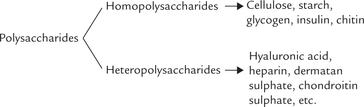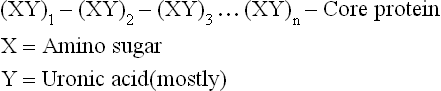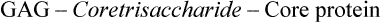Chemistry of Carbohydrates
Carbohydrates are the most abundant organic molecules in nature and the major functional constituents of living cells. The primary source of energy in animal cells, carbohydrates are synthesized in green plants from carbon dioxide, water and solar energy. The term carbohydrate was coined by Karl Schmidt in the mid 19th century, which literally means a hydrate of carbon: a compound with an empirical formula (CH2O)n, where n is an integer of 3 or greater. Structurally carbohydrates have two important features:
more than one hydroxyl group (polyhydroxyl).
a carbonyl group as either an aldehyde group (–CHO–) or a ketone group (–CO–).
Presently it is known that carbohydrates may contain elements other than C, H, and O (e.g. N, S, and P). The hydroxyl groups may be free or substituted, and the carbonyl groups may be present in the free reducing form or in non-reducing form in glycosidic linkages. Moreover, a large number of compounds are classified as carbohydrates even though they do not have the above empirical formula (e.g. deoxyribose, C5H10O4).
This chapter gives a brief account of general characteristics, chemistry, and functions of carbohydrates.
After going through this chapter, the student should be able to understand:
Chemistry, properties, structures, biological significance and classification of carbohydrates; stereo-chemical and reducing properties; isomers, anomers and epimers, the common monosaccharide derivatives and their occurrence.
Nature and type of glycosidic linkage that occur in common disaccharides and homopolysaccharides; and occurrence and role of these compounds.
Structure and role of glycosaminoglycans and various types of mucopolysaccharidoses.
I Biological Significance of Carbohydrates
Carbohydrates are widely distributed in nature, and they perform a vast range of functions. They play a primary role in energy metabolism. For example, glucose is the chief fuel molecule in all tissues and organs. Brain, spinal cord, peripheral nerves and erythrocytes are almost exclusively dependent on glucose. When in surplus, carbohydrates are stored as glycogen or starch.
Besides meeting the major energy requirements of the human body, carbohydrates perform other important roles also. They are components of nucleic acids and are covalently linked with lipids and proteins. Glycoproteins, hybrids of carbohydrates and proteins, form blood group substances and some hormones, and provide recognition elements on cell membranes. As mucopolysaccharides, carbohydrates provide the structural framework for the tissues and organs of the human body and serve as lubricants and support elements of connective tissue. Glycolipids (sphingosine derivatives) are important membrane constituents. Finally, carbohydrates form structural basis of some intracellular messengers, and play a role in intercellular communication.
II Classification of Carbohydrates
Carbohydrates may be classified as monosaccharides, disaccharides, oligosaccharides and polysaccharides (the term saccharide is derived from Greek word for sugar).
A Monosaccharides
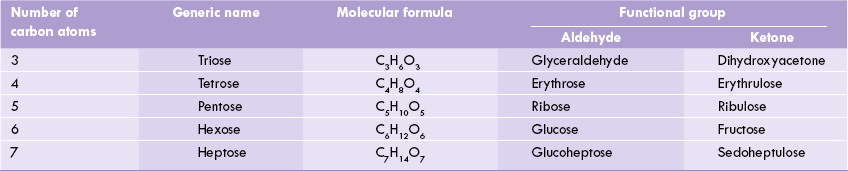
The monosaccharides are the simplest forms consisting of 3 to 9 carbon atoms, which serve as the building blocks of all carbohydrates. They are sub-classified on the basis of (a) number of carbon atoms, e.g. trioses (C-3), tetroses (C-4), pentoses (C-5), and hexoses (C-6), (b) type of the functional group, which is aldehyde in aldoses and ketone in ketoses (Table 2.1, Fig. 2.1 ).
Monosaccharides are linked by covalent linkages (i.e. glycosidic linkages) to yield larger structures such as disaccharides, oligosaccharides and polysaccharides.
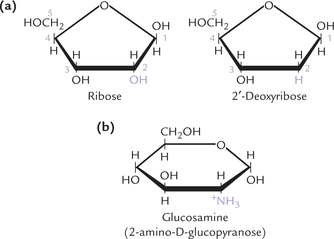
Monosaccharides of greatest biological importance are pentoses, which constitute a part of the nucleic acids (ribose and deoxyribose), and the hexoses, which serve a variety of physiological roles, discussed later.
B Disaccharides
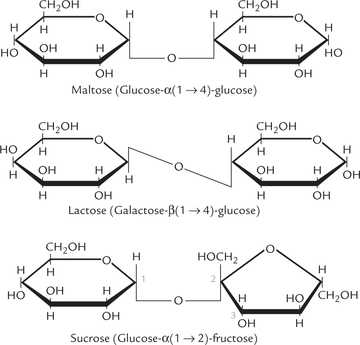
The disaccharides consist of two monosaccharide units linked by a glycosidic bond. They yield two monosaccharide units upon hydrolysis. For example, maltose, a disaccharide, can be cleaved into two glucose molecules. Similarly, lactose is hydrolyzed into galactose and glucose, and sucrose into glucose and fructose.
III Structural Properties
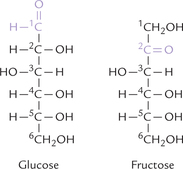
The structures of glucose and fructose shown in Figure 2.2 are referred to as open chain structures. The carbons are numbered, starting with the aldehyde carbon for aldoses, or with the terminal carbon closest to the keto carbon for ketoses. The open chain form accounts for some of the properties of the monosaccharides, as described below.
A Isomerism
Different compounds that have the same molecular formula are called isomers of one another, e.g. glucose and fructose (C6 H12O6) are aldose-ketose isomers (Fig. 2.2). Similarly glyceraldehyde and dihydroxyacetone (C3H6O3) are aldose-ketose isomers (Fig. 2.1).
B Asymmetric Carbon Atom
The carbon atom to which four different substituent groups are attached is called a chiral or asymmetric carbon atom. The second, third, fourth and fifth carbons of glucose are, therefore, asymmetric (Fig. 2.2). Presence of an asymmetric carbon imparts two important properties to the molecule: stereoisomerism and optical activity.
Stereoisomerism
The stereoisomers have same structural formula but arrangement of the substituent groups around the asymmetric carbon atoms is different. Number of the possible stereoisomers is given by 2n, where n is the number of asymmetric carbon atoms. For glyceraldehyde, only two stereoisomers are possible, whereas for glucose, which contains four asymmetric carbon atoms (see Fig. 2.2), the number of stereoisomers is 24 = 16.
Optical Activity
When a plane polarized light is passed through a sugar solution, it may undergo a rotation towards the right or the left direction. The sugars causing the rightward rotation are called dextrorotatory (+), whereas those causing the rotation towards the left are called levorotatory (+). These two forms are referred to as the optical isomers. The optical isomers of a given sugar have identical chemical properties.
C D and L Forms
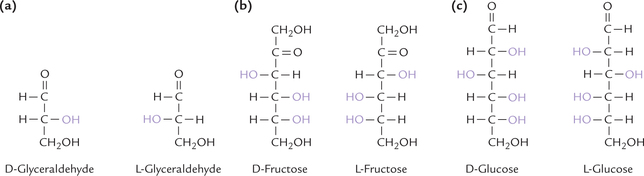
A special type of isomerism is found in the pairs of structures that are mirror images of each other. These mirror images are called enantiomers; the two members of a pair are designated as D and L sugars. They are related to each other like the left hand and the right hand, and therefore, this phenomenon, which seems to offer handedness to a molecule, is called chirality (Greek: “kheir”, meaning hand). Enantiomers of glucose and fructose are shown in Figure 2.3 . Physical and chemical properties of enantiomers of a given sugar are identical, except their optical rotations: + 113° for D- and –113° for L-glucose.
Glyceraldehyde serves as a means of classifying all monosaccharides into the D- and L-series. If the penultimate (next to last) carbon of a sugar has the configuration of D-glyceraldehyde (with hydroxyl group on right-hand side; Fig. 2.3), then it belongs to the D-series. a similar relationship exists between L-glyceraldehyde (with hydroxyl group on left-hand side) and the L-series of monosaccharides. With a few exceptions, the sugars appearing in human metabolism are of the D type.
D Ring Structures of Monosaccharides
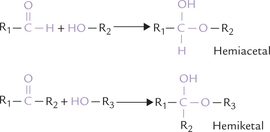
Monosaccharide molecules of 4, 5 or 6 carbons are quite flexible, and this flexibility brings the aldehyde group (or the keto group) in close proximity to other hydroxyl groups on the same molecule. Reaction of aldehyde and ketone with the hydroxyl group may then occur to form hemiacetal and hemiketal respectively. This results in cyclization of the linear forms to stable ring structures. The cyclization is a spontaneous process in solutions.
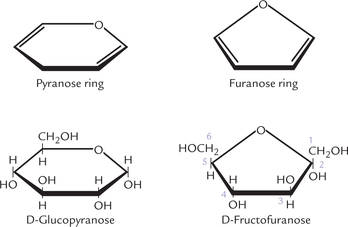
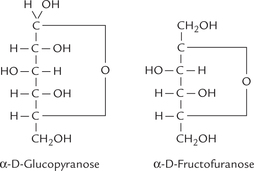
Pyranose and Furanose Ring Structures
If the ring structure formed by cyclization is sixmembered (made of 5 carbons and 1 oxygen), it is called a pyranose ring; if it is five-sided (made of four carbons and one oxygen), it is called a furanose ring (Fig. 2.4 ). Fructose, a ketohexose, is usually found in the furanose form, called fructofuranose, whereas glucose is most stable in the pyranose form. More than 99.7% of the total glucose molecules in solution exist in pyranose form, and termed glucopyranose.
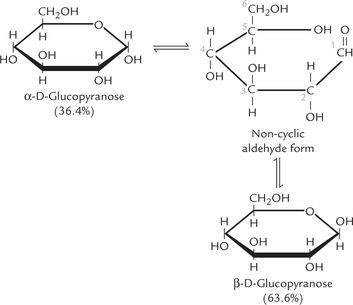
Anomers
Formation of ring structure results in creation of an additional asymmetric carbon, called anomeric carbon (C-1 of an aldose, C-2 of a ketose). For an aldohexose, such as glucose, the number of asymmetric carbon atoms increases to 5 and the number of possible isomers, therefore, increases to 25 = 32. Isomerism as a result of the anomeric carbon in glucose is illustrated in Figure 2.5, where the glucose is shown to be in a pyranose ring form (D-glucopyranose). It may assume either the α- or β- configuration about the anomeric carbon. Note that these two forms differ in the arrangement of the substituents around the carbonyl carbon and are called anomers. Their systematic names are α-D-glucopyranose and β-D-glucopyranose respectively. Together they make up well over 99% of the total sugars present in solution, only about 0.0025% being in the open chain form. Both anomers are present in equilibrium with the open chain (non-cyclic aldehyde) form.
It is important to note that cyclic forms of aldohexoses, with their five asymmetric carbons have total of 32 (25) stereoisomers; and that each of the 16 isomers that belong to D- or L-series has two anomeric forms.
Mutarotation
The anomers are not stable under ordinary conditions and tend to interconvert constantly. The interconversion of the two anomeric forms is referred to as mutarotation (Fig. 2.5). It is caused by spontaneous opening and reclosure of the ring. In case of glucopyranose, the equilibrium between the α- and β-anomers favours the β form (63.6%) over the α form (36.4%). The equilibrium is reached within several hours in neutral α- or β-solutions, but mutarotation is accelerated greatly in the presence of acids.
Mutarotation is followed in a polarimeter by measuring the optical rotation. The α-D-glucopyranose has a specific rotation of +112.2° and the α-D-glucopyranose of + 18.7°. Both undergo mutarotation and over a period of a few hours, the specific rotation changes to attain a stable value of +52°. This change represents the interconversion of the two anomeric forms to yield the equilibrium mixture, consisting of about two-third of the α-form, one-third of the β-form, and a very small amount of the non-cyclic form.
E Diastereomers and Epimers
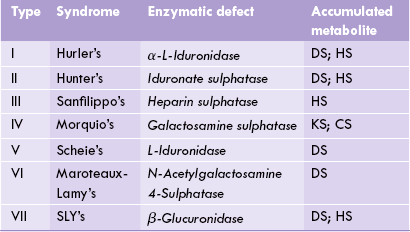
Figure 2.6 shows structures of three sugars, which differ in the arrangement of substituents at one or more (but not all) chiral carbons. They are diastereomers. Glucose and mannose differ in the orientation of substituents around only one of their asymmetric carbons, and are called epimers. Epimers have different physical and chemical properties. Just as mannose differs from glucose on the basis of carbon 2 configuration, another epimer of glucose is galactose, which differs on the basis of carbon 4 configuration. Thus mannose is termed C-2 epimer of glucose and galactose as a C-4 epimer of glucose.
Fig. 2.6 Epimers of glucose: galactose (C-4 epimer) and mannose (C-2 epimer), shown as Howarth projections.
Fischer and Howarth projections
A common way of depicting cyclic structures is shown in Figure 2.7 (these are referred to as Fischer structures). a closer look at them shows that some bonds are short but others are excessively long. But this cannot be the case in nature. Carbohydrate chemists, therefore, more often use the so called Haworth projections, which are fairly representative of reality. Haworth representations of some monosaccharides, including α-D glucose, are shown in Figure 2.6. Note that those groups that are to the right in the Fischer structures appear down in the Haworth structures and those appearing to the left in the Fischer formulas point up in the Haworth structures.
IV Hexoses, Pentoses, and Related Disaccharides and Oligosaccharides
A Hexoses and Pentoses
Among various types of monosaccharides present in human body, hexoses are physiologically the most important. Glucose, galactose, fructose and mannose are the most predominant hexoses. Glucose is important because it is a component of the storage sugars in plants (starch) and animals (glycogen); the blood sugar often mentioned is glucose, which is used as fuel by various tissues for their energy needs. Red blood cells and brain utilize glucose exclusively, although brain in prolonged starvation also utilizes ketone bodies. Roles of hexoses in body are given in Table 2.2 .
Table 2.2
Sources and physiological importance of some hexoses
| Sugar | Source | Importance |
| 1. D-Glucose | Hydrolysis of starch, sugarcane and disaccharides like lactose and maltose. Most fruits contain glucose. | The principal sugar used by the tissues. Carried by the blood to these tissues. |
| 2. D-Galactose | Hydrolysis of the milk sugar (i.e. lactose). | Occurs in glycolipids and glycoproteins. Changes to glucose in the liver and metabolized. Used in the synthesis of milk sugar in the mammary gland. |
| 3. D-Fructose | Fruit juices, honey, sugarcane. | Changes to glucose in the liver (and intestine) and metabolized. |
| 4. D-Mannose | Hydrolysis of plant mannose and gums. | Occurs in glycoproteins, a constituent of prosthetic group of oligosaccharides of albumin, globulins and mucoproteins. |
Pentoses are integral constituents of nucleic acids. Ribose, an aldopentose, is one of the most important molecules in biochemistry, being present in RNA. DNA contains deoxyribose, the corresponding deoxy sugar.
B Disaccharides
Covalent joining of two monosaccharides units by glycosidic bond forms disaccharide; the glycosidic bond links anomeric carbon of a monosaccharide to a hydroxyl group of the other. a number of disaccharides of biological significance contain glucose, fructose and galactose. Maltose, the malt sugar, is a glycoside of two glucose molecules in an α(1 → 4) linkage; lactose, present in milk includes galactose and glucose in β(1 → 4) glycosidic bond; and sucrose, the table sugar includes glucose and fructose in an α(1 → 2) linkage (Fig. 2.8 ). Cellobiose, an isomer of maltose, which can be derived from a plant polysaccharide cellulose, involves β(1 → 4) linkage.
In sucrose, both anomeric carbon atoms are involved in the glycosidic bond. Therefore, the sucrose molecule, in contrast to lactose, maltose, and cellobiose, does not have a reducing end and is called a non-reducing sugar (the reducing end is the end with a free anomeric carbon: free implies not involved with the formation of a glycosidic bond). Benedict’s test, one of the earliest tests to detect presence of sugar in urine, which is based on the reducing properties of the sugar, is therefore, negative with sucrose.
These disaccharides, with the exception of cellobiose, have food value for human beings, and the intestinal tract contains specific glycosidases that can cause their hydrolysis into their constituent monosaccharides. α-Glucosidase (maltase) causes the hydrolysis of maltose to glucose but is inactive with cellobiose. Sucrase catalyzes the hydrolysis of sucrose to glucose and fructose, and α-galactosidase (lactase) converts lactose to galactose and glucose.
Invert sugar
Sucrose is dextrorotatory ( + 66.5°). When it is hydrolyzed, one molecule of glucose ( + 52.5°) and one molecule of fructose (–92°) is formed. As the levorotatory effect of fructose is greater than the dextrorotatory effect of glucose, the hydrolysate is levorotatory. Because the optical rotation is inverted following hydrolysis by sucrase, the enzyme is termed invertase and substrate sucrose is termed invert sugar.
Lactose intolerance, caused by absence of lactase, results in an inability to digest lactose. The undigested lactose moves through the digestive tract to colon, where bacterial fermentation generates large quantities of CO2, H2 and organic acids. These products cause diarrhoea, bloating and painful gastrointestinal upsets in such patients. Lactose intolerance may be the result of a genetic trait, in which case it is absent in infants, who must be given a non-milk artificial formula; or more commonly in adults, in whom lactase disappears after adolescence.
C Oligosaccharides
Oligosaccharides vary from disaccharides to complex branched structures of glycosidically linked monosaccharide units. Considerable variation in the linkage exists: anomeric carbon (C-1 or C-2) of a monosaccharide unit may be linked to either C-1, C-2, C-3, C-4 or C-6 of another, and moreover, this linkage may be in the α- or β-configuration. Each of these linkages forms a different molecule with distinct properties. Comparatively, the number of possible molecules formed by joining two identical amino acids, such as glycine, together is only one.
Such variation in linkage in oligosaccharides is the basis for the mechanism of cell-cell recognition. The number of variations of bonds suggests that it is much easier to create the enormous variability required for identifying different cells by using sugars rather than amino acids.
Glycoprotein
Attachment of oligosaccharide to a protein yields glycoprotein (more appropriately defined as proteins having oligosaccharide attachments). The attachment to the protein usually occurs at a serine or threonine residue (O-linked), but there are also attachments to the amide nitrogen of asparagine residues (N-linked). The remainder of the chain is built stepwise by sequential addition of other monosaccharide units. Monosaccharides like L-fucose and N-acetylglycosamines generally appear at the ends of the chain.
Functions
Glycoproteins are involved in a variety of functions including lubrication, many being secreted, and another large group involved with cell adhesion and cell recognition (for details regarding structure and function of glycoproteins see chapter 5). The functions of the carbohydrate chains of the glycoproteins are diverse: they stabilize the protein against denaturation, protect it from proteolytic degradation, enhance its solubility, or serve as recognition signals to facilitate cell-cell interaction. Moreover, the antigenic sections of a number of glycoproteins, including blood group substances, consist of oligosaccharides.
V Derived Sugars
The term derived sugars is applied to monosaccharides whose structure cannot be represented by the general formula (CH2O)n, or which have some unusual features. Some of the physiologically important derived sugars as described here.
A Acid Sugars
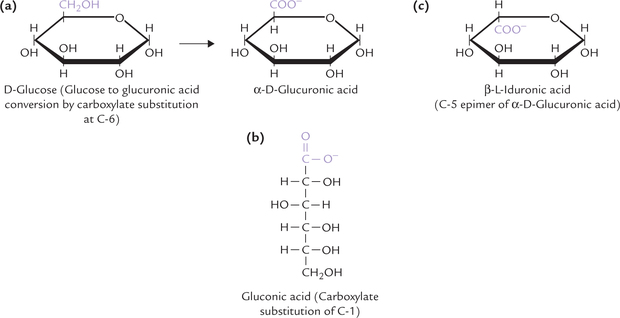
The acid derivatives of the sugars, produced by oxidation of the aldehyde carbon or the hydroxyl carbon or both, are called the acid sugars. For example, D-glucuronic acid is formed by carboxylate group substitution at the sixth carbon position (i.e. by oxidation of the hydroxyl group at C-6; Fig. 2.9a ), and D-gluconic acid at the first carbon (i.e. by oxidation of the aldehyde group at C-1 (Fig. 2.9b). Likewise oxidation of the last carbon of galactose gives galacturonic acid. Iduronic acid is an epimer of glucuronic acid (Fig. 2.9c); both these acid sugars are important constituents of glycosaminoglycans, discussed later. When both the first and the sixth carbons of glucose are oxidized to carboxyl groups, the product is saccharic acid.
B Deoxy Sugars
The deoxy sugars are the ones in which a hydroxyl group of the sugar is replaced by hydrogen atom; for example, the deoxyribose is obtained from ribose (Fig. 2.10a ). Deoxyribose is present in DNA molecules. Deoxyglucose is an important inhibitor of glucose metabolism.
C Amino Sugars
A large group of derived monosaccharides are the amino sugars where an amino group replaces the –OH residue on carbon 2 of the hexose (Fig. 2.10b), such as glucose, galactose and mannose. The corresponding compounds are glucosamine, galactosamine and mannosamine. The amino group is usually acetylated. N-acetylglucosamine and N-acetylgalactosamine are components of connective tissue proteoglycans, glycoproteins, and complex lipids. An unusual amino sugar is N-acetylneuraminic acid (NANA), sometimes called sialic acid, which is derived from N-acetyl mannosamine and pyruvate. It occurs in glycoproteins and complex lipids.
Several antibiotics (e.g. erythromycin and carbomycin) contain amino sugars. Presence of amino sugars is related to the bacteriostatic or the bactericidal activities of these drugs.
D Phosphoric Acid Esters
They are produced by attachment of a phosphate group with hydroxyl group of sugar. The phosphorylated sugars, such as glucose 6-phosphate and glucose 1-phosphate, are thermodynamically favoured to serve as the metabolic intermediates.
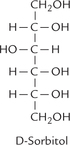
E Sugar Alcohols
Monosaccharides are reduced at their carbonyl group to yield the corresponding polyhydroxy alcohols, which are intermediates of some secondary metabolic pathways. Both aldoses and ketoses can undergo reduction to form the sugar alcohols. For example, the reduced product of glucose (or fructose) is sorbitol. Similarly, mannose yields mannitol and galactose yields galactitol; the latter triggers cataractogenesis (formation of cataract) in galactosaemias.
F Glycosides
When the hemiacetal or hemiketal group of a monosaccharide is linked covalently with an alcohol, sterol or phenol group, the compound so formed is called a glycoside (recall that reaction of aldehyde and ketone with the hydroxyl group forms hemiacetal and hemiketal respectively). It has two components: a carbohydrate and a non-carbohydrate (termed aglycone), and the linkage between the two is called glycosidic linkage. a simple example is a methyl glycoside (Fig. 2.11 ). The term glycosidic means that there is bond to a sugar and in this case the O-glycosidic bond is between a sugar (α-D-glucose) and methyl alcohol (Fig. 2.11).
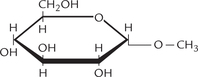
Fig. 2.11 Methyl α-D-glucose is a methyl glycoside formed by covalent linking of α-D-glucose with methyl alcohol (aglycone) by glycosidic linkage.
Medical significance: Glycosides are found in many drugs. Digoxin is a cardiac glycoside, which is used to treat cardiac failure. The glycosidic bond is between a sugar and a steroid (the latter is similar in structure to the oestrogens). Several other medications also have glycosidic bonds as main features of their structures. For example, phlorhizin, a glycoside of glucose and phloretin is used in renal damage. It is obtained from rose bark. Digitonin, a cardiac stimulant (obtained from leaves of foxglove) is formed from galactose and digitogenin. Various antibiotics, such as streptomycin, puromycin and erythromycin, have O-glycosidic bonds.
VI Polysaccharides
Most carbohydrates occur in nature as complex, high molecular weight polysaccharides or glycans, which comprise several monosaccharides or monosaccharidederivatives, linked by glycosidic bonds. They are of two major types: homopolysaccharides, which are made from one particular sugar unit; and heteropolysaccharides, which are made from different sugar units. In both cases there may be linear or branched polymers and the constituent sugar units are linked by glycosidic bonds; the latter link the anomeric carbon of a monosaccharide to the hydroxyl group of another.
Storage of energy: Polysaccharides such as starch and glycogen store the chemical energy intracellularly (i.e. storage polysaccharides). Starch is the principal storage polysaccharide in plants. Its counterpart in the animal cells, namely glycogen, is often referred to as animal starch. Both glycogen and starch are stored intracellularly in the form of cytoplasmic clusters or granules.
Structural elements: Polysaccharides are important constituents of various cell components (i.e. structural polysaccharides). For example, hyaluronic acid is an essential component of connective tissue, and cellulose serves as important structural component of the cell wall. Cellulose is the most abundant extracellular macromolecule.
A Homopolysaccharides
Glycogen is the most important homopolysaccharide in human body. Plant homopolysaccharides include starch, cellulose, inulin, etc. The tough exoskeletons of many lobsters, crabs and several insects consist largely of structural homopolysaccharide, chitin, which is a linear polymer of N-acetylglucosamine.
Starch
Starch is a plant homopolymer of glucose. It is a mixture of two distinct polysaccharide forms: amylose and amylopectin. Amylose is an unbranched polymer of glucose, linked by α(1 →4) bonds, and occurs in helical coiled conformation. Amylopectin, on the other hand, is a branched form; most of the constituent glucose are joined in α(1→4) linkages but additional α(1→6) bonds occur every 2530 residues, creating branch points (Fig. 2.12 ).
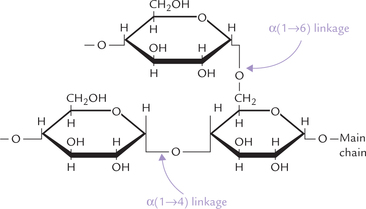
Fig. 2.12 α(1→4) and α(1→6) glycosidic linkages between glucose residues in starch and glycogen. The α(1→4) linkages occur in straight chain, and α(1→6) at branch points.
Like other branched polymers, starch has only one reducing end and multiple non-reducing ends. (The reducing end is the end with free C-1, i.e. the C-1 not involved in the formation of a glycosidic bond.)
Starch hydrolysis
Most plants have two hydrolyzing enzymes: α-amylase and β-amylase; both are specific for α(1→4) linkages in large polymers and have no action on maltose or maltriose. Cleavage by β-amylase is much more common in plants and is characterized by successive removal of maltose units beginning at a non-reducing end (exoglycosidase), until the action of enzyme is blocked at the α(1→6) linkage. Thus, action of β-glycosidase halts at branch points leaving a branched homopolymer called limit dextrin. The α-amylase cleaves internal glycoside bonds (endoglycosidase) randomly, as discussed below.
Starch digestion in humans
It is effected by salivary amylase and pancreatic amylase; both are α-amylases. There is only 1% difference in their composition but the pancreatic amylase is more important from quantitative viewpoint because food does not generally remain long enough in the mouth to be thoroughly digested by salivary amylase. The pancreatic α-amylase, on the other hand with a pH optimum of 6.9 degrades the starch molecule extensively within duodenum where food stays longer. It cleaves α(1→4) linkages that are located internally (endoglycosidase), but has no effect on α(1→6) linkages.
Products formed by action of amylase are α-maltose and maltriose. From amylopectin α-dextrins (oligosaccharides made up of 5–9 glucose units with one α(1→ 6) bond) and linear oligosaccharides are additionally produced. Under physiological conditions, starch is hydrolyzed to produce 40% maltose, 20% maltriose, 30% α-dextrin and 5% linear oligosaccharides.
In acute pancreatitis, the pancreatic amylase levels in serum are highly elevated and this serves as a reliable diagnostic tool.
Glycogen
Glycogen is the storage form of glucose in animals and commonly referred to as animal starch. Structurally, it resembles amylopectin but is more extensively branched: the α(1→6) linkages occur every 10–18, units, whereas in amylopectin they occur about every 25. Thus, it has more reducing ends than amylopectin and its α(1→4) linked oligomers are shorter (Fig. 2.13 ). Moreover, glycogen may have several hundred thousand glucose residues (its molecular weight is up to several millions), whereas amylopectin has only 300–6000 residues.
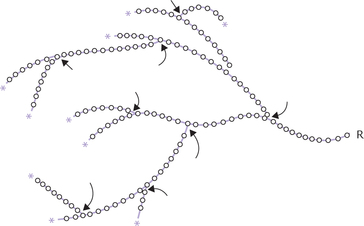
Fig. 2.13 a diagrammatic representation of glycogen. Each circle represents a glucose residue (* = non-reducing ends, O-O = α(1→4) linkage, arrows indicate α(1→6) linkage (branch point), R = remainder of the glycogen molecule).
How does storage of glucose as a polymer (glycogen), rather than simply glucose, offer an advantage? If glucose were stored (as glucose) rather than a polymer, it would cause an enormous osmotic force within the cell. This would cause water to move into the cell and the cell would burst.
Glycogen is stored in cytoplasmic granules in the heptocytes and the muscle cells. Bulk amounts of glycogen are stored in liver and muscles, although most other cells may contain smaller quantities. Highest concentration of glycogen is reached in the liver in the well-fed state (6–8 g/100 g wet weight approximately). The liver glycogen plays a vital role in glucose homeostasis (Chapter 9). Skeletal muscles have much lower glucose concentration (1–2 g/100 g wet weight approximately), but net amount of glycogen stored in muscles (200–300 g) is much more than that in liver (50–100 g). This is because the total muscle mass exceeds that of liver.
Roles of liver and muscle glycogen are different
The muscle glycogen stores provide fuel reserves for the synthesis of ATP during muscle contraction. On the other hand, the liver glycogen serves to maintain the blood glucose concentration within normal range, particularly in the early stages of fasting.
The cytoplasmic granules that store glycogen contain the enzymes of glycogenesis (glycogen synthesis) as well as those of glycogenolysis (glycogen degradation). During muscle contraction or in fasting state, the enzymes of glycogenolysis become active; and conversely, during well-fed state the enzymes of glycogenesis are activated (see Chapter 15).
Celluloses
They are the most abundant organic compounds on earth, being essential components of plants, comprising 20–45% of their cell wall mass. Wood is about 50% cellulose, while cotton is almost pure cellulose. Wood enables trees to attain towering heights and yet it is just a polymer of glucose.
The basic unit of cellulose is a chain of glucose residues linked by β(1→4) glycosidic bonds. The chains are in parallel alignment and held in place by interchain hydrogen bonds. Major variations are in the degree of polymerization which appears to be biphasic: either being less than 500 glucose units or between 2500 and 4500 glucose units per chain. These hydrogen bonded chains are further stabilized by the presence of other polysaccharides, such as hemicellulose, pectin, and lignin, which function as cementing materials.
Cellulose cannot be digested
The nutritional value of cellulose is summarized as: “Glucose everywhere but not a drop to eat”. This is because it is indigestible as humans lack the enzyme cellulase that can cleave the β(1→4) glycosidic bonds. Cellulase is found in snails, bacteria, fungi and insects. Ruminants can digest cellulose because of the presence of bacteria (rumens) in their stomach which hydrolyze the β(1→4) glycosidic bonds. Even with cellulases from bacteria, cows have to chew their cud, which means vomiting out half-digested cellulose and eating it again. (Rabbits are worse as they pass hard pellets initially and eat them to produce the soft pellets).
Despite its nutritional value being nil the indigestible polysaccharides, collectively called dietary fibres (include cellulose hemicellulose, lignin and pectin) are important in our digestive processes because they provide roughage which is believed to be important for keeping the contents of our intestines mobile and in lowering the possibility of developing bowel cancer (Chapter 26).
Cellulose has certain commercial uses as well. Cellulose derivatives are used for manufacturing emulsifiers, plastics and explosives.
Chitin
Composed of N-glucosamine residues linked by β(1→4) glycosidic bonds, chitin is a subtle variation of cellulose. The polymers are held together by hydrogen bonding. It is found in exoskeletons.
The plants and animals contain several other structural and storage homopolysaccharides, as shown in Table 2.3 .
Table 2.3
| Homopolysaccharide | Composition | Occurrence and role |
| Chitin | Polymer of N-acetyl D-glucosamine linked by β(1→4) glycosidic linkages | Present in exoskeleton of invertebrates such as crustaceae, insects and spiders. Integral component of cell walls of most fungi and many algae. |
| Inulin | A fructosan, consisting of D-fructose units, linked covalently | Occurs in tubers and roots of dahlias and dandelion. Used for determination of glomerular filtration rate and to measure the body water volume. |
| Agar | Polymer of galactose units which are sulphated | Occurs in sea weeds. Purified form of agar is used for laboratory culture of bacteria. |
| Dextrins | Consists of α-D-glucose residues linked by α(1→4) and α(1→6) glycosidic bonds | Formed in course of hydrolytic breakdown of starch (limit dextrins are formed first as the degradation reaches branch points). Found in various foodstuffs obtained from starch producing plants. Used as adhesives and binders. |
| Dextran | α(1 →4), α(1→6) and α(1→3) linked D-glucose units, which are interconnected in such a way so as to form a network | Given intravenously it increases plasma volume (i.e. plasma expander), and therefore used in case of blood loss. |
B Heteropolysaccharides
Heteropolysaccharides (also called heteroglycans) are the polymers made from more than one kind of monosaccharides or monosaccharide derivatives. They may exist in the free-state or conjugated (joined) with lipids, peptides or proteins. Study of heteropolysaccharides is one of the most exciting areas of new knowledge in biochemistry and has created immense interest in recent years, much the same way genetics did a decade earlier.
Heteropolysaccharides are involved with viral and bacterial attachment to cells, the immune system, the migration of normal cells, the migration of cancerous cells, the fertilization of ova, and the changes in cells during tissue development. These functions are discussed in detail later in this chapter.
Glycosaminoglycans
Glycosaminoglycans (GAGs)/mucopolysaccharides are a group of high molecular weight, linear polysaccharides, with repeating disaccharide units. Each disaccharide unit comprises of an amino sugar and an acid sugar (uronic acid).
GAGs are the most important group of heteropolysaccharides in humans. They were first isolated from mucin and therefore also called mucopolysaccharides. These complex sugars are major components of the extracellular matrix of connective tissue, including bone and cartilage, synovial fluid of joints, vitreous humor of eye and secretions of mucus-producing cells. The GAGs found in connective tissue may attain extremely large size, with molecular weight of 1–80 millions.
Structure
GAGs are unbranched polysaccharides of disaccharide repeats; each repeat unit comprising a sugar unit linked to a glycosamine, hence called glycosaminoglycans.
• Glycosamine (amino sugar): It is either glucosamine (GluNH2) or galactosamine (GalNH2), both of which are present in their N-acetylated forms. The amino group may be sulphated rather than acetylated in some GAGs.
• Acid sugar unit: Besides glycosamine, the other sugar unit present in a disaccharide repeat is mostly an acid sugar (uronic acid). Usually it is D-glucuronic acid (GluUA), although in some cases it may be L-iduronic acid (IdUA).
GAGs are covalently attached to a protein (termed core protein) to form hybrid molecules, termed proteoglycans; the sugar component constitutes up to 95% of the proteoglycan molecule. Linking of polysaccharide chains to the core protein occurs by a core trisaccharide, Gal-Gal-Xyl.
An exception is hyaluronic acid, the longest GAG, which is not attached to a core protein.
Proteoglycan-monomers and proteoglycan aggregates
The proteoglycan molecules tend to aggregate, forming complex structures: proteoglycan-monomers and proteoglycan-aggregates.
Proteoglycan monomer comprises linear, unbranched polysaccharide chains, covalently linked to their core protein. The chains, each of which may be composed of about 100 monosaccharide units, extend out from the core protein giving an appearance of “bottle brush” (Fig. 2.14a ).
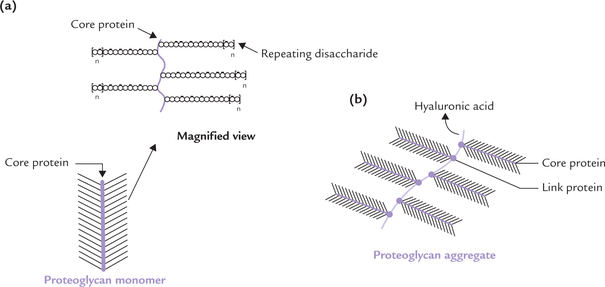
Fig. 2.14 The proteoglycan structure. (a) Bottle brush structure of a proteoglycan monomer: Linear, polysaccharide chains linked to core protein; a magnified segment is also shown, (b) Proteoglycan aggregate showing aggregation of several monomers on a central strand of hyaluronic acid with the help of link proteins ( = represents link protein).
Several proteoglycan monomers associate together to form a proteoglycan aggregate. a molecule of hyaluronic acid is also involved in formation of these aggregates by providing attachment sites for the core proteins (Fig. 2.14b). The association between core proteins and hyaluronic acid is not covalent but occurs through ionic interactions in which a link protein participates. This overall aggregate (also known as aggrecan) is a large complex macromolecule consisting of a three-dimensional array of proteoglycans bound to hyaluronic acid. This creates a stiff matrix in which collagen and other components of the extracellular matrix are embedded.
The proteoglycan aggregates are polyanionic because of the many negative charges of the carboxyl groups of the uronic acids, and the sulphate groups attached to some of the sugars. Being highly hydrophilic, these groups attract water molecules to form a hydrated gel like matrix that forms body’s ground substance.
Proteoglycans versus glycoproteins: It is important to distinguish proteoglycans from glycoprotein. Glycoproteins (i) have short oligosaccharide chains (1–20 sugars in length), which are (ii) highly branched, (iii) generally do not have a repeating sequence and (iv) constitute 1–30% of the hybrid. On the other hand, sugar chains of proteoglycans are longer (100 or more sugars), linear and unbranched, with disaccharide repeats.
Relationship Between Structure and Function
The peculiar structural features of GAGs that explain their suitability to perform a number of specialized functions in the body are described below.
Gel-forming component of extracellular matrix (ECM):
GAGs have polyanionic character, meaning numerous negative charges are present on a single molecule. Because of these charges, GAGs have two important properties:
• The heteropolysaccharide chains repel one another and, therefore, tend to exist in extended conformation in solutions.
• The anionic groups being strongly hydrophilic tend to associate with water.
The special ability of the chains to bind large amounts of water produces the gel-like matrix that forms the body’s ground substance. Due to charge repulsion the chains tend to “slip” past each other, in a way two magnets of the same polarity do. This produces the “slippery” consistency of mucus secretions and synovial fluid.
Structural support to connective tissue
GAGs form a matrix or ground substance that stabilizes and supports the cellular and fibrous components of tissues. The character of connective tissue depends, to a large extent, on the relative proportions of ground substance and embedded fibrous proteins. For example, tendon (having high tensile strength) is composed primarily of fibres, whereas cartilage is rich in ground substance.
Classification
The GAGs are classified in to six major types according to their monomeric compositions, type of glycosidic linkages, and degree and location of their sulphate units (Table 2.4 ). However, all types share some important features:
Table 2.4
Structure and distribution of the proteoglycans
+a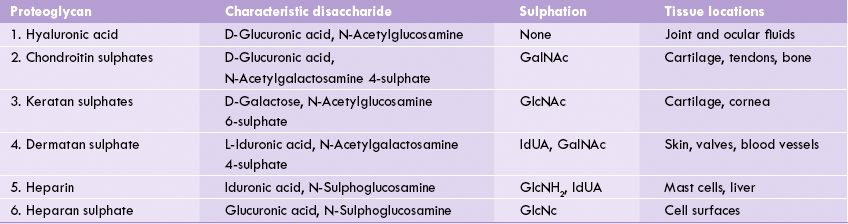
GalNAc = N-acetylgalactosamine, GlcNH2 = glucosamine, GlcUA = D-glucuronic acid, IdUA = L-iduronic acid.
(a) Composition: They contain repeating disaccharide units of an amino sugar (glycosamine) and uronic acid. Keratan sulphate is an exception for it contains galactose in place of uronic acid.
(b) Linkage: With exception of heparin and heparin sulphate, where the linkage between the amino sugar and uronic acid is uniformly 1→4, in all other GAGs it is alternating 1→4/1→3.
(c) Core protein: GAGs are usually attached to a core protein by a core trisaccharide, Gal-Gal-Xyl. The latter is attached to a serine or threonine residue of the core protein by O-glycosidic linkage. Exceptions are hyaluronic acid and keratan sulphate. In case of kertan sulphate, the linkage to core protein is N-glycosidic (discussed in Chapter 5). Hyaluronic acid is a rather unusual GAG for it is not attached to a core protein.
Hyaluronic acid
The repeating disaccharide unit of hyaluronic acid comprises D-glucuronic acid and N-acetylglucosamine, joined by β(1→3) linkage (Fig. 2.15 ). Hyaluronic acid is different from other GAGs in lacking sulphate groups, not being covalently attached to protein and for not being limited to animal tissue (it is found in bacteria also). The polysaccharide chain is longest of all the GAGs, with molecular weight of 1 X 105 to 1 X 107 (250–25,000 repeating disaccharide units). It is a viscous jellylike substance that fills the intercellular spaces of the animal tissues. It is found in synovial fluid of joints, vitreous humor of the eye, umbilical cord and loose connective tissue. It primarily serves as a lubricant and shock absorber. Some pathogenic bacteria secrete an enzyme hyaluronidase, which cleaves the glycosidic bonds of the hyaluronic acid. This renders the tissues more susceptible to invasion by the bacteria. Hyaluronidase also hydrolyzes the outer polysaccharide coat of the ovum and thereby makes the penetration by spermatozoa possible.
Chondroitin 4- and 6-sulphates (CS)
These are the most abundant GAGs in the body that comprise D-glucuronic acid and N-acetylgalactosamine units: the latter are sulphated on either C-4 or C-6 (Fig. 2.15). Chondroitin sulphate is mostly present in the cartilage where it binds collagen and holds fibres in a tight, strong network. Tendons, ligaments, bones and aorta contain relatively smaller amount of this mucopolysaccharide.
Keratan sulphate (KS)
It is the most unusual of all GAGs: the only one that does not contain an acid sugar. The repeating disaccharide unit consists of N-acetylglucosamine and galactose. It is linked to protein by rather unusual linkages (N-glycosidic), that are usually found in glycoproteins. It helps to keep cornea transparent.
Dermatan sulphate (DS)
It was originally isolated from skin, but is also found in blood vessels and heart valves. It helps maintain shape of these tissues. The predominant acid sugar is iduronic acid, though a variable amount of glucuronic acid is also present. The repeating disaccharide unit is made up of iduronic acid and N-acetylgalactosamine 4-sulphate. The L-iduronic acid, a C-5 epimer of D-glucuronic acid, is formed in an unusual reaction by epimerization of the latter, after it has been incorporated into the polymer.
Heparin
The repeating disaccharide unit comprising glucosamine and glucuronic acid (or iduronic acid), is rich in sulphate groups. Almost all glucosamine residues are bound to sulphate group. An average of 2.5 sulphate groups per disaccharide unit is seen. The linkages between the amino sugar and the uronic acid is uniformly 1→4 in heparin (and heparin sulphate). (Alternating 1→4/1→3 linkages seen in other GAGs.) Unlike other GAGs (which are extracellular compounds), heparin is an intracellular component of mast cells that line arteries, especially in liver, lungs, and skin. Heparin is a potent anticoagulant that helps to prevent clotting of the circulating blood. It is therapeutically used to prevent clotting during intravenous therapy, and to inhibit clotting in various pathological conditions, such as following a heart attack.
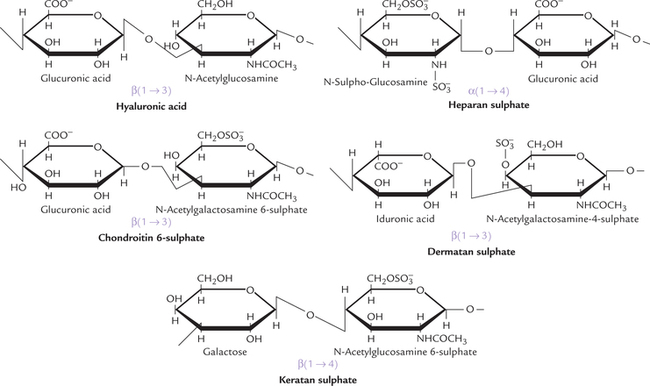
Mucopolysaccharidoses
Mucopolysaccharidoses are a group of hereditary disorders of proteoglycan metabolism that are clinically progressive and characterized by excessive intralysosomal accumulation of GAGs in various tissues. Such accumulation accounts for clinical manifestations of mucopolysaccharidoses such as coarse facial features, thick skin, and corneal opacity. Defective cell function leads to mental retardation, growth deficiency, and skeletal dysplasia.
Biochemical defect
The underlying defect in mucopolysaccharidoses is impaired degradation of mucopolysaccharides by lysosomal enzymes. The lysosomal enzymes are hydrolytic in action and catalyze degradation of the GAGs at an optimum pH of 5.0. Such low optimum pH has a protective value since it prevents destruction of the cellular constituents in case the enzyme leaks out of the lysosomes. Half-life of the GAGs is relatively short (3–10 days), with exception of keratan sulphate (120 days). Therefore, with impaired degradation, the cellular concentration of the GAGs rapidly builds up.
Features of mucopolysaccharidoses
In mucopolysaccharidoses, the tissues that produce GAGs are most affected. In these tissues, degradation of GAGs is impaired. Lysosomal vesicles become swollen with incompletely degraded mucopolysaccharides. a characteristic finding of diagnostic significance is excessive elimination of the GAGs in urine. Diagnosis can be confirmed by measuring the concentration of lysosomal hydrolases.
The mucopolysaccharidoses are autosomal and recessively inherited, with an exception of Hunter’s syndrome which is X-linked. Prenatal diagnosis of these disorders is possible. Children who are homozygous for these disorders are apparently normal at birth. The condition deteriorates gradually, but in severe cases the progression is rapid and death occurs in childhood. Unfortunately, no effective treatment exists at present.
Clinical types
Several types of mucopolysaccharidoses are recognized depending on the lysosomal hydrolase that is deficient (Table 2.5 ).
Exercises
Essay type questions
1. Define carbohydrates and give one example from each class/subclass. Explain why starch can be utilized in humans but not cellulose?
2. What are mucopolysaccharides? Name some and explain their biological significance.
3. How do physical properties of glycosaminoglycans relate to their biological roles?
4. Name some homopolysaccharides and explain their biological significance.
5. Compare and contrast structures and functions of cellulose, chitin, starch and glycogen.
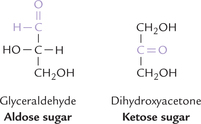
 Monosaccharides, the simplest carbohydrates, are classified as aldoses or ketoses depending on the presence of aldehyde or keto group. Disaccharides contain two, oligosaccharides contain 3–10 and polysaccharides consist of more than 10 monosaccharides linked by glycosidic bonds.
Monosaccharides, the simplest carbohydrates, are classified as aldoses or ketoses depending on the presence of aldehyde or keto group. Disaccharides contain two, oligosaccharides contain 3–10 and polysaccharides consist of more than 10 monosaccharides linked by glycosidic bonds.