Chapter 34 Diseases associated with allergy
Alloimmune hemolytic anemia of the newborn (neonatal isoerythrolysis, isoimmune hemolytic anemia of the newborn)
Equine seasonal allergic dermatitis (queensland itch, sweet itch)
Recurrent airway obstruction (heaves)
Pasture-associated heaves (pasture-associated obstructive pulmonary disease of horses)
Enzootic nasal granuloma of cattle (eng, bovine atopic rhinitis)
ALLOIMMUNE HEMOLYTIC ANEMIA OF THE NEWBORN (NEONATAL ISOERYTHROLYSIS, ISOIMMUNE HEMOLYTIC ANEMIA OF THE NEWBORN)
Etiology Maternal alloantibodies to neonate’s blood group antigens are transferred to the neonate in colostrum and cause lysis of the neonate’s red blood cells.
Epidemiology Disease in progeny of multiparous mares or sows. The dam lacks blood group antigens possessed by the sire and inherited by the foal, calf, or piglet. The majority of cases in foals are due to the presence of Aa or Qa antigens and antibodies. Sows vaccinated with crystal violet vaccine or cows with babesia or anaplasmosis vaccines can be associated with disease in their newborns.
Clinical findings Lethargy, recumbency, tachycardia, tachypnea, icterus, and hemoglobinuria.
Clinical pathology Anemia, hyperbilirubinemia.
Diagnostic confirmation Positive antiglobulin (Coombs test) or hemolysis test using mare’s serum or colostrum and foal’s red blood cells.
Treatment Transfusion of blood or packed red blood cells from suitable donor, or of mare’s washed red blood cells. Supportive care.
Control Identify at-risk mares by blood typing. Examine mare’s serum or colostrum for presence of incompatible antibodies before allowing foal to suckle.
ETIOLOGY
Hemolytic anemia of newborn horse and mule foals, calves, and piglets occurs because of immune-mediated (antibody-dependent cellular cytotoxicity or type II hypersensitivity) destruction of the neonate’s red blood cells by antibodies acquired from the dam. The specific antibodies are present in the colostrum, absorbed by the neonate, and cause lysis and/or agglutination of red blood cells.
The disease is associated with the natural occurrence of inherited blood groups and only occurs if the dam is exposed to red blood cell antigens that she does not possess. In response to such exposure the dam produces antibodies directed against the foreign red blood cell epitopes. If the neonate possesses a blood type against which the dam has developed antibodies and depending on the red blood cell factor involved, red blood cell destruction can occur. The newborn acquires the red blood cell types that are foreign to the dam through inheritance from the sire. Disease occurs after birth because the fetus is not exposed to the antibodies in utero because of the epitheliochorial placentation in mares and sows and the syndesmochorial placentation of cattle. Antibodies in serum are secreted into the colostrum in the peripartum period and are subsequently ingested and absorbed by the newborn. Mares that have anti-red blood cell factor antibodies in their serum almost invariably have the same antibody in their colostrum.1 The serum concentration of anti-Aa and Qa antibodies is highest in the last 3 months of gestation and peaks about 1 week after parturition. For neonatal isoerythrolysis to occur:
• The fetus must have blood group antigens (factors) that the dam does not. These are inherited from the sire
• The dam must be exposed to the foreign blood group antigens that the fetus possesses
• The dam must produce antibodies against the blood group antigens of the fetus. Not all blood group antigens are highly immunogenic or are associated with disease in the neonate
• The newborn must ingest and absorb colostrum that contains antibodies directed against antigens on the newborn’s red blood cells.
The disease occurs naturally in foals and piglets but is usually iatrogenic in calves. Exposure of the dam to foreign red cell epitopes may occur at parturition or during gestation as a result of placental lesions although most incompatible pregnancies do not result in sensitization of the mare.
Mares may also be exposed by transfusion of incompatible blood. While whole blood transfusions to mares or fillies are unusual, plasma transfusions are increasingly being used to treat failure or partial failure of transfer of passive immunity to foals. Because plasma usually contains some red blood cells, transfusion of plasma from donors possessing blood group antigens that the foal does not could immunize the filly against these factors with the potential for disease when the foal matures and gives birth. However, most commercial plasma products are harvested from donors that do not have the blood group antigens identified as being problematic.
32 blood group antigens are recognized in horses:2
Neonatal isoerythrolysis is attributable in over 90% of cases to antibodies directed against either Aa or Qa antigens, although disease due to antibodies against Ab, Dc, Da, Db, Ka, Qb, Qc, Qrs, Pa, and Ua is reported.2,3 The presence in the mare of antibodies against Ca decreases the probability that she will develop anti-Aa antibodies.4
15 blood groups have been identified in pigs and the disease is recorded as occurring spontaneously associated with antibodies to the E group antigens. Historically, the main occurrence of neonatal isoerythrolysis in pigs was manmade and related to repeated vaccination against hog cholera using the pooled blood, inactivated by addition of crystal violet, of affected pigs.
The disease has also occurred in calves whose dams had been vaccinated against babesiosis or anaplasmosis using a vaccine containing bovine blood.5 As a result of vaccination the dam develops lytic antibodies against sire antigens, usually of the A and F blood groups, and antibodies in colostrum cause acute hemolytic anemia in the calves.
EPIDEMIOLOGY
Horses and mules
From 1 to 2% of Thoroughbred and Standardbred mares have antibodies capable of causing neonatal isoerythrolysis.1 The incidence is partially related to the proportion of the mare population at risk. An at-risk mare is one that lacks either the Aa or Qa blood group factor. The proportion of mares that lack one or both of these factors is breed dependent, with 19% of Thoroughbreds lacking either Aa or Qa antigens and 17% of Standardbred mares lacking Aa antigens.1,6 All Standardbred horses lack the Qa factor; neonatal isoerythrolysis in this breed is usually due to antibodies against the Aa factor, with 10% of Standardbred mares having anti-Aa antibodies.1 Only 2% of Thoroughbred mares lack the Aa antigen but this low proportion is important because approximately 50% of these mares will develop anti-Aa antibodies.2 Conversely, 16% of Thoroughbred mares lack the Qa antigen, but only 3% of these mares have the anti-Qa antibody.2 The risk of mares developing antibodies against certain red blood cell types is also related to the prevalence of the antigen in the horse population. For instance, Standardbred mares lack the Qa antigen, but because this antigen is not found in the breed any Standardbred stallion the mare is mated to will not be Qa positive, neither will the foal, and there is no risk of the mare being exposed to the antigen.
The incidence of neonatal isoerythrolysis in mule foals (donkey sire X horse dam) can be 10% and is attributable to the universal presence of a unique blood group antigen, ‘donkey factor’, in jacks and mule foals.7,8 Mares do not possess this factor and therefore all donkey sire X horse dam pregnancies are incompatible.7 Progeny of horse sire X donkey dam matings are not affected.
A feature of naturally occurring neonatal erythrolysis in foals and piglets is that it rarely if ever occurs in offspring of primiparous dams because the induced anti-red blood cell antibody titer is not sufficiently high to induce the disease. Subsequent exposure during later pregnancies elicits an anamnestic response that results in higher anti-RBC antibodies, and disease in the newborn. However, first pregnancy offspring are often affected after vaccination of the dam with blood products.
PATHOGENESIS
The interaction between the antibody and the red cells of the newborn is followed by hemolysis with resultant anemia, hemoglobinuria, and jaundice. Following ingestion and absorption into the systemic circulation, antibodies bind to red blood cell membranes. Parts of the cell membrane of the antibody coated cells are removed from the circulation, probably by the spleen and associated reticulo-endothelial tissues, and affected cells are eventually lysed and release hemoglobin into the circulation. The affected animal develops normovolemic anemia and, if the destruction of red blood cells is sufficient, develops anemic hypoxia and dies. The reaction between red blood cells and antibodies occurs sufficiently quickly that the bone marrow is unable to compensate immediately for the loss of red blood cells. Disseminated intravascular coagulation (DIC) occurs and may contribute to the death.
Permeability of the intestine of the newborn foal to antibody disappears by 36 hours and in most cases much less. Hourly milking of the mare rapidly reduces the antibody content of the colostrum. The duration of the alimentary permeability in piglets has not been determined.
CLINICAL FINDINGS
Horses and mules
Pregnancy and parturition are uneventful and the foal is normal for some hours after birth. Signs appear only if the foal ingests and absorbs colostrum containing anti-red blood cell factor antibody. The severity of disease ranges from clinically inapparent to fulminant with death ensuing soon after birth.
Peracute cases develop within 8–36 hours of birth, and the first indication of the disease may be collapse. Severe hemoglobinuria and pallor are evident but icterus is not apparent initially. The mortality rate is high.
In acute cases signs do not develop until 2–4 days after birth and jaundice is marked, with only moderate pallor and hemoglobinuria.
Subacute cases may not show signs until 4–5 days after birth. Jaundice is marked, but there is no hemoglobinuria and only mild pallor of mucosae. Many subacute cases recover without treatment.
The severity of the disease is related to the type and quantity of antibody ingested. Antibodies against Aa usually produce severe disease apparent within 24 hours of birth, while ingestion of anti-Qa antibodies causes a milder disease apparent at 3–4 days after birth.
General signs include lassitude, weakness, and disinclination to suck. The foal lies down in sternal recumbency for long periods and yawns frequently. There is no febrile reaction but the heart rate is increased up to 120/min. Respiration is normal until severe anemia develops when tachypnea (respiratory rate up to 80/min) and yawning are observed. Terminally, dyspnea and convulsions may develop. Peripheral edema does not occur and there are no signs of involvement of the central nervous system. Bilirubin encephalopathy or kernicterus is a rare complication of neonatal isoerythrolysis. It is apparent as altered mentation and seizures in foals with high serum bilirubin concentration.9
Isoimmune thrombocytopenia of foals and mules may be evident as ecchymotic hemorrhages and a tendency to bleed from relatively minor wounds. A syndrome in foals characterized by ulcerative dermatitis, neutropenia, and thrombocytopenia appears to be related to ingestion of colostral antibodies.10 Affected foals have oral and lingual ulcers, and crusting and erythema around the eyes, muzzle, perineum, trunk, and neck. There are ecchymotic and petechial hemorrhages in mucus membranes. Treatment with corticosteroids and antibiotics is associated with a good prognosis.
Pigs
Piglets show essentially the same syndrome, being normal at birth but developing jaundice at 24 hours and weakness at 48 hours, with most affected pigs dying by the 5th day. Peracute cases occur and piglets may die within 12 hours of birth, showing acute anemia but no jaundice or hemoglobinuria. A proportion of subclinical cases also occurs in which hemolysis can be detected only by hematological examination. Isoimmune thrombocytopenic purpura of piglets may manifest as increased bleeding following routine management procedures such as tail docking.11
Cattle
In calves clinical signs develop within 24–48 hours after birth and the calves die during the first week of life. Surviving calves are returned to normal health in 2–3 weeks. Peracute cases die within 24 hours, and at necropsy examination are characterized by pulmonary edema and splenomegaly.
CLINICAL PATHOLOGY
Hematological examination reveals acute anemia; erythrocyte counts, packed cell volumes, and hemoglobin concentrations are low and there is greatly increased erythrocyte fragility and sedimentation rate. Depending on the severity of the disease and its duration, there can be leukocytosis, attributable to neutrophilia and monocytosis, and the presence of nucleated red blood cells (in piglets and calves but rarely in foals). Affected mule foals, but not horse foals, are often thrombocytopenic.8 Isoimmune thrombocytopenia occurs rarely in foals and is not associated with neonatal isoerythrolysis, as it is in mule foals. In piglets, the erythrocyte count may be as low as 1 million/μL, the hemoglobin level below 2 g/dL, and thrombocytopenia is present. Serum biochemical analysis reveals an increased serum concentration of unconjugated bilirubin.
Diagnostic confirmation is achieved by demonstration of the presence of antibodies in the mare’s serum or colostrum that cause hemagglutination or lysis of foal red blood cells.11 Tests to demonstrate hemagglutination or lysis of foal red blood cells exposed to mare serum or colostrum have been developed. Of these, the standard hemolysis test appears to have the greatest utility.12 However, for practical purposes a positive direct antiglobulin test (direct Coombs’ test), confirming the presence of antibodies on the surface of red blood cells in a foal with anemia, provides a diagnosis of neonatal isoerythrolysis. False negative (foal has the disease but the Coombs’ test is negative) results occur occasionally because of the hemolytic nature of the antibodies. The same principle is applicable to all species. Detection of antibodies on the surface of the neonate’s red blood cells is possible using direct immunofluorescence flow cytometry.13 The test identifies the presence of antibodies on red cells in some instances when the Coombs’ test is negative.
The use of blood typing and other prepartum predictive tests in the prevention of the disease are discussed under control.
NECROPSY FINDINGS
In peracutely affected foals, there is marked pallor but only slight jaundice. The liver may be mildly swollen and friable but the spleen is greatly enlarged and is almost black due to the accumulation of lysed and lysing erythrocytes. In less severe cases jaundice is marked but pallor is only moderate in degree. The kidneys are usually pale and the urine is dark brown. The histopathological changes may include ischemic tubular nephrosis and periacinar hepatic necrosis and degeneration. Erythrophagocytosis is prominent and depending on the clinical course and therapeutic regime, there may be widespread hemosiderin deposition.
Hemoglobinuria is an important sign in piglets, and jaundice or port wine coloration of tissues occur constantly. The presence of blood-stained peritoneal fluid and an enlarged spleen is also typical of the disease in piglets.
TREATMENT
• Prevent the deleterious effects of anemia
• Prevent or treat hemoglobinuric nephrosis
• Prevent ingestion of further colostrum
• Prevent secondary infection in severely ill animals
The treatment of choice for neonatal isoerythrolysis depends on the severity of the disease. The choice of treatment should be based first and foremost on the severity of the clinical signs and secondarily on the hematocrit and red blood cell count. Foals or piglets with mild clinical signs (minimal lethargy, mild tachycardia, slight exercise intolerance) need only protection from environmental and nutritional stresses in order to recover. However, such animals should be carefully monitored to insure that their clinical condition does not worsen.
Severely affected animals need a transfusion of compatible blood to alleviate the anemia and intravenous fluids to insure adequate urine flow and minimize the risk of hemoglobin-nephrosis. In general, the younger the animal at the time the disease is evident, the more severe the disease and the more likely the need for intensive treatment. See Chapter 9 for a discussion of ‘transfusion triggers’.
Foals
Transfusion
Transfusion of an adequate quantity of whole blood or packed red blood cells results in dramatic resolution of clinical signs and anemia. The decision to transfuse blood should be based on the foal’s clinical condition, and not solely on the presence of a low hematocrit or red blood cell count (see Blood transfusion, in Chapter 9). In general, foals that are tachycardic, tachypneic, unable or reluctant to suck, have severe exercise intolerance or are unable to stand should receive blood. These foals will usually have a hematocrit less than 15% (0.15 L/L). Recumbent foals usually have a hematocrit less than 10% (0.10 L/L). Foals that are mildly tachycardic and tachypneic but are able to suckle vigorously and keep up with the mare generally have hematocrits above 15% (0.15 L/L) and do not require transfusion of red blood cells. The hematocrit should be monitored and foals in which the hematocrit is declining rapidly will likely require transfusion of blood or packed red cells.
The volume of blood transfused depends on the clinical condition of the foal and the progression of the anemia. Foals often require transfusion of 1–4 L (20–100 mL/kg body weight) of whole blood or 500 mL (approximately 10 mL/kg body weight) of packed red blood cells, and might require more than one transfusion. Blood should be administered slowly, 1 L/hour, and the foal’s condition monitored closely during the infusion. Packed red cells are preferred for transfusion because of the small volume administered. Transfusion of large quantities of blood should be performed slowly because of the risk of fluid overload of the circulatory system. The half-life for mare erythrocytes transfused into foals is about 5 days.14
The optimal donor is a horse that does not have Aa and Qa blood group factors nor anti-Aa and Qa alloantibodies. The former should not be present as the maternal antibodies against Aa or Qa in the recipient foal’s plasma will destroy the transfused cells. Similarly, donor antibodies against Aa or Qa will cause further lysis of foal red blood cells. Such donors must be identified in advance, because of the time required for the blood type testing, and are only likely to be available on large breeding farms or in specialized veterinary hospitals.
An ideal source of red blood cells is the dam because the maternal alloantibodies in the foal’s plasma will not react with the mare’s red blood cells. However, whole blood transfusions from the dam are contraindicated because of the presence of alloantibodies in the dam’s plasma. This problem can be avoided by transfusing only the mare’s washed red blood cells. Blood is collected from the mare (up to 25 mL/kg) into acid citrate dextrose or sodium citrate (10 mL of a 3.8% solution per 90 mL of blood). The mare’s red cells are then washed by removing the plasma, resuspending the cells in isotonic (0.9%) saline, thorough mixing, and subsequent removal of the saline. Plasma and red cells can be separated by large volume centrifugation or sedimentation. Adequate separation of red cells and plasma occurs by sedimentation within 1–2 hours if the blood is undisturbed.
If an ideal blood-typed donor is not available and the mare’s red cells cannot be washed in time, or are unavailable, then a donor should be chosen based on routine cross-matching. The sire will not be a suitable donor, since the antigens against which the mare’s antibodies are directed were inherited from him. A cross-match should match the foal’s (or dam’s) serum against the donor’s red blood cells, and the donor’s plasma against the foal’s red blood cells. The chance of finding a suitable donor is enhanced by selecting ponies or breeds other than Thoroughbreds, Standardbreds, and Arabians, because of the higher prevalence of Aa and Qa negative animals in these breeds.
Emergency support of severely affected foals can be achieved by administration of a solution containing polymerized bovine hemoglobin.15 This compound increases the hemoglobin concentration of blood thereby increasing oxygen carrying capacity. It is not a replacement for transfusion of blood or packed red cells, but is a useful bridging procedure while a donor is identified and blood collected. The recommended dose rate is 10–30 mL/kg administered slowly (10 mL/kg/h) intravenously. However, the cost of the compound might necessitate the use of lower doses (3–5 mL/kg).
Nutritional support
The foal should not be permitted to nurse the mare until it is >36 hours old. Therefore, nutritional support should provide approximately 100 kcal/kg per day in the form of mare’s milk (10 L per day per 50 kg foal), goat’s milk, or commercial mare’s milk substitutes. If the foal is more than 36 hours old, then it is highly unlikely that either the mare’s milk will still contain a significant quantity of antibodies or that the foal will be able to absorb them, and the foal should be allowed to continue to suckle the mare. In younger foals, an alternative feed should be supplied until the foal is at least 36 hours old. The mare should be milked out every 3–4 hours during this time to remove the colostrum.
The fluid, electrolyte, and acid–base status of moderately to severely ill foals should be assessed and corrected with intravenous administration of balanced polyionic fluids and sodium bicarbonate. Fluid administration should be used to insure an adequate flow of urine to prevent hemoglobinuric nephrosis.
Antibiotics
Broad-spectrum antibiotics should be administered to severely ill foals to prevent secondary infection (see Principles of providing care to the critically ill neonate.
Nursing care should be provided to minimize stress and prevent the development of complications such as pressure sores in recumbent foals.
Piglets
In pigs the prevention of sucking for periods of up to 24 hours does not prevent the disease. The safest procedure is to remove piglets from the sow, feed them artificially for 48 hours, and then return them to the sow. Frozen bovine colostrum collected as soon as possible after calving is a satisfactory substitute for sow colostrum but is improved by the addition of pig serum. When transfusion is necessary the intraperitoneal route is practical and safe.
CONTROL
The principles of control are:
• Identification of incompatible matings by blood group typing
• Identification of at-risk foals by testing of mare serum or colostrum for the presence of alloantibodies directed against blood factors possessed by the foal.
Blood group typing permits the identification of mares that are at risk of developing antibodies against Aa or Qa antigens. If an Aa or Qa negative mare is mated to a stallion that has Aa or Qa factors then there is the potential for neonatal isoerythrolysis. If the stallion is Aa and Qa negative, then there is no risk of the disease caused by antibodies to these blood groups.
Measurement of alloantibodies in the serum or colostrum of at-risk mares is useful in identifying mares at increased risk of having affected foals. Serum from at-risk mares is collected during the last month (preferably 3–5 weeks before expected parturition) of pregnancy and examined for the presence of antibodies against the blood of the sire or, if a sample of the sire’s blood is not available, a range of blood group factors including Aa and Qa. Mares that have such alloantibodies causing hemolysis at >1:16 are not permitted to suckle at risk newborn foals. If the titer is between 1:2 and 1:16, it is measured again 1–2 weeks before anticipated parturition to determine if the titer is rising, in which case the mare is likely carrying a foal with an incompatible blood group. Equine blood typing and detection of isoantibodies is performed by specialized laboratories in a number of countries:
• Australia – www.aegrc.uq.edu.au/services
• New Zealand – www.ivabs.massey.ac.nz/centres/centre_blood.asp
• USA – www.uky.edu/Agriculture/VetScience/textpages/epvrl.HTM
The jaundiced foal agglutination test (JFA) is useful in determining the compatibility of mare’s colostrum and foal’s red blood cells (Table 34.1).16,17 In this test foal red blood cells are added to serial dilutions (1:2 through 1:32) of colostrum and the presence of agglutination examined. Agglutination at dilutions of 1:16 in horses and 1:64 in mules are considered significant and the foal should not be permitted to receive the mare’s colostrum.1 The foal should be fed colostrum from another, compatible, mare or from a colostrum bank. The mare should be milked out every 2–4 hours until the JFA titer is less than 1:16 or for 36 hours, after which the concentration of antibodies in the milk is negligible,1 and the foal can be permitted to suckle its dam. It is critical to the successful use of this test that it is performed before the foal is permitted to suckle the mare. If an incompatibility is detected the foal should be fed colostrum from a mare that does not have a positive jaundiced foal agglutination test to the current foal’s red cells.
Table 34.1 Method for performing the jaundiced foal agglutination test13
Avoidance of vaccines based on whole blood or cellular parts of blood is recommended, and if they have to be used it should be as far away as possible from parturition and should be restricted to one injection and one booster.
1 Bailey E. Am J Vet Res. 1982;4:1917.
2 Bailey E, et al. Am Assoc Equine Pract Proc 33 Ann Conv. 1987:341-353.
3 MacLeay JM. J Am Vet Med Assoc. 2001;219:79.
4 Bailey E, et al. Am J Vet Res. 1988;49:1218.
5 Dowsett KF, et al. Aust Vet J. 1978;54:65.
6 Stormont C, et al. Cornell Vet. 1964;54:439.
7 McClure JJ, et al. Animal Genetics. 1994;25:119.
8 Traub-Dargatz JL, et al. J Am VetMed Assoc. 1995;206:67.
9 David JB, et al. Comp Cont Educ Pract Vet. April, 1998:517.
10 Perkins GA, et al. J Vet Int Med. 2005;19:211.
11 Dimmock CK, et al. Aust Vet J. 1982;59:157.
12 Becht JL, Page EH. Am Assoc Equine Pract Proc 25 Ann Conv. 1980:247.
13 Wilkerson MJ, et al. J Vet Int Med. 2000;14:190.
14 Smith JE, et al. J Vet Int Med. 1992;6:183.
PURPURA HEMORRHAGICA
Etiology Deposition of immune complexes in the walls of capillaries with subsequent vasculitis and extravasation of blood and plasma.
Epidemiology Sporadic disease of horses, and rarely cattle and pigs. The disease in horses is often associated with upper respiratory tract disease, especially Strep. equi infection.
Clinical signs Swellings of the head, limbs, and body. The swellings are usually asymmetrical, not painful on palpation and pit with gentle pressure. Tachycardia and tachypnea are characteristic. Petechial hemorrhages are present in mucosal surfaces. Skin of the limbs may slough.
Clinical pathology None specific. High anti-streptococcal in protein liter in serum thrombocytopenia is not present.
Diagnostic confirmation Clinical signs, skin biopsy.
Treatment Corticosteroids (dexamethasone) and antibiotics. Supportive care.
ETIOLOGY
The disease is acute and non-contagious. The cause of the vasculitis that characterizes purpura hemorrhagica is likely the deposition of complexes of antigen and immunoglobulin in the walls of capillaries and small blood vessels. The disease appears to be immune complex-mediated and due to a type III hypersensitivity reaction. The common association of the disease is with Strep. equi infection of the upper respiratory tract. The high concentrations of antibodies to Strep. equi M protein in affected horses, and the presence of complexes of IgA and streptococcal M protein in sera are evidence that the disease is associated with an immune reaction to streptococcal protein.1-3 The immune complexes are not found in the serum of horses recovering from Strep. equi infection that do not have purpura hemorrhagica. However, in many instances there is no history of streptococcal infection.4 There is a suggestion that the disease might be associated with an adverse reaction to therapeutic drugs. Vaccination using modified live Strep. equi M protein or killed Strep. equi vaccines is strongly suspected of inducing the disease.
EPIDEMIOLOGY
Purpura hemorrhagica is an uncommon, non-contagious, sporadic disease of horses. It has been recorded in pigs and cattle. Estimates of the incidence of the disease are uncommon and imprecise. There was an incidence of 27 cases in 1438 horses housed over a 3-year period in a Swedish Army remount facility.5 All cases of purpura hemorrhagica followed upper respiratory infections, of which 11 were typical of strangles. Only a small proportion of horses are affected but the incidence is highest when extensive outbreaks of strangles occur, possibly because of reinfection with streptococci of horses already sensitized by previous infection.
Of 53 horses with purpura hemorrhagic treated at a referral center, 17 had been exposed to or infected with Streptococcus equi, 5 had been vaccinated with Streptococcus equi M protein, 9 had been infected with Corynebacterium pseudotuberculosis, and 5 had a history of apparently infectious respiratory disease of undiagnosed cause. Fifteen of 53 horses had no history of recent infectious disease.4
There is a strong suspicion among clinicians of an association between purpura hemorrhagica and vaccination.6 However, vaccination against Strep. equi infection has not been clearly demonstrated to be a risk factor for purpura hemorrhagica. Certainly, cases of purpura do occur in horses that have been vaccinated against strangles, but presumably there was a high probability that such horses were at increased risk of developing strangles. The consensus is that vaccination with vaccines containing M protein or avirulent Strep. equi is associated with increased risk of purpura. Edema of the lower limbs does occur after vaccination with streptococcal M protein and may represent a mild form of the disease.7 It is recommended by some authorities that horses with high serum antibody titers to streptococcal M protein not be vaccinated against strangles,6 although definitive data to support this recommendation are not available.
There does not appear to be breed, age, or sex predisposition to the disease. Horses as young as 6 months of age, and possibly younger, can be affected.
The case fatality rate with appropriate treatment is approximately 10%.4 Purpura accounted for 2 and 8% of 2028 and 1245 deaths among horses shipped from Great Britain and the United States, respectively, to South Africa during the Boer War.8 This mortality rate was before the advent of antimicrobials or corticosteroids which have presumably decreased the case fatality rates.
PATHOGENESIS
The basis of the disease process is an aseptic vasculitis of capillary walls that is accompanied by extravasation of plasma and blood into the tissues. Thrombocytopenia does not occur, nor is there a defect in coagulation in most cases. Prolonged clotting times (activated clotting time, partial thrombin time, and thromboplastin time) occur in severely affected horses with infarctive purpura hemorrhagica.3 Skin lesions predominate but other organs, including the kidney, muscles, and gastrointestinal tract, are affected.3,9
CLINICAL FINDINGS
Affected horses are usually depressed and have reduced or absent appetite. The temperature is elevated in approximately 60% of cases,4 as is the heart rate. Extensive subcutaneous edematous swellings are the characteristic sign of the disease. They occur most commonly about the face and muzzle, but are often present on other parts of the body and are not necessarily symmetrical in distribution. The swellings may appear suddenly or develop gradually over several days. They are cold and painless, pit on pressure, and merge gradually into normal tissue without a definite line of demarcation. There is no discontinuity of the skin, although it may be tightly distended and even ooze red-tinged serum. Swellings about the head may cause pressure on the pharynx with subsequent dyspnea and dysphagia. Lesions in the lungs are usually not clinically apparent without radiographic or ultrasonographic examination of the chest. Extensive edema of the limbs occurs in almost all cases.4 Rare cases of the disease in horses do not have edema.10
Submucous hemorrhages occur in the nasal cavities and mouth, and petechiae may be present under the conjunctiva in over 80% of cases. Hemorrhage and edema of the gut wall may cause colic but in most cases there is no diarrhea or constipation. Severely affected skin, and especially that of the legs, may slough and leave granulating wounds.
Infarctive purpura hemorrhagica is an uncommon manifestation of the disease characterized by infarction of multiple tissues including the gastrointestinal tract and muscle.3 Affected horses have signs of colic and muscle swelling. The course is usually over 3–5 days and death, which is the most common outcome, is associated with severe colic and rapidly deteriorating metabolic status.
The course of the disease is usually 1–2 weeks and many animals die from blood loss, dyspnea due to laryngeal or pharyngeal swelling, and secondary bacterial infections. Relapses are uncommon among appropriately treated horses.
CLINICAL PATHOLOGY
There are no characteristic abnormalities detected on routine hematological or biochemical examinations of affected animals. Hematological changes are typically a mild anemia (usually <32% but >20%, <0.32 L/L but >0.20 L/L) with a neutrophilic leukocytosis and hyperfibrinogenemia. The platelet count is normal. Hypergammaglobulinemia can be present. There is an elevation in serum activity of creatine kinase (CK) and aspartate aminotransferase (AST) in affected horses, likely a result of muscle lesions, in approximately 25–30% of cases.4 Horses with infarctive purpura have marked elevations in serum activity of creatine kinase and aspartate aminotransferase, neutrophilia, and in severely affected horses, there is evidence of disseminated intravascular coagulation.3
Diagnostic confirmation is achieved by skin biopsy, especially of early lesions, and reveals leukocytoclastic vasculitis.7 Immunofluorescence staining of sections of skin may reveal the presence of antibodies, antigens, or complement in the walls of small blood vessels.
NECROPSY FINDINGS
Ecchymotic and petechial hemorrhages are present generally throughout the body. The subcutaneous swellings contain plasma which may be blood-stained, or sometimes whole blood. The lungs are edematous and congested. Histologically, the changes are also dominated by bland hemorrhage but a leucocytoclastic vasculitis is usually observed in scattered vessels. Sample of lung, muscle, and gastrointestinal tract, in addition to skin, should be examined via light microscopy to check for the presence of vasculitis.
Horses with infarctive purpura have dark red to black, multifocal coalescing hemorrhages in skeletal muscles.3 Hemorrhages also occur in the lungs and gastrointestinal tract. Histological examination reveals coagulative necrosis of muscle and other tissues. There is inflammation of the blood vessels.3
Horses
Causes of edematous swelling include:
• Equine viral arteritis and equine herpesvirus 1 or 4 infection, which do not have petechiation and are readily distinguished by their epidemiological characteristics and by serological testing
• Equine granulocytic anaplasmosis (ehrlichiosis) which can be differentiated by the presence of granular inclusions in the cytoplasm of neutrophils
• Congestive heart failure, which should be apparent on clinical examination
• Angioneurotic edema, which is not associated with petechiation
TREATMENT
The principles of treatment are to reduce inflammation of the blood vessels, remove the inciting cause, and provide supportive care. Because of the possibility that the disease is due to an adverse drug reaction, administration of any drugs that the horse is receiving at the time the disease develops should be discontinued.
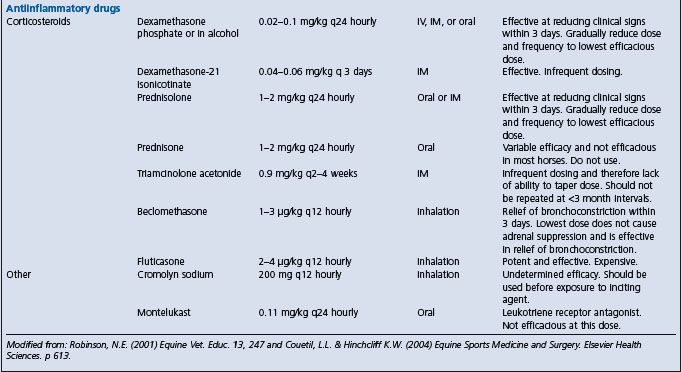
Reduction of inflammation of the blood vessels involves mitigation of the immune response and removal of the source of the antigenic stimulus. The immune response, and its associated inflammatory reaction in blood vessels, should be treated with corticosteroids such as dexamethasone (0.05–0.2 mg/kg, IV or IM every 24 hours) or prednisolone (0.5–1 mg/kg, IM or IV every 24 hours). Prednisolone might not be as effective as dexamethasone. The dose of corticosteroid can be gradually reduced as the clinical signs improve, and the drug can be given orally. Non-steroidal anti-inflammatory drugs (phenylbutazone 2.2 mg/kg orally or IV every 12 hours, or flunixin meglumine 1.1 mg/kg orally or IV every 12 hours) may reduce inflammation and provide some analgesia.
Removal of the source of the antigenic stimulus of the disease is difficult, especially in cases when an antecedent infection or disease is not readily identified. On the assumption that purpura hemorrhagica is often a sequela to Strep. equi infection, and the suspicion that occult Strep. equi infection is present and the source of antigen associated with the disease; affected horses are usually treated with penicillin (procaine penicillin, 20 000 IU/kg, IM every 12 hours, or potassium penicillin, 20000 IU/kg, IV every 6 hours) until the clinical signs resolve. Treatment with antibiotics might need to be continued for as long as 20 days.4
Supportive care includes bandaging of swollen limbs, care of wounds, hydrotherapy, and intravenous fluid administration. Swelling of the head and pharynx may necessitate placement of a nasogastric feeding tube to permit enteral feeding of dysphagic horses. Respiratory distress can develop very rapidly and emergency tracheotomy may be required to relieve respiratory distress and prevent asphyxiation.
CONTROL
There are no specific preventive measures. However, control and prevention of upper respiratory tract infections in horses should lead to a reduction in the incidence of purpura hemorrhagica. Careful consideration should be given to the use of vaccines containing streptococcal M protein or avirulent Strep. equi in horses at low risk of developing strangles. Although the relationship between M protein containing vaccines and purpura hemorrhagica is not definitive, circumstantial evidence and the opinion of authorities in the field support such an association. Measurement of serum antibodies to M protein might be useful in determining the need for vaccination of horses in endemic areas or at high risk.6 Horses with antibody titers >1:3200 should not be vaccinated.6
1 Galan JE, Timoney JF. J Immunol. 1985;135:3134.
2 Heath SE, et al. J Vet Intern Med. 1991;5:263.
3 Kaese HJ, et al. J Am Vet Med Assoc. 2005;226:1893.
4 Pusterla N, et al. Vet Rec. 2003;153:118.
5 Hofer B, et al. Proc 3rd Int Conf Equine Infect Dis 1973; 527.
6 Sweeney CR, et al. J Vet Int Med. 2005;19:123.
7 Morris DD. J Am Vet Med Assoc. 1987;191:460.
8 Smith F. A Veterinary History of the war in South Africa. London: H&W. Brown, 1919;265.
EQUINE SEASONAL ALLERGIC DERMATITIS (QUEENSLAND ITCH, SWEET ITCH)
This is an intensely pruritic dermatitis of horses caused by hypersensitivity to insect bites.
ETIOLOGY
The disease is caused by type I (immediate) hypersensitivity to salivary antigens introduced into the skin by the bites of sandflies and other insects. There may be a lesser role for type IV (cell-mediated) hypersensitivity in the disease. Culicoides brevitarsus is the cause in Australia,1 C. pulicaris in the United Kingdom and Europe,2 and C. obsoletus in Canada.3 Stomoxys calcitrans, the stable fly, and Simulium spp. cause the disease. The distribution of the skin lesions is related to the feeding habits of the inciting insect. For instance, C. pulicaris has a predilection for landing at the mane and tail, and this is where the lesion is most commonly seen.
EPIDEMIOLOGY
The prevalence of the disease varies depending on environmental factors, and possibly characteristics of the local horse population. Up to 60% of horses are reported affected in areas of Queensland, Australia, 22% in Israel, and 18% of Icelandic horses in Norway.4 The prevalence in Switzerland is very low in regions above 1000 m and 1.6% in lower areas.5
The disease is quite common worldwide in areas where hot and humid summer weather favors the causative insects: Sweden, the United Kingdom, Japan, Israel, Hong Kong, North America, Australia, the Philippines, India, and France. Most cases occur during summer and lesions disappear during cooler weather. Lesions disappear when the horses have been stabled in insect-proof barns for several weeks or are moved outside the geographical range of the inciting insect.
The disease is characteristically sporadic and affects only a few of a group of horses. However, because the predilection to the disease is likely inherited, there may be multiple cases among related animals on a farm.5,6 The prevalence of the disease increases with age; 3.4% of Icelandic horses 1–7 years of age compared to 32% of horses older than 14 years were affected.4
PATHOGENESIS
Reaginic antibodies (IgE) produced in response to exposure to proteins in insect saliva bind to mast cells in the skin and, when exposed to the antigen, are associated with degranulation of the mast cell. Horses with sweet itch have IgE antibodies that react with constituents of the salivary gland of Culicoides sp., whereas horses that do not have the disease have IgG, but not IgE, antibodies against Culicoides salivary gland antigens.6 Horses that have not been exposed to Culicoides sp. do not have either antibody to the insect salivary gland antigen.6 Degranulating mast cells and intradermal or subcutaneous lymphocytes release various vasoactive substances and cytokines that cause inflammation and accumulation of eosinophils in the skin of affected areas and eosinophilia.7 The distribution of the lesions on patients reflects the insects’ preferred feeding sites. Ponies with seasonal allergic dermatitis have greater numbers of circulating CD5+ and CD4+ T-lymphocytes than do normal animals.8 Increased numbers of CD3+ T-lymphocytes, most of which are CD4+, and eosinophils are present in the skin of affected ponies after injection of Culicoides antigen.8 Furthermore, eotaxin and monocyte chemoattractant protein (MCP) 1, but not MCP-2 or MCP-4, mRNA expression is upregulated in skin biopsies of sweet itch lesions, demonstrating a mechanism for accumulation of eosinophils and T-2 lymphocytes in the lesions.9
CLINICAL FINDINGS
Lesions are usually confined to the base of the tail, rump, along the back, withers, crest, poll, ears and, less commonly, ventral midline. In severe cases the lesions may extend down the sides of the body and neck and onto the face and legs.
Pruritis is intense, especially at night, and the horse scratches against any fixed object for hours at a time. In the early stages slight, discrete papules, with the hair standing erect, are observed. Constant scratching may cause self mutilation, severe inflammatory lesions, and loss of hair. Scaliness and loss of hair on the ears and tail-base may be the only lesions in mildly affected horses.
CLINICAL PATHOLOGY
Affected animals have eosinophilia and thrombocytosis.
Diagnosis is facilitated by skin biopsy, fungal culture, and parasitological examination of skin scrapings, and intradermal sensitivity testing. Skin biopsy of early lesions, before trauma masks the true picture, reveals edema, capillary engorgement, and eosinophilic and mononuclear perivascular infiltration. Fungal culture and parasitological examination of skin scrapings are useful only in that they rule out dermatophycosis, onchocerciasis, and strongyloidosis. Intradermal skin testing demonstrates immediate and delayed sensitivity reactions to extracts of Culicoides and Stomoxys spp.1
TREATMENT
The principles of treatment are removal of the inciting cause and suppression of the hypersensitivity reaction.
Removal of the inciting cause is achieved by preventing horses from being exposed to the inciting insects. This can be achieved by relocating the horse to a geographical region where the insects do not occur, stabling of the horse in an insect-proof stable during the periods of the day (early evening) when the insects are most active, or applying agents that kill the insect or otherwise prevent them from alighting on and biting the horse.
Suppression of the immediate hypersensitivity reaction or its sequelae can be achieved by administration of corticosteroids (prednisolone, 1 mg/kg every 24 hours initially then reducing to as low a maintenance dose as possible). Theoretically, hyposensitization may be effective, but the only controlled clinical trial to date did not demonstrate a beneficial effect, although the placebo effect on the owners was impressive.10
CONTROL
Prevention of the disease necessitates protection against sandfly bites by stabling in insect-proof quarters. Continuous spraying of the horses with insecticides or repellents may be of some value. A 4% permethrin pour-on gives effective protection. Most horses need only one application a week; others need an application every second day.11
1 Quinn PJ, Baker KP. Equine Vet J. 1983;15:266.
2 Anderson L, Bergman A. Acta Vet Scand. 1980;21:559.
3 Kleider N, Lees MJ. Aust Vet J. 1984;25:26.
4 Halldorsdottir S, Larsen HJ. Equine Vet J. 1991;23:296. 300
5 Marti E, et al. Equine Vet J. 1992;24:113.
6 Wilson AD, et al. Equine Vet J. 2001;33:707.
7 McKelvie J, et al. Res Vet Sci. 2001;70:115.
8 McKelvie J, et al. Equine Vet J. 1999;31:466.
9 Benarafa C, et al. Vet Rec. 2002;151:691.
SEASONAL ALLERGIC DERMATITIS
A disease similar to sweet itch of horses occurs in sheep in the UK.1 The lesions are similar to those in horses and are located principally on the teats, udder, and ventral midline, but also on the tips of the ears, around the eyes, and on the nose and the lips. Because of their appearance only in summer, and in senior ewes, and at a time when midges are plentiful enough to cause signs of insect worry, a cutaneous sensitivity to Culicoides spp., especially C. obsoletus, is suspected. Histologically the lesions represent the changes characteristic of immediate hypersensitivity. Cutaneous sensitivity to ground up Culicoides spp. was demonstrated. A very similar disease occurs in cattle in Japan. It is thought to be due to an allergy to the bite of an external parasite.2
RECURRENT AIRWAY OBSTRUCTION (HEAVES)
Etiology Inhalation of barn and feed dust containing inciting agents which can include particles of molds, endotoxin, mites, plant debris, and inorganic material.
Epidemiology Predominantly a disease of horses stabled in poorly ventilated barns and fed poor quality hay containing molds. Occurs worldwide but more commonly in the northern hemisphere. No breed or sex predilection.
Clinical signs Chronic cough, mucopurulent nasal discharge, poor athletic performance, increased respiratory rate, increased expiratory effort, wheezes on thoracic auscultation, and abundant mucopurulent material in the trachea on endoscopic examination.
Clinical pathology Neutrophilia in tracheal aspirate and bronchoalveolar lavage fluid.
Lesions Bronchiolitis with mononuclear cell infiltration, epithelial, and goblet cell hyperplasia, neutrophil accumulation in airway lumens, and alveolar hyperinflation.
Diagnostic confirmation Clinical signs, examination of bronchoalveolar lavage fluid, and the response to treatment.
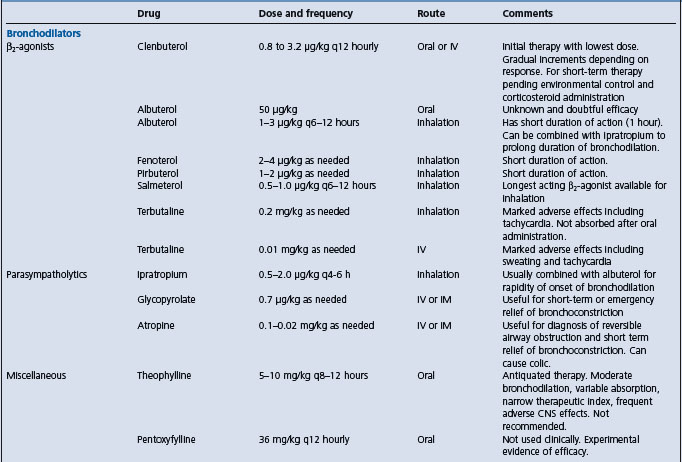
Treatment Remove the inciting cause by providing a dust-free environment, and administer corticosteroids. Bronchodilators are useful for treatment of acute bronchoconstriction.
Control Prevent exposure to inciting cause. Insure optimal air quality in stables or maintain horses at pasture.
Heaves is a recurrent or chronic disease of stabled adult horses (previously known as chronic distructive pulmonary disease) characterized by neutrophilic airway inflammation and airway obstruction manifest clinically by the presence of coughing, excess mucus accumulation in airways, tachypnea and increased respiratory effort, and exercise intolerance. It should be differentiated from the usually transient inflammatory airway disease of young adult horses in which there is no significant impairment of pulmonary function. The disease is included in this section because it is classically considered to have an allergic component, although this assumption is increasingly questioned.
ETIOLOGY
Heaves, previously referred to as chronic obstructive pulmonary disease, is caused by inhalation by susceptible horses of dust particles found in barns, bedding, and feed materials such as dusty hay. The inhaled particles include endotoxin, mites, plant debris, inorganic materials, and conidia and fragments of molds. Faenia rectivirgula (formerly known as Micropolyspora faeni), Aspergillus fumigatus, and Thermoactinomyces vulgaris are molds commonly associated with respiratory disease in susceptible horses, as evidenced by experimental studies involving inhalation of mold or mold fragments by horses.1 Molds contain a number of inflammatory substances including various allergens, glucans, mycotoxins, and proteases and it is not clear which of these agents are the inciting cause of heaves.2 Furthermore, dust containing mold also contains endotoxin. Endotoxin contamination of molds contributes to the airway response to inhalation of preparations of molds used in experimental studies3 and inhalation of endotoxin alone produces airway inflammation and impaired respiratory function in horses in a dose-dependent manner, with heaves-susceptible horses having an exaggerated response at lower doses.4 However, the response to endotoxin is less than that of susceptible horses exposed to hay dust containing endotoxin indicating that endotoxin alone is not sufficient to cause the clinical signs of heaves. Other compounds in hay dust are integral to the development of heaves.5
It is emphasized that there is not one causative agent acting alone but rather a range of agents that, when inhaled in sufficient concentration by susceptible horses, induce airway disease. It is likely that heaves is associated with the potentiating interactions among several agents present in barn or hay dust and is not simply a response to one agent.3,6 The mechanisms underlying development of airway inflammation and respiratory dysfunction are provided under ‘Pathogenesis’. Viral infections and 3-methylindole intoxication are not considered important causes of heaves.7
EPIDEMIOLOGY
Occurrence
Although heaves is one of the more common diseases of horses, and is a major cause of loss of performance and wastage in European horses,8 there are few reports of its epidemiological characteristics. The disease is common in Europe and North America but is rare in Australia. In Germany, 83% of horses believed to be healthy at an auction were found to have clinical evidence of chronic pulmonary disease.8 Inflammatory airway disease is very common in horses, with 96% of racehorses in Hong Kong examined at necropsy and 12% of horses examined in an abattoir in the northern U.S having histological evidence of bronchitis.9,10 Twenty-seven per cent of healthy racehorses in training had an increased proportion (>20%) of neutrophils in tracheal aspirate, indicating inflammatory airway disease.11 However, the airway inflammation common in young athletic horses is not generally considered to be heaves, or a prodrome of heaves. The prevalence of heaves is not well documented.
The case-fatality rate for moderately to severely affected horses is approximately 20% over a 2–4-year period.12 Most mildly to moderately affected horses respond well to treatment and continue to perform at a satisfactory level.12
Risk factors
Animal risk factors
The disease occurs in adult horses and ponies, and animals >7 years of age are 6 to 7 times more likely to be examined because of the disease than are horses <4 years of age.13 There is no apparent breed or sex predisposition with the exception that Thoroughbreds are 3 times more likely to be examined for the disease than are ponies,13 although this could represent a sampling bias in that owners of Thoroughbreds might be more likely to seek veterinary attention than owners of ponies. The finding of increased likelihood of Thoroughbred horses having the disease is not consistent among studies. Horses are approximately 2 times more likely to be examined by a veterinarian because of the disease in winter or spring compared to summer, suggesting a seasonality to the occurrence of the disease13 perhaps as a result of increased stabling during winter.
There are horses that develop the disease and other horses, maintained in an identical situation, that do not. Development of disease is dependent upon the horse being susceptible to the inflammatory effect of inhaled dust but the reasons for this individual susceptibility are poorly understood. A familial predisposition has been suggested based on the observation that Lipizaners and German and Swiss Warmbloods are 3.2 times more likely to have heaves if one parent was affected and 4.6 times as likely if both parents had heaves.14 There is no association between major histocompatability markers (equine leucocyte antigens) and occurrence of heaves.14 However, genetic and environmental factors are often closely associated, in that horses of similar genetic background are often housed in similar situations, making elucidation of inherited susceptibility to heaves difficult.
Exposure to inciting agents is associated with a variety of environmental factors, including potentially outdoor concentrations of aeroallergens and climatic factors,15 but most importantly housing and feeding practices.
Environmental risk factors
There is a clear association between housing, feeding of hay, and development of the disease.16,17 Typically, susceptible horses are clinically normal when at pasture and develop signs of disease within hours to days of being housed in stables and fed dusty hay. Moving affected horses to pasture, or improving air quality by increasing ventilation and feeding processed feedstuffs, results in resolution of the disease.16
Development of disease is related to inhalation of respirable particles that gain access to the lower respiratory tract. Respirable particles are less than 5 μm diameter, the principal source of these particles in stalls is hay, and the majority of particles are fungal spores.18 The concentration of particles in air of the stable is determined by the rate of release of particles from hay, which is dependent in large part on the quality of the hay, concentration of fungal spores in the hay, and the rate of clearance of dust from the stable, a function of the ventilation rate.19.20 Concentrations of respirable dust particles in the breathing zone of stabled horses can be as high as 20 mg/m3.3,21 The severity of increases in neutrophil count and proportion and decreases in pulmonary function in experimental models of heaves are related in a dose-dependent fashion to the amount of dust inhaled.4 The presence of dust particles, and not the soluble products in hay dust, is responsible for most of the airway neutrophilia induced by inhalation of hay dust.5
Hay is the usual original source of spores in stable air. However, decomposing wood shavings are also a source of spores of fungi that multiply during degradation of plant-based materials, and housing horses in poorly ventilated stalls deeply bedded with wood shavings may be detrimental to their respiratory health.22 Spores from hay enter the bedding either directly or after dispersal through the air and multiply in the bedding if it is not removed regularly. Diced paper and wood shavings, when fresh, usually contain very few spores. Barley and wheat straw are usually free of any small spores such as A. fumigatus or M. faeni.19 Bedding horses on fresh wood shavings, and feeding a nutritionally complete pelleted ration, results in a respirable dust burden 3% of that of horses fed hay and bedded with straw.23 Dust burdens measured in the air of the stall underestimate the respirable particle challenge of horses because of the high concentration of particles in hay and bedding, areas from which the horse inhales while eating.23
Respiratory health of horses is related to stable design and ventilation, with horses in poorly ventilated barns having more respiratory disease than horses in well-ventilated barns.22 See ‘Control’ for recommendations regarding stable design.
PATHOGENESIS
Susceptible horses, when exposed to adequate concentrations of respirable dust in the breathing zone, develop airway inflammation, excess mucus accumulation, and respiratory dysfunction within hours to days of exposure. The putative inciting agents include fungal spores and debris, endotoxin, and particulate matter; however, the exact role for each of these agents is unclear, partly because of the difficulty in obtaining uncontaminated material. For instance, fungal material is commonly contaminated with endotoxin which has a synergistic interaction with hay dust in inducing airway neutrophilia in susceptible horses.3
The mechanisms underlying these responses to inhalation of dust are not well defined but can be considered in the contexts of immune and inflammatory responses, mucus secretion, and pulmonary dysfunction.
Inflammatory and immune responses
Inflammation is associated with excessive mucus production, airway swelling, and abnormal lung function.6,24 The inflammatory response in horses with heaves is neutrophilic, with lesser numbers of mast cells and rarely eosinophils. The mechanisms underlying this inflammatory response have not been fully elucidated, although it involves activation of nuclear factor kappaB and there is support for an acquired immune-mediated process.25-28
The presence of allergen-specific IgE antibodies in bronchoalveolar lavage fluid is supportive of a hypersensitivity reaction, although others have proposed type 3 and type 4 immune reactions as the basis of the disease.25 One proposed explanation is that heaves-susceptible horses exhibit a Th2-like immune response to inhalation of hay or barn dust characterized by increased expression of interleukins 4 and 5 and decreased expression of interferon-γ in cells obtained by bronchoalveolar lavage.29,30 Others have not detected a pure Th2-like cytokine profile finding instead a mixed inflammatory response including increases in expression (mRNA) in cells obtained from bronchoalveolar lavage fluid of affected horses of interferon-γ, tumor necrosis factor-α, interleukins 1β and 4, and interleukins 8 and 17 (potent attractors of neutrophils) but not interleukins 2, 5, and 10.31-35 However, all the studies cited above were performed on crude preparations of cells obtained by bronchoaveolar lavage and the results could have been influenced by the varying proportions of types of cells in these preparations.32 A study examining just CD4 and CD8 lymphocytes in blood and bronchoalveolar lavage fluid of heaves-affected horses demonstrated a general down regulation in expression of interferon-γ, and interleukins 4, 5, and 13 and no evidence of a cytokine profile consistent with either sole or predominate Th1 or Th2-like responses.26 The magnitude of the inflammatory response varies depending on the challenge (i.e. nature of the inhaled material) with responses to endotoxin characteristically being less than that of hay dust.35a Regardless of the underlying mechanism, exposure to inciting agents results in airway inflammation and interference with normal respiratory function.
Following inhalation of inciting agents there is recruitment of neutrophils, but not eosinophils or platelets, into the lungs in most horses that develop changes in lung function.36,37 Histologically there is peribronchiolar accumulation of lymphocytes and luminal accumulations of neutrophils in affected horses. The entry of neutrophils into the airways is mediated at least in part by IL-8 and IL-17.31,33 The neutrophils of horses during episodes of heaves, but not when the horses are asymptomatic, have increased adherence in vitro to protein coated plastic suggesting a mechanism for the increased migration of neutrophils into airways of affected horses.38 Inhibition of neutrophil phosphodisesterase-4 activity does not alleviate clinical signs of heaves or decrease neutrophil numbers in bronchoalveolar lavage fluid in affected horses suggesting that neutrophils are not primarily involved in the genesis of airway obstruction.39,40 The extent to which neutrophils in the airways are activated has not been determined and their role in the development of respiratory dysfunction is unclear given that glucocorticoid administration attenuates the respiratory dysfunction but not airway neutrophilia in horses with heaves (see under ‘Treatment’).41
Airway inflammation is associated with increases in concentration of inflammatory mediators including leukotriene B4, prostanoids including thromboxane, and proteases.42 Activity of matrix metalloproteinase-9 is higher in horses with heaves than in unaffected horses and is induced in a dose-dependent manner by inhalation of inciting substances including hay dust and endotoxin.42-45 MMP-9 is likely important in the inflammatory process associated with heaves through excessive gelatinolytic proteolysis that can contribute to lung injury, and through a role in lung remodelling.43 Inflammation is also associated with increased oxidative stress in lungs of horses with heaves as indicated by elevated concentrations of epi-PGF2a and redox ratio of glutathione in pulmonary lavage fluid.24,46
Mucus
Accumulation of excessive quantities of mucus in the large airways is characteristic of horses affected by heaves and can contribute to nonbronchospastic airway obstruction.6,47 Accumulation of mucus is attributable to decreased clearance and increased production.48,49 The mucus in horses with heaves differs in both composition and viscoelasticity from that of clinically normal horses63,65 and this might contribute to its decreased clearance. The viscosity of mucus can increase threefold in heaves susceptible horses stabled and exposed to hay dust.48 Increased production of mucus is associated with up-regulation of the equine MUC5AC gene, which is responsible for production of mucin, in particular in small airways of horses with heaves.49
Airway function and gas exchange
Inhalation of inciting agents causes changes in lung function characterized by an increase in pulmonary resistance, lower dynamic compliance, altered distribution of ventilation, impaired gas exchange, increased functional residual capacity, and an altered breathing strategy.7 Airway obstruction is a result of bronchospasm, inflammatory thickening of airways, and accumulation of mucus and cells in the airways. Bronchospasm is largely relieved by administration of bronchodilator drugs or removal of the inciting cause, but residual effects on lung function remain and are attributable to inflammation and fibrosis and bronchoconstriction of small airways.7 Bronchoconstriction in both normal and affected horses is caused by parasympathetic activity and release of acetylcholine that reacts with muscarinic receptors on airway smooth muscle. However, the response is exaggerated in horses with heaves. Stimulation of airway sensory receptors results in an exaggerated bronchoconstrictive response, possibly because of the action of inflammatory mediators and/or by-products. The exaggerated bronchoconstrictive response is not specific for allergens, and any substance that activates airway sensory receptors may incite bronchoconstriction once the sensitivity of the receptors is enhanced by inhalation of the inciting allergens. Exaggerated airway responsiveness to inhaled irritants persists for up to 3 days after a single exposure to the inciting agent and is likely important in the development of clinical signs of the disease.50 Bronchoconstriction increases work of breathing but hypoventilation probably contributes little to the hypoxemia of affected horses, given that PaCO2 is rarely increased.51
Hypoxemia, which can be severe (<60 mmHg, 8 kPa), is due to ventilation– perfusion mismatches and increased dead space ventilation.51 The increased minute ventilation of affected horses, a result of maintained tidal volume and increased respiratory rate, mainly supplies dead space and regions with high V/Q ratios.51 Pulmonary hypertension in affected horses is probably due to hypoxia and perhaps inflammatory mediators with vasoconstrictor activity.7,51
The elevated functional residual capacity and characteristic breathing strategy of affected horses is due to airway obstruction. Airway obstruction causes trapping of air in alveoli and a higher end-inspiratory volume. The high end-inspiratory volume maximizes airway diameter and facilitates the high expiratory and inspiratory flow rates necessary for affected horses to maintain a normal tidal volume while increasing their respiratory rate.
Bronchiectasis (irreversible dilation and deformation of bronchi or bronchioles) occurs in some horses affected with heaves for a prolonged duration.52 Neutrophilic inflammation is essential for the development of bronchiectasis.
CLINICAL FINDINGS
The degree to which horses are affected varies considerably. Minimally affected horses have airway inflammation evident on endoscopic or cytological examination of the airways, but few other signs on physical examination, whereas severely affected horses have very obvious clinical signs.
The usual history is that of chronic cough in a stabled horse.12 Typically, the disease is precipitated by exposure to hay and stabling, and disease remission occurs in most horses when pastured and removed from hay. There may be a history of reduced exercise tolerance.
Affected horses are usually bright and alert and have a normal appetite and rectal temperature. Severely affected horses appear anxious and have a greatly increased respiratory effort.
Coughing is common in horses with heaves, although it is neither particularly specific nor sensitive as an indicator of the disease. Coughing may consist of a single cough every few seconds to minutes or there may be a paroxysm of coughing. The cough can also be elicited by digital massage of the larynx and proximal part of the trachea because horses with airway inflammation have increased sensitivity of the cough reflex. Stimulation of the larynx or proximal trachea by digital massage does not elicit coughing in normal horses. The cough becomes more pronounced and wheezing with exercise. It also occurs more frequently when the horse is exposed to cold air, physical activity, excitement, and when placed in a dusty environment, or if dusty feed is offered. The amount of coughing, which must be counted over at least 15 minutes and preferably 1 hour for accurate determination of its severity, correlates closely with the amount of mucus in airways, maximal change in pleural pressure (a measure of bronchoconstriction), and neutrophil count in bronchoalveolar lavage fluid.41 Coughing is more frequent in horses with heaves, and affected horses often have paroxysmal coughing especially after barn cleaning and feeding.
An intermittent, bilateral mucopurulent to serous nasal discharge is a common sign in affected horses.
The resting respiratory rate is increased from a normal of 12/min up to 24–36/min. There is a pronounced effort during expiration and markedly affected horses have an obvious abdominal component to respiration. Normal horses have a biphasic pattern of airflow during inspiration and expiration while affected horses lack the second phase of respiration.53 Longstanding cases develop a ‘heave-line’ in the flank due to hypertrophy of the abdominal oblique musculature. It is evident as a trough or furrow along the costal arch. In advanced cases the nostrils may be visibly dilated during inspiration and the force of the expiratory effort causes the anus to protrude.
Heart rate is commonly within the normal range or only slightly increased. In horses with heaves, the heart rate is significantly higher during exercise than in healthy horses.
Abnormal lung sounds are one of the most frequent abnormalities detected on clinical examination and the sensitivity of this finding can be increased from 70% to almost 90% by auscultating the thorax while the horse breathes for 60 to 120 seconds with an airtight plastic bag over its nostrils.12 The bag should be large enough to enable the horse to breathe unhindered (10–15 L) and should not leak. Accumulation of carbon dioxide in the bag increases the horse’s respiratory rate and tidal volume and accentuates lung sounds. Auscultation of the lungs in the early stages of the disease may reveal only a slight increase in the amplitude of normal breath sounds. Abnormal lung sounds become audible as the disease progresses. Wheezing and crackling sounds occur at the end of inspiration and the end of expiration. These abnormal sounds are audible over most of the lung but are usually easiest to detect over the upper one-half of both lung fields. Auscultation of the trachea usually reveals moist sounds characteristic of fluid in the trachea. Some affected horses have quieter than expected lung sounds.
Percussion of the thorax may reveal an increase in the area of resonance by as much as one to two intercostal spaces caudally. However, the area of resonance delineated by percussion is too labile and ill-defined to be of diagnostic value.
Endoscopic examination of the upper airways, trachea, and bronchi reveals an abundance of mucopurulent material in the trachea which, in severe cases, is also present in the nasopharynx. The amount of mucus can be graded on a 0–5 scale41:
• Grade 1 – small blobs of mucus that are not confluent
• Grade 2 – multiple blobs of mucus some of which are confluent
• Grade 3 – mucus confluent in a stream in the ventral aspect of the trachea or multiple large blobs around the circumference of the lumen
• Grade 4 – large pool of mucus in the ventral aspect of the airway
• Grade 5 – Profuse amounts of mucus occupying more than 25% of the tracheal lumen.
Observation of tracheal mucus of grade 4 or 5 has a high specificity (92%) but low sensitivity (52%) for detection of heaves.47
Radiographic examination of the thorax usually reveals evidence of bronchial disease with some evidence of interstitial disease. Radiography is more useful in ruling out other diseases, such as granulomatous or interstitial pneumonia, than in confirming heaves.
Sophisticated techniques for measuring pulmonary function, such as determination of tidal flow–volume loops, nitrogen washout or forced expiratory flow–volume loops, may identify mildly or subclinically affected animals but have limited day-to-day clinical utility.53-55
Measurement of pleural pressure changes by insertion of an esophageal balloon is relatively simple and may be useful in monitoring response to treatment. Affected horses have pleural pressure changes during respiration greater than 6 cm H2O.54 Administration of atropine (0.02 mg/kg, IM or IV), isoproterenol (isoprenaline), or a β2-adrenergic agonist such as terbutaline (0.04 mg/kg, PO) reduces the maximal change in pleural pressure of horses with heaves.
The course of the disease is dependent on the removal or continual presence of the precipitating cause. If the cause is removed in the early stages, complete recovery can occur. In the continual presence of the precipitating cause relapses occur commonly or the disease becomes progressive and affected horses become severely incapacitated. Bronchiectasis, evident on radiographic examination of the thorax, develops in horses with heaves of prolonged duration.52 With conscientious management and adequate housing, breeding animals and hunters or showjumpers with heaves can remain useful for many years.56
CLINICAL PATHOLOGY AND SPECIAL EXAMINATIONS
There are no significant changes in the hemogram or serum biochemistry of affected horses. The PaO2 is below normal in moderately to severely affected horses and the PaCO2 is usually normal although it may be increased in severely affected horses. Blood oxygen tension measurements should be corrected for the temperature of the animal and the altitude. At approximately sea level, PaO2 values of normal horses are usually greater than 90 mmHg (12 kPa), whereas affected horses have PaO2 less than 82 mmHg (10.9 kPa). With increases in altitude the values in both normal and affected horses decrease.54 The normal hypoxemia that occurs in horses during intense exercise is exacerbated by heaves.
Bronchoalveolar lavage fluid from affected horses during symptomatic episodes has a relative neutrophil count greater than 5 to 10%, and usually over 50%, of the absolute nucleated cell count.12,57,58 It is recommended that horses not be considered to have airway inflammation unless >15% of cells in bronchoalveolar lavage fluid are neutrophils. During periods of remission, the bronchoalveolar lavage fluid of previously affected horses is not different from that of normal horses. Absolute nucleated cell counts in bronchoalveolar lavage fluid of affected horses are reported12,57,58 but the values depend on the collection technique used.59 The relative proportions of macrophages and lymphocytes in bronchoalveolar lavage fluid of affected horses are lower than those of normal horses. Eosinophil numbers in bronchoalveolar or tracheal aspirate fluid of affected horses may be mildly elevated (up to 10%), but are usually low (<3–5%). Higher values should raise the index of suspicion for Dictyocaulus arnfieldi or Parascaris equorum infestation. Aspirates of tracheal fluid reveal a profound neutrophilia (>90%).57
Measurement or identification of precipitins in serum of horses is not useful in identifying horses with heaves.60
Intradermal testing using putative allergens has been investigated as a means of identifying horses with heaves or of identifying antigens with which to hyposensitize affected horses with variable or undetermined efficacy.1,61,62 Retrospective examination of records of horses with a history of heaves suggests that they are more likely to react, and react to a larger number, of intra-dermally injected allergens than horses without a history of heaves.63 However, reactions to individual allergens cannot be used to determine hypersensitivity to particular allergens, although it is suggested that overall patterns of reactivity, with a history of exposure of the horse to these allergens, might be useful in guiding management of affected horses.63 Contrary to these results, a prospective study demonstrated that horses with heaves did not have a greater rate of reaction to intradermal skin tests than did horses not affected by heaves.64 Intradermal testing did not distinguish clinically relevant reactions from those that were not clinically relevant.64 Horses with heaves have greater sensitivity to intradermal injection of histamine, which is commonly used as a positive control, than horses without heaves.65 Overall, intradermal skin testing is neither useful in detecting horses with heaves nor in determining hypersensitivity to particular allergens in individual horses. Results of such testing might be useful in management of horses, but this has not been demonstrated. The usefulness of intradermal skin testing and subsequent administration of preparations of antigens selected on the basis of intradermal testing, in an effort to hyposensitize horses with heaves, has not been determined. The apparent lack of efficacy of intradermal testing might be because the extent of reactivity to intradermal injection of mold preparations does not correlate with the severity of pulmonary dysfunction after inhalation of the same preparation in horses with heaves.1
Lung biopsy demonstrates peribronchiolar lymphoplasmacytic inflammation, goblet cell metaplasia, alveolar fibrosis, and bronchial lumen exudate and neutrophils.12 The severity of bronchiolar neutrophil and mast cell infiltration correlates well with the severity of the clinical signs.12
NECROPSY FINDINGS
The major findings are restricted to the lungs, which are pale, voluminous, and do not collapse when the chest cavity is opened. The tissue damage is primarily centered on airways which are less than 2 mm in diameter. Microscopically, a variable degree of alveolar emphysema is accompanied by a chronic bronchiolitis featuring diffuse epithelial hyperplasia, goblet cell metaplasia, peribronchiolar fibrosis, and cellular infiltration by lymphocytes, plasma cells, mast cells, and sometimes eosinophils. Plugs of mucus with entrapped neutrophils often occlude bronchiolar lumina.
DIAGNOSTIC CONFIRMATION
confirmation of the disease is based on the presence of a history and clinical signs consistent with the disease, in particular the response to stabling and pasturing, and demonstration of reversible airway obstruction. Objective confirmation can be achieved by measuring the response of maximal changes in pleural pressure in response to bronchodilator drug (atropine or glycopyrolate) administration.
Horses with respiratory distress may have:
• Pulmonary or mediastinal neoplasia including leiomyosarcoma66
TREATMENT
The principles of treatment are:
Heaves is an inflammatory disease caused by inhalation exposure to inciting agents. Bronchoconstriction is secondary to inflammation. Control of the disease is based upon preventing inhalation of inciting agents and suppression of inflammation by administration of corticosteroids. Relief of bronchoconstriction should be necessary only during acute exacerbations of the disease, and administration of bronchodilatory drugs for more than several days is not optimal treatment in most horses. Drugs used in the treatment of heaves are summarized in Table 34.2.
It is essential that the horse is not exposed to the inciting agents and irritant substances that could provoke or worsen the disease. Even relatively brief exposure of susceptible horses to the inciting agents, such as can occur if a horse is brought into a poorly ventilated barn to be fed, can result in airway hypersensitivity and the development or maintenance of clinical signs. Affected horses should be moved to a clean environment, ideally pasture, in which the concentration of airborne allergens is reduced to an absolute minimum. If the horse cannot be kept at pasture, then it should be housed in a well ventilated barn (see ‘Control’ for details), bedded with clean wood shavings or shredded paper, and fed a complete pelleted ration. If affected horses are fed hay, it should be thoroughly wetted to minimize the release of spores. Remission of clinical signs can be expected in 4–21 days if the environmental changes are adequate.67 This may be all that is necessary to control the disease in many horses.
Antiinflammatory drugs
The disease is essentially one of inflammation of the airways and therefore one of the mainstays of treatment is administration of anti-inflammatory drugs. Non-steroidal anti-inflammatory drugs such as phenylbutazone and flunixin meglumine are not effective. Corticosteroids including dexamethasone, prednisolone, triamcinolone, and betamethasone are effective in controlling the disease. Dexamethasone (0.04–0.1 mg/kg, intravenously, intramuscularly or orally every 24–48 hours) can be given to control the acute signs of the disease, and then the dose reduced and eventually discontinued as environmental alterations have their effect. Similarly, prednisolone (1–2 mg/kg, orally once daily), but not prednisone68,69 can be given initially, then the dose reduced by approximately one half every 5–10 days as the disease is controlled. Often prednisolone or dexamethasone sodium phosphate is effective when administered every second day when the disease has been controlled. Dexamethasone-21-isonicotinate (0.04 mg/kg, intramuscularly) is effective when administered every 3 days, but not when administered only once.69,70 Isoflupredone (0.03 mg/kg, intramuscularly once daily) is as effective as dexamethasone in alcohol in control of exacerbations of heaves, although it does cause hypokalemia.71 Triamcinolone (0.09 mg/kg, intramuscularly) administered once provides long-term (weeks) relief of signs in some horses.72
Inhaled corticosteriods, such as betamethasone, beclomethasone, or fluticasone, are useful in controlling the disease.73,74 Both inhaled and parenterally administered corticosteroids suppress adrenal function of horses75 but 500 μg of beclomethasone proprionate inhaled twice daily effectively alleviated signs of heaves and causes less adrenal suppression than doses of 1000 μg or 1500 μg.76 It is important to reiterate that the use of glucocorticoids should only be as an adjunct to control of the horse’s environment and reduction in the inhaled particle burden.
Clebuterol decreases the production of inflammatory cytokines by cells obtained from bronchoalveolar lavage fluid of horses with heaves, suggesting that clenbuterol can have anti-inflammatory effects in such horses.35 The clinical applicability of this finding remains to be determined.
In summary, a number of different corticosteroid preparations are useful in the control of heaves. Drugs administered by inhalation appear to have a reduced potential for adverse effects including adrenal suppression, but are more difficult to administer and require more frequent administration than drugs administered orally, intravenously, or intramuscularly. Improvements in respiratory effort and clinical signs are evident in approximately 3 days and persist for the duration of treatment. Cell counts and the neutrophilia in bronchoalveolar lavage fluid are not reduced by administration of corticosteroids. Affected horses, after institution of appropriate measures to control inhalation of hay and barn dust, should be treated with the lowest dose that controls the disease and only for as long as necessary. The dose of corticosteroid can be reduced gradually and the frequency of administration decreased from once daily to once every second or third day (or greater, depending on the preparation) to achieve this end. Administration of the lowest effective dose is important because of the effects of corticosteroids in suppressing immune and adrenal function. It is suggested that dexamethasone and triamcinolone increase the likelihood of horses developing laminitis, but this relationship has not been conclusively demonstrated.
Bronchodilator drugs
Bronchodilator drugs might be needed to provide acute relief of airway obstruction but should not be used as maintenance therapy. Atropine (0.02–0.04 mg/kg, intramuscularly) can be used to provide short-term relief of bronchoconstriction, but its use is associated with gastrointestinal side-effects, including colic, that preclude its long-term use. Glycopyrolate (0.1 mg/kg, intramuscularly every 8–12 hours) is a potent bronchodilator with minimal gastrointestinal effects. Ipratropium bromide, a parasympatholytic drug with minimal extrapulmonary effects when given by inhalation, is very effective in relieving airway obstruction in severely affected horses.77
β2-adrenergic agonists are potent bronchodilators frequently used in the management of horses with heaves. They can be administered orally or by inhalation.73,78 Clenbuterol hydrochloride is used as maintenance therapy at a dose of 0.8–3.2 μg/kg, orally every 12 hours, and is effective in controlling signs in 75% of affected horses.79 The lower dose should be used initially and then increased in 0.8 μg/kg increments until the desired effect is achieved or side-effects of tachycardia, muscle fasciculation, and sweating are apparent. Gradual, incremental increases in dose lessen the frequency and severity of side-effects. Terbutaline is not absorbed after oral administration to horses.80 Terbutaline and clenbuterol can also be given intravenously, at a dose one-tenth of that given orally, to severely affected horses in which the need for bronchodilation is urgent. Side-effects of β2-agonist administration include tachycardia, sweating, and apprehension.79 Prolonged administration of clenbuterol is associated with potentially adverse effects on cardiac structure, alterations in body composition, and an impaired response to training.81-83 Delayed parturition may occur in mares treated in late pregnancy. β2-adrenergic agonists may transiently exacerbate hypoxemia in severely affected horses. Intra-tracheal administration does not produce detectable bronchodilation.84
Bronchodilators administered by inhalation to horses include the β2-agonists Albuterol, salbutamol, and salmeterol, and the parasympatholyitc ipratropium. The efficacy and duration of action of each of these drugs varies somewhat, but all are effective in producing bronchodilation in affected horses. Salmeterol produces bronchodilation in horses with heaves that persists for up to 6 hours, although onset of action requires 30–60 minutes.85 Similarly, ipratropium reduces pleural pressure changes and attenuates clinical signs of airway obstruction in horses with heaves.77,86
Theophylline (aminophylline) is a non-adrenergic bronchodilator given at a dose of 10–12 mg/kg, orally every 12 hours. Signs of toxicity include tachycardia, excitement, and convulsions. Theophylline is not a drug of first choice for the treatment of heaves and is now used infrequently because of the availability of efficacious anti-inflammatory drugs and other bronchodilators.
Other drugs
Sodium cromoglycate is useful for the prophylaxis of heaves, but has no direct bronchodilatory activity.55 Its mechanism of action is unclear, but it may act to prevent the degranulation of mast cells. It can be given at a dose of 20–30 mg per 425 kg horse by inhalation once daily for 4 days, and then repeated in 1–2 weeks.87
Pentoxyfylline at high doses improves respiratory function, but not bronchoalveolar lavage fluid cytology, of horses with heaves.88 However, bioavailability after oral administration is quite variable, contributing to variations in the responses of horses to the drug.
Drugs that reduce leukotriene production or activity do not appear to be useful in the treatment of heaves. An experimental leukotriene D4 receptor antagonist was not effective in relieving signs of heaves.89 Similarly, montelukast did not improve respiratory function in 5 horses with heaves.88
Mucolytics are often used but their efficacy is not established and is doubtful. Cough suppressants should not be used as they may impair clearance of mucopurulent material from the airways.
Antibiotics are often given to affected horses but are probably not necessary in the vast majority of cases.
Acupuncture is not effective in the treatment of heaves.90
Administration of large quantities of isotonic electrolyte solution intravenously is associated with a decrement in respiratory function in both normal and heavy horses and is not recommended as a treatment for heaves.91
Integrated therapy
Initial treatment of affected horses usually involves changes to the horse’s environment and feed in combination with administration of corticosteroids. Corticosteroids and β2-adrenergic agonists can be given as combined therapy to severely affected horses until the disease is controlled, at which time therapy should consist of environmental control and, if needed, administration of the lowest effective dose of corticosteroids. Bronchodilators are sometimes used as sole therapy, but their use without correction of the housing and feeding factors, and attempts to control inflammation, is not rational. Long-term administration of bronchodilators is not optimal therapy and, given the documented adverse effects, is not recommended. Long-term control of heaves is achieved by environmental management and administration of corticosteroids.
CONTROL
Housing horses in stalls with good air quality is essential in reducing the occurrence of the disease. Adequate ventilation is critical in maintaining good air quality in stalls. Few horse housing units have adequate ventilation19,22 although a well-designed individual box stall can meet the needs of the horse both for air hygiene and thermal comfort.19 Many horse barns have inadequate open space for ventilation in still air conditions when the doors are closed at both ends of the building. When the release rate of spores is low, ventilation rates of 4 air changes per hour are satisfactory. However, suggested minima are 8–10 air changes per hour, airspace of 44 m3/head, and floor space of 9.2 m2/head.23 In practical terms, if the upper half of the stable door is open, and faces open air and not into a barn, the natural ventilation should exceed the minimum specifications.23 Hay and dusty feed materials should not be stored above stalls or in the same airspace as horses. Bedding should be changed frequently, preferably daily. Use of cardboard as bedding material is effective as part of an overall regimen to improve air quality.92
A portable slit sampler is an accurate, quick, and simple semiquantitative method of assessing the mold contamination of source materials such as hay, straw, and other feeds and bedding collected from stables.18 The health hazard posed by any moldy source material depends on the types of organisms present and their abundance. The size of the respirable challenge from heated hays and straws arises from the prolificacy of the species involved and their small spore size. The highest respirable challenges are from the presence of thermotolerant and thermophilic mold species. The most critical factors in determining the microbial development in plant-based materials are water content and thermal environment. Hay baled at 15–20% moisture heats little, it is virtually dust-free and contains few spores. Baling hay with 20–30% moisture leads to temperatures of up to 35–45°C. At these temperatures, hazardous contamination may develop with the appearance of thermotolerant fungi and actinomycetes.18 The heaviest contamination of hay and straw occurs with baling at 35–50% moisture when spontaneous heating up to 50–60°C may occur. Microscopically, these hays show large numbers of fungal spores in the 2–5 μm size range.
1 McGorum BC, et al. Equine Vet J. 1993;25:273.
2 McGorum BC. Proc Am Coll Vet Int Med. 2004;22:186.
3 Pirie RS, et al. Clin Exp Allergy. 2003;33:676.
4 Pirie RS, et al. Equine Vet J. 2002;34:337.
5 Pirie RS, et al. Equine Vet J. 2002;34:343.
6 Robinson NE, et al. Equine Vet Educ. 2001;13:247.
7 Robinson NE, et al. Br Vet J. 1996;152:283.
8 Bracker V, et al. Equine Vet J. 1991;23:136.
9 Larson VL, Busch RH. Am J Vet Res. 1985;46:144.
10 Mason DK, et al. Equine Exerc Physiol. 1982;1:57.
11 Sweeney C, et al. Am J Vet Res. 1992;53:1172.
12 Naylor JM, et al. Can Vet J. 1992;33:591.
13 Couetil LL, Ward MP. J Am Vet Med Assoc. 2003;223:1645.
14 Marti E, et al. Equine Vet J. 1991;23:457.
15 Ward MP, Couetil LL. Am J Vet Res. 2005;66:818.
16 Thomson JR, McPherson EA. Equine Ve J. 1983;16:35.
17 Derksen FJ, et al. J ApplPhysiol. 1985;58:598.
18 Clarke AF, Madelin T. Equine Vet J. 1987;19:442.
19 Webster AJF, et al. Equine Vet J. 1987;19:448.
20 McGorum BC, et al. Equine Vet J. 1998;30:430.
21 Woods PS, et al. Equine Vet J. 1993;25:208.
22 Clarke AF, et al. Equine Vet J. 1987;19:524.
23 Jones RD, et al. Equine Vet J. 1987;19:454.
24 Kirschvink N, et al. Equine Vet J. 2002;34:563.
25 Halliwell REW, et al. Vet Immunol Immunopathol. 1993;38:201.
26 Sandersen C, et al. Vet Immunol Immunopathol. 2001;80:315.
27 Bureau F, et al. Am J Resp Crit Care Med. 2000;161:1314.
28 Bureau F, et al. J Immunol. 2000;165:5822.
29 Lavoie J, et al. Am J Resp Crit Care Med. 2001;164:1410.
30 Cordeau M, et al. 2004; Vet Immunol Immunopathol. 2001;97:87.
31 Ainsworth DM, et al. Vet Immunol Immunopathol. 2003;96:83.
32 Giguere S, et al. Vet Immunol Immunopathol. 2002;85:147.
33 Debrue M, et al. Vet Immunol Immunopathol. 2005;105:25.
34 Tamarinde TJM, et al. J Vet Int Med. 2005:19.
35a Laan TTJM, et al. Am J Vet Res. 2005;66:1584.
35b Loan TT, et al. Vet J. 2006;171:429.
36 Fairbairn SM, et al. Clin ExpAllergy. 1993;23:821.
37 Marr KA, et al. Res Vet Sci. 1997;62:253.
38 Marr K, et al. Equine Vet J. 2002;34:65.
39 Rickards KJ, et al. Vet Immunol Immunopathol. 2000;76:319.
40 Lavoie JP, et al. J Vet Int Med. 2006:20.
41 Robinson NE, et al. Am J Vet Res. 2003;64:550.
42 Simonen-Jokinen T, et al. Equine Vet J. 2005;37:155.
43 Raulo SM, et al. Equine Vet J. 2001;33:128.
44 Brazil TJ, McGorum BC. Equine Vet J. 2001;33:113.
45 Nevalainen M, et al. Equine Vet J. 2002;34:150.
46 Art T, et al. Equine Vet J. 1999;31:397.
47 Gerber V, et al. J Vet Int Med. 2004;18:92.
48 Gerber V, et al. Equine Vet J. 2000;32:411.
49 Gerber V, et al. Equine Vet J. 2003;34:252.
50 Fairbairn SM, et al. J Vet Pharmacol Therap. 1993;16:469.
51 Nyman G, et al. Equine Vet J. 1991;23:253.
52 Lavoie JP, et al. J Vet IntMed. 2004;18:757.
53 Gallivan GJ, et al. Can J Vet Res. 1990;54:99.
54 McPherson EA, et al. Equine Vet J. 1978;10:47.
55 Couetil LL, et al. J Appl Physiol. 2000;88:1870.
56 Aviza GA, et al. Equine Vet Educ. 2001;13:243.
57 Derksen FJ, et al. Equine Vet J. 1989;21:23.
58 Tremblay GM, et al. Equine Vet J. 1993;25:194.
59 Sweeney CR, et al. Am J Vet Res. 1992;53:1376.
60 Madelin TM, et al. Equine Vet J. 1991;23:247.
61 Evans AG, et al. Am J Vet Res. 1992;53:203.
62 McPherson EA, et al. Equine Vet J. 1979;11:159.
63 Jose-Cunilleras E, et al. J Am Vet Med Assoc. 2001;219:1115.
64 Lorch G, et al. Am JVet Res. 2001;62:389.
65 Wong DM, et al. Am J Vet Res. 2005;66:1341.
66 Rossdale PD, et al. Equine Vet Educ. 2004;16:21.
67 Jackson CA, et al. Equine Vet J. 2000;32:432.
68 Peroni DL, et al. Equine Vet J. 2002;34:283.
69 Robinson NE, et al. Equine Vet J. 2002;34:17.
70 Couetil LL, et al. J Vet Int Med. 2006:20.
71 Picandet V, et al. Equine Vet J. 2003;35:419.
72 LaPointe JM, et al. Am J Vet Res. 1993;54:1310.
73 Hoffman AM. Vet ClinNorth Am Equine Pract. 1997;13:519.
74 Rush BR, et al. Am J Vet Res. 1998;59:1039.
75 Rush BR, et al. Am J Vet Res. 1998;59:1044.
76 Rush BR, et al. Am J Vet Res. 2000;217:359.
77 Duvivier DH, et al. Vet J. 1997;154:149.
78 Duvivier DH, et al. Vet J. 1997;154:189.
79 Erichsen DF, et al. Equine Vet J. 1994;26:331.
80 Torneke MK, et al. Am J Vet Res. 2000;61:761.
81 Kearns CF, McKeever KH. Med Sci Sports Exerc. 2002;34:1976.
82 Sleeper MM, et al. Med Sci Sports Exerc. 2002;34:643.
83 Beekley MD, et al. Vet J. 2003;165:234.
84 Harkins JD, et al. J VetPharmacol Therap. 2000;23:251.
85 Hendrikson SL, Moore BR. J Am Vet Med Assoc. 1961;218.
86 Bayly WM, et al. Equine Vet J. 2002;34:36.
87 Thomson JR, McPherson EA. Equine Vet J. 1981;13:243.
88 Kolm G, et al. Vet Rec. 2003;152:804.
89 Lavoie JP, et al. Am J Vet Res. 2002;63:579.
90 Wilson DV, et al. Equine Vet J. 2004;36:489.
PASTURE-ASSOCIATED HEAVES (PASTURE-ASSOCIATED OBSTRUCTIVE PULMONARY DISEASE OF HORSES)
Summer pasture-associated heaves occurs in horses in the southeastern region of the United States and in Great Britain.1-3 It appears to be a disease of adult horses that are on pasture most of the time in the summer. It occurs most commonly in the warm, humid summer months of June to September. Affected horses gradually recover during the cooler months of winter and early spring, and the disease may recur in the same horse each successive summer. Most severe signs occurred during late spring and early summer during times of high airborne pollen counts. It has been suggested that pulmonary allergy to pollens is a factor but this has not been validated. Affected horses have increased expression of interleukin-4 and interferon-? in cells of bronchoalveolar lavage fluid and peripheral blood mononuclear cells4 but not increased concentrations of IgE in bronchoaveolar lavage fluid.5 Affected horses have clinical findings typical of heaves including nasal discharge, coughing, tachypnea, labored expiratory effort, and crackles and wheezes on auscultation.6 There is moderate-to-severe accumulation of mucus in the large airways evident on endoscopic examination. Lung function testing is consistent with bronchoconstrictio onchoalveolar lavage fluid contains large numbers of nondegenerate neutrophils and lesser numbers of lymphocytes and mast cells.6 Necropy reveals overinflated lungs that do not collapse when the chest is opened and that retain the impressions made by the ribs.6 The predominant histologic finding is accumulation of mucus in small airways. Inflammation is not severe and most inflammatory cells present are neutrophils and lymphocytes in peribronchial tissues.6 Treatment includes stabling and administration of corticosteroids and bronchodilators, as discussed for heaves and Table 34.2).1,2,7
MILK ALLERGY
Signs of allergy, principally urticaria, are often manifested by cows during periods of milk retention.1,2 Most of these occur as the cow is being dried off. The Channel Island breeds of cattle are most susceptible and the disease is likely to recur in the same cow at subsequent drying off periods; it is almost certainly inherited as a familial trait.
The important clinical signs relate to the skin. There is urticaria which may be visible only on the eyelids or be distributed generally. Local or general erection of the hair may also be seen. A marked muscle tremor, respiratory distress, frequent coughing, restlessness to the point of kicking at the abdomen and violent licking of themselves, and even maniacal charging with bellowing may occur. Other cows may show dullness, recumbency, shuffling gait, ataxia, and later inability to rise. The temperature and pulse rates are usually normal or slightly elevated but the respiratory rate may be as high as 100/min.
Diagnosis of milk allergy can be made by the intradermal injection of an extract of the cow’s own milk. A positive reaction occurs with milk diluted as much as 1 in 10 000, and the edematous thickening is present within minutes of the injection. Other clinicopathological observations include the development of eosinopenia, neutrophilia, and hyperphosphatemia during an attack.
Spontaneous recovery is the rule but antihistamines are effective, especially if administered early and repeated at short intervals for 24 hours. Prevention is usually a matter of avoiding milk retention in susceptible cows, but in many cases it is preferable to cull them.
ENZOOTIC NASAL GRANULOMA OF CATTLE (ENG, BOVINE ATOPIC RHINITIS)
Of the three known clinical types of chronic nasal obstruction in cattle, two have been identified etiologically and have clinical or epidemiological features that distinguish them from enzootic nasal granuloma. One is recorded predominantly in beef cattle appears to be caused by a fungus, most commonly Rhinosporidium spp., or Drechslera spp.1,2 Another is caused by the parasite Schistosoma nasalis.3 The third type, enzootic nasal granuloma, occurs commonly in southern Australia, less commonly in New Zealand and is recorded as a sporadic disease in South America and an occasional disease in North America, Britain, and Europe.4,5
Enzootic nasal granuloma occurs sporadically in some herds but may reach an incidence of 30%. In one area, as much as 75% of herds may have the disease. Animals aged between 6 months and 4 years are most commonly affected, and the chronic disease may or may not be preceded by an attack of acute rhinitis. Most cases commence in the summer and autumn months. It is apparent that nasal granuloma develops as a continuous and progressive response to acute episodes of hypersensitivity to an allergen present in the summer months. This accords with the gradual development of the stertorous respiration6 and the observations, in biopsies of nasal mucosa, of the presence of mast cells in all seasons, but the regression of eosinophils in the winter months.4
An extensive survey of Australian dairying areas showed that 22% of cattle had lesions, and that the prevalence was greater in areas where the average annual rainfall was over 70 cm, than where it was less than 70 cm; the prevalence varied between 4% and 48%; Jerseys were more commonly affected than Friesians. In New Zealand, 40% of farms and 36% of culled cattle were affected, whereas only 3.6% of young beef cattle showed lesions.
The disease has been identified as an allergic rhinitis and has been produced experimentally.7 Specific antigens have not been identified as the cause but cows with nasal granuloma are much more sensitive to a number of common allergens in the environment than are unaffected cows. Bovine herpesvirus 1 is often isolated from lesions of ENG and is thought by some to predispose animals to the development of the disease. Additional possible causes include infestation of the nasal cavities with pasture mites (Tyrophagus palmarum). An allied condition has been described in the United States as maduromycosis but there are nasal granulomas plus multiple granulomatous lesions of the skin of the ears, tail, vulva, and thigh.8 The granulomas contain many eosinophils and fungal elements identified provisionally as Helminthosporium sp.
In enzootic nasal granuloma acute cases are characterized by a sudden onset of bilateral ocular and nasal discharge and swelling of the nasal mucosa causing difficult, noisy breathing. Affected animals shake their heads and snort and rub their noses in hedges. As a result they commonly block their nostrils with twigs. This form of the disease is commonest in cattle of the Channel Island breeds and their crossbreeds. The nasal discharge in these breeds is usually yellow to orange in color.
Established cases of enzootic nasal granuloma have lesions, consisting of granulomatous nodules 1–4 mm in diameter and height, in both nostrils. The lesions extend from just inside the nostril posteriorly for 5–8 cm. They may be few in number or be packed closely together. Their texture is firm and the mucosa over them is normal. They have a characteristic histopathology of epithelial metaplasia and hyperplasia, and contain large numbers of eosinophils and mast cells.4,9
The predominant clinical sign is respiratory stertor and dyspnea caused by obstruction to the air flow. The severity of these signs may fluctuate but in general they progress slowly over several months and then remain static. Although the respiratory distress may be sufficiently severe to cause a loss of condition and marked reduction in milk yield, affected animals do not die. A good proportion of them have to be culled as uneconomic units.
The clinical picture in mycotic nasal granuloma is superficially similar with respect to noisy breathing, respiratory distress, and nasal discharge, but there is no seasonal association. Also, the visible and palpable lesions in the anterior part of the nasal cavities are polyps up to 5 cm in diameter which occur singly or in confluent masses. Their cut surfaces are yellow to green and they are sometimes ulcerated.4,10 Histologically the lesions are eosinophilic granulomas containing fungal spores and sometimes hyphae. Fungi (Drechslera rostrata) have been isolated from the lesions.
Equine allergic rhinitis is a clinically and epidemiologically similar condition, but without mucosal lesions, recorded in horses as a common cause of persistent head shaking.11
1 Penrith ML, et al. J South. Afr Vet Med Assoc. 1994;65:179.
2 Conti Diaz IA, et al. Rev Instit Med Trop St. Paulo.. 2003;43:163.
3 Agrowal MC, Alwar VS. Helminthol Abstr. 1992;61:373.
4 de Bosschere H, et al. Vet Rec. 2000;146:322.
5 Rivero R, et al. Veterinaria Uruguay. 1987;24:5.
6 Pemberton DH, White WE. Aust Vet J. 1974;50:85.
7 Pemberton DH, et al. Aust Vet J. 1977;53:201.
8 Patton CS. Cornell Vet. 1977;67:236.
9 Carbonell PL. Vet Pathol. 1979;16:60.