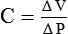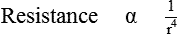Pulmonary Ventilation
Introduction
As we know, respiration, to be more precise, the external respiration (supply of O2 from atmosphere to body tissues and removal of CO2 from the body to atmosphere) involves three major processes:
• Pulmonary ventilation, i.e. exchange of gases between the environment and lungs.
• Pulmonary diffusion, i.e. transfer of gases from the alveoli to capillary blood across the respiratory membrane.
• Transport of gases from the blood to tissue cells and back.
In this chapter following aspects and concepts related to pulmonary ventilation are discussed:
Mechanics of pulmonary ventilation
Mechanism of breathing
Pulmonary ventilation is accomplished by two processes:
• Inspiration refers to the inflow of atmospheric air into the lungs. This obviously occurs when the intrapulmonary pressure falls below the atmospheric air pressure.
• Expiration refers to the outflow of air from the lungs into the atmosphere. This obviously occurs when the intrapulmonary pressure rises above the atmospheric air pressure.
• Changes in intrapulmonary pressure, which govern the respiratory cycle of inspiration and expiration, are related to changes in the intrapleural pressure.
• Changes in intrapleural pressure are brought about by the changes in the size of thoracic cavity. Expansion of thoracic cage leads to fall in the intrapleural pressure and decrease in the size of thoracic cavity leads to rise in the intrapleural pressure.
• Changes in size of the thoracic cavity are brought about by the actions of respiratory muscles:
– Muscles of normal tidal inspiration are diaphragm and external intercostal muscles.
– Accessory muscles of inspiration are scaleni, sternomastoid, serratus anterior and alae nasi.
– Muscles of expiration are internal intercostal muscles and abdominal muscles (abdominal recti muscles, transverse abdominis muscles and internal oblique muscles).
Mechanism of tidal respiration
Inspiration
Inspiration is an active process normally produced by contraction of the inspiratory muscles (negative-pressure breathing). During tidal inspiration (quiet breathing) the diaphragm and external intercostal muscles contract and cause increase in all the three dimensions of thoracic cavity.
Role of diaphragm. The diaphragm is a dome-shaped, musculotendinous partition between thorax and abdomen. The convexity of this dome is directed towards the thorax. When the diaphragm contracts following changes occur:
• The dome becomes flattened and the level of diaphragm is lowered, thereby increasing the vertical diameter of the thoracic cavity (Fig. 5.2-1A). During quiet breathing the descent of diaphragm is about 1.5 cm and during forced inspiration it increases to 7 cm.
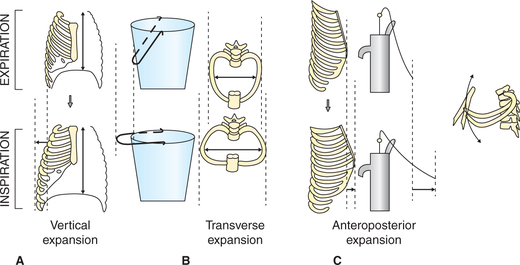
Fig. 5.2-1 Mechanism of increase in diameter of thoracic cavity: A, increase in vertical diameter (descent of diaphragm); B, increase in transverse diameter (bucket handle effect) and C, increase in anteroposterior diameter (pump handle effect).
• Contraction of diaphragm also lifts the lower ribs causing thoracic expansion laterally and anteriorly (the bucket handle and pump handle effect, respectively) (Fig. 5.2-1B and C). In tidal inspiration (quiet breathing) 70%–75% of expansion of chest is caused due to contraction of diaphragm.
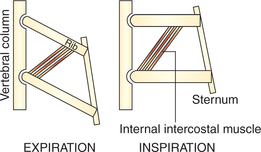
Role of external intercostal muscles. The fibres of external intercostal muscles slope downward and forward. They are attached close to the vertebral ends of the upper ribs than the lower ribs (Fig. 5.2-2). From pivot-like joint with the vertebrae the ribs slope obliquely downwards and forwards. So, when the external intercostal muscles contract (because of the lever effect) the ribs are elevated causing lateral and anteroposterior enlargement of thoracic cavity due to the so-called bucket handle and pump handle effects, respectively (Fig. 5.2-1B and C).
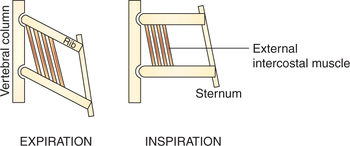
Fig. 5.2-2 Contraction of external intercostal muscle favours elevation of ribs due to mechanical leverage effect of the attachments of its fibres close to the pivot on the upper ribs as compared to the lower ribs.
Role of laryngeal muscles. The abductor muscles of the larynx contract during inspiration pulling the vocal cords apart.
Mechanism of forced respiration
Forced inspiration
Forced inspiration is characterized by:
1. Forceful contraction of diaphragm leading to descent of diaphragm by 7–10 cm as compared to 1–1.5 cm during quiet inspiration.
2. Forceful contraction of external intercostal muscles causing more elevation of ribs leading to more increase in transverse and anteroposterior diameter of thoracic cavity.
3. Contraction of accessory muscles of inspiration which cause the following effects:
Forced expiration
Forced expiration is required when respiration is increased during exercise or in the presence of severe respiratory disease. It is an active process caused as follows.
1. Contraction of abdominal muscles causes
• Downward pull on the lower ribs and thus decreases the anteroposterior diameter of the thoracic cavity.
• Fixation of the lower ribs so that internal intercostal muscles act more effectively.
2. Contraction of the internal intercostal muscles causes the effect which is just opposite to that of the external intercostal muscles. This is because of the leverage mechanism of the direction of the muscle fibres which slope downward and backward (Fig. 5.2-3). Hence, their contraction tends to pull all the ribs downwards, thereby reducing anteroposterior diameter (because of falling of pump handle effect) as well as the transverse diameter (because of action of ribs like falling of bucket handle) of the thoracic cavity.
Note. Besides their role in deep breathing, the expiratory muscles are also involved in other forced expiratory efforts, e.g. in coughing, vomiting, defaecation and Valsalva manoeuvre, etc.
Pressure and volume changes during respiratory cycle
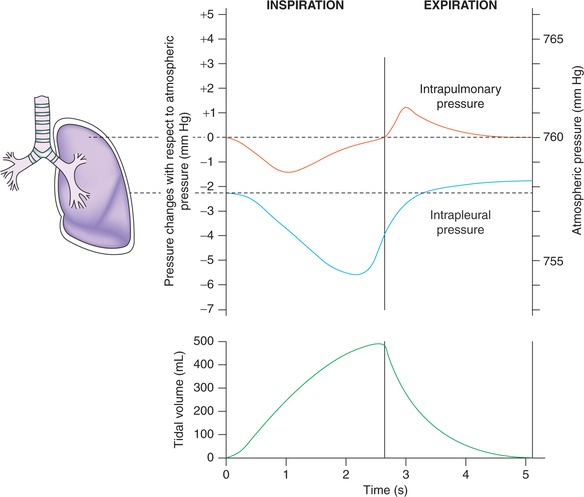
Intrapulmonary pressure changes during respiratory cycle (Fig. 5.2-4)
The movement of air in and out of the lungs depends primarily on the pressure gradient between the alveoli and the atmosphere (i.e. transairway pressure). Intrapulmonary or alveolar pressure is the air pressure inside the lung alveoli.
At end expiration and end inspiration, i.e. when the glottis is open and there is no movement of air, the pressures in all parts of the respiratory tree are equal to atmospheric pressure; the intrapulmonary pressure is considered to be 0 mm Hg.
During inspiration in quiet breathing, the pressure in the alveoli decreases to about −1 mm Hg, which is sufficient to suck in about 500 mL of air into the lungs within 2 s period of inspiration. At the end inspiration the intrapulmonary pressure again becomes zero.
During forced inspiration against a closed glottis (Muller's manoeuvre) the intrapulmonary pressure may be as low as −80 mm Hg below the atmospheric pressure.
During expiration in quiet breathing the elastic recoil of the lungs causes the intrapulmonary pressure to swing slightly to the positive side (+1 mm Hg), which forces the 500 mL of inspired air out of the lungs during 2–3 s of expiration. At the end expiration, once again the alveolar pressure regains the atmospheric pressure (0 mm Hg).
Forceful expiration against the closed glottis (Valsalva's manoeuvre) may produce intrapulmonary pressure of as much as 100 mm Hg.
Intrapleural (pleural) pressure changes during respiratory cycle (Fig. 5.2-4)
Pleural pressure is the pressure of fluid in the space between the visceral pleura and parietal pleura.
Normal pleural pressure when the respiratory muscles are completely relaxed and the airways are open is about −2.5 mm Hg.
• The negative pleural pressure (−2.5 mm Hg) is the amount of suction required to hold the lungs at their equilibrium volume or the functional residual capacity (FRC). FRC is the lung volume at the end of normal (eupnoeic), relaxed expiration, and is about 2–2.5 L of gas.
• The negative pleural pressure (−2.5 mm Hg) is the result of balance of two opposite forces, the recoil tendency of the lungs and the recoil tendency of thoracic cage.
• Recoil tendency of the lungs (continuous tendency to collapse) is caused by:
– The presence of many elastic fibres in the alveolar walls which are under constant stretch in the inflated lungs, and
– Surface tension of the fluid lining the alveoli due to which the alveoli tend to become progressively smaller and collapse.
• Recoil tendency of the thoracic cage, i.e. a constant tendency to expand (to pop outward) is because of the fact that the chest wall is an elastic structure which is normally partially pulled inward. The elastic property of the thoracic cage is because of the elastic nature of ribs, muscles and tendons.
During inspiration due to expansion of the chest wall the pleural pressure becomes still more negative (−6 mm Hg) and pulls the surface of lungs with greater force creating negative intrapulmonary pressure.
During expiration. At the end of inspiration, the inspiratory muscles relax and the recoiling force of lungs begins to pull the chest wall back to expiratory position. At endexpiratory position, where the recoil force of the lungs and recoil force of thoracic cage balance, the pleural pressure returns back to −2.5 mm Hg.
Factors affecting pleural pressure
1. Deep inspirations. The pleural pressure may become as low as −30 mm Hg during deep inspiration.
2. Valsalva manoeuvre or when expiratory muscles are working against closed glottis, there occur marked decrease in the thoracic volume causing deflation of lungs. Under such circumstances the intrapleural pressure can become positive by 60–70 mm Hg (greater than atmospheric pressure), e.g. during defaecation and coughing.
3. Gravity. The pleural pressure in the standing position is more negative (–7 mm Hg) at the apices of the lungs as compared to the bases (–2 mm Hg). Because of this fact lungs are less expanded at the base, and even the airway at the base may become closed at the end of expiration. This is why during the first part of inspiration more of the inspired gas goes to the apices than bases of lungs.
4. Injury to the chest wall causing exposure of pleural cavity to the exterior causes air to enter the pleural cavity (pneumothorax) and the pleural pressure rises from subatmospheric level to atmospheric level. Since there is no opposing force to elastic recoil of the lung, so in pneumothorax the lung is collapsed.
5. Emphysema is a disease of the lungs in which lung elasticity is decreased or lost. Due to decrease in the recoiling force of lungs the intrapleural pressure becomes less negative (i.e. increases). Because of this reason in this disease the lung alveoli expand, and chest also expands and becomes barrel shaped.
Lung volume changes during respiratory cycle
• During tidal inspiration, the volume of air in the lungs increases by 500 mL (tidal volume).
• During tidal expiration, the elastic forces compress the gas in the lungs which starts flowing out, and at the end of expiration the volume of air in the lungs decreases by 500 mL (Fig. 5.2-4).
Lung volumes and capacities
Static lung volumes and capacities
Static lung volumes and capacities are not dependent on time factors hence expressed either in mL or L.
Lung volumes
The maximum volume to which a lung can be expanded has been divided into four non-overlapping volumes (Fig. 5.2-5).
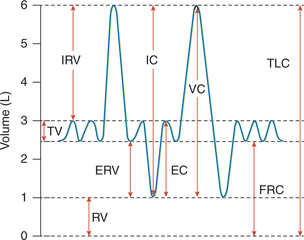
Fig. 5.2-5 A spirogram showing various lung volumes and capacities: RV = residual volume; TV = tidal volume; IRV = inspiratory reserve volume; ERV = expiratory reserve volume; FRC = functional residual capacity; TLC = total lung capacity; IC = inspiratory capacity; VC = vital capacity and EC = expiratory capacity.
1. Tidal volume (TV). It is the volume of air inspired or expired with each breath during normal quiet breathing. It is approximately 500 mL in normal adult male.
2. Inspiratory reserve volume (IRV). It is the extra volume of air that can be inhaled by a maximum inspiratory effort over and beyond the normal TV. It is about 3000 mL in a normal adult male.
3. Expiratory reserve volume (ERV). It is the extra volume of air that can be exhaled by the maximum forceful expiration over and beyond the normal TV (i.e. after the end of normal passive expiration). It is approximately 1100 mL in a normal adult male.
4. Residual volume (RV). It is the volume of the air that still remains in the lungs after the most forceful expiration. It is about 1200 mL in a normal adult male.
Lung capacities
Lung capacities are combination of two or more pulmonary volumes and include (Fig. 5.2-5).
1. Inspiratory capacity (IC). This is the maximum volume of the air that can be inspired after normal tidal expiration. Therefore, it equals the tidal volume plus inspiratory reserve volume (TV + IRV), and is approximately 3500 mL in a normal adult male.
2. Expiratory capacity. It is the maximum volume of air that can be expired after normal tidal inspiration. It equals tidal volume plus expiratory reserve volume (TV + ERV), and is approximately about 1600 mL in a normal adult male.
3. Functional residual capacity. It is the volume of the air remaining in the lungs after normal tidal expiration. Therefore, it equals the expiratory reserve volume plus the residual volume (ERV + RV), and is about 2300 mL in a normal adult male.
Significance of FRC. The FRC (RV + ERV) of about 2300 mL represents the air that remains in the lungs most of the times. Even after the most forceful expiration about 1200 mL (RV) air is always present in the lungs. This has several advantages:
(i) Continuous exchange of gases is possible due to the presence of some air always in the lungs, and thereby concentration of O2 and CO2 in blood are maintained constant.
(ii) Breath holding is made possible due to the FRC.
(iii) Dilution of toxic inhaled gases occurs due to the reserve of 2300 mL of air in the lungs (FRC) most of the times.
(iv) Load on respiratory mechanism and left ventricle would have been much more if there was no FRC, since:
Factors affecting FRC. Hyperinflation of the lungs seen in following conditions may be associated with increased FRC:
4. Vital capacity (VC). This is the maximum amount of air a person can expel from the lungs after the deepest possible inspiration. Therefore it equals tidal volume plus inspiratory reserve volume plus expiratory reserve volume (TV + IRV + ERV), and is about 4600 mL in a normal adult male.
Significance of vital capacity
• Estimation of VC allows assessment of maximum inspiratory and expiratory efforts, and thus gives useful information about strength of the respiratory muscles.
• VC also provides useful information about other aspects of pulmonary functions through forced expiratory volume (FEV) (see page 217).
Factors affecting vital capacity
(i) Size of the thoracic cavity. VC is more in males (2.6 L/m2 body surface area) because of large chest size and more muscle power than females.
(ii) Age. In old age VC is decreased due to decrease in elasticity of the lungs.
(iii) Strength of respiratory muscles. In swimmers and divers VC is more because of the increased strength of the respiratory muscles.
(iv) Gravity. In standing position VC is more than in sitting and lying position because of:
• Increased size of thoracic cavity in standing position (diaphragm moves down) and
• Reduced pulmonary blood flow due to decreased venous return.
(v) In pregnancy VC is reduced due to pushing up of diaphragm and reduced capacity of the thoracic cavity.
(vi) In ascites (accumulation of fluid in the abdominal cavity) VC is reduced due to same reason as in pregnancy.
(vii) Pulmonary diseases like pulmonary fibrosis, emphysema, respiratory obstruction, pulmonary oedema, pleural effusion and pneumothorax are associated in decreased VC.
5. Total lung capacity (TLC). It is the volume of air present in the lungs after the maximal inspiration. It equals the vital capacity plus the residual volume (VC + RV), and is about 5800 mL in a normal adult male.
All volumes and capacities except RV, FRC and TLC are recorded by a spirometer. FRC is determined by nitrogen washout method or helium dilution method, and then residual volume and total lung capacity are calculated.
Recording of lung volumes and capacities are the important lung function tests.
Dynamic lung volumes and capacities
Dynamic lung volume and capacities are dependent on time factor, therefore expressed as mL/min, L/min.
Timed vital capacity (TVC) or forced vital capacities (FVC)
Forced VC is the volume of the air that can be expired rapidly with a maximum force following a maximum inspiration. The volume of air expired can be timed by recording the VC on a spirograph moving at the known speed.
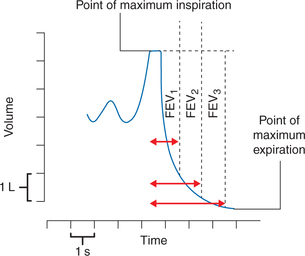
Components of TVC or FVC (Fig. 5.2-6)
(i) Forced expiratory volume in 1 s (FEV1). It represents the volume expired in the first second of an FVC.
• Estimation of FEV1 is the most commonly used screening test for airway diseases.
• The FEV1 is actually a flow rate; its unit are L/s.
• FEV1% is the percent of FVC expired in 1 s (i.e. FEV1% = FEV1/FVC × 100); normally FEV1% is about 80% of the FVC (Fig. 5.2-7A).
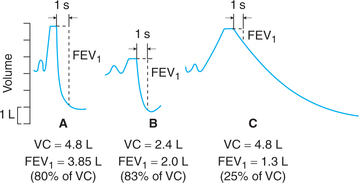
Fig. 5.2-7 Forced expiratory volume in first second (FEV1) component of timed vital capacity: A, in normal subject; B, in a patient with restrictive lung disease (RLD) and C, in a patient with obstructive lung disease (OLD).
Clinical application. Useful in distinguishing between restrictive and obstructive lung diseases.
• Patients with restrictive lung disease (e.g. kyphoscoliosis and ankylosing spondylitis) have a reduced FVC but are able to achieve relatively high flow rates; therefore their FEV1% exceeds 80% (Fig. 5.2-7B).
• Patients with obstructive lung disease (e.g. bronchial asthma) have low flow rates as a result of high airway resistance, therefore their FEV1% is abnormally low (Fig. 5.2-7C).
(ii) Forced expiratory volume in 2 s (FEV2). It represents the volume of air expired in first 2 s of FVC; FEV2% is about 90% of FVC under normal condition.
(iii) Forced expiratory volume in 3 s (FEV3). It represents the volume of air expired in first 3 s. Normally, FEV3% is 98%–100% of FVC.
Minute ventilation (MV) or pulmonary ventilation (PV)
It is the volume of air inspired or expired per minute. It equals the tidal volume multiplied by respiratory rate (TV × RR). The TV at rest averages 500 mL (0.5 L) and the normal respiratory rate is 12–15 breaths/min; therefore normal minute volume is 6–7.5 L/min.
Maximum breathing capacity (MBC) or maximum voluntary ventilation or maximum ventilation volume (MVV)
It is the maximum volume of air that can be ventilated on command during a given interval.
This index of ventilatory function depends upon the complete co-operation of the subject, who is asked to breathe as rapidly and deeply as he/she can for a 15 s interval. The volume of the air moved is either recorded by a spirometer fitted with a writing point or is collected in a Douglas bag; the result is expressed in L/min.
Normal adult male can attain a MVV of 80–170 L/min (average 120 L/min).
MVV is profoundly reduced in patients with emphysema, airway obstruction and very poor respiratory muscle strength.
Measurement of static lung volumes and capacities
Spirometry
Spirometry refers to recording of volume changes during various clearly defined breathing manoeuvres. It can be performed using a simple spirometer, a modified spirometer called respirometer or computerized spirometer.
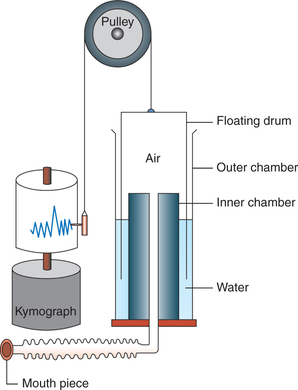
Simple spirometer (Fig. 5.2-8) is made of metal. It consists of following parts:
• Outer chamber or container which is filled with water.
• Floating drum or a gas bell with 6 L capacity floats in the water in an inverted manner. It is attached to a chain which passes over a pulley bearing a balancing weight and a writing needle (pen). The needle (pen) moves with the movement of the floating drum. The floating drum is thus counterpoised and has very little inertia and friction.
• Inner chamber is open at the top end which lies above the water level in outer chamber and is connected to a tube at the bottom end. At the end of tube a mouth piece is attached through which the subject is made to respire.
• Kymograph is a recording drum on which the movements of the needle are recorded.
Pulmonary elastance and compliance
Pulmonary elastance
Elastance refers to the recoil (retractive) tendency of a structure. Both the thoracic cage and lungs have elastance.
Elastance of thoracic cage
Elastance or recoil tendency of the thoracic cage refers to the constant tendency of the thoracic cage to expand (to pop outward). The elastic property of the thoracic cage is because of the elastic nature of ribs, muscles and tendons.
Elastance of lungs
Elastance or recoil tendency of the lungs refers to the constant tendency of the lungs to collapse. The recoil forces in the lungs are generated by tissue and surface forces.
1. Tissue forces. These are due to the presence of many elastic tissues such as smooth muscle, elastic and collagen in the lung parenchyma which are kept under constant stretch in the inflated lungs.
2. Surface forces. These are generated at the alveolar surface lined by fluid (alveolar surface tension) due to which the alveoli tend to become progressively smaller and tend to collapse.
Alveolar surface tension
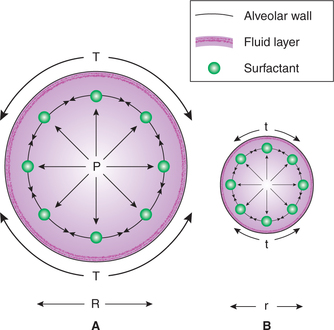
Alveolar surface tension is generated because of the unbalanced attraction of the liquid molecules at the surface of alveolar membrane. A phase change occurs between the alveolar gas and the surface of the alveolar membrane. Alveolar surface tension increases the tendency of the lungs to deflate. An increased transmural pressure is necessary to counteract the effects of surface tension. According to the law of Laplace, in a spherical structure like alveoli, the transmural pressure generated equals two times the (surface) tension divided by radius, i.e. P = 2T/r. Therefore, small alveoli tend to become still smaller whereas large alveoli tend to become still larger (Fig. 5.2-9). Thus, surface tension tends to produce collapse of the alveoli.
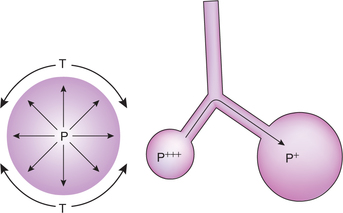
Fig. 5.2-9 Pressure in the alveoli (P) is directly proportionate to the surface tension (T) and inversely proportionate to the radii (r). Therefore small alveoli tend to become still smaller and large alveoli tend to become still larger.
Pulmonary surfactant
Pulmonary alveolar surface tension opposes the expansion of the lungs during inspiration. However, the presence of pulmonary surfactant in the fluid lining the alveoli reduces the surface tension markedly.
Source. Pulmonary surfactant is secreted by type II alveolar epithelial cells (granular pneumocytes).
Composition. Pulmonary surfactant is a complex mixture of several phospholipids, proteins and ions. Dipalmitoylphosphatidylcholine (DPPC) along with several other phospholipids is responsible for reducing surface tension.
Surface tension with normal alveolar fluid lining without surfactant is about 50 dynes/cm2, and with surfactant it varies between 5 and 30 dynes/cm2, depending upon the concentration of surfactant.
Functions of pulmonary surfactant
1. Reduces the tendency of alveoli to collapse. The surface tension in a thin-walled sphere like alveolus tends to make the sphere smaller to collapse. Surfactant, by reducing surface tension, decreases the tendency to collapse.
2. Reduces work of breathing. According to the Laplace's law, due to reduction in the surface tension the mean alveolar radius is increased. This reduces the transmural pressure required for expanding the alveoli. As alveoli are easily expanded, so work of breathing is reduced. The low surface tension also facilitates the reopening of collapsed airway and alveoli.
3. Prevents pulmonary oedema. Surface tension is retracting force which not only pulls alveolar wall to the centre of alveolus but also pulls fluid from the capillaries into the interstitial space surrounding the alveoli and into the alveoli leading to pulmonary oedema. Surfactant prevents this phenomenon by lowering the surface tension.
4. Alveolar stabilization. Surfactant causes stability of alveoli, i.e. it maintains almost uniform size of alveoli.
Pulmonary surfactant causes alveolar stabilization by following mechanisms.
• In the presence of surfactant the surface tension developed in the alveoli is inversely proportional to the concentration of surfactant per unit area.
• In the smaller alveolus the surfactant molecules form a thick layer while in a larger alveolus the surfactant molecules are scattered on the larger surface (as number of molecules is limited) (Fig. 5.2-10).
• Thus, in a smaller alveolus the tendency to develop more pressure due to smaller radius will be neutralized by the tendency to develop less pressure due to more concentration of surfactant per unit area; and the reverse will occur in larger alveolus. In this way there will not be much pressure gradient between the two alveoli helping to maintain the size of alveolar sac constant. Thus, due to the presence of surfactant, stability of alveoli is maintained.
Compliance
Definition and normal value
• Compliance (C) refers to change in lung volume (ΔV) per unit change in transpulmonary pressure (ΔP), i.e.
Transpulmonary pressure is the difference in the pressure between the alveolar pressure and the pleural pressure.
• Compliance expresses the distensibility (expansibility) of the lung and chest wall.
• Normal value of compliance for the lungs and chest wall combined is 0.13 L/cm of H2O and for the lung alone it is 0.22 L/cm H2O.
Static versus specific lung compliance
Static compliance. The static compliance of any system is dependent on its size. Thus the lung compliance depends upon the amount of functional lung tissue. Thus, static compliance is not a good measure of absolute distensibility.
Specific compliance is the compliance of the lung at relaxtion volume (the point at the end of a tidal expiration), i.e. the FRC. The specific compliance is expressed per litre of FRC. It is a measure of the absolute distensibility of a structure.
Factors affecting lung compliance
Lung compliance is inversely proportional to the lung elastance (elastic recoil force). Therefore, lung compliance is determined on the basis of the following elastic forces.
Elastic forces of the lung tissues due to elastic and collagen fibres contribute smaller amount of elasticity.
Elastic forces caused by surface tension within the alveoli account for about two-third of total elastic forces in the lungs.
Alveolar surface tension plays a major role in generating elastance forces in the lungs, and thus is a very important factor affecting lung compliance.
Changes in the lung compliance
Decreased compliance of the lungs can be caused by lung diseases (e.g. tuberculosis, silicosis) that produce scarring or fibrosis of the lungs, destruction of the functional lung tissue or both. Reduced compliance produces a condition termed restrictive lung disease (RLD). Patients with RLD must generate greater than the normal forces to expand the lungs.
Increased compliance is produced by the pathologic processes that occur in emphysema as well as from the aging process. Alveolar septa which provide some of the retractive forces in lungs are destroyed in both conditions, but emphysema causes a much more extensive loss of septa than the normal aging process.
Work of breathing
The contraction of respiratory muscles causes the expansion of thoracic cage, and thereby causes expansion of lungs and fall in intra-alveolar pressure and allows atmospheric pressure to push air into the lungs during inspiration. Thus, to move the air into the lungs the respiratory muscles have to do work to overcome the following resistances.
Resistance to breathing
Tissue resistance. It is the resistance offered by the tissues as they expand or contract. Tissue resistance comprises of the following:
1. Elastic resistance. It is the sum of forces of elastic recoil exerted by the lung and chest wall. The elastic recoil of the lungs is due to the presence of elastic fibres in the lungs and due to the alveolar surface tension.
2. Viscous resistance is the resistance offered by the nonelastic tissues in the lungs.
Airway resistance. It is the resistance caused by the friction of gas molecules between themselves and the walls of the airways. Factors affecting airway resistance are given below:
1. Rate of gas flow. Greater the rate of gas flow, greater is the resistance. Rate of gas flow is highest in the intermediatesized bronchi as they have highest cross-sectional area; so the highest resistance to flow occurs in this part of tracheobronchial tree.
2. Airway radius. It is the most powerful determinant of resistance. Smaller the radius of airway, greater is the resistance. According to the Poiseuille–Hagen formula (see page 161):
In other words, if radius decreases by half (keeping the other factors constant), the resistance increases by 16 times.
Airway radius increases during inspiration and decreases during expiration. Therefore, airway resistance is high during expiration as compared to inspiration. Because of this reason in bronchial asthma patients (where bronchoconstriction develops) inspiration is possible but there is extreme difficulty in expiration.
3. Length of airway. It does not change much during respiration or in lung diseases and, therefore, is not an important factor.
4. Type of air flow. The airway resistance is more in a turbulent flow (e.g. during rapid respiration) than in a laminar flow or streamline flow in quiet breathing.
Components of work of breathing
The total work done by the respiratory muscles during quiet breathing may be divided into following components:
The work done during normal inspiration can be divided into three fractions.
Compliance work refers to the work done by respiratory muscles to inflate the lungs against the elastic resistance of chest wall and lungs. Thus most of the work done (65%) is used to overcome elastic resistance.
Non-elastic resistance work is done to overcome the nonelastic resistance. It includes the work done to overcome:
Thus only a small amount (7%) of the work done is used to overcome the viscosity of the lungs and 28% of the work done is utilized to overcome the resistance of air flow through the respiratory passages.
Work done during expiration
Since in quiet breathing expiration is a passive process so no work is done during expiration. This stored energy can compress the alveolar gas and create expiratory flow. When the lungs are recoiling back some energy is required to overcome non-elastic resistance, i.e. the airway resistance plus viscous tissue resistance. In normal quiet expiration stored energy is utilized to overcome this resistance, thus no extra work is done.
 Mechanism of breathing
Mechanism of breathing