Butcher’s broom
Synonyms
Knee holly, box holly, pettigree, R. ponticus Woron., R. hyrcanus Stankov & Taliev, Mäusedorn (Ger), petit houx (Fr).
What is it?
The butcher’s broom (Ruscus aculeatus) is a small evergreen shrub native to western Europe.1,2 The tough leaf-like branches (known as phylloclades) have been used to assemble makeshift brooms, hence the common name. However, the part used therapeutically is the rootstock and rhizome.
In recent times, butcher’s broom has been the subject of several scientific investigations, including clinical trials. The research has focused on its anti-oedema and venotonic properties, which make it an effective therapy for symptoms associated with varicose veins and haemorrhoids (similar to the horsechestnut with which it combines well). In Europe, proprietary preparations of butcher’s broom are commonly used for varicose veins and haemorrhoids. The extract is often combined with other ingredients, especially hesperidin methylchalcone and ascorbic acid. (Hesperidin is a flavonoid that has beneficial activity on capillaries.)
Traditional view
Butcher’s broom root was traditionally regarded in Western herbal medicine as a diuretic, diaphoretic, laxative and expectorant, and was used to treat dropsy, urinary obstruction or gravel, dysuria, oedema, ascites, jaundice, difficult breathing and for the removal of phlegm. For the treatment of haemorrhoids it was used both orally and locally.3,4 The oedema-reducing effect of this plant is clearly highlighted in the traditional literature, with reference to butcher’s broom as ‘much recommended by Dioscorides and other ancient physicians as an aperient and diuretic’.3
Can be used for
Preparations
Decoction of the dried root, liquid extract and tablets or capsules for internal use. Extracts of the herb can also be applied topically as a cream, ointment or gel.
Dosage
• 1.5 to 3 g/day of dried root
• Butcher’s broom tablets or capsules (200 mg of a 4:1 concentrate standardised to contain 20 mg of saponins expressed as ruscogenin), two to three per day
• 3 to 6 mL/day of 1:2 liquid extract; 7.5 to 15 mL/day of 1:5 tincture
In most of the clinical trials, butcher’s broom extract was used in combination. The typical daily doses of the components used in many trials were 300 mg of butcher’s broom extract, 300 mg of hesperidin methylchalcone and 200 mg of ascorbic acid (corresponding usually to two capsules per day).
Summary assessment of safety
In rare cases the herb has been implicated in the development of lymphocytic colitis after long-term use. However, these cases were all recorded for the combination product and the identification of butcher’s broom root and rhizome as the causative agent has not been confirmed.
Technical data
Botany
A sub-shrub which, in some instances, may grow to 1 m. It has a fibrous, oblique, whitish rhizome from which emerge green, striate stems. The branches take on a leaf-like form and are known as cladodes or phylloclades; they are green, leathery and ovate-lanceolate with a spine at the top. The true leaves are represented by scales. The greenish-white, six-petalled flowers are borne on a lanceolate bract on the first half of the cladode. The fruit is an almost spherical, red berry with one to two seeds and a viscous pulp. This unusual type of shrub can often be seen wild throughout Britain and Europe on the outskirts of woods and in moist, uncultivated ground. It is collected in September, but as it is a protected plant special permission is needed.2
Key constituents
The rhizome contains steroidal compounds (0.5% to 1.5%) comprising the aglycones ruscogenin and neoruscogenin and their glycosides (which are of the spirostanol and furostanol saponin types).5 One study has also identified the presence of triterpene and sterol compounds.6
Pharmacodynamics
A preparation containing butcher’s broom extract, hesperidin methylchalcone and ascorbic acid has been extensively studied in recent decades. Throughout this monograph this formulation will be referred to as ‘butcher’s broom extract combination’.
A 1994 review of published pharmacological studies indicated that butcher’s broom extract exerted activity on the three circulatory compartments involved in chronic venous insufficiency. The venoconstrictor effect is explained by its peripheral post-synaptic alpha-noradrenergic action. This action also possibly explains the lymphatic activity observed in experimental models assessing lymphatic flow. A microcirculatory activity is also involved: this combines an inhibitory effect on capillary permeability, a protective action on the endothelium against hypoxia via stimulation of mitochondrial enzymes, and an inhibition of the endothelial leukocyte adhesion observed in models of ischaemia/reperfusion. The other components in the proprietary butcher’s broom extract combination, namely hesperidin methylchalcone and ascorbic acid, exert their effects mainly by reducing capillary permeability and increasing capillary resistance.7
Anti-inflammatory activity
In research conducted in the 1950s and 1960s, butcher’s broom extract demonstrated activity against experimental paw inflammation and peritonitis in vivo.8
Saponin constituents isolated from butcher’s broom displayed anti-inflammatory activity on rat paw oedema, but did not influence capillary fragility. A strong vasoconstrictor activity was observed on isolated blood vessels and the ruscogenins also decreased capillary permeability in a rabbit model.9 However, the ruscogenins failed to inhibit hyaluronidase activity in vitro, but did exhibit a remarkable anti-elastase activity.10 An in vivo study in mice found that ruscogenin significantly suppressed zymosin A-evoked leukocyte migration, possibly through an anti-adhesive activity via inhibition of the NF-kappaB pathway.11
Venotonic activity
In an experimental microcirculation model, butcher’s broom extract caused constriction in venules, but not arterioles, after intravenous administration.12 The inhibitory effect of butcher’s broom extract on histamine-induced microvascular permeability was attributed to the blocking of calcium and a selective activation of alpha1-adrenoceptors (and to a lesser extent alpha2-receptors).12–14 Butcher’s broom extract potentiated the activity of norepinephrine (noradrenaline) released at nerve endings.15 The vasoconstriction is independent of the endothelium and, as indicated above, is mediated by the activation of smooth muscle adrenergic receptors, but not by endothelin receptors.16 This adrenergic action of butcher’s broom extract enhances venous return.17
The venoconstriction induced by butcher’s broom in isolated vessels was increased by heat.12,18 This effect of temperature on the response to butcher’s broom resembled that noted during partial alpha1-adrenergic activation.19 A venotonic effect was also observed in diabetic animals, and treatment with butcher’s broom extract combination reversed an experimentally impaired venoarteriolar reflex (which is a hallmark of diabetic microangiopathy).20
Studies utilising isolated human veins indicated that butcher’s broom extract caused dose-dependent contractions in leg veins from patients with primary varicosity. Varicose tributaries were more sensitive to the contractile effects of butcher’s broom extract than segments of the greater saphenous vein removed from the same patient. Contractions following butcher’s broom exposure were independent of the endothelium and mediated by the activation of adrenergic receptors on the smooth muscle, but not endothelin-A.21
Butcher’s broom extract increased the cyclic AMP concentration in isolated human varicose veins, but did not affect cyclic GMP levels. In the presence of the extract, the ratios of 6-ketoprostaglandin-F1a to thromboxane B2 in varicose veins were rendered identical to normal vein tissue. (Segments of vein were obtained from patients who had undergone surgery.)22 Contractions initiated by butcher’s broom extract were enhanced following chronic exposure to progesterone, an effect that could be reversed by oestrogen. These contractions were mediated by an adrenergic and a non-adrenergic component. The adrenergic component was enhanced by progesterone and decreased by oestrogen.23
A venoconstrictive activity of a butcher’s broom cream under orthostatic conditions was demonstrated by ultrasonography in a randomised, double blind study involving 18 healthy volunteers. Within 2.5 h of applying a quantity of butcher’s broom cream containing 64 to 96 mg of extract, the diameter of the femoral vein decreased by an average of 1.25 mm, compared with a diameter increase of 0.5 mm for the placebo cream (p<0.05).24
As noted earlier, the mechanism of action of the combination of butcher’s broom extract and hesperidin methylchalcone in the treatment of venous diseases is thought to involve an increase in venous tone (by butcher’s broom extract) and protection of capillary integrity (by hesperidin methylchalcone). In a double blind study involving 20 healthy volunteers, the efficacy of the individual agents, their combination and a placebo were assessed. Butcher’s broom decreased venous capacity (p<0.01), reduced the blood pool in the lower leg under orthostatic conditions and decreased tissue volume in the foot and ankle (p<0.01). Hesperidin methylchalcone lowered the capillary filtration rate (p<0.01) and increased the blood pool (due to dehydration of the lower leg tissue). Blood volume after use of the combination was between the values for butcher’s broom and hesperidin methylchalcone alone, and effects on other parameters corresponded to those obtained for the individual substances.25 Some of the 20 volunteers administered the butcher’s broom extract combination showed a significantly reduced venous capacity, whereas others developed an increase in venous storage ability.
Haemorheological and haemostasis parameters
The influence of combined treatment with butcher’s broom extract (450 mg/day containing 11.16 mg ruscogenin) and hesperidin methylchalcone (450 mg/day) on fibrinolytic activity of the vein wall was investigated ex vivo in patients with venous insufficiency.26 Patients received treatment for 14 days in a double blind, placebo-controlled design: 10 were given active treatment and 10 were given placebo. The fibrinolytic activity of the intima of the greater saphenous vein was significantly increased in the active group (p<0.01). Activity in the adventitia layer also showed a significant increase after an incubation time of 60 minutes (p<0.05). These results suggest that the combination might reduce the risk of venous thrombosis in patients with varicose veins.
The effect of an extract of butcher’s broom on haemorheological parameters was also investigated under double blind, placebo-controlled conditions.27 Forty-five participants took part in the clinical trial. There were 20 volunteers who acted as a normal, untreated control group, 13 patients with venous insufficiency who received oral doses of butcher’s broom extract (amount not specified) and another 12 patients who were given a placebo. Venous blood samples were collected before and after 30 days of treatment, each time before and after 10 minutes of venous stasis induced by a hyperpressure of 10 mmHg. Results demonstrated that butcher’s broom treatment was accompanied by a significant improvement in several rheological parameters under these conditions of venous stasis, including a decrease in plasma viscosity (p<0.01), a reduction in red cell deformability (p<0.001), a reduction in red cell aggregation (p<0.01), an increase in red cell aggregation time (p<0.01) and a reduction in the red cell disaggregation shear rate (p<0.05).
Anti-oedema activity
Oral administration of the butcher’s broom extract combination inhibited histamine-induced plasma exudation in hamsters with mild diabetes, without affecting blood glucose.28 In addition to reducing experimentally induced capillary permeability, a combination of butcher’s broom extract, hesperidin methylchalcone, methylesculetin and ascorbic acid decreased experimentally induced hyperaemia, UV erythema and dextran or carrageenin local oedemas after either oral dosing or intraperitoneal injection.29
The anti-oedematous effect of a combination containing butcher’s broom extract and hesperidin methylchalcone was tested in a randomised, double blind study using water plethysmography.30 Forty patients with permanent oedema caused by chronic venous insufficiency were treated for 15 days orally with 450 mg butcher’s broom extract and 450 mg hesperidin methylchalcone (n=20) or placebo (n=20). After placebo, the oedema volume caused by orthostatic stress increased slightly (16.3 mL). However, in patients receiving active treatment the extent of the provoked venous oedema was significantly reduced (53.4 mL, p<0.01).
Other activity
Butcher’s broom extract inhibited the activation of endothelial cells induced by hypoxia (which mimics venous blood stasis), as evidenced by its effect on several parameters. Hesperidin methylchalcone was also able to inhibit the hypoxia-induced decrease in ATP content.31 Butcher’s broom extract combination and each of its components demonstrated a dose-dependent protection against hypoxia in endothelial cells in vitro. A synergistic effect between the components was observed.32
Pharmacokinetics
The oral bioavailability of butcher’s broom extract was found to be ‘good’ after administration of a radiolabelled extract in an experimental model. The sapogenins were excreted mainly in the urine.33 The relative bioavailability of butcher’s broom extract following oral, intravenous or topical administration was assessed in rats. Absorption was estimated at 65% by the oral route and 25% after topical application. Biliary excretion was also observed, with enterohepatic cycling.34,35
The major spirostanol glycosides of butcher’s broom (degluconeoruscin and deglucoruscin) were detected in human plasma after oral administration of 1 g of butcher’s broom extract. Three healthy volunteers took part in the study.36
Clinical trials
Chronic venous insufficiency/varicose veins
More than 30 clinical trials over the past 30 years have demonstrated the benefit of the butcher’s broom extract combination in the management of chronic venous insufficiency. A recent meta-analysis included 20 placebo-controlled, randomised, double blind studies, five studies randomised against a comparison treatment and six single-arm surveillance studies.37 (Several of the individual studies included in this meta-analysis are also reviewed below.) In total there was information from 10 246 patients. On a 4-point symptom severity scale, the butcher’s broom extract combination significantly reduced pain severity (0.44 ± 0.12), cramps (0.26 ± 0.08), heaviness (0.53 ± 0.11) and paraesthesia (0.29 ± 0.10) compared with placebo. There was also a significant reduction in venous capacity of 0.7 ± 0.19 mL/100 mL. Reductions in the severity of oedema and decreases in calf and ankle circumference did not achieve statistical significance. The authors concluded that their analysis was a strong and objective demonstration of the clinical efficacy of the butcher’s broom extract combination in chronic venous insufficiency.
A study published after this meta-analysis examined the activity of butcher’s broom extract alone. In a well-designed multicentre, double blind, randomised, placebo-controlled trial, 166 women suffering from chronic venous insufficiency received either butcher’s broom extract (72 to 75 mg/day of a 15 to 20:1 extract) or a placebo for 12 weeks.38 Analysis of the 148 women who completed the trial revealed the herbal treatment significantly reduced leg volume, ankle and leg circumferences (all p<0.001) and subjective symptoms such as heavy tired legs (p=0.0022). Tolerability of both treatments was assessed as good to very good. The authors concluded that butcher’s broom was a safe and effective treatment for patients suffering chronic venous insufficiency.
Significant decreases in the venous diameter of deep veins (for example p=0.02 for the common femoral vein), a significant increase in flow parameters in these veins (p=0.05) and a non-significant decrease in the flow parameters in superficial veins was observed when 12 patients with primary varicose vein disease were treated with butcher’s broom extract combination and assessed in the standing position.39 Measurements were taken using ultrasonography at baseline, 2 h after the intake of the combination on the first day of the study and at the end of the 7-day study period. (Most of the above venotonic outcomes occurred at the 2-h measurement.) In terms of the primary endpoint (venous diameter of the greater saphenous vein in the supine position after 7 treatment days), the study failed to demonstrate a significant effect. (Two hours was assumed to be the time of the peak concentration of the herbal treatment.)
During a 2-month treatment with butcher’s broom extract combination, a significant decrease or regression of clinical symptoms and a reduction in ankle circumference was observed.40 This randomised, double blind, placebo-controlled trial involved 60 patients with uncomplicated chronic venous insufficiency. Clinical symptoms that were significantly reduced included heavy legs (p<0.01 at 30 and 60 days), tired legs (p<0.01 at 30 and 60 days), sensation of evening oedema (p<0.01 at 30 and 60 days, p<0.05 at 15 days), pain (p<0.01 at 30 and 60 days), pruritus (p<0.01 at 60 days) and cramp (p<0.05 at 30 and 60 days). The reduction in average ankle circumference for the treatment group compared with placebo was significant at the end of the trial (p<0.05). Global assessment of efficacy in the 30 patients in the butcher’s broom group showed an excellent result in 15 patients, good in 13 and satisfactory in two. Of the 30 patients receiving placebo, results were rated as excellent in four, good in 17, satisfactory in eight and insufficient in one. The tolerability of butcher’s broom extract combination was found to be similar to the placebo.
In a double blind, placebo-controlled, crossover trial, 40 patients with chronic venous disorders of the lower limbs evaluated butcher’s broom extract combination.41 The trial involved two treatment periods of 2 months, with an interim washout phase of 15 days. During active treatment, patients received capsules each day containing butcher’s broom extract (99 mg), hesperidin (450 mg) and ascorbic acid (300 mg). An overall tendency for improvement occurred that was more distinct during active treatment than during placebo treatment. Pruritus and plethysmographic parameters changed significantly with active treatment compared to placebo (p<0.01 or p<0.05, depending on the parameter). Tolerability was deemed to be excellent.
A significant improvement in venous tone (p<0.05) was measured after 2 weeks in a randomised, double blind, placebo-controlled trial involving 50 patients with varicose veins.42 Active treatment consisted of the butcher’s broom extract combination. To assess venous function, venous capacity, venous distensibility and expelled blood volume were measured by plethysmography. The authors suggested that, despite the improvements observed, the treatment period was probably too short to obtain a full therapeutic effect since not all parameters achieved statistical significance.
In patients with post-thrombotic syndrome, the median blood velocity in the femoral vein increased by 24% on the diseased side 2 h after the oral administration of butcher’s broom extract combination.43 The ratio of the middle arterial inflow to venous outflow velocities showed a favourable decrease of 40% after medication (p<0.05). Measurements were conducted using ultrasound and the study was an open design, with results compared against baseline.
In a double blind, placebo-controlled trial involving 100 patients, butcher’s broom extract combination for a period of 1 to 3 months improved the following symptoms: heavy and painful legs, nocturnal cramps, oedema of the lower limbs.44 The overall improvement was significant, as measured by global assessment (p<0.05).
A clinical trial comparing the therapeutic efficacy of butcher’s broom extract combination with micronised diosmin found the two treatments produced similar effects by the conclusion of the trial (2 months).45 The onset of action for the butcher’s broom extract combination appeared to be earlier, since benefits were observed at 15 days.
In a randomised, multicentre study, butcher’s broom extract combination was more effective than hydroxyethyl rutoside after 90 days of treatment for chronic venous insufficiency.46,47 More rapid and complete regression of symptoms occurred for the butcher’s broom group (p<0.01). A significant reduction in the affected limb size was observed in both groups, but only persisted for 90 days with the butcher’s broom extract combination (p<0.01).
Several other double blind, placebo-controlled or comparative trials published in the 1980s or early 1990s demonstrated significant clinical benefit for the butcher’s broom extract combination in the management of chronic venous insufficiency.48–51 Similarly, a group of more recent open label trials from Mexico and South America have shown positive clinical outcomes in patients with chronic venous disorders.52–55
The safety and efficacy of the butcher’s broom extract combination was evaluated in a multicentre surveillance study involving 886 cases.56 After 30 and 60 days of treatment, the number of patients reporting mild and absent symptoms (pain, cramping, paraesthesia, heaviness) was substantially increased. Tolerance was rated as excellent for 85.4% of patients and side effects were reported in 5.6%. These were essentially minor digestive troubles or transient problems that did not necessitate the cessation of treatment except in three cases.
Topical and oral use during pregnancy
A significant reduction in the dilatation of the femoral vein (p<0.05) in pregnancy was observed in an uncontrolled study in 18 women that assessed the use of a butcher’s broom cream.57 Similar beneficial results were also observed in an earlier study.58
A therapeutic trial involving 20 pregnant women (21 to 24 weeks) found that the oral use of butcher’s broom extract combination improved symptoms of venous insufficiency and was innocuous to the fetus, as assessed by the usual clinical and ultrasonographic criteria of pregnancy surveillance, including umbilical artery flow and the state of the infant and placenta following birth.59 In a much larger earlier study, the efficacy and tolerability of the butcher’s broom extract combination were assessed in an uncontrolled multicentre clinical trial.60 The product was administered for 2 months to 214 women who presented with chronic venous insufficiency as a result of their pregnancy. Symptoms (including pain, cramps and paraesthesia) and signs (such as varices and oedema) were significantly improved compared to baseline at 30 and 60 days of treatment (no p values were provided). Tolerability was rated as excellent, with only nine patients reporting mild gastrointestinal or skin reactions. No information regarding pregnancy outcomes was provided.
Varicose ulcers
In a randomised, double blind trial, 23 patients with venous leg ulcers were treated over a period of 6 weeks with six capsules daily of the butcher’s broom extract combination (n=12) or a placebo (n=11).61 The treatment was in addition to basic ulcer therapy. In the placebo group five patients did not show any improvement or healing of the ulcer during the observation period, whereas in 11 of 12 patients treated with the active combination the ulcer area was markedly reduced (p<0.001). Parallel to the change in the ulcer size, venous haemodynamics were improved and venous drainage increased significantly (p<0.05). The results showed that this additional therapy favourably affects the healing rate of venous ulcers.
Lymphoedema
Fifty-seven patients with secondary lymphoedema of the upper limb after previous treatment for breast cancer participated in a double blind, placebo-controlled trial of butcher’s broom extract combination for a period of 3 months. All patients also underwent manual lymphatic drainage twice a week for at least 1 month. A significant reduction in the volume of arm oedema (12.9%, p<0.01) was observed in the treatment group compared with placebo. Decreased oedema was more marked in the forearm compared to the upper arm, where there was increased fat deposition.62
Haemorrhoids
The efficacy of butcher’s broom extract combination for the treatment of acute attacks of haemorrhoids was investigated in an open label multicentre study.63 One hundred and twenty-four patients were treated for 1 week. The treatment protocol was six capsules of the combination daily for the first 3 days, then four capsules daily. Each capsule contained 150 mg of butcher’s broom extract, 150 mg of hesperidin methylchalcone and 100 mg of ascorbic acid. Parameters studied were painful symptoms (discomfort, sensation of heaviness, burning, pruritus, tenesmus), accompanying symptoms (rectal bleeding, altered intestinal motility, abdominal pains), local signs (prolapse, congestive state, inflammation), overall severity score of symptoms and clinical efficacy and safety. Analysis of results demonstrated a statistically significant decrease in all scores between day 0 and day 7. The main results were: an average decrease of 4.9 points in painful symptoms (p=0.0001 compared against baseline), a decrease of 1.3 points in the assessment of accompanying symptoms (p=0.0001), a decrease of 3.6 points in local signs (p=0.0001) and a decrease of 9.8 points in the overall severity score of the symptoms (p=0.0001). Sixty-nine per cent of the patients rated the efficacy of the treatment as good or excellent (>75% for the physicians’ rating).
Premenstrual syndrome
In a randomised, double blind trial, 40 women suffering premenstrual syndrome received either butcher’s broom extract combination (n=20) or an identical placebo (n=20) for 90 days.64 Mastalgia (p<0.02), menstrual pain (p<0.05) and mood (p<0.05) were significantly improved in the treatment group compared with placebo.
Orthostatic hypotension
It has been postulated that butcher’s broom may counter the blood pooling in the lower limbs associated with orthostatic hypotension by virtue of its vasoconstrictive and venotonic properties.65
Diabetic retinopathy
The effects of a combination of buckwheat leaf (1.5 g/day) and troxerutin (90 mg/day) or butcher’s broom extract (75 mg/day) on ophthalmological and biochemical parameters in patients with non-proliferative diabetic retinopathy were compared with that of the synthetic rutin derivative troxerutin (1 g/day).66 During the study period of 3 months, 60 diabetic patients were divided into three equal groups: group I received troxerutin, group II received butcher’s broom and group III received buckwheat plus troxerutin. The amplitude of oscillating potentials decreased in patients receiving only the troxerutin, and increased in group II and III patients. Regression of changes in the fundus of the eye was demonstrated in 23.1% to 27.8% of all treated patients; however, deterioration in 5.6% of patients in group I, 3.3% of those in group III and none in group II was also observed. Troxerutin seemed to be less effective than the herbs, especially when oscillating potentials were considered.
Toxicology and other safety data
Toxicology
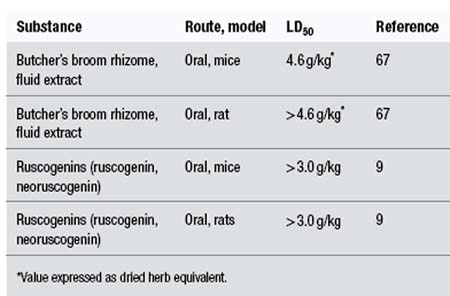
The following LD50 data have been recorded for butcher’s broom extract and its constituents:
Intravenous administration of dried butcher’s broom extract to dogs resulted in a slowing of the heart rate, a marked drop in arterial pressure, and at high doses increased respiration and blood glucose levels. Intraperitoneal doses of 1.5 to 2.0 g/kg of dried ethanolic extract of butcher’s broom were lethal in guinea pigs. The authors estimated that the upper level of a safe dose for a human would be about 10 g of extract by injection.68
Prolonged oral administration of high doses (300 mg/kg) of saponosides, prosapogenins and ruscogenins isolated from butcher’s broom were well tolerated by rats.9
Contraindications
Because of the irritant effect of the saponins, butcher’s broom should not be applied to broken or ulcerated skin.
Special warnings and precautions
The use of herbs rich in saponins is possibly inappropriate in coeliac disease, fat malabsorption and vitamin A, D, E and K deficiency, and some upper digestive irritations. Saponin-containing herbs are best kept to a minimum in patients with pre-existing cholestasis.
Use in pregnancy and lactation
Category B1 – no increase in frequency of malformation or other harmful effects on the fetus from limited use in women. No evidence of increased fetal damage in animal studies.
Animal studies have not produced any evidence of embryotoxic activity for butcher’s broom extract. Butcher’s broom combined with sweet clover (Melilotus officinalis) or hesperidin methylchalcone has been used topically to treat varicose veins in pregnant women.57,67 An uncontrolled trial (noted earlier) observed that oral administration of butcher’s broom combined with hesperidin methylchalcone and ascorbic acid to pregnant women (21 to 24 weeks’ gestation) had no adverse effect on their infants. The 20 women involved in the study received the herbal treatment for at least 6 weeks and not more than 13 weeks. Postprandial digestive heaviness was noted by eight patients and was ascribed to the herbal treatment.59
Side effects
As with all herbs rich in saponins, oral use may cause irritation of the gastric mucous membranes and reflux. The use of an enteric-coated preparation is preferred in sensitive patients.
Contact allergy to ruscogenins occurs rarely, but may be underestimated. In the 10 years to 1998 eight cases were reported to the French medical authorities.69 Contact allergy to butcher’s broom extract has also been reported.70,71 In one of these cases the cream contained butcher’s broom extract and the excipient thimerosal, and positive patch test results were obtained for both ingredients.70
Adverse reactions associated with the oral intake of butcher’s broom extract combined with hesperidin methylchalcone and ascorbic acid have been reported. In particular, lymphocytic colitis (one case associated with ileal villous atrophy and most with chronic diarrhoea) were observed, many after long-term use of the product.72–75 Faecal fluid measurements were not consistent with an osmotic mechanism.75 Other cases of chronic diarrhoea76–79 including diarrhoea without mucus or blood,80 and watery diarrhoea mimicking coeliac disease, have been reported.81 These earlier reports are probably also cases of lymphocytic colitis that were recorded before this association was more widely recognised. The lymphocytic colitis may be secondary to a chronic activation of the mucosal immune system by one or several components of the combination.82 It has not been determined if the butcher’s broom extract is implicated in this adverse reaction.
A case of cytolytic hepatitis associated with the ingestion of a preparation containing ruscogenins, hesperidin, ascorbic acid and aesculetol has been reported.83
All the clinical trials noted previously indicate that the risk of side effects for butcher’s broom is relatively low and that such side effects are likely to be minor.
Regulatory status in selected countries
In the UK butcher’s broom is not included on the General Sale List. In Germany it is covered by a positive Commission E Monograph. Butcher’s broom is official in the European Pharmacopoeia 2006.
In the USA butcher’s broom does not have GRAS status. However, it is freely available as a ‘dietary supplement’ in the USA under DSHEA legislation (Dietary Supplement Health and Education Act of 1994).
In Australia butcher’s broom is not included in Part 4 of Schedule 4 of the Therapeutic Goods Regulations and is freely available for sale.
References
1. Mabberley DJ. The Plant Book, 2nd ed. Cambridge: Cambridge University Press, 1997. p. 628
2. Chiej R. The Macdonald Encyclopedia of Medicinal Plants. London: Macdonald; 1984. Entry No. 269
3. Grieve M, A Modern Herbal, New York, Dover Publications, 1971;Vol 1. pp. 128–129
4. Leclerc H. Précis de Phytothérapie, 5th ed. Paris: Masson, 1983. pp. 46–47
5. Wagner H, Bladt S. Plant Drug Analysis: A Thin Layer Chromatography Atlas, 2nd ed. Berlin: Springer-Verlag, 1996. p. 309
6. Dunouau C, Bellé R, Oulad-Ali A, et al. Planta Med. 1996;62:189–190.
7. Domange JR, Bougaret S, Yubero L. Clin Hemorheol. 1994;14(suppl 1):S7–S13.
8. Chevillard L, Ranson M, Senault B. Med Pharmacol Exp. 1965;12(2):109–114.
9. Capra C. Fitoterapia. 1972;43(4):99–113.
10. Facino RM, Carini M, Stefani R, et al. Arch Pharm (Weinheim). 1995;328(10):720–724.
11. Huang YL, Kou JP, Ma L, et al. J Pharmacol Sci. 2008;108(2):198–205.
12. Bouskela E, Cyrino FZ, Marcolon G. J Cardiovasc Pharmacol. 1993;22(2):221–224.
13. Bouskela E, Cyrino FZ, Marcolon G. J Cardiovasc Pharmacol. 1994;24(2):281–285.
14. Bouskela E, Cyrino FZ, Marcolon G. J Cardiovasc Pharmacol. 1994;24(1):165–170.
15. Marcelon G, Vanhoutte PM. Phlebology. 1988;3(suppl 1):51–54.
16. Miller VM, Rud K, Gloviezki P. Clin Hemorheol. 1994;14(suppl 1):S34–S37.
17. Anon. Dtsch Apoth Ztg. 1985;125(6):295.
18. Rubanyi G, Marcelon G, Vanhoutte PM. Gen Pharmacol. 1984;15(5):431–434.
19. Barton D, Ollis WD, eds. Advances in Medicinal Phytochemistry. London: John Libbey Eurotext, 1986. pp. 187–194
20. Bouskela E, Cyrino FZ, Bougaret S. Clin Hemorheol Microcirc. 1997;17(5):351–356.
21. Miller VM, Rud K, Gloviczki P. Clin Hemorheol. 1994;14(suppl 1):S37–S45.
22. Nemcova S, Gloviczki P, Rud KS, et al. J Vasc Surg. 1999;30(5):876–883.
23. Miller VM, Marcelon G, Vanhoutte PM. Phlebology. 1991;6(4):261–268.
24. Berg D. Fortschr Med. 1990;108(24):473–476.
25. Rudofsky C. Fortschr Med. 1989;107(19):52. 55–58
26. Haas S, Lill G, Stiller A, et al, Vanhoutte PM, ed. Return Circulation and Norepinephrine: An Update, Paris-London, John Libbey Eurotext, 1991:157–162.
27. Le Devehat C, Khodabandehlou T, Vimeux M, Bondoux G, Vanhoutte PM, ed. Return Circulation and Norepinephrine: An Update, Paris-London, John Libbey Eurotext, 1991:225–236.
28. Svensjo E, Bouskela E, Cyrino FZ, et al. Clin Hemorheol Microcirc. 1997;17(5):385–388.
29. Tarayre JP, Lauressergues H. Ann Pharm Franc. 1976;34:375–382.
30. Strauss AL, Rieger H. Phlebologie. 1992;21:247–251.
31. Bouaziz N, Michiels C, Janssens D, et al. Int Angiol. 1999;18(4):306–312.
32. Baurain R, Dom G, Trouet A. Clin Hemorheol. 1994;14(suppl 1):S15–S21.
33. Benard P, Cousse H, Rico AG, et al. Ann Pharm Fr. 1986;43(6):573–584.
34. Chanal JL, Mbatchi B, Sicart MT, et al. Trav Soc Pharm Montpellier. 1981;41(4):263–272.
35. Chanal JL, Cousse H, Sicart MT, et al. Trav Soc Pharm Montpellier. 1978;38(1):43–48.
36. Rauwald HW, Grunwidl J. Planta Med. 1991;57(suppl 2):A75–A76.
37. Boyle P, Diehm C, Robertson C. Int Angiol. 2003;22(3):250–262.
38. Vanscheidt W, Jost V, Wolna P, et al. Arzneimittelforschung. 2002;52(4):243–250.
39. Jager K, Eichlisberger R, Jeanneret Ch, et al. Clin Drug Invest. 1999;17(4):265–273.
40. Parrado F, Buzzi A. Clin Drug Invest. 1999;18(4):255–261.
41. Cappelli R, Nicora M, Di Perri T. Drugs Exp Clin Res. 1988;14(4):277–283.
42. Weindorf N, Schultz-Ehrenburg U. Z Hautkr. 1987;62(1):28–38.
43. Marshall M. Fortschr Med. 1984;102(29–30):772–774.
44. Elbaz C, Nebot F, Reinharez D. Phlebology. 1976;29(1):77–84.
45. Monteil-Seurin J. C R Ther Pharmacol Clin. 1993;109:3–7.
46. Beltramino R, Penenory A, Buceta AM. Int Angiol. 1999;18(4):337–342.
47. Beltramino R, Penenory A, Buceta AM. Angiology. 2000;51(7):535–544.
48. Rudofsky G, Diehm C, Gruss JD, et al. MMW. 1990;132:205–210.
49. Le Devehat C, Lemoine A, Roux E, et al. Angiologie. 1984;34(3):119–122.
50. Lozes A, Boccalon H. Int Angiol. 1984;3:99–102.
51. Braun R, Hirche H, Van Laak H-H. Z Allgemeinm. 1985;61:309–313.
52. Guex JJ, Avril L, Enrici E, et al. Int Angiol. 2010;29(6):525–531.
53. Guex JJ, Enriquez Vega DM, Avril L, et al. Phlebology. 2009;24(4):157–165.
54. Porto CL, Milhomens AL, Pires CE, et al. Int Angiol. 2009;28(3):222–231.
55. Guex JJ, Enrici E, Boussetta S, et al. Dermatol Surg. 2008;34(12):1666–1675.
56. Leutenegger E, Martinaggi P. Gazette Med. 1988;95(13):66–69.
57. Berg D. Fortschr Med. 1992;110(3):67–68. 71–72
58. Berg D, Vanhoutte PM, ed. Return Circulation and Norepinephrine: An Update, Paris, John Libbey Eurotext, 1991:55–61.
59. Baudet JH, Collet D, Aubard Y, et al, Vanhoutte PM, ed. Return Circulation and Norepinephrine: An Update, Paris, John Libbey Eurotext, 1991:63–71.
60. Leutenegger E, Martinaggi P. Gazette Med. 1988;95(33):83–85.
61. Leyh F. Therapiewoche. 1988;36:2325–2331.
62. Cluzan RV, Alliot F, Ghabboun S, et al. Lymphology. 1996;29(1):29–35.
63. Bennani A, Biadillah MC, Cherkaoui A, et al. Phlebologie. 1999;52(1):89–95.
64. Monteil-Seurin J, Ladure Ph. Efficacy of Ruscus extract in the treatment of the premenstrual syndrome. In: Vanhoutte PM, ed. Return Circulation and Norepinephrine: An Update. Paris: John Libbey Eurotext; 1991:43–53.
65. Redman DA. J Altern Complement Med. 2000;6(6):539–549.
66. Archimowicz-Cyrylowska B, Adamek B, Drozdzik M, et al. Phytother Res. 1996;10(8):659–662.
67. Seidenberger AV, Muller I, Heindl HJH. Therapiewoche. 1974;24(8):866–881.
68. Caujolle F, Meriel P, Stanislas E. Ann Pharm Fr. 1953;11:109–120.
69. Elbadir S, El-Sayed R, Renaud F, et al. Rev Fr Allergol. 1998;38(1):37–40.
70. Breuil K, Patte F, Meurice JC, et al. Rev Fr Allergol Immunol Clin. 1989;29(4):215.
71. Landa N, Aguirre A, Goday J, et al. Contact Dermatitis. 1990;22(5):290–291.
72. Dharancy S, Dapvril V, Dupont-Evrard F, et al. Gastroenterol Clin Biol. 2000;24(1):134–135.
73. Pierrugues R, Saingra B. Gastroenterol Clin Biol. 1996;20(10):916–917.
74. Bouaniche M, Chassagne P, Landrin I, et al. Rev Med Interne. 1996;17(9):776–778.
75. Ouyahya F, Codjovi P, Machet MC, et al. Gastroenterol Clin Biol. 1993;17(1):65–66.
76. Mornet M, Boiserie P, Jonville AP, et al. Therapie. 1991;46(3):254.
77. Anon. Presc Int. 1993;2(7):123.
78. Gendreau-Tranquart C, Barbieux JP, Furet Y, et al. Presse Med. 1989;18(29):1439.
79. Oliver JM, Bacq Y, Dorval ED, et al. Gastroenterol Clin Biol. 1991;15(2):160–162.
80. Thomas-Anterion C, Guy C, Vial F, et al. Rev Med Interne. 1993;14(4):215–217.
81. Widgren S, De Peyer R, Geissbuhler P, et al. Schweiz Med Wochenschr. 1994;124(8):313–318.
82. Beaugerie L, Luboinski J, Brousse N, et al. Gut. 1994;35(3):426–428.
83. Sgro C, Taque R, Zoll A, et al. J Hepatol. 1995;22(2):251.