Chronic obstructive pulmonary disease (COPD)
Although chronic bronchitis and pulmonary emphysema are distinct disorders, they often coexist in the patient and it can be difficult to determine the relative importance of each condition in the individual case. The term ‘COPD’ often applies to a combination of the two. In emphysema, the fine architecture of the alveoli is damaged, leading to impairment of ventilatory capacity. There is probably little that can be done to reverse this destruction (although some clinicians feel that bioavailable silica and herbs rich in this mineral, such as Equisetum and Urtica can help restore lung architecture).
In contrast, chronic bronchitis is a syndrome that can develop in response to long-term exposure to various types of irritants to the bronchial mucous membranes. These include cigarette smoke, dust and automobile or industrial air pollution, especially in conjunction with a damp climate. Acute infection is usually a precipitating or aggravating factor and chronic infection is usually present, with regular acute episodes. Hence, there are many factors in chronic bronchitis that are treatable, and long-term herbal treatment can dramatically alter the course of chronic bronchitis.
In chronic bronchitis, ventilatory capacity is reasonably preserved but hypoxia, pulmonary hypertension and right ventricular failure occur early – ‘the blue bloater’. In emphysema, the impairment of ventilatory capacity and exertional dyspnoea lead to the sufferer being labelled a ‘pink puffer’. A mixed syndrome is most common and all patients should be treated along the following lines, regardless of their clinical label. The treatment outcome will, however, depend on how much the changes in their lungs can be reversed.
Treatment
In chronic bronchitis there is overactivity of the mucus-secreting glands and goblet cells. The vast excess of mucus coats the bronchial walls and clogs the bronchioles. Exacerbating this, many ciliated columnar cells are replaced by goblet cells in response to the chronic irritation. Therefore the excessive mucus is also less able to be cleared from the lungs. Hence, the use of expectorants is emphasised in the treatment of chronic bronchitis, despite the fact that an easily productive cough can be a feature of this disease. (In some patients sputum may be scanty and tenacious, which also requires treatment with expectorants.)
• Bronchial irritation must be avoided. Giving up smoking and a change in occupation or climate may be necessary. Mucus-producing foods such as dairy products and bananas should be reduced
• Any chronic infection should be treated and acute infections prevented by immune-enhancing herbs, especially Echinacea root and Astragalus (discontinue Astragalus during acute febrile infections). The role here of Panax ginseng has been supported in at least one clinical trial.27 Many chronic bronchitis patients are constitutionally cold, so the cold herbs Picrorrhiza and Andrographis are best avoided. Heating herbs such as cinnamon may be helpful and could possibly be used in conjunction with these cold herbs
• Expectorant herbs, such as Inula helenium, Thymus vulgaris, Polygala and other saponin-containing herbs, Foeniculum (fennel), Pimpinella (aniseed) and Marrubium (white horehound) can be prescribed throughout the course of the disorder. The diffusive stimulant properties of Zingiber will potentiate the activity of expectorants
• Respiratory antiseptic herbs that also have expectorant or mucolytic properties are particularly indicated, such as Inula helenium, Thymus vulgaris and Allium sativum
• Since the goblet cells are oversecreting, anticatarrhal herbs such as Verbascum, Plantago lanceolata and Hydrastis can help to reduce this excessive secretion
• If there is an unproductive cough at night, a separate formula containing demulcents such as Althaea glycetract and Glycyrrhiza, and antitussives such as Glycyrrhiza and Bupleurum, may be prescribed (see below)
• Inhalation of peppermint and eucalyptus oils combined can help loosen mucus and dilate airways to make breathing easier
• Bronchodilating herbs such as Coleus and Lobelia may be helpful. Ephedra should probably be avoided. Those with expectorant activity such as Grindelia can also be selected
• Since chronic inflammation is present, anti-inflammatory herbs such as Glycyrrhiza, Bupleurum and Rehmannia will be of value, as well as omega-3 fatty acids
• Support for the heart and general circulation with Crataegus and Ginkgo may be required.
Demulcent/antitussive formula
The following demulcent formula can also be used to relieve an irritable cough:
| Althaea officinalis glycetract | 1:5 | 80 mL |
| Glycyrrhiza glabra (high in glycyrrhizin) | 1:1 | 20 mL |
| total | 100 mL |
Dose: 4 mL sipped undiluted (that is no water is added) as required up to 6 times a day.
Case history
A male patient, 66 years, has received herbal treatment for chronic bronchitis for 7 years. During this time there has been considerable improvement in the patient’s condition and friends often now comment on how well he looks. The frequency of acute episodes has substantially reduced and his lung function parameters have improved. Although treatment varied over this time period, a representative herbal treatment is as follows.
| Echinacea angustifolia/purpurea root | 1:2 | 45 mL |
| Arctium lappa | 1:2 | 15 mL |
| Achillea millefolium | 1:2 | 20 mL |
| Withania somnifera | 1:2 | 20 mL |
| total | 100 mL |
Dose: 5 mL with water three times a day.
| Glycyrrhiza glabra | 1:1 | 15 mL |
| Inula helenium | 1:2 | 20 mL |
| Zingiber officinale | 1:2 | 10 mL |
| Foeniculum vulgare | 1:2 | 15 mL |
| Thymus vulgaris | 1:2 | 20 mL |
| Grindelia camporum | 1:2 | 20 mL |
| total | 100 mL |
Bronchiectasis
‘Bronchiectasis’ describes an abnormal dilatation of the bronchi that becomes a focus for chronic infection. In most cases it develops as a complication of a severe bacterial infection and then follows a chronic course. Clinical features include chronic cough, often with copious purulent sputum, and febrile episodes with malaise and night sweats that can last from a few days to weeks, and sometimes haemoptysis. The disorder can be debilitating. Although continual use of antibiotics is inadvisable, patients can often receive this regime.
Treatment
Essential aspects of the treatment of bronchiectasis are as follows:
• Immune-enhancing herbs such as Echinacea root, Andrographis and Astragalus
• Respiratory antiseptic herbs such as Inula helenium, Thymus vulgaris and Allium sativum
• Diaphoretics such as Asclepias tuberosa (pleurisy root) during the febrile episodes
• Tonics such as Panax, Rhodiola, Eleutherococcus and/or Withania if debility is present
• Anticatarrhal herbs, including Verbascum, Plantago lanceolata and Hydrastis
• Expectorant herbs such as Inula helenium, Thymus vulgaris, Polygala and other saponin-containing herbs, Foeniculum (fennel), Pimpinella (aniseed) and Marrubium (white horehound)
• Note that Astragalus, Panax and Eleutherococcus should be discontinued during any acute febrile phases.
Example liquid formula
| Echinacea purpurea/angustifolia root | 1:2 | 40 mL |
| Inula helenium | 1:2 | 25 mL |
| Glycyrrhiza glabra (high in glycyrrhizin) | 1:1 | 15 mL |
| Hydrastis canadensis | 1:3 | 30 mL |
| total | 110 mL |
Dose: 8 mL with water twice daily.
Case history
A male patient, 59 years, with bronchiectasis. He was initially coughing up an egg cup of sputum every morning and experiencing occasional febrile episodes and acute viral infections. This patient has now been maintained on phytotherapy for more than 15 years. Apart from the occasional winter respiratory infection he remains well. His sputum production is minimal and he freely claims that herbs have ‘kept me alive’.
• Herbal treatment consisted of the following: Echinacea purpurea/angustifolia root tablets (1.275 g), two tablets one to two times daily. The Echinacea liquid disagreed with this patient, hence the tablets. The higher dose is taken during febrile episodes and acute infections.
| Aesculus hippocastanum | 1:2 | 15 mL |
| Foeniculum vulgare | 1:2 | 10 mL |
| Thymus vulgaris | 1:2 | 30 mL |
| Astragalus membranaceus | 1:2 | 25 mL |
| Inula helenium | 1:2 | 20 mL |
| total | 100 mL |
The Aesculus was mainly for circulatory problems but it also has expectorant properties due to its saponin content.
Asthma
In terms of phytotherapy, asthma is probably the most complex of the respiratory disorders. The successful management of asthma hence embodies most of the principles already discussed. Asthma can be defined as the occurrence of dyspnoeic bronchospasmodic crises linked to an airways hyper-responsiveness (AHR). Like autoimmune disease it is a chronic disturbance of immunological function that can be controlled to some extent, but not eradicated by modern drug therapy. In other words asthma is not just the attacks (crises); it is a chronic disturbance of the immune system with the attacks being the ‘tip of the iceberg’. Hence any treatment aimed only at relaxing airways and relieving symptoms, be it orthodox or herbal, is superficial and will not change the chronicity of the disease.
Recent research has identified many factors that contribute to the aetiology and morbidity of asthma. Traditional herbal medicine also recognises the role of inefficient digestion, poor immunity, stress, diet and unhealthy mucous membranes in the development of the disease. In order to treat asthma more effectively with phytotherapy, it is necessary to have an understanding of the causative and sustaining factors contributing to the condition. For each individual it is likely that the disease process has been precipitated by a unique and complex interaction of contributive events. A multi-factorial model that allows the individualisation of the patient, yet at the same time incorporates the most likely factors operating in asthma, is discussed below. This in turn requires the synthesis of traditional herbal understanding with the latest research findings, which is fundamental to the practice of modern phytotherapy.
Asthma can be classified as extrinsic (allergic asthma) or intrinsic asthma. Although there has been some confusion with the terms, and some medical scientists feel that the classification is meaningless,28 the differentiation is quite clear. Patients with extrinsic asthma comprise the majority of cases and exhibit a positive skin test to common aeroallergens and foods. Serum IgE levels are usually raised. However, extrinsic asthmatics can still be exacerbated by non-specific stimuli such as cold air and exercise. Intrinsic asthmatics have negative skin tests, and chronic infection and other factors are thought to play a role in the disease process. Intrinsic asthma is usually later in onset and more severe.29 Aspirin-sensitive asthma (ASA) is a form of intrinsic asthma. Both types of asthma show an increased family occurrence.30
Pathophysiology
Asthmatic lungs are characterised by epithelial cell loss, goblet cell hyperplasia, increased collagen deposition, mast cell degranulation and inflammatory cell infiltration.31 Asthma is now primarily classified as an inflammatory disorder. The desquamation allows allergens to penetrate more easily and exposes irritant receptors.
There has been an increased understanding over the past two decades that asthma is a chronic, immunologically mediated condition with a disturbance of the normal airway repair mechanism, which results in inflammatory changes and airway remodelling.32 The airway inflammation and remodelling together likely explain the clinical manifestations of asthma. The mechanisms by which the external environmental cues, together with the complex genetic actions, propagate the inflammatory process that characterise asthma are beginning to be understood. There is also an evolving awareness of the active participation of structural elements, such as the airway epithelium, airway smooth muscle and endothelium in this process. In tandem with this has come the realisation that inflammatory cells respond in a coordinated, albeit dysfunctional, manner via an array of complex signalling pathways that facilitate communication between these cells; these structural elements within the lung and the bone marrow serve as reservoirs for and the source of inflammatory cells and their precursors. Although often viewed as separate mechanistic entities, innate and acquired immunity often overlap in the propagation of the asthmatic response.32
Classically, asthma, specifically allergic asthma, has been attributed to a hyperactive Th2 cell immune response. However, the Th2 cell-mediated inflammation model has failed to adequately explain many of the clinical and molecular aspects of asthma.33 In addition, the outcomes of Th2-targeted therapeutic trials have been disappointing. Thus, asthma is now believed to be a complex and heterogeneous disorder, with several molecular mechanisms underlying the airway inflammation and AHR that is associated with it. The original classification of Th1 and Th2 pathways has recently been expanded to include additional effector Th cell subsets. These include Th17, Th9 and Treg cells. Emerging data highlight the involvement of these new T helper cell subsets in the initiation and augmentation of airway inflammation and asthmatic responses.33
That regulatory T cells (Tregs) have a crucial role in controlling allergic diseases such as asthma is now undisputed. The cytokines most commonly implicated in Treg-mediated suppression of allergic asthma are transforming growth factor-beta (TGF-beta) and interleukin (IL)-10. In addition to naturally occurring Tregs, adaptive Tregs, induced in response to foreign antigens, have been shown in recent studies. The concept of inducible/adaptive Tregs (iTregs) has considerable significance in preventing asthma, if such cells are generated early enough in life.34
Other inflammatory cells implicated in asthma include natural killer T cells, although their role is controversial,35,36 mast cells, neutrophils and eosinophils.37 Platelets and endothelial cells also play a role.
As touched on above, some experts are arguing for a re-evaluation of the therapeutic implications informed by a pathophysiological understanding of asthma. A case is made that asthma has its origins in the airways themselves, involving defective structural and functional behaviour of the epithelium in relation to environmental insults, with wall thickening.38 Specifically, a defect in barrier function and an impaired innate immune response to viral infection may provide the substrate upon which allergic sensitisation takes place. Once sensitised, the repeated allergen exposure will lead to disease persistence. These mechanisms could also be used to explain the observed airway wall remodelling and the susceptibility of the asthmatic lung to exacerbations provoked by respiratory viruses, air pollution episodes and exposure to biologically active allergens. It seems that the problem lies in placing allergy at the centre of disease pathogenesis, when in practice other environmental factors may be equally if not more important in the induction and then progression of asthma. Instead a defect of epithelial barrier function exists that, as in atopic dermatitis, allows greater access of environmental allergens, microorganisms, and toxicants to the airway tissue. Evidence indeed indicates that both the physical and functional barrier of the airway epithelium is defective in asthma with disrupted tight junctions, reduced antioxidant activity, and impaired innate immunity. Viewing asthma primarily as an epithelial disease, with the conceptual adoption of a chronic wound scenario, also provides a route to understand the observed airway wall remodelling and the varying asthma phenotypes over its life course.39
A host of inflammatory mediators have been identified in the pathophysiology of asthma. However some mediators may be more involved in triggering the inflammatory process than others. In this context, important mediators are the Th2 cytokines and probably histamine, platelet activating factor (PAF), major basic protein, leukotrienes especially (leukotriene B4)40 and to a lesser extent prostaglandins.37 Patients with ASA have increased PAF responsiveness, reduced prostaglandin levels and increased leukotrienes compared with normal controls.41,42 TGF-beta is a key player in airway remodelling.
The host of inflammatory cells and mediators involved in the pathogenic process means that treatment directed at a single mediator or cell is unlikely to be successful. A multi-faceted approach to treatment is required. This is compatible with herbal therapy, which has traditionally used combinations of plants to treat diseases.
The above considerations also explain the current preference in conventional medicine for steroid use in asthma. These drugs have a broad suppressing effect on many inflammatory mechanisms. In this context the ‘magic bullet’ is more like an ordinary shotgun.
Factors associated with asthma
Differentiation should be made between the causes that initiate or sustain the underlying condition, which are probably factors that result in injury to the lining of the lungs, and the triggers that precipitate the asthmatic attack. While avoidance of the latter group is of course important, it is only attention to the former that will reduce the progression of the disease. There is no better illustration of this issue than the subject of dairy products. Traditional knowledge suggests that consumption of dairy products can lead to a state of unhealthy mucous membranes in sensitive patients. However, these patients may not give a positive skin test to dairy products, and these products may not provoke an acute asthmatic attack. In the classical sense there is no allergy to dairy products. Yet the avoidance of this food group will, in time, give appropriate patients considerable relief from their asthmatic condition. Key factors amenable to treatment are reviewed below.
Allergens
The most significant allergen in the long-term development of asthma is now considered to be the house dust mite. However, this does not necessarily mean that the degree of house dust mite exposure will correlate with the day-to-day severity of asthma. This is because sensitivity to the house dust mite feeds the underlying pathological process. Unfortunately, chemical and physical methods aimed at reducing exposure to house dust mite allergens have yielded disappointing results.43
Other common factors involved in asthma development may include cats, cockroaches, grass pollens and molds, but the situation is complex, as for dust mite.44 Association with severe asthma in children was seen between non-feather bedding, especially foam pillows and the current ownership of furry pets, or ownership at birth.45 A Finnish study of school children aged 7 to 13 found that moisture and mold problems in the school building were linked to respiratory infections and asthma.46
Of course, all the above allergens can trigger an asthma attack, as can many foods, especially dairy products, eggs and nuts. Dust mite contamination of wheat flour caused anaphylactic reactions and cooking had no effect. It was suggested that flour be stored in the refrigerator.47 Royal jelly should be used cautiously as it is now a well-known trigger of asthma attacks.
Air quality
Maternal or parental smoking has been linked to asthma incidence and severity.48 Air pollution parameters such as NO2, ozone and particulates have also been associated with the incidence of asthma.49
Exposure to dust, irritants and allergens at the workplace can also cause asthma. Usually withdrawal from the irritant or allergen results in remission, but in some cases where exposure is prolonged the asthma becomes self-sustaining despite such withdrawal.
Sinusitis
Sinusitis has been associated with asthma in several studies,50,51 although this has received less attention recently. This is not considered to be due to aspiration of sinus contents.52 In one study 79% of asthma cases had chronic rhinitis or rhinosinusitis.53 In 69% of cases the nasal symptoms coincided with or preceded the onset of asthma. In 59% of associated cases, nasal symptoms coincided with acute asthmatic episodes. Treatment of the nasal condition improved the asthma. The link between sinusitis and asthma is strongest in patients with intrinsic asthma. Evidence supports the concept that rhinosinusitis and asthma may be the expression of an inflammatory process that appears in different sites of the respiratory tract at different times.54
Poor digestion
A number of researchers in the 1930s found a high incidence of hypochlorhydria in asthmatic patients.55 In these studies the test meal method was used. This method is now considered inappropriate for the assessment of histological hypochlorhydria. However it does assess for functional hypochlorhydria, that is a deficiency of vagal stimulation of acid production. Hydrochloric acid therapy improved the asthma.55 Since this kind of gastric deficiency is due to deficient vagal output, the use of bitter herbs that act through a vagal reflex to increase gastric digestion is indicated (see Chapter 2).
Gastro-oesophageal reflux (GOR) has been linked to asthma in several studies. In fact there is evidence that GOR is an important aetiological factor for some asthmatics. Monitoring of oesophageal pH revealed GOR in seven out of nine patients with persistent asthma.56 In another study 61% of patients with intrinsic asthma exhibited GOR.57 From studies on children it was concluded that asthma symptoms were more often elicited by exposure of the distal oesophagus to gastric acid, possibly by a vagal reflex, than by aspiration of gastric juice.58 However, other studies have implicated the importance of aspiration.59,60 In support of the reflex hypothesis, subjects with GOR (but not asthma) showed a significantly greater bronchial reactivity to histamine than normal matched controls.61
Treatment of GOR by surgery or drugs can result in improvement or cure of asthma, although results are mixed and suggest that targetting of individual patients is necessary.62 (Also see Chapter 2, under Mucilages.)
Diet
Particular foods are well-known causes of asthma attacks. However, the contribution of diet to the underlying pathophysiological processes of asthma is not well studied. It has been postulated that dietary changes may have contributed to the rise in asthma mortality.63 In particular, increased consumption of polyunsaturated fatty acids has resulted in the doubling from 8% to 15% of the linoleic content of body fat.63 Such a change could be regarded as pro-inflammatory.
Coffee consumption was found to be inversely correlated to the prevalence of bronchial asthma in an Italian study.64 Epidemiological studies found that consumption of oily fish may protect against asthma in childhood65 and adults,66 and a connection between low magnesium intake and increased asthma risk has also been suggested.67 Consumption of apples (five per week) was found to improve lung function.68 This benefit was attributed to the flavonoids found in apples.
A systematic review and meta-analysis found weak but supportive protective evidence with respect to dietary vitamins A, D and E, zinc, fruits and vegetables and a Mediterranean diet.69
Infection and infestation
Viral infections are known to exacerbate asthma. Viral infection in the upper airways frequently triggers deterioration in airways hyperreactivity in asthmatics.70 Most hospital admissions for asthma occur over the winter months and soon after the start of school terms.70 Viral infections also worsen asthma in adults.71,72
There is also some evidence to suggest that viral infections may also contribute to the development of asthma, especially in children. About 92% of children hospitalised with respiratory syncytial virus (RSV) bronchiolitis in the first year of life subsequently developed symptoms suggestive of asthma within 5 years.73 Of this group, 71% had clinical evidence of asthma.73 Recurrent upper respiratory tract infections are associated with asthma risk in children74 and 37% of children with viral lower respiratory tract infections in infancy subsequently developed asthma.75 With the development of molecular diagnostics, human rhinovirus wheezing illnesses have been recognised more recently as a stronger predictor of school-age asthma than RSV.76
In contrast, bacterial infection has been linked to adult-onset asthma. The first study linking repeated or prolonged exposure to Chlamydia pneumoniae with wheezing, asthmatic bronchitis and asthma was published in 1991.77 Patients with evidence of C. pneumoniae exposure comprised 81% of 26 patients with asthmatic bronchitis, 100% of asthmatic bronchitics 40 years and older, and 8 out of 10 patients with asthma. However, findings from later studies have been inconsistent.78 Associations between Mycoplasma species and chronic asthma are relatively well-established.78
A group of 12 adult patients with asthma and chronic fungal skin infection were found to have hypersensitivity to Trichophyton spp.79 Several patients had many of the features of late-onset intrinsic asthma. A Russian study in children with bronchial asthma living near a microbiological factory found that most were hypersensitive to Candida.80 Asthma has also been associated with parasitic infestation with Strongyloides stercoralis.81
Salt intake and dehydration
Regional sales of table salt in England and Wales are strongly correlated with deaths from asthma in men and children.82 A study of 138 men found a close relation between AHR and 24 h urinary sodium excretion.83 Other studies have yielded conflicting results. A large randomised controlled trial of slow sodium supplementation showed an increase in AHR in men, but not women,84 and a low salt intake was correlated with improved asthma in men.85 Several mechanisms for this association have been postulated, such as an increase in circulating Na,K-ATPase inhibitors or a decrease in catecholamine concentration.86 Data supporting dietary salt restriction for reducing AHR in asthmatics are encouraging.87
Exercise-induced asthma may be related to dehydration of the intrathoracic airways during hyperpnoea.88 A high salt intake may interfere with rehydration of the airways. Mouth breathing as a result of sinusitis may also cause airway dehydration.
Hormonal factors
Glucocorticoid insufficiency related to various adrenal and extra-adrenal mechanisms has been associated with asthma in a Russian study.89 Reduced nocturnal catecholamine and cortisol levels, which are a natural part of circadian rhythm, may be linked to the nocturnal exacerbation commonly associated with asthma.90 Stress-induced corticosteroid resistance or insensitivity in asthma is receiving research attention,91 as is hyporesponsiveness of the HPA (hypothalamic-pituitary- adrenal) axis (see also below).92
It was postulated that an increase in brain norepinephrine can reflect depression of the HPA axis.93 In asthmatic children such an increase was found compared with controls.93
Premenstrual asthma has been observed, which was improved by progesterone injection.94 Dynamic changes in hormone concentrations during the perimenstrual period are thought to be responsible for the rise in emergency admissions of asthmatic women at this time. Hormonal variation may prove to be a significant and independent risk factor for acute exacerbations of asthma.95 Although the observed 4-fold increase in emergency admissions suggested that hormonal variation may influence the timing of an asthma attack, the severity of symptoms was no worse than that among women who presented at any other time in their menstrual cycle.
Stress
Low-income patients were more likely to report exacerbation of asthma when upset or anxious.96 Parents of children who developed asthma were more likely to have problems in coping and parenting.97
Stress and associated muscular tension might influence lung function. One study suggested that an intrinsic impairment of the ability of inspiration to stretch airway smooth muscle is a major feature of asthma.98 In other words, defective deep breathing could be a factor in airway narrowing. This would suggest a role for relaxation and breathing techniques in asthma.
Antioxidant status
The role of oxidative stress in asthma is gaining increasing research attention.99 Activation of the Nrf2/ARE pathway could prove to be of therapeutic benefit.100 Patients with acute asthma had decreased antiperoxide plasma activity compared with normal controls.101 Moreover the severity of the asthma was inversely associated with the above parameter. In patients with steroid-dependent asthma the free radical process was more intensive.101 Low selenium concentrations in whole blood have been correlated to increased asthma risk in New Zealand.102
Traditional factors associated with asthma
Poor digestion
Floyer in his Treatize of the Asthma published in 1698 wrote:
Some writers … have observed the hypochondriac symptoms in the stomach and conclude the asthma … wants digestives …. It is commonly observed that fullness of diet, and all debauches render the fits most severe, and a temperate diet makes the fits more easy …. The defect of digestion and mucilaginous slime in the stomach are very obvious and observed by writers ….
… the precursory symptoms are connected with the stomach and consist of loss of appetite, flatulence, costiveness and certain peculiar uneasy sensations in the epigastrium; but here I think we have something more than mere premonitory signs; I think the relation of these symptoms to the spasm which follows is often that of cause and effect.
Unhealthy mucous membranes and diet
Mucus secreted by mucous membranes (MM) is normal and has many important physiological functions. When MM become unhealthy, the nature and the quantity of the mucus changes. This can be referred to as a catarrhal condition. The degree of catarrhal congestion of the lungs can vary in asthma, and is best assessed by auscultation and case history. The association of chronic sinusitis with asthma is indicative of unhealthy MM. Despite the excessive mucus, catarrhal MM are thought to provide less protection than healthy MM, and in the case of asthma render the lungs more prone to damaging environmental factors such as allergens and pathogens. This traditional concept ties in well with the newer understanding of asthma as primarily a defect of airway epithelium, as described above.
Diet is a significant factor in causing a catarrhal state of the respiratory MM. Excessive protein, refined carbohydrate or salt consumption can lead to excessive and unhealthy mucus production. In some individuals, particular food groups especially dairy and/or wheat can also contribute to this process.
Catarrhal MM can also be regarded as a vicarious elimination due to inefficient detoxification and elimination in the body. Hence in traditional terms the detoxifying and eliminative processes need to be supported and stimulated.
Stress
Traditionally asthma has been regarded as imbalance in the autonomic nervous system. Stress and nervous anxiety contribute to this imbalance which may also cause muscular tension in the diaphragm and disturb the rhythmic nature of the breathing apparatus.It has been postulated that asthma results from a disturbance of the rhythmic activity of the respiratory centre in the brain.103 This disorder is then reflected in the airways and the respiratory muscles through their respective innervations to cause a subclinical template of asthma. This rhythmic disturbance is reflected in the EEG of asthmatic patients, which generally contain certain abnormalities.103
Treatment
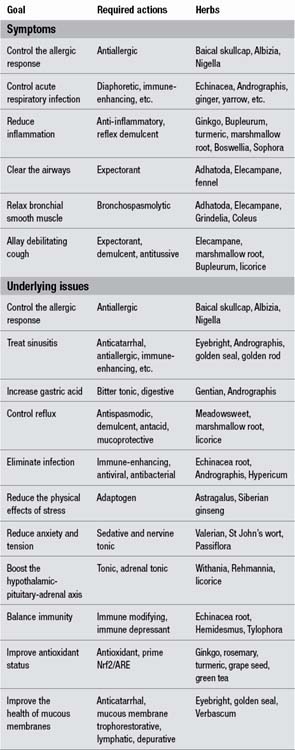
Treatment goals will obviously vary according to the needs of the individual case at the particular time of treatment. However, based on the above considerations they can be divided into two main categories: symptomatic treatment and treatment of the underlying issues (Table 9.1). The required actions related to the specific treatment goals and relevant herbs are also provided. Some treatment goals, such as resolving sinusitis, are complete therapeutic subjects in themselves and are covered elsewhere in this chapter.
This approach is informed by modern research and contrasts with the traditional approach that merely focussed on bronchodilator and expectorant activities (Table 9.2).
Table 9.2 Major herbs for asthma according to the British Herbal Pharmacopoeia 1983
| Herb | Action |
|---|---|
| Datura | Spasmolytic |
| Drosera | Bronchodilator, expectorant |
| Ephedra | Bronchodilator (antiallergic) |
| Euphorbia | Spasmolytic, expectorant |
| Grindelia | Antispasmodic, expectorant |
| Lobelia | Spasmolytic, expectorant |
Some key herbs in asthma
Some plants have specialised effects on the inflammatory or allergic mechanisms known to occur in asthma. Since some of these effects have only recently been discovered or studied, a discussion of the research findings follows.
Cepaenes and thiosulfinates from Allium cepa (onion) are dual inhibitors of arachidonic acid metabolism, as are the gingerols from Zingiber officinale (ginger). See also the monograph on turmeric regarding its relevant anti-inflammatory and antiasthmatic activities. Boswellia has been successfully trialled in asthma on the basis of its anti-inflammatory activity (see monograph).
Isothiocyanates, cepaenes and thiosulfinates from Allium cepa have demonstrated an asthma-protective effect in animal models. The thiosulfinates counter platelet activating factor (PAF) and histamine-induced bronchoconstriction. In a human experiment, allergen-induced asthma attacks were almost completely inhibited by an Allium cepa extract.104
In vitro studies in the late 1980s discovered that the ginkgolides from the Ginkgo biloba leaf are potent and specific PAF antagonists (see monograph). In vivo animal studies (oral and injected routes) confirmed this activity.105 Ginkgo and ginkgolides have also shown some beneficial clinical activity in asthma (see monograph).
Tylophora indica (Tylophora asthmatica) is a potent antiasthmatic herb that depresses cell-mediated immunity.106 It also stimulates the adrenal cortex, increasing plasma steroid levels and antagonising steroid-induced suppression of adrenal activity.107 Several poorly designed clinical trials using Tylophora have shown benefits.108
Flavonoids from Scutellaria baicalensis have marked antiallergic activity. Baicalin and baicalein demonstrated antiallergic and antiasthmatic activity in several animal models.109 For example oral administration of baicalin to egg-white-sensitised guinea pigs protected them against allergic reaction from re-inspiration of the antigen. Both compounds suppressed cutaneous allergy in guinea pigs.109 A soluble derivative of baicalein was antiallergic after oral administration110 and demonstrated more extensive antiallergic activity than sodium cromoglycate in vitro.111 Other flavonoids from Baical skullcap were active in inhibiting the histamine release from rat peritoneal mast cells in vitro,112 and baicalein inhibited basophil histamine content and growth in vitro.113
Saiboku-To (TJ-96) is a Kampo medicine comprising 10 herbs including Baical skullcap. It has been used in Japan for glucocorticoid-dependent asthmatic patients with the aim of reducing the dose of administered glucocorticoids. The antiallergic action of Saiboku-To is based on the suppression of type I and IV allergic reaction, which has been confirmed in animal studies.114
Albizia lebbek has mast cell stabilising activity.115 Its antiallergic effects may also be mediated by suppression of lymphocyte function.116
Adhatoda vasica is a small evergreen, subherbaceous bush that grows on the plains of India, in the lower Himalayan ranges and in Sri Lanka, Burma and Malaysia. Adhatoda leaf has been used extensively in the Ayurvedic system for over 2000 years.117 It has antiasthmatic, bronchodilating and expectorant activities and is traditionally used for the treatment of asthma, bronchitis cough and common cold.118 In the World Health Organization publication The Use of Traditional Medicine in Primary Health Care: A Manual for Health Workers in South-East Asia, Adhatoda was said to facilitate breathing and to make sputum more fluid, thereby facilitating its removal. Adhatoda preparations are recommended for long-term use in adults and children.119 However, it is contraindicated during pregnancy.
Key constituents of Adhatoda leaf are the quinazoline alkaloids (0.5% to 2%). The major alkaloid is vasicine present at levels of 45% to 95%. Minor alkaloids include vasicinine, vasicinone, oxyvasicinine, deoxyvasicine, deoxyvasicinone and vasicinol.120,121 The drug bromhexine was developed from vasicine in Europe prior to the 1960s and is still used as an aid to expectoration by reducing the viscosity of secretions.122 Oral administration of a mixture of vasicine and vasicinone (25 mg, three times per day) showed good bronchodilating activity in asthma patients. Seventy per cent demonstrated clinical improvement and improvement in spirometry.123
Uncontrolled clinical trials conducted in India as early as 1925 suggested that Adhatoda had an expectorant action. In acute bronchitis Adhatoda provided relief, especially where the sputum was thick and tenacious. In patients with chronic bronchitis cough was relieved and the sputum thinned, which facilitated removal. Mild relief was achieved for asthma.118
There are approximately 40 to 60 species of Grindelia native to temperate, mostly arid and semi-arid regions of North and South America. Species of Grindelia (commonly called gumweeds or gumplant) are not well differentiated and the taxonomy of the genus is still poorly understood. The resins produced by Grindelia consist mostly of labdane-type diterpenoid resin acids, similar in chemistry and physical properties to those obtained from pine trees. Resins from various species of Grindelia have been patented for use in adhesives, rubber, coatings and textiles.124
Several species of Grindelia are used in phytotherapy including Grindelia camporum, G. robusta, G. squarrosa and G. humilis. Constituents of the medicinal Grindelia spp. include the resin together with phenolic acids, flavonoids, an essential oil and small amounts of saponins.125
Grindelia is an expectorant herb with bronchospasmolytic activity. It is traditionally recommended for the treatment of spasmodic respiratory conditions such as asthma and bronchitis. The British Herbal Pharmacopoeia 1983 lists the specific indication as bronchial asthma with tachycardia.126 Eclectic physicians also utilised Grindelia for asthma.127
The Chinese herb Sophora flavescens, both on its own and in a formulation, has yielded promising results in asthma management. Its pharmacological properties include diuretic, antiviral, antitumour, sedative and anti-inflammatory activities. The main active components are matrine-type alkaloids, particularly matrine and oxymatrine.128 It has been suggested that the Sophora alkaloids can act as modulators of cell membrane excitability. This is based on its CNS effects, anti-arrhythmic activity and inhibition of glutamate-induced responses.128 On the hypothesis that an excitatory modulator (especially in the context of glutamate responses) may be beneficial in asthma, an open trial of Sophora in refractory asthma was initiated.128
From February 1997 to December 2005, 14 patients with moderate to severe asthma (six men and eight women) aged 22 to 70 years were treated. These patients had been diagnosed with asthma by their allergists and had been receiving medication for asthma for 3 to 6 years. Despite years of moderate to high doses of inhaled corticosteroids and beta-2 agonists, they still suffered episodes of dyspnoea, expectoration, coughing, wheezing or chest tightness more than two times a week and waking up at night with asthma symptoms more than two times a week.128 The patients were given a dried powder of a hot water extract of S. flavescens root, which contained a high content of matrine and oxymatrine (1.8% to 3.2%). The extract was provided in capsules, with a dose equal to 4 g of dried root, three times daily for 3 months and two times daily for 6 months and once daily for 27 months thereafter. Since the study was open, non-randomised and selective, the results below are summarised based on the records of the diary card of symptoms, PEF (peak expiratory flow), medication use and quality of life.128
After 4 weeks, the daytime asthma symptoms were reduced by 78%, and the night-time symptoms by 75%. Beta-2 agonist dose was reduced by 72% and the dose of corticosteroid inhaler reduced by 45%. The mean PEF rate improved by 12%. Two patients had remarkable improvements of eczema symptoms. No side effects were observed.128 At 1 year, the daytime symptoms of asthma reduced by 94% and night-time symptoms by 95%. Beta-2 agonist use was reduced by 95%; corticosteroid inhaler was reduced by 92%. The mean PEF had increased by 18%. After 3 years, the daytime symptoms of asthma were reduced by 97%, night-time symptoms by 98%. The dose of beta-2 agonist was reduced by 97% and no patients inhaled corticosteroids. The mean PEF had increased by 21%. At 3 years, nine of the patients had achieved a symptom-free, medication-free and also an asthma-free condition, meaning they did not develop asthma symptoms when they were exposed to the previous triggers of their asthma attacks. Two of the patients with functional and radiological evidence of emphysema achieved an improvement in both breathing capacity and airway fibrosis (emphysema) to the extent that they were no longer considered to have emphysema. Two patients had complete remission of their eczema.128
Despite these striking results, they must be viewed with caution since this was an open label trial and there was no placebo group. Hence, it is encouraging to learn that Sophora in conjunction with two other traditional Chinese herbs (Glycyrrhiza uralensis (licorice) and Ganoderma lucidum) was found to be active in a controlled trial.129 The efficacy and tolerability of this formula was investigated in 91 patients with moderate to severe asthma in a double blind, randomised trial. The herbal treatment was compared with oral prednisone therapy.129
Patients in the herbal group received oral capsules (3.6 g/day of the mixture) and placebo tablets similar in appearance to prednisone. Those in the prednisone group received oral prednisone tablets (20 mg/day in the morning) and ‘herbal placebo capsules’, for 4 weeks. Treatment was administered daily over a period of 4 weeks. No medications other than rescue beta-2 agonists were allowed. This study found that post-treatment lung function (FEV1 and PEF values) was significantly improved in both groups. The improvement was slightly but significantly greater in the prednisone group (p<0.05). There was a significant and a similar degree of reduction in clinical symptom scores in both treated groups.129
Of interest, the Th2 cytokines IL-5 and IL-13 were significantly reduced in both treated groups (p<0.001 for each). Strikingly, serum interferon (IFN)-gamma and cortisol levels were significantly decreased in the prednisone group (p<0.001), but significantly increased in the herbal group (p<0.001). In addition, the herbal treatment had no significant effect on body weight (increases in body weight post-therapy, 2.8 kg in the prednisone group versus 0.8 kg in the herbal group). No significant side effects were observed.
Prescription construction
Asthma is a deep-seated protracted condition that requires herbal treatment in pharmacological doses. Hence a high dosage protocol is proposed. Based on the treatment goals outlined above it is suggested that two formulations, each of four to six herbs, are developed for the individual patient, or otherwise one formulation and a herbal tablet or capsule.
One should be a long-term treatment aimed at treating the underlying factors behind the asthmatic condition. The second can be aimed at the symptoms and sustaining causes. (A third formula could be developed which is only to be taken to alleviate acute attacks, but these days most patients will resort to a bronchodilating drug in these circumstances.)
The herbs in the two formulations should be chosen so that there is as much overlap of the required actions as possible. This reduces the number of herbs needed to give a broad range of required actions (as informed by Table 9.1). For example:
The adult patient is then prescribed an 8 mL dose of each formula two to three times a day. Dosages are adjusted for children based on their body weight.
The treatment should be varied over time depending on the patient’s response. Also not every factor can necessarily be treated at the one time, so particular treatment goals may need to be changed from time to time. This should be a dynamic and interactive approach. The following case histories illustrate some aspects of this strategy.
Case history
The patient, 16-year-old teenage girl, had had asthma since the age of 6. She had been hospitalised several times, including recently. She had marked upper respiratory allergy and ‘had a constant cold all last year’. Medication was inhaled bronchodilators and steroids. The patient was advised to eliminate all dairy products from her diet.
The following herbal tablets were prescribed:
• A tablet containing Echinacea angustifolia root 500 mg, Ocimum tenuiflorum 500 mg, Andrographis paniculata 1000 mg and Ocimum tenuiflorum leaf essential oil 10 mg. Dose 4/day
• A tablet containing Boswellia serrata 1.9 g, Apium graveolens 1000 mg, Zingiber officinale 300 mg and Curcuma longa 2 g. Dose 2/day
• A tablet containing Echinacea angustifolia root 600 mg and Echinacea purpurea root 675 mg. Dose 1/day
• A tablet containing Scutellaria baicalensis 800 mg, Albizia lebbek 800 mg and Tanacetum parthenium 50 mg. Dose 2/day.
Over a 6-month treatment period the patient’s condition improved dramatically. The need for inhaled conventional drugs was reduced to almost zero, her sinuses cleared and there was a considerable enhancement of general well-being.
The following case illustrates how herbal tablets can be effectively used to manage asthma in patients who have difficulty negotiating tinctures and liquid extracts. Here the tablets were largely aimed at controlling inflammation and allergy, and supporting immune function.
Case history
A 75-year-old female patient developed late onset asthma following a bout of lower respiratory infections and was prescribed prednisone by her medical doctor (now stopped). She also had pronounced sinus congestion and was under stress because of selling and moving house.
The following herbal treatments were instituted:
| Euphrasia officinalis | 1:2 | 25 mL |
| Ginkgo biloba (standardised extract) | 2:1 | 25 mL |
| Echinacea angustifolia/purpurea root | 1:2 | 25 mL |
| Eleutherococcus senticosus | 1:2 | 25 mL |
| total | 100 mL |
Dose: 8 mL with water twice a day
Three herbal tablets per day containing: Scutellaria baicalensis 500 mg, Adhatoda vasica 750 mg, Grindelia camporum 300 mg, Curcuma longa 1000 mg, Ginkgo biloba leaf 1000 mg and Foeniculum vulgare essential oil 5 mg.
After 4 weeks, the patient reported that her asthma had substantially improved. This improvement was also sustained by the next consultation 4 weeks later. The patient was then subsequently maintained with just the herbal tablets, which according to her reports provided substantial relief for her asthma on an ongoing basis.