Chapter 55 NURSING ASSESSMENT: nervous system
1. Describe the functions of neurons and neuroglia.
2. Explain the electrochemical aspects of nerve impulse transmission.
3. Explain the anatomical location and functions of the cerebrum, brainstem, cerebellum, spinal cord, peripheral nerves and cerebrospinal fluid.
4. Identify the major arteries supplying the brain.
5. Describe the functions of the 12 cranial nerves.
6. Compare the functions of the two divisions of the autonomic nervous system.
7. Describe age-related changes in the neurological system and differences in assessment findings.
8. Identify the significant subjective and objective data related to the nervous system that should be obtained from a patient.
9. Describe the techniques used in the physical assessment of the nervous system.
10. Differentiate between normal and common abnormal findings from a physical assessment of the nervous system.
11. Describe the purpose, significance of results and nursing responsibilities related to diagnostic studies of the nervous system.
Structure and function of the nervous system
The human nervous system is a highly specialised system responsible for the control and integration of the body’s many activities. The nervous system can be divided into the central nervous system and the peripheral nervous system. The central nervous system (CNS) consists of the brain and spinal cord. The peripheral nervous system (PNS) consists of the cranial and spinal nerves and the peripheral components of the autonomic nervous system (ANS). Before considering higher-order structures and their functions, cellular elements and nerve impulse transmission are discussed.
CELLS OF THE NERVOUS SYSTEM
The nervous system is made up of two types of cells: neurons and neuroglia. Although neuroglial cells are more numerous, they mainly support the neurons (the primary functional units of the nervous system). Neurons are generally non-mitotic; that is, they do not replicate. However, it is thought that damaged neurons can regenerate if the cell bodies remain intact; this, however, remains unproven.1 Neuroglia are mitotic and can replicate themselves. In general, when neurons are destroyed, the tissue is replaced by the proliferation of neuroglia cells.
Neurons
Neurons come in many different shapes and sizes but they all share common characteristics: (1) excitability, or the ability to generate a nerve impulse; (2) conductivity, or the ability to transmit the impulse to other portions of the cell; and (3) the ability to influence other neurons, muscle cells and glandular cells by transmitting nerve impulses to them.
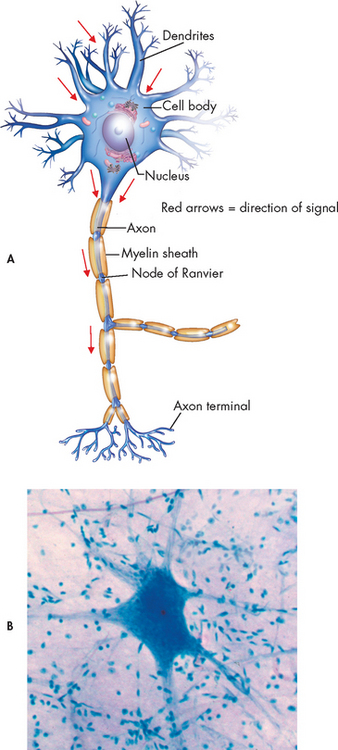
A typical neuron consists of a cell body, an axon and several dendrites (see Fig 55-1). The cell body, which contains the nucleus and cytoplasm, is the metabolic centre of the neuron. Dendrites are short processes extending from the cell body. They receive nerve impulses from the axons of other neurons and conduct impulses towards the cell body. The nerve axon projects varying distances from the cell body, ranging from several micrometres to more than a metre. Its function is to carry nerve impulses to other neurons or to end organs. The end organs are smooth and striated muscles and glands. Axons may be myelinated or unmyelinated. Many axons present in the CNS and PNS are covered by a segmentally interrupted myelin sheath composed of a white, lipid substance that acts as an insulator for the conduction of impulses. Generally, the smaller fibres are unmyelinated.
Neuroglia
Neuroglia, or glial cells, provide support, nourishment and protection to neurons. They constitute almost half the brain and spinal cord mass and are 5–10 times more numerous than neurons. Different types of glial cells, including oligodendrocytes, astrocytes, ependymal cells and microglia, have specific functions.
Oligodendrocytes are specialised cells that produce the myelin sheath of nerve fibres in the CNS (Schwann cells myelinate the nerve fibres in the periphery) and are primarily found in the white matter of the CNS. Astrocytes provide structural support to neurons and their delicate processes, form the blood–brain barrier with the endothelium of the blood vessels and play a role in synaptic transmission (conduction of impulses between neurons). They are found primarily in grey matter. When the brain is injured, astrocytes act as phagocytes for neuronal debris. They help restore the neurochemical milieu and provide support for repair. Proliferation of astrocytes contributes to the formation of scar tissue (gliosis) in the CNS. Ependymal cells line the brain’s ventricles and aid in the secretion of cerebrospinal fluid (CSF). Microglia, a type of macrophage, are relatively rare in normal CNS tissue. They are phagocytes and are important in host defence.
Brain tumours are generally classified according to the cell of origin.2 Most primary CNS tumours involve neuroglia. Primary malignancies involving neurons are rare because these cells are usually not mitotic. Brain tumours are discussed in more detail in Chapter 56.
NERVE REGENERATION
If the axon of the nerve cell is damaged, the cell attempts to repair itself. When damaged, all nerve cells attempt to grow back to their original destinations by sprouting many branches from the damaged ends of their axons. Unfortunately, axons in the CNS are less successful than peripheral axons in regenerating. The situation is more complicated because many more axons are likely to be involved; astrocytes produce scar tissue that can prevent axon growth; and astrocytes also produce chemicals that block the regrowth of axons.3 Regenerating nerve fibres grow 4 mm per day.
In the PNS (outside the brain and spinal cord), injured nerve fibres can regenerate successfully by growing within the protective myelin sheath of the supporting Schwann cells, if the cell body is intact. The result of nerve regeneration depends on the number of axon sprouts that join with the appropriate Schwann cell columns and re-innervate appropriate end organs.
NERVE IMPULSE
The purpose of a neuron is to initiate, receive and process messages about events both inside and outside the body. The initiation of a neuronal message (nerve impulse) involves the generation of an action potential. Once an action potential is initiated, a series of action potentials travels along the axon. When the impulse reaches the end of the nerve fibre, it is transmitted across the junction between nerve cells (the synapse) by a chemical interaction involving neurotransmitters. This chemical interaction generates another set of action potentials in the next neuron. These events are repeated until the nerve impulse reaches its destination.
Action potential
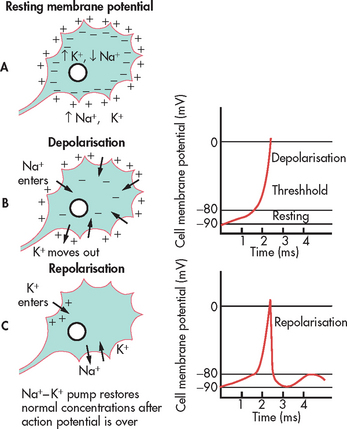
When nerve cells are in a resting (non-active) state, the inside of the cell carries a negative electric charge relative to the outside of the cell. Sodium ions (Na+) are in high concentration outside the cell and potassium ions (K+) are in high concentration inside the cell. The difference in electric charge across the cell membrane is termed the resting membrane potential (see Fig 55-2). An action potential occurs when a stimulus is of sufficient magnitude to alter the membrane potential.
During the action potential, the cell membrane becomes more permeable to Na+, allowing it to move readily into the cell. The resulting change in the voltage across the cell membrane is called depolarisation. The inside of the cell temporarily becomes positive relative to the outside. After rapid depolarisation, repolarisation (the inside of the cell becoming negative relative to the outside) is facilitated by a slower increase in K+ permeability, which in turn is caused by the depolarisation associated with entry of Na+ into the cell. The whole process of depolarisation and repolarisation of the nerve cell membrane takes only 1–2 milliseconds. With repeated action potentials, the cells accumulate Na+. An active metabolic process within the cell is required to move Na+ out of, and K+ back into, the cell. This metabolic process is accomplished by the Na+–K+ pump, which requires energy from the breakdown of adenosine triphosphate (ATP).
The action potential has an all-or-none quality—that is, once the cell depolarises enough to cause an action potential, the size of the action potential is independent of the strength of the stimulus. When an action potential is initiated at one point of a neuron, it is transmitted along the axon without losing its intensity.
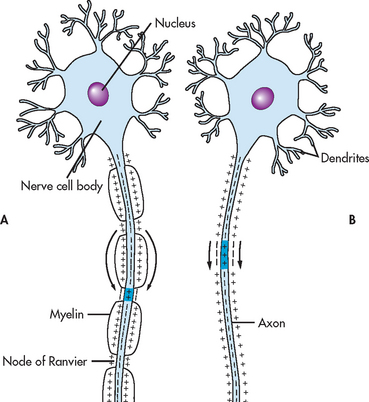
Because of its insulating capacity, myelination of nerve axons facilitates the conduction of an action potential. Many peripheral nerve axons have gaps, termed nodes of Ranvier, at regular intervals in the myelin sheath surrounding them. An action potential travelling down one of these axons hops from node to node without traversing the insulated membrane segment between nodes, making the action potential travel much faster than it would otherwise. This is called saltatory (hopping) conduction. In an unmyelinated fibre, the wave of depolarisation traverses the entire length of the axon, with each portion of the membrane becoming depolarised in turn. Figure 55-3 compares nerve impulse transmission of myelinated and unmyelinated fibres.
Synapse
A synapse is the structural and functional junction between two neurons. It is the point at which the nerve impulse is transmitted from one neuron to another or from neuron to glands or muscles. The essential structures of synaptic transmission are a presynaptic terminal, a synaptic cleft and a receptor site on the postsynaptic cell (see Fig 55-4). There are two types of synapses: electrical and chemical. In an electrical synapse an action potential moves from neuron to neuron directly by allowing electrical current to flow between neurons. In a chemical synapse an action potential reaches the end of the axon (presynaptic terminal); then it causes the release of a chemical substance (neurotransmitter) from tiny vesicles within the axon terminal. This release depends on the influx of calcium, initiated by depolarisation of the nerve terminal. The neurotransmitter then crosses the microscopic space (synaptic cleft) between the two neurons and attaches to receptor sites of the receiving (postsynaptic) neuron. This causes a change in the permeability of the postsynaptic cell membrane to specific ions such as Na+ and K+ and a change in the electric potential of the membrane.
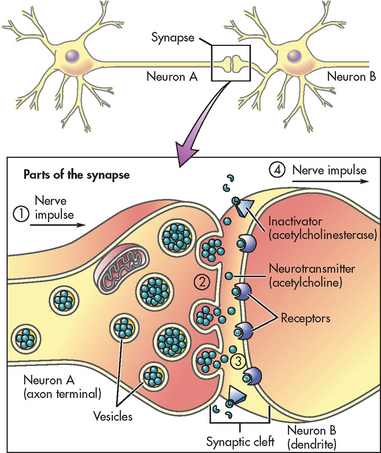
Figure 55-4 The synapse is located in the space between neuron A and neuron B. Parts of the synapse include the neurotransmitters, inactivators and receptors. Neurotransmitters are located in the vesicles of neuron A. Inactivators are located on the membrane of neuron B. Receptors are located on the membrane of neuron B.
Neurotransmitters
A neurotransmitter is a chemical agent involved in the transmission of an impulse across the synaptic cleft. Some neurotransmitters are excitatory: they cause an increase in Na+ permeability at the postsynaptic cell membrane, increasing the likelihood that an action potential will be generated. This type of synaptic input results in an excitatory postsynaptic potential. Other neurotransmitters are inhibitory: they cause an increase in the permeability of K+ and chloride ions (Cl−), decreasing the likelihood that an action potential will be generated. This type of synaptic input results in an inhibitory postsynaptic potential.
Each of the hundreds to thousands of synaptic connections of a single neuron has an influence on that neuron. The net effect of the input is sometimes excitatory and sometimes inhibitory. In general, the net effect depends on the number of presynaptic neurons that are releasing neurotransmitters on the postsynaptic cell. A presynaptic cell that releases an excitatory neurotransmitter does not always cause the postsynaptic cell to depolarise enough to generate an action potential. However, when many presynaptic cells release excitatory neurotransmitters on a single neuron, the sum of their input is enough to generate an action potential. The presynaptic input can be summed by the number of presynaptic cells firing (spatial summation) or by the frequency of firing of a single presynaptic cell (temporal summation). Summation usually occurs by both events.
The effect of an excitatory or inhibitory neurotransmitter depends on which ion channels in the postsynaptic membrane are influenced by that neurotransmitter. The neurotransmitters that are known generally to have an excitatory influence are acetylcholine, noradrenaline, serotonin, dopamine, glutamate and histamine. The neurotransmitters that generally have an inhibitory influence are gamma-aminobutyric acid (GABA) and glycine.
Neurotransmitters continue to combine with the receptor sites at the postsynaptic membrane until they are inactivated by enzymes, are taken up by the presynaptic endings or diffuse away from the synaptic region. In addition, neurotransmitters can be affected by drugs and toxins, which can modify their function or block their attachment to receptor sites on the postsynaptic membrane. Encephalins and endorphins are also considered neurotransmitters. These substances have opiate-like properties. They are found in multiple areas of the CNS and PNS and act to inhibit pain perception (see Ch 8).
CENTRAL NERVOUS SYSTEM
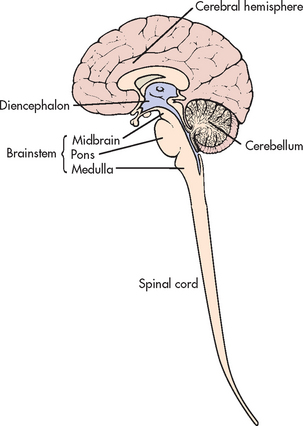
Major structural components of the CNS are the spinal cord and brain. The brain consists of the cerebrum (cerebral hemisphere), the brainstem and the cerebellum.
Spinal cord
The spinal cord is continuous with the brainstem and exits from the cranial cavity through the foramen magnum. A cross-section of the spinal cord reveals grey matter that is centrally located in an H shape and is surrounded by white matter (see Fig 55-5). The grey matter contains the cell bodies of voluntary motor neurons and preganglionic autonomic motor neurons, as well as cell bodies of association neurons (interneurons). The white matter contains the axons of the ascending sensory and the descending (suprasegmental) motor fibres. The myelin surrounding these fibres gives them their white appearance. Specific ascending and descending pathways in the white matter can be identified. The spinal pathways or tracts are named for the point of origin and the point of destination (e.g. spinocerebellar tract [ascending], corticospinal tract [descending]). The major spinal pathways are presented in Figure 55-5, B.
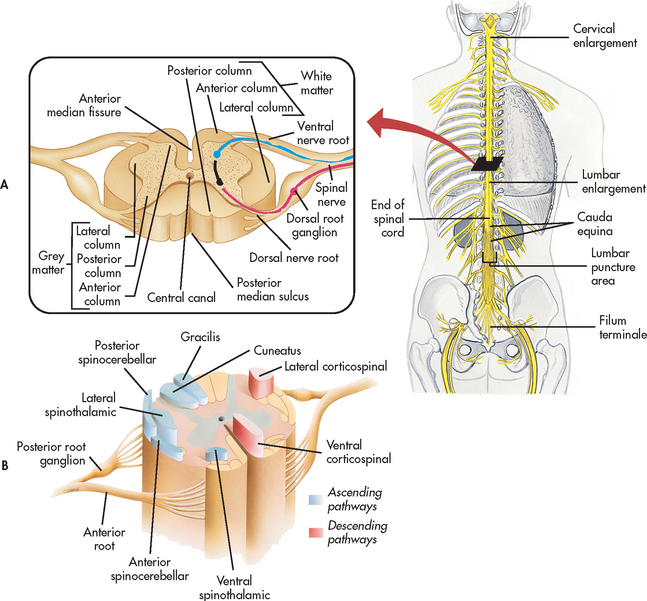
Figure 55-5 Spinal cord. A, Inset illustrates a transverse section of the spinal cord shown in a broader view. B, Cross-section of the spinal cord showing the horns, pathways (nerve tracts) and roots.
Ascending tracts
In general, the ascending spinal tracts carry specific sensory information to higher levels of the CNS. This information comes from special sensory endings (receptors) in the skin, muscles and joints, viscera and blood vessels and enters the spinal cord by way of the dorsal roots of the spinal nerves. The fasciculus gracilis and the fasciculus cuneatus (commonly called the dorsal or posterior columns) carry information and transmit impulses concerned with touch, deep pressure, vibration, position sense and kinaesthesia (appreciation of movement, weight and body parts). The spinocerebellar tracts carry subconscious information about muscle tension and body position to the cerebellum for coordination of movement. This information is not consciously perceived. The spinothalamic tracts carry information about pain and temperature sensations. Therefore, the ascending tracts are organised by sensory modality, as well as by anatomy.
Although the functions of these pathways are generally accepted, other ascending tracts may also carry sensory modalities. The symptoms of various neurological diseases suggest that additional pathways for touch, position sense and vibration exist.
Descending tracts
Descending spinal tracts carry impulses that are responsible for muscle movement. Among the most important descending tracts are the corticobulbar and corticospinal tracts, collectively termed the pyramidal tract. These tracts carry volitional (voluntary) impulses from the cortex to the cranial and peripheral nerves, respectively. Another group of descending motor tracts carries impulses from the extrapyramidal system, which includes all motor systems (except the pyramidal system) concerned with voluntary movement. It includes descending pathways originating in the brainstem, basal ganglia and cerebellum. The motor output exits the spinal cord by way of the ventral roots of the spinal nerves.
Lower and upper motor neurons
Lower motor neurons (LMNs) are the final common pathway through which descending motor tracts influence skeletal muscle, which is the effector organ for movement. The cell bodies of LMNs, which send axons to innervate the skeletal muscles of the arms, trunk and legs, are located in the anterior horn of the corresponding segments of the spinal cord (e.g. cervical segments contain LMNs for the arms). LMNs for skeletal muscles of the eyes, face, mouth and throat are located in the corresponding segments of the brainstem. These cell bodies and their axons make up the somatic motor components of the cranial nerves. LMN lesions generally cause weakness or paralysis, denervation atrophy, hyporeflexia or areflexia and decreased muscle tone (flaccidity).
Upper motor neurons (UMNs) originate in the cerebral cortex and project downwards. The corticobulbar tract ends in the brainstem and the corticospinal tract descends into the spinal cord. These neurons influence skeletal muscle movement. UMN lesions generally cause weakness or paralysis, disuse atrophy, hyperreflexia and increased muscle tone (spasticity).
Reflex arc
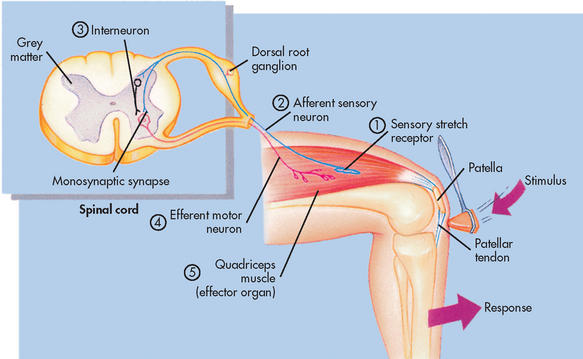
A reflex is defined as an involuntary response to a stimulus. The components of a monosynaptic reflex arc (the simplest kind of reflex arc) are a receptor organ, an afferent neuron, an effector neuron and an effector organ (e.g. skeletal muscle). The afferent neuron synapses with the efferent neuron in the grey matter of the spinal cord. A reflex arc is shown in Figure 55-6. Reflex arcs that are more complex have other neurons (interneurons) in addition to the afferent neuron influencing the effector neuron. In the spinal cord, reflex arcs play an important role in maintaining muscle tone, which is essential for body posture.
Brain
The brain can be divided into three major components: the cerebrum, the brainstem and the cerebellum.
Cerebrum
The cerebrum is composed of the right and left hemispheres. Both hemispheres can be further divided into four major lobes: frontal, temporal, parietal and occipital (see Fig 55-7). These divisions are useful to delineate portions of the neocortex (grey matter), which makes up the outer layer of the cerebral hemisphere. Neurons in specific parts of the neocortex are essential for various highly complex and sophisticated aspects of mental functioning, such as language, memory and appreciation of visual–spatial relationships.
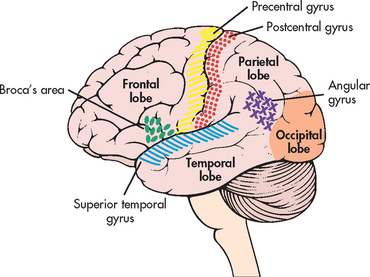
Figure 55-7 Left hemisphere of the cerebrum, lateral surface, showing the major lobes and areas of the brain.
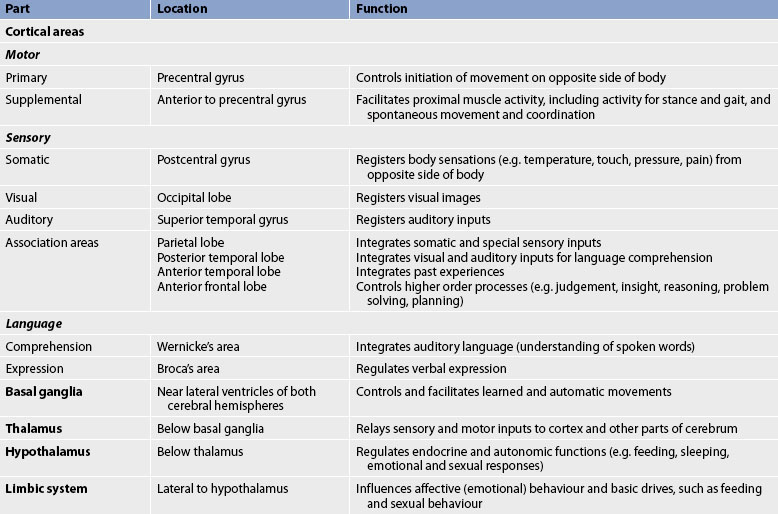
The functions of the cerebrum are multiple and complex. Specific areas of the cerebral cortex are associated with specific functions. Table 55-1 summarises the location and function of the parts of the cerebrum. The basal ganglia, thalamus, hypothalamus and limbic system are also located in the cerebrum. The basal ganglia are a group of paired structures located centrally in the cerebrum and midbrain; most of them are on both sides of the thalamus. The function of the basal ganglia is to modulate the initiation, execution and completion of voluntary movements and automatic movements associated with skeletal muscle activity, such as swinging of the arms while walking, swallowing saliva and blinking.
The thalamus (part of the diencephalon) lies directly above the brainstem (see Figs 55-8 and 55-9) and is the major relay centre for sensory and other afferent (i.e. cerebellar) inputs to the cerebral cortex.
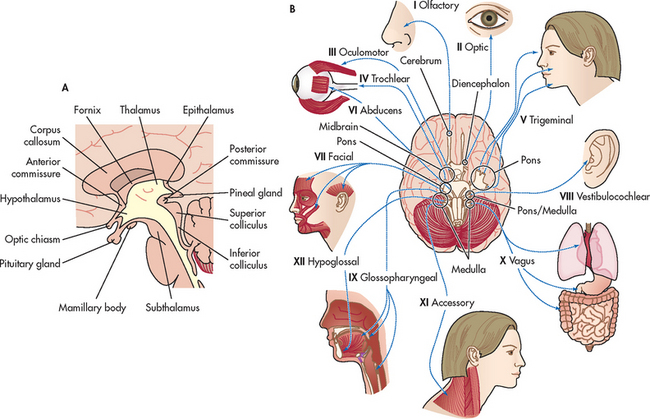
Figure 55-9 A, Diencephalon (thalamus and hypothalamus). B, Twelve pairs of cranial nerves. The cranial nerves are numbered according to the order in which they leave the brain.
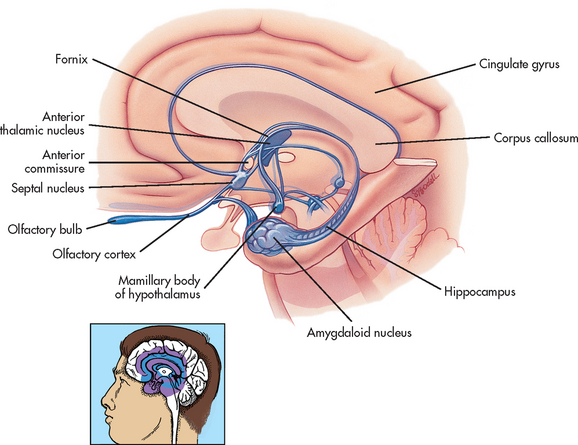
The hypothalamus is located just inferior to the thalamus and slightly in front of the midbrain. It regulates the ANS and the endocrine system. The limbic system is, phylogenetically, an old part of the human cerebrum. It is located near the inner surfaces of the cerebral hemisphere (see Fig 55-10) and is concerned with emotion, aggression, feeding behaviour and sexual response.
Projection fibres link the cerebral cortex to the diencephalon, brainstem, cerebellum and spinal cord. The entire collection of these fibres is known as the internal capsule.3 All the motor fibres serving one side of the body are gathered together in the internal capsule. Injury to this area will therefore cause paralysis (hemiplegia) on the same side (ipsilateral) of the body.4
Brainstem
The brainstem includes the midbrain, pons and medulla (see Fig 55-8). Ascending and descending fibres pass through the brainstem going to and from the cerebrum and cerebellum. The cell bodies, or nuclei, of cranial nerves III–XII are in the brainstem. Also located in the brainstem is the reticular formation, a diffusely arranged group of neurons and their axons that extend from the medulla to the thalamus and hypothalamus. The functions of the reticular formation include relaying sensory information, influencing excitatory and inhibitory control of spinal motor neurons, and controlling vasomotor and respiratory activity. The reticular activating system is part of the reticular formation and is the regulatory system for arousal, a component of consciousness.
The medulla contains the vasomotor centre, which controls blood vessel diameter; the cardiac centre, which controls myocardial contractility and heart rate; and the medullary rhythmicity centre, which controls the rhythm of breathing. The pons also contains two areas that control breathing: the pneumotaxic and apneustic areas.5 In the medulla, motor fibres cross from one side to the other. This is known as the decussation of the pyramidal tracts and explains why motor areas on one side of the brain control skeletal movements on the opposite side of the body.4 The midbrain connects the pons and cerebellum to the cerebrum. It contains the cerebral aqueduct, a narrow channel connecting the third and fourth ventricles.6 The brainstem also contains the centres for sneezing, coughing, hiccupping, vomiting, sucking and swallowing.
Cerebellum
The cerebellum is located in the posterior part of the cranial fossa, along with the brainstem, under the occipital lobe of the cerebrum. The function of the cerebellum is to coordinate voluntary movement and to maintain trunk stability and equilibrium. It influences motor activity through its axonal connections to the motor cortex, brainstem nuclei and their descending pathways. To perform these functions, the cerebellum receives information from the cerebral cortex, muscles, joints and inner ear.
Ventricles and cerebrospinal fluid
Several supporting structures located within the CNS are important in regulating neuronal function and physical support of the brain. The ventricles are four fluid-filled cavities within the brain that connect with one another and with the spinal canal. The lower portion of the fourth ventricle becomes the central canal in the lower part of the brainstem. The spinal canal is located in the centre and extends the full length of the spinal cord. Figure 55-11 shows the ventricles and the flow of cerebrospinal fluid in the CNS.
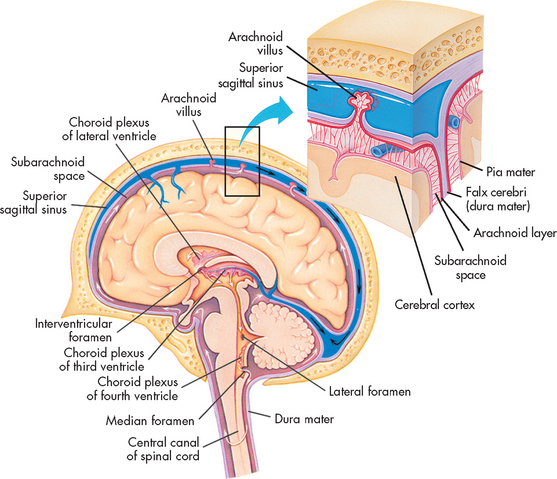
Figure 55-11 Flow of the cerebrospinal fluid. The fluid produced by filtration of blood by the choroid plexus of each ventricle flows inferiorly through the lateral ventricles, intraventricular foramen, third ventricle, cerebral aqueduct, fourth ventricle and subarachnoid space to the blood.
Cerebrospinal fluid (CSF) circulates within the subarachnoid space surrounding the brain, the brainstem and the spinal cord. This fluid provides cushioning for the brain and spinal cord, allows fluid shifts from the cranial cavity to the spinal cavity and carries nutrients. The formation of CSF in the choroid plexus in the ventricles involves both passive diffusion and active transport of substances. CSF resembles an ultrafiltrate of blood. It is produced at a rate of 0.4 mL/min.2 Although CSF is continually being formed, many physiological factors influence its rate of absorption and formation. The ventricles and central canal are normally filled with an average of 135 mL of CSF.
The CSF circulates throughout the ventricles and seeps into the subarachnoid space surrounding the brain and spinal cord. It is absorbed primarily through the arachnoid villi (tiny projections into the subarachnoid space), into the intradural venous sinuses and eventually into the venous system. The analysis of CSF composition provides useful diagnostic information relating to CNS diseases. CSF pressure is sometimes measured in patients with actual or suspected intracranial diseases. Normal intracranial pressure (ICP) is 0–15 mmHg.7 Increases in ICP, indicated by increased CSF pressure, can lead to herniation of the brain and compression of vital brainstem structures. The signs marking this event are part of the herniation syndrome (see Ch 56). The consequences of raised ICP are discussed in detail in Chapter 56.
PERIPHERAL NERVOUS SYSTEM
The PNS includes all the neuronal structures that lie outside the CNS. It consists of the spinal and cranial nerves, their associated ganglia (groupings of cell bodies) and portions of the ANS.
Spinal nerves
The spinal cord can be seen as a series of spinal segments, one on top of another. In addition to the cell bodies, each segment contains a pair of dorsal (afferent) sensory nerve fibres or roots and ventral (efferent) motor fibres or roots, which innervate a specific region of the neck, trunk or limbs. This combined motor–sensory nerve is called a spinal nerve (see Fig 55-12). The cell bodies of the voluntary motor system are located in the anterior horn of the spinal cord grey matter. The cell bodies of the autonomic (involuntary) motor system are located in the anterolateral portion of spinal cord grey matter. The cell bodies of sensory fibres are located in the dorsal root ganglia just outside the spinal cord.
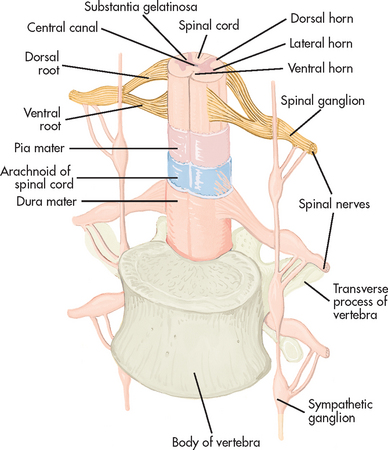
Figure 55-12 Cross-section of the spinal cord showing spinal nerve attachments and spinal cord coverings.
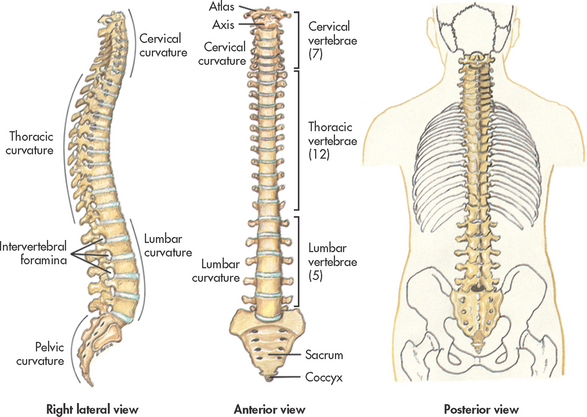
There are 31 pairs of spinal nerves (8 cervical, 12 thoracic, 5 lumbar, 5 sacral, 1 coccygeal).1 On exiting the spinal column, each spinal nerve divides into ventral and dorsal rami, which are a collection of motor and sensory fibres that eventually go to peripheral structures (e.g. skin, muscles, viscera). The sympathetic ganglia are attached to the ventral rami of the spinal nerves by grey and white rami communicantes. The terminal or inferior end of the spinal cord is known as the cauda equina. This is because the ventral and dorsal roots resemble a horse’s tail.3
A dermatome is the area of skin innervated by the sensory fibres of a single dorsal root of a spinal nerve. The dermatomes give a general picture of somatic sensory innervation by spinal segments. A myotome is a muscle group innervated by the primary motor neurons of a single ventral root. These are simple components in the embryonic stage of human development. However, the dermatomes and myotomes of a given spinal segment overlap with those of adjacent segments because of the development of ascending and descending collateral branches of nerve fibres.
Cranial nerves
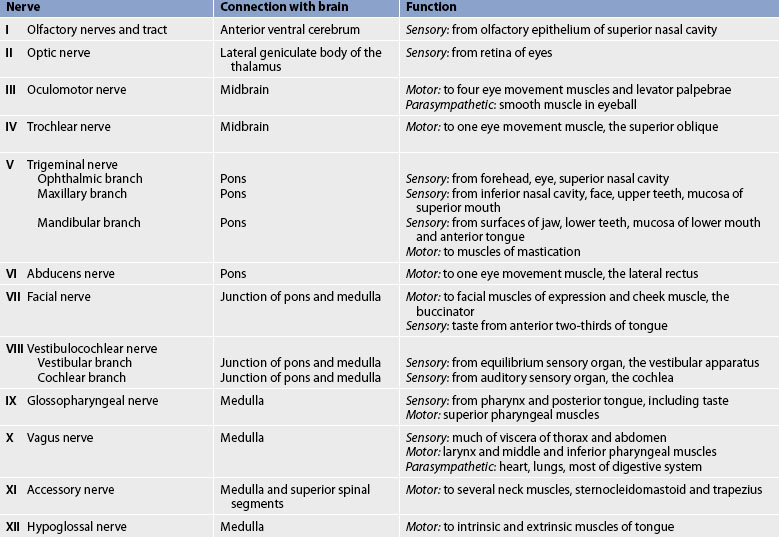
The cranial nerves (CNs) are 12 paired nerves composed of cell bodies with fibres that exit from the cranial cavity (see Fig 55-9, B). Unlike the spinal nerves, which always have both afferent sensory and efferent motor fibres, some CNs have only afferent fibres, some have only efferent fibres and others have both. Table 55-2 summarises the motor and sensory components of the CNs. Figure 55-9, B shows the position of the CNs in relation to the brain and spinal cord. Just as the cell bodies of the spinal nerves are located in specific segments of the spinal cord, so the cell bodies (nuclei) of the CNs are located in specific segments of the brain. Exceptions are the nuclei of the olfactory and optic nerves. The primary cell bodies of the olfactory nerve are located in the nasal epithelium and those of the optic nerve are in the retina. CN XI is a spinal nerve and its efferent fibres migrate upwards before exiting the neuroaxis at the level of the medulla.
Autonomic nervous system
The ANS governs involuntary functions of cardiac muscle, smooth (involuntary) muscle and glands. It is divided into two components, sympathetic and parasympathetic, which are anatomically and functionally different. These two systems function together to maintain a relatively balanced internal environment. The ANS is both an efferent and an afferent system. It consists of preganglionic nerves and postganglionic nerves.
The preganglionic cell bodies of the sympathetic nervous system (SNS) are located in spinal segments T1–L2. The sympathetic ganglia, which contain the cell bodies of the postganglionic neurons, lie close to the spinal column, along the vertebral bodies in the rami communicantes. These ganglia and the connecting nerves are called the paravertebral chain. The major neurotransmitter released by the postganglionic fibres of the SNS is noradrenaline; acetylcholine is released by the preganglionic fibres.
In contrast, the preganglionic cell bodies of the parasympathetic nervous system (PSNS) are located in the brainstem and in the sacral spinal segments (S2–S4). The parasympathetic ganglia are located in or near the structures that they innervate. Acetylcholine is the neurotransmitter released at both preganglionic and postganglionic nerve endings.
The ANS provides dual and often reciprocal innervation to many structures. For example, the SNS increases the rate and force of heart contraction and the PSNS decreases the rate and force. The SNS dilates bronchi and bronchioles of the lungs, and the PSNS constricts them. Some structures are innervated by only one system (e.g. the hair follicles and the sweat glands, which are innervated by only the SNS). Table 55-3 compares the SNS and PSNS.
TABLE 55-3 Effect of sympathetic and parasympathetic nervous systems
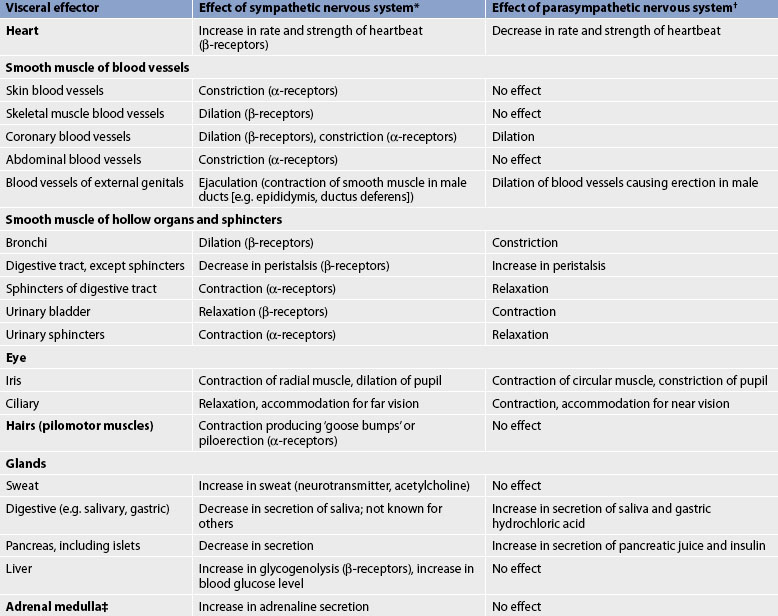
* Neurotransmitter is noradrenaline unless otherwise stated.
† Neurotransmitter is acetylcholine unless otherwise stated.
‡ Sympathetic preganglionic axons terminate in contact with secreting cells of the adrenal medulla. Thus, the adrenal medulla functions as a ‘giant sympathetic postganglionic neuron’.
Source: modified from thibodeau GA, Patton KT. Anatomy and physiology. 6th edn. St louis: mosby; 2006.
The result of SNS stimulation is activation of mechanisms required for the fight or flight response that occurs throughout the body. In contrast, the PSNS is geared to act in localised and discrete regions. It serves to conserve and restore the energy stores of the body.
CEREBRAL CIRCULATION
The blood supply of the brain arises from the internal carotid arteries (anterior circulation) and the vertebral arteries (posterior circulation), which are shown in Figure 55-13. Knowledge of the distribution of the major arteries of the brain and the area supplied is essential for understanding and evaluating the signs and symptoms of cerebrovascular disease and trauma.
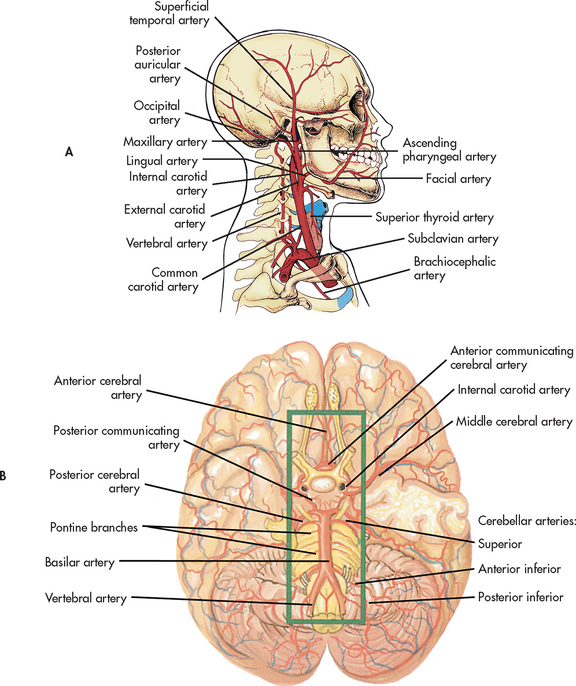
Figure 55-13 Arteries of the head and neck. A, Brachiocephalic artery, right common carotid artery, right subclavian artery and their branches. The major arteries to the head are the common carotid and vertebral arteries. B, Inferior view of the brain showing the vertebral, basilar and internal carotid arteries and their branches.
Each internal carotid artery supplies the ipsilateral hemisphere, whereas the basilar artery, formed by the junction of the two vertebral arteries, supplies structures within the posterior fossa (cerebellum and brainstem). The circle of Willis arises from the basilar artery and the two internal carotid arteries (see Fig 55-14). This vascular circle may act as a safety valve when differential pressures are present in these arteries. It may also function as an alternative pathway when occlusion of a major artery on one side of the brain occurs. In general, the two anterior cerebral arteries supply the medial portion of the frontal lobes. The two middle cerebral arteries supply the outer portions of the frontal, parietal and superior temporal lobes. The two posterior cerebral arteries supply the medial portions of the occipital and inferior temporal lobes. Figure 55-13 shows the major cerebral arteries. Venous blood drains from the brain through the dural sinuses, which form channels that drain into the two jugular veins. Syndromes resulting from occlusion of the main cerebral arteries are discussed in Chapter 56.
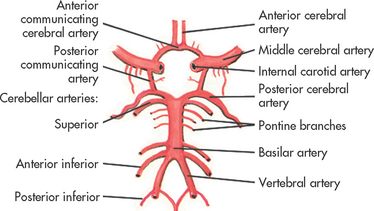
Figure 55-14 Arteries of the base of the brain. The arteries that compose the circle of Willis are the two anterior cerebral arteries joined to each other by the anterior communicating cerebral arteries and to the posterior arteries by the posterior communicating arteries.
Blood–brain barrier
The blood–brain barrier is a physiological barrier between the blood capillaries and brain tissue. The structure of brain capillaries differs from that of other capillaries. Some substances that normally pass readily into most tissues are prevented from entering brain tissue. This barrier protects the brain from certain potentially harmful agents, while allowing nutrients and gases to enter. Because the blood–brain barrier affects the penetration of drugs, only certain ones can enter the CNS from the bloodstream. Lipid-soluble compounds enter the brain easily, whereas water-soluble and ionised drugs enter the brain and spinal cord slowly. Damage to the blood–brain barrier can result in the uncontrolled penetration of drugs and other substances into brain tissue.
PROTECTIVE STRUCTURES
Meninges
The meninges are three layers of protective membranes that surround the brain and spinal cord. The thick dura mater forms the outermost layer, with the arachnoid layer and pia mater being the next two layers. The falx cerebri is a fold of the dura mater that separates the two cerebral hemispheres and prevents expansion of brain tissue in situations such as the presence of a rapidly growing tumour or acute haemorrhage. The expanding brain must squeeze under this structure, causing displacement towards the side opposite the lesion. The tentorium cerebelli is a fold of dura that separates the cerebral hemispheres from the posterior fossa (which contains the brainstem and cerebellum). Expansion of mass lesions in the cerebrum forces the brain to herniate through the opening created by the brainstem. This is termed tentorial herniation.
The arachnoid layer is a delicate, impermeable membrane that lies between the thick dura mater and the pia mater. The subarachnoid space lies between the arachnoid layer and the pia mater. This space is filled with CSF. Structures passing to and from the brain and the skull or its foramina (holes through which blood vessels and nerves enter and exit the intracranial compartment) must pass through the subarachnoid space. All cerebral arteries and veins lie in this space, as do the CNs. A larger subarachnoid space is present in the region of the third and fourth lumbar vertebrae, which is the area penetrated to obtain CSF during a lumbar puncture. The spinal cord itself ends between the first and second lumbar vertebrae.
Skull
The bony skull protects the brain from external trauma. It is composed of 8 cranial bones and 14 facial bones. The structure of the skull cavity explains the physiology of head injuries. Although the top and sides of the inside of the skull are relatively smooth, the bottom surface is uneven. It has many ridges, prominences and foramina, which can tear the inferior surface of the brain during physically traumatic events. The largest hole in the skull is the foramen magnum, through which the brainstem extends to the spinal cord. This foramen offers the only major space for the expansion of brain contents when increased ICP occurs. However, herniation of the brainstem through the foramen magnum is usually fatal as it results in compression of the vital centres controlling respiratory and cardiac function.
Vertebral column
The vertebral column protects the spinal cord, supports the head and provides flexibility. The vertebral column is made up of 33 individual vertebrae: 7 cervical, 12 thoracic, 5 lumbar, 5 sacral (fused into one) and 4 coccygeal (fused into one). Each vertebra has a central opening through which the spinal cord passes. The vertebrae are held together by a series of ligaments. Intervertebral discs occupy the spaces between vertebrae. Figure 55-15 shows the vertebral column in relation to the trunk.
Gerontological considerations: effects of ageing on the nervous system
Several parts of the nervous system are affected by ageing. In the CNS, loss of neurons occurs in certain areas of the brainstem, cerebellum and cerebral cortex. This gradual process begins in early adulthood. With loss of neurons, there is widening or enlargement of the ventricles. Brain weight also decreases as a result of neuron loss. Cerebral blood flow decreases and CSF production declines.
In the PNS, there are changes in the anterior horn cells and peripheral nerves, as well in as the target organ or muscle. Degenerative changes in myelin cause a decrease in nerve conduction. Coordinated neuromuscular activity, such as the maintenance of blood pressure in response to changing from a lying to a standing position, is altered with ageing. As a result, older adults are more vulnerable to problems with orthostatic hypotension. Similarly, coordination of neuromuscular activity to maintain body temperature is also less efficient with ageing. Older adults are less able to adapt to extremes in environmental temperature and are more vulnerable to both hypothermia and hyperthermia.
Other physiological effects of ageing include short-term memory loss, altered motor coordination, decreased muscle tone and strength, slower response to verbal and motor stimuli, decreased ability to process new information and increased sensitivity to sedation.8 Sensory changes, including decreases in taste and smell perception, may result in decreased dietary intake in the older adult. Reduced hearing and vision can result in perceptual confusion. Problems with balance and coordination can put the older adult at risk of falls and subsequent fractures.
Changes in assessment findings result from age-related alterations in the various components of the nervous system. Most physiological functions decline at a rate of 1% per year, beginning at age 30. However, the time at which senescent changes occur varies greatly in individuals.9 Age-related changes in the nervous system and differences in assessment findings are presented in Table 55-4.
GERONTOLOGICAL DIFFERENCES IN ASSESSMENT
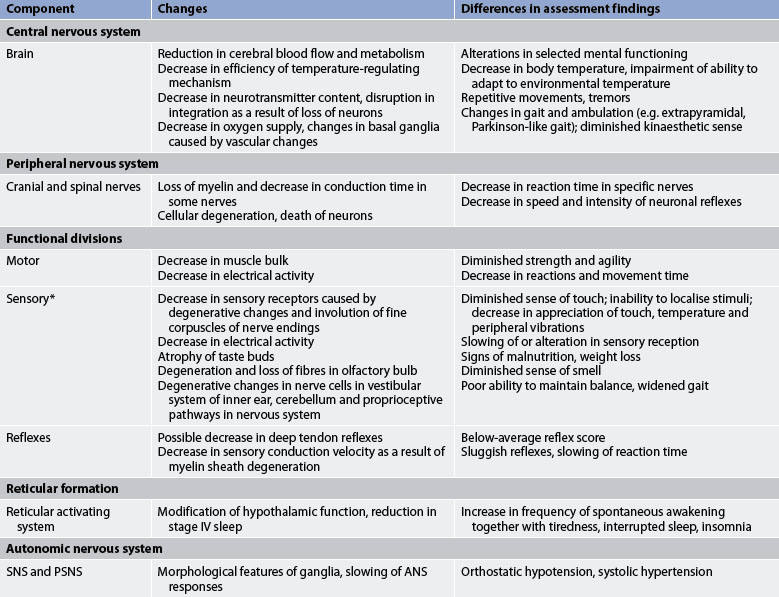
* Specific changes related to the eye are given in Table 20-1 and specific changes related to the ear are given in Table 20-6. ANS, autonomic nervous system; PSNS, parasympathetic nervous system; SNS, sympathetic nervous system.
Assessment of the nervous system
The foundation of managing patients who have a disturbance in neurological function is a comprehensive health assessment, including a detailed assessment of the nervous system.
SUBJECTIVE DATA
Important health information
Health history
Three points should be considered in taking the history of a patient with neurological problems. First, the nurse should avoid suggesting certain symptoms to the patient or asking leading questions such as, ‘Is your headache throbbing?’ or ‘Are you weak on the right side?’ It is better to ask open-ended questions such as: ‘What is your headache like?’ or ‘Is there anything about your right side that bothers you?’ Second, the mode of onset and the course of the illness are especially important aspects of the history. Often the nature of a neurological disease process can be described by these facts alone and the nurse should obtain all pertinent data in the history of the present illness, especially data related to the characteristics and progression of the symptoms. Third, because many neurological diseases affect the patient’s mental functioning, mental status must be assessed accurately before assuming that the history is factual. If the patient is not considered a reliable historian, the history should be obtained from a person who has first-hand knowledge of the patient’s problems and complaints. Many times a health history cannot be obtained and the nurse must proceed with only objective data.
The health history helps guide the approach for the neurological examination; that is, it can direct the healthcare provider towards the parts of the nervous system that need to be closely assessed. For example, if the patient’s primary complaint is dizziness, the examination may be focused on visual, vestibular and cerebellar functions rather than on somatic motor and sensory functions.
Medications
Special attention should be given to obtaining a careful medication history, especially the use of sedatives, narcotics, tranquillisers and other mood-altering drugs. Many other drugs can also cause neurological adverse effects.
Surgery or other treatment history
The nurse should inquire about any previous surgery involving any part of the nervous system, such as the head, spine or sensory organs. If a patient has had surgery, the date, cause, procedure, recovery and current health status should be investigated.
The perinatal history may reveal exposure to toxic agents, such as viruses, alcohol, tobacco, drugs and radiation, which are known to influence adversely the development of the nervous system. The history may reveal a difficult labour and delivery, which can cause brain damage because of hypoxia, forceps delivery or Rh incompatibility.
Growth and developmental history can be important in ascertaining whether nervous system dysfunction was present at an early age. The nurse should inquire about major developmental tasks, such as walking and talking. Successes at school or identified problems in an educational setting are other important developmental data to gather. Often this information is not available when the older patient is interviewed.
Functional health patterns
Key questions to ask a patient with a neurological problem are presented in Table 55-5.
• What are your usual daily activities?
• Do you use any recreational drugs?*
• What safety practices do you perform in a car? On a motorcycle? On a bicycle?
• Do you have hypertension? If so, is it controlled?
• Have you ever been hospitalised for a neurological problem?*
• How does it affect your daily living?
• Do you have any problems getting adequate nutrition because of chewing or swallowing difficulties, facial nerve paralysis or poor muscle coordination?*
• Are you able to feed yourself?
• Do you have incontinence of bowel or bladder? If yes, explain in detail the onset and pattern of the problem. What measures have you used to control the incontinence?
• Do you ever experience problems with hesitancy, urgency, retention?*
• Do you postpone defecation?*
• Does a neurological problem make it difficult to reach a toilet when needed?
• Do you take any medication to manage neurological problems? If so, what?
• Describe any problems you experience with usual activities and exercise as a result of a neurological problem.
• Do you have weakness or lack of coordination caused by a neurological problem?*
• Does a neurological problem keep you from performing your personal hygiene needs independently?*
• Describe any problems you have with sleep.
• If you have trouble falling asleep, what do you do about it? (Ask specifically about use of sleep-inducing drugs.)
• Have you noticed any changes in your memory?*
• Do you experience vertigo, heat or cold sensitivity, numbness or tingling?*
• Describe any pain you have experienced during the past 6 months.
• Do you have any difficulty with verbal or written communication?*
Self-perception–self-concept pattern
• What effect has your neurological problem had on how you feel about yourself? Your abilities? Your body?
• Describe your general emotional pattern.
• Have you experienced changes in roles such as spouse, parent or breadwinner because of neurological disease?*
• How do you feel about these changes?
Sexuality–reproductive pattern
• Are you satisfied with your sexual functioning? Describe any problems you experience related to your sexuality and sexual functioning.
• Are problems related to your sexual functioning causing tension in an important relationship?*
• Do you feel the need for professional counselling related to your sexual functioning?*
• Do you use alternative methods of achieving sexual satisfaction?
Coping–stress tolerance pattern
• Describe your usual coping pattern.
• Do you think your present coping pattern is adequate to meet the stressors of your neurological problem?*
• Is your support system adequate to meet your needs? If not, what needs are unmet?
• Describe any culturally specific beliefs and attitudes that may influence the treatment of this neurological problem.
Health perception–health management pattern
The nurse should ask about the patient’s health practices related to the nervous system, such as history of alcohol intake and smoking, maintenance of adequate nutrition, participation in physical and recreational activities, use of seat belts and helmets, and control of hypertension. The nurse should ask about previous hospitalisations for neurological problems. A careful family history may determine whether the neurological problem has a hereditary or congenital background.
If the patient has an existing neurological problem, the nurse should ask about how it affects daily living and the ability to carry out self-care. After a careful review of information, the nurse should ask someone who knows the patient well whether any mental or physical changes have been noticed in the patient. It is often helpful to obtain details of the history from the patient’s friends, relatives or a witness; it is vital to do this if the patient is a child or if there is impairment of conscious state or memory disturbances.2
Nutritional–metabolic pattern
Neurological problems can result in problems of inadequate nutrition. Problems related to chewing, swallowing, facial nerve paralysis and muscle coordination could make it difficult for the patient to ingest adequate nutrients. Also, certain vitamins such as thiamine (B1), niacin and pyridoxine (B6) are essential for the maintenance and health of the CNS. Deficiencies in one or more of these vitamins could result in such non-specific complaints as depression, apathy, neuritis, weakness, mental confusion and irritability.
Elimination pattern
Bowel and bladder problems are often associated with neurological problems, such as stroke, head injury, spinal cord injury, multiple sclerosis and dementia. It is important to determine if the bowel or bladder problem was present before or after the neurological event to plan appropriate interventions. Incontinence of urine and faeces and urinary retention are the most common elimination problems associated with a neurological problem. Careful documentation of the details of the problem, such as number of episodes, accompanying sensations or lack of sensations, and measures to control the problem, is important.
Activity–exercise pattern
Many neurological disorders can cause problems in the patient’s mobility, strength and coordination. These problems can result in changes in the patient’s usual activity and exercise patterns. Falls can also result from such problems. Many aspects of daily living, such as getting out of a bed or chair, ambulating, preparing meals and performing personal hygiene, can be affected and should be assessed. The ability to perform fine motor tasks may be affected, which increases the possibility of personal injury.
Sleep–rest pattern
Sleep can be disrupted by many neurologically related factors. Discomfort from pain and inability to move and change to a position of comfort because of muscle weakness and paralysis could interfere with sound sleep. Hallucinations resulting from dementia or drugs can also interrupt sleep. The nurse should carefully document the sleep problem and the patient’s methods of dealing with the problem.
Cognitive–perceptual pattern
Because the nervous system controls cognition and sensory integration, many neurological disorders affect these functions. The nurse should assess memory, language, calculation ability, problem-solving ability, insight and judgement. Often a structured mental status questionnaire is used to evaluate these functions and provide baseline data.
Information about sensory changes related to hearing, sight and touch should be sought. The patient should also be asked about problems with vertigo and sensitivity to heat and cold. Ability to both use and understand language is a cognitive function that the nurse should assess. Appropriateness of responses is a useful indicator of cognitive status.
Pain is a common event associated with many health problems. It is often the reason a patient seeks healthcare. The patient’s pain should be assessed carefully (see Ch 8).
Neurological problems and their treatment can be complex and confusing. The patient’s understanding of and ability to carry out necessary treatments should be determined. Cognitive changes associated with the problem can also interfere with understanding and compliance.10
Self-perception–self-concept pattern
Neurological disease can drastically alter control over one’s life and create dependency on others for daily needs. In addition, the patient’s physical appearance and emotional control can be affected. The nurse should ask about the patient’s evaluation of self-worth, perception of abilities, body image and general emotional pattern.
Role–relationship pattern
The patient should be asked if changes in roles, such as spouse, parent or breadwinner, resulting from a neurological problem have occurred. Physical impairments, such as weakness and paralysis, can alter or limit participation in usual roles and activities. However, cognitive changes can permanently change a person’s ability to maintain previous roles, and these changes can dramatically affect both the patient and significant others. Dependent relationships may develop.
Sexuality–reproductive pattern
The patient’s ability to participate in sexual activity should be assessed because many nervous system disorders can affect sexual response. Cerebral lesions may inhibit the desire phase or the reflex responses of the excitement phase. Brainstem and spinal cord lesions may partially or completely interrupt the connections between the brain and effector systems necessary for intercourse. Neuropathies and spinal cord lesions that affect sensation, especially in the erogenous zones, may decrease desire. Autonomic neuropathies and lesions of the sacral cord and cauda equina may prevent reflex activities of the sexual response. The nurse should determine whether the patient and spouse or significant other are satisfied with their sexual activity. If not, the use or need for alternative methods of achieving sexual satisfaction should be explored. Despite neurologically related changes in sexual functioning, many people can achieve satisfying expression of intimacy and affection.
Coping–stress tolerance pattern
The physical sequelae of a neurological problem can seriously strain a patient’s coping patterns. Often the problem is chronic and may require the patient to learn new coping skills. The nurse should assess the patient’s usual coping pattern to determine whether coping skills are adequate to meet the stress of a problem.
When the problem is a decrease in cognitive functioning, both the patient and the carer can be seriously stressed. The nurse should assess for the potential for suicide, abuse and burnout. The presence of an adequate support system in this type of situation should be assessed.
Value–belief pattern
Many neurological problems have serious, long-term, life-changing effects. These effects can strain the patient’s belief system and they should be assessed. The nurse should also determine whether any religious or cultural beliefs could interfere with the planned treatment regimen.
OBJECTIVE DATA
Physical examination
The standard neurological examination helps determine the presence, location and nature of disease of the nervous system. The examination assesses six categories of functions: mental status, function of CNs, motor function, cerebellar function, sensory function and reflex function. The choice of particular parts of the examination depends on the purpose for which it is done. If a comprehensive baseline assessment of neurological functioning is desired, all components of the examination are done. However, if a specific problem is to be evaluated, only certain components may be assessed. For example, if a patient’s primary complaint is lack of feeling in the feet, the examination may be focused only on movement and sensation of the lower limbs. Similarly, if a patient comes into the emergency department after a severe head injury, a limited neurological examination (e.g. Glasgow Coma Scale and pupillary response) is conducted, as priority must also be given to airway, breathing and circulation, and the prevention of secondary injury.11
The primary purpose of the nursing neurological examination is to determine the effects of neurological dysfunction on daily living in relation to the patient’s and family’s ability to cope with the neurological deficits. A full neurological examination does not need to be performed on every patient,12 but neurological observations must be performed on any patient who is in danger of deterioration in CNS functioning.13 Clinical problems need to be addressed in order of priority. For example, maintaining an unconscious patient’s airway and investigating the cause of their unconsciousness is far more important than assessing the impact of the patient’s arm weakness on their activities of daily living. If a modified neurological assessment is performed for nursing purposes, it should include assessment of:
• arousal or level of consciousness
• vital signs (including airway, breathing, circulation).6
Mental status
Assessment of mental status (cerebral functioning) gives an indication of how the patient is functioning as a whole and adapting to the environment. It involves determination of complex and high-level cerebral functions that are governed by many areas of the cerebral cortex. Much of the area covered in this part of the examination is assessed during the history and therefore does not need to be evaluated further. For example, language and memory can be assessed when the patient is asked for details of the illness and significant past events. The patient’s cultural and educational background should be taken into account when evaluating mental status.
The components of the mental status examination are as follows:
• General appearance and behaviour. This component includes motor activity, body posture, dress and hygiene, facial expression and speech.
• State of consciousness. The patient must be conscious before other functions can be determined. The nurse should note orientation to time, place, person and situation, as well as memory, general knowledge, insight, judgement, problem solving and calculation. Common questions are ‘Who is the Prime Minister?’, ‘What day and month is it?’, ‘What is 100 minus 7?’ The nurse should consider whether the patient’s plans and goals match their physical and mental capabilities. Problems with memory may have implications for the ability to retain patient teaching. There are no universally accepted definitions of coma. It is therefore best to describe the activity of the patient, the type of stimulus used for arousal and the response to stimulation.9 The tool most commonly used to assess level of consciousness is the Glasgow Coma Scale. This scale assesses three areas: eye opening, motor response and verbal response. It is discussed in more detail in Chapter 56.
• Mood and affect. The nurse should note agitation, anger, depression or euphoria and the appropriateness of these states. Questions should be directed to bring out the patient’s feelings.
• Thought content. The nurse should note illusions, hallucinations, delusions or paranoia.
• Intellectual capacity. The nurse should note retardation, dementia and intelligence.
Cranial nerves
Testing of each CN is an essential component of the neurological examination (see Table 55-2).
Olfactory nerve
After determining that both nostrils are patent, the olfactory nerve (CN I) is tested by asking the patient to close one nostril, close both eyes and sniff from a bottle containing coffee, spice, soap or some other readily recognised odour. The same is done for the other nostril. Generally, olfaction is not tested unless the patient has some disturbance with smell. Chronic rhinitis, sinusitis and heavy smoking can often decrease the sense of smell. Disturbance in the ability to smell may be associated with a tumour involving the olfactory bulb or it may be the result of a basilar skull fracture that has damaged the olfactory fibres as they pass through the delicate cribriform plate of the skull.
Optic nerve
Visual fields and visual acuity are assessed to test the function of the optic nerve (CN II). Visual fields are assessed by confrontation. The examiner, positioned directly opposite the patient, asks the patient to close one eye, look directly at the bridge of the examiner’s nose and indicate when an object (finger, pencil tip, head of pin) presented from the periphery of each of the four visual fields is seen (see Fig 55-16). The same test is repeated for the other eye. The examiner is used as a control because both examiner and patient are sharing the same visual field. It is important to remember that the nasal side of the visual field is narrower because of the nasal bridge. Visual field defects may arise from lesions of the optic nerve, optic chiasm or tracts that extend through the temporal, parietal or occipital lobes. Visual field changes resulting from brain lesions are usually either a hemianopsia (half of the visual field is affected), a quadrantanopsia (quarter of the visual field is affected) or monocular (one eye).
Visual acuity is tested by asking the patient to read a Snellen chart from 6 metres away. The number on the lowest line that the patient can read with 50% accuracy is recorded. The patient who wears glasses should wear them during testing unless they are used only for reading. The eyes should be tested individually and together. If a Snellen chart is not available, the patient should be asked to read newsprint for a gross assessment of acuity. The distance from the patient to the newsprint required for accurate reading should be recorded. Acuity may not be testable by these means if the patient does not read English or is aphasic.
Funduscopy reveals the physical condition of the optic disc (head of the optic nerve), as well as the retina and blood vessels. This procedure is routinely performed when the optic nerve is tested. Optic nerve atrophy and papilloedema can be detected by this method.
Oculomotor, trochlear and abducens nerves
Because the oculomotor (CN III), trochlear (CN IV) and abducens (CN VI) nerves all help move the eye, they are tested together. The patient is asked to follow the examiner’s finger as it moves horizontally and vertically (making a cross) and diagonally (making an X). If there is weakness or paralysis of one of the eye muscles, the eyes do not move together and the patient has a disconjugate gaze. The presence and direction of nystagmus (fine, rapid jerking movements of the eyes) are observed at this time, even though this condition most often indicates vestibulocerebellar problems.
Other functions of the oculomotor nerve are tested by checking for pupillary constriction and for convergence (eyes turning inwards) and accommodation (pupils constricting with near vision). To test pupillary constriction, the examiner shines a light into the pupil of one eye and looks for ipsilateral constriction of the same pupil and contralateral (consensual) constriction of the opposite eye. The size and shape of the pupils are also noted. The optic nerve must be intact for this reflex to occur. Testing for pupillary constriction is an important component of the neurological assessment of patients at risk of herniation syndrome (see Ch 56). Because the oculomotor nerve exits at the top of the brainstem at the tentorial notch, it can be easily compressed by expanding mass lesions in the cerebral hemispheres. The result is a pupil that does not constrict to light; it may become dilated because the sympathetic input to the pupil acts unopposed. Convergence and accommodation are tested by asking the patient to focus on the examiner’s finger as it moves towards the patient’s nose. Another function of the oculomotor nerve is to keep the eyelid open. Damage to the nerve can cause ptosis (drooping eyelid), pupillary abnormalities and eye muscle weakness.
Trigeminal nerve
The sensory component of the trigeminal nerve (CN V) is tested by having the patient identify light touch (cotton bud) and pinprick in each of the three divisions (ophthalmic, maxillary and mandibular) of the nerve on both sides of the face. The patient’s eyes should be closed during parts of this examination. The motor component is tested by asking the patient to clench the teeth and palpating the masseter muscles just above the mandibular angle. The corneal reflex test evaluates CN V and CN VII simultaneously. It involves applying a cotton bud strand to the cornea. The sensory component of this reflex (corneal sensation) is innervated by the ophthalmic division of CN V. The motor component (eye blink) is innervated by the facial nerve (CN VII). This reflex is not normally tested in patients who are awake and alert because other tests evaluate these two nerves. However, for patients with a decreased level of consciousness, the corneal reflex test provides an opportunity to evaluate the integrity of the brainstem at the level of the pons because the fibres of CN V and CN VII have connections in this area.
Facial nerve
The facial nerve (CN VII) innervates the muscles of facial expression. Its function is tested by asking the patient to raise the eyebrows, close the eyes tightly, purse the lips, draw back the corners of the mouth in an exaggerated smile, and frown. The examiner should note any asymmetry in the facial movements because they can indicate damage to the facial nerve. Although taste discrimination of salt and sugar in the anterior two-thirds of the tongue is a function of this nerve, it is not routinely tested unless a peripheral nerve lesion is suspected.
Acoustic nerve
The cochlear portion of the acoustic (vestibulocochlear) nerve (CN VIII) is tested by asking the patient to close the eyes and indicate when a ticking watch or the rustling of the examiner’s fingertips is heard as the stimulus is brought closer to the ear. Each ear is tested individually and the distance from the patient’s ear to the sound source when first heard is recorded. This test identifies only gross deficits in hearing. For more precise assessment of hearing, an audiometer is used (see Ch 20). The vestibular portion of this nerve is not routinely tested unless the patient complains of dizziness, vertigo or unsteadiness, or has auditory dysfunction. If this is the case, caloric testing, which is beyond the scope of routine testing, may be done.
Glossopharyngeal and vagus nerves
The glossopharyngeal and vagus nerves are tested together because both innervate the pharynx. The glossopharyngeal nerve (CN IX) is primarily sensory. In the gag reflex (bilateral contraction of the palatal muscles initiated by stroking or touching either side of the posterior pharynx or soft palate with a tongue blade), the sensory component is mediated by CN IX and the major motor component by the vagus nerve (CN X). It is important to assess the gag reflex in patients who have a decreased level of consciousness, a brainstem lesion or a disease involving the throat musculature. If the reflex is weak or absent, the patient is in danger of aspirating food or secretions. The strength and efficiency of swallowing are important to test in these patients for the same reason. Another test for the awake, cooperative patient is to have the patient phonate by saying ‘ah’ and to note the bilateral symmetry of elevation of the soft palate. Any asymmetry can indicate weakness or paralysis. Swallowing is also assessed by lightly holding the examiner’s hands on either side of the patient’s throat and asking the patient to swallow. Any asymmetry is noted.
Spinal accessory nerve
The spinal accessory nerve (CN XI) is tested by asking the patient to shrug the shoulders against resistance and to turn the head to either side against resistance. There should be smooth contraction of the sternomastoid and trapezius muscles. Asymmetry, atrophy or fasciculation of the muscle should also be noted.
Hypoglossal nerve
The hypoglossal nerve (CN XII) is tested by asking the patient to protrude the tongue. It should protrude in the midline. The patient should also be able to push the tongue to either side against the resistance of a tongue blade. Again, any asymmetry, atrophy or fasciculation should be noted.
Motor system
The motor system examination includes assessment of the bulk, tone and power of the major muscle groups of the body, as well as assessment of balance and coordination. The examiner tests strength by asking the patient to push and pull against the resistance of the examiner’s arm as it opposes flexion and extension of the patient’s muscle. The patient should be asked to offer resistance at the shoulder, elbow, wrist, hips, knees and ankles. The patient’s grip strength can also be tested. Mild weakness of the upper extremities may be tested by asking the patient to extend both arms forwards at shoulder height with palms up while the eyes are closed. Mild weakness of the arm is demonstrated by downward drifting of the arm or pronation of the palm (pronator drift). When performing motor examinations, bilateral extremities must be assessed at the same time. This allows for comparison of function and identification of subtle differences.14
Tone is tested by passively moving the limbs through their range of motion; there should be a slight resistance to these movements. Abnormal tone is described as hypotonia (flaccidity) or hypertonia (spasticity). Involuntary movements (e.g. tics, tremor, myoclonus [spasm of muscles], athetosis [slow, writhing, involuntary movements of extremities], chorea [involuntary, purposeless, rapid motions] or dystonia [impairment of muscle tone]) should be noted.
Cerebellar function is tested by assessing balance and coordination. A good screening test for both balance and muscle strength is to observe the patient’s stature (posture while standing) and gait. The examiner should note the pace and rhythm of the gait and observe the arm swing (the arms should move symmetrically and in the opposite direction of the leg on the same side). The patient’s ability to ambulate is a key factor in determining the amount of nursing care that is needed and the risk of injury from falling. A patient with cerebellar disease may have an ataxic or staggering gait, in which the feet are placed wide apart and the steps are unsteady.
Coordination can be tested easily in several ways. The finger-to-nose test involves having the patient alternately touch their nose with their index finger and then touch the examiner’s finger. The examiner repositions the finger while the patient is touching the nose so that the patient must adjust to a new distance each time the examiner’s finger is touched. These movements should be performed smoothly and accurately. Other tests include asking the patient to pronate and supinate both hands rapidly and to do a shallow knee bend, first on one leg and then on the other. Dysarthria or slurred speech should be noted during physical examination because it is a sign of incoordination of the speech muscles.
The heel-to-shin test involves having the patient place one heel on the opposite shin below the knee and moving the heel down the shin to the ankle. This is repeated for the other leg. These movements should flow smoothly without jerking or hesitation.
Sensory system
Several modalities are tested in the somatic sensory examination. Each modality is carried by a specific ascending pathway in the spinal cord before it reaches the sensory cortex.
There are some general guidelines for performing the sensory examination. The patient should always have the eyes closed to avoid visual clues. The examiner should avoid giving verbal cues such as, ‘Is this sharp?’ The sensory stimulus should be applied in such a way that the patient does not expect it; that is, the examiner should avoid rhythmic application of the stimulus. In the routine neurological examination, sensory testing of the four extremities is sufficient. However, if a disturbance in sensory function of the skin is identified, the boundaries of that dysfunction should be carefully delineated.
Light touch
Light touch is usually tested first. The examiner gently strokes a cotton bud over each of the four extremities and asks the patient to indicate when the stimulus is felt by saying ‘touch’ (the sensory examination of the trigeminal nerve may be delayed until this time because the same material is used for testing sensation).
Pain and temperature
Pain is tested by touching the skin with the sharp end of a pin. This stimulus is irregularly alternated with a simple touch stimulus with the dull end of the pin to determine whether the patient can distinguish the two stimuli. Extinction or inhibition is assessed by simultaneously stimulating opposite sides of the body symmetrically with either a pain or a touch stimulus. Normally, the simultaneous stimuli are perceived (sensed); perception of only one may indicate a parietal lobe lesion.
The sensation of temperature is tested by applying containers of warm and cold water to the skin and asking the patient to identify the stimuli with the eyes closed. If pain sensation is intact, assessment of temperature sensation may be omitted because both sensations are carried by the same ascending pathways.
Vibration sense
Vibration sense is assessed by applying a vibrating C128-Hz tuning fork to the fingernails and the bony prominences of the hands, legs and feet with the patient’s eyes closed. The examiner asks the patient if the vibration or ‘buzz’ is felt. The examiner then asks the patient to indicate when the vibration ceases. The examiner stops the vibration with the hand as desired.
Position sense
Position sense (proprioception) is assessed by the examiner placing their thumb and forefinger on either side of the patient’s forefinger or great toe and gently moving the patient’s finger or toe up or down. The patient is asked to say the direction in which the digit is moved.
Another test of position sense of the lower extremities is the Romberg test. The patient is asked to stand with the feet together and then close their eyes. If the patient is able to maintain balance with the eyes open but sways or falls with the eyes closed (i.e. a positive Romberg test), this may indicate disease in the posterior columns of the spinal cord. It is important to be aware of patient safety during this test.
Cortical sensory functions
Several tests evaluate cortical integration of sensory perceptions (which occurs in the parietal lobes). Two-point discrimination is assessed by placing the two points of a calibrated compass on the tips of the fingers and toes. The minimum recognisable separation is 4–5 mm in the fingertips and a greater degree of separation elsewhere. This test is important in diagnosing diseases of the sensory cortex and peripheral nerve disease.
Graphaesthesia (ability to feel writing on skin) is tested by asking the patient to identify numbers traced on the palm of the hands. Stereognosis (ability to perceive the form and nature of objects) is tested by asking the patient to identify the size and shape of easily recognised objects (e.g. coins, keys, a safety pin) placed in the hands. Sensory extinction or inattention is evaluated by touching both sides of the body simultaneously. An abnormal response occurs when the patient perceives the stimulus only on one side. The other stimulus is ‘extinguished’.
Reflexes
Tendons attached to skeletal muscles have receptors that are sensitive to stretch. A reflex contraction of the skeletal muscle occurs when the tendon is stretched. A simple muscle stretch reflex is initiated by briskly tapping the tendon of a stretched muscle, usually with a reflex hammer (see Fig 55-17). The response (muscle contraction of the corresponding muscle) is measured as follows: 0/5 absent, 1/5 weak response, 2/5 normal response, 3/5 exaggerated response, 4/5 hyperreflexia with clonus. Clonus, an abnormal response, is a continued rhythmic contraction of the muscle with continuous application of the stimulus.
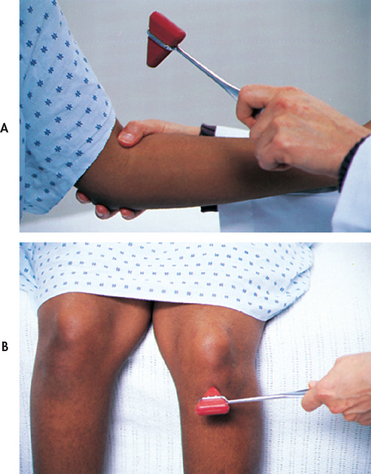
Figure 55-17 The examiner strikes a swift blow over a stretched tendon to elicit a stretch reflex. A, Biceps reflex. B, Patellar reflex.
In general, the biceps, triceps, brachioradialis, patellar and Achilles tendon reflexes are tested. The examiner elicits the biceps reflex by placing their thumb over the biceps tendon in the antecubital space and striking the thumb with a hammer. The patient should have the arms partially flexed at the elbow with the palms up. The normal response is flexion of the arm at the elbow or contraction of the biceps muscle that can be felt by the examiner’s thumb.
The triceps reflex is elicited by striking the triceps tendon above the elbow while the patient’s arm is flexed. The normal response is extension of the arm or visible contraction of the triceps.
The brachioradialis reflex is elicited by striking the radius 3–5 cm above the wrist while the patient’s arm is relaxed. The normal response is flexion and supination at the elbow or visible contraction of the brachioradialis muscle.
The patellar reflex is elicited by striking the patellar tendon just below the patella. The patient can be sitting or lying as long as the leg being tested hangs freely. The normal response is extension of the leg with contraction of the quadriceps.
The Achilles tendon reflex is elicited by striking the Achilles tendon while the patient’s leg is flexed at the knee and the foot is dorsiflexed at the ankle. The normal response is plantar flexion at the ankle.
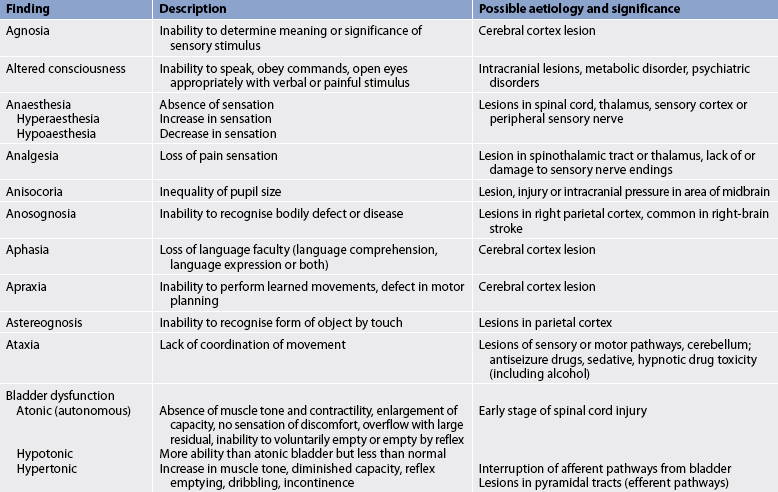
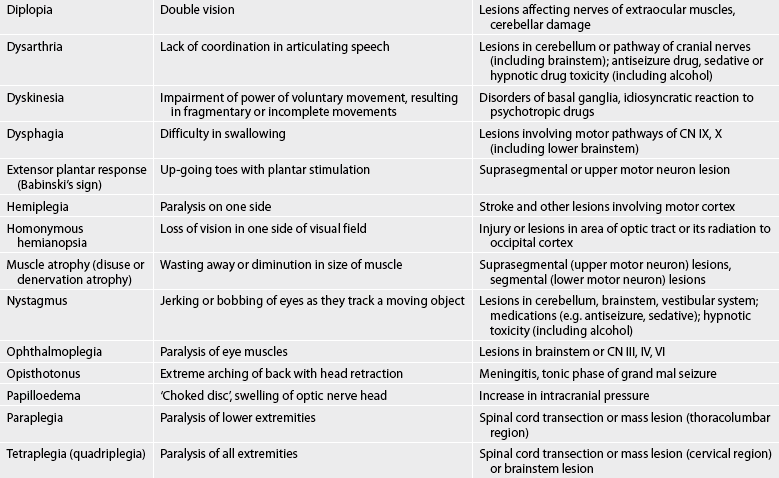
Box 55-1 shows an example of a normal neurological assessment. Common abnormal assessment findings of the neurological system are presented in Table 55-6.
BOX 55-1 Normal physical assessment of the nervous system*
Cranial nerves†
Smell intact to soap and coffee; visual fields full to confrontation; visual acuity 6/6 in both eyes; intact extraocular movements; no nystagmus; pupils equal, round, reactive to light and accommodation (PERRLA); intact facial sensation to touch and pinprick; facial movements full; intact gag and swallow reflexes; symmetrical elevation of soft palate; full strength with head turning and shrugging of shoulders against resistance; midline protrusion of tongue
Motor system
Normal gait and station; normal tandem walk; negative Romberg test; normal and symmetrical muscle bulk, tone, strength; smooth performance of finger–nose, heel–shin movements
Sensory system
Intact sensation to light touch, position sense, vibration, pinprick, heat and cold, two-point discrimination; intact stereognosis and graphaesthesia
Reflexes‡
Biceps, triceps, brachioradialis, patellar and Achilles tendon reflexes 2+ bilaterally; down-going toes with plantar stimulation
Vital signs
The assessment of vital signs is an essential part of every neurological examination, particularly for the patient experiencing an acute neurological event (e.g. head injury, cerebral haemorrhage). Neurological conditions can be immediately life-threatening by causing a problem with the airway, breathing or circulation.15 The essential parameters to assess or measure are:
Although assessing a patient’s vital signs will not provide ‘crucial’ neurological information, collectively the vital signs provide an overall picture of the patient’s stability.16 Any acute change in the patient’s vital signs should make the nurse suspicious. For example, vital sign changes suggesting a neurological injury can include bradycardia, hypertension then hypotension, and loss of thermal control with hypothermia or hyperthermia.17
Diagnostic studies of the nervous system
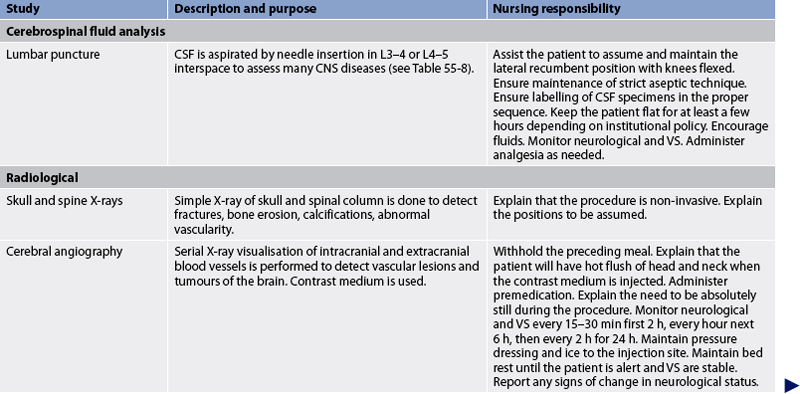
Diagnostic studies provide important information to the nurse in monitoring the patient’s condition and planning appropriate interventions. These studies are considered objective data. Diagnostic studies used to assess the nervous system are presented in Table 55-7.
CEREBROSPINAL FLUID ANALYSIS
CSF analysis gives information about a variety of CNS diseases. Normal CSF is clear, colourless, free of red blood cells and has little protein. Normal values are listed in Table 55-8.
TABLE 55-8 Normal cerebrospinal fluid values
| Parameter | Normal value |
|---|---|
| Specific gravity | 1.007 |
| PH | 7.35 |
| Appearance | Clear, colourless |
| RBCs | None |
| WBCs | <5 × 109/L |
| Protein | |
| Lumbar | 0.15–0.45 g/L |
| Cisternal | 0.15–0.25 g/L |
| Ventricular | 0.05–0.15 g/L |
| Glucose | 2.5–4.2 mmol/L |
| Microorganisms | None |
| Opening pressure with lumbar puncture | 60–150 mm H2o |
RBCs, red blood cells; WBCs, white blood cells.
Lumbar puncture is the most common method of obtaining CSF for analysis. It is contraindicated in the presence of increased ICP or infection at the site of puncture. It is commonly performed to assist with the diagnosis of meningitis, encephalitis, Guillain-Barré syndrome and subarachnoid haemorrhage.18–20
Nurses often assist in this procedure because it is usually performed in the patient’s room. Before the procedure, the nurse should make sure that the patient empties the bladder. The patient should lie in the lateral recumbent position, with the back as near as possible to the edge of the bed. The nurse should assist the patient to draw up the knees to the abdomen and flex the head to the chest. This helps separate the vertebrae so that the needle can be inserted more easily.
Using strict sterile technique, the doctor inserts a long needle below the third lumbar vertebra. This may cause some local discomfort. There is no danger of injuring the spinal cord because the cord terminates between the first and second lumbar vertebrae. However, the patient may have some pain radiating down the leg or muscle twitching if the needle irritates the spinal root. The nurse can assure the patient that this is temporary and that the patient is not in danger of being paralysed.
A manometer is attached to the needle and CSF pressure is determined after the patient is asked to relax and extend the legs. If this is not done, the pressure appears abnormally high. CSF is withdrawn in a series of tubes and sent for analysis. Some examiners believe that the patient should be kept lying flat for at least a few hours after the procedure to avoid a spinal headache, which is presumed to be caused by loss of the cushioning effect of CSF as a result of leakage of CSF at the puncture site. The prone position may be effective in preventing CSF leakage. Others do not believe that the lying position is necessary because headache seems to develop in some patients despite precautions. Meningeal irritation (nuchal rigidity) or signs and symptoms of local trauma (e.g. haematoma, pain) may develop in some patients.
RADIOLOGICAL STUDIES
Cerebral angiography
Cerebral angiography is indicated when vascular lesions or tumours are suspected. A catheter is inserted into the femoral (sometimes brachial) artery. It is then passed up the artery to the aortic arch and into the base of a carotid or a vertebral artery for injection of radio-opaque contrast medium. A series of X-rays is taken in a timed sequence so that pictures of the arteries, smaller vessels and veins can be obtained (see Fig 55-18). This study can help to localise and determine the presence of abscesses, aneurysms, haematomas, arteriovenous malformations, arterial spasm and certain tumours.
Since this is an invasive procedure, adverse reactions may occur. The patient may have an allergic (anaphylactic) reaction to the contrast medium, so they or a relative should be asked whether there are any known allergies. This reaction usually occurs immediately after injection of the contrast medium and may require emergency resuscitation measures in the procedure room. The most common precaution for nurses to take in caring for the patient after return to the room is observation for bleeding at the catheter puncture site (usually the groin). A pressure dressing and ice are usually placed on the site to promote haemostasis and prevent swelling.
Computed tomography
Computed tomography (CT) is a non-invasive procedure, although intravenous injection of contrast medium may be used to enhance visualisation of the blood vessels and identify disruptions in the blood–brain barrier. Therefore, it is necessary to ask about any known allergies. CT scans can be done on an outpatient basis. A number of X-rays scanning different levels of the brain are compiled with computer assistance and presented in a series of black-and-white pictures; these pictures, which illustrate ‘slices’ of the brain, can show haemorrhages, tumours, cysts, oedema, infarction, brain atrophy and hydrocephalus. CT scans do not illustrate structures in the posterior fossa and the base of the brain as clearly as does magnetic resonance imaging (MRI).
Magnetic resonance imaging
MRI involves two kinds of magnetism. The patient is placed within a giant magnetic field that aligns the protons of the hydrogen ions in the cells of the body (see Fig 55-19). Bursts of radiofrequency magnetism are introduced to flip the protons out of alignment. When the radiofrequency magnetism is turned off, the protons realign. The resulting magnetic field change is picked up by the machine and processed by a computer. Vivid black-and-white pictures are then produced.
Figure 55-19 A, Clinical setting for magnetic resonance imaging (MRI). B, Midline sagittal view of the brain using MRI.
Source: Photolibrary/Photo Researchers/Gustoimages.
MRI is useful in evaluating brain and spinal cord oedema, haemorrhage, infarction, blood vessels, tumours, herniation and bone lesions. It is used in the detection of early strokes and multiple sclerosis. Intravenous injection of gadolinium can enhance the images obtained with MRI. Because MRI yields greater contrast in the images of soft-tissue structures than the CT scan does, it is the diagnostic test of choice for many neurological diseases. For example, it may be used in the detection or diagnosis of stroke, multiple sclerosis, tumours, trauma or herniation.21 Zones of demyelination, meningeal thickening and early changes associated with Alzheimer’s disease can also be visualised.22
Positron emission tomography
Positron emission tomography (PET) is used to determine regional metabolism in the brain. PET is a non-invasive means of determining biochemical processes that occur in the brain. It is of particular use in studying the relationship between cerebral blood flow, oxygen utilisation and extraction in focal ischaemia or infarction.2 PET involves the production of isotopes that emit positrons, which are then used to label substances (e.g. oxygen, glucose) that the target organ needs.23 There is increasing clinical use of PET scanning to monitor selected patients with stroke, Alzheimer’s disease, seizure disorders, epilepsy, tumours and Parkinson’s disease. For example, it can be used to evaluate the metabolic features of brain tumours (e.g. glucose metabolism, blood flow and oxygen consumption).24
Myelography
Myelography is used to visualise the spinal column and the subarachnoid space when a spinal lesion is suspected. The most common lesion for which this test is used is a herniated or protruding intervertebral disc. Other lesions include spinal tumours, adhesions, syringomyelia, bony deformations and arteriovenous malformations. The test involves taking X-rays of the spinal column after contrast medium has been injected into the subarachnoid space via a catheter. Water-soluble iodine contrast materials, such as iopamidol, are used most often because they are absorbed into the bloodstream and excreted by the kidneys.
Preparation for this procedure is the same as for lumbar puncture. Also, before the contrast material is injected, patients must be asked whether they have any allergies—specifically, whether they have had any anaphylactic or hypotensive episodes from other contrast media.
After myelography, the patient should lie flat for a few hours in a quiet, comfortable environment. Headache is the most common complaint after the procedure, and it may be accompanied by nausea and occasionally by vomiting. The nurse should observe the patient for any changes in neurological status.
ELECTROGRAPHIC STUDIES
Electroencephalography
The technique of electroencephalography (EEG) involves recording the electrical activity of the surface cortical neurons of the brain using 8–16 electrodes placed on specific areas of the scalp (see Fig 55-20). This test is done to evaluate not only cerebral disease but also the CNS effects of many metabolic and systemic diseases, and it may be used to determine brain death. Among the cerebral diseases assessed by EEG are epilepsy, mass lesions (e.g. tumour, abscess, haematoma), cerebrovascular lesions and brain injury. The procedure is non-invasive. Patients sometimes have the misconception that the recording electrodes will give them an electric shock. They should be assured that this is not true and that the procedure is similar to electrocardiography (ECG).
Electromyography and nerve conduction studies
Electromyography (EMG) is the recording of electrical activity associated with innervation of skeletal muscle. The recording is displayed on a computer screen and may be played on a loudspeaker for simultaneous analysis. Needle electrodes are inserted into the muscle to record specific motor units, because recording from the skin is not sufficient. Normal muscle at rest shows no electrical activity. Typical electrical activity occurs when the muscle contracts. This activity may be altered in diseases of muscle itself (e.g. myopathic conditions) or in disorders of muscle innervation (e.g. segmental or LMN lesions, peripheral neuropathic conditions). Fibrillations are spontaneous, independent contractions of individual muscle fibres that can be detected only by EMG. They appear on EMG 1–3 weeks after a muscle has lost its nerve supply.
Nerve conduction studies involve application of a brief electrical stimulus to a distal portion of a sensory or mixed nerve and recording the resulting wave of depolarisation at some point proximal to the stimulation. For example, a stimulus can be applied to the forefinger and a recording electrode placed over the median nerve at the wrist. The time between the onset of the stimulus and the initial wave of depolarisation at the recording electrode is measured. This is termed nerve conduction velocity. Damaged nerves have slower conduction velocities. It is useful for diagnosing peripheral nerve disorders such as nerve compression and polyneuropathies.18
Evoked potentials
Evoked potentials are recordings of electrical activity associated with nerve conduction along sensory pathways. The activity is generated by a specific sensory stimulus related to the type of study (e.g. checkerboard patterns for visual evoked potentials, clicking sounds for auditory evoked potentials, mild electrical pulses for somatosensory evoked potentials). Electrodes placed on specific areas of the skin and scalp record the electrical activity, which is stored and averaged by a computerised instrument. A wave pattern appears on a screen and it is printed on paper. Peaks in the wave pattern correspond to conduction of the stimulus through certain points along the sensory pathway (e.g. peripheral nerve, brainstem, cortical areas). Increases in the normal time from stimulus onset to a given peak (latency) indicate slowed nerve conduction or nerve damage. This technique is useful in diagnosing abnormalities of the visual or auditory systems because it reveals whether a sensory impulse is reaching the appropriate part of the brain. Indications for these tests include evaluation of the optic nerve in conditions such as multiple sclerosis (optic neuritis) and the vestibulocochlear nerve in acoustic neuroma.
COMBINED DOPPLER AND ULTRASOUND (DUPLEX) STUDIES
Carotid duplex
A duplex study uses combined ultrasound and pulsed Doppler technology. The technician places a probe on the patient’s skin over the carotid artery and slowly moves the probe along the course of the common carotid to the bifurcation of the external and internal carotid arteries. The ultrasound signal emitted from the probe reflects off the moving blood cells within the vessel. The frequency of the reflected signal corresponds to the blood velocity. This response is amplified and registered on a graphic record and as audio sound. The graphic record registers blood velocity. Increased blood flow velocity can indicate stenosis of a vessel. Duplex scanning is a non-invasive study that evaluates the degree of stenosis of the carotid and vertebral arteries.
Transcranial Doppler sonography
Transcranial Doppler (TCD) sonography uses the same technology as a duplex study except that it records blood flow velocities of the intracranial blood vessels. The probe is placed on the skin at various ‘windows’ in the skull (areas in the skull that have only a thin bony covering) to register the velocities of the middle cerebral artery, anterior cerebral artery, posterior cerebral artery, terminal carotid artery and occasionally the anterior and posterior communicating arteries. The temporal, orbital and suboccipital sites are used. The ultrasound signal received is recorded graphically as a waveform. Peak blood flow velocities and systolic/diastolic ratios can be calculated from this information. TCD sonography is a non-invasive technique that is useful in assessing vasospasm associated with subarachnoid haemorrhage, altered intracranial blood flow dynamics associated with occlusive vascular disease, the presence of emboli and cerebral autoregulation.
1. In a patient with a disease that affects the myelin sheath of nerves, such as multiple sclerosis, the glial cells that are affected are the:
2. A state of hypoxia alters the repeated action potentials necessary for transmission of nerve impulses because energy is required for:
3. Drugs or diseases that impair the function of the extrapyramidal system may cause loss of:
4. An obstruction of the anterior cerebral arteries will affect functions of:
5. Paralysis of lateral gaze indicates a lesion of cranial nerve:
6. A result of stimulation of the parasympathetic nervous system is:
7. Assessment of muscle strength of older adults cannot be compared with that of younger adults because:
8. Data about mobility, strength, coordination and activity tolerance are important for the nurse to obtain because:
9. During neurological testing the patient is able to perceive pain elicited by pinprick. Based on this finding, the nurse may omit testing for:
10. A patient’s eyes jerk as they follow the nurse’s moving finger. The nurse records this finding as:
1 Allan D. The central nervous system. In Montague S, Watson R, Herbert R, eds.: Physiology for nursing practice, 3rd edn., Edinburgh: Elsevier, 2005.
2 Kaye A. Essential neurosurgery, 3rd edn. Oxford: Blackwell Publishing, 2005.
3 Martini F, Nath J. Fundamentals of anatomy and physiology, 8th edn. San Francisco: Pearson/Benjamin Cummings, 2010.
4 Watson R. Anatomy and physiology for nurses, 12th edn. Edinburgh: Elsevier, 2005.
5 Rizzo D. Fundamentals of anatomy and physiology, 3rd edn. New York: Delmar, 2010.
6 Dawson D, Shah S. Neurological care. In Sheppard M, Wright M, eds.: Principles and practice of high dependency nursing, 2nd edn., Edinburgh: Elsevier, 2006.
7 Elliot D, Aitken L, Chaboyer W. ACCCN’s critical care nursing. Sydney: Elsevier, 2007.
8 Tracy M. Assessment of critically ill patients and families. In Chulay M, Burns S, eds.: AACN essentials of critical care nursing, 2nd edn., New York: McGraw-Hill, 2010.
9 Hodbell E, Stewart-Amidei C, McNair N, et al. Assessment. In Bader M, Littlejohns L, eds.: AANN core curriculum for neuroscience nursing, 5th edn., St Louis: Saunders, 2010.
10 Hickey J. Neurological and neurosurgical nursing, 6th edn. Philadelphia: Lippincott Williams & Wilkins, 2010.
11 Fulde G, ed. Emergency medicine—the principles of practice, 5th edn., Sydney: Elsevier, 2009.
12 Shin RK, Porter NC. The neurological examination. In Weiner W, Goetz C, eds.: Neurology for the non-neurologist, 6th edn., Philadelphia: Lippincott, 2010.
13 Tollefson J. Clinical psychomotor skills: assessment tools for nursing students, 4th edn. Melbourne: Social Science Press, 2009.
14 Barker E. Neuroscience nursing—a spectrum of care, 3rd edn. St Louis: Mosby, 2007.
15 Grey J, Gavin C. Assessment and management of neurological problems (1). Emerg Med J. 2005;22:440–445.
16 Wolff AM, Taylor SA, McCabe JF. Using checklists and reminders in clinical pathways to improve hospital inpatient care. Med J Aus. 2004;181(8):428–431.
17 Crimlisk JT, Grande MM. Neurologic assessment skills for the acute medical surgical nurse. Orthop Nurs. 2004;23(1):3–9.
18 Pentland B, Statham P, Olson J. The nervous system including the eye. In Douglas G, Nicol F, Robertson C, eds.: Macleod’s clinical examination, 12th edn., Edinburgh: Elsevier, 2009.
19 Cooper N, Cramp P, Forest K. Essential guide to acute care. London: John Wiley, 2006.
20 Baird B, Keen J, Swearingen P. Manual of critical care nursing: nursing interventions and collaborative management, 6th edn. St Louis: Elsevier, 2011.
21 Christensen B, Kockrow E. Adult health nursing, 6th edn. St Louis: Mosby, 2010.
22 Evans D, Evans C, Evans R. Special tests: the procedure and meaning of common tests in hospital, 15th edn. London: Mosby, 2003.
23 Eberhardie C. Patients requiring neurosurgery. In Pudner R, ed.: Nursing the surgical patient, 3rd edn., Edinburgh: Elsevier, 2010.
24 Morales RE, Shah DG. Neuroradiology. In Weiner W, Goetz C, eds.: Neurology for the non-neurologist, 6th edn., Philadelphia: Lippincott, 2010.
American Association of Neuroscience Nurses. www.aann.org
Australasian Neuroscience Nurses’ Association. www.anna.asn.au
British Association of Neuroscience Nurses. www.bann.org.uk
National Institute for Clinical Excellence. www.nice.org.uk
National Institute of Neurological Disorders and Stroke. www.ninds.nih.gov
World Federation of Neuroscience Nurses. www.wfnn.nu