Specialized services
 Pharmacy aseptic compounding services and the range of medicines prepared in them
Pharmacy aseptic compounding services and the range of medicines prepared in them
 Equipment and procedures used in centralized cytotoxic reconstitution services
Equipment and procedures used in centralized cytotoxic reconstitution services
 Occupational health risks of cytotoxic drugs and the effective management of these risks
Occupational health risks of cytotoxic drugs and the effective management of these risks
 Benefits of centralized, pharmacy-operated aseptic compounding services
Benefits of centralized, pharmacy-operated aseptic compounding services
 Scope and operation of a centralized intravenous additive service (CIVAS)
Scope and operation of a centralized intravenous additive service (CIVAS)
Introduction
This chapter describes the specialized services provided by hospital pharmacy departments in the provision of various aseptic dosage forms. These services may include: chemotherapy reconstitution services, centralized intravenous additive services (CIVAS), radiopharmacy services (see Ch. 45) and home-care services. In each case, the service involves the provision of aseptically-prepared medicines which are often tailored to the specific needs of individual patients. This chapter introduces the scope, practice and pharmaceutical challenges of aseptic compounding services.
Cancer chemotherapy
The majority of chemotherapy doses are administered as injections or infusions. This offers the advantages of assured bioavailability, control over the rate and sequence of drug administration and also the ability to stop administration immediately in the event of adverse effects. However, the parenteral route may be associated with complications such as infection, extravasation and thromboembolism. The majority of these drugs are given in the hospital setting.
Cytotoxic drugs are available as sealed vials containing freeze-dried powders or sterile, concentrated solutions and are designed to provide an adequate shelf-life (usually>2 years). The freeze-dried powders require reconstitution with an appropriate diluent. Either may then require further dilution before being filled into syringes, infusion bags or infusion devices for administration.
Ambulatory infusion devices can be filled for use in the community by patients receiving home chemotherapy.
Occupational exposure risks
Cytotoxic drug exposure has been associated with various acute toxicities including headache, rash, nausea and dizziness. However, the more serious risks are related to the potential mutagenic, carcinogenic and teratogenic effects. Routes of exposure include ingestion, inhalation, needle-stick injury and skin contact – the most significant risk for occupational exposure.
In the UK, the requirements for safe handling of cytotoxic drugs are set out and enforced by the Health and Safety Executive. Pharmacy staff preparing cytotoxic agents must be fully trained in the necessary aseptic and safe handling techniques and must be fully aware of the potential health risks and the precautions that are required. Current opinion suggests that resources should be invested in appropriate equipment, staff training and competency assessment.
Published guidelines include the following areas of safe practice:
Useful guidelines on cytotoxic handling include The Cytotoxics Handbook and further references are available in Appendix 4.
Provision of a pharmacy-based chemotherapy preparation service
Protection of both the product and the staff involved in its preparation is technically demanding and requires carefully developed systems and procedures together with extensive validation, which must be integrated with the principles of good pharmaceutical manufacturing practice (GMP). Capacity plans should ensure that the service is capable of meeting current and future demand; for example, the rising demand for targeted therapies.
Despite the challenges outlined above, it is important that pharmacy staff ‘own’ chemotherapy preparation services and take a clear lead. In the UK, from NPSA Alert 20 on injectable medicines, it is clear that application of the risk assessment guidelines places all cytotoxic drugs in the high-risk category. It is therefore essential that these medicines are prepared by specialized hospital pharmacy aseptic units or by appropriate commercial providers. Pharmacy staff offer a unique combination of skills and expertise, including the practice of aseptic technique, a wide clinical knowledge of cancer chemotherapy, familiarity with formulation and drug stability issues, the application of GMP, quality assurance (QA) and quality control (QC) to aseptic preparation and considerable experience in working with SOPs, batch documentation and checking procedures. These are key attributes that help to ensure the provision of safe, effective chemotherapy and contribute towards minimizing the risks of occupational exposure to drugs used in the treatment of cancer.
Training required for staff preparing cytotoxics
All personnel involved in preparing and handling cytotoxics require training and competency assessment in the appropriate techniques. On a practical level, all staff must be aware of the following, over and above standard aseptic technique and the application of GMP to aseptic preparation:
Validation of operator techniques
Prior to commencing work on reconstitution of cytotoxics, an operator’s competence in this field must be assessed.
 The operator is asked to carry out broth transfer simulations where solutions are transferred from one vial or container to another
The operator is asked to carry out broth transfer simulations where solutions are transferred from one vial or container to another
 The broth-filled vials can then be incubated and examined for microbiological growth
The broth-filled vials can then be incubated and examined for microbiological growth
 Operators must achieve negative results (no growth after incubation) on each occasion before they are deemed capable of preparing cytotoxic agents
Operators must achieve negative results (no growth after incubation) on each occasion before they are deemed capable of preparing cytotoxic agents
 Typically, each operator and each process would be re-validated at least every 3 months and training procedures should be reviewed on a regular basis
Typically, each operator and each process would be re-validated at least every 3 months and training procedures should be reviewed on a regular basis
 Operators routinely incorporate environmental monitoring tests such as settle plates and finger-dab plates into the production schedule as part of the QA process
Operators routinely incorporate environmental monitoring tests such as settle plates and finger-dab plates into the production schedule as part of the QA process
 Expert guidance on the validation and monitoring of aseptic compounding has been published by the NHS
Expert guidance on the validation and monitoring of aseptic compounding has been published by the NHS
 Operator technique in the safe handling of cytotoxic drugs can be assessed by simulating aseptic transfer processes using a sterile solution containing a fluorescent dye as splashes or spillage can be visualized using a portable ultraviolet lamp
Operator technique in the safe handling of cytotoxic drugs can be assessed by simulating aseptic transfer processes using a sterile solution containing a fluorescent dye as splashes or spillage can be visualized using a portable ultraviolet lamp
 As with assessment of aseptic technique, safe handling should be evaluated using a combination of simulation and expert observation.
As with assessment of aseptic technique, safe handling should be evaluated using a combination of simulation and expert observation.
Documentation required for cytotoxics
On receipt of a prescription for a cytotoxic agent, a number of procedures must be undertaken. Figure 46.1 shows the areas of work in which a pharmacist may have involvement.
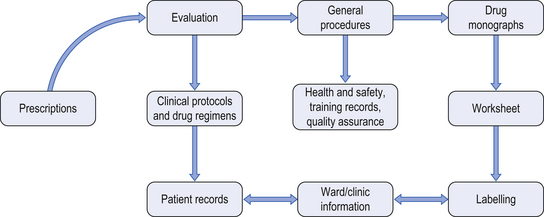
Figure 46.1 Documentation required for cytotoxic services. (From Allwood et al. 2002, reproduced with permission.)
When the prescription is received, it is clinically checked by an experienced oncology pharmacist. The prescription must be validated against an approved chemotherapy protocol. It is essential that patients receive the correct number of cycles of treatment at the correct intervals. The British Oncology Pharmacy Association Standards for Prescription Verification for Systemic Anti Cancer Therapy provides detailed guidance.
Information from the prescription is transferred to a worksheet or batch document and details of medicine(s) required, diluent and volume for reconstitution are recorded together with the number of drug vials required. Details of batch numbers and expiry date for each component used, all dose and dilution calculations, preparation methods, container(s) to be used, time and date of preparation, and expiry of the final product are also required. Additionally, a sample label is attached to the worksheet. Most chemotherapy preparation units use preprinted worksheets for each chemotherapy protocol, with a pharmacist-approved master document from which copies are made. Alternatively, some units use computer-based systems which contain a database of all approved chemotherapy protocols and produce batch documents and labels.
Labels should include the following information:
 Patient’s name, hospital number and ward or clinic name
Patient’s name, hospital number and ward or clinic name
 Drug name, total quantity and final volume of infusion
Drug name, total quantity and final volume of infusion
 Vehicle in which the drug is prepared (e.g. 0.9% sodium chloride)
Vehicle in which the drug is prepared (e.g. 0.9% sodium chloride)
 Batch number, expiry date and storage conditions required
Batch number, expiry date and storage conditions required
 Hospital pharmacy name and address
Hospital pharmacy name and address
When the worksheet is complete, the materials required are collected together and then subjected to an initial check before transfer to the designated clean room. After preparation has been completed, the finished product(s) and used or part-used vials are returned in the tray, together with batch documents, for labelling, inspection and release. Some cytotoxic agents require protection from light and are sealed in opaque plastic overwraps which will also require labelling. The pharmacist responsible for the release of the prepared medicines will check all details on the worksheets and will reconcile the number of drug vials used in the preparation. If correct, the pharmacist will sign the worksheet or batch documents to signify approval, and the medicines are dispatched. All documents must be retained for up to 13 years after the date of preparation.
Cytotoxic preparation areas
In the UK, and in many parts of Europe, pharmaceutical isolators are used for cytotoxic preparation. In addition to providing aseptic conditions for preparation of the product, isolators are designed to protect the operator and the clean room environment from cytotoxic contamination. To achieve this, many isolators operate under negative pressure with respect to the clean room, and the exhaust air is externally ducted via a high-efficiency particulate air (HEPA) filter. All isolators should be located in a classified clean room.
It is generally accepted that isolators offer greater operator protection than open-fronted Class II safety cabinets. The main disadvantages of isolators include limited access for equipment and difficulties in cleaning and removing cytotoxic residues. Gas sterilizable isolators enable sterilization of the outer surface of vials and components used in the preparation process. Gases such as vapourized hydrogen peroxide are pumped into the isolator to sterilize the inside of the isolator and the outer surface of components in situ, prior to manipulation.
Techniques and precautions
When handling cytotoxics, it is vital that the appropriate protective clothing is worn. Operators using clean room facilities must wear appropriate clean room clothing, with the addition of chemotherapy gowns or armlets. These garments are non-shedding and have an absorbent surface and impermeable backing. Full clean room suits are worn beneath the chemotherapy gown so it is important to ensure that the clean room temperature is carefully controlled. Gloves designed specifically for cytotoxic handling are available and these are normally fabricated from a nitrile material. Gloves should be worn for handling cytotoxic drug vials outside the clean rooms as these can be contaminated with cytotoxic residues on the outer surface.
Product segregation is crucial in all aseptic work to avoid any risk of product mix up and only one product or one batch of product, is prepared at any one time.
Reconstitution procedures
When carrying out reconstitution procedures, certain precautions must be taken:
 Vials and outer packs of consumables should be sprayed with sterile 70% alcohol and wiped with a sterile swab before being introduced into the clean room and the process repeated before introducing these materials into the isolator or Class II cabinet workstation. Rubber stoppers on vials should be swabbed with a sterile swab prior to removal of liquid
Vials and outer packs of consumables should be sprayed with sterile 70% alcohol and wiped with a sterile swab before being introduced into the clean room and the process repeated before introducing these materials into the isolator or Class II cabinet workstation. Rubber stoppers on vials should be swabbed with a sterile swab prior to removal of liquid
 Transfer of liquids to and from vials requires the insertion of a venting needle with hydrophobic filter into the vial or the use of a vented reconstitution device. These devices ensure pressure equalization and reduce the risk of aerosol generation
Transfer of liquids to and from vials requires the insertion of a venting needle with hydrophobic filter into the vial or the use of a vented reconstitution device. These devices ensure pressure equalization and reduce the risk of aerosol generation
 Luer lock syringes with wide-bore needles should be used for all procedures to allow free flow in the fluid pathway and to avoid the risk of syringes and needles becoming disconnected during fluid transfer
Luer lock syringes with wide-bore needles should be used for all procedures to allow free flow in the fluid pathway and to avoid the risk of syringes and needles becoming disconnected during fluid transfer
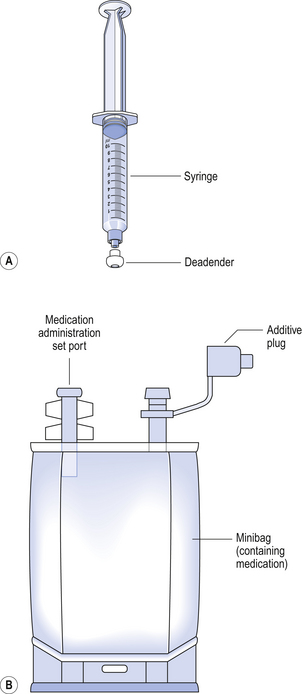
 To ensure that no further additions are made outside the pharmacy preparation area, all completed products in syringe form should be sealed with a blind hub before removal from the cytotoxic cabinet (Fig. 46.2). An additive plug or cap must be placed on each minibag once additions are complete.
To ensure that no further additions are made outside the pharmacy preparation area, all completed products in syringe form should be sealed with a blind hub before removal from the cytotoxic cabinet (Fig. 46.2). An additive plug or cap must be placed on each minibag once additions are complete.
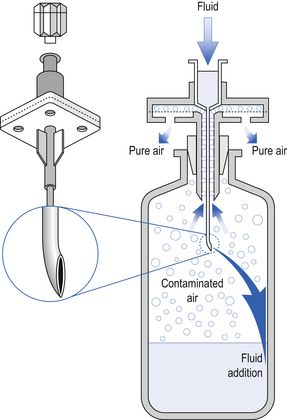
The vials that contain cytotoxic agents are effectively a closed system which contains either a powder requiring reconstitution or a drug concentrate requiring withdrawal from the vial into a syringe. In each case, equalization of pressure within the vial is required to allow withdrawal from it and is achieved by inserting a sterile 0.2 mm hydrophobic filter venting needle into the vial to facilitate liquid transfer. Ordinary needles with no hydrophobic filter must not be used for venting due to the risk of leakage of cytotoxic solution from the needle. Alternatively, reconstitution devices are available to help with the reconstitution process. Some of these devices consist of a small plastic spike with an integral hydrophobic filter. These devices are useful for rapid transfer of solutions, but the large needle bore can produce large holes in the rubber bung of cytotoxic vials, thus increasing the risk of leakage. The CytoSafe needle is a commonly used example of this type of product. This device consists of a needle which is vented to allow equilibrium of pressure between the vial and the syringe. It is useful for reconstitution of large vials or when more than one vial is required for a dose (Fig. 46.3). However, care must be taken when withdrawing or adding liquid to a vial as the filter may become blocked.
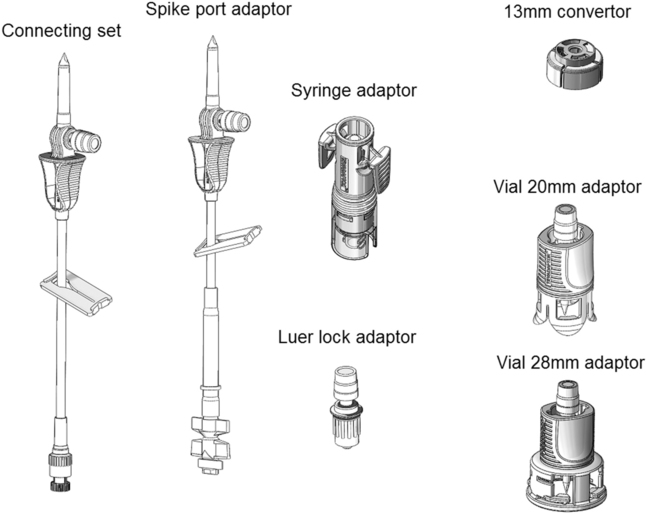
‘Closed systems’ using needle-free technologies have been designed for cytotoxic handling. These devices virtually eliminate the risks of cytotoxic aerosol formation and operator needle-stick injuries. An example of this type of device is the Tevadaptor (Fig. 46.4). This system comprises a vial adaptor to access the drug vial, and a syringe adaptor which fits securely onto a Luer lock syringe and enables needle-free docking with the vial adaptor. The closed reconstitution systems have been shown in studies to be effective in reducing cytotoxic contamination.
Dealing with cytotoxic spillage
In the event of a cytotoxic spillage, the problem should be dealt with immediately to prevent the spread of contamination. A written policy on dealing with spillages should be prepared. Most policies are based on a spillage kit which contains all the required materials to deal with a spill. Spillage kits should be available in pharmacy preparation units, in wards/clinics and in transport vehicles.
If the spillage has come in contact with the skin, the contaminated area should be washed thoroughly with soap and water. Contact with eyes should be dealt with by irrigation with a sodium chloride eyewash and medical help sought. In the event of a needle-stick injury, the puncture wound should be encouraged to bleed and the area should again be thoroughly washed. All incidents should be reported.
Disposal of cytotoxic waste
Cytotoxic waste materials are regarded as ‘hazardous waste’ and should be placed in a purple coloured plastic bag, sealed and labelled with a cytotoxic warning label ready for disposal by incineration. Sharp objects including needles, syringes, ampoules and vials should be placed in a sharps bin which is made of rigid plastic and does not allow leakage of cytotoxic waste. When the sharps bin is full, it should be sealed with ‘cytotoxic’ warning tape and disposed of by incineration.
Packaging of cytotoxic infusions
Cytotoxic infusions should be packaged in a labelled, hermetically sealed overwrap. This has two functions: containment of any leak from the infusion and protection of staff from any cytotoxic residues. Ideally, the infusions should be transported in a rigid, closed plastic box to protect from any mechanical trauma.
Management of the chemotherapy workload
It is evident from the above text that chemotherapy preparation is very labour-intensive. There is a clear tendency to move from in-patient treatment of cancer patients on hospital wards to chemotherapy outpatient clinics. This can place significant workload pressures on pharmacy chemotherapy units, partly because several patients often arrive for treatment at the same time, and also because blood test results and other patient-specific data are required before the oncologist is able to confirm the chemotherapy dose and allow treatment to proceed.
Various strategies have been employed to manage these problems. In many centres, it is possible to organize patients’ blood samples 2 days before the patient is due to visit the outpatient clinic for treatment. This enables prescriptions to be ‘pre-written’ so that pharmacy can prepare batch documents and tray-up consumables on the day before treatment.
Many oncology centres have adopted the approach of ‘dose-banding’. Individual patient doses are calculated in the normal way, but the dose is then fitted to predefined dose ranges or ‘bands’. These standard doses are provided from a limited range of standard pre-filled syringes or infusion bags, either singly or in combination. In practice, five or six standard pre-fills are needed to provide the required range of standard doses.
There is no doubt that managing chemotherapy services is a very challenging task. Operating a patient-focused service that meets clinical needs within the confines of limited resources requires innovation, organization and regular communication with medical and nursing colleagues. The service should be carefully monitored and key outcomes such as errors and patient waiting times should be audited on a regular basis.
Centralized intravenous additive service (CIVAS)
The Breckenridge Report (1976) made recommendations that IV infusions should be prepared, where possible, by hospital pharmacies. The provision of an IV additive service did not commence until the 1980s and then only in a limited number of hospitals.
Currently, a large proportion of hospital pharmacists in the UK and many European countries provide a CIVAS, and this is augmented by a growing number of commercial compounding units. Despite these developments, it is estimated that of all infusions prepared in UK hospitals, less than 40% are prepared in pharmacy CIVAS units.
Scope of a CIVAS
A CIVAS is set up to provide a range of parenteral dosage forms suitable for administration to patients. A CIVA service can provide the following:
 IV antibiotics, antivirals, antifungals and steroids
IV antibiotics, antivirals, antifungals and steroids
 Patient-controlled analgesia, and other opioid infusions for postoperative analgesia and palliative care
Patient-controlled analgesia, and other opioid infusions for postoperative analgesia and palliative care
 Ambulatory infusion devices for various IV therapies at home
Ambulatory infusion devices for various IV therapies at home
 Electrolyte infusions (that are not commercially available).
Electrolyte infusions (that are not commercially available).
Given that CIVAS are resourced to provide only a proportion of the IV additive/compounding needs of a hospital, they normally prioritize the services offered according to clinical risk. Accordingly, CIVAS-produced infusions often include antibiotics for neonates and paediatric patients which require extensive dilution to the required doses. Economic factors can also influence which infusions are prepared in CIVAS units. Many IV medicines contain no preservative and are designed for single use only. However, subject to validated infusion stability, it is possible for a CIVAS unit to prepare a batch of infusions for several days’ use, or even longer, so reducing or eliminating drug wastage.
Provision of a CIVAS
The main goals of providing a CIVAS should include:
 Improved use of hospital resources
Improved use of hospital resources
 Improved services to patients, particularly with home infusions
Improved services to patients, particularly with home infusions
 Improved pharmacy control and reduced risk of medication errors.
Improved pharmacy control and reduced risk of medication errors.
Although there are compelling reasons for the provision of pharmacy CIVAS in all major hospitals, it is important in presenting a balanced argument to be aware of potential disadvantages to the hospital and healthcare system:
 Increased pharmacy expenditure diverting funds from other healthcare services
Increased pharmacy expenditure diverting funds from other healthcare services
 Pharmacy CIVAS, once established, should be available 24 hours a day, including weekends and public holidays
Pharmacy CIVAS, once established, should be available 24 hours a day, including weekends and public holidays
 There is a potential risk of de-skilling ward staff in the preparation of drug infusions
There is a potential risk of de-skilling ward staff in the preparation of drug infusions
 Some wards and departments may be difficult to service, e.g. accident and emergency, because doses of some drugs may be required urgently
Some wards and departments may be difficult to service, e.g. accident and emergency, because doses of some drugs may be required urgently
 The capacity of the CIVAS is not infinite but there will always be pressures to add new drugs to the service as soon as these enter clinical use.
The capacity of the CIVAS is not infinite but there will always be pressures to add new drugs to the service as soon as these enter clinical use.
Many of these problems can be overcome through good communication, both within the pharmacy department and with medical and nursing staff. A service level agreement is a useful device for ensuring that all stakeholders know their responsibilities and that the service operates within the confines of the available resources.
Quality assurance
All procedures used during preparation of CIVAS doses must be fully validated and documented. Procedures must also be audited and be subject to in-process monitoring. Staff preparing IV products should complete appropriate batch documents and adhere to authorized SOPs and published guidelines. As with the chemotherapy preparation service described previously, there are various elements to be considered as part of the decision-making process for the release of CIVAS medicines for administration to patients. Clean room over-pressures should be recorded at least daily, and HEPA filter integrity checks should be carried out at least on an annual basis or in the event of any deviation from defined operating conditions.
Records will be kept for all IV medicines prepared and will include the batch numbers of products used during reconstitution procedures. This ensures that in the event of a product recall or any problems with an IV medicine, a full audit trail documenting all aspects of the process can be reviewed.
Infusion stability and shelf-life assignment
The assignment of a shelf-life or expiry date to any aseptically prepared medicine is a rigorous, evidence-based process which requires expert interpretation of physical and chemical stability data and a clear understanding of the level of protection afforded to prevent microbiological contamination during the aseptic preparation process.
In the UK, aseptic medicines prepared requiring pharmacist supervision of the process, are restricted to a maximum shelf-life of 7 days, and then only if there is evidence to support this. On the other hand, aseptic medicines made under a manufacturer’s ‘specials’ licence issued by the MHRA can be assigned any reasonable shelf-life providing this is supported by rigorous evidence.
Stability data for CIVAS infusions, including cytotoxic drugs, can be sourced from a number of reference books. In many cases, it will be necessary to search the scientific and professional literature for original stability study reports and, on some occasions, the drug manufacturer may be willing to share extended stability data. Whatever the source of information, stability data should always be subjected to critical appraisal before it is used in the assignment of infusion shelf-lives.
Assessment of infusion stability is a key responsibility of any pharmacist managing a CIVAS. In the case of infusions which are outsourced from commercial compounding units, it is essential that clear and robust evidence is available to support the assigned shelf-life. Care must be exercised when attempting to extrapolate published stability data to infusions with variations in the concentration, diluents or container used. Expert review and a written justification are required to validate a shelf-life where there is any variation to the conditions under which the stability data were obtained.
Consideration must also be given to the transportation and storage of aseptically prepared medicines. Cold chain transport systems must be fully validated to ensure that the stability of infusions is not compromised and the storage temperatures should be monitored at least daily, but preferably by continuous monitoring and data logging.
Key Points
 Cancer chemotherapy can include treatment with cytotoxic drugs and biological ‘targeted’ therapies
Cancer chemotherapy can include treatment with cytotoxic drugs and biological ‘targeted’ therapies
 There are significant occupational exposure risks to healthcare workers who handle cytotoxic drugs
There are significant occupational exposure risks to healthcare workers who handle cytotoxic drugs
 Compliance with safe handling guidelines, combined with use of protective equipment, containment facilities and validated technique can minimize unplanned cytotoxic exposure
Compliance with safe handling guidelines, combined with use of protective equipment, containment facilities and validated technique can minimize unplanned cytotoxic exposure
 Use of strict aseptic conditions minimizes the risk of microbiological contamination during preparation
Use of strict aseptic conditions minimizes the risk of microbiological contamination during preparation
 Detailed SOPs for preparation and administration of chemotherapy must be prepared and implemented
Detailed SOPs for preparation and administration of chemotherapy must be prepared and implemented
 All personnel involved in provision of a centralized cytotoxic reconstitutions service must be trained and validated in all relevant procedures and techniques
All personnel involved in provision of a centralized cytotoxic reconstitutions service must be trained and validated in all relevant procedures and techniques
 Transfer of solutions between cytotoxic drug vials and syringes must be carried out using a hydrophobic venting pin or a purpose designed transfer device to avoid the generation of aerosols
Transfer of solutions between cytotoxic drug vials and syringes must be carried out using a hydrophobic venting pin or a purpose designed transfer device to avoid the generation of aerosols
 Drug stability is a key issue in assigning infusion shelf-lives, particularly those used for home infusion
Drug stability is a key issue in assigning infusion shelf-lives, particularly those used for home infusion
 Increased use of chemotherapy outpatient or day-case clinics can place significant pressures on the workload of the pharmacy chemotherapy unit
Increased use of chemotherapy outpatient or day-case clinics can place significant pressures on the workload of the pharmacy chemotherapy unit
 New strategies such as ‘dose-banding’ are useful to manage chemotherapy workload
New strategies such as ‘dose-banding’ are useful to manage chemotherapy workload
 A CIVAS can provide a wide range of aseptically prepared medicines for hospital and domiciliary use
A CIVAS can provide a wide range of aseptically prepared medicines for hospital and domiciliary use
 Potential benefits of CIVAS include reduced risk of medication errors, improved use of resources, better services to patients and pharmacy control
Potential benefits of CIVAS include reduced risk of medication errors, improved use of resources, better services to patients and pharmacy control
 Most doses are provided from CIVAS as either pre-filled syringes or minibags
Most doses are provided from CIVAS as either pre-filled syringes or minibags