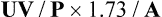Tests For Thyroid, Adrenal And Kidney Functions
Endocrine organs like thyroid, adrenals, kidneys, liver, and pancreas perform important biological roles. The diseases involving these organs cause derangement of their functions, and cause several alterations in the metabolic milieu. Assessment of the functional status of these organs requires, in addition to clinical assessment, a number of laboratory investigations. No single test suffices to assess the functional status of a given organ and generally a battery of tests has to be performed. Some of the common function tests are discussed in this chapter.
After going through this chapter the student should be able to:
I Thyroid Function Tests
A Assay of Hormones
Estimation of T4 (thyroxine) and T3 (triiodothyronine) by radioimmunoassay or ELISA is the mainstay of diagnosis of thyroid diseases. Both are decreased in hypothyroid-ism, but increased in hyperthyroidism. In general, changes in serum T3 are proportionally greater than the changes of T4 in most states of hypo- and hyperthyroidism.
In cases where the results of total serum T4 and T3 estimation are borderline or conflicting, free hormone levels are estimated. Recall that thyroid hormones in blood are in two forms: (a) protein bound, and (b) unbound or free form. Elevation or fall of free T3 and free T4 are true reflections of hyperthyroidism and hypothyroidism, because it is the free hormone that induces metabolic and biological effects in target cells.
B Radioactive Iodide Uptake (RAIU)
The RAIU assay is used to measure the ability of the thyroid gland to trap iodide. A small dose of radioactive iodine (5 µc of Na131I) is ingested by mouth and the radioactivity is counted at various lengths of time, usually 4-6 hours and again at 24 hours. Normally, 10-30% of the ingested dose can be detected within 24 hours. The uptake is increased in hyperthyroidism and decreased in hypothyroidism.
C Serum TSH
Estimation of serum TSH is useful for differentiating the thyroid disorders of hypothalamic or pituitary origin from those of thyroid origin. For example, in hypothyroidism of thyroid origin (primary hypothyroidism), TSH level is elevated because of lack of feedback effect, but in secondary hypothyroidism (of pituitary or hypothalamic origin) the TSH level is low.
Likewise, in primary hyperthyroidism, serum TSH is decreased due to negative feedback by T3 and T4’ whereas in hyperthyroidism of pituitary cause, high TSH is observed.
D Thyroid Hormone Binding Proteins
Levels of hormone-binding proteins, such as thyroxine-binding globulins (TBG), effect the plasma concentration of T4 and T3. Because most of the circulating T3 and T4 is bound to TBG, quantitative changes in this protein are clinically important (Table 34.1 ). The total amount of thyroid hormones transported in plasma will increase with increased TBG, or decrease with decreased TBG. Causes of abnormalities in TBG levels are summarized in Table 34.1.
Table 34.1
Causes of abnormalities in thyroxine-binding globulins (TBG)
| Quantitative | Qualitative |
| Increased TBG serum levels | Genetic |
| • Oral contraceptives | • Genetically determined increased binding-affinity |
| • Pregnancy | • Genetically determined decreased binding affinity |
| • Neonatal period | |
| • Genetic TBG excess | |
| • Oestrogen therapy | |
| Decreased TBG serum levels | Induced by drugs competing for TBG-binding sites |
| • Genetic deficiency (X-linked) | • Aspirin |
| • Anabolic agents | • Dicumarol |
| • Androgens | • Heparin |
| • Protein malnutrition | • Phenytoin |
| • Phenylbutazone |
In addition to absolute changes in TBG levels mentioned above (i.e. the quantitative changes), qualitative changes in this protein are also important. These changes refer to alterations of the hormone-binding affinity. Decreased affinity, for example, liberates thyroid hormones from TBG-binding sites, resulting in decreased total serum T4 and T3, and increased free T4 and free T3.
E TRH Stimulation Test
Administration of TRH causes increased release of TSH, and consequently of thyroid hormones, provided hypothalamo-pituitary-thyroid axis is normal. An abnormal response is obtained in the following conditions:
1. In hypothyroidism, higher-than-normal increments of serum TSH (after TRH stimulation) is observed. This is because of low feedback inhibition by deceased levels of circulating thyroid hormones.
2. In hyperthyroidism, feedback inhibition by elevated levels of thyroid hormones overrides the stimulating effect of TRH, resulting in a blunted TSH response.
3. When receptors for TRH on pituitary are defective, there is lower-than-normal response of serum TSH to TRH stimulation. In such cases, the TSH response (to TRH) is said to be blunted.
II Adrenal Function Tests
Glucocorticoids (primarily cortisol) are mainly involved in the regulation of carbohydrate, lipid and protein metabolism, and the mineralocorticoids (primarily aldosterone) are chiefly concerned with regulation of the extracellular fluid volume and electrolyte metabolism.
A Tests for Glucocorticoid Functions
Tests to Establish Diagnosis
Assessment of Diurnal Rhythm
Plasma cortisol levels are about 10 times higher in the early morning hours than at midnight. The normal range for plasma cortisol level is 8-26 µg/100 mL at 08.00 a.m. to less than 10 µg/100 mL at 12.00 a.m. Loss of this diurnal rhythmicity is an early indication of a lesion at any point in the hypothalamic-pituitary-adrenal axis. Stress such as trauma, pain, apprehension, fever and hypoglycaemia can also override this diurnal rhythm.
The hormone levels are most commonly estimated by radioimmunoassay (Box 34.1).
Estimation Urinary Free Cortisol
Another useful initial screening test is the estimation of urinary free cortisol in a 24-hour urine sample. The test should be performed on three different collection samples. Normal daily excretion of free cortisol in urine ranges from 10 µg to 100 µg. It rises in hyperadrenalism and falls in hypoadrenalism (Case 34.1).
Low Dose Dexamethasone Suppression Test
This test is recommended if results of urinary free cortisol tests are abnormal. Dexamethasone is a potent suppressor of pituitary ACTH secretion and cortisol level, causing about 50% fall in serum cortisol with a dose as low as 2 mg. The patient takes dexamethasone tablet at night and plasma cortisol is determined at 8.00 a.m. the following morning. A morning cortisol level less than 5 µg/100 mL usually excludes any cause of hypercortisolism. If the cortisol level is not suppressed, however, the next step is to identify the aetiology.
Tests to Establish Aetiology
Measurement of Plasma ACTH
Because cortisol and ACTH interact in a feedback loop, low levels of serum cortisol associated with high plasma ACTH level would indicate primary adrenocortical insufficiency. Likewise, increased plasma cortisol will be associated with suppression of ACTH in primary adrenal lesion.
In contrast, increased cortisol due to an ACTH-producing pituitary adenoma (Cushing’s disease) or due to ectopic production of ACTH will be associated with increased plasma ACTH levels.
High Dose Dexamethasone Suppression Test
The test is carried out with administration of 2 mg dexamethasone every 6 hours for 2 days. This dose suppresses urinary 17-OH steroids as well as plasma cortisol in Cushing’s disease. If these parameters are not suppressed, adrenal tumours producing high levels of cortisol or ectopic ACTH-producing tumours are usually the aetiology.
ACTH Stimulation Tests
These tests are useful in assessing adrenal reserve capacity and for documenting the existence of hormonal deficiency state. The test uses a synthetic form of ACTH (synacthen) consisting of the first 24 amino acids of ACTH, which is injected intravenously or intramuscularly. A short ACTH stimulation test (250 µg Synacthen administered intramuscularly or intravenously) is followed by a rise in serum cortisol within a few minutes, whereas a person with primary adrenal failure does not respond. Long ACTH stimulation test uses a lower daily dose of 1 µg administrated over several days. This dose is apparently more effective in stimulating adrenals than single administration of 250 µg which is considered supraphysiological. Thus, long ACTH test successfully assesses the chronic adrenal insufficiency.
Metyrapone Stimulation Test
This test is used to delineate cause of Cushing’s syndrome. Metyrapone is a potent inhibitor of the 11-hydroxylase enzyme, therefore oral administration of metyrapone blocks cortisol synthesis. This removes the negative feedback effect on pituitary. The excess of ACTH secretion continues to drive steroid biosynthesis, but with the pathway of cortisol synthesis blocked, the initially formed precursors (and progesterone) stop at 11-deoxycortisol (Fig. 32.8). Therefore, the serum levels of progesterone and these initial precursors rise following metapyrsone administration. Likewise, the urinary excretion of 17-hydroxycorticosteroids also increases.
In Cushing’s syndrome caused by a pituitary tumour, the ACTH response remains intact, and 11-deoxycortisol levels show marked rise (> 200 nmol/L). Levels of 11-deoxycortisol that are less than this are consistent with adrenal tumour or ectopic ACTH.
III Renal Function Tests
The renal function tests may be broadly divided into (a) tests that assess glomerular functions, and (b) tests that assess tubular functions. In addition, routine urine examination is still held as an important aspect of the overall assessment.
A Routine Urinalysis
It involves a careful physical examination and qualitative tests for detecting any abnormal urinary constituents, such as reducing sugars, proteins, ketone bodies, bile salts, bile pigments, or blood. A careful performance and interpretation of routine urine examination provides useful information about the presence of active lesion in the urinary tract. For example, presence of proteins (proteinuria) indicates glomerular lesion and a need for performing detailed glomerular function tests.
B Glomerular Function Tests
These include measurement in blood of waste products (e.g. urea and creatinine) that accumulate once renal failure sets in, and the clearance tests.
Serum Urea
Urea makes up the majority (> 75%) of the non-protein waste products excreted daily as a result of the oxidative catabolism of proteins. Urea is excreted solely by kidneys: it is filtered at glomeruli and about 40-60% of filtered urea is reabsorbed in collecting ducts. Therefore, impaired glomerular filtration results in retention of urea and its concentration in blood rises (normal is 15-45 mg/dL).
However, it is not a sensitive test because serum urea concentration begins to rise only after the filtration rate has fallen substantially. It is non-specific as well because a rise in serum urea is seen in various non-renal conditions also, such as hypovolaemia and poor perfusion due to cardiovascular failure, prostatic hypertrophy, and prolonged starvation.
In spite of these limitations, serum urea is a widely used test.
Serum Creatinine
Creatinine is a small compound (MW 113) readily filtered by the glomerulus, and unlike urea, is not reabsorbed by the tubules or collecting ducts. Elevated serum creatinine concentration is a more sensitive indicator of glomerular damage than serum urea.
Clearance Tests
Clearance is that volume of plasma from which a measured amount of substance can be completely eliminated into urine per unit time. Measurement of clearance can detect much earlier stages of renal damage. This is a definite advantage over measurement of nitrogenous waste substances (urea and creatinine), because either of these substances begin to rise in blood only after renal failure is quite advanced, with only about 20-30% of the nephrons still functioning.
If a substance is completely filtered by glomeruli, but neither secreted into nor reabsorbed from the tubule, its clearance is equal to glomerular filtration rate (GFR). Inulin, and to a lesser extent creatinine, meet the above criteria. Hence, GFR can be estimated by measuring inulin clearance or creatinine clearance.
Creatinine Clearance
Creatinine is a nearly ideal substance for the measurement of clearance for various reasons:
• It is an endogenous metabolic product synthesized at a constant rate for a given individual.
• It is cleared essentially only by glomerular filtration (not reabsorbed and only slightly secreted by the proximal tubules).
• It can be analyzed inexpensively by colorimetric method.
• It is completely filtered at glomeruli, and there is little of tubular handling, therefore, its clearance is closer to GFR.
Specimen collection includes both a 24-hour urine specimen and a serum sample, preferably taken at the midpoint of the 24-hour urine collection. Volume of urine is carefully measured and concentration of creatinine in both serum and urine is determined. Creatinine clearance is calculated using the general clearance formula UV/P, where,
U = urine creatinine concentration,
V = urine volume excreted in 24 hours,
P = serum creatinine concentration
Although the general clearance formula (UV/P) is mostly used, correction for body surface area must be included in the formula because creatinine excretion varies with regard to lean body mass. A normalization factor (1.73/A) is used for the purpose, where 1.73 is the generally accepted average body surface in square meters and A is patient’s body surface area. Accordingly, the creati-nine clearance modified for surface area is calculated as:
Reference values: The normal range for creatinine clearance is 100-125 mL/min in males and 90-115 mL/min in females. When corrected for surface area, the creatinine clearance value becomes the same for both sexes, which is about 100 mL/min. 1.73 square meter.
Urea Clearance
Urea is filtered at glomeruli and 40-60% is reabsorbed by tubules and collecting ducts. For this reason it does not provide full clearance assessment. Moreover, urea clearance is influenced by protein content of the diet, and therefore, relatively insensitive for assessing glomerular functions. Though it was one of the first clearance tests performed, it has no relevance in modern medicine and is rarely, if ever, used.
Urea is completely filtered, but is reabsorbed at the tubular level, therefore, its clearance is less than GFR.
Value of urea clearance varies with the rate of urinary output:
Inulin Clearance
Inulin is a homopolysaccharide made up of fructose residues (Chapter 2). It is neither reabsorbed nor secreted by renal tubules, so its clearance is equal to GFR. It is administered intravenously to measure GFR. However, it is practically more convenient to estimate clearance of substances already present in blood, and for this reason creatinine clearance is preferred over inulin clearance.
C Tubular Functions
Urine Concentration Test
One of the earliest manifestations of renal tubular damage is the inability to concentrate the urine. It involves measurement of specific gravity (SG) of urine from samples collected in the morning after an overnight fast. Generally, the first three urine specimens passed in the morning are collected, and their SG is measured. If it exceeds 1.022 in at least one of the samples, it implies that ability to concentrate urine is intact, and accordingly the tubular functions are considered as normal.
Dilution Test
Following intake of excess fluids, tubular reabsorption of water is decreased to get rid off the excess water load, and therefore dilute urine is excreted. Test is done in the morning after an overnight food and water deprivation. The bladder is emptied in the morning, a water load is given (1200 mL within 30 minutes) and urine samples are collected every hour for the next 4 hours. In normal subjects, most of the water load will be eliminated within these 4 hours and SG of at least one sample should fall to 1.003 or below. If SG of all samples is above 1.003, it indicates impairment of tubular function. The dilution test is more sensitive than the concentration test.
PSP Excretion Test
Phenolsulphonaphthalein (PSP) is not filtered by glomeruli but secreted by proximal renal tubules. PSP excretion in urine is used to test the efficiency of the tubular secretory capacity. The patient is fasted overnight and asked to drink two glasses of water in the morning (to ensure adequate urine output) and empty the bladder after 15 minutes. This is followed by an IV injection of 6 mg of PSP in 1 mL solution. Urine is collected after 15 minutes, 30 minutes, 60 minutes and 120 minutes after the injection and the amount of PSP in each sample is measured. In a normal subject, 20-25% of the injected dose should be excreted within 15 minutes, and 60-70% should be excreted in the first 2 hours. The test effectively detects early stages of renal tubular damage.
Para-amino Hippurate (PAH) Clearance Test
Renal clearance of PAH equals renal plasma flow (RPF). This is because this analyte is completely filtered by glomeruli and extensively secreted in tubules so much so that the entire PAH present in blood is removed by the kidneys in a single passage.
Acidification Test
The capacity of the kidneys to produce acidic urine is studied following stimulation by giving ammonium chloride (or hydrochloric acid). The following procedure is that of Davies and Wrong.
The subject is imposed an overnight fast but is encouraged to drink water till 8 a.m. Bladder is emptied and ammonium chloride, 0.1g per kilogram, in gelatin coated capsules is given orally. Bladder is emptied again and this sample is discarded. All the urine specimens passed during the next 6 hours are collected in sterile bottles, and the bladder is emptied at the end of that period. pH of the specimens and total ammonia of the combined urine is measured.
In healthy persons, urine pH falls below 5.3 in one of the specimens and total ammonia excretion is between 30-90 µEq/min. In most forms of renal failure, the pH falls in the same way but ammonia excretion is low. In renal tubular acidosis, the pH remains between 5.7 and 7.0; and the ammonia excretion is also low.
D Other Blood Determinations in Renal Diseases
Calcium and Phosphorus
In chronic renal failure there is impairment of phosphate excretion, resulting in hyperphosphataemia, which causes a reciprocal fall in serum calcium concentration. The hypocalcaemia is aggravated by reduced calcium absorption from gut as a result of impaired calcitriol production.
Uric Acid
Like urea and creatinine, the renal retention of uric acid with renal insufficiency causes increased serum concentration of this analyte. Its concentration rises to a significant extent only in an advanced stage of chronic renal failure, but this rarely results in gout.
Serum Electrolytes
Serum concentration of potassium increases (hyperkalaemia) in acute or chronic renal failure due to decreased excretion. Decreased sodium concentration (hyponatraemia) can occur due to an increased extracellular fluid volume, resulting from the inability of the kidneys to excrete water. The concentration of chloride parallels that of sodium.
 Quantitative as well as qualitative changes in thyroid hormone-binding proteins, such as thyroxine-binding globulin, affect total plasma concentration of T4 and T3.
Quantitative as well as qualitative changes in thyroid hormone-binding proteins, such as thyroxine-binding globulin, affect total plasma concentration of T4 and T3.