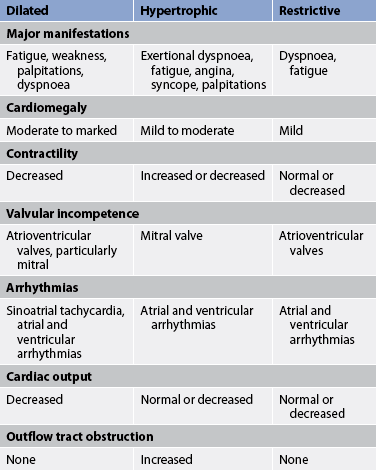
Chapter 36 NURSING MANAGEMENT: inflammatory and structural heart disorders
1. Describe the aetiology, pathophysiology and clinical manifestations of infective endocarditis and pericarditis.
2. Discuss the multidisciplinary care and nursing management of infective endocarditis and pericarditis.
3. Explain the importance of prophylactic antibiotic therapy in infective endocarditis.
4. Explain the aetiology, clinical manifestations, multidisciplinary care and nursing management of myocarditis.
5. Describe the aetiology, pathophysiology and clinical manifestations of rheumatic fever and rheumatic heart disease.
6. Discuss the multidisciplinary care and nursing management of the patient with rheumatic fever and rheumatic heart disease.
7. Identify the aetiologies of acquired valvular heart diseases.
8. Discuss the pathophysiology, clinical manifestations and diagnostic studies for the various types of valvular heart disease.
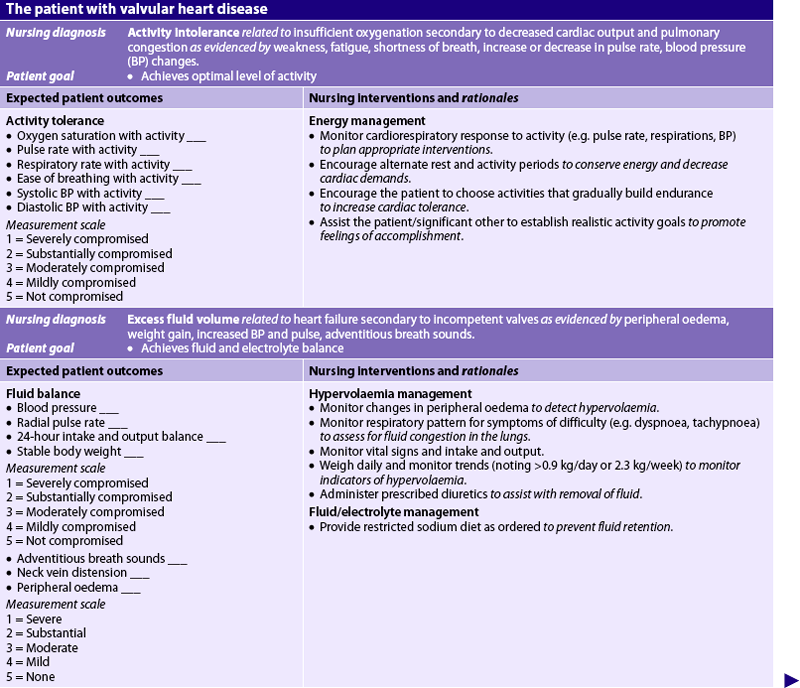
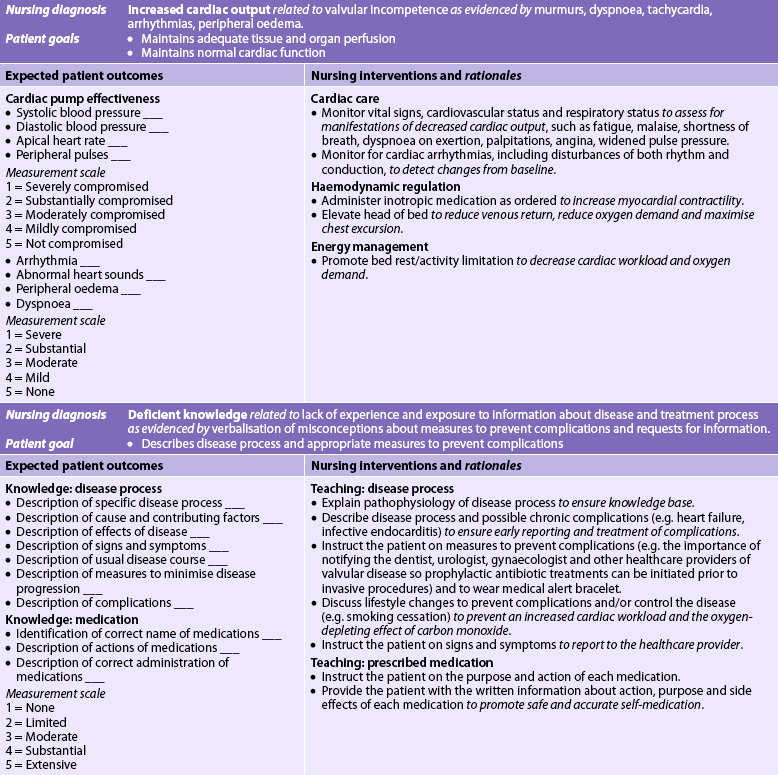
9. Describe the multidisciplinary care and nursing management of the patient with valvular heart disease.
10. Describe interventions used in the management of the patient with valvular heart disease.
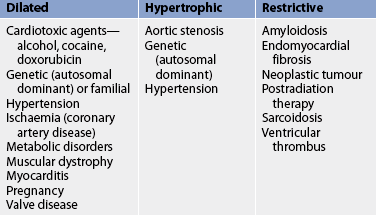
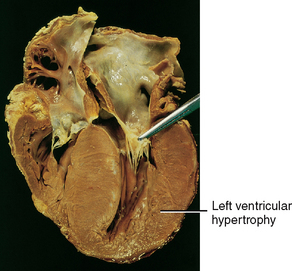
11. Describe the pathophysiology and clinical manifestations of the different types of cardiomyopathies.
12. Discuss the nursing and collaborative management of patients with different types of cardiomyopathies.
Infective endocarditis
Infective endocarditis (IE), previously known as bacterial endocarditis, is an infection of the endocardial surface of the heart. The endocardium, the innermost layer of the heart (see Fig 36-1), is contiguous with the valves of the heart. Therefore, inflammation from IE also affects the cardiac valves.
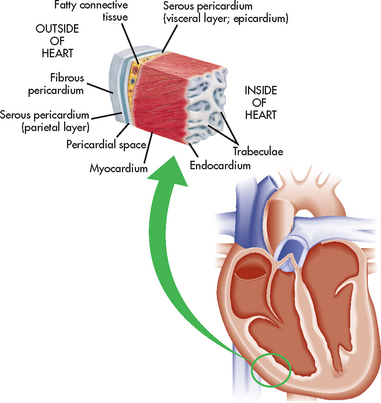
Figure 36-1 Layers of the heart muscle and pericardium. The section of the heart wall shows the fibrous pericardium, the parietal and visceral layers of the serous pericardium (with the pericardial sac between them), the myocardium and the endocardium.
Treatment of IE with penicillin therapy has improved the prognosis of this disease. For example, the mortality rate of IE from Streptococcus viridans is less than 10%. Although IE is relatively uncommon in developed countries, Indigenous Australians, Māori and Pacific Islanders continue to have some of the highest prevalence rates in the world, mostly due to acute rheumatic fever.1
CLASSIFICATION
Two forms of IE, subacute and acute, have been described. The subacute form typically affects those with pre-existing valve disease and has a clinical course that may extend over months. In contrast, the acute form typically affects those with healthy valves and presents as a rapidly progressive illness. Although this classification system has been used historically, clinicians prefer to classify IE based on the cause (e.g. intravenous drug abuse [IVDA], fungal endocarditis) or site of involvement (e.g. prosthetic valve endocarditis [PVE]).
AETIOLOGY AND PATHOPHYSIOLOGY
The most common causative organisms of IE, Staphylococcus aureus and S. viridans, are bacterial (see Box 36-1). Other possible pathogens include fungi and viruses. Newly identified pathogens, which are difficult to cultivate (e.g. Bartonella, Tropheryma whipplei) have been found to cause IE. Resistant organisms (e.g. methicillin-resistant S. aureus) also cause IE and are challenging conventional antibiotic therapy.2
BOX 36-1 Aetiological organisms associated with infective endocarditis
IE occurs when blood flow turbulence within the heart allows the causative organism to infect previously damaged valves or other endothelial surfaces. This can occur in individuals with a variety of underlying cardiac conditions. The principal risk factors for IE are prior endocarditis, prosthetic valves, acquired valvular disease and cardiac lesions. Several non-cardiac conditions and procedures also can allow large numbers of organisms to enter the bloodstream and initiate the infectious process (see Boxes 36-2 and 36-3).
BOX 36-2 Predisposing conditions for the development of infective endocarditis
Cardiac conditions
Acquired valve disease (e.g. mitral valve prolapse with murmur, calcified aortic stenosis)
Cardiac lesions (e.g. ventricular septal defect)
Procedure-associated risks
Intravascular devices (e.g. pulmonary artery catheters)
Procedures listed in Box 36-3
BOX 36-3 Procedures requiring antibiotic prophylaxis to prevent endocarditis*
Oropharyngeal
All dental procedures likely to produce gingival or mucosal bleeding (not simple adjustment of orthodontic appliances or shedding of deciduous teeth), including professional cleaning
Gastrointestinal
Abdominal surgeries involving intestinal mucosa
*This box lists selected procedures and is not all-inclusive.
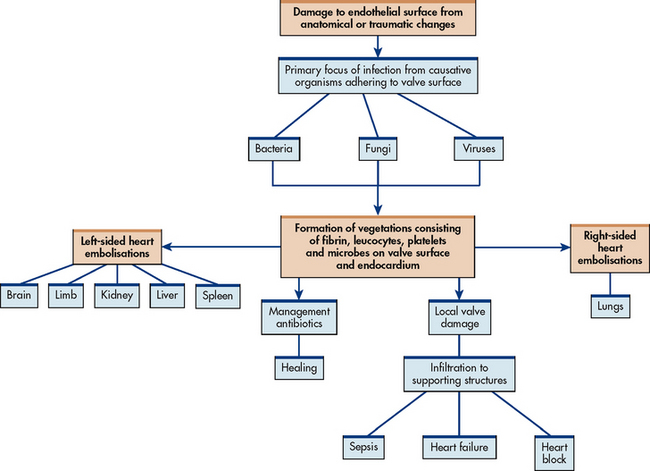
Vegetation, the primary lesion of IE, consists of fibrin, leucocytes, platelets and microbes that adhere to the valve surface or endocardium (see Fig 36-2). The loss of portions of this friable vegetation into the circulation results in embolisation. As many as 22–50% of patients with IE will experience systemic embolisation. This occurs from left-sided heart vegetation, progressing to various organs (particularly the brain, kidneys and spleen) and extremities causing limb infarction. Right-sided heart lesions embolise to the lungs. The infection may spread locally to cause damage to the valves or to their supporting structures. This results in arrhythmias, valvular incompetence and eventual invasion of the myocardium leading to heart failure (HF), sepsis and heart block (see Fig 36-3).
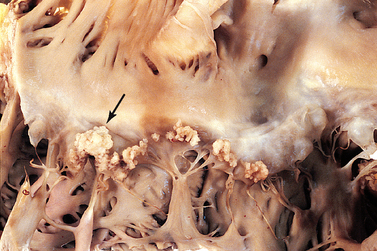
Figure 36-2 Bacterial endocarditis of the mitral valve. The valve is covered with large, irregular vegetations (arrow).
Rheumatic heart disease was, at one time, the most common cause of IE; it now accounts for less than 20% of cases. However, in Indigenous Australians, Māori and Pacific Islanders, rheumatic heart disease remains the primary cause.1,3 The incidence of rheumatic heart disease among the Indigenous population in the Northern Territory and the northern part of Queensland remains one of the highest in the world.1,2 Factors contributing to acute rheumatic fever include poverty and overcrowding, poor sanitary conditions, lack of education and limited access to medical care. In the general population, the main contributing factors include: (1) ageing (more than 50% of older people have calcified aortic stenosis); (2) IVDA; (3) use of prosthetic valves; (4) proliferation of intravascular device placement, resulting in nosocomial infections; and (5) renal dialysis.4
Left-sided endocarditis is more common in patients with bacterial infections and underlying heart disease. The primary cause of right-sided endocarditis is IVDA. However, there has been an increase in left-sided valves being affected, especially with cocaine abuse. S. aureus is the most common aetiological organism in IVDA IE.
CLINICAL MANIFESTATIONS
The findings in IE are non-specific and can involve multiple organ systems. Low-grade fever occurs in more than 90% of patients. Other non-specific manifestations include chills, weakness, malaise, fatigue and anorexia. Arthralgias, myalgias, back pain, abdominal discomfort, weight loss, headache and clubbing of the fingers may occur in subacute forms of endocarditis.
Vascular manifestations of IE include splinter haemorrhages (black longitudinal streaks) that may occur in the nail beds. Petechiae may occur as a result of fragmentation and microembolisation of vegetative lesions and are common in the conjunctivae, the lips, the buccal mucosa, the palate and over the ankles, the feet and the antecubital and popliteal areas. Osler’s nodes (painful, tender, red or purple pea-sized lesions) may be found on the fingertips or toes. Janeway lesions (flat, painless, small red spots) may be found on the palms and soles. Funduscopic examination may reveal haemorrhagic retinal lesions called Roth spots.
The onset of a new or changing murmur is noted in most patients with IE, with the aortic and mitral valves most commonly affected. The mitral murmur of endocarditis is generally a mid-to-late systolic regurgitant type. The aortic murmur may be early diastolic. Murmurs are often absent in tricuspid endocarditis because right-sided heart pressures are too low to be heard. HF occurs in up to 80% of patients with aortic valve endocarditis and in approximately 50% of patients with mitral valve endocarditis.5
Clinical manifestations secondary to embolisation in various body organs may also be present. Embolisation to the spleen may result in sharp, left upper quadrant pain and splenomegaly. Local tenderness and abdominal rigidity may be present. Embolisation to the kidneys may cause pain in the flank, haematuria and azotaemia. Emboli may lodge in small peripheral blood vessels of the arms and legs and may cause gangrene. Embolisation to the brain may cause neurological damage resulting in hemiplegia, ataxia, aphasia, visual changes and change in the level of consciousness. Pulmonary emboli may occur in right-sided endocarditis.
DIAGNOSTIC STUDIES
The patient’s recent health history is important in assessing IE. Inquiry should be made regarding any recent (within the past 3–6 months) dental, urological, surgical or gynaecological procedures, including normal or abnormal obstetric delivery. Previous history of IVDA, heart disease, recent cardiac catheterisation, cardiac surgery, intravascular device placement, renal dialysis or infections (e.g. skin, respiratory, urinary tract) should be documented.
Laboratory data, especially blood cultures, should also be assessed. Two blood cultures taken 30 minutes apart will be positive in more than 90% of patients. Culture-negative endocarditis is often associated with antibiotic usage within the previous 2 weeks or due to a pathogen not easily detected by standard culture procedures (e.g. Bartonella species). Negative cultures should be kept for 3 weeks if the clinical diagnosis remains endocarditis because of the possibility of a slow-growing, causative organism.
A mild leucocytosis occurs in acute endocarditis (uncommon in subacute) with average white blood cell (WBC) counts of 10–11 × 109/L. The erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels may be elevated.
Major criteria to diagnose IE have been established by Duke University Medical Center and include at least two of the following: positive blood cultures, new or changed cardiac murmur, intracardiac mass or vegetation noted on echocardiology, embolic event of unknown origin.6 Echocardiography is valuable in the diagnostic examination of a patient with IE when the blood cultures are negative or for the patient who is a surgical candidate and has an active infection. Transoesophageal echocardiograms and digital imaging using two- or three-dimensional transthoracic echocardiograms can detect vegetation on valves.7
A chest X-ray examination is done to detect the presence of cardiomegaly (an enlarged heart). An electrocardiogram (ECG) may show first- or second-degree atrioventricular (AV) block because the cardiac valves lie in proximity to cardiac conductive tissue, especially the AV node. Cardiac catheterisation may be used to evaluate valve functioning and to assess the status of the coronary arteries when surgical intervention is being considered for patients with IE.
MULTIDISCIPLINARY CARE
Prophylactic treatment
Antibiotic prophylaxis is recommended for patients with specific cardiac conditions before they undergo certain procedures (see Box 36-4).8 Procedures that require endocarditis prophylaxis are summarised in Box 36-3. Specific antibiotic regimens are recommended for dental, respiratory tract, gastrointestinal (GI) and genitourinary (GU) procedures. Antibiotic prophylaxis should also be used for high-risk patients who: (1) are to undergo removal or drainage of infected tissue; (2) receive renal dialysis; or (3) have ventriculoatrial shunts for management of hydrocephalus.9
BOX 36-4 Cardiac and non-cardiac conditions requiring antibiotic prophylaxis to prevent infective endocarditis*
Conditions associated with risk of infective endocarditis
High risk†
Surgically constructed systemic pulmonary shunts
Low/negligible risk‡
Vascular graft material (6 months after implantation)
Central nervous system ventricular shunts
Pacemakers and implantable cardiac defibrillators
Previous coronary bypass graft surgery
Mitral valve prolapse without valvular degeneration
Source: Gerber MA, Baltimore RS, Eaton CB et al. Prevention of rheumatic fever and diagnosis and treatment of acute Streptococcal pharyngitis. Circulation 2009; 119(11):1541–1551
Drug therapy
Accurate identification of the infecting organism is the key to successful treatment of IE. Long-term treatment is necessary to kill dormant bacteria clustered within the valvular vegetations. Complete eradication of the organism generally takes weeks to achieve and relapses are common. Initially, patients are hospitalised and intravenous (IV) antibiotic therapy is started. Table 36-1 outlines suggested antibiotic regimens for patients with IE from various organisms and with different clinical situations.5
TABLE 36-1 Treatment of infective endocarditis with antibiotic therapy*
| Aetiological agent | Antibiotic regimen options |
|---|---|
| Streptococcal endocarditis involving native valve | IV penicillin G or IV/IM ceftriaxone; IV penicillin G or IV/IM ceftriaxone plus IV/IM gentamicin; or IV vancomycin |
| Enterococcal endocarditis involving native or prosthetic valve | IV ampicillin; or IV penicillin G plus IV/IM gentamicin; or IV vancomycin plus IV/IM gentamicin |
| Staphylococcal endocarditis in absence of prosthetic materials | IV gentamicin or IV vancomycin |
| Fungal endocarditis in native or prosthetic valves | IV amphotericin B |
*this table lists common drug regimens, but it is not all-inclusive. IM, intramuscular; IV, intravenous.
Subsequent blood cultures may be performed to evaluate the effectiveness of antibiotic therapy. Blood cultures that remain positive indicate inadequate or inappropriate antibiotic administration, aortic root or myocardial abscess or the wrong diagnosis (e.g. an infection elsewhere). Serum antibiotic drug levels are often monitored to establish therapeutic doses. Finally, renal function is monitored to establish doses for antibiotics that are nephrotoxic (e.g. vancomycin) and/or for patients with poor kidney function.
Fungal infection and PVE respond poorly to antibiotic therapy alone. Early valve replacement followed by prolonged (>6 weeks) drug therapy is recommended in these situations.4 Valve replacement has become an important adjunct procedure in the management of IE. It is used in more than 25% of cases. (Valve replacement is discussed on p 957.)
Fever may persist for several days after treatment has been started and can be treated with aspirin, paracetamol, ibuprofen, fluids and rest. Complete bed rest is usually not indicated unless the temperature remains elevated or there are signs of HF. Endocarditis coupled with HF responds poorly to both drug therapy and valve replacement and is often life-threatening.
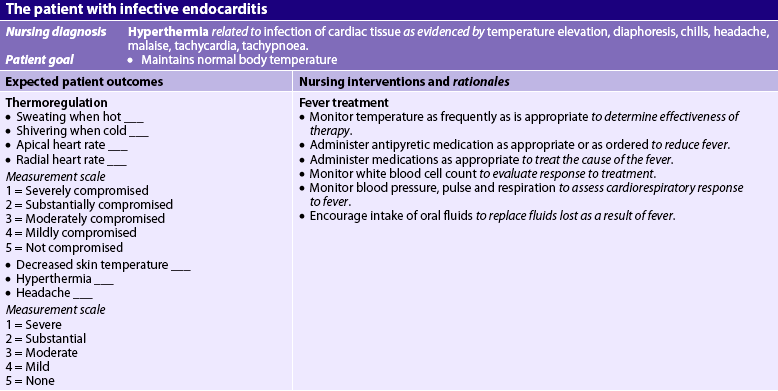
 NURSING MANAGEMENT: INFECTIVE ENDOCARDITIS
NURSING MANAGEMENT: INFECTIVE ENDOCARDITIS
 Nursing assessment
Nursing assessment
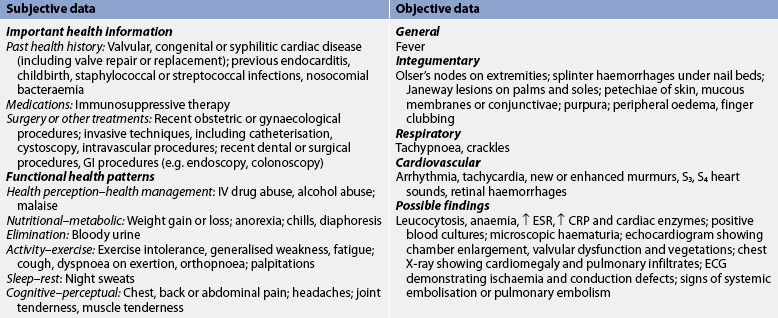
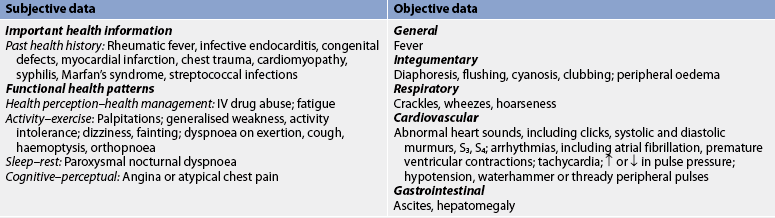
Subjective and objective data that should be obtained from a patient with IE are presented in Table 36-2. Heart sounds should be assessed together with vital signs to detect a murmur or a change in the character of a pre-existing murmur and the presence of extradiastolic sounds. Arthralgia is common; it may involve multiple joints and be accompanied by myalgias. The patient should be assessed for joint tenderness, decreased range of motion (ROM) and muscle tenderness. The oral mucosa, conjunctivae, upper chest and lower extremities should be examined for petechiae. A general systems assessment should be completed to facilitate recognition of haemodynamic and embolic complications.
 Nursing diagnoses
Nursing diagnoses
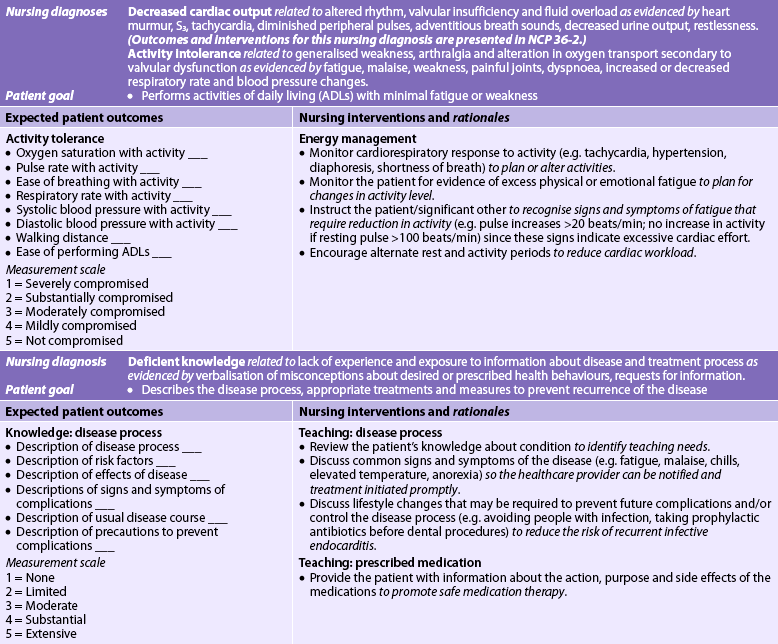
Nursing diagnoses for the patient with IE may include, but are not limited to, those presented in NCP 36-1.
 Planning
Planning
The overall goals for the patient with IE include: (1) normal or baseline cardiac function; (2) performance of activities of daily living (ADLs) without fatigue; and (3) knowledge of the therapeutic regimen to prevent recurrence of endocarditis.
 Nursing implementation
Nursing implementation
 Health promotion
Health promotion
The incidence of IE can be decreased by identifying individuals who are at risk of developing endocarditis (see Boxes 36-2 and 36-4). Assessment of the patient’s history and an understanding of the disease process are crucial for planning and implementing appropriate health promotion strategies.
Teaching the patient who is at risk of or has had IE helps reduce the incidence and recurrence of the disease. Teaching is crucial for the patient’s understanding of and adherence to the planned treatment regimen. The patient should understand the need to avoid people with infection, especially upper respiratory tract infection, and to report cold, flu and cough symptoms. The importance of avoiding excessive fatigue and the need to plan rest periods before and after activity should be carefully explained to the patient. Good oral hygiene, including daily care and regular dental visits, is also important. The patient must inform all healthcare providers performing dental, medical or surgical procedures of the history of IE and should understand the significance of the prescribed prophylactic antibiotic therapy before any invasive procedure. Patients with a history of IVDA should be referred for drug treatment.
 Ambulatory and home care
Ambulatory and home care
The patient with IE has many problems that require nursing management (see NCP 36-1). IE generally requires treatment with antibiotics for 4–6 weeks, depending on the results of blood cultures. After initial treatment in the hospital, the patient may continue treatment in the home setting if haemodynamically stable and compliant. The adequacy of the home environment in terms of in-home support and hospital access must be determined for successful management. Patients who receive outpatient IV antibiotics will require vigilant home nursing care.
Assessment findings are often non-specific (see Table 36-2) but can help assist with the treatment plan. Fever, chronic or intermittent, is a common early sign. Patients and families need instructions about the importance of monitoring body temperature because persistent, prolonged temperature elevations may mean that the drug therapy is ineffective. Patients with IE are at risk for life-threatening complications, such as cerebral emboli, pulmonary oedema and HF, and patients and their families must be taught to recognise signs and symptoms of these complications (e.g. change in mental status, dyspnoea, chest pain).
The patient with IE needs adequate periods of physical and emotional rest. Bed rest may be necessary when fever is present or when there are complications (e.g. heart damage). Otherwise the patient may ambulate and perform moderate activity. To prevent problems because of immobility, the patient should wear elastic compression stockings, perform ROM exercises and cough and deep breathe every 2 hours. The patient may experience anxiety and fear associated with the illness: the nurse must recognise this and implement strategies to help the patient cope with the illness.
Laboratory data should be monitored to determine the effectiveness of the antibiotic therapy. Ongoing monitoring of the patient’s blood cultures is necessary to ensure eradication of the infecting organism. IV lines should be monitored for patency and antibiotics should be given when scheduled. The patient should be monitored continuously for adverse drug reactions.
During the course of therapy in either the home or the hospital setting, management will also focus on teaching the patient about the nature of the disease and on reducing the risk of reinfection. The nurse must explain to the patient the relationship of follow-up care, good nutrition and early treatment of common infections (e.g. colds) to maintain good health. The patient should be instructed about symptoms that may indicate recurrent infection, such as fever, fatigue, malaise and chills. If any of these symptoms occur, the patient should be aware of the importance of notifying the healthcare provider. Finally, the patient must be instructed about the need for and importance of prophylactic antibiotic therapy before invasive procedures (see Box 36-3).
Acute pericarditis
Pericarditis is a condition caused by inflammation of the pericardial sac (the pericardium), which may occur on an acute basis. The pericardium is composed of the inner serous membrane (visceral pericardium), which closely adheres to the epicardial surface of the heart, and the outer fibrous (parietal) layer (see Fig 36-1). The pericardial space is the cavity between these two layers and, in the normal state, it contains 10–30 mL of serous fluid. Although the pericardium may be congenitally absent or surgically removed, it serves a useful anchoring function, provides lubrication to decrease friction during systolic and diastolic heart movements, and assists in preventing excessive dilation of the heart during diastole.
AETIOLOGY AND PATHOPHYSIOLOGY
The common causes of acute pericarditis are listed in Box 36-5. Acute pericarditis is most often idiopathic, with a variety of suspected viral causes. The coxsackievirus B group is the most commonly identified virus. In addition to idiopathic or viral pericarditis, other causes of this condition include uraemia, bacterial infection, acute myocardial infarction (MI), tuberculosis, neoplasm and trauma.10 Pericarditis in the acute MI patient may be described as two distinct syndromes: acute pericarditis, which may occur within the initial 48–72 hours after an MI; and Dressler’s syndrome (late pericarditis), which appears 4–6 weeks after an MI (see Ch 33).
BOX 36-5 Aetiologies of pericarditis
Infectious
Viral: coxsackievirus A and B, echovirus, adenovirus, mumps, hepatitis, Epstein-Barr, varicella zoster, human immunodeficiency virus
Bacterial: pneumococci, staphylococci, streptococci, Neisseria gonorrhoeae, Legionella pneumophila, septicaemia from Gram-negative organisms
Non-infectious
Neoplasms: lung cancer, breast cancer, leukaemia, Hodgkin’s lymphoma, non-Hodgkin’s lymphoma
Trauma: thoracic surgery, pacemaker insertion, cardiac diagnostic procedures
Hypersensitive or autoimmune
Delayed post-myocardial–pericardial injury
Post-myocardial infarction (Dressler’s syndrome)
Drug reactions (e.g. procainamide, hydralazine)
Rheumatological diseases: rheumatoid arthritis, systemic lupus erythematosus, systemic sclerosis (scleroderma), ankylosing spondylitis
An inflammatory response is the characteristic pathological finding in acute pericarditis. There is an influx of neutrophils, increased pericardial vascularity and eventually fibrin deposition on the visceral pericardium (see Fig 36-4).
CLINICAL MANIFESTATIONS
Clinical manifestations found in acute pericarditis include progressive, frequently severe chest pain that is sharp and pleuritic in nature. The pain is generally worse with deep inspiration and when lying supine. It is relieved by sitting. The pain may radiate to the neck, arms or left shoulder making it difficult to differentiate from angina. One distinction is that the pain from pericarditis can be referred to the trapezius muscle (shoulder, upper back) as the phrenic nerve innervates these two regions.11 The dyspnoea that accompanies acute pericarditis is related to the patient’s need to breathe in rapid, shallow breaths to avoid chest pain and may be aggravated by fever and anxiety.
The hallmark finding in acute pericarditis is the pericardial friction rub. The rub is a scratching, grating, high-pitched sound believed to arise from friction between the roughened pericardial and epicardial surfaces. It is best heard with the stethoscope diaphragm firmly placed at the lower left sternal border of the chest. The pericardial friction rub does not radiate widely or vary in timing from the heartbeat, but it may require frequent auscultation to identify because it may be elusive and transient. Timing the pericardial friction rub with the pulse (and not respirations) will help distinguish it from pleural friction rub (e.g. from pleurisy).
COMPLICATIONS
Two major complications that may result from acute pericarditis are pericardial effusion and cardiac tamponade. Pericardial effusion is an accumulation of excess fluid in the pericardium. It can occur rapidly (e.g. chest trauma) or slowly (e.g. tuberculous pericarditis). Large effusions may compress adjoining structures. Pulmonary tissue compression can cause cough, dyspnoea and tachypnoea. Phrenic nerve compression can induce hiccups, and compression of the recurrent laryngeal nerve may result in hoarseness. Heart sounds are generally distant and muffled, although blood pressure (BP) is usually maintained by compensatory mechanisms.
Cardiac tamponade develops as the pericardial effusion increases in volume causing an increase in intrapericardial pressure. This results in compression of the heart. The speed of fluid accumulation affects the severity of clinical manifestations. Cardiac tamponade can occur acutely (e.g. rupture of heart, trauma) or subacutely (e.g. secondary to uraemia, malignancy). The patient with cardiac tamponade may report chest pain and is often confused, anxious and restless. As the compression of the heart increases, heart sounds become muffled and the pulse pressure is narrowed. The patient will develop tachypnoea, tachycardia and a decreased cardiac output (CO). The neck veins are usually markedly distended because of jugular venous pressure elevation and a significant pulsus paradoxus is present. Pulsus paradoxus is a decrease in systolic BP with inspiration that is exaggerated in cardiac tamponade. (See Box 36-6 for measurement technique.) In a patient with a slow onset of a cardiac tamponade, dyspnoea may be the only clinical manifestation.
BOX 36-6 Measurement of pulsus paradoxus
1. Make determination during quiet breathing with stable rhythm.
2. Determine systolic blood pressure.
3. Inflate blood pressure cuff until no sounds are heard with stethoscope.
4. Deflate cuff slowly until systolic sounds are heard on expiration and note the pressure.
5. Deflate cuff until systolic sounds are heard throughout the respiratory cycle and note the pressure.
6. Determine the difference between the measurements taken in steps 4 and 5. This will equal the amount of paradox:
| Sounds heard on expiration at | 110 mmHg |
| Sounds heard throughout cycle at | 82 mmHg |
| Amount of paradox | 28 mmHg |
The difference is normally less than 10 mmHg. If the difference is greater than 10 mmHg, cardiac tamponade may be present.
DIAGNOSTIC STUDIES
The ECG may be normal, or it may exhibit non-specific or specific and diffuse changes. If specific ECG changes do develop, they will evolve over a period of hours to days or weeks and can include PR segment depression, ST segment elevation and T wave flattening and inversion. These changes are believed to be caused by superficial myocardial inflammation under the pericardium. (See Ch 35 for more information on ECG monitoring.)
Chest X-ray findings are generally normal, but cardiomegaly may be seen in a patient who has a large pericardial effusion (see Fig 36-5). Echocardiographic findings are more useful in determining the presence of a pericardial effusion or cardiac tamponade. Newer methods, such as tissue Doppler imaging and colour M-mode of early left-ventricular flow, help to assess diastolic function and diagnose constrictive pericarditis (see p 948).11 Computed tomography (CT) and magnetic resonance imaging (MRI) provide for visualisation of the pericardium and pericardial space.
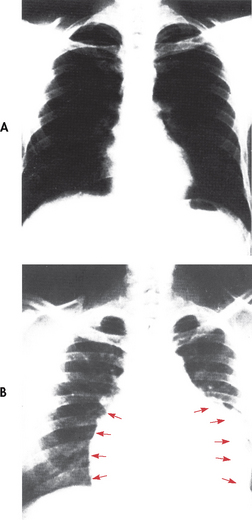
Figure 36-5 A, X-ray of a normal chest. B, Pericardial effusion is present and the cardiac silhouette is enlarged with a globular shape (arrows).
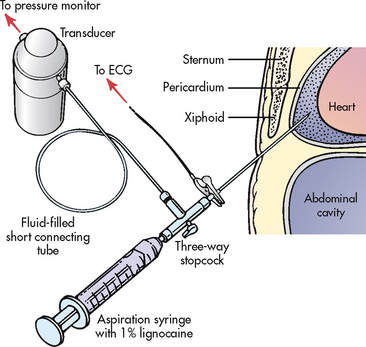
Common laboratory findings include leucocytosis and elevation of CRP and ESR. Troponin levels may be elevated in patients with ST segment elevation and acute pericarditis, which would indicate concurrent myocardial damage. The fluid obtained during pericardiocentesis (see Fig 36-6) or the tissue from a pericardial biopsy may be analysed to determine the cause of the pericarditis.
MULTIDISCIPLINARY CARE
Management of acute pericarditis is directed towards identification and treatment of the underlying problem (see Box 36-7). Antibiotics should be used to treat bacterial pericarditis. Corticosteroids are generally reserved for patients with pericarditis secondary to systemic lupus erythematosus, patients already taking corticosteroids for a rheumatological or other immune system condition or patients who do not respond to non-steroidal anti-inflammatory drugs (NSAIDs). When necessary, prednisone is given using a tapering dosage schedule. Corticosteroids are administered cautiously because of their numerous side effects, such as upper GI bleeding, sodium retention, hyperglycaemia, hypokalaemia and Cushing’s syndrome (see Ch 49).
MULTIDISCIPLINARY CARE
The pain and inflammation of acute pericarditis are usually treated with NSAIDs. High-dose salicylates (e.g. aspirin) or NSAIDs (e.g. ibuprofen) are commonly used. Colchicine, an anti-inflammatory agent used for gout, may be considered for patients who have recurrent pericarditis.
Pericardiocentesis is usually performed for pericardial effusion with acute cardiac tamponade, purulent pericarditis and a high suspicion of a neoplasm (see Fig 36-6). Haemodynamic support for the patient being prepared for pericardiocentesis may include administration of volume expanders and inotropic agents (e.g. dopamine) and the discontinuation of any anticoagulants. The procedure is performed rapidly and safely using a percutaneous approach that is guided by ECG and echocardiography. If surgical drainage is necessary, a 16- to 18-gauge needle is inserted into the pericardial space to remove fluid for analysis and to relieve cardiac pressure. Complications from pericardiocentesis include arrhythmias, further cardiac tamponade, pneumomediastinum, pneumothorax, myocardial laceration and coronary artery laceration.
 NURSING MANAGEMENT: ACUTE PERICARDITIS
NURSING MANAGEMENT: ACUTE PERICARDITIS
The management of the patient’s pain and anxiety during acute pericarditis is a primary nursing consideration. Assessment of the amount, quality and location of the pain is important, particularly in distinguishing the pain of myocardial ischaemia (angina) from the pain of pericarditis. Pericarditic pain is usually located in the praecordium or left trapezius ridge and has a sharp, pleuritic quality that increases with inspiration. Pain is often relieved by sitting or leaning forwards and worsened when lying supine. ECG monitoring can aid in distinguishing these types of pain because ischaemia usually involves localised ST segment changes, as compared to the diffuse ST segment changes present in acute pericarditis.
Pain relief measures include maintaining the patient on bed rest with the head of the bed elevated to 45° and providing an over-bed table for support. Anti-inflammatory medications will help to alleviate the patient’s pain. However, because of the potential for upper GI bleeding with the use of high doses of these medications, nursing interventions should be directed towards management of this potential problem. Specific interventions include administering these drugs with food or milk and instructing the patient to avoid any alcoholic beverages while taking the medications. Other drugs, such as misoprostol, may be ordered to protect the gastric mucosa.
Anxiety-reducing measures include providing simple, complete explanations of all procedures performed and the possible cause of the pain. These explanations are particularly important for patients whose diagnosis of acute pericarditis is being established and for patients who have already experienced angina or an acute MI.
The potential for decreased CO exists for the patient with acute pericarditis because of the possibility of cardiac tamponade. Monitoring for the signs and symptoms of tamponade and making preparations for possible pericardiocentesis are important nursing responsibilities.
Chronic constrictive pericarditis
AETIOLOGY AND PATHOPHYSIOLOGY
Chronic constrictive pericarditis results from scarring with consequent loss of elasticity of the pericardial sac. It usually begins with an initial episode of acute pericarditis (often secondary to idiopathic causes, cardiac surgery or radiation) and is characterised by fibrin deposition with a clinically undetected pericardial effusion. Reabsorption of the effusion slowly follows with progression towards the chronic stage of fibrous scarring, thickening of the pericardium from calcium deposition and eventual obliteration of the pericardial space. The fibrotic, thickened and adherent pericardium encases the heart, thereby impairing the ability of the atria and ventricles to stretch adequately during diastole.
CLINICAL MANIFESTATIONS
Manifestations of chronic constrictive pericarditis occur over an extended time period and mimic those of HF and cor pulmonale. Many of the clinical manifestations are related to decreased CO. They include dyspnoea on exertion, peripheral oedema, ascites, fatigue, anorexia and weight loss. The most prominent finding at the physical examination is jugular venous distension. Unlike cardiac tamponade, the presence of significant pulsus paradoxus is uncommon. Auscultatory findings include a pericardial knock, which is a loud early diastolic sound often heard along the left sternal border.
DIAGNOSTIC STUDIES
ECG changes are often non-specific in chronic constrictive pericarditis. The cardiac silhouette on the chest X-ray may be normal or enlarged depending on the degree of pericardial thickening and the presence of a coexisting pericardial effusion. Two-dimensional echocardiography findings may reveal a thickened pericardium, but without the presence of a large pericardial effusion. Colour M-mode and tissue Doppler imaging are used to confirm constrictive pericarditis. CT and MRI provide measurement of pericardial thickness and assessment of diastolic filling patterns.
 NURSING AND COLLABORATIVE MANAGEMENT: CHRONIC CONSTRICTIVE PERICARDITIS
NURSING AND COLLABORATIVE MANAGEMENT: CHRONIC CONSTRICTIVE PERICARDITIS
Unless the patient is free of symptoms or the condition is inoperable, the treatment of choice for chronic constrictive pericarditis is a pericardiectomy, which usually involves complete resection of the pericardium through a median sternotomy with the use of cardiopulmonary bypass. After surgery some patients show immediate improvement, but others may take weeks. The postoperative prognosis is improved when the surgery is performed before the development of severe clinical disability.
Myocarditis
AETIOLOGY AND PATHOPHYSIOLOGY
Myocarditis is a focal or diffuse inflammation of the myocardium. Possible causes include viruses, bacteria, fungi, radiation therapy and pharmacological and chemical factors. Viruses, particularly coxsackievirus types A and B, are the most common aetiological agents. Autoimmune disorders (e.g. polymyositis) have also been associated with the development of myocarditis. In some cases, myocarditis may occur when no causative agent or factor can be identified (i.e. idiopathic). Myocarditis is frequently associated with acute pericarditis, particularly when it is caused by coxsackievirus B strains.12
When the myocardium becomes infected, the causative agent invades the myocytes and causes cellular damage and necrosis. The immune response is activated and cytokines and oxygen-free radicals are released. As the infection progresses, an autoimmune response is activated, resulting in further destruction of myocytes. Myocarditis results in cardiac dysfunction and has been linked to the development of dilated cardiomyopathy (discussed on p 960).
CLINICAL MANIFESTATIONS
The clinical features of myocarditis are variable, ranging from a benign course without any overt manifestations to severe heart involvement or sudden cardiac death (SCD). Fever, fatigue, malaise, myalgias, pharyngitis, dyspnoea, lymphadenopathy, and nausea and vomiting are early systemic manifestations of the viral illness. Early cardiac manifestations appear 7–10 days after viral infection. These include pleuritic chest pain with a pericardial friction rub and effusion because pericarditis often accompanies myocarditis. Late cardiac signs relate to the development of HF and may include an S3, crackles, jugular venous distension, syncope, peripheral oedema and angina.
DIAGNOSTIC STUDIES
The ECG changes for a patient with myocarditis are often non-specific but may reflect associated pericardial involvement (e.g. diffuse ST segment abnormalities). Arrhythmias and conduction disturbances may be present. Laboratory findings are also often inconclusive. They may include mild-to-moderate leucocytosis and atypical lymphocytes, increased ESR and CRP levels, elevated levels of myocardial markers such as troponin and elevated viral titres (the virus is generally present in tissue and fluid samples only during the initial 8–10 days of illness).
Histological confirmation of myocarditis is done through endomyocardial biopsy (EMB). This technique involves removing several small pieces of myocardial tissue percutaneously from the right ventricle with a special instrument called a bioptome and microscopically examining the samples.13 A biopsy done during the initial 6 weeks of acute illness is most diagnostic because this is the period in which lymphocytic infiltration and myocyte damage indicative of myocarditis are present. Other studies include the use of echocardiography, nuclear scans and MRI to evaluate cardiac function.
MULTIDISCIPLINARY CARE
The specific treatment for myocarditis has yet to be established and usually consists of managing associated cardiac decompensation. Digoxin is often used to treat ventricular failure because it improves myocardial contractility and reduces the ventricular rate. Digoxin should be used cautiously in patients with myocarditis because of the increased sensitivity of the heart to the adverse effects of this drug (e.g. arrhythmias) and the potential toxicity with minimal doses. Diuretics may be used to reduce fluid volume and decrease preload. If hypotension is not present, intravenous medications such as nitroprusside and milrinone are used to reduce afterload and improve CO by decreasing systemic arterial resistance. The use of anticoagulation therapy may be considered in patients with a low ejection fraction who are at risk of thrombus formation from blood stasis in the cardiac chambers.
Based on the infectious–immune theory of myocarditis, immunosuppressive therapy, with agents such as prednisone, azathioprine and cyclosporin, has been used to reduce myocardial inflammation and to prevent irreversible myocardial damage. Evidence of the success of this therapy remains inconclusive and the use of corticosteroids for the treatment of myocarditis remains controversial.12
Intravenous immunoglobulin (IVIG) is being used on an experimental basis to treat myocarditis. It has been associated with improved left ventricular function and improved survival.12 Antiviral agents (e.g. ribavirin, α-interferon) are undergoing clinical investigation for the treatment of acute viral myocarditis.12
Oxygen therapy, bed rest and restricted activity are general supportive measures used for the management of myocarditis. In cases of severe HF, the use of intraaortic balloon pump therapy and ventricular assist devices may be required (see Ch 66).
 NURSING MANAGEMENT: MYOCARDITIS
NURSING MANAGEMENT: MYOCARDITIS
Decreased CO is an ongoing nursing diagnosis in the care of the patient with myocarditis. Interventions focus on assessment for the signs and symptoms of HF. Important nursing measures to decrease cardiac workload include the use of the semi-Fowler position, the spacing of activity and rest periods, and provisions for a quiet environment. Prescribed medications that increase the heart’s contractility and decrease preload, afterload or both require careful monitoring. Ongoing evaluation of the effectiveness of these interventions is necessary.
The patient may be anxious about the diagnosis of myocarditis, recovery from myocarditis and the therapeutic plan. Nursing measures include assessing the level of anxiety, instituting measures to decrease anxiety and keeping the patient and family informed about therapeutic measures.
The patient who receives immunosuppressive therapy has additional problems of alteration in the immune response with the potential for infection and complications related to the therapy. Guidelines for care include monitoring for complications and providing the patient with a clean, safe environment by following proper infection control procedures.
Most patients with myocarditis recover spontaneously although some may develop dilated cardiomyopathy. If severe HF occurs, the patient may require heart transplantation.
Rheumatic fever and heart disease
Rheumatic fever is an inflammatory disease of the heart potentially involving all layers (endocardium, myocardium and pericardium) of the heart. Rheumatic heart disease (RHD) is a chronic condition resulting from rheumatic fever that is characterised by scarring and deformity of the heart valves.
AETIOLOGY AND PATHOPHYSIOLOGY
Acute rheumatic fever (ARF) is a complication that occurs as a delayed sequela (usually after 2–3 weeks) of a group A streptococcal pharyngitis.13 Manifestations of ARF appear to be related to an abnormal immunological response to group A streptococcal cell membrane antigens. ARF affects the heart, joints, central nervous system (CNS) and skin.14 The sequela of ARF, rheumatic heart disease, is found primarily in young adults.
The world’s highest prevalence rates of ARF and RHD are found in Indigenous Australians and Pacific Islanders.1 ARF has declined in non-Indigenous Australians and in developed countries due to improved living conditions and the effective use of antibiotics to treat streptococcal infections.
Cardiac lesions and valvular deformities
About 40% of ARF episodes are marked by carditis, meaning that all layers of the heart (endocardium, myocardium and pericardium) are involved. This generalised involvement gives rise to the term rheumatic pancarditis.
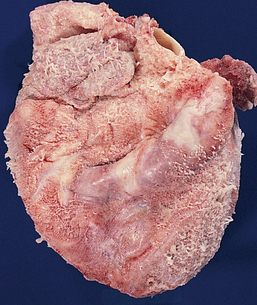
Rheumatic endocarditis is found primarily in the valves, with swelling and erosion of the valve leaflets. Vegetation forms from deposits of fibrin and blood cells in areas of erosion (see Fig 36-7). The lesions initially create fibrous thickening of the valve leaflets, fusion of commissures and chordae tendineae, and fibrosis of the papillary muscle. Valve leaflets may fuse and become thickened or even calcified, resulting in stenosis. Reduction in the mobility of valve leaflets may occur with failure of the leaflets to close properly, resulting in regurgitation. The mitral and aortic valves are most commonly affected.
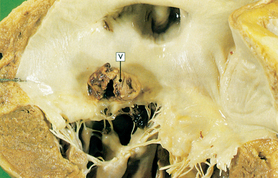
Figure 36-7 Mitral stenosis and clumps of vegetation (V). Mitral leaflets are thickened and fused and have clumps of vegetation containing platelets and fibrin.
Myocardial involvement is characterised by Aschoff bodies, which are histological nodules formed by a reaction to inflammation with accompanying swelling and fragmentation of collagen fibres. As the Aschoff bodies age, they become more fibrous and scar tissue is formed in the myocardium. In addition to Aschoff bodies, a diffuse cellular infiltrate is present in interstitial tissues. Rheumatic pericarditis affects both layers of the pericardium, which become thickened and covered with a fibrinous exudate, and a serosanguineous pericardial effusion may develop. When healing occurs, fibrosis and adhesions develop that partially or completely obliterate the pericardial sac, but constrictive pericarditis does not occur.
These pathophysiological changes in the heart may occur as a result of an initial attack of rheumatic fever. However, recurrent infections contribute to further structural damage.
CLINICAL MANIFESTATIONS
The diagnosis of ARF is suggested by a clustering of signs and symptoms as well as from laboratory findings. The National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand have released guidelines for the diagnosis of acute rheumatic fever (see Table 36-3).15 The presence of two major criteria or one major criterion and two minor criteria plus evidence of a preceding group A streptococcal infection indicates a high probability of ARF.
TABLE 36-3 Australian guidelines for the diagnosis of ARF
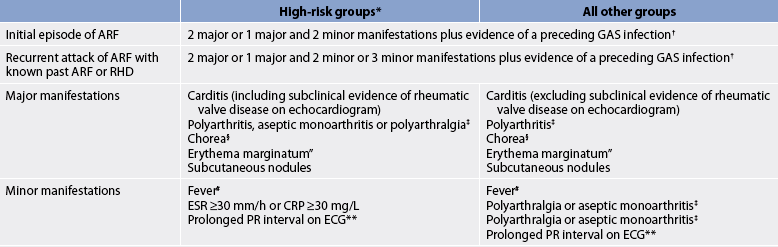
Note: All categories assume that other more likely diagnoses have been excluded.
ARF, acute rheumatic fever; CRP, C-reactive protein; ECG, electrocardiogram; ESR, erythrocyte sedimentation rate; GAS, group A streptococci; RHD, rheumatic heart disease.
† Elevated or rising anti-streptolysin o or other streptococcal antibody, or a positive throat culture or rapid antigen test for GAS.
‡ A definite history of arthritis is sufficient to satisfy this manifestation. Note that if polyarthritis is present as a major manifestation, polyarthralgia or aseptic monoarthritis cannot be considered an additional minor manifestation in the same person.
§ Chorea does not require other manifestations or evidence of preceding GAS infection, provided other causes of chorea are excluded.”Care should be taken not to label other rashes, particularly non-specific viral exanthemas, as erythema marginatum.
# Oral, tympanic or rectal temperature >38°C on admission or documented during the current illness.
** If carditis is present as a major manifestation, a prolonged PR interval cannot be considered an additional minor manifestation. Source: national Heart foundation of Australia and Cardiac Society of Australia and new Zealand. Guidelines for diagnosis of acute rheumatic fever. Available at www.heartfoundation.org.au/document/nHf/ARf_RHD_PP-592_Diag_QuickRefguide_0606.pdf.
Major criteria
Carditis is the most important manifestation of ARF and results in three signs: (1) an organic heart murmur or murmurs of mitral or aortic regurgitation or mitral stenosis; (2) cardiac enlargement and HF secondary to myocarditis; and (3) pericarditis resulting in muffled heart sounds, chest pain, pericardial friction rub or signs of effusion.
Monoarthritis and polyarthritis are the most common findings in ARF. The inflammatory process affects the synovial membranes of the joints, causing swelling, heat, redness, tenderness and limitation of motion. The larger joints are most frequently affected, particularly the knees, ankles, elbows and wrists.
Chorea (Sydenham’s chorea) is the major CNS manifestation of ARF and is often a delayed sign occurring several months after the initial infection. It is characterised by involuntary movements, especially of the face and limbs, muscle weakness and disturbances of speech and gait.
Erythema marginatum lesions are a less common feature of ARF. The bright pink, non-pruritic map-like macular lesions occur mainly on the trunk and proximal extremities and may be exacerbated by heat (e.g. warm bath).
Subcutaneous nodules, usually associated with severe carditis, are firm, small, hard, painless swellings located over extensor surfaces of the joints, particularly the knees, wrists and elbows.
Minor criteria
Minor clinical manifestations (see Table 36-3) are frequently present and are helpful in diagnosing the disease. The minor criteria are used as supplemental data to confirm the presence of rheumatic fever when fewer than two major criteria are present.
Evidence of infection
In addition to the major and minor criteria, there must also be evidence of a preceding group A streptococcal infection. Box 36-8 lists the various laboratory tests used to confirm evidence of infection.
BOX 36-8 Diagnostic tests for rheumatic fever
Recommended for all cases
• Erythrocyte sedimentation rate
• Electrocardiogram (repeat in 2 weeks, then 2 months if prolonged PR interval or other rhythm abnormality)
• Chest X-ray if clinical or echocardiographic evidence of carditis
• Echocardiogram (consider repeating after 1 month if negative)
• Throat swab (preferably before giving antibiotics)—culture for group A streptococci
• Anti-streptococcal serology: both anti-streptolysin O and anti-DNase B titres, if available (repeat 10–14 days later if first test not confirmatory)
Source: Reproduced with permission from Diagnosis of acute rheumatic fever. Quick Reference Guide for Health Professionals. © 2006 National Heart Foundation of Australia.
COMPLICATIONS
A complication that can result from ARF is chronic rheumatic carditis. It results from changes in valvular structure that may occur months to years after an episode of ARF. Rheumatic endocarditis can result in fibrous tissue growth in valve leaflets and chordae tendineae with scarring and contractures. The mitral valve is most frequently involved. Other valves that may be affected are the aortic and tricuspid valves.
DIAGNOSTIC STUDIES
No single diagnostic test exists for ARF (see Box 36-8). An echocardiogram may show valvular insufficiency and pericardial fluid or thickening. A chest X-ray may show an enlarged heart if HF is present. The most consistent ECG change is delayed AV conduction as evidenced in prolongation of the PR interval.
MULTIDISCIPLINARY CARE
Treatment consists of drug therapy and supportive measures (see Box 36-9). Antibiotic therapy does not modify the course of the acute disease or the development of carditis, but it will eliminate residual group A streptococci remaining in the tonsils and pharynx and prevent the spread of organisms to close contacts. Salicylates, NSAIDs and corticosteroids are the anti-inflammatory agents most widely used in the management of ARF. All are effective in controlling the fever and joint manifestations. Salicylates or NSAIDs are used when arthritis is the main manifestation, and corticosteroids are used if severe carditis is present.
 NURSING MANAGEMENT: RHEUMATIC FEVER AND HEART DISEASE
NURSING MANAGEMENT: RHEUMATIC FEVER AND HEART DISEASE
 Nursing assessment
Nursing assessment
Subjective and objective data that should be obtained from a patient with rheumatic fever and heart disease are presented in Table 36-4. It is important to note that rheumatic fever is more likely to recur in a person with a previous history of rheumatic fever than in the general population.
TABLE 36-4 Rheumatic fever and rheumatic heart disease
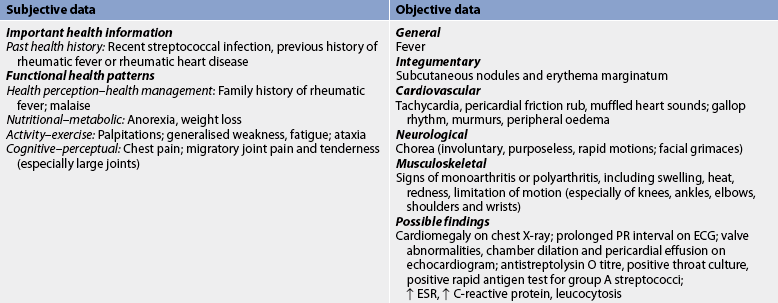
Ecg, electrocardiogram; esr, erythrocyte sedimentation rate.
The patient’s skin should be assessed for subcutaneous nodules and erythema marginatum. The procedure involves palpation for subcutaneous nodules over all bony surfaces and along extensor tendons of the hands and feet. The nodules range in size from 1 to4 cm and are hard, painless and freely movable. Erythema marginatum can occur on the trunk and inner aspects of the upper arm and thigh. The erythematous map-like maculae do not itch and are not raised. The possible presence of these bright pink maculae should be assessed in good light because the rash is difficult to observe, especially if the patient is dark-skinned.
 Nursing diagnoses
Nursing diagnoses
Nursing diagnoses for the patient with ARF and RHD may include, but are not limited to, the following:
• activity intolerance related to arthralgia secondary to joint pain, pain from pericarditis and heart failure
• decreased cardiac output related to valve dysfunction, heart failure
• ineffective therapeutic regimen management related to lack of knowledge concerning the need for long-term prophylactic antibiotic therapy and possible disease sequelae.
 Planning
Planning
The overall goals for the patient with rheumatic fever include: (1) normal or baseline heart function; (2) resumption of daily activities without joint pain; and (3) verbalisation of the ability to manage the disease.
 Nursing implementation
Nursing implementation
 Health promotion
Health promotion
Rheumatic fever is one of the cardiovascular diseases that is preventable. While strategies for controlling RHD are proven, simple, cheap and cost-effective, they are not adequately implemented in populations with the most need. To meet this need, the National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand have released guidelines for programs to control RHD.16 Prevention involves early detection and immediate treatment of group A streptococcal pharyngitis. Adequate treatment of streptococcal pharyngitis prevents initial attacks of rheumatic fever. Treatment consists of intramuscular (IM) injection of benzyl penicillin as a single dose or oral penicillin once daily for 10 days. If the patient is allergic to penicillin, erythromycin or azithromycin may be substituted. Oral therapy requires faithful adherence to the full course of treatment, which is often difficult in people who live in remote communities with little access to health services. The nurse’s role is to educate the community to seek medical attention for symptoms of streptococcal pharyngitis and to emphasise the need for adequate treatment of this infection. Prevention involves injections of benzyl penicillin monthly for long periods, making adherence difficult. These injections are painful and nurses need to work hard to educate patients about the ongoing need for preventative care (see the section on home care below).
 Acute intervention
Acute intervention
The primary goals of managing the patient with ARF are to control and eradicate the infecting organism, prevent cardiac complications and relieve joint pain, fever and other symptoms. The nurse should administer antibiotics as ordered to treat the streptococcal infection and teach the patient that oral antibiotic therapy requires faithful adherence to the full course of therapy. Antipyretics, NSAIDs and corticosteroids should be administered as prescribed and fluid intake monitored.
Promotion of optimal rest is essential to reduce the cardiac workload and to diminish the metabolic needs of the body. Relief of joint pain is an important nursing goal. Painful joints should be positioned for comfort and proper alignment. Heat may be applied and salicylates or NSAIDs administered to relieve joint pain.
After the acute symptoms have subsided, the patient without carditis should ambulate. If the patient has carditis with HF, bed rest restrictions should be applied. (See Ch 34 for care of the patient with HF.) Non-strenuous activities should be encouraged once recovery has begun.
 Ambulatory and home care
Ambulatory and home care
Secondary prevention aims at preventing the recurrence of rheumatic fever. The patient with a previous history of rheumatic fever should be taught about the disease process, possible sequelae and the continual need for prophylactic antibiotics. Prior history of rheumatic fever makes the patient more susceptible to a second attack after a streptococcal infection. The best prevention is monthly injections of long-acting penicillin. Alternative treatment is administration of oral penicillin or erythromycin one or two times a day. Rheumatic fever without carditis after age 18 may require only 5 years of prophylactic antibiotic therapy, or therapy may continue indefinitely in patients with frequent exposure to group A streptococci. Prophylactic treatment should continue for life in individuals who develop RHD.
The dosage of antibiotics used in maintenance prophylaxis of rheumatic fever is not adequate to prevent IE when invasive procedures are performed. Additional prophylaxis is necessary if a patient with known RHD has dental or surgical procedures involving the upper respiratory, GI (e.g. endoscopy) or GU tracts (see Box 36-3). The nurse must explain the difference between these two prophylactic programs.
Patient teaching should encourage good nutrition and hygienic practices and explain the importance of receiving adequate rest. The patient should be cautioned about the possibility of developing valvular heart disease and taught to seek medical attention if symptoms such as excessive fatigue, dizziness, palpitations or exertional dyspnoea develop.
VALVULAR HEART DISEASE
The heart contains two atrioventricular valves (the mitral and tricuspid valves) and two semilunar valves (the aortic and pulmonic valves), which are located in four strategic locations to control unidirectional blood flow. Valvular heart disease is defined according to the valve or valves affected and the type of functional alteration, stenosis or regurgitation (see Fig 36-8). The pressure on either side of an open valve is normally equal. However, in a stenotic valve, the valve orifice is smaller, impeding the forward flow of blood and creating a pressure-gradient difference across the open valve. The degree of stenosis (constriction or narrowing) is seen in the pressure-gradient differences (i.e. the higher the gradient, the greater the stenosis). In regurgitation (also called valvular incompetence or insufficiency) incomplete closure of the valve leaflets results in the backward flow of blood.
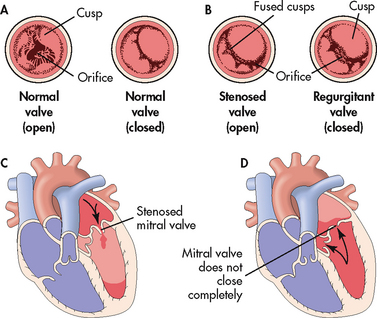
Figure 36-8 Valvular stenosis and regurgitation. A, Normal position of the valve leaflets or cusps, when the valve is open and closed. B, Open position of a stenosed valve (left) and position of closed regurgitant valve (right). C, Haemodynamic effect of mitral stenosis. The stenosed valve is unable to open sufficiently during left atrial systole, inhibiting left ventricular filling. D, Haemodynamic effect of mitral regurgitation. The mitral valve does not close completely during left ventricular systole, permitting blood to re-enter the left atrium.
Valvular disorders occur in children and adolescents primarily from congenital conditions such as tricuspid atresia, pulmonary stenosis and aortic stenosis. Valvular heart disease has remained prevalent because of the increased number of older adults, many of whom have some form of cardiovascular disease. Aortic stenosis and mitral regurgitation are common valvular disorders in the elderly. In the last 20 years, new causes of valve disease have emerged, including valvular disorders related to acquired immunodeficiency syndrome (AIDS) and the use of some antiparkinsonian drugs (e.g. pergolide).17
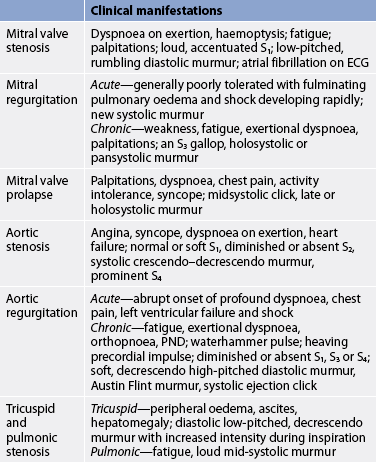
Mitral valve stenosis
AETIOLOGY AND PATHOPHYSIOLOGY
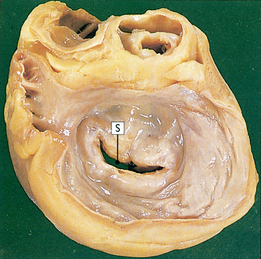
Most cases of adult mitral valve stenosis result from RHD: rheumatic mitral stenosis is endemic in developing countries and in Indigenous Australians, Māori and Pacific Islanders.1,18 Less common causes are congenital mitral stenosis, rheumatoid arthritis and systemic lupus erythematosus. Rheumatic endocarditis causes scarring of the valve leaflets and chordae tendineae. Contractures and adhesions develop between the commissures (the junctional areas) and the stenotic mitral valve takes on a ‘fish mouth’ shape because of the thickening and shortening of the mitral valve structures (see Fig 36-9). These structural deformities cause obstruction of blood flow and create a pressure difference between the left atrium and the left ventricle during diastole. Left atrial pressure and volume elevations cause increased pulmonary vasculature pressure and subsequent hypertrophy of the pulmonary vessels. In chronic mitral stenosis, pressure overload occurs in the left atrium, the pulmonary bed and the right ventricle.
CLINICAL MANIFESTATIONS
The primary symptom of mitral valve stenosis is exertional dyspnoea due to reduced lung compliance (see Table 36-5). Fatigue and palpitations from atrial fibrillation may also occur. Heart sounds include a loud first heart sound and a low-pitched, rumbling diastolic murmur (best heard at the apex with the stethoscope bell). Less frequently, patients may have hoarseness (from atrial enlargement pressing on the laryngeal nerve), haemoptysis (from pulmonary hypertension), chest pain (from decreased CO) and seizures or a stroke (from emboli). Emboli can arise from blood stasis in the left atrium.
Mitral regurgitation
AETIOLOGY AND PATHOPHYSIOLOGY
Mitral valve function depends on intact mitral leaflets, mitral annulus, chordae tendineae, papillary muscles, left atrium and left ventricle. Any defect in any of these structures can result in regurgitation. Most cases of mitral regurgitation (MR) are caused by MI, chronic rheumatic heart disease, mitral valve prolapse, ischaemic papillary muscle dysfunction and IE. MI with left ventricular failure increases the risk of rupture of the chordae tendineae and acute MR.
MR allows blood to flow backwards from the left ventricle to the left atrium due to incomplete valve closing during systole. The left ventricle and left atrium both work harder to preserve an adequate CO. In chronic MR, the additional volume load results in atrial enlargement, ventricular dilation and eventual ventricular hypertrophy. In acute MR, the left atrium and ventricle do not abruptly dilate. The sudden increase in pressure and volume is transmitted to the pulmonary bed, resulting in pulmonary oedema and shock.
CLINICAL MANIFESTATIONS
The clinical course of MR is determined by the nature of its onset (see Table 36-5). Patients with acute MR will have thready, peripheral pulses and cool, clammy extremities. A low CO may obscure a new systolic murmur. Rapid assessment (e.g. cardiac catheterisation) and intervention (e.g. valve repair or replacement) are critical for a positive outcome.
Patients with chronic MR may remain asymptomatic for many years. Initial symptoms of left ventricular failure may include weakness, fatigue, palpitations and dyspnoea that gradually progress to orthopnoea, paroxysmal nocturnal dyspnoea and peripheral oedema. Accentuated left ventricular filling leads to an audible third heart sound (S3), even with normal left ventricular function. The murmur is a loud holo- or pansystolic murmur at the apex radiating to the left axilla. Patients with asymptomatic MR should be monitored carefully and surgery (valve repair or replacement) should be considered before significant left ventricular failure or pulmonary hypertension develops.19
Mitral valve prolapse
AETIOLOGY AND PATHOPHYSIOLOGY
Mitral valve prolapse (MVP) is an abnormality of the mitral valve leaflets and the papillary muscles or chordae that allows the leaflets to prolapse or buckle back into the left atrium during systole (see Fig 36-10). The aetiology of MVP is unknown but is related to diverse pathogenic mechanisms of the mitral valve apparatus. The use of the term prolapse is unfortunate because it is used even when the valve is functionally normal. MVP is the most common form of valvular heart disease in developed countries occurring twice as frequently in women as in men.
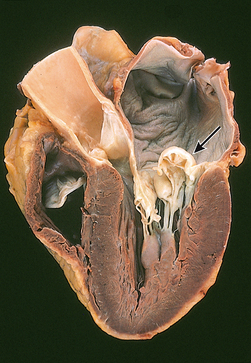
Figure 36-10 Mitral valve prolapse. In this valvular abnormality the mitral leaflets have prolapsed back into the left atrium. They also demonstrate hooding (arrow). The left ventricle is on the right.
MVP is usually benign, but serious complications can occur, including MR, IE, SCD and cerebral ischaemia. There is an increased familial incidence (autosomal dominant) in some patients. MVP in this group results from a connective tissue defect affecting only the valve or as part of Marfan’s syndrome or other hereditary conditions that affect the structure of collagen in the body. In many patients the abnormality found by echocardiography is not accompanied by any other clinical manifestations of cardiac disease and the significance of the finding is unclear.
CLINICAL MANIFESTATIONS
MVP covers a broad spectrum of severity. Most patients are asymptomatic and remain so for their entire lives. A characteristic of MVP is a murmur from regurgitation that gets more intense through systole. This could be a late or holosystolic murmur. Another major sign is one or more clicks usually heard in mid to late systole. MVP does not alter S1 or S2 sounds. Severe MR is an uncommon complication of MVP.
M-mode echocardiography confirms MVP by demonstrating late-systolic prolapse and two-dimensional echocardiography reveals leaflet billowing into the left atrium. Arrhythmias, most commonly ventricular premature contractions, paroxysmal supraventricular tachycardia and ventricular tachycardia, may cause palpitations, light-headedness and dizziness. IE may occur in patients with mitral regurgitation associated with MVP.
Patients may or may not have chest pain. The cause of the chest pain is not known, but it may be due to abnormal tension on the papillary muscles. If chest pain occurs, it tends to occur in clusters, especially during periods of emotional stress. Dyspnoea, palpitations and syncope may occasionally accompany the chest pain and do not respond to antianginal treatment (e.g. nitrates). β-adrenergic blockers may be prescribed to control palpitations and chest pain.
Patients with MVP generally have a benign, manageable course unless problems associated with mitral regurgitation are present. A teaching plan for patients with MVP is presented in Box 36-10.
BOX 36-10 Mitral valve prolapse
PATIENT & FAMILY TEACHING GUIDE
1. Teach the patient the importance of antibiotic prophylaxis for endocarditis before undergoing certain dental or surgical procedures if the patient has MVP with regurgitation (see Box 36-3).
2. Instruct the patient to take medications as prescribed (e.g. β-adrenergic blockers to control palpitations, chest pain).
3. Advise the patient to adopt healthy eating patterns and to avoid caffeine because it is a stimulant and may exacerbate symptoms.
4. Counsel the patient who uses diet pills or other over-the-counter drugs to check for common ingredients that are stimulants (e.g. caffeine, ephedrine) as these will exacerbate symptoms.
5. Help the patient to develop and implement an exercise program to maintain optimal health.
6. Instruct the patient to call 000 (Australia) or 111 (New Zealand) or the healthcare provider if symptoms develop or worsen (e.g. palpitations, fatigue, shortness of breath, anxiety).
Aortic stenosis
AETIOLOGY AND PATHOPHYSIOLOGY
Congenitally abnormal stenotic aortic valves are generally discovered in childhood, adolescence or young adulthood. In older patients, aortic stenosis (AS) is a result of rheumatic fever or senile fibrocalcific degeneration that may have an aetiology similar to coronary artery disease.19,20 In rheumatic valvular disease, fusion of the commissures and secondary calcification cause the valve leaflets to stiffen and retract, resulting in stenosis. If it does occur due to RHD, mitral valve disease accompanies aortic stenosis. Isolated AS is almost always non-rheumatic in origin. The incidence of rheumatic aortic valvular disease has been decreasing, but senile or degenerative stenosis is expected to increase as the population ages.
AS causes obstruction of flow from the left ventricle to the aorta during systole. The effect is left ventricular hypertrophy and increased myocardial oxygen consumption because of the increased myocardial mass. As the disease progresses and compensatory mechanisms fail, reduced CO leads to pulmonary hypertension and HF.
CLINICAL MANIFESTATIONS
Symptoms of AS (see Table 36-5) develop when the valve orifice becomes about one-third its normal size. Symptoms include the classic triad of angina, syncope and exertional dyspnoea, reflecting left ventricular failure.19,20 The prognosis is poor for a patient with symptoms and whose valve obstruction is not relieved. Glyceryl trinitrate is contraindicated because it would reduce the preload necessary to help open the stiffened aortic valve. Auscultation of AS typically reveals a normal or soft S1; a diminished or absent S2; a systolic, crescendo–decrescendo murmur that ends before S2; and a prominent fourth heart sound (S4).
Aortic regurgitation
AETIOLOGY AND PATHOPHYSIOLOGY
Aortic regurgitation (AR) may be the result of primary disease of the aortic valve leaflets or the aortic root, or both. Acute AR is caused by IE, trauma or aortic dissection and constitutes a life-threatening emergency. Chronic AR is generally the result of RHD, a congenital bicuspid aortic valve, syphilis or chronic rheumatic conditions such as ankylosing spondylitis or Reiter’s syndrome.
AR causes retrograde blood flow from the ascending aorta into the left ventricle during diastole, resulting in volume overload. The left ventricle initially compensates for chronic AR by dilation and hypertrophy. Myocardial contractility eventually declines and blood volumes increase in the left atrium and pulmonary bed. This results in pulmonary hypertension and right ventricular failure.
CLINICAL MANIFESTATIONS
Patients with acute AR have sudden manifestations of cardiovascular collapse (see Table 36-5). The left ventricle is exposed to aortic pressure during diastole. The patient develops severe dyspnoea, chest pain and hypotension indicating left ventricular failure and shock that constitute a medical emergency.
Patients with chronic, severe AR develop a waterhammer pulse (a strong quick beat that collapses immediately). Heart sounds may include a soft or absent S1, the presence of S3 or S4 and a soft, decrescendo high-pitched diastolic murmur. A systolic ejection click may also be heard, as well as a low-frequency diastolic murmur known as an Austin Flint murmur.
Patients with chronic AR generally remain asymptomatic for years and are seen with exertional dyspnoea orthopnoea and paroxysmal nocturnal dyspnoea only after considerable myocardial dysfunction has occurred (see Table 36-5). Angina occurs less frequently than in AS.
TRICUSPID AND PULMONIC VALVE DISEASE
AETIOLOGY AND PATHOPHYSIOLOGY
Diseases of the tricuspid and pulmonic valves are uncommon, with stenosis occurring more frequently than regurgitation. Tricuspid valve stenosis occurs almost exclusively in patients with rheumatic mitral stenosis, in IV drug abusers or in patients treated with a dopamine agonist (e.g. cabergoline).17 Pulmonary stenosis is almost always congenital.
Tricuspid and pulmonic stenosis result in the backward flow of blood to the right atrium and right ventricle, respectively. Tricuspid stenosis results in right atrial enlargement and elevated systemic venous pressures, while pulmonic stenosis results in right ventricular hypertension and hypertrophy (see Table 36-5).
DIAGNOSTIC STUDIES FOR VALVULAR HEART DISEASE
Diagnosis of valvular heart disease is generally based on the results of the history, physical examination, echocardiogram and cardiac catheterisation (especially if surgery is considered; see Box 36-11). Chest X-ray results, ECG findings and the clinical manifestations exhibited by the patient also aid in establishing the correct diagnosis. An echocardiogram reveals valve structure, function and chamber size. Transoesophageal echocardiography and Doppler colour-flow imaging are valuable in diagnosing and monitoring the progression of valvular heart disease. Real-time three-dimensional echocardiography may be helpful in qualitative assessments of mitral valve and congenital heart disease.21 Cardiac catheterisation detects pressure changes in the cardiac chambers, measures pressure gradients across the valves and quantifies the size of valve openings. An ECG shows heart rate and rhythm and provides information about any ischaemia or chamber enlargement. Chest X-ray reveals the heart size, alterations in pulmonary circulation and calcification of valves.
BOX 36-11 Valvular heart disease
MULTIDISCIPLINARY CARE
Collaborative therapy
Non-surgical
Prophylactic antibiotic therapy
• Infective endocarditis (see Box 36-3)
Medications to treat/control HF:
• vasodilators (e.g. nitrates,* ACE inhibitors)
Anticoagulation therapy (see Table 37-6)
*Contraindicated in aortic stenosis.
MULTIDISCIPLINARY CARE OF VALVULAR HEART DISEASE
Conservative therapy
An important aspect of conservative management of valvular heart disease is prevention of recurrent rheumatic fever and IE (see Box 36-11). Treatment depends on the valve involved and the severity of the disease. It focuses on preventing exacerbations of HF, acute pulmonary oedema, thromboembolism and recurrent endocarditis. If manifestations of HF develop, vasodilators, positive inotropes, β-adrenergic blockers, diuretics and a low-sodium diet are recommended (see Ch 34).
Anticoagulant therapy is used to prevent and treat systemic or pulmonary embolisation and is also used prophylactically in patients with atrial fibrillation. Arrhythmias, especially atrial arrhythmias, are common and are treated with digoxin, antiarrhythmic drugs or electrical cardioversion. β-adrenergic blockers may be used to slow the ventricular rate in patients with atrial fibrillation. (Arrhythmias are discussed in Ch 35.)
CLINICAL PRACTICE
Situation
A 68-year-old man has been admitted for a second mitral valve surgery and possible coronary artery bypass. He did not adhere to the treatment plan following his original surgery 7 years ago. The nurse worried about his future compliance with medications, diet and exercise. His kidneys are failing and he is on dialysis, but not tolerating it well. Both the patient and his family want complete therapeutic treatment and refuse to discuss not-for-resuscitation (NFR) orders.
Important points for consideration
• Non-adherence with the treatment plan in the past does not always indicate the patient will not follow the plan of care in the future.
• A competent patient can decide whether he or she wants continued treatment.
• Healthcare providers have an obligation to respect the patient’s request for treatment unless there is no clear benefit to continued treatment. Patients’ choices to continue or end treatment are based on their values and beliefs, which may not always coincide with those of the healthcare provider or team.
• NFR orders should reflect the patient’s expressed wishes, either through conversation with the patient or advanced directives, or a surrogate decision maker.
• NFR orders should be re-evaluated periodically with the patient and family, especially prior to major diagnostic procedures or treatments.
• If the healthcare provider does not agree with a patient’s treatment choice, a referral should be made to an Ethics Committee or similar professional group.
Percutaneous transluminal balloon valvuloplasty
An alternative treatment for some patients with valvular heart disease is percutaneous transluminal balloon valvuloplasty (PTBV), which splits open the fused commissures. Balloon valvuloplasty is used for mitral, tricuspid and pulmonic stenosis and less often for aortic stenosis. The procedure, performed in the cardiac catheterisation laboratory, involves threading a balloon-tipped catheter from the femoral artery or vein to the stenotic valve so that the balloon may be inflated in an attempt to separate the valve leaflets. A single- or double-balloon technique may be used for the procedure. Currently, the use of a single Inoue balloon with hourglass configuration allows sequential inflation. This technique is the most popular as it is easy and has good results and fewer complications (e.g. left ventricular perforation).22 PTBV is generally indicated for older adult patients and for patients who are poor surgery candidates. The procedure has fewer complications than valve replacement. The long-term results of PTBV are similar to surgical commissurotomy.21
Surgical therapy
The decision for surgical intervention is based on the clinical state of the patient as assessed using the New York Heart Association classification system for functional disability (see Box 34-1). The type of surgery depends on the valves involved, the valvular pathology, the severity of the disease and the patient’s clinical condition. All types of valve surgery are palliative, not curative, and patients will require lifelong healthcare.
Valve repair is typically the surgical procedure of choice. It is often used in mitral or tricuspid valvular heart disease and has a lower operative mortality rate than replacement. Mitral commissurotomy (valvulotomy) is the procedure of choice for patients with pure mitral stenosis. The less precise closed method of commissurotomy has generally been replaced by the open method in New Zealand and Australia. The direct vision or open procedure requires the use of cardiopulmonary bypass, removal of thrombi from the atrium, excision of the left atrial appendage, commissure incision and, as indicated, separation of fused chordae, splitting of underlying papillary muscle and debriding the valve of calcification. In contrast, the closed procedure is usually performed with the aid of a transventricular dilator inserted through the apex of the left ventricle into the ostium of the mitral valve.
Open surgical valvuloplasty involves repair of the valve by suturing the torn leaflets, chordae tendineae or papillary muscles. It is primarily used to treat mitral or tricuspid regurgitation. Valve repair avoids the risks of replacement but may not establish total valve competence. Minimally invasive valvuloplasty surgery, using ministernotomy or parasternal approaches, has shown results comparable to the open procedure in addition to decreased length of stay, blood transfusions and postoperative atrial fibrillation.23
Further repair or reconstruction of the valve may be necessary and can be achieved by annuloplasty, a procedure also used in cases of mitral or tricuspid regurgitation. Annuloplasty entails reconstruction of the annulus, with or without the aid of prosthetic rings (e.g. a Carpentier ring).
Prosthetic valves
Valvular replacement may be required for mitral, aortic, tricuspid and occasionally pulmonic valvular disease. The surgical treatment of choice for combined AS and AR is valvular replacement. A wide variety of prosthetic valves are available for use. Desirable valves are non-thrombogenic and durable and create minimal stenosis. Prosthetic valves are categorised as mechanical or biological (see Table 36-6 and Fig 36-11).
Figure 36-11 Various valve replacements. A, Porcine bioprosthesis. B, Mechanical valve prosthesis of the tilting disc variety replacing the native mitral valve. C, Mechanical valve prosthesis of the older ball-and-cage variety.
Source: Klatt EC. Robbins and Cotran atlas of pathology. 2nd edn. Philadelphia: Saunders; 2010.
Mechanical valves are manufactured from synthetic materials and consist of combinations of metal alloys, pyrolite carbon and Dacron. Biological valves are constructed from bovine, porcine and human cardiac tissue and usually contain some synthetic materials. Innovations in freezing and thawing techniques have enabled human grafts to be preserved for extensive periods while retaining viability. Mechanical prosthetic valves are more durable and last longer than biological valves. However, they have an increased risk of thromboembolism, necessitating long-term anticoagulation therapy. The main complication of mechanical valves is haemorrhage from the use of anticoagulants.24,25 Biological valves do not require anticoagulation therapy due to their low thrombogenicity. However, they are less durable due to the tendency for early calcification, tissue degeneration and stiffening of the leaflets. Problems with either type of prosthetic valves include paravalvular leaks and endocarditis.
Long-term anticoagulation is recommended for all patients with mechanical valves and for those with biological valves who have atrial fibrillation. Some patients with biological valves or annuloplasty with prosthetic rings may need anticoagulation for the first few months after surgery until the suture lines are covered by endothelial cells (endothelialised).
The choice of valve depends on many factors. For example, if a patient cannot take an anticoagulant (e.g. women of childbearing age), a biological valve may be considered. A mechanical valve may be best for a younger patient because it is more durable. For patients over 65 years of age, durability is less important than the risks of haemorrhage from anticoagulants.
 NURSING MANAGEMENT: VALVULAR DISORDERS
NURSING MANAGEMENT: VALVULAR DISORDERS
 Nursing assessment
Nursing assessment
Subjective and objective data that should be obtained from a patient with valvular disease are presented in Table 36-7.
 Nursing diagnoses
Nursing diagnoses
Nursing diagnoses for the patient with valvular disease may include, but are not limited to, those presented in NCP 36-2.
 Planning
Planning
The overall goals for the patient with valvular heart disease include: (1) normal cardiac function; (2) improved activity tolerance; and (3) an understanding of the disease process and health maintenance measures.
 Nursing implementation
Nursing implementation
 Health promotion
Health promotion
Diagnosing and treating streptococcal infections and providing prophylactic antibiotics for patients with a history of rheumatic fever are critical to prevent acquired rheumatic valvular disease. The patient at risk of endocarditis and any patient with valvular heart disease must also be treated with prophylactic antibiotics (see Box 36-4).
The patient must adhere to recommended therapies. The individual with a history of rheumatic fever, endocarditis and congenital heart disease should know the symptoms suggestive of valvular heart disease so that early medical treatment may be obtained.
 Acute intervention and ambulatory and home care
Acute intervention and ambulatory and home care
The patient with progressive valvular heart disease may require hospitalisation or outpatient care for management of HF, endocarditis, embolic disease or arrhythmias. HF is the most common reason for ongoing medical care.
The role of the nurse is to implement and evaluate the effectiveness of therapeutic interventions. Activity should be designed after considering the patient’s limitations. An appropriate exercise plan can increase cardiac tolerance. However, activities that regularly produce fatigue and dyspnoea should be restricted and an explanation should be provided to the patient. Strenuous physical exercise should be avoided because damaged valves may not be able to handle the demand for an increase in CO. Tobacco use should be discouraged. The patient should be assisted in planning ADLs, with an emphasis on conserving energy, setting priorities and taking planned rest periods. Referral to a vocational counsellor may be necessary if the patient has a physically or emotionally demanding job.
Auscultation of the heart should be performed to monitor the effectiveness of digoxin, β-adrenergic blockers and antiarrhythmic drugs. Teaching regarding the actions and side effects of drugs is important to achieve compliance. The patient must understand the importance of prophylactic antibiotic therapy to prevent IE (see Box 36-3). If the valve disease was caused by rheumatic fever, ongoing prophylaxis to prevent recurrence is necessary.
When valvular heart disease can no longer be managed medically, surgical intervention is necessary (see Ch 33 for care of the patient having cardiac surgery). The patient who is on anticoagulation therapy after surgery for valve replacement must have the international normalised ratio (INR) checked regularly (usually monthly) to assess the adequacy of therapy. The INR is a standardised system of reporting the prothrombin time. Values of 2.5–3.5 are therapeutic for patients with mechanical valves.
Teaching instructions related to anticoagulation therapy are found in Box 37-5. The patient must realise that valve surgery is not a cure and that regular follow-up with a healthcare provider will be required. The nurse also must teach the patient about when to seek medical care. Any manifestations of infection or HF, any signs of bleeding and any planned invasive or dental procedures require the patient to notify the healthcare provider. Finally, the patient should be encouraged to wear a medical alert identification or bracelet.
 Evaluation
Evaluation
The expected outcomes for the patient with valvular heart disease are addressed in NCP 36-2.
CARDIOMYOPATHY
Cardiomyopathy (CMP) constitutes a group of diseases that directly affect the structural or functional ability of the myocardium. A diagnosis of CMP is made based on the patient’s clinical manifestations and non-invasive and invasive diagnostic procedures.
CMP can be classified as primary or secondary. Primary CMP refers to those conditions in which the aetiology of the heart disease is unknown (idiopathic). The heart muscle in this case is the only portion of the heart involved and other cardiac structures are unaffected. In secondary CMP the cause of the myocardial disease is known and is secondary to another disease process. Common causes of secondary CMP are listed in Table 36-8.
The World Health Organization has classified CMP into three major types: dilated, hypertrophic and restrictive.26 Each type has its own pathogenesis, clinical presentation and treatment protocols (see Table 36-9 and Box 36-12). Cardiomyopathies can lead to cardiomegaly and HF and are the leading cause for heart transplants.
Dilated cardiomyopathy
AETIOLOGY AND PATHOPHYSIOLOGY
Dilated cardiomyopathy is the most common type of CMP. It causes HF in 25–40% of cases, occurs more frequently in men and has a genetic link in 30% of cases.26 Dilated CMP is characterised by a diffuse inflammation and rapid degeneration of myocardial fibres that results in ventricular dilation, impairment of systolic function, atrial enlargement and stasis of blood in the left ventricle. Cardiomegaly results from ventricular dilation (see Fig 36-12) and causes contractile dysfunction in spite of an enlarged chamber size. In contrast to HF, the walls of the ventricles do not hypertrophy (see Fig 36-13).
Figure 36-12 Dilated cardiomyopathy. The dilated left ventricular wall has thinned and the chamber size and volume are increased.
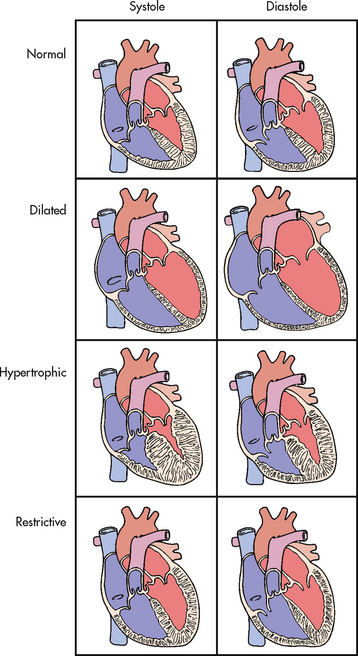
Figure 36-13 Types of cardiomyopathies and the differences in ventricular diameter during systole and diastole, compared with a normal heart.
Dilated CMP often follows an infectious myocarditis. Other common causes of dilated CMP are listed in Table 36-8.
CLINICAL MANIFESTATIONS
The signs and symptoms of dilated CMP may develop acutely after an infectious process or insidiously over a period of time. Most people eventually develop HF. Symptoms can include decreased exercise capacity, fatigue, dyspnoea at rest, paroxysmal nocturnal dyspnoea and orthopnoea. As the disease progresses the patient may experience dry cough, palpitations, abdominal bloating, nausea, vomiting and anorexia. Signs can include an irregular heart rate with an abnormal S3 and/or S4, tachycardia or bradycardia, pulmonary crackles, oedema, weak peripheral pulses, pallor, hepatomegaly and jugular venous distension. Heart murmurs and arrhythmias are common. Decreased blood flow through an enlarged heart promotes stasis and blood clot formation and may lead to systemic embolisation.
DIAGNOSTIC STUDIES
The diagnosis of dilated CMP is made on the basis of the patient’s history and by ruling out other conditions that cause HF. Doppler echocardiography provides the basis for the diagnosis of dilated CMP in the majority of patients and distinguishes dilated CMP from other structural abnormalities. The chest X-ray may show cardiomegaly with signs of pulmonary venous hypertension, as well as pleural effusion. The ECG may reveal tachycardia, bradycardia and arrhythmias with conduction disturbances. Laboratory studies may reveal elevated serum levels of B-type natriuretic peptide in the presence of HF.
Cardiac catheterisation is done to confirm or rule out coronary artery disease and multiple gated radionuclide angiocardiographies to determine the ejection fraction (EF). EFs less than 20% are associated with a 50% mortality within one year. Endomyocardial biopsy may be done at the time of the right-sided heart catheterisation to detect viral antigens in myocardial tissue.
 NURSING AND COLLABORATIVE MANAGEMENT: DILATED CARDIOMYOPATHY
NURSING AND COLLABORATIVE MANAGEMENT: DILATED CARDIOMYOPATHY
Interventions focus on controlling HF by enhancing myocardial contractility and decreasing afterload. This is similar to the treatment of chronic HF. Evidence-based practice guidelines from the National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand base treatment of HF on the specific stage of disease progression (see Ch 34).27 Treatment of patients with a class IV, stage D HF is more palliative than curative.
Several different types of drugs are used to manage HF (see Box 34-5). Nitrates (e.g. glyceryl trinitrate) and loop diuretics (e.g. frusemide) are used to decrease preload, and angiotensin-converting enzyme (ACE) inhibitors (e.g. catopril) are used to reduce afterload. β-adrenergic blockers (e.g. metoprolol) and aldosterone antagonists (e.g. spironolactone) are used to control the neurohormonal stimulation that occurs in HF. Digoxin is used to treat atrial fibrillation but must be used with caution because of increased susceptibility to digoxin toxicity in these patients. Other arrhythmias are treated with antiarrhythmics (e.g. amiodarone) as indicated (see Ch 35). Anticoagulation therapy is initiated to reduce the risk of systemic embolisation from clots that may form in the heart chambers.27
Drug and nutritional therapy and cardiac rehabilitation may help to alleviate symptoms of HF and improve CO and quality of life. A patient with secondary dilated CMP must be treated for the underlying disease process. For example, the patient with alcohol-related dilated CMP must abstain from all alcohol intake. (See Ch 34 for a complete discussion of HF.)
Unfortunately, dilated CMP does not respond well to therapy and patients may experience multiple episodes of HF. Intermittent dobutamine or milrinone infusions can be used. The patient is admitted to the hospital for a continuous infusion of dobutamine or milrinone followed by aggressive diuresis. Sometimes these infusions are undertaken as an outpatient treatment or in the home under supervision of a home care nurse. After infusion, many patients experience an improvement in symptoms that lasts several weeks after therapy.
Patients may also benefit from non-pharmacological therapies, such as a ventricular assist device (VAD), to allow the heart to rest and recover from acute HF or as a bridge to heart transplantation. Additionally, cardiac resynchronisation therapy and an implantable cardioverter–defibrillator may be considered in appropriate patients (see Ch 35).28
The patient with terminal end-stage CMP may be considered for heart transplantation or destination therapy with a permanent or implantable VAD (see Ch 34). Currently, approximately 50% of heart transplants are performed for treatment of CMP. Cardiac transplant recipients have a good prognosis for survival. However, donor hearts are difficult to obtain and many patients with dilated CMP die while awaiting transplantation.
Patients with dilated CMP are very ill people with a grave prognosis who need expert nursing care. The patient’s family must learn cardiopulmonary resuscitation (CPR) and how to access emergency care. The nurse should include family members and other support systems when planning the patient’s care.
Home health and hospice nursing can provide the patient and family with the continuous assessments and therapeutic interventions that are required to maximise and maintain functional status or to prepare for a peaceful death. Observing for signs and symptoms of worsening HF, arrhythmias and embolic formation is paramount, as is monitoring drug responsiveness. The goal of therapy is to keep the patient at an optimal level of function and out of the hospital.
Hypertrophic cardiomyopathy
AETIOLOGY AND PATHOPHYSIOLOGY
Hypertrophic cardiomyopathy (HCM), formerly called idiopathic hypertrophic subaortic stenosis, is asymmetrical left ventricular hypertrophy without ventricular dilation. In one form of the disease, the septum between the two ventricles becomes enlarged and obstructs the blood flow from the left ventricle. This is termed hypertrophic obstructive cardiomyopathy (HOCM) or asymmetric septal hypertrophy (ASH). HCM can be idiopathic, although about half of all cases have a genetic basis characterised by inappropriate myocardial hypertrophy (see Table 36-8). HCM occurs less commonly than dilated CMP and is more common in men aged 30–40 than in women.25 One study in the US found that HCM occurred more frequently in young African American male athletes compared with young white male athletes.29
The four main characteristics of HCM are: (1) massive ventricular hypertrophy; (2) rapid, forceful contraction of the left ventricle; (3) impaired relaxation (diastole); and (4) obstruction to aortic outflow (not present in all patients). Ventricular hypertrophy is associated with a thickened intraventricular septum and ventricular wall (see Figs 36-13 and 36-14). The end result is impaired ventricular filling as the ventricle becomes noncompliant and unable to relax. The primary defect of HCM is diastolic dysfunction from left ventricular stiffness. Decreased ventricular filling and obstruction to outflow can result in decreased CO, especially during exertion. HCM is the most common cause of SCD in otherwise healthy young people. It is usually diagnosed in young adulthood and is often seen in active, athletic individuals.29
CLINICAL MANIFESTATIONS
Patients with HCM may be asymptomatic or may have exertional dyspnoea, fatigue, angina and syncope. The most common symptom is dyspnoea, which is caused by an elevated left ventricular diastolic pressure. Fatigue occurs because of the resultant decrease in CO and in exercise-induced flow obstruction. Angina can occur and is most often caused by the increased left ventricular muscle mass or compression of the small coronary arteries by the hypercontractile ventricular myocardium. The patient may also have syncope, especially during exertion. Syncope is most often caused by an increase in obstruction to aortic outflow during increased activity, resulting in decreased CO and cerebrovascular circulation. Syncope can also be caused by arrhythmias. Common arrhythmias include supraventricular tachycardia, atrial fibrillation, ventricular tachycardia and ventricular fibrillation. Any of these arrhythmias may lead to loss of consciousness or SCD (see Ch 35).
DIAGNOSTIC STUDIES
Clinical findings on examination may be unremarkable. However, on palpation of the chest there may be a forced apical impulse that may be displaced laterally. Auscultation may reveal an S4 heart sound and a systolic ejection murmur between the apex and the sternal border at the fourth intercostal space. ECG findings usually indicate ventricular hypertrophy, ST–T wave abnormalities, prominent Q waves in the inferior or praecordial leads, left-axis deviation, and ventricular and atrial arrhythmias (see Ch 35).
The echocardiogram is the primary diagnostic tool to confirm the classic feature of HCM, which is left ventricular hypertrophy. The echocardiogram may also demonstrate wall motion abnormalities and diastolic dysfunction. Cardiac catheterisation may also be helpful in the diagnosis of HCM.
 NURSING AND COLLABORATIVE MANAGEMENT: HYPERTROPHIC CARDIOMYOPATHY
NURSING AND COLLABORATIVE MANAGEMENT: HYPERTROPHIC CARDIOMYOPATHY
The goals of intervention are to improve ventricular filling by reducing ventricular contractility and relieving left ventricular outflow obstruction. These can be accomplished with the use of β-adrenergic blockers, such as metoprolol, or calcium channel blockers, such as verapamil. Digoxin preparations are contraindicated unless they are used to treat atrial fibrillation. Antiarrhythmics, such as amiodarone or sotalol, are effective medications for arrhythmias, but their use has not been shown to prevent SCD. For patients at risk of SCD, the implantation of a cardioverter–defibrillator is recommended (see Ch 33).28
It has been found that atrioventricular pacing can be beneficial for patients with HCM and outflow obstruction. By pacing the ventricles from the apex of the right ventricle, septal depolarisation occurs first, allowing the septum to move away from the left ventricular wall and reducing the degree of obstruction of the outflow tract.28
Some patients may be candidates for surgical treatment of their hypertrophied septum. The indications for surgery include severe symptoms refractory to therapy with marked obstruction to aortic outflow. The surgery is termed a ventriculomyotomy and myectomy. It involves incision of the hypertrophied septal muscle and resection of some of the hypertrophied ventricular muscle. Most patients have good symptomatic improvement after surgery and improved exercise tolerance.
An alternative non-surgical procedure to reduce symptoms and the left ventricular outflow obstruction is alcohol-induced, percutaneous transluminal septal myocardial ablation (PTSMA). This procedure consists of administering alcohol into the first septal artery branching off the left anterior descending artery, which causes ischaemia and septal wall myocardial infarction. Ablation of the septal wall decreases the obstruction to flow and the patient’s symptoms will decrease. The procedure improves HF symptoms and exercise capacity 3 months after ablation.30 Mortality rates for the procedure are approximately 1% depending on the age and condition of the patient.31 Long-term effects of PTSMA-treated patients are lacking because of the newness of the procedure. Potential complications of PTSMA include conduction disturbances (e.g. heart block) and myocardial infarction beyond the intended septum.
Nursing interventions focus on relieving symptoms, observing for and preventing complications, and providing emotional and psychological support. Teaching should focus on helping patients to adjust their lifestyle to avoid strenuous activity and dehydration. Any activity or procedure that causes an increase in systemic vascular resistance (thus increasing the obstruction to forward flow) is dangerous and should be avoided. Patients with HCM who experience chest pain are managed by rest and elevation of the feet to improve venous return to the heart. Vasodilators such as glyceryl trinitrate may worsen the chest pain by decreasing venous return to the heart, which would further increase obstruction of blood flow from the heart.
Restrictive cardiomyopathy
AETIOLOGY AND PATHOPHYSIOLOGY
Restrictive cardiomyopathy is the least common of the cardiomyopathic conditions. It is a disease of the heart muscle that impairs diastolic filling and stretch (see Fig 36-13). Systolic function remains unaffected. Although the specific aetiology of restrictive CMP is unknown, a number of pathological processes may be involved in its development. Myocardial fibrosis, hypertrophy and infiltration produce stiffness of the ventricular wall with loss of ventricular compliance. Secondary causes of restrictive CMP include amyloidosis, endocardial fibrosis, sarcoidosis, fibrosis of different aetiology and radiation to the thorax. With restrictive CMP, the ventricles are resistant to filling and therefore demand high diastolic filling pressures to maintain CO.
CLINICAL MANIFESTATIONS
Classic symptoms of restrictive CMP are fatigue, exercise intolerance and dyspnoea because the heart cannot increase CO by increasing the HR without further compromising ventricular filling. Additional symptoms may include angina, orthopnoea, syncope and palpitations. The patient may have signs of HF including dyspnoea, peripheral oedema, ascites, hepatomegaly and jugular venous distension.
DIAGNOSTIC STUDIES
The chest X-ray may be normal or it may show cardiomegaly from right and left atrial enlargement. Pleural effusions and pulmonary congestion may be evident in patients with progression to HF. The ECG may reveal a mild tachycardia at rest. The most common arrhythmias are supraventricular (atrial fibrillation) or atrioventricular blocks. Echocardiogram may reveal a left ventricle that is normal in size with a thickened wall, a slightly dilated right ventricle and dilated atria. Endomyocardial biopsy, CT scan and nuclear imaging may be helpful in the diagnosis.
 NURSING AND COLLABORATIVE MANAGEMENT: RESTRICTIVE CARDIOMYOPATHY
NURSING AND COLLABORATIVE MANAGEMENT: RESTRICTIVE CARDIOMYOPATHY
Currently no specific treatment for restrictive CMP exists. Interventions are aimed at improving diastolic filling and the underlying disease process. Treatment includes conventional therapy for HF and arrhythmias. Heart transplant may also be a consideration. Nursing care is similar to care of the patient with HF. As in the treatment of patients with HCM, the patient should be taught to avoid situations that impair ventricular filling, such as strenuous activity, dehydration and increases in SVR. Nursing care also includes individualised teaching based on the patient’s clinical manifestations. All patients with CMP are at risk of IE from any procedure that may cause bacteraemia and should be instructed on the need for prophylactic antibiotics (see Box 36-3). A general patient guide is presented in Box 36-13.
PATIENT & FAMILY TEACHING GUIDE
1. Instruct the patient to take all medications as prescribed and to follow-up with the healthcare provider.
2. Encourage the patient to follow a low-sodium diet (if ordered) and to read all product labels (food and over-the-counter drugs) for sodium content.
3. Unless fluids are restricted, encourage the patient to drink 6–8 glasses of water a day.
4. Encourage the patient to achieve and maintain a reasonable weight and avoid large meals.
5. Advise the patient to avoid alcohol, caffeine, diet pills and over-the-counter cold medicines that may contain stimulants.
6. Teach the patient to balance activity and rest periods.
7. Instruct the patient to avoid heavy lifting or vigorous isometric exercises and to check with the healthcare provider for exercise guidelines.
8. Encourage the use of stress reduction activities: relaxation to relieve tension, guided imagery, diversional activities.
9. Instruct the patient to report any signs of heart failure to the healthcare provider, including weight gain, oedema, shortness of breath and increased fatigue.
10. Suggest that family members learn CPR because of the potential for sudden cardiac arrest.
11. Instruct the patient to notify the healthcare provider or dentist before any invasive medical/dental procedures, as patients with cardiomyopathy are at risk of endocarditis (see Box 36-3).
The patient with valvular heart disease
CASE STUDY
Patient profile
Mrs George, a 32-year-old Indigenous Australian, is admitted to the hospital for treatment of valvular heart disease.
CRITICAL THINKING QUESTIONS
1. Identify the cause and course of this patient’s disease based on her history and current examination.
2. What other factors should be considered when thinking about this patient’s care and the likely progression of her condition?
3. Differentiate between acute and chronic mitral regurgitation.
4. What surgical procedure will this patient probably require as her condition worsens?
5. Identify three priority nursing interventions for this patient.
6. On the basis of the assessment data provided, identify at least three more priority nursing diagnoses.
1. A patient with a history of intravenous cocaine use has acute infective endocarditis. The nurse assesses the patient for:
2. Nursing assessment findings for acute pericarditis include:
3. Prophylactic antibiotics are indicated to prevent infective endocarditis for at-risk individuals who are:
4. The most common underlying cause of myocarditis is:
5. Teaching the patient with rheumatic fever about the disease, the nurse explains that rheumatic fever is:
6. A patient with rheumatic fever should be taught about the need for:
7. A common cause of aortic stenosis in older adults is:
8. Which of the following findings is indicative of left ventricular overload in a patient with chronic aortic regurgitation?
9. A patient hospitalised with aortic stenosis has a nursing diagnosis of activity intolerance related to insufficient oxygen secondary to decreased cardiac output. An appropriate nursing intervention for this patient is to:
10. Which of the following assessment findings would the nurse expect in a patient with dilated cardiomyopathy?
1 Australian Institute of Health & Welfare (AIHW). Australia’s health, 2010. Canberra: AIHW; 2010. Available at www.aihw.gov.au/publications accessed 20 August 2010.
2 Murdoch D, Corey G, Hoen B, et al. Clinical presentation, etiology and outcome of infective endocarditis in the 21st century. Arch Intern Med. 2009;169(5):463.
3 Australian Institute of Health & Welfare (AIHW). Rheumatic heart fever and rheumatic heart disease in Australia. Available at www.aihw.gov.au/cvd/rheumatic_heart_disease.cfm. accessed 2 September 2010.
4 Murdoch DR, Corey GR, Hoen B. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century. The International Collaboration on Endocarditis–Prospective Cohort Study Arch Intern Med. 2009;169(5):463–473.
5 Nishimura RA, Carabello BA, Faxon DP, et al. ACC/AHA 2008 guideline update on valvular heart disease: focused update on infective endocarditis. J Am Coll Cardiol. 2008;52(8):676–685.
6 The Task Force on the Prevention, Diagnosis, Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version, 2009), Eur Heart J. 2009;30:2369–2413. Available at www.escardio.org/guidelines-surveys/esc-guidelines/GuidelinesDocuments/guidelines-IE-FT.pdf accessed 3 January 2011.
7 Moulds RFW, Currie B, Daly CG, et alInfective Endocarditis Prophylaxis Expert Group. Prevention of endocarditis. 2008 update from Therapeutic guidelines: antibiotic version 13, and Therapeutic guidelines: oral and dental version 1. Melbourne: Therapeutic Guidelines Limited; 2008. Available at www.tg.org.au/uploads/PDFs/Prevention%20of%20endocarditis.pdf accessed 3 January 2011.
8 Gerber MA, Baltimore RS, Eaton CB, et al. Prevention of rheumatic fever and diagnosis and treatment of acute Streptococcal pharyngitis. Circulation. 2009;119(11):1541–1551.
9 Imazio M, Trinchero R. Triage and management of acute pericarditis. Int J Cardiol. 2007;118(3):286–294.
10 Khandaker MH, Espinosa RE, Nishimura RA, et al. Pericardial disease: diagnosis and management. Mayo Clin Proc. 2010;85(6):572–593.
11 Wisnieski A. Muscle up your knowledge of myocarditis. Nursing. 2004;34(10):17.
12 Simmons-Holcomb S. Recognizing and managing different types of carditis. Nursing. 2006;36(suppl):4–9.
13 Root-Bernstein R, Vonck J, Podufaly A. Antigenic complementarity between coxsackie virus and streptococcus in the induction of rheumatic heart disease and autoimmune myocarditis. Autoimmunity. 2009;42(1):1.
14 Lang S. Getting to the heart of the problem: serological and molecular techniques in the diagnosis of infective endocarditis. Future Microbiol. 2008;3(3):341.
15 National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand. Guidelines for diagnosis of acute rheumatic fever: quick reference guide for health professionals. Available at www.heartfoundation.com.au/index.cfm?page=43. accessed 26 October 2010.
16 National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand. Rheumatic heart disease control programs: quick reference guide for health organisations. Available at www.heartfoundation.com.au/downloads/ARF_RHD_PP-595_RHD_Control_QuickRefGuide_0606.pdf. accessed 20 August 2010.
17 Horvath J, Fross RD, Kleiner-Fisman G, et al. Severe multivalvular heart disease: a new complication of the ergot derivative dopamine agonists. Mov Disord. 2004;19(6):656–662.
18 Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun B, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med. 2005;352:875–883.
19 Moser D, Riegel B. Cardiac nursing: a companion to Braunwalds’ heart disease. St Louis: Saunders/Elsevier, 2008.
20 Freeman RV, Crittenden G, Otto C. Acquired aortic stenosis. Expert Rev Cardiovasc Ther. 2004;2(1):107–116.
21 Abdulla J, Sivertsen J, Kofoed KF, et al. Evaluation of aortic valve stenosis by cardiac multislice computed tomography compared with echocardiography: a systematic review and meta-analysis. J Heart Valve Dis. 2009;18(6):634–643. Review
22 Wong MC, Clark DJ, Horrigan MC, et al. Advances in percutaneous treatment for adult valvular heart disease. Intern Med J. 2009;39(7):465–474.
23 McRae M, Rodger M, Bailey B. Transcatheter and transapical aortic valve replacement. Crit Care Nurse. 2009;29(2):22.
24 Moffatt-Bruce SD, Jamieson WR. Long-term performance of prostheses in mitral replacement. J Cardiovasc Surg (Torino). 2004;45(5):427–447.
25 De Caterina R. The current role of anticoagulants in cardiovascular medicine. J Cardiovasc Med (Hagerstown). 2009;10(8):595–604. Review
26 World Health Organization (WHO). International classification of functioning, disability and health (ICF). Geneva: WHO. Available at www.who.int/classifications/icf/en/. accessed 28 October 2010
27 Cruickshank S, Mason N. Symptoms and management options in cardiomyopathy. Nurs Times. 2005;101(45):26–29.
28 National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand. Guidelines on the contemporary management of the patient with chronic heart failure in Australia. Available at www.heartfoundation.org.au/SiteCollectionDocuments/CHF%202006%20Guidelines%20NHFA-CSANZ%20WEB.pdf, 2006. accessed 3 January 2011.
29 Al-Mohammad A, Mant J, Laramee P, Swain S. Diagnosis and management of adults with chronic heart failure: summary of updated NICE guidance. Chronic Heart Failure Guideline Development Group. BMJ. 2010;341:c4130.
30 Epstein AE, DiMarco JP, Ellenbogen KA, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. J Am Coll Cardiol. 2008;51(21):e1–e62.
31 Malek LA, Chojnowska L, Klopotowski M, et al. Long-term exercise capacity in patients with hypertrophic cardiomyopathy treated with percutaneous transluminal septal myocardial ablation. Eur J Heart Fail. 2008;10(11):1123–1126.
Australian Institute of Health & Welfare. www.aihw.gov.au
Hypertrophic Cardiomyopathy Association. www.4hcm.org
National Heart Foundation of Australia. www.heartfoundation.org.au
National Heart Foundation of New Zealand. www.heartfoundation.org.nz