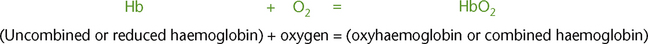CHAPTER 23 Oxygenation
At the completion of this chapter and with some further reading, students should be able to:
• Describe the structure of the respiratory system
• State the functions of the respiratory system
• Describe the structures of the cardiovascular system
• Describe the position of the heart and the function of the circulatory system
• State the factors that affect respiratory function
• Describe the major manifestations of respiratory system disorders
• Describe the major manifestations of circulatory system disorders
• Briefly describe the specific disorders of the cardiac and respiratory systems outlined in this chapter
• Assist in planning and implementing nursing care for the client with a cardiac and respiratory system disorder
• Apply relevant principles in the planning and implementation of nursing actions to assist a client receiving oxygen therapy
The human body relies on oxygen to survive and it is the role of the cardiac and respiratory systems to supply the body’s oxygen demands. The two systems work together to achieve and maintain homeostasis. Cardiopulmonary physiology involves the delivery of oxygenated blood from the lungs to the left side of the heart out to the tissues, and deoxygenated blood from the tissues to the right side of the heart out to the pulmonary circulation for re-oxygenation. Blood is oxygenated through the processes of ventilation, perfusion and transport of respiratory gases.
The overall function of the cardiovascular system, which includes the lymphatic system, is to move blood around the body. The heart pumps the blood for circulation around the body through blood vessels. These blood vessels transport oxygen, nutrients and other substances to the cells and transport waste away from the cells. Blood also assists in protecting the body against infection and distributing heat evenly throughout the body, and prevents its own loss by means of a built-in clotting mechanism.
The respiratory system provides the body with the ability to absorb oxygen and excrete carbon dioxide and other waste products from the body. Oxygen from the atmosphere is delivered to the bloodstream and carbon dioxide diffused out from the bloodstream. This is achieved through the capillary alveoli membrane in the lungs.
Ventilation is the method of delivering air into and out of the lungs and is achieved through inhalation and exhalation. Respiration involves both the respiratory and circulatory system and is the exchange of gases.
An adequate supply of blood is necessary for the normal function of every cell. Homeostasis depends on the ability of the heart to adequately circulate the required volume of blood and oxygen to the tissues. Cells temporarily deprived of blood or oxygen will not function normally, and continued disruption of blood supply causes irreversible damage or cell death. Any disorder that interferes with the distribution or delivery of blood to tissues or the uptake or excretion of gases in respiration is a potential harm to body cells and may have permanent effects on a part, or all, of the body.
The most common complication of a respiratory disorder is carbon dioxide retention. This can be a result of alveolar hypoventilation, or a cardiovascular disorder altering the ventilation or the perfusion of the lungs and other tissues.
Nursing care related to oxygenation requires an understanding of how the three systems work together to achieve oxygenation within the body. Knowledge of how to maintain and restore a clear airway is an essential component to nursing care. This includes measures directed at removing secretions by the use of suction via the nasal, oropharyngeal or endotracheal routes or by tracheostomy. The patency of airways can be assisted by the use of humidification, nebulisation and physiotherapy using isotonic or hypotonic solutions or certain medications. Education is essential to promote exercise, which maintains optimal circulation of blood, and deep breathing and coughing exercises are encouraged to minimise the retention of secretions and secondary infections. Circulation can be assisted by changes in diet, fluid, exercise, medications and positioning.
Although disorders of the cardiovascular and the respiratory system are common in most communities, the incidence of cardiac and respiratory disease is controlled to some extent by: legislation to minimise airborne irritants; immunisation programs; and health education regarding risks such as bad diet, smoking, hypertension and environmental pollution.
Damian, a 62-year-old man, was brought to the emergency department by ambulance after complaining of heavy chest pain that radiated down his shoulder. He arrived at hospital with oxygen therapy delivered via facemask at 8 L/min. As the emergency nurse caring for Damian I conducted a detailed history and examination and immediately took an ECG and some blood for testing. The ECG and his blood results showed that Damian was having a large anterior myocardial infarction. Thrombolytics were immediately administered but Damian quickly developed worsening shortness of breath. His oxygen saturation levels were sitting at around 92% on now 10 L/min oxygen. It was suggested to place Damian on a BiPAP machine. BiPAP delivers positive and negative pressure ventilation and would hopefully improve his oxygen levels. However, after 10 minutes on this machine he became more confused, semi-conscious and his blood pressure dropped. He was moving quickly into respiratory arrest. I immediately laid Damian flat, inserted an oral airway and began using a BVM to start bagging him. The decision was quickly made to intubate Damian. I assisted the doctor with inserting the endotracheal tube. Damian immediately stabilised.
STRUCTURE OF THE RESPIRATORY SYSTEM
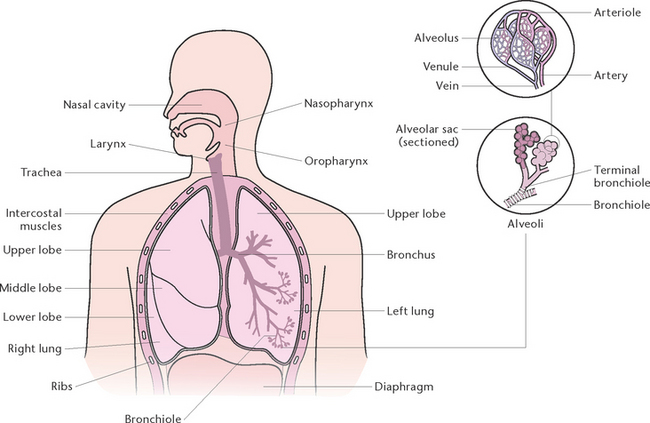
The function of the respiratory system (Fig 23.1) is to deliver oxygen from the atmosphere to the bloodstream and to deliver carbon dioxide from the bloodstream to the atmosphere. The structures that make up the respiratory tract constitute the means by which this exchange of gases occurs. The respiratory system consists of cavities and conducting airways that begin at the nasal and oral cavity and end at the alveoli, the functional unit of the respiratory system. The larger airways are composed of cartilage and smooth muscle that maintain their patency, and are gradually replaced with smooth muscle in the terminal airways, which allows alterations in airway diameter and ventilation. The two lungs are located in the thoracic cavity, encased by a double membrane known as the pleura, and are separated by the mediastinal cavity that contains the heart and great vessels. The thoracic cavity has ribs that aid in ventilation and protect the lungs from damage. The diaphragm and the internal and external intercostal regions are composed of skeletal muscle and constitute the main muscles of ventilation; other muscles are used when required for more forceful inhalation or expiration (Berman et al 2012).
Upper airways
Nasal cavities
The nose is a bony cartilaginous structure divided into a right and left nasal cavity by the nasal septum. The anterior portion of the septum is cartilage and the posterior portion is bone, formed by the vomer and part of the ethmoid bone. Inside each nostril (nares) is a vestibule lined by skin containing sebaceous and sweat glands and coarse hairs that act as filters. Apart from the vestibules, all other areas of the nasal cavity are lined by mucous membrane. In most of the cavity the membrane is covered by ciliated epithelium with many ‘goblet’ cells. Mucous cells are also present in the underlying connective tissue. The nose consists of these chambers, with specific structures and cells that have the following functions:
• Hairs and cilia line the nasal cavities and filter foreign particles and pathogens from the inhaled air
• Mucus secreted by the mucosa traps substances in inhaled air, and the cilia move particles of mucus towards the pharynx to be swallowed or expectorated
• Inhaled air is warmed and moistened as it passes over the mucosa. The three nasal turbinate bones in each cavity cause air flow to become turbulent, which enhances contact of air with the mucosa
• Sensory organ for the sense of smell (olfaction) (Marieb & Hoehn 2010).
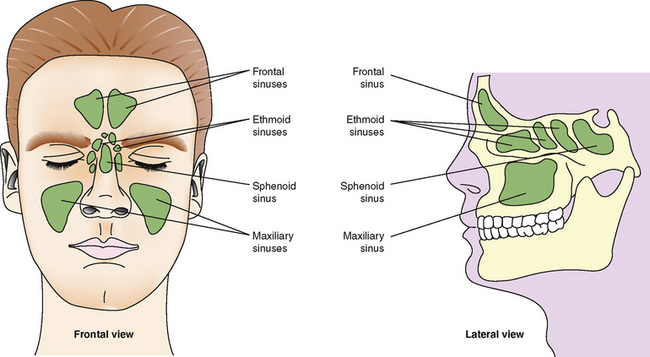
Paranasal sinus
A sinus is a cavity within a bone. There are four air-filled paranasal sinuses (Fig 23.2), frontal, maxillary, sphenoid and ethmoid sinuses.
These sinuses are important resonance chambers and aid in voice production (Marieb & Hoehn 2010).
The pharynx
The pharynx is a muscular tube about 13 cm long, lying in front of the cervical vertebrae and behind the nose, mouth and larynx. It is lined with mucous membrane and has three sections:
Nasopharynx
The nasopharynx, which is continuous with the nasal cavity above and with the orpharynx below. Its functions include:
Oropharynx
This section lies behind the mouth and is separated from the cavity of the mouth by two folds of mucous membrane (the fauces). Between these folds lie the oral tonsils, which are patches of lymphoid tissue involved in the immune system. The oropharynx provides a common passage for air, food and fluids. The uvula is a muscular projection of the soft palate in the middle of the arch formed by the fauces, and prevents the entry of food and fluid into the nasal cavity (Marieb & Hoehn 2010).
The larynx
The larynx is situated in the upper region of the neck and extends from the pharynx above to the trachea below. It is composed of pieces of cartilage connected by membranes and provides a passageway for air between the pharynx and trachea. As air passes through, it is further moistened, warmed and filtered. The main cartilages that form the larynx are:
• The thyroid cartilage, which is the largest and forms a prominence known as the ‘Adam’s apple’
• The epiglottis, a leaf-shaped cartilage attached to the upper part of the thyroid cartilage. During swallowing, the larynx rises and the epiglottis covers its opening, directing food and fluid into the oesophagus and preventing entry into the trachea and subsequent aspiration into the lungs
• The pitch of the voice depends on the length and tightness of the cords, and the air sinuses in the skull bones influence the resonance of the voice. The vowels and consonants that make up speech are formed by various positions of the lips and tongue. Speaking requires coordination of the larynx, mouth, lips, tongue, throat, lungs and abdomen (Marieb & Hoehn 2010).
Lower airway
The trachea
The trachea is about 12 cm long and lies in front of the oesophagus, extending from the larynx to the mid-thorax, where it divides into a right and a left bronchus. The trachea consists of 15–20 C-shaped rings of cartilage joined by involuntary muscle and fibrous tissue. Posteriorly the trachea lacks cartilage and is replaced with smooth muscle to enable the oesophagus to expand, while the cartilages maintain the patency of the airway. It thus provides a permanently open passageway for air travelling to and from the lungs. The trachea is lined with ciliated epithelium containing mucus-secreting goblet cells. The cilia sweep the mucus, cell debris and any foreign particles that enter the trachea up into the pharynx to be swallowed or expectorated. During swallowing, the larynx rises and the epiglottis covers its opening, directing food and fluid into the oesophagus and preventing its entry into the trachea and subsequent aspiration into the lungs.
The cricoid cartilage lies below the thyroid cartilage, and is shaped like a wide, banded ring to provide attachments for the various muscles, cartilages and ligaments involved in opening and closing the airway and in speech production. The larynx is lined with mucous membrane, which becomes ciliated in the lower part. In the upper part, two folds of membrane containing embedded fibrous and elastic tissue form the vocal cords. The vocal cords extend from the anterior wall to the posterior wall of the larynx to form the glottis, or voice box, which produces sounds. The nerve supply to the larynx is from the laryngeal and recurrent laryngeal nerves, which are branches of the vagus nerve (Marieb & Hoehn 2010).
Voice production
The vocal cords are apart during normal breathing. Contraction of muscles attached to the cords brings them closer together, and expired air is used to cause vibration of the cords. The brain, tongue, lips, nasal cavity and facial muscles all help to convert the resultant sounds into speech. The pitch of the voice depends on the length and tightness of the cords, and the air sinuses in the skull bones influence the resonance of the voice. Speaking requires coordination of the larynx, mouth, lips, tongue, throat, lungs and abdomen.
Bronchi
At about the middle of the thorax, the trachea divides to form the right and left bronchus. The bronchi enter the lungs; the right bronchus dividing into three, and the left bronchus dividing into two branches. There are three lobes in the right lung and two lobes in the left lung; therefore, one branch of each bronchus enters each lobe. The left bronchus is longer than the right because of the position of the heart. Smooth involuntary muscles surround the airways to allow for alteration in airway diameter (Marieb & Hoehn 2010).
Bronchioles
Bronchioles are the smallest branches of the bronchi, and their walls consist of involuntary muscle with elastic fibrous tissue, allowing for expansion and constriction. They divide to form terminal bronchioles that give rise to microscopic alveolar ducts, which terminate in clusters of air sacs called alveoli.
Lungs
The two lungs lie in the thoracic cavity on either side of the mediastinum. The mediastinal cavity contains the heart, major blood vessels and the oesophagus. The lungs are light and spongy and consist of the bronchioles, alveoli and blood vessels and are supported by areolar tissue. There is also a great deal of elastic tissue to enable the lungs to expand and recoil freely during respiration. The base of each lung rests on the diaphragm and the apex of each extends to just above each clavicle. The right lung has three lobes and is shorter and wider than the left lung, which has two lobes. Each lobe is made up of lobules, each with its own blood, nerve and lymph supply. On the medial side of each lung is a depression called the hilus, through which the bronchi, lymphatic vessels and blood vessels enter and exit (Marieb & Hoehn 2010).
Alveoli
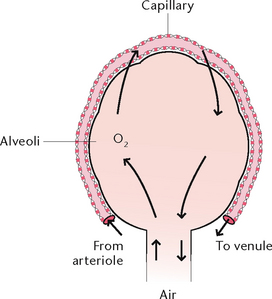
Alveoli are microscopic air sacs in the lungs. Their walls are composed of one layer of type I, simple squamous epithelial cells, and type II cells that produce surfactant which maintains alveolar expansion by reducing surface tension. Macrophages are present and their role is to phagocytose cell debris and pathogens. The alveoli form a surface area of about 70 m2 for semi-permeable membrane diffusion of gases. The alveoli are surrounded by networks of capillaries, arising from the pulmonary arteries and their tributaries. The function of alveoli is the interchange of oxygen and carbon dioxide between the air in the alveoli and the blood in the capillaries (Fig 23.3).
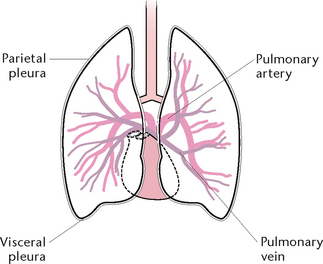
Pleura
The pleura (Fig 23.4) comprise a double layer of serous membrane, consisting of the visceral pleura, which adheres to the surface of the lungs, and the parietal pleura, which lines the thoracic cavity and covers the superior surface of the diaphragm. The pleura secrete a thin film of serous fluid, maintained at about 50 mL, which lies between the two layers and prevents friction between the surfaces. The pressure within the pleura is 2 mmHg below atmospheric pressure to prevent lung collapse.
Muscles of ventilation
The main muscles responsible for ventilation are the diaphragm and internal and external intercostal muscles. During difficult or forced breathing, accessory muscles are used, such as the muscles of the neck, thorax (e.g. sternocleidomastoid, anterior serrate, scalene) and abdominal muscles, including the rectus abdominus and transverse abdominus (Marieb & Hoehn 2010).
SCIENTIFIC PRINCIPLES OF VENTILATION AND RESPIRATION
The major purpose of respiration in to supply oxygen to the body and remove carbon dioxide from the cells. This is accomplished by the process of ventilation (movement of air in and out of the lungs), external respiration (exchange of gases between the atmosphere and the pulmonary capillaries) and internal respiration (exchange of gases between the systematic capillaries and the cell). An understanding of pressure relationships and key principles of respiration will assist in understanding the process of ventilation and respiration.
Pressure
Pressure may be defined as force (or stress) per unit area applied to a surface. The concept of pressure, or force, applies equally to solids, liquids and gases. Described below are some of the principles and concepts relating to pressure that are relevant to nursing.
Atmospheric pressure
Atmospheric pressure arises by virtue of the weight of the air above the earth. Atmospheric pressure decreases as altitude increases because of the reduced amount of air above. Even at a particular altitude, atmospheric pressure is not constant, but varies according to atmospheric conditions. The total pressure exerted by the atmosphere is about 6.8 kg per 25 mm2 of surface area at sea level. The atmosphere consists principally of nitrogen (N2) 79.03%, oxygen (O2) 20.93% and carbon dioxide (CO2) 0.0004%, which totals 99.9604%. Other gases such as carbon monoxide (CO) are present in minute quantities. Oxygen, a colourless and odourless gas, is essential in sustaining most forms of life.
Water vapour and gases in the atmosphere have weight and, at sea level, exert a pressure defined as 1 atmosphere of pressure (‘atmospheric pressure’) equivalent to 760 mmHg. The terms negative and positive pressure are used to compare a pressure to normal atmospheric pressure at sea level. Any pressure above normal atmospheric pressure is regarded as a positive pressure, and any pressure below normal atmospheric pressure is regarded as a negative pressure.
The following three laws of physics define the characteristics of gases:
1. Pascal’s principle, which states that a confined liquid transmits pressure, applied to it from an external force, equally in all directions
2. Boyle’s law, which states that the volume of a given mass of gas is inversely proportional to the pressure to which it is subjected, provided that the temperature remains constant
3. Charles’ law, which states that the volume of a given mass of gas is directly proportional to its absolute temperature, provided that the pressure remains constant; for example, as the temperature of a gas is increased (at constant pressure) the gas expands.
The combined effects of atmospheric pressure and the application of the gas laws above provide the basis for the operation of many common devices, and also of the lungs. Boyle’s law refers to pressure differentials and is able to be applied to the process of breathing. By changing the volume of the thoracic cavity, the air pressure in the lungs can be made lower or higher than atmospheric pressure, leading to inhalation or exhalation, respectively (Marieb & Hoehn 2010).
Sensory mechanisms of the respiratory system
Every cell in the human body requires oxygen for normal metabolism and must excrete the metabolic waste product, carbon dioxide. To maintain homeostasis, cells in different locations of the body react to changes in oxygen and CO2 levels. Sensory cells include chemoreceptors, pressorreceptors and baroreceptors.
Chemoreceptors
Chemoreceptors are specialised cells located centrally in the upper medulla of the brainstem and peripherally in bodies located in the carotid and aortic arteries. They respond to slight increases in arterial or cerebrospinal fluid CO2 pressure (PCO2) and acidity (an increased concentration of hydrogen [H+] ions). Regulation of ventilation depends mainly on the level of CO2 in the blood. A slight increase in CO2 concentration stimulates chemoreceptors to increase the respiratory rate and depth until the excess CO2 is eliminated. Conversely, a decreased CO2 level slows the ventilatory rate. Oxygen levels are normally sensed by the carotid bodies, which are sensitive to a fall in oxygen concentration of less than 50%. Stimulation of the carotid receptors increases the respiratory rate, the exception being clients with chronic hypercapnia such as in emphysema.
Pressorreceptors
Pressorreceptors, or mechanoreceptors, are stretch receptors present in lung tissue and within the thoracic wall. The bronchioles and alveoli also have stretch receptors that respond to extreme over-inflation as well as extreme deflation. When over-inflation occurs, impulses are transmitted from the stretch receptors to the medulla by the vagus nerve, the expiratory centre is activated and exhalation occurs. When extreme deflation occurs impulses from the lungs activate the inspiratory centre, and inhalation occurs.
Baroreceptors
Baroreceptors are cells sensitive to blood pressure, which normally monitor changes in blood pressure. When blood pressure increases, impulses are sent to the respiratory centres to cause a decrease in respiratory rate. Rate, depth and rhythm of respirations are further affected by reflex responses, chemical signals and voluntary control; for example, during actions such as swallowing, impulses from gustatory centres are conveyed to the respiratory centre, and breathing stops temporarily.
Any abnormal mechanical disturbance, for example, the presence of chemical substances such as cigarette smoke, causes excitement of the lung irritant receptors, which induces hyperventilation and a reflex bronchoconstriction. Information from other parts of the body may also be received by the respiratory centres; for example, a rise in body temperature initiates an increase in the rate of ventilation, while a sudden cooling of the body induces a sudden inhalation followed by hyperventilation.
The respiratory centres
Respiration is controlled both voluntarily and involuntarily. The automatic control of breathing is regulated by three respiratory centres, known as the medullary centre, located in the medulla oblongata, the apneustic centre in the pons and the pneumotaxic centre in the upper pons of the brainstem. These centres receive stimuli from sensory cells described above, and from each other. Their function is to control the rate, rhythm and depth of ventilation. Impulses travel from the respiratory centres along separate nerves that exit the spinal cord at different levels to separately innervate and control the diaphragm and internal and external intercostal muscles. Impulses are also transmitted to the other centres and cause stimulation of the respiratory muscles via the phrenic nerves, to stimulate the diaphragm to contract, and the intercostal nerves, which stimulate the intercostal muscles.
Ventilation and respiration
Respiration is the term used to describe an interchange of gases. The main purpose of respiration is to supply the body with oxygen and dispose of carbon dioxide. The four processes involved are:
1. Ventilation: movement of air containing different gases into the respiratory tract
2. External respiration: exchange of oxygen and CO2 between the blood and the alveoli (Fig 23.3)
3. Internal respiration: exchange of oxygen and CO2 between the bloodstream and the tissues
Ventilation
Ventilation has two phases (Fig 23.5): inhalation and exhalation.
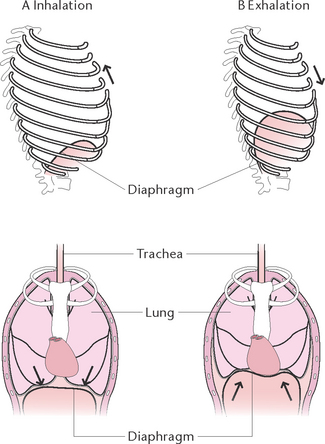
Figure 23.5 Rib cage and diaphragm positions during breathing A: At the end of normal inhalation: chest expanded (top left) and diaphragm depressed (bottom left) B: At the end of a normal exhalation: chest depressed (top right) and diaphragm elevated (bottom right)
Inhalation
During inhalation the diaphragm contracts and flattens, enlarging the thoracic cavity lengthwise, particularly in males. In females normal inhalations occur primarily by contraction of the external intercostal muscles, which raise the ribs and sternum, thus increasing the size of the thoracic cavity from side to side and front to back. As the chest wall moves up and outwards, the parietal pleura moves with it and, because of the 2 mmHg negative pressure within the pleura, the visceral pleura follows the parietal pleura. This causes stretching of the lungs, which expand to fill the enlarged thorax, and air is pushed into the respiratory passages.
Exhalation
Exhalation is normally a more passive process than inhalation except in exercise and respiratory conditions in which active expiration occurs via internal intercostal and accessory muscles. During exhalation the diaphragm relaxes, thus decreasing the size of the thoracic cavity. The external intercostal muscles also relax, allowing the ribs and sternum to return to their former position, further decreasing the size of the thoracic cavity. The elastic tissue of the lungs allows for recoil, further forcing air out of the respiratory passages.
Respiration
External respiration
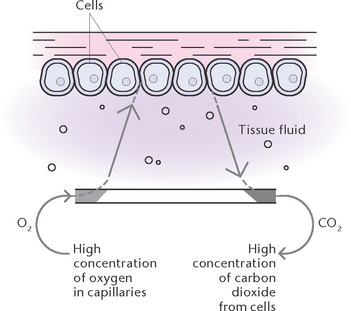
External respiration is the exchange of gases between air in the alveoli and the blood travelling through the capillaries surrounding the alveoli. Branches of the pulmonary artery bring deoxygenated blood to the capillaries surrounding each alveolus. During gas exchange, gases normally diffuse through the semi-permeable walls of the alveoli and capillaries to the area of lowest concentration of each gas, as each gas diffuses independently of other gases, until the pressure is equal on both sides. Thus, oxygen moves from an area of higher concentration in the alveoli to an area of lower concentration in the blood capillaries, while CO2 moves from an area of a higher concentration in blood capillaries to an area of lower concentration in the alveolar air. Pulmonary venules then collect the blood rich in oxygen from the capillaries and unite to form the two pulmonary veins which leave each lung to enter the left atrium of the heart. Table 23.1 illustrates the approximate composition of inspired and expired air.
Table 23.1 Composition of inhaled and exhaled air (approximate)
| Substance | Inhaled air | Exhaled air |
|---|---|---|
| Nitrogen | 78.62% | 74.5% |
| Oxygen | 20.84% | 15.7% |
| Carbon dioxide | 0.04% | 3.6% |
| Water vapour | 0.50% | 6.2% |
| Total | 100.0% | 100.0% |
Internal respiration
Internal respiration (Fig 23.6) is the exchange of gases between the bloodstream and the tissues. During this exchange, the gases diffuse through the semi-permeable walls of the capillaries to equalise the concentration of gases on both sides. Oxygen moves from the blood into the tissues, down a concentration gradient, to replenish oxygen used in cellular metabolism. Carbon dioxide moves from the tissues into the blood, down a concentration gradient, to rid the tissues of waste produced by cellular metabolism.
Factors necessary for ventilation and respiration
The passage of oxygen from the atmosphere to the alveoli in the lungs, and the passage of carbon dioxide from the alveoli to the atmosphere, requires an unobstructed airway. In addition, the process of respiration requires:
• Adequate oxygen in the atmosphere
• A patent functioning respiratory tract
• Functioning thoracic muscles and nerves to control the thoracic cage and diaphragm
• Capillaries in close proximity to the cells to allow the exchange of gases
• A functioning cardiovascular system that contains adequate amounts of plasma and normal erythrocytes and haemoglobin to transport the gases (Marieb & Hoehn 2010).
Transport of oxygen
Oxygen is carried in the blood in two ways:
• Dissolved in the plasma: only 2–3% of oxygen is carried in this way as oxygen is not very soluble. Oxygen dissolved in plasma is measured as PaO2.
• Bound to haemoglobin in the red blood cells: 95–98% of oxygen is carried in this way and is measured by the percentage of oxygen saturated (SaO2).
Haemoglobin is composed of haem (iron) and globulin (protein). Oxygen and haemoglobin combined together form oxyhaemoglobin (Fig 23.7).
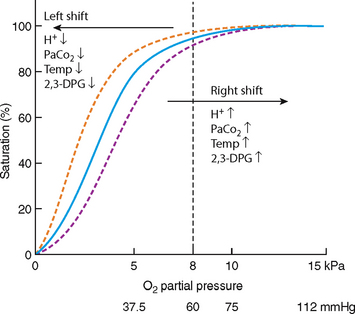
Hb that is not combined with oxygen is referred to as reduced haemoglobin. Hb that is completely converted to HbO2 is referred to as fully saturated haemoglobin. Hb that has a mixture of Hb and HbO2 is referred to as partially saturated haemoglobin. The percentage saturation of haemoglobin is the percentage of HbO2 in the total haemoglobin. The percentage saturation of haemoglobin with oxygen is illustrated in the oxygen–haemoglobin dissociation curve (Fig 23.8).
The bond between haemoglobin and oxygen are affected by various physiological factors that shift the oxygen dissociation curve to the left or to the right. When the PaO2 of blood is high, haemoglobin binds with large amounts of oxygen and is almost fully saturated. When the PaO2 is low, the haemoglobin binds with smaller amounts of oxygen and is partially saturated.
Factors affecting the release of oxygen from haemoglobin
Several factors affect the amount of oxygen being released from haemoglobin so that the oxygen dissociation curve is said to shift to the left or to the right. When a shift occurs to the right there is reduced binding of oxygen to haemoglobin and oxygen is released more easily into the tissues. When a shift occurs to the left there is an increase in the binding of oxygen to the haemoglobin, resulting in oxygen being less easily released into the tissues. Cellular hypoxia can occur.
pH
Oxygen is released more readily from haemoglobin in an acid environment. This condition occurs often during an acute illness. During an acute illness an increase in PaO2 is usually present, resulting in a lower pH. This acidic environment allows the oxygen to separate more readily from the haemoglobin allowing the tissues to have more oxygen.
Temperature
Within limits, the amount of oxygen released from haemoglobin increases as temperature increases, therefore hyperthermia causes a rightward shift while hypothermia causes a leftward shift.
Organic phosphates
2,3-diphosophoglycerate (DPG) is a primary organic phosphate. It is an intermediate compound formed in red blood cells during the conversion of glycogen to glucose. The production of 2,3-DPG is likely an important adaptive mechanism because the production increases in several conditions where there is diminished peripheral tissue O2 availability, such as hypoxaemia, chronic lung disease, anaemia and congestive heart failure. High levels of 2,3-DPG shift the curve to the right, while low levels of 2,3-DPG cause a leftward shift, seen in states such as septic shock and hypophosphataemia.
Carbon dioxide excretion
Carbon dioxide is carried through the venous system and breathed out through the lungs. The normal level of carbon dioxide in the blood is 3.5–5.3 kPa. Carbon dioxide has a direct effect on the respiratory centre in the brain. As carbon dioxide levels rise and diffuse from the blood into the cerebrospinal fluid, the CO2 is hydrated and carbonic acid is formed. The role of the respiratory system is to excrete carbonic acid from the lungs during expiration. Carbon dioxide stimulates respiratory rate and so high carbon dioxide levels result in a higher respirations rate.
STRUCTURE OF THE CARDIOVASCULAR SYSTEM
The structures that make up the cardiovascular system are the:
• Heart, which acts as a pump to circulate the blood through the body
• Blood, which carries essential substances to cells and carries wastes away from cells
• Blood vessels, which contain and transport the blood throughout the body
• Lymphatic system, which transports tissue fluid containing electrolytes, proteins and some waste products, recognises and destroys pathogens before they reach the bloodstream and delivers nutrients from the digestive tract into the cardiovascular system.
Blood
Blood, which is classed as a connective tissue, constitutes about one-twelfth of the weight of the body. It is a viscous substance composed of a fluid portion (plasma) and formed elements (cells and cell fragments). Depending on the weight of the individual, the average total volume of blood is about 5–6 L. Blood varies in colour, from bright red when it has a high oxygen content, to dark red when the oxygen content is low. Arterial blood normally has a pH range of 7.35 to 7.45 (Marieb & Hoehn 2010).
Functions of blood
Blood has the following functions:
• Transporting oxygen, nutrients, water and ions to all tissue cells
• Removing waste materials to excretory organs
• Transporting hormones to cells
• Supplying materials from which cells and glands make their secretions
• Protecting the body against infection by means of the leucocytes and antibodies
• Regulating body temperature by distributing heat evenly throughout the body
• Preventing loss of body fluid and blood cells by means of its clotting mechanism.
Constituents of blood
Plasma and formed elements make up the components of blood. Plasma, the fluid part of blood, is a straw-coloured watery fluid in which blood cells are suspended. Plasma forms about 55% of the blood volume and contains:
• Water: about 90–92% of the plasma is composed of water, which is important in the maintenance of all body fluids and in the production of secretions
• Proteins: albumin, globulin, fibrinogen, prothrombin and heparin are some of the proteins found in plasma. The liver normally produces proteins, with the exception of serum globulin, which is derived from lymphocytes. Plasma proteins have several important functions: they assist in retaining water in the plasma and interstitial tissue; factors such as prothrombin and fibrinogen are essential for blood clotting; proteins such as heparin help to prevent abnormal clotting of blood in the blood vessels
• Mineral salts: the main mineral salts found in blood plasma are sodium chloride, iodine, potassium, phosphorus, calcium, iron, magnesium and copper. Mineral salts are necessary for the regulation of cellular functioning and electropotentials and maintenance of the blood pH
• Nutrients: those found in blood plasma are numerous and include amino acids, glucose, fatty acids, glycerol and vitamins. They have been reduced to their simplest form by the digestive processes and absorbed from the alimentary tract into the blood and lymph for circulation to the cells
• Waste products resulting from fat and protein metabolism, including urea, uric acid and creatinine
• Gases, including oxygen, nitrogen and carbon dioxide. Oxygen and nitrogen enter the bloodstream after inhalation of air, and carbon dioxide is an end product of oxidation in the cells
• Hormones: chemical substances secreted directly into the bloodstream by endocrine glands and carried to the areas of the body where they are required to stimulate activity
• Antibodies and antitoxins: complex protein substances produced by the body in response to an invasion by a foreign protein (antigen). They are part of the body’s defence mechanism
• Enzymes: produced by the body, which initiate or accelerate chemical reactions.
Blood group types
Human blood is grouped into four classifications based on immune reactivity. The groups are O, A, B, AB. The Rhesus factor (either negative or positive) is also determined. Eighty-five per cent of the population has Rh antibodies on the surface of the red blood cell (that is RH positive). The blood of any one group is essentially incompatible with the blood group of another. Therefore blood transfusions should be an exact match to the client’s blood group and Rh factor. When blood transfusions occur with mismatched blood a haemolytic reaction can occur. See Procedural Guideline 23.1 for preparing and monitoring a client undergoing a blood transfusion.
Procedural Guideline 23.1 Preparing and monitoring a client undergoing a blood transfusion
| Review and carry out the standard steps for all nursing procedures/interventions |
Blood cells
The blood cells and fragments are suspended in the plasma and are called formed elements. The three types of blood cells or fragments of cells are erythrocytes, leucocytes and thrombocytes.
Erythrocytes
Erythrocytes (red cells) are biconcave non-nucleated discs measuring about 7 microns in diameter. In adults, erythrocytes are produced in the red bone marrow of cancellous bone tissue, where they pass through several stages of development. They begin as large nucleated cells but when mature (after they have produced haemoglobin) they lose the nucleus and are liberated into the circulation. Haemoglobin is a complex protein composed of four different ‘haem’ chains, each containing a central atom of iron and a globulin protein. It has a strong affinity for both oxygen and carbon monoxide and gives the blood its colour. The normal haemoglobin level is about 14–16 g/100 mL of blood.
The number of erythrocytes is about 5 000 000/mm3 of blood, and their average life span is 100–120 days. As their nucleus is absent, they are unable to repair damage and become worn out in circulation, and are destroyed in the spleen and liver. The haemoglobin is split; the liver stores the iron for future use and the liver uses the pigment in the production of bile. The primary function of erythrocytes is to carry oxygen. In the lungs, oxygen combines with haemoglobin to form oxyhaemoglobin, making the blood bright red in colour. As blood circulates through the tissues, the oxygen is released, forming deoxyhaemoglobin, and the blood becomes dark red in colour.
Leucocytes
Leucocytes (white cells) measure about 10 microns in diameter. They differ from erythrocytes in that they are larger, possess a nucleus and are less numerous. They also have the power of independent movement, known as diapedis, or emigration, which erythrocytes do not possess. There are two main types of leucocytes: granulocytes and agranular leucocytes. Granulocytes contain granules of enzymes and are classified as neutrophils, basophils or eosinophils. Neutrophils are the most numerous of the leucocytes and are important to the body in defence against bacteria, as they have the ability to engulf phagocytose and digest them. Neutrophils also play an important part in the inflammatory response. Injured tissues, and other leucocytes, secrete substances that stimulate the bone marrow to release increased numbers of neutrophils. Basophils release substances in infected tissue that are toxic to many microorganisms. They also play a part in the allergic response and act to limit the inflammatory response. Eosinophils are also involved in phagocytosis, as they ingest antigen–antibody complexes and parasites. They also play a role in clot retraction.
Agranular leucocytes lack granules of enzymes and are classified as monocytes or lymphocytes. Monocytes have the ability to move into the tissues, where they become macrophages and are capable of phagocytosis. They also secrete a variety of substances involved in the body’s defence, and play a role in the immune response. Lymphocytes are either T lymphocytes or B lymphocytes, both of which divide when stimulated by antigens. T lymphocytes are responsible for cellular immunity, and adhere to cells identified as foreign to the body. They secrete cytotoxic substances that kill the foreign cells. B lymphocytes are involved in humoral immunity, as they produce antibodies and are also responsible for immunoglobulin production. While the life span for granular leucocytes is only about 21 days, lymphocytes may survive for up to 100 days.
The total number of leucocytes is about 8000–10 000 mm3 of blood, but this number increases considerably (leucocytosis) when there is any infection in the body. The life span of a leucocyte is variable and depends to some extent on the degree of activity.
Thrombocytes
Thrombocytes (platelets) are colourless microscopic fragments of the megakaryocyte cell. Measuring about 3 microns in diameter, they do not possess a nucleus. Thrombocytes are produced in the red bone marrow, which is present in cancellous bone tissue. The number of thrombocytes is about 250 000–300 000/mm3 of blood, and the average life span of a thrombocyte is 5–9 days. The function of thrombocytes is to play a major role in the clotting of blood to reduce blood loss when a vessel wall is injured. The process involves many substances (clotting factors) which are produced by the liver and circulate in the plasma, as well as some substances released by the platelets and injured tissues. Normally a blood clot will form within 2–6 minutes after a blood vessel wall has been damaged.
The mechanism of clotting (haemostasis) involves three phases: vasoconstriction, formation of a temporary platelet plug and formation of a clot. When a small vessel becomes damaged:
• Local vasoconstriction occurs, which reduces blood flow and therefore blood loss
• The vessel wall becomes ‘sticky’ and platelets adhere to the damaged area
• The platelets release serotonin and adenosine diphosphate (ADP), which attract other thrombocytes, leading to the formation of a temporary platelet plug
• The temporary platelet plug is converted into a clot by the deposition of fibrin, which is formed from fibrinogen. The conversion of fibrinogen to fibrin involves a ‘cascade’ of reactions that requires a number of plasma factors (numbered I to XIII). A series of reactions culminate in the conversion of prothrombin to thrombin, which converts fibrinogen to fibrin. This conversion requires the presence of platelets, Factor V, Factor X and calcium ions. Vitamin K is also necessary for the conversion of Factors VII, IX and X
• The fibrin forms a meshwork of fibres that traps the erythrocytes and forms the basis of a clot
• The clot plugs the injured blood vessel, drawing the edges together.
The clotting mechanism is a complex one that will not occur if any of the necessary elements are reduced, defective or missing (Marieb & Hoehn 2010).
Blood vessels
Blood is circulated throughout the body (Fig 23.9) within vessels that form a closed continuous system.
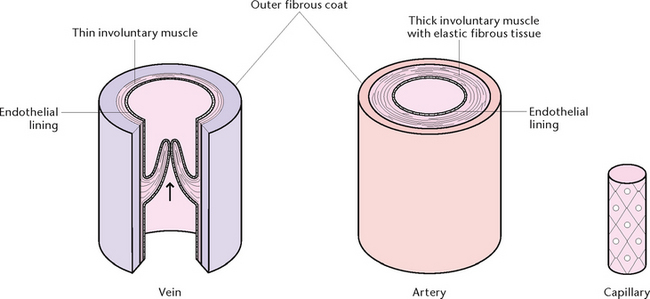
The walls of blood vessels have three layers: an outer coat of fibrous tissue, a thick middle layer of involuntary muscle with elastic fibrous tissue and an inner lining of endothelium to form a smooth surface for contact with blood (Fig 23.10). Blood vessels include the arteries, veins and capillaries.
Arteries
Arteries carry blood away from the heart (efferent). All arteries carry oxygenated (bright red) blood, with the exception of the two pulmonary arteries which carry deoxygenated (dark red) blood from the heart to the lungs. Arteries vary in size, and large arteries divide to form smaller arteries (Fig 23.11). Further division, or branching, occurs to form the smallest arteries, called arterioles, which divide into capillaries. Arteries and arterioles have the same tissue structure that allows them to stretch and recoil as the heart pumps the blood into them.
Veins
Veins carry blood towards the heart (afferent). All veins carry deoxygenated (dark red) blood, with the exception of the four pulmonary veins, which carry oxygenated blood from the lungs to the heart. Veins vary in size, and large veins divide to form smaller veins. The smallest veins are called venules, which divide into capillaries (Fig 23.11). Venules carry deoxygenated blood away from the capillary beds and unite to form veins. The walls of veins are composed of the same three layers as those of arteries, but the walls are thinner and have less elastic and muscular tissue. Veins join up until the two largest veins are formed—the superior and inferior vena cavae. These two veins empty their contents into the right atrium of the heart.
The larger veins possess pocket-like valves on their inner surfaces. These valves aid the unidirectional flow of blood towards the heart, and prevent a backward flow of blood. Skeletal muscle activity also helps venous return. As the muscles surrounding the veins contract and relax, the blood is ‘milked’ through the veins towards the heart.
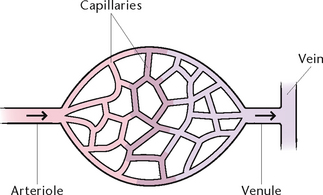
Capillaries
Capillaries are microscopic vessels about 5–7 microns in diameter and are composed of a single layer of endothelium, with little surrounding connective tissue. They form closed networks through all tissues and are structurally adapted for their role in the rapid diffusion of substances between the plasma and interstitial fluid. This allows water, oxygen, nutrients and other essential substances to pass rapidly from the blood to the tissue cells, and waste products from the tissue cells pass through the capillary walls to the blood.
The heart
The heart is a hollow, conical muscular organ situated obliquely in the thoracic cavity between the lungs and behind the sternum. One-third of the heart lies to the right, and two-thirds lie to the left of the median plane. Its base is uppermost and points towards the right shoulder, and its apex is below, pointing to the left. The adult heart is about 12 cm × 8 cm × 6 cm and weighs about 300 g.
Structure of the heart
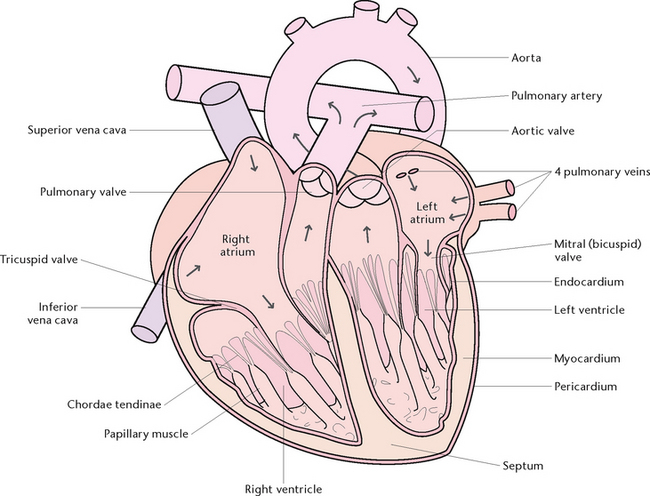
The heart is divided into a right and a left side by a muscular partition called the septum. Each side is further divided into an upper receiving chamber, the atrium, and a lower distributing chamber, the ventricle (Fig 23.12).
The walls of the heart consist of the pericardium, myocardium and endocardium:
• The pericardium is the outer coat, consisting of two layers of serous membrane. The pericardium secretes a small amount of serous fluid to moisten the surfaces in contact with each other, so that the heart can beat with minimal friction
• The myocardium is the middle muscular layer consisting of cardiac muscle, which is a highly specialised type of muscle tissue present only in the heart. It is of varying thickness, being thicker in both ventricles than in the atria, and thicker in the left ventricle than in the right
• Endocardium is the innermost lining of the heart, and provides a smooth surface for the flow of blood. Folds of endocardium help to form the valves of the heart.
Valves of the heart
Heart valves consist of flaps of fibrous tissue covered by endocardium, which allow blood to flow in one direction only, thus preventing a backward flow. The valves are the:
• Bicuspid (or mitral) valve, between the left atrium and left ventricle
• Tricuspid valve, between the right atrium and right ventricle
• Aortic valve, between the left ventricle and the aorta
• Pulmonary valve, between the right ventricle and the pulmonary artery.
Fine cords of tendons (chordae tendinae) are attached from the mitral and tricuspid valves to small projections from the muscle walls of the ventricles called papillary muscles. Contraction of the papillary muscles closes the valves, preventing blood from escaping back into the atria.
Blood vessels
Several blood vessels either enter or leave the heart. The blood vessels that enter the heart are the:
• Inferior vena cava, which carries deoxygenated blood collected from the lower part of the body to the right atrium
• Superior vena cava, which carries deoxygenated blood collected from the upper part of the body to the right atrium
• Four pulmonary veins, two from each lung, which carry oxygenated blood into the left atrium.
The blood vessels that leave the heart are the aorta, which carries oxygenated blood from the left ventricle for distribution to all the systems and tissues of the body, and the pulmonary artery, which leaves the right ventricle then divides into two branches that carry deoxygenated blood from the heart to each lung. Thus, the right side of the heart deals only with deoxygenated blood, and the left side deals only with oxygenated blood.
Blood supply to the heart
As the aorta leaves the heart it gives off two branches called the coronary arteries. These arteries pass into the heart wall to supply mainly the myocardium with blood. The coronary arteries divide into smaller and smaller branches, until networks of capillaries are formed in the heart wall. Venules collect the deoxygenated blood from the tissues in the heart wall and unite to form a vein (coronary sinus), which opens directly into the right atrium.
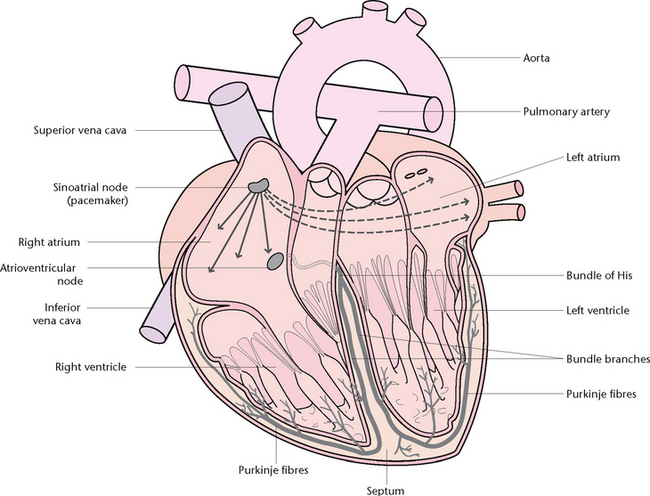
The conducting system of the heart
The heart’s conducting system (Fig 23.13) ensures that it contracts in a coordinated and synchronised series of events. The sinoatrial (SA) node is located in the upper part of the right atrium and acts as the pacemaker of the heart, continuously initiating impulses that innervate the rest of the heart. The atrioventricular (AV) node lies in the lower part of the interatrial septum of the heart. The AV node is connected to the bundle of His and is the only natural pathway for the impulse to travel from the atria to the ventricles. The bundle of His divides into the right and left bundle branches, which in turn divide off into tiny fibres termed Purkinje fibres. These fibres rapidly conduct the impulse throughout the myocardium from the apex to the base.
Functions of the heart
The function of the heart is to act as a pump: it pumps deoxygenated blood to the lungs to excrete carbon dioxide and pick up oxygen, and pumps oxygenated blood to all other parts of the body.
The cardiac cycle is the series of pressure changes, valve actions and electrical potentials that bring about the movement of blood through the heart during one complete heart beat. The cardiac cycle takes about 0.8 of a second and consists of two phases, systole (the contraction phase) and diastole (the relaxation phase). During systole, both atria contract at the same time, emptying their contents into the ventricles. The two ventricles then contract simultaneously, forcing their contents into the aorta and pulmonary artery. Diastole follows after each contraction of the heart.
Cardiac output is the volume of blood pumped out by each ventricle during 1 minute. It is the product of the volume of blood pumped at each beat (stroke volume) and the number of beats during 1 minute (heart rate).
The heartbeat is controlled by the cardioregulatory centre in the central nervous system. The vagus nerve slows it and reduces the force of the beat, while sympathetic nerves quicken the beat and increase its force.
Each cardiac muscle cell is capable of spontaneous, rhythmic self-excitation known as autorhythmia. To be effective as a pump, the action of the whole heart must be coordinated. Coordination of the rhythmic movements is brought about by the specialised cells of the sinoatrial (SA) node (pacemaker).
Blood pressure
The term blood pressure refers to the pressure, or force, exerted by blood on the walls of the blood vessels. Pressure is highest in the arteries, which receive blood from the ventricles of the heart at about 120 mmHg. As the vessels divide, their cross-sectional area increases, causing the pressure to progressively reduce so that there is only very slight pressure in the capillaries (about 35 mmHg and 15 mmHg in the venules). Systolic blood pressure is the pressure registered in a large artery as blood is forced out of the ventricle during the contracting period of the cardiac cycle. Diastolic blood pressure is the pressure registered during the relaxing period of the cardiac cycle, when there is no ejection of blood into the arteries. It is therefore lower than the systolic pressure.
CIRCULATION OF BLOOD
Deoxygenated blood from all body regions is transported via the veins to the superior and inferior vena cavae, which enter the right atrium. The coronary sinus drains venous blood from the myocardium into the right atrium. At first, blood flows passively into the right ventricle as the tricuspid valve is open, then contraction of the right atrium (atrial systole) occurs to empty its entire contents. After the tricuspid valve closes, the right ventricle contracts (ventricular systole), and blood is ejected through the pulmonary valve into the pulmonary artery.
The pulmonary artery divides into the right pulmonary artery, which carries deoxygenated blood to the right lung and the left pulmonary artery, which carries deoxygenated blood to the left lung. In the lungs, oxygen is exchanged for carbon dioxide from the blood, and the oxygenated blood returns to the left atrium via four pulmonary veins. The left atrium contracts and blood passes through the mitral (bicuspid) valve into the left ventricle. After the mitral valve closes, the left ventricle contracts and blood is ejected into the aorta via the aortic valve. The aorta branches off to supply all areas of the body with oxygenated blood. Blood is therefore in constant circulation around the body, and the system of circulation can be divided into three parts:
The systemic circulation
The systemic circulation is the distribution of oxygenated blood to all tissues, and the return of deoxygenated blood from all tissues to the heart. When the left ventricle contracts it forces blood into the aorta under pressure. The elastic walls of the aorta distend to receive the blood. When the left ventricle relaxes the walls of the aorta recoil and, with the aortic valve closed, the blood is driven onwards through the aorta. Branches from the aorta also distend and recoil as the blood travels through them, and this wave of distension and recoil is felt as the pulse wherever a superficial artery crosses a hard structure such as a bone.
Arterioles supply networks of capillaries with oxygenated blood, and the hydrostatic pressure behind the blood causes water and other essential substances to be pushed through the capillary walls and wash over the tissue cells to become part of the tissue fluid. Tissue cells allow certain substances they require to enter, and excrete their waste products into the tissue fluid. The pressure within the capillaries will allow only a small amount of the fluid to return through the capillary and venule wall, back into the blood. The remainder of the fluid reaches the blood via the lymphatic system, which is discussed later in this chapter.
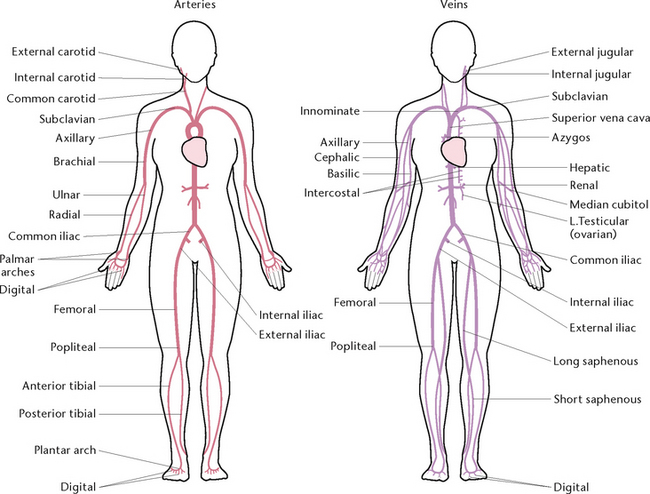
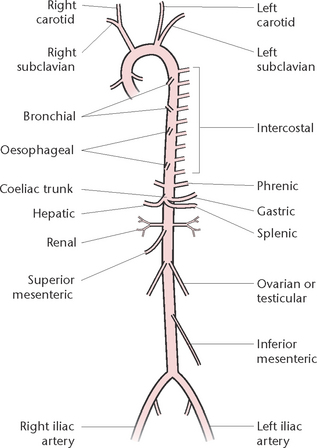
Arteries
The aorta (Fig 23.14) has four sections, each of which has a number of branches. Two coronary arteries to the heart wall branch from the ascending aorta. From the aortic arch branch the left common carotid artery to the head and neck; the left subclavian artery to the left upper limb; and the right innominate artery, which divides into the right common carotid and right subclavian arteries. From the descending thoracic aorta branch the bronchial arteries to the lungs; the oesophageal artery to the oesophagus; and 10 pairs of intercostal arteries to the intercostal muscles. From the abdominal aorta, branch the:
• Phrenic arteries to the diaphragm
• Coeliac trunk, which divides into the gastric artery to the stomach, the hepatic artery to the liver and the splenic artery to the pancreas and spleen
• Superior mesenteric artery to the small intestine
• Renal arteries to the kidneys
• Ovarian or testicular arteries to the ovaries or testes
• Inferior mesenteric artery to the large intestine
• Two common iliac arteries to the pelvic organs and the lower limbs.
Veins
There are two groups of veins: superficial veins, some of which can be seen as bluish lines under the skin; and deep veins, which run beside arteries and often have the same name as the arteries. Veins rely on the squeezing action of skeletal muscles to assist in pushing blood towards the heart, and on respiratory movements, which have a milking effect on the inferior vena cava as it passes through the diaphragm.
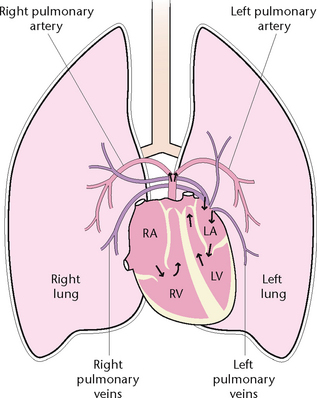
Pulmonary circulation
The pulmonary circulation (Fig 23.15) involves the transport of deoxygenated blood from the heart to the lungs and the return of oxygenated blood from the lungs to the heart. The pulmonary artery leaves the right ventricle and divides into the right and left pulmonary arteries, which carry deoxygenated blood to the lungs. In the lungs, the arteries divide until capillaries are formed. Venules collect the oxygenated blood from the capillaries and unite to form the two pulmonary veins, which leave each lung and enter the left atrium of the heart.
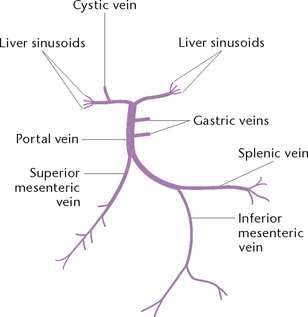
Portal circulation
The portal circulation (Fig 23.16) is responsible for carrying blood that is deoxygenated, but rich in digested nutrients, from some of the abdominal organs to the liver.
The splenic, gastric, inferior and superior mesenteric veins unite to form a large vein called the portal vein, which enters the liver. The liver converts the nutrients brought by the blood into a form to be either used by tissues throughout the body or stored for future use. The liver thus receives blood from two sources: the hepatic artery, which supplies it with oxygenated blood; and the portal vein, carrying deoxygenated blood rich in nutrients. When the oxygen has been extracted from the former and the nutrients from the latter have been processed, the blood leaves via the three hepatic veins.
STRUCTURE OF THE LYMPHATIC SYSTEM
The lymphatic system (Fig 23.17) is closely connected with the circulation of blood and consists of an additional set of vessels through which some of the tissue fluid passes before reaching the large veins and entering the blood. This system consists of lymphatic capillaries, lymphatic vessels, lymphatic nodes and lymphatic ducts. The fluid in the system is called lymph.
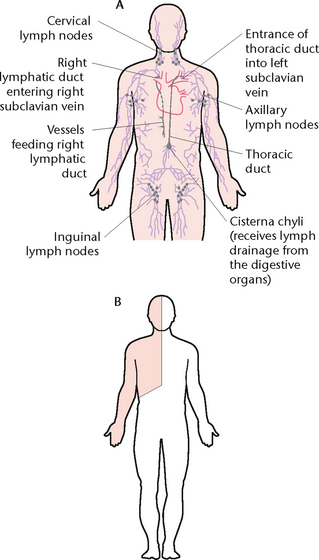
Figure 23.17 The lymphatic system A: The distribution of lymphatic vessels and nodes B: Areas drained by the right lymphatic duct (shaded) and the thoracic duct (unshaded)
The lymphatics serve an important function in preventing oedema, as the tiny vessels collect fluid and proteins from the interstitial spaces and promote their return to the blood circulation. They also collect the larger digested fat particles from the digestive system and empty them into the circulation. In addition, the lymphatics play a key role in the body’s defence against microorganisms. They collect microorganisms in the interstitial spaces and carry them to the lymph nodes, where the lymphocytes (and macrophages) remove them from the lymph.
Lymph
When fluid leaks out of the capillaries from the cardiovascular system it accumulates in the tissue spaces. When this fluid is drained from the tissues and collected by the lymphatic system, it is called lymph and has a composition similar to the blood plasma. Lymph is normally a colourless fluid, although lymph absorbed from the intestines is saturated with fats and is milky in colour. Lymph travels slowly through the lymphatic system—total lymph flow is about 2–4 L/day.
Lymphatic capillaries
Lymphatic capillaries are similar in size and structure to blood capillaries. They unite to form larger lymphatic vessels similar in structure to veins and, like veins, they have valves to prevent a backward flow of lymph. All lymphatic vessels pass through one or more lymph nodes. Afferent lymphatic vessels carry lymph to a node. Efferent lymphatic vessels carry lymph away from the node and empty it into the lymphatic ducts.
Lymph nodes
Lymph nodes are found mainly in groups in many parts of the body, such as the neck, thorax, abdomen, groin and the limbs. Lymphatic nodes vary in size and consist of lymphatic tissue. The functions of lymphatic nodes are to filter and destroy bacteria from the lymph passing through the node; to produce lymphocytes, which are added to the lymph; and to produce antibodies and antitoxins.
Other areas containing lymphatic tissue
In addition to the nodes already described, lymphatic tissue is found in several other anatomical structures, including the tonsils in the oropharynx, adenoids in the nasopharynx, the appendix attached to the intestines, the thymus gland in the thorax, Peyer’s patches in the small intestine and the spleen in the abdomen.
Tonsils
The tonsils form part of a protective ring of tissue at the entrance to the respiratory and digestive tracts. Lymphatic vessels leave the tonsils and enter the cervical nodes.
Thymus gland
The thymus gland is a soft grey-pink gland present in the thorax behind the sternum and in front of the heart. It is large in infants and children, reaching its maximum size at puberty. After puberty this gland gradually shrinks, until in adulthood there is only a small piece of tissue left. The thymus gland functions in the production of lymphocytes.
Spleen
The spleen is a purplish half-moon-shaped organ in the left hypochondriac region of the abdomen. It lies below the diaphragm and behind the lower ribs and is mainly composed of lymphoid tissue enclosed in a fibrous capsule. The functions of the spleen are to:
• Produce lymphocytes, some of which enter the bloodstream to carry out their phagocytic action
• Destroy worn-out erythrocytes, producing bile pigments and iron
• Produce antibodies and antitoxins
• Provide a storage area for erythrocytes needed in emergency situations (if haemorrhage occurs, the spleen vessels contract and empty blood into the circulation in an attempt to restore normal blood volume).
Lymphatic ducts
The two lymphatic ducts receive the lymph from lymphatic vessels and empty it into the bloodstream via the subclavian vein (Fig 23.17). They are the thoracic duct and the right lymphatic duct.
Thoracic duct
The thoracic duct, the larger of the two lymphatic ducts, begins in the abdominal cavity at lumbar level as a dilated sac called the cisterna chyli. The duct passes upwards through the aortic opening behind the diaphragm into the thorax, where it empties its contents into the left subclavian vein so that the lymph rejoins the bloodstream. Lymphatic vessels from all parts of the body below the diaphragm, and the left side of the body above the diaphragm, empty their contents into the thoracic duct.
Right lymphatic duct
The right lymphatic duct is very small (about 1 cm long) and lies in the root of the neck. It receives lymph from the right side of the head and neck, the right side of the thorax and the right upper limb. The right lymphatic duct empties its contents into the right subclavian vein.
FACTORS AFFECTING THE RESPIRATORY SYSTEM
Oxygen concentration
Oxygen makes up approximate 21% of the air, which is normally sufficient to meet the needs of the body. A decrease in this amount of oxygen can cause problems. Two instances in which the available oxygen may be deficient are:
• High altitude: the total pressure of all gases in the air decreases as altitude increases. As the total pressure decreases, the oxygen pressure decreases proportionately, and the individual will experience difficulty in maintaining adequate tissue oxygenation (hypoxia). Acclimatising is a homeostatic response where initially the ventilatory rate is increased in an attempt to supply the body with sufficient oxygen, then later the bone marrow is stimulated to increase erythrocyte production (polycythaemia) to carry more oxygen
• Presence of noxious gases: some noxious gases such as carbon monoxide have a higher affinity for haemoglobin than does oxygen, which is displaced by them, causing a reduction in oxygen availability to tissues.
Regulating mechanisms
Any factor that interferes with the respiratory centres in the brainstem or the nerves that transmit messages to and from them may cause ventilatory difficulties. Respiratory depression can be caused by increased intracranial pressure such as cerebral oedema, due to conditions such as hypercapnia, cerebral bleeds, meningitis, encephalitis, hydrocephalus, tumours, hypoalbuminaemia and ketoacidosis. Clinical Interest Box 23.1 discusses the effect pyrexia (fever) has on oxygen regulation mechanisms. Other factors include certain medications such as analgesics (e.g. morphine), and various anticonvulsant drugs (e.g. clonazepam). Ventilation increases when the pH of the blood is lowered (a respiratory response to rid the body of the excess acid) whereas ventilation decreases when the pH increases (to retain acid). (See respiratory alkalosis and acidosis later in this chapter.)
CLINICAL INTEREST BOX 23.1 Oxygen requirements and pyrexia
Many respiratory conditions cause pyrexia. Historically clients have been treated with antipyretics or other methods to cool their skin, such as tepid sponges, fans and removing clothing. Pyrexia is the body’s homeostatic mechanism for providing a hostile environment for pathogens and for increasing the immune response. Clients normally feel hot to touch on the ‘up’ state of the fever but state that they feel cold, and may shiver, while the ‘down’ state normally is the reverse.
Cooling a client’s skin may actually increase the pyrexial state, as the body shivers to keep warm. Medical treatment of pyrexia is increasingly aimed at allowing the body’s immune system to maintain the pyrexia, without nursing or medical interference, drugs or other care. The pyrexia is still investigated and the cause treated, and antipyretics administered if a client’s other symptoms or past history (e.g. febrile convulsion) deem it safer, or their discomfort may be alleviated by antipyretics.
Most at risk of pyrexial complication are some clients with congenital or chronic respiratory or cardiac conditions in which they have a diminished ‘reserve’ or ability for their bodies to supply the extra 10% of oxygen needed per 1ºC of body temperature increase. In these clients not only are antipyretics commonly used but also supplemental oxygen is administered while the pyrexial state exists.
Exchange of gases during ventilation and respiration
The efficiency of ventilation and respiration can be affected by any factor that interferes with the patency of the respiratory tract or the actions of the ventilatory muscles. For example, an accumulation of secretions may result from respiratory conditions such as asthma, bronchitis or a reduced cough reflex. Ventilations may also be reduced by factors that affect the actions of the ventilatory muscles, such as brainstem or spinal cord injury or motor neuron diseases such as multiple sclerosis or Guillain–Barré syndrome. These conditions can restrict the movements of the diaphragm and/or intercostal muscles. Chest expansion and ventilation can also be affected by deformities of the chest wall or skeleton, such as scoliosis, flail chest (multiple rib fractures causing instability in part of the chest wall and paradoxical breathing movements, the part of the lung underlying the injured area contracts on inspiration and bulges on expiration) and pectus excavatum (a skeletal abnormality of the chest that is characterised by a depressed sternum).
Diffusion of oxygen and CO2
Any dysfunction of the lungs that affects the alveolar capillary membrane thickness or causes a reduction in their surface area will affect respiratory function. Conditions such as pulmonary oedema, alveolitis, pneumonia or chronic obstructive airways disease may reduce the diffusion of oxygen and CO2. Information on these and other respiratory disorders is provided later in this chapter.
Transport of oxygen and CO2 to and from the cells
Any condition affecting the efficiency of the heart, blood vessels or blood can interfere with the transportation of oxygen to the cells or CO2 away from the cells. Such conditions include congestive cardiac failure, atherosclerosis, carbon monoxide poisoning and anaemia. Information on these and other cardiovascular disorders is provided later in this chapter.
Influences on the rate, depth and rhythm of breathing
Several factors influence the characteristics of breathing, such as pyrexia and physical activity. Oxygen requirements are greatest during exertion and least during sleep. The rate and depth of ventilations vary in response to the body’s production of CO2; for example, during strenuous exercise the volume of air drawn into the lungs with each breath may be increased from a normal tidal volume of 500 mL to as much as 2300 mL. Changes in mood, emotion and pain may also affect the rate, depth and rhythm of ventilation; for example, the ventilation rate is commonly increased during fear, anxiety or apprehension. Chronic irritation by inhaled irritants such as smoke or dust can also affect breathing and cause short-term effects such as coughing and shortness of breath, or long-term effects such as severe dyspnoea resulting from emphysema.
Inadequate ventilation (hypoventilation)
Hypoventilation and alveolar hypoventilation is a reduction in the ventilation of the alveoli. The causes are many and varied and include airway obstruction by oedema, inhaled foreign bodies, retained secretions (mucus, casts), polyps, tumours, bronchospasm, emphysema, neuromuscular or skeletal abnormalities and central nervous system disorders. Hypoventilation occurs when the volume of air entering the alveoli is not adequate for the metabolic needs of the body. The reduction in ventilation causes an increase in the partial pressure of CO2 in arterial blood (PaCO2), termed hypercapnia, and may also cause hypoxaemia, a decrease in the partial pressure of oxygen in arterial blood (PaO2).
Impaired diffusion
Diffusion is the process by which oxygen and CO2 molecules are transported between the alveoli and the capillary network. Diffusion abnormalities can interfere with the passage of oxygen into the blood. Such diffusion abnormalities can result from: a thickening of the alveoli capillary walls, for example, in pulmonary oedema; a reduced amount of functioning lung tissue, such as in emphysema; or fibrosis of the alveolar walls, as seen in alveolitis and pneumonitis.
Impaired perfusion
Not only is an adequate intake of oxygen by ventilation essential, but for oxygenation of the body tissues to occur, adequate perfusion of lung tissue with blood is essential. Any condition that decreases the circulation of blood to lung tissue may lead to ventilation–perfusion mismatches (V/Q principle) and hypoxaemia. Such conditions include decreased blood volume, pulmonary embolism, cardiac disorders and chronic obstructive pulmonary disease.
In addition to abnormalities of the lungs or respiratory structures, dysfunction of other body systems can adversely affect respiratory function. For example, a disease process that affects the nervous system may adversely affect respirations. When the spinal cord is damaged, the nervous system may greatly impair respiratory function. Cardiovascular dysfunction can affect respiratory function; for example, right-sided heart failure may affect the volume of pulmonary blood circulation. A deformity of the skeletal system, such as scoliosis, may restrict movement of the thoracic cage and thus alter respiratory function. Inadequate lung expansion can also occur after abdominal surgery, as pain in the operation site may inhibit deep breathing and coughing. Not only can abnormalities of other body systems adversely affect the respiratory system, but respiratory abnormalities generally affect all other body systems.
Additional factors
Numerous other factors can modify the rate and depth of ventilation, for example, involuntary and reflex mechanisms such as exercise, pain, hiccupping, sneezing, sighing and emotions, and conscious voluntary actions such as voluntary breath-holding, inhalation and exhalation. Clinical Interest Box 23.2 discusses the alteration to respirations in the older adult. Other factors that affect functioning include:
• Digestive system disorders and diminished appetite, potentiating an alteration in nutritional status
• Changes in renal function, which can affect erythropoietin production
CLINICAL INTEREST BOX 23.2 Respirations and the older client
Involutional changes that can occur with increasing age include: loss of elasticity of the lung tissue, costal cartilage calcification, kyphosis, weakening and loss of skeletal muscle fibre, reduction in cilia numbers and beating speed, and thickening of alveolar membranes. These factors and many more diminish the respiratory system’s ventilatory capacity and the ability to excrete CO2 and absorb oxygen, increasing the resting respiratory rate from the adult rate of about 20/min to 25/min for an older client.
These may all combine to affect blood cell production, with subsequent anaemias. In clients who are more sedentary such combinations of factors and subsequent alveolar hypoventilation increase the risk of primary or secondary infections. To avoid the complications of ageing, all clients can be educated to:
PATHOPHYSIOLOGY RELATED TO THE RESPIRATORY SYSTEM
The signs and symptoms manifested by a client with a respiratory disorder vary with the location and severity of the disorder. Detailed below are some common clinical features of respiratory disease.
Chest pain
Disorders of the respiratory system such as pleurisy can result in chest pain. During ventilation, friction occurs between the inflamed pleura. Chest pain may be localised or may be experienced only when the client breathes deeply, and can vary from a continuous aching pain to a stabbing knife-like pain. Pain associated with respiratory disorders may be retrosternal, lateral or posterior and is exacerbated by deep inhalation.
Cough
A cough is a common symptom of many respiratory disorders and may result from irritation or from retained secretions that obstruct some part of the airway. If sputum is swallowed, expectorated or the cough sounds moist, it is described as productive. A non-productive, or dry, cough is one that sounds dry or irritating and no sputum is expectorated or swallowed. Sputum is the result of excessive mucus production and may result from inflammation, infection or congestion.
Haemoptysis (the coughing or expectoration of blood) may occur in some lung diseases. Blood-streaked sputum frequently occurs in some respiratory tract conditions, such as infections (e.g. bronchitis, tuberculosis), pulmonary oedema or bronchogenic carcinoma. The expectoration of bright red or frothy blood indicates a more serious disorder, such as pulmonary embolism or lung abscess.
Voice changes, ranging from hoarseness to aphonia (no speech), may result from numerous causes, including viral upper respiratory tract infections (e.g. laryngitis), vocal cord polyps and laryngeal tumour. Unilateral or bilateral vocal cord paralysis may also result from congenital defects, intubation or other damage to the recurrent nerve, for example, after thyroid or cardiac surgery.
Dyspnoea (difficult or laboured breathing) may result from disorders affecting either the upper or the lower respiratory tract or surrounding structures. Disorders of the upper respiratory tract that may cause dyspnoea include obstruction of the airway by inflammation, a tumour or foreign body. Disorders of the lower respiratory tract that cause dyspnoea include airway inflammations or infections, asthma, pneumonia, pneumonitis, carcinoma of the lung and chronic obstructive pulmonary disease. Any disorder affecting the thorax, such as trauma to the chest wall, commonly causes dyspnoea. Laboured breathing may be accompanied by nasal flaring, the use of the neck and accessory chest muscles and increased ventilation rate.
Changes in breathing patterns and sounds
Any disorder that affects the respiratory system may produce changes in the pattern of breathing. Examples include tachypnoea (increased respiratory rate), bradypnoea (decreased respiratory rate) and airway obstruction; for example, due to emphysema, which can result in prolonged forceful expiration and pursed-lip breathing. Certain disorders also result in abnormal breathing sounds, such as wheezing or ‘grunting’ ventilations. Information on abnormal breathing sounds is provided later in this chapter.
Hypoxia
Hypoxia is a deficiency of oxygen in the tissues and may be due to lung disorders that prevent adequate supplies of oxygen from reaching the blood (hypoxaemia). Hypoxia may also be due to hypoventilation, anaemia or impaired tissue utilisation of oxygen. The initial manifestations of hypoxia include tachycardia, tachypnoea, breathlessness, pallor, lethargy or agitation, followed by increasing confusion and deepening cyanosis.
Thoracic abnormalities
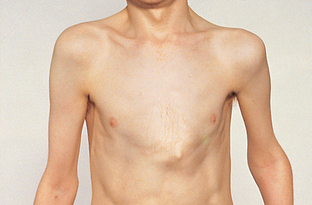
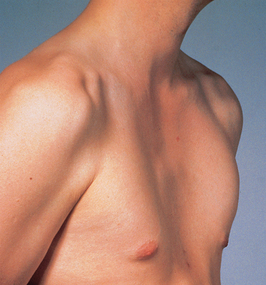
Chronic respiratory system disorders may alter chest configuration; for example, a ‘barrel chest’ is characteristic of chronic obstructive conditions such as cystic fibrosis and emphysema. Abnormal curvatures of the spine, such as severe scoliosis, and congenital chest deformities such as pigeon chest (Fig 23.18) and pectus excavatum (‘funnel chest’) (Fig 23.19) may also affect pulmonary ventilation. Asymmetrical chest expansion can result from conditions such as flail chest, haemothorax, pneumothorax, retained secretions or inhaled foreign bodies causing atelectasis.
Other manifestations
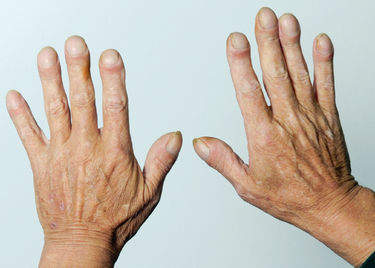
Depending on the type and severity of the disorder, other manifestations may be evident. For example, infections of the respiratory tract generally result in pyrexia, headaches, aching muscles and lethargy. Difficulty in swallowing (dysphagia) may be present in disorders such as pharyngitis and tonsillitis. In certain chronic disorders of the respiratory system, clubbing of the fingers (Fig 23.20) may be evident. The distal portions of the fingers are abnormally enlarged by anastamosis of blood vessels in response to peripheral hypoxaemia. The nails have an increased curvature and the angle of the nail bed increases to over 85 degrees.
SPECIFIC DISORDERS OF THE RESPIRATORY SYSTEM
Disorders of multiple cause
Certain disorders of the respiratory system have more than one cause and may be related to structural or functional changes, environmental conditions or a combination of factors. It is beyond the scope of this text to provide in-depth information on the various conditions related to the respiratory system. Listed below are some examples of respiratory conditions:
Infectious disorders
Infectious disorders can be classed as upper or lower respiratory tract infections. Bacteria or viruses cause infections of the upper respiratory tract while a variety of microorganisms can cause lower respiratory tract infections. For more information review the references and further reading list at the end of this chapter.
Clinical Interest Box 23.3 looks at a viral infection, sudden acute respiratory syndrome (SARS). Listed below are some examples of respiratory conditions:
CLINICAL INTEREST BOX 23.3 Sudden acute respiratory syndrome (SARS)
Sudden acute respiratory syndrome (SARS) first appeared in the southern Chinese province of Guangdong in 2002 and by 2003 had been classified as an epidemic by the World Health Organization. Symptoms of SARS, which is believed to be spread mainly through droplet infection, include pyrexia, rigors, cough, headache, aches and pains and dyspnoea. These same symptoms are common for many other respiratory infections, making the positive diagnosis difficult.
The number of deaths to June 2003 was about 800 people worldwide, and the number of people infected since 2002 about 8500. In 2002 and 2003 about 14% of those infected died from SARS, usually 5–6 weeks after becoming ill.
The virus was found to be a coronavirus, and the genome was sequenced in April 2003 by the US Centers for Disease Control and Prevention. After relaxation of SARS infection-control measures in Canada, a further outbreak occurred, due probably to transmission by an unrecognised SARS carrier, and affected five clients in a rehabilitation hospital. As a result, infection-control departments worldwide have introduced the most stringent infection-control policies for medical and paramedical workers seen to date, to limit the spread of SARS.
Obstructive disorders
Obstructive disorders are lung diseases that cause a persistent obstruction of bronchial air flow. Airway obstruction can also be due to the inhalation of a foreign body, or one or both bronchi may become obstructed by a benign or malignant tumour. Some of the more common types of airways obstruction are grouped under the heading of chronic obstructive pulmonary disease (COPD) or chronic obstructive airways disease (COAD). Common forms of COAD include asthma, emphysema, bronchiectasis and chronic bronchitis. Asthma and emphysema are discussed briefly here.
Asthma
Asthma is a disease manifested by difficulty in breathing caused by generalised narrowing of the airways. Asthma is characterised by recurring episodes of paroxysmal dyspnoea, wheezing on expiration, coughing and tenacious, mucoid bronchial secretions. A person having an asthma attack commonly experiences anxiety, diaphoresis, tachycardia and elevated blood pressure. The increased effort of breathing can cause extreme fatigue.
Episodes of asthma may be precipitated by inhalation or ingestion of allergens or pollutants, infection, or it may be exercise induced or occur in response to changes in air temperature or emotional stress. The severity and duration of asthma attacks vary; treatment may control the attack rapidly, or the symptoms can become increasingly severe and prolonged.
A severe and prolonged asthma attack that resists treatment is referred to as status asthmaticus (indicated by a PaCO2 of over 50%, or extreme exhaustion). Unless treatment reverses the condition, the client may develop respiratory failure.
Clinical Interest Box 23.4 provides an overview of the current incidence of childhood asthma.
CLINICAL INTEREST BOX 23.4 Childhood asthma
The incidence of children suffering from asthma has increased. Studies comparing the incidence of asthma in city-living and farming communities found that children living in a ‘clean environment’ were more at risk of asthma. They cited that the difference may be due to the obsession-like trend towards a germ-free house and environment. They indicated that children in farming communities had more exposure to pathogens, which stimulated their immune system. However, a significant proportion of asthma in children in the 1–5-year-old age bracket is due to pathogens.
A study done on wheezing-associated respiratory infections reported that the identified pathogens were, in order of occurrence: respiratory syncytial virus (RSV), parainfluenza virus (PIV-1 and PIV-2) and influenza virus A and B. Children in the 6–15-year age bracket were affected more by Mycoplasma pneumoniae, influenza virus A and B and rhinovirus. Lower respiratory infections such as those mentioned above accounted for up to 30% of visits to the local medical officer in the first year of life, later dropping to 5% by age 9.
Commonly, city living exposes children to larger class sizes, crèches, child care centres and places of interest, all of which increase the contact with other people and pathogens.
Emphysema
Emphysema (pulmonary) is a chronically progressive disease characterised by over-distension and destruction of alveolar walls, resulting in a loss of lung elasticity and surface area for diffusion. The predisposing causes are the same as those for chronic bronchitis, with cigarette smoking being the major factor. Initially the peripheral bronchioles become inflamed and the subsequent narrowing of the airways traps air in the alveoli. As the disease progresses the alveolar walls become over-inflated and rupture. Because of loss of lung elasticity the terminal bronchioles tend to collapse prematurely during exhalation, making expulsion of air from the lungs more difficult.
Symptoms of chronic emphysema include shortness of breath, dyspnoea, cyanosis and cough. Individuals will commonly exhale through pursed lips to prolong expiration and reduce the tendency of the airways to collapse. As the disease becomes more severe, they may use their accessory muscles to breathe. The anteroposterior width of the chest usually increases because of expansion of the chest wall and loss of lung elasticity, giving the chest a barrel-shaped appearance. Distension of the neck veins may be present, as may clubbing of the fingers. In advanced emphysema, the person fights for every breath of air.
Neoplastic disorders
Benign or malignant neoplasms may affect the upper or lower respiratory tract. Tumours can affect the normal functioning of the respiratory tract and, if malignant, can cause extensive tissue damage by infiltration. The signs and symptoms of neoplastic disease vary depending on the location and extent of the lesion.
Nasal polyps are masses of hypertrophied mucosa that commonly form in response to recurrent swelling of the nasal mucosa. Obstruction of the nasal passages develops gradually as the benign polyps multiply and enlarge. The client experiences difficulty in breathing through the nose, and the voice may have a nasal quality.
Laryngeal polyps are growths that arise from the mucous membrane of the vocal cords. The major symptom is hoarseness. Laryngeal polyps are usually benign but they may become malignant.
Laryngeal carcinoma is a malignant neoplasm arising from or around the vocal cords. Persistent hoarseness is the major symptom, and heavy cigarette smoking is believed to be a major causative factor. If the lesion is large, dysphagia may be present.
Lung cancer commonly affects a bronchus. It arises from the bronchial epithelium and rapidly invades lung tissue, causing parts of the lung to collapse. It may spread through the lymphatic network and bloodstream to form metastases in other parts of the body, such as the liver, bones or brain. The incidence of lung cancer is related to several factors, the most important being the inhalation of cigarette smoke. Other factors include exposure to atmospheric pollution and occupational pollutants.
Unfortunately, the physical manifestations of lung cancer do not generally appear until the disease is well advanced. Symptoms include persistent cough, dyspnoea, purulent or blood-streaked sputum, chest pain and repeated attacks of bronchitis or pneumonia. Sometimes the initial symptoms are associated with organs that are the sites of metastasis, such as the liver, bones or brain. Cancer of the lung may also occur secondary to a primary malignant tumour elsewhere in the body, as a result of metastasis.
Traumatic disorders
Injury to part of the respiratory tract can result from a variety of causes.
Laryngotracheal trauma
Laryngotracheal trauma can be minor and cause hoarseness and some dysphagia, or it may be severe, as a result of laryngeal or cricoid fracture. In a severe injury, oedema of the larynx may occur and be accompanied by signs of respiratory distress.
Flail chest
Flail chest occurs when multiple rib fractures result in ‘floating’ of a segment of the rib cage. As a consequence there may be instability in part of the chest wall and paradoxical breathing. Paradoxical breathing is characterised by the injured chest wall collapsing in during inhalation, and moving out during exhalation. The lung underlying the injury contracts on inhalation and bulges on exhalation. If uncorrected, ventilation is impaired, which may lead to hypoxia and respiratory failure. Manifestations of a flail chest are paradoxical motion of the chest wall during breathing, severe pain, dyspnoea, tachycardia and cyanosis.
Pneumothorax
Pneumothorax is a collection of air in the pleural space causing the lung on the side of the injury to collapse. A pneumothorax may be open or closed. In an open pneumothorax, an injury creates an opening in the chest wall, allowing air to flow into the pleural cavity. In a closed, or spontaneous, pneumothorax, the chest wall is intact and air enters the pleural space from an opening on the surface of the lung.
Depending on the severity, a pneumothorax may cause severe dyspnoea, hypoxaemia, tachypnoea and associated pain, along with ipsilateral diminished chest expansion and breath sounds. There may be subcutaneous emphysema in the neck and upper chest, and a ‘sucking’ sound may be heard in the region of an open pneumothorax. Tracheal deviation to the contralateral side may be observed in severe cases in some clients.
Tension pneumothorax is a particularly severe form that occurs when air escapes into the pleural cavity. As a result, continuously increasing air pressure in the pleural cavity causes progressive collapse of the lung tissue. Emergency aspiration of air from the pleural cavity is necessary.
Haemothorax
Haemothorax is the accumulation of blood in the pleural space, usually as a result of trauma. Manifestations of haemothorax include dyspnoea and chest tightness, and may include signs of hypovolaemia if bleeding continues. If not treated, haemothorax can lead to shock from haemorrhage, and severe pain, or respiratory failure.
Haemopneumothorax is a collection of air and blood in the pleural cavity. Symptoms include those of both pneumothorax and haemothorax.
FACTORS AFFECTING THE CARDIOVASCULAR SYSTEM
Changes in cardiac function
Cardiac failure occurs when the heart is unable to maintain an output of blood sufficient to meet the body’s requirements and, as a result, the body tissues may become ischaemic.
Cardiac failure may result from mechanical failure due to disease of valves, obstruction to the blood flow, congenital heart disease, arteriosclerosis or hypertension. Cardiac failure can also occur as a consequence of a disease process, such as cardiomyopathy or myocardial infarction, or as the result of normal ageing. Consequently, the heart’s ability to pump blood may be diminished.
When the heart fails to meet the requirements of the body, compensatory mechanisms occur in an attempt to improve cardiac output and to maintain the blood pressure. Compensatory responses include the sympathetic response, the renal response and myocardial hypertrophy. The sympathetic response is stimulated by reduced cardiac output and results in increased heart rate, dilation of the coronary and cerebral arterioles and constriction of the renal and skin arterioles. As a result, essential life functions are maintained. The renal response results in the secretion of substances that stimulate the production of aldosterone, causing vasoconstriction and retention of sodium and water. This response causes an increase in blood volume and peripheral resistance, which increases the workload of the heart.
Myocardial hypertrophy results from prolonged increase in myocardial wall tension and, while this initially maintains cardiac output, eventually cardiac output and tissue perfusion are decreased.
Changes in the blood vessels
Disturbances in the ability of the arteries to stretch and recoil as blood is pumped from the heart, or changes in the ability of the veins to return the blood to the heart, result in ischaemia of the tissues. The ability of arteries to stretch and recoil may be affected by conditions such as arteriosclerosis, obstruction from inflammation or arterial spasms or from excessive external pressure on an artery. When an artery is narrowed or constricted it is unable to transport sufficient blood to the area it supplies, resulting in ischaemia and possible tissue death (necrosis).
The ability of veins to return blood to the heart may be affected by impaired valves, a sedentary lifestyle, reduced skeletal muscle usage that normally assists venous flow or from excessive external pressure on a vein. When blood flow through a vein is impeded, pooling of blood in the vein occurs. The hydrostatic pressure inside the vein increases and causes oedema in the surrounding tissues. Chronic venous insufficiency can result in thrombophlebitis, stasis cellulitis and stasis ulcers.
The formation of an embolus can cause obstruction of an artery or a vein. The most common embolus is a blood clot (thrombus), although an embolus can also consist of air or fat or foreign bodies. When the thrombus, or part of it, becomes dislodged from the vessel wall it travels in the bloodstream until it reaches a blood vessel that is too narrow for its passage. As a result, blood flow beyond that area is obstructed, and the ultimate consequence may be death of the tissues deprived of adequate oxygen and nutrition.
Changes in the blood
Haemoglobin in the erythrocytes is responsible for transporting oxygen in the bloodstream. Any condition that affects the normal production or function of erythrocytes may decrease the supply of oxygen to the tissues. Conditions that may impede erythrocyte formation or their ability to carry oxygen include smoking, bone marrow aplasia, metabolic abnormalities, nutritional deficiencies, chronic or acute blood loss, drugs, living at high altitudes, flying, toxins, ionising radiation and genetic abnormalities. Tissue hypoxia results in a compensatory increased production of erythrocytes (polycythaemia), which causes the blood to become viscous and increases the risk of thrombi formation.
Leucocytes protect the body from infection through phagocytosis and the production of antibodies. Any disorder that decreases the production or maturation of leucocytes renders the client susceptible to overwhelming infection. Conditions that may impede leucocyte production or function include inadequate blood cell production, proliferation of immature leucocytes, viruses, drug reactions, radiation, nutritional deficiencies and bone marrow hypoplasia.
Thrombocytes are necessary for the clotting of blood. Any disorder that impairs thrombocyte production or function renders the client susceptible to bleeding. A decrease or increase in the formation of thrombocytes generally occurs in association with other disorders. Thrombocytopenia (decrease in thrombocyte number) is commonly due to viral infections but may be idiopathic or may result from bone marrow disease. It may also result from a condition that causes thrombocyte destruction, such as cirrhosis of the liver, or drug toxicity or continued bleeding. Thrombocythaemia (increase in thrombocyte number) is frequently idiopathic, but it may also accompany some disorders such as polycythaemia or chronic myeloid leukaemia.
Changes in the lymphatic system
The lymphatic system removes fluid and particles from the interstitial spaces, filters the lymph and returns it to the circulation. Impaired lymphatic function may result from obstruction or inflammation of the lymphatic vessels or nodes, or from neoplastic disease. When lymphatic function is impaired, fluid accumulates in the interstitial spaces, and oedema results. As the function of lymphocytes is the key factor in immune responses, diseases of the lymphatic system, such as Hodgkin’s disease, may seriously impair the immune processes. The client with immunodeficiency is vulnerable to infection and other pathological processes that would normally be inhibited by a healthy immune system.
PATHOSPHYSIOLOGY RELATED TO THE CIRCULATORY SYSTEM
The manifestations of disorders of the circulatory system vary depending on whether the disorder is one that affects the heart, the blood vessels or the blood or blood-forming organs.
Manifestations of cardiac disorders
Dyspnoea
Dyspnoea (difficult or laboured breathing) is the most common (and often the earliest) symptom of cardiac disease. Typically the dyspnoea occurs with exertion although, as cardiac disease progresses, the client may experience dyspnoea at rest. Paroxysmal nocturnal dyspnoea, which is associated with congestive cardiac failure, occurs during sleep; the client wakes suddenly with difficulty in breathing and a sensation of suffocation.
Chest pain
Chest pain (Table 23.2) may result from myocardial ischaemia or from pericarditis. Chest pain can also be caused by conditions not associated with cardiac disease, such as oesophagitis, reflux, pleurisy, musculoskeletal disorders or stress and anxiety. Ischaemic pain is the result of a deficiency of blood to the myocardium, caused by a blocked or constricted coronary blood vessel. Angina (pectoris) is pain that results from diminished supply of oxygen to the heart, and is basically a reversible ischaemic process. Acute myocardial infarction represents the point when ischaemia becomes irreversible; blood flow to part of the heart is inadequate and unrelieved by rest, causing cardiac muscle necrosis.
Table 23.2 Typical patterns of cardiac pain
| Angina | Myocardial infarction |
|---|---|
| Gradual or sudden onset | Sudden onset |
| Episodic and temporary, usually lasting 3–15 minutes | Lasts longer than 15 minutes |
| Substernal or anterior, not sharply localised. Radiates to back, neck, arms and jaw | Substernal, midline or anterior. Radiates to jaw, neck, back, shoulders or one or both arms |
| Sensation of mild to moderate pressure. Described as lightness, squeezing or crushing | Persistent sensation of severe pressure. Described as crushing, heavy, vice-like, squeezing |
| Precipitated by exertion, stress, ingestion of food, exposure to cold | Not necessarily related to exertion or emotion, and may occur at rest |
| Accompanied by dyspnoea, diaphoresis, nausea, apprehension | Accompanied by nausea, vomiting, dyspnoea, apprehension, diaphoresis, a sensation of ‘impending doom’, pallor, cold clammy skin |
| Client keeps still to relieve the pain | Client moves about in search of a comfortable position |
| Relieved by rest and/or nitroglycerine | Not relieved by rest or glyceryl trinitrate |
Signs and symptoms that are textbook characteristics of angina or myocardial infarction, and any chest pain, should be suspected to be a myocardial infarction until proven otherwise. However, many myocardial infarctions may not follow the classic pattern of symptoms and as such may not be diagnosed and treated.
Palpitations
Palpitations are a sensation of fluttering in the chest or an awareness of the heart’s action. The person may describe the heart’s action as racing, pounding, stopping or skipping beats. Palpitations may be due to rhythm disturbances such as premature contractions (extra heartbeat), but they can also result from anxiety, stress, caffeine or nicotine, cough and cold medications and fatigue.
Cough
Cough may be associated with certain cardiovascular and most respiratory diseases. If cardiovascular disease is suspected it may be caused by an accumulation of fluid in the lungs (pulmonary oedema) often made worse at night or when lying in bed.
Fatigue
Fatigue frequently accompanies cardiac dysfunction and is related to inadequate cardiac output resulting in insufficient blood flow to the brain and skeletal tissues.
Cyanosis
Cyanosis (blue discolouration of the skin or mucous membranes) appears when haemoglobin oxygen saturation is greatly reduced below 92%. Central cyanosis is evident in all areas of the body, particularly in the lips, mucous membranes and nail beds, whereas peripheral cyanosis is evident mainly in the extremities.
Syncope
Syncope (transient loss of consciousness) may result when cardiac dysfunction causes an inadequate flow of blood to the brain. Sudden loss of consciousness due to heart block is known as Stokes–Adams syndrome. Heart block is defined as ‘impairment of conduction in heart excitation; often applied specifically to atrioventricular heart block’ (Hawley et al 2012).
Oedema
Oedema (local or generalised accumulation of fluid in the tissues) may result from certain cardiac diseases. Peripheral or systemic oedema generally develops from right-sided cardiac failure and is first noticed in the lowest, or dependent, parts of the body, such as the legs, fingers, sacral area, periorbital area, in the abdomen (ascites) and intestines (causing constipation). As venous stasis increases, oedema increases. In left-sided cardiac failure fluid accumulates in the lungs (pulmonary oedema). In advanced cardiac failure total body oedema may develop. The severity of the oedema will depend on the degree to which venous return and/or cardiac output are reduced.
Pulse abnormalities
Pulse rate, volume or rhythm may be abnormal in the presence of cardiac dysfunction. Tachycardia may accompany cardiac failure, while heart block commonly results in bradycardia. A low cardiac output, and therefore a reduced pulse volume, is associated with cardiac failure and acute myocardial infarction. Certain cardiac disorders cause dysrhythmias accompanied by an irregular pulse.
Manifestations of peripheral blood vessel disorders
Intermittent claudication
Intermittent claudication is cramping pain in a muscle of the leg, brought on by exercise but relieved by rest. Commonly the pain is experienced in the calf muscle during walking and is thought to be due to accumulation of lactic acid in the tissues, rather than to ischaemia of the contracting muscle. Generally, it results from blockage of the superficial femoral artery; for example, due to atherosclerosis.
Rest pain
Rest pain is leg pain experienced even when resting and occurs when chronic arterial occlusive disease is advanced, or when a vessel is blocked by a thrombus or embolus. As a result, the blood supply to the surrounding tissues is diminished, causing ischaemic pain.
Pale cold extremities
Pale cold extremities indicate impaired blood flow to the limb. If an artery is suddenly occluded by a thrombus or embolus, there will be numbness and absence of distal pulses as well as coldness and pallor.
Altered peripheral pulses
Altered peripheral pulses may be present when arterial blood flow is impeded. Diminished or absent pulses suggest partial or total occlusion of an artery; for example, if the popliteal pulse is absent, the superficial femoral artery may be occluded.
Gangrene
Gangrene, which causes death of tissue, may occur as a result of chronic arterial insufficiency and is the consequence of severe and prolonged ischaemia. Gangrene first develops in the most distal parts of the lower limbs.
Leg ulcers
Leg ulcers or cellulitis may be present. Chronic occlusion of arterioles and small arteries results in ischaemia, skin breakdown and ulceration. Pooling of venous blood in the tissues of the extremities, for example, as a result of varicose veins, may lead to venous stasis ulcers. Aching or a feeling of fullness or heaviness in the legs is associated with venous insufficiency or valve incompetence. Homans’ sign may be positive in the presence of thrombophlebitis. A positive Homans’ sign is present when dorsiflexion of the foot (with the knee bent) produces pain in the calf. Tenderness, firmness and swelling in the calf are also suggestive of deep-vein thrombophlebitis.
Manifestations of blood and blood-forming organ disorders
Bruising and bleeding
Bruising and bleeding may occur when there is abnormal thrombocyte production or function or reduced levels of clotting factors in the plasma. The appearance of any bruising or haemorrhagic spots in the absence of injury is suggestive of a blood disorder. Types of bruising and bleeding include:
• Purpura: haemorrhagic areas under the skin and in the mucous membranes. If the haemorrhages are small they are termed petechiae; larger purpuric areas are called bruises or ecchymoses
• Petechiae: red-brown pinpoint haemorrhages in the skin. Petechiae can occur over any part of the skin but are most common where pressure has been applied to a body part; seen in menigococcal meningitis and anthrax as well as common viral infections
• Ecchymoses: haemorrhagic spots larger than petechiae. They may be precipitated by an injury or may occur spontaneously
• Gastrointestinal bleeding: which appears as haematemesis and/or melaena and may occur in certain disorders of the blood such as thrombocytopenia, but may also occur as a result of liver failure, oesophageal varices, gastrointestinal ulcerations and drug therapies
• Menorrhagia: which may occur in haemorrhagic disorders as well as gynaecological disorders
• Haematuria: which may occur in haemorrhagic disorders as well as renal disorders
• Neurological changes (e.g. headaches, blurred vision, disorientation or altered consciousness): which may occur if there is bleeding within the central nervous system.
Changes in the skin may accompany disorders of the blood or blood-forming organs. Changes that may occur include pallor, rudor, jaundice, pruritus, thickened nails and ulcerations.
SPECIFIC DISORDERS OF THE CIRCULATORY SYSTEM
Disorders of the circulatory system may be congenital or due to multiple causes, pathogens or chemicals, or they may be drug related, neoplastic, obstructive, degenerative or the result of trauma. It is beyond the scope of this text to provide in-depth information on the various conditions related to the circulatory system. Listed below are some examples of circulatory system conditions.
Congenital disorders
Congenital disorders are conditions that exist and are which are ‘present at and existing from the time of birth’ (Hawley et al 2003). These disorders include: ventricular septal defect; atrial septal defect; coarctation of the aorta; patent ductus arteriosus; tetralogy of Fallot; transposition of the great vessels; thalassaemias (sickle-cell anaemia); and bleeding disorders (haemophilia A, haemophilia B, Von Willebrand’s disease).
Disorders of multiple cause
It is beyond the scope of this text to provide in-depth information on the various conditions related to the disorders of multiple causes; only a few of the most common conditions are briefly discussed here.
Hypertension
High arterial blood pressure over 135/85 mmHg is generally classified as either primary (‘essential’) or secondary hypertension. Primary hypertension is the most common form and, while the cause is often unknown, many factors have been implicated as contributing to its development, including high sodium intake, obesity, diabetes, hypercholesterolaemia, genetic factors, alcohol, cigarette smoking and psychosocial factors.
Secondary hypertension is caused by either disease or certain medications. Diseases that result in secondary hypertension include those in which renal, vascular, endocrine or neurological mechanisms are involved, such as renal artery stenosis or intercranial lesions. Medications that may lead to secondary hypertension include oral contraceptives, corticosteroids and monoamine oxidase inhibitors.
Malignant hypertension is the term used to describe primary hypertension when there is a rapid rise of blood pressure to a very high level, such as 250/150 mmHg. It is accompanied by severe headache, visual disturbances and oliguria. If untreated, death may occur rapidly from cardiac or renal failure or cerebrovascular accident.
Hypertension is frequently asymptomatic until the client experiences a major problem such as cerebral haemorrhage, renal failure or myocardial infarction. Symptoms that may be due to hypertension include dizziness, chest pain, palpitations, epistaxis, headaches and brief episodes of memory loss (transient ischaemic attacks).
Coronary artery (ischaemic heart) disease
This is a disorder in which the arteries that supply blood to the heart muscle become diseased and fail to supply the heart with sufficient blood. Arteriosclerosis is the most common cause of coronary artery disease, causing disturbances of blood flow within the coronary arteries that give rise to altered myocardial perfusion and disruption of the electrical cycle controlling heart rhythm. Atherosclerosis is one type of arteriosclerosis in which narrowing of the arteries occurs as a result of deposits of lipids in and around the smooth muscle, roughening of the endothelial lining and loss of elasticity, with fibrosis and calcification. Eventually the artery becomes occluded, inelastic and incapable of dilating.
Although the precise cause of arteriosclerosis and coronary artery disease is unclear, there is general agreement that many factors contribute to its development, including genetic influences, gender (males are more commonly affected), hypertension, lack of exercise, cigarette smoking, stress, metabolic or endocrine disorders, obesity, diabetes mellitus, hypercholesterolaemia and dietary factors. The dietary factors that are considered to contribute to coronary artery disease are salt, saturated fats and lack of dietary fibre. Coronary artery disease may be asymptomatic until the client experiences angina or a myocardial infarction, which may result in sudden death.
Myocardial infarction
Myocardial infarction is the death of part of the myocardium as a result of severe or total deprivation of its blood supply. Blood flow to the myocardium may be obstructed by arteriosclerosis or by thrombus formation within an atheromatous coronary artery. The most common site of infarction is the anterior surface of the left ventricle, resulting from occlusion of the left coronary artery. An infarction may affect some or all of the layers of the heart.
The major complications of a myocardial infarction are left ventricular failure, pericarditis and arrhythmias, which together account for a large percentage of deaths following myocardial infarction. It is generally recognised that the risk of death is greatest in the first few hours after myocardial infarction, with the risk decreasing after that time. Depression is common after recovery from the infarction and can result in non-compliance with treatment regimens and further infarctions.
A myocardial infarction may be asymptomatic (a silent myocardial infarction). More commonly, it may manifest with pain in the centre of the chest, arms, neck, jaw or back lasting longer than five minutes, pallor, sweating, anxiety, shortness of breath, nausea or vomiting or sudden collapse.
Other disorders of multiple cause include: dysrhythmias, valvular heart disease, heart failure, cardiogenic shock, cardiac arrest, Raynaud’s disease, disseminated intravascular coagulation, idiopathic thrombocytopenic purpura, agranulocytosis and Buerger’s disease.
Anaemias
Anaemias are a group of disorders characterised by reduced oxygen-carrying capacity of the blood. Causes of anaemia are numerous and are related to the altered production or destruction of erythrocytes, and to blood loss. Anaemias can thus be classified as due to haemopoietic, haemolytic or haemorrhagic causes.
Aplastic anaemia is caused by injury or destruction of the haematopoietic cells in bone marrow, resulting in reduced or abnormal erythrocyte production. In this disorder the normal haematopoietic tissue is replaced by fatty bone marrow. Aplastic anaemia may be idiopathic or it may be caused by medications, toxic agents, radiation or immunological factors. Manifestations of aplastic anaemia are related to pancytopenia (abnormal depression of the cellular components of blood). They include pallor, tiredness, repeated infections and bleeding tendencies. Bleeding may present as petechiae, ecchymoses, haemorrhage from the mucous membranes, such as the gums, or gastrointestinal haemorrhage.
Pernicious anaemia is characterised by a metabolic defect involving the absence of intrinsic factor, which is secreted by the parietal cells of the gastric mucosa and is essential for vitamin B absorption in the terminal ileum. Pernicious anaemia is thought to result from an autosomal dominant defect. Other causes include gastric cancer, gastrectomy and malabsorption disorders involving the ileum. Manifestations of pernicious anaemia include pallor, tiredness, sore tongue and numbness and tingling in the extremities. Because of vitamin B deficiency, demyelination of nerves and degeneration of nerve tissue occurs, producing neurological effects such as ataxia, altered vision, poor memory, depression and paralysis.
Iron-deficiency anaemia is characterised by small and pale erythrocytes because of reduced haemoglobin concentration. The two most common causes of iron-deficiency anaemia are chronic blood loss and an inadequate dietary intake of iron. Manifestations include pacophagia (craving for ice), pallor, chronic tiredness, tachycardia and shortness of breath on exertion.
Folate deficiency anaemia results from an inadequate dietary intake of folate, a disorder of the small intestine, where folate is absorbed, or from altered metabolism. Manifestations are similar to those associated with pernicious anaemia.
Acute blood-loss anaemia is a condition that results from sudden loss of erythrocytes and, consequently, depletion of haemoglobin and iron. Acute blood-loss anaemia may result from severe trauma, postoperative haemorrhage, invasive neoplasm, ruptured peptic ulcer, ruptured aneurysm or coagulation defects. Acute blood loss itself produces features associated with hypovolaemia and hypoxia, such as pallor, faintness, restlessness, anxiety, hypotension and a weak rapid pulse.
If anaemia is persistent and the client’s haemoglobin levels decrease, the client may require a transfusion of blood.
Neoplastic and obstructive disorders
Tumours may occur in any of the chambers of the heart and may affect one or all of the layers of the heart. Secondary metastatic tumours that infiltrate the heart are more common than primary cardiac tumours. Manifestations are related to which part of the heart is affected, and include signs of heart failure, dysrhythmias, angina, heart block and infarction. It is beyond the scope of this text to provide in-depth information on the various conditions related to neoplastic and obstructive disorders and only a few of the most common conditions are briefly discussed here.
Leukaemia
Leukaemia is a neoplastic disorder characterised by an accumulation and proliferation of abnormal cells in the bone marrow. Cells fail to develop and are unable to function normally, and the accumulation of leukaemic cells in the bone marrow prevents normal haematopoiesis. The precise cause of leukaemia is unknown but several factors have been implicated in its development including chromosome abnormality, exposure to radiation from power lines, viruses or chemicals, such as certain weed killers or insecticides. Leukaemia occurs either in acute forms, which involve the proliferation of immature cells, or in chronic forms which involve the proliferation of mature cells. The four most common forms of leukaemia are acute myeloid, chronic myeloid, acute lymphocytic and chronic lymphocytic.
Although manifestations of leukaemia vary according to the particular form of the disorder, there is a similarity in the signs and symptoms, which are related to the lack of normal haematopoiesis in the bone marrow. Bone marrow dysfunction results in:
• Anaemia, which may present as pallor, lethargy and shortness of breath
• Thrombocytopenia, which commonly manifests as petechiae, easy bruising, bleeding gums and haemorrhage (e.g. as occult haematuria)
• Leucopenia, which renders the client susceptible to recurrent infections. There is generally splenic enlargement, lymphadenopathy and bone pain.
Central nervous system involvement may be present in any of the leukaemias, giving rise to symptoms such as nausea and vomiting, irritability, headache and blurred vision.
Hodgkin’s disease
Hodgkin’s disease is a malignant disorder of the lymph node macrophages, characterised by painless and progressive enlargement of the lymph nodes, spleen and other lymphoid tissue. Untreated, Hodgkin’s disease metastasises via the lymphatics to sites outside the lymphatic system. The precise cause of the disorder is unknown, but both genetic and environmental factors seem to be implicated in its development. Manifestations of Hodgkin’s disease are painless enlargement of the lymph nodes, especially the cervical nodes, pruritus, night sweats, malaise and weight loss. Other symptoms depend on the degree and location of systemic involvement.
Other conditions related to neoplastic and obstructive disorders include: arteriosclerosis obliterans, acute arterial obstruction, thrombophlebitis, chronic venous insufficiency, Burkitt’s lymphoma, multiple myeloma and malignant lymphomas.
Degenerative disorders
Cardiomyopathy
Cardiomyopathy results from extensive damage to the myocardial muscle fibres, causing hypertrophy of the entire heart, especially of the septum. Although the heart is enlarged, the ventricular chambers are small and are resistant to filling during diastole. Cardiomyopathy leads to congestive cardiac failure, arrhythmias and, frequently, sudden death.
The cause of most cardiomyopathies is unknown but the condition is thought to be genetically transmitted. Some forms of cardiomyopathy result from hypertension, congenital defects and myocardial destruction by toxic, infectious or metabolic agents. The most common manifestation of cardiomyopathy is dyspnoea, as a result of congestive cardiac failure. Angina, fatigue and syncope may occur because of inadequate cardiac output. As cardiac failure progresses, peripheral cyanosis, oedema, liver enlargement and jugular venous distension become evident.
Aortic aneurysm
Aortic aneurysm is a dilation of the wall of the aorta. There are several types of aneurysm:
• Saccular: an outpouching of one side of the arterial wall
• Fusiform: a spindle-shaped enlargement of the entire circumference of the artery
• Dissecting: a haemorrhagic separation between the medial and internal layers of the artery.
The most common cause of an aneurysm is arteriosclerosis, which weakens the aortic wall and gradually distends the lumen at the weakened area. Other causative factors include congenital defects, infection, hypertension and trauma.
Manifestations of an aortic aneurysm depend on its location, and may not develop until enlargement of the aneurysm exerts pressure on nearby structures. Depending on the location, an aortic aneurysm may manifest as dyspnoea; chest pain; dysphagia; dilated superficial veins on the chest, neck and arms; prominent abdominal pulsation; or dull abdominal or low back pain. A dissecting aneurysm may produce a sudden ‘tearing’ pain accompanied by pallor, shortness of breath, sweating and syncope. The main complication of an aortic aneurysm is rupture and, without immediate surgical intervention, the client may bleed to death.
Varicose veins
Varicose veins are dilated, tortuous branches of the saphenous veins. They result from incompetent valves, which cause a back-flow of venous blood. Varicose veins may result from congenital weakness of the valves, from injury or thrombophlebitis, or from conditions that produce venous stasis, such as pregnancy or occupations that necessitate standing for long periods. Superficial varicose veins may be unsightly but produce no symptoms. Deeper varicose veins may produce mild to severe leg symptoms, such as a feeling of heaviness, cramps, dull aching and discomfort that increases with prolonged standing. Over time, dilation of the veins results in venous stasis, with oedema and changes in skin pigmentation. Visible and palpable protrusions frequently occur along the veins, resulting in disfigurement of the leg(s).
Infectious and inflammatory disorders
Pericarditis
Pericarditis (inflammation of the pericardium) may be an acute or chronic condition. Acute pericarditis may be accompanied by a purulent, serous or haemorrhagic exudate, which can produce further complications. Chronic pericarditis is characterised by fibrous pericardial thickening. As well as being caused by infection, pericarditis may result from trauma, radiation, neoplasms, cardiac surgery or myocardial infarction. The prime manifestation is chest pain that increases with deep inhalation, and decreases when the person sits up and leans forward. Other manifestations include dyspnoea and the signs of a systemic infection.
Myocarditis
Myocarditis (inflammation of the myocardium) may be an acute or chronic condition. Myocarditis may result from viral or bacterial infections, radiation, chemicals or metabolic disorders. Infective myocarditis usually causes non-specific symptoms that reflect a systemic infection. Myocarditis sometimes produces manifestations of severe congestive cardiac failure.
Endocarditis
Endocarditis (inflammation or infection of the endocardium) may result from invasion by microorganisms or from non-infective injury to the lining of the heart, or via intravenous (IV) cannulas, dental surgery or any other invasive procedure. Infective endocarditis involves the endocardium of the heart valves more frequently than the endocardium lining the heart chambers. The microorganisms stimulate the deposit of fibrin around them, producing vegetative growth on the endocardium.
Early manifestations are commonly non-specific, and the symptoms of acute endocarditis resemble those associated with influenza: pyrexia, sweats, anorexia, headaches and musculoskeletal aches. If a heart murmur develops, the pulse rate may be rapid and, if vegetations become dislodged, there may be manifestations of embolisation, producing the features of splenic, renal, cerebral, pulmonary or peripheral vascular occlusion.
Rheumatic heart disease
Rheumatic heart disease refers to the cardiac manifestations of rheumatic fever and includes pericarditis, myocarditis, endocarditis and chronic valvular disease. Rheumatic fever is associated with the type A beta-haemolytic streptococcus and is thought to be immunological in origin. It may be as long as 10 years after an attack of rheumatic fever before signs of heart valve disease become evident. The end result of the disease progression is stenosis of a heart valve, inability of the valve to close properly, or valve incompetence, which leads to regurgitation of blood through the valve during systole. Manifestations of rheumatic heart valve disease depend on the valve affected and on the degree of valve dysfunction. There may be signs of reduced cardiac output, pulmonary congestion, cardiac enlargement, heart failure and the presence of heart murmurs.
Lymphangitis
Lymphangitis is an acute or chronic inflammation of the lymphatic vessels, which generally results from a streptococcal infection of an extremity. The accompanying lymph node enlargement (lymphadenopathy) may be localised or generalised. Lymphangitis is characterised by red, warm, tender streaks spreading up a limb from a focal point of infection. The regional lymph nodes become enlarged and tender, and the client experiences pyrexia and malaise.
DIAGNOSTIC TESTS
Respiratory diagnostic tests
Certain tests may be used to assist or confirm the diagnosis and severity of respiratory disorders.
Pulmonary function studies
These studies are used to determine the presence and degree of respiratory dysfunction and are a measure of the functional ability of the lungs. Spirometry or plethysmography is used to assess the client’s lung volume by measuring and recording the volume of inhaled and exhaled air. The values are then compared with the normal values against predicted values for a client of the same sex, weight, height and age. Table 23.3 lists types of pulmonary function tests and the normal expected values.
Table 23.3 Pulmonary function tests
| Tests | Explanation | Normal value |
|---|---|---|
| Tidal volume | Amount of air inhaled or exhaled during normal breathing | 500 mL |
| Total lung capacity | Total volume of the lungs when maximally inflated | 5800 mL |
| Vital capacity | Total volume of air that can be forcibly exhaled after a maximum inhalation | 3000–6000 mL |
| Functional residual capacity | Amount of air remaining in the lungs after normal exhalation | 2300 mL |
| Inspiratory capacity | Amount of air that can be inhaled after normal exhalation | 3500 mL |
| Expiratory reserve volume | Amount of air that can be exhaled after normal exhalation | 1200 mL |
| Forced expiratory volume (in one second [FEVl]) | Maximal amount of air that can be forcibly exhaled, in one second, after full inhalation | 3000–5000 mL |
| Residual volume | Amount of air remaining in the lungs after a maximal forced exhalation | 1200 mL |
Polysomnography
This test is used to measure upper obstruction and pattern of respirations during sleep, using various pieces of equipment.
Chest x-ray
A chest x-ray is one of the most common procedures used to evaluate the lungs, and generally involves posterior, anterior and lateral views. Abnormal findings that may be evident on a chest x-ray include areas of density, presence of a mass or accumulation of fluid.
Ventilation/perfusion scan
A ventilation/perfusion scan involves the administration of a radioactive gas. The radioactive particles are distributed and trapped in the pulmonary capillary bed, and the lung scan produces a visual image of pulmonary blood flow. Tissue that does not pick up the radionuclide will show light coloured area, indicating lack of adequate lung perfusion. Conditions such as pulmonary oedema, lung cancer or COPD may cause abnormal perfusion.
Cultures
A specimen of secretions is obtained, for example, via a nose swab or nasopharyngeal aspirate or throat swab. Care must be taken to ensure that only the back of the throat is swabbed, which represents microorganisms in the respiratory tract. Samples are sent to the laboratory so that any microorganisms present can be identified by use of a special growth medium (the culture). Sensitivity studies may then be done to determine which drug is effective against the specific microorganism.
Sputum cytology
A specimen of sputum is obtained and sent to the laboratory, where it is examined to detect the presence of pus, pathogenic microorganisms or malignant cells.
Skin tests
A common skin test performed is the Mantoux test, used in the detection of tuberculosis (TB). A medical officer or registered nurse (RN) qualified in intradermal administration injects intradermally a 0.1 mL of solution containing old tuberculin. A positive reaction may be defined as an area of redness or induration of at least 5 mm in diameter appearing within 48–72 hours. The greater the reaction size the greater the exposure to antibodies to the tubercle bacillus.
Bronchoscopy
Bronchoscopy involves the direct viewing of the trachea and bronchi by means of an instrument called a bronchoscope. A bronchoscopy is used in the diagnosis of respiratory tract disorders and may be used to remove foreign bodies or flush out secretions in the airways and to obtain a specimen of secretions or tissue for microscopic examination.
Thoracentesis
In thoracentesis the thoracic wall is punctured with a needle to obtain a specimen of pleural fluid for analysis. The procedure may also be performed to relieve pulmonary compression caused by a pleural effusion. A local anaesthetic is injected into the skin before the needle is inserted.
Arterial blood gas analysis
Blood gas analysis shows how well a client’s lungs are delivering oxygen to the bloodstream and eliminating carbon dioxide. Blood is collected for analysis of pH, PaCO2, PaO2, bicarbonate and base levels.