Standard operating procedures
Introduction
Standard operating procedures (SOPs) are written procedures or protocols to describe the way that tasks and activities within the pharmacy must be carried out. The NHS, and organizations contracted to them, must continually improve the quality of their services and ensure they are safeguarding the public by providing high standards of care. This is known as clinical governance (see Ch. 9). Every pharmacy must have an identifiable clinical governance lead, who is responsible for applying clinical governance principles to the delivery of all services. This will include the use of SOPs; recording, reporting and learning from incidents; participation in clinical audit and continuing professional development (CPD, see Ch. 6); and assessing patient satisfaction through surveys. Therefore, within clinical governance, SOPs are a legal requirement of the community pharmacy contract and of hospitals in England, Scotland, Wales and Northern Ireland, to ensure a high quality of service and patient safety.
Background to SOPs
In January 2005, it became a requirement for pharmacists to establish and operate within SOPs. These SOPs were to be used in both hospital and community to cover the dispensing processes, including all the tasks that take place from the time that prescriptions are received in the pharmacy until the items are transferred to the patient.
In England, Wales and Northern Ireland, these requirements currently sit within essential services under clinical governance for core pharmacy activities. SOPs should, also, be produced to cover all advanced and enhanced services. In Scotland, SOPs are required within each element of the four core services and any local services. In hospitals, SOPs are the responsibility of the local trust.
It was recognized that there is a great deal of variance between each pharmacy, therefore no specific set of SOPs could be developed to cover all pharmacies. Each pharmacy can have individual SOPs, although larger companies may have a single SOP for each activity that covers all of their premises.
Regulations
The standards for owners and superintendent pharmacists of retail pharmacy businesses, published by the GPhC, specifies that SOPs should be in place for all aspects of the safe and effective provision of pharmacy services and that they must be maintained and regularly reviewed. Procedures must respect and protect confidential information about patients and employees in accordance with current legislation, relevant codes of practice and professional guidelines. The Pharmaceutical Society of Northern Ireland requires SOPs for dispensing services that the pharmacist provides or is responsible for.
Responsible pharmacists in the UK must ensure that pharmacy procedures are established (where not already established) and SOPs are in place, to ensure the safe and effective running of the pharmacy. These procedures need to be maintained and regularly reviewed.
Reducing risk to patient safety
Set up as part of the process of assuring clinical governance, SOPs should be designed to reduce risk and the chance of harm to patients. They should allow for continuing improvement to the standards of service offered to the public. Pharmacists should be able to ensure that their teams are working consistently to the safest method possible. SOPs should enable pharmacists to delegate and therefore, fully use the expertise of all pharmacy team members. The qualifications and capabilities of individual members of staff will determine the extent to which individual tasks can be delegated. This may in turn create efficiencies to free up time for other tasks and for talking to patients about their care. SOPs will also help to advise locums and new members of the pharmacy team where guidance is needed, which will reduce the chance of mistakes happening.
Content of the SOP
SOPs should be pharmacy specific and will always be dependent on the level of competence of the team members working in the pharmacy. A set of SOPs will be required even where no dispensary support staff are employed and the pharmacist is working single-handedly. The SOPs should cover all aspects of the dispensing process from receiving the prescription to transfer of products to the patient, including delivery. Patient confidentiality must be maintained at all times. SOPs can be drafted in many different formats, e.g. detailed information, algorithm-based or bulleted points.
 A full and clear description of each part of the process and how it should be carried out
A full and clear description of each part of the process and how it should be carried out
 Activities that must be carried out by the pharmacist
Activities that must be carried out by the pharmacist
 Activities that can be delegated to identified and competent support staff
Activities that can be delegated to identified and competent support staff
 Any contingency plans, where appropriate, to specify any changes to the SOP in the event of any situation that leads to reduced staffing levels
Any contingency plans, where appropriate, to specify any changes to the SOP in the event of any situation that leads to reduced staffing levels
 Other useful information including, e.g. the auditing of the SOP or references to any documents or computer-based information
Other useful information including, e.g. the auditing of the SOP or references to any documents or computer-based information
It is good practice to keep a record of those members of the team that have read the SOPs and been signed off as competent by the pharmacist. As new members join the team they should read the SOPs for the activities they will be undertaking as a priority. The pharmacist should always check with each team member after they have read the SOPs to ensure they have fully understood the procedure. By asking questions or observing the team member, they can confirm that they are satisfied that the procedure will be adhered to in day-to-day practice. Where the team member shows a lack of competence, further training must be given to ensure compliance. Similar arrangements should be made for any amended SOPs and it may provide a good opportunity to define roles within the team and is good for staff development.
Regular reviews must take place to ensure that SOPs are current and relevant to the practice in the pharmacy. These reviews should take place regularly to allow for changes to practice or circumstances. These changes may be due to a change in the premises or team members, or to external factors, e.g. changes to legislation or new services. In the absence of any obvious changes, these reviews should take place at least once every 2 years.
Non-conformance
Once SOPs are in place, there may be circumstances where it is necessary to work outside of an established SOP, either by changing the process or by asking a member of the team with different qualifications to complete the activity. A responsible pharmacist must always use professional judgement in these situations to minimize risk to the patient.
A responsible pharmacist should always be aware that any amendments to a SOP may introduce risk and all team members must be made aware of the necessary changes.
An example of this could be where a pharmacist decides to allow an untrained counter assistant to put the dispensary delivery to shelf on a day they are low on staff. Although the counter assistant is not named on the SOP, due to the lack of time the pharmacist allows the counter assistant to put the stock away without any training or reading the SOP. The counter assistant does not realize the difference between different drugs and strengths, and the medicines with similar packaging become mixed on the shelves. As the staff are busy in the pharmacy, the dispenser selects the wrong medication and the error is missed in the final check. The patient takes the medication and it causes harm. This is just one example of how non-compliance with SOPs can introduce significant risk to the patient.
Where possible, contingency plans should be written in to the SOP to ensure the safest alternative is always used. On those occasions where it is necessary and appropriate to amend a procedure, it is good practice to record the incident. These amendments can then be used during the audit to review the SOP.
Examples of SOPs
How to dispense a prescription in both a community and hospital setting can be split into a number of stages:
2. Clinical and legal checking
3. Interventions and problem-solving
4. Labelling and assembly of the product(s)
6. Transferring the prescription item(s) to the patient or their representative
7. Dealing with ‘owings’, where the full amount of medication prescribed is not in stock and cannot be supplied, and any procedures to be followed during the second dispensing.
Each stage will need a separate SOP and individual issues within the stages may need a separate SOP, e.g. the supply of ‘specials’, dealing with queries from another healthcare professional or additional safety guidance for high-risk medicines.
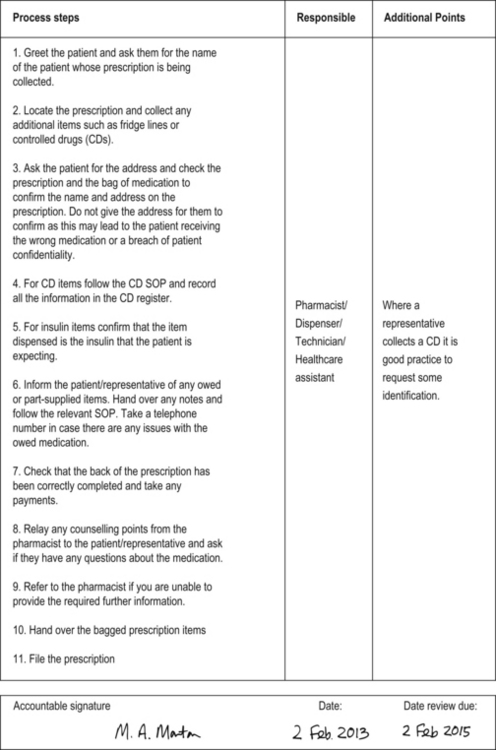
Figure 11.1 contains an example of an SOP for handing out dispensed medications to the patient or their representative, variations of which could be used in both hospital and community settings.
SOPs, in just a few years since their introduction, have become an integral part of pharmacy practice.
Key Points
 SOPs are written procedures or protocols to describe the way that all tasks and activities within the pharmacy must be carried out
SOPs are written procedures or protocols to describe the way that all tasks and activities within the pharmacy must be carried out
 SOPs are a requirement in both hospital and community pharmacy to ensure the safe and effective provision of pharmacy services
SOPs are a requirement in both hospital and community pharmacy to ensure the safe and effective provision of pharmacy services
 SOPs should be pharmacy specific and will always be dependent on the level of competence of the team members working in the pharmacy
SOPs should be pharmacy specific and will always be dependent on the level of competence of the team members working in the pharmacy
 Regular reviews must take place to ensure that SOPs are current and relevant to the practice in the pharmacy
Regular reviews must take place to ensure that SOPs are current and relevant to the practice in the pharmacy