Endocrinal Functions of Hypothalamus and Pituitary Gland
Introduction and functional anatomy
• Gross anatomy and development of pituitary gland
• Histological structure of pituitary gland
Endocrinal aspects of hypothalamus
*. Applied aspects: abnormalities of anterior pituitary hormones
Introduction and functional anatomy
The hypothalamic–pituitary unit forms a unique component of the entire endocrine system that regulates growth, lactation, fluid homeostasis and the functions of thyroid gland, adrenal glands and gonads.
Gross anatomy and development of pituitary gland
Pituitary gland, also called hypophysis cerebri, is a small gland, weighs about 0.5 g and is approximately 1 cm in diameter. It is situated in the hypophyseal fossa (sella turcica) of the sphenoid bone.
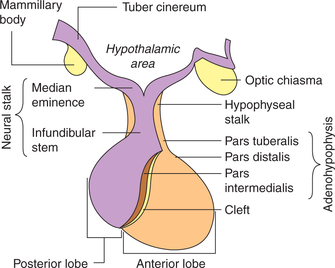
Physiologically, the pituitary gland consists of three distinct parts or lobes (Fig.8.2-1):
Development of pituitary gland
Anterior pituitary is ectodermal in origin. It develops from the Rathke's pouch, which is an embryonic upward outpouching from the roof of the primitive oral cavity.
Posterior pituitary or neurohypophysis develops from a lowered outpouching of neuroectodermal tissue from the central areas of the hypothalamus (tuber cinereum and median eminence).
From the above, it is quite clear that the anterior and posterior pituitary develops independently from widely different origins, and it is only a coincidence that when fully formed, they happen to lie so close together that they are considered parts of the same organ.
Parts of pituitary gland
Adenohypophysis. The glandular anterior lobe of the pituitary gland is called adenohypophysis. It constitutes about 80% of the pituitary gland. It can be further divided into three parts (Fig.8.2-1):
Neurohypophysis. The posterior lobe of the pituitary is a neural structure and hence called neurohypophysis. It consists of three parts (Fig.8.2-1):
• Pars posterior. It is also called infundibular process and forms the main bulk of neurohypophysis.
• Infundibular stem. It is the funnel-shaped extension arising from the median eminence at the floor of third ventricle.
• Median eminence. It is a small protrusion from the base of hypothalamus (tuber cinereum). It is situated just beneath the third ventricle and is highly vascular
Intermediate lobe of pituitary gland is rudimentary in humans as well as in a few other mammalian species. In certain lower animals, this lobe secretes melanocyte-stimulating hormone (MSH) in response to changes in exposure to light and other environmental factors.
Histological structure of pituitary gland
Adenohypophysis
Pars distalis consists of cords of cells separated by fenestrated sinusoids. The cells can be divided into two main types: the chromophobes and chromophils.
1. Chromophobes. These are agranular cells and it is considered that the chromophils are derived from the chromophobes.
2. Chromophils. These are granular cells, constitute 50% of the cells of anterior pituitary. Chromophils are further classified as: acidophils (35%) and basophils (15%).
(i) Acidophilic cells (a cells). The granules of these cells are acidophilic. These cells secrete growth hormone (GH) or somatotropin (STH), prolactin (PRL) or lactogenic hormone (LTH).
(ii) Basophilic cells (b cells). The granules of these cells are basophilic. These secrete ACTH and β-lipoprotein, thyrotropic hormone (thyrotropin or TSH), which stimulates the activity of thyroid gland and follicular stimualting hormone (FSH) and luteinizing hormone (LH).
3. Folliculostellate cells. These cells send processes between the secretory cells. Recently, it has been demonstrated that these contain and secrete the cytokine 1L-6, but their physiological role is still not clear.
Pars tuberalis. It mainly consists of undifferentiated cells with few acidophils and basophils.
Pars intermedia. It contains β cells, few secretory cells and chromophobe cells.
Neurohypophysis
Histologically, posterior pituitary contains following structures:
1. Unmyelinated nerve fibres. These are the axons of the neurons located in the supraoptic and paraventricular nuclei of the hypothalamus. These carry precursor of posterior pituitary hormones and end as close terminals near the blood capillaries (Fig.8.2-2).
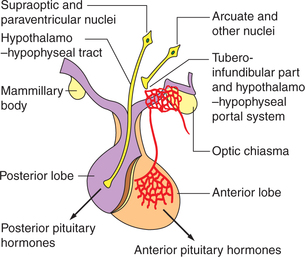
Fig. 8.2-2 Anatomical and functional relationship between the hypothalamus and pituitary gland through hypothalamo–hypophyseal tract and hypothalamo–hypophyseal portal system.
2. Pituicytes are the special type of supporting cells having long dendritic processes. These are present in between the axons.
3. Glial cells like astrocytes and oligodendrocytes are also seen.
Blood supply of pituitary gland
Arterial supply
The arterial blood to the pituitary gland is supplied by the branches of:
• Internal carotid arteries (superior and inferior hypophyseal branches),
Hypothalamo–hypophyseal portal system (Figs.8.2-2 and 8.2-3)
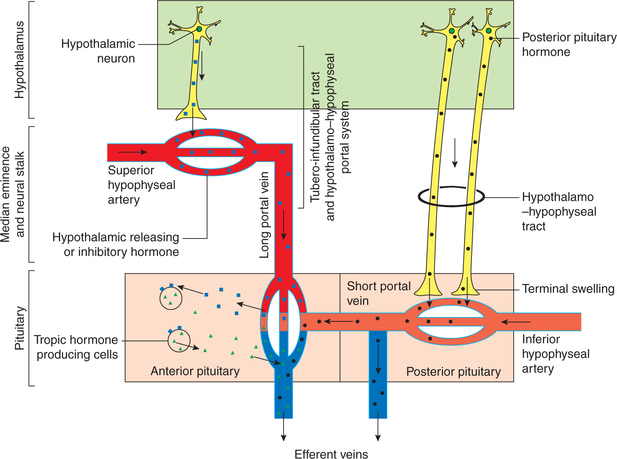
Fig. 8.2-3 Schematic diagram to explain anatomical and functional relationship between the hypothalamus and pituitary gland.
• The branches from superior hypophyseal artery form a ring around the upper part of the pituitary stalk and are further branched to form a capillary network.
• The blood from this capillary network is drained by long portal veins in the infundibulum.
• Then in the anterior lobe, these long portal veins break up into another set of capillary network and represented as sinusoids of pars anterior. This arrangement is called hypothalamo–hypophyseal portal system.
• The inferior hypophyseal (branch of internal carotid artery) branches to form a capillary network at the lower end of the infundibulum stem.
• The short portal vessels arise from this capillary network and supply blood mainly to the posterior pituitary and some part of the anterior pituitary.
• The short portal vessels provide link between the anterior and the posterior pituitary.
Venous drainage
The blood from the anterior pituitary is drained to the cavernous sinus and then into the jugular vein.
Note. The anterior pituitary lies outside the blood—brain barrier, hence it is accessible to influences from general circulation (hormones and neurotransmitter by the brain and hormones secreted in the general circulation).
Hypothalamic–pituitary relationship
The influences from the hypothalamus are conveyed to the pituitary gland by two different tracts:
1. Hypothalamo–hypophyseal tract. It is composed of axons of the large neurosecretory cells of the supraoptic andparaventricular nuclei of the hypothalamus. These fibres pass to neurohypophysis through the infundibular stem and form a series of dilated terminals known as Herring bodies (Fig.8.2-2). The neurosecretory cells of supraoptic and paraventricular nuclei secrete peptide hormones (vasopressin and oxytocin), which travel down their axons in the neurosecretory granules to be stored in the nerve terminals lying the neurohypophysis. Upon stimulation of the cell bodies, the granules are released from the axonal terminals by exocytosis.
2. Tubero-infundibular tract and hypothalamo–hypophyseal portal system. The cell bodies of hypothalamic neurons synthesize certain releasing and inhibiting hormones, which are conveyed by the tubero-infundibular tract to the median eminence region where they are stored in the nerve terminals. After these hypothalamic neurons are stimulated by nerve impulses, the releasing or inhibiting hormones are discharged into the median eminence and enter the capillary plexus of the superior hypophyseal artery. From here they are transported down the portal vessels (long portal veins) and then exit from the secondary capillary plexus to reach the specific endocrine target cells in the adenohypophysis where they regulate the secretion of tropic hormones of anterior pituitary (Fig.8.2-3).
Endocrinal aspects of hypothalamus
Functional anatomy
Hypothalamus is a specialized centre in the brain that functions as a master co-ordinator of hormonal action. It is a part of the brain situated below the thalamus and is very closely connected to the pituitary gland as described above. Thus hypothalamus provides an important link between the endocrine system and the nervous system. Before proceeding further see details of functional anatomy of hypothalamus at page 482.
Endocrinal functions of hypothalamus
The hypothalamus serves its endocrinal functions through the neurosecretory cells, which are arranged in different nuclei of hypothalamus.
The main endocrinal functions of hypothalamus are:
1. Control of anterior pituitary function
Hypothalamus controls the functioning of anterior pituitary through various hypothalamic–hypophysiotropic hormones, inhibiting hormones, which are released in response to neural stimuli.
Various hypothalamic-releasing and -inhibiting hormones include:
2. Control of posterior pituitary function
The large (magnocellular) neurosecretory cells forming the supraoptic and the paraventricular nuclei of the hypothalamus are responsible for synthesis of the two posterior pituitary peptide hormones oxytocin and antidiuretic hormone (ADH). These hormones reach the posterior pituitary through hypothalamic–hypophyseal tract described above (Figs.8.2-2 and 8.2-3).
Anterior pituitary hormones
Anterior pituitary is truly the master endocrine organ. The hormones of adenohypophysis are broadly classified into three categories:
I. Hormones of growth hormone family Growth hormone (GH) prolactin (PRL) are secreted from the anterior pituitary and human chorionic somatomammotropin (HCS) belong to this family is secreted from placenta.
II. Glycoprotein hormone family. The hormones of glycoprotein family secreted by the anterior pituitary include:
III. Pro-opiomelanocortin peptides. The hormones of this group are derived from a single precursor, the proopiomelanocortin (POMC), consisting of 241 amino acids. The precursor molecule (POMC) is then cleaved into three fragments which include:
• Adrenocorticotropic hormone (ACTH),
• Melanocyte-stimulating hormone (MSH), α-MSH and β-MSH, are produced in the intermediary lobe (which is rudimentary in humans),
Physiological aspects of growth hormone are discussed in detail in this chapter. Other hormones are discussed in different chapters (e.g. TSH with thyroid hormone and ACTH with adrenal gland).
Growth hormone
Growth hormone (GH), also called somatotropin, is the most important hormone for postnatal growth and development to adult size. It also helps to maintain lean body mass and bone mass in adults.
Structure and synthesis
Structure. GH consists of a single unbranched chain containing 191 amino acids. Its molecular weight is 2000.
Synthesis. GH is synthesized by acidophilic cells called somatotrophs of anterior pituitary.
Plasma levels, binding and metabolism
Plasma levels
Basal plasma GH level varies from 2 to 4 ng/mL. After every 1–2 h interval there is rise in plasma GH level, i.e. GH is released in 10–20 pulses per day. This pulsatility arises from some combination of the pulsatile release of GHRH and of GRIH into the portal blood.
Diurnal variation in plasma levels of GH is noted. The nocturnal peak occurs 1–2 h after deep sleep. These secretory bursts are greater in children and decrease with age.
Variation in plasma GH levels with age
• From birth to early childhood, plasma GH levels increase progressively.
• Children versus adults. In general, children have only slightly higher plasma GH levels than adults.
• Puberty is associated with a peak period of plasma GH levels.
• Senescence is associated with a reduction in GH secretion in response to GHRH and other stimuli.
Circulation, half-life and metabolism
Circulation. Circulating GH is bound to a plasma protein (GH binding protein).
Half-life of circulating GH in humans is 0–20 min, and the daily GH output has been calculated to be 0.2–1.0 mg/day in adult.
Metabolism. Growth hormone is rapidly metabolized, probably at least in part in the liver. Metabolic clearance daily urinary GH excretion correlates well with the integrated 24 h plasma GH profile.
Regulation of gh secretion
1. Hypothalamic control
Hypothalamus controls GH secretion by releasing two hormones, growth-hormone-releasing hormone and growthhormone- release-inhibiting hormone (fig.8.2-4).
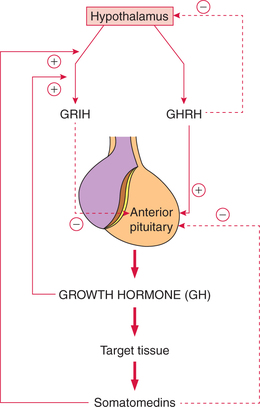
Fig. 8.2-4 Control of growth hormone secretion. GHRH (growth-hormone-releasing hormone) and GRIH (growthhormone-release-inhibiting hormone).
Growth-hormone-releasing hormone. It is a polypeptide with 44 amino acids. It stimulates the secretion of GH from the anterior pituitary.
Growth-hormone-release-inhibiting hormone, also called somatostatin, is a polypeptide with 14 amino acids. It inhibits the release of GH from the anterior pituitary.
2. Negative feedback control of GH secretion
The negative feedback control mechanism for GH involves the role of somatomedins, GH and GHRH (fig.8.2-4).
(i) Negative feedback control by somatomedins. Somatomedins are insulin-like growth factors (IGFs) that are produced when growth hormone acts on the target tissues. Somatomedins inhibit the secretion of GH directly or by stimulating the secretion of somatostatin from the hypothalamus.
(ii) Negative feedback control by GH. Growth hormone also inhibits its own secretion by stimulating the secretion of somatostatin from the hypothalamus.
(iii) Negative feedback control by GHRH. GHRH inhibits its own secretion from the hypothalamus. This mechanism is called ultrashort feedback loop.
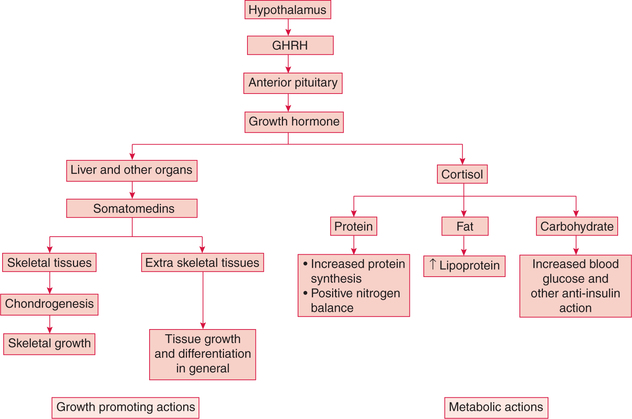
Actions of growth hormone
Growth hormone promotes growth and also influences the normal metabolism; therefore, besides acting on one specific organ, its actions are generalized (fig.8.2-5).
1. Growth promoting actions of GH
Growth hormone promotes linear growth of an individual by its effects on the bone, cartilage and other connective tissues.
(i) Effects on cartilage. GH stimulates the proliferation of chondrocytes (cartilage cells) present in the epiphyseal end plates of long bones.
(ii) Effects on bone. GH stimulates the osteoblastic activity which converts cartilage into bone. This process continues up to adolescence till there is fusion of the epiphyseal end plate with shaft of the bone. The bone mass also increases during this period.
2. Metabolic actions of GH
(i) Effects on protein metabolism. Growth hormone has an anabolic effect on the protein metabolism. It promotes the protein deposition in the tissues by following effects:
• Increases the rate of amino acid uptake into the cells.
The overall effect of GH on the protein metabolism is positive nitrogen balance that leads to an increase in the body weight. In addition, GH also decreases protein breakdown as well as the rate of amino acid degradation for energy purposes.
(ii) Effects on fat metabolism. GH promotes lipolysis in adipose tissue (catabolic effect) and then increases fat utilization for energy.
(iii) Effects on carbohydrate metabolism. GH is antagonistic to insulin and produces hyperglycaemia by following effects on the carbohydrate metabolism:
• Increases gluconeogenesis, i.e. increases hepatic glucose output,
• Decreases the uptake as well as utilization of glucose by the tissues for energy production and
• Inhibits glycolysis and thus glycogen stores tend to increase. This occurs as a consequence of increased mobilization and use of FFA for energy production.
(iv) Effects on mineral metabolism. Growth hormone promotes bone mineralization in growing children.
Human prolactin
Structure, secretion and plasma concentration
Structure and secretion. Human prolactin is a single peptide chain, secreted by acidophilic cells of anterior pituitary gland.
Plasma concentration. The prolactin secretion is pulsatile, shows diurnal variations (secretion increases about one hour after the onset of sleep and continues throughout the sleep period).
During pregnancy, prolactin secretion starts rising from eighth weeks onwards and peak value (200–400 ng/mL) is reached at term. The sources of prolactin during pregnancy are placenta, amniotic fluid and maternal anterior pituitary gland. The prolactin secretion during pregnancy and during lactation is affected by oestrogen.
Control of prolactin secretion
Hypothalamic control. Secretion of prolactin from anterior pituitary is controlled by the hypothalamus. A PIF formed in the arcuate nucleus of the hypothalamus is transported through hypothalamo–hypophyseal portal system to anterior pituitary, where it checks the synthesis and release of prolactin.
Physiological effects of prolactin
1. Breast growth. During pregnancy, it increases the breast growth particularly of alveolar tissue.
2. Lactogenic effect. Prolactin acts on the alveolar epithelium and stimulates the secretory activity.
During pregnancy, the lactogenic effect is suppressed by high concentration of oestrogen and progesterone.
After parturition, the lactogenic effect of prolactin is enhanced because of following reasons:
Note. The women who do not wish to feed their babies or when baby dies immediately after birth, in these situations, oestrogen is administered to stop lactation.
3. Suppression of ovarian cycle in nursing mothers. Prolactin inhibits the secretion of gonadotropin-releasing hormone from the hypothalamus. Therefore, gonadotropin (FSH and LH) secretion from the anterior pituitary also decreases. Thus in nursing mothers due to low levels of gonadotropins the ovarian cyclic changes do not occur. For details see page 450.
Applied aspects: abnormalities of anterior pituitary hormones
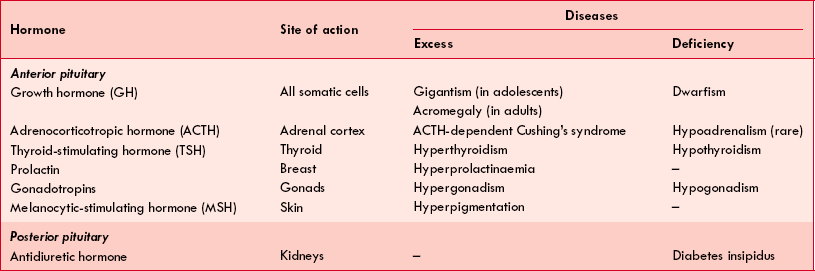
The abnormalities related to pituitary hormones occur either due to excess or deficiency of the hormones secreted. The most common causes of pituitary hormone disturbances are pituitary tumours, which may cause symptoms of excess of one or more hormones and simultaneous deficiency of other hormones, hence a mixed picture may evolve. The various hormones of pituitary, their site of action and disease produced by them are given in Table8.2-1.
Table. 8.2-1
Pituitary hormones: site of action and diseases associated with their deficiency and excess
Pituitary disorders seen in clinical practice are:
Hypopituitarism
Hypopituitarism is a clinical condition of hyposecretion of one or more pituitary hormones. Hypopituitarism can be due to hypothalamic causes or pituitary causes. Since anterior pituitary has a large reserve, the endocrine abnormalities are produced only when large part of pituitary is destroyed.
Abnormalities of growth hormone secretion
The abnormalities of growth hormone secretion include:
Hypersecretion of GH
Hypersecretion of GH occurs in tumours of acidophilic cells (particularly of somatotrophs) of the anterior pituitary. Depending upon the age of an individual, excess of GH may cause:
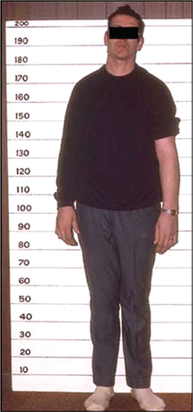
It is a clinical condition resulting from the hypersecretion of GH in growing children before the closure of epiphysis of long bones. The main features of gigantism are given below (Fig.8.2-6):
It is a clinical condition that occurs due to excess of GH in adults (after epiphyseal closure of long bones has occurred) and causes excessive growth in those areas where cartilage persists.
Clinical features are (Fig.8.2-7):
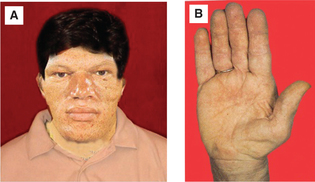
Fig. 8.2-7 Clinical features of acromegaly: A, note coarse facial features, broad thick nose, prognathism, prominent eyebrows and thickened skin and B, large spade-like hand with short, thick wide fingers.
• Acromegalic face, which is characterized by thick lips, macroglossia, broad and thick nose, prominent eyebrows, thickened skin and coarse facial features.
• Prognathism, i.e. protrusion of the lower jaw due to elongation and widening of mandible associated with increased spacing of the teeth.
• Acral part of abnormalities include large spade-like hands, thick wide fingers, large feet with an increase in size of the shoes. Height is normal, build is stout and stocky.
• Kyphosis may occur due to improper vertebral growth.
• Excessive growth of internal organs, i.e. cardiomegaly, hepatomegaly, splenomegaly and renomegaly may be associated.
• Increased sympathetic activity may cause increased sweating and hypertension.
Hyposecretion of GH
Deficiency of GH in childhood leads to stunted growth or dwarfism.
Deficiency of GH in adulthood results in mild anaemia, which is refractory to usual treatment with haematinics like iron.
Dwarfism. Short stature or dwarfism may be due to endocrinal or non-endocrinal causes.
Endocrinal causes of dwarfism are:
• Growth hormone deficiency (pituitary dwarf),
• Hypothyroid dwarf (see page 385) and
• Cushing's syndrome (see page 404).
Non-endocrinal causes of dwarfism include:
Growth-hormone-related dwarfism
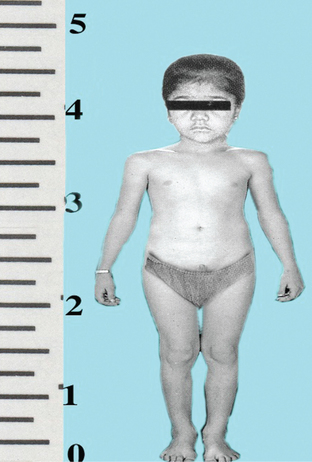
Pituitary dwarfism occurs due to deficiency of GH in early childhood.
Characteristic features. Deficiency of GH causes retardation of growth in all parts of the body proportionately. Consequently, a pituitary dwarf with a chronological age of 20 years has the body structure like that of a normal child of 7–10 years of age. Thus a pituitary dwarf has following features (Fig.8.2-8):
Posterior pituitary hormones
The two important hormones released from posterior pituitary are:
Antidiuretic hormone
Antidiuretic hormone (ADH), as the name indicates, prevents diuresis and is chiefly concerned with the conservation of body water. Since it also causes vasoconstriction, it is also called vasopressin or more precisely arginine vasopressin (AVP).
Structure, synthesis, storage, release,transport and metabolism
Structure, synthesis, storage and release of ADH and oxytocin (OTC) being similar are discussed together.
Structures. ADH and OTC both are homologous neurohormones, polypeptide in nature, containing 9 amino acids each, but the amino acids in position 3 and 8 differ in the two hormones.
Synthesis. ADH as well as OTC are synthesized in the cell bodies of magnocellular neurons of both paraventricular and supraoptic nuclei of the hypothalamus.
Storage. The axons of ADH- and OTC-synthesizing neurons end in the posterior pituitary gland as terminal swelling. The secretory granules containing hormone precursors, known as Herring bodies, are transported down the axons by axoplasmic flow to the nerve endings in the posterior pituitary.
Secretion. Antidiuretic hormone and OTC are released when a nerve impulse is transmitted from the cell body in the hypothalamus down the axon, where it depolarizes the neurosecretory vesicles.
Transport. The hormone and other secreted products separately enter the closely adjacent capillary. The hormone then reaches the target cells and also to the anterior pituitary by circulatory interconnections.
Biological half-life of ADH is 16–20 min after its release into the circulation.
Metabolism. The circulating vasopressin is rapidly inactivated in the liver and kidney.
Actions of ADH
Vasopressin receptors
Three types of vasopressin receptors are recognized:
• V1–A receptors. These are involved in the vasoconstrictor effect of ADH.
• V1–B receptors. These are involved in the action of ADH on the anterior pituitary.
• V2 receptors. These are involved in the action of ADH on kidney.
1. Action on kidney. The main role of ADH is regulation of water balance in the body by acting on the kidney, where it decreases the excretion of free water (i.e. antidiuretic and concentrating effect on kidney).
Site of action. The ADH-responsive cells line the distal convoluted tubules and collecting ducts of the renal nephron. ADH increases the permeability of these cells to water.
2. Vasoconstrictor effect. ADH in large doses cause vasoconstriction and leads to rise in blood pressure. Haemorrhage is a potent stimulus to ADH secretion, and it has been reported that ADH plays important role in blood pressure homeostasis by causing constriction of splanchnic vascular bed. The ADH exerts its vasoconstrictor effect by binding to the V1–A receptors on the arterial smooth muscles and causing it to constrict.
3. Action on anterior pituitary. ADH travels to the anterior pituitary via the portal veins, combines with the V1–B receptors and causes increased ACTH secretion.
Regulation of ADH secretion
The main factors which regulate the ADH secretion are:
1. Effective osmotic pressure of plasma or plasma osmolality
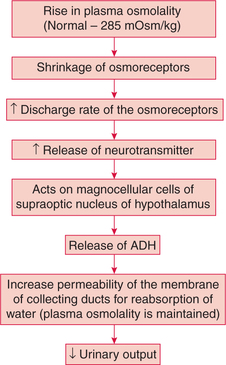
Plasma osmolality in normal individuals is maintained very close to 285 mOsm/kg by ADH. In other words, change in plasma osmolality is a very potent regulator of ADH secretion. Thus, water deprivation (which increases plasma osmolality) stimulates ADH secretion. On the other hand, a water load (which decreases plasma osmolality) decreases ADH secretion.
Mechanism of action. Changes in plasma osmolality affect the osmoreceptors.
• Osmoreceptors refer to a group of neurons located in the anterior hypothalamus.
• The rise in plasma osmolality even by 1%–2% results in the shrinkage of osmoreceptors causing an increased rate of discharge and reflexly increased ADH secretion and thus maintained plasma osmolality (Fig.8.2-9).
• The substances like Na+, mannitol and sucrose are potent stimulators for ADH secretion.
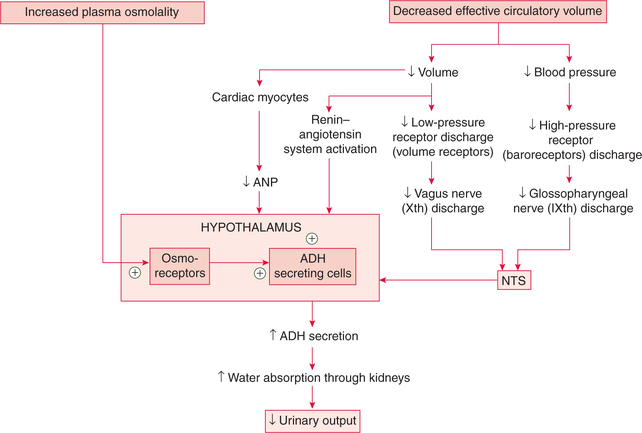
2. Changes in blood volume
Changes in the circulating blood volume, central blood volume, cardiac output and blood pressure affect the secretion of ADH.
3. Other factors affecting ADH secretion
Factors, other than the two major stimuli (i.e. hypovolaemia and plasma osmolality), which affect ADH secretion are:
• Stress of pain, chronic emotional stress and surgical procedures cause increase in ADH secretion leading to reduction in urine formation under these conditions.
• Adrenaline decreases the ADH. Hence one experiences an increased frequency of micturition during acute emotional stresses such as interviews or examinations.
Summary of factors regulating ADH secretion is given in Figure.8.2-10.
Abnormalities of adh secretion
Abnormalities of ADH secretion include:
Syndrome of inappropriate hypersecretion of ADH
Syndrome of inappropriate hypersecretion of antidiuretic hormone (SIADH) refers to a condition in which ADH secretion is increased despite the presence of hypoosmolality. Excessive ADH secretion leads to water intoxication, i.e. overhydration, and because of this SIADH is also called dilution syndrome.
Diabetes insipidus
Diabetes insipidus refers to a clinical condition of polyuria that occurs either due to deficiency of ADH (vasopressin) release or failure of renal response to ADH.
Characteristic features. The diabetes insipidus is characterized by decreased renal absorption of water leading to following features:
1. Polyurea, i.e. passage of large amount of urine, up to 3–20 L, is the most important single feature of diabetes insipidus.
2. Polydipsia. Polyuria is followed by obligatory polydipsia (drinking of large amount of water). It occurs due to the stimulation of thirst mechanism.
3. Dehydration may occur in severe cases. Its signs and symptoms include dry tongue, dry mouth, fall of blood pressure and loss of consciousness may be seen in acute severe cases.
Oxytocin
Structure, synthesis, storage and release of oxytocin
Structure, synthesis, storage and release of oxytocin have been described along with ADH (see page 376).
Actions of oxytocin
In humans, oxytocin acts mainly on the uterus and breasts.
Action on breast. Oxytocin causes contraction of myoepithelial cells and thus plays an important role in milk ejection. For details see page 461.
Action on uterus. Oxytocin causes contraction of uterine smooth muscles and thus plays an important role during parturition (labour). For details see page 458. Oxytocin also acts on non-pregnant uterus and facilitates the transport of sperm in the female genital tract.
 Vasopressin receptors
Vasopressin receptors