Physiology of Excretory System
 Tubular reabsorption and secretion
Tubular reabsorption and secretion
 Renal handling of common solutes and water
Renal handling of common solutes and water
Urine formation
Processes concerned with urine formation
The main function of the kidneys is to clear waste products from the blood and excrete them in the urine. The kidneys accomplish their excretory function by the formation of urine.
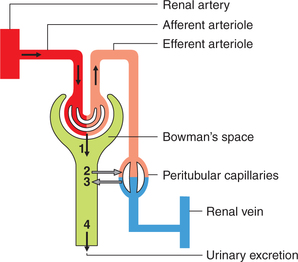
Three processes are involved in the urine formation (Fig. 6.2-1):
Glomerular filtration
Glomerular filtration refers to the process of ultrafiltration of plasma from the glomerular capillaries into the Bowman's capsule.
Composition of glomerular filtrate
The unique characteristic features of the glomerular filtration membrane determine the composition of the glomerular filtrate; in that it is like that of the plasma except for absence of proteins (colloids) and cells. Filtration membrane permeability alteration in diseases, however, may alter diffusibility of colloids and cells. As a result, filtration of proteins is increased and albumin appears in the urine in significant amount (albuminuria or proteinuria).
Dynamics of glomerular filtration
The forces which determine the bulk flow or ultrafiltration of protein-free plasma across the glomerular membrane are the same, which determine the formation of tissue fluid.
The glomerular filtration rate (GFR) will depend upon the balance of Starling forces (Fig. 6.2-2). According to the Starling hypothesis, the GFR can be expressed as:
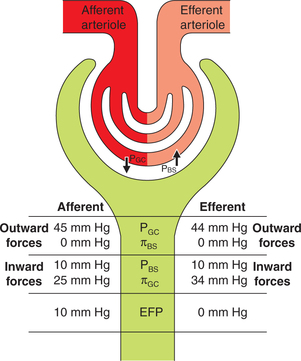
Fig. 6.2-2 Depiction of the Starling forces across the glomerular filtration membrane. (PGC = Glomerular capillary hydrostatic pressure; PBS = Bowman's space hydrostatic pressure; πGC = glomerular capillary oncotic pressure; πBS = Bowman's space oncotic pressure.)
GFR is the filtration across the glomerular membrane.
Kf is the filtration coefficient of the glomerular membrane.
The filtration coefficient (Kf) normally equals 12.5 m2/min/mm Hg.
PGC is glomerular capillary hydrostatic pressure. Its normal value is about 45 mm Hg.
PBS or Bowman's space hydrostatic pressure. Its normal value is about 10 mm Hg.
πGC or glomerular capillary oncotic pressure. Its normal value is 25 mm Hg.
πBSor Bowman's space oncotic pressure. Its normal value is zero because glomerular filtrate contains no proteins.
Normal glomerular filtration rate
The normal GFR in an average sized man is about 125 mL/ min (range 90–140 mL/min). The values in women are 10% lower than those in men.
Factors affecting glomerular filtration rate
1. Filtration coefficient (Kf). Increased Kf raises GFR and decreased Kf reduces GFR. As mentioned earlier, Kf is the product of permeability and filtration area of the glomerular capillary membrane.
2. Hydrostatic pressure in Bowman's space fluid (PBS) opposes filtration and therefore GFR is inversely related to it. It is increasing in an acute obstruction of urinary tract (e.g. a ureteric obstruction by stone).
3. Glomerular capillary hydrostatic pressure (PGC). GFR is mainly dependent on arterial pressure, renal blood flow, afferent arteriolar resistance and efferent arteriolar resistance.
4. Glomerular capillary oncotic pressure (πGC). GFR is inversely proportional to πGC. In hyperproteinaemia and in haemoconcentration, the πGC is raised leading to a decrease in GFR. Conversely in hypoproteinaemia and haemodilution, the πGC is reduced leading to increased GFR.
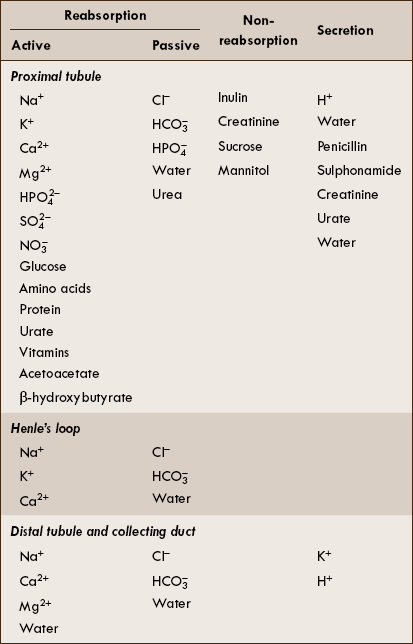
Tubular reabsorption and secretion
Of the 180 L glomerular filtrate formed per day, about 1.5 L (i.e. less than 1%) per day is excreted as urine. The different segments of the renal tubule, viz. proximal tubule, loop of Henle, distal tubule and collecting duct determine the composition and volume of the urine by process of selective reabsorption of solutes and water and selective secretion of solutes.
Tubular reabsorption denotes the active transport of solutes and passive movement of water from the tubular lumen into the peritubular capillaries. In other words, reabsorption is the removal of substances of nutritive value, such as glucose, amino acids, electrolytes (Na+, K+, Cl–, 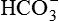 ) and vitamins from the glomerular filtrate. Small proteins and peptide hormones are reabsorbed in the proximal tubules by endocytosis.
) and vitamins from the glomerular filtrate. Small proteins and peptide hormones are reabsorbed in the proximal tubules by endocytosis.
Tubular secretion refers to the transport of solutes from the peritubular capillaries into the tubular lumen, i.e. it is the addition of a substance to the glomerular filtrate.
Active secretion of substances occurs into the tubular fluid with the help of certain non-selective carriers.
The carrier which secretes para-aminohippuric (PAH) acid can also secrete uric acid, bile acids, oxalic acid, penicillin, probenecid, cephalothin and furosemide.
Transport across different segments of renal tubule
The substances transported across the different segments of renal tubules are described below and are enlisted in Table 6.2-1.
Transport across proximal tubule
The proximal tubule reabsorbs:
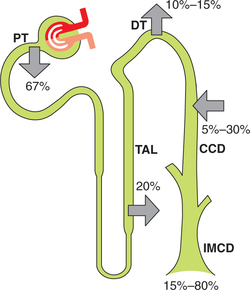
• Approximately 67% of the filtered water, Na+, Cl−, K+ and other solutes.
• Almost all the glucose and amino acids filtered by the glomerulus.
The proximal tubule does not reabsorb inulin, creatinine, sucrose and mannitol.
The proximal tubule secretes H+, PAH, urate, penicillin, sulphonamides and creatinine.
Renal handling of common solutes and water
Renal handling of common solutes and water which include:
• Renal handling of sodium and water,
• Renal handling of potassium,
Renal handling of sodium and water
Percentage reabsorption of the filtered sodium in different segments of the renal tubule is:
| Proximal tubule: | 67% |
| Loop of Henle (mainly thick ascending limb): | 20% |
| Distal tubule: | 7% |
| Cortical collecting duct: | 5% |
Sodium reabsorption
The process of sodium reabsorption in the proximal tubule is isosmotic, i.e. the reabsorption of sodium and water are exactly proportional.
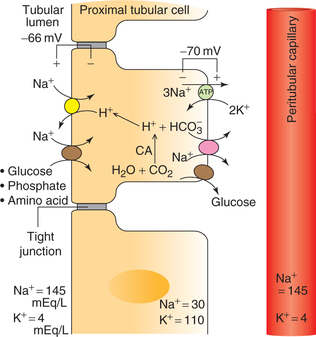
Mechanisms of Na+ reabsorption
Na+ is reabsorbed by co-transport with H+ or organic solutes (glucose, amino acids, phosphate and lactate). The Na+ absorption is a two-step process (Fig. 6.2-3).
Across the basolateral membrane, Na+ moves against an electrochemical gradient via Na+–K+–ATPase pump, which pumps Na+ into the paracellular spaces and lowers the intracellular Na+ concentration.
Across the apical membrane, the sodium moves down an electrochemical gradient as above. The entry of Na+ is mediated by the specific antiporter and symporter proteins, and not by diffusion through channels.
Water reabsorption
Site and mechanism of reabsorption
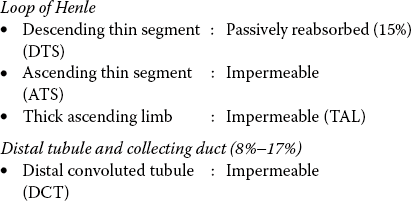
Water is absorbed passively by osmosis in response to a transtubular osmotic gradient. Rapid diffusion of water across the cell membrane occurs through water channels made up of proteins called Aquaporins. Different types of aquaporins are aquaporin-1, 2, 5 and 9. Mostly, these aquaporins are present in the kidney. In the collecting ducts, reabsorption of water is controlled by ADH. Renal handling of water by different segments of renal tubule is given below.
Proximal tubule: Passively reabsorbed (67%).
Obligatory and facultative reabsorption of water
Obligatory reabsorption. About 85% of the filtered water is always reabsorbed, irrespective of the body water balance. This reabsorption occurs by osmosis in response to a transtubular osmotic gradient and is called obligatory (must occur) reabsorption.
• About 67% of obligatory reabsorption occurs in the proximal tubules.
• About 15%−18% of obligatory reabsorption occurs in the descending thin segment of loop of Henle.
Facultative reabsorption. The remaining 15%–18% of the water may or may not be absorbed depending upon the body water balance. It is called facultative (optional) reabsorption.
Facultative reabsorption of water occurs from the collecting tubule and is under the control of ADH.
Renal handling of potassium
Functions of K+
Potassium (K+) is one of the most abundant cations in the body. It is the principal intracellular cation, and is equally important in the extracellular fluid for specific functions. Potassium is required for the following biochemical functions:
• Maintenance of intracellular osmotic pressure.
• Optimal activity of enzyme pyruvate kinase (of glycolysis).
• Proper synthesis of DNA and proteins by ribosomes.
• Transmission of nerve impulse.
• Generation of cell membrane potential and muscle contraction.
• Extracellular K+ influences cardiac muscle activity.
• Regulation of acid-base balance and water balance in the cells.
Transport of potassium across major nephron
Filtration occurs freely across the glomerular capillaries; potassium is not bound to plasma proteins.
Tubular reabsorption and secretion
As shown in Fig. 6.2-4, 67% of the filtered K+ is reabsorbed in the proximal tubule, 20% in the loop of Henle and approximately 10% is delivered to the early distal tubule. In contrast to proximal tubule and loop of Henle, which are capable of only reabsorbing K+, the distal tubule (DT) and collecting duct (CD) are able to either reabsorb or secrete K+. The role of reabsorption or secretion by DT and CD depends on a variety of hormones and factors.
Reabsorption of K+ by proximal tubule
In proximal tubule, approximately 7% of the filtered K+ is reabsorbed passively in proportion to the H2O reabsorption (solvent drug) and about 60% of the filtered K+ is reabsorbed actively by the paracellular transport mechanism
The K+ that exits from the basolateral membrane is immediately absorbed in the peritubular capillaries.
Reabsorption of K+ by loop of Henle
Twenty percent of the filtered K+ is reabsorbed in the thick ascending limb (TAL) of loop of Henle along with Na+ reabsorption by two mechanisms:
Reabsorption and secretion of K+ by distal tubule and collecting duct (Fig. 6.2-5)
Normally, K+ is secreted in the distal convoluted tubule. However, when there is need to conserve body K+ (e.g. during K+ depletion), K+ is reabsorbed. Both secretion as well as reabsorption occurs in the same cell, i.e. distal tubular cell, depending upon the status of K+ balance in the body.
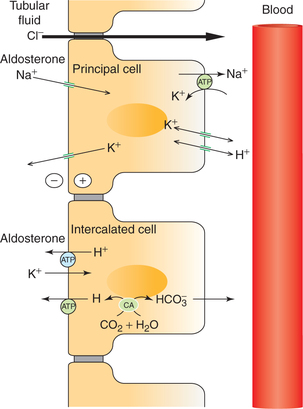
Fig. 6.2-5 Mechanism of transport in principal cells and intercalated cells of the late distal tubule and collecting duct. (CA = Carbonic anhydrase.)
Reabsorption of K+ occurs only when the dietary intake is very low (i.e. during K+ depletion). Under these circumstances K+ excretion can be as low as 1% of the filtered load because the kidneys conserve as much K+ as possible.
Secretion of K+ is variable and accounts for the wide range of urinary K+ excretion, depending upon the dietary K+ intake, aldosterone levels, acid-base status and urine flow rate.
Factors that regulate urinary K+ excretion
Hormones and other factors involved in the regulation of K+ tubular secretion and thus of urinary K+ excretion include the following.
1. Plasma K+ level, which mainly depends on:
Dietary intake of K+ is an important determinant of K+ secretion by principal cells (Fig. 6.2-6). Hyperkalaemia, resulting from a high K+ diet or any other factor (e.g. rhab-domyolysis) stimulates K+ secretion within minutes.
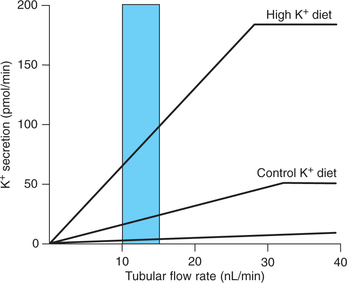
Fig. 6.2-6 Effect of dietary intake of K+ on the relationship between tubular flow rate and K+ secretion by the distal tubule and collecting duct.
Hypokalaemia, resulting from a low K+ diet or other factors (e.g. diarrhoea) decreases K+ secretion by mechanisms opposite to those described for hyperkalaemia.
2. Aldosterone. Aldosterone by its effect on principal cell increases Na+ reabsorption and increases K+ secretion. About 2% of overall Na+ absorption is affected by aldosterone (Fig. 6.2-5).
Aldosterone also increases H+ secretion by intercalated cells by stimulating the K+-H+-ATPase (in addition to its actions on the principal cells).
Renal handling of glucose
Glucose reabsorption
Glucose is freely filtered into the glomerular filtrate. Filtration load of glucose increases in direct proportion to the plasma glucose concentration (P glucose). Filtered load of glucose = GFR × P glucose (PG).
Mechanism of tubular reabsorption
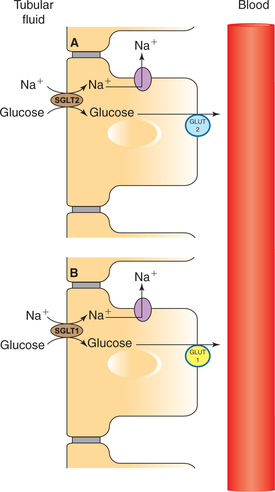
All the filtered glucose is completely reabsorbed into the proximal tubule by an active transport mechanism (Fig. 6.2-7).
Carrier-mediated Na+–glucose co-transport. Carrier protein located at the apical membrane in the proximal tubule reabsorbs glucose from tubular fluid into the blood.
• The carrier protein for glucose in early and late proximal tubule is called SGLT-2 and SGLT-1, respectively (SGLT = sodium-dependent glucose transporter).
Facilitated diffusion moves the glucose out of the cell through the basolateral membrane. The carrier for facilitated diffusion across the basolateral membrane in early and late proximal tubule is called GLUT-2 and GLUT-1, respectively (GLUT = glucose transporter).
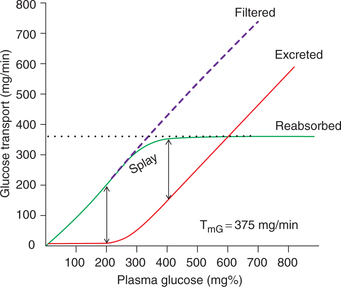
Characteristics of glucose transport and glucose excretion
Glucose is reabsorbed by a transport maximum process, i.e. there are limited number of Na+–glucose carriers. The characteristics of glucose transport and glucose excretion can be elicited from the glucose titration curve.
Glucose titration curve (Fig. 6.2-8) depicts that:
Filtered load increases with the plasma glucose concentration (PG).
Renal threshold, i.e. the plasma glucose concentration at which glucose first appears in the urine (glycosuria) is about 180–200 mg%. At plasma levels below renal threshold, the reabsorption of glucose is complete (100%).
Transport maximum As shown in Fig. 6.2-8, beyond plasma glucose concentration of 350 mg% (TmG) the reabsorption rate does not increase, i.e. becomes constant and is independent of PG. Therefore, as the TmG is reached, the urinary excretion rate increases linearly with increase in plasma glucose concentration.
Splay refers to the region of the glucose curve between threshold and TmG, i.e. between PG 180 and 350 mg%. It represents the excretion of glucose in urine before the TmG is fully achieved. Note that in the region of splay, the reabsorption curve is rounded indicating that though the reabsorption rate is increasing with increase in PG, but reabsorption is less than filtration. Similarly, the excretion curve is also rounded in the region of splay, indicating that though the urinary excretion is increasing with increase in PG, but there is no linear relation.
Renal handling of proteins, peptides and amino acids
Protein reabsorption
Normally, only a small amount of proteins is filtered by the glomerulus (40 mg/L). However, because of high GFR (180 L/day) the total amount of protein filtered per day is significant (180 L/day × 40 mg/L = 7.2 g/day). Normally, the proteins are completely taken into the cells of proximal tubules by process of endocytosis. Once inside the cells, enzymes digest the proteins and peptides into their constituent amino acids, which exit across the basolateral membrane and return to the blood in the peritubular capillaries.
When the amount of filtered proteins increases (due to disruption of glomerular filtration barrier in kidney diseases), the reabsorbing mechanisms saturate and the proteins may appear in the urine (proteinuria).
Concentration and dilution of urine
The kidneys possess unique property of regulating the volume and osmolality of the urine by concentrating and diluting it as per need of the body.
Purpose of concentration and dilution of urine. The main purpose is to maintain the osmolality and volume of the body fluids within a narrow range, which is accomplished by kidneys in concert with other systems by regulating the excretion of water and NaCl, respectively.
The kidney can produce urine with osmolality as low as 30 mOsm/kg H2O to as high as 1400 mOsm/kg H2O by changing the water excretion as high as 23.3 L/day to as low as 0.5 L/day, respectively.
Principal factors. Principal factors responsible for mechanism of concentration and dilution of urine are:
• Antidiuretic hormones (see page 376) and
• Hyperosmolality and osmolality gradient in medullary interstitium of kidneys.
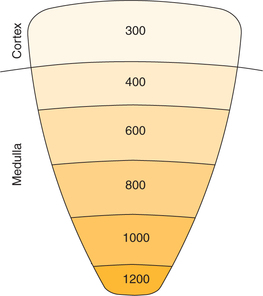
Medullary hyperosmolality and medullary gradient
The interstitial fluid of the medulla is critically important in concentrating the urine because the osmotic pressure of this fluid provides the driving force for reabsorbing water from both the descending thin segment (DTS) and the collecting duct.
Normal osmolality of plasma and other body fluids is about 300 mOsm/kg H2O. The interstitial fluid of the renal cortex has the same osmolality as that of plasma, and virtually all osmoles attributable to NaCl. The osmolality of the renal medulla is higher than the plasma (i.e. hyperosmolar) and that it goes on increasing progressively from about 300 mOsm/kg H2O at corticomedullary junction to about 1200 mOsm/kg H2O at papilla (medullary gradient), where a maximally concentrated urine is excreted (Fig. 6.2-9). This hyperosmolality and medullary gradient is generated and maintained by the so-called counter current system.
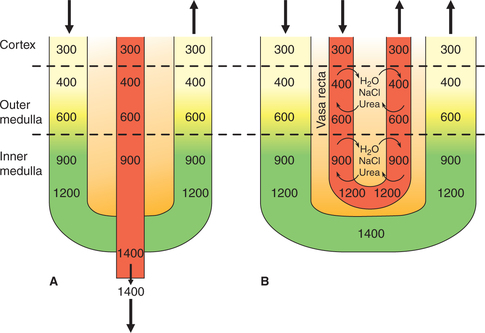
Counter current system
A counter current system refers to a system in which the inflow runs parallel to, counter to, and in close proximity to the outflow for some distance. The counter current flow system is formed by U-shaped tubules.
In the kidney, the structures which form the counter current system are loop of Henle and the vasa recta. The counter current system of kidney consists of two components:
• Counter current multiplier, which is formed by the operation of loop of Henle and is responsible for production of hyperosmolality and a gradient in renal medulla.
• Counter current exchanger, which is formed by the operation of the vasa recta and is responsible for the maintenance of the medullary gradient and hyperosmolality.
Counter current multipliers
The working of a counter current multiplier, which operates in the loop of Henle and generates hyperosmolarity and medullary gradient, can be best understood by describing it as two processes:
Origin of single effect in inner medulla
The origin of single effect for the development of medullary gradient and hyperosmolality occur in the inner medullary interstitium due to:
• Passive transport of sodium ions from the ascending thin segment into the interstitium,
• Active transport of sodium from the inner medullary collecting duct and
• Diffusion of urea from the collecting duct into the medullary interstitium.
Role of urea. Urea plays an important role in the development of medullary osmotic gradient, especially when concentration of ADH is high in the blood. Under such circumstances, the inner medullary collecting duct absorbs large amount of urea, which plays an important role in the counter current system.
Multiplication of the single effect
The hyperosmolality and medullary gradient is in fact generated by the multiplication of the single effect by the counter current multiplier. The main characteristics of the components of counter current multiplier which play role in multiplication of single effect are as follows:
• High permeability of the descending thin segment to water.
• Impermeability to water and ability to actively absorb solutes by TAL.
• In renal medulla, all other tubular structures (except ascending limb) are in osmotic equilibrium. The descending limb, therefore acquires the increased osmolality of the surrounding fluid. The effect is multiplied as new iso-smolar filtrate arrives at the descending limb and forces the concentrated tubular contents towards the tip of loop of Henle (hair-pin band).
Counter current exchanger
Vasa recta (the counter current exchanger). If the vasa recta would have been a straight blood vessel, the osmotic gradient in the medullary pyramid would not last long, as the Na+ and urea in the interstitial spaces would have been removed by the circulation (Fig. 6.2-10). However, because of the hair pin (U-shaped) anatomical arrangement of the vasa recta operates as counter current exchanger and retains these solutes in the medullary interstitium. Thus, the counter current exchanger formed by the vasa recta is responsible for the maintenance of the hyperosmolality medullary gradient generated by the counter current multiplier.
Mechanism of urine dilution and concentration
Production of diluted urine
Conditions in which dilute urine is formed
Dilute urine is called hypoosmotic urine in which urine osmolality is less than blood osmolality. It is produced under following circumstances:
• When circulating levels of ADH are low (e.g. after water drinking), central diabetes insipidus (see page 378) or
• When ADH is ineffective (e.g. nephrogenic diabetes insipidus).
Principal factors governing formation of dilute urine
As mentioned earlier, the principal factors governing formation of dilute and concentrated urine are hyperosmolality medullary gradient and the presence or absence of ADH. The renal medullary osmotic gradient is smaller in the absence of ADH. This is because ADH stimulates both counter current multiplication and urea cycling.
Production of concentrated urine
Conditions in which concentrated urine is formed
Concentrated urine is also called hyperosmotic urine, in which urine osmolality is more than that of blood. It is produced when circulating ADH levels are high, e.g.:
• Syndrome of inappropriate antidiuretic hormone, i.e. SIADH (see page 377).
Urine volume and osmolality changes in response to water intake and water deprivation
Characteristic features
• Water diuresis begins about 15 min after ingestion of the water and reaches its maximum in 40 min.
• Urine output may be increased to 20 L/day.
• Urine formed is diluted, osmolality is always below 30 mOsm/kg H2O and may be as low as 50 mOsm/kg H2O.
Osmotic diuresis refers to an increased urine output because of an osmotic effect.
Pathophysiology. Presence of large quantities of unreab-sorbed solutes in the proximal tubules exerts an appreciable osmotic effect. Osmotic diuresis may occur under following circumstances:
• When the filtered load of naturally occurring substances such as Na+, glucose (e.g. in diabetes mellitus), urea, etc. exceeds the maximum capacity of the tubules to reabsorb (Tm).
• Following administration of compounds such as mannitol and related polysaccharides that are filtered but not reabsorbed.
• Following infusion of large amounts of sodium chloride and urea.
Acidification of urine
The pH of urine is variable depending upon the concentration of H+ ions. Under normal circumstances the pH of urine is acidic (~6.0). This clearly indicates that kidneys have contributed to the acidification of urine when it is formed from the plasma (pH 7.4). In other words, H+ ions generated in the body in the normal circumstances are eliminated by the acidified urine. Thus, the role of kidneys in the maintenance of acid-base balance of the body (blood pH) is highly significant.
The kidneys regulate the blood pH by three main mechanisms:
• Reabsorption of the filtered 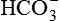 .
.
• Generation of 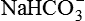 of the alkali reserve of the body.
of the alkali reserve of the body.
• Excretion of acid in the form of titrable acid and ammonium ions.
• All these mechanisms are accomplished through the process of H+ secretion by the nephron.
Hydrogen ion secretion
The tubular cells of the proximal tubule, distal tubule and collecting ducts are capable of secreting H+. The kidney is the only organ which can eliminate the fixed acids by active secretion of H+.
Mechanism of H+ secretion by proximal tubule
Steps involved are (Fig. 6.2-11) discussed below.
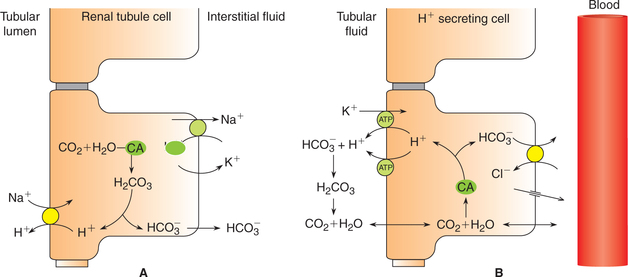
Figure 6.2-11 Cellular mechanism for secretion of H+ by proximal tubular cell (A) and intercalated cells of late tubule (B).
• Formation of carbonic acid. Carbonic acid (H2CO3) is first formed in the cells of proximal tubules from CO2 and H2O by a reaction that is catalyzed by the intracellular carbonic anhydrase.
• Dissociation of carbonic acid (H2CO3) then occurs in H+ and 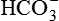 .
.
• Secretion of H+ into the lumen occurs via Na+–H+exchange mechanism in the luminal membrane by Na+– H+–antiporter (Figure11Fig. 6.2-11).
• The secreted H+, in the lumen, combines with the filtered 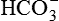 and helps its reabsorption (as described below). Therefore, this process does not result in net secretion of H+.
and helps its reabsorption (as described below). Therefore, this process does not result in net secretion of H+.
• 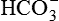 formed in the cell (from dissociation of H2CO3) diffuses into the interstitial fluid. Thus, for each H+ secreted one Na+ ion and one
formed in the cell (from dissociation of H2CO3) diffuses into the interstitial fluid. Thus, for each H+ secreted one Na+ ion and one 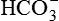 ion enter the interstitial fluid. The later adds up to the alkali reserve of the body.
ion enter the interstitial fluid. The later adds up to the alkali reserve of the body.
Mechanism of H+ secretion by distal tubules and collecting ducts. In the distal tubule and collecting ducts, H+ secretion occurs independent of Na+.
• H+, K+–ATPase is also responsible for secretion of some of the H+ coupled with reabsorption of K+ in these parts of renal tubules.
Fate of H+ secreted in the renal tubule. The secretion of H+ in the renal tubule can continue only if the H+ is immediately buffered in the luminal fluid.
Generation of new 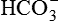
As discussed above, the kidneys play an important role in the maintenance of acid-base balance of the body by completely reabsorbing the filtered 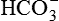 . However, in reality
. However, in reality 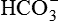 reabsorption alone does not replenish the
reabsorption alone does not replenish the 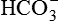 lost during the titration of non-volatile acids, which are daily added to the plasma from the diet and produced by metabolism. Therefore, to maintain acid–base balance, the kidneys replace this lost
lost during the titration of non-volatile acids, which are daily added to the plasma from the diet and produced by metabolism. Therefore, to maintain acid–base balance, the kidneys replace this lost 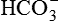 with new
with new 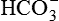 by following processes:
by following processes:
Excretion of H+ as titrable acid
Excretion of H+ as titrable acid refers to the excretion of secreted H+ along with the primary urinary buffer—the dibasic phosphate (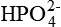 ). This reaction occurs in the distal tubules and collecting ducts (Fig. 6.2-12).
). This reaction occurs in the distal tubules and collecting ducts (Fig. 6.2-12).
As a result of H+ excretion in the form of a titrable acid, the pH of urine is progressively decreased (from 7.4, that of blood). The acidification of the urine may lower its pH to a minimum of 4.5.
Excretion of H+ as ammonium ion
Excretion of H+ as 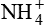 is another mechanism of excretion of secreted H+ and formation of new
is another mechanism of excretion of secreted H+ and formation of new 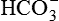 . The amount of H+ excreted as
. The amount of H+ excreted as 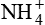 depends upon both the amount of NH3 synthesized by renal cells and the urine pH.
depends upon both the amount of NH3 synthesized by renal cells and the urine pH. 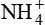 is a major urine acid. It is estimated that about half to two-thirds of body acid load is eliminated in the form of
is a major urine acid. It is estimated that about half to two-thirds of body acid load is eliminated in the form of 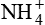 ions. The process by which the kidneys excrete NH4 is complex.
ions. The process by which the kidneys excrete NH4 is complex.
 Factors affecting glomerular filtration rate
Factors affecting glomerular filtration rate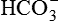
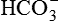
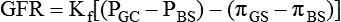
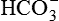 (i.e. consumed in reabsorption of filtered
(i.e. consumed in reabsorption of filtered 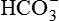 , vide infra).
, vide infra).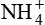 , vide infra).
, vide infra).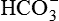
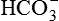 .
.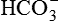 , mainly in the region of TAL.
, mainly in the region of TAL.
 .
.