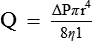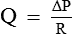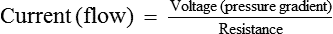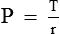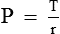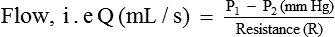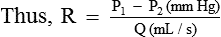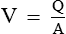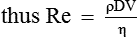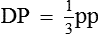Dynamics of Circulation: Pressure and Flow of Blood and Lymph
Functional organization and Structure of vascular system
Pressure and flow in various Functional segments of systemic Vascular tree
Introduction
Dynamics of circulation is concerned with flow of blood and lymph and also the pressure in the various segments of the vascular system of the body. For descriptive purposes, the ‘dynamics of circulation’ is discussed under following headings:
Functional organization and structure of vascular system
Organization of vascular system
The vascular system is organized into two separate circulations, systemic and pulmonary, arranged in series. In addition, parallel to the circulation of blood is disposed circulation of lymph, which helps the blood circulation to perform its various functions.
Systemic circulation supplies blood to various systemic organs through parallel distribution channels (Fig. 4.4-1).
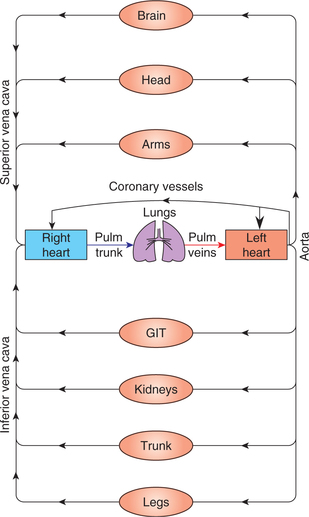
Fig. 4.4-1 A schematic illustration of the organization of cardiovascular system depicting in series arrangement of pulmonary and systemic circulation and parallel arrangement of vessels supplying blood to the organs.
This parallel arrangement of vessels ensures the supply of blood of the same arterial composition (i.e. same O2 and CO2 tension, pH, glucose level and essentially the same arterial pressure) to the various body organs. In systemic circulation, from the left ventricle, blood is pumped through the arteries and arterioles to the capillaries, where it equilibrates with the interstitial fluid. The capillaries drain through the venules into the veins and ultimately to the right atrium.
Pulmonary circulation is meant for oxygenation of blood. Since pulmonary circulation is arranged in series with systemic circulation, so it receives the same amount of blood over any significant time period. In pulmonary circulation, from the right ventricle, blood is pumped through the pulmonary arteries to the pulmonary capillaries. In the pulmonary capillaries the blood equilibrates with the O2 and CO2 of alveolar air. The capillaries then drain the oxygenated blood through venules and then pulmonary veins into the left atrium.
Lymphatic circulation, which is disposed in parallel to the circulation of blood can be considered a third type of circulation. Some tissue fluid enters the lymphatic channels as lymph, which is ultimately drained into the venous system via the thoracic lymphatic duct and the right lymphatic duct.
Systemic vascular tree
For descriptive purposes and from functional point of view, the systemic vascular tree can be divided into following types of blood vessels:
• Large elastic arteries (windkessel vessels) include aorta and its main branches, such as carotid, iliac and axillary arteries.
• Large muscular arteries (distribution vessels) which include most of the arteries of the body, e.g. arteries like radial, ulnar, popliteal.
• Arterioles and precapillary sphincters (resistance vessels).
• Meta-arterioles and capillaries (exchange vessels).
• Venules (postcapillary resistance vessels).
• Veins (capacitance vessels).
• Arteriovenous anastomoses (shunt or thoroughfare vessels).
Structure of blood vessels
Structural characteristics
General structural characteristics
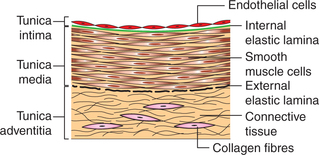
Histologically, walls of most of the blood vessels except the capillaries consist of three coats. General structural characteristics of a large artery are as follows (Fig. 4.4-2).
1. Tunica intima. It is the innermost coat of the vessel wall. In large arteries, from inside to outside, it consists of:
• Endothelial lining, which is very smooth and silky, and consists of a single layer of cells. It lies in contact with the blood.
• Basal lamina is a thin layer of glycoprotein which lines the external aspect of the endothelium.
• Subendothelial connective tissue is a delicate layer of connective tissue which lies outside the basal lamina.
• Internal elastic lamina is a thin membrane formed by the elastic fibres.
2. Tunica media. It is the middle, thickest coat of the vessel wall. It consists of smooth muscles and elastic tissue. The ratio of these two tissues varies from vessel to vessel. On the outside, tunica media is limited by a membrane formed by the elastic fibres called the external elastic lamina.
3. Tunica adventitia. It is the outermost coat of the vessel wall. It is made of connective tissue in which collagen fibres are prominent. This layer prevents undue stretching or distension of the blood vessel.
Essential characteristics
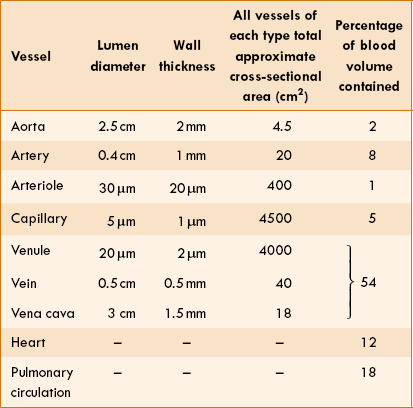
Essential characteristics of blood vessels like lumen diameter, wall thickness, approximate total cross-sectional area and percentage of blood volume contained are shown in Table 4.4-1.
Innervational characteristics
• Smooth muscles of the blood vessels are innervated by the sympathetic fibres. These muscles contain α adrenergic receptors. Therefore, noradrenaline causes contraction of muscle fibres leading to vasoconstriction.
• Sympathetic fibres exert tonic effect even at rest, resulting in the existence of vasomotor tone in the blood vessels.
• Stimulation of sympathetics increases the vasomotor tone; as a result the vessels are constricted and narrowed.
• Inhibition of sympathetic discharge results in a decreased vasomotor tone and hence vasodilatation.
• Skeletal muscle arterioles also contain β2 receptors in addition to the α receptors; therefore adrenaline causes dilatation of these vessels.
• Besides α adrenergic receptors, smooth muscles of the blood vessels are also stimulated by other agents like O2 tension, lactic acid, etc.
Haemodynamics
Haemodynamics, which refers to the study of blood flow in various segments of the vascular system, can be discussed under following headings:
• General principles governing (factors affecting) blood flow,
• Distribution of blood flow to various regions of the body and
General principles governing (factors affecting) blood flow
Flow–pressure–resistance relationship
Relationship between the flow of a fluid with the pressure and resistance offered to it through a rigid tube was studied by a French physiologist Poiseuille in 1842 and Hagen. This relationship is known as Poiseuille's law or Poiseuille–Hagen law.
Poiseuille's law
Poiseuille's law expressing the relation between the flow (Q) and pressure gradient (ΔP) in a long narrow tube of length (L), the viscosity of fluid (η) and the radius (r) of the tube is as:
Thus, according to the Poiseuille's law, the flow (Q) of a Newtonian fluid through a rigid tube is determined by the following.
1. Pressure gradient (ΔP). i.e. difference in the pressure between the two ends of the tube. In other words, fluid always flows from an area of high pressure (P1) to one of lower pressure (P2), and rate of flow (Q) is determined by the pressure gradient (P1 − P2), i.e. Q ∝ ΔP or (P1 − P2).
2. Radius of tube. The flow of fluid varies directly as the fourth power of radius (r4). Thus if the radius is halved, the flow will decrease by 16 times and vice versa. Thus this factor is very important for the flow of blood through the blood vessels.
3. Viscosity of fluid (η) The flow of fluid varies inversely with the viscosity of fluid, i.e. greater the viscosity, lesser the flow and vice versa.
4. Length of the tube (L) The flow is inversely proportional to the length of the tube. This is easily understandable, as every segment of the tube is offering resistance to the flow; therefore, longer the length greater will be the total resistance offered.
Resistance (R) According to mathematical calculation in principles of physics, resistance (R) is represented by 8 Ln/πr4. By replacing 8 Ln/πr4 with R in the Poiseuille's law, it becomes:
Thus, the Poiseuille's law can be considered analogous to the Ohm's law of current in which:
Hence, the rate of flow (Q) is inversely proportional to the R.
The Poiseuille's law is valid for straight rigid tubes with Newtonian fluid flowing through them. Since, the blood vessels are not rigid and the blood is not a Newtonian fluid, therefore, strictly speaking the Poiseuille's law does not apply to flow of blood through the vascular system. Nevertheless, the important principles relating flow, pressure gradient and resistance remain applicable, so they are discussed in relation to blood flow as:
Blood flow and pressure gradient relationship
According to the Poiseuille's law, fluid always moves from an area of higher pressure to the one of lower pressure. This downhill movement of blood (i.e. along the pressure gradient) occurs in the vascular system. In the systemic circulation, pressure at the beginning of aorta is about 100 mm Hg and is nearly zero near the terminal portion of inferior vena cava (Fig. 4.4-3). Therefore, P1 − P2 = (100 − 0) = 100 mm Hg. Note the progressive decrease in pressure from the left ventricle through the systemic circulation until the blood enters the right ventricle. It is important to note that greatest pressure drop occurs in the arterioles, which represent the highest resistance segment of the systemic circulation.
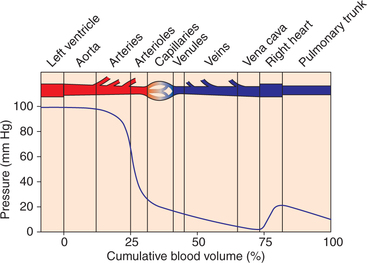
Fig. 4.4-3 Mean lateral pressure in various components of vascular system and cumulative blood volume.
Critical closing pressure
Since, the flow–pressure relationship in a rigid tube is linear, so flow will cease only if the pressure is zero (Fig. 4.4-4A). However, in a blood vessel the flow ceases when the blood pressure is 20 mm Hg or even more (Fig. 4.4-4B). The pressure value at which the vessel collapses, its lumen closes and flow ceases is called critical closing pressure (CCP).
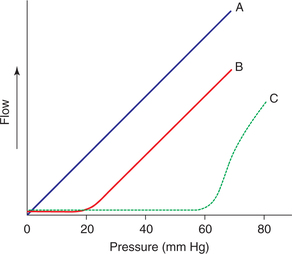
Fig. 4.4-4 Relationship of pressure and flow in a rigid tube A, in a blood vessel; B, and on sympathetic stimulation; C, depicting critical closing pressure.
The blood flow ceases when the blood pressure falls below the CCP because:
• Certain amount of intramural pressure is essentially required to push the RBCs (with average diameter of 7.5 μm) through the capillaries (average diameter 5 μm).
• Further, the tissue pressure exerted over the vessels also causes their collapse. So, certain amount of intramural pressure is must to counteract the tissue pressure and thus to keep the vessels patent and maintain the blood to flow.
Equilibrium of factors at critical closing pressure
• In general, vasomotor tone of the blood vessels tries to constrict the vessels.
• Intramural pressure tries to dilate the blood vessels up to a certain limit. When a point is reached where the intramural pressure (P) is not able to maintain an equilibrium with tension (T) in the vessel wall. At this point, the blood vessel closes and the pressure at which it occurs is called CCP. Laplace law described below helps to explain this equilibrium relationship.
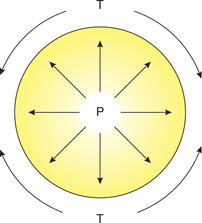
Law of Laplace
Law of Laplace governs the relation between the distending pressure and tension in the wall of a distensible viscus including blood vessels (Fig. 4.4-5). According to this law, the distending pressure (P) in a distensible hollow object is equal at equilibrium to the tension in wall (T) divided by the two principal radii of curvature of the object (r1 and r2):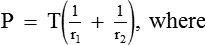
• In a sphere, r1 = r2. Therefore,
• In a cylinder such as a blood vessel, one radius is infinite. Therefore,
Physiological applications of law of Laplace
This law applies to all the hollow viscous structures in the body. Some of its important applications are discussed below.
In vascular system. As described above, for the blood vessels Laplace equation is P = T/r. This equation shows that smaller is the radius of a blood vessel, lesser is the tension (T) on the walls of the blood vessel required to balance the distending pressure or force (P). For example:
• In aorta the tension at normal pressure is 1,70,000 dynes/cm.
• In inferior vena cava it is about 21,000 dynes/cm.
• But in the capillaries, it is approximately 16 dynes/cm. This explains, why capillaries being so thin walled and delicate are not prone to rupture.
In heart. Law of Laplace also explains the disadvantage faced by a dilated heart. When the radius of a cardiac chamber is increased, a greater tension must be developed in the myocardium to produce any given pressure; consequently, a dilated heart must do more work than a non-dilated heart.
Flow and resistance relationship
From the Poiseuille's law, it can be derived that the flow is inversely proportional to the resistance, i.e. Q ∝ 1/R; and that resistance is represented by 8 Lη/πr4, i.e. resistance depends upon the length (L) and fourth power of radius (r4) of the tube and viscosity of the fluid (η).
Peripheral resistance unit (PRU)
As discussed above, the Poiseuille's law can be considered analogous to the Ohm's law, so:
Total peripheral resistance (TPR)
At rest, the resistance (R) of the entire systemic circulation is called ‘total peripheral resistance’. Its values are:
Factors that affect resistance to blood flow
The major factors that determine the resistance to flood flow are:
I Blood viscosity and resistance
Definition and unit of viscosity
Viscosity was described by Isaac Newton in 1713 as an internal friction to flow in a fluid or lack of slipperiness. These terms emphasize that when fluid moves along a tube, laminae in the fluid slip on one another and move at different speeds thereby causing a velocity gradient in a direction perpendicular to the wall of the tube. Thus, resistance met by the fluid moving through a tube in a streamlined flow is due to the friction between adjacent laminae and not due to the friction between vessel wall and fluid. Thus, greater the internal friction, greater is difference in velocity (shear rate) between two laminae and greater the coefficient of viscosity.
Unit of viscosity is Poise (after Poiseuille). A fluid of 1 poise viscosity has a force of 1 dyne/cm2 of contact between layers when flowing with a velocity gradient of 1 cm/s. One Poise is considered to be consisting of 100 centipoise (CP). Viscosity of water at 21°C is 0.01 poise or 1 centipoise.
Relative viscosity is a more often used term and refers to the viscosity of a fluid relative to the viscosity of water at body temperature (37°C).
II Radius of blood vessels and resistance
As mentioned earlier, the rate of flow is proportional to the fourth power of the radius (r4) of the blood vessels. Thus, even a small change in the calibre of the blood vessels will produce a marked change in the blood flow. For example, at a pressure head of 100 mm Hg, the change in the flow rate with change in the radius:
| Radius | Flow rate |
| 1 mm | 1 mL/min |
| 2 mm | 16 mL/min |
| 4 mm | 256 mL/min |
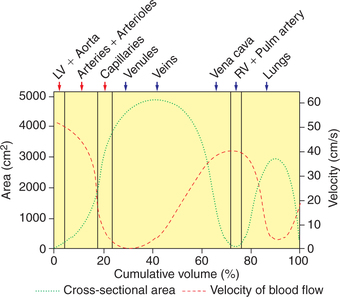
Among the blood vessels, in aorta and large arteries, there is little resistance. Arterioles are the major seat of vascular resistance.
Velocity of blood flow
The velocity of blood flow refers to the displacement of blood per unit time, i.e. cm/s, while, the rate of blood flow is the amount of blood flowing per unit time, i.e. cm3/s. The physiologically important aspects to be considered in relation to velocity of blood flow and cross-sectional area are as follows.
Velocity and cross-sectional area relationship
• The relationship between velocity (V), quantity of blood flow (Q) and cross-sectional area (A) of the blood vessel is:
• Thus, if quantity of blood flow (Q) remains constant and the cross-sectional area (A) increases, then the velocity of blood flow will decrease.
• Since, the cross-sectional area of capillaries is 1000 times as that of the aorta, so the velocity of blood flow in the capillaries is approximately 1 mm/s as compared to 40 cm/s in the aorta.
• The total cross-sectional area of various types of blood vessels, the velocity of blood flow and pressure in the various segments of the cardiovascular system plotted as a function of the cumulative blood volume is shown in Fig. 4.4-6 and Table 4.4-1
Blood flow: types and distribution
Types of blood flow
Blood flow in the vascular system is of two types:
Laminar blood flow
Blood flow in the blood vessels is normally in streamline, like the flow of liquids in narrow rigid tubes. Such a blood flow is called laminar blood flow and is considered to consist of a series of thin laminae slipping over one another.
• The outermost lamina, i.e. an infinitely thin layer of blood in contact with the wall of the blood vessel, does not move and the next lamina has some velocity. The subsequent inner layers have progressively increasing velocity, and thus the innermost lamina, i.e. the core of the blood stream, has the maximum velocity (Fig. 4.4-7).
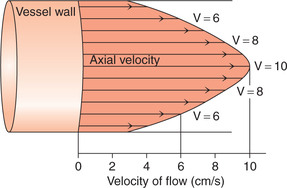
Fig. 4.4-7 Laminar blood flow showing different velocities of the different laminae resulting in a parabolic distribution of velocities. Note that the central core of blood stream has greatest velocity.
• The laminar blood flow being streamlined is noiseless and within physiological limits shows a linear relationship with the pressure.
Turbulent blood flow
The above described laminar blood flow occurs up to a certain velocity, at or above which the blood flow becomes turbulent. The velocity of flow at which blood flow becomes turbulent is called critical velocity.
• In turbulent blood flow, the blood moves in an irregular varying paths continuously mixing within the vessel and colliding with the vessel wall. This causes a greater energy loss as compared to the laminar flow.
• The turbulent blood flow is noisy and does not show a typical linear relationship with the pressure.
• Normally, none of the small vessels show turbulent flow. In humans, the critical velocity sometimes exceeds in the aorta at the peak of systole.
The chances of blood flow becoming turbulent are determined by the probability of turbulence, which is denoted as Re (Reynold number), named after the person who described it.
• Directly proportional to the:
• Blood flow is usually not turbulent when Re is less than 2000.
• Chances of blood flow becoming turbulent are increased when Re exceeds 2000.
When Re is more than 3000, turbulence is almost always present.
Conditions associated with turbulent blood flow
• Constriction of the artery by an atherosclerotic plaque or by any other cause, e.g. application of external pressure while measuring the blood pressure with a sphygmomanometer is associated with a blood flow velocity, which exceeds critical velocity and thus causes the turbulent blood flow. The turbulent flow generates vibrations (sounds) which can be heard over the artery by a stethoscope, e.g. Korotkoff sounds heard while recording the blood pressure or the murmur heard over a constricted artery.
Distribution of blood flow to various regions of the body
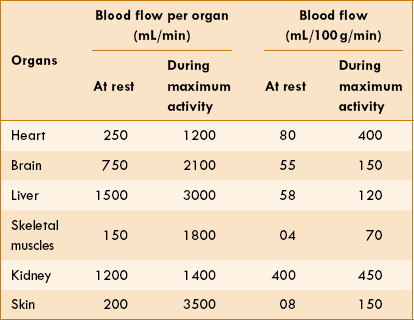
At rest, about 5 L of blood enters aorta per minute. In terms of tissue weight, blood flow to liver, brain and heart is very high. Kidney has a high blood flow because it is related to the excretory function rather than the metabolic requirement. The distribution of blood flow to various organs and regions of the body during resting conditions and during maximum activity conditions is shown in Table 4.4-2.
• At rest, at least 50% of the circulating blood volume is in the systemic veins.
• Twelve percent is in the heart cavities, and 18% in the lower pressure pulmonary circulation. Only 2% in the aorta, 8% in the arteries, 1% in the arterioles and 5% in the capillaries.
• When extra blood is administered by transfusion, less than 1% of it is distributed in the arterial system (the high pressure system) and all the rest in the systemic veins, pulmonary circulation and heart chambers other than the left ventricle (the ‘low pressure system’).
Pressure and flow in various functional segments of systemic vascular tree
Pressure and flow functions of elastic arteries
The large elastic arteries (windkessel vessels) include aorta and its main branches, such as carotid, iliac and axillary arteries. These vessels contain elastic tissue in their walls in abundance, which provides them two properties of distensibility and elastic recoil. The effect of these two properties of the elastic arteries on pressure and flow of blood is given below.
Distensibility
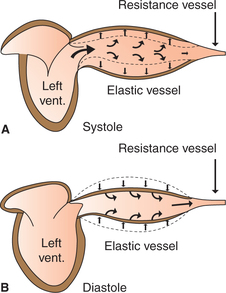
As we know, the heart acts as a pump and ejects about 70 mL of blood into the aorta with each systole. The distensibility (compliance) of the elastic arteries allows them to accommodate the stroke volume of heart with only a moderate increase in pressure (from 80 to 120 mm Hg) (Fig. 4.4-8A). Due to distension of these vessels a part of energy released from the heart is stored as the potential energy in the wall of aorta.
Elastic recoil
During diastole, the stretched elastic wall of the aorta recoils and the potential energy stored in the wall is released onto the blood. This causes the blood to flow during diastole also; in this way the pressure in the aorta does not fall below 80 mm Hg (Fig. 4.4-8B). In other words, the elastic recoil of big arteries acts as a subsidiary pump for a continuous blood flow. This recoil effect is called windkessel effect. Windkessel is a German word meaning elastic reservoir.
Functions of elastic vessels
1. They reduce the velocity of blood flow to some extent during the ventricular contraction (systole) due to property of distensibility.
2. They cause increase in the velocity of blood flow to some extent during the ventricular diastole by elastic recoil. Thus, the windkessel effect reduces the energy expenditure of heart.
3. Pumping action of the heart along with the elastic recoil of aorta together constitutes a driving force for blood to move forward (towards periphery). This force is called vis-a-tergo force and is an important determinant for the venous return.
4. Conversion of pulsatile blood flow from the heart to a steady continuous flow. The elastic vessels act together with arterioles (resistance vessels) to convert this pulsatile flow into a steady continuous flow in the tissue capillaries, which allows maximum exchange between the blood and tissues.
Clinically applied aspects
1. Due to age-related degenerative changes, the elasticity of large vessels is decreased and so is the windkessel effect. Therefore, in old age systolic blood pressure increases due to loss of distensibility and diastolic blood pressure decreases (due to loss of elastic recoil). Thus, in normal healthy individual aged about 70 years, typical blood pressure is 160/70 mm Hg. That is, there occurs systolic hypertension with an increased pulse pressure (SBP-DBP).
2. Atherosclerotic changes in small blood vessels are also common in old age. These produce essential hypertension, i.e. an increase in the systolic as well as the diastolic blood pressure.
Pressure and flow functions of muscular arteries
The muscular arteries comprise most of the named arteries in the body, such as radial artery, facial artery, ophthalmic artery and so on. These arteries serve as the distributing channels to the organs.
Pressure and flow functions of arterioles
Structural characteristics
Each arteriole is only a few millimeter long and branches many a times to supply about 10−100 capillaries. The characteristic features of arterioles are given below.
• A thick muscular wall having a profuse vasomotor (sympathetic) innervations.
• A relatively narrow lumen (30 μm) because of which these vessels are considered the major site of peripheral resistance (PR). The arterial pressure drops by about 50 mm Hg while passing through a few millimetre long arterioles.
Functions of arterioles
1 Control of blood flow to the organs
The arterioles play a major role in the control of blood flow to the organs or tissues. So, they are considered stopcocks (valves) of circulation. The constriction of the arterioles increases the resistance and decreases the blood flow, whereas dilation of arterioles decreases the resistance and increases the blood flow. The arterioles control blood flow to the organs by the following two mechanisms.
Control of blood flow during alterations in blood pressure (autoregulation)
Autoregulation. It is the ability of an organ or tissue to adjust its vascular resistance and maintain a relatively constant blood flow over a wide range of arterial pressure (Fig. 4.4-9). Autoregulation is well developed in the kidney, brain, heart, skeletal muscle and mesentery. Two theories have been put forward to explain the phenomenon of autoregulation.
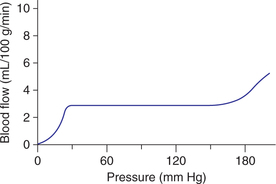
Fig. 4.4-9 Autoregulation of blood flow. Note: The blood flow remains relatively constant over a wide range of arterial pressure. This is accomplished by change in resistance proportionate to change in arterial pressure.
Metabolic theory proposed that an increased arterial blood pressure initially increases the blood flow to a tissue or organ. This increased blood flow washes out the vasodilator substances such as CO2, H+, nitric oxide, adenosine, prostaglandins, K+, phosphate ions and low oxygen levels in the area. As a result, the arterioles constrict, the vascular resistance increases and the blood flow returns to normal.
Myogenic theory. According to this theory, the vascular smooth muscle (VSM) responds to wall tension. An increase in the arterial pressure initially stretches the smooth muscle fibres, in response to which the VSM contracts, increases the resistance and compensates for the higher arterial pressure, returning the blood flow to control levels.
Microcirculation
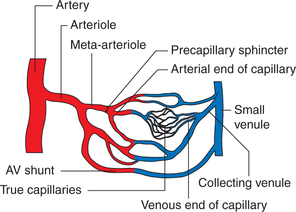
Architecture of microcirculation
The microcirculation involves a meshwork of vessels that are less than 100 μm in diameter. These include small arterioles, meta-arterioles, capillaries, postcapillary venules and arteriovenous shunts (Fig. 4.4-10).
Meta-arterioles. The arterioles divide into smaller muscles walled vessels, sometimes called meta-arterioles, and these in turn feed into capillaries.
Precapillary sphincters refer to a cuff of smooth muscle cells that surround the origin of capillaries. These determine the size of the capillary exchange area at one particular moment in the tissue. For example, increase in the sphincter patency increases number of open capillaries. Precapillary sphincters respond to local or circulating vasoconstrictor substances.
Capillaries arise directly from the arterioles or metaarterioles. These vessels allow easy exchange of gases and nutritive substances across them and so are also called as exchange vessels. Capillaries constitute the most important segment of the circulatory system.
Postcapillary venules, which measure 20−60 μm in diameter, are the most permeable part of the microcirculation.
Arteriovenous anastomosis (shunt or thoroughfare vessels). These are short, low-resistance connections between the arterioles and veins, bypassing the capillaries. These are abundantly innervated by the vasomotor sympathetic fibres. These vessels are especially found in the skin of fingers, toes and ear lobes, where they are involved in the regulation of body temperature.
Structural characteristics of capillaries
Each capillary has an average diameter of 5 μm, length of 50 μm, wall thickness of 1 μm and cross-sectional area of 40 μm2.
The capillary wall essentially consists of a single layer of endothelial cells which are lined on the outside by a basal lamina (glycoprotein), overlying the basal lamina there may be isolated branching perivascular cells called pericytes.
The endothelial structure of capillaries varies in different organs depending on the function of the particular tissue. Under the electron microscope, three types of capillaries have been identified.
1. Continuous capillaries are characterized by a single layer of endothelial cells which are almost continuous, except for small clefts of 6−7 nm in size in between the cells. It is believed that most of the water-soluble ions (Fig. 4.4.11A) and molecules pass across the capillary through these clefts (or slit pores). These are the most common type of capillaries and are found in most of the body tissues, viz. skeletal muscle, adipose tissue, connective tissue, pulmonary circulation and so on.
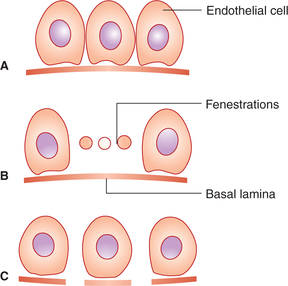
Fig. 4.4-11 Structure of different types of capillaries: A, continuous; B, fenestrated and C, discontinuous.
2. Fenestrated capillaries consist of thin endothelial cells with large fenestrations (20−100 nm in diameter) in between, which are bridged by a thin basement membrane that surrounds the endothelial cells (Fig. 4.4-11B). The fenestrations permit the passage of relatively large molecules and make the capillaries porous. Fenestrated capillaries are found in the renal glomeruli, intestinal villi and most endocrinal glands.
3. Discontinuous capillaries are characterized by large gaps (600−3000 nm in diameter) between endothelial cells that are not closed by the basement membrane (Fig. 4.4-11C). Through these gaps even formed elements of blood can pass freely. Such capillaries are also called sinusoids and are found in the bone marrow, liver and spleen.
Functions of capillaries
The primary function of circulation is to transport nutrients to the tissues and remove waste products—occur in the capillaries. About 10 billion capillaries which have a total surface area 500–700 m2 provide this function for the body. The cross-sectional area of capillary bed when fully patent is 2800 times that of aorta.
In active tissues, the meta-arterioles and the precapillary sphincters dilate and the blood flows through all the capillaries (active capillaries).
The opening and closing of the precapillary sphincters is controlled mostly by the local metabolic vasodilators and possibly also through sympathetic innervation.
Transcapillary exchange
The capillary blood brings oxygen, electrolytes and nutrients to the tissues and removes the waste products of cellular metabolism. The exchange of these substances occurs across the thin membrane formed by the endothelial cells.
Mechanisms of transcapillary exchange
Exchange of substances occurs primarily in the capillaries and postcapillary venules. The major mechanisms of exchange are diffusion and filtration (bulk flow). Some substances also pass through the cells by the vesicular transport.
Filtration and reabsorption across microvascular endothelium
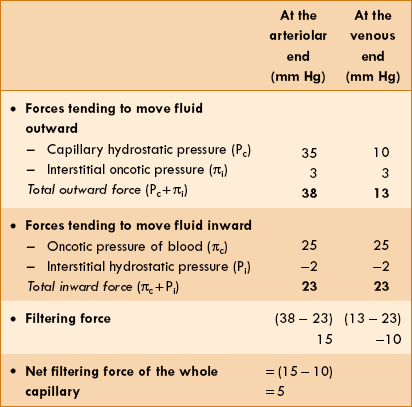
The rate of filtration and absorption at any point along the capillary wall depends on the balance of forces known as Starling forces. According to the Starling hypothesis, the filtration absorption is expressed as: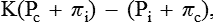 K = the permeability-surface area coefficient, Pc = the hydrostatic capillary pressure, Pi = the hydrostatic interstitial pressure, πc = oncotic pressure of blood and πi = oncotic pressure of the interstitium.thus, Pc − Pi, represents the hydrostatic pressure gradient and πc − πi, represents the oncotic pressure gradient. Starling forces are defined as follows.
K = the permeability-surface area coefficient, Pc = the hydrostatic capillary pressure, Pi = the hydrostatic interstitial pressure, πc = oncotic pressure of blood and πi = oncotic pressure of the interstitium.thus, Pc − Pi, represents the hydrostatic pressure gradient and πc − πi, represents the oncotic pressure gradient. Starling forces are defined as follows.
1. Hydrostatic capillary pressure (Pc) tends to force the fluid out through the capillary membrane. The values of hydrostatic capillary pressure in most of the tissues are:
2. Hydrostatic interstitial pressure (Pi) tends to force fluid inward through the capillary membrane. It is about −2 mm Hg in the subcutaneous tissue.
3. Oncotic pressure of blood or plasma colloid osmotic pressure (πc) results from the osmotic pressure of plasma proteins. It tends to pull fluid inward through the capillary membrane. It is about 25−27 mm Hg.
4. Oncotic pressure of the interstitium (πi) is due to the presence of proteins in the interstitial space. It tends to pull fluid out of the capillary membrane. The effective oncotic pressure in the interstitium is estimated to range between 5–10 mm Hg (average 8 mm Hg).
Calculation of net filtration at the capillaries
From the above description, the net forces acting on the fluid at the arteriolar and venous end of a typical muscle capillary can be calculated (Table 4.4-3).
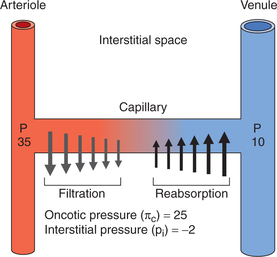
However, over the length of the capillary, the hydrostatic pressure gradually declines to zero near the middle of capillary. From here the inward forces become dominant and reabsorption process starts to reach its maximum at the venular end (Fig. 4.4-12).
Lymphatic circulation
Lymph: formation and composition
Formation. As discussed in capillary exchange, most (90%) of the fluid filtered at the arterial end of the capillary is reabsorbed at its venous end, the remaining 10% enters the circulation through the lymphatics and is called lymph. Thus, the lymph is a transudate formed from the blood in the tissue spaces, i.e. it is derived from the interstitial fluid.
Composition of lymph is similar to the plasma except that its protein content is usually lower than that of the plasma (2–5 g%).
Protein content of the lymph varies with the region it drains:
Fat content. Since, the lymphatic system also provides a route of absorption of long-chained fatty acids and cholesterol from the intestine (in the form of chylomicrons); therefore, after a fatty meal these fat globules may be so numerous that lymph becomes milky and is then called chyle.
Cellular content. Suspended in the lymph are cells that are chiefly lymphocytes. Most of these lymphocytes are added to the lymph as it passes through the lymph nodes.
Lymphatic vessels
The lymphatic system constitutes an accessory route for the removal of interstitial fluid. The small lymph vessels are called lymph capillaries, the large lymph vessels are called lymphatic trunks and the largest lymph vessel is thoracic duct.
Lymph capillaries
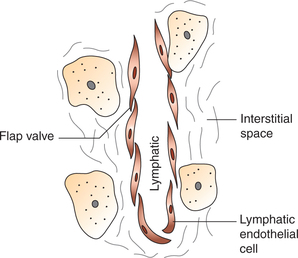
The lymph capillaries originate as closed endothelial tubes that are permeable to fluid and high-molecular weight compounds.
Lymph capillaries (Fig. 4.4-13) are basically similar to that of the blood capillaries. The junctions between endothelial cells are open. In fact, the edges of the endothelial cells overlap in such a way that they form minute flap valves.
Larger lymph vessels
The lymphatic capillaries join to form larger lymph vessels which ultimately form lymphatic trunks and lymphatic ducts as:
Thoracic duct is the largest lymph vessel in the body. It carries lymph from both sides of the body below the diaphragm and from the left side above the diaphragm.
The thoracic duct ends by opening into the junction of the left subclavian vein and the internal jugular vein.
Right lymphatic duct drains lymph from the right half of the body above the diaphragm. It ends by opening into the right subclavian vein.
Structure of larger lymph vessels is similar to that of the veins:
Lymph flow
Functions of lymph flow
1. Returns proteins from tissue spaces to blood. The lymphatic system recovers approximately 200 g of protein daily that has been lost from the microcirculation.
2. Absorption of nutrients, especially fats from the gastrointestinal tract.
3. Acts as a transport mechanism to remove red blood cells that have lost into the tissues as a result of haemorrhage.
4. Supplies nutrients and oxygen to those parts where blood cannot reach.
5. Role in defence mechanism. Lymph nodes associated with lymphatic system act as efficient filters. They have sinuses lined with phagocytic cells that engulf bacteria, red cells and other particulate material.
Venous circulation
Structural characteristics of veins
Walls of veins, as compared to arteries, at equivalent levels in the vascular tree are thin walled and contain small amount of elastic tissue and smooth muscle, and have larger lumen. Because of their structural characteristics, they are more distensible and collapsible.
Lumen of the veins is larger than the equivalent arteries.
Valves are present in the veins of the dependent parts of the body (Fig. 4.3-9) that prevent the back flow of venous blood.
Venous pressure and flow
Central venous pressure
Central venous pressure refers to the pressure in the right atrium because all the systemic veins open into the right atrium. The normal right atrial pressure is about 0 mm Hg, (i.e. equal to atmospheric pressure) but it can rise to as high as 20−30 mm Hg under abnormal conditions, such as heart failure and massive blood transfusion. The right atrial pressure can decrease to as low as −3 to −5 mm Hg when the heart (right atrium) is pumping with vigour or when venous return is greatly depressed.
Peripheral venous pressure
The pressure in the venules is about 10 mm Hg. As the veins approach the heart, there is a gradual decrease in the venous pressure. In the great veins near the heart, venous pressure is approximately 5 mm Hg.
Factors affecting peripheral venous pressure
1. Effect of gravitational pressure. Peripheral venous pressure, like arterial pressure, is affected by this gravitational hydrostatic pressure. When an adult person stands absolutely still the pressure in the body veins because of the effect of hydrostatic pressure is (Fig. 4.4-14):
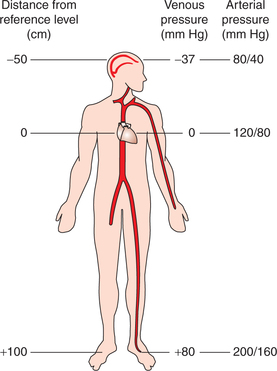
Fig. 4.4-14 Effect of hydrostatic pressure of blood on arterial and venous pressure in an upright position.
• At the level of heart, it is 0 mm Hg.
• At the level of ankles, it is approximately 180 mm Hg.
• At other levels in the body, from heart to feet, the venous pressure varies between 0 and 80 mm Hg. It increases by 0.77 mm Hg for each centimetre below the right atrium. Therefore, if the venous valves are incompetent, as in varicose veins, it results in venous pooling, i.e. accumulation of blood in the lower parts of the body.
• Pressure in the veins above the right atrium decreases by 0.77 mm Hg for each centimetre. Therefore, neck veins completely collapse due to atmospheric pressure on the outside of neck, and pressure inside the vein almost remains zero.
• Subdural venous sinuses in the skull cannot collapse because of the tough non-collapsible nature of dura mater; therefore, a negative hydrostatic pressure (−10 mm Hg) exists in them. Because of this reason, if a dural sinus is opened during a neurosurgical procedure with the patient seated, air is sucked into the sinus, resulting in air embolism.
2. Effect of venous resistance. Increased venous resistance can raise peripheral venous pressure to some extent. This is specially useful in postural changes. For example, sudden change of posture from lying down to standing position results in peripheral pooling of the blood in veins of legs and feet due to effect of gravity. Therefore, venous return to heart decreases, systemic blood pressure falls and may cause dizziness. However, normally the compensatory mechanism operated via baroreceptor reflex prevents any fall in blood pressure (see page 183).
3. Effect of central venous pressure. Peripheral venous pressure to a great extent depends upon the central venous pressure. Therefore, any factor that increases central venous pressure (i.e. right atrial pressure) will also increase the peripheral venous pressure. Common causes associated with rise in right atrial pressure are:
Venous flow and venous return
As we know, the blood flows in the veins towards the heart due to a pressure gradient which exists between the right atrial pressure (0 mm Hg) and the peripheral veins (6−7 mm Hg).
Venous return has been discussed in detail on page 155.
Blood pressure
Definitions (terminology)
Blood pressure
Blood pressure is the lateral pressure exerted by the flowing blood on the walls of the vessels. It is usually measured in mm Hg. Without any further qualification the term blood pressure denotes the arterial pressure. While describing the pressure exerted by the blood column in other types of blood vessels, the type of vessels is also mentioned, e.g. capillary pressure and venous pressure.
Systolic blood pressure
• The maximum arterial pressure during the systole is called systolic blood pressure and occurs during the ventricular ejection.
• The systolic blood pressure is a function of the cardiac output (CO), i.e. it represents the extent of work done by the heart.
• Normal systolic blood pressure in a young adult is 120 mm Hg (range: 105−135 mm Hg).
• Systolic blood pressure undergoes considerable fluctuations, e.g. it is increased during excitement, exercise and meals, and is decreased during sleep and rest.
Diastolic blood pressure
• Diastolic blood pressure refers to the minimum arterial pressure during diastole and occurs just before the onset of the ventricular ejection.
• Normal diastolic blood pressure in a young adult is 80 mm Hg (range 60–90 mm Hg).
• The diastolic pressure is the function of TPR and indicates the constant load against which the heart has to work. It undergoes much less fluctuations.
Conventional expression of blood pressure
Conventionally, systolic and diastolic blood pressures are denoted as numerator and denominator, respectively. For example, blood pressure of a normal person is written as 120/80 mm Hg.
Pulse pressure
• Pulse pressure (PP) is the arithmetic difference between the systolic and the diastolic blood pressures.
• Normally, average PP is 40 mm Hg.
• PP determines the pulse volume.
• The high PP is an indicative of the systolic hypertension and indirectly determines a decrease in elasticity of blood vessels.
Mean arterial pressure
• Mean arterial pressure (MAP) is the average of all pressure measured millisecond by millisecond throughout the cardiac cycle.
• Since, the duration of cardiac systole is shorter than the duration of diastole, so the MAP is not equal to the algebraic mean of the systolic and diastolic blood pressures, i.e. it is not equal to (systolic pressure + diastolic pressure).½
• Practically, MAP is roughly equal to the diastolic pressure (DP) plus one-third of PP, i.e.
• MAP is same for each organ and determines the pressure head. Thus, the regional blood flow through an organ depends on it.
Determinants of (factors affecting) arterial blood pressure
• The arterial blood pressure is a function of the product of cardiac output (CO) and TPR, i.e.
Arterial blood pressure = CO × PR
• Therefore, the arterial blood pressure is affected by conditions that affect either cardiac output or peripheral resistance.
• Changes in the cardiac output affect the systolic pressure more than the diastolic pressure while changes in the peripheral resistance affect diastolic pressure more than the systolic pressure.
• As discussed in the section on cardiac output (page 152), the cardiac output is a function of heart rate and stroke volume, so these two are important determinants of the blood pressure.
• The peripheral resistance (page 163) depends upon the viscosity of blood, elasticity of the vessel wall and velocity of the blood flow. Thus, these factors are also important determinants of the blood pressure.
Variations in blood pressure
Physiological factors affecting blood pressure
1. Age. In healthy humans, both systolic and diastolic pressures rise with age.
• At birth, systolic blood pressure is 40 mm Hg (range: 20–60 mm Hg). It then rises rapidly up to 1 month of age.
• At 1 month of age, the systolic blood pressure becomes about 80 mm Hg and then rises slowly.
• At about 17 years of age, normal adult level of blood pressure is 120/80 mm Hg.
• At about 70 years of age, the normal value of blood pressure is 160/90 mm Hg. The increase in blood pressure associated with advancing age is due to increase in rigidity of vessel wall.
2. Sex. Before menopause, females have little lower (4−6 mm Hg) systolic blood pressure than males of corresponding age. After menopause, systolic blood pressure in females is little higher (4−6 mm Hg) than males of same age group.
3. Effect of meals. Systolic blood pressure increases by 4−6 mm Hg after meals and this effect lasts for about 1 h.
Diastolic blood pressure either remains unchanged or decreases slightly due to the vasodilatation in splanchnic vessels.
4. Emotions. Increased sympathetic activity during emotional situations leads to increase in the systolic blood pressure.
5. Climatic temperature. Exposure to cold produces rise in the blood pressure, while exposure to hot temperature lowers the blood pressure.
6. Diurnal variation. Systolic blood pressure shows a diurnal variation of about 6−10 mm Hg—the values being lower in the morning and higher in the afternoon. In night workers, however, a reverse rhythm is observed.
7. Exercise. In muscular exercise, generally systolic blood pressure rises and diastolic blood pressure falls.
8. Effect of gravity. In standing position, due to hydrostatic (gravitational) effect of the blood column, the pressure in the vessels below heart level is increased and in the vessels above heart level it is decreased (Fig. 4.4-14). For every centimetre below or above the heart level the pressure increases or decreases by 0.77 mm Hg, respectively. Therefore, for clinical recording blood pressure should always be checked at the heart level.
9. Effect of change in posture. Immediately on standing, there occurs peripheral pooling of blood in dependent parts leading to decreased venous return and decreased cardiac output, and momentary fall in the systolic blood pressure. Fall in systolic pressure immediately decreases baroreceptor discharge via vasomotor centre leading to an increased diastolic blood pressure. On standing, there also occurs an increase in the peripheral resistance and momentary increase in diastolic blood pressure.
Thus, immediately after standing from lying down posture a rise in diastolic blood pressure can be recorded for about 30–60s. Later on due to decrease in baroreceptor discharge, the blood pressure comes back to normal and no symptoms are experienced by the normal individuals. The sequence of events are summarized in Fig. 4.4-15.
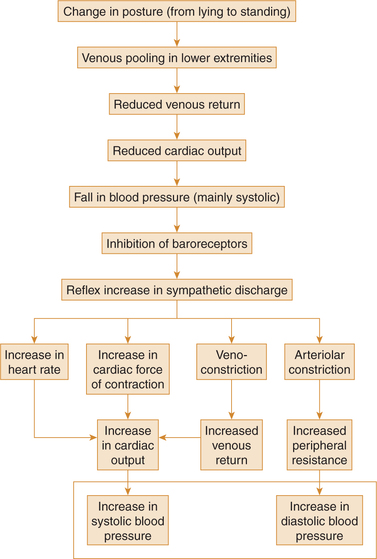
Fig. 4.4-15 Summary of events maintaining normal blood pressure during change of posture from lying to standing.
10. Sleep. In complete relaxed state during early hours of sleep there occurs fall in blood pressure up to 15–20 mm Hg. However, in disturbed sleep blood pressure increases due to increased sympathetic discharge.
11. Body built. Systolic blood pressure is slightly higher in obese individuals as compared to thin-built individuals. This usually occurs because there is more tissue between the cuff and artery and so, some of the cuff pressure is dissipated. Therefore, use of a cuff that is wider than the standard arm cuff is recommended in the obese individuals.
Pathological variations in blood pressure
Hypertension
Definition. Hypertension (HT) refers to a condition in which value of systolic blood pressure is persistently more than 140 mm Hg and/or that of diastolic blood pressure is above 90 mm Hg. If there is increase only in the systolic blood pressure, it is called systolic hypertension in which pulse pressure is raised.
Types of hypertension It is of two types: primary and secondary hypertension.
1. Primary hypertension, also known as essential hypertension, is characterized by a raised blood pressure without any underlying disease. Risk factors for primary HT include heredity, obesity, mental tension and smoking.
2. Secondary hypertension refers to a condition in which blood pressure is raised due to some other underlying disease. Common causes of secondary hypertension are:
• Cardiovascular diseases, e.g. atherosclerosis.
• Renal diseases, e.g. glomerulonephritis and tumour of juxtaglomerular cells leading to formation of excess of angiotensin II.
• Endocrinal disorders like hyperaldosteronism (excessive secretion of aldosterone from adrenal cortex), phaeochromocytoma (tumour of adrenal medulla) and Cushing's syndrome (excessive secretion of glucocorticoids from adrenal cortex).
• Neurologic disorders which may produce hypertension include raised intracranial pressure.
• Pregnancy-induced hypertension (PIH) is noticed in some of the pregnant women. Its exact cause is not known.
Hypotension
Hypotension refers to a condition in which values of blood pressure are below the normal range. Clinically, when the systolic blood pressure is less than 90 mm Hg, it is considered hypotension. It is of following types:
• Primary hypotension, also known as essential hypotension, is a disorder of unknown aetiology.
• Secondary hypotension, occurs secondary to some other underlying diseases, such as myocardial infarction, neurogenic shock, haemorrhagic shock, hypoactivity of pituitary gland and hypoactivity of adrenal glands.
• Postural hypotension refers to the sudden fall in blood pressure when patients stand up from lying down posture. It occurs due to some dysfunction of autonomic nervous system.
Measurement of blood pressure
Direct method
Direct method of measuring blood pressure is used in the experimental studies. In it, a cannula or T-tube is inserted into an artery and connected to either
• Mercury manometer and pressure is recorded on the kymograph or
• Pressure transducer (strain gauge) which in turn is connected to Polyrite for recording.
The record will show fluctuations as depicted in Fig. 4.4-16, upper level of which indicates systolic blood pressure and lower level indicates diastolic blood pressure.
Indirect method
In human beings, blood pressure is measured indirectly by using sphygmomanometer.
Sphygmomanometer
Commonly called blood pressure apparatus, it is the instrument used to measure blood pressure (Fig. 4.4-17).
Procedure
The blood pressure may be tested with subject lying supine or sitting, but should be physically and mentally relaxed and free from excitation.
The blood pressure can be measured by an auscultatory method described by Korotkoff in 1905, is the most useful technique.
• The cuff of the blood pressure apparatus is applied on the upper arm with the centre of the rubber bag lying over the brachial artery, which lies medially, and its lower edge should be about 3 cm above the elbow.
• Raise the pressure in the cuff by 30–40 mm above the level, when the radial artery pulse disappears.
• Then diaphragm of stethoscope is placed on the brachial artery in the cubital space and is kept in a position with the help of thumb and fingers of left hand.
• Pressure in the cuff is lowered slowly by opening the leak valve of the air pump with right hand. While doing so, initially no sound is heard.
However, when mercury column is lowered further a tap sound is heard. The character and quality of sound goes on changing while further lowering the mercury column by deflating the cuff, and ultimately the sound disappears. These sounds are called Korotkoff sounds and from these the levels of systolic and diastolic blood pressure are noted.
Mechanism of Korotkoff's sounds
• Normally, the blood flow through the arteries is streamlined or laminar, and no sounds are heard over them when auscultated.
• While testing blood pressure, when the cuff pressure is raised above the expected systolic pressure level, the blood flow in the brachial artery completely ceases and no sounds are heard. When the cuff pressure is reduced gradually, a time comes when, at the peak of each systole, the intra-arterial pressure just exceeds the cuff (extra-arterial) pressure. The small amounts of blood that are ejected at a high velocity (exceeding the critical velocity) through the partially narrowed artery result in intermittent turbulence which produces the sounds. These sounds are called Korotkoff sounds.
• As the cuff pressure falls further, the blood flow becomes laminar once again and the sounds disappear.
Regulation of blood pressure
Arterial blood pressure is controlled by several mechanisms which under physiological conditions maintain the normal MAP within a narrow range of 95–100 mm Hg. The different mechanisms concerned with regulation of blood pressure have been discussed in detail in Chapter 4.5 on Cardiovas cular Regulation and have been briefed here just for orientation about control of blood pressure. Each mechanism performs a specific function. Various mechanisms controlling arterial pressure can be grouped as:
Rapid blood pressure control mechanism (nervous regulating mechanism)
Rapid blood pressure control mechanism or the so-called short-term control mechanism primarily includes the following three nervous reflexes:
1. Baroreceptor reflexes (see page 183).
2. Central nervous system ischaemic response (see page 186), and
3. Chemoreceptor reflexes (see page 186).
Salient features. The salient features of the nervous reflexes are discussed below.
• These act very rapidly, i.e. within seconds to few minutes of alterations in the blood pressure.
• These are short-term mechanisms, i.e. these act for few hours to few days and are thus insignificant in the longterm regulation of blood pressure.
• These are useful in preventing acute decreases in blood pressure (e.g. during severe haemorrhage) as well as in preventing excessive increases in blood pressure (e.g. as might occur in response to excessive blood transfusion).
Intermediate blood pressure control mechanisms
The intermediate blood pressure control mechanisms that are important in the blood pressure control after several minutes of acute pressure changes are:
• Renin–angiotensin vasoconstrictor mechanism (for details see page 187).
• Stress relaxation and reverse stress relaxation mechanism.
Salient features of intermediate blood pressure control mechanisms are:
• These mechanisms come into play after several minutes of acute pressure changes and reach full function within a few hours.
• These mechanisms play their role from few days to few weeks.
• All these mechanisms basically try to control the alterations in the blood pressure by altering the blood volume.
Stress relaxation and reverse stress relaxation mechanisms
Stress relaxation mechanism refers to vasodilatation occurring due to stress on the vascular smooth muscles. When pressure in the vessels become too high (e.g. following massive slow intravenous transfusion), the vessels become stretched and continue to stretch for minutes or hours. This causes relaxation of blood vessels simply by a vascular tone adjustment. This leads to an increase in the capacity of the arterial system with a concomitant fall in blood pressure.
Reverse stress relaxation mechanism operates when the blood pressure is low due to less stress on the vessels walls and tries to restore it back to normal. For example, when blood pressure falls due to prolonged slow bleeding, there occurs tightening of blood vessel walls by vascular tone adjustment secondary to less stress on the vessel wall (reverse stress relaxation mechanism). This mechanism tries to restore the blood pressure back to normal. This mechanism can correct up to 15% change in blood volume below normal.
Capillary fluid shift mechanism
Capillary fluid shift mechanism helps in restoring both low and high blood pressure back to normal.
When blood pressure is raised, the mean capillary pressure is also high resulting in shift of fluid from circulation to the interstitial fluid compartments. This reduces the blood volume to restore the arterial pressure.
When blood pressure is lowered, the mean capillary pressure is also low, resulting in absorption of fluid from the interstitial compartments to circulation. Thus the blood volume is increased which helps to return the blood pressure back to normal.
The capillary fluid shift mechanism is about two times more effective than baroreceptor reflex mechanism in controlling the blood pressure, but it acts much more slowly (intermediate acting mechanism) than baroreceptor mechanism (rapid acting mechanism).
Long-term blood pressure control mechanisms
Kidneys play main role in the long-term control of blood pressure by the following mechanisms.
1. Direct mechanism, i.e. ‘Renal body fluid feedback mechanism’.
2. Indirect mechanisms control kidney functions indirectly via following hormonal mechanisms:
For details see page 187.
Renal-body fluid system for arterial pressure control
The most important mechanism for the long-term control of blood pressure is linked to control of circulatory volume by the kidney, a mechanism known as the renal-body fluid feedback system. In fact, it is similar to the capillary fluid shift mechanism except that only the renal glomerular capillaries are involved in the process.
Modes of operation of renal-body fluid feedback system
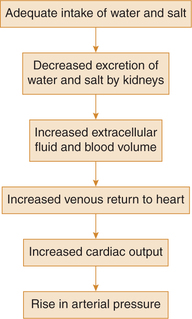
The renal-body fluid system corrects the blood pressure by causing appropriate changes in the blood volume through diuresis and natriuresis.
When blood pressure rises too high, the kidneys excrete increased quantities of sodium and water because of pressure natriuresis and pressure diuresis, respectively. As a result of increased renal excretion, the extracellular fluid volume and blood volume both decrease until blood pressure returns to normal and the kidneys excrete normal amounts of sodium and water.
When the blood pressure falls too low, the kidneys reduces the rate of sodium and water excretion, and over a period of hours to days, if the person drinks enough water and eats enough salt to increase blood volume, the blood pressure will return to its previous level. This mechanism, being very slow to act, is not of major importance in the acute control of arterial pressure. However, it is by far the most potent of all long-term arterial pressure controllers.
The sequence of events in order of occurrence during control of blood pressure by this mechanism is summarized as:
Salient features of renal-body fluid feedback mechanism
The salient features of the renal-body fluid feedback mechanism can be summarized as:
• The renal-body fluid feedback mechanism takes several hours to show any significant response.
• These mechanisms operate very powerfully to control arterial pressure over days, weeks and months.
• The effectiveness of these mechanisms becomes steadily greater with time.
• These mechanisms, if given sufficient time, control arterial pressure at the level that provides normal output of salt and water by the kidneys.
 Organization of vascular system
Organization of vascular system