PULMONARY HEMORRHAGE
Pulmonary hemorrhage is uncommon in farm animals but does occur occasionally in cattle, and exercise-induced pulmonary hemorrhage (EIPH) occurs in 45–75% of exercised horses. Pulmonary hemorrhage also occurs in horses with pulmonary abscesses, tumors or foreign bodies. Tracheobronchoscopic, radiographic and ultrasonographic examinations are useful in identifying the site and cause of the hemorrhage.
Cattle
In cattle the most common cause is erosion of pulmonary vessels adjacent to lesions of embolic pneumonia associated with vena caval thrombosis and hepatic abscessation. The onset of hemorrhage may be sudden and affected animals hemorrhage profusely and die after a short course of less than 1 hour. Marked epistaxis and hemoptysis, severe dyspnea, muscular weakness and pallor of the mucous membranes are characteristic. In other cases, episodes of epistaxis and hemoptysis may occur over a period of several days or a few weeks along with a history of dyspnea.
EXERCISE-INDUCED PULMONARY HEMORRHAGE OF HORSES (EIPH, BLEEDERS)
Epidemiology
Present in most (> 80%) Thoroughbred and Standardbred racehorses, although clinical signs are less common. Occurs worldwide in any horse that performs strenuous exercise. Rarely causes death
Pathogenesis
Probably associated with rupture of pulmonary capillaries by the high pulmonary vascular pressures generated during exercise. There may be a contributory role for inflammation and obstruction of small airways, and tissue damage caused by large and rapid changes in intrathoracic pressure
Clinical signs
Epistaxis is an uncommon but very specific sign of EIPH in horses that have just exercised. Affected horses may cough or suddenly slow during a race. Endoscopic examination of the trachea and bronchi reveals blood
Clinical pathology
Presence of hemosiderin-laden macrophages in tracheal aspirates or bronchial lavage fluid
Lesions
Fibrosis and discoloration of the caudodorsal regions of the lungs. Fibrosis, accumulation of hemosiderin-laden macrophages in interstitial tissue, inflammation and bronchial artery angiogenesis. Horses dying acutely have blood-filled airways and heavy, wet lungs
Epidemiology
EIPH is primarily a disease of horses, although it has been reported in racing camels.1 EIPH occurs in horses worldwide and there does not appear to be any geographical distribution. It is a disorder of horses that run at high speed, such as Thoroughbred or Standardbred racehorses. The disorder is uncommon in endurance horses or draft breeds, although it does occur in horses used for these activities. As a general rule, the more intense the exercise or the higher the speed attained, the greater the proportion of horses with EIPH.
The prevalence of EIPH varies with the method used to detect it and the frequency with which horses are examined, as discussed later in this section. Epistaxis associated with exercise is almost always attributable to pulmonary hemorrhage and occurs only in a small proportion of racehorses.2-5 Epistaxis occurs in only 3% of horses that have blood detected in the trachea by endoscopic examination performed within 2 hours of racing.5 The prevalence of epistaxis in racehorses varies between 0.1 and 9.0%, with the frequency depending on the breed, age and sex of horses selected for study, the type of racing and the timing and frequency of observation of horses after racing. Epistaxis is more common in older horses.2,3 There are conflicting reports of a sex predisposition, although epistaxis may be more common in female Thoroughbreds.2,3 Epistaxis is more common after races of less than 1600 m than in longer races,2 although not all sources agree on this point.3,6 However, horses in steeplechase races, which are typically longer than 2000 m, are at greater risk of epistaxis than are horses in flat races.2,6 Epistaxis is relatively uncommon and most horses with EIPH do not have epistaxis.
There are a variety of other methods of detecting EIPH, including endoscopic examination of the airways and microscopic examination of tracheal aspirates or bronchoalveolar lavage fluid.
Almost all Thoroughbred racehorses in active training have hemosiderophages in bronchoalveolar lavage fluid, indicating that all have some degree of EIPH.7 The prevalence of EIPH decreases when diagnosis is based on endoscopic examination of horses after exercise or racing.
Exercise-induced pulmonary hemorrhage is very common in Thoroughbred racehorses, with estimates of prevalence, based on a single endoscopic examination of the trachea and bronchi, of 43–75%.6,8-10 The prevalence increases with the frequency of examination, with over 80% of horses having evidence of EIPH on at least one occasion after examination after each of three consecutive races.11 The prevalence of EIPH in Standardbred racehorses is assumed to be lower, with 26–34% of horses reported to have blood in the trachea after racing.12,13 However, these studies were based on a single examination and one12 only reported as positive those horses with blood covering more than one half the tracheobronchial tree. When examined after each of three races, 87% of Standardbred racehorses have evidence of EIPH on at least one occasion,14 suggesting that EIPH is as common in Standardbred racehorses as it is in Thoroughbred racehorses.
Exercise-induced pulmonary hemorrhage occurs in approximately 62% of racing Quarter horses, and has been observed in Quarter horses used for barrel racing.15 The disorder occurs in racing Appaloosa horses.16 Approximately 11% of polo ponies are affected with EIPH.17 The disease occurs in draft horses but is not well documented.
Age is considered a risk factor for EIPH, with the prevalence of the disorder being higher in older horses.8-10 There is no consistent association of sex with prevalence of EIPH.8-1013 Among Thoroughbred racehorses the prevalence of EIPH increases with increasing speed,10,18 being greater in Thoroughbreds after racing than after breezing (galloping). Lesions of EIPH are not detected in young Thoroughbred racehorses that have trained at speeds of less than 7 m/s.10,18
Pathogenesis
The cause of EIPH is rupture of alveolar capillary membranes with subsequent extravasation of blood into interstitial and alveolar spaces.19 The source of blood in such instances is the pulmonary circulation. Bleeding from bronchial circulation during exercise has been suggested, based on histological evidence of bronchial angiogenesis in horses that have experienced previous episodes of EIPH,20 but contribution of the bronchial circulation to EIPH has not been demonstrated. Regardless of the contribution of bronchial circulation to blood in the airways, the likely initial lesion is in capillaries associated with the pulmonary circulation. Hemorrhage into the interstitial space and alveoli, with subsequent rostral movement of blood in the airways, results in blood in the trachea and bronchi.
Rupture of alveolar capillaries occurs secondary to an exercise-induced increase in transmural pressure (pressure difference between the inside of the capillary and the alveolar lumen). If the transmural stress exceeds the tensile strength of the capillary wall, the capillary ruptures.19 The proximate cause of alveolar capillary rupture is the high transmural pressure generated by positive intracapillary pressures, which are largely attributable to capillary blood pressure, and the lower intra-alveolar pressure generated by the negative pleural pressures associated with inspiration.
During exercise, the absolute magnitudes of both pulmonary capillary pressure and alveolar pressure increase, with a consequent increase in transmural pressure. Strenuous exercise is associated with marked increases in pulmonary artery pressure in horses.22-24 Values for mean pulmonary arterial pressure at rest of 20–25 mmHg increase to more than 90 mmHg during intense exercise because of the large cardiac output achieved by exercising horses. The increases in pulmonary artery pressure, combined with an increase in left atrial pressure during exercise, probably result in an increase in pulmonary capillary pressure. Combined with the increase in pulmonary capillary pressure is a marked decrease (more negative) in pleural, and therefore alveolar, pressure during exercise. The pleural pressure of normal horses during inspiration decreases from approximately –0.7 kPa (–5.3 mmHg) at rest to as low as –8.5 kPa (64 mmHg) during strenuous exercise.25 Together, the increase in pulmonary capillary pressure and decrease (more negative) in intrapleural (alveolar) pressure contribute to a marked increase in stress in the alveolar wall. Although the alveolar wall and pulmonary capillaries of horses are stronger than those of other species, rupture may occur because the wall stress in the alveolus exceeds the mechanical strength of the capillary.26
Other theories of the pathogenesis of EIPH include: small-airway disease, upper airway obstruction, hemostatic abnormalities, changes in blood viscosity and erythrocyte shape, intrathoracic sheer forces associated with gait, and bronchial artery angiogenesis.20,27 It is likely that the pathogenesis of EIPH involves several processes, including pulmonary hypertension, lower alveolar pressure and changes in lung structure, that summate to induce stress failure of pulmonary capillaries.
Obstruction of either the upper or lower airways has been proposed as a cause of EIPH. Inspiratory airway obstruction results in more negative intrapleural, and therefore alveolar, pressures. This effect is exacerbated by exercise, with the result that alveolar transmural pressure is greater in horses with airway obstruction.28,29 The higher transmural pressure in such horses may increase the severity of EIPH, although this has not been demonstrated. Moreover, while inspiratory airway obstruction may predispose to EIPH, the prevalence of this condition is much less than that of EIPH, indicating that it is not the sole factor inducing EIPH in most horses.
Horses with moderate to severe EIPH have histological evidence of inflammation of the small airways,18,30 and there is a clear association between the presence of EIPH and inflammatory changes in bronchoalveolar or tracheal aspirate fluid.6 However, instillation of autologous blood into the airways induces a marked inflammatory response in normal horses,31 and it is therefore unclear whether inflammation alone induces or predisposes to EIPH or whether the inflammation is a result of EIPH. Theoretically, small-airway inflammation and bronchoconstriction have the potential to produce intrathoracic airway obstruction and, therefore, a more negative alveolar pressure. Given that small-airway disease is common in horses, there is the potential for an important effect of factors, such as viral infections, air pollution and allergic airway disease, to contribute to the initiation or propagation of EIPH.
The characteristic location of lesions of EIPH in the caudodorsal lung fields has led to the proposal that hemorrhage is a result of tissue damage occurring when waves of stress, generated by forelimb foot strike, are focused and amplified into the narrowing cross-sectional area of the caudal lung lobes.27 According to the theory, the locomotor impact of the forelimbs results in transmission of forces through the scapula to the body wall, from where they pass into the lungs and caudally and dorsally. As the wave of pressure passes into the narrower caudodorsal regions of the lungs it generates progressively greater shearing forces that disrupt tissue and cause EIPH. However, studies of intrapleural pressures have not demonstrated the presence of a systemic pressure wave passing through the lung and do not provide support for this hypothesis.32
Horses with EIPH have been suspected of having defects in either hemostasis or fibrinolysis. However, while exercise induces substantial changes in blood coagulation and fibrinolysis, these is no evidence that horses with EIPH have defective coagulation or increased fibrinolysis.33,34
Regardless of the cause, rupture of pulmonary capillaries and subsequent hemorrhage into airways and interstitium causes inflammation of both airways and interstitium with subsequent development of fibrosis and alteration of tissue compliance. Heterogeneity of compliance within the lungs, and particularly at the junction of normal and diseased tissue, results in the development of abnormal shear stress with subsequent tissue damage. These changes are exacerbated by inflammation and obstruction of small airways, with resulting uneven inflation of the lungs.35 The structural abnormalities, combined with pulmonary hypertension and the large intrathoracic forces associated with respiration during strenuous exercise, cause repetitive damage at the boundary of normal and diseased tissue with further hemorrhage and inflammation. The process, once started, is lifelong and continues for as long as the horse continues to perform strenuous exercise.20
Clinical findings
Poor athletic performance or epistaxis are the most common presenting complaints for horses with EIPH. While poor performance may be attributable to any of a large number of causes, epistaxis associated with exercise is almost always secondary to EIPH.
Epistaxis due to EIPH occurs during or shortly after exercise and is usually first noticed at the end of a race, particularly when the horse is returned to the paddock or winner’s circle and is allowed to lower its head. It is usually bilateral and resolves within hours of the end of the race. Epistaxis may occur on more than one occasion, especially when horses are raced or exercised at high speed soon after an initial episode.
Exercise-induced pulmonary hemorrhage and performance
Failure of racehorses to perform to the expected standard (poor performance) is often, accurately or not, attributed to EIPH. Many horses with poor performance have cytological evidence of EIPH on microscopic examination of tracheobronchial aspirates or bronchoalveolar lavage fluid or have blood evident on endoscopic examination of the tracheobronchial tree performed 30–90 minutes after strenuous exercise or racing.7,36 However, it is important to recognize that EIPH is very common in racehorses and it should be considered the cause of poor performance only after other causes have been eliminated. Severe EIPH undoubtedly results in poor performance and, on rare occasions, death of Thoroughbred racehorses.37 Thoroughbred horses with EIPH racing in Victoria, Australia have impaired performance compared to unaffected horses. Affected horses have a lower likelihood of finishing in the first three places, are less likely to be elite money earners and finish further behind the winner than do unaffected horses.5
Results of studies in Standardbred racehorses indicate either a lack of effect of EIPH on performance or an association between EIPH and superior performance. There was no relationship between presence of EIPH and finishing position in 29 Standardbred racehorses with intermittent EIPH examined on at least two occasions,14 nor in 92 Standardbred racehorses examined on one occasion.13 However, of 965 Standardbred racehorses examined after racing, those finishing first or second were 1.4 times more likely (95% confidence interval 0.9–2.2) to have evidence of EIPH on tracheobronchoscopic examination than were horses that finished in seventh or eighth position.38
Physical examination
Apart from epistaxis in a small proportion of affected horses, there are few abnormalities detectable on routine physical examination of horses with EIPH. Rectal temperature and heart and breathing rates may be elevated as a consequence of exercise in horses examined soon after exercise, but values of these variables in horses with EIPH at rest are not noticeably different from horses with no evidence of EIPH. Affected horses may swallow more frequently during recovery from exercise than do unaffected horses, probably as a result of blood in the larynx and pharynx. Coughing is common in horses recovering from strenuous exercise and after recovery from exercise; horses with EIPH are no more likely to cough than are unaffected horses. Other clinical signs related to respiratory abnormalities are uncommon in horses with EIPH. Respiratory distress is rare in horses with EIPH and, when present, indicates severe hemorrhage or other serious lung disease such as pneumonia, pneumothorax or rupture of a pulmonary abscess. Lung sounds are abnormal in a small number of EIPH-affected horses and when present are characterized by increased intensity of normal breath sounds during rebreathing examination. Tracheal rales may be present in horses with EIPH but are also heard in unaffected horses.
Tracheobronchoscopy
Observation of blood in the trachea or large bronchi of horses 30–120 minutes after racing or strenuous exercise provides a definitive diagnosis of EIPH. The amount of blood in the large airways varies from a few small specks on the airway walls to a stream of blood occupying the ventral one-third of the trachea. Blood may also be present in the larynx and nasopharynx. If there is a strong suspicion of EIPH and blood is not present on a single examination conducted soon after exercise, the examination should be repeated in 60–90 minutes. Some horses with EIPH do not have blood present in the rostral airways immediately after exercise, but do so when examined 1–2 hours later. Blood is detectable by tracheobronchoscopic examination for 1–3 days in most horses, with some horses having blood detectable for up to 7 days.
Bronchoscopic examination can be used to estimate the severity of EIPH through the use of a grading system.39,40 The interobserver repeatability of tracheobronchoscopic assessment of severity of EIPH using a 0–4 grading scale is excellent:40
• Grade 0: No blood detected in the pharynx, larynx, trachea or main stem bronchi
• Grade 1: Presence of one or more flecks of blood or ≤ 2 short (< quarter the length of the trachea) narrow (< 10% of the tracheal surface area) streams of blood in the trachea or main stem bronchi visible from the tracheal bifurcation
• Grade 2: One long stream of blood (> half the length of the trachea) or > 2 short streams occupying less than one-third of the tracheal circumference
• Grade 3: Multiple, distinct streams of blood covering more than one-third of the tracheal circumference. No blood pooling at the thoracic inlet
• Grade 4. Multiple, coalescing streams of blood covering > 90% of the tracheal surface with pooling of blood at the thoracic inlet.
It is assumed that a higher score represents more severe hemorrhage, but while the repeatability of this scoring system has been established, the relationship between the amount of blood in the large airways and the actual amount of hemorrhage has not been established.
Radiography
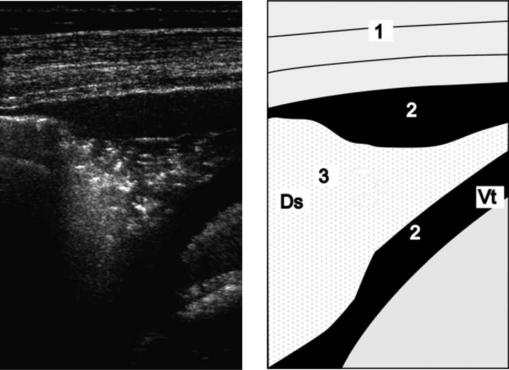
Thoracic radiography is of limited use in detecting horses with EIPH. Radiographs may demonstrate the presence of densities in the caudodorsal lung fields of some horses but many affected horses have minimal to undetectable radiographic abnormalities.41 Examination of thoracic radiographs of horses with EIPH may be useful in ruling out the presence of another disease process, such as a pulmonary abscess, contributing to the horse’s pulmonary hemorrhage or poor athletic performance.
Prognosis
Horses that have experienced one episode of epistaxis are more likely to have a second episode. For this reason most racing jurisdictions do not permit horses with epistaxis to race for a period of weeks to months after the initial instance, with more prolonged enforced rest after a subsequent episode of epistaxis and retirement from racing after a third bout. The recurrence rate after one episode of epistaxis in Thoroughbred horses is approximately 13.5% despite affected horses not being permitted to race for 1 month after the initial episode.2 This high rate of recurrence suggests that the inciting pulmonary lesions have not healed.
Clinical pathology
Examination of airway secretions or lavage fluid
The presence of red cells or macrophages containing either effete red cells or the breakdown products of hemoglobin (hemosiderophages) in tracheal or bronchoalveolar lavage fluid provides evidence of EIPH. Detection of red cells or hemosiderophages in tracheal aspirates or bronchoalveolar lavage fluid is believed to be both sensitive and specific in the diagnosis of EIPH.7 Examination of airway fluids indicates the presence of EIPH in a greater proportion of horses than does tracheobronchoscopic examination after strenuous exercise or racing. The greater sensitivity of examination of airway fluid is probably attributable to the ability of this examination to detect the presence of small amounts of blood or its residual products and the longevity of these products in the airways. While endoscopic examination may detect blood in occasional horses up to 7 days after an episode of EIPH, cellular evidence of pulmonary hemorrhage persists for weeks after a single episode.42 Red blood cells and macrophages containing red cells are present in bronchoalveolar lavage fluid or tracheal aspirates for at least 1 week after strenuous exercise or instillation of autologous blood into airways and hemosiderophages are present for at least 21 days and possibly longer.42
Recent studies have reported on the use of red cell numbers in bronchoalveolar lavage fluid as a quantitative indicator of EIPH. However, this indicator of EIPH severity has not been validated nor demonstrated to be more reliable or repeatable than tracheobronchoscopic examination and visual scoring. Furthermore, considerable concern exists over the suitability of red cell counts in bronchoalveolar lavage fluid for assessment of severity of EIPH given that an unknown area, although presumably small, of the lung is examined by lavage and that there is a risk that this area of lung may not be representative of the lung as a whole, similar to the situation of examination of bronchoalveolar lavage fluid of horses with pneumonia. Bronchoalveolar lavage of sections of both lungs, achieved using an endoscope, may obviate some of these concerns.
Tracheal aspirates may be obtained any time after exercise by aspiration either during tracheobronchoscopic examination or through a percutaneous intratracheal needle. Aspirates obtained through an endoscope may not be sterile, depending on the collection technique. Bronchoalveolar lavage fluid can be obtained through either an endoscope wedged in the distal airway or a cuffed tube inserted blindly into a distal airway. Collection of fluid through an endoscope has the advantage of permitting examination of the distal airways and selection of the area of lung to be lavaged. However, it does require the use of an endoscope that is longer (2 m) than those readily available in most equine practices. Use of a commercial bronchoalveolar lavage catheter does not require use of an endoscope and this procedure can be readily performed in field situations.
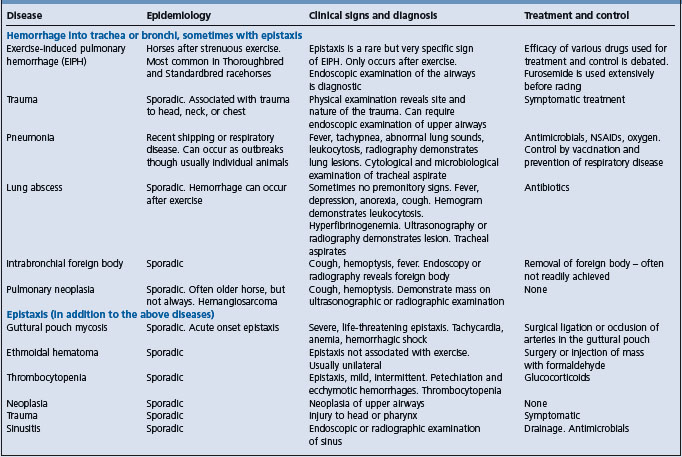
Epistaxis and hemorrhage into airways can occur as a result of a number of diseases (Table 10.5).
Necropsy
Exercise-induced pulmonary hemorrhage is a rare cause of death of racehorses, but among race horses that die during racing for reasons other than musculoskeletal injuries, EIPH is common.37 Necropsy examination of horses is usually incidental to examination for another cause of death. Pertinent abnormalities in horses with EIPH are restricted to the respiratory tract. Grossly, horses examined within hours of strenuous exercise, such as horses examined because of catastrophic musculoskeletal injuries incurred during racing, may have severe petechiation in the caudodorsal lung fields. Horses with chronic disease have blue/gray or blue/brown discoloration of the visceral pleural surfaces of the caudodorsal lung fields that is often sharply demarcated, especially on the diaphragmatic surface. The discoloration affects both lungs equally with 30–50% of the lung fields being discolored in severe cases. Affected areas do not collapse to the same extent as unaffected areas and, in the deflated lung, have a spleen-like consistency. On cut surface, the discolored areas of lung are predominantly contiguous with the dorsal pleural surface and extend ventrally into the lung parenchyma. Areas of affected lung may be separated by normal lung. There is proliferation of bronchial vessels, predominantly arteries and arterioles, in affected areas. Histologically, affected areas exhibit bronchiolitis, hemosiderophages in the alveolar lumen and interstitial spaces, and fibrosis of interlobular septa, pleural and around vessels and bronchioles.
Treatment
Therapy of EIPH is usually a combination of attempts to reduce the severity of subsequent hemorrhage and efforts to minimize the effect of recent hemorrhage. Treatment of EIPH is problematic for a number of reasons. Firstly, the pathogenesis of EIPH has not been determined although the available evidence supports a role for stress failure of pulmonary capillaries secondary to exercise-induced pulmonary hypertension. Secondly, there is a lack of information using large numbers of horses under field conditions that demonstrates an effect of any medication or management practice (with the exception of bedding) on EIPH. There are numerous studies of small numbers of horses (< 40) under experimental conditions but these studies often lacked the statistical power to detect treatment effects and, furthermore, the relevance of studies conducted on a treadmill to horses racing competitively is questionable. Treatments for EIPH are usually intended to address a specific aspect of the pathogenesis of the disease and will be discussed in that context.
Prevention of stress failure of the pulmonary capillaries
There is interest in reducing the pressure difference across the pulmonary capillary membrane in an effort to reduce EIPH. Theoretically, this can be achieved by reducing the pressure within the capillary or increasing (making less negative) the pressure within the intrathoracic airways and alveolus.
Reducing pulmonary capillary pressure
Furosemide administration as prophylaxis of EIPH is permitted in a number of racing jurisdictions worldwide, most notably Canada, the USA, Mexico and most of the South American countries. Within the USA and Canada, almost all Thoroughbred, Standardbred and Quarter horse racing jurisdictions permit administration of furosemide before racing.
The efficacy of furosemide in treatment of EIPH is uncertain. While field studies of large numbers of horses do not demonstrate an effect of furosemide on the prevalence of EIPH,43 studies of Thoroughbred horses running on a treadmill provide evidence that furosemide reduces the severity of EIPH.44 Under field conditions, based on tracheobronchoscopic evaluation of the severity of bleeding, furosemide has been reported to reduce or have no influence on the severity of bleeding.43,45 This apparent inconsistency may be attributable to measurement of red blood cell counts in bronchoalveolar lavage fluid of horses that have run on a treadmill not being representative of effects of furosemide under field conditions. The weight of evidence, albeit unconvincing, from field studies does not support a role for furosemide in preventing or reducing the severity of EIPH.
The mechanism by which furosemide may reduce the severity of EIPH is unknown, although it is speculated that furosemide, by attenuating the exercise-induced increase in pulmonary artery and pulmonary capillary pressure of horses, reduces the frequency or severity of pulmonary capillary rupture.
Furosemide is associated with superior performance in both Thoroughbred and Standardbred racehorses,46,47 which further complicates assessment of its efficacy in treating EIPH.
An increase in pulmonary capillary pressure secondary to altered rheostatic properties of blood during exercise has been suggested as a possible contributing factor for EIPH.48
Increasing alveolar inspiratory pressure
Airway obstruction, either intrathoracic or extrathoracic, increases airway resistance and results in a more negative intrathoracic (pleural) pressure during inspiration to maintain tidal volume and alveolar ventilation. Causes of extrathoracic airway obstruction include laryngeal hemiplegia and other abnormalities of the upper airway, whereas intrathoracic obstruction is usually a result of bronchoconstriction and inflammatory airway disease. Horses with partial extrathoracic inspiratory obstruction or bronchoconstriction and airway inflammation associated with recurrent airway obstructive disease (heaves) have pleural (and hence alveolar) pressures that are lower (more negative) than those in unaffected horses or in horses after effective treatment.
Partial inspiratory obstruction, such as produced by laryngeal hemiplegia, exacerbates the exercise-induced decrease in intrapleural pressures with a consequent increase in transmural capillary pressures.28,29 These changes may exacerbate the severity of EIPH although an association between upper airway obstructive disease and EIPH has not been demonstrated. Surgical correction of airway obstruction is expected to resolve the more negative intrapleural pressure, but its effect on EIPH is unknown.
Recently, the role of the nares in contributing to upper airway resistance, and hence lowering inspiratory intrapleural pressure during intense exercise, has attracted the attention of some investigators. Application of nasal dilator bands (Flair® strips) reduces nasal resistance by dilating the nasal valve and reduces red cell count of bronchoalveolar lavage fluid collected from horses after intense exercise on a treadmill.44 Furthermore, application of the nasal dilator strips to horses in simulated races reduces red cell count in bronchoalveolar lavage fluid of some, but not all, horses.49
The role of small-airway inflammation and bronchoconstriction in the pathogenesis of EIPH is unclear. However, horses with EIPH are often treated with drugs intended to decrease lower airway inflammation and relieve bronchoconstriction. Beta-adrenergic bronchodilatory drugs such as clenbuterol and albuterol (salbutamol) are effective in inducing bronchodilation in horses with bronchoconstriction, but their efficacy in preventing EIPH is either unknown or, in very small studies, is not evident. Corticosteroids, including dexamethasone, fluticasone and beclomethasone administered by inhalation, parenterally or enterally reduce airway inflammation and obstruction but have no demonstrated efficacy in preventing EIPH. Cromolyn sodium (sodium cromoglycate) has no efficacy in preventing EIPH.
Water vapor treatment (inhalation of water-saturated air) has been proposed as a treatment for EIPH because of its putative effect on small-airway disease. However, water vapor treatment has no effect on EIPH.
The use of bedding of low allergenic potential (shredded paper) to prevent EIPH has no apparent effect on prevalence of the condition.50 While it is suggested that preventing or minimizing small-airway disease may reduce the severity of EIPH, studies to demonstrate such an effect have not been reported. However, optimizing the air quality in barns and stables and preventing infectious respiratory disease appear sensible precautions.
Interstitial inflammation and bronchial angiogenesis
Hemorrhage into interstitial tissues induces inflammation with subsequent development of fibrosis and bronchial artery angiogenesis.30,42 The role of these changes in perpetuating EIPH in horses is unclear but is probably of some importance. Treatments to reduce inflammation and promote healing with minimal fibrosis have been proposed. Rest is an obvious recommendation and many racing jurisdictions have rules regarding enforced rest for horses with epistaxis. While the recommendation for rest is intuitive, there is no information that rest reduces the severity or incidence of EIPH in horses with prior evidence of this disorder.
Similarly, corticosteroids are often administered, either by inhalation, enterally or parenterally, in an attempt to reduce pulmonary inflammation and minimize fibrosis. Again, the efficacy of this intervention in preventing or minimizing severity of EIPH has not been documented.
Excessive bleeding
Coagulopathy and fibrinolysis
Exercise induces substantial changes in blood coagulation and fibrinolysis. However, there is no evidence that horses with EIPH have defective coagulation or increased fibrinolysis.33,34 Regardless, aminocaproic acid, a potent inhibitor of fibrin degradation, has been administered to horses to prevent EIPH. The efficacy of aminocaproic acid in preventing EIPH has not been demonstrated. Similarly, estrogens are given to horses with the expectation of improving hemostasis, although the effect of estrogens on coagulation in any species is unclear. There is no evidence that estrogens prevent EIPH in horses.
Vitamin K is administered to horses with EIPH, presumably in the expectation that it will decrease coagulation times. However, as EIPH is not associated with prolonged bleeding times, it is unlikely that this intervention will affect the prevalence or severity of EIPH.
Platelet function
Aspirin inhibits platelet aggregation in horses and increases bleeding time. Seemingly paradoxically, aspirin is sometimes administered to horses with EIPH because of concerns that increased platelet aggregation contributes to EIPH. There is no evidence that aspirin either exacerbates or prevents EIPH.
Capillary integrity
Capillary fragility increases the risk of hemorrhage in many species. Various bioflavonoids have been suggested to increase capillary integrity and prevent bleeding. However, hesperidin and citrus bioflavonoids have no efficacy in prevention of EIPH in horses. Similarly, vitamin C is administered to horses with EIPH without scientific evidence of any beneficial effect.
Summary of treatment options
Selection of therapy for horses with EIPH is problematic. Given that most horses have some degree of pulmonary hemorrhage during most bouts of intense exercise, the decision must be made not only as to the type of treatment and its timing but also which horses to treat. Moreover, the apparently progressive nature of the disease with continued work highlights the importance of early and effective prophylaxis and emphasizes the need for studies of factors such as air quality and respiratory infections in inciting the disorder.
The currently favored treatment for EIPH is administration of furosemide before intense exercise. Its use is permitted in racehorses in a number of countries. Increasingly persuasive laboratory evidence of an effect of furosemide in reducing red cell count in bronchoalveolar lavage fluid collected from horses soon after intense exercise supports the contention that furosemide is effective in reducing the severity of EIPH in race horses. However, it should be borne in mind that neither the relationship between severity of EIPH and red cell count in bronchoalveolar lavage fluid nor the efficacy of furosemide in reducing severity of EIPH in race horses in the field has been demonstrated. In fact, there is evidence that furosemide does not reduce the prevalence of EIPH and other evidence that it does not reduce the severity of EIPH under field conditions. The association between furosemide administration and superior performance in Standardbred and Thoroughbred racehorses should be borne in mind when recommending use of this drug.
Prevention and control
There are no documented preventive strategies. Rest is an obvious recommendation for horses with EIPH, but the hemorrhage is likely to recur when the horse is next strenuously exercised. The duration of rest and the optimal exercise program to return horses to racing after EIPH is unknown, although some jurisdictions require exercise no more intense than trotting for 2 months. Firm recommendations cannot be made on duration of rest because of a lack of objective information.
Although a role for lower airway disease (either infectious or allergic) in the genesis of EIPH has not been demonstrated, control of infectious diseases and minimization of noninfectious lower airway inflammation appears prudent.
Concern about the role of impact waves in the genesis of EIPH has led to discussion of ‘low-stress’ training protocols, but these have not been adequately evaluated.
1 Akbar SJ, et al. Vet Rec. 1994;135:624.
2 Takahashi T, et al. J Am Vet Med Assoc. 2001;218:1462.
3 Weideman H, et al. J S Afr Vet Assoc. 2003;74:127.
4 Williams R, et al. Equine Vet J. 2001;33:478.
5 Hinchcliff KW, et al. J Am Vet Med Assoc. 2005;227:768.
6 Newton JR, Wood JLN. Equine Vet J Suppl. 2002;34:417.
7 McKane SA, et al. Aust Vet J. 1993;70:401.
8 Mason DK, et al. Snow DH, Persson SGB, Rose RJ, editors. Equine exercise physiology. Cambridge: Granta. 1983:57.
9 Pascoe J, et al. Am J Vet Res. 1981;42:703.
10 Raphel CF, Soma LR. Am J Vet Res. 1982;43:11237.
11 Sweeney CR, et al. Am J Vet Res. 1990;51:772.
12 MacNamara B, et al. J Am Vet Med Assoc. 1990;196:443.
13 Speirs VC, et al. Aust Vet J. 1982;59:38.
14 Lapointe JM, et al. Equine Vet J. 1994;26:482.
15 Hillidge CJ, et al. J Equine Vet Sci. 1984:4.
16 Hillidge CJ, et al. J Equine Vet Sci. 1986;5:351.
17 Voynick BT, Sweeney CR. J Am Vet Med Assoc. 1986;188:301.
18 Oikawa M. J Comp Pathol. 1999;121:339.
19 West JB, et al. J Appl Physiol. 1993;75:1097.
20 Pascoe JR. Proc Am Assoc Equine Pract. 1996;42:220.
21 West JB, Mathieu-Costello O. Equine Vet J. 1994;26:441.
22 Birks EK, et al. J Appl Physiol. 1997;82:1584.
23 Langsetmo I, et al. Equine Vet J. 2000;32:379.
24 Manohar M, et al. Br Vet J. 1993;149:419.
25 Art T, et al. Respir Physiol. 1990;82:279.
26 Birks EK, et al. Respir Physiol. 1994;97:235.
27 Schroter RC, et al. Equine Vet J. 1998;30:186.
28 Hackett RP, et al. Am J Vet Res. 1999;60:485.
29 Ducharme NG, et al. Equine Vet J Suppl. 1999;30:27.
30 O’Callaghan MW, et al. Equine Vet J. 1987;19:411.
31 McKane SA, Slocombe RF. Equine Vet J Suppl. 2002;34:451.
32 Jones JH, et al. Equine Vet J Suppl. 2002;34:391.
33 Bayly WM, et al. Snow DH, Persson SGB, Rose RJ, editors. Equine exercise physiology. Cambridge: Granta. 1983:64.
34 Johnstone IB, et al. Can J Vet Res. 1991;55:101.
35 Robinson NE, Derksen FJ. Proc Am Assoc Equine Pract. 1980;26:421.
36 Martin BBJr, et al. J Am Vet Med Assoc. 1999;214:673.
37 Boden LA, et al. Equine Vet J. 2005;37:269.
38 Rohrbach BW. J Am Vet Med Assoc. 1990;196:1563.
39 Pascoe JR, et al. Am J Vet Res. 1981;42:703.
40 Hinchcliff KW, et al. Am J Vet Res. 2005;66:596.
41 Pascoe JR, et al. Vet Rad. 1983;24:85.
42 McKane S, Slocombe R. Equine Vet J. 1999;30:126.
43 Birks EK, et al. Equine Vet J Suppl. 2002;34:375.
44 Geor RJ, et al. Equine Vet J. 2001;33:577.
45 Pascoe JR, et al. Am J Vet Res. 1985;46:2000.
46 Gross DK, et al. J Am Vet Med Assoc. 1999;215:670.
47 Soma LR, et al. Equine Vet J. 2000;32:334.
48 Fedde MR, Erickson HH. Equine Vet J. 1998;30:329.