Chapter 34 NURSING MANAGEMENT: heart failure
1. Compare the pathophysiology of systolic and diastolic ventricular failure.
2. Discuss the compensatory mechanisms involved in heart failure.
3. Explain the clinical manifestations and diagnostic criteria of heart failure.
3. Identify nursing and collaborative management of the patient with acute decompensated heart failure and pulmonary oedema.
4. Analyse the multidisciplinary care and nursing management, including drug and nutritional therapy, of the patient with chronic heart failure.
5. Explore the indications for cardiac transplantation and the nursing management of cardiac transplant recipients.
Heart failure
Heart failure (HF) is an abnormal clinical condition involving impaired cardiac pumping. It results in the characteristic pathophysiological changes of vasoconstriction and fluid retention. Heart failure, formerly called congestive heart failure, is the terminology preferred today because not all patients with HF have pulmonary congestion. HF is not a disease; however, it is associated with numerous types of heart disease, particularly with longstanding hypertension and coronary artery disease (CAD) and myocardial infarction (MI). HF is a major burden on the community characterised by ventricular dysfunction, reduced exercise tolerance, diminished quality of life and premature death of those affected.1
Cardiovascular disease is the major cause of death in both New Zealand and Australia. The incidence dramatically increases with advancing age and, as the elderly population increases, HF incidence and prevalence will increase too. Based on the most recent National Health Survey self-reports, 1.3% of the population have HF, with women making up more than two-thirds of that number. HF accounts for approximately 2% of all deaths and is the third largest cause of cardiovascular-related death.2
HF is associated with high rates of morbidity, mortality and economic costs.1,2 Direct health costs for cardiovascular disease are estimated to be about 12% of total healthcare costs, and HF is estimated to account for around 11% of cardiovascular disease costs in both New Zealand and Australia.1,2 HF is one of the most common reasons for hospital admission and GP consultations in people aged 70 years and over.1 The lifetime risk of developing HF has been estimated at around 20% for Western countries, with a prevalence of more than 50% in people aged 85 years and over. The increase in the prevalence of HF in Australia and New Zealand has been attributed to the ageing of the population, improved survival rates from heart attack, the increased prevalence of diabetes mellitus and obesity in the population, and the wider use of sensitive diagnostic technology. Men and women tend to experience HF failure in different ways (see Health disparities box) and nurses need to be aware of these possible differences when talking to patients about heart failure.
HEALTH DISPARITIES
Women
• Women experience diastolic dysfunction more frequently than men.
• Women have a higher risk of ACE inhibitor-related cough than men.
• Benefits of long-term use of digoxin in women may not justify the risks (e.g. drug-related death) when compared to men.
• Women with heart failure experience major depression more frequently than men.
In New Zealand, mortality and hospitalisation rates for HF are up to eight times higher for Māori than for non-Māori in the 45–65 age group.2 Indigenous Australians are hospitalised for heart, stroke and vascular diseases at younger ages than are non-Indigenous Australians, with 59% of these hospitalisations occurring before the age of 65 compared with 23% for non-Indigenous Australians. Deaths from heart failure among Indigenous Australians have been found to be almost three times higher than those of non-Indigenous Australians.3 Māori, Pacific Islanders, people from the Indian subcontinent and Indigenous Australians have a pattern of cardiovascular disease prevalence that is equivalent that of non-Indigenous Australians and New Zealanders of European descent who are 10 years older.3
AETIOLOGY AND PATHOPHYSIOLOGY
Although CAD and advancing age are the primary risk factors for HF, there are other factors, including hypertension, diabetes mellitus, cigarette smoking, obesity and high serum cholesterol levels. Hypertension is a major contributing factor, increasing the risk of HF approximately threefold. The risk of HF increases progressively with the severity of hypertension, and systolic and diastolic hypertension equally predict risk. Diabetes predisposes an individual to HF regardless of the presence of concomitant CAD or hypertension. Diabetes is more likely to predispose women than men to HF.4
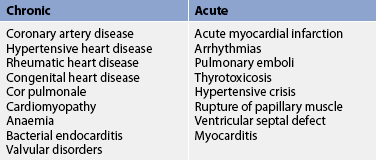
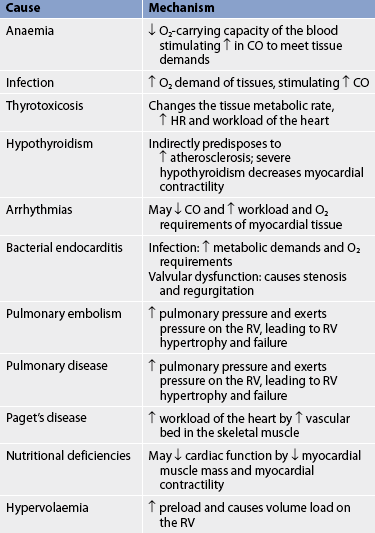
HF may be caused by any interference with the normal mechanisms regulating cardiac output (CO). CO depends on: (1) preload; (2) afterload; (3) myocardial contractility; (4) heart rate (HR); and (5) the metabolic state of the individual. (Preload and afterload are discussed in Ch 31.) Any alteration in these factors can lead to decreased ventricular function and the resultant manifestations of HF.4 The major causes of HF may be divided into two subgroups: (1) primary causes (see Table 34-1); and (2) precipitating causes (see Table 34-2). Precipitating causes often increase the workload of the ventricles, causing a decompensated condition that leads to decreased myocardial function.5
Pathophysiology of ventricular failure
HF is classified as systolic or diastolic failure (or dysfunction), or mixed systolic and diastolic failure.
Systolic failure
Systolic failure, the most common cause of HF, results from an inability of the heart to pump blood. It is a defect in the ability of the ventricles to contract (pump). The left ventricle (LV) loses its ability to generate enough pressure to eject blood forwards through the high-pressure aorta. The hallmark of systolic dysfunction is a decrease in the LV ejection fraction (EF; the fraction of total ventricular filling volume that is ejected during each ventricular contraction). Normal ejection fraction is greater than 55% of the ventricular volume. Systolic failure is caused by impaired contractile function (e.g. myocardial infarction [MI]), increased afterload (e.g. hypertension), cardiomyopathy and mechanical abnormalities (e.g. valvular heart disease).6
Diastolic failure
Diastolic failure, or heart failure with preserved systolic function (HFPSF), is an impaired ability of the ventricles to relax and fill during diastole.1 Approximately 20–40% of patients with HF have diastolic failure with a normal EF and systolic function in the presence of HF symptoms. Decreased filling of the ventricles will result in decreased stroke volume. In diastolic failure there is normal systolic function. Diastolic failure is characterised by high filling pressures and resultant venous engorgement in both the pulmonary and the systemic vascular systems. The diagnosis of diastolic failure is made on the basis of the presence of pulmonary congestion, pulmonary hypertension, ventricular hypertrophy and a normal EF.
Diastolic failure is usually the result of LV hypertrophy from chronic systemic hypertension, aortic stenosis or hypertrophic cardiomyopathy. Diastolic failure is commonly seen in older adults as a result of myocardial fibrosis and hypertension.7 However, the majority of patients who present with HF and normal systolic function do not have an identifiable heart disease. Less common is isolated right ventricular diastolic failure. This results from pulmonary hypertension (chronic or acute) and causes reduced right ventricular emptying, resulting in a low left ventricular filling pressure and reduced CO. Acute right ventricular failure can cause rapid cardiac demise despite a normal LV.
Mixed systolic and diastolic failure
Systolic and diastolic failure of mixed origin is seen in disease states such as dilated cardiomyopathy (DCM), a condition in which poor systolic function (weakened muscle function) is further compromised by dilated left ventricular walls that are unable to relax. Such patients often have extremely poor ejection fractions, high pulmonary pressures and biventricular failure (both ventricles may be dilated and have poor filling and emptying capacity).
The patient with ventricular failure of any type has low systemic arterial blood pressure (BP), low CO and poor renal perfusion. Poor exercise tolerance and ventricular arrhythmias are also common. Whether a patient arrives at this point acutely from an MI or chronically from worsening cardiomyopathy or hypertension, the body’s response to this low CO is to mobilise its compensatory mechanisms to maintain CO and BP.
Compensatory mechanisms
HF can have an abrupt onset, as with acute MI, or it can be an insidious process resulting from slow, progressive changes. The overloaded heart resorts to certain compensatory mechanisms to try to maintain adequate CO. The main compensatory mechanisms include: (1) sympathetic nervous system (SNS) activation; (2) neurohormonal response; (3) ventricular dilation; and (4) ventricular hypertrophy.
Sympathetic nervous system activation
SNS activation is often the first mechanism triggered in low-CO states. However, it is the least effective compensatory mechanism. Because there is inadequate stroke volume and CO, there is increased SNS activation, resulting in the increased release of adrenaline and noradrenaline. This results in an increased HR, myocardial contractility and peripheral vascular constriction. Initially this increase in HR and contractility improves CO. However, over time these factors act in a detrimental fashion by increasing myocardial need for oxygen and the workload of the already failing heart. The vasoconstriction causes an immediate increase in preload, which may initially increase CO. However, an increase in venous return to the heart, which is already volume overloaded, actually worsens ventricular performance.
Neurohormonal response
As the CO falls, blood flow to the kidneys decreases, causing decreased glomerular blood flow. This is sensed by the juxtaglomerular apparatus in the kidneys as decreased volume. In response, the kidneys release renin, which converts angiotensinogen to angiotensin I. The stimulation of renin and the resulting conversion of angiotensinogen to angiotensin I and II is known as the renin–angiotensin system. Angiotensin II causes: (1) the adrenal cortex to release aldosterone, which causes sodium retention; and (2) increased peripheral vasoconstriction, which increases the arterial BP. This response is known as the renin–angiotensin–aldosterone system (RAAS).
Low CO causes a decrease in cerebral perfusion pressure. The posterior pituitary then secretes antidiuretic hormone (ADH). ADH increases water reabsorption in the renal tubules, causing water retention and therefore increased blood volume. Thus, the blood volume is increased in a person who is already volume overloaded.
Other factors also contribute to the development of HF. The production of endothelin, produced by the vascular endothelial cells, is stimulated by ADH, catecholamines and angiotensin II. Endothelin results in further arterial vasoconstriction and an increase in cardiac contractility and hypertrophy.5
Locally, proinflammatory cytokines are released by cardiac myocytes in response to various forms of cardiac injury (e.g. MI). The cytokines, tumour necrosis factor (TNF) and interleukin-1 (IL-1), further depress cardiac function by causing cardiac hypertrophy, contractile dysfunction and myocyte cell death.4 Over time, a systemic inflammatory response is also mounted and accounts for the cardiac wasting, muscle myopathy and fatigue that accompanies advanced HF.5,6
Activation of the SNS and the neurohormonal response lead to elevated levels of noradrenaline, angiotensin II, aldosterone, ADH, endothelin and proinflammatory cytokines. Together, these factors result in an increase in cardiac workload, myocardial dysfunction and ventricular remodelling. Remodelling involves hypertrophy of the cardiac myocytes, resulting in large, abnormally shaped contractile cells. This eventually leads to increased ventricular mass, changes in ventricular shape and impaired contractility. Although the ventricles become larger, they become less effective pumps. All of these factors are over-expressed in HF and eventually perpetuate the downward spiral of progressive HF syndrome.5,6
In addition to the SNS and renin–angiotensin system, other factors also contribute to the development of HF. Endothelin is produced by the endothelial cells primarily in the lining of blood vessels in response to shear stress (stretch). Endothelin is a potent vasoconstrictor, which may contribute to hypertension. Circulating cytokines are also released in response to cardiac failure.
Dilation
Dilation is an enlargement of the chambers of the heart. It occurs when pressure in the heart chambers (usually the LV) is elevated over time. The muscle fibres of the heart stretch in response to the volume of blood in the heart at the end of diastole. The degree of stretch is directly related to the force of the contraction (systole) (Starling’s law). Initially this increased contraction leads to increased CO and maintenance of arterial BP and perfusion. In the early stages dilation is an adaptive mechanism to cope with increasing blood volume. Eventually this mechanism becomes inadequate because the elastic elements of the muscle fibres are overstretched and can no longer contract effectively, thereby decreasing the CO.5
Hypertrophy
In chronic HF, hypertrophy is an increase in the muscle mass and cardiac wall thickness in response to overwork and strain. It occurs slowly because it takes time for this increased muscle tissue to develop. Hypertrophy generally follows persistent or chronic dilation and thus further increases the contractile power of the muscle fibres. This will lead to an increase in CO and maintenance of tissue perfusion. However, hypertrophic heart muscle has poor contractility, requires more oxygen to perform work, has poor coronary artery circulation (tissue becomes more easily ischaemic) and is prone to ventricular arrhythmias.
Counter-regulatory mechanisms
The body’s ability to try to maintain balance is demonstrated by several counter-regulatory processes. Natriuretic peptides (atrial natriuretic peptide [ANP] and B-type natriuretic peptide [BNP]) are hormones produced by the heart muscle that promote venous and arterial vasodilation (thus reducing afterload and preload). Natriuretic peptides are endothelin and aldosterone antagonists and enhance diuresis by increasing glomerular filtration rates (thus reducing preload and volume stress) and blocking the effects of the RAAS. In addition, they inhibit the development of cardiac hypertrophy and may have anti-inflammatory effects. ANP is produced by the atrium and is primarily triggered by increases in volume. BNP is produced by the ventricles and is primarily triggered by increased pressure. Prolonged atrial and ventricular distension (during HF) leads to a depletion of these factors.7 Nitric oxide (NO) is another substance released from the vascular endothelium in response to the compensatory mechanisms activated in HF. Like the natriuretic peptides, NO works to relax the arterial smooth muscle resulting in vasodilation and decreased afterload.5
Stimulation of the SNS and renin–angiotensin system leads to elevated levels of noradrenaline, angiotensin II, aldosterone and vasopressin. The effects of these mediators are vasoconstriction, increased blood volume, increased HR and increased contractility, all of which increase myocardial oxygen consumption.
Cardiac compensation occurs when compensatory mechanisms succeed in maintaining an adequate CO that is needed for tissue perfusion. Cardiac decompensation occurs when these mechanisms can no longer maintain adequate CO and inadequate tissue perfusion results.
TYPES OF HEART FAILURE
HF is usually manifested by biventricular failure, although one ventricle may precede the other in dysfunction. Normally the pumping actions of the left and right sides of the heart complement each other, producing a continuous flow of blood. However, as a result of pathological conditions, one side may fail while the other side continues to function normally for a period of time. Because of the prolonged strain, both sides of the heart will eventually fail, resulting in biventricular failure.
Left-sided failure
The most common form of initial heart failure is left-sided failure (see Fig 34-1). Left-sided failure results from LV dysfunction, which causes blood to back up through the left atrium and into the pulmonary veins. The increased pulmonary pressure causes fluid extravasation from the pulmonary capillary bed into the interstitium and then the alveoli, which is manifested as pulmonary congestion and oedema.
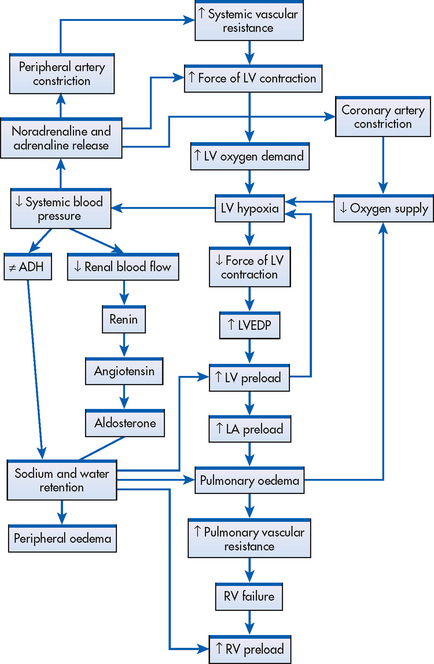
Figure 34-1 Left-sided heart failure from elevated systemic vascular resistance. Left-sided heart failure leads to right-sided failure. Systemic vascular resistance and preload are exacerbated by renal and adrenal mechanisms. ADH, antidiuretic hormone; LA, left atrium; LV, left ventricle; LVEDP, left ventricular end-diastolic pressure; RV, right ventricle.
Right-sided failure
Right-sided failure causes a backup of blood flow into the right atrium and venous circulation. Venous congestion in the systemic circulation results in peripheral oedema, hepatomegaly, splenomegaly, vascular congestion of the gastrointestinal (GI) tract and jugular venous distension. The primary cause of right-sided failure is left-sided failure. In this situation, left-sided failure results in pulmonary congestion and increased pressure in the blood vessels of the lung (pulmonary hypertension). Eventually, chronic pulmonary hypertension results in right-sided hypertrophy and failure. Cor pulmonale (right ventricular dilation and hypertrophy caused by pulmonary pathology) can also cause right-sided failure. (Cor pulmonale is discussed in Ch 28.) Right ventricular infarction may also cause right ventricle (RV) failure.
CLINICAL MANIFESTATIONS
Acute decompensated heart failure
Regardless of aetiology, acute decompensated HF (ADHF) typically manifests as pulmonary oedema, an acute, life-threatening situation in which the lung alveoli become filled with serosanguineous fluid (see Fig 34-2). The most common cause of pulmonary oedema is acute LV failure secondary to CAD.
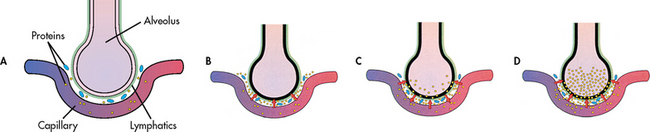
Figure 34-2 As pulmonary oedema progresses, it inhibits oxygen and carbon dioxide exchange at the alveolar–capillary interface. A, Normal relationship. B, Increased pulmonary capillary hydrostatic pressure causes fluid to move from the vascular space into the pulmonary interstitial space. C, Lymphatic flow increases in an attempt to pull fluid back into the vascular or lymphatic space. D, Failure of lymphatic flow and worsening of left heart failure result in further movement of fluid into the interstitial space and into the alveoli.
In most cases of ADHF, there is an increase in the pulmonary venous pressure caused by decreased efficiency of the LV. This results in engorgement of the pulmonary vascular system. As a result, the lungs become less compliant and there is increased resistance in the small airways. In addition, the lymphatic system increases its flow to help maintain a constant volume of the pulmonary extravascular fluid. This early stage is clinically associated with a mild increase in the respiratory rate and a decrease in arterial oxygen tension (PaO2).
If pulmonary venous pressure continues to increase, the increase in intravascular pressure causes more fluid to move into the interstitial space than the lymphatics can drain. Interstitial oedema occurs at this point. Severe tachypnoea is present. If the pulmonary venous pressure increases further, the tight alveoli lining cells are disrupted and a fluid containing red blood cells (RBCs) moves into the alveoli (alveolar oedema). As the disruption becomes worse from further increases in the pulmonary venous pressure, the alveoli and airways are flooded with fluid (see Fig 34-2). This is accompanied by a worsening of the blood gas values (i.e. lower PaO2 and possible increased arterial carbon dioxide tension [PaCO2] and progressive respiratory acidaemia).
Clinical manifestations of pulmonary oedema are unmistakable. The patient may be agitated, pale and possibly cyanotic. The skin is clammy and cold from vasoconstriction caused by stimulation of the SNS. The patient has severe dyspnoea, as evidenced by the obvious use of accessory muscles of respiration, a respiratory rate greater than 30 breaths per minute and orthopnoea. There may be wheezing and coughing with the production of frothy, blood-tinged sputum. Auscultation of the lungs may reveal bubbling crackles, wheezes and rhonchi throughout the lungs. The patient’s HR is rapid and BP may be elevated or decreased, depending on the severity of the oedema.
Chronic heart failure
The clinical manifestations of chronic HF depend on the patient’s age, the underlying type and extent of heart disease, and which ventricle is failing to pump effectively. Table 34-3 lists the manifestations of right-sided heart failure and left-sided heart failure. The patient with chronic HF will probably have manifestations of biventricular failure.
TABLE 34-3 Clinical manifestations of heart failure
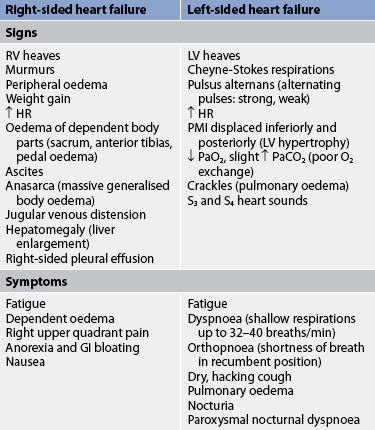
GI, gastrointestinal; HR, heart rate; LV, left ventricle; PMI, point of maximal impulse; RV, right ventricle.
Fatigue
Fatigue is one of the earliest symptoms of chronic HF. The patient notices fatigue after activities that normally are not tiring. The fatigue is caused by decreased CO, impaired perfusion to vital organs, decreased oxygenation of the tissues and anaemia. Anaemia can result from poor nutrition, renal disease or drug therapy (e.g. angiotensin-converting enzyme [ACE] inhibitors).
Dyspnoea
Dyspnoea (shortness of breath) is a common manifestation of chronic HF. It is caused by increased pulmonary pressures secondary to interstitial and alveolar oedema. Dyspnoea can occur with mild exertion or at rest. Orthopnoea is shortness of breath that occurs when the patient is in a recumbent position. Paroxysmal nocturnal dyspnoea (PND) occurs when the patient is asleep. It is caused by the reabsorption of fluid from dependent body areas when the patient is recumbent. The patient awakens in a panic, has feelings of suffocation and has a strong desire to seek relief by sitting up. Careful questioning of patients often reveals adaptive behaviour, such as sleeping with two or more pillows, to aid breathing. Because there are increased pulmonary pressures and fluid accumulation in the lung tissues, the patient may have a persistent, dry cough, unrelieved with position changes or over-the-counter cough suppressants. A dry, hacking cough may be the first clinical symptom of HF.
Tachycardia
Tachycardia is an early clinical sign of HF. One of the body’s first mechanisms to compensate for a failing ventricle is to increase the HR. Because of diminished CO, there is increased SNS stimulation, which increases the HR. However, many patients with chronic HF take β-blocker medications and may not show an increase in response to SNS stimulation.
Oedema
Oedema is a common sign of HF. It may occur in dependant body areas (peripheral oedema), the liver (hepatomegaly), abdominal cavity (ascites) or lungs (pulmonary oedema and pleural effusion). If the patient is in bed, sacral oedema (dependent oedema) may develop. Pressing the oedematous skin with the finger may leave a transient indentation (pitting oedema). The development of dependent oedema or a sudden weight gain of 1.5 kg or more in 2 days is often indicative of exacerbated HF.
Nocturia
A person with chronic HF who has decreased CO will also have impaired renal perfusion and decreased urinary output during the day. However, when the person lies down at night, fluid movement from interstitial spaces back into the circulatory system is enhanced. This causes increased renal blood flow and diuresis. The patient may complain of having to void six or seven times during the night.
Skin changes
Because tissue capillary oxygen extraction is increased in a person with chronic HF, the skin may appear dusky. It may also be cool to the touch from diaphoresis. Often the lower extremities are shiny and swollen, with diminished or absent hair growth. Chronic swelling may result in pigment changes, causing the skin to appear brown or brawny in areas covering the ankles and lower legs.
Behavioural changes
Cerebral circulation may be impaired with chronic HF secondary to decreased CO. The patient or family may report unusual behaviour, including restlessness, confusion and decreased attention span or memory. This may also be secondary to poor gas exchange and worsening HF.
Chest pain
HF can precipitate chest pain because of decreased coronary perfusion from decreased CO and increased myocardial work. Anginal-type pain may accompany either ADHF or chronic HF.
Weight changes
Many factors contribute to weight changes. Initially there may be a progressive weight gain from fluid retention. However, over time the patient is often too sick to eat. Abdominal fullness from ascites and hepatomegaly frequently causes anorexia and nausea. Renal failure may also contribute to fluid retention. In many cases the muscle and fat loss is masked by the patient’s oedematous condition. The actual weight loss may not be apparent until after the oedema subsides.
COMPLICATIONS OF HEART FAILURE
Pleural effusion
Pleural effusion (see Ch 28) results from increasing pressure in the pleural capillaries. A transudation of fluid occurs from these capillaries into the pleural space.
Arrhythmias
Chronic HF causes enlargement of the chambers of the heart. This enlargement (stretching of the atrial and ventricular tissues) may cause an alteration in the normal electrical pathway and atrial fibrillation may result. When numerous sites in the atria fire spontaneously and rapidly, an organised spread of depolarisation can no longer take place. Thus, the atria cannot contract normally. This can promote thrombus formation within the atria, which may break loose and form emboli. Patients with atrial fibrillation require treatment with antiarrhythmics and anticoagulants. (Arrhythmias are discussed in Ch 35.)
Patients with HF and an EF less than 35% have a high risk of fatal arrhythmias: nearly half experience sudden cardiac death, usually due to ventricular ischaemia, ventricular tachycardia and fibrillation. (Sudden cardiac death is discussed in Ch 33.)
Left ventricular thrombus
With ADHF or chronic HF, the enlarged LV and decreased CO combine to increase the chance of thrombus formation in the LV. Current Cardiac Society guidelines in Australia and New Zealand1 recommend anticoagulation therapy in patients with HF and atrial fibrillation or very poor LV function (e.g. EF <20%). Once a thrombus has formed, it may also decrease LV contractility, decrease CO and further worsen the patient’s perfusion. The development of emboli from the thrombus is also a possibility and can result in a stroke.
Hepatomegaly
HF can lead to severe hepatomegaly, especially with RV failure. The liver lobules become congested with venous blood. The hepatic congestion leads to impaired liver function. Eventually liver cells die, fibrosis occurs and cirrhosis can develop (see Ch 43).
Renal failure
The decreased CO that accompanies chronic HF results in a decrease in perfusion to the kidneys and can lead to renal insufficiency or failure (see Ch 46).
CLASSIFICATION OF HEART FAILURE
The New York Heart Association (NYHA) has developed internationally recognised and used functional guidelines for classifying people with HF. These guidelines are used in New Zealand and Australia. The classification is based on the person’s tolerance to physical activity (see Box 34-1).
BOX 34-1 New York Heart Association functional classification of cardiac disease
Class I
No limitation of physical activity. Ordinary physical activity does not cause fatigue, dyspnoea, palpitations or anginal pain.
Class II
Slight limitation of physical activity. No symptoms at rest. Ordinary physical activity results in fatigue, dyspnoea, palpitations or anginal pain.
DIAGNOSTIC STUDIES
Diagnosing HF is often difficult since neither patient signs nor patient symptoms are highly specific and may mimic many other medical conditions, such as anaemia or lung disease. The primary goal in diagnosis is to determine the underlying aetiology of HF. Diagnostic measures to assess the cause and degree of HF include physical examination, chest X-ray, electrocardiogram (ECG), laboratory data (cardiac enzymes, BNP, serum chemistry, liver function studies, thyroid function studies and full blood count), haemodynamic assessment, echocardiogram, stress testing and cardiac catheterisation. EF can be measured using echocardiography and/or nuclear studies and can be used to differentiate between systolic and diastolic HF. (A normal EF is >50%.) BNP levels are used to assist in the diagnosis of HF. In general, levels correlate positively with the degree of LV dysfunction and can help to differentiate dyspnoea caused by HF from other causes of dyspnoea (e.g. exacerbation of chronic obstructive pulmonary disease).6 Diagnostic studies used for the patient with ADHF are presented in Box 34-2, and those for the patient with chronic HF are presented in Box 34-3.
BOX 34-2 Acute decompensated heart failure and pulmonary oedema
MULTIDISCIPLINARY CARE
Collaborative therapy
Drug therapy: morphine IV; diuretics IV (frusemide, bumetanide); digoxin IV; glyceryl trinitrate IV; sodium nitroprusside IV; inotropic therapy (see Box 34-5)
BP, HR, RR, PAWP, urinary output at least every 1 h
Endotracheal intubation and mechanical ventilation
ABGs, arterial blood gases; BP, blood pressure; ECG, electrocardiogram; HR, heart rate; IV, intravenous; PAWP, pulmonary artery wedge pressure; RR, respiratory rate.
BOX 34-3 Chronic heart failure
MULTIDISCIPLINARY CARE
Diagnostic studies
History and physical examination
Determination of underlying cause
A complete history, physical, diagnostic and laboratory assessment is necessary to identify the cause of HF, aggravating factors, potential risk factors that may influence outcomes and current clinical status. The patient’s coexisting conditions, especially active chronic conditions, may act as exacerbating factors affecting the plan of care and influencing the timing and intensity of therapies.
 NURSING AND COLLABORATIVE MANAGEMENT: ACUTE HEART FAILURE AND PULMONARY OEDEMA
NURSING AND COLLABORATIVE MANAGEMENT: ACUTE HEART FAILURE AND PULMONARY OEDEMA
With the addition of new pharmaceutical agents and device therapies, the management of ADHF and chronic HF has dramatically changed in the last 5 years. The goals of therapy for both ADHF and chronic HF are to decrease patient symptoms, reverse ventricular remodelling, improve quality of life and decrease mortality and morbidity. Management therapies are similar for both clinical conditions.
The challenge of planning and providing nursing care that promotes the best possible clinical outcomes for the patient with ADHF remains a complex task. Because of the large number of patients and the high cost of care associated with hospital readmissions, acute and critical care nurses must develop and implement strategies that are associated with improved outcomes. The Joint Commission (formerly the Joint Commission of Accreditation of Healthcare Organisations) recently established four core measures in the acute management of patients with HF, which are used in Australia and New Zealand to promote adherence to basic standards of evidence-based care (see Box 34-4).
BOX 34-4 Core measures for heart failure
• Written discharge instructions or educational material must be given to the patient or caregiver and include all of the following: activity level, diet, discharge medications, follow-up appointment, weight monitoring and symptom management.
• Left ventricular function must be documented in the hospital record to indicate that it was assessed before or during hospitalisation or will be assessed after discharge.
• Patients with known systolic dysfunction of moderate to severe impairment (ejection fraction <40%) and without contraindication to angiotensin-converting enzyme (ACE) inhibitor will be prescribed an ACE inhibitor at hospital discharge. An angiotensin receptor blocker is an acceptable alternative for patients with contraindication to ACE inhibitors.
• Patients who are current smokers or former smokers who quit in the past 12 months will be given smoking cessation advice or counselling during the hospital stay.
The optimal treatment of ADHF remains a challenge and an important area of research today. The use of various diuretic regimens, vasoactive drugs, newer pharmacological agents, ultra-filtration and novel device therapy are being explored. Data from the Acute Decompensated Heart Failure National Registry (ADHERE) indicate that patients receiving early treatment with IV diuretics and vasoactive drugs have better outcomes and shorter hospital and intensive care stays than patients who have delays in treatment. This has led to new protocols from the ACC/AHA regarding the treatment of ADHF.7 Diuretics have long been the standard drugs used for decreasing preload and improving the patient’s signs and symptoms. This single therapeutic approach is now under reconsideration due to the findings from the ADHERE. Thus treatment strategies should include improving LV function by decreasing intravascular volume, decreasing venous return (preload), decreasing afterload, controlling HR and rhythm, improving gas exchange and oxygenation, increasing CO, reducing anxiety, preserving target organ function (e.g. kidneys) and decreasing progression of the disease.7,8 This would include the use of oxygen therapy, diuretics, vasodilators and possibly inotropic agents. The use of circulatory assist devices may also be used for the acutely ill patient to preserve heart and target organ function. Box 34-3 lists the major components of the therapeutic approach.
 Decreasing intravascular volume
Decreasing intravascular volume
Decreasing intravascular volume with the use of diuretics reduces venous return. A loop diuretic (e.g. frusemide, bumetanide) may be used to decrease volume because it may be administered by IV bolus and its action within the kidneys occurs rapidly. By decreasing venous return to the LV and thereby reducing preload, the overfilled LV may contract more efficiently and improve CO. This increases LV function, decreases pulmonary vascular pressures and improves gas exchange.
The use of a loop diuretic may be delayed until the patient is assessed for haemodynamic and renal function and started on other vasodilator agents. The use of IV loop diuretic therapy alone has been shown to be associated with an increased risk of fatal dysrhythmias, aggravated renal dysfunction and enhanced activation of the RAAS and SNS. Overall, the effects of loop diuretics actually increase systemic vascular resistance (afterload), decrease preload and cause electrolyte imbalances.9
Ultrafiltration, or aquapheresis, is an option for the patient with volume overload. Ultrafiltration has generally been achieved through haemodialysis or central venous access. Newer technology allows removal of up to 500 mL of fluid per hour through either a peripheral or a central venous line without significantly changing mean arterial pressure. When ultrafiltration is performed, volume is removed similar to haemodialysis but without haemodynamic instability. This therapeutic modality may be an appropriate adjunctive therapy for patients with HF and renal failure. Current research trials are ongoing to evaluate this modality for patients with ADHF.8 (Ultrafiltration is discussed in Ch 46.)
 Decreasing venous return
Decreasing venous return
Decreasing venous return (preload) reduces the amount of volume returned to the LV during diastole. This can be accomplished by placing the patient in a high Fowler position with the feet horizontal in the bed or dangling at the bedside. This position helps decrease venous return because of the pooling of blood in the extremities. This position also increases the thoracic capacity, allowing improved ventilation.
IV glyceryl trinitrate is a vasodilator used in the treatment of ADHF. It reduces circulating volume by decreasing preload and also increases coronary artery circulation by dilating the coronary arteries. Therefore, glyceryl trinitrate not only reduces preload it also slightly reduces afterload (in high doses) and increases myocardial oxygen supply.10 When titrating IV glyceryl trinitrate, BP is monitored frequently (every 5–10 minutes) to avoid symptomatic hypotension.
 Decreasing afterload
Decreasing afterload
Afterload is the resistance against which the LV must pump; that is, it is the amount of work the LV has to produce to eject blood into the systemic circulation. Systemic vascular resistance (SVR) is a determinant of afterload, as is LV filling. If afterload is reduced, the CO of the LV improves and thereby decreases pulmonary congestion.
IV sodium nitroprusside is a potent vasodilator that reduces preload and afterload. Because of its potent effects on the vascular system, it is the drug of choice for patients with pulmonary oedema. By reducing both preload and afterload (by arteriolar and venous dilation), myocardial contraction improves, increasing CO and reducing pulmonary congestion. Complications of IV sodium nitroprusside include: (1) hypotension, which may require the use of dobutamine IV to maintain a mean arterial BP greater than or equal to 60 mmHg; and (2) thiocyanate toxicity, which can develop after 48 hours of use. Patients receiving sodium nitroprusside must have their BP monitored frequently (every 5–10 minutes) during the titration to avoid profound hypotension.
Morphine sulphate also reduces preload and afterload and is frequently used in the treatment of ADHF and pulmonary oedema. It dilates both the pulmonary and the systemic blood vessels, a goal in decreasing pulmonary pressures and improving gas exchange. Morphine sulphate also reduces anxiety and may assist in reducing dyspnoea.
Nesiritide is an IV vasoactive therapy for ADHF. It is a recombinant form of BNP and causes both arterial and venous dilation. The main haemodynamic effects of nesiritide include: (1) a reduction in pulmonary artery wedge pressure (PAWP); and (2) an increase in CO without increasing myocardial oxygen consumption or the occurrence of arrhythmias.11 In addition, renal perfusion is enhanced, thus protecting the kidneys. Nesiritide also has direct renal effects, resulting in the inhibition of the RAAS and the promotion of natriuresis and diuresis.10 Although classified as a vasodilator, nesiritide is also referred to as a neurohormonal blocking agent and is indicated for short-term treatment of ADHF. The main adverse effect of nesiritide is symptomatic hypotension, and BP should be closely monitored.
 Improving gas exchange and oxygenation
Improving gas exchange and oxygenation
Gas exchange may be improved by several measures. IV morphine decreases oxygen demands, which may be raised as a result of anxiety and subsequent increased musculoskeletal and respiratory activity. Administration of oxygen helps increase the percentage of oxygen in inspired air. (Oxygen therapy is discussed in Ch 27.) In severe pulmonary oedema the patient may need non-invasive ventilatory support (e.g. bilevel positive airway pressure [BiPAP]) or continuous positive airway pressure (CPAP) or intubation and mechanical ventilation (ventilatory support is discussed in Ch 67). BiPAP and CPAP are widely available in Australia and New Zealand and are valuable tools to improve alveolar function; they do this by reducing the fluid in the alveolar space and thereby improving gas exchange.1
 Improving cardiac function
Improving cardiac function
When patients become haemodynamically unstable—due to hypotension, an abnormally fast or slow HR or the development of arrhythmias—they become hypoxic with cool and clammy skin. The nursing care becomes more urgent and treatment protocols may require aggressive, complex therapies. The use of diuretics, morphine sulphate and vasodilators may not be sufficient to control symptoms. The addition of inotropic therapy may be warranted, as well as the initiation of haemodynamic monitoring to evaluate the effectiveness of interventions. Once a pulmonary artery catheter is in position, accurate measurement of CO, pulmonary artery pressure and PAWP may be made and therapy instituted and titrated to maximise CO. A PAWP of 14–18 mmHg will generally achieve the goal of increasing CO. (Haemodynamic monitoring is discussed in Ch 66.)
Digoxin is a positive inotrope that improves LV function. Digoxin increases contractility but also increases myocardial oxygen consumption. Because digoxin requires a loading dose and time to accomplish haemodynamic improvement, it is not recommended for the initial treatment of ADHF.
Other positive inotropes that can be considered include the β-adrenergic agonists (e.g. dopamine, dobutamine, adrenaline, noradrenaline). Stimulation of β-adrenergic receptors results in an increase in cyclic adenosine monophosphate (cAMP) within the myocardial cells and an increase in contractility (inotropic effect) and HR. The β-adrenergic agonists are typically used as a short-term treatment of ADHF in the intensive care unit (ICU) and, most recently, in step-down or intermediate care units with ECG monitoring capability. Dopamine is used for treatment of severe ADHF and cardiogenic shock. In addition to increasing myocardial contractility and SVR, activation of dopamine receptors in the kidneys dilates the renal blood vessels and enhances urine output.12 Unlike dopamine, dobutamine is a selective β-adrenergic agonist and works selectively on the receptors in the heart. Dobutamine does not increase SVR and may be preferred for short-term treatment of ADHF.12
Potential problems related to long-term treatment with β-adrenergic agonists include tolerance, increased ventricular irritability and increased need for oxygen by the myocardium. In addition, these drugs are only available for IV use.
Inamrinone and milrinone are two phosphodiesterase inhibitors that have been called inodilators because they increase myocardial contractility (inotropic effect) and promote peripheral vasodilation. Inhibition of phosphodiesterase increases cAMP, which enhances calcium entry into the cell and improves myocardial contractility. Inamrinone and milrinone increase CO and reduce arterial pressure (decrease afterload). Studies have shown inamrinone to be superior to dobutamine or dopamine in improving cardiac function.12 Like dopamine and dobutamine, these drugs are only available for IV use. Adverse effects include arrhythmias, thrombocytopenia and hepatotoxicity.
Although these inotropic agents can effectively increase CO, reduce filling pressures and lead to short-term clinical improvement, some data suggest that their use may be associated with increased overall mortality.11 Currently, inotropic therapy is recommended for use only in the short-term management of patients with ADHF who have not responded to conventional pharmacotherapy (e.g. diuretics, vasodilators, morphine sulphate).7
 Reducing anxiety
Reducing anxiety
Reduction of anxiety is an important nursing function, since anxiety may increase the SNS response and further increase myocardial workload. Reducing anxiety may be facilitated by a variety of nursing interventions (see NCP 34-1) and the use of sedative medications (e.g. benzodiazepines, morphine sulphate). When morphine sulphate is used, the patient often experiences relief from dyspnoea and, consequently, the anxiety that is often associated with dyspnoea. Though morphine-induced respiratory depression is rare, the patient’s respiratory rate should be monitored.
Once the patient is more stable, determination of the cause of pulmonary oedema is important. Diagnosis of systolic or diastolic failure will then determine further management protocols. Aggressive drug therapy may continue with IV forms of diuretics, vasodilators and inotropes. Nursing care focuses on continual physical assessment, haemodynamic monitoring and evaluation of the patient’s response to treatment.
MULTIDISCIPLINARY CARE: CHRONIC HEART FAILURE
The main goal in the treatment of chronic HF is to treat the underlying cause and contributing factors, maximise CO, provide treatment to alleviate symptoms, improve ventricular function, improve quality of life, preserve target organ function and improve mortality and morbidity (see Box 34-4). The management of arrhythmias is discussed in Chapter 35, hypertension in Chapter 32, valvular disorders in Chapter 36 and coronary artery disease in Chapter 33.
In a person with HF, oxygen saturation of the blood is reduced because the blood is not adequately oxygenated in the lungs. Administration of oxygen improves saturation and assists greatly in meeting tissue oxygen needs. Thus, oxygen therapy helps relieve dyspnoea and fatigue. Optimally, either arterial blood gases (ABGs) or pulse oximetry is used to monitor the effectiveness of oxygen therapy.
Physical and emotional rest allows the patient to conserve energy and decreases the need for additional oxygen. The degree of rest recommended depends on the severity of HF. A patient with severe HF may be on bed rest with limited activity. A patient with mild-to-moderate HF can be ambulatory with a restriction of strenuous activity. The patient should be instructed to participate in limited activities with adequate recovery periods.
Non-pharmacological therapies are now used in the management of HF patients who are receiving maximum medical therapy, continue to have NYHA class III or IV symptoms and have a widened QRS interval. One therapy is biventricular pacing. Traditional pacemakers pace one or two chambers (e.g. atrium and/or ventricle), but cardiac resynchronisation therapy (CRT) coordinates right and left ventricle contractility through biventricular pacing. The ability to have normal electrical conduction within the right and left ventricles increases LV performance and CO. This additional therapy allows patients to increase their exercise capacity and decrease their overall symptoms. CRT does not prolong life, but it does improve quality of life in patients with NYHA class III or IV HF.13 CRT therapy can be combined with traditional pacing capability as well as defibrillator technology. If the patient has ischaemia-induced HF and an EF of less than 35%, the implementation and use of an implantable cardioverter–defibrillator (ICD) with CRT may be warranted.13 Life-threatening ventricular arrhythmias (e.g. ventricular tachycardia) are a complication of the ischaemic myocardium and can cause sudden cardiac death. The addition of an ICD in these patients has reduced the overall mortality. (Pacemakers and defibrillators are discussed in Ch 35.)
Cardiac transplantation is one form of treatment for ADHF and chronic HF. However, the lack of donor hearts and the challenges of care make it an option for only a small number of patients with HF. Stringent criteria are necessary to select the few patients with advanced HF who can even hope to receive a transplanted heart. (Cardiac transplantation is discussed on p 910.)
Several mechanical options are available to sustain HF patients with deteriorating conditions, especially those awaiting cardiac transplantation. The intraaortic balloon pump (IABP) is frequently employed in the setting of myocardial infarction or perioperatively during cardiac surgery. The IABP can be useful in the haemodynamically unstable HF patient because it decreases SVR, PAWP and pulmonary artery pressure by as much as 25%, leading to improved CO.14 However, the limitations of bed rest, infection and vascular complications preclude long-term use. (IABPs are discussed in Ch 66.) Ventricular assist devices (VADs) provide highly effective long-term support for up to 2 years and have become standard care in many heart transplant centres. VADs are used as a bridge to transplantation, effectively increasing cardiac function until a donor heart becomes available for the patient. The use of a permanent, implantable VAD, known as destination therapy, is an option for patients with advanced NYHA class IV HF who are not candidates for heart transplantation.15 (VADs are discussed in Ch 66.)
DRUG THERAPY: CHRONIC HEART FAILURE
General therapeutic objectives for drug management of chronic HF include the following: (1) identification of the type of HF and underlying causes; (2) correction of sodium and water retention and volume overload; (3) reduction of cardiac workload; (4) improvement of myocardial contractility; and (5) control of precipitating and complicating factors (see Box 34-5). The aims of treating HF are to improve symptoms, minimise side effects of treatment, prevent morbidity and prolong survival.10 Current therapeutic approaches stress the role of ACE inhibitors, diuretics, inotropic agents and vasodilator drugs (see Box 34-5).11
DRUG THERAPY
Angiotensin-converting enzyme (ACE) inhibitors (see Table 32-5)
Diuretics (see Table 32-5)
Antiarrhythmic drugs (see Box 35-3)
ACE inhibitors
The benefits of ACE inhibitors in the treatment of all stages of heart failure have been well documented.12,13 ACE inhibitors are useful in both systolic and diastolic HF and they are the first-line therapy in the treatment of HF. Examples of ACE inhibitors include captopril and enalapril. Other examples are discussed in Chapter 32 and listed in Table 32-6.
The conversion of angiotensin I to the potent vasoconstrictor angiotensin II requires the presence of ACE. ACE inhibitors exert their effects by blocking this enzyme, resulting in decreased levels of angiotensin II. As a result, plasma aldosterone levels are also reduced. Because CO is dependent on afterload in chronic HF, the reduction in SVR seen with the use of ACE inhibitors produces a significant increase in CO. Furthermore, with the use of ACE inhibitors, although BP may be decreased, tissue perfusion is maintained or increased as a result of the improvement of CO and redistribution of regional blood flow. Other haemodynamic changes include a reduction in: (1) pulmonary artery pressure; (2) right arterial pressure; and (3) LV filling pressure.
Side effects of ACE inhibitors include symptomatic hypotension, chronic cough and renal insufficiency (when ACE inhibitors are used in high doses). Ageing and baseline renal insufficiency slow the metabolism of ACE inhibitors and may therefore lead to increased serum drug levels.14,15 It is recommended that these drugs be started at the lowest dose and that BP and renal function be monitored at regular intervals. Overall, ACE inhibitors are well tolerated by patients.
In patients who are unable to tolerate ACE inhibitors because of angio-oedema or cough, angiotensin II receptor blockers, such as candesartan, eprosartan, irbesartan and telmisartan, may be used (see Table 32-6).16
Diuretics
Diuretics are used in HF to mobilise oedematous fluid, reduce pulmonary venous pressure and reduce preload (see Table 32-6). If excess extracellular fluid is excreted, blood volume returning to the heart can be reduced and cardiac function improved.
Diuretics act on the kidneys by promoting excretion of sodium and water. Many varieties of diuretics are available and some have specific indications for use. Thiazide diuretics may be the first choice in chronic HF because of their convenience, safety, low cost and effectiveness. They are particularly useful in treating oedema secondary to HF and in controlling hypertension. The thiazides inhibit sodium reabsorption in the distal tubule, thus promoting excretion of sodium and water.
Loop diuretics (e.g. frusemide, bumetanide) are potent diuretics. These drugs act on the ascending loop of Henle to promote the excretion of sodium, chloride and water. Frusemide is more commonly used in acute HF and pulmonary oedema because it is slightly more predictable in its response. Problems in using loop diuretics include a reduction in serum potassium levels, ototoxicity and a possible allergic reaction in the patient who is sensitive to sulfur-type drugs.
Spironolactone is a potassium-sparing diuretic that promotes sodium and water excretion but blocks potassium excretion by blocking the action of aldosterone. The drug is inexpensive and effective in patients with advanced HF.16 Aldosterone receptor antagonism with spironolactone has been proven effective in the treatment of patients with HF. Spironolactone appears to be additive to the benefits of ACE inhibitors and is appropriate to use while renal function is adequate. A combination of diuretics may be administered for maximum potential.
Inotropic drugs
The use of inotropic drugs in patients with HF is directed at improving cardiac contractility to increase CO, decrease LV diastolic pressure and decrease SVR. Types of inotropic agents are listed in Box 34-5.
Digoxin
Digoxin (cardiac glycoside) has been the mainstay of treatment of HF and has been used for more than 200 years, but its use has recently become controversial because it has never been shown to reduce mortality rates. It does, however, seem to offer some benefit in moderate-to-severe HF by reducing hospitalisations and symptoms (see Evidence-based practice box). It is particularly useful in the treatment of HF accompanied by atrial flutter and/or fibrillation with a rapid ventricular rate. Digoxin increases the force or strength of cardiac contraction (inotropic action). It also decreases the conduction speed within the myocardium and slows the HR (chronotropic action). This action allows more complete emptying of the ventricles, thus diminishing the volume remaining in the ventricles during diastole. CO increases because of an increased stroke volume from improved contractility.
An individual receiving digoxin is subject to digoxin toxicity (see Box 34-6). Some of the earliest symptoms of toxicity are anorexia, nausea and vomiting. Visual disturbances, such as ‘yellow’ vision, can also occur. Arrhythmias are a common indication of digoxin toxicity. Although almost any arrhythmia can occur, the types most frequently found are premature beats, atrial fibrillation and first-degree heart block.
BOX 34-6 Manifestations of digoxin toxicity
DRUG THERAPY
EVIDENCE-BASED PRACTICE
Clinical question
In patients with normal sinus rhythm (P), is digoxin (I) effective in treating heart failure (HF) (O)?
Implications for nursing practice
• If a patient with HF is in sinus rhythm, digoxin should be used as a second-line drug.
• The drugs being taken by a patient with HF must be evaluated to assess for effectiveness and possible interactions.
P, patient population of interest; I, intervention or area of interest; O, outcome(s) of interest.
Hypokalaemia is one of the most common causes of digoxin toxicity, resulting in arrhythmias because low serum potassium levels enhance ectopic pacemaker activity. Monitoring the serum potassium levels of patients receiving both digoxin and potassium-losing diuretics (e.g. thiazides, loop diuretics) is essential. Other electrolyte imbalances, such as hyperkalaemia, hypercalcaemia and hypomagnesaemia, can also precipitate toxicity.
Diseases of the kidney and liver increase susceptibility to digoxin toxicity because most of the preparations are metabolised and eliminated by these organs. Older adults are especially prone to digoxin toxicity because digoxin accumulation occurs sooner with decreased liver and kidney function and slowed body metabolism, which occur with ageing.
β-adrenergic agonists
β-adrenergic agonists include dopamine, dobutamine, adrenaline and noradrenaline. Stimulation of β-adrenergic receptors results in an increase in cAMP within the myocardial cells and an increase in contractility (inotropic effect). The β-adrenergic agents are typically used as a short-term treatment of acute exacerbations of HF in the coronary care unit (CCU). However, their role in long-term therapy of HF is controversial. Potential problems related to long-term treatment with β-adrenergic agonists include tolerance, increased ventricular irritability and increased need for oxygen by the myocardium.
Dopamine is an adrenergic agonist used for therapy of severe HF and cardiogenic shock. In addition to increasing myocardial contractility, it also increases blood flow to the renal, mesenteric, coronary and cerebrovascular beds. The action of dopamine is highly effective in patients with HF because it increases CO (contractility) as well as urine output (decreases preload).
Phosphodiesterase inhibitors
Inhibition of phosphodiesterase increases cAMP, which enhances calcium entry into the cell and improves myocardial contractility. Phosphodiesterase inhibitors are also potent vasodilators. They increase CO and reduce arterial pressure (decrease afterload). These drugs are not currently available in oral form and therefore are limited to short-term use in the critical care setting.
Milrinone increases myocardial contraction, increases CO, promotes peripheral vasodilation and decreases SVR, thus augmenting performance of the LV. Adverse reactions include arrhythmias, thrombocytopenia and GI effects.
Vasodilator drugs
Vasodilator drugs are a class of drugs that have been clearly shown to improve survival in overt HF. The goals of vasodilator therapy in the treatment of HF include: (1) increasing venous capacity; (2) improving the EF through improved ventricular contraction; (3) slowing the process of ventricular dysfunction; (4) decreasing heart size; and (5) avoiding stimulation of the neurohormonal responses initiated by the compensatory mechanisms of HF.
Sodium nitroprusside
Sodium nitroprusside is the most commonly used IV vasodilator in the management of acute HF and pulmonary oedema (see Ch 28).
Nitrates
Nitrates cause vasodilation by acting directly on the smooth muscle of the vessel wall. Their effects primarily involve increasing venous capacitance, dilating the pulmonary vasculature and improving arterial compliance. The major haemodynamic effect of nitrates is to decrease preload. Nitrates are of particular benefit in the management of myocardial ischaemia related to HF because they promote vasodilation of the coronary arteries. One specific deterrent to the use of nitrates in HF is nitrate tolerance.
β-adrenergic blockers
β-adrenergic blockers are of increasing importance in the management of HF. Marked improvement in patient survival occurs with the use of β-adrenergic blockers, specifically carvedilol and long-acting metoprolol. β-adrenergic blockers directly block the SNS’s negative effects on the failing heart, such as increased HR. They are used in combination with ACE inhibitors, digoxin and diuretics. β-adrenergic blockers must be started gradually, increasing the dosage slowly every 2 weeks as tolerated by the patient. Patient teaching and close monitoring help identify side effects of β-adrenergic blockers, such as dizziness or oedema.
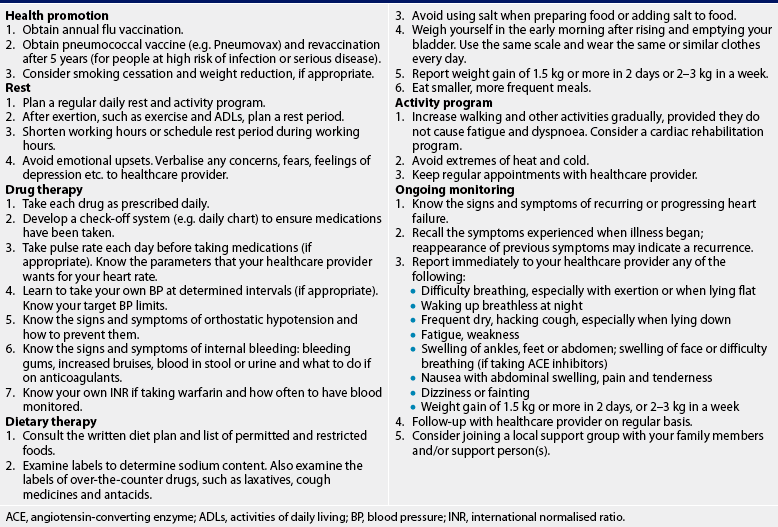
NUTRITIONAL THERAPY: CHRONIC HEART FAILURE
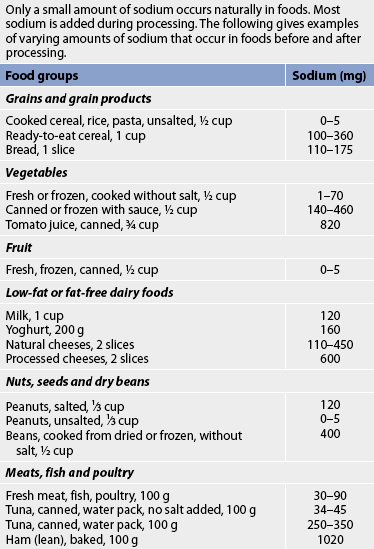
Diet education and weight management are critical to the patient’s control of chronic HF. The nurse or dietician should obtain a detailed diet history, determining not only what foods the patient eats and when but also the sociocultural value of food. This database can be used to assist the patient in solving problems and developing an individual diet plan. The patient should be taught what foods are low and high in sodium and ways to enhance food flavours without using salt (e.g. substituting lemon juice and various spices).
The oedema of chronic HF is often treated by dietary restriction of sodium. The degree of sodium restriction depends on the severity of the HF and the effectiveness of diuretic therapy. Diets that are severely restricted in sodium are rarely prescribed because they are unpalatable and patient compliance is poor. The Dietary Approaches to Stop Hypertension (DASH) diet is effective as a first-line therapy for many individuals with isolated systolic hypertension (see Table 32-4). This diet is now widely recommended in Australia and New Zealand for the patient with HF, with or without hypertension.1,17
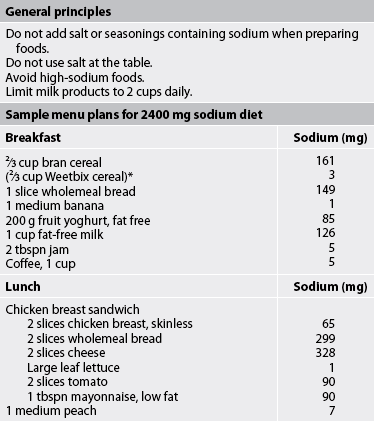
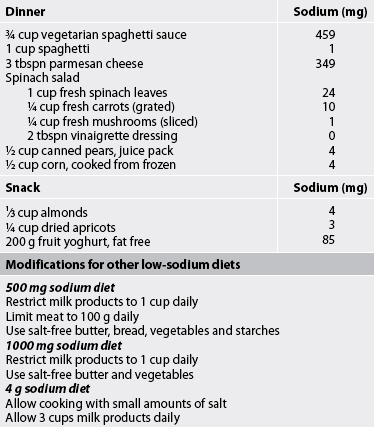
The normal daily dietary intake of sodium ranges from 3 g to 7 g. A commonly prescribed diet for patients with mild HF is a 2 g sodium diet (see Table 34-4). All foods high in sodium should be eliminated (see Tables 34-5 and 34-6). For more severe HF, sodium intake is restricted to 500–1000 mg (see Table 34-4). On this diet, milk, cheese, bread, cereals, tinned soups and some tinned vegetables must be eliminated. The patient and family must be instructed on how to read labels to look for sodium as an ingredient.
TABLE 34-5 Sodium label language
| Phrase | What it means |
|---|---|
| Sodium free or salt free | Less than 5 mg per serving |
| Very low sodium | 35 mg or less of sodium per serving |
| Low sodium | 120 mg or less of sodium per serving |
| Low-sodium meal | 140 mg or less of sodium |
| Reduced or less sodium | At least 25% less sodium than regular |
| Light in sodium | 50% less sodium than regular |
| Unsalted or no salt added* | No salt added to the product during processing |
*Use caution with products advertised as no salt replacements—they may contain high potassium.
Fluid restrictions are not commonly prescribed for patients with mild-to-moderate HF. Diuretic therapy and digoxin act as effective diuretics to promote fluid excretion. However, in moderate-to-severe HF and renal insufficiency, fluid restrictions are usually implemented.
Instructing patients to weigh themselves daily is important for monitoring fluid retention, as well as weight reduction. Patients should be instructed to weigh themselves at the same time each day, preferably before breakfast, while wearing the same type of clothing. This helps ensure valid comparisons from day to day and helps identify early signs of fluid retention. If a patient experiences a weight gain of 1.5 kg or more over 2–5 days, the healthcare provider should be notified.
 NURSING MANAGEMENT: CHRONIC HEART FAILURE
NURSING MANAGEMENT: CHRONIC HEART FAILURE
 Nursing assessment
Nursing assessment
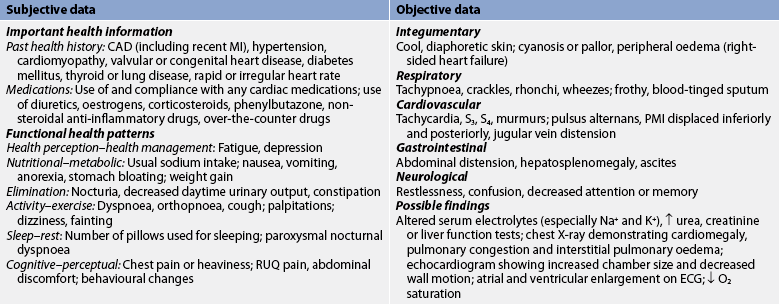
Subjective and objective data that should be obtained from a patient with HF include those presented in Table 34-7.
 Nursing diagnoses
Nursing diagnoses
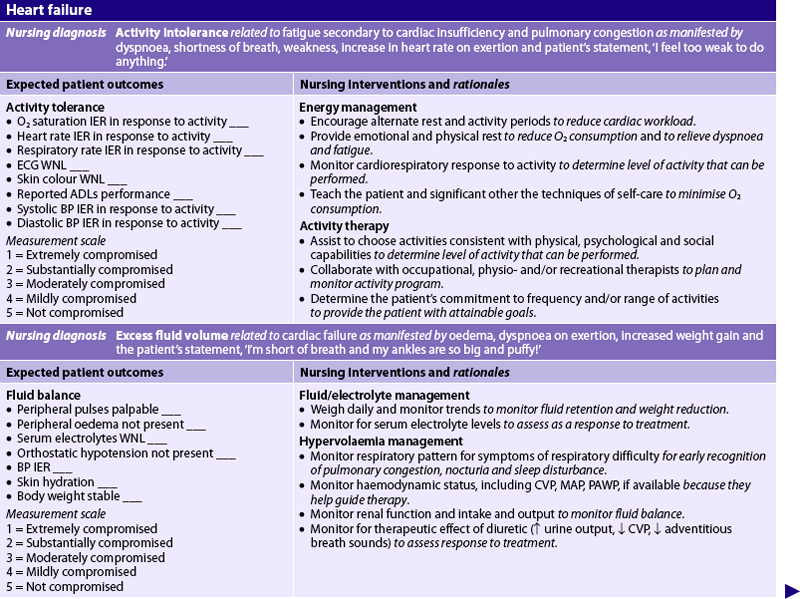
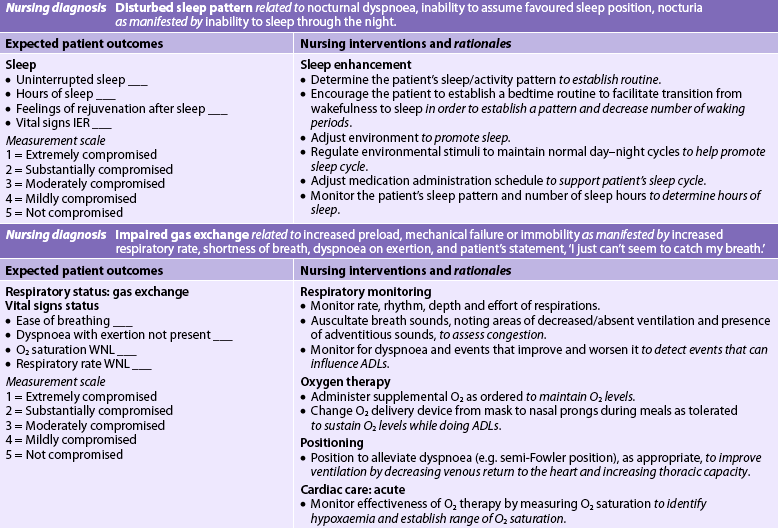
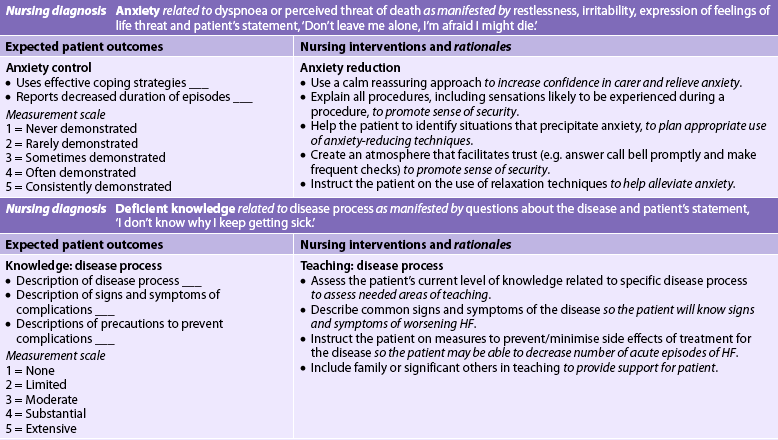
Nursing diagnoses for the patient with HF include, but are not limited to, those presented in NCP 34-1.
 Planning
Planning
The overall goals are that the patient with HF will have: (1) decreased peripheral oedema; (2) decreased shortness of breath; (3) increased exercise tolerance; (4) compliance with the medication regimen; and (5) no complications related to HF.
 Nursing implementation
Nursing implementation
 Health promotion
Health promotion
An important measure used to prevent HF is the treatment or control of the underlying heart disease. For example, in valvular disease, valve replacement should be planned before lung congestion develops. Coronary revascularisation procedures should be performed in patients with CAD. Another important preventative measure concerns early and continued treatment of hypertension. Hyperlipidaemic states in patients with CAD should be managed with diet, exercise and medication. The use of antiarrhythmic agents or pacemakers is indicated for people with serious arrhythmias or conduction disturbances. In addition, patients with HF should be counselled to obtain vaccinations against the flu and pneumonia.
When a patient is diagnosed with HF, preventative care should focus on slowing the progression of the disease. Knowledge of the importance of following the medication, diet and exercise regimens and cardiac rehabilitation is essential. The in-hospital nurse may request home nursing care for the patient and family to provide follow-up care and to monitor the patient’s response to treatment. Early detection of signs and symptoms of worsening failure may help modify care and prevent an acute episode requiring further hospitalisation.18
 Acute intervention
Acute intervention
Successful HF management depends on several important principles: (1) HF is a progressive disease and treatment plans are established with quality-of-life goals; (2) symptom management is controlled by the patient with self-management tools (daily weighing, medication regimen, exercise plan); (3) salt and water must be restricted; (4) exercise and energy must be conserved; and (5) support systems are essential to the success of the entire treatment plan.19,20
Many patients with HF do not experience an acute episode. If they do, they are usually initially managed in a CCU and later transferred to a general unit when their condition has stabilised. The nursing care plan for the patient with HF (see NCP 34-1) applies to the patient with stabilised ADHF or chronic HF.
 Ambulatory and home care
Ambulatory and home care
HF is a chronic illness for most people. Important nursing responsibilities are: (1) teaching the patient about the physiological changes that have occurred; (2) assisting the patient to adapt to both the physiological and the psychological changes; and (3) integrating the patient and the patient’s family or support system in the overall care plan.21 Research has revealed that patients with NYHA class III or IV HF have a high risk of anxiety and depression. In one large study, major depression was found to be more prevalent in female patients and patients younger than 60 years of age.22,23 It must be emphasised to the patient that it is possible to live productively with this chronic health problem. Specialist HF nurse-monitored health action plans can prevent or limit future unplanned hospital admissions. Specialist HF nursing care provided in the patient’s home should be followed up with ongoing clinical assessment, monitoring of vital signs and monitoring the response to therapy. Managing patients out of the hospital is a priority of care to improve quality of life and restore control of HF symptoms. A patient and family teaching guide for the patient with HF is presented in Table 34-8.
ACE, angiotensin-converting enzyme; ADLs, activities of daily living; BP, blood pressure; INR, international normalised ratio.
Does nutritional counselling help heart failure symptoms?
EVIDENCE-BASED PRACTICE
Clinical question
For patients with heart failure (P), does referral to a nutritionist (I) decrease levels of oedema and fatigue (O)?
Critical appraisal and synthesis of evidence
Implications for nursing practice
• Refer patients with HF for nutritional counselling.
• Reinforce fluid intake between 1.4 and 1.9 L per day, depending on symptoms (i.e. oedema, fatigue, shortness of breath).
P, patient population of interest; I, intervention or area of interest; O, outcome(s) of interest.
American Dietetic Association (ADA). ADA heart failure: evidence-based nutrition practice guideline. Chicago: American Dietetic Association; 2008. Available at www.adaevidencelibrary.com/topic.cfm?cat=3249
Patients with HF are usually required to take medication for the rest of their lives. This often becomes difficult because a patient may be asymptomatic when HF is under control. The patient needs to understand that the disease is chronic and that medication must be continued to keep the HF under control.
The patient should evaluate the action of the prescribed drug and be taught to recognise the manifestations of drug toxicity. The patient should also be taught how to take their pulse rate and to know under what circumstances drugs, especially digoxin and β-adrenergic blockers, should be withheld and a healthcare provider consulted. The pulse rate should always be taken for 1 full minute. A pulse rate lower than 50–60 beats per minute may be a contraindication to taking digoxin, unless specified otherwise by the healthcare provider. However, in the absence of primary heart block or the development of ventricular ectopy, a pulse rate of 60 beats per minute or less may not be a contraindication to taking digoxin or β-adrenergic blockers. A pulse rate of 50 beats per minute (especially in a patient who is also taking β-adrenergic blockers) may be acceptable.
It may also be appropriate to instruct patients in home BP monitoring, especially for those patients with hypertension. Patients should also be taught the symptoms of hypokalaemia if diuretics that cause potassium excretion are being taken. (Manifestations of hypokalaemia are discussed in Ch 16.) Hypokalaemia sensitises the myocardium to digoxin. Consequently, toxicity may develop from an ordinary dose of digoxin. Frequently the patient who is taking thiazide or loop diuretics is given supplemental potassium.
The nurse, physiotherapist or occupational therapist can instruct the patient in energy-saving and energy-efficient behaviours after an evaluation of daily activities has been done. For example, once the nurse understands the patient’s daily routine, suggestions can be made for simplification of work or modification of an activity. Frequently, the patient needs a prescription for rest after an activity. Many hard-working people need the ‘permission’ to not feel ‘lazy’. Sometimes an activity that the patient enjoys may need to be eliminated. In such situations the patient should be helped to explore alternative activities that cause less physical and cardiac stress. The physical environment may require modification in situations in which there is an increased cardiac workload demand (e.g. frequent climbing of stairs). The nurse can help the patient identify areas where outside assistance can be obtained. Referral to a supervised exercise program, coordinated by an exercise physiologist or physiotherapist, is recommended as it can improve the patient’s deconditioned physical state and functional capacity.
The specialist HF nurse is essential in the care of the HF patient, family and carers. Advanced practice nurses—such as nurse practitioners—have established a valued and expert role in providing home, clinic and hospital-based HF care alongside cardiologists and other members of the multi-disciplinary health team. Frequent physical assessments, including vital signs and weight, are extremely important. Specialist HF nurses frequently work within protocols set up with the patient’s healthcare team. The protocols may enable the nurse and patient to identify problems, such as an increase in weight and HR, as evidence of worsening HF and institute interventions to prevent hospitalisation. This may include altering medications and fluid restrictions and recommending an appropriately supervised exercise program. Education provided by specialist HF nurses to patients for disease and symptom management of HF is paramount in reducing the number of hospitalisations, increasing functional capacity and increasing quality of life.22
 Future directions
Future directions
Increasingly, the focus is on managing patients within their own homes. (See Ch 69 for discussion on chronic disease management.) To do this requires the use of technology to monitor patients remotely (e.g. electronic scales, BP cuff and pulse oximeter) to collect physiological data. The technology can also be programmed to audibly ask the patient questions such as, ‘Are you short of breath today?’ Results are then transmitted electronically to the healthcare provider. Once received, the data are reviewed and, based on the information, a follow-up call may be made to the patient to further assess the situation or an unplanned visit may be scheduled.