for this purpose.
Catheterization of the right side of the heart is a comparatively simple procedure in large animals but is not without risk to the animal. Catheterization is done in the standing position, and descriptions are available for horses1,2 and cattle.3-7 Flow-directed catheters are used and can be introduced through a needle inserted into the jugular vein. Balloon-tipped catheters aid the flow of the catheter into the pulmonary artery. Catheterization of the left side of the heart is more complicated and less commonly performed. Left heart catheterization is usually performed under general anesthesia and requires the use of a stiff catheter that is introduced into the carotid or femoral artery by surgical methods and subsequently manipulated to the left ventricle.
The systematic determination of the pressure within each area of the heart and in the inflow and outflow vessels can allow a determination of the type of abnormality that is present. Valvular stenosis or incompetence is associated with abnormal pressure differences across the affected valve during systole or diastole. Cardiac hypertrophy is generally accompanied by an increase in pressure during systole of the affected chamber. With high-fidelity equipment, pressure waveforms can also have diagnostic value.
The right atrium is usually used as the reference point for pressure comparison and is arbitrarily assigned a reference pressure of zero when recording using a fluid-filled catheter system. The scapulohumeral joint (point of the shoulder) is taken as the anatomical equivalent reference height in the standing animal.4 A simultaneously recorded base–apex electrocardiogram assists in interpretation of the pressure tracings. Numerous publications have reported cardiovascular values for conscious awake horses, adult cattle and calves, and representative values are presented in Table 8.2.
During catheterization blood may be withdrawn through the catheter and subjected to blood gas analysis. In right-sided catheterization an increase in oxygen saturation in the right ventricle or pulmonary artery can be diagnostic for the presence of a left-to-right shunt due to an atrial septal defect, a ventricular septal defect or a patent ductus arteriosus. The normal maximum increase in venous oxygen content between the right heart chambers and pulmonary artery in humans is 0.9 mL O2/dL from the right atrium to right ventricle and 0.5 mL O2/dL blood from the right ventricle to the pulmonary artery.3 It is a reasonable assumption that similar changes in oxygen content (due to streaming of blood flow and variability in sampling site within the right atrium and ventricle) exist in large animals. Not only can blood gas analysis indicate the presence of a left-to-right to shunt; sequential blood gas analysis can be used to quantify the magnitude of the shunt by calculating the pulmonary-to-systemic flow ratio and therefore assist in prognosis.
The pulmonary blood flow and systemic blood flow are approximately equivalent in healthy individuals, with the exception of a small amount of right-to-left shunt (physiological shunt) caused by venous blood from coronary and bronchial blood flow draining into the left ventricle, left atrium or pulmonary veins. The pulmonary-to-systemic flow ratio should therefore approximate 1.0.8 In animals with a left-to-right shunt, the pulmonary to systemic flow ratio (Qp/Qs) quantifies the magnitude of the left-to-right shunt across the defect. The pulmonary-to-systemic flow ratio is calculated using the Fick method from measurements of Sao2 (oxygen content of arterial blood), MVO2 (oxygen content of mixed venous blood, which is the pulmonary artery in animals without a shunt, the right ventricle in animals with a patent ductus arteriosis, the right atrium in animals with a ventricular septal defect, and the vena cava in animals with an atrial septal defect), Pvo2 (oxygen content of pulmonary venous blood), and Pao2 (oxygen content of pulmonary artery blood), such that: (Qp/Qs) = (Sao2 − MVO2)/(Pvo2 − Pao2).8 This method assumes the animal is in steady state and that cardiac output does not change during blood sampling.8 Oxygen content (in mL O2/dL blood) is calculated from the measured values for blood hemoglobin concentration ([Hb], in g/dL), oxygen tension and percentage O2 saturation, such that O2content = [Hb] × 1.39 × O2 saturation/100 + 0.003 × Po2.8 In clinical cases at sea level, it is assumed that that saturation of pulmonary venous blood and arterial blood = 97.5% and that Po2 = 90 mmHg. Application of this equation and assumptions to data from a 2-year-old Holstein–Friesian cow with a ventricular septal defect, atrial fibrillation and pulmonary hypertension (mean pressure 67 mmHg) indicated that (Qp/Qs) = (8.67 − 5.89)/(8.67 − 8.04) = 4.4, using the right atrial content as the mixed venous sample because the right ventricle contained a large volume of oxygenated blood from the left ventricle. This calculation indicated the presence of an extremely large left-to-right shunt (shunt = Qp − Qs = Qp(1 − 1/4.4) = 0.77 × Qp); in other words, 77% of the blood flowing through the lungs was from the left heart. Such a large shunt into the right ventricle was suspected based on the large step up in O2 content from the right atrium to right ventricle (2.1 mL O2/dL; Table 8.3) which exceeded the maximal normal value of 0.9 mL O2/dL. A 2 cm diameter ventricular septal defect was confirmed at necropsy.
Table 8.3 Results of sequential blood gas analysis from a 2-year-old Holstein-Friesian heifer with a large ventricular septal defect. Blood was obtained from passing a fluid filled catheter from the jugular vein through the right atrium, right ventricle, and into the pulmonary artery. The O2 content of arterial and pulmonary venous blood was calculated to be 8.67 mL O2/dL blood.
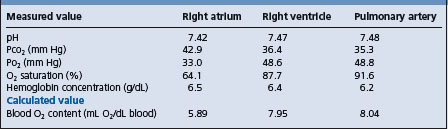
Shunts can also be demonstrated by dye or thermodilution techniques,.8 but these are much more complicated to analyze than blood gas analysis of sequential blood samples obtained from a fluid-filled catheter during a pullback from the pulmonary artery through the right ventricle into the right atrium.
Echocardiography can provide information that, while different, may be of equivalent diagnostic value to that obtained by cardiac catheterization and, because it is noninvasive and technically a much easier procedure, echocardiography has largely supplanted cardiac catheterization in the examination of cardiac disease in large animals.
1 Brown CM, Holmes JR. Equine Vet J. 1978;10:188.
2 Brown CM, Holmes JR. Equine Vet J. 1978;10:207.
3 Manohar M, et al. J Am Vet Med Assoc. 1973;163:351.
4 Amory H, et al. Vet Res Commun. 1992;16:391.
5 Amory H, et al. Vet Rec. 1993;132:426.
6 Constable PD. Shock. 1999;12:391.
7 Wagner AE, et al. Am J Vet Res. 1990;51:7.
8 Yang SS, et al. From cardiac catheterization data to hemodynamic parameters, 2nd ed., Philadelphia, PA: FA Davis; 1978:209-231.
RADIOGRAPHIC AND ANGIOCARDIOGRAPHIC EXAMINATION
Because of the size of horses and cattle these methods of examination are largely confined to neonates of these species except in teaching hospitals. Angiocardiography can be a diagnostic method of examination in congenital cardiac defects where the passage of contrast media through abnormal routes can be detected.
Arrhythmias (dysrhythmias)
Variations in cardiac rate and rhythm include tachycardia (increased rate), bradycardia (decreased rate), arrhythmia or dysrhythmia (irregularity in rate and rhythm) and gallop rhythms. The rate and rhythm of the heart is influenced primarily by the integrity of the pacemaker, the conducting system and the myocardium, and also by the influence of the autonomic nervous system. Variation in the rate and rhythm can occur in normal animals due to strong or varying autonomic influence but can also be a reflection of primary myocardial disease. Other factors such as acid–base and electrolyte imbalance can influence rate and rhythm. These factors must be taken into consideration in the assessment of apparent abnormalities detected on clinical examination of the cardiovascular system.
The majority of arrhythmias and conduction disturbances can be detected on clinical examination. However, some may be unsuspected on clinical examination and be found only on electrocardiographic examination. The occurrence of conduction and myocardial disturbances is probably more common than generally recognized, because an electrocardiogram is usually only taken from animals in which there have been prior clinical indications of conduction abnormalities. Because of the importance of electrocardiography in the diagnosis of arrhythmias the salient electrocardiographic findings are given in the sections below.
The common conduction disturbances and arrhythmias in large animals are listed in Table 8.4. A large scale cross sectional study of 952 healthy dairy cattle aged 1 or more years produced the following prevalence of arrhythmias:1 sinus arrhythmia, 8.5%; first-degree atrioventricular block, 1.6%; ventricular premature complexes, 0.6%; atrial premature complexes, 0.4%; sinus bradycardia, 0.2% and ventricular escape beats, 0.1%. Atrial fibrillation was not observed in healthy cattle in this study.1
Table 8.4 Common arrhythmias and conduction disturbances in the horse and cow
| Horses | Cattle |
|---|---|
| Second-degree atrioventricular block | First-degree atrioventricular block |
| Atrial fibrillation | Atrial premature complexes |
| Atrial premature complexes | Ventricular premature complexes |
| Ventricular premature complexes | Atrial fibrillation |
| First degree atrioventricular block | |
| Sinoatrial block |
The treatment of arrhythmic heart disease generally relies on the treatment of the underlying clinical condition causing the problem. This may vary from electrolyte and acid–base disturbance and toxicities to primary myocardial disease resulting from myocarditis, myocardial ischemia and changes resulting from heart failure or myopathies resulting from nutritional deficiency. These are detailed in later sections in this text. Racing and work horses should be rested for periods up to 3 months following evidence of myocardial disease. Frequently a course of corticosteroids, or a nonsteroidal anti-inflammatory drug (NSAID) such as flunixin meglumine, is given to attempt to reduce the severity of myocarditis if this is not contraindicated by the initiating cause. Specific antiarrhythmic therapy may be applied in certain conditions and is detailed below.
It is important to be able to recognize those forms of arrhythmia that are not indicative of pathological heart disease but are normal physiological variations. These occur commonly in the horse and most can be differentiated on physical examination. It is also important to understand the difference between a premature beat or contraction and a premature complex. A beat or contraction is a mechanical event that can be clinically detected by auscultation, palpation of an artery or visual examination of the jugular venous pulse, or recorded by pressure measurements. A complex is an electrical event that is detected by an electrocardiograph. A beat is always associated with a complex; however, a complex can be unaccompanied by a beat, particularly in electromechanical dissociation. The terms beat or contraction should therefore be used to describe an arrhythmia that is detected by auscultation, palpation or recording of the arterial pulse, whereas the term complex should be used when the arrhythmia is detected electrocardiographically.
Hilwig RW. Cardiac arrhythmias in the horse. J Am Vet Med Assoc. 1977;170:153-163.
McQuirk SM, Muir WW. Diagnosis and treatment of cardiac arrhythmias. Symposium on Cardiology. Vet Clin North Am Equine Pract. 1985;1:353-370.
Fregin GF. Medical evaluation of the cardiovascular system. Vet Clin North Am Equine Pract. 1992;8:329.
Mitten LA. Cardiovascular causes of exercise intolerance. Vet Clin North Am Equine Pract. 1996;12:729-746.
SINUS TACHYCARDIA, SINUS BRADYCARDIA AND PHYSIOLOGICAL DYSRHYTHMIAS
The heart rate results from the discharge of impulses from the sinoatrial node, which has its own intrinsic rate of discharge but which is also modified by external influences, particularly the vagus nerve.
SINUS TACHYCARDIA
The term sinus tachycardia or simply tachycardia is used to describe an increase in heart rate caused by detectable influences such as pain, excitement, exercise, hyperthermia, a fall in arterial blood pressure or the administration of adrenergic drugs. The heart rate returns to normal when the influence is removed or relieved.
It needs to be stated that sinus tachycardia indicates an increase in heart rate that is initiated by the sinoatrial node in the right atrium (hence the sinus modifier). This means that the term sinus tachycardia should be reserved for use when electrocardiography has been performed and the sinus node has been determined to be the dominant pacemaker. For comparison, the term tachycardia should be used when an increased heart rate is detected by auscultation or palpation of the pulse and the origin of the pacemaker has not been determined. In resting horses and cattle that are used to being handled heart rates are not usually elevated above 48 and 80 beats/min respectively and rates above this are usually classified as tachycardia (Figure 8.1). In the cow and horse it is rare for the causes of sinus tachycardia to elevate the heart rate above 120 beats/min in the resting animal, and at heart rates above this an intrinsic pathological tachycardia should be sought.
SINUS BRADYCARDIA
Sinus bradycardia or simple bradycardia is used to describe a decrease in heart rate due to a decreased rate of discharge from the sinoatrial node. Sinus bradycardia is most commonly associated with highly trained, fit animals and can be differentiated from the pathological bradycardias by its abolition by exercise or the administration of atropine. Obviously sinus bradycardia, like sinus tachycardia, requires electrocardiographic confirmation that the sinus node is the dominant pacemaker.
Bradycardia may also occur in association with an increase in arterial blood pressure, space-occupying lesions of the cranium and increased intracranial pressure, pituitary abscess, hyperkalemia (Figure 8.1) hypothermia and hypoglycemia and following the administration of alpha-2-adrenergic agonists such as xylazine or detomidine. Bradycardia is sometimes associated with vagus indigestion and diaphragmatic hernia in cattle and also occurs in cattle deprived of food.1 Bradycardia has also been reported in cattle with bovine spongiform encephalopathy,2 although this probably reflects inappetence rather than damage to the vagal nucleus in the brain stem; the latter would be expected to increase, rather than decrease, heart rate. Bradycardia can be induced in young ruminants by forceful elevation of the tail. Sinus arrhythmia is usually present in animals with sinus bradycardia.
The resting heart rate seldom falls below 22 beats/min in adult horses and 44 beats/min in adult cattle. Rates below this are suggestive of pathological bradycardias, and hypothermia, hypothyroidism or an intrinsic cardiac problem should be suspected. However, a general rule is that resting heart rates are inversely proportional to body weight, and large, fit horses and cattle have apparently low heart rates.
PHYSIOLOGICAL ARRHYTHMIAS
There are several dysrhythmias that can occur in the absence of heart disease and that appear to result from excess vagal tone. These occur especially in the horse and include:
These physiological arrhythmias occur in animals at rest and can frequently be induced by the application of a nose twitch in horses or by forceful elevation of the tail in young ruminants. There is some debate as to the significance of these arrhythmias in animals but it is generally believed that if they are abolished by exercise or excitement and if there is no evidence of cardiac insufficiency they are not of pathological significance and do not require further investigation.
Perinodal myocardial fibrosis and microvascular abnormality have been reported in horses with sinoatrial and atrioventricular block, and considered as the excitatory cause.3 However, because myocardial fibrosis is common in horses, being present in 79% of horse hearts examined at random,4 it remains likely that these arrhythmias are physiological in horses. All animals with evidence of arrhythmic heart disease should be examined following exercise, as should any animal in which cardiac disease is suspected.
The occurrence of cardiac irregularities following exercise is highly indicative of serious cardiac disease.
A high frequency of arrhythmia has been recorded in newborn foals immediately following birth.5 48 of 50 foals had some form of arrhythmia; atrial premature complexes were recorded in 30 foals, atrial fibrillation in 15 foals and ventricular premature complexes in 10 foals. Other arrhythmias were recorded with less frequency. It was concluded that the arrhythmias resulted from transient physiological hypoxemia during birth and that their occurrence should be considered as part of the normal adaptive process to extrauterine life, as normal sinus rhythm was recorded by 5 minutes following birth and the foals subsequently developed normally.5
Cardiac arrhythmias also occur commonly in association with gastrointestinal disorders in the dairy cow6 and less commonly in the horse7 and resolve without specific antiarrhythmic treatment when the primary gastrointestinal disorder is corrected. Atrial premature complexes, ventricular premature complexes and atrial fibrillation have been detected in apparently healthy dairy cattle by serial monitoring;8 however, ventricular premature complexes should be assumed to indicate the presence of organic heart disease.
1 McQuirk SM, et al. J Am Vet Med Assoc. 1990;196:894.
2 Austin AR, et al. Vet Rec. 1997;141:352.
3 Kiri K, et al. Jpn J Vet Sci. 1985;47:45.
4 Dudan F, et al. Schweiz Arch Tierheilkd. 1985;127:319.
5 Yamamoto K, et al. Equine Vet J. 1992;23:169.
6 Constable PD, et al. J Am Vet Med Assoc. 1990;197:1163.
7 Cornick JL, Seahorn TL. J Am Vet Med Assoc. 1990;197:1054.
ARRHYTHMIAS WITH NORMAL HEART RATES OR BRADYCARDIA
SINUS ARRHYTHMIA
Sinus arrhythmia is a normal physiological arrhythmia that occurs at slow resting heart rates and is associated with variation in the rate of discharge from the sinoatrial node associated with variation in the intensity of vagal stimulation. It is commonly correlated with respiration so that the discharge rate and heart rate increase during inspiration and decrease during expiration. In the horse, sinus arrhythmia unassociated with respiration also occurs. In the majority of large animals, sinus arrhythmia is much less overt than in the dog and generally it is not detected except on very careful clinical examination or examination of the electrocardiogram. Sinus arrhythmia is more clinically obvious in tame sheep and goats and in the young of all species and is correlated with respiration. It is abolished by exercise or by the administration of atropine.
In the electrocardiogram sinus arrhythmia is detected by variations in the P–P intervals (greater than 10% of the mean heart rate) with or without variation in the P–R interval and is frequently associated with a wandering pacemaker. This is associated with differences in the site of discharge from the sinoatrial node with subsequent minor variations in the vector of atrial depolarization with subsequent minor variations in the configuration of the P wave. In the horse there may be an abrupt change in the contour of the P wave so that the normal biphasic positive P wave in lead II, for example, changes to one with an initial negative deflection. There may or may not be a change in P–R interval. This is not pathological and is present in as many as 30% of normal horses at rest. If sinus arrhythmia is not abolished by exercise it is considered pathological. Sinus arrhythmia may be induced in the early stages of hypercalcemia during treatment for milk fever in cattle.
SINOATRIAL BLOCK
In sinoatrial block the sinus node fails to discharge or its impulse is not transmitted over the atrial myocardium.1 It is associated with the complete absence of heart sounds, of jugular atrial wave and of an arterial pulse for one beat period. The underlying rhythm is regular unless sinus arrhythmia is present. In the electrocardiogram there is complete absence of the P, QRS and T complex for one beat. The distance between the preblock and postblock P waves is twice the normal P–P interval or sometimes slightly shorter. This arrhythmia is not uncommon in fit racing horses at rest and can be induced in horses and cattle by procedures that increase vagal tone. Provided it does not persist during and following exercise it is considered as a physiological variant of normal rhythm.
ATRIOVENTRICULAR BLOCK
Atrioventricular block is divided into three categories depending upon the degree of interference with conduction at the atrioventricular node.
First-degree atrioventricular block
This is an electrocardiographic diagnosis and cannot be detected clinically. It occurs when conduction is delayed at the atrioventricular node. The P–R interval is prolonged beyond normal limits (conventionally > 400 ms in the horse) and the condition may be transient because of waxing and waning vagal tone. First-degree atrioventricular block is generally considered to have little significance.
Second-degree atrioventricular block
Also called partial heart block, this occurs when there is periodic interference with conduction at the atrioventricular node so that some atrial contractions are not followed by ventricular contraction (Figure 8.1). This may occur apparently at random or occur in a regular pattern, for example at every third or fourth beat. At the blocked beat there is complete absence of the first and second heart sounds and no palpable pulse. The underlying rhythm is still sinus in origin and is thus regular. In horses the presence of a fourth heart sound can be a valuable aid to diagnosis as with careful auscultation it can be heard during the block period in the manner of du LUBB DUPP, du …, du LUBB DUPP. This is diagnostic for this condition. An atrial jugular impulse can also be detected during the block period. The intensity of the first sound in the immediate postblock beat is usually intensified.
The electrocardiogram shows the presence of a P wave but complete absence of the subsequent QRS and T waves at the blocked beat. There may be variations in the P–R intervals preceding and following the block. With Mobitz type 1 (Wenkebach) second degree atrioventricular block there is a gradual increase in the PQ interval up to the point of the blocked conduction. With Mobitz type 2 block the PQ interval remains unchanged. In most species, Mobitz type 1 is a normal physiological response reflecting changes in vagal tone, whereas Mobitz type 2 always indicates the presence of organic heart disease such as myocarditis. However, many second-degree atrioventricular blocks in horses do not fit these two categories and the PQ interval increases until the immediate preblock complex in which it may be decreased. The clinical significance of these variations in the horse has not been established.
Second-degree atrioventricular block is extremely common in horses and occurs as a normal physiological variation due to variations in vagal tone.1 The application of a twitch to the upper lip of a horse will frequently slow the heart rate and allow the expression of second-degree heart block. It is more common in Thoroughbreds and Standardbreds than in heavy horses and may be detected in approximately 20% of racehorses. Frequency is highest when they are examined in quiet surroundings at rest, at night or early in the morning.2 Second degree atrioventricular block can be abolished by exercise or the administration of atropine.
Second-degree atrioventricular block can be associated with myocarditis in the horse and its presence has been associated with decreased racing performance by some clinicians. Second-degree atrioventricular block at fast heart rates has also been associated with the syndrome of duodenitis–proximal jejunitis in horses and was correlated with high serum bicarbonate concentrations in this condition.3 Atrioventricular conduction disturbances can be associated with electrolyte imbalance in all species, overdosing with calcium salts, digoxin toxicity, cardiomyopathy and myocarditis associated with nutritional and infectious disease.
Methods for the clinical differentiation of physiological (Mobitz type 1) versus pathological (Mobitz type 2) second-degree heart block in the horse have not been established. However, the persistence of the arrhythmia at heart rates above resting normal values should be considered to be abnormal. In all other species the presence of Mobitz type 2 atrioventricular block should probably be considered as an indication of myocardial disease.
There is usually no necessity to treat this arrhythmia specifically and treatment is generally directed at the underlying cause. In cases where the block is frequent and syncopal episodes are likely, atropine may give some alleviation of the frequency of the block; however, this is only short-term therapy. Second-degree heart block may progress to third degree (complete) heart block.
Third-degree or complete heart block
This occurs rarely in large animals, or perhaps is seen only infrequently because it is almost invariably fatal. In complete heart block there is no conduction at the atrioventricular node. The ventricle establishes a pacemaker in the nodal or conducting system and the atria and ventricles beat independently. The ventricular rate is regular but very slow. Bradycardia is the prominent feature and it is unresponsive to exercise or atropine. Atrial contractions are much faster than the ventricle. Atrial contraction sounds are rarely heard on auscultation but evidence of the rate may be detected by examination of the jugular inlet. Periodically, as the atrium contracts during the period that the atrioventricular valves are closed, atrial cannon waves may occur up the jugular vein. There is usually variation in the intensity of the first heart sound due to variation in ventricular filling. Affected animals show extremely poor exercise tolerance and usually have evidence of generalized heart failure. There is frequently a history of syncopal attacks.
The electrocardiogram shows a slow and independent ventricular rate characterized by QRS complexes that are completely dissociated from the faster P waves.
The prognosis in complete heart block is extremely grave unless it is associated with a correctable electrolyte imbalance. The animal should be kept at rest in quiet surroundings while every effort is made to correct the underlying cause. Corticosteroids and dextrose are usually given intravenously in an attempt to reduce the severity of the initiating myocardial lesion. Isoproterenol (isoprenaline) may stimulate higher nodal tissue and may increase the heart rate. Isoproterenol is usually infused intravenously at a concentration of 1 mg/L of infusion fluid and the rate of infusion is adjusted to effect. This is not a practical treatment in most situations. The use of an internal pacemaker has been reported in the horse but would clearly make the animal unsuitable for athletic endeavors.
Atrioventricular block and atrioventricular dissociation may develop during anesthesia and can be associated with arrhythmogenic anesthetic drugs, hypercapnia, hypoxia and electrolyte and acid–base imbalances.4,5 In these circumstances, the administration of regular doses of atropine (0.02 mg/kg) may not alleviate the arrhythmia. Dopamine HCl infusions (3– μg/kg per min) have been effective.6
The Wolff–Parkinson–White syndrome is recorded as a rare observation in cattle.7
PREMATURE COMPLEXES
Premature complexes or extrasystoles arise by the discharge of impulses from irritable foci within the myocardium. They are classified according to the site of their origin as atrial, junctional and ventricular premature complexes. It is often not possible to distinguish between these by physical examination, particularly at fast heart rates. However, auscultation of an animal with premature beats usually reveals an occasionally irregular rhythm.
Atrial premature complexes
These arise from the discharge of an ectopic atrial pacemaker outside the sinus node. Atrial premature contractions are difficult to detect on physical examination if they do not affect ventricular rhythm. If the stimulus from the atrial premature complex falls outside the refractory period of the ventricle it will initiate a ventricular complex that occurs earlier than expected. Ventricular contractions initiated by atrial premature complexes have lower intensity because of lower diastolic filling, and the associated arterial pulse amplitude is decreased.
Two main patterns occur. In some instances the sinus node becomes reset from the atrial premature complex so that a regular rhythm is established from this contraction. In this case atrial premature complexes are characterized by the occurrence of periods of regular rhythm interrupted by beats with exceptionally short interbeat periods. In other instances the sinus node is not reset following the atrial premature complexes and if its discharge occurs during the refractory period of the atrium then no atrial or subsequent ventricular contraction will occur. This will be detected electrocardiographically as an early ventricular complex followed by a pause following which normal rhythm is continued. This character is identical to that produced by many ventricular premature complexes.
At slow heart rates the presence of atrial premature beats is suggested by periodic interruption of an underlying sinus rhythm and by the occurrence of a ‘dropped pulse’. The prime differentiation is from sinoatrial block and second-degree atrioventricular block, which have distinguishing electrocardiographic characteristics.
On the electrocardiogram the P wave of the premature beat occurs earlier than expected from the basic rhythm and is abnormal in configuration. (Figure 8.1) QRS complexes associated with atrial premature beats are normal in configuration because this is a supraventricular arrhythmia and the pathway for ventricular depolarization is not altered.
Junctional premature complexes
These are also called atrioventricular nodal premature complexes arise from the region of the atrioventricular node or conducting tissue. They produce a premature ventricular contraction, which is usually followed by a compensatory pause due to the fact that the following normal discharge from the sinus node usually falls upon the ventricle during its refractory period.
Junctional premature complexes produce QRS configurations that are similar to those of normal beats but they may produce a P wave that has a vector opposite to normal.
Ventricular premature complexes
Ventricular premature complexes may arise from an irritable process anywhere within the ventricular myocardium. The normal rhythm is interrupted by a beat that occurs earlier than expected but the initial rhythm is established following a compensatory pause. This can be established by tapping through the arrhythmia as described earlier. The heart sounds associated with the premature beat are usually markedly decreased in amplitude while the first sound following the compensatory pause is usually accentuated. Occasionally, ventricular premature complexes may be interpolated in the normal rhythm and not followed by a compensatory pause. If the diastolic filling period preceding the premature beat is short the pulse associated with it will be markedly decreased in amplitude or even absent.
On the electrocardiogram ventricular premature complexes are characterized by bizarre QRS morphology (Figure 8.1). Conduction over nonspecialized pathways results in a complex of greater duration and amplitude to normal and the complex slurs into a T wave that is also of increased duration and magnitude. The vector orientation depends on the site of the ectopic foci initiating the contraction but it is invariably different from that of normal contractions. Electrocardiographic examination allows a differentiation of the site of origin of premature complexes and further subclassification within the categories.
Premature complexes of all site origins are indicative of myocardial disease, the one exception being the occurrence of atrial premature complexes accompanying cases of gastrointestinal disease in cattle. Atrial premature complexes occur commonly in cattle with gastrointestinal disease and their presence should be suspected whenever there is a variation in the intensity of the first heart sound with or without an underlying detectable cardiac irregularity.8 Atrial premature complexes can progress to atrial fibrillation in these cases where there is excessive vagal tone.9,10
Horses in which premature beats are detected or suspected should be examined after careful exercise, which will usually increase the occurrence and severity of the arrhythmia. Premature beats are most easily detected during the period of slowing of heart rate after exercise.1
1 Holmes JR. In Pract. 1980;2:15.
2 Scheefer CWJ, et al. Vet Rec. 1995;137:371.
3 Cornick JL, Seahorn TL. J Am Vet Med Assoc. 1990;197:1054.
4 Greene SA, et al. J Vet Pharmacol Ther. 1988;11:295.
5 Grubb TL, et al. J Am Vet Med Assoc. 1996;208:349.
6 Whitton DL, Trim CM. J Am Vet Med Assoc. 1985;187:1357.
7 Endo Y, et al. Jpn J Vet Sci. 1990;52:1155.
8 Constable PD, et al. J Am Vet Med Assoc. 1990;197:1163.
ARRHYTHMIAS WITH TACHYCARDIA
An excitable focus within the myocardium may spontaneously discharge and cause depolarization of the remaining myocardium. If the discharge rate approaches or exceeds that of the sinus node the focus may transiently take over as the pacemaker of the heart.
PAROXYSMAL TACHYCARDIA
Paroxysmal tachycardia may arise from an irritant focus within the atria or the ventricles but in large animals ventricular paroxysmal tachycardia is more common. Atrial paroxysmal tachycardia (Figure 8.1) and atrial flutter are rare and are transients leading to atrial fibrillation.
In paroxysmal tachycardia the increase in heart rate is abrupt and the fall to normal is equally sudden. This characteristic usually serves to distinguish this arrhythmia from the transient increases in heart rate that may normally follow such factors as excitement. Also the heart rate is elevated to a rate far in excess of that which would be normally expected from such stimuli.
More commonly the excitable focus discharges repetitively over a long period of time to produce more continual ventricular tachycardia associated with ventricular extrasystoles. Sustained tachycardia is not normal and can lead to myonecrosis; three parturient dairy cows with sustained tachycardia (heart rate >120 beats/min) had multifocal areas of necrosis throughout the myocardium characterized by myofibrillar lysis and disarray.
VENTRICULAR TACHYCARDIA
Ventricular tachycardia may produce either a regular heart rate or an irregular heart rate and rhythm.
When the discharge rate of the irritant focus far exceeds that of the sinoatrial pacemaker, the ectopic focus will take over completely as the pacemaker of the heart and on examination of the cardiovascular system a rapid but regular heart rate and pulse is detected and there is no irregularity of rhythm or of pulse amplitude or intensity of heart sounds. This is known as ventricular tachycardia with atrioventricular dissociation. This abnormality is easily overlooked clinically but should be suspected in any adult horse or cow where the heart rate exceeds 90 beats/min1 and is frequently the cause of heart rates in excess of 120 beats/min. Ventricular tachycardia should also be suspected where the heart rate is elevated to a level that is higher than that expected from the animal’s clinical condition.
An electrocardiogram gives the diagnosis based on the occurrence of multiple regular QRS complexes with abnormal amplitude and duration of the QRS and T complexes and the T wave oriented in a direction opposite to the QRS complex (Figure 8.1).2 P waves may be detected on the electrocardiogram but they have no relationship to the QRS–T complex and are frequently lost within them.
When the discharge rate of the irritant focus within the myocardium is similar to that of the sinoatrial node the ventricular tachycardia can be manifested by a gross irregularity in rhythm. This is a common manifestation in large animals. In this situation many of the discharges which originate in the sinus node are transmitted to the ventricle during a refractory period from a previous ectopic foci, but some reach the ventricle when it is not in a refractory state and are conducted normally. At some periods ventricular complexes may be initiated by the discharges from both sites.
The varying influence of each pacemaker on ventricular contraction produces a marked irregularity in cardiac rhythm and it is frequently not possible to establish clinically a regular pattern to the heart rhythm. Variations between beats in the degree of atrial filling and in the diastolic filling period will result in a marked variation in the intensity of the heart sounds and in the amplitude of the pulse. Frequently at fast heart rates there is a pulse deficit. Cannon atrial waves can be observed in the jugular vein when atrial contraction occurs at the same time as a ventricular extrasystole.
The electrocardiogram shows runs of extrasystoles interspersed with normally conducted complexes and usually the presence of fusion beats.
Ventricular tachycardias are evidence of severe cardiac disease and are usually accompanied by signs of acute heart failure. They may result from primary myocarditis, nutritional cardiomyopathy, or myocardial neoplasia3 or be secondary to valvular disease and myocardial ischemia. Ventricular arrhythmias are common in certain plant poisonings and other toxicities, and in severe electrolyte and acid–base disturbance, and commonly occur in the final stages of heart failure. If uncorrected, ventricular tachycardia may lead to ventricular fibrillation and death and frequently specific antiarrhythmic therapy is indicated during the period that the prime cause is being corrected.
Treatment
Lidocaine is the drug of first choice for treating hemodynamically important ventricular arrhythmias in large animals. Lidocaine is an antiarrhythmic agent of class 1b of Vaughan-Williams’s classification, and slows intracardiac conduction by blocking the fast sodium channel while shortening the refractory period of myocardial tissue. Typically, lidocaine is given as an intravenous bolus at 0.5–1.0 mg/kg BW every 5 minutes for a total of four treatments (total dose 2–4 mg/kg BW).4,5 Lidocaine has the advantages of widespread availability, low cost and low cardiovascular toxicity, with the major disadvantage being its very short duration of action (half-life is 40 minutes in the horse). The most commonly seen initial sign of lidocaine toxicity is muscle fasciculations, which occur at a serum lidocaine concentration of 1.9–4.5 mg/L.5 If infusion is continued, sedation and altered visual function are apparent, the latter being manifest as rapid eye blinking, anxiety and attempts to inspect closely located objects. Temporary recumbency, excitement, sweating and convulsions occur with higher doses.4
Quinidine sulfate is the drug of second choice for use in horses. Quinidine is an antiarrhythmic agent of class 1a of Vaughan-Williams’s classification, and slows intracardiac conduction by blocking the fast sodium channel and prolongs the action potential duration. An initial dose of 20 mg/kg is given orally, followed by a dose of 10 mg/kg given every 8 hours. The drug is not effective until 1–2 hours following administration. Intravenous quinidine gluconate (0.5–2.2 mg/kg BW bolus every 10 minutes to a total of 12 mg/kg BW) may be of greater value in those rare instances when oral quinidine is not indicated. Serum quinidine concentrations of 4 mg/L appear effective in treatment of cattle with ventricular tachycardia but serum concentrations following an oral dose of 20 mg/kg vary widely between cows and slow intravenous infusion is the preferred method of therapy.6 There is a very narrow therapeutic index in cattle and death can occur in some cows at doses that are therapeutically effective in others.6 Quinidine treatment in cattle should be approached with caution.
Phenytoin sodium is a good alternative to quinidine sulfate, and has been effective in treating ventricular arrhythmias in horses.7 Phenytoin is an antiarrhythmic agent of class 1b of Vaughan-Williams’s classification (same as lidocaine), and slows intracardiac conduction by blocking the fast sodium channel while shortening the refractory period of myocardial tissue. The recommended dosage protocol for the horse requires an initial oral dose of 20 mg/kg BW every 12 hours for four doses, followed by a maintenance oral dose of 10–15 mg/kg BW every 12 hours, with monitoring of phenytoin plasma concentrations. Plasma concentrations of 5–10 mg/L appear to be effective in treatment of horses with ventricular tachycardia. High plasma phenytoin concentrations are associated with sedation, recumbency and excitement,7 and the dosage protocol should be altered in horses that appear sedated. The major advantage of phenytoin over lidocaine is its long duration; conversely, its major disadvantage is the initial time required (2–6 h) to exert an antiarrhythmic effect. An intravenous form of phenytoin sodium has been administered to a pony with digitalis-induced ventricular arrhythmias, but the alkaline pH of the infused solution carries a high risk of thrombophlebitis.8 Magnesium sulfate (0.004 mg/kg BW boluses intravenously at 5-minute intervals to a maximum dose of 0.05 mg/kg BW) has also been successful in treating ventricular arrhythmias either alone or in combination with other antiarrhythmic agents; however, clinical experience with magnesium sulfate administration in horses is minimal.
The severity of ventricular tachycardia is augmented by factors that increase sympathetic tone, and affected animals should be kept in quiet surroundings.
VENTRICULAR FIBRILLATION
Ventricular fibrillation is not usually observed clinically. It occurs in the terminal stages of most suddenly fatal diseases, including lightning stroke, plant poisonings such as acute Phalaris toxicity, overdose with anesthetics, severe toxemia and in the terminal phases of most acquired cardiac diseases. There is complete absence of the pulse and heart sounds, the blood pressure falls precipitously and the animal rapidly becomes unconscious and dies within a minute or two of onset. Treatment is usually impractical although deaths during anesthesia may be prevented by immediate and aggressive external cardiac massage. Electrical defibrillation is not feasible in large animals due to the bulk of the animal and the current required. Intracardiac injections of epinephrine are often used in acute cardiac arrest but do not correct fibrillation and are of little value.
ATRIAL FIBRILLATION
In atrial fibrillation atrial depolarization is characterized by numerous independent fronts of excitation that course continuously and haphazardly through the atria. There is no synchronous atrial contraction and atrioventricular nodal stimulation occurs in an irregular and random fashion. The effects within the atria cannot be appreciated on auscultation and the clinical detection of this arrhythmia occurs through its effects on ventricular function. The random stimulation of the ventricles produces a heart rate and pulse that is irregularly irregular. It is not possible to establish any basic rhythm by tapping out this arrhythmia and the rate varies from period to period.
Because there is no atrial contraction, filling of the ventricles is entirely passive and very much dependent on diastolic filling time. Some contractions occur very quickly following the preceding contraction with little time for diastolic filling and this produces a marked variation in the intensity of the heart sounds and in the amplitude of the pulse. At fast heart rates there will be a pulse deficit. There is no fourth heart sound (S4) or atrial wave at the jugular inlet because there is no coordinated atrial contraction, but the third heart sound is usually grossly accentuated. The degree of cardiac insufficiency that results from this arrhythmia varies and depends upon the general rate at which the ventricles beat at rest. This is determined primarily by vagal activity.
On the electrocardiogram there are no P waves discernible but the baseline shows multiple waveforms (f waves) that occur with a frequency of between 300 and 600 beats/min (Figure 8.1). QRS–T complexes are normal in configuration but there is wide variation and no pattern in the Q–Q intervals. Atrial fibrillation is one of the more common arrhythmias in large animal species.
Atrial fibrillation in the horse
Horses with atrial fibrillation fall into two categories. In one category, sometimes called ‘benign fibrillators’,9 there is no evidence of underlying heart disease whereas in the other, there is.
Benign fibrillation
In cases that are benign fibrillators the vagal tone may be high and conduction through the atrioventricular node is suppressed to result in heart rates in the region of approximately 26–48 beats/min. At this rate there is no cardiac insufficiency at rest and hemodynamic parameters are normal.10 The horse can elevate its heart rate with exercise to allow moderate performance, although it will never perform satisfactorily as a racehorse. This is the most common manifestation in this species and it is typified by a gross irregularity in rate, rhythm and intensity of the heart sounds and by the occurrence, at rest, of occasional periods lasting for 3–6 seconds where there is no ventricular activity. At very slow rates periodic syncope may occur.
The benign form of atrial fibrillation occurs not infrequently in draft horses and is also seen in racehorses. A survey of 106 cases of atrial fibrillation in horses11 found the disease most commonly in Standardbred and Thoroughbred horses under 7 years of age, with a high proportion under 4 years of age, which may have been a reflection of the admissions to the clinic rather than real age incidence.
Exercise intolerance was the most common clinical history. All horses had an irregular heart rate and rhythm and the pulse and intensity of the heart sounds were variable. A separate study of 67 horses12 showed a significantly higher prevalence in Standardbreds and Thoroughbreds than other breeds of horse and a significant difference in the mean age at diagnosis between Standardbreds (4 years) and Thoroughbreds (9 years).
Racehorses commonly have a history of normality at rest but poor exercise tolerance following a race in which the horse ran well for the first 200–300 m but subsequently faded badly and finished a long way behind the field. Paroxysmal atrial fibrillation has also been observed in the horse under these circumstances. Horses with paroxysmal atrial fibrillation show atrial fibrillation when examined immediately following the race, but convert to normal sinus rhythm shortly after and have normal cardiovascular function if the examination as to cause of poor racing performance is delayed. A large scale study of 39 302 racehorses undergoing 404 090 race starts estimated a minimum prevalence of atrial fibrillation of 0.29%.13 The estimated prevalence was higher (1.39%) in horses that finished slowly or did not finish, and the prevalence increased markedly with age. Atrial fibrillation was paroxysmal in most horses, with 93% of horses with atrial fibrillation spontaneously converting to sinus rhythm within 24 hours of the race. Attempted conversion of horses with atrial fibrillation should therefore be delayed for at least a couple of days following a race, because most will convert spontaneously without treatment.
There is debate as to the cause of the benign form of atrial fibrillation and whether myocardial and vascular lesions are present in the atria of a significant proportion of animals with this arrhythmia. However, the high rate at which atrial fibrillation converts spontaneously or by treatment to be followed by successful racing performance suggests that this arrhythmia frequently occurs in young horses in the absence of significant atrial pathology. Benign atrial fibrillation in young racing horses therefore has many similarities to atrial fibrillation in lactating dairy cattle with abdominal disease, in that it is likely that most cases do not have underlying heart disease. The increased prevalence of atrial fibrillation in race horses with age13 suggests, however, that underlying heart disease does predispose to developing atrial fibrillation during a race.
Underlying disease
Horses may develop atrial fibrillation at fast heart rates in response to underlying cardiovascular disease. Commonly this is mitral valve insufficiency, tricuspid valve insufficiency or a combination of both but any acquired or congenital lesion that results in atrial hypertrophy has this risk.
Where there is underlying heart disease the ventricular rate at rest is much higher and the arrhythmia presents as a tachycardia. It has been suggested that a heart rate greater than 60 beats/min is indicative of underlying cardiac disease in cases of atrial fibrillation.12 In horses with atrial fibrillation ventricular filling is impaired at heart rates above 70 beats/min4 and at resting heart rates above 80–100 beats/min there is severe cardiac inefficiency and the animal rapidly develops signs of cardiac failure. At fast heart rates, atrial fibrillation presents with a syndrome clinically similar to ventricular tachycardia associated with multiple ventricular extrasystoles and electrocardiographic differentiation is required.
Primary pulmonary hypertension as a cause of atrial fibrillation is also recorded in horses, the increased resistance to right ventricular outflow leading to ventricular hypertrophy and dilatation, stretching of the right atrioventricular annulus, atrial dilatation and subsequent atrial fibrillation.14
Paroxysmal atrial fibrillation has been observed in newborn foals showing signs of respiratory distress and with birth anoxia.15
Atrial fibrillation in the cow
Atrial fibrillation in the cow may occur secondary to myocardial disease or endocarditis resulting in atrial enlargement, but more commonly is functional in occurrence and traditionally has not been associated with clinically detectable cardiac lesions.16,17 However, a recent histopathological study in nine Holstein-Friesian cows with atrial fibrillation and 12 healthy controls in sinus rhythm indicated that multifocal or large areas of myocardial fibrosis were present more frequently and with greater severity in cattle with atrial fibrillation than healthy controls.18 Interestingly, the atrial lesions were largely confined to the dorsal regions of the cranial lateral and medial regions of the right atrium. Organic heart disease therefore appears to predispose cattle to the development of atrial fibrillation, and an atrial fibrillation prevalence of 2.5% was recorded in apparently healthy lactating dairy cows over an 18 month period.17 In a large cross sectional study, atrial fibrillation was not observed during a 3–5 minute ECG recording in 952 of dairy cattle aged 1 or more years.19
In sick cattle, atrial fibrillation most commonly occurs in association with gastrointestinal disease, abnormalities causing abdominal pain and metabolic disease. Abnormalities as diverse as acute enteritis, left displacement of the abomasum and torsion of the uterus may be accompanied by this arrhythmia. Heightened excitation of the atria, in association with electrolyte and acid–base disturbances or due to change in vagal tone, has been postulated as a cause, and atrial premature complexes are also seen in the same types of clinical case.16,20 (Figure 8.1) The administration of neostigmine to cattle with gastrointestinal disease may precipitate the occurrence of atrial fibrillation.21 The arrhythmia usually converts spontaneously to sinus rhythm with correction of the abdominal disorder.
Atrial fibrillation in the sheep and goat
This may occur as a result of incompetence of the tricuspid or mitral valves, the presence of myocarditis or, in goats, as a sequel to interstitial pneumonia along with cor pulmonale. The presenting signs are those of respiratory distress and heart failure. Ascites is prominent and there is marked jugular distension with an irregular jugular pulse.
Treatment of atrial fibrillation
Ruminants
Ruminants with atrial fibrillation are not in general treated with specific antiarrhythmic drugs as the heart will usually revert to sinus rhythm following the correction of the underlying abdominal disorder and sufficient time (at least 1 week after return to normal physical health). However, the intravenous administration of quinidine (49 mg of quinidine sulfate/kg BW, at 0.20 (mg/kg)/min) was successful in converting seven of nine cows to normal sinus rhythm at a mean plasma quinidine concentration of 3.6 μg/mL.22 Oral quinidine administration is not effective in ruminants because of the poor oral bioavailability. Side effects of intravenous quinidine administration in cattle include depression, ataxia, blepharospasm, diarrhea and increased frequency of defecation.22 Response to treatment in sheep and goats is poor, although one ram was successfully converted to normal sinus rhythm using electrical cardioversion using 360 J and paddles placed over the right heart base (behind the triceps muscles) and the left cardiac apex close to the sternum.23
Horses
Horses with atrial fibrillation at high heart rates are generally not treated successfully as serious cardiac pathology is usually present. Digoxin and quinidine sulfate are used. The decision to treat a horse with atrial fibrillation at low heart rates depends upon the requirement for the horse to perform work, because horses with this arrhythmia can be retired and will live for several years. They may be used successfully as brood mares.
Horses with benign atrial fibrillation can be converted to normal sinus rhythm with subsequent return to successful racing or other performance.24,25 Oral quinidine sulfate is usually used. Quinidine is an antiarrhythmic agent of class 1a of Vaughan-Williams’s classification, slows intracardiac conduction by blocking the fast sodium channel and prolongs the action potential duration. Several dose regimens have been used, but the administration of an oral dose of 22 mg/kg every 2 hours until conversion is achieved or toxicity is manifest has proved effective.11,24 In the majority of cases, conversion will occur before the total dose exceeds 40 g. Toxicity is likely when the total dose exceeds 60 g and the decision to continue with therapy once this dose has been reached should be considered carefully. The plasma quinidine concentration required for cardioversion ranges from 2–4 μg/mL and toxicosis has been reported at μg/mL.
Toxicity is not uncommon with quinidine therapy and separate studies report 48% and 28% of horses with some form of adverse reaction.11,24 Depression, lassitude, anorexia, urticaria, congestion of the mucous membranes, colic and death are recorded. Prolongation of the QRS interval to 25% greater than pretreatment values has been considered a monitor for cardiovascular toxicity. The toxic effects of quinidine may be corrected by intravenous administration of sodium bicarbonate in an attempt to increase the percentage of quinidine bound to protein. Such treatment runs the risk of inducing hypokalemia, which may exacerbate quinidine toxicity. Some prefer to digitalize the horse intravenously prior to medication with quinidine in an attempt to reduce tachyarrhythmias at the point of conversion and those associated with quinidine toxicity. Nephrotoxicity with uremia and diarrhea can occur at lower doses. Nephrotoxicity is transient and repairs rapidly following withdrawal of the drug but the serum urea nitrogen concentration and urine should be monitored during therapy in addition to cardiovascular function.
There is a much greater success rate with conversion in young horses and when it is attempted shortly following the onset of the arrhythmia. If the arrhythmia has been present for more than 4 months, successful conversion is much less common, and side effects with therapy are more common.24 Following cardioversion the horse should be rested for 3 months. In some horses the conditions recur after a period of racing and repeated conversions with quinidine are possible. Conversion by intravenous quinidine gluconate is reported in the horse using an initial dose of 1.0–1.5 mg/kg, given over a period of 1 minute and repeated every 5–10 minutes until sinus rhythm is restored or the QRS interval increases 25% over baseline, ventricular rate exceeds 90 beats/min, signs of toxicity occur or a total dose of 11 mg/kg has been administered.25,26 Conversion by quinidine and atrial pacing is also recorded in the horse.27
Oral and intravenous flecainide has been used with mixed success to convert horses in atrial fibrillation. Flecainide is an antiarrhythmic agent of class 1c of Vaughan-Williams’s classification, slows intracardiac conduction by blocking the fast sodium channel and shortens the refractory period of the Purkinje fibers. Intravenous administration of flecainide acetate (1–2 mg/kg BW) infused at 0.2 (mg/kg BW)/min was effective in converting experimentally-induced atrial fibrillation in six horses and naturally occurring atrial fibrillation in two horses.28 The plasma flecainide concentration at the time of conversion was 1.3 mg/L. Oral administration of flecainide acetate (4–6 mg/kg BW) also produced plasma flecainide concentrations that approximated 1.3 mg/L28 for a number of hours. However, in a subsequent study in 10 horses with naturally occurring atrial fibrillation, intravenous flecainide failed to convert nine horses with long-standing atrial fibrillation to sinus rhythm, but did convert one horse who had been in atrial fibrillation for 12 days.29 Orally administered quinidine sulfate subsequently converted eight of the nine horses to normal sinus rhythm. Two horses administered flecainide developed potentially dangerous ventricular arrhythmias during treatment.
One horse was converted from atrial fibrillation using rectilinear biphasic electrical cardioversion, which is safer than conventional monophasic electrical cardioversion.30 General anesthesia is induced using agents that produce minimal cardiovascular depression (such as intravenous induction with guaifenesin, diazepam and ketamine and maintenance with sevoflurane). The front legs of the horse were extended and cardioversion–defibrillation pads were placed over both sides of the shaved thorax, directly over the atria, the position of which had been determined ultrasonographically. The horse converted to normal sinus rhythm after delivering 200 J in conjunction with a small amount of intravenous quinidine. Three horses were converted from atrial fibrillation using transvenous electrical cardioversion via placement of a custom-length 6.5 French bipolar catheter using ultrasonographic guidance.31 Catheter placement was manipulated so that one electrode was in the pulmonary artery and the other electrode in the vicinity of the right atrium. Cardioversion was accomplished at 125–300 J using a biphasic truncated exponential shock delivered to be not coincident with the T wave. Concurrent use of antiarrhythmic medications was not required. The use of electrical cardioversion should be considered in cases of atrial fibrillation refractory to oral or intravenous quinidine administration.
McQuirk SM, Muir WW. Diagnosis and treatment of cardiac arrhythmias. Symposium on Cardiology. Vet Clin North Am Equine Pract. 1985;1:353-370.
Bertone JJ, Wingfield WE. Atrial fibrillation in horses. Compend Contin Educ Pract Vet. 1987;9:763.
Fregin GF. Medical evaluation of the cardiovascular system. Vet Clin North Am Equine Pract. 1992;8:329.
Collatos C. Treating atrial fibrillation in the horse. Compend Contin Educ Pract Vet. 1995;17:243-245.
Marr CM. Cardiac emergencies and problems of the critical care patient. Vet Clin North Am Equine Pract. 2004;20:217-230.
1 Nielsen IJ. Aust Vet J. 1990;67:140.
2 Reimer JM, et al. J Am Vet Med Assoc. 1992;201:1237.
3 Delesalle C, et al. J Vet Intern Med. 2002;16:612.
4 Muir WW, McQuirk SM. Vet Clin North Am Equine Pract. 1985;1:335.
5 Meyer GA, et al. Equine Vet J. 2001;33:434.
6 Takemura N, et al. Jpn J Vet Sci. 1989;51:515.
7 Wijnberg ID, Ververs FFT. J Vet Intern Med. 2004;18:350.
8 Wijnberg ID, et al. Vet Rec. 1999;144:259.
9 Bertone JJ, Wingfield WE. Compend Contin Educ Pract Vet. 1987;9:763.
10 Muir WW, McQuirk SM. J Am Vet Med Assoc. 1986;184:965.
11 Deem DA, Fregin GF. J Am Vet Med Assoc. 1982;180:261.
12 Reef VB, et al. J Vet Intern Med. 1988;2:1.
13 Ohmura H, et al. J Am Vet Med Assoc. 2003;223:84.
14 Gelberg HB, et al. J Am Vet Med Assoc. 1991;198:679.
15 Machida N, et al. Equine Vet J. 1989;21:66.
16 Brightling P, Townsend HGG. Aust Vet J. 1983;24:331.
17 Machida N, et al. J Vet Med A. 1993;40:233.
18 Machida N, Kiryu K. J Vet Med Sci. 2001;63:873.
19 Rezakhani A, et al. Rev Med Vet. 2004;155:159.
20 Constable PD, et al. J Am Vet Med Assoc. 1990;197:1163.
21 Constable PD, et al. J Am Vet Med Assoc. 1990;196:329.
22 McGuirk SM, et al. J Am Vet Med Assoc. 1983;182:1380.
23 Moresco A, et al. J Am Vet Med Assoc. 2001;218:1264.
24 Reef VB, et al. J Vet Intern Med. 1988;2:1.
25 Collatos C. Compend Contin Educ Pract Vet. 1995;17:243.
26 Muir WW, et al. J Am Vet Med Assoc. 1990;197:1607.
27 Van Loon G, et al. Vet Rec. 1998;142:301.
28 Ohmura H, et al. J Vet Med Sci. 2001;63:511.
29 Van Loon G, et al. Equine Vet J. 2004;36:609.
Diseases of the heart
MYOCARDIAL DISEASE AND CARDIOMYOPATHY
Etiology Certain bacterial, viral, parasitic infections, some nutritional deficiencies and toxic agents
Epidemiology Specific to causative agent
Clinical findings Reduction of cardiac reserve and decreased exercise tolerance, cardiac arrhythmias, congestive heart failure or acute heart failure
Clinical pathology Electrocardiography, echocardiography and serum cardiac troponin I concentrations. Other examinations directed at determining the specific cause
Necropsy findings Myocarditis, myocardial degeneration
Treatment For cardiac insufficiency. Specific therapy, if available, for specific cause
ETIOLOGY
A number of diseases are accompanied by inflammation, necrosis or degeneration of the myocardium. These include several bacterial, viral or parasitic infections and some nutritional deficiencies and toxicities. In most cases, the involvement of the myocardium is only part of the total spectrum of these diseases, although the cardiac manifestations may be clinically pre-eminent. The term cardiomyopathy is generally restricted to those diseases where myocardial damage is the prime manifestation. Causes of myocardial dysfunction include the following.
Viral myocarditis
Parasitic myocarditis
This is primarily associated with Strongylus spp. (migrating larvae) cysticercosis, Sarcocystis spp. and Neospora caninum (in the neonatal calf). In a postmortem study of over 2000 equine hearts, 15% showed myocardial fibrosis in association with occlusive angiopathic change.1 No age association was found, but recent infarcts were more common in yearlings. It was postulated that these lesions result from thromboemboli from verminous plaques in the proximal thoracic aorta.
Toxicity
• Inorganic poisons – selenium, arsenic, mercury, phosphorus, thallium
• Gossypol from cotton seed cake
• The mycotoxin fumonisin when ingested by pigs and horses
• Fluoroacetate (1080) and poisoning by Acacia georgina, Gastrolobium and Oxylobium spp., Dichapetalum cymosum
• Plants and weeds, including members of Ixiolena, Pachystigma, Pavette, Asclepias, Geriocarpa, Cryptostigia, Albizia, Cassia, Digitalis, Urechites, Pimelea, Astragalus, Fadogia, Cicuta, Colchicum, Karwinskia, Vicia, Cicuta, Trigonella, Bryophyllum, Palicourea, Lupinus, Lantana, Kalanchoe, Homeria, Hymenoxys, Eupatorium spp.
• Trees, including gidgee, yew, oleander, avocado
• Grasses, including Phalaris tuberosa, corynetoxins in Lolium rigidum infested with nematodes and Corynebacterium spp. (also tunicamycin in rain-damaged infected wheat, pigs), cantharidin in hay infested with blister beetles (horses)
• Drugs including succinylcholine, catecholamines, xylazine (ruminants) monensin – especially in horses, but also cattle, sheep, and pigs – lasalocid and salinomycin in horses, pigs, cattle and sheep, maduramicin in cattle and sheep fed poultry litter, and Adriamycin (used experimentally to produce cardiomyopathy). Overdosing with doxycycline in veal calves
• Vitamin D and myocardial and endocardial calcification following ingestion of Cestrum diurnum, Solanum malacoxylon, Trisetum flavescens (see enzootic calcinosis); calcification also occurs with hypomagnesemia in milk-fed calves.
Inherited
• Malignant hyperthermia of swine
• Hypertrophic cardiomyopathy in swine
• Congenital cardiomyopathy of Polled Hereford and Horned Hereford calves with dense curly coats, and Japanese Black calves
• Inherited cardiomyopathy in adult cattle occurring in Red Holstein–Simmental crossbred cattle in Switzerland and Austria, Red Danish dairy cattle in Denmark, Holstein–Friesian cattle in the UK, Austria, Denmark, Sweden, Japan, Canada and Australia (see cardiomyopathy – inherited as an autosomal recessive gene)
• Glycogen storage disease – α-1,4-glucosidase deficiency in Shorthorn and Brahman cattle and Corriedale sheep.
Unknown or uncertain etiology
• Myocardial necrosis and hemorrhage secondary to acute lesions in the central nervous system3
• Exertional rhabdomyolysis of horses, capture myopathy of wild ruminants, restraint stress in swine
• Sudden death in young calves associated with acute heart failure and myocardial necrosis and precipitated by periods of intense excitement such as that experienced at feeding time4,5
• Myocardial lipofuscinosis (brown atrophy) in aged or cachectic cattle, especially Ayrshires, but often found in healthy animals at slaughter6
• Myocardial disease following mild upper respiratory disease in horses, especially when training or exercise is continued through the respiratory disease episode.
PATHOGENESIS
The primary effect of any myocardial lesion is to reduce cardiac reserve and limit compensation in circulatory emergencies. Minor lesions may only reduce performance efficiency while more severe lesions may produce greater clinical effect.
Most commonly, myocardial disease results in arrhythmias and conduction disturbances from primary involvement of the conduction system or establishment of excitatory foci within the myocardium. While the animal is at rest there may be minimal evidence of cardiac disease but catastrophic disturbances in cardiac conduction may occur under the adrenergic influences of exercise or excitement. The effects of pharmacological cardiotoxic agents in poisonous plants are frequently also initially manifest when the animals are moved or otherwise excited.
Endogenous or synthetic catecholamines, in their own right, can produce multifocal myocardial necrosis, especially in the left ventricle.7 Sympathetic overactivity and local catecholamine release in the myocardium has been postulated as the cause of myocardial disease accompanying acute brain lesions in domestic animals and myocardial disease associated with some forms of stress and overexertion.3,8
Myocardial disease may also result in congestive heart failure through its primary effect on the myocardium and the function of the heart as a pump.
CLINICAL FINDINGS
In early cases, or cases with mild or moderate myocardial damage, a decreased exercise tolerance is the usual initial presenting sign. This is usually accompanied by an increase in heart rate and heart size, although the latter may only be detectable by echocardiography. There may be clinically recognizable arrhythmia, particularly tachyarrhythmias associated with multiple ventricular ectopic foci. The characteristics of the pulse and heart sounds are also changed (see arrhythmias).
Animals with suspect myocardial disease but with no or minimal arrhythmic disturbances at rest can be judiciously exercised, which will frequently result in the expression of conduction or arrhythmic abnormality. Exercise or excitement should be avoided in animals with overt arrhythmias at rest.
In the late stages, or in cases with more severe myocardial damage, there may be sudden death or attacks of cardiac syncope due to acute heart failure, or severe dyspnea or general edema due to congestive heart failure. Details of the clinical findings associated with conduction disturbances, arrhythmias and heart failure have been given earlier.
CLINICAL PATHOLOGY
Electrocardiography and echocardiography are used in special examination. Hematological examination, blood culture and serology may be of value in determining the cause of myocardial disease and a full biochemical profile is advisable to determine if multisystemic problems are present. Myocardial infarction and necrosis may be associated with the release of cell enzymes into the bloodstream during the acute phase and the determination of the serum activities of lactate dehydrogenase, creatine kinase and aspartate aminotransaminase are of value.9,10
The cardiospecific isoenzyme troponin I provides the most sensitive and specific indication of cardiac necrosis (see chronic heart failure section) whereas the predictive value of serum creatinine kinase and lactate dehydrogenase activities is much lower.11,12 Toxicological examination and tests for nutritional trace element deficiencies may be indicated.
NECROPSY FINDINGS
Bacterial infections may cause discrete abscesses or areas of inflammation in the myocardium but viral infections and degeneration due to nutritional deficiencies and poisonings usually produce a visible pallor of the muscle, which may be uniform or present as streaks between apparently normal bundles of muscle. In acute cases, there may be petechial or linear hemorrhages in the myocardium. Calcification may occur in areas of myocardial damage and with enzootic calcinosis and vitamin D toxicity. The nature and distribution of myocardial damage within the heart can vary according to the inciting agent and this can be an aid to diagnosis. The degenerated muscle may also be present in only the inner layers of the wall, leaving the external layers with a normal appearance.
In coronary thrombosis infarction of a large area of the wall may have occurred but this is not visible unless the animal survives for at least 24 hours afterwards. Careful examination of the coronary arteries is usually necessary to detect the causative embolus. In horses infarction occurs most commonly in the right atrium.
The terminal stage of myocardial degeneration or myocarditis is often fibrous tissue replacement of the damaged tissue. The heart is flabby and thin-walled and shows patches of shrunken, tough fibrous tissue. Rupture of the atrial walls may result, with sudden death occurring as a result of the pressure of blood in the pericardial sac. The lesions of lymphomatosis are characteristic of this disease: large, uneven masses of pale, firm, undifferentiated tissue with the consistency of lymphoid tissue.
Focal myocardial fibrosis, possibly resulting from microembolism from strongyle-induced endarteritis, is common in healthy horses but has also been ascribed as the predisposing factor to conduction disturbances such as atrial fibrillation and heart block.13
The diagnosis and differential diagnosis of the specific etiology of myocardial disease rests with the epidemiological and other considerations of the individual causes and may require specific bacteriological and virological examinations, toxicological and nutritional analyses or an examination of the environment.
TREATMENT
The primary cause must be treated and details are given under the individual headings of the specific diseases listed above. When possible, the primary cause of the myocardial damage must be corrected or treated, and details are given elsewhere for the various etiologies listed above. The treatment of conduction disturbances, arrhythmias and heart failure is given elsewhere in this chapter.
1 Baker JR, Ellis CE. Equine Vet J. 1981;123:43. 47
2 Nout YS, et al. J Vet Intern Med. 2003;17:588.
3 King JM, et al. J Am Vet Med Assoc. 1982;180:144.
4 Rogers PAM, Poole DBR. Vet Rec. 1978;103:366.
5 Mee JF. Ir Vet J. 1990;43:61.
6 Bradley R, Duffell SJ. J Comp Pathol. 1982;92:85.
7 Van Vleet JF, et al. Am J Vet Res. 1977;38:991.
8 Van Vleet JF, Ferrans VJ. Am J Pathol. 1986;124:98.
9 Fujii Y, et al. Bull Equine Res Inst. 1983;20:87.
10 Zust J, et al. Vet Rec. 1996;139:391.
11 Marr CM. Equine Vet Educ. 1990;2:18.