Chapter 8 Pain management
2. Describe the neural mechanisms of pain and pain modulation.
3. Differentiate between nociceptive and neuropathic types of pain.
4. Explain the physical and psychological effects of unrelieved pain.
5. Interpret the subjective and objective data that are obtained from a comprehensive pain assessment.
6. Describe effective multidisciplinary pain management techniques.
7. Explore drug and non-drug methods of pain relief.
8. Explain your role and responsibility in pain management.
9. Discuss ethical and legal issues related to pain and pain management.
10. Evaluate the influence of one’s own knowledge, beliefs and attitudes about pain assessment and management.
Pain is a complex, multidimensional experience. For many people, it is a major problem that causes suffering and reduces their quality of life. It is important for nurses to understand the physiological and psychosocial dimensions of pain to effectively assess and manage patients with pain. Nurses also need to know what therapies are available for pain to successfully help patients in pain. This chapter presents evidence-based information to enable nurses to assess and manage pain successfully as part of a multidisciplinary team.
Magnitude of the pain problem
It is estimated that 3.1 million adult Australians (1.4 million males and 1.7 million females) experience chronic pain.1 Women in Australia seem more likely to experience chronic pain than men: a telephone survey of adults in New South Wales found that 20% of females and 17% of males experienced chronic pain in the 6 months prior to the interview.2 There are no recent studies from New Zealand that examine the incidence and impact of pain on the population, although in the 2006/2007 National Health Survey one in six people reported experiencing chronic pain (there were no differences between the numbers of men and women reporting pain).3 The New Zealand Ministry of Health recently examined the direct and indirect costs of long-term conditions on society and, although chronic pain was not one of the conditions specifically examined, the estimated costs were assessed as being more than $100 million per annum per condition.4 The estimated cost of chronic pain in Australia was found to be $34.3 billion in 2007 (or $10,847 for each person affected).1
In broad terms, pain can be divided into five categories:
• acute pain, defined as a normal and time-limited response to trauma or other ‘noxious’ experience, including pain related to medical procedures and acute medical conditions (e.g. acute shingles)
• pain that is progressing towards chronic pain, but this progression may be prevented (‘subacute’ pain)
• recurrent pain (e.g. migraine)
• chronic (non-cancer) pain, defined as constant daily pain for a period of 3 months or more in the last 6 months (sometimes the term ‘persistent pain’ is also used)
• cancer-related pain.5
There are currently no data in Australia or New Zealand regarding the incidence of chronic pain in children and adolescents, but international research has found that the incidence of chronic pain in this population is large enough to be of concern and has long-lasting and negative effects on self-esteem, quality of life and education.6,7 It is particularly difficult for people with chronic pain to obtain effective care and support because chronic pain is poorly understood by many health professionals. For people who live in rural and remote areas, access to pain services is even more challenging. For example, Indigenous Australians generally have even less access to evidence-based pain services and community support than the general population.8
Pain experience
Pain is the main reason that people seek healthcare. One in five Australians and one in six New Zealanders, including children and adolescents, will suffer chronic pain in their lifetime, and up to 80% of people living with chronic pain are missing out on treatment that could help them to live with less pain.1,5 Chronic pain is generally more common with increasing age, and people with chronic pain, especially those with higher levels of pain-related disability, use healthcare services more than people without pain.5,9 It is estimated that 50–75% of hospital patients with acute pain receive inadequate treatment of their pain and up to 80% experience unnecessary postoperative pain.10 τhe prevalence of pain in patients at all stages of cancer is 53% and, of those surveyed, one-third grade their pain as moderate or severe.11 In cancer survivors after curative treatment, 13–60% experience ongoing pain.5 This is despite the availability of current techniques to relieve more than 90% of acute and cancer pain.12,13
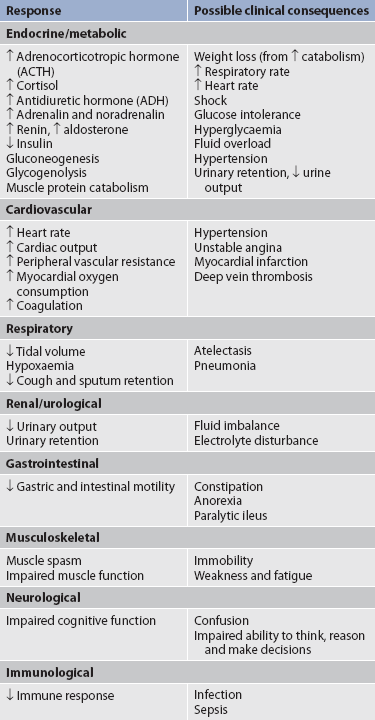
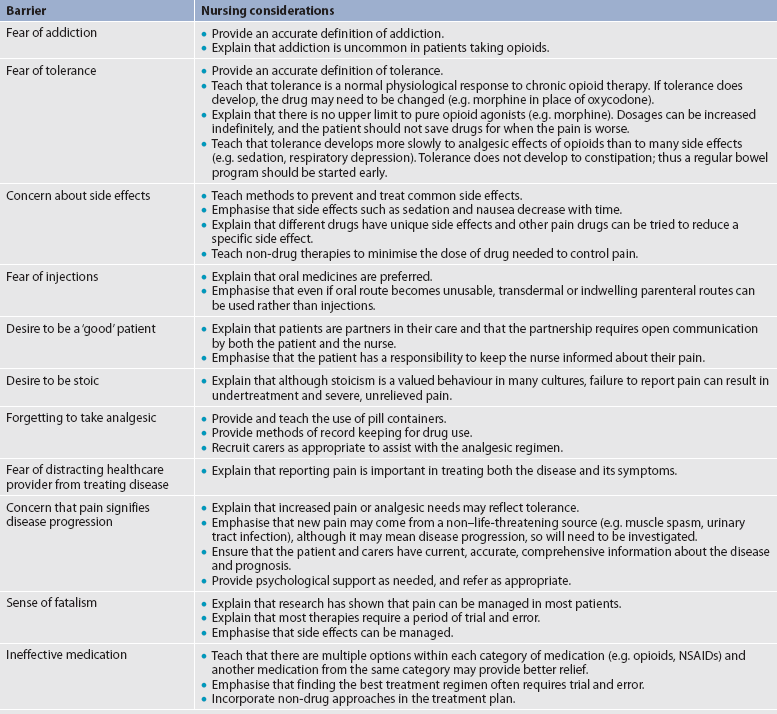
The consequences of untreated pain include unnecessary suffering, physical and psychosocial dysfunction, impaired recovery from acute illness and surgery, immunosuppression and sleep disturbances. In acutely ill patients, unrelieved pain can cause increased morbidity due to respiratory dysfunction, increased heart rate and cardiac workload, increased muscular contraction and spasm, decreased gastrointestinal (GI) motility and transit, and increased breakdown of body energy stores (catabolism).5,14 Table 8-1 describes the harmful effects of unrelieved acute pain. The reasons for the undertreatment of pain are varied. Among healthcare providers, these include: (1) inadequate knowledge and skills to assess and treat pain; (2) unwillingness to believe patients’ report of pain; (3) lack of time, expertise and perceived importance of making regular pain assessments; and (4) inaccurate and inadequate information about addiction, tolerance, respiratory depression and other side effects of opioids.14 In addition, some healthcare providers fear that aggressive pain management may hasten or cause death.15 Among patients and family carers, attitudes towards pain and opioids play a major role in the underreporting and undertreatment of pain. Fear of addiction, tolerance and side effects often make patients reluctant to report pain or comply with a regimen that involves opioid drugs. Other barriers include the belief that pain is the inevitable result of worsening disease and the expectation that drugs will not relieve pain anyway. The belief that pain is inevitable and the desire to be a ‘good’ patient who does not complain have also been seen in patients, particularly in older adults.14,16
Definitions and dimensions of pain
More than 40 years ago, Margo McCaffery, a nurse and pioneer in pain management, defined pain as ‘whatever the person experiencing the pain says it is, existing whenever the person says it does’.17 The International Association for the Study of Pain (IASP) defines pain as ‘an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage’.18 Note that these definitions emphasise the subjective nature of pain, in which the patient’s self-report is the most valid means of assessment. Although these definitions are useful, and they emphasise the subjective nature of pain, they do not include the experience of patients who are comatose or who suffer from dementia, patients who have mental disabilities and patients with expressive aphasia who may possess varying ability to report pain. In these instances, nonverbal information, such as behaviours, needs to be incorporated into pain assessment. In defining pain as a human experience, successful pain assessment and treatment must incorporate multiple dimensions and therefore must include all members of the healthcare team.
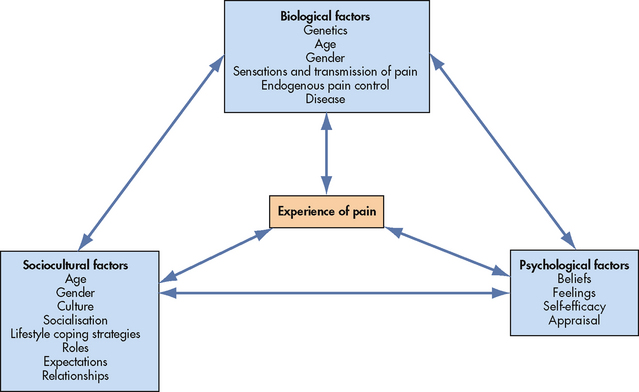
A number of theories have been proposed to explain the experience of pain. The traditional biomedical theory of pain, dominant until the mid-1960s, assumed that pain was the result of a direct and inevitable relationship between the stimulation of pain receptors and the sensation of pain.5 For example, if you bruise your arm by banging it against a hard object, you experience pain through the activation of pain receptors in the skin and underlying tissue. The biomedical theory came under challenge when it was recognised that people experience different levels of pain regardless of similar degrees of tissue damage.19 As our own everyday experiences show, a person’s thoughts and feelings, together with the context and the meaning of the pain, all impact on the level of pain experienced. Psychological factors that influence the experience of pain include the processes of attention, other cognitive processes (e.g. memory/learning, thought processing, beliefs, mood), behavioural responses and interactions with the environment.19 As a result of this knowledge, a biopsychosocial theory of pain was developed to explain the complexity of a person’s experience of pain.20 This model includes the physiological, affective, cognitive, behavioural and sociocultural dimensions of pain. The concept map shown in Figure 8-1 highlights the relationship between psychological, sociocultural and biological factors. The linking arrows highlight interactions between factors. For example, biological factors of age and disease may impact on psychological feelings, such as fear and depression, and on sociocultural aspects, such as relationships.
Cognitive, affective, behavioural and sociocultural responses to pain
Suffering is defined in terms of a person’s cognitive and affective response to pain. Suffering is described as the total experience that combines pain intensity with emotional factors and specific behaviours relating to each pain experience. The degree of suffering is determined by a range of factors, from type, site, duration and controllability of pain, to an individual’s personal and health history and sociocultural factors. Both pain and the degree of suffering will impact on pain behaviours.21
COGNITIVE FACTORS
A person with any pain, but particularly unresolved or chronic pain, develops a set of beliefs to explain the pain experience, beliefs about the cause and onset of pain, the meaning of symptoms, ability to control pain and the impact of pain, now and in the future.5 Pain can be seen as a stressor that is appraised like any other stressor.
Appraisal involves a consideration of the threat the pain poses and the coping strategies available to cope with it. How the pain is appraised is influenced by the person’s beliefs and previous experiences and will impact on how the person deals with the pain, from ignoring it and maintaining usual activities to taking on the sick role. Beliefs have been found to be central to the functioning of the patient with pain (especially chronic pain), impacting on psychological and physical functioning, coping efforts, behavioural responses and responses to treatment.5 Self-efficacy is defined as the belief that one is capable of doing what is required in a particular situation. Self-efficacy appears to be important in the perception of pain, adjustment to it and level of disability.5 If you believe that your thoughts and behaviours can have a positive impact on your pain, then you are more likely to try a range of strategies to moderate the pain and to persevere in your efforts and have a sense of control. For example, those taking some control of analgesia and who are active rather than passive show less distress and disability.5,19 If patients are less distressed they are likely to experience less physiological arousal and bodily tension, which act to exacerbate pain sensation.
AFFECTIVE FACTORS
Fear and anxiety are an inevitable and primary aspect of pain because pain signals danger requiring an immediate response. In chronic pain, in particular, fear and anxiety will be ongoing because the patient’s efforts at responding often have little effect.
Previous pain experiences impact on current pain episodes, influencing the intensity of pain and pain behaviours. Patients may:
• fear that their pain will not be well managed
• fear that they will be a nuisance to busy nurses
• fear that too much analgesia may cause dependence and believe that opioids (e.g. morphine) are only for cancer pain
• fear that healthcare professionals will not understand their pain or believe the intensity of their pain—this is particularly true of people who suffer from chronic pain who may be admitted for a reason not associated with their chronic pain state.
Depression is commonly present in adult patients with chronic pain who present at pain clinics and can be caused by the disabling consequences of pain and its impact on quality of life.22 Anger may be associated with depression. Anger may be an outcome of severe frustration at the impact on quality of life or may be more specifically directed at failed treatments and healthcare professionals. Ongoing fear, depression and/or anger will interfere with the patient’s ability to engage with a range of pain therapies and these feelings should be addressed as part of the overall pain management regimen.22,23
Attention and vigilance to pain are likely to occur when the threat of pain is ongoing. Heightened attention and hyper-vigilance to ongoing pain is, however, counterproductive as a long-term coping strategy. In addition, it is associated with high levels of disability and distress and lower pain thresholds, anxiety and poor concentration.22,23 Catastrophic thinking about pain appears to be best understood as an extreme type of worrying about pain and is also seen as a manifestation of depression.21
BEHAVIOURAL FACTORS
What people believe and feel about their pain will impact on their behaviour. Intervening to change thoughts and feelings can help change behaviour in positive ways, improving the sense of wellbeing and decreasing pain and disability. Similarly, changing behaviour, for example by increasing activity, can have a positive impact on thoughts and feelings.5,16
In addition to cognitive and affective influences on behaviour, learning mechanisms, such as operant conditioning, have been shown to have an impact on pain behaviour. Operant conditioning defines pain behaviour as behaviour like any other in that its manifestation, strength and frequency may come under the control of external stimuli, which may be rewarding or reinforcing—for example, attention from a partner or nurse. Escape from punishing or negative reinforcement, such as avoidance of some painful movements, will also maintain pain behaviour.
SOCIOCULTURAL FACTORS
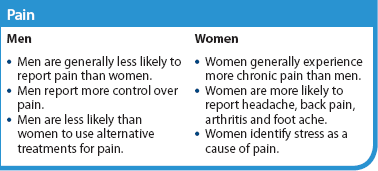
In relation to gender, women are generally said to report more frequent and severe pain of longer duration than men.5 In New Zealand, the 2006/2007 National Health Survey found that men and women reported the same incidence of chronic pain, but when the data were adjusted for age, Māori men were found to have a significantly increased incidence of chronic pain compared to men in the total population. Pacific Islander women, Asian men and Asian women were significantly less likely to report chronic pain.3
Families and carers influence the patient’s response to pain through their beliefs and behaviours.24 People learn behaviours appropriate to their culture by a process of socialisation. These behaviours include pain behaviours and some differences have been reported in the cultural expression, but not experience, of pain.25 For example, in Australia, in one study Aboriginal patients were reported as having a higher pain threshold than non-Aboriginal patients26 and to be less likely to complain of pain. The same study reported that men in particular did not complain as they did not want to appear weak. Many traditional Aboriginal people may be quiet and reserved when experiencing pain, and may be labelled by health professionals as not needing pain relief.27 A study of Aboriginal women after surgery found that they had culturally appropriate ways of expressing and managing pain that were not well-understood by non-Aboriginal nurses.28 It was reported that the Aboriginal women were generally silent about their pain, even when asked, and that, in part at least, this arose from fear of pain (including its origin and significance in relation to themselves and the external world).19 Many traditional Aboriginal people believe that nurses will know about the pain they are experiencing, so that there is no need to tell them.28 Nurses need to realise that they may underestimate the pain of those who are from a culture different from their own and whose language is different.
Some people cope with pain by distracting themselves, whereas others convince themselves that the pain is permanent, untreatable and overwhelming. Research has shown that people who believe that their pain in uncontrollable and overwhelming are more likely to have poorer clinical outcomes.21,22
Pain mechanisms
Nociception is the physiological process by which information about tissue damage is communicated to the central nervous system (CNS). It involves four processes: (1) transduction; (2) transmission; (3) perception; and (4) modulation (see Fig 8-2). (The nervous system is described in Ch 55.)
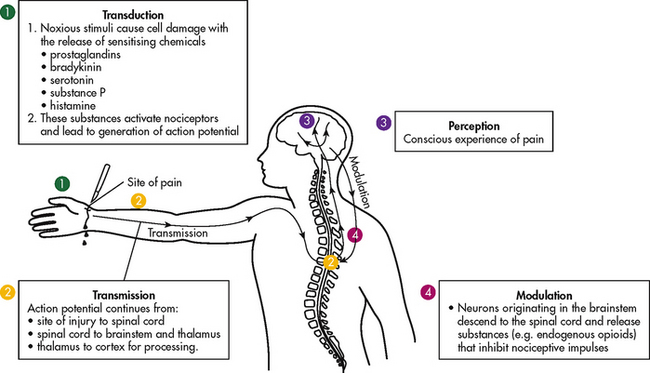
Figure 8-2 Nocioceptive pain originates when the tissue is injured. 1, Transduction occurs when there is release of chemical mediators. 2, Transmission involves the conduct of the action potential from the periphery (injury site) to the spinal cord and then to the brainstem, thalamus and cerebral cortex. 3, Perception is the conscious awareness of pain. 4, Modulation involves signals from the brain going back down the spinal cord to modify incoming impulses.
TRANSDUCTION
Transduction involves the conversion of a noxious mechanical, thermal or chemical stimulus into an electrical signal called an action potential. Noxious (tissue-damaging) stimuli, including thermal (e.g. sunburn), mechanical (e.g. surgical incision) and chemical stimuli (e.g. toxic substances), cause the release of numerous chemicals such as hydrogen ions, substance P and adenosine triphosphate (ATP) into the damaged tissues. Other chemicals are released from mast cells (e.g. serotonin, histamine, bradykinin and prostaglandins) and macrophages (e.g. bradykinin, interleukins and tumour necrosis factor [TNF]). These chemicals activate nociceptors, which are specialised receptors or free nerve endings that respond to painful stimuli. Activation of nociceptors results in an action potential that is carried from the nociceptors to the spinal cord, primarily via small, rapidly conducting, myelinated A-delta fibres and slowly conducting unmyelinated C fibres.
In addition to stimulating nociceptors to fire, inflammation and the subsequent release of chemical mediators promote lowered nociceptor thresholds. As a result, nociceptors may fire in response to stimuli that previously were insufficient to elicit a response; they may also fire in response to non-noxious stimuli, such as light touch. This increased susceptibility to nociceptor activation is called peripheral sensitisation. Cyclooxygenase (COX), an enzyme produced in the inflammatory response, plays an important role in peripheral sensitisation,29 and leukotrienes, prostaglandins, cytokines and substance P are also involved in peripheral sensitisation. A clinical example of this process is sunburn. This thermal injury causes inflammation that results in a sensation of pain when the affected skin is touched lightly. Peripheral sensitisation also amplifies signal transmission, which in turn contributes to central sensitisation (discussed under dorsal horn processing later in the chapter).
The pain produced from activation of peripheral nociceptors is called nociceptive pain. There is a second source of pain-related action potentials arising from abnormal processing of stimuli by the nervous system. This kind of pain is called neuropathic pain. Both types of pain are described later in the chapter.
Therapies that alter either the local environment or the sensitivity of the peripheral nociceptors can prevent transduction and initiation of an action potential. Decreasing the effects of chemicals released at the periphery is the basis of several drug approaches to pain relief. For example, non-steroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen and naproxen, and corticosteroids, such as dexamethasone, exert their analgesic effects by blocking pain-sensitising chemicals. NSAIDs block the action of COX, thereby interfering with the production of prostaglandins. Corticosteroids block the action of phospholipase, thereby reducing the production of both prostaglandins and leukotrienes (see Ch 12). Drugs that stabilise the neuronal membrane and inactivate peripheral sodium channels inhibit the production of the nerve impulse. These medications include local anaesthetics (e.g. injectable or topical lignocaine, bupivacaine and ropivacaine) and antiseizure drugs (e.g. carbamazepine and lamotrigine).
TRANSMISSION
Transmission is the process by which pain signals are relayed from the periphery to the spinal cord and then to the brain. The nerves that carry pain impulses from the periphery to the spinal cord are called primary afferent fibres and include A-delta fibres and C-fibres, each of which is responsible for a different pain sensation (see Fig 8-3). A-delta fibres are small, myelinated fibres that conduct pain rapidly and are responsible for the initial sharp pain that accompanies tissue injury. C-fibres are small, unmyelinated fibres that transmit painful stimuli more slowly and produce pain that is typically aching or throbbing in quality. Primary afferent fibres terminate in the dorsal horn of the spinal cord, which contains the cell bodies for afferent nerve fibres. Activity in the dorsal horn integrates and modulates pain inputs from the periphery. The propagation of pain impulses from the site of transduction to the brain is shown in Figure 8-2. Three segments are involved in nociceptive signal transmission: (1) transmission along the peripheral nerve fibres to the spinal cord; (2) dorsal horn processing; and (3) transmission to the thalamus and cerebral cortex.
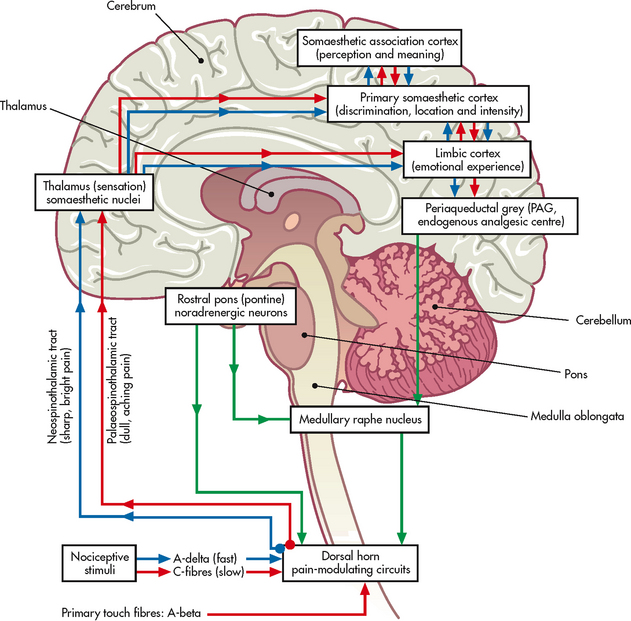
Figure 8-3 Possible ascending and descending pathways of A-delta fibres and C-fibres from the dorsal horn in the spinal cord to the thalamus and other centres. These pathways are not definitive and may cross over.
Transmission to the spinal cord
The first-order neuron extends the entire distance from the periphery to the dorsal horn of the spinal cord with no synapses. For example, an afferent fibre from the great toe travels from the toe through the fifth lumbar nerve root into the spinal cord; it is one cell. Once generated, an action potential travels all the way to the spinal cord unless it is blocked by a sodium channel inhibitor (e.g. local anaesthetic) or disrupted by a lesion at the central terminal of the fibre (e.g. by a dorsal root entry zone [DREZ] lesion).
The manner in which nerve fibres enter the spinal cord is central to the notion of spinal dermatomes. Dermatomes are areas on the skin that are innervated primarily by a single spinal cord segment. The distinctive pattern of the rash caused by herpes zoster (shingles) across the back and trunk is determined by dermatomes.
Dorsal horn processing
Once a nociceptive signal arrives in the CNS, it is processed within the dorsal horn of the spinal cord. Neurotransmitters released from the afferent fibre bind to receptors on nearby cell bodies and dendrites of cells. Some of these neurotransmitters produce activation (e.g. glutamate, aspartate, substance P), whereas others inhibit activation of nearby cells (e.g. gamma-aminobutyric acid [GABA], serotonin, noradrenalin). In this area, exogenous and endogenous opioids also play an important role by binding to opioid receptors and blocking the release of neurotransmitters, particularly substance P. Endogenous opioids, which include encephalin and β-endorphin, are chemicals that are synthesised and secreted by the body. They are capable of producing analgesic effects similar to those of exogenous opioids such as morphine.
When enhanced excitability occurs in spinal neurons, it is termed central sensitisation. Peripheral tissue damage or nerve injury can cause central sensitisation, and continued nociceptive input from the periphery is necessary to maintain it. Central sensitisation plays a crucial role in the pathogenesis of chronic pain.13 It is defined by an increase in the excitability of neurons within the CNS, so that normal sensory inputs cause abnormal sensing and responses to painful and other stimuli. This explains why some people experience significant pain from touch or tactile stimulation in and around the areas of tissue or nerve injury. With central sensitisation, the central processing circuits are disrupted. Some aspects of central sensitisation can persist after peripheral inputs cease; however, peripheral and central neural mechanisms involved in causing central sensitisation can be long-lasting.30
With ongoing stimulation of slowly conducting unmyelinated C-fibre nociceptors, firing of specialised dorsal horn neurons gradually increases. These inputs create many problems, including the sprouting of wide dynamic range (WDR) neurons and the induction of glutamate-dependent N-methyl-d-aspartate (NMDA) receptors. WDR neurons respond to both nociceptive and non-nociceptive inputs that are of varying levels of stimulus intensity. When sprouting of these neurons occurs, they grow into areas where pain-receiving nerve cell bodies are located. This results in the capacity to transmit a broader range of stimuli-producing signals, which are then passed up the spinal cord and brain. This process is known as ‘windup’ and is dependent on the activation of NMDA receptors. NMDA receptor antagonists, such as ketamine, are agents that can potentially interrupt or block mechanisms that lead to or sustain central sensitisation. Windup, like central sensitisation and hyperalgesia (increased pain responses to noxious stimuli), is induced by C-fibre inputs. Windup, however, is different as it can be short-lasting, whereas central sensitisation and hyperalgesia persist over time.31
It is important for nurses to appreciate that acute unrelieved pain can lead to chronic pain through the process of central sensitisation.5,19 Acute tissue injury produces a cascade of events that involve the release of certain excitatory neurotransmitters (e.g. glutamate) and neuropsychological responses. Even brief intervals of acute pain are capable of inducing long-term neuronal remodelling and sensitisation (plasticity), chronic pain and lasting psychological distress. Neuroplasticity refers to an intricate group of processes that allow neurons in the brain to compensate for injury and adjust their responses to new situations or changes in their environment.32 Neuroplasticity contributes to adaptive mechanisms for reducing pain but also can result in maladaptive mechanisms that enhance pain. Genetic make-up and variability among individuals may play important roles in affecting the plasticity of the CNS.33 Understanding this phenomenon helps to explain individual differences in responses to pain, and why some patients develop chronic pain conditions while others do not.
Clinically, central sensitisation of the dorsal horn results in: (1) hyperalgesia; (2) painful responses to normally innocuous stimuli (termed allodynia); (3) prolonged pain after the original noxious stimulus ends (called persistent pain); and (4) the extension of tenderness or increased pain sensitivity outside of an area of injury to include uninjured tissue (i.e. referred pain or secondary hyperalgesia).34
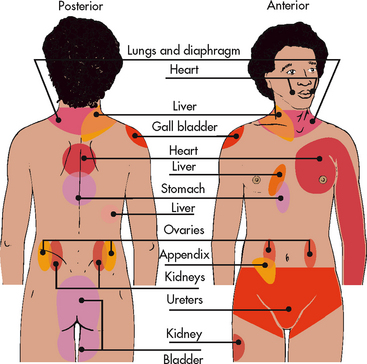
Referred pain must be considered when interpreting the location of pain reported by the patient with injury to or disease involving visceral organs. The location of a tumour may be distant from the pain location reported by the patient (see Fig 8-4). For example, pain from liver disease is frequently located in the right upper abdominal quadrant, but can also be referred to the anterior and posterior neck region and to a posterior flank area. If referred pain is not considered when evaluating a pain location report, diagnostic tests and therapy could be misdirected.
Transmission to the thalamus and cortex
From the dorsal horn, nociceptive stimuli are communicated to the third-order neuron, primarily in the thalamus, and several other areas of the brain. Fibres of dorsal horn projection cells enter the brain through several pathways, including the spinothalamic tract (STT) and the spinoreticular tract (SRT). Distinct thalamic nuclei receive nociceptive input from the spinal cord and have projections to several regions in the cerebral cortex, where the perception of pain is presumed to occur.
Therapeutic approaches that target pain transmission include opioid analgesics that bind to opioid receptors on primary afferent and dorsal horn neurons. These agents mimic the inhibitory effects of endogenous opioids. Another medication, baclofen, inhibits transmission by binding to GABA receptors, thus mimicking the inhibitory effects of GABA.
PERCEPTION
Perception occurs when pain is recognised, defined and responded to by the individual experiencing the pain. In the brain, nociceptive input is perceived as pain. There is no single, precise location where pain perception occurs. Instead, pain perception involves several brain structures. For example, it is believed that the reticular activating system (RAS) is responsible for warning the individual to attend to the pain stimulus; the somatosensory system is responsible for the localisation and characterisation of pain; and the limbic system is responsible for the emotional and behavioural responses to pain. Cortical structures are also crucial to constructing the meaning of the pain. Therefore, behavioural strategies such as distraction and relaxation are effective pain-reducing therapies for many people. By directing attention away from the pain sensation, patients can reduce the sensory and affective components of pain. Opioids are one class of analgesic drugs that modify pain perception, and one mechanism of action is through their ability to activate descending pathway inhibition of pain. Other classes of analgesics such as some antiseizure drugs and antidepressants act in a similar manner.
MODULATION
Modulation involves the activation of descending pathways that exert inhibitory or facilitatory effects on the transmission of pain (see Fig 8-2). Depending on the type and degree of modulation, nociceptive stimuli may or may not be perceived as pain. Modulation of pain signals can occur at the level of the periphery, spinal cord, brainstem and cerebral cortex. Descending modulatory fibres release chemicals such as serotonin, noradrenalin, GABA and endogenous opioids that can inhibit pain transmission.
Several antidepressants exert their effects through the modulatory systems. For example, tricyclic antidepressants (e.g. amitriptyline) and serotonin noradrenalin reuptake inhibitors (SNRIs) (e.g. venlafaxine and duloxetine) are used in the management of chronic non-malignant and cancer pain. These agents interfere with the reuptake of serotonin and noradrenalin, thereby increasing their availability to inhibit noxious stimuli. Noradrenalin appears to play a greater role in centrally inhibiting pain than does serotonin, which explains why SNRIs have greater analgesic effects than selective serotonin reuptake inhibitors (SSRIs) (e.g. fluoxetine, paroxetine and sertraline).35
Classification of pain
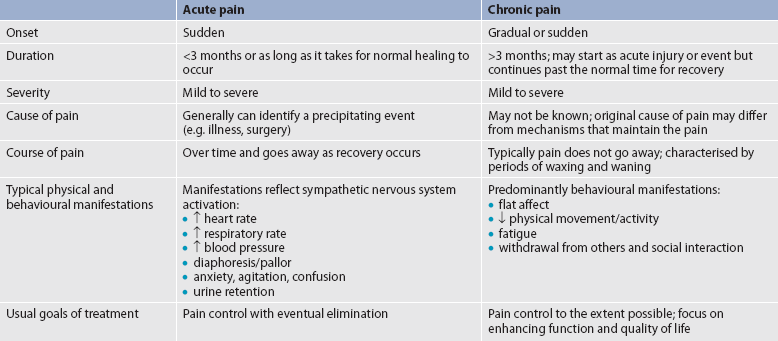
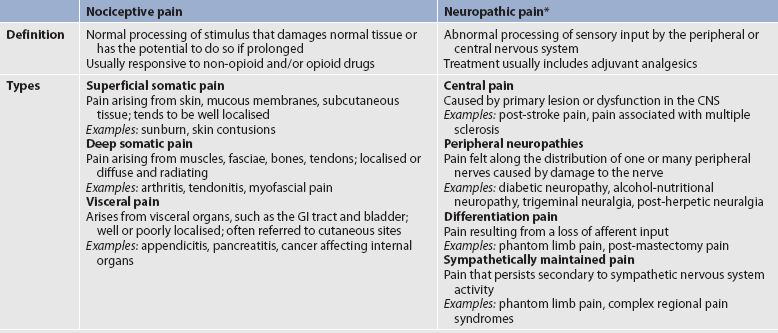
Pain can be categorised in several ways. Most commonly, it is categorised as nociceptive or neuropathic based on the underlying pathology (see Table 8-2). Another useful scheme is to classify pain as acute or chronic (see Table 8-3).
TABLE 8-2 Comparison of nociceptive and neuropathic pain
Available at www.ninds.nih.gov/disorders/reflex_sympathetic_dystrophy/detail_reflex_sympathetic_dystrophy.htm, accessed 9 April 2011.
* Some types of neuropathic pain (e.g. post-herpetic neuralgia) are caused by more than one neuropathological mechanism.
Source: Adapted from national Institute of neurological Disorders and Stroke. Complex regional pain syndrome fact sheet.
NOCICEPTIVE PAIN
Nociceptive pain is caused by damage to somatic or visceral tissue. Somatic pain is often further categorised as superficial or deep. Superficial pain arises from skin, mucous membranes and subcutaneous tissues, and is often described as sharp, burning or prickly. Deep pain is often characterised as deep, aching or throbbing and originates in bone, joint, muscle, skin or connective tissue. Visceral pain comes from the activation of nociceptors in the internal organs and lining of the body cavities such as the thoracic and abdominal cavities. Visceral nociceptors respond to inflammation, stretching and ischaemia. Stretching of hollow viscera in the intestines and bladder that occurs from tumour involvement or obstruction can produce intense cramping pain. Examples of nociceptive pain include pain from a surgical incision, a broken bone, arthritis, pancreatitis and inflammatory bowel disease. Nociceptive pain is generally responsive to non-opioid medications, such as NSAIDs, as well as opioids.
NEUROPATHIC PAIN
Neuropathic pain is caused by damage to peripheral nerves or structures in the CNS. Typically described as numbing, hot-burning, shooting, stabbing, sharp or electric shock–like in nature, neuropathic pain can be sudden, intense, short-lived or lingering. Paroxysmal firing of injured nerves is responsible for shooting and electric shock–like sensations. Common causes of neuropathic pain include trauma, inflammation (e.g. secondary to a herniated disc inflaming the adjacent nerve and dorsal root ganglion), metabolic diseases (e.g. diabetes mellitus), alcoholism, infections of the nervous system (e.g. herpes zoster, human immunodeficiency virus), tumours, toxins and neurological diseases (e.g. multiple sclerosis).
Deafferentation pain results from loss of afferent input secondary to either the peripheral nerve injury or CNS disease. Sympathetically maintained pain is associated with dysregulation of the autonomic nervous system and central pain is caused by CNS lesions or dysfunction. Painful peripheral polyneuropathies (pain felt along the distribution of multiple peripheral nerves) and painful mononeuropathies (pain felt along the distribution of a damaged nerve) arise from damage to peripheral nerves and generate pain that may be described as burning, paroxysmal or shock-like. Examples of neuropathic pain include postherpetic neuralgia, phantom limb pain, diabetic neuropathies and trigeminal neuralgia.
One particularly debilitating type of neuropathic pain is complex regional pain syndrome (CRPS). Typical features include dramatic changes in the colour and temperature of the skin over the affected limb or body part, accompanied by intense burning pain, skin sensitivity, sweating and swelling. CRPS type I is frequently triggered by tissue injury; the term describes all patients with the above symptoms but with no underlying nerve injury. CRPS type II is associated with diverse sympathetic dysfunction.35
Often, neuropathic pain is not well controlled by opioid analgesics alone. Treatment is typically augmented with adjuvant therapies including tricyclic antidepressants (e.g. amitriptyline, nortriptyline, desipramine), SNRIs (e.g. venlafaxine, duloxetine, bupropion), antiseizure drugs (e.g. gabapentin, pregabalin), and α2-adrenergic agonists (e.g. clonidine). More recently, NMDA receptor antagonists such as ketamine have shown promise in alleviating neuropathic pain refractory to other drugs.36
ACUTE AND CHRONIC PAIN
Acute pain and chronic pain are different as reflected in their cause, course, manifestations and treatment (see Table 8-3). Examples of acute pain include postoperative pain, labour pain, pain from trauma (e.g. lacerations, fractures, sprains), infection (e.g. dysuria from cystitis) and angina. For acute pain, treatment includes analgesics for symptom control and treatment of the underlying cause (e.g. splinting for a fracture, antibiotic therapy for an infection). Normally, acute pain diminishes over time as healing occurs. However, acute pain that persists can ultimately lead to disabling chronic pain states. For example, pain associated with herpes zoster (shingles) subsides as the acute infection resolves, usually within a month. However, sometimes the pain persists and develops into a chronic pain state called postherpetic neuralgia (PHN).
Chronic pain, or persistent pain, lasts for longer periods—often defined as longer than 3 months or past the time when an expected acute pain or acute injury should subside.5 The severity and functional impact of chronic pain is frequently disproportionate to objective findings. Whereas acute pain functions as a signal, warning the person of potential or actual tissue damage, chronic pain does not appear to have an adaptive role. Chronic pain can be disabling and is often accompanied by anxiety and depression. As previously discussed, untreated acute pain leads to chronic pain through central sensitisation and neuroplasticity. Consequently, it is imperative to treat acute pain aggressively and effectively to prevent chronic pain.
Chronic pain is strongly associated with markers of social disadvantage, such as lower levels of completed education, not having private health insurance, receiving a disability benefit or unemployment benefit and being unemployed for health reasons.5
Pain assessment
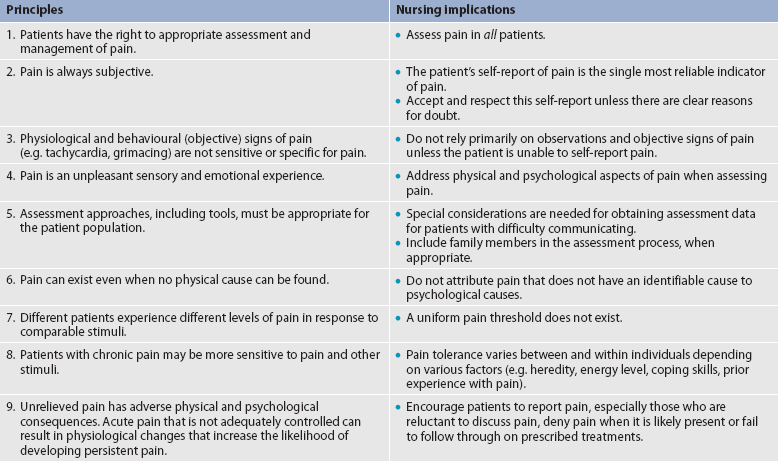
Assessment is an essential, though often overlooked, step in pain management. Pain should be considered the fifth vital sign. The key to accurate and effective pain assessment is to consider the core principles of pain assessment (see Table 8-4).
The goals of a nursing pain assessment are to: (1) describe the patient’s multidimensional pain experience for the purpose of identifying and implementing appropriate pain management; (2) explore the pain management techniques used by the patient; and (3) identify the patient’s goal for therapy and resources for self-management.
ELEMENTS OF A PAIN ASSESSMENT
Most components of a pain assessment involve direct interview or observation of the patient. Diagnostic studies and physical examination findings complete the initial assessment. Although the assessment will differ according to the clinical setting, patient population and point of care (i.e. whether it is part of an initial assessment or a reassessment of pain following therapy), the evaluation of pain should always be multidimensional. The first assessment is the presence or absence of pain (see Fig 8-5, which shows an initial pain assessment tool).
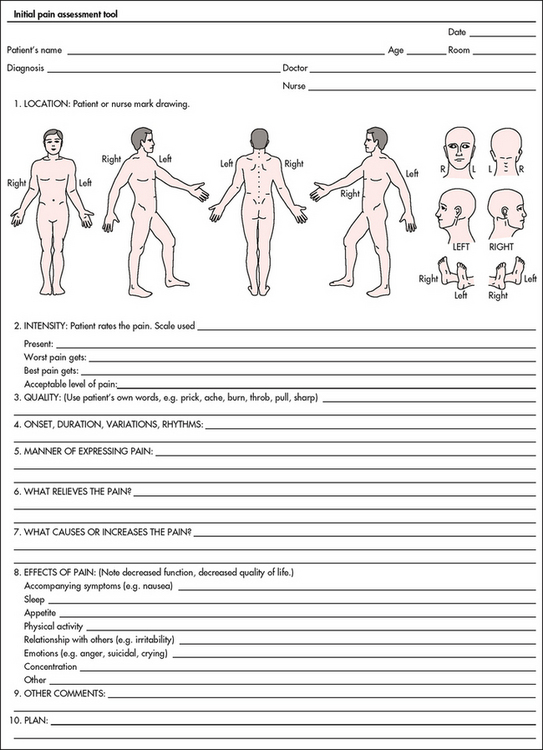
Figure 8-5 Initial pain assessment tool. (May be duplicated for use in clinical practice.)
Source: McCaffery M, Pasero C. Pain: clinical manual. 2nd edn. St Louis: Mosby; 1999:60. Copyright © 1999, Mosby.
Before beginning any assessment, the nurse needs to recognise that patients may use words other than ‘pain’. For example, older adults may deny that they have pain but respond positively when asked if they have soreness or aching. The nurse should document the specific words that the patient uses to describe pain, then consistently ask the patient about pain using those words.
Pain pattern
Pain onset involves determining when the pain started. Patients with acute pain resulting from injury, acute illness or treatment (e.g. surgery) typically know exactly when their pain began. Those with chronic pain resulting from a failure of the body to heal properly or from a chronic progressive illness may be less able to identify when the pain started. Establishing how long the pain has lasted, or its duration, will help to determine whether the pain is acute or chronic and assist in identifying the aetiology of the pain. For example, a patient with advanced cancer who also has chronic low back pain from spinal stenosis reports a sudden, severe pain in the back that began 2 days ago. Knowing the onset and duration can lead to a diagnosis that may reveal new metastatic disease in the spine.
Pain pattern also provides clues about the cause of the pain and directs its treatment. Many types of chronic pain (e.g. arthritis pain) wax and wane over time. A patient may have pain all the time (constant, around-the-clock pain), as well as discrete periods of intermittent pain. Breakthrough pain is transient, moderate to severe pain that occurs in patients whose pain is otherwise well controlled. Typically it is associated with cancer pain. End-of-dose failure is breakthrough pain that occurs before the duration of pain relief that is expected with a specific analgesic. For example, in a patient on transdermal fentanyl patches the typical duration of action is 72 hours. An increase in pain after 48 hours on the medicine would be characterised as end-of-dose failure. End-of-dose failure signals the need for changes in the dose or scheduling of the analgesic. Episodic, procedural or incident pain is a transient increase in pain caused by a specific activity or event that precipitates the pain. Examples include dressing changes, movement, eating, position changes and procedures such as catheterisation.
Pain location
The area or location of pain assists in identifying possible causes and treatment. Some patients may be able to specify the precise location(s) of their pain, whereas others may describe very general areas, or comment that they hurt all over. The location of the pain may be referred from its origin to another site (see Fig 8-4); it may also radiate from its origin to another site. For example, angina pectoris can radiate from the chest to the jaw or down the left arm. Sciatica is pain that follows the course of the sciatic nerve. It may originate from joints or muscles around the back or from compression or damage to the sciatic nerve. The pain is projected along the course of the peripheral nerve, causing painful shooting sensations down the back of the thigh and inside of the leg to the foot.
The nurse should obtain information about the location of pain by asking the patient to: (1) describe the site(s) of pain; (2) point to painful areas on the body; or (3) mark painful areas on a pain map (see Fig 8-5). Because many patients have more than one site of pain, it is important to make certain that the patient describes every location.
Pain intensity
Assessing the severity, or intensity, of pain provides a reliable measure to determine the type of treatment and its effectiveness. Pain scales help patients to communicate pain intensity. Scales must be adjusted to age and cognitive development. Most adults can rate the intensity of their pain using numeric scales (e.g. 0 = no pain, 10 = the worst pain) or verbal descriptor scales (e.g. none, a little, moderate, severe). These tools are sometimes easier for patients to use if they are oriented vertically or include a visual component. The pain thermometer scale is an example of this type of scale (see Fig 8-6).
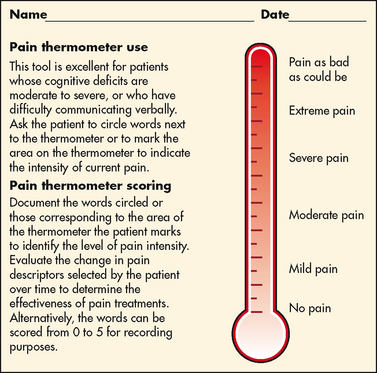
Figure 8-6 Pain thermometer scale. The patient is asked to circle words next to the thermometer or to mark the area on the thermometer to indicate the intensity of pain.
Used with permission of Keela Herr, PhD, RN, AGSF, FAAN, The University of Iowa.
Although intensity is an important factor in determining analgesic approaches, it is essential not to dose patients with opioids solely based on reported pain scores. Opioid ‘dosing by numbers’ without taking into account a patient’s sedation level and respiratory status can lead to unsafe practices and serious adverse events. Safer analgesic administration can be achieved by balancing the patient’s pain with analgesic side effects. Adjustments in therapy can be made to promote better pain control and minimise adverse outcomes.
Pain quality
Pain quality refers to the nature or characteristics of the pain. For example, patients often describe neuropathic pain as burning, numbing, shooting, stabbing or electric shock-like, or as an itchy sensation. Nociceptive pain may be described as sharp, aching, throbbing, dull or cramping.
Associated symptoms
Associated symptoms such as anxiety, fatigue and depression may exacerbate or be exacerbated by pain. The nurse should ask about activities and situations that increase or alleviate pain. For example, musculoskeletal pain can be exacerbated with movement and ambulation. In contrast, resting or immobilising a painful body part can decrease pain.
Management strategies
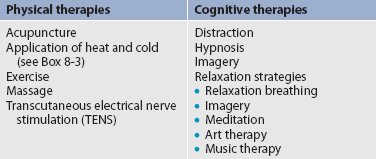
As people experience and live with pain, they try different strategies to manage it. Some are successful and others are not. To maximise the effectiveness of the pain treatment plan, patients should be asked what they are using now to control pain and what they have used in the past. Strategies include prescription and non-prescription drugs and non-drug therapies such as hot and cold applications, complementary and alternative therapies (e.g. herbal products, acupuncture) and relaxation strategies (e.g. imagery). All strategies must be documented, both those that work and those that are ineffective.
Impact of pain
Pain can have a profound influence on a patient’s quality of life and functioning. The assessment needs to include the effect of the pain on the patient’s ability to sleep, enjoy life, interact with others, perform work and household duties, and engage in physical and social activities. The nurse should also assess the impact of pain on the patient’s mood.
Patient beliefs, expectations and goals
Patient and family beliefs, attitudes and expectations influence responses to pain and pain treatment. The nurse should assess for attitudes and beliefs that may hinder effective treatment (e.g. the belief that opioid use will result in addiction) and ask about expectations and goals for pain management.
In an acute care setting, time limitations may dictate an abbreviated assessment. At a minimum, the nurse should assess the effects of the pain on the patient’s sleep and daily activities, relationships with others, physical activity and emotional wellbeing. In addition, the nurse should include the ways in which the patient describes the pain and the strategies used to accept and control the pain.
In some clinical settings, additional assessment information is necessary to ensure effective treatment. This is particularly true when working with chronic pain patients. Initial evaluation for these patients typically includes items shown in Table 8-5.
| subjective data |
| Important health information |
| Health history: Pain history includes onset, location, intensity, quality, patterns and expression of pain; coping strategies; past treatments and their effectiveness; pain triggers; review of healthcare use related to the pain problem (e.g. emergency department visits, treatment at pain clinics, visits to primary healthcare provider and specialists) |
| Medications: Use of any prescription or over-the-counter, illicit or herbal products for pain relief; alcohol use |
| Functional health patterns |
| Health perception–health management: Social and work history; mental health history; smoking history; effects of pain on emotions, relationships, sleep and activities; interviews with family members; records from psychiatric treatment related to the pain |
| Elimination: Constipation related to opioid drug use |
| Activity-exercise: Fatigue, limitations in activities, pain related to muscle use |
| Sexuality-reproductive: Decreased libido |
| Coping–stress tolerance: Psychological evaluation using standardised measures to examine coping style, depression, anxiety |
| Objective data |
DOCUMENTATION
Documentation of the pain assessment is critical to ensure effective communication between team members and appropriate care planning and implementation. Many healthcare facilities and agencies have adopted specific tools to record the initial pain assessment, treatment and reassessment. There also are many multidimensional pain assessment tools. Fig 8-7 illustrates an assessment chart for patients postoperatively. Fig 8-8 illustrates the Abbey pain scale, which was developed for people with dementia who are unable to report their pain.37
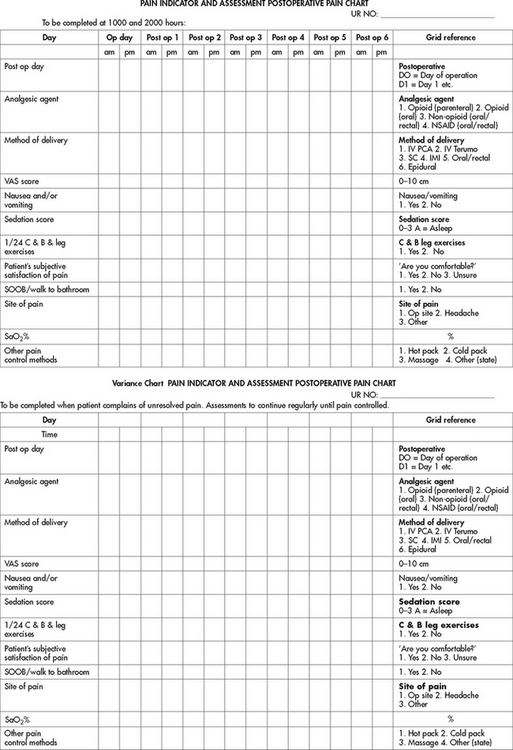
Figure 8-7 Pain indicator and assessment (PINDA) chart. C & B, coughing and breathing; IMI, intramuscular injection; IV, intravenous; NSAID, non-steroidal anti-inflammatory drug; OP site, operation site; PCA, patient-controlled analgesia; SaO2%, saturation percentage; SC, subcutaneous; SOOB, sit out of bed; VAS, visual analogue scale.
REASSESSMENT
It is critical that the nurse reassess the patient’s pain at appropriate intervals. For example, reassessment for a postoperative patient is done within 30 minutes of an intravenous (IV) dose of an analgesic. In a long-term care facility, residents with chronic pain are reassessed at least quarterly or with a change in condition or functional status. The frequency and scope of reassessment are guided by factors such as pain severity, physical and psychosocial condition, and institutional policy.
Pain treatment
BASIC PRINCIPLES
All pain treatment plans are based on the following principles and practice standards:
1. Follow the principles of pain assessment. These principles are listed in Table 8-4. It is important to remember that pain is a subjective experience. Patients are not only the best judge of their own pain, but also the experts on the effectiveness of each pain treatment.
2. Every patient deserves adequate pain management. Many patient populations, including people who may be of different ethnicity to the nurses caring for them, older adults and people with past or current substance abuse, are at risk for inadequate pain management. Healthcare providers need to be aware of their own biases and ensure that all patients are treated respectfully.
3. Base the treatment plan on the patient’s goals. Discussion about the patient’s goals for pain treatment should occur at the initial pain assessment. Although this goal can be described in terms of pain intensity (e.g. the desire for average pain to decrease from an ‘8/10’ to a ‘3/10’), with chronic pain conditions functional goal setting should be encouraged (e.g. the patient sets a goal of performing certain daily activities, such as socialising and hobbies). Over the course of prolonged therapy, these goals should be reassessed and progress towards meeting them should be documented. The patient, rather than the healthcare team, determines new goals. (Ch 69 discusses the principles of self-management.) If the patient has unrealistic goals for therapy, such as wanting to be completely rid of all chronic arthritis pain, the nurse will need to work with the patient to establish a more realistic goal.
4. Use both drug and non-drug therapies. Although drugs are often considered the mainstay of therapy, particularly for moderate to severe pain, self-care activities and non-drug therapies should be incorporated to increase the overall effectiveness of therapy and to allow for the reduction of drug dosages to minimise adverse drug effects.38
5. When appropriate, a multimodal approach to analgesic therapy should be used. Multimodal analgesia is the use of two or more classes of analgesic medications to take advantage of the various targets throughout the pain pathways. This approach has been shown to achieve superior pain relief, enhance patient satisfaction and decrease adverse effects of individual drugs.39 Multimodal analgesia has long been an accepted practice for the management of chronic pain and is gaining acceptance in the treatment of acute pain.
6. Address pain using a multidisciplinary approach. The expertise and perspectives of a multidisciplinary team are necessary to provide effective evaluation and therapies for patients with pain, especially those with chronic pain. As well as nurses, multidisciplinary teams frequently include psychologists, physiotherapists, occupational therapists, pharmacists, spiritual carers and multiple medical specialties such as neurology, pain, palliative care, oncology, surgery and anaesthetics. Some pain services also include complementary therapy practitioners such as massage therapists, music therapists, acupuncturists and art therapists.
7. Evaluate the effectiveness of all therapies to ensure that they are meeting the patient’s goals. Therapy must be individualised for each patient. Achievement of an effective treatment plan often requires trial and error. Adjustments in drug, dosage or route are common to achieve maximal benefit while minimising adverse effects. This trial-and-error process can become frustrating for the patient and carer. They need to be reassured that pain relief, if not pain cessation, is possible and that the healthcare team will continue to work with them to achieve adequate pain relief.
8. Prevent and/or manage medication side effects. Side effects are a major reason for treatment failure and non-adherence. Side effects are managed in one of several ways, as described in Box 8-1. Nurses play a key role in monitoring for, and treating, side effects, and in patient and family teaching to minimise these effects.
9. Incorporate patient and family teaching throughout assessment and treatment. Content should include information about the cause(s) of the pain, pain assessment methods, treatment goals and options, expectations of pain management, instruction regarding the proper use of drugs, the management of side effects, and non-drug and self-help pain relief measures. Teaching should be documented, and include evaluation of patient and family comprehension.
BOX 8-1 Managing side effects of pain medications
DRUG THERAPY
Side effects can be managed using one or more of the following methods.
• Decrease the dose of analgesic by 10–15%.
• Change to a different medication in the same class.
• Add a drug to counteract the adverse effect of the analgesic (e.g. initiate a bowel regimen using a gentle stimulant laxative and a stool softener for patients experiencing opioid-induced constipation or use an antiemetic to reduce nausea).
• Use an administration route that minimises drug concentrations at the site of the side effect (e.g. intraspinal administration of opioids is sometimes used to minimise high drug levels that produce sedation, nausea and vomiting).
DRUG THERAPY FOR PAIN
Pain medications are generally divided into three categories: non-opioids, opioids and adjuvant drugs. Treatment regimens may include medications from one or more of these groups. Mild pain may be relieved using non-opioids alone. Moderate to severe pain usually requires an opioid. Certain types of pain, such as neuropathic pain, often require adjuvant drug therapy alone or in combination with an opioid or another class of analgesics. Pain caused by specific medical conditions, such as cancer and rheumatoid arthritis, may be treated with curative or disease-modifying therapies (e.g. chemotherapy for cancer; TNF antagonists such as etanercept for rheumatoid arthritis), as well as pain medications.
Non-opioids
Non-opioid analgesics include paracetamol, aspirin and other salicylates, and NSAIDs (see Table 8-6). These agents are characterised by the following: (1) there is an analgesic ceiling to their analgesic properties—that is, increasing the dose beyond an upper limit provides no greater analgesia; (2) they do not produce tolerance or physical dependence; and (3) many are available without a prescription. It is important to monitor over-the-counter analgesic use to avoid serious problems related to drug interactions, side effects and overdose. Non-opioids are effective for mild to moderate pain. They are often used in conjunction with opioids because they allow for effective pain relief using lower opioid doses (thereby causing fewer opioid side effects); this phenomenon is called the opioid-sparing effect.
TABLE 8-6 Selected non-opioid analgesics and NSAIDS
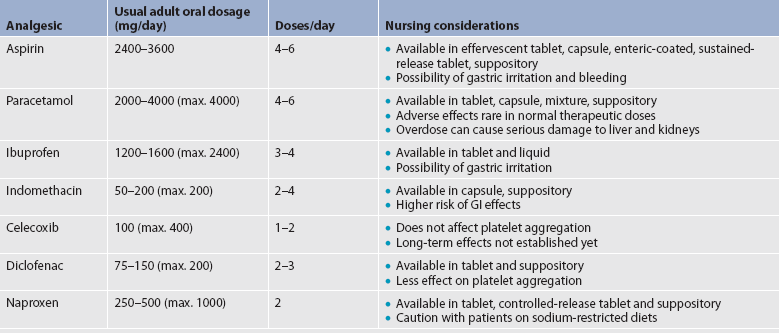
GI, gastrointestinal; NSAIDS, non-steroidal anti-inflammatory drugs.
Aspirin is effective for mild pain but its use is limited by its common side effects, including increased risk for platelet dysfunction and bleeding, especially GI bleeding. Other salicylates such as choline magnesium trisalicylate cause fewer GI disturbances and bleeding abnormalities. Like aspirin, paracetamol has analgesic and antipyretic effects, but, unlike aspirin, it has no antiplatelet or anti-inflammatory effects. Although paracetamol is well tolerated, it is metabolised by the liver; chronic dosing greater than 4 g/day, acute overdose or use by patients with severe pre-existing liver disease may result in hepatotoxicity.
NSAIDs represent a broad class of drugs with varying efficacy and side effects. All NSAIDs inhibit COX, the enzyme that converts arachidonic acid into prostaglandins and related compounds. There are two forms of this enzyme: COX-1 and COX-2. COX-1 is found in almost all tissues and is responsible for several protective physiological functions. In contrast, COX-2 is produced mainly at the sites of tissue injury, where it mediates inflammation (see Fig 8-9). Inhibition of COX-1 causes many of the untoward effects of NSAIDs, such as impairment of renal function, bleeding tendencies, GI irritation and ulceration. Inhibition of COX-2 is associated with the therapeutic, anti-inflammatory effects of NSAIDs. Older NSAIDs, such as ibuprofen, inhibit both forms of COX and are referred to as non-selective NSAIDs. In the late 1990s, NSAIDs that selectively inhibit COX-2 were introduced. These medications, which include celecoxib, are called COX-2 inhibitors.
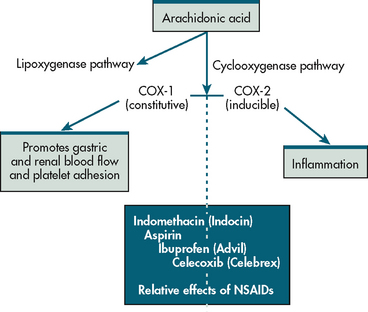
Figure 8-9 Arachidonic acid is oxidised by two different pathways: lipoxygenase and cyclooxygenase. The cyclooxygenase pathway leads to two forms of the enzyme cyclooxygenase: COX-1 and COX-2. COX-1 is known as ‘constitutive’ (always present) and COX-2 is known as ‘inducible’ (meaning its expression varies markedly depending on the stimulus). NSAIDs differ in their actions, with some having more effects on COX-1 and others more on COX-2. Indomethacin acts primarily on COX-1, whereas ibuprofen is equipotent on COX-1 and COX-2. Celecoxib primarily inhibits COX-2.
Some NSAIDs possess analgesic efficacy equal to that of aspirin, whereas others have better efficacy profiles. Patients vary greatly in their responses to a specific NSAID, so when one NSAID does not provide relief, another should be tried. NSAIDs are associated with many side effects, including GI problems ranging from dyspepsia to life-threatening ulceration and haemorrhage. Renal insufficiency and hypertension also occur. Serious side effects of NSAIDs result in thousands of hospitalisations every year.40 Thus the use of NSAIDs should be limited in those at highest risk for adverse effects, including the elderly and patients with a history of peptic ulcer disease. If NSAIDs are used in patients at risk for GI bleeding, they should have concomitant therapy with a misoprostol or a proton pump inhibitor (PPI) such as omeprazole. NSAIDs should not be administered concurrently with aspirin, as the risk for bleeding and GI adverse events in increased.
When the COX-2 inhibitors were first introduced, they were thought to be much safer than non-selective NSAIDs, but ultimately two have been withdrawn from the market. There is an increased risk of cardiovascular events associated with both selective COX-2 inhibitors and non-selective NSAIDs41 and the prescribing practitioner needs to discuss the risks of taking these medications with the patient.
Opioids
Opioids produce their effects by binding to receptors in the CNS. This results in: (1) inhibition of the transmission of nociceptive input from the periphery to the spinal cord; (2) altered limbic system activity; and (3) activation of the descending inhibitory pathways that modulate transmission in the spinal cord. Thus opioids act on several nociceptive processes.
Types of opioids
Opioid analgesics (also known as narcotic analgesics) are a group of both synthetic and natural agonist drugs that have morphine-like properties and interact in the brain with specific opioid receptors (mu, kappa, delta) to lessen the sensation of pain.41 (Agonist drugs are site-specific drugs that trigger cellular activity; antagonist drugs act to block cellular activity.) Although nociceptive pain appears to be more responsive to opioids than neuropathic pain, opioids are used to treat both types of pain. Pure opioid agonists include morphine, oxycodone, hydrocodone, codeine, methadone and hydromorphone (see Table 8-7). These drugs are effective for moderate to severe pain because they are potent, have no analgesic ceiling and can be administered through several routes.

* Neuraxial anaesthesia pertains to local anaesthetics placed around the nerves of the central nervous system such as spinal and epidural anaesthesia.
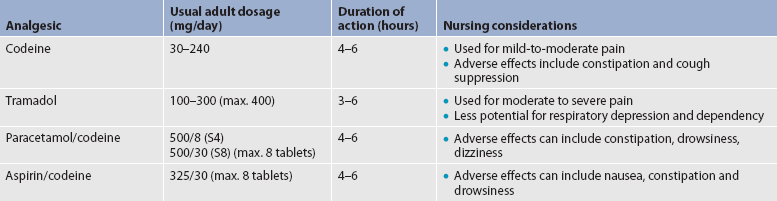
When opioids are prescribed for moderate pain, they are usually combined with a non-opioid analgesic such as paracetamol. Table 8-8 describes selected weak opioids and combination analgesics.
Pethidine is a synthetic opioid that acts on mu receptors. It is commonly prescribed in Australia and New Zealand because the analgesic effects are good when used for postoperative pain and the adverse effects (e.g. constipation and urinary retention) are less than they are with other opioids.41 However, it is not recommended for use in the US as it has been associated with neurotoxicity (e.g. seizures) caused by accumulation of its metabolite, normeperidine.42
Side effects of opioids
Common side effects of opioids include constipation, nausea and vomiting, sedation, respiratory depression and pruritus. With continued use, many side effects diminish, with the exception of constipation. Less common side effects include urinary retention, myoclonus, dizziness, confusion, delirium and hallucinations.
Constipation is the most common side effect. Because tolerance to opioid-induced constipation does not occur, a bowel regimen should be instituted at the beginning of opioid therapy and continued for as long as the patient takes opioids. Although dietary roughage, fluids and exercise should be encouraged to the extent possible, these measures alone may not be sufficient. Most patients should use a gentle stimulant laxative (e.g. senna) plus a stool softener (e.g. docusate sodium). Other agents (e.g. milk of magnesia, bisacodyl or lactulose) can be added if necessary.
Nausea is often a problem in opioid-naive patients (i.e. those who have never or rarely used opioids). The use of antiemetics such as metoclopramide, transdermal scopolamine or a phenothiazine (e.g. prochlorperazine) can prevent or minimise opioid-related nausea and vomiting until tolerance develops, which usually occurs within a week. Metoclopramide is particularly effective when the patient reports a feeling of gastric fullness. Opioids delay gastric emptying, and this effect can be reduced by metoclopramide. If nausea and vomiting are severe and persistent, changing to a different opioid may be necessary. In this case, a serotonergic (5HT3) antagonist (e.g. ondansetron) may be used.
Concerns about sedation and respiratory depression are two of the most common fears associated with opioids. Sedation is usually seen in opioid-naive patients in the treatment of acute pain. Hospitalised patients receiving opioid analgesics for acute pain should be monitored regularly, especially in the first few days after surgery. Nurses need to be aware that for postoperative patients the risk for sedation is greatest within 4 hours after leaving the recovery unit. Opioid-induced sedation resolves with the development of tolerance. Persistent sedation with chronic opioid use can be effectively treated with psychostimulants such as caffeine, dextroamphetamine, methylphenidate or the anticataleptic drug modafinil.
The risk of respiratory depression is also higher in opioid-naive hospitalised patients who are treated for acute pain. Clinically significant respiratory depression is rare in opioid-tolerant patients and when opioids are titrated to analgesic effect.43 Patients most at risk for respiratory depression include those who are elderly, have underlying lung disease or a history of sleep apnoea, or are receiving other CNS depressants (e.g. sedatives, benzodiazepines, antihistamines). For postoperative patients, the greatest risk for opioid-related respiratory adverse events is within the first 24 hours after surgery.43 Clinically significant respiratory depression cannot occur in patients who are awake. Thus, the sedation level must be monitored in addition to the respiratory rate. A sedation scale can be used for monitoring (see Box 8-2). The patient should be roused and asked to take deep breaths.
BOX 8-2 Sedation assessment scale
1 = Anxious, agitated or restless
2 = Calm, cooperative to tranquil (normal baseline without sedation)
3 = Quiet, drowsy, responds to verbal commands
4 = Asleep, brisk response to forehead tap or loud verbal stimuli
5 = Asleep, sluggish response to increasingly vigorous stimuli
6 = Unresponsive to painful stimuli
Moderate sedation = sedation score of 4
Source: Pain care facts. Available at http://prc.coh.org/pdf/Resp%20Dep-FF%2012-08.pdf, accessed 9 April 2011.
For patients who are excessively sedated or unresponsive, naloxone (an opioid antagonist that rapidly reverses the effects of opioids) can be administered. Naloxone can be given intravenously or subcutaneously every 2 minutes. If the patient has been taking opioids regularly for more than a few days, naloxone should be used judiciously and titrated carefully because it can precipitate severe, agonising pain, profound withdrawal symptoms and seizures. Naloxone’s half-life is shorter than that of most opiates, so the patient’s respiratory rate should be monitored, because it can drop again 1–2 hours after naloxone administration.
Pruritus (itching) occurs most frequently when opioids are administered via neuraxial (i.e. epidural, intrathecal) routes. Management of opioid-induced pruritus typically involves low-dose infusions of naloxone, mixed agonist-antagonists (e.g. nalbuphine) or a 5HT3 antagonist such as ondansetron.44
A rare but concerning problem with long-term and even short-term use of opioids is the development of opioid-induced hyperalgesia (OIH). OIH is a state of nociceptive sensitisation caused by exposure to opioids. It is characterised by a paradoxical response in which patients actually become more sensitive to certain painful stimuli and report increased pain with opioid use. The exact mechanism for this phenomenon is not clearly understood, but it is believed to be related to neuroplastic changes that lead to sensitisation of pronociceptive pathways.45 This may explain why opioids tend to lose their effectiveness in certain patients over time.
Adjuvant analgesic therapy
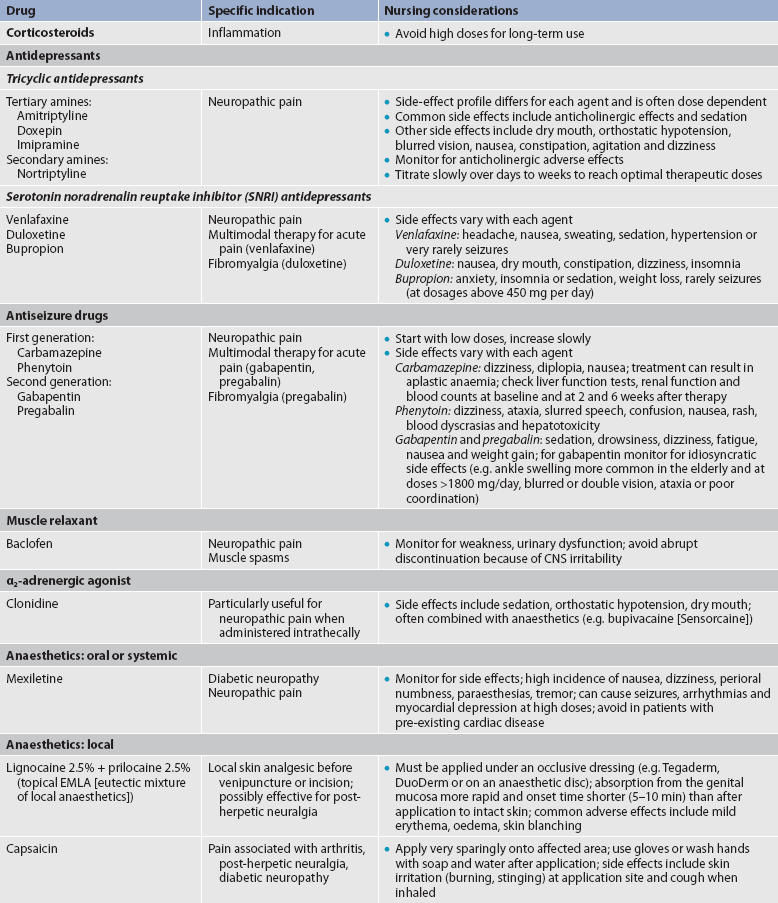
These medications comprise classes of drugs that can be used alone or in conjunction with opioid and non-opioid analgesics. Generally, these agents were developed for other purposes (e.g. antiseizure drugs, antidepressants) and found later to be effective for pain. Commonly used analgesic adjuvants are listed in Table 8-9.
Corticosteroids
These drugs, which include dexamethasone, prednisone and methylprednisolone, are used for management of acute and chronic cancer pain, pain secondary to spinal cord compression and inflammatory joint pain syndromes. Mechanisms of action are unknown but may be due to the ability of corticosteroids to decrease oedema and inflammation. They also may decrease activation of an inflamed neuron. Because of this effect, corticosteroids are useful when injected epidurally for acute or subacute disc herniations. Corticosteroids have many side effects, especially when given chronically in high doses. Adverse effects include hyperglycaemia, fluid retention, dyspepsia and GI bleeding, impaired healing, muscle wasting, osteoporosis, adrenal suppression and susceptibility to infection. Because they act through the same final pathway as NSAIDs, corticosteroids should not be given at the same time as NSAIDs.
Antidepressants
Tricyclic antidepressants (TCAs) enhance the descending inhibitory system by preventing the cellular reuptake of serotonin and noradrenalin. Higher levels of serotonin and noradrenalin in the synaptic cleft inhibit the transmission of nociceptive signals in the CNS. Other potential beneficial actions of TCAs include sodium channel modulation, α1-adrenergic antagonist effects and a weak NMDA receptor modulation. They appear to be effective for a variety of pain syndromes, especially neuropathic pain syndromes. However, side effects such as sedation, dry mouth, blurred vision and weight gain limit their usefulness. Antidepressants that selectively inhibit reuptake of serotonin and noradrenalin (SSRIs and SNRIs) are effective for many neuropathic pain syndromes and have a better side effect profile than the TCAs. These agents include venlafaxine, duloxetine and bupropion.46
Antiseizure drugs
Antiseizure drugs affect both peripheral nerves and the CNS in several ways, including sodium channel modulation, central calcium channel modulation and changes in excitatory amino acids and other receptors. Agents such as gabapentin, lamotrigine and pregabalin are valuable adjuvant agents in chronic pain therapy and are being increasingly used in the treatment of acute pain.
GABA receptor agonists
Baclofen, an analogue of the inhibitory neurotransmitter GABA, can interfere with the transmission of nociceptive impulses and is mainly used for muscle spasms. It crosses the blood–brain barrier poorly and is much more effective for spasticity when delivered intrathecally.
α2-adrenergic agonists
Currently, clonidine is the most widely used α2-adrenergic agonist. α2-adrenergic agonists are thought to work on the central inhibitory α-adrenergic receptors and may also decrease noradrenalin release peripherally. They are used for chronic headache and neuropathic pain.
Local anaesthetics
For acute pain from surgery or trauma, local anaesthetics such as bupivacaine and ropivacaine can be administered epidurally by continuous infusion, and also by intermittent or continuous infusion with regional nerve blocks. Topical applications of local anaesthetics are used to interrupt transmission of pain signals to the brain.
Mixed mu agonist opioid and NE/5HT reuptake inhibitors
Some analgesics have two distinct actions, or dual mechanisms. Tramadol is a weak mu agonist and also inhibits the reuptake of noradrenalin and serotonin. It is effective in low back pain, osteoarthritis, diabetic peripheral neuropathic pain, polyneuropathy and post-herpetic neuralgia. The most common side effects are similar to those of other opioids, including nausea, constipation, dizziness and sedation. As with other medications that increase serotonin and noradrenalin, this agent should be avoided in patients with a history of seizures because it lowers seizure threshold.
Tapentadol is the newest dual-mechanism agent. It is available by restricted prescription in Australia and New Zealand for treatment of chronic disabling pain that does not respond to other analgesics. It acts via mu opioid receptors and also by inhibition of serotonin and noradrenalin reuptake. In clinical trials, tapentadol showed comparable pain relief to oxycodone for surgical pain and has been shown to be effective for non-surgical joint and back pain.47 The side effects are similar to conventional opioids, except that it is associated with less nausea and constipation.
Cannabinoids
Cannabinoid-derived medications have been approved for use in Canada, the UK, the US and New Zealand, but are not currently approved by the Therapeutic Goods Administration (TGA) for therapeutic use in Australia. Cannabinoid-derived medications show promise in the treatment of certain pain syndromes and symptoms, but these preparations have sparked considerable controversy, prejudice and confusion, mostly because cannabinoids have some relationship to the cannabis plant—also known as marijuana. Smoking marijuana or cannabis rapidly increases plasma levels of tetrahydrocannabinol (THC), but the amount is highly dependent on the composition of the marijuana cigarette and inhalation technique, so this form of use is associated with highly variable results in relief of pain and symptoms.48 With commercially available oral preparations, the absorption and bioavailability are much more reliable and predictable.
Cannabinoids exert their analgesic effects primarily mediated through the cannabinoid-l (CB1) receptor in nociceptive areas in the periphery and CNS. CB1 stimulation modulates neurotransmission in the serotoninergic, dopaminergic and glutamatergic systems, as well as other systems. It is also believed that cannabinoids enhance the endogenous opioid system. Other beneficial effects include alleviation of nausea and increased appetite. Cannabinoids may also have opioid-sparing effects, possibly reduce opioid tolerance and even ameliorate symptoms of opioid withdrawal.49
Administration
Scheduling
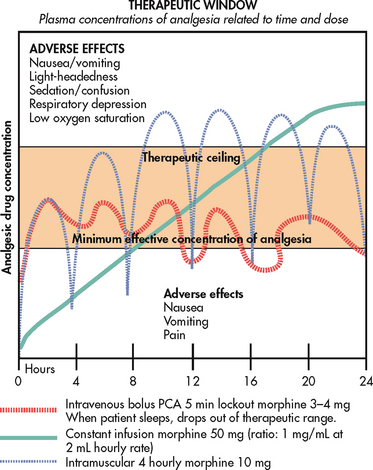
Appropriate analgesic scheduling focuses on prevention or control of pain, rather than the provision of analgesics after the patient’s pain has become severe. A patient should be premedicated before painful procedures and activities that are expected to produce pain. Similarly, a patient with constant pain should receive analgesics around the clock rather than on an ‘as needed’ (prn) basis. These strategies control pain before it starts and usually result in lower analgesic requirements. Fast-acting drugs should be used for incident or breakthrough pain, whereas long-acting analgesics are more effective for constant pain. Examples of fast-acting and sustained-release analgesics are described later in this section.
Titration
Analgesic titration is dose adjustment based on assessment of the adequacy of analgesic effect versus the side effects produced. There is wide variability in the amount of analgesic needed to manage pain, and titration is an important strategy in addressing this variability. An analgesic can be titrated upwards or downwards, depending on the situation. For example, in a postoperative patient the dose of analgesic generally decreases over time as the acute pain resolves. On the other hand, opioids for chronic, severe cancer pain may be titrated upwards many times over the course of therapy to maintain adequate pain control. The goal of titration is to use the smallest dose of analgesic that provides effective pain control with the fewest side effects.
Equianalgesic dosing
The term equianalgesic dose refers to a dose of one analgesic that is equivalent in pain-relieving effects to another analgesic. This equivalence permits substitution of analgesics in the event that a particular drug is ineffective or causes intolerable side effects. Generally, equianalgesic doses are provided for opioids and are important because there is no upper dosage limit for many of these drugs. Equianalgesic charts and conversion programs are widely available in pharmacology textbooks, clinical guidelines, healthcare facility pain protocols and on the internet. They are useful tools, but healthcare providers need to understand their limitations. Equianalgesic dosages are estimates, and some are based on small, single-dose studies on healthy volunteers.50 Differences exist among various published charts. For these reasons, all changes in opioid therapy must be monitored carefully and adjusted for the individual patient. When possible, healthcare providers should use equianalgesic conversions that have been approved for their facility or clinic and, if concerned, should consult an expert in pain control or a pharmacist before making changes.
Administration routes
Opioids and other analgesic agents can be delivered via many routes. This flexibility allows the healthcare provider to: (1) target a particular anatomical source of the pain; (2) achieve therapeutic blood levels rapidly when necessary; (3) avoid certain side effects through localised administration; and (4) provide analgesia when patients are unable to swallow. The following discussion highlights the uses and nursing considerations for analgesic agents delivered through a variety of routes.
Oral route
Generally, oral administration is the route of choice for the patient with a functioning GI system. Most pain medications are available in oral preparations, such as liquid and tablet formulations. For opioids, larger oral doses are needed to achieve the equivalent analgesia as doses administered intramuscularly (IM) or intravenously. For example, 10 mg of parenteral morphine is equivalent to approximately 30 mg of oral morphine.50 The reason larger doses are required is related to the first-pass effect of hepatic metabolism. This means that oral opioids are absorbed from the GI tract into the portal circulation and shunted to the liver. Partial metabolism in the liver occurs before the drug enters the systemic circulation and becomes available to peripheral receptors or can cross the blood–brain barrier and access CNS opioid receptors, which is necessary to produce analgesia. Oral opioids are as effective as parenteral opioids if the dose administered is large enough to compensate for the first-pass metabolism.
Many opioids are available in short-acting (immediate-release) and long-acting (sustained-release) oral preparations. Immediate-release products are effective in providing rapid, short-term pain relief. Sustained-release preparations generally are administered every 8–12 hours. As with other sustained-release preparations, these products should not be crushed, broken or chewed.
Sublingual and buccal routes
Opioids can be administered under the tongue or held in the mouth and absorbed into the systemic circulation, which exempts them from the first-pass effect. Although morphine is commonly administered to patients with cancer pain via the sublingual route, little of the drug is actually absorbed from the sublingual tissue. Instead, most of the drug is dissolved in saliva and swallowed, making its metabolism the same as that of oral morphine.
Fentanyl citrate is administered transmucosally. The fentanyl dose is embedded in a flavoured lozenge on a stick. The drug is absorbed by the permeable buccal mucosa after being rubbed actively over it (not sucked as a lollipop), allowing the drug to enter the bloodstream and travel directly to the CNS. Pain relief typically occurs within 5–7 minutes after administration. This agent should be used only for patients who are already receiving and who are tolerant to opioid therapy.
An oromucosal spray delivery of cannabinoid extract (Sativex) shows promise in treating chronic, neuropathic pain conditions. It has been approved in Canada for the treatment of pain in multiple sclerosis.
Intranasal route
Intranasal administration allows delivery of medication to highly vascular mucosa and avoids the first-pass effect. Butorphanol is one of the few intranasal analgesics currently available. This drug is indicated for acute headache and other intense, recurrent types of pain. Intranasal delivery of other opioids is being investigated.
Rectal route
The rectal route is often overlooked but is particularly useful when the patient cannot take an analgesic by mouth, such as those patients with severe nausea and vomiting. Analgesics that are available as rectal suppositories include hydromorphone, oxymorphone, morphine and paracetamol. If rectal preparations are not available, many oral formulations can be given rectally if the patient is unable to take medications by mouth.
Transdermal route
Fentanyl is available as a transdermal patch system for application to non-hairy skin. This delivery system is useful for the patient who cannot tolerate oral analgesic drugs. Absorption from the patch is slow and it takes 12–17 hours to reach full effect with the first application. Therefore, transdermal fentanyl is not suitable for rapid dose titration, but it can be effective if the patient’s pain is stable and the dose required to control it is known. Patches may need to be changed every 48 hours rather than the recommended 72 hours based on individual patient responses. Rashes caused by the patch adhesive may be reduced by preparing the skin 1 hour before placement with a weak corticosteroid cream. Bio-occlusive dressings are available if the patch keeps falling off because of excessive sweating.
Transdermal medications are also available over-the-counter. Creams and lotions containing capsaicin available in Australia and New Zealand include Zostrix, which is available in two strengths: capsaicin 0.025%w/w topical cream and capsaicin 0.075%w/w topical cream. Derived from red chilli pepper, capsaicin acts on the vanilloid heat receptors of the C-fibres. If used regularly three or four times a day for 4–6 weeks, it will cause the C-fibres to become inactive because of excessive calcium entering the nerve cell. The result is neuronal resistance to painful stimuli. Capsaicin can control pain associated with post-herpetic neuralgia, diabetic neuropathy and arthritis.
Parenteral routes
The parenteral route includes subcutaneous, intramuscular and intravenous administration. Single, repeated or continuous dosing (subcutaneous or IV) is possible via parenteral routes. Although it is frequently used, the IM route is not recommended because injections cause significant pain, result in unreliable absorption and, with chronic use, can result in abscesses and fibrosis. Onset of analgesia following subcutaneous administration is slow and thus the subcutaneous route is rarely used for acute pain management. However, continuous subcutaneous infusions are effective for pain management at the end of life. This route is especially helpful for people with abnormal GI function and limited venous access. IV administration is the best route when immediate analgesia and rapid titration are necessary. Continuous IV infusions provide excellent steady state analgesia through stable blood levels.
Intraspinal delivery
Intraspinal (epidural or intrathecal) opioid therapy involves inserting a catheter into the subarachnoid space (intrathecal delivery) or the epidural space (epidural delivery) (see Fig 8-10). Analgesics are injected by either intermittent bolus doses or continuous infusion.
Figure 8-10 Spinal anatomy. The spinal cord extends from the foramen magnum to the first or second lumbar vertebral space. The subarachnoid space (intrathecal space) is filled with cerebrospinal fluid that continually circulates and bathes the spinal cord. The epidural space is a potential space filled with blood vessels, fat and a network of nerve extensions.
Percutaneously placed temporary catheters are used for short-term therapy (2–4 days) and surgically implanted catheters are used for long-term therapy. Although the lumbar region is the most common site of placement, epidural catheters may be placed at any point along the spinal column (cervical, thoracic, lumbar or caudal). The tip of the epidural catheter is placed as close to the nerve supplying the painful dermatome as possible. For example, a thoracic catheter is placed for upper abdominal surgery and a high lumbar catheter is used for lower abdominal surgery. Fluoroscopy is used to ensure correct placement of the catheter.
Intraspinally administered analgesics are highly potent because they are delivered close to the receptors in the spinal cord dorsal horn. Thus much smaller doses of analgesics are needed when compared with other routes, including IV. For example, 1 mg of intrathecal morphine is approximately equivalent to 10 mg of epidural morphine, 100 mg of IV morphine and 300 mg of oral morphine. Drugs that are delivered intraspinally include morphine, fentanyl, hydromorphone, ziconotide (a calcium channel receptor modulator for use in neuropathic pain syndromes) and clonidine. Nausea, itching and urinary retention are common side effects of intraspinal opioids.
Complications of intraspinal analgesia include catheter displacement and migration, accidental infusions of neurotoxic agents, and infection. Clinical manifestations of catheter displacement or migration depend on catheter location and the drug being infused. A catheter that migrates out of the intrathecal or epidural space will cause a decrease in pain relief with no improvement, even with additional boluses or increases in the infusion rate. If an epidural catheter migrates into the subarachnoid space, an increase in side effects will become quickly apparent. Somnolence, confusion and an increased anaesthesia (if the infusate contains an anaesthetic) occur. Correct placement of an intrathecal catheter can be checked by aspirating cerebrospinal fluid (CSF). Migration of a catheter into a blood vessel may cause an increase in side effects because of systemic drug distribution.
Many drugs and chemicals are highly neurotoxic when administered intraspinally. These include many preservatives such as alcohol and phenol, antibiotics, chemotherapy agents, potassium and parenteral nutrition. To avoid inadvertent injection of IV drugs into an intraspinal catheter, the catheter should be clearly marked as an intraspinal access device, and only preservative-free drugs should be injected.
Infection is a rare but serious complication of intraspinal analgesia. The skin around the exit site should be assessed carefully for inflammation, drainage or pain. Signs and symptoms of an intraspinal infection include diffuse back pain, pain or paraesthesia during bolus injection, and unexplained sensory or motor deficits in the lower limbs. Fever may or may not be present. Acute bacterial infection (meningitis) is manifested by photophobia, neck stiffness, fever, headache and altered mental status (see Ch 56). Infection is avoided by providing regular, meticulous wound care and using sterile technique when caring for the catheter and injecting drugs.
Long-term epidural catheters may be placed for terminal cancer patients or patients with certain pain syndromes that are unresponsive to other treatments. If a long-term indwelling epidural catheter is used, double bacterial filters are recommended. Because the highest infective risk occurs when the medication bags are changed, the concentration and volume in the infusing bag should be optimised to reduce the number of bag changes.
Implantable pumps
Intraspinal catheters can be surgically implanted for long-term pain relief. The surgical placement of an intrathecal catheter to a subcutaneously placed pump and reservoir allows for the delivery of drugs directly into the intrathecal space. The pump, which is normally placed in a pocket made in the subcutaneous tissue of the abdomen, may be programmable or fixed. Changes are made by either reprogramming the pump or changing the mixture or concentration of drug in the reservoir. The pump is refilled every 30–90 days depending on flow rate, mixture and reservoir size.
Patient-controlled analgesia
A specific type of IV delivery system is patient-controlled analgesia (PCA), or demand analgesia. It can also be connected to an epidural catheter (patient-controlled epidural analgesia [PCEA]). With PCA, a dose of opioid is delivered when the patient decides a dose is needed. PCA uses an infusion system in which the patient pushes a button to receive a bolus infusion of an analgesic. PCA is used widely for the management of acute pain, including postoperative pain and cancer pain.
Opioids such as morphine and hydromorphone are commonly administered for IV PCA therapy for both acute and chronic pain management. Fentanyl is used less often for acute pain. Sometimes IV PCA is administered with a continuous or background infusion called a basal rate depending on the patient’s opioid requirement. For acute pain (e.g. postoperative pain), basal rates are not recommended when initiating therapy in opioid-naive patients. The addition of a basal rate does not improve pain control, reduce the number of demand doses or improve sleep. Furthermore, the addition of a basal rate in opioid-naive patients and those at risk for adverse respiratory outcomes (e.g. patients who are older, who have obstructive sleep apnoea or existing pulmonary disease) may lead to serious respiratory events.19
Use of PCA begins with patient teaching. The nurse should help the patient to understand the mechanics of getting a drug dose and how to titrate the drug to achieve good pain relief. The patient should be taught to self-administer the analgesic before pain intensity is greater than the patient’s desired pain intensity goal. Patients should be assured that they cannot ‘overdose’ because the pump is programmed to deliver a maximum number of doses per hour. The dose of medication is ordered to achieve what is known as a therapeutic window—that is, the ideal plasma concentration needed to achieve effective pain relief (see Fig 8-11). Pressing the button after the maximum dose is administered will not result in additional analgesic. If the maximum dose is inadequate to relieve pain, the pump can be reprogrammed to increase the amount or frequency of dosing. In addition, bolus doses can be given if they are ordered. To make a smooth transition from infusion PCA to oral drugs, the patient should receive increasing doses of oral drug as the PCA analgesic is tapered.
Interventional therapy
Therapeutic nerve blocks
Nerve blocks generally involve one-time or continuous infusion of local anaesthetics into a particular area to produce pain relief. These techniques are also called regional anaesthesia. Nerve blocks interrupt all afferent and efferent transmission to the area and thus are not specific to nociceptive pathways. They include local infiltration of anaesthetics into a surgical area (e.g. chest incisions, inguinal hernia, joints) and injection of anaesthetic into a specific nerve (e.g. occipital or pudendal nerve) or nerve plexus (e.g. brachial or coeliac plexus). Nerve blocks can be used during and after surgery to manage pain. For longer-term relief of chronic pain syndromes, local anaesthetics can be administered via a continuous infusion.
The adverse effects of nerve blocks are similar to those for local anaesthetics delivered via other systemic routes and include arrhythmias, confusion, nausea and vomiting, blurred vision, tinnitus and metallic taste. Temporary nerve blocks affect both motor function and sensation and typically last 2–24 hours depending on the agent and site of injection. Motor ability generally returns before sensation.51
Neuroablative techniques
Neuroablative interventions are performed for severe pain that is unresponsive to all other therapies. Neuroablative techniques destroy nerves, thereby interrupting pain transmission. Destruction is accomplished by surgical resection or thermocoagulation, including radiofrequency coagulation. Neuroablative interventions that destroy the sensory division of a peripheral or spinal nerve are classified as neurectomies, rhizotomies and sympathectomies. Neurosurgical procedures that ablate the lateral spinothalamic tract are classified as cordotomies if the tract is interrupted in the spinal cord, or tractotomies if the interruption is in the medulla or the midbrain of the brainstem. Figure 8-12 identifies the sites of neurosurgical procedures for pain relief. Both cordotomy and tractotomy can be performed with the aid of local anaesthesia by a percutaneous technique under fluoroscopy.
Neuroaugmentation
Neuroaugmentation involves electrical stimulation of the brain and the spinal cord. Spinal cord stimulation is performed much more often than deep brain stimulation. Recent advances have allowed the use of multiple leads and multiple electrode terminals to stimulate large areas. The most common use of spinal cord stimulation is for chronic back pain secondary to nerve damage that is unresponsive to other therapies. Other uses include complex regional pain syndrome, spinal cord injury pain and interstitial cystitis. Potential complications include those related to the surgery (bleeding and infection), migration of the generator (which is usually implanted in the subcutaneous tissues of the upper gluteal or pectoralis area) and nerve damage.52
Non-drug therapies for pain
Non-drug strategies play a critical role in pain management (see Table 8-10). They can reduce the dose of an analgesic required to relieve pain and thereby minimise side effects of drug therapy. Moreover, they can increase the patient’s sense of personal control about managing pain and bolster coping skills. Some strategies are believed to alter ascending nociceptive input or stimulate descending pain modulation mechanisms. Some non-drug therapies are used independently by patients as self-management strategies. They are especially important in the treatment of chronic pain.53
Physical pain relief strategies
Massage
Massage is a common therapy for pain. There is good evidence to support the use of massage for acute and chronic pain.54 Many different massage techniques exist, including moving the hands or fingers over the skin slowly or briskly with long strokes or in circles (superficial massage) or applying firm pressure to the skin to maintain contact while massaging the underlying tissues (deep massage). Another example is trigger point massage. A trigger point is a circumscribed hypersensitive area within a tight band of muscle. It is caused by acute or chronic muscle strain and can often be felt as a tight knot under the skin. Trigger point massage is performed by applying strong sustained digital pressure, deep massage or gentle massage with ice followed by muscle heating. (Massage is discussed in Ch 7.)
Exercise
Exercise is a critical part of the treatment plan for patients with chronic pain, particularly those with musculoskeletal pain. Research supports the effectiveness of many types of exercise for a variety of painful conditions.55,56 Many patients become physically deconditioned as a result of their pain, which in turn leads to more pain. Exercise acts via many mechanisms to relieve pain. It enhances circulation and cardiovascular fitness, reduces oedema, increases muscle strength and flexibility, and enhances physical and psychosocial functioning. An exercise program should be tailored to the physical needs and lifestyle of the patient and should include aerobic exercise, stretching and strengthening exercises. The program should be supervised by trained personnel (e.g. exercise physiologist, physiotherapist). Examples of exercise programs include yoga, Tai Chi and water aerobics.
Transcutaneous electrical nerve stimulation
Transcutaneous electrical nerve stimulation (TENS) involves the delivery of an electric current through electrodes applied to the skin surface over the painful region, at trigger points or over a peripheral nerve. A TENS system consists of two or more electrodes connected by lead wires to a small battery-operated stimulator (see Fig 8-13). Initially, a physiotherapist may be responsible for administering TENS therapy, although nurses can be trained in the technique. TENS may be used for acute pain, including postoperative pain and pain associated with physical trauma.57 The effects of TENS on chronic pain are less clear but the procedure may be effective for some chronic pain patients.58
Acupuncture
Acupuncture is a technique of traditional Chinese medicine in which very thin needles are inserted into the body at designated points. Acupuncture is used for many different kinds of pain. (Acupuncture is discussed in Ch 7.)
Heat therapy
Heat therapy is the application of moist or dry heat to the skin. Heat therapy can be either superficial or deep. Superficial heat can be applied using an electric heating pad (dry or moist), a hot pack, hot moist compresses, warm wax (paraffin) or a hot water bottle. For exposure to large areas of the body, patients can immerse themselves in a hot bath, shower or whirlpool. Physiotherapy departments provide deep-heat therapy through such techniques as short-wave diathermy, microwave diathermy and ultrasound therapy. Patient teaching regarding heat therapy is outlined in Box 8-3.
PATIENT & FAMILY TEACHING GUIDE
Include the following instructions when teaching the patient and/or family about superficial heat techniques.
• Do not use heat on an area that is being treated with radiation therapy, is bleeding, has decreased sensation or has been injured in the past 24 hours.
• Do not use any menthol-containing products (e.g. Dencorub, Vicks, Icy Hot) with heat applications because this may cause burns.
• Cover the heat source with a towel or cloth before applying to the skin to prevent burns.
Include the following instructions when teaching the patient and/or family about superficial cold techniques.
• Cover the cold source with a cloth or towel before applying to the skin to prevent tissue damage.
• Do not apply cold to areas that are being treated with radiation therapy, have open wounds or have poor circulation.
• If it is not possible to apply the cold directly to the painful site, try applying it just above or below the painful site or on the opposite side of the body on the corresponding site (e.g. left elbow if the right elbow hurts).
Cold therapy
Cold therapy involves the application of either moist or dry cold to the skin. Dry cold can be applied by means of an ice pack, moist cold by means of towels soaked in ice water, cold hydrocollator packs or immersion in a bath or under running cold water. Icing with ice cubes or blocks of ice made to resemble ice blocks is another technique used for pain relief. Cold therapy is believed to be more effective than heat for a variety of painful conditions, including acute pain from trauma or surgery, acute flare-ups of arthritis, muscle spasms and headache. Patient teaching regarding cold therapy is described in Box 8-3.
Cognitive therapies
Techniques to alter the affective, cognitive and behavioural components of pain include a variety of cognitive strategies and behavioural approaches. Some of these techniques require little training and are often adopted independently by the patient. For others, a trained therapist is necessary.5
Distraction
Distraction involves redirecting attention away from the pain and onto something else. It is a simple but powerful strategy to relieve pain. Distraction can be achieved by engaging the patient in any activity that can hold their attention (e.g. watching TV or a movie, conversing, listening to music, playing a game). It is important to match the activity with the patient’s energy level and ability to concentrate.
Hypnosis
Hypnotherapy is a structured technique that enables the patient to achieve a state of heightened awareness and focused concentration that can be used to alter the patient’s pain perception. Hypnosis should be administered and monitored only by specially trained clinicians.59
Relaxation strategies
Relaxation strategies are varied, but their goal is to reach a state that is free from anxiety and muscle tension. Relaxation reduces stress, decreases acute anxiety, distracts from pain, alleviates muscle tension, combats fatigue, facilitates sleep and enhances the effectiveness of other pain relief measures.34 Relaxation strategies include relaxation breathing, music, imagery, meditation and progressive muscle relaxation.
 NURSING AND COLLABORATIVE MANAGEMENT: PAIN
NURSING AND COLLABORATIVE MANAGEMENT: PAIN
Nurses are important members of the multidisciplinary pain management team. Nursing roles include planner, educator, patient advocate, interpreter and supporter of the patient and carer. Because patients in any care setting can experience pain, nurses need to be knowledgeable about current therapies and flexible in trying new approaches to pain management. Many nursing roles have been described earlier, including pain assessment, administering therapies, monitoring for side effects, and teaching patients and carers. However, the success of these actions depends on the nurse’s ability to establish a trusting relationship with the patient and carer and to address their concerns about pain and its treatment.
 Effective communication
Effective communication
Because pain is a subjective experience, patients need to feel confident that their reporting of pain will be believed and will not be perceived as ‘complaining’. The patient and carer also need to know that the members of the healthcare team consider the pain significant and understand that pain may profoundly disrupt a person’s life. Nurses should communicate concern and commitment to helping the patient obtain pain relief and to cope with any unrelieved pain. Nurses need to support the patient and carer through the period of trial and error that may be necessary to implement an effective therapeutic plan. It is also important to clarify responsibilities in pain relief. Nurses can help the patient to understand the role of the members of the healthcare team, as well as the roles and expectations of the patient.
In addition to addressing specific aspects of pain assessment and treatment, the nurse needs to evaluate the impact that the pain has on the lives of the patient and carer. Box 8-4 lists possible nursing diagnoses that may be appropriate for the patient in pain. Box 8-5 addresses the teaching needs of patients and carers related to pain management.
PATIENT & FAMILY TEACHING GUIDE
Include the following information in the teaching plan for the patient with pain.
• Unrelieved pain can have negative consequences.
• It is important to maintain a record of pain level and the effectiveness of treatment.
• Pain should be treated with drugs or non-drug therapies before it becomes severe.
• Medication may stop working after it is taken for a period of time and dosages may need to be adjusted.
• Potential side effects and complications associated with therapy/therapies can include nausea and vomiting, constipation, sedation and drowsiness, itching, urinary retention and sweating.
• It is important to report when pain is not relieved to tolerable levels.
 Barriers to effective pain management
Barriers to effective pain management
Pain is a complex, subjective experience and its management is influenced greatly by psychosocial, sociocultural and legal-ethical factors. These factors include the emotions, behaviours, beliefs and attitudes of patients and carers about pain and the use of pain therapies. Achieving effective pain management requires careful consideration of these factors. Common barriers are tolerance, dependence and addiction. Patients, carers and healthcare providers often share these concerns. It is important for nurses to understand and to be able to explain the differences among these various concepts.
 Tolerance
Tolerance
Tolerance occurs with prolonged exposure to a variety of drugs. In the case of opioids, tolerance is characterised by the need for an increased opioid dose to maintain the same degree of analgesia. The incidence of clinically significant opioid tolerance in chronic pain patients is unknown, since dosage needs may also increase as a disease such as cancer progresses. Although tolerance is not as common as once thought, it is essential to assess for increased analgesic needs in patients on long-term therapy. The healthcare team needs to evaluate and rule out other causes of increased analgesic needs, such as disease progression or infection. If significant tolerance to opioids develops and it is believed that an opioid is losing its effectiveness, or if there are intolerable side effects associated with escalation of doses, the practice of opioid rotation may be considered.60 This involves switching from one opioid to another, assuming that the new opioid will be more effective at lower equianalgesic doses. As previously discussed, very high opioid doses can result in hyperalgesia rather than pain relief. Thus further increase in the dose can lead to higher pain levels.
 Physical dependence
Physical dependence
Like tolerance, physical dependence is an expected physiological response to ongoing exposure to drugs (this is not the same as addiction, which is described below). It is manifested by a withdrawal syndrome that occurs when blood levels of the drug are abruptly decreased. Symptoms of opioid withdrawal are listed in Table 8-11. When opioids are no longer needed to provide pain relief, a tapering schedule should be used in conjunction with careful monitoring. A typical tapering schedule begins with calculating the 24-hour dose used by the patient and dividing by 2. Of this decreased amount, 25% is given every 6 hours. After 2 days, the daily dose is reduced by an additional 25% every 2 days until the 24-hour oral dose is 30 mg (morphine equivalent) per day. After 2 days on this minimum dose, the opioid is then discontinued.
 Addiction
Addiction
Addiction is a complex neurobiological condition characterised by aberrant behaviours arising from a drive to obtain and take substances for reasons other than the prescribed therapeutic value (see Ch 10). Tolerance and physical dependence are not indicators of addiction. Rather, they are normal physiological responses to chronic exposure to certain drugs, including opioids.
The risk of developing addiction is associated with certain factors, including younger age, personal or family history of substance abuse, and mood disorders. The risk of addiction, however, should not prevent clinicians from using opioids to treat moderate to severe acute and chronic pain. Several professional organisations and government agencies have issued joint statements about the roles and responsibilities of healthcare professionals in the appropriate use of opioids in the treatment of pain.61
Assessing and treating pain in people with current or a history of drug and alcohol abuse is challenging, particularly when therapy involves medications that may themselves be abused (e.g. opioids). However, these individuals have the right to receive effective pain management. A comprehensive pain assessment is imperative, including a detailed history, physical examination, psychosocial assessment and diagnostic assessment to determine the cause of the pain. Screening tools are available to assess risk for substance abuse and to evaluate abnormal behaviours in patients with chronic pain.62
Opioids may be used effectively and safely in patients with substance dependence when indicated for pain control. Withholding opioids from chemically dependent patients with pain has not been shown to increase the likelihood of recovery from addiction. Opioid agonists-antagonists (e.g. pentazocine, butorphanol) should not be used in this patient group because they may precipitate withdrawal. The use of ‘potentiators’ and psychoactive drugs that do not have analgesic properties should be avoided. In individuals who are tolerant to CNS depressants, larger doses of opioids or increased frequency of drug administration is necessary to achieve pain relief.
Pain management for people with addiction is challenging and requires a multidisciplinary team approach. When possible, the team should include pain management and addiction specialists. Team members need to be aware of their own attitudes and misconceptions about people with substance abuse problems, which may result in undertreatment of pain.
In addition to the fears about addiction, physical dependence and tolerance, other barriers hinder effective pain management. Table 8-12 lists these and other barriers and includes strategies to address the barriers.
Institutionalising pain education and management
Besides patient and carer barriers, other major barriers to effective pain management include inadequate healthcare provider education and lack of organisational support. Traditionally, medical and nursing school curricula spent little time teaching future doctors and nurses about pain and symptom management. This lack of emphasis has contributed to the problem of a lack of knowledge and skills to treat pain adequately. However, over recent decades medical and nursing programs have increased the amount of time spent addressing pain and there are now a number of specialty postgraduate programs available. In 1999 a Faculty of Pain Medicine (FPM) was established within the Australian and New Zealand College of Anaesthetists (ANZCA), incorporating multidisciplinary representation from the Royal Australasian College of Physicians (RACP), the Royal Australasian College of Surgeons (RACS), the Royal Australian and New Zealand College of Psychiatrists (RANZCP) and the Australasian Faculty of Rehabilitation Medicine (AFRP).5
In addition, ANZCA and the FMP have published and updated evidence-based guidelines for assessing and managing pain.19 In 2010 ANZCA, in collaboration with the FPM, the Australian Pain Society and Chronic Pain Australia, produced a national pain strategy that was endorsed by the Commonwealth Department of Health and Ageing.5 Many hospitals and healthcare organisations now have dedicated pain teams, which include specialised clinical nurse consultants. In addition, accreditation guidelines have standards that specifically address the quality of pain management within healthcare institutions. Under the standards, healthcare facilities are required to: (1) recognise the patient’s right to appropriate assessment and management of pain; (2) identify pain in patients during their initial assessment and as needed during ongoing, periodic reassessments; (3) educate healthcare providers about pain assessment and management and ensure competency; and (4) educate patients and their families about pain management.
Ethical issues in pain management
FEAR OF HASTENING DEATH BY ADMINISTERING ANALGESICS
A common concern of healthcare professionals and carers is that providing sufficient drugs to relieve pain will precipitate the death of a terminally ill person. Despite the fear, however, there is no scientific evidence that opioids can hasten death, even among patients at the very end of life. Moreover, nurses have a moral obligation to provide comfort and pain relief at the end of life. Even if there is a concern about the possibility of hastening death, the rule of double effect provides ethical justification. This rule states that if an unwanted consequence (i.e. hastened death) occurs as a result of an action taken to achieve a moral good (i.e. pain relief), the action is justified if the nurse’s intent is to relieve pain and not to hasten death.63
REQUESTS FOR EUTHANASIA
Unrelieved pain is one of the reasons that patients make requests for euthanasia (assisted suicide). In some states in the US and in the Netherlands, Belgium and Switzerland, euthanasia is legal. However, it is currently illegal in New Zealand and Australia. The Death with Dignity Bill 2003 was introduced into the New Zealand Parliament on 6 March 2003. The Bill’s purpose was to provide terminally and/or incurably ill people with the opportunity of requesting assistance from a medically qualified person to end their lives, but the Bill was defeated on 30 July 2003. Voluntary euthanasia was legal in the Northern Territory from 1996 to 1997, and since then several legislative attempts have been made to legalise euthanasia in parts of Australia. However, at present it remains unlawful. Euthanasia has been the subject of much moral, religious, philosophical, legal and human rights debate in both Australia and New Zealand. At the core of this debate is how to reconcile competing values: the desire of individuals to choose to die with dignity when suffering, and the need to uphold the inherent right to life of every person. Currently it is believed that aggressive and adequate pain management will solve the needs of people who are suffering. Euthanasia is a complex issue that extends beyond pain and pain management and the scope of this chapter. Nurses need to be aware of the laws that surround this issue.
Persistent pain is a common problem in older adults and is often associated with significant physical disability and psychosocial problems. Estimates of the prevalence of chronic pain among community-dwelling older adults exceed 50%, and among elderly nursing home patients the prevalence is around 80%. The most common painful conditions among older adults are musculoskeletal conditions such as osteoarthritis, low back pain and previous fracture sites. Chronic pain often results in depression, sleep disturbance, decreased mobility, increased use of healthcare, and physical and social role dysfunction. Despite the high prevalence of pain in the elderly, it is often inadequately assessed and treated.68 Chronic non-cancer pain is not the only problem: cancer is the second most common cause of death in older people, with pain occurring in more than 70% of cancer sufferers.69 In addition, older individuals have higher rates of hospital admissions and medical procedures, many of which are associated with acute pain.5
There are several barriers to pain assessment in older patients. In addition to the barriers discussed earlier in the chapter, older patients and their healthcare providers often believe that pain is a normal, inevitable part of ageing. They may also believe that nothing can be done to relieve the pain. Older adults may not report pain for fear of being a ‘burden’ or a ‘complainer’. They may have greater fears of taking opioids than other age groups, and they are more likely to use words such as ‘aching’, ‘soreness’ or ‘discomfort’ rather than ‘pain’. For all these reasons, nurses need to be persistent in asking older adults about pain and to carry out the assessment in an unhurried, supportive manner.
Another barrier to pain assessment in older adults is the relatively high prevalence of cognitive, sensory-perceptual and motor problems that interfere with a person’s ability to process information and to communicate. Examples of these problems include dementia and delirium, post-stroke aphasia and paraplegia, and language barriers. Hearing and vision deficits may complicate assessment. Therefore, pain assessment tools may need to be adapted for older adults (see Fig 8-8 for a tool that has been adapted for this population) or it may be necessary to use a large-print pain intensity scale. Although there is some concern that older adults have difficulty using pain scales, many older adults, even those with mild to moderate cognitive impairment, can use quantitative scales accurately and reliably.
In older patients with chronic pain, a thorough physical examination and history should be performed to identify causes of pain, possible therapies and potential problems. Because depression and functional impairments are common among older adults with pain, they also must be assessed.
Treatment of pain in older patients is complicated by several factors. First, older adults metabolise drugs more slowly than younger patients and thus are at greater risk for higher blood levels and adverse effects. For this reason, the adage ‘start low and go slow’ needs to be applied to analgesic therapy in this age group. Second, the use of NSAIDs in the elderly is associated with a high frequency of serious GI bleeding. For this reason, paracetamol should be used whenever possible. Third, older adults often take many drugs for one or more chronic conditions, and the addition of analgesics can result in dangerous drug interactions and increased side effects. Fourth, cognitive impairment and ataxia can be exacerbated when analgesics such as opioids, antidepressants and antiseizure drugs are used. This requires that healthcare providers titrate drugs slowly and monitor carefully for side effects.
Treatment regimens for older adults must incorporate non-drug modalities. Exercise and patient teaching are particularly important non-drug interventions for older adults with chronic pain. Family and paid carers must be included in the treatment plan.
USE OF PLACEBOS IN PAIN ASSESSMENT AND TREATMENT
Although their use has declined, placebos are sometimes still used to assess and to treat pain. Using a placebo involves deceiving patients by making them believe that they are receiving an analgesic (usually an opioid) when in fact they are typically receiving an inert substance such as saline. The use of placebos to assess or treat pain is condemned by several professional organisations.64
Patients unable to self-report pain
Although patient self-report is the gold standard of pain assessment, many illnesses and conditions affect a patient’s ability to report pain. These diagnoses and conditions include advanced dementia and other progressive neurological diseases such as Parkinson’s disease and multiple sclerosis, cerebrovascular disease, psychosis and delirium. For these patients, behavioural and physiological changes may be the only indicators that they are in pain. Therefore, the nurse must be astute at recognising behavioural symptoms of pain.
An assessment guide is presented in Box 8-6. Several scales have been developed to assess pain-related behaviours in non-verbal patients, particularly those with advanced dementia.50 Several excellent reviews of these tools have been published,65,66 and evaluations for several pain assessment tools for people with dementia are available at the City of Hope Pain & Palliative Care Resource Center website (see the Resources on p 157).
BOX 8-6 Assessing pain in non-verbal patients
The following assessment techniques are recommended.
1. Obtain a self-report when possible (never assume a person is unable to give a verbal report).
2. Investigate potential causes of pain.
3. Observe patient behaviours that indicate pain (e.g. grimacing, frowning, rubbing a painful area, groaning and restlessness).
4. Obtain surrogate reports of pain from professional and family carers.
5. Try to use analgesics and reassess the patient to observe for a decrease in pain-related behaviours.
Source: Position Statement from the American Society for Pain Management Nursing (ASPMN). Modified from Herr K, Coyne PJ, Key T et al. Pain assessment in the nonverbal patient: position statement with clinical practice recommendations. Pain Manag Nurs 2006; 7:44.
International perspectives
In 2010 the International Association for the Study of Acute Pain declared that October 2010 to October 2011 would be the Global Year Against Acute Pain.67 This strategy was designed to improve knowledge about pain assessment and management, to overcome identified gaps in treatment and ensure that all people, whether or not they live in developed or developing countries, have access to adequate pain relief. In 2010 the delegates to the International Pain Summit of the International Association for the Study of Pain declared that access to pain management is a fundamental human right (the Montreal Declaration). They acknowledged the intrinsic dignity of all persons and stated that withholding of pain treatment is profoundly wrong, leading to unnecessary suffering, which is harmful. The Montreal Declaration states that the following human rights must be recognised throughout the world:
Article 1. The right of all people to have access to pain management without discrimination.
Article 2. The right of people in pain to acknowledgement of their pain and to be informed about how it can be assessed and managed.
Article 3. The right of all people with pain to have access to appropriate assessment and treatment of the pain by adequately trained healthcare professionals.67
The effective recognition and treatment of pain is a responsibility of all healthcare professionals. Nurses have an ethical and professional responsibility to understand the mechanisms and causes of pain and to be aware of ways that pain can be managed. This chapter has provided an overview of current thinking about pain and pain management, but because this is a relatively new and growing area of research and practice there is much more to learn.
The older patient with chronic pain
CASE STUDY
Patient profile
Jean Davis, a 72-year-old woman, has been suffering chronic pain from osteoarthritis for many years. This limits her mobility, especially in the early morning, as she finds long periods of sitting or lying exacerbate pain in her hips and lumbar and cervical spine. She has been admitted to hospital for elective surgery for a total left hip replacement resulting from this disease. Her general health is satisfactory and there are no other medical concerns for this patient.
CRITICAL THINKING QUESTIONS
1. Explain what nursing history and assessment you would take on admission.
2. Describe what pain management education you would provide for this patient.
3. What specific nursing interventions would you incorporate to ensure that this patient’s pain is well controlled postoperatively?
4. What factors need to be considered in her nursing care plan to ensure successful pain control?
5. What non-pharmacological therapies could you employ to complement pharmacological therapies for this patient?
2. A patient is receiving a PCA infusion following surgery to repair a hip fracture. She is sleeping soundly but awakens when the nurse speaks to her in a normal tone of voice. Her respirations are 8 breaths per minute. The most appropriate nursing action in this situation is to:
3. Which of the following words is most likely to be used to describe neuropathic pain?
5. A cancer patient who reports ongoing, constant moderate pain with short periods of severe pain during dressing changes:
6. An example of a distraction to provide pain relief is:
7. An appropriate non-opioid analgesic for mild pain is:
8. An important nursing responsibility related to pain is to:
9. Providing opioids to a dying patient who is experiencing moderate to severe pain:
10. A nurse believes that patients with the same type of tissue injury should have the same amount of pain. This statement reflects:
1 MBF Foundation. The high price of pain: the economic impact of chronic pain in Australia in 2007. Report by Access Economics in collaboration with the University of Sydney. Pain Management Research Institute, November 2007.
2 Lovell M. Cancer pain education for patients. Sydney: University of Sydney, 2009.
3 New Zealand Ministry of Health (NZMOH). A portrait of health: key results of the 2006/2007 New Zealand Health Survey. Wellington: NZMOH.
4 New Zealand Ministry of Health (NZMOH). Report on New Zealand cost-of-illness studies on long-term conditions. Wellington: NZMOH, 2009.
5 Australian and New Zealand College of Anaesthetists, Faculty of Pain Medicine, Australian Pain Society. Chronic pain Australia: national pain strategy. Melbourne: Faculty of Pain Medicine; 2010. Available www.painsummit.org.au/strategy/Strategy-NPS.pdf/view accessed March 2011.
6 Mulvaney S, Lambert EW, Garber J, Walker LS. Trajectories of symptoms and impairment for pediatric patients with functional abdominal pain: a 5-year longitudinal study. J Am Acad Child Adolesc Psychiatry. 2006;45(6):737–744.
7 van Dijk A, McGrath PA, Pickett W, Van Den Kerkhof EG. Pain prevalence in nine- to 13-year-old school children. Pain Res Manag. 2006;11(4):234–240.
8 Cheffins TE, Blackman R, Veitch C. Rural GPs’ management of vehicle related trauma. Aus Fam Phys. 2007;36(9):782–784.
9 Blyth FM, March LM, Brnabic AJM, Cousins MJ. Chronic pain and frequent use of health care. Pain. 2004;111:51–58.
10 Breivik H, Stubhaug A. Management of acute postoperative pain: still a long way to go!. Pain. 2008;137:233–234.
11 van den Beuken-van Everdingen MHJ, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18(9):1437–1449.
12 Walsh NE, Brooks P, Mieke Hazes JM, Walsh RM, et al. Standards of care for acute and chronic musculoskeletal pain: the bone and joint decade (2000–2010). Arch Phys Med Rehabil. 2009;89:1830–1845.
13 Deyo RA, Mirza SK, Turner JA, Martin BI. Over treating chronic back pain: time to back off? J Am Board Fam Med. 2009;22:62–68.
14 Ersek M. Overview of pain types and prevalence. In: Dahl J, Gordon DE, Paice JA, eds. Pain resource nurse (PRN) program curriculum and planning guide. Madison: Wisconsin Pain Initiative, Resource Center of the Alliance of State Pain Initiatives, 2008.
15 Hospice and Palliative Nurses Association. Position statement: the ethics of opiate use within palliative care. Available at www.hpna.org/DisplayPage.aspx?Title=Position%20Statements. accessed March 2011.
16 International Association for the Study of Pain. Global Year Against Acute Pain, 2011. Why the gaps between evidence and practice? Available at www.painsummit.org.au/strategy/. accessed April 2011.
17 McCaffery M. Nursing practice theories related to cognition, bodily pain and man-environmental interactions. Los Angeles: UCLA Students Store, 1968. (Seminal)
18 Merskey H, Bugduk N, eds. Classification of chronic pain, descriptions of chronic pain syndromes and definitions of pain terms, 2nd edn., Seattle: IASP Press, 1994. (Seminal)
19 Macintyre PE, Schug SA, Scott DA, Visser EJ, Walker SM. APM: SE Working Group of the Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine. Acute pain management: scientific evidence, 3rd edn. Melbourne: ANZCA & FPM, 2010.
20 Gatchel RJ, Peng YB, Peters ML, et al. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007;133:581.
21 Hasenbring MI, Verbunt JA. Fear-avoidance and endurance-related responses to pain: new models of behaviour and their consequences for clinical practice. Clin J Pain. 2010;26:747–753.
22 Edwards RR, Bingham CO, Bathon J, et al. Catastrophizing and pain in arthritis, fibromyalgia, and other rheumatic diseases. Arthritis Rheum. 2006;55:325.
23 Stewart ST, Woodward RM, Rosen AB, Cutler DM. The impact of symptoms and impairments on overall health in US National Health Data. Med Care. 2008;46:954–962.
24 DiMatteo MR. Social support and patient adherence to medical treatment: a meta-analysis. Health Psychol. 2004;23(2):207–218.
25 Narayan MC. Culture’s effect on pain assessment and management. Aus J Nurs. 2010;110(4):38–47.
26 McGrath P. ‘The biggest worry…’: research findings on pain management for Aboriginal peoples in Northern Territory, Australia. Rural and Remote Health. 2006;6(3):549.
27 Mungabareena Aboriginal Corporation, Wodonga Institute of TAFE and Mercy Health Service Albury. Providing culturally appropriate palliative care to Aboriginal and Torres Strait Islander peoples. Canberra: Department of Health & Ageing; 2004. Available at www.vaccho.org.au/documents/PracticePrinciples.pdf accessed April 2011.
28 Fenwick C, Stevens J. Postoperative pain experiences of central Australian Aboriginal women. What do we understand? Aust J Rural Health. 2004;12(1):22–27.
29 Wang H, Ehnert C, Brenner GJ, et al. Bradykinin and peripheral sensitization. Biol Chem. 2006;387:11.
30 Woolf CJ. Central sensitization: uncovering the relation between pain and plasticity. Anesthesiology. 2007;106:864.
31 Staud R, Craggs JG, Peristein WM. Brain activity associated with slow temporal summation of C-fiber evoked pain in fibromyalgia patients and controls. Eur J Pain. 2008;12:1078.
32 Basbaum AI, Bautista DM, Scherrer G, et al. Cellular and molecular mechanisms of pain. Cell. 2009;139:267.
33 Gjerstad J. Genetic susceptibility and development of chronic non-malignant back pain. Rev Neurosci. 2007;18:83.
34 Berry P, Covington EC, Dahl JL, et al. Pain: current understanding of assessment, management and treatments. Available at www.npcnow.org/App_Themes/Public/pdf/Issues/pub_related_research/pub_quality_care/Pain-Current-Understanding-of-Assessment-Management-and-Treatments.pdf, 2006. accessed March 2011.
35 Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. (4):2007. CD005454.
36 National Institute of Neurological Disorders and Stroke. Complex regional pain syndrome fact sheet. Available at www.ninds.nih.gov/disorders/reflex_sympathetic_dystrophy/detail_reflex_sympathetic_dystrophy.htm. accessed March 2011.
37 Abbey J, Do Bellis A, Piller N, et al. Pain assessment in nursing home residents with dementia. Gunn grant report, 1997–98. Adelaide: JH & JD Gunn Medical Research Foundation, 1998. (Seminal)
38 Chou R, Huffman LH. Nonpharmacological therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492.
39 Guindon J, Walczak JS, Beaulieu P. Recent advances in the pharmacologicalal management of pain. Drugs. 2007;67:2121.
40 Recommendations for use of selective and nonselective nonsteroidal antiinflammatory drugs: an American College of Rheumatology white paper. Arthritis Rheum. 2008;59:1058.
41 Tiziani A. Havard’s nursing guide to drugs. Sydney: Mosby-Elsevier, 2010.
42 Boothby LA, Wang L-J, Mayher S, Chestnutt L. Academic detailing of meperidine at a teaching hospital, Hospital Pharmacy. 2003;38(1):30–35. Available at www.ciap.health.nsw.gov.au/nswtag/publications/otherdocs/academic_detailing_Boothby_etal.pdf accessed April 2011.
43 Taylor S, Kirton OC, Staff I, et al. Postoperative day one: a high risk period for respiratory events. Am J Surg. 2005;190:752.
44 Ganesh A, Maxwell LG. Pathophysiology and management of opioid-induced pruritus. Drugs. 2007;67:2323–2333.
45 Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain. 2008;24:479.
46 Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacological management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237.
47 Stegmann JU, Weber H, Steup A, et al. The efficacy and tolerability of multiple-dose tapentadol immediate release for the relief of acute pain following orthopedic (bunionectomy) surgery. Curr Med Res Opin. 2008;24:3185.
48 Hosking RD, Zajicek JP. Therapeutic potential of cannabis in pain medicine. Br J Anaesth. 2008;101:59.
49 McCarberg BH, Barkin RL. The future of cannabinoids as analgesic agents: a pharmacological, pharmacokinetic, and pharmacodynamic overview. Am J Ther. 2007;14:475.
50 Gammaitoni AR, Fine P, Alvarez N, et al. Clinical application of opioid equianalgesic data. Clin J Pain. 2003;19:286.
51 McCamant KL. Peripheral nerve blocks: understanding the nurse’s role. J Perianesth Nurs. 2006;21:16.
52 Falowski S, Celii A, Sharan A. Spinal cord stimulation: an update. Neurotherapeutics. 2008;5:86.
53 Reid MC, Papaleontiou M, Ong A, et al. Self-management strategies to reduce pain and improve function among older adults in community settings: a review of the evidence. Pain Med. 2008;9:409.
54 Frey Law LA, Evans S, Knudtson J, et al. Massage reduces pain perception and hyperalgesia in experimental muscle pain: a randomized, controlled trial. J Pain. 2008;9:714.
55 Little P, Lewith G, Webley F, et al. Randomised controlled trial of Alexander technique lessons, exercise, and massage (ATEAM) for chronic and recurrent back pain. BMJ. 2008;337:a884.
56 Busch AJ, Barber KA, Overend TJ, et al. Exercise for treating fibromyalgia syndrome. Cochrane Database Syst Rev. (4):2007. CD003786.
57 Lang T, Barker R, Steinlechner B, et al. TENS relieves acute posttraumatic hip pain during emergency transport. J Trauma. 2007;62:184.
58 Nnoaham KE, Kumbang J. Transcutaneous electrical nerve stimulation (TENS) for chronic pain. Cochrane Database Syst Rev. (3):2008. CD003222.
59 Elkins G, Jensen MP, Patterson DR. Hypnotherapy for the management of chronic pain. Int J Clin Exp Hypn. 2007;55:275.
60 de Leon-Casasola OA. Current developments in opioid therapy for management of cancer pain. Clin J Pain. 2008;24(suppl 10):S3.
61 American Society for Pain Management Nursing. Pain management in patients with addictive disease. Available at www.aspmn.org/Organization/position_papers.htm. accessed March 2011.
62 Passik SD, Kirsh KL. The interface between pain and drug abuse and the evolution of strategies to optimize pain management while minimizing drug abuse. Exp Clin Psychopharmacol. 2008;16:400.
63 Beauchamp T, Childress J. Principles of biomedical ethics. New York: Oxford University Press, 2009.
64 American Society for Pain Management Nursing. Position statement on the use of placebos in pain management. Available at www.aspmn.org/pdfs/Use%20of%20Placebos.pdf. accessed March 2011.
65 Li D, Puntillo K, Miaskowski C. A review of objective pain measures for use with critical care adult patients unable to self-report. J Pain. 2008;9:2.
66 Herr K, Bjoro K, Decker S. Tools for assessment of pain in nonverbal older adults with dementia: a state-of-the-science review. J Pain Symptom Manage. 2006;31:170.
67 International Association for the Study of Pain. Global Year Against Acute Pain: Montreal Declaration. Available at www.iasp-pain.org/AM/Template.cfm?Section=Declaration_of_MontrandNum233_al, 3 September 2010. accessed March 2011.
68 Gibson SJ. IASP global year against pain in older persons: highlighting the current status and future perspectives in geriatric pain. Expert Rev Neurother. 2007;7:627.
69 van den Beuken-van Everdingen MHJ, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18(9):1437–1449.
Acute Pain Management Scientific Evidence. www.anzca.edu.au/resources/books-and-publications/acutepain.pdf
American Academy of Pain Management. www.aapainmanage.org
American Pain Society. www.ampainsoc.org
American Society for Pain Management Nursing. www.aspmn.org
Australasian Cochrane Centre. http://aaa.cochrane.org.au
Australian and New Zealand College of Anaesthetists, Faculty of Pain Medicine. www.fpm.anzca.edu.au
Australian Pain Society. www.apsoc.org.au
British Pain Society. www.britishpainsociety.org
City of Hope Pain & Palliative Care Resource Center. http://prc.coh.org
International Association for the Study of Pain. www.iasp-pain.org
National Institute of Clinical Studies. www.nicsl.com.au
New Zealand Pain Society Inc. www.nzps.org.nz
North Coast Area Health Service: pain management guidelines. www.ncahs.nsw.gov.au/pain
The Oxford Pain Internet Site. www.medicine.ox.ac.uk/bandolier/booth/painpag
World Health Organization: pain ladder. www.who.int/cancer/palliative/painladder/en/