Management of cervical spine disorders
A neuro-orthopaedic perspective
Introduction
This chapter aims to provide appropriate information to allow the clinician to assess and treat common pain disorders affecting the cervical spine by using a solid clinical reasoning approach. These disorders include whiplash-associated disorder (WAD), headache and cervical nerve root lesion. The epidemiology will be discussed within a bio-psychosocial paradigm demonstrating how features of each individual's pain experience can then be identified in clinical interview and physical examination. Treatment will then be considered with reference to the disorders above.
Epidemiology of neck, head and facial pain
The incidence of neck pain varies significantly from fairly rare diagnoses of disc herniation with radiculopathy (0.055 per 1000 persons) to more common self-reported neck pain (213 per 1000 persons). From 30 to 50% of neck pain sufferers report ongoing symptoms up to 12 months after onset with an increased prevalence among women, peaking in middle age (Hogg-Johnson et al. 2009). This indicates that we should be more effective with our treatment and consider why such a large proportion of people have ongoing pain.
Localized neck pain appears to be fairly rare and neck pain is almost always reported as part of either regional or widespread pain states (Natvig et al. 2010). Those suffering with neck pain as part of a more widespread pain state are also more likely to have concurrent reduction of function. This suggests that the clinician should maintain a global or holistic perspective within their treatment approach.
In WAD, up to 50% of people have ongoing neck pain 1 year after the accident. In terms of the risk factors following injury, greater initial pain, more symptoms and greater initial disability predicted slower recovery. There are very few prognostic factors, however, that actually relate to the collision. Psychological factors such as passive coping style, depressed mood and fear of movement all relate to slower or less complete recovery (Carroll et al. 2009a).
A large variation exists in the prevalence of neck pain in a working population with 27.1% reporting symptoms in Norway and 47.8% in Quebec. In Quebec between 11 and 14.1% of workers had to limit their activities as a result of their neck pain (Côté et al. 2009). Recovery is not influenced by physical demands from the job or other workplace characteristics. Those with a poorer prognosis were shown to: have little influence on their work situation; were blue collar workers as opposed to white collar workers; or had experienced prior neck pain or had taken previous sick leave, again pointing to the need for the practitioner to maintain a bio-psychosocial perspective and truly understand the individual (Carroll et al. 2009b).
Interestingly, no prevention strategies aimed at changing workstation set-up or ergonomics have been shown to reduce the incidence of neck pain in workers (Côté et al. 2009). There are, however, psychological protective factors. Having a job which allows for decision-making and where there is empowering leadership helps to reduce the incidence of neck pain (Christensen & Knardahl 2010). This health focused or salutogenic approach (Antonovsky 1996) considers the positive attributes in the person with neck pain and fits well with a collaborative approach that a clinician and their patient can utilize.
There is a prevalence of neck pain in people with metabolic syndrome (Mäntyselkä et al. 2010) and it is unsurprising that around 34% of people with head and neck cancer experience neuropathic pain, breakthrough pain and pain of non-malignant origin in the neck region (Williams et al. 2010). Clinicians should maintain strong clinical reasoning skills to incorporate this information and understand the multifactorial components of a pain state at all times (see Chapter 2).
Common syndromes of the cervical region and their presentations
Whiplash-associated disorders (WAD)
WAD are a common and sometimes disabling condition as a consequence, generally, of a motor vehicle accident. These conditions may be seen in the acute or chronic stages with many variations between. The full spectrum of physical and psychological impairments is given in the proposed adaptation of the Quebec Task force (QTF) classification (Sterling 2004). Jull and colleagues (2008) suggest that WAD is one of the most controversial musculoskeletal conditions, due to its physical and psychological complexity and that precise patho-anatomical diagnosis is commonly not available even with current imaging techniques.
Symptoms occur predominately in the posterior region of the neck, but may radiate to the head, shoulder, arm, interscapular and lumbar region (Barnsley et al. 1998). Headache, dizziness, loss of balance, visual disturbance, paraesthesia, anaesthesia and cognitive disturbance are common (Treleaven et al. 2003). Hypersensitivity is also a familiar symptom and can be present in a local form suggesting a relationship to nociceptive input, or over widespread body sites when the central nervous system (CNS) is implicated (Sterling et al. 2003a).
Headache
Headache is a common complaint. There are many proposed causes (The International Headache Society (IHS) 2004). It may be a primary disorder such as migraine, tension type or cluster type, or cervicogenic headache, referred to as a secondary kind of disturbance.
The main origins of cervicogenic headache are thought to be patho-anatomical and pathophysiological events occurring in the neuromusculoskeletal structures, but there may be considerable overlap with the primary types of headaches (Vincent 2011). Central nervous system sensitivity also plays an important role in all types of headache pain. In the differentiation procedure cervicogenic headache is usually unilateral and side-consistent while migraine can change sides within or between attacks (HIS 2004).
Cervical nerve root lesion
Cervical nerve root injuries are a common problem particularly in contact sports where they may be attributed to the ‘stinger’ or burner injury (Safran 2004). The mechanism of injury is considered to be tensile or compressive forces acting on either the nerve root or brachial plexus (Standaert & Herring 2009). As a result of this type of injury, there can be partial or total loss of motor, sensory and autonomic functions of the damaged nerves (Navarro et al. 2007). This means that this type of problem may present with varied symptomology.
It was commonly thought that in nerve root lesions causing radiculopathy, the underlying problem was due to compressive forces acting on the nerve root through disc herniation or foraminal stenosis (Levitz et al. 1997). Although this can happen it appears that the presence of new disruption is likely in only a small percentage of people suffering with radicular symptoms (Caragee et al. 2006). More recently it has been shown that a chemical influence may be more critical in the development of these symptoms (Winkelstein & DeLeo 2004), whereby the nerves become highly sensitized due to a local immune response (e.g. inflammation).
Clinical reasoning and the bio-psychosocial model
It is obvious from the epidemiological information on neck pain that the clinician needs to consider a broader reasoning approach than the traditional biomedical model will allow. Although the biomedical model is not wrong, as a paradigm it is insufficient to fully understand each person's neck pain experience (as shown in the differing epidemiology of neck pain). This is due to the differences that exist in genetic make-up, previous experiences, cultural backgrounds and socioeconomic situations, to name a few. The bio-psychosocial model takes on board all of this information, allowing the clinician to synthesize information from many different areas in a person's pain experience. The bio-psychosocial model requires that we consider the different interacting variables across biological, psychological and social domains (Engel 1978). This will allow some appreciation of the variety of responses to treatment of what may seem similar pathophysiology.
Using the bio-psychosocial paradigm to clinically reason requires the consideration of interactions of systems at many levels. For example, a person who experiences ongoing headache may notice a change in their mood. This could be as a result of the availability of one of the chemicals acting in their CNS, such as serotonin. The change in mood may affect how they function in society. They may avoid going out or it may affect the dynamics of their family. On another level the change in serotonin availability may have an effect on their overall sensitivity, exhibited by increased sensitivity to different external stimuli like bright lights or loud noises. This demonstrates how relatively small changes at one system level can have repercussions at many other levels. A more holistic view of our patients and identification of the effect of their illness behaviours ties into the current appreciation of the WHO International classification of functioning, disability and health. This allows the integration of the knowledge that all biological systems and every system level are functionally interrelated in a hierarchical continuum, i.e. from microscopic to macroscopic and beyond.
To apply clinical reasoning to the patient on an individual basis requires a depth of knowledge and skills beyond anatomy, biomechanics and tissue healing. It should embrace knowledge from other models such as a current understanding of pain mechanisms and with that the mature organism model (Gifford 1998; Fig. 4.1) and the neuromatrix paradigm (Melzack 1989, 1990). In addition, understanding of psychology models would be beneficial. The fear-avoidance models have value in understanding relevant thoughts and beliefs in ongoing pain states (Vlaeyen & Linton 2000) and ties in with the idea of both education and the notion of graded exposure, which is often applied by movement therapists in rehabilitation. Information from models such as the evolutionary biology model may enhance the thought process for both the clinician and patient. An example of the clinical reasoning approach for WAD is given in Box 4.1.
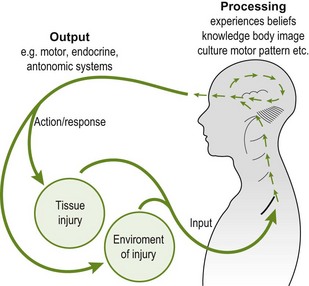
Figure 4.1 The mature organism model (Gifford 1998), showing the input, processing and output domains and interactions in a circular model.
Working with an underlying conceptual model can guide treatment by allowing the synthesis of information and evidence into a unifying principle. This helps provide some understanding of the different enigmatic pain situations such as pain in the absence of nociception; the absence of pain in the presence of tissue damage; variability and unpredictability of individual responsiveness to identical treatments; and the lack of predictable relationships between pain, impairment and disability. Understanding more fully the relationship of interacting variables from biological, psychological and social domains should allow a more effective clinical diagnosis and appropriate treatment plan and intervention (Smart et al. 2008).
Despite several guidelines published for the management of different neck disorders it is necessary in the clinic for the clinician to apply these in the context of the individual and their current presentation by using high level reasoning, evaluation and therapeutic skills (Jull 2009). By recognizing the heterogeneity of our patients alternate pathways can be created to understand how best to individualize intervention. This may be achieved by grouping individuals into classification systems such as recognition of bio-psychosocial variables or through the development of clinical predication rules for certain conditions (Beneciuk et al. 2009a). Despite progress in the research of these scientific principles it is important that the therapist maintains a high level of independent reasoning regarding each individual presenting to them.
A definition for pain
Pain has been described as ‘an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms as such’ (Merskey & Bogduk 1994). However, with continuing understanding of pain, this definition could be considered insufficient to relate the full extent of what a neck pain experience encompasses. As a pain experience always involves the brain, it is necessary that clinicians and the general public become aware and are ready to accept that. A newer working definition that should allow this transition in understanding has been proposed, stating:
Pain is a multiple system output activated by an individual pain neuromatrix. This pain neuromatrix is activated whenever the brain considers that the tissues are in danger and action is required…and that pain is allocated an anatomical reference in the virtual body.
Therefore, pain is a response produced by the neuromatrix as a consequence of potential or perceived threats to the individual's tissues. This means that the different brain areas (and other parts of the neuroimmune system) that communicate together to process this information subsequently create a response that includes the conscious perception of pain. Pain is not the only output from the neuromatrix; there will be changes in activity of other response systems. For example, there may be changes in the motor system seen through altered movements or in the sympathetic nervous system with abnormal sweating or increased heart rate. Many of these changes in the response systems or deviations from the expected norms can be picked up through a comprehensive assessment – both subjective and objective.
Moseley's pain definition also includes an understanding that brain changes have been measured in different pain states, such as in the primary somatosensory cortex (e.g. Flor et al. 1997a). These changes may be reflected in an alteration of the body's representation of the symptomatic body part in the brain (i.e. a change in the virtual body). Therefore, if we looked at the brain of someone with neck pain we are likely to find changes in many areas that represent the neck and neck movements. These changes in representation of the virtual body may also correlate with certain assessment techniques.
Pain mechanisms
Pain mechanisms have been categorized to allow a better understanding of the neurobiological changes responsible for generating or maintaining a pain experience (Gifford & Butler 1997). Through the recognition of different signs and symptoms present during clinical interview and physical examination and the use of clinical reasoning skills the clinician should be able to pick up the dominant pain mechanisms (Smart et al. 2010). These include:
It is important to understand some of the basic neurophysiology underpinning these categories in order to recognize them in your patient. As suggested in the known epidemiology and presentations of the common cervical problems, there may be varying elements of any/all pain mechanisms in each individual.
Placing pain mechanisms into a reasoning framework
The mature organism model (Gifford 1998), sometimes referred to as ‘the circular model’ (Butler 2000), allows integration of pain mechanisms into an understandable framework. It describes the continuous processing of information that allows us to be comfortable at any time and in any given environment. The model consists of multiple sensory inputs into the central nervous system. The CNS then processes this incoming information. Finally, there is an output or response created by the brain that may include changes in homeostatic regulation such as subtle changes in hormone levels or maybe the perception of pain. This correlates with the working definition of pain proposed by Moseley (2003), which incorporates the neuromatrix paradigm that will be discussed later.
Pain may be initiated and often maintained in any or all components of the model, i.e. input, processing or output (Box 4.2). Ongoing input from an unhealthy nerve, changes in thought processes regarding movement, or the response to circulating hormones can all contribute in maintaining a pain experience. This model is helpful for clinical reasoning and subsequent therapeutic education. It can be used as a tool to emphasize the different areas of a problem that need addressing and highlights where a particular treatment technique may specifically act.
Input dominant mechanisms
Nociception is essentially the processing of noxious or dangerous stimuli, be it intense heat, strong mechanical pressure or chemicals in the local tissues. This is partly due to the transduction of those different types of stimuli by specific ion channels and receptors found on the end of peripheral nerves in our tissues (nociceptors). These nerves are generally known as nociceptive neurons due to their ability to pick up the noxious stimuli (Bear et al. 2001). By processing this potentially damaging stimuli and communicating this information (transmission) to the brain, nociceptive neurons allow some defense against threatening inputs to our systems (Woolf & Ma 2007). It is the brain that ultimately decides whether it is necessary to create a pain experience from this information – nociceptive pain. It is useful to remember that pain is often not an end product of nociception.
Types of nociceptive neurons
Sensory stimulation is communicated by a range of afferent neurons: Aβ, Aδ and C fibres. Noxious stimuli is generally transmitted on the slow conducting, relatively unmyelinated C fibres and thinly myelinated Aδ fibres (Woolf & Ma 2007), although some larger myelinated and faster conducting Aβ fibres may also act as nociceptive neurons. It may be beneficial to know the different classes and their relative responses to chemical mediators and growth factors and their central connectivity (Snider & McMahon 1998).
Location of nociceptive neurons
Nociceptive neurons are present in most body tissues, including skin, muscle, joints, internal organs, blood vessels and nerve connective tissue. They are most notably absent in the brain itself, except the meninges (the connective tissues of the central nervous system).
Activation of nociceptive neurons
Nociceptive neurons are activated by stimuli that have the potential to cause tissue damage. Tissue damage can result from strong mechanical stimulation, extremes of temperature, oxygen deprivation and exposure to certain chemicals. The membrane of nociceptive neurons contains ion channels and receptors (nociceptors) that are activated by these types of stimuli.
Nociceptors and nociception
Potentially damaging extremes of heat are picked up by different nociceptors located at the nerve endings (Dhaka et al. 2006). The most common being the TRPV1 channel, which under normal circumstances picks up noxious heat temperatures above 43°C and is responsible for the feelings brought about by touching chili peppers!
For conduction of an electrical impulse to occur there must be sufficient nociceptors opened through the stimulation, e.g. heat, to allow enough positively charged ions to flow into the nerve cell, thus causing depolarization and the creation of an electrochemical message, the action potential or, in the case of nociception, a danger message. Greater stimulation of a nociceptor causes greater intensity of its firing. Ultimately, nociception could be referred to as danger signalling or messaging.
Speed of messaging
The action potentials (nociception or danger messages) bring relative information towards the CNS about the site and intensity of the stimulus. Aδ and C fibres conduct to the CNS at different speeds due to the differences in myelination. It is usually considered that there are two distinct sensory perceptions when creating nociception (e.g. when stubbing your toe very hard): a fast, sharp, first pain caused by activation of Aδ fibres followed by a duller, longer-lasting second pain due to activation of C fibres (Costigan et al. 2009). See Table 4.1 for a summary of the features of nerve fibres.
Transmission of messages via second order neurons
Primary afferent neurons (including the peripheral nociceptive neurons) transmit electrochemical impulses towards the dorsal horn of the spinal cord where they synapse with second order neurons. These second order neurons may be either nociceptive specific neurons or wide dynamic range neurons depending on the lamina that they synapse at and communicate nociceptive or non-nociceptive messages, respectively.
There are many ascending pathways that contribute to the messaging of nociceptive information that ultimately project to cortical and subcortical regions (Almeida et al. 2004). There is huge complexity of neuroanatomy that helps to form the sensory-discriminatory part of perception.
Mechanical nociception
With sufficient pressure or stretch, local mechanoreceptors will be activated causing nociception. It is not clear yet what the exact high threshold mechanotransducers are, although it is clear that noxious mechanical stimuli can activate nociceptors (Hu et al. 2006). Under normal circumstances when you stub your toe the nociceptors will be activated and send a message to the brain via second order nociceptive specific neurons. Many routine clinical tests may create mechanical nociception, especially where overpressure is used to assess joint range or stability.
Ischaemic nociception
Following sustained postures there will be local changes in oxygenation of the tissues. This ultimately leads to a rise in hydrogen ions that are picked up by local acid sensing ion channels and TRPV1 receptors. These are found on the peripheral terminals of nociceptive neurons and if sufficiently stimulated will create nociception. This is often enough to motivate a change in behaviour without being aware of it, i.e. move. The motivation to move and promote blood flow and normalization of tissue oxygenation will reduce the ischaemic nociceptive process. For someone with neck pain that is exacerbated by maintaining prolonged postures, for example at their computer workstation, they can be easily helped by recommending regular movement, which will reduce the nociception caused by the ischaemia.
Inflammatory nociception
Following injury to tissues there will be an immune-mediated healing response. Local immune cells, macrophages, neutrophils and mast cells are excited and move into the damaged area. They release immune-signalling chemicals like tumour necrosis factor-α and interleukin-1β. Some of the immediate effects of the immune system will cause the blood vessels to become leakier and allow more immune cells to access the damaged area. They cause mast cells to break down releasing histamine, bradykinin, ATP and various proinflammatory cytokines (Costigan et al. 2009). This ultimately means that there are numerous chemicals circulating in the damaged area. This is commonly referred to as an inflammatory soup.
The various chemicals that make up the inflammatory soup activate their specific receptors on the nerve endings causing nociception. There are other cellular effects that include the alteration in the ion channel type, number and kinetics. This can create sensitivity in the nerves supplying the damaged tissue. This phenomenon is called primary hyperalgesia. Now less stimulation is required to activate the neuron (Juhl et al. 2008), i.e. the damaged area becomes more sensitive. This can be seen when jumping in a warm shower when you are sun-burned. It feels excessively hot on the damaged/burned area! This is likely to be due to the lowering of the heat threshold necessary to activate the TRPV1 receptors on the ends of the sensitized nociceptive neurons.
Along with changes to sensitivity in the damaged tissues (primary hyperalgesia) there will also be an increase in sensitivity outside of the damaged area. This is known as secondary hyperalgesia. This indicates changes in CNS processing (Huang et al. 2006, Woolf & Salter 2000). If you examined sensibility to pin-prick examination in these areas it would be perceived as much more intense even though there is no underlying tissue damage.
Neurogenic contributions to inflammation: When the peripheral terminal of a particular C fibre, the peptidergic C fibre, is excited it creates an axon reflex. This is essentially a spread of activity (transmission of impulses) to neighbouring nerve endings. There is subsequent release of chemicals into the tissues, known as neurogenic inflammation. Neurogenic inflammation adds to the immune-mediated inflammatory process. It can sometimes be seen as redness and swelling away from the damaged area, in an innervation field for the excited nerve. There are obviously implications to ongoing tissue health if neurogenic inflammation persists. (See Butler (2000) for a more in-depth summary of these events.)
Summary of clinical patterns from inflammation:
Box 4.3 summarizes the nociceptive patterns occurring in WAD.
Clinical detection of nociceptive mechanisms
Nociceptive pain has generally been considered to have a fairly close stimulus/response relationship such that aggravating/easing factors are reasonably well defined and testable on examination. It is considered that pain localized to the area of injury or dysfunction, localized pain on palpation and antalgic postures or abnormal movement patterns are more predictive of a dominant nociceptive pain process (Smart et al. 2010).
It is important to remember that nociception is not sufficient for a pain experience. This was suggested with ischaemic nociception due to sustained postures, where nociception often occurs without ever coming into our consciousness.
Pain associated with changes in the nervous system
The definition of neuropathic pain is controversial (Bennett 2003). For our purpose peripheral neuropathic pain is the term used to describe situations where pain originates with an identified lesion of the peripheral somatosensory system, including the nerve roots or peripheral nerve trunks (Merskey & Bogduk 1994).
Nee & Butler (2006) described the clinical manifestation of peripheral neuropathic pain in terms of positive and negative symptoms. Positive symptoms reflect an abnormal level of excitability in the nervous system including pain, paraesthesia, dysaesthesia and spasm. Negative symptoms indicate reduced impulse conduction in neural tissues and include hypoesthesia, anaesthesia and weakness.
Neuropathic pain is typically described as a deep, burning or aching and has been attributed to an increase in the sensitivity of the nervous system. Dysaesthesic symptoms are also often reported with more unfamiliar or abnormal sensations such as tingling or crawling (Asbury & Fields 1984).
Nerves aren't normally that sensitive
Nerves are designed to move, slide and glide. They are happy to be squeezed and stretched a bit. They can also cope with a lack of blood supply for short periods without complaint. This is mostly the same for the nerve root complex, which will be irritated (i.e. evoke nociception) by a lack of blood – ischaemia – but will generally not cause any lasting symptoms. This is certainly not the case in the presence of local inflammation. In this instance there will be increased responsiveness of the peripheral nerves and in particular the nerve root complex to movements and ischaemic changes (e.g. Dilley et al. 2005). This means that moving and placing pressure through the excited nerves will more easily cause nociception. The irritated nerve root complex will also create a greater intensity of firing or nociception. It is, therefore, unsurprising that this can cause pain out of all proportions to the lesion or injury.
Injuries to peripheral nerves
Injuries to the peripheral nerves cause changes in both the injured and the uninjured axons. These changes take place not only where the nerve has been injured but also along the axon of the nerve, at the central terminals, dorsal root ganglion and higher centers (Costigan et al. 2009).
There are many changes that underpin a pain state following injury to a nerve. These are mediated by the immune system (Thacker et al. 2007). Assuming that there is no loss of conduction there will ultimately be an increase in excitability and a reduction in the ability to control this (i.e. a loss of descending modulation) – essentially the brain wants to know what is happening and therefore arranges to receive more messages that are also much louder.
One important neurophysiological change present in nerve injury is the development of ectopic impulse generating sites. These are areas where ion channels insert into portions of the axon membrane devoid of myelin. Myelin is broken down during injury and results in different types and amount of channels and receptors being inserted into the membrane. This allows the generation of messages to many different kinds of stimuli in areas that do not normally have this ability, hence ectopic (Devor & Seltzer 1999). Therefore, these ‘hot spots’ may now signal nociception from areas along the nerve as opposed to just the peripheral terminals.
Ectopic impulse generating sites have been consistently shown to be present not only in nerve injury but following inflammation within the nerve. They are part of the neurophysiology that causes the nerve to become mechanosensitive (Dilley et al. 2005) and may be picked up during nerve palpation or movement testing such as with neurodynamics. Ectopic impulse generating sites can be present along the length of the axon following nerve injury and are in part dependent on the health of the nerve and the local axoplasmic flow (Dilley & Bove 2008).
Blood flow
Nerves are bloodthirsty. A pressure gradient exists around and in neural tissue to ensure the maintenance of adequate nutrition. The gradient must exist so that blood can flow into neural tissue and then out of the tunnel surrounding the nerve. This can obviously be perturbed (e.g. through inflammation, scar tissue or an overactive muscle) and as such the clinician may want to evaluate the state of the surrounding tissues in order to understand the impact to the local blood flow.
The dimensions of neural tunnels can diminish from encroachment of surrounding structures. In the example of the cervical intervertebral foramina, the extraneural narrowing of the foramina can result from swelling of the facet joints, protrusion or degeneration of the intervertebral disc, abnormal posture producing a ‘closing’ effect on the foramina, such as the ‘poking chin’ position where the upper cervical spine is kept in extension. Intraneural swelling will also reduce the space available for unhindered neural movement and blood flow.
Axoplasmic flow
Axoplasmic flow (Fig. 4.2) is the mechanism that allows cell components (e.g. ion channels, neurotransmitters held in pouches and mitochondria) to be transported to their functional sites and returned to the nerve cell for re-cycling. As with blood flow, unhindered axoplasmic flow is critical for nerve health (Delcomyn 1998). The thixotropic properties of axoplasm require regular bodily movements to maintain the flow. If there is insufficient movement then the axoplasm becomes thicker and the flow rate slows down, which will ultimately have an effect on the speed of recovery from nerve damage. This has obvious clinical implications i.e. the need to maintain some movement of the nervous system to maintain normal blood and axoplasmic flow.
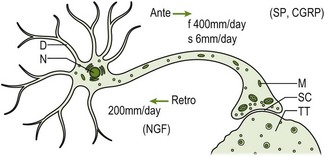
Figure 4.2 Axoplasmic transport system: diagram. Axoplasmic transport system within a single neuron. D: dendrite; N: nucleus; M: mitochondria; SC: synaptic cleft; TT: target tissue; NGF: nerve growth factor; SP: substance; CGRP: calcitonin gene-related peptide (adapted from Butler 1991, p. 25 with permission).
Clinical detection of peripheral neuropathic pain
Clinical tools such as the PainDETECT questionnaire and the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) have been found to be beneficial in helping to diagnose peripheral neuropathic pain (Bennett et al. 2007). These highlight the differences in the quality and behaviour of pain and dysesthesias as well as the signs and symptoms of allodynia, hyperalgesia and hyperpathia. However, other clinical signs have been suggested such as pain referred in a dermatomal/cutaneous distribution, pain/symptom provocation associated with subjective aggravating/easing factors and clinical tests associated with disturbance of neural tissue (e.g. neurodynamic testing) and pain/symptom provocation on palpation of relevant neural tissues (Smart et al. 2010).
Centrally mediated mechanisms, such as the development of central sensitization or representational changes in the brain, are also important in the generation and maintenance of neuropathic pain.
Centrally mediated mechanisms
One consequence of both inflammation and nerve injury is the onset of the phenomenon central sensitization. This explains some of the common clinical signs that are present in cervical conditions such as the presence of allodynia (pain on normally non-painful stimulation) and secondary hyperalgesia (increased responsiveness to a normally painful stimulus outside of the original area of injury). If the clinician is able to pick up on this information then it will not only help to guide their treatment but minimize negative responses to treatment.
Central sensitization was proposed as a model to describe the different responses found to noxious stimulation in animals that had received prior noxious stimulation (Woolf 1994). It appeared they were more sensitive after repetitive testing and therefore the term sensitization was adopted to describe this phenomenon. The primary focus was originally of changes in the spinal cord but the same changes are known to occur in higher centers too.
The changes brought about by ongoing nociceptive stimulation include a lowering of the activation threshold at the pre- and post-synaptic membranes (i.e. primary afferent and second order neurons) at the dorsal horn, reduced descending inhibitory modulation and an increase in the descending facilitation. Ultimately this leads to an increase in the response to normal stimuli or an amplification of the signaling in the nociceptive system (Latremoliere & Woolf 2009). Therefore, the brain is receiving louder/more intense messages to normal stimulation, as illustrated by the case in Box 4.4.
From a clinical point of view this means that normal movement can become painful even when there is no damage to the particular area. Also, neurosensory testing will likely reveal changes to light touch and pin-prick examination. Other proposed signs and symptoms are that the pain is diffuse, there is a distortion in the stimulus-response characteristics, spontaneous pain and pain associated with emotional and cognitive change. Also, previous failed interventions and tenderness on palpation are more signs of the presence of central sensitization (Smart et al. 2010).
Central sensitization has been found to be present in headache, WAD and neuropathic pain and musculoskeletal disorders with the presence of generalized pain hypersensitivity. It is also a phenomenon closely linked to irritable bowel disorder, depressive symptoms, chronic fatigue and joint pains (Woolf 2011). This suggests that picking up on these co-morbidities during the clinical interview will also act as guidance towards the diagnosis of the underlying pain mechanism.
The brain and pain
The CNS is the ultimate representational device. It has the ability to represent the whole body, embracing anatomy, physiology, movements, emotions and diseases. An understanding of pain and the role of the CNS in terms of the body-self neuromatrix have been proposed (Melzack 1990). The neuromatrix can be considered as a vast, interconnecting, highly flexible, plastic network of groups of neurons in the brain activated and sculpted by any and every lifetime activity and experience.
Essentially there is input into the body-self neuromatrix from sensory, cognitive and emotional influences. The consequent output from the brain is commonly called the neurosignature and involves firing in and between many different and individual brain areas. This subsequent neurosignature, also called a neurotag (Butler & Moseley 2003), includes changes in the regulation of many homeostatic, behavioural and perceptual systems. This fits with our current understanding of pain that considers pain to be a multiple output response (Moseley 2003) and the mature organism model that has been used to incorporate pain mechanisms (Gifford 1998).
Imaging studies demonstrates there is no single ‘pain centre’. Many areas alight almost simultaneously during a pain experience (Tracey & Bushnell 2009) and wide variability exists within and between individuals (Ingvar 1999). See Box 4.5 for an example of the brain areas that make up the neuromatrix in an acute nociceptive experience.
Brain changes in pain
Recent studies have shown that there may be several distinct changes in the brain during a pain state. These include:
• Changes in the brain's representation of the body e.g. in the primary somatomotor (Tsao et al. 2008) and somatosensory cortices (Flor et al. 1997a)
• Decreased areas of gray-matter in different cortical areas e.g. dorsolateral prefrontal cortex (Apkarian et al. 2004)
• Altered resting brain dynamics e.g. changes in the default mode network (Baliki et al. 2006)
• Altered levels of neurotransmitters and/or receptors, e.g. in the brainstem in descending modulation (D'Mello & Dickenson 2008)
• Changes in immune activity, e.g. ongoing activation of microglia in the thalamus in neuropathic pain (Banati et al. 2001).
The changes expected as being present in neck pain, for instance in the somatosensory cortex, may be picked up through careful assessment. It may be that the patient describes an inability to imagine the symptomatic part clearly, which may reflect in a change in the representation of the neck. Other clinical tools such as two-point discrimination, left/right discrimination tasks or reasoning tasks such as the Iowa Gambling Task may become important in understanding what underlying brain changes are present and will give the clinician objective markers to use during treatment to document the progression of these changes (Box 4.6).
Other clinical changes such as an alteration in movement can be picked up through careful assessment or are often noticeable while just observing the patient. These may be related to both changes in the motor representations and alterations at spinal and tissue levels. More changes in the brain's output include altered mood, concentration, emotion, thoughts and activity of the stress systems. Each pain experience will be individual and as such the level of involvement of each of the response systems will vary widely both between individuals and as a pain state progresses.
Mirror neurons and context change
Mirror neurons are a huge revelation in terms of our understanding of the development of language, imitation and learning and are likely to be important in the assessment and treatment of our patient in the clinic. Essentially they are neurons that fire to both the observation of movement (i.e. mirror the movement) and to the execution of the same movement (Rizzolatti et al. 2001). Picking up changes in mood or movement may feel natural to some clinicians and this is likely to be a result of the activation of our mirror neuron system.
Different populations of neurons represent the same movement depending on the context that the movement is performed in or the desired goal of that movement (Iacoboni & Mazziotta 2007). This means that if a particular movement activates a pain neurotag then it could be possible that the same movement performed in a different context, and therefore activating a different population of neurons within the neuromatrix, will not activate a pain neurotag. In this case the brain is running the same movement but without creating a pain experience. Box 4.7 gives examples of how to change the context in which a movement is performed.
Output mechanisms
The brain is constantly making small adjustments to the regulatory/homeostatic systems in order to keep us in maximal comfort within our environment. This is part of what we understand from the mature organism model and neuromatrix paradigm. It monitors the levels of different hormones and chemicals (interoception), thoughts, emotions, sensory inputs and perceptual inputs including pain experiences coming into the neuromatrix. The subsequent alterations in activity of the different regulatory systems (neurosignature) will be influenced by the perceived threat of the situation (Moseley 2007). If someone feels threatened, even at an unconscious level, then the brain will act by changing the activity in the different systems, affecting various physiological mechanisms and behavioural adaptive responses in order to deal with the stressor (Chrousos 2009). This may be through a change in movement, mediated through the prefrontal and motor cortices or increase in heart rate due to altered activity in the sympathetic nervous system. As mentioned previously, in a pain state there will be measurable changes in many systems that are apparent as brain changes too. The common output systems that a clinician will be considering during assessment and related to a pain experience include:
• Sympathetic nervous system (SNS)
• Parasympathetic nervous system (PNS)
Some of these will be apparent on observation of your patient, others may be more obvious during careful questioning, and others still may require specific examination.
Sympathetic nervous system
It is normal to be stressed during daily life and the SNS helps deal with these stresses. It is a fast acting system that works via two pathways: the sympatho-adrenal and sympathoneural axes.
The sympatho-adrenal axis works by activating the release of adrenaline/noradrenaline via the adrenal medulla. This results in systemic action and, as such, the effects will generally be widespread. The sympathoneural axis works as an efferent system via the peripheral nervous system to effect change locally by releasing adrenaline directly into the target tissues (including visceral organs) and so has a fast acting effect. Essentially, the SNS helps deliver blood to the necessary systems and it can be seen how mood is intimately linked with the body (see Gifford & Thacker 2002 for more in-depth analysis).
Adrenaline/noradrenaline does not cause pain in itself, but can magnify the sensitivity of alarm signals (Devor & Seltzer 1999). In chronic inflammation, nerve damage (ectopic impulse generating sites) and nerve root irritation there is an increase of ion channels picking up the local availability of circulating adrenaline (Navarro et al. 2007).
There are recent interesting developments which appear to show that it is not so much the centrally mediated changes which are controlled by the SNS but local changes in channel responses to circulating adrenaline. This means that the same amount of circulating adrenaline can have more potent effects on the tissues it supplies.
Endocrine response
Together with the SNS, the endocrine system is the other key stress response system. Sympathetic reactions are rapid and of short duration, the endocrine response can take slightly longer to respond due to the systemic, hormonal effect. Higher centres stimulate the hypothalamus (particularly during perceived threat), which releases corticotropin-releasing hormone. This in turn stimulates the pituitary gland to release the adrenocorticotropic hormone (ACTH) into the blood stream. ACTH activates the adrenal cortex to release glucocorticoids such as cortisol, a famous stress hormone, into the bloodstream.
A summary of the general actions caused by an increase in activation of the stress systems is generally summed up as a ‘fight or flight’ response (although this is a fairly simplistic view). This includes: increasing cardiovascular tone and respiration, increasing oxygenation, nutrition of the brain, heart and skeletal muscles, increased metabolism (including inhibition of reproduction and growth), facilitation of arousal, alertness, cognition and attention (Chrousos 2009). See also Box 4.8 for examples of the effects of stress response.
The general effects from the stress response systems are therefore to help liberate energy and deliver it to the areas and organs that most need them. With ongoing activation of these stress systems there is likely to be deleterious effects on the health of tissues and organs.
Parasympathetic nervous system (PNS)
The PNS is concerned with slowing and conserving energy. It helps digestion, storing of energy, cellular replenishment and reproduction (Butler & Moseley 2003). The PNS is involved in tissue healing, rest and repair.
In persistent pain states there are changes to the representation of the PNS in the brain (Thayer & Sternberg 2010) – as has been found in sensory and motor areas. This will have knock-on effects on the function of the parasympathetic nervous system and could impact on general health and the balance between the stress systems.
Breathing exercises, e.g. the Buteyko technique, will promote a longer exhalation or pause before inspiration. This will preferentially activate the PNS and could be one way of trying to actively balance the activity in the sympathetic and parasympathetic nervous system. This will have direct effects on blood pressure, heart rate and respiratory rate (Kaushik et al. 2006).
The immune system
The immune system is a powerful protective system especially in disease and trauma. It also plays an important role in persistent pain. Glial cells in the brain (e.g. astrocytes and microglia) contribute to the onset and maintenance of pain states, particularly neuropathic pain (Thacker et al. 2007). In sickness there is a clear immune/pain link (Watkins & Maier 2000).
Communicating chemicals, such as the proinflammatory cytokines interleukin-1β, interleukin-6 and tumour necrosis factor-α, collectively mediate inflammatory responses. They are also important signalling proteins between the immune and central nervous system and may mediate effects through parasympathetic ganglia on vagal afferents. It is through this signalling that the brain can promote changes in behaviours and sensitivity (such as illness/sickness behaviour) in order to best manage the current experience.
Motor system
Changes in the motor system can sometimes be seen even before your introduction to the patient. This may manifest as holding the neck in a forward position, protection of a limb, the facial expressions shown or the voice of the patient (Box 4.9).
In chronic pain states where central sensitivity is a dominant process, there will usually be unhealthy and unfit muscles, which may be a source of nociception. This may be maintained through an ongoing local immune response, altered axoplasmic flow of the nerve or a persistent axon reflex creating neurogenic inflammation creating ill-health of the tissues or from altered use and firing of the muscles.
Descending modulatory control
During a pain experience there are changes to the descending modulatory pathways. This was referred to as one of the processes responsible for central sensitization. There is tonic and phasic facilitatory and inhibitory modulation of the afferent and efferent pathways. There may be an increase in the facilitation of afferent processing and a reduction in the inhibition (disinhibition). This will cause a general amplification of the incoming signals to the brain, which will then respond accordingly (Mason 2005). A similar effect occurs in the efferent pathways, exciting the motor cells and maintaining muscle activity. This may, for example, be seen in a brisk response to reflex testing.
Some of the therapeutic methods employed by manual therapists include techniques that aim to effect a change in descending modulation (Box 4.10). This may be through traditional manual therapy techniques or appropriate education to effect a change in the facilitatory tone. These act via a top-down process, possibly through changes in thoughts or beliefs as a result of experiencing some pain relief or hearing reassuring information. By considering these components of the pain experience and the concept of affecting change directly on the outputs generated by the brain it can be helpful in guiding the therapist towards an appropriate treatment plan.
There are many other changes in the response systems including mood changes, altered cognitions and changes language to name a few but are beyond the scope of this chapter.
Examination of the cervical region
The most important element of the subjective examination is the communication between the patient and therapist. Maitland had many attributes but communication was, considered by many, to be the greatest of all. This topic is described in great detail elsewhere (e.g. Jones & Rivett 2004). The subjective examination is a great opportunity for the patient to tell their own story, in any way they choose. It is the therapist's job to listen attentively to the patient and make them feel comfortable telling their story.
Where necessary you may have to ask essential questions yet unanswered or direct the conversation (see Box 4.11 for examples). Ultimately you should remain empathetic and open. It is unwise to lead the patient along your own questioning path, as this is likely to run towards a favourite clinical hypothesis or miss valuable information.
By the end of the subjective examination the clinician should have a working hypothesis with which to guide their physical examination. It is likely that the patients will also have formulated their own hypothesis related to the questioning and it is beneficial to ask for their thoughts on this.
Planning the physical examination
Before commencing the physical examination the key points highlighted during the subjective interview must be considered:
• What will be the extent of the examination related to severity and irritability of the problem?
• Where irritability is the main factor, minimal (if any) tests will be performed with the principle desire to find easing or relieving postures, movements or other self-administered management such as medication or the application of heat. It is likely that some education will be required or a consideration of that person's current beliefs regarding their problem
• It would be counterproductive to perform a physical examination that aggravates the problem. Therefore, the physical assessment could be considered in terms of titrating the appropriate dose of assessment for your patient. It is important to understand this concept in light of the potential latency of symptoms found in neuropathic pain problems or that creating sensory input will amplify the present state of central sensitization. The patient may be fine following your assessment but their symptoms may flare up days, or even a few weeks, later, purely as a result of an excessive dose of physical assessment. Hopefully the therapist will no longer ask what the patient did to aggravate themselves under the assumption that because they were fine immediately after the assessment, it wasn't due to the assessment itself!
• The severity of the problem helps identify those people who are more likely to proceed into chronicity. This has been shown to be the case in WAD where ongoing moderate/severe symptoms correlate with changes in physical parameters and psychological distress (Sterling et al. 2006)
• Consider the minimum amount of physical examination required to provide relevant data to prove or disprove a current hypothesis of an underlying pain state, for example, neurosensory testing to confirm the presence of central sensitization. Box 4.12 outlines some considerations for physical examination of underlying pain mechanisms.
Physical examination
The aims of the physical examination are to:
1. Support or reject hypotheses identified in the subjective examination in terms of the likely underpinning pain mechanisms, e.g.:
a. recognizing the presence of a mechanical nociceptive contribution originating in the cervical spine or a local inflammatory problem
b. adaptive/maladaptive movement or behaviour due to a change in the motor output
c. a change in mechanosensitivity in the nervous system indicative of a peripheral neurogenic pain mechanism
d. the contribution of CNS sensitivity
e. consideration of other output mechanisms and their effect on the pain problem e.g. activation of the sympathetic nervous system during testing
2. Find the least provoking postures and movements
3. Look at current function and functional limitation
4. Confirm or rule out the need for caution and decide when physical intervention is inappropriate.
Starting out the physical examination
Find out whether the patient is happy to undergo a physical examination and briefly what this is likely to entail. Make sure your patient understands the importance of their feedback from the testing and that they should keep you informed regarding their responses. It is often wise to begin with symptom-free movements when possible. Try not to give them too much information as this may bias their responses. When movement is unnecessary/unwise then some form of neurological assessment will give information to reason the underlying pain mechanisms, such as an increase in sensitivity.
Observation
Observation of your patient begins during your introductions or possibly before. If they have adopted a particular posture is this to alleviate their symptoms or just normal for them (remember the role of your mirror neurons in understanding both the movement of the patient and their current emotional state)? In which case would you want to alter it? This should guide your assessment and ultimately your treatment by showing the most comfortable position.
It is sad to think how many patients have been forced to alter an antalgic posture e.g. retracting the chin in order to regain what is thought by the therapist to be an optimal posture, when they are appropriately resting in their safe/comfortable and relieving position due to an irritable nerve root lesion i.e. adopting a poking chin with some ipsilateral side flexion of the neck and elevation of the shoulder. An antalgic posture provides a huge amount of relevant information for the judicial therapist.
Functional assessment
This is an ideal time for the patient to demonstrate their main functional problems while respecting the symptoms. Ultimately this is what brought them to see you, e.g. the difficulty in turning their head whilst reversing the car. It is unnecessary to evaluate every possible movement and in some instances is likely to make your patient worse. It will also show your patient that you are interested in their problem and have listened thoroughly. It is possible to incorporate context change strategies within this part of the assessment.
Testing positions
The testing positions will relate to the need to respect the severity and irritability of the disorder but also the functional considerations. Contextual change of a movement is thought to cause different neuronal populations within the neuromatrix to fire with the consequent result being a different neurotag that no longer involves pain as one of the outputs.
Ongoing analysis of your patient and reassessment
During your patient assessment you are constantly monitoring their response. This may be a verbal response but often takes the form of non-verbal reactions such as withdrawing a limb, grimacing, an increase in sweating and colouration of the skin. All of these are important signs guiding your evaluation to assessment and firming your clinical reasoning. These clinical signs or outputs are likely to be what you monitor during reassessment/re-evaluation.
It is not necessary to reassess function immediately after treatment. Assuming you have chosen the appropriate technique there should be a positive change. The change you are most interested in is one that has been maintained beyond the first seconds following treatment. This may mean reassessing 15–20 minutes after treatment or waiting until your follow-up appointment. There are occasions when a judicial and professional examination with appropriate feedback will reduce the fear or worry of the complaint and therefore shows as a reduction of sensitivity, possibly mediated as a reduction in the descending facilitation.
Physical examination of the nervous system
Data retrieved through physical examination of the nervous system will help in refining your current hypothesis regarding your patient's current diagnosis, including an understanding of the underlying pain mechanisms (Dworkin et al. 2007). Clinicians with good manual skills and strong clinical reasoning should be able to identify pathophysiological changes (Greening & Lynn 1998). Mechanosensitivity of nervous system may be assessed through palpation of the peripheral nerves and passive and active neurodynamic testing. These can be combined with the assessment and comparison of the sensitivity and health of adjacent tissues. This will guide the clinician's understanding of possible nociceptive elements driven by neighbouring tissues, local sensitivity of the nervous system or the presence of central sensitization.
Conduction abilities of the nervous system are traditionally assessed through manual muscle, reflex and sensibility testing. It is also possible to examine cranial nerve function or the representation of the body in the brain through assessment of two-point discrimination and testing of left/right discrimination. See Box 4.13 for a brief summary of common neurological examination techniques.
Due to a lack of a gold standard test for most disorders, a battery of tests including motor and sensory elements should be the most valid way of identifying these changes (Novak & MacKinnon 2005). The next section will concentrate on palpation of the peripheral nerves and neurodynamic testing.
Palpation of peripheral nerves
Nerves are hard, rounded and, due to their outer covering of mesoneurium, feel slippery to the touch. Some nerves are visible, especially when the adjacent joint component is positioned to load the nerve. Many nerves are sufficiently prominent to allow direct palpation. The three trunks of the brachial plexus are an example of easily identifiable and superficial peripheral nerves that the clinician may decide to palpate. Abnormal or increased sensitivity is a frequent finding in this area following WAD and nerve root lesions and may require sensitive handling.
Nerves can also be palpated indirectly where muscle and fascial tissues cover them. This is possible when palpating the median nerve through the wrist flexor muscles in the forearm.
Palpation can be performed transversely over the nerve or with static pressure directly onto the nerve. This depends on the size and location of the nerve and a reasoned approach. Placing the nerve on some slight load, as with a neurodynamic test position, may allow it to become more prominent and places a greater mechanical load and pressure change through the nerve trunk. This method can also be used to differentiate a local nociceptive process from nerve trunk mechanosensitivity whereby a perceived change in response to nerve palpation with altered neurodynamic positioning is more indicative of mechanosensitivity of the nervous system than local nociceptive processes.
Response to nerve palpation
The response should be compared to the asymptomatic/less symptomatic side. Palpation in the lower limbs can be used when central sensitization is suspected or the pain problem is highly irritable or severe. This should give a wider perspective of the mechanosensitivity of the nervous system.
Normal responses to palpation of a nerve will naturally vary amongst and within individuals through the day but will also vary due to internal and external factors. The same nerve trunk may also vary slightly in its response at different sites due to the change in relative connective tissue or conductive tissue present (Butler 2000).
A relevant increased response may present as feeling more marked or may even provoke a change in behaviour, such as withdrawal of the limb, a grimace or exclamation. When the response reflects negative symptoms then the feeling is less marked or absent in comparison to the asymptomatic side.
Palpation related to peripheral neuropathic pain
There is some experimental support for nerve palpation as a diagnostic aid towards finding the primary source of a neurological lesion (Durkan 1991). Injured nerves often hurt when subjected to mechanical forces; this must be due a combination of local – for example the presence of an ectopic impulse generating site – and central changes, such as central sensitization. This mechanosensitivity can be present even without an identified nerve lesion when there is local inflammation surrounding the nerve (Dilley et al. 2005). Sensitivity to palpation must be seen as a reflection of processes throughout the nervous system, including thoughts and beliefs such as the expectation of the consequences of touching a nerve.
Palpation of the upper limb nerve trunks and brachial plexus has reasonable reliability (Jepsen et al. 2006; Schmid et al. 2009). Someone with a painful cervical radiculopathy is highly likely to have an increased response to nerve trunk palpation (Hall & Quintner 1996). Increased response to palpation of the median nerve along its tact has been documented in people with WAD (Greening et al. 2005). Unilateral migraine and tension-type headache also display mechanosensitivity to nerve palpation of the supraorbital nerve on the symptomatic side along with increased sensitivity to palpation of nerve trunks bilaterally in the upper limbs, indicating a predominance of central changes indicative of central sensitization (Fernández-de-las-Peñas et al. 2008, 2009).
Palpation of the nerves of the head, neck and upper limb
The clinician is likely to be guided by the distribution of the symptoms in terms of which nerves/nerve trunks they palpate. Headache symptoms may require palpation of the facial and occipital nerves, whereas a nerve root lesion may require assessment of the peripheral nerve trunks of the upper limb.
To understand widespread sensitivity and central sensitization is appropriate to assess palpation away from the symptomatic site such as in the lower limbs prior to assessment over the symptomatic area. This helps to give some understanding of what to expect for the patient. See Box 4.14 for an outline of clinical symptoms found in headache.
The trigeminal nerve (V)
The trigeminal nerve is the sensory supply to the face, the greater part of the scalp, the oral and nasal cavities and motor supply to the masticatory muscles. Fibres in the sensory root are mainly axons of the trigeminal (semilunar) ganglion. From the ganglion three nerves arise: the ophthalmic, maxillary and mandibular nerves.
Palpation of the trigeminal nerve is most effective where it becomes superficial as shown in Figure 4.3. It is useful to consider palpating these nerves with headache and facial pain problems.
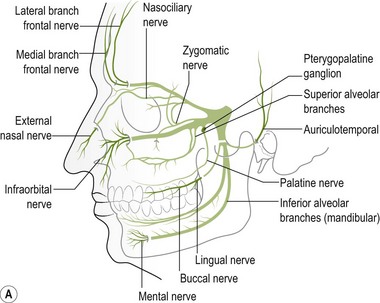


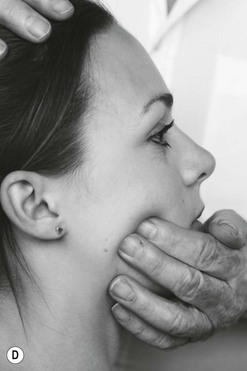

Figure 4.3 A Schematic diagram of the trigeminal nerve (reproduced from Clemente 1975 with permission). B Palpation of lacrimal nerve (a branch of the ophthalmic nerve) at the supraorbital fissure.
C Palpation of the frontal (supraorbital) nerve. D Palpation of the maxillary nerve at the infraorbital fossa. E Palpation of the mandibular nerve at the mental foramen.
The ophthalmic nerve becomes superficial through the supraorbital fissure.
The greater occipital nerve becomes superficial at the superior nuchal line lateral to the greater occipital protruberance and the lesser occipital nerve can be located more laterally. Palpation of these nerves can be considered for both headache and WAD. See Figures 4.4 and. 4.5 and Box 4.15.
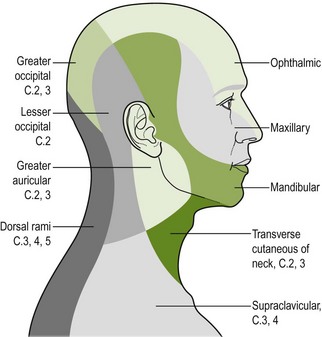
Figure 4.4 Cutaneous nerve supply of the face, scalp and neck (reproduced from Williams et al. (1989) Gray's Anatomy, 37e, with permission from Elsevier). This can provide useful guidance when performing neurosensory testing.
Palpation of the cervical nerve roots and the brachial plexus
Palpation of the intervertebral foraminal area is achieved by the identification of the pointed tubercles on the end of the transverse processes of the cervical vertebrae. The application of the classical anteroposterior unilateral vertebral pressure technique is ideal for this purpose (Fig. 4.6). The sternocleidomastoid muscle should be displaced during the procedure. The actual nerve root is difficult to identify in this location, as it lies between scalenus anterior and medius, so the principle of indirect nerve palpation may be applied.
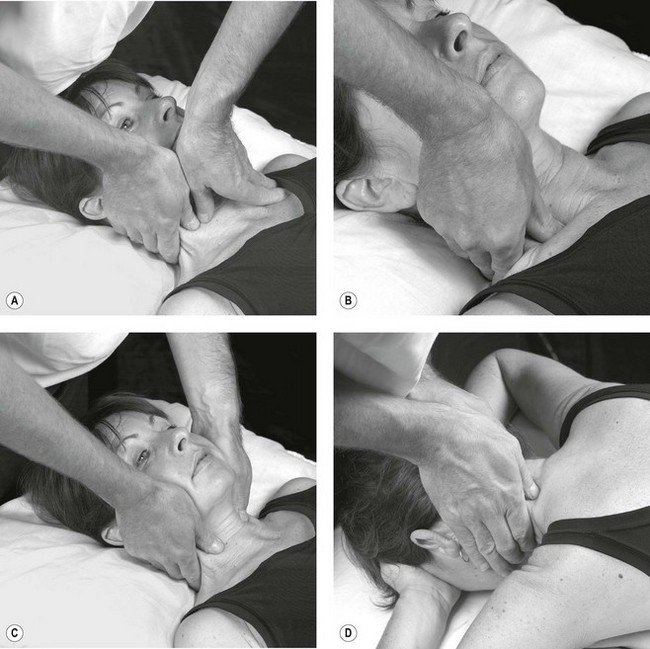
Figure 4.6 A unilateral anteroposterior vertebral pressure. B Bilateral anteroposterior vertebral pressure. C Anteroposterior unilateral vertebral pressure in upper thoracic area. D Anteroposterior unilateral vertebral pressure.
The three trunks of the brachial plexus are clearly detected between scalenus anterior and medius to where the trunks pass under the medial one-third of the clavicle. By applying contralateral lateral flexion to the neck or shoulder girdle depression the nerve trunks become more prominent (Fig. 4.7).
The cords of the brachial plexus can be discovered and palpated in the axilla as they encapsulate the axillary artery. It is therefore useful to be able to palpate pulsatile structures to help use as a guide for locating the nerves (Fig. 4.8).
Palpation of the median and ulnar nerves in the upper arm next to the brachial artery is illustrated in Figure 4.9. The median nerve lies anterior to the ulnar nerve in this situation.
Palpations of the major nerves of the upper extremity are illustrated in Figures 4.10–4.13 and are described in detail in The sensitive nervous system (Butler 2000).
Neurodynamic testing
Another technique used to assess the mechanosensitivity of the nervous system is neurodynamic testing (Dilley et al. 2005). In contrast to the assessment of the nervous system's ability to accept pressure exerted on nerves through palpation, neurodynamic testing examines mechanosensitivity to specific movements. More recently it has also been suggested that neurodynamic tests assess the representation for specific movements of body parts, i.e. cortical representation or the movement neurotag. Therefore the mechanosensitivity found during testing may be mediated peripherally or centrally or, likely, both.
There are many base tests that have been designed in order to bias an emphasis towards specific nerve tracts and movements. However, it is necessary to be able to adapt these to make the assessment individual for the patient with neck pain. This may include changes in the start position, order of movements of the test and the use of structural differentiation.
Responses to neurodynamic testing
During testing it is important to compare the range and quality of movement and compare, where possible, the symptomatic with the asymptomatic/less symptomatic side. A change in the available movement is often due not to specific restriction in the nerve but to a protective motor response, possibly brought on by the increase in mechanosensitivity to stretch and pressure (van der Heide et al. 2001).
It is important to watch for antalgic postures and subtle changes in the muscles as these may be the first overt signs which help you to understand the patient's willingness to be moved. This may include a slight withdrawal of the limb but is mostly a noticeable increase in resistance to the movement.
Other signs that may be apparent are the reproduction of symptoms, which needs to be monitored carefully and should be noted after the test. Be open with your questioning and allow your patient to inform you of the response and the area of symptoms. This will allow some comparison with what you know to be normal. The aim is not always to reproduce pain, especially for someone with more severe or irritable symptoms. In this case, neurodynamic testing may be used to explore relieving positions and allow the patient to have some control over their problem. This works especially well during a functional assessment.
Using structural differentiation in neurodynamics
A change in response to structural differentiation, i.e. an addition or subtraction of a joint component away from the symptomatic area, can help support or refute a clinical diagnosis. It is thought that a change in response, whether increased or decreased, reflects a change in the load through the nerve and subsequent mechanosensitivity (Coppieters et al. 2005). This should be used as a guide and not by itself to confirm the presence of peripheral neuropathic pain or more correctly, mechanosensitivity of the nervous system.
Neurodynamics relating to cervical conditions
Neurodynamic testing is a great assessment technique for ruling out cervical radiculopathy due to its high sensitivity and low specificity (Rubinstein et al. 2007). Positive responses to upper limb neurodynamic testing of the median nerve have also been shown in people with WAD (Greening et al. 2005, Sterling et al. 2003b, Sterling & Pedler 2009). The responses to testing slump in a long sitting position are significant for migraine and headache (von Piekartz et al. 2007).
Furthermore, it has been shown that pain catastrophizing predicts the pain intensity perceived by the individual during neurodynamic testing (Beneciuk et al. 2010) and provides another link to the need to consider a bio-psychosocial approach with your patient.
Neurodynamic testing for people with unilateral arm and/or neck pain is moderately reliable (Schmid et al. 2009). There is also a high reliability between trials in terms of symptom reproduction and onset of symptoms during testing (van der Heide et al. 2001). We believe that if neurodynamic examination is performed well as a part of a clinical reasoning approach it can be an extremely useful tool in ascertaining the underlying mechanosensitivity of the nervous system. It can be used as a good clinical guide to document changes in mechanosensitivity over a period of time. Neurodynamic tests for the craniocervical nerves are shown in Figure 4.14 and for the occipital and auricular nerves in Figure 4.15.
The nerve roots, trunks and cords of the brachial plexus
The classical upper limb neurodynamic tests (ULNT) with structural differentiation are shown in Figures 4.16–4.19.
Altering the test and/or start position
It may be appropriate, especially with more severe pain problems such as a nerve root lesion, to adapt the base test and find ways to help reduce the symptoms. A cradle position allows support for the arm and is useful when the patient is unhappy with it flat on the bed (Fig. 4.20).
Pre-cervical spine treatment screening – implications for examination
Cervical arterial dysfunction (CAD)
As part of the clinical interview, therapists are asked to identify any precautions or contraindications for treatment. Screening for vertebrobasilar artery insufficiency is commonly suggested in the presence of specific subjective symptoms (Box 4.17). The presence of CAD, which may be attributed to upper cervical instability, is considered to be one risk of manual therapy techniques to the cervical spine.
Guidelines for screening patients for risk of neurovascular complications of manual therapy have been available for many years (Taylor & Kerry 2010). In spite of the large number of papers devoted to CAD, there is no consensus of opinion as to the validity and reliability of the guidelines. Due to the medicolegal implications and the need to follow clinical guidelines, despite the lack of evidence, functional testing may be necessary – the minimum comprising a judicious sustained rotation of the cervical spine. If in doubt the patient must be referred for further medical tests before commencing the treatment (Kerry et al. 2008). However, extreme caution should be shown when presented with a case where there is subjective data that is likely to prove CAD. It is the authors' opinion to err on the side of the caution with CAD testing.
Clinically, the type of tests selected will closely relate to the patients description of the aggravating factors (e.g. end of range cervical rotation or extension). The intensity of the tests should be taken only to, or just before, the onset of symptoms, in the first instances. These tests constitute important physical ‘markers’.
Craniovertebral instability
Craniovertebral instability has been attributed as one possible cause of CAD and as such the discerning clinician should be aware of it. If you encounter patients displaying the symptoms noted below then craniovertebral instability is suggested as a necessary measure. A good depth of knowledge and use of clinical reasoning should guide the clinician when confronted with these symptoms, for example, craniovertebral instability testing could be a consideration for headache and WAD.
Symptoms and signs of cervical instability
Symptoms and signs of instability include (Gibbon & Tehan 2006):
1. Facial paraesthesia secondary to dysfunction of the connections of the hypoglossal nerve. Dysfunction ventral ramus as a cause of neck–tongue syndrome (Lance & Anthony 1980) and the dorsal ramus producing facial numbness
2. C1–C2 instability causing abnormal pressure on cervical nerves
3. Vertebrobasilar artery compromise (Savitz & Caplin 2005, Thanvi et al. 2005)
4. Cord compression (Rao 2002).
Clinical testing of craniovertebral instability
In the past certain classical tests have been proposed to test for cervical instability. These include the Sharp-Purser for the transverse ligament, the lateral flexion rotatory tests for the alar ligaments and the longitudinal cranial assessment for the tentorial membrane (Fig. 4.21). It is the authors' opinion that if there is a suspicion of cervical instability then it would be unwise to use these clinical tests due to the inherent danger to the patient.
Examination of the cervical spine through mobilization techniques
Manual examination techniques have a place in the physical examination of cervical pain disorders. Traditional passive joint palpation techniques, such as passive accessory intervertebral movements (PAIVMS), can assist in the clinical reasoning process. For instance, a unilateral posteroanterior palpation of a C5 that elicits a pain response should be viewed with consideration of the relative stimulus-response predictability i.e. what is the relationship between the extent of the response and how this varies with each repetition of the palpation? If there is fairly close stimulus/response predictability then it is more indicative of underlying nociceptive mechanisms. It should also encompass prior reasoning of the possible underlying pain mechanisms i.e. whether central sensitization is likely to be present.
The testing position for cervical palpation can be changed according to patient comfort or the need to mimic functional movement. It would be far more predictive of a local nociceptive process if the same posteroanterior palpation evoked the same response in both a supine and a sitting position. If there is some variance then the therapist should be considering how this could be incorporated into their treatment, in terms of context change and altered firing of the pain neurotag.
Mobilization linked to context change
One further way of analyzing movement of the cervical spine is through passive physiological techniques, such as passive physiological intervertebral motion (PPIVMS). Again these can help to cement a reasoning process. It may be that these physiological techniques alter the current pain neurotag and can be used as one method within a graded exposure approach in the treatment of a functional impairment.
Changing the context of a movement, as previously suggested, may give weight to a hypothesis and guide treatment. Painful and restricted cervical rotation assessed in a traditional supine position may be eased with a SNAG performed in sitting (see Fig. 4.24). This could be due to altered descending modulation, firing of a neurotag, or possibly relief through segmental inhibition.

Figure 4.24 A, B Example of active cervical left rotation with the addition of a transverse mobilization of T1 to the right as an assessment or treatment technique for someone struggling to turn his head.
Mobilization techniques have a role to play in the diagnosis and treatment of cervical pain disorders but should be viewed in relation to how the fit into the bio-psychosocial perspective of the individual.
The treatment of the cervical region
Ultimately treatment is not about sticking to set algorithms. Due to the individual nature of pain, no single treatment will be sufficient for each common complaint. It is therefore necessary to remain flexible. If pushing on a certain point on the neck relieves the patient's pain or by them holding a particular area then you should have the flexibility to accept that and incorporate it into your treatment. This is used to great effect with Brian Mulligan's SNAGs (sustained natural apophyseal glides; 2010). These techniques ultimately empower the patient to have some self-control over their pain problem. These techniques also lead well from the assessment into an individually tailored treatment approach.
Understanding the local pathophysiology and interacting components within each person's pain experience and maintaining a bio-psychosocial perspective will help to determine the specific intervention that will be most appropriate and beneficial. This next section documents some of these treatment approaches that will help the common cervical conditions and their underlying pain neurobiology.
Information and communication
Part of the rehabilitation process is to promote the patients' health literacy skills. Health literacy is the degree to which individuals have the capacity to obtain, process and understand basic health information and services needed to make appropriate health decisions. In general those with lower health literacy have a lower health status, which ultimately impacts on the health economics and both the prevalence and prognosis of neck pain. Improving health literacy could enhance the ability and motivation of the patient to solve their pain problems by applying their knowledge of health and accessing healthcare in a self-efficacious and appropriate manner (Ishikawa & Kiuchi 2010).
The information you impart and how you go about communicating it is vitally important. Communication is not only your verbal instructions but also the non-verbal aspects. These include your handling skills, touch of the patient and the careful way you perform techniques. This should allow continued positive feedback to your patient that will be apparent in their presentation and will enable them to understand their pain problem more fully.
Shaping beliefs through pain education
Part of a clinical reasoning approach incorporates the use of psychology models or the concept of cognitive and affective domains within the neuromatrix paradigm. This includes a need to understand a person's attitudes and beliefs regarding their pain experience. Some of the factors that commonly relate to the disability and chronicity of a disorder are catastrophization regarding their pain and attributing it to a patho-anatomical cause, which in the light of known pain neurobiology is insufficient and sometimes incorrect.
Interest in the provision of education regarding pain neurobiology, helped by the book Explain pain (Butler & Moseley 2003), has grown recently. With suitable pain education it is possible for someone to change their belief that pain is a good indicator of tissue damage and can lessen their pain catastrophization (Meeus et al. 2010). Along with these changes in pain attitudes and beliefs there are likely to be improvements in physical markers that relate to both the underlying mechanosensitivity and the subsequent motor response, even without any physical interventions (Moseley 2004a, 2004b). This ultimately shows a beneficial change in the outputs from the brain; possibly reflecting altered descending modulation and a change in the activation of motor processing areas.
Brief education has a positive effect on the health literacy in people suffering acute WAD (Oliveira et al. 2006). This includes a change in the use of medication, seeking ongoing interventions and a person's self-efficacy. Benefit is also seen when education is provided to people with chronic WAD (Van Oosterwijck et al. 2011).
However, with the advent of promising results following pain education, there appears to have been a worrying trend for some therapists to consider education sufficient in the treatment of people with pain problems. They have essentially become ‘hands off’ therapists. This is not only sad for manual therapy but shows a distinct lack of understanding of the benefit that certain therapeutic techniques have in aiding the diagnosis of underlying pain mechanisms (e.g. as seen in the use of neurodynamic techniques) or that can be directed towards affecting changes of specific elements of a pain experience (e.g. passive mobilization techniques creating hypoalgesic effects).
Passive mobilization techniques
Passive cervical mobilization techniques have been found to create hypoalgesic effects. They also activate the sympathetic nervous system (Vicenzino et al. 1998, Sterling et al. 2001, Skyba et al. 2003, Schmid et al. 2008). Both can effect modulation of nociceptive processing. The modulatory effects created are extrasegmental and can last up to 24 hours following the intervention. This suggests that passive mobilization can be directed to segments above and below the most painful area and help to reduce the impact of ongoing nociception (Box 4.18).
These responses to passive mobilization techniques are likely to be mediated by higher centres creating descending inhibition (Wright 2002, Souvlis et al. 2004). There appears to be less of a direct effect on the motor system and it is thought that changes shown in it are more of a reflection on the modulation of pain.
Given the extrasegmental effects it would be beneficial to begin mobilization above or below the most painful area, in the knowledge that there should still be a modulatory effect on their neck pain. The dismissal of more peripheralistic viewpoints regarding the effects of passive joint mobilization allow for the therapist to have flexibility in treating their patient effectively, i.e. it is more than moving a stiff segment.
Specific mobilization treatments
The cervical lateral glide technique (Fig. 4.26) has been used as a treatment technique due to its capacity to elicit hypoalgesia in painful musculoskeletal conditions (Sterling et al. 2010, Vicenzino et al. 1996, 1998, Coppieters et al. 2003). The technique should not be provocative (Elvey 1986).
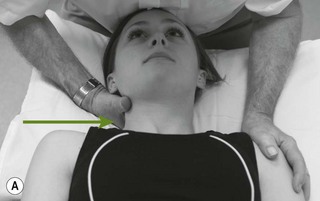
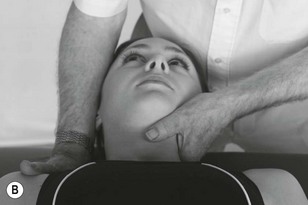
Figure 4.26 A Lateral glide. This technique may be performed either as a basic localized translation or as a pulling technique whereby the therapist hooks their fingers onto the opposite side of the neck and draws the neck towards the position of their hand. B Lateral glide alternate grip. The fingers hook around the neck to draw towards the side of the mobilizing arm.
This technique may be performed either as a basic localized translation or as a pulling technique whereby the therapist hooks their fingers onto the opposite side of the neck and draw the neck towards the position of their hand.
Selecting the correct technique
Ultimately the selection of the most appropriate specific passive mobilization technique will be dictated by the patient's requirements and the clinical skills of the therapist. It may be necessary for the patient to focus on the area that is being treated; however, there is always a possibility that this may reinforce any hypervigilance.
When using these techniques to help a pain experience the treatment should be comfortable. This may allow the subconscious to reduce the vigilance maintained by the perception of an ongoing threat (e.g. pain that is always associated with this area and the belief that the pain is a good indicator of tissue damage). If the perceived threat reduces then there will likely be changes in descending modulatory control and the subsequent neurotag relating to the neck. However, there are still a lot of unknowns regarding the possible processes that affect mobilization techniques and that may become apparent in the future.
The correct testing position
Finding the correct testing position depends very much on patient comfort and functional considerations. It may be impossible to assess or treat them in prone, as is commonly suggested, particularly when they have severe pain or breathing difficulties in this position. Conversely, this might be a position of comfort and as such would be a sensible starting position.
For example, it may be appropriate to begin a mobilization technique for someone with a nerve root lesion in an upright/supported sitting position, avoiding neck extension. In essence this incorporates the principle of context change and is likely to cause a change in the output from the neuromatrix – i.e. a different neurotag!
Incorporating context change into treatment
Brian Mulligan (2010) defined SNAGS as sustained natural apophyseal gliding movements (see Fig. 4.27). They are accessory mobilizations performed through movement in order to provide a pain-free movement. They provide an ideal opportunity to assess someone with neck pain in a functional position, e.g. cervical rotation in sitting that correlates with that person's current functional impairment of turning the neck when reversing a car.
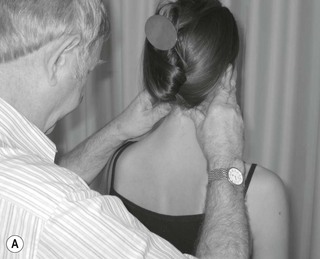
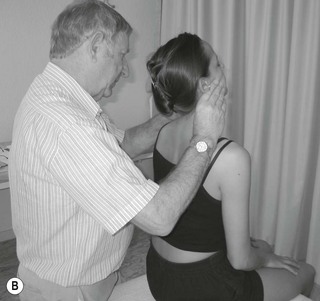
Figure 4.27 A, B The examiner performs a SNAGS technique in the upper cervical region in order to facilitate a pain-free cervical extension.
SNAGS and other similar techniques are ideal to offer as a self-treatment strategy, therefore boosting the individual's control of their pain problem.
Manual therapy and central sensitization
It is important to understand whether central sensitization is a dominant mechanism underpinning a pain experience prior to the use of manual therapy. By applying more afferent stimulation through already sensitized nociceptive pathways it is likely that the therapist will only serve to amplify the current sensitization and underlying changes (Nijs & van Houdenhove 2009). It would be more appropriate in these circumstances to consider other treatment approaches aimed at reducing the central sensitization prior to the application of local manual therapy. This may include education and drug therapy aimed at reducing the amplification of the nociceptive system or techniques that aim to create lateral cortical inhibition such as sensory-discrimination and left/right discrimination training.
Manipulation
There is a high incidence of adverse events experienced by patients following cervical manipulation, with up to one in five patients experiencing mild or transient effects following treatment (Kerry et al. 2008). Cerebral vascular accidents are five times more likely to occur in someone who has received cervical manipulation up to one week prior to the event. Also, people suffering from vertebral artery stroke are five times more likely to have received cervical manipulation up to 1 week prior to the stroke. This information suggests the judicial evaluation of the use of manipulation prior to the use of cervical manipulation and as such is an approach that the authors would not choose.
However, the grade V manipulative techniques advocated by Maitland, which consider all aspects of safety, may be practised by physiotherapists. They are appropriate to use when activation of a local neuroimmune response or neural silencing are desired; the proviso being that when the nerve begins to fire following the silent period, there may be an increase in the symptoms due to nociceptive firing.
Treatment with reference to neurodynamics
One of the objectives of treatment of abnormal neurodynamics is to improve and maintain the health and mobility of the tissues comprising the containers, in combination with direct mobilization of the nervous system. The neural containers are considered to be the interfacing tissues of the nervous system, commonly known as the nerve bed (i.e. the tissues in which the nerve lies). This consists of any structures adjacent to the nervous system, such as muscles, tendons, bones, ligaments, fascia and blood vessels (Shacklock 2005).
From the neurodynamic aspect, nerves slide through the tunnels, while tunnels move over nerves. Movements of the nerve bed such as stretching, shortening, bending, twisting and turning produce similar effects on the nervous system. The nerve can also move independently from the nerve bed and requires appropriate movement to maintain health, e.g. axoplasmic and blood flow.
It may be necessary to treat the container tissues prior to commencing any neurodynamic techniques such as sliders or tensioners. This is particularly the case when the interfacing tissues directly affect the movement, pressure changes or health of the nerves (e.g. through local inflammation, muscle spasm or scarring).
Neural mobilization techniques
In recent times, neurodynamic mobilizing techniques have been divided into ‘sliders’ and ‘tensioners’ (Coppieters & Butler 2008). Sliders consist of simultaneously increasing the load of the nervous system at one joint whilst decreasing the load at another joint (Butler 2000, Coppieters et al. 2004, Shacklock 2005). During these manoeuvres there is large amplitude of movement of the nerve (Coppieters et al. 2009) and, most likely, little change in intraneural pressure. These techniques are generally chosen for more sensitive conditions where the objective is to get some movement and health without increasing symptoms in the nervous system. This may create hypoalgesia or local sympathetic activation and should be less threatening for the patient. They can also be seen as another form of context change that will alter the neurotag.
Tensioners are the more traditional and aggressive techniques where movements place progressive load through the nervous system. These would be equivalent to using movements of the base test as a treatment and are more likely to be used in the later stages of rehabilitation where the patient needs to become accustomed to more load through the system. Tensioners have been shown to cause a change in thermal sensitivity and possible hypoalgesic effects and should be used with these effects in mind (Beneciuk et al. 2009b).
Slider technique for the median nerve (ULNT1) is illustrated in Figure 4.28. As elbow extension is added there is simultaneous subtraction of wrist extension. This is then repeated in the reverse direction in a slow, oscillatory manner. This may be useful for nerve root disorders in which there is sensitivity of the upper limb nerve trunks.
Figure 4.29 shows how the base ULNT1 test can be incorporated into a neural mobilization treatment as a tensioner. The movement will still be oscillatory, depending on the desired needs of the patient. It will enable progressive loading through the nerve trunks and a graduated exposure to these positions.
When the patient is recovering from adverse neurodynamic problems, a combination of slider and tensioner techniques can be performed since the normal nervous system continually functions through these means, i.e. both sliding through the nerve bed and loading the nerve when placed in a stressed position. It is important for the clinician to clinically reason when this may be appropriate. A slider technique is illustrated in Figure 4.30.
Massage
Massage should be considered an important part of your treatment modalities. This may include massage directly over nerve trunks in order to create a gating/inhibitory effect of the pain problem. The same caution should be shown in the presence of allodynia as with other manual techniques. However, massage or desensitization techniques on the border of a sensitized or allodynic area may provide beneficial inhibitory effects.
More vigorous/stronger techniques may be more appropriate after any sensitivity has reduced sufficiently to be able to cope with them or as a progression when the patient is getting used to accepting greater force or load being placed on their tissues.
Self-treatment and management
Self-treatment and management will be included in most of the treatment options. This will promote self-efficacy and hopefully enhance the beneficial effects proposed through the various treatment approaches. Ideally the patient should clearly understand what they are doing and why. This means spending sufficient time educating them and ensuring that the self-treatment is performed appropriately. It is imperative that the patient feels they can ask any further questions and is a good idea to ask what their understanding of the problem is following the delivery of treatment.
Figure 4.31 shows a self-mobilizing slider technique for the radial nerve using the neck ipsilateral rotation with elbow extension and can be used to maintain health of the nervous system.

Figure 4.31 A, B Self-mobilizing slider technique for the radial nerve using the neck ipsilateral rotation with elbow extension.
Figures 4.32 and 4.33 illustrate self-management of cervical rotation using a towel or the hand to change the context of the movement and hence the motor output from the neuromatrix.
Treatment dose and ongoing intervention
In the same way that a doctor would prescribe medication and consider the appropriate dose, they would also think about the possible side-effects or adverse events caused by taking the medication. This should be no different for the manual therapist delivering a particular intervention. These calculations should be based on the current hypothesis and understanding of the mechanisms underpinning the pain problem. With a highly sensitive condition you need to deliver far less treatment (lower dose). This means less time and fewer repetitions of a treatment and most likely a less forceful technique.
For a more stable condition it may be prudent to deliver far more. To gain lasting effects, it will be beneficial to give the patient some self-management techniques or to see them for ongoing appointments at reasonably regular time intervals. Although one risk factor for someone proceeding onto a chronic condition is the over-reliance of a passive intervention, i.e. they don't take ownership of their own care.
For the more severe and sensitized states, there is no benefit in seeing the patient numerous times for the delivery of manual therapy. In this case it is far more important to gain some stability in the condition first. This may come through education, drug therapy and appropriate self-management.
Graded exposure in order to progress treatment
Progressing treatment can be achieved through a graded exposure approach. This essentially includes some form of pacing within contextual changes and challenges. It should encompass the functional limitations and goals of the individual. The ultimate aim is to change the current pain response (neurotag) and gradually expose the individual (and hence the brain) to more challenging activities. These activities include changes in how a movement is performed and recognizing that changing the emotional context or thoughts and beliefs regarding the movement may be a part of achieving this. This obviously ties into the concept of the neuromatrix paradigm and the different input domains. The end goal for the patient would be for them to have the freedom and flexibility to run any movement without creating a pain experience. An example of graded exposure treatment for WAD can be found in Box 4.19.
There are many other treatment options and techniques that are at the disposal of manual therapists and we encourage clinicians to maintain a healthy interest in these but hope that treatment will include strong clinical reasoning skills and an understanding of the underlying pain mechanisms.
References
Almeida, TF, Roizenblatt, S, Tufik, S. Afferent pain pathways: a neuroanatomical review. Brain Res. 2004;1000:40–56.
Antonovsky, A. The salutogenic model as a theory to guide health promotion. Health Promot Int. 1996;11(1):11–18.
Apkarian, AV, Sosa, Y, Krauss, BR, et al. Chronic pain patients are impaired on an emotional decision-making task. J Neurosci. 2004;24(46):10410–10415.
Asbury, A, Fields, H. Pain due to peripheral nerve damage: an hypothesis. Neurol. 1984;34:1587–1590.
Asmundson, G, Wright, K, Hadjistavropoulos, D. Hypervigilence and attentional fixedness in chronic musculoskeletal pain: consistency of findings across modified stroop and dot-probe tasks. J Pain. 2005;6(8):497–506.
Baliki, M, Chialvo, DR, Geha, PY, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26(47):12165–12173.
Banati, R, Cagnin, A, Brooks, DJ, et al. Long-term trans-synaptic glial responses in the human thalamus after peripheral nerve injury. NeuroReport. 2001;12(16):3439–3442.
Barnsley, L, Lord, S, Bogduk, N. The pathophysiology of whiplash. Spine. 1998;12:209–242.
Bear, MF, Connors, BW, Paradiso, MA, Neuroscience: exploring plain. ed 2. 2001.
Bechara, A, Damasio, AR, Damasio, H, et al. Insensitivity to future consequences following damage to human prefrontal cortex. Cogn. 1994;50:7–15.
Beneciuk, JM, Bishop, MD, George, SZ. Clinical prediction rules for physical therapy interventions: a systematic review. Phys Ther. 2009;89:114–124.
Beneciuk, J, Bishop, MD, George, SZ. Effects of upper extremity neural mobilization on thermal pain sensitivity: a sham-controlled study in asymptomatic participants. J Orthop and Sport Phys Ther. 2009;39(6):428–438.
Beneciuk, J, Bishop, MD, George, SZ. Pain catastrophizing predicts pain intensity during a neurodynamic test for median nerve in healthy participants. Man Ther. 2010.
Bennett, G. Neuropathic pain: a crisis of definition. Anaesth Analg. 2003;97:619.
Bennett, MI, Attal, N, Miroslav, MB, et al. Using screening tools to identify neuropathic pain. Pain. 2007;127:199–203.
Butler, DS. Mobilisation of the nervous system. Melbourne: Churchill Livingstone; 1991.
Butler, DS. The sensitive nervous system. Adelaide, Australia: NOI Publications; 2000.
Butler, DS, Moseley, GL. Explain pain. Adelaide, Australia: NOI Publications; 2003.
Caragee, E, Alamin, T, Cheng, I, et al. Are first-time episodes of serious low back pain associated with new MRI findings? Spine J. 2006;6:624–635.
Carroll, LJ, Holm, LW, Hogg-Johnson, S, et al. Course and prognostic factors for neck pain in whiplash-associated disorders (WAD). J Manipulative Physiol Ther. 2009;32(2S):S97–S107.
Carroll, LJ, Hogg-Johnson, S, Côté, P, et al. Course and prognostic factors for neck pain in workers. J Manipulative Physiol Ther. 2009;32(2S):S109–S116.
Christensen, JO, Knardahl, S. Work and neck pain: a prospective study of psychological, social, and mechanical risk factors. Pain. 2010;151:162–173.
Chrousos, G. Stress and disorders of the stress system. Nature Rev Endocrinol. 2009;5:374–381.
Clemente, C. Anatomy. Urban and Schwarzenbuerg: Munich; 1975.
Coppieters, M, Strappaerts, K, Wouters, L, et al. The immediate effects of a cervical lateral glide treatment technique in patients with neurogenic cervicogenic pain. J Orthop Sports Phys Ther. 2003;33:369–378.
Coppieters, MW, Barthomeeusen, KE, Strappaerts, KH. Incorporating nerve-gliding techniques in the conservative treatment of cubital tunnel syndrome. J Manipulative Physiol Ther. 2004;27(9):560–568.
Coppieters, MW, Kurz, K, Mortensen, TE, et al. The impact of neurodynamic testing on the perception of experimentally induced muscle pain. Man Ther. 2005;10:52–60.
Coppieters, MW, Butler, DS. Do ‘sliders’ slide and ‘tensioners’ tension? An analysis of neurodynamic techniques and consideration regarding application. Man Ther. 2008;13(3):213–221.
Coppieters, M, Hough, A, Dilley, A. Different nerve-gliding exercises induce different magnitudes of median nerve longitudinal excursion: an in-vivo study using dynamic ultrasound imaging. J Orthop Sports Phys Ther. 2009;39:3.
Costigan, M, Scholz, J, Woolf, C. Neuropathic pain: a maladaptive response of the nervous system. Annu Rev Neurosci. 2009;32:1–32.
Côté, P, van der Velde, G, Cassidy, JD, et al. The burden and determinants of neck pain in workers. J Manipulative Physiol Ther. 2009;32:S70–S86.
Dehghani, M, Sharpe, L, Nicholas, M. Selective attention to pain-related information in chronic musculoskeletal pain patients. Pain. 2003;105:37–46.
Delcomyn F: Foundations of neurobiology WH Freeman, New York, 1998.
Dellon, A, MacKinnon, S, Crosby, P. Reliability of two-point discrimination measurements. J Hand Surg (Am). 1987;12(5 Pt.1):693–696.
Devor, M, Seltzer, Z. Pathophysiology of damaged nerves in relation to chronic pain. In: Wall PD, Melzack R, eds. Textbook of pain. Edinburgh: Churchill Livingstone, 1999.
Dhaka, A, Viswanath, V, Patapoutian, A. TRP ion channels and temperature sensation. Annu Rev Neurosci. 2006;29:135–161.
Dick, J. The deep tendon and abdominal reflexes. J Neurol Neurosurg and Psychiatry. 2003;74:150–153.
Dilley, A, Bove, G. Disruption of axoplasmic transport induces mechanical sensitivity in intact rat C fibre nociceptors axons. J Neurophysiol. 2008;586:593–604.
Dilley, A, Lynn, B, Pang, SJ. Pressure and stretch mechanosensitivity of peripheral nerve fibres following local inflammation of the nerve trunk. Pain. 2005;117:462–472.
D'Mello, R, Dickenson, AH. Spinal cord mechanisms of pain. Br J Anaesth. 2008;101(1):8–16.
Durkan, J. A new diagnostic test for carpal tunnel syndrome. J Jt and Bone Surg. 1991;73A:536–538.
Dworkin, R, Jensen, M, Gammaitoni, A, et al. Symptom profiles differ in patients with neuropathic versus non-neuropathic pain. J Pain. 2007;8(2):118–126.
Elvey, RL. Treatment of arm pain associated with abnormal brachial plexus tension. Aust J Physiother. 1986;32:224–230.
Engel, G. The biopsychosocial model and the education of health professionals. Annals NY Academy of Sciences; 1978.
Farmer, J, Wisneski, R. Cervical nerve root compression. An analysis of neuroforaminal pressure with varying head and arm positions. Spine. 1994;19(16):1850–1855.
Fast, A, Pirakh, S, Marin, E. The shoulder abduction relief sign in cervical radiculopathy. Arch Phys Med Rehabil. 1989;70(5):402–403.
Fernández-de-las-Peñas, C, Coppieters, MW, Cuadrado, M, et al. Patients with chronic tension-type headache demonstrated increased mechano-sensitivity of the supra-orbital nerve. Headache. 2008;48:570–577.
Fernández-de-las-Peñas, C, Arendt-Nielsen, L, Cuadrado, M, et al. Generalized mechanical pain sensitivity over nerve tissues in patients with strictly unilateral migraine. Clin J Pain. 2009;25:401–440.
Flor, H, Braun, C, Elbert, T, et al. Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neurosci Lett. 1997;224:5–8.
Flor, H, Knost, B, Birbaumer, N. Processing of pain- and body-related verbal material in chronic pain patients: central and peripheral correlates. Pain. 1997;73:413–421.
Gibbon, P, Tehan, P. HVLA thrust techniques: what are the risks? Int J Osteopath Med. 2006;9(1):4–12.
Gifford, L. Pain, the tissues and the nervous system: a conceptual model. Physiotherapy. 1998;84:27–33.
Gifford, L. Acute low cervical nerve root conditions: symptom presentation and pathological reasoning. Man Ther. 2001;6(2):106–115.
Gifford, L, Butler, D. The integration of pain science into clinical practice. Hand. 1997;10(2):86–95.
Gifford, L, Thacker, M. A clinical overview of the autonomic nervous system, the supply to the gut and mind-body pathways. In: Gifford L, ed. Topical Issues in Pain 3. Falmouth: CNS Press, 2002.
Greening, J, Lynn, B. Minor peripheral nerve injuries: an underestimated source of pain? Man Ther. 1998;3(4):187–194.
Greening, J, Dilley, A, Lynn, B. In vivo study of nerve movement and mechanosensitivity of the median nerve in whiplash and non-specific arm pain patients. Pain. 2005;115:248–253.
Hall, T, Quintner, J. Responses to mechanical stimulation of the upper limb in painful cervical radiculopathy. Aust J Physiother. 1996;42:277–285.
HIS (The International Headache Society). Headache Classification Subcommittee. The International Classification of Headache Disorders. Cephalalgia. 2004;24(suppl. 1):1–151.
Hogg-Johnson, S, van der Velde, G, Carroll, LJ, et al. The burden and determinants of neck pain in the general population. J Manipulative Physiol Ther. 2009;32:S46–S60.
Hu, J, Milenkovic, N, Lewin, GR. The high threshold mechanotransducer: a status report. Pain. 2006;120:3–7.
Huang, J, Zhang, X, McNaughton, PA. Inflammatory pain: the cellular basis of heat hyperalgesia. Curr Neuropharmacol. 2006;4:197–206.
Iacoboni, M, Mazziotta, JC. Mirror neuron system: basic findings and clinical applications. Ann Neurol. 2007;62:213–218.
Ingvar, M. Pain and functional imaging. Philos Trans Roy Soc Lond B: Biol Sci. 1999;354(1387):1347–1358.
Ishikawa, H, Kiuchi, T. Health literacy and health communication. Biopsychosocial Med. 2010;4:18.
Jepsen, J, Laursen, L, Hagert, C-G, et al. Diagnostic accuracy of the neurological upper limb examination 1: Inter-rater reproducibility of selected findings and patterns. BMC Neurol. 2006;6:8.
Jones, M, Rivett, D. Clinical reasoning for manual therapists. Edinburgh: Butterworth Heinemann; 2004.
Juhl, GI, Jensen, TS, Northholt, SE, et al. Central sensitisation phenomena after third molar surgery: a quantitative sensory testing study. Eur J Pain. 2008;12:116–127.
Jull, G. the primacy of clinical reasoning and clinical practical skills. Man Ther. 2009;14:353–354.
Jull, G, Sterling, M, Falla, D, et al. Whiplash, headache and neck pain. Edinburgh: Churchill Livingstone Elsevier; 2008.
Kaushik, RM, Kaushik, R, Mahajan, SK, et al. Effects of mental relaxation and slow breathing in essential hypertension. Complement Ther Med. 2006;14:120–126.
Kerry, R, Taylor, A, Mitchell, J, et al. Manipulation Association of Chartered Physiotherapists. CAD information document. Nottingham: University of Nottingham; 2007.
Kerry, R, Taylor, A, Mitchell, J, et al. Cervical arterial dysfunction and manual therapy: A critical literature review to inform professional practice. Man Ther. 2008;13:278–288.
Lance, J, Anthony, M. Neck-tongue syndrome on sudden turning of the head. J Neurol, Neurosurg Psychiatry. 1980;43(2):97–101.
Latremoliere, A, Woolf, C. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926.
Levitz, C, Reilly, P, Torg, J. The pathomechanics of chronic, recurrent cervical nerve root neurapraxia: the chronic burner syndrome. Am J Sports Med. 1997;25(1):73–76.
Lotze, M, Moseley, GL. Role of distorted body image in pain. Curr Rheumatol Rep. 2007;9:488–496.
Lundborg, G, Rosen, B. The two-point discrimination test – time for a reappraisal? J Hand Surg (Br). 2004;29B(5):418–422.
Mäntyselkä, P, Kautiainen, H, Vanhala, M. Prevalence of neck pain in subjects with metabolic syndrome – a cross-sectional population-based study. BMC Musculoskelet Disord. 2010;11:171.
Mason, P. Deconstructing endogenous pain modulation. J Neurophysiol. 2005;94:1659–1663.
Meeus, M, Nijs, J, Van Oosterwijck, J, et al. Pain physiology education improves pain beliefs in patients with chronic fatigue syndrome compared with pacing and self-management education: a double-blind randomized controlled trial. Arch Phys Med Rehabil. 2010;91:1153–1159.
Melzack, R. Phantom limbs, the self and the brain. Can Psychol. 1989:1–16.
Melzack, R. Phantom limbs and the concept of a neuromatrix. Trends Neurosci. 1990;13:88–92.
Merskey, H, Bogduk, N. Classification of chronic pain, ed 2, IASP Task Force on Taxonomy. Seattle: IASP Press; 1994.
Moriarty, O, McGuire, B, Finn, D. The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol. 2011;93:385–404.
Moseley, GL. A pain neuromatrix approach to patients with chronic pain. Man Ther. 2003;8(3):130–140.
Moseley, GL. Why do people with complex regional pain syndrome take longer to recognise their affected hand? Neurol. 2004;62:2182–2186.
Moseley, GL. Evidence for a direct relationship between cognitive and physical change during an education intervention in people with chronic neck pain. Eur J Pain. 2004;8:39–45.
Moseley, GL. Reconceptualising pain according to modern pain science. Phys Ther Rev. 2007;12:169–178.
Moseley, GL. I can't find it! Distorted body image and tactile dysfunction in patients with chronic back pain. Pain. 2008;140:239–243.
Mulligan, B. Manual therapy, NAGS, SNAGS, MWMS etc., ed 6. Minneapolis: Orthopedic Physical Therapy Products; 2010.
Natvig, B, Ihlebæk, C, Grotle, M, et al. Neck pain is often part of widespread pain and is associated with reduced functioning. Spine. 2010;35(23):E1285–E1289.
Navarro, X, Vivó, M, Valero-Cabré, A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201.
Nee, RJ, Butler, D. Management of peripheral neuropathic pain: integrating neurobiology, neurodynamics and clinical evidence. Phys Ther Sport. 2006;7:36–49.
Nee, R, Vicenzino, B, Jull, G, et al. Neural tissue management provides immediate clinically relevant benefits without harmful effects for patients with nerve-related neck and arm pain: a randomised trial. J Physiother. 2012;58(1):23–31.
Nijs, J, Van Houdenhove, B. From acute musculoskeletal pain to chronic widespread pain and fibromyalgia: Application of pain neurophysiology in manual therapy practice. Man Ther. 2009;14:3–12.
Novak, C, MacKinnon, S. Evaluation of nerve injury and nerve compression in the upper quadrant. J Hand Ther. 2005;18:230–240.
Oliveira, A, Gevirtz, R, Hubbard, D. A psycho-educational video used in the emergency department provides effective treatment for whiplash injuries. Spine. 2006;31:1652–1657.
Pleger, B, Ragert, P, Schwenkreis, P, et al. Patterns of cortical reorganization parallel impaired tactile discrimination and pain intensity in complex regional pain syndrome. NeuroImage. 2006;32:503–510.
Rao, R. Neck pain, cervical radiculopathy and cervical myelopathy. J Bone Joint Surg. 2002;840(10):1872–1881.
Rizzolatti, G, Leonardo Fogassi, L, Gallese, V. Neurophysiological mechanisms underlying the understanding imitation of action. Nat Rev Neurosci. 2001;2:661–670.
Rubinstein, SM, Pool, JJM, van Tulder, MW, et al. A systematic review of the diagnostic accuracy of provocative tests of the neck for diagnosing cervical radiculopathy. Eur Spine J. 2007;16:307–319.
Safran, M. Nerve injury about the shoulder in athletes, part 2: long thoracic nerve, spinal accessory nerve, burners/stingers, thoracic outlet syndrome. Am J Sports Med. 2004;32:1063–1076.
Savitz, S, Caplan, L. Vertebrobasilar disease. N J Med. 2005;352:2618–2626.
Schmid, A, Brunner, F, Wright, A, et al. Paradigm shift in manual therapy? Evidence for a central nervous system component in the response to passive cervical joint mobilisation. Man Ther. 2008;13:387–396.
Schmid, A, Brunner, F, Luomajoki, H, et al. Reliability of clinical tests to evaluate nerve function and mechanosensitivity of the upper limb peripheral nervous system. BMC Musculoskelet Disord. 2009;10:11.
Shacklock, M. Clinical neurodynamics: a new system of musculoskeletal treatment. Edinburgh: Butterworth Heinemann Elsevier; 2005.
Siao, P, Cros, D. Quantitative sensory testing. Phys Med Rehabil Clin N Am. 2003;14:261–285.
Skyba, D, Radhakrishnan, R, Rohlwing, L, et al. Joint manipulation reduces hyperalgesia by activation of monoamine receptors but not opioid or GABG receptors in the spinal cord. Pain. 2003;106:159–168.
Smart, KM, O'Connell, NE, Doody, C. Towards a mechanisms-based classification of pain in musculoskeletal physiotherapy? Phys Ther Rev. 2008;13(1):1–10.
Smart, KM, Blake, C, Staines, A, et al. Clinical indicators of ‘nociceptive’, ‘peripheral neuropathic’ and ‘central’ mechanisms of musculoskeletal pain. A Delphi survey of expert clinicians. Man Ther. 2010;15:80–87.
Snider, WD, McMahon, SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20:629–632.
Souvlis, T, Vicenzino, B, Wright, A. Neurophysiological effects of spinal manual therapy. In: Boyling JD, et al, eds. Grieve's modern therapy. Edinburgh: Churchill Livingstone; 2004:367–379.
Standaert, C, Herring, S. Expert opinion and controversies in musculoskeletal and sports medicine: stingers. Arch Phys Med Rehabil. 2009;90:402–406.
Sterling, M. A proposed new classification system for whiplash associated disorders – implications for assessment and treatment. Man Ther. 2004;9:60–70.
Sterling, M, Jull, G, Wright, A. Cervical mobilisation: concurrent effects on pain, sympathetic nervous system activity and motor activity. Man Ther. 2001;6:72–81.
Sterling, M, Jull, G, Vicenzino, B, et al. Sensory hypersensitivity occurs soon after whiplash and is associated with poor recovery. Pain. 2003;104:509–517.
Sterling, M, Jull, G, Vicenzino, B, et al. Development of motor system dysfunction following whiplash injury. Pain. 2003;103:65–73.
Sterling, M, Jull, G, Kenardy, J. Physical and psychological factors maintain long-term predictive capacity post-whiplash injury. Pain. 2006;122:102–108.
Sterling, M, Pedler, A. A neuropathic pain component is common in acute whiplash and associated with a more complex clinical presentation. Man Ther. 2009;14:173–179.
Sterling, M, Pedler, A, Chan, C, et al. Cervical lateral glide increases nociceptive threshold but not pressure and thermal pain in chronic whiplash associated disorders: A pilot randomised controlled trial. Man Ther. 2010;15(2):149–153.
Taylor, AJ, Kerry, R. A ‘system based’ approach to risk assessment of the cervical spine prior to manual therapy. Int J Osteopath Med. 2010;13:85–93.
Thacker, MA, Clark, AK, Marchand, F, et al. Pathophysiology of peripheral neuropathic pain: immune cells and molecules. Anesth Analg. 2007;105:838–847.
Thanvi, B, Munshi, SK, Dawson, SL, et al. Carotid and vertebral artery dissection syndrome. Postgrad Med J. 2005;81(956):383–388.
Thayer, JF, Sternberg, EM. Neural aspects of immunomodulation: focus on the vagus nerve. Brain Behav Immun. 2010;24:1223–1228.
Tracey, I, Bushnell, MC. How neuroimaging studies have challenged us to rethink: is chronic pain a disease? J Pain. 2009;10(11):1113–1120.
Treleaven, J, Jull, G, Sterling, M. Dizziness and unsteadiness following whiplash injury –characteristic features and relationships with cervical joint position error. J Rehabil. 2003;34:1–8.
Tsao, H, Galea, MP, Hodges, PW. Reorganization of the motor cortex is associated with postural control deficits in recurrent low back pain. Brain. 2008;131:2161–2171.
van der Heide, B, Allison, G, Zusman, M. Pain and muscular responses to a neural tissue provocation test in the upper limb. Man Ther. 2001;6(3):154–162.
Van Oosterwijck, J, Nijs, J, Meeus, M, et al. Pain neurophysiology education improves cognitions, pain thresholds, and movement performance in people with chronic whiplash: a pilot study. J Rehabil Res Dev. 2011;48(1):43–58.
Vlaeyen, JWS, Linton, SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85:317–332.
Vincent, MB. Headache and neck. Curr Pain Headache Rep. 2011;15:324–331.
Vicenzino, B, Collins, D, Wright, A. The initial effects of cervical spine physiotherapy treatment on the pain and dysfunction of lateral epicondylalgia. Pain. 1996;68:69–74.
Vicenzino, B, Collins, D, Benison, H, et al. An investigation of the interrelationship between manipulative therapy induced hypoalgesia sympathoexcitation. J Manipulative Physiol Ther. 1998;21:448–453.
von Piekartz, HJM, Schouten, S, Aufdemkampe, G. Neurodynamic responses in children with migraine or cervicogenic headache versus a control group. A comparative study. Man Ther. 2007;12:153–160.
Watkins, LR, Maier, SF. The pain of being sick: implications of immune-to-brain communication for understanding pain. Annu Rev Psychol. 2000;51:29–57.
Williams, JE, Yen, JT, Parker, G, et al. Prevalence of pain in head and neck cancer out-patients. J Laryngol Otol. 2010;124(7):767–773.
Williams PL, Warwick R, Dyson M, et al, eds. Gray's anatomy, 37e. Edinburgh: Churchill Livingstone, 1989.
Winkelstein, B, DeLeo, J. Mechanical thresholds for initiation and persistence of pain following nerve root injury: mechanical and chemical contributions at injury. J Biomech Eng. 2004;126(2):258–263.
Woolf, C. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–S15.
Woolf, CJ. The dorsal horn: state dependent sensory processing and the generation of pain. Textbook of Pain. P. D. Wall and R. Melzack. Edinburgh: Churchill Livingstone; 1994.
Woolf, C, Ma, Q. Nociceptors: noxious stimulus detectors. Neuron. 2007;55:353–364.
Woolf, C, Salter, MW. Neural plasticity: increasing the gain in pain. Science. 2000;288:1765–1769.
Wright, A. Pain-relieving effect of cervical manual therapy. In: Grant R, ed. Physical therapy of the cervical and thoracic spine. New York: Churchill-Livingstone; 2002:217–238.

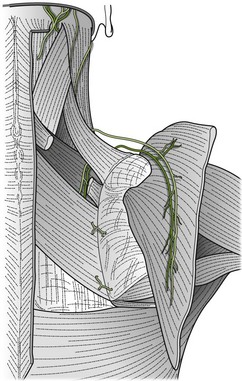

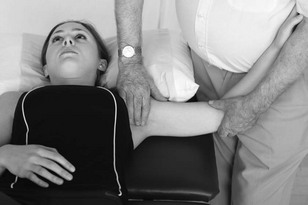
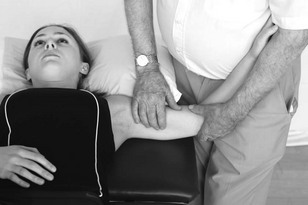
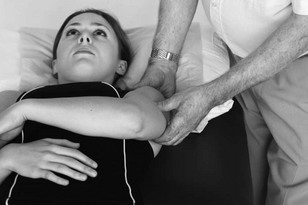






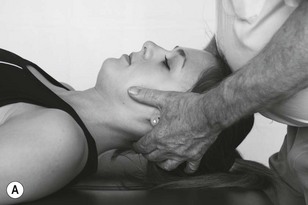
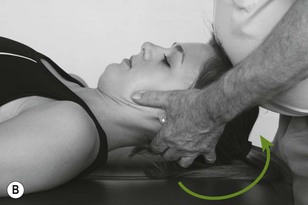
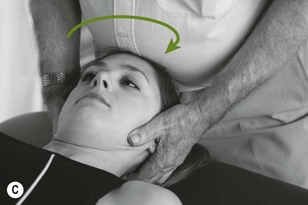


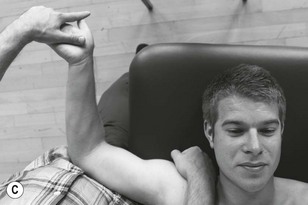




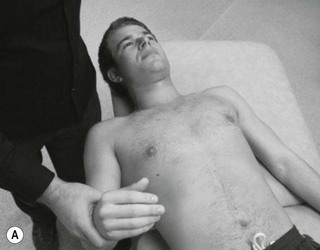

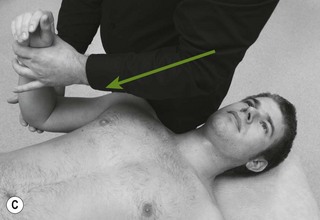
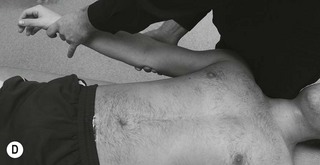









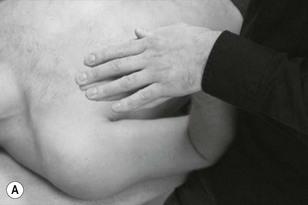
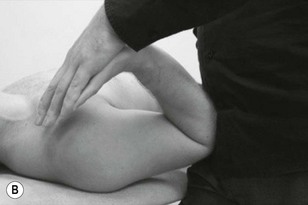
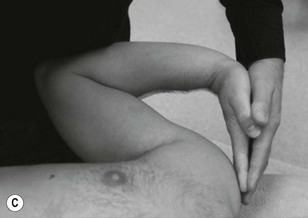


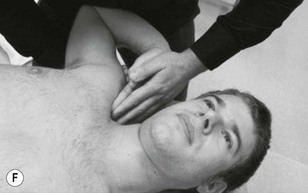
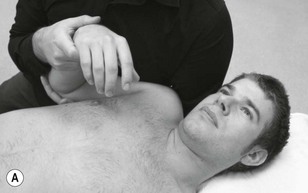



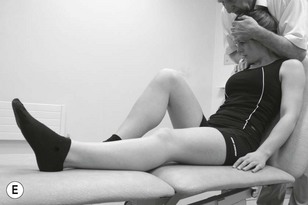
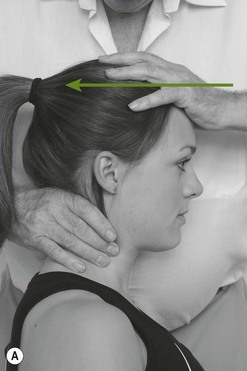
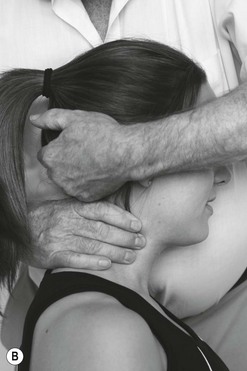
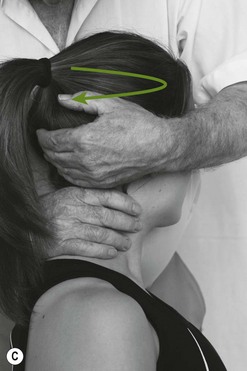
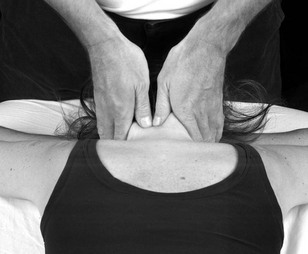
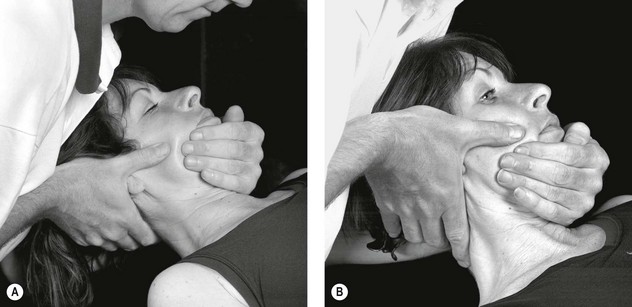
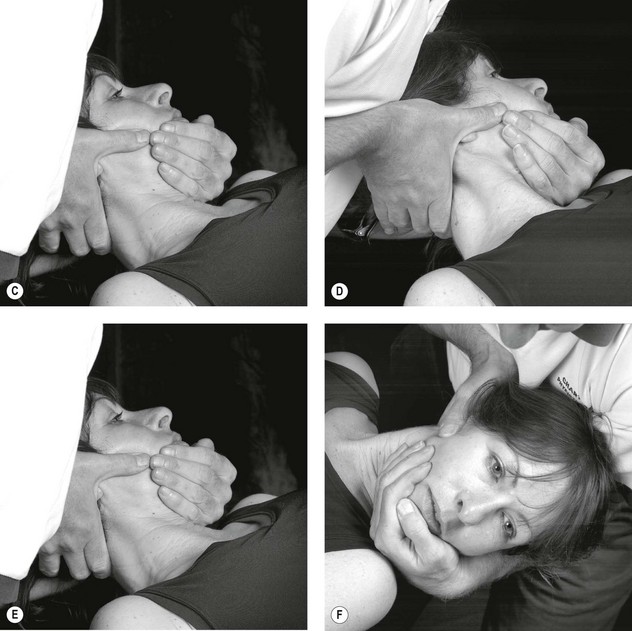




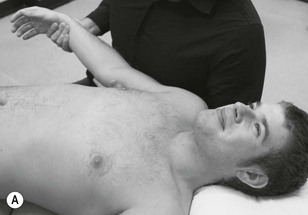
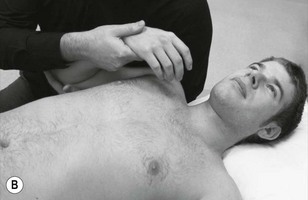
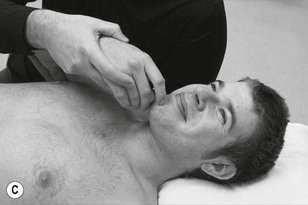


 came on with a jolt as a low flying Tornado jet flew overhead. Initially the pain subsided within 2 days or so but returned 2 weeks later. This time the neck pain got worse, spreading into the left scapular region, as a dull ache
came on with a jolt as a low flying Tornado jet flew overhead. Initially the pain subsided within 2 days or so but returned 2 weeks later. This time the neck pain got worse, spreading into the left scapular region, as a dull ache  and a heavy achy feeling in the left upper limb
and a heavy achy feeling in the left upper limb  with pins and needles in the hand
with pins and needles in the hand  . Pat reported the neck becoming stiffer and increasingly painful to move. Refer to the body chart in
. Pat reported the neck becoming stiffer and increasingly painful to move. Refer to the body chart in