Physiological Activities in Stomach
Functional anatomy
Gross anatomy
Stomach is a J-shaped, hollow, muscular bag connected to the oesophagus at its upper end and to the duodenum at the lower end.
Parts of stomach
The stomach can be divided into five anatomic regions (Fig. 7.3-1):
• Cardia is the narrow conical portion of the stomach immediately distal to the gastroesophageal junction.
• Fundus is the dome-shaped proximal portion of the stomach.
• Body or corpus is the main part of the stomach that extends up to the incisura angularis.
• Pyloric antrum extends from the incisura angularis to the pyloric canal.
• Pyloric canal or pylorus is the distal most 1-inch long tubular part of stomach.
Note. Anatomically, the antrum and pylorus are continuous and respond to nervous control as a unit. Functionally, first part of duodenum is associated with the pyloric part of stomach.
Structural characteristics
The gastric wall consists of mucosa, submucosa, muscular coat and serosa (serous layer).
Histological features
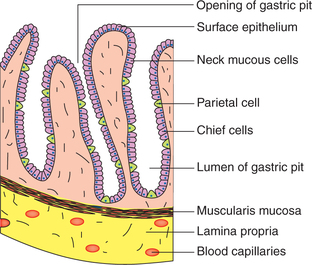
Gastric mucosa comprises (Fig. 7.3-2) the following:
Surface foveolar cells are tall columnar mucin-secreting cells, which line the entire gastric mucosa as well as the gastric pits.
Mucous neck cells are present deeper in the gastric pits. These cells are thought to be the progenitors of both, the surface epithelium and the cells of gastric glands.
Glandular cells form the gastric glands. There are three types of gastric glands.
1. Main gastric glands are found in the body and fundus of stomach. These are simple tubular glands (Fig. 7.3-2). The alveoli of main gastric glands contain two types of cells:
• Chief cells, also known as peptic or zymogen cells, are basophilic. These cells are concentrated at the base of main gastric glands and secrete proteolytic proenzymes, pepsinogen I and II.
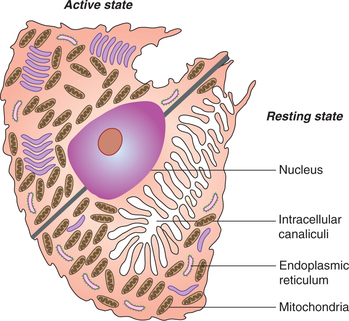
• Parietal cells (oxyntic cells) are acidophilic. These cells line predominantly the upper half of glands and have an extensive intracellular canalicular system. These secrete hydrochloric acid (HCl) and the intrinsic factor.
2. Cardiac tubular glands are found in the mucosa of cardia. These secrete soluble mucus.
3. Pyloric (antral) glands are found in the antrum and pylorus region of the stomach. These glands contain two types of cells:
Physiology of gastric secretion
The gastric secretions include:
• Exocrine secretions, i.e. gastric juice and
• Endocrine secretions, i.e. gastrin hormone (see regulation of gastric secretion).
Gastric juice
Composition
Gastric glands secrete about 2–2.5 L of gastric juice in the lumen of stomach per day. It is acidic with a pH varying from 1 to 2. Important constituents of gastric juice are water and solids.
Solids – 0.55%, which include the following.
Electrolytes, such as Na+, K+, Mg2+, Cl−, 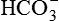 ,
, 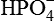 and
and 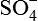 . The electrolyte content of gastric juice varies with the rate of secretions. At low secretory rates, Na+ concentration is high and H+ concentration is low, but as acid secretion increases Na+ concentration falls.
. The electrolyte content of gastric juice varies with the rate of secretions. At low secretory rates, Na+ concentration is high and H+ concentration is low, but as acid secretion increases Na+ concentration falls.
Enzymes present in the gastric juice are:
• Pepsin is a proteolytic enzyme, which is secreted by the chief cells of gastric glands in an inactive form pepsinogen.
• Gastric lipase is a weak fat-splitting enzyme. It is of little importance in fat digestion except in pancreatic insufficiency.
• Gastric gelatinase liquefies gelatin, a protein contained in the connective tissue.
Mucin or mucus is of two types:
• Soluble mucus secreted by the mucus cells of pyloric and cardiac glands and
• Insoluble mucus secreted by the surface foveolar cells (tall columnar mucin secreting cells) lining the entire gastric mucosa.
Intrinsic factor is secreted by the parietal cells of gastric glands.
Secretion of gastric juice
Secretion of HCl
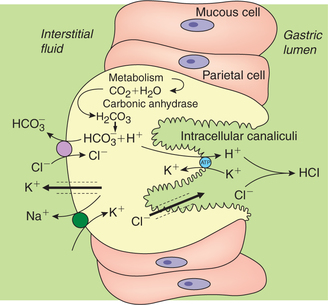
Hydrochloric acid (HCl) is secreted by the parietal cells (also called oxyntic cells). These cells show, under electron microscope, a complex network of intracellular canaliculi (Fig. 7.3-3) into which HCl is secreted.
Various theories have been put forward to explain the origin of H+ of HCl. The hypothesis more widely accepted is shown in Fig. 7.3-4. Hydrochloric acid is made up of hydrogen (H+) and chloride ions (Cl−), therefore its secretion can be described in two steps, i.e. secretion of H+ and secretion of Cl−.
Secretion of H+
• The H+ ions are believed to be generated inside the parietal cell from metabolic CO2 and H2O present in the cell. The enzyme carbonic anhydrase present in abundance in the parietal cells is essential for the secretion. It accelerates the formation of 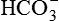 , which dissociates to release H+ and
, which dissociates to release H+ and 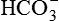 as
as
• The H+ ions generated by the above reaction are then secreted into the lumen of the canaliculi in exchange for K+ by a primary active transport mediated by a H+–K+–ATPase pump (Fig. 7.3-4).
Note. The drug omeprazole used to decrease HCl formation inhibits the H+–K+–ATPase and blocks H+secretion.
• The MATHML ions produced in the parietal cell are transported by an antiport in the serosal (basolateral) membrane into the blood in exchange of Cl− by an active transport.
Secretion of Cl−. Because of the high intracellular negativity, the Cl− present in the parietal cell is forced out into the lumen of gland through the Cl− channels located on the apical membrane of the cell.
Pepsinogen secretion
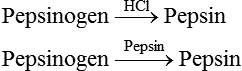
Pepsinogen is an inactive precursor (proenzyme) of pepsin. It is mainly secreted by the chief cells of the main gastric glands. A small amount of pepsinogen is also secreted by the pyloric glands. The pepsinogen secreted by the chief cells is called pepsinogen I and that secreted by the pyloric glands is called pepsinogen II.
Pepsinogen is converted to pepsin (the active form) by the action of HCl or preformed pepsin.
Secretion of mucus
Mucus is of two types: insoluble and soluble.
Insoluble mucus is secreted by the mucous-secreting cells lining the entire gastric mucosa. The insoluble mucus is such a viscid that it forms a gel-like coat over the mucosa.
Soluble mucus is secreted by the mucus cells of pylorus and cardiac glands.
Secretion of intrinsic factor
Intrinsic factor (IF), a glycoprotein, is secreted by the parietal cells of gastric mucosa, chiefly by those in the fundus.
Functions. The intrinsic factor is essential for the absorption of Vitamin B12. It forms a complex with B12, which is carried to the terminal ileum where the vitamin is absorbed.
Regulation of gastric secretion
Mechanisms regulating the gastric secretion include neural control and chemical control.
Neural control
Neural control over the gastric glands is exerted by a local enteric plexus involving cholinergic neurons and impulses from the central nervous system via vagal (extrinsic) innervation.
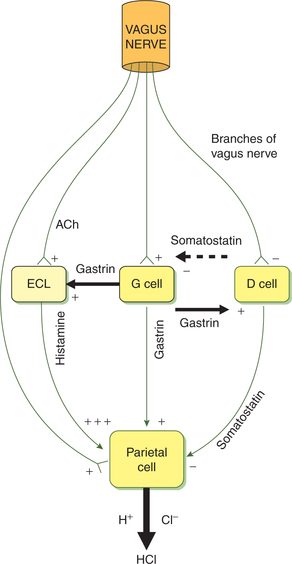
Vagal stimulation increases the secretion of HCl by the parietal cells and pepsin by the chief cells. Vagal stimulation increases H+ secretion by a direct and an indirect path (Fig. 7.3-5):
• In the direct path, the vagus nerve fibres innervating the parietal cells stimulate H+ secretion by releasing neurotransmitter acetylcholine (ACh), which acts on the muscarinic receptors on the parietal cells. In addition, ACh also potentiates the effects of histamine on H2receptors of parietal cells.
• In the indirect path, the vagus nerve innervates G cells and stimulates the release of gastrin into circulation through gastrin-releasing peptide (GRP). The gastrin in turn stimulates H+ secretion.
• Further, vagal stimulation also inhibits the release of somatostatin, and thus indirectly stimulates H+ secretion by removing the inhibitory effect of somatostatin on the parietal cells.
• ACh released on the vagal stimulation also acts on the enterochromaffin-like (ECL) cells, which release histamine. Histamine increases H+ secretion by acting on H2receptors on the parietal cells.
Chemical control
Chemical control on the gastric glands is exerted mainly through:
• Gastrin, a hormone secreted by G cells;
• Histamine, a paracrine agent released from the mast cells of gastric mucosa;
• Somatostatin, secreted by D-cells;
• Low pH (<3.0) in stomach acts in a negative feedback mechanism and
• Local hormones released in the duodenum inhibit the gastric secretion in addition to promoting the secretion of pancreatic juice and bile.
1 Role of gastrin
Gastrin, a hormone, is secreted by the G cells into the blood circulation (and not into gastric juice). It reaches the stomach through the arterial circulation and stimulates secretory activity of the parietal cells and chief cells.
• The main function of gastrin is to stimulate HCl secretion by binding with the cholecystokinin (CCK)-β gastrin receptors on the parietal cell. It acts to open Ca2+channels and to release Ca2+ from the intracellular stores. The Ca2+ along with cyclic AMP acts via protein kinase to increase the transport of H+ into the gastric lumen by H+-K+-ATPase (Fig. 7.3-6).
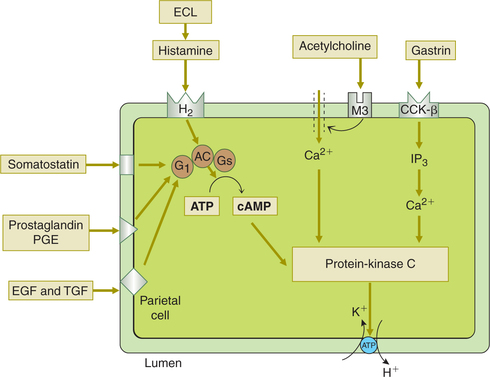
Fig. 7.3-6 Control of HCl secretion at the parietal cell level. Acetylcholine (ACh), histamine and gastrin act agonists of HCl secretion by distinct mechanism (for details see text). Somatostatin, prostaglandins (PGE), epidermal growth factor (EGF) and transforming growth factor (TGF) act as endogenous antagonists of HCl secretion by inhibiting adenylyl cylase.
Gastrin also increases the HCl secretion by stimulating secretion of histamine from the ECL cells present in the body of the stomach.
2 Role of histamine
Histamine is released from the ECL cells found in the base of the gastric gland. ECL cells bear both gastrin receptors and ACh receptors. They release histamine in response to both circulating gastrin as well as ACh released by vagal fibres. The histamine released stimulates HCl secretion from the parietal cells by acting on H2 receptors. The H2receptors increase intracellular cAMP via Gs. The cAMP acts as a second messenger to activate cAMP-dependent protein kinase. H2 receptor-blocking drugs, such as cimeti-dine and ranitidine, inhibit H+ secretion by blocking the stimulatory effect of histamine.
3 Role of somatostatin
• Somatostatin is secreted by D cells located adjacent to the G cells or the parietal cells in the gastric glands.
• Somatostatin inhibits HCl secretion in two ways:
• Somatostatin, prostaglandins, EGF and TGF act on the parietal cells to inhibit HCl secretion by inhibiting adenylyl cyclase (Fig. 7.3-6).
Phases of gastric secretion and their regulation
Meal-related gastric secretion can be divided into three phases:
7.3.1 Cephalic phase
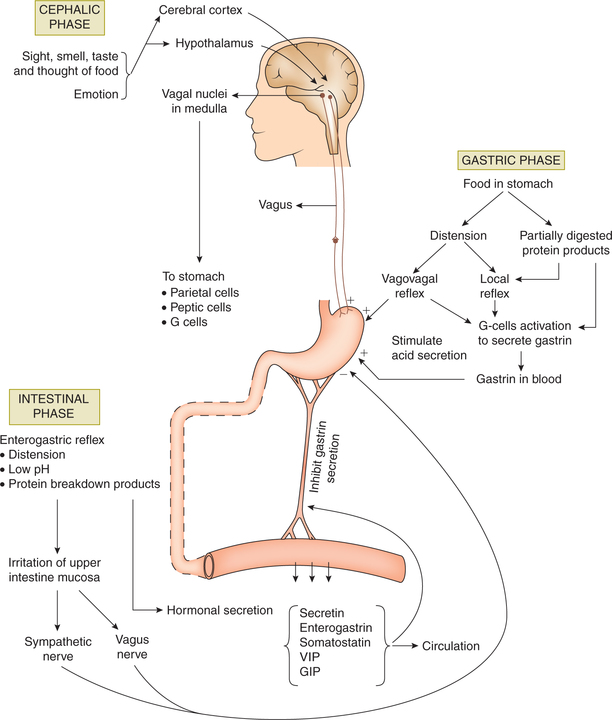
• Cephalic phase of the gastric secretion occurs before the entry of food into the stomach.
• The secretion is initiated by the thought, sight, smell or taste of food. Neurogenic signals originate in the cere bral cortex and hypothalamus. The impulses are trans mitted to dorsal vagal nuclei and from there through vagii to the stomach (Fig. 7.3-7).
• Emotions also influence this vagally mediated gastric secretion. Anger and hostility are associated with an increased gastric secretion. Fear and depression decrease the gastric secretion and motility.
7.3.2 Gastric phase
• Gastric phase of the gastric secretion occurs when food enters the stomach.
• The presence of food in the stomach induces gastric secretion by following mechanisms:
– Distension of the body of stomach acting through local myenteric and vagovagal reflexes results in an increase in HCl secretion.
– Distension of the antrum initiates vagally mediated and local reflexes that result in gastrin release from the antral G-cells. Gastrin release is inhibited when pH becomes low (< 3).
– Products of partial protein digestion also stimulate gas trin secretion, and this increases mainly secretion of gastric acid.
7.3.3 Intestinal phase
• Intestinal phase of gastric secretion begins as the chyme begins to empty from the stomach into the duodenum.
• Intestinal factor inhibits gastric secretion by following mechanisms:
– Enterogastric reflex is initiated by the distension of small intestine, presence of acid or protein break down products in the upper intestine, and irritation of mucosa.
– Hormonal mechanism. Presence of acid, fat, hyper- or hypotonic solution and irritating factors in the upper small intestine release several hormones, such as secretin, CCK, GIP, VIP and somatostatin, which inhibit gastric secretion.
• The inhibitory influences discussed above help to termi nate the gastric secretion when all the food has left stomach.
Physiology of gastric motility
General considerations
Gastric motility is the function of gastric musculature which consists of three layers of smooth muscle fibres: an outer longitudinal layer, middle circular layer and an inner oblique layer.
Initiation of gastric motility
Basal electrical rhythm
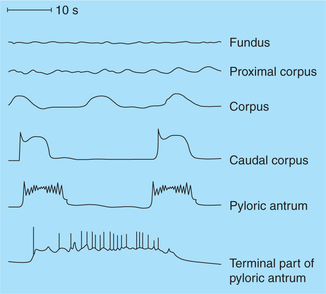
• The musculature of stomach, being a single unit smooth muscle, has its only rhythmic contractile myogenic tone called the basic electrical rhythm (BER) or gastric slow waves.
• The gastric slow waves are initiated by the pacemaker cells located near the fundus on the greater curvature of the stomach.
• Gastric slow waves consist of an upstroke and a plateau phase (Fig. 7.3-8) and occur at a rate of approximately 3–4 waves/min.
Types of gastric motility
Gastric motility can be described as follows:
Motility of empty stomach
Migrating motor complexes
• Migrating motor complex (MMC) is the name given to the peristaltic wave that begins in the oesophagus and travels through the entire gastrointestinal tract (migra tory motor activity) during interdigestive period.
• The MMCs remove any food remaining in the stom ach and intestines during interdigestive period in the preparation for the next meal because of this they have been called the interdigestive housekeepers.
• The MMC are abolished immediately after the entry of food in the stomach.
Gastric motility related to meals
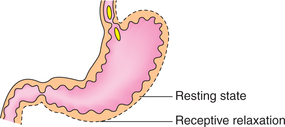
Receptive relaxation and accommodation
• Storage function of stomach is accomplished by the receptive relaxation and accommodation.
• As the food enters the fundus and upper part of body of stomach, i.e. the oral region relaxes to accommodate the ingested food without any increase in intramural pressure. This phenomenon is called receptive relaxation (Fig. 7.3-9).
• The passage of each bolus of food stimulates the stretch receptors of oral region and produces relaxation. By the end of meal about 1–2 L of food can be accommodated.
• Receptive relaxation is a vagovagal reflex initiated by distension of stomach.
Mixing peristaltic waves
The presence of food in the caudal region (distal body and antral part) of stomach increases the contractile activity of this part of stomach. This enhanced contractile activity (a combination of peristalsis and retropulsion) is called mixing waves, which mix the food with stomach acid and enzymes, and break it into smaller and smaller pieces. When the food is mixed into a pasty consistency it is called chyme.
Mixing mechanism of peristalsis and retropulsion
• Peristaltic contractions begin in the mid stomach (Fig. 7.3-10A) and proceed caudally.
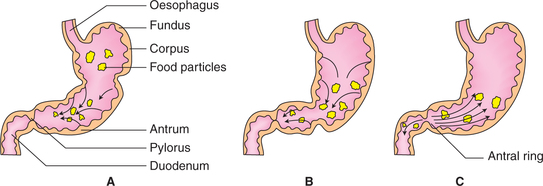
Fig. 7.3-10 Mixing peristaltic waves of stomach: A, peristaltic contractions begin in the mid stomach and pushes the food towards pylorus; B, when food reaches the pylorus it strikes against closed pyloric sphincter and C, antral contents forced back (retropulsion).
• The food particles also move towards pylorus (Fig. 7.3-10B) along with the deep wave of contraction.
• When the food reaches the pylorus, it strikes against the closed pyloric sphincter with a force. As a result, most of the antral contents are forced back into the body of stomach, and only a small amount of chyme passes into the duodenum (Fig. 7.3-10C). The backward movement of the food is called retropulsion.
Gastric emptying
• Gastric emptying results from a progressive wave of forceful contraction, which sequentially involves antrum, pylorus (pyloric sphincter) and proximal duodenum; thus all the three function as a unit.
• Each time the chyme is pushed against the pyloric sphincter. Therefore, chyme is pumped in a bit (2–7 mL) at a time into the small intestine.
Factors regulating the gastric emptying
After a normal meal, the emptying time is 2–3 h. The gastric emptying is regulated by various factors.
1. Fluidity of the chyme. The rate of gastric emptying of liquids is much faster than solids.
2. Gastric factors, which affect emptying, are:
• Volume of food in the stomach. Greater the volume of food in the stomach, greater is the stretching of stomach wall leading to increased rate of gastric emptying.
• Gastrin hormone. Gastrin promotes gastric emptying.
• Type of food ingested (present in the stomach) affects the gastric emptying as:
3. Duodenal factors, which inhibit gastric emptying, are the following.
• Enterogastric reflex. The enterogastric reflex is initiated in the duodenum and passes to the stomach through the myenteric plexus and also through the extrinsic nerves to inhibit or even stop emptying.
Size of duodenal osmoreceptors. High osmolality of the chyme causes shrinkage and low osmolality increases the size of osmoreceptors. In both the conditions the rate of gastric emptying decreases.
• Enterogastric hormones. A variety of intestinal hor mones, collectively called enterogastrones, inhibit gastric contractions. Some of the hormones which have been identified are:
Functions of stomach
After studying the physiological activities of stomach, its functions can be summarized as follows.
1. Mechanical or motor functions include:
• Slow emptying of food into the duodenum occurs to provide proper time for digestion and absorption by the small intestine.
2. Digestive functions. Only small amounts of foods are digested in stomach as:
• Carbohydrate digestion in the stomach depends on the action of salivary amylase, which remains active until halted by the low pH of stomach.
• Protein digestion. About 10% of ingested protein is bro ken down completely in the stomach. Gastric pepsin facil itates later digestion of protein by breaking protein into peptone.
• Fat digestion in stomach is minimal due to the restriction of gastric lipase activity to triglycerides containing short-chain (<10 carbon) fatty acids.
3. Absorptive function. Stomach contributes little in absorptive function:
4. Reflex functions. Various reflexes initiated from the stomach are:
• Presence of food in the stomach reflexly stimulates secretion of pancreatic juice and expulsion of bile.
5. Antiseptic action. HCl present in the gastric juice kills the bacteria and other harmful substances.
Applied aspects
Important applied aspects of stomach which need special attention are:
Gastric mucosal barrier and pathophysiology of peptic ulcer
Gastric mucosal barrier
The gastric mucosal barrier protects the gastric mucosa from damage by the intraluminal HCl, i.e. autodigestion. It is created by the following:
• Mucin secretion. The thin layer of surface mucus in the stomach and duodenum prevents the direct contact of acid- and pepsin-containing fluid with surface epithelial cells.
• Bicarbonate secretion. Surface epithelial cells in both the stomach and the duodenum secrete bicarbonate, which create an essentially pH-neutral microenvironment immediately adjacent to the cell surface.
• Epithelial barrier. Intercellular tight junctions provide a barrier to the back-diffusion of H+.
• High mucosal blood flow. It rapidly carries away any acid that penetrates the cellular lining, and also provides oxy gen, bicarbonate and nutrients to the epithelial cells.
• Prostaglandins are responsible for maintaining the gas tric mucosal barrier.
Pathophysiology of peptic ulcer
Peptic ulcer refers to an excavation of mucosa of duodenum or pyloric part of stomach caused by the digestive action of gastric juice. Peptic ulcer can be caused by either of two ways.
1. Diminished ability of the gastroduodenal mucosal barrier to protect against the digestive properties of the acid-pepsin complex. Factors that disturb mucosal barrier include:
• Bacterial infection by Helicobacter pylori. At least 75% patients with peptic ulcer have recently been found to have chronic infection by H. pylori. The bacterium releases digestive enzymes that liquefy the barrier, which allows gastric secretion to digest the epithelial cells lead ing to peptic ulceration.
• Other factors, which can disrupt the mucosal barrier are ethyl alcohol, vinegar, bile salts, cigarette smoking and non-steroidal anti-inflammatory drugs (NSAIDs), such as aspirin and ibuprofen.
2. Excessive secretion of gastric acid
Hyperacidity leads to ulcer formation in the duodenum and pyloric part of stomach. Hyperacidity may occur due to:
Physiologic basis of management of peptic ulcer
Commonly employed measures for treatment of peptic ulcer are the following.
Antacids. These form a gel that coats the mucosa and neutralizes the acid.
• H2-receptor blocking drugs, such as cimetidine.
• M1-muscarinic receptor blocking drugs, such as atropine.
• Gastric H+-K+-ATPase inhibiting drugs, such as omeprazole.
Bilateral vagotomy combined with resection of gastrin-producing pyloric part of stomach is performed in very severe cases of duodenal and pyloric ulcers.
Physiology of vomiting
Vomiting refers to the forceful expulsion of contents from stomach and intestine.
Initiation of vomiting
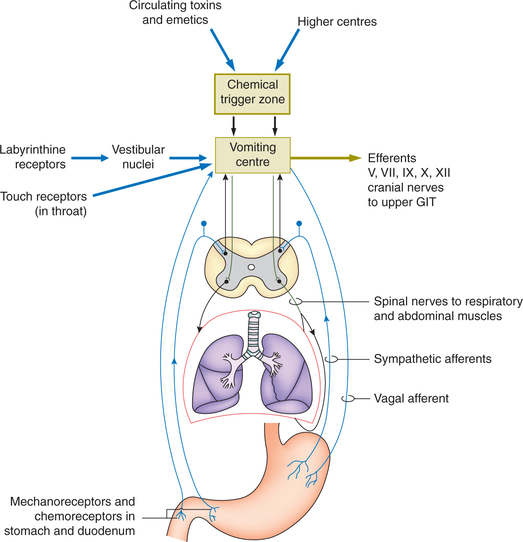
Vomiting may be initiated by either activation of vomiting centre or by an activation of the chemoreceptor-trigger zone.
1. Activation of vomiting centre. Vomiting centre is situated in the reticular formation of medulla oblongata near the vagal nucleus. It may be activated directly or through afferents.
(i) Direct activation of the vomiting centre occurs due to an injury to the area or by raised intracranial pressure.
(ii) Afferent impulses activating vomiting centre includes (Fig. 7.3-11):
• Visceral afferent pathway in the sympathetic and vagi relay impulses arising due to the irritation of mucosa of upper GIT.
• Afferent impulses from the vestibular nuclei mediate nausea and vomiting of motion sickness.
• Afferents from higher centres (diencephalon and limbic system) mediate emetic response to stimuli, such as nauseating smell, sickening sights and memory, etc.
2. Activation of chemoreceptor trigger zone. Chemore-ceptor trigger zone (CTZ) is located in the areapostrema, a V-shaped band of tissue on the lateral walls of fourth ventricle near the obex, which is outside the blood-brain barrier and is thus more permeable to many substances than the underlying medulla (Fig. 7.3-11).
Sequence of mechanical events of vomiting
Vomiting is a complex process and consists of following phases.
 Gastric juice
Gastric juice Migratory motor complexes
Migratory motor complexes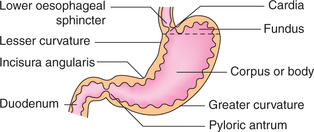
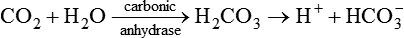
 Important note
Important note