Chapter Twenty-three Eyes
INTRODUCTION
As the eye is the major source of sensory information, the brain is heavily involved in the function of vision. Processing visual information takes place in the cerebral cortex and the visual association area occupies the most of the occipital lobe. It is the largest of all the cortical sensory areas in the occipital lobe (Marieb and Hoehn, 2010). In this chapter we review the external and internal anatomy of the eye and accessory muscles, visual pathways and visual fields and visual light reflexes. This information is also relevant to neurological system assessment (see Ch 22).
EXTERNAL ANATOMY
Because this sense is so important to humans, the eye is well protected by the bony orbital cavity, which is surrounded with a cushion of fat. The eyelids are like two movable shades that further protect the eye from injury, strong light and dust. The upper eyelid is the larger and more mobile one. The eyelashes are short hairs in double or triple rows that curve outwards from the lid margins, filtering out dust and dirt.
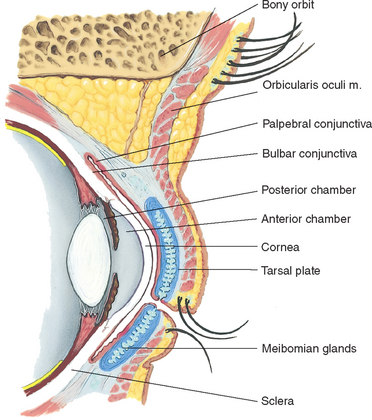
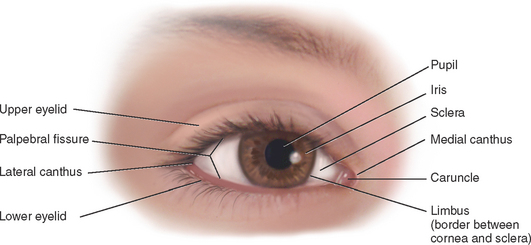
The palpebral fissure is the elliptical open space between the eyelids (Fig 23.1). When closed, the lid margins approximate completely. When open, the upper lid covers part of the iris. The lower lid margin is just at the limbus, the border between the cornea and sclera. The canthus is the corner of the eye, the angle where the lids meet. At the inner canthus the caruncle is a small fleshy mass containing sebaceous glands.
Within the upper lid, tarsal plates are strips of connective tissue that give it shape (Fig 23.2). The tarsal plates contain the meibomian glands, modified sebaceous glands that secrete an oily lubricating material onto the lids. This stops the tears from overflowing and helps to form an airtight seal when the lids are closed.
The exposed part of the eye has a transparent protective covering, the conjunctiva. The conjunctiva is a thin mucous membrane folded like an envelope between the eyelids and the eyeball. The palpebral conjunctiva lines the lids and is clear, with many small blood vessels. It forms a deep recess and then folds back over the eye. The bulbar conjunctiva overlays the eyeball, with the white sclera showing through. At the limbus the conjunctiva merges with the cornea. The cornea covers and protects the iris and pupil.
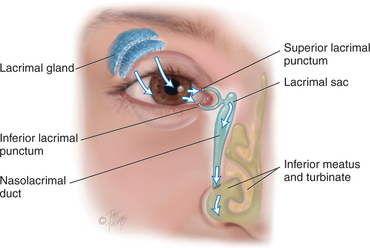
The lacrimal apparatus provides constant irrigation to keep the conjunctiva and cornea moist and lubricated (Fig 23.3). The lacrimal gland, in the upper outer corner over the eye, secretes tears. The tears wash across the eye and are drawn up evenly as the lid blinks. The tears drain into the puncta, visible on the upper and lower lids at the inner canthus. The tears then drain into the nasolacrimal sac, through the 7.5 cm nasolacrimal duct and empty into the inferior meatus inside the nose. A tiny fold of mucous membrane prevents air from being forced up the nasolacrimal duct when the nose is blown.
Extraocular muscles.
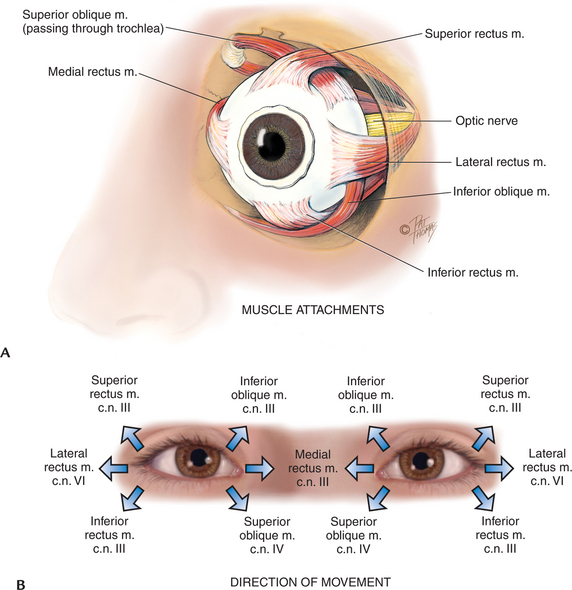
Six muscles attach the eyeball to its orbit (Fig 23.4) and serve to direct the eye to points of the person’s interest. These extraocular muscles give the eye both straight and rotary movement. The four straight, or rectus, muscles are the superior, inferior, lateral and medial rectus muscles. The two slanting, or oblique, muscles are the superior and inferior muscles.
Each muscle is coordinated, or yoked, with one in the other eye. This ensures that when the two eyes move, their axes always remain parallel (called conjugate movement). Parallel axes are important because the human brain can tolerate seeing only one image. Although some animals can perceive two different pictures through each eye, human beings have a binocular, single-image visual system. This occurs because our eyes move as a pair. For example, the two yoked muscles that allow looking to the far right are the right lateral rectus and the left medial rectus.
Movement of the extraocular muscles (below) is stimulated by three cranial nerves. Cranial nerve VI, the abducens nerve, innervates the lateral rectus muscle (which abducts the eye); cranial nerve IV, the trochlear nerve, innervates the superior oblique muscle; and cranial nerve III, the oculomotor nerve, innervates all the rest—the superior, inferior and medial rectus and the inferior oblique muscles. Note that the superior oblique muscle is located on the superior aspect of the eyeball, but when it contracts it enables the person to look downwards and inwards.
INTERNAL ANATOMY
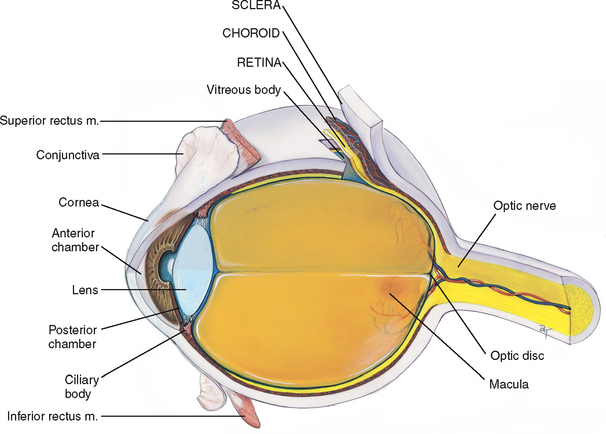
The eye is a sphere composed of three concentric coats: (1) the outer fibrous sclera, (2) the middle vascular choroid and (3) the inner nervous retina (Fig 23.5). Inside the retina is the transparent vitreous body. The only parts accessible to examination are the sclera anteriorly and the retina through the ophthalmoscope.
The outer layer.
The sclera is a tough, protective, white covering. It is continuous anteriorly with the smooth, transparent cornea, which covers the iris and pupil. The cornea is part of the refracting media of the eye, bending incoming light rays so that they will be focused on the inner retina.
The cornea is very sensitive to touch; contact with a wisp of cotton stimulates a blink in both eyes, called the corneal reflex. The trigeminal nerve (cranial nerve V) carries the afferent sensation into the brain, and the facial nerve (cranial nerve VII) carries the efferent message that stimulates the blink.
The middle layer.
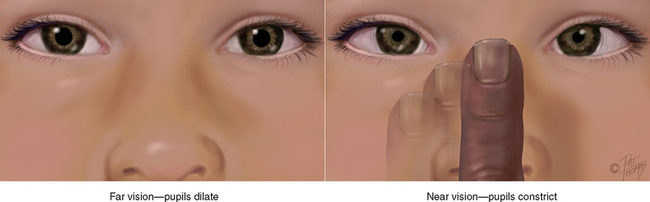
The choroid has dark pigmentation to prevent light from reflecting internally and is heavily vascularised to deliver blood to the retina. Anteriorly, the choroid is continuous with the ciliary body and the iris. The muscles of the ciliary body control the thickness of the lens. The iris functions as a diaphragm, varying the opening at its centre, the pupil. This controls the amount of light admitted into the retina. The muscle fibres of the iris contract the pupil in bright light and to accommodate for near vision, and dilate the pupil when the light is dim and for far vision. The colour of the iris varies from person to person.
The pupil is round and regular. Its size is determined by a balance between the parasympathetic and sympathetic chains of the autonomic nervous system. Stimulation of the parasympathetic branch, through cranial nerve III, causes constriction of the pupil. Stimulation of the sympathetic branch dilates the pupil and elevates the eyelid. As mentioned earlier, the pupil size also reacts to the amount of ambient light and to accommodation, or focusing an object on the retina.
The lens is a biconvex disc located just posterior to the pupil. The transparent lens serves as a refracting medium, keeping a viewed object in continual focus on the retina. Its thickness is controlled by the ciliary body; the lens bulges for focusing on near objects and flattens for far objects.
The anterior chamber is posterior to the cornea and in front of the iris and lens. The posterior chamber lies behind the iris to the sides of the lens. These contain the clear, watery aqueous humour that is produced continually by the ciliary body. The continuous flow of fluid serves to deliver nutrients to the surrounding tissues and to drain metabolic wastes. Intraocular pressure is determined by a balance between the amount of aqueous produced and resistance to its outflow at the angle of the anterior chamber.
The inner layer.
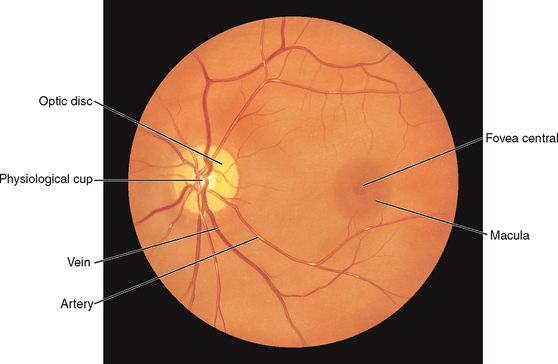
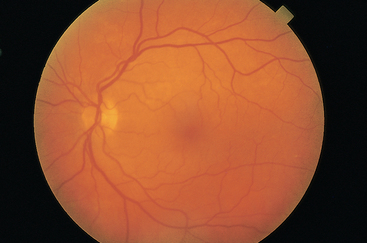
The retina is the visual receptive layer of the eye in which light waves are changed into nerve impulses. The retina surrounds the soft gelatinous vitreous humour. The retinal structures viewed through the ophthalmoscope are the optic disc, the retinal vessels, the general background and the macula (Fig 23.6).
The optic disc (or optic papilla) is the area in which fibres from the retina converge to form the optic nerve. Located towards the nasal side of the retina, it has these characteristics: a colour that varies from creamy yellow-orange to pink; a round or oval shape; margins that are distinct and sharply demarcated, especially on the temporal side; and a physiological cup, the smaller circular area inside the disc where the blood vessels exit and enter.
The retinal vessels normally include a paired artery and vein extending to each quadrant, growing progressively smaller in calibre as they reach the periphery. The arteries appear brighter red and narrower than the veins, and the arteries have a thin sliver of light on them (the arterial light reflex). The general background of the fundus varies in colour, depending on the person’s skin colour. The macula is located on the temporal side of the fundus. It is a slightly darker pigmented region surrounding the fovea centralis, the area of sharpest and keenest vision. The macula receives and transduces light from the centre of the visual field.
VISUAL PATHWAYS AND VISUAL FIELDS
Objects reflect light. The light rays are refracted through the transparent media (cornea, aqueous humour, lens and vitreous body) and strike the retina. The retina transforms the light stimulus into nerve impulses that are conducted through the optic nerve and the optic tract to the visual cortex of the occipital lobe.
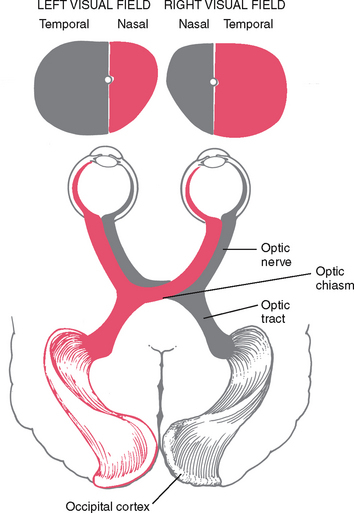
The image formed on the retina is upside down and reversed from its actual appearance in the outside world (Fig 23.7). That is, an object in the upper temporal visual field of the right eye reflects its image onto the lower nasal area of the retina. All retinal fibres collect to form the optic nerve, but they maintain this same spatial arrangement, with nasal fibres running medially and temporal fibres running laterally.
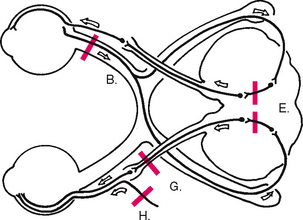
At the optic chiasm, nasal fibres (from both temporal visual fields) cross over. The left optic tract now has fibres from the left half of each retina, and the right optic tract contains fibres only from the right. Thus the right side of the brain looks at the left side of the world.
VISUAL REFLEXES
Pupillary light reflex.
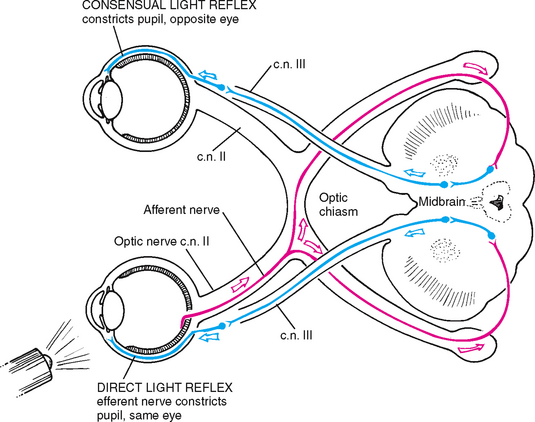
The pupillary light reflex is the normal constriction of the pupils when bright light shines on the retina (Fig 23.8). It is a subcortical reflex arc (i.e. a person has no conscious control over it); the afferent link is cranial nerve II, the optic nerve, and the efferent path is cranial III, the oculomotor nerve. When one eye is exposed to bright light, a direct light reflex occurs (constriction of that pupil) as well as a consensual light reflex (simultaneous constriction of the other pupil). This happens because the optic nerve carries the sensory afferent message in and then synapses with both sides of the brain. For example, consider the light reflex in a person who is blind in one eye. Stimulation of the normal eye produces both a direct and a consensual light reflex. Stimulation of the blind eye causes no response because the sensory afferent in cranial nerve II is destroyed.
Fixation.
This is a reflex direction of the eye towards an object attracting a person’s attention. The image is fixed in the centre of the visual field, the fovea centralis. This consists of very rapid ocular movements to put the target back on the fovea, and somewhat slower (smooth pursuit) movements to track the target and keep its image on the fovea. These ocular movements are impaired by drugs, alcohol, fatigue and inattention.
Accommodation.
This is adaptation of the eye for near vision. It is accomplished by increasing the curvature of the lens through movement of the ciliary muscles. Although the lens cannot be observed directly, the components of accommodation that can be observed are convergence (motion towards) of the axes of the eyeballs and pupillary constriction.
DEVELOPMENTAL CONSIDERATIONS
Infants and children
At birth, eye function is limited, but it matures fully during the early years. Peripheral vision is intact in the newborn infant. The macula, the area of keenest vision, is absent at birth but is developing by 4 months and is mature by 8 months. Eye movements may be poorly coordinated at birth. By 3 to 4 months of age, the infant establishes binocularity and can fixate on a single image with both eyes simultaneously. Most neonates (80%) are born farsighted; this gradually decreases after 7 to 8 years of age.
In structure, the eyeball reaches adult size by 8 years. At birth, the iris shows little pigment, and the pupils are small. The lens is nearly spherical at birth, growing flatter throughout life. Its consistency changes from that of soft plastic at birth to rigid glass in old age.
Late adulthood (65+ years)
Internationally, over 161 million people are visually impaired. Australian statistics show that common causes for blindness and vision loss are related to changes associated with ageing (Australian Institute of Health and Welfare (AIHW), 2007). It is estimated that 9.4% of the Australian population aged over 55 years have vision impairment, of which 1.2% are blind (AIHW, 2005).
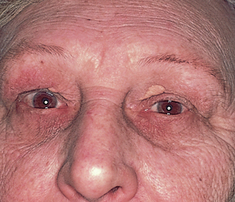
Changes in eye structure contribute greatly to the distinct facial changes of the ageing person. The skin loses its elasticity, causing wrinkling and drooping; fat tissues and muscles atrophy; and the external eye structures appear (see Fig 23.16 below). Lacrimal glands involute, causing decreased tear production and a feeling of dryness and burning.
Inside the globe, floaters appear in the vitreous as a result of debris that can accumulate because the vitreous is not continuously renewed as the aqueous humour is. Visual acuity may diminish gradually after 50 years of age, and even more so after 70 years.
On the globe itself, the cornea may show an infiltration of degenerative lipid material around the limbus. (This is known as arcus senilis; see Fig 23.17 below.) As the person grows older pupil size decreases and the lens progressively hardens and loses its ability to change shape (accommodate); this leads to a decreased ability to focus on close objects with no coinciding changes to distance vision. This is known as presbyopia. As these changes occur progressively with age, symptoms will usually manifest in the early to mid-40s (Smith, Neely and Twyford, 2008). As the adult ages the normally transparent fibres of the lens begin to thicken and yellow. This is nuclear sclerosis, or the beginning of a senile cataract. The eye also needs more light to see because of a decreased adaptation to darkness, and this condition may affect the function of night driving.
Over 90% of vision problems in the older adult are attributed to uncorrected refractive errors, cataracts, glaucoma, macular degeneration and diabetic retinopathy (AIHW, 2005). These are explained as:
1. Cataract formation, or lens opacity, resulting from a clumping of proteins in the lens. Cataract formation can be anticipated in all individuals over the age of 70. Between the ages of 65 and 74, 50% of people will have cataract formation, and in those over 75 years of age, the prevalence increases to 70% (Bradford, 2004). In Australia in 2005–06, senile cataract was responsible for 14.7% of hospital admissions for eye diseases/disorders (AIWH, 2008).
2. Glaucoma is damage to the intraocular structures caused by elevation of intraocular pressure (Wotton, 2009). This disease of the optic nerve involves loss of retinal ganglion cells that manifests in a typical pattern of optic neuropathy (AIWH, 2008). In 2004 the prevalence of glaucoma was 2.3% of the Australian population aged 55 years and over (AIHW, 2005). Chronic open-angle glaucoma, also called chronic glaucoma, is the most common type and involves a gradual loss of peripheral vision that is often unnoticed. As damage becomes permanent, symptoms such as tunnel vision are noticeable. It is worth noting that while high intraocular pressure has been associated with glaucoma, this is now considered a risk factor as opposed to a criterion for diagnosis (Glaucoma Information Sheet, 2007).
3. Age-related macular degeneration (ARMD) is the breakdown of cells in the macula of the retina, causing sudden blurred central vision with no cure available. It is the most common form of visual impairment in individuals over the age of 50. This is also the most common cause of blindness in the older adult (Wotton, 2009). Peripheral vision is not affected, so the person can manage self-care and will not become completely disabled.
4. Retinopathy refers to microvascular damage of the retina which may develop slowly or rapidly, leading to blurred vision and progressive vision loss. Retinopathy is most often associated with chronic hypertension and diabetes mellitus (Smith, Neely and Twyford 2008). Diabetic retinopathy is caused by microvascular occlusion and leakage in the small blood vessels that feed the retina (Tabandeh and Goldberg, 2009). Microvascular occlusion can be caused by increased platelet adhesion, thickening of the capillary basement membrane, abnormal proliferation of capillary endothelium, increased blood viscosity and defective fibrinolysis (Tabandeh and Goldberg, 2009). Occurrence of diabetic retinopathy is most often associated with chronic diabetes mellitus, poor glycaemic control, chronic hypertension, pregnancy, smoking and diabetic nephropathy (Smith, Neely and Twyford, 2008; Tabandeh and Goldberg, 2009). There are two types of diabetic retinopathy: non-proliferative retinopathy, the most common form, and proliferative retinopathy, the most severe form. If the condition is left untreated over 50% of individuals will experience blindness (Smith, Neely and Twyford, 2008). Eighty per cent of people who have type 2 diabetes mellitus and all of those with type 1 diabetes mellitus for over 15 years will experience a degree of retinal disease (Smith, Neely and Twyford, 2008).
CULTURAL AND SOCIAL CONSIDERATIONS
Australia and New Zealand are culturally diverse societies and the health practitioner will need to be familiar with specific variations and disease incidences that affect different cultural groups. Structural differences are evident in the palpebral fissures and epicanthic fold in individuals’ eyes. Examples of this include narrowed palpebral fissures in persons of Asian origin, which in non-Asian individuals may be diagnostic of a serious congenital anomaly like Down syndrome (see Table 23.2).
TABLE 23.2 Abnormalities in the eyelids
 |
| Periorbital oedema |
| Lids are swollen and puffy. Lid tissues are loosely connected so excess fluid is easily apparent. This occurs with local infections; crying; and systemic conditions such as congestive heart failure, renal failure, allergy, hypothyroidism (myxoedema). |
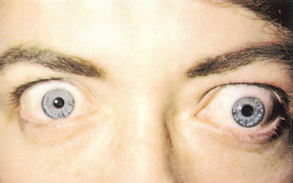 |
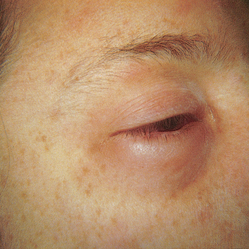
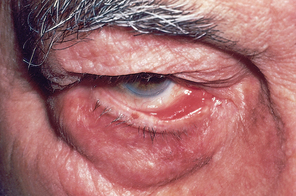
| Exophthalmos (protruding eyes) |
| Exophthalmos is a forward displacement of the eyeballs and widened palpebral fissures. Note ‘lid lag’, the upper lid rests well above the limbus and white sclera is visible. Acquired bilateral exophthalmos is associated with thyrotoxicosis. |
| Enophthalmos (sunken eyes) (not illustrated) |
| A look of narrowed palpebral fissures shows with enophthalmos, in which the eyeballs are recessed. Bilateral enophthalmos is caused by loss of fat in the orbits and occurs with dehydration and chronic wasting illnesses. |
 |
| Ptosis (drooping upper lid) |
| Ptosis occurs from neuromuscular weakness (e.g. myasthenia gravis with bilateral fatigue as the day progresses), oculomotor cranial nerve III damage or sympathetic nerve damage (e.g. Horner’s syndrome). It is a positional defect that gives the person a sleepy appearance and impairs vision. |
 |
| Upward palpebral slant |
| Although normal in many children, when combined with epicanthal folds, hypertelorism (large spacing between the eyes) and Brushfield spots (light-coloured areas in outer iris), indicates Down syndrome. |
 |
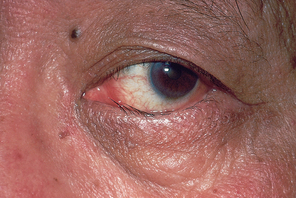
| Ectropion |
| The lower lid is loose and rolling out, does not approximate to eyeball. Puncta cannot siphon tears effectively, so excess tearing results. The eyes feel dry and itchy because the tears do not drain correctly over the corner and towards the medial canthus. Exposed palpebral conjunctiva increases risk for inflammation. Occurs in ageing as a result of atrophy of elastic and fibrous tissues but may result from trauma. |
 |
| Entropion |
| The lower lid rolls in because of spasm of lids or scar tissue contracting. Constant rubbing of lashes may irritate cornea. The person feels a ‘foreign body’ sensation. |
Variability exists in the colour of the iris and in retinal pigmentation, with darker irides having darker retinas behind them. Individuals with light retinas generally have better night vision but can have pain in an environment that has too much light.
Variations in disease according to culture and ethnicity
Specific populations within Australia and New Zealand are at higher risk for certain diseases that affect the eye. These groups include: Aboriginal and Torres Strait Islanders, Māori, the older adult, people with diabetes mellitus, those with a family history of eye disease and those who are considered marginalised and disadvantaged (Commonwealth of Australia, 2005).
Prevalence of eye disease is up to ten times higher in the Australian Indigenous community compared with the non-Indigenous community (Vision 2020 Australia, nd). Cataract, diabetic retinopathy, under-corrected refractive error and trachoma are the leading causes of blindness and vision impairment in Australian Indigenous communities (Vision 2020 Australia, nd). Trachoma within the Aboriginal community is a significant factor in ocular morbidity (Mott, 2009). Reporting eye disease is an issue in Australia with Aboriginal and Torres Strait Islander populations being three times more likely to experience vision loss due to cataracts compared to non-Indigenous Australians, and four times less likely to have cataract surgery (Vision 2020 Australia, nd). This higher rate of vision loss in these populations can be attributed to barriers to access and delays in diagnosis (Laforest, Durkin, Selva et al, 2006). Barriers to accessing eye healthcare include: health service barriers (infrastructure and systems, cost, transport and distance, interpreters, escorts, consent, trust of specialists); community (family influence, community influence, culture and beliefs); individual (ignorance, stoicism, fear, beliefs, demographics, other illnesses) (Wearne, 2007). Similarly, in the Māori and Pacific Islander populations, there are higher self-reported incidence rates of cataract and blindness (Simmons, Clover, Hope, 2007).
Over 97 000 Australians are affected by diabetic retinopathy. Retinopathy is the third highest eye disease with higher rates evident in those of Fijian, Melanesian, Māori, African and South Asian decent (Simmons, Clover and Hope, 2007). Diabetic retinopathy in Australia has a higher prevalence in Aboriginal and Torres Strait Islander populations due to higher levels of diabetes mellitus (Harvey, 2010). This is similar in the Māori population in New Zealand, who experience complications of diabetic retinopathy and nephropathy (Lim, Chellumuthi, Crook et al, 2008).
SUBJECTIVE DATA
Vision enables people to perform daily tasks and to learn about the world that surrounds them. The eye is the sensory organ of vision, which is the major source of sensory information. Disorders of the eye can affect so many parts of an individual’s functional life. Accurate, systematic assessment can identify issues and provide focus for early treatment that may lead to sight preservation.
Subjective data can lead the nurse to focus more specifically on aspects of the eye examination, particularly in identifying symptoms that will not necessarily be evident in objective assessment. Other body systems that can affect the eye which you might consider when focusing your specific assessment questions include the cardiovascular, neurovascular and neurological systems. Eye assessment can also detect abnormalities that impact on other body systems such as the musculoskeletal system. Subjective data assessment focuses on the following:
| Assessment guidelines | Clinical signi.cance and clinical alerts |
|---|---|
| 1 Vision difficulty (decreased acuity, blurring, blind spots). | |
| • Any difficulty seeing or any blurring? Does this begin suddenly or progress slowly? In one eye or both? | |
| • Do spots move in front of your eyes? Are there one or are there many? In one or both eyes? | Floaters are common with myopia or after middle age as a result of condensed vitreous fibres. Usually not significant, but acute onset of floaters (‘shade’ or ‘cobwebs’) may occur with retinal detachment. |
| • Are there any halos/rainbows around objects? Or rings around lights? | Halos around lights occur with acute narrow-angle glaucoma. |
| • Is there a blind spot? Does it move as you shift your gaze, or is it like looking through a tunnel? Any loss of peripheral vision? | Scotoma, a blind spot in the visual field surrounded by an area of normal or decreased vision, occurs with glaucoma, with optic nerve and visual pathway disorders. |
| • Is there any night blindness? | Night blindness occurs with optic atrophy, glaucoma or vitamin A deficiency. Preliminary studies are investigating the gene in Maori children that causes stationary night blindness. |
| 2 Pain. | |
| Sudden onset of eye symptoms (pain, floaters, blind spot, loss of peripheral vision) may be an emergency (such as detached retina, corneal abrasion, foreign body). Refer immediately. | |
| • Quality of pain. Is burning or itching present? Is it sharp, stabbing pain or pain with bright light? | Photophobia is inability to tolerate light. |
| • Is there a foreign body sensation? Or deep aching? Or headache in the brow area, or behind the eye? | Note: some common eye diseases cause no pain (e.g. refractive errors, cataract, glaucoma). |
| 3 Strabismus, diplopia. | |
| • Is there any history of crossed eyes at present or in the past? Does this occur with eye fatigue? | Strabismus is a deviation in the anteroposterior axis of the eye. |
| • Do you ever see double? Is this constant, or does it come and go? In one eye or both? | |
| 4 Redness, swelling. Any redness or swelling in the eyes? | |
| • Have you had any infections presently or in the past? When do/did these occur? Was this in a particular time of year or only with the use of contact lenses? | Eye infections may be a result of bacterial or viral sources. Foreign-body-introduced infections are common from contact lens use, injuries, seasonal variations (pollens) and environmental factors (e.g. swimming in contaminated water). Eye infections are very contagious and require immediate treatment by a medical practitioner. Advice should be given to the person regarding infection control measures to protect the unaffected eye and household members. |
| 5 Watering, discharge. | |
| • Have you noticed watering or excessive tearing? | Lacrimation (tearing) and epiphora (excessive tearing) are due to irritants or obstruction in drainage of tears. |
| Purulent discharge is thick and yellow. Crusts often form at night. Assess hygiene practices and knowledge of cross-contamination. More common in children or people who use contact lenses. | |
| 6 History of ocular problems. | |
| Allergens may cause irritation of conjunctiva or cornea (e.g. make-up, contact lens solution). | |
| 7 Glaucoma. | |
| Glaucoma is associated with increased intraocular pressure. Where there is a family history of glaucoma the person should be advised to have regular eye examinations. | |
| • If you have glaucoma, how do you manage your eye drops? | Compliance may be a problem if symptoms are absent. Assess ability to administer eye drops. |
| 8 Use of glasses or contact lenses. | |
| • Do you wear glasses or contact lenses? How do they work for you? How long have you needed these? | |
| • Has your prescription been stable? When was the last time your prescription was checked? Was it changed? | |
| • If you wear contact lenses, are there any problems such as pain, photophobia, watering or swelling? | |
| Assess self-care behaviours as below. Some of these questions will only need to be asked once. | |
| • Do you remove them/wear them for certain activities? | |
| 9 Self-care behaviours. | |
| • When was your last vision test? Who tested it? | |
| • Have you ever been tested for colour vision? | |
| • Are there any environmental conditions at home or at work that may affect your eyes? For example, flying sparks, metal shards, smoke, dust, chemical fumes? If so, do you wear goggles to protect your eyes? | Work-related eye disease (e.g. an auto mechanic with a foreign body from metal working or radiation damage from welding). |
| 10 Medications. | |
| Some medications have ocular side effects, e.g. prednisone may cause cataracts or increased intraocular pressure. Others can dilate or constrict the pupil thus making it difficult to accommodate changes in light. | |
| 11 Coping mechanisms for vision loss. | |
| Be alert to patients in a hospital setting or new to residential care who are vision impaired. They may require additional supports until they find their way around in new surroundings. They are at significant risk of injury. | |
| A constant spatial layout eases navigation through the home. | |
| Additional history for infants and children (Questions for parents) | |
| Additional history for the adult over 65 years | |
| • Have you noticed any visual difficulty with climbing stairs or driving or any problem with night vision? | Any loss of depth perception or central vision can be significant for age-related macular degeneration. The person should be referred to a medical practitioner. |
| • Is there a history of cataracts? Any loss or progressive blurring of vision? Have you had cataract surgery? | |
| • Do your eyes ever feel dry or burn? What do you do for this? | Decreased tear production may occur with ageing. |
| • Have you had to decrease any of your usual activities, such as reading or sewing? | Age-related macular degeneration causes a loss in central vision acuity. |
OBJECTIVE DATA
Objective data assists the nurse to complete the eye assessment by collecting verifiable data which is data gained by physical examination and any investigations. Generalist nurses do not usually perform the majority of physical assessment techniques related to eye examination. However nurses working in specialist centres, emergency departments and community settings may develop advanced skill in using more complex assessment techniques such as use of eye assessment equipment (e.g. tonometers, Snellen charts etc). Documentation of this data also takes a specific form and needs to follow a logical sequence (e.g. visual acuity documented as 6/6 means the patient can read to line 6 on the Snellen chart at 6 metres distance from the chart). Visual acuity is often the focal point of the objective data collected.
| Preparation | Equipment needed |
|---|---|
| Have person sitting comfortably in an upright position where possible. The examiner should sit or stand opposite the person so that they can maintain a straight back. | Opaque card or occluder Penlight |
| Procedures and normal. ndings | Abnormal. ndings and clinical alerts | |
|---|---|---|
| INSPECT EXTERNAL OCULAR STRUCTURES | ||
| Begin with the most external points, and logically work your way inwards. | ||
| General | ||
| Already you will have noted the person’s ability to move around the room, with vision functioning well enough to avoid obstacles and respond to your directions. Also note the facial expression, looking specifically for squinting, grimacing and so on. | Note groping with hands while walking and squinting or craning forwards. | |
| The palpebral fissures are horizontal in non-Asians, whereas Asian people normally have an upward slant to the eye. | Ptosis is drooping of upper lid. For examples of periorbital oedema and lesions see Tables 23.1 and 23.3. | |
| Note that the eyelashes are evenly distributed along the lid margins and curve outwards. | Ectropion occurs when lower lid turns outwards, entropion when the lower lid turns inwards. Both of these findings are abnormal. Both of these problems can predispose the person to corneal damage and eye infection (see Table 23.2). Xanthelasma is raised yellowish plaques on eyelids/nasal portion, often associated with lipid disorders but can be a normal finding. |
|
| Eyeballs | ||
| The eyeballs are aligned normally in their sockets with no protrusion or sunken appearance. | Exophthalmos(protruding eyes) and enophthalmos (sunken eyes) (see Table 23.2). | |
| Conjunctiva and sclera | ||
| Ask the person to look up. Using your thumbs, slide the lower lids down along the bony orbital rim. Take care not to push against the eyeball. Inspect the exposed area (Fig 23.9). The eyeball looks moist and glossy. Numerous small blood vessels normally show through the transparent conjunctiva. Otherwise, the conjunctivae are clear and show the normal colour of the structure below– pink over the lower lids and white over the sclera. Note any colour change, swelling or lesions. | Note abnormal findings such as: general reddening (see Table 23.6) cyanosis of the lower lids. Pallor near the outer canthus of the lower lid may indicate anaemia (the inner canthus normally contains less pigment). |
|
| The sclera is china white, although those with dark pigmented skin occasionally have a grey-blue or ‘muddy’ colour to the sclera. Also you normally may see small brown macules (like freckles) on the sclera, which should not be confused with foreign bodies or petechiae. | Scleral icterus is an even yellowing of the sclera extending up to the cornea, indicating jaundice. | |
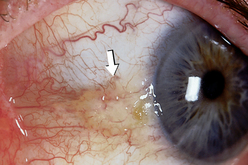
| Note any tenderness, foreign body, discharge, lesions or pinguecula (growth on conjunctiva due to chronic ultraviolet light or other environmental exposure–see Fig 23.10). | ||
| INSPECT ANTERIOR EYEBALL STRUCTURES | ||
| Cornea and lens | ||
| Shine a light from the side across the cornea, and check for smoothness and clarity. This oblique view highlights any abnormal irregularities in the corneal surface. There should be no opacities (cloudiness) in the cornea, the anterior chamber or the lens behind the pupil. Do not confuse an arcus senilis with an opacity. The arcus senilis is a normal finding in ageing persons and is illustrated below. | A corneal abrasion causes irregular ridges in reflected light, producing a shattered look to light rays (see Table 23.7). Note pterygium formation (triangular growth over the cornea and sclera commonly thought to be an extension of a pinguecula, a degenerative lesion related to ultraviolet radiation/exposure). |
|
| Iris and pupil | ||
| The iris normally appears flat, with a round regular shape and even colouration. Note the size, shape and equality of the pupils. Normally the pupils appear round, regular and of equal size in both eyes. In the adult, resting size is from 3 to 5 mm. A small number of people (5%) normally have pupils of two different sizes, which is termed anisocoria. | ||
| To test the pupillary light reflex, darken the room and ask the person to gaze into the distance. (This dilates the pupils.) Advance a light in from the side and note the response. Normally you will see (1) constriction of the same-sided pupil (a direct light reflex) and (2) simultaneous constriction of the other pupil (a consensual light reflex). | Unequal or no response to light (see Table 23.4). Clinical alert: Always advance the light in from the side to test the light reflex. If you advance from the front, the pupils will constrict to accommodate for near vision. Thus you do not know what the pure response to the light would have been. |
|
| TEST VISUAL FIELDS | ||
| Confrontation test | ||
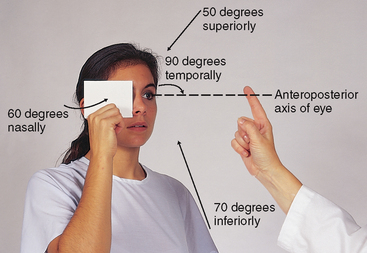
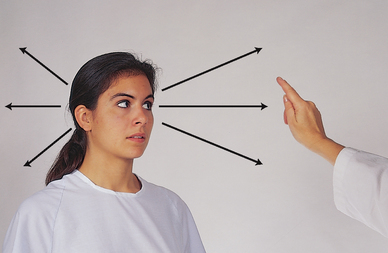
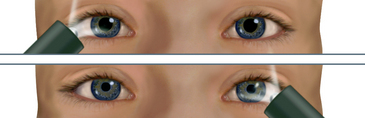
| This is a gross measure of peripheral vision. It compares the person’s peripheral vision with your own, assuming yours is normal (Fig 23.11). Position yourself at eye level with the person, about 60 cm away. Direct the person to cover one eye with an opaque card, and with the other eye to look straight at you. Cover your own eye opposite to the person’s covered one. You are testing the uncovered eye. Hold a pencil or your flicking finger as a target midline between you and the other person, and slowly advance it in from the periphery in several directions. | ||
| Ask the person to say ‘now’ as the target is first seen; this should be just as you see the object also. (This works with all but the temporal visual field, with which you would need a 1.8 m arm to avoid being seen initially! With the temporal direction, start the object somewhat behind the person.) Estimate the angle between the anteroposterior axis of the eye and the peripheral axis where the object is first seen. Normal results are about 50 degrees upwards, 90 degrees temporal, 70 degrees down and 60 degrees nasal (Fig 23.12). | Abnormalities in peripheral vision can occur following stroke or head injury or with abnormalities of the eye. (See Table 23.5) Visual field loss can lead to risk of injury due to bumping into objects or falling. |
|
| DEVELOPMENTAL CONSIDERATIONS | ||
| Infants and children (birth to 12 years) | ||
The eye examination is often deferred at birth because of transient oedema of the lids from birth trauma. The eyes should be examined within a few days and at every well child visit thereafter. Currently in Australia there is limited consistency in how and when visual screening is performed in children. This includes inconsistencies in number of vision checks recommended, age screening is undertaken, the tools and procedures used, the personnel conducting screening and referral and follow-up of the screening (Centre for Community Child Health, 2008). |
Clinical alert: prevalence of common visual conditions in Australian children is inconsistent. Amblyopia (poor or indistinct vision in an eye that is otherwise physically normal) affects 1.4–3.6% of children. Refractive error affects 1.0–14.7% (Centre for Community Child Health, 2008). |
|
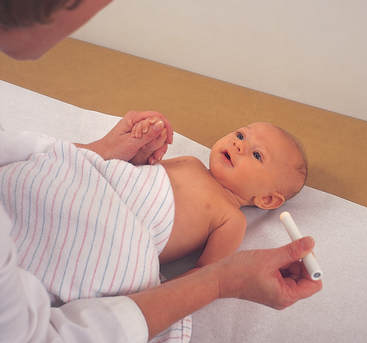
| Test light perception using the blink reflex; the neonate blinks in response to bright light (Fig 23.13). Also, the pupillary light reflex shows that the pupils constrict in response to light. These reflexes indicate that the lower portion of the visual apparatus is intact. But you cannot infer that the infant can see; that requires later observation to show that the brain has received images and can interpret them. | ||
| As you introduce an object to the infant’s line of vision, note these attending behaviours: | ||
| Birth to 2 weeks—Refusal to reopen eyes after exposure to bright light; increasing alertness to object; infant may fixate on an object. | Clinical alert: Look for absence of blinking, and pupillary light reflex, especially after 3 weeks, as this may indicate blindness. | |
| By 2 to 4 weeks—Infant can fixate on an object. | ||
| By 1 month—Infant can fixate and follow a light or bright toy. | ||
| By 3 to 4 months—Infant can fixate, follow and reach for the toy. | ||
| By 6 to 10 months—Infant can fixate and follow the toy in all directions. | Centre for Community Child Health (2008) advises that available evidence suggests screening for vision should be undertaken from 18 months of age and no later than 5 years of age. Exceptions are made for newborns with existing congenital vision conditions. | |
| External eye structures. Inspect the ocular structures as described in the earlier section. A neonate usually holds the eyes tightly shut. Do not attempt to pry them open; that just increases contraction of the orbicularis oculi muscle. Hold the newborn supine and gently lower the head; the eyes will open. Also, the eyes will open when you hold the infant at arm’s length and slowly turn the infant in one direction (Fig 23.14). In addition to inspecting the ocular structures, this also tests the vestibular function reflex. That is, the baby’s eyes will look in the same direction as the body is being turned. When the turning stops, the eyes will shift to the opposite direction after a few quick beats of nystagmus. Also termed ‘doll’s eyes’, this reflex disappears by 2 months of age. | ||
| Eyelids and lashes. Normally the upper lids overlie the superior part of the iris. In newborns the setting-sun sign is common. The eyes appear to deviate down, and you see a white rim of sclera over the iris. It may show as you rapidly change the neonate from a sitting to a supine position. | ||
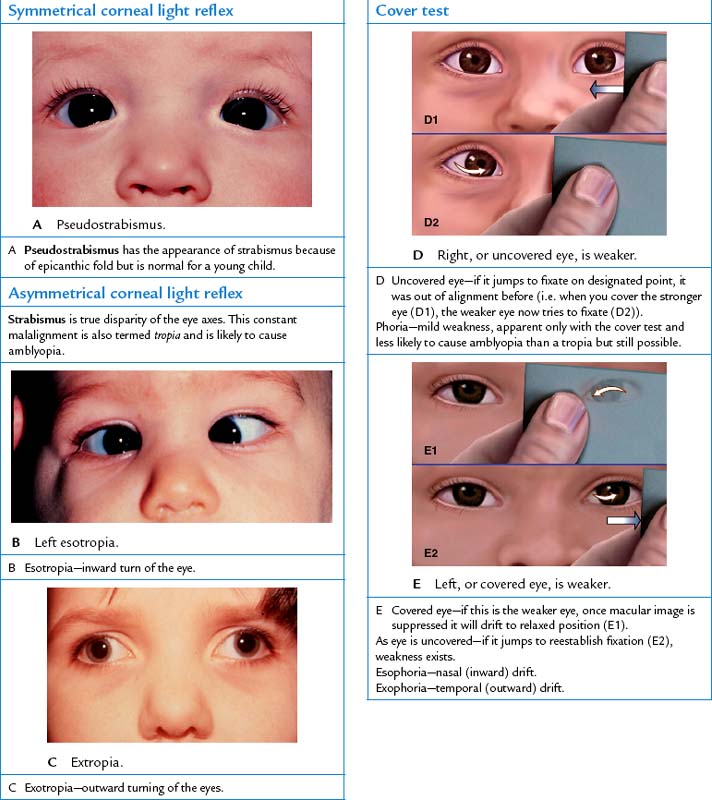
| Many infants have an epicanthal fold, an excess skinfold extending over the inner corner of the eye, partly or totally overlapping the inner canthus. It occurs frequently in children of Asian descent and in 20% of other children. In children of non-Asian descent it disappears as the child grows, usually by 10 years of age. While they are present, epicanthal folds give a false appearance of mal-alignment, termed pseudostrabismus (Fig 23.15). Yet the corneal light reflex is normal. | ||
| Infants of Asian descent normally have an upward slant of the palpebral fissures. Entropion, a turning inward of the eyelid, is found normally in some children of Asian descent. If the lashes do not abrade the corneas, it is not significant. | An upward lateral slope together with epicanthal folds and hypertelorism (large spacing between eyes) occurs with Down syndrome. | |
| Conjunctiva and sclera. The sclera should be white and clear, although it may have a blue tint as a result of thinness at birth. The lacrimal glands are not functional at birth. | Clinical alert: Ophthalmia neona-torum (conjunctivitis of the newborn) is a purulent discharge caused by a bacterial or viral agent from the birth canal. | |
| Iris and pupils. The iris normally is blue or slate grey in light-skinned newborns and brown in dark-skinned infants. By 6 to 9 months, the permanent colour is differentiated. Brushfield’s spots, or white specks around the edge of the iris, occasionally may be normal. | ||
| A searching nystagmus is common just after birth. The pupils are small but constrict to light. | Clinical alert: Abnormality: Constant nystagmus, prolonged setting-sun sign, marked strabismus and slow lateral movements suggest vision loss. | |
| Late adulthood (65+ years) | ||
| Ocular structures. The eyebrows may show a loss of the outer one-third to one-half of hair because of a decrease in hair follicles. The remaining brow hair is coarse (Fig 23.16). As a result of atrophy of elastic tissues, the skin around the eyes may show wrinkles or crow’s feet. The upper lid may be so elongated as to rest on the lashes, resulting in a pseudoptosis. | ||
| The eyes may appear sunken from atrophy of the orbital fat. Also, the orbital fat may herniate, causing bulging at the lower lids and inner third of the upper lids. | Look for ectropion (lower lid dropping away) and entropion (lower lid turning in) (see Table 23.2). | |
| The lacrimal apparatus may decrease tear production, causing the eyes to look dry and lustreless and the person to report a burning sensation.Pingueculae commonly show on the sclera (see Fig 23.12). These yellowish elevated nodules are due to a thickening of the bulbar conjunctiva from prolonged exposure to sun, wind and dust. Pingueculae appear at the 3 and 9 o’clock positions—first on the nasal side, then on the temporal side. | Distinguish pinguecula from the abnormal pterygium, also an opacity on the bulbar conjunctiva, but one that grows over the cornea (see Table 23.7). | |
| The cornea may look cloudy with age. An arcus senilis is commonly seen around the cornea (Fig 23.17). This is a grey-white arc or circle around the limbus; it is due to deposition of lipid material. As more lipid accumulates, the cornea may look thickened and raised, but the arcus has no effect on vision. | ||
| Xanthelasma are soft, raised yellow plaques occurring on the lids at the inner canthus (Fig 23.18). They commonly occur around the fifth decade of life and more frequently in women. They occur with both high and normal blood levels of cholesterol and have no pathological significance. | ||
| Pupils are small in old age, and the pupillary light reflex may be slowed. The lens loses transparency and looks opaque. | ||
FURTHER OBJECTIVE ASSESSMENT FOR ADVANCED PRACTICE
The assessments described in the following sections require advanced skill and scope of practice. Nurses working in specialist eye hospitals and clinics and some community nurses need to develop these skills.
TABLE 23.3 Lesions on the eyelids
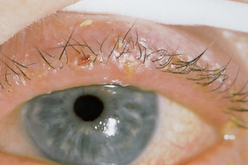 |
| Blepharitis (inflammation of the eyelids) |
| Red, scaly, greasy flakes and thickened, crusted lid margins occur with staphylococcal infection or seborrhoeic dermatitis of the lid edge. Symptoms include burning, itching, tearing, foreign body sensation and some pain. |
 |
| Chalazion |
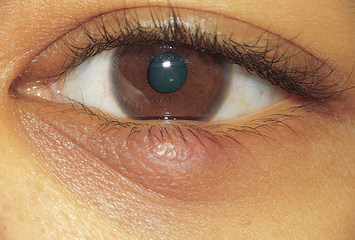
| A beady nodule protruding on the lid, chalazion is an infection or retention cyst of a meibomian gland. It is a nontender, firm, discrete swelling with freely movable skin overlying the nodule. If it becomes inflamed, it points inside and not on lid margin (in contrast with stye). |
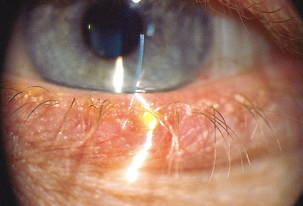 |
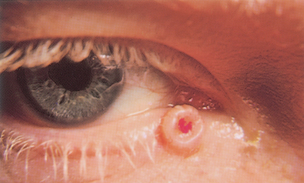
| Hordeolum (stye) |
| Hordeolum is a localised staphylococcal infection of the hair follicles at the lid margin. It is painful, red and swollen–a pustule at the lid margin. Rubbing the eyes can cause cross-contamination and development of another stye. |
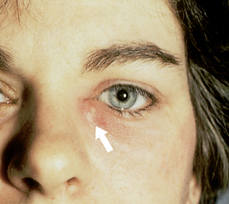 |
| Dacryocystitis (inflammation of the lacrimal sac) |
| Dacryocystitis is infection and blockage of sac and duct. Pain, warmth, redness and swelling occur below the inner canthus towards nose. Tearing is present. Pressure on sac yields purulent discharge from puncta. |
 |
| Basal cell carcinoma |
| Carcinoma is rare, but it occurs most often on the lower lid and medial canthus. It looks like a papule with an ulcerated centre. Note the rolled-out pearly edges. Metastasis is rare but should be referred for removal. |
| Dacryoadenitis (inflammation of the lacrimal gland) (not illustrated) |
| Dacryoadenitis is an infection of the lacrimal gland. Pain, swelling and redness occur in the outer third of upper lid. It occurs with mumps, measles and infectious mononucleosis, or from trauma. |
TABLE 23.4 Abnormalities in the pupil
 |
 |
| A Unequal pupil size—anisocoria |
| Although this exists normally in 5% of the population, consider central nervous system disease. |
 |
| B Monocular blindness |
| When light is directed to the blind eye, no response occurs in either eye. When light is directed to normal eye, both pupils constrict (direct and consensual response to light) as long as the oculomotor nerve is intact. |
 |
| C Constricted and fixed pupils—miosis |
| Miosis occurs with the use of pilocarpine drops for glaucoma treatment, the use of narcotics, with iritis and with brain damage of pons. |
 |
| D Dilated and fixed pupils—mydriasis |
| Enlarged pupils occur with stimulation of the sympathetic nervous system, reaction to sympathomimetic drugs, use of dilating drops, acute glaucoma, past or recent trauma. Also, they herald central nervous system injury, cardiorespiratory arrest or deep anaesthesia. |
 |
| E Argyll Robertson pupil |
| No reaction to light, pupil does constrict with accommodation. Small and irregular bilaterally. Argyll Robertson pupil occurs with central nervous system syphilis, brain tumour, meningitis and chronic alcoholism. |
 |
| F Tonic pupil (Adie’s pupil) |
| Sluggish reaction to light and accommodation. Tonic pupil is usually unilateral, a large regular pupil that does react, but sluggishly after long latent time. No pathological significance. |
 |
| G Cranial nerve III damage |
| Unilateral dilated pupil with no reaction to light or accommodation, occurs with oculomotor nerve damage. May also have ptosis with eye deviating down and laterally. |
 |
| H Horner’s syndrome |
| Unilateral, small, regular pupil does react to light and accommodation. Occurs with Horner’s syndrome, a lesion of the sympathetic nerve. Also, note ptosis and absence of sweat (anhidrosis) on same side. |
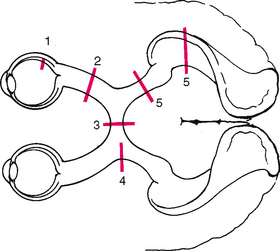 |
TABLE 23.6 Vascular disorders of the external eye
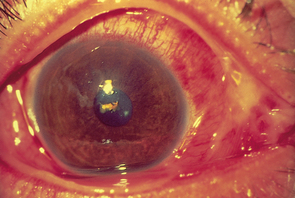 |
| Conjunctivitis |
| Infection of the conjunctiva, ‘red eye’, has red beefy-looking vessels at periphery but usually clearer around iris (although here it is severe). This is common from bacterial or viral infection, allergy or chemical irritation. Purulent discharge accompanies bacterial infection. Preauricular lymph node is often swollen and painful, with a history of upper respiratory infection. Symptoms include itching, burning, foreign body sensation and eyelids stuck together on awakening. |
 |
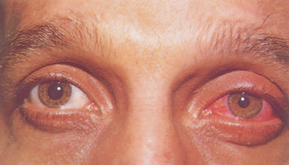
| Subconjunctival haemorrhage |
| A red patch on the sclera, subconjunctival haemorrhage looks alarming but is usually not serious. The red patch has sharp edges like a spot of paint, although here it is extensive. It occurs from increased intraocular pressure from coughing, sneezing, weight lifting, labour during childbirth, straining at stool or trauma. |
 |
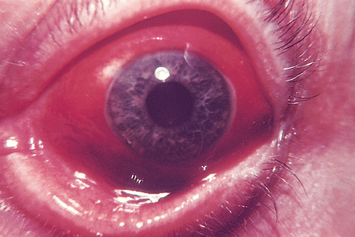
| Iritis (circumcorneal redness) |
| Deep dull red halo around the iris and cornea. Note red is around iris, in contrast with conjunctivitis, in which redness is more prominent at the periphery. Pupil shape may be irregular from swelling of iris. Person also has marked photophobia, constricted pupil, blurred vision and throbbing pain. Requires immediate medical intervention. |
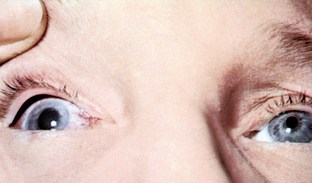 |
| Acute glaucoma |
| Acute, narrow-angle glaucoma shows a circumcorneal redness around the iris, with a dilated pupil. Pupil is oval, dilated; cornea looks ‘steamy’; and anterior chamber is shallow. Acute glaucoma occurs with sudden increase in intraocular pressure from blocked outflow from anterior chamber. The person experiences a sudden clouding of vision, sudden eye pain and halos around lights. This requires emergency treatment to avoid permanent vision loss. |
TABLE 23.7 Abnormalities on the cornea and iris
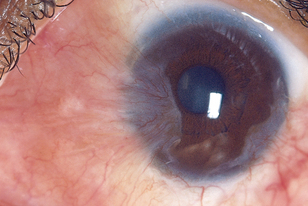 |
| Pterygium |
| A triangular opaque wing of bulbar conjunctiva overgrows towards the centre of the cornea. It looks membranous, translucent and yellow to white, usually invades from nasal side and it may obstruct vision as it covers pupil. Occurs usually from chronic exposure to hot, dry, sandy climate, which stimulates the growth of a pinguecula (see above) into a pterygium. |
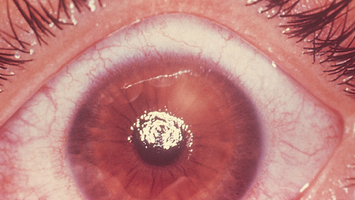 |
| Corneal abrasion |
| This is the most common result of a blunt eye injury, but irregular ridges usually visible only when fluorescein stain reveals yellow-green branching. Top layer of corneal epithelium removed, from scratches or poorly fitting or overworn contact lenses. Because the area is rich in nerve endings, the person feels intense pain, a foreign body sensation and lacrimation, redness and photophobia. |
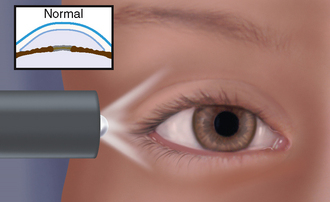 |
| Normal anterior chamber (for contrast) |
| A light directed across the eye from the temporal side illuminates the entire iris evenly because the normal iris is flat and creates no shadow. |
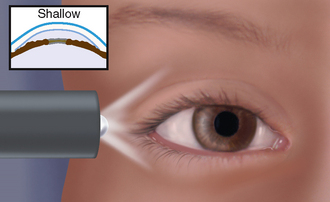 |
| Shallow anterior chamber |
| The iris is pushed anteriorly because of increased intraocular pressure. Because direct light is received from the temporal side, only the temporal part of iris is illuminated; the nasal side is shadowed, the ‘shadow sign’. This may be a sign of acute angle-closure glaucoma; the iris looks bulging because aqueous humour cannot circulate. |
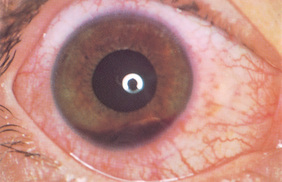 |
| Hyphaema |
| Blood in anterior chamber is a serious result of blunt trauma (a fist or a tennis ball) or spontaneous haemorrhage. Suspect scleral rupture or major intraocular trauma. Note that gravity settles blood. |
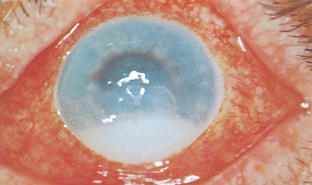 |
| Hypopyon |
| Purulent matter in anterior chamber occurs with iritis and with inflammation in the anterior chamber. |
| Preparation | Equipment needed |
| The person should be positioned at the distance specified from the eye chart (e.g. 3 m or 6 m) |
| Procedures and normal. ndings | Abnormal. ndings and clinical alerts | |
|---|---|---|
| Inspect extraocular muscle function | ||
| Corneal light reflex (the Hirschberg test) | ||
| Assess the parallel alignment of the eye axes by shining a light towards the person’s eyes. Direct the person to stare straight ahead as you hold the light about 30 cm away. Note the reflection of the light on the corneas; it should be in exactly the same spot on each eye. See the bright white dots in Fig 23.15 for symmetry of the corneal light reflex. | Asymmetry of the light reflex indicates deviation in alignment from eye muscle weakness or paralysis. If you see this, perform the cover test. | |
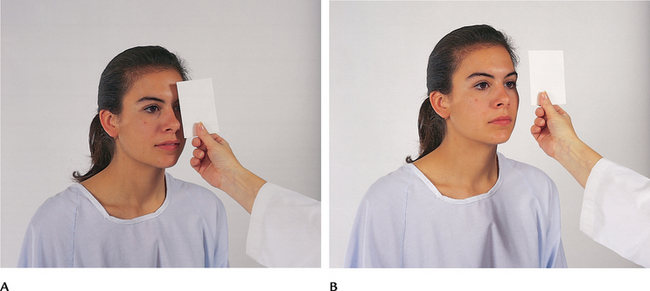
| Cover test | ||
| This test detects small degrees of deviated alignment by interrupting the fusion reflex that normally keeps the two eyes parallel. Ask the person to stare straight ahead at your nose even though the gaze may be interrupted. With an opaque card, cover one eye. As it is covered, note the uncovered eye. A normal response is a steady fixed gaze (Fig 23.19, A). | ||
| Now uncover the eye and observe it for movement. It should stare straight ahead (Fig 23.19, B). If it jumps to reestablish fixation, eye muscle weakness exists. Repeat with the other eye. | A phoria is a mild weakness noted only when fusion is blocked. Tropia is more severe—a constant malalignment of the eyes (see Table 23.1). | |
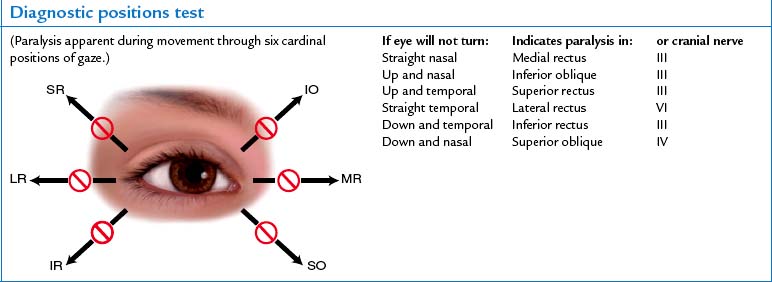
| Diagnostic positions test | ||
| Leading the eyes through the six cardinal positions of gaze will elicit any muscle weakness during movement (Fig 23.20). Ask the person to hold the head steady and to follow the movement of your finger, pen or penlight only with the eyes. Hold the target back about 30 cm so the person can focus on it comfortably, and move it to each of the six positions, hold it momentarily, then back to centre. Progress clockwise. A normal response is parallel tracking of the object with both eyes. | When eye movement is not parallel there is an abnormality. Failure to follow in a certain direction indicates weakness of an extraocular muscle (EOM) or dysfunction of the cranial nerve innervating it (see cranial nerves for each direction in Fig 23.4). | |
| In addition to parallel movement, note any nystagmus, a fine oscillating movement best seen around the iris. Mild nystagmus at extreme lateral gaze is normal; nystagmus at any other position is not. | ||
| Finally, note that the upper eyelid continues to overlap the superior part of the iris, even during downward movement. You should not see a white rim of sclera between the lid and the iris. If noted, this is termed ‘lid lag’. | Lid lag occurs with hyperthyroidism. | |
| Test central visual acuity | ||
| Clinical alert: Visual acuity of 6/6 does not exclude a serious eye condition. | ||
| Snellen eye chart | ||
| The Snellen alphabet chart is the most commonly used and accurate measure of visual acuity. It has lines of letters arranged in decreasing size. | Clinical alert: Ensure that no pressure is applied to the ocular surface during testing. | |
| Place the Snellen alphabet chart in a well-lit spot at eye level. Position the person on a mark exactly 6 m from the chart. Hand the person an opaque card with which to shield one eye at a time during the test; inadvertent peeking may result when shielding the eye with the person’s own fingers (Fig 23.21). If the person wears glasses or contact lenses, leave them on. Remove only reading glasses because they will blur distance vision. Ask the person to read through the chart to the smallest line of letters possible. Encourage the person to try the next smallest line also. (Note: Use a Snellen picture chart for people who cannot read letters.) | Note hesitancy, squinting, leaning forwards, misreading letters. | |
| Visual acuity is a ratio recorded as a fraction. This is not a percentage of vision, but a measurement that is affected by the eye chart that is used (e.g. 6 m or 3 m chart). The ratio = recorded distance measured/smallest line that the person can read the majority of (Sehu and Eye Emergency Manual Steering Committee, 2009). | ||
| Normal visual acuity is 6/6 using a 6 m chart and means ‘You can read at 6 m what the normal eye could have read at 6 m’. | Impaired vision may be due to refractive error, opacity in the media (cornea, lens, vitreous) or disorder in the retina or optic pathway. | |
| If the person is unable to see even the largest letters, shorten the distance to the chart until it is seen and record that distance (e.g. ‘4/60’). If visual acuity is even lower, assess whether the person can count your fingers when they are spread in front of the eyes or distinguish light perception from your penlight. | ||
| Pinhole test | ||
A pinhole test is a test performed on a person who has diminished visual acuity to distinguish a refractive error caused by organic disease. Use a pinhole occluder (solid occluder with multiple 19 gauge needle holes over the eye) to test visual acuity if visual acuity is reduced in the above testing (i.e. using Snellen chart). Test each eye as above but note that the pinholes are used to look through. |
A test using the pinhole mask can identify those people with poor vision who may need spectacles to improve their vision. The mask has very small holes in the area in front of the pupil. People who have visual acuity improved with the pinhole should be referred for examination and treatment by an eye care practitioner as their visual acuity is due to refractive error rather than a disease process. | |
| Near vision | ||
For people over 40 years of age or for those who report increasing difficulty reading, test near vision with a handheld vision screener with various sizes of print (e.g. a Jaeger card) (Fig 23.22). Hold the card in good light about 35 cm from the eye. Test each eye separately with glasses on. Record the visual acuity that relates to the smallest line of print the person reads comfortably. A normal result is J1, meaning the person can read 4-point type at 35 cm. Moderately impaired = J10. This means that 14-point type can be read at 35 cm. The person should be able to read without hesitancy and without moving the card closer or further away. |
||
| Inspect external ocular structures | ||
| Eversion of the upper lid | ||
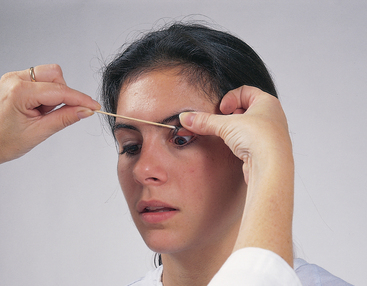
This manoeuvre is not part of the normal examination, but it is useful when you must inspect the conjunctiva of the upper lid, as with eye pain or suspicion of a foreign body. Most people are apprehensive of any eye manipulation. Enhance their cooperation by using a calm and gentle, yet deliberate, approach. 1 Ask the person to keep both eyes open and look down. This relaxes the eyelid, whereas closing it would tense the orbicularis muscle. 2 Slide the upper lid up along the bony orbit to lift up the eyelashes. 3 Grasp the lashes between your thumb and forefinger and gently pull down and outwards. 4 With your other hand, place the tip of an applicator stick on the upper lid above the level of the internal tarsal plates (Fig 23.23). |
||
| 5 Gently push down with the stick as you lift the lashes up. This uses the edge of the tarsal plate as a fulcrum and flips the lid inside out. Take special care not to push in on the eyeball. | ||
| 6 Secure the everted position by holding the lashes against the bony orbital rim (Fig 23.24). | ||
| 7 Inspect for any colour change, swelling, lesion or foreign body. | ||
| 8 To return to normal position, gently pull the lashes outwards as the person looks up. | ||
| Lacrimal apparatus | ||
| Ask the person to look down. With your thumbs, slide the outer part of the upper lid up along the bony orbit to expose under the lid. Inspect for any redness or swelling. | Swelling of the lacrimal gland may show as a visible bulge in the outer part of the upper lid. | |
| Normally the puncta drain the tears into the lacrimal sac. Presence of excessive tearing may indicate blockage of the nasolacrimal duct. Check this by pressing the index finger against the sac, just inside the lower orbital rim, not against the side of the nose (Fig 23.25). Pressure will slightly evert the lower lid, but there should be no other response to pressure. | ||
| Inspect anterior eyeball structures | ||
| Test for accommodation by asking the person to focus on a distant object (Fig 23.26). This process dilates the pupils. Then have the person shift the gaze to a near object, such as your finger held about 7 to 8 cm from the nose. A normal response includes (1) pupillary constriction and (2) convergence of the axes of the eyes. | Note any absence of constriction or convergence or an asymmetric response. | |
| Inspect the ocular fundus | ||
The ophthalmoscope enlarges your view of the eye so that you can inspect the media (anterior chamber, lens, vitreous) and the ocular fundus (the internal surface of the retina). It accomplishes this by directing a beam of light through the pupil to illuminate the inner structures. The ophthalmoscope should function as an appendage of your own eye. This takes some practice. Practise holding the instrument and focusing at objects around the room before you approach a ‘real’ person. |
Hold the ophthalmoscope right up to your eye, braced firmly against the cheek and brow. Extend your index finger onto the lens selector dial so that you can refocus as needed during the procedure without taking your head away from the ophthalmoscope to look. Now look about the room, moving your head and the instrument together as one unit. Keep both your eyes open; just view the field through the ophthalmoscope. | |
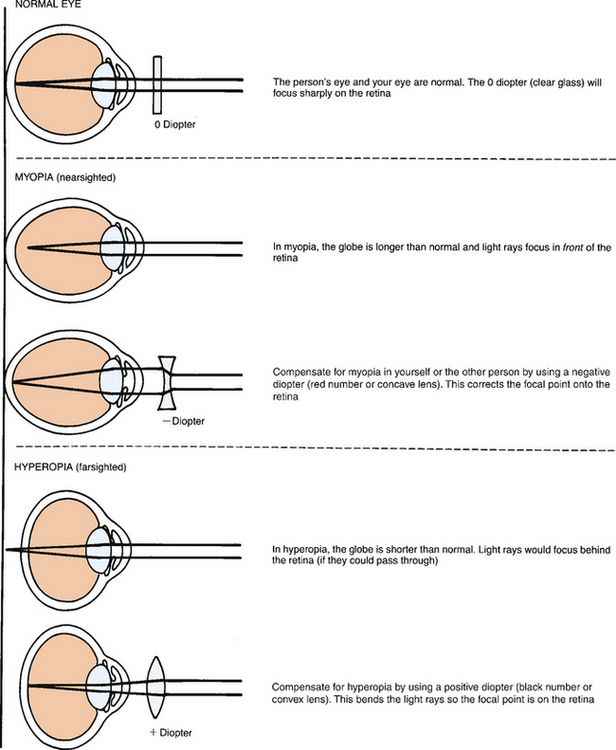
| The ophthalmoscope contains a set of lenses that control the focus (Fig 23.27). The unit of strength of each lens is the diopter. The black numbers indicate a positive diopter; they focus on objects nearer in space to the ophthalmoscope. The red numbers show a negative diopter and are for focusing on objects further away. | ||
| To examine a person, darken the room to help dilate the pupils. Remove eyeglasses from yourself or the other person; they obstruct close movement and you can compensate for their correction by using the diopter setting. Contact lenses may be left in; they pose no problem as long as they are clean. | Clinical alert: Dilating eye drops are not needed during a screening examination. When indicated, they dilate the pupils for a wider look at the fundus background and macular area. Eye drops are used only when glaucoma can be completely ruled out, because dilating the pupils in the presence of glaucoma can precipitate an acute episode. | |
| Select the large round aperture with the white light for the routine examination. If the pupils are small, use the smaller white light. The light must have maximum brightness; replace old or dim batteries. | Although the instrument has other shape and coloured apertures, these are rarely used in a screening examination. | |
| Tell the person, ‘Please keep looking at that light switch (or mark) on the wall across the room, even though my head will get in the way’. | Staring at a distant fixed object helps to dilate the pupils and to hold the retinal structures still. | |
| Match sides with the person. That is, hold the ophthalmoscope in your right hand up to your right eye to view the person’s right eye. You must do this to avoid bumping noses during the procedure. Place your free hand on the person’s shoulder or forehead (Fig 23.28, A). This helps orient you in space, because once you have the ophthalmoscope in position, you have only a very narrow range of vision. Also, your thumb can anchor the upper lid and help prevent blinking. | ||
| Begin about 25 cm away from the person at an angle about 15 degrees lateral to the person’s line of vision. Note the red glow filling the person’s pupil. This is the red reflex, caused by the reflection of your ophthalmoscope light off the inner retina. | Keep sight of the red reflex, and steadily move closer to the eye. If you lose the red reflex, the light has wandered off the pupil and onto the iris or sclera. Adjust your angle to find it again. | |
| As you advance, adjust the lens to +6 and note any opacities in the media. These appear as dark shadows or black dots interrupting the red reflex. Normally, none are present. | Cataracts appear as opaque black areas against the red reflex (see Table 23.8). | |
| Progress towards the person until your foreheads almost touch (Fig 23.28, B). Adjust the diopter setting to bring the ocular fundus into sharp focus. If you and the person have normal vision, this should be at 0. Moving the diopters compensates for nearsightedness or farsightedness. Use the red lenses for nearsighted eyes and the black for farsighted eyes (Fig 23.29). | ||
| Moving in on the 15-degree lateral line should bring your view just to the optic disc. If the disc is not in sight, track a blood vessel as it grows larger and it will lead you to the disc. Systematically inspect the structures in the ocular fundus: (1) optic disc, (2) retinal vessels, (3) general background and (4) macula (Fig 23.30). | Note the illustration here shows a large area of the fundus. Your actual view through the ophthalmoscope is much smaller–slightly larger than 1 disc diameter. | |
| Optic disc | ||
| The most prominent landmark is the optic disc, located on the nasal side of the retina. Explore these characteristics: | ||
| 1 Colour | Creamy yellow-orange to pink. | Note pallor, hyperaemia (excess of blood flow to the area). |
| 2 Shape | Round or oval. | Irregular shape. |
| 3 Margins | Distinct and sharply demarcated, although the nasal edge may be slightly fuzzy. | Look for blurred margins. |
| 4 Cup-disc ratio | Distinctness varies. When visible, physiological cup is a brighter yellow-white than rest of the disc. Its width is not more than one-half the disc diameter. | Cup extending to the disc border (see Table 23.9). |
| Two normal variations may occur around the disc margins. A scleral crescent is a grey-white new moon shape (Fig 23.31). It occurs when pigment is absent in the choroid layer and you are looking directly at the sclera. A pigment crescent is black; it is due to accumulation of pigment in the choroid. | ||
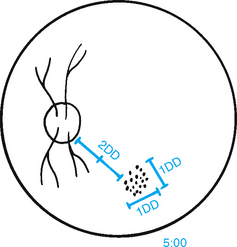
| The diameter of the disc, or DD, is a standard of measure for other fundus structures (Fig 23.32). To describe a finding, note its clock-face position as well as its relationship to the disc in size and distance (e.g. ‘… at 5:00, 3 DD from the disc’). | ||
| Retinal vessels | ||
| This is the only place in the body where you can view blood vessels directly. Follow a paired artery and vein out to the periphery in the four quadrants (see Fig 23.30), noting these points: | Clinical alert: Many systemic diseases that affect the vascular system show signs in the retinal vessels. | |
| 1 Number | A paired artery and vein pass to each quadrant. Vessels look straighter at the nasal side. | Absence of major vessels. |
| 2 Colour | Arteries are brighter red than veins. Also, they have the arterial light reflex, with a thin stripe of light down the middle. | |
| 3 A:V ratio | The ratio comparing the artery-to-vein width is 2:3 or 4:5. | Arteries too constricted. Veins dilated. |
| 4 Calibre | Arteries and veins show a regular decrease in calibre as they extend to periphery. | Focal constriction. Neovascularisation. |
| 5 A-V (arterio-venous) crossing | An artery and vein may cross paths. This is not significant if within 2 DD of disc and if no sign of interruption in blood flow is seen. There should be no indenting or displacing of vessel. | Crossings more than 2 DD away from disc. Nicking or pinching of underlying vessel. Vessel engorged peripheral to crossing (see Table 23.10). |
| 6 Tortuosity | Mild vessel twisting when present in both eyes is usually congenital and not significant. | Extreme tortuosity or marked asymmetry in two eyes. |
| 7 Pulsations | Present in veins near disc as their drainage meets the intermittent pressure of arterial systole. (Often hard to see.) | Absent pulsations. |
| General background of the fundus | ||
| The colour normally varies from light red to dark brown-red, generally corresponding with the person’s skin colour. Your view of the fundus should be clear; no lesions should obstruct the retinal structures. | Abnormal lesions: haemorrhages, exudates, microaneurysms. | |
| Macula | ||
| The macula is 1 DD in size and located 2 DD temporal to the disc. Inspect this area last in the funduscopic examination. A bright light on this area of central vision causes some watering and discomfort and pupillary constriction. Note that the normal colour of the area is somewhat darker than the rest of the fundus but is even and homogeneous. Clumped pigment may occur with ageing. | Clumped pigment occurs with trauma or retinal detachment. | |
| Within the macula, you may note the foveal light reflex. This is a tiny white glistening dot reflecting your ophthalmoscope light. | Haemorrhage or exudate in the macula occurs with senile macular degeneration. | |
| ADVANCED PRACTICE SKILLS FOR INFANTS AND CHILDREN (BIRTH TO 12 YEARS) | ||
| Visual fields. Assess peripheral vision with the confrontation test in children older than 3 years when the preschooler is able to stay in position. As with the adult, the child should see the moving target at the same time as your normal eyes do. Often a young child forgets to say ‘now’ or ‘stop’ as the moving object is seen. Rather, note the instant the child’s eyes deviate or head shifts position to gaze at the moving object. Match this nearly automatic response with your own sighting. | Usually only performed by advance practice nurses (see section below regarding advanced eye examination). | |
| Extraocular muscle function. Testing for strabismus (squint, crossed eye) is an important screening measure during early childhood. Strabismus causes disconjugate vision because one eye deviates off the fixation point. To avoid diplopia or unclear images, the brain begins to suppress data from the weak eye (a suppression scotoma). Then visual acuity in this otherwise normal eye begins to deteriorate from disuse. Early recognition and treatment are essential to restore binocular vision. Diagnosis after 6 years of age has a poor prognosis. Test malalignment by the corneal light reflex and the cover test. | Untreated strabismus can lead to permanent visual damage and can impact on a child’s general development and learning. The resulting loss of vision from disuse is amblyopia ex anopsia. | |
| Check the corneal light reflex by shining a light towards the child’s eyes. The light should be reflected at exactly the same spot in the two corneas (see Fig 23.15). Some asymmetry (where one light falls off centre) under 6 months of age is normal. | Asymmetry in the corneal light reflex after 6 months is abnormal and must be referred. | |
| Perform the cover test on all children as described above. Some examiners omit the opaque card and place a hand on the child’s head. The examiner’s thumb extends down and blocks vision over the eye without actually touching the eye. You can use a familiar character puppet to attract the child’s attention. The normal results are the same as those listed in the adult section. | ||
| Function of the extraocular muscles during movement can be assessed during the early weeks by the child following a brightly coloured toy as a target. An older infant can sit on the parent’s lap as you move the toy in all directions. After 2 years of age, direct the child’s gaze through the six cardinal positions of gaze. You may stabilise the child’s chin with your hand to prevent them from moving the entire head. | ||
Visual acuity. The child’s age determines the screening measures used. With a newborn, test visual reflexes and attending behaviours. Until children reach school age, naming letters on visual acuity charts such as the Snellen chart is usually not possible. Testing may include: • Nonsense symbol recognitions. A study in 1986 in Australia showed that the linear illiterate E test was the test of choice for young children aged 3–5 years. The test that children found most enjoyable is the Stycar test and the Lighthouse test most useful for the frightened/uncooperative child (Livanes, Greaves and Bevan, 1986). The Stycar test can also be used with children with extreme visual acuity deficits. This test uses sets of large white letters and other symbols on black grounds for the child to name, copy or match. The Lighthouse test uses standardised symbols for visual testing; these are presented on cards at 40 cm distance. The letter size progression is indicated beside the shapes. |
Clinical alert: At-risk groups for further eye problems include those born prematurely, those with multiple disabilities and children in remote Indigenous communities. For the children in remote communities education and health assessment needs to be tailored towards detection and prevention of trachoma (Centre for Community Child Health, 2008). | |
| However, the Sheridan-Gardiner Test remains the gold standard for testing visual acuity in children who cannot yet read or those who are illiterate (Centre for Community Child Health, 2008). This test is an evolution of the Stycar letter test. The Sheridan-Gardiner test is conducted by providing the child with a seven-letter card and the examiner with a set of single letter cards corresponding in size to Snellen letters. The child is then asked to point on their card to the letter shown to them by the examiner. For children above 3 years of age, the examiner may use cards containing lines of letters. | ||
| Colour vision. Colour blindness (red–green) is an inherited recessive X-linked trait affecting about 8% of males and 0.4% of females. Only 5% of people who are colour blind have blue colour blindness—for these people this is not inherited but a change in the chromosome during development. In assessment this may be identified by children not being able to recognise different colours and separate items by colour. | Clinical alert: ‘Colour deficient’ is a more accurate term, because the condition is relative and not disabling. Often, it is just a social inconvenience, although it may affect the person’s ability to discern traffic lights, or it may affect school performance when colour is a learning tool. | |
| The ocular fundus. The amount of data gathered during the funduscopic examination depends on the child’s ability to hold the eyes still and on your ability to glean as much data as possible in a brief period of time. | ||
A complete funduscopic examination is difficult to perform on an infant, but the red reflex could be checked when the infant fixates at the bright light for a few seconds. Note any interruption. Position the infant (up to 18 months) lying on the table. The fundus appears pale, and the vessels are not fully developed. There is no foveal light reflection because the macula area will not be mature until 1 year. Inspect the fundus of the young child and school-age child as described in the preceding section on the adult. Allow the child to handle the equipment. Explain why you are darkening the room and that you will leave a small light on. Assure the child that the procedure will not hurt. Direct the young child to look at an appealing picture, perhaps a toy or an animal, during the examination. |
Clinical alert: An interruption in the red reflex indicates an opacity in the cornea or lens. An absent red reflex occurs with congenital cataracts or retinal disorders. Papilloedema is rare in the infant because the fontanels and open sutures will absorb any increased intracranial pressure if it occurs. |
|
| ADVANCED PRACTICE SKILLS FOR THE ADULT OVER 65 YEARS | ||
| Visual acuity. Perform the same examination as described in the adult section. Central acuity may decrease, particularly after 70 years of age. Peripheral vision may be diminished. | ||
| The ocular fundus. Retinal structures generally have less shine. The blood vessels look paler, narrower and attenuated. Arterioles appear paler and straighter, with a narrower light reflex. More arteriovenous crossing defects occur. | ||
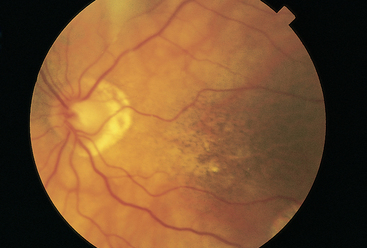
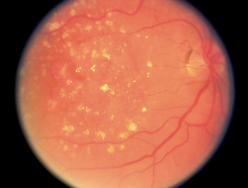
| A normal development on the retinal surface is drusen, or benign degenerative hyaline deposits (Fig 23.33). They are small, round, yellow dots that are scattered haphazardly on the retina. Although they do not occur in a pattern, they are usually symmetrically placed in the two eyes. They have no effect on vision. | Drusen are easily confused with the abnormal finding hard exudates, which occur with a more circular or linear pattern (see Table 23.10). Also, drusen in | |
| the macular area occur with age-related macular degeneration. | ||
TABLE 23.8 Opacities in the lens
| Senile cataracts |
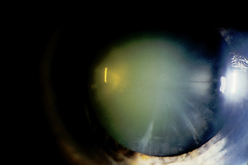 |
| Central grey opacity—nuclear cataract |
| Nuclear cataract shows as an opaque grey surrounded by black background as it forms in the centre of lens nucleus. Through the ophthalmoscope, it looks like a black centre against the red reflex. It begins after age 40 years and develops slowly, gradually obstructing vision. |
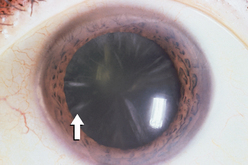 |
| Star-shaped opacity—cortical cataract |
| Cortical cataract shows as asymmetrical, radial, white spokes with black centre. Through ophthalmoscope, black spokes are evident against the red reflex. This forms in outer cortex of lens, progressing faster than nuclear cataract. |
TABLE 23.9 Abnormalities in the optic disc
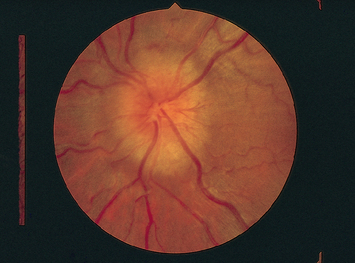 |
| Optic atrophy (disc pallor) |
| Optic atrophy is a white or grey colour of the disc as a result of partial or complete death of the optic nerve. This results in decreased visual acuity, decreased colour vision and decreased contrast sensitivity. |
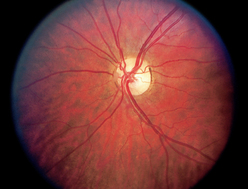 |
| Papilloedema |
| Increased intracranial pressure causes venous stasis in the globe, showing redness, congestion and elevation of the disc; blurred margins; haemorrhages; and absent venous pulsations. This is a serious sign of intracranial pressure, usually caused by a space-occupying mass (e.g. a brain tumour or haematoma). Visual acuity is not affected. |
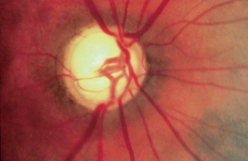 |
| Excessive cup–disc ratio |
| With primary, open-angle glaucoma, the increased intraocular pressure decreases blood supply to retinal structures. The physiological cup enlarges to more than half of the disc diameter, vessels appear to plunge over edge of cup and the vessels are displaced nasally. This is asymptomatic, although the person may have decreased vision or visual field defects in the late stages of glaucoma. |
TABLE 23.10 Abnormalities in retinal vessels and background
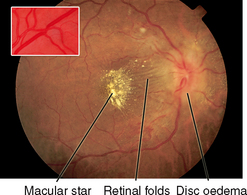 |
| Arteriovenous crossing (nicking) |
| Arteriovenous crossing with interruption of blood flow. When vein is occluded, it dilates distal to crossing. This person also has disc oedema and hard exudates in a macular star pattern that occur with acutely elevated (malignant) hypertension. With hypertension, the arteriole wall thickens and becomes opaque so that no blood is seen inside it (silverwire arteries). |
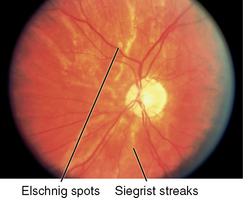 |
| Narrowed (attenuated) arteries |
| This is a generalised decrease in diameter. The light reflex also narrows. It occurs with severe hypertension (shown here) and with occlusion of central retinal artery and retinitis pigmentosa. |
| Vessel nicking |
| Nicking is a localised narrowing in vein caused by arteriole crossing. It is seen with hypertension and atherosclerosis. |
| Diabetic retinopathy |
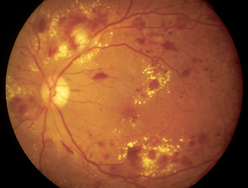 |
| Microaneurysms |
| Microaneurysms are round punctate red dots that are localised dilatations of a small vessel. Their edges are smooth and discrete. The vessel itself is too small to view with the ophthalmoscope; only the isolated red dots are seen. This occurs with diabetes. |
| Intraretinal haemorrhages |
| Dot-shaped haemorrhages are deep intraretinal haemorrhages that look splattered on. They may be distinguished from microaneurysms by the blurred irregular edges. Flame-shaped haemorrhages are superficial retinal haemorrhages that look linear and spindle shaped. They occur with hypertension. |
| Exudates |
| Soft exudates or ‘cotton wool-like’ areas. They are arteriolar microinfarctions that envelop and obscure the vessels. They occur with diabetes, hypertension, subacute bacterial endocarditis, lupus and papilloedema of any cause. Hard exudates are numerous small yellow-white spots, having distinct edges and a smooth, solid-looking surface. They often form a circular pattern, clustered around a venous microinfarction. They also may form a linear or star pattern. (This is in contrast with drusen, which have a scattered haphazard location (see Fig 23.33).) |
Promoting a healthy lifestyle Screening for glaucoma
Preventing glaucoma
Glaucoma is one of the leading cause of preventable blindness in Australia (2.3%—AIWH, 2005) and New Zealand. Glaucoma is a condition in which the optic nerve is damaged, usually as a result of increasing pressure within the eye. In Australia, New Zealand and the United States glaucoma is the second leading cause of permanent blindness. The risk of glaucoma increases with age, but it can occur in anyone in any age group. Glaucoma is not curable and an individual’s vision, once lost, cannot be regained. However, with medication or surgery, it is possible to halt further loss of vision. Screening for glaucoma is the first step in early diagnosis and intervention. However, many individuals do not realise they are at risk and never think about being screened. Screening for glaucoma is part of the comprehensive adult medical eye evaluation, starting at age 20 years with subsequent frequency depending on an individual’s age and other risk factors. Risk factors for glaucoma include:
2. Increased intraocular pressure (IOP) above 21 mmHg
3. Family history of glaucoma (hereditary)—for those with a family history the age of higher risk reduces to 40 years and over
4. Severe myopia (nearsightedness)
5. Conditions such as diabetes and hypertension (therefore many Australian/New Zealand Indigenous communities are at higher risk due to the high prevalence of these diseases)
8. Thin central cornea thickness
9. Use of certain other medications, including antihypertensives, antihistamines, anticholinergics and antidepressants.
There are two main types of glaucoma. To understand the difference, it is helpful to review the basic structure of the eye. The anterior and posterior chambers of the eye are filled with fluid called aqueous humour. This fluid is produced in the posterior chamber and then passes into the anterior chamber through the pupil. The fluid moves out of the eye and into the bloodstream through a drainage area located in front of the iris. This drainage area is located in the angle formed between the iris and the point at which the iris appears to meet the inside of the cornea. If the flow of aqueous humour is blocked, intraocular pressure increases. This pressure eventually damages the optic nerve and results in a permanent loss of vision because, in the retina, neurons are not regenerated once they are lost.
Open-angle glaucoma is the most common type of glaucoma, accounting for more than 90% of all cases. With open-angle glaucoma, the angle between the iris and the cornea is open, but the fluid is slow to drain, creating a build-up of pressure. There are virtually no symptoms. Vision loss begins with the peripheral vision, which often goes unnoticed because individuals learn to compensate intuitively by turning their heads. As the condition progresses, individuals have a decreasing field of peripheral vision until eventually they may not be able to see anything to either side. This is referred to as tunnel vision.
Closed-angle glaucoma occurs when the space between the cornea and iris is narrower than normal. Anything that causes the pupil to dilate can block the drainage of fluid, such as dim light, eye drops or certain medications. Ageing and injury also contribute to and can block the drainage of fluid. Closed-angle glaucoma causes sudden attacks of increased pressure that result in blurred vision, sensitivity to light, nausea and halos around lights. These individuals need to be treated immediately.
Regular comprehensive eye examinations are extremely important because glaucoma does not have symptoms in its early stages. These examinations should include:
Although those individuals who wear glasses appear to have more regular eye examinations, healthcare providers need to remind all individuals of the risk for glaucoma and the need for regular comprehensive eye examinations (Smith, Neely and Twyford, 2008).
DOCUMENTATION AND CRITICAL THINKING
FOCUSED ASSESSMENT: CLINICAL CASE STUDY: BLUNT TRAUMA
Emma is a 34-year-old married female brought to the emergency department by police after a reported domestic quarrel.
Subjective
States husband struck her about the face and eyes with his fists about 1 hour before presenting to the emergency department. ‘He didn’t like what I cooked for dinner. I can’t do anything right’. Pain in left cheek and both eyes felt immediately and continues at time of assessment. Emma is distressed about ‘bright red blood on eyeball’. Vision was intact immediately after trauma. Emma now states she has difficulty opening eye lids. Pain 6/10 in both globes and surrounding structures using numerical pain intensity scale (1 = least pain, 10 = highest pain level).
Objective
Patient is sitting quietly and hunched over, hands over eyes. Voice sounds tired and flat. Left cheek swollen and discoloured, no lacerations evident on face. No bleeding from eye area or cheek visible. Lids oedematous and discoloured on both eyes. Left eye lid swollen—almost completely closed. Left eye—round 1-mm bright red colouring over lateral aspect of globe. No active bleeding out of eye, iris intact. Right eye—conjunctiva clear, sclera white, cornea and iris intact. PERRLA. Pupils R 4/1 = 4/1 L.
Australian Institute of Health and Welfare (AIHW). A guide to Australian eye health data. Canberra: AIHW, 2007. Cat. no. PHE 86
Australian Institute of Health and Welfare (AIHW). Eye health in Australia: a hospital perspective. Canberra: AIHW, 2008. Cat. no. PHE 100
Australian Institute of Health and Welfare (AIHW). Vision problems among older Australians. Canberra: AIHW, 2005. Bulletin no. 27. Cat. no. AUS60
Bradford CA. Basic ophthalmology, 8th edn. San Francisco: American Academy of Ophthalmology, 2004.
Centre for Community Child Health. National children’s vision screening project discussion paper. Melbourne: Centre for Community Child Health, 2008.
Commonwealth of Australia. Eye health in Australia—a background paper to the national framework for action to promote eye health and prevent avoidable blindness and vision loss. Canberra: Commonwealth of Australia, 2005.
Glaucoma Information Sheet. Available at www.vision2020 australia.org.au/assets/…/Fact-Sheet-Glaucoma.rtf, 2007.
Harvey N. Sensory perception. In: Berman A, Snyder SJ, Kozier B, et al. Kozier and Erb’s Fundamentals of Nursing Volume III. Frenchs Forest, NSW: Pearson, 2010.
Laforest C, Durkin S, Selva D, et al. Aboriginal versus non-Aboriginal ophthalmic disease: admission characteristics at the Royal Adelaide Hospital. Clinical Experimentation Ophthalmology. 2006;34(4):324.
Lim S, Chellumuthi C, Crook N, et al. Low prevalence of retinopathy, but high prevalence of nephropathy among Māori with newly diagnosed diabetes—Te Wai o Rona: diabetes prevention strategy. Diabetes Research and Clinical Practice. 2008;80:271–274.
Livanes A, Greaves D, Bevan J. Pre-school visual acuity tests. Clinical & Experimental Optometry. 1986;69(4):145–148.
Marieb EN, Hoehn K. Human anatomy & physiology, 8th edn. San Francisco: Benjamin Cummings Pearson Education, 2010.
Mott S, Sensory alterations. Crisp J, Taylor C. Potter & Perry’s fundamentals of nursing, 3rd edn., Chatswood: Mosby Elsevier, 2009.
Sehu W, Eye Emergency Manual Steering Committee. Eye emergency manual: an illustrated guide, 2nd edn. North Sydney NSW: Department of Health, 2009.
Simmons D, Clover G, Hope C. Ethnic differences in diabetic retinopathy. Diabetic Medicine. 2007;24:1093–1098.
Smith SC, Neely S, Twyford K, Nursing assessment: visual and auditory systems. Brown D, Edwards H, Lewis SL, et al. Lewis’s medical–surgical nursing, 2nd edn., Marrickville: Mosby Elsevier, 2008.
Tabandeh H, Goldberg MF. The retina in systemic disease. New York: Thieme, 2009.
Vision 2020 Australia, nd: Facts on eye health: Australia (including Aboriginal and Torres Strait Islanders) (Facts sheet). Available at http://www.vision2020australia.org.au/assets/content/1308/WSD-Fact-Sheet-Aust-ATSI.pdf.
Wearne SM. Remote Indigenous Australians with cataracts: they are blind and still can’t see. Medical Journal of Australia. 2007;187(6):353–356.
Wotton K, Health assessment and physical examination. Crisp J, Taylor C. Potter & Perry’s Fundamentals of nursing, 3rd edn., Chatswood: Mosby Elsevier, 2009.