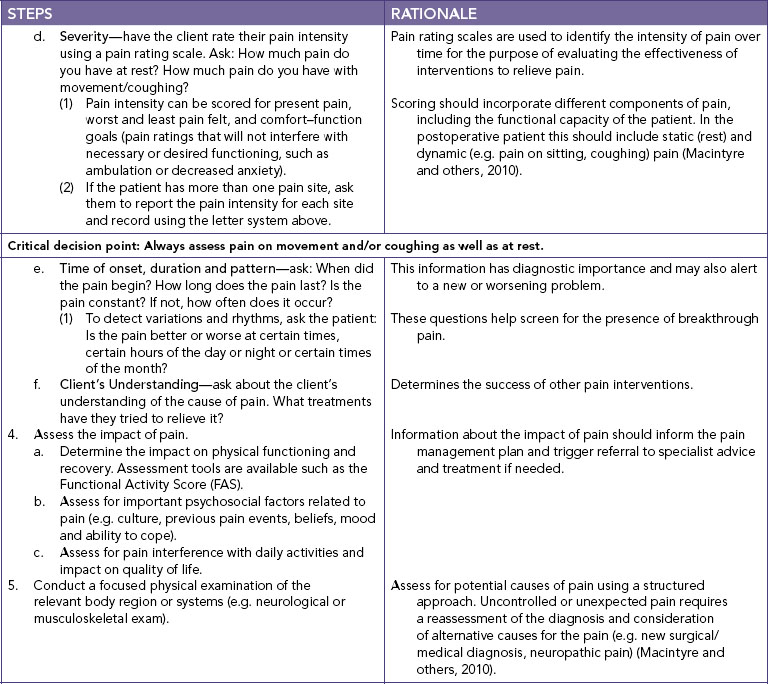
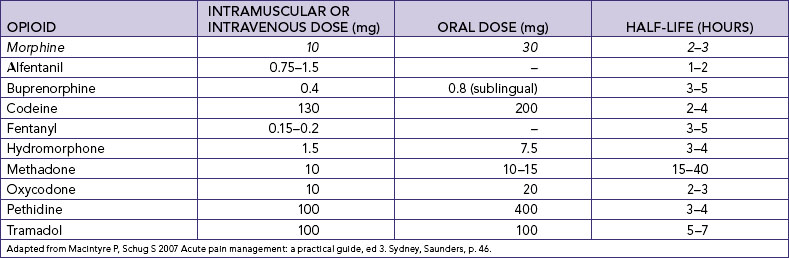
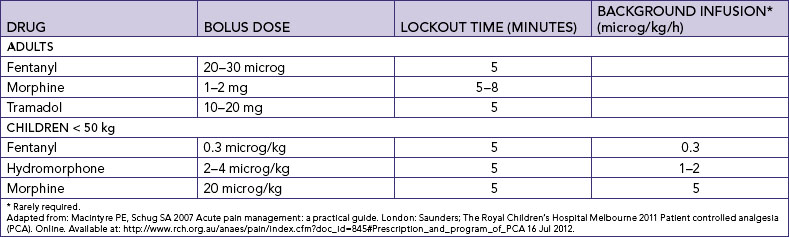
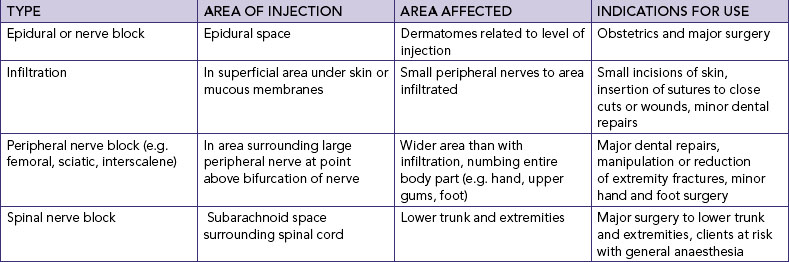
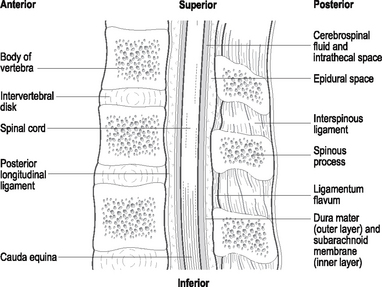
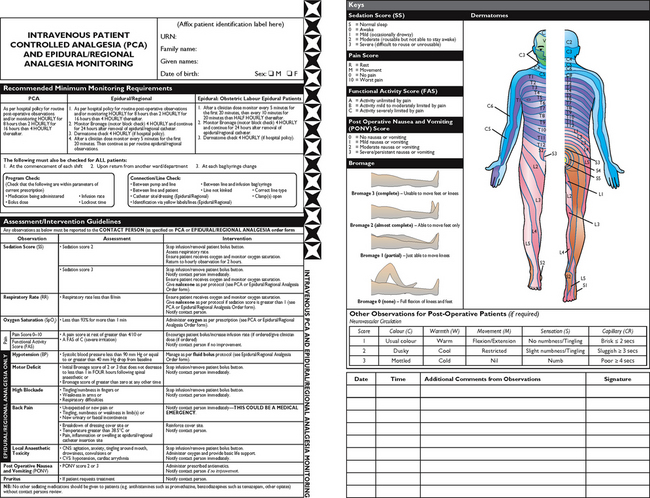
Chapter 41 Pain management
Mastery of content will enable you to:
• Define pain and other key terms.
• Review key pain models, including the biopsychosocial model of pain.
• Describe the key neural mechanisms of pain.
• Describe some key psychosocial contributors of pain.
• Perform a nursing assessment of a client experiencing pain.
• Apply a rational and evidence-based approach to pain management.
• Explain the various pharmacological approaches to treating pain.
• Describe interventions for preventing and treating opioid-related side effects.
• Describe the use of non-pharmacological pain interventions.
• Describe advanced pain techniques and the nursing responsibilities when they are used.
Pain management nursing
Regardless of the setting in which they work, nurses are responsible for the assessment and management of clients with pain. Pain is a key consideration in all patient care, and nurses play a critical role in pain management. Indeed, throughout history nurses have made important contributions to our understanding and management of pain through research and clinical practice. This chapter aims to provide an introduction to some key concepts in pain management and to encourage you to reflect on some of your own assumptions about pain.
Scientific knowledge base
Effective pain management requires knowledgeable and skilled practitioners. Nurses are uniquely positioned to improve patient outcomes through effective pain assessment and management, and yet there is ample evidence over several decades of poor pain assessment and under-treatment of patients in pain (Pasero and McCaffery, 2011). Despite the rapidly evolving scientific knowledge base for pain management, the data suggests that patients in pain remain poorly managed. Why might this be so?
Nelson (2007) argues that part of the problem may be that nurses view scientific and technical knowledge of pain management as less important than the human and relational skills that they bring to the client encounter. Yet in failing to engage with the science of pain, nurses risk becoming a key contributor to poorly managed pain and its sequelae, including chronic pain. Moreover, when nurses do not possess a scientific knowledge base of pain assessment and management, they are unable to participate fully as members of the interprofessional team, which is so important to collaborative pain management.
‘A REAL PAIN’ BY PAOLA SCAMPERLE, RN
As a neurosurgical nurse I’ve got plenty to do, including helping patients with some of the things some doctors don’t do well—one of which is effectively managing patients’ pain. Let me give you an example. I recently took care of a patient whose surgeon insisted that he didn’t need much nursing care. The patient had been operated on for a herniated disc and had only been out of surgery for 1 day. Interestingly, the patient himself was a senior doctor, a gastroenterologist who’d come to our hospital and unit because of the spine surgeon’s reputation.
As soon as I began to take care of him, the patient-doctor asked me to tell him about the normal course of postoperative recovery for this kind of operation. I told him we usually discharged patients 2 days after their surgery and that we gave them a prescription for pain medication. He was very concerned about pain meds because he’d been in unbearable pain before his surgery and took pain pills daily. After the operation he continued to take these pills, but was still complaining about his pain. Plus, he had a lot of pain that we hadn’t expected from the wound site.
When his well-respected surgeon came in to do a quick exam, the patient told him that he was in pain. Although this surgeon is very competent and self-confident, pain management is not one of his strengths. He just doesn’t understand that pain is an exquisitely personal experience and that each patient experiences it differently.
So what was the doctor’s response to this particular patient? He insisted the patient was somehow ‘imagining’ his pain, not really having it. The surgeon told the patient that he’d checked on his surgery, all was well, and he should no longer be in pain. So even though I spoke with him about the patient’s pain, he didn’t come up with a different pain management strategy.
Not surprisingly, when it came time for his discharge two days later, the patient was still in so much pain that he couldn’t leave the hospital. When the surgeon and a medical student made their rounds that day, the patient again asked them about his pain. Once again, they insisted that the patient’s pain could not be as severe as he said it was.
By now, I’d been with the patient over several days. I watched how he walked and knew how uncomfortable he was. I knew I had to do something to manage his pain and recognised that ordinary pain-killers just wouldn’t work.
The first thing I did was talk to the patient. In a careful tone, I said: ‘Look, Dr X, your surgeon is very knowledgeable and competent. Most of the time patients don’t have a lot of pain. But I know from experience that the kind of inflammatory pain you’re having doesn’t resolve within the first day or two. What you need is some cortisone prescribed in a single dose in the morning for a few days. I have seen this work in other cases with worse pain than yours.’
The patient-doctor thought about this. He thanked me for the suggestion and asked me to talk to the surgeon and suggest that he order the medication. I waited until the surgeon was out of the operating room and talked with him. He’d become so frustrated with the patient that he quickly agreed. He ordered the medication, and I gave the cortisone to the patient. Pretty soon the patient was comfortable and appeared not only relaxed but serene. He approached me in the hall and said, ‘I want to thank you so much for your patience, your availability and most of all your competence and professional attitude you showed me these past days. If it hadn’t been for you, I would still be in pain. I would have been suffering the same pain that made my life intolerable and led me to have this surgery in the first place. I want to thank you for dealing with this and all the needs I’ve had since I came to the hospital.’
Nurses do these kinds of things every day. Isn’t it about time we advertised them?
From Gordon S editor 2010 When chicken soup isn’t enough: stories of nurses standing up for themselves, their patients, and their profession. New York, Cornell University Press.
Read the following clinical example and think about how the registered nurse (RN) draws on her knowledge and experience to advocate for the client and effectively manage his pain.
Defining pain
The most oft-cited definition of pain in the literature and accepted by the International Association for the Study of Pain (IASP; Merskey, 1986:51) is:
Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.
This definition underscores the inherent subjectivity of pain and acknowledges the importance of emotional as well as sensory factors in the pain experience. Moreover, it highlights that although pain is usually considered as a warning signal of actual or potential tissue damage, pain can occur in the absence of tissue damage, even though the experience may be described as if the damage has occurred.
The most widely accepted and clinically useful definition of pain, however, was developed by a leading nursing expert on pain, Margo McCaffery (1968:95):
• CRITICAL THINKING
Take a minute to think about McCaffery’s clinical definition of pain. Do you agree with this definition? Who is the authority about a client’s pain? Whose pain is it?
Acute pain
In evolutionary terms, acute pain can be understood as an important biological protective mechanism to warn the body of injury or disease. It directs immediate attention to the situation, promotes reflexive withdrawal and fosters other actions that prevent further damage and enhance healing. Acute pain usually stops long before healing has occurred, which may take days or a few weeks.
Acute pain seriously threatens a client’s recovery and should be one of the priorities in the client’s care. Acute postoperative pain delays healing and leads to systemic complications (see Table 41-1). Rehabilitation may be delayed and hospitalisation may be prolonged if acute pain is not controlled. Moreover, unrelieved acute pain can lead to the development of chronic pain problems.
TABLE 41-1 THE HARMFUL EFFECTS OF UNRELIEVED PAIN
| DOMAINS AFFECTED | SPECIFIC RESPONSES TO PAIN |
|---|---|
| Endocrine | ↑ Adrenocorticotrophic hormone (ACTH), ↑ cortisol, ↑ antidiuretic hormone (ADH), ↑ adrenaline, ↑ noradrenaline, ↑ growth hormone (GH), ↑ catecholamines, ↑ renin, ↑ angiotensin II, ↑ aldosterone, ↑ glucagon, ↑ interleukin-1, ↓ insulin, ↓ testosterone |
| Metabolic | Gluconeogenesis, hepatic glycogenolysis, hyperglycaemia, glucose intolerance, insulin resistance, muscle protein catabolism, ↑ lipolysis |
| Cardiovascular | ↑ Heart rate, ↑ cardiac workload, ↑ peripheral vascular resistance, ↑ systemic vascular resistance, hypertension, ↑ coronary vascular resistance, ↑ myocardial oxygen consumption, hypercoagulation, deep-vein thrombosis |
| Respiratory | ↓ Flows and volumes, atelectasis, shunting, hypoxaemia, ↓ cough, sputum retention, infection |
| Genitourinary | ↓ Urinary output, urinary retention, fluid overload, hypokalaemia |
| Gastrointestinal | ↓ Gastric and bowel motility |
| Musculoskeletal | Muscle spasm, impaired muscle function, fatigue, immobility |
| Cognitive | Reduction in cognitive function, mental confusion |
| Immune | Depression of immune response |
| Developmental | ↑ Behavioural and physiological responses to pain, altered temperaments, higher somatisation, infant distress behaviour; possible altered development of the pain system, ↑ vulnerability to stress disorders, addictive behaviour and anxiety states |
| Future pain | Debilitating chronic pain syndromes: postmastectomy pain, post-thoracotomy pain, phantom pain, post-herpetic neuralgia |
| Quality of life | Sleeplessness, anxiety, fear, hopelessness, ↑ thoughts of suicide |
↓ = decreased; ↑ = increased.
From Pasero C, McCaffery M 2011 Pain assessment and pharmacologic management. St Louis, Mosby, p. 11. Data from Cousins M 1994 Acute postoperative pain. In Wall PD, Melzack R, editors, Textbook of pain, ed 3. New York, Churchill Livingstone; Kehlet H 1998 Modification of responses to surgery by neural blockade. In Cousins MJ, Bridenbaugh PO, editors, Neural blockade. Philadelphia, Lippincott-Raven; Mcintyre PE, Ready LB 1996 Acute pain management: a practical guide. Philadelphia, Saunders.
• CRITICAL THINKING
A 50-year-old man is under your care on a surgical ward after a fall that resulted in multiple rib fractures. He has an intercostal catheter to correct a pneumothorax. His respirations are shallow and he cannot cough effectively. Think about further assessment issues you might consider, given that he now has a temperature of 39°C. What complications could poorly controlled acute pain cause in this situation? How could this be prevented?
Chronic pain
In contrast to acute pain, chronic pain persists constantly or intermittently past the normal time of healing and serves no biological purpose. The terms chronic, chronic non-malignant and chronic benign pain are all found in the literature. Examples of chronic non-malignant pain include arthritis, low back pain, myofascial pain, headache and peripheral neuropathy.
Chronic pain is a major cause of physical and psychosocial disability, leading to problems such as loss of employment, inability to perform daily activities, mood disorders and social isolation from family and friends. The broader economic impact of chronic pain on the Australian community is also significant, costing approximately $34.3 billion annually (Access Economics, 2007).
Nociceptive pain
Although pain is often classified by its duration, another common way to categorise pain problems is by their underlying mechanisms. Nociceptive (physiological) pain is sustained by ongoing activation of the sensory system that subserves the perception of noxious stimuli. It implies the existence of damage to somatic or visceral tissues sufficient to activate the nociceptive system.
Neuropathic pain
Neuropathic (pathophysiological) pain is sustained by injury or dysfunction of the peripheral or central nervous system (CNS). Neuropathic pain problems are common in diseases affecting the nervous system, such as diabetes mellitus, herpes zoster and multiple sclerosis. It may also result from surgery and/or trauma to nervous tissue.
Evolution of pain theories
Early pain theories
Philosophical debate concerning the nature of pain has existed for thousands of years. Historically, pain has been dichotomised as either purely a psychological or a sensory phenomenon. For example, Aristotle (384–322 BC) viewed pain as an emotion, and the Stoic philosophers taught that pain could be overcome by ‘rational repudiation’ through logic and reasoning (Turk and Holzman, 1986). In contrast, pain has been understood as a specific sensation, having a direct relationship to the degree of noxious sensory stimuli. The latter has been the dominant conceptualisation of pain and has informed clinical practice over the last several hundred years.
The basic ideas of this sensory–physiological view of pain are most often attributed to the work of 17th-century philosopher René Descartes (1596–1650). Descartes attempted to explain pain in purely mechanistic terms using the scientific knowledge base of the time. Cartesian physiology held that nerves were hollow tubes that functioned with the flow of ‘animal spirits’ and contained fine filaments or threads that reached from the peripheries to the brain (Benini and DeLeo, 1999). Descartes argued in 1664 that the experience of the pain pathway was analogous to two ends of a direct communication line, ‘just as by pulling at one end of a rope [peripheral nerves] one makes to strike at the same instant a bell which hangs at the other end [brain]’ (cited in Melzack and Wall, 1996:150). To elaborate on this idea, he offered the analogy of a foot coming into contact with fire (see Figure 41-1). He proposed that a flame sets particles in the foot into activity and the motion is transmitted up the leg and back and into the head where pain becomes conscious (through the pineal gland) and elicits a reflexive response (Melzack and Wall, 1996). Thus, Descartes’ conception of pain was that of a straight-through channel from the skin to the brain. It assumed a direct and invariant relationship between stimulus intensity and perceived pain.

FIGURE 41-1 Descartes’ 1664 model of pain.
From Melzack R, Wall PD 1996 The challenge of pain, ed 2. London, Penguin Books, p. 150.
The legacy of biomedical reductionism inherited from Cartesian theory has had a profound impact on the way pain has been conceptualised and treated over at least the past three centuries. Modern pain theorists (19th and early 20th centuries) embraced this new paradigm to the extent that the neurophysiological basis for pain perception became the dominant model across disciplines and was often taught as fact rather than theory (Melzack and Wall, 1996).
The first of these theories to reach significant popularity was the specificity theory of pain as advocated by Müller (1842) and von Frey (1894). Specificity theory hypothesises that pain is a primary sensation like olfaction or taste. It assumes that there is a specific pain modality that can be stimulated only by painful stimuli, and therefore that these impulses are always felt specifically as pain and only pain. Essentially, it postulates that ‘pain receptors’ (designated as free nerve endings) generate pain impulses that are carried by specific ‘pain fibres’ (e.g. A-delta and C fibres) in peripheral nerves to the spinal cord. These impulses synapse in the spinal cord and travel via a ‘pain pathway’ (e.g. the spinothalamic tract) to a ‘pain centre’ in the brain (e.g. the thalamus or sensory cortex) where they are perceived as pain. Thus, pain is perceived as having specific central, as well as peripheral, mechanisms similar to other bodily senses. Such a model is clearly similar to the conceptual nervous system described by Descartes centuries earlier.
Based on extensive laboratory evidence, the basic assumptions of specificity theory are now viewed as wholly oversimplified. In addition to a lack of clear scientific support, specificity theory has also been unable to account for the wide variability of individuals’ subjective experiences and responses to pain and its treatment. As Turk and Flor (1999:19) have cogently argued, ‘pain in the absence of pathology, pathology in the absence of pain, individual differences in response to identical treatments, failure of neurosurgical procedures and potent analgesic agents to consistently eliminate pain, and the low association between impairment and disability fail to conform to a model of pain that presumes a direct transmission from the periphery to central nervous system structures.’
• CRITICAL THINKING
It is easy to assume that pain severity is closely tied to the amount of tissue damage based on our personal experiences of pain, but can you think of exceptions to this idea? Can you think of any examples of injury without pain, or vice versa? Why might two clients having the same surgery report different pain severity postoperatively?
In response to the inadequacies of specificity theory, several other conceptualisations of pain were proposed. Together these are grouped as variants of the pattern theory of pain, originating from the work of Goldscheider (1894). The basic premise of these models is that the transmission of nerve impulse patterns (spatial and temporal) which are produced and coded by receptors are the primary determinants of pain. Thus, there was a rejection of the earlier emphasis on identifying physiological structures in favour of defining sensory patterns or the balance of sensory input as the cause of pain. Peripheral pattern theorists (e.g. Sinclair, 1955; Weddell, 1955) suggested that, for example, all nerve fibre endings are similar (apart from those that innervate hair cells) and that pain occurs when particular spatial or temporal patterns of impulses are generated in afferent nerves by intense stimulation of non-specific receptors. None of these models, however, provided an adequate general theory of pain. Psychological aspects, when considered, were dismissed as reactions to pain.
Gate-control theory of pain
Based on a critique of existing models, Melzack and Wall (1965) formulated their revolutionary gate-control theory of pain. The original proposition was that pain is not simply the net result of defined peripheral input. Rather, Melzack and Wall drew on converging lines of evidence to establish that pain perception is a dynamic, multidimensional experience involving psychophysiological mechanisms. According to gate-control theory, ‘the experience of pain is an ongoing sequence of activities, largely reflexive in nature at the outset, but modifiable even in the earliest stages by a variety of excitatory and inhibitory influences, as well as the integration of ascending and descending CNS activity’ (Turk and Monarch, 2002:4). Thus, from the gate-control perspective, pain perception is considered a complex interactive product of afferent sensory activity and central (psychological) processes. It was this radical shift in thinking about pain perception that is perhaps the most important contribution of the gate-control theory.
The fundamental and most original feature of the gate-control theory is the proposed neural gating system, postulated to exist in the dorsal horns of the spinal cord which modulates the transmission of nociceptive information from the periphery to the CNS on the basis of the diameters of active peripheral fibres, as well as the dynamic action of brain processes (see Figure 41-2). Specifically, the gate-control theory is based on the following hypotheses (Melzack and Wall, 1965, 1982):
• The transmission of nerve impulses from afferent fibres to spinal cord transmission cells (coined ‘T cells’) is modulated by a ‘spinal gating mechanism’ in the substantia gelatinosa of the dorsal horn.
• The spinal gating mechanism is influenced by the relative amount of activity in large-diameter (A-beta) and small-diameter (A-delta and C) fibres, so that activity in large fibres tends to inhibit transmission (i.e. ‘closes the gate’) whereas small-fibre activity tends to facilitate transmission (i.e. ‘opens the gate’).
• The spinal gating mechanism is also influenced by nerve impulses that descend from the brain.
• A specialised system of large-diameter, rapidly conducting fibres (the ‘central control trigger’) activates selective cognitive processes (e.g. meaning of pain) that then influence, by way of descending fibres, the modulating properties of the spinal gating mechanism. Thus, the central control system could modulate the spinal gate and thereby selectively alter certain afferent sensory patterns before they reach the brain.
• When the output of the spinal cord T cells exceeds a critical level, it activates the ‘action system’ or those areas of the brain that are responsible for the behavioural responses to pain and the perceptual experience of pain itself.
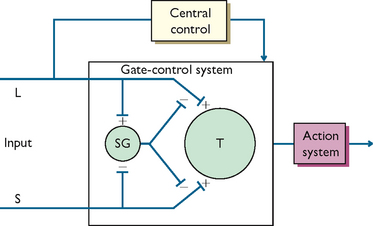
FIGURE 41-2 Schematic model of the gate-control theory.
L = large-diameter fibre; S = small-diameter fibre; SG = spinal gating mechanism; T = spinal cord transmission cell.; Redrawn from Melzack R, Wall PD 1965 Pain mechanisms: a new theory. Science 150:971.
• CRITICAL THINKING
Take a minute to think about the ideas proposed by the gate-control theory. How might it explain why rubbing an injured area on the body helps to reduce pain, or how a sportsperson can continue a game after serious injury yet report no pain?
Shortly after first publication, the theory was expanded upon by Melzack and Casey (1968) to describe the neural systems beyond the gate. The extended theory differentiated three systems related to the processing of nociceptive information, all of which are argued to contribute to the subjective experience of pain. Melzack and Casey suggested that the neospinothalamic projecting system serves to process the sensory–discriminative dimension of pain, such as location, intensity and nature of the stimulus, whereas impulses that course through the paleospinothalamic tract and paramedial ascending system activate reticular and limbic structures that provide the motivational–affective response to pain, such as aversive drive and unpleasant affect. The neocortical or higher CNS processes were predicted to further influence the sensory and motivational systems through cognitive–evaluative appraisal of input in terms of attention, meaning, past experience, and so on. Because of the continuous interactive nature of these parallel systems, considerable potential for shaping the pain experience is implied (Turk and Monarch, 2002).
Post-gate-control theories
The neuroanatomical and physiological assumptions of the gate-control theory have since been challenged, and it has been suggested that the theory is incomplete (e.g. see Sufka and Price, 2002). While additional mechanisms have been suggested to revise the theory in order to account for chronic pain (Melzack and Wall, 1996), Melzack (1996, 1999) himself points out that the gate-control theory still fails to explain certain phenomena such as phantom-limb pain. Melzack’s most recent work has been to shift the focus of pain theory from the peripheries and spinal mechanisms to understand how the brain creates the experience of pain in the proposed neuromatrix theory of pain.
Biopsychosocial model of pain
One of the most useful contemporary models for nursing practice which brings together much of what we know about pain is the biopsychosocial model of pain. The biopsychosocial model highlights the multidimensional nature of pain, attending to the multiple factors that both contribute to and are affected by pain. Although the gate-control theory provided an integrative model of pain perception, it focused primarily on the neurophysiology of pain. The biopsychosocial model, in contrast, builds on this model by also emphasising the influence of psychosocial and behavioural components of pain (Turk and Monarch, 2003).
The conceptual view of the biopsychosocial model of pain is presented in Figure 41-3. This nested circles model demonstrates the interdependent relationships among processes that culminate in the person’s perception of pain and overt pain behaviours. Biological factors may initiate and maintain nociceptive input, psychological factors influence the appraisal and perception of pain, and social factors shape the person’s behavioural responses.
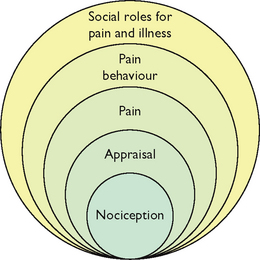
FIGURE 41-3 Biopsychosocial model of pain.
From Turk DC, Burwinkle TM 2006 Coping with chronic pain. In Carr A, McNulty M, editors, The handbook of adult clinical psychology: an evidence-based practice approach. London, Routledge, p. 647.
Following this biopsychosocial model, this chapter now discusses some of the key physical and psychosocial contributors to the pain experience.
Physiology of pain
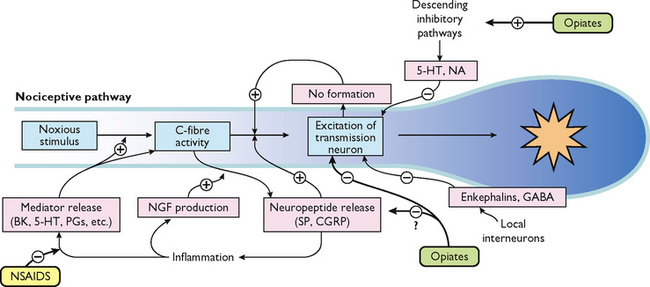
What is presented here is a brief overview of the normal neural mechanisms involved in the pain experience, called nociception. Between the stimulus of tissue injury and the subjective experience of pain is a series of complex electrical and chemical events. To understand this, it is useful to conceptualise four distinct physiological processes involved: transduction, transmission, perception and modulation (see Figure 41-4).

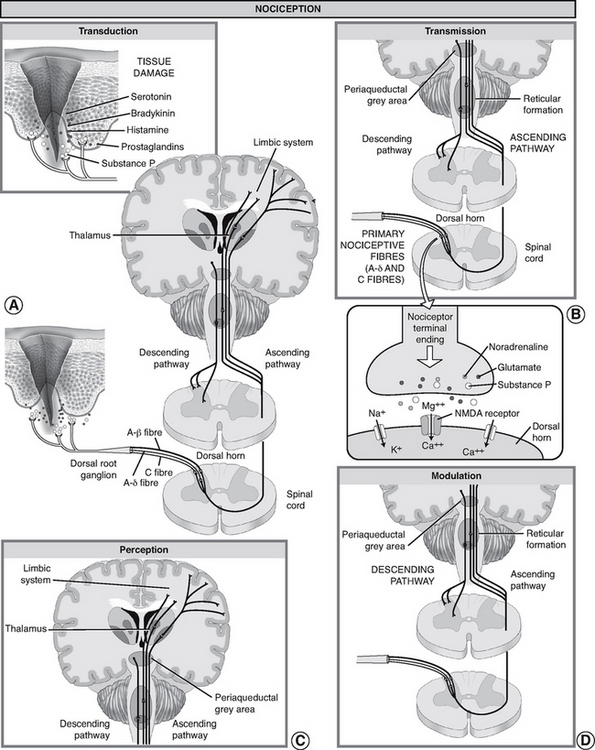
FIGURE 41-4 Nociception: ‘normal’ pain transmission.
From Pasero C, McCaffery M, editors, 2011 Pain assessment and pharmacologic management. St. Louis, Mosby, p. 5.
Transduction
Transduction refers to the processes by which noxious stimuli activate nociceptors, which are primary afferent neurons that exist throughout the body (skin, subcutaneous tissue and visceral or somatic structures). Nociceptors have free nerve endings which respond selectively to mechanical (e.g. incision, tumour growth), thermal (e.g. burn, frostbite) or chemical (e.g. toxins, chemotherapy) stimuli, which are converted into a neuronal action potential.
Tissue damage and inflammation also cause the release of chemical mediators from injured cells, inflammatory cells (e.g. mast cells, lymphocytes) and primary afferent neurons which lower the excitability threshold of nociceptors and sensitise them to further sensory input. A partial list of these chemicals includes bradykinin, prostaglandins, serotonin, histamine and substance P, known collectively as the ‘inflammatory soup’. These chemicals stimulate or sensitise the nociceptors to send impulses to the spinal cord.
Transmission
Transmission of action potentials generated from effective transduction occurs along two types of nociceptor fibres towards the CNS. A-delta fibres are thinly myelinated, large-diameter, fast-conducting fibres (5–30 metres/second), which transmit well-localised sensations of sharp pain. C fibres are unmyelinated, small-diameter, slow-conducting fibres (0.5–2 metres/second), which transmit pain that is poorly localised, dull and aching. Other types of sensory fibres such as A-beta fibres do not usually transmit nociceptive information, but respond to touch, movement and vibration.
For example, after spraining an ankle a person initially feels a sharp, localised pain, which is a result of A-delta fibre transmission. Within a few seconds, the pain becomes more diffuse and widespread, until the whole foot aches because of C-fibre activation. The C fibres remain exposed to the chemicals released when cells are damaged.
Transmission continues along the primary afferent fibres until they synapse in the dorsal horn of the spinal cord. Within the dorsal horn, neurotransmitters (such as glutamate, neurokinins and substance P) are released, causing a synaptic transmission from the primary afferent neurons to second-order neurons in the spinal cord which project to the brainstem and thalamus via multiple ascending pathways. Of these pathways, the spinothalamic and spinoreticular tracts are particularly important. These second-order fibres cross the midline and ascend in the anterolateral quadrant of the spinal cord.
Perception
Perception is the point at which a person is consciously aware of pain. It requires the activation of higher brain structures, presumably including the cortex. It involves both awareness and related cognitions and the occurrence of emotions and drives associated with pain (Pasero and Portenoy, 2011).
The mechanisms of pain perception remain poorly understood. From the thalamus, third-order neurons transmit impulses to various areas of the brain. However, the exact location in the brain where pain becomes a conscious experience remains unclear. Studies using functional magnetic resonance imaging (fMRI) have consistently demonstrated involvement of the thalamus, primary and secondary somatosensory cortex (S1, S2), insula, forebrain and anterior cingulate cortex (ACC) during painful stimulation. Data suggests that these areas process different aspects of pain. For example, while the perception of sensory–discriminative aspects (e.g. intensity, quality, location and pattern) is associated with the S1 and S2 cortices, the ACC and insula seem to be associated with the affective–motivational (e.g. fear, anxiety) processing of pain (Apkarian and others, 2005).
Modulation
Modulation (inhibition or facilitation) of afferent input can also occur anywhere along nociceptive pathways, particularly in the spinal cord as suggested by the gate-control theory discussed earlier. When activated, descending efferent pathways originating in the brain release a number of chemicals capable of altering the processing of nociceptive input. Of the descending inhibition systems, the periaqueductal grey (PAG) region is particularly important; this projects to the dorsal horn of the spinal cord.
These descending pathways release substances such as endogenous opioids (endorphins and encephalin), serotonin and noradrenaline which can inhibit the transmission of noxious stimuli and produce analgesia. For example, when released in the spinal dorsal horn these substances can bind to receptors to prevent the release of neurotransmitters from primary afferent nociceptors such as substance P to second-order neurons, thus blocking transmission of the impulse.
• CRITICAL THINKING
The discussion so far has been about the normal physiological processes involved in nociceptive pain, but what mechanisms can explain the development of pathophysiological pain problems such as chronic inflammatory and neuropathic pain? A key development in our understanding of pain mechanisms is the concept of neuroplasticity of the nervous system, which refers to the ability of neurons throughout the peripheral and central nervous systems to change their structure and function because of nociceptive input. Explore what the terms peripheral sensitisation and central sensitisation mean and how they might contribute to the pathophysiology of acute and chronic pain problems.
From nociception to pain
Tracing the physiological processes involved in pain perception from peripheral receptor to cortex, it is easy to assume a simple and reproducible relationship among stimulus intensity, nociceptive transmission cell activity and the perceived intensity of pain. However, despite its intuitive appeal, a physiological understanding of pain is still incomplete. As discussed above, evidence clearly demonstrates there is no one-to-one relationship between tissue injury and the experience of pain.
It is useful here to make the distinction between nociception and pain. Nociception is a physiological phenomenon that involves activation of sensory transduction in nerves by thermal, mechanical or chemical energy impinging on specialised nerve endings. The nerves involved convey information about tissue damage to the CNS, but such sensations are not yet considered pain until they are subjected to higher-order psychological and cognitive processing that involves appraisals. Hence, nociception is a sensory process with nociceptive stimuli capable of producing pain. Pain, however, is a subjective, perceptual experience that results from the nociceptive input and is modulated on a number of different levels in the CNS (Turk and Monarch, 2002). Thus, nociception may be necessary for pain to occur but it is not sufficient to account for pain as a clinical phenomenon, which is always a perceptual experience.
The discussion now considers the important role of some key psychosocial factors in shaping the pain experience.
Psychosocial factors influencing pain
Psychological, environmental and behavioural factors play a central role in the experience of pain. Together they shape the way an individual makes sense of and gives meaning to pain. They also influence the way in which the individual will cope with pain and the extent to which it will interfere with the roles and responsibilities of daily life. They are therefore important considerations in the assessment of acute and chronic pain and holistic planning for client care.
• CRITICAL THINKING
Consider the following: ‘Here is perhaps the most difficult thought to accept about pain. We experience pain only and entirely as we interpret it. It seizes us as if with an unseen hand, sometimes stopping us in mid-sentence or mid-motion, but we too capture and reshape it’ (Morris, 1991:29).
Age
The age and developmental stage of a client is an important variable that influences the experience of pain, particularly in children and older adults. Unfortunately, these groups are also at risk for inadequate pain management because of common misconceptions about pain perception, appropriate pain assessment and effective and safe use of analgesics (see Table 41-2).
| MISCONCEPTION | CORRECTION |
|---|---|
| Infants are incapable of feeling pain | Infants have developed the anatomy and physiology for pain processing by mid to late gestation |
| Infants are less sensitive to pain than older children and adults | |
| Infants are incapable of expressing pain | Although infants cannot verbalise pain, they demonstrate it with observable cues, both behavioural and physiological |
| Infants must learn about pain from previous painful experiences | Pain requires no prior experience; it need not be learned from earlier painful experience. Pain is present from the first injury |
| Pain cannot be accurately assessed in infants | Behavioural cues (i.e. facial expressions, crying, body movements) and physiological indicators of pain can be reliably and validly assessed either alone (univariate approach) or in combination (multivariate approach). The most valid univariate approach is facial expression. The most valid multivariate approach is through the use of a composite pain measure |
| Infants are incapable of remembering pain | Studies have shown that infants who are exposed to painful stimuli at an early age respond more vigorously to subsequent painful stimuli |
| Analgesics and anaesthetics cannot be safely given to infants and neonates because of their immature capacity to metabolise and eliminate drugs and their sensitivity to opioid-induced respiratory depression | Infants older than 1 month of age metabolise drugs in the same manner as older infants and children. Careful selection of the agent, dosage, administration route and time, frequent monitoring for desired and undesired effects and drug titration and weaning can minimise the adverse effects of opioids and non-opioids for pain management in neonates |
Adapted from McCaffery M, Pasero C 1999 Pain: clinical manual, ed 2. St Louis, Mosby.
Young children have difficulty understanding pain and the procedures nurses administer that may cause pain. Young children who have not developed full vocabularies also have difficulty describing and expressing pain to parents or caregivers. Cognitively, toddlers and preschoolers are unable to recall explanations about pain, or associate pain with experiences that can occur in various situations. With these developmental considerations in mind, the nurse must adapt approaches for assessing a child’s pain (including what to ask and the behaviours to observe for) and how to prepare a child for a painful procedure.
While older adults may be at greater risk of having painful conditions, pain is not an inevitable part of ageing. Once an older client suffers pain, there can be serious impairment of functional status. Mobility, activities of daily living, social activities outside the home and activity tolerance can all be reduced. The presence of pain in the older adult requires aggressive assessment, diagnosis and management.
Chronic pain is a problem for many elderly people living in both community and extended-care facilities. When they are admitted to hospital, they often have acute pain superimposed on chronic pain problems. There are also many myths about pain in older adults that need to be corrected in order to provide effective nursing care (see Table 41-3).
TABLE 41-3 PAIN IN OLDER ADULTS
Adapted from McCaffery M, Pasero C 1999 Pain: clinical manual, ed 2. St Louis, Mosby.
The ability of older clients to interpret pain can be complicated by the presence of multiple diseases with vague symptoms that may affect similar parts of the body. It is important to take enough time to complete a thorough assessment of pain and pain-related concerns.
Gender
Considerable evidence suggests that women and men differ in their experience of pain, as well as in their response to pain treatments (Fillingim, 2009). Women are at greater risk of a variety of chronic pain conditions such as headache, facial pain, abdominal pain and musculoskeletal pain (LeResche, 1999; Unruh, 1996). Experimental evidence also suggests that women may be more sensitive to pain, less tolerant of pain and more able to discriminate among different levels of pain than men (Riley and others, 1998). Interestingly, although women were traditionally excluded from many clinical trials examining the efficacy of drugs including analgesics, recent evidence suggests there may be important sex differences. A recent systematic review, for example, demonstrated sex differences for morphine analgesia in both experimental pain studies and clinical patient-controlled analgesia (PCA) studies, with greater morphine efficacy in women (Niesters and others, 2010).
Biological and psychosocial factors are both important in explaining these differences, yet more research is needed to elucidate the mechanisms underlying sex differences in pain. Hormonal and genetic factors may contribute to sex differences in pain perception and response to analgesics (Fillingim, 2009). Important psychosocial factors also include differences in cognitive appraisals, coping strategies and social role expectancies in response to pain (e.g. men encouraging and rewarding stoicism and punishing overt pain expression, whereas women are encouraged to be aware of pain and express it).
Culture
The multicultural nature of the Australasian population means that nurses will encounter a variety of responses to pain in their work environment. For example, Indo-Chinese clients may often be quite stoic, not requesting pain relief themselves. They may instead delegate a member of their family to approach the nurse for assistance. People of Middle Eastern origin may express pain through facial expression, body posture and moaning or soft cries. Some may believe, however, that pain is something to be endured to facilitate cleansing of the soul and for this reason may not seek analgesia. Filipino patients tend to focus their illness around their religious beliefs. Regardless of how bad their pain is, they tend to not complain and instead focus on praying (Schmidt, 2005).
Aboriginal and Torres Strait Islander people tend to be very orientated towards their families and are frequently not willing to share personal information with strangers. For this reason, these people may appear withdrawn and non-communicative until a level of trust and confidence has been established with hospital staff. The nurse again needs to be astute in assessing these clients in an effort to determine the presence and severity of pain as well as the most appropriate method of pain relief that may be implemented.
It is important to note that observed cultural differences are more likely to concern the way in which pain is regarded and the extent to which a person is permitted to express pain and under what conditions. These differences in pain behaviour are not evidence of differences in pain threshold and tolerance. They signify differences in the way people from different cultures have learned to behave when they are in pain.
While it is important that nurses are knowledgeable about cultural differences in response to pain, we need to be careful not to stereotype clients based on their cultural background. Although an individual may identify with a cultural group, there is also diversity within each culture.
Providing culturally safe care means being aware of your own cultural values and beliefs about pain and reflecting on how this might influence practice. The nurse, the client and the family must work together to facilitate communication about the assessment and management of pain. Finding a common assessment tool and communicating that tool to other healthcare providers is important.
• CRITICAL THINKING
Smithy is a 55-year-old Indigenous Australian who has a fractured femur resulting from the impact of a cow in the cattle yards. He is a long way from home and family members. As his female RN, you are having difficulty assessing his pain and he avoids eye contact with you.
What issues might you consider in terms of ensuring cultural safety and a positive nurse–client relationship?
Meaning of pain
The meaning that a person associates with pain affects the experience and impact of pain. This can be closely associated with the person’s cultural background. A person will perceive pain differently if it suggests a threat, loss, punishment or challenge. For example, a woman in labour will perceive pain differently from a woman with a history of cancer who is experiencing a new pain and fearing recurrence. The degree and quality of pain perceived by a client are related to the meaning of pain.
Anxiety
The relationship between pain and anxiety is complex. Anxiety often increases the perception of pain, but pain may also cause feelings of anxiety. Chapman and Turner (2001) report that studies have determined that arousal of the noradrenergic brain pathways is a major mechanism of anxiety and stress. The noradrenergic brain pathways are stimulated by biological (e.g. pain), psychological (e.g. fear) and psychosocial (e.g. isolation) threats.
Critically ill or injured clients, who often perceive a lack of control over their environment and care, may have high anxiety levels. This anxiety, if it has gone unnoticed in the high-tech environment of an intensive care unit, can lead to serious pain-management problems. The challenge is to relieve the pain in a client who is anxious in any setting (long-term care, acute care or home care). Although pharmacological and non-pharmacological approaches to the management of anxiety are appropriate, anxiolytic medications should not be a substitute for analgesia.
Attention
The degree to which a client focuses attention on pain can influence pain perception. Diverting attention away from pain perception reduces pain distress and increases pain tolerance in both acute and chronic conditions. This concept is one that nurses apply in various pain-relief interventions such as relaxation, guided imagery and massage. By focusing a client’s attention and concentration on other stimuli, the nurse places pain on the periphery of awareness.
Fatigue
Fatigue heightens the perception of pain. The sense of exhaustion intensifies pain and decreases coping abilities. This can be a common problem with any person experiencing chronic illness or who has fatigue as a result of treatment. If fatigue occurs along with sleeplessness, the perception of pain may be even greater.
Pain beliefs
As active processors of information, people with pain problems develop underlying beliefs, attitudes and assumptions in an attempt to make sense of their pain condition. These include attributions about the cause, meaning and appropriate treatment of pain, as well as perceptions of control over pain and personal coping efficacy (Thorn, 2004). Although certain beliefs may be adaptive and promote positive adjustment, others are likely to contribute to heightened pain, distress and disability. A growing body of research shows that pain-related beliefs are strongly associated with various measures of pain severity and physical and psychosocial functioning (Thorn, 2004). These beliefs, appraisals and expectancies held by individuals regarding the possible consequences of pain and their abilities to deal with them are hypothesised to affect functioning directly by influencing mood as well as indirectly by influencing coping efforts (Jensen and others, 1991).
Coping strategies
Faced with chronic pain, individuals also learn and utilise a variety of strategies to help them cope or deal with their pain. Pain coping is defined as purposeful cognitive and behavioural efforts to manage or negate the negative impact of pain (Jensen and others, 1991). Much of the pain literature broadly classifies coping strategies as active or passive. In general, studies have found active coping strategies (efforts to function in spite of pain or to distract oneself from pain, such as activity or ignoring pain) to be associated with adaptive functioning, and passive coping strategies (withdrawal or surrendering control to an external source, such as resting or medication use) to be related to greater pain and depression (Boothby and others, 1999). Interestingly, although maladaptive strategies are strongly associated with negative outcomes, adaptive strategies generally show only modest correlations with positive outcomes (Geisser and others, 1999).
One specific maladaptive coping response that has consistently demonstrated robust associations with various measures of adjustment is termed catastrophising. It refers to ‘a method of cognitively coping with pain characterised by negative self-statements and overly negative thoughts and ideas about the future’ (Keefe and others, 1989:51). Greater endorsement of catastrophic thinking when in pain has been consistently associated with higher levels of pain, distress and disability among diverse populations (Keefe and others, 2004).
Research findings concerning the relationship between other specific pain coping strategies and adjustment to chronic pain have been somewhat more inconsistent (Boothby and others, 1999). When significant associations are found, greater use of coping self-statements (such as ‘I tell myself I can overcome the pain’) is generally related to positive adjustment, whereas praying/hoping, pain-contingent rest and wishful thinking are frequently associated with greater dysfunction. Ignoring pain, reinterpreting pain and distraction/diverting attention, in contrast, rarely predict functioning among people with chronic pain (Boothby and others, 1999).
Family and social support
Another factor that can significantly influence a person’s pain response is the presence and attitudes of significant others. People in pain often depend on family members or close friends for support, assistance or protection. Although pain still exists, the presence of a loved one can minimise loneliness and fear. An absence of family or friends can often make the pain experience more stressful. The presence of parents is especially important for children experiencing pain.
The social environment can also significantly contribute to adjustment to chronic pain. Pain behaviours which are initially adaptive (e.g. avoidance of activity believed to exacerbate pain) are subject to the influence of environmental or interpersonal factors so that over time these behaviours may come to be under the control of operant conditioning (Fordyce, 2001). For example, positive reinforcement of pain behaviour may inadvertently occur through solicitous attention from family members so that disability is maintained. In addition, negative reinforcement such as avoidance of a stressful work setting or unpleasant interpersonal obligations and reduction of pain through limiting activity may also contribute to persistence of pain behaviour.