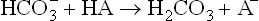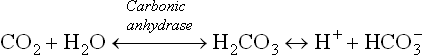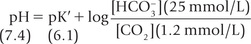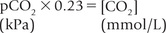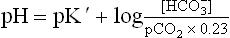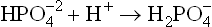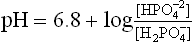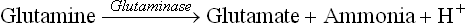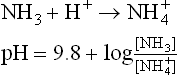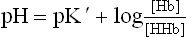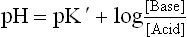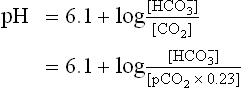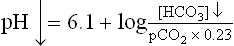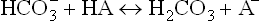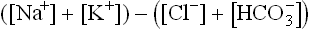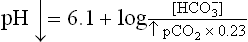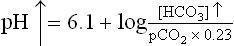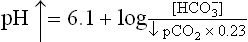Water, Buffers And Acid-Base Chemistry
Water is the most abundant compound in the living world. It makes up 70% or more of the body weight in living systems. Water is required for various intra- and extracellular functions, such as transfer of chemical energy, and transport of nutrients. It is the medium in which various enzyme-catalyzed biochemical reactions take place.
The ability of water to carry out diverse functions is due to its unique properties. These properties, followed by acids, bases, buffers are described in this chapter. The basic concepts of acid-base chemistry are also dealt with.
After going through this chapter, the student will be able to understand:
Electric dipolar nature of water and some of its unusual properties.
Differences between (a) an acid and a base, and (b) a strong acid/base and a weak acid/base; and interpret titration curve of a weak acid
pH expression and the Henderson—Hasselbalch equation and the behaviour of buffers.
Three-tier defense against imminent pH alterations and causes of various types of acid-base disorders.
Compensatory mechanisms in each type of acid-base disorder, and the biochemical alterations in acid-base parameters in arterial blood in both the compensated and the un-compensated cases.
I Water as Principal Biological Fluid
A Ionic Properties of Water
Dissociability
Water molecule can dissociate to yield a proton (H+) and a hydroxyl ion (OH–). The disassociation is reversible.
These two products influence properties of several important constituents of cells. For example, H+ concentration determines pH of the medium, which in turn influences catalytic activities of various cellular enzymes.
Polarity
Though water molecule appears to be neutral, it is polar with distinct positive and negative poles, therefore it is referred to as an electric dipole. Polar nature of water accounts for its role as a transport medium for a variety of other polar molecules such as plasma proteins, and ions like K+ and Na+.
B Electric Dipolar Nature of Water
The electric dipolar nature of water molecule is due to its structure which consists of two hydrogen atoms linked covalently to an oxygen atom (Fig. 1.1 ).
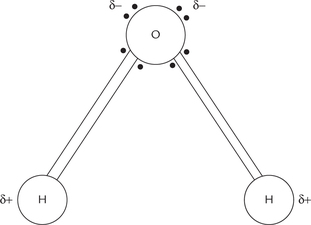
Fig. 1.1 Dipolar nature of water molecule. The two unshared electron pairs of the oxygen atom give it a localized partial negative charge, and the strong electron-withdrawing tendency of oxygen gives the two hydrogen atoms partial positive charges.
Each of the hydrogen atom shares a pair of electrons with the oxygen atom. Oxygen has a strong electron-withdrawing tendency, and therefore, these electrons tend to lie more towards oxygen and away from hydrogen. Consequently hydrogen atom acquires a localized partial positive charge. Oxygen has two more pairs of electrons which remain unshared. These electrons impart partial negative charge to oxygen.
C Hydrogen Bonds
Opposite poles of the adjacent water molecules attract each other, i.e. positive pole of one molecule attracts negative pole of the adjacent one and vice versa. This type of electrostatic attraction is called hydrogen bond (Fig. 1.2 ). As shown in Figure 1.2, arrangement of electrons around oxygen is nearly tetrahedral. Consequently, each water molecule can theoretically form hydrogen bonds with as many as four neighbouring water molecules. Since these molecules are in a stage of continuous motion, the hydrogen bonds are continuously being broken and reformed. At room temperature, each water molecule forms hydrogen bonds with three or four water molecules, at any given instant. In ice, however, this motion is restricted and four hydrogen bonds are possible, resulting in formation of crystal lattice.
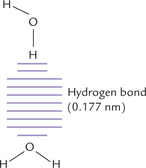
Fig. 1.2 Hydrogen bonding (designated by dashes) between the hydrogen atom of the upper water molecule and the oxygen atom of the lower water molecule.
Hydrogen bonds (bond energy = 4.5 kcal/mole) are much weaker than the covalent bond (bond energy = 110 kcal/mole). However, the hydrogen bonds are present in very large numbers and their collective strength confers great internal cohesion to the liquid water. Half-life of a hydrogen bond is very short—less than 1 × 10 –9 sec. Consequently, liquid water is not viscous but very fluid in nature.
D Properties of Water
On account of its polar nature and consequent hydrogen bonding, water possesses the following unusual properties.
Higher Melting and Boiling Points
Hydrogen bonding between adjacent water molecules cause strong intermolecular forces of attraction. Large amount of thermal energy is required to overcome these cohesive forces. As a result, melting and boiling points of water are relatively higher than other common liquids (Table 1.1 ). No such intermolecular attraction is present among molecules of non-polar liquids like benzene or hexane resulting in lower melting and boiling points.
Solvent Properties
Water is a better solvent than other common liquids. Most crystalline salts (e.g. sodium chloride), which are nearly insoluble in non-polar solvents, readily dissolve in water. Better solvent properties are because of dipolar character of water. For example, the crystal lattice structure of sodium chloride is held together by strong electrostatic attractions between alternating positive sodium and negative chloride ions. When this compound is exposed to water, the negative pole of the dipolar water is attracted towards Na+ and the positive pole is attracted towards Cl–. Thus, water hydrates both the component ions of the crystal and then pulls them away from the lattice (Fig. 1.3 ). Consequently, the crystal lattice is rapidly broken, which accounts for its high solubility in water.
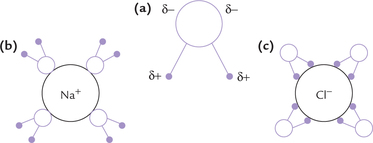
Fig. 1.3 High solubility of NaCl in water. Water hydrates Na+ and Cl– ions and pulls them away from the lattice. (a) Structure of water, (b) Hydration of Na+, (c) Hydration of Cl–.
Similarly, water tends to dissolve several other organic compounds with polar functional groups, such as sugars, alcohols, aldehydes and ketones. Solubility of these substances is also due to tendency of water molecules to form hydrogen bonds with the polar groups (hydroxyl, carbonyl or alcohol) of these substances.
In addition to crystalline salts and organic compounds, the third category of compounds with which water interacts in a characteristic way are the amphipathic compounds. These compounds have both hydrophobic (water repelling) and hydrophilic (water attracting) portions within the same molecule. An example is sodium salt of the long chain fatty acid, also called soap; its long hydrocarbon tail forms the hydrophobic portion and the negatively charged carboxyl group forms the hydrophilic portion (Fig. 1.4a ). Soap molecules are readily dispersed by water to form aggregates called micelles (Fig. 1.4b). In a micelle, the hydrophilic carboxylate groups are exposed to the aqueous exterior and the hydrophobic hydrocarbon chains move toward the interior. Movement of the non-polar hydrophobic tails towards the interior of the micelle is because the surrounding water molecules tend to associate more with the hydrophilic carboxylate group than with the hydrophobic portions of the molecule. Towards the core, the hydrophobic tails tend to associate with one another; their association is known as hydrophobic interactions.
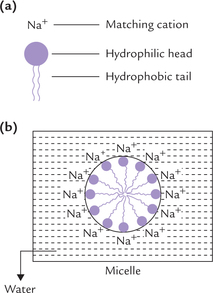
Fig. 1.4 (a) An amphipathic soap molecule with a hydrophilic head and hydrophobic tail, (b) Interaction of water with soap to form a micelle.
Thus, the driving forces for the formation and stability of micelles are: (a) Tendency of the surrounding water molecules to form hydrogen bonds with each other and with the negative carboxylate groups, and (b) hydropho-bic interactions between the hydrocarbon tails.
Besides soap, micellar structures are also formed by other amphipathic molecules, e.g. phospholipids, nucleic acids and certain proteins. Micellar arrangement of these molecules forms the core of biological membranes.
E Ionization Behaviour of Water
Equilibrium Constant
Degree of ionization of a dissociable compound is indicated by its equilibrium constant. Let us consider a reversible reaction:
The equilibrium constant of this reaction can be derived by making use of the law of mass action which states that rate of a chemical reaction depends, among other factors, on concentrations of the substrate molecules. Therefore, rate of the forward reaction (VF) depends on concentration of A, B or both. Conversely, rate of the reverse reaction (VR) depends on concentration of C, D or both
where KF and KR are the proportionality constants for the forward and the reverse reactions respectively (the square brackets indicate the molar concentrations).
Equilibrium is defined as a condition when rate of the forward reaction (VF) equals the rate of the reverse reaction (VR). Therefore, at equilibrium the following equality exists:
The ratio of the two constants, KF /KR, is also known as equilibrium constant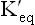 of the reaction. It indicates relative concentrations of the reactants and the products (i.e. the composition of the equilibrium mixture of a reaction). It has a characteristic value for a given chemical reaction at a given temperature. It does not depend on the starting concentrations of either the reactants or the products. Using the above principle, the equilibrium constant or the dissociation constant of water is expressed as:
of the reaction. It indicates relative concentrations of the reactants and the products (i.e. the composition of the equilibrium mixture of a reaction). It has a characteristic value for a given chemical reaction at a given temperature. It does not depend on the starting concentrations of either the reactants or the products. Using the above principle, the equilibrium constant or the dissociation constant of water is expressed as:
Ion Product of Water
Dissociation of water proceeds to a limited extent only. At 25°C, only about 1 out of 10 million water molecules is in an ionized state. Therefore, the molar concentration of water at equilibrium is equal to the number of grams of water in one liter (1000 gm), divided by the gram molecular weight of water (i.e. 1000/18 = 55.5). The equation (6) can, therefore, be rewritten as:
The value of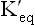 has been determined by electrical conductivity measurement of water and found to be 1.8 × 10–16 mol/L at 25°C. Substitution of this value in equation (7) yields
has been determined by electrical conductivity measurement of water and found to be 1.8 × 10–16 mol/L at 25°C. Substitution of this value in equation (7) yields
The symbol KW used to designate the product of H+ and OH– concentrations is called the ion product of water. Its value always remains the same, i.e. 10–14 M in any aqueous solution. Thus, the concentration of H+ can be calculated if concentration of OH– is known or vice versa as shown below:
In acidic solution, concentration of H+ exceeds 10–7 M. Therefore, a corresponding decreases in the concentration of OH– must occur, so that product of two remains 1 × 10–14. Conversely, in the alkaline solutions concentration of OH– is high (> 10–7 M) and that of H+ must be correspondingly low.
pH Scale or Measure of H+ Concentration
The term pH is defined as negative logarithm of the hydrogen ion concentration, expressed in moles.
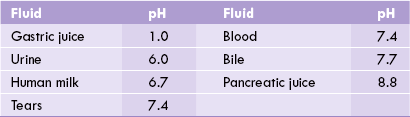
pH measurement is a convenient way of designating the actual concentration of H+, and thus of OH– in any aqueous solution. Mean pH values of body fluids are given in Table 1.2 . The pH value of a neutral solution having an H+ concentration of 10–7 M is 7 as calculated below:
In acidic solutions, where H+ concentration if greater than 10–7 M, the pH values becomes less than 7. Conversely, in the alkaline solutions pH is greater than 7 because concentration of H+ is less than 10–7 M.
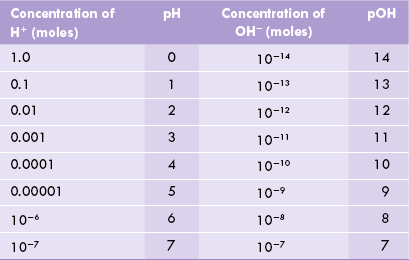
Any two solutions, pH values of which differ by one unit, differ with respect to their H+ concentrations by ten-folds. Thus, a solution with pH value of 5.0 has 10 times higher H+ concentration compared to the one with a pH of 6.0. Another expression analogous to pH, though less commonly used, is pOH. It denotes concentration of the hydroxyl ions and is defined as the negative logarithm of the OH– ion concentration, expressed in moles (pOH = - log [OH–]).
Since product of molar concentrations of H+ and OH– equals 1 × 10–14, the expressions pH and pOH are inversely related to each other, so that their sum always equals 14. The pH scale is represented in Table 1.3 .
pH measurement:
pH of a solution is measured by indicator dyes, indicator papers, or more accurately, by the pH meter.
Indicator dyes:
Chemically an indicator (designated as HI) is a weak acid.
The indicators most commonly used are phenol red or phenolphthalein. Action of an indicator is based on the principle that its colour in the dissociated from (I–) is different from its colour in the undissociated form (HI). Which one of the two forms—dissociated or undissociated—predominates at a given instant depends on pH of the surrounding medium. Thus, colour of the indicator changes as pH of the surrounding medium changes.
In other words, at a given pH, colour of an indicator depends on relative proportions of the dissociated and the undissociated forms.
Note:
A weak acid exists predominantly in an undissociated form at a pH value lower than its pK' (pK' is the negative logarithm of the equilibrium constant: pK' = -log K’). When pH rises above the pK' value, the dissociated from predominates; and both the forms are present in equal concentrations when pH equals pK’.
Indicator papers (pH papers):
Principles of action of the indicator papers is same as that of the indicator dyes. Like the indicator dyes, the indicator papers are useful in a specific pH range. This pH range, which occurs around its pK' value, brings about a visible change in colour of the indicator.
pH meter:
It is used for accurate measurement of pH. The basic components of a pH meter are shown in Figure 1.5 .
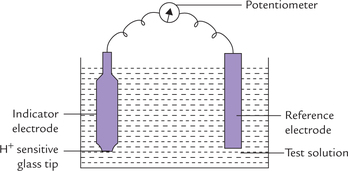
Fig. 1.5 pH meter. The reference electrode generates a stable electric potential and the indicator electrode generates a potential based on H+ concentration of the test solution.
• Reference electrode: It consists of a metal and its salt (in contact with a solution containing the same anion). Mercury/murcurous chloride or Ag/AgCl are most commonly used reference electrodes. The reference electrode generates a stable electrical potential.
• Indicator electrode: It consists of a silver wire coated with AgCl, immersed into solution of AgCl. These are placed into a tube containing a special glass membrane tip, which is sensitive only to hydrogen ions.
When the indicator electrode is dipped into a solution of unknown pH, a potential difference is established across the glass membrane tip. The magnitude of potential difference varies with the hydrogen ion concentration (a measure of pH) of the test solution. The potential difference generates a signal (i.e. electromotive force) which is read against the fixed potential of the reference electrode.
Presently, pH meters which use single electrode are also available.
II Acids, Bases and Conjugate Acid-Base Pairs
A Acids
Acids are defined as substances that are ionized in dilute aqueous solutions to liberate protons (H+). They are commonly referred to as proton donors.
Acids such as hydrochloric acid (HCl), nitric acid (HNO3) and sulphuric acid (H2SO4), which have a strong tendency to liberate protons, are called strong acids. They undergo near complete ionization in dilute aqueous solutions. Therefore, their equilibrium lies far towards the right. At the equilibrium point, concentration of the disassociated form far exceeds that of the undissociated form.
Conversely, the weak acids are only partially ionized in water. Their equilibrium lies towards the left. At equilibrium point, concentration of the undissociated form exceeds that of the dissociated form. In living systems, behaviour of weak acids is more important than that of the strong acids. Acetic acid (CH3COOH) is an example of weak acid; it mildly dissociates to yield an acetate anion and a proton.
Since degree of ionization is greater in case of strong acids, equilibrium constant of strong acids is higher than that of weak acids (Table 1.4 ).
pK' value of an acid is defined as the negative logarithm of its equilibrium constant 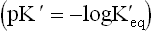 . It is an inverse relationship. Hence, stronger acids having larger
. It is an inverse relationship. Hence, stronger acids having larger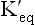 values have relatively lower pK' values. Conversely, weak acids have relatively low
values have relatively lower pK' values. Conversely, weak acids have relatively low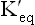 values and high pK' values.
values and high pK' values.
B Bases
Bases are defined as are substances that accept protons in aqueous solutions, and commonly known as proton acceptors. For example, Cl–,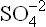 , CH3COO– accept a proton (reactions 10-12) and therefore, are bases.
, CH3COO– accept a proton (reactions 10-12) and therefore, are bases.
Stronger bases have greater tendency to accept protons, whereas the weaker bases have weaker tendency for the same. For instance, equilibrium of the dissociation of acetic acid (reaction 12) lies towards the left, which implies that acetate ion has a strong tendency to accept proton. It is therefore, a strong base. Cl– and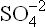 have weaker tendency to accept protons and are therefore weak bases.
have weaker tendency to accept protons and are therefore weak bases.
Acids can donate protons and bases can accept protons. The strength of an acid is expressed as its pK’: it corresponds to the pH value at which the ionizable group is half-protonated.
C Conjugate Acid-Base Pairs
In ionization reactions (discussed above), there is a proton donor-proton acceptor pair.
This pair is known as conjugate acid-base pair.
Conjugate base of a weak acid is always a strong base. For example, acetic acid is a weak acid because of its weak dissociability, while acetate, its conjugate base, has a strong tendency to accept a proton.
On the other hand, conjugate base of a strong acid is a weak base (reactions 10 and 11).
Titration Curve of Weak Acids
Titration is a procedure that is used to quantitatively determine the amount of acid in a given solution. In this procedure, an acid reacts with an alkali of known concentration till the point of neutralization (end point).
Titration curve is drawn by plotting the pH against the amount of alkali added. It indicates the pH changes of the acid that occur when alkali is added in small amounts.
Example: Titration curve of acetic acids vs sodium hydroxide of shown in Figure 1.6 :
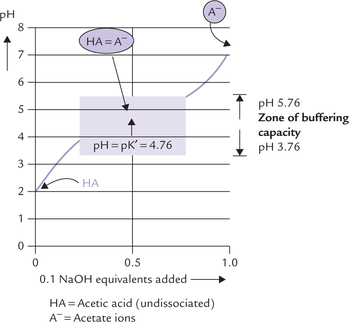
Fig. 1.6 Titration curve of acetic acid (HA), showing predominant ionic forms at various pH points. At midpoint of the titra-tion, the concentration of acetic acid and acetate are equal, and pH is numerically equal to pH of acetic acid. The zone of buffering capacity is shaded.
1. At the start of the titration, acetic acid is present pre-dominantly in undissociated state (HA). As the alkali (sodium hydroxide; 0.1N) is added, it reacts with the acid molecules to form acetate ions (A–). Thus, there is a progressive rise in the concentration of base (i.e. acetate) with a concomitant fall in the concentration of the acid.
Note: Acetate exists in the form of its sodium salt, sodium acetate (CH3COONa), though the two have been written separately in the above equation.
2. At the midpoint of titration about half of the acetic acid loses its proton to form acetate. Thus, concentrations of the acetic acid and its conjugate base (acetate) are equal at this point.
3. Towards the end of the titration, most of acetic acid loses its proton, so that the predominant ionic species present is acetate.
It may be observed that at the start and towards the end, the titration curve is relatively steep, indicating that addition of alkali causes significant alteration in pH. However, at the midpoint, the curve is relatively flat, which indicates that small additions of alkali do not elevate the pH significantly. Therefore, the flat zone represents the buffering zone because of the resistance it offers to any imminent alteration of pH with addition of alkali (or acid). The following important relationships exist at the midpoint of this zone:
The latter relationship is obtained by using the Henderson-Hasselbalch equation:
Hence, the Henderson-Hasselbalch equation can be rewritten as:
To learn more about the Henderson-Hasselbalch equation, including derivation and significance, refer Box 1.1.
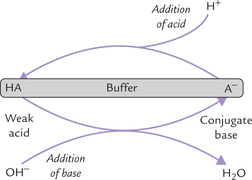
D Buffers
Buffer is a system that resists any alteration in its pH when a small amount of acid or alkali is added to it. It comprises two major components: a weak acid (HA) and its conjugate base (A–). A buffer system is most effective when (a) these two components are present in equimolar concentrations, and (b) pH (of the medium) equals pK' (of the acid-base pair).
A buffer remains effective when pH is within the range of pK' ± 1.
Buffers resist changes in pH within about one pH unit of the pK' of the buffering species (pK' ± 1 pH unit).
Mechanism of Action of a Buffer System
When a small amount of acid is added, it is taken up by the base component of the buffer (A–), and any pH change is averted.
Similarly, the acid component of the buffer system (HA) is capable of reacting with any OH– that is added.
Thus, buffering action is the net result of capacity of the base component (of the acid-base pair) to neutralize the added acid, and of the acid component to neutralize the added base. During these reactions, the sum of the acid and the base does not change, only their ratio may change. For example, when H+ is added, the buffer base (A–) is consumed, but an equivalent amount of acid (HA) is generated. Conversely, with addition of a base, the decrease in acid component of the buffer system is balanced by a corresponding increase in the base component.
Major Body Buffers
Cellular metabolism predominantly generates acids. Body buffers avert imminent pH change by these acids. Thus, buffers are first-line of defense against acid load. The important ones are bicarbonate buffer, phosphate buffer, proteins, etc.
Bicarbonate Buffer
It is the principal extracellular buffer, comprising carbonic acid (the proton donor) and bicarbonate (the proton acceptor). It functions in the same way as other conjugate acid-base pairs. However, there are important differences:
1. The base constituent, bicarbonate (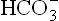 ) is regulated by kidneys.
) is regulated by kidneys.
2. The acid component (H2CO3) is regulated by pulmonary ventilation.
Thus, bicarbonate buffer is subject to regulation by kidneys and lungs. It is part of a three-tier defense, described later, that also includes kidney and lungs.
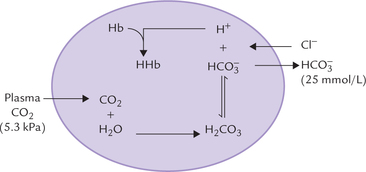
The acid component of this buffer (also called respiratory component) is generated from dissolved carbon dioxide [CO2(d)] and water, by the reaction shown below. The reaction is catalyzed by the enzyme carbonic anhydrase.
The dissolved carbon dioxide in blood circulation is in equilibrium with the gaseous carbon dioxide [CO2(g)] in the air space of the lungs. As a result, concentration of carbonic acid is ultimately dependent on the partial pressure of carbon dioxide in the gas phase. Carbonic acid can dissociate to yield bicarbonate.
Thus, reversible equilibria exist between the gaseous carbon dioxide in the lungs and the bicarbonate ions in blood plasma, as shown below:
Action of the bicarbonate buffer involves these equilibria. For instance, when an acid is added to blood, concentration of H+ rises. The latter is taken up by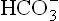 resulting in the rise of concentration of carbonic acid (Step 1).
resulting in the rise of concentration of carbonic acid (Step 1).
This causes the Step 2 to go forward, and the concentration of carbon dioxide (d) in the blood rises. This in turn results in an increase in the pressure of carbon dioxide in the gas phase in the lungs (Step 3), and the extra carbon dioxide is exhaled through increased rate of breathing.
Reverse series of reactions occur when an alkali (OH–) is added. It is taken up by carbonic acid to form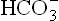 .
.
Concentration of carbonic acid falls momentarily, but is quickly replenished from large pool of gaseous carbon dioxide. Rate of breathing decreases under these circumstances so that the carbon dioxide is retained and dissolved in water to form carbonic acid.
Bicarbonate buffer is highly effective: Bicarbonate buffer system is an effective physiological buffer because of its equilibration with a large reserve of gaseous carbon dioxide in the air space of the lungs. Since pK' of carbonic acid is 6.1, the bicarbonate buffer should be most effective at or around pH of 6.1 (i.e. 6.1 ± 1) as a buffer is most effective when pH equals pK’. However, bicarbonate buffer is highly effective at the physiological pH of 7.4 also because of its equilibration with gaseous carbon dioxide.
Carbonic anhydrase is the principle enzyme that catalyzes generation of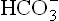 . Decreased activity of this enzyme, therefore, results in decreased plasma bicarbonate concentration. Consequently, the ratio of bicarbonate to carbonic acid (normally 20) tends to fall, resulting in a fall of pH (see Henderson-Hasselbalch equation). A state of primary bicarbonate deficit is therefore associated with the fall in physiological pH.
. Decreased activity of this enzyme, therefore, results in decreased plasma bicarbonate concentration. Consequently, the ratio of bicarbonate to carbonic acid (normally 20) tends to fall, resulting in a fall of pH (see Henderson-Hasselbalch equation). A state of primary bicarbonate deficit is therefore associated with the fall in physiological pH.
Phosphate Buffer
It is the major intracellular buffer. Its pK' value of 6.86 is near the intracellular pH of 7.0. Therefore, this buffer is very effective intracellularly. It consists of the following components:
Proteins
They may act as buffers because many of them have amino acids which behave like weak acids. For example, histidine acts as a buffer because of presence of imidaz-ole group (Chapter 4). It has a pK' value of 6.0 which is close to the physiological pH. Therefore, it is very effective in living systems.
Most of the buffering capacity of blood is due to haemoglobin (Hb), which is present within RBCs. Hb is more effective than plasma proteins because of (a) its high content of histidine, and (b) its higher concentration (12-16 g/dL). As a result, buffering capacity of Hb is much higher than that of the plasma proteins.
The intracellular fluid (ICF) proteins serve as major buffers. Since ICF volume is around 60% of the total body fluid volume, proteins may be considered as most abundant buffers in the body. However, the interstitial fluid, which forms more than 70% of the extracellular fluid (ECF) lacks proteins.
The acetate buffer is ineffective in the body since its pK' is 4.76, which is far removed from the physiological pH. Similarly ammonium buffer with pK' of 9.25 is not effective at the physiological pH.
III Acid-Base Balance: Applied Aspects
A Overview
Various body fluids have nearly fixed pH values (Table 1.2), which lie within definite range. For example, pH of blood is 7.4 and it remains within a range of 7.357.45. Intracellular pH is taken to be around 7.0, although its value varies among different organelles. Deviations from normal values disrupt structural and functional integrity of body proteins, including enzymes. Properties of nucleic acids, cell membranes and other cellular constituents are also adversely affected by alteration of pH. These changes may have hazardous effects on the body, and are often incompatible with life. Therefore, it is important that the regulation of pH is given utmost priority. Failure of pH regulation occurs in a number of disorders, collectively known as acid-base disorders.
B The Three-tier Defense
Normal body metabolism poses a constant threat to pH because it generates various products that can alter the blood pH. For example, carbon dioxide is the major acidic end product of normal metabolism that tends to lower the pH. In addition, certain abnormal metabolic states and disorders are also capable of causing alteration in pH: the pH may fall (i.e. acidosis) or rise (i.e. alkalosis). A three-tier defense system, comprising buffers, lungs, and kidneys therefore, remains constantly in operation to guard against any changes in pH. Various components may act at different times, but their overall effect is well synchronized.
(a) Buffers serve as the first-line of defense against acid load and, as mentioned earlier, bicarbonate buffer is most important for being regulated by pulmonary ventilation and renal adjustments.
(b) Pulmonary ventilation takes from a few minutes to few hours to become operational. It has a direct bearing on acid-base balance of the body because carbon dioxide is an acidic substance. Since carbon dioxide is exhaled during expiration, increase in respiratory activity reduces the acidity of body fluids.
The pulmonary ventilation system operates by negative feedback. For instance, fall in pH of ECF stimulates peripheral chemoreceptors and central chemoreceptors. The reflex response to these stimulations is an increase in the rate and depth of respiration. This removes the excessive carbon dioxide, thereby leading to a compensatory rise in pH. Thus, the decreased pH is brought back to normal by hyperventilation. Conversely, increased pH is compensated by hypoventilation.
(c) Renal adjustments take from several hours to few days to become effective, but provide a long-term solution by supplementing buffer action. For instance, the non-volatile acids (HA) produced endogenously are initially neutralized by the bicarbonate buffer.
(The anions of these acids (A–) pass on to Na+, which is the predominant extracellular cation.)
Though this reaction averts immediate risk of a pH fall, it is accompanied by another imbalance, i.e. fall of bicarbonate ion concentration. The latter must be replenished in order to preserve the buffering capacity. Renal adjustments accomplish this by generating bicarbonate ions (see Fig. 1.7 ). Under normal circumstances, kidneys excrete about 50 mmol of H+ in urine and simultaneously generate about 50 mmol of bicarbonate ions. However, in acidosis, kidneys can eliminate as much as 500 mmol of H+ per day; in alkalosis excretion of H+ falls markedly.
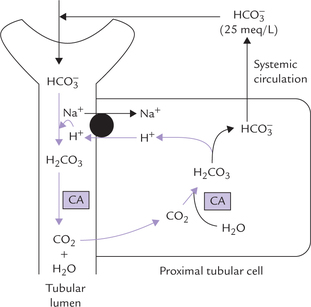
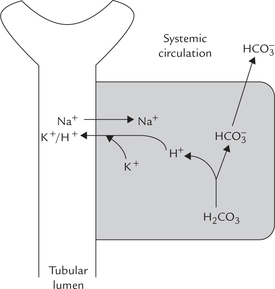
Fig. 1.7 Reclamation of bicarbonate by proximal tubular cell (CA = carbonic anhydrase, • = Na+ — K+ antiport).
Ability of kidneys to excrete variable amounts of acids and to generate variable amounts of bicarbonate ions depending on body requirements, makes them act as final defense mechanism against any imminent pH alterations.
C Mechanisms for Control of Bicarbonate
Renal tubular cells and erythrocytes possess a specific system, called carbonate dehydratase system, which enables them to use carbon dioxide for generating bicarbonate. Under normal circumstances, the erythrocyte mechanism merely makes fine adjustments in the plasma bicarbonate concentration in response to specific body requirement while the kidneys play a major role in maintaining the bicarbonate concentration.
Carbonate Dehydratase System
The principle enzyme of this system is carbonic anhydrase, which catalyzes the first reaction of the following chain:
Cells remove one of the final products of the chain, i.e. H+. Consequently, both the reactions are pulled towards right to generate bicarbonate ions. For each H+ removed from the cell, one bicarbonate ion is generated. Rise in intracellular concentration of H+ and carbon dioxide accelerates these reactions. A fall in plasma concentration of bicarbonate also enhances the reaction sequence.
In a normal human being, at a plasma pCO2 of 5.3 kPa (equivalent to CO2 concentration of about 1.2 mmol/L), erythrocytes and renal tubular cells maintain the extracellular bicarbonate concentration at about 25 mmol/L. Thus, value of the extracellular [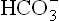 /[CO2] ratio (both in mmol/L) is 20 : 1. Taking pK' value of carbonic acid to be 6.1, it can be calculated from the Henderson-Hasselbalch equation that this ratio represents a pH of about 7.4.
/[CO2] ratio (both in mmol/L) is 20 : 1. Taking pK' value of carbonic acid to be 6.1, it can be calculated from the Henderson-Hasselbalch equation that this ratio represents a pH of about 7.4.
In practice, the partial pressure of carbon dioxide (pCO2) is measured and its concentration in plasma is obtained by multiplying the pCO2 with the solution constant for carbon dioxide.
where 0.23 is the solution constant. The equation can be rewritten as:
Role of Kidneys in Bicarbonate Homeostasis
Kidneys play an important role in bicarbonate homeo-stasis through the following mechanisms:
1. Reabsorption of the filtered bicarbonate, i.e. bicarbonate reclamation.
2. Generation of bicarbonate ions, termed new bicarbonate generation.
Both these actions depend on the carbonate dehydra-tase system. As indicated earlier, this system is operative in renal tubular cells and possesses considerable reserve capacity.
Bicarbonate Reclamation
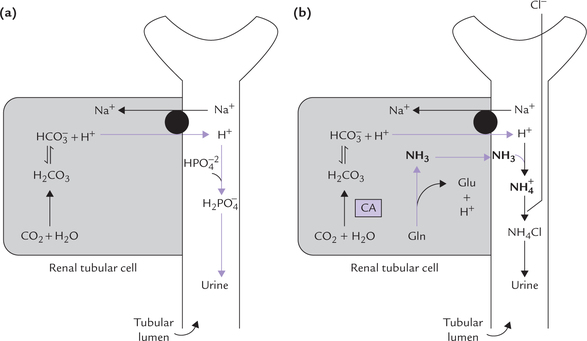
The unmodified glomerular filtrate contains bicarbonate ions in the same concentration as plasma (i.e. 25 mmol/L). About 4000 mmol of ions are filtered by glomeruli each day. If all the filtered bicarbonate were lost in urine, severe bicarbonate deficit would soon develop. However, specific mechanisms exist in proximal tubular cells which permit conservation (reclamation) of all the filtered bicarbonate. The most important of these mechanisms is the Na+-H+ antiport system. This system operates in the renal tubular cells.
Na+ – H+ antiport system: Bicarbonate ions (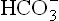 ) and H+ are produced by the carbonate dehydratase system, as shown in Figure 1.7. The H+ ions are transported into the tubular lumen in exchange for Na+ present in tubular fluid. The exchange occurs through mediation of the Na+ – H+ antiport. Within the tubular lumen, the H+ reacts with the filtered bicarbonate to form carbon dioxide and water. This reaction needs carbonic anhydrase, located in the brush border of the proximal tubular cells.
) and H+ are produced by the carbonate dehydratase system, as shown in Figure 1.7. The H+ ions are transported into the tubular lumen in exchange for Na+ present in tubular fluid. The exchange occurs through mediation of the Na+ – H+ antiport. Within the tubular lumen, the H+ reacts with the filtered bicarbonate to form carbon dioxide and water. This reaction needs carbonic anhydrase, located in the brush border of the proximal tubular cells.
The luminal membrane of the tubular cell allows the carbon dioxide to diffuse into the cell. At the same time, the bicarbonate, the other product of carbonate dehydra-tase system, moves across the basolateral membrane of the renal tubular cell to enter systemic circulation. Movement of bicarbonate in this direction is favoured by (a) increased intracellular concentration of bicarbonate, (b) electrical potential, and (c) impermeability of the luminal membrane to bicarbonate.
Through this process of reclamation the loss of the filtered bicarbonate ions in urine is prevented. These ions are reabsorbed, though indirectly, in the form of carbon dioxide; direct reabsorption is not possible since the luminal membrane is impermeable to bicarbonate ions. Further, this mechanism permits excretion of H+ and prevents loss of Na+ in urine.
Control: The carbonate dehydratase mechanism may be stimulated by a rise in pCO2 or a fall of bicarbonate concentration within the tubular cells.
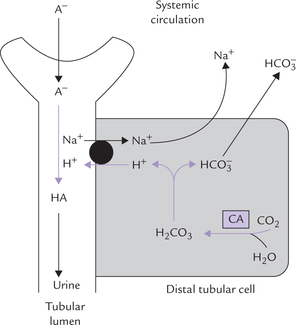
New Bicarbonate Generation
This mechanism operates in the later pat of distal convoluted tubules (DCT) and the collecting ducts. In these segments, H+ secretion is associated with new bicarbonate generation (Fig. 1.8 ). The carbonate dehydratase system generates equimolar amounts of H+ and bicarbonate. The hydrogen ions are secreted into the tubular lumen and combine with A– (A– represents a filtered anion of an acid, e.g. lactate, acetoacetate or sulphate). HA thus formed is eliminated in urine.
The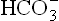 ions, the other product of the carbonate dehydratase system, is absorbed into systemic circulation together with Na+. Thus, this mechanism serves to increase alkali reserve of the body.
ions, the other product of the carbonate dehydratase system, is absorbed into systemic circulation together with Na+. Thus, this mechanism serves to increase alkali reserve of the body.
Urinary Buffers
Hydrogen ion excretion requires presence of suitable buffer systems in urine. The H+ secreted into the tubular lumen causes acidification of urine. The minimum pH of urine is about 4.5 which is equivalent to 0.03 mEq/L of the H+. At this H+ concentration, sufficiently high concentration gradient is built up across the luminal cell membrane which prevents further secretion of H+. Increase in the excretion of H+ occurs only when the secreted H+ is taken up by a luminal buffer system. The buffer takes up the secreted H+, thus preventing the building of the concentration gradient. In normal urine, the most important buffer is phosphate buffer (Fig. 1.9a ). Ammonia, produced in renal tubular cells, also acts as urinary buffer (Fig. 1.9b). Together, they permit excretion of 30-40 mmol of hydrogen ions every 24 hours.
Phosphate is present in the glomerular filtrate; about 4/5th of it is in the form of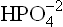 . This combines with the secreted H+ and is converted to the monovalent anion,
. This combines with the secreted H+ and is converted to the monovalent anion,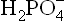 :
:
Thus,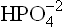 and
and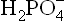 , form a conjugate acid-base (i.e. buffer) pair.
, form a conjugate acid-base (i.e. buffer) pair.
where 6.8 is pK' of this system.
As more H+ is secreted, more and more of the divalent form is converted to monovalent form until, at pH below 5.5, most of this is in monovalent form.
Ammonia, the other important urinary buffer, is produced by deamination of glutamine in renal tubular cell (Fig. 1.9b).
Glutaminase, present in tubular cells is responsible for this conversion. Its activity is induced in chronic acido-sis, thus permitting increased ammonia production, which can take up H+ in tubular lumen. Ammonia and ammonium form a buffer pair
where 9.8 is pK' of this system.
This buffer system allows increased hydrogen ion excretion via ammonium ions. Ammonia can readily diffuse across cell membranes, but ammonium ions cannot do so and are, therefore, eliminated in urine. As the urine becomes more acidic, it contains increasing amount of ammonium ions. The ammonia-ammonium buffer pair becomes active only after the buffering power of phosphate is exhausted.
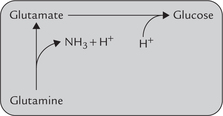
Ammonia buffer produces an H+ intracellularly during the conversion of glutamine to glutamate (Fig. 1.10 ). This apparently negates the effect of ammonia buffering system since an H+ is generated in the cell for each H+ that is buffered in the tubular lumen. Glutamate enters gluconeogenesis, a process requiring input of H+. The H+ generated earlier is probably incorporated in glucose during gluconeogenesis.
Role of Erythrocytes
Erythrocytes are also capable of generating bicarbonate ions, as shown in Figure 1.11 .
Since erythrocytes lack aerobic pathways, they are incapable of generating substantial amount of carbon dioxide. The plasma carbon dioxide diffuses into the cell along a concentration gradient, where it generates bicarbonate ions through the carbonate dehydratase system. The other product, H+ is buffered by haemoglobin. As the concentration of bicarbonate ions rises intracellularly, it diffuses into the extracelllular fluid along a concentration gradient. To maintain electroneutrality, diffusion of chloride ions occurs in the opposite direction, i.e. chloride shift.
D The Acid-Base Disorders
A number of acid-base disorders are known which are classified on the basis of changes in the components of bicarbonate-carbonic acid buffer. The three components (pH,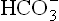 and H2CO3 are related as per the Henderson-Hasselbalch equation.
and H2CO3 are related as per the Henderson-Hasselbalch equation.
Base = bicarbonate ions; Acid = carbonic acid.
Considering the fact that plasma H2CO3 equilibrates with the dissolved as well as the gaseous carbon dioxide, the equation can be rewritten as:
where pCO2 is the partial pressure of CO2 and 0.23 is the constant. (Concentrations of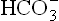 and CO2 are measured in mmol/L, and pCO2 is expressed in kPa.)
and CO2 are measured in mmol/L, and pCO2 is expressed in kPa.)
From this equation, it may be observed that decreased pH value (i.e. acidosis) can result mainly due to two reasons: (a) primary decrease in bicarbonate ion (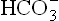 ) concentration, and (b) primary increase in pCO2. The primary decrease of bicarbonate ions [
) concentration, and (b) primary increase in pCO2. The primary decrease of bicarbonate ions [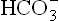 ], is called metabolic acidosis and primary increase of pCO2 is called respiratory acidosis. Conversely, increased pH, i.e. alkalosis may result either due to primary increase in
], is called metabolic acidosis and primary increase of pCO2 is called respiratory acidosis. Conversely, increased pH, i.e. alkalosis may result either due to primary increase in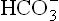 concentration (metabolic alkalosis), or to primary decrease of pCO2 (i.e. respiratory alkalosis).
concentration (metabolic alkalosis), or to primary decrease of pCO2 (i.e. respiratory alkalosis).
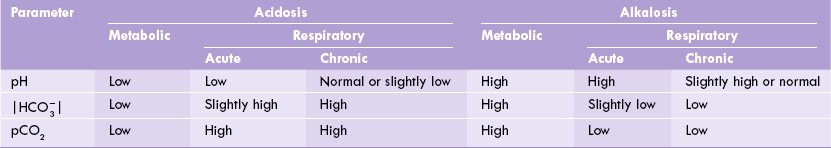
Acidosis
Metabolic Acidosis
A disorder resulting from a primary fall in bicarbonate level (<24 mmol/L) resulting in a decreased pH of arterial plasma (because of decreased ratio for bicarbonate concentration to pCO2 in the Henderson-Hasselbalch equation) is metabolic acidosis
Causes:
Some common causes of bicarbonate depletion are given below:
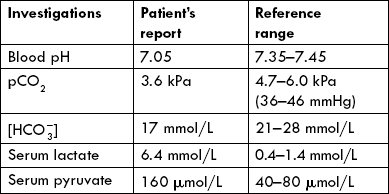
1. Excessive production of organic acids: When an acid metabolite (HA) enters blood, it is taken up by the base component of the bicarbonate buffer (i.e.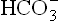 ). Consequently, the concentration of these ions falls, giving rise to a bicarbonate deficit, therefore metabolic acidosis occurs. A mild tendency of acidosis produced in this manner is almost a normal occurrence. This is because several body reactions continually produce non-volatile acids. Some examples include oxidation of the -SH group of cysteine to sulphate and hydrolysis of phosphates. However, these reactions do not cause any serious disorder in the body because the acids are neutralized by bicarbonate buffer. The mild bicarbonate deficit so produced is promptly reverted by the renal and the pulmonary mechanisms. In other words, the amount of acid generated is too small to override body’s defenses. On the other hand, certain processes release acids into the blood in very large quantities which cannot be taken care of by body’s defenses. As a result, severe bicarbonate deficit leading to a fall in pH occurs (Case 1.1).
). Consequently, the concentration of these ions falls, giving rise to a bicarbonate deficit, therefore metabolic acidosis occurs. A mild tendency of acidosis produced in this manner is almost a normal occurrence. This is because several body reactions continually produce non-volatile acids. Some examples include oxidation of the -SH group of cysteine to sulphate and hydrolysis of phosphates. However, these reactions do not cause any serious disorder in the body because the acids are neutralized by bicarbonate buffer. The mild bicarbonate deficit so produced is promptly reverted by the renal and the pulmonary mechanisms. In other words, the amount of acid generated is too small to override body’s defenses. On the other hand, certain processes release acids into the blood in very large quantities which cannot be taken care of by body’s defenses. As a result, severe bicarbonate deficit leading to a fall in pH occurs (Case 1.1).
Most common processes that release acids are lactic acidosis and ketoacidosis.
Lactic acidosis arises due to generation of excessive lactic acid from anaerobic glycolysis, e.g. during intense muscular exercise.
Ketoacidosis results due to excessive production of ketone bodies (acetone, acetoacetate and β-hydroxy-butyrate) in liver under certain abnormal metabolic states such as starvation and uncontrolled diabetes mellitus.
In addition to acute acidosis causing conditions mentioned above, certain conditions lead to the development of chronic acidosis. These are: phenylketonuria, hyperammonaemias or maple syrup urine disease. But in these disorders acidosis is less important than other metabolic consequences.
2. Excessive loss of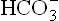 from the body: Duodenal fluid, with a bicarbonate concentration about twice that of plasma, is alkaline. Loss of this fluid may occur through intestinal fistula. If the rate of loss exceeds that of the renal ability to regenerate bicarbonate ions, the plasma bicarbonate may fall enough to cause acidosis. For the same reason, excessive loss of bicarbonate ions in severe diarrhoea causes acidosis.
from the body: Duodenal fluid, with a bicarbonate concentration about twice that of plasma, is alkaline. Loss of this fluid may occur through intestinal fistula. If the rate of loss exceeds that of the renal ability to regenerate bicarbonate ions, the plasma bicarbonate may fall enough to cause acidosis. For the same reason, excessive loss of bicarbonate ions in severe diarrhoea causes acidosis.
3. Generalized renal tubular dysfunction: It may cause loss of a mixture of ions in urine. Renal bicarbonate reclamation and generation are also impaired due to damaged Na+-H+ antiport.
4. Poisoning or overdose: Production of acid metabolites in poisoning and overdoses also lead to metabolic acidosis. For example, in methanol poisoning, formate is produced; and in ethylene glycol poisoning, production of oxalate occurs.
5. Increase in chloride ion: In the acidosis-causing conditions discussed so far, no change in the concentration of chloride ion occurs. However, in some conditions, such as renal tubular acidosis and acetazolamide therapy, fall of plasma bicarbonate concentration is accompanied by a simultaneous increase in chloride ion concentration. In short, bicarbonate is substituted by chloride and a state of hyperchloraemic acidosis results.
Compensation of metabolic acidosis:
In metabolic acidosis, the primary bicarbonate deficit results in the fall of bicarbonate to pCO2 ratio. Thus, the compensatory mechanisms are aimed at elevation of this ratio (a normal ratio of 20 is crucial for maintaining the blood pH at 7.4). This is accomplished by compensatory hyperventi-lation which washes off carbon dioxide to decrease pCO2. Consequently, the above-stated ratio is elevated.
Usually, the metabolic acidosis is because of excessive acid (HA) production. Acceleration of the following reaction occurs under such circumstances:
The H2CO3 so produced dissociates to water and carbon dioxide. Though carbon dioxide increases temporarily in this manner, it induces hyperventilation which washes off the CO2. Consequently, the pCO2 (and hence [CO2]) tends to fall, which reverts the ratio of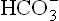 : pCO2 to normal. In this manner, hyperventilation serves as the major compensatory process in metabolic acidosis. But the pulmonary compensation is said to be incomplete because (a) concentration of
: pCO2 to normal. In this manner, hyperventilation serves as the major compensatory process in metabolic acidosis. But the pulmonary compensation is said to be incomplete because (a) concentration of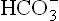 gets reduced because there is no new generation of
gets reduced because there is no new generation of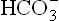 consumed earlier, and (b) the anion of the acid metabolite (A–) is still present in extracellular fluid.
consumed earlier, and (b) the anion of the acid metabolite (A–) is still present in extracellular fluid.
These abnormalities must be corrected for complete restoration of the acid-base status. The bicarbonate concentration is self-correcting: decreased bicarbonate concentration causes stimulation of renal carbonate dehydratase, which in turn enhances bicarbonate reclamation and bicarbonate generation. Consequently, the depleted bicarbonate concentration slowly rises towards normal. The anion of the acid metabolite (A–) is eliminated by renal excretion. In some cases, the acid metabolite (e.g. lactate) is slowly metabolized. With these processes, the compensation is complete and acid-base status returns to normal.
Plasma findings in metabolic acidosis:
1. Bicarbonate concentration is always low.
2. pCO2 is usually low (compensatory change).
3. pH is low in uncompensated (or partially compensated) cases and normal in fully compensated cases.
4. Concentration of chloride ions is normal in most cases. However, raised value of this ion is seen in renal tubular acidosis or acetazolamide therapy.
Anion gap:
Cause of metabolic acidosis is usually apparent from clinical history of the patient. Knowledge of anion gap serves as an additional tool in delineating the cause. Anion gap is estimated by measuring the difference between the sums of the concentrations of principal cations (Na+ and K+) and principal anions (Cl– and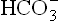 ).
).
Average reference values of these ions are: Na+, 140; K+, 4; Cl–, 100; and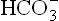 , 25 (all values are in mmol/L). Therefore, in healthy individuals the anion gap has an average value of 19 mmol/L.
, 25 (all values are in mmol/L). Therefore, in healthy individuals the anion gap has an average value of 19 mmol/L.
The anion gap refers to the discrepancy between the measured electrolytes, but in reality no such gap exists. It merely represents unmeasured net negative charge on plasma proteins. In short, the negatively charged amino acid side chains on the proteins account for the apparent discrepancy between the measured electrolytes.
In metabolic acidosis, deviations from the above-stated normal values occur. The anion gap can increase or remain normal depending on the underlying cause of metabolic acidosis.
Increased anion gap: When excessive production of acids is the cause of metabolic acidosis, concentration of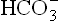 decreases but that of Cl– remains unaffected. Consequently, anion gap is increased.
decreases but that of Cl– remains unaffected. Consequently, anion gap is increased.
Normal anion gap: In renal tubular acidosis or acet-azolamide therapy, fall of bicarbonate is accompanied by increase in chloride ion concentration. Hence, anion gap does not change in these conditions, which are, therefore, called normal anion gap acidosis or hyperchlorae-mic acidosis.
Respiratory Acidosis
It results from a decrease in alveolar ventilation, causing decreased elimination of CO2 by the lungs. The consequent increase in pCO2 is the primary event in the acid-base disturbance.
Decreased alveolar ventilation, may result in a number of conditions (Table 1.5 ).
Table 1.5
Causes of respiratory acidosis
| Airway obstruction | Respiratory centre depression |
| Asthma | Sedatives |
| Chronic obstructive airway disease | General anaesthesia |
| Pulmonary diseases | Neuromuscular diseases |
| Respiratory distress syndrome | Tetanus |
| Severe pneumonia | Neurotoxins |
| Thoracic diseases | |
| Kypnoscoliosis | |
| Flail chest |
Compensation of respiratory acidosis:
In respiratory acidosis, the ratio of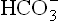 : pCO2 is reduced, and so the role of compensatory mechanisms is primarily to retain more of bicarbonate ions. In contrast to metabolic acidosis, where respiratory compensation is important, in this type of acidosis the primary defect being in lungs, the physiological response of lungs cannot be expected to play any significant role. Renal response plays an important role in bringing the acid-base status back to normal. Acceleration of the carbonate dehydratase mechanism in erythrocytes is also important for the short-term compensation.
: pCO2 is reduced, and so the role of compensatory mechanisms is primarily to retain more of bicarbonate ions. In contrast to metabolic acidosis, where respiratory compensation is important, in this type of acidosis the primary defect being in lungs, the physiological response of lungs cannot be expected to play any significant role. Renal response plays an important role in bringing the acid-base status back to normal. Acceleration of the carbonate dehydratase mechanism in erythrocytes is also important for the short-term compensation.
Renal response:
In respiratory acidosis, the renal mechanism compensates for the disturbance by generating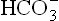 ions and by eliminating H+ (Figs 1.7 and 1.8). The carbonate dehydratase mechanism in renal tubular cells is accelerated by increased pCO2. As a result, most (or all) of the filtered
ions and by eliminating H+ (Figs 1.7 and 1.8). The carbonate dehydratase mechanism in renal tubular cells is accelerated by increased pCO2. As a result, most (or all) of the filtered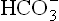 is reabsorbed and more of new
is reabsorbed and more of new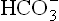 is generated. The urine becomes acidic as more of H+ is secreted. Renal response develops slowly. In cases of chronic carbon dioxide retention renal compensation is maximum.
is generated. The urine becomes acidic as more of H+ is secreted. Renal response develops slowly. In cases of chronic carbon dioxide retention renal compensation is maximum.
The H+ secreted during respiratory acidosis is exchanged with the luminal Na+, thus preventing urinary loss of the latter.
Plasma findings in respiratory acidosis:
The biochemical changes in acute and chronic respiratory acidosis differ from each other (Table 1.6 ). This is because of the fact that in acute cases there is insufficient time for the renal mechanisms to generate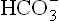 and add them into plasma. Slight rise in plasma [
and add them into plasma. Slight rise in plasma [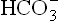 ] that occurs in acute stages is mostly derived from erythrocytes. This mild degree of compensation is inadequate to prevent fall in pH. In chronic respiratory acidosis, on the other hand, the renal mechanisms are sufficiently speeded up to elevate the levels of
] that occurs in acute stages is mostly derived from erythrocytes. This mild degree of compensation is inadequate to prevent fall in pH. In chronic respiratory acidosis, on the other hand, the renal mechanisms are sufficiently speeded up to elevate the levels of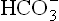 to such extent that pH fall is averted.
to such extent that pH fall is averted.
Alkalosis
The body’s defenses against alkalosis are less effective than those against acidosis. This is because the biological systems have little capacity to buffer the OH– ions, and the bicarbonate buffer is far less effective when the pH rises above 7.4. Fortunately, alkalosis is far less common than acidosis because the body metabolites are acidic, not alkaline.
Metabolic Alkalosis
It is characterized by an increased serum bicarbonate concentration as a result of either an excessive loss of acid or abnormal retention of bicarbonate. In accord with the Henderson-Hasselbalch equation, the primary rise in bicarbonate results in increase in pH.
Causes:
Some of the major causes of metabolic alkalosis are:
1. Excessive renal generation of bicarbonate: In distal part of the renal tubules (distal convoluted tubules), K+ and H+ ions compete with each other for secretion into the tubular lumen in exchange for Na+ (Fig. 1.12 ). Because of this reciprocal relation, changes in potassium-balance influence the H+ balance. If the plasma potassium level is low (i.e. hypokalaemia), less of this ion is available intracellularly for secretion. Hence, more of H+ ions are secreted and the urine becomes acidic. However, H+ secretion is accompanied by equimolar generation of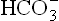 . The latter passes into ECF to raise the
. The latter passes into ECF to raise the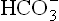 concentration, and therefore, the extracellular pH rises. This type of alkalosis is termed hypokalaemic alkalosis.
concentration, and therefore, the extracellular pH rises. This type of alkalosis is termed hypokalaemic alkalosis.
2. Loop diuretics: These diuretics inhibit the pumping of sodium in the loop of Henle and, therefore, increase sodium load on DCT and collecting ducts. This results in increased Na+ - K+ exchange at these sites resulting in excessive K+ loss and hence hypokalaemia. Thus, in this case alkalosis is a consequence of hypokalaemia.
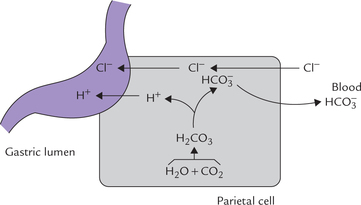
3. Pyloric stenosis: In this condition, excessive loss of H+ and Cl– occurs in vomitus. This necessitates increased secretion of H+ into the gastric lumen by the parietal cells. As shown in Figure 1.13 secretion of H+ and Cl– is accompanied by the generation of equivalent amount of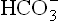 by these cells. The
by these cells. The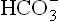 ions, thus generated, move into the extracellular fluid resulting in elevated pH.
ions, thus generated, move into the extracellular fluid resulting in elevated pH.
The narrowing at pylorus has further hazardous consequences: (a) it prevents loss of alkaline duodenal contents, which could have served as a safeguard against alkalosis, and (b) it causes volume depletion (hypovolumia), which is a known cause of alkalosis, as noted below.
4. Hypovolumia: It triggers a series of events that ultimately result in increased secretion of aldosterone from adrenal cortex. Since aldosterone causes K+ elimination through urine, hypokalaemia results. Hypokalaemia in turn leads to alkalosis.
5. Alkali ingestion: Massive quantities of ingested alkali cause alkalosis. Inappropriate treatment of acidotic conditions and chronic alkali ingestion can lead to this state.
Compensation of metabolic alkalosis:
The compensatory mechanism of metabolic alkalosis must bring down the ratio of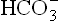 : pCO2. This occurs by respiratory response which involved depression of the respiratory centre by elevated plasma
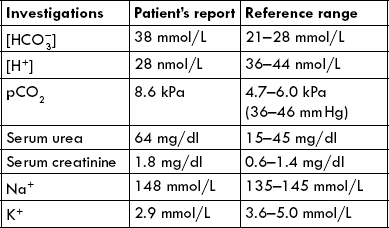
: pCO2. This occurs by respiratory response which involved depression of the respiratory centre by elevated plasma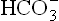 concentration. It leads to under-breathing; and thereby tends to build up the pCO2 (Case 1.2). This depresses above-mentioned ratio, which in turn leads to decrease of the elevated pH towards normal. However, this response has limited capacity because underbreathing leads to oxygen deficit. Moreover, the alkaline state is not capable of sufficiently depressing the respiratory centre.
concentration. It leads to under-breathing; and thereby tends to build up the pCO2 (Case 1.2). This depresses above-mentioned ratio, which in turn leads to decrease of the elevated pH towards normal. However, this response has limited capacity because underbreathing leads to oxygen deficit. Moreover, the alkaline state is not capable of sufficiently depressing the respiratory centre.
Respiratory Alkalosis
The primary abnormality in respiratory alkalosis is the decrease of arterial pCO2 and, therefore, rise of pH.
Causes:
Fall in the arterial pCO2 is due to hyperventila-tion resulting from tissue hypoxia, high altitude, anaemia or bacterial sepsis. Hysterical overbreathing, assisted breathing, or certain nervous system disorders (encephalitis, head injury) may also lower pCO2 and hence cause alkalosis.
The compensation is aimed at lowering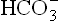 concentration, which will revert the
concentration, which will revert the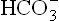 to pCO2 ratio (thereby causing correction of pH). The compensatory mechanism is triggered by decreased pCO2 which slows down the carbonate dehydratase mechanism in renal tubular cells and RBCs. Consequently, decrease in the
to pCO2 ratio (thereby causing correction of pH). The compensatory mechanism is triggered by decreased pCO2 which slows down the carbonate dehydratase mechanism in renal tubular cells and RBCs. Consequently, decrease in the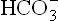 reclamation and generation occurs. The compensation is slow to develop, like in the case of respiratory acidosis. If a steady low pCO2 is maintained, maximal compensation develops within 36-72 hours.
reclamation and generation occurs. The compensation is slow to develop, like in the case of respiratory acidosis. If a steady low pCO2 is maintained, maximal compensation develops within 36-72 hours.
The biochemical findings in arterial blood in various types of acidosis and alkalosis are summarized in Table 1.6.
Exercises
Essay type questions
1. What is role of carbonic anhydrase system and renal mechanisms in the maintenance of blood pH?
2. Describe the various mechanisms by which are responsible for maintenance of pH of the body fluids.
3. Classify the acid-base disorders. Discuss their causes and characteristic biochemical parameters.
4. Mention the biochemical determinants of metabolic and respiratory acidosis. Why is bicarbonate buffer system especially important as a blood buffer?
5. Describe how excess acid (H+) is excreted chiefly in urine, highlighting role of urinary buffers.
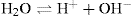
 Water molecule forms a dipole because oxygen atom has high electron density (hence having partial negative charge) and hydrogen atoms are relatively electron deficient (hence having partial positive charges).
Water molecule forms a dipole because oxygen atom has high electron density (hence having partial negative charge) and hydrogen atoms are relatively electron deficient (hence having partial positive charges).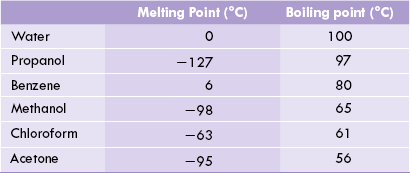
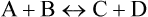
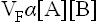
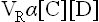
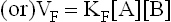
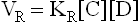
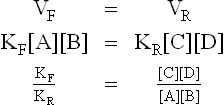
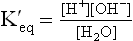
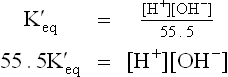
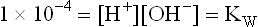
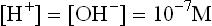
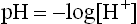
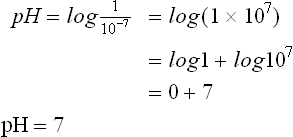
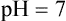
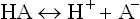

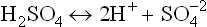
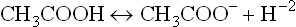
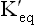 and pK' values of a strong acid and a weak acid
and pK' values of a strong acid and a weak acid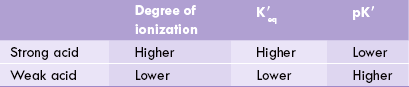
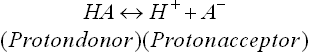
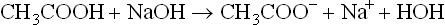
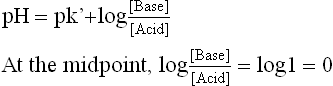
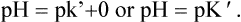
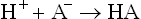
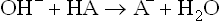
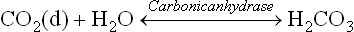
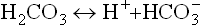
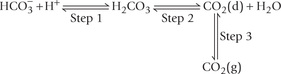
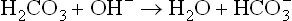
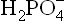 as the proton donor (i.e. the acid component).
as the proton donor (i.e. the acid component).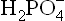 as the proton acceptor (i.e. the base component).
as the proton acceptor (i.e. the base component).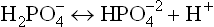
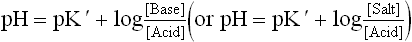
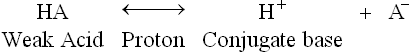
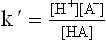
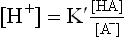
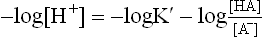
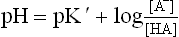
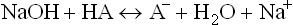
 occurs, causing changes in pH of the medium, as can be seen from the equation.
occurs, causing changes in pH of the medium, as can be seen from the equation.