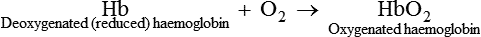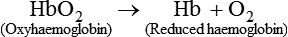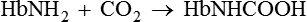Red Blood Cells and Anaemias
Characteristic features of red blood cells functional morphology
Functional morphology
The red blood cells (RBCs) (mature erythrocytes) form one of the important constituent of the cellular elements of the blood. Each RBC like any other cell in the body is bounded by a cell membrane but is non-nucleated and lacks the usual cell organelles. The cytoplasm of the RBC contains a special pigmented protein called the haemoglobin (Hb), which forms 90% of the weight of the erythrocytes. The red colour of the RBCs and thus of the blood is due to the presence of Hb.
Normal size, shape and counts of rbcs
Normal shape
The RBCs are circular, biconcave discs (Fig. 3.2-1).
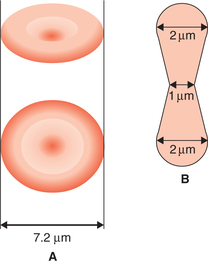
Fig. 3.2-1 Size and shape of a normal red blood cell: A, biconcave disc (diameter 7.2 μm) and B, thickness (2 μm at the periphery and 1 μm in the centre).
Advantages of biconcave shape are:
• It renders the red cells quite flexible so that they can pass through the capillaries whose minimum diameter is 3.5 μm (Fig. 3.2-2).
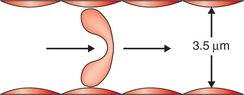
Fig. 3.2-2 Diagram showing how flexibility of red blood cell allows it to pass through smaller capillaries (diameter 3.5 μm).
• The biconcavity provides greater surface area as compared to volume, which allows considerable alterations in the cell volume. Thus, the RBCs can withstand considerable changes of osmotic pressure. In this way, the RBCs can resist haemolysis to certain extent when placed in the hypotonic solution.
• Greater surface area allows easy exchange of O2 and CO2, and rapid diffusion of other substances.
Variations in counts of RBCs
Physiological increase in the RBC count (physiological polycythaemia) is seen in the following circumstances:
• Age. At birth, the RBC count is 6–7 million/mm3 of blood. After about 10 days of birth the count decreases due to the destruction of cells. This is the cause of physiological jaundice of newborn. In infants, the RBC count is slightly more than the adults.
• Sex. RBC count in the adult females (average 4.8 million/mm3) is lower than the adult males (average 5.5 million/mm3).
• High altitude. RBC count of the individuals residing in high altitude areas (above 10,000 feet from the sea level) have high RBC count (7 million/mm3) because of the hypoxic stimulation of erythropoiesis.
• Excessive exercise. Mild hypoxia and spleen contraction causes temporary increase in the RBC count.
• Emotional conditions like anxiety are associated with the temporary increase in the RBC count due to sympathetic stimulation.
• Increase in atmospheric temperature causes a temporary elevation of RBC count.
Polycythaemia or pathological increase in the RBC count (above 7 million/μL) is of two types:
• Primary polycythaemia or polycythaemia vera occurs in myeloproliferative disorder-like malignancies of the bone marrow. The RBC count is persistently above 14 million/mm3 and is always associated with high white blood cell (WBC) count.
• Secondary polycythaemia occurs due to certain conditions producing a state of chronic hypoxia in the body such as:
Physiological decrease in RBC count is seen in the following conditions:
• At high barometric pressure, the RBC count is decreased slightly due to high O2 tension of the blood.
• After sleep, the RBC count is decreased slightly.
• In pregnancy, there occurs relative reduction in RBC count due to haemodilution caused by increase in the plasma volume.
Anaemia. In anaemia, there may occur marked reduction in the RBC count or the Hb level or both (see page 85).
Packed cell volume and red cell indices
Packed cell volume
Packed cell volume (PCV) refers to the percentage of the cellular elements (RBCs, WBCs and platelets) in the whole blood. Since the volume of WBCs and platelets is very less, so for all practical purposes the PCV is considered equivalent to the volume of packed red cells or the so-called haematocrit value. The normal values of the PCV in males are about 45% and in females about 42%. The PCV is increased in polycythaemia and decreased in anaemia.
Red cell indices
The red cell indices defined below are calculated taking normal values of the RBC count 5 million/μL, PCV 45% and Hb level of 15 g%.
The mean corpuscular volume (MCV) refers to the average volume of a single RBC. It is calculated by dividing the PCV by the red cell count.
Mean cell haemoglobin (MCH) refers to the average weight of the Hb contained in each RBC. It is calculated by dividing the amount of Hb in 1 L of blood by the red cell count in 1 L of blood.
3 Mean cell haemoglobin concentration
The MCH concentration (MCHC) refers to the amount of Hb expressed as a percentage of the volume of a RBC. It is calculated by dividing the amount of Hb in g% by the volume of packed cells in 100 mL of blood and then multiplying by 100.
• Normal value of MCHC is 33.3% (range 30–38%). RBCs with normal values of MCHC are called normochromic.
• In hypochromic RBCs the values of MCHC are less than the normal, as is seen in the iron-deficiency anaemia.
• Hyperchromia is very rare, high levels of MCHC (> 38%) cannot occur, since the RBCs cannot hold Hb beyond the saturation point.
• Since MCHC is independent of the RBC count and the size of RBCs, it is considered to be of greater clinical significance as compared to other absolute values.
Rouleaux formation and erythrocyte sedimentation rate
Rouleaux formation
• Rouleaux formation refers to the tendency of the RBCs to pile one over the other like a pile of coins (Fig. 3.2-3). The discoid shape and protein coating of red cells play a major role in the rouleaux formation. Rouleaux formation does not occur in the normal circulation under physiological conditions, as the moving cells show little or no tendency to adhere.
• This is a reversible phenomenon, but it promotes sedimentation of the RBCs. It should not be confused with agglutination where the cells are irreversibly clumped.
• Albumin decreases the rouleaux formation, while fibrinogen, globulin and other products of tissue destruction increase rouleaux formation.
Erythrocyte sedimentation rate
Erythrocyte sedimentation rate (ESR) is the rate at which the RBCs sediment (settle down) when the blood containing an anticoagulant is allowed to stand in a vertically placed tube. It is expressed in millimetre at the end of first hour.
• Normal values of ESR by Westergren's method vary from 3 to 7 mm in males and from 5 to 9 mm in females in first hour.
• Values of ESR are raised in a large number of pathological conditions, so it has got no specific diagnostic value. However, raised levels of ESR do suggest presence of some chronic inflammatory conditions in the body.
• Estimation of ESR is more useful as a prognostic test, i.e. to judge the progress of the disease in patients under treatment.
• Rouleaux formation. Increased tendency of the rouleaux formation raises the ESR. Fibrinogen and the proteins, which enter the plasma in inflammatory (globulins) and neoplastic diseases, favour rouleaux formation and thus increase the ESR.
• Size of the RBCs. Increase in the size of the RBCs (macrocytosis) raises the ESR.
• Number of RBCs. When the number of RBCs is increased, the ESR is decreased and when the number of RBCs is decreased (as in anaemia), the ESR is increased.
• Viscosity of blood. ESR is increased when the viscosity of blood is decreased and vice versa.
• Sex. ESR is greater in the females (5–9 mm) than the males (3–7 mm).
• Menstruation. ESR is slightly raised during menstruation in the females.
• Pregnancy. ESR is raised in pregnancy from third month to parturition, and returns to normal after 3–4 weeks of delivery.
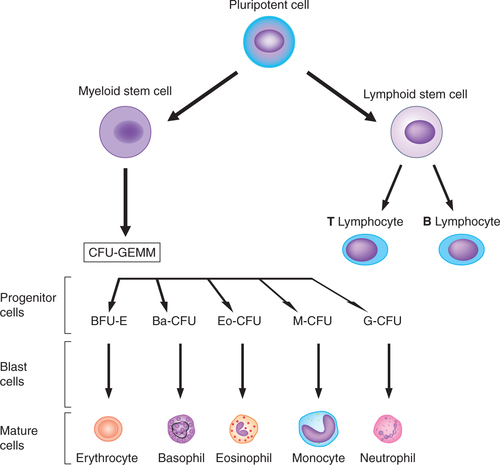
Formation of red blood cells
Formation of RBCs is a part of the process of development of blood cells (RBCs, WBCs and platelets) called haemopoiesis, which includes:
• Erythropoiesis, i.e. development of RBCs,
• Leucopoiesis, i.e. development of WBCs and
• Thrombopoiesis or megakaryocytopoiesis, i.e. development of platelets.
Sites of haemopoiesis
• In the first two months of gestation, the yolk sac is the main site of haemopoiesis.
• From third months of gestation, liver and spleen become the main sites of blood formation and continue to do so till birth. Spleen makes small contribution as compared to the liver.
• From 20th week of gestation, haemopoiesis begins in the bone marrow and by seventh or eighth month it becomes the main site.
• At birth (in normal full-term), almost whole of the haemopoiesis occurs in the bone marrow.
• In young children, active haemopoietic bone marrow is found in both axial skeleton and bones of extremities. The active haemopoietic bone marrow is red in colour due to marked cellularity, and hence is called red bone marrow. However, during this period there occurs a progressive fatty replacement throughout the long bones converting red bone marrow into the so-called yellow bone marrow.
• In adults, therefore, haemopoietic (red) bone marrow is confined to the axial skeleton (skull, vertebrae, sternum, ribs, sacrum and pelvis) and the proximal ends of long bones (humerus, femur and tibia). Even in these haemopoietic areas, about 50% the bone marrow consists of fat.
• In adults, during pathological conditions, when there is an increased demand of blood cells, the non-haemopoietic (yellow) marrow is capable of reverting back to active haemopoiesis.
• During pathological conditions, when the increased demand of blood cells cannot be met by the hyperactivity of bone marrow alone, even the liver and spleen resume their fetal role of haemopoiesis, as the stem cell retains its potential haemopoietic activity. Such a situation is referred as extramedullary haemopoiesis.
Blood cell precursors
Stem cells
The monophyletic theory of haemopoiesis is now widely accepted, according to which all blood cells originate from the pluripotent or multipotent stem cell. Stem cells possess two fundamental properties:
Progenitor cells
The stem cells after a series of divisions differentiate into progenitor cells:
Pluripotent progenitor cells, which can give rise to any type of blood cells.
Lymphoid (immune system) stem cells, which ultimately develop into lymphocytes.
Myeloid (trilineage) stem cells, which later differentiate into three types of cell lines:
• Granulocyte–monocyte progenitors, which produce all leucocytes except the lymphocytes.
Morphologically the progenitor cells present in the bone marrow cannot be differentiated from the stem cells, as they both look alike. However, they can be differentiated by immunological techniques, taking advantage of the different types of molecules present on their cell membrane.
Progenitor cells possess the ability to give rise to clones (group of cells), so they are also called colony forming cells or colony forming units (CFUs). The three types of progenitor cells are given:
• CFU-GEMM (colony forming unit–granulocyte, erythroid, megakaryocyte and macrophage) refers to a multipotent progenitor cell, i.e. myeloid progenitor cells.
• BFU-E (burst forming unit–erythroid) form large colonies of erythroid series.
• CFU-E (colony forming unit–erythroid) develop into erythrocytes.
• Ba–CFU refers to the basophil colony forming units.
• Eo–CFU are the eosinophil colony forming units.
The broad outlines of haemopoiesis discussed above are summarized in Fig. 3.2-4. Further details of erythropoiesis are discussed in this chapter and details of development of other blood cells are discussed in the relevant chapters.
Stages of erythropoiesis
The RBCs develop from the burst forming unit–erythrocyte (BFU-E) and colony forming unit–erythrocyte (CFU-E), which are derived from the committed progenitor cells.
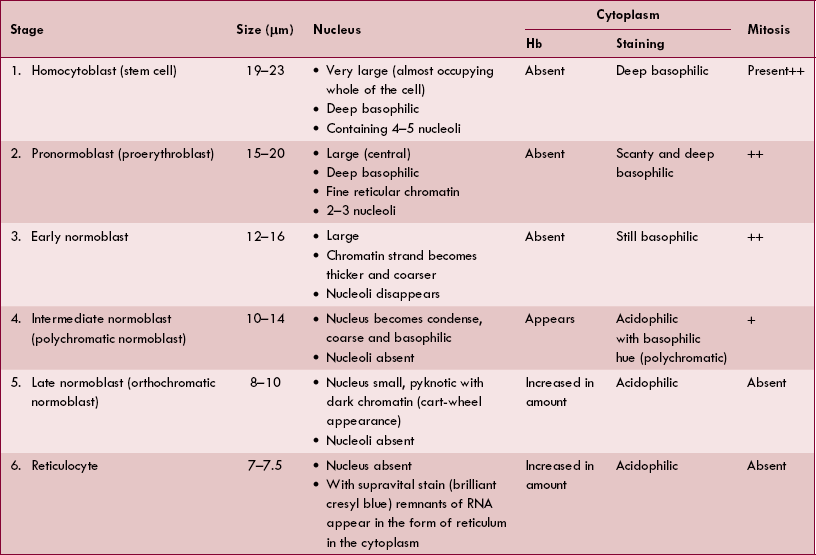
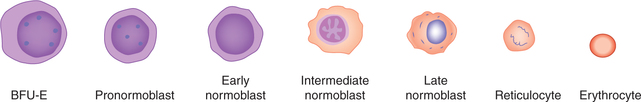
The well-defined and readily recognisable lineage of nucleated red cells (erythroid series), which form different stages of erythropoiesis (Fig. 3.2-5), are summarized in Table 3.2-1.
Maturation of a reticulocyte into an erythrocyte
A reticulocyte spends 1–2 days in bone marrow and circulates for 1–2 days in the peripheral blood before maturing in the spleen to become a biconcave red cell. The mature red cell has lost its nucleus as well as the ribosomes and mitochondria. It thus cannot synthesize Hb and any other protein. Normally only mature blood cells are able to enter the circulation. The reticu locytes are also found normally in the peripheral blood. Normal range of the reticulocytes in healthy adults is 0.5%–2% and in infants it is 2%–6%. Abnormal increase in the circulating reticulocytes is called reticulocytosis. This is seen when the rate of erythropoiesis is very high, as it occurs in the haemolytic anaemia and following the treatment of deficiency anaemias.
Reticulocytes can be counted in the laboratory by vital staining with dyes, such as new methylene blue or brilliant cresyl blue.
Summary of changes occurring in the cells of erythroid series during maturation
It takes 7 days for the formation and maturation of RBCs. Till the stage of reticulocyte, it takes 5 days and to become matured red cell from reticulocyte, it takes 2 days. Various changes which occur during maturation from the stage of pronormoblast to erythrocyte are summarized below:
Size of the cell (from 15–20 μm of pronormoblast) goes on decreasing with subsequent stages till it reaches about 7 μm.
Nucleus first condenses, then becomes pyknotic and finally disappears at the stage of reticulocyte formation.
Haemoglobin synthesis starts at the stage of intermediate normoblast and then its content increases progressively.
Cytoplasm staining. Initially, before the appearance of Hb the cytoplasm is basophilic. When Hb starts appearing cytoplasm becomes polychromatic, i.e. stained both by acidic and basic dyes. In the stage of late normoblast when Hb synthesis is almost completed, cytoplasm is stained by an acidic dye.
Mitosis is seen up to the stage of intermediate normoblast. During these stages, 3–5 cell divisions occur. In this way, each pronormoblast gives rise to 8–320 late normoblasts. From the stage of late normoblast onwards, the mitosis ceases and cell only matures.
Regulation of erythropoiesis
Erythropoietin
Erythropoietin is a hormone, which regulates the process of erythropoiesis. It is a glycoprotein having molecular weight of 34,000.
Site of formation. Erythropoietin is mainly (85%) produced by the juxta glomerular apparatus of kidney. Extrarenal sources like liver and cells of tissue macrophage system produce about 15% of erythropoietin, especially when hypoxia is marked.
Stimulus for secretion. A certain basal level of the hormone is necessary for the normal rate of erythropoiesis. The main function of the RBCs is to supply oxygen to the tissues. Therefore, whenever, there is hypoxia or decrease in the number of RBCs (e.g. after haemorrhage or in haemolytic anaemia), there occurs a release of renal erythropoietic factor from juxta glomerular cells of kidney. Renal erythropoietic factor acts on the plasma alpha globulin called erythropoietinogen to form the erythropoietin (Fig. 3.2-6). Thus the levels of erythropoietin vary with degree of hypoxia or number of circulating RBCs. This explains how polycythaemia (increased RBC count) is observed in hypoxic states, such as in normal individuals residing at high altitude or in patients suffering from cardiopulmonary disorder.
Actions of erythropoietin. Erythropoietin increases erythropoiesis because of its following actions:
• Erythropoietin exerts its chief effect on the stem cells causing them to differentiate.
• It promotes Hb synthesis by increasing globulin synthesis and potentiating δ-amino laevulinic acid synthetase.
• It also promotes every stage of maturation from pronormoblast to the mature red cells.
• Erythropoietin also promotes release of RBCs from bone marrow into the peripheral circulation.
Factors increasing erythropoietin secretion. Other factors which increase secretion of erythropoietin are:
1. Hormones which increase erythropoietin secretion are the following:
• Other hormones which increase erythropoietin secretion are growth hormone, prolactin, ACTH and adrenocortical steroids.
2. Haemolysates, i.e. the products released following RBC destruction, also increase erythropoietin secretion.
3. Nucleotides which enhance erythropoietin secretion include cAMP, NAD and NADP.
4. Vasoconstrictor drugs produce renal hypoxia, which in turn affects erythropoietin secretion.
Factors decreasing erythropoietin secretion are the following:
Factors necessary for erythropoiesis
Factors necessary for erythropoiesis can be divided in three groups:
I General factors
The main general factors necessary for the process of erythropoiesis are optimum level of the hormone erythropoietin, and the efficient feedback mechanism controlling the secretion of erythropoietin have been discussed in the regulation of erythropoiesis.
II Special maturation factors
Special factors which are essential for maturation of RBC include vitamin B12, intrinsic factor of Castle and folic acid.
Vitamin B12 and intrinsic factor of Castle
Vitamin B12 (cyanocobalamin), also known as extrinsic factor, is essential for maturation of red cells.
Daily requirement of vitamin B12 in adults is 1–2 μg. Since its deficiency causes pernicious anaemia, it is also called antipernicious factor.
Role of vitamin B12. It is required for the synthesis of DNA and maturation of nucleus and cell. Deficiency of vitamin B12 leads to:
Folic acid (pteroylglutamic acid) and related compounds are known as folates—play an important role in the synthesis of DNA.
Daily requirement of folate for a normal healthy adult is 100 μg.
Folate deficiency causes megaloblastic anaemia (see page 87).
III Factors necessary for haemoglobinization
Various factors necessary for Hb formation in the RBCs are described on page 81.
Haemoglobin
The cytoplasm of erythrocytes (RBCs) contains an oxygen-binding protein called haemoglobin. Erythrocyte precursors synthesize haemoglobin. While the mature erythrocytes lose the property of synthesizing haemoglobin. The inclusion of haemoglobin within the erythrocytes is most effective for functional purposes since it avoids the following disadvantages which would have occurred if the haemoglobin was present in the plasma as free haemoglobin:
• Increase in blood viscosity causing a rise in blood pressure.
• Increase in the osmotic pressure.
• Rapid destruction of haemoglobin by the reticuloendothelial system.
In pathologic states, e.g. acute haemolytic disorder, haemoglobin appears in the plasma and may lead to abovementioned consequences.
Normal blood haemoglobin
The normal blood haemoglobin concentration at different ages is given below:
• In fetus, just before birth, the haemoglobin concentration of blood from the umbilical cord ranges from 16.5 to 18.5 g/dL.
• After birth, the haemoglobin concentration increases rapidly and may reach up to 23 g/dL. This occurs due to the transfusion of cells from the placenta to infant.
• At the end of 3 months. After 2 days of birth, the haemoglobin levels start falling and stabilize at the end of 3 months to 10.5 g/dL.
• At 1 year of age. The concentration then rises gradually to reach 12 g/dL at 1 year of age.
• In adult males the mean blood Hb concentration is 15.5 g/dL (range 14–18 g/dL).
• In adult females the mean Hb concentration is 14 g/dL (range 12–15.5 g/dL).
Structure of haemoglobin
Haemoglobin is a globular molecule having a molecular weight of 68,000. It consists of the protein globin combined with iron-containing pigment called haem.
Structure of globin
The protein globin, present in the Hb, is made of four polypeptide chains. Haemoglobin A (HbA) consists of the following four polypeptide chains:
Therefore, the normal adult haemoglobin A is written as HbA (α2β2).
Structure of haem
The haem is an iron–porphyrin complex called iron– protoporphyrin IX, i.e. it consists of a porphyrin nucleus and the iron. The structural characteristics of the haem (iron–protoporphyrin IX) are given below (Fig. 3.2-7A–C):
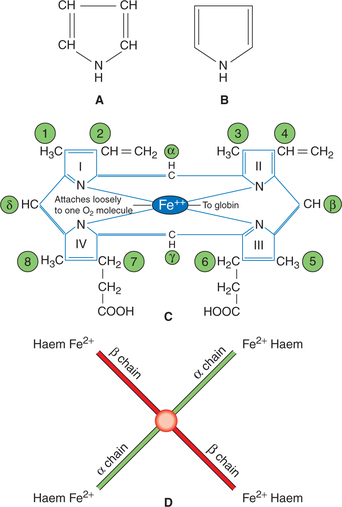
Fig. 3.2-7 Chemistry of haemoglobin: A, structure of a pyrrole ring; B, conventional outline of a pyrrole ring; C, arrangement of pyrrole rings in one unit of haem (iron protoporphyrin IX) and D, arrangement of four units of haem in one molecule of haemoglobin.
• The porphyrin nucleus consists of four pyrrole rings numbered I, II, III and IV, i.e. porphyrins are tetrapyrroles.
• The pyrrole rings are joined together by four methine bridges (=CH−). The carbon atoms of methine bridges are labelled α, β, γ and δ.
• Eight side chains are attached to the pyrrole ring at positions labelled 1–8. These are:
One molecule of Hb contains four units of haem, each attached to one of the four polypeptide chains constituting globin (Fig. 3.2-7D). As there are four units of haem in one molecule of Hb, so there are four iron atoms in one molecule of Hb, which can carry four molecules (eight atoms) of oxygen.
Functions of haemoglobin
1 Transport of O2 from lungs to tissues
• In the lungs, one molecule of O2 is attached loosely and reversibly at the sixth covalent bond of each iron atom of the Hb to form oxyhaemoglobin represented as HbO2:
• Oxygenation of first haem molecule in the Hb increases the affinity of second haem for oxygen, which in turn increases the affinity of third haem and so on. In this way, the affinity of Hb for fourth oxygen molecule is many times that for the first molecule. Because of this:
– Hb reacts with oxygen very rapidly taking less than 0.01 s. Similarly, deoxygenation of Hb is also very rapid.
– The oxygen–Hb dissociation curve becomes sigmoid shape (see page 232).
• The affinity of Hb for oxygen is influenced by pH, temperature and concentration of 2,3-diphosphoglycerate, i.e. 2,3-DPG (a product of metabolism of glucose) in the RBCs.
2 Transport of CO2 from the tissues to the lungs
Hb also transports CO2 from the tissues to the lungs.
• It is important to note that the CO2 from the tissues is transported by combining with amino acids of the globin part as shown below and not in combination with Fe2+ atom like O2.
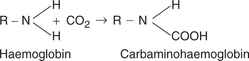
• Deoxygenated Hb forms carbamino-Hb more readily than oxygenated Hb. That is why venous blood becomes more suitable for the transport of CO2 from the tissues to the lungs.
Varieties of haemoglobin
Several varieties of Hb occur in human beings. In all varieties of Hb the haem moiety remains the same. It is the composition of polypeptide chains of globin part of the Hb which differs in various varieties of Hb. Various varieties of Hb can be grouped as under:
Physiological varieties of haemoglobin
Adult haemoglobin Adult Hb is of two types.
(i) Haemoglobin A [HbA (α2β2)]. It is the main form of normal adult Hb. As described on page 78, its globin part consists of two α and two β polypeptide chains.
(ii) Haemoglobin A2 [HbA2 (α2δ2)]. It is a minor component (about 2.5% of the total Hb) in normal adults. Its globin part consists of two α and two δ polypeptide chains. δ chains have slightly different amino acid composition (out of 146, 10 amino acids are different) as compared to β chains.
Fetal haemoglobin or haemoglobin F [HbF (α2γ2)], as the name indicates, refers to the Hb present in the fetal RBCs and gradually disappears 2–3 months after birth.
Structure of HbF is similar to that of HbA, except that its globin part consists of two α and two γ polypeptide chains (in place of β chains). γ chains also have 146 amino acids but its 37 amino acids are different than that of β chains.
Special features of HbF are given below:
• Affinity for oxygen in case of HbF is more than that of HbA, i.e. it can take more oxygen than HbA at low oxygen pressure. It is owing to poor binding of 2,3-DPG by the γ polypeptide chain. Because of this, movement of oxygen from maternal to fetal circulation is facilitated. At PO2 20 mm Hg, HbF is 70% saturated while HbA is only 30%–35% saturated.
• Resistance to action of alkalies is more in HbF than HbA. This property is used in a photoelectric calorimetric method to estimate HbF in the presence of HbA.
Haemoglobinopathies
Haemoglobinopathies, i.e. abnormal formation of Hb occurs due to the disorders of globin synthesis; haem synthesis being normal. Disorders of the globin synthesis are of two main types:
• Formation of abnormal polypeptide chains due to substitution of an abnormal amino acid chain in the HbA. Example of such a disorder is haemoglobin S (HbS).
• Suppression of synthesis of polypeptide chain of globin as seen in thalassaemia.
1. Sickle cell haemoglobin or HbS is the most important haemoglobinopathy.
• It occurs in 10%–20% of Negroes. Sickle cell gene has originated in the black population in Africa.
• Sickle trait is inherited as Mendelian dominant trait but the full blown disease is autosomally recessive. Heterozygous individual with sickle cell trait rarely has severe symptoms but homozygous develop full blown disease.
• HbS is formed due to substitution of valine for glutamic acid at position 6 in the β chain of HbA.
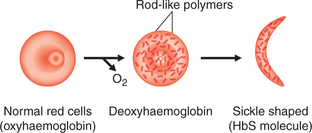
• When HbS is reduced (e.g. in low-O2 tension or when pH at tissue level is low), it becomes much less soluble and precipitates into crystals within the RBCs. The crystals elongate producing changes in shape of the cells from biconcave to sickle-shaped cells (sickling) (Fig. 3.2-8).
• The cells containing HbS are less flexible as compared to the RBCs containing HbA, hence leading to a blockade of microcirculation.
• Sickle-shaped cells greatly increase blood viscosity, thereby decreasing the blood flow to tissues.
• Sickle-shaped cells are more fragile and are very liable to undergo haemolysis producing the so-called sickle cell anaemia.
• The individual with sickle cell trait has resistance to one type of malaria.
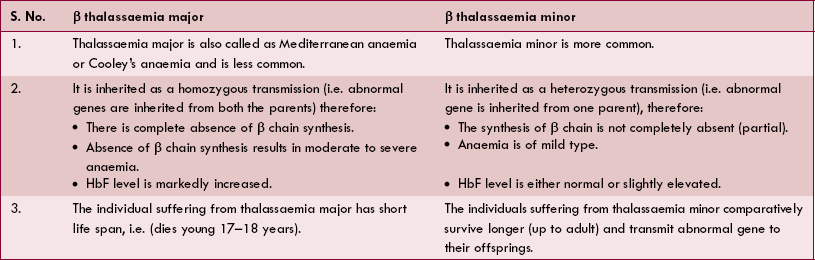
2. Thalassaemia (Mediterranean anaemia) is a haemoglobinopathy characterized by following features:
• Cause. Thalassaemia results due to defect in the synthesis of polypeptide chain α and β of HbA.
• Types. Depending upon whether α or β chains are not synthesized, α thalassaemia or β thalassaemia may occur, respectively. β thalassaemia is more common and is further of two types: thalassaemia major and thalassaemia minor.
• Features of thalassaemia major and minor are depicted in Table 3.2-2.
Derivatives of haemoglobin (reactions of haemoglobin)
Haemoglobin has the property to readily react with any gas, other substance to form the so-called derivatives of Hb. These include:
1. Oxyhaemoglobin. Hb reacts readily with oxygen to form oxyhaemoglobin, which is an unstable and reversible compound, i.e. oxygen can be released from this compound. In this compound iron remains in the ferrous state.
2. Reduced haemoglobin or deoxygenated Hb is formed when oxygen is released from the oxyhaemoglobin.
3. Carbaminohaemoglobin is a compound of Hb with carbon dioxide.
4. Carboxyhaemoglobin or carbon monoxyhaemoglobin is a compound of Hb with carbon monoxide (CO):
The affinity of Hb for CO is much more (200–250 times) than its affinity for oxygen. Because of this, the CO displaces oxygen from Hb, thereby reducing the oxygen carrying capacity of the blood.
5. Methaemoglobin. When reduced or oxygenated Hb is treated with an oxidizing agent, e.g. potassium ferricyanide, the ferrous Fe2+, is oxidized to ferric (Fe3+); the sixth bond is attached to OH to form the compound methaemoglobin. Methaemoglobin is represented as HbOH.
Disadvantages of methaemoglobin are:
• It cannot unite reversibly with gaseous oxygen; the O2 of the attached OH is not given off in a vacuum.
6. Glycosylated haemoglobin is a derivative of HbA present in very small amount, e.g. haemoglobin A1C (HbA1C), in which glucose is attached to terminal valine in the β chains. The level of glycosylated Hb in the blood increases in poorly controlled patients of diabetes mellitus.
Synthesis of haemoglobin
Haemoglobin is synthesized in the cytoplasm of intermediate normoblasts.
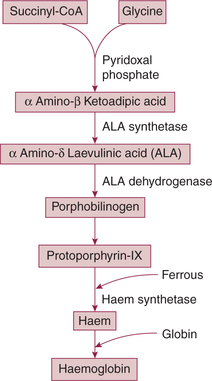
Synthesis of haem
Haem is synthesized in the mitochondria.
• Succinyl-CoA (derived from the citric acid cycle in mitochondria) and glycine are the starting substances in the synthesis of haem. Steps of synthesis are shown in Fig. 3.2-9.
• The protoporphyrin IX is then formed after a series of reactions promoted by other enzymes.
• Finally, ferrous ion is introduced into the protoporphyrin IX molecule to form haem in a reaction catalyzed by the enzyme haem synthetase.
Factors controlling haemoglobin formation
Whipple's standard anaemic dog studies show that iron as well as dietary proteins, both play very important role in synthesis of Hb. In addition, certain other minerals and vitamins also control the Hb formation.
1. Role of proteins. First class proteins provide amino acids required for the synthesis of globin part of the Hb. A low protein intake retards Hb regeneration even in the presence of excess iron; the limiting factor being lack of globin.
2. Role of iron. Iron is necessary for formation of the haem part of Hb. In addition to dietary iron, the iron released by degradation of RBCs is also reused for the synthesis of Hb.
• Copper is essential for the Hb synthesis, as it promotes the absorption, mobilization and utilization of iron.
• Cobalt. Its role in humans is not evidenced, but in some species cobalt increases the production of erythropoietin, which in turn stimulates RBC formation.
• Calcium reported to help indirectly by conserving iron and its subsequent utilization.
4. Role of vitamins. Vitamin B12, folic acid, and vitamin C help in synthesis of nucleic acid which in turn is required for the development of RBCs. Vitamin C also helps in absorption of iron from the gut.
5. Role of bile salts. Presence of bile salts in the intestine is necessary for proper absorption of metals like copper and nickel which in turn are essential factors for synthesis of Hb.
Red cell fragility
Red cell fragility refers to the susceptibility of red cell membrane to get broken or bursted. The process of breaking of RBCs and release of Hb into the plasma is called haemolysis. Red cell fragility depending upon the underlying mechanism is of two types:
Osmotic red cell fragility
Osmotic red cell fragility refers to the susceptibility of red cell membrane to get lysed due to changes in the osmotic pressure of the solution in which they are suspended (see page 18).
Effect of osmotic pressure of solution on the RBCs
• Isotonic solutions have tonicity as that of plasma. These include 0.9% NaCl, 5% glucose, 10% mannitol and 20% urea. When RBCs are placed in these solutions, they remain suspended in them, i.e. neither swell nor shrink. This is because the osmotic pressure of the isotonic solutions is equal to the osmotic pressure within the RBCs.
• Hypertonic solutions (e.g. >0.9% NaCl, 20% mannitol) have osmotic pressure more than the osmotic pressure within the RBCs. Therefore, when the RBCs are placed in such solutions, fluid goes out of the cells and they shrink.
• Hypotonic solutions (e.g. <0.9% NaCl) have osmotic pressure less than the osmotic pressure within the RBCs. Therefore, when the RBCs are placed in such solutions, they swell up by absorbing water from outside and finally burst, i.e. get haemolysed.
Normal values (index of fragility)
• Onset of haemolysis (fragility) in normal RBCs occurs in 0.48% NaCl.
• Completion of haemolysis (ending of fragility) occurs in 0.35% NaCl.
• Explanation. Normally only the older red cells with comparative fragile membrane are haemolysed in around 0.48% of NaCl solution since these cells cannot withstand this hypotonicity. But, younger cells are not affected. In 0.35% NaCl even the youngest cells are haemolysed and so haemolysis is completed.
Increase in osmotic fragility index occurs in following conditions:
Decrease in osmotic fragility index occurs when the RBCs become slender, e.g. in iron-deficiency anaemia. In this condition the onset of haemolysis occurs at 0.36% NaCl and is completed at 0.24% NaCl solution.
Mechanical red cell fragility
The red cells are subjected to a mechanical stress and trauma as they pass through the capillaries and trabeculae of spleen some 3,00,000 times during their life span of 120 days. They are made more brittle due to unusual mechanical stress. The red cells can become more rigid as a result of the pathological changes in the membrane or in the cell contents caused by a number of red cell disorders. The cells thus become mechanically more fragile, i.e. less liable to tolerate deforming stresses than the normal healthy red cell.
Life span and fate of red blood cells
Fate of RBCs
The cell membrane of old RBCs (after about 120 days) becomes more fragile due to decreased NADPH activity. The younger RBCs can easily pass through the capillaries which have diameter smaller than the red cells. The older cells with fragile membrane are destroyed when the cells try to squeeze through the capillaries. The destruction of red cells occurs mostly in the capillaries of spleen because they have very thin lumen. Because of this, spleen is also called the graveyard of RBCs. The Hb released after the haemolysis of red cells is taken up by the tissue macrophages.
The tissue macrophage system (reticuloendothelial system) includes the following phagocytic cells:
• In the bone marrow these cells form part of the lining of the blood sinuses (littoral cells).
• In the liver they lie at intervals along the vascular capillaries (Kupffer cells).
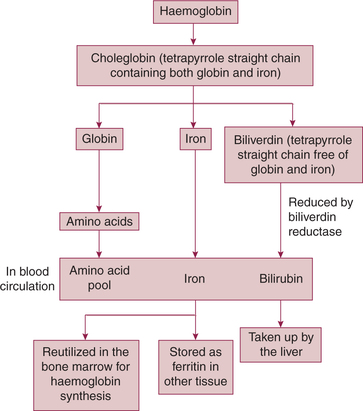
Fate of haemoglobin (Fig. 3.2-10)
• In the macrophages, the haem part of the Hb molecule is altered by oxidation of one of its methine (=CH) bridges. As a result of this chemical change, the green ironcontaining compound choleglobin is formed. As the name implies, the choleglobin molecule still contains the original globin.
• Next, the choleglobin splits off into globin, iron and biliverdin (tetrapyrrole straight chain gets free from globin and iron).
• Globin is degraded into amino acids, joins the amino acid pool of plasma and is released.
• Iron released into the circulation is:
– carried into the bone marrow for reutilization, and
– in the other tissues it combines with apoferritin to form the ferritin (storage form of iron).
• Biliverdin (tetrapyrrole straight chain free from globin and iron) is converted into bilirubin (by the enzyme biliverdin reductase) and is released into the blood.
Bilirubin and jaundice
Bilirubin formation and its fate
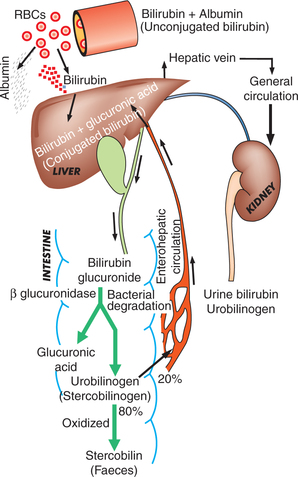
As discussed above, the bilirubin is formed in the macrophages. It undergoes the following changes (Fig. 3.2-11):
1. Uptake of bilirubin. Macrophages release the bilirubin into circulation. This bilirubin is called free or unconjugated bilirubin. It is lipid soluble, in the plasma it is bound to the albumin (protein conjugated), which prevents its excretion by the kidneys.
2. Conjugation of bilirubin. The unconjugated bilirubin (bound to albumin) from the circulation is taken up by the liver. In the liver the bilirubin is split off from the albumin and enters the hepatic cells. In the hepatic cells, it is conjugated with uridine diphosphate glucuronic acid (UDP-glucuronic acid) making it a water-soluble conjugated bilirubin.
3. Excretion of bilirubin. The conjugated bilirubin from the hepatic cells is excreted and enters the intestine. Some of it escapes into general circulation and is excreted by the kidneys in urine as urine bilirubin.
4. Formation and excretion of urobilinogen. The conjugated bilirubin which enters the intestine is degraded by the intestinal bacteria in the terminal ileum and the large intestine. The bacterial enzyme β-glucuronidase splits off the glucuronide and converts bilirubin into the urobilinogen (stercobilinogen), which is a colourless compound.
• Some urobilinogen (20%) from the intestine is reabsorbed and goes via the portal system to the liver. From the liver some urobilinogen escapes into general circulation and some are re-excreted into the bile (enterohepatic circulation).
• From general circulation, the urobilinogen is filtered off by the kidney and is excreted in the urine.
Bilirubin
The normal serum bilirubin level ranges from 0.3 to 1.0 mg/100 mL. The total serum bilirubin includes conjugated as well as unconjugated bilirubin. The Van den Bergh test described below is helpful in determining the type of bilirubin present in the serum.
Van den Bergh test
Van den Bergh test is performed using the diazo reagent (mixture of sulphanilic acid, hydrochloric acid and sodium nitrite). It is of two types:
Direct Van den Bergh reaction. When diazo reagent is added to the serum containing conjugated bilirubin (water soluble) a reddish brown colouration is obtained within 30 s. This is called direct positive Van den Bergh reaction.
Indirect Van den Bergh reaction. When diazo reagent is added to the serum containing mainly unconjugated bilirubin (water insoluble), no colour is obtained. However, if some solvent like alcohol (which dissolves the unconjugated bilirubin) is added, the reddish brown colouration is obtained. This is called indirect positive Van den Bergh reaction.
Jaundice
Jaundice (icterus) refers to the yellow appearance of the skin, sclera and mucous membranes resulting from an increased bilirubin concentration (hyperbilirubinaemia) in the body fluids. Clinically, jaundice is detectable when the plasma bilirubin exceeds 2–3 mg/100 mL.
Mechanisms producing jaundice
Hyperbilirubinaemia producing jaundice can result from the following mechanisms:
1. Excessive breakdown (haemolysis) of RBCs produces the so-called haemolytic jaundice or pre-hepatic jaundice. Unconjugated hyperbilirubinaemia occurs since it is being produced in excess of what can be conjugated by the liver.
2. Damage to the liver cells (infective or toxic) produces the so-called hepatic or hepatocellular jaundice. Both unconjugated as well as conjugated bilirubin is increased in serum.
3. Obstruction to bile ducts produces the obstructive or post-hepatic or cholestatic jaundice. Conjugated hyperbilirubinaemia results due to impaired flow of bile.
Physiological jaundice of newborn
Physiological jaundice of newborn is also called neonatal jaundice.
Mechanism of production. A hyperbilirubinaemia producing jaundice may be seen normally in the newborn. It appears within 2–5 days of birth and usually disappears in 2 weeks. Its mechanism of production includes:
• Excessive destruction of RBCs occurs in first few days after birth causing increase in the serum bilirubin.
• Hepatic immaturity in the first few (7–10) days after birth also contributes to increased serum bilirubin.
• Prevention. Neonatal jaundice can be prevented by the administration of hepatic microsomal enzyme inducers (e.g. phenobarbital) to the pregnant mother or newborn. The microsomal enzyme inducers increase the activity of glucuronyl transferase in liver.
• Treatment. Neonatal jaundice can be effectively treated by phototherapy. Exposure of the skin to white light causes photoisomerization of bilirubin to water-soluble lumirubin, which can be rapidly excreted in bile without requiring any conjugation.
Anaemias
Definition and classification
Definition
Anaemia is not a single disease but a group of disorders in which Hb concentration of blood is below the normal range for the age and sex of the subject. Therefore anaemia is labelled when the Hb concentration is less than:
Low RBC count (less than 4 million/mm3) is usually, but not always, associated with low Hb levels in anaemia.
Grading of anaemia depending upon the level of Hb has somewhat arbitrarily been made as:
Classification
Aetiological (Whitby's) classification
Types of anaemia depending upon the causative mechanism are:
• Megaloblastic anaemia (pernicious anaemia) due to deficiency of vitamin B12
B. Blood loss anaemias or haemorrhagic anaemias are commonly known and can be:
C. Haemolytic anaemias. These are relatively uncommon and occur in conditions associated with increased destruction of RBCs. These can be:
1. Hereditary haemolytic anaemias, e.g. as seen in:
2. Acquired haemolytic anaemias such as Immunohaemolytic anaemia (due to antibodies against RBCs):
D. Aplastic anaemia. It occurs due to the failure of bone marrow to produce RBCs.
E. Anaemia due to chronic diseases. It is seen in tuberculosis, chronic infections, malignancies, chronic lung diseases, etc.
Morphological (Wintrobe's) classification
Based on the mean cell volume (MCV), i.e. cell size and the MCHC, i.e. haemoglobin saturation of RBCs, the anaemias can be classified as:
1. Normocytic normochromic anaemias. These are characterized by normal MCV (78–94 μm3 or 78–94 μL) and normal MCHC (30%–38%). Such a morphological picture is seen in:
2. Microcytic hypochromic anaemias. These are characterized by reduced MCV (< 78 μm3) and reduced MCHC (< 30%). Examples of such anaemias are:
3. Macrocytic normochromic anaemia. It is characterized by increased MCV (> 94 μm3) and normal MCHC (30–38%). Examples are:
General clinical features of anaemia
General clinical manifestations of anaemia which occur either due to tissue hypoxia or due to compensatory mechanisms are:
Iron-deficiency anaemia
Iron-deficiency anaemia is the commonest nutritional deficiency disorder present throughout the world, but its prevalence is higher in the developing countries. In India, iron-deficiency is the commonest cause of anaemia. Irondeficiency anaemia is much more common:
• In women between 20–45 years than in men,
• At periods of active growth in infancy, childhood and adolescence.
Daily requirement. Only 10% of the dietary intake of iron is absorbed. Therefore, daily requirement in the adult males is 5–10 mg/day and in females is 20 mg/day (to compensate the menstrual loss). Pregnant and lactating women require about 40 mg of iron per day.
Causes of iron-deficiency anaemia
Causes of iron-deficiency vary with age, sex and country of residence of patient. In general, the causes of irondeficiency anaemia can be grouped as:
1. Inadequate dietary intake of iron as in:
2. Increased loss of iron (as blood loss) from the body, e.g.
• Uterine bleeding in females in the form of excessive menstruation, repeated miscarriages, post-menopausal bleeding, etc.
• Gastrointestinal bleeding due to peptic ulcer, haemorrhoids, ulcerative colitis, etc.
• Renal tract bleeding, e.g. haematuria.
Clinical features, laboratory findings and treatment
Clinical features of anaemia
Megaloblastic anaemia
Megaloblastic anaemias are characterized by the abnormally large cells of erythrocyte series. These are caused by defective DNA synthesis due to deficiency of vitamin B12 and/or folic acid (folate).
I Megaloblastic anaemia due to vitamin B12deficiency
Causes of vitamin B 12 deficiency are:
1. Inadequate dietary intake may occur in:
2. Malabsorption of vitamin B12 is more often the cause of deficiency and may be due to the deficiency of intrinsic factors such as an autoimmune cause of failure of secretion of intrinsic factor (Addisonian pernicious anaemia), gastrectomy and congenital lack of intrinsic factor.
Clinical features of megaloblastic anaemia
B Characteristic features of megaloblastic anaemia
1. Blood picture and red cell indices
• RBCs are larger in size (macrocytosis) but contain a normal concentration of Hb (normochromia).
• MCV increases to 95–160 μm3 (normal 78–94 μm3).
• MCHincreases to 50 pg (normal 28–32 pg).
• MCHC usually normal (35 ± 3%) because both MCV and MCH increase. In late stages MCHC may decrease.
• Peripheral smear shows nucleated RBCs.
• Reticulocyte count increases to more than 5% (normal less than 1%).
• Life span of RBCs is decreased.
• WBCs and platelets decrease because of encroachment of megaloblastic tissue.
Bone marrow shows megaloblastic hyperplasia characterized by presence of:
• Serum bilirubin increases more than 1 mg/dL (normal 0.2–0.8 mg/dL) due to excessive destruction of RBCs in spleen, liver and bone marrow, and jaundice occurs.
• Urine urobilinogen excretion may increase due to increased serum bilirubin.
• Serum iron and ferritin is usually increased because iron is not utilized by the immature RBCs.
• Serum vitamin B12 levels are decreased (normal 200–900 pg/mL) in patients with megaloblastic anaemia due to vitamin B12 deficiency.
• Serum folate levels are decreased in the patients with megaloblastic anaemia due to folic acid deficiency.
 Functional morphology
Functional morphology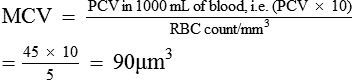
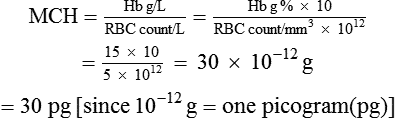
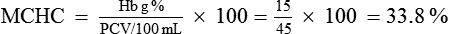

 Important note
Important note