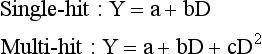Deterministic Effects of Radiation
At the completion of this chapter, the student should be able to do the following:
1 Describe the three acute radiation syndromes.
2 Identify the two stages that lead to acute radiation lethality.
4 Discuss local tissue damage after high-dose irradiation.
5 Review the cytogenetic effects of radiation exposure.
6 Describe the three features of a deterministic radiation effect.
DURING THE 1920s and the 1930s, it would not have been unusual for a radiologic technologist to visit the hematology laboratory once a week for a routine blood examination. Before the introduction of personnel radiation monitors, periodic blood examination was the only way to monitor x-ray workers.
There was great concern over the danger of occupational radiation exposure. Today’s occupational radiation exposures are quite low. Unfortunately, patient radiation dose is on the rise, including doses high enough to cause injury. That is why the radiologic technologist must understand the deterministic effects of high radiation doses.
This chapter explores such deterministic effects from the most severe (death) to the most worrisome today (skin effects). The chapter also reviews hematologic and cytogenetic effects.
To produce a radiation response in humans within a few days to months, the dose must be substantial. Such a response is called an early effect of radiation exposure. A dose of this magnitude is rare in diagnostic radiology.
Deterministic radiation responses are those that exhibit increasing severity with increasing radiation dose. Furthermore, there is a dose threshold, and the dose-response relationship is nonlinear.
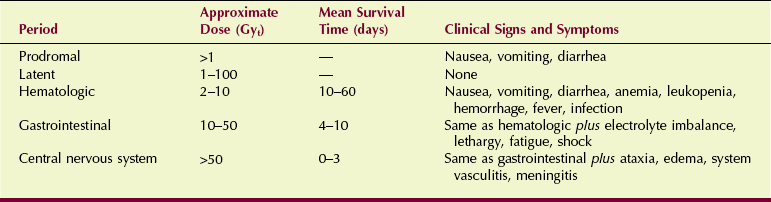
These early effects have been studied extensively with laboratory animals, and some data have been obtained from observations of humans. This chapter considers only the more important effects as identified in Table 33-1 along with the minimum radiation dose necessary to produce each.
TABLE 33-1
Principal Deterministic Effects of Radiation Exposure on Humans and the Approximate Threshold Dose
| Effect | Anatomic Site | Threshold Dose |
| Death | Whole body | 2 Gyt (200 rad) |
| Hematologic depression | Whole body | 250 mGyt (25 rad) |
| Skin erythema | Small field | 2 Gyt (200 rad) |
| Epilation | Small field | 3 Gyt (300 rad) |
| Chromosome aberration | Whole body | 50 mGyt (5 rad) |
| Gonadal dysfunction | Local tissue | 100 mGyt (10 rad) |
Acute Radiation Lethality
Death, of course, is the most devastating human response to radiation exposure. No cases of death after diagnostic x-ray exposure have ever been recorded, although some early x-ray pioneers died from the stochastic effects of x-ray exposure. In each of these cases, however, the total radiation dose was extremely high by today’s standards.
Acute radiation-induced human lethality is of only academic interest in diagnostic radiology. Diagnostic x-ray beams are neither intense enough nor large enough to cause death.
Some accidental exposures of persons in the nuclear weapons and nuclear energy fields have resulted in immediate death, but the number of such accidents has been small considering the length and activity of the atomic age. The unfortunate incident at Chernobyl in April 1986 is the one notable exception.
Thirty people at Chernobyl experienced the acute radiation syndrome and died. A number of minor late effects have been observed. No one died or was even seriously exposed in the March 1979 incident at the nuclear power reactor at Three Mile Island, Pennsylvania. And no acute lethality was observed at the tsunami-induced nuclear reactor meltdown at Fukushima, Japan, in 2011.
Employment in the nuclear power industry is a safe occupation.
The sequence of events that follow high-level radiation exposure leading to death within days or weeks is called the acute radiation syndrome. There are, in fact, three separate syndromes that are dose related and that follow a rather distinct course of clinical responses.
These syndromes are hematologic death, gastrointestinal (GI) death, and central nervous system (CNS) death. The clinical signs and symptoms of each are outlined in Table 33-2. CNS death requires radiation doses in excess of 50 Gyt (5000 rad) and results in death within hours. Hematologic death and GI death follow lower exposures and require a longer time for death to occur.
In addition to the three lethal syndromes, two periods are associated with acute radiation lethality. The prodromal period consists of acute clinical symptoms that occur within hours of exposure and continue for up to a day or two. After the prodromal period has ended, there may be a latent period, during which the subject is free of visible effects.
Prodromal Period
At radiation doses above approximately 1 Gyt (100 rad) delivered to the total body, signs and symptoms of radiation sickness may appear within minutes to hours. The symptoms of early radiation sickness most often take the form of nausea, vomiting, diarrhea, and a reduction in the white blood cells of the peripheral blood (leukopenia).
The prodromal period may last from a few hours to a couple of days. The severity of the symptoms is dose related; at doses in excess of 10 Gyt (1000 rad), symptoms can be violent. At still higher doses, the duration of the prodromal syndrome becomes shorter until it is difficult to separate the prodromal syndrome from the period of manifest illness.
Latent Period
After the period of initial radiation sickness, a period of apparent well-being occurs, which is called the latent period. The latent period extends from hours or less (at doses in excess of 50 Gyt) to weeks (at doses from 1 to 5 Gyt).
The latent period is sometimes mistakenly thought to indicate an early recovery from a moderate radiation dose. It may be misleading, however, because it gives no indication of the extensive radiation response yet to follow.
Manifest Illness
The dose necessary to produce a given syndrome and the mean survival time are the principal quantitative measures of human radiation lethality (see Table 33-2). Although ranges of dose and resultant mean survival times are given, there is rarely a precise difference in the dose and time-related sequence of events associated with each syndrome. At very high radiation doses, the latent period disappears altogether. At very low radiation doses, there may be no prodromal period at all.
Hematologic Syndrome
Radiation doses in the range of approximately 2 to 10 Gyt (200–1000 rad) produce the hematologic syndrome. The patient initially experiences mild symptoms of the prodromal syndrome, which appear in a matter of a few hours and may persist for several days.
The latent period that follows can extend as long as 4 weeks and is characterized by a general feeling of wellness. There are no obvious signs of illness, although the number of cells in the peripheral blood declines during this time.
The period of manifest illness is characterized by possible vomiting, mild diarrhea, malaise, lethargy, and fever. Each of the types of blood cells follows a rather characteristic pattern of cell depletion. If the dose is not lethal, recovery begins in 2 to 4 weeks, but as long as 6 months may be required for full recovery.
If the radiation injury is severe enough, the reduction in blood cells continues unchecked until the body’s defense against infection is nil. Just before death, hemorrhage and dehydration may be pronounced. Death occurs because of generalized infection, electrolyte imbalance, and dehydration.
Gastrointestinal Syndrome
Radiation doses of approximately 10 to 50 Gyt (1000–5000 rad) result in the GI syndrome. The prodromal symptoms of vomiting and diarrhea occur within hours of exposure and persist for hours to as long as a day. A latent period of 3 to 5 days follows, during which no symptoms are present.
The manifest illness period begins with a second wave of nausea and vomiting followed by diarrhea. The victim experiences a loss of appetite (anorexia) and may become lethargic. The diarrhea persists and becomes more severe, leading to loose and then watery and bloody stools. Supportive therapy cannot prevent the rapid progression of symptoms that ultimately leads to death within 4 to 10 days of exposure.
Intestinal cells are normally in a rapid state of proliferation and are continuously being replaced by new cells. The turnover time for this cell renewal system in a normal person is 3 to 5 days.
Radiation exposure kills the most sensitive cells—stem cells; this controls the length of time until death. When the intestinal lining is completely denuded of functional cells, fluids pass uncontrollably across the intestinal membrane, electrolyte balance is destroyed, and conditions promote infection.
At doses consistent with the GI syndrome, measurable and even severe hematologic changes occur. It takes a longer time for the cell renewal system of the blood to develop mature cells from the stem cell population; therefore, there is not enough time for maximum hematologic effects to occur.
Central Nervous System Syndrome
After a radiation dose in excess of approximately 50 Gyt (5000 rad) is received, a series of signs and symptoms occur that lead to death within a matter of hours to days. First, severe nausea and vomiting begins, usually within a few minutes of exposure.
During this initial onset, the patient may become extremely nervous and confused, may describe a burning sensation in the skin, may lose vision, and can even lose consciousness within the first hour. This may be followed by a latent period that lasts up to 12 hours, during which earlier symptoms subside or disappear.
The latent period is followed by the period of manifest illness, during which symptoms of the prodromal stage return but are more severe. The person becomes disoriented; loses muscle coordination; has difficulty breathing; may go into convulsive seizures; experiences loss of equilibrium, ataxia, and lethargy; lapses into a coma; and dies.
Regardless of the medical attention given the patient, the symptoms of manifest illness appear rather suddenly and always with extreme severity. At radiation doses high enough to produce CNS effects, the outcome is always death within a few days of exposure.
The CNS syndrome is characterized by increased intracranial pressure, inflammatory changes in the blood vessels of the brain (vasculitis), and inflammation of the meninges (meningitis). At doses sufficient to produce CNS damage, damage to all other organs of the body is equally severe. The classic radiation-induced changes in the GI tract and the hematologic system cannot occur because there is insufficient time between exposure and death for them to appear.
LD50/60
If experimental animals are irradiated with varying doses of radiation—for example, 1 to 10 Gyt (100–1000 rad)—the plot of the percentage that dies as a function of radiation dose would appear as in Figure 33-1. This figure illustrates the radiation dose-response relationship for acute human lethality.
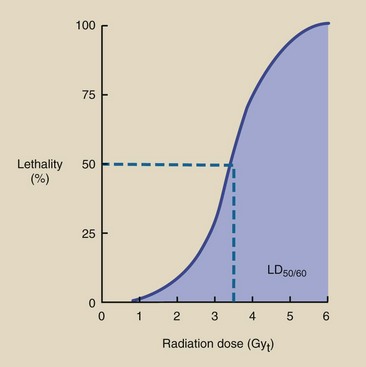
FIGURE 33-1 Radiation-induced death in humans follows a nonlinear, threshold dose-response relationship.
At the lower dose of approximately 1 Gyt (100 rad), no one is expected to die. Above approximately 6 Gyt (600 rad), all those irradiated die unless vigorous medical support is available. Above 10 Gyt (1000 rad), even vigorous medical support does not prevent death.
If death is to occur, it usually happens within 60 days of exposure. Acute radiation lethality is measured quantitatively by the LD50/60, which is approximately 3.5 Gyt (350 rad) for humans. With clinical support, humans can tolerate much higher doses; the maximum is reported to be 8.5 Gyt (850 rad). Table 33-3 lists values of LD50/60 for various species.
TABLE 33-3
Approximate LD50/60 for Various Species After Whole-Body Radiation Exposure
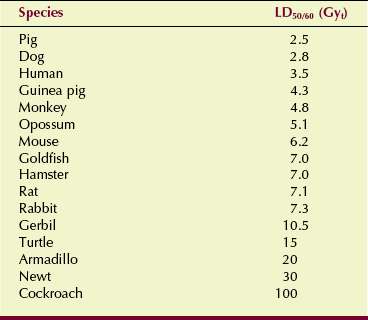
LD50/60, Dose of radiation to the whole body that causes 50% of irradiated subjects to die within 60 days.
| Question: | From Figure 33-1, estimate the radiation dose that will produce 25% lethality in humans within 60 days. |
| Answer: | First, draw a horizontal line from the 25% level on the y-axis until it intersects the S curve. Now, drop a vertical line from this point to the x-axis. This intersection with the x-axis occurs at the LD25/60, which is approximately 2.5 Gyt (250 rad). |
Mean Survival Time
As the whole-body radiation dose increases, the average time between exposure and death decreases. This time is known as the mean survival time. A graph of radiation dose versus mean survival time is shown in Figure 33-2. This graph depicts three distinct regions associated with the three radiation syndromes.
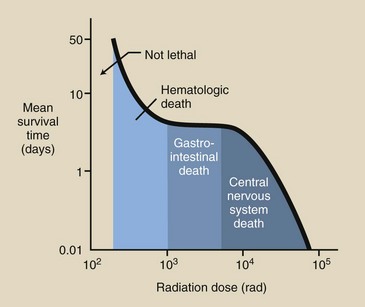
FIGURE 33-2 Mean survival time after radiation exposure shows three distinct regions. If death is attributable to hematologic or central nervous system (CNS) effects, the mean survival time will vary with dose. If gastrointestinal (GI) effects cause death, it occurs in approximately 4 days.
As the radiation dose increases from 2 to 10 Gyt (200–1000 rad), the mean survival time decreases from approximately 60 to 4 days; this region is consistent with death resulting from the hematologic syndrome. Mean survival time is dose dependent with the hematologic syndrome.
In the dose range associated with the GI syndrome, however, the mean survival time remains relatively constant, at 4 days. With larger doses, those associated with the CNS syndrome, the mean survival time is again dose dependent, varying from approximately 3 days to a matter of hours.
Local Tissue Damage
When only part of the body is irradiated, in contrast to whole-body irradiation, a higher dose is required to produce a response. Every organ and tissue of the body can be affected by partial-body irradiation. The effect is cell death, which results in shrinkage of the organ or tissue. This effect can lead to total lack of function for that organ or tissue, or it can be followed by recovery.
There are many examples of local tissue damage immediately after radiation exposure. In fact, if the dose is high enough, any local tissue will respond. The manner in which local tissues respond depends on their intrinsic radiosensitivity and the kinetics of cell proliferation and maturation. Examples of local tissues that can be affected immediately are the skin, gonads, and bone marrow.
All deterministic radiation responses—local tissue damage is a good example—follow a threshold-type dose-response relationship. A minimum dose is necessary to produce a deterministic response. When that threshold dose has been exceeded, the severity of the response increases with increasing dose in a nonlinear fashion.
Effects on the Skin
The tissue with which we have had the most experience is the skin. Normal skin consists of three layers: an outer layer (the epidermis), an intermediate layer of connective tissue (the dermis), and a subcutaneous layer of fat and connective tissue.
The skin has additional accessory structures, such as hair follicles, sweat glands, and sensory receptors (Figure 33-3). All cell layers and accessory structures participate in the response to radiation exposure.
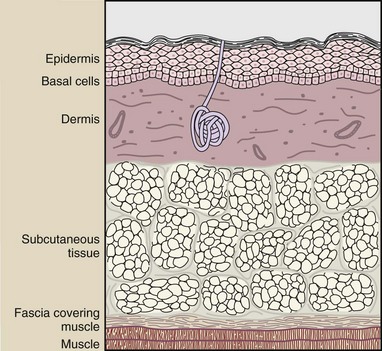
FIGURE 33-3 A sectional view of the anatomic structures of the skin. The basal cell layer is most radiosensitive.
The skin, similar to the lining of the intestine, represents a continuing cell renewal system, only with a much slower rate than that experienced by intestinal cells. Almost 50% of the cells lining the intestine are replaced every day, but skin cells are replaced at the rate of only approximately 2% per day.
The outer skin layer, the epidermis, consists of several layers of cells; the lowest layer consists of basal cells. Basal cells are the stem cells that mature as they migrate to the surface of the epidermis. When these cells arrive at the surface as mature cells, they are slowly lost and have to be replaced by new cells from the basal layer.
In earlier times, the tolerance of the patient’s skin determined the limitations of radiation oncology with orthovoltage x-rays (200–300 kVp x-rays). The object of x-ray therapy was to deposit energy in the tumor while sparing the surrounding normal tissue. Because the x-rays had to pass through the skin to reach the tumor, the skin was necessarily subjected to higher radiation doses than the tumor. The resultant skin damage was seen as erythema (a sunburn-like reddening of the skin) followed by desquamation (ulceration and denudation of the skin), which often required interruption of treatment.
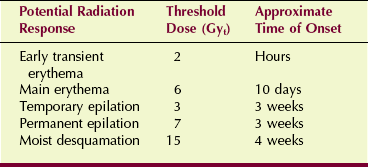
After a single dose of 3 to 10 Gyt (300–1000 rad), an initial mild erythema may occur within the first or second day. This first wave of erythema then subsides, only to be followed by a second wave that reaches maximum intensity in about 2 weeks.
At higher doses, this second wave of erythema is followed by a moist desquamation, which in turn may lead to a dry desquamation. Moist desquamation is known as clinical tolerance for radiation therapy.
During radiation therapy, the skin is exposed according to a fractionated scheme, usually approximately 2 Gyt/day (200 rad/day, 5 days a week). To assist the radiation oncologist in planning patient treatment, isoeffect curves have been generated that accurately project the dose necessary to produce skin erythema or clinical tolerance after a prescribed treatment routine (Figure 33-4). Contemporary radiation oncology uses high-energy x-radiation from linear accelerators; this protects the skin from radiation damage.
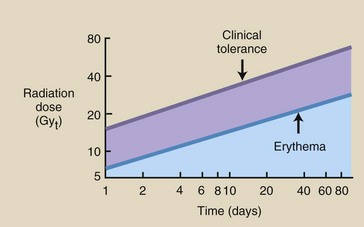
FIGURE 33-4 These isoeffect curves show the relationship between the number of daily fractions and the total radiation dose that will produce erythema or moist desquamation. As the fractionation of the dose increases, so does the total dose required.
Erythema was perhaps the first observed biologic response to radiation exposure. Many of the early x-ray pioneers, including Roentgen, sustained skin burns induced by x-rays.
One of the hazards to the patient during the early years of radiology was x-ray–induced erythema. During those years, x-ray tube potentials were so low that it was usually necessary to position the tube very close to the patient’s skin; exposures of 10 to 30 minutes were required. Often, the patient would return several days later with an x-ray burn.
These skin effects follow a nonlinear, threshold dose-response relationship similar to that described for radiation-induced lethality. Small doses of x-radiation do not cause erythema. Extremely high doses of x-radiation cause erythema in all persons so irradiated.
Whether intermediate radiation doses produce erythema depends on the individual’s radiosensitivity, the dose rate, and the size of the irradiated skin field. Analysis of persons irradiated therapeutically with superficial x-rays has shown that the skin erythema dose required to affect 50% of those irradiated (SED50) is about 5 Gyt (500 rad).
Before the Roentgen was defined and accurate radiation-measuring apparatus was developed, the skin was observed, and its response to radiation was used in formulating radiation protection practices. The unit used was the SED50, and permissible radiation exposures were specified in fractions of SED50.
Another response of the skin to radiation exposure is epilation, or loss of hair. For many years, soft x-rays (10–20 kVp), called grenz rays, were used as the treatment of choice for persons with skin diseases, such as tinea capitis (ringworm).
Tinea capitis of the scalp, which is common in children, was successfully treated by grenz radiation; unfortunately, the patient’s hair would fall out for weeks or even months. Sometimes an unnecessarily high dose of grenz rays resulted in permanent epilation.
High-dose fluoroscopy has focused more attention on the response of the skin to x-rays. The longer fluoroscopy times required for cardiovascular and interventional procedures, coupled with allowed exposure rates twice the previous normal, are of great concern. Injuries to patients have been reported, and steps are being taken to establish better control over such exposures. Table 33-4 summarizes the potential effects of high-dose fluoroscopy.
Effects on the Gonads
Human gonads are critically important target organs. As an example of local tissue effects, they are particularly sensitive to radiation. Responses to doses as low as 100 mGyt have been observed. Because these organs produce the germ cells that control fertility and heredity, their response to radiation has been studied extensively.
Much of what is known about the types of radiation response and about dose-response relationships has been derived from numerous animal experiments. Significant data are also available from human populations. Radiotherapy patients, radiation accident victims, and volunteer convicts all have provided data; this has resulted in a rather complete description of the gonadal response to radiation.
The cells of the testes (the male gonads) and the ovaries (the female gonads) respond differently to radiation because of differences in progression from the stem cell to the mature cell. Figure 33-5 illustrates this progression, indicating the most radiosensitive phase of cell maturation.
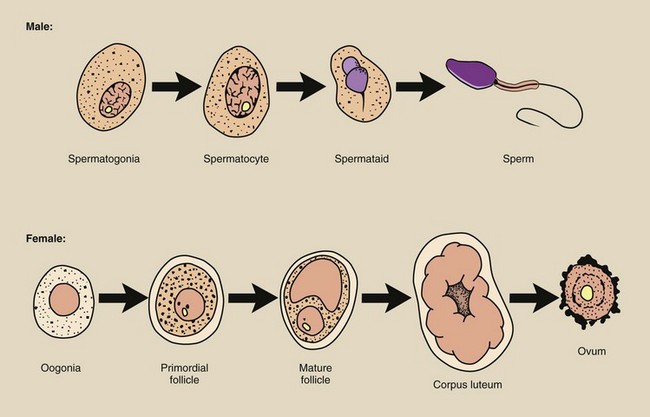
FIGURE 33-5 Progression of germ cells from the stem cell phase to the mature cell. The asterisk indicates the most radiosensitive cell.
Germ cells are produced by both ovaries and testes, but they develop from the stem cell phase to the mature cell phase at different rates and at different times. This process of development is called gametogenesis.
The stem cells of the ovaries are the oogonia, and they multiply in number only before birth during fetal life. The oogonia reach a maximum number of several million and then begin to decline because of spontaneous degeneration.
During late fetal life, many primordial follicles grow to encapsulate the oogonia, which become oocytes. These follicle-containing oocytes remain in a suspended state of growth until puberty. By the time of prepuberty, the number of oocytes has been reduced to only several hundred thousand.
Commencing at puberty, the follicles rupture with regularity, ejecting a mature germ cell, the ovum. Only 400 to 500 such ova are available for fertilization (number of years of menstruation times 13 per year).
The germ cells of the testes are continually being produced from stem cells progressively through a number of stages to maturity, and similar to the ovaries, the testes provide a sustaining cell renewal system.
The male stem cell is the spermatogonia, which matures into the spermatocyte. The spermatocyte in turn multiplies and develops into a spermatid, which finally differentiates into the functionally mature germ cell, the spermatozoa or sperm. The maturation process from stem cell to spermatozoa requires 3 to 5 weeks.
Ovaries
Irradiation of the ovaries early in life reduces their size (atrophy) through germ cell death. After puberty, such irradiation also causes suppression and delay of menstruation.
Radiation effects on the ovaries depend somewhat on age. At fetal life and in early childhood, the ovaries are especially radiosensitive. They decline in radiosensitivity, reaching a minimum in the age range of 20 to 30 years, and then increase continually with age.
Doses as low as 100 mGyt (10 rad) may delay or suppress menstruation in a mature female. A dose of approximately 2 Gyt (200 rad) produces temporary infertility; approximately 5 Gyt (500 rad) to the ovaries results in permanent sterility.
In addition to the destruction of fertility, irradiation of the ovaries of experimental animals has been shown to produce genetic mutations. Even moderate doses, such as 250 to 500 mGyt (25–50 rad), have been associated with measurable increases in genetic mutations. Evidence also indicates that oocytes that survive such a modest dose can repair some genetic damage as they mature into ova.
Testes
The testes, similar to the ovaries, atrophy after high doses of radiation. A large volume of data on testicular damage has been gathered from observations of volunteer convicts and patients treated for carcinoma in one testis while the other was shielded. Many investigators have recorded normal births in such patients, whose remaining functioning testis received a radiation dose up to 3 Gyt (300 rad).
The spermatogonial stem cells signify the most sensitive phase in the gametogenesis of the spermatozoa. After irradiation of the testes, maturing cells, spermatocytes, and spermatids are relatively radioresistant and continue to mature. Consequently, no significant reduction in spermatozoa occurs until several weeks after exposure; therefore, fertility continues throughout this time, during which irradiated spermatogonia would have developed into mature spermatozoa had they survived.
Radiation doses as low as 100 mGyt (10 rad) can reduce the number of spermatozoa (Table 33-5) in a manner reminiscent of the radiation response of the ovaries. With increasing dose, the depletion of spermatozoa increases and extends over a longer period.
Two Gray (200 rad) produces temporary infertility, which commences approximately 2 months after irradiation and persists for up to 12 months. Five Gray (500 rad) to the testes produces permanent sterility. Even after doses sufficient to produce permanent sterility, the male patient normally retains his ability to engage in sexual intercourse.
Male gametogenesis is a self-renewing system; some evidence suggests that the most hazardous mutations are the genetic ones induced in surviving postspermatogonial cells. Consequently, after testicular irradiation of doses exceeding approximately 100 mGyt (10 rad), the male patient should refrain from procreation for 2 to 4 months until all cells that were in the spermatogonial and postspermatogonial stages at the time of irradiation have matured and disappeared.
This reduces but probably does not eliminate any increase in genetic mutations caused by the persistence of the stem cell. Evidence from animal experiments suggests that genetic mutations undergo some repair even when the stem cell is irradiated.
Hematologic Effects
If you were a radiologic technologist in practice during the 1920s and the 1930s, you might have visited the hematology laboratory once a week for a routine blood examination. Before the introduction of personnel radiation monitors, periodic blood examination was the only monitoring performed on x-ray and radium workers. This examination included total cell counts and a white blood cell (leukocyte) differential count.
Most institutions had a radiation safety regulation such that, if the leukocytes were depressed by greater than 25% of normal level, the employee was given time off or was assigned to nonradiation activities until the count returned to normal.
What was not entirely understood at that time was that the minimum whole-body dose necessary to produce a measurable hematologic depression was approximately 250 mGyt (25 rad). These workers were being heavily irradiated by today’s standards.
Hemopoietic System
The hemopoietic system consists of bone marrow, circulating blood, and lymphoid tissue. Lymphoid tissues are the lymph nodes, spleen, and thymus. With this system, the principal effect of radiation is a depressed number of blood cells in the peripheral circulation. Time- and dose-related effects on the various types of circulating blood cells are determined by the normal growth and maturation of these cells.
All cells of the hemopoietic system apparently develop from a single type of stem cell (Figure 33-6). This stem cell is called a pluripotential stem cell because it can develop into several different types of mature cells.
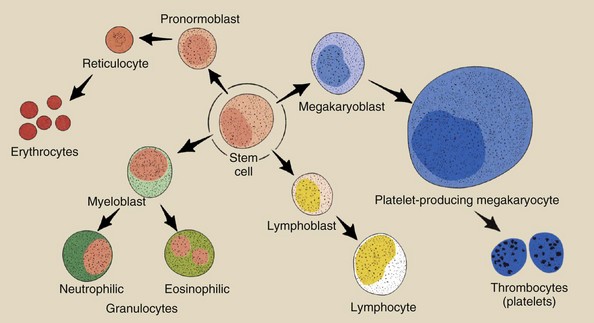
FIGURE 33-6 Four principal types of blood cells—lymphocytes, granulocytes, erythrocytes, and thrombocytes—develop and mature from a single pluripotential stem cell.
Although the spleen and the thymus manufacture one type of leukocyte (the lymphocyte), most circulating blood cells, including lymphocytes, are manufactured in the bone marrow. In a child, the bone marrow is rather uniformly distributed throughout the skeleton. In an adult, the active bone marrow responsible for producing circulating cells is restricted to flat bones, such as the ribs, sternum, and skull, and ends of long bones.
From the single pluripotential stem cell, a number of cell types are produced. Principally, these are lymphocytes (those involved in the immune response), granulocytes (scavenger type of cells used to fight bacteria), thrombocytes (also called platelets and involved in the clotting of blood to prevent hemorrhage), and erythrocytes (red blood cells that are the transportation agents for oxygen). These cell lines develop at different rates in the bone marrow and are released to the peripheral blood as mature cells.
While in the bone marrow, the cells proliferate in number, differentiate in function, and mature. Developing granulocytes and erythrocytes spend about 8 to 10 days in the bone marrow. Thrombocytes have a lifetime of approximately 5 days in the bone marrow.
Lymphocytes are produced over varying times and have varying lifetimes in the peripheral blood. Some are thought to have lives measured in terms of hours and others in terms of years. In the peripheral blood, granulocytes have a lifetime of only a couple of days. Thrombocytes have a lifetime of approximately 1 week and erythrocytes a lifetime of nearly 4 months.
The hemopoietic system, therefore, is another example of a cell renewal system. Normal cell growth and development determine the effects of radiation on this system.
Hemopoietic Cell Survival
The principal response of the hemopoietic system to radiation exposure is a decrease in the numbers of all types of blood cells in the circulating peripheral blood. Lethal injury to the stem cells causes depletion of these mature circulating cells.
Figure 33-7 shows the radiation response of three circulating cell types. Examples are given for low, moderate, and high radiation doses, showing that the degree of cell depletion increases with increasing dose. These figures are the results of observations on experimental animals, radiotherapy patients, and the few radiation accident victims.
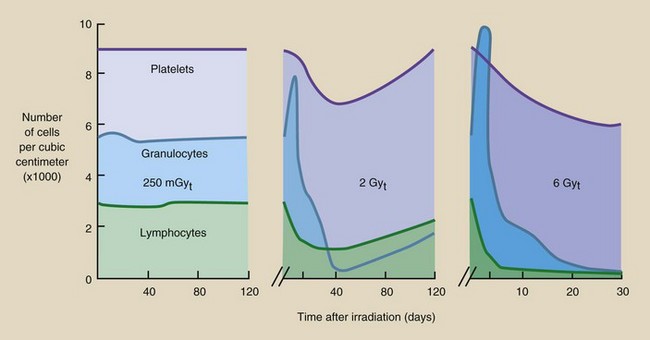
FIGURE 33-7 Graphs showing the radiation response of the major circulating blood cells. A, 25 rad. B, 200 rad. C, 600 rad.
After exposure, the first cells to become affected are the lymphocytes. These cells are reduced in number (lymphopenia) within minutes or hours after exposure, and they are very slow to recover. Because the response is so immediate, the radiation effect is apparently a direct one on the lymphocytes themselves rather than on the stem cells.
Granulocytes experience a rapid rise in number (granulocytosis) followed first by a rapid decrease and then a slower decrease in number (granulocytopenia). If the radiation dose is moderate, then an abortive rise in granulocyte count may occur 15 to 20 days after irradiation. Minimum granulocyte levels are reached approximately 30 days after irradiation. Recovery, if it is to occur, takes approximately 2 months.
The depletion of platelets (thrombocytopenia) after irradiation develops more slowly, again because of the longer time required for the more sensitive precursor cells to reach maturity. Thrombocytes reach a minimum in about 30 days and recover in approximately 2 months, similar to the response of granulocytes.
Erythrocytes are less sensitive than the other blood cells, apparently because of their very long lifetime in the peripheral blood. Injury to these cells is not apparent for a matter of weeks. Total recovery may take 6 months to a year.
Cytogenetic Effects
A technique developed in the early 1950s contributed enormously to human genetic analysis and radiation genetics. The technique calls for a culture of human cells to be prepared and treated so that the chromosomes of each cell can be easily observed and studied. This has resulted in many observations on radiation-induced chromosome damage.
The photomicrograph shown in Figure 33-8 shows the chromosomes of a human cancer cell after radiation therapy. The many chromosome aberrations represent a high degree of damage.
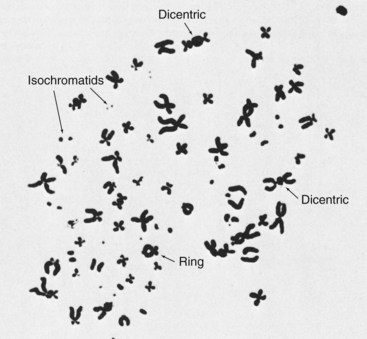
FIGURE 33-8 Chromosome damage in an irradiated human cancer cell. (Courtesy Neil Wald, University of Pittsburgh.)
Radiation cytogenetic studies have shown that nearly every type of chromosome aberration can be radiation induced and that some aberrations may be specific to radiation. The rate of induction of chromosome aberrations is related in a complex way to the radiation dose and differs among the various types of aberrations.
Attempts to measure chromosome aberrations in patients after diagnostic x-ray examination have been largely unsuccessful. However, some studies involving high-dose fluoroscopy have shown radiation-induced chromosome aberrations soon after the examination was performed.
Without question, high doses of radiation cause chromosome aberrations. Low doses no doubt also do so, but it is technically difficult to observe aberrations at doses that are less than approximately 100 mGyt (10 rad). An even more difficult task is to identify the link between radiation-induced chromosome aberrations and latent illness or disease.
When the body is irradiated, all cells can sustain cytogenetic damage. Such damage is classified here as an early response to radiation because, if the cell survives, the damage is manifested during the next mitosis after the radiation exposure.
Human peripheral lymphocytes are most often used for cytogenetic analysis, and these lymphocytes do not move into mitosis until stimulated in vitro by an appropriate laboratory technique.
Cytogenetic damage to the stem cells is sustained immediately but may not be manifested for the considerable time required for that stem cell to reach maturity as a circulating lymphocyte.
Although chromosome damage occurs at the time of irradiation, it can be months and even years before the damage is measured. For this reason, chromosome abnormalities in circulating lymphocytes persist in some workers who were irradiated in industrial accidents 20 years ago.
Normal Karyotype
The human chromosome consists of many long strings of DNA mixed with a protein and folded back on itself many times. Refer to Figure 29-11, which shows a normal chromosome as it would appear in the G1 phase of the cell cycle when only two chromatids are present and in the G2 phase of the cell cycle after DNA replication. The chromosome structure of four chromatids represented for the G2 phase is that which is visualized in the metaphase portion of mitosis.
For certain types of cytogenetic analysis of chromosomes, photographs are taken and enlarged so that each chromosome can be cut out like a paper doll and paired with its sister into a chromosome map, which is called a karyotype (Figure 33-9).
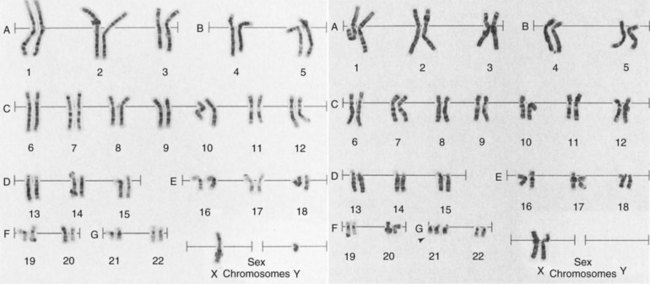
FIGURE 33-9 A photomicrograph of the human cell nucleus at metaphase shows each chromosome distinctly. The karyotype is made by cutting and pasting each chromosome similar to paper dolls and aligning them largest to smallest. The left karyotype is male, and the right is female. (Courtesy Carolyn Caskey Goodner, Identigene, Inc.)
Structural radiation damage to individual chromosomes can be visualized without constructing a karyotype. These are the single- and double-hit chromosome aberrations. Reciprocal translocations require a karyotype for detection. Point genetic mutations are undetectable even with karyotype construction.
Single-Hit Chromosome Aberrations
When radiation interacts with chromosomes, the interaction can occur through direct or indirect effect. In either mode, these interactions result in a hit. The hit, however, is somewhat different from the hit described previously in radiation interaction with DNA.
The DNA hit results in an invisible disruption of the molecular structure of the DNA. A chromosome hit, on the other hand, produces a visible derangement of the chromosome. Because the chromosomes contain DNA, this indicates that such a hit has disrupted many molecular bonds and has severed many chains of DNA.
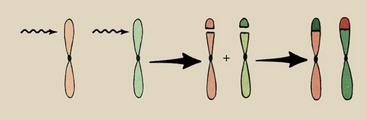
Single-hit effects produced by radiation during the G1 phase of the cell cycle are shown in Figure 33-10. The breakage of a chromatid is called chromatid deletion. During S phase, both the remaining chromosome and the deletion are replicated.
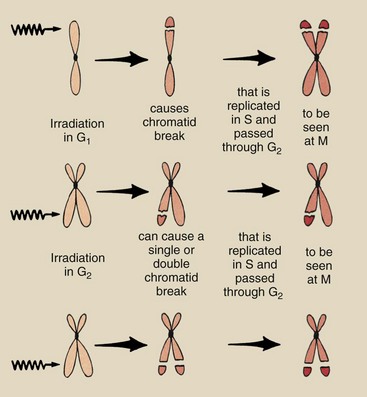
FIGURE 33-10 Single-hit chromosome aberrations after irradiation in G1 and G2. The aberrations are visualized and recorded during the M phase.
The chromosome aberration visualized at metaphase consists of a chromosome with material missing from the ends of two sister chromatids and two acentric (without a centromere) fragments. These fragments are called isochromatids.
Chromosome aberrations also can be produced by single-hit events during the G2 phase of the cell cycle (see Figure 33-10). The probability that ionizing radiation will pass through sister chromatids to produce isochromatids is low. Usually, radiation produces a chromatid deletion in only one arm of the chromosome. The result is a chromosome with an arm that is obviously missing genetic material and a chromatid fragment.
Multi-Hit Chromosome Aberrations
A single chromosome can sustain more than one hit. Multi-hit aberrations are not uncommon (Figure 33-11).
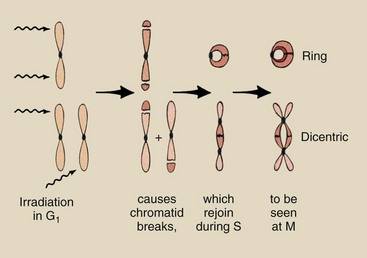
FIGURE 33-11 Multi-hit chromosome aberrations after irradiation in G1 result in ring and dicentric chromosomes in addition to chromatid fragments. Similar aberrations can be produced by irradiation during G2, but they are rarer.
In the G1 phase of the cell cycle, ring chromosomes are produced if the two hits occur on the same chromosome. Dicentrics are produced when adjacent chromosomes each sustain one hit and recombine. The mechanism for the joining of chromatids depends on a condition called stickiness that is radiation-induced and appears at the site of the severed chromosome.
Similar aberrations can be produced in the G2 phase of the cell cycle; however, such aberrations again require that (1) either the same chromosome be hit two or more times or (2) adjacent chromosomes be hit and joined together. However, these events are rare.
Reciprocal Translocations
The multi-hit chromosome aberrations previously described represent rather severe damage to the cell. At mitosis, the acentric fragments are lost or are attracted to only one of the daughter cells because they are unattached to a spindle fiber. Consequently, one or both of the daughter cells can be missing considerable genetic material.
Reciprocal translocations are multi-hit chromosome aberrations that require karyotypic analysis for detection (Figure 33-12). Radiation-induced reciprocal translocations result in no loss of genetic material, simply a rearrangement of the genes. Consequently, all or nearly all genetic codes are available; they simply may be organized in an incorrect sequence.
Kinetics of Chromosome Aberration
At very low doses of radiation, only single-hit aberrations occur. When the radiation dose exceeds approximately 1 Gyt (100 rad), the frequency of multi-hit aberrations increases more rapidly.
The general dose-response relationship for production of single- and multi-hit aberrations is shown in Figure 33-13. Single-hit aberrations are produced with a linear, nonthreshold dose-response relationship. Multi-hit aberrations are produced following a nonlinear, nonthreshold relationship. A number of investigators have experimentally characterized these relationships.
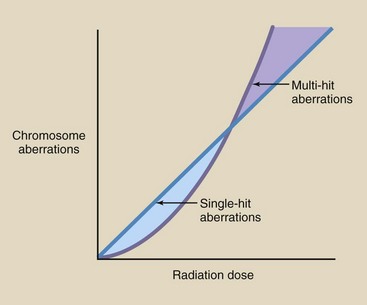
FIGURE 33-13 Dose-response relationships for single-hit aberrations are linear, nonthreshold, but those for multi-hit aberrations are nonlinear, nonthreshold.
Multi-hit aberrations are considered to be the most significant in terms of latent human damage. If the radiation dose is unknown yet is not life threatening, the approximate chromosome aberration frequency is two single-hit aberrations per 10 mGyt per 1000 cells and one multi-hit aberration per 100 mGyt per 1000 cells.
The Human Genome
After approximately 10 years of scientific investigation, in the year 2000, the human genome was mapped. This was a worldwide project involving many different laboratories. Humans have about 35,000 genes distributed along the DNA of the 46 chromosomes.
Many human health effects have now been associated with aberrations identified for specific genes and researchers are finding ways to correct these genetic defects or replace them. A wonderful example is brca1 and brca2, located on chromosomes 17 and 13, respectively, that are associated with breast cancer.
It is now possible to perform molecular genetic counseling and advise patients of their risk for breast cancer, other cancers, and other health risks. It is hoped that we will soon be able to identify radiation-induced aberrations and alert patients and radiation workers to possible future risk.
Summary
After exposure to a high radiation dose, humans can experience a response within a few days to a few weeks. This immediate response is called a deterministic effect of radiation exposure. Such early effects are deterministic because the severity of response is dose related, there is a dose threshold, and the dose-response relationship is nonlinear.
The sequence of events that follows high-dose radiation exposure leading to death within days or weeks is called the acute radiation syndrome, which includes the hematologic syndrome, the GI syndrome, and the CNS syndrome. These syndromes are dose related.
LD50/60 is the dose of radiation to the whole body in which 50% of subjects will die within 60 days. For humans, this dose is estimated at 3.5 Gyt (350 rad). As radiation dose increases, the time between exposure and death decreases.
When only part of the body is irradiated, higher doses are tolerated. Examples of local tissue damage include effects on the skin, gonads, and bone marrow. The first manifestation of radiation injury to the skin is damage to the basal cells. Resultant skin damage occurs as erythema, desquamation, or epilation.
Radiation of the male testes can result in a reduction of spermatozoa. A dose of 2 Gyt (200 rad) produces temporary infertility. A dose of 5 Gyt (500 rad) to the testes produces permanent sterility. In males as in females, the stem cell is the most radiosensitive phase.
The hemopoietic system consists of bone marrow, circulating blood, and lymphoid tissue. The principal effect of radiation on this system is fewer blood cells in the peripheral circulation. Radiation exposure decreases the numbers of all precursor cells; this reduces the number of mature cells in the circulating blood. Lymphocytes and spermatogonia are considered the most radiosensitive cells in the body.
The study of chromosome damage from radiation exposure is called cytogenetics. Chromosome damage takes on the following different forms: (1) chromatid deletion, (2) dicentric chromosome aberration, and (3) reciprocal translocations.
1. Define or otherwise identify the following:
2. What is the minimum dose that results in reddening of the skin?
3. Explain the prodromal syndrome.
4. Clinical signs and symptoms of the manifest illness stage of acute radiation lethality are classified into what three groups?
5. During which stage of the acute radiation syndrome is recovery stimulated?
6. What dose of radiation results in the GI syndrome?
7. Why does death occur with the GI syndrome?
8. Identify the cause of death from the CNS syndrome.
9. Describe the stages of gametogenesis in a female. Identify the most radiosensitive phases.
10. What cells of the hemopoietic system arise from pluripotential stem cells?
11. Discuss the maturation of basal cells in the epidermis.
12. What two cells are the most radiosensitive cells in the human body?
13. Describe the changes in mean survival time associated with increasing dose.
14. What are the approximate values of LD50/60 and SED50 in humans?
15. What are the four principal blood cell lines, and what is the function of each?
16. Diagram the mechanism for the production of a reciprocal translocation.
17. List the clinical signs and symptoms of the hematologic syndrome.
18. What mature cells form from the omnipotential stem cell?
19. If the normal incidence of single hit–type chromosome aberrations is 0.15 per 100 cells and the dose coefficient is 0.0094, how many such aberrations would be expected after a dose of 380 mGyt?
20. If the normal incidence of multi-hit chromosome aberrations is 0.082 and the dose coefficient is 0.0047, how many dicentrics per 100 cells would be expected after a whole-body dose of 160 Gyt?
The answers to the Challenge Questions can be found by logging on to our website at http://evolve.elsevier.com.