Odontogenic epithelium with odontogenic ectomesenchyme with or without hard tissue formation
Ameloblastic Fibroma (Soft mixed odontogenic tumor, soft mixed odontoma, fibroadamantoblastoma)
The ameloblastic fibroma is a relatively uncommon neoplasm of odontogenic origin which is characterized by the simultaneous proliferation of both epithelial and mesenchymal tissue without the formation of enamel or dentin. Thus this may be viewed as an example of a true mixed tumor.
Some investigators have suggested; however, that the ameloblastic fibroma represents an immature complex odontoma and that, if the tumor is left undisturbed, it will ultimately differentiate or mature further into a lesion known as an ameloblastic fibro-odontoma (q.v.) and then continue maturation into a completely differentiated odontoma. In contrast, Eversole and his coworkers, in a discussion of the histogenesis of odontogenic tumors, have proposed that the mixed odontogenic tumors are solely and totally dependent upon the presence of differentiation factors which are or are not elaborated by a particular tumor. Thus, they have concluded that there is little probability of sequential differentiating events resulting in the progression of an immature entity such as the ameloblastic fibroma into a highly differentiated entity such as the complex odontoma. As Eversole and his associates discussed, and as Slootweg has emphasized, if these three lesions—the ameloblastic fibroma, the ameloblastic fibro-odontoma and the odontoma—were simply stages in a continuum, clinical data on each of the lesions should support this. For example, the ameloblastic fibroma should occur in younger patients, the odontoma in somewhat older patients and the ameloblastic fibro-odontoma in an intermediate age group. In addition, the gender predilection and distribution of all lesions should be the same. Slootweg has investigated this problem utilizing data from 55 reported cases of ameloblastic fibroma, 50 cases of ameloblastic fibro-odontoma, 77 cases of complex odontoma and 48 cases of compound odontoma. While correction had to be made for the fact that the odontogenic apparatus is active in various parts of the jaws at various ages, Slootweg concluded that the ameloblastic fibroma represents a separate specific neoplastic entity that does not develop into a more differentiated odontogenic lesion. He also concluded from his data that the ameloblastic fibro-odontoma does represent an immature complex odontoma, a hamartomatous rather than neoplastic odontogenic lesion.
Clinical Features
The ameloblastic fibroma, arising most commonly in the molar region of the mandible, is similar in location to the simple ameloblastoma. Nearly 75% of the 55 cases reviewed by Slootweg occurred in this location. There is a considerable difference, however, in the age group of patients most commonly affected. Whereas the simple ameloblastoma occurs typically in middle-aged persons, the average age of the patient at the time of discovery being approximately 33 years according to Small and Waldron, the ameloblastic fibroma occurs in much younger persons. In the review of 55 cases, Slootweg found that the average age of patients with the ameloblastic fibroma was 14.6 years, with 40% of the patients under the age of 10 years. He also reported that there was a very slight predilection for occurrence in males.
This tumor exhibits some what slower clinical growth than the simple ameloblastoma and does not tend to infiltrate between trabeculae of bone. Instead it enlarges by gradual expansion so that the periphery of the lesion often remains smooth. It will frequently cause no complaint on the part of the patient and has been discovered accidentally during radiographic examination. Pain, tenderness or mild swelling of the jaw may induce the I patient to seek aid from the dentist, however.
Radiographic Features
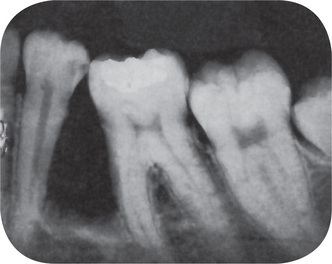
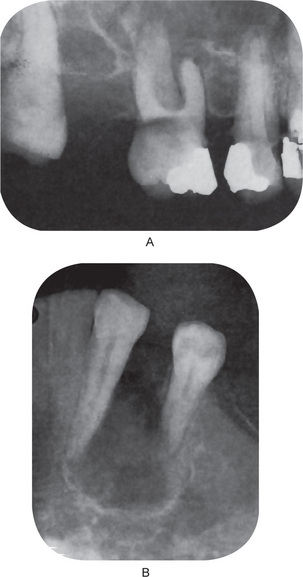
No constant significant differences between the appearance of the simple ameloblastoma and that of the ameloblastic fibroma are found. The later is manifested as a unilocular or multilocular radiolucent lesion which has a rather smooth outline, often with a sclerotic border, and which may or may not produce evident bulging of bone (Fig. 4-32). In the study of 24 cases by Trodahl, most of the lesions were associated with unerupted teeth. In addition, he found considerable variation radiographically in the size of lesions, there being a range of 1–8.5 cm in diameter.
Histologic Features
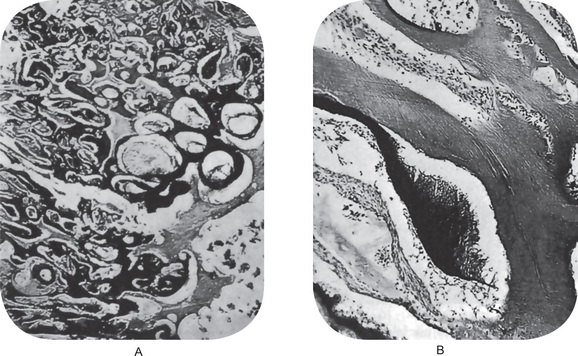
The microscopic appearance of this odontogenic neoplasm is characteristic. The ectodermal portion consists of scattered islands of epithelial cells in a variety of patterns, including rosettes, long finger like strands, nest and cords. These epithelial cells are usually of a cuboidal or columnar type and bear close resemblance to primitive odontogenic epithelium. Mitotic activity is not common. Because the pattern of these cells is one of strands and cords, tissue resembling stellate reticulum is often not seen. However, occasional cases occur in which some of these tumor islands are found ‘opening,’ with the formation of stellate reticulum. The resemblance to the dental lamina is far more striking in this lesion than in the simple ameloblastoma (Fig. 4-33 A, B).
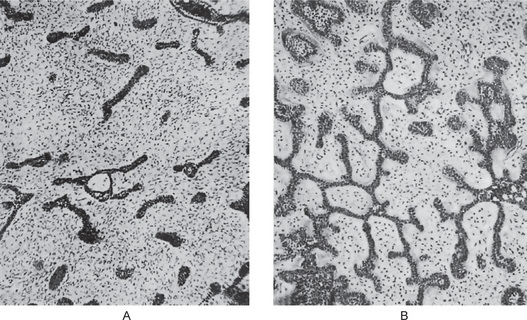
Figure 4-33 Ameloblastic fibroma. Within a stroma of primitive mesoderm there are interlacing thin strands of odontogenic epithelium, from which budding outgrowths arise. The epithelial buds have a peripherally placed row of more columnar cells and centrally placed more stellate cells.The septae of fibrous tissue may give the tumor a lobular pattern.
The mesenchymal component is made up of a primitive connective tissue that, in some cases, shows closely intertwining fibrils interspersed by large connective tissue cells closely resembling those of the dental papilla. There may be a paucity of blood vessels, and juxtaepithelial hyalinization of areas of the connective tissue occurs. On occasion, this may even resemble dysplastic dentin. Electron microscopic studies have suggested that this apparent hyalinization may, instead, actually represent exuberant basal lamina.
Treatment and Prognosis
The treatment of the ameloblastic fibroma has been somewhat more conservative than that of the simple ameloblastoma, since it does not appear to infiltrate bone as actively or as widely as the ameloblastoma. It also tends to separate from the bone more readily.
At one time the ameloblastic fibroma was regarded as exhibiting little tendency for recurrence, even following the most conservative surgical removal. This was based on part on the review by Gorlin and his associates of 35 documented cases with only two instances of recurrence (approximately 6%). In contrast, Trodahl subsequently reported 10 recurrences in his series of 24 cases (approximately 44%). Since these two series, numerous other instances of recurrent lesions have been reported, and these have been reviewed and discussed by Zallen and his colleagues, who noted a total cumulative recurrence rate of 18.3% for this lesion. In addition, there have been a surprising number of cases reported of ameloblastic fibrosarcoma originating, at least in some instances, in a recurrent ameloblastic fibroma, as in the cases of Leider and his associates. Thus, it would seem that a somewhat more aggressive surgical removal, rather than simple curettage, should be considered for this lesion.
Ameloblastic Fibro-odontoma
The ameloblastic fibro-odontoma (AFO) has been confused in the earlier literature with a lesion of similar nomenclature, the ameloblastic odontoma (q.v.), although a clear distinction between the two was finally drawn by Hooker in 1967 at the annual meeting of the American Academy of Oral Pathology. It has been emphasized that some investigators, furthermore, have believed that the ameloblastic fibroma (q.v.) represents an early, undifferentiated complex odontoma and that the ameloblastic fibro-odontoma is simply a further differentiated maturing odontoma. After investigating this problem, Slootweg concluded that the ameloblastic fibroma is a true neoplasm and does not differentiate into an ameloblastic fibro-odontoma. This evidence has been discussed in the section on ameloblastic fibroma. He also concluded that the ameloblastic fibroodontoma to be discussed here did, in fact, represent an immature complex odontoma, and therefore, represented a hamartomatous rather than a neoplastic odontogenic lesion.
Clinical Features
The original report by Hooker consisted of 26 cases of ameloblastic fibro-odontoma. Of these 26 cases, there were 20 male and six female for a ratio of 3.3 : 1 in favor of the males. However, of the 50 cases reviewed by Slootweg, which did not include the 26 cases of Hooker, there were 28 males and 22 females. The age range of the cases in the series of Hooker was 0.5–39 years with the mean being 11.5 years of age. Nineteen of the 26 cases occurred under the age of 15, only two cases over the age of 20. In the Slootweg series, the mean age was 8.1 years, with 62% of the patients under the age of 10 years. Hooker’s cases were evenly divided between the maxilla and mandible with 13 each. Nine of the 13 cases in the maxilla, and 10 of the 13 in the mandible occurred in the molar area. All cases were associated with an impacted tooth and three cases involved the maxillary sinus. Of Slootweg’s cases, 38% were in the maxilla and 62% in the mandible, with 54% occurring in the posterior mandible.
Clinical manifestations of the lesion are often absent. The two most common presenting complaints are swelling and failure to tooth eruption.
Radiographic Features
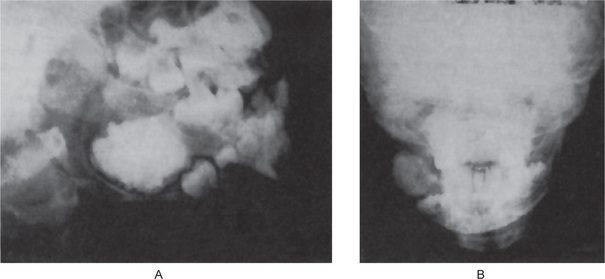
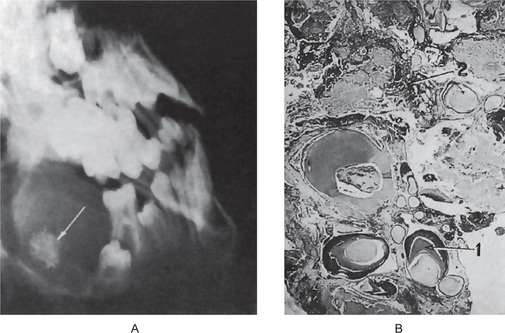
The ameloblastic fibro-odontoma is almost invariably a well-circumscribed lesion, presenting as an expansile radiolucency generally containing either a solitary radiopaque mass or multiple small opacities representing the odontoma portion of the lesion (Fig. 4-34). Some of the lesions are relatively small when first detected, measuring not over 1–2 cm in diameter, while others may be exceedingly large, involving a considerable portion of the body of the mandible and even extending into the ramus. Occasionally, these lesions will become huge. A case was illustrated by Miller and his associates in their series that showed the calcified mass alone to measure 6 cm by 7 cm and that had produced a terrible facial deformity.
Histologic Features
The lesion consists of the same apparent type of tissue as seen in the ameloblastic fibroma. Thus, cords, fingers, strands and rosettes of primitive odontogenic columnar or cuboidal epithelial cells, often resembling dental lamina, are found. The mesenchymal component is an embryonic fibrous connective tissue with delicate fibrils interspersed by large primitive fibroblasts, all resembling dental papilla. In addition, typical composite odontoma is found (Fig. 4-35 A, B).
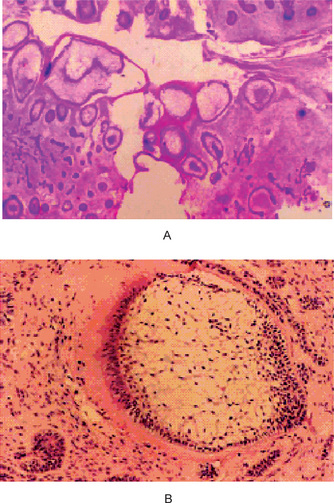
Figure 4-35 Ameloblastic fibro-odontoma. (A) Ameloblastic fibro-odontoma exhibiting rudimentary tooth buds (H&E stain, original magnification x10). (B) AFO exhibiting dentinoid material around odontogenic epithelial island resembling a developing tooth bud (H&E stain, original magnification x 40) (Courtesy of Dr DM Cohen).
Treatment and Prognosis
The ameloblastic fibro-odontoma is treated by curettage, since it does not appear to locally invade bone. There appears to be little tendency for recurrence of the ameloblastic fibro-odontoma. Tsagaris reviewed a total of 77 cases, including Hooker’s 26 cases from the Armed Forced Institute of athology, and of 29 cases which he was able to follow up, found only one recurrence.
Odontoma
The term 'odontoma', by definition alone, refers to any tumor of odontogenic origin. Through usage, however, it has come to mean a growth in which both the epithelial and the mesenchymal cells exhibit complete differentiation, with the result that functional ameloblasts and odontoblasts form enamel and dentin. This enamel and dentin are usually laid down in an abnormal pattern because the organization of the odontogenic cells fails to reach a normal state of morphodifferentiation. Most authorities accept the view today that the odontoma represents a hamartomatous malformation rather than a neoplasm. This lesion is composed of more than one type of tissue, and for this reason, has been called a composite odontoma. In some composite odontomas the enamel and dentin are laid down in such a fashion that the structures bear considerable anatomic resemblance to normal teeth, except that they are often smaller than typical teeth. They have been termed compound composite odontomas when there is at least superficial anatomic similarity to normal teeth. On the other hand, when the calcified dental tissues are simply an irregular mass bearing no morphologic similarity even to rudimentary teeth, the term complex composite odontoma is used. The complex form of odontoma is less common than the compound type.
Etiology
The etiology of the odontoma is unknown. It has been suggested that local trauma or infection may lead to the production of such a lesion. This is entirely possible, but it would appear more likely that in such an event hypoplasia would be the end-result, depending upon the stage of odontogenesis. There is no seeming predilection for occurrence of the odontoma in particular sites of the oral cavity; it does not appear to be associated especially with supernumerary teeth, as might be suggested were it to occur with great frequency between the maxillary central incisors or distal to the maxillary third molar.
It has been suggested by Hitchin that odontomas are either inherited or are due to a mutant gene or interference, possibly postnatal, with the genetic control of tooth development. On the other hand, Levy has reported the experimental production of this lesion in the rat by traumatic injury.
Clinical Features
The odontoma may be discovered at any age in any location of this dental arch, maxillary or mandibular. Budnick has compiled an analysis of 149 cases of odontoma (76 complex and 73 compound) from the literature (65 cases) and from the files of Emory University (84 cases). While it may be discovered at any age, from the very young to the very elderly, he found the mean age of detection to be 14.8 years, with the most prevalent age for diagnosis and treatment being the second decade of life. He also found a slight predilection for occurrence in males (59%) compared with females (41%).
Of all odontomas combined, 67% occurred in the maxilla and 33% in the mandible. The compound odontoma had a predilection, in this study, for the anterior maxilla (61%), whereas only 34% of complex odontomas occurred here. In general, complex odontomas had a predilection for the posterior jaws (59%) and lastly the premolar area (7%). Interestingly, both types of odontomas occurred more frequently on the right side of the jaw than on the left (compound, 62%; complex 68%). The odontoma usually remains small, the diameter of the mass only occasionally greatly exceeding that of a tooth. Occasionally, it does become large and may produce expansion of bone with consequent facial asymmetry. This is particularly true if a dentigerous cyst develops around the odontoma.
Most odontomas are asymptomatic, although occasionally signs and symptoms relating to their presence do occur. These generally consist of unerupted or impacted teeth, retained deciduous teeth, swelling and evidence of infection.
Radiographic Features
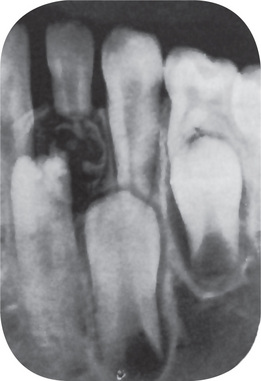
The radiographic appearance of the odontoma is characteristic. Since most odontomas are clinically asymptomatic and are discovered by routine radio-graphic examination, the dentist should be familiar with their appearance. They are often situated between the roots of teeth and appear either as an irregular mass of calcified material surrounded by a narrow radiolucent band with a smooth outer periphery (Fig. 4-36), or as a variable number of tooth like structures with the same peripheral outline. This latter type of odontoma may contain only a few structures resembling teeth, or it may contain several dozen (Fig. 4-36). Both forms of odontoma are frequently associated with unerupted teeth. It is of interest that the majority of odontomas in the anterior segments of the jaws are compound composite in type, while the majority in the posterior areas are complex composite. This latter odontoma may appear also as a calcified mass overlying the crown of an unerupted or impacted tooth. A devel-oping odontoma may be discovered on the routine radiograph and present difficulty in diagnosis because of the lack of calcification (Fig. 4-37).
Histologic Features
The histologic appearance of the odontoma is not spectacular. One finds normal-appearing enamel or enamel matrix, dentin, pulp tissue and cementum which may or may not exhibit a normal relation to one another (Fig. 4-38). If the morphologic resemblance to teeth does exist, the structures are usually single-rooted. The connective tissue capsule around the odontoma is similar in all respects to the follicle surrounding a normal tooth.
One additional interesting feature is the presence of ‘ghost’ cells in odontomas. These are the same cells described in the calcifying odontogenic cyst (q.v.) and have been reported by Levy to be present in nearly 20% of a series of 43 odontomas which he investigated. This has been substantiated by Sedano and Pindborg, although they could find no significance attached to the presence of these cells regarding prognosis or treatment of the odontoma.
Treatment and Prognosis
The treatment of the odontoma is surgical removal, and there is no expectancy of recurrence. Since both the ameloblastic odontoma and the ameloblastic fibro-odontoma bear great resemblance to the common odontoma, particularly on the radiograph, it is suggested that all odontomas be sent to a qualified oral pathologist for microscopic examination.
Ameloblastic Odontoma (Odontoameloblastoma)
The ameloblastic odontoma is an odontogenic neoplasm characterized by the simultaneous occurrence of an ameloblastoma and a composite odontoma. As judged by the reported cases reviewed by Frissell and Shafer, it is a rare clinical entity. It should not be inferred that this tumor represents two separate neoplasms growing in unison; there exists, rather, a peculiar proliferation of tissue of the odontogenic apparatus in an unrestrained pattern, including complete morphodifferentiation, as well as apposition and even calcification. The lesion is unusual in that a relatively undifferentiated neoplastic tissue is associated with a highly differentiated tissue, both of which may show recurrence after inadequate removal.
Clinical Features
So few cases of this tumor have been reported that any statistical information may not be valid. However, the ameloblastic odontoma appears to occur at any age, but more frequently in children, and is somewhat more common in the mandible than in the maxilla. It is a slowly expanding lesion of bone which produces considerable facial deformity or asymmetry if left untreated. Since it is a central lesion, considerable destruction of bone occurs. Mild pain may be a presenting complaint, as well as delayed eruption of teeth.
Radiographic Features
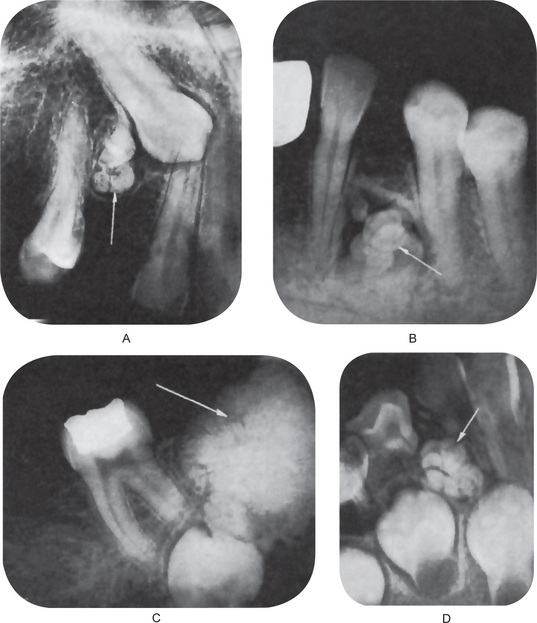
Central destruction of bone with expansion of the cortical plates is prominent. The character-istic feature is the presence within the lesion proper of numerous small radiopaque masses which may or may not bear resemblance to formed, albeit miniature, teeth (Fig. 4-39 A). In other instances there is only a single, irregular radiopaque mass of calcified tissue present. Thus the radiographic appearance of the ameloblastic odontoma is identical with that of the composite odontoma of one form or another.
Histologic Features
The microscopic appearance of this tumor is both unusual and characteristic. It consists of a great variety of cells and tissue in a complex distribution, including columnar, squamous and undifferentiated epithelial cells, as well as ameloblasts, enamel and enamel matrix, dentin, osteodentin, dentinoid and osteoid material, stellate reticulum-like tissue, dental papilla, bone and cementum as well as stromal connective tissue (Fig. 4-39 B). Many structures resembling normal or atypical tooth germs may be found, with or with-out the presence of calcified dental tissues. In addition, an outstanding characteristic is the presence of sheets of typical ameloblastoma of one or another of the recognized types — usually basal cell, follicular or plexiform. Few mitotic figures are present even though the proliferative tendencies of the epithelial cells are obvious.
Treatment and Prognosis
The treatment of the ameloblastic odontoma is controversial, probably because there are few published cases. Some investigators believe that recurrence, associated with continued destruction of tissue, is common after conservative curettage or enucleation and that a more radical approach is necessary. Resection of the jaw, if possible preserving the inferior border of the mandible when this area is uninvolved, will certainly result in a permanent cure. The general behavior of this lesion is the same as that of the ameloblastoma component, and therefore, the same philosophy of management would also apply here as for the ameloblastoma.
Calcifying Odontogenic Cyst and Dentinogenic Ghost Cell Tumor
The calcifying odontogenic cyst was first described as a distinct clinicopathologic entity by Gorlin and his colleagues in 1962. Gold (1963) chose a similar, but not identical term for the lesion, namely ‘keratinizing and calcifying odontogenic cyst’. Perhaps the Gorlin-Gold cyst would be a fitting term for this interesting odontogenic lesion (Tomich CE, 2004). Kurt Thoma and Henry Goldman considered the lesions to be odontogenic tumors of ectodermal and mesodermal origin. They were not far from being correct. All of the investigators noted the unusual ‘ghost cell’ keratinization of the odontogenic epithelium which is the striking histologic feature of the entity. Gorlin and his associates suggested that the calcifying odontogenic cyst might be the oral analog of the ‘calcifying epithelioma of Malherbe’ (now preferably termed a ‘pilomatricoma’); a well-recognized lesion of skin. The lesions have in common the peculiar abnormal keratinization of odontogenic and metrical (hair) epithelial cells that is termed ‘ghost cell’ or ‘shadow cell’ keratinization. Actually, the calcifying odontogenic cyst shares more histologic features with the rare, intracranial neoplasm known as ‘craniopharyngioma.’
As more and more cases were studied, it became apparent that the lesion was not always a cyst; sometimes it was a solid lesion. Furthermore, it became apparent that the lesion could present centrally within the jaws or as an extraosseous gingival lesion. This raises the question: ‘‘Is the Gorlin cyst a cyst or is it a tumor that is frequently cystic?’’ In view of these findings, investigators suggested different names for this lesion. Fejerskov and Krogh suggested the term ‘calcifying ghost cell odontogenic tumor’ and Freedman and his associates suggested the name ‘cystic calcifying odontogenic tumor’. Neither of these terms met with approval, possibly because of their similarity to the well-recognized lesion called the ‘calcifying epithelial odontogenic tumor’ or Pindborg tumor. The issue of ‘cyst versus tumor’ or ‘cystic versus solid’ has not been resolved totally. The calcifying odontogenic cyst can be classified mainly into two type:
A third malignant counterpart of the neoplastic lesion may be added.
Clinical Features
The calcifying odontogenic cyst is not a common lesion; the dentinogenic ghost cell tumor is even less common and should be considered rare. Despite the uncommon occurrence of the calcifying odontogenic cyst and the rare dentinogenic ghost cell tumor, the former has gained wide recognition and a large series of cases have been reported in the literature. Although the calcifying odontogenic cyst may be seen in any decade of life, studies have shown predilection for persons in the second and third decades. The cyst occurs in both genders with almost equal frequency. Buchner’s extensive review of 215 cases included 110 women and 105 men. The lesion has been seen in many racial groups. Buchner reported that in Asians there was a predilection for the maxilla, whereas in whites there was a 62% predisposition in the mandible.
Radiographic Features
The calcifying odontogenic cyst that occurs centrally in the jaws may present as a painless expansile lesion or it is discovered frequently on routine radiographic examination. The lesions may present as radiolucencies or radiolucencies with foci of opacification, particularly in the case of those that are associated with odontomas. Expansion may be evident in some cases. The dentinogenic ghost cell tumor is known to present as an expansile radiopacity. The lesions that occur peripherally (extraosseous) on the gingiva present as painless swellings or nodules. There are no pathognomonic clinical features that are diagnostic of the lesion.
Histologic Features
The main histopathologic criteria for the diagnosis of the calcifying odontogenic cyst are well established. The cyst lining should show proliferation to the point that it resembles ameloblastoma (i.e. columnar cells over which are stellate and spindled cells in an arrangement that suggest stellate reticulum). Within this proliferation of epithelium, cells undergo the characteristic ‘ghost cell’ keratiniza-tion. The calcifying odontogenic cyst is often encountered in association with an odontoma which may be identified in jux-taposition to the proliferative lining epithelium or intermixed with the ghost cells. This has been called ‘dentinoid’. When this material is formed in abundance and the lesion is ‘solid’ rather than ‘cystic’, the lesion may be termed a ‘dentinogenic ghost cell tumor’. Dystrophic calcification of the ghost cells may be seen; however, it is one of the less common and least important of the histologic features (Fig. 4-40). The presence of ghost cells within the proliferative odontogenic epithelium is the essential characteristic for the diagnosis. The presence of ghost cell keratinization alone is not sufficient enough for the diagnosis nor is it pathognomonic. Ghost cell keratinization may be observed in odontomas, ameloblastomas, ameloblastic fibro-odontomas, and in ameloblastic odontomas. Thus, one must observe ghost cells to make the diagnosis of the Gorlin cyst (Fig. 4-41).
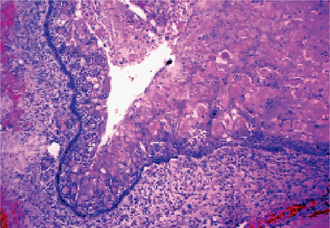
Figure 4-40 Calcifying odontogenic cyst. This calcifying odontogenic cyst shows the proliferation of the lining epithelium with palisaded, hyperchromatic columnar nuclei, stellate reticulum like area, and sheets of ghost cells (100x H&E stain) (Courtesy of Dr CE Tomich).
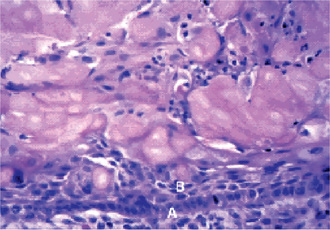
Figure 4-41 Dentinogenic ghost cell tumor. Aggregates of ghost cells are seen in association with odontogenic epithelium. Low columnar cells (A) and spindle cells (B) are seen in association with groups of ghost cells (45 X, H&E stain) (Courtesy of Dr CE Tomich).
The ghost cells contained nuclear remnants, remnants of cytoplasmic organelles, and numerous tonofilaments. The ghost cells differ from normal keratotic squames in that they are larger, often vacuolated, and the remnants of the nuclear membranes are more prominent. This may be due to intracellular edema and the presence of dilated degenerated membranous organelles.
Although Gorlin and his coworkers suggested that the calcifying odontogenic cyst is the oral counterpart of the cutaneous calcifying epithelioma of Malherbe (pilomatricoma), it is more likely to be the oral counterpart of the intracranial craniopharyngioma due to immunologic similarity. Both lesions demonstrated similar immunoreactivity to low and high molecular weight cytokeratins and involucrin—a protein that is characteristic of terminally-differentiated keratinocytes.
Treatment and Prognosis
The calcifying odontogenic cyst, with or without an associated odontoma, can be treated successfully by enucleation or thorough curettage. Peripheral lesions should be treated by conservative excision. The central (intraosseous) dentinogenic ghost cell tumor is treated by surgical excision. The removal of the tumor may require block excision or segmental resection, depending upon its size or anatomic extent. Recurrence following conservative treatment has been reported.
A rare malignant counterpart is recognized which was first reported in the Spanish language literature in 1965 by Astacio and Martinez. This tumor has been known by a variety of terms, including malignant calcifying odontogenic cyst, odontogenic ghost cell carcinoma, and aggressive epithelial ghost cell odontogenic tumor, to name a few. The designation odontogenic ghost cell carcinoma seems to be descriptive and specific. These tumors are unusual and rare lesions which develop in previously diagnosed calcifying odontogenic cysts or as de novo tumors. They are capable of locally aggressive behavior as well as distant metastasis.
Odontogenic ectomesenchyme with or without included odontogenic epithelium
Peripheral Odontogenic Fibroma
There are a variety of different types of focal proliferative lesions which may occur on the gingiva, some neoplastic but others inflammatory, including such entities as the peripheral giant cell granuloma, pyogenic granuloma, giant cell fibroma, the simple fibroma, and the peripheral ossifying fibroma (q.v.). At one time, the terms ‘peripheral ossifying fibroma’ and ‘peripheral odontogenic fibroma’ were used quite interchangeably for one of these lesions. However, WHO, in its classification of odontogenic tumors, has used the term ‘peripheral odontogenic fibroma’ for a specific entity, quite separate from the peripheral ossifying fibroma, and designated specifically in the past also as an ‘odontogenic gingival epithelial hamartoma’ by Baden and his coworkers and as a ‘peripheral ameloblastic fibrodentinoma’ by numerous oral pathologists such as McKelvy and Cherrick.
The peripheral ossifying fibroma has been separated here from the peripheral odontogenic fibroma and is discussed in Chapter 2 in the Benign Connective Tissue Tumors section. The peripheral odontogenic fibroma, sometimes characterized as the ‘WHO type’, is considered here as an odontogenic tumor. An attempt at clarifying the distinction between these two conditions has been published recently by Gardner.
Clinical Features
The peripheral odontogenic fibroma, in distinct contrast to the peripheral ossifying fibroma, is a rare lesion. The largest series of cases reported has been that of Farman, who found only five cases in an extensive review of the English-language literature and added 10 new cases. There was no gender predilection in the 15 reported cases, while the ages of the patients ranged from five to 65 years, spaced throughout the various decades. There did seem to be a predilection for occurrence in the mandible, which was the site of 11 cases compared with only four cases in the maxilla.
The lesions appear to be slow growing, often present for a number of years. They are generally described as a solid, firmly attached gingival mass, sometimes arising, between teeth and sometimes displacing teeth (Fig. 4-42 A). Some lesions are found to contain a calcified stalk at the time of I surgery and this, or other islands of calcified material, may be seen as radiopaque flecks on the radiograph.
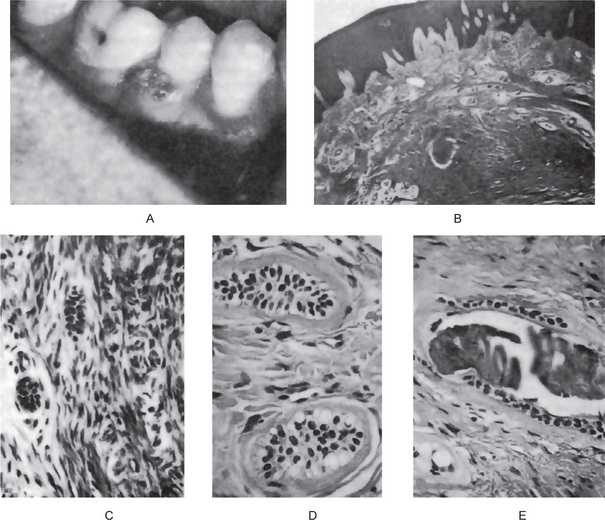
Figure 4-42 Peripheral odontogenic fibroma. The mass on the gingiva (A) is characterized histologically by a cellular connective tissue containing islands of odontogenic epithelial cells, some of which show a clear vacuolated cytoplasm, and few foci of calcifications (B, C, D, E). (A, Courtesy of Dr Richard Henry; D and E, Courtesy of Dr Howard Goldstein).
Histologic Features
The peripheral odontogenic fibroma consists of a markedly cellular fibrous connective tissue parenchyma, not the usual bland, acellular collagenous stroma of many tumors, with non-neoplastic islands, strands and cords of columnar or cuboidal, sometimes vacuolated odontogenic epithelium ranging from scanty to numerous. When numerous, the peripheral odontogenic fibroma has been occasionally mistaken for an epithelial neoplasm such as a peripheral ameloblastoma (Fig. 4-42B–E). This epithelium is usually deep in the lesion, away from the surface epithelium and is sometimes found ‘cuffing’ calcifications. Calcified tissue may or may not be present in the peripheral odontogenic fibroma. If found, it may resemble trabeculae of bone or osteoid, dentin or osteodentin (sometimes described as dysplastic dentin), or cementum like material. Mature fibrous connective tissue stroma is present and is sometimes highly vascularized, particularly in the less cellular areas. Myxomatous changes may also be found in the stroma, and the presence of inflammation is variable.
Central Odontogenic Fibroma
The odontogenic fibroma is a central tumor of the jaws which is seen so infrequently that little is known about this neoplasm. Of all the odontogenic tumors, this lesion has the most poorly defined parameters. The major explanation for the uncertainty regarding this tumor, as discussed by Gardner in his attempt at unification of the concept of the central odontogenic fibroma, is the lack of unanimity of definition of the lesion.
Three basic concepts have existed concerning this tumor:
• It is a lesion around the crown of an unerupted tooth re-sembling a small dentigerous cyst, although most inves-tigators regard this as simply a hyperplastic dental follicle and not an odontogenic tumor.
• It is a lesion of fibrous connective tissue, with scattered islands of odontogenic epithelium, bearing some resem-blance to the dental follicle but because of the size which it may attain appearing to constitute a neoplasm.
• It is a lesion which has been described by WHO as a fibro-blastic neoplasm containing varying amounts of odontogenic epithelium, and in some cases, calcified material resembling dysplastic dentin or cementum like material; thus, except for location, it is histologically identical to the peripheral odontogenic fibroma as described by the WHO group.
Since the occurrence of the latter two lesions in the jaws is undeniable even though their histogenesis is uncertain, Gardner has suggested referring to the tumor made up of connective tissue and odontogenic islands resembling dental follicle as the simple central odontogenic fibroma and to the tumor described by the WHO as the WHO-type central odontogenic fibroma. Until more cases of each are reported to clarify their histogenesis and their relationship, if any, this approach appears reasonable.
The simple central odontogenic fibroma has been discussed by Wesley and his associates, who reviewed eight cases, including one of their own, which they believed met the histologic criteria for this form of the lesion, while Dahl and her associates have added two more cases. Farman has reviewed eight cases of the WHO type of central odontogenic fibroma from the literature in his paper dealing basically with the peripheral form of this neoplasm. Thus, it may be seen that too few examples of either type of central odontogenic fibroma have been reported from which one may draw any significant conclusions regarding specific clinical features, treatment or prognosis.
Clinical Features
The odontogenic fibroma appears to occur more frequently in children and young adults, although a few cases have been reported in older persons, and has a predilection for occurrence in the mandible. It is generally asymptomatic except for swelling of the jaw.
Radiographic Features
This tumor sometimes produces an expansile, multilocular radiolucency similar to that of the ameloblastoma (Fig. 4-43 A).
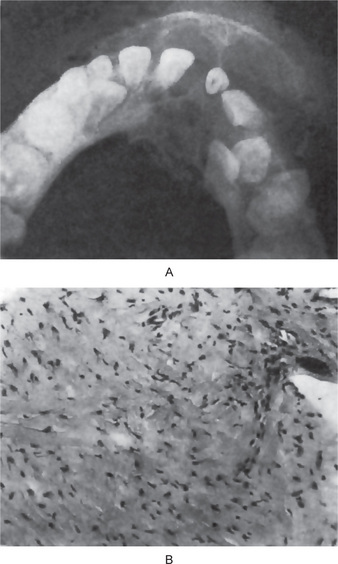
Figure 4-43 Central odontogenic fibroma. The radiograph (A) exhibits expansion of the mandible with thinning of the cortical plates. The radiographic appearance is suggestive of ameloblastoma. The photomicrograph (B) shows the bundles of collagen fibers and an epithelial rest (Courtesy of Dr Charles A Waldron).
Histologic Features
The odontogenic fibroma of both types has been described above. The simple type is characterized by a tumor mass made up of mature collagen fibers interspersed usually by many plump fibroblasts that are very uniform in their placement and tend to be equidistant from each other. Small nests or islands of odontogenic epithelium that appear entirely inactive are present in variable but usually in quite minimal amounts (Fig. 4-43 B).
The WHO type also consists of relatively mature but quite cellular fibrous connective tissue with few to many islands of odontogenic epithelium. Osteoid, dysplastic dentin or cementum like material is also variably present.
Care must be exercised not to misdiagnose other fibrous lesions of the jaws as an odontogenic fibroma. Lesions to be considered in the differential diagnosis include neurofibroma and desmoplastic fibroma.
Odontogenic Myxoma (Odontogenic fibromyxoma or myxofibroma)
The odontogenic myxoma is a tumor of the jaws which apparently arises from the mesenchymal portion of the tooth germ, either the dental papilla, the follicle or the periodontal ligament. Previous reports of this tumor have been discussed by many investigators, most recently White and his colleagues, who summarized pertinent data on over 90 individual and series of cases of odontogenic myxoma of the jaws, including their own group of nine cases. Absolute proof of origin from the odontogenic apparatus is lacking for this lesion, but it appears most likely because of the frequent occurrence of this lesion in the jaws and almost universal absence in any other bone of the skeleton. For example, in a study by McClure and Dahlin of 6,000 cases of bone tumors at the Mayo Clinic up to 1976, no cases involving bones other than the jaws were found.
Clinical Features
The odontogenic myxoma occurs most frequently in the second or third decade of life, the average age in the various reported series ranging from 23–30 years. It rarely occurs before the age of 10 years or after the age of 50. There is no particular gender predilection in the occurrence of this tumor, and there is a slight predilection for occurrence in the mandible. Occasional cases occur outside the tooth-bearing areas, several cases having been reported in the condyle or neck of the condyle. In the series reported by Thoma and Goldman, nearly every case was associated with missing or embedded teeth. However, in many instances this is not the case.
The odontogenic myxoma is a central lesion of the jaws which expands the bone and may cause destruction of the cortex. It is not a rapidly growing lesion, and pain may or may not be a feature.
Radiographic Features
The radiograph may present a mottled or honeycombed appearance of bone in some cases, while others may appear as a destructive, expanding radiolucency which sometimes has a multilocular pattern (Fig. 4-44 A, B). Displacement of teeth by the tumor mass is a relatively common finding, but root resorption is less frequent. The tumor is often extensive before being discovered. Invasion of the antrum occurs frequently in lesions of the maxilla.
Histologic Features
The myxoma is made up of loosely arranged, spindle-shaped and stellate cells, many of which have long fibrillar processes that tend to intermesh. The loose tissue is not highly cellular, and those cells present do not show evidence of significant activity (pleomorphism, prominent nucleoli or mitotic figures). The intercellular substance is mucoid. The tumor is usually interspersed with a variable number of tiny capillaries and occasionally strands of collagen. Nests of odontogenic epithelium may be found infrequently (Fig. 4-45).
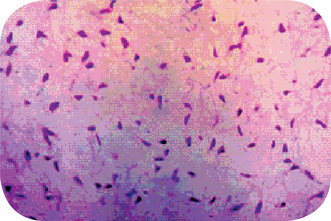
Figure 4-45 Odontogenic myxoma. High-power view of randomly arranged, spindle-shaped, stellate, and rounded cells in a loose myxoid stroma (hematoxylin and eosin, original magnification x10) (Courtesy of Dr RB Brannon).
Hodson and Prout have reported the presence of two acid mucopolysaccharides in the odontogenic myxoma: relatively large quantities of hyaluronic acid and lesser amounts of chondroitin sulfate. They have suggested that this high hyaluronic acid component may be a significant factor in the neoplastic behavior of the tumor.
Histochemical studies by Mori and his associates have shown that the tumor cells with long anastomosing processes in the odontogenic myxoma exhibited high alkaline phosphatase and lactate dehydrogenase activity, while there was low acid phosphatase, glucose-6-phosphate dehydrogenase and isocitrate dehydrogenase activity. These enzymatic reactions differed from those present in osteogenic sarcomas or even benign proliferating fibrous lesions such as osteogenic fibroma or a fracture callus. This suggested to the investigators that the tumor cells of the myxoma are not as biologically active as other tumors of the jaws.
Ultrastructural studies have also been reported by several investigators. White and his associates described ‘pale’ cells which probably represented active secretory cells, the ‘secretion’ actually representing transportation of synthesized material though to be nonsulfated mucopolysaccharide, the myxoid material, into the intercellular compartment. The other cell type, the ‘dark’ cell, contained collagen fibrils, and this suggested a disturbance in the secretory process of collagen molecules so that they became crystallized into fibrils intracellularly.
The ultrastructural studies of Goldblatt have also led him to conclude that the myxoma cell is not a typical fibroblast, that there is an attempt at collagen fibrillogenesis with little ultimate success and that prominent secretory activity within tumor cells appears to result in excess production of acid mucopolysaccharide matrix. He also pointed out that, according to other investigators, there may be actual phagocytosis by tumor cells of the small amount of collagen present. Finally, he concluded that, while the myxoma cells showed many characteristics of fibroblasts of the odontogenic apparatus, tumor origin from nonodontogenic mesenchyme cannot be ruled out by existing ultrastructural studies.
Care must be used in distinguishing the odontogenic myxoma histologically from a variety of other myxoid lesions, including myxoid neurofibroma, myxoid liposarcoma, and myxoid chondrosarcoma.
Treatment and Prognosis
The treatment of odontogenic myxomas is surgical excision, followed by cautery. Extensive lesions may require resection to eradicate the tumor. Although this is a benign neoplasm, it frequently exhibits insidious local invasion, making its complete removal difficult, a problem augmented by the loose, gelatinous nature of the tissue itself. The prognosis is good despite unpredictable recurrence. The tumor is not sensitive to X-ray radiation.
A frankly malignant form of this tumor, an odontogenic myxosarcoma, is known but is exceedingly rare.
Malignant odontogenic tumors
Odontogenic malignancies are very uncommon tumors. Presently the diagnosis of odontogenic malignancies is based on clinicoradiographic findings and subjective histopathologic evaluation. To be regarded as ‘odontogenic’, the tumor must arise in the gingiva or jaws (Table 4-3).
Table 4-3
Classification: Odontogenic malignancies
Odontogenic carcinomas
1. Metastasizing ameloblastoma
2. Ameloblastic carcinoma
(a) Carcinoma ex ameloblastoma
(b) De novo
3. Primary intraosseous carcinom
(a) Solid
(b) Cystic (ex odontogenic cyst)
(c) Central mucoepidermoid carcinoma
4. Ghost cell odontogenic carcinoma
5. Clear cell odontogenic carcinoma
Odontogenic sarcoma
1. Ameloblastic fibrosarcoma
Odontogenic Carcinomas
Metastasizing Ameloblastoma
Traditionally, ameloblastoma has been regarded as a benign tumor that can be locally aggressive but occasionally can metastasize and kill the patient. The prospect that all ameloblastomas could represent low-grade malignant neoplasms deserves consideration. The term ‘metastasizing ameloblastoma’ is used to describe a tumor that shows histologic features of classic ameloblastoma in the gingiva or jaw and has metastatic deposits elsewhere.
Clinical Features
Typically, the primary ameloblastoma arises in the mandible of a young adult. The average age at presentation is 30 years, but 33% of patients are younger than 20 years of age. After a mean interval of approximately 11 years, metastatic nodules develop in the lung (80%), cervical lymph nodes (15%), or extragnathic bones. Typically, the pulmonary metastases are multifocal and involve both lungs. The median survival after discovery of the metastatic lesion is about two years. Innocuous lung ‘granulomas’ that are seen on routine chest films of a patient who has ameloblastoma can prove to be silent metastases. Kunze et al (1985) noted that most patients had multiple recurrences of the jaw ameloblastoma. The multiple recurrences could result from an intrinsically more aggressive tumor or from surgery-associated tumor ‘spillage’ into adjacent tissue or tumor embolization into lymphatic or blood vessels. The primary ameloblastoma can become locally aggressive (with parapharyngeal and cranial base invasion) before pulmonary metastases arise.
Histologic Features
Ameloblastoma with metastatic potential is histologically indistinguishable from conventional ameloblastoma. The metastatic ameloblastoma usually does not show any greater cytologic atypia or mitotic activity than that seen in the primary. Metastasizing ameloblastoma, ambiguously termed ‘malignant ameloblastoma’, clearly demonstrates the biologic behavior of a well-differentiated low-grade carcinoma.
Treatment
Metastatic ameloblastoma is managed best by surgery, but there is no evidence that it improves survival. Cervical lymph node metastases are managed by neck dissection. If adequate pulmonary function can be preserved, lung metastases can be excised by lobectomy. At surgery, more numerous pulmonary metastatic deposits are often identified than were apparent in diagnostic imaging studies. Generally, chemotherapy has been ineffective, but a short-term partial response is possible.
Ameloblastic Carcinoma
Ameloblastic carcinoma is a malignant epithelial proliferation that is associated with an ameloblastoma (carcinoma exameloblastoma) or histologically resembles an ameloblastoma (de novo ameloblastic carcinoma). Ameloblastic carcinoma subjectively demonstrates greater cytologic atypia and mitotic activity than ameloblastoma. Some ameloblastomas exhibit basilar hyperplasia and an increased mitotic index. These findings might warrant a designation of ‘atypical ameloblastoma’ or ‘proliferative ameloblastoma’, but they are probably insufficient to permit a diagnosis of ameloblastic carcinoma in the absence of nuclear pleomorphism, perineural invasion, or other histologic evidence of malignancy.
Ameloblastic carcinoma is an aggressive neoplasm that is locally invasive and can spread to regional lymph nodes or distant sites, such as lung and bones. Usually, ameloblastic carcinoma is managed as a squamous cell carcinoma with attempted complete surgical excision, elective or therapeutic neck dissection, and postoperative radiation therapy. The prognosis is poor.
Carcinoma ex Ameloblastoma
A carcinoma directly contiguous with an ameloblastoma is appropriately termed a ‘carcinoma arising from an ameloblastoma’ (carcinoma ex ameloblastoma, carcinoma in ameloblastoma) (Fig. 4-46). The carcinoma arises when an ameloblastoma undergoes ‘dedifferentiation’ (i.e. when a less-differentiated proliferative clone arises within an ameloblastoma). This aggressive clone overgrows the ameloblastoma and becomes the dominant component. A follicular or plexiform ameloblastoma blends through a narrow transition zone with a hypercellular poorly differentiated carcinoma that demonstrates sheets of disordered mitotically active small basaloid cells with hyperchromatic nuclei, larger squamoid or polygonal cells with pale vesicular nuclei or elongated spindled epithelial cells. The carcinomatous cells show mild to moderate nuclear pleomorphism. A low-grade spindled ameloblastic carcinoma also has been described, in which ameloblastomatous epithelium is associated with hypercellular stroma that displays elongated fibroblastic cells that show little cytologic atypia and scattered mitotic figures; the ‘fibroblastic cells’ display immunoreactivity for cytokeratin (Slater LJ, 2004). Hamakawa et al (2000) reported a squamous cell carcinoma that arose adjacent to an ameloblastoma with no apparent transition between the two components. They interpreted it as a probable collision tumor, two separate primary tumors—a squamous cell carcinoma of gingival or odontogenic epithelial origin that abutted a conventional ameloblastoma.
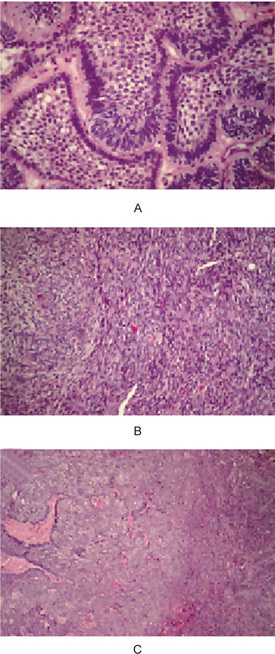
Figure 4-46 Carcinoma ex ameloblastoma. (A) A 66-year-old woman who had a maxillary ameloblastoma that involved antrum; the ameloblastoma shows little cytologic atypia. (B) Pale islands and sheets of ameloblastoma (left and center) merge with dark sheets of spindled carcinoma cells (right). (C) Sheets of sarcomatoid spindled carcinoma cells at higher magnification (Courtesy of Dr LJ Slater (Reprinted from Oral and Maxfac Surg Clin N Am 16: 411, 2004 with permission).
De novo Ameloblastic Carcinoma
If a carcinoma lacks a component of conventional ameloblastoma, its unequivocal categorization as an ameloblastic carcinoma is considerably less secure (Fig. 4-47). The diagnosis of de novo ameloblastic carcinoma is based on subjective interpretation. The tumor should demonstrate vague ameloblastomatous features: a plexiform architecture that exhibits budding and anastomosing epithelial processes with peripheral palisaded cuboidal to columnar cells and central polygonal to angular cells. Tumors that lack evidence of reverse polarization, the so-called ‘Vick-ers-Gorlin’ changes that are indicative of ameloblastic differentiation, can be accepted as examples of de novo ameloblastic carcinoma; the stringent criterion of reverse polarity has not been required.
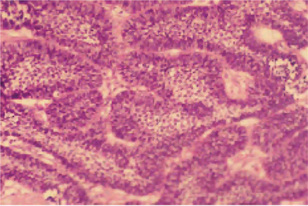
Figure 4-47 Ameloblastic carcinoma. Budding and anastomosing epithelial trabecula that demonstrate peripheral palisaded tall cells, basilar hyperplasia (expansion of the proliferative compartment), and moderate nuclear pleomorphism; the central pale zones resemble stellate reticulum. It has the architecture of an ameloblastoma and sufficient cytologic atypia to warrant a diagnosis of carcinoma.
The possibility of a variant of squamous cell carcinoma that shows peripheral palisaded cells should be considered before a diagnosis of ameloblastic carcinoma is rendered. But the distinction is of academic interest only, because squamous cell carcinoma and ameloblastic carcinoma are treated the same way.
Primary Intraosseous Carcinoma
Primary intraosseous carcinoma (PIOC) is a squamous cell carcinoma that occurs in the jaw bone. It is called ‘primary’ because it is not a secondary deposit; metastasis from a distant primary to the jaw must be excluded. It is termed ‘intraosseous’ because it develops centrally within bone; origin from surface stratified squamous or sinonasal epithelium must be ruled out. It lacks evidence of ameloblastic differentiation, otherwise it would be classified as a de novo ameloblastic carcinoma. If the intraosseous carcinoma demonstrates mucous cells, then a diagnosis of central mucoepidermoid carcinoma is appropriate.
Primary intraosseous carcinoma presents clinically as a diffuse enlargement of the jaw; if mucosal ulceration or mucosal tumefaction is present, the diagnosis of PIOC should be questioned. PIOC epithelium can become confluent with gingival surface stratified squamous epithelium after erosion of bone. Such confluence does not indicate necessarily that the carcinoma is of gingival origin if the tumor’s overall growth pattern suggests an intraosseous origin. CT images can be useful in assessing whether a putative PIOC could have originated from a gingival squamous cell carcinoma, as well as buccal and lingual cortical destruction and trismusinducing invasion of the masticator space before involving surface oral mucosa.
Primary intraosseous carcinoma is believed to arise from central odontogenic epithelium, but in rare cases, origin from incisive canal epithelium has been proposed. Cervical metastases have been identified synchronously or metachronously with a PIOC.
Solid Primary Intraosseous Carcinoma
Thomas et al, thoroughly reviewed the clinicoradiographic attributes of this rare tumor. The typical solid PIOC presents as a painful mass in the posterior mandible. Although the mean age at presentation is 52 years, 20% of patients are younger than 34 years and 39% are older than 65 years. Clinically, patients may present with pain, swelling and paresthesia.
Radiographically, 42% of patients demonstrate a cup-shaped radiolucent lesion, 26% demonstrate a poorly-circumscribed ‘moth eaten’ radiolucency, and 10% exhibit a well-circumscribed radiolucency. At presentation, 31% of patients have evidence of cervical metastases; however, the survival rate of patients who have regional metastases is apparently no worse than those who do not.
Most often, the tumor is treated by surgical excision and postoperative radiation therapy. Most deaths occur within two years of therapy. The overall five-year survival rate is 38%.
Cystic Primary Intraosseous Carcinoma
Cystic PIOC (PIOC arising in an odontogenic cyst, PIOC ex odontogenic cyst) is squamous cell carcinoma that demonstrates a cystic component with a lumen that contains fluid or keratin and a lining of stratified squamous epithelium that exhibits cytologic atypia (intraepithelial neoplasia, epithelial dysplasia). If the cystic component lacks cytologic atypia, the possibilities of a gingival carcinoma that eroded bone and collided with an odontogenic cyst or of a PIOC that arose adjacent to a benign cyst might be considered.
Intraosseous carcinomas that show a cystic and a solid component can mimic an odontogenic cyst radiographically.
Squamous cell carcinoma can exhibit a predominantly cystic architecture. The theoretic possibility of a squamous cell carcinoma metastasizing to the jaw as a cystic lesion, has not been reported.
Carcinoma ex Dentigerous Cyst
The most common odontogenic cyst to show carcinomatous changes is the dentigerous cyst. Intraepithelial neoplasia that involves sulcular gingival epithelium can mimic carcinoma ex dentigerous cyst histologically. It typically presents as an asymptomatic or painful pericoronal radiolucent lesion that is associated with an impacted mandibular third molar. The mean age of occurrence is 59 years with male predominance. Some lesions can cause osseous destruction. Most carcinomas that arise in dentigerous cysts occur in the mandibular molar area; occasionally they are associated with impacted canine teeth.
Histologically, the lesions demonstrate membranous connective tissue that is lined by stratified squamous epithelium that exhibits evidence of intraepithelial neoplasia (epithelial dysplasia) and is associated with an invasive well-differentiated or moderately-differentiated squamous cell carcinoma. The lining epithelium is derived from dental follicular epithelium or gingival sulcular epithelium (if the tooth is partially erupted).
Carcinoma ex Odontogenic Keratocyst
Makowski et al (2001) identified 15 previously reported cases of squamous cell carcinoma that arose in an odontogenic keratocyst (OKC). In most cases, radiographic findings are those of a benign OKC.
In the maxilla, OKC lining epithelium can fuse with buccal vestibular surface stratified squamous epithelium to form a chronic sinus tract that drains pus-like keratinaceous material; this ‘automarsupialized’ cyst develops thickened nonkeratinized lining epithelium that is similar to that seen in a surgically decompressed OKC. PIOC that arises in a cyst that is lined by dysplastic thickened parakeratinized stratified squamous epithelium that lacks convincing features of classic OKC or orthokeratinized odontogenic cyst cannot be classified assuredly as carcinoma ex OKC.
Usually, the carcinomas have been treated by resection and postoperative radiation therapy, and infrequently with chemotherapy.
Intraosseous Mucoepidermoid Carcinoma
Intraosseous mucoepidermoid carcinoma (central mucoepidermoid carcinoma, PIOC with mucous cells) arises from odontogenic epithelium or an odontogenic cyst. It represents a cystic primary intraosseous carcinoma that shows squamous differentiation and mucous cells. If a rare central mucoepidermoid carcinoma arises from ectopic salivary gland tissue that was enclaved within the mandible during development, it would be classified as a primary intraosseous carcinoma. A low-grade mucoepidermoid carcinoma that presents as a retromolar mucosal mass with an underlying broad ‘cupped-out’ radiolucency of the mandible may represent a conventional mucoepidermoid carcinoma derived from retromolar salivary glands that has secondarily eroded the mandible. With this presentation, a diagnosis of ‘central mucoepidermoid carcinoma’ should be questioned because central tumors do not present as mucosal masses. Some apparent maxillary intraosseous mucoepidermoid carcinomas probably arise from seromucous glands that are associated with sinonasal mucosa. Most tumors are low-grade cystic lesions that grow in a broad ‘pushing’ front and demonstrate a well-circumscribed unilocular or multilocular radiolucent lesion (Fig. 4-48). As for all neoplasms, the prognosis depends on the extent of disease (clinical stage) and the histologic grade of the tumor. High-grade tumors display a destructive permeative growth pattern and have metastatic potential. Typical low-grade tumors have a favorable prognosis. Death eventuates from uncontrolled local disease rather than from metastases (Slater LJ, 2004).
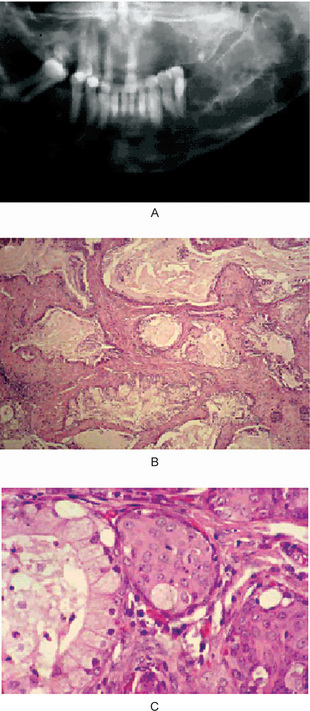
Figure 4-48 Central low-grade mucoepidermoid carcinoma. (A) A 36-year-old woman had a painful expansile lesion of the left posterior mandible; radiographically, a multiloculated radiolucency is observed. (B) Multiple adjacent cystic structures contain mucus and are lined by epithelium that demonstrates numerous mucous cells. (C) At higher magnification, a cystic space is lined by mucous cells (left) and mural islands of epidermoid cells (center and right) are seen (Courtesy of Dr LJ Slater) (Reprinted from Oral Maxfac Surg Clin N Am 16: 415, 2004 with permission).
Varying surgical treatments have been used, including: curettage, partial resection, en bloc resection, and hemimandibulectomy. In several cases, curettage resulted in no evidence of disease after a follow-up period of 3–19 years.
Ghost Cell Odontogenic Carcinoma (Odontogenic ghost cell carcinoma, malignant epithelial odontogenic ghost cell tumor, aggressive [malignant] epithelial odontogenic ghost cell tumor, dentinogenic ghost cell tumor)
Ghost cell odontogenic carcinoma is an ameloblastic carcinoma that shows evidence of ghost cell keratinization. As such, it can be considered to be a variant of ameloblastic carcinoma. The biologic behavior of the lesion cannot be predicted from the presence of ghost cells. The tissue that surrounds the ghost cells gives the prognosis. For example, if a benign cyst contains ghost cells, its biologic behavior is that of a benign cyst; if an ameloblastoma contains ghost cells, its biologic behavior is that of an ameloblastoma; and if a carcinoma contains ghost cells, its biologic behavior is that of a carcinoma (Fukushima M, 1983).
Ghost cells are immunoreactive for amelogenin, a protein that is unique to enamel matrix; therefore, the presence of ghost cells in jaw tumors represents evidence of ameloblastic differentiation. As with all primary intraosseous carcinomas, the possibility of a metastasis should be excluded; carcinomas that demonstrate ghost cells include cutaneous pilomatrix carcinoma and visceral carcinomas.
The mean age at presentation was 38 years, but the age range was from 13–72 years; 75% arose in men. About 66% of cases occur in the maxilla. Ghost cell odontogenic carcinoma seems to be disproportionately more frequent in Asians.
Radiographically, it presents as an expansile multiloculated to poorly delineated radiolucent lesion; the radiographic features are not characteristic.
Ghost cell odontogenic carcinomas have a biologic behavior that is similar to that of an ameloblastoma or a low-grade ameloblastic carcinoma. The tumor can be locally aggressive (e.g. maxillary tumors can invade the orbit). Apparently, the tumor has little metastatic potential.
Most of the ghost cell odontogenic carcinomas arise de novo. Most often, the carcinomas demonstrate sheets of small basaloid cells that show intraepithelial islands; stratified squamous epithelium that exhibit ghost cell keratinization. Approximately 50% have a prominent cystic component. Palisaded columnar cells at the periphery of tumor islands can create an ameloblastomatous pattern. Lesional tissue subjectively reveals features that warrant a malignant interpretation, including cytologic atypia, increased mitotic activity, an infiltrative growth pattern (perineural or intravascular invasion), and necrosis (Fig. 4-49), (Slater LJ, 2004).
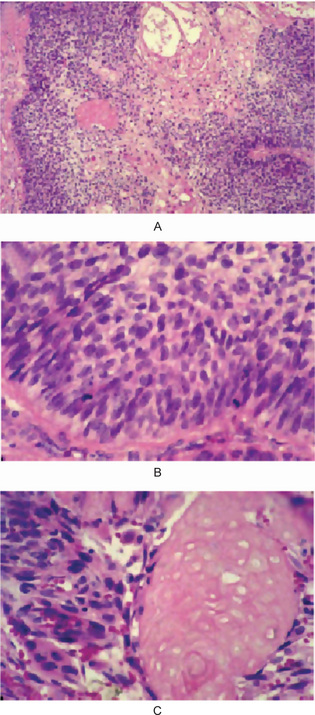
Figure 4-49 Ghost cell odontogenic carcinoma. (A) Sheets of malignant epithelial cells demonstrate disordered dark basaloid cells at the periphery (left and right), central squamous differentiation with cystic change (top center), and an intraepithelial oval island of ghost cells (in center of left half of photograph). (B) Higher magnification of peripheral basaloid cells. (C) Higher magnification of an island of pink squamous epithelium that exhibits ghost cell keratinization (right half of photograph) (Reprinted from Oral Maxfac Surg Clin N Am. 16: 416, 2004 with permission).
It is treated by surgical excision, postoperative radiation therapy, and sometimes chemotherapy, as well.
Clear Cell Odontogenic Carcinoma (Clear cell ameloblastic carcinoma, clear cell ameloblastoma, clear cell odontogenic tumor)
Clear cell odontogenic carcinoma is a low-grade carcinoma that is composed of cells that show uniform nuclei and clear cytoplasm. Approximately 90% of cases arise in the mandible— half in the anterior portion and half in the posterior segment. About 70% of patients are women with an age range of 17–89 years.
The tumor has a varied radiographic appearance. Often, it presents as a unilocular expansile radiolucent lesion with an indistinct periphery; however, some cases are multiloculated and well-circumscribed.
The classic case of clear cell carcinoma demonstrates budding and branching cords, islands, and sheets of neoplastic polygonal epithelial cells. Lesional cells exhibit central to eccentric small dark uniform nuclei; little evidence of nuclear pleomorphism or mitotic activity; abundant pale cytoplasm, ‘clear cells’; and distinct cell borders. Tumor islands can display peripheral ameloblastomatous palisaded columnar cells; however, no evidence of central stellate reticulum, squamous differentiation, or cystic change is observed (Fig. 4-50). The clear cells contain periodic acid Schiff–positive diastase-sensitive glycogen, but they are mucicarmine negative. Recurrent lesions may be more proliferative than the original tumor, with cells showing greater mitotic index than those in the original tumor. The histologic differential diagnosis includes the clear cell variant of calcifying epithelial odontogenic tumor, metastatic renal cell carcinoma, the clear cell variant of mucoepidermoid carcinoma and clear cell squamous cell carcinoma. Salivary gland clear cell carcinomas are indistinguishable from clear cell odontogenic carcinoma based on histologic features, special stains, and immunohistochemical assays (Muramatsu T et al 1996).
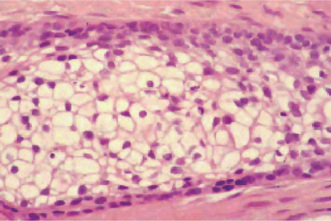
Figure 4-50 Clear cell odontogenic carcinoma. Central large pale cells have abundant clear cytoplasm, small dark uniform nuclei, and distinct cytoplasmic borders. Peripheral basaloid cells display little cytologic atypia (Courtesy of LJ Slater).
The tumor is refractory to curettage. Resected tumors had a high recurrence rate; 10 of 21 (48%) resected tumors recurred following a median interval of 6.3 years. Approximately 20% of tumors show cervical lymph node metastases; 17% display lung metastases; and about 20% of patients die of disease (after a median period of 14 years). In case of extensive extraosseous extension postoperative radiation therapy might be useful.
Ameloblastic Fibrosarcoma (Odontogenic sarcoma, ameloblastic sarcoma)
Ameloblastic fibrosarcoma is a malignant proliferation of connective tissue cells that contains benign odontogenic epithelium that is similar to that seen in ameloblastic fibroma. Approximately 80% of cases occur in the mandible, predominantly the posterior segment; 60% occur in men. Often, it demonstrates an expansile radiolucency with indistinct margins and evidence of extraosseous soft tissue extension.
About 35% of cases arise in an ameloblastic fibroma. Although sarcoma ex ameloblastic fibroma presents in patients with a mean age of 33 years, de novo ameloblastic fibrosarcoma presents in younger patients with an average age of 22 years. Approximately 37% of patients have one or more recurrence and 19% die of disease. Typically, the patients do not develop metastases; they die of a locally aggressive neoplasm.
Varying radiographic patterns can be seen, that include destructive/permeative, unilocular, or multilocular sometimes, associated with an impacted molar.
Ameloblastic fibrosarcoma has the histologic architecture of an ameloblastic fibroma. Slender budding and branching epithelial cords of bland cuboidal to columnar cells with uniform nuclei or epithelial islands that are indistinguishable from those that are seen in follicular ameloblastoma are separated widely by hypercellular connective tissue that exhibits plump polygonal to fusiform stromal cells that show mild to moderate cytologic atypia and numerous mitotic figures in a pale hypocollagenous myxoid extracellular matrix. If the tumor recurs, it tends to display greater stromal cell cellularity, increased mitotic activity, more pronounced nuclear atypia, and less evidence of odontogenic epithelium with each recurrence. In some cases, the sarcomatous component completely overgrows the epithelial component; such recurrent tumors are devoid of epithelium (Fig. 4-51). Ameloblastic fibrosarcoma can demonstrate focal evidence of dentin formation or dentin and enamel formation; and this insignificant dental hard tissue component has resulted in the terms ‘ameloblastic dentinosarcoma’ and ‘ameloblastic odontosarcoma’, respectively. The histologic differential diagnosis includes a low-grade spindled ameloblastic carcinoma (Philips VM et al 1998).
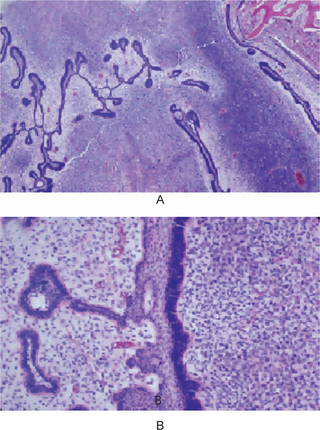
Figure 4-51 Ameloblastic fibrosarcoma. (A) The neoplasm has the architecture of an ameloblastic fibroma that demonstrates slender budding and anastomosing cords that ‘open up’ to form central pale stellate reticulum; the dark band of sarcoma cells (lower right) is particularly hypercellular. (B) The pale connective tissue to the left of the epithelium is less cellular than the obviously sarcomatous dark polygonal cells at the right (Courtesy of Dr LJ Slater).
The sarcoma is treated most often by a wide surgical excision and postoperative radiation therapy, without elective neck dissection.
References
Abiko, Y., Murata, M., Ito, Y., Taira, T. Immunohistochemical localization of amelogenin in human odontogenic tumors, using a polyclonal antibody against bovine amelogenin. Med Electron Microsc. 2001; 34(3):185–189.
Abrams, A.M., Melrose, R.J., Howell, F.V. Adenoameloblastoma: a clinical pathologic study of ten new cases. Cancer. 1968; 22(1):175–185.
Abrams, A.M., Howell, F.V. Calcifying epithelial odontogenic tumors: report of four cases. J Am Dent Assoc. 1967; 74:1231.
Abrams, A.M., Kirby, J.W., Melrose, R.J. Cementoblastoma. Oral Surg. 1974; 38:394.
Ackerman, G., Cohen, M., Altini, M. The paradenatal cyst: a clinicopathologic study of 50 cases. Oral Surg Oral Med Oral Patholo. 1987; 64:308–312.
Agazzi C, Belloni L. Gli odontomi duri dei mascellari. Arch Ital Otol, LXIV (Suppl XVI): 1953
Aguilo, L., Cibrian, R., Bagan, J.V., Gia, J.L. Eruption cysts: retrospective clinical study of 36 cases. ASDC J Dent Child. 1998; 65(2):102–106. [Mar-Apr].
Ahlfors, E., Larsson, A., Sjogren, S. The odontogenic keratocyst: a benign cystic tumor? J Oral Maxillofac Surg. 1984; 42(1):10–90.
Aisenberg, M.S. Adamantinohemangiom. Oral Surg. 1950; 3:798.
Ajagbe, H.A., Daramola, J.O., Junaid, T.A., Ajagbe, A.O. Adenomatoid odontogenic tumor in a black African population: report of thirteen cases. J Oral Maxillofac Surg. 1985; 43(9):683–687.
Altini, M., Smith, I. Ameloblastic dentinosarcoma (a case report). Int J Oral Surg. 1976; 5:142.
Ameerally, P., McGurk, M., Shaheen, O. Atypical ameloblastoma: report of 3 cases and a review of the literature. Br J Oral Maxillofac Surg. 1996; 34(3):235–239.
Anand, V.K., Arrowood, J.P., Jr Krolls. Odontogenic keratocyst: a study of 50 patients. Laryngoscope. 1995; 105:14–16.
Anderson, R.A. Eruption cysts: a retrograde study. ASDC J Dent Child. 1990; 57(2):124–127. [Mar-Apr].
Anderson, H.C., Byunghoon, K., Minkowitx, S. Calcifying epithelial odontogenic tumor of Pindborg: an electron microscopic study. Cancer. 1969; 24:585.
Angelopoulou, E., Angelopoulos, A.P. Lateral periodontal cyst: review of the literature and report of a case. J Periodontol. 1990; 62(2):126–131. [Feb].
Antoh, M., Hasegawan, H., Kawakami, T. Hyperkeratosis and atypical proliferation appearing in the lining epithelium of a radicular cyst: report of a case. J Craniomaxillofac Surg. 1993; 21(5):210–213. [Review, Jul].
Areen, R.G., McClatchey, K.D., Baker, H.L. Squamous cell carcinoma developing in an odontogenic keratocyst. Arch Otolaryngol. 1981; 107:568.
Arotiba, G.T., Arotiba, J.T., Olaitan, A.A., Ajayi, O.F. The adenomatoid odontogenic tumor: an analysis of 57 cases in a black African population. J Oral Maxillofac Surg. 1997; 55(2):146–148.
Ashman, S.G., Corio, R.L., Eisele, D.W., Murphy, M.T. Desmoplastic ameloblastoma: a case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1993; 75(4):479–482.
Awange, D.O. Adenomatoid odontogenic tumour (adenoameloblastoma)–a review. East Afr Med J. 1991; 68(3):155–163.
Baden, E. Odontogenic tumors. Pathol Ann. 1971; 6:475–568.
Baden, E. Terminology of the ameloblastoma: history and current usage. J Oral Surg. 1965; 23:40.
Bajaj, M.S., Mahindrakar, A., Pushker, N. Dentigerous cyst in the maxillary sinus: a rare cause of nasolacrimal obstruction. Orbit. 2003; 22(4):289–292. [Dec].
Baker, P.L., Dockerty, M.B., Coventry, M.B. Adamantinoma (so-called) of the long bones. J Bone Joint Surg. 1954; 36(A):704.
Baker, R.D., D’Onofrio, E.D., Corio, R.L., Crawford, B.E. Squamous-cell carcinoma arising in a lateral periodontal cyst. Oral Surg. 1979; 47:495.
Barreto, D.C., Gomez, R.S., Bale, A.E., Boson, W.L., De Marco, L. PTCH gene mutations in odontogenic keratocysts. J Dent Res. 2000; 79(6):1418–1422.
Barros, R.E., Dominguez, F.V., Cabrini, R.L. Myxoma of the jaws. Oral Surg. 1969; 27:225.
Basu, M.K., Matthews, J.B., Sear, A.J., Browne, R.M. Calcifying epithelial odontogenic tumor: a case showing features of malignancy. J Oral Pathol. 1984; 13:310–319.
Bataineh, A.B., Rawashdeh, M.A., Al Qudah, M.A. The prevalence of inflammatory and developmental odontogenic cysts in a Jordanian population: a clinicopathologic study. Quintessence. 1984; 5(10):815–819. [Int].
Becker, M.H., Lopf, A.W., Lande, A. Basal cell nevus syndrome: its roentgenologic significance. Am J Roentgenol, Radium Ther Nucl Med. 1967; 99:817.
Beden, E., Moskow, B.S., Moskow, R. Odontogenic gingival epithelial hamartoma. J Oral Surg. 1968; 26:702.
Bernier, J.L., Tiecker, R.W. Adenoameloblastoma. J Oral Surg. 1950; 8:259–261.
Bernier, J.L., Tiecke, R.W. Adenoameloblastoma: report of nine cases. Oral Surg. 1965; 84:304–317.
Bernier, J.L., Thompson, H.C. The histogenesis of the cementoma. Am J Orthod Oral Surg. 1946; 32:543.
Bernier, J.L. Ameloblastoma: report of 34 cases. J Dent Res. 1942; 21:529.
Bernstein, B. The residual radicular dental cyst: a case report and discussion. New York State Dent J. 1976; 42(9):548–551. [Nov].
Bezerra, S., Costa, I. Oral conditions in children from birth to 5 years: the findings of a children’s dental program. J Clin Pediatr Dent Fall. 2000; 25(1):79–81.
Bhaskar, S.N. Adenoameloblastoma: its histogenesis and report of 15 new cases. J Oral Surg. 1964; 22:218–226.
Bhaskar, S.N., Jacoway, J.R. Peripheral fibroma and peripheral fibroma with calcification: report of 376 cases. J Am Dent Assoc. 1966; 73:1312.
Bhaskar, S.N., Laskin, D.M. Gingival cysts. Oral Surg. 1955; 8:803.
Blake, H., Blake, F.S. Ameloblastic odontoma: report of case. J Oral Surg. 1951; 9:240.
Bodner, L., Goldstein, J., Sarnat, H. Eruption cysts: a clinical report of 24 new cases. Bradley JL. Multiple cementoma. J Oral Surg. 1944; 2:278.
Brandon, S.A. Adamantinoma of the left maxillary area: report of case. J Oral Surg. 1949; 7:252.
Brannon, R.B. The odontogenic keratocyst: a clinicopathological study of 312 cases Part I: clinical features. Oral Surg. 1976; 42:54–71.
Bredenkamp, J.K., Zimmerman, M.C., Mickel, R.A. Maxillary ameloblastoma: a potentially lethal neoplasm. Arch Otolaryngol Head Neck Surg. 1989; 115(1):99–104.
Breuer, W., Geisel, O., Linke, R.P., Hermanns, W. Light microscopic, ultrastructural and immunohistochemical examination of two calcifying epithelial odontogenic tumors in a dog and a cat. Vet Pathol. 1994; 31:415–420.
Brondum, N., Jensen, V.J. Recurrence of keratocysts and decompression treatment: a long-term follow-up of forty-four cases. Oral Surg Oral Med Oral Pathol. 1991; 72:265–269.
Broume, R.M. The odontogenic keratocyst-histological features and their correlation with clinical behavior. Br Dent J. 1971; 131:249–259.
Browne, R.M., Gough, N.G. Malignant change in the epithelium lining odontogenic cysts. Cancer. 1972; 29:1199.
Browne, R.M. The odontogenic keratocyst: clinical aspects. Br Dent J. 1970; 1238:255.
Bruce, R.A., Jackson, I.T. Ameloblastic carcinoma: report of an aggressive case and review of the literature. J Craniomaxillofac Surg. 1991; 19(6):267–271.
Buchner, A., Hansen, L.S. The histomorphologic spectrum of the gingival cyst in the adult. Oral Surg. 1979; 48:532.
Budnick, S.D. Compound and complex odontomas. Oral Surg. 1976; 42:501.
Byrne, M.P., Kosmala, R.L., Cunningham, M.P. Ameloblastoma with regional and distant metastases. Am J Surg. 1974; 128(1):91–94.
Cahn, L.R. The dentigerous cyst as a potential adamantinoma. Dent Cosmos. 1933; 75:889.
Cairo, F., Rotundo, R., Ficarra, G. A rare lesion of the periodontium: the gingival cyst of the adult— a report of three cases. Int J Periodontics Restorative Dent. 2002; 22(1):79–83. [Feb].
Carinci, F., Francioso, F., Piattelli, A., Rubini, C. Genetic expression profiling of six odontogenic tumors. J Dent Res. 2003; 82(7):551–557.
Carr, R.F., Halperin, V. Malignant ameloblastomas from 1953 to 1966—review of the literature and report of a case. Oral Surg. 1968; 26:514.
Cataldo, E., Berkman, M.D. Cysts of the oral mncosa in newborns. Am J Dis Child. 1968; 116:44.
Cavanha, A.O. Enamel pearls. Oral Surg. 1965; 19:373.
Changus, G.W., Speed, J.S., Stewart, F.W. Malignant angioblastoma of bone; reappraisal of adamantinoma of long. Cancer. 1957; 10:540.
Chaudhry, A.P., Spink, J.H., Gorlin, R.J. Periapical fibrous dysplasia (cementoma). J Oral Surg. 1985; 16:483.
Chen, C-H, Lin, C-C. Clinical and histopathological study of the odontogenic keratocyst-a follow-up study of 16 cases. Kaohsiung J Med Sci. 1986; 2:601–607.
Chen, S-Y, Fantasia, J.E., Miller, A.S. Hyaline bodies in the connective tissue wall of odontogenic cysts. J Oral Pathol. 1981; 10:147.
Cherrick, H.M., King, O.H., Jr Lucatorto, F.M., Suggs, D.M. Denign cementoblastoma. Oral Surg. 1974; 37:54.
Churchill, H.R. Histological differentiation between certain dentigerous cysts and ameloblastomata. Dent Cosmos. 1934; 76:1173.
Ciment, L.M., Ciment, A.J. Malignant ameloblastoma metastatic to the lungs 29 years after primary resection: a case report. Chest. 2002; 121(4):1359–1361.
Cina, M.T., Dahlin, D.C., Gores, R.J. Ameloblastic adenomatoid tumors: a report of four new cases. Am J Clin Pathol. 1963; 39:59–65.
Colgan, C.M. Paradental cysts: a role for food impaction in the pathogenesis? A review of cases from Northern Ireland. Br J Oral Maxillo Surg. 2002; 40(2):163–168.
Corio, R.L., Crawford, B.E., Schaberg, S.J. Benign cementoblastoma. Oral Surg. 1976; 41:524.
Couch, R.D., Morris, E.E., Vellios, F. Granular cell ameloblastic fibroma: report of two cases in adults, with observations on its similarity to congential epulis. Am J Clin Pathol. 1962; 37:398.
Courtney, R.M., Kerr, D.A. The odontogenic adenomatoid tumor. Oral Surg. 1975; 39:424–435.
Craig, G.T. The paradental cyst. A specific inflammatory odontogenic cyst. Br Dent J. 1976; 141:9.
Critechley, M., Ironside, R.N. The pituitary adaman Critchley M, Ironside RN. The pituitary damantinomata. 1926; 49:473.
Crowley, T.E., Kaugars, G.E., Gunsolley, J.C. Odontogenic keratocysts: a clinical and histologic comparison of the parakeratin and orthokeratin variants. J Oral Maxillofac Surg. 1992; 50:22–26.
Cundiff EJ. Peripheral ossifying fibroma: a review of 365 cases. MSD Thesis, Indiana University, 1972.
Dachi, S.F., Howell, F.V. A survey of 3,875 routine full-mouth radiographs II—a study of impacted teeth. Oral Surg. 1961; 14:1165.
Dahl, E.C., Wolfson, S.H., Haugen, J.C. Central odontogenic fibroma: review of literature and report of cases. J Oral Surg. 1981; 39:120.
Darlington, C.G., Ehrlich, H.E., Seldin, H.M. Malignant transformation of odontogenic cyst: report of case. J Oral Surg. 1953; 11:64.
Datta, R., Winston, J.S., Diaz-Reyes, G., Loree, T.R. Ameloblastic carcinoma: report of an aggressive case with multiple bony metastases. Am J Otolaryngol. 2003; 24(1):64–69.
Del Rosario, R.N., Barr, R.J., Jensen, J.L., Cantos, K.A. Basal cell carcinoma of the buccal mucosa. Am J Dermatopathol. 2001; 23(3):203–205.
Economopoulou, P., Patrikiou, A. Glandular odontogenic cyst of the maxilla: report of a case. J Oral Maxillofac Surg. 1995; 53:834–837.
el Sedfy, B.N. An ectopic odontome in the cheek. Oral Surg Oral Med Oral Pathol. 1977; 43(4):583–584.
Eliasson, A.H., Moser III, R.J., Tenholder, M.F. Diagnosis and treatment of metastatic ameloblastoma. South Med J. 1989; 82(9):1165–1168.
El-Labban, N.G., Lee, K.W. Vascular degeneration in adenomatoid odontogenic tumour: an ultrastructural study. J Oral Pathol. 1988; 17(6):298–305.
El-Labban, N.G. The nature of the eosinophilic and laminated masses in the adenomatoid odontogenic tumor: a histochemical and ultrastructural study. J Oral Pathol Med. 1992; 21(2):75–81.
El-Labban, N. Electron microscopic investigation of hyaline bodies in odontogenic cysts. J Oral Pathol. 1979; 8:81.
Elzay, R.P. Primary intraosseous carcinoma of the jaws: review and update of odontogenic carcinomas. Oral Surg. 1982; 54:299.
Epstein, A. Ueber Epithelperlen in der Mundhohle Neurgeborener Kinder Ztsch fur Heilkunde. 1880; 1:59.
Eversole, L.R., Leider, A.S., Hansen, L.S. Ameloblastomas with pronounced desmoplasia. J Oral Maxillofac Surg. 1984; 42(11):735–740.
Eversole, L.R., Tomich, C.E., Cherrick, H.M. Histogenesis of odontogenic tumors. Oral Surg. 1971; 32:569.
Extensive radicular cyst of the maxilla: case report. Rev Dent Liban, 23(3): 83–87, Sep, 1973.
Fantasia, J.E. Lateral periodontal cyst. Oral Surg. 1979; 48:273.
Farman, A.G., Kohler, W.W., Nortje, C.J., Van Wyk, C.W. Cementoblastoma: report of a case. J Oral Surg. 1979; 37:198.
Farman, A.G. The peripheral odontogenic fibroma. Oral Surg. 1975; 40:82.
Feinberg, S.E., Steinberg, B. Surgical management of ameloblastoma: current status of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996; 81(4):383–388.
Field, H.J., Ackerman, A.A. Calcifying fibro-adamantoblastoma. Am J Orthod Oral Surg. 1942; 28:543.
Forssell, K., Forssell, H., Kahnberg, K.E. Recurrence of keratocysts: a long-term followup study. Int J Oral Maxillofac Surg. 1988; 17:25–28.
Forssell, K., Sainio, P. Clinicopathological study of keratinized cysts of the jaws. Proc Finn Dent Soc. 1979; 75:36.
Forssell, K., Kallioniemi, H., Sainio, P. Microcysts and epithelial islands in primordial cysts. Proc Finn Dent Soc. 1979; 75:99.
Forssell, K., Sorvari, T.E., Oksala, E. An analysis of the recurrence of odontogenic keratocysts. Proc Finn Dent Soc. 1974; 70:135.
Forssell K. The primodial cyst: a clinical and radiographic study: academic dissertation, Turku, Finland, 1980.
Fowler, C.B., Brannon, R.B. The paradental cyst: a clincopathologic study of six new cases and review of the literature. J Oral Maxillo Surg. 1989; 47:243–248.
Franklin, C.D., Pindborg, J.J. The calcifying epithelial odontogenic tumor. Oral Surg. 1976; 42:753.
Freedman, P.D., Lumerman, H., Gee, J.K. Calcifying odontogenic cyst. Oral Surg. 1975; 40:93.
Frissell, C.T., Shafer, W.G. Ameloblastic odontoma: report of case. Oral Surg. 1953; 6:1129.
Frissell CT. Ameloblastoma: a statistical study of two hundred sixty-one cases, including fifteen previously unreported from the Indiana University Medical Center. MS Thesis, Indiana University, 1952.
Fromma, A. Epstein’s pearls, Bohn’s nodules and inclusioncysts of the oral cavity. J Dent Child. 1967; 34:275.
Fukushima, M. Simple ameloblastoma with ghost cell and granular cell components. Acta Pathol Jpn. 1983; 33:1215–1221.
Gabell, D., James, W.W., Payne, J.L. Report on Odontomes. London, 1914 as cited by Sprawson E. Odontomes. Brit Dent J. 1937; 74:178–201.
Gallini, G., Merlini, C., Martelossi, L., Benetti, C. Peripheral developmental odontogenic cysts: neonatal cysts and gingival cysts. Dent Cadmos, 15. 1991; 59(6):70–72. [75–77, Apr].
Gao, Y.H., Yang, L.J., Yamaguchi, A. Immunohistochemical demonstration of bone morphogenetic protein in odontogenic tumors. J Oral Pathol Med. 1997; 26(6):273–277.
Gardner, D.G., Kessler, H.P., Morency, R., Schaffner, D.L. The glandular odontogenic cyst: an apparent entity. J Oral Pathol. 1988; 17:359–366.
Gardner, D.G., Morency, R. The glandular odontogenic cyst: a rare lesion that tends to recur. J Can Dent Assoc. 1993; 59:929–930.
Gardner, D.G. A pathologist’s approach to the treatment of ameloblastoma. J Oral Maxillofac Surg. 1984; 42(3):161–166.
Gardner, D.G. Critique of the review by Reichart et al, of the biologic profile of 3677 ameloblastomas. Oral Oncol. 1999; 35(4):443–449.
Gardner, D.G. Some current concepts on the pathology of ameloblastomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996; 82(6):660–669.
Gardner, D.G. The concept of hamartomas: its relevance to the pathogenesis of odontogenic lesions. Oral Surg. 1978; 45(6):884–886.
Gardner, A.F. Proliferation of dental lamina in the wall of a primordial cyst. Oral Surg. 1955; 8:510.
Gardner, D.G., Pecak, A.M.J. The treatment of ameloblastoma based on pathologic and anatomic principles. Cancer. 1980; 46:2514.
Gardner, D.G., Michaels, L., Kiepa, E. Calcifying epithelial odontogenic tumor: an amyloid-producing neoplasm. Oral Surg. 1968; 54:812.
Gardner, D.G., Smith, F.A., Weinberg, S. Ameloblastic fibroma: a benign tumour treatable by curettage. J Can Dent Assoc. 1969; 35:306.
Gardner, D.G. Peripheral ameloblastoma: a study of 21 cases, including 5 reported as basal cell carcinoma of the gingiva. Cancer. 1977; 39:1625.
Geist, S.M., Mallon, H.L. Adenomatoid odontogenic tumor: report of an unusually large lesion in the mandible. J Oral Maxillofac Surg. 1995; 53(6):414–417.
Ghatak, N.R., Hirano, A., Zimmerman, H.M. Ultrastructure of a craniopharyngioma. Cancer. 1971; 27:1465.
Giansanti, J.S., Someren, A., Waldron, A. Odontogenic adenomatoid tumor (adenoameloblastoma): survey of 3 cases. Oral Surg. 1970; 30(1):69–88.
Gilhar, A., Winterstein, G., Godfried, E. Gingival cysts of the newborn. Int J Dermatol. 1988; 27(4):261–262.
Glickman, I., Robinson, L. Destruction of calcified dental tissue in an ovarian dermoid cyst. Oral Surg. 1949; 2:902.
Goldblatt, L.I., Brannon, R.B., Ellis, G.L. Squamous odontogenic tumor: report of five cases and review of the literature. Oral Surg. 1982; 54:187.
Goldblatt, L.I. Ultrastructural study of an odontogenic myxoma. Oral Surg. 1976; 42:206.
Gonzalez-Olaguer, H. Spontaneous disappearance of an eruption cyst. J Philipp Dent Assoc. 1999; 50(4):21–26. [Mar-May].
Gorlin, R.J., Chaudhry, A.P., Pindborg, J.J. Odontogenic tumors: classification, histopathology, and clinical behavior in man and domesticated animals. Cancer. 1961; 14:73–101.
Gorlin, R.J., Chaudhry, A.P. Adenoameloblastoma. Oral Surg. 1958; 11:762–768.
Gorlin, R.J., Meskin, L.H., Brodey, R. Odontogenic tumors in man and animals: pathologic classification and clinical behavior—a review. Ann New York Acad Sci. 1963; 108:722–771.
Gorlin, R.J., Goltz, R.W. Multiple nevoid basal-cell epithelioma, jaw cysts and bifid rib. New Engl J Med. 1960; 262:908.
Gorlin, R.J., Chaudhry, A.P. The ameloblastoma and the craniopharyngioma: their similarities and differences. Oral Surg. 1959; 12:199.
Gorlin, R.J., Chaudhry, A.P., Pindborg, J.J. Odontogenic tumors classification, histopathology, and clinical behavior in man and domesticated animals. Cancer. 1961; 14:73.
Gorlin, R.J., Meskin, L.H., Brodey, R. Odontegenic tumors in man and animals: pathologic classification and clinical behavior—a review. Ann New York Acad Sci. 1963; 108:722.
Gorlin, R.J., Pindborg, J.J., Clausen, F.P., Vickers, R.A. The calcifying odontogenic cyst: a possible analog of the cutaneous calcifying epithelioma of Malherbe. Oral Surg. 1962; 15:1235.
Gorlin, R.J., Pindborg, J.J., Redman, R.S., Williamson, J.J. The calcifying odontogenic cyst: a new entity and possible analog of the cutaneous calcifying epithelioma of Malherbe. Cancer. 1964; 17:723.
Gorlin, R.J., Vickers, R.A., Kelln, E., Williamson, J.J. The multiple basal-cell nevi syndrome. An analysis of a syndrome consisting of multiple nevoid basalcell carcinoma, Jaw cysts, skeletal anomalies, Medulloblastoma, and hyporesponsiveness to parathormone. Cancer. 1965; 18:89.
Gorlin, R.J. Potentialities of oral epithelium manifest by mandibular dentigerous cysts. Oral Surg. 1957; 10:271.
Gould, A.R., Farman, A.G., DeJean, E.K., Van Arsdall, L.R. Peripheral ameloblastoma: an ultrastructural analysis. J Oral Pathol. 1982; 11:90.
Greer, R.O., Jr., Hammond, W.S. Extraosseous ameloblastoma: light microscopic and ultrastructural observations. Oral Surg. 1978; 36:553.
Grimes, O.F., Stephen, H.B. Adamantinoma of the maxilla metastatic to the lung. Ann Surg. 1948; 128:999.
Gunzl, H.J., Horn, H., Vesper, M., Hellner, D. Diagnosis and differential diagnosis of sialo-odontogenic (glandular odontogenic) cyst. Pathologe. 1993; 14:346–350.
Hamner, J.E., III., Pizer, M.E. Ameloblastic odontoma: report of two cases. An J Dis Child. 1968; 115:332.
Hamner, J.E., III., Scofield, H.H., Cornyn, J. Benign fibro-osseous jaw lesions of periodontal membrane origin: an analysis of 249 cases. Cancer. 1968; 22:861.
Harbitz, F. On cystic tumors of the maxillae, and especially on adamantine cystadenomas (adamantomas). Dent Cosmos. 1915; 57:1081–1093.
Haring, J.I., Van Dis, M.L. Odontogenic keratocysts: a clinical, radiographic, and histopathologic study. Oral Surg Oral Med Oral Pathol. 1988; 66:145–153.
Hartman, K.S. Granular-cell ameloblastoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1974; 38(2):241–253.
Hatakeyama, S., Suzuki, A. Ultrastructural study of adenomatoid odontogenic tumor. J Oral Pathol. 1978; 7(5):295–300.
Henderson, J.M., Sonnet, J.R., Schlesinger, C., Ord, R.A. Pulmonary metastasis of ameloblastoma: case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999; 88(2):170–176.
Hietanen, J., Calonius, P.E.B., Collan, Y., Poikkeus, P. Histology and ultrastructure of an ameloblastic fibroma: a case report. Proc Finn Dent Soc. 1973; 69:129.
Hitchin, A.D. The etiology of the calcified composite odontomes. Br Dent J. 1971; 130:475.
Hjorting-Hansen, E., Andreasen, J.O., Robinson, L.H. A study of odontogenic cysts with special reference to location of keratocysts: Part 1. Br J Oral Surg. 1969; 7:15.
Hodson, J.J., Prout, R.E.S. Chemical and histochemical characterization of mucopolysaccharides in a jaw myxoma. J Clin Pathol. 1968; 21:582.
Hoke, H.F., Jr., Harrelson, A.B. Granular cell ameloblastoma with metastasis to the cervicular vertebrae: observations on the origin of the granular cells. Cancer. 1967; 20:991.
Hooker, S.P. Ameloblastic odontoma: an analysis of twenty-six cases. Oral Surg. 1967; 24:375. [(Abst)].
Houston, G., Davenport, W., Keaton, W., Harris, S. Malignant (metastatic) ameloblas-toma: report of a case. J Oral Maxillofac Surg. 1993; 51(10):1152–1155.
Houston, G.D., Fowler, C.B. Extraosseous calcifying epithelial odontogenic tumor: report of two cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997; 83:577–583.
Howell, R.M., Burkes, E.J., Jr. Malignant transformation of ameloblastic fibro-odontoma to ameloblastic fibrosarcoma. Oral Surg. 1977; 43:391.
Huvos, A.G., Marcove, R.C. Adamantinoma of long bones: a clinicopathological study of fourteen cases with vascular origin suggested. J Bone Joint Surg. 1975; 57:148.
Ide, F., Shimoyama, T., Horie, N., Shimizu, S. Intraosseous squamous cell carcinoma arising in association with a squamous odontogenic tumour of the mandible. Oral Oncol. 1999; 35(4):431–434.
Ingham, G.G. Dentinoma. Oral Surg. 1952; 5:353.
Ishikawa, G., Mori, K. A histopathological study on the adenomatoid ameloblastoma—report of four cases. Acta Odont Scand. 1962; 20:419–432.
Ishkawa, T., Yamamoto, H. Case of calcifying epithelial odontogenic tumor in a dog. J Small Anim Pract. 1996; 37:597–599.
James, W., Forbes, J.G. An epithelial odontome. Proc R Soc Med. 1909; 2:166–175.
Ko, K.S.C., Dover, D.G., Jordan, R.C.K. J Can Dent Assoc. 1999; 65:49–51.
Kahn, L.B. Adamantinoma, osteofibrous dysplasia and differentiated adamantinoma. Skeletal Radiol. 2003; 32(5):245–258.
Kao, S.Y., Pong, B.Y., Li, W.Y., Gallagher, G.T. Maxillary odontogenic carcinoma with distant metastasis to axillary skin, brain, and lung: case report. Int J Oral Maxillofac Surg. 1995; 24(3):229–232.
Kaplan, I., Buchner, A., Calderon, S., Kaffe, I. Radiologic and clinical features of calcifying epithelial odontogenic tumor. Dentomaxillofac Radiol. 2001; 30:22–28.
Kawai, T., Kishino, M., Hiranuma, H., Sasai, T. A unique case of desmoplastic ameloblastoma of the mandible: report of a case and brief review of the English language literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999; 87(2):258–263.
JY, de Magalhaes RP, Sousa SC, Magalhaes MH. Management of a large dentigerous cyst occurring in a six-year-old boy.
Kemper, J.W., Root, R.W. Adamanto-odontoma: report of case. Am J Orthod Oral Surg. 1944; 30:709.
Kerezoudis, N.P., Donta-Bakoyianni, C., Siskos, G. The lateral periodontal cyst: aetiology, clinical significance and diagnosis. Endod Dent Traumatol. 2000; 16(4):1445–1450. [Review, Aug].
Kessler, H.P., Schwartz-Dabney, C., Ellis, E., III. Recurrent left mandibular enlargement. J Contemp Dent Pract. 2003; 3(4):127–137.
Keszler, A., Paparella, M.L., Dominguez, F.V. Desmoplastic and non-desmoplastic ameloblastoma: a comparative clinicopathological analysis. Oral Dis. 1996; 2(3):228–231.
Khan, M.Y., Kwee, H., Schneider, L.C., Saber, I. Adenomatoid odontogenic tumor resembling a globulomaxillary cyst: light and electron microscopic studies. J Oral Surg. 1977; 35(9):739–742.
Kimm, H.T. The radiosensitivity of adamantinoma. Chin Med J. 1941; 59:497.
Kramer IRH, Pindborg JJ, Shear M. Histological typing of odontogenic tumors (2nd edn). Springer-Verlag, New York, 1992.
Kreshover SJ. The incidence and pathogenesis of gingival cysts: presented at 35th General Meeting, International Association for Dental Research, March, 1957.
Krikos, G.A. Histochemical studies of mucins of odontogenic cysts exhibiting mucous metaplasia. Arch Oral Biol. 1966; 11:633.
Krolls, S.O., Pindborg, R.J. Calcifying epithelial odontogenic tumor: a survey of 23 cases and discussion of histomorphologic variations. Arch Pathol. 1974; 98:206.
Kunze, E., Donath K Luhr, H.G., Engelhardt, W. Biology of metastasizing ameloblastoma. Pathol Res Pract. 1985; 180(5):526–535.
Larsson, A., Forsberg, O., Sjogren, S. Benign cementoblastoma—Cementum analog of benign osteoblastoma. J Oral Surg. 1978; 36:299.
Laughlin, E.H. Metastasizing ameloblastoma. Cancer. 1989; 64(3):776–780.
Lee, K.W. A light and electron microscopic study ofthe adenomatoid odontogenic tumor. Int J Oral Surg. 1974; 3(4):183–193.
Lee, R.E., White, W.L., Totten, R.S. Ameloblastoma with distant metastases. Arch Pathol. 1959; 68:23.
Lehrhaupt, N.B., Brownstein, C.N., Deasy, M.J. Osseous repair of a lateral periodontal cyst. J Periodontol. 1997; 68(6):608–611. [jun].
Leider, A.S., Nelson, J.F., Trodahl, J.N. Ameloblastic fibrosarcoma of the jaws. Oral Surg. 1972; 33:559.
Levanat, S., Gorlin, R.J., Fallet, S., Johnson, D.R., Fantasia, J.E., Bale, A.E. A two-hit model for developmental defects in Gorlin’s syndrome. Nat Genetics. 1996; 12(1):85–87.
Levy, B.A. Effects of experimental trauma on developing first molar teeth in rats. J Dent Res. 1968; 47:323.
Li, T.J., Kitano, M., Arimura, K., Sugihara, K. Recurrence of unicystic ameloblastoma: a case report and review of the literature. Arch Pathol Lab Med. 1998; 122(4):371–374.
Lucas, R.B., Pindborg, J.J., Odontogenic tumours and tumour-like lesionsCohen, B., eds. Scientific foundations of dentistry. William Heinemann Medical Books, London, 1976:240–250. [Kramer IRH (eds)].
Lucas, R.B. A tumor of enamel epithelium. Oral Surg. 1957; 10:652–656.
Lucas, R.B. Neoplasia in odontogenic cysts. Oral Surg. 1954; 7:1227.
Lurie, H.I. Congenital melanocarcinoma, melanotic adamantinoma, retinal anlage tumor, progonoma, and pigmented epulis of infancy. Cancer. 1961; 14:1090.
Madras, J., Lapointe, H.. Keratocystic odontogenic tumor. 2000;74(2):165 www.cda-adc.ca/jcda.
Maher, W.P., Swindle, P.F. Etiology and vascularization of dental lamina cysts. Oral Surg. 1970; 29(2):590.
Main, D.M.G. Epithelial jaw cysts: a clinicopathological reappraisal. Br J Oral Surg. 1970; 8:1141–1149.
Main, D.M.G. The enlargement of epithelial jaw cysts. Odontol Revy. 1970; 21:29.
Maiorano, E., Renne, G., Tradati, N., Viale, G. Cytologic features of calcifying epithelial odontogenic tumor with abundant cementum-like material. Virchows Arch. 2003; 442:107–110.
Marx, R.E.Oral and maxillofacial pathology. Chicago: Quintessence, 2003.
Mass, E., Kaplan, F., Hirshberg, K. A clinical and histopathological study of radicular cysts associated with primary molars. J Oral Pathol Med. 1995; 24:4584–4661.
McClure, D.K., Dahlin, D.C. Myxoma of bone: report of three cases. Mayo Clin Proc. 1977; 52:249.
McGowan, R.H. Primary intra-alveolar carcinoma: a difficult diagnosis. Br J Oral Surg. 1980; 18:259.
Mcguff, H.S., Alderson, G.L., Jones, A.C. Oral and maxillofacial pathology case of the month: gingival cyst of the adult. Tex Dent J. 2003; 120(1):108–112. [Jan].
Mckelvy, B.D., Cherrick, H.M. Peripheral ameloblastic fibrodentinoma. J Oral Surg. 1976; 34:826.
Mehlisch, D.R., Dahlin, D.C., Masson, J.K. Ameloblastoma: a clinicopathologic report. J Oral Surg. 1972; 30:9.
Melrose, R.J. Benign epithelial odontogenic tumors. Semin Diagn Pathol. 1999; 16:271–287.
Mendis, B.R., Macdonald, D.G. Adenomatoid odontogenic tumour: a survey of 21 cases from Sri Lanka. Int J Oral Maxillofac Surg. 1990; 19(3):141–143.
Mesquita, R.A., Lotufo, M.A., Sugaya, N.N., De Araujo, N.S. Peripheral clear cell variant of calcifying epithelial odontogenic tumor: report of a case and immunohistochemical investigation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003; 95:198–204.
Miles, A.E.W. A cystic complex composite odontome. Proc R Soc Med. 1951; 44:51–55.
Miller, A.S., Lopez, C.F., Pullon, P.A., Elzay, R.P. Ameloblastic fibro-odontoma. Oral Surg. 1976; 41:354.
Mori, M., Yamada, K., Kasai, T., Yamada, T. Immunohistochemical expression of amelogenins in odontogenic epithelial tumours and cysts. Virchows Arch A Pathol Anat Histopathol. 1991; 418(4):319–325.
Mori, M., Murakami, M., Hirose, I., Shimozato, T. Histochemical studies of myxoma of the jaws. J Oral Surg. 1975; 33:529.
Moro, I., Okamura, N., Okuda, S., Komiyama, K., Umemura, S. The eosinophilic and amyloid-like materials in adenomatoid odontogenic tumor. J Oral Pathol. 1982; 11(2):138–150.
Moskow, B.S., Siegel, K., Zegarelli, E.V., Kutscher, A.H. Gingival and lateral periodontal cysts. J Periodontol. 1970; 41(5):249–260.
Muramatsu, T., Hashimoto, S., Inoue, T., Shimono, M. Clear Cell odontogenic carcinoma in the mandible: histochemical and immunohistochemical observations with a review of the literature. J Oral Pathol Med. 1996; 25:516–521.
Murata, M., Cheng, J., Horino, K., Hara, K. Enamel proteins and extracellular matrix molecules are co-localized in the pseudocystic stromal space of adenomatoid odontogenic tumor. J Oral Path Med. 2000; 29:483–490.
Naclerio H, Simoes WA, Zindel D, Chilvarquer I et al. Dentigerous cyst associated with an upper permanent central incisor: case report and literature review.
Nagai, N., Yamachika, E., Nishijima, K., Inoue, M. Tumours and human fetal tooth germs. Eur J Cancer B Oral Oncol. 1994; 30B(3):191–195.
Neville BW, Damm DD, Allen CM, Bouquot JE. Oral and Maxillofacial Pathology (2nd ed). Saunders, Philadelphia, 611–19, 2002.
Nichamin, S.J., Kaufman, M. Gingival microcysts in infancy. Pediatrics. 1963; 31:412.
Nishimura, M., Hori, Y. Adamantinoid basal cell carcinoma: an ultrastructural study. Arch Pathol Lab Med. 1991; 115(6):624–626.
Nishimura, N., Sakurai, K., Noguchi, K., Urade, M. Keratotic basal cell carcinoma of the upper gingiva with cervical lymph node metastasis: report of a case. J Oral I Maxillofac Surg. 2001; 59(6):677–680.
Nxumalo, T.N., Shear, M. Gingival cyst in adults. J Oral Pathol Med. 1992; 21(7):309–313.
Odukoya, O. Odontogenic tumors: analysis of 289 Nigerian cases. J Oral Pathol Med. 1995; 24:454–457.
Oehlers, F.A.C. An unusual pleomorphic adenoma-like tumor in the wall of a dentigerous cyst: report of a case. Oral Surg. 1956; 9:411–417.
Ohmachi, T., Taniyama, H., Nakade, T., Kaji, Y. Calcifying epithelial odontogenic tumors in small domesticated carnivoes: histological, immunohistochemical and electron microscopic studies. J Comp Pathol. 1996; 114:305–314.
Onerci, M., Yilmaz, T., Dogan, R., Sungur, A. Pulmonary metastasectomy in the treatment of recurrent ameloblastoma of the maxilla and mandible: a case report. Eur Arch Otorhinolaryngol. 2001; 258(1):25–27.
Ord, R.A., Blanchaert, R.H., Jr., Nikitakis, N.G., Sauk, J.J. Ameloblastoma in children. J Oral Maxillofac Surg. 2002; 60(7):762–771.
Padayachee, A., Van Wyk, C.W. Two cystic lesions with features of both the botryoid odontogenic cyst and the central mucoepidermoid tumor: sialo-odontogenic cyst. J Oral Pathot. 1987; 16:4995–5004.
Panders, A.K., Hadders, H.N. Solitary keratocysts of the jaws. J Oral Surg. 1969; 27:931–938.
Patron, M., Colmenero, C., Larranri, J. Glandular odontogenic cyst: clinicopathologic analysis of 3 cases. Oral Surg Oral Med Oral Pathol. 1991; 72:71–74.
Payne, T.P. An analysis of the clinical and histopathologic parameters of the odontogenic keratocyst. Oral Surg. 1972; 53:538.
Peterson, T.C., Gorlin, R.J. Possible analogous cutaneous and odontogenic tumors. Arch Dermatol. 1964; 90:255.
Philips, V.M., Grotepass, F.W., Hendricks, R. Ameloblastic odontosarcoma with epithelial atypia: a case report. Br J Oral Maxillofac Surg. 1998; 26:45–51.
Philipsen, H.P., Birn, H. The adenomatoid odontogenic tumor. Acta Pathol Microbiol Scand. 1969; 75:375–398.
Philipsen, H.P., Reichart, P.A., Nikai, H., Takata, T. Peripheral ameloblastoma: biological profile based on 160 cases from the literature. Oral Oncol. 2001; 37(1):17–27.
Philipsen, H.P., Reichart, P.A., Zhang, K.H., Nikai, H. Adenomatoid odontogenic tumor: biologic profile based on 499 cases. J Oral Pathol Med. 1991; 20:149–158.
Philipsen, H.P., Reichart, P.A. Adenomatoid odontogenic tumor: facts and figures. Oral Oncol. 1998; 35:125–131.
Philipsen, H.P., Reichart, P.A. Revision of the 1992-edition of the WHO histological typing of odontogenic tumours. a suggestion. J Oral Pathol Med. 2002; 31(5):253–258.
Philipsen, H.P., Reichart, P.A. The adenomatoid odontogenic tumour: ultrastructure of tumour cells and noncalcified amorphous masses. J Oral Pathol Med. 1996; 25(9):491–496.
Philipsen, H.P., Reichart, P.A. Unicystic ameloblastoma: a review of 193 cases from the literature. Oral Oncol. 1998; 34(5):317–325.
Philipsen, H.P., Reichert, P.A. Calcifying epithelial odontogenic tumour: biological profile based on 181 cases from the literature. Oral Oncol. 2000; 36:17–26.
Philipsen, H.P. Om keratocyster (kolesteatom) I kaekberne. Tandlaegegebladet. 1956; 60:963–981.
Philipsen, H.P., Birn, H. The adenomatoid odontogenic tumour: ameloblastic, adenomatoid tumour or adeno-ameloblastoma. Acta Pathol Microbiod Scand (A). 1969; 75:375.
Pincock, L.D., Bruce, K.W. Odontogenic fibroma. Oral Surg. 1954; 7:307.
Pindborg, J.J., Clausen, F. Classification of odontogenic tumors: a suggestion. Acta Odont Scand. 1958; 16:293–301.
Pindborg, J.J., Hansen, J. Studies on odontogenic cyst epithelium II: clinical and roentgenologic aspects of odontogenic keratocysts. Acta Pathol Microbiol Scand. 1963; 58:283–294.
Pindborg, J.J., Vedtofte, P., Reibel, J., Praetorious, F. The calcifying epithelial odontogenic tumor. A review of recent literature and report of a case. APMIS Suppl. 1991; 23:152–157.
Pindborg, J.J. A calcifying epithelial odontogenic tumor. Cancer. 1958; 11:838–843.
Pindborg, J.J. Calcifying epithelial odontogenic tumors. Acta Pathol Microbiol Scand Suppl. 1955; 111:71.
Pindborg, J.J., Clausen, F. Classification of odontogenic tumors. Acta Odontol Scand. 1958; 16:293.
Pindborg, J.J., Kramer, I.R.H., Torloni, H.Histological Typing of Odontogenic Tumours, Jaw Cysts, and Allied Lesions: International Histological Classification of Tumours(5). Geneva: World Health Organisation, 1971.
JJ, Philipsen HP, Henriksen J. Studies on odontogenic cyst epithelium in: Fundamentals of Keratinization Publication (70). American Association for the Advancement of Science, Washington DC, 151–60, 1962.
Poulson, T.C., Greer, R.O., Jr. Adenomatoid odontogenic tumor: clinicopathologic and ultrastructural concepts. J Oral Maxillofac Surg. 1983; 41(12):818–824.
Praetorius, F., Hjoting-Hansen, E., Gorlin, R.J., Vickers, R.A. Calcifying odontogenic cyst: range, variations neoplastic potential. Acta Odontol Scand. 1981; 39:227.
Pullon, P.A., Shafer, W.G., Elzay, R.P., Kerr, D.A. Squamous odontogenic tumor. Oral Surg. 1975; 40:616.
Punniamoorthy, A. Gigantiform cementoma: a review of the literature a case report. Br J Oral Maxillo Surg. 1980; 18:221.
R, Martinez-Conde. Paradental cyst of the second molar: report of a bilateral case. J Oral Maxillo Surg. 1995; 53(10):1212–1214. [Octo].
Raghoebar, G.M., Vissink, A. Gingival cysts in a newborn infant. Ned Tijdschr Tandheelkd. 2001; 108(12):500. [Dutch, Dec].
Ramadas, K., Jose, C.C., Subhashini, J., Chandi, S.M., Viswanathan, F.R. Pulmonary metastases from ameloblastoma of the mandible treated with cisplatin, adriamycin, and cyclophosphamide. Cancer. 1990; 66(7):1475–1479.
Ramon Boj, J., Garcia-Godoy, F. Multiple eruption cysts: report of case. ASDC J Dent Child. 2000; 67(4):282–284. [232. Review, Jul-Aug].
Ranlov, P., Pindborg, J.J. The amyloid nature of the homogeneous substance in the calcifying epithelial odontogenic tumor. Acta Pathol Microbiol Scand (A). 1966; 68:169.
Rater, C.J., Selke, A.C., Van Epps, E.F. EF Basal cell nevus syndrome. Am J Roentgenol Radium Ther Nucl Med. 1968; 103:589.
Raubenheimer, E.J., Seeliger, J.E., van Heerden, W.F., Dreyer, A.F. Adenomatoid odontogenic tumour: a report of two large lesions. Dentomaxillofac Radiol. 1991; 20(1):43–45.
Reeve, C.M., Levy, B.P. Gingival cysts: a review of the literature and a report of four cases. Period. 1968; 6:115.
Regezi, J.A. Odontogenic cysts, odontogenic tumors, fibroosseous, and giant cell lesions of the jaws. Mod Pathol. 2002; 15(3):331–341.
Regezi, J.A., Kerr, D.A., Courtney, R.M. Odontogenic tumors: analysis of 706 cases. J Oral Surg. 1978; 36:771.
Reichart, P.A., Philipsen, H.P., Sonner, S. Ameloblastoma: biological profile of 3677 cases. Eur J Cancer B Oral Oncol. 1995; 31(2):86–99. [B].
Reichart, P.A., Philipsen, H.P.Odontogenic tumors and allied lesions. London: Quintessence, 2004.
Richardson, J.F., Balogh, K., Merk, F., Booth, D. Pigmented odontogenic tumor of jawbone. A previously undescribed expression of neoplastic potential. 1974; 34:1244. [Cancer].
Rick, G.M., Reibel, J., Wewer, U., Basement membraneproteins in adenomatoid odontogenic tumors [abstract No. 40]Abstracts of the 38th Annual Meeting of the American Academy of Oral Pathology. Boston: American Academy of Oral Pathology, 1984.
Ritchey, B., Orban, B. Cysts of the gingiva. Oral Surg. 1953; 6:765.
Robinson HBG. Primordial cyst versus keratocyst. Oral Surg, 975, 40: 362–66.
Robinson, H.B.G. Proceedings of the Fifth Annual Meeting of the American Academy of Oral Pathology. Oral Surg. 1952; 5:177–178.
Robinson, L., Martinez, M. Unicystic ameloblastoma: a prognostically distinct entity. Cancer. 1977; 40(5):2278–2285.
Robinson, H.B.G., Koch, WE Jr. Diagnosis of cysts of the jaw. J Mo Dent Assoc. 1941; 21:187.
Robinson, H.B.G., Koch, WE Jr, Kolas, S. Radiographic interpretation of oral cysts. Dent Radiogr Photogr. 1956; 29:61.
Robinson, H.B.G. Ameloblastoma: a survey of 379 cases from the literature. Arch Pathol. 1937; 23:831.
Robinson, L., Martinez, M.G. Unicystic ameloblastoma: a prognostically distinct entity. Cancer. 1977; 40:227.
Rodini, C.O., Lara, V.S. Study of the expression of CD68+ macrophages and CD8+T cells in human granulomas and periapical cysts. Oral Surg Oral Medi Oral Patholo Oral Radiol and Endodon. 2001; 92(2):221–222. [Aug].
Rodini, Co, Batista, A.C., Lara, V.S. Comparative immunohistochemical study of the presence of mast cells in apical granulomas and periapical cysts: possible role of mast cells in the course of human periapical lesions. Oral Surg Oral Medi Oral Patho Oral Radiol Endod. 2004; 97(1):59–63. [Jan].
Rosai, J. Admantinoma of the tibia: electron microscopic evidence of its epithelial origin. Am J Clin Pathol. 1969; 51:786–##.
Rud, J., Pindborg, J.J. Odontogenic keratocysts: a follow-up study of 21 cases. J Oral Surg. 1969; 27:323–330.
Sadeghi, E.M., Weldon, L.L., Kwnn, P.H., Sampson, E. Mucoepidermoid odontogenic cyst. Int J Oral Maxillofac Surg. 1991; 20:142–143.
Saku, T., Okabe, H., Shimokawa, H. Immunohistochemical demonstration of enamel proteins in odontogenic tumors. J Oral Pathol Med. 1992; 21(3):113–119. [An aggressive dentigerous cyst in a seven-year-old child. ASDC J Dent Child, 68(4): 268-71. Review, Jul-Aug, 2001.Scannell JM Jr. Cementoma. Oral Surg, 2: 1169, 1949].
Scannell, JM Jr. Cementoma. Oral Surg. 1949; 2:1169.
Schafer, D.R., Thompson, L.D.R., Smith, B.C., Wenig, B.M. Primary ameloblastoma of the sinonasal tract: a clinicopathologic study of 24 cases. Cancer. 1998; 82(4):667–674.
Schlosnagle, D.C., Someren, A. The ultrastructure of the adenomatoid odontogenic tumor. Oral Surg Oral Med Oral Pathol. 1981; 52(2):154–161.
Schweitzer, F.C., Barnfield, W.F. Ameloblastoma of the mandible with metastasis to the lungs; report of a case. J Oral Surg. 1943; 1:287.
Sciubba, J.J., Zola, M.B. Odontogenic epithelial hamartoma. Oral Surg. 1978; 45:261.
Sedano, H.O., Pindborg, J.J. Ghost cell epithelium in odontomas. J Oral Pathol. 1975; 4:27.
Seeman, G.F. Sacrococcygeal cystic teratoma with traumatic hemorrhage. Oral Surg. 1948; 1:308.
Seemayer, T.A., Blundell, J.S., Wiglesworth, F.W. Pituitary craniopharyngioma with tooth formation. Cancer. 1972; 29:423.
Sehdev, M.K., Huvos, A.G., Strong, E.W., Gerold, F.P. Ameloblastoma of maxilla and mandible. Cancer. 1974; 33(2):324–333.
Seward, M.H. Eruption cyst: an analysis of its clinical features. J Oral Surg. 1973; 31:31.
Shade, N.L., Carpenter, W.M., Delzer, D.D. Gingival cyst of the adult. Case report of a bilateral presentation. J Periodontol. 1987; 58(11):796–799.
Shafer, W.G., Hine, M.K., Levy, B.M. Tumors and cysts of odontogenic origin: in a textbook of oral pathology, 2, Philadelphia: WB Saunders; 1963:218–219.
Shafer, W.G., Frissell, C.T. The melano-ameloblastoma and retinal anlage tumors. Cancer. 1953; 6:360.
Shafer, W.G., Waldron, C.A.Fibro-osseous Lesions of the Jaws. Reno, Nevada: American Academy of Oral Pathology Continuing Education Course, 1982. [May].
Shafer, W.G. Ameloblastic fibroma. J Oral Surg. 1955; 13:317.
Sharffetter, K., Balz-Herrmann, C., Lagrange, W., Koberg, W. Proliferation kinetics–study of the growth of keratocysts. J Cranio-Max-Fac Surg. 1989; 17:226–233.
Shear, M., Pindborg, J.J. Microscopic features of the lateral periodontal cyst. Scand J Dent Res. 1975; 83(2):103–110.
Shear, M. The aggressive nature of the odontogenic keratocyst: is it a benign cystic neoplasm? Oral Oncology. 2002; 38:407–415.
Shear, M. The histogenesis of the tumor of enamel organ epithelium. Br Dent J. 1962; 112(12):494–498.
Shear, M. Cysts of the oral regions, 3. Boston: Wright, 1992.
Shear, M., Altini, M. Malignant odontogenic tumours. J Dent Assoc S Afr. 1982; 37:547.
Shear, M., Pindborg, J.J. Microscopic features of the lateral periodontal cyst. Scand J Dent Res. 1975; 83:103.
Shear, M. Primary intra-alveolar epidermoid carcinoma of the jaw. J Pathol. 1969; 97:645.
Sherman, R.S., Caumartin, H. The roentgen appearance of adamantinoma of the mandible. Radiology. 1955; 65:361.
Shimono, M., Shimono, Y., Hashimoto, S., Yamane, H. Intercellular junctions in an adenomatoid odontogenic tumor. Bull Tokyo Dent Coll. 1984; 25(4):145–157.
Shohat, A., Buchner, S. Taicher and Mibular buccal bifurcation cyst: enucleation without extraction Int J Oral Maxillof Surg. 2003; 32(6):610–613. [Dec].
Siar, C.H., Ng, K.H., Murugasu, P. Adenomatoid odontogenic tumour: gross and histological examination of 45 cases. Singapore Med J. 1987; 28(2):180–189.
Singh, S., Singh, M., Chhabra, N., Nagar, Y. Dentigerous cyst: a case report. J Indian Soc Pedod Prev Dent. 2001; 19(3):123–126.
Sivapathasundharam, B., Einstein, A., Syed, R.I. Desmoplastic ameloblastoma in Indians: report of five cases and review of literature. Indian J Dent Res. 2007; 18(4):218–221.
Slater, L.J. Odontogenic malignancies. Oral Maxillofa Surg Clin N Am. 2004; 16:409–424.
Slootweg, P.J., Muller, H. Malignant ameloblastoma or ameloblastic carcinoma. Oral Surg Oral Med Oral Pathol. 1984; 57(2):168–176.
Slootweg, P.J. An analysis of the interrelationship of the mixed odontogenic tumors— ameloblastic fibroma, ameloblastic fibro-odontoma, and the odontomas. Oral Surg. 1981; 51:266.
Small, I.A., Waldron, C.A. Ameloblastomas of the jaws. Oral Surg. 1955; 8:281.
Smith, J.F. The controversial ameloblastoma. Oral Surg. 1968; 26:45–75.
Smith, R.R., Olson, J.L., Hutchins, G.M., Crawley, W.A. Adenomatoid odontogenic tumor: ultrastructural demonstration of two cell types and amyloid. Cancer. 1979; 43(2):505–511.
Smith, I., Shear, M. Radiological features of mandibular primordial cysts. J Max-Fac Surg. 1978; 6:147.
Snead, M.L., Luo, W., Hsu, D.D., Melrose, R.J. Human ameloblastoma tumors express the amelogenin gene. Oral Surg. 1992; 74(1):64–72.
Soskolne, W.A., Shear, M. Observations on the pathogenesis of primordial cysts. Br Dent J. 1967; 123:321.
Spouge, J.D. The adenoameloblastoma. Oral Surg. 1967; 23(4):470–482.
Sriram, G., Shetty, R.D. Odontogenic tumors: a study of 250 case in an Indian teaching hospital. Oral Surg Oral Med Oral Pathol Oral Radiol Endo. 2008; 105(6):14–21.
Stafne, E.C. Epithelial tumors associated with developmental cysts of the maxilla: report of three cases. Oral Surg. 1948; 1:887–894.
Stafne, E.C. Periapical osteofibrosis with formation of cementoma. J Am Dent Assoc. 1934; 21:1822.
Standish, S.M., Shafer, W.G. The lateral periodontal cyst. J Periodontol. 1958; 29:27.
Stanley, H.R., Diehl, D.L. Ameloblastoma potential of follicular cysts. Oral Surg. 1965; 20:260.
Stasinopoulos, M. Mixed calcifiedodontogenic tumours. Br J Oral Surg. 1970; 8:93.
Stockdale, Cr, Chandler, N.P. The nature of the periapical lesion — a review of 1108 cases. J Dentis. 1988; 16(3):123–129.
Stoelinga, P.J. Long-term follow-up on keratocysts treated according to a defined protocol. Int J Oral Maxillofac Surg. 2001; 30:14–25.
Stoelinga, Pjw, Bronkhorst, F.B. The incidence, multiple presentation and recurrence of aggressive cysts of the jaws. J Cranio-Max-Fac Surg. 1988; 16:184–195.
Stoelinga, P.J.W., Peters, J.H. A note on the origin of keratocysts of the jaws. Int J Oral Surg. 1973; 2:37.
Struthers, P., Shear, M. Root resorption by ameloblastomas and cysts of the jaws. Int J Oral Surg. 1976; 5:128.
Swinson, T.W. An extraosseous adenomatoid odontogenic tumor. Br J Oral Surg. 1977; 15:32.
Tagaki, M. Adenomatoid ameloblastoma: an analysis of nine cases by histopathological and electron microscopic study. Bull Tokyo Med Dent Univ. 1967; 14:487–506.
Takahashi, K., Yoshino, T., Hashimoto, S. Unusually large cystic adenomatoid odontogenic tumour of the maxilla: case report. Int J Oral Maxillofac Surg. 2001; 30(2):173–175.
Takata, T., Zhao, M., Uchida, T., Kudo, Y. Immunohistochemical demonstration of an enamel sheath protein, sheathlin, in odontogenic tumors. Virchows Arch. 2000; 436(4):324–329.
Takeda, Y. Glandular odontogenic cyst mimicking a lateral periodontal cyst: a case report. Int J Oral Maxillofac Surg. 1994; 23:96–97.
Takiguchi, M.T., Fujiwara, S., Sobire, T., Uoshimq. Radicular cysts associated with a primary molar following pulp therapy: a case report. Int J Pediatr Dent. 2001; 11:452–455.
Tandler, B., Rossi, E.P. Granular cell ameloblastoma: electron microscopic observations. J Oral Pathol. 1977; 6:401.
Tatemoto, Y., Tanaka, T., Okada, T., Mori, M. Adenomatoid odontogenic tumour: coexpression of keratin and vimentin. Virchows Arch A Pathol Anat Histopathol. 1988; 413(4):341–347.
Teuscher, G.W. 128 years of public service. ASDC J Dent Child. 2000; 67(5):365–368. [Sep Oct].
Thoma, K.H., Goldman, H.M. Odontogenic tumors: a classification based on observations of the epithelial, mesenchymal, and mixed varieties. Am J Pathol. 1946; 22:433–471.
Thoma, K.H. A contribution to the knowledge of the development of the submaxillary and sublingual salivary glands in human embryos. J Dent Res. 1919; 1:95–142.
Thoma, K.H. Adenoameloblastoma. Oral Surg. 1955; 8:441–444.
Thoma, K.H. Oralpathology, ed. Tumors of odontogenic origin. CV Mosby, St Louis, 1941:945–946.
Thoma, K.H. Cementoblastoma. Int J Orthod. 1937; 23::1127.
Tiecke, R.W., Bernier, J.L. Melanotic ameloblastoma. Oral Surg. 1956; 9::1197.
Toida, H., Nakashima, E., Okumura, Y., Tatematsu, N. Glandular odontogenic cyst: a case report and literature review. J Oral Maxillofac Surg. 1994; 52::1312–1316.
Toller, P. Origin and growth of cysts of the jaws. Ann R Coll Surg, Engl. 1967; 40::306.
Toller, P.A. Protein substances in odontogenic cyst fluids. Br Dent J. 1970; 128::317.
Tratman, E.K. Classification of odontomas. Br Dent J. 1951; 91::167.
Trodahl, J.N. Ameloblastic fibroma. A survey of cases from the Armed Forces Institute of Pathology. Oral Surg. 1972; 33::547.
Tsagaris, G.T.A review of the ameloblastic fibro-odontoma MS Thesis. Washington DC: George Washington University, 1972.
Tsaknis, P.J. Carpenter WM, Shade NL. Odontogenic adenomatoid tumor: report of case and review of the literature. J Oral Surg. 1977; 35(2):146–1449.
Tsaknis, P.J., Nelson, J.F. The maxillary ameloblastoma: an analysis of 24 cases. J Oral Surg. 1980; 38(5):336–342.
Tsukada, Y., de la Pava, S., Pickren, J.W. Granularcell ameloblastoma with metastasis to the lungs. Report of a case and review of the literature. Cancer. 1965; 18::916.
Turner, J.G. A note on enamel nodules. Br Dent J. 1945; 78:39.
Unni, K.K., Dahlin, D.C., Beabout, J.W., Ivins, J.C. Adamantinomas of long bones. Cancer. 1974; 34:1796.
Ustuner, E., Fitoz, S., Atasoy, C., Erden, I., Akyar, S. Bilateral maxillary dentigerous cysts: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003; 95(5):632–635.
Valderhaug, J., Zander, H.A. Relationship of “epithelial rests of Malassez” to other periodontal structures. J AM Soc Periodontol. 1967; 5:254.
Van der Waal, I., Van der Dwast, W.A.M. A case of gigantiform cementoma. Int J Oral Surg. 1974; 3:440.
van-Heerden, W.F., Raubenheimer, E.J., Turner, M.L. Glandular odontogenic cyst. Head Neck. 1992; 14:316–320.
Vap, D.R., Dahlin, D.C., Turlington, E.G. Pindborg tumor: the so-called calcifying epithelial odontogenic tumor. Cancer. 1970; 25:629.
Vedtofte, p, Praetorious, F. Recurrence of the odontogenic keratocyst in relation to clinical and histological features. Int J Oral Surg. 1979; 8:412–420.
Vedtofte, P., Praetorius, F. The inflammatory paradental cyst. Oral Surg Oral Med Oral Pathol. 1989; 68:182–188.
Vickers, R.A., Gorlin, R.J. Ameloblastoma: delineation of early histopathologic features of neoplasia. Cancer. 1970; 26(3):699–710.
Vickers, R.A., Dahlin, D.C., Gorlin, R.J. Amyloid containing odontogenic tumors. Oral Surg. 1965; 20:476.
Villa, V.G. Ameloblastic sarcoma in the mandible: report of case. Oral Surg. 1955; 8:123.
Vindenes, H., Nilsen, R., Gilhuus-Moe, O. Benign cementoblastoma. Int J Oral Surg. 1979; 8:318.
Waldron, C.A., Giansanti, J.S., Browand, B.C. Sclerotic cemental masses of the jaws. Oral Surg. 1975; 39:590. [(socalled chronic sclerosing ostemoyelitis, sclerosing osteitis, multiple enostosis, and gigantiform cementoma)].
Walton, R.E. The radicular cyst: Does it exist? Oral Surg Oral Med Oral Pathol. 1996; 82:471.
Wang, S.Z., Chen, X.M., Li, Y. Clinicopathologic analysis of glandular odontogenic cyst. Chung Hua Kou Chiang Hsueh Tsa Chih. 1994; 29:329–331.
Weathers, D.R., Waldron, C.A. Unusual multilocular cysts of the jaws. Oral Surg. 1973; 36:235. [(botryoid odontogenic cysts)].
Wertheimer, F.W., Fullmer, H.M., Hansen, L.S. A histochemical study of hyaline bodies in odontogenic cysts and a comparison to the human secondary dental cuticle. Oral Surg. 1962; 15:1466.
Wertheimer, F.W., Zielinski, R.J., Weskey, R.K. Extraosseous calcifying epithelial odontogenic tumor. Int J Oral Surg. 1977; 6:266.
Wesley, R.K., Wysocki, G.P., Mintz, S.M. The central odontogenic fibroma clinical and morphologic studies. Oral Surg. 1975; 40:235.
Wessberg, G.A. Unusual presentations of dentigerous cysts. Hawaii Dent J. 1998; 29(9):12–14. [Nov–Dec].
Wettan, H.L., Patella, P.A., Freedman, P.D. Peripheral ameloblastoma: review of the literature and report of recurrence as severe dysplasia. J Oral Maxillofac Surg. 2001; 59(7):811–815.
White, D.K. Preface. Oral and Maxillofacial Surgery Clinics. 2004; 16:IX–XI.
White, D.K., Chen, S-Y, Mohnac, S.M., Miller, A.S. Odontogenic myxoma. Oral Surg. 1975; 39:901.
Willis, R.A.Teratomas Atlas of Tumor Pathology, Section III, Fascicle 9. Washington DC: Armed Forces Institute of Pathology, 1951.
Winer, H.J., Goepp, R.A., Olsen, R.E. Gigantiform cementoma resembling—Paget’s disease: report of a case. J Oral Surg. 1972; 30:517.
Witterick, I.J., Parikh, S., Mancer, K., Gullane, P.J. Malignant ameloblastoma. Am J Otolaryngol. 1996; 17(2):122–126.
Wohl, M.G. Tooth germ cysts of the jaw. Ann Surg. 1916; 64:672–679.
Woo, S.B., Smith-Williams, J.E., Sciubba, J.J., Lipper, S. Peripheral ameloblastoma of the buccal mucosa: case report and review of the English literature. Oral Surg Oral Med Oral Pathol. 1987; 63(1):78–84.
Wright, J.M. The odontogenic keratocyst: orthokeratinized variant. Oral Surg. 1981; 51:609–618.
Wright, B.A., Jennings, E.H. Oxytalan fibers in peripheral odontogenic fibromas: a histochemical study of eighteen cases. Oral Surg. 1979; 48:451.
Wright, J.M., Jr. Squamous odontogenic tumor like proliferations in odontogenic cysts. Oral Surg. 1979; 47:354.
Wysocki, G.P., Sapp, J.P. Scanning and transmission electron microscopy of odontogenic keratocysts. Oral Surg. 1975; 40:494.
Wysocki, G.P., Brannon, R.B., Gardner, D.G., Sapp, P. Histogenesis of the lateral periodontal cyst and the gingival cyst of the adult. Oral Surg. 1980; 50:327.
Yamamoto, H., Kozawa, Y., Hirai, G., Hagiwara, T. Adenomatoid odontogenic tumor: light and electron microscopic study. Int J Oral Surg. 1981; 10(4):272–278.
Yasuhiro, Morimoto. Inflammatory paradental cyst (IPC) in the mandibular premolar region in children Oral Surg, Oral Med Oral Pathol Oral Radiol Endo. 2004; 97(2):286–293. [February].
Zachariades, N., Papanicolaou, S., Triantafyllou, D. D. Odontogenic keratocysts: review of the literature and report of sixteen cases. J Oral Maxillofac Surg. 1985; 43:177–182.
Zallen, R.D., Preskar, M.H., McClary, S.A. Ameloblastic fibroma. J Oral Maxillofac Surg. 1982; 40:513.
Zegarelli, E.V., Ziskin, D.E. Cementomas: a report of 50 cases. Am J Orthod Oral Surg. 1943; 29:285.
Zegarelli, E.V., Napoli, N., Hoffman, P. The cementoma: a study of 230 patients with 435 cementomas. Oral Surg. 1964; 17:219.
Zwahlen, R.A., Gratz, K.W. Maxillary ameloblastomas: a review of the literature and of a 15-year database. J Craniomaxillofac Surg. 2002; 30(5):273–279.
Zwahlen, R.A., Vogt, P., Fischer, F.S., Gratz, K.W. Case report: myocardial metastasis of a maxillary malignant ameloblastoma. J Oral Maxillofac Surg. 2003; 61(6):731–734.