Adderley U, Smith R. Topical agents and dressings for fungating wounds, Cochrane Database Syst Rev. 2007;(2). doi: 10.1002/14651858. CD003948. CD003948.pub2.
Alexander S. An intense and unforgettable experience: the lived experience of malignant wounds from the perspectives of patients, caregivers and nurses. Int Wound J. 2010;7:456–465.
Araujo T. Managing the patient with venous ulcers. Ann Int Med. 2003;138:326.
Australian Bureau of Statistics (ABS). 3222.0 Population projections, Australia, Table B9. Canberra: ABS, 2008.
Australian Centre for Diabetes Strategies (Prince of Wales Hospital, Sydney) for the Diabetes Australia Guideline Development Consortium. Detection and prevention of foot problems in type 2 diabetes, part 6. Canberra: National Health and Medical Research Council, 2005. Online Available at www.nhmrc.gov.au/_files_nhmrc/publications/attachments/di12.pdf?q=publications/synopses/_files/di12.pdf 8 Jun 2012.
Australian Institute of Health and Welfare (AIHW). Australia’s health 2008, Cat. no. AUS 99. Canberra: AIHW, 2008.
Australian Wound Management Association (AWMA). Clinical practice guidelines for the prediction and prevention of pressure ulcers. Perth: Cambridge Publishing, 2001.
Australian Wound Management Association (AWMA). Bacterial impact on wound healing: from contamination to infection. Perth: AWMA, 2011. Online Available at www.awma.com.au/publications/2011_bacterial_impact_position_1.5.pdf 20 Nov 2011.
Australian Wound Management Association. Pan Pacific clinical practice guideline for the prevention and management of pressure injury. Osborne Park, WA: Cambridge Media, 2012;12. Online. Available at www.awma.com.au/publications/2012_AWMA_Pan_Pacific_Guidelines.pdf 4 Jul 2012.
Ayello E, Cuddigan J. Debridement: controlling the necrotic/cellular burden. Wound Repair Regen. 2004;17:66–78.
Banwell P, Teot L. Topical negative pressure: the evolution of a novel wound therapy. J Wound Care. 2003;12:22–28.
Beldon P. What you need to know about skin grafts and donor site wounds. Wound Essentials. 2007;2:149–155.
Bergqvist D, Lindholm C, Nelzen O. Chronic leg ulcers: the impact of venous disease. J Vasc Surg. 1999;29:752–755.
Carville K, Smith J. A report on the effectiveness of comprehensive wound assessment and documentation in the community. Primary Intention. 2004;12:41–44. 46–8.
Carville K. Wound care manual, ed 5. Osborne Park, WA: Silver Chain Foundation, 2007.
Celik S. Surgical wound infections in the intensive care unit: the nurse’s role. J Wound Ostomy Cont Nurs. 2007;34:499–504.
European Wound Management Association (EWMA). Position document: hard-to-heal wounds—a holistic approach. London: MEP Ltd, 2008.
Fernandez R, Griffiths R. Water for wound cleansing. Cochrane Database Syst Rev. 15(2), 2012. CD003861
Flanagan M. Wound management. New York: Churchill Livingstone, 1997.
Franz M, Robson M, Steed D, et al. Guidelines to aid healing of acute wounds by decreasing impediments of healing. Wound Repair Regen. 2008;16:723–748.
Gauglitz G, Korting H, Pavicic T, et al. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Molec Med. 2011;17:113–125. Online. Available at http://molmed.org/pdfstore/09_153_Gauglitz.pdf 20 Apr 2011.
Gethin G, Cowman S, Kolbach DN. Debridement for venous leg ulcers. Cochrane Database Syst Rev. (7):2010. CD008599. DOI: 10.1002/14651858.CD008599.
Goldman R. Hyperbaric oxygen therapy for wound healing and limb salvage: a systematic review. Phys Med Rehab. 2009;1(5):471–489.
Grey J, Enoch S, Harding K. Wound assessment. Br Med J. 2006;332:285–288.
Griggs K. Evidence summary: chronic wound management. JBI COnNECT. http://www.jbiconnect.org/connect/docs/jbi/cis/connect_gu_view_summary.php?SID=6977, 2008.
Harding K. Chronic wounds and their management and prevention is a significant public health issue. Wounds Int. 2010;7:125–126.
Harvey C. Wound healing. Orthopaed Nurs. 2005;24:143–157.
Hess C. The art of skin and wound care documentation. Adv Skin Wound Care. 2005;18:43–53.
Hopf H, Ueno C, Aslam R. Guidelines for the treatment of arterial insufficiency ulcers. Wound Repair Regen. 2006;14:693–710.
Joanna Briggs Institute (JBI). Solutions, techniques and pressure in wound cleansing. Best practice 10. Adelaide: JBI, 2006. Online Available at http://connect.jbiconnectplus.org/ViewSourceFile.aspx?0=4341 8 Jun 2012.
Joanna Briggs Institute (JBI). Burn wound management: primary care facility; evidence summary. Adelaide: JBI, 2010.
Joanna Briggs Institute (JBI). Cavity wounds: evidence summary. Adelaide: JBI, 2011.
Kaur S, Pawar M, Banerjee N, et al. Evaluation of the efficacy of hyperbaric oxygen therapy in the management of chronic nonhealing ulcer and role of periwound transcutaneous oximetry as a predictor of wound healing response: a randomised prospective controlled trial. J Anaesth Clin Pharm. 2012;28(1):70–75.
Kirshen C, Woo K, Ayello E, et al. Debridement: a vital component of wound bed preparation. Adv Skin Wound Care. 2006;19:506–517.
Kranke P, Bennett MH, Debus SE, et al. Hyperbaric oxygen therapy for chronic wounds, Cochrane Database Syst Rev. 2004;(1). doi: 10.1002/14651858.CD004123.pub2. CD004123.
Krishnamoorthy L, Morris H, Harding K. A dynamic regulator: the role of growth factors in tissue repair. J Wound Care. 2001;10:99–101.
Lawton S. Addressing the skin-care needs of the older person. Br J Commun Nurs. 2007;12:203–210.
Lewellyn M, Jones J, Hopkins A, et al. Challenging wounds. In: Harding K, Harker J, eds. Essential wound management for day-to-day practice. London: Medical Education Partnership, 2002.
Lindholm C, Bjellerup M, Christensen O. A demographic survey of leg and foot ulcer patients in a defined population. Acta Dermatol Venereol. 1992;72:227–230.
Lo S, Hu W, Hayter M, et al. Experiences of living with a malignant fungating wound: a qualitative study. J Clin Nurs. 2008;17(20):2699–2708.
Lo S, Hayter M, Hu W, et al. Symptom burden and quality of life in patients with malignant fungating wounds. J Adv Nurs. 2012;68(6):1312–1321.
McCulloch J, Kloth L, Feedar J. Wound healing: alternatives in management, ed 2. Philadelphia: FA Davis, 1995.
Moffatt C, Morrison M, Pina E. Wound bed preparation for venous leg ulcers. In: European Wound Management Association (EWMA) position document: wound bed preparation in practice. London: Medical Education Partnership; 2008:12–15.
Mulligan S, Scott L, Prentice J, et al. WoundsWest wound prevalence survey 2009: state-wide report overview. Perth: Ambulatory Care Services, Department of Health, 2009. Online Available at www.health.wa.gov.au/woundswest/docs/WWWPS_09_state_overview.pdf 8 Jun 2012.
Naylor W. Part 1: symptom control in the management of fungating wounds. World Wide Wounds. Online. Available at www.worldwidewounds.com/2002/march/Naylor/Symptom-Control-Fungating-Wounds.html, 2002. 20 Apr 2011.
Queensland Health. Pressure ulcer prevention and management resource guidelines 2009. Brisbane: Queensland Health Patient Safety Centre, 2009.
Ratliff C. Under pressure: get current on best practices for wound care. Nurs Crit Care. 2006;1:37–43.
Regan P. The impact of cancer and its treatment on wound healing. Wounds UK. 2007;3:87–95.
Royal College of Nursing (RCN). Clinical practice guidelines: the nursing management of patients with venous leg ulcers: recommendations. London: Royal College of Nursing, 1998.
Schultz G, Sibbald G, Falanga V, et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen. 2003;11:1–28.
Shores J. Skin substitutes and alternatives: a review. Adv Skin Wound Care. 2007;20:493–508.
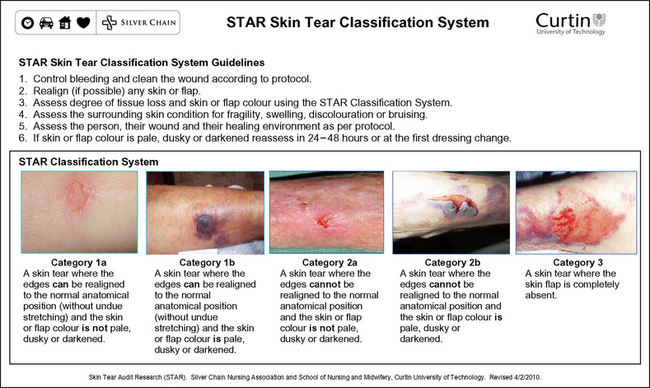
Silver Chain Nursing Association, Curtin University of Technology (School of Nursing and Midwifery). (revised 2010) STAR (Skin Tear Audit Research) skin tear classification system. Online. Available at www.silverchain.org.au/assets/files/STAR-Skin-Tear-tool-04022010.pdf, 2007. 20 Apr 2011.
Terrill P. Management of skin grafts and donor sites. Woundcare Network. 2003;11:1–5.
Traversa B, Sussman G. The role of growth factors, cytokines and proteases in wound management. Primary Intention. 2001;9:161–167.
Whitney A, Phillips L, Aslam R. Guidelines for the treatment of pressure ulcers. Wound Repair Regen. 2006;14:663–679.
Williams D, Enoch S, Miller D, et al. Effect of sharp debridement using curette on recalcitrant nonhealing venous leg ulcers: a concurrently controlled, prospective cohort study. Wound Repair Regen. 2005;13:131–137.
World Union of Wound Healing Societies (WUWHS). Principles of best practice: minimising pain at wound-dressing-related procedures. A consensus document. London: Medical Education Partnerships, 2004.
World Union of Wound Healing Societies (WUWHS). Principles of best practice: wound exudate and the role of dressings. A consensus document. London: Medical Education Partnerships, 2007.
Xue Y. Evidence summary: wound dressings. JBI COnNECT. Available at: http://www.jbiconnect.org/connect/docs/jbi/cis/connect-gen-user-view.php?IID=723&qu=1&p=1&e=1&r=1&o=1, 2008.
Young T, Fowler A. Nursing management of skin grafts and donor sites. Br J Nurs. 1998;7:324–334.
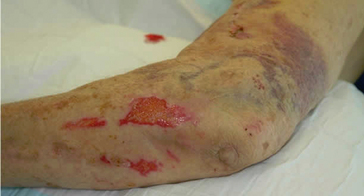
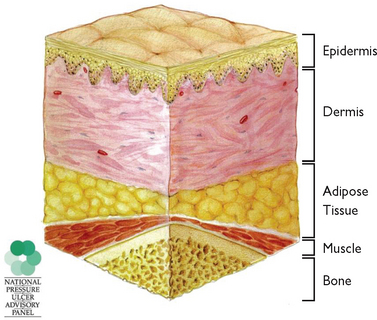
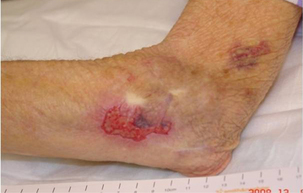 Skin tear
Skin tear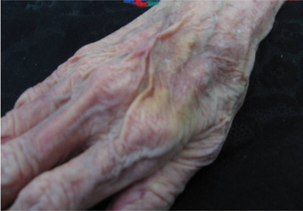 Decreased tissue turgor
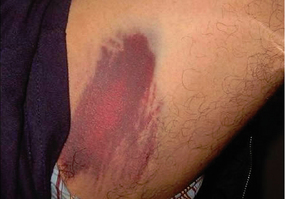
Decreased tissue turgor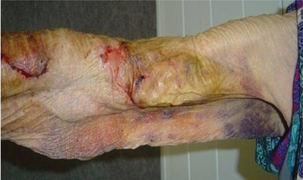 Loss of subcutaneous tissue
Loss of subcutaneous tissue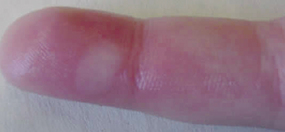
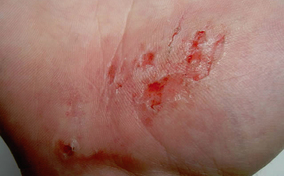
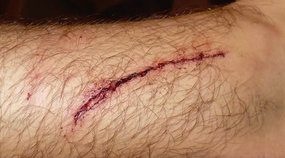
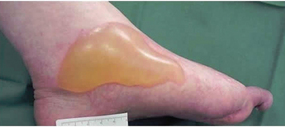


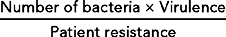
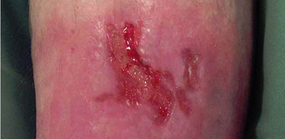
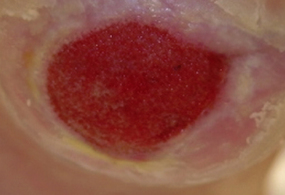
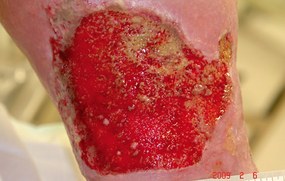 Bumpy granulation tissue
Bumpy granulation tissue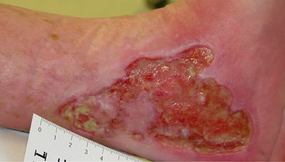 Contraction of wound edges and islands of new epithelial tissue
Contraction of wound edges and islands of new epithelial tissue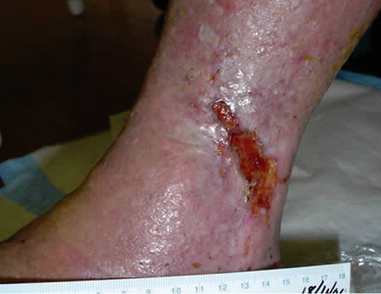
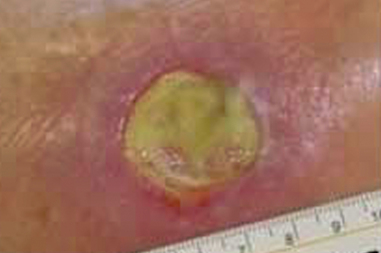
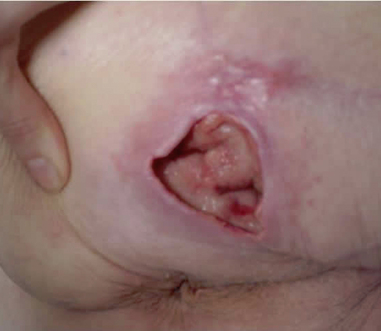
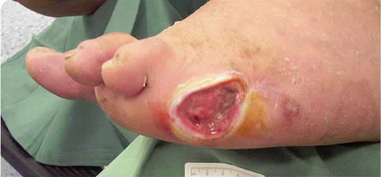
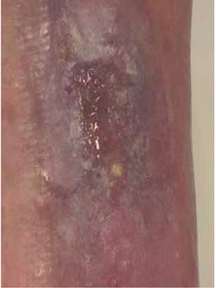
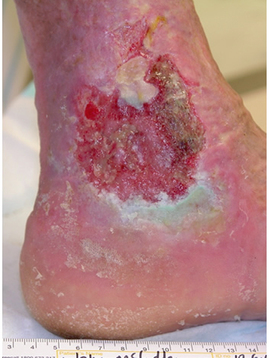
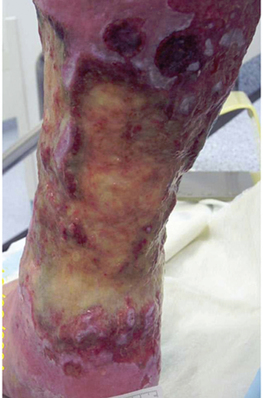
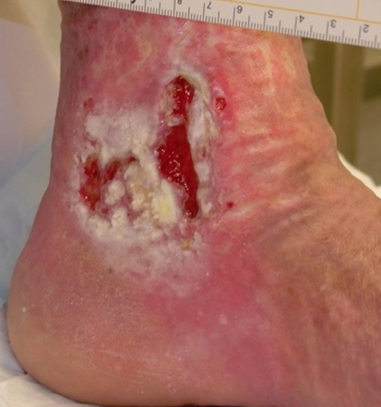

 Step 8 Remove gloves over contaminated dressing
Step 8 Remove gloves over contaminated dressing