Diseases associated with Salmonella species
SALMONELLOSIS (PARATYPHOID)
Etiology Salmonella spp. Cattle: Salmonella typhimurium, Salmonella dublin, Salmonella newport. Sheep and goats: S. typhimurium, S. dublin. Horses: S. typhimurium. Pigs: Salmonella choleraesuis
Epidemiology Worldwide. Important zoonosis and foodborne illness. Prevalence of infection in healthy animals varies according to species and country. Incidence of clinical disease lower than prevalence; outbreaks occur precipitated by stressors. Spread by direct or indirect means; infected animal source of organism, which contaminates feed and water supplies.
Disease may become endemic on farm. Carrier animals shed organism and may introduce infection into herd. Deprivation of feed and water, transportation, drought, intensive grazing and housing, mixing animals from different sources contribute to onset of disease. Antimicrobial resistance major public health problem. Subclinical infection in pigs potential zoonosis
Signs Septicemia in neonatal ruminants and foals, and in pigs up to 4 months of age with high case fatality rate. Acute diarrhea and dysentery, fibrinous fecal casts, fever, marked dehydration, toxemia; chronic enteritis; abortion; dry gangrene of extremities; arthritis and foci of osteomyelitis. Severe diarrhea and dehydration characteristic in horse
Clinical pathology Culture organism from feces; detect organism with special tests; hematology for changes in leukon and clinical chemistry electrolyte changes
Lesions Septicemic hemorrhages. Mucoenteritis to marked fibrinohemorrhagic necrotic enteritis; enlarged mesenteric lymph nodes. Kidney petechiation in pigs. Foci of necrosis and thickened intestinal wall in chronic enteritis. Culture organism from blood, spleen, liver, lymph nodes
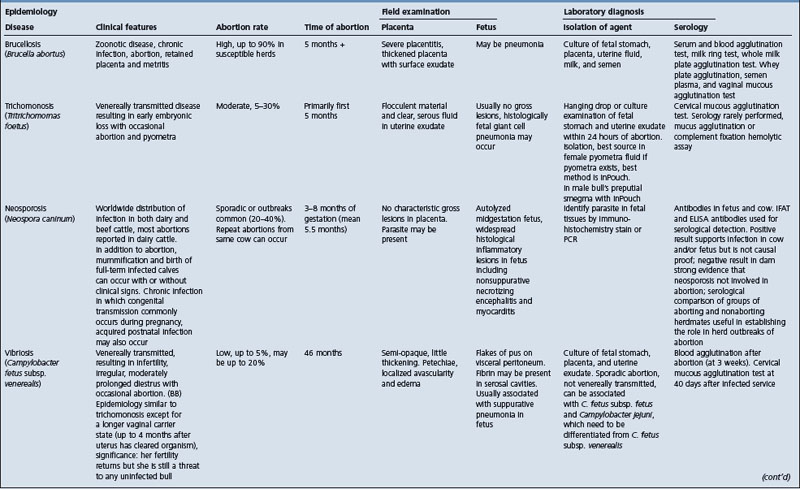
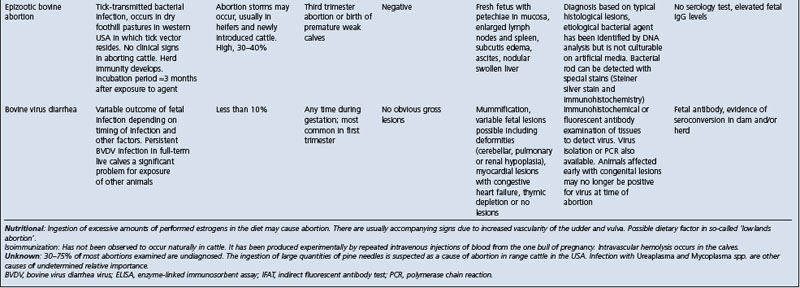
• Abortion: see Table 18.7
Treatment Antimicrobials. Supportive fluid and electrolyte therapy
Control Prevent introduction of infection into herd. Limit spread of infection within herd by identification of carrier animals, prophylactic antimicrobials, restricting movement of animals, clean water supply, hygiene and disinfection of buildings. Avoid spread of infection in veterinary clinics, dispose of infective materials. Vaccines for immunization are available but not effective
ETIOLOGY
The genus Salmonella belongs to the family Enterobacteriaceae. Currently there are 2463 serotypes (serovars) of Salmonella.1 The antigenic formulae of Salmonella serotypes are defined and maintained by the World Health Organization (WHO) Collaborating Centre for Reference and Research on Salmonella at the Pasteur Institute, Paris, France, and new serotypes are listed in annual updates of the Kaufmann–White scheme.
In 1986, the Subcommittee of Enterobacteriaceae of the International Committee on Systematic Bacteriology recommended that the type species for Salmonella be changed to Salmonella enterica. However, Salmonella typhi and Salmonella choleraesuis were maintained as type species.1 The literature on Salmonella nomenclature has been reviewed.1
According to the current system used by the Centers for Disease Control, the genus Salmonella contains two species, each of which contains multiple serotypes.1 The two species are S. enterica, the type species, and Salmonella bongori. S. enterica subsp. enterica is divided into six subspecies. S. enterica subsp. enterica I is divided into serotypes, for example serotypes enteriditis, typhimurium, typhi, and choleraesuis. At the first citation of a serotype the genus name is given followed by the word ‘serotype’ or the abbreviation ‘ser.’ and then the serotype name (e.g. Salmonella serotype or ser. typhimurium). Subsequently, the name may be written with the genus followed directly by the serotype name (e.g. Salmonella typhimurium or S. typhimurium). The majority (59%) of the 2463 Salmonella serotypes belong to S. enterica subsp. I (S. enterica subsp. enterica).
Serovars of S. enterica subsp. I are associated mainly with warm-blooded vertebrates and are responsible for most Salmonella infections in humans and domesticated animals. Salmonella serovars differ in the range of hosts they can infect and in the nature of disease that may result: this difference is referred to as serovar–host specificity. Some Salmonella serovars, for example typhimurium and enteritidis, can infect a wide range of hosts and are termed ubiquitous. They are usually associated with a relatively mild enteric disease, although in some hosts, such as mice, the disease can be systemic and severe. Other serovars are very restricted in their host range, causing severe systemic disease in only one host. For example, S. typhi is restricted to infection in humans, Salmonella abortusovis to infections in sheep, Salmonella dublin in cattle, Salmonella abortusequi in horses, and Salmonella choleraesuis in pigs.
A third group of serovars is associated predominantly with disease in one species but may also infect a limited number of other hosts. For example, S. dublin is usually associated with cattle but natural infection by this serovar may also occur in other animals, including humans and sheep. The nature of disease associated with this third group of serovars is variable, depending on the specific combination of serovar and host, although in the predominant serovar–host combination the disease is usually systemic.
The serotypes (serovars) that most commonly cause salmonellosis in farm animal species are as follows:
• Cattle: S. typhimurium, S. dublin, Salmonella newport
• Sheep and goats: S. abortusovis, S. typhimurium, S. dublin, Salmonella anatum
• Pigs: S. typhimurium, S. choleraesuis
• Horses: S. typhimurium, S. abortusequi, S. anatum, S. newport, S. enteritidis, Salmonella heidelberg, Salmonella arizona, Salmonella angona.
The molecular methods are now available for epidemiological investigation of S. enterica subsp. enterica infections.2
EPIDEMIOLOGY
The epidemiology of salmonellosis is complex, which often makes control of the disease difficult. The epidemiological patterns of prevalence of infection and incidence of disease differ greatly between geographical areas depending on climate, population density, land use, farming practices, food harvesting and processing technologies, and consumer habits. In addition, the biology of the serovars differs so widely that considerations of salmonellosis, Salmonella infections or Salmonella contamination are inevitably complex.
Prevalence of infection
Surveys from various countries indicate a 13–15% infection rate in dairy cows in New Zealand, with similar rates in calves and sheep, and 4% in beef cattle. In the Netherlands, the infection rate is 25% in healthy pigs at abattoirs but similar investigations elsewhere record 10% (New Zealand) and 6% (UK). American figures indicate a 10–13% infection rate. Salmonellas were isolated from the mesenteric lymph nodes and cecal contents of 84% of slaughtered sows in a Minnesota abattoir. These data are based on abattoir material and should be viewed with caution because of the very rapid increase in infection rate which occurs when animals are held over in yards for several days.
A national survey of the prevalence of fecal carriage of Salmonella in healthy pigs, cattle, and sheep at slaughter, and of pig carcass contamination with Salmonella in Great Britain found the carriage rate in prime slaughter cattle and sheep was very low compared with pigs.3 In pigs, the cecal carriage rate was 23.0%, although carcasses were only moderately contaminated at 5.3%. The meat juice ELISA results indicated that 15.2% of tissue fluid samples were positive at the 40% cutoff level and 35.7% at the 10% experimental cutoff level. This indicates that pigs are exposed to a relatively high level of Salmonella during the weeks prior to slaughter. The carriage rate in cattle and sheep was very low, ranging from 0.1–1.7%.
In the UK, S. dublin and S. typhimurium account for nearly 90% of bovine salmonellosis. S. typhimurium is endemic in calves, especially those purchased at livestock markets and raised for beef or veal. In the USA, the organism is shed by calves on 16% of farms sampled; in Ontario, calves on 22% of farms surveyed were found to shed Salmonella spp. In a survey of 47 dairy farms in Ohio, of 452 calves sampled, 10 calves from seven farms were culture-positive.4 Salmonella serotypes isolated were S. dublin, S. typhimurium, S. enteritidis, S. agona, Salmonella mbandaka, and Salmonella montevideo. Bulk milk tank filters from these dairies were also submitted for culture; Salmonella spp. were isolated from one of 50 filters and two calves from this herd were found to be shedding Salmonella spp. of the same serotype.
The geographical distribution of the serotypes differs: S. typhimurium has a universal distribution; S. dublin has a more patchy habitat. In the USA, up until 1948, it was limited to California and as recently as 1971 it had not been reported in cattle east of the Rocky Mountains. In 1980, the first case of S. dublin occurred in Indiana. The movement of infected adult cattle and calves is responsible for the introduction of infection to areas where it had not previously been diagnosed. In a California survey of 60 dairy herds, milk samples and serum samples tested with an ELISA for antibodies against Salmonella serogroup B, C1, and D1 antigens found that 75% of dairy herds surveyed had cows with serological evidence of recent exposure to salmonellas, especially S. typhimurium and S. dublin. The prevalence of fecal shedding of Salmonella by cull dairy cattle marketed in Washington state is estimated to be 4.6 per 1000 head. Neonatal calves under 28 days of age may be shedding Salmonella without any evidence or recent herd history of clinical disease. Australia has had little S. dublin but there has been a marked increase in outbreaks of abortions, gastroenteritis and calf deaths due to S. dublin infection in Queensland dairy cattle since 1983. South Africa, South America, the UK, and Europe have had S. dublin as the principal pathogen for cattle for some time. It has also come to surpass S. abortusovis as a cause of abortion in sheep.
In Danish pig herds, Salmonella infections are usually subclinical. A survey in 1993–94 found that 22% of 1368 larger herds were infected with Salmonella. The most prevalent serotypes were S. typhimurium (62% of infected herds), Salmonella infantis (10%), Salmonella 4.12:b (8%), and Salmonella panama (5%). Phage typing of isolates of S. typhimurium from pigs and humans reveals that pigs are probably a major source of the infection in humans in Denmark.
In Sweden the prevalence of salmonellosis in food-producing animals is low because of the Salmonella control programs, which started in 1961 with the aim of keeping meat-producing animals free from Salmonella in Sweden.5 When Sweden joined the European Union in 1995, surveillance of Salmonella in cattle, pigs, and poultry at slaughter was included in the control programs. Any finding of Salmonella from animals or in feeds or feed production, regardless of serotype, is notifiable to the Swedish Board of Agriculture. The occurrence of Salmonella in animals and in the feed production in Sweden remained relatively stable from 1993–97.5
The incidence of salmonellosis in animals and humans may change within a geographical area over a period of years.6 In Scotland, the incidence of S. typhimurium DT104 in cattle peaked in 1996 and then decreased annually to 2001.6 Similar declines have been observed in its incidence in sheep and pigs. In humans, the number of reports of S. enteritidis PT4 peaked in 1997 and then declined to a low level by 2001.
S. infantis and S. typhimurium persist among cattle in Finland, with a low prevalence rate of 2% of farms, but these serovars play a major role in human salmonellosis.7 Molecular epidemiology is now used to determine if strains isolated from cattle are the same as those affecting humans.2,6 Multiple genetic typing of S. typhimurium isolates of different phage types (DT104, U302, DT204B, and DT 49) from animals and humans in England, Wales, and Northern Ireland identified different degrees of polymorphism.8 A prevalent genomic clone, as well as a variety of less frequent clones, is present for each of the phage types. Molecular epidemiology of S. typhimurium isolates from wild-living birds, domestic animals and the environment was investigated in Norway using pulse-field gel electrophoresis.9 Passerines (perching birds) constitute an important source of infection for humans in Norway, whereas gulls and pigeons represent only a minor source of human S. typhimurium infections. Passerines, gulls, and pigeons may constitute a source of infection of domestic animals and feed plants. Some isolates from cattle were confirmed as mr S. typhimurium DT104 for the first time.
The serotype and phage type distribution of Salmonella strains isolated from humans, cattle, pigs, and chickens in the Netherlands from 1984–2001 were determined.10 The most prevalent serotypes were as follows: in humans, serovars typhimurium and enteritidis; in cattle, serovars typhimurium and dublin; in pigs, serovar typhimurium; and in chickens, serovars enteritidis, infantis, and typhimurium. In general, similar sero- and phage types were found in humans and animals, although distinct types were more common in animals.
Monitoring of the population and herd Salmonella seroprevalence in finishing pigs and sows provided a baseline for the success of future intervention and control strategies for Salmonella in pork. The seroprevalence of Salmonella in sows and finishing pigs in the Netherlands was determined using indirect ELISA on blood samples collected at the abattoirs.11 The population prevalence for finishing pigs in 1996 and 1999 was 23.7% and 24.5%, respectively, and for sows 40.5% and 60.4%, respectively. The prevalence in free-ranging finishing pigs was higher, at 44.6%, than in intensively housed finishing pigs. In 46 multiplying sow herds, the average herd prevalences were 54, 44, and 19%, respectively. The prevalence of Salmonella in Danish pork decreased from 3.5% in 1993 to 0.7% in 2000 following introduction of a national program to reduce the prevalence of salmonellas in pork.12
Occurrence
Salmonellosis occurs universally in all species.
Cattle
The disease has assumed major importance because of outbreaks in dairy cattle and the occurrence of infections in humans. Of major concern is the increased incidence of outbreaks of salmonellosis in dairy cattle and humans associated with S. typhimurium definitive type (DT)104 in the USA6 and the UK. This strain is multiple-antibiotic-resistant and is classified as R-type ACSSuT by being resistant to ampicillin, chloramphenicol, streptomycin, sulfonamides, and tetracycline. The strain is now the second most prevalent Salmonella serotype in humans in England and Wales and the most common Salmonella serotype isolated from cattle. A case-control study identified the following risk factors associated with infection with the multiple-resistant S. typhimurium DT104:
• A high population density of feral cats around the farm
A high frequency of antibiotic-resistant phage types of S. typhimurium has been reported from Australia. The intensification of cattle production and management has played a large part, but the emergence of S. dublin as a common pathogen has been most important. In recent years there has also been a large increase in notifications of exotic species of Salmonella such as S. agona and S. newport, which have mostly originated from the use of unusual food materials of animal and fish origin.
Horses
The incidence of salmonellosis has been increasing in the horse population, particularly where horses are assembled at large clinical centers and breeding farms. Moreover, nosocomial salmonellosis is an important problem for horses in veterinary hospitals. It is also possible that many of the unidentified enteritides of horses may have been associated with Salmonella spp.
Pigs
In the midwestern USA, salmonellosis associated with the host-adapted facultative intracellular S. choleraesuis is an important cause of economic loss in pig herds because of death and reduced productivity. It is the most frequent serotype recovered from pigs and is isolated from more than 95% of porcine salmonellosis outbreaks in Iowa. The incidence has been increasing in some geographical areas. S. typhimurium causes enterocolitis in young pigs.
The majority of Salmonella infections are subclinical, associated with a large number of serotypes. A national US survey for fecal Salmonella shedding by pigs most frequently found S. enterica serotypes derby, agona, typhimurium, brandenburg, mbendaka, and heidelberg.13
Sheep
Salmonellosis is commonly encountered when sheep are assembled at high stocking rates. It was one of the main contributing causes of death in sheep exported by sea from Australia to the Middle East, although inanition is usually the primary cause.
In the 1990s there was an increase in the incidence of salmonellosis in sheep associated with S. enterica subsp. diarizonae serovar 61:k:1,514 in the UK10,15 and Norway.16,17
Morbidity and case fatality
The morbidity rate in outbreaks of salmonellosis in pigs, sheep, and calves is usually high, often reaching 50% or more. Morbidity and mortality are usually highest in calves under 12 weeks of age. In all species the case fatality rate often reaches 100% if treatment is not provided. In outbreaks in out-wintered suckler cattle herds, the morbidity varied from 14–60% and mortality in adult cattle from 0–14%. In a review of 40 cases of clinical salmonellosis in horses that were diagnosed in one clinic, the case-fatality rate was 60%. Epidemics of salmonellosis affecting up to 40% of foals under 8 days of age on one Thoroughbred horse farm have been reported.
Methods of transmission
Salmonellas are spread by direct or indirect means. Infected animals are the source of the organisms; they excrete them and infect other animals, directly or indirectly by contamination of the environment, primarily feed and water supplies. The farm animal may be infected in different ways: by animal-to-animal transmission, especially of host-adapted serovars; by contaminated animal feed; and by a contaminated environment (soil, birds, rodents, insects, water supplies). Liquid wastes from infected animals may contaminate the environment directly, including streams, rivers, and pastures. Bacteria may also be disseminated during the transport of infected animals and during the holding of animals in a lairage prior to slaughter. In both situations, the excretion of salmonellas is exacerbated by the stress imposed.
In the UK, surplus calves from dairy farms are sold at public market and moved to rearing farms. The mixing of young susceptible calves and their subsequent transportation is an efficient mechanism for the rapid dissemination of Salmonella. The dealers’ premises can serve as reservoirs of infection despite cleaning and disinfection. Many vehicles and markets are contaminated with Salmonella, and S. typhimurium DT204C, the most common Salmonella isolated from calves, is the most frequent isolation.
The introduction of infected carrier animals into a herd is a common cause of outbreaks of clinical salmonellosis in dairy herds that are expanding in size.
The organism can persist for an average of 14 months in the environment where calves are reared. Salmonella does not survive for more than 5 days in bovine urine not mixed with feces but will survive in dried bovine feces for up to 6 years. After a clinical outbreak of salmonellosis, for example in a dairy herd raising its own replacements, the premises cannot be declared to be Salmonella-free solely on the basis of freedom from clinical cases over the next few years or on the basis of comparatively high herd performance. In large dairy herds with modern free-stalls that recycle water in their manure flush systems, it may be possible to isolate Salmonella serovars for several years following an outbreak of clinical salmonellosis. The organisms may be found in recycled water samples, bulk tank milk filters and the feces of calves and adult cows.
Salmonellas can be isolated from piggery waste water after orthodox pondage treatment, and the recirculation of contaminated water through the piggery serves as a constant source of the organism. Housing of finishing-age pigs in barns with open-flush gutters may contribute to increased shedding of Salmonella compared to pigs housed on partially slotted floors. Methanogenic fermentation in waste ponds does not eliminate Salmonella from piggery waste; acidogenic fermentation with the production of free acid can destroy salmonellas and other potential pathogens.
During slaughter, fecal contamination of the carcass commonly occurs and can be carried through all slaughter procedures up to the processing of the raw products. Milk can be contaminated directly by cows that excrete the organism in the udder, especially those cattle infected with S. dublin and Salmonella muenster,18 both of which have adapted to colonize the bovine mammary gland. Although S. typhimurium is not usually excreted in the milk, except during the febrile stage of clinical disease, it has been reported to have been persistently isolated from the milk of a healthy cow. S. enteritidis has been isolated from ill humans, milking filters, milk from a bulk tank and milk from one quarter of a 5-year-old dairy cow that persistently shed the organism in the milk for several months. Milk is most likely to become contaminated by feces, either from an animal with clinical salmonellosis or from a healthy carrier animal, during the milking process. Additional sources of contamination during milking are use of polluted water or contaminated equipment. Workers who lack personal hygiene skills and have clinical salmonellosis or are chronic shedders of the organism may also contaminate milk supplies.
Airborne transmission can be a primary mode of infection of S. typhimurium. Studies have shown that the organism can survive in air sufficiently long to present a significant hazard of air-borne spread.
The carrier state
Because salmonellas are facultative intracellular organisms that survive in the phagolysosome of macrophages, they can evade the bactericidal effects of antibody and complement. Thus, persistence of infection in animals and in the environment is an important epidemiological feature of salmonellosis. When an animal is infected with S. dublin it may become a clinical case or an active carrier, shedding organisms constantly or intermittently in the feces. It may also become a latent carrier with infection persisting in lymph nodes or tonsils but no salmonellas in the feces, or even a passive carrier which is constantly acquiring infection from pasture or the calf-pen floor. But invasion of tissues may not occur and when the animal is removed from the environment the infection disappears. However, these animals probably multiply the salmonellas without becoming permanent carriers. The importance of the latent carriers is that they can become active carriers or even clinical cases under stress, especially at calving time. The cattle themselves then become the means by which the infection is retained in the herd for long periods. A major problem with the control of S. dublin infection is that latent carriers of the organism, unlike persistent excreters, cannot be readily identified by fecal culture or serological methods. In a 3-year study of one dairy herd, the organism was isolated occasionally from the feces of adult cattle, from some cattle after parturition, and from some calves within 24 hours after birth. In some dairy herds, the organism may persist for many years with a low incidence rate of clinical disease.
For S. typhimurium the donor can be any domestic animal species, including humans, or any wild animal or bird. Although all infected adults become carriers it is rarely for any length of time, and calves rarely become carriers. In sheep and cattle the carrier state may persist for as long as 10 weeks, and in horses up to 14 months.
Experimental infection of pigs at 7–8 weeks of age with a single oral dose of S. typhimurium can persist continually at least until market age. Regardless of the route of infection, S. choleraesuis can persist in the tonsil and ileocolic lymph nodes, ileocolic junction and colon, and can be excreted in the feces of experimentally infected pigs for at least 12 weeks. The amount of shedding and persistence of infection is dose dependent. Low doses of S. choleraesuis can be easily cleared, moderate doses can persist for at least 2 months, and high doses result in long-term carrier states. After intranasal inoculation of S. typhimurium, the organism rapidly appears in the intestines, suggesting that the tonsils and lungs may be important sites for invasion and dissemination of Salmonella species. Experimental infection with a zoonotic strain of S. newport can also be established in pigs at 7 weeks of age to persist until market age. Long-term persistence of infection is limited generally to the palatine tonsils, the intestinal tract caudal to the mid-jejunum and their lymph nodes. The prevalence of the organism in pigs creates a reservoir of infection for animals and humans. The transmission of salmonellosis in pigs can occur in a few days. Exposure to relatively low levels of S. choleraesuis may result in high morbidity and initiate a severe outbreak in naive pigs within several days of being exposed to infected pigs. Only a small fraction of carrier pigs are responsible for the maintenance of the pathogen in a pig population.
Risk factors predisposing to clinical disease
The clinical characteristics of salmonellosis in large animals vary depending on the various management systems used, the intensity of stocking, whether or not the animals are housed, and the epidemiological characteristics of the different Salmonella species. Thus, salmonellosis in cattle is a very serious and persistent disease in areas where it is caused principally by S. dublin. But where it is associated with S. typhimurium the disease is sporadic and, even though it is highly fatal to individual animals, it is not a serious disease. Although there are probably similar differences with the other species, they are not particularly well defined. The difference between the diseases associated with S. dublin and S. typhimurium is the marked tendency for S. dublin to persist in adult cattle and create a significant reservoir of carrier animals. S. typhimurium does not do so as much, so that the disease is likely to subside after an initial exposure and to recur only when the source of infection, from rodents or feedstuffs, or sewage or slurry, reappears. This does not of course preclude the disease from persisting in a flock or herd for long periods. S. typhimurium infection persisted in a large dairy herd for 3.5 years. While the incidence rate of clinical disease declined over the study period, the organism could still be cultured from the bulk tank milk filters, which may have been associated with one cow identified as a milk excretor. Three associated human disease incidents occurred following the consumption of raw milk.
Animal risk factors
Except in the newborn, especially foals, infection with a Salmonella sp. is usually not a sufficient cause of clinical salmonellosis. The response to infection with a Salmonella sp. varies depending on the size of the challenge dose and the immunological status of the animal, itself dependent on colostrum intake in neonates, previous exposure to infection and exposure to stressors, particularly in older animals. It is generally accepted that the intervention of some precipitating factor such as transport, intercurrent disease, anesthesia and surgery, dosing with antimicrobials or anthelmintics, acute deprivation of food, or parturition is usually necessary to cause the disease salmonellosis, as distinct from infection with Salmonella sp.
The portal of infection in salmonellosis is almost always the mouth, so that the severity of the disease in an individual, or of an outbreak in a group, depends on the degree of contamination and the environmental conditions of temperature and dryness that determine the survival time of the salmonellas. Just as important is the influence of the host on the outcome of the infection. Many animals become infected naturally and are passive carriers; they shed salmonellas in their feces without clinical disease but only for the duration of their cohabitation with other infected animals. It is also possible to reproduce salmonellosis experimentally in most animals using a sufficiently large dose of a virulent strain of the organism. There still remains the common occurrence of the animal that is a subclinical carrier of the infection but develops clinical salmonellosis when exposed to stressors such as long transportation, hospitalization, severe feed deprivation or parturition.
Genetic resistance to salmonellosis in domestic animals
There is evidence of a strong genetic association with resistance to salmonellosis in several economically important domestic animal species.19 However, as yet, selective breeding for resistance traits is not utilized in control of diseases or the carriage of Salmonella in any of these species. The value of a particular resistance trait in reduction of disease must be balanced against other factors such as productivity of meat and milk. The control of Salmonella colonization of the gastrointestinal tract of food animals, particularly where intensive rearing occurs, as in pig units, would appear to be a particularly useful objective with enormous potential public health benefits. There may be a role for several inherited immunological traits, including polymorphonuclear leukocyte function and lecithin-induced mitogenic proliferation.
The interrelationships between the risk factors of the host, the environment, and the pathogen are described here according to species differences.
Dairy cattle
In calves, the disease is usually endemic on a particular farm, although outbreaks can occur. In adult cattle at pasture the disease is less common. This is particularly so with S. typhimurium infections, but S. dublin affects both young and adult cattle. Spread between calves in communal pens is by fecal–oral contamination. Infection of the newborn calf may be from the dam because many cows that are latent shedders become active shedders at parturition. The calves are not infected at birth but become infected from the environment. In adult cattle, S. dublin is the common infection and occurs sporadically, but as outbreaks when stressors occur. Spread is usually by the oral route and in cattle at pasture is greatly enhanced by persistently wet conditions. Wild mice are potential reservoirs of S. dublin in dairy herds.
Risk factors identified in dairy and beef cattle herds with clinical salmonellosis in Virginia included herd size, exposure to wild geese, rodent activity in housing and feed areas and spreading manure on bordering property.20 Previous antimicrobial treatment of cattle with laboratory-confirmed Salmonella infections increases the probability of isolating salmonellas.21
In cattle, deprivation of feed and water is a common risk factor, usually as a result of transportation, but recent calving, sudden changes in the composition of the diet, vaccination with a living vaccine that produces a systemic reaction, treatment with irritant compounds such as carbon tetrachloride for fluke, and fluke infestation can precipitate clinical disease. However, prior infection of calves can provide resistance to experimental infection. In some herds there are sporadic cases in cows as they calve, usually within 1 week afterwards. In grazing cattle there is a distinct seasonal incidence in late summer, fall, and early winter, probably because of greater exposure to infection at pasture.
The pH of rumen contents has been shown to affect the number of salmonellas surviving passage through the rumen. A high volatile fatty acid content and a low pH, such as prevails when a ruminant is on full feed, is unfavorable to salmonellas passing through the forestomachs.
The epidemiological and biological characteristics of three dairy herds in California have been examined and several risk factors were identified that varied between dairies.22 The various sources of salmonellas in dairy farms indicate that they are part of a larger ecosystem. The prevalence of fecal shedding indicated the magnitude of environmental contamination possible. Animals were exposed to many Salmonella serotypes via feed contaminated through irrigation of crops with effluent or dairy wastes. Salmonella contamination of irrigation water by human sewage was identified as a potential source of exposure with S. agona, S. montevideo, and Salmonella manila. Nutritional stress caused by transition diets and heat stress was associated with outbreaks in some herds. Salmonellas were isolated from aseptically collected composite milk samples, and from bulk-tank milk and inline milk filters. Such contamination can result in contamination of human dairy products. A large number of Salmonella serotypes were present in cull dairy cows at slaughter with S. montevideo being most common.23 The overall prevalence of Salmonella spp. in cull dairy cows at slaughter across the USA was 23.1%, with a range accounting for location and season between 4.3% and 54.5%.24 This could burden the Hazard Analysis Critical Points Programs implemented in abattoirs. Procedures to reduce Salmonella infection at the farm and during transport to slaughter could reduce the risk of spread during the slaughter process.
The water supplies of dairy calves in California dairy herds may be contaminated with S. typhimurium.25 Water offered to weaned dairy calves in a continuous water-tank-filling method was a risk factor compared to a valve on demand and a water pH of more than 8.
Subclinical fecal shedding of Salmonella can persist in dairy herds for up to 18 months with no measurable effects on health or production on individual cows.26 Large herd size and intensive management may provide an environment conducive to Salmonella shedding and chronic dairy herd infection.27 Salmonella spp. can be isolated from the environment of free stalls in dairy herds in Wisconsin without any history of clinical salmonellosis.28 Birds that commonly inhabit California dairy farms harbor Salmonella organisms but the low prevalence of infection in birds and the serotypes isolated are not important reservoirs of infection.29
The risk factors for fecal shedding of Salmonella in US dairy herds were herd size, region of country, use of flush water systems, and feeding brewers’ products to lactating cows.30 The estimated population attributable risks for all four factors combined was 0.95. These factors can be used to predict the presence of Salmonella shedders in a herd. Salmonella can be isolated from more than 90% of dairy farms but 25% of farms account for more than 75% of Salmonella-positive samples.31 Concentrating control efforts on farms with a high prevalence may be the most effective means of control of the infection in dairy herds.
The risk factors for becoming a carrier of S. dublin in dairy cattle in Denmark include heifers infected between the age of 1 year of age and first calving, and cows infected around the time of calving.32 The risk was higher in the first two quarters of the year (late winter to spring). Herds with the highest risk of carrier development were those with clinical disease outbreaks.
Beef cattle herds and feedlots
While salmonellosis can cause significant economic losses in beef herds and feedlots it is not as important as in dairy cattle. Low numbers of beef cattle are found to shed Salmonella at the time of slaughter and beef cattle do not appear to be a major risk of carcass contamination. In Australian cattle, the prevalence of Salmonella in the feces of cattle at slaughter was 6.8%.33 In grass-fed cattle the prevalence was 4.5% and not much different to that found in feedlot cattle. In the US, fewer than 7% of cattle in feedlots shed Salmonella in their feces.34 In European cattle, the prevalence of Salmonella in feces has ranged from less than 1% to 42%.33 S. montevideo has been the cause of large economic losses due to abortion and cow mortality in an outwintered beef herd in Scotland.35 Up to 25% of the cows aborted and the overall herd mortality was 7%. The organism had been the cause of abortion in a neighboring sheep flock.
Case-control studies of risk factors for clinical salmonellosis in cattle herds in Virginia, USA, found that larger herd size, exposure to wild geese, rodent activity in housing and feed areas, and spreading poultry manure on bordering property had positive associations with the occurrence of the disease.20 Case farms were less likely than control farms to have calves born primarily in a building and had smaller percentage changes in the number of mature cows during the previous year. In contrast, in the UK, farms with housed cattle had increased risk of S. typhimurium DT104. Feeding recycled poultry bedding that had been stored and stacked properly for 51 days prior to feeding, to feedlot cattle did not increase the prevalence of detectable Salmonella in calves.36
Changes in the incidence of shedding Salmonella in the feces of cattle being transported from the farm to slaughter plant have been examined.37 In feedlot cattle, fecal shedding remains fairly constant before and after transport (3–5%); in adult cattle the shedding rate increased from 1% to 21%. Contamination of hides increased for both animal types from 18–20% to 50–56%. Nineteen percent of feedlot cattle carcasses and 54% of adult cattle tested positive for Salmonella. Feed withdrawal, transport stress, and the commingling of animals prior to slaughter can affect the number of cattle that are contaminated with bacterial pathogens such as Salmonella. However, none of the risk factors evaluated prior to or throughout the transport process had an impact on fecal shedding, hide contamination or carcass contamination.
Feedlot playas (temporary shallow lakes) are frequently contaminated with many Salmonella serotypes.38 Using playas as a source of water for feedlots can be a source of Salmonella and they should not be used to cool cattle in the summer months, or for dust abatement, or for irrigation of crops. Wildlife, birds, and migratory waterfowl have access to these bodies of water and, because of their size and number, there is little which can be done to prevent them from becoming contaminated.
Sheep
Salmonellosis in sheep may occur with a range of different syndromes of variable severity, depending mainly on the particular serovar involved. Serovars abortuovis, dublin, and typhimurium, which each have different degrees of host restriction, are associated with disease in sheep. Serovar dublin can cause both enteritis and abortion in adult sheep, and the disease is often associated with metritis, anorexia, and loss of wool. Newborn lambs may develop diarrhea with a high mortality rate. Serovar typhimurium is associated with acute disease, enteritis but not usually abortion.39
A new strain of Salmonella brandenburg has affected livestock and humans in the South Island of New Zealand.40 The strain has caused abortions in sheep, abortions in cattle and gastroenteritis in calves and adult cattle. The same strain also caused disease in horses, goats, deer, pigs, and humans. Spread of the disease on farms was strongly associated with aborting ewes, which resulted in considerable environmental contamination. During the abortion season, black-backed gulls appeared to spread the disease to other farms. Other potential sources of infection were carrier sheep, contaminated water sources, and contaminated sheep dust.
Salmonella infections of sheep and human food poisoning are rare. However, outbreaks of food poisoning in Iceland associated with Salmonella were traced to the consumption of singed sheep heads.41 The organism could be isolated from 20% of the specimens sampled. The prevalence of infection of the sheep population in Iceland is low, at 1.3%. Infection occurs on mountain pastures, which may be contaminated by wild birds, especially gulls.
In range sheep, the commonest occurrence of the disease is during a drought when sheep are concentrated in small areas of surviving grass heavily contaminated by feces. Sheep held in holding yards or transport vehicles previously occupied by sheep for long periods are also susceptible to clinical disease. This is most likely to occur when they drink from puddles of water, especially in heavily contaminated yards, or when they are exposed to recycled dip wash. In sheep, the disease is commonly associated with deprivation of feed when animals are assembled for vaccination, anthelmintic administration or shipment over long distances. Lambs in feedlots are susceptible to salmonellosis within a few weeks after arrival in the lot.
The modern development of pen-lambing in which ewes about to lamb are brought into small pens is also a means of potentiating spread from a chronic shedder. In all these situations feed stress by deprivation is likely to contribute to susceptibility. Field outbreaks in range sheep have been recorded. In some instances they have been caused by the use of unsterilized bone meal as a phosphorus supplement. Outbreaks occurring in sheep on a number of farms in the same area at the same time have been ascribed to contamination of drinking water by birds eating carrion. Heavy dosing with zinc oxide as a prophylaxis against facial eczema is also credited with precipitating outbreaks of salmonellosis in young sheep.
Goats
Outbreaks in goats occur in the same circumstances as in other ruminant species. Transportation and capture are additional stressors in feral goats used for embryo transplantation.
Pigs
The epidemiology of S. choleraesuis infection in pigs is well documented and has changed remarkably since the mid-1960s, when explosive outbreaks occurred that could easily be mistaken for hog cholera. The morbidity and mortality rates were high and the disease spread rapidly through commercial pig-finishing units. These outbreaks are now rare and small in scope, largely because of the restriction of garbage feeding, much less movement and mixing of pigs through public auction marts, and disease-prevention strategies such as the use of specific pathogen-free pigs, an all-in/all-out policy in commercial finishing units, and the vertical integration of pig-producing enterprises. This insures a constant supply of disease-free growing hogs to finishing units and the assumption of a pyramid-type responsibility at all levels of the enterprise. The marked decline in the prevalence of swine salmonellosis coincided with the decline in and eradication of hog cholera. However, modern methods of raising pigs in multiple-site production systems, using all-in/all-out management of finishing pigs, appear to have no benefit in reducing the prevalence of Salmonella compared with conventional farrow–finish systems.
S. enterica does not normally cause clinical disease in pigs but subclinical infections constitute an important food safety problem throughout the world.42 Comprehensive longitudinal studies of two multiple-site pig production systems in the USA revealed considerable temporal variability in Salmonella prevalence between cohorts of pigs.43 Cohorts of sows and individually identified growing pigs from their litters were serially sampled to determine the prevalence and serotypes of Salmonellas in each stage of production based on fecal culture, and feed and environmental samples. A total of 15 different serotypes were isolated from the two systems. Pig prevalence estimates ranged from 0–48.1%. Environmental contamination was frequently encountered despite cleaning and disinfection. Feed was only rarely contaminated. The prevalence of infection within and among cohorts of pigs was highly variable, which indicates that point estimates of Salmonella prevalence and serotypes are not reliable indicators of the Salmonella status on farms, and that uncontrolled studies of interventions to control Salmonella on pig farms may yield misleading results.43
In the USA, new regulations regarding the safety of meat products have been implemented in response to public concerns about food-borne disease outbreaks. The salient features of the regulations are requirements for approved systems of microbiological monitoring of S. enterica, E. coli O157:H7, and generic E. coli as an indicator of contamination by gastrointestinal contents. From the perspectives of public health, regulatory compliance and international competitiveness, S. enterica is the most important foodborne pathogen for the US pig industry. This has resulted in longitudinal epidemiological studies of fecal shedding of S. enterica in both breeding and growing pig populations.43
A quantitative risk analysis model simulating Salmonella prevalence in growing pigs and at slaughter would be of great value for food safety. However, sampling strategies for input information to a model are difficult to establish as the relationship between subclinical infections at the levels of the herd, the individual pig, and at slaughter is complex. The onset and duration of Salmonella shedding and the patterns of transmission between individual pigs and between different age groups during the growing period all have influence. Bacteriology and serology can be used to assess this relationship but repeated sampling in different cohorts of animals is required to correctly assess the infection dynamics.78
Longitudinal studies of S. typhimurium infection in farrow–finish pig herds in Denmark reveal that Salmonella occurrence varies between and within age groups within herds, even in herds with an apparent moderate-to-high infection level. Salmonella was predominant in weaners, growers, and finishers, and was only occasionally detected in sows and gilts. In Denmark, Salmonella is typically detected in the nursery and rarely in the sow unit, suggesting that the infection level among sows is low. This is contrary to the results of studies in the USA, where Salmonella was found to be common in sows.44 In the Danish study, there was a rapid increase in Salmonella prevalence in the nursery, which may be associated with the stressors of weaning such as change in feed, commingling of litters and piglets being deprived of the antibodies in sow’s milk before activation of their own immune response. The observation that no piglets were shedding Salmonella just before weaning but 3–4 weeks later in the nursery between 5% and 50% of the piglets were shedding suggests that horizontal transmission occurred in the nursery.42 During the finishing period Salmonella shedding decreased, but with considerable variation. Some pigs cleared themselves of the infection whereas others continued shedding. Average shedding time was estimated to be 18–26 days. Seroprevalence peaked approximately 60 days after peak prevalence in culture. At slaughter there is a marked increase in the prevalence of Salmonella infection. This increase may be due to rapid cross-contamination during transport and lairage.45 Rapid infection during transport, and particularly during holding, is a major reason for increased Salmonella prevalence in pigs.46 A high degree of carcass contamination occurs at slaughter due to the delivery of Salmonella-positive pigs and cross-contamination from the slaughterhouse environment.47 Contaminated feed trucks may also serve as a potential source of Salmonella contamination.48 The withdrawal of feed from pigs prior to slaughter does not increase the prevalence of Salmonella colonization or the risk of carcass contamination.49
Risk factors associated with serological Salmonella prevalence in finishing pig herds in the Netherlands have been examined.50 Feeding a complete liquid feed containing fermented byproducts and the omission of disinfection after pressure washing a compartment as part of an all-in/all-out procedure were both associated with a lower Salmonella seroprevalence. A small to moderate herd size (<800 finishing pigs), a previous diagnosis of clinical Salmonella infection in the herd, the use of tylosin as an antimicrobial growth promoter in finishing feed, and herds that have more than 16% of their pigs’ livers condemned at slaughter because of white spots were associated with a higher Salmonella seroprevalence. There was no effect on experimental Salmonella infection of the use of tylosin as an antimicrobial growth promoter.51
In those herds where the disease does occur, introduction is usually associated with the importation of infected carrier pigs. However, it is possible for the infection to be spread by flies and the movement of inanimate objects such as cleaning equipment and utensils. Feedstuffs do not provide a favorable environment for S. choleraesuis, so food-borne infection is not common. Survival in soil and water is approximately 6 months and in slurry up to 5 weeks. Persistence in streams fouled by piggery effluent is unlikely. Susceptibility to salmonellosis in pigs is thought to be increased by intercurrent disease, especially hog cholera, nutritional deficiency of nicotinic acid, and other nutritional stress such as a sudden change in diet.
Horses
Based on the culture of single fecal samples from horses on 972 operations in 28 states, the national prevalence of fecal shedding of Salmonella spp. among horses in the US horse population was 0.8%. The overall prevalence of operations positive for fecal shedding of Salmonella was 0.8%. Based on feed samples taken from the same operations, the prevalence of Salmonella spp. in grain and other concentrates used for horse feed was 0.4%.52
In adult horses, most clinical salmonellosis occurs after the stress of transport and mostly in horses that are overfed before shipment, receive little or no food or water for the duration of a protracted journey, and are fed excessively on arrival, cases appearing 1–4 days later. Groups of horses that have been exposed to a contaminated environment, such as saleyards or railroad yards, may experience outbreaks in which up to 50% are affected. As with other species, the presence of an asymptomatic carrier in a group of horses is often credited with initiating an outbreak, but the search for the carrier is always laborious and often fruitless. At least five negative cultural examinations of feces should be made before acquitting a suspected donor. On the other hand, the cultural examination of large numbers of horses often reveals up to 50% of the population to be carriers. Multiple serotypes of S. enteritidis have been isolated from the mesenteric lymph nodes of 71% of healthy horses examined at an abattoir, which indicates that extraintestinal infection occurs in the horse as it does in other species. In the light of the high carrier rate in this species it is surprising that there are not more outbreaks.
The occurrence of salmonellosis in horses hospitalized for another disease has become a major problem for veterinary teaching hospitals and private equine practices that provide surgical veterinary care. In these circumstances there is a constant reintroduction of carriers of the disease, a persisting contamination of the environment, and a large population of horses, all of which are under physiological stress because of anesthesia, surgical invasion, or intercurrent disease and many of which are exposed to oral and parenteral treatment with antibiotics, which appears to greatly increase their chances of acquiring salmonellosis. Horses in which nasogastric tubes were passed were at 2.9 times greater risk of having salmonellas isolated than horses that did not undergo this procedure. Horses treated with antibiotics parenterally were at 6.4 times greater risk, and those treated with antibiotics orally and parenterally were at 40 times greater risk of developing salmonellosis, compared with horses not receiving such treatment. In hospitalized horses, the factors found to be associated with fecal shedding of salmonellas included diarrhea at the time of admission, fever while hospitalized, and a change of diet while hospitalized.
The extent of environmental contamination with Salmonella enterica in a veterinary teaching hospital in Colorado was examined.53 The results indicate that environments in veterinary teaching hospitals can frequently be contaminated with S. enterica near where infected animals are managed and fecal specimens containing the bacteria are processed for culture in a laboratory. The bacteriological culture of environmental samples collected with electrostatic wipes is an effective method of detecting contamination in a veterinary teaching hospital and may be beneficial as part of surveillance activity for other veterinary and animal-rearing facilities.
Outbreaks of nosocomial salmonellosis among horses in a veterinary teaching hospital have been described.54 Case fatality rates may be high, necessitating closure of the hospital for complete disinfection and systematic sampling of the environment to detect the presence of persistent Salmonella. Strict isolation of hospitalized horses that have been shedding Salmonella is necessary. The organisms can be isolated from the feces of hospitalized horses and many different environmental surfaces in the hospital.55 Salmonella may be detected in 5.5% of hospitalized horses. The planning and implementation of infectious disease control throughout the hospital is then necessary. Bleach is the most effective disinfectant on the largest number of surfaces.
Several variables have been associated with nosocomial Salmonella infections in hospitalized horses:56 the mean number of horses in the hospital shedding Salmonella krefeld during the first 4 days prior to and the day of admission, the mean number of horses shedding S. typhimurium during this period, a diagnosis of large colon impaction, withholding feed, the number of days fed bran mash, the duration of treatment with potassium penicillin G, and the mean daily ambient temperature.56
The factors potentially associated with Salmonella shedding among horses hospitalized for colic at a veterinary teaching hospital were examined.57 Salmonella spp. were detected in the feces 9% of patients at least once during hospitalization. They were more likely to shed Salmonella if diarrhea was evident 6 hours or less after hospitalization and duration of hospitalization exceeded 8 days (OR 20.3), laminitis developed during hospitalization (OR 12.0), results of nasogastric intubation were abnormal (OR 4.9), leukopenia was evident 6 hours or less after hospitalization (OR 4.6), or travel time to the teaching hospital exceeded 1 hour (OR 3.5). Horses treated with a probiotic did not differ from control horses in likelihood of fecal shedding of Salmonella (OR 1.5) or prevalence of clinical signs.
Occasionally, outbreaks occur in young horses at pasture when they are heavily infested with worms. Salmonellosis is also one of the common neonatal septicemias of foals, and the disease may occur as endemic on particular studs or there may be outbreaks with many foals being affected at the one time. The common management strategy on ‘visiting stud-farms’ of bringing mares and newborn foals to communal studs and then bringing them daily to a central point for observation and teasing is also likely to facilitate spread of an infection through a group of foals.
Immune mechanisms
Most information on the mechanisms of immunity to Salmonella, including the safety and immunogenicity of most Salmonella vaccines, has been done experimentally in mice. In primary infections in mice, early bacterial growth in the reticuloendothelial system is controlled by the contribution of both macrophages and polymorphonuclear cells and is affected by the virulence of the strain.58 In lethal infections, the early growth of the bacteria is the tissues results in high bacterial numbers that lead to death of the animal. Following natural infection with Salmonella antibody responses to lipopolysaccharides and protein determinants can be detected.58 Anti-Salmonella IgM appear in serum early after infection followed by IgG. T-cells have a critical role in the later stages of primary infection.
Environmental and management risk factors
Farming practice in general
Intensification of husbandry in all species is recognized as a factor contributing significantly to an increase in the new infection rate. A typical example is the carrier rate of 54% observed in intensive piggeries in New Guinea compared to the 9% in village pigs. Any significant change in management of the herd or a group of animals can precipitate the onset of clinical salmonellosis if the infection pre-exists in those animals.
Intensive pasture utilization
The means of infection is principally ingestion of feed, especially pasture, contaminated by the feces of an infected animal, so that the new infection rate is dependent on all those factors that govern the bacterial population in the environment.
Temperature and wetness are most important, as salmonellas are susceptible to drying and sunlight. S. typhimurium can remain viable on pasture and in soil, still water, and feces for up to 7 months. Survival times of the bacteria in soil are influenced by too many variables to make any overall statement meaningful.
As well as infection of pasture by cattle or other domestic animal species, the use of ‘slurry’ as a means of disposal of animal manure from cow housing or zero grazing areas has led to a highly efficient means of spreading Salmonella infections. The chance of cows becoming infected increases considerably if they are grazed soon after the slurry is applied, and is less likely during dry, sunny periods and when there is sufficient pasture growth to avoid it being eaten right down to the ground surface. The survival time of Salmonella spp. in cold liquid manure depends on several factors, including pH of the slurry and the serotype of the organism. It can be as long as 28 weeks.
Pasture contaminated by human sewage, especially septic tank or sewage plant effluent or sludge, is also credited with being a potential source of Salmonella infection for cattle, but there are a number of reports that do not support this view. Drinking water can remain infected for long periods, as long as 9 months, and in cattle at extensive pasture infected drinking water in stagnant ponds is a significant source of infection.
Housed animals
In housed animals the same factors apply to the spread of infection as apply to pastured animals. Thus, infection can be introduced by infected domestic animal carriers. For example, in large-scale calf-rearing units, where the disease is often of diabolical severity, many of the calves are infected when they are picked up from their home farms and, if they are penned in groups, all calves in the group are soon infected. The infection can spread among calves penned individually, which suggests that aerosol spread may occur. S. typhimurium type 204C can survive for several months in calf-rearing premises despite depopulation, cleansing, and disinfection. However, because of the failure of most calves to continue as carriers, they are usually free of infection within 6 weeks of arrival.
The premixing of food into a liquid form for pumping to feeding stations in piggeries and calf-rearing units is an effective way of spreading salmonellosis if infection is present in the feedstuffs and the mix is allowed to stand before feeding. The feeding of medicated milk replacer and hay to dairy heifers from 24 hours of age until weaning was associated with a reduced risk of Salmonella shedding, as was calving in an individual area within a building.
Contaminated feedstuffs
Housed animals are generally more susceptible to infection from purchased feeds containing animal byproducts than are pastured animals, which are again more susceptible to animal-product-based fertilizers. Organic feedstuffs, including bonemeal, are being increasingly incriminated in the spread of salmonellosis and although the usual figure, for example in the UK, is 23% of consignments being infected, the figure may be as high as 70%. Most of the contamination of meat and bonemeal occurs after heat sterilization, especially if the material is left in digester tanks. Fishmeal is one of the most frequently and badly contaminated feedstuffs. For example, most of a recent increase in reported isolations of salmonellas in the USA was due to S. agona introduced in Peruvian fish meal. These feed meals need to be heated at 82°C (180°F) for an hour to be sterilized. The infection of these materials may derive from antemortem infections in the animals used to make the byproduct, but soiling of the material at the preparation plant or abattoir or during storage may also occur. Stored feed not of animal origin, especially grain, is also commonly contaminated by the droppings of rodents that infest it and this can lead to sharp outbreaks of salmonellosis due to S. typhimurium. Of special importance is colostrum stored without refrigeration. If the colostrum is contaminated initially, multiplication of salmonellas may occur and transmission of the infection is likely. Dried milk products appear to be relatively safe.
A case-control study of an outbreak of salmonellosis due to Salmonella menhaden in dairy herds was associated with one particular feed mill and feeding animal fat.
Some Salmonella serotypes such as S. typhimurium have been isolated from 2.8% of pig feed and feed ingredient samples and from 46% of farm feed samples tested. S. choleraesuis was not isolated from pig feed.
Introduction of the infection to a farm
Contaminated feedstuffs, carrier animals and infected clothing of visitors and casual workers are the most common methods of introducing infection. Less common methods include free-flying birds such as the herring gull, and nematode larvae that are already infected with the salmonellas. Salmonellas have been isolated from a wide variety of wild animals, which could act as reservoirs for infection of domestic animals under certain conditions.
Pathogen risk factors
Salmonellas are facultative intracellular organisms that survive in the phagolysosome of macrophages and can therefore evade the bactericidal effect of antibody. Compared to other organisms of the same family, salmonellas are relatively resistant to various environmental factors. They multiply at temperatures between 8° and 45°C, at water activities above 0.94, and in a pH range of 4–8. They are also able to multiply in an environment with a low level of or no oxygen. The bacterium is sensitive to heat and will not survive temperatures above 70°C. It is sensitive to pasteurization. Salmonellas have been shown to be resistant to drying, even for years, especially in dried feces, dust and other dry materials such as feeds and certain foods. Prolonged survival in water and soil has also been described. They are quite sensitive to beta- and gamma-irradiation. The O-antigen lipopolysaccharide of salmonellas is toxic and an important virulence factor, and immunity directed against the lipopolysaccharide is thought to be of major importance in the host defense against salmonellosis.
Fimbrial antigens of some Salmonella species have been described and characterized.59 The fimbriae mediate a variety of virulence factors important for the maintenance and survival of the organisms in the host and environment, including initiation and stabilization of the organism to epithelial cells, colonization of the organism to receptor sites, maintenance of persistent infection in the host by mediating selective bacterial trapping by phagocytic cells, and evasion of the host’s specific immunological defense mechanisms. The fimbriae are also useful in diagnostic tests.
Naturally occurring strains with varying virulence factors and antimicrobial susceptibility patterns can be identified in herds with endemic infection. Strains of S. dublin with distinct antimicrobial susceptibility patterns and/or plasmid profiles have been repeatedly isolated from calves in a calf-rearing facility over a period of years.60 Some of the strains were isolated from numerous calves during outbreaks of clinical salmonellosis, while other strains were not.
The literature on the molecular basis of Salmonella-induced enteritis has been reviewed.61 Most of the Salmonella virulence genes have been identified. The effector proteins of S. typhimurium, which act in concert to induce experimental diarrhea in calves, have been characterized.62 There are several differences between S. dublin and S. typhimurium in the phenotypes, caused by inactivation of genes encoding effector proteins. The literature on the molecular methods for epidemiological investigations of S. enterica infections has been reviewed.2
Antimicrobial resistance of Salmonella
Strains of Salmonella spp. with resistance to antimicrobials are now widespread in both developed and developing countries.63 Since 1990 there have been dramatic increases in the occurrence of multiply-resistant strains of Salmonella spp. in many developed countries. Of particular note has been the epidemic spread of S. typhimurium DT104, which now has a worldwide distribution. Antimicrobial resistance in zoonotically transmitted salmonellas is an undesirable but almost inevitable consequence of the use of antimicrobials in food animals. In general, such use is legitimate. Recommendations have been made that new antimicrobials with cross-resistance to those used in human medicine should not be used for prophylaxis in food animal production. For example, it is argued that the use of antimicrobials in food animals has been a major factor in the development of decreased susceptibility to antibiotics such as ciprofloxacin in zoonotically transmitted salmonellas.63
Antimicrobial resistance of salmonellas has been a major controversial concern in veterinary medicine and human public health.64 Antimicrobials are used in food-producing animals for the treatment of infectious diseases and for growth-promoting effects. Their continued use has long been incriminated as a major cause of selective pressure that leads to the appearance and persistence of resistant strains. The resistance is usually to multiple antimicrobials and its existence is considered as a potential risk factor. The significance of antimicrobial resistance is most obvious in its impact on the treatment of human infections. If the frequency of drug resistance increases, the choice of antimicrobials for the treatment of systemic salmonellosis in humans becomes more limited. There is also an association between drug-resistant salmonellas and the routine clinical use of antimicrobials for infections other than salmonellosis. Antimicrobial-resistant Salmonella infections can complicate antimicrobial therapy of other infections; prior antimicrobial therapy allows fewer numbers of antimicrobial-resistant salmonellas to cause symptomatic infections, and an increase in the proportion of salmonellas that are antimicrobial-resistant will increase the overall frequency of salmonellosis. S. anatum isolates from horses have developed multiple drug resistance; this is a public health concern because the serotype has been isolated from humans, and individuals who have contact with infected horses are at risk of becoming infected.
Infections in humans associated with antimicrobial-resistant salmonellas are increasing and have become a cause for public health concern.14 Prospective studies in the USA claim to show that human infections with antimicrobial-resistant salmonellas are increasing, and that these resistant strains can be traced to foods of animal origin. There are wide variations from country to country in the percentage of Salmonella isolates that are antimicrobial-resistant. In general, antimicrobial resistance among salmonellas is much higher in the USA than in other countries. In the UK, over a period of about 20 years, little change has occurred in the antimicrobial resistance patterns of salmonellas isolated from animals. S. dublin remains predominantly sensitive to most antimicrobials. Most of the resistance in S. typhimurium is associated with phage type DT204C. Serotypes other than S. dublin and S. typhimurium show low levels of resistance to most antimicrobials, with the exception of sulfonamides and tetracyclines, to which resistance is increasing. The prevalence of antimicrobial resistance among salmonellas in New Zealand is low relative to many other countries.
The occurrence of the same clone of tetracycline- and streptomycin-resistant S. typhimurium in several dairy herds closely related geographically, and within a few months, suggests that spread of a single clone can occur quickly and may have been introduced into the herds by feed, wild animals, use of the same machinery, or a human vector.
Antimicrobial resistance in salmonellas in the UK has been monitored since 1970 using disk diffusion tests. A total of 76% of all Salmonella isolates are still sensitive to all 16 antimicrobials used for testing. Most antimicrobial resistance is encountered in bovine isolations of S. typhimurium phage type DT204C. This phage type, which was initially resistant to at least seven antimicrobials, has become more sensitive in recent years. Ninety-eight percent of S. dublin strains from cattle are still sensitive to all the antimicrobials used for testing. Treatment of calves with apramycin has selected for apramycin resistance in E. coli and S. typhimurium in the intestine, and plasmid transfer from the E. coli to S. typhimurium is suspected because the plasmids were similar.25
The incidence of antimicrobial resistance in strains of Salmonella isolated from Canadian agricultural products and imported fish has increased over specified study periods and emphasizes the need to reassess the benefits of subtherapeutically medicated feeds in current animal management practices.
Multi-drug-resistant S. newport has been spreading on an epidemic scale in both animals and humans throughout the USA.14 In addition to the resistance to five drugs found in S. typhimurium DT104, S. newport, called Newport MDR-AmpC, is also resistant to amoxicillin–clavulinic acid, cephalothin, cefoxitin, and ceftiofur and exhibits decreased susceptibility to ceftriaxone. The emergence of Newport MDR-AmpC strains in humans has coincided with the emergence of Newport MDRAmpC infections in cattle.65 In Massachusetts, the prevalence of the strain among S. newport isolates obtained from humans increased from 0% in 1998 to 53% in 2001. Case-control studies found an association with exposure to a dairy farm. Isolates from both humans and cattle had indistinguishable or closely related antibiograms and pulse-field gel electrophoresis patterns. The data document the widespread emergence of Newport MDR-AmpC strains in the USA and show that the fivefold increase, between 1998 and 2001, in the prevalence of Salmonella resistant to expanded-spectrum cephalosporins is primarily due to the emergence of that MDR-AmpC strain. The strain was isolated from humans, cattle, or ground beef in 27 states.
Molecular epidemiology of cephalosporin-resistant S. newport from animals in Pennsylvania, some from a single farm that experienced an outbreak of clinical salmonellosis in periparturient dairy cows, found integrons in about 38% of the isolates.66 (Integrons are potentially independently mobile DNA elements that encode a recA-independent, site-specific integration system responsible for the acquisition of multiple small mobile elements called gene cassettes that, in turn, encode antibiotic resistance genes. Integrons have been shown to be integrated within the chromosome in S. typhimurium DT104 and have also been described on plasmid DNA in S. enteritidis.) There is also evidence that common plasmids have been transferred between animal-associated Salmonella and E. coli and identical CMY-2 genes carried by similar plasmids have been identified in humans, suggesting that the CMY-2 plasmid has undergone transfer between different bacterial species and may have been transmitted between food animals and humans.67 The prevalence of Salmonella resistant to extended-spectrum cephalosporins from food-producing animals in Canada is very low.68
The antimicrobial susceptibilities of Salmonella strains isolated from humans, cattle, pigs, and chickens in the Netherlands from 1984–2001 have been monitored.69 Resistance was most common in S. typhimurium and among the strains from humans, pigs, and chickens, the level of resistance to tetracycline, ampicillin, chloramphenicol, and trimethoprim– sulfamethoxazole increased over the 17 years. The increase could be attributed to the emergence of S. typhimurium DT104. Among the strains from cattle, resistance levels were high in the 1980s and then declined during the study period to the levels of the other species from 1996–2001. This indicates that the levels and patterns of resistance differ considerably among serovars isolated from one host species. A similar finding occurred in England and Wales from 1988–1999.70
Since their introduction into veterinary medicine in Europe in the late 1980s and early 1990s, the susceptibility of several bacterial species to fluoroquinolones has increasingly been reported to be decreasing and their resistance to quinolones has been reported to be increasing.63 The incidence of quinolone resistance in strains of Salmonella isolated from poultry, cattle, and pigs in Germany between 1998 and 2001 has increased.71
In Canada, resistance to S. typhimurium isolated from animals, animal food products and the environment of animals to each of seven antibiotics – ampicillin, chloramphenicol, kanamycin, neomycin, streptomycin, sulfisoxazole, and tetracycline – increased persistently during each of the years 1994–9772 but none of the isolates showed decreased sensitivity to ciprofloxacin.
Studies on one dairy calf farm rearing animals for dairy–beef production found that 70% of the fecal samples were positive for Salmonella, and also found high rates of resistance to several antibiotics commonly used for the treatment of calf diarrhea.73
The susceptibility patterns of Salmonella isolates from feedlot cattle across the USA have been examined. In general, with the exception of tetracycline and sulfamethoxazole, most isolates have been susceptible to all antimicrobials tested.34 Also, resistance was not related to the use of antimicrobials in the rations being fed. The prevalence of Salmonella on beef animal hides and carcasses was 15.4% and 1.4%, respectively, and the percentage of isolates resistant to commonly used antimicrobials was low.74,75
The prevalence of S. typhimurium and S. choleraesuis isolates from pigs and humans that are fluoroquinolone-resistant and multi-drug-resistant has increased in Taiwan and the isolates have become widespread across the country.76 The S. choleraesuis isolates from humans and pigs were closely related genotypically, suggesting the nationwide dissemination of the organism from pigs to humans.
During an 8-year period, 232 Salmonella strains from horses with salmonellosis in the Netherlands were studied.77 S. typhimurium was the predominant serovar, accounting for 71%, followed by S. enteritidis at 8%. Resistance was common against tetracycline and ampicillin. S. typhimurium DT104 was most frequent and was more resistant to antimicrobials than other serovars, and had the pentadrug resistance pattern of ASSuT. The most common S. typhimurium phage type in horses corresponded with those found in humans, pigs, and cattle during the same period in the Netherlands.
Zoonotic implications
Salmonellosis, a common human intestinal disorder primarily associated with Salmonella-contaminated meats and poultry, is estimated to cost Americans about US$1 billion or more annually. The Centers for Disease Control report approximately 40 000 confirmed cases of salmonellosis annually.1 A Canadian study estimated the total cost of salmonellosis in humans at US$100 million per year in Canada; this included hospital and medical costs, lost production, lost leisure, investigating costs and loss of life. Contaminated poultry is a common source of human infection.78 The cumulative losses are due to medical costs, productivity losses and absenteeism, pain and suffering, lost leisure time, and chronic disease costs. The costs of food safety regulatory programs and costs to the food industry for product recalls and plant closures due to food-borne salmonellosis outbreaks, if included, would also increase the size of the estimates.
The disease has assumed increasing importance in recent years because of the much more frequent occurrence of human salmonellosis, with animal salmonellosis as the principal reservoir.78 Although transmission to humans does occur via contaminated drinking water, raw milk, and meat, particularly sausage, the important pathway today has become that through pigs and poultry. In Denmark, this was an important source of human salmonellosis until control measures were instituted. In most instances the increase in human infections is with ‘exotic’ serotypes other than S. typhimurium that come by animal feedstuffs to pigs and chicken, and then to humans through pork and chicken products. The most serious risk is that the transmitted bacteria will have acquired resistance to specific antibiotics because the animals from which they originate have been treated with the particular antibiotics repeatedly or over a long period.
An epidemic of salmonellosis associated with S. typhimurium DT 160 in wild birds and humans in New Zealand has been described.79 Sparrows and other birds usually die of an acute septicemia and the organism is considered to be a serious zoonotic risk.
The USDA-FSIS issued the Pathogen Reduction: Hazard Analysis and Critical Control Points Systems regulation to encourage effective pathogen reduction systems in meat and poultry processing facilities. This has been successful and is being followed by measures to reduce the numbers of Salmonella entering processing plants through live animals.1
Salmonella serovar typhimurium DT104
The increasingly common isolation of S. typhimurium DT104 (definitive phage type) is of major concern for public health officials.4 S. typhimurium DT104 was first reported in the UK in 1984 and emerged in the 1990s as an increasing cause of Salmonella infections in humans and animals in England, Wales, Scotland, other European countries such as Germany, France, Austria, and Denmark, and Canada.80 A wide range of potential reservoirs is associated with this infectious strain, from humans to the traditional food animals such as poultry, cattle, sheep,81 and pigs. Over a 1-year period in Scotland it was the predominant Salmonella isolated from nine species of animal (cattle, pigs, sheep, chickens, pigeons, horses, cats, dogs, and rabbits). All isolates were resistant to at least one antimicrobial and 98% were resistant to multiple antimicrobials, with R-type ACTSp being the predominant resistance pattern. In the UK, a clonal strain of multiply resistant (mr)DT104 that is resistant to at least five antimicrobials (ampicillin, chloramphenicol, streptomycin, sulfonamides, and tetracycline) (R-type ACSSu T) was detected in humans in 1984 and cattle in 1988.4
The organism has emerged as an important cause of diarrhea in horses in Ontario82 and is a public health concern because of its multiple drug resistance and the close relationship of the horse with humans.
The organism has been found in a variety of human foods, including salami, sausages, chicken, burgers, oysters, and vegetables. Human infections may result from contact with farm animals (cattle and sheep transmit infections to humans) and from consumption of contaminated foods such as chicken, pork, sausages, meat pastes, and beef. The organism is widespread in the USA. It has been isolated from elementary school children in a Nebraska farming community after they experienced an episode of diarrhea. Farm families are particularly at risk of acquiring infection by contact with infected animals or drinking unpasteurized milk.80 The organism’s ecology, its precise reservoirs, and its distribution in the human food chain are unclear. Clinical signs in humans infected with DT104 include diarrhea, fever, headache, nausea, and vomiting. Septicemia may develop in a small percentage of cases with potential complications of meningitis and foci of infection in bones and joints.
The antimicrobial resistance factor of DT104 is a major concern. Resistance to ampicillin, chloramphenicol, streptomycin, tetracyclines, and sulfisoxazole is characteristic of the organism. There is now evidence that DT104 is developing resistance to trimethoprim and fluoroquinolones such as ciprofloxacin, the drug of choice for treating human adult Salmonella infections. A large outbreak of salmonellosis due to mrDT104 occurred in people in England who had consumed milk from a dairy that received raw milk supplied by two farms. DT104 was isolated from the milk filter and failure of on-farm pasteurization was thought to be the cause. Strains of the organism from humans, the dairy cattle, and the milk filter showed decreased susceptibility to ciprofloxacin.83
Control and prevention of infection with DT104 will depend on increasing surveillance activities, investigating outbreaks, and identifying vehicles and risks of infections.
Contamination of milk usually occurs after the milk leaves the cow, even though the organism can be excreted into the milk during the acute phase of the disease, and occasionally by carrier animals. In a Canadian study of families from dairy farms with and without Salmonella-positive bulk tank filters, 41 of 43 of participating families regularly consumed nonpasteurized milk. Of 22 farms with Salmonella-positive milk filters, five had at least one farm family member shedding Salmonella sp. In all cases, the organism shed was of the same serotype, biotype, antimicrobial susceptibility pattern, and plasmid profile as the organism isolated from the filters. Of the individuals in these five families, 63% were shedding Salmonella sp. There is also the chance that contact between animals and humans in agriculture, and in a companion animal relationship, especially with horses, can cause interspecies spread. An unusual but predictable transmission from lambs to humans occurs when sick lambs are foster-fed, especially by children.
Various clinical forms of salmonellosis can occur in veterinarians working with Salmonella-infected animals. Gastroenteritis, bacteremia and other systemic abnormalities can occur. Cutaneous salmonellosis has been reported in veterinarians attending to infected cattle at the time of parturition. The disease was characterized by pustular dermatitis from which Salmonella virchow and S. dublin were isolated. Veterinarians may develop skin lesions after obstetric deliveries, even after hygienic precautions and the use of abundant amounts of disinfectant creams and careful washing of the arms and hands.
Economic importance
Salmonellosis is a significant cause of economic loss in farm animals because of the costs of clinical disease, which include deaths, diagnosis and treatment of clinical cases, diagnostic laboratory costs, the costs of cleaning and disinfection, and the costs of control and prevention. In addition, when the disease is diagnosed in a herd it can create considerable apprehension in the producer because of the difficulty in identifying infected animals. The veterinarian is also often in a difficult position because the diagnosis, treatment, and control of the disease are less than reliable and it is difficult to provide advice with confidence. An estimation of the economic impact of an outbreak of S. dublin infection in a calf-rearing unit indicated that the cost of disease represented a substantial proportion of the gross margin of rearing calves. The losses incurred by livestock producers include reduced feed efficiency, and reduced weight gains or deaths because of salmonellosis.
PATHOGENESIS
The pathogenesis of salmonellosis is a complex and multifactorial phenomenon. The nature of the disease that occurs following infection is dependent on the specific combination of serovar and host known as serovar–host specificity. A range of infections is included in the term ‘salmonellosis’. The most common type of infection is known as ‘the carrier state’, in which carriage of the organism is not accompanied by clinical abnormalities or clinical disease. In production animals, these carriers are of importance because they may serve as reservoirs for further spread of infection through shedding and may be present as contaminated food products.
The evolution of host-specific Salmonella serovars is considered to be associated with an increase in pathogenicity for the specific host.84 The hypothesis is based on the fact that broad-range serovars (typhimurium and enteritidis) are generally associated with severe disease only in young animals, whereas host-restricted serovars cause high mortality in both young and adult hosts.
The pathogenesis of different Salmonella serovars possessing different degrees of host restriction have been studied in young lambs to evaluate the basis of the serovar–host specificity in sheep.39 Infection with S. abortusovis resulted in clinical signs of salmonellosis, including a fever and bacterial dissemination to systemic tissues. This confirms the virulence of the strain with sheep. Salmonella gallinarum caused relatively mild disease but is virulent in chickens. S. dublin was virulent in sheep, confirming its association with ovine salmonellosis. The apparent specificity of a serovar for a particular host or range of hosts as defined by epidemiological data is influenced not only by bacterial virulence but also by the ability of the serovar to circulate within the population of the host.
Infection
Salmonella infects animals and humans by the oral route. Following ingestion, a proportion of the organisms resist the low pH of the stomach, reach the distal ileum and the cecum, invade the mucosa, and replicate in the submucosa and Peyer’s patches.
In young animals, and in adults whose resistance has been lowered, spread beyond the mesenteric lymph nodes occurs and the infection is established in the reticuloendothelial cells of the liver; from there it invades the bloodstream. These steps in the infection process can occur very rapidly. For example, in newborn calves, S. dublin when taken by mouth can be found in the bloodstream 15 minutes later. In older calves bacteria can be isolated from the intestinal lymph nodes 18 hours after their oral administration. Provided a sufficient number of a sufficiently pathogenic serotype is used, the disease is reproducible with pure cultures, for example of S. typhimurium in lambs, S. choleraesuis in pigs, S. dublin, S. typhimurium, and S. enteritidis in calves, and S. typhimurium in horses. Once systemic infection has been established, salmonellosis as a disease can develop. Its principal manifestations are as septicemia, enteritis, abortion, and a group of localizations in various tissues as a result of bacteremia.
Septicemia, bacteremia and the carrier state
After invasion of the bloodstream occurs a febrile reaction follows in 24–48 hours, and the acute phase of the disease, similar to that seen in natural cases, is present 3–9 days later. The early septicemia may be rapidly fatal. If the systemic invasion is sufficient to cause only a bacteremia, acute enteritis may develop, and abortion is a common final sequel in sheep and cattle. Many animals survive this stage of the disease but localization of the salmonellas occurs in mesenteric lymph nodes, liver, spleen, and particularly the gallbladder. In experimental S. typhimurium infection in pigs, the organism can persist from 6–8 weeks of age until market age with long-term persistence in the palatine tonsils, gastrointestinal tract, and adjacent lymph nodes. In healthy adults there may be no clinical illness when infection first occurs but there may be localization in abdominal viscera. In either instance the animals become chronic carriers and discharge salmonellas intermittently from the gallbladder and foci of infection in the intestinal wall into the feces and occasionally into the milk. For this reason they are important sources of infection for other animals and for humans. Carrier animals may also develop an acute septicemia or enteritis if their resistance is lowered by environmental stresses or intercurrent infection. Salmonellas can reside intracellularly where they are able to escape antibody-mediated killing, and the numbers of organisms are controlled by cellular defense mechanisms involving the macrophages in which they reside.
Septicemia in pigs associated with S. choleraesuis can cause pneumonia in pigs similar to the pneumonia in pasteurellosis and infection with Actinobacillus pleuropneumoniae, hepatitis, enterocolitis, and encephalitis.
S. arizonae may colonize the upper respiratory tract of sheep and induce a mild proliferative response in lambs.
Enteritis
Enteritis may develop at the time of first infection or at some other time in carrier animals. The best information available on the pathogenesis of enteritis is derived from the experimentally produced disease. In most instances the disease is produced by the administration of massive doses of bacteria, and this may result in the production of a different syndrome from that which occurs naturally. The pathogenesis of enteric salmonellosis is much more complex than cholera, involving an increase in mucosal cell cyclic AMP content and prostaglandin concentration, as well as an inflammatory response to the invading bacteria. Intestinal invasion is a characteristic feature of Salmonella pathogenesis. Within minutes of injecting ileal loops in calves, Salmonella can be seen to invade both M cells and enterocytes that overlie domed villi associated with lymphoid follicles and absorptive villi.61 The organism must invade the intestinal mucosal epithelium to cause disease.
S. typhimurium requires a functional type III secretion system encoded by Salmonella pathogenicity island I (SPII) to cause diarrhea.61,62 The SP II secretion system mediates the translocation of secreted effector proteins into target epithelial cells. These effector proteins are key virulence factors required for Salmonella intestinal invasion and the induction of fluid secretion and inflammatory responses.
After oral infection with S. dublin, invasion occurs through the intestinal wall in the terminal ileum and cecum and progresses as far only as the mesenteric lymph nodes. Progress beyond this point, and the development of the disease, salmonellosis, is determined by factors such as immune status and age of the animal, whether or not it is exposed to stress, and the virulence of the strains. A number of characteristics of the bacteria influence their virulence, including the presence of adhesin-pili and flagellae, cytotoxin, enterotoxin, lipopolysaccharide and the inflammatory response that they initiate in the intestinal wall. The effects of some of these factors are not limited to the intestinal tract and also contribute to the systemic complications of salmonellosis. The S. dublin virulence plasmid mediates systemic infection in cattle by causing macrophage dysfunction.
S. dublin infections in calves have been used to create the disease experimentally. In calves 6–7 weeks of age, an oral dose of the organisms is fatal within 24 hours, with the animals dying of septicemia and an acute necrotizing panenteritis. Calves 12–14 weeks of age developed a progressive fatal diarrhea within 1 week following infection. Experimental infection of ligated ileal loops from calves with S. typhimurium results in an acute neutrophilic inflammatory response associated with invasion of Peyer’s patches.85
In calves, infection is initiated by bacterial invasion of the mucosal epithelium of the distal ileum or proximal colon causing extensive local tissue damage that leads to shortening of the villi and degeneration of the enterocyte layer. Salmonella invasion induces potent inflammatory response characterized by a massive infiltrate of polymorphonuclear cells into the lamina propria and submucosa, and secretion of fluid into the intestinal lumen.86 Damage to the enterocyte layer and the secretion of fluid into the intestinal lumen results in diarrhea, and the fever is due to circulating inflammatory cytokines. The molecular basis of Salmonella-induced enteritis has been described.61
Experimentally induced Salmonella infection in calves results in an increase in serum haptoglobin levels within 3 days of challenge.87 By day 3 after experimental infection the serum haptoglobin levels increased to a median level of 212 μg/mL while placebo controls had median levels of 0 μg/mL. The increased levels closely reflected the clinical findings of infection and are considered useful markers of infection severity in salmonellosis in calves.
In sheep, the experimental disease produced by oral dosing with S. typhimurium includes an early acute enteritis of the small intestine at 24 hours. At 5–8 days there is hemorrhagic and necrotic typhlitis and the infection is established in mesenteric lymph nodes and the liver. Experimental S. dublin infection of the mammary gland of dairy cattle results in a persistent infection associated with a chronic active mastitis similar to carriers with naturally acquired S. dublin infection.
In ponies with experimental infection with S. typhimurium orally, there is much variation in the time after infection that the various signs appear. Pyrexia, neutropenia, and high fecal Salmonella counts coincided on the second and fourth days, but diarrhea occurred in only some ponies and then on the third to 11th days after inoculation. Positive agglutination tests were recorded from day 1 but were mostly during the period 6–12 days postinoculation. The neutropenia of the early stages of the disease is transient, and neutrophilia occurs when diarrhea commences.
The characteristic fever and leukopenia of equine salmonellosis have been attributed to the release of endotoxin from the bacteria during invasion of, and replication within, the intestinal epithelium. The equine colonic mucosa can respond to cholera toxin, which causes an increased secretion of chloride, sodium, and water into the intestinal lumen. The enterotoxin activity of S. typhimurium of equine origin has been compared to cholera enterotoxin.
Although there is sufficient obvious enteritis to account for the diarrhea that characterizes the disease, there appear to be other factors involved. For example, it has been shown experimentally that in Salmonella enteritis there is stimulation of active chloride secretion combined with inhibition of sodium absorption, but invasion of the mucosa is not essential for these changes to occur. These observations are of interest in the light of the known hyponatremia that characterizes the disease. Studies of calves with salmonellosis have shown that the fluid loss associated with the diarrhea of this disease is much greater than in other calf diarrheas. This, together with a large solid matter output, contributes to the significant weight loss occurring in salmonellosis. In pigs and to a lesser extent in cattle, ulcerative lesions may develop in the intestinal mucosa and may be of sufficient size to cause chronic intermittent diarrhea. In pigs it has also been observed that villous atrophy is a sequel to infection with S. choleraesuis.
In pigs, most clinical cases of salmonellosis are associated with S. choleraesuis or typhimurium. S. choleraesuis is host-adapted to pigs, causing a systemic, typhoid-like disease. S. typhimurium is not host-adapted to pigs, and infection results in a localized enterocolitis.
In the pig the development of enteritis associated with S. choleraesuis begins 36 hours after infection with the appearance of erosions and edema of the cecal mucosa. At 64 hours the wall is thickened and there is diffuse caseation overlying the erosions. The necrotic membrane sloughs at 96 hours and at 128 hours all function is lost and the entire intestinal wall is involved in the inflammatory process, the muscular coat being obliterated by 176 hours. The colon is usually the major organ affected in S. typhimurium infections in pigs, causing either focal or diffuse necrotizing colitis. The organisms proliferate in the intestine, invade the intestinal epithelium, stimulate fluid secretion and disseminate from the intestine to mesenteric lymph nodes and other organs. S. choleraesuis invades enterocytes by penetration of the brush border, resulting in focal loss of microvilli, and the bacteria are endocytosed into membrane-bound vacuoles. Experimental infection of ileal–gut loops of pigs with S. enterica results in preferential bacterial adherence to microfold cells (M cells) within 5 minutes, and by 10 minutes bacterial invasion of the apical membrane occurs in M cells, goblet cells, and enterocytes.88 Experimental perfusion of porcine livers with polysaccharide or live S. choleraesuis results in the release of mediators that mediate biological activities that have an important role in reducing the severity of bacterial infections. The comparison between early invasion of ileal loops by S. enterica and S. choleraesuis in the pig has been described.89
Abomasitis
S. typhimurium DT104 has been associated with some independent outbreaks of abomasitis in veal calves.90 Abomasitis was reproduced experimentally by oral infection of calves.
Abortion
Abortion is a common manifestation of salmonellosis in cattle between days 124 and 270 of gestation. When infection is associated with S. dublin, the organism multiplies in the placenta, having been seeded there from a primary lesion in other maternal tissues. Fetal death has already occurred in many cases, because of its invasion by bacteria, but live calves also occur, suggesting that the placental lesion is the critical one. S. montevideo has been associated with a significant number of outbreaks of abortion in ewes.
CLINICAL FINDINGS
The disease is most satisfactorily described as three syndromes, classified arbitrarily according to severity as septicemia, acute enteritis, and chronic enteritis. These are described first but the differences between the animal species are sufficiently significant to justify describing the disease separately in each of them. There are no significant differences between infections associated with the different Salmonella species.
Septicemia
This is the characteristic form of the disease in newborn foals and calves, and in young pigs up to 4 months old. Commonly, there is profound depression, dullness, prostration, high fever (40.5–42°C, 105–107°F) and death within 24–48 hours.
Acute enteritis
This is the common form in adult animals of all species. There is a high fever (40–41°C, 104–106°F) with severe, fluid diarrhea, sometimes dysentery, and occasionally tenesmus. The fever often subsides precipitously with the onset of diarrhea. The feces have a putrid smell and contain mucus, sometimes blood, fibrinous casts, which may appear as complete tubular casts of intestine, and intestinal mucosa in sheets or casts. There is complete anorexia but in some cases increased thirst. The heart rate is rapid, the respirations are rapid and shallow and the mucosae are congested. Pregnant animals commonly abort. The case fatality rate without early treatment may reach 75%. In all species, severe dehydration and toxemia occur and the animal loses weight, becomes weak and recumbent, and dies in 2–5 days. Newborn animals that survive the septicemic state usually develop severe enteritis, with diarrhea becoming evident at 12–24 hours after the illness commences. If they survive this stage of the illness, residual polyarthritis or pneumonia may complicate the recovery phase.
Chronic enteritis
This is a common form in pigs following a severe outbreak, and occurs occasionally in cattle and adult horses. In calves there is intermittent or persistent diarrhea, with the occasional passage of spots of blood, mucus and firm fibrinous casts, intermittent moderate fever (39°C, 102°F), and loss of weight leading to emaciation. Although chronic enteritis may occur initially it usually succeeds an acute episode.
Bovine salmonellosis
The disease associated with S. dublin is usually endemic on a particular farm, with sporadic cases occurring when individual animals are exposed to stress. Severe outbreaks are rare but do occur when there is severe stress, usually acute nutritional deprivation, applied to the entire herd.
When S. typhimurium is the cause, it is usual to have a single animal or a small number of animals affected at one time. When the disease is in the calf population it is usual for it to be much more severe, with many affected, either as a point outbreak or, when there is a succession of calves, a continuing occurrence of the disease. The emphasis, therefore, is generally on the occurrence of individual, sporadic cases in newborn calves and recently calved cows. S. muenster in a dairy herd has been associated with abortions, diarrhea in adults and calves, and shedding of the organism in the milk of about 8% of the cows.18
Septicemia is the common form of the disease in newborn calves under a few weeks of age. There is depression, toxemia, fever, dyspnea, and weakness; nervous signs, including incoordination and nystagmus, may occur. Diarrhea and dysentery may occur but are not common.
Calves older than a week, and adults, are usually affected by acute enteritis, followed in survivors by abortion in pregnant cows and polyarthritis in calves. In severe cases of enteritis, there is often dysentery, with whole blood passed in large clots, and complete agalactia in lactating cows. Abdominal pain, with kicking at the abdomen, rolling, crouching, groaning, and looking at the flanks, may occur in adult cattle. Rectal examination at this stage usually causes severe distress.
Chronic enteritis with inappetence, reduced weight gain, and unthriftiness may follow an attack of acute enteritis or be the only manifestation of the disease. Abortion is a common sequel in pregnant cows that survive an attack of acute enteritis. However, infection with S. dublin is also a significant cause of abortion in cattle without there having been any clinical signs other than retained placenta. A sequel to some cases of apparent enteric salmonellosis is the development of terminal dry gangrene due to endarteritis of the extremities, including eartips, tailtip, and the limbs from the fetlock down.
Terminal dry gangrene of the extremities of calves is characterized by lameness, swelling of the hindlimbs below the fetlocks, and separation of the skin above the fetlock. The distal portion of the limb is cool, not painful, and the skin is dry or moist. There is a clear line of demarcation of the skin at the level of the fetlock joints between the normal proximal skin and the distal necrotic tissue. The phalanges may be separated from the metatarsus. The tips of the ears may be indurated and deviated medially and the distal aspect of the tail may be dry and shriveled.
Abortion due to S. dublin may occur spontaneously without any previous clinical evidence of salmonellosis in the herd. Abortion has occurred from days 124 to 270 of gestation. Cows that abort may be ill with a fever, anorexia and hypogalactia and some will retain fetal membranes. In some cases, calves may be born shortly before term and die in the perinatal period. S. muenster has also been implicated in abortions in a dairy herd.18
The experimental disease produced by infecting adult cattle with S. dublin by mouth varies from no clinical illness to fatal dysentery. Abortion occurs in some pregnant females. Many suffer pyrexia, anorexia, and mild diarrhea. Experimental infection of calves with S. typhimurium has the same general effect, with more severe syndromes occurring in younger calves. Chronic cases may develop bone lesions, including osteoperiostitis and osteomyelitis, sometimes with epiphyseal separation. Experimental infection with S. enteritidis causes profuse yellow diarrhea, fever, dehydration, frequent cough, and a mucopurulent nasal discharge.
Ovine and caprine salmonellosis
The only recognized form of the disease in sheep is acute enteritis on a flock scale. However, in the early stages of the outbreak there may be some cases of the septicemic form. After experimental infection of sheep with S. dublin, fever and diarrhea are followed in pregnant ewes by abortion. Abortion is also common in the naturally occurring disease and has come to exceed S. abortusovis as a cause of abortion in sheep in the UK. Some ewes die after abortion and many of the lambs born alive die subsequently. Fever and diarrhea, followed by abortion, have also been produced experimentally in sheep by the administration of S. dublin.
In goats, naturally occurring cases are not often reported. S. dublin is the usual pathogen in those countries where it is a resident, but S. typhimurium is also recorded as a cause. Peracute septicemia, in newborn animals, and acute enteritis occur with signs and lesions similar to those in cattle.
Porcine salmonellosis
In pigs, the disease varies widely and, although all forms occur in this species, there is often a tendency for one form to be more common in any particular outbreak. In the septicemic form in pigs affected by S. choleraesuis a dark red to purple discoloration of the skin is evident, especially on the abdomen and ears, and subcutaneous petechial hemorrhages may also be visible. Nervous signs, including tremor, weakness, paralysis, and convulsions, may be prominent and occur in a large proportion of affected pigs. The case-fatality rate in this form is usually 100%.
A semispecific entity occurring in pigs up to 4 weeks old is manifested by meningitis and clinical signs of prostration and clonic convulsions.
In the acute form there is also a tendency for pulmonary involvement to occur, but the main feature of the disease is enteritis, with pneumonia and occasionally encephalitis present as only secondary signs. In some situations pigs dying of septicemia more commonly yield S. choleraesuis, while those with acute enteritis are usually infected with S. typhimurium. Acute pneumonia is a common accompaniment of this form of the disease in pigs, and nervous signs and cutaneous discoloration as described in the septicemic form may also be present. Meningitis due to S. typhimurium DT104 in 1-week-old piglets has been reported.91 Incoordination, paralysis, opisthotonos, paddling, and polyarthritis resulting in runts and deaths were common. Bronchopneumonia resembling pasteurellosis, and pleuropneumonia resembling A. pleuropneumoniae infection can be associated with S. choleraesuis in pigs.
A syndrome of rectal stricture occurs in feeder pigs as a sequel to enteric salmonellosis associated with S. typhimurium and is described under that heading.
Equine salmonellosis
The disease in horses usually occurs in a single animal and sporadically. However, outbreaks do occur in newborn foals, in groups of horses recently transported, and in horses hospitalized in veterinary clinics. Analysis of spatial and temporal clustering of horses with salmonellosis in an intensive care unit of a veterinary teaching hospital suggested that affected horses were grouped in time. Experimental infection of horses by oral administration of S. typhimurium produces a disease similar to the natural disease. The incubation period may be as short as 24 hours. Four syndromes occur:
• Asymptomatic shedding of S. typhimurium in feces intermittently or continuously for short periods of 4–6 days
• A subacute enteric form in adult horses on farms where the disease is endemic, with fever, depression, anorexia but without severe diarrhea, although the feces may have the consistency of soft bovine feces. There is no other obvious intestinal abnormality. There may be a neutropenia with a left shift
• Severe, acute fulminating enteritis with diarrhea, fever, dehydration, and neutropenia. There is abdominal pain, which may be sufficiently severe to stimulate violent actions. This is the common form of the disease, occurring commonly in adults that are exposed to stress in one form or another. Newborn and young foals up to 8 days of age also often have this form of the disease,36 characterized by depression, anorexia, and diarrhea
• In foals up to about 2 days of age there is a highly fatal septicemia. Localization in survivors includes lesions in the brain, causing meningo-encephalitis, polyarthritis, and many other sites. Fatal meningo-encephalomyelitis due to S. agona has been described in a 7-day-old foal.92 Clinical findings included head tilt, seizures, and diarrhea.
CLINICAL PATHOLOGY
A definitive etiological diagnosis of salmonellosis depends on culture of the organism from feces, blood, milk, and other body fluids or tissues. Feed and water samples may also be cultured to determine the source of the organism. Numerous serological tests are available but lack sensitivity and specificity.
The three aspects of cases of salmonellosis that profit by clinicopathological support are:
• Diagnosis in the individual animal, when its treatment and prognosis depend on a definitive diagnosis
• Diagnosis of a herd problem to insure that expensive herd-wide control measures are not implemented unnecessarily
• Monitoring the biochemical status of a sick animal in order to determine most accurately its requirements for supportive therapy, especially fluid and electrolytes.
The diagnostic techniques available are as follows.
Bacterial culture and detection
This is the only way of making a definitive etiological diagnosis of salmonellosis and of exactly determining the serotype. However, culturing the organism is unreliable for various factors including the method used to collect samples, the amount of sample submitted, variation in the shedding of the organism, and the bacteriological method used. A major complicating factor is the occurrence of apparently healthy carriers, which shed the organism intermittently in the feces, and silent carriers, which do not shed but harbor the organism in mesenteric lymph nodes or in the mucosa of the cecum and colon. The difficulty varies according to genotype. In cattle with S. dublin infections, the bacteria are present in the blood and milk for a very brief period during the bacteremic phase and before diarrhea commences.
The organism can be cultured from fecal samples, bulk tank milk, milk filters, water and feed sources, and environmental sites.93 When sampling dairy farms weekly for 7–8 weeks, the prevalence of fecal shedding from different groups of cattle may vary widely among herds, indicating that herds with infected cattle may be classified incorrectly if only one group is tested. Cows near calving are most likely to be shedding Salmonella in the feces. Testing environmental sample sources is more efficient for identifying infected premises than using individual cattle fecal samples.
Fecal culture
The culturing of salmonellas from feces is done commonly but can be unreliable. This difficulty is noticeable with S. dublin infection in cattle, S. choleraesuis infections in pigs and S. typhimurium infection in horses. The discrepancy in S. dublin infections in calves may be as great as 55% accuracy only and in horses only 50%. The difficulties relate to dilution by diarrhea and the heavily contaminated nature of the sample; a sample of fluid feces collected in a container is superior to a fecal swab. Clinical laboratories generally require at least 48 hours for presumptive diagnosis of Salmonella spp. in feces. Biochemical and serological confirmation of the genotype and the antibiogram may require an additional 24–48 hours. The use of extended enrichment of fecal samples with tetrathionate broth is superior to primary enrichment for detection of salmonellas from cattle.
A special feature of S. dublin is the tendency to produce persistent infections without clinical manifestations in some infected cattle – also called carriers. The organism is harbored in lymph nodes and other internal organs, and is only periodically shed in milk and/or feces. The rate of fecal culture of S. dublin from known carrier cows and calves is low even when sampled over a period of 12 months – 3.35–17.26%.94 Carriers frequently have continuously high immunoglobulin levels in serum and milk and ELISAs are used as an alternative.94
Because culture of a large number of individual fecal samples is expensive and time-consuming, pooled fecal samples from individual animals provides excellent agreement for detection of S. infantis when the number of samples per pool is 20 or less.95 With the kappa test, the agreement ranged in two groups of pooled fecal samples from 0.81–0.98.
Multiple fecal cultures
Multiple cultures at 24-hour intervals are superior to single fecal cultures for the diagnosis of clinical salmonellosis in horses.40 Fecal samples should be cultured directly on Brilliant Green Agar and an additional sample inoculated in Selenite broth and incubated for 24–48 hours followed by culture on Brilliant Green Agar.54 Simultaneous culture of a pinch biopsy of rectal mucosa may increase the number of isolations of salmonellas in cattle and horses.
Antigen-capture enzyme-linked immunosorbent assay
An antigen-capture ELISA with enrichment culture for detection of salmonellas from fecal samples is more rapid than routine culture techniques, with a test sensitivity of 69% and specificity of 97%.
Polymerase chain reaction
A PCR assay is a highly specific and sensitive test for the detection of salmonellas in fecal samples from horses.96 The test is genotype specific, is much more sensitive than microbiological culture for detecting the organism from fecal swab specimens, requires fewer samples, and can provide results 24 hours after receipt of fecal samples. Direct detection is done by amplification of part of omp C after extraction of DNA from feces.96 Salmonella DNA was detected in 40% of fecal samples, while Salmonella was cultured from only 2% of the samples. The PCR assay has been used to detect Salmonella DNA persisting in the environment of a veterinary teaching hospital.54 Pulse-field gel electrophoresis can be used to characterize the types of S. enterica isolate.54
A real-time PCR assay uses enrichment of fecal specimens, followed by genomic DNA extraction to detect Salmonella ssp.-specific DNA segment.97 Relative sensitivity was 100% and specificity 98.2% compared with bacterial culture.
Bulk tank milk filters
The bulk tank milk filter is collected immediately after milking and submitted for culture. Samples of bulk tank milk are also submitted.
DNA probes
The use of the DNA probe encoding a well-conserved virulence gene of the Salmonella virulence plasmid is a sensitive method for screening large numbers of samples to detect potentially virulent Salmonella spp.
Serial blood culture
In the early stages of the disease when the animal is likely to be bacteremic this is not a practicable technique because of the need to collect serial samples and the cost of blood cultures.
Serology
Serum enzyme-linked immunosorbent assay
Serological testing using ELISA tests on serum or milk can be used in herds to identify S. dublin carriers, which can then be culled.94 The test is based on immunoglobulins to the O antigens of the lipopolysaccharide of the organism. The superior sensitivity and negative predictive value of the serum ELISA is preferable to fecal culture as an initial screening test and for herds not infected with S. dublin.94 It may be able to differentiate between uninfected, recently infected recovered, and milk-shedding (mammary-gland-infected) carrier cows; it can also be used for assessing infection rates and vaccine responses. ELISA testing of individual milk samples can be used for surveillance of herds infected with S. typhimurium or S. dublin.
An ELISA test using lipopolysaccharide antigen is highly O-antigen-specific and predictable. A serum with a positive result on the screening antigen can then be tested on the other antigens to determine the specific serotype that has infected the animal. The ELISA is suitable for screening for the presence of infection with S. typhimurium or S. infantis on a herd basis.
Using a variety of ELISA tests, muscle fluid samples from cattle taken at slaughter can be used as an alternative to serum to detect antibodies to Salmonella polysaccharide. Bulk tank milk can be tested for antibodies to S. dublin and used as a national screening diagnostic aid.98 The S. dublin ELISA had a high sensitivity (97%) and specificity (97%) for muscle fluid samples, compared with serum samples; the relationship for S. typhimurium was also good.
Meat juice enzyme-linked immunosorbent assay: Danish mix-ELISA
The Danish mix-ELISA (DME) is a combination of lipopolysaccharide extractions of S. choleraesuis (O antigens 6 and 7) and typhimurium (O antigens 1, 4, 5, and 12), used to assay serum samples collected from live animals on the farm or from meat juice (collected when a meat sample from the carcass is frozen and thawed).12 The DME was designed for surveillance and is recommended for monitoring herds and detecting high levels of Salmonella infection.99 The test has been the basis for national Salmonella control programs in Denmark (SALINPORK), Germany, and the UK and is being considered in the Netherlands and Belgium. In a series of studies using pigs experimentally infected with either S. typhimurium or S. infantis, the sensitivity of the DME was more than 95% and the specificity 100% when compared to culture used to determine the positive or negative status of the pigs.12 There is a strong association between herd serology and the prevalence of Salmonella measured at three sampling sites: cecal content, pharynx, and carcass surface.100
Indirect tests
These include a total and differential white cell count. A leukopenia, neutropenia and severe degenerative left shift are highly suggestive. There is also a marked hyponatremia and a mild hypokalemia. These tests are well established in horses, and the leukopenia has been observed in acute salmonellosis in cattle. The fecal leukocyte count is also a worthwhile supportive test in the search for salmonellosis. A high count is strongly suggestive, but many horses with acute or severe diarrhea have high fecal leukocyte counts in the absence of salmonellas in the feces.
Laboratory diagnosis in a suspected sick animal
A positive diagnosis depends on culture of the organism, usually from feces but possibly from blood in the septicemic stage. If serological diagnosis is available a serum sample should also be submitted. Indirect tests are very valuable and, if laboratory availability is good, a total white cell count and estimation of serum sodium levels should be undertaken urgently. A presumptive diagnosis is often all that can be stated, and this may be supported by a herd diagnosis – a diagnosis that the disease or infection is present in the herd and that it is presumed that the subject case is one of the group.
Herd diagnosis
A serological examination of a sample of animals is a first step. A completely negative serological test would indicate that the infection is not present. Positive results indicate a need for further examination, and periodic fecal cultures at 15-day intervals using enriching media should be undertaken. When S. typhimurium is the causative bacteria, the feces of other species of animals on the farm should be examined, because ducks, dogs, horses, pigs, sheep, and cattle may be sources of infection for each other. It is always advisable to examine the drinking water and feed for evidence of infection.
Detection of clinically normal carrier animals
The most difficult diagnostic problem in salmonellosis is the detection of the clinically normal carrier animal. The recommended procedure is to do fecal cultures on all cows at 14-day intervals for 3 examinations, and repeat the examination on the day of calving. At that time, swabs are taken from feces and the vagina of the cow, and the feces of the calf. The sampling should preferably be done when the cows are tied in stanchions and not grazing pasture, because of the large number of passive carriers of the infection in the latter circumstance.
The reliability of diagnosis based solely on culture of fecal swabs is not high and represents the major difficulty in detecting carriers. A combination of fecal culture and serological tests offers some improvement in accuracy, but even with the agglutination or complement fixation tests accuracy is insufficient.
Determination of prevalence of infection in population of animals
In food-producing animals it is particularly important to determine the prevalence of Salmonella infection in a population of cattle or pigs. Pork and pork products are important sources of nontyphoidal Salmonella for humans consuming these products if they are not handled with care. Pigs entering the abattoir that are carriers of Salmonella are the most important source of carcass and product contamination. In order to be able to estimate the number of infected animals entering the abattoir and estimate the size of the Salmonella problem in pig herds, the population and herd level prevalence of Salmonella have to be investigated. An estimation of the prevalence of S. enterica infection in finishing pigs in Iowa was done using on-farm fecal cultures, culture of on-farm necropsy and abattoir-collected samples, and serum ELISA using serum exudate (meat juice).101 Fecal samples collected on the farm detected only 13.3% of all positive pigs necropsied on the farm. Abattoir and on-farm results combined, the fecal sample detected 57.4% of positive pigs. Abattoir-collected samples provided prevalence estimates much higher than on-farm collected samples (39.9 vs 5.3%). Thus fecal samples have a low sensitivity for detecting infected pigs and abattoir-collected samples overestimate the on-farm S. enterica prevalence. Pigs can become infected during routine testing or holding periods during marketing when exposed to relatively low numbers of Salmonella in the preslaughter environment. Intervention this step on the production process may have a major impact on the safety of pork products.
The probability of detecting Salmonella in seropositive pig herds and thereby correlation between serological and fecal culture results were examined in pig herds as part of an international research program sponsored by the European Commission, Salmonella on Pork.102 Samples were examined from herds in Denmark, the Netherlands, Greece, and Germany. The serological herd status was determined by blood-sampling 50 finishing pigs. There was an increased probability of recovering Salmonella with increasing within-herd seroprevalence but the correlation was only moderate.
NECROPSY FINDINGS
Septicemia
There may be no gross lesions in animals that have died peracutely but extensive submucosal and subserosal petechial hemorrhages are usually evident. In pigs, the petechiae are very prominent and may give the kidney the ‘turkey-egg’ appearance usually associated with hog cholera. A rhomboidal area of gastric mucosal infarction is usually present in pigs. Congestion and hepatization of lung tissue may also be present in this species. Skin discoloration is marked in pigs and, depending on the severity of the case, this varies from extreme erythema with hemorrhage, to plaques and circumscribed scabby lesions similar to those of swine pox. In some cases the necropsy findings may include splenomegaly and pinpoint white foci in the liver (paratyphoid nodules). The histological lesions are nonspecific, with the exception of the somewhat granulomatous character of the older paratyphoid nodules. The placentas of cattle and sheep aborting due to Salmonella spp. often contain very large numbers of intravascular bacteria.
Acute enteritis
Some of the changes associated with the septicemic form are often present but the most consistent damage is found in the large and small intestines. The character of the inflammation here varies from a mucoenteritis with submucosal petechiation to diffuse hemorrhagic enteritis. Similar lesions may be present in the abomasum, and in S. dublin infections in calves multiple mucosal erosions and petechiation of the abomasal wall are common. In porcine enteric salmonellosis, congestion and infarction of the gastric mucosa is often seen. Infections with S. typhimurium are characterized by severe necrotic enteritis in the ileum and large intestine. The intestinal contents are watery, have a putrid odor and may contain mucus or whole blood. In cases that have survived for longer periods, superficial necrosis and fibrin exudation may proceed to the development of an extensive diphtheritic pseudomembrane and fibrin casts. The mesenteric lymph nodes are enlarged, edematous, and hemorrhagic. The wall of the gallbladder may be thickened and inflamed. In pigs, survivors of the septicemic and acute enteric forms of salmonellosis may develop rectal strictures.
Chronic enteritis
In cattle, the chronic form is usually manifested by discrete areas of necrosis of the wall of the cecum and colon. The wall is thickened and covered with a yellow-gray necrotic material overlying a red, granular mucosal surface. In pigs the lesion is similar but usually more diffuse. Less commonly the lesions are discrete in the form of button ulcers, occurring most frequently in the cecum around the ileocecal valve. The mesenteric lymph nodes and the spleen are swollen. In all species, chronic pneumonia and a variety of other localized inflammatory processes such as polyarthritis and osteomyelitis may be found.
Salmonellas are present in the heart, blood, spleen, liver, bile, mesenteric lymph nodes, and intestinal contents in both septicemic and acute enteric forms. In the chronic form, the bacteria may be isolated from the intestinal lesions and less commonly from other viscera. Culture is more successful if enrichment media such as tetrathionate broth are employed. In pigs experimentally infected with S. typhimurium and S. choleraesuis var. kunzendorf the organisms can be detected with peroxidase–antiperoxidase immunoenzymetric labeling and immunogold techniques.51 Surveys that set out to determine the percentage of carriers in animal populations by examining abattoir material show that by far the largest number of isolations are made from the lymph nodes draining the cecum and lower small intestine.
Samples for confirmation of diagnosis
• Bacteriology – ileocecal lymph node, ileum, colon, spleen, lung, liver, culture swab from gall bladder (CULT)
• Histology – formalin-fixed samples from these tissues plus kidney, stomach, brain (LM).
Note the zoonotic potential of these organisms when handling carcasses and submitting specimens.
The clinical diagnosis of salmonellosis is difficult because of the number of other diseases that resemble each form of the disease. Salmonellosis is characterized by septicemia in young animals and acute and chronic enteritis in adults, although acute enteritis can occur in neonates. Thus the septicemic form of the disease must be differentiated from all other causes of septicemia, and the enteric forms from all other causes of diarrhea in both young and adult animals. At necropsy the isolation of salmonellas from tissues and intestinal contents, although suggestive of the presence of salmonellosis, does not of itself confirm the diagnosis, and care must be taken to ascertain whether other disease is present.
Cattle
Septicemia
The septicemic form of salmonellosis in calves resembles coliform septicemia and differentiation is possible only by bacteriological examination of blood, feces, and tissues. Salmonellosis occurs most commonly during the second and third weeks of life in contrast to coliform septicemia which occurs most commonly in the first few days of life. Both are characterized by weakness, depression, polypnea, tachycardia, fever or hypothermia, scleral injection and hemorrhages, diarrhea, and rapid death.
Acute enteritis
Acute enteric salmonellosis in adult cattle or calves is characterized by fever, anorexia, toxemia, abdominal pain, diarrhea and dysentery, excessive mucus and fibrinous casts and strands in the feces, and dehydration.
• Coccidiosis occurs most commonly in young cattle 2–8 months of age and is characterized by diarrhea with frank blood in the feces, tenesmus, only occasionally systemic signs of dehydration and anemia, and spontaneous recovery in a few days; rarely there are nervous signs and death
• Acute intestinal obstruction is characterized by abdominal pain, scant or absent feces, blood-stained feces, tenesmus, anorexia, and palpable abnormalities on rectal examination
• Winter dysentery occurs in explosive outbreaks in housed adult cattle; the feces are gray with flecks of blood, there is no toxemia, no dehydration, and the disease is self-limiting in 24–48 hours
• Mucosal disease is characterized by typical oral erosions, anorexia, fever, persistent diarrhea, dehydration, lesions in the interdigital clefts and a high case-fatality rate
• Bracken fern poisoning is characterized by dysentery, scleral hemorrhages and a history of access to the bracken plant
• Other poisonings, especially arsenic and to a lesser extent lead and a number of miscellaneous weeds, may cause a similar acute enteritis.
Sheep
Diarrhea associated with infections with coccidia or Campylobacter spp. or by parasitic infestation may resemble enteric salmonellosis in sheep but the latter is usually more acute and more highly fatal.
Horses
Septicemia
Septicemic salmonellosis in foals may resemble the septicemias associated with E. coli and Actinobacillus equuli.
Acute enteritis
Acute enteric salmonellosis in adult horses causes profuse diarrhea, dehydration, severe depression, and weakness. A history of recent transportation often helps in suggesting the diagnosis of salmonellosis in adult horses, in which colitis X is the important differential diagnosis.
Idiopathic equine colitis X is a severe enterocolitis of adult horses characterized by profuse diarrhea, marked dehydration and a high case-fatality rate in spite of intensive fluid therapy. Many cases are considered to be enteric salmonellosis but the definitive etiological diagnosis is often not obtained.
Pigs
Septicemic salmonellosis occurs in pigs 1–4 months of age and is characterized by fever, depression, skin color changes, diarrhea, and rapid death.
• Hog cholera, ASF coliform gastroenteritis of recently weaned pigs, and pasteurellosis may resemble septicemic salmonellosis very closely and laboratory examination is usually necessary for identification
• Acute erysipelas is characterized by typical skin lesions, fever, swollen joints, and typical lesions at necropsy
• Swine dysentery is characterized by mucoid feces with dysentery and typical lesions of the large intestine.
TREATMENT
Primary treatment – antimicrobial therapy
The use of antimicrobials for the treatment of clinical salmonellosis is controversial and different approaches to the problem exist among veterinarians. The controversy centers on two parts of the response to treatment, and which view is taken depends to a large extent on the experience one has with respect to them.
The first issue is that of the success of treatment in saving the lives of clinically affected animals. It is our experience that early treatment with broad-spectrum antimicrobials is highly effective in reducing mortality and returning animals to normal function. It is generally agreed that treatment must be early, because delay means loss of the integrity of intestinal mucosa. A common pattern of response to treatment in a herd is that the first one or two cases are regarded lightly by the owner and they are treated 24–48 hours after diarrhea begins. When these cases die, a more prompt regimen is instituted in which the farmer has the approved drug on-hand and begins treatment as soon as diarrhea with fever is observed. The cure rate is then likely to be of the order of 100%, except in the case of foals and calves, in which a fulminating septicemia is apt to defeat even the best treatment program.
The second issue in the controversy about antimicrobial therapy for salmonellosis is the risk of inducing ‘carrier’ animals. In humans and in animals there is some evidence that antimicrobials can prolong the duration of the period after clinical recovery during which the causative bacteria can be isolated from the intestine. It is accepted that this can occur and that the use of antimicrobials can theoretically contribute to the spread of disease. However, because of the way in which animals are kept, and because they constantly ingest contaminated pasture or other feed, there is an almost universal carrier segment in animal populations, and to regard another survivor from salmonellosis as a significant contributor to the carrier frequency seems an exaggeration. In many situations this appears to be the correct view, but in other situations an animal can become infected, for example, in a veterinary hospital or at an exhibition or show, recover clinically with treatment and, after returning to its parent herd, initiate an outbreak of fatal and debilitating salmonellosis. Both epidemiological patterns occur, and they seem to occur in different places, so that the most appropriate attitude to take seems to be the one that fits local circumstances. In an area where only sporadic cases of the disease occur in herds, it would be professionally negligent not to treat infected animals with appropriate antimicrobials. In endemic areas, recovered animals should not be sent into herds until they are known not to be carriers.
Other related issues are the creation of drug-resistant strains of the bacteria and the effect on the normal intestinal flora that results from oral medication. The problem with resistant strains would not have become a significant one if only individual animals had been treated, but mass medication of in-contact animals and prophylactic treatments have generally resulted in a large population of resistant strains.
Oral treatment in cattle and pigs is recognized as a satisfactory treatment but it is not recommended in horses in which an immediate worsening of the diarrhea, or its prolongation as a persisting chronic diarrhea, may be encountered. It is thought that both sequelae result from an alteration of the normal population of intestinal microflora resulting from the 8–10 times greater concentration of drug that occurs in the intestine after oral treatment, compared to the concentration resulting from parenteral injection.
In summary, antimicrobials are recommended for all clinically affected animals as set out below. The choice of antimicrobials depends on a test of drug sensitivity in each case or outbreak but failing this the following generalizations can be applied.
Ruminants
Currently there are no antimicrobials labeled for treatment of bovine salmonellosis in the USA. As a result, treatment of salmonellosis in cattle is largely empirical and extralabel use of certain antimicrobials is common in veterinary practice.103 Ceftiofur at 5 mg/kg BW intramuscularly/24 hours is effective for the treatment of experimental salmonellosis in neonatal calves.103 It promotes animal welfare, reduces fecal shedding of Salmonella, and may prolong clearance of Salmonella infections when plasma ceftiofur concentrations are maintained above MICs.
In calves with S. dublin infections trimethoprim–sulfadoxine is recommended, given parenterally daily until clinical recovery occurs. Ampicillin and amoxicillin are also effective. Oral dosing is satisfactory in preruminant calves but it is much less effective when given to grazing ruminants. Trimethoprim and sulfadiazine are very effective for the treatment of experimental salmonellosis in calves with S. dublin. There is marked synergism of the two drugs and both parenteral and oral therapy are effective. Sulfadimidine and framycetin are also widely used and recommended. Chloramphenicol was once a commonly used antimicrobial for salmonellosis but is now banned for use in food-producing animals in many countries. Nitrofurazone given orally to calves and adult cattle affected with salmonellosis also was used commonly but is now similarly banned.
Horses
Antimicrobial therapy in equine salmonellosis should be based on drug sensitivity of the organisms isolated. Based on some studies of isolates from horses, gentamicin at 3 mg/kg BW combined with ampicillin at 20 mg/kg BW given intravenously at 8–12-hour intervals is reommended. An alternative is trimethoprim–sulfonamide given twice daily intravenously at a combined dose of 30 mg/kg BW. Sulfadiazine, sulfadoxine, and sulfamethoxazole are the best sulfonamides to combine with trimethoprim for salmonellosis in the horse. In a double-blind prospective study, 220 horses undergoing surgery were given a probiotic orally once daily for 7 days postoperatively and fecal cultures for Salmonella were done daily for 10 days. The commercial probiotics had no effect on Salmonella shedding, prevalence of diarrhea, length of antimicrobial therapy, or length of hospitalization.104
Foals
Foals with septicemic salmonellosis are usually treated both systemically and orally with antimicrobials, sometimes a different one by each route. Treatment must be given at least at 6-hourly intervals and accompanied by a supportive fluid therapy. Antimicrobials recommended include gentamicin (250 mg intravenously, twice daily), ampicillin (1 g, 6-hourly), and chloramphenicol (20 mg/kg BW intravenously, 6-hourly). Care needs to be exercised when treating adult horses for salmonellosis because of the tendency for antimicrobials, especially tetracyclines, to precipitate attacks of diarrhea. Parenteral treatment with ampicillin or sulfonamide combinations is recommended.
Pigs
For pigs with septicemic salmonellosis, trimethoprim–sulfadoxine is recommended, along with a combination of mass medication of the water supply with chlortetracycline and sulfamethazine (75 mg of each per liter of water). Where large numbers of pigs are affected, mass medication via the feed or drinking water is usually practiced. Because sick pigs do not eat, water treatment is necessary and if drugs are unpalatable individual treatment is the last recourse. Drugs that dissolve readily and are palatable are therefore in demand. Experimental disease of pigs with Salmonella typhisuis can be controlled by the inclusion of low concentrations of chlortetracycline, penicillin, and sulfamethazine in the feed.
Supportive therapy
Horses
Fluid and electrolyte therapy
In adult horses affected with acute salmonellosis, the dehydration, acidosis and loss of electrolytes are severe. The loss of sodium is most serious, followed by potassium and chloride in that order. A solution of 5% sodium bicarbonate at the rate of 5–8 L/400 kg BW given intravenously over a period of 2 hours as the initial electrolyte replacement therapy will usually help to convert the hyponatremia and acidosis. The hypertonic solution is considered necessary to correct the severe loss of sodium. Following this initial therapy, equal mixtures of isotonic saline (0.9%) and isotonic sodium bicarbonate (1.3%) may be given as maintenance fluid and electrolyte therapy in amounts as indicated. The hypokalemia may be severe in some horses and is recognized clinically by muscular weakness and trembling. It may be corrected by adding potassium chloride to the saline and bicarbonate solution at the rate of 1–2 g/L for a total of 4–6 g of potassium chloride given over 2–4 hours. Concentrated solutions of potassium must be given slowly and the heart monitored for evidence of arrhythmia. However, provided that renal function has been restored, the hyperkalemia that may result from electrolyte therapy or that which could occur following correction of the acidosis should not be hazardous, since the kidney will excrete excess potassium. A safer method would be the oral administration of 30 g of potassium chloride in 8 L of water given twice daily.
Under practical conditions, without the aid of a laboratory for serial evaluation of serum electrolytes, the field veterinarian is faced with using hypertonic solutions as described above or balanced electrolyte solutions and careful clinical monitoring for evidence of overhydration or electrolyte imbalances.
The administration of electrolyte solutions by the oral route is gaining popularity because of the ease of administration and relative safety. Large quantities of fluids (10–30 L) can be administered orally, either all at once, for example in a mature cow, or in smaller quantities (5–10 L) three to four times daily in mature horses. This has been discussed in more detail under fluid therapy in Chapter 2.
CONTROL
Prevention of introduction of infection (biosecurity)
Avoidance of infection is the major objective but is not easily achieved. The principal sources of infection are carrier animals and contaminated feeds containing foodstuffs of animal origin. There is a critical need to develop methods to control the spread of Salmonella infections on dairy farms by instituting biosecurity and biocontainment practices in addition to enhanced farm management. This would result in a reduction in the use of excessive antibiotic treatment of individual animals or herds.
A closed herd minimizes the risk of infection but is not a practicable procedure for the types of animal producer for which salmonellosis is a major problem – the calf-rearer and the commercial pig fattener. For such producers the following rules apply:
• Introduce the animals directly from the farm of origin. Avoid auction marts, saleyards and public transport, all of which are likely to be sources of infection. Insure that the farm of origin is free of salmonellosis
• If possible, purchase animals when they are older, such as 6 weeks of age for calves, to provide an opportunity for specific and nonspecific immunity to develop. Animals from vaccinated herds are desirable
• The premises of dealers, saleyards and transport vehicles must be under close surveillance and the need for frequent vigorous disinfection must be stressed. The infection rate in calves delivered to calf-dealers’ yards in the UK was less than 1% but the infection rate increased to 36% if the calves were kept on the premises over the weekend
• Introduce only those animals likely not to be carriers. Unfortunately the detection of carriers is inaccurate and expensive. To have any confidence in the results, fecal samples for culture must be submitted on at least three occasions. Even then, occasional carriers with lesions in the gallbladder or tonsils will escape the net and be capable of reviving the disease on the farm or transferring it to another one.
For the control of multiple drug-resistant S. typhimurium DT104 in cattle herds, the risk factors over which the farmer can exert a level of control and that are effective in reducing the incidence of disease include purchasing replacement stock from direct sources rather than dealers, quarantine of purchased cattle for a 4-week period, housing sick animals in dedicated isolation areas and preventing wild bird access to cattle feed supplies.
Management practices to reduce the risk of S. brandenburg on a sheep farm include reducing stocking density; avoiding strip grazing; maintaining adequate nutrition; minimizing yarding of ewes and the time spent in yards; dampening down yards prior to yarding; providing stock with a fresh clean source of drinking water; avoiding the purchase and/or grazing of stock from known affected farms, as they may contain carrier animals; preventing dogs from scavenging; and preventing scavenging by black-backed gulls by removing and burying aborted fetuses frequently during the lambing season.40
Limitation of spread within a herd
When an outbreak occurs, procedures for limiting spread, as set out below, need to be strictly enforced, and medication of affected groups, and of susceptible groups at high risk, must be carried out. The drugs to be used are those listed under treatment, the choice of the individual drug depending on its efficiency and cost.
• Identify carrier animals and either cull them or isolate and treat them vigorously. Treated animals should be resampled subsequently to determine whether a ‘clean’ status has been achieved
• The prophylactic use of antimicrobials such as oxytetracycline in the feed at the rate of 10 g/tonne, or chlortetracycline in the drinking water at the rate of 55 mg/L, is used but not recommended because results are poor and there is a risk of developing resistant strains. Probiotics intended for the prevention of shedding of Salmonella in the postoperative period in horses with colic have been evaluated and found to be ineffective104
• Restrict the movement of animals around the farm and limit the infection to the smallest group. Pasture and permanent buildings are both important, although the major source of infection in most cases is the drinking water
• The water supply should be provided in troughs that are not susceptible to fecal contamination. Static drinking water or pasture may remain infected for as long as 7 months
• Rigorous disinfection of buildings is important. An all-in/all-out policy should be adopted and steam cleaning and chemical sterilization performed after each batch of animals. Piglets can be reared free of Salmonella infections up to 6 weeks of age by removing the piglets from infected herds to isolation facilities when they are weaned at 10–21 days of age. The movement of pigs either at weaning, from the nursery, or from the grower unit to newly built or rigorously cleaned and disinfected finishing units with known history of Salmonella infection is highly successful.102 If economics permit, individual pens for calves are beneficial. Where calves are reared indoors they are common and economical. Pig houses need especially careful treatment. Dirt yards present a problem, especially those used for sheep and calves, but, provided they can be kept dry and empty, two sprayings, 1 month apart, with 5% formalin is recommended
• The control of salmonellosis in veterinary clinics and veterinary teaching hospitals requires special attention to the possible sources of infection and containing and preventing the spread of infection. Following the diagnosis of the disease in a clinic, an environmental survey should be carried out using bacteriological culturing of stalls, wall padding, stomach pumps, nasogastric tubes, alleyways, water drains, and other equipment used routinely.105 This is followed by a thorough cleaning and disinfection of the entire animal-holding premises. A power water sprayer is used, followed by application of 10% solution of hypochlorite for at least 15 minutes. The surfaces are then recultured to determine the presence of residual contamination. Medical and surgical equipment are cleaned and gas-sterilized. Traffic flow patterns in the clinic are reviewed and modified accordingly. Use of disposable gloves and thorough washing of hands after handling suspect animals are recommended. Stalls in which horses with salmonellosis were housed should only be used to accommodate newly hospitalized horses after sample (collected after two cycles of cleaning and disinfection) from stall drains, cracks and corners yield negative results on bacteriological culture.105 Using PCR assay for Salmonella DNA, samples from floor drains and drainpipes yield the greatest proportion of positive results. The PCR results should be confirmed by bacteriological culture because a positive PCR in itself is not considered to pose a risk of salmonellosis to hospitalized horses. When a hospitalized horse leaves its stall permanently, it should be cleaned of organic matter using a cold water hose and scrubbed with a steel wool mop. This is followed by an application of generic bleach solution. This is then followed 24 hours later by another cleaning and disinfection with a peroxygen solution (Virkon) and allowed to dry. Virkon is a balanced stabilized blend of peroxygen compounds, surfactants, organic acids, and inorganic buffer system. Active ingredients are potassium peroxymonosulfate, sodium chloride, and other ingredients. It is effective against a wide range of bacteria, virus, and fungi, including: Streptococcus pyogenes, Campylobacter pyloridis, Klebsiella pneumoniae, E. coli, and S. typhimurium
• Suitable construction of housing is important. Impervious walls to stop spread from pen to pen, pen design to permit feeding without entering the pen, avoidance of any communal activity and slatted floors to provide escape routes for manure all assist in limiting the spread of enteric diseases. Deep litter systems are satisfactory provided they are kept dry and plenty of bedding is available. With pigs the opportunity for oral–fecal cycling of the organism and buildup and spread of infection within and between groups must be kept to a minimum. Pen design and the environment should be such as to encourage proper eliminative behavior and good pen hygiene. Drinkers should be sited at one end of the pen, preferably on a narrow end with oblong pens, to encourage defecation in this area. Wet or damp areas of the floor in other parts of the pen will encourage defecation and urination there and should be eliminated. Drinkers of the nipple type rather than bowls are preferable for hygienic reasons. Communal dunging alleys increase the possibility of spread, especially during the cleaning procedure, and the trend is towards slatted or meshed areas over a channel. A totally slatted or mesh floor for pigs from weaning until 10–12 weeks of age will markedly reduce the opportunity of oral–fecal cycling of organisms in this age group, which is especially susceptible to enteric disease. Feeders should allow the ingress of the pig’s head and should be constructed to avoid fecal and other contamination of feed. Pigs need to be grouped according to size, and overcrowding, which may result in improper pen hygiene, must be avoided. Space requirements vary according to pen and housing design but generally fall in the region of 0.3 m2 for recently weaned piglets to 0.6–1 m2 for market-size pigs. In conventionally floored or partially slatted floored pens, approximately two-sevenths of the area should be available for the dunging area. The construction of the pen should allow for easy and efficient cleaning. In problem herds an especial vigilance for the occurrence of enteric disease is needed following the breakdown of pen hygiene on very hot days
• Heat treatment of feed is an effective procedure for pigs. Heating during pelleting greatly reduces the bacterial content of feed and special treatment is worthwhile because of the very high proportion of animal-derived feeds that are infected. The availability of such feeds guaranteed to be Salmonella-free would be an advantage
• Disposal of infective material should be done with care. Carcasses should be burned or, better still, sent to an institution for diagnosis, rather than to a rendering plant to be converted into still more contaminated bone meal. Slurry and manure for disposal should be placed on crops rather than on pasture. Slurry does not constitute a danger via hay, and salmonellas do not survive silage making. When slurry is used on pasture it should be stored for at least a month beforehand and even longer if silo effluent is included. Slurried pasture should not be grazed for 1 month, and for young animals a 6-month delay is recommended. Pig slurry is most dangerous and should always be avoided
• All persons working on infected premises should be warned of the hazards to their own health. Other peripatetic species, especially dogs, should be kept under close restraint.
Principles of infectious disease control for prevention of nosocomial gastrointestinal and respiratory diseases in large-animal hospitals
The principles of an infectious disease control (IDC) program for the prevention of gastrointestinal and respiratory diseases in a large-animal hospital have been described and are applicable to the control of salmonellosis.106 The three basic strategies are reducing exposure to pathogens, avoiding increasing susceptibility to pathogens, and monitoring effectiveness of the IDC program. The major procedures are summarized here.
Recommended steps in developing an effective infectious disease control program (IDC) for a large-animal hospital
An effective IDC program is necessary for all large-animal veterinary teaching hospitals and private veterinary clinics. The recommended steps are outlined here.106
• Have all clinicians work together to develop and approve the IDC program, as grassroots buy-in is vital
• Develop a specific, written IDC program and disseminate it widely among staff members
• Identify a veterinarian who is active in the large-animal hospital to serve as the IDC officer; this individual will oversee the IDC program and should report to the hospital director and practice partners
• Provide the resources, both human and monetary, needed for the IDC officer to effectively carry out the approved IDC program; prevention costs less than the alternatives
• Make students, residents, and staff aware of the key points of the IDC program and the importance that clinicians place on compliance
• Teach the barn crew, particularly those actually responsible for cleaning, disinfecting and feeding, about the goals of the IDC program and the methods to be used
• Monitor the effectiveness of cleaning and sanitation by means of bacterial culture of environmental samples and give regular feedback to the barn crew, staff, students, and clinicians
• Hold a seminar at least yearly to distribute written information about the IDC program and results of monitoring.
Animals being transported
These are a special case. They should be unloaded or exercised at least once every 24 hours and given water and feed, the feed being provided first and at least 2 hours before watering. Hay or chopped hay is preferred to succulent feeds. All railroad cars and feeding and watering troughs should be properly cleaned and disinfected between shipments. Horses that are to be transported should be yarded and hand-fed on hard feed for 4–5 days beforehand. If the disease is likely to occur, prophylactic feeding with sulfonamides or antimicrobials has been shown to decrease the incidence in all species. Apart from the risk that this practice will produce resistant bacteria, there has been a suggestion that it may so change the normal bacterial flora of the gut as to encourage the proliferation of salmonellas and lead to the development of the clinical disease.
Immunization
Salmonella vaccinology
The literature on Salmonella vaccines has been reviewed.58 Host resistance to Salmonella relies initially on the production of inflammatory cytokines leading to the infiltration of activated inflammatory cells in the tissues. Thereafter, T- and B-cell-dependent specific immunity develops, allowing the clearance of Salmonella from the tissues and the establishment of long-lasting acquired immunity to reinfection. The increased resistance that develops after primary infection or vaccination requires T cells, cytokines such as interferon gamma, tumor necrosis factor and interleukin 2, in addition to opsonizing antibody. Seroconversion and/or the presence of detectable T-cell memory do not always correlate with the development of acquired resistance to infection.
Immunization with live salmonellas induces early resistance rechallenge with virulent organisms that appears 1 day after infection or vaccination with live but not killed organisms. Early protection is nonspecific and effective against different Salmonella serotypes. Long-term immunity using live attenuated vaccines is serotype-specific and involves the recall of immunological immunity. Killed vaccines induce strong antibody responses but trigger insufficient T helper 1 (Th1)-cell responses.
Vaccination can decrease the number of bacteria shed in feces and the number of blood-culture-positive calves, thus decreasing the number of carriers and reducing environmental contamination. Many types of vaccine have been developed and tested in cattle and pigs. If vaccination is combined with the hygienic precautions described, the vaccines are an aid to management. Killed bacterins and live attenuated vaccines are available. Either can be used as a prenatal vaccine to provide passive immunization of the newborn. It is now generally accepted that live Salmonella vaccines are more effective immunogens in calves than are killed vaccines.
Cattle
In cattle, S. dublin is the infection likely to be endemic in a herd and a commercial vaccine, to be effective, must have a strong S. dublin component. Live organisms are better able to stimulate antilipopolysaccharide antibodies and to stimulate cell-mediated immunity. Calves vaccinated at 1–3 weeks of age with a modified-live aromatic-dependent S. dublin bacterin have detectable antilipopolysaccharide immunoglobulins after immunization. Safe live oral vaccines against S. typhimurium and S. dublin have been constructed and shown to confer protection against experimental infection with virulent wild-type strains of the organism. Vaccination of calves orally with a genetically altered stable nonreverting aro-S. dublin as a modified-live vaccine provided a measurable systemic immune response but the vaccine volume makes it unlikely to be practical for field use. Vaccinated calves responded with increases in humoral-mediated immunity and cell-mediated immunity, as measured by ELISA and skin testing. It is claimed that the combination of humoral immunity and cell-mediated immunity stimulated by live-organism vaccines provides superior protection. Other genetically altered vaccines consisting of hybrid strains derived from S. dublin and S. typhimurium are being evaluated. An avirulent live S. choleraesuis vaccine is efficacious experimentally against salmonellosis due to S. dublin infection in calves.
The vaccine strain 51, produced in the UK from a rough variant strain of this organism, has been found to be efficient and safe, and provides good protection against S. typhimurium as well as S. dublin. It has the disadvantages of a living vaccine but calves can be vaccinated successfully at 2–4 weeks of age. In limited experiments other living, attenuated and killed, adjuvanted vaccines have given calves protection, and a comprehensive program of vaccination, hygiene, and adoption of a closed herd policy has been successful in controlling the disease. Reports on killed S. typhimurium vaccines used in calves indicated good results provided the antigenic mass in the vaccine is kept high, but commercial killed vaccines are of doubtful value.
Attenuated S. typhimurium (strain SL1479) given orally or intramuscularly has shown good efficiency, and attenuated S. dublin (strain SL1438) has been similarly effective. The S. typhimurium vaccine also gives some protection against S. dublin.
The autogenous bacterin, which must be precipitated on aluminum hydroxide to have any significant effect, is given as two injections 2 weeks apart. Good immunity is produced but calves and pigs less than 6 weeks of age are refractory, and anaphylactic reactions may cause the loss of a significant number of animals. To protect young calves the best program is to vaccinate the cows during late pregnancy. This will give passive protection to the calves for 6 weeks, provided they take sufficient colostrum, and the calves can be vaccinated at that time if danger still exists. Vaccination of pregnant cattle with a formalin-killed S. typhimurium vaccine approximately 7 and 2 weeks before parturition protected their calves against experimental infection. Reports of results have not been enthusiastic but if proper attention is given to the detail of the program it has been sufficient, in our hands, to provide almost complete protection. A similar observation has been made with respect to vaccination of calves against S. typhimurium.
Pigs
A commercial vaccine containing living, attenuated S. choleraesuis has also been shown to protect neonatal pigs after vaccination of sows and weaned pigs. Because of the early age at which pigs need to be immune, it is recommended that sows be vaccinated three times at 7–14-day intervals. The young pigs are vaccinated at 3 weeks of age. A live avirulent S. choleraesuis vaccine has been developed and evaluated for protection against experimental challenge. Vaccinated pigs were able to maintain normal body weight gains during a 4-week observation period following challenge inoculation with a high dose of a virulent strain. It has consistently been safe and efficacious in pigs as young as 3 weeks and provides protection for at least 20 weeks.
Most cases of salmonellosis in pigs are subclinical and due to S. typhimurium. The ideal vaccine against S. typhimurium would prevent colonization, shedding of the organism in the environment, development of carriers and clinical salmonellosis, and promote elimination of the organism from infected animals.107 Live vaccine strains are considered to provide superior protection compared to inactivated vaccines.
Horses
In horses, a similar regimen with a booster dose for all mares in late pregnancy appears to be effective. In foals, an autogenous S. typhimurium bacterin has been used in several bad field situations and has been credited with preventing further clinical cases and with reducing environmental contamination, in spite of continued poor hygiene and management practices.
Nationwide surveillance and control programs
In 1993, the Ministry of Food, Agriculture and Fisheries of Denmark and the Danish Bacon and Meat Council initiated an ambitious program to eliminate pork as an important source of human salmonellosis. In the early 1990s pork had become recognized as an increasingly important source of human salmonellosis in Denmark. In Denmark, the proportion of human salmonellosis attributable to pork was estimated to be 10–15% in 1997 and 1998.108 In the Netherlands, it was estimated that approximately 15% of human cases of salmonellosis were associated with the consumption of contaminated pork.
The Danish Salmonella Surveillance and Control Program for pigs operates at all stages of the production chain and has been applied nationally since 1995.108 As a result of the program the level of Salmonella in Danish pork declined from 3.5% in 1993 to 0.7% in 2000. Simultaneously, the number of human cases of salmonellosis due to pork declined from approximately 1444 in 1993 to 166 in 2000.109
The control program is integrated from ‘feed to food’. It is based on routine testing and classification of slaughter pig herds and subsequent slaughter of pigs according to the inherent risk, as measured by the continual test program.109
Basically, the level of Salmonella is controlled at various stages.
Feedstuffs
Compounded feeds are heat treated at 81°C to eliminate Salmonella. The national program requires mandatory Salmonella testing in all plants producing animal feeds. In 2000, the level of Salmonella spp. in final products was only 0.3%.
Breeder and multiplier herds
Each month all herds are blood sampled and examined for Salmonella antibodies.110 Based on the level of antibodies, a Salmonella index is calculated. If the index exceeds 5, pen fecal samples must be taken and examined for the presence of Salmonella spp. When the index exceeds 15, a sales ban on breeding pigs is imposed until the index has declined below 15 again.
Weaner producers
If a sow herd sells weaners to a Salmonella level 2 or 3 finishing herd, pen fecal samples must be taken and examined for Salmonella.
Slaughter pigs
Slaughter herd pigs are monitored continuously by serological testing of ‘meat juice’. Meat samples are frozen, and meat juice (harvested after thawing) is examined for specific antibodies against S. enterica using an ELISA.111 The ELISA combines several S. enterica O antigens and allows detection of antibody response after a variety of serovar infections. The meat samples for testing are collected at the slaughter line, and the number of samples and frequency of sampling are determined by the size of the herd. Herds sending fewer than 200 pigs to slaughter per year are not examined, which amounts to 1.6% of slaughter pigs not examined. The herds are categorized in four levels based on the proportion of seropositive meat juice samples during the previous 3 months. Based on the optical density per cent (OD%) of the ELISA test, the herds are classified into:
Level 0: Herds having only seronegative over 3 months or more
Level 1: Herds with acceptable low Salmonella prevalence
Level 2: Herds with moderate Salmonella prevalence
Level 3: Herds with unacceptable high Salmonella prevalence.
A herd categorized as level 2 or 3 must receive an advisory visit by a practicing veterinarian and a local extension specialist, and certain management precautions must be adopted. In a herd with level 3, the finishing pigs must be slaughtered under special hygiene conditions.
The proportion of serologically positive meat-juice samples collected during 1995 ranged from a mean of 2.9% in small herds to 6.1% in large herds.
Danish National Surveillance Program for Salmonella dublin
A national surveillance program for S. dublin in dairy cattle was begun in 2002 by initiative of both the Danish Veterinary and Food Administration and the Danish Cattle Federation. The short-term goal was to screen both dairy and beef cattle herds for S. dublin infection, and to classify the herds according to the estimated level of infection in order to provide a control scheme. The long-term goal was to reduce the prevalence of S. dublin in Danish cattle and reduce the risk of human infection associated with the consumption of meat and milk from Danish cattle.112 All Danish cattle herds are screened by the use of ELISA assay of bulk tank milk collected from dairy herds every 3 months and of three yearly blood samples from other herds.
Akkina JE, Hogue AT, Angulo FJ, et al. Epidemiologic aspects, control, and importance of multiple-drug resistant Salmonella typhimurium DT 104 in the United States. J Am Vet Med Assoc. 1999;214:790-798.
Brenner FW, Villar RG, Angulo FJ, et al. Salmonella nomenclature. J Clin Microbiol. 2000;38:2465-2467.
Mastroeni P, Chabalgoity JA, Dunstan SJ, et al. Salmonella: immune responses and vaccines. Vet J. 2000;161:132-164.
Wallis TS, Galyov EE. Molecular basis of Salmonella-induced enteritis. Mol Microbiol. 2000;36:997-1005.
Wray C, Wray A, editors. Salmonella in domestic animals. New York: CABI Publishing, 2000.
Van der Wolf PJ, Hensel A. The Fourth International Symposium on Epidemiology and Control of Salmonella and other Foodborne Pathogens in Pork, September 2–5, 2001, Leipzig, Germany. Berl Munch Tierärztl Wochenschr. 2001;114:321-408.
Liebana E. Molecular tools for epidemiological investigations of S. enterica subspecies enterica infections. Res Vet Sci. 2002;72:169-175.
Lo Fo, Wong DMA, Hald T, van der Wolf PJ, Swanenberg M. Epidemiology and control measures for Salmonella in pigs and pork. Livestock Prod Sci. 2002;76:215-222.
Sanchez S, Hofacre CL, Lee MD, et al. Animal sources of salmonellosis in humans. J Am Vet Med Assoc. 2002;221:492-497.
Threlfall EJ. Antimicrobial drug resistance in Salmonella: problems and perspectives in food- and water-borne infections. FEMS Microbiol Rev. 2002;26:141-148.
Wegener HC. Antibiotics in animal feed and their role in resistance development. Curr Opin Microbiol. 2003;6:439-445.
Wegener HC, Hald T, Lo Fo, Wong D, et al. Salmonella control programs in Denmark. Emerg Infect Dis. 2003;9:774-780.
Smith BP, House JK, Magdesian KG, et al. Principles of an infectious disease control program for preventing nosocomial gastrointestinal and respiratory tract diseases in large animal veterinary hospitals. J Am Vet Med Assoc. 2004;225:1186-1195.
Wigley P. Genetic resistance to Salmonella infection in domestic animals. Res Vet Sci. 2004;76:165-169.
1 Brenner FW, et al. J Clin Microbiol. 2000;38:2465.
2 Liebana E. Res Vet Sci. 2002;72:169.
3 Beloeiel PA, et al. Prev Vet Med. 2003;60:207.
4 Akkina JE, et al. J Am Vet Med Assoc. 1999;214:790.
5 Boqvist S, et al. Acta Vet Scand. 2003;44:181.
6 Smith-Palmer A, et al. Vet Rec. 2003;153:517.
7 Linquist N, et al. J Clin Microbiol. 2003;40:3648.
8 Liebana E. J Clin Microbiol. 2002;40:4450.
9 Refsum T, et al. Appl Environ Microbiol. 2002;68:5600.
10 Van Duijkeren E, et al. J Clin Microbiol. 2002;40:3980.
11 Van der Wolf PJ, et al. Vet Microbiol. 2001;80:171.
12 Harris IT. J Swine Health Prod. 2003;11:247. 300
13 Rabsch W, et al. Torrence ME, Isaacson RE, editors. Microbial food safety in animal agriculture: current topics. Oxford: Blackwell. 2003:97-106.
14 Zhao S, et al. J Clin Microbiol. 2003;41:5366.
15 Davies RH, et al. Vet Rec. 2001;149:555.
16 Alvseike O, Skjerve E. Prev Vet Med. 2002;52:277.
17 Sandberg M, et al. Prev Vet Med. 2002;52:267.
18 Radke BR, et al. Can Vet J. 2002;43:443.
19 Wigley P. Res Vet Sci. 2004;76:165.
20 Warnick LD, et al. Prev Vet Med. 2001;49:259.
21 Warnick LD, et al. Prev Vet Med. 2003;56:285.
22 Anderson RJ, et al. J Am Vet Med Assoc. 2001;219:310.
23 Galland JC, et al. J Am Vet Med Assoc. 2001;219:1216.
24 Troutt HF, et al. J Am Vet Med Assoc. 2001;219:1212.
25 Kirk J, et al. Prev Vet Med. 2002;54:169.
26 Huston CL, et al. J Am Vet Med Assoc. 2002;220:650.
27 Huston CL, et al. J Am Vet Med Assoc. 2002;220:645.
28 Peek SF, et al. J Am Vet Med Assoc. 2004;225:574.
29 Kirk JH, et al. J Am Vet Med Assoc. 2002;220:359.
30 Kabagambe EK, et al. Prev Vet Med. 2000;43:177.
31 Fossler CP, et al. J Am Vet Med Assoc. 2004;225:567.
32 Nielsen LR, et al. Prev Vet Med. 2004;65:47.
33 Fegan N, et al. J Appl Microbiol. 2004;97:892.
34 Dargatz DA, et al. J Appl Microbiol. 2003;95:753.
35 Taylor DW, et al. Cattle Pract. 2001;9:217.
36 Capucille DJ, et al. Bovine Pract. 2002;36:15.
37 Beach JC, et al. J Food Prot. 2002;65:1687.
38 Purdy CW, et al. Am J Vet Res. 2004;65:40.
39 Uzzau S, et al. Infect Immun. 2001;69:3092.
40 Clark RG, et al. N Z Vet J. 2004;52:26.
41 Hjartardottir S, et al. Acta Vet Scand. 2002;43:43.
42 Kranker S, et al. J Clin Microbiol. 2003;41:2282.
43 Funk JA, et al. Vet Microbiol. 2001;83:45.
44 Davies PR, et al. J Am Vet Med Assoc. 1998;212:1925.
45 Hurd HS, et al. Appl Environ Microbiol. 2002;68:2376.
46 Stark KDC, et al. Prev Vet Med. 2002;53:7.
47 Bottledorn N, et al. J Appl Microbiol. 2003;95:891.
48 Fedorka-Gray PJ, et al. J Swine Health Prod. 1997;5:189.
49 Morrow WEM, et al. J Am Vet Med Assoc. 2002;220:497.
50 Van der Wolf PJ, et al. Vet Microbiol. 2001;78:205.
51 Baggesen DL, et al. Am J Vet Res. 1999;60:120.
52 Traub-Dargatz JL, et al. J Am Vet Med Assoc. 2000;217:226.
53 Burgess BA, et al. J Am Vet Med Assoc. 2004;225:1344.
54 Schott HCII, et al. J Am Vet Med Assoc. 2001;218:1152.
55 Ewart SL, et al. J Am Vet Med Assoc. 2001;218:1145.
56 House JK, et al. J Am Vet Med Assoc. 1999;214:1511.
57 Kim LM, et al. J Am Vet Med Assoc. 2001;218:740.
58 Mastroeni P, et al. Vet J. 2000;161:132.
59 Thorns CJ. Br Vet J. 1995;151:643.
60 Rice DH, et al. Vet Microbiol. 1997;56:111.
61 Wallis TS, Galyov EE. Vet Microbiol. 2000;36:997.
62 Zhang S, et al. Infect Immun. 2002;70:3843.
63 Threlfall EJ. FEMS Microbiol Rev. 2002;26:141.
64 Wegener HC. Curr Opin Microbiol. 2003;6:439.
65 Gupta A, et al. J Infect Dis. 2003;188:1707.
66 Rankin SC, et al. J Clin Microbiol. 2002;40:4679.
67 Winokur PL, et al. Antimicrob Agents Chemother. 2001;45:2716.
68 Allen KJ, Poppe C. Am J Vet Res. 2002;66:137.
69 Van Duijkeren E, et al. J Clin Microbiol. 2003;41:3574.
70 Jones YE, et al. Vet Rec. 2002;150:649.
71 Malorny B, et al. Vet Rec. 2003;153:643.
72 Poppe C, et al. Microb Resist. 2001;7:197.
73 Bischoff KM, et al. Lett Appl Microbiol. 2004;38:140.
74 Bacon RT, et al. J Food Prot. 2002;65:284.
75 Beach JC, et al. J Food Prot. 2002;65:1694.
76 Hsueh PR, et al. Emerg Infect Dis. 2004;10:60.
77 Van Duijkeren E, et al. Vet Microbiol. 2002;86:203.
78 Sanchez S, et al. J Am Vet Med Assoc. 2002;221:492.
79 Alley MR, et al. N Z Vet J. 2002;50:170.
80 Poppe C, et al. Can Vet J. 1998;39:559.
81 Gough J, McEwen B. Aust Vet J. 2000;41:413.
82 Weese SJ, et al. Can Vet J. 2001;42:788.
83 Walker RA, et al. Vet Rec. 2000;147:395.
84 Baumler AJ, et al. Infect Immun. 1998;66:4567.
85 Santos RL, et al. Vet Pathol. 2002;39:200.
86 Wallis TS, et al. Infect Immun. 1995;63:2755.
87 Deignan T, et al. Res Vet Sci. 2000;69:153.
88 Meyerholz DK, et al. Vet Pathol. 2002;39:712.
89 Meyerholz DK, Stabel TJ. Vet Pathol. 2003;40:371.
90 Carlson SA, et al. Vet Microbiol. 2002;85:233.
91 Van der Wolf PJ, et al. Vet Q. 2001;23:199.
92 Patterson-Kane JC, et al. N Z Vet J. 2001;49:159.
93 Warnick LD, et al. Prev Vet Med. 2003;60:195.
94 House JK, et al. Am J Vet Res. 1993;54:1391.
95 Kivela SL, et al. Bovine Pract 33:74.
96 Amavisit P, et al. Vet Microbiol. 2001;79:63.
97 Kurowski PB, et al. Am J Vet Res. 2002;63:1255.
98 Wedderkopp A, et al. Can J Vet Res. 2001;65:15.
99 Alban L, et al. Prev Vet Med. 2002;53:133.
100 Sorensen LL, et al. Vet Microbiol. 2004;101:131.
101 Hurd HS, et al. Epidemiol Infect. 2003;132:127.
102 Lo Fo, Wong DM, et al. Vet Microbiol. 2003;97:201.
103 Fecteau M-E, et al. Am J Vet Res. 2003;64:918.
104 Parraga ME, et al. J Vet Intern Med. 1997;11:36.
105 Alinovi CA, et al. J Am Vet Med Assoc. 2003;223:1640.
106 Smith BP, et al. J Am Vet Med Assoc. 2004;225:1186.
107 Haesebrouck F, et al. Vet Microbiol. 2004;100:255.
108 Lo Fo, Wong DMA, et al. Livestock Prod Sci. 2002;76:215.
109 Nielsen B, et al. Berl Munch Tierärztl Wochenschr. 2001;114:323.
110 Wegener HC, et al. Emerg Infect Dis. 2003;9:774.
111 Nielsen LR, et al. J Appl Microbiol. 2004;96:311.
112 Nielsen LR. Salmonella Dublin in dairy cattle. Use of diagnostic tests for investigation of risk factors and infection dynamics. PhD Thesis, Department of Animal Science and Animal Health, Royal Veterinary and Agricultural University, Frederiksberg, Denmark, 2003.