Physiology of Acid–Base Balance
General considerations
Acids and bases
Acids and bases. Acid refers to a substance that acts as proton (H+) donor while base refers to a substance that accepts proton (H+). Examples of a few acids and their corresponding base are:
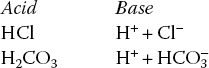
Alkalies refer to the metallic hydroxides, e.g. NaOH and KOH. These compounds do not directly satisfy the criteria of bases. However, they dissociate to form metallic ion and OH−, which being the base accepts H+ ions. Therefore, for all practical purposes, alkalies are considered bases.
Strong acid and bases. Acid or base having strong tendency to dissociate into ions is called strong acid or strong base; and the acid or base having weak tendency to dissociate into ions is called weak acid or weak base. In general, a strong acid has a weak base, while a weak acid has a strong base. For example, strong acid HCl has weak base Cl− and weak acid HCN has a strong base CN−.
Ampholytes refer to the substances that can act both as acids and bases. Water is the best example of ampholyte.
Concept of pH and H+ concentration
H+ ion concentration. The acidic or basic nature of a solution is measured by H+ ion concentration. Since the concentration of H+ ions in the biological fluids is exceedingly low, the conventional units such as mEq/L or moles/L, etc. are not commonly used to express H+ concentration. Therefore, pH is the term suggested to express H+ ion concentration. pH is defined as the negative logarithm of H+ concentration.
It is important to note that pH and H+ are inversely related. For example, pH of plasma with a H+ ion concentration of 0.00004 mEq/L is 7.4, while pH of HCl with H+ ion concentration of 150 mEq/L is 0.8.
Neutral pH, acidic pH and alkaline pH. Pure water has an equal concentration of H+ and OH− ions, i.e. 10−7M each. Thus pure water has pH of 7, which is neutral. Therefore, solutions with pH less than 7 are considered acidic and those with more than 7 are considered alkaline.
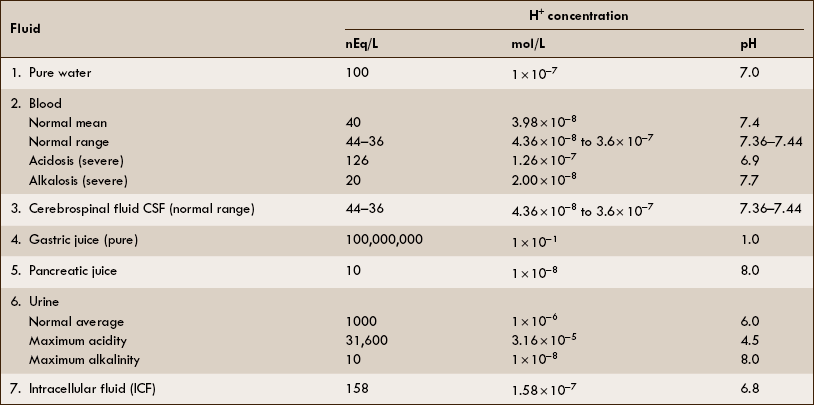
H+ concentration and pH of biological fluids
The H+ concentration and pH of some biological fluids are depicted in Table 6.3–1.
Maintenance of blood pH
General considerations
Blood and plasma pH
• The term blood pH always refers to the plasma pH.
• Normal plasma pH is 7.4 (H+ concentration −40 mEq/L), which is higher than the intracellular pH of the erythrocyte (7.2).
• Plasma pH compatible with life varies from 7.7 to 6.9 (H+ concentration is from 20 to 120 nEq/L).
• Normally, the pH of extracellular fluid (ECF) is maintained between a narrow range of 7.35–7.45.
Dietary and metabolic production of acids and bases
The daily consumed diet contains many acids and alkalies. In addition, cellular metabolism produces a number of acidic and alkaline substances that have an impact on the body pH.
Acid production by the body
The metabolic activities of the body are accompanied by production of two types of acids.
1.Volatile acids. CO2 is the volatile acid produced from the aerobic metabolism of cells. It is also major end product in the oxidation of carbohydrates, fats and amino acids. CO2 accounts for over 12,000 mEq/L of H+ per day. It is considered acid because CO2 combines with H2O [by a reaction catalyzed by carbonic anhydrase, i.e. (CA)] to form weak acid H2CO3, which dissociates into H+ and 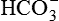 by the following reaction:
by the following reaction:
It is called volatile because it is a gas, and under normal circumstances almost all the CO2 is excreted by lungs.
Defences against changes in H+ concentration
There are three lines of defence to regulate the body's acid–base balance and maintain the blood pH (around 7.4).
I Buffer systems of body fluid
• Immediately combine with H+ to prevent changes in H+concentration and form a temporary measure to control changes in the H+ concentration.
Buffer system: primary defence
Buffers. A buffer is a solution consisting of a weak acid and its salt with strong base that prevents a change in pH when H+ ions are added to or removed from a solution. It must be born in mind that a buffer cannot remove H+ ions from the body. It temporarily acts as a shock absorbant to reduce the free H+ ions. The H+ have to be ultimately eliminated by the renal mechanism.
When acid is added to a buffer solution its H+ ion concentration is increased and the reaction is forced towards right leading to an increase in undissociated molecules; therefore, increase in H+ concentration is less.
When base is added to a buffer, reaction shifts towards left; more H+ ions are released from the buffer to combine with base, thereby limiting the decrease in H+ concentration.
Henderson–Hasselbalch equation which is used to calculate the pH in a buffer system can be derived as: The general equation for a buffer is
A− represents any anion from a buffer (i.e. H+ acceptor) and HA the undissociated acid from a buffer (i.e. H+ donor). By the law of mass action, at equilibrium
(K = Dissociation constant of the acid HA)
The equation to represent free H+ ion in a solution can be rewritten as
By taking the reciprocals and logarithms (for log, multiplication becomes addition).
The equation (3) may be rewritten as
From this equation, it is evident that buffering capacity of a buffer system is greatest when the amount of anions [A−] and undissociated acid [HA] is same, i.e.
thus, pH = pK. Therefore, most effective buffers in the body are those with pK close to the pH in which they operate.
Classification of the buffer systems
Buffer systems in the body can be classified as follows.
Bicarbonate versus non-bicarbonate buffers
1. Bicarbonate buffer forms 53% of the buffering in the whole body. Out of it:
2. Non-bicarbonate buffers form remaining 47% of the buffering in the whole body. With a contribution from:
| • Haemoglobin and oxyhaemoglobin | 35% |
| • Plasma proteins | 7% |
| • Organic phosphate | 3% |
| • Inorganic phosphate | 2% |
Major buffer systems of the body
The major buffer systems involved in the maintenance of body pH are:
1 Bicarbonate buffer system
The carbonic acid-sodium bicarbonate (H2CO3–NaHCO3) is the most predominant buffer system of the extracellular fluid, particularly the plasma H2CO3 in the body is formed by CO2 and H2O:
This reaction is catalyzed by the enzyme carbonic anhydrase, which is present in the RBCs, walls of the lungs, alveoli and epithelial cells of renal tubules.
Dynamics of bicarbonate buffer system
Carbonic acid dissociates into hydrogen and bicarbonate ions.
According to the Henderson-Hasselbalch equation for this system:
The pK1 of this system (6.1) is still low relative to the pH of the blood (7.4), but the system is one of the most effective buffer systems in the body because the amount of dissolved CO2 is controlled by respiration and plasma concentration of 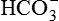 is regulated by the kidney. Therefore, pH of ECF can be precisely controlled.
is regulated by the kidney. Therefore, pH of ECF can be precisely controlled.
2 Phosphate buffer system
Inorganic orthophosphate buffer system
Inorganic orthophosphate buffer system is formed by sodium dihydrogen phosphate and disodium hydrogen phosphate (NaH2PO4 ~ Na2HPO4), which exist in at a plasma pH of 7.4 in a concentration ratio of 1:4.
Sites of operation of NaH2PO4–Na2HPO4 buffer
1. In ECF (plasma and interstitial fluid), the 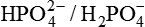 buffer exists in a small concentration (0.66 mmol/L) and thus contributes little to the buffering capacity of plasma.
buffer exists in a small concentration (0.66 mmol/L) and thus contributes little to the buffering capacity of plasma.
2. In intracellular fluid (ICF), 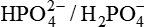 forms an important buffer pair because:
forms an important buffer pair because:
3. In renal tubules, the 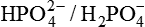 forms an effective extracellular buffer because:
forms an effective extracellular buffer because:
The 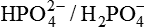 system is a major elimination route for H+ via the urine.
system is a major elimination route for H+ via the urine.
3 Protein buffer system
The protein buffer system of the blood is constituted by the plasma proteins and haemoglobin combinedly.
Plasma proteins buffer system accounts for 15% of the buffering capacity of the whole blood. Plasma proteins are effective buffers because both their free carboxyl and free amino groups dissociate
Because of their amphoteric nature, plasma proteins can combine with acids and bases as:
Haemoglobin buffer system (Hb/HHb and 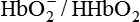 ) accounts for 35% of the total buffering capacity of the whole blood. It mainly buffers the fixed acids, besides being involved in the transport of gases (O2 and CO2).
) accounts for 35% of the total buffering capacity of the whole blood. It mainly buffers the fixed acids, besides being involved in the transport of gases (O2 and CO2).
Deoxyhaemoglobin (Hb) is better buffer than oxyhaemoglobin (HbO2) because the imidazole groups of Hb dissociate less than those of HbO2, making Hb a weaker acid.
Respiratory mechanism for pH regulation
Second line of defence against acid-base disorders is formed by the respiratory mechanism, which provides a short-term but rapid control. It acts via respiratory centre located in the medulla to regulate removal of CO2 and therefore carbonic acid (H2CO3) concentration in the blood.
Renal mechanism for pH regulation
The kidneys regulate pH through three main processes (see page 285).
Acid-base disorders
Acidosis refers to a decline in blood pH, while alkalosis refers to a rise in blood pH. As described above, our body has been provided with an efficient system for the maintenance of acid–base equilibrium with a result that the pH of blood is almost constant (7.4). The blood pH compatible to life is 6.8–7.8, beyond which life cannot exist.
Simple acid–base disorders
The physiological aspects of single acid-base disorders are summarized in Table 6.3–2 and are described as follows.
Table 6.3-2
Summary of characteristics of simple acid–base disorders
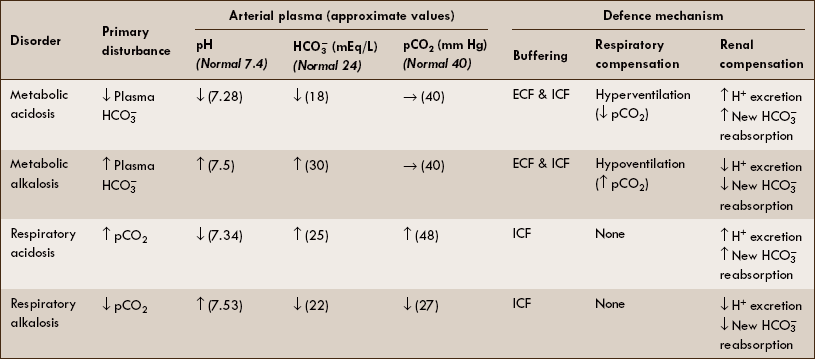
ICF = Intracellular fluid; ECF = extracellular fluid; ↑ = increased; ↓ = decreased; → = normal.
Metabolic acidosis
Physiological disturbance that produces metabolic acidosis is either increased net non-volatile acid load or loss of base 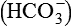 .
.
Primary disturbance in metabolic acidosis is decreased plasma 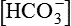 producing a low plasma pH.
producing a low plasma pH.
Causes of metabolic acidosis include:
When metabolic acidosis is produced by the non-renal factors, the respiratory and renal compensatory mechanisms tend to minimize the change in pH of blood. In renal failure, only respiratory compensation is possible.
1. Respiratory compensation. Increased H+ stimulates the respiratory centre through peripheral chemoreceptors and produces hyperventilation (Kussmaul breathing), which in turn decreases the arterial pCO2 value and minimizes the degree of acidosis.
2. Renal compensation is slow but an effective mechanism to control metabolic acidosis. It consists of:
Metabolic alkalosis
Physiological disturbance that produces metabolic alkalosis is either addition of non-volatile alkali or loss of H+from the body.
Primary disturbance in metabolic alkalosis is increased plasma 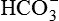 , producing a high plasma pH.
, producing a high plasma pH.
Causes of metabolic alkalosis include:
• Vomiting (H+ is lost from the stomach) and
• Hyperaldosteronism (increased H+ secretion by distal tubule).
1. Respiratory compensation. Increased pH (or decreased H+) inhibits the respiratory centre through peripheral chemoreceptors and produces hypoventilation, which in turn elevates pCO2 and thus normalizes plasma pH.
Note. It is important to note that the magnitude of respiratory compensation is limited by the fact that hypoventilation results in decreased arterial pO2, which stimulates the respiratory centres via peripheral chemoreceptors.
Respiratory acidosis
Primary disturbance in respiratory acidosis is increased pCO2, which by mass action causes an increase in H+ and thus lowers the blood pH.
Causes. The pCO2 is increased due to decreased gas exchange across the alveoli because of following causes:
Respiratory alkalosis
Primary disturbance in the respiratory alkalosis is decreased pCO2 associated with low (H+) and thus an elevated plasma pH.
Causes. The pCO2 is decreased due to increased gas exchange in the lungs because of increased ventilation.
Note. There is no respiratory compensation for respiratory alkalosis.
Renal compensation. Decreased pCO2 causes a deficit of H+ in the renal cells for secretion which leads to:
The resulting decrease in serum (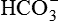 ) helps to normalize the pH. In this way, the alkalosis is mostly but not completely compensated by the renal mechanism.
) helps to normalize the pH. In this way, the alkalosis is mostly but not completely compensated by the renal mechanism.
 Acids and bases
Acids and bases Bicarbonate buffer system
Bicarbonate buffer system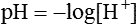
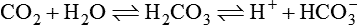
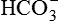 produced by the metabolism of organic anions (e.g. citrate) offsets non-volatile acid production to some degree.
produced by the metabolism of organic anions (e.g. citrate) offsets non-volatile acid production to some degree.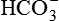 , generating new
, generating new 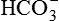 , and excreting H+ as titrable acid and ammonium ion.
, and excreting H+ as titrable acid and ammonium ion.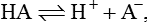
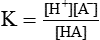
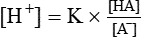
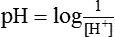
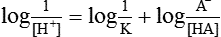
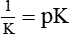
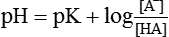
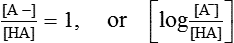
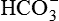 contributes 35% and
contributes 35% and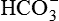 contributes 18%.
contributes 18%.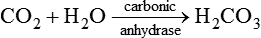
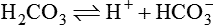
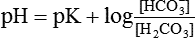
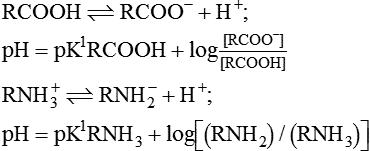
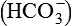 .
. .
. ).
).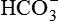 , which replenishes the
, which replenishes the 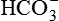 used in buffering the added fixed H+.
used in buffering the added fixed H+.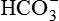 excretion as the filtered load of
excretion as the filtered load of 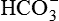 exceeds the ability of renal tubule to reabsorb it. The urinary loss of
exceeds the ability of renal tubule to reabsorb it. The urinary loss of 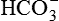 decreases the plasma level of
decreases the plasma level of 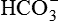 , thereby restoring the pH of blood to near normal.
, thereby restoring the pH of blood to near normal.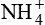 ,
,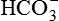 and
and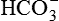 .
.