Black cohosh
Synonyms
Cimicifuga racemosa (L.) Nutt. (botanical synonym), bugbane, black snakeroot (Engl), Cimicifugae rhizoma (Lat), schwarzes Wanzenkraut, Cimicifugawurzelstock (Ger), cimicaire, actée à grappes (Fr), sølvlys (Dan).
What is it?
Black cohosh is still widely referred to by its former botanical name, Cimicifuga racemosa. However, a taxonomic revision published in 1998 reclassified the genus Actaea to include Cimicifuga.1 The common names of black snakeroot and rattle snakeroot refer to its former use in North America, where it is native, to treat snakebite including that of rattlesnake. The old generic name Cimicifuga comes from the Latin ‘to chase insects away’ and reflects on a reputed use of the European species. Black cohosh rhizome is a popular treatment for menopausal symptoms, and proprietary medicines based on black cohosh are registered in Germany. The part used therapeutically consists of the fresh or dried rhizome with attached roots.2,3
Effects
Suppresses luteinising hormone (LH), allays inflammation, promotes fertility, improves bone density.
Traditional view
Black cohosh, a favourite of the Eclectic physicians, was used for myalgia, neuralgia (not of spinal origin), chorea, female reproductive tract disorders (amenorrhoea, dysmenorrhoea, ovarian pain and menorrhagia) and rheumatic conditions (arthralgia, muscular rheumatism). Other conditions treated included whooping cough, tinnitus and mastitis.4,5 Black cohosh is also used to treat premenstrual syndrome and secondary amenorrhoea in Germany.3,6
Preparations
Dried or fresh rhizome for decoction, liquid extract or solid dose forms for internal use.
Dosage
Typical adult dosage ranges used by Western herbalists are:
• 0.9 to 6 g/day of dried root and rhizome or by decoction
• 0.9 to 6 mL/day of a 1:1 liquid extract
• 1.5 to 3 mL/day of a 1:2 liquid extract, 3.0 to 7.5 mL/day of the 1:5 tincture, or equivalent doses in tablet or capsule form.
However, herbalists typically now recommend doses at the lower end of these ranges. In addition doses at the higher end have been linked to adverse reactions such as headaches (see Overdosage section).
Typical adult dosage ranges used in most clinical trials of German products:2
Duration of use
May be taken long term within the recommended dosage, although the Commission E recommends not for more than 6 months.
Summary assessment of safety
Generally no adverse effects are expected from ingestion of black cohosh when used at the lower end of the recommended dosage. Dosage at the higher end may cause headaches. Black cohosh has been associated with rare cases of liver damage, although this link is disputed. It is not recommended during pregnancy except for assisting childbirth, although there is no strong indication of harm.
Technical data
Botany
Black cohosh is a member of the Ranunculaceae (buttercup) family and grows to an average height of 150 cm.7 It produces white blossoms in slender, feathery drooping racemes and a dry fruit containing numerous seeds. The leaves have three-pointed, trilobate leaflets. The fleshy, dark brown/black rhizome is a creeping underground stem, from which follow dark brown roots.2,8
Adulteration
Other Actaea/Cimicifuga species, particularly C. americana, have been unintentionally mixed with black cohosh due to the similarity in aboveground appearance. Occasionally black cohosh is adulterated with the underground portions of baneberry (Actaea pachypoda and A. rubra).2 In August 1998, Australian manufacturers were alerted to the possibility of substitution of black cohosh by other Cimicifuga spp. In May 2001 the Therapeutic Goods Administration Laboratories advised that 35% of the Australian products they tested indicated the presence of species other than Cimicifuga racemosa. Manufacturers were advised to verify their raw materials to ensure the correct Cimicifuga species is used. Other medicinal Cimicifuga species were implicated such as C. foetida, C. dahurica, C. heracleifolia and C. simplex.9 See also the findings of Health Canada regarding adulteration (cited under Side effects in the safety section later in this monograph).
Key constituents
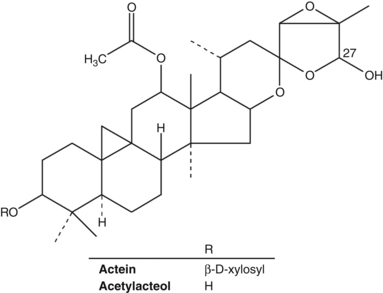
Constituents of black cohosh include at least 20 triterpene glycosides (saponins) of the cycloartane type, including actein, 23-epi-26-deoxyactein, cimiracemoside A and cimicifugoside.
Other constituents include aromatic acids (including ferulic, isoferulic and acyl caffeic acids), resins (cimicifugin), tannins and fatty acids.10,11 Despite earlier reports, the herb does not contain significant quantities of isoflavones (see below).
Pharmacodynamics
The pharmacology of black cohosh is poorly understood. The question of whether the herb has oestrogenic activity in particular remains unresolved and conflicting results from many studies on this topic are available. While the majority of more recent studies indicate that black cohosh extracts do not contain compounds that are potent agonists at either of the two known oestrogen receptors, black cohosh appears to possess several pharmacological effects that are consistent with oestrogen-like activity, including lowering of LH levels (by injection) and protection against menopausal bone loss. Meanwhile, other potential mechanisms of action have emerged such as serotonergic, dopaminergic and opioid receptor activity, all of which could potentially play a part in the ameliorating effects of black cohosh on menopausal symptoms. In addition, anti-inflammatory activity has been demonstrated in vitro, supporting some traditional uses of the herb. Any oestrogenic effects from the herb are likely to be complex and mild.
Hormonal activity
In early research, prolonged injections of black cohosh extract in rats and mice increased the weight of the uterus and established menstrual cycles in juvenile and climacteric animals.12 Black cohosh extract demonstrated a selective reduction of serum LH in ovariectomised rats. FSH (follicle-stimulating hormone) and prolactin levels were unchanged. Intraperitoneal administration of black cohosh extract (24 mg dried extract per day) resulted in significant inhibition of LH secretion after the third day. Fractionated extraction of black cohosh and subsequent testing with three fractions indicated that the LH-suppressive substances resided in the dichloromethane extract. The intact glycosidal components of this extract were more active with regard to LH suppression than the aglycone form. Oral administration of the non-hydrolysed extract demonstrated a significant inhibition of LH, although much lower than that achieved by injection.13 (This does not necessarily mean the aglycone is inactive, as the glycoside may provide enhanced bioavailability with cleavage of the sugars yielding an active aglycone.)14
A subsequent study by the same group found serum levels of LH in ovariectomised rats were reduced after intraperitoneal administration of a trichloromethane fraction of a black cohosh methanolic extract, whereas the ethanolic fraction of the extract did not affect LH levels. The trichloromethane fraction also demonstrated an ability to bind to oestrogen receptors in vitro. This fraction was further separated into three subfractions, two of which suppressed LH secretion in vivo and displaced oestrogen in vitro. This indicates that at least two groups of compounds may be responsible for the endocrine activity of black cohosh.15 One active compound identified was the isoflavone formononetin. However, five more recent studies have failed to detect formononetin in black cohosh,2 a fact that casts some doubt on the extract used in the study.
In other early research, water and chloroform fractions prepared from a black cohosh methanol extract were tested in ovariectomised rats by intraperitoneal injection over several days (chronic administration). The chloroform fraction demonstrated a strong LH-suppressing effect after 3 days; the water fraction was inactive. Further fractionation of the chloroform extract led to the conclusion that the LH-suppressive effect of black cohosh extract was caused by at least three different synergistically acting compounds.16
Another later study in ovariectomised rats showed that a dichloromethane (lipophilic) extract (60 mg subcutaneously for 7 days) produced effects similar to those of oestradiol on LH levels and other biomarkers influenced by oestrogen receptors.17
Two commercial black cohosh extracts were tested for their ability to compete with oestradiol for antigen binding sites on an antibody (IgG) directed against oestradiol (radioimmunoassay). Both extracts ran parallel with the displacement curve obtained with oestradiol, which supports the presence of oestrogenic compounds in black cohosh.18 However, no oestrogenic activity was found after oral or subcutaneous administration of black cohosh extract (6, 60, 600 mg/kg) to groups of immature mice and ovariectomised rats respectively.19
A 50% aqueous ethanolic extract of black cohosh did not bind to oestrogen or progesterone receptors of human breast cancer cell lines (MCF7 and T47D) in vitro (100 μg/mL) and did not affect cell proliferation.20 Similarly, a methanolic extract (200 μg/mL) did not bind to human recombinant alpha and beta oestrogen receptors in vitro.21 Gene expression studies in MCF7 cells found that a lipophilic black cohosh extract had no oestrogenic activity, but instead exerted anti-proliferative and pro-apoptotic effects at the transcriptional level.22 (See also below under Effects on human breast cancer cells.)
Only the lipophilic subfraction of a dry, hydroethanolic extract of black cohosh was able to activate the human alpha oestrogen receptor, but neither the total extract nor the lipophilic sub-fraction showed any in vivo uterotrophic effects in 21-day-old rats.23
One hundred and ten women experiencing menopausal symptoms, who had received no hormone replacement therapy (HRT) for at least 6 months, received either a standardised black cohosh extract or placebo. After 8 weeks of treatment, LH levels were significantly reduced (p<0.05) in the black cohosh group, but FSH was unchanged.16
Effects on menopausal bone loss
Several studies have indicated that black cohosh might protect against menopausal bone loss (see also under Clinical trials).
An in vitro study investigated the effects of an ethanolic black cohosh extract on bone nodule formation in mouse pre-osteoblast cells.24 The extract did not stimulate osteoblast proliferation, but significantly increased bone nodule formation (500 ng/mL), an effect that was shown to result from enhanced gene expression of osteocalcin and Runx2 (a transcription factor involved in osteoblast differentiation). Interestingly, co-treatment with a selective oestrogen receptor antagonist abolished the effects on gene expression, demonstrating the involvement of an oestrogen receptor-dependent mechanism.
An experimental model was designed to understand the mechanism of action of black cohosh on bone tissue and to compare its effects with oestrogen and testosterone.25 RANK (receptor activator of nuclear factor-kappaB, NF-kappaB) and its ligand RANKL largely regulate osteoclast activity and hence bone breakdown. Crosslaps (or Ratlaps in rats), specifically the metabolic products of bone-specific collagen-1 alpha1, are markers of such bone degradation. When black cohosh, oestradiol and testosterone were given to castrated rats of both sexes, oestradiol and black cohosh reduced levels of RANKL and Ratlaps, the latter parameter only in female rats. The authors suggested that the bone sparing effect of black cohosh is therefore partly mediated by inhibition of RANKL. However, the receptors involved in mediating this effect are not thought to be oestrogen receptors.
In a 35-day study of metaphyseal fracture healing in ovariectomised rats with early stage osteoporosis, the effects of a black cohosh supplemented diet (dried aqueous ethanolic extract, average 24.9 mg/day) was compared with oestrogen treatment (average 0.03 mg/kg/day).26 A high rate of metaphyseal callus formation was observed in the animals receiving black cohosh, but oestrogen improved fracture healing more, with the authors commenting that the 5-week treatment period was possibly too short for black cohosh to demonstrate its full potential.
The triterpenoid glycoside 25-acetylcimigenol xylopyranoside isolated from black cohosh was shown in vitro to potently block osteoclastogenesis induced by tumour necrosis factor-alpha (TNF-alpha) and a related cytokine. The compound was also found to reduce bone loss induced by TNF-alpha in vivo.27
Effects on human breast cancer cells
Because of the potential oestrogen-like action of black cohosh, the issue of its safety in oestrogen-dependent tumours is of significant clinical interest, and a number of studies have been undertaken in an attempt to clarify this issue.
Unlike oestradiol, black cohosh extract did not stimulate growth of mammary tumour cells in vitro. In fact, a dosage of 2.5 mg/mL led to a strong inhibition of proliferation.28 The simultaneous incubation of tumour cells with tamoxifen (anticarcinogenic agent, oestrogen antagonist) and black cohosh displayed a much stronger inhibition of growth than for either substance alone.29 (Oestrogen is contraindicated in patients with oestrogen receptor-positive breast carcinoma, since it promotes the growth of the tumour cells.)
Several other studies have demonstrated that black cohosh does not promote the growth of human breast cancer cells in vitro. A 50% aqueous ethanolic extract (100 μg/mL) did not promote cell proliferation of T47D cells.20 In another study, a methanol-water extract was fractionated. A fraction rich in triterpenoid glycosides inhibited the growth of two human breast cancer cell lines (IC50 values of 10 to 20 μg/mL).30 The same fraction also caused cell cycle arrest at G1 (30 μg/m and G2 (60 μg/mL), suggesting that different compounds are involved in causing cell cycle arrest. The triterpene glycosides actein, 23-epi-26-deoxyactein and cimiracemoside A were shown to be involved in these effects.
In a subsequent gene expression study conducted by the same group, actein was shown to activate transcription factors that enhance apoptosis.31 It also repressed cell cycle genes and acted synergistically with two chemotherapy agents, doxorubicin and 5-fluorouracil, in causing growth inhibition of the breast cancer cell line MDA-mB 453.32 The same study also showed that actein enhanced the induction of apoptosis by paclitaxel, 5-fluorouracil and doxorubicin.
A review of the safety and efficacy of black cohosh for cancer patients assessed data from clinical (n=5) and pre-clinical (n=21) studies as well as case reports.33 The five clinical studies all involved women with breast cancer or a history of the disease. None of the adverse effects reported from these trials (including one case of breast cancer recurrence) was linked to the black cohosh treatment. The authors of the review concluded that black cohosh does not have phyto-oestrogenic activity, appears to be safe for breast cancer patients and may potentially be protective against breast cancer, as it appears to inhibit the growth of tumour cells in vitro (See also under Clinical trials and Toxicology.)
Effects on prostate cancer cells
In vitro studies have demonstrated that black cohosh can inhibit proliferation of prostate cancer cells. In one study, the cell growth inhibitory effects of an isopropanolic extract of black cohosh on androgen-sensitive LNCaP and androgen-insensitive PC-3 and DU 145 prostate cancer cells were investigated. Results showed the extract caused a significant dose- and time-dependent downregulation of all prostate cancer cell lines after 72 h (IC50 values between 37.1 and 62.7 μg/mL). Further, the study demonstrated that the extract killed prostate cancer cells by induction of apoptosis and activation of caspases, regardless of the hormone responsive status of the cells.34
A phenolic compound isolated from black cohosh, petasiphenone, was studied in vitro with regard to its effects on the proliferation of LNCaP cells (incubated with 10 nM oestradiol, 1 nM dihydrotestosterone, or without either) and their secretion of prostate-specific antigen (PSA). A dose-dependent inhibition of the LNCaP cells was observed regardless of hormone treatment, while PSA release was not altered.35
In an in vivo study, a black cohosh extract was tested in immunodeficient mice inoculated sc with LNCaP cells. Inoculation with the prostate cancer cells resulted in formation of solid tumours in 12 of 18 control animals, compared with only five of 18 animals treated with dietary black cohosh extract (2 to 2.8 mg extract/day). After 10 weeks, the amount of tumour tissue in the black cohosh-treated animals was significantly less than in controls. Serum testosterone levels were not significantly affected by the treatment.36
Other activity possibly contributing to menopausal effects
While the nature and degree of any oestrogenic activity of black cohosh remains unresolved, other potential mechanisms of action have emerged. That several mechanisms might be at play in a chemically complex herbal medicine is of course unsurprising.
A commercial black cohosh extract demonstrated in vitro dopaminergic activity in the dopamine receptor D2 assay,37 and there are suggestions that serotonergic effects might play a role in the therapeutic effects of black cohosh. Researchers at the University of Illinois at Chicago working on black cohosh extract did not find evidence of oestrogen receptor binding or oestrogenic effects in animals, but did observe strong binding to several serotonin receptor subtypes in vitro, with partial agonist activity at the 5-HT7 receptor.38 The same group has also shown that black cohosh acts as a mixed competitive ligand and partial agonist at the human mu opioid receptor.39
Central opioid activity has also been shown in a mechanistic study involving 11 postmenopausal women.40 The study employed two methods to examine the effects on central opioid function: naloxone challenge (n=6) and positron emission tomography with a selective micro-opioid receptor radioligand (n=5), before and after 12 weeks of standard treatment with a black cohosh isopropanolic extract (40 mg/day). Treatment did not affect oestrogen levels or spontaneous LH pulsatility, but administration of naloxone (a competitive mu opioid receptor antagonist) caused suppression of mean LH pulse frequency. This was most marked during sleep, when the mean interpulse interval was prolonged by 90 minutes. Positron emission tomography showed significant increases in mu opioid receptor binding potential in parts of the brain involved with emotional and cognitive function.
Because of the close relationship between the central nervous and hormone systems, it is plausible that mechanisms involving serotonergic, dopaminergic and opioid receptors are at least partly responsible for the effects of black cohosh on menopausal symptoms.
Anti-inflammatory and antioxidant activities
Several recent in vitro studies have lent some support to the traditional use of black cohosh in the treatment of inflammatory conditions. An aqueous extract (up to 6 mg/mL) was found to inhibit the generation of nitric oxide in lipopolysaccharide (LPS)-stimulated macrophages in a concentration-dependent manner. The mechanism for this was shown to be the reduced expression of inducible nitric oxide synthase (iNOS) rather than inhibition of iNOS activity. The triterpenoid glycoside 23-epi-26-deoxyactein was identified as the active compound.41
Another black cohosh compound, cimiracemate A (140 µM), has been shown to suppress the in vitro production of the inflammatory cytokine TNF-alpha induced by LPS in macrophages.42
Aqueous black cohosh extracts (3 and 6 mg/mL) have also been shown to reduce the release of TNF-alpha and the interleukin IL-6 (another inflammatory cytokine) and almost completely block the release of interferon-gamma in LPS-stimulated whole human blood. In contrast, IL-8 (also an inflammatory chemokine) was stimulated. Among five prevalent compounds isolated from the extract, isoferulic acid was found to be responsible for the inhibition of TNF-alpha and IL-6, but not for the stimulation of IL-8.43
Black cohosh showed potent antioxidant activity in the oxygen radical absorption capacity (ORAC) assay in a study that applied a sequential three solvent (ethyl acetate, methanol and 50% aqueous methanol) extraction process and combined the antioxidant activity of the three extracts. Black cohosh was the second most potent of 53 medicinal plants tested, being second only to olive leaf.44
Another in vitro study found black cohosh to be an effective scavenger of 1,1-diphenyl-2-picrylhydrazyl free radicals. Nine compounds with activity in this assay were isolated and six of these (methyl caffeate>caffeic acid>ferulic acid>cimiracemate A>cimiracemate B>fukinolic acid) were found to also reduce menadione-induced DNA damage in cultured S30 breast cancer cells.45
Other activity
Pretreatment with cimicifugoside inhibited blastogenesis in mouse splenic lymphocytes and brought about a decrease in the number of plaque-forming colonies using sheep erythrocytes (SRBC). The anti-SRBC response in the plaque-forming assay was suppressed in mice after pretreatment by intraperitoneal administration, and delayed hypersensitivity was suppressed after intravenous administration. The immunosuppressive activity of cimicifugoside is directed toward B-cell function, with larger doses being required for suppression of T-cell function.46
Black cohosh extract, and several fractions obtained from it, demonstrated hypotensive activity after intravenous administration at 1 mg/kg to rabbits. The hypotensive activity was observed in particular with the resinous part and may be due to actein. A hypotensive effect was not observed in human volunteers (by intravenous administration), although a peripheral vasodilatory effect was evident, even in subjects with peripheral arterial disease.47 Oral administration of black cohosh extract (2 g/kg) inhibited 5-hydroxytryptophan-induced diarrhoea in mice.48
Pharmacokinetics
The triterpene glycoside actein has been shown to be bioavailable in rats when administered by gastric intubation (37.5 mg/kg). Serum levels of actein peaked after 6 h at 2.4 μg/mL, dropping to 0.1 μg/mL 24 h post administration. The urine level of actein at 24 h was 0.8 μg/mL.49
The pharmacokinetics of one of the most abundant triterpenoid glycosides in black cohosh, 23-epi-26-deoxyactein, was studied in 15 healthy, postmenopausal women. They received single oral doses of a 75% ethanolic extract containing 1.4, 2.8 or 5.6 mg of 23-epi-26-deoxyactein. Serial blood and 24 h urine samples were collected and analysed. Peak plasma levels (2.2 to 12.4 ng/mL) and area under the time-concentration curve (a measure of bioavailability) of 23-epi-26-deoxyactein increased proportionally with dosage, and the half-life was 2 to 3 h. Less than 0.01% of the compound was recovered in the urine 24 h after administration, but metabolites were not detected.50
Clinical trials
Menopause
In phytotherapy, black cohosh is predominantly used in the treatment and management of menopausal symptoms. Menopause is the permanent cessation of menstruation and fertility defined as the absence of ovarian follicular activity for at least 12 months. The symptom picture associated with the transition from peri- to postmenopause can range from mild to debilitating, and the duration of symptoms can range from a few months up to 10 years.
Experts on menopause refer to a wide variety of physical symptoms including hot flushes, cardiac complaints, fatigue, vertigo, sweating, muscle pain, muscle spasm, joint pain, urinary incontinence, vaginal dryness and vaginal epithelium atrophy, and psychological symptoms: depression, anxiety, nervousness, irritability, forgetfulness, sleep disturbances and decreased libido. Vasomotor symptoms of hot flashes and night sweats are the most common physical manifestation, occurring in up to 80% of menopausal women.51 Women who experience vasomotor symptoms are also more likely to suffer sleep disturbances and depressive symptoms and experience a negative impact on their quality of life.
A broad array of treatments including hormone therapy, antidepressant medications, herbal and nutritional therapies, and diet and lifestyle modifications are utilised by women to ameliorate unwanted menopausal symptoms.
Black cohosh is the herbal medicine most commonly recommended for managing psychological and physical symptoms of menopause. Standardised black cohosh extract was used with success in the 1950s and 1960s for the treatment of menopausal symptoms, menstrual disturbances in young women (secondary but not primary amenorrhoea) and symptoms arising from ovarian dysfunction or insufficiency.
Research interest in black cohosh has gained momentum since the publication of the Women’s Health Initiative study in 2002. This study found an association between the long-term use of HRT and increased breast cancer and cardiovascular risk.52 As a result of these findings, prescribers and users of HRT began to explore non-hormonal alternatives, the most common being black cohosh. Black cohosh is prescribed alone in a number of proprietary formulas, but is also used in combination with other herbs and nutrients in formulations for menopausal symptoms.
Black cohosh for menopausal symptoms has been investigated extensively in clinical trials, but not all trials have been convincing. This may in part be due to poor trial design, including small sample size and variability in the dose used. Many trials (including several with positive outcomes) have used an extract dose equivalent to 40 mg/day of dried herb, a dose which is low in comparison to the more traditional one recommended in the British Herbal Pharmacopoeia 1983 (0.9 to 6 g/day dried root and rhizome).4
A number of recent comprehensive monographs,53 systematic reviews54,55 and a meta-analysis56 have examined the efficacy and safety of black cohosh for menopausal symptoms; this body of literature represents the most rigorous and global assessment of black cohosh currently available.
A systematic review published in 2008 included six double blind, randomised, controlled trials (n=1112) of black cohosh for the relief of menopausal symptoms.55 Trials that included women suffering medically induced menopause were excluded. Each of the included trials focused on menopausal symptoms, and used a placebo or a standard drug treatment as control. Three of the six trials used an isopropanolic extract in a daily dose equivalent to 40 mg herb, one trial used 160 mg of a 70% ethanolic extract (equivalent to 5 mg of triterpene glycosides), another used a 58% ethanolic extract equivalent to 40 mg of herb, and the sixth used 6.5 mg of a 60% ethanolic extract. Despite the authors’ conclusion that the evidence for the efficacy of black cohosh was still inconclusive, five of the six trials produced some positive outcomes. Details of these six studies are provided below.
In an early trial, Stoll used a randomised, double blind design in 75 women with symptoms of menopause. The 3-month study compared treatment with black cohosh with conjugated oestrogens and placebo. The Kupperman Menopausal Index (KMI), Hamilton anxiety scale, and proliferation status of vaginal epithelium were the outcome measures, and results demonstrated that black cohosh improved all parameters compared with placebo.57
Another 3-month randomised, double blind, multicentre trial involving 62 postmenopausal women also compared black cohosh (equivalent to 40 mg/day), conjugated oestrogens and placebo. Outcome measures included the Menopause Rating Scale (MRS), markers of bone formation and degradation, and endometrial thickness. Results of this trial showed that both active treatments were effective in reducing menopausal symptoms and both had beneficial effects on bone metabolism. Unlike treatment with conjugated oestrogens, black cohosh did not affect endometrial thickness.58
Osmers and co-workers conducted a 12-week randomised, double blind, placebo-controlled multicentre trial involving 304 women with menopausal symptoms. Women in the black cohosh group received extract equivalent to 40 mg/day, and the MRS was again the primary outcome measure. The women receiving black cohosh experienced significantly greater improvement in menopausal symptoms, especially hot flushes, compared with the placebo group. Women in early menopause benefited the most.59
A multicentre, randomised, placebo-controlled, double blind study was conducted by Frei-Kleiner and co-workers in 122 menopausal women experiencing at least three hot flushes daily. They were treated over 12 weeks with a black cohosh extract (equivalent to 42 mg herb) or placebo. Outcome measures were a weekly score for hot flushes, the Kupperman Index and the MRS. Overall the results showed no significant difference between the two groups. However, significant benefits of black cohosh were evident in women with more severe symptoms (Kupperman Index score of at least 20), and the effects of black cohosh over placebo almost reached significance (p=0.052) for the subgroup comprising perimenopausal women.60
In the HALT study, a 12-month randomised, double blind, trial was undertaken with 351 peri or postmenopausal women. They received either black cohosh (160 mg of a 70% ethanolic extract equivalent to 5 mg triterpene glycosides daily), a multi-botanical preparation (alfalfa, chaste tree, dong quai, false unicorn, licorice, oats, pomegranate, Siberian ginseng, boron; 200 mg daily), the multi-botanical plus telephone counselling to increase dietary soy intake, conjugated oestrogens with or without medroxyprogesterone acetate, or placebo. The main outcome measures were frequency and intensity of vasomotor symptoms. The study found no difference between the herbal interventions and placebo at 3, 6 or 12 months, whereas the HRT resulted in a significant decrease in vasomotor symptoms compared with placebo (p<0.001).61
Bai and colleagues conducted a 3-month double blind, multicentre, non-inferiority trial in China in 244 women with menopausal symptoms. They were randomised to receive either black cohosh extract (equivalent to 40 mg/day) or the synthetic steroid drug tibolone (2.5 mg/day), which has been shown in numerous placebo-controlled trials to be effective in the treatment of menopausal symptoms. The main outcome measures were the KI and the frequency of adverse events. The effects of the two treatments were similar and clinically relevant: in the black cohosh group mean KI decreased from 24.7 at baseline to 11.2 and 7.7 after 4 and 12 weeks, respectively, while the corresponding scores for the tibolone group were 11.2 and 7.5. These results demonstrated that the black cohosh treatment was as effective as tibolone treatment (non-inferiority was statistically significant, p=0.002), with the benefit-risk balance for black cohosh being significantly superior to tibolone (p=0.01).62
A 2010 meta-analysis by researchers at McGill University in Montreal included nine randomised, placebo-controlled trials of black cohosh-containing preparations for menopausal symptoms. Four of the included trials have been reviewed above; the additional five trials used black cohosh in combination with other active ingredients such as St John’s wort, isoflavones, lignans or other herbs. Overall, the authors of this meta-analysis found that six of the nine studies demonstrated a significant effect for the black cohosh-containing intervention over placebo. Combining the data from seven trials, they calculated an estimated improvement in menopausal vasomotor symptoms of 26% (95% CI 11–40%) with black cohosh treatment.56 However, the value of comparing different treatments in a meta-analysis is questionable. The five trials of black cohosh in combination with other active ingredients are reviewed below.
The efficacy of black cohosh in combination with St John’s wort was assessed in 89 symptomatic peri- and postmenopausal women in a 12-week double blind, randomised, placebo-controlled, multicentre study. The main outcome measure was the Kupperman Index (KI); biochemical parameters (hormone levels and lipid profiles) and pathology (vaginal atrophy) were also measured. The active treatment consisted of a 264 mg tablet containing 0.0364 mL of extract from black cohosh rhizome (equivalent to 1 mg terpene glycosides) and 84 mg of dried extract from Hypericum perforatum, equivalent to 0.25 mg hypericin. Treatment with the herbal preparation resulted in significantly greater reduction in KI scores after 4 (p=0.002) and 12 (p<0.001) weeks, compared with placebo. There were no other clinically significant differences between the two groups (although HDL levels decreased marginally in the placebo group). The results demonstrated this combination of black cohosh and St John’s wort to be effective in alleviating menopausal symptoms.63
A second study also examined the efficacy of the fixed combination of black cohosh and St John’s wort in women with menopausal symptoms. The double blind, randomised, placebo-controlled study included 301 women experiencing menopausal symptoms with a pronounced psychological component. They were treated with ethanolic St John’s wort extract and isopropanolic black cohosh extract or placebo for 16 weeks. The MRS and the Hamilton Depression Rating Scale were used to measure outcomes. The mean MRS score decreased 50% (0.46 to 0.23) in the treatment group and 19.6% (0.46 to 0.37) in the placebo group, whereas the Hamilton Depression Rating Scale total score decreased 41.8% (18.9 to 11.0) in the treatment group compared with 12.7% (18.9 to 16.5) in the placebo group. The herbal treatment was significantly (p<0.001) better than placebo for both measures.64
Another randomised, double blind, placebo-controlled trial in 50 healthy peri- and postmenopausal women assessed a formula containing standardised extracts of black cohosh, dong quai, milk thistle, red clover, American ginseng and chaste tree berry for 3 months. Frequency and intensity of menopausal symptoms were monitored by way of a structured questionnaire, administered weekly. Biochemical tests, breast checks and transvaginal ultrasonography were also performed. Women receiving the herbal formula reported a significant and progressive reduction in menopausal symptoms over the placebo group. After 3 months there was a 73% decrease in hot flushes and a 69% reduction of night sweats, with a decrease in symptom intensity and improved sleep quality in the herbal group. Complete cessation of hot flushes was reported by 47% of women in the herbal group compared with 19% in the placebo group. Vaginal ultrasonography, hormone levels (oestradiol, FSH), liver enzymes or thyroid-stimulating hormone showed no change in either group.65
A study by an Italian group evaluated the short-term effects of a combination of black cohosh with isoflavones and lignans on acute menopause-related symptoms in postmenopausal women using a double blind, randomised, placebo-controlled design. Eighty healthy, postmenopausal women were randomly assigned to receive either the combination formula or a placebo (calcium supplement). The groups were similar at baseline, but after 3 months of treatment the KI was significantly lower (p<0.05) in the black cohosh-phyto-oestrogen group compared with the placebo group.66
A supplement containing black cohosh and soy isoflavones was studied in a randomised, placebo-controlled, double blind multicentre 12-week trial involving 124 women who experienced at least five vasomotor symptoms every 24 h. They were randomised to receive daily either the black cohosh-isoflavone supplement or placebo. The modified KI and the Greene Climacteric Scale were used to measure outcomes. After 6 and 12 weeks of treatment, all scores had improved in both groups compared with baseline, and there was no statistically significant difference between the supplement and placebo groups.67
A large scale, observational study comparing the effect of black cohosh alone and black cohosh in combination with St John’s wort also deserves mention. The study was conducted in Germany between March 2002 and March 2004, and included 6141 women with any menopausal symptoms and who had not taken HRT in the 4 weeks preceding the study. The participants had mostly mild to moderate symptoms and were treated with recommended doses of black cohosh monotherapy (isopropanolic extract equivalent to 20 mg herb per tablet) or combination therapy (isopropanolic extract of black cohosh equivalent to 30 mg herb plus St John’s wort extract equivalent to 245 to 350 mg herb per tablet). The treating physician determined the choice and dose of treatment. Patients were evaluated at baseline, 3 and 6 months, with some patients electing to continue for a further 6 months. The primary outcome measures were the MRS and the PSYCHE sub-score of the MRS. In women receiving black cohosh monotherapy, total MRS scores declined from baseline by 0.10 and 0.14 after 3 and 6 months, respectively, whereas the corresponding decreases were 0.12 and 0.18 for the combination therapy, suggesting that the addition of St John’s wort provided an additional effect. Similarly, the changes on the PSYCHE sub-score were significantly greater with the combination therapy than with black cohosh alone (p<0.001). However, the data are potentially confounded by the fact that baseline scores for the two groups were not equal and doses were not fixed. Improvements were sustained by both treatments at 6 and 12 months.68
In a small double blind, placebo-controlled trial, black cohosh had no significant anxiolytic activity in women with anxiety disorder due to menopause.69
Breast cancer patients with menopausal symptoms
Black cohosh used in the management of hot flushes in patients with breast cancer has also been studied in clinical trials. Two such studies, with conflicting results, are summarised below. In an open label randomised trial, 136 breast cancer survivors (35 to 52 years) were treated over 12 months with a black cohosh extract for hot flushes caused by tamoxifen therapy. The patients had all been treated with segmental or total mastectomy, chemotherapy and radiation therapy and were receiving tamoxifen 20 mg/day (n=46) or tamoxifen 20 mg/day plus black cohosh (equivalent to 20 mg herb per day) (n=90). Results revealed those in the intervention (black cohosh) group had fewer and less severe hot flushes compared with controls. After 12 months’ treatment nearly half of those receiving black cohosh were completely free of hot flushes, while 24.4% of the black cohosh group versus 73.9% of the tamoxifen group were still reporting severe hot flushes (p<0.01).70
An earlier randomised, placebo-controlled trial involving 85 breast cancer survivors stratified for tamoxifen use (59 on tamoxifen and 26 not on tamoxifen) did not find any effect of black cohosh over placebo in terms of number and severity of hot flushes. The dose of black cohosh used in this trial was not provided in the report. Subjects completed a 4-day hot flush diary at baseline and at 30 and 60 days, as well as a menopause symptom questionnaire at baseline and at the final visit. FSH and LH levels were measured in a subset of patients at the first and final visits, but no significant differences were detected between the two groups.71
Bone loss prevention
Bone mineral loss (osteoporosis) is a major consideration for postmenopausal women, as it is a major age-related source of morbidity and mortality. A 12-week double blind study in 62 postmenopausal women compared the effects of a black cohosh preparation (equivalent to 40 mg herb per day), conjugated oestrogens or placebo on bone metabolism and other parameters. Black cohosh and conjugated oestrogens were found to have beneficial effects of similar magnitude on bone metabolism. Black cohosh treatment significantly increased serum levels of bone-specific alkaline phosphatase, indicative of increased osteoblast activity. Treatment with conjugated oestrogens, however, did not produce this effect, but appeared to decrease the activity of osteoclasts. Hence, while the net effects on bone of the two treatments were comparable, it appeared that the mechanism of action differed.58
This effect of black cohosh on bone density parameters appears to be mild and, on current evidence, it could not be credibly proposed as a treatment for osteoporosis. However, it probably should play a role as part of an appropriate diet, supplementation and lifestyle regime for the prevention of osteoporosis in both men and women, especially those with borderline osteopenia.
A study in 128 women found that, while an exercise programme favourably affected bone health, adjuvant supplementation with black cohosh (40 mg/day) did not enhance this effect.72 Perhaps a higher dose might have been necessary for this experimental design.
Arthritis
In a randomised, double blind, placebo-controlled trial, 82 male and female patients with osteoarthritis and rheumatoid arthritis received a licensed over-the-counter (OTC) herbal medicine (two tablets/day) or placebo for a period of 2 months. The formula contained black cohosh, willow bark, guaiacum resin, sarsaparilla and poplar bark. Although there was no significant difference between the two groups for most symptoms, a significant decrease (p<0.05) in pain scores occurred for those taking the herbal formula. Many patients reported a decline in health related to the cold, damp, windy weather experienced near the end of the trial, which may have altered the findings. A relative improvement in mood scores was also noted for those taking the herbal tablets.73 The authors of this study advised that the results may not be relevant to the activity of black cohosh, as the formulation predominantly comprised herbs containing salicylate derivatives.
Infertility
Unexplained infertility is typically defined as the failure to conceive over 1 year for couples exhibiting no apparent abnormalities. It is believed to occur in 15% to 30% of couples trying to conceive. Medically it is often treated with the fertility drug clomiphene citrate, which can be used in conjunction with gonadotropins to help trigger ovulation. In a controlled clinical trial, patients with unexplained infertility who had not responded to clomiphene therapy alone were randomly divided into two groups. The first group was given black cohosh dry extract at 120 mg/day (500 mg of dried root) from days 1 to 12 of the cycle. Both groups received clomiphene from days 3 to 7 and human chorionic gonadotropin (HCG) injection close to ovulation. All were recommended to have timed intercourse every day for the corresponding week. Endometrial thickness was measured on the day of the HCG injection and was found to be significantly thicker for the group receiving black cohosh (8.9±1.4 mm versus 7.5±1.3 mm, p<0.001). Serum progesterone concentrations measured in the luteal phase (days 21 to 23) were significantly higher in the black cohosh group (13.3±3.1 ng/mL versus 9.3±2.0 ng/mL, p<0.01). The pregnancy rate was also significantly higher for the group given black cohosh (36.7% versus 13.6%, p<0.01), even after one treatment cycle. These results suggest a benefit to including black cohosh in the medical management of infertility.74
A follow-up trial by the same group using a similar design found that follicular-phase black cohosh exerted a better activity than ethinyloestradiol in terms of improving cycle characteristics in 134 infertile women treated with clomiphene citrate. No significant difference was found regarding clinical pregnancy rates.75
Toxicology and other safety data
Toxicology
No toxic effects were observed from oral administration of standardised black cohosh extract (up to 5 g/kg/day) for 26 weeks in rats.76 A constituent isolated from the chloroform fraction of black cohosh extract, likely to be actein, did not provoke acute toxicity when administered by intragastric and hypodermic routes to rabbits. The minimum lethal dose of this constituent was greater than 500 mg/kg (ip) in mice, 1000 mg/kg (oral) in rats and 70 mg/kg (iv) in rabbits. In subchronic toxicity studies over 30 days, the minimum lethal dose was greater than 10 mg/kg (ip) in mice and 6 mg/kg (oral) in rabbits.77
Standardised black cohosh extract did not show mutagenic activity in the Ames test.78 Scientists from Duquesne University observed that the incidence of metastasis increased in sexually mature female transgenic (MMTV-neu, genetically engineered) mice fed black cohosh (at amounts said to reflect the normal human dose, about 0.3 mg/mouse) for 12 months. The incidence of mammary tumours was not increased.79 This experimental model, in which female mice spontaneously develop mammary tumours through the activation of an oncogene common in human breast cancer, is still highly controversial in terms of providing reproducible and relevant results. The experimental conditions were highly artificial (for example, feeding black cohosh to mice for 12 months is the equivalent of a woman taking it continuously for at least 30 years).
The relevance of using a mouse model to assess the safety of a treatment that is already widely used in the community can be queried; it could be that this in vivo study is nothing more than a scientific curiosity. The best way to assess any risks associated with black cohosh consumption is to study the health of women already taking it.
Such studies have now been published. The association between a range of ‘hormone-related supplements’ (including black cohosh) containing ‘phyto-oestrogens’ and breast cancer incidence was reassessed in a retrospective case-control epidemiological study.80 The US study examined 949 cases of women with breast cancer and 1524 controls and specifically targeted use of black cohosh, American ginseng (Panax quinquefolius), red clover (Trifolium pratense), dong quai (Angelica sinensis) and yam products (Dioscorea species). After adjusting for variables such as age, education, age at full-term pregnancy, menopause status, family history of breast cancer and use of HRT, intake of the above herbal products (as a group) was associated with a reduced incidence of breast cancer (adjusted odds ratio (OR) 0.65, 95% confidence interval (CI): 0.49 to 0.87). However, it was only black cohosh that demonstrated a highly significant breast cancer protective effect (adjusted OR 0.39, 95% CI: 0.22 to 0.70). The authors concluded that additional confirmatory studies are required to determine whether black cohosh could be used as a treatment to prevent breast cancer.
Within a German case-control study, associations between patterns of herbal product use and incidence of breast cancer were investigated in 10 121 postmenopausal women.81 Use of herbal products was inversely associated with invasive breast cancer (OR 0.74) in a dose-dependent manner. The two black cohosh subgroups (isopropanolic and other types of extracts) demonstrated moderate protection, but it was most marked for chaste tree (Vitex agnus-castus, OR 0.4). As part of the VITAL epidemiological study, 35 016 postmenopausal women were queried on their use of dietary supplements and followed for up to 7 years.82 Black cohosh use was not found to be associated with an increased risk of invasive breast cancer.
However, questions remain as to whether black cohosh can be safely used by women with diagnosed breast cancer. While more information is required, findings from one clinical study strongly imply that black cohosh lacks any oestrogenic activity in breast or endometrial tissue. This was a prospective, open, uncontrolled safety study in which baseline status was compared by blinded observers with status after 6 months of treatment.83 A total of 74 women were treated with black cohosh extract daily (40 mg/day), and 65 women completed the study. Mammograms were performed and breast cells were collected by percutaneous fine needle aspiration biopsies at baseline and after 6 months. Breast cell proliferation was assessed using the Ki-67/MIB-1 monoclonal antibody (cells positive for this marker are in a state of active proliferation). Safety was monitored by adverse event reporting, laboratory assessments and measurement of the endometrium by vaginal ultrasound.
None of the women showed any increase in mammographic breast density. Furthermore, there was no increase in breast cell proliferation. The mean change in the proportion of Ki-67-positive cells was 0.5%±2.4% for paired samples. The mean change in endometrial thickness was 0.0±0.9 mm. A modest number of adverse events were possibly related to treatment, but none of these was serious. Laboratory findings and vital signs were normal. The findings suggest that the isopropanolic extract of black cohosh tested did not cause adverse effects on breast tissue. Furthermore, the data did not indicate any endometrial or general safety concerns during 6 months of treatment.
This finding was supported by another study published at around the same time that found 12 weeks of black cohosh given to postmenopausal women had no impact on oestrogen markers in serum and no effect on pS2 (a potential marker of breast cancer activity) or cellular morphology in nipple aspirate fluid.84
An observational retrospective cohort study investigated breast cancer patients treated at German institutions.85 Of 18 861 patients, a total of 1102 had received therapy with an isopropanolic extract of black cohosh. The mean overall observation time was 3.6 years. Black cohosh was not linked with an increased risk of recurrence, but instead was associated with prolonged disease-free survival (hazard ratio 0.83).
Contraindications
A traditional contraindication is pregnancy and lactation (see below), except to assist with childbirth.
A systematic review of the safety and efficacy of black cohosh in patients with cancer concluded that black cohosh appears to be safe in breast cancer patients without risk for liver disease, although further research is needed.33
Black cohosh is contraindicated in patients with pre-existing liver disease.
Special warnings and precautions
As noted above, caution should be exercised in patients with oestrogen-sensitive malignant tumours, especially when using doses at the higher end of the range.
Patients on long-term black cohosh therapy should be monitored for signs and symptoms of liver damage.
Interactions
The antiproliferative effect of black cohosh extract in combination with tamoxifen was assessed in vitro on 17-beta-oestradiol-stimulated MCF-7 human breast cancer cells.86 Dilutions of black cohosh extract in the range 10–3 to 10–5 augmented the antiproliferative action of 10–5 tamoxifen. Whether this interaction also applies in vivo has not been established.
Although a black cohosh extract and six triterpenoid glycosides isolated from it were shown to inhibit the key drug metabolising cytochrome P450 enzyme CYP3A4 in vitro,87 two human drug interaction studies have indicated this does not occur in people. One study found that a black cohosh extract (40 mg twice daily, standardised to 2.5% triterpene glycosides) did not affect CYP3A, which is involved in the metabolism of about half of all pharmaceutical drugs.88 The other study screened a black cohosh extract (1090 mg twice daily, each capsule standardised to 0.2% triterpene glycosides) for effects on the drug metabolising enzymes CYP3A4/5, CYP1A2, CYP2E1 and CYP2D6 in 12 healthy volunteers who took the extract for 28 days. There was a statistically significant inhibition of only CYP2D6, but the magnitude of the effect was small (around 7%) and deemed unlikely to be of clinical relevance.89 A subsequent study conducted by the same group, in which 18 healthy volunteers took a standardised black cohosh extract for 14 days, found no evidence for an effect on CYP2D6.90 A separate drug interaction study (using digoxin as a probe drug) found no evidence for effects on the drug transporter P-glycoprotein in 16 healthy volunteers who took a black cohosh extract (equivalent to 40 mg herb per day) for 2 weeks.91
The potential for black cohosh to alter the response to radiation therapy and four common anticancer drugs (docetaxel, doxorubicin, cisplatin and 4-hydroperoxycyclophosphamide – 4-HC, an analogue of cyclophosphamide that is active in cell culture) was studied in vitro in a mouse breast cancer cell line. Black cohosh extract increased the cytotoxicity of docetaxel and doxorubicin, decreased the cytotoxicity of cisplatin and did not alter the effects of 4-HC or radiation.92 The relevance of these findings to the human use of black cohosh is uncertain.
Use in pregnancy and lactation
Category B2 – no increase in frequency of malformation or other harmful effects on the fetus from limited use in women. Animal studies are lacking.
The traditional position is generally that black cohosh should not be taken during pregnancy except to assist with birth. According to the British Herbal Compendium black cohosh is contraindicated in pregnancy;93 however, this restriction is not listed in the Commission E.94 Black cohosh was widely used by the Eclectics in traditional Western herbal medicine as a partus preparator, if taken in the last weeks of pregnancy.5
In a 1999 survey of certified nurse-midwives in the US, black cohosh was used by 45% of the 90 respondents who used herbal medicine to stimulate labour. Adverse effects attributed to use of blue cohosh and black cohosh were not separately assigned and included nausea, increased meconium-stained fluid and transient fetal tachycardia.95
After a normal labour, a female infant was not able to breathe spontaneously and sustained CNS hypoxic-ischaemic damage. A midwife had attempted induction of labour using a combination of blue cohosh and black cohosh given orally (dosage undefined) at around 42 weeks’ gestation.96 It was not possible to identify the herbal preparation as the causative agent; however, this reaction may have been due to the blue cohosh (Caulophyllum thalictroides) rather than to the black cohosh (see Chapter 5).
A prospective, epidemiological study investigated the influence of first trimester use of medications and vaccines in the 1950s on the occurrence of congenital malformations and fetal survival in approximately 3200 pregnancies. Black cohosh was used in 1 of 266 pregnancies where a malformation occurred and in 2 of 532 pregnancies from the comparison groups. The dose and duration of use of black cohosh and the nature of the malformation were not specified.97 Black cohosh could not be identified as a causative agent.
Black cohosh use should be strongly discouraged during breastfeeding. This consideration is based on a possible oestrogenic effect. The British Herbal Compendium contraindicates black cohosh during lactation.93 However, the Commission E does not list this restriction.94
Side effects
General side effects
High doses of black cohosh can cause a frontal headache, with a dull, full or bursting feeling. This headache is the most characteristic effect observed when giving even therapeutic doses.98 A review published in 2000 found that mild gastrointestinal upset was the most frequent minor adverse event reported in clinical studies (average of 5.6% of patients across five studies). Other minor adverse events reported in clinical studies included headache, vertigo, weight gain, mastalgia, heavy feeling in the legs and a stimulant effect. Vaginal bleeding has also been reported.2
Two reviews published in 2003 confirm that adverse events with black cohosh are rare, mild and reversible. Gastrointestinal upsets and rashes are the most common adverse events. There is record of a few serious adverse events, including hepatic and circulatory conditions, but causality could not be determined.99,100 Details of some of the case reports follow.
A case was reported in 2001 of a woman diagnosed with grade 1 endometrioid adenocarcinoma of the endometrium ‘whose history was notable for extensive use of supplemental phytoestrogens’. Herbs included chaste tree, dong quai, black cohosh and licorice.101 No causality was demonstrated.
A 45-year-old woman who had been taking separate bottled products of black cohosh, Vitex agnus-castus and evening primrose oil for 4 months had three nocturnal seizures within a 3-month period. The patient had also consumed one to two beers 24 to 48 h prior to each incident.102 It was not established whether the herbal preparations caused the seizures.
A 26-year-old woman presented at a hospital with chest pain. Her heart rate and blood pressure dropped temporarily during the course of monitoring. Her urine digoxin level was ‘elevated’ at 0.9 ng/mL (but within the normal therapeutic range – 0.5 to 2.0 ng/mL). However, she was not taking digoxin. In addition to the contraceptive pill, she was taking a herbal preparation containing black cohosh, skullcap (Scutellaria lateriflora), lousewort (Pedicularis canadensis), hops (Humulus lupulus), valerian (Valeriana officinalis) and cayenne pepper (Capsicum annuum). The product was not available for analysis. The chest pain had started during her shift as a topless dancer, during which she had consumed four alcoholic drinks, but no illicit drugs.103 This study inappropriately speculated on ‘digoxin-like factors’ with cardiotonic activity claimed to be ‘commonly’ found in herbal teas. The source of the patient’s symptoms remains a mystery, but factors that interfere with digoxin assays, yet are without cardiotonic activity, have been reported in some herbs. (Refer to the Ginseng (Eleutherococcus senticosus) monograph by way of example.)
A case report exists of a 54-year-old woman who developed severe asthenia and high blood levels of creatine phosphokinase (230 to 237 U/L), lactate dehydrogenase (504 to 548 U/L) and total cholesterol, while taking a supplement derived from black cohosh for the management of vasomotor symptoms related to menopause. Notably the woman had previously taken the product for 12 months with no alteration in biochemistry and had restarted the product after a 3-month break. The black cohosh tablets contained 20 mg of dried rhizome and root extract. No other cause could be identified for her symptoms and she was advised to discontinue the product, after which a progressive normalisation of biochemical parameters and improvement in clinical symptoms occurred.104
A case report described a 56-year-old woman diagnosed with cutaneous pseudolymphoma after taking a black cohosh product for 12 months. The localised erythematous plaques on her arms and legs appeared after 6 months and completely disappeared with withdrawal of the product for 3 months.105
Other adverse reactions attributed to black cohosh use include cutaneous vasculitis106 and coagulation activation with fluid retention (secondary to a transient autoimmune hepatitis).107
Liver injury
On the 9 February 2006, the Australian TGA announced the following:
The Therapeutic Goods Administration (TGA) reviewed the safety of Black cohosh (Cimicifuga racemosa) following reports of possible liver problems internationally and in Australia. At the time of the review, there were 47 cases of liver reactions worldwide, including 9 Australian cases. In Australia, 4 patients were hospitalised, including two who required liver transplantation. Although some reports are confounded by multiple ingredients, by more than one medication or by other medical conditions, there is sufficient evidence of a causal association between Black cohosh and serious hepatitis. However, considering the widespread use of Black cohosh, the incidence of liver reaction appears to be very low. Following the safety review, the TGA has decided that medicines containing Black cohosh should include the following label statement: ‘Warning: Black cohosh may harm the liver in some individuals. Use under the supervision of a healthcare professional’.
On July 18, 2006, the Medicines and Healthcare Products Regulatory Agency (MHRA) in the UK issued a press release stating that all black cohosh products sold there should carry the following label warning: ‘Black cohosh may rarely cause liver problems. If you become unwell (yellowing eyes/skin, nausea, vomiting, dark urine, abdominal pain, unusual tiredness) stop taking immediately and seek medical advice. Not suitable for patients with a previous history of liver disease.’
Notwithstanding the Australian TGA’s claim at the time concerning the number of cases of liver damage linked to black cohosh (which include the adverse reaction reports filed with the UK MHRA and other health authorities) only five papers or letters had been published purporting to demonstrate a link between black cohosh (Actaea racemosa) ingestion and subsequent liver injury. It is important to closely examine these published reports since, because of the process of peer review, these represent the best-documented evidence of any association with liver injury. The first publication, from doctors at the Princess Alexandra Hospital in Brisbane, Australia, described six patients with evidence of severe hepatitis that was linked to taking a range of herbal products.108 Two of these patients were taking black cohosh, although one was also taking other herbs including skullcap, a herb which can be substituted by Teucrium species, a known hepatotoxic genus.109
The one case attributed to black cohosh alone (Case 1) was truly dramatic. Of the cases reported, the most serious illness occurred in this 47-year-old woman who was taking black cohosh for menopausal symptoms. She required liver transplantation even though, according to the publication, the patient had been taking the black cohosh for just 1 week. Histological examination of her liver confirmed severe hepatitis and early fibrosis. The patient did not exhibit eosinophilia and had no signs of any systemic disturbance. Serology for hepatitis A, B and C was negative, but rechallenge with the herb was not performed ‘for ethical reasons’. The dose of black cohosh taken was not specified, neither was the product.
The second publication, also from Australia, describes a 52-year-old woman with acute liver failure (Case 2).110 She had been taking an herbal formula containing 1:1 liquid extracts prescribed by a pharmacist. Black cohosh 1:1 was 10% of the mixture and the daily dose of the combination was 7.5 mL twice a day. The patient underwent successful liver transplantation. Although the authors stated that: ‘Extensive investigation excluded other recognised causes of liver failure’, they provided no details of what these investigations were. Analysis by the TGA was said to confirm the presence of golden seal, black cohosh and Ginkgo in the herbal mixture. Other stated ingredients were ground ivy and oats seed.
The phenomenon of idiosyncratic hepatic reactions to drugs is well documented. It also appears that this reaction does occur to certain herbs, for example chaparral (Larrea tridentata) and germander (Teucrium species). By definition, such reactions are rare and unpredictable and are not dose related. There are two types of idiosyncratic hepatic injury: hypersensitivity and aberrant metabolism. The former develops 1 to 5 weeks after exposure to the drug and, since it is immune-mediated and acute, also involves a systemic reaction including rash, fever and eosinophilia. The latter takes weeks to months to develop and symptoms are confined to the liver. Diagnosis of drug-induced idiosyncratic liver injury (DILI) is very difficult and relies largely on circumstantial evidence. Factors taken into account include a temporal association, exclusion of other possible causes, a consistent latency period to those described above, presence or absence of hypersensitivity (systemic) features, positive response to drug removal (dechallenge), positive response to rechallenge and a positive lymphocyte stimulation test (this last factor is quite controversial). Complicating this is the fact that DILI can mimic every known human liver disease.111 There are many confounding factors that could lead to incorrect associations between ingested medications or herbs and idiosyncratic liver injury. Many viruses that cause liver disease are still to be identified112 and there are no tests for them. Even known viruses are not always tested for. For example, a Dutch study published this year found that hepatitis E virus was a significant cause of unexplained hepatitis.113 Occult coeliac disease has been suggested as a cause of unexplained raised ALT and AST.114 Rare liver diseases may not be excluded.115,116 Other environmental factors could be implicated.
The experience of a liver transplant unit highlights some key issues behind the history and incidence of severe acute hepatitis (fulminant hepatic failure, FHF). All adult cases of FHF presenting to the Victorian Liver Transplant Unit (Australia) from 1988 to 2002 were analysed. Eighty patients (mostly female) were referred, at a rate of approximately one case per million population per year. Mean age was about 38 years. Most cases were due to paracetamol poisoning (36%) or idiopathic hepatitis (34%).110 Only five of the 80 cases were classified as drug induced, making this causality a rare factor. Other main causes included hepatitis A (three cases), hepatitis B (eight cases) and Wilson’s disease (six cases). The 27 cases (34%) of hepatitis due to unknown causes (idiopathic) is a surprising rate. These cases are also described as non-A non-B hepatitis, since patients are not positive for hepatitis A or B. In the USA one study found that the most common cause of FHF was non-A non-B (idiopathic) hepatitis.117 (Note that this US study was published in 1995, well before the dramatic rise in herbal use in that country.) Presumably unidentified infectious or environmental factors could cause these cases of idiopathic hepatitis. However, the authors of the Australian study state: ‘The strong female predominance of cases argues against a viral cause and raises the possibility that hormonal factors are involved, or that the condition is linked to autoimmune liver diseases. There is clearly a need for large, detailed, multicentre epidemiological studies, to provide further clues to a possible aetiology/ies of this syndrome.’
The demographics of idiopathic hepatitis (female, late 30s to early 50s) and black cohosh use strongly overlap. Hence, there is a distinct possibility that some patients who develop idiopathic hepatitis might also be coincidentally taking black cohosh. The herb could then be mistakenly attributed as the cause.
Since these initial series of cases attributing idiosyncratic hepatotoxicity to black cohosh, more cases have been reported. There have also been several publications analysing these and the earlier case reports, especially from the team headed by Teschke.
In 2009 the group rigorously analysed all 69 reported cases (at the time) and found no likelihood of causality in 68.118 Most cases were marred by confounding variables, misreported data and a lack of critical information.119 In particular, there was a lack of identification of the herb involved in the initial cases.120
Of high relevance here are the findings of Health Canada. From January 2005 to March 2009, Health Canada received six domestic reports of liver adverse reactions suspected of being associated with black cohosh. Analysis of three products associated with these reports revealed that they did not contain authentic black cohosh. Their phytochemical profiles were consistent with the presence of other related herbal species. A review of the authenticity of all licensed products containing black cohosh resulted in the voluntary withdrawal of several products not containing authentic black cohosh, including the products reported in four of the adverse reaction cases.121
Studies in black cohosh users have also sought to understand its impact on the liver. A prospective, longitudinal study recruited 100 healthy postmenopausal women from a hospital in Egypt. The women received black cohosh extract (40 mg/day) for relief of menopausal symptoms and were followed up for 12 months. Eighty-seven women completed the study, which included evaluation of total hepatic blood flow and liver function. The study sought to investigate potential mechanisms of hepatotoxicity: compromise of blood flow to the liver or a direct toxic effect on liver cells. The following results were obtained after 12 months of treatment:
• No significant changes in hepatic artery blood flow, portal vein blood flow or total hepatic blood flow
• No significant changes in any liver function tests
• A significant reduction in the prevalence, daily frequency and severity of hot flushes, compared with baseline.122
A meta-analysis of five trials involving 1117 women found no evidence that the isopropanolic extract of black cohosh has an adverse effect on liver function.123
Overdosage
According to early data, ingestion of 5 g of the herb or 12 g of the fluid extract can cause nausea, vomiting, violent headache, vertigo, joint pain, red eyes and weak pulse. Visual and nervous disturbances have also been noted.8,124,125 Some of these effects may have been due to the past adulteration of black cohosh with the poisonous plants red baneberry (Actaea spicata) and white cohosh or white baneberry (A. panchypoda (A. alba)).126 However, in the absence of any further information such doses of black cohosh are not recommended.
Safety in children
No information available, although the Eclectics did describe use in children for fever.5
Regulatory status in selected countries
Black cohosh is covered by a positive Commission E monograph with the following applications: neurovegetative complaints of premenstrual, dysmenorrhoeic or climacteric origin.
Black cohosh is on the UK General Sale List, with a maximum single dose of 200 mg. However, it does require a warning statement as noted above. Black cohosh products have achieved Traditional Herbal Registration in the UK with the traditional indication of relief of symptoms of the menopause.
Black cohosh does not have GRAS status. However, it is freely available as a ‘dietary supplement’ in the USA under DSHEA legislation (1994 Dietary Supplement Health and Education Act). Black cohosh has been present in OTC menstrual drug products. The FDA advises: ‘based on evidence currently available, there is inadequate data to establish general recognition of the safety and effectiveness of these ingredients for the specified uses’.
Black cohosh is not included in Part 4 of Schedule 4 of the Therapeutic Goods Act Regulations of Australia and is freely available for sale provided products carry a warning regarding possible liver damage.
References
1. Compton JA, Culham A, Jury SL. Taxon. 1998;47:593–634.
2. American Herbal Pharmacopoeia. Black cohosh rhizome: Actaea racemosa L. syn. Cimicifuga racemosa (L.) Nutt.: Analytical, Quality Control, and Therapeutic Monograph. Santa Cruz: American Herbal Pharmacopoeia, 2002.
3. German Federal Minister of Justice. German Commission E for Human Medicine Monograph, Bundes-Anzeiger (German Federal Gazette) no. 43, dated 02.03.1989.
4. British Herbal Medicine Association. British Herbal Pharmacopoeia. Cowling: BHMA, 1983. p. 66
5. Felter HW, Lloyd JU. King’s American Dispensatory, 18th ed., rev 3. Portland: 1905. Reprinted by: Eclectic Medical Publications, 1983;1:528–533.
6. Harnischfeger G, Stolze H. Erfahrungsheilkunde. 1981;30(6):439–444.
7. Chiej R. The Macdonald Encyclopedia of Medicinal Plants. London: Macdonald, 1984. Entry no. 87
8. Grieve MA, A Modern Herbal, New York, Dover Publications, 1971;vol 1. p. 211
9. Information on file. Warwick, Queensland 4072, Australia: MediHerb Pty Ltd; 2003.
10. Hostettmann K, Marston A. Chemistry & Pharmacology of Natural Products: Saponins. Cambridge: Cambridge University Press, 1995. p. 280
11. Wagner H, Bladt S. Plant Drug Analysis: A Thin Layer Chromatography Atlas, 2nd ed. Berlin: Springer-Verlag, 1996. p. 336
12. Gizycki H. Z Exp Med. 1944;113:635–644.
13. Jarry H, Harnischfeger G. Planta Med. 1985;51(1):46–49.
14. Jarry H, Ch Gorkow, Wuttke W, Loew D, Rietbrock N, eds. Phytopharmaka in Forschung und klinischer Anwendung, Darmstadt, Steinkopff (Verlag), 1995:104.
15. Jarry H, Harnischfeger G, Düker EM. Planta Med. 1985;51(4):316–319.
16. Duker EM, Kopanski L, Jarry H, et al. Planta Med. 1991;57(5):420–424.
17. Jarry H, Leonhardt S, Duls C, et al. 23rd International LOF-Symposium on Phyto-Oestrogens. Belgium: University of Gent, 1999.
18. Jarry H, Ch Gorkow, Wuttke W, Loew D, Rietbrock N, eds. Phytopharmaka in Forschung und klinischer Anwendung, Darmstadt, Steinkopff (Verlag), 1995:109–110.
19. Einer-Jensen N, Zhao J, Andersen KP, et al. Maturitas. 1996;25(2):149–153.
20. Zava DT, Dollbaum CM, Blen M. Proc Soc Exp Biol Med. 1998;217(3):369–378.
21. Liu J, Burdette JE, Xu H, et al. J Agric Food Chem. 2001;49(5):2472–2479.
22. Gaube F, Wolfl S, Pusch L, et al. BMC Pharmacol. 2007;7:11.
23. Bolle P, Mastrangelo S, Perrone F, et al. J Steroid Biochem Mol Biol. 2007;107(3–5):262–269.
24. Chan BY, Lau KS, Jiang B, et al. Bone. 2008;43(3):567–573.
25. Seidlová-Wuttke D, Jarry H, Wuttke W. Planta Med. 2007;73:995.
26. Kolios L, Schumann J, Sehmisch S, et al. Planta Med. 2010;76(9):850–857.
27. Qiu SX, Dan C, Ding LS, et al. Chem Biol. 2007;14(7):860–869.
28. Nesselhut T, Schellhase C, Dietrich R, et al. Arch Gynecol Obstet. 1993;254(1–4):817–818.
29. Nesselhut T. Expert Forum on Remifemin®: Report and Results From Endocrinology Expert Forum in Luneburg. Salzgitter (Ringelheim): Schaper & Brummer GmbH & Co., 1993.
30. Einbond LS, Shimizu M, Xiao D. Breast Cancer Res Treat. 2004;83(3):221–231.
31. Einbond LS, Su T, Wu HA. Int J Cancer. 2007;121(9):2073–2083.
32. Einbond LS, Shimizu M, Nuntanakorn P, et al. Planta Med. 2006;72(13):1200–1206.
33. Walji R, Boon H, Guns E, et al. Support Care Cancer. 2007;15:913–921.
34. Hostanska K, Nisslein T, Freudenstein J, et al. Anticancer Res. 2005;25(1A):139–147.
35. Jarry H, Stromeier S, Wuttke W, et al. Planta Med. 2007;73(2):184–187.
36. Seidlová-Wuttke D, Thelen P, Wuttke W. Planta Med. 2006;72(6):521–526.
37. Jarry H, Metten M, Spengler B, et al. Maturitas. 2003;44(Suppl 1):S31–S38.
38. Burdette JE, Liu J, Chen SN, et al. J Agric Food Chem. 2003;51(19):5661–5670.
39. Rhyu MR, Lu J, Webster DE, et al. J Agric Food Chem. 2006;54(26):9852–9857.
40. Reame NE, Lukacs JL, Padmanabhan V, et al. Menopause. 2008;15(5):832–840.
41. Schmid D, Gruber M, Woehs F, et al. J Pharm Pharmacol. 2009;61(8):1089–1096.
42. Yang CL, Chik SC, Li JC, et al. J Med Chem. 2009;52(21):6707–6715.
43. Schmid D, Woehs F, Svoboda M, et al. Can J Physiol Pharmacol. 2009;87(11):963–972.
44. Wojcikowski K, Stevenson L, Leach D, et al. J Altern Complement Med. 2007;13(1):103–109.
45. Burdette JE, Chen SN, Lu ZZ, et al. J Agric Food Chem. 2002;50(24):7022–7028.
46. Hemmi H, Ishida N. J Pharmacobiodyn. 1980;3(12):643–648.
47. Genazzani E, Sorrentino L. Nature. 1962;194:544–545.
48. Yoo JS, Jung JS, Lee TH, et al. Korean J Pharmacogn. 1995;4(26): 355
49. Einbond LS, Soffritti M, Esposti DD, et al. Fundam Clin Pharmacol. 2009;23(3):311–321.
50. van Breemen RB, Liang W, Banuvar S, et al. Clin Pharmacol Ther. 2010;87(2):219–225.
51. Bachmann GA. J Reprod Med. 2005;50(3):155–165.
52. Rossouw JE, Anderson GL, Prentice RL, et al. JAMA. 2002;288(3):321–333.
53. Knöss W. Assessment Report on Cimicifuga racemosa (L.) Nutt., rhizoma. London: European Medicines Agency, 2010.
54. Palacio C, Masri G, Mooradian AD. Drugs Aging. 2009;26(1):23–36.
55. Borrelli F, Ernst E. Pharmacol Res. 2008;58(1):8–14.
56. Shams T, Setia MS, Hemmings R, et al. Altern Ther Health Med. 2010;16(1):36–44.
57. Stoll W. Therapeuticum. 1987;1:23–31.
58. Wuttke W, Seidlová-Wuttke D, Gorkow C. Maturitas. 2003;44(suppl 1):S67–S77.
59. Osmers R, Friede M, Liske E, et al. Obstet Gynecol. 2005;105(5 Pt 1):1074–1083.
60. Frei-Kleiner S, Schaffner W, Rahlfs VW, et al. Maturitas. 2005;51(4):397–404.
61. Newton KM, Reed SD, LaCroix AZ, et al. Ann Intern Med. 2006;145(12):869–879.
62. Bai W, Henneicke-von Zepelin HH, Wang S, et al. Maturitas. 2007;58(1):31–41.
63. Chung DJ, Kim HY, Park KH, et al. Yonsei Med J. 2007;48(2):289–294.
64. Uebelhack R, Blohmer JU, Graubaum HJ, et al. Obstet Gynecol. 2006;107(2 Pt 1):247–255.
65. Rotem C, Kaplan B. Gynecol Endocrinol. 2007;23(2):117–122.
66. Sammartino A, Tommaselli GA, Gargano V, et al. Gynecol Endocrinol. 2006;22(11):646–650.
67. Verhoeven MO, van der Mooren MJ, van de Weijer PH, et al. Menopause. 2005;12(4):412–420.
68. Briese V, Stammwitz U, Friede M, et al. Maturitas. 2007;57(4):405–414.
69. Amsterdam JD, Yao Y, Mao JJ, et al. J Clin Psychopharmacol. 2009;29(5):478–483.
70. Hernández Muñoz G, Pluchino S. Maturitas. 2003;44(suppl 1):S59–S65.
71. Jacobson JS, Troxel AB, Evans J, et al. J Clin Oncol. 2001;19(10):2739–2745.
72. Bebenek M, Kemmler W, von Stengel S, et al. Menopause. 2010;17(4):791–800.
73. Mills SY, Jacoby RK, Chacksfield M, et al. Br J Rheumatol. 1996;35(9):874–878.
74. Shahin AY, Ismail AM, Zahran KM, Makhlouf AM. Reprod Biomed Online. 2008;16(4):580–588.
75. Shahin AY, Ismail AM, Shaaban OM. Reprod Biomed Online. 2009;19(4):501–507.
76. Korn WD. Six-month Oral Toxicity Study with Remifemin Granulate in Rats Followed by an Eight-week Recovery Period. Hannover, Germany: International Bioresearch, 1991.
77. Genazzani E, Sorrentino L. Nature. 1962;194(4828):544–545.
78. Beuscher N. Z Phytother. 1995;16:301–310.
79. Davis VL, Jayo MJ, Ho A, et al. Cancer Res. 2008;68(20):8377–8383.
80. Rebbeck TR, Troxel AB, Norman S, et al. Int J Cancer. 2007;120:1523–1528.
81. Obi N, Chang-Claude J, Berger J, et al. Cancer Epidemiol Biomarkers Prev. 2009;18(8):2207–2213.
82. Brasky TM, Lampe JW, Potter JD, et al. Cancer Epidemiol Biomarkers Prev. 2010;19(7):1696–1708.
83. Hirschberg AL, Edlund M, Svane G, et al. Menopause. 2007;14(1):89–96.
84. Ruhlen RL, Haubner J, Tracy JK, et al. Nutr Cancer. 2007;59(2):269–277.
85. Zepelin HH, Meden H, Kostev K, et al. Int J Clin Pharmacol Ther. 2007;45(3):143–154.
86. German Patent DE 196 52 183 CI. Verwendung eines Extraktes aus Cimicifuga racemosa: Schaper & Brümmer.
87. Tsukamoto S, Aburatani M, Ohta T. Evid Based Complement Alternat Med. 2005;2(2):223–226.
88. Gurley B, Hubbard MA, Williams DK, et al. J Clin Pharmacol. 2006;46(2):201–213.
89. Gurley BJ, Gardner SF, Hubbard MA, et al. Clin Pharmacol Ther. 2005;77(5):415–426.
90. Gurley BJ, Swain A, Hubbard MA, et al. Mol Nutr Food Res. 2008;52(7):755–763.
91. Gurley BJ, Barone GW, Williams DK, et al. Drug Metab Dispos. 2006;34(1):69–74.
92. Rockwell S, Liu Y, Higgins SA. Breast Cancer Res Treat. 2005;90(3):233–239.
93. British Herbal Medicine Association. British Herbal Compendium. Bournemouth, BHMA; 1992. pp. 34–36.
94. Blumenthal M, ed. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. Austin: American Botanical Council, 1998.
95. McFarlin BL, Gibson MH, O’Rear J, et al. J Nurse Midwifery. 1999;44(3):205–216.
96. Gunn TR, Wright IM. N Z Med J. 1996;109(1032):410–411.
97. Mellin GW. Am J Obst Gynec. 1964;90(7, Pt 2):1169–1180.
98. Felter HW. The Eclectic Materia Medica, Pharmacology and Therapeutics, 1922. Portland: Eclectic Medical Publications, 1983. pp. 466–469
99. Dog TL, Powell KL, Weisman SM. Menopause. 2003;10(4):299–313.
100. Huntley A, Ernst E. Menopause. 2003;10(1):58–64.
101. Johnson EB, Muto MC, Yanushpolsky EH, et al. Obstet Gynecol. 2001;98(5 Pt 2):947–950.
102. Shuster J. Hosp Pharm. 1996;31(12):1553–1554.
103. Scheinost ME. J Am Osteopathic Assoc. 2001;101(8):444–446.
104. Minciullo PL, Saija A, Patafi M, et al. Phytomedicine. 2006;13(1–2):115–118.
105. Meyer S, Vogt T, Obermann EC, et al. Dermatology. 2007;214(1):94–96.
106. Ingraffea A, Donohue K, Wilkel C, Falanga V. J Am Acad Dermatol. 2007;56(suppl 5):S124–S126.
107. Zimmermann R, Witte A, Voll RE, et al. Climacteric. 2010;13(2):187–191.
108. Whiting PW, Clouston A, Kerlin P. MJA. 2002;177(8):440–443.
109. Mills S, Bone K. The Essential Guide to Herbal Safety. USA: Churchill Livingstone, 2005. pp. 581–584
110. Lontos S, Jones RM, Angus PW, et al. MJA. 2003;179(7):390–391.
111. Pishvaian AC, Trope BW, Lewis JH. Curr Opin Gastroenterol. 2004;20(3):208–219.
112. Bowden D, Moaven LD, Locarnini S. MJA. 1996;164:87–89.
113. Waar K, Herremans M, Vennema H. J Clin Virol. 2005;33(2):145–149.
114. Bardella M, Vecchi M, Conte D. Hepatology. 1999;29(3):654–657.
115. Tordjmann T, Grimbert S, Genestie C. Gastroenterol Clin Biol. 1998;22(3):305–310.
116. Gaya D, Thorburn D, Oien K. J Clin Pathol. 2003;56(11):850–853.
117. Hoofnagle J, Carithers R, Shapiro C. Hepatology. 1995;21:240–252.
118. Teschke R, Bahre R, Genthner A, et al. Maturitas. 2009;63(4):302–314.
119. Teschke R. Menopause. 2010;17(2):426–440.
120. Teschke R, Schwarzenboeck A, Schmidt-Taenzer W, et al. Ann Hepatol. 2011;10(3):249–259.
121. Black cohosh products and liver toxicity: update. Canadian Adverse Reaction Newsletter. 2010:1–2.
122. Nasr A, Nafeh H. Fertil Steril. 2009;92(5):1780–1782.
123. Naser B, Schnitker J, Minkin MJ, et al. Menopause. 2011;18(4):366–375.
124. Mills SY. The A-Z of Modern Herbalism. London: Thorsons, 1989. p. 39
125. Blaschek W, Ebel S, Hackenthal E, et al. HagerROM 2002: Hagers Handbuch der Drogen und Arzneistoffe. Heidelberg: Springer, 2002.
126. Grieve M, A Modern Herbal, New York, Dover Publications, 1971;vol 1. pp. 81–82