Diseases of the Pulp and Periapical Tissues
Diseases of Dental Pulp
The dental pulp is a delicate connective tissue liberally interspersed with tiny blood vessels, lymphatics, nerves, and undifferentiated connective tissue cells. Like other connective tissues throughout the body, it reacts to bacterial infection or to other stimuli by an inflammatory response known as pulpitis, which is the most common cause of odontalgia or toothache. Certain anatomic features of this specialized connective tissue; however, tend to alter the nature and the course of this response. The enclosure of the pulp tissue within the rigid calcified walls of the dentin precludes the excessive swelling of tissue that occurs in the hyperemic phases of inflammation in other tissues. The fact that the blood vessels supplying the pulp tissue must enter the tooth through the tiny apical foramina precludes the development of an extensive collateral blood supply to the inflamed part.
The diseases of the dental pulp to be considered in this section are those occurring chiefly as sequelae of dental caries. Those reactions following various physical and chemical injuries are discussed in Chapter 12 on Physical and Chemical Injuries of the Oral Cavity. The sequential conditions are almost exclusively inflammatory and do not differ basically from inflammation elsewhere in the body.
Etiologic Factors
Most cases of pulpitis are primarily a result of dental caries in which bacteria or their products invade the dentin and pulp tissue. Dental caries is usually obvious unless it extends under the edge of a restoration. Brannstrom and Lind, among others, have reported that changes in the pulp may occur even with very early dental caries represented by demineralization limited to the enamel alone, appearing as white spots without actual cavitations.
Occasionally, there is bacterial invasion in the absence of caries, as in cases of tooth fracture due to trauma or cracked tooth syndrome that expose the dental pulp to the oral environment. In cracked tooth syndrome, a tooth, usually a restored premolar may split under masticatory stress. These cracks are often minute and invisible clinically, and they allow the bacteria to enter into the pulp. Bacterial invasion may also occur as a result of a bacteremia and septicemia. Pulpitis may rarely follow chronic periodontal disease wherein the microorganisms enter through the accessory canals of the exposed root surface especially through lateral canals in furcation areas of molars.
The significance of microorganisms in the etiology of pulpitis has been confirmed by Kakehashi and his associates, who produced surgical pulp exposures in germ-free rats. It was found that no devitalized pulps or periapical infections developed even when gross food impactions existed. By contrast, conventional animals rapidly developed complete pulpal necrosis.
Pulpitis may also arise as a result of chemical irritation of the pulp caused by erosion or use of acidic restorative materials. This may occur not only in an exposed pulp to which some irritating medicament is applied but also in intact pulps beneath deep or moderately deep cavities into which some irritating filling material is inserted. This is undoubtedly a result of penetration of the irritating substances into the pulp via the dentinal tubules. In many instances; however, the pulp may respond to the irritation either by dentinal sclerosis or by forming reparative dentin rather than progressing to pulpitis.
Severe thermal change in a tooth may also produce pulpitis. Polishing procedures, tooth restored with exothermic restorative materials, or large metallic restorations, particularly, in which there is inadequate insulation between the restoration and the pulp are more prone to pulpal inflammation.
Heat produced by over-rapid tooth preparation or without sufficient coolant may also cause pulpal irritation. Heat and more particularly, cold are transmitted to the pulp, often producing pain, and if the stimulus is prolonged and severe, leading to actual pulpitis. Mild thermal changes are most apt to stimulate only the formation of reparative dentin, and this is a relatively common phenomenon.
When two dissimilar metallic restorations are present, the saliva acts as an electrolyte and there will be formation of a galvanic current. This may be transmitted to the pulp through metallic restoration and may thus initiate pulpitis.
It is apparent that pulpitis may be caused by a variety of circumstances and the nature of the etiologic agent or agents can usually be found through study of the clinical or microscopic features of the condition or both.
Classification of Pulpitis
Pulpitis has been classified in a variety of ways, the simplest being a division into acute and chronic pulpitis. Furthermore, some investigators classify both acute and chronic pulpitis in several different ways. There may be a partial pulpitis or a subtotal pulpitis, depending upon the extent of involvement of the pulp. If the inflammatory process is confined to a portion of the pulp, usually a portion of the coronal pulp such as a pulp horn, the condition has been called partial or focal pulpitis. If most of the pulp is diseased, the term total or generalized pulpitis has been used. But this is of marginal clinical significance.
Another classification of both acute and chronic pulpitis is based upon the presence or absence of a direct communication between the dental pulp and the oral environment, usually through a large carious lesion. The term open pulpitis (pulpitis aperta) has been used to describe those cases of pulpitis in which the pulp obviously communicates with the oral cavity, whereas the cases in which no such communication exists are described as closed pulpitis (pulpitis clausa). In both the clinical and the histologic features of open and closed pulpitis, differences do exist that are referable to the presence or absence of drainage, which in turn determine the degree of pain present. The basic process is the same in each case, but the classification has been used as an aid in understanding the variations in clinical features that occur in different cases.
In this section, pulpitis will be discussed under the two chief types of the disease: acute and chronic. In addition, attention will be drawn to those differences in clinical and histologic features that are dependent upon the extent of the inflammation and upon whether drainage can occur.
Focal Reversible Pulpitis
One of the earliest forms of pulpitis is the condition known as focal reversible pulpitis. At one time, this was often referred to as pulp hyperemia. However, it is known that vascular dilatation can occur artefactually from the ‘pumping’ action during tooth extraction as well as pathologically as a result of dentinal and pulpal irritation. Therefore, this early mild transient pulpitis, localized chiefly to the pulpal ends of irritated dentinal tubules, is now known as focal reversible pulpitis.
Clinical Features
A tooth with focal pulpitis is sensitive to thermal changes, particularly to cold. The application of ice or cold fluids to the tooth results in pain, but this disappears upon removal of the thermal stimuli or restoration of the normal temperature. It will be found also that such a tooth responds to stimulation by the electric pulp tester at a lower level of current, indicating a lower pain threshold (or a greater sensitivity) than that of adjacent normal teeth. Teeth in which this condition exists usually show deep carious lesions, large metallic restorations (particularly without adequate insulation), or restorations with defective margins.
Histologic Features
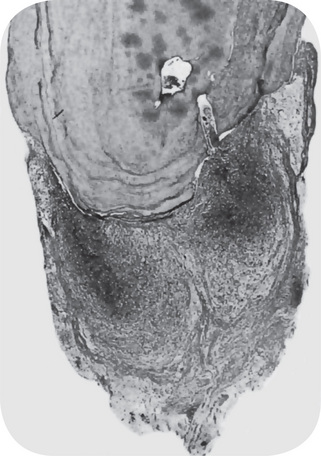
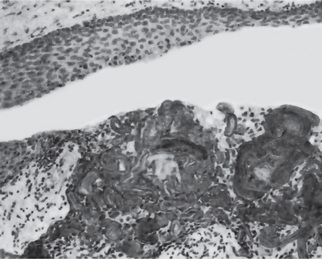
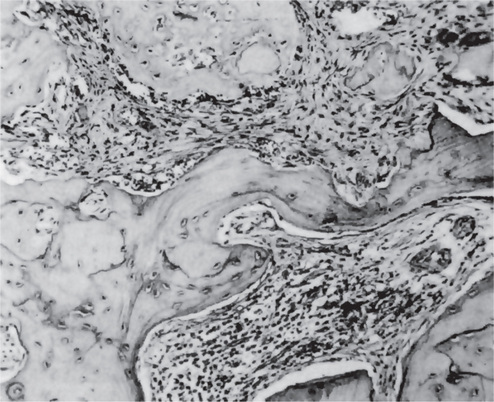
Focal pulpitis is characterized microscopically by dilatation of the pulp vessels (Fig. 10-1). Edema fluid may collect because of damage to the capillary walls, allowing actual extravasation of red blood cells or some diapedesis of white blood cells. Slowing of the blood flow and hemoconcentration due to transudation of fluid from the vessels conceivably could cause thrombosis. The belief has prevailed also that self-strangulation of the pulp may occur as a result of increased arterial pressure occluding the vein at the apical foramen. Boling and Robinson argued that this belief is incorrect because the pulp may have several afferent and efferent vessels and several foramina, making self-strangulation unlikely.
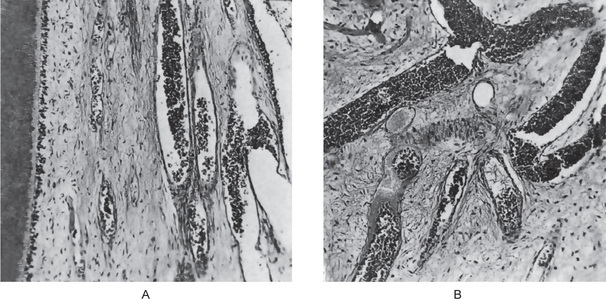
Figure 10-1 Focal reversible pulpitis.
The photomicrographs show vasodilatation but no extravasation of red or white blood cells.
Supporting this, in clinical practice, when a necrosed pulp chamber is opened, there may be vital tissue in the root canal of some teeth proving that total necrosis does not always occur. Reparative dentin may be noted in the adjacent dentinal wall.
Treatment and Prognosis
Focal pulpitis is generally regarded as a reversible condition, provided the irritant is removed before the pulp is severely damaged. Thus, a carious lesion should be excavated and restored or a defective filling replaced as soon as it is discovered. If the primary cause is not corrected, extensive pulpitis eventually results, with subsequent ‘death’ of the pulp.
Acute Pulpitis
Extensive acute inflammation of the dental pulp is a frequent immediate sequela of focal reversible pulpitis, although it may also occur as an acute exacerbation of a chronic inflammatory process. Significant differences in both the clinical and microscopic features are found between acute and chronic pulpitis.
Clinical Features
Acute pulpitis usually occurs in a tooth with a large carious lesion or restoration, commonly a defective one around which there has been ‘recurrent caries’. Even in its early stages when the inflammatory reaction involves only a portion of the pulp, usually in the area just beneath the carious lesion, relatively severe pain is elicited by thermal changes, particularly when taking ice or cold drinks. Characteristically, this pain persists even after the thermal stimulus has disappeared or been removed. However, a clinicopathologic study by Mitchell and Tarplee provided evidence that evaluation of the type or degree of pulpitis is present by sensitivity to either heat or cold is fallacious, since in their study most patients with any type of pulpitis exhibited increased sensitivity to both heat and cold. This has been confirmed by Seltzer and his associates, who have also shown that the severity of the pain is only partially related to the severity of the inflammatory response.
Other factors include establishment of drainage, the patient’s previous experiences, emotions, and so forth.
As a greater proportion of the pulp becomes involved with intrapulpal abscess formation, the pain may become even more severe and is often described as lancinating or throbbing type. The pain generally lasts for 10–15 minutes but may be more or less continuous, and its intensity may be increased when the patient lies down. The application of heat may cause an acute exacerbation of pain. The tooth reacts to the electric pulp vitality tester at a lower level of current than adjacent normal teeth, indicating increased sensitivity of the pulp. When necrosis of the pulp tissue occurs, this sensitivity is lost.
Severe pain is more likely to be present when the entrance to the diseased pulp is not wide open. The pulpal pain is not only caused by the pressure built-up due to lack of escape of inflammatory exudates but also by the pain producing substances released by the inflammatory reaction. Caviedes-Bucheli J and coworkers have demonstrated that, the receptors for substance P, a neurotransmitter in the pain fiber system, increases during inflammation of pulp. Soon there is rapid spread of inflammation throughout the pulp with pain and necrosis. Until this inflammation or necrosis extends beyond root apex, the tooth is not particularly sensitive to percussion. When a large open cavity is present, there is no opportunity for a build-up of pressure. Thus the inflammatory process does not tend to spread rapidly throughout the pulp. In such a case the pain experienced by the patient is a dull, throbbing ache, but the tooth is still sensitive to thermal changes. Mobility and sensitivity to percussion are usually absent.
The patient with a severe acute pulpitis is extremely uncomfortable and at least mildly ill. He/she is usually apprehensive and desirous of immediate attention from the dentist.
Early in the course of the disease, the polymorphonuclear leukocytes are confined to a localized area, and the remainder of the pulp tissue appears relatively normal. The rise in pressure in the pulp associated with an inflammatory exudate causes local collapse of the venous part of the circulation. This leads to local tissue hypoxia and anoxia, which in turn may lead to localized destruction and the formation of a small abscess, known as a pulp abscess, which contains pus arising from breakdown of leukocytes and bacteria as well as from digestion of tissue (Figs. 10-2, 10-3). This necrotic zone contains polymorphonuclear leukocytes and histiocytes. Abscess formation is most likely to occur when the entrance to the pulp is a tiny one and there is lack of drainage. The chemical mediators released from the necrotic tissue lead to further inflammation and edema. Hepatocyte growth factor (HGF), a multifunctional cytokine mediates epithelial mesenchymal interaction, and is involved in the development and regeneration of various tissues including teeth. Ohnishi T et al, have reported the presence of HGF during acute inflammation of the pulp. Interleukin-8 level in the exudate of the acute pulpitis is higher than that in chronic pulpitis as shown by Guo X et al.
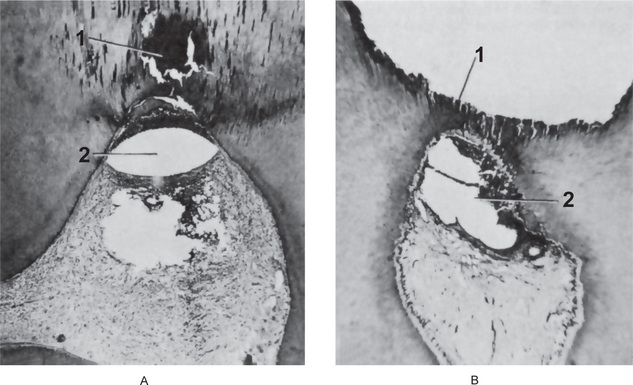
Figure 10-2 Acute pulpitis with pulp abscess formation.
There is diffuse inflammation of the pulp chamber in A beneath the carious lesion (1) with the formation of a circumscribed focus of suppuration, a pulp abscess (2). In B, the carious lesion (1) has evoked only a focal inflammation of the pulp with abscess formation (2).
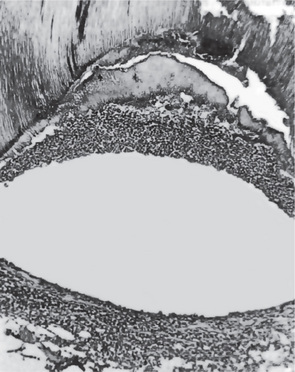
Figure 10-3 Pulp abscess.
The high-power photomicrograph of Figure 10-2 A, shows the void caused by the loss of the suppurative contents of the abscess and the limiting band of leukocytes.
Eventually, in some cases in only a few days, the acute inflammatory process spreads to involve most of the pulp so that neutrophilic leukocytes fill the pulp. The entire odontoblastic layer degenerates. If the pulp is closed to the outside, there is considerable pressure formed, and the entire pulp tissue undergoes rather rapid disintegration. Numerous small abscesses may form, and eventually the entire pulp undergoes liquefaction and necrosis. This is sometimes referred to as acute suppurative pulpitis (Fig. 10-4).
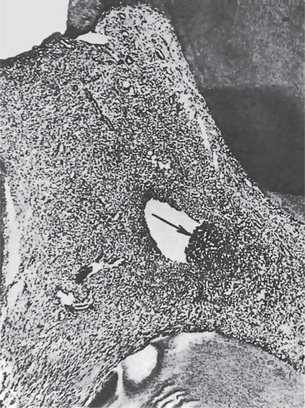
Figure 10-4 Acute pulpitis.
The entire pulp is involved (total pulpitis), and a focal area of suppuration is present.
The pulp, especially in the later stages of pulpitis following carious invasion, contains large numbers of bacteria. These microorganisms are usually a mixed population and consist essentially of those found normally in the oral cavity.
Treatment and Prognosis
There is no successful treatment of an acute pulpitis involving most of the pulp that is capable of preserving the pulp. Once this degree of pulpitis occurs, the damage is irreparable. Occasionally, acute pulpitis—especially with an open cavity—may become quiescent and enter a chronic state. This is unusual; however, and appears to occur most frequently in persons who have a high tissue resistance or in cases of infection with microorganisms of low virulence. In very early cases of acute pulpitis involving only a limited area of tissue, there is some evidence to indicate that pulpotomy (removal of the coronal pulp) and placing a bland material that favors calcification, such as calcium hydroxide, over the entrance to the root canals may result in survival of the tooth. This technique is also used in cases of mechanical pulp exposures without obvious infection (Fig. 10-5). Teeth involved with acute pulpitis may be treated by filling the root canals with an inert material, provided the pulp chamber and root canals can be sterilized.
Chronic Pulpitis
Chronic pulpitis may arise on occasion through quiescence of a previous acute pulpitis, but more frequently it occurs as the chronic type of disease from the onset. As in most chronic inflammatory conditions, the signs and symptoms are considerably milder than those in the acute form of the disease. A special form of chronic pulpitis known as chronic hyperplastic pulpitis has characteristic features and will be described separately.
Clinical Features
Pain is not a prominent feature of chronic pulpitis, although sometimes the patient complains of a mild, dull ache, which is more often intermittent than continuous. The reaction to thermal change is dramatically reduced in comparison to that in acute pulpitis. Because of the degeneration of nerve tissue in the affected pulp, the threshold for stimulation by the electric pulp vitality tester is often increased. The general features of chronic pulpitis are not distinctive, and serious involvement of the pulp may be present in the absence of significant symptoms. Even in cases of chronic pulpitis with wide-open carious lesions and with exposure of the pulp to the oral environment, there is relatively little pain. The exposed pulp tissue may be manipulated by a small instrument, but though bleeding may occur, pain is often absent. As Selzer and his associates have emphasized, pulps may become totally necrotic without pain.
Histologic Features
Chronic pulpitis is characterized by infiltration of the pulp tissue by varying numbers of mono-nuclear cells, chiefly lymphocytes and plasma cells (Fig. 10-6) and more vigorous connective tissue reaction. Bacterial products may act as antigens and the dendritic cells of the pulp capture the antigens, migrate to lymph nodes and present them to lymphocytes. These activated T cells then leave the lymph nodes and reach the pulp. Capillaries are usually prominent; fibroblastic activity is evident; and collagen fibers are seen, often gathered in bundles. There is sometimes an attempt by the pulp to ward off the infection through deposition of collagen fibers around the inflamed area. The tissue reaction may resemble the formation of granulation tissue. When this occurs on the surface of the pulp tissue in a wide-open exposure, the term ulcerative pulpitis is applied. With bacterial stains, microorganisms may be found in the pulp tissue, especially in the area of a carious exposure. In some cases, the pulpal reaction vacillates between an acute and a chronic phase. This holds true not only for diffuse inflammation but also for that form of pulp disease characterized by pulp abscess formation. Thus a pulp abscess may become quiescent and be surrounded by a fibrous connective tissue wall, which is known as the pyogenic membrane.
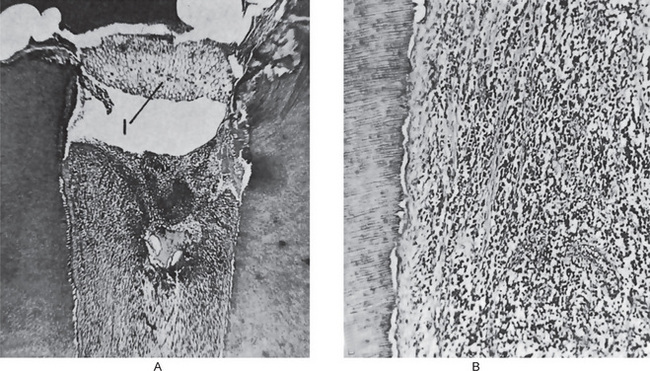
Figure 10-6 Chronic pulpitis.
(A) The pulp of this tooth shows diffuse involvement by chronic inflammatory cells. The entrance to the pulp chamber is wide open (open pulpitis), but contains food debris (1). (B) In the high-power photomicrograph there is seen diffuse infiltration of lymphocytes and plasma cells with fibrosis and loss of the odontoblastic layer.
In nearly all cases, the pulp is eventually involved in its entirety by the chronic inflammatory process, although this may take a long time to present few clinical symptoms.
Chronic Hyperplastic Pulpitis: (Pulp polyp)
Chronic hyperplastic pulpitis is a unique form of pulpitis wherein the inflamed pulp, instead of perishing by continued suppuration, reacts by excessive and exuberant proliferation. It occurs either as a chronic lesion from the onset or as a chronic stage of a previously acute pulpitis.
Clinical Features
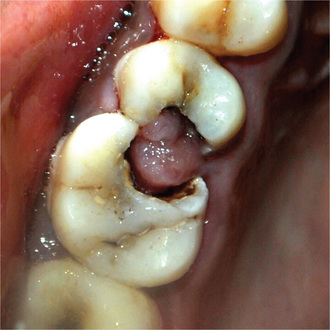
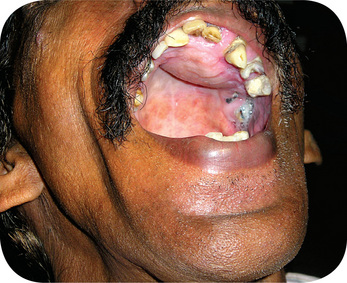
Chronic hyperplastic pulpitis occurs almost exclusively in children and young adults who possess a high degree of tissue resistance and reactivity, and readily respond to proliferative lesions. It involves teeth with large, open carious lesions. A pulp so affected appears as a pinkishred globule of tissue protruding from the pulp chamber and not only fills the caries defect but also extends beyond (Fig. 10-7). Because the hyperplastic tissue contains few nerves, it is relatively insensitive to manipulation. However, Southam and Hodson have found that sometimes innervation of polyps may be quite rich and have stated that the number of nerve fibers in pulp polyps cannot be presumed to be directly related to the sensory acuity found on clinical examination. They have even noted innervation of the epithelium in epithelialized polyps in some instances. The lesion may or may not bleed readily, depending upon the degree of vascularity of the tissue and epithelialization.
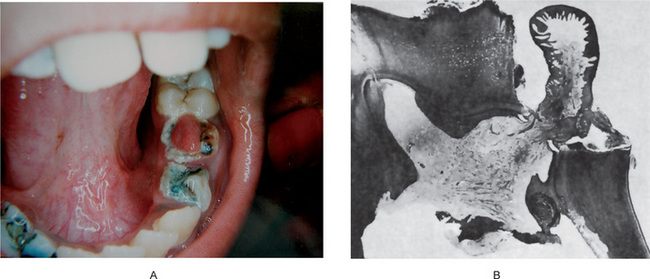
Figure 10-7 Chronic hyperplastic pulpitis.
(A) There is a mass of tissue protruding from the pulp chamber into the carious lesion. (B) In the photomicrograph this is seen to be continuous with the pulp and covered by stratified squamous epithelium (A, Courtesy of Dr S Karthigakannan, Department of Oral Medicine, Sree Moogambica Dental College, Tamil Nadu).
The teeth most commonly involved by this phenomenon are the deciduous molars and the first permanent molars. These have an excellent blood supply because of the large root opening, and this coupled with the high tissue resistance and reactivity in young persons, accounts for the unusual proliferative property of the pulp tissue.
Histologic Features
The hyperplastic tissue is basically granulation tissue made up of delicate connective tissue fibers interspersed with variable numbers of small capillaries (Fig. 10-7). Inflammatory cell infiltration, chiefly lymphocytes and plasma cells, sometimes admixed with polymorphonuclear leukocytes, is common. In some instances fibroblast and endothelial cells proliferation is prominent.
This granulation tissue commonly becomes epithelialized and the origin of these epithelial cells is a matter of controversy. The epithelium is stratified squamous in type and closely resembles the oral mucosa, even to the extent of developing well formed rete ridges. The grafted epithelial cells are thought to be normally desquamated cells carried to the surface of the pulp by the saliva. Most desquamated epithelial cells in the saliva are degenerated superficial squames, which have lost their dividing capacity. For the polyp to become epithelialized, the cells should have the capacity to divide and differentiate into stratified squamous epithelium. So, such cells must come from the region of the basal cell layer and might be released from trauma or from the gingival sulcus. In some instances, the buccal mucosa may rub against the hyperplastic tissue mass, and epithelial cells become transplanted directly. Southam and Hodson have reported that polyps from deciduous teeth were epithelialized far more frequently (82% of 56 polyps) than those of permanent teeth (44% of 77 polyps). It should be appreciated that the tissue reaction here is an inflammatory hyperplasia and does not differ from inflammatory hyperplasia occurring elsewhere in the oral cavity as well as in other parts of the body. In time, organization of the tissue leads to decreased vascularity and increased fibrosis. M Sattari, AK Haghighi, and HD Tamijani showed that presence and concentration of IgE, histamine and IL-4 were higher in pulp polyps than in normal pulps, and suggested that type I hypersensitivity reaction being involved in pulp polyp’s pathogenesis.
Diseases of Periapical Tissues
Once infection has become established in the dental pulp, spread of the process can be in only one direction—through the root canals and into the periapical region. Here a number of different tissue reactions may occur, depending upon a variety of circumstances.
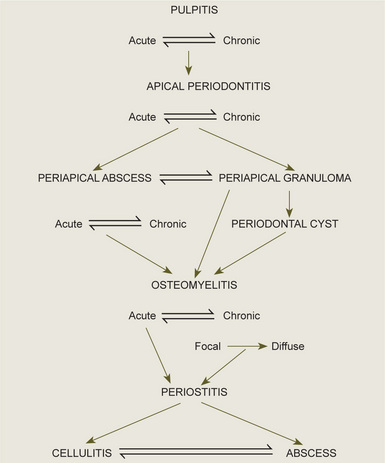
It is important to realize that these periapical lesions do not represent individual and distinct entities, but rather that there is a subtle transformation from one type of lesion into another type in most cases. Furthermore, it should be appreciated that a certain degree of reversibility is possible in some lesions. The interrelations between the types of periapical infection must be clearly understood, and the schematic diagram shown in Figure 10-9 will aid in clarification of this.
Apical Periodontitis
Apical periodontitis is the inflammation of the periodontal ligament around the root apex. Though the inflammatory process here is similar to that occurring elsewhere, there may be resorption of the periapical bone and sometimes the root apex. This process may be acute or chronic depending upon the virulence of the microorganisms involved, the type and severity of the physical or chemical irritants, and host resistance. The common causes of apical periodontitis include spread of infection following pulp necrosis, occlusal trauma from a high restoration or biting suddenly on a hard object, inadvertent endodontic procedures such as over instrumentation, pushing the infected material into the apical portion or chemical irritation from root canal medicaments.
Acute Apical Periodontitis
Patients suffering from acute apical periodontitis usually give the history of previous pulpitis. Thermal change does not induce pain as in pulpitis. Due to the collection of inflammatory edema in the periodontal ligament, the tooth is slightly elevated in its socket and causes tenderness while biting or even to mere touch. The external pressure on the tooth forces the edema fluid against already sensitized nerve endings and results in severe pain. Radiographic appearance is essentially normal at this stage except for a slight widening of periodontal ligament space.
Histologic Features
The periodontal ligament shows signs of inflammation characterized by vascular dilatation and infiltration with polymorphonuclear leukocytes. Initially, these changes are localized around the root apex, as this area is richly vascular. The inflammation is transient if it is caused by acute trauma. If the irritant is not removed, it progresses with resorption of the surrounding bone. Abscess formation may occur if it is associated with bacterial infection and is known as acute periapical abscess or alveolar abscess.
Chronic Apical Periodontitis
Chronic apical periodontitis, also known as periapical granuloma, is a low-grade infection and one of the most common of all sequelae of pulpitis or acute periapical periodontitis. If the acute process is left untreated, it is incompletely resolved and becomes chronic. The acute inflammatory process is an exudative response whereas the chronic one is proliferative. Periapical granuloma is essentially a localized mass of chronic granulation tissue formed in response to the infection (Fig. 10-10). But the use of this term is not totally accurate since it does not shows true granulomatous inflammation microscopically.
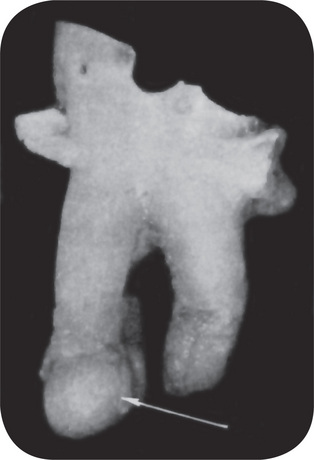
Figure 10-10 Periapical granuloma.
The granuloma often remains attached to the root when the tooth is extracted.
It should be pointed out here that the spread of pulp infection is usually, but not always, in a periapical direction. The presence of lateral or accessory root canals opening on the lateral surface of the root at any level is a well-recognized anatomic deviation along which the infection may spread. This would give rise to a ‘lateral’ granuloma or related inflammatory lesion.
Clinical Features
The involved tooth is usually nonvital and may be slightly tender to percussion, and percussion may produce a dull sound instead of a normal metallic sound because of the presence of granulation tissue around the root apex. Patients may complain of mild pain on biting or chewing on solid food. In some cases, the tooth feels slightly elongated in its socket and may actually be so. The sensitivity is due to hyperemia, edema, and inflammation of the apical periodontal ligament. The early or even the fully developed chronic periapical granuloma seldom presents any more severe clinical features than those just described.
Actually, many cases are entirely asymptomatic. There is usually no perforation of overlying bone and oral mucosa with the formation of a fistulous tract unless the lesion undergoes an acute exacerbation.
Radiographic Features
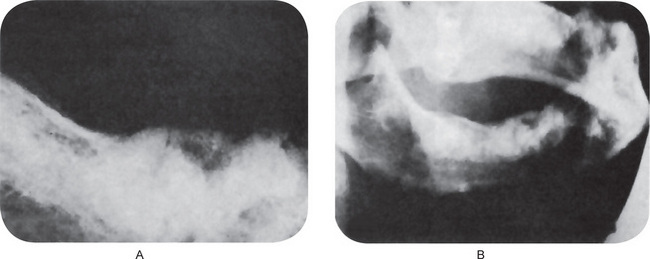
The earliest periapical change in the periodontal ligament appears as a thickening of the ligament at the root apex (Fig. 10-11). As proliferation of granulation tissue and concomitant resorption of bone continues, the periapical granuloma appears as a radiolucent area of variable size seemingly attached to the root apex (Fig. 10-12). In some cases, this radiolucency is a well-circumscribed, definitely demarcated from the surrounding bone. In these instances a thin radiopaque line representing a zone of sclerotic bone may sometimes be seen outlining the lesion. This indicates that the periapical lesion is a slowly progressive and long standing one that has probably not undergone an acute exacerbation.
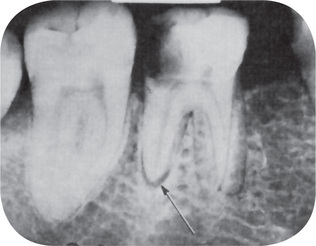
Figure 10-11 Early apical periodontitis.
There is radiographic evidence of thickening of the apical periodontal membrane as a result of the large carious lesion involving the dental pulp.
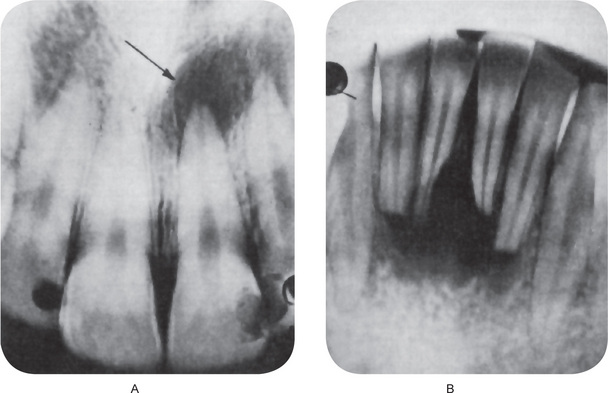
Figure 10-12 Periapical granuloma.
The periapical radiolucencies signify destruction of bone and replacement by granulation tissue. The maxillary central incisor (A) has a carious lesion of the distal surface that involves the pulp. The mandibular incisors (B) have sustained traumatic injury with loss of pulp vitality and subsequent formation of diffuse periapical granulomata.
The periphery of granulomas in other instances appears on the radiograph as a diffuse blending of the radiolucent area with the surrounding bone. This difference in radiographic appearance is due to the difference in cellular activity around the margins of the lesion and cannot be used to distinguish between different forms of periapical disease. Although the diffuse radiolucency might suggest a more acute phase of disease or a more rapidly expanding lesion, this is not necessarily the case. In addition, some degree of root resorption is occasionally observed.
Histologic Features
The periapical granuloma that arises as a chronic process from the onset and does not pass through an acute phase begins as a hyperemia and edema of the periodontal ligament with infiltration of chronic inflammatory cells. The inflammation and locally increased vascularity of the tissue are associated with resorption of the supporting bone adjacent to this area. Occasionally, microscopic or even macroscopic resorption of the root apex occurs, but this is not usually an early finding. As the bone is resorbed, there is proliferation of both fibroblasts and endothelial cells and the formation of more tiny vascular channels as well as numerous delicate connective tissue fibrils (Figs. 10-13, 10-14). The new capillaries are usually lined by swollen endothelial cells. It is a relatively homogeneous lesion composed predominantly of macrophages, lymphocytes, and plasma cells, and less frequently with mast cells and eosinophils, thus qualifying as an immune-type granuloma. As Athanassiades and Spears, and Page and his associates have pointed out, immune granulomas have more lymphocytes and plasma cells than non-immune granulomas, which are relatively pure collections of macrophages and giant cells with only a rare plasma cell.
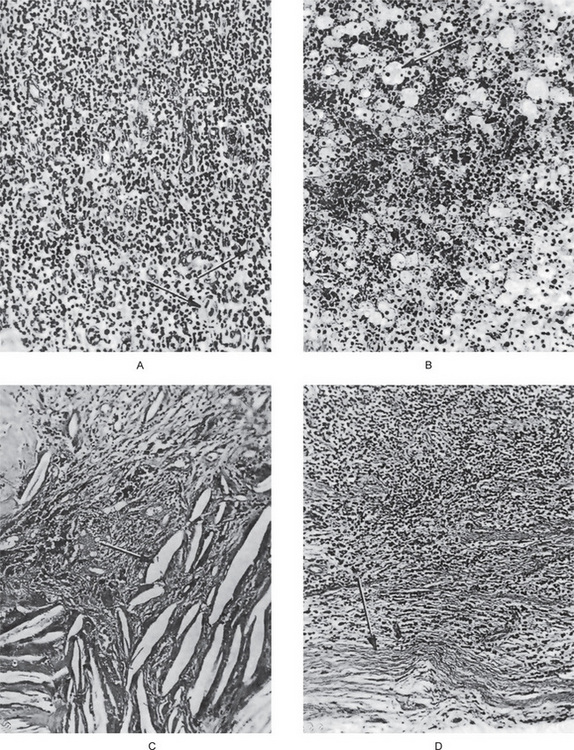
Figure 10-14 Periapical granuloma.
The photomicrographs represent various histologic features, although all such features need not be present in a single granuloma. The typical periapical granuloma shows the delicate fibrillar stroma with intense lymphocytic and plasma cell infiltration and sometimes polymorphonuclear leukocytes as well as many small capillaries (A), collections of macrophages that are often filled with lipoid material (B), and cholesterol slits in the tissue (C). The typical granuloma is usually surrounded by a connective tissue ‘capsule’ (D).
The vast majority of the small lymphocytes (81%) was not associated with immunoglobulin production and was designated non-B lymphocytes. 19% of the lymphocytes contained immunoglobulins, of which the majority (74%) produced IgG. IgA was found in 20% of the lymphocytes, while IgE and IgM were found in 4% and 2%, respectively. Many of the plasma cells contained Russell bodies, some of which were subsequently extruded and could be found extracellularly. The non-B lymphocytes are probably the T cells of the cellular arm of the immune system, thus making the lesion an expression of delayed type hypersensitivity. There is ample evidence to indicate that T cell activity could account for much of the bone and tooth resorption through the production of osteoclast activating factor (OAF). Since T cells also produce various cytotoxic lymphokines, collagenase and other enzymes, and destructive lymphokines, they may be responsible for much of the destructive potential of the periapical lesion. On the other hand, the presence of antibody-producing lymphocytes and plasma cells in periapical granulomas is very important because antibodies are known to be modulators of disease activity. In addition, their specificity may provide clues to the antigens and thus to the causes of periapical granulomas and cysts.
Macrophages and other mononuclear phagocytes are the hallmarks of granulomatous inflammation, a specific form of chronic inflammation.
In periapical granuloma substantial amount of dendritic cells are present. As per the study by Kaneko T et al, these dendritic cells are associated with local defense reactions as stronger antigen presenting than macrophages.
Compared with granulomatous inflammation, banal chronic inflammation lacks organization. Rather it is a diffuse heterogeneous collection of cells, usually dominated by mononuclear cells other than macrophages, as Adams has noted. The cause of differentiation from solid to cystic periapical lesions cannot be deduced from alterations in the inflammatory cell populations of granulomas and cysts. There were no differences in inflammatory cell composition, immunoglobulin production, or immunoglobulin distribution between granulomas and cysts.
In some granulomas, large numbers of phagocytes will ingest lipid material and become collected in groups, forming sheets of so-called foam cells (Fig. 10-14B). Abundant mast cells may also be found. Deposits of cholesterol as well as hemosiderin are often present and both are probably derived from the breakdown of extravasated red blood cells. Cholesterol crystals appear microscopically as clear needlelike spaces or clefts owing to the dissolving of the contained cholesterol by the agents used in the preparation of the tissues for histologic examination (Fig. 10-14C) and are almost invariably associated with multinucleated giant cells of the foreign body type.
Connective tissue activity is usually most prominent on the periphery of the granuloma, and the bundles of collagen become condensed there, apparently as a result of the slow expansion of the soft-tissue mass, to form a continuous capsule separating the granulation tissue from the bone (Fig. 10-14D).
Another important feature noted in the chronic periapical granuloma is the presence of epithelium.
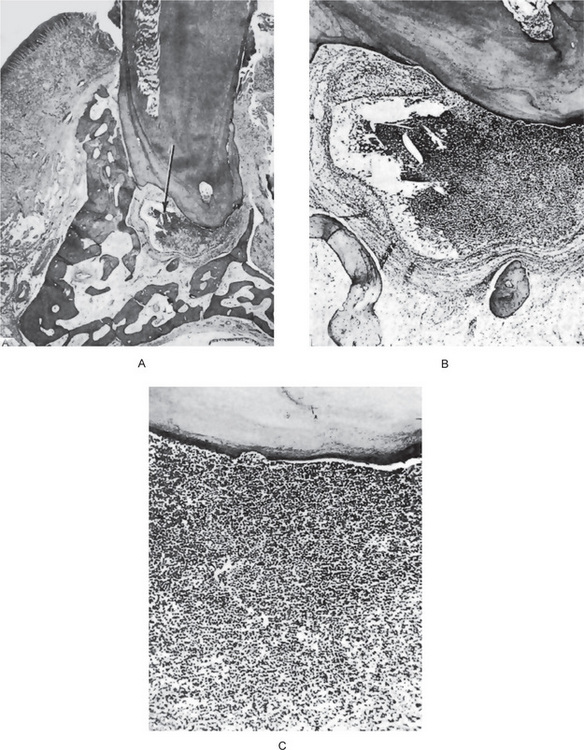
In early periapical granulomas, the epithelium is confined to the immediate vicinity of the periodontal ligament. In course of time, the epithelium undergoes proliferation by the inflammatory stimuli, in an attempt to wall off the irritant coming out through the apical foramen, which becomes extensive, and presents as sheets of stratified squamous epithelial cells as well as anastomosing cords (Fig. 10-15). It is this epithelium that gives rise to the apical periodontal cyst, and a sharp dividing line between granuloma and cyst cannot always be drawn because of the tendency for degeneration of individual epithelial cells that could be considered precystic (Fig. 10-16).
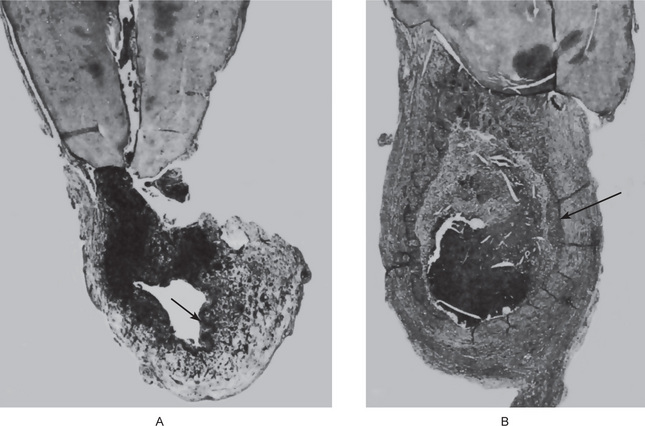
Figure 10-15 Epithelial proliferation in a periapical granuloma attached to the tooth root.
Arrow indicates sheets of proliferating epithelial cells, derived from the epithelial cell rests of Malassez.
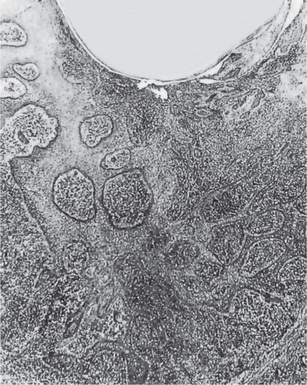
Figure 10-16 Precystic epithelial proliferation in a periapical granuloma.
A lumen has just formed and may be considered the beginning of an apical periodontal cyst.
That epithelium is uniformly present in all periapical granuloma has been substantiated by numerous workers. Its demonstration is dependent, in many cases, upon serial section of the tissue specimens, but this procedure will reveal its presence. Thus every periapical granuloma may potentially form a periodontal cyst if it is left undisturbed and if the inflammatory reaction persists to stimulate the epithelium.
One additional interesting finding in occasional periapical granulomas is a condition described by Dunlap and Barker as giant-cell hyaline angiopathy. This consists of inflammatory cell infiltration, collections of foreign body type giant cells, and the presence of ringlike structures known as Rushton bodies, composed of an eosinophilic material resembling hyalinized collagen. There may also be seen as fragments of foreign material, sometimes resembling vegetable matter such as legumes, which suggested the use of the term ‘pulse granuloma’ for this lesion by King and by Mincer and his coworkers. Dunlap and Barker believed that the earliest change was an acute vasculitis with subsequent thickening and hyalinization of vessel walls. This tissue finding is not confined to periapical granulomas but has also been reported in granulomas in edentulous jaws, in a nasopalatine duct cyst, and in chronic periostitis.
This has been studied by both electron microscopy and immunoperoxidase procedure by Chen and his colleagues, who concluded that the hyaline bodies are probably endogenous in origin. The true nature of this lesion still remains uncertain but is of little apparent clinical significance.
Microbiologic Features. Bacteria were found not only in the periapical abscesses but also in granulomas and cysts. One of the factors that has made this a difficult area of investigation is the inability of the dentist to extract a tooth without microbial contamination of the periapical area or the granuloma.
The majority of studies have been based upon bacteriologic cultures taken after extraction of the tooth. Pre-extraction cultures have been made in a few instances through the root canal or the alveolar plate, and these have been relatively free of actual contamination. The microorganisms that have been isolated by such techniques, e.g. in the studies of Burket, were those generally found in the oral cavity, such as Streptococcus viridans, Streptococcus hemolyticus, nonhemolytic streptococci, Staphylococcus aureus, Staphylococcus albus, Escherichia coli, and pneumococci. Seldom can microorganisms actually be demonstrated histologically in the periapical granuloma.
Some investigators have even suggested that the dental granuloma is usually a sterile lesion. However, the body of evidence now forming indicates that many of these lesions may indeed be infected before and after endodontic treatment. Iwu et al, in a study showed 88% or 14 of 16 periapical granulomas were bacteria positive when they were homogenized and cultured. Mixed infections are the rule, as the organisms present being generally found in the mouth. It has not been possible to associate particular types of microorganisms with specific periapical lesions, based upon either clinical or histologic evaluation.
Sebati M and Slots J claimed on their study on 34 periapical lesions, that most teeth with necrosed pulp and periapical lesions harbored human cytomegalovirus and Epstein-Barr virus in periapical granulomatous tissue, and these viruses along with the endodontopathic bacteria may play an important role in etiopathogenesis of aggressive type of periapical pathosis in human.
Slots J and coworkers in their study involving 25 symptomatic and 19 nonsymptomatic periapical lesions found human cytomegalovirus (HCMV) in 100% of symptomatic and 37% of non-symptomatic periapical lesions and Epstein-Barr virus (EBV) only in lesions infected with HCMV. They concluded that periapical pathosis may be caused by HCMV and EBV by induction of cytokine and lymphokine induction from inflammatory or connective tissue cells or by impairing the local host defenses which increase the virulence of resident bacterial pathogens.
Treatment and Prognosis
The treatment of the periapical granuloma consists in extraction of the involved teeth, or under certain conditions, root canal therapy with or without subsequent apicoectomy. If left untreated, the periapical granuloma may ultimately undergo transformation into an apical periodontal cyst.
Apical Periodontal Cyst: (Radicular cyst, periapical cyst, root end cyst)
The apical periodontal cyst is the most common odontogenic cyst encountered in a dental clinic. It is the usual but not inevitable sequela of the periapical granuloma originating as a result of bacterial infection and necrosis of the dental pulp, nearly always following carious involvement of the tooth.
It is a true cyst, since the lesion consists of a pathologic cavity that is lined by epithelium and is often fluid-filled (Fig. 10-15). The epithelial lining is derived from the epithelial rests of Malassez, which proliferate as a result of the inflammatory stimulus in a pre-existing granuloma. As pointed out in the section on the periapical granuloma, the epithelium may be derived in some cases from:
• Respiratory epithelium of the maxillary sinus when the periapical lesion communicates with the sinus wall
• Oral epithelium from a fistulous tract
• Oral epithelium proliferating apically from a periodontal pocket.
Pathogenesis
This type of periodontal cyst exhibits a lumen that almost invariably lined by stratified squamous epithelium, while the wall is made up of condensed connective tissue. Although the stimulus for the proliferation of epithelium in the periodontal cyst is recognized to be the inflammation in the periapical granuloma, the reason that all such granulomas do not eventually develop into cysts is not known. This is particularly puzzling, since rests of Malassez are universally present in the periodontal ligament areas of all teeth. It might be that if all periapical granulomas persisted for a sufficiently long time, they would eventuate in cysts.
The actual mode of development of the apical periodontal cyst is an interesting phenomenon. The initial reaction leading to cyst formation is a proliferation of the epithelial rests in the periapical area involved by the granuloma. This epithelial proliferation is induced by the keratinocyte growth factor elaborated by the stromal cells of the periodontal ligament, or inflammatory stimulus. Gao et al, speculated that activated T cells in the periapical granulomas produce lymphokines that may act on the rest of Malassez causing proliferation and altered differentiation leading to cyst formation. This epithelial proliferation follows an irregular pattern of growth and occasionally presents a frightening picture because of the pseudo-invasiveness and inflammatory altered appearance of the cells (Fig. 10-16). As this proliferation continues, the epithelial mass increases in size by division of the cells on the periphery, corresponding to the basal layer of the surface epithelium, and the cells in the central portion of the mass become separated further and further from their source of nutrition, the capillaries and tissue fluid of the connective tissue. As these central cells fail to obtain sufficient nutrients, they eventually degenerate, become necrotic, and liquefy (Fig. 10-17). This creates an epithelium-lined cavity filled with fluid, the apical periodontal cyst.
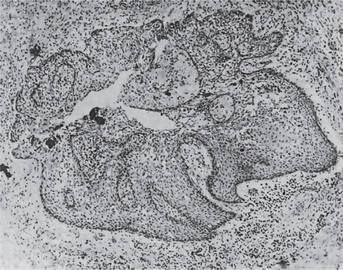
Figure 10-17 Precystic epithelial proliferation in a periapical granuloma.
A lumen has just formed and may be considered the beginning of an apical periodontal cyst.
It is conceivable also that a cyst may form through proliferation of epithelium to line a preexisting cavity formed through focal necrosis and degeneration of connective tissue in a periapical granuloma. But the finding of epithelium or epithelial proliferation near an area of necrosis is not common, so that the formation of a cyst in this manner is presumably unlikely.
Once begun, the cyst increases its size by various mechanisms, namely osmosis, local fibrinolysis, and continued epithelial proliferation.
Clinical Features
The majority of cases of apical periodontal cysts are asymptomatic and present no clinical evidence of their presence. They are commonly seen between the ages of 20 and 60 years, but the involvement of deciduous dentition is not uncommon. The most commonly involved teeth are maxillary anteriors. The associated tooth is non-vital or shows deep carious lesion or a restoration which is seldom painful or even sensitive to percussion. This type of cyst is only infrequently of such a size that it destroys much bone, and even more rarely does it produce expansion of the cortical plates. The apical periodontal cyst is a lesion that represents a chronic inflammatory process and develops only over a prolonged period of time. In some cases, such a cyst of long standing may undergo an acute exacerbation of the inflammatory process and develop rapidly into an abscess that may then proceed to a cellulitis or form a draining fistula. The cause of such a sudden flare up is not known, but it may be a result of loss of local or generalized tissue resistance.
Radiographic Features
The Radiographic appearance of the apical periodontal cyst is identical in most cases with that of the periapical granuloma. Since the lesion is a chronic progressive one, developing in a pre-existing granuloma, the cyst may be of greater size than the granuloma by virtue of its longer duration, but this is not invariably the case (Fig. 10-18). Priebe and his coworkers showed that it is impossible to distinguish between a periapical granuloma and a cyst by radiographic means alone. These workers reported that the oral surgeon and radiologist were able to diagnose correctly only 13% of a group of 55 cases of periodontal cysts by means of radiographs alone. Of the group of 46 periapical granulomas and abscesses, 59% were correctly diagnosed. The actual diagnoses were established by histologic examination of the tissue after its removal. Occasionally the apical periodontal cyst exhibits a thin, radiopaque line around the periphery of the radiolucent area, and this indicates a reaction of the bone to the slowly expanding mass. The granuloma also presents such a phenomenon in many instances. Carillo C et al, based on their study on 70 cases concluded that neither the radiographic size nor the presence of radiopaque lamina helps in the typing of periapical lesion. Simon JH et al, claim cone beam computed tomography may provide a more accurate diagnosis than biopsy in differentiating cyst from granuloma based on their study on 17 cases.
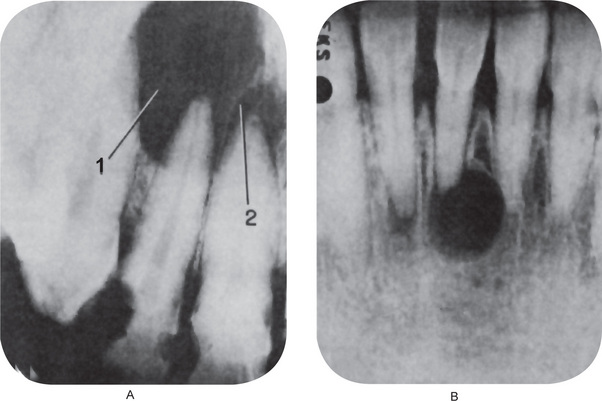
Figure 10-18 Apical periodontal cyst.
(A) This cyst (1) developed in a pre-existing periapical granuloma such as that involving the apex of the maxillary central incisor (2). The two conditions cannot be differentiated by the radiograph—only histologically. (B) This cyst developed after traumatic injury to the mandibular incisors with loss of pulp vitality. There is mild apical root resorption associated with the development of the periapical lesion.
Priebe and his associates indicated the fallacy of attempting to distinguish between the granuloma and the cyst, although such a distinction may have important endodontic implications. Thus periapical radiolucent areas will fill in with bone, apparently healing, after root canal therapy of some teeth. In other instances, even after clinically identical treatment, healing does not take place. The latter cases may represent examples of periodontal cysts, which would, of course, heal slowly if at all after endodontic therapy.
Histologic Features
The apical periodontal cyst is histologically identical with the periapical granuloma, from which it is actually derived, except for the presence of the epithelium-lined lumen. The epithelium lining the apical periodontal cyst is usually stratified squamous in type (Fig. 10-19). The only exception to this is in those rare cases of periapical lesions of maxillary teeth that involve the maxillary sinus. In occasional instances the cyst may then be lined with a pseudostratified ciliated columnar or respiratory type of epithelium. The usual squamous epithelium seldom exhibits keratin formation. This lining epithelium varies remarkably in thickness. In newly formed cysts the epithelial thickness is uneven and often shows hyperplasia while in established cysts it is of regular appearance and of fairly even thickness. It may be only a few cells thick, or exceedingly thick with a great deal of proliferation into the adjacent connective tissue. Actual rete ridge formation sometimes occurs. The epithelial lining many times is discontinuous, frequently missing over areas of intense inflammation. Despite the presence of long-standing inflammation, alterations in individual epithelial cells, such as dyskeratosis, are uncommon (Fig. 10-20). Shear has reported that there is no apparent relationship between the degree of inflammation present, either in the connective tissue wall or within the epithelium itself, and the thickness of the epithelial lining of the cyst.
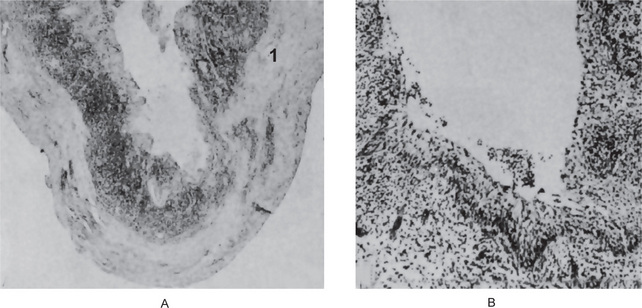
Figure 10-19 Apical periodontal cyst.
The epithelium lining the cavity is relatively thin and distorted by the inflammation but is recognizable as stratified squamous type.
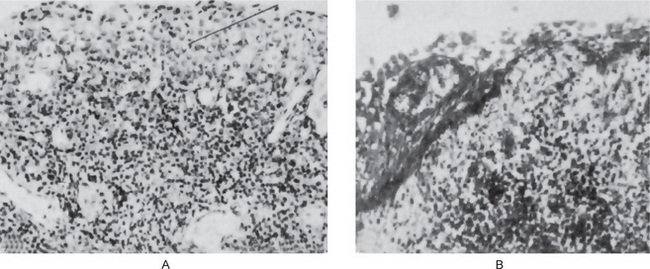
Figure 10-20 Apical periodontal cyst.
The epithelium (1) sometimes shows considerable reaction to the underlying inflammation.
In rare instances, carcinoma has been reported to develop from the lining epithelium of odontogenic cysts, including the apical periodontal cyst. These have been reviewed by Gardner. An interesting and peculiar structure, originally described by Rushton and subsequently reported by Molyneux, Medak and Weinmann, and Shear, is the hyaline body or Rushton body, often found in great numbers in the epithelium of apical periodontal or residual cysts. These hyaline bodies are tiny linear or arc-shaped bodies, generally associated with the lining epithelium, that appear amorphous in structure, eosinophilic in reaction, and brittle in nature, since they evidence fracture in some cases (Fig. 10-21). Their frequency of occurrence in cyst linings ranges between 2.6 and 9.5% of cysts, according to a review by Allison. They appear to have no clinical or diagnostic significance and their origin is unknown, but they may represent some type of epithelial product. However, Sedano and Gorlin have reported a marked morphologic and histochemical similarity between these bodies and red blood cells, suggesting that they arise from thrombus formation in small capillaries, being formed chiefly from these red blood cells—a rouleau phenomenon. Nevertheless, even by electron microscopic study of these hyaline bodies, Allison was unable to shed any further light on their etiology. The connective tissue that makes up the wall of the apical periodontal cyst is composed of parallel bundles of collagen fibers that often appear compressed. Variable numbers of fibroblasts and small blood vessels are also present. A characteristic feature is the almost universal occurrence of an inflammatory infiltrate in the connective tissue immediately adjacent to the epithelium. This infiltrate varies in its composition but is generally made up of lymphocytes and plasma cells, with some admixed polymorphonuclear leukocytes, depending partially upon the intensity of the infection. In some lesions, dystrophic calcifications and collections of cholesterol slits with associated multinucleated giant cells are found in the wall of the lesion. This mass of cholesterol frequently erodes through the lining epithelium and is extruded into the cyst lumen. The source of this cholesterol is not known, although there are numerous theories as reviewed by Shear. It appears that local tissue damage is a prerequisite for the cholesterol accumulation. In other instances, collections of lipid-filled macrophages or even macrophages containing hemosiderin are present. The contents of the cyst lumen vary from watery, straw-colored, blood tinged fluid to semisolid materials, with a low concentration of protein that stains palely eosinophilic. Occasionally, the lumen may contain a great deal of cholesterol which imparts a shimmering effect; in rare instances, limited amounts of keratin are present. Blood is a rare finding except when associated with the surgical procedure involved in removing the cyst. Whitten has reported that cytologic smears on aspirated material from cysts and cystlike lesions of the jaws, including the apical periodontal cyst, frequently permit a provisional diagnosis of the nature of the lesion.
Treatment and Prognosis
The treatment of this type of cyst is similar to that of the periapical granuloma. The involved tooth may be removed and the periapical tissue carefully curetted. Under some conditions root canal therapy may be carried out with apicoectomy of the cystic lesion. The cyst does not recur if surgical removal is thorough. As per the study by Carillo C et al, if the size of the periapical lesion is larger the prognosis is worst, particularly the periapical cyst.
If untreated, the apical periodontal cyst slowly increases in size at the expense of the surrounding bone. The bone undergoes resorption, but seldom is there a remarkable compensating expansion of the cortical plates, as is frequently seen in the case of the dentigerous cyst. Epidermoid carcinoma may develop from the lining epithelium rarely.
Periapical Abscess: (Dentoalveolar abscess, alveolar abscess)
Periapical abscess is an acute or chronic suppurative process of the dental periapical region. It may develop either from acute periapical periodontitis or more commonly from a periapical granuloma. Acute exacerbation of a chronic periapical lesion is known as Phoenix abscess. It usually arises as a result of infection following carious involvement of the tooth and pulp infection, but it also does occur after traumatic injury to the teeth, resulting in necrosis of the pulp, and in cases of irritation of the periapical tissues either by mechanical manipulation or by the application of chemicals in endodontic procedures. It is a mixed infection with the culture of pus yielding to a wide range of different bacterial species.
Clinical Features
The acute periapical abscess presents the features of an acute inflammation of the apical periodontium. The initial stages produce tenderness of the tooth, which is often relieved by application of pressure. In time, the tooth is extremely painful and is slightly extruded from its socket. As long as this abscess is confined to the immediate periapical region, there are seldom severe systemic manifestations, although regional lymphadenitis and fever may be present. However, rapid extension to adjacent bone marrow spaces frequently occurs, producing an actual osteomyelitis, but this is sometimes still considered clinically to be a dentoalveolar abscess. In such cases the clinical features may be severe and serious with swelling of the tissues. The chronic periapical abscess generally presents no clinical features, since it is essentially a mild, well-circumscribed area of suppuration that shows little tendency to spread from the local area (Fig. 10-22).
Radiographic Features
The acute periapical abscess is such a rapidly progressive lesion that, except for slight thickening of the periodontal ligament space, there is usually no radiographic evidence of its presence. The chronic abscess, developing in a periapical granuloma, presents the radiolucent area at the apex of the tooth described previously or the radiolucency may be ill-defined.
Histologic Features
The area of suppuration is composed chiefly of a central area of disintegrating polymorphonuclear leukocytes surrounded by viable leukocytes, occasional lymphocytes, cellular debris, necrotic materials and bacterial colonies. There is dilatation of the blood vessels in the periodontal ligament and adjacent marrow spaces of the bone. These marrow spaces also show an inflammatory cell infiltrate. The tissue around the area of suppuration contains a serous exudate.
Treatment and Prognosis
The principle of treatment of the periapical abscess is the same as for any abscess, drainage must be established. This can be accomplished by either opening the pulp chamber or extracting the tooth. Under some circumstances, the tooth may be retained and root canal therapy is carried out if the lesion can be sterilized.
If the periapical abscess is not treated, it may lead to serious complications through the spread of the infection. These include osteomyelitis, cellulitis, and bacteremia and the ultimate formation of the fistulous tract opening on the skin or oral mucosa. Cavernous sinus thrombosis has also been reported.
Osteomyelitis
Osteomyelitis is defined as the inflammation of bone and its marrow contents. Changes in the calcified tissue are secondary to inflammation of the soft tissue component of the bone. Though the pathologic changes of periapical abscess can even be considered as osteomyelitis as there is involvement of bone, the term ‘osteomyelitis’ is reserved for infections which spread through the bone to a larger extent. The dividing line between osteomyelitis and a localized abscess involving the bone is; however, still unclear. Though osteomyelitis commonly occurs as a complication of dental sepsis, it is also seen in various other situations. Despite antibiotics, it is still not uncommon in developing countries.
Predisposing factors include fractures due to trauma and road traffic accidents; gunshot wounds, radiation damage, Paget’s disease, and osteopetrosis. Systemic conditions like malnutrition, acute leukemia, uncontrolled diabetes mellitus, sickle cell anemia, and chronic alcoholism may also predispose to osteomyelitis. The disease may be acute, subacute, or chronic and presents a different clinical course, depending upon its nature.
Lukošiūnas A, et al, have analyzed the factors associated with the development of osteomyelitis during the treatment of mandibular fractures. They found that the immunity dysfunction, caries-affected teeth at the fracture line, mobile fractured bones, bone fixation after more than a week following trauma, insufficient bone reposition, and bone fixation after 3–7 days following trauma were the factors associated with the development of osteomyelitis.
Acute Suppurative Osteomyelitis
Acute suppurative osteomyelitis of the jaw is a serious sequela of periapical infection that often results in a diffuse spread of infection throughout the medullary spaces, with subsequent necrosis of a variable amount of bone. Clinical features of this form of osteomyelitis, which arises from a dental infection, are the same as those present after infection due to a fracture of the jaw, a gunshot wound, or even hematogenous spread. For this reason, the disease and its clinical aspects will be considered a single entity.
Dental infection is the most frequent cause of acute osteomyelitis of the jaws. It may be a rather well-localized infection of one involving a great volume of bone. A periapical infection (usually an abscess), if it is a particularly virulent one and not walled off, may spread spontaneously throughout the bone. In other instances, a chronic periapical infection such as a granuloma, or even a cyst that is walled off, may undergo an acute exacerbation, especially if the area is traumatized or surgically disturbed without establishing and maintaining drainage.
It is usually a polymicrobial infection. Different types of organisms may be cultured from these lesions; the most common are Staphylococcus aureus and Staphylococcus albus, and various streptococci. Anaerobes such as Bacteroides, Porphyromonas or Prevotella species also predominate. Cases of specific infectious osteomyelitis in tuberculosis, syphilis, actinomycosis, and so forth, are considered in the discussions of these diseases.
Clinical Features
Acute or subacute suppurative osteomyelitis may involve either the maxilla or the mandible. In the maxilla the disease usually remains fairly well localized to the area of initial infection. In the mandible, bone involvement tends to be more diffuse and widespread. The disease may occur at any age. A particular form of acute osteomyelitis, referred to as neonatal maxillitis in infants and young children, is a well recognized entity that is fortunately becoming extremely uncommon nowadays because of antibiotic drugs. In some instances, this osteomyelitis of infants is of hematogenous origin, but at other times it seems to be a result of local oral infection following some minor injury or abrasion. Infants so affected are seriously ill and may not survive the disease. In some cases, the source of the infecting organism cannot be discovered. A series of 24 such cases of maxillary osteomyelitis in infants was reported by Cavanagh, while Norgaard and Pindborg reviewed the literature and discussed the dental implications of this disease.
The adult afflicted with acute suppurative osteomyelitis usually has severe pain, trismus, and paresthesia of the lips in case of mandibular involvement and manifests an elevation of temperature with regional lymphadenopathy. The white blood cell count is frequently elevated. The teeth in the area of involvement are loose and sore so that eating is difficult, if not impossible. Pus may exude from the gingival margin. Until periostitis develops, there is no swelling or reddening of the skin or mucosa.
Radiographic Features
Acute osteomyelitis progresses rapidly and demonstrates little radiographic evidence of its presence until the disease has developed for at least one to two weeks. At this time diffuse lytic changes in the bone begin to appear. Individual trabeculae become fuzzy and indistinct, and radiolucent areas begin to appear (Fig. 10-23A).
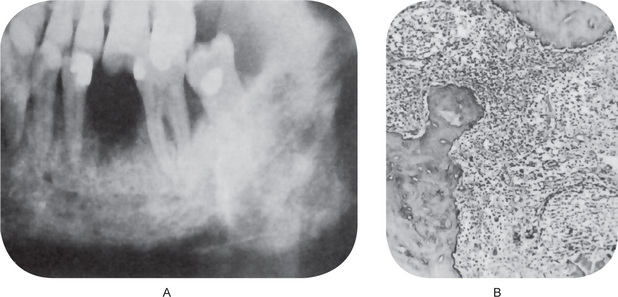
Figure 10-23 Acute osteomyelitis.
The diffuse destruction of bone is evident on the radiograph in a case of acute osteomyelitis of several weeks’ duration. Note the raggedness of the inferior border of the mandible in (A). The inflammatory process in (B) shows both a polymorphonuclear leukocyte, a lymphocyte and plasma cell response. There is also irregular resorption of bone.
Histologic Features
The medullary spaces are filled with inflammatory exudates (Fig. 10-23B). The inflammatory cells are chiefly polymorphonuclear leukocytes, but may show occasional lymphocytes and plasma cells. The osteoblasts bordering the bony trabeculae are generally destroyed, and depending upon the duration of the process, the trabeculae may lose their viability and begin to undergo slow resorption.
Treatment and Prognosis
General principles of management includes debridement, drainage, and antimicrobial therapy. When the intensity of the disease becomes attenuated, either spontaneously or under treatment, the sequestrum begins to separate from the living bone. If the sequestrum is small, it gradually exfoliates through the mucosa. If large, surgical removal may be necessary, since its removal by normal processes of bone resorption would be extremely slow. Sometimes an involucrum forms when the sequestrum becomes surrounded by new living bone. Unless proper treatment is instituted, acute suppurative osteomyelitis may proceed to the development of periostitis, soft-tissue abscess, or cellulitis (Fig. 10-24). Pathologic fracture occasionally occurs because of weakening of the jaw by the destructive process.
Chronic Suppurative Osteomyelitis
Chronic suppurative osteomyelitis may develop in inadequately treated acute osteomyelitis or may arise from a dental infection without a preceding acute stage. Rarely may it occur as a complication of irradiation. Clinical features are similar to those of acute osteomyelitis except that all signs and symptoms are milder. The pain is less severe; the temperature is still elevated, but only mildly; and the leukocytosis is only slightly greater than normal. Teeth may not be loose or sore, so that mastication is at least possible even though the jaw may not be perfectly comfortable.
Acute exacerbations of the chronic stage may occur periodically, and these present all features of acute suppurative osteomyelitis. The suppuration may perforate the bone and overlying skin or mucosa to form a fistulous tract and empty on the surface. This form of the disease should be treated on the same principles as its acute counterpart.
As per Hudson(1991), treatment of both acute and chronic forms of the disease is successful if surgically supported with sustained bacteriocidal antibiotic therapy, especially in the face of potentially refractory virulent microorganisms and compromised regional vascular penetrance. He further stated that hyperbaric oxygen therapy also may be included in the more refractory forms of osteomyelitis of the jaws to enhance the local and regional immune response of the jaws as well as to produce microvascular neoangiogenesis for reperfusion support. With resolution of infection, hard and soft tissue reconstruction may be necessary to augment the reparative process.
Chronic Focal Sclerosing Osteomyelitis: (Condensing osteitis)
Chronic focal sclerosing osteomyelitis is an unusual reaction of the bone to infection: a reaction to mild bacterial infection entering the bone through a carious tooth in persons who have a high degree of tissue resistance and tissue reactivity. In such instances, the tissues react to the infection by proliferation rather than destruction, since the infection acts as a stimulus rather than an irritant.
Clinical Features
This form of osteomyelitis arises most commonly in children and young adults and rarely in older individuals. The tooth most commonly involved is the mandibular first molar, which presents a large carious lesion. There may be no signs or symptoms of the disease other than mild pain associated with an infected pulp.
Radiographic Features
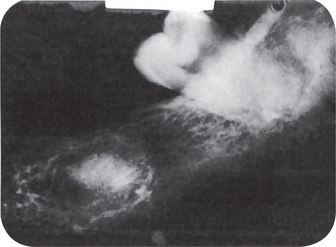
The periapical radiograph demonstrates the pathognomonic, well-circumscribed radiopaque mass of sclerotic bone surrounding and extending below the apex of one or both roots (Fig. 10-25). The entire root outline is nearly always visible, with an intact lamina dura. Periodontal ligament space is widened and this is an important feature in distinguishing it from the benign cementoblastoma (q.v.) that may closely resemble it radiographically. The border of this lesion, abutting the normal bone, may be smooth and distinct or appears to blend into the surrounding bone in contrast to focal cemento-osseus dysplasia, which has radiolucent border. In either case, the radiopacity stands out in distinct contrast to the trabeculation of the normal bone.
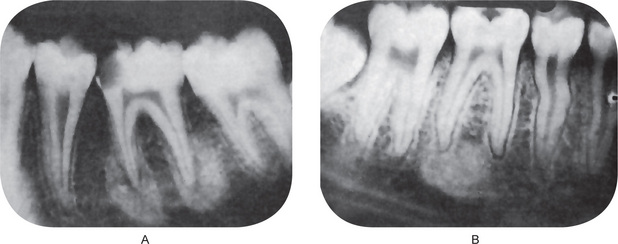
Figure 10-25 Chronic focal sclerosing osteomyelitis.
The apical sclerosis involves both roots of the first molar in (A). Only the distal root of the first molar is involved in (B), while the mesial root shows thickening of the apical periodontal membrane. Note the large carious lesions in the teeth associated with this focal sclerosing osteomyelitis.
Histologic Features
Histologic examination reveals only a dense mass of bony trabeculae with little interstitial marrow tissue (Fig. 10-26). The osteocytic lacunae appear empty. The bony trabeculae exhibit many reversal and resting lines giving pagetoid appearance. If interstitial soft tissue is present, it is generally fibrotic and infiltrated only by small numbers of lymphocytes. Osteoblastic activity may have completely subsided at the time of microscopic study.
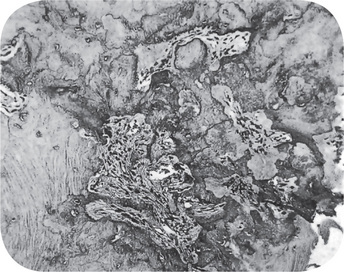
Figure 10-26 Chronic focal sclerosing osteomyelitis.
The lesion consists of dense, irregular bone with some intermingled fibrous tissue.
Condensing osteitis should be differentiated from idiopathic osteosclerosis, which is generally accepted as developmental intraosseous anatomic variation and characterized by the occurrence of asymptomatic, round, elliptical, or irregular radiopaque mass, in the bicuspid, molar region of the mandible.
Treatment and Prognosis
The tooth with which this specific lesion is associated may be treated endodontically or extracted, since the pulp is infected and the infection has spread past the immediate periapical area. The sclerotic bone constituting the osteomyelitis is not attached to the tooth and remains after the tooth is removed. This dense area of bone is sometimes not remodeled but in many cases may be recognized on the radiograph even years later and is referred to as bone scar (Fig. 10-27). For example, Boyne has reported 38 cases of such focal osteosclerotic areas in a review of 927 full-mouth radiographs of male patients between the ages of 22 and 56 years, an incidence of 4%. This incidence is somewhat higher than previously supposed. Since the condition is actually an indication that the body has been able to deal effectively with the infection, surgical removal of the sclerotic lesions is not indicated unless symptomatic.
Chronic Diffuse Sclerosing Osteomyelitis
Chronic diffuse sclerosing osteomyelitis is a condition analogous to the focal form of the disease and also apparently represents a proliferative reaction of the bone to a low-grade infection. In many of these cases, the portal of entry for the infection is not through a carious lesion with subsequent pulp infection, as in chronic focal sclerosing osteomyelitis but rather through diffuse periodontal disease. The basic nature of this condition has been discussed by Shafer and by Bell.
Clinical Features
The diffuse type of sclerosing osteomyelitis, in contrast with the focal type, may occur at any age, but is most common in older persons, especially in edentulous mandibular jaws or edentulous areas and does not exhibit any gender predominance. Often the disease is of such an insidious nature that it presents no clinical indications of its presence. On occasion there is an acute exacerbation of the dormant chronic infection and this results in vague pain, unpleasant taste, and mild suppuration, many times with the spontaneous formation of a fistula opening onto the mucosal surface to establish drainage.
Radiographic Features
The radiographic appearance of chronic diffuse sclerosing osteomyelitis is, as the name suggests, that of a diffuse patchy, sclerosis of bone often described as ‘cotton-wool’ appearance (Fig. 10-28). This radiopaque lesion may be extensive and is sometimes bilateral. In occasional cases, there is bilateral involvement of both the maxilla and the mandible in the same patient. Because of the diffuse nature of the disease, the border between the sclerosis and the normal bone is often indistinct. The pattern may actually mimic Paget’s disease of bone or cemento-osseous dysplasia.
Histologic Features
Microscopic study of tissue taken from the lesion shows dense, irregular trabeculae of bone, some of which are bordered by an active layer of osteoblasts (Fig. 10-29). Focal areas of osteoclastic activity are sometimes seen. The bone in some lesions shows a pronounced ‘mosaic’ pattern, indicative of repeated periods of resorption followed by repair. The soft tissue between the individual trabeculae is fibrous and shows proliferating fibroblasts and occasional small capillaries as well as small focal collections of lymphocytes and plasma cells. Polymorphonuclear leukocytes may be present, particularly if the lesion is undergoing an acute phase. In some lesions, the inflammatory component is completely ‘burned out’, leaving only sclerotic bone and fibrosis, but this does not contravene a diagnosis of chronic sclerosing osteomyelitis.
Treatment and Prognosis
The treatment of chronic diffuse sclerosing osteomyelitis is a difficult problem. Resolution of the adjacent foci of chronic infection often leads to improvement of this lesion. The lesion is usually too extensive to be removed surgically, yet it frequently undergoes acute exacerbations. The most reasonable approach to this disease is a conservative one. Acute episodes are treated with antibiotics. Although the lesion may be slowly progressive, it is not particularly dangerous, since it is not destructive and seldom produces any complications. If a tooth is present in one of these sclerotic areas and must be extracted, the probability of infection and protracted healing must be recognized. Sclerotic bone is hypovascular and responds poorly to any bacterial infection. Bell has recommended tooth extraction only as a last resort, utilizing a surgical approach with removal of liberal amounts of bone to facilitate extraction and increase bleeding. Sclerosed bone may remain as such in some patients even after the resolution of the lesion and may be remodeled in others.
Sclerotic Cemental Masses
A series of 38 cases of lesions of the jaws with striking similarities to those lesions described as chronic diffuse sclerosing osteomyelitis has been reported by Waldron and his coworkers under the term ‘sclerotic cemental masses of the jaws’.
Clinical Features
Just as in chronic diffuse sclerosing osteomyelitis, the majority of cases reported by Waldron and his associates occurred in older black females, who often presented with multiple, symmetric lesions that sometimes produced pain, drainage, or localized expansion. The radiographic appearance was also similar to that of diffuse sclerosis.
Histologic Features
The only significant difference between the two diseases, described in one case as sclerosing osteomyelitis and in the other as sclerotic cemental masses, was in the microscopic appearance of the radiopaque lesional tissue. In sclerosing osteomyelitis, the tissue was essentially sclerotic bone, while in the cemental masses, the tissue usually was interpreted as cementum. In some instances, this cementum was in the form of large solid masses with smooth, lobulated margins, often with a globular accretion pattern. In other cases, variable amounts of bone were admixed. The remarkable similarities between the two diseases suggest very strongly that these represent two closely related facets of the same basic disease process. Supporting this concept, in addition to the clinical and radiographic similarities, is the fact that lesions are commonly seen that exhibit the microscopic features of both diseases in the same lesional tissue: sclerotic bone and sclerotic cementum. For this reason, it appears more appropriate to understand the nature of the reaction of the tissues to injury rather than to adhere to rigid standards of nomenclature.
Chronic Osteomyelitis with Proliferative Periostitis: (Garrè’s chronic nonsuppurative sclerosing osteitis, periostitis ossificans)
This is a distinctive type of chronic osteomyelitis in which there is focal gross thickening of the periosteum, with peripheral reactive bone formation resulting from mild irritation or infection. It is essentially a periosteal osteosclerosis analogous to the endosteal sclerosis of chronic focal and diffuse sclerosing osteomyelitis. The synonym ‘Garrè’s osteomyelitis’ for this lesion is unfortunate as Garrè, in his original publication, neither described the periostitis nor the classical onion skin appearance in the radiograph produced by the cortical duplication.
Clinical Features
This sclerosing osteomyelitis occurs almost entirely in young persons before the age of 25 years and most frequently involves the anterior surface of the tibia. The lesion in this location has been recognized for many years by orthopedic surgeons and pathologists. It was generally overlooked as a distinctive entity affecting the jaws also until the report of Pell and his associates. Since there is probably greater opportunity for infection to enter the bone of the maxilla and the mandible than any other bone of the body, because of the peculiar anatomic arrangement of the teeth situated in and protruding from the bone, it is surprising that the disease has not been described more frequently as a dental complication. The condition in the jaws occurs almost exclusively in the mandible in children and young adults, and most cases occur in the bicuspid and molar region. The maxilla is seldom affected, and the reason for this is not clear.
The patient usually complains of a toothache or pain in the jaw and a bony hard swelling on the outer surface of the jaw. This mass is usually of several weeks duration. Occasionally, this reactive periostitis may develop not as a result of a central dental infection of the jaw that perforates outward but as a result of an overlying soft-tissue infection or cellulitis that subsequently involves the deeper periosteum. A most unusual case of the condition occurring simultaneously in four quadrants of the jaws in an 11-year-old child has been reported by Eisenbud and his coworkers.
Radiographic Features
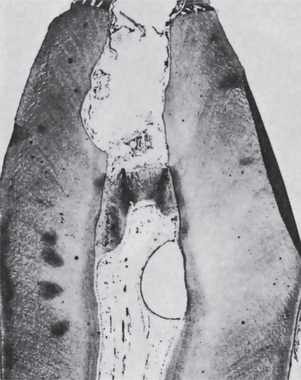
Intraoral radiographs will often reveal a carious tooth opposite the hard bony mass (Fig. 10-30). An occlusal radiograph shows a focal over-growth of bone on the outer surface of the cortex, which may be described as duplication of the cortical layer of bone (Fig. 10-31). This mass of bone is smooth and rather well calcified and may itself show a thin but definite cortical layer.
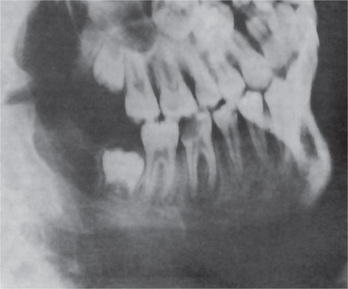
Figure 10-30 Chronic osteomyelitis with proliferative periostitis.
There are caries and periapical involvement of the mandibular first molar, but the periosteal reaction is not evident on the lateral jaw radiograph.
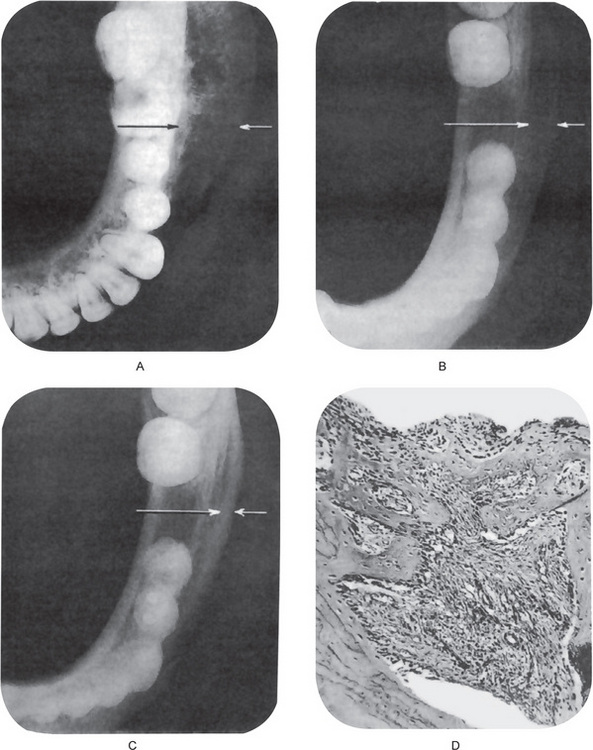
Figure 10-31 Chronic osteomyelitis with proliferative periostitis.
The intense periosteal reaction is seen on the occlusal film (A). The second occlusal film (B) was taken three months after extraction of the first molar, while the third film (C) was taken one year after the extraction and demonstrates the remarkable remodeling that occurred without other treatment. The photomicrograph (D) shows reactive new periosteal bone formed in response to the mild chronic inflammation present.
Histologic Features
This supracortical but subperiosteal mass is composed of much reactive new bone and osteoid tissue, with osteoblasts bordering many of the trabeculae. These trabeculae often are oriented perpendicular to the cortex, with the trabeculae arranged parallel to each other or in a retiform pattern. The connective tissue between the bony trabeculae is rather fibrous and shows a diffuse or patchy sprinkling of lymphocytes and plasma cells (Fig. 10-31D). The periosteal reaction is a result of the infection from the carious tooth perforating the cortical plate and becoming attenuated, stimulating the periosteum rather than producing the usual suppurative periostitis.
Treatment and Prognosis
Chronic osteomyelitis with a proliferative periostitis is treated endodontically or removal of the carious infected tooth, with no surgical intervention for the periosteal lesion except for biopsy to confirm the diagnosis. Pell and his coworkers reported that after extraction of the involved tooth, gradual remodeling of the jaws occurs, restoring the original facial symmetry (Fig. 10-31C).
Periosteal new bone formation or neoperiostosis may occur in a variety of other conditions, and care must be taken to exclude them from the diagnosis. These include infantile cortical hyperostosis (Caffey’s disease), hypervitaminosis A, syphilis, leukemia, Ewing’s sarcoma, metastatic neuroblastoma, and even a fracture callus. This differential diagnosis has been discussed by Eversole and his associates in their review of this disease.
References
Adams, D.O. The granulomatous inflammatory response. Am J Pathol. 1976; 84:164.
Aison, E.L. Osteomyelitis of the jaw. J Am Dent Assoc. 1938; 25:1261.
Allison, R.T. Electron microscopic study of ‘Rushton’ hyaline bodies in cyst linings. Br Dent J. 1974; 137:102.
Athanassiades, T.J., Speirs, R.S. Granuloma induction in the peritoneal cavity: a model for the study of inflammation and plasma-cytopoiesis in nonlymphatic organs. J Reticuloendothel Soc. 1972; 11:60.
Baumgartener, J.C., Falker, W.A., Jr. Bacteria in the apical 5mm of infected root canals. Endodont. 1991; 17:380.
Bell, W.H. Sclerosing osteomyelitis of the mandible and maxilla. Oral Surg. 1959; 12:391.
Besic, F. Fate of bacteria sealed in dental cavities. J Dent Res. 1943; 22:349.
Blair, V.P., Brown, J.B., Moore, S. Osteomyelitis of the jaws. Int J Orthod. 1931; 17:168.
Boling, L.R., Robinson, HBG. The anachoretic effect in pulpitis. Archyj Pathol. 1942; 33:477.
Boling, L.R., Robinson, HBG. Vascular changes in inflamed dental pulp. J Dent Res. 1938; 17:310.
Boulger, E.P. Histologic study of a hypertrophied pulp. J Dent Res. 1931; 11:256.
Bourgoyne, J.R., Quinn, J.H. The periapical abscess. J Oral Surg. 1949; 7:320.
Boyle, P.E. Intracellular bacteria in a dental granuloma. J Dent Res. 1934; 14:297.
Boyne, P.J. Incidence of osteosclerotic areas in the mandible and maxilla. J Oral Surg. 1960; 18:486.
Brannstrom, B., Lind, P.O. Pulpal response to early dental caries. J Dent Res. 1965; 44:1045.
Buchanan, J.C. Oral abscesses and granuloma. Dent Cosmos. 1930; 72:605.
Burket, L. Recent studies relating to periapical infection, including data obtained from human necropsy studies. J Am Dent Assoc. 1938; 25:260.
Cahn, L.R. The role of the pulp in dental caries: a clinicopathological study. Dent Cosmos. 1932; 74:1164.
Cavanagh, F. Osteomyelitis of the superior maxilla in infants. Br Med J. 1960; 1:468.
Caviedes-Bucheli, J., Gutierrez-Guerra, J.E., Salazar, F., Pichardo, D., et al. Substance P receptor expression in healthy and inflamed human pulp tissue. Int Endod J. 2006; 40:106–111.
Carrillo, C., Peñarrocha, M., Bagán, J.V., Vera, F. Relationship between histological diagnosis and evolution of 70 periapical lesions at 12 months, treated by periapical surgery. J Oral Maxillofac Surg. 2008; 66(8):1606–1609.
Cawson, R.A. Essentials of Dental Surgery and Pathology, 4th, Edinburgh: Churchill Livingstone, 1984.
Cawson, R.A., Odell, E.W. Essentials of Oral Pathology and Oral Medicine, 6th, Edinburgh: Churchill Livingstone, 1998.
Chen, S.Y., Fantasia, J.E., Miller, A.S. Hyaline bodies in the connective tissue wall of odontogenic cysts. J Oral Path. 1981; 10:147.
Cohen, M. Osteomyelitis of the mandible in the newborn. Oral Surg. 1949; 2:50.
Cohen, S., Burns, R.C. Pathways of the Pulp, 7th, St. Louis: CV Mosby, 1998.
Cook, T.J. Dental granuloma. J Am Dent Assoc. 1927; 14:2231.
Dachi, S.F. The relationship of pulpitis and hyperemia to thermal sensitivity. Oral Surg. 1965; 19:776.
De, Campos Vidal B. Histochemistry of the dental granuloma. Ann Histochem. 1963; 8:35.
Dunlap, C.L., Barker, B.F. Giant-cell hyalin angiopathy. Oral Surg. 1977; 44:587.
Durbeck, W.E. Mandibular osteomyelitis: its diagnosis and treatment. J Oral Surg. 1946; 4:33.
Eisenbud, L., Klatell, J. Acute alveolar abscess; a review of 300 hospitalized cases. Oral Surg. 1951; 4:208.
Eisenbud, L., Miller, J., Roberts, I.L. Garrè’e proliferative periostitis occurring simultaneously in four quadrants of the jaws. Oral Surg. 1981; 51:172.
El-Labban, N.G., Kramer, R.H. The nature of the hyaline rings in chronic periostitis and other conditions: an ultrastructural study. Oral Surg. 1981; 51:509.
Eversole, L.R., Leider, A.S., Corwin, J.O., Karian, B.K. Proliferative periostitis of Garrè: its differentiation from other neoperiostoses. J Oral Surg. 1979; 37:725.
Fabe, S.S. Acute hematogenous osteomyelitis of the mandible. Oral Surg. 1950; 3:22.
Fish, E.W. The pathology of the dentin and dental pulp. Br Dent J. 1932; 53:351.
Freeman, N. Histopathological investigations of the dental granuloma. J Dent Res. 1931; 11:175.
Frey, H. A contribution to the histopathology of pulp ‘polyp’ especially of temporary teeth. Br Dent J. 1948; 85:225.
Frithiof, L., Hagglund, G. Ultrastructure of the capsular epithelium of radicular cysts. Acta Odontol Scand. 1966; 24:23.
Fullmer, H.M. Observations on the development of oxytalan fibers in dental granulomas and radicular cysts. Arch Pathol. 1960; 70:59.
Gardner, A.F. The odontogenic cyst as a potential carcinoma: a clinicopathologic appraisal. J Am Dent Assoc. 1969; 78:746.
Guo, X., Niu, Z., Xiao, M., Yue, L., Lu, H. Detection of interleukin-8 in exudates from normal and inflamed human dental pulp tissues. Int Endod J. 2000; 33(2):132. [Mar].
Harris, R., Griffin, C.J. Histogenesis of the fibroblasts in the human dental pulp. Arch Oral Biol. 1967; 12:459.
Hasler, J.F., Mitchell, D.F. Analysis of 1628 cases of odontalgia: a corroborative study. J Indianap Dist Dent Soc. 1963; 17:23.
Herbert, W.E. Correlation of clinical signs and symptoms and histologic conditions of pulps of 52 teeth. Br Dent J. 1945; 78:161.
Hill, T.J. Experimental granulomas in dogs. J Am Dent Assoc. 1932; 19:1389.
Hill, T.J. Pathology of the dental pulp. J Am Dent Assoc. 1934; 21:820.
Hill, T.J. The epithelium in dental granuloma. J Dent Res. 1930; 10:323.
Hudson, J.W. Osteomyelitis of the jaws: a 50-year perspective. J Oral Maxillofac Surg. 1993; 51(12)):1294–1301.
Iwu, C., et al. The microbiology of periapical granulomas. Oral Surg. 1990; 69:502.
James, W.W., Counsell, A. A histological study of the epithelium associated with chronic apical infection of the teeth. Br Dent J. 1932; 53:463.
Kader, M.I., Christmas, B.H. Generalized suppurative osteomyelitis of the mandible. Oral Surg. 1951; 4:732.
Kakehashi, S., Stanley, H.R., Fitzgerald, R.J. The effects of surgical exposure of dental pulps in germfree and conventional laboratory rats. Oral Surg. 1965; 20:340.
Kaneko, T., Okiji, T., Kaneko, R., Nör, J.E., Suda, H. Antigen-presenting cells in human radicular granulomas. J Dent Res. 2008; 87(6):553–557.
Kreshover, S.J., Bevelander, G. Histopathology of the dental pulp of dogs following exposure. J Dent Res. 1948; 27:467.
Leonard, E.P., Lunin, M., Provenza, D.V. On the occurrence and morphology of Russell bodies in the dental granuloma. Oral Surg. 1974; 38:584.
Lukošiūnas, A., Kubilius, R., Sabalys, G., Keizeris, T., Sakavi ius, D. An analysis of etiological factors for traumatic mandibular osteomyelitis. Medicina (Kaunas. 2011; 47(7)):380–385.
ius, D. An analysis of etiological factors for traumatic mandibular osteomyelitis. Medicina (Kaunas. 2011; 47(7)):380–385.
Lundy, T., Stanley, H.R. Correlation of pulpal histopathology and clinical symptoms in human teeth subjected to experimental irritation. Oral Surg. 1969; 27:187.
Lutz, J., Cimasoni, G., Held, A.J. Histochemical observations on the epithelial lining of radicular cysts. Helv Odontol Acta. 1965; 9:90.
Mathiesen, A. Preservation and demonstration of mast cells in human apical granulomas and radicular cysts. Scand J Dent Res. 1973; 81:218.
McConnell, G. The histopathology of dental granuloma. J Am Dent Assoc. 1921; 8:390.
McMillan, M.D., Kardos, T.B., Edwards, J.L., Thorburn, D.N., et al. Giant cell hyalin angiopathy or pulse granuloma. Oral Surg. 1981; 52:178.
Medak, H., Weinmann, J.P. Hyaline bodies in dental cysts. Br Dent J. 1960; 109:312.
Melrose, R.J., Abrams, A.M., Mills, B.G. Florid osseous dysplasia. Oral Surg. 1976; 41:62.
Mincer, H.H., McCoy, J.M., Turner, J.E. Pulse granuloma of the alveolar ridge. Oral Surg. 1979; 48:126.
Mitchell, D.F., Tarplee, R.E. Painful pulpitis. Oral Surg. 1960; 13:1360.
Molyneux, G. Hyaline bodies in the wall of dental cysts. Aust Dent J. 1957; 2:155.
Morse, D.R. Immunologic aspects of pulpal-periapical diseases. Oral Surg. 1977; 43:436.
Nørgaard, B., Pindborg, J.J. Acute neonatal maxillitis. Acta Ophthalmol. 1959; 59:52.
Neville, B.W., Damm, D.D., Allen, C.A., Bouquot, J.E. Oral and Maxillofacial Pathology, 2nd, Philadelphia: WB Saunders, an imprint of Elsevier, 2002.
Ohnishi, T., Suwa, M., Oyama, T., Arakaki, N., Torii, M., Daikuhara, Y. Prostaglandin E2 predominantly induces production of hepatocyte growth factor/scatter factor in human dental pulp in acute inflammation. J Dent Res. 2000; 79(2):748.
Orban, B. Contribution to histology of dental pulp and periodontal membrane with special reference to cells of defense of those tissues. J Am Dent Assoc. 1929; 16:695.
Orban, B., Ritchey, B.T. Toothache under conditions simulating high altitude flight. J Am Dent Assoc. 1945; 32:145.
Padgett, E.C. Osteomyelitis of jaws: analysis of 59 patients. Surgery. 1940; 8:821.
Page, R.C., Davies, P., Allison, A.C. Pathogenesis of the chronic inflammatory lesion induced by hroup: a streptococcal cell walls. Lab Invest. 1974; 30:568.
Pell, G.J., Shafer, W.G., Gregory, G.T., Ping, R.S., Spear, L.B. Garrè’s osteomyelitis of the mandible. J Oral Surg. 1955; 13:248.
Pisterna, G.V., Siragusa, M. CD44 Presence in inflamed pulp tissue. J Endod. 2007; 33(10):1203–1207. [Oct].
Priebe, W.A., Lazansky, J.P., Wuehrmann, A.H. The value of the radiographic film in the differential diagnosis of periapical lesions. Oral Surg. 1954; 7:979.
Reeves, R., Stanley, H.R. The relationship of bacterial penetration and pulpal pathosis in carious teeth. Oral Surg. 1966; 22:59.
Robinson, HBG. Pathology of periapical infection. Oral Surg. 1951; 4:1044.
Robinson, HBG. Osseous dysplasia—reaction of bone to injury. J Oral Surg. 1958; 16:483.
Rushton, M.A. Hyaline bodies in the epithelium of dental cysts. Proc R Soc Med. 1955; 48:407.
Russell, W. An address on a characteristic organism of cancer. Br Med J. 1980; 2:1356.
Sabeti, M., Slots, J. Herpesviral-bacterial coinfection in periapical pathosis. J Endod. 2004; 30(2):69–72. [Feb].
Sattari, M., Haghighi, A.K., Tamijani, H.D. The relationship of pulp polyp with the presence and concentration of immunoglobulin E, histamine, interleukin-4 and interleukin-12. Aust Endod J. 2009; 35(3):164–168.
Sedano, H.O., Gorlin, R.J. Hyaline bodies of Rushton: some histochemical considerations concerning their etiology. Oral Surg. 1968; 26:198.
Seltzer, S., Bender, I.B. The Dental Pulp Biologic Considerations in Dental Procedures, 2nd, Philadelphia: JB Lippincott, 1975.
Seltzer, S., Bender, I.B., Ziontz, M. The dynamics of pulp inflammation: correlations between diagnostic data and actual histologic findings in the pulp. Oral Surg. 1963; 16:846–969.
Seltzer, S., Soltanoff, W., Bender, I.B. Epithelial proliferation in periapical lesions. Oral Surg. 1969; 27:111.
Shafer, W.G. Chronic sclerosing osteomyelitis. J Oral Surg. 1957; 15:138.
Shear, M. Cholesterol in dental cysts. Oral Surg. 1963; 16:1465.
Shear, M. Cysts of the oral regions, 3rd, London: Butterworth Heinemann Oxford, 1996.
Shear, M. Inflammation in dental cysts. Oral Surg. 1964; 17:756.
Shear, M. The hyaline and granular bodies in dental cysts. Br Dent J. 1961; 110:301.
Shroff, F.R. Observations on the reactions of the pulp and dentin to advancing caries. NZ Dent J. 1944; 40:103.
Simon, J.H., Enciso, R., Malfaz, J.M., Roges, R., et al. Differential diagnosis of large periapical lesions using cone-beam computed tomography measurements and biopsy. J Endod. 2006; 32(9):833–837.
Siskin M., ed. The Biology of the Human Dental Pulp. St. Louis: CV Mosby, 1973.
Sisman, Y., Ertas, E.T., Ertas, H., Sekerci, A.E. The frequency and distribution of idiopathic osteosclerosis of the jaw. Eur J Dent. 2011; 5(4):409–414.
Slots, J., Nowzari, H., Sabeti, M. Cytomegalovirus infection in symptomatic periapical pathosis. Int Endod J. 2005; 38(11):854. [Nov].
Soames, J.V, Southam, J.C. Oral Pathology, 3rd, London: Oxford University Press, 1999.
Southam, J.C., Hodson, J.J. Neurohistology of human dental pulp polyps. Arch Oral Biol. 1973; 18:1255.
Southam, J.C., Hodson, J.J. The growth of epithelium, melanocytes, and langerhans cells on human and experimental dental pulp polyps. Oral Surg. 1974; 37:546.
Spouge, J.D. Oral Pathology, 1st, St. Louis: CV Mosby, 1973.
Stanley, H.R. The cells of the dental pulp. Oral Surg. 1962; 15:849.
Stephan, R.M. Correlation of clinical tests with microscopic pathology of the dental pulp. J Dent Res. 1937; 16:267.
Stern, M.H., Dreizen, S., Mackler, B.F., Levy, B.M. Antibody-producing cells in human periapical granulomas and cysts. J Endod. 1981; 7:447.
Stern, M.H. Isolation and characterization of inflammatory cells from the human periapical granuloma. J Dent Res. 1982; 61:1408.
Stern, M.H., Dreizen, S., Mackler, B.F., Selbst, A.G., et al. Quantitative analysis of cellular composition of human periapical granuloma. J Endod. 1981; 7:117.
Thoma, K.H. A histologic study of the dental granuloma and diseased root apex. J Am Dent Assoc. 1917; 4:1075.
Thoma, K.H. The condition of the bone in cases of dental granuloma. Dent Items Interest. 1918; 40:421.
Thoma, K.H. A practical discussion of pulp disease based on microscopic study. Dent Items Interest. 1925; 47:637.
Thoma, K.H. Kalil FH. Chronic osteomyelitis of the mandible. Am J Orthod Oral Surg. 1943; 29:536.
Thoma, K.H. The histologic pathology of alveolar abscesses and diseased root ends. Dent Cosmos. 1918; 60:13.
Thoma, K.H. The infected vital dental pulp. J Dent Res. 1928; 8:529.
Toller, P.A. Experimental investigation into factors concerning the growth of cysts of the jaws. Proc R Soc Med. 1948; 41:681.
Torabinejad, M., Bakland, L.K. Immunopathogenesis of chronic periapical lesions. Oral Surg. 1978; 46:685.
Waldron, C.A., Giansanti, J.S., Browand, B.C. Sclerotic cemental masses of the jaws (so-called chronic sclerosing osteomyelitis, sclerosing osteitis, multiple enostosis, and gigantiform cementoma. Oral Surg. 1975; 39:590.
Waldron, C.W. Osteomyelitis of the jaws. J Oral Surg. 1943; 1:317.
Weine, F.S. Endodontic Therapy, 2nd, St. Louis: CV Mosby, 1976.
Wellings, A.W. Early inflammatory reactions of the tooth pulp to bacterial invasion of the dentinal tubules. Br Dent J. 1940; 68:510.
Whitten, J.B., Jr. Cytologic examination of aspirated material from cysts or cystlike lesions. Oral Surg. 1968; 25:710.
 . Diseases of Dental Pulp
. Diseases of Dental Pulp