Adrenal Glands
Functional anatomy
General considerations
• There are two adrenal glands, situated one on either side, at the upper pole of kidney, hence also called ‘suprarenal gland’ (Fig. 8.5-1).
• Normally, each gland weighs about 5 g and consists of two parts (Fig. 8.5-1):
Histological structure
Adrenal cortex
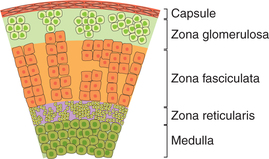
The adrenal gland is covered by a connective tissue capsule from which septa extend into the gland substance. The mature human adrenal cortex consists of three distinct layers or zones of cells (Fig. 8.5-2):
1. Zona glomerulosa, constituting outer one-fifth of cortex, is a small zone present under the capsule. It consists of cells that secrete aldosterone and corticosterone.
2. Zona fasciculata is the widest zone forming middle three-fifths of the cortex. It is made up of cells that are arranged in two cell thick straight columns. Sinusoids intervene between the columns.
3. Zona reticularis forms the inner one-fifth of the cortex. It is made up of a network of compactly arranged cords of cells (hence the name zona reticularis).
Adrenal medulla
Histologically, it is made up of chromaffin cells innervated by preganglionic sympathetic neurons.
Chromaffin cells. The cells forming adrenal medulla show yellow granules in their cytoplasm (i.e. chromaffin reaction) and are hence called chromaffin cells.
• Functionally, these cells are considered to be modified postganglionic neurons which do not have axons.
• Catecholamines are stored in the chromaffin granules. In addition to the catecholamines, the chromaffin granules also contain proteins, lipids and adenine nucleotides (mainly ATP).
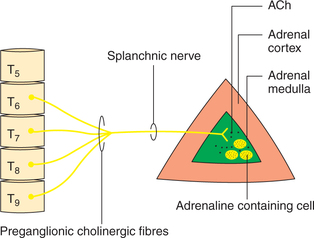
Nerve endings, present in the adrenal medulla, are the cholinergic preganglionic sympathetic fibres that synapse directly on the chromaffin cells. These fibres traverse the splanchnic nerve and are myelinated, emanating mainly from the lower thoracic segments (T5 and T9) of the ipsilateral intermediolateral grey column of the spinal cord (Fig. 8.5-3).
Blood supply
Arterial blood supply. The arterial blood to the gland reaches the outer capsule from the superior suprarenal artery (a branch of the inferior phrenic artery), middle suprarenal artery (a branch of the abdominal aorta) and inferior suprarenal artery (a branch of the renal artery). The arterial blood enters sinusoidal capillaries in the cortex and then drains into the medullary veins, which supply blood to medulla and thus form a portal system. This arrangement of portal circulation exposes the medulla to relatively high concentrations of corticosteroids from the cortex.
Venous drainage. The venous blood drains via single central vein. The right suprarenal vein drains into the inferior vena cava and left suprarenal vein into the left renal vein.
Hormones of adrenal cortex
Hormones secreted by the adrenal cortex, called corticosteroids, can be grouped as:
• Glucocorticoids, which include cortisol and corticosterone, have widespread effect on glucose and protein metabolism.
• Mineralocorticoids. Aldosterone is the chief mineralocorticoid. It regulates sodium balance and extracellular fluid (ECF) volume in the body.
• Adrenal sex steroids. These include Dehydroepiandrosterone (DHEA) and its sulphate ester.
Glucocorticoids
Synthesis
The glucocorticoids are synthesized largely by the cells forming zona fasciculata with a small contribution by the cells of zona reticularis of adrenal cortex.
Plasma levels, transport, metabolism and excretion of glucocorticoids
Plasma levels
Plasma levels of glucocorticoids and other corticosteroids are shown in Table 8.5-1. The plasma levels of total cortisol show diurnal fluctuation and range from 10 to 25 μg/dL with an average of 14 μg/dL. The rate of secretion of cortisol, which is about 15 mg/day under normal condition, may increase to 300–400 mg/day under conditions of severe stress.
Transport
Cortisol. In the plasma cortisol circulates in two forms: bound (90%) and free (10%).
Bound form. Most of the plasma cortisol is bound to specific corticosteroid binding α2-globulin (CGB), which is a glycoprotein and is also called transcortin. A small amount (15%) is bound to albumin.
Free form of cortisol constitutes only 5%–10% of the total plasma cortisol.
Mechanism of action of glucocorticoids
Like other steroid hormones, the glucocorticoids act through effect on gene expression by binding with specific intracellular receptors called glucocorticoid receptor (GR). For details of mechanism of action see page 366.
Actions of glucocorticoids
Glucocorticoids are essential for survival.
I Metabolic effects of glucocorticoids
Cortisol has major effects on protein glucose and fat metabolism. The different metabolic effects of glucocorticoids are:
1. Effects on carbohydrate metabolism. Glucocorticoids exert an anti-insulin effect, which leads to hyperglycaemia by following actions:
(i) Increased gluconeogenesis. Glucocorticoids increase the rate of glucose production from non-carbohydrate sources by as much as sixfold to tenfold.
(ii) Decreased utilization of glucose in peripheral tissues.
2. Effects on protein metabolism exerted by glucocorticoids are:
• Catabolic effect. Cortisol enhances the release of amino acids by proteolysis in the skeletal muscle and other extrahepatic tissues.
• Antianabolic effect. It is the ability of the glucocorticoids to inhibit the de novo synthesis of protein, probably at the translational level.
3. Effects on fat metabolism are complex and include:
(i) Lipolytic effects. Although cortisol itself has only a slight lipolytic activity, its presence is necessary for epinephrine, growth hormone and other lipolytic substances to stimulate hydrolysis of stored triglycerides at maximal rates.
Fatty acid synthesis is inhibited in the liver by cortisol— an effect which not observed in the adipose tissue.
(ii) Lipogenic role. Glucocorticoids stimulate lipogenesis by increasing adipocyte lipoprotein lipase and glucose-6- phosphate dehydrogenase activity. Lipogenic effect varies in different regions of the body. Therefore, in cortisol excess there occurs a selective accumulation of fat in the abdomen, trunk and above (trunked obesity) sparing the extremities which become thin due to loss of muscle mass.
4. Effects on electrolyte and water metabolism. Glucocorticoids control distribution of body water and electrolytes by their opposing actions:
II Physiological actions on various organs and systems
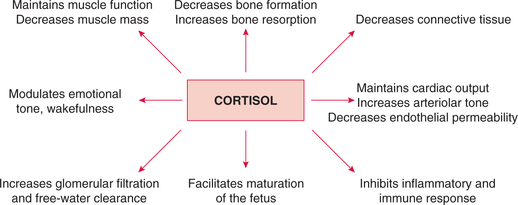
In addition to the metabolic effects noted above, the glucocorticoids affect various organs and systems throughout the body (Fig. 8.5-4):
(i) Contractility and work performance of skeletal and cardiac muscle are maintained by the cortisol.
(ii) Decrease in muscle mass and strength is caused by an excess of cortisol. This occurs due to the decrease in muscle protein synthesis and increase in muscle catabolism.
Note. Because of the above effects on bone, osteoporosis results in skeletal deformity.
3. Effects on connective tissue. Cortisol decreases collagen synthesis producing thereby:
• Thinning of walls of capillaries, which leads to their easy rupture and to intracutaneous haemorrhage.
4. Effects on vascular system. Cortisol is essential for maintaining normal blood pressure by:
• Sustaining myocardial performance and
• Enhancing the vasopressure effect (responsiveness of arterioles to constrictive effect) of catecholamines (especially norepinephrine) and angiotensin II, and maintaining normal blood volume.
• Increase in GFR by increasing glomerular plasma flow,
• Rapid excretion of water load and
• Increase in calcium and phosphate excretion by decreasing their reabsorption in the proximal tubules.
6. Effects on central nervous system. GR are present in various parts of the brain, especially in the limbic system. Through these receptors the glucocorticoids modulate excitability behaviour and mood.
7. Effects on gastrointestinal tract. The glucocorticoids increase gastric acid secretion and can lead to peptic ulceration following long-term use of cortisol.
8. Effects on blood cells and lymphatic organs. The excess of glucocorticoids lead to:
III Anti-inflammatory and anti-allergic effects
These effects are not produced by the glucocorticoids, which are normally secreted physiologically, but are produced by their large doses when administered therapeutically, and are thus called the pharmacological actions of glucocorticoids.
1. Anti-inflammatory effects of glucocorticoids are produced by following actions:
• Cortisol inhibits the activity of phospholipase A2
• Cortisol stabilizes the lysosomal membrane
• Cortisol inhibits migration of circulating leucocytes to the site of inflammation
2. Anti-immunity effect. Cortisol inhibits both cellular and humoral immunity by decreasing the proliferation of T cells (involved in cellular immunity) and B cells (involved in humoral immunity) response.
3. Anti-allergic effect. Cortisol reduces the number of circulating basophils and protects against the release of secretory products of granulocytes, mast cells and macrophages, which have vesicles containing serotonin, histamine and hydrolases.
Regulation of glucocorticoid secretion
The glucocorticoid secretion is regulated by hypothalamicanterior pituitary–adrenal cortex axis, which exerts its effect through (Fig. 8.5-5):
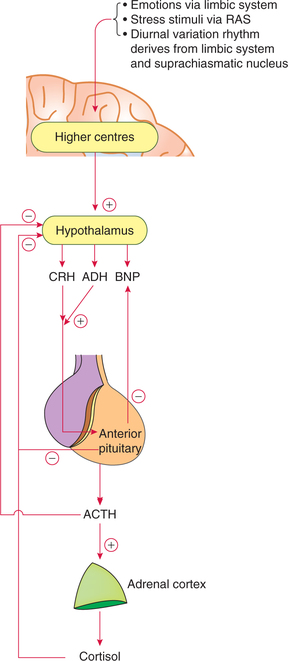
Fig. 8.5-5 Hypothalamic–anterior pituitary–adrenal cortex axis and negative feedback mechanism controlling glucocorticoid secretion.
Note. Since CRH and ACTH are related to regulation of glucocorticoid release, they are described in detail here rather than along with other hormones of hypothalamus and anterior pituitary, respectively.
1 Role of corticotropin-releasing hormone
Corticotropin-releasing hormone is secreted by the small cells of paraventricular nucleus of hypothalamus. Corticotropinreleasing hormone is a polypeptide with 41 amino acids.
Actions of CRH
Actions of ACTH
(i) Actions on adrenal cortex. ACTH is primarily concerned with growth and functions of the adrenal cortex.
• It promotes conversion of cholesterol to pregnenolone, which is the precursor of synthesis of all the hormones of adrenal cortex.
• It stimulates the secretion of glucocorticoids and the adrenal androgens.
(ii) Extra adrenal actions of ACTH occur only with very high levels, which are seen in abnormal conditions.
Regulation of ACTH secretion
(i) Hypothalamic control on ACTH secretion is mainly exerted through CRH (see above). The hypothalamic control is responsible for following characteristics of ACTH secretion:
• Diurnal variation in the levels of ACTH (and thus of cortisol) is due to variation in CRH release. As shown in Fig. 8.5-6, a large peak in the levels of ACTH and cortisol occurs in the morning (6–8 AM) during awakening (plasma level of ACTH ranges between 20 and 100 pg/mL, with an average of 50 pg/mL). Thereafter, the average level decreases markedly (5 pg/mL), just before or after the subject falls asleep. In night workers, the rhythm is reversed. The biological clock responsible for diurnal variation in CRH, ACTH and cortisol levels is located either in the limbic system or the suprachiasmatic nucleus of the hypothalamus.
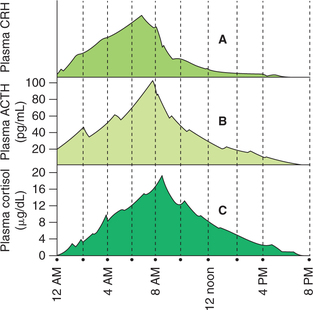
Fig. 8.5-6 Diurnal variations and pulsatility in secretion of CRH (A), ACTH (B) and cortisol (C). Note the ACTH peak follows CRH peak and cortisol peak follows ACTH peak by 10 min.
• Pulsatile release of ACTH is also due to pulsatile release of CRH. Up to three pulses per hour occur and each pulse lasts about 20 min. Cortisol pulses follow the ACTH pulses.
(ii) Negative feedback inhibition of ACTH is caused by (Fig. 8.5-5):
3 Negative feedback control of glucocorticoid secretion
Chronically, elevated plasma levels of free cortisol (and not the total cortisol, i.e. free plus bound form) exert a direct negative feedback action on its own secretion. This effect is exerted at two levels (Fig. 8.5-5):
Mineralocorticoids
The mineralocorticoids include:
• Aldosterone. It is the chief mineralocorticoid.
• 18-hydroxy-deoxycorticosterone (18-OH-DOC) is secreted in small amount and has some mineralocorticoid activity.
Since aldosterone is the major mineralocorticoid, so discussion in this section is limited to it.
Synthesis
Aldosterone, the chief mineralocorticoid, is synthesized exclusively by the zona glomerulosa cells.
Plasma levels, transport, metabolism and excretion
Plasma levels. Depending upon the dietary intake of sodium, the aldosterone secretion ranges from 50 μg/day (with dietary sodium intake of 150 mEq) to 250 μg/day (with dietary sodium intake of 10 mEq).
Transport. In the plasma, 40% aldosterone circulates in free form and 60% in bound form to the specific aldosteronebinding globulin to transcortin and albumin.
Metabolism and excretion. 90% of aldosterone, like the glucocorticoids, is degraded in the liver and excreted in the urine.
Actions of aldosterone
A Primary actions of aldosterone
Aldosterone acts on late distal tubules and collecting ducts of kidney, and causes following effects:
(i) Sodium reabsorption from the tubular fluid into the renal tubular epithelial cells.
(ii) Potassium excretion. In the kidney, the active reabsorption of Na+ occurs in exchange of K+ and H+. Thus, aldosterone not only causes reabsorption of Na+, but also excretion of K+ by renal tubular epithelial cells.
(iii) H+ excretion. Aldosterone also enhances the tubular secretion of H+ as Na+ is reabsorbed.
2 Effects on sweat glands, salivary glands and colon
Sweat glands and salivary glands produce primary secretions which contain a large amount of sodium chloride. The sodium chloride is absorbed as the secretion passes through the ducts, and in turn K+ and 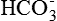 are excreted. Thus aldosterone decreases the loss of Na+ and Cl− in sweat and salivary secretion.
are excreted. Thus aldosterone decreases the loss of Na+ and Cl− in sweat and salivary secretion.
Colon. The aldosterone stimulates sodium reabsorption from the colon, while enhancing potassium excretion in the faeces.
Regulation of aldosterone secretion
Aldosterone secretion is controlled by following factors (Fig. 8.5-7):
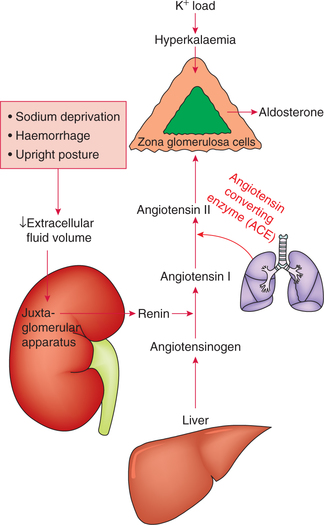
1 Renin–angiotensin system
The secretion of aldosterone is influenced by changes in the circulating fluid volume, which are sensed in the kidney. The signals arising from the kidney increase aldosterone secretion when ECF volume is decreased and vice versa.
Conditions associated with decreased ECF are:
Steps involved in the secretion of aldosterone by renin– angiotensin system are:
• Decrease in ECF volume leads to decrease in the renal arterial blood flow and pressure.
• Decrease in renal perfusion pressure causes the juxtaglomerular cells of the afferent arterioles to secrete renin.
• Renin catalyzes the conversion of angiotensinogen (α 2-globulin substrate present in the plasma) to angiotensin I.
• Angiotensin I is converted into angiotensin II by the action of angiotensin-converting enzyme present in the endothelium of blood vessels, especially in the lungs.
• Angiotensin II binds to specific plasma membrane receptors in adrenal's zona glomerulosa cells and increases the secretion of aldosterone.
Aldosterone secretion may be increased to fourfold to eightfold by renin–angiotensin system.
Adrenal sex steroids
• Dehydroepiandrosterone (DHEA). Its sulphate ester (DHEA-S) and androstenedione are the major androgenic precursor products of adrenal cortex.
• Oestrogen and progesterone are produced in very small amounts.
Synthesis
The adrenal sex steroid precursors are synthesized in the zona reticularis.
The 17-hydroxylated derivatives of pregnenolone and progesterone are the starting points for synthesis of androgen precursors.
Plasma levels and contribution towards sex steroids
Plasma levels. Normal plasma level of DHEA is 150– 200 μg/dL at 25 years of age in both sexes.
Contribution of adrenal glands towards sex steroids and their functions are:
During fetal life, adrenal cortex is hyperplastic and secretes a large amount of DHEA, which acts as the main precursor for synthesis of oestrogen by placenta.
In adult women, the adrenal glands supply 50%–60% of the androgenic hormone requirement. DHEA-S contributes to increased muscle mass, growth of pubic and axillary hair, and libido.
In adult man, since testes produce a large quantity of testosterone, the adrenal androgen precursors are of little biological importance. However, they may be partly responsible for the development of male sex organs in childhood.
Applied aspects
The important applied aspects in relation to the adrenal cortex which need mention include:
• An integrated response to stress (see page 407),
• Hyperactivity of adrenal cortex and
• Hypoactivity of adrenal cortex (see page 405).
Hyperactivity of adrenal cortex
Disorders of hyperactivity of adrenal cortex include:
• Cushing's syndrome (hypercortisol state),
• Conn's syndrome (hyperaldosteronism) and
• Adrenogenital syndrome (excessive secretion of adrenal androgens).
1 Cushing's syndrome
Cushing's syndrome refers to the group of clinical conditions occurring due to prolonged excessive levels of glucocorticoids.
Characteristic features
Characteristics features of Cushing's syndrome are (Fig. 8.5-8):
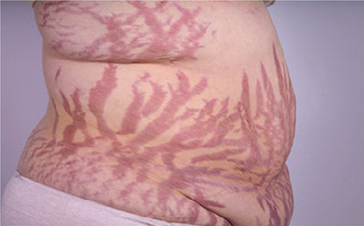
Fig. 8.5-8 Photograph of a patient with Cushing's syndrome showing truncal obesity and purple striae on the abdomen.
1. Truncal or centripetal obesity. It occurs due to redistribution of body fat from extremities (which is in the abdominal wall, back and face) producing following characteristic features:
• Buffalo hump, due to collection of fat at upper back;
• Moon face, due to fat collection on the face and
• Purple striae or cutaneous abdominal striae or livid stretch marks. The skin and subcutaneous tissue becomes thin due to protein catabolism. The stretching of abdominal skin due to excessive subcutaneous fat deposition causes rupture of subdermal tissues producing reddish purple striae.
2. Muscle weakness and backache due to protein catabolism.
3. Sodium and water retention may cause weight gain, oedema and hypertension.
4. Hyperglycaemia occurs due to gluconeogenesis and inhibition of peripheral utilization of glucose. It may lead to glycosuria and adrenal diabetes.
5. Hirsutism and menstrual irregularity may occur due to increased adrenal androgens.
6. Susceptibility to osteoporosis and bone fracture is increased due to protein depletion and bone resorption.
7. Susceptibility to infections is increased due to immunosuppression.
8. Psychological, emotional and personality changes may occur due to CNS effects of glucocorticoids.
9. Blackening of skin may occur due to pigmentation caused by MSH-like effects of excessive ACTH.
2 Hyperaldosteronism
Hyperaldosteronism refers to overproduction of the hormone aldosterone—a major sodium-retaining hormone.
Characteristic features of hyperaldosteronism are:
• Sodium and water retention leading to hypertension and oedema.
• Hypokalaemia may occur due to an increased potassium excretion producing muscle weakness.
• Metabolic alkalosis may occur due to secretion of more amount of H+ into renal tubules. Metabolic alkalosis may produce hypocalcaemia causing tetany.
3 Adrenogenital syndrome
As mentioned earlier, the androgen precursors secreted by the adrenal cortex are of little biological importance under normal circumstances. However, when secreted in large amounts, as in tumour of zona reticularis of adrenal cortex, the following abnormal features may be produced:
• In pre-pubertal males, the excessive androgens produce precocious pseudopuberty.
• In males, the oestrogen-producing cells may produce female-like secondary sexual characters, such as enlargement of breasts (gynaecomastia), atrophy of testes, loss of libido and feminine body.
• In females, they cause development of male secondary sexual characteristics, such as beard, muscular body, breaking of voice, male-type hair growth, enlargement of clitoris and amenorrhoea.
Note. In virilized children or adult women, a large increase in urinary 17-ketosteroid excretion almost always indicates an adrenal abnormality.
Hypoactivity of adrenal cortex
Adrenocortical deficiency occurs due to involvement of adrenal cortex and is associated with high ACTH levels due to feedback mechanism. The conditions producing adrenocortical deficiency include:
Addison's disease
Addison's disease occurs due to chronic deficiency of hormones secreted by all the three zones of adrenal cortex. Therefore:
1. Glucocorticoid insufficiency produces weight loss, malaise, anorexia, nausea, vomiting, weakness and diarrhoea. Since glucocorticoids are essential for adaptation to stress, therefore in Addison's disease exposure to any type of stress, e.g. even mild infection, may be fatal.
2. Mineralocorticoid deficiency produces hyponatraemia, hyperkalaemia, acidosis and decreased ECF volume with hypotension.
3. Loss of androgens causes sparse hair in females.
4. Increased ACTH secretion occurs due to feedback mechanism and causes diffuse pigmentation of the skin and mucous membranes (because of its MSH like actions).
Congenital adrenal hyperplasia
Causes. Congenital adrenal hyperplasia is caused by congenital deficiency of 21-hydroxylase deficiency and deficiency of 11-hydroxylase enzymes.
Characteristic features are virilism and excessive body growth.
In boys, it is characterized by:
• Precocious body growth leading to stocky appearance called infant hercules.
• Precocious sexual development with enlarged penis even at age of 4 years.
In female fetus, high plasma androgen levels cause masculinized pattern of development (virilism). Sometimes the female fetus may be born with male-type external genitalia. This condition is called pseudohermaphroditism.
Hormones of adrenal medulla
The adrenal medulla secretes catecholamines which include epinephrine, norepinephrine and dopamine. About 80% of adrenal medullary catecholamine is epinephrine and rest is norepinephrine. Apart from catecholamines, the adrenal medulla also contains small amounts of dynorphins, neurotensin, encephalin, somatostatin and substance P. The functions of these adrenal peptides are not clear.
Synthesis of catecholamine hormones
Synthesis of catecholamines
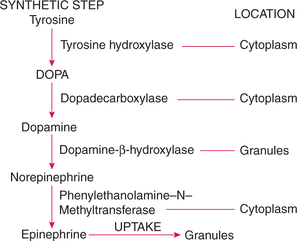
Epinephrine and norepinephrine are synthesized in different cells. The biosynthetic pathway originates with L-tyrosine. Conversion of tyrosine to epinephrine occurs in four steps (Fig. 8.5-9).
1. Conversion of tyrosine to DOPA. The conversion of tyrosine to DOPA (dihydroxyphenylalanine) is catalyzed by the enzyme tyrosine hydroxylase. This reaction occurs in the chromaffin cell cytoplasm.
2. Conversion of DOPA to dopamine in the cytosol is catalyzed by the enzyme dopa decarboxylase.
3. Conversion of dopamine to norepinephrine. Dopamine from the cytosol enters the chromaffin granules, where it is converted to epinephrine by the enzyme dopamine-bhydroxylase.
4. Conversion of norepinephrine to epinephrine. In about 20% of chromaffin cells, the norepinephrine is end product, and in about 80% of chromaffin cells, norepinephrine diffuses back into the cytoplasm, where it is converted to epinephrine by the enzyme phenylethanolamine N-methyltransferase (PNMT).
Regulation of catecholamine secretion
Nervous control of secretion
The catecholamine secretion is entirely controlled by the splanchnic nerves supplying the medulla. These nerves comprise preganglionic sympathetic fibres emerging mainly from lower thoracic segments (T5–T9) of ipsilateral intermediolateral grey column of the spinal cord. These fibres, when stimulated, act by releasing acetylcholine close to the adrenal medullary chromaffin cells.
Physiological and psychological stimuli for release
The adrenal medullary activation occurs as a part of generalized sympathetic response to any emergency situation. Therefore, this has also been called sympathetic alarm reaction. The various sensory stimuli associated with the rapid release of epinephrine (and probably norepinephrine) from adrenal medulla include:
Circulation, metabolism and excretion
Circulation
• Secreted epinephrine and norepinephrine from the adrenal medulla is in the ratio of 4:1.
• Basal plasma levels (in recumbent humans) of free epinephrine are 30 pg/mL and that of free norepinephrine are 300 pg/mL.
• Variation in plasma levels of catecholamines according to physiological or pathological states are quite common.
Metabolism and inactivation of circulating catecholamines
Circulating catecholamines (epinephrine and norepinephrine) are metabolized predominantly in the liver and kidney by the enzymes, monoamine oxidase (MAO) and catechole- O-methyltransferase (COMT). The metabolites are excreted in the urine and bile as vanillylmandelic acid (VMA) and methoxyhydroxyphenyl glycol (MOPG).
Adrenergic receptors and actions of catecholamines
Adrenergic receptors
The adrenergic receptors are of two types:
Alpha (α) receptors. These are further of two types (α1 and α2). The α-adrenergic receptors are sensitive to both epinephrine and norepinephrine. These receptors are associated with most of the excitatory functions of the body but have one major inhibitory function (i.e. inhibition of intestinal motility).
Beta (β) receptors. These are further of three types: β1, β2 and β3. β-adrenergic receptors respond to epinephrine and, in general, are relatively insensitive to norepinephrine. These receptors are associated with most of the inhibitory functions of the body but have an important excitatory function (i.e. excitation of myocardium).
Relative potency of two catecholamines varies with each receptor type. In general, epinephrine tends to repeat more strongly with β receptors and norepinephrine with α receptors but overlap is considerable.
Actions of catecholamines
I Metabolic actions of catecholamines
Epinephrine affects metabolic functions more than norepinephrine, via α and β receptors.
1. General metabolic effects of epinephrine. This includes:
• Increased O2 consumption (by 20%–40%) and increased CO2 output.
• Raised basal metabolic rate (BMR) and respiratory quotient (RQ).
• Increased heat production due to stimulation of cellular oxidative processes.
2. Effect on carbohydrate metabolism. Epinephrine produces hyperglycaemia and makes the glucose available for the brain and other tissues to meet the emergency by its following effects:
• Glycogenolysis is stimulated in the liver.
• Glycogenesis is reduced in the liver by inhibition of the enzyme glycogen synthase.
• Gluconeogenesis, i.e. hepatic production of glucose from lactate, amino acids and glycerol is increased.
• Insulin secretion is inhibited.
• Glucagon secretion is stimulated. This amplifies the hyperglycaemic effects of epinephrine.
• ACTH secretion is stimulated, which then stimulates cortisol secretion. Cortisol is a potent gluconeogenic hormone.
3. Effects on fat metabolism. Norepinephrine has more potent action on the lipid metabolism.
II Physiological actions of catecholamines
1 Effects on cardiovascular system
The net effects of epinephrine and norepinephrine are (Table 8.5-2):
On CNS, catecholamines via β receptors activate reticular activating system (RAS) and thus lead to arousal and alerting responses producing anxiety, apprehension and coarse tremors of extremities.
On GIT, epinephrine via β receptors causes relaxation of smooth muscles of wall of the gut, decreasing its tone and motility. Via α receptors epinephrine causes contraction of sphincters of gut; the net result is production of constipation.
On urinary bladder. Epinephrine produces retention of urine by relaxing the detrusor muscles.
On skin. Catecholamines act on pilomotor muscle producing piloerection of hair.
On skeletal muscle. During exercise, epinephrine via β2 receptors increases blood supply (by causing vasodilation).
On eyes. Epinephrine causes dilation of the pupil (mydriasis) by contracting dilator pupillae (radial) muscle.
On respiration. Epinephrine via β2 receptors relaxes smooth muscles of bronchioles producing bronchodilation. It also increases rate and force of respiration.
On blood. Epinephrine produces following effects:
• Reduces blood coagulation time by increasing activity of factor V.
• Increases RBC count, haemoglobin content and packed cell volume (PCV) due to release of RBCs in circulation by causing contraction of spleen.
• Increases plasma protein concentration by movement of fluid out of circulation.
• Neutrophilia occurs due to release of sequestrated neutrophils into the circulation.
On secretion of other hormones. Catecholamines regulate secretion of a number of hormones:
• Insulin and somatostatin secretion is decreased via α2 receptors (by decreasing cAMP).
• Glucagon and pancreatic peptide secretion is increased via β2 receptors.
• Thyrotropin-releasing hormone (TRH)-induced secretion of TSH from thyrotrophs is decreased via α2 receptors.
• Thyroid hormone secretion is enhanced by catecholamines under certain circumstances and peripheral conversion of T4–T3 is stimulated via β2 receptors.
On renin secretion and Na+ and K+ movement. Catecholamines increase renin secretion by stimulation of β receptors in the kidney. The increase in renin in turn increases aldosterone secretion, which in turn enhances sodium retention.
An integrated response to stress
Stress, may it be emotional, physical or biological, evokes an integrated response of sympathoadrenal medullary system and hypothalamic–pituitary–adrenal cortex axis.
Steps involved in stress adaptation by an integrated response of the above system are (Fig. 8.5-10):
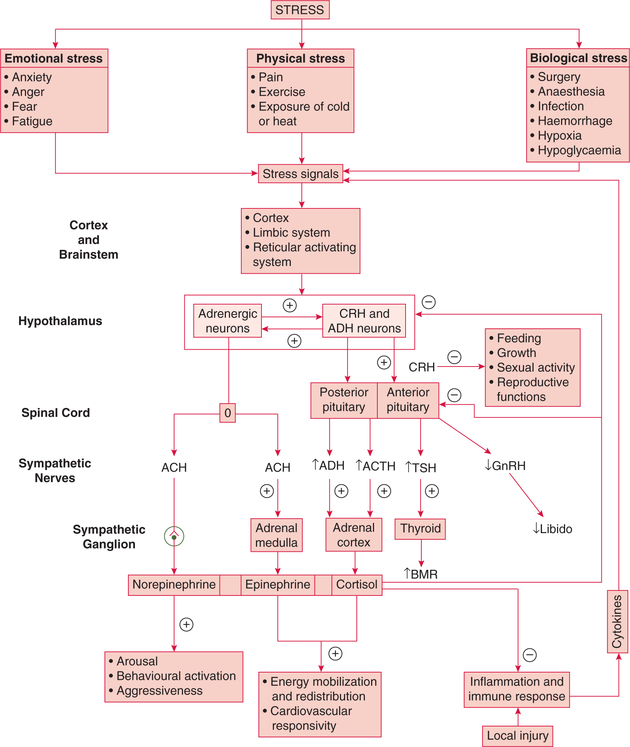
Fig. 8.5-10 Steps involved in the adaptation to stress by an integrated response of hypothalamic–pituitary–adrenal cortex axis and sympathoadrenal medullary system.
Perception of stress signals. Stress is perceived by many areas of the brain, from the cortex down to brainstem including limbic system and RAS.
Stimulation of hypothalamus. Major stresses activate the CRH and ADH neurons in the paraventricular nucleus and adrenergic neurons.
Activation of hypothalamic–pituitary–adrenal axis. Corticotropin-releasing hormone and antidiuretic hormone (ADH) release stimulates ACTH release and ultimately elevates plasma cortisol levels.
Activation of sympathoadrenal medullary system. Sudden exposure to any type of stress initially produces the sympathetic alarm reaction. Stimulation of adrenergic neurons of hypothalamus ultimately leads to a release of epinephrine from adrenal medulla and norepinephrine from the sympathetic ganglia.
Integrated role of hormones released by hypothalamic– pituitary–adrenal axis and sympathoadrenal medullary system in stress adaptation. Together these hormones help in adaptation to stress by their following actions:
• Increase in glucose production. Catecholamines rapidly raise plasma glucose by activating glycogenolysis and cortisol acts more slowly by providing amino acid substrate for gluconeogenesis. Together they shift glucose utilization towards the central nervous system away from the peripheral tissues.
• Free fatty acid supply. Epinephrine rapidly augments the supply of free fatty acids to heart and muscles, and cortisol facilitates the lipolytic role.
• Cardiovascular adjustments. Catecholamines and cortisol raise blood pressure and cardiac output, and they improve the delivery of substrates to tissues that are critical to the immediate defence of the organism.
• Inhibition of activities that are not useful during stress, and divert individuals and their resources from defensive responses to danger is an important part of adaptation to stress. For example, CRH input to the hypothalamic neurons inhibits growth hormone, gonadotropin release and sexual activity.
• Interaction with immune system. The hormones produced during stress interact with the immune system to produce a balance between useful local cytokine production at threatened sites and potentially dangerous systemic effects of these immune system products.
 Glucocorticoids
Glucocorticoids
 Important note
Important note