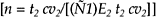COPPER DEFICIENCY
Etiology Primary copper deficiency due to inadequate levels in diet. Secondary copper deficiency due to conditioning factors such as excess molybdenum and sulfur in the diet.
Epidemiology Primarily in young pastured ruminants (cattle, sheep, goats, and farmed deer) in spring and summer. Primary deficiency occurs in sandy soil and heavily weathered areas; secondary in peat or muck soil areas. Feed and water supplies may contain molybdenum, sulfate and iron salts, which interfere with copper metabolism. May be congenital in newborn lambs (swayback) if ewes deficient or delayed in nursing lambs (enzootic ataxia). Some breeds of sheep highly susceptible.
Signs Herd problem. Young growing ruminants on pasture. Unthriftiness, changes in hair color, chronic diarrhea in molybdenosis (secondary deficiency), chronic lameness, neonatal ataxia, anemia later stages of deficiency and falling disease in adult cattle.
Clinical pathology Low serum and hepatic copper. Ceruloplasmin. Anemia.
Necropsy findings Anemia, emaciation, hemosiderosis, osteodystrophy, demyelination in enzootic ataxia, myocardiopathy.
Diagnostic confirmation Low serum and hepatic copper and response to treatment.
Copper deficiency must be differentiated from herd problems associated with the following clinical findings:
• Unthriftiness due to intestinal parasitism
• Malnutrition due to energy-protein deficiency
• Lameness caused by osteodystrophy due to calcium, phosphorus, and vitamin D imbalance
• Neonatal ataxia in lambs (congenital swayback and enzootic ataxia) from border disease; cerebellar hypoplasia (daft lamb disease); hypothermia; meningitis
Treatment Copper sulfate orally; copper glycinate parenterally.
Control Provide source of copper by oral dosing or dietary supplementation in feed or on pasture. Parenteral administration of copper at strategic times. Copper oxide needles orally for prolonged effectiveness. Controlled-release boluses. Genetic selection. Removal of sulfates from water supply.
ETIOLOGY
Copper deficiency may be primary, when the intake in the diet is inadequate, or secondary (conditioned) when the dietary intake is sufficient but the utilization of the copper by tissues is impeded.
Primary copper deficiency
The amount of copper in the diet may be inadequate when the forage is grown on deficient soils or on soils in which the copper is unavailable.
Secondary copper deficiency
In secondary copper deficiency, the amount of copper in the diet is adequate, but conditioning dietary factors interfere with the utilization of the copper. Such secondary copper deficiencies are summarized in Table 30.2. The administration of copper is preventive and curative. The conditioning factor is known only in some instances, but a dietary excess of molybdenum is one of the most common. A high molybdenum intake can induce copper deficiency even when the copper content of the pasture is quite high and a higher copper intake can overcome the effect of the molybdenum. Conversely, supplementation of the diet with molybdenum can be used to counteract the copper intake when its content in the diet is dangerously high. There are species differences in response to high copper and molybdenum intake; sheep are much more susceptible to copper poisoning, cattle to excess molybdenum.
Zinc, iron, lead, and calcium carbonate are also conditioning factors and in New Zealand, the administration of selenium to sheep on copper-deficient pastures increases copper absorption and improves the growth rate of lambs. The use of zinc sulfate for the control of facial eczema may cause a depression of plasma copper levels, which can be alleviated by the injection of copper glycinate.
Dietary inorganic sulfate in combination with molybdenum has a profound effect on the uptake of copper by ruminants. Sheep consuming a complete diet, low in sulfur and molybdenum and with a modest 12–20 mg copper/kg dry matter (DM), may die from copper toxicity, while others grazing pasture of similar copper content but high in molybdenum and sulfur can give birth to lambs affected with the copper deficiency disease swayback.1 An increase of sulfate concentration in a sheep diet from 0.1% to 0.4% can potentiate a molybdenum content as low as 2 mg/kg (0.02 mmol/kg) to reduce copper absorption to below normal levels. Additional sulfate in the diet also has a depressing effect on the absorption of selenium so that areas of a country with marginal copper and selenium levels in the soil may produce deficiency syndromes in animals if sulfate is added; this is likely to happen when heavy dressings of superphosphate are applied. Such combined deficiencies are becoming more common. The possibility of interaction between copper and selenium must also be considered because of the reported failure of animals to respond to treatment unless both elements are provided.
EPIDEMIOLOGY
Occurrence
Copper deficiency is endemic in ruminants worldwide and causes diseases of economic importance that may be severe enough to render large areas of otherwise fertile land unsuitable for grazing by ruminants of all ages, but primarily young, growing ruminants. Based on serum copper surveys of cattle herds in Britain, copper deficiency constitutes a serious problem requiring vigilance. It is estimated that characteristic clinical signs of copper deficiency develop annually in about 0.9% of the cattle population in the UK. In some surveys, the lowest levels of serum copper were in heifers being reared as heifer replacements. Although heavy mortalities occur in affected areas, the major loss is due to failure of animals to thrive. Enzootic ataxia may affect up to 90% of a lamb flock in badly affected areas and most of these lambs die of inanition. In falling disease, up to 40% of cattle in affected herds may die.
Copper deficiency is the most common trace element deficiency in farmed deer in New Zealand.2 The deficiency in deer is widespread and is a problem in many herds. It can be diagnosed from clinical signs such as enzootic ataxia and osteochondrosis.
Geographical distribution
Primary copper deficiency
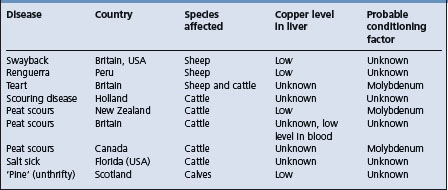
The diseases caused by a primary deficiency of copper in ruminants are enzootic ataxia of sheep in Australia, New Zealand, and the USA, licking sickness, or liksucht of cattle in Holland and falling disease of cattle in Australia.
Copper deficiency occurs naturally in grazing livestock in many parts of the world. It has long been recognized as an endemic disease in the Salado’s river basin in Buenos Aires Province, Argentina, affecting over 50% of the cattle population.3 Some 81% of this wide area of 56 000 km2 is devoted to breeding over 6.5 million head of beef cattle. The copper content in the grass is inadequate for most of the year and is most critical in autumn.4
A concurrent deficiency of both copper and cobalt occurs in Australia (coast disease) and Florida in the USA (salt sickness) and is characterized by the appearance of clinical signs of both deficiencies in all species of ruminants. The disease is controlled by supplementation of the diet with copper and cobalt.
In the USA, copper deficiency is not restricted to a single geographic region.5 In a survey of 2007 beef cows and heifers from 256 herds, 1.7% were deficient and 38% were marginally deficient. In herds, 36% were marginally deficient and 0.8%, deficient. Approximately 50% of the producers reported use of copper supplements, but a significant portion of cattle from those herds were classified as marginally deficient or deficient.
A survey in Saskatchewan, Canada, found that 67% of slaughter cattle had liver levels lower than 10 mg copper/kg on a wet weight (WW). A survey of the copper status of the fetuses and livers from adult animals found that 20% of steers, 54% of pregnant cows, 52% of heifers, and 77% of non-pregnant cows had liver levels <25 mg/kg DM. The concentrations of copper in the liver of the fetuses were directly proportional to the liver copper concentrations in the dams. Liver copper levels of fetuses from dams with liver copper >25 mg/kg DM were higher than those in fetuses from dams with liver copper levels <25 mg/kg DM. During gestation, the level of copper progressively increased in the fetal liver and decreased in the maternal liver. The concentration of copper in fetal livers increased with increasing fetal age and at term, the newborn calf has high levels of liver copper to meet postnatal requirements because cows’ milk is a poor source of copper. The magnitude of copper deficiency in some areas is extensive and emphasizes the importance of adequate copper nutrition in pregnant cattle in order to maintain adequate fetal levels of copper.
Copper deficiency has been diagnosed in Canada in a herd of captive musk-oxen, which had originated in the Northwest Territories.6
Copper deficiency may also cause anemia in sucking pigs and reduced growth rate and cardiac disease in growing pigs. Adult horses are unaffected, but abnormalities of the limbs and joints of foals reared in copper-deficient areas do occur. Osteochondrosis is associated with a copper deficiency in young, farmed red deer and wapiti X red deer hybrids in New Zealand.7
Secondary copper deficiency
The diseases caused by secondary copper deficiency, mostly due to high dietary intakes of molybdenum and sulfate, are listed in Table 30.2. They include syndromes characterized by diarrhea or by unthriftiness. ‘Yellow calf’, a disease of nursing calves occurs on Hawaii’s rangeland where copper content of forages ranges from 2.6 to 11.8 mg/kg and the molybdenum from <1 to 39 mg/kg. Swayback of lambs in the UK has been classed as a secondary copper deficiency, but no conditioning factor has been determined. While swayback is a naturally occurring disease caused by a primary deficiency of copper, identical lesions occur experimentally by feeding molybdenum and sulfate to the ewes. There is some evidence that heavy lime dressing of a pasture may predispose to swayback. A wasting disease similar to peat scours and preventable by the administration of copper and unthriftiness (‘pine’) of calves, occur in the UK, but in both instances the copper and molybdenum intakes are normal. Molybdenum appears to be the conditioning agent in enzootic ataxia in the USA. A dietary excess of molybdenum is known to be the conditioning factor in the diarrheic diseases, peat scours in New Zealand, California, and Canada and ‘teart’ in Britain.
High concentrations of molybdenum in forage (21–44 mg/kg DM) have been identified in several reclaimed mining areas in British Columbia but cattle have grazed these areas for 12-week periods yearly for 3 years without developing secondary copper deficiency.8 One-half of the animals received a copper supplement and there were no differences in weight gain, liver molybdenum, serum copper, and molybdenum and milk copper and molybdenum between the two groups. The results indicated that the upper tolerable dietary concentrations of 5–10 mg molybdenum described by the National Research Council and the minimum safe copper to molybdenum ratio of 2:1 are not universal.8
Moose sickness is a disease of moose (Alces alces L.) in Sweden.9 The disease has also been known as ‘Alvsborg disease’ and ‘wasting disease’. About 4–5% of the moose population is affected annually. The appearance of the disease coincided with intensified liming of wetlands, lakes, and forests during the 1980s, undertaken to counteract the deleterious effects of acid rain. The increase in pH caused by the liming affected the availability of nutrients in the soil, reducing copper availability and increasing molybdenum.
Copper deficiency may be a factor contributing to the population decline of moose in North-western Minnesota.10 In moose found dead, the copper concentrations based on criteria set for cattle, were deficient in 39.5% of livers, marginally deficient in 29.5% and adequate in 31%.10 The lower concentrations of copper in moose from bog and forest areas compared with the agricultural and prairie areas of North-western Minnesota coincide with a lower calf-to-cow ratio in the north-west forest area compared with the northwestern prairies.
Seasonal incidence
Both primary and secondary copper deficiency occur most commonly in spring and summer coinciding with the lowest levels of copper in the pasture.
Large monthly variations occur in the serum levels of copper in both beef and dairy cattle and are commonly correlated with the rainfall; the higher the rainfall the lower the copper level.
The incidence of secondary copper deficiency may be highest at other times, depending upon the concentration of the conditioning factor in the forage. For example, the molybdenum content may be highest in the autumn when rains stimulate a heavy growth of legumes.
Risk factors
Several factors influence the plasma and tissue concentrations of copper, particularly in ruminants, including:
Animal factors
Age susceptibility
Young animals are more susceptible to primary copper deficiency than adults. Calves on dams fed deficient diets may show signs at 2–3 months of age. As a rule, the signs are severe in calves and yearlings, less severe in 2-year-olds and of minor degree in adults. Enzootic ataxia is primarily a disease of sucking lambs whose dams receive insufficient dietary copper. Ewes with a normal copper status take some time to lose their hepatic reserves of copper after transfer to copper-deficient pastures and do not produce affected lambs for the first 6 months. The occurrence of the disease in sucklings and its failure to appear after weaning, point to the importance of fetal stores of copper and the inadequacy of milk as a source of copper. Milk is always a poor source of copper and when it is the sole source of nourishment the intake of copper will be low. Milk from normal ewes contains 20–60 μg/dL (3.1–9.4 μmol/L) copper, but under conditions of severe copper deficiency this may be reduced to 1–2 μg/dL (0.16–0.31 μmol/L).
Breed and species susceptibility
There are marked genetic differences in copper metabolism between breeds of sheep. The Welsh Mountain ewe can absorb copper 50% more efficiently than the Scottish blackface,11 and the Texel cross blackface 145% more efficiently than pure blackface lambs.11 The susceptibility to, or protection from, the effects of copper deficiency and also copper poisoning, is influenced from birth by genetic effects. These affect copper status of the lamb at birth, through the maternal environment controlled by the dam’s genes and through the effect of the lamb’s own genes. Later in life, the animal’s own genes become the predominant influence determining its copper status on any given nutritional regimen. These genetic differences have physiological consequences reflected in differences in the incidence of swayback, both between and within breeds and in effects on growth and possibly on reproduction. The differences observed are due to genetic differences in the efficiency of absorption of dietary copper.
The genetic effects determining the copper status of the lamb are already present in utero and the effects are not controlled by the lamb’s own genotype but by that of its dam. The maternal effect is still present at weaning at 9 weeks of age, but disappears after weaning when the genetic differences are due to the sheep’s own genotype.
The existence of genes determining plasma copper has been shown by the successful continued selection for high and low concentrations in closed lines of a single breed type. Ram selection is made on the basis of plasma copper concentrations at 18 and 24 weeks of age. The proportion of the normal variation in plasma copper that is heritable is 0.3. The high-line female sheep retain more copper in the liver than the low-line females, caused by a positive correlation between the concentration of copper in plasma and the efficiency of absorption.
The genetic variation in the copper metabolism of sheep has important physiological consequences. Breeds show wide variation in their susceptibility to swayback; the incidence of swayback may vary from 0 to 40% between breeds within one flock and the incidence according to breed type is closely related to the differences in the concentration of copper in the liver than in blood. When these high and low female lines are placed on improved and limed pasture, which can induce a severe copper deficiency, soon after birth there are indications of swayback, general dullness, lack of vigor and mortality in the lambs. By 6 weeks of age, the mortality rate is higher in the lambs from the low copper line than in those from the high copper line. In addition, at 6 weeks of age, lambs from the low line are 2 kg lighter than those in the high line.
There are significant differences in the copper requirements and tolerance between goats and sheep.12 The dietary copper requirements of goats are uncertain but may be higher than in sheep. Dietary levels of copper which could cause copper toxicity in sheep, do not cause toxicity in goats. Some limited data on growth performance indicates a stimulatory effect of 100–300 pap copper in the diet of Nubian goats. Extra copper accumulated in liver and to a lesser extent in other tissues and was excreted through the biliary system and into the feces.
Certain breeds of cattle, e.g. the Simmental and Charolais, may have higher copper requirements than other breeds, e.g. Angus and these differences may be related to differences in copper absorption in the gastrointestinal tract. Angus heifers have a lower minimal copper requirement than Simmental.13 Based on liver copper, the control diets containing 4.4 mg or 6.4 mg of copper/kg DM did not meet the copper requirement of either breed during gestation and lactation or growth, Addition of 7 mg of copper/kg DM to the control diets met the copper requirements of both breeds.
Fetal liver copper
During gestation, the copper concentration increases progressively in the ovine and bovine fetal liver and decreases in the maternal liver. The developing bovine fetus obtains its copper by placental transfer and at birth, the liver concentration of copper is high and declines postnatally to adult levels within the first few months. Placental transfer is less efficient in sheep and lambs are commonly born with low liver reserves, making the neonatal lambs susceptible to copper deficiency. In copper-deficient cattle, the accumulation of liver copper in the fetus continues independent of the dam’s liver copper until the fetus is about 180 days, then a gradual decline in fetal liver copper occurs. The liver copper concentration in fetuses from dams on a copper-adequate diet continues to increase and not decline at 180 days of gestation. All of this indicates an increase in copper requirements by the dam during pregnancy; during the last month of pregnancy, the daily requirement for copper in cattle increases to approximately 70% above the maintenance requirements, which means that the dietary allowance of 10 mg/kg DM needs to be increased up to 25 mg/kg DM during pregnancy. The concentrations of copper, iron, manganese, and zinc are consistently lower than normal in the livers of aborted fetuses, indicating a non-specific change in trace element status which is probably an effect of abortion and not a cause.
Colostrum is rich in copper, allowing the newborn with its preferential ability to absorb copper to increase hepatic stores. Later, the copper content of milk declines rapidly so that it is usually insufficient to meet the requirements of the sucking neonate for copper. The young milk-fed animal is able to absorb about 80% of its copper intake, but the efficiency of absorption declines with age as the rumen becomes functional, when only 2–10% of available copper is absorbed.
Dietary factors
Pasture composition
The absorption (or availability) of copper is influenced by the type of diet, the presence of other substances in the diet such as molybdenum, sulfur, and iron, the interaction between the type of diet and the chemical composition of the diet and the genetic constitution of the animals. Copper is well-absorbed from diets low in fiber, such as cereals and Brassicas, but poorly absorbed from fresh forage. Conservation of grass as hay or silage generally improves its availability. This explains why copper deficiency is a problem of the grazing animal and seen only rarely in housed ruminants receiving diets that are commonly adequate in copper.
Molybdenum and sulfur
Only small increases in the molybdenum and sulfur concentration of grass will cause major reductions in the availability of copper. This is especially notable in ruminants grazing improved pastures in which the molybdenum and sulfur concentrations were increased. The copper content of feedstuffs should be expressed in terms of available copper concentration, using appropriate equations, which permits a more accurate prediction of clinical disease and can be used for more effective control strategies.
The effect of changes in molybdenum and sulfur concentrations in grass on the availability of copper is changed by conservation. At a given concentration of sulfur, the antagonistic effect of molybdenum is proportionately less in hay than in fresh grass. At a low concentration of molybdenum, the effect of sulfur is more marked in silage than in fresh grass. The use of formaldehyde as a silage additive may weaken the copper sulfur antagonism and yield material of high availability. Thus, fields of herbage high in molybdenum should be used for conservation when possible and sulfuric acid should not be used as an additive for silage unless accompanied by a copper salt because it significantly raises the sulfur concentration of the silage.
Copper in diet
For general purposes, pasture containing <3 mg/kg DM of copper will result in signs of deficiency in grazing ruminants. Levels of 3–5 mg/kg DM can be considered as dangerous and levels >5 mg/kg DM (preferably 7–12) are safe unless complicating factors cause secondary copper deficiency. The complexity of minimum copper requirements, affected as they are by numerous conditioning factors, necessitates examination under each particular set of circumstances. For example, plant molybdenum levels are related directly to the pH reaction of the soil. Grasses grown on strongly acidic molybdenum-rich soils are characterized by low molybdenum values (<3 mg/kg DM), whereas those associated with alkaline molybdenum-poor soils may contain up to 17 mg/kg DM. Thus, it seems likely that conditioned copper deficiency can be related to regionally enhanced levels of plant available rather than soil molybdenum. Heavily limed pastures are often associated with a less than normal copper intake and a low copper status of sheep grazing them. Secondary copper deficiency is also recorded in pigs whose drinking water contains very large amounts of sulfate.
Dietary iron
A dietary intake of iron can interfere with copper metabolism.14 Dietary levels of iron in the range of 500–1500 mg/kg DM, within the range of their fluctuation in silage and forage and higher levels, are a risk of inducing copper deficiency in ruminants, especially when the copper intake is marginal. Ruminants obtain iron from ingested soil and mineral supplements and, in areas where hypocuprosis is likely to occur, the risk can be minimized by avoiding the use of mineral supplements of high iron content, minimizing the use of bare winter pasture and avoiding the excessive contamination of silage with soil during harvesting.
Molybdenum-induced secondary copper deficiency in cattle occurred when motor oil containing molybdenum bisulfide was spilled on a pasture located on the side of a railway bed near the farm.
Stored feeds
Livestock that are housed have a slightly different dietary intake to those on pasture. Concentrates and proprietary feeds usually contain adequate copper. Pasture is less likely to contain sufficient copper, especially in early spring when the grass growth is lush and silage and haylage may be deficient. Hay is more mature and usually contains more of all minerals, so that animals housed for the winter are protected against copper deficiency for a few weeks after they come out onto pasture in the spring. Young, growing animals will be first affected. These comments should not be interpreted to mean that housed or feedlot animals cannot be affected by hypocuprosis; they can if the locally produced feed is copper-deficient, or more likely has a high concentration of molybdenum. Both are likely to be prevented, or less severe, if there is some supplementary feeding.
Soil characteristics
Copper deficiency
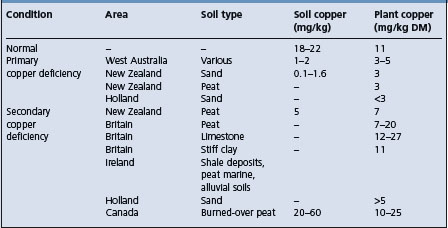
In general, there are two types of soil on which copper-deficient plants are produced. Sandy soils, poor in organic matter and heavily weathered, such as on the coastal plains of Australia and in marine and river silts, are likely to be deficient in copper as well as other trace elements, especially cobalt.
The second important group of soils is ‘peat’ or muck soils reclaimed from swamps and are soils more commonly associated with copper deficiency in the USA, New Zealand, and Europe. Such soils may have an absolute deficiency of copper, but more commonly, the deficiency is relative in that the copper is not available and the plants growing on the soils do not contain adequate amounts of the element.
The cause of the lack of availability of the copper is uncertain, but is probably the formation of insoluble organic copper complexes. An additional factor is the production of secondary copper deficiency on these soils due to their high content of molybdenum. A summary of the relevant levels of copper in soils and plants is given in Table 30.3.
Molybdenum excess
Pastures containing <3 mg/kg DM of molybdenum are considered to be safe, but disease may occur at 3–10 mg/kg DM if the copper intake is low. Pastures containing >10 mg/kg DM of molybdenum are dangerous unless the diet is supplemented with copper. Excess molybdenum may occur in soils up to levels of 10 and even 100 mg/kg. Perhaps more dangerous is the risk that overzealous application of molybdenum to pasture to increase bacterial nitrogen fixation may have similar effects, which are likely to be long-lasting.
In the UK, appreciable land is underlain by marine black shales rich in molybdenum, resulting in a high content of molybdenum in the soil and pastures and in a secondary copper deficiency that, potentially, limits livestock performance. Secondary (conditioned) copper deficiency is now recognized in cattle in many parts of Canada. Large areas of west-central Manitoba are underlain by molybdeniferous shale bedrocks and the soil contain up to 20 mg/kg of molybdenum. However, in the same geographical location, hypocupremia may be associated with a primary deficiency of copper in the forage, or a secondary copper deficiency due to molybdenum in the forages.
In New Zealand, soil types have been identified which produce pastures that have molybdenum concentrations, varying from 3.5 to 20 mg/kg DM.2 In some deer herds, copper deficiency may be molybdenum induced rather than due to low copper intake alone. Increasing the pasture molybdenum concentrations from 2 mg/kg DM to 4.6 mg/kg DM significantly reduced serum and liver copper concentrations in grazing red deer.2 Reduced growth rate occurred when pasture molybdenum >10 mg/kg DM.
PATHOGENESIS
Effects on tissues
The consequences of hypocuprosis include a failure of copper metalloenzymes, many of which form part of the antioxidant defense system such as copper/zinc superoxide dismutase (Cu/Zn SOD) and ceruloplasmin.15 Copper, as well as other essential trace elements, is an atypical antioxidant because it functions indirectly. Copper is a catalytic cofactor for Cu/Zn SOD and ceruloplasmin. Cu/Zn SOD catalyzes dismutation of the superoxide anion, producing molecular oxygen and hydrogen peroxide, with the latter product usually metabolized by glutathione peroxidase and catalase. The ferroxidase activity of ceruloplasmin mediates the oxidation of ferrous ions to the ferric state, thereby preventing ferrous ion-dependent formation of hydroxyl radicals via the Fenton reaction. Thus, in enabling Cu/Zn SOD and ceruloplasmin to function as described, copper can be classified as part of the antioxidant defense system of cells.
Copper deficiency can affect the antioxidant defense system resulting in oxidative damage to cellular components. The activity of Cu/Zn SOD and glutathione peroxidase is decreased in animals with copper deficiency. Copper deficiency in cattle has been associated with a decrease in Cu/Zn SOD, ceruloplasmin and cytochrome oxidase activity and with an increase in lipid peroxidation. Collectively, this indicates that copper deficiency weakens the antioxidant defense systems.15
Ceruloplasmin is the copper-containing enzyme through which copper exerts its physiological function. The pathogenesis of most of the lesions of copper deficiency has been explained in terms of faulty tissue oxidation because of failure of these enzyme systems. This role is exemplified in the early stages of copper deficiency by the changes in the wool of sheep.
Chromosomal abnormalities
The association between copper deficiency and DNA damage in cattle has been examined.3,15 The Comet assay is a sensitive, reliable and rapid method for the detection of DNA double- and single-strand breaks and alkali-labile sites detection.16 In naturally-occurring copper deficiency in Aberdeen Angus cattle in Argentina, cytogenetic analysis of peripheral lymphocyte cultures showed a significant increase in the frequency of abnormal metaphases in moderate to severe copper deficient groups. Thus, copper deficiency in cattle is associated with an increase in the frequency of chromosomal aberrations (clastogenic effect) as well as in DNA migration.
Wool
The straightness and stringiness of this wool is due to inadequate keratinization, probably due to imperfect oxidation of free thiol groups. Provision of copper to such sheep is followed by oxidation of these free thiol groups and a return to normal keratinization within a few hours.
Body weight
In the later stages of copper deficiency, the impairment of tissue oxidation causes interference with intermediary metabolism and loss of condition or failure to grow.
Diarrhea
The pathogenesis of copper deficiency in causing diarrhea is uncertain and there is little evidence that a naturally-occurring primary copper deficiency will cause diarrhea. There are no histological changes in gut mucosa, although villous atrophy is recorded in severe, experimentally produced cases. Diarrhea is usually only a major clinical finding in secondary copper deficiency associated with molybdenosis.
Anemia
The known importance of copper in the formation of hemoglobin accounts for the anemia in copper deficiency. The presence of hemosiderin deposits in tissues of copper-deficient animals suggests that copper is necessary for the reutilization of iron liberated from the normal breakdown of hemoglobin. There is no evidence of excessive hemolysis in copper-deficiency states. Anemia may occur in the later stages of primary copper deficiency, but is not remarkable in the secondary form unless there is a marginal copper deficiency, as occurs in peat scours in New Zealand. The unusual relationship in New Zealand between copper deficiency and postparturient hemoglobinuria is unexplained. Heinz body anemia in lambs with deficiencies of copper or selenium and moved from improved pasture to rape (Brassica napus) has been reported.
Bone
The osteoporosis that occurs in some natural cases of copper deficiency is caused by the depression of osteoblastic activity.11 In experimentally induced primary copper deficiency, the skeleton is osteoporotic and there is a significant increase in osteoblastic activity. There is a marked overgrowth of epiphyseal cartilage, especially at costochondral junctions and in metatarsal bones. This is accompanied by beading of the ribs and enlargement of the long bones. There is also an impairment of collagen formation. When the copper deficiency is secondary to dietary excesses of molybdenum and sulfate, the skeletal lesions are quite different and characterized by widening of the growth plate and metaphysis and active osteoblastic activity.
Copper deficiency in foals causes severe degenerative disease of cartilage, characterized by breaking of articular and growth plate cartilage through the zone of hypertrophic cells, resulting in osteochondrosis of the articular-epiphyseal complex (A–E complex).11 The incidence and severity of osteochondrosis in foals can be decreased by supplementation of the diets of mares during the last 3–6 months of pregnancy and the first 3 months of lactation. Foals from non-supplemented mares have separation of the thickened cartilage from the subchondral bone. Clinical, radiographic, and biochemical differences occur between copper-deficient and copper-supplemented foals and there may be a relationship between low copper intakes in rapidly growing horses, inferior collagen quality, biomechanically weak cartilage, and osteochondritis.17
Copper is essential for metalloenzyme lysyl oxidase, which produces aldehydic groups on hydroxylysine residues as a prerequisite for eventual cross-link formation in collagen and elastin. Similar lesions in foals have been attributed to zinc toxicity from exposure of affected animals to pasture polluted by smelters. Experimentally, the addition of varying amounts of zinc to the diet of foals containing adequate copper will result in zinc-induced copper deficiency, but there are no effects with zinc intakes up to 580 ppm and it is suggested that 2000 ppm or higher are necessary to affect copper absorption in horses.18 Similar lesions of osteochondrosis have occurred in young farmed red deer and wapiti X red deer hybrids in New Zealand.7
Connective tissue
Copper is a component of the enzyme lysyl oxidase, secreted by the cells involved in the synthesis of the elastin component of connective tissues and has important functions in maintaining the integrity of tissues such as capillary beds, ligaments, and tendons.
Heart
The myocardial degeneration of falling disease may be a terminal manifestation of anemic anoxia, or be due to interference with tissue oxidation. In this disease, it is thought that the stress of calving and lactation contribute to the development of heart block and ventricular fibrillation when there has already been considerable decrease in cardiac reserve. Experimentally induced copper deficiency in piglets causes a marked reduction in growth and hematocrit and cardiac pathology and electrical disturbances.
Blood vessels
Experimentally produced copper deficiency has also caused sudden death due to rupture of the heart and great vessels in a high proportion of pigs fed a copper-deficient diet. The basic defect is degeneration of the internal elastic laminae. There is no record of a similar, naturally occurring disease. A similar relationship appears to have been established between serum copper levels and fatal rupture of the uterine artery at parturition in aged mares.
Pancreas
Lesions of the pancreas may be present in normal cattle with a low blood copper status. The lesions consist of an increase in dry matter content and a reduction in the concentrations of protein and copper in wet tissue. The cytochrome oxidase activity and protein:RNA ratio are also reduced. There are defects in acinar basement membranes, splitting, and disorganization of acini, cellular atrophy and dissociation and stromal proliferation.
Nervous tissue
Copper deficiency halts the formation of myelin and causes demyelination in lambs, probably by a specific relationship between copper and myelin sheaths. Defective myelination can commence as early as the midpoint of the fetus’s uterine life. The focus of lesions in the white matter shifts from the cerebrum in lambs affected at birth (congenital swayback) to the spinal cord in delayed cases, which may reflect respective peaks of myelin development at those sites at 90 days’ gestation and 20 days after birth. The postnatal development of delayed swayback has been confirmed through its control by copper supplementation after birth. In experimental animals, it has been shown that copper deficiency does interfere with the synthesis of phospholipids. While anoxia is a cause of demyelination, an anemic anoxia is likely to occur in highly deficient ewes and anemic ewes produce a higher proportion of lambs with enzootic ataxia, there is often no anemia in ewes producing lambs with the more common subacute form of the disease. Severely deficient ewes have lambs affected at birth and in which myelin formation is likely to have been prevented. The lambs of ewes less severely deficient have normal myelination at birth and develop demyelination in postnatal life.
Reproductive performance
There is no evidence that copper deficiency causes reproductive failure in dairy cows. Copper glycinate given to dairy cattle does not affect the average interval in days between calving and first observed heat, services per conception, or first service conception rate compared with untreated cows in the same population. Experimentally, the addition of molybdenum to the diet of heifers delayed the onset of puberty, decreased the conception rate and caused anovulation and anestrus in cattle without accompanying changes in copper status or in live weight gain. Thus, the presence of molybdenum rather than low copper status may affect reproductive performance of cattle. Geochemical data indicate that approximately 10% of the cultivated area of England and Wales has soils that may result in forage molybdenum concentrations similar to those used in the above experimental diet. It appears inadvisable to ascribe poor reproductive performance to subclinical hypocuprosis on the evidence of blood copper analysis alone. Other factors, such as management and energy and protein intake, should be examined.
Immune system
Copper is an essential trace mineral with an important role in the immune response but the precise mechanism is not well understood. In experimental secondary copper deficiency in cattle induced by molybdenum at 30 ppm and sulfate at 225 ppm, the intracellular copper content of peripheral blood lymphocytes, neutrophils, and monocyte-derived macrophages was reduced between 40% and 70%.19 In copper deficient animals, the serum ceruloplasmin activity decreased to 50% of control values. Both the copper-zinc-superoxide dismutase and the cytochrome c oxidase activities are significantly reduced in leukocytes. Thus, copper deficiency alters the activity of several enzymes, which mediate antioxidant defenses and ATP formation. These effects may impair cell immune function, affecting the bactericidal capacity and making the animals more susceptible to infection.
Copper deficiency results in decreased humoral and cell-mediated immunity, as well as decreased non-specific immunity regulated by phagocytic cells, such as macrophages and neutrophils.20,21 The decreased resistance to infection in sheep is amenable to treatment with copper and genetic selection. In lambs genetically selected for low and high concentrations of plasma copper, the mortality from birth to 24 weeks of age in the high line was half that in the low line. Most of the losses were due to a variety of microbial infections. Experimental viral and bacterial infections of cattle can cause a rapid, though transient, increase in serum ceruloplasmin and plasma copper in copper-replete animals, suggesting a major protective role for copper in infectious diseases. These changes in copper metabolism evolve from an interleukin-1 mediated increase in hepatic synthesis and release of ceruloplasmin, an acute phase protein. Copper concentrations in organs involved in immune regulations such as liver, spleen, thymus, and lung are substantially reduced by copper deficiency, suggesting that copper-deficient animals are at greater risk for infection than copper-adequate animals. However, experimental low copper diets with or without supplemental molybdenum does not alter the specific immunity of stressed cattle.22
The severity of copper depletion needed for immune dysfunction is less than required to induce clinical signs of copper deficiency and endogenous copper may contribute to the regulation of both non-immune and immune inflammatory responses. Low molecular weight complexes may have an anti-inflammatory effect in animal models of inflammation and it is postulated that the elevation of plasma copper-containing components during inflammatory disease represents a physiological response.
In experimental coliform mastitis in dairy cattle, copper in the diet at 20 ppm reduced the clinical response but not duration of the mastitis compared with animals receiving 6.5 ppm beginning 60 days prepartum through 42 days of lactation.23 Liver copper in the supplemented group was 162 and 33 ppm at calving and 256 and 45 ppm at 42 days post partum, respectively.
Copper deficiency in heifers in Northern India was associated with significant reduction in the candidacidal activity of neutrophils compared with copper supplemented animals.24
Sequence of clinical signs development
In experimental copper deficiency in calves, beginning at 6 weeks of age, subclinical and clinical abnormalities appear after the following intervals: hypocupremia at 15 weeks, growth retardation from 15 to 18 weeks, rough hair coat at 17 weeks, diarrhea at 20 weeks and leg abnormalities at 23 weeks. These signs correlate well with the onset of hypocupremia and are indicative of a severe deficiency. Even with these signs of deficiency, the histological abnormalities may be only minor in degree.
In experimental primary copper deficiency in calves, beginning at 12 weeks of age, clinical signs of the deficiency may not become apparent for about 6 months. Musculoskeletal abnormalities include a stilted gait, a ‘knock-kneed’ appearance of the forelimbs, overextension of the flexors, splaying of the hooves and swellings around the metacarpophalangeal and carpometacarpal joints. Changes in hair pigmentation occur after about 5 months and diarrhea between 5 and 7 months. The diarrhea ceased 12 h after oral administration of a small amount (10 mg) of copper.
Copper–molybdenum–sulfate relationship
The interaction between copper, molybdenum, and sulfur in ruminant nutrition is unique in its effects on health and production. Copper, molybdenum, and sulfur from organic or inorganic sources can combine in the rumen to form an unabsorbable triple complex, copper tetrathiomolybdate and deplete the host tissues of copper.1
Secondary or conditioned copper deficiency occurs when the dietary intake of copper is adequate, but absorption and utilization of the copper are inadequate because of the presence of interfering substances in the diet.1
Molybdenum and sulfate alone or in combination can affect copper metabolism and the mechanisms by which this occurs are now being clarified. This effect also operates in the fetus and interferes with copper storage in the fetal liver. Besides the relationship with molybdenum, an interaction between the absorption of copper and selenium has been demonstrated, the administration of selenium to sheep on copper-deficient pastures causing an improvement in copper absorption.
The toxicity of any level of dietary molybdenum is affected by the ratio of the dietary molybdenum to dietary copper. The critical copper:molybdenum ratio in animal feeds is 2 and feeds or pasture with a lower ratio may result in conditioned copper deficiency. In some regions of Canada, the copper:molybdenum ratio will vary from 0.1 to 52.7. Higher critical ratios closer to 4.1–5.1 have been recommended for safety. The influence of dietary molybdenum on copper metabolism in ponies has been examined experimentally.
The copper status of growing calves can also be affected to a similar degree by the inclusion of appropriate levels of supplementary iron or molybdenum in the diet. Following such inclusion, the liver and plasma concentrations of copper will decline within 12–16 weeks to levels indicating severe copper deficiency. The clinical signs of copper deficiency, as indicated by reduced growth rate and changes in the hair texture and color, are evident after 16–20 weeks only in animals supplemented with molybdenum. The reduced growth rate was accompanied by a decreased feed intake and reduced efficiency of feed utilization.
Copper absorption
On the basis of a response to copper injections and no response to copper administered orally to sheep on a high molybdenum intake, it is suggested that interference occurs with the absorption of copper from the gut.
It is proposed that thiomolybdates form in the rumen from the reaction of dietary molybdenum compounds with sulfides produced from the reduction of dietary sulfur compounds by rumen bacteria. The thiomolybdates reduce the absorption of dietary copper from the intestine and also inhibit a number of copper-containing enzymes, including ceruloplasmin, cytochrome oxidase, superoxide dismutase and tyrosine oxidase.
Copper utilization
Sulfate and molybdate can interfere with mobilization of copper from the liver, inhibition of copper intake by the tissues, inhibition of copper transport both into and out of the liver and inhibition of the synthesis of copper-storage complexes and ceruloplasmin.
The clinical signs of hypocuprosis (such as steely wool) can occur in sheep on diets containing high levels of molybdenum and sulfate, even though blood copper levels are high. This suggests that under these circumstances copper is not utilizable in tissues and the blood copper rises in response to the physiological needs of the tissues for the element. In pigs, a copper– molybdenum complex can exist in animals and that in this form the copper is unavailable. This would interfere with hepatic metabolism of copper and the formation of copper-protein complexes such as ceruloplasmin.
Hepatic storage
The copper status of the liver depends on whether the animals are receiving adequate dietary copper. With adequate dietary levels, the liver copper levels are less in the presence of molybdate and sulfate. If the animals are receiving a copper-deficient diet such that copper is being removed from the liver, then the molybdate plus sulfate animals retain more copper in their liver than copper-deficient animals not receiving sulfate plus molybdate. This supports the hypothesis that molybdate and sulfate together impair the movement of copper into or out of the liver, possibly by affecting copper transport. Sulfate alone exerts an effect. An increase in intake reduces hepatic storage of both copper and molybdenum.
Phases of copper deficiency
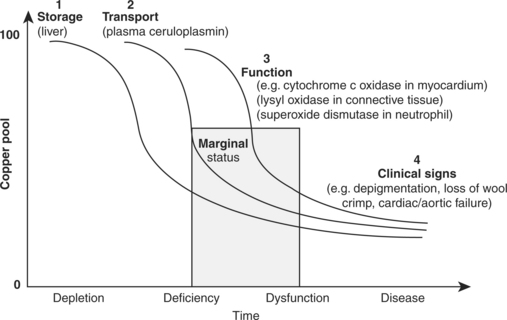
The development of a deficiency can be divided into four phases:
During the depletion phase, there is loss of copper from any storage site, such as liver, but the plasma concentrations of copper may remain constant. With continued dietary deficiency, the concentrations of copper in the blood decline during the phase of marginal deficiency. However, it may be some time before the concentrations or activities of copper-containing enzymes in the tissues begin to decline and it is not until this happens that the phase of dysfunction is reached. There may be a further lag before the changes in cellular function are manifested as clinical signs of disease.
Summary
The overall effect of these interactions is as follows. Molybdate reacts with sulfides to produce thiomolybdates in the rumen. The subsequent formation of copper-thiomolybdate complexes isolates the copper from being biologically available.1 The thiomolybdates reduce the effectiveness of enzymes containing copper and there are some significant interactions between copper, zinc, and iron.
CLINICAL FINDINGS
The general effects of copper deficiency are the same in sheep and cattle, but in addition to these general syndromes, there are specific syndromes more or less restricted to species and to areas. What follows is a general description of the disease caused by copper deficiency, in turn followed by the specific syndromes of enzootic ataxia, swayback, falling disease, peat scours, teart, and unthriftiness (pine).
Cattle
Subclinical hypocuprosis
No clinical signs occur, blood copper levels are marginal or below 57 mg/dL (9.0 mmol/L) and there is a variable response in productivity after supplementation with copper. Some surveys in copper-deficient areas found that about 50% of beef herds and 10% of dairy herds within the same area have low blood levels of blood copper associated with low copper intake from natural forages. The deficiency is likely to be suspected only if production is monitored and found to be suboptimal.
A perplexing feature of subclinical hypocuprosis is the wide variation in improved growth rate obtained when cattle of the same low copper status are given supplementary copper under field conditions.
General syndrome
Primary copper deficiency
Primary copper deficiency causes unthriftiness, loss of milk production, and anemia in adult cattle. The coat color is affected, red and black cattle changing to a bleached, rusty red and the coat itself becomes rough and staring. In severely deficient states, which are now uncommon, calves grow poorly and there is an increased tendency for bones to fracture, particularly the limb bones and the scapula. Ataxia may occur after exercise, with a sudden loss of control of the hindlimbs and the animal falling or assuming a sitting posture. Normal control returns after rest. Itching and hair-licking are also recorded as manifestations of copper deficiency in cattle. Although diarrhea may occur, persistent diarrhea is not characteristic of primary copper deficiency and its occurrence should arouse suspicion of molybdenosis or helminthiasis. In some affected areas, calves develop stiffness and enlargement of the joints and contraction of the flexor tendons causing the affected animals to stand on their toes. These signs may be present at birth or occur before weaning. Paresis and incoordination are not evident.
An increased occurrence of postparturient hemoglobinuria is also recorded, but only in New Zealand and may be unrelated to copper deficiency.
Secondary copper deficiency
This syndrome includes the signs of primary copper deficiency, except that anemia occurs less commonly, probably due to the relatively better copper status in the secondary state, anemia being largely a terminal sign in primary copper deficiency. For example, anemia occurs in peat scours of cattle in New Zealand, but in this instance, the copper intake is marginal. In addition to the other signs, however, there is a general tendency for diarrhea to occur, particularly in cattle. Because diarrhea is not a major sign in naturally occurring primary copper deficiency it is possible that it is due to the conditioning factor, which reduces the availability of copper. For example, the severity of the diarrhea is roughly proportional to the level of intake of molybdenum.
Falling disease
The characteristic behavior in falling disease is for cows in apparently good health to throw up their heads, bellow, and fall. Death is instantaneous in most cases, but some fall and struggle feebly on their sides for a few minutes with intermittent bellowing and running movement attempts to rise. Rare cases show signs for up to 24 h or more. These animals periodically lower their heads and pivot on the front legs. Sudden death usually occurs during one of these episodes.
Peat scours (‘teart’)
Persistent diarrhea with the passage of watery, yellow-green to black feces with an inoffensive odor occurs soon after the cattle go on to affected pasture, in some cases within 8–10 days. The feces are released without effort, often without lifting the tail. Severe debilitation is common, although the appetite remains good. The hair coat is rough and depigmentation is manifested by reddening or gray flecking, especially around the eyes, in black cattle. The degree of abnormality varies a great deal from season to season and year to year and spontaneous recovery is common. Affected animals usually recover in a few days following treatment with copper.
Unthriftiness (pine) of calves
The earliest signs are a stiffness of gait and unthriftiness. The epiphyses of the distal ends of the metacarpus and metatarsus may be enlarged and resemble the epiphysitis of rapidly growing calves deficient in calcium and phosphorus or vitamin D. The epiphyses are painful on palpation and some calves are severely lame. The pasterns are upright and the animals may appear to have contracted flexor tendons. The unthriftiness and emaciation are progressive and death may occur in 4–5 months. Grayness of the hair, especially around the eyes in black cattle, is apparent. Diarrhea may occur in a few cases.
Sheep
General syndrome
Primary copper deficiency
Abnormalities of the wool are the first observed signs and may be the only sign in areas of marginal copper deficiency. Fine wool becomes limp, glossy and loses its crimp, developing a straight, steely appearance. Black wool shows depigmentation to gray or white, often in bands coinciding with the seasonal occurrence of copper deficiency. The straight, steely defect may occur in similar bands and the staple may break easily. There appear to be some differences between breeds in susceptibility to copper deficiency, Merino sheep appearing to have a higher copper requirement than mutton sheep. The fleece abnormalities of Merino sheep in Australia have not been observed in Romney Marsh sheep in copper-deficient areas in New Zealand, but this may be due in part to the difficulty of detecting abnormality in wool that is normally rather straight and steely. Anemia, scouring, unthriftiness and infertility may occur in conditions of extreme deficiency, but in sheep, the characteristic findings are in the lamb, the disease enzootic ataxia being the major manifestation. Retardation of growth, diarrhea, delay to marketing, and increased mortality are common clinical findings in lambs genetically selected for low plasma copper and placed on improved and limed upland pastures. Osteoporosis, with increased tendency of the long bones to fracture, has also been recorded under conditions of copper deficiency insufficient to cause enzootic ataxia.
Swayback and enzootic ataxia in lambs and goat kids
These diseases have much in common, but there are differences in epidemiology and some subtle clinical ones.
Swayback is the only authentic manifestation of a primary nutritional deficiency of copper in the UK. The incidence can vary greatly among breeds of sheep, reflecting the genetic differences in copper metabolism both between and within breeds of sheep. The disease occurs in several forms.
A congenital form, cerebrospinal swayback, occurs only when the copper deficiency is extreme. Affected lambs are born dead or weak and unable to stand and suck. Incoordination and erratic movements are more evident than in enzootic ataxia and the paralysis is spastic in type. Blindness also occurs occasionally. There is softening and cavitation of the cerebral white matter and this probably commences about day 120 of gestation.
Progressive (delayed) spinal swayback begins to develop some weeks after birth with lesions and clinical signs appearing at 3–6 weeks of age.
Postnatal acute fatal swayback may be a third form of the disease and appears to occur only in Wales. It resembles the more usual delayed form, but develops suddenly. There is a sudden onset of recumbency with death occurring 1–2 days later due to acute swelling of the cerebrum.
Enzootic ataxia affects only unweaned lambs. In severe outbreaks, the lambs may be affected at birth, but most cases occur in the 1–2-month age group. The severity of the paresis decreases with increasing age at onset. Lambs affected at birth or within the first month usually die within 3–4 days. The disease in older lambs may last for 3–4 weeks and survival is more likely, although surviving lambs always show some ataxia and atrophy of the hindquarters. The first sign to appear in enzootic ataxia is incoordination of the hindlimbs, appearing when the lambs are driven. Respiratory and cardiac rates are also greatly accelerated by exertion. As the disease progresses, the incoordination becomes more severe and may be apparent after walking only a few yards. There is excessive flexion of joints, knuckling over of the fetlocks, wobbling of the hindquarters and finally falling. The hindlegs are affected first and the lamb may be able to drag itself about in a sitting posture. When the forelegs eventually become involved recumbency persists and the lamb dies of inanition. There is no true paralysis, the lamb being able to kick vigorously even in the recumbent stage. The appetite remains unaffected.
Goats
Enzootic ataxia due to copper deficiency has been reported in young goat kids. The disease is similar in most respects to the disease in lambs. Kids may be affected at birth, or the clinical signs may be delayed until the animals are several weeks of age. Cerebellar hypoplasia is a frequent finding in goats.
Other species
Deer
Enzootic ataxia in red deer is remarkably different from the disease in lambs in that it develops in young adults well past weaning age, and in adults. The clinical signs include ataxia, swaying of the hindquarters, a dog-sitting posture and, eventually, inability to use the hindlimbs. Spinal cord demyelination and midbrain neuronal degeneration are characteristic. Osteochondrosis of young, farmed deer with copper deficiency is characterized by lameness, one or more swollen joints and an abnormal ‘bunny-hopping’ gait or ‘cow-hocked’ stance.7 Copper deficiency in red deer in Australia during a period of drought caused loss of weight in lactating hinds after calving and steely hair coats (the hair had a lustre resembling that of so-called steely wool of copper-deficient sheep). Both adult and yearling stags had normal hair coats but those of the yearling hinds were patchy, with large areas of harsh, light colored, steely hair.21 The high sulfur content of the diet and possible accidental iron ingestion from being fed on the ground may have resulted in secondary copper deficiency.
Pigs
Naturally occurring enzootic ataxia has occurred in growing pigs 4–6 months of age. Posterior paresis progresses to complete paralysis in 1–3 weeks. Dosing with copper salts had no effect on the clinical conditions, but hepatic copper levels were 3–14 mg/kg (0.05–0.22 mmol/kg). Copper deficiency in piglets 5–8 weeks of age has been reported and was characterized clinically by ataxia, posterior paresis, nystagmus, inability to stand, paddling movements of the limbs and death in 3–5 days. Demyelination of the spinal cord and degenerative lesions of the elastic fibers of the walls of the aorta and pulmonary arteries are present.
The inclusion of copper sulfate, at levels of 125–250 mg/kg of copper, in the diets of pigs 11–90 kg live weight and fed ad libitum, results in slight improvements in growth rate and feed efficiency, but has no significant effect on carcass characteristics. The supplemental copper causes a marked increase in liver copper concentration which poses a potential hazard and it is recommended that copper supplementation be limited to starter and grower diets fed to pigs weighing less than 50 kg live weight.
Horses
Adult horses are unaffected by copper deficiency, but there are unconfirmed reports of abnormalities of limbs of foals. Foals in copper-deficient areas may be unthrifty and slow-growing, with stiffness of the limbs and enlargement of the joints. Contraction of the flexor tendons causes the animal to stand on its toes. There is no ataxia or indication of involvement of the central nervous system. Signs may be present at birth or develop before weaning. Recovery occurs slowly after weaning and foals are unthrifty for up to 2 years.
Geophagia or soil eating in horses in Australia has been associated with larger concentrations of iron and copper in soil samples compared to paired control samp les, suggesting that these elements provide the stimulus for geophagia.25
CLINICAL PATHOLOGY
The laboratory evaluation of the copper status of farm animals is complex because the biochemical values are often difficult to interpret and to correlate with the clinical state of the animal. Interpretation of the copper status of an individual animal is more difficult than of a herd.
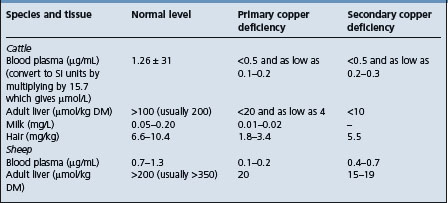
The guidelines for the laboratory diagnosis of primary and secondary copper deficiency in cattle and sheep are summarized in Table 30.4.
The diagnosis of copper deficiency in a herd of animals is based on a combination of collection and interpretation of the history, clinical examination of the affected animals, laboratory tests on serum and liver samples, and examination of the environment including analysis of the feed and water supplies and perhaps soil analysis.11
It is necessary to be especially careful when collecting specimens for copper analysis to avoid contamination by needles, copper distilled water, vial caps, cans for liver specimens and other possible sources of copper. An additional problem is the possible effect of intercurrent disease on plasma levels of copper.
A comparison of health and production variables in a group of animals treated with copper and a similar group not treated with copper, is also desirable. Variables include calf growth rates, calf mortality and reproductive performance.26
In order to assess the copper status of herd, a standard practice is to take blood samples at random from at least 10% of clinically affected animals and from 10% of normal animals. However, this may be inappropriate when there may be a wide variation in the serum copper concentration within a herd. In some cases, a 10% sample may be too large and in other cases too small. The minimal sample size for random samples from a finite population of a normal continuously distributed variable has been calculated as follows:
Where n = minimal sample size; N = herd size; t = Student’s t value; cv = coefficient of variation; and E = allowable error.
Initial testing can be used to estimate variability of serum copper concentration within a herd and a minimal sample size may be calculated. Each class of animal according to age groups, diet and production status should also be sampled. Follow-up samples should be taken from the same animals following therapy or the institution of control measures.
Laboratory diagnosis
Historically, the laboratory diagnosis of copper deficiency in cattle and sheep centered on the determination of serum or plasma copper and liver copper. However, serum copper levels alone are not reliable as indicators of copper status and liver samples collected either by liver biopsy or at slaughter should be used to accurately assess copper status in cattle. Clinically normal animals may have marginal levels of serum copper, or unthrifty animals may have marginal or deficient serum levels of copper. Furthermore, when either the normal animals with the marginal levels of copper or the unthrifty animals with the marginal or deficient levels are treated with copper there may or may not be an improvement in weight gain as might be expected in the former, or improvement in clinical condition in the latter.
Phases
The development of a deficiency can be divided into four phases11 (Fig. 30.1):
During the depletion phase, there is loss of copper from any storage site, such as liver, but the plasma concentrations of copper may remain constant. With continued dietary deficiency, the concentrations of copper in the blood will decline during the phase of marginal deficiency. However, it may be some time before the concentrations or activities of copper-containing enzymes in the tissues begin to decline and it is not until this happens that the phase of dysfunction is reached. There may be a further lag before the changes in cellular function are manifested as clinical signs of disease.
Interpretation of laboratory results
The three principles governing the interpretation of biochemical criteria of trace element status include:
• The relationships between the concentration of the marker and the intake of the element
From these principles, the concentrations of liver copper are insensitive indices of deficiency, but good indicators of excess. Plasma copper <57 μg/dL (9 μmol/L) is a good index of marginal deficiency, but values may have to fall to below 19 μg/dL (3 μmol/L) before there is a risk of dysfunction and loss of production in sheep and cattle. However, these are only guidelines. The range of values and the cut-off levels above which animals are normal, or below which they are deficient, have not been well-established. There is considerable biological variation dependent on the species, the breed of animal, the length of time over which the depletion has occurred and the presence of intercurrent disease.
Serum copper concentration in cattle is fairly specific for detection of low liver copper but only marginally sensitive when serum copper concentration of 0.45 μg/g is used as a test endpoint.27 The value of serum copper concentration as a diagnostic indicator depends on the prevalence of copper deficiency in the particular area.
The interpretation of serum copper can change depending on what liver copper concentration is considered low.28 With a liver copper <20 μg/g DM as indicative of copper deficiency, serum copper concentrations =9 mmol/L will be a good indicator of copper deficient status but concentrations >9 mmol/L will be a poor indicator of copper sufficient status. If 10 μg/g DM is used as liver copper cut-off, then serum concentrations >9 mmol/L are reliable as indicative of copper sufficient status but not on concentrations =9 mmol/L as indicative of copper deficiency.
Concentrations of copper in liver and blood may be of diagnostic value but should be interpreted with caution since clinical signs of copper deficiency may appear before there are significant changes in the levels of copper in the blood and liver. Conversely, the plasma levels of copper may be very low in animals that are otherwise normal and performing well. There is a tendency to overestimate the presence of copper deficiency because veterinarians use a diagnostic threshold for copper deficiency that is too high.11,26 Among veterinary laboratories, there is a wide variation in the normal range currently used for equine serum copper values.
Very low levels of both blood and liver copper in a group of animals need not be associated with clinical abnormalities. In a study in the Netherlands, in a group of dairy heifers, the copper status was determined at regular intervals over an 18-month period.29 One group was supplemented with copper sulfate and the other was not. The copper and molybdenum levels in the grass were within normal limits as accepted in the Netherlands; copper 7–15 mg/kg DM and molybdenum <5 mg/kg DM. The levels of copper in both the blood and liver were much below the reference ranges used in the Netherlands (6–15 μmol/L in blood and >30 mg/kg DM in liver). No clinical signs of copper deficiency occurred and there were no differences in growth rate and reproductive performance.
Plasma and liver copper levels
Cattle and sheep
In cattle and sheep, plasma copper levels between 19 μg/dL and 57 μg/dL (3.0 and 9.0 μmol/L) represent marginal deficiency and levels below 19 μg/dL (3 μmol/L) represent functional deficiency or hypocuprosis. The internationally recognized threshold to assess copper deficiency is 9.4 μmol/L. In both species a value for plasma or serum of 11.0 μmol/L can be associated with a liver concentration from 789 to 3786 μmol/kg DM (50–240 mg/kg). By contrast, a value of 9.3 μmol/L will usually be associated with liver copper values of 315–789 μmol (20–50 mg/kg DM), which are regarded as marginally inadequate. Plasma copper levels of 49.9 μg/dL (7.85 μmol/L) or less are indicative of low liver copper levels. Plasma copper levels above 90.2 μg/dL (14.2 μmol/L) are usually associated with liver levels above 38.1 mg/kg (0.6 mmol/kg) DM. Of the two estimations, that on liver is the most informative as levels in blood may remain normal for long periods after liver copper levels commence to fall and early signs of copper deficiency appear. Levels of copper in adult liver above 200 mg/kg DM (3.14 mmol/kg) in sheep and above 100 mg/kg DM (1.57 mmol/kg) in cattle are considered to be normal. Levels of less than 80 mg/kg DM (1.5 mmol/kg) in sheep and less than 30 mg/kg DM (0.5 mmol/kg) in cattle are classed as low. Liver copper levels in fetuses and neonates are usually much higher than in adults and normal foals have had levels of 219 mg/kg (3.4 mmol/kg DM) compared with a normal of 31 mg/kg (0.49 mmol/kg DM) in adults.
Liver copper
Because the liver is a storage compartment for copper, the concentrations of liver copper indicate the state of depletion rather than deficiency. There is no particular threshold value for liver copper below which the performance and health of livestock are likely to be impaired. A broad range of values may, for example, coincide with the marginally deficient state, e.g. 5.1–20.3 mg (0.08–0.32 mmol) copper/kg liver DM. The concentration of hepatic copper in sheep is uniform and a single biopsy sample should be representative of the whole liver. The technique of liver biopsy for assessing the copper status of sheep has been evaluated. Frequency of biopsy does not affect copper concentration, the variability between successive samples is small and the biopsy procedure does not reduce body weight or rate of gain. Copper concentrations in the kidney cortex may be of more diagnostic value because concentrations are normally within a narrow range of 12.7–19.0 mg/kg DM (0.2–0.3 mmol/kg DM). Thus, concentrations below 12.7 mg/kg DM (0.2 mmol/kg DM) in the kidney may be a more reliable indicator of dysfunction than liver copper concentration.
The concentrations of copper in the livers of calves vary according to age and production class (dairy or beef) with no evidence of copper toxicosis or deficiency. In calves submitted for necropsy, the liver copper concentrations were as much as 60 mg/kg WW higher in dairy calves than beef calves.30 The concentration increased for the first 2 months of age, then declined until 9 months of age, after which it began to increase. Thus, the diagnosis of copper imbalances based on liver copper concentration in calves should take into account the diagnostic covariates of age and production class.
Ceruloplasmin
The difficulty of interpreting plasma levels of copper led to the estimation of plasma levels of copper-protein complexes, especially ceruloplasmin. Ceruloplasmin contains greater than 95% of the circulating copper in normal animals. There is a highly significant correlation between plasma copper levels and plasma ceruloplasmin activity, which is a less complicated and more rapid procedure than plasma copper. The regression analyses indicate a strongly positive correlation coefficient of ceruloplasmin with serum of cattle and sheep of 0.83 and 0.92, respectively. The correlation between serum ceruloplasmin activity and hepatic copper concentrations in cattle was only 0.35, indicating an unreliable relationship. Normal plasma ceruloplasmin levels in sheep are in the region of 45–100 mg/L. Normal levels of serum ceruloplasmin activity in cattle range from 120 to 200 mg/L. The mean copper and ceruloplasmin levels are higher in plasma than serum; the percentage of copper associated with ceruloplasmin is less in serum (55%) than in plasma (66%). Normal plasma ceruloplasmin levels in sheep range from 4.5 to 10 mg/dL. In experimental primary copper deficiency in calves, rapid decreases occur in plasma ceruloplasmin activity at least 80 days before overt clinical signs of deficiency.
Erythrocyte dismutase
The measurement of the activity of erythrocyte superoxide dismutase (ESOD), a copper-containing enzyme, is now being evaluated as a procedure for the diagnosis of copper deficiency. The activity of this enzyme decreases more slowly than plasma or liver copper in copper-deficient animals and may be more closely correlated with the presence of imminence of hypocuprosis. In marginal deficiency, the ESOD value ranges from 2 to 5 U/mg hemoglobin and in functional deficiency the value is below 2.
Milk and hair copper
The levels of copper in milk and hair are also lower in deficient than in normal cattle and estimation of the copper content of hair is now acceptable as a diagnostic aid. It has the advantage of providing an integrated progressive record of nutritional intake. The levels of copper in bovine hair are more markedly depressed when extra molybdenum is fed.
Horses
A threshold level of plasma copper of 16 μmol/L is used to distinguish between the normal and subnormal values.31 Liver copper from horses sampled at slaughter vary widely about a mean of 113.7 μmol/kg WW.31 The threshold of 52.5 μmol/kg WW of copper in liver is proposed to distinguish deficient from marginal liver copper status. Many healthy horses have serum values between 12 and 16 μmol/L.
The mean hepatic copper concentrations of horses fed diets containing 6.9–15.2 mg copper/kg DM were 17.1–21.0 μg/g DM (0.27–0.33 μmol/g DM) tissue. The plasma copper concentrations ranged from 3.58 to 4.45 μg/dL (22.8–28.3 μmol/L). There was no simple mathematical relationship between plasma and hepatic copper concentrations. The range of serum copper concentrations in Thoroughbred horses at grass was 63–196 μg/dL (9.91–30.85 mmol/L) and in stabled Thoroughbreds the range was 47–111 μg/dL (7.40–17.47 mmol/L).
Farmed red deer (Cervus elaphus)
The suggested reference ranges for serum and liver copper concentrations to categorize the copper status of deer are: serum concentrations (μmol/L): <5, deficient; 5–8 marginal and; >8, adequate; and liver copper concentrations (μmol/kg weight wet, WW): <60, deficient; 60–100, marginal and >100, adequate.32 Enzootic ataxia and osteochondrosis occur when liver copper concentrations are <60 μmol/kg fresh tissue and serum copper concentrations are below 3–4 μmol/L.33 Growth responses to copper supplementation are equivocal when blood copper concentrations are <3–4 μmol/L, but are significant when mean blood copper concentrations are 0.9–4.0 μmol/L. No antler growth or body weight response to copper supplementation occurs when blood ceruloplasmin (ferroxidase) levels averaged 10–23 IU/L (equivalent to serum copper concentrations of 6–13 μmol/L) and liver concentrations averaged 98 μmol/kg fresh tissue. This suggests deficient, marginal and adequate ranges for serum copper concentrations should be <5, 5–8 and >8 μmol/L, respectively and those for liver copper concentrations should be <60, 60–100 and >100 μmol/kg, respectively.33
Hematology
Anemia may occur in advanced cases of primary copper deficiency, hemoglobin levels being depressed to 50–80 g/L and erythrocytes to 2–4 × 1012/L. A high proportion of cows in problem herds may have a Heinz-body anemia without evidence of hemoglobinuria and the severity of the anemia will be related to the hypocupremia.
NECROPSY FINDINGS
The characteristic gross findings in copper deficiency of ruminants are those of anemia and emaciation. Hair and wool abnormalities may be present as already described. Extensive deposits of hemosiderin can cause darkening of the liver, spleen and kidney in most cases of primary copper deficiency and in the secondary form if the copper status is sufficiently low. In lambs, there may be severe osteoporosis and long bone fractures. Osteoporosis is less evident in cattle, but can be confirmed radiographically and histologically. In naturally occurring secondary copper deficiency in cattle, associated with high dietary molybdenum and sulfate, there is widening of the growth plates due to abnormal mineralization of the primary spongiosa, resulting in a grossly rachitic appearance to the bones.
The most significant finding in enzootic ataxia is the degeneration of axons and myelin within the cerebellar and motor tracts in the spinal cord, a change only evident at the microscopic level. Chromatolysis of neurons in a variety of locations within the central nervous system is usually detectable. In a few extreme cases and in most cases of swayback, the myelin loss also involves the cerebrum, where there is destruction and cavitation of the white matter. There is marked internal hydrocephalus in such cases and the convolutions of the cerebrum are almost obliterated. Acute cerebral edema with marked brain swelling and cerebellar herniation, reminiscent of polioencephalomalacia, may also accompany the more typical myelopathy and multifocal cerebral leukomalacia in lambs with hypocuprosis.
In falling disease, the heart is flabby and pale. There is generalized venous congestion and the blood may appear watery. The liver and spleen are enlarged and dark. Histological examination reveals atrophy of the cardiac muscle fibers and considerable cardiac fibrosis. Deposits of hemosiderin are present in the liver, spleen and kidney.
Necropsy findings associated with copper deficiency in non-ruminant species are not well-documented. Degenerative changes with subsequent rupture of the aorta have been experimentally induced in pigs, but this has not been described as a naturally occurring disease. A myelopathy with white matter changes similar to those of enzootic ataxia has also been reported in 4–5-month-old copper-deficient pigs. Musculoskeletal changes similar to those described for calves have also been reported in foals with hypocuprosis.
Necropsy examinations should include assay of copper in viscera. The levels of copper in liver are usually low (see Table 30.4) and in secondary copper deficiency, there may be a high level of copper in the kidney and high levels of molybdenum in the liver, kidney and spleen. Copper levels in body tissues and fluids in primary and secondary copper deficiency are listed in Table 30.4.
Samples for confirmation of diagnosis
• Toxicology – 50 g liver, kidney (ASSAY (Cu) (Mo))
• Histology – formalin-fixed samples of: long bone (including growth plate), skin, liver, spleen. Enzootic ataxia/swayback: half of midsagittally-sectioned brain, lumbar and cervical spinal cord. Falling disease: heart (several sections), bone marrow, spleen (LM).
The clinical findings which are common in young, growing ruminants, include a herd problem of unthriftiness and progressive loss of weight, changes in hair coat color or texture of wool, chronic lameness, neonatal ataxia in lambs and kids and terminal anemia. In adult cattle on pasture with excess molybdenum, chronic diarrhea is characteristic. A combination of serum and liver copper and serum molybdenum, are major diagnostic aids in distinguishing between copper deficiency and the other diseases.
Several disease complexes that are herd or flock problems in cattle and sheep may resemble both primary and secondary copper deficiency. The emphasis is on many animals being affected at about the same time, with a chronic debilitating disease complex, under the same dietary and seasonal circumstances.
A scheme for the differential diagnosis of mineral and vitamin responsive disorders in beef cattle herds with suboptimal performance should include three major directions: malnutrition (lack of feed); chronic infectious disease; and, lack of specific micronutrients.34
Unthriftiness and progressive weight loss may be due to protein-energy malnutrition and examination of the diet will reveal the cause.
Changes in hair coat color in young growing cattle is caused only by copper deficiency.
Chronic lameness in young growing cattle may be caused by a calcium, phosphorus and vitamin D imbalance, which is determined by examination of the diet and radiography of the long bones. The radiographic changes in cattle with secondary copper deficiency consist of widened irregular epiphyseal plates with increased bone density in the metaphysis and metaphyseal lipping. These findings are similar to those described for rickets and secondary nutritional hyperparathyroidism in cattle.
Chronic diarrhea in young cattle may be due to intestinal parasitism and fecal examination and response to therapy are diagnostic. Diarrhea in a group of adult cattle on pasture known to be high in molybdenum is probably due to secondary copper deficiency and response to therapy is diagnostic.
Winter dysentery of cattle, salmonellosis, coccidiosis and mucosal disease are acute diseases characterized by diarrhea but are accompanied by other signs and clinicopathological findings which facilitate their identification. Many poisons, particularly arsenic, lead and salt, cause diarrhea in ruminants but there are usually additional diagnostic signs and evidence of access to the poison. Assay of feed and tissues helps to confirm a diagnosis of poisoning.
A diagnosis of peat scours is usually made if there is an immediate response to oral dosing with a copper salt.
Falling disease occurs only in adult cattle and must be differentiated from other causes of sudden death. Poisoning by the gidgee tree (Acacia Georginae) produces a similar syndrome in cattle.
Unthriftiness and abnormal wool or hair as a flock or herd problem are characteristic of copper deficiency in sheep and goats, which must be differentiated from protein-energy malnutrition, intestinal parasitism, cobalt deficiency, and external parasites.
Lameness in a group of lambs several weeks of age must be differentiated from nutritional osteodystrophy due to a calcium, phosphorus and vitamin D deficiency or imbalance, stiff lamb disease due to enzootic muscular dystrophy.
Neonatal ataxia caused by congenital swayback and enzootic ataxia in newborn lambs and kids due to maternal copper deficiency must be differentiated from border disease of newborn lambs, characterized by an outbreak of newborn lambs with hairy fleece and tremors, cerebellar hypoplasia (daft lamb disease) and hypothermia.
TREATMENT
The treatment of copper deficiency is relatively simple, but if advanced lesions are already present in the nervous system or myocardium complete recovery will not occur. Oral dosing with 4 g of copper sulfate for calves from 2 to 6 months of age and 8–10 g for mature cattle given weekly for 3–5 weeks is recommended for the treatment of primary or secondary copper deficiency. Parenteral injections of copper glycinate may also be used and the dosages are given under control.
The diet of affected animals should also be supplemented with copper. Copper sulfate may be added to the mineral-salt mix at a level of 3–5% of the total mixture. A commonly recommended mixture for cattle is 50% calcium–phosphorus mineral supplement, 45% cobalt-iodized salt, and 3–5% copper sulfate. This mixture is offered free of choice or can be added to a complete diet at the rate of 1% of the total diet.
CONTROL
Dietary requirements
The minimum dietary requirement for copper for cattle is 10 mg copper/kg DM and 5 mg/kg DM for sheep.
The requirement necessary to prevent subclinical or clinical copper deficiency will depend on the presence of interfering substances such as molybdenum, sulfur, and iron in the diet and possibly the genotype of the animal. Copper sulfate is considered a better supplement than copper oxide or injectable copper for cattle consuming diets containing excess molybdenum or molybdenum plus sulfur. Although there is a marked difference between breeds of sheep in their susceptibility to hypocuprosis, this would not seem to have an immediate practical application. The estimated copper requirement in the diet of mature ponies is 3.5 mg/kg DM. The levels of copper in liver samples of 50% of cull ewes and 40% of market lambs were high to toxic indicating that monitoring the dietary levels of copper is essential.35
The copper requirements may also vary according between breeds within a species. Angus heifers have a lower minimal copper requirement than Simmental heifers.13 Based on liver copper, diets containing copper at 4.4 or 6.4 mg/kg DM, did not meet the requirement of either breed during gestation and lactation or growth. Supplementation of copper at 7 mg/kg DM to the control diets provided the requirements of both breeds.
There are significant differences in the copper requirements and tolerance between goats and sheep.12 The dietary copper requirements of goats are uncertain but may be higher than in sheep. Dietary levels of copper which could cause copper toxicity in sheep, do not cause toxicity in goats. Some limited data on growth performance indicates a stimulatory effect of 100–300 ppm copper in the diet of Nubian goats. Extra copper accumulated in liver and to a lesser extent in other tissues and was excreted through the biliary system and into the feces.
Supplementation of diets with copper of feedlot cattle under some circumstances can affect performance. As little as 20 mg/kg DM of supplemental copper can reduce performance in finishing steers.36 The addition of 10 or 20 mg/kg DM of supplemental copper to a high-concentrate diet containing 4.9 mg/kg DM alters lipid and cholesterol metabolism in steers but does not alter ruminal fermentation.37 Decreasing cholesterol and altering fatty acid composition (saturated to unsaturated) in beef produced for human consumption has potential health benefits.
Copper can be supplied by several different methods as outlined below. The dose rates given are those recommended for the control of primary copper deficiency and these may have to be increased or treatment given more frequently in some instances of secondary copper deficiency. In these circumstances it is often necessary to determine the most satisfactory dosing strategy by a field trial.
Copper toxicity
The incidence of copper toxicity in dairy cattle in the UK has increased recently.38 Apparent subclinical hepatopathy, with no clinical disease, due to excess copper intake in lactating dairy cattle has been described. On average, each cow received 963 mg copper daily from the mineral supplement alone. Calculation of the total dietary copper found that high producing cow had an estimated intake of 1325 mg copper daily, while each low producing cow received 1250 mg daily. The estimated copper requirements of the cows were 290 and 217 mg/cow per day, respectively. Thus oversupplementation of dairy cows with copper may be a significant problem in dairy herds without overt clinical signs of toxicity.
Copper sulfate
Oral dosing
Oral dosing with copper sulfate (5 g to cattle, 1.0 g to sheep, weekly) is adequate as prophylaxis and will prevent the occurrence of swayback in lambs if the ewes are dosed throughout pregnancy. Lambs can be protected after birth by dosing with 35 mg of copper sulfate every 2 weeks. However, regular oral dosing with copper sulfate is laborious and time-consuming and is no longer widely practiced.
Dietary supplementation
The copper sulfate may be mixed with other minerals into a mineral premix, which is then incorporated into the concentrate part of the ration. The final concentration of copper is usually adjusted to provide an overall intake of at least 10 ppm of copper in the DM of the final ration. If the forage components of the ration contain much less than 10 ppm, the concentrate part of the ration may need to contain much larger concentrations of copper. Where a secondary copper deficiency is due to molybdenum in the forage, up to 1200 mg copper (approximately 5 g of hydrated copper sulfate) is added to the concentrate daily. When sheep are grazing toxic lupin stubble, the signs of lupinosis may be exacerbated by the supplementation of only 10 mg copper/kg DM as copper sulfate and therefore the supply of copper in the absence of suitable amounts of molybdenum and sulfur should be kept to a minimum.39
If animals are not receiving concentrates containing copper, an alternative is to provide free access to a mineral mixture or salt-lick containing 0.25–0.5% of copper sulfate for sheep and 2% for cattle, which will supply sufficient copper provided an adequate intake of the mixture is assured. The mineral mixture usually contains iodized salt, cobalt, calcium, phosphorus, and other trace minerals.
In some deficient areas, an effective method of administering copper is by the annual top-dressing of pasture with 10 kg/ha copper sulfate, although the amount required may vary widely with the soil type and the rainfall. Top-dressing may cause copper poisoning if livestock are turned onto pasture while the copper salt is still adherent to the leaves. Treated pasture should be left unstocked for 3 weeks or until the first heavy rain. It is also possible that chronic copper poisoning may result if the copper status of the soil increases sufficiently over a number of years.
Top-dressing grazing pastures of farmed red deer was compared with oral administration of copper oxide wire particles to some deer.40 Top-dressing pastures with copper sulfate at a rate of 12 kg/ha, but not 6 kg/ha, in mid-March was effective increasing the copper status of weanling hinds; while pastures top dressed the 12 kg/ha copper sulfate in mid-March and dosing hinds with 10 g copper oxide in late July were effective in increasing the copper status of pregnant hinds and in the case of the yearling hinds, significantly improved the copper status of their progeny from birth to weaning.
Drinking water supplementation.
Addition of copper salts to drinking water is usually impractical because the solution corrodes metal piping and maintenance of the correct concentration of copper in large bodies of water is difficult. However, if the need is great, some way around these difficulties can usually be found and a system has been devised for automatic supplementation for short periods via the drinking water and has been effective in controlling copper deficiency in cattle. Copper pellets which provide 2–3 mg copper/L of water have been recommended for cattle. Calves can tolerate copper in milk replacers at a concentration of 50 ppm but there is no advantage in providing more than 10 ppm.
Molasses based mineral supplements.
Copper can be provided in molasses-based supplements. However, the high sulfur concentrations in the molasses may affect the availability, through the formation ruminal thiomolybdates and result in lower liver copper concentrations. A dietary copper concentration greater than 10 ppm may be necessary to ensure absorption in beef cattle fed molasses-based supplements.41
Removal of sulfates
The removal of sulfates from drinking water by water purification, using a process of reverse osmosis, may have a positive effect on the copper status of beef cows. Cows drinking desulfated water had an increased availability of copper compared with those drinking water containing a large concentration of sulfates.
Parenteral injections of copper
To overcome the difficulty of frequent individual dosing or top-dressing of pasture, periodic parenteral injection of copper compounds that release copper gradually has given good results. They can be given at strategic times depending on the circumstances. They also have the advantage of avoiding fixation of copper by molybdenum in the alimentary tract. Injectable preparations of copper are now the method of choice for the prevention of swayback in lambs. The following have been evaluated under field conditions:
• Copper calcium ethylenediamine tetra-acetate (copper calcium edetate)
The criteria used to judge these injections are minimal damage at the site of injections, satisfactory liver storage (90–100%) of the administered dose and a safe margin between therapeutic and toxic doses. The dose of copper in any of the compounds for cattle is 400 mg and for sheep 150 mg.
Copper heptonate at the rate of 25 mg of copper in 2 mL of preparation given by IM injection to ewes in mid-pregnancy was successful in preventing swayback in lambs. The IM injection of 1 or 2 mg Cu/kg BW as copper heptonate does not result in any signs of toxicity such as weakness, lethargy, or icterus. The copper is lost from the injection site within 7 days and most is transferred to the liver with little or no deposition in the skeletal muscle. The higher dose raised mean liver copper values to within the range of 13–52 mmol/kg DM, which is associated with copper toxicity.
In sheep on pasture of high molybdenum content, a single IM injection of copper heptonate providing 37.5 mg copper to adults or 25 mg copper to weaners increases the liver copper reserves for at least 9 and 3 months, respectively and is considered an acceptable alternative to copper oxide wire particles for preventing copper deficiency in sheep in southern Australia.42
Copper calcium edetate has the advantage of giving maximum copper storage very quickly – 1 week after injection – and blood levels are elevated within a few hours.
Because of the rapidity of the absorption, toxic effects can be encountered unless proper dose levels are observed. As well as deaths from serious overdosing, some deaths occur in groups of sheep for unexplained reasons. It is suggested that stress be minimized and simultaneous other therapy be avoided.
A marked local reaction occurs at the site of injection so that SC injection is preferable in animals to be used for meat, although to avoid an unsightly blemish, breeding animals should receive an IM injection. The injections are a small risk for precipitating blackleg in cattle on farms where this disease occurs. For sheep, a single injection of 45 mg of copper as copper glycinate in mid-pregnancy is sufficient to prevent swayback in the lambs.
The SC injection of copper calcium edetate or copper oxyquinoline sulfonate into sheep results in a rapid increase in the concentration of copper in whole blood, serum, and urine within the first 24 h. Following the injection of copper methionate, the concentration of copper in blood and serum rises steadily over a period of 10 days and there is no detectable increase in urinary copper. After the injection of any of the three compounds, there is a steady increase in serum ceruloplasmin activity over a period of 10–20 days, followed by a slow fall to preinjection activity by 40 days. The lower toxicity of copper injected as methionate compared with that as copper calcium edetate or copper oxyquinoline sulfonate is due to the slower absorption and transport of the copper to the liver and kidney. Death has occurred in sheep following the parenteral administration of diethylamine oxyquinoline sulfonate at recommended doses. Affected sheep manifested signs of hepatic encephalopathy and at necropsy, there was acute, severe, generalized, centrilobular hepatocellular necrosis. The use of copper disodium edetate at recommended doses in calves has also resulted in deaths associated with liver necrosis and clinical signs of hepatic encephalopathy.
Injectable copper glycinate is an excellent source of supplementary copper for increasing the concentration of copper in the serum of copper-deficient cattle and maintaining grazing cattle in an adequate copper status. One dose of copper glycinate will maintain adequate copper levels for about 60–90 days. The recommended dose in beef herds is 120 mg of copper for adult cattle and 60 mg of copper for calves. A supplemental source of copper is required for the calf during the pasture season because milk is a poor source of copper, particularly from copper-deficient cows and calves do not have the opportunity to increase or maintain body stores of copper while grazing. When the dam is severely hypocupremic in the spring, the calf is also severely hypocupremic or copper-deficient. Insufficient copper is secreted into the milk of copper-treated cows. Therefore, where the dam has not received an adequate copper intake during pregnancy, direct treatment of the calf will be required in early life. The copper reserves of newborn calves are increased in fetal liver at the expense of copper stores in the dam’s liver, which are dependent on the availability of dietary or supplemental copper to the dam. Calves usually have sufficient liver copper at birth and do not need an injection of 50 mg until they are 6 weeks old. Because of the higher requirements for copper during the last trimester of pregnancy (demands of the fetal liver), a program of copper supplementation should involve the use of copper supplements, throughout the year as required.
One dose of copper glycinate is sufficient when cattle are grazing forage that contains no more than 3 mg/kg DM of molybdenum and 3 g/kg DM of sulfur. With higher levels of molybdenum and sulfur, repeated injections of copper glycinate are recommended. The injectable copper may be supplemented by the use of copper sulfate in a mineral supplement at a level of 1%. The inclusion of copper sulfate in the mineral supplement may be adequate for cows, but the calves may not consume an adequate amount of mineral and injectable copper. The level of supplementation required to prevent a drop in serum copper over the pasture season will depend upon the concentration of dietary molybdenum and sulfur and their effect upon the coefficient of absorption of copper.
Injectable copper complex compounds have been evaluated as supplementary copper for grazing beef cattle under Canadian conditions. Copper edetate at 100 mg of copper, copper glycinate at 120 mg, and copper methionate at 120 mg were used and were equally effective in improving copper status of copper-deficient cattle and maintaining them in an adequate copper status for 90 days. The copper methionate was least acceptable because of the incidence and severity of reactions at the site of injection.
The use of injectable copper edetate in horses has been investigated as a method of increasing liver copper in foals at birth to reduce the incidence and severity of developmental bone and joint disease in newborn foals. The administration of 100 mg and 250 mg copper edetate IM to mares during the 9th and 10th months of gestation had no effect on the liver concentration of their foals at birth.43
Copper phenylalanine in a single injection to dairy cows in Uruguay maintained serum copper levels at an adequate level for at least 100 days.44
Controlled-release glass
Death due to poisoning is one of the dangers of parenteral administration because it is difficult to control the rate at which the supplement releases the copper, especially if the controlling mechanism is chemical binding. Methods used to control the release include the development of soluble controlled-release glass for oral administration to sheep and cattle. The copper is slowly released, absorbed, and stored in the liver. Initial field evaluations indicate that the boluses may not contain sufficient copper to maintain normal levels of copper for a sufficient length of time compared to the use of copper oxide needles.
Boluses of a soluble copper-containing controlled-release glass have been developed and evaluated. The boluses are based on a phosphate-type glass into which appropriate quantities of trace elements are incorporated. The boluses lodge in the rumen and release copper at a slow rate. They can provide additional supplies of copper to ruminants at an almost uniform rate for many months. One commercial product contains selenium and cobalt and in one experiment increased ceruloplasmin activity for at least 1 year. In one field study, the administration of two commercial soluble glass boluses containing copper and selenium, the selenium levels were increased from marginal to adequate, but adequate copper levels were not maintained.
A soluble glass bolus containing copper, cobalt, and selenium given to extensively grazed sheep in the North East of Scotland, under two different situations: lowland finishing lambs and upland sheep in their non-productive year between being a lamb and being a productive ewe (gimmer) was able to prevent or correct deficient and/or marginal cobalt and selenium status of sheep throughout the trial period. The bolus has little measured effect on the already adequate blood indicators of copper status, although the liver copper concentrations of the bolused sheep were higher.45
Copper oxide needles
Copper oxide needles or wire particles (fragments of oxidized copper wire up to 8 mm in length and 0.5 mm in diameter) are used for oral dosing and one of the most effective and safest methods for the control of copper deficiency in ruminants. Its major advantages are prolonged effectiveness and low cost. A single treatment can be effective for an entire summer or winter season. The needles are retained in the forestomachs and abomasum for up to 100 days or more and the copper is slowly released, absorbed and stored in the liver.
Sheep
A dose of 0.1 g/kg live weight (5 g) in sheep is safe and does not induce copper toxicity in the susceptible North Ronaldsay breed. The response in liver copper concentrations is dose-dependent. In sheep given doses ranging from 2.5 to 20 g per animal, the liver copper concentrations will peak 10 weeks after administration and will thereafter decline in a linear fashion over the next 40 weeks.
The administration of a single dose of 2 g cupric oxide needles orally to lambs between 3 and 5 weeks of age is an effective method for the prevention of induced hypocuprosis manifested as ill-thrift in lambs grazing pastures improved by liming and reseeding. The treatment maintained the lambs in normocupremia, provided adequate liver copper reserves, prevented clinical signs of hypocuprosis and produced a live weight gain advantage. The administration of the needles to ewes in the first half of pregnancy is also effective for the prevention of swayback in their lambs. The administration of cupric oxide needles to ewes at parturition is effective in preventing hypocupremia for up to 17 weeks in animals on pasture previously shown to cause a molybdenum-sulfur-induced copper deficiency. The treatment of the ewes at parturition also resulted in higher concentrations of copper in the milk in the initial weeks of lactation.
However, this increase in milk copper will not be effective in preventing hypocupremia and hypocuprosis in the lambs, which can be treated with cupric oxide needles at 6 weeks of age. Because some breeds of sheep may have a propensity to concentrate excess quantities of copper in the liver, it is important to adhere to the recommended dosage. Cupric oxide needles at a dose of 4 g per animal have also been used for the prevention of swayback in goats and to maintain liver copper levels for up to 5 months in farmed red deer grazing on a marginally copper-deficient pasture.
Copper oxide needles given to ewes in early pregnancy increases their liver copper status through gestation and early lactation and the copper status of their lambs from birth to 36 days old.46 Serum copper concentration was not affected by treatment but a marked rise was observed in all lambs between birth and 10 weeks of age.
Cattle
A single dose of 20 g of copper oxide needles to hypocupremic suckler cows was sufficient to maintain adequate copper status for at least 5 months. The use of 20 g of copper oxide needles to young cattle weighing 190 kg effectively prevented growth retardation and severe hypocupremia, which occurred in an undosed control over a 70-day trial period. The currently recommended doses for beef cattle are 5 g for calves, 10 g for yearlings and 20 g for heavier or adult cattle, which will give protection for at least 6 months. A single oral dose of 20 g of copper oxide needles at the beginning of the grazing season is effective in increasing or maintaining stores of copper in the liver of grazing cows and calves consuming low-copper, high-molybdenum forage, and high-sulfate water supplies. The use of 50 g of needles in adult cows (55 kg BW) sustained higher levels of plasma concentrations than the SC injection of copper glycinate and 100, 200, or 300 g of needles given orally did not cause clinical effects.
Farmed red deer
The administration of 20 g boluses of copper-oxide wire particles to rising 2-year-old red deer stags did not significantly alter velvet antler weight, daily velvet antler growth rate, days from casting to removal, grade or value, or stag live weight gain.47 Copper supplementation increased mean serum ceruloplasmin (ferroxidase) concentrations by approximately 10 IU/L. Mean liver copper concentrations in control deer was 99 μmol/kg and ranged from 194 to 386 μmol/kg in the treated groups.
Field trials on New Zealand deer farms using 5 g of copper oxide wire particles given to deer 4–7 months old found no effect on live weight gain despite evidence of hypocupremia in 38% of non-supplemented animals, which gained weight at similar rates to those which had adequate plasma copper levels.48 This suggests that the extent of the hypocupremia was either not sufficiently severe, or not maintained for a long enough period to cause copper deficiency resulting in live weight gain. This indicates that deer farmers need to reassess the need for copper supplementation in young deer.
Copper oxide powder
Copper oxide powder administered in the form of experimental, sustained-release rumen boluses significantly increased blood and liver copper concentrations in growing sheep, in out-wintered suckler cows during late pregnancy and early lactation and in growing cattle at grass in the summer periods over periods of at least 170 and 123 days, respectively.
Genetic selection
It is now possible to manipulate trace element metabolism by genetic selection in farm animals. Within a period of 5 years, selection of sheep based on plasma concentration of copper resulted in two divergent sets of progeny, one with a high level of copper status, the other with a low level, which resulted in clinical manifestations of copper deficiency in the low level and protection in the high level.
General guidelines
Several rules of thumb are important and useful.
• A dietary intake of copper equivalent to 10 mg/kg DM will prevent the occurrence of primary copper deficiency in both sheep and cattle
• Diets containing less than 5 mg/kg DM will cause hypocuprosis
• Diets with copper:molybdenum ratios of less than 5:1 are conducive to conditioned (secondary) hypocuprosis
• The newborn calf is protected against neonatal hypocuprosis by donations from the dam, but newborn lambs assume the same copper status as the ewe
• Cattle are more susceptible to copper deficiency than are sheep.
Underwood EJ, Suttle NF. Copper. In The mineral nutrition of livestock, 3rd ed., Wallingford, Oxon: CAB International; 1999:283-342.
Lee J, Masters DG, White CL, Grace ND, Judson GJ. Current issues in trace element nutrition of grazing livestock in Australia and New Zealand. Aust J Agric Res. 1999;50:1341-1364.
Frank A. A review of the ‘Mysterious’ wasting disease in Swedish Moose (Alces alces L) related to molybdenosis and disturbances in copper metabolism. Biol Trace Element Res. 2004;102:143-159.
Spears JW. Micronutrients and immune function in cattle. Proc Nutr Soc. 2000;59:587-594.
Mattioli GA, et al. Characterization of cattle copper deficiency in the Magdalena district. Livestock Prod Sci. 1996;47:7.
Wildman REC, et al. Aspects of cardiomyopathy in copper-deficient pigs. Electrocardiography, echocardiography and ultrastructural findings. Biol Trace Element Res. 1996;55:55.
Judson GJ, Babidge PJ. An assessment of the safety of copper heptonate for parenteral therapy in sheep. Aust Vet J. 2004;82:75.
1 Suttle NF. Ann Rev Nutr. 1992;11:121.
2 Grace ND, et al. N Z Vet J. 2005;53:137.
3 Abba M, et al. Mutation Res. 2000;466:51.
4 Spears JW. Proc Nutr Soc. 2000;59:587.
5 Dargatz DA, et al. J Am Vet Med Assoc. 1999;215:1828.
6 Blakley BR, et al. Can Vet J. 1998;39:293.
7 Thompson KG, et al. N Z Vet J. 1994;42:137.
8 Gardner WC, et al. Can J Anim Sci. 2003;83:479.
9 Frank A. Biol Trace Element Res. 2004;102:143.
10 Custer TW, et al. Sci Total Environ. 2004;330:81.
11 Underwood EJ, Suttle NF. Copper. In The mineral nutrition of livestock, 3rd ed., Wallingford, Oxon: CAB International; 1999:283-342.
12 Solaiman SG, et al. Small Rum Res. 2001;41:127.
13 Mullis LA, et al. J Anim Sci. 2003;81:865.
14 Schonewille JT, et al. Vet Q. 1995;17:14.
15 Picco SJ, et al. Mutagenesis. 2004;19:453.
16 Picco SJ, et al. Mutation Res. 2001;498:1.
17 Hurtig M, et al. Equine Vet J. 1993;16(Suppl):66.
18 Hoyt ZK, et al. J Equine Vet Sci. 1995;15:357.
19 Cerone SI, et al. Biol Trace Element Res. 2000;73:269.
20 Minatel I, Carfagnini JC. Nutr Res. 2000;30:1519.
21 Fyffe JJ. Aust Vet J. 1996;73:188.
22 Ward JD, Spears JW. J Anim Sci. 1999;77:230.
23 Scalleti RW, et al. J Dairy Sci. 2003;86:1240.
24 Sharma MC, et al. Res Vet Sci. 2005;79:113.
25 McGreevy PD, et al. Appl Anim Behav Sci. 2001;71:119.
26 Laven RA, Livesey CT. Cattle Pract. 2004;13:55.
27 Tessman RK, et al. J Am Vet Med Assoc. 2001;218:756.
28 Minatel L, Carfagnini JC. Prev Vet Med. 2002;53:1.
29 Wentink GH, et al. Vet Rec. 1999;145:258.
30 Puschner B, et al. J Vet Diagn Invest. 2004;16:382.
31 Suttle NF, et al. Equine Vet J. 1996;28:497.
32 Grace ND, Wilson PR. N Z Vet J. 2002;50:252.
33 Wilson PR, Grace ND. N Z Vet J. 2001;49:126.
34 Suttle NF. Cattle Pract. 2003;11:161.
35 Menzies PI, et al. Can Vet J. 2003;44:898.
36 Engle TE, Spears JW. J Anim Sci. 2000;78:2446.
37 Engle TE, Spears JW. J Anim Sci. 2000;78:2452.
38 Laven RA, et al. Vet Rec. 2004;155:120.
39 White CL, et al. Aust J Agric Res. 1994;45:279.
40 Grace ND, et al. N Z Vet J. 2004;53:31.
41 Arthington JD, et al. J Anim Sci. 2003;81:1357.
42 Judson GJ, Babidge PJ. Aust Vet J. 2002;80:630.
43 Gee EK, et al. Aust Vet J. 2000;78:347.
44 Torre MH, et al. Livestock Prod Sci. 2005;95:45.
45 Kendall NR, et al. Livestock Prod Sci. 2005;95:45.
46 Grace ND, et al. N Z Vet J. 2004;52:189.