Tumors of the Salivary Glands
Tumors of the salivary glands constitute a heterogeneous group of lesions of great morphologic variation, and for this reason, present many difficulties in classification. Since these tumors are relatively uncommon, early investigators were handicapped by insufficient material for study. With the publication of several large series of cases, accompanied by serious discussions of the nature of tumors of the salivary glands based upon a cumulative clinical experience of many years, considerable progress has been made in broadening our knowledge of these lesions. Among these studies have been those of Evans and Cruickshank, Thackray and Lucas, and WHO.
Foote and Frazell were among the first investigators to provide a usable classification of salivary gland tumors. Spiro and his associates, Thackray and Sobin, and Batsakis have proposed classifications for practical use that are based on clinical behavior or specific histologic criteria. However, Eversole has proposed a histogenetic classification of salivary gland tumors, implicating two cell types as possible progenitors: the intercalated duct cell and the excretory duct reserve cell. The various types of salivary gland tumors are best distinguished by their histologic patterns. The clinical behavior of these various lesions may be based, as in most tumors, on the type of tumor as well as on the method of treatment utilized.
It is important to recognize that neoplasms may arise not only from the major salivary glands, but also from any of the numerous, diffuse, intraoral accessory salivary glands. Thus one may expect to see tumors originating from the glands in the lip, palate, tongue, buccal mucosa, floor of the mouth and retromolar area. Salivary gland tumors are much more common on the hard palate than on the soft, probably because there are a greater number of gland aggregates on the hard palate than on the soft palate. With only occasional exceptions, any type of tumor which occurs in a major salivary gland may also arise in an intraoral accessory gland. Thus, in the following discussion, the general features described under each tumor will hold true for both major and minor salivary gland lesions. There appear to be no truly specific, recognized tumors native only to the intraoral glands. Annual incidence of salivary gland tumors around the world is stated to be 1–6.5 cases per 100,000 people. Most studies have shown that minor salivary gland tumors are more common in females than males, with a ratio range from 1.2 : 1–1.9 : 1. Eneroth has presented data on over 2,300 tumors of the major salivary glands (Table 3-1), while a complete review of the intraoral minor salivary gland tumors has been published by Chaudhry and his coworkers, with much valuable information obtained from the analysis of over 1,300 cases (Table 3-2).
Table 3-1
Occurrence of major salivary gland tumors
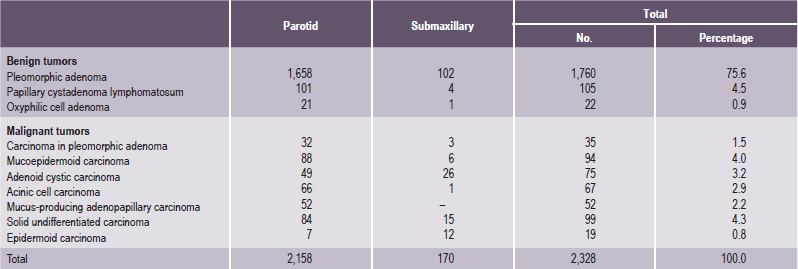
Data from CM Eneroth: Salivary gland tumors in the parotid gland, submandibular gland and the palate region. Cancer, 27: 1415, 1971.
Table 3-2
Occurrence of intraoral accessory salivary gland tumors
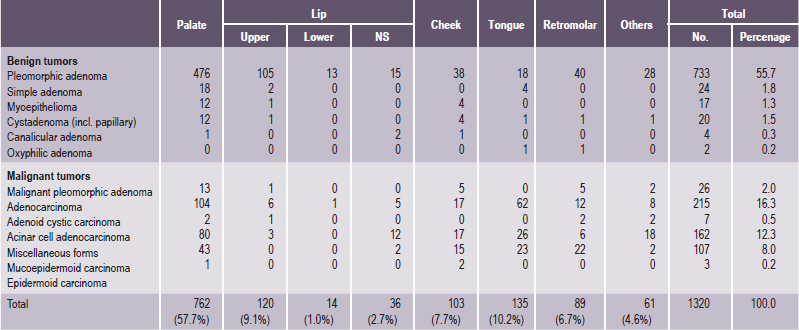
Data from AP Chaudhry, RA Vickers, and RJ Gorlin: Intraoral minor salivary gland tumors. Oral Surg, 14: 1194, 1961.
Benign tumors of the salivary gland need no treatment other than surgical removal. Malignant tumors, on the other hand, may require radiation or chemotherapy or both after surgery. Surgery for salivary gland tumors removes all or most of the affected glands, not just the tumor. When performing surgery on these glands, great care is taken to identify and protect the nerves that pass through or near these glands and supply the muscles of the face, mouth and tongue. These nerves are stretched during surgery, which results in a temporary weakness on part of the face in up to 15% of patients. This weakness is temporary and usually disappears in one to three months. Occasionally, malignant tumors invade the nerves that supply part or all of the muscles of the face. When this occurs, a portion of the nerve is surgically removed with the tumor, and a nerve graft is used to rebuild the nerve.
Benign Tumors of the Salivary Glands
Pleomorphic Adenoma (Mixed tumor)
Pleomorphic adenoma is a benign neoplasm consisting of cells exhibiting the ability to differentiate to epithelial (ductal and nonductal) cells and mesenchymal (chondroid, myxoid and osseous) cells. This tumor has been referred to by a great variety of names through the years (e.g. mixed tumor, enclavoma, branchioma, endothelioma, enchondroma), but the term ‘pleomorphic adenoma’ suggested by Willis characterizes closely the unusual histologic pattern of the lesion. It is almost universally agreed that this tumor is not a ‘mixed’ tumor in the true sense of being teratomatous or derived from more than one primary tissue. Its morphologic complexity is the result of the differentiation of the tumor cells, and the fibrous, hyalinized, myxoid, chondroid and even osseous areas are the result of metaplasia or are actually products of the tumor cells per se. Pleomorphic adenoma is the most common salivary gland tumor (Table 3-3).
Table 3-3
Histological classification of salivary gland tumors (WHO 1991)
1. Adenomas
• Pleomorphic adenoma
• Myoepithelioma (myoepithelial adenoma)
• Basal cell adenoma
• Warthin’s tumor (adenolymphoma)
• Oncocytoma (oncocytic adenoma)
• Canalicular adenoma
• Sebaceous adenoma
• Ductal papilloma
▫ Inverted ductal papilloma
▫ Intraductal papilloma
▫ Sialadenoma papilliferum
• Cystadenoma
▫ Papillary cystadenoma
▫ Mucinous cystadenoma
2. Carcinomas
• Acinic cell carcinoma
• Mucoepidermoid carcinoma
• Adenoid cystic carcinoma
• Polymorphous low grade adenocarcinoma (terminal duct adenocarcinoma)
• Epithelial-myoepithelial carcinoma
• Basal cell adenocarcinoma
• Sebaceous carcinoma
• Papillary cystadenocarcinoma
• Mucinous adenocarcinoma
• Oncocytic carcinoma
• Salivary duct carcinoma
• Adenocarcinoma
• Malignant myoepithelioma (myoepithelial carcinoma)
• Carcinoma in pleomorphic adenoma (malignant mixed tumor)
• Squamous cell carcinoma
• Small cell carcinoma
• Undifferentiated carcinoma
• Other carcinomas
3. Nonepithelial tumors
4. Malignant lymphomas
5. Secondary tumors
6. Unclassified tumors
7. Tumor like lesions
• Sialadenosis
• Oncocytosis
• Necrotizing sialometaplasia (salivary gland infarction)
• Benign lymphoepithelial lesion
• Salivary gland cysts
• Chronic sclerosing sialadenitis of submandibular gland (Küttner tumor)
• Cystic lymphoid hyperplasia in AIDS
Adapted from: Seifert G. Histological typing of salivary gland tumors, 2nd ed. Berlin, Springer-Verlag, 1991.
Histogenesis
Numerous theories have been advanced in explaining the histogenesis of this bizarre tumor. Currently, these center around the myoepithelial cell and a reserve cell in the intercalated duct. Ultrastru ctural studies have confirmed the presence of both ductal and myoepithelial cells in pleomorphic adenomas. It follows that possibly either or both may play active roles in the histogenesis of the tumor. Hubner and his associates have postulated that the myoepithelial cell is responsible for the morphologic diversity of the tumor, including the production of the fibrous, mucinous, chondroid and osseous areas. Regezi and Batsakis postulated that the intercalated duct reserve cell can differentiate into ductal and myoepithelial cells and the latter, in turn, can undergo mesenchymal metaplasia, since they inherently have smooth muscle like properties. Further differentiation into other mesenchymal cells then can occur. Batsakis has discussed salivary gland tumorigenesis, and while still implicating the intercalated duct reserve cell as the histogenetic precursor of the pleomorphic adenoma, stated that the role of the myoepithelial cell is still uncertain and that it may be either an active or passive participant histogenetically. Finally, Dardick and his associates have questioned the role of both ductal reserve and myoepithelial cells. They state that a neoplastically altered epithelial cell with the potential for multidirectional differentiation may be histogenetically responsible for the pleomorphic adenoma.
Pleomorphic adenomas have shown consistent cytogenetic abnormalities, chiefly involving the chromosome region 12q13-15. The putative pleomorphic adenoma gene (PLAG1) has been mapped to chromosome 8q12. Many other genes have also been implicated; however, cytogenetic or molecular studies do not as yet have an established role in the diagnosis of pleomorphic adenoma.
Clinical Features
Pleomorphic adenoma is the most common tumor of salivary glands. The parotid gland is the most common site of the pleomorphic adenoma; 90% of a group of nearly 1,900 such tumors reported by Eneroth. It may occur in any of the major glands or in the widely distributed intraoral accessory salivary glands; however its occurrence in the sublingual gland is rare. In the parotid this tumor most often presents in the lower pole of the superficial lobe of the gland, about 10% of the tumors arise in the deeper portions of the gland. Approximately 8% of pleomorphic adenomas involve the minor salivary glands, the palate is the most common site (60–65%) of minor salivary gland involvement. It occurs more frequently in females than in males, the ratio approximating 6 : 4. The majority of the lesions are found in patients in the fourth to sixth decades with the average age of occurrence of about 43 years, but they are also relatively common in young adults and have been known to occur in children. The clinical behavior of this tumor in children is similar to that in adults (Table 3-4).
Table 3-4
TNM/AJCC 1997 staging (clinical staging)
TX: primary tumor cannot be assessed
• T0: No evidence of primary tumor
• T1: Tumor 2 cm or less in greatest dimension without extraparenchymal extension*
• T2: Tumor > 2 cm but < 4 cm in greatest dimension without extraparenchymal extension*
• T3: Tumor having extraparenchymal extension* without seventh nerve involvement and/or more than 4 cm but no more than 6 cm in greatest dimension
• T4: Tumor invades base of skull, seventh nerve, and/or exceeds 6 cm in greatest Dimension
• N0: No regional node metastasis
• Nx: Regional nodes cannot be assessed
• N1: Single ipsilateral node, < 3 cm
• N2a: Single ipsilateral node, > 3 cm and < 6 cm
• N2b: Multiple ipsilateral nodes, < 6 cm
• N2c: Contralateral or bilateral nodes, < 6 cm
• N3: Node > 6 cm
• MO: No distant metastasis
• Mx: Metastasis cannot be assessed
Minor salivary gland tumors are staged according to their site of origin.
Microscopic evidence alone does not constitute extraparenchymal extension for classification purpose.
*Extraparenchymal extension is clinical or macroscopic evidence of invasion of skin, soft tissues, bone or nerve.
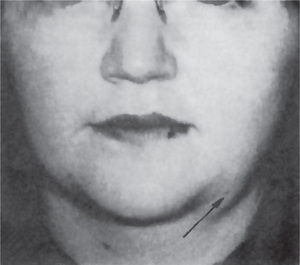
The history presented by the patient is usually that of a small, painless, quiescent nodule which slowly begins to increase in size, sometimes showing intermittent growth (Fig. 3-1). The pleomorphic adenoma, particularly of the parotid gland, is typically a lesion that does not show fixation either to the deeper tissues or to the overlying skin (Fig. 3-2 A). It is usually an irregular nodular lesion which is firm in consistency, although areas of cystic degeneration may sometimes be palpated if they are superficial. The skin seldom ulcerates even though these tumors may reach a fantastic size, lesions having been recorded which weighed several kilograms (Fig. 3-2 B). Pain is not a common symptom of the pleomorphic adenoma, but local discomfort is frequently present. Facial nerve involvement manifested by facial paralysis is rare.
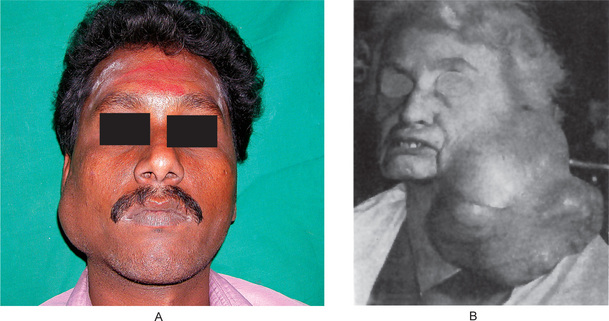
Figure 3-2 Pleomorphic adenoma of parotid gland.
(A) Typical appearance of pleomorphic adenoma of parotid gland. (B) The lesion here is used not to illustrate the usual clinical appearance of a pleomorphic adenoma of the parotid gland, but to demonstrate the size which these tumors may attain. This lesion was present for eighteen years A, Courtesy of Dr Neelakandan RS, Department of Oral and Maxillofacial Surgery, Meenakshi Ammal Dental College, Chennai.
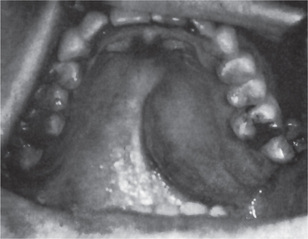
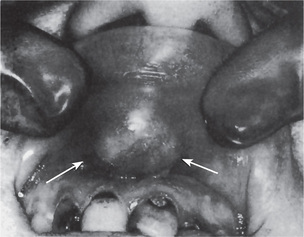
The pleomorphic adenoma of intraoral accessory glands seldom is allowed to attain a size greater than 1–2 cm in diameter. Because this tumor causes the patient difficulties in mastication, talking and breathing, it is detected and treated earlier than tumors of the major glands. The palatal glands are frequently the site of origin of tumors of this type (Fig. 3-3), as are the glands of the lip (Fig. 3-4) and occasionally other sites. Except for size, the intraoral tumor does not differ remarkably from its counterpart in a major gland. The palatal pleomorphic adenoma may appear fixed to the underlying bone, but is not invasive. In other sites the tumor is usually freely movable and easily palpated. Recurrent lesions, however, occur as multiple nodules and are less mobile than the original tumor (Table 3-5).
Histologic Features
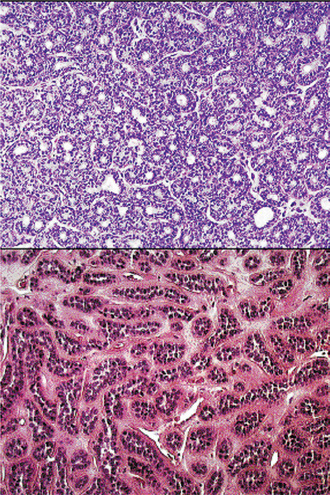
The mixed tumors generally appear as an irregular to ovoid mass with well-defined borders. The tumors in major glands have either an incomplete fibrous capsule or are unencapsulated whereas in the minor glands they are unencapsulated. The cut surface may be rubbery, fleshy, mucoid or glistening with a homogeneous tan or white color. Areas of hemorrhage and infarction may be noted occasionally.
Morphologic diversity is the most characteristic feature of this neoplasm. Microscopically, benign mixed tumors are characterized by variable, diverse, structural histologic patterns, seldom do individual cases resemble each other, considerable variation is also seen within a single tumor. Pleomorphic adenomas demonstrate combinations of glandular epithelium and mesenchyme like tissue and the proportion of each component varies widely among individual tumors. Foote and Frazell (1954) categorized the tumor into the following types:
Table 3-6
Typical features of benign and malignant salivary gland tumors
| Benign salivary gland tumors | Malignant salivary gland tumors |
| • Slow growing | • Sometimes fast growing |
| • Soft or rubbery consistency | • Sometimes hard consistency |
| • 85% of parotid tumors are benign | • 45% of minor glands are malignant |
| • Do not ulcerate | • May ulcerate and invade bone |
| • No associated nerve signs | • May cause cranial nerve palsies (e.g. parotid tumor causes facial palsy, adenoid cystic carcinoma can cause multiple nerve lesions especially of lingual, facial or hypoglossal nerves) |
The epithelial component forms ducts and small cysts that may contain an eosinophilic coagulum, the epithelium may also occur as small cellular nests, sheets of cells, anastomosing cords and foci of keratinizing squamous or spindle cells. Myoepithelial cells are a major component of pleomorphic adenoma. They have a variable morphology, sometimes appearing as angular or spindled, while some cells are more rounded with eccentric nuclei and hyalinized eosinophilic cytoplasm resembling plasma cells (earlier referred to as hyaline cells) (Fig. 3-5). Myoepithelial-cells are also responsible for the characteristic mesenchyme like changes; these changes are brought about by extensive accumulation of mucoid material around individual myoepithelial cells giving a myxoid appearance. Vacuolar degeneration of these myoepithelial cells then results in a cartilaginous appearance (Fig. 3-6). Foci of hyalinization, bone and even fat can be noted in the connective tissue stroma of many tumors. When the pleomorphic pattern of the stroma is absent, and the tumor is highly cellular, it is often referred to as a ‘cellular adenoma’. When myoepithelial proliferation predominates, the diagnosis of ‘myoepithelioma’ (q.v.) is generally made.
Treatment and Prognosis
The accepted treatment for this tumor is surgical excision. The intraoral lesions can be treated somewhat more conservatively by extracapsular excision. Since these tumors are radioresistant, the use of radiation therapy is of little benefit and is therefore contraindicated.
Rarely, a malignant tumor may arise within this tumor, a phenomenon known as carcinoma ex pleomorphic adenoma. There is a second class of tumors which are called metastasizing benign mixed tumors. These tumors have a histologically benign appearance but usually have a history of multiple local recurrences. Metastases occur several years after the initial diagnosis and may occur to the lungs, regional lymph nodes, skin, and bone. The usual clinical course is good but there are cases which have an aggressive clinical course leading to death in 22% of cases. Fortunately this last category of tumors is very rare.
Myoepithelioma: (Myoepithelial adenoma)
The term myoepithelioma was first used by Sheldon in 1943. The myoepithelioma is an uncommon salivary gland tumor which accounts for less than 1% of all major and minor salivary tumors. Nonetheless, it is important in that the component cell constitutes a prominent place in salivary gland neoplasia. Many authorities, including Batsakis, consider the myoepithelioma to be a ‘one-sided’ variant at the opposite end of the spectrum from the pleomorphic adenoma.
Clinical Features
There are no clinical features which can serve to separate the myoepithelioma from the more common pleomorphic adenoma. It occurs in adults with an equal gender distribution. The parotid gland is most commonly involved and the palate is the most frequent intraoral site of occurrence. Sciubba and Brannon reported lesions in the retromolar glands and the upper lip.
Histologic Features
The tumor is composed exclusively, or almost exclusively, of neoplastic myoepithelial cells. The neoplastic cells are predominantly spindle-shaped or plasma-cytoid. Epithelioid or clear cells may also be present (Figs. 3-7, 3-8). Either a single cell type predominates in a tumor or there may be a combination of cell types. The tumor is often difficult to diagnose definitively at the light microscopic level. Myoep-ithelioma does not contain the characteristic chondromyxoid stroma of pleomorphic adenoma. Myoepithelioma consisting predominantly of spindle cells tends to be more cellular than the tumor consisting of predominantly plasmacytoid cells. Definitive diagnosis lies in the ultrastructural identification of myoepithelial cells. The myoepithelial cell exhibits a basal lamina and fine intracytoplasmic myofilaments. Desmosomes are encountered between adjacent cells.
Basal Cell Adenoma
Basal cell adenoma is a neoplasm of a uniform population of basaloid epithelial cells arranged in solid, trabecular, tubular, or membranous patterns. The basal cell adenoma was first reported as distinct entity by Kleinsasser and Klein in 1967. Batsakis is credited with reporting the first case in the American literature in 1972, and suggested that the intercalated duct or reserve cell is the histogenetic source of the basal cell adenoma.
Clinical Features
Basal cell adenomas tend to occur primarily in the major salivary glands, particularly the parotid gland. In the series reported by Batsakis and associates, 48 of 50 tumors were in the parotid gland and two were in the submaxillary gland. The tumors are usually painless and are characterized by slow growth. They occur chiefly in adults, the average age of the patients is 57.7 years with the peak of incidence seen in the sixth decade. However, the tumor can occur in younger persons, Canalis and his coworkers reported a basal cell adenoma in the submaxillary gland of a newborn male. There is a 2 : 1 female predilection for the occurrence of this tumor. These tumors appear as a firm swelling which may be cystic and compressible. These tumors are clinically indistinguishable from mixed tumors and their greatest dimension is usually less than 3 cm.
Histologic Features
Basal cell adenoma occurs as a single well-defined nodule, the membranous type may be multifocal. Tumors in major salivary gland have a well defined capsule, whereas intraoral tumors are less well defined. The cut surface often is homogeneous with gray to brown in color, and may have cystic areas.
The basal cells that make up this lesion are fairly uniform and regular; two morphologic forms can be seen. One is a small cell with scanty cytoplasm and round deeply basophilic nucleus. The other cell is large with eosinophilic cytoplasm and an ovoid pale staining nucleus. Basal cell adenomas can be divided on the basis of their morphologic appearances into four subtypes:
The most common type of basal cell adenoma is the solid variant. The basaloid cells form islands and cords that have a broad, rounded, lobular pattern. These cells are sharply demarcated from the connective tissue stroma by basement membrane. This feature contrasts with the melting type of growth characteristic of pleomorphic adenoma.
This pattern exhibits multiple small, round duct like structures. These tubules are lined by two distinct layers of cells, with inner cuboidal ductal cells surrounded by an outer layer of basaloid cells. The tubular variant is the least common; however, tubule formation either alone or with basal cell masses, can be found in most basal cell adenomas, at least focally (Fig. 3-9).
This subtype has the same cytologic features as the solid type, but the epithelial islands are narrower and cord like and are interconnected with one another, producing a reticular pattern (Fig. 3-10).
This is a distinct subtype of basal cell adenoma characterized by the presence of abundant, thick, eosinophilic hyaline layer that surrounds and separates the epithelial islands. Electron microscopy has shown that this hyaline material is reduplicated basement membrane. The epithelial islands are arranged in large lobules and appear to mould to the shape of other lobules to resemble a jigsaw puzzle pattern.
Warthin’s Tumor: (Papillary cystadenoma lymphomatosum, adenolymphoma)
Warthin’s tumor is the second most common tumor in the salivary glands. This tumor was first recognized by Albrecht in 1910 (quoted by Ellis and Auclair 1991) and later described by Warthin in 1929. This unusual type of salivary gland tumor occurs almost exclusively in the parotid gland, although occasional cases have been reported in the submaxillary gland. The intraoral accessory salivary glands are rarely affected.
Histogenesis
Numerous theories have been advanced to account for the peculiar nature of this tumor. The currently accepted theory is that the tumor arises in salivary gland tissue entrapped within paraparotid or intraparotid lymph nodes during embryogenesis. However, Allegra has suggested that the Warthin’s tumor is most likely a delayed hypersensitivity disease, the lymphocytes being an immune reaction to the salivary ducts which undergo oncocytic change. Hsu and coworkers recently studied the tumor immunohistochemically and have suggested that the lymphoid component of the tumor is an exaggerated secretory immune response.
A strong association between development of this tumor and smoking is documented. The exact mechanism by which smoking may predispose patients to Warthin’s tumor is unclear. However, several studies have shown that a high percentage of Warthin’s tumor patients smoke. Epstein-Barr virus has also been implicated in the pathogenesis of this tumor; however there are many conflicting reports.
Clinical Features
Warthin’s tumor was traditionally considered a disease of men. However, recent reports have identified a substantial percentage of patients who are women. This tumor commonly presents in the sixth and seventh decades and average age of the patients at the time of diagnosis of the lesion was 62 years. The tumor is generally superficial, lying just beneath the parotid capsule or protruding through it. Seldom does the lesion attain a size exceeding 3–4 cm in diameter. It is not painful, is firm to palpation and is clinically indistinguishable from other benign lesions of the parotid gland.
Histologic Features
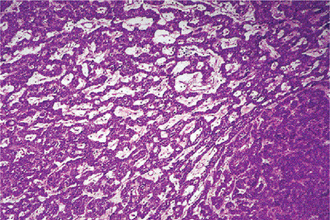
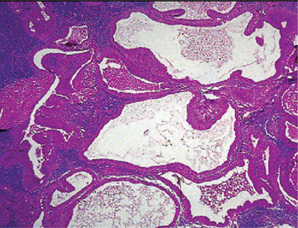
It is a smooth, some what soft parotid mass and is well encapsulated when located in the parotid. The tumor contains variable number of cysts that contains a clear fluid. Areas of focal hemorrhage may also be seen.
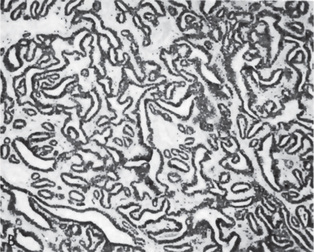
This tumor is made up of two histologic components: epithelial and lymphoid tissue. As the name would indicate, the lesion is essentially an adenoma exhibiting cyst formation, with papillary projections into the cystic spaces and a lymphoid matrix showing germinal centers (Fig. 3-11). The cysts are lined by papillary proliferations of bilayered oncocytic epithelium. The inner layer cells are tall columnar with finely granular and eosinophilic cytoplasm due to presence of mitochondria and slightly hyperchromatic nuclei. The outer layer cells are oncocytic triangular and occasionally fusiform basaloid cells. Focal areas of squamous metaplasia and mucous cell prosoplasia may be seen. There is frequently an eosinophilic coagulum present within the cystic spaces, which appears as a chocolate-colored fluid in the gross specimen. The abundant lymphoid component may represent the normal lymphoid tissue of the lymph node within which the tumor developed or it may actually represent a reactive cellular infiltrate which involves both humoral and cell mediated mechanisms (Fig. 3-12).
Treatment and Prognosis
The accepted treatment of the papillary cystadenoma lymphomatosum is surgical excision. This can almost invariably be accomplished without injury to the facial nerve, particularly since the lesion is usually small and superficial. These tumors are well encapsulated and seldom recur after removal.
Malignant transformation is exceedingly rare in either the epithelial or lymphoid component. Cases of mucoepidermoid carcinoma involving Warthin’s tumor of the parotid gland have been reported; however, a direct transition from Warthin’s tumor to mucoepidermoid carcinoma was not identified.
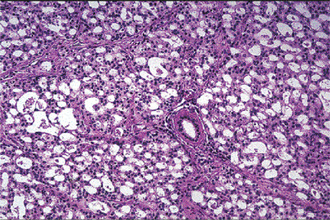
Oncocytoma: (Oncocytic adenoma, oxyphilic adenoma, acidophilic adenoma)
Oncocytoma is a rare benign tumor composed of oncocytes with granular eosinophilic cytoplasm and a large number of atypical mitochondria. This rare salivary gland tumor is a small benign lesion, which usually occurs in the parotid gland. Except that it does not generally attain any great size, it does not differ in its clinical characteristics from other benign salivary gland tumors. For this reason, a clinical diagnosis is difficult if not impossible to establish. The name ‘oncocytoma’ is derived from the resemblance of these tumor cells to apparently normal cells which have been termed ‘oncocytes’ and which are found in a great number of locations, including the salivary glands, respiratory tract, breast, thyroid, pancreas, parathyroid, pituitary, testicle, fallopian tube, liver and stomach. These cells are predominantly seen in duct linings of glands in elderly persons, but little is actually known of their mode of development or significance (Fig. 3-13). Electron microscopic studies have shown that the cytoplasm of the oncocyte is choked with mitochondria. Ionizing radiation is the predisposing condition.
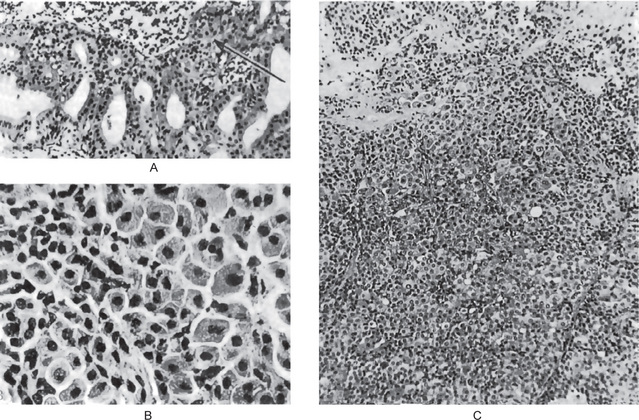
Figure 3-13 Oxyphilic adenoma.
(A) Normal oncocytes in accessory salivary gland ducts of elderly patient. (B) High-power photomicrograph. (C) Low-power photomicrograph of oncocytoma.
Clinical Features
The oncocytoma is somewhat more common in women than in men and occurs almost exclusively in elderly persons. Chaudhry and Gorlin, who reviewed the literature, found 29 cases of oncocytoma and added four new cases to those reported. Only occasionally does this tumor arise before the age of 60 years, 80% of cases occurring between the ages of 51 and 80 years. The tumor usually measures 3–5 cm in diameter and appears as a discrete, encapsulated mass which is sometimes nodular. Pain is generally absent.
An interesting condition called diffuse multi nodular oncocytoma or ‘oncocytosis’ of the parotid gland has been described by Schwartz and Feldman. This condition is characterized by nodules of oncocytes which involve the entire gland or large portions of it.
Histologic Features
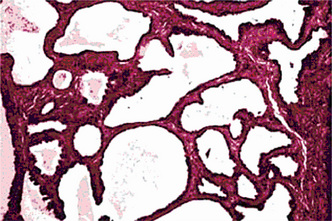
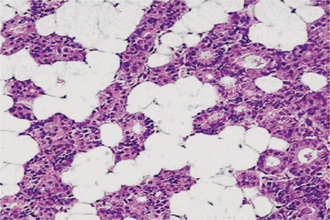
The oxyphilic adenoma is characterized microscopically by large cells which have an eosinophilic cytoplasm and distinct cell membrane and which tend to be arranged in narrow rows or cords (Fig. 3-13). The oncocytes are arranged in sheets or nests and cords, which form alveolar or organoid pattern. Some degree of cellular atypia, nuclear hyperchromatism and pleomorphism is accepted as compatible with benignancy in oncocytoma. These cells, exhibiting few mitotic figures, are closely packed, and there is little supportive stroma (Fig. 3-14). Lymphoid tissue is frequently present, but does not appear to be an integral part of the lesion. Ultrastructural studies of parotid oncocytomas by Tandler and associates and Kay and Still have shown that the cells are engorged with enlarged and morphologically altered mitochondria.
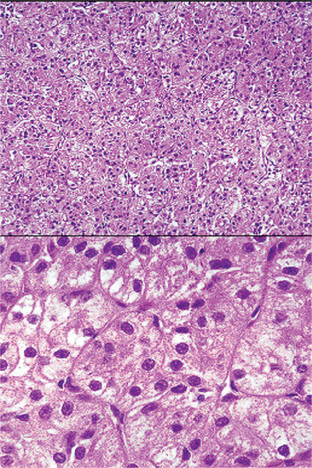
Figure 3-14 Oncocytoma alveolar pattern.
Illustrated by clusters of oncocytes that are supported by thin, fibrous connective tissue septa and small blood vessels. Note oncocytes have clear cytoplasm that is interspersed with cytoplasmic granularity.
A variant of the oxyphilic adenoma is sometimes seen in intraoral salivary glands, particularly in the buccal mucosa and upper lip. This has been termed, an oncocytic cystadenoma since it is a tumor like nodule composed chiefly of numerous dilated duct like or cyst like structures lined with oncocytes. In recurrent tumors, there may be marked clear cell change and these tumors may be referred to as clear-cell oncocytoma. Treatment and Prognosis. The treatment of choice is surgical excision, and the tumor does not tend to recur. Malignant transformation is uncommon, but malignant oncocytoma is now a well-established entity. Johns and associates reviewed the literature on malignant oncocytomas and reported three additional cases. Other well-documented cases have been those of Lee and Roth, and Gray and his coworkers.
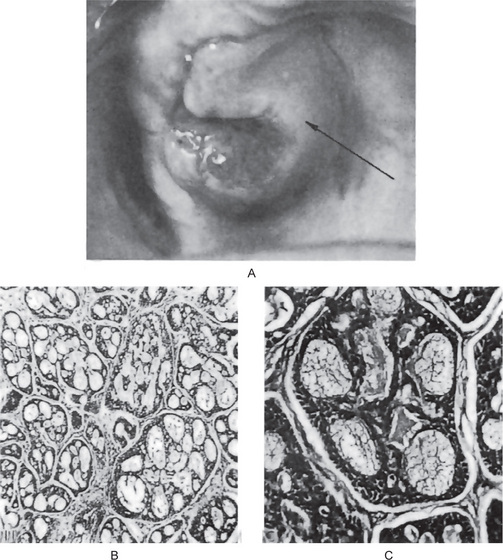
Canalicular Adenoma
Canalicular adenoma is an uncommon neoplasm composed of columnar epithelial cells arranged in a single or double layer forming branching cords in a loose stroma. The bilayer shows an intermittent separation, which forms structures resembling lumen or canaliculi.
Clinical Features
This lesion originates primarily in the intraoral accessory salivary glands, and in the vast majority of cases, it occurs in the upper lip. However, cases are known in which the lesion occurred in the palate, buccal mucosa and lower lip. Only one was noted in the parotid gland in the series reported by Nelson and Jacoway. The tumor occurs in adults far more common in patients of age 34–65 years; this tumor is seen more commonly in females with a ratio of 1.8 : 1. The tumor generally presents as a slowly growing, well-circumscribed, firm nodule which, particularly in the lip, is not fixed and may be moved through the tissue for some distance. Occasionally, two separate and distinct tumors may occur in the upper lip of an individual.
Histologic Features
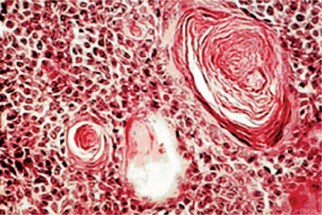
The canalicular adenoma has a strikingly characteristic picture. It is composed of long columns or cords of cuboidal or columnar cells in a single layer. These single layers of cells are parallel, forming long canals. Some-times rows of cells are closely approximated and appear as a double row of cells showing a ‘party wall’. In some instances, cystic spaces of varying sizes are enclosed by these cords. The cystic spaces are usually filled with an eosinophilic coagulum. The supporting stroma is loose and fibrillar with delicate vascularity (Fig. 3-15). The tumor has, at times, been mistaken for an adenoid cystic carcinoma and care should be taken to prevent this error (Fig. 3-16). As Mader and Nelson have pointed out, the adenoid cystic carcinoma rarely occurs in the upper lip and is seldom freely movable. Occasionally the tumor may be multifocal and foci of tumor cells are found outside the main lesion.
Sebaceous Adenoma
Sebaceous adenoma is a rare benign tumor that accounts for 0.1% of all salivary gland neoplasms and slightly less than 0.5% of all salivary adenomas. The mean age at initial clinical presentation was 58 years (range 22–90 years). This tumor is more common in men. In a series of cases reported by Ellis et al (1991), 12 tumors were located in the parotid gland, 4 in the buccal mucosa, two in the submandibular gland, and three in the area of lower molars or retromolar region. The tumors ranged in size from 0.42–3.0 cm in diameter.
The tumors are commonly encapsulated or sharply circumscribed, and they vary in color from grayish-white to pinkish-white to yellow or yellowish gray. These tumors are composed of sebaceous cell nests with minimal atypia and pleomorphism and no tendency to invade local structures. Many tumors are microcystic or may be composed predominantly of ectatic salivary ducts with focal sebaceous differentiation. The sebaceous glands may vary markedly in size and in tortuosity and are usually embedded in a fibrous stroma. Occasionally tumors demonstrate marked oncocytic metaplasia, and histiocytes and/or foreign body giant cells may be seen focally. Lymphoid follicles, cytologic atypia, cellular necrosis, and mitosis are not observed in sebaceous adenomas.
Conservative excision seems to be the treatment of choice. No recurrences have been reported.
Ductal Papilloma
The term ductal papilloma is used to identify a group of three rare benign papillary salivary gland tumors. They represent adenomas with unique papillary features and arise from the salivary gland duct system. There are three types with unique histopathological features. These are inverted ductal papilloma, intraductal papilloma, and sialadenoma papilliferum.
Inverted Ductal Papilloma
Inverted ductal papilloma was first described by White et al, in 1982. It is a very rare tumor and has been described only in minor salivary glands of adults. The lower lip is the most frequently involved site followed by buccal vestibular mucosa. Inverted ductal papillomas appear to arise from the excretory ducts near the mucosal surface. Clinically, these tumors are seen as submucosal nodules which may have a pit or indentation in the overlying surface mucosa. These tumors do not show any gender predilection.
Histologically, it consists of basaloid and squamous cells arranged in thick, bulbous papillary proliferations that project into the ductal lumen. The lumen of the tumor is often narrow and in some tumors communicates with the exterior of the mucosal surface through a constricted opening.
Intraductal Papilloma
Intraductal papilloma is an ill-defined lesion that often is confused with papillary cystadenoma. It usually occurs in adults, with a mean age of occurrence being 54 years and is common in the minor salivary glands. The lower lip is the most frequently involved site followed by upper lip, palate and buccal mucosa. No gender predilection has been noted. Intraductal papilloma presents clinically as a submucosal swelling. These tumors appear to arise from the excretory ducts at a deeper level than the inverted ductal papilloma.
Microscopically, it exhibits a unicystic dilated structure. The cyst wall is lined by a single or double row of cuboidal and columnar cells, which extend into the cyst lumen as papillary projections having thin fibrovascular cores.
Sialadenoma Papilliferum
Sialadenoma papilliferum most commonly involves the minor salivary gland. This has a more complex histology, with a biphasic growth pattern of exophytic papillary and endophytic components. This tumor usually affects adults, the average age of the patients is 56 years. A male predilection is seen. The clinical presentation is unique as nearly all salivary gland neoplasms manifest as subsurface nodular swellings whereas sialadenoma papilliferum occurs as an exophytic, papillary surface lesion. The clinical impression in most cases is squamous papilloma of the mucosa.
Microscopically, these tumors display both an exophytic and an endophytic proliferation of ductal epithelium. The surface of the lesion is formed of papillary projections of epithelium supported by fibrovascular cores, covered by parakeratotic stratified squamous epithelium. The fibrovascular cores have an inflammatory cell infiltrate of lymphocytes, plasma cells and neutrophils. The ductal epithelium continues downwards into the deeper connective tissues. Multiple ductal lumina are seen, which are lined by a double row of cells consisting of a luminal layer of tall columnar cells resting on a cuboidal basal layer.
Cystadenoma
Cystadenomas of the salivary glands are benign neoplasms in which the epithelium demonstrates adenomatous proliferation that is characterized by formation of multiple cystic structures. Several morphologic variants of cystadenoma have been described of which papillary cystadenoma and mucinous cystadenoma are important. The papillary cystadenoma (PC) is defined as a cystadenoma in which the cystic space is filled with papillary projections. WHO described papillary cystadenoma as “a tumor that closely resembles Warthin’s tumor but without the lymphoid elements, constituting multiple papillary projections and a greater variety of epithelial lining cells.” If mucous cells predominate in the cell population of the lining epithelial cells, the tumor is termed as mucinous cystadenoma.
Clinical Features
Cystadenoma is widely distributed among major and minor salivary glands. Most of the minor salivary gland tumors are seen in lips, buccal mucosa, palate and the tonsillar area. This is more common in females than males (2:1) and occurs in older age, most common in the eighth decade of life. Clinically, it presents as a slow growing painless slightly compressible swelling. Some of the nodules are clinically similar to mucocele.
Histologic Features
Epithelial proliferation (Fig. 3-17) results in various sized cystic structures. The lining of these cystic structures varies from flattened to tall columnar cells, and cuboidal, mucous and oncocytic cells may also be seen. The lining thickness varies from one to three epithelial cells. Limited papillary growth with central connective tissue core is seen. Eosinophilic or slightly hematoxyphilic secretions are seen in the stroma. Dense fibrous connective tissue stroma with scattered inflammatory cells is present.
Malignant Tumors of the Salivary Glands
Acinic Cell Carcinoma: (Acinar cell or serous cell adenoma, adenocarcinoma)
Most salivary gland tumors arise from the epithelium of the duct apparatus, but occasionally lesions seem to show acinar cell differentiation. Acinic cell carcinoma is a malignant epithelial neoplasm in which the neoplastic cells express acinar differentiation. Some authors advocate the existence of both benign and malignant acinic cell neoplasms, whereas others are of the opinion that all acinic cell neoplasms are malignant. Unfortunately, the criteria for distinguishing between benign and malignant acinar cell tumors, if such a distinction exists, have not been clearly established. In an extensive study of acinic cell tumors of the major salivary glands by Abrams and his coworkers, it was concluded that most investigators believe that all tumors of this type have at least a low-grade malignant potential. By conventional use, the term acinic cell carcinoma is defined by cytologic differentiation towards serous acinar cells (as opposed to mucous acinar cells), whose characteristic feature is cytoplasmic PAS-positive zymogen-type secretory granules. In AFIP data of salivary gland neoplasms, acinic cell carcinoma is the third most common malignant salivary gland epithelial neoplasm after mucoepidermoid carcinoma and adenocarcinoma. In this data, acinic cell carcinoma comprised 17% of primary malignant salivary gland tumors or about 6% of all salivary gland neoplasms (cited by Ellis GL, Auclair PL, Gnepp DR, 1991).
Clinical Features
The acinic cell carcinoma closely resembles the pleomorphic adenoma in gross appearance, tending to be encapsulated and lobulated. Although this tumor has been reported occurring chiefly in the parotid, with more than 80% of the cases occurring in the parotid gland, it does occur occasionally in the other major glands and in the accessory intraoral glands (Fig. 3-18). The most common intraoral sites are the lips and buccal mucosa. The acinic cell carcinoma occurs predominantly in persons in middle age or somewhat older, the mean age being 44 years. It has also been encountered in 12% of the patients before the age of 20 years. Women were affected more than men (3 : 2). This tumor presents as a slowly growing, mobile or fixed mass of various durations. Usually asymptomatic but pain or tenderness is seen in over one third of the patients. Facial muscle weakness may be seen. Patients with bilateral synchronous tumors have been reported.
Histologic Features
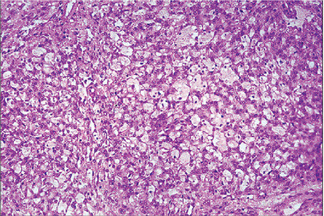
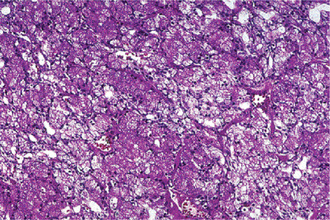
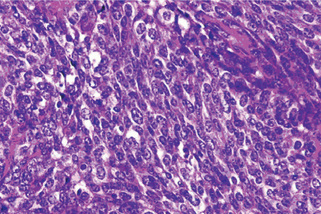
The acinic cell carcinoma, which is frequently surrounded by a thin capsule, may be composed of cells of varying degrees of differentiation. Well-differentiated cells bear remarkable resemblance to normal acinar cells, whereas less differentiated cells resemble embryonic ducts and immature acinar cells. Abrams and his associates have described four growth patterns: (1) solid, (2) papillary-cystic, (3) follicular, and (4) microcystic. In general, one pattern predominates, although combinations can occur. The most characteristic cell seen has the features of the serous acinar cells, with abundant granular basophilic cytoplasm and a round darkly stained eccentric nucleus. Other cells seen are the intercalated duct like cells, which are smaller and the vacuolated cells which seem to be unique to acinic cell carcinomas among salivary gland neoplasms.
Connective tissue stroma is delicately fibro vascular collagenous tissue. Lymphoid elements are commonly found in parotid acinic cell carcinomas, a feature which is helpful in the diagnosis. Such features are not found in the intraoral tumors. Apparently the acinic cell carcinoma can arise from embryologically entrapped salivary gland tissue in lymph nodes in or near the parotid compartment. Although ‘clear cells,’ have been described in acinic cell carcinomas, they most likely represent cells altered by fixation or they may actually represent the component cells of a clear cell carcinoma (q.v.), a recently recognized entity (Figs. 3-19, 3-20).
Treatment and Prognosis
The treatment of the acinic cell carcinoma in most cases has been surgical. Perzin and LiVolsi recommend total excision of parotid gland tumors with preservation of the facial nerve unless it is involved. Lymph node dissection is indicated only in the presence of clinical involvement and not as a routine procedure. Radiation therapy has not been shown to be of therapeutic value. Intraoral tumors are treated by surgical excision. Poor prognostic features included pain or fixation; gross invasion; and microscopic features of desmoplasia, atypia, or increased mitotic activity. Neither morphologic pattern nor cell composition was a predictive feature.
Mucoepidermoid Carcinoma
Mucoepidermoid carcinoma is a malignant epithelial tumor, first studied and described as a separate entity by Stewart, Foote and Becker in 1945. As the name implies, the tumor is composed of both mucus-secreting cells and epidermoid-type cells in varying proportions. Columnar and clear cells are also seen, and often demonstrate prominent cystic growth. It is the most common malignant neoplasm observed in the major and minor salivary glands. Mucoepidermoid carcinoma represents 29–34% of malignant tumors originating in both major and minor salivary glands. This carcinoma of the salivary glands accounts for 5% of all salivary gland tumors. The parotid gland is the most common site of occurrence. Intraorally, mucoepidermoid carcinoma shows a strong predilection for the palate.
Clinical Features
Mucoepidermoid carcinoma occurs with a slight female predilection. It occurs primarily in the third or fifth decades of life, with an average age of 47 years, but can occur in virtually all decades. It is the most common malignant salivary gland tumor of children. Prior exposure to ionizing radiation appears to substantially increase the risk of developing mucoepidermoid carcinoma.
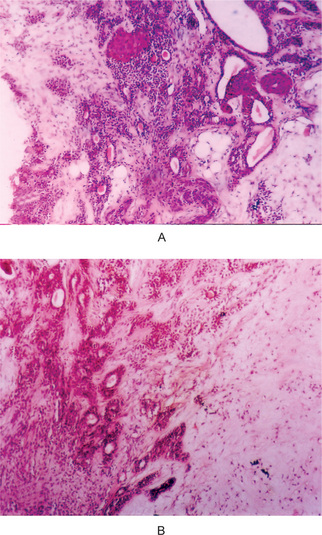
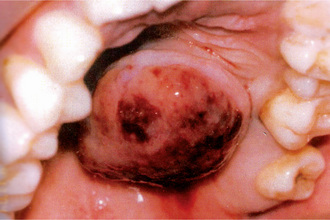
The tumor of low-grade malignancy usually appears as a slowly enlarging, painless mass which simulates the pleomorphic adenoma. Unlike the pleomorphic adenoma; however, the low-grade mucoepidermoid carcinoma seldom exceeds 5 cm in diameter, is not completely encapsulated and often contains cysts which may be filled with a viscid, mucoid material. In addition to palate intraoral tumors occur on the buccal mucosa, tongue and retromolar areas. Because of their tendency to develop cystic areas, these intraoral lesions may bear close clinical resemblance to the mucous retention phenomenon or mucocele, especially those in the retromolar area (Fig. 3-21).
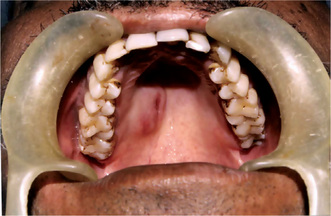
Figure 3-21 Mucoepidermoid carcinoma of palate. Courtesy of Dr Neelakandan RS, Department of Oral and Maxillofacial Surgery, Meenakshi Ammal Dental College, Chennai.
The tumor of high-grade malignancy grows rapidly and does produce pain as an early symptom. Facial nerve paralysis is frequent in parotid tumors. The patient may also complain of trismus, drainage from the ear, dysphagia, numbness of the adjacent areas and ulceration, noted particularly in tumors of the minor salivary glands. The mucoepidermoid carcinoma is not encapsulated, but tends to infiltrate the surrounding tissue, and in a large percentage of cases, it metastasize to regional lymph nodes. Distant metastases to lung, bone, brain and subcutaneous tissues are also common.
Histologic Features
The mucoepidermoid carcinoma is composed of mucous secreting cells, epidermoid type (squamous) cells and intermediate cells. The mucous cells are of various shapes and have abundant, pale, foamy cytoplasm that stains positively for mucin stains. The epidermoid cells have squamoid features, demonstrate a polygonal shape, intercellular bridges and rarely keratinization. A population of cells that is often more important in recognizing mucoepidermoid carcinoma is a group of highly prolific, basaloid cells referred to as the intermediate cells. These cells are larger than basal cells and smaller than the squamous cells and are believed to be the progenitor of epidermoid and mucous cells. Occasionally clusters of clear cells can be present. These clear cells are generally mucin and glycogen free. Epidermoid cells, together with intermediate and mucous cells line cystic spaces or form solid masses or cords. Epidermoid and mucous cells may be arranged in a glandular pattern. The cysts may rupture liberating mucus which may pool in the connective tissue and evoke an inflammatory reaction (Fig. 3-22).
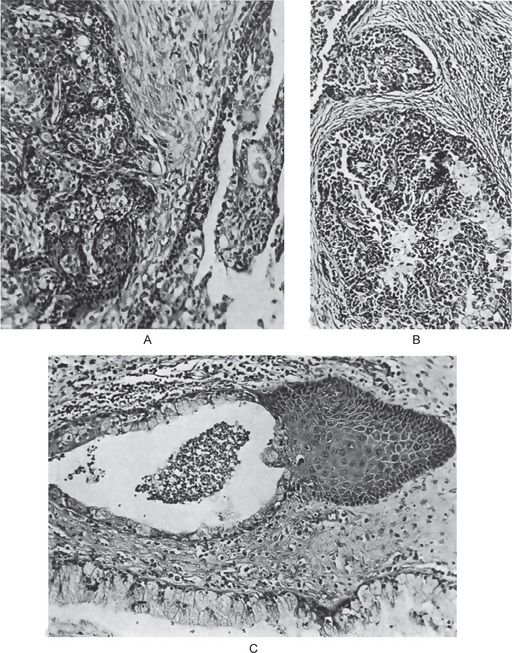
Figure 3-22 Mucoepidermoid carcinoma.
(A, B) Photomicrographs illustrate the association of the pale-staining, mucus-containing cells associated with darker staining epidermoid cells in a moderately high-grade mucoepidermoid carcinoma. (C) In one duct like structure the lining consists partially of squamous epithelium and partially of mucous cells From F Vellios: Am J Clin Pathol, 25: 147, 1955.
Mucoepidermoid carcinomas are graded as low-grade, intermediate-grade, and high-grade.
Low-grade tumors show well formed glandular structures and prominent mucin filled cystic spaces, minimal cellular atypia and a high proportion of mucous cells (Fig. 3-23).
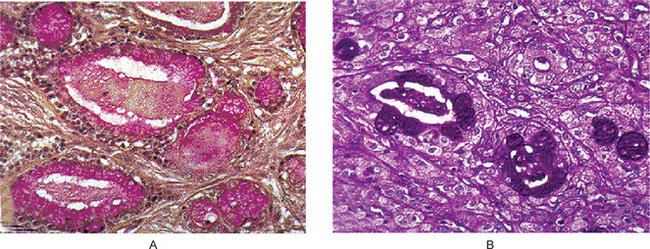
Figure 3-23 Mucoepidermoid carcinoma.
(A) Mucicarmine stain. (B) PAS stain. The two components of the tumor are large, pale, mucous secreting cells, which typically surround large or small cystic spaces and sheets of epidermoid cells.
Intermediate-grade tumors have solid areas of epidermoid cells or squamous cells with intermediate basaloid cells. Cyst formation is seen but is less prominent than that observed in low-grade tumors. All cell types are present, but intermediate cells predominate.
High-grade tumors consist of cells present as solid nests and cords of intermediate basaloid cells and epidermoid cells. Prominent nuclear pleomorphism and mitotic activity is noted. Cystic component is usually very less (<20%). Glandular component is rare although occasionally it may predominate. Necrosis and perineural invasion may be present.
Variants of Tumor
Sclerosing mucoepidermoid carcinoma
Although mucoepidermoid carcinoma is the most common primary malignancy of the salivary glands, the sclerosing morphologic variant of this tumor is extremely rare, with only six reported cases. As its name suggests, sclerosing mucoepidermoid carcinoma is characterized by an intense central sclerosis that occupies the entirety of an otherwise typical tumor, frequently with an inflammatory infiltrate of plasma cells, eosinophils, and/ or lymphocytes at its peripheral regions.
The sclerosis associated with these tumors may obscure their typical morphologic features and result in diagnostic difficulties. Tumor infarction and extravasation of mucin resulting in reactive fibrosis are two mechanisms that have been suggested as the cause of this morphologic variant.
Intraosseous mucoepidermoid carcinoma
Mucoepidermoid carcinoma may originate within the jaws. This tumor type is known as central mucoepidermoid carcinoma. It is thought to form by the malignant transformation of the epithelial lining of odontogenic cysts. The tumor presents as an asymptomatic radiolucent lesion and is histologically of low-grade malignancy. The mandible is three times more commonly affected than the maxilla.
Treatment and Prognosis
Conservative excision with preservation of the facial nerve, if possible, is recommended for low- and intermediate-grade mucoepidermoid carcinomas of the parotid gland. The affected submandibular gland should be removed entirely. Radical neck dissection is performed in patients with clinical evidence of cervical node metastasis and is considered in any patient with a T3 lesion. Treatment for the minor glands is also primarily surgical. Some investigators have recommended postoperative irradiation only for high-grade malignancies, including high-grade mucoepidermoid carcinomas of the parotid glands. Few studies have assessed the role of chemotherapy and showed that high-grade mucoepidermoid carcinoma may show sensitivity similar to that of squamous cell carcinoma. Low-grade lesions had a five-year cure rate of 92%, whereas the intermediate-grade and high-grade lesions had a 49% 5-year cure rate.
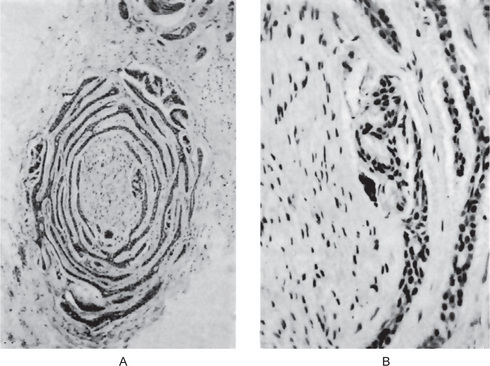
Adenoid Cystic Carcinoma: (Cylindroma, adenocystic carcinoma, adenocystic basal cell carcinoma, pseudoadenomatous basal cell carcinoma, basaloid mixed tumor)
Adenoid cystic carcinoma (formerly known as ‘cylindroma’) is a slow-growing but aggressive neoplasm with a remarkable capacity for recurrence. It is characterized by proliferation of ductal (luminal) and myoepithelial cells in cribriform, tubular, solid and cystic patterns. In a review of its case files, the AFIP found adenoid cystic carcinoma to be the fifth most common malignant epithelial tumor of the salivary glands after mucoepidermoid carcinoma; adenocarcinoma, acinic cell carcinoma; and polymorphous low-grade adenocarcinoma (PLGA). However, other series report adenoid cystic carcinoma to be the second most common malignant tumor.
Clinical Features
The salivary glands most commonly involved by this tumor are the parotid, the submaxillary and the accessory glands in the palate and tongue (Fig. 3-24). The adenoid cystic carcinoma occurs most commonly during the fifth and sixth decades of life, but it is by no means rare even in the third decade. It is seen more commonly in females. Many of the patients exhibit clinical manifestations of a typical malignant salivary gland tumor: early local pain, facial nerve paralysis in the case of parotid tumors, fixation to deeper structures and local invasion. Some of the lesions, particularly the intraoral ones, exhibit surface ulceration. There may be clinical resemblance in some cases to the pleomorphic adenoma. Adenoid cystic carcinoma has a marked tendency to spread through perineural spaces and usually invades well beyond the clinically apparent borders.
Histologic Features
Adenoid cystic carcinoma is composed of myoepithelial cells and ductal cells which have a varied arrangement. Morphologi cally, three growth patterns have been described: cribriform (classic), tubular, and solid (basaloid). The tumors are categorized according to the predominant pattern. The cribriform pattern shows basaloid epithelial cell nests that form multiple cylindrical cyst like patterns resembling a Swiss cheese or honey comb pattern, which is the most classic and best recognized pattern. The lumina of these spaces contain periodic acid-Schiff (PAS) positive mucopoly-saccharide secretion. The tubular pattern reveals tubular structures that are lined by stratified cuboidal epithelium. The solid pattern shows solid groups of cuboidal cells with little tendency towards duct or cyst formation. The cribriform pattern is the most common, whereas the solid pattern is the least common. Solid adenoid cystic carcinoma is a high-grade lesion with reported recurrence rates of up to 100% compared with 50–80% for the tubular and cribriform variants.
Variants
Dedifferentiation of adenoid cystic carci noma
Dedifferentiated adenoid cystic carcinomas are a recently defined, rare variant of adenoid cystic carcinoma characterized histologically by two components: conventional low-grade adenoid cystic carcinoma and high-grade ‘dedifferentiated’ carcinoma. Because of frequent recurrence and metastasis, the clinical course is short, similar to that of adenoid cystic carcinomas with a predominant solid growth pattern. Histologically, the low-grade adenoid cystic carcinoma merges gradually into an extensive dedifferentiated component that is composed of solid sheets and cords of anaplastic tumor cells with focal gland formation. Immuno histochemically, the dedifferentiated component (but not the adenoid cystic carcinoma component) shows strong overexpression of p53 protein and cyclin D1, as well as a higher Ki67 index. Molecular studies confirmed the presence of p53 gene mutation selectively in the dedifferentiated component, suggesting a pivotal role of p53 gene alteration in the dedifferentiation process of adenoid cystic carcinoma.
Treatment and Prognosis
The treatment of the adenoid cystic carcinoma is chiefly surgical, although in some cases surgery has been successfully coupled with X-ray radiation. Radiation alone is not recommended. In general, this tumor is a slowly growing lesion which tends to metastasize only late in its course. The cure rate for patients with this disease, though varying somewhat from series to series, is discouragingly low. Factors influencing prognosis are the site of occurrence and the histologic pattern of the tumor (Fig. 3-25). Conley and Dingman (1974) found that there is a marked difference in the clinical behavior of major and minor gland adenoid cystic carcinomas. Only 28% of 78 patients with minor gland tumors were alive with no evidence of disease in a 4–14-year follow-up study. Sixty-four percent of 54 patients with major gland tumors studied over a comparable period were alive and free of disease.
Polymorphous Low-Grade Adenocarcinoma
Polymorphous low-grade adenocarcinoma (PLGA) is a malignant epithelial tumor that is essentially limited in occurrence to minor salivary gland sites and is characterized by bland, uniform nuclear features; diverse but characteristic architecture; infiltrative growth; and perineural infiltration. This is a recently recognized type of salivary gland tumor first described in 1983. Evans and Batsakis first used the term polymorphous low-grade adenocarcinoma in 1984 to describe this tumor. This tumor includes those entities, which were previously termed as terminal duct carcinoma, lobular carcinoma, papillary carcinoma and trabecular carcinoma.
Clinical Features
In minor gland sites polymorphous lowgrade adenocarcinoma is twice as frequent as adenoid cystic carcinoma, and, among all benign and malignant salivary gland neoplasms, only pleomorphic adenoma and mucoepidermoid carcinoma are more common. The average age of patients is reported to be 59 years, with 70% of patients between the ages of 50 and 79 years. The female to male ratio is about 2 : 1. In the AFIP case files, over 60% of tumors occurred in the mucosa of either the soft or hard palates, approximately 16% occurred in the buccal mucosa, and 12% in the upper lip. Polymorphous low-grade adenocarcinoma typically presents as a firm, nontender swelling involving the mucosa of the hard and soft palates (often at their junction), the cheek, or the upper lip. Discomfort, bleeding, telangiectasia, or ulceration of the overlying mucosa may occasionally occur.
Histologic Features
Microscopically polymorphous low-grade adenocarcinoma is characterized by infiltrative growth with diverse morphology and uniform cytologic features. The tumors are well circumscribed but unencapsulated and infiltrate into adjacent structures. The polymorphic nature of the lesion refers to the variety of growth patterns it assumed which includes solid, ductal, cystic and tubular. In some tumors, a cribriform pattern can be produced, which resembles adenoid cystic carcinoma. The tumor is composed of cuboidal to columnar isomorphic cells that have uniform ovoid to spindle- shaped nuclei. Scant to moderate amounts of eosinophilic cytoplasm can be seen. The tumor stroma varies from mucoid to hyaline and in some cases, tumor nests are separated by fibrovascular stroma. Perineural invasion is common that makes this tumor mistaken as adenoid cystic carcinoma.
Treatment
Polymorphous low-grade adenocarcinoma is best treated by conservative wide surgical excision. This neoplasm typically runs a moderately indolent course. Although the tumor can recur, sometimes multiple times over many years, distant metastases have not been reported. The overall prognosis is good. Perineural invasion does not appear to affect the prognosis.
Epithelial-Myoepithelial Carcinoma: (Adenomyoepithelioma, clear cell adenoma, tubular solid adenoma, monomorphic clear cell tumor, glycogen-rich adenoma, glycogen-rich adenocarcinoma)
Epithelial-myoepithelial carcinoma is an uncommon, biphasic low-grade epithelial neoplasm composed of variable proportions of ductal and large, clear-staining, differentiated myoepithelial cells. It comprises approximately 1% of all epithelial salivary gland neoplasms.
Clinical Features
This is predominantly a tumor of the parotid gland. In the AFIP case files, the mean age of patients is about 60 years and women are more often affected than men. Localized swelling with a history of steady increase in size over a period of time is the common symptom, but occasionally patients experience facial weakness or pain. Nasal obstruction and facial deformity may represent major complaints of patients with maxillary involvement. Some studies have suggested that patients with these tumors are at increased risk for a second primary malignancy—either in the salivary glands or in a separate site (breast and thyroid have been reported).
Histologic Features
The histologic features of this tumor may vary greatly from solid lobules that are separated by bands of hyalinized fibrous tissue to irregular, papillary cystic arrangements with tumor cells which partially or completely fill cystic spaces but most tumors show a multinodular growth pattern with islands of tumor cells separated by dense bands of fibrous connective tissue. The islands of tumor cells are composed of small ducts lined by cuboidal epithelium that is surrounded by clear cells which interface with a thickened, hyaline-like basement membrane. The inner luminal cuboidal cells have finely granular, dense eosinophilic cytoplasm and central or basally located nucleus. The outer, clear myoepithelial cells vary in shape from columnar to ovoid and have a vesicular nucleus located towards the basement membrane.
Basal Cell Adenocarcinoma: (Basaloid salivary carcinoma, carcinoma ex monomorphic adenoma, malignant basal cell adenoma, malignant basal cell tumor, basal cell carcinoma)
Basal cell adenocarcinoma of salivary glands is an uncommon and recently described entity occurring almost exclusively at the major salivary glands. It is a low-grade malignant neoplasm that is cytologically similar to basal cell adenoma, but is infiltrative and has a small potential for metastasis.
Clinical Features
In AFIP case files spanning almost 11 years, basal cell carcinoma comprised 1.6% of all salivary gland neoplasms and 2.9% of salivary gland malignancies. Nearly 90% of tumors occurred in the parotid gland. The average age of patients is reported to be 60 years. Basal cell adenocarcinoma predominantly occurs at the seventh decade without gender preference. The tumors affecting the minor salivary glands occur most frequently at the oral cavity (buccal mucosa, palate) and the upper respiratory tract. Similar to most salivary gland neoplasms, swelling is typically the only sign or symptom experienced. A sudden increase in size may occur in a few patients.
Histologic Features
As seen with basal cell adenoma, basal cell adenocarcinoma can also be histologically divided into four subtypes: (1) solid, (2) ductal, (3) trabecular, and (4) membranous.
The prevalent histologic tumor pattern is represented by solid neoplastic aggregates with a peripheral cell palisading arrangement frequently delineated by basement membrane like material. Few areas with trabecular or membranous arrangement may be seen. The neoplastic clusters are formed by two cell populations: the small dark cell type, which is predominant and a large pale cell type. Generally, the small dark cells are present peripherally to the larger paler cells. The tumor extends into the surrounding tissues by local infiltration as nodules, nests and cords. Perineural and vascular invasion is noticed in a few cases.
Treatment and Prognosis
Basal cell carcinomas are low-grade carcinomas that are infiltrative, locally destructive, and tend to recur. They only occasionally metastasize. Surgical excision with a wide enough margin to ensure complete removal of the tumor is the primary treatment. Regional lymph node dissection is recommended only if there is evidence of metastatic disease. The overall prognosis for patients with this tumor is good.
Sebaceous Carcinoma
Sebaceous carcinoma is a malignant neoplasm consisting chiefly of sebaceous cells, which are arranged in sheets and/or nests with different degrees of pleomorphism, nuclear atypia and invasiveness.
Clinical Features
This tumor shows bimodal age distribution, peak incidence of tumor occurrence is found in the third decade and seventh and eighth decades of life (range 17–93 years). The male and female incidence of occurrence is almost equal. Most of the reported cases have arisen in the parotid gland. The chief complaint is that of painful masses, with varying degrees of facial nerve paralysis, and occasionally, fixation of the skin is present. Tumors range in size from 0.6–8.5 cm in greatest dimension.
Histologic Features
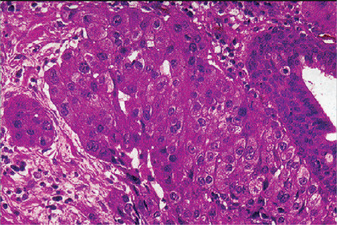
Tumors are well circumscribed or partially encapsulated, with pushing or locally infiltratingmargins. Cellular pleomorphism and cellular atypia are uniformly present and are more prevalent than in sebaceous adenomas. Tumor cells may be arranged in multiple lare focior in sheets and have hyperchromatic nuclei surrounded by abundant clear to eosinophilic cytoplasm (Fig. 3-26 A). Areas of cellular necrosis and fibrosis are commonly found. Perineural invasion has been observed in more than 20% of tumors. Vascular invasion is extremely unusual. Rare oncocytes and foreign body giant cells with histiocytes may be observed.
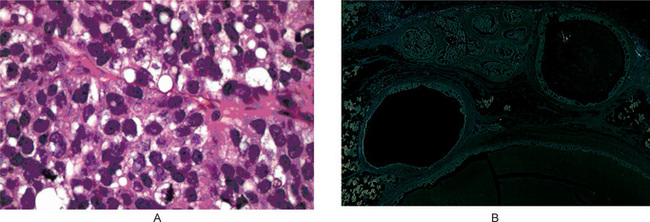
Figure 3-26 Sebaceous cell adenocarcinoma and cystadenocarcinoma.
(A) Sebaceous cell adenocarcinoma. Well differentiated sebaceous carcinoma composed of an area resembling sebaceous adenoma admixed with sheets of carcinoma cells. (B) Cystadenocarcinoma. Cystic neoplasm showing irregular cysts with great variation in size and frequent intraluminal papillary process. Invasion into the parotid parenchyma is noticed (upper field) Courtesy of Christopher DM Fletcher.
Papillary Cystadenocarcinoma: (Cystadenocarcinoma, malignant papillary cystadenoma, mucus-producing adenopapillary [nonepidermoid] carcinoma, low-grade papillary adenocarcinoma of palate, papillary adenocarcinoma)
Cystadenocarcinoma is the malignant counterpart of cystadenoma. It is a rare malignant epithelial tumor characterized histologically by prominent cystic and, frequently, papillary growth but lacking features that characterize cystic variants of several more common salivary gland neoplasms.
Clinical Features
Cystadenocarcinoma is considered to be a low-grade neoplasm. Most of the cases, about 65%, occurred in the major salivary glands (primarily in the parotid). Among the reported cases men and women are found to be affected equally and the average age at presentation is about 59 years. Patients present with a slowly growing asymptomatic mass. Clinically, this neoplasm is rarely associated with pain or facial paralysis.
Histologic Features
A cystic growth pattern must dominate the histologic appearance for the diagnosis to be considered (Fig. 3-26 B). These cystic spaces vary in size between tumors as well as within the same tumor. These neoplasms may appear circumscribed or reveal haphazard growth throughout the gland. The lumina often are filled with mucus and hemorrhage and dystrophic calcifications are sometimes evident focally. The lining cells vary from cuboidal to tall columnar, and often a single tumor contains basaloid, oncocytic, clear, and occasionally, mucus cells that form adenomatous or nodular, solid epithelial areas. These solid areas usually occupy the space between the cystic structures. Nuclear hyperchromatism and nuclear variability are subtle, and only rare mitotic figures are present. Nucleoli may be obvious. Encapsulation is incomplete, and infiltration into either salivary gland parenchyma or fibrous or adipose tissue is seen. The tumor may infiltrate either as cyst like structures or as solid islands. Although the vast majority of cystadenocarcinomas are low-grade lesions, moderate/intermediate-grade tumors do exist.
Treatment and Prognosis
No information is currently available that pertains specifically to the prognosis of the salivary gland cystadenocarcinomas. Currently the treatment principles for low-grade cystadenocarcinomas are similar to the principles applied for other low-grade salivary gland adenocarcinomas. These include consideration of the site, the clinical extent of the disease and the histologic grade prior to institution of therapy. Radical neck dissection should be preserved only to deal with obvious metastases.
Mucinous Adenocarcinoma
Mucinous adenocarcinoma is a rare malignant neoplasm characterized by large amount of extracellular epithelial mucin that contains cords, nests, and solitary epithelial cells. The incidence is unknown. Limited data indicate that most, if not all, occur in the major salivary glands with the submandibular gland as the predominant site. These tumors may be associated with dull pain and tenderness. This neoplasm may be considered to be low-grade.
Clinical Features
Clinically, these lesions are soft, spongy masses that may be thought to be cysts.
Histologic Features
The gross specimens are very mucoid, with a slimy texture and they may actually ooze mucoid material. These tumors are circumscribed but not encapsulated. Low magnification reveals islands and cords of tumor cells that appear to be floating within pools of pale staining mucin. The pools of mucin may be divided into irregular lobules by fibrous connective tissue septa that course through the tumor, which is unencapsulated. The tumor cells are moderately large, cuboidal, and polygonal cells with eosinophilic to amphophilic cytoplasm. The nuclei are vesicular, and scattered mitotic figures may be found. The tumor islands are surrounded by pale-staining mucoid substance. The mucoid substance stains with mucicarmine, periodic acid-Schiff, and alcian blue at pH 2.0. Mucicarmine stain also reveals that many tumor cells contain intracytoplasmic mucin.
Oncocytic Carcinoma: (Oncocytic adenocarcinoma)
Oncocytic carcinoma is a rare, predominantly oncocytic neoplasm whose malignant nature is reflected both by its abnormal morphologic features and infiltrative growth. Bauer and Bauer reported the first case in 1953. Although focal oncocytic features are seen in a wide variety of salivary neoplasms, both benign and malignant oncocytic neoplasms are extremely rare.
Clinical Features
Oncocytic carcinoma may be considered to be a high-grade carcinoma. Most cases occur in the parotid gland but recent reports have described tumors that involved the submandibular gland and minor glands of the palate, nasal cavity, and ethmoid and maxillary sinuses. The average age of patients in one series was 63 years. Patients usually develop parotid masses that cause pain or paralysis. The skin overlying the gland is occasionally discolored or wrinkled.
Histologic Features
All types of benign or malignant salivary gland tumors may have foci of oncocytic cells, but the oncocytic component usually contains such a small portion that it is unlikely to be confused with oncocytic carcinoma. Tumors with a significant oncocytic component include Warthin’s tumor, oncocytoma, and oncocytic carcinoma. They are characterized by oncocytes with marked cellular atypia, frequent mitosis, destruction of adjacent structures, perineural or vascular invasion, and distant or regional lymph node metastasis. Histochemical or electron microscopic confirmation of the oncocytic (mitochondrial) nature of the cytoplasm is necessary because cytoplasmic accumulation of smooth endoplasmic reticulum, lysosomes, or secretory granules may have a similar appearance.
Treatment and Prognosis
Oncocytic carcinoma is considered to be a high-grade neoplasm and aggressive initial surgery seems to have a significantly better overall prognosis. Radiation does not appear to favorably alter the biologic behavior of this tumor. Prophylactic neck dissection may be indicated for tumors that are larger than 2 cm in diameter.
Salivary Duct Carcinoma: (Salivary duct adenocarcinoma)
Salivary duct carcinoma is a rare, typically high-grade malignant epithelial neoplasm composed of structures that resemble expanded salivary gland ducts. A low-grade variant exists. Incidence rates vary depending upon the study cited. In the AFIP files, salivary duct carcinomas represent only 0.2% of all epithelial salivary gland neoplasms. More than 85% of cases involve the parotid gland and approximately 75% of patients are men. The peak incidence is reported to be in the seventh and eighth decades of life.
Clinical Features
Parotid swelling is the most common sign. Facial nerve dysfunction or paralysis occurs in over one-fourth of patients and may be the initial manifestation. The high-grade variant of this neoplasm is one of the most aggressive types of salivary gland carcinomas and is typified by local invasion, lymphatic and hematogenous spread with poor prognosis.
Histologic Features
The infiltrative tumor elements are typically composed of clusters of tumor cells that may have small lumina or cribriform arrangements, but solid, irregularly shaped tumor cell aggregates are frequently present. The neoplastic epithelial cells are cuboidal and polygonal with a moderate amount of eosinophilic cytoplasm and are accompanied by a dense fibrous connective tissue stroma that may be hyalinized in some areas. Invasion of the nerves and blood vessels is frequent in addition to infiltration of salivary gland lobules and extra-salivary gland tissues, such as fat, muscle, and bone. Mucicarmine and Alcian blue stains are generally negative, except, perhaps, for a small amount of luminal staining. Immuno histochemical and ultrastructural studies have identified ductal cells but no myoepithelial cells.
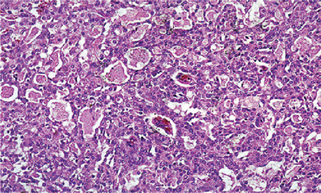
Adenocarcinoma
Adenocarcinomas of the salivary glands are rare but aggressive tumors.
Clinical Features
They tend to be present in patients over 40 years of age and occur with nearly equal frequency in men and women. About half of these tumors present in the parotid glands, the minor salivary glands, particularly the palate, lip and tongue are the next most commonly affected sites. Clinical presentation again most often involves an enlarging mass. Adenocarcinoma is different from other salivary gland neoplasms in that as many as 25% of patients will complain of pain or facial weakness at presentation.
Histologic Features
Gross pathology reveals a firm mass with irregular borders and infiltration into surrounding tissue. It is generally a solid tumor without any cystic spaces. These malignancies can demonstrate a wide range of growth patterns, and for this reason, can be somewhat difficult to classify. However, all adenocarcinomas have in common the formation of glandular structures and they are described as grades I, II or III based upon the degree of cellular differentiation. Grade I lesions have well formed ductal structures while grade III lesions have a more solid growth pattern with few glandular characteristics (Figs. 3-27, 3-28).
Treatment and Prognosis
Because these are more aggressive tumors, treatment for adenocarcinoma is aggressive. Complete local excision is the mainstay of therapy. In the parotid this may include facial nerve sacrifice. In the minor salivary glands, a portion of the maxilla or mandible may have to be resected with the tumor. Postoperative radiation therapy does seem to be of some benefit. Lymph node metastasis is not uncommon and in patients with palpable neck disease neck dissection is warranted. Local recurrence rates vary in the literature but have been cited as high as 51%.
Malignant Myoepithelioma: (Myoepithelial carcinoma)
Ellis GL (1991) defined myoepithelial carcinoma as a malignant epithelial neoplasm whose tumor cells demonstrate cytologic differentiation towards myoepithelial cells and lack ductal or acinar differentiation. Myoepithelial carcinoma is a rare, malignant salivary gland neoplasm in which the tumor cells almost exclusively manifest myoepithelial differentiation. This neoplasm represents the malignant counterpart of benign myoepithelioma. A majority of the tumors occur in the parotid gland. The mean age of patients is reported to be 55 years.
Clinical Features
The majority of patients present with the primary complaint of a painless mass. This is an intermediateto high-grade carcinoma. Histologic grade does not appear to correlate well with clinical behavior; tumors with a low-grade histologic appearance may behave aggressively.
Histologic Features
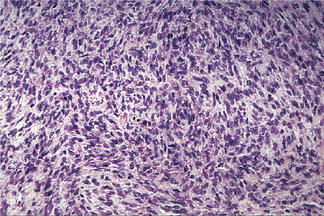
The cytologic features of individual cells and the general morphologic features resemble tumor cells in benign myoepithelioma and the myoepithelial cells of mixed tumor. The tumor cells may be spindle shaped or plasmacytoid cells (Fig. 3-29). The cell types are often intermixed but usually one or the other cell type predominates. The tumors may be quite cellular and more suggestive of sarcoma than carcinoma. The stroma in other areas of the tumors may be more conspicuous and myxoid. These tumors are distinguished from benign myoepithelial neoplasms by their infiltrative, destructive growth. They usually demonstrate increased mitotic activity and cellular pleomorphism. Some tumor cell should be immunoreactive for cytokeratin, S100 protein, smooth muscle actin, and occasionally, glial fibrillary acidic protein.
Carcinoma in Pleomorphic Adenoma: (Malignant mixed tumor)
Malignant mixed tumors include three distinct clinicopathologic entities: carcinoma ex pleomorphic adenoma, carcinosarcoma, and metastasizing mixed tumor. Carcinoma ex pleomorphic adenoma constitutes the vast majority of cases, whereas carcinosarcoma (true malignant mixed tumor) and metastasizing mixed tumor are extremely rare.
Carcinoma ex Pleomorphic Adenoma (Carcinoma exmixed tumor)
Carcinoma ex pleomorphic adenoma is the most common of the three salivary neoplasms that are broadly referred to as malignant mixed tumors. It occurs when a carcinoma develops from the epithelial component of a preexisting pleomorphic adenoma. Diagnosis requires the identification of benign tumor in the tissue sample. The incidence or relative frequency of this tumor may vary considerably depending on the study cited. A review of material at the AFIP showed carcinoma ex pleomorphic adenoma to comprise 8.8% of all mixed tumors and 4.6% of all malignant salivary gland tumors, ranking it as the sixth most common malignant salivary gland tumor after mucoepidermoid carcinoma; adenocarcinoma; acinic cell carcinoma; polymorphous low-grade adenocarcinoma; and adenoid cystic carcinoma.
Clinical Features
The neoplasm occurs primarily in the major salivary glands. It occurs most often in the parotid, followed by the submandibular gland and palate. It presents in the sixth to eighth decade of life with patients averaging 10 years older than those with pleomorphic adenomas. Presentation is usually a painless mass but some patients will report recent rapid enlargement of a long-standing nodule. Approximately one-third of patients may experience facial paralysis.
Histologic Features
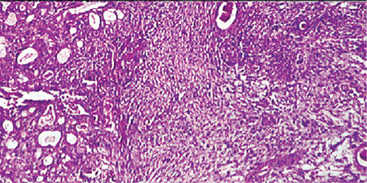
Gross pathology of carcinoma ex pleomorphic adenoma often shows a poorly circumscribed, infiltrative, hard mass. Micro scopically malignant appearing cells are present adjacent to a typical appearing pleomorphic adenoma. The malignant portion of the tumor can take the form of any epithelial malignancy except acinic cell (Fig. 3-30). Most commonly this will be in the form of an undifferentiated carcinoma (30%) or adenocarcinoma (25%). This tumor tends to be more aggressive than other salivary malignancies and about 25% of patients will have lymph node metastasis on presentation.
Treatment and Prognosis
Treatment includes radical surgical resection, often in conjunction with neck dissection, and postoperative radiation therapy. Prognosis appears to be related to the local extent of disease and the histologic type of the carcinoma component.
Carcinosarcoma (True malignant mixed tumor)
Carcino-sarcoma is a rare malignant salivary gland neoplasm that contains both carcinoma and sarcoma components. In a carcinosarcoma, the metastatic lesions contain both the stromal and epithelial elements. Some carcinosarcomas develop de novo while others develop in association with benign mixed tumor.
This neoplasm is rare in occurrence; there are only eight cases in the AFIP case files. Only 11 cases were recorded at MD Anderson over a 32-year period. The majority of tumors occur in the major salivary glands. The parotid is the most frequent site of occurrence. Average age at presentation is about 60 years and men and women appear to be equally affected. Clinically, swelling, pain, nerve palsy, and ulceration have been frequent findings.
Histologic Features
Microscopically, these tumors have both sarcomatous and carcinomatous elements. In the majority, the sarcoma is the dominating component and chon-drosarcoma is the most common cell type. The carcinoma element is usually an undifferentiated or high-grade ductal adenocarcinoma.
Treatment and Prognosis
This is also an aggressive tumor. In the largest series reported (12 cases), the average survival period was 3.6 years and it is not uncommon for patients to have distant metastasis on presentation. Currently, recommended treatment includes radical surgery, neck dissection for palpable nodes and postoperative radiotherapy. Although efficacy has yet to be proven, chemotherapy is likely to have a role in the treatment of this disease given the high rate of distant metastasis.
Metastasizing Mixed Tumor
Metastasizing mixed tumor is a very rare histologically benign salivary gland neoplasm. The meta stasizing mixed tumor refers to an otherwise benign acting pleomorphic adenoma that develops metastatic deposit. There is often a long interval between the diagnosis of the primary tumor and the metastases. The histologic features are within the spectrum of features that typify pleomorphic adenoma. The majority occur in the major salivary glands. The primary neoplasm is typically a single, well-defined mass. Recurrences, which may be multiple, have been reported to occur up to 26 years after excision of the primary neoplasm.
Squamous Cell Carcinoma
Primary squamous cell carcinoma is a malignant epithelial neoplasm of the major salivary glands that is composed of squamous (epidermoid) cells (Fig. 3-31). This diagnosis is not made in minor salivary glands because distinction from the more common mucosal squamous cell carcinoma is not possible. Primary squamous cell carcinoma of the salivary glands is quite rare, accounting for about 1.6% of salivary gland neoplasms. In order to make this diagnosis, high-grade mucoepidermoid carcinoma, metastatic squamous cell carcinoma to the gland or intraglandular nodes and direct extension of a squamous cell carcinoma must first be excluded.
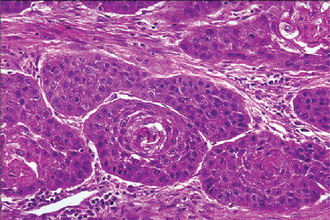
Figure 3-31 Squamous cell carcinoma.
Primary squamous cell carcinoma of salivary gland showing neoplastic squamous cells, metaplastic in origin, with varying degrees of nuclear atypia.
Clinical Features
The reported frequency of this tumor among all major salivary gland tumors has varied from 0.9–4.7%. There is a 2 : 1 male-to-female ratio of occurrence and patients are usually over age 60. This neoplasm occurs in the parotid gland almost nine times more often than in the submandibular gland. This tumor is graded in a way similar to the extra salivary lesions according to the degree of differentiation (low, intermediate, and high). Previous exposure to ionizing radiation appears to increase the risk for developing this neoplasm. The median time between irradiation and diagnosis of the neoplasm is approximately 15.5 years. These tumors present as firm enlarging masses that are not uncommonly fixed to surrounding tissue and associated with pain or facial weakness.
Histologic Features
The gross and microscopic appearance is similar to squamous cell carcinoma of other primary sites and varies from well-differentiated to poorly differentiated. Salivary gland squamous cell carcinoma displays aggressive behavior with rapid growth and early spread to regional lymph nodes (Fig. 3-32).
Treatment and Prognosis
Treatment consists of surgical resection, neck dissection and postoperative radiation. The prognosis for this neoplasm is poor. In a 30-year retrospective analysis of 50 cases of squamous cell carcinoma of the salivary glands, survival rates at five and 10 years were 24% and 18%, respectively.
Small Cell Carcinoma
Small cell carcinoma of the salivary gland was first described in 1972. Small cell carcinomas arising in salivary glands are extremely rare, high-grade malignant tumors and are subclassified into neuroendocrine and ductal types.
Histologic Features
Microscopically, the tumor cells have oval, hyperchromatic nuclei and scant amount of cytoplasm and are organized in sheets, strands, and nests. Small cell carcinoma of major salivary glands is a highly aggressive tumor, although the prognosis may be better than that for extra-salivary neoplasms. Studies also suggest that most salivary gland small cell carcinomas exhibit neuroendocrine differentiation. Neuroendocrine carcinomas are more frequently found in the minor salivary glands and have a better survival rate compared with small cell carcinomas of the lung. The undifferentiated counterpart of this neoplasm is the small cell undifferentiated carcinoma.
Treatment and Prognosis
The treatment of choice is wide surgical excision, possibly with radiation and/or chemotherapy. The prognosis for patients with small cell carcinomas arising in the major or minor salivary glands is better than that for patients with small cell carcinomas of the lung or larynx.
Undifferentiated Carcinoma
Undifferentiated carcinomas of salivary glands are a group of uncommon malignant epithelial neoplasms that lack the specific morphologic features of other types of salivary gland carcinomas. These carcinomas are histologically similar to undifferentiated carcinomas that arise in other organs and tissues. Accordingly, metastatic carcinoma is a primary concern in the differential diagnosis of these neoplasms. This group includes the following three tumors:
Small Cell Undifferentiated Carcinoma (Extrapulmonary oat cell carcinoma)
Small cell undifferentiated carcinoma is a rare, primary malignant tumor that, with conventional light microscopy, is composed of undifferentiated cells, and with ultrastructural or immunohistochemical studies, does not demonstrate neuroendocrine differentiation. This is the undifferentiated counterpart of anaplastic small cell carcinoma.
Large Cell Undifferentiated Carcinoma
Large cell undifferentiated carcinoma is a tumor in which features of acinar, ductal, epidermoid, or myoepithelial differentiation are absent under light microscopy, although, occasionally, poorly formed duct like structures are found. Rapid growth of a parotid swelling is a common clinical presentation. This is a high-grade neoplasm that frequently metastasizes and has a poor prognosis.
Lymphoepithelial Carcinoma (Undifferentiated carcinoma with lymphoid stroma, carcinoma ex lymphoepithelial lesion)
Lymphoephithelial carcinoma is an undifferentiated tumor that is associated with a dense lymphoid stroma. There is an exceptionally high incidence of this tumor in the Eskimo and Inuit populations. This neoplasm has been associated with Epstein-Barr virus infection. Over 80% occur in the parotid gland. In addition to the presence of a parotid or submandibular mass, pain is a frequent symptom, and facial nerve palsy occurs in up to 20% of patients. Over 40% of patients have metastases to cervical lymph nodes at initial presentation, and 20% develop distant metastases within three years following therapy.
Salivary Adenocarcinoma, NOS
Adenocarcinoma, NOS, (not otherwise Specified) is a salivary gland carcinoma that shows glandular or ductal differentiation but lacks the prominence of any of the morphologic features that characterize the other, more specific carcinoma types. The diagnosis of adenocarcinoma, NOS, is essentially one of exclusion. In the Armed Forces Institute of Pathology (AFIP) review of cases, adenocarcinoma, NOS, was second only to mucoepidermoid carcinoma in frequency among malignant salivary gland neoplasms. Other series have reported an incidence of 4–10 % (Speight PM, Barret AW, 2002). In AFIP files, the mean patient age was 58 years. Approximately 40–60% of tumors occurred in the major and minor salivary glands, respectively. Among the major salivary gland tumors, 90% occurred in the parotid gland. Adenocarcinoma, NOS is graded in a similar way to extrasalivary lesions according to the degree of differentiation. Tumor grades include low grade, intermediate grade, and high grade categories (Ellis GL, Auclair PL, 1996).
Patients with tumors in the major salivary glands typically present with solitary, painless masses. Two retrospective studies indicate that survival is better for patients with tumors of the oral cavity than for those with tumors of the parotid and submandibular glands (Spiro RH, Huvos AG, Strong EW 1982, Matsuba HM, Mauney M et al, 1988). These studies differ regarding the prognostic significance of tumor grade.
Other Carcinomas
Adenosquamous Carcinoma
Adenosquamous carcinoma of the salivary gland is a controversial neoplasm that is not included in many classifications of salivary gland tumors. This is a malignant epithelial neoplasm that has histomorphologic features of both adenocarcinoma and squamous cell carcinoma.
Sebaceous Lymphadenocarcinoma
Sebaceous lymphadenocarcinoma is the malignant counterpart of sebaceous lymphadenoma and represents carcinoma arising in sebaceous lymphadenoma. These tumors appear to be low-grade malignant tumors that have the ability to recur locally. Lymph node or distant metastases may develop late in the clinical course of these tumors.
Nonepithelial Tumors
Mesenchymal Neoplasms
Mesenchymal neoplasms account for 1.9–5% of all neoplasms that occur within the major salivary glands. These classifications pertain to major salivary gland tumors. Because the minor salivary glands are small and embedded within fibrous connective tissue, fat, and skeletal muscle, it is impossible to determine the origin of a mesenchymal neoplasm from stroma. The types of benign mesenchymal salivary gland neoplasms include hemangioma, lipoma, and lymphangioma. Malignant mesenchymal salivary gland neoplasms include malignant schwannoma, hemangiopericytoma, malignant fibrous histiocytoma, rhabdo myosarcoma, and fibrosarcoma, among others; in the major salivary glands, these neoplasms represent approximately 0.5% of all benign and malignant salivary gland tumors and approximately 1.5% of all malignant tumors. It is important to establish a primary salivary gland origin for these tumors by excluding the possibilities of metastasis and direct extension from other sites. In addition, the diagnosis of salivary gland carcinosarcoma should be excluded. Primary salivary gland sarcomas behave like their soft tissue counterpart in which prognosis is related to sarcoma type, histologic grade, tumor size, and stage.
Malignant Lymphomas
Lymphomas of the major salivary glands are characteristically of the non-Hodgkin’s type. In AFIP review of case files, non-Hodgkin’s lymphoma accounted for 16.3% of all malignant tumors that occurred in the major salivary glands; disease in the parotid gland accounted for about 80% of all cases.
Patients with benign lymphoepithelial lesion (Mikulicz’s disease), a manifestation of the autoimmune disease, Sjogren’s syndrome, are at an increased risk for development of non-Hodgkin’s lymphoma (Fig. 3-33).
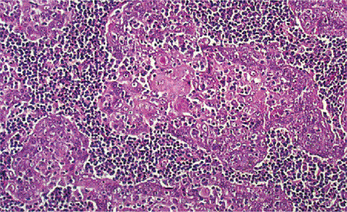
Figure 3-33 Malignant lymphoma—mucosa associated lymphoid tissue (MALT) type.
Note the myoepithelial islands suspended in a background of lymphoid tissue. The lymphoid element is neoplastic with varying degrees of malignancy.
Secondary Tumors
Malignant neoplasms whose origins lie outside the salivary glands may involve the major salivary glands by:
1. Direct invasion from cancers that lie adjacent to the salivary glands
2. Hematogenous metastases from distant primary tumors
3. Lymphatic metastases to lymph nodes within the salivary gland.
Direct invasion of nonsalivary gland tumors into the major salivary glands is principally from squamous cell and basal cell carcinomas of the overlying skin.
Tumor Like Lesions
Sialadenosis
Sialadenosis is the name given to nonneoplastic, noninflammatory enlargement of salivary glands, particularly the parotid gland. The enlargement is usually bilateral and may manifest recurrence or pain, or both. The condition is almost always found in association with systemic disorders; this association forms the basis for classification of sialadenosis.
Clinical Features
Characterized mainly by the presence of chronic, afebrile salivary gland enlargement, usually of the parotid glands. The enlargement is described as slowly evolving, indolent, undulating, and recurrent. Persons in the later decades of life (4th decade or beyond) are most afflicted. De-creased salivary secretion occurs, and sialochemistry generally demonstrates increased levels of potassium and decreased levels of sodium. Hypertrophy of acinar cells crowds and compresses the finer terminal ducts, thereby yielding the sialographic ‘leafless tree’ pattern (Table 3-7).
Table 3-7
Classification of sialadenosis
Hormonal sialadenosis
Sex hormonal sialadenosis
Diabetic sialadenosis
Thyroid sialadenosis
Hypophyseal and adrenal cortical disorders
Neurohumoral sialadenosis
Peripheral neurohumoral sialadenosis
Central neurogenous sialadenosis
Dysenzymatic sialadenosis
Hepatogenic sialadenosis
Pancreatogenic (exocrine) sialadenosis
Nephrogenic sialadenosis
Dysproteinemic sialadenosis
Malnutritional sialadenosis
Mucoviscidosis
Drug-induced sialadenosis
Histologic Features
The parotid swelling is due to acinar enlargement. The diameter of the acinar cell increases two to three times that of normal. The nuclei tend to be basally situated, and the cytoplasm tends to be packed with granules. Inflammatory cells are absent but individual fat cells may be seen in the interstitial tissue. Batsakis (1988) suggests that long standing uncorrected autonomic neuropathy, such as occurs in alcoholism or diabetes mellitus eventually leads to acinar atrophy and replacement with fat.
Oncocytosis
Oncocytosis is a metaplastic, sometimes hyperplastic, developmental or reactive process that is characterized by focal replacement of normal glandular tissue with enlarged eosinophilic epithelial cells with granular cytoplasm. These swollen epithelial cells were first described by Shafer (1897) and later the term ‘onkocyte’ was applied by Hamperl (1931) (cited by Ellis GL and Auclair PL, 1991). Oncocytosis is seen with greater frequency in older people and is considered a sequela of aging. The majority of cases occur in the parotid but it may be found in any major or minor gland. The histologic distinction between oncocytosis and oncocytoma, which is the benign neoplasm of oncocytes, may occasionally be difficult.
Histologic Features
Oncocytosis may present as scattered foci of enlarged, eosinophilic epithelial cells or a solitary focus of metaplastic oncocytes. The cells may retain an acinar arrangement and form sheets, trabeculae, or duct like structures surrounded by fine collagen septa. The cytologic features consist of large, eosinophilic, polyhedral epithelial cells with fine granular cytoplasm and a centrally placed pyknotic nucleus (Fig. 3-34). Oncocytes occasionally show transition to clear cells as a result of intracytoplasmic glycogen.
Ultrastructural findings are characterized by cytoplasm that is packed with large, pleomorphic mitochondria containing filamentous, tubular, and vesicular cristae. The oncocytes have been considered a product of degenerative changes.
Necrotizing Sialometaplasia: (Salivary gland infarction)
Necrotizing sialometaplasia (NS) is a non-neoplastic inflammatory condition of the salivary glands. In 1973, Abrams et al, first reported this condition. The clinical and histopathologic features of NS often simulate those of malignancies such as squamous cell carcinoma or mucoepidermoid carcinoma.
Etiology
Most cases of NS appear to arise spontaneously, whereas others are associated with a history of trauma, radiation therapy, or surgery. In most cases of NS, the etiology is believed to be related to vascular ischemia. The association of adjacent neoplasia that results in ischemic necrosis of the glandular elements and the histologic features of NS supports this pathogenic mechanism. Tobacco use is suggested as a possible etiologic risk factor for NS.
Clinical Features
NS is generally reported to involve the minor salivary glands of the oral cavity, particularly those of the palate. Reports of this entity in the minor glands of the retromolar pad area, buccal mucosa, tongue, incisive canal, and labial mucosa have also been reported. In addition, NS is recognized in the parotid and submandibular salivary glands, minor mucous glands in the lung, nasal cavity, larynx, trachea, nasopharynx, and maxillary sinus. Similar lesions are identified in the breast; the condition is referred to as posttraumatic lobular metaplasia of the breast. Lesions in the skin are referred to as syringometaplasia. The male-to-female ratio is approximately 2 : 1. The average age of patients with NS is 47.9 years, with a range of 17–80 years.
The lesions of NS often are painless; less frequently, they cause pain and numbness. NS manifests as a swelling with or without ulceration in anatomic sites that have mucous or serous glandular tissue. The typical clinical presentation of NS is that of a crateriform ulcer of the palate that simulates a malignant process. These ulcerated lesions are 1–3 cm and are usually unilateral, but bilateral synchronous lesions and metachronous lesions can occur. Some lesions of NS may present as a submucosal swelling, without ulceration of the overlying mucosa. An intact surface mucosa may be noted in an evolving lesion at the time of diagnosis, although most cases are accompanied by mucosal ulceration. Erosion of the palatal bone may occur in either ulcerated or nonulcerated lesions. Examination of a biopsy specimen is usually required to establish the correct diagnosis and to exclude a malignant or infectious process or an inflammatory condition such as Wegener granulomatosis. Extranodal lymphoma also may be considered in the clinical differential diagnosis of a palatal swelling or ulceration.
Histologic Features
The microscopic features of NS include coagulative necrosis of glandular acini and squamous metaplasia of its ducts. Mucin pooling is present, and an associated inflammatory infiltrate consists of macrophages, neutrophils, and less commonly, lymphocytes, plasma cells, and eosinophils (Fig. 3-35).
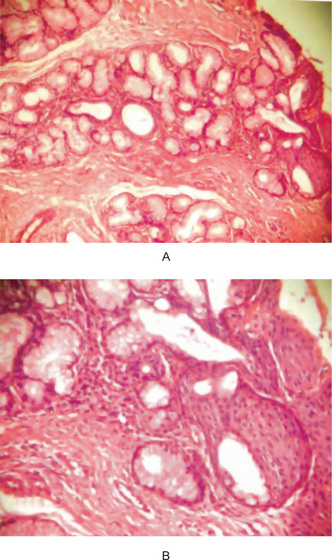
Figure 3-35 Sialometaplasia.
Squamous metaplasia of salivary ducts. Lobular architecture of the glands is intact.
Pseudoepitheliomatous hyperplasia of the overlying mucosa can also be present, but the cytologic features of the epithelial atypia are usually absent. Occasionally, isolated mucous cells may be entrapped within the squamous islands; these cells should not be confused with those of mucoepidermoid carcinoma. The microscopic differential diagnosis for NS includes mucoepidermoid carcinoma and squamous cell carcinoma. Some believe that subacute necrotizing sialadenitis is yet another entity that occurs within the spectrum of NS; it should be distinguished from NS.
Treatment and Prognosis
NS resolves spontaneously. No treatment is necessary. Surgical care consists of incisional biopsy for diagnostic purposes. Periodic evaluation of the affected site is recommended until spontaneous resolution occurs. The prognosis for NS is excellent. Spontaneous resolution usually occurs within weeks. The average healing time for NS of the minor salivary glands of the hard and soft palates is approximately five weeks. The size of the lesion and whether or not bony perforation has occurred are clinical parameters that may influence the healing time.
Benign Lymphoepithelial Lesion: (Mikulicz syndrome, dacryosialoadenopathia, Mikulicz-Radecki syndrome, Mikulicz-Sjögren syndrome, von Mikulicz syndrome)
Mikulicz disease is a chronic condition characterized by the abnormal enlargement of the salivary and lacrimal glands. The tonsils and other glands in the soft tissue of the face and neck may also be involved. In 1888 Johann Mikulicz, a German surgeon first reported a case of chronic bilateral lacrimal gland enlargement associated with enlargement of the salivary glands. Subsequently many cases of bilateral salivary or lacrimal gland enlargement were reported as Mikulicz disease. But as most of these cases were caused by tuberculosis, sarcoidosis or lymphoma, this led to confusion regarding the terminology related to Mikulicz disease. Hence it was proposed that the term Mikulicz disease should be used if the cause is unknown and the Mikulicz syndrome be reserved for cases if the enlargement is associated with a known disease. People who have Mikulicz disease are at heightened risk for developing lymphomas.
Etiology
The exact cause of Mikulicz disease is not known, although it is suspected to be an autoimmune disorder. The symptoms of Mikulicz disease may occur due to the excessive accumulation of lymphocytes into the involved glands.
Clinical Features
Mikulicz disease affects more females than males and most often presents during the middle adult years. It often occurs in combination with Sjögren syndrome. Mikulicz disease is characterized by the sudden onset of xerostomia that may lead to difficulty in swallowing and result in tooth decay. Other symptoms include enlarged lacrimal glands, leading to absent or decreased tears; painless swellings (tumefactions) of the salivary glands (parotid, submaxillary) are noticed. The symptoms of Mikulicz disease are very similar to those of Sjögren syndrome and some researchers suspect that they may be the same disorder. Some people with Mikulicz disease may experience recurring fevers. The fever may be accompanied by dry eyes, diminished lacrimation, and uveitis. Lacrimal gland enlargement, parotid gland enlargement, dry mouth and dry eyes are the classic signs.
Histologic Features
The disease is characterized by an orderly lymphocytic infiltration of the salivary gland tissue, destroying or replacing the acini, with the persistence of islands of epithelial cells which probably represent residua of gland ducts (Fig. 3-36 B). Although the lymphoid element is usually diffuse, actual germinal centers are occasionally present. The epithelium may consist of ducts showing cellular proliferation and loss of polarity, or as the disease persists, solid nests or clumps of poorly defined epithelial cells which Morgan and Castleman termed ‘epimyoepithelial islands’. These sometimes seem to form a syncytium. It has been suggested that such islands arise as a result of proliferation of both ductal cells and peripheral myoepithelial cells. A characteristic change also found in advanced lesions is the deposition of eosinophilic, hyaline material in the epithelial islands.
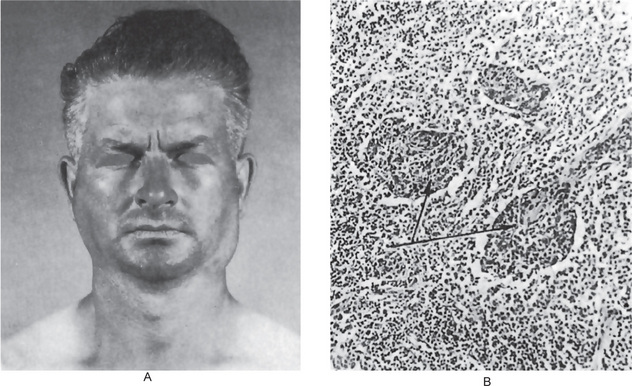
Figure 3-36 Benign lymphoepithelial lesion (Mikulicz’s disease).
(A) There is enlargement of the parotid, submaxillary and lacrimal glands. (B) The photomicrograph illustrates the diffuse lymphocytic inFiltrate and the typical epimyoepithelial islands Courtesy of Dr Frank Vellios.
Great care must be taken in differentiating between the benign lymphoepithelial tumor and a malignant lymphoma involving the salivary glands. In the latter disease, epimyoepithelial islands are not present, the lymphoid element is atypical, and there is infiltration of the interlobular septa by lymphoid tissue. The epithelial islands, on the other hand, may be mistaken for metastatic carcinoma. Other histologically similar lesions which must be considered in the differential diagnosis are chronic sialadenitis, papillary cystadenoma lymphomatosum and uveoparotitis.
Treatment and Prognosis
Biopsy of one of the swollen glands is key to the diagnosis of Mikulicz disease. An ultrasonographic examination of the area may help to rule out other reasons for gland swelling. Treatment of this disorder is symptomatic. Artificial tears may be used to maintain moisture in the eyes, and artificial saliva may be used to treat oral symptoms. Some individuals with Mikulicz disease may be instructed to follow a soft moist diet. This may help to reduce the pain caused by chewing and swallowing.
Sjögren’s Syndrome: (Sicca syndrome, Gougerot-Sjögren syndrome)
Sjögren’s syndrome is a condition originally described by Henrik Sjögren in 1933, as a triad consisting of keratoconjunctivitis sicca, xerostomia and rheumatoid arthritis. Subsequently, it has been found that some patients present only with dry eyes and dry mouth (sicca complex or primary Sjögren’s syndrome), while others also develop systemic lupus erythematosus, polyarteritis nodosa, polymyositis or scleroderma, as well as rheumatoid arthritis (secondary Sjögren’s syndrome). As Sjögren pointed out, cases of xerostomia and arthritis without keratoconjunctivitis sicca have been observed.
Etiology
Various causes of this disease have been suggested: genetic, hormonal, infectious and immunologic, among others. It may well be that a combination of factors, both extrinsic and intrinsic, play a role in the etiology of this condition. Most authorities consider an altered immunologic response to be the main intrinsic factor which is responsible for the disease. Laboratory findings support the autoimmune etiologic role (q.v.). Bertram has reported that 75% of a series of 35 patients with Sjögren’s syndrome had in their sera antisalivary duct antibody. Similar antibody was found in the sera of 24% of a group of 29 patients with systemic lupus erythematosus, a documented autoimmune disease. In addition, the sicca complex and Sjogren’s syndrome have been found to be associated with the HLA system, specifically HLA-DR3 and HLA-B8 which are associated with primary form of the disease and HLA-DRw52 seen to be associated with both the forms of Sjögren’s syndrome. Cytomegalovirus, paramyxo virus and Epstein-Barr virus have all been implicated in the pathogenesis of this condition but have not been proven conclusively.
Clinical Features
This disease occurs predominantly in women over 40 years of age, although children or young adults may be affected. The female: male ratio is approximately 10 : 1. The typical features of the disease are dryness of the mouth and eyes as a result of hypofunction of the salivary and lacrimal glands. This often results in painful, burning sensations of the oral mucosa. In addition, various secretory glands of the nose, larynx, pharynx and tracheobronchial tree (buccopharyngolaryngitis sicca), as well as of the vagina, are involved with this dryness. Schall and his associates have evaluated the degree of xerostomia by means of sequential salivary scintigraphy. Chisholm and Mason have quantified saliva production in patients with the sicca complex and Sjögren’s syndrome. Reduced salivary flow was noted in both groups. Sialochemistry studies by Ben-Aryeh and coworkers have demonstrated significantly elevated levels of IgA, potassium and sodium in the saliva of patients with the sicca complex. Moutsopoulos has reported that 80% of patients with primary Sjögren’s syndrome have parotid enlargement in contrast to only 14% with secondary Sjögren’s syndrome. Lymph adenopathy is more than twice as common in the primary form of the disease.
Rheumatoid arthritis, as mentioned, is an integral part of secondary Sjögren’s syndrome. It has been shown that patients with Sjögren’s syndrome with rheumatoid arthritis have certain different clinical manifestations than patients with sicca complex, despite similar histologic findings and some laboratory findings. In this regard, patients without rheumatoid arthritis, that is, sicca complex or primary Sjögren’s syndrome, more frequently manifest parotid gland enlargement, lymphadenopathy, purpura, Raynaud’s phenomenon, kidney involvement and myositis.
Histologic Features
Three types of histologic alterations in the major salivary glands have been described. In one case, there may be intense lymphocytic infiltration of the gland replacing all acinar structures although the lobular architecture is preserved. In another, there may be proliferation of ductal epithelium and myoepithelium to form ‘epimyoepithelial islands’. Both of these histologic changes are identical with those occurring in the benign lymphoepithelial lesion in Mikulicz’s disease. The third alteration may be simply an atrophy of the glands sequential to the lymphocytic infiltration.
Interestingly, Bertram and Hjoting-Hansen have reported that 85% of a group of patients with Sjögren’s syndrome exhibited alterations in the accessory salivary glands of the lip characteristically similar to those in the major glands; they also have suggested biopsy of the labial mucosa as an aid in establishing the diagnosis of the disease. Similar findings in accessory glands, as well as the demonstration of antisalivary duct antibody by an immunofluorescent technique, have been reported by Tarpley, Anderson and White.
Laboratory Findings
Over 75% of patients with primary Sjögren’s syndrome have a polyclonal hyperglobulinemia and many develop cryoglobulins. Multiple organ- or tissue-specific antibodies are found, including antisalivary duct antibodies, rheumatoid factor and antinuclear antibodies. An increased sedimentation rate is present in 80% of these patients. Interestingly, the presence of antisalivary duct antibody is three times more common in those with secondary Sjögren’s syndrome as compared to those with the sicca complex.
Radiographic Features
Sialography may be of diagnostic value in Sjögren’s syndrome. Sialographs demonstrate the formation of punctate, cavitary defects which are filled with radiopaque contrast media. These filling defects have been said to produce a ‘cherry blossom’ or ‘branchless fruit-laden tree’ effect radiographically. Som and his associates have suggested that the contrast material actually extravasates through the weakened salivary gland ducts to produce the sialographic features. Poor elimination of contrast media is noted, as might be expected, with retention of the material for over a month.
Treatment and Prognosis
There is no satisfactory treatment for Sjögren’s syndrome. Most patients are treated symptomatically. Keratocon junctivitis is treated by instillation of ocular lubricants such as artificial tears containing methylcellulose, and xerostomia is treated by saliva substitutes such as those used in the treatment of person with xerostomia secondary to radiation therapy. Extensive dental caries is a complication which is quite common, and scrupulous oral hygiene and frequent fluoride application is indicated to reduce this problem. There is no specific treatment for enlargement of the salivary glands. Surgery has been employed but is generally recommended only in patients with discomfort. Although radiation therapy has been recommended in the past, its use is not advocated currently.
A major complicating factor in patients with Sjögren’s syndrome is the development of pseudolymphoma and malignant lymphoma. In an extensive National Institutes of Health (NIH) study, 136 patients with Sjögren’s syndrome were followed for an average period of over eight years. Nonlymphoma malignancies were no more common than would be expected, whereas lymphomas were observed in nearly 44 times the expected incidence rate. The risk of lymphoma in Sjögren’s patients is 6.4 cases per 100 cases per year. Most lymphomas are non-Hodgkin’s types and are B-cell in origin. Macroglobulinemia of Waldenstrom also has been noted to develop in these patients.
The relation between the benign lymphoepithelial lesion, Mikulicz’s disease and Sjögren’s syndrome is possibly a very close one. Mikulicz’s disease, but not Mikulicz’s syndrome, is probably identical with the lymphoepithelial lesion. This entity shares several features in common with Sjögren’s syndrome. Both diseases are manifested, often but not invariably, by a swelling of the major salivary glands and lacrimal glands, singly or in pairs. In both diseases the patient has xerostomia, which probably is related to the displacement and destruction of acinar tissue. Finally, both diseases occur chiefly in middleaged or elderly women.
It is likely that the benign lymphoepithelial lesion is a mild form of Sjögren’s syndrome, but it should not be inferred that lymphoepithelial lesions will eventually terminate in Sjögren’s disease. More aptly, the two diseases may be considered two forms of the same disease with a probably common etiology.
Salivary Gland Cysts
Most cystic lesions of the major salivary glands are cystic neoplasms. Benign cyst is much less common and accounts for approximately 2–5% of parotid gland lesions. They are rare in the other major salivary glands. Benign cyst (sialocyst) of the parotid gland can be conveniently classified into the following three types: lymphoepithelial cyst, salivary duct cyst, and dysgenetic cyst. Polycystic (dysgenetic) disease of the parotid gland is considered a developmental malformation of the ductal system.
Salivary Duct Cyst
Salivary duct cyst may be acquired or congenital. The majority; however, are acquired and most of these are probably secondary to obstruction. Some authors, therefore, prefer the term retention cyst to designate these lesions. Still others prefer the term simple cyst.
Clinical Features
Salivary duct cysts of the parotid gland are unilateral painless swellings with no involvement of facial nerve and no fixation to the overlying skin. The majority of the affected patients are over 40 years of age. The cysts range in size from 0.8–10.0 cm with an average size of approximately 1–3 cm.
Histologic Features
Typically, these unilocular cysts are lined by single or multilayered cuboidal or columnar epithelium. Occasional mucus containing goblet cells and areas of oncocytic differentiation may be seen. It may be completely or partially lined by squamous epithelium. A sparse to moderate lymphocytic infiltration is present in the cyst wall.
Chronic Sclerosing Sialadenitis of Submandibular Gland: (Küttner tumor)
Kuttner tumor or chronic sclerosing sialadenitis is a benign inflammatory condition of the submandibular gland that mimics a malignant neoplasm clinically because of presentation as a hard mass. This entity is poorly documented in the surgical pathology and cytology literature.
Histologic Features
Histologic examination of the excised submandibular glands revealed preserved lobular architecture, thickening of interlobular septa by sclerotic tissue, dense lymphoplasmacytic infiltrate, preservation of ducts with periductal fibrosis, and variable loss of acini. Fine-needle aspiration cytologic findings were characterized by scattered tubular ductal structures often enveloped by collagen bundles or lymphoplasmacytic infiltrate, isolated fragments of fibrous stroma, a background rich in lymphoid cells, and paucity or absence of acini.
Cystic Lymphoid Hyperplasia in AIDS
Cystic lesions of the parotid gland have been reported in patients at risk for acquired immuno deficiency syndrome (AIDS). The cysts in many of these cases have features of lymphoepithelial cysts but differ from those described before the AIDS epidemic in that they are more frequently multiple and bilateral. In addition to the cysts, the parotid gland in many of the patients at risk of AIDS exhibits features suggestive of benign lymphoepithelial lesions. Such lesions, without associated cysts, may also occur in patients at risk of AIDS. The parotid lymphoid tissue in some of these cystic and noncystic lesions may exhibit features seen in the lymph nodes of patients with AIDS-related complex with persistent generalized lymphade nopathy. Transformation to malignant lymphoma may occur.
Bibliografia
Abbondanzo, S.L. Extranodal marginal-zone: B-cell lymphoma of the salivary glands. Ann Diagn Pathol. 2001; 5(4):246–254.
Abrams, A.M., Melrose, R.J., Howell, F.V. Necrotizing sialometaplasia: a disease simulating malignancy. Cancer. 1973; 32(1):130–135. [Jul].
Abrams, A.M. Necrotizing sialometaplasia of the nasal cavity. Otolaryngol Head Neck Surg. 1986; 94(3):416. [Mar].
Abrams, A.M., Finck, F.M. Sialadenoma papilliferum: a previously unreported salivary gland tumor. Cancer. 1969; 24:1057.
Abrams, A.M., Melrose, R.J. Acinic cell tumors of minor salivary gland origin. Oral Surg. 1978; 46:220.
Abrams, A.M., Cornyn, J., Scofield, H.H., Hansen, L.S. Acinic cell adenocarcinoma of the major salivary glands. Cancer. 1965; 18:1145.
El-Naggar, Adel K, et al. Cytogenetic analysis of a primary salivary gland myoepithelioma. Cancer Genetics and Cytogenetics. 1999; 113(a):49–53.
Adriano, Piattelli, et al. Intraduct papilloma of the palate: report of a case. Oral Oncology. 2002; 38(4):398–400. [June].
Aframian, D., Milhem, I.I., Kirsch, G., Markitziu, A. Necrotizing sialometaplasia after silastic ring vertical gastroplasty: case report and review of the literature. Obesity Surgery. 1995; 5:179–182.
Allegra, S.R. Warthin’s tumor: a hypersensitivity disease? Ultrastructural, light and immunofluorescent study. Hum Pathol. 1971; 2:403.
Allenspach, E.J., Maillard, I., Aster, J.C., et al. Notch signaling in cancer. Cancer Biol Ther. 2002; 1(5):466–476. [Sep-Oct].
Anneroth, G., Hansen, L.S. Necrotizing sialometaplasia: the relationship of its pathogenesis to its clinical characteristics. Int J Oral Surg. 1982; 11(5):283–291. [Oct].
Arthaud, J.B. Anaplastic parotid carcinoma (malignant lymphoepithelial lesion) in seven: alaskan natives. Am J Clin Pathol. 1972; 57:275.
Assor, D. Bilateral carcinoma of the parotid, one cancer arising in a Warthin’s tumor. Am J Clin Pathol. 1974; 61:270.
Auclair, P.L., Ellis, G.L., Gnepp, D.R., et al, Salivary gland neoplasms: general considerationsEllis, G.L., Auclair, P.L., Gnepp, D.R., eds. Surgical Pathology of the Salivary Glands. Philadelphia, WB Saunders, 1991:135–164. [eds].
Auclair, P.L., Ellis, G.L., Nonlymphoid sarcomas of the major salivary glandsEllis, G.L., Auclair, P.L., Gnepp, D.R., eds. Surgical Pathology of the Salivary Glands. Philadelphia, WB Saunders, 1991:514–527.
Auclair, P.L., Goode, R.K., Ellis, G.L. Mucoepidermoid carcinoma of intraoral salivary glands: evaluation and application of grading criteria in 143 cases. Cancer. 1992; 69(8):2021–2030.
Auclair, P.L., Langloss, J.M., Weiss, S.W., et al. Sarcomas and sarcomatoid neoplasms of the major salivary gland regions: a clinicopathologic and immunohistochemical study of 67 cases and review of the literature. Cancer. 1986; 58(6):1305–1315.
Baden, E., Pierce, M., Selman, A.J., Roberts, T.W., et al. Intraoral papillary cystadenoma lymphomatosum. J Oral Surg. 1976; 34:533.
Barnes, L., Rao, U., Krause, J., et al. Salivary duct carcinoma Part I: a clinicopathologic evaluation and DNA image analysis of 13 cases with review of the literature. Oral Surg Oral Med Oral Pathol. 1990; 78(1):64–73.
Batsakis, J.G., Bautina, E. Metastases to major salivary glands. Ann Otol Rhinol Laryngol. 1990; 99(6 Pt 1):501–503.
Batsakis, J.G., Naggar, A.K., Luna, M.A. Epithelial-myoepithelial carcinoma of salivary glands. Ann Otol Rhinol Laryngol. 1992; 101(6):540–542.
Batsakis, J.G., Luna, M.A., Luna, A. Histopathologic grading of salivary gland neoplasms III—adenoid cystic carcinomas. Ann Otol Rhinol Laryngol. 1990; 99(12):1007–1009.
Batsakis, J.G., Luna, M.A. Undifferentiated carcinomas of salivary glands. Ann Otol Rhinol Laryngol. 1991; 100(1):82–182.
Batsakis, J.G., Manning, J.T. Necrotizing sialometaplasia of major salivary glands. J Laryngol Otol. 1987; 101(9):962–966. [Sep].
Batsakis, J.G. Pathology consultation: sialadenosis. Ann Otol Rhinol Laryngol. 1988; 97:94–95.
Batsakis, J.C. Basal cell adenoma of the parotid gland. Cancer. 1972; 29:226.
Batsakis, J.G., Regezi, J.A. Selected controversial lesions of salivary tissues. Otolaryngol Clin North Am. 1977; 10:309.
Batsakis, J.G., Brannon, R.B., Sciubba, J.J. Monomorphic adenomas of major salivary glands: a histologic study of 96 tumours. Clin Otolaryngol. 1981; 6:129.
Batsakis, J.G., Wozniak, K.J., Regezi, J.A. Acinous cell carcinoma: a histogenetic hypothesis. J Oral Surg. 1977; 35:904.
Bauer, W.H., Bauer, J.D. Classification of glandular tumors of salivary glands: study of one hundred forty-three cases. Arch Pathol. 1953; 55:328–346.
Bell, G.W., Loukota, R.A. Necrotizing sialometaplasia coincident with ipsilateral infarcted antral polyps. Br J Oral Maxillofac Surg. 1996; 34(1):129–131. [Feb].
Belsky, J.L., Tachikawa, K., Cihak, R.W., Yamamoto, T. Salivary gland tumors in atomic bomb survivors, Hiroshima-Nagasaki, 1957 to 1970. J Am Med Assoc. 1972; 219:864.
Ben-Aryeh, H., Scharf, J., Gutman, D., Szargel, R., et al. Sialochemistry of KCS patients. J Oral Surg. 1978; 7:172.
Ben-Izhak, O., Ben-Arieh, Y. Necrotizing squamous metaplasia in herpetic tracheitis following prolonged intubation: a lesion similar to necrotizing sialometaplasia. Histopathology. 1993; 22(3):265–269. [mar].
Bernier, J.L., Bhaskar, S.N. Lymphoepithelial lesions of salivary glands: histogenesis and classification based on 186 cases. Cancer. 1958; 11:1156.
Bertram, U., Hjoting-Hansen, E. Punch-biopsy of minor salivary glands in the diagnosis of Sjogren’s syndrome. Scand J Dent Res. 1970; 78:295.
Bertram, U. Xerostomia. Acta Odontol Scand. 1967; 25(Suppl):49.
Bhaskar, S.N., Bernier, J.L. Mucoepidermoid tumors of major and minor salivary glands. Cancer. 1962; 15:801.
Bhaskar, S.N. Acinic-cell carcinoma of salivary glands: report of twenty-one cases. Oral Surg. 1964; 17:62.
Borg, M.F., Benjamin, C.S., Morton, R.P., et al. Malignant lympho-epithelial lesion of the salivary gland: a case report and review of the literature. Australas Radiol. 1993; 37(3):188–191.
Bosch, J.D., Kudryk, W.H., Johnson, G.H. The malignant lymphoepithelial lesion of the salivary glands. J Otolaryngol. 1988; 17(4):187–190.
Brandwein, M.S., Ferlito, A., Bradley, P.J., et al. Diagnosis and classification of salivary neoplasms: pathologic challenges and relevance to clinical outcomes. Acta Otolaryngol. 2002; 122(7):758–764.
Brandwein, M.S., Ivanov, K., Wallace, D.I., et al. Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol. 2001; 25(7):835–845.
Brannon, R.B., Fowler, C.B., Hartman, K.S. Necrotizing sialometaplasia: a clinicopathologic study of sixty-nine cases and review of the literature. Oral Surg Oral Med Oral Pathol. 1991; 72(3):317–325. [sep].
Brooks, D.G., Hottinger, H.A., Dunstan, R.W. Canine necrotizing sialometaplasia: a case report and review of the literature. J Am Anim Hosp Assoc. 1995; 31(1):31–131. [Jan-Feb].
Brookstone, M.S., Huvos, A.G. Central salivary gland tumors of the maxilla and mandible: a clinicopathologic study of 11 cases with an analysis of the literature. J Oral Maxillofac Surg. 1992; 50(3):229–236.
Browand, B.C., Waldron, C.A. Central mucoepidermoid tumors of the jaws. Oral Surg. 1975; 40:613.
Buchner, A., David, R., Hansen, L.S. ‘Hyaline cells’ in pleomorphic adenoma of salivary gland origin. Oral Surg. 1981; 52:506.
Bullerdiek. Cytogenetic subtyping of 220 salivary gland pleomorphic adenomas: correlation to occurrence, histological subtype and in vitro cellular behavior. Cancer Genet Cytogenet. 1993; 65:27–31.
Burke, J.S. Waldeyer’s ring, sinonasal region, salivary gland, thyroid gland, central nervous system, and other extranodal lymphomas and lymphoid hyperplasias. In: Knowles D.M., ed. Neoplastic Hemato pathology. williams and Wilkins: Baltimore; 1992:1047–1079.
Canalis, R.F., Mok, M.W., Fishman, S.M., Hemenway, W.G. Congenital basal cell adenoma of the submandibular gland. Arch Otolaryngol. 1980; 106:284.
Cantera, J.M., Hernandez, A.V. Bilateral parotid gland metastasis as the initial presentation of a small cell lung carcinoma. J Oral Maxillofac Surg. 1989; 47(11):1199–1201.
Castro, E.B., Huvos, A.G., Strong, E.W., Foote, F.W., Jr. Tumors of the major salivary glands in children. Cancer. 1972; 29:312.
Chaudhary, A.P., Vickers, R.A., Gorlin, R.J. Intraoral minor salivary gland tumors. Oral Surg. 1961; 14:1194.
Chaudhry, A p, Gorlin, R.J. Papillary cystadenoma lymphomatosum (adenolymphoma): a review of the literature. Am J Surg. 1958; 95:925.
Chaudhry, A P, Satchidanand, S., Peer, R., Cutler, L.S. Myoepithelial cell adenoma of the parotid gland: a light and ultrastructural study. Cancer. 1982; 49:288.
Chen, K.T. Necrotizing sialometaplasia of the nasal cavity. Am J Otolaryngol. 1982; 3(6):444–446. [Nov-Dec].
Chen, S-Y., Brannon, R.B., Miller, A.S., White, D.K., et al. Acinic cell adenocarcinoma of minor salivary glands. Cancer. 1987; 42:678.
Cheuk, W. Salivary gland tumors. In: Fletcher C.D.M, ed. Diagnostic Histopathology of Tumors. London: Churchill Livingstone; 2000:231–311.
Chisholm, D.M., Mason, D.K. Salivary gland function in Sjogren’s syndrome: a review. Br Dent J. 1973; 135:393.
Chisholm, D.M., Waterhouse, J P, Kraucunas, E., Sciubba, J.J. A quantitative ultrastructural study of the pleomorphic adenoma (mixed tumor) of human minor salivary glands. Cancer. 1974; 34:1631.
Cleary, K.R., Batsakis, J.G. Undifferentiated carcinoma with lymphoid stroma of the major salivary glands. Ann Otol Rhinol Laryngol. 1990; 99(3):236–238.
Martins, C. Cytogenetic characterisation of Warthin’s tumour. Oral Oncol. 1997; 33(5):344–347. [Sept].
Collina, G., Gale, N., Visonà, A., et al. Epithelial-myoepithelial carcinoma of the parotid gland: a clinico-pathologic and immunohistochemical study of seven cases. Tumori. 1991; 77(3):257–263.
Conley, J., Dingman, D.L. Adenoid cystic carcinoma in the head and neck (cylindroma). Arch Otolaryngol. 1974; 100:81–90.
Corio, R.L., Sciubba, J.J., Brannon, R.B., Batsakis, J.G. Epithelial-myoepithelial carcinoma of intercalated duct origin: a clinicopathologic and ultrastructural assessment of sixteen cases. Oral Surg. 1982; 53:280.
Cossman, J., Deegan, M.J., Batsakis, J.G. Warthin tumor B-lymphocytes within the lymphoid infiltrates. Arch Pathol Lab Med. 1977; 101:354.
Cummings, N.A. Sjogren’s syndrome: newer aspects of research, diagnosis and therapy. Ann Intern Med. 1971; 75:937. [(mod)].
Daley, T.D., Wysocki, G P, Smout, M.S. Epithelial-myoepithelial carcinoma of salivary glands. Oral Surg Oral Med Oral Pathol. 1984; 57(5):512–519.
Dardick, I., van Nostrand, P., Phillips, M.J. Histogenesis of salivary gland pleomorphic adenoma (mixed tumor) with an evaluation of the role of the myoepithelial cell. Hum Pathol. 1982; 13:62.
David, C., Augusto, F. Basaloid tumors of the salivary glands. Ann Diagn Pathol. 2002; 6(6):364–372.
Dean, A., et al. Malignant myoepithelioma of the salivary glands: clinicopathological and inmunohistochemical features. Br J Oral Maxillofa Surg. 1999; 37(1):64–66.
Delgado, R., Klimstra, D., Albores-Saavedra, J. Low grade salivary duct carcinoma: a distinctive variant with a low grade histology and a predominant intraductal growth pattern. Cancer. 1996; 78(5):958–967.
DiGiuseppe, J.A., Corio, R.L., Westra, W.H. Lymphoid infiltrates of the salivary glands: pathology, biology and clinical significance. Curr Opin Oncol. 1996; 8(3):232–237.
Dunlap, C.L., Barker, B.F. Necrotizing sialometaplasia. Oral Surg. 1974; 37:722.
Maiorano, E., et al. Warthin’s tumour: a study of 78 cases with emphasis on bilaterality, multifocality and association with other malignancies. Oral Oncology. 2002; 38(1):35–40. [Jan].
Sakai, E., et al. Pathologic and imaging findings of an oncocytoma in the deep lobe of the left parotid gland. Int J Oral Maxillofa Surg. 2003; 32(5):563–565. [oct].
Echevarria, R.A. Ultrastructure of the acinic cell carcinoma and clear cell carcinoma of the parotid gland. Cancer. 1967; 20:563.
Edmund, M. Collins: Papillary cystadenoma of accessory salivary gland. Am J Surg. 1958; 96(6):749–750. [Dec].
Ellis, G.L., Auclair, P.L., Gnepp, D.R., et al, Other malignant epithelial neoplasmsEllis, G.L., Auclair, P.L., Gnepp, D.R., eds. Surgical Pathology of the Salivary Glands. WB Saunders, Philadelphia, 1991:455–488.
Ellis, G.L., Auclair, P.L., Tumors of the Salivary GlandsArmed Forces Institute of Pathology, Atlas of Tumor Pathology. Washington DC: Atlas of Tumor Pathology, 1996. [(3)].
Ellis, G.L., Wiscovitch, J.G. Basal cell adenocarcinomas of the major salivary glands. Oral Surg Oral Med Oral Pathol. 1990; 69(4):461–469.
El-Naggar, A.K., Lovell, M., Killary, A.M., et al. A mucoepidermoid carcinoma of minor salivary gland with t(11;19)(q21;p13.1) as the only karyotypic abnormality. Cancer Genet Cytogenet. 1996; 87(1):29–33.
Evans, H.L., Batsakis, J.G. Polymorphous low grade adenocarcinoma of minor salivary glands. A study of 14 cases of a distinctive neoplasm. Cancer. 1996; 53:935–942.
Evans, H.L., Luna, M.A. Polymorphous low-grade adenocarcinoma: a study of 40 cases with long-term follow up and an evaluation of the importance of papillary areas. Am J Surg Pathol. 2000; 24(10):1319–1328.
Evans, R.W., Cruickshank, A.H.Epithelial Tumors of the Salivary Glands. Philadelphia: WB Saunders, 1970.
Fantasia, J.E., Neville, B.W. Basal cell adenomas of the minor salivary glands: aclinicopathologic study of seventeen new cases and a review of the literature. Oral Surg. 1980; 50:433.
Foote, F.W., Jr., Frazell, E.L.Tumors of the major salivary glands. Atlas of Tumor Pathology, Section I V,Fascicle 11, 1st Series. Washington DC: Armed Forces Institute ofPathology, 1954.
Foote, F.W., Jr., Frazell, E.L. Tumors of the major salivary glands. Cancer. 1953; 6:1065.
Forney, S.K., Foley, J.M., Sugg, W.E., Oatis, G.W., Jr. Necrotizing sialometaplasia of the mandible. Oral Surg Oral Med Oral Pathol. 1977; 43(5):720–726. [May].
Fowler, C.B., Brannon, R.B. Subacute necrotizing sialadenitis: report of 7 cases and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000; 89(5):600–609. [May].
Fox, R.I., Kang, H-I. Sjögren’s Syndrome. In: Kelley W.N., Harris E.D., Ruddy S., et al, eds. Textbook of Rheumatology. 4. Philadelphia: WB Saunders; 1993:931–941.
Franchi, A., Gallo, O., Santucci, M. Pathologic quiz case 1: necrotizing sialometaplasia obscuring recurrent well-differentiated squamous cell carcinoma of the maxillary sinus. Arch Otolaryngol Head Neck Surg. 1995; 121(5):584–586. [May].
Freedman, P.D., Lumerman, H. Sialadenoma papilliferum. Oral Surg. 1978; 45:88.
Friedman, M., Hall, J.W. Radiation-induced squamous metaplasis and hyperplasia of the normal mucous glands of the oral cavity. Radiology. 1950; 55:848.
Friedrich, R.E., Bleckmann, V. Adenoid cystic carcinoma of salivary and lacrimal gland origin: localization, classification, clinical pathological correlation, treatment results and long-term follow-up control in 84 patients. Anticancer Res. 2003; 23(2):931–940. [Mar-Apr].
Seifert, G., Donath, K. Multiple tumours of the salivary glands—terminology and nomenclature. Oral Oncology. 1996; 1(32):3–7. [Jan].
Zajicek, G. The histogenesis of glandular neoplasia. Medical Hypotheses. 1981; 7(10):1241–1251. [Oct].
Gardner, D.G., Bell, M.E.A., Wesley, R.K., Wysocki, G.P. Acinic cell tumors of minorsalivary glands. Oral Surg. 1980; 50:545.
Gaughan, R.K., Olsen, K.D., Lewis, J.E. Primary squamous cell carcinoma of the parotid gland. Arch Otolaryngol Head Neck Surg. 1992; 118(8):798–801.
Gerughty, R.M., Hennigar, G.R., Brown, F.M. Adenosquamous carcinoma of the nasal,oral and laryngeal cavities: a clinicopathologic survey of ten cases. Cancer. 1968; 22:1140.
Gerughty, R.M., Scofield, H.H., Brown, F.M., Hennigar, G.R. Malignant mixed tumors of salivary gland origin. Cancer. 1969; 24:471.
Gleeson, M.J., Bennett, M.H., Cawson, R.A. Lymphomas of salivary glands. Cancer. 1986; 58(3):699–704.
Gnepp, D.R., Brannon, R. Sebaceous neoplasms of salivary gland origin: report of 21 cases. Cancer. 1984; 53(10):2155–2170.
Gnepp, D.R., Corio, R.L., Brannon, R.B. Small cell carcinoma of the major salivary glands. Cancer. 1986; 58(3):705–714.
Gnepp, D.R., Wick, M.R. Small cell carcinoma of the major salivary glands: an immunohistochemical study. Cancer. 1990; 66(1):185–192.
Gnepp, D.R. Malignant mixed tumors of the salivary glands: a review. Pathol Annu. 1993; 28(1):279–328.
Gnepp, D.R. Metastatic disease to the major salivary glands. In: Ellis G.L., Auclair P.L., Gnepp D.R., eds. Surgical Pathology of the Salivary Glands. Philadelphia: WB Saunders; 1991:560–609.
Gnepp, D.R. Sebaceous neoplasms of salivary gland origin: a review. Pathol Annu. 1983; 18(1):71–102.
Gnepp, D.R. Warthin tumor exhibiting sebaceous differentiation and necrotizing sialometaplasia. Virchows Arch A Pathol Anat Histol. 1981; 391(3):267–273.
Godwin, J.T., Foote, J.W., Frazell, E.L. Acinic cell adenocarcinoma of the parotid gland: report of twenty seven cases. Am J Pathol. 1954; 30:465.
Godwin, J.T. Benign lymphoepithelial lesion of the parotid gland (adenolymphoma, chronic inflammation, lymphoepithelioma, chronic inflammation, lymphoepithelioma, lymphocytic tumor, Mikulicz disease): report of eleven cases. Cancer. 1952; 5:1089.
Goode, R.K., Auclair, P.L., Ellis, G.L. Mucoepidermoid carcinoma of the major salivary glands: clinical and histopathologic analysis of 234 cases with evaluation of grading criteria. Cancer. 1998; 87(7):1217–1224.
Goode, R.K., Corio, R.L. Oncocytic adenocarcinoma of salivary glands. Oral Surg Oral Med Oral Pathol. 1988; 65(1):61–68.
Goto, T.K., Shimizu, M., Kobayashi, I., et al. Lymphoepithelial lesion of the parotid gland. Dentomaxillofac Radiol. 2002; 31:198–203.
Granich, M.S., Pilch, B.Z. Necrotizing sialometaplasia in the setting of acute and chronic sinusitis. Laryngoscope. 1981; 91(9):1532–1535. Pt 1 Sep
Granick, M.S., Solomon, M.P., Benedetto, A.V., et al. Necrotizing sialometaplasia masquerading as residual cancer of the lip. Ann Plast Surg. 1988; 21(2):152–154. [Aug].
Gray, S.R., Cornog, J.L., Jr., Seo, I.S. Oncocytic neoplasms of salivary glands: a report of fifteen cases including two malignant oncocytomas. Cancer. 1976; 38:1306.
Greenspan, J.S., Daniels, T.E., Talal, N., Sylvester, R.A. The histopathology of Sjogren’s syndrome in labial salivary gland biopsies. Oral Surg. 1974; 37:217.
Guang-yan, Y.U., et al. Histogenesis and development of membranous basal cell adenoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endodonto. 1998; 86(4):446–451.
Guzzo, M., Andreola, S., Sirizzotti, G., et al. Mucoepidermoid carcinoma of the salivary glands: clinicopathologic review of 108 patients treated at the National Cancer Institute of Milan. Ann Surg Oncol. 2002; 9(7):688–695.
Guzzo, M., Di Palma, S., Grandi, C. Salivary duct carcinoma: clinical characteristics and treatment strategies. Head Neck. 1997; 19(2):126–133.
Hamilton-Dutoit, S.J., Therkildsen, M.H., Neilsen, N.H., et al. Undiffe rentiated carcinoma of the salivary gland in Greenlandic Eskimos: demonstration of Epstein-Barr virus DNA by in situ nucleic acid hybridization. Hum Pathol. 1991; 22(8):811–815.
Hamper, K., Lazar, F., Dietel, M., et al. Prognostic factors for adenoid cystic carcinoma of the head and neck: a retrospective evaluation of 96 cases. J Oral Pathol Med. 1990; 19(3):101–107.
Harris, N.L. Extranodal lymphoid infiltrates and mucosa-associated lymphoid tissue. A unifying concept. Am J Surg Pathol. 1991; 15(9):879–884. [(MALT)].
Harris, N.L. Lymphoid proliferations of the salivary glands. Am J Clin Pathol. 1999; 111(1):94–103.
Harusachi, Kanazawa, et al. Oncocytoma of an intraoral minor salivary gland: Report of a case and review of literature. J Oral Maxillofac Surg. 2000; 58(8):894–897. [Aug].
Healey, W.V., Perzin, K.H., Smith, L. Classification, clinical-pathologic correlation, and results of treatment, Mucoepidermoid carcinoma of salivary gland origin. Cancer. 1970; 26:368.
Hochberg, M.C. Sjögren’s Syndrome. In: Bennett J.C., Plum F., eds. Cecil Textbook of Medicine. 20. Philadelphia: WB Saunders; 1996:1488–1490.
Horsman, D.E., Berean, K., Durham, J.S. Translocation (11;19)(q21;p13.1) in mucoepidermoid carcinoma of salivary gland. Cancer Genet Cytogenet. 1995; 80(2):165–166.
Hubner, G., Klein, H.J., Schiefer, H.G. Role of myoepithelial cells in the development of salivary gland tumors. Cancer. 1971; 27:1255.
Hui, K.K., Luna, M.A., Batsakis, J.G., et al. Undifferentiated carcinomas of the major salivary glands. Oral Surg Oral Med Oral Pathol. 1990; 69(1):76–83.
Hurt, M.A., Diaz-Arias, A.A., Rosenholtz, M.J., et al. Posttraumatic lobular squamous metaplasia of breast. An unusual pseudocarcinomatous metaplasia resembling squamous (necrotizing) sialometaplasia of the salivary gland. Mod Pathol. 1988; 1(5):385–390. [Sep].
Hus, S-M, Hsu, P-L, Nayak, R.N. Warthin’s tumor: an immunohistochemical study of its lymphoid stroma. Hum Pathol. 1981; 12:251.
Hyman, G.A., Wolff, M. Malignant lymphomas of the salivary glands. Review of the literature and report of 33 new cases, including four cases associated with the lymphoepithelial lesion. Am J Clin Pathol. 1976; 65:421.
Ihrler, S., Baretton, G.B., Menauer, F., et al. Sjögren’s syndrome and MALT lymphomas of salivary glands: a DNA-cytometric and interphase-cytogenetic study. Mod Pathol. 2000; 13(1):4–12.
Imbery, T.A., Edwards, P.A. Necrotizing sialometaplasia: literature review and case reports. J Am Dent Assoc. 1996; 127(7):1087–1092. [Jul].
Jainkittivong, A., Sookasam, M., Philipsen, H.P. Necrotizing sialometaplasia: review of 127 cases. J Dent Assoc Thai. 1989; 39(1):11–16. [Jan-Feb].
Jao, W., Keh, P.C., Swerdlow, M.A. Ultrastructure of the basal cell adenoma of parotid gland. Cancer. 1976; 37:1322.
Jason, L Hornick, Christopher, DM Fletcher. Cutaneous myoepithelioma: a clinicopathologic and immunohistochemical study of 14 cases. Hum Pathol. 2004; 35(1):14–24.
Jean, E., Lewis, M.D., et al. Carcinoma ex pleomorphic adenoma: pathologic analysis of 73 cases. Hum Pathol. 2001; 32:596–604.
Jensen, J.L. Idiopathic diseases. In: Ellis G.L., Auclair P.L., Gnepp D.R., eds. Surgical Pathology of the Salivary Glands. Philadelphia: WB Saunders; 1991:60–82.
John, B Alexis, Victor, Dembrow. Papillary cystadenoma of a minor salivary gland. J Oral Maxillofac Surg. 1995; 53(1):70–72. [Jan].
John, P Leonetti, Sam, J Marzo, Guy, J Petruzzelli. Recurrent pleomorphic adenoma of the parotid gland. Otolaryngol Head Neck Surg. 2004; 131(2):65–66.
Johns, M.E., Regezi, J.A., Batsakis, J.G. Oncocytic neoplasms of salivary glands: an ultrastructural study. Laryngoscope. 1977; 87:862.
Kassan, S.S., Thomas, T.L., Moutsopoulos, H.M., et al. Increased risk of lymphoma in sicca syndrome. Ann Intern Med. 1978; 89(6):888–892.
Kay, S., Still, W.J.S. Electron microscopic observations on a parotid oncocytoma. Arch Pathol. 1973; 96:186.
Kessler, H.S. A laboratory model for Sjogren’s syndrome. Am J Pathol. 1968; 52:671.
Kimiko, Takezawa, et al. Molecular characterization of Warthin tumor. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998; 85(5):569–575. [May].
King, D.T., Barr, R.J. Syringometaplasia: mucinous and squamous variants. J Cutan Pathol. 1979; 6(4):284–291. [Aug].
Koss, L.G., Spiro, R.H., Hajdu, S. Small cell carcinoma of minor salivary gland origin. Cancer. 1972; 30(3):737–741. [(oat cell)].
Krolls, S.O., Boyers, R.C. Mixed tumors of salivary glands. Long-term follow-up.Cancer. 1972; 30:276.
Krolls, S.O., Hicks, J.L. Mixed tumors of the lower lip. Oral Surg. 1973; 35:212.
Krolls, S.O., Trodahl, J.N., Boyers, R.C. Salivary gland lesions in children: a survey of 430 cases. Cancer. 1972; 30:459.
Kunio, Tsurumi, et al. Papillary oncocytic cystadenoma of palatal minor salivary gland: a case report. J Oral Maxillo Surg. 2003; 61(5):631–633. [May].
Lapes, M., Antoniades, K., Gartner, W., Jr., Vivacqua, R. Conversion of a benign lymphoepithelial salivary gland lesion to lymphocytic lymphoma during Dilantin therapy: correlation with Dilantin-induced lymphocyte transformation in vitro. Cancer. 1976; 38:1318.
Lee, S.C., Roth, L.M. Malignant oncocytoma of the parotid gland: a light and electron microscopic study. Cancer. 1976; 37:1607.
Guedes Queiroz, Lélia Maria, et al. A rare salivary gland neoplasm: multiple canalicular adenoma: a case report. Auris Nasus Larynx. 2004; 31(2):189–193. [June].
Leung, A.K.C. Benign Lymphoepithelial Lesion. In: NORD Guide to Rare Disorders. Philadelphia: JB Lippincott, Williams and Wilkins; 2003:17.
Leung, S.Y., Chung, L.P., Yuen, S.T., et al. Lymphoepithelial carcinoma of the salivary gland: in situ detection of Epstein-Barr virus. J Clin Pathol. 1995; 48(11):1022–1027.
Lewis, J.E., Olsen, K.D., Weiland, L.H. Acinic cell carcinoma: clinicopathologic review. Cancer. 1991; 67(1):172–179.
Linhartová, A. Sebaceous glands in salivary gland tissue. Arch Pathol. 1974; 98(5):320–324.
Little, J.W. The histogenesis of papillary cystadenoma lymphomatosum. Oral Surg. 1966; 22:72.
LiVolsi, V.A., Perzin, K.H. Malignant mixed tumors arising in salivary glands I: carcinomas arising in benign mixed tumors: a clinicopathologic study. Cancer. 1977; 39(5):2209–2230.
Lomax-Smith, J.D., Azzopardi, J.G. The hyaline cell: a distinctive feature of mixed salivary tumours. Histopathology. 1978; 2:77.
Loreti, et al. Diffuse hyperplastic oncocytosis of the parotid gland. Br J Plast Surg. 2002; 55(2):151–152. [Mar].
Luna, M.A., Tortoledo, M.E., Ordóñez, N.G., et al. Primary sarcomas of the major salivary glands. Arch Otolaryngol Head Neck Surg. 1991; 117(3):302–306.
Luna, M.A., Mackay, B., Gamez-Araujo, J. Myoepithelioma of the palate: report of a case with histochemical and electron microscopic observations. Cancer. 1973; 32:1429.
Lynch, D.P., Crage, C.A., Martinez, M.G., Jr. Necrotizing sialometaplasia: a review of the literature and report of two additional cases. Oral Surg. 1979; 47:63.
Machado de, Sousa SO, et al. Immunohistochemical aspects of basal cell adenoma and canalicular adenoma of salivary glands. Oral Oncology. 2001; 37(4):365–368.
Mader, C.L., Nelson, J.F. Monomorphic adenoma of the minor salivary glands. J Am Dent Assoc. 1981; 201:657.
Major and minor salivary glands. In: Rosai J., ed. Ackerman’s Surgical Pathology. 8th. St. Louis: CV Mosby; 1996:815–856.
Mandel, L., Kaynar, A., DeChiara, S. Necrotizing sialometaplasia in a patient with sickle-cell anemia. J Oral Maxillofac Surg. 1991; 49(7):757–759. [July].
Manikkapurath, Hemachandran, et al. Basal cell adenoma—an unusual presentation. Ann Diagn Pathol. 2003; 7(5):292–295.
Martinez-Mora, J., Boix-Ochoa, J., Tresserra, L. Vascular tumors of the parotid region in children. Surg Gynecol Obstet. 1971; 133:973.
Matsuba, H.M., Mauney, M., Simpson, J.R., et al. Adenocarcinomas of major and minor salivary gland origin: a histopathologic review of treatment failure patterns. Laryngoscope. 1988; 98(7):784–788.
Matsumoto, T., Kuwabara, N., Shiotsu, H., et al. Necrotizing sialometaplasia in the mouth floor secondary to reconstructive surgery for tongue carcinoma. Acta Pathol Jpn. 1991; 41(9):689–693. [Sep].
McGavran, M.H., Bauer, W.C., Ackerman, L.V. Sebaceous lymphadenoma of the parotid salivary gland. Cancer. 1960; 13:1185.
Melrose, R.J., Abrams, A.M., Howell, F V. Mucoepidermoid tumors of the intraoral minor salivary glands: a clinicopathologic study of 54 cases. J Oral Pathol. 1973; 2:314.
Merwin, G.E., Duckert, L.G., Pollak, K. Necrotizing sialometaplasia of the nasopharynx. Ann Otol Rhinol Laryngol. 1979; 88(3 Pt 1):348–351. [May-Jun].
Mesa, M.L., Gertler, R.S., Schneider, L.C. Necrotizing sialometaplasia: frequency of histologic misdiagnosis. Oral Surg Oral Med Oral Pathol. 1984; 57(1):71–73. [Jan].
Milchgrub, S., Gnepp, D.R., Vuitch, F., et al. Hyalinizing clear cell carcinoma of salivary gland. Am J Surg Pathol. 1994; 18(1):74–82.
Miller, A.S., McCrea, M.W. Sebaceous gland adenoma of buccal mucosa. J Oral Surg. 1968; 26:593.
Miller, A.S., Winnick, M. Salivary gland inclusion in the anterior mandible. Oral Surg. 1971; 31:790.
Mintz, G.A., Abrams, A.M., Melrose, R.J. Monomorphic adenomas of the major and minor salivary glands. Oral Surg. 1982; 53:375.
Morgan, W.S., Castleman, B. A clinicopathologic study of Mikulicz’s disease. Am J Path. 1953; 29:471.
Moutsopoulos, H.M. Sjogren’s syndrome (sicca syndrome): current issues. Ann Intern Med. 1980; 92:212.
Muller, S., Barnes, L. Basal cell adenocarcinoma of the salivary glands: report of seven cases and review of the literature. Cancer. 1996; 78(12):2471–2477.
Naeim, F., Forsberg, M.I., Jr., Waisman, J., Coulson, W.F. Mixed tumors of the salivary glands: growth pattern and recurrence. Arch Pathol Lab Med. 1976; 100:271.
Nagao, K., Matsuzaki, O., Saiga, H., Sugano, I., et al. Histopathologic studies on carcinoma in pleomorphic adenoma of the parotid gland. Cancer. 1981; 48:113.
Nelson, J.F., Jacoway, J.R. Monomorphic adenoma (canalicular type): report of 29 cases. Cancer. 1973; 31:1511.
Neville, B.W., Damm, D.D., Weir, J.C., et al. Labial salivary gland tumors. Cancer. 1988; 61(10):2113–2116.
Nilsen, R., Bernhoft, C.H., Gilhuus-Moe, O. Necrotizing sialometaplasia. Int J Oral Surg. 1978; 7(6):580–584. [Dec].
Noel, S., Brozna, J.P. Epithelial-myoepithelial carcinoma of salivary gland with metastasis to lung: report of a case and review of the literature. Head Neck. 1992; 14(5):401–406. [Sep-Oct].
Nordkvist, A., Gustafsson, H., Juberg-Ode, M, et al. Recurrent rearrangements of 11q14–22 in mucoepidermoid carcinoma. Cancer Genet Cytogenet. 1994; 74(2):77–83.
Ogawa, I., Nikai, H., Takata, T., et al. Clear cell tumors of minor salivary gland origin: an immunohistochemical and ultrastructural analysis. Oral Surg Oral Med Oral Pathol. 1991; 72(2):200–207.
Osaki, T., Hirota, J., Ohno, A., et al. Mucinous adenocarcinoma of the submandibular gland. Cancer. 1990; 66(8):1796–1801.
Perez-Ordonez, B., Caruana, S.M., Huvos, A.G., et al. Small cell neuroendocrine carcinoma of the nasal cavity and paranasal sinuses. Hum Pathol. 1998; 29(8):826–832.
Perez-Ordonez, B. Selected topics in salivary gland tumour pathology. Curr Diagn Pathol. 2003; 9(6):355–365.
Perzin, K.H., Gullane, P., Clairmont, A.C. Adenoid cystic carcinomas arising in salivary glands: a correlation of histologic features and clinical course. Cancer. 1978; 42(1):265–282.
Perzin, K.H., LiVolsi, V. Acinic cell carcinomas arising in salivary glands: a clinicopathologic study. Cancer. 1979; 44:1434.
Peter, Zbären, et al. Recurrent pleomorphic adenoma of the parotid gland. Am J Surg. 2005; 189(2):203–207.
Gomes, P.N., et al. Sialadenoma papilliferum: immunohistochemical study. Inter J Oral Maxillo Surg. 2004; 33(6):621–624. [Sep].
Poulson, T.C., Greer, R.O., Jr., Ryser, R.W. Necrotizing sialometaplasia obscuring an underlying malignancy: report of a case. J Oral Maxillofac Surg. 1986; 44(7):570–574. [Jul].
Pulse, C.L., Lebovics, R.S., Zegarelli, D.J. Necrotizing sialometaplasia: report of a case after lower lip mucocele excision. J Oral Maxillofac Surg. 2000; 58(12):1419–1421. [Dec].
Capone, Randolph B., et al. Oncocytic neoplasms of the parotid gland: a 16–year institutional review. Otolaryngol Head Neck Surg. 2002; 126(6):657–662. [June].
Regezi, J.A., Batsakis, J.G. Histogenesis of salivary gland neoplasms. Otolaryngol Clin North Am. 1977; 10:297.
Rice, D.H., Batsakis, J.G., McClatchey, K.D. Postirradiation malignant salivary gland tumor. Arch Otolaryngol. 1976; 102:699.
Brannon, Robert B., et al. Ductal papillomas of salivary gland origin: a report of 19 cases and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radio Endod. 2001; 92(1):68–77. [July].
Röijer, E., Nordkvist, A., Ström, A.K., et al. Translocation, deletion/amplification, and expression of HMGIC and MDM2 in a carcinoma ex pleomorphic adenoma. Am J Pathol. 2002; 160(2):433–440.
Romagosa, V., Bella, M.R., Truchero, C., Moya, J. Necrotizing sialometaplasia (adenometaplasia) of the trachea. Histopathology. 1992; 21(3):280–282. [Sep].
Rossie, K.M., Allen, C.M., Burns, R.A. Necrotizing sialometaplasia: a case with metachronous lesions. J Oral Maxillofac Surg. 1986; 44(12):1006–1008. [Dec].
Russell, J.D., Glover, G W, Friedmann, I. Necrotizing sialometaplasia. J Laryngol Otol. 1992; 106(6):569–571. [June].
Rye, L.A., Calhoun, N.R., Redman, R.S. Necrotizing sialometaplasia in a patient with Buerger’s disease and Raynaud’s phenomenon. Oral Surg Oral Med Oral Pathol. 1980; 49(3):233–236. [May].
Saksela, E., Tarkkanen, J., Wartiovaara, J. Parotid clear-cell adenoma of possible myoepithelial origin. Cancer. 1972; 30:742.
Salhany, K.E., Pietra, G.G. Extranodal lymphoid disorders. Am J Clin Pathol. 1993; 99(4):472–485.
Santis, H.R., Kabani, S P, Roderiques, A., Driscoll, J.M. Necrotizing sialometaplasia: an early, nonulcerative presentation. Oral Surg Oral Med Oral Pathol. 1982; 53(4):387–390. [Apr].
Savera, A.T., Sloman, A., Huvos, A.G., et al. Myoepithelial carcinoma of the salivary glands: a clinicopathologic study of 25 patients. Am J Surg Pathol. 2000; 24(6):761–774.
Saw, D., Lau, W.H., Ho, J.H., et al. Malignant lymphoepithelial lesion of the salivary gland. Hum Pathol. 1986; 17(9):914–923.
Schall GL, Anderson LG, Wolf RO, Herdt JR et al. Xerostomia in Sjogren’s syndromE. Evaluation by sequential salivary scintigraphy. J Am Med Assoc, 216: 2109, 1971.
Scher, R.L., Feldman, P.S., Levine, P.A. Small-cell carcinoma of the parotid gland with neuroendocrine features. Arch Otolaryngol Head Neck Surg. 1988; 114(3):319–321.
Schmid, U., Helbron, D., Lennert, K. Primary malignant lymphomas localized in salivary glands. Histopathology. 1982; 6(6):673–687.
Schmidt, Westhausen A, Philipsen, H.P., Reichart, P.A. Necrotizing sialometaplasia of the palate: literature report of 3 new cases. Dtsch Z Mund Kiefer Gesichtschir. 1991; 15(1):30–34. [Jan-Feb].
Schneider, A.B., Favus, M.J., Stachura, M.E., et al. Salivary gland neoplasms as a late consequence of head and neck irradiation. Ann Intern Med. 1977; 87(2):160–164.
Schoning, H., Emshoff, R., Kreczy, A. Necrotizing sialometaplasia in two patients with bulimia and chronic vomiting. Int J Oral Maxillofac Surg. 1998; 27(6):463–465. [Dec].
Schwartz, I.S., Feldman, M. Diffuse multinodular oncocytoma oncocytosis of the parotid gland. Cancer. 1969; 23:636.
Sciubba, J.J., Brannon, R.B. Myoepithelioma of salivary glands: report of 23 cases. Cancer. 1982; 49:562.
Seifert, G., Donath, K. Hybrid tumours of salivary glands: definition and classification of five rare cases. Eur J Cancer B Oral Oncol. 1996; 32(4):251–259.
Seifert, G., Hennings, K., Caselitz, J. Metastatic tumors to the parotid and submandibular glands—analysis and differential diagnosis of 108 cases. Pathol Res Pract. 1986; 181(6):684–692.
Seifert, G., Oehne, H. Mesenchymal (non-epithelial) salivary gland tumors: analysis of 167 tumor cases of the salivary gland register. Laryngol Rhinol Otol (Stuttg). 1986; 65(9):485–491.
Seifert, G.Diseases of the Salivary Glands: Pathology, Diagnosis, Treatment, Facial Nerve Surgery; translated by Stell PM. Stuttgart. Germany: Thieme, 1986.
Seifert, G. Tumour-like lesions of the salivary glands: the new WHO classification. Pathol Res Pract. 1992; 188(7):836–846. [Oct].
Sheldon, W.H. So-called mixed tumors of salivary glands. Arch Pathol. 1943; 35:1–20.
Shemen, L.J., Huvos, A.G., Spiro, R.H. Squamous cell carcinoma of salivary gland origin. Head Neck Surg. 1987; 9(4):235–240. [Mar-Apr].
Sherif, Said, John, Campana. Myoepithelial carcinoma ex pleomorphic adenoma of salivary glands: a problematic diagnosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endodo. 2005; 99(2):196–201.
Shigematsu, H., Shigematsu, Y., Noguchi, Y., Fujita, K. Experimental study on necrotizing sialometaplasia of the palate in rats: role of local anesthetic injections. Int J Oral Maxillofac Surg. 1996; 25(3):239–241. [June].
Shodayu, Takashima. Warthin’s tumor of the parotid gland with extension into the parapharyngeal space. European J Radio. 1997; 24(3):227–229. [May].
Silverglade, L.B., Alvares, O.F., Olech, E. Central mucoepidermoid tumors of the jaws. Cancer. 1968; 22:650.
Simpson, R.H., Clarke, T.J., Sarsfield, P.T., et al. Epithelial-myoepithelial carcinoma of salivary glands. J Clin Pathol. 1991; 44(5):419–423.
Simpson, R.H., Sarsfield, P.T., Clarke, T., et al. Clear cell carcinoma of minor salivaryglands. Histopathology. 1990; 17(5):433–438.
Simpson, R.H.W. Myoepithelial tumours of the salivary glands. Current Diagnostic Pathology. 2002; 8(5):328–337.
Sjogren, H. Some problems concerning keratoconjunctivitis sicca and the sicca-syndrome. Acta Opthalmol. 1951; 29–33.
Smith, R.L., Dahlin, D.C., Waite, D.E. Mucoepidermoid carcinomas of the jawbones. J Oral Surg. 1968; 26–387.
Sneige, N., Batsakis, J.G. Necrotizing sialometaplasia. Ann Otol Rhinol Laryngol. 1992; 101(3):282–284. [Mar].
Som, P.M., Shugar, J.M.A., Train, J.S., Biller, H.F. Manifestations of parotid gland enlargement: radiographic, pathologic, and clinical correlations Part I: the autoimmune pseudosialectasias. Radiology. 1981; 141–415.
Speight, P.M., Barrett, A.W. Salivary gland tumours. Oral Dis no.5. 2002; 8:229–240.
Spiro, R.H., Huvos, A.G., Berk, R., et al. Mucoepidermoid carcinoma of salivary gland origin: a clinicopathologic study of 367 cases. Am J Surg. 1978; 136(4):461–468.
Spiro, R.H., Huvos, A.G., Strong, E.W. Adenocarcinoma of salivary origin: clinicopathologic study of 204 patients. Am J Surg. 1982; 144(4):423–431.
Spiro, R.H., Huvos, A.G. Stage means more than grade in adenoid cystic carcinoma. Am J Surg. 1992; 164(6):623–628.
Spiro, R.H. Salivary neoplasms: overview of a 35–year experience with 2,807 patients. Head Neck Surg. 1986; 8(3):177–184. [Jan-Feb].
Spiro, R.H., The controversial adenoid cystic carcinoma: clinical considerationsMcGurk, M., Renehan, A.G., eds. Controversies in the Management of Salivary Gland Disease; eds. Oxford University Press, Oxford, UK, 2001:207–211.
Spiro, R.H., Huvos, A.G., Strong, E.W. Acinic cell carcinoma of salivary origin: a clinicopathologic study of 67 cases. Cancer. 1978; 41:41–924.
Spiro, R.H., Huvos, A.G., Berk, R., Strong, E.W. Mucoepidermoid carcinoma of salivary gland origin: a clinicopathologic study of 367 cases. Am J Surg. 1978; 136:461.
Spiro, R.H., Koss, L.G., Hajdu, S.I., Strong, E.W. Tumors of minor salivary origin: a clinicopathologic study of 492 cases. Cancer. 1973; 31:117.
Spitz, M.R., Batsakis, J.G. Major salivary gland carcinoma: descriptive epidemiology and survival of 498 patients. Arch Otolaryngol. 1984; 110(1):45–145.
Stafford, R.F., Sonis, S.T., Shklar, G. Bilateral necrotizing sialometaplasia: a case report. J Oral Med. 1981; 36(2):28–30. [Apr-Jun].
Standish, S.M., Shafer, W.G. Serial histologic effects of rat submaxillary and sublingual salivary gland duct and blood vessel ligation. J Dent Res. 1957; 36:866.
Standish, S.M. Early histologic changes in induced tumors of the submaxillary salivary glands of the rat. Am J Pathol. 1957; 33:671.
Stephen, J., Batsakis, J.G., Luna, M.A., et al. True malignant mixed tumors (carcinosarcoma) of salivary glands. Oral Surg Oral Med Oral Pathol. 1986; 61(6):597–602.
Sterman, B.M., Kraus, D.H., Sebek, B.A., et al. Primary squamous cell carcinoma of the parotid gland. Laryngoscope. 1990; 100(2):146–148.
Smullin, Steven E, et al. Canalicular adenoma of the palate: case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endodont. 2004; 98(1):32–36. [July].
Stewart, F.W., Foote, F.W., Becker, W.F. Mucoepidermoid tumors of salivary glands. Ann Surg. 1945; 22:820.
Sugimoto, T., Wakizono, S., Uemura, T., et al. Malignant oncocytoma of the parotid gland: a case report with an immunohistochemical and ultrastructural study. J Laryngol Otol. 1993; 107(1):69–74.
Tabibzadeh, S. Salivary gland tumors: clinical and pathological features. Frontiers in Bioscience: Lecture Series. 3(129), 1998. [Jan 1].
Izutsu, T., et al. Sebaceous adenoma in the retromolar region: report of a case with a review of the English literature. Inter J Oral Maxillo Surg. 2003; 32(4):423–426.
Tandler, B., Hutter, R.V.P., Erlandson, R.A. Ultrastructure of oncocytoma of the parotid gland. Lab Invest. 1970; 23:567.
Tandler, B. Warthin’s tumor. Arch Otolaryngol. 1966; 84:68.
Tarpley, T.M., Jr., Giansanti, J.S. Adenoid cystic carcinoma. Oral Surg. 1976; 41:484.
Tarpley, T.M., Jr., Anderson, L.G., White, C.L. Minor salivary gland involvement in Sjogren’s syndrome. Oral Surg. 1974; 37:64.
Taxy, J.B. Necrotizing squamous/mucinous metaplasia in oncocytic salivary gland tumors: a potential diagnostic problem. Am J Clin Pathol. 1992; 97(1):40–45. [Jan].
Thackray, A.C., Lucas, R.B.umors of the Major Salivary Glands Fascicle 10, Second Series. Washington DC: Armed Forces Institute of Pathology, 1974.
Thackray, A.C., Sobin, L.H.Histological Typing of Salivary Gland Tumours Geneva. World Health Organization, 1972. Thackray AC, LH., 1972
Lowry, Thomas R., Heichel, David J. Pleomorphic adenoma of the hard palate. Otolaryngol Head Neck Surg. 2004; 131(5):793.
Tomich, C.E., Adenoid cystic carcinomaEllis, G.L., Auclair, P.L., Gnepp, D.R., eds. surgical Pathology of the Salivary Glands. Philadelphia, WB Saunders, 1991:333–349.
Tonon, G., Modi, S., Wu, L., et al. t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet. 2003; 33(2):208–213.
van der Wal, J.E., van der Waal, I. Necrotizing sialometaplasia: report of 12 new cases. Br J Oral Maxillofac Surg. 1990; 28(5):326–328. [Oct].
Vellios, F., Davidson, D. The natural history of tumors peculiar to the salivary glands. Am J Clin Pathol. 1995; 25:147.
Vellios, F., Shafer, W.G. Tumors of the intraoral accessory salivary glands. Surg Gynecol Obstet. 1959; 108:450.
Vincent, S.D., Hammond, H.L., Finkelstein, M.W. Clinical and therapeutic features of polymorphous low-grade adenocarcinoma. Oral Surg Oral Med Oral Pathol. 1994; 77(1):41–47.
Waldron, C.A., el-Mofty, S.K., Gnepp, D.R. Tumors of the intraoral minor salivary glands: a demographic and histologic study of 426 cases. Oral Surg Oral Med Oral Pathol. 1988; 66(3):323–333.
Walker, G.K., Fechner, R.E., Johns, M.E., Teja, K. Necrotizing sialometaplasia of the larynx secondary to atheromatous embolization. Am J Clin Pathol. 1982; 77(2):221–223. [Feb].
Warthin, A.S. Papillary cystadenoma lymphomatosum: rare teratiod of the parotid region. J Cancer Res. 1929; 13:116.
Weiss, S.W., Goldblum, J.R. Enzinger and Weiss’s Soft Tissue Tumors, 4th. St Louis: CV Mosby, 2001.
Wenig, B.M., Hitchcock, C.L., Ellis, G.L., et al. Metastasizing mixed tumor of salivary glands: a clinicopathologic and flow cytometric analysis. Am J Surg Pathol. 1992; 16(9):845–858.
Wenig, B.M. Necrotizing sialometaplasia of the larynx: a report of two cases and a review of the literature. Am J Clin Pathol. 1995; 103(5):609–613. [May].
White, D.K., Miller, A.S., Mc Daniel, R.K., Rothman, B.N. Inverted ductal papilloma: a distinctive lesion of minor salivary gland. Cancer. 1982; 49:519–524.
White, D.K., Miller, A.S., McDaniel, R.K., Rothman, B.N. Inverted ductal papilloma: a distinctive lesion of minor salivary gland. Cancer. 1982; 49:519.
Williams, R.F. Necrotizing sialometaplasia after bronchoscopy. J Oral Surg. 1979; 37(11):816–818. [Nov].
Xin, W., Paulino, A.F. Prognostic factors in malignant mixed tumors of the salivary gland: Correlation of immunohistochemical markers with histologic classification. Ann Diagn Pathol. 2002; 6(4):205–210.
Okamoto, Y., et al. Expression of cytokeratins in Warthin’s tumour (adenolymphoma) of parotid glands: specific detection of individual cytokeratin types by monoclonal antibodies. Oral Oncology. 1996; 32(5):352–358. [Sep].
Nelson, Z.L., et al. Bilateral multifocal canalicular adenomas of buccal minor salivary glands: a case report. Br J Oral Maxillo Surg. 1995; 33(5):299–301. [Oct].
Zschoch, H. Mucus gland infarct with squamous epithelial metaplasia in the lung: a rare site of so-called necrotizing sialometaplasia. Pathologe. 1992; 13(1):45–48. [Feb].
 . Benign Tumors of the Salivary Glands
. Benign Tumors of the Salivary Glands