Biological Mechanisms in Orthodontic Tooth Movement
Sunil Kapila, Gregory J. King
The goal of this chapter is to examine the relationships between orthodontic biomechanics and the underlying biological processes. The topics discussed include the factors affecting the rate of tooth movement, anchorage considerations, causes of relapse, and root resorption. All relevant biological principles underlying orthodontic tooth movement can be characterized as tissue remodeling. The process of orthodontic tooth movement is a resultant dynamic change in the shapes and composition of the investing bone and soft tissues. The dental and peridental tissues (dentin, cementum, periodontal ligament [PDL], and alveolar bone) all have active reparative mechanisms and will adapt under the normal forces of orthodontic appliances. At the most basic level, extrinsic forces set up localized areas of “pressure” and “tension” in the tissues adjacent to teeth and the subsequent responses satisfy the principles of Wolff's law of bone remodeling.1
When orthodontists use fixed appliances to apply force on teeth, predictable tooth movement is anticipated. This is accompanied by transiently increased tooth mobility and, occasionally, radiographic evidence of mild root resorption. Experienced clinicians also expect a certain amount of relapse to occur following orthodontic treatment. Other types of natural tooth migration commonly encountered are eruption of primary and succedaneous teeth as well as mesial or distal drifting of teeth. These physiological processes are not necessarily stimulated by biomechanical signals.
In rare instances teeth fail to erupt or move in response to forces (i.e., ankylosis). Each of these common clinical findings can be explained with a better understanding of the underlying biological principles that determine tooth movement.
Tooth Movement
Clinical Responses
Kinetics of Orthodontic Tooth Movement
From a clinical perspective orthodontic tooth movement has three distinct phases: (1) displacement phase, (2) delay phase, and (3) acceleration and linear phase (Fig. 5-1).
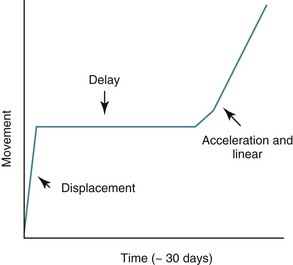
Figure 5-1 Phases of orthodontic tooth movement. The classic curve has three phases: an initial displacement that reflects the viscoelastic properties of the tooth supporting structures; a delay or lag period characterized by no movement; and a postlag phase with linear tooth movement. Minimal tooth movement occurs during the first two phases and most of the tooth movement occurs during the acceleration and linear phases, when alveolar bone remodeling occurs. The timeline is approximate, with considerable individual variation due to mechanical and biological differences.
Displacement Phase.
The initial reaction of a tooth following force application is almost instantaneous (within a fraction of a second) and reflects the immediate movement of the tooth within the viscoelastic PDL cradle. These movements are generally predictable by biophysical principles and typically do not involve extensive amounts of tissue remodeling or deformation of the investing alveolar bone.2 The fluid compartments within the PDL play an important role in the transmission and damping of forces acting on teeth.3 The magnitude of the displacement response is also dependent on root length and alveolar bone height, which are factors that determine the location of a tooth's center of resistance and center of rotation (see Chapter 4).4,5 For example, loss of alveolar bone results in a more apically positioned center of resistance, which affects the nature of both the initial displacement and net tooth movement (Fig. 5-2). Age is another factor affecting displacement. Young's modulus of the PDL has been shown to be greater in adults than in adolescents and this difference in biomechanical properties correlates to an equivalent or somewhat increased stress level in the PDL in adults. It is suggested that this might reduce the biological response of the PDL and thus delay tooth movement in adults.6 The displacement capacity of a tooth can change even within the same individual; the elasticity of the PDL and alveolar bone has the potential to be substantially reduced at the end of tooth movement.7
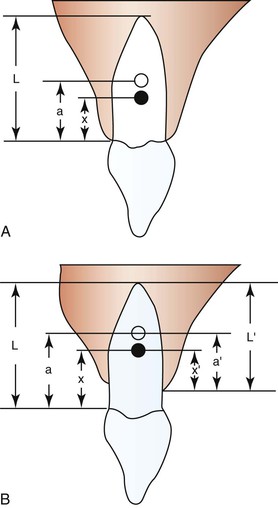
Figure 5-2 Degree of freedom within the viscoelastic periodontal ligament (PDL) apparatus (displacement) is affected by root lengths and alveolar bone heights. A, Varying root length (L) will cause shifts in the positions of the distance of the center of rotation (CROT) to the cervix (a) and the distance of the center of resistance (CRES) to the cervix (x). B, Alveolar bone height changes can affect CROT and CRES. (a, distance of the CROT to the cervix; a′, distance of the CROT to the alveolar crest; L, average root length; L′, varying alveolar bone heights; x, distance of the CRES to the cervix; x′, distance of the CRES to the alveolar crest.) Ultimately the patterns of tooth displacement will be determined by the change in the position of CRES produced by changes in alveolar bone height or root length. (Modified with permission from Tanne K, Nagataki T, Inoue Y, Sakuda M, Burstone CJ. Patterns of initial tooth displacements associated with various root lengths and alveolar bone heights. Am J Orthod Dentofacial Orthop. 1991;100:66–71.)
Delay Phase.
The second phase of the orthodontic tooth movement cycle is characterized by the absence of clinical movement and is generally referred to as the delay or latency phase. During this period there is no tooth movement but extensive remodeling occurs in all tooth-investing tissues. The absolute amount of force applied is not as relevant as the relative force applied per unit area. Depending on the localized compression of the PDL, there can be either (1) a partial occlusion of the blood vessels in the area or (2) an absolute occlusion of blood vessels when high excessive forces have been applied. In cases of partial blockage, the blood vessels delivering nutrients to the area have the capacity to adapt to the new environment and can undergo angiogenesis to bypass occluded areas. However, complete occlusion of vascular flow leads to temporary necrosis of the immediate area and follows a completely different pathway of tooth movement, which is slower to be initiated, starting after approximately 1 to 2 weeks. In either situation, structural and biochemical changes initiate a cascade of cellular mechanisms required for bone remodeling.
Aging has been shown to substantially affect the proliferative activity of the PDL cells and subsequent tooth movement, particularly during the delay phase.8 However, some studies on molar movement in animal models have shown faster initial tooth movement in young subjects than in adults. Yet once tooth movement had reached the linear phase, the rate of tooth movement became equal in both groups. This indicates that the clinically observed increase in orthodontic treatment time for adults can be attributed primarily to the delay phase prior to the onset of tooth movement but the rate of migration is equally efficient once tooth movement has started.9
Acceleration and Linear Phase.
The third phase of the orthodontic tooth movement cycle is characterized by rapid tooth displacement. Tooth movement is initiated in deference to the adaptation of the supporting PDL and alveolar bone changes. Studies on the bone-resorptive osteoclast response following orthodontic appliance activation indicate that when appliance reactivation occurs during the appearance of reactivation osteoclasts, a second cohort of osteoclasts can be recruited immediately. This causes immediate, significant tooth movement with no greater risk of root resorption.10 The force magnitude directly affects the rate of tooth movement. High forces in excess of 100 g used in conventional orthodontic therapy to retract canine teeth have been shown to produce a lag phase of up to 21 days before tooth movement. Lower forces can induce tooth translation without a lag phase at rates that are still clinically significant.11 The difference in rates of tooth movements can be explained by the different biological responses (frontal resorption versus undermining resorption) discussed later in the chapter. Equally as important as magnitude, however, is the timing of the force application. The force regimen has more influence on the rate of orthodontic tooth movement than the force magnitude.12 Light continuous forces are much more conducive to orthodontic tooth movement because the cell biology system remains in a constantly responsive state. Conversely the application of intermittent forces creates a fluctuating environment of cellular activity and quiescence (Fig. 5-3, A). Additionally, it is recognized that very low forces produce lower rates of tooth movement then higher forces (Fig. 5-3, B) up to a specific optimal threshold. Exceeding this optimal force does not result in substantially greater rates of tooth movement. This threshold may differ between individuals as demonstrated in experiments on beagle dogs where it was noted that 25 cN of force caused greater tooth movement than 10 cN of force in one animal but not in another (Fig. 5-3, B and C).
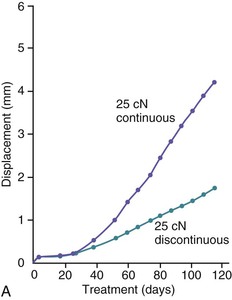
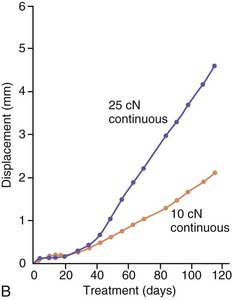
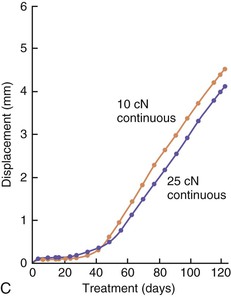
Figure 5-3 Time displacement curves for premolar tooth movement in a beagle dog experimental model demonstrating that (A) light continuous forces of 25 cN are more effective in tooth movement than light discontinuous forces and that (B) continuous forces of 25 cN produce greater movement than 10 cN in one animal, while (C) in another animal the two forces produce equal amounts of tooth movement, demonstrating individual variation and a plateau effect in the latter animal. (Reproduced with permission from van Leeuwen EJ, Maltha JC, Kuijpers-Jagtman AM. Tooth movement with light continuous and discontinuous forces in beagle dogs. Eur J Oral Sci. 1999;197:468–474.)
Ankylosis
In rare cases a tooth may not move at all, regardless of the amount of external force applied on it. A likely cause of this is a phenomenon known as “ankylosis” where the PDL fibers are conspicuously absent and therefore cannot serve as an intermediary between the root structure and the alveolar bone. The contact point is a direct fusion of the cementum layer to the cortical bone of the tooth socket. Apart from idiopathic ankylosis, the primary cause of ankylosis is extrinsic localized trauma.13 In cases of severe dental trauma, such as avulsion or intrusion, there is injury to the periodontal membrane resulting in a direct fusion of the alveolar bone with the tooth. Consequences of this condition include progressive resorption of the root with replacement by bone (replacement resorption) and arrested growth of the alveolar process in growing patients. Individuals with congenitally missing succedaneous permanent teeth characteristically exhibit infraocclusion and overretention of ankylosed deciduous teeth.14 Partial ankylosis can occur when only limited areas of the teeth are fused to the bone. If these localized regions of bone–tooth attachment can be overcome with sufficient force application, the remainder of the tooth that does have PDL support can proceed toward a normal pattern of tissue remodeling and tooth movement.
Principles of Anchorage in Orthodontics
The biomechanics of orthodontics are not always designed for the purpose of moving teeth. In certain cases the intent of the clinician may be to hold the position of certain teeth in the arch or to use groups of teeth comprising an “anchor unit” to serve as a foundation for pushing or pulling other teeth. Several types of anchorage are used in orthodontics: (1) extraoral anchorage appliances (e.g., headgear) where external skeletal structures are used, (2) intraoral osseointegrated implants and temporary anchorage devices (TADs) that are physically interlocked within the bone and therefore are very stable, and (3) dental anchorage, which is essentially the preparation and consolidation of teeth into units for use in pushing or pulling the rest of the dentition.
Dental anchorage is a term applied to the intentional minimization of migration of specific teeth through the supporting alveolar bone structure. The following section elaborates on dental anchorage since it is based on the premise of biological adaptation to orthodontic forces. Dental anchorage can be increased either by increasing the number of teeth consolidated into the anchor unit or by intentionally angulating specific teeth to better resist movement, or both. In general, teeth with greater root surface area tend to move less when used to move teeth with less root surface area. This occurs because the ability to resist movement is directly related to the periodontal fibers and bone surface area engaged in withstanding tooth movement. When forces are light and distributed over large surface areas, the compression on underlying periodontal structures leads to a partial vascular occlusion of the system and a transient ischemia. Although limited, there is still oxygenation to the area, enabling the microsystem to adapt and recruit new blood vessels for initiation of frontal resorption to occur. Movement of teeth with frontal resorption occurs within 3 to 4 days. However, when hyalinization of bone occurs in areas of periodontal compression during force application, there is a significant retardation of tooth movement while undermining resorption occurs. In this case the resistance to tooth movement is due to complete vascular occlusion in the compression area causing localized necrosis of bone and undermining resorption. When this happens the teeth start moving only after 12 to 15 days of bone remodeling. Therefore anchorage preparation is affected by the magnitude of forces applied, the total root surface area of the teeth upon which the forces are applied, and the angulation of the teeth.
Increasing numbers of adults are seeking orthodontic treatment today and in these cases anchorage becomes a critical concern. Extraoral anchorage appliances are not usually a feasible alternative for these individuals. Therefore the clinician must maximize all available resources, such as the engagement and colligation of second molars (and third molars, if present) into the dental anchorage unit as well as the use of palatal anchorage devices such as the Nance acrylic button appliance. Also in multidisciplinary cases, implants and other fixed restorative devices can and should be incorporated into the treatment plan for use as anchorage units during orthodontics. Finally, the introduction of TADs has provided substantial advantages for anchorage preservation in adults and adolescents alike and has opened up novel approaches to orthodontic biomechanics in complex cases.15
Histological Responses
In general, teeth can move through their investing tissues with or without histological evidence of tissue injury. There is no evidence that the intra-alveolar phase of tooth movement during physiological eruption, drift, or relapse is mediated by pathological processes.16–18 However, most studies of orthodontic tooth movement have described pathological processes at sites of compression, including vascular collapse, compensatory hyperemia, and tissue necrosis. Hyperemic changes are not restricted to the periodontal tissues adjacent to compression but have also been variously described in the adjacent marrow spaces and the dental pulp.
Tooth Movement Without Injury
The most obvious course of physiological tooth movement is the intra-alveolar eruption of teeth. As the crown of a tooth completes mineralization and begins its process of migration through the alveolar bone, it becomes enclosed in a crypt. This crypt is translated bodily by a combined effort of osteoclastic bone resorption along the path of eruption and osteoblastic bone formation on the path that the crown has already taken. The rate-limiting factor of the earliest (intraosseous) stage of tooth eruption is bone resorption and eruption can be accelerated or retarded by the local delivery of factors that alter the rate of osteoclastic activity.19 Certain hormones, such as parathyroid hormone–related protein (PTHrP), have been shown to be crucial in the normal tooth eruption processes and cementogenesis.20,21 Pathological systemic conditions with dysfunctional PTH/PTHrP or their cognate receptors can lead to the prevention of normal tooth eruption and inhibition of normal cementogenesis.
As teeth erupt into the oral cavity, and even throughout life, there is a natural tendency for them to continue moving along the path of least resistance until they encounter an obstacle of resistance. Usually this barrier comes in the form of interproximal contact from an adjacent tooth or occlusal contact from a tooth in the opposing arch. In the absence of this resistance there will be continued mesiodistal tipping or supraeruption, depending on the location of deficient contact. Studies have shown that mesial drifting of a tooth can have clinical significance on its morphological composition. In the process of mesial migration, tensional forces on the distal root surfaces may account for the increased cementum thickness on the distal surfaces of mesially drifted teeth.22 As for supraeruption, in a mouse model where the opposing molar was extracted to induce occlusal hypofunction, histological staining showed that after 15 days of hypofunction the PDL was significantly narrowed and the fibrous nature was very disorganized for a minimum of 30 days and up to 3 months. There was concurrent deposition of disorganized woven bone at the top of the interradicular septa, at the bottom of the sockets, and along the modeling sides.23 Thus it is evident that loading is an integral part of the maintenance of the supporting structures surrounding the tooth.
Tooth Movement with Injury
Necrotic lesions at compression sites in the PDL space have been described in the early literature documenting the histological changes accompanying orthodontic tooth movement.24 These areas are referred to as “hyalinization zones” because of their similarity in appearance to hyaline cartilage. Modern morphological techniques have elucidated that these so-called “hyalinizations” are in fact areas of focal tissue necrosis.25 As long as these lesions persist, orthodontic tooth movement does not occur. This period is coincident with the delay phase of the tooth movement cycle. Specialized phagocytic cells are recruited and migrate to the site to remove these necrotic lesions. These cells remove the injured tissue from the periphery, resulting in the resorption of not only the necrotic soft tissue lesion but also the adjacent alveolar bone and cementum.26
The tissue responses at sites of tension are consistent with those that have been described for other sites where soft tissue separates bone. In addition to the PDL, such sites can be found naturally in the craniofacial complex at sutures and artificially at sites of osteodistraction. Tensile forces are known to initiate an exuberant osteogenic response at these locations, with the first bone being deposited on the stretched soft tissue scaffold (Fig. 5-4). Through remodeling processes new compact bone is eventually deposited at these sites. This so-called consolidation process is slow to occur and therefore tends to lag behind the tissue removal activity that is simultaneously occurring at compression sites. The clinical result is the prevalence of increased mobility in teeth that are actively being treated orthodontically. This difference in timing between tissue removal and osteogenesis also accounts for the need to retain teeth that have recently been moved.
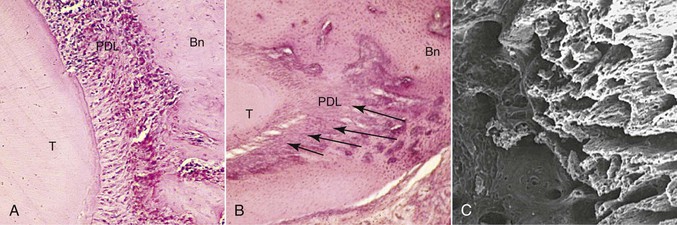
Figure 5-4 Morphological changes on the tension side during orthodontic tooth movement. A, Initial changes are characterized by stretching of the principal periodontal ligament (PDL) fibers, seen here as linear orientation of cell nuclei adjacent to the tooth. B, Later changes show deposition of bone on the stretched PDL fibers, oriented perpendicular to the tooth and socket wall (arrows). Bn, Alveolar bone; T, tooth root. C, The three-dimensional organization of these initial bony spicules can be appreciated with a scanning electron micrograph of the alveolar bone on the socket wall after removal of the tooth and PDL. The micrograph is looking into the socket with the tension socket wall on the right.
In addition to bone remodeling, histological evidence has shown that initial root resorption occurs in the peripheries of the necrotic PDL following orthodontic treatment (Fig. 5-5). This is a result of mononucleated nonclast macrophage-like and fibroblast-like cellular activity.27,28 Minor resorptive lacunae created on the root surface by cementoclasts can be repaired gradually over time. However, heavy forces create extensive defects on the root surface, which exceed reparative capacity and lead to craterlike topography on the root surface and at the apex (Fig. 5-6).
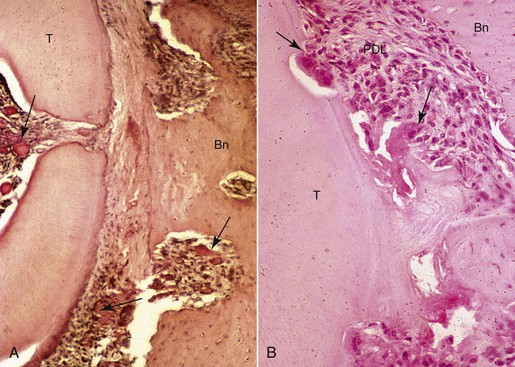
Figure 5-5 Morphological changes on the compression side showing tissue and cellular responses leading to root resorption during orthodontic tooth movement. A, Initial changes are characterized by focal areas of periodontal ligament (PDL) necrosis, so-called hyalinization, seen as the clear area in the PDL running vertically down the center of this micrograph. Areas of vascular congestion can be seen adjacent (pulp, PDL, and marrow spaces) to the necrotic PDL (arrows). B, Later changes show removal of the necrotic PDL and adjacent tissues, including the root cementum and dentin from the periphery by osteoclasts, cementoclasts, and macrophages (arrows). The remaining necrotic PDL is seen as the clear pink area in the lower middle of the micrograph. Vital adjacent PDL can be seen as the highly cellular areas above and below the necrosis. Bn, Alveolar bone; T, tooth root.
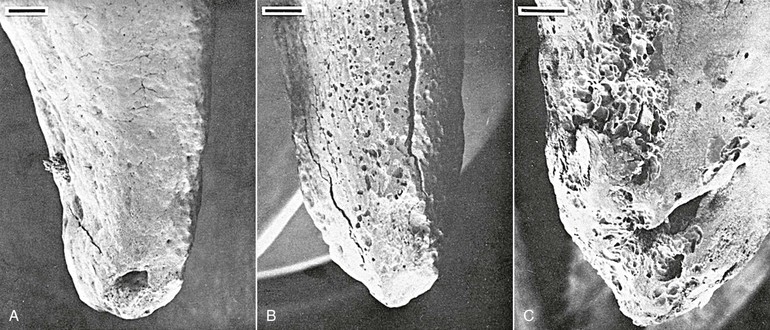
Figure 5-6 Tooth injury resulting in root resorption following bone remodeling. A, Apical third of the lingual root surface of the mandibular left bicuspid control tooth demonstrating the absence of resorption. B, Apical resorption and loss of root length association with a multitude of resorption pits over the lingual root surface as a result of 2 weeks' application of a continuous 10-g intrusive force. Many of the resorption loci have coalesced to form extensive invasive lesions. C, Lingual aspect of a maxillary right bicuspid showing early apical resorption caused by 14 days' intrusion with 50 g. Original magnifications for A and B are ×20 (bar = 300 mm) and for C is ×40 (bar = 200 mm). (Reproduced with permission from Harry MR, Sims MR. Root resorption in bicuspid intrusion: a scanning electron microscope study. Angle Orthod. 1982;52:235–258.)
Bone Turnover in Orthodontic Tooth Movement
Osteogenesis and Bone Modeling and Remodeling
Bone structure can be changed in three principle ways: (1) osteogenesis, (2) bone modeling, and (3) bone remodeling. Osteogenesis is when bone is formed on soft tissue and generally occurs during embryonic development, the early stages of growth, and healing. There are two major subclassifications: intramembranous ossification and endochondral ossification, in which bone is formed on soft fibrous tissue and, usually, cartilage, respectively. Osteoblasts are a differentiation product from mesenchymal cells and act independently of osteoclasts, resulting in a large potential to create significant amounts of bone.
Modeling is characterized by bone formation on existing bone tissue over extended surface areas for significant periods of time. This type of bone turnover is prevalent during craniofacial growth and development and leads to change in shape of the structure or translation of the surface. For example, the mandibular alveolar process increases in length by resorptive modeling on the anterior surface of the ramus and by formative modeling on its posterior surface. From an orthodontics standpoint, modeling is important in normal growth of the craniofacial structure as well as changes in alveolar size and shape during tooth movement.
Remodeling is a reparative mechanism and involves a series of cellular events that occur cyclically throughout life (Fig. 5-7). It is the only physiological mechanism for maintaining and repairing the structural integrity of bone. The bone remodeling cycle begins with a period referred to as activation that is characterized by the recruitment and activation of osteoclasts at the site to be remodeled. This is followed by a resorptive phase when a “packet” of bone is removed. After a finite amount of time the resorptive process ceases. This phase is termed reversal. Reversal is followed by a formative phase characterized by the recruitment of bone formative cells to the site and the active repair of the defect created during the resorptive phase. Once the cycle is complete, the bone surface returns to a resting state. In healthy adults bone surfaces are primarily in a resting state, although a small fraction of the cell population can be seen to progress through the other phases. Remodeling is important in calcium homeostasis as well as in producing changes in bone matrices that modify the mechanical properties of the bone in response to altered loading. Remodeling and modeling of bone are differentiated by the fact that while osteoblast and osteoclast activities are found on the same site in remodeling, they occur on different sites in modeling, which enables morphological changes in the bone.
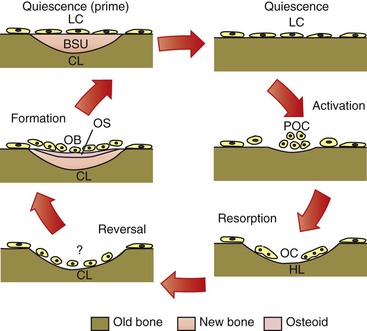
Figure 5-7 Cellular quiescence phase and five phases of cellular activity in remodeling of trabecular bone. BSU, Bone structural unit or newly formed bone structure; CL, closed lacunae; HL, Howship lacunae/resorption pit; LC, lining cells; OB, osteoblast; OC, osteoclasts; OS, osteoid; POC, osteoclast precursors.
Cellular and Molecular Mechanisms of Bone Modeling and Remodeling
Skeletal integrity is the result of a dynamic interaction between bone-forming osteoblasts and bone-resorbing osteoclasts. The rate of remodeling is defined primarily by cells of the osteoblast lineage, which in addition to bone formation are also responsible for the activation and recruitment of osteoclast precursors.29–31 The basis of communication between osteoblasts and osteoclasts was unclear until several groups independently identified the presence of an intermediary factor on the surface of osteoblasts that was responsible for the induction of osteoclastogenesis. This factor is a member of the tumor necrosis factor (TNF) superfamily and was termed receptor activator of nuclear factor κB ligand (RANKL).32,33 Binding of RANKL to its cognate receptor, receptor activator of nuclear factor κB (RANK), expressed on the surface of osteoclast progenitor cells, induces osteoclastogenesis and activates osteoclasts (in the presence of macrophage colony-stimulating factor), resulting in increased bone resorption.34,35 However, RANKL also has the potential to bind to osteoprotegerin (OPG), a soluble decoy receptor protein that competitively binds to cell surface membrane–bound RANKL proteins and inhibits RANKL activation of osteoclastogenesis. The interaction of RANKL with OPG therefore decreases bone resorption (Fig. 5-8).36 The ratio of RANKL/OPG expression by osteoblasts is believed to be a key determinant of the rate of recruitment and activation of immature osteoclasts. In dentistry these genes have already been strongly implicated as the causative factors of alveolar bone changes. RANKL and OPG protein production has been detected in human periodontal cells.37 Pathologically, lymphocytes and macrophages in periodontitis tissues show correlations with RANKL protein production and endothelial cells have associations with OPG production.38 From an orthodontic perspective, it is very likely that pressure changes in the microenvironment of the tooth socket may cause up-regulation and down-regulation of the RANKL and OPG genes as a means of modulating protein production and ultimately bone remodeling.
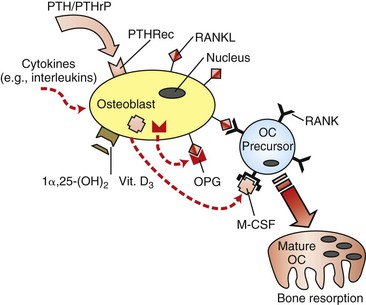
Figure 5-8 Regulation of osteoclastogenesis by osteoblasts. Receptor activator of nuclear factor κB ligand (RANKL) induces immature osteoclast (OC) precursors to differentiate into mature and functional osteoclasts, while osteoprotegerin (OPG) is a decoy receptor that acts as a competitive binding inhibitor of RANKL. Since RANKL is a cell surface–bound receptor protein, cell–cell interaction is required. Macrophage colony-stimulating factor (M-CSF) is also an essential co-factor. Vitamin D3 and various cytokines have been demonstrated to have down-regulatory effects on OPG gene expression and up-regulatory effects on RANKL gene expression. PTH, Parathyroid hormone; PTHRec, PTH receptor; PTHrP, PTH-related protein.
Besides the prominent role of the RANKL/OPG ratio in the regulation of osteoclasts by osteoblasts, the rate of bone remodeling is controlled by other local and systemic mechanisms. Local, or paracrine, mechanisms involve numerous inflammatory cytokines (e.g., interleukins, TNFs, and growth factors) that have biological activities influencing individual phases of the cycle (Fig. 5-9).39 In addition, there is evidence that alterations in the genetic expression of bioactive agents can occur directly on bone cells. Systemic control of bone remodeling occurs through several endocrine mechanisms, including the calciotropic hormones (e.g., parathyroid hormone [PTH] and 1α,25-(OH)2 vitamin D3) and the sex steroids (e.g., estrogen).40–42 These factors act on osteoblasts as an intermediary to regulate osteoblast-osteoclast equilibrium and can either up-regulate or down-regulate a cascade of downstream signaling pathways that ultimately affect the expression of specific genes necessary to synthesize proteins involved in bone remodeling. For example, estrogen inhibits bone resorption, at least in part, by regulating the production of several cytokines, including interleukin-6 (IL-6), IL-1, RANKL, and OPG by cells of the osteoblastic lineage.43 The potential implications and more recent findings on the applications of this knowledge to orthodontics are discussed at the end of the chapter.
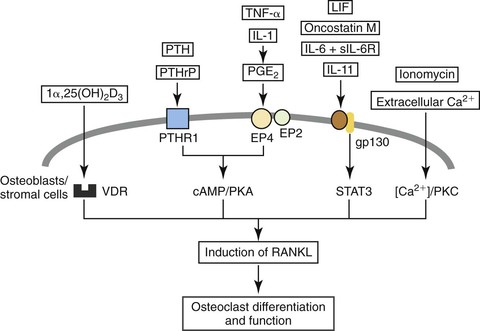
Figure 5-9 Paracrine and endocrine regulation of receptor activator of nuclear factor κB ligand (RANKL) in osteoblasts or stromal cells, which in turn induces osteoclastogenesis and results in the alteration of the osteoblast-osteoclast equilibrium. Hormones and cytokine factors: 1α,25(OH)2D3; 1α, vitamin D receptor; PTH/PTHrP, parathyroid hormone/PTH-related protein receptor; PTHR1, parathyroid hormone receptor type 1; TNF-α, tumor necrosis factor-α; IL-1, IL-6, sIL-6R, IL-11, interleukin-1, interleukin-6, soluble IL-6 receptor, interleukin-11; PGE2, prostaglandin E2; LIF, leukemia inhibitory factor. Pathways: VDR, Vitamin D receptor; cAMP/PKA, cyclic adenosine monophosphate/protein kinase A pathway; STAT3, signal transducer and activator of transcription 3 (important for the signal transduction of IL-6 and related cytokines); [Ca2+]/PKC, calcium concentration/protein kinase C pathway. (Reproduced with permission from Takahashi N, Udagawa N, Takami M, Suda T. Cells of bone: osteoclast generation. In: Bilezikian JP, Raisz LG, Rodan GA, eds. Principles of Bone Biology. San Diego, CA: Academic Press; 2002:109–126.)
The human bone remodeling cycle takes about 4 months and is characterized by a rapid period of resorption followed by a fairly slow period of formation. In a healthy adult bone resorption is coupled to formation so that there is no net loss or gain. However, in certain disease conditions this coupling can be lost, resulting in a net loss or gain of bone. Because it takes significantly more time to complete bone formation than it does to complete resorption, stimulation of large amounts of remodeling often leads to a net bone loss. This can be a temporary condition if the level of remodeling activity returns to normal, permitting formation to “catch up” with resorption as characterized by fracture healing. However, if high levels of bone remodeling persist, bone can be lost permanently, as in postmenopausal osteoporosis.
Conversely, increased bone density resulting in osteopetrosis is a clinical manifestation of certain syndromes, such as Albers-Schönberg disease and Paget disease, where there is an excessive hyperactivity of the osteoclasts resulting in a concurrent compensatory excessive deposition of bone. Rates of bone remodeling may be increased up to 20-fold, but this coupling is imperfect. Since the newly formed bone is laid down so quickly, it is irregular and chaotic, resulting in a composite mixture of lamellar and woven bone, which compromises the quality of the bone. The clinical consequences of this abnormal bone cell activity include diffuse sclerosis of the whole skeleton accompanied by pathological bone fragility and delayed physical development, profound intractable myelophthisic anemia, neurological deficits, and osteomyelitis, especially of the jaws and skull. Eruption and development of the dentition can be impeded and delayed due to the irregular bone remodeling adjacent to the tooth bud and crown.
Biological Responses in Clinical Treatment
In orthodontic tooth movement, sites of tension display osteogenesis over an extensive surface area, a framework consistent with modeling (Fig. 5-10, A). However, sites of compression undergo phases of a remodeling cycle (Fig. 5-10, B).44 Since large amounts of remodeling are initiated at these sites, there is a net loss of alveolar bone over a short term, which subsequently returns to pre-treatment levels over the course of orthodontic treatment. This ultimately leads to the clinical characterization of teeth being actively moved (i.e., radiographic evidence of widening of the PDL and clinical evidence of increased tooth mobility). This, along with the persistence of stretched ligament and gingival fibers, leads to rapid relapse of orthodontically moved teeth and necessitates stabilization, at least until bone formation is able to return to pre-treatment levels.45 Recent advances in the potential utility of biological mediators in enhancing anchorage46 and post-treatment stability47 are discussed later in the chapter.
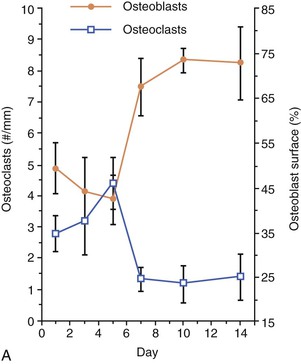
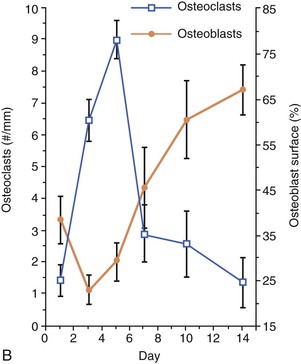
Figure 5-10 Cell dynamics associated with orthodontic tooth movement. A, Tension sites are characterized by an increase in osteoblasts and a reduction in resident osteoclasts in the latter part of the tooth movement. B, Compression sites are characterized by an influx of osteoclasts in the early part of the tooth movement followed by their reversal to baseline levels, followed by an increase in osteoblasts in the latter part of the tooth movement.
There is a predictable relationship between mechanical strain and bone turnover. Increasing amounts of strain stimulate osteogenesis and conversely the lack of strain on bone (e.g., weightlessness during space flight) leads to osteopenia. Superficially this seems to contradict what happens in orthodontic tooth movement because sites of compression lose bone and sites of tension gain it. However, it is important to realize that the PDL is loaded in tension sites and unloaded in compression sites. The latter also exhibit evidence of tissue damage and infiltration of inflammatory cells releasing cytokines, which can also stimulate large amounts of bone resorption. Recent studies show that alveolar bone at sites distant from the tooth socket wall contribute to the bone remodeling response, and this is consistent with what would be predicted by the bone literature (i.e., bony trabeculae adjacent to compression sites are osteogenic and those next to tension sites are not).48 Evidence also indicates that tissues are directly sensitive to deformations and strains in their immediate environment in addition to pressure or tension.49
Biological data are helpful in answering several clinically relevant questions in orthodontic tooth movement. How does bone density alter tooth movement? What is the relationship between force level and clinical response? How do intermittently applied forces compare to constant forces? What is the most effective rhythm for appliance reactivations? There is some controversy regarding the relationship between alveolar bone density and orthodontic tooth movement. Moreover, the extent to which bone density affects root resorption is not fully understood. Some approaches to orthodontic treatment recommend taking advantage of the more dense cortical bone to enhance anchorage. This approach assumes that teeth would move more slowly through more dense alveolar bone. Although this appears to be a reasonable assumption, clinical studies have not been able to support the effectiveness of this approach. Some studies have found a relationship between root resorption and movement of teeth through dense cortical bone, while others have not. Orthodontic tooth movement in animal models has been shown to be accelerated when alveolar bone density is experimentally reduced, which also results in reduced risk of root resorption.50 Today, bioactive agents show promise of being able to reduce localized bone density and the use of such agents in conjunction with conventional fixed appliances may provide more rapid tooth movement of individual teeth with less risk of root resorption.
The relationship between tooth movement and force magnitude is also controversial. Some data suggest that a force optimum exists, where there is a direct linear relationship at low force levels and an inverse one at high forces. Animal data have confirmed the direct linear relationship at low force levels but seem to suggest that there is a plateau at higher levels. Despite the persistence of biomechanical approaches to orthodontic treatment that assume the existence of an optimal force for orthodontic tooth movement, there is no direct evidence for it. Clinical studies aimed at addressing the concept are technically quite complex because of the difficulty of accurately measuring tooth movement and force magnitude and distribution in a clinical environment. As technology develops, intraoral biomechanical sensors with data storage capacities should become available. Highly sensitive methods for measuring orthodontic tooth movement in three dimensions using modern imaging instrumentation are also rapidly becoming a reality.11,51 Together, these two advances should make clinical studies of this relationship feasible in the near future.
Orthodontists realize that the biomechanics can be quite complex. Most appliances can be considered to be slowly dissipating. The goal of constant force may not be routinely achieved even with the use of superelastic wires. Clinicians also commonly use appliances with interrupted and intermittent durations of force application. Superimposed on the mechanics are the usual forces associated with oral physiology (e.g., chewing, deglutition, and speech). Most studies have attempted to model this complex mechanical environment by asking simple questions or creating simplified models about its component parts.
What does it take to initiate orthodontic tooth movement? The bone literature suggests that significant amounts of osteogenesis can be stimulated by short exposures to dynamic strains.52 Orthodontists expect to see tooth movement in response to less than constant force application (e.g., headgears and functional appliances). This is further supported by animal studies that have shown tooth movement with part-time exposure to mechanical signals.53 A relevant question would be how long do the tissue changes stimulated during orthodontic tooth movement continue following appliance removal? Despite rapid relapse under these conditions, there is evidence that the cellular changes persist for a period of time, which would presumably be reflected in a more rapid response to a second appliance activation following a rest interval.54 Some clinical studies have reported no difference in tooth movement when comparing constant and intermittent force protocols. Animal studies have suggested that the key factor may be the duration of force application, with forces for about one quarter of the time being effective, although other animals studies have shown that jiggling forces of extremely short duration can stimulate large numbers of osteoclasts and the tooth mobility characteristic of orthodontics.55,56 This may be the case because intermittent protocols with short durations of force application provide long periods of time for significant amounts of relapse to occur.
Because most orthodontic appliances are slowly dissipating, clinical treatment can be viewed as a series of intermittent force applications. Experimental data from animal models suggest that reactivating an orthodontic appliance over longer intervals stimulates more efficient tooth movement than reactivations over short intervals.10 Biochemical analysis of known markers of active bone metabolism have demonstrated that significant amounts of alveolar bone turnover continue for an indeterminate period following appliance decay.57 Studies of tooth movement and tissue responses following reactivations indicate that linear tooth movement and rapid recruitment of osteoclasts can be achieved if reactivation is timed to coincide with the latter part of the bone remodeling cycle initiated by the first activation.58 Clinical data on reactivation rhythm are extremely important to improving the efficiency and safety of treatment. Again, in the absence of sensitive means to monitor biomechanics and tooth movement in three dimensions, these experiments will be difficult to accomplish (Fig. 5-11).
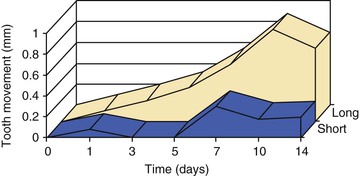
Figure 5-11 Orthodontic tooth movement curves following short (1 day) and long (10 days) interval reactivation schedules. Note the greater overall tooth movement without a delay period.
Related to the concept of reactivation timing is how the circadian rhythm factors into the biology of tooth movement. Since there is a decreased metabolism at night during sleep, the question arises as to whether this becomes more favorable or less desirable for tooth movement. A research study was carried out on subjects divided into three groups: appliances were activated over 21 days either continuously throughout the experimental period, only during the light (0700 to 1900 hours) period, or only during the dark (1900 to 0700 hours) period.59 Results demonstrated that the tooth movement in the whole-day and light-period groups was about twice that in the dark-period group. Histological evaluation of the periodontal tissues confirmed that there was greater new bone formation on the tension side in the whole-day and light-period groups and more osteoclastic activity on the pressure side compared to the dark-period group. Overall, the light-period group exhibited decreased hyalinization of the PDL compared to the whole-day group. Therefore it is obvious that diurnal rhythms in bone metabolism and physiology have significant ramifications for orthodontic tooth movement.
The desirable goal of expediting tooth movement remains a perplexing and complex issue to resolve. Several historical approaches and some novel ones have recently been proposed to facilitate this goal. Prominent among these approaches are those dependent on physical or mechanical stimulation (vibrations, low-level laser, electrical current, and pulsed electromagnetic fields) and surgically facilitated orthodontic therapy (SFOT) (corticotomy, dentoalveolar distraction, periodontal distraction). Most of these have yet to be shown to expedite orthodontic tooth movement or, as in the case of corticotomies, appear to do so only transiently.60,61 All of these approaches in principle attempt to modulate biological processes nonspecifically rather than to rationally use knowledge of bone biology to achieve the desired expedited tooth movement. Thus, for example, corticotomies result in short-term acceleration of tooth movement, which may be related to its modulation of a transient increase in RANKL61 but has the caveat of significant patient morbidity and costs. In contrast, a more logical approach to achieving the goal of faster tooth movement would entail directly harnessing the biological regulation of bone turnover without large surgical procedures. Indeed, the local gene transfer of RANKL that results in a sustained overexpression of RANKL over time is accompanied by greater amounts and rates of tooth movement in experimental rats than in control rats or in rats subjected to corticotomies.61,62 A consistent increase in RANKL expression and osteoclastogenesis occurs locally in the RANKL-vector injected side without either local inflammation at the injection site or systemic bony effects in the tibia. Thus local gene transfer or possibly local administration of pro-osteoclastic factors may prove to be a more attractive option than surgery or other options currently in use in enhancing tooth movement.
Root Resorption
Root resorption is a relatively common sequel of orthodontic treatment. Consequences range from slight tooth mobility resulting from mild amounts of root resorption to complete loss of teeth due to excessive root resorption. The type and magnitude of root resorption vary substantially from mild apical root blunting in a large number of cases, to lateral root resorption, to, infrequently but unfortunately, excessive root loss (Fig. 5-12). Because of the potential for significant clinical and legal implications of root resorption, assessment of the biological and mechanical basis of root resorption has been the subject of extensive research and discussion for almost a century. Yet because of the multiple factors contributing to this process, the research findings are inconclusive and remain a highly debated topic in the literature.63,64
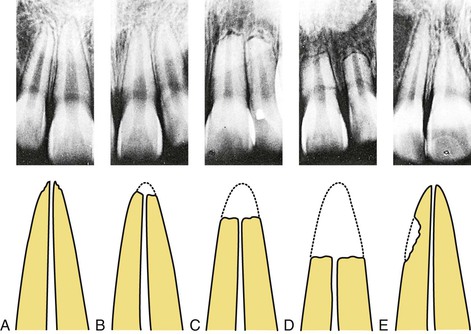
Figure 5-12 Types of root resorption observed in orthodontic patients. These are depicted radiographically (upper panel) and diagrammatically (lower panel) and range from (A) very mildly irregular apical root contours, to (B) mild apical root blunting, to (C) moderate apical root resorption, to (D) severe apical root resorption, to (E) lateral root resorption. (Modified from Goldson L, Malmgren O. Orthodontic treatment of traumatized teeth. In: Andreasen JO, ed. Traumatic Injuries of the Teeth. 2nd ed. Philadelphia, PA: WB Saunders; 1981:395.)
As early as 1914 clinical observers suggested a direct link between orthodontic treatment and root resorption.65 In the late 1920s radiographic evidence demonstrating differences in root morphology before and after orthodontic treatment was presented.66,67 Since then numerous potential causal relationships and contributory factors have been proposed and studied but definitive explanations of why root resorption occurs and what factors contribute to its occurrence have remained controversial. A comprehensive understanding of this problem has remained elusive because of the difficulty in comparing the results and conclusions of various studies that have used different experimental designs, patient populations, treatment mechanics, and analyses. Additionally, the variability in radiographic techniques and materials in different studies further contributes to the discrepant findings among studies. Finally, because of inherent differences in the characteristics of both the patient and the treatment, far too many potential etiologic factors have been considered in these studies, making it impossible to derive any meaningful conclusions. A better understanding of this important clinical problem requires controlled studies that are designed to examine the association between a limited set of variables and root resorption. The verification of the reliability of three-dimensional cone beam computed tomography (CBCT) relative to two-dimensional radiographs for assessing root resorption will be beneficial in better deciphering the variables associated with or contributing to root resorption.68–72
While the causative or contributory factors of orthodontic root resorption remain unknown, several studies have proposed a spectrum of factors that may predispose patients to root resorption. A hereditary component for orthodontic root resorption has been suggested by findings showing a significantly higher co-occurrence of root resorption among siblings than among nonsiblings (Fig. 5-13).73 In the context of genetic predisposition to root resorption, findings also support the notion of an association, albeit small, between IL-1α, TNF-α, and osteopontin polymorphisms and root resorption.74–76 Other studies have explored the relationship of force strength, as well as rate and direction of tooth movement, to root resorption with inconsistent findings.77,78 A positive association between duration of treatment and root resorption has been demonstrated.79,80 Root morphology, specifically abnormally shaped or dilacerated roots, also appears to influence the severity of root resorption.81,82 Root resorption of varying degrees has been observed in the sagittal, transverse, and vertical planes of tooth movement.83,84 Previous trauma to teeth treated orthodontically may also be a significant risk factor for root resorption.85 However, because of the low incidence of trauma to teeth, this factor possibly accounts for root resorption in relatively few patients.
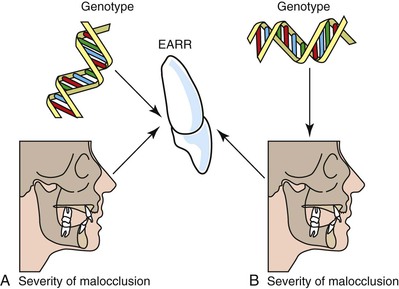
Figure 5-13 Competing models for the pathway through which an individual's genotype modulates the extent of external apical root resorption (EARR) experienced during the course of orthodontic treatment. A, The first paradigm suggests that the amount of EARR during treatment depends on an individual's genotype and, independently, on malocclusion. Genotype and malocclusion both have a modulating effect on the extent of EARR but they operate along separate biological and biomechanical pathways. With this model, a patient's genotype would have a direct effect on the extent of EARR and incorporating measures of severity of malocclusion into statistical design would not affect the estimate of heritability (h2, the proportion of the total variance due to common genetic influences). B, The alternative paradigm is that genotype has an indirect effect acting through malocclusion. This model acknowledges that craniometric studies show that facial size and shape have moderately high genetic components. Because siblings share similar craniofacial relationships, the genetic influence on EARR would be modulated through malocclusion. With this model, inclusion of skeletodental covariates would alter h2 estimates, although it is not clear a priori that estimates should necessarily increase or decrease. (Reproduced from Harris EF, Kineret SE, Tolley EA. A heritable component for external apical root resorption in patients treated orthodontically. Am J Orthod Dentofacial Orthop. 1997;111:301–309.)
In general, much of the focus in previous studies has been on the mechanical variables, with little attention being paid to the potential contribution of biological factors to orthodontic root resorption. One factor that may be associated with orthodontic root resorption is trabecular bone density. The hypothesis that trabecular bone density may be associated with orthodontic root resorption is based on the assumption that bone and roots with similar levels of calcification are likely to undergo comparable amounts of degradation when exogenous forces are applied. While there is no conclusive evidence to support this hypothesis, some studies have provided indirect evidence for a potential association between bone density and root resorption. For example, it has been demonstrated that strong forces applied to teeth in less dense alveolar bone cause the same amount of root resorption seen in roots in dense alveolar bone subjected to much weaker forces.78 Additionally, teeth being moved in close proximity to dense cortical bone undergo greater levels of root resorption than those in trabecular bone.86 More appropriately, animal studies show that calcium-deficient rats exhibiting very low alveolar density have markedly low levels of root resorption following tooth movement (Fig. 5-14).50 Similarly, studies on hypocalcemic rats have also demonstrated a proportional relationship between bone density and magnitude of root resorption.87 These studies provide important insights into the potential association between bone density and root resorption but this association requires more definitive validation.
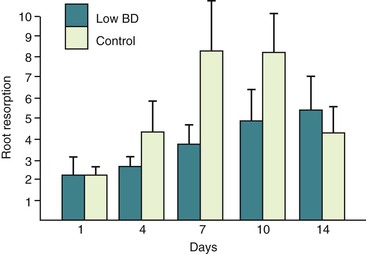
Figure 5-14 Root resorption comparison between normal (control) and low bone density (low BD). Note that there is less root resorption in the low-density group. (Reproduced from Harris EF, Kineret SE, Tolley EA. A heritable component for external apical root resorption in patients treated orthodontically. Am J Orthod Dentofacial Orthop. 1997;111:301–309.)
Finally, the contribution of biological mediators to orthodontic root resorption is coming under increasing scrutiny. In addition to the link between root resorption and gene polymorphisms for IL-1α, TNF-α, and osteopontin74–76 that can generate varied gene products, some of which likely have greater predisposition to resorption, the RANK/RANKL/OPG pathway that is activated during orthodontic tooth movement may contribute to orthodontic root resorption.88 When compressed in vitro, PDL cells from cases with severe external apical root resorption (EARR) produce higher levels of RANKL and lower amounts of OPG than cells from control subjects.89,90 Also, RANKL immunoreactivity has been shown in tissues associated with root resorption as early as day 7 of tooth movement.91 Finally, rats with higher pre-treatment levels of serum RANKL show more root resorption after tooth movement than rats with lower initial concentrations of RANKL,92 suggesting that systemic levels of RANKL may be predictive of future root resorption. It is likely that findings such as these will lead to novel approaches to minimize orthodontic root resorption or to help identify patients who may be at a greater risk of root resorption, thereby enabling the clinician to proactively modify treatment objectives and plans.
Orthodontic Relapse
Many biological and mechanical factors dictate the rate of tooth movement in orthodontics. Similarly this complex dynamic physiological environment also dictates the amount of relapse, which will occur following the completion of orthodontic treatment. Relapse can be defined as a natural tendency for a tooth or teeth to migrate back to their original pre-treatment position and angulation in the dental arch. In general, the amount of active tooth movement correlates strongly with the relapse potential (i.e., greater distances moved or greater rotations corrected result in a greater tendency for relapse).93 Factors involved in clinical orthodontic relapse include force duration, distance of tooth movement, and mechanical management to minimize relapse occurrence. The role of gingival and transseptal fibers and the period of bone remodeling following orthodontic tooth movement also play significant roles in the ultimate magnitude of the relapse that occurs.
The potential for relapse is great during a critical period immediately after orthodontic treatment when the teeth and roots have been moved into the desired final position (Fig. 5-15). In the process of tooth movement, bone resorption occurs in areas of pressure and bone deposition occurs in areas of tension, as defined by Wolff's law.1 The type of bone that is deposited in the area of tension is a rather soft, unorganized osteoid matrix. This bone is subsequently remodeled into organized lamellar bone architecture to provide a stronger alveolar support, a slow process that can take upward of 6 months.54 At the same time, there are changes in the transseptal fibers attached to the roots of the teeth. The tensile forces cause transseptal fibers to adjust their length by rapid remodeling and this change continues to occur, as demonstrated by increased collagenous protein turnover within the middle third of the transseptal fibers following release of orthodontic force.94 In all cases it is apparent that the retention mechanism used immediately after removal of the orthodontic appliances is a crucial component of the treatment plan.
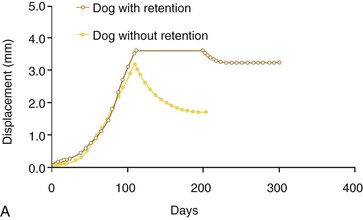
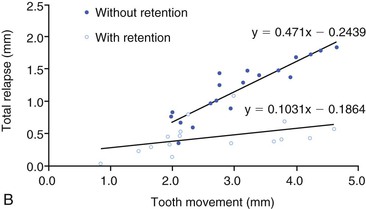
Figure 5-15 Retention enhances stability following orthodontic tooth movement and larger movements correlate with increased relapse, particularly in the absence of retention. A, Time–displacement curves of two dog premolars, one with retention and one without. B, Relationship between active tooth movement and the total amount of relapse with or without retention. The plots represent linear regression lines for the two different conditions. (Reproduced with permission from van Leeuwen EJ, Maltha JC, Kuijpers-Jagtman AM, van't Hof MA. The effect of retention on orthodontic relapse after the use of small continuous or discontinuous forces: an experimental study in beagle dogs. Eur J Oral Sci. 2003;111:111–116.)
Orthodontic relapse occurs when teeth are permitted to migrate out of position during postorthodontic remodeling of the supporting structures. Histological and electron micrograph studies have shown that rapid remodeling of the PDL and surrounding alveolar bones is the main cause of tooth relapse and that hyalinization formed subsequent to compression and/or mineralized tissues subjected to compression are rapidly resorbed by osteoclasts and macrophage-like and fibroblast-like cells.18 Biological studies have provided evidence that teeth show a greater relapse tendency when continuous forces are used compared to intermittent forces.95 This is because continuous forces are more effective in moving teeth and therefore the teeth are displaced farther from their original positions. The result of this is an increase in the potential of the teeth to revert to their initial positions. This “relapse energy” phenomenon can also be observed in situations where teeth require rotational correction.
As the tooth is rotated, the PDL is stretched (Fig. 5-16).96 The greater the magnitude of rotation, the further these fibers become elongated. In its new milieu the PDL is able to reorganize and invest its fibrils in alveolar bone and cementum during the deposition of new bone and cementum. However, this process is slow and requires significant time.97 Acceleration of this reorganization is facilitated by gingivectomy or circumferential supracrestal fiberotomy (CSF) aimed at surgically releasing the stretched free gingival fibers and allowing reattachment of these fibers in a less stressed or less stretched configuration.98,99 Scanning electron microscopy and histological evaluation of postfiberotomy teeth confirm that there is an eventual reparative reattachment of the collagen fibers of the PDL (Figs. 5-17 and 5-18). Long-term evaluation at 4 to 6 years and 12 to 14 years after active treatment validates that CSF is effective in improving retention of tooth position.45 This study also validated that there was neither a clinically significant increase in the periodontal sulcus depth nor a decrease in the labially attached gingiva of the CSF teeth observed at 1 and 6 months following the surgical procedure. However, recent biochemical studies propose that the rotational relapse of a tooth is not due to “stretched” collagen fibers but rather is a result of a change in the elastic properties of the whole gingival tissue.100 Unlike bone and PDL, which regain their original structure after the removal of force, the gingival tissue does not regain its pre-treatment structure and it may be this tension that contributes to orthodontic relapse.101
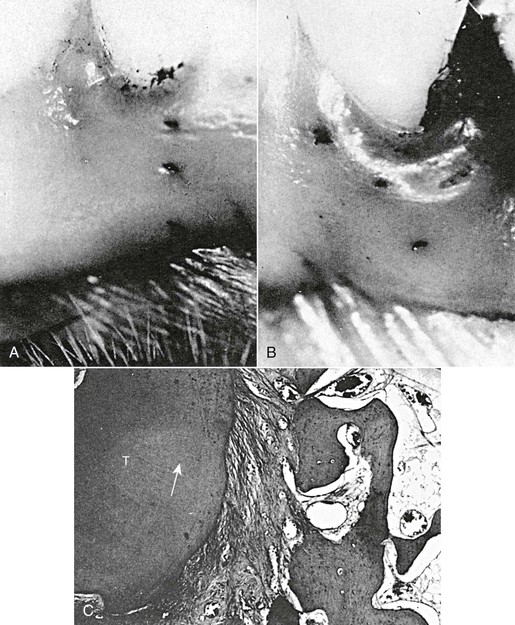
Figure 5-16 Stretching of periodontal ligament (PDL) and gingival tissue by orthodontic rotation of teeth. A, Tattoo marks on gingiva before rotation of the tooth. B, Tattoo marks on gingiva show deviation in direction of rotational movement of the tooth. C, PDL stretched and deviated (arrow) during rotation of the tooth (T). Magnification of original is ×64. (Reproduced from Edwards JG. A study of the periodontium during orthodontic rotation of teeth. Am J Orthod. 1968;54:441–461.)
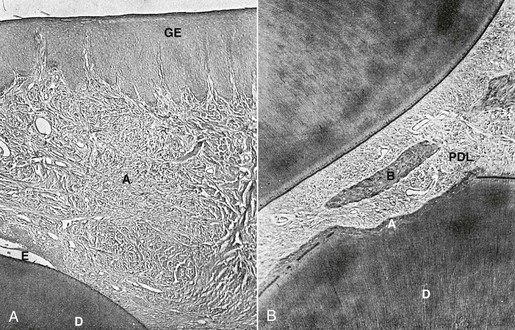
Figure 5-17 Histological changes of periodontal ligament (PDL) fiber adaptations in the presence and absence of surgical intervention. A, Shows the PDL arrangement of an incisor with total repair 42 days following surgical intervention. Overall, there is good orientation of the PDL ligaments as they slowly start to reattach and reorganize into the more familiar parallel collagen bundles. The supragingival tissue shows a zone of healing where the fibers are much sparser than the adjacent fiber bundles. Fiber organization outside the area of healing is very similar to the fiber arrangement observed in comparable areas of unrotated incisors. B, Histological section of a nonsurgical control incisor with a similar degree of tooth rotation as in A. PDL shows areas of compression and damage on the distolingual aspect and a widening on the mesiolabial aspect. Observation of the supragingival tissues displays a generalized discontinuity of the fiber pattern, areas of hemorrhage, and fiber disorganization. No evidence of any area of scarring or healing is observed. A, Area of resorption undergoing healing; B, bone; D, dentin; GE, gingival epithelium. (Modified from Brain WE. The effect of surgical transsection of free gingival fibers on the regression of orthodontically rotated teeth in the dog. Am J Orthod. 1969;55:50–70.)
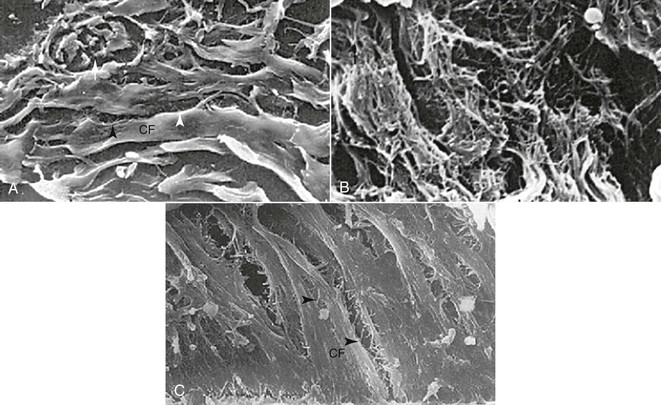
Figure 5-18 Teeth that have undergone surgical release of the gingival fibers demonstrate greater stability after orthodontic treatment. This is primarily due to the reattachment capability of the collagen fibers of the periodontal ligament (PDL). A, Observations with scanning electron microscopy (SEM) of supraalveolar fibers in palatal, buccal, and transseptal gingival areas reveal well-organized, parallel, and densely packed collagen fiber (CF) bundles with thin fibers connecting the large fiber bundles (arrowheads). B, SEM analysis of the different gingival regions after rotation and retention reveal disorganized, torn, and ripped collagen fibers. C, After gingival fiberotomy and release of retention the teeth remain stable in their rotated positions. SEM shows large, parallel, and densely packed collagen fibers. There is normalization of the longitudinally sectioned parallel and densely packed CF bundles interconnected with thinner fibers (arrowheads), resembling untreated controls. (Reproduced from Redlich M, Rahamin E, Gaft A, Shoshan S. The response of supraalveolar gingival collagen to orthodontic rotation movement in dogs. Am J Orthod Dentofacial Orthop. 1996;110:247–255.)
Biological enhancement of bone maturation or diminishing its degradation by osteoclasts could provide a possible basis for enhancing post-treatment stability. Indeed a recent study47 demonstrated that the local administration of the RANKL decoy ligand, OPG, inhibits postorthodontic relapse in an animal model of orthodontic tooth movement. Specifically, OPG injections significantly diminished postorthodontic relapse from 63% of total movement in vehicle control rats to as low as 24% in animals receiving OPG 24 days after appliance removal. Histologically the bone and periodontal tissue around teeth appeared normalized as early as 8 days after OPG administration, while the vehicle control group showed only partial tissue recovery 24 days following tooth movement. Quantitative bone measures derived from micro-computed tomography had fully recovered to pre-tooth-movement values in OPG-treated animals, while these parameters had recovered only partially or had not recovered in the vehicle control rats 24 days after orthodontic treatment. The profound decrease in postorthodontic relapse by local OPG administration indicates that osteoclasts are critical to bone maturation following tooth movement and points to the potential pharmacological use of OPG or other RANKL inhibitors for orthodontic retention.
The factors noted above are considered intrinsic factors of orthodontic relapse related to the immediate supporting structures of PDL and alveolar bone surrounding the teeth. These relapse factors should be differentiated from extrinsic factors, such as continual craniofacial skeletal growth, external forces from facial muscles, and occlusal interdigitation. Despite the numerous studies over the years defining all of the variables and contributory factors related to orthodontic relapse, the degree of postretention anterior crowding is still both unpredictable and variable and no pre-treatment variables, from clinical findings, casts, or cephalometric radiographs before or after treatment, seem to be useful predictors.
Future Uses of Biological Principles in Orthodontic Treatment
In the future, the principles gleaned from molecular biology and tissue engineering are expected to become part of the therapeutic regimen used during orthodontic treatment. This paradigm of orthodontic treatment may involve the delivery of bioactive agents in combination with conventional biomechanics. Numerous agents that have activities that should be of significance in accelerating or preventing tooth movement already exist and indeed have been shown to modulate experimental tooth movement in animal models (Table 5-1). For example, localized application of calciotropic or osteoclastic agents has significant effects on local bone remodeling dynamics and may be used to facilitate tooth movement, such as retraction of canines into extraction spaces,61,62 to impair tooth movement in cases with high anchorage needs,46 or to enhance retention.47 The increasing knowledge about the biological mechanisms in orthodontic tooth movement combined with smart local pharmaceutical delivery systems will facilitate the utility of these biomolecules in orthodontic therapy. Data on efficacy and safety, as well as means of delivery in the context of orthodontic treatment, will likely be forthcoming.
TABLE 5-1
Bioactive Agents That Have Been Shown to Enhance or Decrease Rates of Orthodontic Tooth Movement in Animal Models
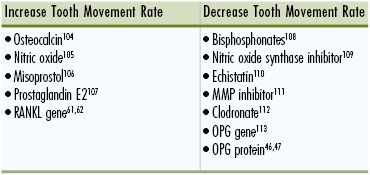
MMP, Matrix metalloproteinase; OPG, osteoprotegerin; RANKL, receptor activator of nuclear factor κB ligand.
Advances in technological bioassays may also facilitate the biological diagnostics and monitoring of bone turnover and metabolism as well as markers of root resorption. Medical researchers have already attempted to establish relationships between molecular biochemical markers of bone remodeling (e.g., osteocalcin, alkaline phosphatase, and procollagen I) and the metabolic rate of bone turnover in clinical subjects.102 However, the correlation and interpretation of results are complicated by numerous variables, such as age, pubertal stage, growth velocity, mineral accrual, hormonal regulation, nutritional status, and circadian variation. Yet the rapid progress in developing more sensitive and specific assays, as well as recent discoveries of more specific metabolic bone markers or bioactive agents associated with root resorption, is making this potential assessment of bone and root turnover a likely reality in the near future.74–76,92,103
References
1. Wolff J. The Law of Bone Remodeling (trans.). Springer-Verlag: Berlin, Germany; 1986.
2. Dorow C, Krstin N, Sander FG. Experiments to determine the material properties of the periodontal ligament. J Orofac Orthop. 2002;63:94–104.
3. van Driel WD, van Leeuwen EJ, Von den Hoff JW, Maltha JC, Kuijpers-Jagtman AM. Time-dependent mechanical behaviour of the periodontal ligament. Proc Inst Mech Eng H. 2000;214:497–504.
4. Tanne K, Nagataki T, Inoue Y, Sakuda M, Burstone CJ. Patterns of initial tooth displacements associated with various root lengths and alveolar bone heights. Am J Orthod Dentofacial Orthop. 1991;100:66–71.
5. Yoshida N, Jost-Brinkmann PG, Koga Y, Mimaki N, Kobayashi K. Experimental evaluation of initial tooth displacement, center of resistance, and center of rotation under the influence of an orthodontic force. Am J Orthod Dentofacial Orthop. 2001;120:190–197.
6. Tanne K, Yoshida S, Kawata T, Sasaki A, Knox J, Jones ML. An evaluation of the biomechanic response of the tooth and periodontium to orthodontic forces in adolescent and adult subjects. Br J Orthod. 1998;25:109–115.
7. Tanne K, Inoue Y, Sakuda M. Biomechanic behavior of the periodontium before and after orthodontic tooth movement. Angle Orthod. 1995;65:123–128.
8. Kyomen S, Tanne K. Influences of aging changes in proliferative rate of PDL cells during experimental tooth movement in rats. Angle Orthod. 1997;67:67–72.
9. Ren Y, Maltha JC, van't Hof MA, Kuijpers-Jagtman AM. Age effect on orthodontic tooth movement in rats. J Dent Res. 2003;82:38–42.
10. King GJ, Archer L, Zhou D. Later orthodontic appliance reactivation stimulates immediate appearance of osteoclasts and linear tooth movement. Am J Orthod Dentofacial Orthop. 1998;114:692–697.
11. Iwasaki LR, Haack JE, Nickel JC, Morton J. Human tooth movement in response to continuous stress of low magnitude. Am J Orthod Dentofacial Orthop. 2000;117:175–183.
12. van Leeuwen EJ, Maltha JC, Kuijpers-Jagtman AM. Tooth movement with light continuous and discontinuous forces in beagle dogs. Eur J Oral Sci. 1999;107:468–474.
13. Andersson L, Malmgren B. The problem of dentoalveolar ankylosis and subsequent replacement resorption in the growing patient. Aust Endod J. 1999;25:57–61.
14. Kurol J. Infraocclusion of primary molars: an epidemiological, familial, longitudinal, clinical and histological study. Swed Dent J. 1984;21(suppl):1–67.
15. Nanda R. Temporary Anchorage Devices in Orthodontics. Mosby Elsevier: St. Louis, MO; 2009.
16. King GJ, Keeling SD, McCoy EA, Ward TH. Measuring dental drift and orthodontic tooth movement in response to various initial forces in adult rats. Am J Orthod Dentofacial Orthop. 1991;99:456–465.
17. Marks SC Jr, Schroeder HE. Tooth eruption: theories and facts. Anat Rec. 1996;245:374–393.
18. Yoshida Y, Sasaki T, Yokoya K, Hiraide T, Shibasaki Y. Cellular roles in relapse processes of experimentally-moved rat molars. J Electron Microsc (Tokyo). 1999;48:147–157.
19. Marks SC Jr. The basic and applied biology of tooth eruption. Connect Tissue Res. 1995;32:149–157.
20. Philbrick WM, Dreyer BE, Nakchbandi IA, Karaplis AC. Parathyroid hormone–related protein is required for tooth eruption. Proc Natl Acad Sci USA. 1998;95:11846–11851.
21. Ouyang H, McCauley LK, Berry JE, Saygin NE, Tokiyasu Y, Somerman MJ. Parathyroid hormone–related protein regulates extracellular matrix gene expression in cementoblasts and inhibits cementoblast-mediated mineralization in vitro. J Bone Miner Res. 2000;15:2140–2153.
22. Dastmalchi R, Polson A, Bouwsma O, Proskin H. Cementum thickness and mesial drift. J Clin Periodontol. 1990;17:709–713.
23. Levy GG, Mailland ML. Histologic study of the effects of occlusal hypofunction following antagonist tooth extraction in the rat. J Periodontol. 1980;51:393–399.
24. Rygh P. Ultrastructural changes in pressure zones of human periodontium incident to orthodontic tooth movement. Acta Odontol Scand. 1973;31:109–122.
25. Rygh P. Hyalinization of the periodontal ligament incident to orthodontic tooth movement. Nor Tannlaegeforen Tid. 1974;84:352–357.
26. Rygh P. Elimination of hyalinized periodontal tissues associated with orthodontic tooth movement. Scand J Dent Res. 1974;82:57–73.
27. Brudvik P, Rygh P. The initial phase of orthodontic root resorption incident to local compression of the periodontal ligament. Eur J Orthod. 1993;15:249–263.
28. Brudvik P, Rygh P. Non-clast cells start orthodontic root resorption in the periphery of hyalinized zones. Eur J Orthod. 1993;15:467–480.
29. Suda T, Takahashi N, Martin TJ. Modulation of osteoclast differentiation. Endocrinol Rev. 1992;13:66–80.
30. Takahashi N, Akatsu T, Udagawa N, et al. Osteoblastic cells are involved in osteoclast formation. Endocrinology. 1988;123:2600–2602.
31. Udagawa N, Takahashi N, Akatsu T, et al. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci USA. 1990;87:7260–7264.
32. Anderson DM, Maraskovsky E, Billingsley WL, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179.
33. American Society for Bone and Mineral Research President's Committee on Nomenclature. Proposed standard nomenclature for new tumor necrosis factor family members involved in the regulation of bone resorption: American Society for Bone and Mineral Research President's Committee on Nomenclature. J Bone Miner Res. 2000;15:2293–2296.
34. Hsu H, Lacey DL, Dunstan CR, et al. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci USA. 1999;96:3540–3545.
35. Nakagawa N, Kinosaki M, Yamaguchi K, et al. RANK is the essential signaling receptor for osteoclast differentiation factor in osteoclastogenesis. Biochem Biophys Res Commun. 1998;253:395–400.
36. Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319.
37. Hasegawa T, Yoshimura Y, Kikuiri T, et al. Expression of receptor activator of NF-kappa B ligand and osteoprotegerin in culture of human periodontal ligament cells. J Periodontal Res. 2002;37:405–411.
38. Crotti T, Smith MD, Hirsch R, et al. Receptor activator NF kappaB ligand (RANKL) and osteoprotegerin (OPG) protein expression in periodontitis. J Periodontal Res. 2003;38:380–387.
39. Takahashi N, Udagawa N, Takami M, Suda T. Cells of bone: osteoclast generation. Bilezikian JP, Raisz LG, Rodan GA. Principles of Bone Biology. Academic Press: San Diego, CA; 2002:109–126.
40. Huang JC, Sakata T, Pfleger LL, et al. PTH differentially regulates expression of RANKL and OPG during osteoblast development. J Bone Miner Res. 2004;19:235–244.
41. Suda T, Ueno Y, Fujii K, Shinki T. Vitamin D and bone. J Cell Biochem. 2003;88:259–266.
42. Bord S, Ireland DC, Beavan SR, Compston JE. The effects of estrogen on osteoprotegerin, RANKL, and estrogen receptor expression in human osteoblasts. Bone. 2003;32:136–141.
43. Cheung J, Mak YT, Papaioannou S, Evans BA, Fogelman I, Hampson G. Interleukin-6 (IL-6), IL-1, receptor activator of nuclear factor kappaB ligand (RANKL) and osteoprotegerin production by human osteoblastic cells: comparison of the effects of 17-beta oestradiol and raloxifene. J Endocrinol. 2003;177:423–433.
44. King GJ, Keeling SD, Wronski TJ. Histomorphometric study of alveolar bone turnover in orthodontic tooth movement. Bone. 1991;12:401–409.
45. Edwards JG. A long-term prospective evaluation of the circumferential supracrestal fiberotomy in alleviating orthodontic relapse. Am J Orthod Dentofacial Orthop. 1988;93:380–387.
46. Dunn MD, Park CH, Kosteniuk PJ, Kapila S, Giannobile WV. Local delivery of osteoprotegerin inhibits mechanically mediated bone modeling in orthodontic tooth movement. Bone. 2007;41:446–455.
47. Hudson JB, Hatch N, Hayami T, et al. Local delivery of recombinant osteoprotegerin prevents post-orthodontic relapse. Calcif Tissue Int. 2012;90:330–342.
48. Verna C, Zaffe D, Siciliani G. Histomorphometric study of bone reactions during orthodontic tooth movement in rats. Bone. 1999;24:371–379.
49. Mundy GR. Cellular and molecular regulation of bone turnover. Bone. 1999;24(5 suppl):35S–38S.
50. Goldie RS, King GJ. Root resorption and tooth movement in orthodontically treated, calcium-deficient, and lactating rats. Am J Orthod. 1984;85:424–430.
51. Ashmore JL, Kurland BF, King GJ, Wheeler TT, Ghafari J, Ramsay DS. A 3-dimensional analysis of molar movement during headgear treatment. Am J Orthod Dentofacial Orthop. 2002;121:18–30.
52. Judex S, Boyd S, Qin YX, et al. Adaptations of trabecular bone to low magnitude vibrations result in more uniform stress and strain under load. Ann Biomed Eng. 2003;31:12–20.
53. Gibson JM, King GJ, Keeling SD. Long-term orthodontic tooth movement response to short-term force in the rat. Angle Orthod. 1992;62:211–216.
54. King GJ, Keeling SD. Orthodontic bone remodeling in relation to appliance decay. Angle Orthod. 1995;65:129–140.
55. Proffit WR, Sellers KT. The effect of intermittent forces on eruption of the rabbit incisor. J Dent Res. 1986;65:118–122.
56. Konoo T, Kim YJ, Gu GM, King GJ. Intermittent force in orthodontic tooth movement. J Dent Res. 2001;80:457–460.
57. King G, Latta L, Rutenberg J, Ossi A, Keeling S. Alveolar bone turnover and tooth movement in male rats after removal of orthodontic appliances. Am J Orthod Dentofacial Orthop. 1997;111:266–275.
58. Gu G, Lemery SA, King GJ. Effect of appliance reactivation after decay of initial activation on osteoclasts, tooth movement, and root resorption. Angle Orthod. 1999;69:515–522.
59. Miyoshi K, Igarashi K, Saeki S, Shinoda H, Mitani H. Tooth movement and changes in periodontal tissue in response to orthodontic force in rats vary depending on the time of day the force is applied. Eur J Orthod. 2001;23:329–338.
60. Long H, Pyakurela U, Wang Y, Liaoa L, Zhoua Y, Laic W. Interventions for accelerating orthodontic tooth movement: a systematic review. Angle Orthod. 2013;83:164–171.
61. Iglesias-Linares A, Moreno-Fernandez AM, Yañez-Vico R, Mendoza-Mendoza A, Gonzalez-Moles M, Solano-Reina E. The use of gene therapy vs corticotomy surgery in accelerating orthodontic tooth movement. Orthod Craniofac Res. 2011;14:138–148.
62. Kanzaki H, Chiba M, Arai K, et al. Local RANKL gene transfer to the periodontal tissue accelerates orthodontic tooth movement. Gene Ther. 2006;13(8):678–685.
63. Baumrind S, Korn EL, Boyd RL. Apical root resorption in orthodontically treated adults. Am J Orthod Dentofacial Orthop. 1996;110:311–320.
64. Goldson L, Henrikson CO. Root resorption during Begg treatment: a longitudinal roentgenologic study. Am J Orthod. 1975;68:55–66.
65. Ottolengui R. The physiological and pathological resorption of tooth roots. Items Interest. 1914;36:332–362.
66. Ketcham AH. A preliminary report of an investigation of apical root resorption of vital permanent teeth. Int J Orthod. 1927;13:97–127.
67. Ketcham AH. A progress report of an investigation of apical root resorption of vital permanent teeth. Int J Orthod. 1929;15:310–328.
68. Alqerban A, Jacobs R, Fieuws S. Comparison of two cone beam computed tomographic systems versus panoramic imaging for localization of impacted maxillary canines and detection of root resorption. Eur J Orthod. 2011;33:93–102.
69. Alqerban A, Jacobs R, Souza PC, et al. In-vitro comparison of two cone-beam computed tomography systems and panoramic imaging for detecting simulated canine impaction-induced external root resorption in maxillary lateral incisors. Am J Orthod Dentofacial Orthop. 2009;136 [764-e1–764-e11] .
70. Durack C, Patel S, Davies J, Wilson R, Mannocci F. Diagnostic accuracy of small volume cone beam computed tomography and intraoral periapical radiography for the detection of simulated external inflammatory root resorption. Int Endod J. 2011;44:136–147.
71. Ponder S, Benavides E, Kapila S, Hatch N. Quantification of external root resorption by low- vs. high-resolution cone beam computed tomography and periapical radiography: a volumetric and linear analysis. Am J Orthod Dentofacial Orthop. 2012;143:77–91.
72. Ren H, Chen J, Deng F, Zheng L, Liu X, Dong Y. Comparison of cone-beam computed tomography and periapical radiography for detecting simulated apical root resorption. Angle Orthod. 2013;83:189–195.
73. Harris EF, Kineret SE, Tolley EA. A heritable component for external apical root resorption in patients treated orthodontically. Am J Orthod Dentofacial Orthop. 1997;111:301–309.
74. Al-Qawasmi RA, Hartsfield JK Jr, Everett ET, et al. Genetic predisposition to external apical root resorption. Am J Orthod Dentofacial Orthop. 2003;123:242–252.
75. Al-Qawasmi RA, Hartsfield JK Jr, Everett ET, et al. Genetic predisposition to external apical root resorption in orthodontic patients: linkage of chromosome-18 marker. J Dent Res. 2003;82:356–360.
76. Iglesias-Linares A, Yañez-Vico R, Moreno-Fernández A, et al. Osteopontin gene SNPs (rs9138, rs11730582) mediate susceptibility to external root resorption in orthodontic patients. Oral Dis. 2013.
77. McFadden WM, Engstrom C, Engstrom H, Anholm JM. A study of the relationship between incisor intrusion and root shortening. Am J Orthod Dentofacial Orthop. 1989;6:390–396.
78. Reitan K. Initial tissue behavior during apical root resorption. Angle Orthod. 1974;44:68–82.
79. Dermaut LR, De Munck A. Apical root resorption of upper incisors caused by intrusive tooth movement: a radiographic study. Am J Orthod Dentofacial Orthop. 1986;90:321–326.
80. Taithongchai R, Sookkorn K, Killiany DM. Facial and dentoalveolar structure and the prediction of apical root shortening. Am J Orthod Dentofacial Orthop. 1996;110:296–302.
81. Mirabella AD, Artun J. Risk factors for apical root resorption of maxillary anterior teeth in adult orthodontic patients. Am J Orthod Dentofacial Orthop. 1995;108:48–55.
82. Newman WG. Possible etiologic factors in external root resorption. Am J Orthod. 1975;67:522–539.
83. Harry MR, Sims MR. Root resorption in bicuspid intrusion: a scanning electron microscope study. Angle Orthod. 1982;52:235–258.
84. Wainwright WM. Faciolingual tooth movement: its influence on the root and cortical plate. Am J Orthod. 1973;64:278–302.
85. Linge L, Linge BO. Patient characteristics and treatment variables associated with apical root resorption during orthodontic treatment. Am J Orthod Dentofacial Orthop. 1991;99:35–43.
86. Horiuchi A, Hotokezaka H, Kobayashi K. Correlation between cortical plate proximity and apical root resorption. Am J Orthod Dentofacial Orthop. 1998;114:311–318.
87. Engstrom C, Granstrom G, Thilander B. Effect of orthodontic force on periodontal tissue metabolism: a histologic and biochemical study in normal and hypocalcemic young rats. Am J Orthod Dentofacial Orthop. 1988;93:486–495.
88. Tyrovola JB, Spyropoulos MN, Makou M, Perrea D. Root resorption and the OPG/RANKL/RANK system: a mini review. J Oral Sci. 2008;50:367–376.
89. Nishijima Y, Yamaguchi M, Kojima T, Aihara N, Nakajima R, Kasai K. Levels of RANKL and OPG in gingival crevicular fluid during orthodontic tooth movement and effect of compression force on releases from periodontal ligament cells in vitro. Orthod Craniofac Res. 2006;9:63–70.
90. Yamaguchi M, Aihara N, Kojima T, Kasai K. RANKL increase in compressed periodontal ligament cells from root resorption. J Dent Res. 2006;85:751–756.
91. Nakano Y, Yamaguchi M, Fujita S, Asano M, Saito K, Kasai K. Expressions of RANKL/RANK and M-CSF/c-fms in root resorption lacunae in rat molar by heavy orthodontic force. Eur J Orthod. 2011;33:335–343.
92. Tyrovola JB, Perrea D, Halazonetis DJ, Dontas I, Vlachos IS, Makou M. Relation of soluble RANKL and osteoprotegerin levels in blood and gingival crevicular fluid to the degree of root resorption after orthodontic tooth movement. J Oral Sci. 2010;52:299–311.
93. Reitan K. Principles of retention and avoidance of posttreatment relapse. Am J Orthod. 1969;55:776–790.
94. Row KL, Johnson RB. Distribution of 3H-proline within transseptal fibers of the rat following release of orthodontic forces. Am J Anat. 1990;189:179–188.
95. van Leeuwen EJ, Maltha JC, Kuijpers-Jagtman AM, van't Hof MA. The effect of retention on orthodontic relapse after the use of small continuous or discontinuous forces: an experimental study in beagle dogs. Eur J Oral Sci. 2003;111:111–116.
96. Edwards JG. A study of the periodontium during orthodontic rotation of teeth. Am J Orthod. 1968;54:441–461.
97. Reitan K. Tissue rearrangement during retention of orthodontically rotated teeth. Angle Orthod. 1959;29:105–113.
98. Boese LR. Increased stability of orthodontically rotated teeth following gingivectomy in Macaca nemestrina. Am J Orthod. 1969;56:273–290.
99. Brain WE. The effect of surgical transsection of free gingival fibers on the regression of orthodontically rotated teeth in the dog. Am J Orthod. 1969;55:50–70.
100. Redlich M, Rahamim E, Gaft A, Shoshan S. The response of supraalveolar gingival collagen to orthodontic rotation movement in dogs. Am J Orthod Dentofacial Orthop. 1996;110:247–255.
101. Redlich M, Shoshan S, Palmon A. Gingival response to orthodontic force. Am J Orthod Dentofacial Orthop. 1999;116:152–158.
102. Szulc P, Seeman E, Delmas PD. Biochemical measurements of bone turnover in children and adolescents. Osteoporos Int. 2000;11:281–294.
103. Woitge HW, Seibel MJ. Biochemical markers to survey bone turnover. Rheum Dis Clin North Am. 2001;27:49–80.
104. Hashimoto F, Kobayashi Y, Mataki S, Kobayashi K, Kato Y, Sakai H. Administration of osteocalcin accelerates orthodontic tooth movement induced by a closed coil spring in rats. Eur J Orthod. 2001;23:535–545.
105. Shirazi M, Nilforoushan D, Alghasi H, Dehpour A-R. The role of nitric oxide in orthodontic tooth movement in rats. Angle Orthod. 2002;72:211–215.
106. Sekhavat AR, Mousavizadeh K, Pakshir HR, Aslani FS. Effect of misoprostol, a prostaglandin E1 analog, on orthodontic tooth movement in rats. Am J Orthod Dentofacial Orthop. 2002;122:542–547.
107. Seifi M, Eslami B, Saffar AS. The effect of prostaglandin E2 and calcium gluconate on orthodontic tooth movement and root resorption in rats. Eur J Orthod. 2003;25:199–204.
108. Igarashi K, Mitani H, Adachi H, Shinoda H. Anchorage and retentive effects of a bisphosphonate (AHBuBP) on tooth movements in rats. Am J Orthod Dentofacial Orthop. 1994;106:279–289.
109. Hayashi K, Igarashi K, Miyoshi K, Shinoda H, Mitani H. Involvement of nitric oxide in orthodontic tooth movement in rats. Am J Orthod Dentofacial Orthop. 2002;122:306–309.
110. Dolce C, Vakani A, Archer L, Morris-Wiman JA, Holliday LS. Effects of echistatin and an RGD peptide on orthodontic tooth movement. J Dent Res. 2003;82:682–686.
111. Holliday LS, Vakani A, Archer L, Dolce C. Effects of matrix metalloproteinase inhibitors on bone resorption and orthodontic tooth movement. J Dent Res. 2003;82:687–691.
112. Liu L, Igarashi K, Haruyama N, Saeki S, Shinoda H, Mitani H. Effects of local administration of clodronate on orthodontic tooth movement and root resorption in rats. Eur J Orthod. 2004;26:469–473.
113. Kanzaki H, Chiba M, Takahashi I, Haruyama N, Nishimura M, Mitani H. Local OPG gene transfer to periodontal tissue inhibits orthodontic tooth movement. J Dent Res. 2004;83:920–925.