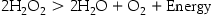Ophthalmic products
 The formulation, preparation and uses of ophthalmic preparations
The formulation, preparation and uses of ophthalmic preparations
 The packaging and labelling requirements for ophthalmic preparations
The packaging and labelling requirements for ophthalmic preparations
 Advising patients on the use of eye medication and on any adverse effects
Advising patients on the use of eye medication and on any adverse effects
 The anatomy and physiology of the eye in relation to the administration of medication and the wearing of contact lenses
The anatomy and physiology of the eye in relation to the administration of medication and the wearing of contact lenses
 The properties of contact lenses in relation to their physicochemical composition
The properties of contact lenses in relation to their physicochemical composition
 The wearing of and caring for contact lenses and the various products available to facilitate comfort, effectiveness, convenience and safety
The wearing of and caring for contact lenses and the various products available to facilitate comfort, effectiveness, convenience and safety
 The role of antimicrobial preservatives in ophthalmic products
The role of antimicrobial preservatives in ophthalmic products
 Advising patients on the possible adverse effects of concurrent medication and the sensible use of cosmetics when wearing contact lenses
Advising patients on the possible adverse effects of concurrent medication and the sensible use of cosmetics when wearing contact lenses
Introduction
The human eye is a remarkable organ and the ability to see is one of our most treasured possessions. Thus, the highest standards are necessary in the compounding of ophthalmic preparations and the greatest care is required in their use. It is necessary that all ophthalmic preparations are sterile and essentially free from foreign particles.
These preparations may be categorized as follows:
 Eye drops including solutions, emulsions and suspensions of active medicaments for instillation into the conjunctival sac
Eye drops including solutions, emulsions and suspensions of active medicaments for instillation into the conjunctival sac
 Eye lotions for irrigating and cleansing the eye surface, or for impregnating eye dressings
Eye lotions for irrigating and cleansing the eye surface, or for impregnating eye dressings
 Eye ointments, creams and gels containing active ingredient(s) for application to the lid margins and/or conjunctival sac
Eye ointments, creams and gels containing active ingredient(s) for application to the lid margins and/or conjunctival sac
 Contact lens solutions to facilitate the wearing and care of contact lenses
Contact lens solutions to facilitate the wearing and care of contact lenses
 Parenteral products for intracorneal, intravitreous or retrobulbar injection
Parenteral products for intracorneal, intravitreous or retrobulbar injection
 Ophthalmic inserts placed in the conjunctival sac and designed to release active ingredient over a prolonged period
Ophthalmic inserts placed in the conjunctival sac and designed to release active ingredient over a prolonged period
Medicaments contained in ophthalmic products include:
 Anaesthetics used topically in surgical procedures
Anaesthetics used topically in surgical procedures
 Anti-infectives such as antibacterials, antifungals and antivirals
Anti-infectives such as antibacterials, antifungals and antivirals
 Anti-inflammatories such as corticosteroids and antihistamines
Anti-inflammatories such as corticosteroids and antihistamines
 Antiglaucoma agents to reduce intraocular pressure, such as beta-blockers
Antiglaucoma agents to reduce intraocular pressure, such as beta-blockers
 Astringents such as zinc sulphate
Astringents such as zinc sulphate
 Diagnostic agents such as fluorescein which highlight damage to the epithelial tissue
Diagnostic agents such as fluorescein which highlight damage to the epithelial tissue
 Miotics such as pilocarpine which constrict the pupil and contract the ciliary muscle, increasing drainage from the anterior chamber
Miotics such as pilocarpine which constrict the pupil and contract the ciliary muscle, increasing drainage from the anterior chamber
 Mydriatics and cycloplegics such as atropine, which dilate the pupil and paralyse the ciliary muscle and thus facilitate the examination of the interior of the eye.
Mydriatics and cycloplegics such as atropine, which dilate the pupil and paralyse the ciliary muscle and thus facilitate the examination of the interior of the eye.
Anatomy and physiology of the eye
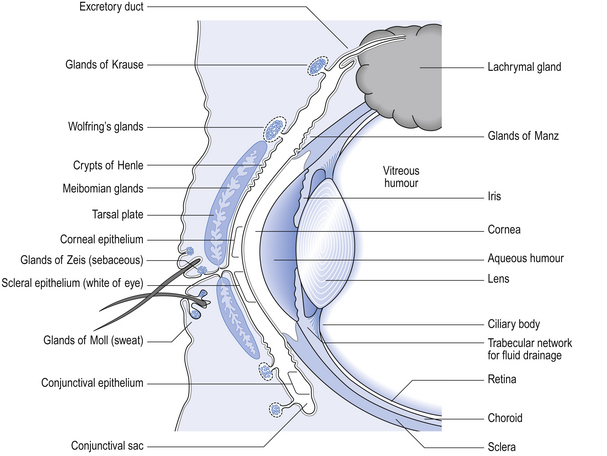
Figure 42.1 gives an indication of the relevance of the external structures of the eye and the structure of the eyelids to the application of medication and the wearing of contact lenses (see p.406 also).
Formulation of eye drops
The components of an eye drop formulation are given below:
 Active ingredient(s) to produce desired therapeutic effect
Active ingredient(s) to produce desired therapeutic effect
 Vehicle, usually aqueous but occasionally may be oil
Vehicle, usually aqueous but occasionally may be oil
 Antimicrobial preservative to eliminate any microbial contamination during use and thus maintain sterility; it should not interact adversely with the active ingredient(s)
Antimicrobial preservative to eliminate any microbial contamination during use and thus maintain sterility; it should not interact adversely with the active ingredient(s)
 Adjuvants to adjust tonicity, viscosity or pH in order to increase the ‘comfort’ in use and to increase the stability of the active ingredient(s); they should not interact adversely with other components of the formulation
Adjuvants to adjust tonicity, viscosity or pH in order to increase the ‘comfort’ in use and to increase the stability of the active ingredient(s); they should not interact adversely with other components of the formulation
 Suitable container for administration of eye drops which maintains the preparation in a stable form and protects from contamination during preparation, storage and use.
Suitable container for administration of eye drops which maintains the preparation in a stable form and protects from contamination during preparation, storage and use.
The single most important requirement of eye drops is that they are sterile. Historically, instances of microbially contaminated eye drops have been reported; the contaminating organism, Pseudomonas aeruginosa, is difficult to treat successfully and can cause loss of the eye.
Antimicrobial preservatives
Multiple-dose eye drops contain an effective antimicrobial preservative system, which is capable of withstanding the test for efficacy of antimicrobial preservatives of the British Pharmacopoeia (BP 2007). This ensures that the eye drops are maintained sterile during use and will not introduce contamination into the eyes being treated. Normal healthy eyes are quite efficient at preventing penetration by microorganisms. Eyes that have damaged epithelia are compromised and may be colonized by microorganisms. This has to be guarded against. The lack of vascularity of the cornea and certain internal structures of the eye make it very susceptible and difficult to treat once infection has been established.
No single substance is entirely satisfactory for use as a preservative for ophthalmic solutions. The systems that have been used, based on work of the author and others in the 1960s, have formed the basis of effective preservation over the subsequent years.
Eye drops specifically formulated for use during intraocular surgery should not contain a preservative because of the risk of damage to the internal surfaces of the eye. Diagnostic dyes should preferably be supplied as single-dose preparations. Preservatives which are suitable for a selection of eye drops are given in Box 42.1.
Benzalkonium chloride
This quaternary ammonium compound is the preservative of choice. It is in over 70% of commercially produced eye drops and over a third of these also contain disodium edetate, usually at 0.1% w/v.
Benzalkonium chloride is not a pure material, but is a mixture of alkylbenzyldimethyl ammonium compounds. This permits a mixture of alkyl chain lengths containing even numbers of carbon atoms between 8 and 18 and results in products of different activities. The longer the carbon chain length, the greater the antibacterial activity but the less the solubility. Therefore the manufacturer should seek to maximize the activity within the constraints of solubility. This means maximizing the proportions of C12, C14 and C16. It should be noted that Benzalkonium Chloride BP contains 50% w/v benzalkonium chloride.
Benzalkonium chloride is well tolerated on the eye up to concentrations of 0.02% w/v but is usually used at 0.01% w/v. It is stable to sterilization by autoclaving. The compound has a rapid bactericidal action in clean conditions against a wide range of Gram-positive and Gram-negative organisms. It destroys the external structures of the cell (cell envelope). It is active in the controlled aqueous environment and pH values of ophthalmic solutions. Activity is reduced in the presence of multivalent cations (Mg2+, Ca2+). These compete with the antibacterial for negatively charged sites on the bacterial cell surface. It also has its activity reduced if heated with methylcellulose or formulated with anionic and certain concentrations of non-ionic surfactants. Benzalkonium chloride is incompatible with fluorescein (large anion) and nitrates and is sorbed from solutions through contact with rubber.
The antibacterial activity of benzalkonium is enhanced by aromatic alcohols (benzyl alcohol, 2-phenylethanol and 3-phenylpropanol) and its activity against Gram-negative organisms is greatly enhanced by chelating agents such as disodium edetate. These agents chelate the divalent cations, principally Mg2+, of Gram-negative cells. These ions form bridges and bind the polysaccharide chains which protrude from the outer membrane of these cells. Thus, the integrity of the membrane is compromised and the benzalkonium chloride activity enhanced. This is particularly valuable in preserving against contamination with Pseudomonas aeruginosa.
The surface activity of benzalkonium chloride may be used to enhance the transcorneal passage of non-lipid-soluble drugs such as carbachol. Care must be taken since the preservative can solubilize the outer oily protective layer of the precorneal film. This film has an internal mucin layer in contact with the corneal and scleral epithelia, a middle aqueous layer and an outer oily layer. The oil prevents excessive aqueous evaporation and protects the inner surface of the lids from constant contact with water. The blink reflex helps maintain the integrity of the precorneal film. For these reasons, it is important not to use benzalkonium chloride to preserve local anaesthetic eye drops which abolish the blink reflex. The combined effect of the two agents causes drying of the eye surface and irritation of the cornea.
Chlorhexidine acetate or gluconate
Chlorhexidine is a cationic biguanide bactericide with antibacterial properties in aqueous solution similar to benzalkonium chloride. Its activity is often reduced in the presence of other formulation ingredients. It is used at 0.01% w/v. Its antibacterial activity against Gram-negative bacteria is enhanced by aromatic alcohols and by disodium edetate. Activity is antagonized by multivalent cations. Stability is greatest at pH 5–6 but it is less stable to autoclaving than benzalkonium chloride. Chlorhexidine salts are generally well tolerated by the eye although allergic reactions may occur.
Chlorobutanol
This chlorinated alcohol is used at 0.5% w/v and is effective against bacteria and fungi. Chlorobutanol is compatible with most ophthalmic products. The main disadvantages are its volatility, absorption by plastic containers and lack of stability at autoclave temperatures.
Organic mercurials
Phenylmercuric acetate and nitrate and thiomersal are organic mercurials. They are slowly active, at concentrations of 0.001–0.004% w/v, over a wide pH range against bacteria and fungi. Absorption by rubber is marked.
The organic mercurials should not be used in eye drops which require prolonged usage because this can lead to intraocular deposition of mercury (mercurialentis). Allergy to thiomersal is also possible.
Tonicity
Where possible, eye drops are made isotonic with lachrymal fluid (approximately equivalent to 0.9% w/v sodium chloride solution). In practice, the eye will tolerate small volumes of eye drops having tonicities in the range equivalent to 0.7–1.5% w/v sodium chloride. Nevertheless it is good practice to adjust the tonicity of hypotonic eye drops by the addition of sodium chloride to bring the solution to the tonicity of the lachrymal fluid. (See Ch. 19 for methods for calculating the amount of sodium chloride required.) Some preparations are themselves hypertonic and so no adjustment should be made.
Viscosity enhancers
There is a general assumption that increasing the viscosity of an eye drop increases the residence time of the drop in the eye and results in increased penetration and therapeutic action of the drug. Most commercial preparations have their viscosities adjusted to be within the range 15–25 millipascal seconds (mPas). However, gently pressing downwards on the inside corner of the closed eye restricts the drainage channel into the nasal cavity and prolongs contact time. This has been recommended to increase the therapeutic index of antiglaucoma medications. Under normal conditions, a large proportion of a typical 50 μL drop will have drained from the conjunctival sac (capacity 25 μL) within 30 s. There will be no trace of the drop after 20 min.
Hypromellose
The hydroxypropyl derivative of methylcellulose is the most popular cellulose derivative employed for enhancing viscosity. It has good solubility characteristics (soluble in cold but insoluble in hot water) and good optical clarity. Typical concentrations in eye drop formulations are 0.5–2.0% w/v.
Polyvinyl alcohol
This is used at 1.4% w/v as a viscosity enhancer. It has a good contact time on the eye surface and good optical qualities. It withstands autoclaving and it can be filtered through a 0.22 μm filter.
Polyvinylpyrrolidone, polyethylene glycol and dextrin have also been used as viscolizing agents.
pH adjustment
The best compromise is required after considering the following factors:
 The pH offering best stability during preparation and storage
The pH offering best stability during preparation and storage
Most active ingredients are salts of weak bases and are most stable at an acid pH but most active at a slightly alkaline pH.
The lachrymal fluid has a pH of 7.2–7.4 and also possesses considerable buffering capacity. Thus a 50 μL eye drop which is weakly buffered will be rapidly neutralized by lachrymal fluid. Where it is possible, very acidic solutions, such as adrenaline acid tartrate or pilocarpine hydrochloride, are buffered to reduce a stinging effect on instillation. Suitable buffers are shown in Box 42.2.
Antioxidants
Reducing agents are preferentially oxidized and are added to eye drops in order to protect the active ingredient from oxidation. Active ingredients requiring protection include adrenaline (epinephrine), proxymetacaine, sulfacetamide, tetracaine, phenylephrine and physostigmine.
Sodium metabisulphite and sodium sulphite
Both may be used as antioxidants at 0.1% w/v. The former is preferred at acid pH and the latter at alkaline pH. Both are stable in solution when protected from light. Sodium metabisulphite possesses marked antimicrobial properties at acid pH and enhances the activity of phenylmercuric nitrate at acid pH. It is incompatible with prednisolone phosphate, adrenaline (epinephrine), chloramphenicol and phenylephrine.
Chelating agents
Traces of heavy metals can catalyse breakdown of the active ingredient by oxidation and other mechanisms. Therefore, chelating agents such as disodium edetate may be included to chelate the metal ions and thus enhance stability. Disodium edetate is a very useful adjuvant to ophthalmic preparations at concentrations of up to 0.1% w/v to enhance antibacterial activity and chemical stability. It has also been used at higher concentrations as an eye drop for the treatment of lime burns in cattle.
Bioavailability
The effect of pH on the therapeutic activity of weak bases such as atropine sulphate has already been indicated under the section on pH adjustment. At acid pH, these bases exist in the ionized hydrophilic form. In order to penetrate the cornea, the bases need to be at alkaline pH so that they are in the unionized lipophilic form. Thus at tear pH (7.4) they are able to penetrate the outer lipid layer of the lipid–water–lipid sandwich, which constitutes the physicochemical structure of the cornea. Once inside the epithelium the undissociated free base will partially dissociate. The water-soluble dissociated moiety will then traverse the middle aqueous stromal layer of the cornea. When the dissociated drug reaches the junction of the stroma and the endothelium it will again partially associate, forming the lipid-soluble moiety and thus cross the endothelium. Finally, the drug will dissociate into its water-soluble form and enter the aqueous humour. From here it can diffuse to the iris and the ciliary body which are the sites of its pharmacological action (see Fig. 42.1). Thus, the most effective penetration of the lipophilic–hydrophilic–lipophilic corneal membrane is by active ingredients having both hydrophilic and lipophilic forms. For example, highly water-soluble steroid phosphate esters have poor corneal penetration but the less water-soluble, more lipophilic steroid acetate has much better corneal penetration.
Storage conditions
To minimize degradation of eye drop ingredients, storage temperature and conditions must be considered at the time of formulation. The stability of several drugs used in eye drops is improved by refrigerated storage (2–8°C), e.g. chloramphenicol.
Containers for eye drops
Containers should protect the eye drops from microbial contamination, moisture and air. Container materials should not be shed or leached into solution, neither should any of the eye drop formulation be adsorbed or absorbed by the container. If the product is to be sterilized in the final container, all parts of the container must withstand the sterilization process.
Containers may be made of glass or plastic and may be single- or multiple-dose containers. The latter should not contain more than 10 mL. Both single-dose and multiple-dose packs must have tamper-evident closures and packaging.
Single-dose containers
The ‘Minims’® range is the most widely used type of single-dose eye drop container in the UK. It consists of an injection-moulded polypropylene container which is sealed at its base and has a nozzle sealed with a screw cap. This container is sterilized by autoclaving in an outer heat-sealed pouch with peel-off paper backing.
Plastic bottles
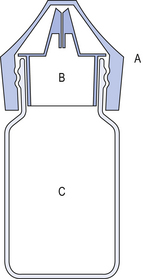
Most commercially prepared eye drops are supplied in plastic dropper bottles similar to the illustration in Figure 42.2. Bottles are made of polyethylene or polypropylene and are sterilized by ionizing radiation prior to filling under aseptic conditions with the previously sterilized preparation.
Glass bottles
Most extemporaneously prepared eye drops are supplied in 10 mL amber partially ribbed glass bottles.
The components of the eye dropper bottle are illustrated in Figure 42.3.
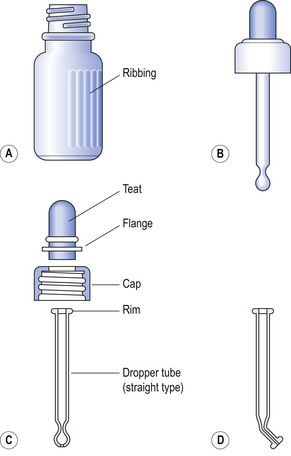
Figure 42.3 Eye dropper bottle. (A) Bottle. (B) Assembled closure. (C) Components of closure. (D) Dropper tube (angled type).
Bottles can be made of neutral glass and can be autoclaved more than once, or soda glass which has had the internal surfaces treated during manufacture to reduce the release of alkali when in contact with aqueous solutions, but can only be autoclaved once. The teat can be made of good-quality natural or synthetic rubber. The former will withstand autoclaving at 115°C for 30 min but will not withstand the high temperatures of dry-heat sterilization. The latter teats, made from silicone rubber, will withstand dry-heat sterilization and are suitable for use with oily eye drops. Silicone rubber is permeable to water vapour and for this reason aqueous eye drops in bottles having silicone rubber teats are given a limited shelf-life of 3 months. This can be lengthened by supplying the sterile eye drops in an eye drop bottle sealed with an ordinary screw cap together with a separately wrapped and sterilized silicone rubber dropper unit. The dropper is carefully substituted for the cap when the eye drops are about to be used.
Teats and caps are used once only. All components are thoroughly washed with filtered distilled or deionized water, dried and stored in a clean area until required.
Rubber teats sorb preservatives and antioxidants during autoclaving and storage. It is necessary that individual studies are undertaken during formulation to help counteract preservative and antioxidant loss.
Preparation of eye drops
Extemporaneous preparation of eye drops involves the following:
Preparation of the solution
The aqueous eye drop vehicle containing any necessary preservative, antioxidant, stabilizer, tonicity modifier, viscolizer or buffer should be prepared first. Then the active ingredient is added and the vehicle made up to volume.
Clarification
The BP has stringent requirements for the absence of particulate matter in eye drop solutions. Sintered glass filters or membrane filters of 0.45–1.2 μm pore sizes are suitable. The clarified solution is either filled directly into the final containers which are sealed prior to heat sterilization or temporarily filled into a suitable container prior to filtration sterilization. Clarified vehicle is used to prepare eye drop suspensions which are filled into final containers and sealed prior to sterilization.
Sterilization
 Autoclaving at 115°C for 30 min or 121°C for 15 min
Autoclaving at 115°C for 30 min or 121°C for 15 min
 Filtration through a membrane filter having a 0.22 μm pore size into sterile containers using strict aseptic technique. Filling should take place under Grade A laminar airflow conditions (see Ch. 46)
Filtration through a membrane filter having a 0.22 μm pore size into sterile containers using strict aseptic technique. Filling should take place under Grade A laminar airflow conditions (see Ch. 46)
 Dry-heat sterilization at 160°C for 2 h is employed for non-aqueous preparations such as liquid paraffin eye drops. Silicone rubber teats must be used.
Dry-heat sterilization at 160°C for 2 h is employed for non-aqueous preparations such as liquid paraffin eye drops. Silicone rubber teats must be used.
Immediately following sterilization, the eye drop containers must be covered with a readily breakable seal, such as a viskring, to distinguish between opened and unopened containers.
Labelling of containers
Labelling requirements are summarized in Table 42.1 and Box 42.3.
Table 42.1
Labelling requirements for eye drop and eye ointment containers at the time of dispensing
| Requirement | Include on label |
| State route of administration | ‘For use in the eye only’ |
| Fully identify the product | The name and concentration of the active ingredient(s) |
| Statement on preservation | Confirm presence or absence of preservative |
| Directions for use | e.g. ‘Add one drop to each eye morning and evening’ |
| State an ‘in use’ expiry date | Day, month, year |
| Storage requirements | ‘Store in a cool place’ or ‘Protect from light’ |
| Identify patient | Patient’s name |
| Date of dispensing | Day, month, year |
Note: When the stability of the final preparation requires it, eye drops may be provided in two containers as a dry powder and an aqueous vehicle. The labels should state ‘Powder for eye drops’ on one container and the directions for the preparation of the eye drops on the other package or container.
Based on the Department of Health guidance HSC(IS)122 1975, revised by the Royal Pharmaceutical Society of Great Britain 2001.
Instillation of eye drops
Patients who have not used eye drops before need an explanation of how to instil the drops satisfactorily.
 Tilt head back and with one hand gently pull down lower eyelid to form a pouch between the eye and the eyelid
Tilt head back and with one hand gently pull down lower eyelid to form a pouch between the eye and the eyelid
 Hold dropper bottle (or separate eye dropper containing eye drops) above the eye and drop a single drop into the preformed pouch. Do not touch the dropper on the eye or eyelid. (Using a well illuminated mirror will help.) Administration aids are available to assist the self-administration of eye drops contained in plastic eye dropper bottles
Hold dropper bottle (or separate eye dropper containing eye drops) above the eye and drop a single drop into the preformed pouch. Do not touch the dropper on the eye or eyelid. (Using a well illuminated mirror will help.) Administration aids are available to assist the self-administration of eye drops contained in plastic eye dropper bottles
 Release lower lid. Try not to blink more than usual as this removes the medicine from the eye
Release lower lid. Try not to blink more than usual as this removes the medicine from the eye
Formulation of eye lotions
The purpose of eye lotions is to assist in the cleaning of the external surfaces of the eye. This might be to help remove a non-impacted foreign body or to clean away conjunctival discharge. Eye lotions may also be used to impregnate eye dressings. Eye lotions intended for use in surgical or first-aid procedures should not contain antimicrobial preservatives and should be supplied in single-use containers. In keeping with their simple requirements these preparations should have simple formulations and the most common eye lotion consists of sterile normal saline. This preparation typifies the requirements of an eye lotion which are:
Labels
 Title identifying the product and concentration of contents
Title identifying the product and concentration of contents
Preserved eye lotion would need the additional labelling:
 ‘Avoid contamination of contents during use’
‘Avoid contamination of contents during use’
 ‘Discard remaining solutions not more than 4 weeks after first opening’.
‘Discard remaining solutions not more than 4 weeks after first opening’.
The lotions should be supplied in coloured bottles and sealed to exclude microorganisms.
Powders for the preparation of eye drops and powders for the preparation of eye lotions
These powders are supplied in a dry, sterile form for dissolving or suspending in an appropriate vehicle at the time of use to provide a solution or suspension which complies with the requirements for eye drops or eye lotions as appropriate. The powders may contain suitable excipients to aid dissolution or dispersion, to adjust the tonicity and to improve stability. Unless an exception has been authorized, eye drops in the form of a suspension must pass the same particle size limit test as that applied to the size of particles in eye ointments (see below). In addition, single-dose powders for eye drops and eye lotions should either comply with the test for the uniformity of dosage of the European Pharmacopoeia (EP), or where appropriate, with the tests for uniformity of content and/or uniformity of mass.
Formulation of eye ointments
Eye ointments are popular and duplicate many of the therapeutic options offered by eye drops. Ointments have the disadvantage of temporarily interfering with vision, but have the advantage over liquids of providing greater total drug bioavailability. However, ointments take a longer time to reach peak absorption.
Eye ointments must be sterile and may contain suitable antimicrobial preservatives, antioxidants and stabilizers. The United States Pharmacopoeia (USP 25) requires these ointments to contain one of the following antimicrobials: chlorobutanol, the parabens or the organic mercurials. In addition such ointments should be free from particulate matter that could be harmful to the tissues of the eye. The EP and BP (2007) have limits for the particle size of incorporated solids. Each 10 μg of active solid should have no particles>90 μm, not more than 2 particles>50 μm and not more than 20 particles>25 μm.
The basic components of an eye ointment are:
| Liquid paraffin | 1 part |
| Wool fat | 1 part (to facilitate incorporation of water) |
| Yellow soft paraffin | 8 parts |
Hard paraffin may be substituted as necessary to maintain an appropriate consistency in hot climates.
Containers for eye ointments
Eye ointments should be supplied in small sterilized collapsible tubes made of metal or a suitable plastic. The tube should not contain more than 10 g of preparation and must be fitted or provided with a nozzle of a suitable shape to facilitate application to the eye and surrounds without allowing contamination of the contents. The tubes must be suitably sealed to prevent microbial contamination.
Preparation of eye ointments
Eye ointments are normally prepared using aseptic techniques to incorporate the previously sterilized finely powdered active ingredient or a sterilized concentrated solution of the medicament into the sterile eye ointment basis. Immediately after preparation, the eye ointment is filled into the sterile containers which are then sealed so as to exclude microorganisms. The screw cap should be covered with a readily breakable seal.
All apparatus used in the preparation of eye ointments must be scrupulously clean and sterile. Certain commercial eye ointments may be sterilized in their final containers using ionizing radiation.
Preparation of eye ointment basis
The paraffins and the wool fat are heated together and filtered, while molten, through a coarse filter paper in a heated funnel into a container which can withstand dry-heat sterilization temperatures. The container is closed to exclude microorganisms and together with contents, is maintained at 160°C for 2 h.
Ophthalmic inserts
These are sterile solid or semi-solid preparations for insertion in the conjunctival sac. They contain a reservoir of active material which is slowly released from a matrix or through a rate-controlling membrane over a known time period. Ophthalmic inserts each have their own sterile container which is labelled to state the total quantity of active substance per insert and, where applicable, its rate of release. The EP requires that in the manufacturing of ophthalmic inserts appropriate product dissolution behaviour is demonstrated.
Monitoring of eye preparations for adverse effects
Pharmacists should be available to counsel patients on the use of their eye medication and advise them about any adverse effects they may experience while using their medicines. Failing to use eye medication appropriately may also have serious consequences. It is important that the pharmacist is able to support the patient in using their medicine correctly. The pharmacist should also be alert to notice any signs/symptoms of adverse effects, that the patient may be experiencing resulting from medication, in order to give appropriate and timely advice. Table 42.2 indicates the signs/symptoms of adverse effects which may occur with eye preparations used in the treatment of primary open angle glaucoma. In addition to adverse effects associated with the eye it should be noted that undesirable systemic effects can also occur with eye medication. Such systemic effects have been reported for certain potent ophthalmic medicines. This is due to excess solution draining from the eye surface through two small channels, the lachrymal canaliculi, into the lachrymal sac and on via the nasolachrymal duct and the gastrointestinal tract. Consequently, it is necessary to seek to avoid the instilling of excess eye drops.
Table 42.2
Signs/symptoms of adverse effects which may occur with treatment for primary open angle glaucoma
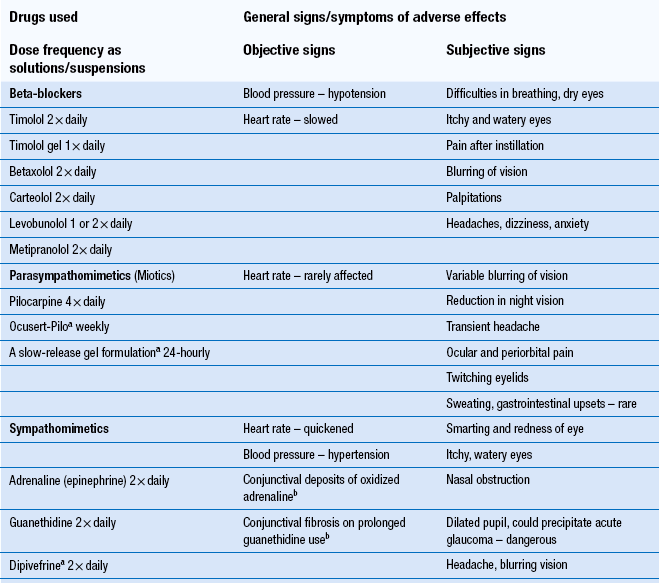
aThese formulations can reduce adverse effects.
bThese are specific effects.
Patients who are using an eye drop preparation for a chronic condition may become sensitive to the preservative in the formulation. This may happen with contact lens products also. Changing to a formulation having the same active ingredient but having a different or no preservative should solve the problem.
Contact lenses and their solutions
The ready accessibility of the eye and its external structures facilitates the fitting and wearing of lenses on the precorneal film and on the surface of the eye. Optometrists prescribe and fit contact lenses and monitor their use. Pharmacists should refer patients having persistent problems with wearing their lenses to their optometrist.
Popularity, problems, risks
The popularity of contact lenses results from their cosmetic appeal, optical advantages and their usefulness in sporting activities. Many prefer extended-wear soft lenses to daily-wear soft and hard lenses because of their relative convenience.
The problems that occur with the wearing of contact lenses result from inadequate education of the wearer about lens care. Extended-wear lenses in particular have been marketed in a manner which maximizes the volume of sales at the expense of adequate consumer education. That is, the marketing of lenses has overemphasized the convenient and carefree aspects of overnight lenses to the extent of trivializing the wearing of contact lenses. This has often resulted in poor patient compliance with suggested regimes of lens wear and care. It is estimated that more than 50% of those who wear contact lenses care for them unhygienically.
The risks associated with the wearing of contact lenses include recurrent corneal abrasions, corneal scarring and corneal vascularization. However, the most serious complication is microbial ulcerative keratitis or corneal ulcer, caused by microbial invasion of the cornea. Left untreated this can lead to loss of vision. Fortunately, the natural defences of the cornea are very effective and the normal cornea resists microbial infection as long as the surface epithelium is intact.
It has been shown that the risk of corneal ulcers is 9–15 times greater for extended-wear lenses worn overnight than for daily-wear soft lenses worn only during the day. The risk increases with the number of consecutive days that lenses are worn without removal.
A serious, but fairly rare, complication that can arise from using non-sterile water in the care of lenses is infection with the free-living opportunistic pathogen Acanthamoeba. This is found in most soil and water habitats. Acanthamoeba keratitis is hard to diagnose and to treat and can lead to serious loss of vision. Acanthamoeba infection has also resulted from wearing soft lenses while bathing in a Jacuzzi; consequently this practice is contraindicated.
The aim of formulators and providers of contact lens systems must be to supply the safest possible system with known and acceptable risks; that is, both convenience and safety must be the aim.
Relevant properties of the eye
Anatomy and physiology
Figure 42.1 indicates the structures of the eye which are particularly relevant to the use of topical medications, contact lenses and contact lens products. First, it is important to note that the cornea, the lens and the humour compartments are avascular and that this property facilitates the transmission of light and vision. Second, exchange of nutrients and waste products in these situations takes place almost entirely by diffusion processes through the aqueous humour, through the lens and cornea and through the lachrymal fluid. Contact lenses reduce the diffusion of oxygen to the cornea and thus can affect corneal metabolism.
Secretions
The secretions of the eye have an important role and influence on the wearing of contact lenses. Lachrymal fluid, commonly known as tears, performs the important functions of lubricating, hydrating, cleaning and disinfecting the anterior surface of the eye. The latter function is performed by the enzyme lysozyme (1,4-N-acetylglycosaminidase) which catalyses the hydrolysis of 1,4-glycosidic linkages between N-acetyl muramic acid and N-acetyl-glucosamine in the peptidoglycan layer of the bacterial cell wall. The peptidoglycan layer of Gram-positive cells is accessible to the action of lysozyme.
The fluid forming the precorneal film is produced by differing groups of glands. It contains mucus (Henle and Manz), water (Krause and Wolfring) and oil (Meibomian, Moll and Zeis). These fluids are stratified in three distinct layers. The surface-active mucoid layer spreads on the corneal surface and associates with the intermediate aqueous layer externally. The aqueous layer is surfaced with an oily layer which lubricates and protects the mucous membranes of the internal lid surfaces.
Tear electrolyte content
This is broadly similar to that of serum except that the potassium ion is approximately four to six times greater (24 mEq/L compared with 4–6 mEq/L in serum). The protein content of tears is mainly albumin and globulin and is approximately a tenth of that in serum (0.7% compared with 7%).
Tear production
Tears are produced by the lachrymal glands in response to four distinct types of stimuli: emotional via psychological factors, sensory via external irritants, continuous via automatic nervous control and systemic via chemicals in the bloodstream affecting the nerves innervating the lachrymal glands.
Tear pH
This is slightly alkaline at 7.2. Tears have sufficient buffering capacity to adjust rapidly the pH of small volumes of weakly buffered solutions to pH 7.2.
Eyelids
These perform a protecting and a cleaning function. The outer margins of the eyelids close slightly before the inner margins and sweep the fluids across the eye towards the lachrymal canaliculi at the inner angle of the eye from where it can pass via the lachrymal sac into the nasal cavity and gastrointestinal tract. Systemic absorption of excess eye medicament may take place through this mechanism.
Bacterial flora
There is a common misconception that lachrymal fluid is sterile. It has been known since 1908 that staphylococci and diphtheroids can be found regularly in normal conjunctiva. Gram-negative enteric bacilli have also been isolated from the conjunctivas and lids of about 5% of people. This shows that care is necessary when wearing contact lenses to avoid abrading the corneal epithelium.
Contact lenses
Sir John Herschel used a refractive glass shell in 1823 to protect the cornea from a diseased lid. Dr Eugen Fickfirst used the term ‘contact lens’ in 1887. Fick’s blown glass lenses were intended to correct defective vision. In 1948, Tuohy introduced the hydrophobic hard plastic corneal lens and in 1962, soft pliable lenses were introduced as the result of work in Prague University. These lenses have been very popular. Gas-permeable hard lenses have also been introduced which allow oxygen perfusion to the cornea. These lenses are more comfortable than the original hard lenses. The first extended-wear lenses were introduced in 1981.
The aim in making contact lenses is to produce lenses which will:
 Maintain their position on the eye
Maintain their position on the eye
 Allow respiration of the cornea
Allow respiration of the cornea
 Permit free flow of tears round or through the lens
Permit free flow of tears round or through the lens
 Not introduce microbial contamination
Not introduce microbial contamination
Hard lenses
Polymethylmethacrylate (PMMA) or ‘Perspex’ has optical properties similar to spectacle crown glass. PMMA has hydrophobic properties conferred by the large proportion of methyl groups compared with hydrophilic carboxy ester groups. This means that lachrymal fluid does not readily wet lenses made of this material. Therefore, the lenses need to be wetted before mounting on the precorneal film to reduce or eliminate patient discomfort. Hence the need for a wetting solution to facilitate wear and the need for a storage, hydrating, decontaminating solution to facilitate care of the lenses when not being worn. The original hard lens composition had some major disadvantages for the wearer. Free passage of oxygen and carbon dioxide to and from the corneal epithelium could not take place. Corneal oedema and distortion were a common result. Thus, modern lenses have been designed to be gas permeable. These lenses are physiologically more user-friendly and have greater wearer acceptance.
The original gas-permeable lenses consisted of cellulose acetate butyrate (CAB), which was readily wettable and proved quite acceptable. More recently, lenses based on silicone and fluorine have been produced which have greater gas permeability. Silicone methacrylate copolymers are very popular. The silicone composition controls the permeability properties and the PMMA composition controls the degree of rigidity. Similarly, fluorosilicone methacrylate copolymers, which have very high oxygen permeability properties and good wetting properties, are proving to be popular. These gas-permeable lenses are cared for using hard lens solutions. These lenses are less subject than soft lenses to deposits of lipids, protein and other substances from the lachrymal fluid. They also have better optical qualities and are generally easier to care for.
Soft lenses
Soft lenses are made from the hydroxyethyl ester of polymethacrylic acid (poly-HEMA). The large number of polar hydroxyl groups confers hydrophilic properties to the polymer. Poly-HEMA is flexible and can absorb about 47% of its own weight of water. Thus, lenses of this material are comfortable and easy to wear but more difficult to care for than hard lenses. A particular problem is uptake of antibacterial preservatives and subsequent release and irritancy during wear. Although a wetting solution is not needed, cleaning, storing, hydrating and decontaminating functions are required of solutions.
Copolymers of poly-HEMA with vinylpyrrolidine (VP) are also produced which can absorb up to 80% by weight of water depending on the HEMA/VP ratio. The higher water content lenses have the advantage of greater gas permeability and comfort than the poly-HEMA lenses which may occasionally cause corneal oedema. However, they are more fragile and difficult to care for than poly-HEMA, have a greater tendency to attract deposits, more solution problems and less precise optical properties.
Disposable lenses
Disposable lenses may be discarded after 1 month, 1 week or even 1 day. The latter would obviate the need for the use of solutions and theoretically increase the safety and acceptability of lens wear. However, the original intention of these lenses was for extended wear without removal. It has already been pointed out that the additional risks that are associated with extended wear makes this unattractive and even a dangerous practice. These lenses would seem to offer the greatest advantage to those people who wear lenses on an irregular basis for social and sporting activities and for those children who may need soft lenses.
Hard lens solutions
A ‘wetting solution’ and a ‘soaking/storing/decontaminating solution’ are required for the wear and routine care of hard lenses. The first is suitable for placing in the eye but the second must not have contact with the eye.
Soft lens solutions
Storing solutions
Formulation
Hydrogen peroxide – was introduced into commercially available care systems in 1984. Hydrogen peroxide is a powerful oxidizer and this is the source of its antimicrobial activity. It has good activity against Acanthamoeba.
Decomposition is more rapid at alkaline than acid pH and many substances catalyse the reaction. These properties are utilized in the formulation of storage, disinfecting and cleaning solutions. For example, a solution containing 3% hydrogen peroxide at acid pH is used to disinfect lenses over a period of 6 h. This is then followed by suitable inactivation with sodium pyruvate, platinum or the enzyme catalase, to facilitate subsequent safe wearing of the lenses. The procedure is referred to as the two-step system because a separate neutralization step follows the disinfecting step. One-step systems have also been developed for greater patient convenience. The inactivating substance is incorporated with the 3% hydrogen peroxide and the lenses in the disinfecting solution. This system needs to be calibrated to slowly neutralize hydrogen peroxide, but to allow it to have antimicrobial activity over a 6 h period to ensure effectiveness against Acanthamoeba cysts. Some commercial systems may neutralize the effect of the hydrogen peroxide over a period of 30 min or so, which is too rapid to guarantee effectiveness.
Polyquad – a polyquaternium compound is used as an antimicrobial in soft lens solutions because it is not sorbed by lenses and it has low toxicity to corneal and ocular tissues.
(Thermal disinfection is an alternative disinfection process and the American FDA stipulates heating the lenses in a suitable solution in a lens case at a minimum of 80°C for 10 min. Heating reduces the life of the lens and it is also inconvenient.)
Enzyme protein digest
All-purpose solutions
The all-purpose solutions initially represented a compromise for hard lens wearers finding it difficult to comply with a two-solution regimen. Single-solution lens care systems are now widely available for use with soft lenses, which incorporate an enzyme cleaner combined with a disinfection solution. For example, the serine protease subtilisin A, obtained from the bacterium Bacillus subtilis, is used in the presence of hydrogen peroxide to remove protein contamination from contact lenses. Certain all-purpose lens solutions incorporate polyhexamide (polyhexamethylene biguanide) 0.00006–0.0004% as the antimicrobial agent. It is reported to be active against a wide range of bacteria and against Acanthamoeba.
All-purpose solutions for soft lenses have become very popular.
Containers
Contact lens solutions are usually packed in plastic containers. It is imperative that the low concentrations of antimicrobials present in these products are not reduced to ineffective levels due to sorption effects with the plastic.
Contact lens storage cases are also of importance to the contact lens wearer. It is important that these containers are kept in a hygienic condition by keeping them scrupulously clean and using the disinfecting/storage solutions strictly in accordance with the manufacturers’ instructions. Storage cases should be changed periodically.
Advice to patients
General considerations
Contact lens wearers presenting at the pharmacy with a persistent red eye indicating an infection should not be recommended antibacterial eye drops. They should be referred to an ophthalmologist. This is to guard against the possibility that the person has an infection with Acanthamoeba. Such an infection would be more difficult to diagnose after partial treatment.
Disease states leading to a dry eye syndrome such as Sjögren syndrome, which is mostly confined to menopausal women having osteoarthritis, will also adversely affect the ability of a person to wear contact lenses.
Hard lenses and to a lesser extent soft lenses interrupt the oxygen supply to the cornea and with prolonged wear produce increasing hypoxia. After approximately 16 h of wear, this corneal hypoxia results in a dip in the corneal glycogen level with resultant oedema. Irritation, itchiness, photophobia and blurred vision can result. The patient should be advised not to over-wear the lenses and they may also be recommended to instil sterile sodium chloride 2% w/v every 3–4 h, after the lenses have been removed, until the oedema has resolved. They should be warned that the hypertonic drops may cause temporary stinging on instillation.
Adverse effects of medicines
Pharmacists should be aware that many medicines taken systemically can also cause problems for wearers of contact lenses and be prepared to offer appropriate counselling.
Certain medicines can affect the eye surface and lachrymal fluid production and thereby influence the comfort of contact lens wear. Medication having anticholinergic properties such as sedative antihistamines, chlorphenamine, antispasmodics, hyoscine, tricyclic antidepressants and neuroleptics can all reduce lachrymal fluid production. Diuretics will also reduce tear volume and topical timolol can cause transitory dry eyes. The consequent lack of lubrication may cause lens discomfort and increased lens deposits.
Oral contraceptives may cause corneal oedema, decreased aqueous and increased mucus and protein production and thus lead to lens intolerance. Pregnancy may also be associated with increased lens awareness and discomfort possibly associated with reduced tear flow and changes in corneal thickness and the curvature of the eye. Clomifene and primidone have also been reported to cause lid and corneal oedema.
Cholinergic drugs and also ephedrine and reserpine will increase tear volume. Aspirin produces low concentrations of salicylic acid in the lachrymal fluid. This can be absorbed by soft lenses and subsequently cause irritation. Isotretinoin may cause conjunctival inflammation and consequently cause discomfort to contact lens wearers.
Discolouration, via the lachrymal fluid, particularly with soft lenses, may occur with the administration of certain medicines such as labetalol, nitrofurantoin, phenothiazines, phenolphthalein, rifampicin, sulfasalazine and tetracyclines. Rifampicin for example will stain the lenses and tears orange.
Lenses must be removed before diagnostic dyes such as fluorescein are instilled. In fact it is a general rule that patients should be counselled not to place any ophthalmic preparation on to the eyelids or surface of the eye, while contact lenses are in place. Certain eye drops may be instilled while hard lenses are being worn. Sterile ‘Comfort drops’ may be instilled while lenses are being worn to help maintain the hydration and lubrication of the eye surfaces and lenses when required. Numerous commercial solutions are available. The basic requirements are that the drops should be isotonic, have good wetting properties and be slightly viscous.
Concurrent use of cosmetics
Soft lenses should always be inserted before applying eye makeup but rigid gas-permeable lenses may be put on after. All lenses should be put on before applying nail polish, hand creams, perfumes or using nail polish remover. Aerosol products should be used with caution so that spray does not get between the lens and the eye. All eye make-up should be water based and powders should be avoided. Mascara (not waterproof) should only be applied to the tips of the eyelashes.
The pharmacist should be aware of the various situations mentioned above when offering advice and discussing customers’/patients’ questions.
Key Points
 Ophthalmic preparations must be sterile
Ophthalmic preparations must be sterile
 Eye drops may be solutions, suspensions or emulsions and contain:
Eye drops may be solutions, suspensions or emulsions and contain:
 Liquid vehicle free from particulate matter; particle size limits for suspensions
Liquid vehicle free from particulate matter; particle size limits for suspensions
 Adjuvants: tonicity, viscosity, buffering, antioxidants, chelating, dispersing, emulsifying
Adjuvants: tonicity, viscosity, buffering, antioxidants, chelating, dispersing, emulsifying
 Eye drops are contained in a glass or plastic bottle
Eye drops are contained in a glass or plastic bottle
 Properties of the eye affecting formulation of products include:
Properties of the eye affecting formulation of products include:
 Contact lens solutions may be:
Contact lens solutions may be:
 Hard lenses – (i) wetting and cleaning; (ii) storing and disinfecting or (iii) all purpose
Hard lenses – (i) wetting and cleaning; (ii) storing and disinfecting or (iii) all purpose
 Soft lenses – (i) cleaning; (ii) storing and disinfecting or (iii) all purpose
Soft lenses – (i) cleaning; (ii) storing and disinfecting or (iii) all purpose