Cysts and Tumors of Odontogenic Origin
Tumors derived from the odontogenic tissues constitute an unusually diverse group of lesions. This multiformity reflects the complex development of the dental structures, since these tumors all originate through some aberration from the normal pattern of odontogenesis. An understanding of the pathogenesis of the odontogenic tumors is predicated upon an understanding of the histogenesis of the tooth.
Certain of the lesions discussed in this chapter represent only minor alterations in odontogenesis and not true neoplasms. The odontogenic cysts are included because they too represent an aberration at some stage of odontogenesis, and in fact, may be intimately associated with the development of certain of the odontogenic tumors. All of the various lesions are grouped here because of their common origin from a uniquely specialized group of tissues, and their classification is based upon this origin from the various germ layers.
Odontogenic Cysts
Cysts of the jaws can be classified as:
The odontogenic cysts are derived from epithelium associated with the development of the dental apparatus. The type of epithelium can vary with most lesions having stratified squamous but some developmental or fissural cysts in the maxilla may have respiratory epithelium.
Several types of odontogenic cysts may occur, dependent chiefly upon the stage of odontogenesis during which they originate. Various investigators have attempted to devise a classification and system of nomenclature of the lesions. Some of these classifications have not been entirely satisfactory because they generally failed to recognize the mode of origin and development of the cysts and did not unite the views of the oral surgeon, the radiologist and the pathologist.
The following classification of odontogenic cysts (Table 4-1) is based on etiology and tissue of origin.
Table 4-1
Classiication of odontogenic cysts
Classification by etiology
Dentigerous cyst
Eruption cyst
Odontogenic keratocyst
Gingival cyst of newborn
Gingival cyst of adult
Lateral periodontal cyst
Calcifying odontogenic cyst
Glandular odontogenic cyst
Inflammatory:Result of inflammation
Periapical cyst
Residual cyst
Paradental cyst
Classification by tissue of origin
Derived from rests of Malassez
Periapical cyst
Residual cyst
Derived from reduced enamel epithelium
Dentigerous cystt
Eruption cyst
Derived from dental lamina (rests of Serres)
Odontogenic keratocyst
Gingival cyst of newborn
Gingival cyst of adult
Lateral periodontal cyst
Glandular odontogenic cyst
Unclassified
Paradental cyst
Calcifying odontogenic cyst
Dentigerous Cyst (Follicular cyst)
Dentigenous cyst can be defined as an odontogenic cyst that surrounds the crown of an impacted tooth; caused by fluid accumulation between the reduced enamel epithelium and the enamel surface, resulting in a cyst in which the crown is located within the lumen. This is one of the most common types of developmental odontogenic cyst, estimated to be about 20% of all jaw cysts. It is estimated that about 10% of impacted teeth have formed a dentigerous cyst. Their frequency in the general population has been estimated at 1.44 cyst for every 100 unerupted teeth. The dentigerous cyst nearly always involves or is associated with the crown of a normal permanent tooth. Seldom is a deciduous tooth involved.
Clinical Features
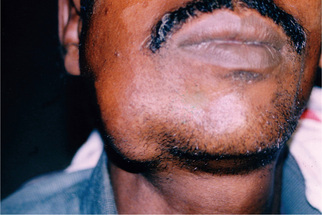
This cyst is always associated initially with the crown of an impacted, embedded or unerupted tooth (Fig. 4-1). A dentigerous cyst may also be found enclosing a complex compound odontoma or involving a supernumerary tooth. The most common sites of this cyst are the mandibular and maxillary third molar and maxillary cuspid areas, since these are the most commonly impacted teeth. Most lesions present in second and third decades, with slight male predilection (M:F–3:2). Most dentigerous cysts are solitary. Bilateral and multiple cysts are usually found in association with a number of syndromes including cleidocranial dysplasia and Maroteaux–Lamy syndrome. In the absence of these syndromes, bilateral dentigerous cysts are rare. The dentigerous cyst is potentially capable of becoming an aggressive lesion. Expansion of bone with subsequent facial asymmetry, extreme displacement of teeth, severe root resorption of adjacent teeth and pain are all possible sequelae brought about by continued enlargement of the cyst. Cystic involvement of an unerupted mandibular third molar may result in a ‘hollowing-out’ of the entire ramus extending up to the coronoid process and condyle as well as in expansion of the cortical plate due to the pressure exerted by the lesion. Associated with this reaction may be displacement of the third molar to such an extent that it sometimes comes to lie compressed against the inferior border of the mandible. In the case of a cyst associated with a maxillary cuspid, expansion of the anterior maxilla often occurs and may superficially resemble an acute sinusitis or cellulitis. There is usually no pain or discomfort associated with the cyst unless it becomes secondarily infected.
Radiographic Features
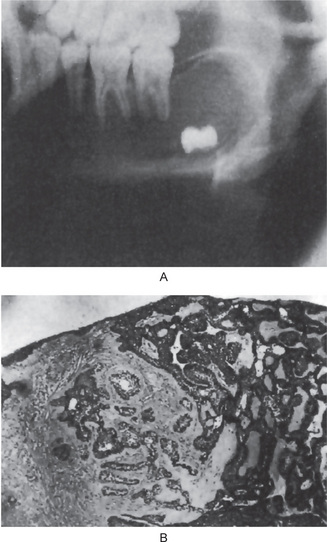
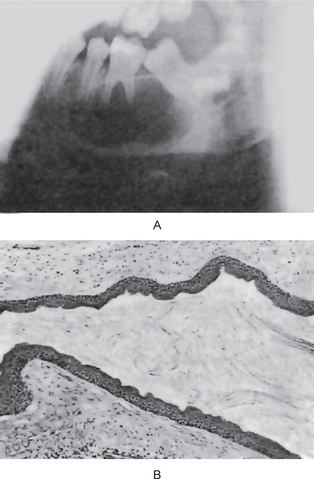
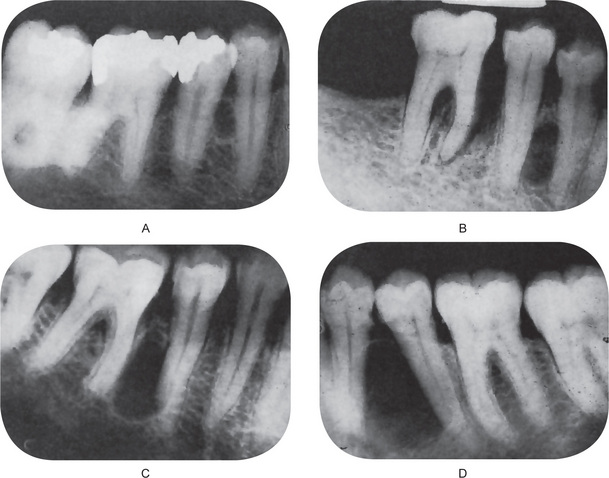
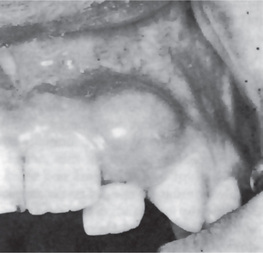
Radiographic examination of the jaw involved by a dentigerous cyst will reveal a radiolucent area associated in some fashion with an unerupted tooth crown (Fig. 4-2A). The impacted or otherwise unerupted tooth crown may be surrounded symmetrically by this radiolucency, although the distinction between a small dentigerous cyst and an enlarged dental follicle or follicular space is quite arbitrary, especially since the small cyst and the enlarged follicle would be histologically identical. While a normal follicular space is 3–4 mm, a dentigerous cyst can be suspected when the space is more than 5 mm. Only when the size of the radiolucency is grossly pathologic can the distinction be made with assurance.
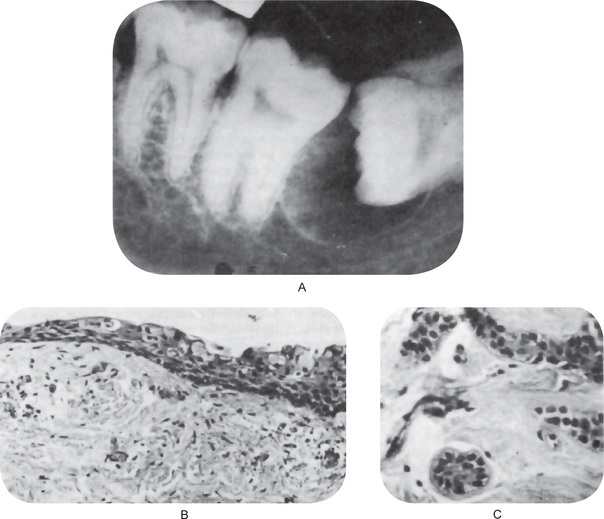
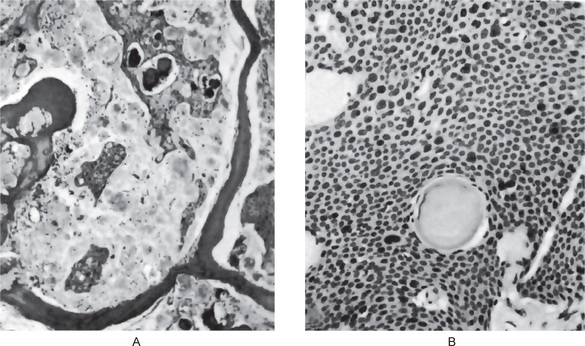
Figure 4-2 Dentigerous cyst. The radiograph (A) demonstrates a large radiolucent area associated with the crown of the impacted mandibular third molar. The photomicrograph (B) shows this cyst to be lined by a thin layer of stratified squamous epithelium similar in appearance to the primordial cyst. Occasional mucuscontaining cells are present in the epithelium. Small islands of odontogenic epithelium (C) are often present in the connective tissue wall.
Three radiological variations of the dentigerous cyst may be observed. In the central variety, the crown is enveloped symmetrically. In these instances, pressure is applied to the crown of the tooth and may push it away from its direction of eruption. In this way, mandibular third molars may be found at the lower border of the mandible or in the ascending ramus and a maxillary canine may be forced into the maxillary sinus as far as the floor of the orbit. The lateral type of dentigerous cyst is a radiographic appearance which results from dilatation of the follicle on one aspect of the crown. This type is commonly seen when an impacted mandibular third molar is partially erupted so that its superior aspect is exposed. The so-called circumferential dentigerous cyst results when the follicle expands in a manner in which the entire tooth appears to be enveloped by cyst. The dentigerous cyst is usually a smooth, unilocular lesion, but occasionally one with a multilocular appearance may occur. In actuality, the various compartments are all united by the continuous cystic membrane. Sometimes the radiolucent area is surrounded by a thin sclerotic line representing bony reaction. In cases of apparently multiple dentigerous cysts, care should be taken to rule out the possible occurrence of the odontogenic keratocyst, basal cell nevus, bifid rib syndrome (q.v.).
Histologic Features
There are no characteristic micro-scopic features which can be used reliably to distinguish the dentigerous cyst from the other types of odontogenic cysts. It is usually composed of a thin connective tissue wall with a thin layer of stratified squamous epithelium lining the lumen (Fig. 4-2B). Rete peg formation is generally absent except in cases that are secondarily infected. The connective tissue wall is frequently quite thickened and composed of a very loose fibrous connective tissue or of a sparsely collagenized myxomatous tissue, each of which has been sometimes mistakenly diagnosed as either an odontogenic fibroma or an odontogenic myxoma. A hyperplastic dental follicle is not necessarily associated with inflammation. An additional feature of the connective tissue wall of both normal dental follicles and dentigerous cysts is the presence of varying numbers of islands of odontogenic epithelium (Fig. 4-2C). These are sometimes very sparse and obviously inactive, while at other times they are present in sufficient numbers to be mistaken for an amelo-blastoma. While this latter odontogenic tumor can originate in this situation, care must be taken to differentiate between it and simply odontogenic epithelial rests. Inflammatory cell infiltration of the connective tissue is common although the cause for this is not always apparent. An additional finding, especially in cysts which exhibit inflammation, is the presence of Rushton bodies within the lining epithelium. These are peculiar linear, often curved, hyaline bodies with variable stainability which are of uncertain origin, questionable nature and unknown significance. Even electron microscopic studies, such as those of El-Labban, have only been of partial help in determining that the structures are probably of hematogenous origin, although it is not clear why they have such an intimate relationship to epithelium. The content of the cyst lumen is usually a thin, watery yellow fluid, occasionally blood tinged.
It was reported by Brannon in his excellent clinicopathologic study of 312 cases of odontogenic keratocysts that 8.5% of a series of 1,850 dentigerous cysts were odontogenic keratocysts (q.v.) with the characteristic histologic findings in the epithelium of a parakeratinized, corrugated surface, a remarkable uniformity of a 6- to 10-cell thickness without rete peg formation, and a polarized and palisaded basal layer of cells. This percentage of dentigerous cysts which are odontogenic keratocysts is in close agreement with the findings of Pindborg and his coworkers (7.1%) and Payne (8.5%).
The pluripotentialities of the epithelium in mandibular dentigerous cysts has been further emphasized by Gorlin, who described mucus-secreting cells in the lining stratified squamous epithelium, respiratory epithelial lining, sebaceous cells in the connective tissue wall, and lymphoid follicles with germinal centers.
Treatment
The treatment of the dentigerous cyst is usually dictated by the size of the lesion. Smaller lesions can be surgically removed in their entirety with little difficulty. The larger cysts which involve serious loss of bone and thin the bone dangerously are often treated by insertion of a surgical drain or marsupialization. Such a procedure is often necessary because of the potential danger of fracturing the jaw if complete surgical removal were attempted. Recurrence is relatively uncommon.
Potential Complications
Several relatively serious potential complications exist stemming from the dentigerous cyst, besides simply the possibility of recurrence following incomplete surgical removal. These include:
• The development of an ameloblastoma either from the lining epithelium or from rests of odontogenic epithelium in the wall of the cyst.
• The development of epidermoid carcinoma from the same two sources of epithelium.
• The development of a mucoepidermoid carcinoma, basically a malignant salivary gland tumor, from the lining epithelium of the dentigerous cyst which contains mucussecreting cells, or at least cells with this potential, most commonly seen in dentigerous cysts associated with impacted mandibular third molars.
It is of great clinical significance that numerous cases of ameloblastoma have been reported developing in the wall of dentigerous cysts from the lining epithelium or associated epithelial rests (Fig. 4-3). Stanley and Diehl have reviewed a series of 641 cases of ameloblastoma and have found that at least 108 cases of this neoplasm, approximately 17%, were definitely associated with an impacted tooth and/or a follicular or dentigerous cyst. The disposition for neoplastic epithelial proliferation in the form of an ameloblastoma is far more pronounced in the dentigerous cyst than in the other odontogenic cysts. The formation of such a tumor manifests itself as a nodular thickening in the cyst wall, the mural ameloblastoma, but this is seldom obvious clinically. Therefore, it is not only good clinical practice, but also an absolute requisite that all tissue from dentigerous cysts be submitted to a qualified oral pathologist for thorough gross and microscopic examination. In reviewing the histologic changes sought by the oral pathologist which occur in the dentigerous cyst as a premonitory manifestation of ameloblastoma, Vickers and Gorlin have stressed that hyperchromatism of basal cell nuclei, palisading with polarization of basal cells and cytoplasmic vacuolization with intercellular spacing of the lining epithelium, when observed together, are manifestations of impending neoplasia. These findings may occur individually in other rather harmless conditions. The presence of sprouting or budding and protruding of epithelial islands from lining epithelium has been claimed to be evidence of neoplastic transformation, but this in itself was not considered such an indication by Vickers and Gorlin.
The development of epidermoid carcinoma from the lining epithelium of the dentigerous cyst also has been adequately documented in the literature reviewed by Gardner, who reported eight acceptable cases among 25 cases of carcinoma developing in odontogenic cysts of all types combined. Browne and Gough have reported two additional cases of malignant transformation in dental cysts and suggested that keratin metaplasia in long-standing cyst lining appears to precede the development of the carcinomatous change, although there is little evidence that the odontogenic keratocyst is associated with malignant change more commonly than other types of odontogenic cysts. The predisposing factor and mechanism of development of this malignancy are unknown, but its occurrence appears unequivocal (Fig. 4-4).
Finally, the development of a mucoepidermoid carcinoma, a type of salivary gland tumor, is less well documented than the epidermoid carcinoma of this origin, but it also appears to be a potentiality. The inclusion of normal salivary gland tissue in the posterior portion of the body of the mandible has been reported, and undoubtedly, some central salivary gland tumors in this location originate from this source. However, cases of central mucoepidermoid carcinoma (q.v.) have been discovered in association with dentigerous cysts involving impacted mandibular third molars, and considering the frequency with which mucus-secreting cells are found in this lining epithelium indicative of the pluripotentiality of this epithelium, this very distinct possibility must always be considered. These findings comprise most of the medical rationale for removal of impacted third molars with pericoronal radiolucencies; however, impacted teeth with small pericoronal radiolucencies (suggesting the presence of normal dental follicle rather than dentigerous cyst) also may be monitored with serial radiographic examination. Any increase in the size of the lesion should prompt removal and histopathologic examination. Any lesion that appears larger than a normal dental follicle indicates removal and histopathologic examination.
Eruption Cyst
Eruption cyst is defined as an odontogenic cyst with the histologic features of a dentigerous cyst that surrounds a tooth crown that has erupted through bone but not soft tissue and is clinically visible as a soft fluctuant mass on the alveolar ridges.
An eruption cyst or ‘eruption hematoma’ is in fact a dentigerous cyst occurring in the soft tissues (Shear, 1992). Whereas the dentigerous cyst develops around the crown of an unerupted tooth lying in the bone, the eruption cyst occurs when a tooth is impeded in its eruption within the soft tissue overlying the bone. The pathogenesis is probably very similar to that of the dentigerous cyst. The difference is that the tooth in the case of the eruption cyst is impeded in the soft tissue of gingiva rather than in the bone. The presence of particularly dense fibrous tissue in the overlying gingiva could be responsible.
Clinical Features
Shear (1992) had recorded a 0.8% frequency. It is likely that they occur more frequent clinically and as some rupture spontaneously, these cysts go unnoticed and are not submitted for histological examination.
These cysts are found in children of different ages, and occasionally in adults if there is delayed eruption. Deciduous and permanent teeth may be involved, most frequently anterior to the first permanent molar. Clinically, the lesion appears as a circumscribed, fluctuant, often translucent swelling of the alveolar ridge over the site of the erupting tooth. When the circumcoronal cystic cavity contains blood, the swelling appears purple or deep blue; hence the term ‘eruption hematoma’. It is usually painless unless infected.
Sometimes more than one cyst may be present. There is often a brief history of about three to four weeks duration during which they enlarge to approximately 1–1.5 cm.
Radiographic Features
It may show a soft tissue shadow since the cyst is confined within it and there is usually no bone involvement.
Histologic Features
The superficial aspect is covered by the keratinized stratified squamous epithelium of the overlying gingiva. This is separated from the cyst by a strip of dense connective tissue of varying thickness which usually shows a mild chronic inflammatory cell infiltrate. Inflammatory cell infiltration is common. The follicular connective tissue is more densely cellular, less collagenous and has a basophilic hue, presumably because of a higher content of acid mucopolysaccharide in the ground substance. Odontogenic epithe-lial cell nests may be present in the connective tissue.
In noninflamed areas, the epithelial lining of the cysts is characteristically of reduced enamel epithelial origin, consisting of two or three cell layers of squamous epithelium with a few foci where it may be a little thicker. The adjacent corium is hyperemic and is the seat of a chronic inflammatory cell infiltrate (Fig. 4-5).
Odontogenic Keratocyst
A cyst derived from the remnants (rests) of the dental lamina, with a biologic behavior similar to a benign neoplasm, with a distinctive lining of six to 10 cells in thickness, and that exhibits a basal cell layer of palisaded cells and a surface of corrugated parakeratin.
This is the most interesting of jaw cysts. The term ‘odontogenic keratocyst’ was first used by Philipsen in 1956, while Pindborg and Hansen in 1963 described the essential features of this type of cyst. It is named keratocyst because the cyst epithelium produces so much keratin that it fills the cyst lumen. Furthermore, flattening of the basement membrane and palisading of the basal epithelial cells, reminiscent of odontogenic epithelium, are characteristics of odontogenic keratocyst.
They are unique odontogenic lesions that have the potential to behave aggressively, that can recur, and can be associated with the nevoid basal cell carcinoma syndrome. Toller (1967) suggested that OKCs might be regarded as benign cystic neoplasms. Whether they are developmental or neoplastic continues to be debated.
Studies indicate that a significant number of OKCs show clonal loss of heterozygosity of common tumor suppressor genes. The finding of clonal deletion mutations of genomic DNA in these cysts supports the hypothesis that they are neoplastic rather than developmental in origin. The odonto-genic keratocyst is regarded as a distinctive entity because of its characteristic histology, proliferation kinetics, and behavior. Therefore, although keratinization may be present in many other types of cysts, the specific histologic pattern of the odontogenic keratocyst separates it from all others.
Differences in cytokeratin, epithelial membrane antigen (EMA) and carcinoembryonic antigen (CEA) immunoreactivity between the parakeratinized OKC and the orthokeratinized variety have been demonstrated and the suggestion made that the latter having a considerably less aggressive behavior is different entity and should bear a different name orthoke-ratinized odontogenic cyst (Shear M, 2002).
There is general agreement that the origin of the odontogenic keratocyst comes from dental lamina remnants in the mandible and maxilla. However, the origin of this cyst from the extension of basal cells of the overlying oral epithelium has also been suggested.
Reclassification of the Odontogenic Keratocyst to Tumor: Keratocystic Odontogenic Tumor (KOT)
In 1967, Toller suggested that the OKC may best be regarded as a benign neoplasm rather than a conventional cyst based on its clinical behavior. The WHO has reclassified the lesion as a tumor based on several factors, that formed the basis of this decision.
• Behavior: The KOT is locally destructive and recurrence rate is very high.
• Histopathology: The basal epithelial layer of KOT shows proliferation and budding into the underlying connective tissue in the form of daughter cysts and mitotic figures are frequently found in the suprabasal layers of the lesional epithelium.
• Genetics: PTCH (patched), a tumor suppressor gene involved in both syndrome associated and sporadic KOTs, occurs on chromosome 9q22.3 – q31. Normally, PTCH forms a receptor complex with the oncogene SMO (smoothened) for the SHH (sonic hedgehog) ligand. PTCH binding to SMO inhibits growth signal transduction. SHH binding to PTCH releases this inhibition. If normal functioning of PTCH is lost, the proliferation-stimulation effects of SMO are permitted to predominate.
Evidence has shown that the pathogenesis of syndrome associated and sporadic KOTs involves a ‘two hit mechanism’, with allelic loss at 9q22. The ‘two hit mechanism’ refers to the process by which a tumor suppressor gene is inactivated. The first hit is a mutation in one allele, which, although it can be dominantly inherited, has no phenotypic effect. The second hit refers to loss of the other allele and is known as ‘loss of heterozygosity’ (LOH). In KOTs, this leads to the dysregulation of the oncoproteins cyclin D1 and p53. LOH in the 9q22.3–q31 region has been reported for many epithelial tumors, including basal cell carcinomas, squamous cell carcinomas, and transitional cell carcinomas; and LOH is by definition a feature of tumorigenic tissue.
Clinical Features
The largest and most detailed series of cases of odontogenic keratocyst has been published by Brannon, and his data are probably most representative of this lesion. The cyst may occur at any age, from the very young to the very elderly, although Brannon found it to be exceedingly rare under the age of 10 years, there being only two such patients in his series of 283 persons. The peak incidence is in the second and third decades of life, with a gradual decline thereafter. The data of Browne (104 patients) and of Forssell (119 patients) are virtually identical. In all series, there is a predilection for occurrence in males, ranging from 1.44:1 (Brannon), 1.46:1 (Browne) to 1.79:1 (Forssell).
The mandible is invariably affected more frequently than the maxilla: in the series of Brannon, 65% versus 35%, in the series of Browne, 79% versus 21%, and in that of Forssell, 78% versus 22%. In the mandible, the majority of the cysts occur in the ramus-third molar area, followed by the first and second molar area and then the anterior mandible. In the maxilla, the most common site is the third molar area followed by the cuspid region.
Multiple odontogenic keratocysts occur with some frequency. Lesions found in children are often reflective of multiple odontogenic keratocysts as a component of the nevoid basal cell carcinoma syndrome. However, at other times, these multiple cysts are independent of the syndrome. A rather remarkable parallelism between the odontogenic keratocyst and the ameloblastoma with respect to mean age of occurrence, predilection for site of occurrence, radiographic findings and recurrence rate has also been pointed out by Browne.
There are no characteristic clinical manifestations of the keratocyst, although about 50% of the patients in Brannon’s series were symptomatic prior to seeking treatment. Among the more common features are pain, soft-tissue swelling and expansion of bone, drainage and various neurologic manifestations such as paresthesia of the lip or teeth. The maxillary OKC tends to be secondarily infected with greater frequency than the mandibular ones, due to its vicinity to the maxillary sinus.
Radiographic Features
Radiographically most OKCs are unilocular, presenting a well-defined peripheral rim. Scalloping of the border is also a frequent finding and this represents variations in the growth pattern of the cyst. Multilocular radiolucent OKC is also observed, generally representing a central cavity having satellite cysts. When it is multilocular and especially if located in the third mandibular molar area, it may be confused radiographically with an ameloblastoma. Occasionally OKC may mimic a dentigerous cyst and contain the crown of a retained tooth within its lumen. The final diagnosis of any cystic cavity within the jaw bones will be achieved only after biopsy of the surgical specimen. Multilocularity (20%) is often present and tends to be seen more frequently in larger lesions. Most lesions, however, are unilocular, with as many as 40% noted adjacent to the crown of an unerupted tooth (dentigerous cyst position). Approximately 30% of maxillary and 50% of man-dibular lesions produce buccal expansion. Mandibular lingual enlargement is occasionally seen. Proximity to the roots of adjacent normal teeth sometimes causes resorption of these roots, although displacement is more common. Sometimes these cysts displace the neurovascular bundle.
Histologic Features
The odontogenic keratocyst wall is usually rather thin unless there has been superimposed inflammation. The lining epithelium is highly characteristic, and is composed of:
• A parakeratinized surface which is typically corrugated, rippled or wrinkled.
• A remarkable uniformity of thickness of the epithelium, usually ranging from 6 to 10 cells thick.
• A prominent palisaded, polarized basal layer of cells often described as having a ‘picket fence’ or ‘tombstone’ appearance.
Histologically, these cysts are formed with a stratified squamous epithelium that produces orthokeratin (10%), parakeratin (83%), or both types of keratin (7%). No rete ridges are present; therefore, the epithelium often sloughs from the connective tissue (94% of the time). The epithelium is thin, and mitotic activity is frequent; therefore, OKC grows in a neoplastic fashion and not in response to internal pressure. In the presence of an intense inflammatory process, the adjacent epithelium loses its keratinized surface, may thicken and develop rete processes or may ulcerate.
The connective tissue wall often shows small islands of epithelium similar to the lining epithelium; some of these islands may be small cysts. In at least some cases, the apparent islands of epithelium and small satellite or ‘daughter’ cysts actually represent the ends of folds of the lining epithelium of the main cystic cavity which have been cut in cross-section; the linings of these cysts are very commonly folded. Forssell and his associates have studied this problem using serial sections of cysts and found microcysts in the wall in 20% and epithelial islands in 50% of their cases. In 35% of the cases, apparent microcysts were found to be part of the main cyst by the serial sections while pseudo islands of epithelium were found in 75% of the cases.
The lumen of the keratocyst may be filled with a thin straw colored fluid or with a thicker creamy material. Sometimes the lumen contains a great deal of keratin, while at other times it has little. Cholesterol, as well as hyaline bodies at the sites of inflammation, may also be present. The electrophoretic measurement of fluid from these cysts has been reported by Toller to show that it contains a very low content of soluble protein compared with the patient’s own serum (Fig. 4-6).
Dysplastic and neoplastic transformation of the lining epithelium in the odontogenic keratocyst is an uncommon occurrence but has been reported. Of the 312 keratocysts studied by Brannon, only two exhibited cellular atypia. Occasional other cases have also been described in the literature. Areen and his associates have recently reported a case of epidermoid carcinoma developing in an odontogenic keratocyst, and in reviewing the literature, have emphasized the necessity for careful microscopic examination of all such cysts.
The variant of OKC that produces only orthokeratin acts somewhat differently than other OKCs. These almost always are found in a dentigerous association, usually around the mandibular third molar, and they are much less aggressive. They do not have a hyperchromatic basal layer; in fact, the basal layer is flattened. They are not associated with basal cell nevus syndrome (orthokeratinized odontogenic cyst).
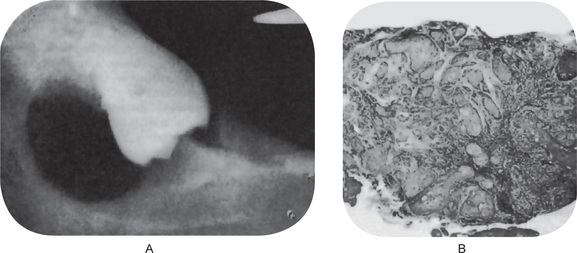
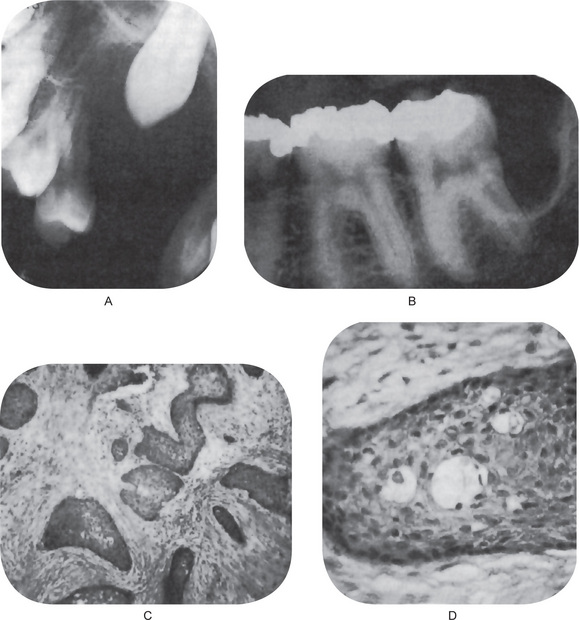
Finally, the highly characteristic nature of the parakeratinized lining epithelium and its relationship to the high recurrence rate have been emphasized by a report dealing with orthokeratinized odontogenic cysts and their recurrence rate. Wright investigated 59 cases of orthokeratinized odontogenic cysts and found that they showed a predilection for occurrence in males, most commonly in the second to fifth decades. These cysts were located predominantly in the posterior mandible, where they most typically appeared as dentigerous cysts. The thin, uniform lining epithelium was covered with orthokeratin and showed a prominent granular layer and a cuboidal to flattened basal layer. Follow-up of 24 of these patients revealed only one case of recurrence. This difference in biologic behavior would further underscore the necessity for very strict application of the definition of the term odontogenic keratocyst in diagnosis of the lesion (Fig. 4-7).

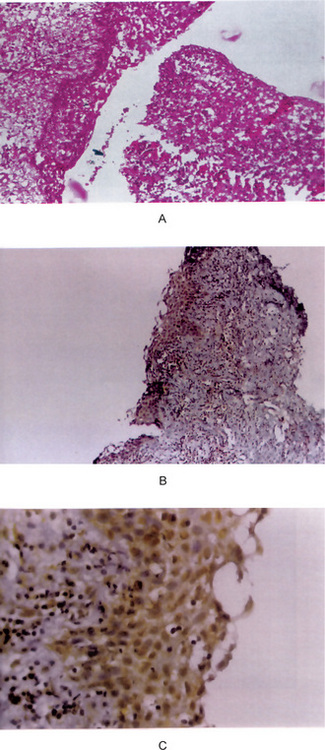
Figure 4-7 Odontogenic keratocyst. (A) The epithelial lining is uniformly thin, generally ranging from 8–10 cell layers thick. The basal layer exhibits a characteristic palisaded pattern with polarized and intensely stained nuclei of uniform diameter. The luminal epithelial cells are parakeratinized and produce an uneven or corrugated profile. (B) Odontogenic keratocyst stained using proliferating cell nuclear antigen (PCNA) antibodies (x 10).
Treatment and Prognosis
The odontogenic keratocyst should be surgically excised. However, clinical experience has shown that complete eradication of the cyst may be difficult because the wall of the cyst is very thin and friable and may easily fragment. In addition, perforation of cortical bone, particularly in lesions involving the ramus, is common and this complicates total removal.
The most important feature of the odontogenic keratocyst is its extraordinary recurrence rate. This has been reported as being between 13 and 60%. A review of 763 cases of odontogenic keratocysts in 13 different reported series of cases has shown the average recurrence rate to be 26%, with the majority occurring within five years of the surgical procedure. Forssell, Forssell and Kahnberg (1988) observed that recurrences were more frequent (63%) with cysts in patients with the nevoid basal cell carcinoma syndrome than with cysts in patients without the syndrome (37%). Keratocysts enucleated in one piece recurred significantly less often than cysts enucleated in several pieces, and the recurrence rate in cases with a clinically observable infection, a fistula or with a perforated bony wall was higher. The size of the cyst did not seem to influence its prognosis after surgery, but those whose radiographic appearance was multilocular had a higher recurrence rate than those with a unilocular appearance.
Browne found no significant differences in recurrence rate following three basic methods of treating the lesions:
Furthermore, recurrence does not appear related to the presence of satellite cysts. On this basis, Browne concluded that recurrence of the keratocyst is due to the nature of the lesion itself, i.e. the presence of additional remnants of dental lamina from which a cyst may develop, and is not related to its method of treatment. Since recurrence may be long delayed in this lesion, follow-up of any case of odontogenic keratocyst with annual radiographs is essential for at least five years after surgery. It is also essential that patients with an odontogenic keratocyst, especially if multiple, be evaluated medically to rule out the possibility of the jaw cyst-basal cell nevus-bifid rib syndrome (q.v.).
Jaw Cyst-Basal Cell Nevus, Biid Rib Syndrome (Basal cell nevus syndrome, hereditary cutaneomandibular polyoncosis, Gorlin and Goltz syndrome)
This syndrome, first described by Binkley and Johnson in 1951, has been thoroughly reviewed by Gorlin and his coworkers. A hereditary condition, it is transmitted as an autosomal dominant trait, with high penetrance and variable expressivity. It is caused by mutations in patched (PTCH), a tumor suppressor gene that has been mapped to chromosome 9q22.3–q31.
Clinical Features
The syndrome is very complex and includes a great variety of possible abnormalities. These may be briefly summarized as follows:
• Cutaneous anomalies, including basal cell carcinoma, other benign dermal cysts and tumors, palmar pitting, palmar and plantar keratosis and dermal calcinosis.
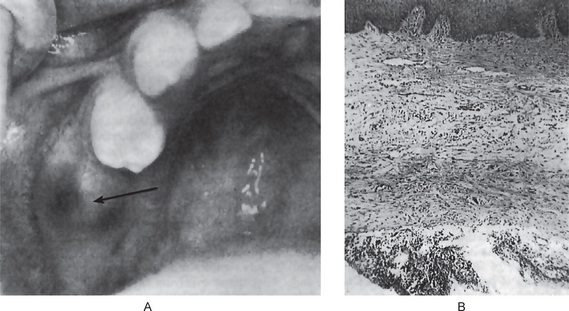
• Dental and osseous anomalies, including odontogenic keratocysts (often multiple), mild mandibular progna-thism, rib anomalies (often bifid), vertebral anomalies and brachymetacarpalism (Fig. 4-8).
• Ophthalmologic abnormalities, including hyper-telorism with wide nasal bridge, dystopia canthorum, congenital blindness and internal strabismus.
• Neurologic anomalies, including mental retardation, dural calcification, agenesis of corpus callosum, congenital hydrocephalus and occurrence of medulloblastomas with greater than normal frequency.
• Sexual abnormalities, including hypogonadism in males and ovarian tumors.
Some of these patients have shown a lack of response to parathormone as judged by the lack of phosphate diuresis which, coupled with shortened fourth metacarpals in some patients, has suggested that there may be some relationship to pseudohypoparathyroidism.
Oral Manifestations
The odontogenic keratocysts are indistinguishable from those previously described by that term which are not associated with this syndrome (Fig. 4-8). Because they often develop early in life, deformity and displacement of developing teeth may occur. However, they may not develop until middle age even though the basal cell skin tumors have occurred early in life.
Treatment and Prognosis
Several cases of ameloblastoma have developed in cysts of this syndrome, thus emphasizing the importance of surgical removal of the cysts and their histologic examination. Whenever a diagnosis of odontogenic keratocyst is received by the dentist, he must be certain to rule out the presence of this syndrome because of the many associated problems which these patients ultimately will face.
Dental Lamina Cyst of Newborn (Gingival cyst of newborn, Epstein’s pearls, Bohn’s nodules)
Dental lamina cyst of the newborn are multiple, occasionally solitary, superficial raised nodules on edentulous alveolar ridges of infants that resolve without treatment; derived from rests of the dental lamina and consisting of keratin-producing epithelial lining. Bohn’s nodules and Epstein’s pearls are two similar lesions with which gingival cysts sometimes may be confused; however, the location and etiology of these lesions are somewhat different. As originally described, Epstein’s pearls are cystic, keratin-filled nodules found along the midpalatine raphe, probably derived from entrapped epithelial remnants along the line of fusion (q.v.). Bohn’s nodules are keratin-filled cysts scattered over the palate, most numerous along the junction of the hard and soft palate and apparently derived from palatal salivary gland structures (q.v.). Discussions of these various types of cysts in the newborn have been published by Fromm and by Cataldo and Berkman.
In studying sections of the maxillae and mandibles of 17 infants, Kreshover reported finding 65 examples of gingival cysts (38 multiple and 27 single). These cysts were localized in the corium below the surface epithelium. Those in the anterior portion of the jaws were usually displaced lingually with respect to the deciduous incisors and cuspids. Those in the posterior portion of the jaw were found occlusal to the crown of the molars. Kreshover stated that in all instances the cystic lesions were seen to arise from cells of the dental lamina. The etiology of these cysts has been thoroughly discussed by Maher and Swindle.
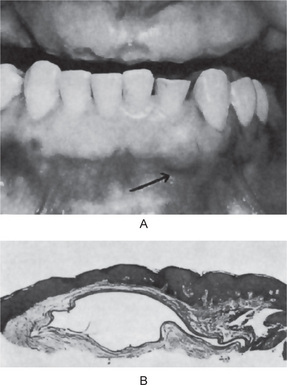
Clinical Features
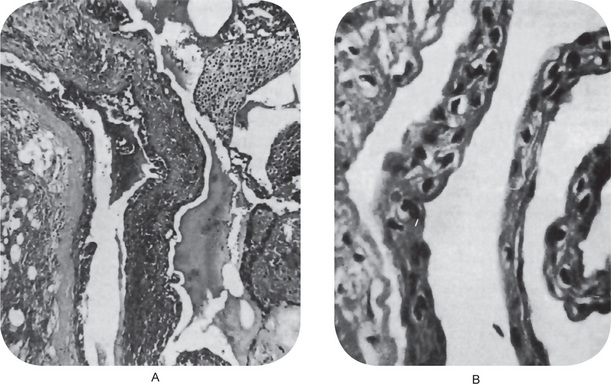
Occasionally these dental lamina cysts in infants become sufficiently large to be clinically obvious as small discrete white swellings of the alveolar ridge, sometimes appearing blanched as though from internal pressure (Fig. 4-9A). These probably correspond to those structures described in the older literature as the ‘predeciduous dentition’. These lesions appear to be asymptomatic and do not seem to produce discomfort in the infants.
Histologic Features
These are true cysts with a thin epithelial lining which lacks rete processes and show a lumen usually filled with desquamated keratin, occasionally containing inflammatory cells (Fig. 4-9B). Interestingly, dystrophic calcification and hyaline bodies of Rushton (q.v.), commonly found in dentigerous cysts, are also sometimes found in this lesion.
Gingival Cyst of Adult
A small developmental odontogenic cyst of the gingival soft tissue derived from the rests of the dental lamina, containing a lining of embryonic epithelium of cuboidal cells and distinctive focal thickenings similar to the lateral periodontal cyst.
The gingival cyst of the adult is an uncommon cyst of gingival soft tissue, occurring in either the free or attached gingiva.
The etiology and pathogenesis of this lesion have been reviewed by Ritchey and Orban, who suggested possible sources of cystic formation as:
• Heterotopic glandular tissue.
• Degenerative changes in a proliferating epithelial peg.
• Remnants of the dental lamina, enamel organ or epithelial islands of the periodontal membrane.
Of these possibilities, only the last two appear valid, and on this basis there do appear to be two recognizable forms of gingival cyst:
• That arising from cystic transformation of dental lamina or the ‘glands’ or rests of Serres.
• That arising from traumatic implantation of surface epithelium (and, therefore, not truly an odontogenic cyst).
The origin of the gingival cyst of the adult has been evaluated by Wysocki and his colleagues, who concluded that it does arise from postfunctional rests of dental lamina and thus represents the extraosseous counterpart of the lateral periodontal cyst, with which it shares a common histogenesis. Buchner and Hansen have reported essentially the same conclusions. The similarities between the lateral periodontal cyst and the gingival cyst of the adult in clinical behavior, morphologic appearance, anatomic site of occurrence and age predilection are too striking to be coincidental.
The vast majority of these gingival cysts appear to originate in the fashion described from dental lamina, including a soft tissue counterpart of the multilocular botryoid odontogenic cyst. However, an implantation type of cyst can occur lined by a more mature keratinizing stratified squamous epithelium lining derived from surface mucosal epithelium. Finally, as suggested by Buchner and Hansen, there is some evidence that a dental lamina cyst of the newborn may persist into adulthood, as judged by the finding of cysts packed with orthokeratin that appear identical to those in the newborn.
Clinical Features
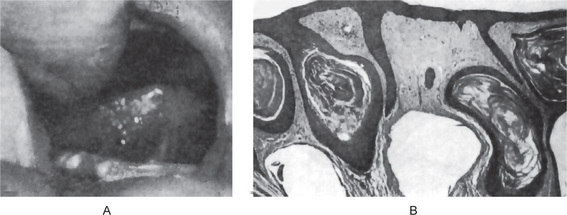
The gingival cyst may occur at any age but is most common in adults. In the review of the literature by Reeve and Levy, the majority of patients were over 40 years of age. The mean age in the 33 cases reported by Buchner and Hansen was 48 years and that of the 10 cases reported by Wysocki and his associates was 51 years. The location of the lesion closely follows that of the lateral periodontal cyst. Thus, all except one cyst in the series of Wysocki and his coworkers were in the mandibular bicuspid-cuspid incisor area, the one exception being in the maxillary lateral incisor area. The locations in the series of Buchner and Hansen were virtually identical except that they had several cases also in the maxillary arch from cuspid to first molar.
This lesion presents generally as a small, well-circumscribed, painless swelling of the gingiva, sometimes closely resembling a superficial mucocele (Fig. 4-10). The lesion is of the same color as the adjacent normal mucosa and seldom measures over 1 cm in diameter, generally much less. Although this cyst may occur in either the free or attached gingiva, some gingival cysts occur in the gingival papilla itself.
Radiographic Features
The gingival cyst is a soft tissue lesion and does not generally manifest itself on the dental radiograph. If it enlarges to sufficient size, it may cause superficial erosion of the cortical plate of bone, but this is still generally not visible on the radiograph. If a circumscribed, radiolucent cystic lesion of alveolar bone with some swelling of the soft tissue is present, the cyst probably represents a lateral periodontal cyst rather than a gingival cyst.
Histologic Features
The gingival cyst of the adult is a true cyst, since it is a pathologic epithelium lined cavity which usually contains fluid (Fig. 4-10 B). The lining epithelium is generally identical to that found in the lateral periodontal cyst, with the occasional exceptions noted above. The epithelium ranges in thickness from simply one flattened cell to several cells, a thin stratified squamous epithelium. Glycogen-rich clear cells may be present, especially in the focal thickenings or plaques of the lining. Dental lamina rests may also be found in the connective tissue wall and these are commonly composed of the same type of glycogen-rich clear cells. As noted earlier, these lesions may be unicystic or polycystic. Since the cyst lies free in the connective tissue of the gingiva, it may or may not exhibit an associated inflammatory reaction.
In cases of the traumatic or implantation type of gingival cyst, calcification or even ectopic ossification on rare occasions may be associated with the cystic lesion, reminiscent of the ossification occurring after experimental implantation of bladder epithelium into ubcutaneous tissues.
Lateral Periodontal Cyst
A slow growing, nonexpansile developmental odontogenic cyst derived from one or more rests of the dental lamina, containing an embryonic lining of one to three cuboidal cells and distinctive focal thickenings (plaques).
The lateral periodontal cyst, as the name implies, occurs on a lateral periodontal location and it is of developmental origin arising from cystic degeneration of clear cells of the dental lamina. In order to establish the proper diagnosis, an inflammatory origin as well as exclusion of a possible odontogenic keratocyst must be ruled out clinically and histologically.
The lateral periodontal cyst is an uncommon but well-recognized type of developmental odontogenic cyst. The various theories concerning the etiology and pathogenesis of the lesion have been reviewed by Standish and Shafer. These cysts appear to arise in intimate association with the lateral root surface of an erupted tooth, with a predilection for the mandibular bicuspid area. The possibilities which have been offered to explain their origin and mode of development include:
• Origin initially as a dentigerous cyst developing along the lateral surface of the crown, and as the tooth erupts, the cyst assumes a position in approximation to the lateral surface of the root.
• Origin from proliferation of rests of Malassez in the peri-odontal ligament although the stimulus for this proliferation is unknown.
• Origin simply as a primordial cyst of a supernumerary tooth germ, since the predilection for occurrence of the lateral periodontal cyst in the mandibular bicuspid area corresponds well with the known high incidence of supernumerary teeth in this same region.
• Origin from proliferation and cystic transformation of rests of dental lamina, which are in a postfunctional state and therefore have only a limited growth potential that is in accordance with the usual small size of these cysts.
This latter theory, including the suggestion that the lateral periodontal cyst and the gingival cyst of the adult (q.v.) share this common histogenesis from postfunctional dental lamina rests and that these two cysts represent basically the central or intraosseous and peripheral or extraosseous manifestations of the same lesion, has been discussed in detail by Wysocki and his colleagues. At present, it seems the most appropriate one. They have also pointed out the important fact that in many reports of the lateral periodontal cyst in the previous literature, the term has been used to designate any cyst that may be positioned against the lateral root surface of a tooth (e.g. lateral radicular cyst related to pulp infection, odontogenic keratocyst, etc.). This positional use of the term should be avoided and the designation applied only to that specific developmental lesion with characteristic features.
An unusual form of cyst was reported in 1973 by Weathers and Waldron under the term botryoid odontogenic cyst. They described two cases of cysts which had a multilocular pattern apparent radiographically, histologically and even clinically at the time of surgical removal. Additional experience with this cyst, as indicated by Wysocki and his associates, now strongly suggests that this is simply a polycystic variant of the lateral periodontal cyst developing through cystic transformation of multiple islands of dental lamina rests. Then epithelial lining in the two cysts is identical, as are its clinical features, including age of predilection and sites of occurrence. In addition, a multilocular extraosseous form analogous to the gingival cyst of the adult also occurs.
Clinical Features
The lateral periodontal cyst occurs chiefly in adults, according to the series of 39 cases reported by Wysocki and his associates in which there was a mean age of 50 years and an age range of 22–85 years. In this series, there was a predilection for occurrence in males over females, 67: 28 with 5% unknown. The location of the lesion was extremely limited in this study: 67% of cases were in the mandibular bicuspid/cuspid/incisor area, while 33% were in the maxillary lateral incisor area. Lesions were found at no other sites and this has also been the xperience of most other investigators. No rational explanation has been offered for this localization.
The majority of cases have presented no clinical signs or symptoms and have been discovered during routine radiographic examination of the teeth. Occasionally, when the cyst is located on the labial surface of the root, there may be a slight mass obvious, although the overlying mucosa is normal. Unless otherwise affected, the associated tooth is vital. If the cyst becomes infected, it may resemble a lateral periodontal abscess and even seek to establish drainage.
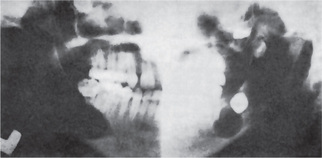
Radiographic Features
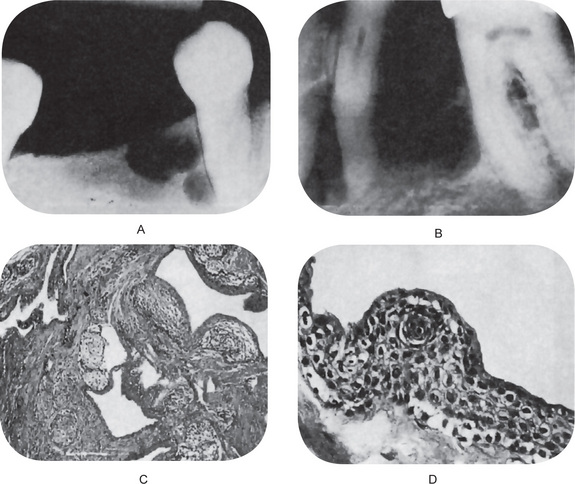
The periapical radiograph discloses the lateral periodontal cyst as a radiolucent area in apposition to the lateral surface of a tooth root (Fig. 4-11A–D). The lesion is usually small, seldom over 1 cm in diameter, and may or may not be well circumscribed. In most cases the border is definitive and is even surrounded sometimes by a thin layer of sclerotic one. The botryoid odontogenic cyst appears similar except that its polycystic nature is often evident through its multilocular pattern on the radiograph (Fig. 4-12).
Histologic Features
Histologically, the lateral periodontal cyst is a distinct type of developmental cyst characterized by a thin, nonkeratinized epithelium usually one to five cell layers thick, which resembles the reduced enamel epithelium. Cuboidal or even columnar cells may be found composing the lining. Many of the lining cells have a clear, vacuolated, glycogen-rich cytoplasm (Fig. 4-13). This lining is incomplete and easily sloughs away. Focal thickened plaques of proliferating lining cells often project into the lumen in areas. These are especially prominent in the botryoid odontogenic cyst (Fig. 4-13). Rests of dental lamina are sometimes found in the connective tissue wall and these similarly are frequently composed of glycogen-rich clear cells. These also appear to be more common in the botryoid-type cyst. Papillary infoldings of the lateral periodontal cyst wall are sometimes seen and inflammatory cells may be present, but this is a secondary reaction. The histologic characteristics of these cysts have been detailed by Wysocki and his colleagues as well as by Shear and Pindborg. The connective tissue subjacent to the epithelium exhibits a zone of hyalinization, consisting of a thick fibrous noninflamed cyst wall. A different histologic appearance has been described for this cyst with the name of botryoid odontogenic cyst. This name reflects the gross similarity of the cystic cavities to that of a cluster of grapes.
Treatment and Prognosis
Provided that the lesion is unilocular on radiographic examination the lateral periodontal cyst is treated by surgical enucleation. Attempts should be made to avoid sacrificing the associated tooth, but this may not always be possible. The botryoid variety, a lesion that is radiographically or histologically multilocular, has an increased risk of recurrence or persistence and patients treated for a botryoid odontogenic cyst should be followed periodically. It is especially important that the diagnosis be established because of the similarity in appearance between this cyst and other more serious lesions such as an early ameloblastoma.
Calcifying Odontogenic Cyst (Keratinizing and/or calcifying epithelial odontogenic cyst, Gorlin cyst, cystic keratinizing tumor)
A rare, well-circumscribed, solid or cystic lesion derived from odontogenic epithelium that resembles follicular ameloblastoma but contains ‘ghost cells’ and spherical calcifications.
The so-called calcifying odontogenic cyst (COC) represents a heterogeneous group of lesions that exhibit a variety of clinicopathologic and behavioral features. Because of this diversity, there has been confusion and disagreement on the terminology and classification of these lesions. The term calcifying odontogenic cyst includes both non-neoplastic cyst and true neoplasms. The 1992 WHO classification includes this cyst and all its variants in the category of odontogenic tumors.
It has become obvious that it is not one lesion but really two—a cystic lesion and a solid neoplastic lesion. A third malignant counterpart of the neoplastic lesion may be added. The calcifying odontogenic cyst may present as an intraosseous cystic lesion or in association with an odontoma. On rare occasions, it may present as a peripheral (gingival) lesion. The neoplasm, more aptly termed a dentinogenic ghost cell tumor, is a rare lesion that may occur in bone or in the gingival soft tissue (for detailed description refer section on odontogenic tumors).
Glandular Odontogenic Cyst (Sialo-odontogenic cyst, mucoepidermoid odontogenic cyst)
An unusually large solitary or multilocular odontogenic cyst probably derived from the rests of dental lamina, consisting of a stratified squamous epithelium containing numerous mucus secreting cells.
The glandular odontogenic cyst (GOC), also known as sialo-odontogenic cyst has many similarities to the lateral periodontal cyst of which it is considered a variant by some authors. Glandular odontogenic cyst occurs in the same location as the lateral periodontal cyst but as a rule it has a multilocular radiolucent appearance. The name of the cyst is not yet established. The term most descriptive of the lesion is probably mucoepidermoid odontogenic cyst because of the presence of both secretory elements and stratified squamous epithelium (Sadeghi et al, 1991). The use of this name might, however, lead to confusion with the mucoepidermoid carcinoma, and is therefore unlikely to find favor (Shear, 1992).
Padayachee and van Wyk, 1987, indicated that the typical features of the cyst are that it is intrabony and multilocular radiographically; that it can recur if not adequately excised; that it is multicystic, with the cystic spaces lined by a nonkeratinized epithelium similar to reduced enamel epithelium, with epithelial thickenings or plaques; that mucous and cylindrical cells form an integral part of the epithelial component; and that mucinous material within the cystic spaces is a prominent feature. Gardner et al, (1988) who favored the name ‘glandular odontogenic cyst’ suggested that its histological features and biological behavior are sufficient for it to be regarded as an entity.
Clinical Features
The glandular odontogenic cyst occurs over a wide age range. A slight male predilection was reported. The common site affected was anterior mandible. The lesions showed slow progressive growth, were painless and locally destructive. The mandible seems to be affected more commonly (87.2%) than the maxilla. The age ranges from 10–90 years with the mean of 49.5 years.
Radiographic Features
The lesions appear well defined with a multilocular pattern but without specific diagnostic features.
Histologic Features
Histologically, glandular odontogenic cyst is lined in parts by a nonkeratinized stratified epithelium of varying thickness (Fig. 4-14). The epithelium has a glandular or pseudoglandular structure, with goblet mucous producing cells as well as intraepithelial crypts or microcysts containing mucus. These microcysts may open onto the surface of the epithelium giving a papillary or corrugated appearance. Some cells may also be ciliated. Occasionally the epithelium is thinner, similar to reduced enamel epithelium. Epithelial thickenings or plaques may be present either in this thin epithelium or in the stratified epithelium. Interface between epithelium and connective tissue is flat. The diagnosis of glandular odontogenic cyst should be considered when observing a lateral periodontal multilocular radiolucency. The diagnosis is essentially microscopic.
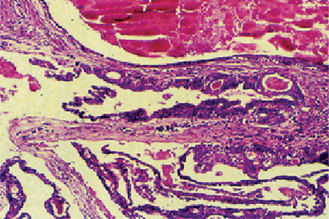
Figure 4-14 Sialo-odontogenic cyst. Nonkeratinized stratified epithelium of varying thickness; cuboidal or columnar ciliated surface lining with goblet cells; polycystic with secretory and epithelial elements; microcysts may form duct like structures and are filled with a PASpositive material surrounded by fibrous capsule.
Inflammatory Cysts
Periapical Cyst (Radicular cyst, apical periodontal cyst, root end cyst)
The periapical (radicular) cyst is the most common odontogenic cyst. The usual etiology is an infected tooth, leading to necrosis of the pulp. Toxins exit at the apex of the tooth, leading to periapical inflammation. This inflammation stimulates the epithelial rests of Malassez, which are found in the apical periodontal ligament, resulting in the formation of a periapical granuloma that may be infected or sterile. Eventually, this epithelium undergoes necrosis caused by a lack of blood supply, and the granuloma becomes a cyst (periapical cyst). The lesions are not usually clinically detectable when small but most often are discovered as an incidental findings on radiographic survey.
Pathogenesis
This cyst is classified as inflammatory, because in the majority of cases it is a consequence to pulpal necrosis following caries, with an associated periapical inflammatory response. Other causes include any event that may result in pulpal necrosis such as tooth fracture and improper restorations, among others. The first line of defense to pulpal necrosis in the periapical area is the formation of a granuloma. A granuloma is a highly vascularized tissue containing a profuse infiltrate of immunologically competent cells, i.e. lymphocytes, macrophages and plasma cells.
The epithelial rests of Malassez, which are pluripotential in nature can differentiate into any type of epithelium, under the proper stimuli. These rests play a central role in the formation of radicular cysts. In the midst of the rich vascular area provided by the periapical granuloma, the rests of Malassez proliferate and eventually form a large mass of cells. With continuous growth, the inner cells of the mass are deprived of nourishment and they undergo liquefaction necrosis. This leads to the formation of a cavity which is located in the center of the granuloma, giving rise to a radicular cyst.
Islands of squamous epithelium which have developed from odontogenic rests of Malassez can also be found in a periapical granuloma without cystic transformation. Endodontists refer to these granulomas as ‘bay cyst’.
Clinical Features
Around 60% of all jaw cysts are radicular or residual cysts. Radicular cysts can occur in the periapical area of any teeth, at any age but are seldom seen associated with the primary dentition. The majority of cases of apical periodontal cysts are asymptomatic. The tooth is seldom painful or even sensitive to percussion. This type of cyst is only infrequently of such a size that it destroys much bone, and even more rarely does it produce expansion of the cortical plates. The apical periodontal cyst is a lesion that represents a chronic inflammatory process and develops only over a prolonged period of time. In some cases, such a cyst of long standing may undergo an acute exacerbation of the inflammatory process and develop rapidly into an abscess (periapical abscess) that may then proceed to a cellulitis or form a draining fistula. The cause of such a sudden flare up is not known, but it may be a result of loss of local or generalized tissue resistance.
Radiographic Features
The radiographic image of the radicular cyst is a peri- or para-apical, round or oval radiolucency of variable size which is generally well delineated and most likely with a marked radiopaque rim. Other lesions, such as granulomas, neoplasms of various origin and some diseases of bone can also present a similar radiolucent periapical appearance. Therefore, a periapical radiolucency cannot be automatically assumed to be a cyst. Several studies have indicated that it is not possible to rely on the radiographic size of a periapical radiolucency to establish the diagnosis of either cyst or granuloma unless the lesion is larger than 2 cm in diameter. Rarely radicular cysts will induce resorption of the root of the affected tooth.
Histologic Features
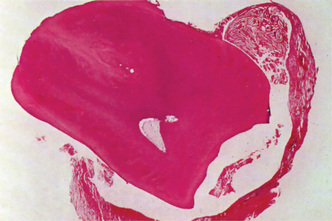
Microscopically a radicular cyst is limited by a mature collagenous connective tissue wall. Abundant fibroblasts can be identified within the cystic wall. The wall generally presents an inflammatory infiltrate of variable degree. Lymphocytes are generally the most prominent cells in the infiltrate and are characterized by their darkly stained nucleus, which occupies most of the cytoplasm. Plasma cells are also abundant in cysts’ walls and mostly seen in long standing (chronic) cysts. They are characterized by an eccentric nucleus with a cartwheel arrangement of the nuclear chromatin. Plasma cells are considered repertoire of immunoglobulins. Other histological findings within the cystic wall are: erythrocytes and areas of hemorrhage, occasional spicules of dystrophic bone, multinucleated giant cells and cholesterol crystals (Fig. 4-15A).
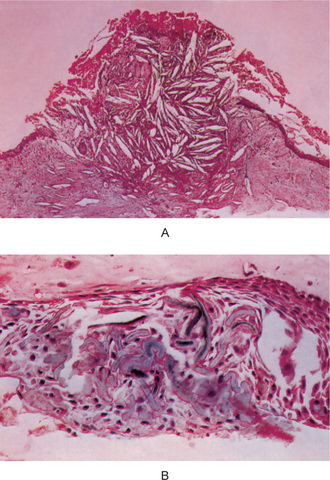
Figure 4-15 Radicular cyst. (A) The cyst cavity is filled with an eosinophilic coagulum containing slit-like spaces which contain cholesterol. These cholesterols may form mucosal nodules; the epithelial lining becomes discontinued where such nodules have formed. (B) Hyaline bodies. Another feature that may be seen within the epithelial linings of radicular cyst is the peculiarly shaped structures which are eosinophilic, and typically, are described as lady’s hairpin shaped (Courtesy of Dr K W Lee).
The cavity of a radicular cyst is generally lined by stratified squamous epithelium. These cysts can be lined by respiratory epithelium, especially if they are in the vicinity of the maxillary sinus. The epithelial lining, many times, is discontinuous, frequently missing over areas of intense inflammation. Rarely radicular cysts may be lined by mucus producing epithelium in either maxillary or mandibular locations. The mucous epithelium is the result of metaplastic transformation of the epithelial rests of Malassez which are pluripotential. In rare instances, carcinoma has been reported developing from the lining epithelium of odontogenic cysts, including the radicular cyst. These have been reviewed by Gardner (1969).
An interesting and peculiar structure, originally described by Rushton and subsequently reported by Molyneux, Medak and Weinmann and Shear, is the hyaline body or Rushton body, often found in great numbers in the epithelium of apical, periodontal or residual cysts (Fig. 4-15 B). These hyaline bodies are tiny linear or arc-shaped bodies, generally associated with the lining epithelium, that appear amorphous in structure, eosinophilic in reaction and brittle in nature, since they evidence fracture in some cases. Their frequency of occurrence in cyst linings ranges between 2.6 and 9.5% of cysts, according to a review by Allison. The etiology, pathogenesis, and significance of these structures are unknown. The lumen of the cyst usually contains a fluid with a low concentration of protein that stains palely eosinophilic. Occasionally the lumen may contain a great deal of cholesterol, and in rare instances, limited amounts of keratin are present.
Treatment
The treatment of the radicular cyst consists of extraction of the involved teeth and careful curettage of the periapical tissue. Under some conditions, root canal therapy may be carried out with apicoectomy of the cystic lesion. The cyst does not recur if surgical removal is thorough. If the cystic sac is badly fragmented, leaving epithelial remnants, or if a periapical granuloma is incompletely removed with epithelial rests remaining, a residual cyst may develop in this area months or even years later. If untreated, the radicular cyst slowly increases in size at the expense of the surrounding bone. The bone undergoes resorption, but seldom is there a remarkable expansion of the cortical plates, as is frequently seen in the case of the dentigerous cyst.
Residual Cyst
Residual cyst is a term of convenience because no teeth are left by which to identify the lesion. Most commonly, these actually are retained periapical cysts from teeth that have been removed. The histology of the lining is a nondescript stratified squamous epithelium.
Theoretically, it could develop in a dental granuloma that is left after an extraction. The residual cyst may be found in any of the tooth bearing areas of the mandible or maxilla. Morphologically, the residual cyst may present as a fairly well defined radiolucency that can vary in size from a few mm to several cm. Clinically, these cysts are usually found on routine radiographic examination of patients. They may have been present for many months or years and only become symptomatic upon secondarily infected (Figs 4-16 A, B, and C). Usually, residual cysts do not expand bone. Treatment is surgical curettage (refer periapical cyst).
Paradental Cyst
A cyst of uncertain origin found primarily on the distal or facial aspect of a vital mandibular third molar, consisting of intensely inflamed connective tissue and epithelial lining.
The paradental cyst is an inflammatory cyst which develops on the lateral surface of a tooth root. Some authors refer to this cyst as an inflammatory periodontal cyst or collateral cyst. This cyst is of rare occurrence and must be radiographically differentiated from the lateral periodontal cyst. It is treated by surgical ablation and does not have a tendency to recur.
It seems clear that the paradental cyst is of inflammatory origin and that it arises from odontogenic epithelium. Craig (1976) has suggested that either the cell rests of Malassez or the reduced enamel epithelium might provide the cell of origin. He favored the latter source, arguing that in his study the rests of Malassez always appeared inactive and that if the Malassez rests were responsible the lesions should be equally distributed around the root surface. His serial sections indicated that the development of paradental cyst may follow hyperplasia and cystic change in reduced enamel epithelium. He suggested that the presence of an extension of reduced enamel epithelium over the enamel projections might be the source, and could explain the frequent buccal location of the cyst.
Pathogenesis
There is no unanimity with regard to pathogenesis. Ackerman, Cohen and Altini (1987) like Craig (1976), favored origin from reduced enamel epithelium but suggested that cyst formation occurs as a result of unilateral expansion of the dental follicle secondary to inflammatory destruction of periodontium and the alveolar bone. Fowler and Brannon (1989) suggested that it may be a variant of the dentigerous cyst or derived from an occluded periodontal pocket. Vedtofte and Praetorius (1989) were satisfied that the cyst was of inflammatory origin, initiated by a pericoronitis at the time of tooth eruption and considered rests of Malassez and reduced enamel epithelium the most likely source of the cyst epithelium.
Clinical Features
In the sample of 2,616 jaw cysts reported by Shear (1992), there were 65 paradental cysts classified over a 32-year period (2.5%). The series of 50 cases reported by Ackerman, Cohen and Altini (1987) represented the 3% of a sample of 1,852 odontogenic cysts observed over a 20-year period. Virtually all the cases in the study by Ackermann, Cohen and Altini occurred between the ages of 10 and 39 with two-thirds of their sample in the third decade; the same as in Craig’s material (cited by Shear, 1992). There was a considerable preponderance of males reported by Ackermann, Cohen and Altini and by Fowler and Brannon but of Vedtofte and Praetorius. There was an equal gender distribution. Ackermann, Cohen and Altini found most of their cyst located distally and distobuccally to the third molar. All the papers emphasized that the involved teeth were vital. Bilateral examples occurred in a number of instances.
Radiographic Features
All authors reported a variable radiographic picture but there are some features which appeared consistently and which seem to be useful in contributing to the diagnosis. These are the non widening of the periodontal ligament space and that the lesion was superimposed on the buccal root face. When there was a distal as well as a buccal radiolucency, the distal element was separate from the distinct distal follicular space (Shear, 1992).
Histologic Features
Histologically, the cysts were lined by a hyperplastic nonkeratinized stratified squamous epithelium. An intense inflammatory cell infiltrate is present associated with the hyperplastic epithelium and in the fibrous capsule adjacent to the epithelium. Histologically the paradental cyst cannot be differentiated from a radicular cyst.
Tumors of odontogenic origin
Odontogenic tumors represent a spectrum of lesions ranging from malignant (rare) and benign neoplasms to dental hamartomas, all arising from odontogenic residues, i.e. odontogenic epithelia and/or ectomesenchyme with variable amounts of dental hard tissues formed generally in the same sequence as in normal tooth development.
Occasionally an odontogenic tumor develops from a preexisting developmental cyst (e.g. adenomatoid odontogenic tumor from a dentigerous cyst) or dental primordium (e.g. ameloblastomas often take the place of lower third molars). In many instances the exact tissue of origin (histogenesis) of an odontogenic tumor may only be inferred from its site and structure.
Classification of Odontogenic Tumors
The WHO classification (1992) divides odontogenic tumors into benign and malignant, with major subdivisions in each category (White, 2004) (Table 4-2). The subdivisions for the benign neoplasms are based on types of odontogenic tissue involved in the process and include:
Table 4-2
Odontogenic tumors (modified WHO classification)
A. Benign
I. Odontogenic epithelium without odontogenic ectome senchyme
1. Ameloblastoma
2. Squamous odontogenic tumor
3. Calcifying epithelial odontogenic tumor (Pind borg tumor)
4. Adenomatoid odontogenic tumor*
II. Odontogenic epithelium with odontogenic ectom esenchyme with or without hard tissue formation
1. Ameloblastic fibroma
2. Ameloblastic fibrodentinoma
3. Ameloblastic fibro-odontoma
4. Odontoameloblastoma
5. Calcifying odontogenic cyst
6. Complex odontoma
7. Compound odontoma
III. Odontogenic ectomesenchyme with or without included odontogenic epithelium
1. Odontogenic fibroma
2. Myxoma (myxofibroma)
3. Cementoblastoma (benign cementoblastoma, true cementoma)
B. Malignant
I. Odontogenic carcinomas
1. Malignant ameloblastoma
2. Primary intraosseous carcinoma
3. Clear cell odontogenic carcinoma**
4. Ghost cell odontogenic carcinoma
II. Odontogenic sarcomas
1. Ameloblastic fibrosarcoma
2. Ameloblastic fibrodentinosarcoma
3. Ameloblastic fibro-odontosarcoma
*Originally classified under II, but in a new revision of the WHO classification it will be classified under I.
**Originally classified as a benign tumor under I (clear cell odontogenic tumor), but it is now recognized as a malignant tumor (clear cell odontogenic carcinoma) and classified accordingly.
• Odontogenic epithelium without odontogenic ectomesenchyme.
• Odontogenic epithelium with odontogenic ectomesenchyme, with or without dental hard tissue formation.
• Odontogenic ectomesenchyme with or without included odontogenic epithelium.
The classic example of odontogenic tumor viz. ameloblastoma is an archetype of a neoplasm where the neoplastic component is epithelial only without contribution from the ectomesenchyme. In the second category, some of the neoplasms are pure epithelial tumors but are capable of inducing dysplastic enamel and dentin formation. Examples include adenomatoid odontogenic tumor, calcifying odontogenic cyst, and odontoameloblastoma. Other tumors in the category exhibit neoplastic cells of both odontogenic epithelium and ectomesenchyme and include ameloblastic fibroma and ameloblastic fibro-odontoma (mixed odontogenic tumors). Yet other entities represent hamartomatous proliferations of both tissues, including compound and complex odontomas. In the third category, odontogenic ectomesenchyme with or without included odontogenic epithelium, the neoplastic cells appears to be of connective tissue origin. Neoplasms include odontogenic fibroma and odontogenic myxoma. Any epithelial component is not considered to be neoplastic.
Ameloblastic carcinoma and odontogenic sarcomas are examples of odontogenic neoplasms which contain a malignant epithelial and connective tissue component as part of the tumor. Calcifying odontogenic cyst has been placed in the odontogenic tumors classification by the WHO because some variants consist of a solid tumor with ameloblastoma like areas, dentinoid, and ghost cells. This form has been designated as dentinogenic ghost cell tumor or odontogenic ghost cell tumor. Benign cementoblastoma has currently been delinked from the WHO classification of odontogenic tumors and is described along with benign osteoblastoma.
Ameloblastoma (Adamantinoma, adamantoblastoma, multilocular cyst)
The ameloblastoma is a true neoplasm of enamel organ type tissue which does not undergo differentiation to the point of enamel formation. It has been described very aptly by Robinson as being a tumor that is ‘usually unicentric, nonfunctional,intermittent in growth, anatomically benign and clinically persistent’.
The term ‘ameloblastoma’ as applied to this particular tumor was suggested by Churchill in 1934 to replace the term ‘adamantinoma’, coined by Malassez in 1885, since the latter term implies the formation of hard tissue, and no such material is present in this lesion. The first neoplasm of this nature reported in the scientific literature is credited to Broca in 1868, although Guzack reported a tumor of the jaw in 1826 which may be the first recorded instance of an ameloblastoma. In any event, the first thorough description of an ameloblastoma is that of Falkson in 1879.
It is the second most common odontogenic neoplasm, and only odontoma outnumbers it in reported frequency of occurrence. Excluding odontoma, the incidence of amelo blastoma is at least equal to the incidence of all the other odontogenic neoplasms combined. However G Sriram and Shetty RP based on an Indian institutional study on 250 odontogenic tumors reported ameloblastoma to be the most common (61.5%) odontoplasmic neoplasm in India. Its incidence, combined with its clinical behavior, makes ameloblastoma the most significant odontogenic neoplasm of concern to oral and maxillofacial surgeons.
Pathogenesis
The earlier workers noted the resemblance between the odontogenic apparatus and the ameloblastoma and suggested that the neoplasm was derived from a portion of this apparatus or from cells potentially capable of forming dental tissue. Malassez described small collections of epithelial cells adjacent to the roots of teeth in the periodontal ligament and suggested that the ‘adamantine epithelioma’ was produced by a proliferation of these cell rests.
Most authorities consider the ameloblastoma to be of varied origin, although the stimulus initiating the process is unknown. Thus the tumor conceivably may be derived from:
• Cell rests of the enamel organ, either remnants of the dental lamina or remnants of Hertwig’s sheath, the epithelial rests of Malassez.
• Epithelium of odontogenic cysts, particularly the dentigerous cyst, and odontomas.
• Disturbances of the developing enamel organ
• Basal cells of the surface epithelium of the jaws.
• Heterotopic epithelium in other parts of the body, especially the pituitary gland.
Presently, it is thought that it is likely the result of alterations or mutations in the genetic material of cells that embryologically preprogrammed for tooth development. Environmental factors and individual patient variables (e.g. general health status, nutritional status) also likely have a role in modulating the incidence of the disease. This theory is demonstrated by the finding that the average age of occurrence of ameloblastoma in industrialized nations is 10–15 years greater than that seen in developing countries (Kessler HP et al, 2003).
Cahn in 1933 reported a case of ameloblastoma originating in the wall of a dentigerous cyst, and numerous cases have subsequently been reco gnized as developing in this fashion. It should be reiterated that Stanley and Diehl, in reviewing 641 cases of ameloblastoma, found that 108 of these tumors, approximately 17%, were definitely associated with an impacted tooth and/or a follicular (dentigerous) cyst. They also noted a marked reduction in the prevalence of such cases after the age of 30, presumably because of the loss of the ameloblastomatous potential of the odontogenic epithelium in impacted tooth follicles and follicular cysts as patients age. Such a significant finding emphasizes the dangerous potential of the denti gerous cyst and the need for careful microscopic examination of every such lesion. This is discussed in greater detail in the section on the dentigerous cyst. Since a dentigerous cyst may develop in association with an odontoma, as well as with an impacted tooth, it is suggested that these too be examined by the pathologist. There is apparently little tendency for the development of the ameloblastoma in the ordinary apical periodontal or radicular cyst.
Clinical Features
A wide age range of occurrence of the tumor from 10 years through 90 years has been reported. The average age at diagnosis is in the range of 33–39 years, and most cases cluster between ages 20 and 60 years. Only about 10% of cases are reported to arise in children, and less than one third of those occur in children younger than 10 years. No significant sex predilection has been reported. There is conflicting evidence on the incidence rates in different races. Although some reports claim an increased incidence of ameloblastoma in black individuals, a large study identifies Asians as the population with the greatest number of affected patients. Because sizeable numbers of cases are reported in every racial group, race does not seem to be a significant defining demographic characteristic of the disease (Reichart PA et al, 1995).
Ameloblastoma occurs in all areas of the jaws, but the mandible is the most commonly affected area (more than 80% of all cases). Within the mandible, the molar angleramus area is involved three times more commonly than are the premolar and anterior regions combined. Statistics on the location of maxillary ameloblastomas are more variable and more difficult to interpret. Some studies report a low incidence in the anterior maxilla, whereas other studies suggest that the incidence in the anterior maxilla is roughly equivalent to the incidence in the maxillary molar region. When comparing large studies, it appears that maxillary tumors tend to occur in slightly older patients than do mandibular lesions. The incidence of occurrence of ameloblastoma in different sites within the jaws has been shown to vary among racial groups. Asians seem to have fewer tumors involving the ramus than do whites or blacks, whereas blacks have an increased frequency of tumors in the anterior mandible compared with the other two groups (Kessler HP et al, 2003).
Peripheral (extraosseous) ameloblas toma
This is a tumor which histologically resembles the typical central or intraosseous ameloblastoma but which occurs in the soft tissue outside and overlying the alveolar bone. In addition, a number of cases of lesions in a similar location and with similar histologic features have been reported under the term ‘basal cell carcinoma of the gingiva’. Many investigators consider these the same basic lesion, including Gardner, who has reviewed the literature, adding seven additional unpublished cases for a total of 21 examples of peripheral ameloblastoma. Occasional other cases have since been reported, such as those of Greer and Hammond and of Gould and his colleagues.
This tumor appears to originate from either surface epithelium or remnants of dental lamina. In some instances, the tumor exhibits one or more areas of continuity with the surface epithelium, while in other cases, even with serial sectioning, no evidence of continuity between the two can be found.
The ages of the patients in the 21 cases reviewed by Gardner ranged between 23 and 82 years, with 10 patients between 30 and 50 years of age. There was a slight predilection for occurrence in males, 13 cases to 8 cases in females. There was an approximately 2 : 1 ratio of occurrence in the mandible over the maxilla. The lesions, all of which appeared as nodules on the gingiva, varied in size from 3 mm to 2 cm in diameter. In only two cases was radiographically evident superficial erosion of bone present.
The peripheral ameloblastoma histologically may exhibit the same pattern seen in the intraosseous ameloblastoma. However, while some lesions appeared to be of the follicular type, the vast majority were acanthomatous, at least in areas. Greer and Hammond have studied the ultrastructural characteristics of their case and found the electron microscopic appearance to be similar to that of the intraosseous ameloblastoma and the cutaneous basal cell carcinoma. In a similar ultrastructural study, Gould and his associates found that the features of their tumor was characteristic of origin from either surface epithelium or odontogenic remnants, thereby precluding definitive conclusions regarding site of origin. In terms of differential diagnosis, one must always consider the possibility of the peripheral odontogenic fibroma, which is also a peripheral lesion of the gingiva with variable amounts of odontogenic epithelium. The distinction on the basis of the connective tissue parenchyma of the odontogenic fibroma is usually not difficult, although cases with features of both lesions may occur, such as that reported by Sciubba and Zola.
One of the most important aspects of the peripheral ameloblastoma, emphasizing the need for its careful identification as a peripheral lesion and separation from the intraosseous counterpart, is the difference in clinical behavior. The peripheral lesion is relatively innocuous, lacks the persistent invasiveness of the intraosseous lesion and has very limited tendency for recurrence. For this reason, it may be excised locally, although follow-up examination is always good practice.
Pituitary ameloblastoma (cranio pharyngioma, Rathke’s pouch tumor)
This is a neoplasm involving the central nervous system which grows as a pseudoencapsulated mass, usually in the suprasellar area but occasionally in the intrasellar area, and often destroys the pituitary gland. The peak incidence is reported to be between 13 and 23 years of age. According to Zulch, the pituitary ameloblastoma is the most common tumor of childhood and adolescence. In his series of 6,000 CNS tumors, it constituted 2.5% of the total in all patients regardless of age.
It is generally thought to originate from the unobliterated portions of the fetal craniopharyngeal duct, which itself is derived from Rathke’s pouch. This pouch is a recess arising as a result of invagination of a portion of the stomodeal ectoderm, and the pituitary gland forms by fusion of this pouch with a process of the forebrain. Epithelial remnants of this craniopharyngeal duct are extremely common in the adult. These cell rests have a certain pluripotential and on occasion may give rise to tumors histologically similar to the ameloblastoma of the jaw.
Microscopic features of the craniopharyngioma, not generally found in the ameloblastoma, include the almost universal occurrence of irregular calcified masses as well as occasional foci of metaplastic bone or cartilage (Fig. 4-17 A). In addition, many investigators have noted the similarity between the craniopharyngioma and the calcifying odontogenic cyst because of the presence in the pituitary lesion of islands and nests of ‘ghost’ cells, as well as the calcifications and the fact that cyst formation is common. Several cases of craniopharyngioma with tooth formation have also been reported, such as that of Seemayer and his associates.
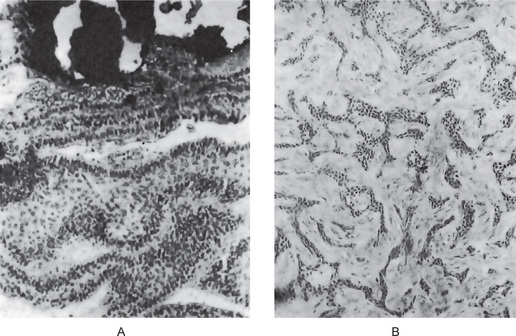
Figure 4-17 Ameloblastoma like tumors in other locations. (A) Craniopharyngioma showing typical ameloblastoma like formation with calcification. (B) Adamantinoma of tibia (Courtesy of Dr William G Sprague and Dr David C Dahlin).
There is great variability in the rate of growth of the pituitary ameloblastoma. Depending upon its exact location, there may be a gamut of clinical features eventually manifested, such as evidence of endocrine disturbance, drowsiness or even toxic symptoms. The treatment of this neoplasm is a neurosurgical problem.
This tumor has been discussed by Baker, Dockerty and Coventry, who reviewed the literature and concluded that the true nature of the lesion is still unknown. The tumor, which bears a superficial microscopic resemblance to the ameloblastoma of the jaws, has occurred in the tibia in approximately 90% of the slightly over 100 reported cases, but also has been recorded in the ulna, femur and fibula (Fig. 4-17B). Changus and his coworkers suggested that the lesion is actually a malignant angioblastoma, and this view is supported by Huvos and Marcove in their investigation of 14 cases. In contrast, Unni and his coworkers studied 29 cases, with ultrastructural microscopy of three of the tumors, and concluded that this provided evidence that the islands of tumor cells were epithelial in origin. Thus, although the histogenetic origin of this tumor is unknown, most authorities agree that it is not related to the ameloblastoma of the jaws, even though they support retention of the term ‘adamantinoma’ because of its acceptance through usage and for lack of a better name.
Radiographic Features
The ameloblastoma has been described classically as a multilocular cyst like lesion of the jaw. This is especially true in advanced cases of ameloblastoma. Here, the tumor exhibits a compartmented appearance with septa of bone extending into the radiolucent tumor mass (Fig. 4-18A). In many cases, however, the lesion is a unilocular one and presents no characteristic or pathognomonic features (Figs. 4-18B, 4-19). The periphery of the lesion on the radiograph is usually smooth, although this regularity may not be borne out at the time of operation. In the advanced lesion producing jaw expansion, thinning of the cortical plate may be seen on the radiograph (Figs. 4-20, 4-21).
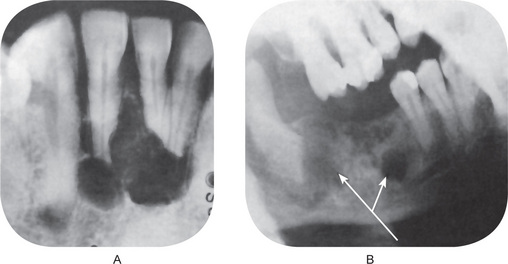
Figure 4-18 Ameloblastoma. (A) The typical loculations which often occur are clearly seen. (B) This lateral jaw radiograph reveals an early lesion with no loculations, but with several focal areas of bone destruction.
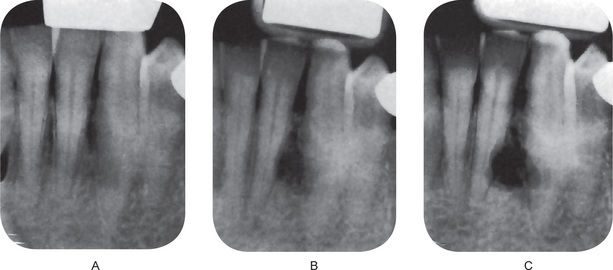
Figure 4-19 Developing ameloblastoma. Each radiograph was taken at intervals of two years. The slow growth of the ameloblastoma over the four-year period is typical (Courtesy of Dr Harry R Kerr Jr and Dr G Thaddeus Gregory).
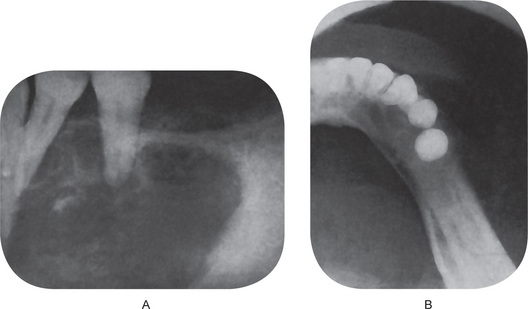
Figure 4-20 Ameloblastoma. The periapical (A) and occlusal (B) radiographs showing the destruction and expansion of bone which frequently occur.
The term ‘cystic ameloblastoma’ is frequently used in referring to certain of these neoplasms. It is important to note that there is no correlation between the term thus used clinically and the appearance of the tumor on the radiograph. The radiographic film does nothing more than indicate the relative presence or absence of calcified tissue, and a variety of lesions may manifest themselves in manner similar to that of the ameloblastoma.
Histologic Features
Six histopathologic subtypes of ameloblastoma are recognized: follicular, acanthomatous, granular cell, basal cell, desmoplastic, and plexiform. Most tumors show a predominance of one pattern, but few lesions are found to be composed purely of one histopathologic subtype. Mixtures of the different patterns commonly are observed. Lesions tend to be subclassified according to the predominant pattern that is present. The literature-based retrospective study by Reichart et al (1995) showed that the histologic subtype may have prognostic implications for recurrence. According to their study, the follicular type of ameloblastoma had the highest rate of recurrence at 29.5%. In contradistinction, the acanthomatous type of ameloblastoma showed only a 4.5% recurrence rate. The plexiform subtype was intermediate between the two extremes and showed a 16.7% recurrence rate. Studies have verified that desmoplastic ameloblastoma shows a tendency to recur, and the rate of recurrence is reported within the range of the other histologic subtypes of ameloblastoma.
A moderately to densely collagenized connective tissue characteristically constitute the stroma. The epithelial component of the neoplasm proliferates in what seems to be disconnected islands, strands, and cords within the collagenized fibrous connective tissue stroma. A prominent budding growth pattern often is seen, with small, rounded extensions of epithelium projecting from larger islands, recapitulating the various stages of enamel organ formation (Kessler HP et al 2003). The islands, strands, and cords may vary considerably in size. In high power magnification, the darkly staining periphery is composed of tall columnar cells with hyperchromatic nuclei. The nuclei tend to be round to oval in shape, and the nuclei of adjacent cells are in roughly the same location within the cytoplasm. This produces a characteristic palisading pattern. The palisaded nuclei are oriented away from the basement membrane area of the cell, and a small clear vacuole can be seen between the nucleus and the basement membrane. This peripheral layer of tall columnar cells with hyperchromasia, reverse polarity of the nuclei, and subnuclear vacuole formation mimic the normal embryologic development of the tooth bud at the stage of enamel matrix production. These classic features of ameloblastoma originally were described by Vickers and Gorlin in 1970 (criteria).
Focally in many ameloblastomas, the proliferating epithelium is seen to exert an inductive effect on the surrounding connective tissue stroma. In these areas, a zone of hyalinization of the collagen is present immediately adjacent to the epithelium. Fibroblasts are almost totally absent within the zone of hyalinization. It is theorized that the ameloblastic epithelium, in an attempt to complete its embryologic function and produce enamel matrix, signals the connective tissue to induce dentin formation; however, the fibroblastic cells in the connective tissue are unable to differentiate into odontoblasts, a required step in dentin and enamel formation. The hyalinized zone most likely represents the end result of this blockade in the normal embryologic sequence of odontogenesis (Kessler HP et al, 2003).
The follicular (simple) ameloblastoma is the most commonly encountered variant (Reichart PA et al, 1995), composed of many small discrete islands of tumor composed of a peripheral layer of cuboidal or columnar cells whose nuclei are generally well polarized. These cells strongly resemble ameloblasts or preameloblasts and these enclose a central mass of polyhedral, loosely arranged cells resembling the stellate reticulum. The terms ‘solid’ and ‘cystic’ have often been applied to the ameloblastoma and have variously referred to the clinical or histologic appearance of the tumor. Clinically, some cases exhibit tiny cysts that are grossly evident when the lesion is excised and examined carefully. In such instances, the stellate reticulum like tissue has undergone complete breakdown or cystic degeneration, and in such cases, there is often flattening of the peripheral columnar cells so that they resemble low cuboidal or even squamous cells. Cyst formation is relatively common in this follicular type of ameloblastoma.
In the plexiform ameloblastoma, the ameloblast like tumor cells are arranged in irregular masses, or more frequently, as a network of interconnecting strands of cells. Each of these masses or strands is bounded by a layer of columnar cells, and between these layers may be found stellate reticulum like cells. Sometimes double rows of columnar cells are lined up back to back. However, the stellate reticulum like tissue is much less prominent in the plexiform type than in the follicular type of ameloblastoma. Areas of cystic degeneration of stroma are also common.
In the acanthomatous ameloblastoma, the cells occupying the position of the stellate reticulum undergo squamous metaplasia, sometimes with keratin formation in the central portion of the tumor islands. This usually occurs in the follicular type of ameloblastoma. On occasion, epithelial or keratin pearls may even be observed.
In the granular cell ameloblastoma, there is marked transformation of the cytoplasm, usually of the stellate reticulum like cells, so that it takes on a very coarse, granular, eosinophilic appearance. This often extends to include the peripheral columnar or cuboidal cells as well. Ultrastructural studies, such as that of Tandler and Rossi, have shown that these cytoplasmic granules represent lysosomal aggregates with no recognizable cellular components. Hartman has reported a series of 20 cases of granular cell ameloblastoma and emphasized that this granular cell type appears to be an aggressive lesion with a marked proclivity for recurrence unless appropriate surgical measures are instituted at the first operation. In addition, several cases of this type have been reported as metastasizing. However, all other clinical features of the lesion appear similar to the other forms of ameloblastoma.
The basal cell type of ameloblastoma bears considerable resemblance to the basal cell carcinoma of the skin. It is believed that this is the rarest histologic subtype and the epithelial tumor cells are more primitive and less columnar, are generally arranged in sheets, more so than in the other tumor types (Fig. 4-22).
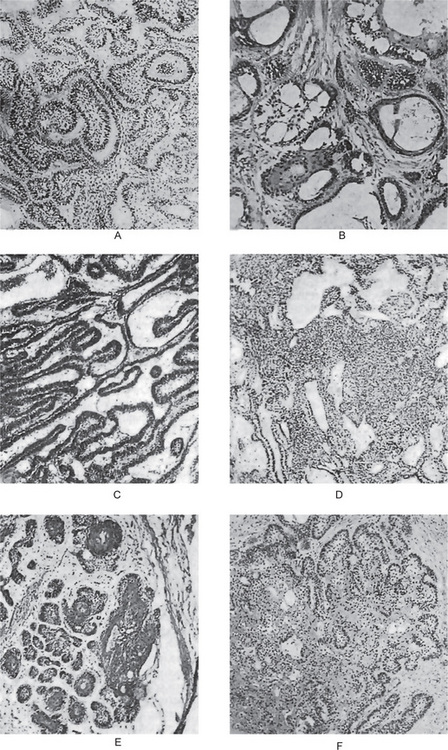
Figure 4-22 Ameloblastoma. (A) Follicular type. (B) Follicular type showing cystic degeneration and squamous metaplasia. (C) Plexiform type. (D) Basal cell type. (E) Acanthomatous type. (F) Granular cell type.
The desmoplastic ameloblastoma, characteristically, is found in a dense collagen stroma that may appear hyalinized and hypocellular. The desmoplastic ameloblastoma has a greater tendency to grow in thin strands and cords of epithelium rather than in an island like pattern. The epithelial proliferation almost seems to be compressed and fragmented by the dense hyalinized stroma. Central cells are often scant in the epithelial proliferation, and the cells making up the periphery of the strands and cords often are flattened or cuboidal rather than tall columnar in appearance. Reverse polarity of nuclei and subnuclear vacuole formation may be difficult to recognize (Fig. 4-23).
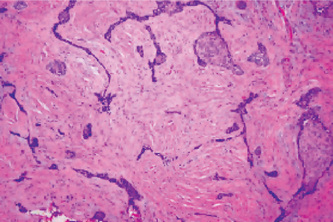
Figure 4-23 Desmoplastic ameloblastoma. Higher magnification details the cord and strand like growth that is characteristic of this histologic subtype (hematoxylin-eosin, original magnification x5) (Courtesy of Dr HP Kessler).
Most desmoplastic ameloblastomas display occasional classic islands of follicular ameloblastoma among the predominant strands and cords. Without these classic islands of ameloblastoma, the diagnosis can be difficult.
The growth pattern of the neoplasm, categorized as conventional or unicystic, is more important than the histopathologic subtype in treatment decision. The desmoplastic ameloblasloma is more important than the histopathologic subtype in treatment decision. Desmoplasia of the stromal connective tissue can be argued to be a maturation stage of the tumor, as similar dense collagenization is seem during maturation of long tumors (Sivapathamundaram et al, 2007). However, these authors failed to explain the off occurrence of these variants in the anterior jaw, unlike in conventional forms.
Unicystic ameloblastoma, the second and far less frequent growth pattern seen in the intraosseous ameloblastoma is the unicystic type. This growth pattern is seen in approximately 6% of ameloblastomas. It tends to occur in a younger population (average age in one large study, 22.1 years) compared with the patient population with conventional ameloblastomas. A high percentage of these lesions are associated with an impacted tooth, and the most commonly cited provisional diagnosis is dentigerous cyst. Cystic areas nearly always are noted grossly at the time of surgery. Recognition of this growth pattern is important, because it is well accepted that the unicystic type has a considerably better overall prognosis and a much reduced incidence of recurrence compared with conventional ameloblastoma (Li TJ et al, 1998).
The unicystic ameloblastoma is characterized by one or more of the following features:
• Lining epithelium exhibiting alterations virtually identical with those described by Vickers and Gorlin as representing early ameloblastomatous changes in the dentigerous cyst (q.v.).
• Nodules of tumor projecting intraluminally.
• Ameloblastomatous lining epithelium proliferating into the connective tissue wall.
• Islands of ameloblastoma occurring isolated in the connective tissue wall (Fig. 4-24).
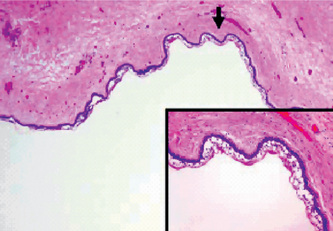
Figure 4-24 Unicystic ameloblastoma. The ameloblastoma shows a cystic architecture with the typical ameloblastic changes confined to the cyst-lining epithelium. The arrow indicates the area enlarged in the inset at lower right (hematoxylin-eosin, original magnification x2). (Inset) Ameloblastic epithelium with hyperchromatic palisaded basal cell layer, thin layer of stellate reticulum like cells, and abrupt transition to a thin parakeratinizing luminal surface (hematoxylin-eosin, original magnification x10) (Courtesy of Dr HP Kessler).
The recurrence rate of this lesion is distinctly lower than that for the characteristic ameloblastoma, thus indicating a less aggressive type of lesion. The epithelium lining the cystic cavity of the neoplasm shows typical cytomorphologic features that are recognizable as ameloblastoma, with a basal cell layer composed of columnar cells displaying hyperchromatic, palisaded nuclei.
Reverse polarity of the nuclei is present, and a subnuclear vacuole is usually noted between the basement membrane and nucleus. A thin overlying layer of stellate reticulum-like cells is seen. A luminal parakeratin layer may or may not be present. When keratinization is present, an abrupt transition from the stellate reticulum-like layer is usually observed. In some instances, the ameloblastic epithelium may be proliferative, with extension of the ameloblastic epithelium into the lumen of the cystic cavity. This feature has been termed intraluminal proliferation, and in many instances, this growth resembles the plexiform type of ameloblastoma. Thus, some lesions have been referred to as plexiform unicystic ameloblastoma (Fig. 4-25).
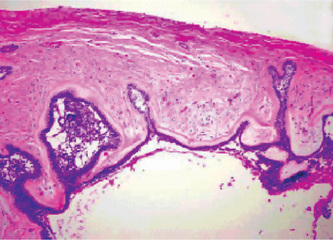
Figure 4-25 Ameloblastoma with mural growth. Ameloblastic epithelium infiltrates the connective tissue of the cyst wall. The infiltrating ameloblastic epithelium remains in direct continuity with the ameloblastic epithelium lining the cystic lesion. The cyst lumen is seen at the bottom (hematoxylin-eosin, original magnification x5).
Treatment and Prognosis
There are some differences of opinion about the preferable method of treatment of the ameloblastoma. The only unanimity centers around the fact that complete removal of the neoplasm, regardless of how it is accomplished, will result in a cure of the patient.
The types of treatment that have been used include both radical and conservative surgical excision, curettage, chemical and electrocautery, radiation therapy or a combination of surgery and radiation. The majority of workers today prefer some form of surgical excision. Curettage is least desirable, since it is associated with the highest incidence of recurrence. The basic principles of treatment have been discussed in detail by Gardner and Mehlisch and his colleagues.
Frissell reviewed the reported cases in which radiation therapy was utilized and found that there was considerable variance of opinion as to its benefit. The report of Kimm, supported by study of serial biopsy, on the treatment of the ameloblastoma by radiation indicated that this neoplasm is generally highly radioresistant and that the use of this form of therapy is not warranted. Wide clinical experience has shown the truth of this finding. Regardless of the form of treatment, long-term follow-up of the patient is an absolute necessity.
Treatment decisions for ameloblastoma are based on the individual patient situation and the best judgment of the surgeon. The surgical plan should be influenced strongly by whether the lesion involves the mandible or maxilla. Maxillary lesions behave distinctly differently from mandibular lesions. The higher cancellous bone percentage in the maxilla facilitates the spread of the ameloblastoma, whereas the density of the cortical plates in the mandible tends to limit spread of the neoplasm. Regardless of which jaw is involved, once an ameloblastoma has recurred, retreatment becomes more challenging. Radical retreatment typically is performed even for suspected unicystic lesions during the initial phase of the disease.
Calcifying Epithelial Odontogenic Tumor (Pindborg tumor)
The calcifying epithelial odontogenic tumor was first described in 1956 by the late Dr Jens J Pindborg. Pindborg tumor is now a universally recognized synonym for this neoplasm. An alternative abbreviation also commonly used is CEOT.
The Pindborg tumor is classified as an uncommon, benign, odontogenic neoplasm that is exclusively epithelial in origin. Some have suggested that the epithelial cells of the Pindborg tumor are reminiscent of the cells in the stratum intermedium layer of the enamel organ in tooth development. Some hypothesize that the Pindborg tumor arises from remnants of the primitive dental lamina found in the initial stage of odontogenesis, and these epithelial rests are the more likely true progenitor cell. The definite etiology of this neoplasm still remains enigmatic.
Clinical Features
A report by Franklin and Pindborg in 1976 shows that 113 cases of this intraosseous tumor have been described in the literature since Pindborg’s original paper. As the number of reported cases continues to increase, we are rapidly improving our knowledge of this lesion.
This tumor occurs most frequently in middle-age. Of the reported cases, the mean age of occurrence at the time of diagnosis was 40 years of age in both men and women, with a range of 8–92 years. There is no significant difference in occurrence between the gender, since 49% of the cases were in men and 51% in women.
There is a predilection for occurrence of the tumor in the mandible over the maxilla by a ratio of 2 : 1, and the prevalence in the molar region is three times that in the bicuspid region, whereas in other sites in the jaws there is a relatively even distribution. If these two respects, i.e. age and site, the Pindborg tumor is very similar to the ameloblastoma.
Most patients with this lesion are asymptomatic and are aware only of a painless swelling. It is significant, however, that 52% of the reported cases have been definitely associated with an unerupted or impacted tooth.
An extraosseous calcifying epithelial odontogenic tumor is also known to occur but is quite rare, with only eight reported cases, according to the review by Wertheimer and his associates in 1977. This extraosseous lesion has had a mean age of occurrence of 35 years and an approximately equal sex distribution. With the exception of one equivocal lesion on the upper lip, all cases have occurred on the gingiva, five mandibular and two maxillary, and almost invariably in the anterior segment (Fig. 4-26). The extraosseous lesion is histologically identical with the intraosseous one.
Radiographic Features
The tumor may show considerable radiographic variation. In some cases, the lesion appears as either a diffuse or a well-circumscribed unilocular radiolucent area, while in other cases there may appear to be a combined pattern of radiolucency and radiopacity with many small, irregular bony trabeculae traversing the radiolucent area in many directions, producing a multilocular or honeycomb pattern. Scattered flecks of calcification throughout the radiolucency have given rise to the descriptive term of a ‘driven snow’ appearance. In some instances, the lesion is totally radiolucent and is in association with an impacted tooth, thus leading to a mistaken clinical diagnosis of dentigerous cyst (Fig. 4-27).
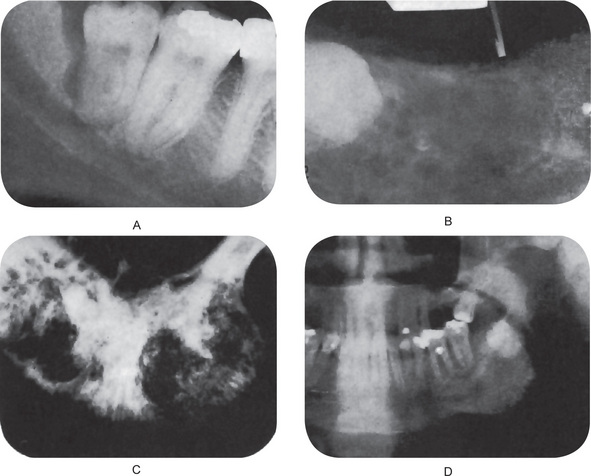
Figure 4-27 Calcifying epithelial odontogenic tumor of Pindborg. Courtesy of Dr Charles Redish, Dr Robert Bresick, Dr Charles Hutton, and Dr Ronald Vincent.
On CT examination, calcifying epithelial odontogenic tumor in the mandible demonstrates expansion and thinning of buccal and lingual cortical bony plates by a well-defined mass containing scattered radiopaque areas of varying size and signal intensity. MRI reveals predominantly a hypointense lesion on T1-weighted images and a mixed hyperintense lesion on T2-weighted images.
Histologic Features
The calcifying epithelial odontogenic tumor is composed of polyhedral epithelial cells, sometimes closely packed in large sheets but other times consisting chiefly of scattered small islands of cells in a bland fibrous connective tissue stroma (Fig. 4-28). Occasionally, the cells are arranged in cords or rows, mimicking adenocarcinoma.
The tumor cells have a well-outlined cell border with a finely granular eosinophilic cytoplasm, and intercellular bridges are often prominent. The nuclei are frequently pleomorphic, with giant nuclei and multinucleation being quite common but mitotic figures rare. The tumor cells in some lesions are characterized by extreme morphologic variation with severe cellular abnormalities, mimicking those often seen in some highly malignant neoplasms, while other cases of the calcifying epithelial odontogenic tumor are composed of very monomorphic, innocuous-appearing tumor cells; yet, to the best of our knowledge, the biologic behavior does not differ between the two.
A well-recognized form of this neoplasm is the clear-cell variant. In this type, the tumor cells exhibit a clear vacuolated cytoplasm rather than an eosinophilic cytoplasm. The nucleus may remain round or oval in the center of the cells or be flattened against the cell membrane. According to Krolls and Pindborg, who have discussed these histomorphologic variations, most of the clear cells are mucicarmine negative, although a few may show a faint tinge. In some tumors, the clear cells comprise the bulk of the tumor cells while, in others, they consist of only a few scattered foci. In as much as a variety of other types of tumors, both primary (e.g. mucoepidermoid carcinoma) and metastatic (e.g. hypernephroma), may exhibit clear cells, great care must be utilized in their interpretation and diagnosis
This tumor has been investigated under the electron microscope by many researchers including Anderson and his coworkers, who have demonstrated that the tumor cells exhibit the features commonly seen in epidermal cells such as intercellular bridges with desmosomes, intracytoplasmic tonofilaments and well-developed hemidesmosomes.
One of the characteristic microscopic features of this tumor is the presence of a homogeneous, eosinophilic substance which has been variously interpreted as amyloid, comparable glycoprotein, basal lamina, keratin or enamel matrix. In at least some instances, this appears to form intracellularly and then is extruded into the extracellular compartment as a result of cell secretion or degeneration. This homogeneous eosinophilic material may be present in large or very limited quantities. In most cases, it stains metachromatically with crystal violet, positively with Congo red, and fluoresces under ultraviolet light with thioflavin T, all in a fashion similar to amyloid (Fig. 4-29). Ultrastructural studies have shown that this amyloid-like material is composed of at least three different types of fibrils, but that they have a smaller size than the fibers of ‘conventional’ amyloid, although this term is a rapidly expanding one. Some forms of amyloid are now suggested to arise from light chain fragments of immunoglobulin molecules, called immunamyloid, while another form is thought to originate from cells of certain endocrine tumors (e.g. medullary carcinoma of the thyroid) which may be derived from the endocrine polypeptide cells of neural crest origin of the amine precursor uptake and decarboxylation (APUD) system, called APUD-amyloid. On the evidence available at present, the exact nature of the amyloid like substance in the CEOT cannot be definitively assessed.
Figure 4-29 Calcifying epithelial odontogenic tumor of Pindborg. Photomicrograph showing apple-green color birefringence of amyloid deposited in fibrous connective tissue stroma (Congo red stain under polarized light source, original magnification x20) (Courtesy of Dr RK Goode).
Another characteristic feature of the Pindborg tumor is the presence of calcification, sometimes in large amounts, and often in the form of Liesegang rings. This calcification actually appears to occur in some instances in globules of the amyloid like material, many of which have coalesced and are transformed from being PAS (periodic acid-Schiff)-negative to PAS-positive during this calcification process. There does not appear to be necessarily a relationship between the amount of amyloid material formed in a given lesion and the amount of calcification occurring.
The source of the epithelial cells comprising this tumor was originally suggested by Pindborg to be the reduced enamel epithelium of the associated unerupted tooth. Today, most investigators believe that the cells originate from the stratum intermedium because of the morphologic similarity of the tumor cells to the normal cells of this layer of the odontogenic apparatus. Unfortunately, this does not explain those cases of tumor apparently occurring without an associated unerupted tooth or those extraosseous cases outside the jaw.
Treatment and Prognosis
There are a variety of alternative surgical treatment methods to successfully manage Pindborg tumors. Small, intrabony lesions with well-defined borders can be treated with enucleation or curettage followed by judicious removal of a thin layer of bone adjacent to the tumor. But some pathologists suggest that maxillary tumors should be treated more aggressively than a similar-sized lesion in the mandible.
Lesions designated recurrences, following conservative approaches like intrabony curettage, may in fact represent persistence of disease. Like ameloblastoma, small infiltrative foci of Pindborg tumor may insinuate along bony trabeculae and appear as uninvolved bone radiographically.
Recurrent or persistent tumors, which over an extended time have become larger and more extensive (greater than 4 cm), would require segmental resections such as partial or hemimandibulectomy or hemimaxillectomy.
Early intervention is advocated for the treatment of histopathologically atypical or frankly malignant calcifying epithelial odontogenic tumors before such a lesion could escape the confines of the involved mandible or maxilla. Radical resection of the affected jaw portion and any associated soft tissues with not less than 1 cm in every direction, as might be performed for any other variant of odontogenic carcinoma is adviced. Adjunctive external beam radiation therapy is advocated following determination of local spread to cervical lymph nodes and adjunctive chemotherapy may play some role in control of distant organ metastasis in some patients. Most studies of Pindborg tumor report a local recurrence rate of between 10 and 20% following conservative but complete removal of the lesion. The incidence of malignant transformation to odontogenic carcinoma ex-Pindborg tumor is so extremely low as to be considered distinctly rare; only three such cases being reported.
Adenomatoid Odontogenic Tumor (Adenoameloblastoma, ameloblastic adenomatoid tumor)
Adenomatoid odontogenic tumor (AOT), generally considered to be an uncommon tumor, occurs mostly in association with an unerupted maxillary cuspid. Some investigators consider it as a benign neoplasm, while others have categorized it as a hamartomatous malformation due to the limited size and to the lack of recurrence of most cases (attributed perhaps to its minimal growth potential). Those who prefer to consider AOT to be a benign neoplasm believe that the limited size of most cases stems from early detection and removal of the lesion. They also point to the considerable size of some reported cases that had gone undetected or untreated for many years and resulted in facial asymmetry and distortion.
Histogenesis
Like all other odontogenic tumors, the specific stimulus that triggers proliferation of the progenitor cells of AOT is unknown. Because of its exclusive occurrence within the tooth-bearing areas of the jaws (most often associated closely with an unerupted or impacted tooth) and its cytologic resemblance to the dental lamina and components of the enamel organ, there is no disagreement that the AOT is of odontogenic origin (Rick GM, 2004).
Clinical Features
The mean age of these patients was approximately 18 years, with a range of 5–53 years. However, 73% of the patient were under 20 years of age. There is a marked predilection for occurrence of the tumor in females — 64% contrasted to 36% developing in males. The site of occurrence is greater in the maxilla (65%) than in the mandible (35%). In contrast to the ameloblastoma, this tumor occurs more frequently in the anterior part of the jaws with 76% developing anterior to the cuspid in the maxilla and mandible. Only very rarely does the lesion occur distal to the premolar area. It is of some interest that in at least 74% of the cases, the tumors were associated with an unerupted tooth, and in over two-thirds of the cases, this tooth was the maxillary or mandibular cuspid.
The vast majority of the lesions measured between 1.5 and 3.0 cm, although large lesions, exceeding 7.0 cm, have been reported. A large proportion of these tumors produced an obvious clinical swelling although they were generally asymptomatic. Five tumors reported in the literature have been extraosseous in their occurrence, according to Swinson.
Giansanti and his coworkers have pointed out that the high percentage of these lesions being associated with unerupted teeth and present as dentigerous cysts would strongly suggest that they are related to some late disturbance in odontogenesis. Since none of the associated teeth were described as morphologically defective, the disturbance must occur after odontogenesis is complete.
Adenomatoid odontogenic tumor may occur within the jaw bones or the gingiva. Peripheral lesions present as a painless, gingival colored mass that ranges from 1–1.5 cm in diameter. They are 10 times more prevalent in the maxillary gingiva than in the mandibular gingiva. The female to male ratio for the gingival lesion is 14: 1 (Philipsen HP et al, 1991).
Adenomatoid odontogenic tumor has to be clinically differentiated from central odontogenic cysts and tumors, benign fibro-osseous lesions and benign mesenchymal neoplasms. Gingival lesions cannot be differentiated clinically from gingival fibromas, peripheral cemento-ossifying fibromas, peripheral giant cell lesions, or from other peripheral odontogenic tumors, such as odontogenic fibroma, ameloblastoma, calcifying odontogenic cyst, and calcifying epithelial odontogenic tumor.
Radiographic Features
Central AOTs present as well-demarcated, almost always unilocular radiolucency that generally exhibit a smooth corticated (and sometimes sclerotic) border.
Most lesions are pericoronal or juxtacoronal but the radiolucency may extend apically beyond the cementoenamel junction on at least one side of the root. Rare, multilocular cases have been reported and a scalloped border is observed occasionally. Most cases are between 1 and 3 cm in greatest diameter. About 65% of reported cases also demonstrate faintly detectable radiopaque foci within the radiolucent lesion. Occasionally, a more obvious intralesional radiopacity may be identified, usually eccentrically positioned within the lesion. Divergence of roots and displacement of teeth occurs more frequently than root resorption. Orbital and maxillary sinus encroachment have been reported. Gingival lesions may cause slight erosion of the underlying alveolar bone cortex.
Histologic Features
Central AOTs macroscopically appears as a soft, roughly spherical mass with a distinct fibrous capsule. Upon gross sectioning, the tumor may exhibit white to tan, solid to crumbly tissue or one or more cystic spaces of varying sizes with yellowish brown fluid or semisolid material; fine, hard ‘gritty’ granular material; and one to several larger calcified masses. Additionally, intact specimens demonstrate the crown of an embedded tooth in the solid tumor mass or projecting into a cystic cavity.
The AOT exhibits diverse histomorphologic features. The tumor is made up of a multinodular proliferation of spindle, cuboidal, and columnar cells in a variety of patterns comprising of scattered duct like structures, eosinophilic material, and calcifications in several forms; delimited by a fibrous capsule of variable thickness. Cytologic atypia is never a prominent feature.
Although not present in all tumors, the most distinctive microscopic feature of AOT is varying numbers of duct like structures with lumina of varying size that are lined by a single layer of cuboidal to columnar epithelial cells that have nuclei that frequently are polarized away from the lumen. These duct like or microcyst lumina frequently are lined by an eosinophilic rim of varying thickness (the so-called hyaline ring).
The stellate reticulum like spindle cells, and occasionally round or polygonal epithelial cells dominate the tissue between the cell-rich nodules. Small amounts of eosinophilic material or calcifications also may be present between these cells. Anastomosing strands of basaloid epithelial cells which resemble cell rests of the dental lamina, are arranged in a plexiform, trabecular, cribriform, or lattice like configuration. They occasionally extend between the cell-rich nodules and usually are present in the peripheral subcapsular area of most tumors. Many AOTs contain a few clusters of well defined calcifying epithelial odontogenic tumor like foci with eosinophilic polyhedral squamous epithelial cells and prominent intercellular bridges, and occasional mild nuclear pleomorphism (Fig. 4-30).
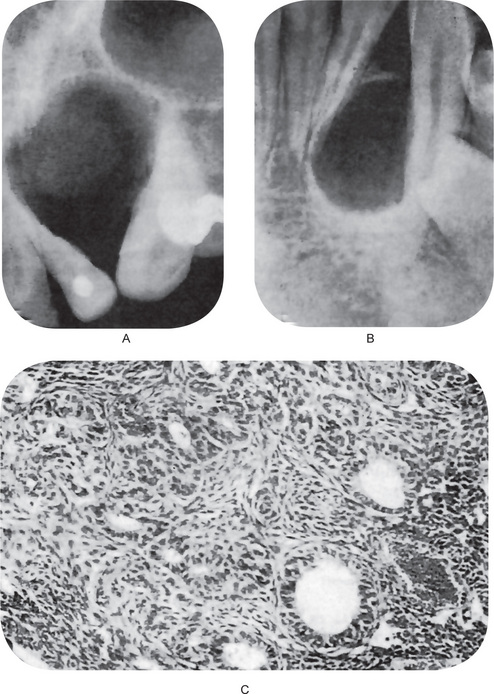
Figure 4-30 Adenomatoid odontogenic tumor. The tumor in (A) radiographically resembled a globulomaxillary cyst. The tumor in (B) was associated with an impacted tooth and superficially resembled a dentigenous cyst. The photomicrograph (C) shows the typical duct like structures (Courtesy of Dr Charles Redish and Dr Charles A Waldron).
A considerable number of AOTs demonstrate cystic component. It is not clear whether this represents pooling of the mucoid stroma or if the tumor developed within or adjacent to a preexisting cyst. Although the cyst lining may occasionally resemble that seen in dentigerous cysts, it more often is similar to the basaloid cells that form the plexiform pattern that was described above. Some tumors exhibit pools of finely fibrillar eosinophilic material at the epithelial–connective tissue interface; this was immunoreactive for the basement membrane component laminin. Some AOTs contain varying amounts of dysplastic dentin, dentinoid, and osteodentin. Irregular to round calcified bodies which may exhibit areas with a concentric layered pattern (Liesegang rings) may be seen in parenchymal or stromal zones. The supporting stroma is loose, hypocellular, and fibrovascular that may show a prominent vascular component.
Squamous Odontogenic Tumor (Benign epithelial odontogenic tumor)
The squamous odontogenic tumor is a lesion which had been recognized as an apparent entity for a number of years but had not been named or reported until 1975, when Pullon and a small group of other oral pathologists combined their material and published six cases. Several additional cases have been reported since the original study, including five further cases by Goldblatt and his colleagues, who also reviewed the literature and commented further on this lesion.
The most important aspect of this lesion is its mistaken histologic identification as an acanthomatous ameloblastoma or as a well-differentiated epidermoid carcinoma. Most investigators believe that it represents a benign odontogenic neoplasm, probably arising from rests of Malassez, although a hamartomatous epithelial proliferation has also been considered.
The histogenesis of squamous odontogenic tumor may be varied. Rests of Malassez are the source of the epithelial proliferation for lesions that are associated with the alveolar process adjacent to the lateral root surface of the teeth, and dental lamina may be the source for lesions that developed in association with the crowns of unerupted or impacted teeth. Surface stratified squamous epithelium and rests of Serres have been cited as the sources of the extraosseous variant.
Clinical Features
The age at discovery of the 16 cases evaluated by Goldblatt and his coworkers ranged from 11 to 67 years with 10 cases being between 19 and 31 years of age. There were six males and 10 females in the series.
There is a slight male preponderance, and the mandible is commonly involved. In the maxilla, lesions centered around the incisor-cuspid area, whereas in the mandible, lesions had a predilection for the bicuspid-molar area. However, several cases exhibited multiple site involvement, including both maxillary and mandibular involvement in the same patient.
The lesions were often asymptomatic but presenting manifestations included mobility of involved teeth, pain, tenderness to percussion, and occasionally, abnormal sensations.
Radiographic Features
There are no radiographic features sufficiently characteristic to suggest the diagnosis of this condition. It presents as a semicircular or roughly triangular radiolucent area, with or without a sclerotic border, usually in association with the cervical portion of the tooth root (Fig. 4-31 A, B).
Histologic Features
The squamous odontogenic tumor is composed entirely of islands of mature squamous epitheliumwithout a peripheral palisaded or polarized columnar layer (Fig. 4-31 C, D). This peripheral layer is usually quite flattened or at least cuboidal. The squamous cells are very uniform and exhibit no pleomorphism, nuclear hyperchromatism or mitotic activity. Occasionally, individual cell keratinization is present but no epithelial pearls. Intercellular bridges are usually seen with no difficulty. Three other variable findings are microcyst formation involving only small portions of the epithelial islands, laminar calcifications in the epithelium and globular, hyalin, eosinophilic structures within the islands, which are not amyloid. The fibrous stroma of the tumor is simply mature bundles of collagen fibers and is devoid of any peri-insular inductive effect.
Squamous odontogenic tumor like proliferations have been reported by Wright in the walls of odontogenic cysts, such as dentigerous or apical periodontal cysts. These proliferations may appear histologically nearly identical with those in the squamous odontogenic tumor, but they do not appear to cause any alteration in the usual biologic behavior of the cyst and do not appear to develop into the tumor. Care must be used, however, in differentiating between these proliferations and the tumor itself.
Although squamous odontogenic tumor exhibits a unique microscopic picture, it may be confused with other conditions, including ameloblastoma and squamous cell carcinoma. The acanthomatous and desmoplastic variants of ameloblastoma have been misdiagnosed as squamous odontogenic tumor. Both variants exhibit squamous differentiation within the tumor islands, but there is demonstrable ameloblastic change of the peripheral cells, including columnar shape, polarization of elongated nuclei away from the basement membrane, and a vacuolated or clear cytoplasm. These changes may be less evident in the desmoplastic variant but can be found on careful and thorough examination of the specimen. These changes are not seen in the squamous odontogenic tumor in which the peripheral cell layer is composed of flat to cuboidal cells. The islands and strands of desmoplastic ameloblastoma are often thin and compressed rather than rounded and broad-based, as seen in squamous odontogenic tumor.
Squamous odontogenic tumor may be confused with well-differentiated squamous cell carcinoma; however, the islands in squamous odontogenic tumor are well defined, and the cells lack variation in cell size, shape and nuclear staining and mitotic figures that are characteristically seen in squamous cell carcinoma. Significant keratin formation also is not typical.
Occasionally, dentigerous and apical periodontal (radicular) cysts and periodontal granulation tissue exhibit foci of squamous odontogenic tumor like proliferation. This has been interpreted as a nonneoplastic, reactive process that is secondary to the cyst formation or inflammation. Foci of squamous odontogenic tumor within the connective tissue wall of odontogenic cysts do not seem to alter the prognosis of the primary cystic process.
 . Inflammatory Cysts
. Inflammatory Cysts